Abstract
Background
Dupuytren's disease is a benign fibroproliferative disorder that causes the fingers to be drawn into the palm via formation of new tissue under the glabrous skin of the hand. This disorder causes functional limitations, but it can be treated through a variety of surgical techniques. As a chronic condition, it tends to recur.
Objectives
To assess the benefits and harms of different surgical procedures for treatment of Dupuytren's contracture of the index, middle, ring and little fingers.
Search methods
We initially searched the following databases on 17 September 2012, then re‐searched them on 10 March 2014 and on 20 May 2015: the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library, the British Nursing Index and Archive (BNI), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE, the Latin American Caribbean Health Sciences Literature (LILACS), Ovid MEDLINE, Ovid MEDLINE‐In‐Process and Other Non‐Indexed Citations, ProQuest (ABI/INFORM Global and Dissertations & Theses), the Institute for Scientific Information (ISI) Web of Science and clinicaltrials.gov. We reviewed the reference lists of short‐listed articles to identify additional suitable studies.
Selection criteria
We included randomised clinical trials and controlled clinical trials in which groups received surgical intervention for Dupuytren's disease of the index, middle, ring or little finger versus control, or versus another intervention (surgical or otherwise). We excluded the thumb, as cords form on the radial aspect of the thumb and thus are not readily accessible in terms of angular deformity. Furthermore, thumb disease is rare.
Data collection and analysis
A minimum of two review authors independently reviewed search results to select studies for inclusion by using pre‐specified criteria, assessed risk of bias of included studies and extracted data from included studies.
We grouped outcomes into the following categories: (1) hand function, (2) other patient‐reported outcomes (e.g. satisfaction, pain), (3) early objective outcomes (e.g. correction of angular deformity), (4) late objective outcomes (e.g. recurrence) and (5) adverse effects.
Main results
We included 14 articles describing 13 studies, comprising 11 single‐centre studies and two multi‐centre studies. These studies involved 944 hands of 940 participants; of these, 93 participants were reported twice in separate articles describing early and late outcomes of one trial. Three papers reported the outcomes of two trials comparing different procedures. One trial compared needle fasciotomy versus fasciectomy (125 hands, 121 participants), and the other compared interposition firebreak skin grafting versus z‐plasty closure of fasciectomy (79 participants). The other 11 studies reported trials of technical refinements of procedures or rehabilitation adjuncts. Of these, three investigated effects of postoperative splinting on surgical outcomes.
Ten studies (11 articles) were randomised controlled trials (RCTs) of varying methodological quality; one was a controlled clinical trial. Trial design was unclear in two studies awaiting classification. All trials had high or unclear risk of at least one type of bias. High risks of performance and detection bias were particularly common. We downgraded the quality of evidence (Grades of Recommendation, Assessment, Development and Evaluation ‐ GRADE) of outcomes to low because of concerns about risk of bias and imprecision.
Outcomes measured varied between studies. Five articles assessed recurrence; two defined this as reappearance of palpable disease and two as deterioration in angular deformity; one did not explicitly define recurrence.
Hand function on the Disabilities of the Arm, Shoulder and Hand (DASH) Scale (scores between 0 and 100, with higher scores indicating greater impairment) was 5 points lower after needle fasciotomy than after fasciectomy at five weeks. Patient satisfaction was better after fasciotomy at six weeks, but the magnitude of effect was not specified. Fasciectomy improved contractures more effectively in severe disease: Mean percentage reduction in total passive extension deficit at six weeks for Tubiana grades I and II was 11% lower after needle fasciotomy than after fasciectomy, whereas for grades III and IV disease, it was 29% and 32% lower.
Paraesthesia (defined as subjective tingling sensation without objective evidence of altered sensation) was more common than needle fasciotomy at one week after fasciectomy (228/1000 vs 67/1000), but reporting of complications was variable.
By five years, satisfaction (on a scale from 0 to 10, with higher scores showing greater satisfaction) was 2.1/10 points higher in the fasciectomy group than in the fasciotomy group, and recurrence was greater after fasciotomy (849/1000 vs 209/1000). Firebreak skin grafting did not improve outcomes more than fasciectomy alone, although this procedure took longer to perform.
One trial investigated four weeks of day and night splinting followed by two months of night splinting after surgery. The other two trials investigated three months of night splinting after surgery, but participants in 'no splint' groups with early deterioration at one week were issued a splint for use. All three studies demonstrated no benefit from splinting. The two trials investigating postoperative night splinting were suitable for meta‐analysis, which demonstrated no benefit from splinting: Mean DASH score in the splint groups was 1.15 points lower (95% confidence interval (CI) ‐2.32 to 4.62) than in the no splint groups. Mean total active extension in the splint groups was 2.21 degrees greater (95% CI ‐3.59 to 8.01 degrees) than in the no splint groups. Mean total active flexion in the splint groups was 8.42 degrees less (95% CI 1.78 to 15.07 degrees) than in the no splint groups.
Authors' conclusions
Currently, insufficient evidence is available to show the relative superiority of different surgical procedures (needle fasciotomy vs fasciectomy, or interposition firebreak skin grafting vs z‐plasty closure of fasciectomy). Low‐quality evidence suggests that postoperative splinting may not improve outcomes and may impair outcomes by reducing active flexion. Further trials on this topic are urgently required.
Plain language summary
Surgery for Dupuytren's disease of the fingers
Review question
We conducted a review of the effects of surgery for people with Dupuytren’s disease of the fingers and found 13 studies with 940 participants; 93 participants were reported twice in separate articles describing early and late outcomes of one trial.
Background
Dupuytren's disease is common. Patients develop scar‐like tissue under the palmar skin of the hand that draws their fingers into the palm and can affect function.
This condition can be surgically treated by cutting out the disease, then stitching the skin back into place (fasciectomy) or replacing it with a graft of skin taken from elsewhere on the body (dermofasciectomy). Alternative approaches involve breaking the cord of disease to straighten the finger. This can be done by moving a needle back and forth through the cord until it snaps, as when rubbing a rope repeatedly over a rock (needle fasciotomy), or by injecting into it an enzyme that digests a piece of the cord (collagenase). This weakens one spot, allowing the surgeon to snap the cord and straighten the finger. As the condition is related in part to genetics, it tends to come back, even after successful treatment. As the latter two treatments leave the broken ends of the cord behind, recurrence may be quicker after these procedures than after traditional excisional surgery. However, recovery might also be quicker. The most effective treatment is unclear.
Study characteristics
After searching for all relevant studies up to May 2015, we found 13 studies (14 articles) that met our inclusion criteria. However, only three compared different operation types. The others compared aspects of one operation type. One study presented early and late outcomes.
Key results
What happens to people with Dupuytren’s disease up to five weeks after needle fasciotomy compared with fasciectomy?
• Hand function may be slightly better after needle fasciotomy than after fasciectomy (low‐quality evidence).
• People who have had needle fasciotomy may be more satisfied than those who have had fasciectomy (low‐quality evidence).
• Fasciectomy probably straightens fingers better than needle fasciotomy in people with advanced disease, but probably no difference is apparent in people with milder disease (low‐quality evidence).
• A feeling of tingling in the fingers is probably more common after fasciectomy than after needle fasciotomy during the first week after treatment (low‐quality evidence).
What happens to people with Dupuytren’s disease five years after needle fasciotomy compared with fasciectomy?
• Satisfaction may be better after fasciectomy than after needle fasciotomy (low‐quality evidence).
• Recurrence may be more common after needle fasciotomy than after fasciectomy (low‐quality evidence).
What happens to people with Dupuytren’s disease up to 36 months after z‐plasty closure of a limited fasciectomy compared with use of small ‘firebreak’ skin grafts (a form of dermofasciectomy)?
• Little or no difference in outcomes is likely between patients who had z‐plasty and those who had small skin grafts, although skin graft procedures take longer to perform (low‐quality evidence).
What happens to people with Dupuytren’s disease who wear a splint at night after surgery?
• Wearing a splint at night after surgery probably does not help to straighten fingers nor to improve hand function, and it may slightly worsen the patient's ability to make a full fist (low‐quality evidence).
Side effects in people with Dupuytren’s disease after surgery and in those who wear a splint at night after surgery
Reporting of complications was variable. We often do not have precise information about side effects and complications, particularly rare but serious side effects. Side effects may include altered feeling in the fingers or reduced ability to make a full fist. Rare complications may include injury to the tendons that pull the fingers into the palm.
Summary of findings
Background
Description of the condition
Dupuytren's disease may affect the hand and the sole of the foot. In the hand, it is characterised by slow but progressive fibroproliferative changes associated with the palmar aponeurosis, which lies beneath the skin of the palm, and its extensions into the fingers (Hurst 2000). Although it most commonly involves the ring and little fingers, this disorder can affect any digit. In early stages, nodules of Dupuytren's tissue are formed in association with palmar aponeurosis. These may coalesce to form cords of Dupuytren's tissue that run to the fingers. The cords may shorten and prevent full extension of the fingers, thus stopping patients from placing their hands flat on a surface. Patients may report difficulty in putting on gloves, washing their face or performing other dextrous tasks. If left untreated, this restriction of finger extension usually progresses, although the rate of progression is unpredictable. Changes are irreversible without treatment (Luck 1959). Loss of motion, particularly functional extension, results in activity limitations and motivates the patient to explore surgical options (Pratt 2009).
The prevalence of Dupuytren's disease varies with geographic location and patient sex and age. It is unusual among individuals younger than 50 years and is more common in men, although this sex difference may diminish with increasing age. Its prevalence is highest in men of Northern European origin, and in British men and women over the age of 75 years may be as high as 18% and 9%, respectively (Early 1962).
The aetiology of Dupuytren's disease is not fully understood. Higher prevalence amongst family members has been accepted for a long time (Yost 1955), and the disorder is associated with diabetes mellitus and smoking (Burge 1997). However, its proposed association with epilepsy is unclear (Geoghegan 2004), and its reported association with socio‐economic factors and manual work remains controversial (Early 1962; Herzog 1951).
Although typical patients experience slow progression of disease and respond well to intervention, some experience aggressive disease progression, often from an early age, and earlier recurrence ‐ a condition referred to as 'Dupuytren's diathesis' (Hueston 1963). Benefits of treatment may vary according to the anatomical location of Dupuytren's disease within the hand. One study reported more improved hand function when investigators compared proximal interphalangeal joint (PIPJ) treatment versus metacarpophalangeal joint (MCPJ) correction (Draviaraj 2004). However, achieving correction at the PIPJ is more difficult: Whereas MCPJ contractures usually can be fully corrected with surgery, PIPJ contractures frequently are incompletely corrected. Consequently, heterogeneity is evident in terms of disease presentation within the digits, response to surgery and functional benefit derived from surgery.
No cure is known for Dupuytren's disease; cure would require removal of Dupuytren's tissue from the palm of the hand and the flexor surfaces of digits, as well as inhibition of subsequent disease formation. Instead, the primary goal of treatment is to excise, divide, break or dissolve cords of Dupuytren's tissue that are preventing full finger extension, with the intention of improving or correcting finger contracture (loss of extension). However, as some cells that produce Dupuytren's tissue are inevitably left throughout the hand and within the region of the treated cord, Dupuytren's contractures can form later at other sites in the hand (disease extension), or a 'recurrent contracture' may develop within the operative site. Treatment usually is offered before the affected finger has contracted so far that hand function is significantly impaired, because small contractures that have developed recently have a better chance of correction than long‐standing severe contractures, which may have allowed secondary joint stiffness to develop in the underlying 'flexed' joints. A 30‐degree MCPJ contracture is often cited as a threshold for offering surgery (BSSH 2010). Such a figure may be chosen, as less significant contractures might not be expected to cause functional impairment, and because some believe that surgery itself might stimulate disease progression (Bisson 2003), although this theory has not been proved scientifically.
Non‐operative strategies include radiotherapy, physical therapy (typically involving splinting) and ultrasonography. The value of radiotherapy for established contractures is uncertain, and outcomes of splinting and ultrasonography are variable (Ball 2002; Stiles 1966). A novel treatment approach consists of injecting collagenase into Dupuytren's cords, causing finger contracture (Hurst 2009). Collagenase synthesised by Clostridium histolyticum degrades collagen within Dupuytren's cords, thus weakening them, so they can be broken by forced extension of the affected finger on the following day. The Food and Drug Administration (FDA) has approved this treatment for use in the United States (FDA 2010), and it is now licenced for use in the European Union, although its effectiveness has not been fully evaluated (Thomas 2010). This topic will be considered in a separate review.
Currently, the mainstay of treatment for Dupuytren's contracture is surgery, and many surgical options for Dupuytren's disease are available, beginning with Baron Dupuytren's description of surgical release of the contracture, performed without anaesthesia in 1831 (Elliot 1999). Common management strategies are presented here by extent of surgery, starting with the least invasive approach. Relative benefits and disadvantages are summarised.
Description of the intervention
Observation
Treatment is not mandatory, and after informed discussion of the natural history of the condition and different treatment options, a patient may elect observation of the hand. Patients with mild disease and no functional impairment may also be observed. A subgroup, labelled "non‐Dupuytren's disease" by one team of authors, may not experience disease progression (Rayan 2005). Observation may be encountered as a comparator intervention rather than as an experimental intervention.
Needle fasciotomy (aponeurotomy)
This involves blind division of the contracture with a hypodermic needle (usually 25‐gauge). This concept dates back to the time of Dupuytren himself, and it has experienced a resurgence in popularity since the 1990s (Badois 1993). Benefits of this procedure include that it can be performed in clinic on an outpatient basis, and so it may be cost‐effective, and it may have a good rapid recovery rate. Disadvantages include that needle fasciotomy may have a significant recurrence rate of 75% or more at five years (van Rijssen 2006a; van Rijssen 2012a), and the procedure carries risks of tendon and digital nerve and artery damage. Although most surgeons agree that this procedure has a role in managing Dupuytren's disease causing contracture of the MCPJ, it is less popular for the treatment of Dupuytren's cords that are causing contracture of the PIPJ, because of associated risk of damage to the digital nerves and flexor tendons in the finger and inability to reliably release contractures of the PIPJ. A six‐week follow‐up study suggested that it might be a reasonable alternative to limited fasciectomy in the short‐term management of selected cases such as those involving elderly patients, with acceptance of significantly higher recurrence after needle fasciotomy compared with fasciectomy and the tendency for quicker recurrence. This was seen in five‐year follow‐up data from the same study (van Rijssen 2012a).
Very limited fasciectomy (segmental aponeurectomy)
Small incisions are made over the portions of the Dupuytren's cord that are causing the contracture, and segments are excised so that the finger straightens (Moermans 1991). No attempt is made to remove all of the cord causing the contracture. Benefits of this procedure are that it is relatively less invasive and involves a quick (two‐week or three‐week) recovery period. However, it is performed in an operating theatre and is thought to be associated with a high rate of recurrence of Dupuytren's contracture ‐ up to 38% (Moermans 1996) ‐ which may occur because significant deposits of Dupuytren's tissue persist in the hand and the finger. Although most surgeons agree that this procedure has a role in Dupuytren's disease in the palm of the hand that is causing contracture of the MCPJ, it is less popular for treatment of cords in the finger itself, which cause contracture of the PIPJ, because it introduces risk of damage to the digital nerves and the inability to reliably release contracture of the PIPJ.
Limited fasciectomy
Through this procedure, the surgeon aims to remove all of the Dupuytren's cord that is causing the finger contracture. Limited fasciectomy has been the most popular treatment for Dupuytren's disease in the recent past, but it carries a significant recurrence rate and involves a relatively long rehabilitation phase (four to six weeks). Furthermore, it carries a small, although significant, risk of complications such as diffuse finger stiffness, which may involve not only the operated finger but the other fingers of the hand as well. Recurrence following limited fasciectomy may exceed 20% at five years (van Rijssen 2012a), possibly because disease‐forming cells retained in the subcutaneous fat and skin may form 'recurrent' contractures.
Dermofasciectomy
This is a more extensive procedure in which all of the Dupuytren's cord causing the contracture is excised. In addition, all subcutaneous fat and skin on the palmar aspect of the proximal and middle pulp spaces of the finger (overlying the cord) are excised, leaving only the flexor tendon sheath and the two neurovascular bundles. The resultant skin defect is covered with a full‐thickness skin graft, which usually is harvested from the medial border of the forearm or upper arm, the front of the elbow or the ulnar aspect of the hand. Proponents of this procedure claim that through excision of skin and subcutaneous fat that may be involved in Dupuytren's disease, the rate of recurrence of a Dupuytren's contracture is reduced (Armstrong 2000). Many surgeons selectively use this procedure in young patients and in those with the 'diathesis', in whom risk of recurrence in later life is high. Specific disadvantages of dermofasciectomy include a longer rehabilitation phase and the need to harvest a skin graft. Complications include loss of the skin graft and, as for limited fasciectomy, the possibility of finger stiffness and complex regional pain syndrome.
How the intervention might work
Surgery, consisting of excision or division of Dupuytren's cord, should allow immediate full extension of affected joints, as long as the underlying joint has not developed a fixed flexion deformity for other reasons (e.g. collateral ligament contraction, checkrein ligament shortening, arthritis).
Why it is important to do this review
Comparative analysis of the outcomes of different surgical treatment options for Dupuytren's disease is needed to investigate whether more invasive procedures, such as dermofasciectomy, have lower 'recurrent contracture' rates, and whether any such benefit is outweighed by a higher rate of adverse events (complications) or an unacceptably longer or more difficult rehabilitation period. Although comparison of different operative techniques is important, it must be recognised that surgery is only part of a complex intervention needed for the treatment of Dupuytren's contracture. The outcome of treatment may not be determined only by which type of surgery is performed, but also by the postoperative rehabilitation regimen provided (splintage and hand therapy) and other treatment factors such as patient selection and site of contracture (MCPJ alone, PIPJ alone or both joints together). Also the outcome of Dupuytren's surgery is usually defined by the 'recurrent contracture rate' (in contrast to 'disease extension' to other digits, the rate of which is not affected by surgery). Only a few studies have assessed outcomes by using patient‐centred outcome tools, or have investigated the severity and length of postoperative recovery from surgery.
Objectives
To assess the benefits and harms of different surgical procedures for treatment of Dupuytren's contracture of the index, middle, ring and little fingers.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs), irrespective of language or sample size.
Types of participants
Adult men and women from all ethnic origins, with or without risk factors for Dupuytren's disease, who had undergone a surgical procedure for primary (not recurrent) Dupuytren's contracture of one or more of the index, middle, ring and little fingers.
Types of interventions
Any surgical intervention, including percutaneous needle fasciotomy (aponeurotomy), very limited fasciectomy, limited fasciectomy and dermofasciectomy. Comparators included alternative surgical procedures, placebo/sham surgery and other active non‐surgical treatments (collagenase injection, hand therapy, physiotherapy, radiotherapy). We did not anticipate studies undertaken to compare active treatment versus observation alone. If we identified such studies, we planned to discuss them.
Types of outcome measures
The validity and reliability of any outcome measures commonly used in Dupuytren's disease have not been well studied. We have listed below outcomes expected to be reported by study investigators. We selected hand function as the top primary outcome, as this represents an important patient‐centred measure. In contrast, angular measurements are objective, surgeon‐centred measurements.
Major outcomes
Level of hand function restored, as assessed by the Disabilities of the Arm, Shoulder and Hand (DASH) Scale (Hudak 1996), the Patient Evaluation Measure (PEM) (Macey 1995), grip strength measures or the Jebsen‐Taylor Hand Function Test (Jebsen 1969). We were uncertain about which standardised outcome instruments we would encounter, but we found that all were reported.
Patient satisfaction and other patient‐rated outcomes (such as pain or health‐related quality of life (HRQoL)). We will report all measures encountered.
Early angle outcomes and other objective outcomes. These may involve (1) improvement in contracture immediately after surgery ‐ differences between finger angle measurements immediately after surgery and preoperative finger angle measurements, (2) residual contracture immediately after surgery ‐ as assessed by angle measurement (goniometry) or (3) early results (as above) at time of discharge from care. Active or passive angles may be reported. Angles may be presented per joint, or per ray.
Recurrence of Dupuytren's disease/contracture in the operated field. As recurrence is time‐dependent, length of follow‐up is not standardised and a universally agreed upon definition of recurrence is not available, we have described recurrence rates and length of follow‐up for each study in narrative format. We planned to perform time‐to‐event analyses when we found appropriate data. However, we did not expect that these data would be available. We would have performed meta‐analyses only for studies with similar definitions of recurrence and providing recurrence data at similar follow‐up times after surgery ('similar definitions of recurrence' would include those with recurrence involving a 20‐degree to 30‐degree increase in angle compared with early discharge data or preoperative data). Minimum length of follow‐up for eligibility in this analysis was 18 months. This was decided on the basis of two considerations: Shorter follow‐up gives insufficient time for recurrence; and no consensus has been reached to define minimum length of follow‐up, which varies widely in published studies, ranging from three weeks to 13 years (Becker 2010).
Adverse effects. Those anticipated included loss of finger flexion, loss of finger sensation due to digital nerve injury, vascular compromise, delayed healing and infection. As the extent of reported adverse events was unknown, we collected and reviewed total adverse effects data. Review authors agreed to focus on five key adverse events, should these prove to be extensive.
Minor outcomes
Economic costs of intervention. When provided, we would assess these costs as total documented costs of the procedure and rehabilitation. When time to recurrence was documented, we would calculate cost per year of recurrence‐free survival. However, we anticipated that these data would not be commonly available.
Search methods for identification of studies
We performed all searches on 17 September 2012. We re‐ran searches on 10 March 2014, and again on 20 May 2015, and we updated the results.
Electronic searches
We searched the following electronic databases to find reports of relevant RCTs and CCTs:
The Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 8).
British Nursing Index and Archive (BNI) ‐ 1985 to September 2012.
Cumulative Index to Nursing and Allied Health Literature (CINAHL) ‐ 1981 to September 2012.
EMBASE ‐ 1980 to September 2012.
Latin American Caribbean Health Sciences Literature (LILACS) ‐ 1982 to September 2012.
Ovid MEDLINE ‐ 1948 to September 2012.
Ovid MEDLINE‐In‐Process and Other Non‐Indexed Citations ‐ 1948 to September 2012.
ProQuest (ABI/INFORM Global and Dissertations & Theses) ‐ all entries to September 2012.
Institute for Scientific Information (ISI) Web of Science.
clinicaltrials.gov.
We provided the full search strategy for CENTRAL in Appendix 1.
We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for Identifying Randomised Trials in MEDLINE: Sensitivity‐ and Precision‐Maximizing Version (2008 revision) (Lefebvre 2011): Ovid format (see Appendix 2 for the full strategy). We combined the EMBASE (Appendix 3) and CINAHL (Appendix 4) searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2011), and we applied no restrictions on the basis of language nor date of publication.
We used variations of the Ovid MEDLINE search strategy to search the other databases listed above (Appendix 5; Appendix 6; Appendix 7; Appendix 8).
Searching other resources
We reviewed the reference lists of short‐listed articles to identify additional suitable studies, and we searched Web of Science to identify studies that cited the items in the short list. We applied no language restrictions, and we translated potentially eligible foreign language studies.
Data collection and analysis
Selection of studies
From the title, abstract or descriptor, two review authors (JR, GB) independently screened all abstracts to identify potential studies for review, using a checklist of the criteria for inclusion (see Criteria for considering studies for this review and Appendix 9). The two review authors compared their lists of potential studies and produced an agreed upon short list. We obtained copies of the full articles of papers on the agreed upon short list.
Two review authors (JR, GB) independently reviewed the full text of abstracts of the 'agreed short list' papers and identified those suitable for inclusion, using the selection checklist (Criteria for considering studies for this review). We resolved disagreements by discussion and by referral to a third review author (TD). We did not mask titles of journals nor names of study authors and supporting institutions.
Data extraction and management
Two review authors (JR, CB) independently extracted data regarding source, study design, intervention, population and outcomes using a piloted form. We resolved disagreements by consensus after additional review by a third review author (TD).
Assessment of risk of bias in included studies
Two review authors (CB, JR) independently used the tool for assessing risk of bias developed by The Cochrane Collaboration (Higgins 2011). We assessed all seven domains (sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other issues) of this tool by allowing classification of domains into 'high risk of bias', 'low risk of bias' or 'unclear risk of bias' (see Appendix 10). We judged by outcome when most of the seven domains were deemed to provide high risk or low risk. We resolved disagreements by discussion and by referral to a third review author (TD). As we had anticipated that few studies might employ blinding, we assessed use of blocked randomisation in unblinded studies as a source of 'other' bias. In blocked randomisation, investigators use sequences of allocation assignment to balance enrolment between trial arms. In an unblinded study, this could contribute to risk of bias similar to that seen with poor allocation concealment. We assessed the quality of evidence by using Grades of Recommendation, Assessment, Development and Evaluation (GRADE) criteria, while taking into account risks of bias.
Measures of treatment effect
If appropriate, we had planned to use standardised mean differences (SMDs) to combine different outcome measures from different trials (Hedges 1982).
In studies that reported dichotomous data, we planned to calculate risk ratios (RRs) with 95% confidence intervals (CIs). For rare events (< 10%), we planned to calculate Peto odds ratios with 95% CIs. We planned to combine results for meta‐analysis using fixed‐effect or random‐effects models, depending on heterogeneity (see Data synthesis).
Unit of analysis issues
Review authors expected that most studies would use the hand as the unit of randomisation. Assessing outcomes such as hand function would not be possible if individual fingers from the same hand were used as the unit of randomisation. We recorded the unit of randomisation (participant, hand, finger or unclear) for each included study.
We did not expect to identify any cross‐over studies, given that the interventions described here are single‐stage definitive treatments. We expected to find no cluster‐randomised studies.
Dealing with missing data
We anticipated two types of missing data: unreported and withdrawn. If data were not reported in included trials, we contacted study authors to request assistance. We planned to attempt no imputation.
Assessment of heterogeneity
If appropriate, we planned to test statistical heterogeneity by visually inspecting graphs and by performing Chi2 and I2 statistical tests. We considered a Chi2 test result with P value < 0.10 to be significant. We classified an I2 test result greater than 50% as showing substantial heterogeneity.
Assessment of reporting biases
To reduce the risk of reporting bias, we searched multiple sources, including ProQuest (ABI/INFORM Global and Dissertations & Theses), to identify all published and unpublished results.
We drew funnel plots to assess risk of publication bias.
We searched ISI Web of Science to identify relevant results that had not been published. If we identified such work, we planned to contact study authors to ask for a copy of the data.
Data synthesis
We compared data from selected studies by using the statistical software of The Cochrane Collaboration, Review Manager (Review Manager 2011). If studies were sufficiently similar, we planned to undertake a meta‐analysis. If we needed to perform meta‐analyses, we planned to use the random‐effects model.
When the same outcome measures were assessed with different scales, we would use SMDs.
However, we anticipated that data from different studies would be difficult to compare, and that a meta‐analysis might be inappropriate. This would be the case particularly for the main outcome of 'recurrence' because of:
differences in length of follow‐up (recurrence rate increases with length of follow‐up); and
differences in the definition of 'recurrence'.
We would use a fixed‐effect model to combine data if outcomes were homogeneous. If results were heterogeneous, we would undertake subgroup analysis to identify the reasons for heterogeneity. We would apply the random‐effects model if we could find no reason for heterogeneity.
If the nature of the included studies did not allow for statistical analysis, we would use narrative (qualitative) summaries to present study results.
Subgroup analysis and investigation of heterogeneity
If we found significant heterogeneity, we would not pool the data and we would present a summary of methodological quality and study results. We would consider reasons for heterogeneity by performing subgroup analysis with regard to:
length of time to follow‐up;
PIPJ and MCPJ outcomes separately, as it is well recognised that MCPJ contractures correct better than PIPJ contractures;
severity of disease before operation (when provided, we expect that this will be given in the form of total passive extension deficit (i.e. sum of the passive extension deficit at the MCPJ and the PIPJ);
number of joints involved; and
postoperative treatment offered.
Sensitivity analysis
Outcome measures (e.g. the definition of recurrence) have been explained differently (Becker 2010). If appropriate, we would perform a sensitivity analysis to examine whether results vary according to different definitions used.
We would undertake sensitivity analysis in cases of missing data (e.g. intention‐to‐treat vs per‐protocol analysis) to examine variations between approaches to analysis.
Results
Description of studies
Results of the search
The search yielded 2464 references (see Figure 1). We removed 103 duplicates before screening abstracts.
1.
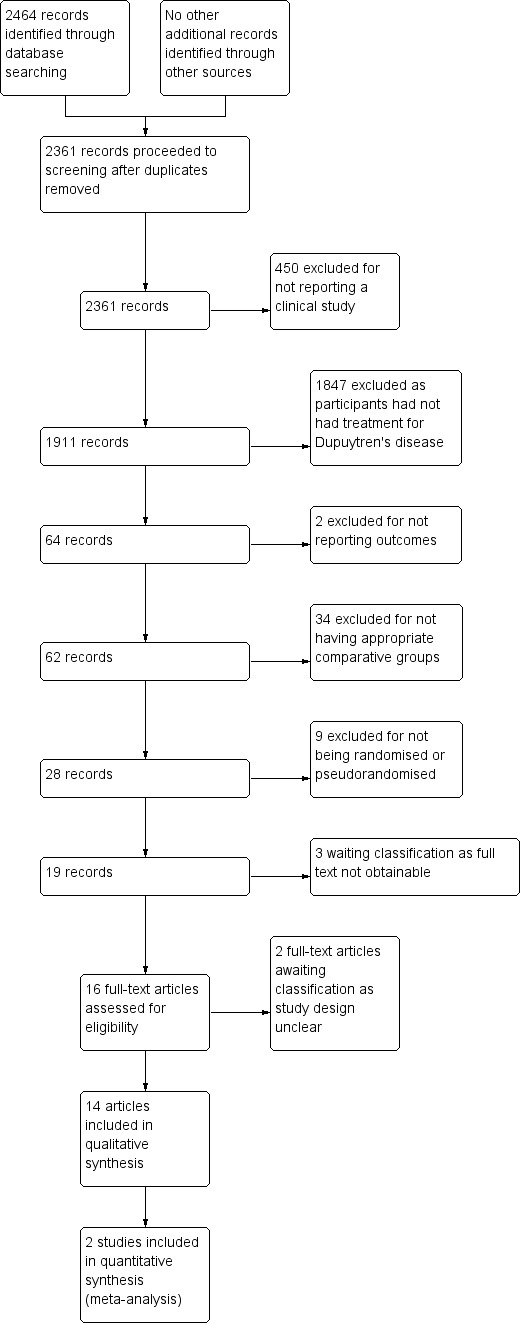
Study flow diagram.
Of the remaining 2361 studies, we reviewed 16 full‐text articles. We could not classify two because the full‐text article did not adequately describe the study design, and it was not clear if studies were randomised or pseudorandomised (Hazarika 1979; Ward 1976). Both articles are over 35 years old, and we could not obtain clarification from study authors.
We included 14 full‐text articles in the review (see Characteristics of included studies and Bhatia 2002; Bulstrode 2004; Chignon‐Sicard 2012; Citron 2003; Citron 2005; Collis 2013; Degreef 2014; Howard 2009; Jerosch‐Herold 2011; Kemler 2012; McMillan 2012; Ullah 2009; van Rijssen 2006; van Rijssen 2012a), and we excluded 2264 studies. These 14 articles described 13 studies, with one study described in both an early outcome paper (van Rijssen 2006) and a late outcome paper (van Rijssen 2012a).
Included studies
Eleven studies were single‐centre studies; seven of these were based in the United Kingdom and one each in Canada, France, Belgium and New Zealand. Two articles were reports of one trial based in the Netherlands. We identified two multi‐centre studies (Jerosch‐Herold 2011; Kemler 2012). All five centres in Jerosch‐Herold 2011 were located in the UK. Both centres in Kemler 2012 were located in the Netherlands. All studies were published in English. One study (Jerosch‐Herold 2011) had an associated publication, which presented the trial protocol (Jerosch‐Herold 2008).
The 14 studies included 940 participants; the 93 reported in van Rijssen 2012a were the same as those described in van Rijssen 2006. Thus 847 individual participants were recruited across all studies.
Interventions studied
We identified no articles in which investigators compared surgery versus observation.
Three articles described the outcomes of two trials that compared different surgical procedures (Ullah 2009; van Rijssen 2006; van Rijssen 2012a). One trial compared use of firebreak full‐thickness skin grafts (a type of dermofasciectomy) versus z‐plasty closure of a limited fasciectomy. Study authors refer to the original description of firebreak grafts in Hueston 1984. Here, firebreak grafts were described as small grafts strategically placed at flexion creases. In contrast, traditional dermofasciectomy may involve resurfacing of much larger areas of palmar skin (Seah 2012), which was achieved by conducting a limited fasciectomy, then excising palmar skin to accommodate the skin graft among those randomly assigned to this cohort. The other two articles reported early and late outcomes, respectively, for a single trial comparing needle fasciotomy versus limited fasciectomy.
Four of the other eleven articles compared surgical incision and wound management options (Bhatia 2002; Citron 2003; Citron 2005; Howard 2009). Bhatia 2002 and Howard 2009 compared staple closure against suture closure, and absorbable versus non‐absorbable suture closures, respectively ‐ both in limited fasciectomy. Citron 2003 and Citron 2005 studied types of incisions used for limited fasciectomy.
Three publications studied adjunctive treatments to surgery: One investigated bathing the operation site in 5‐fluorouracil versus saline before closure (Bulstrode 2004), one compared use of steroid injections in conjunction with needle fasciotomy versus no adjunctive treatment (McMillan 2012) and the other compared tamoxifen versus placebo as neoadjuvant treatment in conjunction with fasciectomy (Degreef 2014).
The other four trials studied non‐invasive adjuncts to surgery: Collis 2013,Jerosch‐Herold 2011 and Kemler 2012 studied use of postoperative splints versus no splints, and Chignon‐Sicard 2012 investigated use of a fibrin‐ and platelet‐rich fibrin plug as a primary dressing versus a conventional low‐adherence dressing for open palm surgery.
These different interventions can be used to classify studies into:
those studying different treatment options;
those refining a treatment option (e.g. limited fasciectomy incisions, closure types, invasive adjuncts, equipment usage); and
those refining rehabilitation.
The first group comprises Ullah 2009, van Rijssen 2006 and van Rijssen 2012a, three other articles describe refining rehabilitation (Collis 2013; Jerosch‐Herold 2011; Kemler 2012) and the remaining eight explore ways to refine intraoperative techniques.
Inclusion and exclusion criteria
Criteria were not always specified. Two articles did not provide inclusion and exclusion criteria (Bhatia 2002; Howard 2009). Of the other 12 studies, four specified age‐related cutoffs for recruitment: younger than 70 years (Bulstrode 2004) and over 18 years of age (Chignon‐Sicard 2012; Jerosch‐Herold 2011; Kemler 2012). One study did not describe the ratio of participant genders (Howard 2009). None of the others explicitly excluded potential participants on the basis of gender, although one comprised only male participants (Bulstrode 2004).
Four studies excluded patients undergoing revision surgery (Citron 2003; Citron 2005; Degreef 2014; McMillan 2012). Of these, one study also excluded patients who had previously undergone other types of hand surgery (McMillan 2012).
Three studies specified site of disease within the hand. Citron 2003 included only patients with palmar disease affecting the MCPJ. In contrast, Ullah 2009 included only those with 30 or more degrees of contracture at the PIPJ. Jerosch‐Herold 2011 excluded thumb and first webspace treatments. Citron 2005 recruited participants with Dupuytren's disease in one ray only.
Some studies used exclusion criteria related to co‐morbidities that might influence outcome: Citron 2005,Ullah 2009,van Rijssen 2006 and van Rijssen 2012a excluded patients with bleeding tendencies. Diabetes mellitus was an exclusion criterion in Chignon‐Sicard 2012 and McMillan 2012.
Some specific criteria were related to study design. For example, in Bulstrode 2004, participants had to receive treatment for two rays in one procedure, as one was randomly assigned to receive 5‐fluorouracil, and the other to receive the control treatment of normal saline. In van Rijssen 2006 (and therefore van Rijssen 2012a), participants had to have well‐defined cords of disease. This is a requirement for suitability for needle fasciotomy. Degreef 2014 excluded premenopausal women, patients taking anti‐inflammatory drugs, those with a history of malignancy and patients with known allergy to tamoxifen.
Unit of analysis
The predicted unit of analysis was that randomisation would be performed by 'hand'. This could lead to enrolment of the same patient twice for surgery to each hand on separate occasions. van Rijssen 2006 and van Rijssen 2012a included four such cases. In contrast, in McMillan 2012, one participant with bilateral disease was entered only once in the trial. Similarly, in Citron 2005, six participants presented for randomisation twice for treatment of bilateral disease. They were enrolled only once in the trial. In the latter studies, the unit of randomisation was the 'participant'. Other studies reported specific individualised methods. Bulstrode 2004 used an internal control, with one digit on a hand randomly assigned to treatment, and another to control.
In terms of reporting recurrence, van Rijssen 2012a presented the number of hands and the number of participants who had developed deformity greater than 20 degrees in one joint. Other studies presented recurrence per participant (Citron 2003; Citron 2005; Degreef 2014). The only other study investigating recurrence was Ullah 2009, in which recurrence was described as the percentage of fingers that showed recurrence, rather than as the proportion of hands or participants.
Outcome measures
Outcomes measured varied between studies (Table 5). Specific outcomes were used for particular studies.
1. Outcomes measured and length of study follow‐up.
| Article | Aspect of care studied | Length of follow‐up, months | Outcomes measured | ||||||||
| Recurrence | Extension deficit | Flexion deficit | Total motion | PROM | Time | Complications as an outcome measure | Hand volume | Other | |||
| Bhatia 2002 | Technical refinement | 0.5 | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | Wound appearance |
| Bulstrode 2004 | Technical refinement | 18 | ‐ | + | ‐ | + | ‐ | + | ‐ | ‐ | ‐ |
| Chignon‐Sicard 2012 | Rehabilitation adjunct | 2 | ‐ | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ |
| Citron 2003 | Technical refinement | 24 | + | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Citron 2005 | Technical refinement | 24 | + | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Collis 2013 | Rehabilitation adjunct | 3 | ‐ | + | + | ‐ | + | ‐ | ‐ | ‐ | Grip strength, composite flexion |
| Degreef 2014 | Technical refinement | 24 | + | + | ‐ | ‐ | + | ‐ | + | ‐ | ‐ |
| Howard 2009 | Technical refinement | 0.5 | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | ‐ |
| Jerosch‐Herold 2011 | Rehabilitation adjunct | 12 | ‐ | + | + | + | + | ‐ | ‐ | ‐ | ‐ |
| Kemler 2012 | Rehabilitation adjunct | 12 | ‐ | + | ‐ | ‐ | + | ‐ | + | ‐ | ‐ |
| McMillan 2012 | Technical refinement | 6 | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ullah 2009 | Procedure type | 36 | + | + | ‐ | + | + | + | ‐ | ‐ | Grip strength |
| van Rijssen 2006 | Procedure type | 1.5 | ‐ | + | + | ‐ | + | ‐ | + | ‐ | ‐ |
| van Rijssen 2012a | Procedure type | 60 | + | + | ‐ | ‐ | + | ‐ | ‐ | ‐ | ‐ |
Length of follow‐up varied between papers. Those investigating rehabilitation and early recovery varied from two‐week follow‐up (Bhatia 2002; Howard 2009) to six‐week follow‐up (van Rijssen 2006). Late outcome papers varied in length of follow‐up from two years (Citron 2003; Citron 2005; Degreef 2014) to five years (van Rijssen 2012a).
Hand function
Several trials presented patient‐reported outcomes. These included previously published patient‐reported outcome measures (PROMs) such as DASH scale scores (Collis 2013; Degreef 2014; Jerosch‐Herold 2011; van Rijssen 2006; van Rijssen 2012a) or the PEM (Ullah 2009). The design of studies such as Bulstrode 2004, with two digits on the same hand randomly assigned to different groups, would have prevented meaningful interpretation of patient‐reported outcomes such as hand function.
Patient satisfaction and other patient‐rated outcomes
Studies comparing procedure types (van Rijssen 2006; van Rijssen 2012a) reported patient satisfaction. However, although statistical significance was presented, investigators did not present full data. Furthermore, they did not describe or reference the development, validity and reliability of tools used to assess satisfaction. Collis 2013, Degreef 2014 and Jerosch‐Herold 2011 also assessed satisfaction. Kemler 2012 assessed patient‐perceived change. Some studies included self reported pain assessed by a visual analogue scale (VAS) (Bhatia 2002; Howard 2009; Kemler 2012). One study (Bhatia 2002) also reported patient‐assessed wound appearance, although development, validity or reliability of the tool used was not described or referenced.
Early angles and other objective outcomes
Angular deformity was presented in different ways. Some investigators presented active finger angles (Bulstrode 2004; Collis 2013; Jerosch‐Herold 2011; Kemler 2012; McMillan 2012), and others presented passive angles (van Rijssen 2006; van Rijssen 2012a). In some studies, it was not clear whether the angles presented were active or passive (Citron 2003; Citron 2005; Degreef 2014; Ullah 2009). Presentation of angular measurements varied between early and late outcomes of the same clinical trial (van Rijssen 2006; van Rijssen 2012a). Other objective outcomes measured included timings. Three studies presented analyses of time taken to perform key tasks involved in surgery or postoperative care (Bhatia 2002; Howard 2009; Ullah 2009). Bulstrode 2004 and Chignon‐Sicard 2012 presented time to healing. Collis 2013 and Ullah 2009 studied grip strength.
Recurrence
Studies that reported comparisons of operative technique considered recurrence in late outcome papers (Citron 2003; Citron 2005; Degreef 2014; Ullah 2009; van Rijssen 2012a) and extension deficit at early outcome points (Ullah 2009; van Rijssen 2006). The definition of recurrence varied from reappearance of palpable disease in the operated field (Citron 2003; Citron 2005) to recurrent angular deformity (Degreef 2014; Ullah 2009; van Rijssen 2012a).
Within trials comparing different procedures, recurrence was defined as an increase in joint angle of 20 or more degrees in van Rijssen 2012a and was not explicitly defined in Degreef 2014 or Ullah 2009. However, researchers discussed 'progressive recurrence of contracture', suggesting that angular deformity rather than reappearance of palpable disease accounted for this.
Adverse effects
Adverse effect reporting varied from not studying complications in a study of rehabilitation adjuncts (Jerosch‐Herold 2011), to describing 'no intraoperative complications' (Bulstrode 2004) or 'no complications' (Citron 2003), to describing and attempting to quantify specific complications (Chignon‐Sicard 2012). No standardisation was evident regarding which adverse effects were studied, even between similar studies.
Cost‐effectiveness
No included studies presented formal cost‐effectiveness analyses, although several articles did assess cost‐effectiveness. However, these studies were not randomised and were not pseudorandomised, so we excluded them. Three studies presented analyses of time taken to perform aspects of surgery or postoperative care (Bhatia 2002; Howard 2009; Ullah 2009) that would be expected to have cost‐effectiveness implications.
Summary
The primary objective of this review was to study trials comparing different treatment options. This group comprises only three papers describing two trials, and these two trials compared different interventions using different inclusion and exclusion criteria. Ullah 2009 compared small firebreak full‐thickness skin grafting versus z‐plasty closure of limited fasciectomy for contractures involving the PIPJ. In contrast, van Rijssen 2006 and van Rijssen 2012a described a trial comparing needle fasciotomy versus limited fasciectomy, with inclusion criteria including contractures that may not necessarily affect the PIPJ.
Among trials refining intraoperative techniques, all compared different interventions. Among trials refining rehabilitation adjuncts, two (Collis 2013; Jerosch‐Herold 2011) investigated the same intervention using comparable measures and time points, allowing meta‐analysis.
Excluded studies
In all, we excluded 450 studies on the basis of Q1 in Appendix 9 (i.e. they did not report the outcome of a clinical trial), and 1847 on the basis of Q2 in Appendix 9 (i.e. study participants had not undergone surgery for Dupuytren's disease of the fingers). We excluded two publications on the basis of Q3 in Appendix 9, as investigators reported a study protocol but no results (Jerosch‐Herold 2008 reported the study protocol for Jerosch‐Herold 2011). We excluded 34 studies on the basis of Q4 in Appendix 9 (i.e. two interventions were not compared, or no control group was included), and nine studies on the basis of Q5 in Appendix 9 (i.e. these studies were not randomised and were not pseudorandomised). We have described the studies in these three categories under Characteristics of excluded studies.
Three studies provided inadequate details in the abstract and references to allow a decision (Gazdzik 1997; Slullitel 1987; Yoshida 1998), and we could not obtain the original paper. We excluded them on this basis.
Risk of bias in included studies
We presented risk of bias for each study under Characteristics of included studies and summarised study results in Figure 2 and Figure 3.
2.
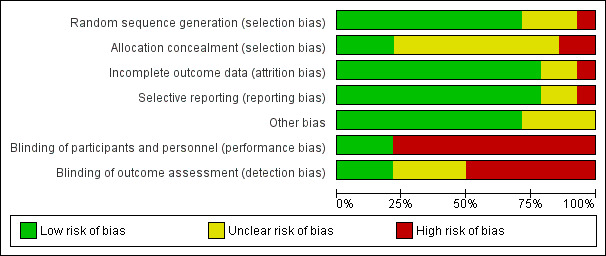
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
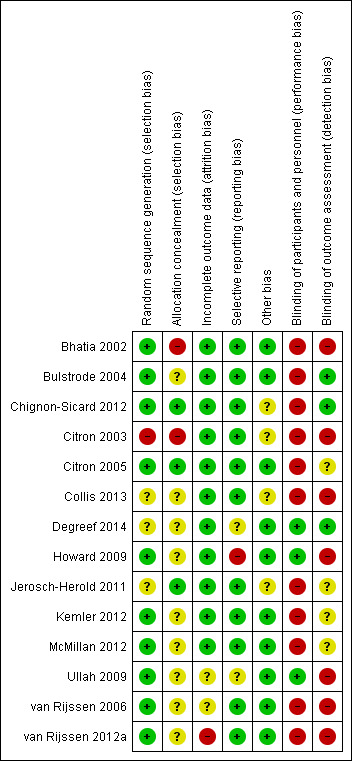
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three trials did not explain the randomisation process used, and one used alternation. The remaining nine studies used acceptable randomisation processes. Allocation concealment was poorly described, with only three studies adequately describing secure processes. Allocation concealment processes were robust in Chignon‐Sicard 2012, Citron 2005 and Jerosch‐Herold 2011 only. The former two of these studies listed here described sealed, sequentially numbered opaque envelopes, whereas Jerosch‐Herold 2011 used telephone randomisation from another site but did not specify how the random sequence was generated. Four other articles used numbered sealed containers (envelopes or boxes) but did not describe whether they were opaque (Degreef 2014; Ullah 2009; van Rijssen 2006; van Rijssen 2012a). Other studies provided similar inadequate details on concealment.
Blinding
As the treatment involved is a surgical procedure, it is acknowledged that many trials are likely to be at high risk of performance bias, as the surgical team performing the procedure cannot always be blinded. In Degreef 2014, double‐blinding was possible, as the intervention was a medical adjunct to surgery. However, trials of wound closure and adjuncts could defer randomisation until after the corrective element of the procedure had been completed. This was done only in Ullah 2009. Several other studies (Bhatia 2002; Bulstrode 2004; Chignon‐Sicard 2012; Howard 2009; McMillan 2012) could have deferred randomisation in this way to reduce the impact of performance bias on other parts of the procedure, but they did not.
Few studies explicitly described blinding of assessment. Bulstrode 2004 employed double‐blinding of the participant as well as the assessor. It is acknowledged that such blinding may be difficult to achieve in comparisons of procedures that leave distinctive and very different scar patterns on the hand (such as needle fasciotomy and fasciectomy). Chignon‐Sicard 2012 also described blinding of outcome assessment.
Incomplete outcome data
Several studies did not formally describe attrition. van Rijssen 2012a, the study with the longest follow‐up period, described significantly different levels of attrition between groups, which could have been influenced by treatment outcomes. Articles classified as having 'unclear' risk did not explicitly describe levels of attrition experienced.
Selective reporting
One study (Howard 2009) excluded outliers despite formal testing of the normality of data distribution and the decision to use non‐parametric statistics. The primary conclusion of the study could become invalid with these outliers included in the analysis. Researchers described no protocol for their exclusion. One further study (Ullah 2009) listed the greatest number of secondary outcomes but did not describe them in detail and presented some only graphically.
Other potential sources of bias
As many studies were expected to be unblinded, we considered risks of blocked randomisation in such studies. This risk was unclear in four articles (Chignon‐Sicard 2012; Citron 2003; Collis 2013; Jerosch‐Herold 2011), which provided inadequate detail to allow exclusion of this risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Summary of findings table 1: comparison of operation types: early results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease.
| Comparison of operation types: early results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease | |||||
|
Patient or population: 125 hands in 121 participants with Dupuytren's disease of the fingers for early outcomes (van Rijssen 2006) Settings: single‐centre Dutch study Intervention: needle fasciotomy Comparison: limited fasciectomy | |||||
| Outcomesa | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riskb | Corresponding risk | ||||
| Limited fasciectomy | Needle fasciotomy | ||||
|
DASH hand function score at 5 weeks Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) |
Mean DASH hand function score in the fasciectomy group was 16 | DASH hand function score in the fasciotomy group was 5 lower than in the fasciectomy group | 97 (1 study) | ⊕⊕⊝⊝ Lowc | P value = 0.017 as quoted in van Rijssen 2006 24/121 participants in the study did not adequately complete the DASH PROM tools Insufficient detail in article to allow calculation of 95% CI (standard deviations not provided) Unclear whether this is the most appropriate time point for study of 'early' outcome |
|
Patient satisfaction at 6 weeks Major outcome group 2 (other PROM) (scores from "0 (no/very negative) to 10 (yes/very positive)") |
See comment | See comment | 121 (1 study) | ⊕⊕⊝⊝ Lowd | Data not described in van Rijssen 2006. Only level of significance provided P value = 0.002 as quoted in van Rijssen 2006 |
|
Early angular outcome at 6 weeks for Tubiana grade I disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 0 to 45 degrees) Early angular outcome at 6 weeks for Tubiana grade II disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 45 to 90 degrees) Early angular outcome at 6 weeks for Tubiana grade III disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 90 to 135 degrees) Early angular outcome at 6 weeks for Tubiana grade IV disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED > 135 degrees) Major outcome group 3 (early objective measurement) |
For Tubiana grade I disease, mean percentage reduction in TPED in the fasciectomy group was 82% For Tubiana grade II disease, mean percentage reduction in TPED in the fasciectomy group was 78% For Tubiana grade III disease, mean percentage reduction in TPED in the fasciectomy group was 75% For Tubiana grade IV disease, mean percentage reduction in TPED in the fasciectomy group was 79% |
For Tubiana grade I disease, mean percentage reduction in TPED in the fasciotomy group was 11% lower than in the fasciectomy group For Tubiana grade II disease, mean percentage reduction in TPED in the fasciotomy group was 11% lower than in the fasciectomy group For Tubiana grade III disease, mean percentage reduction in TPED in the fasciotomy group was 29% lower than in the fasciectomy group For Tubiana grade IV disease, mean percentage reduction in TPED in the fasciotomy group was 32% lower than in the fasciectomy group |
For grade I disease, 57 (1 study) For grade II disease, 70 (1 study) For grade III disease, 27 (1 study) For grade IV disease, 10 (1 study) |
⊕⊕⊝⊝ Lowe | For grade I disease, P value = 0.329 in van Rijssen 2006 For grade II disease, P value = 0.071 in van Rijssen 2006 For grade III disease, P value = 0.000 in van Rijssen 2006 For grade IV disease, P value = 0.004 in van Rijssen 2006 |
| Major outcome group 4 (recurrence) | See comment | See comment | See comment | See comment | Not studied in van Rijssen 2006 |
|
Paraesthesia at 1 week Major outcome group 5 (adverse effects) Defined as "tingling sensations at any part of the treated digit without objective disturbance of sensation at the tip of the digit" per hand |
228 per 1000 | 67 per 1000 | 117 (1 study) | ⊕⊕⊝⊝ Lowf | P value = 0.013 in van Rijssen 2006 Relative effect not calculated as only study available |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DASH: Disabilties of the Arm, Shoulder and Hand Scale; DIPJ: Distal interphalangeal joint; MCPJ: Metacarpophalangeal joint; PIPJ: Proximal interphalangeal joint; PROM: Patient‐reported outcome measures; RR: Risk ratio; TPED: Total passive extension deficit. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aRecurrence was not studied in van Rijssen 2006, as this article considered early outcomes only. Recurrence is a late effect, and recurrence in this trial is considered in the next 'Summary of findings' table.
bAll assumed risks are based on mean values for limited fasciectomy as reported in van Rijssen 2006.
cEvidence downgraded from high to low for DASH at 5 weeks because of significant attrition. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision.
dEvidence downgraded from high to low for patient satisfaction at 6 weeks, as scale used was not validated. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision.
eEvidence downgraded from high to low for early angular outcomes in grade I disease at 6 weeks, as van Rijssen 2006 had significant risk of performance and detection biases, and imprecision.
fParaesthesia at 6 weeks downgraded from high to low, as scale was not validated. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision.
Summary of findings 2. Summary of findings table 2: comparison of operation types: late results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease.
| Comparison of operation types: late results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease | |||||
|
Patient or population: 93 participants (van Rijssen 2012a) Settings: single‐centre Dutch study Intervention: needle fasciotomy Comparison: limited fasciectomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Limited fasciectomy | Needle fasciotomy | ||||
|
DASH hand function score at 5 years Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) |
See comment | See comment | See comment | See comment | Not studied in van Rijssen 2012a |
|
Patient satisfaction at 5 years Major outcome group 2 (other PROM) (scores between "1 (not at all), 10 (excellent)") |
Mean satisfaction score in fasciectomy group was 8.3 | Mean satisfaction score in fasciotomy group was 2.1 lower than in fasciectomy group | 93 (1 study) | ⊕⊕⊝⊝ Lowa | P value < 0.001 as quoted in van Rijssen 2012a Likelihood of selecting treatment again significantly higher after fasciectomy (P value = 0.008) Insufficient detail in article to allow calculation of 95% CI (standard deviations not provided) |
| Major outcome group 3 (early angular outcome)b | See comment | See comment | See comment | See comment | This major outcome group is not relevant to a late outcome comparison |
|
Recurrence at 5 years Major outcome group 4 (recurrence) Defined as reoperation or progressive angular deformity of 20 degrees in a successfully treated joint |
209 per 1000 | 849 per 1000 | 93 (1 study) | ⊕⊕⊝⊝ Lowc | Progressive angular deformity defined in van Rijssen 2006 as an increase in TPED ≥ 30 degrees. In van Rijssen 2012a, different definitions used (increase of 20 degrees in a successfully treated joint) in other studies of Dupuytren's disease, such as Hurst 2009, acknowledged and applied P value < 0.001 in van Rijssen 2012a Relative effect not calculated, as only study available Recurrence rate influenced by the definition of recurrence used, and by length of follow‐up period |
| Major outcome group 5 (adverse effects)d | see comment | see comment | see comment | see comment | Not discussed in van Rijssen 2012a; analysed in van Rijssen 2006 |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DASH: Disabilities of the Arm, Shoulder and Hand Scale; PROM: Patient‐reported outcome measure; RR: Risk ratio; TPED: Total passive extension deficit. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aQuality of evidence for patient satisfaction at 5 years downgraded from high to low because of significant risks of bias in van Rijssen 2012a, and as the result of imprecision. bEarly angular outcomes and adverse effects not considered in this table, as these are relevant to early outcome assessment, and so are included in the previous 'Summary of findings' table.
cQuality of evidence for recurrence at 5 years downgraded from high to low because of significant risks of bias in van Rijssen 2012a, and as the result of imprecision. dEarly angular outcomes and adverse effects not considered in this table, as these are relevant to early outcome assessment, and so are included in the previous 'Summary of findings' table.
Summary of findings 3. Summary of findings table 3: comparison of operation types: firebreak skin grafting vs z‐plasty closure of fasciectomy for Dupuytren's disease.
| Comparison of operation types: firebreak skin grafting vs z‐plasty closure of fasciectomy for Dupuytren's disease | |||||
|
Patient or population: 79 participants (Ullah 2009) Settings: single‐centre UK study Intervention: firebreak skin grafting to close incision Comparison: z‐plasty closure of incision | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| z‐plasty | Firebreak skin grafting | ||||
|
PEM hand function score at 3 years Major outcome group 1 (hand function) (scores between 0 and 77, where 0 represents no impairment in hand function and 77 represents maximum impairment in hand function) |
See comment | See comment | 79 (1 study) |
⊕⊕⊝⊝ Lowa | Data represented graphically only; differences between groups described as not statistically significant; no P value provided |
| Major outcome group 2 (patient satisfaction and other PROM) | See comment | See comment | See comment | See comment | Not studied in Ullah 2009 |
|
Correction of MCPJ and PIPJ deformities at
2 weeks Major outcome group 3 (early angular outcomes) |
All MCPJs fully corrected Mean PIPJ correction 6 degrees in the z‐plasty group |
All MCPJs also fully corrected Mean PIPJ correction no different (also 6 degrees) in the skin graft group from the z‐plasty group |
79 (1 study) |
⊕⊕⊝⊝ Lowb | |
|
Progressive contracture by 3 years Major outcome group 4 (recurrence) |
109 per 1000 | 136 per 1000 | 79 (1 study) |
⊕⊕⊝⊝ Lowc | P value = 0.17 in Ullah 2009 Rates assessed per finger (90 fingers treated among 79 participants) |
|
Hypoaesthesia Major outcome group 5 (adverse effects) |
Radial digital nerve territory: 217 per 1000 Ulnar digital nerve territory: 217 per 1000 |
Radial digital nerve territory: 341 per 1000 Ulnar digital nerve territory: 455 per 1000 |
79 (1 study) |
⊕⊕⊝⊝ Lowd | P value = 0.2 for radial digital nerve territory in Ullah 2009 P value = 0.03 for ulnar digital nerve territory in Ullah 2009 |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MCPJ: Metacarpophalangeal joint; PEM: Patient Evaluation Measure; PIPJ: Proximal interphalangeal joint; PROM: Patient‐reported outcome measure; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aQuality of evidence for PEM hand function score at 3 years downgraded from high to low, as neither data nor P value was provided to support statement, and as the result of imprecision.
b,c,dQuality of evidence downgraded from high to low because of risks of bias and imprecision.
Summary of findings 4. Summary of findings table 4: refining rehabilitation: three months of postoperative night splinting with hand therapy vs hand therapy alone for rehabilitation following surgery for Dupuytren's disease.
| Refining rehabilitation: three months of postoperative night splinting with hand therapy vs hand therapy alone for rehabilitation following surgery for Dupuytren's disease | |||||
|
Patient or population: 210 participants with Dupuytren's disease of the fingers in 2 studies (225 digits reported across all studies) (Collis 2013; Jerosch‐Herold 2011) Settings: multi‐centre UK RCT and single‐centre New Zealand RCT Intervention: three months of night splinting in extension in addition to hand therapy ("splint") Comparison: hand therapy alone ("no splint") | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| No splint | Splint | ||||
|
DASH hand function score at 3 months Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) |
Mean DASH ranged across 'no splint' groups from 10.8 to 11 | Mean DASH in 'splint' groups was 1.15 lower (95% CI ‐2.32 to 4.62) than in 'no splint' groups | 205 participants (2 studies) | ⊕⊕⊝⊝ Lowa | Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Major outcome group 2 (patient satisfaction) | See comment | See comment | See comment | See comment | Not assessed in these studies |
|
Total active extension at 3 months Major outcome group 3 (early objective measurement) Total active extension (TAE) of MCPJ, PIPJ and DIPJ; higher value indicates loss of extension and a worse outcome |
Mean TAE ranged across 'no splint' groups from 24 degrees to 33 degrees | Mean TAE in 'splint' groups was 2.21 degrees higher (95% CI ‐3.59 to 8.01) than in 'no splint' groups | 225 digits (2 studies) | ⊕⊕⊝⊝ Lowb | Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Major outcome group 4 (recurrence) | See comment | See comment | See comment | See comment | Not assessed in these studies |
|
Total active flexion at three months Major outcome group 5 (adverse effects) Total active flexion (TAF) of MCPJ, PIPJ and DIPJ; lower value indicates loss of flexion and a worse outcome |
Mean TAF ranged across 'no splint' groups from 217.6 degrees to 245 degrees | Mean TAF in 'splint' groups was 8.42 degrees lower (95% CI 1.78 to 15.07) than in 'no splint' groups | 225 digits (2 studies) | ⊕⊕⊝⊝ Lowc | Conflicting findings from subgroups Unclear whether this is the most appropriate time point for study of 'early' outcome |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DASH: Disabilities of the Arm, Shoulder and Hand Scale; DIPJ: Distal interphalangeal joint; MCPJ: Metacarpophalangeal joint; PIPJ: Proximal interphalangeal joint; RCT: Randomised controlled trial; TAE: Total active extension; TAF: Total active flexion. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
a,b,cQuality of evidence was downgraded from high to low because of risks of bias and imprecision.
Comparison of procedure types
Needle fasciotomy versus fasciectomy
One trial compared these procedures and reported early and late outcomes separately (van Rijssen 2006; van Rijssen 2012a). The early outcome article (van Rijssen 2006) reported 125 hands in 121 participants. The late outcome article (van Rijssen 2012a) included 93 participants from the original cohort. This comparison involved low‐quality evidence related to study design limitations and imprecision.
Hand function
Low‐quality evidence suggests that hand function, as determined by the DASH PROM, may be statistically significantly less after needle fasciotomy than after fasciectomy at all time points up to five weeks following surgery (P value = 0.017 at five weeks) (van Rijssen 2006). However, only 97 of 121 (80%) participants completed the PROM tool adequately to allow analysis. van Rijssen 2012a provided no evidence describing later functional outcomes.
Patient satisfaction and other patient‐rated outcomes
Low‐quality evidence indicates that patient satisfaction may be significantly greater for the needle fasciotomy group than for the fasciectomy group at six weeks (P value = 0.003 in van Rijssen 2006). Low‐quality evidence also shows that by five years, patient satisfaction had reversed; satisfaction was significantly greater for fasciectomy (P value < 0.001 in van Rijssen 2012a), and overall satisfaction was less among patients with recurrence (P value < 0.001 in van Rijssen 2012a). However, the tools used may not have been robustly developed or validated, as has been discussed (Description of studies), and data presented were incomplete.
Early angles and other objective outcomes
Low‐quality evidence suggests that correction of total passive extension deficit was not different between procedures for milder contractures (Tubiana stages I and II, which equates to total passive extension deficit across all joints less than 90 degrees) by six weeks, but limited fasciectomy achieved significantly better correction for more severe contractures (Tubiana stages III and IV, i.e. over 90 degrees of total passive extension deficit) (see Table 6).
2. Six‐week outcomes described in van Rijssen 2006.
| Tubiana stage preop | % improvement in TPED for needle fasciotomy | % improvement in TPED for fasciectomy | Significance of differences between procedures |
| I | 71 | 82 | 0.329 |
| II | 67 | 78 | 0.071 |
| III | 46 | 75 | 0.000 |
| IV | 47 | 79 | 0.004 |
Recurrence
Low‐quality evidence indicates that recurrence may be significantly greater five years after needle fasciotomy (84.9% of hands after fasciotomy vs 20.9% of hands after fasciectomy; P value < 0.001 in van Rijssen 2012a).
Adverse effects
Low‐quality evidence suggests that complication rates were similar between procedures in terms of infection, haematoma, wound slough, skin fissure, sympathetic dystrophy, altered sensation, digital nerve injury, tendon injury and revision surgery. The incidence of paraesthesia was statistically significantly greater after limited fasciectomy than after fasciotomy one week after treatment (P value = 0.013).
Cost‐effectiveness
No published data were found comparing the cost‐effectiveness of needle fasciotomy and fasciectomy.
Summary
Evidence indicates that needle fasciotomy delivered better satisfaction and function than fasciectomy at early outcomes, although poor rates of completion of the PROM were an issue. Fasciectomy was more effective in correcting severe disease. Recurrence was greater at five years after needle fasciotomy, although functional outcomes had not been described. The cost‐effectiveness of performing multiple needle fasciotomies rather than a single fasciectomy over a given period had not been studied. Study design limitations and imprecision reduced the quality of the evidence. At present, evidence in key areas is insufficient to show which treatment is superior overall.
Dermofasciectomy versus fasciectomy
One study compared firebreak skin grafting versus direct closure of fasciectomy (Ullah 2009) and provided low‐quality evidence. Investigators included 79 participants and presented a large quantity of data only graphically.
Hand function
Hand function, as determined by the PEM PROM, was not different between fasciectomies and firebreak dermofasciectomies at 36 months. Investigators presented earlier time points only graphically.
Patient satisfaction and other patient‐rated outcomes
We found no published data that compared these outcomes for fasciectomies and firebreak dermofasciectomies.
Early angles and other objective outcomes
Grip strength, angular deformity and motion at the PIPJ all correlated at 36 months. No differences between groups were evident throughout the study, although the data supporting this were presented graphically only.
Recurrence
We noted no differences between firebreak dermofasciectomies and fasciectomies in terms of recurrence, defined as progressive contracture, and time to recurrence.
Adverse effects
We found no differences between procedures in terms of antibiotic requirement, skin necrosis, wound dehiscence, radial hypoaesthesia or reflex sympathetic dystrophy. We noted a significantly greater incidence of ulnar hypoaesthesia after firebreak dermofasciectomy.
Cost‐effectiveness
We identified no formal cost‐effectiveness analysis data. However, we noted that firebreak dermofasciectomy took significantly longer to perform than fasciectomy involving z‐plasty closure (79 vs 66 minutes; P value = 0.01).
Summary
Low‐quality evidence indicates that firebreak dermofasciectomy and fasciectomy with z‐plasty closure performed similarly. Given that firebreak dermofasciectomy took longer to perform, evidence does not support its routine use. However, we cannot extend this conclusion to other approaches to dermofasciectomy involving larger skin grafts. We obtained no data on comparison of other approaches versus dermofasciectomy and fasciectomy.
Technical refinements
Type of incision
Two articles studied incisions and included 30 and 100 participants, respectively. The first compared z‐plasty closure versus direct closure of a transverse incision for fasciectomy (Citron 2003). The second compared a zig‐zag (Bruner's) incision with direct closure versus a longitudinal incision with z‐plasty closure for fasciectomy (Citron 2005). Researchers provided low‐quality evidence related to study design limitations and imprecision.
Hand function
We found no data that described this.
Patient satisfaction and other patient‐rated outcomes
We found no data that described this.
Early angles and other objective outcomes
We found no evidence of differences between a zig‐zag incision and a z‐plasty closure in terms of deformity or extension.
Recurrence
Low‐quality evidence suggests that z‐plasty closure of a palmar fasciectomy had significantly less recurrence (reappearance of palpable disease) than was seen with direct closure of a transverse incision (P value < 0.01 when trial recruitment was stopped at the interim analysis point in Citron 2003). Researchers reported no differences in recurrence defined this way between a zig‐zag incision and a z‐plasty closure.
Adverse effects
Investigators in Citron 2003 encountered no complications. In Citron 2005, researchers comparing a zig‐zag incision versus a z‐plasty reported no differences in total complications, algodystrophy and digital nerve injury.
Cost‐effectiveness
We identified no formal cost‐effectiveness analysis data.
Summary
Low‐quality evidence supported z‐plasty closure over direct closure of transverse incisions for MCPJ cords and showed no differences between a zig‐zag incision and a z‐plasty closure for fasciectomy.
Wound closure
Two studies (Bhatia 2002; Howard 2009) investigated wound closure. Bhatia 2002 compared staple closure versus non‐absorbable suture closure in 31 participants. Howard 2009 compared absorbable suture closure versus non‐absorbable suture closure for fasciectomy in 62 participants. Both trials provided low‐quality evidence related to study design limitations and imprecision.
Hand function
We found no data that described this.
Patient satisfaction and other patient‐rated outcomes
Bhatia 2002 found no differences in patient‐reported wound appearance at two weeks between those who received staples and those given non‐absorbable sutures. Removal of staples was more painful than removal of non‐absorbable sutures (P value = 0.008 in Bhatia 2002). Howard 2009 found no differences in visual analogue scale (VAS) pain scores between absorbable and non‐absorbable suture groups at the first postoperative visit.
Early angles and other objective outcomes
Staple closure was quicker to perform than non‐absorbable suture closure (P value < 0.001 in Bhatia 2002). Absorbable suture closure incurred less clinic time for management than non‐absorbable suture closure, once outliers were excluded (P value = 0.003 in Howard 2009). However, exclusion of outliers from a non‐parametric analysis may not have been appropriate and may invalidate this finding.
Recurrence
We found no data that described this.
Adverse effects
Neither study performed formal analyses of differences in complications between groups. However, complication rates were low for both groups in both studies.
Cost‐effectiveness
We identified no formal cost‐effectiveness analysis data. Analysis of timings as presented in Howard 2009 may not be robust, as has been explained.
Summary
Staple closure may be quicker to perform than suture closure and may achieve a comparable early wound appearance. However, removal of staples may be more painful.
In Howard 2009, absorbable sutures achieved early outcomes comparable with those of non‐absorbable sutures and were reported to require less clinic time for postoperative management. However, the evidence was incomplete, and the quality of evidence was low. In particular, the main conclusion in Howard 2009 may not be valid, as the statistical analysis performed may have been inappropriate.
Intraoperative adjuncts
Two studies investigated intraoperative adjuncts to surgery. Each considered a different intervention. Bulstrode 2004 investigated bathing a fasciectomy wound in 5‐fluorouracil compared with control, included 15 participants and provided low‐quality evidence. McMillan 2012 compared a postoperative series of steroid injections as an adjunct to needle fasciotomy versus no injections in 47 participants and provided low‐quality evidence related to study design limitations and imprecision.
One other study (Degreef 2014) considered a medical adjunct spanning the perioperative period. Researchers compared tamoxifen versus placebo administered preoperatively and postoperatively as an adjunct to fasciectomy, included 30 participants and provided low‐quality evidence.
Hand function
Degreef 2014 reported no differences in hand function as assessed by the DASH Scale at one year for tamoxifen versus placebo as an adjunct to fasciectomy. We found no other data that described this.
Patient satisfaction and other patient‐rated outcomes
Degreef 2014 reported that satisfaction was not significantly different between groups at three months. We found no other data that described this.
Early angles and other objective outcomes
Bulstrode 2004 found no differences between 5‐fluorouracil treatment and control treatment in terms of healing time, total active motion and loss of extension. In McMillan 2012, a series of steroid injections resulted in significantly greater percentage improvement in total active extension deficit at all time points. In Degreef 2014, relative improvement in extension deficit, as quantified by the Tubiana index, was significantly better in the tamoxifen group than in the placebo group.
Recurrence
Bulstrode 2004 found no differences between 5‐fluorouracil treatment and control treatment in terms of loss of extension nor total active motion at 18 months. McMillan 2012 reported significantly greater percentage improvement in total active extension deficit at six months (65% correction for steroid group vs 41% for control group; P value = 0.04) and in MCPJs and PIPJs considered separately at six months. In Degreef 2014, only one participant in the tamoxifen group experienced recurrence.
Adverse effects
Bulstrode 2004 and McMillan 2012 reported no complications. Degreef 2014 provided a narrative description of adverse events.
Cost‐effectiveness
We found no data that described this.
Summary
We found no evidence of benefit nor harm resulting from addition of a 5‐fluorouracil bath at completion of a fasciectomy, although function and long‐term outcomes (beyond 18 months) were not studied. Evidence suggests that a series of steroid injections provided after needle fasciotomy may achieve and maintain better correction of contractures than needle fasciotomy alone, although we found no long‐term data, and available evidence was of low quality. Evidence indicates that a perioperative course of tamoxifen may improve early correction of deformity, but that any potential effect was lost by two‐year follow‐up.
Rehabilitation adjuncts
We identified four studies that investigated adjuncts that might aid rehabilitation. Collis 2013 and Jerosch‐Herold 2011 compared three months of static postoperative splinting versus no postoperative splinting. Kemler 2012 also investigated postoperative splinting; the intervention arm underwent day and night splinting for a month, then night splinting for two months, and the study included 154 participants. Chignon‐Sicard 2012 studied application of fibrin‐ and platelet‐rich fibrin plug to open palmar wounds after fasciectomy to identify whether this improved healing. All studies provided low‐quality evidence related to study design limitations and imprecision.
The primary aim of this review had been to study operative techniques. However, as rehabilitation adjuncts are components of hand therapy in Dupuytren's disease, these trials did meet the inclusion criteria specified in the protocol and have been included.
The published article for Collis 2013 did not provide all relevant data. We contacted study authors, who provided the necessary data for per‐protocol analyses.
Hand function
Hand function, as assessed with the DASH PROM, was not affected by postoperative splinting at three months, six months and 12 months (Jerosch‐Herold 2011). Collis 2013 also found no effect when analysing individual time points up to three months postoperatively. In the latter study, investigators combined time points because no differences were found with a mixed‐effect model.
Meta‐analysis of these two studies demonstrated no significant heterogeneity at baseline (Analysis 1.1), and an intention‐to‐treat analysis showed no differences in function between splint and no splint groups at three‐month follow‐up (Analysis 2.1; Figure 4). However, per‐protocol groups were different from intention‐to‐treat groups, as participants in the 'no splint' group who experienced early re‐contracture were then given a splint, and some participants in the splint group were not compliant with splinting (defined as self report of < 50% compliance). No differences between 'splint' and 'no splint' groups were apparent when data were analysed per protocol (Analysis 3.1).
1.1. Analysis.
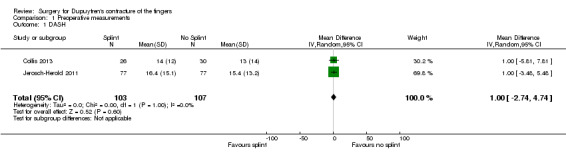
Comparison 1 Preoperative measurements, Outcome 1 DASH.
2.1. Analysis.
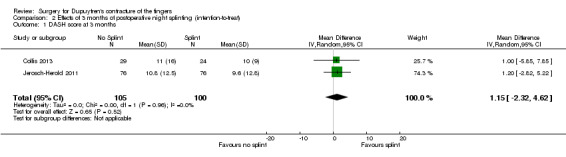
Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 1 DASH score at 3 months.
4.
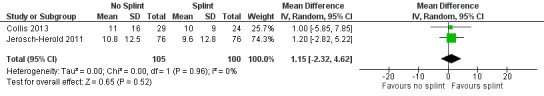
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.1 DASH score at 3 months.
3.1. Analysis.
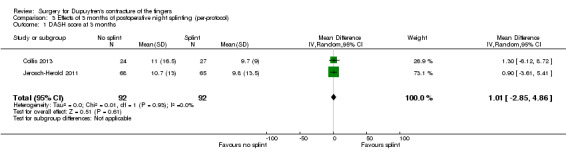
Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 1 DASH score at 3 months.
Patient satisfaction and other patient‐rated outcomes
Patient satisfaction was not different among those receiving postoperative splinting and those not receiving splinting, as assessed by an 11‐point verbal rating scale (Jerosch‐Herold 2011). However, the validity and reliability of this scale were not described or cited. Patient‐perceived changes were not significantly different between groups in Kemler 2012.
Early angles and other objective outcomes
As with hand function, no significant heterogeneity between studies was apparent at baseline (Analysis 1.2;Analysis 1.3). Total active flexion and total active extension were not different among those who received postoperative splinting at three, six or 12 months (Jerosch‐Herold 2011). Collis 2013 found no differences in total active extension or flexion. Meta‐analysis of three‐month follow‐up results from both studies revealed no differences in total active extension between splint and no splint groups (Analysis 2.2;Figure 5) but showed a significant difference in total active flexion, with splint group participants achieving 8.42 degrees less total active flexion than no splint group participants (Analysis 2.3;Figure 6). As discussed in the section on hand function above, intention‐to‐treat analyses were complicated by the fact that some in the 'no splint' group were given a splint if they experienced early re‐contracture, and some in the 'splint' group were non‐compliant. When meta‐analyses were performed on the basis of per‐protocol data, no differences were found between groups in terms of total active extension (Analysis 3.2), but the significant difference in total active flexion shown in Analysis 2.3 was more pronounced (Analysis 3.3).
1.2. Analysis.
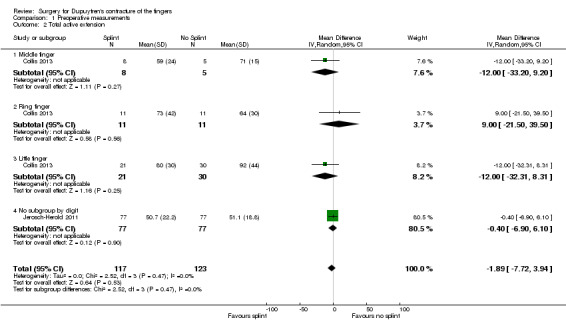
Comparison 1 Preoperative measurements, Outcome 2 Total active extension.
1.3. Analysis.
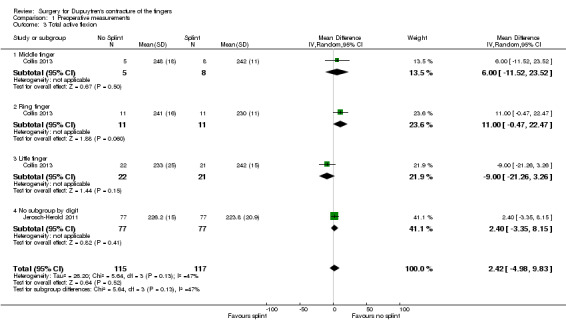
Comparison 1 Preoperative measurements, Outcome 3 Total active flexion.
2.2. Analysis.
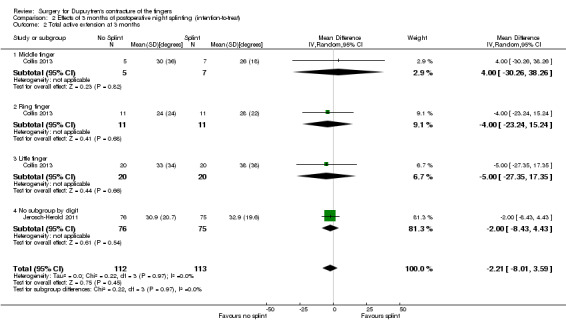
Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 2 Total active extension at 3 months.
5.
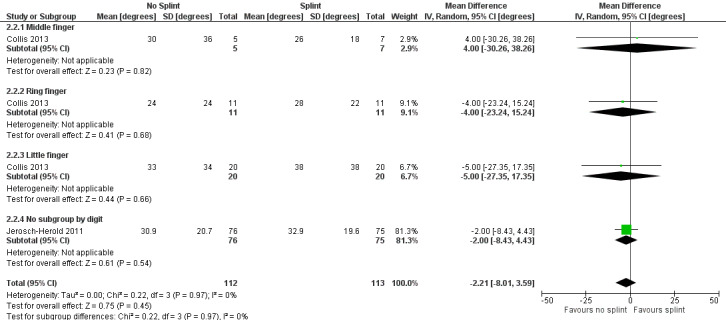
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.2 Total active extension at 3 months [degrees].
2.3. Analysis.
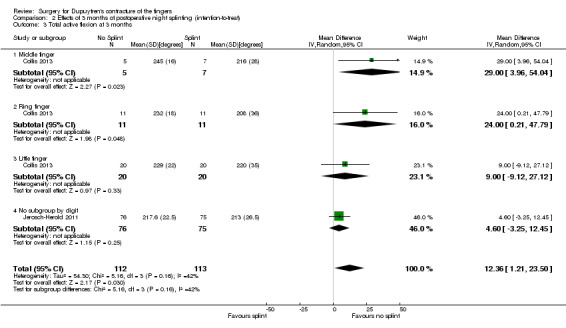
Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 3 Total active flexion at 3 months.
6.

Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.3 Total active flexion at 3 months [degrees].
3.2. Analysis.
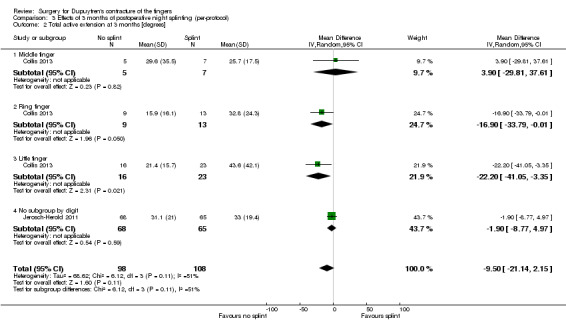
Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 2 Total active extension at 3 months [degrees].
3.3. Analysis.

Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 3 Total active flexion at 3 months [degrees].
Chignon‐Sicard 2012 reported statistically significantly shorter healing delay from application of a fibrin‐ and platelet‐rich fibrin plug to the open palmar wound versus control (median 24 days vs median 29 days) but no differences in secondary endpoints.
Recurrence
Chignon‐Sicard 2012, Collis 2013, Jerosch‐Herold 2011 and Kemler 2012 did not study recurrence.
Adverse effects
Chignon‐Sicard 2012 studied bleeding and exudate and reported no significant differences between intervention and control groups. Investigators discussed other rare adverse events, including wound and chest infection.
Cost‐effectiveness
No articles provided formal cost‐effectiveness analysis data.
Summary
Evidence showing that postoperative splinting improves rehabilitation after fasciectomy or dermofasciectomy is lacking, but available evidence shows that three months of night splinting reduces total active flexion at three months.
Discussion
Summary of main results
Outcomes measured
Review authors noted variation between studies in primary outcome measures assessed. Although some of this might be expected, for example, time taken for staple removal rather than recurrence as a long‐term outcome, the extent of variation within groups of studies limits the usefulness of the data presented, as well as their interpretation. Such variation is seen across lower‐quality studies as well. Providing no definition of recurrence, as was the case in Ullah 2009, is commonplace (Becker 2010) and limits interpretation of data.
Recurrence defined as the reappearance of palpable disease, as in Citron 2003 and Citron 2005, is acknowledged as generating qualitative data (Werker 2012). When detection bias is a risk, as in Citron 2003, the combination of an unblinded assessor and outcomes defined in a binary but subjective manner might be expected to be unsound. Additionally, this outcome is not a sensible option for studying fasciotomy, as palpable disease is never cleared from the treated field in the first place. Therefore, its use in Citron 2003 is not appropriate.
Even within studies that use angular deformity to define outcomes and recurrence, wide variation is evident in what exactly is measured and how it is described and presented (Ball 2013). Angles may be presented as the passive angles obtained by an assessor, as in van Rijssen 2006 and van Rijssen 2012a, or as active extension achieved unsupported by the participant, as in McMillan 2012. Such angles may differ, influencing study outcomes.
Although we could not perform meta‐analysis to compare operation types because of the paucity of comparable trials, it is probable that even if additional data are generated in the future, meta‐analysis would not be possible without standardisation of follow‐up length and outcome measures used. As Dupuytren's disease is a slowly progressive condition (Luck 1959), recurrence is likely to increase with longer periods of follow‐up. Furthermore, an understanding of the natural history of the condition (Luck 1959) suggests that recurrence defined as reappearance of palpable disease is likely to be encountered earlier than recurrence defined as deteriorating angular deformity. These inconsistencies contribute to the wide variation in recurrence rates reported in the existing literature ‐ from 0% to 71% (Becker 2010). Other groups have noted the need for clarity and consistency (Werker 2012). This review reiterates this and calls for more detailed study of the validity and reliability of outcome measures to ensure that the most appropriate outcomes are assessed at consistent and meaningful time points. Furthermore, recurrence of palpable disease and recurrence of angular deformity may not be truly relevant endpoints. Assessment of hand function through patient‐reported and patient‐centred measures may be more appropriate. Some of the studies included in this review used patient‐reported outcomes as a secondary endpoint.
Marked variation is evident in data reported by study authors as secondary outcomes. In part, this is a result of the study question selected, although outcomes handled as 'secondary' measures in studies were classified as appropriate primary outcome measures in this review, for example, patient‐reported hand function. However, trials of procedure types and technical refinement varied in that some recorded numerous secondary measures (Ullah 2009) and others recorded virtually none other than complications (McMillan 2012). In Ullah 2009, not all outcomes were reported fully other than in graphs, raising an unclear possibility of reporting bias. Furthermore, even if the data had been fully reported, the value of capturing all outcomes is not clear. One secondary outcome measure of importance is assessment of health‐related quality of life. Systems for analysing cost‐effectiveness are informed by data describing this, and data are captured by PROMs such as the EuroQol 5 Domain scale (EuroQol‐5D) (NICE 2008). Functional outcomes represent patient‐centred outcomes and may be of pragmatic interest to commissioners of health care. Use of patient‐reported data has been promoted nationally in the UK (Darzi 2008). This review cannot examine which of the range of PROMs available for use in Dupuytren's disease (Ball 2013) is most appropriate for use in future research. Recent reviews have called for further study of outcome measures (Ball 2013; Becker 2010; Werker 2012). To date, only a few studies have included patient‐reported hand function. Data captured by the PEM in Ullah 2009 were not described in detail in the paper. The DASH scale used in Degreef 2014, van Rijssen 2006 and van Rijssen 2012a has been the most popular measure across all studies of Dupuytren's disease (Ball 2013). DASH data presented in van Rijssen 2006 did not support the same conclusions as were reached by measuring angles; needle fasciotomy fared better throughout early rehabilitation in terms of DASH scores, despite the fact that fasciectomy arguably provided better correction of angular deformity in general. Thus, the conclusions drawn in this paper are likely to vary considerably, depending on which outcome is considered to be of primary importance. van Rijssen 2012a did not include corresponding late outcome function data. Given the value of health‐related quality of life data for accepted cost‐effectiveness analyses (NICE 2008), and the wide variation in reporting of angles in the literature (Ball 2013), future pragmatic trials might consider patient‐reported outcomes as the primary outcome measures, with joint angles demoted in importance. Furthermore, such a change might support the design of pragmatic studies. Ullah 2009 measured a variety of secondary outcomes. However, separating the primary outcome from complications may limit the clinical applicability of research findings. If an intervention achieves low rates of recurrence but does so with significant risk of complications such as chronic regional pain syndrome, cold intolerance and loss of grip strength or flexion, it may still fail to achieve meaningful clinical improvement for patients and cost‐effectiveness for commissioning bodies. Reports of early and late outcomes of the trial comparing fasciectomy and needle fasciotomy (van Rijssen 2006; van Rijssen 2012a) considered patient satisfaction, as did a trial on the use of tamoxifen as an adjunct to surgery (Degreef 2014), although the validity and reliability of these assessments were not clear. As already discussed for angular measurements, further work is urgently required to establish the validity and reliability of patient‐reported outcome measures.
Comparison of procedure types
We identified no studies in which surgery was compared with observation. Given that Dupuytren's disease typically is slowly progressive and is not life‐threatening, such comparisons would be informative.
The trial reported in van Rijssen 2006 and van Rijssen 2012a suggests that needle fasciotomy may achieve angular correction comparable with that of limited fasciectomy for milder Tubiana I and II contractures, but inferior correction for Tubiana III and IV contractures. However, this procedure leads to less functional impairment in the early postoperative phase (up to five weeks in van Rijssen 2006), earlier recovery and higher early patient satisfaction. By five years, it results in significantly higher recurrence and lower satisfaction than fasciectomy. The considerable difference in recurrence rates between fasciotomy and fasciectomy may be interpreted as demonstrating that fasciotomy is an inferior treatment. However, attrition bias may have affected late outcomes, and late functional outcomes were not recorded. As fasciotomy is less invasive, with quicker recovery (van Rijssen 2006), recurrence alone may not comprehensively describe late functional outcomes. Patient satisfaction is an important outcome for measurement, but perhaps it should be combined with, rather than used instead of, valid measures of hand function, as it might be influenced by factors besides the functional efficacy of treatment.
It might not be reasonable to expect needle fasciotomy, a demonstrably less invasive procedure that can be repeated for recurrent disease (van Rijssen 2012b), to achieve a durable effect comparable with that achieved by the more invasive fasciectomy. A more pragmatic study might consider early and late functional outcomes in groups randomly assigned to receive one fasciectomy or multiple needle fasciotomies over a period of years, with cost‐effectiveness calculated on the basis of functional outcomes and treatment pathway expenses.
Comparison of fasciectomy with z‐plasty closure versus firebreak skin grafting in Ullah 2009 revealed no differences between groups, other than prolonged operation time for skin grafting. This suggests that firebreak skin grafting may not prevent recurrence better than fasciectomy. However, dermofasciectomy comprises a spectrum, with small skin grafts used as firebreaks in Ullah 2009 at one end, and much more extensive skin grafts at the other end (Seah 2012). Thus, further comparison between limited fasciectomy and dermofasciectomy is needed.
Investigations of postoperative splinting
This was the only area in which meta‐analysis was possible. Recruitment for Collis 2013 began before Jerosch‐Herold 2011 was published. However, earlier publication of the trial protocol for Jerosch‐Herold 2011 (Jerosch‐Herold 2008) facilitated standardisation. Indeed, this was the only published trial protocol that we identified. Advanced publication of trial protocols is encouraged, as this may facilitate future standardisation of outcome assessment.
The functional outcome studied here was absolute DASH score at three months (rather than change in DASH score from preoperative to postoperative state), as preoperative DASH scores were not different between splint and no splint groups in either of the included studies (Collis 2013; Jerosch‐Herold 2011). Furthermore, this final result represents patients' functional performance at that time. Individual splinting results from all three trials showed no beneficial or adverse effects over postoperative splinting, but meta‐analysis of two of these studies showed statistically significant loss of flexion at three months caused by splinting. Whether the magnitude of the difference noted is of clinical significance, or whether it persists later in the rehabilitation period, is unclear. However, given the potentially expanded utilisation of resources needed to produce and maintain splints, we do not support their routine use.
Investigations of other questions
The primary objective of this review was to identify trials comparing different types of procedures. However, we have identified other trials within Dupuytren's disease surgery and have grouped them into trials investigating technical refinements of procedures, and trials investigating rehabilitation adjuncts. Although these studies might be considered tangential to the central aim of this review, we believe that appraising them is important to ensure that this review has been comprehensive, and that aspects of study methods and reporting have contributed to the conclusions presented here. In particular, analysing these studies informs implications for future research in this field. For example, Citron 2005 was the only included study that adequately described a randomisation process that used envelopes to provide adequate allocation concealment. As with studies comparing types of procedures, lack of comparable studies limited the performance of meta‐analysis.
Bhatia 2002 demonstrated that staple closure of fasciectomies may be quicker than suture closer, while showing that it resulted in greater pain at staple removal. However, this finding may be limited by the risk of bias in this study regarding allocation concealment. Citron 2003 was stopped early because a higher rate of recurrence was noted after direct closure than after z‐plasty closure. However, this difference had reached P value < 0.1 rather than the more conventional P value < 0.05; also, this study had been assigned high risk of bias related to use of alternation rather than randomisation, and as the result of performance and detection biases.
Overall completeness and applicability of evidence
This systematic review has identified very few high‐quality studies of Dupuytren's disease surgery. Among the included trials, fewer still compared different procedures, and others studied refinements in practice. Despite the availability of many current treatment options for years or decades, the paucity of studies suggests that research in this field lacks direction.
Quality of the evidence
The quality of methods varied between studies. More modern studies were generally at less risk of bias. Our assessment of performance bias might be controversial. To minimise the potential for the surgeon to influence the quality of the procedure, we included blinding of the surgeon during the procedure. Achieving this blinding may be extremely challenging and may not be possible in some studies. However, clear efforts were made in Ullah 2009 to standardise the surgical procedure as far as possible, with randomisation performed intraoperatively rather than preoperatively, unlike other studies investigating an intervention of relevance to closing stages of the procedure. As a result of these efforts, excision of disease that might be considered the 'correction' portion of the surgery was not subject to lack of blinding, and only wound closure was unblinded. Taking such steps when possible may limit the effects of performance bias.
In addition to risks of bias related to study design limitations, we further downgraded the quality of evidence as the result of imprecision, with most comparisons based on one or two studies with small sample sizes and wide confidence intervals.
Potential biases in the review process
We explained deviations from the published protocol in the section titled Differences between protocol and review. We believe that these differences are minor and have not influenced review outcomes. Although we took explicit steps to review conference proceeding abstracts, we may have missed unpublished data. However, given the paucity of trial data identified across all sources, we believe it is unlikely that a significant volume of relevant data has not been published.
Agreements and disagreements with other studies or reviews
We are not aware of similar reviews on this topic. However, expert opinion supports the need for further research in this area to inform clinical practice.
Authors' conclusions
Implications for practice.
This review has identified insufficient evidence to inform the roles of different procedures (needle fasciotomy, fasciectomy, interposition firebreak skin grafting or z‐plasty closure of fasciectomy) in the surgical treatment of patients with Dupuytren's disease of the fingers, beyond discussion of the limited results of individual studies. Further research in this field is urgently required.
The meta‐analysis performed here questions routine use of splinting following surgery for Dupuytren’s disease, and this warrants further research. Splinting may impair outcomes by reducing active flexion, although this is not clear from the data presented here. Furthermore, given the unclear role of splinting in early re‐contracture, splinting should be considered on an individual patient basis until further evidence becomes available.
Implications for research.
A marked paucity of randomised controlled trials on Dupuytren's disease surgery has been noted. This is the case for comparisons of different treatment procedures, of which several are currently in use, including needle fasciotomy, fasciectomy and dermofasciectomy. The role of each of these treatments in relation to other treatments is not currently supported by high‐quality evidence. Evidence related to collagenase will be considered in a separate review.
Given the need to justify the cost‐effectiveness of treatments in modern healthcare systems, it might be advisable to target research towards comparisons of different procedures. At present, clinical practice in Dupuytren's disease is not informed by high‐quality evidence. Logically, studies aimed at refining treatment might be better conducted only once the effectiveness and roles of different treatments have been established.
To date, design quality and reporting of trials in Dupuytren's disease surgery remain generally poor, with only a few examples showing good practice.
Future trials should ensure that risks of bias are minimised. As acknowledged, performance bias may prove difficult to minimise in some studies. That said, certain components of the studies included here have set precedents for processes by which risk of bias in random sequence generation, allocation concealment and outcome detection can be minimised. Future studies should endeavour to employ such robust processes, and to report them clearly.
Advanced publication of trial methods was encountered only once in this review, but it facilitated standardisation and meta‐analysis. This practice is encouraged.
Before needed trials are undertaken, further study of outcome measures is needed to establish their validity and reliability for use in Dupuytren's disease. Once this has been done, consensus and consistency of outcome choices and time points of assessment are needed to ensure standardisation with other studies.
History
Protocol first published: Issue 10, 2012 Review first published: Issue 12, 2015
| Date | Event | Description |
|---|---|---|
| 14 November 2008 | Amended | Converted to new review format |
Acknowledgements
The authors would like to thank the Cochrane Musculoskeletal Group for support provided, and Tamara Rader and Louise Falzon for assistance in developing the search strategy.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dupuytren Contracture] explode all trees 36
#2 MeSH descriptor: [Fibroma] explode all trees 4
#3 Dupuytren*:ti,ab,kw (Word variations have been searched) 49
#4 Fibromatosis 12
#5 MeSH descriptor: [Fascia] explode all trees 142
#6 palmar fibromatosis 1
#7 viking disease 1
#8 palmar fascia 7
#9 MeSH descriptor: [Fibroblasts] explode all trees 161
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 357
Appendix 2. MEDLINE search strategy: Ovid (numbers of results from original search in 2012)
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1948 to Present>Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Dupuytren Contracture/ (2121)
2 exp Fibroma/ (11134)
3 Fibromatosis.tw. (2482)
4 exp Fascia/ (7945)
5 Fibroblasts/ (93904)
6 (palmar adj3 fascia).tw. (212)
7 Dupuytren*.tw. (2087)
8 (palmar adj3 fibromatosis).tw. (65)
9 (viking adj3 disease).tw. (1)
10 or/1‐9 (115275)
11 randomized controlled trial.pt. (336898)
12 controlled clinical trial.pt. (85168)
13 randomized.ab. (252166)
14 placebo.ab. (139534)
15 clinical trials as topic.sh. (162410)
16 randomly.ab. (184567)
17 trial.ti. (108505)
18 or/11‐17 (807835)
19 exp animals/ not humans.sh. (3780560)
20 18 not 19 (746667)
21 10 and 20 (767)
Appendix 3. EMBASE search strategy
EMBASE Classic+EMBASE <1947 to 2012 September 17> ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Dupuytren contracture/ (3303)
2 fibroma/ (12928)
3 Fibromatosis.tw. (3239)
4 fascia/ (9898)
5 *fibroblast/ (30576)
6 (palmar adj3 fascia).tw. (309)
7 Dupuytren*.tw. (3062)
8 (palmar adj3 fibromatosis).tw. (84)
9 (viking adj3 disease).tw. (1)
10 or/1‐9 (59116)
11 random$.tw. (776280)
12 factorial$.tw. (20394)
13 crossover$.tw. (45803)
14 cross over.tw. (20874)
15 cross‐over.tw. (20874)
16 placebo$.tw. (189116)
17 (doubl$ adj blind$).tw. (140770)
18 (singl$ adj blind$).tw. (12915)
19 assign$.tw. (216631)
20 allocat$.tw. (72946)
21 volunteer$.tw. (171262)
22 crossover procedure/ (35355)
23 double blind procedure/ (115561)
24 randomized controlled trial/ (331643)
25 single blind procedure/ (16422)
26 or/11‐25 (1294294)
27 10 and 26 (1139)
Appendix 4. CINAHL search strategy
CINAHL via Ebscohost ‐ 1985‐2012
| S18 | S5 and S17 |
| S17 | S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 |
| S16 | TX allocat* random* |
| S15 | (MH "Quantitative Studies") |
| S14 | (MH "Placebos") |
| S13 | TX placebo* |
| S12 | TX random* allocat* |
| S11 | (MH "Random Assignment") |
| S10 | TX randomi* control* trial* |
| S9 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) |
| S8 | TX clinic* n1 trial* |
| S7 | PT Clinical trial |
| S6 | (MH "Clinical Trials+") |
| S5 | S1 or S2 or S3 or S4 |
| S4 | "Dupuytren" |
| S3 | (MH "Fascia") |
| S2 | "Fibromatosis" |
| S1 | (MM "Dupuytren's Contracture") |
Appendix 5. LILACS search strategy
LILACS (Latin American and Caribbean Health Sciences) ‐ 1982 to current date
Search terms: dupuytren's contracture
Appendix 6. ProQuest Dissertations & Theses search strategy
ProQuest Dissertations & Theses (PQDT) (all years)
| Set# | Searched for | Databases | Results |
| S1 | dupuytren's contracture | ProQuest Dissertations & Theses (PQDT) | 18 |
| S2 | Fibroma | ProQuest Dissertations & Theses (PQDT) | 256 |
| S3 | Fibromatosis | ProQuest Dissertations & Theses (PQDT) | 118 |
| S4 | palmar fibromatosis | ProQuest Dissertations & Theses (PQDT) | 2 |
| S6 | palmar fascia | ProQuest Dissertations & Theses (PQDT) | 86 |
| S7 | "viking disease" | ProQuest Dissertations & Theses (PQDT) | 0 |
| S8 | ab(viking disease) | ProQuest Dissertations & Theses (PQDT) | 1 |
| S9 | S1 OR S2 OR S3 OR S4 OR S6 OR S7 OR S8 | ProQuest Dissertations & Theses (PQDT) | 462 |
Appendix 7. ISI Web of Science search strategy
ISI Web of Science via Thomson Web of Knowledge Conference Proceedings Citation Index ‐ Science (CPCI‐S) ‐‐ 1990‐present
Topic=(dupuytren contracture)
Refined by: Web of Science Categories=(SURGERY ) AND Document Types=( PROCEEDINGS PAPER OR MEETING ABSTRACT )
Databases=CPCI‐S Timespan=All Years
Lemmatization=On
Appendix 8. Clinicaltrials.gov search strategy
(advanced search screen) Conditions: Dupuytren's contracture
Appendix 9. Study eligibility form
| Study eligibility form ‐ Surgery for Dupuytren's disease | |||
|
Authors _______________________________ Title _______________________________ _______________________________ |
Journal __________________________ Date of publication _______________ |
||
| Q1. Does the paper report the outcome of a clinical study? (i.e. not a review article or just a paper describing an operative technique description)? | Yes Next question |
No Exclude |
Unclear Refer |
|
Q2. Participant Have participants had a surgical intervention for Dupuytren's contracture of a finger? |
Yes Next question |
No Exclude |
Unclear Refer |
|
Q3. Outcomes Did the study report short‐term or long‐term outcomes (recurrence) of surgery? |
Yes Next question |
No Exclude |
Unclear Refer |
|
Q4. Intervention Did participants receive an intervention compared with a control group, or were at least 2 interventions compared? |
Yes Next question |
No Exclude |
Unclear Refer |
|
Q5. Type of study Was the study randomised or quasi‐randomised? |
Yes Include |
No Exclude |
Unclear Refer |
Appendix 10. Assessment of potential for bias in report (tool of The Cochrane Collaboration for assessing risk of bias)
| Paper title | ||
| Paper authors | ||
| Reviewer | ||
| Domain | Support for judgement | Review authors' judgement |
| Selection bias | Insert description, preferably a direct quote from report or correspondence, and add comment | One of : "Low risk", "High risk", "Unclear risk" |
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment |
| Performance bias | ||
|
Blinding of participants and personnel Assessments should be made for each main outcome (or class of outcomes) |
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information related to whether intended blinding was effective | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Detection bias | ||
| Blinding of outcome assessmentAssessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information related to whether intended blinding was effective | Detection bias due to knowledge of allocated interventions by outcome assessors |
| Attrition bias | ||
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes) | Describe completeness of outcome data for each main outcome, including attrition and exclusions from analysis. State whether attrition and exclusions were reported, numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions when reported and any re‐inclusions in analyses performed by review authors | Attrition bias due to amount, nature or handling of incomplete outcome data |
| Reporting bias | ||
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what they found | Reporting bias due to selective outcome reporting |
| Other bias | ||
| Other sources of bias | State any important concerns about bias not addressed in other domains of the tool If particular questions/entries were pre‐specified in the review protocol, responses should be provided for each question/entry |
Bias due to problems not covered elsewhere in the table |
Appendix 11. Paper assessment form
| Surgery for Dupuytren’s disease ‐ Study checklist | ||||||
| Authors and year | ||||||
| Title | ||||||
| Journal/Source if not published | ||||||
| Study ID (Revman) | Date of extraction | |||||
| Study design | ||||||
| Single/Multi‐centre | Study setting (country) | |||||
| Number of participants | Mean (SD:range) age | |||||
| Male:Female | ||||||
| Inclusion criteria | Exclusion criteria | |||||
| Randomisation technique |
|
Concealment of allocation sequence? | Yes (envelopes, etc.) No Unclear |
|||
| Blinding of participant | Yes No Unclear |
Incomplete outcome data (%FU) | Yes No Unclear |
|||
| Interventions | ||||||
| Intervention | Control intervention | |||||
| Length of follow‐up (mean, SD, median, min, max) |
Withdrawals (and why) | |||||
| Number lost to follow‐up | ||||||
| Outcomes assessed | ||||||
| Recurrence | Recurrence | No. Rec. | Total | |||
| Treatment | ||||||
| Control treatment | ||||||
| Pre‐op contracture (mean/SD) | MCP PIP Combined | |||||
| Imm post‐op contracture | MCP PIP Combined | |||||
| Final contracture | MCP PIP Combined | |||||
| Complications of surgery | Infection (definition) Digital nerve injury Tendon injury Other |
|||||
| Other outcomes | ||||||
| Quality of evidence | ||||||
Data and analyses
Comparison 1. Preoperative measurements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DASH | 2 | 210 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.74, 4.74] |
| 2 Total active extension | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐7.72, 3.94] |
| 2.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐33.20, 9.20] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐21.50, 39.50] |
| 2.3 Little finger | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐32.31, 8.31] |
| 2.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.90, 6.10] |
| 3 Total active flexion | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 2.42 [‐4.98, 9.83] |
| 3.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐11.52, 23.52] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 11.00 [‐0.47, 22.47] |
| 3.3 Little finger | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐21.26, 3.26] |
| 3.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐3.35, 8.15] |
Comparison 2. Effects of 3 months of postoperative night splinting (intention‐to‐treat).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DASH score at 3 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐2.32, 4.62] |
| 2 Total active extension at 3 months | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐8.01, 3.59] |
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐30.26, 38.26] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐23.24, 15.24] |
| 2.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐27.35, 17.35] |
| 2.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐8.43, 4.43] |
| 3 Total active flexion at 3 months | 2 | 225 | Mean Difference (IV, Random, 95% CI) | 12.36 [1.21, 23.50] |
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 29.00 [3.96, 54.04] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 24.0 [0.21, 47.79] |
| 3.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐9.12, 27.12] |
| 3.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐3.25, 12.45] |
Comparison 3. Effects of 3 months of postoperative night splinting (per‐protocol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DASH score at 3 months | 2 | 184 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐2.85, 4.86] |
| 2 Total active extension at 3 months [degrees] | 2 | 206 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐21.14, 2.15] |
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐29.81, 37.61] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐16.9 [‐33.79, ‐0.01] |
| 2.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐22.20 [‐41.05, ‐3.35] |
| 2.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐8.77, 4.97] |
| 3 Total active flexion at 3 months [degrees] | 2 | 206 | Mean Difference (IV, Random, 95% CI) | 12.64 [3.68, 21.60] |
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 28.60 [3.79, 53.41] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 21.70 [‐0.80, 44.20] |
| 3.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 13.10 [‐4.61, 30.81] |
| 3.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐1.42, 15.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhatia 2002.
| Methods | Single‐centre UK study Randomised controlled trial Recruitment between June 2000 and March 2001 |
|
| Participants | 31 participants 28:3 male/female ratio Mean age: 61 years Inclusion criteria: not specified Exclusion criteria: not specified |
|
| Interventions | Automated staple device closure vs Polybutester nonabsorbable suture closure |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 2 weeks Low‐quality evidence due to risk of bias regarding allocation concealment and imprecision No funding sources acknowledged; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Not discussed in paper; use of unsecured random numbers table assumed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of 5 endpoints reported with complete data; 1 of 5 not reported (wound appearance category at 2 weeks) |
| Selective reporting (reporting bias) | Low risk | 4 of 5 endpoints reported with complete data; 1 of 5 not reported (wound appearance category at 2 weeks) |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 3 of 5 assessments performed by surgeon or reported by participant; 1 other |
Bulstrode 2004.
| Methods | Single‐centre UK study Randomised controlled trial Recruitment dates not specified |
|
| Participants | 15 participants All male Mean age: 61 years Inclusion criteria: yes: age < 70 years, Luck involutional stage, ≥ 2 rays on hand affected Exclusion criteria: not specified |
|
| Interventions | Intraoperative wound bath in 5‐FU vs Wound bath in normal saline |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 18 months Low‐quality evidence due to risk of bias and imprecision Funded by the RAFT Institute of Plastic Surgery; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unmarked envelopes; unclear whether sealed or opaque |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgeon administering treatment not blinded; study described as double‐blinded: participant and assessor |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measurements performed by blinded therapist |
Chignon‐Sicard 2012.
| Methods | Single‐centre French study Randomised controlled clinical trial Recruitment between 2007 and 2010 |
|
| Participants | 68 participants 54 male:10 female; 4 excluded after randomisation not described Mean age: 61.4 (SD 8.8) years for intervention group; 66.0 (SD 7.7) years for control group Inclusion criteria: yes: "healthy individuals older than 18 years without any comorbidity who had been scheduled for elective McCash (open palm) surgery for Dupuytren disease" Exclusion criteria: yes: "Patients allergic to one of the dressing’s components, with diabetes mellitus type 1, who were undergoing cancer treatment, who were pregnant, or who were unable to participate in follow‐up visits were excluded" |
|
| Interventions | Leucocyte‐ and platelet‐rich fibrin platelet concentrate applied to wound vs Petroleum jelly mesh applied to wound |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 60 days Low‐quality evidence due to risk of bias and imprecision Funding source: academic grant from the French Ministry of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing randomisation from a "predefined randomization list, constructed through random permuted blocks" |
| Allocation concealment (selection bias) | Low risk | "sealed, sequentially numbered, opaque envelopes containing treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% loss to follow‐up; split between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Blocked randomisation employed in single‐blinded study (blinding of outcome assessment only) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Randomisation performed before procedure; surgeon probably unblinded throughout operative procedure, including fasciectomy component of procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessors blinded |
Citron 2003.
| Methods | Single‐centre UK study Pseudorandomised controlled clinical trial Recruitment between 1996 and 2000 |
|
| Participants | 30 participants 24 male:6 female Mean age (at diagnosis): 67 years for treatment group; 66 years for control group Inclusion criteria: yes: "Dupuytren’s contracture of a single ray confined to the palm and affecting only the MCPJ, a single cord of Dupuytren’s tissue, no previous surgery for Dupuytren’s disease in that ray, agreement to surgery" Exclusion criteria: not specified |
|
| Interventions | Longitudinal incision closed with z‐plasty vs Transverse incision |
|
| Outcomes |
|
|
| Notes | Mean length of follow‐up: 2 years (range 2.0 to 3.5) Low‐quality evidence, as pseudorandomised and at high risk of bias and imprecision No funding sources acknowledged; no conflicts of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation rather than randomisation |
| Allocation concealment (selection bias) | High risk | Alternation rather than randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% loss to follow‐up but balanced between groups |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported |
| Other bias | Unclear risk | Unblinded study with alternation rather than randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgeon unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Multiple unblinded assessors throughout study |
Citron 2005.
| Methods | Single‐centre UK study Randomised controlled trial Recruitment between February 1998 and August 2002 |
|
| Participants | 100 participants 63 male:16 female (21 incomplete) Mean age: 65 (SD 10) years Inclusion criteria: yes: Dupuytren’s disease in 1 ray only and any degree of resultant contracture Exclusion criteria: yes: bleeding diathesis, recurrent disease |
|
| Interventions | Bruner incision closed with Y‐V plasties vs Longitudinal incision closed with z‐plasties |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 2 years Low‐quality evidence, as high risk of performance bias, which may have influenced outcomes and imprecision No funding sources acknowledged; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers in envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed sequentially numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgeon unblinded; participant possibly unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Independent observer, but unclear whether blinded |
Collis 2013.
| Methods | Single‐centre New Zealand study Randomised controlled clinical trial Study period: 2010 to 2011 |
|
| Participants | 56 participants 45 male:11 female Mean age: 68 (SD 8) years in intervention group; 67 (SD 9) years in control group Inclusion criteria: yes: "Patients of all ages and surgery types were included, provided they attended their first postoperative hand therapy appointment within 14 days after surgery" Exclusion criteria: yes: "K‐wiring of the proximal interphalangeal joint during surgery or inability to comply with hand therapy" |
|
| Interventions | Night extension orthosis plus standard hand therapy vs Hand therapy alone (apart from participants in this group who had a net loss of ≥ 20 degrees at the PIPJ and/or a net loss of ≥ 30 degrees at the MCPJ of the operated fingers, in which case a splint was given) |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 3 months Low‐quality evidence, as inadequate detail provided on study design and high risk of performance bias and imprecision Funding source: A grant was received through the Clinical Centre for Research and Effective Practice (CCREP) Innovation Fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate detail: "participant selecting a tag from an envelope with group allocation concealed" |
| Allocation concealment (selection bias) | Unclear risk | Inadequate detail: "participant selecting a tag from an envelope with group allocation concealed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition described: split between groups; attrition unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Inadequate details of randomisation in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
Degreef 2014.
| Methods | Single‐centre Belgian study Randomised controlled clinical trial Study period not stated |
|
| Participants | 30 participants 26 male:4 female Mean age: 63.5 (SD 8) years Inclusion criteria: Adult patients scheduled for subtotal fasciectomy to treat Dupuytren's disease were eligible for inclusion if they had a D score > 4 Exclusion criteria: patients undergoing a reintervention for recurrent contractures; patients with a need for skin grafts or flaps; premenopausal women; patients using anti‐inflammatory drugs; patients with a history of malignancy; patients with a known allergy to tamoxifen |
|
| Interventions | Segmental fasciectomy with 80 mg oral tamoxifen daily for 6 weeks before surgery continuing until 12 weeks after surgery vs Segmental fasciectomy with 80 mg oral placebo daily for 6 weeks before surgery continuing until 12 weeks after surgery |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 24 months Low‐quality evidence, as inadequate details on study design and imprecision Funding source: Belgian Orthopaedic Society |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate detail: not described |
| Allocation concealment (selection bias) | Unclear risk | Inadequate detail: boxes used to store allocation, but opacity not described; second copies of allocations stored in envelopes with inadequate details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition described; systematic differences between groups unlikely |
| Selective reporting (reporting bias) | Unclear risk | No data presented for hand function (DASH) at 3 months |
| Other bias | Low risk | Blinded study; hence blocked randomisation not problematic |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome measurements |
Howard 2009.
| Methods | Single‐centre UK study Randomised controlled trial Recruitment dates not specified |
|
| Participants | 62 participants Gender ratio not presented Age data not presented Inclusion criteria: not specified Exclusion criteria: not specified |
|
| Interventions | Absorbable polyglactin suture closure vs Non‐absorbable polypropylene suture closure |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: primary outcome assessed at 10 to 14 days Low‐quality evidence, as review group was concerned that exclusion of outliers despite use of non‐parametric statistics in analysis of primary outcome created significant differences and imprecision No funding sources acknowledged; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Unclear from paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 of 62 missing |
| Selective reporting (reporting bias) | High risk | Protocol for exclusion of outliers not given; non‐parametric statistics used after test of normality described; unclear why outliers excluded; outlier exclusion may have influenced outcome of study |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded until start of procedure |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor unblinded |
Jerosch‐Herold 2011.
| Methods | Multi‐centre (5‐centre) UK study Randomised controlled trial Recruitment between October 2007 and January 2009 |
|
| Participants | 154 participants 120 male:34 female Mean age: 67.2 years in splint group; 67.5 years in no splint group Inclusion criteria: yes: Patients with Dupuytren’s disease affecting ≥ 1 digit of either hand and requiring fasciectomy or dermofasciectomy were invited to participate. Patients had to be over 18 years of age and competent to give fully informed written consent Exclusion criteria: yes: contracture of the thumb or first webspace |
|
| Interventions | Static splint for 3 months postop vs No splint (apart from participants in this group who had a net loss ≥ 15 degrees at the PIPJ and/or a net loss ≥ 20 degrees at the MCPJ of the operated fingers, in which case a splint was given) |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 12 months Low‐quality evidence due to risk of bias and imprecision Funded by Action Medical Research Charity; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process at central site not explained |
| Allocation concealment (selection bias) | Low risk | Central telephone cluster randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition recorded and explained |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Cluster randomisation used in an unblinded study, but unclear it if would have introduced bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Patient‐reported outcome measure unblinded but unclear whether likely to be biased; independent observer measured range of motion but unclear whether blinded |
Kemler 2012.
| Methods | 2‐Centre Dutch study Randomised controlled clinical trial Recruitment between 2007 and 2008 |
|
| Participants | 54 participants 46 male:8 female Mean age: 63 (SD 9) years in intervention group; 64 (SD 11) years in control group Inclusion criteria: yes: "DD (Dupuytren's disease) and a proximal inter‐phalangeal (PIP) joint flexion contracture of at least 30°" Exclusion criteria: yes: "below 18 years of age, had undergone partial amputation or arthrodesis of a digit or were patients with insufficient knowledge of the Dutch language" |
|
| Interventions | 3‐Month splinting protocol together with hand therapy vs Hand therapy alone |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 1 year Low‐quality evidence due to risk of bias and imprecision No specific funding sources declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not specified; allocation concealed from outcome assessor but allocation concealment not clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgeon blinded but therapist and participant not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All assessments by "same independent third party, a resident who had no part in the operative procedure or postoperative treatment", although blinding status not specified |
McMillan 2012.
| Methods | Single‐centre Canadian study Randomised controlled trial Recruitment dates not specified |
|
| Participants | 47 participants 41 male:6 female Mean age: 61.2 years Inclusion criteria: yes: "at least one joint contracture of at least 20°" Exclusion criteria: yes: "diabetes mellitus and those who had previously had hand surgery, including PNA, on the affected hand for any reason" |
|
| Interventions | Steroid injection at end of percutaneous needle fasciotomy, repeated at 6 weeks and 3 months, vs no steroid injection | |
| Outcomes |
|
|
| Notes | Length of follow‐up: 6 months Low‐quality evidence due to risk of bias and imprecision Funded by the Canadian Society of Plastic Surgeons; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions after randomisation |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; no sham injection for control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Ullah 2009.
| Methods | Single‐centre UK study Randomised controlled trial Recruitment dates not specified |
|
| Participants | 79 participants 65 male:14 female Mean age: 62.9 (range 27 to 85) years Inclusion criteria: yes: "primary Dupuytren’s contracture greater than 30° of the PIP joint of a finger" Exclusion criteria: yes: "receiving anticoagulation treatment or were unable to complete questionnaires, give consent or attend for follow‐up" |
|
| Interventions | Firebreak full‐thickness skin graft closure vs z‐plasty closure |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 36 months Low‐quality evidence due to risk of bias and imprecision No sources of funding acknowledged; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via sequential envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed sequential envelopes but unclear whether opaque |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not described |
| Selective reporting (reporting bias) | Unclear risk | 2‐Week postoperative data not presented; some data presented only graphically |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation not performed until after contracture excised |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Independent observer unlikely to be blinded |
van Rijssen 2006.
| Methods | Single‐centre Dutch study Randomised controlled trial Recruitment between August 2002 and January 2005 |
|
| Participants | 125 hands in 121 participants 94 male:19 female; 8 incomplete Mean age: 63 years Inclusion criteria: yes: "(1) a flexion contracture of at least 30° in the MCP, PIP, or DIP joints; (2) a clearly defined pathologic cord in the palmar fascia; and (3) willingness to participate in this trial" Exclusion criteria: yes: "(1) patients with postsurgical recurrence or extension of the disease, (2) patients who were not allowed to stop taking their anticoagulants, (3) patients generally unfit to have surgery, and (4) patients who were not willing to participate in this study or had a specific treatment wish" |
|
| Interventions | Percutaneous needle fasciotomy vs Limited fasciectomy |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 6 weeks Low‐quality evidence due to risk of bias and imprecision No sources of funding acknowledged; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via sealed sequential envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed sequential envelopes but unclear whether opaque |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not different between groups |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
van Rijssen 2012a.
| Methods | (as per van Rijssen 2006) Single‐centre Dutch study Randomised controlled trial Recruitment between August 2002 and January 2005 |
|
| Participants | 93 participants (out of 113 patients studied in van Rijssen 2006) 76 male:17 female Mean age 62.8 years for needle fasciotomy; 63.1 years for limited fasciectomy Inclusion criteria: yes: "(1) a flexion contracture of at least 30° in the MCP, PIP, or DIP joints; (2) a clearly defined pathologic cord in the palmar fascia; and (3) willingness to participate in this trial" Exclusion criteria: yes: "(1) patients with postsurgical recurrence or extension of the disease, (2) patients who were not allowed to stop taking their anticoagulants, (3) patients generally unfit to have surgery, and (4) patients who were not willing to participate in this study or had a specific treatment wish" |
|
| Interventions | Percutaneous needle fasciotomy vs Limited fasciectomy |
|
| Outcomes |
|
|
| Notes | Length of follow‐up: 5 years Low‐quality evidence due to risk of bias and imprecision No sources of funding acknowledged; no conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether envelopes were numbered but were numbered in van Rijssen 2006, in which early outcomes of same study were reported; unclear whether opaque |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significantly different attrition between cohorts possibly because of differences in true outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes presented |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
Abbreviations:
5‐FU: 5‐Fluorouracil.
DASH: Disabilities of the Arm, Shoulder and Hand Scale.
DIPJ: Distal interphalangeal joint.
MCPJ: Metacarpophalangeal joint.
PIPJ: Proximal interphalangeal joint.
PROM: Patient‐reported outcome measure.
TAED: Total active extension deficit.
VAS: Visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atroshi 2014 | Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial |
| Barros 1997 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Bendon 2012 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Braga Silva 1999 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Braga Silva 2002 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Castro 1981 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Cervero 2013 | Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial |
| Craft 2011 | Excluded on the basis of question 5 of Appendix 3; no concurrent control and intervention groups |
| Dias 2013 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Dib 2008 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Dickie 1967 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Erne 2014 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Evans 2002 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Ferry 2013 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Galbiatti 1995 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Glassey 2001 | Excluded on the basis of question 5 of Appendix 3; retrospective service evaluation of participants treated with a splint and those treated without a splint based on clinical grounds rather than randomisation/pseudorandomisation |
| Gomes 1984 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Halliday 1966 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Herrera 2013 | Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial |
| Hovius 2011 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Jerosch‐Herold 2008 | Excluded on the basis of question 3 of Appendix 3; publication reports the protocol of a study; final study is described in Jerosch‐Herold 2011, which is among the Included studies |
| Larson 2012 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Malta 1984 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Malta 2013 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Moraes Neto 1996 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Nancoo 2007 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Nydick 2013 | Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial |
| Orbezo 1999 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Ould‐Slimane 2013 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Pereira 2012 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Pesco 2008 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Pess 2012 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Reuben 2006 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Rives 1992 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Skoff 2004 | Excluded on the basis of question 5 of Appendix 3; no concurrent control and intervention groups |
| van Rijssen 2012b | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| Vollbach 2013 | Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial |
| von Campe 2012 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
| White 2012 | Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
Characteristics of studies awaiting assessment [ordered by study ID]
Gazdzik 1997.
| Methods | Unclear |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hazarika 1979.
| Methods | Single‐centre UK study Unclear study design Recruitment dates not specified |
| Participants | 39 participants 17 male:4 female; 18 incomplete Mean age: not presented, range 46 to 76 years Inclusion criteria: not specified Exclusion criteria: not specified |
| Interventions | Intermittent pneumatic postoperative compression vs Boxing glove dressing and roller towel elevation |
| Outcomes |
|
| Notes | Length of follow‐up: 7 days ‐ "one follow‐up appointment" Low‐quality evidence, as extremely limited details of study design provided Funded by the Department of Health and Social Security; no conflicts of interest declared |
Slullitel 1987.
| Methods | Unclear |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ward 1976.
| Methods | Single‐centre UK study Unclear study design Recruitment dates not specified |
| Participants | 20 participants 13 male:7 female Mean age: not reported (range 42 to 81 years) Inclusion criteria: yes: "previously untreated simple Dupuytren's disease" Exclusion criteria: yes: "any illness or medication that might influence fluid retention" |
| Interventions | Elevated hand table for surgery vs Intraoperative tourniquet |
| Outcomes |
|
| Notes | Length of follow‐up: 28 days Low‐quality evidence No funding sources acknowledged; no conflicts of interest declared |
Yoshida 1998.
| Methods | Unclear |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
As the result of difficulty gaining access, the following resources were not searched.
Cochrane Wounds Group Specialised Register BNI (British Nursing Index and Archive).
Sciverse.
Zetoc.
As the journals listed for handsearching are currently indexed in databases searched electronically, we deemed handsearching to be redundant and we did not perform a handsearch. Two additional resources were searched: ISI Web of Science was chosen as a source of conference abstracts, and clinicaltrials.gov was searched.
Given the comprehensive and inclusive nature of the search strategies listed in Appendix 1 and Appendix 2, and the large number of references retrieved (2464), we believe that this search was comprehensive.
The primary outcomes studied have been reordered to reflect the increasing importance of patient‐reported outcomes among clinical studies since the time the protocol was first written.
Contributions of authors
Jeremy Rodrigues
Contributed to authoring of the protocol. Referenced the protocol. Contributed to the design of the search strategy. Screened abstracts. Assessed risks of bias. Extracted data. Performed meta‐analysis. Co‐authored the main text. Read and approved the final version.
Giles Becker
Contributed to the design of the review. Contributed to the design of the statistical analysis. Screened abstracts. Read and approved the final version.
Cathy Ball
Contributed to the design of the protocol. Contributed to the search strategy. Assessed risks of bias. Extracted data. Read and approved the final version.
Weiya Zhang
Re‐designed the methodology and statistics components of the protocol, following review. Read and approved the final version.
Henk Giele
Contributed to the design of the review. Tested different search strategies to compare effectiveness and appropriateness. Read and approved the final version.
Jonathan Hobby
Contributed to the design of the protocol. In particular, contributed to the design of the statistical analysis. Read and approved the final version.
Anna L. Pratt
Contributed to the interpretation of results, particularly of the meta‐analysis. Read and approved the final version.
Tim Davis
Conceived of the review. Acted as guarantor of the review. Served as primary author of the protocol. Resolved conflicts in study selection, risk of bias assessment and data extraction. Contributed to the authorship of the review. Read and approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
British Society for Surgery of the Hand (BSSH), UK.
Contributed to funding Mr Rodrigues' Research Fellowship
-
Nottingham Hospitals Charity, UK.
Contributed to funding Mr Rodrigues' Research Fellowship
-
Nottingham Orthopaedic Walk, UK.
Contributed to funding Mr Rodrigues' Research Fellowship
-
National Institute for Health and Care Excellence (NICE) Fellows & Scholars Programme, UK.
A scholarship has provided analytical and educational support during Mr Rodrigues' period of full‐time research, and a fellowship has provided further support since.
-
Kennedy Institute Trust for Rheumatology Research and Health Innovation Challenge Fund (Wellcome Trust + Department of Health), UK.
Contributed to funding Catherine Ball
Declarations of interest
None to declare.
New
References
References to studies included in this review
Bhatia 2002 {published data only}
- Bhatia R, Blackshaw G, Barr V, Savage R. Comparative study of "staples versus sutures" in skin closure following Dupuytren's surgery. Journal of Hand Surgery (British & European Volume) 2002;27(1):53‐4. [DOI] [PubMed] [Google Scholar]
Bulstrode 2004 {published data only}
- Bulstrode NW, Bisson M, Jemec B, Pratt AL, McGrouther DA, Grobbelaar A. A prospective randomised clinical trial of the intra‐operative use of 5‐fluorouracil on the outcome of Dupuytren's disease. Journal of Hand Surgery (British & European Volume) 2004;29(1):18‐21. [DOI] [PubMed] [Google Scholar]
Chignon‐Sicard 2012 {published data only}
- Chignon‐Sicard B, Georgiou CA, Fontas E, David S, Dumas P, Ihrai T, et al. Efficacy of leukocyte‐ and platelet‐rich fibrin in wound healing: a randomized controlled clinical trial. Plastic and Reconstructive Surgery 2012;130(6):819e‐29e. [DOI] [PubMed] [Google Scholar]
Citron 2003 {published data only}
Citron 2005 {published data only}
Collis 2013 {published data only}
- Collis J, Collocott S, Hing W, Kelly E. The effect of night extension orthoses following surgical release of Dupuytren contracture: a single‐center, randomized, controlled trial. Journal of Hand Surgery (American Volume) 2013;38(7):1285‐94. [DOI] [PubMed] [Google Scholar]
Degreef 2014 {published data only}
- Degreef I, Tejpar S, Sciot R, Smet L. High‐dosage tamoxifen as neoadjuvant treatment in minimally invasive surgery for Dupuytren disease in patients with a strong predisposition toward fibrosis: a randomized controlled trial. Journal of Bone & Joint Surgery (American Volume) 2014;96(8):655‐62. [DOI] [PubMed] [Google Scholar]
Howard 2009 {published data only}
- Howard K, Simison AJM, Morris A, Bhalaik V. A prospective randomised trial of absorbable versus non‐absorbable sutures for wound closure after fasciectomy for Dupuytren's contracture. Journal of Hand Surgery (British & European Volume) 2009;34(5):618‐20. [DOI] [PubMed] [Google Scholar]
Jerosch‐Herold 2011 {published data only}
- Jerosch‐Herold C, Shepstone L, Chojnowski AJ, Larson D, Barrett E, Vaughan SP. Night‐time splinting after fasciectomy or dermo‐fasciectomy for Dupuytren's contracture: a pragmatic, multi‐centre, randomised controlled trial. BMC Musculoskeletal Disorders 2011;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemler 2012 {published data only}
- Kemler MA, Houpt P, Horst CMAM. A pilot study assessing the effectiveness of postoperative splinting after limited fasciectomy for Dupuytren’s disease. Journal of Hand Surgery (British & European Volume) 2012;37(8):733‐7. [DOI] [PubMed] [Google Scholar]
McMillan 2012 {published data only}
- McMillan C, Binhammer P. Steroid injection and needle aponeurotomy for Dupuytren contracture: a randomized, controlled study. Journal of Hand Surgery (American Volume) 2012;37:1307‐12. [DOI] [PubMed] [Google Scholar]
Ullah 2009 {published data only}
- Ullah AS, Dias JJ, Bhowal B. Does a 'firebreak' full‐thickness skin graft prevent recurrence after surgery for Dupuytren's contracture? A prospective, randomised trial. Journal of Bone and Joint Surgery (British Volume) 2009;91(3):374‐8. [DOI] [PubMed] [Google Scholar]
van Rijssen 2006 {published data only}
- Rijssen AL, Gerbrandy FSJ, Linden HT, Klip H, Werker PMN. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren's disease: a 6‐week follow‐up study. Journal of Hand Surgery (American Volume) 2006;31(5):717‐25. [DOI] [PubMed] [Google Scholar]
van Rijssen 2012a {published data only}
- Rijssen AL, ter Linden H, Werker PM. Five‐year results of a randomized clinical trial on treatment in Dupuytren's disease: percutaneous needle fasciotomy versus limited fasciectomy. Plastic and Reconstructive Surgery 2012;129(2):469‐77. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atroshi 2014 {published data only}
- Atroshi I, Strandberg E, Lauritzson A, Ahlgren E, Walden M. Costs for collagenase injections compared with fasciectomy in the treatment of Dupuytren's contracture: a retrospective cohort study. BMJ Open 2014;4(1):e004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barros 1997 {published data only}
- Barros F, Barros A, Almeida S. Dupuytren's diseases: evaluation of 100 cases [Enfermidade de Dupuytren: avaliaçião de 100 casos]. Revista Brasileira de Ortopedia 1997;32(3):177‐83. [Google Scholar]
Bendon 2012 {published data only}
- Bendon CL, Giele HP. Collagenase for Dupuytren's disease of the thumb. Journal of Bone and Joint Surgery (British Volume) 2012;94(10):1390‐2. [DOI] [PubMed] [Google Scholar]
Braga Silva 1999 {published data only}
- Braga Silva J, Fernandes H, Fridman M. The open palm technique in severe Dupuytren contracture [A técnica da palma aberta na contratura grave de Dupuytren]. Revista Brasileira Ortopedia 1999;34:51‐4. [Google Scholar]
Braga Silva 2002 {published data only}
- Silva Braga J, Kuyven CR, Martins PDE. The open palm technique in severe Dupuytren's contracture. Revista da Sociedade Brasileira de Cirurgia Plástica 2002;17:61‐5. [Google Scholar]
Castro 1981 {published data only}
- Castro O. Dupuytren's contracture: surgical treatment by the open palm technique [Contratura de Dupuytren: tratamento cirurgico pela tecnica "open palm"]. Ceará Médico 1981;3:13‐7. [Google Scholar]
Cervero 2013 {published data only}
- Cervero RS, Ferrando NF, Jornet JP. Use of resources and costs associated with the treatment of Dupuytren's contracture at an orthopedics and traumatology surgery department in Denia (Spain): collagenase Clostridium histolyticum versus subtotal fasciectomy. BMC Musculoskeletal Disorders 2013;14:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Craft 2011 {published data only}
- Craft RO, Smith AA, Coakley B, Casey WJ, Rebecca AM, Duncan SFM. Preliminary soft‐tissue distraction versus checkrein ligament release after fasciectomy in the treatment of Dupuytren proximal interphalangeal joint contractures. Plastic and Reconstructive Surgery 2011;128(5):1107‐13. [DOI] [PubMed] [Google Scholar]
Dias 2013 {published data only}
- Dias JJ, Singh HP, Ullah A, Bhowal B, Thompson JR. Patterns of recontracture after surgical correction of Dupuytren disease. Journal of Hand Surgery (American Volume) 2013;38:1987‐93. [DOI] [PubMed] [Google Scholar]
Dib 2008 {published data only}
- Dib CC, Gervais J, Monteiro CGZ, Mendoza E, Pimentel RAP. Dupuytren’s disease: our conduct [Doença de Dupuytren: nossa conduta]. Revista da Sociedade Brasileira de Cirurgia Plástica 2008;23(4):290‐3. [Google Scholar]
Dickie 1967 {published data only}
- Dickie WRD. Dupuytren's contracture. A review of the late results of radical fasciectomy. British Journal of Plastic Surgery 1967;20:311‐4. [DOI] [PubMed] [Google Scholar]
Erne 2014 {published data only}
- Erne HC. Downgrading severe stages of Dupuytren's contracture to simplify partial aponeurectomy using percutaneous needle fasciotomy. Plastic and Reconstructive Surgery 2014;133(1):79e‐80e. [DOI] [PubMed] [Google Scholar]
Evans 2002 {published data only}
- Evans RB, Dell PC, Fiolkowski P. A clinical report of the effect of mechanical stress on functional results after fasciectomy for Dupuytren's contracture. Journal of Hand Therapy 2002;15:331‐9. [DOI] [PubMed] [Google Scholar]
Ferry 2013 {published data only}
- Ferry N, Lasserre G, Pauchot J, Lepage D, Tropet Y. Characteristics of Dupuytren's disease in woman. A study of 67 cases [Particularités de la maladie de Dupuytren chez la femme. À propos de 67 cas]. Annales de Chirurgie Plastique et Esthetique 2013;58(6):663‐9. [DOI] [PubMed] [Google Scholar]
Galbiatti 1995 {published data only}
- Galbiatti JA, Fiori JM, Mansano RT, Durigan JA. Treatment of Dupuytren's illness using straight longitudinal incision completed with "Z" drawn surgery [Tratamento da moléstia de Dupuytren pela técnica de incisão longitudinal reta, complementada com Z‐plastia]. Revista Brasileira Ortopedia 1995;30:207‐12. [Google Scholar]
Glassey 2001 {published data only}
- Glassey N. A study of the effect of night extension splintage on post‐fasciectomy Dupuytren's patients. British Journal of Hand Therapy 2001;6:89‐94. [Google Scholar]
Gomes 1984 {published data only}
- Gomes CA. Dupuytren's disease: experience of partial fasciectomy in 11 cases [Doenca de Dupuytren: experiencia da fasciectomia parcial em 11 casos]. Ceára Médico 1984;6:79‐81. [Google Scholar]
Halliday 1966 {published data only}
Herrera 2013 {published data only}
- Herrera FA, Benhaim P, Suliman A, Roostaeian J, Azari K, Mitchell S. Cost comparison of open fasciectomy versus percutaneous needle aponeurotomy for treatment of Dupuytren contracture. Annals of Plastic Surgery 2013;70(4):454‐6. [DOI] [PubMed] [Google Scholar]
Hovius 2011 {published data only}
- Hovius SE, Kan HJ, Smit X, Selles RW, Cardoso E, Khouri RK. Extensive percutaneous aponeurotomy and lipografting: a new treatment for Dupuytren disease. Plastic and Reconstructive Surgery 2011;128:221‐8. [DOI] [PubMed] [Google Scholar]
Jerosch‐Herold 2008 {published data only}
- Jerosch‐Herold C, Shepstone L, Chojnowski AJ, Larson D. Splinting after contracture release for Dupuytren's contracture (SCoRD): protocol of a pragmatic, multi‐centre, randomized controlled trial. BMC Musculoskeletal Disorders 2008;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2012 {published data only}
- Larson D. The relative responsiveness of patient‐rated outcome measures in evaluating clinical change after Dupuytren's surgery: a literature review and prospective observational pilot study. Hand Therapy 2012;17(3):52‐9. [Google Scholar]
Malta 1984 {published data only}
- Malta MC, Vianna SE, Schott PC. Open palm technique in Dupuytren's contracture [A tecnica da palma aberta na contratura de Dupuytren]. Revista Brasileira Ortopedia 1984;19:46‐8. [Google Scholar]
Malta 2013 {published data only}
- Malta MC, Alves MdPT, Malta LMdA. Open palm technique in Dupuytren's disease treatment [A técnica da palma aberta no tratamento da doença de Dupuytren]. Revista Brasileira de Ortopedia 2013;48(3):246‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moraes Neto 1996 {published data only}
- Moraes Neto GP, Chambriard C, Knackfuss I, Osório L, Couto P. Percutaneous fasciotomy for the correction of metacarpophalangeal joint derformity on Dupuytren's disease [Fasciotomia percutânea na correção da deformidade da articulação metacarpofalângica na contratura de Dupuytren]. Revista Brasileira Orthopedia 1996;31:347‐50. [Google Scholar]
Nancoo 2007 {published data only}
- Nancoo T, Kang N, Kambhampati B, Floyd D, White J, Grouther DA. Severe Dupuytren's PIP joint contracture treated by a two stage technique: a pilot study. Proceedings of the 10th Congress of the International Federation of Societies for Surgery of the Hand; 7th Congress of the International Federation of Societies for Hand Therapy. Bologna: Medimond International Proceedings, 2007:303‐7.
Nydick 2013 {published data only}
- Nydick JA, Olliff BW, Garcia MJ, Hess AV, Stone JD. A comparison of percutaneous needle fasciotomy and collagenase injection for Dupuytren disease. Journal of Hand Surgery (American Volume) 2013;38(12):2377‐80. [DOI] [PubMed] [Google Scholar]
Orbezo 1999 {published data only}
- Orbezo F, Trueba DC, Alvarez IE, Preciado Aceves ME, Sale H Larrañaga SS, Navarrete Alvarez JM. Dupuytren's diasease: report of 100 cases: experience of surgical treatment [Reporte de 100 casos, experiencia de tratamiento quirúrgico en el Hospital Español de México y el Hospital San Eloy: Vizcaya España]. Revista Mexicana de Ortopedia y Traumatología 1999;13:269‐72. [Google Scholar]
Ould‐Slimane 2013 {published data only}
- Ould‐Slimane M, Guinet V, Foulongne E, Melconian A, Beccari R, Milliez PY, et al. Razemon's lateral digital rotation flap in severe Dupuytren contracture of the fifth finger [Le lambeau de rotation latéro‐digital de Razemon dans les formes graves de la maladie de Dupuytren du cinquième rayon]. Chirurgie de la Main 2013;32(5):317‐21. [DOI] [PubMed] [Google Scholar]
Pereira 2012 {published data only}
- Pereira A, Massada M, Sousa R, Silva C, Trigueiros M, Lemos R. Percutaneous needle fasciotomy in Dupuytren's contracture: is it a viable technique?. Acta Orthopaedica Belgica 2012;78(1):30‐4. [PubMed] [Google Scholar]
Pesco 2008 {published data only}
- Pesco MS. A comparison of palmar static digit extension splinting and dorsal block dynamic digit extension splinting with postoperative Dupuytren's contracture release. Proceedings of the 7th Congress of the Asian Pacific Federation of Societies for Surgery of the Hand. Bologna: Medimond International Proceedings, 2008:25‐30.
Pess 2012 {published data only}
- Pess GM, Pess RM, Pess RA. Results of needle aponeurotomy for Dupuytren contracture in over 1,000 fingers. Journal of Hand Surgery (American Volume) 2012;37(4):651‐6. [DOI] [PubMed] [Google Scholar]
Reuben 2006 {published data only}
- Reuben SS, Pristas R, Dixon D, Faruqi S, Madabhushi L, Wenner S. The incidence of complex regional pain syndrome after fasciectomy for Dupuytren's contracture: a prospective observational study of four anesthetic techniques. Anesthesia and Analgesia 2006;102:499‐503. [DOI] [PubMed] [Google Scholar]
Rives 1992 {published data only}
- Rives K, Gelberman R, Smith B, Carney K. Severe contractures of the proximal interphalangeal joint in Dupuytren's disease: results of a prospective trial of operative correction and dynamic extension splinting. Journal of Hand Surgery (American Volume) 1992;17:1153‐9. [DOI] [PubMed] [Google Scholar]
Skoff 2004 {published data only}
van Rijssen 2012b {published data only}
- Rijssen AL, Werker PMN. Percutaneous needle fasciotomy for recurrent Dupuytren disease. Journal of Hand Surgery (American Volume) 2012;37(9):1820‐3. [DOI] [PubMed] [Google Scholar]
Vollbach 2013 {published data only}
- Vollbach FH, Walle L, Fansa H. Dupuytren's disease ‐ patient satisfaction and functional results one year after partial fasciectomy and injection of collagenase [Morbus Dupuytren – Patientenzufriedenheit und funktionelle Ergebnisse ein Jahr nach partieller Aponeurektomie und Injektion von Kollagenase]. Handchirurgie Mikrochirurgie Plastische Chirurgie 2013;45(5):258‐64. [DOI] [PubMed] [Google Scholar]
von Campe 2012 {published data only}
- Campe A, Mende K, Omaren H, Meuli‐Simmen C. Painful nodules and cords in Dupuytren disease. Journal of Hand Surgery (American Volume) 2012;37(7):1313‐8. [DOI] [PubMed] [Google Scholar]
White 2012 {published data only}
- White JW, Kang SN, Nancoo T, Floyd D, Kambhampati SBS, McGrouther DA. Management of severe Dupuytren's contracture of the proximal interphalangeal joint with use of a central slip facilitation device. Journal of Hand Surgery (European Volume) 2012;37(8):728‐32. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gazdzik 1997 {published data only}
- Gazdzik T, Wasilewski Z. Results of surgical treatment for Dupuytren contracture [Wyniki leczenia operacyjnego przykurczu Dupuytrena]. Chirurgia Narzadow Ruchu i Ortopedia Polska 1997;62(5):375‐9. [PubMed] [Google Scholar]
Hazarika 1979 {published data only}
- Hazarika EZ, Knight MT, Frazer‐Moodie A. The effect of intermittent pneumatic compression on the hand after fasciectomy. The Hand 1979;11(3):309‐14. [DOI] [PubMed] [Google Scholar]
Slullitel 1987 {published data only}
- Slullitel M. Our behaviour in Dupuytren's disease [Nuestro comportamiento ante la enfermedad de Dupuytren / Dupuytren´s disease]. Revista de la Asociación Argentina de Ortopedia y Traumatología 1987;52(1):103‐6. [Google Scholar]
Ward 1976 {published data only}
Yoshida 1998 {published data only}
- Yoshida K, Yamada Y, Hara H, Yamanaka K, Inoue H. The surgical treatment for Dupuytren's contracture. 7th Congress of the International Federation of Societies for Surgery of the Hand. Bologna: Medimond International Proceedings, 1998:307‐11.
Additional references
Armstrong 2000
- Armstrong JR, Hurren JS, Logan AM. Dermofasciectomy in the management of Dupuytren's disease. Journal of Bone and Joint Surgery (British Volume) 2000;82(1):90‐4. [DOI] [PubMed] [Google Scholar]
Badois 1993
- Badois FJ, Lermusiaux JL, Masse C, Kuntz D. Non‐surgical treatment of Dupuytren's disease using needle fasciotomy [Traitement non chirurgical de la maladie de Dupuytren par aponévrotomie à l'aiguille]. Revue du rhumatisme (Ed. française) 1993;60(11):808‐13. [PubMed] [Google Scholar]
Ball 2002
- Ball C, Nanchahal J. The use of splinting as a non‐surgical treatment for Dupuytren's disease. British Journal of Hand Therapy 2002;7(3):76‐8. [Google Scholar]
Ball 2013
- Ball C, Pratt A, Nanchahal J. Optimal functional outcome measures for assessing treatment for Dupuytren’s disease: a systematic review and recommendations for future practice. BMC Musculoskeletal Disorders 2013;14:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2010
- Becker GW, Davis TR. The outcome of surgical treatments for primary Dupuytren's disease ‐ a systematic review. Journal of Hand Surgery (European Volume) 2010;35(8):623‐6. [DOI] [PubMed] [Google Scholar]
Bisson 2003
- Bisson MA, McGrouther DA, Mudera V, Grobelaar OA. The different characteristics of Dupuytren's disease fibroblasts derived from either nodule or cord: expression of alpha‐smooth muscle actin and the response to stimulation by TGF‐beta 1. Journal of Hand Surgery (British & European Volume) 2003;28(4):351‐6. [DOI] [PubMed] [Google Scholar]
BSSH 2010
- British Society for Surgery of the Hand. BSSH Evidence for Surgical Treatment (BEST). 1: Dupuytren's disease. http://www.bssh.ac.uk/education/guidelines/dd_guidelines.pdf (accessed 29 August 2012).
Burge 1997
- Burge P, Hoy G, Regan P, Milne R. Smoking, alcohol and the risk of Dupuytren's contracture. Journal of Bone and Joint Surgery (British Volume) 1997;79(2):206‐10. [DOI] [PubMed] [Google Scholar]
Darzi 2008
- Darzi A. High Quality Care for All: NHS Next Stage Review Final Report. London: TSO, 2008.
Draviaraj 2004
- Draviaraj KP, Chakrabarti I. Functional outcome after surgery for Dupuytren's contracture: a prospective study. Journal of Hand Surgery (American Volume) 2004;29(5):804‐8. [DOI] [PubMed] [Google Scholar]
Early 1962
- Early PF. Population studies in Dupuytren's contracture. Journal of Bone and Joint Surgery (British Volume) 1962;44:602‐13. [Google Scholar]
Elliot 1999
- Elliot D. The early history of Dupuytren's disease. Hand Clinics 1999;15(1):1–19. [PubMed] [Google Scholar]
FDA 2010
- US Food, Drug Administration. FDA approves Xiaflex for debilitating hand condition. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm199736.htm (accessed 29 August 2012).
Geoghegan 2004
- Geoghegan JM, Forbes J, Clark DI, Smith C, Hubbard R. Dupuytren's disease risk factors. Journal of Hand Surgery (British Volume) 2004;29(5):423‐6. [DOI] [PubMed] [Google Scholar]
Hedges 1982
- Hedges LV. Fitting continuous models to effect size data. Journal of Educational Statistics 1982;7(4):245‐70. [Google Scholar]
Herzog 1951
- Herzog EG. The aetiology of Dupuytren's contracture. Lancet 1951;1(6668):1305‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hudak 1996
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG). American Journal of Industrial Medicine 1996;29(6):602‐8. [DOI] [PubMed] [Google Scholar]
Hueston 1963
- Hueston JT. The Dupuytren's diathesis. In: Hueston J editor(s). Dupuytren's Contracture. Edinburgh: E & S Livingstone, 1963:51‐63. [Google Scholar]
Hueston 1984
- Hueston JT. 'Firebreak' grafts in Dupuytren's contracture. Australia and New Zealand Journal of Surgery 1984;54:277‐81. [DOI] [PubMed] [Google Scholar]
Hurst 2000
- Hurst LC, Badalamente MA. Histopathology and cell biology. In: Tubiana R, Leclerq C, Hurst L, Badalamente M, Mackin E editor(s). Dupuytren's Disease. London: Martin Dunitz, 2000. [Google Scholar]
Hurst 2009
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, et al. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. New England Journal of Medicine 2009;361(10):968‐79. [DOI] [PubMed] [Google Scholar]
Jebsen 1969
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation 1969;50:311‐9. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Luck 1959
- Luck JV. Dupuytren's contracture: a new concept of the pathogenesis correlated with surgical management. Journal of Hand Surgery (American Volume) 1959;41(4):635‐64. [PubMed] [Google Scholar]
Macey 1995
- Macey AC, Burke FD. Outcomes of hand surgery. Journal of Hand Surgery (European Volume) 1995;20‐B:841‐55. [DOI] [PubMed] [Google Scholar]
Moermans 1991
- Moermans JP. Segmental aponeurectomy in Dupuytren's disease. Journal of Hand Surgery (British Volume) 1991;16(3):243‐54. [DOI] [PubMed] [Google Scholar]
Moermans 1996
- Moermans JP. Long‐term results after segmental aponeurectomy for Dupuytren's disease. Journal of Hand Surgery (British Volume) 1996;21B(6):797‐800. [DOI] [PubMed] [Google Scholar]
NICE 2008
Pratt 2009
- Pratt AL, Byrne G. The lived experience of Dupuytren's disease of the hand. Journal of Clinical Nursing 2009;18:1793‐802. [DOI] [PubMed] [Google Scholar]
Rayan 2005
- Rayan GM, Moore J. Non‐Dupuytren's disease of the palmar fascia. Journal of Hand Surgery (British and European Volume) 2005;30(6):551‐6. [DOI] [PubMed] [Google Scholar]
Review Manager 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Seah 2012
- Seah MK, Harry L, Davidson D. A three‐digit dermofasciectomy for Dupuytren's disease. Hand Surgery 2012;17:109‐10. [DOI] [PubMed] [Google Scholar]
SIGN 2011
- Scottish Intercollegiate Guidelines Network. Search Filters (last modified: 16/09/11). http://www.sign.ac.uk/methodology/filters.html. SIGN 2011, (accessed 29 August 2012).
Stiles 1966
- Stiles PJ. Ultrasonic therapy in Dupuytren's contracture. Journal of Bone and Joint Surgery (British Volume) 1966;48(3):452‐3. [PubMed] [Google Scholar]
Thomas 2010
- Thomas A, Bayat A. The emerging role of Clostridium histolyticum collagenase in the treatment of Dupuytren disease. Journal of Therapeutics and Clinical Risk Management 2010;6:557‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Rijssen 2006a
- Rijssen AL, Werker PM. Percutaneous needle fasciotomy in Dupuytren's disease. Journal of Hand Surgery (British Volume) 2006;31(5):498‐501. [DOI] [PubMed] [Google Scholar]
Werker 2012
- Werker PM, Pess GM, Rijssen AL, Denkler K. Correction of contracture and recurrence rates of Dupuytren contracture following invasive treatment: the importance of clear definitions. Journal of Hand Surgery (American Volume) 2012;37:2095‐105. [DOI] [PubMed] [Google Scholar]
Yost 1955
- Yost J, Winters T, Fett HC. Dupuytren's contracture: a statistical study. American Journal of Surgery 1955;90(4):568‐71. [DOI] [PubMed] [Google Scholar]


