Abstract
Background
Acquired brain injury (ABI) can result in impairments in motor function, language, cognition, and sensory processing, and in emotional disturbances, which can severely reduce a survivor's quality of life. Music interventions have been used in rehabilitation to stimulate brain functions involved in movement, cognition, speech, emotions, and sensory perceptions. An update of the systematic review published in 2010 was needed to gauge the efficacy of music interventions in rehabilitation for people with ABI.
Objectives
To assess the effects of music interventions for functional outcomes in people with ABI. We expanded the criteria of our existing review to: 1) examine the efficacy of music interventions in addressing recovery in people with ABI including gait, upper extremity function, communication, mood and emotions, cognitive functioning, social skills, pain, behavioural outcomes, activities of daily living, and adverse events; 2) compare the efficacy of music interventions and standard care with a) standard care alone, b) standard care and placebo treatments, or c) standard care and other therapies; 3) compare the efficacy of different types of music interventions (music therapy delivered by trained music therapists versus music interventions delivered by other professionals).
Search methods
We searched the Cochrane Stroke Group Trials Register (January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 6), MEDLINE (1946 to June 2015), Embase (1980 to June 2015), CINAHL (1982 to June 2015), PsycINFO (1806 to June 2015), LILACS (1982 to January 2016), and AMED (1985 to June 2015). We handsearched music therapy journals and conference proceedings, searched dissertation and specialist music databases, trials and research registers, reference lists, and contacted relevant experts and music therapy associations to identify unpublished research. We imposed no language restriction. We performed the original search in 2009.
Selection criteria
We included all randomised controlled trials and controlled clinical trials that compared music interventions and standard care with standard care alone or combined with other therapies. We examined studies that included people older than 16 years of age who had ABI of a non‐degenerative nature and were participating in treatment programmes offered in hospital, outpatient, or community settings. We included studies in any language, published and unpublished.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias of the included studies. We contacted trial researchers to obtain missing data or for additional information when necessary. Where possible, we presented results for continuous outcomes in meta‐analyses using mean differences (MDs) and standardised mean differences (SMDs). We used post‐test scores. In cases of significant baseline difference, we used change scores. We conducted a sensitivity analysis to assess the impact of the randomisation method.
Main results
We identified 22 new studies for this update. The evidence for this update is based on 29 trials involving 775 participants. A music intervention known as rhythmic auditory stimulation may be beneficial for improving the following gait parameters after stroke. We found a reported increase in gait velocity of 11.34 metres per minute (95% confidence interval (CI) 8.40 to 14.28; 9 trials; 268 participants; P < 0.00001; moderate‐quality evidence). Stride length of the affected side may also benefit, with a reported average of 0.12 metres more (95% CI 0.04 to 0.20; 5 trials; 129 participants; P = 0.003; moderate‐quality evidence). We found a reported average improvement for general gait of 7.67 units on the Dynamic Gait Index (95% CI 5.67 to 9.67; 2 trials; 48 participants; P < 0.00001). There may also be an improvement in gait cadence, with a reported average increase of 10.77 steps per minute (95% CI 4.36 to 17.18; 7 trials; 223 participants; P = 0.001; low‐quality evidence).
Music interventions may be beneficial for improving the timing of upper extremity function after stroke as scored by a reduction of 1.08 seconds on the Wolf Motor Function Test (95% CI ‐1.69 to ‐0.47; 2 trials; 122 participants; very low‐quality evidence).
Music interventions may be beneficial for communication outcomes in people with aphasia following stroke. Overall, communication improved by 0.75 standard deviations in the intervention group, a moderate effect (95% CI 0.11 to 1.39; 3 trials; 67 participants; P = 0.02; very low‐quality evidence). Naming was reported as improving by 9.79 units on the Aachen Aphasia Test (95% CI 1.37 to 18.21; 2 trials; 35 participants; P = 0.02). Music interventions may have a beneficial effect on speech repetition, reported as an average increase of 8.90 score on the Aachen Aphasia Test (95% CI 3.25 to 14.55; 2 trials; 35 participants; P = 0.002).
There may be an improvement in quality of life following stroke using rhythmic auditory stimulation, reported at 0.89 standard deviations improvement on the Stroke Specific Quality of Life Scale, which is considered to be a large effect (95% CI 0.32 to 1.46; 2 trials; 53 participants; P = 0.002; low‐quality evidence). We found no strong evidence for effects on memory and attention. Data were insufficient to examine the effect of music interventions on other outcomes.
The majority of studies included in this review update presented a high risk of bias, therefore the quality of the evidence is low.
Authors' conclusions
Music interventions may be beneficial for gait, the timing of upper extremity function, communication outcomes, and quality of life after stroke. These results are encouraging, but more high‐quality randomised controlled trials are needed on all outcomes before recommendations can be made for clinical practice.
Plain language summary
Music interventions for acquired brain injury
Review question
We reviewed the evidence for the effects of music interventions on functional outcomes in adults with acquired brain injury.
Background
Acquired brain injury (brain damage through accident or illness, including stroke, that is unlikely to degenerate further) can cause problems with movement, language, sensation, thinking, or emotion. Any of these can severely reduce a survivor's quality of life. Many new treatments have been developed to help recover lost functions and to prevent depression. Music interventions involve using music to aid rehabilitation. Specific treatments may include using rhythm to aid movement and walking; playing music instruments to improve movement; singing to improve speaking and voice quality; listening to music to improve pain management, mood, or thinking; and playing and composing music to improve a sense of well‐being.
Study characteristics
We aimed to identify research studies that tested music interventions combined with standard care for adults with acquired brain injury who were receiving rehabilitation in hospital or community settings. We looked for research that tested the effects of music interventions on walking, moving, communicating, thinking, emotions, pain, and well‐being. Interventions included moving to music, singing, listening to music, composing, playing musical instruments, or a combination of these. We identified and included 29 trials involving 775 adult participants. The evidence is current to June 2015.
Key results
The results suggest that music interventions using rhythm may be beneficial for improving walking in people with stroke, and this may improve quality of life. Music interventions may be beneficial for improving the speed of repetitive arm movements and communication in people with stroke. Music interventions that use a strong beat within music may be more effective than interventions where a strong beat is used without music. Treatment delivered by a trained music therapist might be more effective than treatment delivered by other professionals. Information was insufficient to examine the effects of music interventions on other outcomes. We found no studies that reported on harmful effects.
Quality of the evidence
The quality of the research was generally low. We found only one study that we considered as having a low risk of bias. The quality of the evidence for walking speed and stride length was moderate. The quality of the evidence for other aspects of walking was low. The quality of the evidence for the speed of repetitive arm movements was very low, as was the quality of the evidence for overall communication. The quality of the evidence for quality of life was low. Further clinical trials are needed.
Summary of findings
Summary of findings for the main comparison. Music compared with standard care for acquired brain injury.
| Music compared with standard care for acquired brain injury | |||
| Patient or population: acquired brain injury Setting: outpatient Intervention: music interventions Comparison: control | |||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
| Gait velocity assessed with: metres/minute | The mean gait velocity in the intervention group was 11.34 metres more (8.4 more to 14.28 more). | 268 (9 RCTs) | ⊕⊕⊕⊝ MODERATE 1, 2, 3, 4 |
| Stride length (affected side) assessed with: metres | The mean stride length (affected side) in the intervention group was 0.12 metres more (0.04 more to 0.2 more). | 129 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1, 2, 5, 6 |
| Gait cadence assessed with: steps/minute | The mean gait cadence in the intervention group was 10.77 steps/minute more (4.36 more to 17.18 more). | 223 (7 RCTs) | ⊕⊕⊝⊝ LOW 1, 2, 4, 7 |
| Stride symmetry | The mean stride symmetry in the intervention group was 0.94 standard deviations more (0.32 fewer to 2.2 more). | 139 (3 RCTs) | ⊕⊕⊝⊝ LOW 2, 6, 8, 9 |
| General upper extremity functioning assessed with: Fugl‐Meyer Assessment | The mean general upper extremity functioning in the intervention group was 3.56 units higher (0.88 lower to 8 higher). | 194 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 4, 6, 10 |
| Overall communication | The mean overall communication in the intervention group was 0.75 standard deviations more (0.11 more to 1.39 more). | 67 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 4, 11 |
| Quality of life assessed with: Stroke Specific Quality of Life Scale |
The mean quality of life in the intervention group was 0.89 standard deviations more (0.32 more to 1.46 more). | 53 (2 RCTs) | ⊕⊕⊝⊝ LOW 2, 4, 11 |
| CI: confidence interval; RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1Most studies were rated as at unclear or high risk of bias 2All point estimates favour the music interventions, although the magnitude of the effect differs across studies 3Results were inconsistent across studies, as evidenced by I2 = 61% 4Wide confidence interval; however, this is due to the fact that some studies reported very large beneficial effects 5Results were inconsistent across studies, as evidenced by I2 = 80% 6Wide confidence interval 7Results were inconsistent across studies, as evidenced by I2 = 83% 8One study was rated as at low, one as at unclear, and one as at high risk of bias 9Results were inconsistent across studies, as evidenced by I2 = 90% 10Results were inconsistent across studies, as evidenced by I2 = 85% 11All studies were at high risk of bias
Background
Description of the condition
Acquired brain damage embraces a range of conditions involving rapid onset of brain injury, including trauma due to head injury or postsurgical damage, vascular event such as stroke or subarachnoid haemorrhage, cerebral anoxia, toxic or metabolic insult such as hypoglycaemia, and infection or inflammation (RCP 2012). Acquired brain injury (ABI) can result in impairments in motor function, language, cognition, sensory processing, as well as emotional disturbances. Hemiplegia and hemiparesis are common and may severely reduce a survivor's quality of life. Consequently, a primary concern in rehabilitation for ABI is the restoration of motor function. The improvement of ambulation and upper extremity function directly affects the level of independence of the person with ABI related to activities of daily living. The affected individual is likely to be left with communication impairments, such as a severely reduced ability to understand, speak, and use spoken and written language, which can result in isolation. Furthermore, brain damage often leads to disturbances in memory, learning, and awareness. Sensory disturbances and neuropathic pain can result from damage to the nervous system. Finally, there may be behavioral implications resulting in disinhibition, apathy, and a lack of motivation. Recovery of lost functions and skills after acquired brain damage is typically incomplete, putting survivors at increased risk for depression. Poststroke depression and apathy are estimated to be as high as 33%, impeding functional recovery (Matsuzaki 2015). Mood disorders are considered to be one of the greatest barriers to reintegration back into the community, affecting motivation to engage in rehabilitation (Giles 2006). Effective treatment of depression may bring substantial benefits by improving medical status, enhancing quality of life, and reducing pain and disability (van de Port 2007; Whyte 2006).
Acquired brain injury causes significant levels of disabilities that tend to result in long‐term problems. There were an estimated 316,080 people living with disabilities stemming from stroke, and a further 170,000 people per year who sustained a traumatic brain injury in the UK in 2013 (NA 2014). Figures from the US exceed those in the UK, with an estimated 3.5 million people sustaining a traumatic brain injury each year (Coronado 2012), of whom 125,000 will be left with long‐term disability (Selassie 2008). Approximately 5.3 million Americans, or 2% of the population of all ages, have long term or lifelong needs for help in performing personal activities of daily living following traumatic brain injury (Selassie 2008; Thurman 1999; Zaloshnja 2008). In 2010, 16.9 million people had a first stroke, and the worldwide prevalence of stroke was 33 million (Mozaffarian 2015).
Global health burden attributed to ABI resulting from stroke and traumatic brain injury is considerable. Furthermore, with the population ageing, even if the stroke incidence stagnates, the number of people with stroke requiring medical and rehabilitation care will rise dramatically (WHO 2014). In Europe alone in 2010, estimated costs were EUR 64.1 billion for stroke and EUR 33.0 billion for traumatic brain injury (Gustavsson 2010). In the USA, traumatic brain injury annual costs are estimated at USD 221 billion, comprising USD 14.6 billion for medical costs, USD 69.2 billion for work loss, and USD 137 billion for lost quality of life (Orman 2011). Acquired brain injury therefore has significant effects on society in terms of human and economic costs.
Description of the intervention
Many innovative therapy methods have been developed to help restore lost functions and aid in the prevention and treatment of depression in ABI. Music therapy has been used in rehabilitation settings to stimulate brain functions involved in movement, cognition, speech, emotions, and sensory perceptions. Music interventions range from the use of rhythmic auditory stimulation (RAS) to aid in the execution of movement and normalisation of gait parameters (Thaut 1993), to music listening and singing to reduce pain (Kim 2005), to the use of music listening, music improvisations, composition, and song discussions to address emotional needs and enhance sense of well‐being (Nayak 2000). While music interventions are traditionally implemented by trained music therapists, other health professionals may also use music to facilitate therapeutic outcomes. For example, music listening has been used by other health professionals in rehabilitation settings to enhance cognitive recovery and to improve mood (Särkämö 2008). Music interventions utilised in therapy are distinguished from passive music listening or recreational music activities when the following components are present: 1) implementation of goal‐directed music interventions by a trained health professional, or 2) the use of music experiences individualised to the need of the person with ABI. In rehabilitation settings, these interventions may include 1) listening and moving to live, improvised, or pre‐recorded music as well as RAS, 2) performing or creating music on an instrument, 3) improvising music spontaneously using voice or instruments or both, 4) singing or vocal activities to music, 5) music‐based speech and language activities, 6) composing music, and 7) music combined with other modalities (e.g. imagery, art) (Dileo 2007; Magee 2006b; Magee 2009). Music therapy (in comparison with music interventions more broadly) is delivered by a professional with specific clinical training in music therapy, who offers a systematic therapeutic process including assessment, treatment, and evaluation. Music therapy treatment involves the presence of a therapeutic process and the use of personally tailored music experiences.
How the intervention might work
Biomedical theories suggest that neurophysiological processes may be activated through musical stimulation and used to affect non‐musical behaviour and encourage neuroplasticity (Thaut 2014a). Following neurological injury, major neural reorganisation is common. Music interventions aim to capitalise on this naturally occurring neuroplastic change by enriching the environment of the person with ABI to promote functional gains (Särkämö 2008).
Music is physiologically arousing, entrains movement, and can motivate exercise and override pain perception. In particular, rhythm in music is a strong driving stimulus for motor function (Clark 2016). This influence of rhythm may be useful in physical rehabilitation, for example gait retraining and upper limb co‐ordination (Thaut 1997; Thaut 2002). Speech and language skills can also be addressed using music interventions. Singing is a motivating way to practice the structured movement behaviours necessary for speech rehabilitation, as it requires controlled deep breathing, phonation, pitch control, rhythmic accuracy, controlled volume, and articulation of lyrics (Baker 2011). Furthermore, melodic intonation therapy uses the unimpaired singing ability of a person with brain injury to rehabilitate impaired language skills (Norton 2009).
Music is processed diffusely in the brain, meaning that music interventions can be targeted to address a wide range of cognitive deficits and behavioural and emotional issues. The repetitive and predictable structures in music can act as cues for learning. For example, songs can chunk information to aid in memory formation and recall (Thaut 2014b). In addition to its utility in physical rehabilitation, music has been reported to have positive effects on mood and social participation (Baker 2006). During music participation the brain releases neurochemicals that increase feelings of pleasure and alertness, and decrease anxiety and stress (Altenmuller 2013). Used in a group setting, music participation can provide opportunities for peer support and building social skills to facilitate increased independence (Nayak 2000).
Why it is important to do this review
Many research studies on the use of music in rehabilitation of ABI have suffered from small sample size, making it difficult to achieve statistically significant results. In addition, differences in factors such as study designs, methods of interventions, and intensity of treatment have led to varying results. The first edition of this review included only music therapy interventions involving a trained professional music therapist. However, in order to fully investigate the effects of music interventions in ABI rehabilitation, in this update we have included music interventions delivered by a music therapist or trainees in a music therapy programme, by other medical professionals, or by other health professionals with training in rehabilitation. This systematic review aimed to gauge more accurately the efficacy of music interventions in rehabilitation for people with ABI as well as to identify variables that may moderate any effects.
Objectives
To assess the effects of music interventions for functional outcomes in people with ABI. We expanded the criteria of our existing review to: 1) examine the efficacy of music interventions in addressing recovery in people with ABI including gait, upper extremity function, communication, mood and emotions, cognitive functioning, social skills, pain, behavioural outcomes, activities of daily living, and adverse events; 2) compare the efficacy of music interventions and standard care with a) standard care alone, b) standard care and placebo treatments, or c) standard care and other therapies; 3) compare the efficacy of different types of music interventions (music therapy delivered by trained music therapists versus music interventions delivered by other professionals).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials and controlled clinical trials with quasi‐randomised or systematic methods of treatment allocation in any language, published and unpublished. We conducted a sensitivity analysis to assess the impact of the randomisation method.
Types of participants
We included people of any gender older than 16 years of age who had acquired brain damage of a non‐degenerative nature and were participating in treatment programmes offered in hospital, outpatient, or community settings at the time that they received the music intervention. This included traumatic brain injury, stroke, anoxia, infection, and any mixed cause. We excluded any condition of a progressive nature. We did not use the site of lesion and stage of rehabilitation as inclusion or exclusion criteria.
Types of interventions
We included all studies in which standard treatment combined with music interventions was compared with: 1) standard care alone, 2) standard care with placebo, or 3) standard care combined with other therapies. We considered studies where the music interventions were delivered by a formally trained music therapist, by trainees in a formal music therapy programme, or by professionals other than trained music therapists. We included studies in which one or more of the following music interventions was used.
Interventions in which musical instruments are played (e.g. clinical improvisation in which participants are involved in active music making in dialogue with the therapist, therapeutic instrumental musical performance, cognitive training with drums).
Singing and music‐based voice interventions (e.g. song‐singing programmes, melodic intonation therapy or modified melodic intonation therapy, vocal intonation therapy, rhythmic speech cueing, and therapeutic singing).
RAS or rhythmic auditory cueing (RAC).
Receptive interventions in which participants listen to music.
Songwriting.
Any combination of the above.
Types of outcome measures
Primary outcomes
Rehabilitation of mobility is crucial in ABI rehabilitation to enhance personal independence. We therefore selected the following primary outcomes for this review.
Improvement in gait, measured by changes in gait velocity, cadence, stride length, stride symmetry, stride timing, general gait, balance.
Improvement in upper extremity function (UEF), measured by general UEF, timing of UEF, range of motion, hand function, upper limb strength, manual dexterity, and elbow extension.
Secondary outcomes
Communication (e.g. language production, speech production, parameters of voice production, speaking fundamental frequency).
Mood and emotions (e.g. depression, anger, anxiety).
Social skills and interactions (e.g. eye contact, non‐verbal interactions).
Pain.
Behavioural outcomes (e.g. participation in treatment, motivation, self esteem).
Cognitive functioning.
Activities of daily living.
Adverse events (e.g. death, fatigue, falls).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers where necessary. We imposed no language restrictions for either searching or trial inclusion.
Electronic searches
We searched the following electronic databases and trials registers. Due to our changed criteria, we updated the previously run searches from our 2010 review; however, we ran searches from the inception of each database. The original searches are detailed in the appendices.
Cochrane Stroke Group Trials Register (last searched by the Managing Editor on 5 January 2016).
Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 6, part of the Cochrane Library (www.thecochranelibrary.com); accessed 11 June 2015; Appendix 1).
MEDLINE (1946 to June 2015; Appendix 2).
Embase (1980 to June 2015; Appendix 3).
CINAHL (1982 to June 2015; Appendix 4).
PsycINFO (1806 to June 2015; Appendix 5).
LILACS (Latin American and Caribbean Health Sciences Literature) (1982 to January 2016; Appendix 6).
AMED (Allied and Complementary Medicine) (1985 to June 2015; Appendix 7).
CAIRSS for Music (Computer‐Assisted Information Retrieval Service System) (December 2015; Appendix 8).
ProQuest Digital Dissertations (1861 to August 2015; Appendix 9).
ClinicalTrials.gov (www.clinicaltrials.gov/) (August 2015; Appendix 10).
Current Controlled Trials (www.controlled‐trials.com/) (December 2015; Appendix 11).
We undertook searches of the following for our previous review; however, we could not renew the searches for this update as the databases are no longer functional, no longer maintained, or have been subsumed by other databases we searched: The National Research Register (NRR) Archive, RehabTrials.org, Indexes to Theses in Great Britain and Ireland, and Music Therapy World. We also conducted a search of the Science Citation Index for our previous review; however, we did not have access to this database for this review update and so did not update that search.
Searching other resources
We handsearched the following music therapy journals and conference proceedings:
Arts in Psychotherapy (1974 to 2015;46);
Australian Journal of Music Therapy (1990 to 2015;26);
Australian Music Therapy Association Bulletin (1977 to 2005; final issue);
British Journal of Music Therapy (1987 to 2015;29(1));
Canadian Journal of Music Therapy (1976 to 2015;21(1));
International Journal of the Arts in Medicine (1993 to 1999;6(2), final issue);
Journal of Music Therapy (1964 to 2015;52(4));
Japanese Journal of Music Therapy (2005 to 2013;13(2; latest issue available with online abstracts));
Music and Medicine (2009 to 2015:17(4));
Musik‐, Tanz‐, und Kunsttherapie (Journal for Art Therapies in Education, Welfare and Health Care) (1999 to 2014;25(3));
Musiktherapeutische Umschau (1980 to 2015;35(4));
Music Therapy (1981 to 1996;14(1), final issue);
Music Therapy Yearbook (1951 to 1962; final issue);
Music Therapy Perspectives (1982 to 2015;33(2));
Nordic Journal of Music Therapy (1992 to 2016;25(1));
Music Therapy Today (online journal of music therapy) (2000 to 2007;3, final issue);
New Zealand Journal of Music Therapy (1987 to 2013;11, latest issue available with online abstracts);
Psychomusicology (1981 to 2015:25(4));
Voices (online international journal of music therapy) (2001 to 2015;15(32));
Canadian Conference Proceedings (2004 to 2006);
The World Music Therapy Congress Proceedings (1993 to 2014);
The European Music Therapy Congress Proceedings (1992 to 1998; 2004 to 2010).
Data collection and analysis
Selection of studies
For this update, four review authors (WM, IC, JT, JB) conducted the searches as outlined in the Search methods for identification of studies. One review author (WM) and a graduate research assistant scanned titles and abstracts of each record retrieved from the search and deleted obviously irrelevant references. When we were uncertain as to whether to reject a title or abstract, we obtained the full article, which two review authors (IC and JT) independently inspected. Both review authors used an inclusion criteria form to assess the trial's eligibility for inclusion. One review author (WM) checked the inter‐rater reliability for trial selection, and in the case of disagreement or uncertainty, consulted a third review author (JB). We kept a record of both the article and the reason for exclusion for all excluded studies.
Data extraction and management
Two authors (WM and JB) independently extracted data from the selected trials using a standardised coding form. Any differences in data extraction were discussed. We extracted the following data.
General information
Author
Year of publication
Title
Journal (title, volume, pages)
If unpublished, source
Duplicate publications
Country
Language of publication
Trial information
Study design (parallel group, cross‐over)
Randomisation
Randomisation method
Allocation concealment
Allocation concealment method
Level of blinding (interventionist, objective outcomes, subjective outcomes)
Attrition (rate, reasons for withdrawal)
Intervention information
Type of intervention (e.g. clinical improvisation, therapeutic instrumental musical performance, singing or music‐based voice interventions, RAS or RAC, receptive interventions, songwriting, combination)
Music preference (participant preferred versus researcher selected in cases of music listening)
Professional delivering the intervention (music therapist or other)
Length of intervention
Intensity of intervention
Comparison intervention
Participant information
Total sample size
Number of experimental group
Number of control group
Gender
Age
Ethnicity
Diagnosis
Site of lesion
Setting
Country
Inclusion criteria
Outcomes
We planned to extract statistical information for the following outcomes (if applicable):
parameters of gait (e.g. velocity, cadence, stride length, stride symmetry, stride timing, general gait, balance);
parameters of UEF (e.g. range of movement, hand function, manual dexterity, upper limb strength, elbow extension);
communication outcomes (e.g. language production; parameters of voice production, speaking fundamental frequency);
mood and emotion outcomes (e.g. depression, anger, anxiety);
social interactions outcomes (e.g. eye contact, non‐verbal interactions);
pain;
cognitive functioning (e.g. memory, attention);
behavioural outcomes (e.g. participation in treatment, motivation);
activities of daily living;
adverse events (e.g. death, fatigue, falls).
Assessment of risk of bias in included studies
Two review authors (WM and JB) independently assessed all included trials for trial quality. We used the following criteria for quality assessment.
1. Random sequence generation
Low risk
Unclear risk
High risk
We rated random sequence generation as low risk if every participant had an equal chance to be selected for either condition and if the investigator was unable to predict to which treatment the participant would be assigned. Use of date of birth, date of admission, or alternation resulted in high risk of bias.
2. Allocation concealment
Low risk methods to conceal allocation included:
central randomisation;
serially numbered, opaque, sealed envelopes;
other descriptions with convincing concealment.
Unclear risk: authors did not adequately report on method of concealment.
High risk (e.g. alternation methods were used).
3. Blinding of participants and personnel
Low risk
Unclear risk
High risk
Participants usually cannot be blinded in a music intervention trial, with the exception of studies where pre‐recorded music is used in a comparative trial that compares different types of music. For this reason, we did not downgrade studies for not blinding the participants. As for the personnel delivering the intervention, in many music intervention studies the professional delivering the intervention cannot be blinded because they are actively making music with the participants or providing music for the intervention. We therefore applied downgrading for not blinding personnel only in studies that used interventions where blinding was possible, for example in studies in which listening to pre‐recorded music was the treatment condition and control group participants were provided with headphones but no music (such as a blank CD). This included studies that examined the use of metronome beat as part of the RAS intervention.
4. Blinding of outcome assessors
-
Low risk:
outcome assessors were blinded; or
particular outcome group (i.e. objective outcomes; subjective outcomes) was not included in the review.
Unclear risk: authors did not adequately report on method of blinding.
-
High risk:
outcome assessors were not blinded; or
self report measures were used and participants were not blinded.
5. Incomplete data
We recorded the proportion of participants whose outcomes were analysed. We coded losses to follow‐up for each outcome as follows.
Low risk: if fewer than 20% of participants were lost to follow‐up, and reasons for loss to follow‐up were similar in both treatment arms.
Unclear risk: if loss to follow‐up was not reported.
High risk: if more than 20% of participants were lost to follow‐up, or reasons for loss to follow‐up differed between treatment arms.
6. Selective reporting
Low risk: reports of the study were free of the suggestion of selective outcome reporting.
Unclear risk: unclear if reports of the study included selective outcome reporting.
High risk: reports of the study suggested selective outcome reporting.
7. Financial conflict of interest
We considered information on potential financial conflicts of interest as a possible source of additional bias.
Low risk: unlikely that other sources of bias influenced the results.
Unclear risk: unclear if other sources of bias may have influenced the results.
High risk: likely that other sources of bias influenced the results.
We used the above criteria to give each article an overall quality rating based on Section 8.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Low risk of bias: all criteria met.
Moderate risk of bias: one or more of the criteria only partially met.
High risk of bias: one or more criteria not met.
We did not exclude studies based on a low quality score.
Measures of treatment effect
We presented all outcomes in this review as continuous variables. We calculated standardised mean differences (SMDs) with 95% confidence intervals (CIs) for outcome measures using results from different scales. When sufficient data were available from various studies using the same measurement instrument, we computed a mean difference (MD) with 95% CI.
Unit of analysis issues
In all studies included in this review, participants were individually randomised to the intervention or the standard‐care control group. We collected and analysed post‐test values or change values on a single measurement for each outcome from each participant.
Dealing with missing data
We analysed data on an endpoint basis, including only participants for whom final data point measurement was obtained (available‐case analysis). We did not assume that participants who dropped out after randomisation had a negative outcome.
Assessment of heterogeneity
We investigated heterogeneity using the I2 test with I2 greater than 50% indicating significant heterogeneity.
Assessment of reporting biases
We tested for publication bias visually in the form of funnel plots (Higgins 2011).
Data synthesis
One review author (JB) entered all trials included in the systematic review into Review Manager 5 (RevMan 2014). JB conducted the data analysis, and WM reviewed the analysis for accuracy. We presented the main outcomes in this review as continuous variables. We calculated SMDs for outcome measures using the results from different scales, and computed MDs for results using the same scales. We calculated pooled estimates using the random‐effects model. We determined levels of heterogeneity using the I2 statistic (Higgins 2002). We calculated 95% CIs for each effect size estimate. This review did not include any categorical variables.
For cross‐over trials, we used the guidelines by Elbourne 2002 for the inclusion of cross‐over trials in meta‐analyses that include both parallel‐group and cross‐over trials. When statistical information regarding the within‐individual comparison of treatment was available, we used or computed estimates of the treatment effects and associated standard errors. If these data were not available, we opted to use data from the first period only if those data were reported separately. A third option was to treat the results as if they came from a study of parallel‐group design. We favoured this option the least, as according to Elbourne and colleagues it ignores the within‐patient correlation and results in an underestimate of the treatment effect (Elbourne 2002).
We made the following treatment comparison: music interventions versus standard care alone.
Subgroup analysis and investigation of heterogeneity
We planned the following subanalyses a priori as described by Deeks 2001 and as recommended in Section 8.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
type of music intervention;
interventionist (music therapist or other);
dosage of music intervention; and
diagnosis.
We performed subanalyses on intervention where possible; however, for most interventions there were not enough studies per outcome to do so. We did not perform subanalyses on diagnosis, as the populations in the studies that examined the same outcomes were heterogenous.
Sensitivity analysis
We examined the impact of group allocation method by comparing the results of including and excluding trials that used inadequate or unclear randomisation methods.
Results
Description of studies
Results of the search
For the original review, the database searches and handsearching of conference proceedings and journals identified 3855 unique citations, of which 94 references were identified for possible inclusion. After further title and abstract scanning, 14 references to seven studies were identified that met all of the inclusion criteria (see Figure 1).
1.
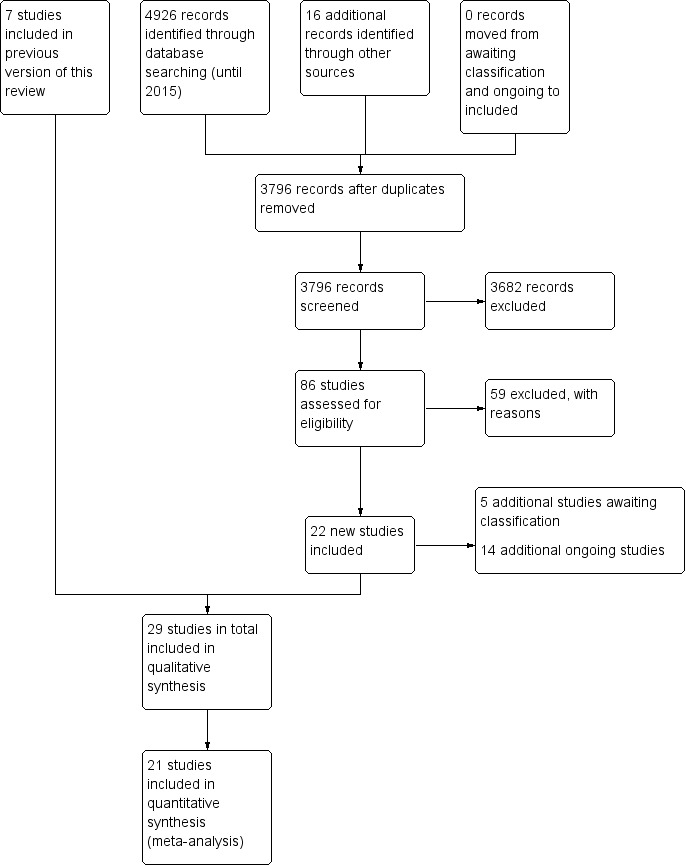
Study flow diagram for the updated review.
The 2016 update of the search, based on the revised inclusion criteria, resulted in 3796 additional citations. One review author (WM) and a graduate research assistant scanned the titles and abstracts and identified 100 references to 86 studies for possible inclusion, which two review authors (IC and JT) independently screened. We consulted another review author (JB) where needed. We included 29 references to 22 new studies in this review update (see Characteristics of included studies) (Baker 2001; Cha 2014a; Cha 2014b; Chouan 2012; Conklyn 2012; Fernandes 2014; Hill 2011; Jeong 2007; Jungblut 2004; Kim 2005; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Mueller 2013; O'Kelly 2014; Park 2010a; Paul 1998; Pool 2012; Särkämö 2008; Schneider 2007; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Tong 2015; Van Delden 2013; van der Meulen 2014; Whitall 2011). We contacted chief investigators to obtain additional information on study details and data where necessary.
The studies that had been classified in our previous review as awaiting assessment (N = 1) and ongoing (N = 3) have now been excluded. We reclassified one study that was previously excluded as included in this review update, given the revised inclusion criteria. In this update, five further studies are awaiting classification and 14 additional studies are ongoing (see Figure 1).
Included studies
We included 29 studies (24 randomised controlled trials (RCTs) and five quasi‐RCTs) with a total of 775 participants. These studies examined the effects of music interventions on gait parameters after stroke (Cha 2014a; Cha 2014b; Chouan 2012; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Park 2010a; Suh 2014; Thaut 1997; Thaut 2007), UEF following stroke (Chouan 2012; Hill 2011; Jeong 2007; Paul 1998; Schneider 2007; Thaut 2002; Tong 2015; Van Delden 2013; Whitall 2011), communication outcomes following stroke (Conklyn 2012; Jungblut 2004; Särkämö 2008; van der Meulen 2014), mood (Jeong 2007; Pool 2012; Särkämö 2008), social skills following stroke (Jeong 2007), pain during exercise following stroke (Kim 2005), behavioural outcomes (Baker 2001; Cha 2014b; Fernandes 2014; Hill 2011; Jeong 2007; O'Kelly 2014), cognitive functioning (Baker 2001; Mueller 2013; Pool 2012; Särkämö 2008), and activities of daily living (Van Delden 2013). Twenty‐five studies involved only participants with stroke (N = 698, 90% of total N). Four studies involved participants with mixed ABI aetiologies, including two studies with participants with disorders of consciousness (N = 47, 6% of total N). Fifty‐seven per cent of the participants were male. The average age of the participants was 58.27 years. We could not compute average time post incident, as times were reported in days, weeks, months, and years. The studies were conducted in 10 different countries: South Korea (Cha 2014a; Cha 2014b; Jeong 2007; Kim 2005; Kim 2011a; Kim 2012a; Kim 2012b; Park 2010a; Suh 2014), the USA (Conklyn 2012; Hill 2011; Mueller 2013; Paul 1998; Thaut 1997; Thaut 2002; Whitall 2011), Germany (Jungblut 2004; Schneider 2007), China (Lichun 2011; Tong 2015), the Netherlands (Van Delden 2013; van der Meulen 2014), the UK (O'Kelly 2014; Pool 2012), Australia (Baker 2001), Finland (Särkämö 2008), India (Chouan 2012), Spain (Fernandes 2014), and the USA and Germany (Thaut 2007). Only four studies reported on the ethnicity of the participants (Baker 2001; Hill 2011; Kim 2005; Tong 2015). Trial sample size ranged from nine to 111 participants (mean 28.3).
Types of interventions: live versus recorded music
Thirteen studies used music therapy interventions as defined by the review authors in the Background section of this review (Baker 2001; Conklyn 2012; Jungblut 2004; Kim 2005; Lichun 2011; Mueller 2013; O'Kelly 2014; Paul 1998; Pool 2012; Särkämö 2008; Thaut 1997; Thaut 2002; Thaut 2007). Nineteen studies used music that was either live or recorded (Baker 2001; Cha 2014b; Conklyn 2012; Fernandes 2014; Jeong 2007; Jungblut 2004; Kim 2005; Lichun 2011; Mueller 2013; O'Kelly 2014; Park 2010a; Paul 1998; Pool 2012; Särkämö 2008; Schneider 2007; Thaut 1997; Thaut 2007; Tong 2015; van der Meulen 2014), and 10 studies used a rhythmic stimulus only without music (Cha 2014a; Conklyn 2012; Hill 2011; Kim 2011a; Kim 2012a; Kim 2012b; Suh 2014; Thaut 2002; Van Delden 2013; Whitall 2011). Twelve studies used live music interventions, eight of which were music therapy studies (Baker 2001; Conklyn 2012; Jungblut 2004; Lichun 2011; Mueller 2013; O'Kelly 2014; Paul 1998; Pool 2012), and four involved rehabilitation professionals (Jeong 2007; Schneider 2007; Tong 2015; van der Meulen 2014). Live music interventions included receptive listening to live music, active music‐making on instruments and electronic devices, songwriting, vocalising to music, and movement to music. Seven studies used recorded music (Cha 2014b; Fernandes 2014; Kim 2005; Park 2010a; Särkämö 2008; Thaut 1997; Thaut 2007), and two used both live and recorded music (Baker 2001; O'Kelly 2014). Ten studies used a rhythmic pulse only without music, employing either a metronome (Cha 2014a; Chouan 2012; Hill 2011; Kim 2011a; Kim 2012a; Kim 2012b; Thaut 2002; Van Delden 2013; Whitall 2011), or single tone series (Suh 2014). Only three studies used participant‐preferred music (Baker 2001; O'Kelly 2014; Särkämö 2008).
Sixteen studies used rhythm‐based methods to address motor disorders including gait and UEF. Fourteen studies used RAS or RAC (Cha 2014a; Cha 2014b; Chouan 2012; Jeong 2007; Hill 2011; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Whitall 2011). RAS and RAC involve the use of rhythmic sensory cueing of the motor system, engaging entrainment principles in which "rhythmic auditory cues synchronize motor responses into stable time relationships. The fast‐acting physiological entrainment mechanisms between auditory rhythm and motor response serve as coupling mechanisms to stabilise and regulate gait patterns" or reaching arm movements (Thaut 2007, p 455). The rhythmic stimulus used in the majority of studies was a beat provided by a metronome, although one study used pitched tones (Suh 2014). Two other studies used modified versions of RAS or RAC: Park 2010a used fast‐tempo RAS, and Van Delden 2013 used modified bilateral arm training with RAC (mBATRAC), which targeted rhythmic flexion and extension movements.
Types of interventions: active versus receptive methods
Six studies evaluated the effects of active music‐making using musical instruments. Three music therapy studies used active music‐making (Mueller 2013; Paul 1998; Pool 2012). Mueller 2013 used instrument playing to train endogenous task shifting; Pool 2012 used simple instrument playing tasks to train attention; and Paul 1998 required participants to actively play electronic music devices that demanded active shoulder flexion and elbow extension and that enabled easy sound manipulation by the participants. Electronic paddle drums were individually set to the maximum range of motion of each participant. This was compared with a control intervention that involved a physical exercise group in which participants were encouraged to reach their affected extremity as far as they could in different directions. Jeong 2007 combined RAS with instrument playing using dynamic rhythmic movements; Schneider 2007 used music‐supported training that addressed fine motor skills through playing a MIDI keyboard or gross motor skills by playing an electronic drum set with eight pads, or both. Music exercises were adapted to participant need and increased incrementally over 10 levels of difficulty. Tong 2015 used an audible percussion instrument in comparison to a muted musical instrument that resembled the audible instrument, but was made of sponge. The muted musical instrument thus inhibited the participants from hearing sound during the music‐supported therapy training.
Other active methods included songwriting to address mood state (Pool 2012), and neurologic music therapy methods to address cognition (Mueller 2013; Pool 2012; Thaut 2014a).
Receptive methods are those in which the participant is directed to listen to recorded music or live music presented by the interventionist, and thus is not required to be actively involved in making the music him or herself. Five studies used receptive methods (Baker 2001; Fernandes 2014; Kim 2005; O'Kelly 2014; Särkämö 2008). Two of these studies involved heavily dependent participants emerging from coma with whom active methods would not be viable (Fernandes 2014; O'Kelly 2014).
Four trials examined the effects of music therapy on communication outcomes (Conklyn 2012; Jungblut 2004; Särkämö 2008; van der Meulen 2014). Each of these used a different music intervention. Jungblut 2004 employed SIPARI, a music therapy method to address aphasia using singing, intonation, prosody embedded in physiologically appropriate breathing. This method also employs instrumental and vocal rhythmic exercises and music improvisations to practice communication scenarios. Särkämö 2008 used receptive methods where participants listened to recordings of participant‐preferred music. Conklyn 2012 and van der Meulen 2014 used melodic intonation therapy, a method that involves repetitive singing of short phrases in conjunction with left hand tapping of the rhythm.
Dosage of interventions and trial designs
Frequency and duration of treatment sessions varied greatly among the studies. The total number of sessions ranged from one to 60. The duration of sessions varied widely due to the range of interventions being used to address a diverse set of outcomes. As interventions were so varied, it was not meaningful to provide a comparison of session durations. The frequency of sessions ranged from once to 10 times weekly. We have included details on frequency and duration of sessions for each trial in the Characteristics of included studies table.
Eight studies used cross‐over designs (Baker 2001; Cha 2014a; Kim 2005; Kim 2011a; O'Kelly 2014; Pool 2012; Thaut 2002; Tong 2015); one study used a wait‐list control design (van der Meulen 2014); and all of the other studies used a parallel‐group design. Not all studies measured all outcomes identified in this review.
Details of the studies included in the review are shown in the Characteristics of included studies table.
Excluded studies
In this update, we identified 80 additional experimental research studies that appeared to be eligible for inclusion. However, we excluded these after closer examination or after receiving additional information from the chief investigators. Reasons for exclusions were:
not an RCT or controlled clinical trial (48 studies);
insufficient data reporting (nine studies);
comparative study of two music interventions with no control (two studies);
control participants did not have ABI (seven studies);
could not locate published report of the research (five studies);
not population of interest (two studies);
outcomes not of interest to this review (four studies); and
the methodological problems employed presented a risk of bias to reported results (three studies).
We have listed details of the excluded trials in the Characteristics of excluded studies table.
Risk of bias in included studies
Only one study received a rating of low risk of bias (Thaut 1997), and two studies received a rating of unclear risk of bias (Cha 2014a; O'Kelly 2014). Twenty‐four studies received a rating of high risk of bias. 'Risk of bias' summaries are reported in Figure 2 and Figure 3, with details about each 'Risk of bias' item for each included study.
2.
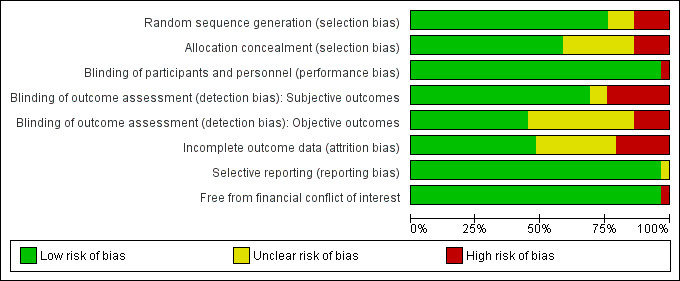
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
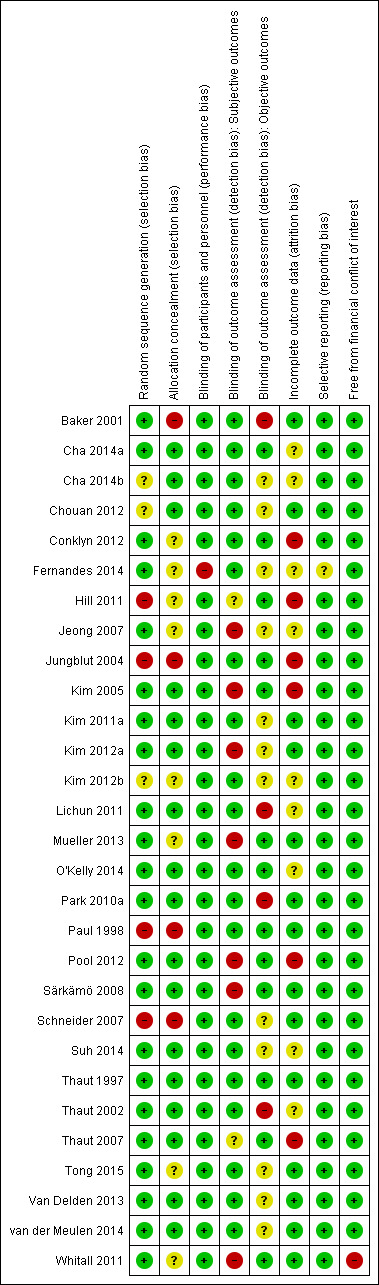
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We included 22 studies that used appropriate methods of randomisation (e.g. computer‐generated random number table, drawing of lots, flipping of coins) (Baker 2001; Cha 2014a; Conklyn 2012; Fernandes 2014; Jeong 2007; Kim 2005; Kim 2011a; Kim 2012a; Lichun 2011; Mueller 2013; O'Kelly 2014; Park 2010a; Pool 2012; Särkämö 2008; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Tong 2015; Van Delden 2013; van der Meulen 2014; Whitall 2011), as well as four studies that used non‐random methods of group assignment (e.g. alternate group assignment) (Hill 2011; Jungblut 2004; Paul 1998; Schneider 2007). The methods used in three studies resulted in a judgement of unclear risk of bias (Cha 2014b; Chouan 2012; Kim 2012b). We examined the impact of method of randomisation by sensitivity analyses.
Seventeen studies used allocation concealment (Cha 2014a; Cha 2014b; Chouan 2012; Kim 2005; Kim 2011a; Kim 2012a; Lichun 2011; O'Kelly 2014; Park 2010a; Pool 2012; Särkämö 2008; Suh 2014; Thaut 1997; Thaut 2002; Thaut 2007; Van Delden 2013; van der Meulen 2014). Allocation concealment was unclear in eight studies (Conklyn 2012; Fernandes 2014; Hill 2011; Jeong 2007; Kim 2012b; Mueller 2013; Tong 2015; Whitall 2011), and not used in the remaining four studies (Baker 2001; Jungblut 2004; Paul 1998; Schneider 2007).
Blinding
In music intervention studies, research participants and interventionists cannot be blinded, with the exception of studies that compare different types of music interventions (blinding of participant) or interventions that use headphones (blinding of outcome assessors and potentially interventionist). For this reason, we did not downgrade studies for not blinding participants. Only one study reported blinding of participants (Suh 2014). We rated one study at high risk for performance bias (Fernandes 2014); music was delivered via headphones to heavily dependent participants, however blinding of interventionists was not reported.
Thirteen studies reported blinding of the outcome assessors for objective measures (Cha 2014a; Conklyn 2012; Hill 2011; Jungblut 2004; Kim 2005; Mueller 2013; O'Kelly 2014; Paul 1998; Pool 2012; Särkämö 2008; Thaut 1997; Thaut 2007; Whitall 2011). In 14 trials the use of blinding for detection bias was unclear (Cha 2014b; Chouan 2012; Fernandes 2014; Jeong 2007; Kim 2011a; Kim 2012a; Kim 2012b; Lichun 2011; Park 2010a; Schneider 2007; Suh 2014; Tong 2015; Van Delden 2013; van der Meulen 2014). Two studies did not blind outcome assessors (Baker 2001; Thaut 2002).
For subjective outcomes (e.g. the Profile of Mood States (POMS)) (Lorr 2003), blinding of the outcome assessor was not possible unless the participants were in studies that compared different types of music interventions. The 'Risk of bias' summary lists 20 studies at low risk of bias for outcome assessment of subjective outcomes (Figure 3). However, these studies did not include subjective outcomes and were therefore not downgraded for this 'Risk of bias' criterion. We assessed seven trials as having a high risk of bias, as subjective outcomes were used and participants were not blinded (Jeong 2007; Kim 2005; Kim 2012a; Mueller 2013; Pool 2012; Särkämö 2008; Whitall 2011). The use of blinding for subjective outcomes was unclear for two trials (Hill 2011; Thaut 2007).
Incomplete outcome data
Just under half of the trials reported attrition, at a rate of between 0% and 17%. Six studies had attrition rates of 20% or higher (20% to 29%) (Conklyn 2012; Hill 2011; Jungblut 2004; Kim 2005; Pool 2012; Thaut 2007). Nine studies did not report attrition adequately (Cha 2014a; Cha 2014b; Fernandes 2014; Jeong 2007; Kim 2012b; Lichun 2011; O'Kelly 2014; Suh 2014; Thaut 2002). We have included detailed information on dropout rates in the Characteristics of included studies table.
Selective reporting
We found evidence of selective reporting by the authors in one study (Fernandes 2014).
We examined publication bias visually in the form of funnel plots for gait velocity (Figure 4). The funnel plot did not show evidence of publication bias.
4.
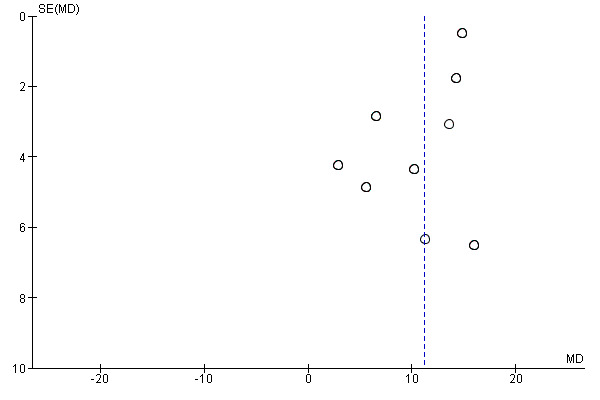
Funnel plot of comparison: 1 Music therapy versus control, outcome: 1.1 Gait velocity [metres/min].
Other potential sources of bias
We assessed one study as having a potential conflict of interest (Whitall 2011).
Effects of interventions
See: Table 1
Primary outcomes
Gait
Ten RCTs with a total of 298 participants examined the effects of RAS versus standard neurodevelopmental therapy (Kim 2012a; Suh 2014; Thaut 1997; Thaut 2007), or versus gait training without auditory stimulation on improvement in gait (Cha 2014a; Cha 2014b; Chouan 2012; Kim 2012b; Lichun 2011; Park 2010a). Improvements in gait were measured by changes in gait velocity (nine studies), cadence (seven studies), stride length (eight studies), stride symmetry (three studies), general gait (two studies), and balance (three studies).
Gait velocity
The pooled estimate of nine RCTs with 268 participants indicated that RAS improved gait velocity by an average of 11.34 metres per minute compared with the control group (95% CI 8.40 to 14.28; P < 0.00001) (Cha 2014a; Cha 2014b; Kim 2012a; Kim 2012b; Lichun 2011; Park 2010a; Suh 2014; Thaut 1997; Thaut 2007). The results were inconsistent across studies (I2 = 61%), with some studies reporting greater effect sizes than others, but all effect sizes were in the desired direction (Analysis 1.1). A subgroup analysis comparing studies conducted by a music therapist versus those conducted by non‐music therapy healthcare professionals indicated that music therapy studies (MD 14.76, 95% CI 13.84 to 15.69; P < 0.00001; I2 = 0%) resulted in a statistically significantly greater improvement (P = 0.0004) in gait velocity than the studies conducted by a non‐music therapy interventionist (MD 8.48, 95% CI 5.16 to 11.80; P < 0.00001; I2 = 11%). Results were consistent across studies within each subgroup (Analysis 1.2).
1.1. Analysis.
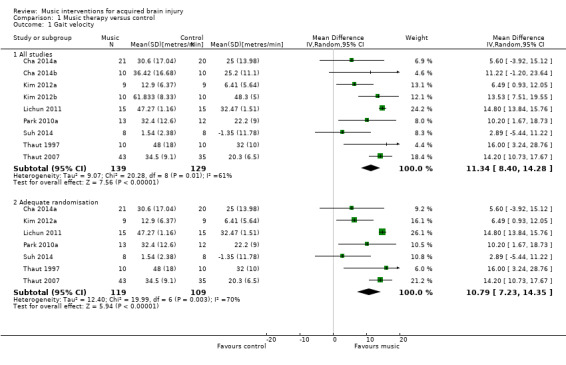
Comparison 1 Music therapy versus control, Outcome 1 Gait velocity.
1.2. Analysis.
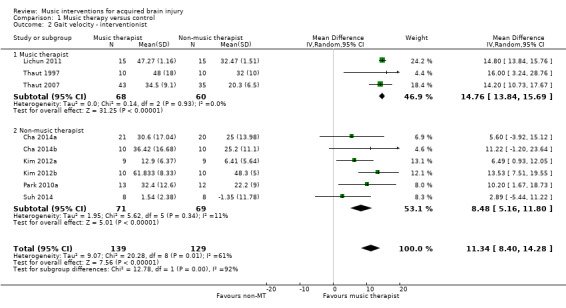
Comparison 1 Music therapy versus control, Outcome 2 Gait velocity ‐ interventionist.
We also conducted a subgroup analysis for the type of auditory stimulation used in the study, namely music versus an auditory stimulus without music (e.g. metronome beat). Results indicated that the use of music led to greater and more consistent improvements in gait velocity (MD 14.69, 95% CI 13.77 to 15.61; P < 0.00001; I2 = 0%) than auditory stimulation without music (MD 7.7, 95% CI 3.03 to 12.38; P = 0.001; I2 = 42%), and this difference was statistically significant (P = 0.004) (Analysis 1.3).
1.3. Analysis.
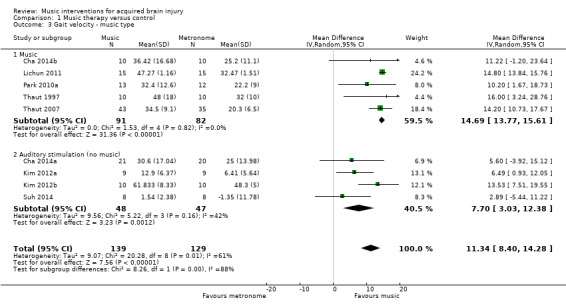
Comparison 1 Music therapy versus control, Outcome 3 Gait velocity ‐ music type.
A sensitivity analysis to examine the impact of randomisation method, excluding the data of two trials for which the randomisation method was not clear (Cha 2014b; Kim 2012b), had minimal impact on the effect size (MD 10.79, 95% CI 7.23 to 14.35; P < 0.00001; I2 = 70%; Analysis 1.1).
Stride length
RAS also resulted in significantly greater improvements in stride length of the affected side in five RCTs (MD 0.12 metres, 95% CI 0.04 to 0.20; P = 0.003; I2 = 80%; N = 129) (Analysis 1.4) (Cha 2014a; Cha 2014b; Kim 2012a; Kim 2012b; Lichun 2011), and stride length of the unaffected side in four studies (MD 0.11 metres, 95% CI 0.01 to 0.22; P = 0.03; I2 = 85%; N = 99; Analysis 1.6) (Cha 2014a; Cha 2014b; Kim 2012a; Kim 2012b). The heterogeneity across studies was due to some studies reporting greater improvements than others, but all treatment effects were in the desired direction. Three studies (186 participants) examined the effects of RAS on stride length but did not specify whether stride length was assessed for the affected or unaffected side or whether an average for both sides was computed (Suh 2014; Thaut 1997; Thaut 2007). The pooled effect size of these three studies was not statistically significant, and the results were inconsistent across studies (MD 0.16 metres, 95% CI ‐0.01 to 0.33; P = 0.07; I2 = 83%; Analysis 1.7).
1.4. Analysis.
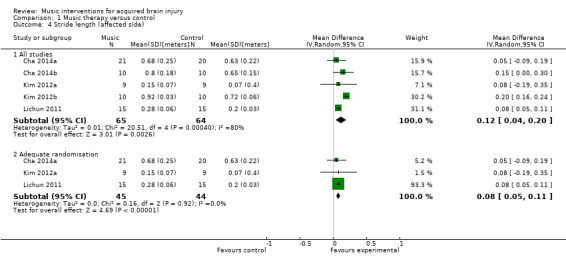
Comparison 1 Music therapy versus control, Outcome 4 Stride length (affected side).
1.6. Analysis.
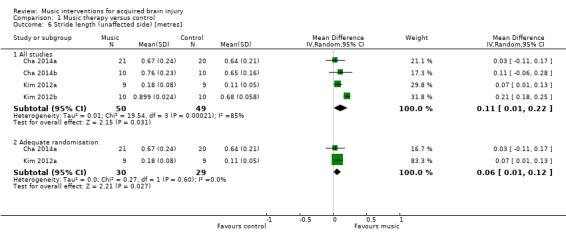
Comparison 1 Music therapy versus control, Outcome 6 Stride length (unaffected side) [metres].
1.7. Analysis.
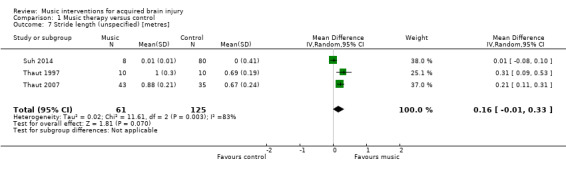
Comparison 1 Music therapy versus control, Outcome 7 Stride length (unspecified) [metres].
Subgroup analysis per music intervention type revealed that there was no statistically significant difference (P = 0.37) between studies that used music (MD 0.08, 95% CI 0.05 to 0.12; P <0.00001; I2 = 0%) and those that used an auditory stimulus without music in terms of stride length (MD 0.14, 95% CI 0.02 to 0.25; P = 0.02; I2 = 55%) (Analysis 1.5).
1.5. Analysis.
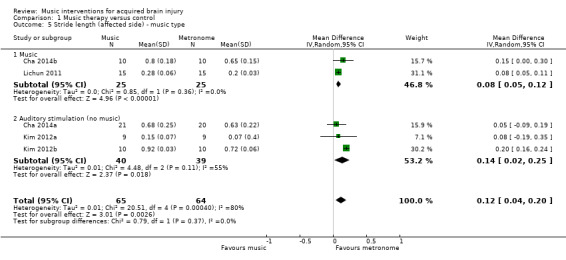
Comparison 1 Music therapy versus control, Outcome 5 Stride length (affected side) ‐ music type.
A sensitivity analysis to examine the impact of randomisation method, excluding the data of two trials for which the randomisation method was not clear (Cha 2014b; Kim 2012b), resulted in a small decrease in effect size, but it greatly reduced the heterogeneity so that the treatment effect was consistent across the studies that used adequate methods of randomisation. Pooling the effects of only those studies that used adequate methods of randomisation resulted in an improvement of stride length by 0.08 metres (95% CI 0.05 to 0.11; P < 0.00001; I2 = 0%) on the affected side (Analysis 1.4) and 0.06 metres (95% CI 0.01 to 0.12; P = 0.03; I2 = 0%) on the unaffected side (Analysis 1.6).
Gait cadence
The pooled estimate of seven RCTs with 223 participants indicated that RAS improved gait cadence by 10.77 steps per minute compared with the control group (95% CI 4.36 to 17.18; P = 0.001; I2 = 83; Analysis 1.8) (Cha 2014a; Cha 2014b; Kim 2012a; Lichun 2011; Suh 2014; Thaut 1997; Thaut 2007). However, the results were inconsistent across studies, with the larger study, Thaut 2007, showing a greater cadence improvement (22.00 steps/minute, 95% CI 16.94 to 27.06; N = 78) than the other studies (ranging from 3.86 to 12.78 steps/minute).
1.8. Analysis.
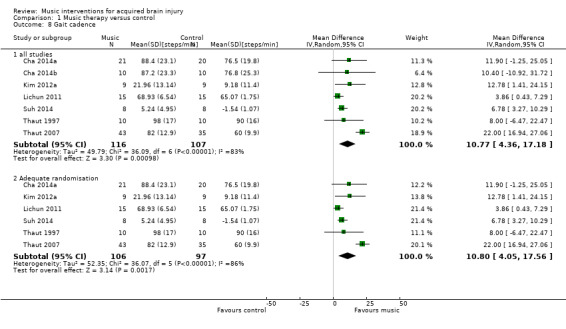
Comparison 1 Music therapy versus control, Outcome 8 Gait cadence.
A subgroup analysis compared studies in which the intervention was delivered by a music therapist, Lichun 2011, Thaut 1997, and Thaut 2007, with studies in which the intervention was delivered by another professional, Cha 2014a, Cha 2014b, Kim 2012a, and Suh 2014. This analysis revealed that studies with music therapist interventionists led to greater improvements (MD 11.51, 95% CI ‐2.57 to 25.60; P = 0.11) than studies with non‐music therapist interventionists (MD 7.65, 95% CI 4.43 to 10.86; P < 0.0001), but this difference was not statistically significant (P = 0.6). The effect size of the music therapist interventionist subgroup was no longer statistically significant. The heterogeneity within the music therapist interventionist subgroup (I2 = 94%) was much larger than that of the non‐music therapist interventionist group (I2 = 0%). This was due to the large effect sizes reported in the Thaut 2007 study (Analysis 1.9).
1.9. Analysis.
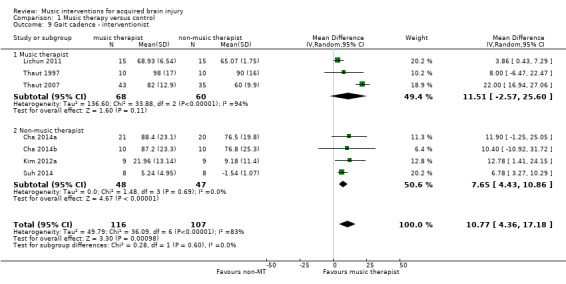
Comparison 1 Music therapy versus control, Outcome 9 Gait cadence ‐ interventionist.
A subgroup analysis comparing studies that used music versus those that used an auditory stimulus without music indicated a larger improvement in the music group (MD 11.34, 95% CI ‐1.05 to 23.74; P = 0.07; I2 = 91%) than in the no‐music auditory stimulation group (MD 7.58, 95% CI 4.33 to 10.83; P < 0.00001; I2 = 0%), but this difference was not statistically significant (P = 0.57) (Analysis 1.10).
1.10. Analysis.
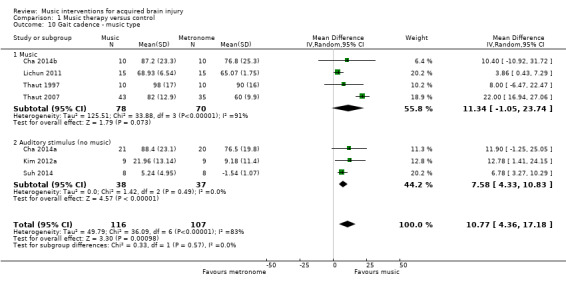
Comparison 1 Music therapy versus control, Outcome 10 Gait cadence ‐ music type.
For gait cadence, one study used unclear randomisation methods (Cha 2014b). Excluding this study from the analysis had little impact on the pooled effect size (MD 10.80, 95% CI 4.05 to 17.56; P = 0.002; I2 = 86%) (Analysis 1.8).
Stride symmetry
Three RCTs involving 139 participants examined the effects of RAS on stride symmetry (defined as the ratio between the swing time of two consecutive steps using the longer step as the denominator) (Cha 2014a; Thaut 1997; Thaut 2007). Their pooled estimate was not statistically significant, and the results were inconsistent across studies (SMD 0.94, 95% CI ‐0.32 to 2.20; P = 0.14; I2 = 90%; Analysis 1.11).
1.11. Analysis.
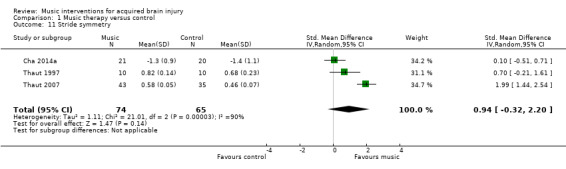
Comparison 1 Music therapy versus control, Outcome 11 Stride symmetry.
General gait
The pooled estimate of two RCTs indicated that RAS improved general gait by 7.67 units on the Dynamic Gait Index compared with the control group (95% CI 5.67 to 9.67; P < 0.00001; I2 = 0%; N = 48; Analysis 1.12) (Chouan 2012; Kim 2012a).
1.12. Analysis.
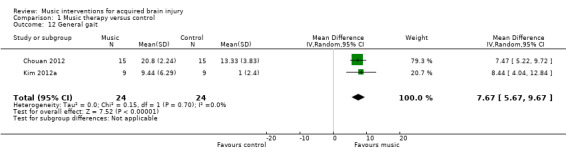
Comparison 1 Music therapy versus control, Outcome 12 General gait.
Balance
Finally, there was no strong evidence for an effect of RAS on balance (SMD 0.31, 95% CI ‐0.48 to 1.09; P = 0.44; I2 = 51%). This evidence was based on three RCTs with small sample sizes resulting in a total sample size of 54 participants (Analysis 1.13) (Cha 2014b; Kim 2012a; Suh 2014). Removing one study for which the method of randomisation was not clear reduced the effect size (SMD 0.13, 95% CI ‐1.1 to 1.37) (Cha 2014b), and the effect size remained not statistically significant (P = 0.84).
1.13. Analysis.
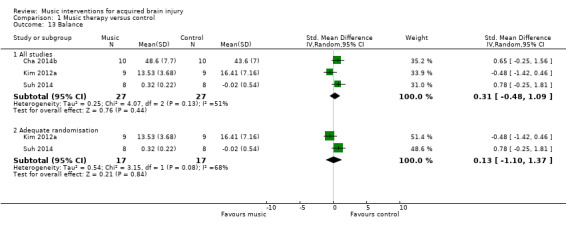
Comparison 1 Music therapy versus control, Outcome 13 Balance.
Other outcomes
RAC was examined as an added music intervention to visual locomotor imagery training and kinaesthetic locomotor imagery training in an RCT with 15 stroke participants (Kim 2011a). This review included only the visual locomotor imagery training as the control condition with added RAC as the music intervention. We measured changes of peak‐to‐peak joint angular displacement using electromyographic analyses, and so we could not include these results in the meta‐analysis. Increased activation in a greater number of lower limb muscles involved in gait and an improvement in lower limb joint angular displacement were reported when auditory step rhythm was integrated into locomotor imagery. During the swing phase there were significant differences for all four muscles for the rhythm condition: quadriceps (F = 3.398; P < 0.05); hamstring (F = 9.324; P < 0.05); tibialis anterior (F = 5.089; P < 0.05); and gastrocnemius (F = 3.639; P < 0.05). Activation was increased significantly during the stance phase in the hamstring (F = 4.815; P < 0.05) and the gastrocnemius (F = 4.087; P < 0.05) for the rhythm intervention. Peak‐to‐peak joint angular displacement was significantly different for the ankle joint with rhythmic auditory cueing (F = 6.519; P < 0.05).
Upper extremity function
Nine studies, comprising six RCTs, Chouan 2012, Jeong 2007, Thaut 2002, Tong 2015, Van Delden 2013, and Whitall 2011, and three quasi‐RCTs, Hill 2011, Paul 1998, and Schneider 2007, with a total of 308 participants, examined the effects of music interventions on UEF. Improvements in UEF were measured by changes in general UEF (five studies), timing of UEF movements (two studies), range of motion (shoulder flexion) (two studies), hand function (two studies), upper limb strength (two studies), manual dexterity (two studies), and elbow extension angle (two studies).
General upper extremity function
Five studies, comprising four RCTs, Chouan 2012, Tong 2015, Van Delden 2013, and Whitall 2011, and one quasi‐RCT (Hill 2011), examined the effect of music‐based interventions on general UEF in 194 participants as measured by the Fugl‐Meyer Assessment (MD 3.56, 95% CI ‐0.88 to 8.00; P = 0.12; Analysis 1.14). Their pooled effect was not statistically significant, and the results were inconsistent across studies (I2 = 85%), with one study reporting a much greater improvement than the other studies (Chouan 2012). Whereas Chouan 2012 used RAS, Van Delden 2013 and Whitall 2011 used modified bilateral arm training with RAC (mBATRAC), and Tong 2015 used music‐supported therapy with audible and mute musical instruments.
1.14. Analysis.
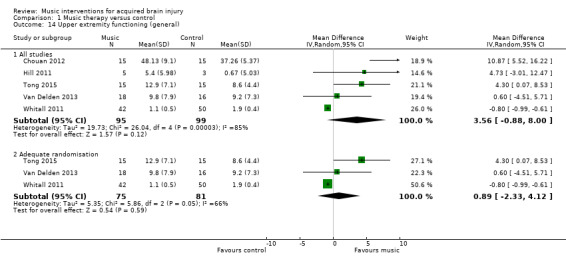
Comparison 1 Music therapy versus control, Outcome 14 Upper extremity functioning (general).
Upper extremity function: time
Two RCTs examined the effects of music interventions on timed upper extremity movements to complete functional tasks using the Wolf Motor Function Test or a validated modified version of this measure (Tong 2015; Whitall 2011). Their pooled effect indicated a statistically significant reduction in time in the music intervention groups (MD ‐1.08, 95% CI ‐1.69 to ‐0.47; P = 0.0006; I2 = 52%; N = 122; Analysis 1.15).
1.15. Analysis.
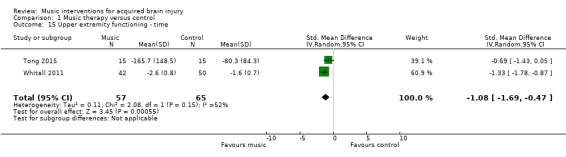
Comparison 1 Music therapy versus control, Outcome 15 Upper extremity functioning ‐ time.
Range of motion: shoulder flexion
There was no evidence of effect of RAS on range of motion (MD 9.81, 95% CI ‐12.71 to 32.33; P = 0.39; I2 = 0%). This evidence was based on only two studies, comprising one RCT, Jeong 2007, and one quasi‐RCT, Paul 1998, that used different types of music interventions to improve shoulder flexion. Jeong 2007 used an "RAS music‐exercise intervention" (p127). Paul 1998 evaluated the effects of electronic music‐making activity using "musical activities that were improvisational … requiring that the participants find a rhythm or beat that was expressive and comfortable for them. Music pieces were designed to elicit steady rhythmic pulses that were engaging to the participant." (p230). Both interventions used rhythm embedded in music as part of instrument playing activities, and thus were similar enough to warrant examination within meta‐analysis. In addition, Jeong 2007 had large standard deviations indicating significant variability in the findings (Analysis 1.16). Both studies used goniometer measures.
1.16. Analysis.
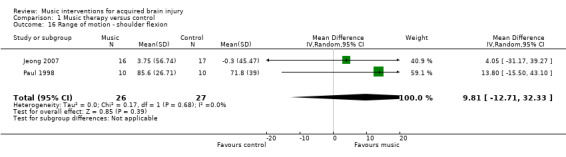
Comparison 1 Music therapy versus control, Outcome 16 Range of motion ‐ shoulder flexion.
Hand function
The pooled estimates of two RCTs, Van Delden 2013 and Whitall 2011, with 113 participants using mBATRAC did not indicate evidence of effect for hand function as measured by the Stroke Impact Scale (MD 0.32, 95% CI ‐0.91 to 1.54; P = 0.61; I2 = 0%; Analysis 1.17) (Duncan 1999).
1.17. Analysis.
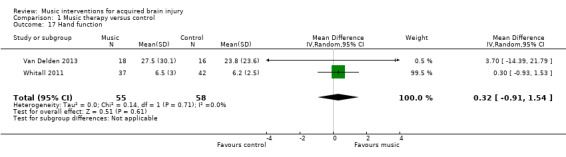
Comparison 1 Music therapy versus control, Outcome 17 Hand function.
Upper limb strength
A pooled estimate of 6.03 (95% CI ‐2.52 to 14.59; I2 = 56%) in two RCTs with 113 participants found upper limb strength favouring the mBATRAC intervention, but this effect was not statistically significant (P = 0.17; Analysis 1.18) (Van Delden 2013; Whitall 2011).
1.18. Analysis.
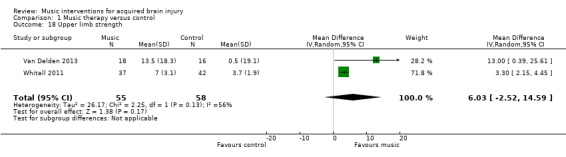
Comparison 1 Music therapy versus control, Outcome 18 Upper limb strength.
Manual dexterity
We found no evidence of effect for manual dexterity (MD 0.47, 95% CI ‐1.08 to 2.01; P = 0.55; I2 = 52%). This evidence was based on the results of two studies, comprising one RCT, Van Delden 2013, and one quasi‐RCT, Schneider 2007, with a total of 74 participants (Analysis 1.19). The effect of music on dexterity was assessed with the Nine‐Hole Peg Test (Kellor 1971).
1.19. Analysis.
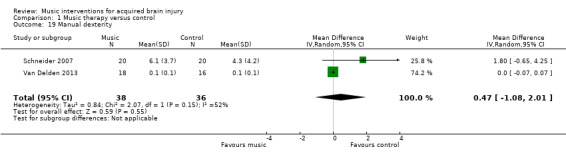
Comparison 1 Music therapy versus control, Outcome 19 Manual dexterity.
Elbow extension angle
Two studies, comprising one RCT, Thaut 2002, and one quasi‐RCT, Paul 1998, measured the effects of music therapy on elbow extension angle in people with hemispheric stroke. However, due to the significant clinical heterogeneity of the studies, we did not pool their effect sizes.
Thaut 2002 examined the effects of RAS on spatio‐temporal control of reaching movements of the paretic arm in 21 participants. Results indicated that RAS increased the elbow extension angle by 13.8% compared with the non‐rhythmic trial, and this difference was statistically significant (P = 0.007). Results further indicated that variability of timing and reaching trajectories were reduced significantly (35% and 40.5%, respectively; P < 0.05).
Paul 1998 evaluated the effects of music‐making activity on elbow extension in 20 participants with hemiplegia. The elbow extension (measured from 135 to 0, with negative numbers expressing limitations) postintervention was ‐29.4 (standard deviation (SD) 29.49) for the experimental group and ‐39.2 (SD 38.19) for the control group. This difference was not statistically significant. Post‐test shoulder flexion data indicated a non‐statistically significant difference (P = 0.44) between the music therapy group (85.6°, SD 26.71) and the control group (71.8°, SD 39).
Secondary outcomes
Communication
Overall communication
Music interventions significantly improved the overall communication of people with aphasia after stroke as indicated by a moderate effect size of 0.75 (95% CI 0.11 to 1.39; P = 0.02; I2 = 31%) (Cohen 1988). This included people with ischaemic stroke (Särkämö 2008; van der Meulen 2014), haemorrhagic stroke or stroke of an unknown type (van der Meulen 2014), and people with chronic expressive and global aphasia (Jungblut 2004). This evidence was based on three studies, comprising two RCTs, Särkämö 2008 and van der Meulen 2014, and one quasi‐RCT (Jungblut 2004), with a total of 67 participants (Analysis 1.20). Each of the three studies used different measures. Overall communication in Särkämö 2008 was measured using repetition and reading subtests from the Finnish version of the Boston Diagnostic Aphasia Examination (Hänninen 1989), verbal fluency and naming subtests from the Consortium to Establish a Registry for Alzheimer’s Disease (Morris 1989), and a shortened version of the Token Test (De Renzi 1978). Overall communication outcomes in van der Meulen 2014 were measured with the Amsterdam‐Nijmegen Everyday Language Test (Blomert 1995). For Jungblut 2004, we used the reported total score from the Aachen Aphasia Test (Hogrefe 1983).
1.20. Analysis.
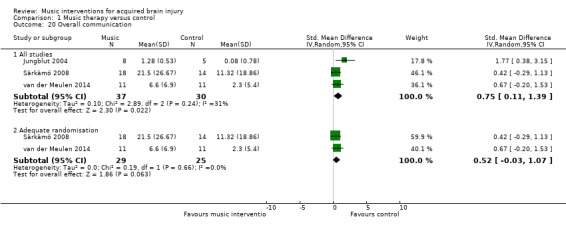
Comparison 1 Music therapy versus control, Outcome 20 Overall communication.
Removing one study considered to be at high risk of bias for randomisation reduced the size of the effect (SMD 0.52, 95% CI ‐0.03 to 1.07), and the resulting effect size was no longer statistically significant (P = 0.06) (Analysis 1.20) (Jungblut 2004).
Naming
The pooled estimate of two small studies, comprising one RCT, van der Meulen 2014, and one quasi‐RCT (Jungblut 2004), with a total of 35 participants, suggested an improvement in naming by 9.79 units on the Aachen Aphasia Test (95% CI 1.37 to 18.21; P = 0.02; I2 = 0%) in participants who received music therapy interventions compared with training without music (Analysis 1.21).
1.21. Analysis.
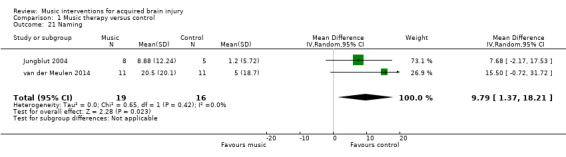
Comparison 1 Music therapy versus control, Outcome 21 Naming.
Repetition
Music interventions also had a beneficial effect on speech repetition as measured by the Aachen Aphasia Test (MD 8.90, 95% CI 3.25 to 14.55; P = 0.002; I2 = 0%). However, this pooled estimate was based on only two studies, comprising one RCT, van der Meulen 2014, and one quasi‐RCT (Jungblut 2004), with a total of 35 participants (Analysis 1.22). A third study, Conklyn 2012, examined the effects of modified melodic intonation therapy on speech repetition using two tasks drawn from the Western Aphasia Battery (Kertesz 1982). Changes were examined over three session visits. Due to high attrition in visit three, we included change scores between visits one and two only for this review and examined total scores only rather than subscale scores. Change scores were used due to large differences in pre‐test scores between the treatment arms. Significant improvements were found in both the control group adjusted total score (change = 4.1; P = 0.03) and the treatment group adjusted total scores (change 8.1; P < 0.01). The improvement in the treatment group was not significantly greater than that in the control group. However, post‐hoc analyses suggested that the control group improved in repetition only, whereas the treatment group improved in both repetition and responsiveness, suggesting a possible carry‐over effect of the modified melodic intonation therapy intervention.
1.22. Analysis.
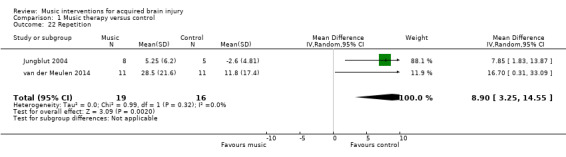
Comparison 1 Music therapy versus control, Outcome 22 Repetition.
Mood
Three RCTs examined mood as measured by the Profile of Mood States (POMS) (Jeong 2007; Pool 2012; Särkämö 2008). However, we could not combine these studies in a meta‐analysis as different versions of the POMS were used, and the scores were reported inconsistently, omitting either total scores or subscale scores. Särkämö 2008 used the shortened Finnish version of the POMS (Hänninen 1989), with 38 items measuring tension, depression, irritability, vigour, fatigue, inertia, confusion, and forgetfulness in eight subscales. Subscale scores were reported, and total scores were provided by the principal investigator. Jeong 2007 reported total scores only for the 34‐item version of the POMS translated and modified into a Korean version (Shin 1996). Mood subscales of the Korean POMS were not reported. Pool 2012 used the bipolar version of the POMS (Lorr 2003), which contains 72 adjectives grouped into six bipolar mood states. Pool 2012 used a shortened version of the POMS with just four subscales (48 items) due to the cognitive deficits of the participants, including composed‐anxious, agreeable‐hostile, elated‐depressed, and energetic‐tired only. Subscale total scores only were available. Although subscale totals were provided in both Särkämö 2008 and Pool 2012, the mood states subscales were different in the two different versions of the POMS, and so these could not be combined meaningfully.
Särkämö 2008 compared the effects of music listening versus no intervention versus audio book listening (not included in this review) on mood states in 60 people in the acute stage after stroke. Significant differences were found between the music intervention and the other groups at three months' poststroke (the time frame examined in this review) for the mood states confusion (F(2, 51) = 3.3; P = 0.045) and depression (F(2, 51) = 3.7; P = 0.031). A post‐hoc test revealed significantly lower scores for depression in the music intervention group (P = 0.024). Scores for confusion were marginally lower in the music intervention group than in the control group (P = 0.061). Tendencies for less depression in the music intervention group were sustained at the six‐month poststroke stage.
Pool 2012 examined the effects of group music therapy interventions versus standard care in 10 people with chronic ABI (mixed aetiologies) on mood. Four bipolar mood states were measured: agreeable‐hostile, composed‐anxious, elated‐depressed, and energetic‐tired. No significant differences were found in mood states between conditions after eight weeks. Mean scores showed that mood states improved slightly following eight weeks of standard care (control) for each mood state but worsened slightly following music therapy intervention at the same time point. Although non‐significant, an improvement in mean mood scores for all moods states was noted after 16 weeks for music therapy intervention beyond the scores for standard care.
Jeong 2007 compared RAS with no intervention in 36 people with stroke. The Korean version of the POMS was used, in which total scores range from 0 to 60, and a higher total score indicates worse depression. There was a significant improvement in mood for both groups (post‐RAS scores: 1.56 (SD 0.82) and post‐control scores: 2.29 (SD 0.77)). However, it should be noted that baseline scores were already very low (RAS: 2.11; control: 2.81), providing a narrow window for change.
Two further RCTs examining physical functioning as the primary outcome also reported on mood subscales in their results, specifically the Stroke Impact Scale emotion subscale (Van Delden 2013; Whitall 2011). However, because mood was not identified as a primary outcome at the outset of the study or discussed in the findings, we did not include these data, as it appeared they were extraneous.
Social skills
Jeong 2007 used the Relationship Change Scale (Shannon 1973), translated into Korean and then further modified to examine the effects of music interventions on social relationships. A significant effect was found for the music intervention, showing improved interpersonal relationships compared with the control group (F = 10.087; P = 0.003), which showed a significant decrease in interpersonal relationships.
Pain
Kim 2005 examined the effects of listening to pre‐recorded music on pain in people with ABI. Pain ratings on a 0‐to‐10 numeric scale indicated no statistically significant difference in pain ratings between the music and the no‐music condition (P = 0.05).
Behavioural outcomes
Agitation
One RCT examined the effects of listening to live music and to recorded music on agitation in 22 people with a severe head injury with a diagnosis of post‐traumatic amnesia (Baker 2001). Listening to live music was effective in reducing agitation scores (as measured by the Agitation Behavior Scale (ABS)) (effect size = 5.01 ABS units; P < 0.0001) (Corrigan 1989). Agitation also decreased after listening to recorded music (6.25 ABS units; P < 0.0001). The difference in effect between live and recorded music was not statistically significant (1.2 ABS units; P = 0.8).
Other behavioural outcomes
Two studies, comprising one RCT, O'Kelly 2014, and one quasi‐RCT, Fernandes 2014, with people with disorders of consciousness reported on other behavioural outcomes. O'Kelly 2014 reported on a range of behavioural outcomes including blinks per minute, eyes closed with or without body movements, eyes open with or without body movements, and respiration rate per minute. Behaviours of 21 participants with disorders of consciousness were observed across conditions of baseline silence, non‐music therapy conditions (white noise, recordings of disliked music), and music therapy conditions (live, participant‐preferred music and live, improvised music entrained to the participant's respiration). Differences in eye blink rate in vegetative participants were significant across conditions (F(2.3, 13.9) = 3.6; P = 0.019), with a peak response during the participant‐preferred live music condition when compared with baseline silence (F(1, 11) = 8.2; P = 0.029). Fernandes 2014 also reported on changes in facial expression, including muscular facial relaxation, eye opening, mouth movements, head movements, yawning, smiling, and eyebrow movements in response to recorded music. However, insufficient data reporting by Fernandes 2014 prevented meta‐analysis on this outcome.
Quality of life
Two RCTs, Cha 2014b and Jeong 2007, looked at the impact of RAS on quality of life (N = 53) using the Stroke Specific Quality of Life Scale (Williams 1999). However, the reported means and standard deviations suggested that the authors computed the total score differently: Cha 2014b appears to have computed the total score by adding the participant's rating of each item, whereas Jeong 2007 computed the total score by averaging all the ratings. We therefore computed a SMD for this meta‐analysis. Their pooled estimate suggested a large effect on quality of life (SMD 0.89, 95% CI 0.32 to 1.46; P = 0.002; I2 = 0%; Analysis 1.25). A third quasi‐RCT examined the effects of auditory rhythmic training on quality of life using the Stroke Impact Scale (Hill 2011); however, due to large baseline differences between the groups in this study, we could not include the data from this study in the meta‐analysis. Computation of a SMD does not allow for combining post‐test scores with change scores.
1.25. Analysis.
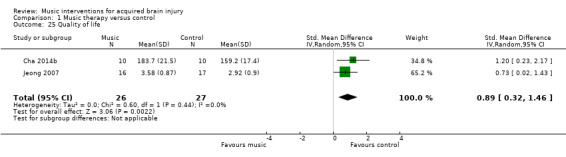
Comparison 1 Music therapy versus control, Outcome 25 Quality of life.
Cognitive functioning
Memory
Two RCTs included memory as an outcome variable (N = 42) (Pool 2012; Särkämö 2008). Särkämö 2008 examined short‐term working memory using the digit span subtest from the Wechsler Memory Scale‐Revised (Wechsler 1987). Pool 2012 used the Rivermead Behavioural Memory Test (Wilson 2008). Their pooled estimate indicated no strong evidence of effect for music interventions on memory (SMD 0.33, 95% CI ‐0.29 to 0.95; P = 0.30; I2 = 0%; Analysis 1.23).
1.23. Analysis.
Comparison 1 Music therapy versus control, Outcome 23 Memory.
Attention
Two RCTs examined the effects of music on attention (N = 39), but their pooled estimate indicated no strong evidence for an effect (SMD 0.30, 95% CI ‐0.34 to 0.94; P = 0.36; I2 = 0%; Analysis 1.24). Pool 2012 used the Test of Everyday Attention (Robertson 1994). Särkämö 2008 used CogniSpeed reaction time software to measure the percentage of correct responses in the vigilance subtest and summed reaction times in the vigilance and simple reaction time subtests (Revonsuo 1995).
1.24. Analysis.
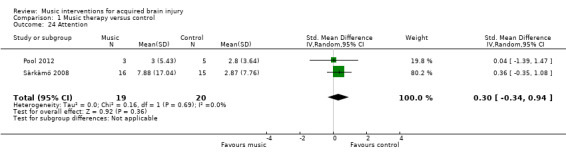
Comparison 1 Music therapy versus control, Outcome 24 Attention.
Mental flexibility
One RCT examined the effects of music‐based endogenous shifting training led by a music therapist on executive functioning of 14 people with stroke or ABI (Mueller 2013). The effects of music training were compared with a control group and a placebo singing group (not included in this review). Mental flexibility was tested using the Trail Making Test Part B (Reitan 1985). No difference was found between the treatment and control conditions (F = 0.81; P = 0.4717). This study also examined working memory; however, we did not include this outcome in the review due to the adapted administration of the test to determine outcomes.
Orientation
One RCT examined the effects of listening to live music and to recorded music on orientation levels in 22 participants with a severe head injury with a diagnosis of post‐traumatic amnesia (Baker 2001). Listening to live music had a significant effect on participant orientation levels (as measured by the Westmead Post‐traumatic Amnesia Scale) compared with the no‐music control condition (effect size = 0.82; P < 0.001) (Shores 1986), and this effect was slightly larger than the effect of listening to recorded music compared to the control condition (effect size = 0.72; P < 0.001).
Activities of daily living
One RCT measured the quality and quantity of spontaneous paretic upper limb use to accomplish 26 activities of daily living outside the laboratory (Van Delden 2013), using the Motor Activity Log (Uswatte 2005). No significant differences in change scores were observed between the groups for amount of use (P = 0.09) or quality of use (P = 0.27).
Adverse events
No studies included adverse event outcomes.
Discussion
Summary of main results
Gait
The results of 10 studies suggest that RAS may have a beneficial effect on gait velocity in people with stroke with an average of 11.34 metres per minute compared with standard treatment. RAS may also improve stride length by about 0.12 metres and general gait by an average of 7.67 units as measured on the Dynamic Gait Index in people with stroke compared with standard treatment. One study found significant improvement in peak‐to‐peak joint angular displacement in the lower limbs during RAC. RAS may have a beneficial effect on gait cadence for people with stroke; however, the degree of improvement across studies was inconsistent. We found no evidence of effect for music interventions on gait symmetry and balance.
Upper extremity function
The music interventions used for UEF varied across nine studies, including rhythm‐based instrument‐playing tasks in music‐making (Paul 1998), RAS within music‐making (Jeong 2007), RAS using rhythmic pulse without music (Chouan 2012; Thaut 2002), fast‐tempo auditory stimulation with and without music (Tong 2015), bilateral arm training with RAC (BATRAC) or a modified version of BATRAC (Van Delden 2013; Whitall 2011), and music‐supported training (Schneider 2007). The results of two studies indicated that music interventions may improve the timing of UEF by about one second. One study found significant improvements in elbow extension angle using RAS with reduced variability of timing (35%) and reduced reaching trajectories (45%) (Thaut 2002). We found no evidence of effect for music interventions for general UEF, range of motion (shoulder flexion), hand function, upper limb strength, and manual dexterity.
Communication outcomes
The results of this review suggest that music interventions may have a moderate effect (SMD = 0.69) on overall communication. This pooled effect size was derived from three studies. The results of two small studies suggested that music interventions may benefit the expressive language outcome of naming (9.79 units on the Aachen Aphasia Test) and the speech outcome of repetition (8.9 units on the Aachen Aphasia Test) for people following stroke (Jungblut 2004; van der Meulen 2014). The studies that examined communication outcomes used diverse music interventions encompassing both receptive (listening) and active (singing and playing) methods.
Mood
Three studies included in our review suggested positive effects of music interventions on mood (Jeong 2007; Pool 2012; Särkämö 2008). Meta‐analysis of these three studies was not possible due to: 1) the use of different versions of the same measure (POMS), and 2) reporting of selected subscales or total score only. Two studies found significant improvements in mood states. One music‐listening study found improvements in depression and confusion, with the positive effects on depression sustained at six months' follow‐up (Särkämö 2008). One study found significant improvements in mood following rhythmic movement to music and active music‐making (Jeong 2007).
Quality of life
Based on the results of Cha 2014b and Jeong 2007, we found a large effect for music interventions on quality of life (SMD = 0.89). The music intervention used in both studies was RAS. A third study that we could not include in the meta‐analysis also used auditory rhythmic training (Hill 2011). More research examining the effects of a wider range of music interventions on quality of life is needed.
Other secondary outcomes
The primary reason noted for referral to music therapy in rehabilitation settings is the rehabilitation of social skills (Magee 2007). However, we identified only one study that measured this as an outcome. Jeong 2007 reported significant improvements in social skills following rhythmic movement to music and active music‐making with stroke participants.
Based on the results of one study, we found no evidence for the effect of music listening on pain for people with ABI (Kim 2005).
One trial reported positive effects for reducing agitation in people with post‐traumatic amnesia following a severe head injury, using both live and recorded music (Baker 2001). Two studies examined the effects of music interventions on a range of behavioural outcomes in people with disorders of consciousness (Fernandes 2014; O'Kelly 2014). We could not combine the results for meta‐analysis due to insufficient data reporting. The severity of injury in this population means that participants are heavily dependent, and only receptive methods can be used. One study reported significant changes in behaviours to music conditions compared with baseline silence (O'Kelly 2014).
Based on two trials, we found no strong evidence for the effect of music interventions on cognitive functioning, specifically memory or attention (Pool 2012; Särkämö 2008). One trial found significant effects for orientation in response to listening to live or recorded music in comparison with no music in participants with post‐traumatic amnesia (Baker 2001). We found no studies that examined activities of daily living or adverse events as outcomes.
More research is needed for all secondary outcomes before reliable conclusions can be drawn.
Overall completeness and applicability of evidence
This review included 29 studies with a total of 775 participants. The results suggest that music interventions may improve gait, communication, and quality of life in people with ABI. While there is much cross‐over in treatments for people with ABI resulting from stroke and traumatic injury, 90% of participants included in this review were stroke survivors, and thus our findings may be more relevant for this population.
Subgroup analyses for gait velocity provide important information about the impact of the type of music intervention and the professional delivering the intervention on the treatment effect. Studies that used trained music therapists to deliver the music interventions resulted in significantly greater improvements in gait velocity than studies in which the intervention was delivered by a non‐music therapy healthcare professional. It should be noted that the subgroup analysis reflects the results of different trials and not direct comparisons of interventionists within a trial. The results of studies that used a trained music therapist were consistent across studies. Furthermore, the subgroup analyses indicated that interventions that use RAS (e.g. metronome beat) embedded within music may be more effective than using non‐music RAS alone. These results provide support for using professionals who are trained in delivering music interventions, such as music therapists, rather than just a metronome. Subanalyses for gait cadence suggested greater improvements when the intervention was delivered by a music therapist, and also when the music was combined with auditory stimulation. Although we had planned to complete a subanalysis for dosage of intervention, there was too much heterogeneity amongst the RAS studies in terms of the number of treatment sessions, the frequency of sessions, the duration of individual sessions, and the total course of treatment to complete this analysis; therefore, recommendations for dosage could not be made. Reporting was problematic for several studies included in this review, particularly concerning blinding of the outcome assessor. The results indicate that interventions implemented by a trained music therapist may result in greater treatment benefits than those delivered by other professionals. This could be explained by the training that music therapists have in delivering interventions using live music that matches the participant's in‐the‐moment physical responses. However, we acknowledge that other factors may have confounded this comparison.
Music interventions may improve the timing of UEF. The findings of this review were influenced by the large variance in the number of participants within studies examining UEF and the variance in reported improvements. Furthermore, one large study reported that there was a large variance in deficit severity of participants (Whitall 2011, N = 92). All of these factors may have contributed to the non‐significant results for general UEF, hand function, and upper limb strength. Rhythmic stimulation appears to induce temporal stability and enhance motor control in walking. It could be that rhythmic cueing has a similar effect on some aspects of UEF, such as timing of movements. Even though functional arm movements, unlike gait, are "discrete, biologically non‐rhythmic, and volitional" (Thaut 2002, p1074), rhythmic stimuli are successfully used to enhance the execution of motor skills in non‐rehabilitation areas such as music performance and sports (Karageorghis 2012a; Karageorghis 2012b).
Although this review included more studies with an increased number of speech and language outcomes than our previous review, the selected subdomains in speech and language outcomes were inconsistent across music intervention studies. This prevented more outcomes being examined in a meta‐analysis. Standardised communication‐specific measures included the Aachen Aphasia Test (Jungblut 2004; van der Meulen 2014), the Amsterdam‐Nijmegen Everyday Language Test (van der Meulen 2014) and the Sabadell (van der Meulen 2014). However, all of these studies examined slightly different subdomains, preventing meta‐analysis of a greater number of outcomes. Similarly, although we were able to report on the effects of music interventions on four cognitive outcomes (memory, attention, mental flexibility, and orientation), we were unable to report on a further 13 cognitive outcomes examined in research studies due to the lack of agreement between studies in the subdomains examined and outcome measures used.
We identified only three studies of sufficient methodological quality that included mood as an outcome. This is surprising given the high incidence of depression following stroke (Matsuzaki 2015), and that mood disorders can affect motivation to engage in rehabilitation and impede re‐integration back into the community (Giles 2006). Two of the three studies reported greater improvements in mood in the music intervention group compared with the control group. However, inconsistent reporting of results prevented meta‐analysis.
Given the importance of improving and maintaining mood after ABI, it is also important to examine the relationship between functional gains and mood during rehabilitation. Several studies tested the effects of music interventions on a functional outcome as well as mood (N = 3) or quality of life (N = 3). Two trials examined cognitive and mood outcomes (Pool 2012; Särkämö 2008). Three trials examined the effects on motor function (gait) and quality of life (Cha 2014b; Hill 2011; Jeong 2007), and one trial examined motor function (gait) and mood (Jeong 2007). Effects on combined domains also reflect clinical practice, which typically aims to address function in combination with mood rather than individual domains alone. Motivating interventions are important for brain‐injured populations, who may experience a loss of motivation due to brain injury.
The benefit of using music as a medium for addressing human function is its flexibility and the range of activities it offers, such as singing, playing, composing, and listening. The music used in therapeutic interventions also can be adapted through varying its multiple components, such as rhythm, tempo, articulation, melodic contour, dynamic range, and harmonic progression, to meet a person’s specific needs (Schneck 2006). This flexibility enables music to be applied in a number of ways within tasks, and it can also be adapted within that task to match or drive the person's level of functioning. Music also provides a motivational force to enhance engagement and participation through stimulating the pleasure and reward networks in the brain (Schneck 2006). However, this flexibility is not advantageous when trying to make meaningful comparisons of interventions and dosage. Given the heterogeneity of interventions across the range of domains that are targeted in ABI rehabilitation, recommendations for dosage cannot be made based on this review. Interventions for motor outcomes (gait and UEF) were relatively homogenous, using rhythm‐based interventions (RAS, variations of RAS, or instrument playing to rhythmic music). However, other interventions for any one outcome were more varied. For example, the interventions addressing mood illustrate the heterogeneity of treatments, ranging from rhythm‐based movement to music (Jeong 2007), receptive listening to participant‐selected recorded music (Särkämö 2008), and active music‐making through songwriting methods (Pool 2012). In order to generate high‐quality evidence, future trials need to standardise and clearly describe details of music‐based methods so that meta‐analysis provides more meaningful information about interventions and dosage.
Quality of the evidence
Overall, the quality of reporting was poor. We judged only one study to be at low risk of bias (Thaut 1997), and two studies as at unclear risk of bias (Cha 2014a; O'Kelly 2014). We judged all of the other studies to be at high risk of bias (N = 26). We have detailed risk of bias for each study in the 'Risk of bias' tables included in the Characteristics of included studies table. Three studies reported the methods of randomisation and allocation concealment, and detailed all levels of blinding (Cha 2014a; O'Kelly 2014; Thaut 1997). We needed to contact the chief investigators of many studies to request more information about methodological issues.
The findings of this review should be interpreted with caution due to the large number of trials rated as having a high risk of bias. We downgraded the quality of many studies because of unclear reporting. We downgraded O'Kelly 2014 and Cha 2014a for not reporting attrition. Four studies reported inadequate methods of randomisation (Hill 2011; Jungblut 2004; Paul 1998; Schneider 2007), and a further three were unclear in reporting randomisation (Cha 2014b; Chouan 2012; Kim 2012b). Four studies did not use allocation concealment (Baker 2001; Jungblut 2004; Lichun 2011; Schneider 2007), and a further seven were unclear in reporting on this criterion (Conklyn 2012; Fernandes 2014; Hill 2011; Jeong 2007; Mueller 2013; Tong 2015; Whitall 2011). Reporting the blinding of participants, interventionists, and outcome assessors needs improving in research trials using music interventions. Blinding of participants in music intervention studies is usually not possible unless two music interventions are being compared (e.g. music listening and music‐making). The lack of participant blinding is problematic when studies examine subjective outcomes such as mood or quality of life. Blinding of interventionists is often not possible in music intervention studies when active music‐making is examined. Where interventionists cannot be blinded, they should be blinded to the purpose of the study where possible. In either case, blinding should be reported or discussed. We found attrition to be problematic, rating it inadequate in six studies and not adequately reported in a further nine studies.
Most of the included trials used small sample sizes (average N = 28; range of sample size 9 to 111), except for Whitall 2011 (N = 111). For the majority of the outcomes measured, results were inconsistent across studies. However, this was due to some studies reporting much larger treatment benefits than other studies. All treatment benefits were in the desired direction. In Table 1, large confidence intervals were reported for gait velocity, gait cadence, general UEF, and overall communication. Small sample sizes, combined with high risk of bias and wide confidence intervals, require that the results of this review be interpreted with caution. In summary, the quality of the evidence was low (Table 1).
Potential biases in the review process
The strength of this review is based in the search of all available databases and a comprehensive number of music therapy journals (English, German, and Japanese). This update omitted an updated search of the Science Citation Index from August 2009; however, given the extensive cross‐referencing between databases, it is unlikely that potential studies would be cited on this database alone. We also checked the reference lists of all relevant trials, contacted relevant experts in order to identify unpublished trials, and included publications in any language. In spite of such a comprehensive search, it is still possible that we missed some published and unpublished trials. We requested additional data for all trials we considered for inclusion where necessary, which allowed us to obtain accurate information on the trial quality and data for most trials, assisting us in making well‐informed trial selection decisions.
It is possible that we did not identify some grey literature; however, it is doubtful that this would have had a significant impact on our results. Grey literature tends to include trials with relatively small numbers of participants and inconclusive results (McAuley 2000).
Agreements and disagreements with other studies or reviews
The aim of this review was to update the previous version examining the effects of music therapy on adults with ABI (Bradt 2010). In this update, we expanded our criteria to include trials that examined the effects of music interventions more broadly, including music interventions delivered by professionals other than trained music therapists, such as other medical or health professionals with training in rehabilitation. This revision enabled the inclusion of a greater number of studies.
In our previous review, we could include only two studies for meta‐analysis. This previous analysis showed significant improvements in gait cadence, stride length, and symmetry. A recent review by Yoo 2016 detailed the findings of 11 trials examining the effects of RAS on motor rehabilitation in people with stroke. Meta‐analyses of outcomes from seven trials examining gait function demonstrated large effect sizes for gait parameters (walking velocity, cadence, and stride length) and UEF. Another recent review by Nascimento 2015 compared the effects of cadence cueing and walking training alone following stroke (seven trials, 211 participants). Meta‐analyses of six trials with 171 participants also demonstrated improvements in walking velocity, cadence, stride length, and gait symmetry. The positive effects of RAS on gait in the current review are consistent with previous reviews (Bradt 2010; Nascimento 2015; Yoo 2016). Our review also provided evidence to support previous findings from Yoo 2016 indicating greater effects from rhythmic cueing combined with music in comparison with metronome cueing alone.
Yoo 2016 also examined the effects of RAS on UEF. Meta‐analysis of Fugl‐Meyer Assessment outcomes reported in three studies yielded large effect sizes for UEF. In our updated review, the pooled effect of five studies examining the effect of music‐based interventions on UEF using the Fugl‐Meyer Assessment was not statistically significant, nor were there significant pooled effects for shoulder flexion, hand function, upper limb strength, manual dexterity, or elbow extension angle.
We also included one additional outcome that is important in brain injury rehabilitation, namely cognitive functioning. However, there were not enough studies at this time to provide strong evidence for an effect of music interventions on cognitive outcomes.
In summary, the results of this review provide new insights and further evidence of the effects of music‐based interventions in ABI rehabilitation.
Authors' conclusions
Implications for practice.
Rehabilitation of mobility is crucial in stroke rehabilitation. Rhythmic auditory stimulation (RAS) may help improve gait velocity, stride length, and general gait in people with stroke, and it may be beneficial for gait cadence. Intervention for gait may be enhanced when a trained music therapist delivers the intervention and the RAS is embedded in music. RAS may also be beneficial for improving the timing of upper extremity function (UEF). Although encouraging, more high‐quality randomised controlled trials (RCTs) are needed before conclusions can be made for clinical practice due to the inconsistent use and heterogeneity of outcome measures. Small sample sizes and high risk of bias also limit the research in this area. Rhythm may be a primary influencing factor in music‐based interventions, facilitating functional gains in motor performance in this population. The results of this review thus suggest that using music with a strong and consistent beat may have a greater effect than RAS without music.
Music interventions may be helpful in improving overall communication, although we are unable to draw conclusions as to whether active or receptive methods are most beneficial. Active methods involving singing may be beneficial for addressing difficulties in naming and repetition, however these conclusions were based on a small number of studies with small sample sizes.
Music interventions may improve mood states. We are unable to draw conclusions about which interventions are most beneficial. Rhythm‐based methods in combination with patient‐preferred music to address gait disorders may also improve quality of life outcomes.
Listening to patient‐preferred music may be most beneficial in improving agitation. Although listening to live patient‐preferred music may be beneficial for orientation, we are unable to make further conclusions about the use of music interventions to improve cognition. Conclusions about optimal frequency, duration, and intensity of any music intervention for people with ABI cannot be made based on the findings of this review.
Implications for research.
This review shows encouraging results for the effects of RAS on gait parameters; however, more RCTs with greater numbers of participants are needed to strengthen the current data. It is important to specify whether the effects of RAS on stride length are measured on the affected or unaffected legs, or to provide a computed average for both. More research on the effects of RAS on gait cadence and gait symmetry is needed.
Since 13 of the studies producing significant results in this review involved rhythm‐based methods to address upper limb and gait functioning, we recommend more RCT investigations of RAS across functional domains. Future research would benefit from improving the consistency of the music interventions used across studies and descriptions of how these interventions are delivered. Rhythm appears to be the important component in music interventions to address UEF. However, it is unclear whether rhythm is optimally used with music or without music in rehabilitation of UEF. Additional RCTs are needed to further examine the potential benefits of RAS on UEF. Although the results of this updated review suggest that there is greater improvement when rhythmic auditory cues are embedded in music, further research is warranted comparing the effectiveness of RAS with and without music.
Continued commitment to researching the efficacy of music interventions for UEF in people with hemiparetic stroke is paramount, with a focus on which music interventions are most effective. Future research needs to report the severity of impairment of participants at baseline, and future systematic reviews should plan to perform subanalyses of deficit impairments that are reported.
More RCTs are needed to examine the effect of music interventions on communication in people with acquired brain injury (ABI). Although six trials reported on speech or language outcomes, or both, in this review, we could include the results of only three trials in meta‐analyses due to the wide range of outcomes examined across trials, which could not be combined. Identifying a core outcome set in clinical trials is a prescient issue (Williamson 2012). This has been noted to be particularly problematic in previous Cochrane reviews examining speech and language therapy for people with aphasia following stroke (Brady 2012), as reflected in this review. Greater consensus is needed as to a core outcome set for the subdomains of both communication and cognition in research on music interventions in ABI.
Greater consistency in the choice of outcome measures in populations with ABI and greater accuracy in reporting on how these are used would also strengthen the research. For example, three studies used the Stroke Impact Scale to examine quality of life (Cha 2014b; Hill 2011; Jeong 2007). However, these studies seem to have used the Stroke Impact Scale in different ways, as the ranges of scores between the studies were highly variable. The Profile of Mood States was used in all three studies examining mood due to its validity for neurological populations (Jeong 2007; Pool 2012; Särkämö 2008). This measure, in its different versions, is formed of several subscales for specific moods. Although the one outcome measure for mood was used across studies, different versions of the measure were used. The subscales of the different versions varied too much to allow comparison. This prevented meaningful combination of outcome data from subscales. Total scores need to be reported for the measures used, as well as scores for the relevant subscales, where appropriate, so that these can be combined for meta‐analysis. The direction of improvement (i.e. a higher score indicates improvement) should also be reported for each subscale and total score to aid with translation to practice.
It is promising that this review update included a small number of trials examining outcomes in the domains of mood and emotions, social skills and interactions, quality of life, and cognitive functioning, all of which were not included in our previous review. Although this review examined gait as the primary outcome in clinical trials examining music interventions with ABI, this is inconsistent with music therapy clinical practice. Communication and psycho‐emotional domains tend to be the primary reason for referral (Magee 2007). More research is needed to examine how music interventions may benefit outcomes in these domains in addition to behavioural and cognitive outcomes. This is particularly relevant for more complex populations such as post‐traumatic amnesia and disorders of consciousness.
Populations with significant impairments following profound brain injury pose considerable challenges for researchers in terms of determining meaningful outcomes and finding appropriate measures. There is a growing number of studies examining the effects of music interventions using neurophysiological and imaging methods with severely impaired brain damaged populations. We thus recommend that a separate review be conducted on the effect of music interventions on these non‐behavioural outcomes of interest.
Further trials are needed to examine how music interventions may have a combined impact on functional outcomes and mood/quality of life, as music has been noted to be physiologically arousing and motivating, and offers a strong driving stimulus for motor functions (Clark 2016). Research examining the effect of music interventions on both motor skills and mood or quality of life, or both, in the same study, is needed.
We did not include any studies that examined activities of daily living and adverse events. Future trials should consider examining the benefits of music interventions on all of these outcomes.
Future RCTs should ensure that the quality criteria absent in previous trials are addressed and also reported, particularly for selection, detection, and attrition biases. Random group allocation should be used, and the method of group allocation should be reported. Blinding of outcome assessors needs to improve in music intervention studies, ensuring that this is incorporated into the design and is reported in publications. Reporting of whether interventionists are blinded to the purpose of the study also needs to be improved in RAS studies. Finally, many studies in this review used a small sample size (eight to 22 participants). Future studies need to include power analyses so that sufficiently large samples are used.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2016 | New citation required and conclusions have changed | The conclusions have changed. Two new authors were added, and three authors from the original review were removed. |
| 31 December 2015 | New search has been performed | Handsearches and searches of electronic sources have been updated. The protocol was revised to include music interventions delivered by non‐music therapists. The title of the review was amended in line with changes to the protocol. The outcomes to be included were revised to include cognitive outcomes. We included 22 new studies, bringing the total number of included studies to 29, involving 775 participants. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 10 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge the Cochrane Stroke Group Editorial Team for advice and support, and Brenda Thomas for her assistance in the design of the search strategy for the original review and updated search strategies for this update. We also acknowledge the following individuals for their help in screening the titles and abstracts and the retrieval of articles as graduate assistants: Patricia Gonzalez and Mike Viega in the original review, and Vern Miller for this update. Lastly, we acknowledge the authors of the original review who were not involved with this update: Cheryl Dileo, Emer McGilloway and Barbara Wheeler.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain +"] or [mh "carotid artery diseases"] or [mh "cerebrovascular trauma"] or [mh "intracranial arterial diseases"] or [mh " intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] or [mh "hypoxia, brain"] |
| #2 | (stroke* or poststroke or "post‐stroke" or apoplex* or cerebral next vasc* or cerebrovasc* or cva or SAH):ti,ab |
| #3 | ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle next cerebr* or mca* or "anterior circulation" or "basilar artery" or "vertebral artery") near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab |
| #4 | ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal next gangli* or putaminal or putamen or "posterior fossa" or hemispher* or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab |
| #5 | [mh ^hemiplegia] or [mh paresis] or [mh aphasia] or [mh "gait disorders, neurologic"] |
| #6 | (hempar* or hemipleg* or paresis or paretic or aphasi* or dysphasi*):ti,ab |
| #7 | [mh "brain damage, chronic"] or [mh ^"brain injuries"] or [mh "brain concussion"] or [mh "brain hemorrhage, traumatic"] or [mh ^"brain injury, chronic"] or [mh ^"diffuse axonal injury"] |
| #8 | [mh ^"craniocerebral trauma"] or [mh "head injuries, closed"] or [mh "intracranial hemorrhage, traumatic"] |
| #9 | [mh "brain abscess"] or [mh "central nervous system infections"] or [mh encephalitis] or [mh meningitis] |
| #10 | (encephalitis or meningitis or head injur*):ti,ab |
| #11 | [mh "brain neoplasms"] |
| #12 | ((brain or cerebr*) near/5 (injur* or hypoxi* or damage* or concussion or trauma* or neoplasm* or lesion* or tumor* or tumour* or cancer* or infection*)):ti,ab |
| #13 | {or #1‐#12} |
| #14 | [mh ^music] or [mh ^"music therapy"] or [mh ^singing] or [mh ^"acoustic stimulation"] |
| #15 | (music* or rhythmic* or melod* or harmon*):ti,ab |
| #16 | ((auditory or acoustic) near/5 (stimulat* or cue*)):ti,ab |
| #17 | (sing or sings or singing or singer* or song* or chant* or compose or composing or improvis*):ti,ab |
| #18 | ((vocal or voice) near/5 intonat*):ti,ab |
| #19 | (gait near/5 (puls* or rhythm*)):ti,ab |
| #20 | {or #14‐#19} |
| #21 | #13 and #20 |
Appendix 2. MEDLINE search strategy
MEDLINE (Ovid) 1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ or exp hypoxia, brain/ 2. (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. exp hemiplegia/ or exp paresis/ or exp aphasia/ or exp gait disorders, neurologic/ 6. (hempar$ or hemipleg$ or paresis or paretic or aphasi$ or dysphasi$).tw. 7. exp brain damage, chronic/ or brain injuries/ or exp brain concussion/ or exp brain hemorrhage, traumatic/ or brain injury, chronic/ or diffuse axonal injury/ 8. craniocerebral trauma/ or exp head injuries, closed/ or exp intracranial hemorrhage, traumatic/ 9. exp brain abscess/ or exp central nervous system infections/ or exp encephalitis/ or exp meningitis/ 10. (encephalitis or meningitis or head injur$).tw. 11. exp brain neoplasms/ 12. ((brain or cerebr$) adj5 (injur$ or hypoxi$ or damage$ or concussion or trauma$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).tw. 13. or/1‐12 14. music/ or music therapy/ or singing/ or acoustic stimulation/ 15. (music$ or rhythmic$ or melod$ or harmon$).tw. 16. ((auditory or acoustic) adj5 (stimulat$ or cue$)).tw. 17. (sing or sings or singing or singer$ or song$ or chant$ or compose or composing or improvis$).tw. 18. ((vocal or voice) adj5 intonat$).tw. 19. (gait adj5 (puls$ or rhythm$)).tw. 20. or/14‐19 21. 13 and 20 22. Randomized Controlled Trials as Topic/ 23. random allocation/ 24. Controlled Clinical Trials as Topic/ 25. control groups/ 26. clinical trials as topic/ 27. double‐blind method/ 28. single‐blind method/ 29. Placebos/ 30. placebo effect/ 31. cross‐over studies/ 32. randomized controlled trial.pt. 33. controlled clinical trial.pt. 34. clinical trial.pt. 35. (random$ or RCT or RCTs).tw. 36. (controlled adj5 (trial$ or stud$)).tw. 37. (clinical$ adj5 trial$).tw. 38. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 39. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 40. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 41. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 42. (cross‐over or cross over or crossover).tw. 43. (placebo$ or sham).tw. 44. trial.ti. 45. (assign$ or allocat$).tw. 46. controls.tw. 47. or/22‐46 48. 21 and 47 49. exp animals/ not humans.sh. 50. 48 not 49
Appendix 3. Embase search strategy
1 stroke/ or cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke patient/ or stroke unit/ 2 (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw. 3 ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4 ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5 hemiparesis/ or hemiplegia/ or paresis/ or exp aphasia/ or dysphasia/ or exp neurologic gait disorder/ 6 (hempar$ or hemipleg$ or paresis or paretic or aphasi$ or dysphasi$).tw. 7 brain injury/ or acquired brain injury/ or brain concussion/ or brain contusion/ or brain damage/ or brain stem injury/ or cerebellum injury/ or diffuse axonal injury/ or postconcussion syndrome/ or traumatic brain injury/ or brain hypoxia/ or head injury/ 8 central nervous system infection/ or exp brain infection/ or exp meningitis/ 9 exp brain tumor/ 10 (encephalitis or meningitis or head injur$).tw. 11 ((brain or cerebr$) adj5 (injur$ or hypoxi$ or damage$ or concussion or trauma$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).tw. 12 or/1‐11 13 exp music/ or music therapy/ or musician/ or singing/ or auditory stimulation/ 14 (music$ or rhythmic$ or melod$ or harmon$).tw. 15 ((auditory or acoustic) adj5 (stimulat$ or cue$)).tw. 16 (sing or sings or singing or singer$ or song$ or chant$ or compose or composing or improvis$).tw. 17 ((vocal or voice) adj5 intonat$).tw. 18 (gait adj5 (puls$ or rhythm$)).tw. 19 or/13‐18 20 12 and 19 21 Randomized Controlled Trial/ 22 Randomization/ 23 Controlled Study/ 24 control group/ 25 clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/ 26 Crossover Procedure/ 27 Double Blind Procedure/ 28 Single Blind Procedure/ or triple blind procedure/ 29 placebo/ 30 (random$ or RCT or RCTs).tw. 31 (controlled adj5 (trial$ or stud$)).tw. 32 (clinical$ adj5 trial$).tw. 33 ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 34 (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 35 ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 36 ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 37 (cross‐over or cross over or crossover).tw. 38 (placebo$ or sham).tw. 39 trial.ti. 40 (assign$ or allocat$).tw. 41 controls.tw. 42 or/21‐41 43 20 and 42 44 (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/) 45 43 not 44
Appendix 4. CINAHL search strategy
Database: CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature, 1982 to June 2015; EBSCO
(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") or (MH "Hypoxia, Brain")
(MH "Stroke Patients") OR (MH "Stroke Units")
TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S6. S4 and S5
TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S7 and S8
(MH "Hemiplegia") or (MH "Aphasia+") OR (MH "Gait Disorders, Neurologic+")
TI ( hemipleg* or hemipar* or paresis or paretic or aphas* or dysphas*) or AB ( hemipleg* or hemipar* or paresis or paretic or aphas* or dysphas*)
(MH "Brain Damage, Chronic") OR (MH "Brain Injuries") OR (MH "Brain Concussion+")
(MH "Head Injuries")
(MH "Central Nervous System Infections+") OR (MH "Encephalitis+") OR (MH "Meningitis+") OR (MH "Meningoencephalitis+")
(MH "Brain Neoplasms+")
TI (encephalitis or meningitis or head injur*) or AB (encephalitis or meningitis or head injur*)
TI ((brain or cerebr*) N5 (injur* or hypoxi* or damage* or concussion or trauma* or neoplasm* or lesion* or tumor* or tumour* or cancer* or infection*))
AB ((brain or cerebr*) N5 (injur* or hypoxi* or damage* or concussion or trauma* or neoplasm* or lesion* or tumor* or tumour* or cancer* or infection*))
S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18
(MH "Music") OR (MH "Music Therapy (Iowa NIC)") OR (MH "Music Therapy") OR (MH "Performing Artists") OR (MH "Singing") OR (MH "Performing Arts") OR (MH "Acoustic Stimulation")
TI (music* or rhythmic* or melod* or harmon*) or AB (music* or rhythmic* or melod* or harmon*)
TI ((auditory or acoustic) N5 (stimulat* or cue*)) or AB ((auditory or acoustic) N5 (stimulat* or cue*))
TI (sing or sings or singing or singer* or song* or chant* or compose or composing or improvis*) or AB (sing or sings or singing or singer* or song* or chant* or compose or composing or improvis*)
TI ((vocal or voice) N5 intonat*) or AB ((vocal or voice) N5 intonat*)
TI (gait N5 (puls* or rhythm*)) or AB (gait N5 (puls* or rhythm*))
S20 OR S21 OR S22 OR S23 OR S24 OR S25
PT randomized controlled trial or clinical trial
(MH "Random Assignment") or (MH "Random Sample+")
(MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies")
(MH "Control (Research)") or (MH "Control Group")
(MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials")
(MH "Placebo Effect") or (MH "Placebos")
(MH "Clinical Research") or (MH "Clinical Nursing Research")
(MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design")
TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
TI ( singl* or doubl* or tripl* or trebl* ) or AB ( singl* or doubl* or tripl* or trebl* )
TI ( blind* or mask*) or AB ( blind* or mask* )
S36 and S37
TI ( crossover or cross‐over or placebo* or control* or factorial or sham ) or AB ( crossover or cross‐over or placebo* or control* or factorial or sham )
TI ( clin* or controlled or intervention* or compar* or experiment* or preventive or therapeutic ) or AB ( clin* or controlled or intervention* or compar* or experiment* or preventive or therapeutic )
TI trial* or AB trial*
S40 and S41
TI (assign* or allocat*) or AB (assign* or allocat*)
TI trial
( TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) ) OR ( AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) )
S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S38 OR S39 OR S42 OR S43 OR S44 OR S45
S19 AND S26 AND S46
Appendix 5. PsycINFO search strategy
Database: PsycINFO (Ovid); 1806 to June Week 1 2015
1 cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2 (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw. 3 ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4 ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5 hemiparesis/ or hemiplegia/ or exp aphasia/ 6 (hempar$ or hemipleg$ or paresis or paretic or aphasi$ or dysphasi$).tw. 7 traumatic brain injury/ or brain damage/ or brain concussion/ or exp head injuries/ 8 exp meningitis/ or exp encephalitis/ or intracranial abscesses/ 9 brain neoplasms/ 10 (encephalitis or meningitis or head injur$).tw. 11 ((brain or cerebr$) adj5 (injur$ or hypoxi$ or damage$ or concussion or trauma$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).tw. 12 or/1‐11 13 exp music/ or music therapy/ or musicians/ or singing/ or tempo/ or music perception/ or musical ability/ or exp rhythm/ or music education/ or exp auditory stimulation/ 14 (music$ or rhythmic$ or melod$ or harmon$).tw. 15 ((auditory or acoustic) adj5 (stimulat$ or cue$)).tw. 16 (sing or sings or singing or singer$ or song$ or chant$ or compose or composing or improvis$).tw 17 ((vocal or voice) adj5 intonat$).tw. 18 (gait adj5 (puls$ or rhythm$)).tw. 19 or/13‐18 20 12 and 19 21 clinical trials/ or treatment effectiveness evaluation/ or placebo/ 22 (random$ or RCT or RCTs).tw. 23 (controlled adj5 (trial$ or stud$)).tw. 24 (clinical$ adj5 trial$).tw. 25 ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 26 (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 27 ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 28 ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 29 (cross‐over or cross over or crossover).tw. 30 (placebo$ or sham).tw. 31 trial.ti. 32 (assign$ or allocat$).tw. 33 controls.tw. 34 or/21‐33 35 20 and 34
Appendix 6. LILACS search strategy
((music*) or (rhythmic stimul*) or (auditory stimulat*) or (rhythmic cue*) or (auditory cue*) or (acoustic stimulat*) or (acoustic cue*) or sing or sings or singing or song* or compose or composing or improvis*) AND (brain or cerebrovascular or cerebral or stroke or hemiplegia or paresis or aphas* or dysphas*)
Appendix 7. AMED search strategy
Database: AMED (Allied and Complementary Medicine) (Ovid)1985 to June 2015
1 cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2 (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw. 3 ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4 ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5 hemiplegia/ or aphasia/ 6 (hempar$ or hemipleg$ or paresis or paretic or aphasi$ or dysphasi$).tw. 7 head injuries/ or brain injuries/ or brain concussion/ or brain disease/ or brain neoplasms/ or encephalitis/ or meningitis/ 8 (encephalitis or meningitis or head injur$).tw. 9 ((brain or cerebr$) adj5 (injur$ or hypoxi$ or damage$ or concussion or trauma$ or neoplasm$ or lesion$ or tumor$ or tumour$ or cancer$ or infection$)).tw. 10 or/1‐9 11 music/ or music therapy/ 12 (music$ or rhythmic$ or melod$ or harmon$).tw. 13 ((auditory or acoustic) adj5 (stimulat$ or cue$)).tw. 14 (sing or sings or singing or singer$ or song$ or chant$ or compose or composing or improvis$).tw. 15 ((vocal or voice) adj5 intonat$).tw. 16 (gait adj5 (puls$ or rhythm$)).tw. 17 or/11‐16 18 10 and 17
Appendix 8. CAIRSS search strategy
1. Brain injur? [as a phrase] OR head injur? [as a phrase] OR skull fracture [as a phrase] 2. Brain damage [as a phrase] OR cerebral trauma [as a phrase] OR brain neoplasm? [as a phrase] 3. Brain tumor? [as a phrase] OR cereb? tumor? [as a phrase] OR brain infarction [as a phrase] 4. cerebrovascular disorder? [as a phrase] OR brain ischemia [as a phrase] OR cerebrovascular accident [as a phrase] 5. intracranial hemorrhage? [as a phrase] OR stroke OR poststroke 6. post‐stroke [as a phrase] OR cva OR cereb? Thrombosis [as a phrase] 7. brain thrombosis [as a phrase] OR brain embolism [as a phrase] 8 hemiplegi? OR paresis OR paretic 9. Aphasi? OR dysphasi?
Appendix 9. ProQuest Digital Dissertations search strategy
ab((music) OR (rhythmic auditory stimulation) OR (acoustic stimulation) OR (rhythmic auditory cueing) OR (therapeutic instrumental) OR (melodic intonation) OR (vocal intonation) OR (therapeutic singing) OR (songwriting)) AND ab((stroke OR head OR brain OR intracranial OR cerebrovascular))
Appendix 10. ClinicalTrials.gov search strategy
(music OR singing OR song OR songs OR (rhythmic auditory stimulation) OR (rhythmic auditory cueing) OR (acoustic stimulation) OR (acoustic cueing) OR melody OR melodic OR vocal) AND (stroke OR head OR brain OR intracranial OR cerebrovascular) | Interventional Studies
Appendix 11. Current Controlled Trials search strategy
music OR (music therapy)
Data and analyses
Comparison 1. Music therapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait velocity | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies | 9 | 268 | Mean Difference (IV, Random, 95% CI) | 11.34 [8.40, 14.28] |
| 1.2 Adequate randomisation | 7 | 228 | Mean Difference (IV, Random, 95% CI) | 10.79 [7.23, 14.35] |
| 2 Gait velocity ‐ interventionist | 9 | 268 | Mean Difference (IV, Random, 95% CI) | 11.34 [8.40, 14.28] |
| 2.1 Music therapist | 3 | 128 | Mean Difference (IV, Random, 95% CI) | 14.76 [13.84, 15.69] |
| 2.2 Non‐music therapist | 6 | 140 | Mean Difference (IV, Random, 95% CI) | 8.48 [5.16, 11.80] |
| 3 Gait velocity ‐ music type | 9 | 268 | Mean Difference (IV, Random, 95% CI) | 11.34 [8.40, 14.28] |
| 3.1 Music | 5 | 173 | Mean Difference (IV, Random, 95% CI) | 14.69 [13.77, 15.61] |
| 3.2 Auditory stimulation (no music) | 4 | 95 | Mean Difference (IV, Random, 95% CI) | 7.70 [3.03, 12.38] |
| 4 Stride length (affected side) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All studies | 5 | 129 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.04, 0.20] |
| 4.2 Adequate randomisation | 3 | 89 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.05, 0.11] |
| 5 Stride length (affected side) ‐ music type | 5 | 129 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.04, 0.20] |
| 5.1 Music | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.05, 0.12] |
| 5.2 Auditory stimulation (no music) | 3 | 79 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.02, 0.25] |
| 6 Stride length (unaffected side) [metres] | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 All studies | 4 | 99 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.01, 0.22] |
| 6.2 Adequate randomisation | 2 | 59 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.12] |
| 7 Stride length (unspecified) [metres] | 3 | 186 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.01, 0.33] |
| 8 Gait cadence | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 all studies | 7 | 223 | Mean Difference (IV, Random, 95% CI) | 10.77 [4.36, 17.18] |
| 8.2 Adequate randomisation | 6 | 203 | Mean Difference (IV, Random, 95% CI) | 10.80 [4.05, 17.56] |
| 9 Gait cadence ‐ interventionist | 7 | 223 | Mean Difference (IV, Random, 95% CI) | 10.77 [4.36, 17.18] |
| 9.1 Music therapist | 3 | 128 | Mean Difference (IV, Random, 95% CI) | 11.51 [‐2.57, 25.60] |
| 9.2 Non‐music therapist | 4 | 95 | Mean Difference (IV, Random, 95% CI) | 7.65 [4.43, 10.86] |
| 10 Gait cadence ‐ music type | 7 | 223 | Mean Difference (IV, Random, 95% CI) | 10.77 [4.36, 17.18] |
| 10.1 Music | 4 | 148 | Mean Difference (IV, Random, 95% CI) | 11.34 [‐1.05, 23.74] |
| 10.2 Auditory stimulus (no music) | 3 | 75 | Mean Difference (IV, Random, 95% CI) | 7.58 [4.33, 10.83] |
| 11 Stride symmetry | 3 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.94 [‐0.32, 2.20] |
| 12 General gait | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 7.67 [5.67, 9.67] |
| 13 Balance | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All studies | 3 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.48, 1.09] |
| 13.2 Adequate randomisation | 2 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐1.10, 1.37] |
| 14 Upper extremity functioning (general) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All studies | 5 | 194 | Mean Difference (IV, Random, 95% CI) | 3.56 [‐0.88, 8.00] |
| 14.2 Adequate randomisation | 3 | 156 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐2.33, 4.12] |
| 15 Upper extremity functioning ‐ time | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.69, ‐0.47] |
| 16 Range of motion ‐ shoulder flexion | 2 | 53 | Mean Difference (IV, Random, 95% CI) | 9.81 [‐12.71, 32.33] |
| 17 Hand function | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.91, 1.54] |
| 18 Upper limb strength | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 6.03 [‐2.52, 14.59] |
| 19 Manual dexterity | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐1.08, 2.01] |
| 20 Overall communication | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 All studies | 3 | 67 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.11, 1.39] |
| 20.2 Adequate randomisation | 2 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.03, 1.07] |
| 21 Naming | 2 | 35 | Mean Difference (IV, Random, 95% CI) | 9.79 [1.37, 18.21] |
| 22 Repetition | 2 | 35 | Mean Difference (IV, Random, 95% CI) | 8.90 [3.25, 14.55] |
| 23 Memory | 2 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.29, 0.95] |
| 24 Attention | 2 | 39 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.34, 0.94] |
| 25 Quality of life | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | 0.89 [0.32, 1.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baker 2001.
| Methods | RCT Cross‐over trial with 3 groups | |
| Participants | Participants with a severe head injury Diagnosis: post‐traumatic amnesia scoring less than or equal to 8 on the Westmead Post‐traumatic Amnesia Scale on the day prior to commencement of the experiment Time since onset: not stated N randomised: 22 N analysed in treatment group (live music): 22 N analysed in treatment group (recorded music): 22 N analysed in control group: 22 Mean age: 34 years (SD 15.34) Sex: 5 (23%) female, 17 (77%) male Ethnicity: 72.7% Australian, 9% Croatian, 4.5% Taiwanese, 4.5% Bangladeshi, 9% Italian Setting: rehabilitation hospital Country: Australia |
|
| Interventions | 3 study groups: 1: Music intervention (live): Participants listened to live music. The music selection was individualised for each participant and comprised 3 music pieces that were chosen from selections suggested by family members. All styles of music were permitted. The researcher was present in the room sitting opposite and facing the participant 2. Music intervention (recorded): Participants listened to recorded music. The same 3 pieces were played during the recorded music condition as were used in the live music condition, and played in the same order. The music was played free‐field on an audio cassette player. To avoid agitating the participant no headphones were used. The researcher was present in the room sitting opposite and facing the participant 3. Control condition: The music therapist was present in the room, but no music was played. Participants were free to do whatever they wanted. As in the music conditions, the verbal interactions were kept to a minimum Number of sessions: 6 in total (2 of each condition) over 6 days Length of sessions: 10 to 12 minutes each |
|
| Outcomes | Agitation (Agitated Behavior Scale): effect size reported Level of orientation (Westmead Post‐traumatic Amnesia Scale): effect size reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | High risk | No allocation concealment used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants was not possible. It was not possible to blind the personnel delivering the interventions. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout because of early resolution of PTA |
| Selective reporting (reporting bias) | Low risk | There were no indications of selective reporting in this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Cha 2014a.
| Methods | RCT Cross‐over trial |
|
| Participants | Participants with first ischaemic CVA Time since onset: at least 6 months post‐CVA N randomised: 41 N analysed at baseline condition: 20 N analysed in RAS condition: 21 Mean age: 60.8 years (SD 19.8) Sex: 17 females (41.5%), 24 males (58.5%) Ethnicity: not reported Setting: rehabilitation centres Country: South Korea |
|
| Interventions | All participants were studied under 5 conditions. Study compared walking with no intervention (baseline) with RAS at 4 different speeds (baseline‐matched RAS, ‐10%, +10%, and +20%). In this review we used baseline‐matched RAS and +20% Number of sessions: not clear Length of sessions: not stated |
|
| Outcomes | Gait parameters: gait velocity (cm/second), gait cadence (steps per minute), stride length‐affected (cm), stride length‐unaffected (cm), stride symmetry. Post‐test scores used | |
| Notes | This study used rhythm delivered by a metronome without music | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Conditions were applied in random order" (p480) All participants received all conditions. We assessed randomisation bias to be low for this reason |
| Allocation concealment (selection bias) | Low risk | Allocation of treatment order not reported. However, all participants received all treatments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The GAITRite system recorded the gait velocity, cadence, stride length, double limb support (% of cycle), and double single limb support (% of cycle)” (p480). As personnel were not involved in entering the data, we rated detection bias as low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported. However, 41 participants were recruited, and the authors report 41 data sets included in the analysis |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Cha 2014b.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with chronic hemiparetic stroke Diagnosis: ischaemic or haemorrhagic stroke Time since onset: at least 6 months N randomised to RAS and intense gait‐training treatment: 10 N randomised to intensive gait training alone (control): 10 N analysed in treatment group: 10 N analysed in control group: 10 Mean age: 61.4 years Sex: 8 females (40%), 12 males (60%) Ethnicity: not reported Setting: inpatient hospital Country: South Korea |
|
| Interventions | 2 study groups: 1. Music intervention group: RAS with intensive gait training 2. Control group: intensive gait training alone Number of sessions: 30 sessions in total over 6 weeks Length of sessions: 30 minutes |
|
| Outcomes | Gait velocity (cm/second), gait cadence (steps/minute), stride length‐affected side (cm), stride length‐unaffected side (cm), balance (Berg Balance Scale), quality of life (Stroke Specific Quality of Life Scale). Pre‐ and post‐test scores | |
| Notes | This study used rhythm delivered by a metronome in combination with recorded music | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to either and [sic] RAS training or control group using sealed envelopes”. Method of randomisation was not reported (p682) |
| Allocation concealment (selection bias) | Low risk | Allocation using sealed envelopes. Quote: "randomly assigned to either and [sic] RAS training or control group using sealed envelopes" (p682) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of the personnel involved in assessing outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported, although 20 were randomised and 20 completed |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported. Quote: "The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article" (p687) |
Chouan 2012.
| Methods | RCT 3‐arm parallel‐group design |
|
| Participants | Participants with middle cerebral artery hemiparetic stroke Time since onset: discharged from hospital at least 3 months earlier N randomised to RAS and standard care: 15 N randomised to standard care: 15 N randomised to visual cueing and standard care: 15 (not included in this review) N analysed in RAS and standard care group: 15 N analysed in standard care (control) group: 15 N analysed in visual cueing and standard care group: 15 (not included in this review) Mean age: 57.40 years (SD 5.18) Sex: 9 females (20%), 36 males (80%) Ethnicity: not reported Setting: multispecialty hospital and research centre Country: India |
|
| Interventions | 3 study groups: 1. Music intervention group: RAS plus conventional treatment 2. Other therapy intervention (not used in this review): visual cueing plus conventional treatment 3. Control group: conventional treatment Number of sessions: RAS given for 9 sessions in total over 3 weeks Length of sessions: 2 hours |
|
| Outcomes | Upper extremity function (Fugl‐Meyer Assessment), general gait (Dynamic Gait Index). Post‐test scores used | |
| Notes | This study used rhythm delivered by a metronome without music | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects selected for the study were randomly allocated using sealed envelopes into 3 groups." (p344). Method of randomisation was not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "The subjects selected for the study were randomly allocated using sealed envelopes into 3 groups." (p396) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of the personnel involved in assessing outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported 0 withdrawals |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Conklyn 2012.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with acute stroke with mild to severe nonfluent aphasia Time since onset: most within 13 days, 2 control and 1 treatment participant were > 60 days N randomised to treatment group at baseline: 16 N randomised to control group at baseline: 14 N analysed in treatment group at visit 1: 14 N analysed in control group at visit 1: 10 N analysed in treatment group at visit 2: 9 N analysed in control group at visit 2: 8 Mean age: 61.51 years (SD 15.49) Sex: 14 females (47%), 16 males (53%) Ethnicity: not reported Setting: inpatient Country: USA |
|
| Interventions | 2 study groups: 1. Music intervention group: received modified melodic intonation therapy (MMIT). This involved a 10‐ to 15‐minute session with the music therapist "consisting of the music therapist teaching the participant a melodic phrase." (p1466) 2. Control group: received a 10‐ to 15‐minute session with the music therapist "who discussed the participant’s impairment, different forms of treatment, different outcomes, and various issues that can result from aphasia, such as depression and withdrawal." (p1466) Number of sessions: 2 in total Length of sessions: 10 to 15 minutes |
|
| Outcomes | 2 tasks similar to Western Aphasia Battery: adjusted total score. Change scores used | |
| Notes | Quote: "The Western Aphasia Battery has two subtests that were deemed appropriate, one for repetition and one for responsiveness; however, both sections are designed to elicit short answers. Because of the length of the phrases utilized in MMIT it was decided not to use the exact subtests from the Western Aphasia Battery, but instead to design two similar tasks that would elicit longer responses." (p465) Outcomes were measured for all 3 visits. However, due to high attrition for visit 3, we only reported change scores between visit 1 and visit 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization table was generated by a biostatistician prior to the start of the study. Random assignment was performed by the music therapist after enrolment by the nursing manager, who had no prior knowledge of the ordering of participants." (p1466) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants was not possible. It was not possible to blind the personnel delivering the interventions |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The evaluators were not present in the room when the treatment or control session was given, and the music therapist, being blinded to the test scores until after the post‐test was completed for each session, was not in the room when the test was administered." (pp1465‐6) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition from baseline to visits 1 and 2 higher than 20% for control group. Attrition from baseline to visit 2 higher than 20% for treatment group. Quote: "Out of the 14 controls, 10 had both pre and post scores at Visit 1, and eight had pre and post scores at Visit 2. For the treatment group, 14 out of the 16 had both pre and post scores at Visit 1, and nine had pre and post scores at Visit 2. Only patients who completed both components (responsive and repetitive) in both pre and post assessments were considered in the following analysis. Data are not given for Visit 3 due to the small number of participants (one control, three treatments)." (pp1466‐7) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Fernandes 2014.
| Methods | Quasi‐RCT 2‐arm parallel‐group design |
|
| Participants | Participants with severe cerebral damage in vegetative state Diagnosis: traumatic brain injury (38%), non‐traumatic origin hypoxic‐ischaemic encephalopathy (35%), acute cerebrovascular accident (20%), central nervous system infections (4%), and central nervous system tumours (4%). Time since onset: > 3 years, (mean 45.9 months; SD 20.5 months) N randomised to treatment group: 13 N randomised to control group: 13 N analysed in treatment group: 13 N analysed in control group: 13 Mean age: 54.05 years (SD 14.37) Sex: 13 females (50%), 13 males (50%) Ethnicity: not reported Setting: inpatient, "Irreversible cerebral damage unit" (p120) Country: Spain |
|
| Interventions | 2 study groups: 1. Music intervention group: Participants were exposed to 3 types of musical/auditory stimuli: classical relaxing music (CRM), relaxing music with nature sounds (RMNS), and radio (various musical genres and commercial messages). CRM and RMNS were played individually using an MP3 player via headphones for a period of 20 minutes. The radio was played as environmental music via a stereo system for 1 hour 2. Control group: The control group was exposed to silence on an MP3 player via headphones Number of sessions: 18 sessions in total. The frequency of sessions is unclear: "18 sessions (six sessions for each musical stimulus), being performed once a day, twice weekly at the same hour" (p119) Length of sessions: CRM and RMNS were played for 20 minutes. "Radio ... was played as environmental music ... for one hour via a stereo system" (p119) |
|
| Outcomes | Vital signs: systolic BP, diastolic BP, heart rate, respiratory rate, oxygen saturation (not included in this review). Facial expressions: muscular facial relaxation, eye opening, mouth movements, head movements, yawning, smiling, eyebrow movements, and sound emission (results not provided for control group) |
|
| Notes | The outcomes of this study were not included in a meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated list of random numbers (personal communication with principal investigator) |
| Allocation concealment (selection bias) | Unclear risk | Allocation was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not reported, however it may be assumed that personnel delivering the interventions were not blinded, as the part of the experimental intervention involved radio played as "environmental music ... via a stereo system" (p119) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding is not reported, however it may be assumed that raters were not blind, as behavioural ratings were taken immediately after live music was played on headphones to heavily dependent participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data were reported to assess the effects of music listening on facial expressions. Objectives at the outset of the research were (quote): "to verify the influence of music listening on patients’ facial expressions" (p117). Although the authors state (quote): "Alterations in facial expression were displayed in each patient" (p117), inadequate information is presented to evaluate whether this outcome has been reported selectively |
| Free from financial conflict of interest | Low risk | Quote: "The authors declare no conflicts of interest." (p117) |
Hill 2011.
| Methods | Quasi‐RCT with alternate group allocation 2‐arm parallel‐group design |
|
| Participants | Participants with chronic stroke and right hemiparesis Time since onset: mean 3.3 years (SD 2.1) N assigned to treatment group: 6 N assigned to control group: 4 N analysed in treatment group: 5 N analysed in control group: 3 Mean age: 60 years (8.74) Sex: 6 females (60%), 4 males (40%) Ethnicity: 70% Caucasian (understood to be white). Otherwise not reported Setting: Not reported. However, the setting seems to be a community outpatient setting. Quote: "Subjects were recruited by local rehabilitation therapists and by subject inquiry regarding current studies" (p729) Country: USA |
|
| Interventions | 2 study groups: 1. Music intervention group: interactive metronome (IM) intervention. Consisted of occupational therapy treatment with 30 minutes of IM session embedded. Interactive metronome consisted of a computer‐based rhythmic and auditory training program. As the computer‐generated reference was heard through headphones, the participants attempted to match the rhythmic auditory beat with repeated limb movements, such as clapping their hands together with a switch in their hand. One IM session consisted of repetitive limb movement lasting 1 to 3 minutes. Sessions took place 3 times per week for 10 weeks. 2. Control group: occupational therapy conventional treatment in 1‐hour sessions, 3 times per week for 10 weeks |
|
| Outcomes | Upper extremity function (FMA, Arm Motor Ability Test, Box and Block Test, Canadian Occupational Performance Measure). Quality of life (Stroke Impact Scale 2.0) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Subjects were enrolled in the study groups by alternating group assignment (i.e. Subject 1 was in the OT group, Subject 2 was in the IM+OT group)" (p729) |
| Allocation concealment (selection bias) | Unclear risk | Allocation is not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants was not possible. It was not possible to blind the personnel delivering the interventions. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | It is unclear whether the SIS for quality of life involved self reports |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All outcomes except the COPM were measured by the same blinded rater 1 week before intervention and within 1 week after intervention" (p729) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition reported at 20%. 1 participant was lost to follow‐up, and 1 withdrew from the study |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Equipment support reported. Quote: "We thank Interactive Metronome for providing the equipment and software for the study" (p737) |
Jeong 2007.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants following infarct (60.6%) and haemorrhagic stroke (39.4%) Diagnosis: 17 with left stroke lesion (51.1%), 15 with right stroke lesion (45.5%), 1 with bilateral stroke lesion (3%) Time since onset: mean 6.39 years (SD 4.96) N randomised to treatment group: 18 N randomised to control group: 18 N received intended treatment in treatment group: 18 N received intended treatment in control group: 18 N analysed in treatment group: 16 N analysed in control group: 17 Mean age: 60.1 years (SD 7.88) Sex: 10 females (30.3%), 23 males (69.7%) Ethnicity: not reported Setting: outpatient. Follow‐up data collected at a "community setting" for experimental group and from individual households for the control group (p127) Country: South Korea |
|
| Interventions | 2 study groups: 1. Music intervention group: RAS music‐movement exercise intervention, which consisted of 4 sections: (a) preparatory activities, (b) main activities, (c) wrap‐up activities, and (d) follow‐up. Quote: "The routines are composed of a series of dynamic rhythmic motions involving the whole body". Other types of dynamic rhythmic movements and rhythm tools that were used in the programme included shaking an egg shaker and playing percussion instruments, such as a small Korean drum or tambourine, to a rhythm after listening to it." (p127) 2. Control group: The intervention involved receiving referral information about available usual care services. Number of sessions: 8 weeks in total. Number of sessions per week unclear Length of sessions: 2 hours per week |
|
| Outcomes | Physiological parameters: upper extremity function, shoulder flexion ROM (goniometer); lower extremity function, ankle flexion ROM (goniometer); lower extremity function, ankle extension ROM (goniometer); shoulder flexibility, upward in affected arm (back‐scratch test); shoulder flexibility, downward in affected arm (back‐scratch test): change scores Psychological outcomes: mood (POMS ‐ Korean version); interpersonal relationships (The Relationship Change Scale); Quality of life (Stroke Specific Quality of Life Scale): pre‐ and post‐scores |
|
| Notes | Intervention described appears to be more similar to therapeutic instrumental performance or patterned sensory enhancement than RAS Total POMS scores reported only; subscale results not reported. Authors used the Korean version of the POMS. However, the total scores were very low (range 1.56 to 2.81 out of a possible 136). We repeatedly attempted to contact the authors to check the POMS data, but were unable to obtain more information. As these data seemed unreliable, we excluded them from the meta‐analysis This RAS study used music in combination with rhythmic stimulation. Participants were encouraged to practice the RAS music‐movement exercises at home each week. "Each week, participants were given a rhythmic music tape that was specifically developed for this study, together with simple instructions for home exercise" (p127) Change scores were computed by 1 review author (JB) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number list. Quote: "Using computer‐generated number cards, the participants were then randomly assigned to one of two groups" (p125) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self report measures were used for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of the outcome assessors for the objective outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although the numbers of withdrawals are reported as less than 20%, the reasons for withdrawal are not given. Quote: "Of the total 36 who were originally recruited, 33 completed the follow‐up data collection. Attrition is less than 20%" (p129) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "This study was supported by the BK21 project (Grant No. 0522‐20010002), the Korea Science and Engineering Foundation (Grant No. R04‐2001‐000‐00197‐0), and the Research Institute of Nursing Science at Seoul National University." (p131) |
Jungblut 2004.
| Methods | Quasi‐RCT with alternate group allocation 2‐arm parallel‐group design | |
| Participants | People with stroke with chronic aphasia (Broca's aphasia and global aphasia) who were no longer receiving speech therapy
Time since onset: mean 11.5 years (since onset of aphasia)
N randomised: 17 N allocated to treatment group: 9 N allocated to control group: 7 N analysed in treatment group: 8 N analysed in control group: 5 Mean age: 63.8 years (experimental group); 67.8 years (control group) Sex: 6 female (46%), 7 male (54%) Ethnicity: not reported Setting: outpatient services Country: Germany |
|
| Interventions | 2 study groups: 1. Music intervention group: rhythmic‐melodic voice training (SIPARI) sessions. SIPARI is a music therapy technique based on specific use of the voice. It actively works with the remaining speech capabilities in the right hemisphere of people with aphasia, namely singing, intonation, prosody embedded in physiologically appropriate breathing. The SIPARI method also employs instrumental and vocal rhythmic exercises and music improvisations to practice communication scenarios. 2. Control group: no treatment Number of sessions: 20 group sessions and 10 individual sessions in total over a period of 7 months Length of sessions: group sessions 60 minutes, individual sessions 45 minutes |
|
| Outcomes | Articulation and prosody, repetition, labelling, speech comprehension, total speech profile (Aachener Aphasie Test/Aachen Aphasia Test): effect size reported | |
| Notes | 1 review author (JB) computed change scores and SD from raw scores received from the principal investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate group allocation |
| Allocation concealment (selection bias) | High risk | No allocation concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Independent outcome assessors were used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23% attrition reported: 1 control and 1 experimental excluded as diagnosis of global or Broca's aphasia was unclear. 2 further participants excluded due to serious illness |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Kim 2005.
| Methods | RCT Cross‐over trial with 3 groups | |
| Participants | Participants with stroke: 8 with severe hemiplegia, 2 with mild hemiplegia
Time since onset: approximately 3 years
N randomised: 10
N analysed: 10 Mean age: not reported, age range: 61 to 73 years Sex: 9 female (90%), 1 male (10%) Ethnicity: 100% South Korean Setting: Daycare centre for seniors Country: South Korea |
|
| Interventions | 3 study groups: 1. Music intervention group: listening to recorded songs with lyrics 2. Music intervention group: listening to karaoke accompaniment without lyrics during upper extremities exercises 3. Control group: no music during upper extremities exercises Number of sessions: 8 sessions in total on a weekly basis Length of sessions: not reported |
|
| Outcomes | Pain (Likert scale). No post‐test means or change scores were reported; only F statistic and significance level. | |
| Notes | The author informed us that she no longer had access to the raw data, therefore we could obtain no means or SD. We did not include extracted data from this study in our review as no other included studies examined pain as an outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | All participants underwent the 3 conditions in random order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self report measures were used for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No objective outcomes were used in this study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 participants (28.5%) withdrew due to health condition or frequent absences (personal communication with author) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "The authors wish to thank the Kwanak Senior Center in Seoul, Korea for its generous support of this research." (p81) |
Kim 2011a.
| Methods | RCT Cross‐over trial with 4 groups |
|
| Participants | Participants with poststroke hemiparesis. No other diagnostic information provided Time since onset: mean 19.40 months (SD 19.49) N recruited: 18 N analysed: 15 Mean age: 60.07 years (SD 11.93) Sex: 7 females (47%), 8 males (53%) Ethnicity: not reported Setting: rehabilitation unit Country: Korea |
|
| Interventions | 4 study groups: 1. Control group: visual locomotor imagery training (used as the control in this review) 2. Music intervention group: visual locomotor imagery training with auditory step rhythm (used as the experimental condition in this review) 3. Other therapy intervention (not used in this review): kinesthetic locomotor imagery training 4. Other therapy intervention (not used in this review): kinesthetic locomotor imagery training with auditory step rhythm Number of sessions: 4 sessions in total over 4 days, with 1 intervention presented in each session Length of sessions: 10 to 12 minutes |
|
| Outcomes | Walking performance (Timed Up‐and‐Go Test, EMG data recorded from the quadriceps, hamstring, tibialis anterior, and gastrocnemius of the affected leg). Change scores were used | |
| Notes | We did not include EMG recording outcomes in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots. Quote: "For randomization, we drew lots with four cards marked with 1, 2, 3 or 4 to determine the order of treatments" (p137) |
| Allocation concealment (selection bias) | Low risk | Drawing of lots. Quote: "Each subject had an envelope containing the four cards; without looking, each drew one card on each occasion" (p137) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of the outcome assessors for the objective outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported at 16.6%. Quote: "Although initially 18 subjects were recruited, 3 subjects were excluded in data analysis owing to spontaneous refusal and irregular participation in intervention sessions" (p137) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Kim 2012a.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with subacute stroke Diagnosis: 8 infarction (40%), 12 haemorrhage (60%) Time since onset: mean 5.22 months (SD 2.02) N randomised to treatment group: 10 N randomised to control group: 10 N analysed in treatment group: 9 N analysed in control group: 9 Mean age: 55.05 years (SD 12.88) Sex: 7 females (35%), 13 males (65%) Ethnicity: not reported Setting: inpatient rehabilitation Country: South Korea |
|
| Interventions | 2 study groups: 1. Music intervention group: RAS 2. Control group: conventional therapy consisting of "one‐on‐one neurodevelopmental therapy between a patient and a therapist. Was composed of sitting up from lying down, sit to stand, and trunk and limb training aimed at learning normal gait patterns" (p1308) Number of sessions: 15 sessions in total with 3 sessions per week Length of sessions: 30 minutes |
|
| Outcomes | Gait velocity (m/minute); gait cadence (steps/minute); stride length (affected side ‐ m); stride length (unaffected side ‐ m); functional gait ability (Dynamic Gait Index); dynamic balance (Four Square Step Test); gait ability (functional ambulation category), sit to stand, walking, stand to sit (Timed Up‐and‐Go Test); spatio‐temporal parameters of gait (up stair time ‐ step/second); spatio‐temporal parameters of gait (down stair time ‐ step/second). Change scores used for all of these outcomes Risk of falls (activities‐specific balance confidence scale). Change scores used Dynamic balance (Timed Up‐and‐Go Test). Post scores used |
|
| Notes | This study used metronome pulse without music, delivered via a smart phone metronome application | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots used (personal correspondence with principal investigator) |
| Allocation concealment (selection bias) | Low risk | Participants drew lots (personal correspondence with principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self report measures were used for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of the outcome assessors for the objective outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported at 10% due to 1 participant from each group (N = 2) leaving the hospital halfway through the study |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Kim 2012b.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with hemiplegic stroke Diagnosis: 14 infarction (70%), 6 haemorrhage (30%) Time since onset: mean 15.5 months N randomised to treatment group: 10 N randomised to control group: 10 N analysed in treatment group: 10 N analysed in control group: 10 Mean age: 64.85 years Sex: not reported Ethnicity: not reported Setting: outpatient Country: South Korea |
|
| Interventions | 2 study groups: 1. Music intervention group: Auditory stimulation with metronome beat. Quote: "over the ground gait training with a metronome beat" (p775) 2. Control group: Quote: "over the ground gait training" (p775) Number of sessions: 18 in total, 3 sessions per week for 6 weeks Length of sessions: 10 minutes |
|
| Outcomes | Gait velocity (km/h); stride length (affected side) (cm); stride length (unaffected side) (cm); stride length asymmetry ratio; single‐support‐time asymmetry; ratio; affected side single support time; non‐affected side single support time m/s. Pre‐ and post‐scores were used | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Contradictory reporting of randomisation procedures. Quote: "At the time of enrolment, the subjects were randomly assigned to the experimental or control groups by a computerized random‐number generator supervised by an independent researcher" (p776) Quote: "The limitations of this study were the lack of randomization" (p777) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the subjects were randomly assigned to the experimental or control groups by a computerized random‐number generator supervised by an independent researcher" (p776) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of the outcome assessors for the objective outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported. Attempts to contact authors were unsuccessful |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Lichun 2011.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with stroke Diagnosis: 15 thrombosis (50%), 15 haemorrhage (50%) Time since onset: mean 8.13 months (SD 2.16) N randomised to treatment group: 15 N randomised to control group: 15 N analysed in treatment group: 15 N analysed in control group: 15 Mean age: 67.4 years (range 40 to 80) Sex: 21 females (70%), 9 males (30%) Ethnicity: not reported Setting: nursing home Country: China |
|
| Interventions | 2 study groups: 1. Music intervention group: RAS with conventional gait training 2. Control group: conventional gait training Number of sessions: 10 in total with 2 sessions per week over 5 weeks Length of sessions: 30 minutes |
|
| Outcomes | Stride length (affected side ‐ cm), affected and unaffected stride difference (cm), stride frequency (steps per minute), max walking speed (m/min). Post scores used | |
| Notes | This study used rhythm delivered by a metronome in combination with live music | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots (personal correspondence with principal investigator) |
| Allocation concealment (selection bias) | Low risk | Drawing of lots (personal correspondence with principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Blinding of the outcome assessors for the objective outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support reported |
Mueller 2013.
| Methods | RCT 3‐arm parallel‐group design |
|
| Participants | Participants with CVA (N = 1; 6.67%) and traumatic brain injury (N = 14; 93.33%) Time since onset: mean 21.56 years (SD 21.93) N randomised to experimental group: 5 N randomised to placebo singing group: 5 N randomised to control group: 4 N analysed in experimental group: 5 N analysed in placebo singing group: 5 N analysed in control group: 4 Mean age: 43.93 years (SD 10.41) Sex: 5 females (36%), 9 males (64%) Ethnicity: not reported Setting: rehabilitation Country: USA |
|
| Interventions | 3 study groups: 1. Music intervention group (used in this review): endogenous shifting training within the context of neurologic music therapy tasks led by a board‐certified music therapist 2. Placebo singing group (not used in this review): group sing‐a‐long sessions, led by the same music therapist 3. Control group: standard care Number of sessions: 5 in total once per day over 5 days Length of sessions: 60 minutes |
|
| Outcomes | Mental flexibility (Trail Making Test parts A and B); executive functioning (Dysexecutive Questionnaire (DEX) of the Behavioural Assessment of the Dysexecutive Syndrome and the Paced Auditory Serial Addition Test) Pre and post scores used |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated number list with stratified random sampling. Quote: "Random assignment was accomplished by assigning numbers to each participant using the online programme RANDOM.org. The numbers were then randomly sorted into three groups using the online randomisation programme, Research Randomizer" (p32) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were provided with information in the consent form that could influence subjective outcomes. Quote: "We hope to show that music therapy makes a positive difference. We hope this research will help insurance companies decide to pay for future music therapy services" (p76) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding of outcome assessment was adequate for the outcomes recommended for inclusion in this review (from the Trail Making Test Part B). Quote: "The psychometrist ... who remained blind to group membership, performed data collection on the Trail Making Test parts A & B scores (time and errors), and scores on the Paced Auditory Serial Addition Test (3 second and 2 second delivery rate). The researcher (neurologic music therapist) collected the data for the AMMA and also distributed and collected the DEX questionnaires" (pp39‐40). Outcomes from the Trail Making Test Part A, the Paced Auditory Serial Addition Test, and the Dysexecutive Questionnaire of the Behavioural Assessment of the Dysexecutive Syndrome were not used in this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 6.67%. Quote: "One participant dropped out due to scheduling conflicts" (p33 and p41) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support was reported |
O'Kelly 2014.
| Methods | RCT Cross‐over trial Quote: "A multiple baseline within subjects protocol was chosen to provide data on a range of contrasting music therapy, and non‐music therapy auditory stimuli." (p38) |
|
| Participants | Participants with disorders of consciousness, grouped into 2 cohorts: 1. Minimally consciousness state (N = 9; 43%) 2. Vegetative state (N = 12; 57%) Healthy normal participants were also included in another cohort not included in this review Cause of brain injury: hypoxic (N = 8; 38%); traumatic brain injury (N = 11; 52%); intracerebral haemorrhage (N = 2; 10%) Time since onset: mean 7.3 months (SD 2.8) N randomised: 21 N analysed: 21 Mean age: 45 years (SD 17.5) Sex: 10 females (48%), 11 males (52%) Ethnicity: not reported Setting: inpatient rehabilitation Country: UK |
|
| Interventions | All participants were studied under 5 conditions on 1 occasion. Treatment order was randomised. 5 minutes of baseline silence was followed by the presentation of 4 contrasting conditions, each condition administered for 3 minutes with a 2‐minute period of silence between each. The 5 conditions were as follows. 1. Baseline (silence) 2. Liked music: live performance by a music therapist of a participant‐preferred song 3. Entrained improvisation: live performance of an improvised vocal melody singing "Hello" and the participant's name, entrained to the participant's respiration 4. Disliked music: recordings of music disliked by the participant 5. White noise Number of sessions: 1 Length of session: 22 minutes |
|
| Outcomes | Behavioural outcomes were rated from video recordings in 10‐second segments: eye blinks per minute, eyes closed with body movements present, eyes closed with no body movements, eyes open with body movements present (not used in this review) Physiological outcomes: respiration rate per minute, respiration amplitude variance, respiration variance, heart rate, heart rate variability (not used in this review) Neurophysiological outcomes: electroencephalogram data across delta, theta, alpha, and beta bandwidths (not used in this review) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation through drawing of lots |
| Allocation concealment (selection bias) | Low risk | Quote: "To control for order effects, the order of stimuli was randomised, with order series placed in opaque sealed envelopes with envelopes selected by an independent observer for each participant." (p40). All participants underwent the 5 conditions in random order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Behavioural data using video recordings of patient sessions were analysed by a trained volunteer, who was blinded by removing audio from recordings." (p41) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "The research detailed in this thesis was funded primarily through a three year full time PhD Mobility Fellowship from the Doctoral School of the Humanities within the Department of Psychology and Communication at Aalborg University. Additional funding was provided by the Royal Hospital for Neuro‐disability and the Music Therapy Charity." (piii) |
Park 2010a.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with unilateral poststroke hemiparesis Diagnosis: haemorrhagic stroke (32%), infarction (68%) Time since onset: mean 15.5 months (SD 5) N randomised to experimental condition (fast‐tempo auditory stimulation (FTAS)): 13 N randomised to wait‐list control: 13 N analysed in FTAS: 13 N analysed in control: 12 Mean age: 59.55 years Sex: 16 females (64%), 9 males (36%) Ethnicity: not reported Setting: rehabilitation unit Country: South Korea |
|
| Interventions | 2 study groups: 1. Music intervention group: FTAS 2. Control group: walking training with no specific auditory stimulation Number of sessions: 20 sessions in total, with sessions twice a day 5 days a week over 2 weeks Length of sessions: 30 minutes |
|
| Outcomes | Gait parameters: gait velocity, gait cadence, stride length, Wisconsin Gait Scale: post‐test scores used. | |
| Notes | This study used rhythm delivered by a metronome in combination with recorded music. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation through drawing of lots (correspondence with principal investigator) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment through drawing of sealed envelopes (correspondence with principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving FTAS or the personnel involved in delivering FTAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Blinding of the outcome assessors for the objective outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was eliminated from the data analysis due to a history of irregular participation in repeated trials. Attrition reported at 3.85%. Quote: "During the study, one CG subject was eliminated from data analysis due to a history of irregular participation in repeated trials" (p296) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support was reported |
Paul 1998.
| Methods | Quasi‐RCT 2‐arm parallel‐group design | |
| Participants | Adults with stroke with unilateral cerebral hemiplegia determined to have reached their maximum capacity of physical function and subsequently discharged from occupational and physical therapies. All participants had at least 10 degrees of limitation in active shoulder flexion and elbow extension. Time since onset: mean 93.4 days (SD 49.5) N randomised to experimental group: 10 N randomised to control group: 10 N analysed in experimental group: 10 N analysed in control group: 10 Mean age: 61.75 years (SD 5.1) Sex: 9 females, 11 males Ethnicity: not reported Setting: nursing/rehabilitation facility Country: USA |
|
| Interventions | 2 study groups: 1. Music intervention group: participants engaged in active music improvisation sessions with the music therapist using electronic music devices that allowed for easy sound manipulation. Improvisations emphasised steady rhythmic pulses. 2. Control group: physical exercise session conducted by recreational therapist for the same duration as the music therapy session Number of sessions: 20 sessions in total with 2 sessions per week over 10 weeks Length of sessions: 30 minutes |
|
| Outcomes | Active shoulder flexion (Jamar goniometer); elbow extension (Jamar goniometer). Post‐test scores were used | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate group allocation |
| Allocation concealment (selection bias) | High risk | No allocation concealment used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind the participants or professionals delivering this intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | 2 occupational therapists who did the goniometric measurements were blinded. Quote: "The therapists were blind to the conditions of each participant” (p229) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "This project was funded by a research grant from the Institute for Music and Neurologic Function, New York, New York" (p236) |
Pool 2012.
| Methods | RCT Cross‐over trial with 2 groups |
|
| Participants | TBI participants in subacute rehabilitation Diagnosis: haemorrhage (N = 5) 50%, stroke (N = 2) 20%, traumatic brain injury (N = 3) 30% Time since onset: mean 11.55 years (138.6 months) N randomised to experimental condition: 5 N randomised to control condition: 5 N analysed in experimental group: 3 N analysed in control group: 5 Mean age: 53.8 years Sex: 6 females (60%), 4 males (40%) Ethnicity: not reported Setting: community day centres Country: UK |
|
| Interventions | 2 study groups: 1. Music intervention group: 8 sessions of music therapy followed by another 8 sessions of music therapy followed by 8 weeks of standard care/follow‐up 2. Control group: 8 weeks of standard care followed by 8 sessions of music therapy followed by another 8 sessions of music therapy followed by 8 weeks of follow‐up Music therapy intervention was musical attention‐training exercises and songwriting In this review we only used the first phase of this study (8 sessions), before the cross‐over Number of sessions: 8 sessions on a weekly basis Length of sessions: 60 minutes |
|
| Outcomes | Cognitive function: Test of Everyday Attention, Immediate Recall subtest from the Rivermead Behavioural Memory Test‐Third Edition Mood: POMS‐Bipolar version, satisfaction of emotional needs (developed for this study) Change scores were used |
|
| Notes | For mood outcomes, this study used the following POMS‐Bipolar form subscales: agreeable‐hostile, composed‐anxious, energetic‐tired, and elated‐depressed only. As total scores were not available, we could not include these outcomes in our meta‐analyses 1 review author (JB) computed change scores |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation through flipping of coin |
| Allocation concealment (selection bias) | Low risk | Allocation concealment through flipping of coin |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self report measures were used for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessors for the objective outcomes were blinded. Quote: "The test administrators were not informed about which time‐point each participant was at in the treatment schedule. Therefore, the administrators were blinded to the treatment conditions for each participant" (p117) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition reported as 2 (20%). Reasons for attrition not given. Quote: "Two subjects dropped out from the total number of ten subjects recruited" (p337) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support was reported |
Schneider 2007.
| Methods | Quasi‐RCT 2‐arm parallel‐group design |
|
| Participants | People with stroke with moderate impairment of upper limb motor function. 20 (50%) with left extremity affected (10 in each group) and 20 (50%) with right extremity affected (10 in each group) Diagnosis: 34 (85%) ischaemic stroke, 6 (15%) haemorrhagic stroke Time since onset: mean 2 months N randomised to experimental group: 20 N randomised to control group: 20 N analysed in experimental group: 20 N analysed in control group: 20 Mean age: 56.3 years Sex: 13 females (33%), 27 males (67%) Ethnicity: all native German speakers Setting: inpatient Country: Germany |
|
| Interventions | 2 study groups: 1. Music intervention group: Music‐supported training (MST). This involved playing either a MIDI keyboard (fine motor skills) or an electronic drum set consisting of 8 pads (gross motor skills), or both. The music exercises were adaptable to the needs of the participants and systematically increased in difficulty according to 10 set levels. All exercises were demonstrated by the instructor first and then repeated by the participant 2. Control group: Conventional therapy Number of sessions (experimental group only): 15 in total over 3 weeks Length of sessions: 30 minutes |
|
| Outcomes | Upper extremity motor functions (Action Research Arm Test; Arm Paresis Score; Box and Block Test; Nine‐Hole Pegboard Test). Analysis of quality and velocity of finger‐tapping and hand‐tapping movements assessed using a computerised movement analysis system (frequency of full cycles per second; number of inversions of velocity profiles per movement segment; average maximum angular velocity) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were assigned pseudo‐randomly" (p1340). We determined through correspondence with author that participants were assigned to groups in blocks using alternate assignment (20 to MST, followed by 20 to control, followed by 12 to MST, followed by 10 to control) |
| Allocation concealment (selection bias) | High risk | Quote: "Patients were assigned pseudo‐randomly by the occupational therapists not involved in the study to two groups" (p1340). However, we determined that there was a high risk of selection bias due to serial block allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcomes were not used in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no drop outs" (p1340) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "Supported by grants from the DFG (AL 269/7‐1) and the BMBF" (p1345) |
Suh 2014.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with hemiplegic stroke Diagnosis: 5 (31.25%) haemorrhagic stroke, 11 (68.75%) ischaemic stroke Time since onset: mean 305.32 days N randomised to experimental group: 8 N randomised to control group: 8 N analysed in experimental group: 8 N analysed in control group: 8 Mean age: 65.82 years Sex: 10 females (62.5%), 6 males (37.5%) Ethnicity: not reported Setting: rehabilitation unit Country: South Korea |
|
| Interventions | 2 study groups: 1. Music intervention group: neurodevelopmental therapy (NDT) gait training with RAS 2. Control group: NDT gait training without RAS Number of sessions: 15 in total, once per day for 3 weeks Length of sessions: 15 minutes |
|
| Outcomes | Gait parameters: gait velocity (m/minute), gait cadence (steps per minute), stride length (m), standing balance (overall stability index). Change scores used | |
| Notes | The RAS employed in this study did not use accompanying music. Quote: "The rhythm stimulation was composed of single tone series in 4/4 time signature" (p195) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number list. Quote: "Patients were randomly assigned to either experimental (N = 8) or control (N = 8) group by a computerized random number generator" (p194) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment reported. Quote: "Random numbers for the allocation‐to‐treatment sequence were concealed from the recruiter and the therapists. Patients were informed of the two possible treatment allocations, but not whether they are in the experimental or control arm." (p194) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blind to treatment allocations. Quote: "Random numbers for the allocation‐to‐treatment sequence were concealed from the recruiter and the therapists. Patients were informed of the two possible treatment allocations, but not whether they are in the experimental or control arm" (p194). It is not possible to blind the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcomes were not used in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "The work was supported by the Ewha Global Top 5 Grant 2012 of Ewha Womans University." (p198) |
Särkämö 2008.
| Methods | RCT 3‐arm parallel‐group design |
|
| Participants | Participants with ischaemic stroke Time since onset: mean 6.2 days N randomised to music listening: 20 N randomised to audio book listening: 20 N randomised to standard care control: 20 N analysed in music listening: 19 N analysed in audio book listening: 19 N analysed in standard care control: 17 Mean age: 58.87 years Sex: 16 females (44%), 20 males (56%) Ethnicity: not reported Setting: neurology unit Country: Finland |
|
| Interventions | 3 study groups: 1. Music intervention group: Music therapists provided participants with portable CD players and CDs of their own favourite music in any musical genre. 2. Language intervention group (not used in this review): Participants were provided with portable cassette players and narrated audio books on cassettes selected by the participants from a collection of the Finnish Celia library for the visually impaired (celia.fi) 3. Control group: No listening material. Number of sessions (experimental group): daily for 2 months Length of sessions: minimum of 60 minutes per day |
|
| Outcomes | Communication function repetition and reading (subtests of the Finnish version of the Boston Diagnostic Aphasia Examination); verbal fluency and naming subtests (Consortium to Establish a Registry for Alzheimer’s Disease battery and a shortened version of the Token Test). Cognitive function (story recall subtest from the Rivermead Behavioural Memory Test, digit span subtest from the Wechsler Memory Scale‐Revised), and a memory interference task (Frontal Assessment Battery). Attention (CogniSpeed reaction time software). Mood (POMS). Change scores used | |
| Notes | The POMS used in this study was "the shortened Finnish version (Hänninen 1989) of the Profile of Mood States (POMS; McNair et al 1981). It contains 38 items that form following eight subscales: tension, depression, irritability, vigour, fatigue, inertia, confusion and forgetfulness." (p868). Scores for the subscales were available from published data, and total scores were made available by the principal investigator in unpublished data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer‐generated number list. Quote: "Randomization was performed with a random number generator" (p867) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed with a random number generator by a researcher not involved in the patient enrollment" (p867)Quote: "The researchers involved in these studies (authors TS and MM) were blinded to the group allocation of the patients" (p868) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self report measures were used for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Clinical neuropsychological assessment was performed on all patients at the baseline (1 week from stroke onset), and repeated again 3 months and 6 months post‐stroke. The researchers involved in these studies (authors TS and MM) were blinded to the group allocation of the patients" (p868) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported with reasons for withdrawal. Quote: "Of the 60 subjects originally recruited in to the study, 55 completed the study up to the 3‐month follow‐up (music group N = 19, language group N = 19 and control group N = 17). Of the five drop‐outs, one was due to false diagnosis (transient Ischaemic attack), one due to a new stroke, one due to dementia and two due to refusal. One further subject died from myocardial infarction before the 6‐month follow‐up (music group N = 18, language group N = 19, and control group N = 17 at the 6‐month stage)" (p867) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "This work was supported by Academy of Finland (project no 77322), Jenny and Antti Wihuri Foundation (Helsinki, Finland), National Graduate School of Psychology and Neurology Foundation (Helsinki, Finland). Funding to pay the Open Access publication charges for this article was provided by Cognitive Brain Research Unit, Department of Psychology, University of Helsinki, Finland." (p874) |
Thaut 1997.
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Participants with hemiparesis following stroke
Time since onset: mean 16.1 days (SD 4) for experimental group, 15.7 days (SD 4) for control group N randomised to experimental group: 10 N randomised to control group: 10 N analysed in experimental group: 10 N analysed in control group: 10 Mean age: 73 years (SD 7) experimental group, 72 years (SD 8) control group Sex: 10 (50%) female, 10 (50%) male Ethnicity: not reported Setting: inpatient Country: USA |
|
| Interventions | 2 study groups: 1. Music intervention group: RAS 2. Control group: standard neurodevelopmental treatment/Bobath Number of sessions: 60 sessions in total, twice daily for 6 weeks Length of sessions: 30 minutes |
|
| Outcomes | Gait parameters: velocity, stride length, cadence, symmetry: pre‐test and post‐test values EMG variability: change score | |
| Notes | The RAS employed in this study used metronome beat in combination with recorded music. Quote: "The rhythmic stimulus in the training sessions consisted of music tapes played over headsets that were prerecorded on a synthesizer/sequencer module. Instrumental music in 4 different styles was prepared (classic, folk, country, jazz). The music was recorded in 2/4 meter to match the rhythm of the step patterns in gait. A metronome beat was overlaid on the strong beat of the music to enhance the rhythmic perception for the patient." (p209) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers (personal communication with principal investigator) |
| Allocation concealment (selection bias) | Low risk | Recruiters did not know group conditions (personal communication with principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcomes were not used in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Participants were assessed by "a physical therapist blind to the experiment" (p208) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant loss (personal communication with principal investigator) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "This research was funded in part by a grant from the Poudre Valley Hospital Foundation and grants RR 07127‐20 and RR 07127‐23 from the National Institutes of Health (NIH)" (p211) |
Thaut 2002.
| Methods | RCT Cross‐over trial with 2 groups | |
| Participants | Participants with left hemispheric stroke Time since onset: mean 11.4 months (SD 5.2) Diagnosis: 19 (90%) ischaemic stroke (15 in the middle cerebral artery distribution and 4 in the anterior cerebral artery distribution); 2 (10%) intracerebral haemorrhage related to a cerebral aneurysm N randomised: 21 N analysed: 21 Mean age: 52.7 years (SD 13.7) Sex: 8 (38%) female, 13 (62%) male Setting: outpatient services Country: USA |
|
| Interventions | 2 study groups: 1. Music intervention group: RAS 2. Control group: non‐cued repetitive training Number of sessions: 2 in total: 1 session with RAS and 1 session without external time cueing Length of sessions: 30 minutes each |
|
| Outcomes | Arm timing, variability of movement timing, wrist trajectories, wrist trajectory variability, elbow range of motion. Pre‐test and post‐test scores used | |
| Notes | The RAS employed in this study did not use accompanying music. Quote: "The auditory rhythm consisted of a metronome‐like 1000 Hz square wave tone with a 50 ms plateau time produced by a computerized MIDI‐sequencing sound software" (p1075) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers (personal communication with principal investigator) |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes (personal communication with principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or the personnel involved in delivering RAS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcomes were not used in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant withdrawals were not reported |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "This research was supported in part by a grant from the Deutsche Forschungsgesellschaft, Sonderforschungsbereich 194 to Thaut and Hoemberg (DFG: German Research Council, Special Research Section 194)" (p1079) |
Thaut 2007.
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Participants with subacute hemiparetic stroke Diagnosis: 65 (83%) middle cerebral artery stroke; 8 (11%) internal capsule stroke; 4 (5%) basal ganglia/thalamus stroke; 1 (1%) subdural haematoma Time since onset: approximately 21 days N randomised to experimental group: 43 N randomised to control group: 35 N analysed in experimental group: 43 N analysed in control group: 35 Mean age: 69.2 years (SD 11.5) experimental group; 69.7 years (SD 11.2) control group Sex: 37 (47%) female, 41 (53%) male Ethnicity: not reported Setting: 2 research centres Country: USA and Germany |
|
| Interventions | 2 study groups: 1. Music intervention group: RAS 2. Control group: standard neurodevelopmental therapy/Bobath Number of sessions: 15 sessions in total, once daily for 5 days over 3 weeks Length of sessions: 30 minutes |
|
| Outcomes | Gait parameters: velocity, stride length, cadence, symmetry: post‐test scores were used Participant satisfaction with treatment: F statistic and P values used | |
| Notes | The RAS employed in this study used metronome beat in combination with recorded music. Quote: "RAS training followed established protocols using a metronome and specifically prepared music tapes in digital MIDI format to ensure temporal precision and tempo stability as well as full capacity for frequency modulation of the stimulus based on patient needs" (p456) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind participants receiving RAS or the personnel involved in delivering RAS. Quote: "Therapists were not blinded to the treatment conditions of the study. However, because both conditions are considered full treatment conditions, no performance bias was expected." (p456) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Subjective outcomes included participant satisfaction, however the measures used and the methods of data collection were not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “Both groups were assessed by blinded physical therapists” (p456) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23% dropouts in German centre, 10% in US centre (absolute numbers are not reported) Reasons: hospital transfer, early discharge, medical complications, unspecified personal reasons |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | No funding support was reported |
Tong 2015.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with light to moderate motor impairment in the upper extremity following stroke Diagnosis: 15 (50%) haemorrhagic stroke, 15 (50%), ischaemic stroke Time since onset: mean 5.35 months N randomised to experimental group: 15 N randomised to control group: 15 N analysed in experimental group: 15 N analysed in control group: 15 Mean age: 49.35 years Sex: 4 females (62.5%), 26 males (37.5%) Ethnicity: Chinese Setting: rehabilitation unit Country: China |
|
| Interventions | 2 study groups: Music‐supported therapy involving 2 conditions: 1. Music intervention group: audible music group involving the playing of musical instruments that were audible/not muted 2. Control group: mute music group involving the playing of musical instruments that resembled the audible musical instruments used in the music intervention group but that were made of sponge Number of sessions: 20 in total over 4 weeks Length of sessions: 30 minutes |
|
| Outcomes | Upper extremity function (Wolf Motor Function Test, FMA): change scores used | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using random number table. Quote: "Randomisation was performed by assigning random numbers from random number tables" (p2) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective outcomes were not used in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of outcome assessors for the objective outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was reported as 9%. Quote: "Three patients in the CG dropped out because of training boredom" (p4) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | The authors declare no conflict of interest. Quote: "This work was partially supported by China Rehabilitation Research Center (CRRC) fund (no. 2008‐19)." (p6) |
Van Delden 2013.
| Methods | RCT 3‐arm parallel‐group design |
|
| Participants | Participants with stroke with light to moderate motor impairment in the upper extremity Diagnosis: stroke Time since onset: mean 9.37 weeks N randomised to modified bilateral arm training with rhythmic auditory cueing (mBATRAC) group: 19 N randomised to DMCT control group: 19 N randomised to modified constraint‐induced movement therapy (mCIMT) control group: 22 N analysed in mBATRAC group: 18 N analysed in DMCT control group: 16 N analysed in mCIMT control group: 21 Mean age: 59.75 years Sex: not reported Ethnicity: not reported Setting: rehabilitation unit Country: Netherlands |
|
| Interventions | 3 study groups: 1. Music intervention group: mBATRAC, which involved a modification of the original bilateral arm training with rhythmic auditory cueing protocol that targeted rhythmic flexion and extension movements about the wrist rather than movements of proximal parts of the upper limb 2. Control group: Conventional treatment (DMCT) was an exercise therapy based on existing guidelines for upper limb rehabilitation after stroke, discarding specific elements of the 2 experimental conditions 3. 2nd intervention group (not used in this review): mCIMT, which involved repetitive task practices and shaping of the desired movements, with an emphasis on increased control of wrist and finger extensors Number of sessions: 18 sessions in total with 3 sessions per week over 6 weeks Length of sessions: 60 minutes |
|
| Outcomes | Upper extremity function (Action Research Arm Test, Motricity Index, Nine‐Hole Peg Test, Fugl‐Meyer Motor Assessment, Erasmus modifications of the Nottingham Sensory Assessment) Communication function, cognitive function, mood (all using the Stroke Impact Scale) Change scores used |
|
| Notes | RAC in this study followed the protocol for mBATRAC, which was not defined in this article. However, the BATRAC protocol has been defined elsewhere as moving "in time to a metronome" (McCombe Waller 2005, p546) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized in permuted blocks and allocated to 1 of the 3 intervention groups" (p2164) |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed allocation was effectuated online using the minimization method" (p2164) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Althoughsubjective outcomes were examined in this study, these outcomes were not included in this systematic review, as they had not been specified as outcomes of interest at the outset of the study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | The study is reported as a single‐blind trial, so presumably the data collector was blind. However, blinding is not described and is therefore unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported as 15.8%. 19 enrolled in mBATRAC; 19 enrolled in DMCT; follow‐up 17 in mBATRAC and 15 in DMCT groups. Descriptions of withdrawals: 1 refused after allocation; 3 moved away; 2 did not appear for follow‐up |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "This study was funded by the Dutch Scientific College of Physiotherapy of the Royal Dutch Society for Physical Therapy." (p2615) |
van der Meulen 2014.
| Methods | RCT with a wait‐list control group | |
| Participants | Participants with stroke with aphasia Diagnosis: 1 (7%) haemorrhagic stroke, 14 (86%) ischaemic stroke, 1 (7%) stroke type unknown Time since onset: mean 10.6 months N randomised to melodic intonation therapy (MIT): 16 N randomised to wait‐list control: 11 N analysed in MIT: 11 N analysed in wait‐list control: 11 Mean age: 52.55 years Sex: 16 females (60%), 11 males (40%) Ethnicity: not reported Setting: hospitals, rehabilitation centres, and nursing homes Country: Netherlands |
|
| Interventions | 2 study groups: 1. Music intervention group: intensive melodic intonation therapy (MIT) for the first 6‐week period (between T1 and T2), and then received "regular therapy" for the second 6‐week period (between T2 and T3) 2. Control group: received "intensive control treatment" between T1 and T2, and then received delayed MIT between T2 and T3 Number of sessions: unclear. 5 hours a week over 6 weeks Length of sessions: unclear. 3 hours minimum face‐to‐face intervention and 2 hours of "homework" using recorded videos |
|
| Outcomes | Communication function (Aachen Aphasia Test, Amsterdam‐Nijmegen Everyday Language Test, Semantic Association Task, Sabadell story retelling task (connected speech), MIT repetition task). Change scores used | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number list. Quote: "A computer‐generated random allocation sequence was used” (p537) |
| Allocation concealment (selection bias) | Low risk | Used opaque, sealed envelopes. Quote: "a computer‐generated random allocation sequence was used and the results placed in consecutively numbered sealed envelopes” (pp537‐8) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention. Quote: "participants and speech‐language therapists (SLTs) could not be blinded for treatment condition" (p538) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Blinding of the outcome assessors for the objective outcomes was not achieved in all cases. Quote: "The researchers administering and scoring the assessments at each test moment were blinded for group allocation. In a few cases, blinding could not be maintained because the patients spontaneously informed the researcher about their therapy allocation" (p538) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exact attrition rate is unclear as there is a lack of congruence between the text and the CONSORT diagram. Text suggests that there was a 14.8% attrition rate, due to early discharge and refusal to participate. Quote: "A total number of 27 patients were included in the study: 16 were allocated to the experimental group and 11 to the control group. Four patients withdrew from MIT after 1 or 2 weeks, because they felt uncomfortable with the therapy or were disappointed by their progress." (p539) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | Low risk | Quote: "The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Stichting Rotterdams Kinderrevalidatie Fonds Adriaanstichting (Grant No. 2007/0168 JKF/07.08.31KFA)" (p543) |
Whitall 2011.
| Methods | RCT 2‐arm parallel‐group design |
|
| Participants | Participants with unilateral stroke Diagnosis: Locations of strokes reported as follows. Brainstem: 6 (6%) Cerebellar: 2 (2%) Cortex: 39 (42%) Multiple: 3 (3%) Subcortical: 19 (20%) Unknown/missing: 24 (26%) Time since onset: > 6 months N randomised to BATRAC group: 55 N randomised to control (dose‐matched therapeutic exercises (DMTE)): 56 N analysed in BATRAC group: 42 N analysed in control group: 50 Mean age: 59.8 years Sex: 42 females (46%), 50 males (54%) Ethnicity: not reported Setting: outpatient Country: USA |
|
| Interventions | 2 study groups: 1. Music intervention group: BATRAC 2. Control group: dose‐matched therapeutic exercises (DMTE) consisting of 4 exercises based on neurodevelopmental principles including thoracic spine mobilisation with weight shifting, scapular mobilisation, weight bearing with the paretic arm (elbow fixed), and opening the hand with finger extension Number of sessions: 18 in total with 3 sessions per week over 6 weeks Length of sessions: 1 hour, which included 20 minutes active participation and 4 minutes rest |
|
| Outcomes | Motor impairment (Fugl‐Meyer Assessment of the Upper Extremity, Wolf Motor Function Test (time), Stroke Impact Scale, isokinetic strength of elbow flexion/extension arm movements) | |
| Notes | Total N of participants adds up to 83, not 92 as reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized after B2 to receive either BATRAC or DMTE using a stratified block allocation scheme based on initial function (NIH Stroke Scale with 2 as cutoff) and motor dominance of stroke." (pp121‐2) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind the participants or professionals delivering the intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self report measures were used for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Testing was conducted in a separate location from the training site by trained testers blinded to group assignment." (p122) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition at post‐training analysis (6‐week time point): 17%. 55 allocated to BATRAC; 56 allocated to DMTE. N analysed in BATRAC = 42; N analysed in DMTE = 50. Descriptions of withdrawals: 12 for medical reasons (BATRAC, N = 8; DMTE, N = 4); 7 for personal reasons (BATRAC, N = 5; DMTE, N = 2) |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest | High risk | Quote: "The author(s) declared a potential conflict of interest (e.g. a financial relationship with the commercial organizations or products discussed in this article) as follows: As inventors of the subject technology, Jill Whitall and Sandy McCombe Waller anticipate receiving licensing income from their institution (UMB), under its Intellectual Property Policy." (p127) |
BATRAC: bilateral arm training with rhythmic auditory cueing BP: blood pressure COPM: Canadian Occupational Performance Measure CVA: cerebrovascular accident EMG: electromyography FMA: Fugl‐Meyer Assessment POMS: Profile of Mood States PTA: post‐traumatic amnesia RAC: rhythmic auditory cueing RAS: rhythmic auditory stimulation RCT: randomised controlled trial ROM: range of motion SD: standard deviation SIS: Stroke Impact Scale TBI: traumatic brain injury
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Mahasneh 1991 | Insufficient reporting on intervention and design. Attempts to obtain additional data from authors were unsuccessful. |
| Amengual 2013 | Control group used healthy participants, and not RCT. |
| Baker 2004 | Not RCT or CCT |
| Baker 2005 | Not RCT or CCT |
| Barnes 2006 | Not RCT or CCT No control group |
| Beatty 1995 | Control group used healthy participants. |
| Bonakdarpour 2003 | Not RCT or CCT Single‐subject design |
| Bossert 2012 | Insufficient reporting of results: only means are reported, no SDs. Attempts to obtain additional data from authors were unsuccessful. The authors use a standardised measure (12‐Item Short Form Health Survey) for physical and mental health, but all other outcomes (e.g. body awareness, emotional awareness, relational quality) are measured by self developed questionnaires. |
| Breitenfeld 2005 | The published results of this study examine outcomes not included in this review. |
| Carlisle 2000 | Not RCT or CCT |
| Chen 2013 | Not RCT or CCT Within‐subject design |
| Cofrancesco 1985 | Not RCT or CCT |
| Cohen 1992 | Unacceptable treatment allocation method |
| Cohen 1995 | Compared rhythmically cued speech, melodically cued speech, and verbal speech of participants who had been receiving music therapy No standard‐treatment group Insufficient data reporting |
| Conklyn 2010 | Not population of interest (multiple sclerosis) |
| Dellacherie 2011 | Control group used healthy participants. |
| Eslinger 1997 | We could not locate any published results. Attempts to obtain additional data from authors were unsuccessful. |
| Ford 2007 | Not RCT or CCT |
| Gerlichova 2014 | Not RCT or CCT |
| Goh 2001 | Planned to be conducted as RCT, however only 2 participants enrolled |
| Gollaher 1993 | Not RCT or CCT Within‐subject design |
| Grossman 1981 | Not RCT or CCT Within‐subject design |
| Hald 2012 | Standardised outcome measures had been adapted, and adaptations had not been validated. |
| Hayden 2009 | Not RCT or CCT Wait‐list design with no control group |
| Hitchen 2007 | Insufficient data collection (personal communication) |
| Hurt 1998 | Not RCT or CCT |
| Hébert 2003 | Not RCT or CCT Single‐subject design with healthy controls |
| Johannsen 2010 | An intervention using rhythmic auditory stimulation was used as a control condition, therefore control condition did not qualify as a 'no‐music' condition. |
| Jun 2013 | Extremely large standard deviations indicate that the data was not normally distributed. |
| Kasai 2014 | Not RCT or CCT |
| Kim 2008 | Not RCT or CCT Protocol description |
| Kim 2011b | No randomisation or quasi‐randomisation |
| Kim 2012c | Not population of interest (cerebral palsy) |
| Kim 2013 | Not RCT or CCT Within‐subject design using pre and post measures |
| Lee 2012 | Single‐group design with no randomisation |
| Li 2002 | The research question was not relevant to this review. |
| Lin 2007 | Not RCT or CCT |
| Magee 2002 | Comparative study of 2 music therapy interventions |
| Magee 2006a | Not RCT or CCT |
| Malcolm 2009 | Not RCT or CCT |
| Mandel 1990 | Further details are required about the randomisation process. Attempts to obtain additional data from authors were unsuccessful. We could not locate the authors through an internet search for the facility. Given the age of this article, we have excluded it from our review. |
| McCombe Waller 2005 | Not RCT or CCT |
| Moon 2008 | Not RCT or CCT (personal communication with author's project advisor) |
| Nayak 2000 | Not RCT or CCT Participants were assigned to music therapy group individually or in groups of varying sizes, as this was the only way they were available to the researchers, compromising the randomisation procedures (personal communication). |
| Nie 2014 | Cannot access this publication through interlibrary searching |
| Park 2010b | Cross‐over design that examined 2 conditions (preferred music with classical music) and used baseline data as the "control" No control data reported |
| Popovici 1992 | We could not determine whether randomisation had been used in this study. Attempts to obtain additional data from authors were unsuccessful as we were unable to obtain author contact information. |
| Prassas 1997 | Not RCT or CCT |
| Puggina 2011 | Inconsistent reporting of research design, treatment conditions, and dosage We contacted the authors on several occasions but received no response. |
| Purdie 1997 | Not RCT or CCT |
| Richards 2008 | Not RCT or CCT No control group |
| Roerdink 2009 | Control group used healthy participants. |
| Scalha 2010 | Not RCT or CCT No randomisation (personal communication with author) |
| Schauer 1996 | Control group used healthy participants. |
| Schauer 2003 | Inadequate methodological information |
| Schinner 1995 | Outcomes are not of interest to this review. |
| Schneider 2010 | Not RCT or CCT Study was designed as a 2‐group parallel study, and the control group was added to the research at a later stage. |
| Shafshak 2013 | Unable to retrieve publication |
| Sinclair 2013 | Used matched healthy controls |
| Stahl 2011 | Not RCT or CCT |
| Studebaker 2007 | Not RCT or CCT |
| Särkämö 2010a | This study is part of the Särkämö 2008 study, however it only reports on brain imaging outcomes, which are not outcomes of interest to this review. |
| Särkämö 2010b | Not RCT or CCT This study does not examine outcomes of interest to this review (amusia). |
| Thaut 1992 | Control group used healthy participants. |
| Thaut 1993 | Not RCT or CCT |
| Thaut 1997b | Not RCT or CCT |
| Thaut 1999 | Not RCT or CCT |
| Thaut 2009 | Not RCT or CCT No randomisation or quasi‐randomisation Results present within‐group comparisons rather than between‐group comparisons. |
| Thompson 1986 | Not RCT or CCT Single‐subject design with multiple baselines Intervention does not seem to include a musical condition, and so is not an intervention of interest to this review. |
| Tsai 2013a | Not RCT or CCT Within‐subject design |
| Tsai 2013b | Not RCT or CCT Single‐subject design |
| Tseng 2014 | Not RCT or CCT Single‐subject design |
| van Nes 2006 | Not RCT or CCT No control intervention Comparison of 2 interventions: somatosensory stimulation and "exercise therapy on music" |
| Wallace 1985 | Not RCT or CCT |
| Walworth 2008 | Unable to determine methods of randomisation We contacted the authors on several occasions but received no response. |
| Wan 2014 | Not RCT or CCT No randomisation or quasi‐randomisation |
| Whitall 1999 | Not RCT or CCT |
| Whitall 2000 | Not RCT or CCT |
| Zazula 1984 | Unable to retrieve publication |
| Zhao 2010 | Unable to retrieve publication |
CCT: controlled clinical trial RCT: randomised controlled trial SD: standard deviation
Characteristics of studies awaiting assessment [ordered by study ID]
Bayat 2014.
| Methods | RCT 4‐arm parallel‐group design |
| Participants | Participants with stroke with hemiparesis Time since onset: unknown N randomised: 60 Age range: unknown Sex: unknown Ethnicity: unknown Setting: unknown Country: Iran |
| Interventions | 4 study groups: 1. Program‐based computer software use 2. Listening to Mozart Sonata K448 3. Software use plus listening to Mozart Sonata K448 4. Control: no intervention Length of intervention: 6 months Number of sessions: unclear Length of sessions: 1 hour per night |
| Outcomes | Magnetic resonance spectroscopy Fugl‐Meyer Assessment of Physical Performance Mini Mental State Exam |
| Notes |
John 2010.
| Methods | RCT 3‐arm parallel‐group design |
| Participants | Participants with subacute stroke Time since onset: unknown N randomised: 60 Age range: 50 to 70 years, mean unknown Sex: 22 females (37%), 38 males (63%) Ethnicity: unknown Setting: unknown Country: unknown |
| Interventions | 3 study groups: 1: Listening to film and classical songs plus conventional management 2. Meditation plus conventional management 3. Conventional management only (control) Length of intervention: 6 weeks Number of sessions: total unknown Length of sessions: unknown |
| Outcomes | Hamilton Rating Scale for Depression Berg Balance Scale Barthel Activities of Daily Living Index Fatigue Severity Scale |
| Notes |
Oiga 2014.
| Methods | RCT 3‐arm parallel‐group design |
| Participants | Participants with stroke Time since onset: unknown N randomised: 16 Mean age: unknown Sex: unknown Ethnicity: unknown Setting: tertiary inpatient medical centre Country: Philippines |
| Interventions | 3 study groups: 1. Control: white noise background 2. Rhythm: metronome ‐ 100 beats per minute 3. Music: "Pomp and Circumstance" Length of intervention: unknown Number of sessions: unknown Length of sessions: unknown |
| Outcomes | Functional Independence Measure Hand dynamometer |
| Notes |
Poćwierz‐Marciniak 2014.
| Methods | RCT 2‐arm parallel‐group design |
| Participants | Participants with stroke Time since onset: unknown N randomised to intervention: 8 N randomised to control: 11 Mean age: unknown Sex: unknown Ethnicity: unknown Setting: unknown Number of sessions: unknown Length of intervention: unknown Length of sessions: unknown |
| Interventions | Music therapy |
| Outcomes | Health‐related quality of life Anxiety, depression, irritation, and anger Quality of life (anxiety, acceptance of condition, sense of control) |
| Notes |
Renna 2012.
| Methods | RCT 2‐arm parallel‐group design |
| Participants | Adults following stroke Time since onset: first 12 weeks' poststroke N randomised: unknown Mean age: unknown Sex: unknown Ethnicity: unknown Setting: unknown Number of sessions: unknown Length of intervention: unknown Length of sessions: unknown |
| Interventions | 70 hours of preferred music listening over 12 weeks via MP3 players and logged in diaries |
| Outcomes | Not specified, but describes mood and cognition as primary outcomes, and function and quality of life as secondary outcomes |
| Notes | Prospective abstract describing study protocol |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Ala‐Ruona 2010.
| Trial name or title | Examining the effects of active music therapy on post‐stroke recovery: a randomised controlled cross‐over trial |
| Methods | RCT Cross‐over trial Computer‐generated randomisation |
| Participants | 45 participants with stroke |
| Interventions | Experimental music therapy condition: 2 (60‐minute) weekly sessions of active music therapy in individual setting over a period of 3 months The music therapy includes a combination of structured musical exercises with different levels of difficulty, interactive clinical improvisation, rhythmic dynamic playing with changing movement sequences, music‐assisted relaxation, and therapeutic discussion Control condition: standard care according to the Finnish Current Care guidelines for stroke |
| Outcomes | Functional disability and activities of daily living independency (BI), level of impairment (NIHSS), disability grade (mRS), neglect (BIT), and motor function of upper extremity (ARAT) |
| Starting date | |
| Contact information | Contact: Professor Esa Ala‐Ruona, email: esa.ala‐ruona@jyu.fi |
| Notes |
NCT00903266.
| Trial name or title | Melodic‐intonation‐therapy and speech‐repetition‐therapy for patients with non‐fluent aphasia |
| Methods | RCT Parallel assignment |
| Participants | Adults with aphasia following first‐time ischaemic left‐hemispheric stroke or CVA |
| Interventions | Music condition: melodic intonation therapy Active comparator: speech repetition therapy Control: no therapy |
| Outcomes | Primary outcomes: language outcomes (correct information units) Secondary outcomes: language, speech, functional and structural brain changes |
| Starting date | February 2008 |
| Contact information | Contact: Gottfried Schlaug, MD, PhD, email: gschlaug@bidmc.harvard.edu Andrea Norton, email: aphasia_recovery@yahoo.com |
| Notes | This study is currently recruiting participants. Estimated study completion date: December 2016 |
NCT01372059.
| Trial name or title | The effects of a rhythm and music‐based therapy program and therapeutic riding in late recovery phase following stroke |
| Methods | RCT Parallel assignment |
| Participants | Adults aged 50 to 75 years who are 1 to 5 years' poststroke Estimated enrolment: 123 |
| Interventions | Music condition: rhythm and music therapy Active comparator: therapeutic riding Control: receives no intervention |
| Outcomes | Primary: degree of participation (Stroke Impact Scale, version 2) Secondary: self reported fatigue, perceived physical functioning, self rated perceived mental functioning, cognitive function, body function, environmental factors, personal factors |
| Starting date | January 2010 |
| Contact information | Contact: Lina Bunketorp Kall, PhD, email: Lina.Bunketorp‐Kall@neuro.gu.se |
| Notes | The results of this study are being prepared for publication (correspondence with principal investigator). Estimated study completion date: December 2015 |
NCT01455155.
| Trial name or title | Creative therapy to affect stroke outcomes |
| Methods | RCT Parallel assignment |
| Participants | Adults with stroke more than 1 month prior |
| Interventions | Music condition: creative therapy (art and music therapy) Control condition: conventional physical therapy |
| Outcomes | Primary outcome: cognition (Abbreviated Mental Test Score) Secondary outcomes: physical function (BI), mood (Hospital Anxiety and Depression Scale), quality of life (Pictorial Thai Quality of Life) |
| Starting date | November 2011 |
| Contact information | Contact: Vilai Kuptniratsaikul, MD, email: sivkp@mahidol.ac.th |
| Notes | The recruitment status of this study is unknown because the information has not been recently verified. Estimated study completion date: May 2014 |
NCT01721668.
| Trial name or title | Improving arm and hand functions in chronic stroke |
| Methods | RCT Parallel assignment |
| Participants | Adults who sustained first‐time unilateral middle cerebral artery stroke more than 6 months prior. Estimated enrolment: 60 |
| Interventions | Music condition: music‐supported rehabilitation using musical exercises to improve hand and arm motor functioning Control: conventional upper extremity therapy |
| Outcomes | Primary: arm and hand functions: ARAT; Chedoke Arm and Hand Activity Inventory; Stroke Impact Scale Secondary: brain structure and brain function |
| Starting date | November 2012 |
| Contact information | Contact: Deirdre R Dawson, PhD, email: ddawson@research.baycrest.org |
| Notes | This study is ongoing, but not recruiting participants. Estimated study completion date: December 2015 |
NCT01749709.
| Trial name or title | Music listening and stroke recovery |
| Methods | RCT Factorial assignment |
| Participants | Adults with stroke. Estimated enrolment: 60 |
| Interventions | Music condition 1: daily listening to instrumental music Music condition 2: daily listening to vocal music Control condition: standard rehabilitation |
| Outcomes | Primary outcomes: physiological stress indicators, neuropsychological performance, brain MRI |
| Starting date | December 2012 |
| Contact information | Contact: Seppo Soinila, MD, email: seppo.soinila@tyks.fi |
| Notes | The recruitment status of this study is unknown because the information has not been recently verified. Estimated study completion date: December 2014 |
NCT01769326.
| Trial name or title | Influence of timing on motor learning |
| Methods | RCT Parallel assignment |
| Participants | Adults with CVA. Estimated enrolment: 40 |
| Interventions | Music condition: MusicGlove group Active comparator for MusicGlove: conventional hand exercise Experimental: resonating arm exerciser Active comparator for experimental: conventional arm exercise |
| Outcomes | Motor and strength: Box and Block Test; Fugl‐Meyer Assessment |
| Starting date | September 2012 |
| Contact information | Principal investigator: Steven Cramer, MD, University of California, Irvine |
| Notes | This study is ongoing, but not recruiting participants. Estimated study completion date: June 2015 |
NCT01956136.
| Trial name or title | Efficacy and neural basis of music‐based neurological rehabilitation for traumatic brain injury (MUBI) |
| Methods | RCT Cross‐over trial |
| Participants | Adults with traumatic brain injury. Estimated enrolment: 60 |
| Interventions | Music condition: music‐based neurological rehabilitation with standard care Control condition: standard care |
| Outcomes | Primary outcomes: cognition (executive functions; focused and sustained attention; verbal working memory and learning; verbal and non‐verbal reasoning) Secondary outcomes: upper extremity motor function; depression; quality of life; emotional well‐being; structural and functional neuroplasticity |
| Starting date | March 2014 |
| Contact information | Contact: Susanna Melkas, MD, PhD, email: susanna.melkas@hus.fi |
| Notes | This study is currently recruiting participants. Estimated study completion date: December 2017 |
NCT02208219.
| Trial name or title | Music therapy to restore motor deficits after stroke (NEUROMUSIC) |
| Methods | RCT Parallel assignment |
| Participants | Adults aged 30 to 75 with motor deficits following a first stroke |
| Interventions | Music condition 1: music‐supported therapy Music condition 2: home‐based music‐supported therapy Control condition: conventional treatment |
| Outcomes | Primary outcome: performance of movements with the paretic upper extremity (ARAT) Secondary outcomes: motor function; cognitive function; emotional and quality of life change; changes in brain activation |
| Starting date | November 2013 |
| Contact information | Contact: Antoni Rodríguez‐Fornells, PhD, email: antoni.rodriguez@icrea.cat |
| Notes | Currently recruiting participants. Estimated study completion date: April 2016 |
NCT02259062.
| Trial name or title | Listening for leisure after stroke (MELLO) |
| Methods | RCT Parallel assignment |
| Participants | Adults with ischaemic stroke, ≤ 14 days poststroke at time of recruitment. Estimated enrolment: 100 |
| Interventions | Music condition: music listening with brief mindfulness Active comparator: music listening Placebo comparator: audio book intervention |
| Outcomes | Neuropsychological assessment of cognition and mood |
| Starting date | October 2014 |
| Contact information | Contact: Jonathan Evans, PhD, email: jonathan.evans@glasgow.ac.uk Satu Baylan, PhD, email: satu.baylan@glasgow.ac.uk |
| Notes | Currently recruiting participants. Estimated study completion date: September 2016 |
NCT02310438.
| Trial name or title | Music therapy for the rehabilitation of upper limb with stroke patients |
| Methods | RCT Cross‐over trial |
| Participants | Estimated enrolment: 12 |
| Interventions | Experimental music condition: early‐intervention music therapy Active comparator: delayed‐intervention music therapy |
| Outcomes | Primary outcomes: ARAT Secondary outcomes: Nine‐Hole Peg Test |
| Starting date | January 2014 |
| Contact information | Contact: Alexander J Street, email: alex.street@anglia.ac.uk |
| Notes | This study is currently recruiting participants. Estimated study completion date: September 2016 |
NCT02328573.
| Trial name or title | The impact of group singing on patients with stroke and their personal caregivers |
| Methods | RCT Parallel assignment |
| Participants | Adults with stroke. Estimated enrolment: 80 |
| Interventions | Music condition: communal singing Control: no intervention |
| Outcomes | Primary: change in mood and quality of life as indicated through saliva (cortisol and melatonin sampling) Secondary: change in language aphasia |
| Starting date | April 2014 |
| Contact information | Contact: Joanne Loewy, DA, email: jloewy@chpnet.org Marie Grippo, email: mgrippo@chpnet.org |
| Notes | Currently recruiting participants. Estimated study completion date: April 2018 |
NCT02410629.
| Trial name or title | To determine the therapeutic effect of the Music Glove and conventional hand exercises to subacute stroke patients |
| Methods | RCT Cross‐over trial |
| Participants | Adults with CVA. Estimated enrolment: 40 |
| Interventions | Music condition: MusicGlove Active comparator: conventional hand exercise programme |
| Outcomes | Primary: Box and Block Test Secondary: Fugl‐Meyer Assessment of the Upper Extremity; ARAT; Nine‐Hole Peg Test |
| Starting date | March 2015 |
| Contact information | Contact: Vicky Chan, email: vchan2@uci.edu Renee Augburger, email: raugsbur@uci.edu |
| Notes | Currently recruiting participants. Estimated study completion date: June 2016 |
NTR1961.
| Trial name or title | The efficacy of Melodic Intonation Therapy (MIT) in aphasia rehabilitation |
| Methods | RCT |
| Participants | Adults with aphasia after left hemisphere stroke |
| Interventions | Music condition: melodic intonation therapy (MIT) Control condition (postacute group): non‐MIT condition Control condition (chronic group): no treatment |
| Outcomes | Primary outcome: language (Sabadell) Secondary outcomes: language (ANELT; Aachen Aphasia Test; repetition of trained and untrained items) |
| Starting date | October 2009 |
| Contact information | Contact: Dr van der Meulen, email: ivandermeulen@rijndam.nl |
| Notes | See van der Meulen 2014 for results of MIT in the postacute group. This study examined the efficacy of MIT in the chronic phase of stroke. The results of the chronic phase are being prepared for publication (correspondence with principal investigator). |
ANELT: Amsterdam‐Nijmegen Everyday Language Test ARAT: Action Research Arm Test BI: Barthel index BIT: Behavioral Inattention Test CVA: cerebrovascular accident MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial
Differences between protocol and review
We planned to update our search of the Science Citation Index electronic database. However, this database was omitted in the initial search by our search specialist. Although we attempted to correct this omission when we updated our searches in January 2016, a change in search specialist personnel resulted in no specialist who was available to undertake this search at that time. Although Science Citation Index is a major database, we believe that research relating to the topic under investigation (health and music) is most likely to have been published on primarily healthcare databases, for which searches were performed.
Contributions of authors
Wendy Magee (WM), Imogen Clark (IC), Jeanette Tamplin (JT), Joke Bradt (JB)
Co‐ordinating the review: WM
Revision of the background, objectives, criteria for considering studies for this update: WM, IC, JT, JB
Search strategies, methods: JB
Undertaking manual searches: WM, IC, JT, and graduate assistants
Searches: WM
Screening search results: WM and graduate assistant
Retrieval of papers: WM
Screening retrieved papers against inclusion criteria: IC, JT
Appraising the quality of the papers: IC, JT (in cases of disagreement, WM, JB)
Abstracting data from papers: WM, JB
Writing to authors of all trials (published and unpublished) for additional information: WM
Providing and screening additional data on all studies (published and unpublished): WM
Data management for the review: WM
Entering data into Review Manager 5: JB
Review Manager 5 statistical data and all other statistical data: JB
Double entry of data: JB, WM
Interpretation of data: JB, WM
Statistical inferences: JB
Writing the review: WM, IC, JT, JB
Obtaining funding for the review: WM for the update
Person responsible for reading and checking the review before submission: WM
Sources of support
Internal sources
-
Temple University, USA.
Partial support for this update provided by a Boyer College Vice Provost for the Arts Grant
External sources
-
State of Pennsylvania Formula Fund, USA.
Partial support for the original review (Bradt 2010)
Declarations of interest
All four of the review authors (WM, IC, JT, JB) are music therapists. WM was involved in the design, conduct, and publication of two of the studies included in this review (O'Kelly 2014; Pool 2012).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Baker 2001 {published and unpublished data}
- Baker F. The Effects of Live and Taped Music on the Orientation and Agitation Levels of People Experiencing Post‐Traumatic Amnesia (Masters thesis). Melbourne, Australia: University of Melbourne, 1999. [Google Scholar]
- Baker F. The effect of live and taped music on agitation and orientation levels of people experiencing PTA. 5th European Music Therapy Congress; 2001 April 21‐24; Napoli, Italy. 2001.
- Baker F. The effects of live, taped, and no music on people experiencing posttraumatic amnesia. Journal of Music Therapy 2001;38(3):170‐92. [DOI] [PubMed] [Google Scholar]
Cha 2014a {published data only}
- Cha Y, Kim Y, Chung Y. Immediate effects of rhythmic auditory stimulation with tempo changes on gait in stroke patients. Journal of Physical Therapy Science 2014;26:479‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cha 2014b {published data only}
- Cha Y, Kim Y, Hwang S, Chung Y. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: a pilot randomized controlled study. NeuroRehabilitation 2014;35:681‐8. [DOI] [PubMed] [Google Scholar]
Chouan 2012 {published data only}
- Chouan S, Kumar S. Comparing the effects of rhythmic auditory cueing and visual cueing in acute hemiparetic stroke. International Journal of Therapy and Rehabilitation 2012;20:125‐31. [Google Scholar]
Conklyn 2012 {published data only}
- Conklyn D, Novak E, Boissy A, Bethoux F, Chemali K. The effects of modified melodic intonation therapy on nonfluent aphasia: a pilot study. Journal of Speech, Language, and Hearing Research 2012;55:1463‐71. [DOI] [PubMed] [Google Scholar]
Fernandes 2014 {published data only}
- Fernandes Ribeiro AS, Ramos A, Bermejo E, Casero M, Corrales JM, Grantham S. Effects of different musical stimuli in vital signs and facial expressions in patients with cerebral damage: a pilot study. Journal of Neuroscience Nursing 2014;46:117‐24. [DOI] [PubMed] [Google Scholar]
Hill 2011 {published data only}
- Hill V, Dunn L, Dunning K, Page SJ. A pilot study of rhythm and timing training as a supplement to occupational therapy in stroke rehabilitation. Topics in Stroke Rehabilitation 2011;18(6):728‐37. [DOI] [PubMed] [Google Scholar]
Jeong 2007 {published data only (unpublished sought but not used)}
- Jeong S, Kim MT. Effects of a theory‐driven music and movement program for stroke survivors in a community setting. Applied Nursing Research 2007;20:125‐31. [DOI] [PubMed] [Google Scholar]
Jungblut 2004 {published and unpublished data}
- Jungblut M. Music therapy for people with chronic aphasia: a controlled study. Music Therapy and Neurological Rehabilitation. Performing Health. London: Jessica Kingsley Publishers, 2005:189‐211. [Google Scholar]
- Jungblut M, Aldridge D. The music therapy intervention SIPARI with chronic aphasics ‐ research findings [Musik als brücke zur sprache – die musik‐therapeutische behandlungsmethode »SIPARI®« bei langzeitaphasikern]. Neurologie und Rehabilitation 2004;10(2):69‐78. [Google Scholar]
Kim 2005 {published data only}
- Kim SJ, Koh I. The effects of music on pain perception of stroke patients during upper extremity joint exercises. Journal of Music Therapy 2005;42(1):81‐92. [DOI] [PubMed] [Google Scholar]
Kim 2011a {published data only}
- Kim J, Oh D, Kim S, Choi J. Visual and kinesthetic locomotor imagery training integrated with auditory step rhythm for walking performance of patients with chronic stroke. Clinical Rehabilitation 2011;25:134‐45. [DOI] [PubMed] [Google Scholar]
Kim 2012a {published data only}
- Kim JH, Park SG, Lim HJ, Park GC, Kim MH, Lee BH. Effects of the combination of rhythmic auditory stimulation and task‐oriented training on functional recovery of subacute stroke patients. Journal of Physical Therapy Science 2012;24:1307‐13. [Google Scholar]
Kim 2012b {published data only}
- Kim JS, Oh DW. Home‐based auditory stimulation training for gait rehabilitation of chronic stroke patients. Journal of Physical Therapy Science 2012;24:775‐7. [Google Scholar]
Lichun 2011 {unpublished data only}
- Lichun L, Gao T. Effect of music therapy rhythmic auditory stimulation on gait of stroke patients. Programme of the 13th World Congress of Music Therapy; 2011 July 5‐9; Seoul, South Korea. World Federation of Music Therapy, 2011:46.
Mueller 2013 {published data only}
- Mueller C. Training Endogenous Task Shifting Using Neurologic Music Therapy (Masters thesis). Fort Collins, USA: Colorado State University, 2013. [Google Scholar]
O'Kelly 2014 {published and unpublished data}
- O'Kelly J. [The development of evidence based music therapy with disorders of consciousness]. Cultural Diversity in Music Therapy Practice, Research and Education. Collection of Abstracts of the 14th World Congress of Music Therapy; 2014 July 7‐12; Vienna and Krems an der Donau, Austria. Krems/Austria: IMC University of Applied Sciences Krems, 2014:P131.
- O'Kelly J. The Development of Evidence Based Music Therapy With Disorders of Consciousness (PhD thesis). Aalborg, Denmark: Aalborg University, 2014. [Google Scholar]
- O'Kelly J, James L, Palannappan R, Taborin J, Fachner J, Magee W. Neurophysiological and behavioral responses to music therapy in vegetative and minimally conscious states. Frontiers in Human Neuroscience 2013;7(884):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2010a {published data only}
- Park IM, Oh DW, Kim SY, Choi JD. Clinical feasibility of integrating fast‐tempoauditory stimulation with self‐adopted walking training for improving walking function in post‐stroke patients: a randomized, controlled pilot trial. Journal of Physical Therapy Science 2010;22:295‐300. [Google Scholar]
Paul 1998 {published data only}
- Paul S, Ramsey D. The effects of electronic music‐making as a therapeutic activity for improving upper extremity active range of motion. Occupational Therapy International 1998;5(3):223‐37. [Google Scholar]
Pool 2012 {unpublished data only}
- Pool J. Brief Group Music Therapy for Acquired Brain Injury: Cognition and Emotional Needs (PhD thesis). Cambridge, UK: Anglia Ruskin University, 2013. [Google Scholar]
- Pool J. Time‐limited music therapy to address functional gains and emotional needs of people with acquired brain injury. Programme of the 13th World Congress of Music Therapy; 2011 July 5‐9; Seoul, South Korea. World Federation of Music Therapy, 2011:34.
Särkämö 2008 {published data only}
- Särkämö T, Tervaniemi M, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 2008;131:866‐76. [DOI] [PubMed] [Google Scholar]
Schneider 2007 {published data only}
- Altenmüller E, Marco‐Pallares J, Münte TF, Schneider S. Neural reorganization underlies improvement in stroke‐induced motor dysfunction by music‐supported therapy. Annals of the New York Academy of Sciences 2009;1169(1):395‐405. [DOI] [PubMed] [Google Scholar]
- Altenmüller E, Schneider S, Marco‐Pallares J, Münte TF. Learning to play piano supports fine motor rehabilitation after stroke. Neurorehabilitation & Neural Repair 2010;26(6):19. [Google Scholar]
- Schneider S, Münte T, Rodriguez‐Fornells A, Sailer M, Altenmüller E. Music‐supported training is more efficient than functional motor training for recovery of fine motor skills in stroke patients. Music Perception: An Interdisciplinary Journal 2010;27(4):271‐80. [Google Scholar]
- Schneider S, Schönle PW, Altenmüller E, Münte TF. Using musical instruments to improve motor skill recovery following a stroke. Journal of Neurology 2007;254(10):1339‐46. [DOI] [PubMed] [Google Scholar]
Suh 2014 {published data only}
- Suh JH, Han SJ, Jeon SY, Kim HJ, Lee JE, Yoon TS, et al. Effect of rhythmic auditory stimulation on gait and balance in hemiplegic stroke patients. NeuroRehabilitation 2014;34:193‐9. [DOI] [PubMed] [Google Scholar]
Thaut 1997 {published data only}
- McIntosh GC, Thaut MH, Rice RR, Prassas SG. Auditory rhythmic cuing in gait rehabilitation with stroke patients. Canadian Journal of Neurological Sciences 1993;20:168. [Google Scholar]
- Mcintosh GC, Rice RR, Prassas SG, Thaut MH. Rhythmic auditory‐motor entrainment as gait rehabilitation technique with stroke patients. International Congress on Stroke Rehabilitation; 1993 November 21‐24; Berlin. Berlin, Germany: German Society for Neurological Rehabilitation, 1993.
- Thaut MH, McIntosh GC, Rice RR. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. Journal of the Neurological Sciences 1997;151(2):207‐12. [DOI] [PubMed] [Google Scholar]
- Thaut MH, McIntosh GC, Rice RR, Miller RA. Rhythmic‐auditory motor training in gait rehabilitation with stroke patients. Journal of Stroke and Cerebrovascular Disease 1995;5:100‐1. [Google Scholar]
Thaut 2002 {published data only}
- Thaut MH, Hoemberg B, Hurt CP, Kenyon GP. Rhythmic entrainment of paretic arm movements in stroke patients. Annual Meeting of the Society for Neuroscience; 1998 November 7‐12; Los Angeles. 1998.
- Thaut, MH, Kenyon GP, Hurt CP, McIntosh, GC, Hoemberg V. Kinematic optimization of spatiotemporal patterns in paretic arm training with stroke patients. Neuropsychologia 2002;40(7):1073‐81. [DOI] [PubMed] [Google Scholar]
Thaut 2007 {published data only}
- Argstatter H, Hillecke TH, Thaut M, Bolay HV. Music therapy in motor rehabilitation. Evaluation of a musico‐medical gait training program for hemiparetic stroke patients [Musiktherapie in der neurologischen rehabilitation. Evaluation eines musikmedizinischen behandlungskonzepts für die gangrehabilitation von hemiparetischen patienten nach schlaganfall]. Neurologie und Rehabilitation 2007;13(3):159‐65. [Google Scholar]
- Thaut MH, Leins AK, Rice RR, Argstatter H, Kenyon GP, McIntosh GC, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near‐ambulatory patients early poststroke: a single‐blind, randomized trial. Neurorehabilitation and Neural Repair 2007;21(5):455‐9. [DOI] [PubMed] [Google Scholar]
Tong 2015 {published data only}
Van Delden 2013 {published data only}
- Delden AEQ, Peper CE, Nienhuys KN, Zijp NI, Beek PJ, Kwakkel G. Unilateral versus bilateral upper limb training after stroke: the upper limb training after stroke clinical trial. Stroke 2013;44:2613‐6. [DOI] [PubMed] [Google Scholar]
van der Meulen 2014 {published data only}
- Meulen I, Sandt‐Koenderman WME, Heijenbrok‐Kal MH, Visch‐Brink EG, Ribbers GM. The efficacy and timing of melodic intonation therapy in subacute aphasia. Neurorehabilitation and Neural Repair 2014;28(6):536‐44. [DOI] [PubMed] [Google Scholar]
Whitall 2011 {published data only}
- Luft AR, McCombe‐Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA 2004;292:1853‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitall J, McCombe Waller S, Sorkin JD, Forrester LW, Macko RF, Hanley DF, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single‐blinded randomized controlled trial. Neurorehabilitation and Neural Repair 2011;25(2):118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Mahasneh 1991 {published data only}
- Al‐Mahasneh SM. Nursing Interventions to Reduce Unilateral Neglect in Right Hemisphere Stroke Patients (PhD thesis). Ann Arbor, MI: University of Michigan, 1991. [Google Scholar]
Amengual 2013 {published data only}
- Amengual JL, Rojo N, Veciana de las Heras M, Marco‐Pallarés J, Grau‐Sánchez J, Schneider S, et al. Sensorimotor plasticity after music‐supported therapy in chronic stroke patients revealed by transcranial magnetic stimulation. PLoS One 2013;8(4):e61883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2004 {published data only}
- Baker F, Wigram T. The immediate and long‐term effects of singing on the mood states of people with traumatic brain injury. British Journal of Music Therapy 2004;2:55‐64. [Google Scholar]
Baker 2005 {published data only}
- Baker F, Wigram T, Gold C. The effects of a song‐singing programme on the affective speaking intonation of people with traumatic brain injury. Brain Injury 2005;19(7):519‐28. [DOI] [PubMed] [Google Scholar]
Barnes 2006 {published data only}
- Barnes CL, Smith MB, Harriet E, Kunisch A, Little C, Modica J. A pilot study of bilateral arm training with repetitive auditory cueing in subjects with low functioning upper limb hemiparesis as a result of chronic stroke. Journal of Neurologic Physical Therapy 2006;30(4):221. [Google Scholar]
Beatty 1995 {published data only}
- Beatty WF, Scott JG, Moreland VJ, Rankin EJ. Head injury effects on a new measure of remote memory: The Famous Tunes Test. Journal of Head Trauma Rehabilitation 1995;10(3):63‐6. [Google Scholar]
Bonakdarpour 2003 {published data only}
- Bonakdarpour B, Eftekharzadeh A, Ashayeri H. Melodic intonation therapy in Persian aphasic patients. Aphasiology 2003;17(1):75‐95. [Google Scholar]
Bossert 2012 {unpublished data only}
- Bossert S, Marz J, Pöpel A. Treatment for Patients With Psychological Disturbances After Accident in Neurological Rehabilitation. Pfäffikon, Zurich, Switzerland: Zurich University of Arts in co‐operation with derInterkantonalen College of Special Education, Service Training Music Therapy, 2012. [Google Scholar]
- Pöpel A, Bossert S, Marz J. Music therapy in patients in neuro‐rehabilitation: a randomized control trial. Cultural Diversity in Music Therapy Practice, Research and Education. Collection of Abstracts of the 14th World Congress of Music Therapy; 2014 July 7‐12; Vienna and Krems an der Donau, Austria. Krems, Austria: IMC University of Applied Sciences Krems, 2014:366.
Breitenfeld 2005 {published and unpublished data}
- Antić S, Galinović I, Lovrenčić‐Huzjan A, Vuković V, Jurašić MJ, Demarin V. Music as an auditory stimulus in stroke patients. Collegium Antropologicum 2008;32(1):19‐23. [PubMed] [Google Scholar]
- Antić S, Morović S, Kes VB, Zavoreo I, Jurašić MJ, Demarin V. Enhancement of stroke recovery by music. Periodicum Biologorum 2012;114(3):397‐401. [Google Scholar]
- Breitenfeld T, Jergovic K, Vargek Solter V, Demarin V. Music therapy in aphasic stroke patients: a pilot study. European Journal of Neurology 2005;12 Suppl 2:55. [Google Scholar]
- Breitenfeld T, Vargek Solter V, Breitenfeld D, Supanc V, Jergovic K, Demarin V. Is there a benefit for aphasic stroke patients treated with music therapy?. Cerebrovascular Diseases 2005;19 Suppl 2:92‐3. [Google Scholar]
Carlisle 2000 {published data only}
- Carlisle BJ. The Effects of Music‐Assisted Relaxation Therapy on Anxiety in Brain Injury Patients (Masters thesis). East Lansing, MI: Michigan State University, 2000. [Google Scholar]
Chen 2013 {published data only}
- Chen MC, Tsai PL, Huang YT, Lin KD. Pleasant music improves visual attention in patients with unilateral neglect after stroke. Brain Injury 2013;27(1):75‐82. [DOI] [PubMed] [Google Scholar]
Cofrancesco 1985 {published data only}
- Cofrancesco EM. The effect of music therapy on hand grasp strength and functional task performance in stroke patients. Journal of Music Therapy 1985;22(3):129‐45. [Google Scholar]
Cohen 1992 {published and unpublished data}
- Cohen NS. The effect of singing instruction on the speech production of neurologically impaired persons. Journal of Music Therapy 1992;29(2):87‐102. [Google Scholar]
Cohen 1995 {published data only}
- Cohen NS, Ford J. The effect of musical cues on the nonpurposive speech of persons with aphasia. Journal of Music Therapy 1995;32(1):46‐57. [Google Scholar]
Conklyn 2010 {published data only}
- Conklyn D, Stough D, Novak E, Paczak S, Chemali K, Bethoux F. A home‐based walking program using rhythmic auditory stimulation improves gait performance in patients with multiple sclerosis: a pilot study. Neurorehabilitation and Neural Repair 2010;24(9):835‐42. [DOI] [PubMed] [Google Scholar]
Dellacherie 2011 {published data only}
- Dellacherie D, Bigand E, Molin P, Baulac M, Samson S. Multidimensional scaling of emotional responses to music in patients with temporal lobe resection. Cortex 2011;47(9):1107‐15. [DOI] [PubMed] [Google Scholar]
Eslinger 1997 {unpublished data only}
- Eslinger PJ, Stauffer JW, Rohrbacher M, Grattan LM. Music therapy and psychosocial adjustment to brain injury. Stroke Trials Registry 1997. [R21RR09415]
Ford 2007 {published data only}
- Ford M, Wagenaar R, Newell K. The effects of auditory rhythms and instruction on walking patterns in individuals post stroke. Gait and Posture 2007;26:150‐5. [DOI] [PubMed] [Google Scholar]
Gerlichova 2014 {published data only}
- Gerlichova M. The effects of music therapy during neurorehabilitation with persons after brain injury. Cultural Diversity in Music Therapy Practice, Research and Education. Collection of Abstracts of the 14th World Congress of Music Therapy; 2014 July 7‐12; Vienna and Krems an der Donau, Austria. Krems, Austria: IMC University of Applied Sciences Krems, 2014:398.
Goh 2001 {unpublished data only}
- Goh M. The role of music therapy in the rehabilitation of people who have had strokes, specifically focusing on depression. National Research Register, Issue 1 2001.
Gollaher 1993 {published data only}
- Gollaher KK. The Effect of Music on Task Performance in Stroke Patients. UMI Dissertation Services, University Microfilms International, 1993. [Google Scholar]
Grossman 1981 {published data only}
- Grossman M, Shapiro BE, Gardner H. Dissociable musical processing strategies after localized brain damage. Neuropsychologia 1981;19(3):425‐33. [DOI] [PubMed] [Google Scholar]
Hald 2012 {unpublished data only}
- Hald S. Music Therapy, Acquired Brain Injury and Interpersonal Communication Competencies: Randomized Cross‐Over Study on Music Therapy in Neurological Rehabilitation (PhD thesis). Aalborg, Denmark: Aalborg University, 2012. [Google Scholar]
- Hald S. Active music therapy, ABI, and interpersonal communication competencies. Programme of the 13th World Congress of Music Therapy; 2011 July 5‐9; Seoul, South Korea. World Federation of Music Therapy, 2011:194‐5.
Hayden 2009 {published data only}
- Hayden R, Clair AA, Johnson G, Otto D. The effect of rhythmic auditory stimulation (RAS) on physical therapy outcomes for patients in gait training following stroke: a feasibility study. International Journal of Neuroscience 2009;119(12):2183‐95. [DOI] [PubMed] [Google Scholar]
Hébert 2003 {published data only}
- Hébert S, Racette A, Gagnon L, Peretz I. Revisiting the dissociation between singing and speaking in expressive aphasia. Brain 2003;126(8):1838‐50. [DOI] [PubMed] [Google Scholar]
Hitchen 2007 {published and unpublished data}
- Hitchen H, Magee WL. A comparison of the effects of verbal de‐escalation techniques with music based de‐escalation techniques on agitation levels in patients with neuro‐behavioural disorders. National Research Register 2007. [N0204175715]
Hurt 1998 {published data only}
- Hurt CP, Rice RR, McIntosh GC, Thaut MH. Rhythmic auditory stimulation in gait training for patients with traumatic brain injury. Journal of Music Therapy 1998;35:228‐91. [DOI] [PubMed] [Google Scholar]
Johannsen 2010 {published data only}
- Johannsen L, Wing AM, Pelton T, Kitaka K, Zietz D, Brittle N, et al. Seated bilateral leg exercise effects on hemiparetic lower extremity function in chronic stroke. Neurorehabilitation and Neural Repair 2010;24:243‐53. [DOI] [PubMed] [Google Scholar]
Jun 2013 {published data only}
- Jun EM, Roh YH, Kim MJ. The effect of music‐movement therapy on physical and psychological states of stroke patients. Journal of Clinical Nursing 2013;22:22‐31. [DOI] [PubMed] [Google Scholar]
Kasai 2014 {published data only}
- Kasai F, Wada S, Mizuma M. The effects of playing electronic musical instruments during at‐home rehabilitation on hemiplegic upper limb function. Stroke 2014;95(10):e19. [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim M, Tomaino CM. Protocol evaluation for effective music therapy for persons with nonfluent aphasia. Topics in Stroke Rehabilitation 2008;15(6):555‐69. [DOI] [PubMed] [Google Scholar]
Kim 2011b {published data only}
- Kim DS, Park YG, Choi JH, Im SH, Jung KJ, Cha YA, et al. Effects of music therapy on mood in stroke patients. Yonsei Medical Journal 2011;52(6):977‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012c {published data only}
- Kim SJ, Kwak EE, Park ES, Cho SR. Differential effects of rhythmic auditory stimulation and neurodevelopmental treatment/Bobath on gait patterns in adults with cerebral palsy: a randomized controlled trial. Clinical Rehabilitation 2012;26(10):904‐14. [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim SJ, Jo U. Study of accent‐based music speech protocol development for improving voice problems in stroke patients with mixed dysarthria. NeuroRehabilitation 2012;32(1):185‐90. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee SH, Lee KJ, Song CH. Effects of rhythmic auditory stimulation (RAS) on gait ability and symmetry after stroke. Journal of Physical Therapy Science 2012;24(4):311‐4. [Google Scholar]
Li 2002 {published data only}
- Li YM. The effect of feeling music therapy on the rehabilitation of post‐stroke depression. Zhongguo Zuchi Goncheng Yanjiu 2002;6(19):2952. [Google Scholar]
Lin 2007 {published and unpublished data}
- Lin SI. Effect of rhythmic auditory cues on gait of stroke patients. Cerebrovascular Diseases 2007;23 Suppl 2:128. [Stroke Trial Registry Ref 12104] [Google Scholar]
Magee 2002 {published data only}
- Magee WL, Davidson JW. The effect of music therapy on mood states in neurological patients: a pilot study. Journal of Music Therapy 2002;39(1):20‐9. [DOI] [PubMed] [Google Scholar]
Magee 2006a {unpublished data only}
- Magee WL. Music therapy for adults with acquired brain injury. National Research Register 2006.
Malcolm 2009 {published data only}
- Malcolm MP, Massie C, Thaut MH. Rhythmic auditory‐motor entrainment improves hemiparetic arm kinematics during reaching movements: a pilot study. Topics in Stroke Rehabilitation 2009;16(1):69‐79. [DOI] [PubMed] [Google Scholar]
Mandel 1990 {published data only}
- Mandel AR, Nymark JR, Balmer SJ, Grinnell DM, O'Riain MD. Electromyographic versus rhythmic positional biofeedback in computerized gait retraining with stroke patients. Archives of Physical Medicine and Rehabilitation 1990;71:649‐54. [PubMed] [Google Scholar]
McCombe Waller 2005 {published data only}
- McCombe Waller S, Whitall J. Hand dominance and side of stroke affect rehabilitation in chronic stroke. Clinical Rehabilitation 2005;19(5):544‐51. [DOI] [PubMed] [Google Scholar]
Moon 2008 {published and unpublished data}
- Moon SY. The effects of piano‐playing music therapy on motor coordination of stroke patients using midi‐based computer analysis. Neurorehabilitation and Neural Repair 2008;22(5):593. [Google Scholar]
- Moon SY, Grocke DE. Piano playing focused music therapy and MIDI analysis in neurological rehabilitation. Programme of the 12th World Congress of Music Therapy; 2008 July 22‐26; Buenos Aires. Buenos Aires, Argentina: World Federation of Music Therapy, 2008:29.
Nayak 2000 {published and unpublished data}
- Nayak S, Wheeler BL, Shiflett SC, Agostinelli S. Effect of music therapy on mood and social interaction among individuals with acute traumatic brain injury and stroke. Rehabilitation Psychology 2000;45(3):274‐83. [Google Scholar]
- Wheeler BL, Shiflett SC, Nayak S. Effects of number of sessions and group or individual music therapy on the mood and behavior of people who have had strokes or traumatic brain injuries. Nordic Journal of Music Therapy 2003;12(2):139‐51. [Google Scholar]
Nie 2014 {published data only}
- Nie DA, Chen JP, Xing YL, Liu LC. Effect of music rhythm stimulation on the lower limb motor function in ischemic stroke patients with hemiplegia. Chinese Journal of Cerebrovascular Diseases 2014;11:80‐3. [Google Scholar]
Park 2010b {published data only}
- Park S. Effect of Preferred Music on Agitation After Traumatic Brain Injury (PhD thesis). Ann Arbor, MI: University of Michigan, 2010. [DOI] [PubMed] [Google Scholar]
Popovici 1992 {published data only}
- Popovici M, Mihailescu L. Melodic intonation in the rehabilitation of Romanian aphasics with bucco‐lingual apraxia. Revue Roumaine de Neurologie et Psychiatrie 1992;30(2):99‐113. [PubMed] [Google Scholar]
Prassas 1997 {published data only}
- Prassas SG, Thaut MH, McIntosh GC, Rice RR. Effect of auditory rhythmic cuing on gait kinematic parameters in hemiparetic stroke patients. Gait and Posture 1997;6:218‐23. [Google Scholar]
Puggina 2011 {published data only}
- Puggina ACG. Analysis of Vital, Facial and Muscular Responses Front to Music or Message in Coma, Vegetative State or Sedated Patients (PhD thesis). São Paulo, Brazil: Universidade de São Paulo, 2011. [Google Scholar]
- Puggina ACG. Use of music and voice stimulus on patients with disorders of consciousness. Journal of Neuroscience Nursing 2011;43(1):E8‐16. [Google Scholar]
Purdie 1997 {published data only}
- Purdie H, Hamilton S, Baldwin S. Music therapy: facilitating behavioral and psychological change in people with stroke ‐ a pilot study. International Journal of Rehabilitation Research 1997;20(3):325‐7. [PubMed] [Google Scholar]
Richards 2008 {published data only}
- Richards LG, Senesac CR, Davis SB, Woodbury ML, Nadeau SE. Bilateral arm training with rhythmic auditory cueing in chronic stroke: not always efficacious. Neurorehabilitation and Neural Repair 2008;22(2):180‐4. [DOI] [PubMed] [Google Scholar]
Roerdink 2009 {published data only}
- Roerdink M, Lamoth CJ, Kordelaar J, Elich P, Konijnenbelt M, Kwakkel G, et al. Rhythm perturbations in acoustically paced treadmill walking after stroke. Neurorehabilitation and Neural Repair 2009;23(7):668‐78. [DOI] [PubMed] [Google Scholar]
Särkämö 2010a {published data only}
- Särkämö T, Pihko E, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al. Music and speech listening enhance the recovery of early sensory processing after stroke. Journal of Cognitive Neuroscience 2010;22(12):2716‐27. [DOI] [PubMed] [Google Scholar]
Särkämö 2010b {published data only}
- Särkämö T, Tervaniemi M, Soinila S, HeSilvennoinen TA, Laine M, Hietanen M, et al. Auditory and cognitive deficits associated with acquired amusia after stroke: a magnetoencephalography and neuropsychological follow‐up study. PLoS One 2010;5(12):e15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scalha 2010 {published data only}
- Scalha TB, Souaz VMG, Suzuki SS, Oberg TD, Vieira Lima NMF. Effects of the task oriented and auditory cues for chronic stroke patients. Revista Terapia Manual 2010;8(39):441‐7. [Google Scholar]
Schauer 1996 {published data only}
- Schauer M, Steingruber W, Mauritz KH. Effect of music on gait symmetry of stroke patients on a treadmill. Biomedizinische Technik 1996;41(10):291‐6. [PubMed] [Google Scholar]
Schauer 2003 {published data only}
- Schauer M, Mauritz KH. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clinical Rehabilitation 2003;17(7):713‐22. [DOI] [PubMed] [Google Scholar]
Schinner 1995 {published data only}
- Schinner KM, Chisholm AH, Grap MJ, Siva P, Hallinan M, LaVoice‐Hawkins A. Effects of auditory stimuli on intracranial pressure and cerebral perfusion pressure in traumatic brain injury. Journal of Neuroscience Nursing 1995;27(6):348‐54. [DOI] [PubMed] [Google Scholar]
Schneider 2010 {published data only}
- Schneider S, Münte T, Rodriguez‐Fornells A, Sailer M, Altenmüller E. Music‐supported training is more efficient than functional motor training for recovery of fine motor skills in stroke patients. Music Perception 2010;27(4):271‐80. [Google Scholar]
Shafshak 2013 {published data only}
- Shafshak TS. The effect of repetitive bilateral ARM training with rhythmic auditory cueing on motor performance and central motor changes in patients with chronic stroke. 12th Congress of European Forum for Research in Rehabilitation. Journal of Physical Medicine & Rehabilitation Sciences/Fiziksel Tup ve Rehabilitasyon Bilimleri Dergisi 2013;16:8. [Google Scholar]
Sinclair 2013 {published data only}
- Sinclair KL, Ponsford JL, Rajaratnam SMW, Anderson C. Sustained attention following traumatic brain injury: use of the Psychomotor Vigilance Task. Journal of Clinical and Experimental Neuropsychology 2013;35(2):210‐24. [DOI] [PubMed] [Google Scholar]
Stahl 2011 {published data only}
- Stahl B, Kotz SA, Henseler I, Turner R, Geyer S. Rhythm in disguise: why singing may not hold the key to recovery from aphasia. Brain 2011;134:3083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Studebaker 2007 {unpublished data only}
- Studebaker S. The Effect of a Music Therapy Protocol on the Attentional Abilities of Stroke Patients (Masters thesis). Lawrence, KS: University of Kansas, 2007. [Google Scholar]
Thaut 1992 {published data only}
- Thaut MH, McIntosh GC, Prassas S, Rice R. Effects of auditory rhythmic pacing on normal gait and gait in stroke, cerebellar disorder and transverse myelitis. International Symposium on Postural and Gait Research 1992;2:437‐40. [Google Scholar]
Thaut 1993 {published data only}
- Thaut MH, McIntosh CG, Rice R, Prassas S. Effect of rhythmic cuing on temporal stride parameters and EMG patterns in hemiparetic gait of stroke patients. Journal of Neurological Rehabilitation 1993;7:9‐16. [Google Scholar]
Thaut 1997b {published data only}
- Thaut MH, Hurt CP, Mcintosh GC. Rhythmic entrainment of gait patterns in traumatic brain injury rehabilitation. Journal of Neurological Rehabilitation 1997;11:131. [Google Scholar]
Thaut 1999 {published data only}
- Thaut MH, Ueno K, Hurt CP, Hoemberg V. Bilateral limb entrainment and rhythmic synchronization in paretic arm movements of stroke patients. Annual Meeting of the Society for Neuroscience; 1999 October 23‐28; Miami Beach, Florida. 1999.
Thaut 2009 {published data only}
- Thaut MH, Gardiner JC, Holmberg D, Horwitz J, Kent L, Andrews G. Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Annals of the New York Academy of Sciences 2009;1169(1):406‐16. [DOI] [PubMed] [Google Scholar]
Thompson 1986 {published data only}
- Thompson CK, McReynolds LV. Wh interrogative production in agrammatic aphasia: an experimental analysis of auditory‐visual stimulation and direct‐production treatment. Journal of Speech, Language, and Hearing Research 1986; Vol. 29, issue 2:193‐206. [PubMed]
Tsai 2013a {published data only}
- Tsai PL, Chen MC, Huang YT, Lin KC, Chen KL, Hsu YW. Listening to classical music ameliorates unilateral neglect after stroke. American Journal of Occupational Therapy 2013;67:328‐35. [DOI] [PubMed] [Google Scholar]
Tsai 2013b {published data only}
- Tsai PL, Chen MC, Huang YT, Lin KC. Effects of listening to pleasant music on chronic unilateral neglect: a single‐subject study. NeuroRehabilitation 2013;32:33‐42. [DOI] [PubMed] [Google Scholar]
Tseng 2014 {published data only}
- Tseng CE, Lin CP, Tsai PC, Yip BS, Lin CM, Yang FP. Melodic intonation therapy in stroke patients with aphasia: a DTI study. Cerebrovascular Diseases 2014;38 (Suppl 1):40. [Google Scholar]
van Nes 2006 {published data only}
- Nes IJ, Latour H, Schils F, Meijer R, Kuijk A, Geurts AC. Long‐term effects of 6‐week whole‐body vibration on balance recovery and activities of daily living in the postacute phase of stroke: a randomized, controlled trial. Stroke 2006;37(9):2331‐5. [DOI] [PubMed] [Google Scholar]
Wallace 1985 {published data only}
- Wallace GL, Canter GJ. Comprehension of neutral, melodically intoned, and affectively toned sentences by adults with aphasia. Journal of Communication Disorders 1985;18(5):321‐7. [DOI] [PubMed] [Google Scholar]
Walworth 2008 {published data only}
- Walworth D, Rumana CS, Nguyen J, Jarred J. Effects of live music therapy sessions on quality of life indicators, medications administered and hospital length of stay for patients undergoing elective surgical procedures for brain. Journal of Music Therapy 2008;45:349‐59. [DOI] [PubMed] [Google Scholar]
Wan 2014 {published data only}
- Wan CY, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain and Language 2014;136:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitall 1999 {published data only}
- Whitall J, McCombe Waller S, Gordes K, Kelsey C, Montgomery C, Silver K. Locomotor training with and without rhythmic auditory stimulation in patients with chronic stroke. Neurology Report 1999;23(5):190. [Google Scholar]
Whitall 2000 {published data only}
- Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke 2000;31(10):2390‐5. [DOI] [PubMed] [Google Scholar]
Zazula 1984 {published data only}
- Zazula TO. Perception and Production of Intonation in Moderate and Severe Head Injuries (PhD thesis). New York, NY: City University of New York, 1984. [Google Scholar]
Zhao 2010 {published data only}
- Zhao J, Guo D, Guo L, Wu H. The therapy of anxiety after stroke. Cerebrovascular Diseases 2010;30:210. [Google Scholar]
References to studies awaiting assessment
Bayat 2014 {published data only}
- Bayat G, Dastgheib S, Shoeibi A. The impact of neurorehabilitation software versus Mozart's music on hemiparetic patients using SPECT imaging: a randomized control trial study. International Journal of Stroke 2014;9 Suppl 3:218. [Google Scholar]
- Dastgheib S, Bayat G, Shoeibi A. The impact of neurorehabilitation software versus Mozart's music on hemiparetic patients using SPECT imaging: a randomized control trial study. International Journal of Stroke 2014;9:220. [Google Scholar]
John 2010 {published data only}
- John S, Khanna GL, Kotwal P. Effect of music therapy and meditation along with conventional physiotherapy management in sub‐acute stroke patients. British Journal of Sports Medicine 2010;44 Suppl 1:i14. [Google Scholar]
Oiga 2014 {published data only}
- Oiga L. The effect of music and rhythmic auditory stimulation on upper motor strength rehabilitation of hemiparetic stroke patients in a tertiary hospital: a randomized controlled study. International Journal of Stroke 2014;9 Suppl 3:237. [Google Scholar]
Poćwierz‐Marciniak 2014 {published data only}
- Poćwierz‐Marciniak I. Music Therapy Impact on the Quality of Life and Other Aspects of Psychological Functionality in Patients after Stroke (PhD thesis). Gdansk, Poland: University of Gdansk, 2014. [Google Scholar]
- Poćwierz‐Marciniak I. Music therapy with patients with strokes as a way of coping with the illness ‐ a pilot study [Zastosowanie muzykoterapii u pacjentów po udarze mózguw celu emocjonalnego poradzenia sobie z chorobą – badania pilotażowe]. Conference programme: Music, therapy, education. Practice, education, art 2014.
Renna 2012 {published data only}
- Renna L, Frkovic N, Spear M, Ruddell K. Stroke sounds: music listening in stroke rehabilitation. International Journal of Stroke 2012;7 Suppl 1:58. [Google Scholar]
References to ongoing studies
Ala‐Ruona 2010 {unpublished data only}
- Ala‐Ruona E, Bamberg H, Suhonen J, Fachner J, Erkkilä J, Parantainen H, et al. Examining the effects of active music therapy on post‐stroke recovery: a randomised controlled cross‐over trial. The Third Arts and Quality of Life Research Center Conference, February 2010, Temple University, Philadelphia (USA).
NCT00903266 {unpublished data only}
- NCT00903266. Melodic‐intonation‐therapy and speech‐repetition‐therapy for patients with non‐fluent aphasia. clinicaltrials.gov/ct2/results?term=NCT00903266 (first received 15 May 2009).
NCT01372059 {published data only}
- Bunketorp Kall L, Blomstrand C, Lundgren‐Nilsson A. Is it possible to improve the life situation among community‐dwelling individuals in the late phase of stroke through a rhythm and music method and therapeutic riding? Study protocol for a three‐armed randomized controlled trial. Neurorehabilitation and Neural Repair 2012;26(6):734. [Google Scholar]
- Bunketorp Käll L, Lundgren‐Nilsson Å, Blomstrand C, Pekna M, Pekny M, Nilsson M. The effects of a rhythm and music‐based therapy program and therapeutic riding in late recovery phase following stroke: a study protocol for a three‐armed randomized controlled trial. BMC Neurology 2012;12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01372059. The effects of a rhythm and music‐based therapy program and therapeutic riding in late recovery phase following stroke. clinicaltrials.gov/ct2/show/NCT01372059 (first received 26 May 2011).
NCT01455155 {unpublished data only}
- NCT01455155. Creative therapy to affect stroke outcomes. clinicaltrials.gov/ct2/results?term=NCT01455155 (first received 15 October 2011).
NCT01721668 {unpublished data only}
- NCT01721668. Improving arm and hand functions in chronic stroke. clinicaltrials.gov/ct2/show/NCT01721668 (first received 1 November 2012).
NCT01749709 {unpublished data only}
- NCT01749709. Music listening and stroke recovery. clinicaltrials.gov/ct2/results?term=NCT01749709 (first received 12 December 2012).
NCT01769326 {unpublished data only}
- NCT01769326. Influence of timing on motor learning. clinicaltrials.gov/ct2/results?term=NCT01769326 (first received 16 November 2012).
NCT01956136 {unpublished data only}
- NCT01956136. Efficacy and neural basis of music‐based neurological rehabilitation for traumatic brain injury (MUBI). clinicaltrials.gov/ct2/show/NCT01956136 (first received 12 September 2013).
NCT02208219 {unpublished data only}
- NCT02208219. Music therapy to restore motor deficits after stroke (NEUROMUSIC). clinicaltrials.gov/ct2/results?term=NCT02208219 (first received 18 July 2014).
NCT02259062 {unpublished data only}
- NCT02259062. Listening for leisure after stroke (MELLO). clinicaltrials.gov/ct2/results?term=NCT02259062 (first received 3 October 2014).
NCT02310438 {unpublished data only}
- NCT02310438. Music therapy for the rehabilitation of upper limb with stroke patients. clinicaltrials.gov/ct2/results?term=NCT02310438 (first received 4 December 2014).
NCT02328573 {unpublished data only}
- NCT02328573. The impact of group singing on patients with stroke and their personal caregivers. clinicaltrials.gov/ct2/results?term=NCT02328573 (first received 23 June 2014).
NCT02410629 {unpublished data only}
- NCT02410629. To determine the therapeutic effect of the music glove and conventional hand exercises to subacute stroke patients. clinicaltrials.gov/ct2/show/NCT02410629 (first received 2 April 2015).
NTR1961 {unpublished data only}
- NTR1961. The efficacy of Melodic Intonation Therapy (MIT) in aphasia rehabilitation. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1961 (first received 19 Aug 2009).
Additional references
Altenmuller 2013
- Altenmuller E, Schlaug G. Neurobiological aspects of neurologic music therapy. Music and Medicine 2013;5(4):210‐6. [Google Scholar]
Baker 2006
- Baker FA, Tamplin J. Music Therapy Methods in Neurorehabilitation: A Clinician's Manual. London, Philadelphia: Jessica Kingsley Publishers, 2006. [Google Scholar]
Baker 2011
- Baker F, Tamplin J. Coordinating respiration, vocalization, and articulation: rehabilitating apraxic and dysarthric voices of people with neurological damage. In: Baker F, Uhlig S editor(s). Voicework in Music Therapy: Research and Practice. London: Jessica Kingsley Publishers, 2011:189‐205. [Google Scholar]
Blomert 1995
- Blomert L, Koster C, Kean ML. Amsterdam‐Nijmegen Everyday Language Test [Amsterdam‐Nijmegen Testvoor Alledaagse Taalvaardigheid]. Lisse, the Netherlands Swets & Zeitlinger 1995.
Brady 2012
- Brady MC, Kelly H, Godwin J, Enderby P. Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD000425.pub3] [DOI] [PubMed] [Google Scholar]
Clark 2016
- Clark IN, Baker FA, Taylor NF. The modulating effects of music listening on health related exercise and physical activity in adults: a systematic review and narrative synthesis. Nordic Journal of Music Therapy 2016;25(1):76‐104. [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
Coronado 2012
- Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD, et al. Trends in traumatic brain injury in the US and the public health response: 1995–2009. Journal of Safety Research 2012;43(4):299‐307. [DOI] [PubMed] [Google Scholar]
Corrigan 1989
- Corrigan JD. Development of a scale for assessment of agitation following TBI. Journal of Clinical and Experimental Neuropsychology 1989;11(2):261‐77. [DOI] [PubMed] [Google Scholar]
De Renzi 1978
- Renzi E, Faglioni P. Normative data and screening power of a shortened version of the Token Test. Cortex 1978;14:41–9. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Dileo 2007
- Dileo C, Bradt J. Music therapy: applications to stress management. In: Lehrer P, Woolfolk R editor(s). Principles and Practice of Stress Management. 3rd Edition. New York: Guilford Press, 2007. [Google Scholar]
Duncan 1999
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30(2):2131‐40. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Giles 2006
- Giles GM, Manchester D. Two approaches to behavior disorder after traumatic brain injury. Journal of Head Trauma Rehabilitation 2006;21(2):168‐78. [DOI] [PubMed] [Google Scholar]
Gustavsson 2010
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. Cost of disorders of the brain in Europe 2010. European Neuropsychopharmacology 2011;21(10):718‐79. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hogrefe 1983
- Hogrefe CJ. Aachen Aphasia Test [Aachener aphasie test (AAT)]. Verlag für Psychologie 1983.
Hänninen 1989
- Hänninen H. Profile of Mood States: Finnish version [Neurotoksisten haittojen seulonta ‐ oirekyselyt ja psykologisettestit]. Helsinki, Finland: Työterveyslaitos 1989.
Karageorghis 2012a
- Karageorghis CI, Priest DL. Music in the exercise domain: a review and synthesis (Part I). International Review of Sport Exercise Psychology 2012;5(1):44‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karageorghis 2012b
- Karageorghis CI, Priest DL. Music in the exercise domain: a review and synthesis (Part II). International Review of Sport Exercise Psychology 2012;5(1):67‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kellor 1971
- Kellor M, Frost J, Silderberg N, IversenI, Cummings R. Hand strength and dexterity. American Journal of Occupational Therapy 1971;25:77‐83. [PubMed] [Google Scholar]
Kertesz 1982
- Kertesz A. Western Aphasia Battery. Western Aphasia Battery. 1st Edition. San Antonio, TX: The Psychological Corporation, 1982. [Google Scholar]
Lorr 2003
- Lorr M, McNair DM, Heuchert JWP. Profile of Mood States: Bi‐polar manual supplement. New York: Multi‐Health Systems Inc 2003.
Magee 2006b
- Magee W, Wheeler BL. Music therapy for patients with traumatic brain injury. In: Murrey GJ editor(s). Alternative Therapies in the Treatment of Brain Injury and Neurobehavioral Disorders: A Practical Guide. Binghamton, NY: Haworth Press, 2006:51‐73. [Google Scholar]
Magee 2007
- Magee WL, Andrews K. Multi‐disciplinary perceptions of music therapy in complex neuro‐rehabilitation. International Journal of Therapy and Rehabilitation 2007;14(2):70‐5. [Google Scholar]
Magee 2009
- Magee WL, Baker M. The use of music therapy in neuro‐rehabilitation of people with acquired brain injury. British Journal of Neuroscience Nursing 2009;5(4):150‐6. [Google Scholar]
Matsuzaki 2015
- Matsuzaki S, Hashimoto M, Yuki S, Koyama A, Hirata Y, Ikeda M. The relationship between post‐stroke depression and physical recovery. Journal of Affective Disorders 2015;176:56‐60. [DOI] [PubMed] [Google Scholar]
McAuley 2000
- McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta‐analyses?. Lancet 2000;356:1228‐31. [DOI] [PubMed] [Google Scholar]
Morris 1989
- Morris JC, Heyman A, Mohs RC, Hughes JP, Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989;39:1159–65. [DOI] [PubMed] [Google Scholar]
Mozaffarian 2015
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics ‐ 2015 update: a report from the American Heart Association. Circulation 2015;131:e29‐e322. [DOI] [PubMed] [Google Scholar]
NA 2014
- Neurological Alliance. Neuro Numbers: A Brief Review of the Numbers of People in the UK With a Neurological Condition. London: Neurological Alliance, 2014. [Google Scholar]
Nascimento 2015
- Nascimento LR, Quel de Oliveira C, Ada L, Michaelsen SM, Teixeira‐Salmela LF. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. Journal of Physiotherapy 2015;61:10‐5. [DOI] [PubMed] [Google Scholar]
Norton 2009
- Norton A, Zipse L, Marchina S, Schlaug G. Melodic intonation therapy: shared insights on how it is done and why it might help. Annals of the New York Academy of Sciences 2009;1169(1):431‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orman 2011
- Orman JAL, Kraus JF, Zaloshnja E, Miller T. Epidemiology. In: Silver JM, McAllister TW, Yudofsky SC editor(s). Textbook of Traumatic Brain Injury. 2nd Edition. Washington, DC: American Psychiatric Publishing, 2011. [Google Scholar]
RCP 2012
- Royal College of Physicians. National Clinical Guideline for Stroke. 4th Edition. London: Royal College of Physicians, 2012. [Google Scholar]
Reitan 1985
- Reitan RM, Wolfson D. The Halstead–Reitan Neuropsychological Test Battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press, 1985. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Revonsuo 1995 [Computer program]
- Revonsuo A, Portin R. CogniSpeed: the computer based measurement of cognitive processing. Turku, Finland: AboaTech, 1995.
Robertson 1994
- Robertson IH, Ward T, Ridgeway V, Nimmo‐Smith I. The Test of Everyday Attention: Manual. London: Pearson Assessment, 1994. [Google Scholar]
Schneck 2006
- Schneck DJ, Berger DS. The Music Effect: Music Physiology and Clinical Applications. London, Philadelphia: Jessica Kingsley Publishers, 2006. [Google Scholar]
Selassie 2008
- Selassie AW, Zaloshnja E, Langolis JA, Miller T, Jones P, Steiner C. Incidence of long‐term disability following traumatic brain injury hospitalization, United States, 2003. Journal of Head Trauma Rehabilitation 2008;23(2):123‐31. [DOI] [PubMed] [Google Scholar]
Shannon 1973
- Shannon J, Guerney B Jr. Interpersonal effects of interpersonal behavior. Journal of Personality and Social Psychology 1973;26(1):142–50. [DOI] [PubMed] [Google Scholar]
Shin 1996
- Shin YH. A study on verification of the Profile of Mood States (POMS) for Korean elders. Journal of Korean Academy of Nursing 1996; Vol. 26, issue 4:743–58.
Shores 1986
- Shores EA, Marosszeky JE, Sandanam J, Batchelor J. Preliminary validation of a clinical scale for measuring the duration of PTA. Medical Journal of Australia 1986;144:569‐72. [DOI] [PubMed] [Google Scholar]
Thaut 2014a
- Thaut MH, Hoemberg V. Handbook of Neurologic Music Therapy. London: Oxford University Press, 2014. [Google Scholar]
Thaut 2014b
- Thaut MH, Peterson DA, McIntosh GC, Hoemberg V. Music mnemonics aid verbal memory and induce learning‐related brain plasticity in multiple sclerosis. Frontiers in Human Neuroscience 2014;8:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thurman 1999
- Thurman D, Alverson C, Dunn K, Guerrero J, Sniezek J. Traumatic brain injury in the United States: a public health perspective. Journal of Head Trauma Rehabilitation 1999;14(6):602‐15. [DOI] [PubMed] [Google Scholar]
Uswatte 2005
- Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper‐extremity Motor Activity Log‐14 for measuring real‐world arm use. Stroke 2005;36:2493‐6. [DOI] [PubMed] [Google Scholar]
van de Port 2007
- Port IG, Kwakkel G, Bruin M, Lindeman E. Determinants of depression in chronic stroke: a prospective cohort study. Disability and Rehabilitation 2007;29(5):353‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wechsler 1987
- Wechsler D. Wechsler Memory Scale‐Revised manual. San Antonio: The Psychological Corporation 1987.
WHO 2014
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization, 2014. [Google Scholar]
Whyte 2006
- Whyte EM, Mulsant BH, Rovner BW, Reynolds CF. Preventing depression after stroke. International Review of Psychiatry 2006;18(5):471‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 1999
- Williams LS, Weinberger MW, Harris LE, Clark OE, Biller J. Development of a stroke‐specific quality of life scale. Stroke 1999;30:1362–9. [DOI] [PubMed] [Google Scholar]
Williamson 2012
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2008
- Wilson BA, Greenfield E, Clare L, Baddeley A, Cockburn J, Watson P, et al. The Rivermead Behavioural Memory Test: Administration and Scoring Manual. 3rd Edition. London: Pearson Assessment, 2008. [Google Scholar]
Yoo 2016
Zaloshnja 2008
- Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long‐term disability from traumatic brain injury in the civilian population of the United States, 2005. Journal of Head Trauma Rehabilitation 2008;23(6):394‐400. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bradt 2010
- Bradt J, Magee WL, Dileo C, Wheeler BL, McGilloway E. Music therapy for acquired brain injury. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD006787.pub2] [DOI] [PubMed] [Google Scholar]


