Abstract
Background
Proper sedation for neonates undergoing uncomfortable procedures may reduce stress and avoid complications. Midazolam is a short‐acting benzodiazepine that is used increasingly in neonatal intensive care units (NICUs). However, its effectiveness as a sedative in neonates has not been systematically evaluated.
Objectives
Primary objecive
To assess the effectiveness of intravenous midazolam infusion for sedation, as evaluated by behavioural and/or physiological measurements of sedation levels, in critically ill neonates in the NICU.
Secondary objectives
To assess effects of intravenous midazolam infusion for sedation on complications including the following.
1. Incidence of intraventricular haemorrhage (IVH)/periventricular leukomalacia (PVL).
2. Mortality.
3. Occurrence of adverse effects associated with the use of midazolam (hypotension, neurological abnormalities).
4. Days of ventilation.
5. Days of supplemental oxygen.
6. Incidence of pneumothorax.
7. Length of NICU stay (days).
8. Long‐term neurodevelopmental outcomes.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5), MEDLINE via PubMed (1966 to 16 June 2016), Embase (1980 to 16 June 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 16 June 2016). We searched clinical trials databases, conference proceedings and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We selected for review randomised and quasi‐randomised controlled trials of intravenous midazolam infusion for sedation in infants aged 28 days or younger.
Data collection and analysis
We abstracted data regarding the primary outcome of level of sedation. We assessed secondary outcomes such as intraventricular haemorrhage, periventricular leukomalacia, death, length of NICU stay and adverse effects associated with midazolam. When appropriate, we performed meta‐analyses using risk ratios (RRs) and risk differences (RDs), and if the RD was statistically significant, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH), along with their 95% confidence intervals (95% CIs) for categorical variables, and weighted mean differences (WMDs) for continuous variables. We assessed heterogeneity by performing the I‐squared (I2) test.
Main results
We included in the review three trials enrolling 148 neonates. We identified no new trials for this update. Using different sedation scales, each study showed a statistically significantly higher sedation level in the midazolam group compared with the placebo group. However, none of the sedation scales used have been validated in preterm infants; therefore, we could not ascertain the effectiveness of midazolam in this population. Duration of NICU stay was significantly longer in the midazolam group than in the placebo group (WMD 5.4 days, 95% CI 0.40 to 10.5; I2 = 0%; two studies, 89 infants). One study (43 infants) reported significantly lower Premature Infant Pain Profile (PIPP) scores during midazolam infusion than during dextrose (placebo) infusion (MD ‐3.80, 95% CI ‐5.93 to ‐1.67). Another study (46 infants) observed a higher incidence of adverse neurological events at 28 days' postnatal age (death, grade III or IV IVH or PVL) in the midazolam group compared with the morphine group (RR 7.64, 95% CI 1.02 to 57.21; RD 0.28, 95% CI 0.07 to 0.49; NNTH 4, 95% CI 2 to 14) (tests for heterogeneity not applicable). We considered these trials to be of moderate quality according to GRADE assessment based on the following outcomes: mortality during hospital stay, length of NICU stay, adequacy of analgesia according to PIPP scores and poor neurological outcomes by 28 days' postnatal age.
Authors' conclusions
Data are insufficient to promote the use of intravenous midazolam infusion as a sedative for neonates undergoing intensive care. This review raises concerns about the safety of midazolam in neonates. Further research on the effectiveness and safety of midazolam in neonates is needed.
Plain language summary
Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit
Review question: For sick babies admitted to a neonatal intensive care unit (NICU), how effective is midazolam, given by continuous intravenous drip, as a sedative for reducing stress, as measured by changes in behaviour and vital signs?
Background: Proper sedation for babies undergoing uncomfortable procedures while receiving intensive care may reduce stress and avoid complications. Midazolam is a sedative that is used increasingly in NICUs. However, researchers have not systematically reviewed the evidence to see if it is effective and safe for babies in this setting.
Study characteristics: We have selected for inclusion in this review randomised controlled trials of continuous intravenous drip of midazolam as a sedative in babies aged 28 days or younger.
Key results: We included three clinical trials in this review. Using different scales to measure level of sedation, each study showed that midazolam was effective in providing sedation to babies. However, the validity of the sedation scales used in these studies has not been proven in babies; therefore, we cannot be certain that midazolam is, in fact, an effective sedative for babies. Moreover, one study showed that babies who received midazolam had a significantly higher risk of death or brain injury, and combined results of two studies showed that midazolam use may prolong length of stay in the NICU.
Industry: One of the studies included in this review received support from industry, and for the other two studies, industry provided all study drugs.
Quality of evidence: We assessed the quality of the evidence on the outcomes of mortality during hospital stay, length of stay in the NICU, pain, and neurological outcomes at 28 days of life and found the evidence to be of moderate quality, as there was not enough evidence available. Therefore, we conclude there is not enough evidence to support the use of midazolam as a sedative for babies undergoing intensive care. Additional research is needed to address the safety and effectiveness of midazolam in this population.
Summary of findings
Background
Description of the condition
Term and preterm infants are capable of perceiving pain and stress (Anand 1987). In the neonatal intensive care unit (NICU), supportive and investigative management of sick infants frequently requires painful and uncomfortable procedures. However, because pain and stress are subjective phenomena and are difficult to evaluate in preverbal infants, use of appropriate analgesia and sedatives is often overlooked by care providers. It has been suggested that responses to pain may compromise clinical conditions (Anand 1992), and that adequate sedation during mechanical ventilation may decrease stress (Quinn 1993) and facilitate effective ventilation, so that complications such as pneumothoraces and intraventricular haemorrhage (IVH) may be prevented (Greenough 1983; Perlman 1985).
Description of the intervention
Benzodiazepines, administered as intravenous infusions or as intravenous boluses, can be used to provide sedation, but not analgesia, in many clinical settings. Midazolam is a short‐acting benzodiazepine that has been used increasingly in the NICU.
How the intervention might work
Benzodiazepines are a class of sedatives that act on specific receptors in the central nervous system. These receptors, which are present in the foetus from seven weeks' gestation (Hebebrand 1988), potentiate the neuronal inhibitory pathways mediated by gamma‐aminobutyric acid (GABA) (Jacqz‐Aigrain 1996). Researchers have studied the pharmacokinetics of midazolam in neonates. Midazolam is preferred over other benzodiazepines because of its water solubility and rapid clearance (Jacqz‐Aigrain 1992). Although its elimination half‐life is significantly shorter than that of other benzodiazepines, such as diazepam, its elimination is delayed in preterm neonates compared with older infants and children (Lee 1999). Functional immaturity of hepatic and renal systems in preterm neonates probably accounts for the slower elimination of midazolam. In a recent very large cohort study conducted in Europe, midazolam was given to 576 (9%) of the total cohort of 6680 neonates and to 536 (25%) of 2142 neonates who were tracheally ventilated (Carbajal 2015). Overall, midazolam was by far the sedative most commonly used. It was given to 25% of neonates who had tracheal ventilation, and its use ranged from 0% to 73% across European countries, despite few clinical data to lend support for midazolam sedation in neonates.
It is of note that in an animal model, Koch 2008 reported paradoxical effects of midazolam on nociception and sedation in rats between postnatal days 3 and 10. Midazolam failed to sedate young rats and instead caused an excitatory effect by sensitising their flexor reflex activity. Investigators did not observe the sedative effects of midazolam in supraspinal centres until later in life, after maturation. These results highlight the need for better understanding of the ontogeny of pharmacological effects of drugs such as midazolam that are used routinely in NICUs.
Why it is important to do this review
The effectiveness of intravenous midazolam as a sedative in neonates has not been systematically reviewed. Moreover, its safety at the currently recommended dosage in critically ill neonates has not been well established.
Objectives
Primary objective
To assess the effectiveness of intravenous midazolam infusion for sedation, as evaluated by behavioural and/or physiological measurements of sedation levels, in critically ill neonates in the NICU.
Secondary objectives
To assess effects of intravenous midazolam infusion for sedation on complications including the following.
Incidence of intraventricular haemorrhage (IVH)/periventricular leukomalacia (PVL).
Mortality.
Occurrence of adverse effects associated with the use of midazolam (hypotension, neurological abnormalities).
Days of ventilation.
Days of supplemental oxygen.
Incidence of pneumothorax.
Length of NICU stay (days).
Long‐term neurodevelopmental outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials and quasi‐randomised trials in which the use of intravenous midazolam infusion was compared with placebo or other sedatives in neonates undergoing intensive care.
Types of participants
We included infants aged 28 days or younger who were admitted to the NICU requiring sedation for medical interventions.
Types of interventions
Interventions included continuous intravenous infusion of midazolam administered at a dose of 20 microgram/kg/h to 60 microgram/kg/h for at least 24 hours for sedation during mechanical ventilation and radiological investigative procedures. We excluded studies that used a combination of midazolam and an analgesic for neonates undergoing painful procedures. We also excluded studies that investigated the use of intravenous bolus doses of midazolam, unless the bolus was followed by an infusion; and studies examining use of midazolam as an anaesthetic induction agent or as an anticonvulsant.
Types of outcome measures
Primary outcomes
The primary outcome was level of sedation, evaluated by:
behavioural measures: facial actions, excitability, muscle tone, physical movements and respiratory behaviour, which may be evaluated by age‐appropriate scoring systems; and
physiological parameters: changes in heart rate, respiratory rate, blood pressure, oxygen saturation and plasma cortisol or catecholamine levels, measured at baseline and at regular intervals during midazolam administration.
Secondary outcomes
IVH (defined by classification of Papile et al (Papile 1978)).
PVL (defined as periventricular cysts on brain imaging, with exclusion of subependymal or choroid plexus cysts).
Mortality (death within 28 days of life).
Adverse effects associated with use of midazolam: hypotension (significant drop from baseline compared with controls), neurological abnormalities (epileptiform activities, movement disorders, myoclonus, hypertonia, hypotonia).
Days of mechanical ventilation.
Days of supplemental oxygen use.
Pneumothorax.
Days of NICU stay.
Neurodevelopmental outcomes, as evaluated by a validated developmental assessment tool.
Neurobehavioural Assessment of Preterm Infants (NAPI) (Snider 2005).
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1966 to 16 June 2016); Embase (1980 to 16 June 2016); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 16 June 2016), using the following search term: (midazolam), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for full search strategies for each database). We applied no language restrictions. We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); the ISRCTN Registry).
For previous editions of this review, we identified randomised and quasi‐randomised controlled trials of intravenous midazolam in infants from the Cochrane Central Register of Controlled Trials (CENTRAL; 2012, Issue 3) in the Cochrane Library; MEDLINE (from 1985 to March 2012); Embase (1980 to 2012) and CINAHL (1980 to 2012), using the medical subject headings (MeSH): midazolam; infant; newborn. We searched for abstracts published in Pediatric Academic Societies Meetings Abstract Archives from 1990 to 2011. We imposed no language restrictions. We attempted to contact investigators of studies meeting the inclusion criteria to gather additional data for analysis. We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; controlled‐trials.com; who.int/ictrp), and we searched the Web of Science to identify any trial that quoted the earliest study that we identified (Jacqz‐Aigrain 1994).
Searching other resources
In addition, we manually searched bibliographies of articles and personal files and imposed no language restrictions. We attempted to contact investigators of studies meeting the inclusion criteria to gather additional data for analysis. We did not attempt to identify unpublished studies. We excluded studies involving neonates and older infants and children if we could not extract data for neonates.
Data collection and analysis
We used the standard method of the Cochrane Neonatal Review Group for performing systematic reviews (http://neonatal.cochrane.org/resources‐review‐authors).
Selection of studies
We included randomised and quasi‐randomised controlled trials involving neonates aged 28 days or younger that included a treatment group and a placebo group. We accepted for the review studies that reported outcome measures including physiological, behavioural and hormonal changes, as well as adverse neurological outcomes.
We excluded studies involving neonates and older infants and children if we could not extract data for neonates.
Two review authors (EN, AT) independently decided to include or exclude a specific study. When discrepancies occurred, the three review authors (EN, AT, AO) made the decision by consensus.
Data extraction and management
We created a data collection form on which we abstracted the following data from the included studies: demographics of participants, age at enrolment into the study, inclusion and exclusion criteria, sample size, treatment and control group regimens and outcomes. Two review authors (EN, AT) independently abstracted the data and resolved differences by consensus.
Assessment of risk of bias in included studies
Two review authors (EN, AO) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains: selection bias, performance bias, attrition bias, reporting bias and any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 2 for a detailed description of risks of bias for each domain.
Measures of treatment effect
We performed statistical analyses using Review Manager 5.1 software. We analysed categorical data using risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial (NNTB) or harmful outcome (NNTH). We analysed continuous data by using weighted mean difference (WMD) and reported the 95% confidence interval (CI) for all estimates.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting forest plots (if we included at least 10 trials in one analysis) and quantified the impact of heterogeneity by using the I2 statistic. If we detected statistical heterogeneity, we planned to explore possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments) by performing post hoc subgroup analyses.
Data synthesis
When we identified at least two RCTs that evaluated the effectiveness of intravenous midazolam infusions by examining the same outcome measures, we pooled the results to obtain an overall estimate of effect size using RevMan 5.1.4 (RevMan 2011). We used the Mantel‐Haenszel method for estimates of typical RR and RD, and the inverse variance method for measured quantities. We used the fixed‐effect model for all meta‐analyses.
Quality of the evidence
For the 2016 update, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schunemann 2013), to assess the quality of evidence for the following clinically relevant outcomes: mortality during hospital stay, length of NICU stay, adequacy of analgesia as measured by the Premature Infant Pain Profile (PIPP) (Stevens 1996) and poor neurological outcomes by 28 days' postnatal age. In terms of the primary outcome of level of sedation, none of the sedation scales used in these studies had been validated in preterm infants; therefore, we could not ascertain the effectiveness of midazolam in this population as reported by these studies and did not subject this outcome to GRADE assessment.
We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (and two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates and presence of publication bias.
The GRADE approach assesses the quality of a body of evidence and assigns one of four grades.
High: We are very confident that the true effect lies close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Two review authors (EN, AO) independently assessed the quality of the evidence for each of the outcomes above. We used the GRADEpro Guideline Development Tool to create ‘Summary of findings’ tables to report the quality of evidence. See Appendix 3 for details on assessment of quality of the evidence.
Subgroup analysis and investigation of heterogeneity
We prospectively planned no subgroup analyses.
Results
Description of studies
Results of the search
We presented results of the search in the study flow diagram for the review update provided in Figure 1. We identified six RCTs on the use of intravenous midazolam in infants; we included three of these and excluded three. Literature searches conducted in September 2009, March 2012 and June 2016 identified no additional trials.
1.
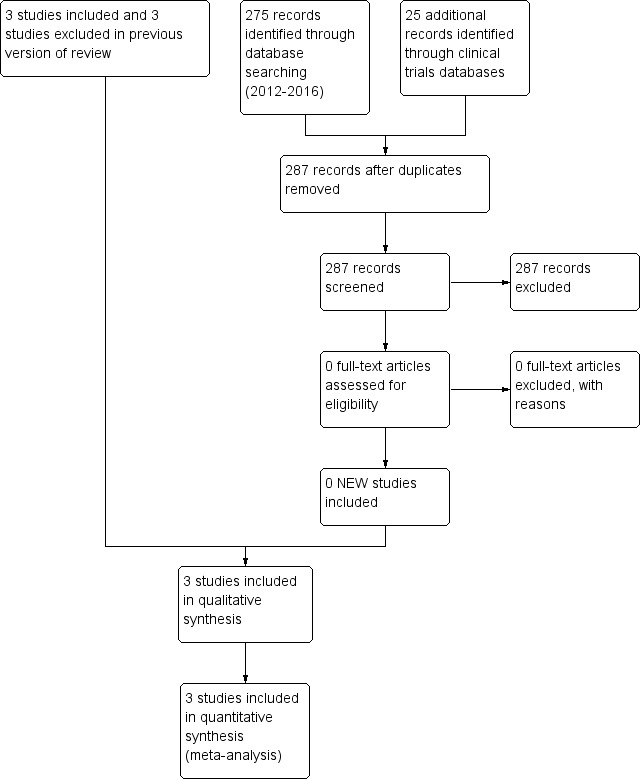
Study flow diagram: review update.
Included studies
For details, see Characteristics of included studies.
Jacqz‐Aigrain 1994 randomly assigned 46 preterm infants (25 at < 33 weeks' gestation and 21 at ≥ 33 weeks' gestation) ≤ 48 hours of age to receive midazolam infusion or manufactured placebo for five days while mechanically ventilated for respiratory distress syndrome. Twenty‐four infants received midazolam and 22 received placebo infusions. Researchers withdrew one infant in the midazolam group because of major neurological abnormalities at 24 hours of age; and two infants from the midazolam group and two from the placebo group within 72 hours owing to rapid clinical improvement. They noted contamination in one infant in the placebo group (midazolam was detectable in the serum at 24 hours). Baseline characteristics did not differ between groups. Severity of illness, as measured by mean airway pressure (MAP) while ventilated and fraction of inspired oxygen (FiO2) from the time of enrolment to the end of the study, was not significantly different between groups. Investigators administered midazolam as an infusion at 60 microgram/kg/h for up to five days in infants at ≥ 33 weeks' gestation, and at 60 microgram/kg/h for one day followed by 30 microgram/kg/h for up to a total of five days in infants at < 33 weeks' gestation. They did not report the duration of the infusion. Weaning of sedatives was allowed after at least 48 hours of administration, but investigators did not specify a weaning protocol. The primary outcome was adequacy of sedation as measured by a behavioural score adapted from the clinical neurological and behavioural scoring system of Barrier (Barrier 1989) and by changes in physiological variables (heart rate and blood pressure). The sedation score consisted of five items that assessed facial expression, sucking, spontaneous motor activity, excitability/responsiveness to stimulation and excessive flexion, with scores ranging from 0 (sedation) to 5 (inadequate sedation). Care providers measured the sedation score four times per day during treatment (nurses twice, physicians twice). Secondary outcomes included days of ventilation support, days of supplemental oxygen, surfactant use, duration of NICU stay and common complications of preterm birth (e.g. pneumothorax, pulmonary interstitial emphysema, hypotension, chronic lung disease, necrotising enterocolitis, intracranial haemorrhage, persistent pulmonary hypertension of the newborn, death). Researchers reported outcomes for all 46 infants.
In a multi‐centre randomised pilot study (Anand 1999), investigators assigned 67 preterm infants of 24 to 32 weeks' gestation who were ≤ 72 hours of age and who were ventilated for less than eight hours to receive midazolam infusion, morphine infusion or dextrose placebo infusion for as long as sedation was considered necessary up to a maximum of 14 days. Twenty‐two infants received midazolam infusion, 24 received morphine infusion and 21 received dextrose placebo. The three groups did not differ significantly in baseline characteristics. Severity of illness at birth, as assessed by the Clinical Risk Index for Babies (CRIB) score (The International Neonatal Network), showed no significant differences among groups at birth (P = 0.24). However, severity of illness as measured by the Neonatal Medical Index (NMI) (Korner 1993) on the basis of response variables during the hospital stay showed significant differences in the distribution of risk categories among the three groups at discharge (P = 0.01). Researchers administered midazolam at 200 microgram/kg loading dose followed by an infusion of 20 microgram/kg/h, 40 microgram/kg/h or 60 microgram/kg/h for infants of gestational age 24 to 26 weeks, 27 to 20 weeks or 30 to 33 weeks, respectively. They administered morphine at 100 microgram/kg loading dose followed by an infusion of 10 microgram/kg/h, 20 microgram/kg/h or 30 microgram/kg/h for infants of gestational age 24 to 26 weeks, 27 to 29 weeks or 30 to 33 weeks, respectively. Duration of the infusion was not different among groups (5.1 days vs 3.4 days vs 5.0 days in the midazolam, morphine and placebo groups, respectively; P = 0.37). If necessary, they provided additional boluses of morphine and documented the frequency and amount given as measures of inadequate sedation. Investigators used a standardised protocol in weaning sedatives. The primary outcome was the incidence of adverse neurological events (defined as neonatal death, grade III or IV IVH or PVL). Researchers measured the adequacy of sedation by obtaining the COMFORT score, an eight‐item behavioural and physiological measurement of distress in the paediatric intensive care unit (Ambuel 1992). This score includes assessment of alertness, calmness/agitation, respiratory response, physical movement, mean arterial blood pressure, heart rate, muscle tone and facial tension, with scores ranging from 8 (sedated) to 40 (not adequately sedated). They measured adequacy of analgesia by obtaining the Premature Infant Pain Profile (PIPP) (Stevens 1996) in response to tracheal suctioning. The PIPP score includes assessment of gestational age, behavioural state, heart rate, oxygen saturation, brow bulge, eye squeeze and nasolabial furrow, with scores ranging from 0 (adequate analgesia) to 21 (inadequate analgesia). They obtained the two scores on all infants at baseline, after 24 hours of infusion and at 10 to 12 hours after discontinuation of the infusion. Other secondary outcomes included days of mechanical ventilation, continuous positive airway pressure, supplemental oxygen use, incidence of pneumothorax, duration of NICU and hospital stay, days to full enteral (full strength, full gavage, full oral) feeds, daily weight gain and neurodevelopmental outcomes at 36 weeks' corrected age as measured by Neurobehavioral Assessment of the Premature Infant (NAPI) examination cluster scores (Korner 1991). Researchers reported outcomes for all 67 infants.
Arya 2001 randomised 33 infants with birth weight < 2000 grams and requiring mechanical ventilation during the first week of life to receive midazolam or placebo infusion for sedation. Seventeen infants received midazolam and 16 received placebo. The two groups were similar in baseline characteristics. Severity of respiratory illness, as measured by peak inspiratory pressure (PIP), MAP, oxygenation index (OI) and the alveolar‐arterial oxygen gradient (AaDO2), was similar between the two groups at the time of enrolment. Investigators administered midazolam intravenously at 200 microgram/kg loading dose followed by an infusion of 60 microgram/kg/h. Infants in both groups also received morphine infusion at 10 microgram/kg/h during the study period. The study concentrated on the first 48 hours of midazolam infusion and did not report on duration of benzodiazepine use nor on method of weaning. Three infants in each group did not complete the first 24 hours of the study, and four in each group did not complete 48 hours of the study. Reasons for withdrawal were death (13 infants) and extubation (one infant). Researchers included these infants in the analyses on an intention‐to‐treat basis. The primary outcome was adequacy of sedation as measured by a behavioural score adapted from the clinical neurological and behavioural scoring system of Barrier (Barrier 1989). This is the same scoring system used in Jacqz‐Aigrain 1994. Study authors assessed infants for adequacy of sedation before midazolam administration, then every six hours over the 48‐hour study period. Other measured outcomes included changes in physiological variables (heart rate and blood pressure), changes in oxygen requirement (FiO2) and ventilation requirement (PIP, positive end‐expiratory pressure (PEEP), ventilator rate) and arterial blood gas as measured by mean daily values. Investigators documented complications related to mechanical ventilation (air leak, IVH) and potential adverse effects of midazolam (epileptiform movements, hypotension, tachycardia and oliguria) while reporting the duration of ventilation. They reported no long‐term outcomes but described outcomes for all 33 infants in the study.
Excluded studies
For details, see Characteristics of excluded studies. We excluded one trial that used a single bolus dose of intravenous midazolam (McCarver‐May 1996) and another trial that used intravenous midazolam for anaesthetic induction (Kawakami 1998). The third excluded trial (Parkinson 1997) used midazolam for sedation in individuals from one day to 15 years of age, and we could not extract data pertaining to neonates. The three trials included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) reported on the effectiveness of midazolam infusion and included a total of 146 infants.
Risk of bias in included studies
For details, see the Risk of bias in included studies table, Figure 2 and Figure 3.
2.
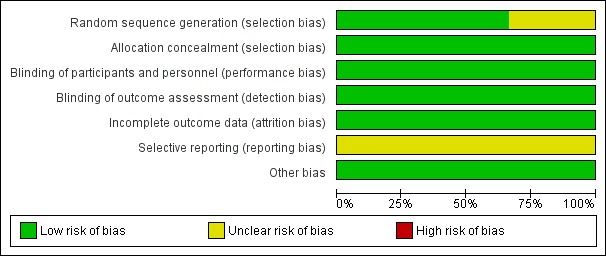
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
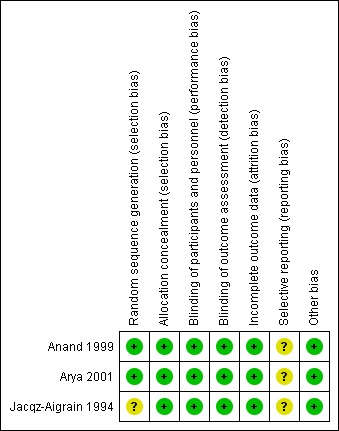
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of bias for random sequence generation was low in two studies (Anand 1999; Arya 2001) and unclear in one study (Jacqz‐Aigrain 1994). All three studies adequately concealed allocation (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994).
Blinding
Risk of performance and detection was low in all three studies (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994).
Incomplete outcome data
Investigators reported outcomes for all randomised infants.
Selective reporting
A protocol was not available for any of the three studies, so we could not judge whether deviations from the protocol occurred.
Other potential sources of bias
Included studies appeared free of other sources of bias. Anand 1999 received support from industry, and Arya 2001 and Jacqz‐Aigrain 1994 received midazolam and placebo from a pharmaceutical company.
Only Arya 2001 performed sample size calculation, and Anand 1999 stated that the study was a pilot trial. All three studies performed statistical analyses using an intention‐to‐treat approach.
Effects of interventions
for the main comparison.
| Midazolam infusion compared with placebo for sedation in neonates | ||||||
|
Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: placebo infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Midazolam | |||||
|
Mortality during hospital stay |
High‐risk population |
RR 0.79 (0.40 to 1.56) |
122 (3) | ⊕⊕⊕⊝ Moderate | Risk of bias for these 3 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 3 studies were conducted in the target population of newborn infants. |
|
| 220 per 1000 | 165 per 1000 | |||||
| Length of NICU stay (days) | Mean length of NICU stay ranged across control groups from 9 to 37.5 days. | WMD of NICU stay for intervention groups was 5.4 days longer. | WMD 5.4 days (0.4 to 10.5) | 89 (2) | ⊕⊕⊕⊝ Moderate | Risk of bias for these 2 studies was low. We noted no heterogeneity in the results (I2 = 0%). Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. The 2 studies were conducted in the target population of newborn infants. |
|
PIPP score during drug infusion Range of scale 0‐21 for infants < 28 weeks' PMA and 0‐18 for infants > 36 weeks' PMA. Lower score = less pain (Stevens 1996) |
Mean PIPP score in the control group was 12.7. | Mean PIPP score in the intervention group was lower at 8.9. | MD ‐3.80 (‐5.93 to ‐1.67) | 43 (1) | ⊕⊕⊕⊝ Moderate | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 1.34 (0.50 to 3.56) | 43 (1) | ⊕⊕⊕⊝ Moderate | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
|
| 230 per 1000 | 310 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NICU: neonatal intensive care unit; PIPP: Premature Infants Pain Profile; PMA: postmenstrual age; RR: risk ratio; WMD: weighted mean difference. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
2.
| Midazolam infusion compared with morphine infusion for sedation in neonates | ||||||
|
Patient or population: neonates requiring intubation and ventilation Setting: neonatal intensive care unit Intervention: midazolam infusion Comparison: morphine infusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Morphine | Midazolam | |||||
|
PIPP score during drug infusion Range of scale 0‐21 for infants < 28 weeks' PMA and 0‐18 for infants > 36 weeks' PMA. Lower score = less pain (Stevens 1996) |
Mean PIPP score in the control group was 7.9. | Mean PIPP score in the intervention group was 8.9. | MD 1.00 (‐0.66 to 2.66) | 46 (1) | ⊕⊕⊕⊝ Moderate | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
| Poor neurological outcome by 28 days' postnatal age | High‐risk population | RR 7.64 (1.02 to 57.21) | 46 (1) | ⊕⊕⊕⊝ Moderate | Risk of bias for this study was low. As we identified only 1 study, tests for heterogeneity were not applicable. Precision for the point estimate was low, so we downgraded the quality of the evidence 1 step. This study was conducted in the target population of newborn infants. |
|
| 318 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PIPP: Premature Infants Pain Profile; PMA: postmenstrual age; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Midazolam infusion versus placebo (comparison 1)
Anand 1999 evaluated outcomes by performing analysis of variance to detect statistically significant differences among midazolam, morphine and placebo groups. For this review, we performed comparisons between the midazolam group and the placebo group on relevant continuous outcome variables using information provided by the publication (sample size, mean, standard deviation (SD), standard error of the mean). For the current update of this review, we included an additional comparison of midazolam versus morphine infusion for the outcome of PIPP during drug infusion, as well as for poor neurological outcome by 28 days' postnatal age.
Primary outcomes
Primary outcomes involved level of sedation, as evaluated by:
behavioural measures: facial actions, excitability, muscle tone, physical movements and respiratory behaviour, which may be evaluated by age‐appropriate scoring systems; and
physiological parameters: changes in heart rate, respiratory rate, blood pressure, oxygen saturation and plasma cortisol or catecholamine levels, measured at baseline and at regular intervals during midazolam administration.
In Additional Table 3 ('Sedation scores used in included studies'), we present some basic information about measures of sedation used. All investigators applied the scores to infants and children, most of whom were older than one month, and did not include preterm newborn infants. None of the tools used to assess sedation had been validated for use in newborn infants. We therefore reported the results of each included study separately, as did the authors of the included trials. We did not enter data for sedation scores into RevMan analyses. For the same reasons, we did not include sedation scores in the 'Summary of findings' tables.
1. Sedation scores used in included studies.
| Study reference for included trial | Name of score used | Reference for the score | Age of infants/children subjected to the score | Score validated in newborns? |
| Anand 1999 | COMFORT Scale | Ambuel 1992; Marx 1994 |
Ambuel 1992 ‐ 37 participants (age newborn to 204 months (mean 37.1; SD 52.7)) Marx 1994 ‐ children (age 0 to 102 months (mean age > 1 year) |
No |
| Arya 2001 | Sedation score | Barrier 1989 | Barrier 1989 ‐ 23 infants (age 1 to 7 months) | No |
| Jacqz‐Aigrain 1994 | Behaviour score | Craig 1984; Barrier 1989; Robieux 1991 |
Craig 1984 ‐ 30 children (age 2 to 24 months) Barrier 1989; Robieux 1991 ‐ 41 infants and toddlers (age 3 to 36 months) |
No |
Sedation scores (behavioural measures)
Jacqz‐Aigrain 1994 enrolled and reported on 46 newborn infants. Sedation scores (behavioural measures) (Barrier 1989; Craig 1984; Robieux 1991) were not different between groups at baseline. The midazolam group had consistently lower scores (more sedated) than the placebo group on all days, as assessed by both nurses and physicians (P < 0.05). Investigators observed significant decreases in sedation scores from baseline (mean (SD) score 1.9 (0.4)) to day 1 (score 1.1 (0.3); P < 0.01), day 2 (score 0.8 (0.2); P < 0.010) and day 3 (score 1.1 (0.3); P < 0.05) in the midazolam group (per nurses' scores) and significant increases in the placebo group from baseline (mean (SD) score 1.7 (0.3)) to day 1 (score 2.6 (0.3); P < 0.01) (per physicians' scores).
Anand 1999 enrolled and reported on 67 preterm infants and found statistically significantly lower COMFORT scores (more sedated) in the midazolam group during the infusion (mean (SD) score 14.9 (4.6) vs 17.5 (4.2); P = 0.04), although they noted no statistically significant differences in scores between the two groups before infusion and 12 hours after the infusion was stopped (mean (SD) score 15.9 (3.8) vs 15.6 (3.2); P = 0.8, before the infusion; and 15.8 (4.7) vs 16.2 (4.1); P = 0.76, after the infusion). In response to tracheal suctioning, the midazolam group had significantly lower PIPP scores (more sedated) during the infusion compared with the placebo group (mean (SD) score 8.9 (3.3) vs 12.7 (3.8); P < 0.001). The requirement for additional morphine was not statistically different between midazolam and placebo groups, but midazolam groups tended to require fewer additional morphine doses than placebo groups.
Arya 2001 enrolled and reported on 33 preterm newborns. Sedation scores were not significantly different between the two groups at baseline. The midazolam group had statistically significantly lower sedation scores (more sedated) compared with the placebo group from 18 hours after the start of infusion (median (range) score 0 (0 to 3) vs 1 (0 to 4); P < 0.05). This trend continued for the study duration (up to 48 hours), with statistically significant differences noted at 36 (median (range) score 0 vs 1 (0 to 3); P < 0.05), 42 (0 (0‐3) vs 1 (0‐3); P < 0.05) and 48 hours (0 (0 to 2) vs 1 (0 to 3); P < 0.05) of study drug infusion.
Even though Jacqz‐Aigrain 1994 and Arya 2001 used the same sedation score, we could not combine results on adequacy of sedation by meta‐analysis, as Arya 2001 presented sedation scores as median values and ranges, whereas Jacqz‐Aigrain 1994 presented results as means and SDs.
Sedation scores (physiological parameters)
Jacqz‐Aigrain 1994 enrolled and reported on 46 newborn infants. The physiological parameters heart rate and blood pressure did not differ between groups at baseline but were significantly lower in the midazolam group than in the placebo group on days 1 and 2. These trends continued through to day 5, although they were not statistically significant. One infant in the midazolam group and seven in the placebo group (P < 0.05) were inadequately sedated and required fentanyl and muscle relaxants within 72 hours. Two infants in the midazolam group received fentanyl within 72 hours (Jacqz‐Aigrain 1994).
Secondary outcomes
Intraventricular haemorrhage (outcome 1.1)
Neither Jacqz‐Aigrain 1994 nor Anand 1999 found a statistically significant difference between midazolam and placebo groups in the incidence of IVH. Arya 2001 observed no intracranial haemorrhage during the 48‐hour study period in all enrolled neonates. Meta‐analysis of results of the three studies (n = 122) showed no statistically significant differences in the incidence of IVH of any grade (typical RR 1.68, 95% CI 0.87 to 3.24; typical RD 0.12, 95% CI ‐0.02 to 0.26; I2 = 0% (none) for RR but 64% (moderate) for RD; Analysis 1.1).
1.1. Analysis.
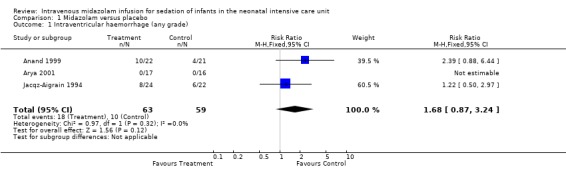
Comparison 1 Midazolam versus placebo, Outcome 1 Intraventricular haemorrhage (any grade).
Mortality (outcome 1.2)
Neither Jacqz‐Aigrain 1994 nor Anand 1999 found a statistically significant difference in mortality between midazolam and placebo groups. Arya 2001 did not report mortality as an outcome measure. However, six infants in the midazolam group and seven in the placebo group died before completing the 48‐hour study period. Meta‐analysis of results of the three studies showed no evidence of effect (typical RR 0.79, 95% CI 0.40 to 1.56; typical RD ‐0.05, 95% CI ‐0.18 to 0.09; I2 = 0% (none for both RR and RD); Analysis 1.2).
1.2. Analysis.
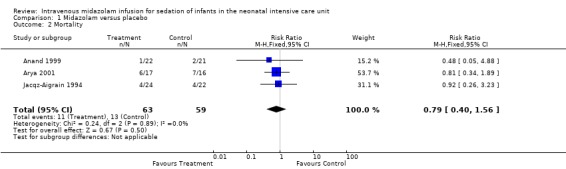
Comparison 1 Midazolam versus placebo, Outcome 2 Mortality.
Days on ventilation (outcome 1.3)
Combined results of Jacqz‐Aigrain 1994 and Anand 1999 showed no statistically significant difference in days of ventilation (WMD 3.6 days, 95% CI ‐0.2 to 7.4 days; I2 = 0% (none); Analysis 1.3).
1.3. Analysis.
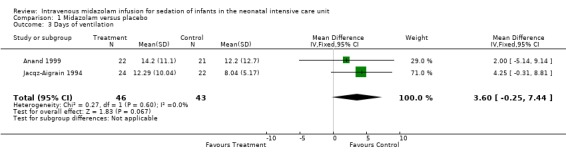
Comparison 1 Midazolam versus placebo, Outcome 3 Days of ventilation.
Arya 2001 presented data on days of ventilation as median values and ranges, so we could not combine these data with data from the other two studies. Median duration of ventilation (range) was 53 hours (7 to 216) in the midazolam group and 59 hours (13 to 194) in the placebo group.
Days on supplemental oxygen use (outcome 1.4)
Combined results of Jacqz‐Aigrain 1994 and Anand 1999 showed no statistically significant difference in days of supplemental oxygen use (WMD 0.6 days, 95% CI ‐5.3 to 6.6 days; I2 = 0% (none); Analysis 1.4).
1.4. Analysis.
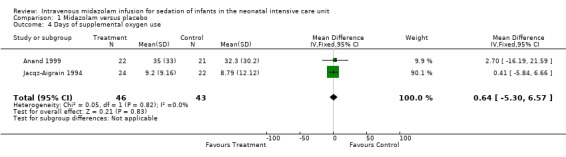
Comparison 1 Midazolam versus placebo, Outcome 4 Days of supplemental oxygen use.
Pneumothorax (outcome 1.5)
All three studies (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) reported on pneumothorax (n = 122) and observed no significant effect of midazolam versus placebo for this outcome (typical RR 1.08, 95% CI 0.41 to 2.84; typical RD 0.01, 95% CI ‐0.10 to 0.12; I2 = 0% (none for both); Analysis 1.5).
1.5. Analysis.
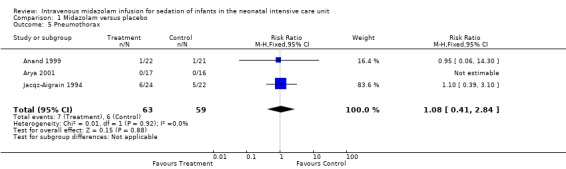
Comparison 1 Midazolam versus placebo, Outcome 5 Pneumothorax.
Length of NICU stay (outcome 1.6)
Jacqz‐Aigrain 1994 and Anand 1999 reported that length of NICU stay was not statistically significantly different between midazolam and placebo groups. Meta‐analysis of these data showed that the midazolam group had a statistically significantly longer length of stay in the NICU than the placebo group (WMD 5.4 days, 95% CI 0.4 to 10.5 days; I2 = 0% (none); Analysis 1.6; Figure 4).
1.6. Analysis.
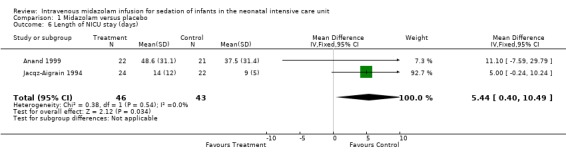
Comparison 1 Midazolam versus placebo, Outcome 6 Length of NICU stay (days).
4.
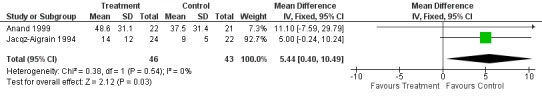
Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.6 Length of NICU stay (days).
Arya 2001 did not report on length of NICU stay.
Average NAPI score at 36 weeks' postmenstrual age (outcome 1.7)
Anand 1999 reported average NAPI score at 36 weeks' postmenstrual age and found no significant difference between midazolam and placebo (dextrose) groups (MD ‐2.10, 95% CI ‐14.38 to 10.18; tests for heterogeneity not applicable; Analysis 1.7).
1.7. Analysis.
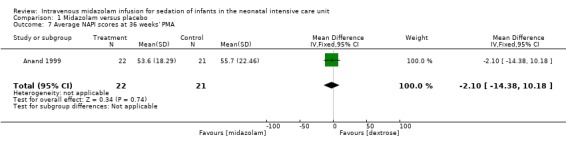
Comparison 1 Midazolam versus placebo, Outcome 7 Average NAPI scores at 36 weeks' PMA.
Poor neurological outcome up to 28 days' postnatal age (IVH grade III or IV, PVL or death at 28 days or sooner without discharge from the NICU) (outcome 1.8)
Anand 1999 reported on this outcome and described no statistically significant difference between midazolam and placebo (dextrose) groups (RR 1.34, 95% CI 0.50 to 3.56; RD 0.08, 95% CI ‐0.19 to 0.35; tests for heterogeneity not applicable; Analysis 1.8).
1.8. Analysis.
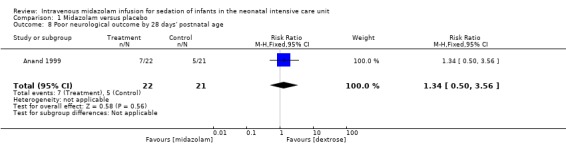
Comparison 1 Midazolam versus placebo, Outcome 8 Poor neurological outcome by 28 days' postnatal age.
PIPP score during drug infusion (outcome 1.9)
Anand 1999 reported on this outcome and found a statistically significant difference between midazolam and placebo (dextrose) groups favouring the midazolam group (MD ‐3.80, 95% CI ‐5.93 to ‐1.67; tests for heterogeneity not applicable; Analysis 1.9; Figure 5).
1.9. Analysis.
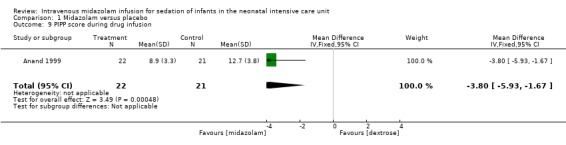
Comparison 1 Midazolam versus placebo, Outcome 9 PIPP score during drug infusion.
5.
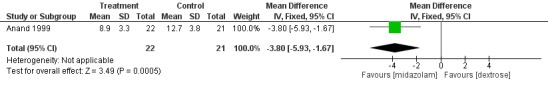
Forest plot of comparison: 1 Midazolam versus placebo, outcome: 1.9 PIPP score during drug infusion.
Occurrence of adverse effects associated with midazolam administration
Jacqz‐Aigrain 1994 observed no adverse neurological effects, but investigators excluded one infant in the midazolam group from the study within 24 hours owing to major neurological abnormalities. Researchers provided no details of this case and reported no statistically significant differences between groups in the incidence of hypotension requiring albumin or vasoactive drugs (8/24 vs 6/22).
Anand 1999 noted no adverse neurological effects associated with midazolam administration and did not report the incidence of hypotension.
Arya 2001 described no adverse neurological effects associated with midazolam administration. Researchers noted epileptiform movements of unknown cause in two infants in the placebo group and noted no significant hypotension in any infant during the study period.
Neurodevelopmental outcome
Jacqz‐Aigrain 1994 and Arya 2001 did not report long‐term neurodevelopmental outcomes.
Midazolam infusion versus morphine (comparison 2)
PIPP score during drug infusion (outcome 2.1)
Anand 1999 reported on this outcome and described no statistically significant differences between midazolam and morphine groups (MD 1.00, 95% CI ‐0.66 to 2.66; tests for heterogeneity not applicable).
Poor neurological outcome by 28 days' postnatal age (IVH grade III or IV, PVL or death at 28 days or sooner without discharge from the NICU) (outcome 2.2)
Arya 2001 reported on this outcome in 46 infants. Investigators observed statistically significantly increased risk of poor neurological outcome by 28 days' postnatal age compared with infants treated with morphine (RR 7.64, 95% CI 1.02 to 57.21; RD 0.28, 95% CI 0.07 to 0.49; NNTH 4, 95% CI 2 to 14; test for heterogeneity not applicable; Analysis 2.2; Figure 6).
2.2. Analysis.
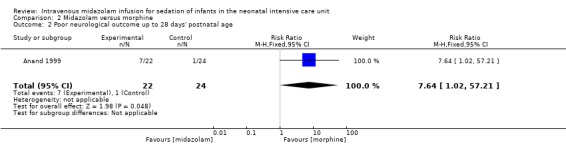
Comparison 2 Midazolam versus morphine, Outcome 2 Poor neurological outcome up to 28 days' postnatal age.
6.

Forest plot of comparison: 2 Midazolam versus morphine, outcome: 2.2 Poor neurological outcome up to 28 days' postnatal age.
Discussion
Summary of main results
Since midazolam was introduced into the neonatal intensive care unit (NICU) in the 1980s, little information has been published on its effectiveness and safety when administered to critically ill neonates. Most reports to date are case series and case reports of midazolam use in patients of diverse age groups (from three days to 18 years of age), given at variable doses (from 0.025 mg/kg to 0.3 mg/kg administered as a bolus, to 24 microgram/kg/h to 400 microgram/kg/h administered as an infusion) (Hartwig 1991; Pellier 1999; Rosen 1991; Stenhammar 1994). The three studies included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) are the only randomised controlled trials (RCTs) conducted to date on the use of midazolam infusion for sedation in infants. Repeated literature searches in September 2009, March 2012 and June 2016 yielded no additional trials.
Tools to measure level of sedation in preterm infants are few (AAP/CPS 2000). Sedation level in such infants is currently measured by scales previously validated in older infants and children. Whether these scales are appropriate in preterm infants is unknown. Therefore, in the three RCTs included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994), although intravenous infusion of midazolam appeared to provide an effective sedative compared with placebo, investigators could draw no definitive conclusions on its effectiveness as a sedative in preterm infants. Anand 1999 assessed level of sedation using the COMFORT score, a composite scale based on eight behavioural and physiological items used to assess distress (Ambuel 1992). Although these items are applicable to preterm infants, this score has been validated only in older infants and children (mean age, 37.1 months). Jacqz‐Aigrain 1994 and Arya 2001 used a sedation scale adapted from the scoring system of Barrier (Barrier 1989), which had not been validated in preterm infants, by selecting five of 10 items from the scoring system. The validity of such an adapted score in assessing sedation level in neonates is unknown.
Jacqz‐Aigrain 1994 showed similar incidences of intracranial haemorrhage between midazolam and control groups. However, the midazolam‐treated infant who was excluded within 24 hours for major neurological abnormalities raises concern about the safety of midazolam. In Anand 1999, the incidence of poor neurological outcomes (death, severe intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL)) was higher in the midazolam group than in placebo and morphine groups (32% vs 24% vs 4%, respectively; P = 0.03). It should be noted, however, that the morphine group included a higher percentage of female infants with slightly higher birth weight and more mature gestational age. These baseline characteristics may have contributed to the differences in neurological outcomes noted in these groups.
Overall completeness and applicability of evidence
Today, only 146 neonates have been enrolled in three trials comparing midazolam versus placebo or morphine. The studies included in this review observed adverse neurological events more frequently, although possibly multi‐factorial in origin, among midazolam‐treated infants.
Quality of the evidence
The three trials included in this review (Anand 1999; Arya 2001; Jacqz‐Aigrain 1994) had small sample sizes but low risk of bias for most of the items included in the risk of bias tables. We rated risk of bias as unclear for selective reporting (reporting bias), as the protocols for all three studies were not available to us. We assessed these trials as having moderate quality according to GRADE Working Group grades of evidence. Thus further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Most important, none of the sedation scales used in these trials had been validated in newborns, and we could include only a few outcomes in the 'Summary of findings' tables (Table 1; Table 2).
Potential biases in the review process
We are aware of no biases in our review process. However, although we used a robust search method, we cannot exclude the possibility that we could have missed literature evidence.
Agreements and disagreements with other studies or reviews
The literature has reported adverse neurological effects associated with midazolam in term and preterm neonates (Adams 1997; Bergman 1991; Collins 1991; Magny 1994; Ng 2002; van den Anker 1992). Investigators have reported a variety of transient neurological effects after boluses or infusions, or both, of midazolam, including impaired level of consciousness, lack of visual following, hypertonia, hypotonia, choreic movements, dyskinetic movements, myoclonus and epileptiform activity. They have noted abnormalities on electroencephalograms in some cases. In all cases, effects were transient, although researchers have not reported long‐term neurodevelopmental outcomes. Two studies (Harte 1997; van Straaten 1992) found a significant decrease in middle cerebral artery blood flow velocity among preterm infants administered a single bolus injection of midazolam. This effect lasted up to one hour and was directly related to a drop in mean arterial blood pressure. Thus, it appears that the neurological effects of midazolam may be related at least in part to transient cerebral hypoperfusion. Long‐term sequelae of these effects remain unknown.
The mechanism of midazolam‐induced hypotension was thought to be vasodilation related to levels of extravascular prostanoids and calcium (Modanlou 1997). Jacqz‐Aigrain 1994 found that the number of infants with haemodynamic instability was not significantly different between midazolam and placebo groups (eight vs six, respectively), although infants in the midazolam group had significantly lower blood pressure than infants in the placebo group. Other investigators (Burtin 1991; Ng 2002; van den Anker 1992) observed significant hypotension in several preterm infants after bolus doses and infusions of midazolam that required volume resuscitation or vasoactive drugs. In most cases, providers had administered fentanyl concomitantly.
Authors' conclusions
Implications for practice.
This systematic review can provide no definitive conclusions about the effectiveness and safety of midazolam infusion (in the dose range of 30 microgram/kg/h to 60 microgram/kg/h) as a sedative in preterm neonates. Authors of studies included in this review observed adverse neurological events, although possibly multi‐factorial in origin, more frequently in midazolam‐treated infants. These adverse effects cannot be dismissed in light of previous case reports of serious neurological and haemodynamic effects provided by non‐randomised uncontrolled studies as well as studies on effects of midazolam on cerebral artery blood flow velocity. Therefore, evidence is currently insufficient to support routine use of intravenous midazolam infusion for sedation of newborn infants in the NICU.
Implications for research.
Reliable, valid and clinically useful scales are needed to measure level of sedation in preverbal infants. When such scales are developed, investigators must conduct further research on the effectiveness of sedatives such as midazolam infusion on term and preterm infants. With regards to safety of midazolam use in infants, researchers must undertake additional studies on associated short‐term and long‐term adverse effects.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2020 | Amended | Arne Ohlsson deceased. |
History
Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 29 July 2016 | New search has been performed | We updated this review in July 2016 and identified no new trials. We expanded on the risk of bias tables and activated new headings in several sections of the review. We included 'Summary of findings' tables. |
| 29 July 2016 | New citation required but conclusions have not changed | We identified no new trials for this update and did not change review conclusions. |
| 22 July 2014 | Amended | We made minor amendments. |
| 22 March 2012 | New citation required but conclusions have not changed | We identified no new trials and made no changes to review conclusions. |
| 22 March 2012 | New search has been performed | This updates the review, "Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit", published in the Cochrane Database of Systematic Reviews (Ng 2003). We performed a literature search in March 2012. |
| 9 September 2009 | New search has been performed | This updates the review, "Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit", published in the Cochrane Library (2003, Issue 1) (Ng 2003). We identified no new trials. |
| 2 November 2008 | Amended | We converted the review to new review format. |
| 1 August 2006 | New search has been performed | In an updated search conducted in August 2006, we found no additional studies. We made no changes to review conclusions as a result of this update. |
| 17 September 2002 | New citation required and conclusions have changed | We made substantive amendments. We conducted a new search in September 2002 and identified 1 additional randomised controlled trial for inclusion. We made no changes to review conclusions as a result of this update. |
Acknowledgements
We acknowledge Joseph Beyene, Biostatistician, University of Toronto, Ontario, Canada, for performing statistical analyses of the data provided by Anand 1999 in previous versions of this review.
Appendices
Appendix 1. Standard search method
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
The following issues were evaluated and entered into the risk of bias table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we categorised the method used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel; and
low risk, high risk or unclear risk for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
5. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (when not all of the study’s prespecified outcomes have been reported; when one or more reported primary outcomes that were not prespecified outcomes of interest are reported incompletely and so cannot be used; when study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
6. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Appendix 3. Quality of the evidence assessment
In cases where we considered the risk of bias arising from inadequate concealment of allocation, randomised assignment, complete follow‐up or blinded outcome assessment to reduce our confidence in the effect estimates, we downgraded the quality of evidence accordingly. We evaluated consistency via similarity of point estimates, extent of overlap of confidence intervals and statistical criteria including measurement of heterogeneity (I²). We downgraded the quality of evidence when large and unexplained inconsistency was present across study results (i.e. some studies suggest important benefit and others no effect or harm without a clinical explanation). We assessed precision according to the 95% confidence interval (CI) around the pooled estimation. When trials were conducted in populations other than the target population, we downgraded the quality of evidence because of indirectness.
Data and analyses
Comparison 1. Midazolam versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intraventricular haemorrhage (any grade) | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.87, 3.24] |
| 2 Mortality | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Days of ventilation | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐0.25, 7.44] |
| 4 Days of supplemental oxygen use | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐5.30, 6.57] |
| 5 Pneumothorax | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.41, 2.84] |
| 6 Length of NICU stay (days) | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 5.44 [0.40, 10.49] |
| 7 Average NAPI scores at 36 weeks' PMA | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐14.38, 10.18] |
| 8 Poor neurological outcome by 28 days' postnatal age | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.50, 3.56] |
| 9 PIPP score during drug infusion | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐3.80 [‐5.93, ‐1.67] |
Comparison 2. Midazolam versus morphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score during drug infusion | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.66, 2.66] |
| 2 Poor neurological outcome up to 28 days' postnatal age | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.64 [1.02, 57.21] |
2.1. Analysis.
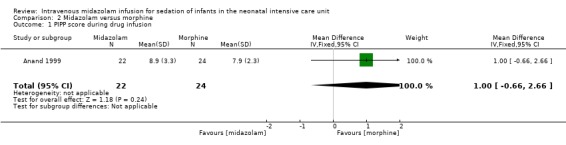
Comparison 2 Midazolam versus morphine, Outcome 1 PIPP score during drug infusion.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anand 1999.
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled pilot study (NOPAIN trial) 1. Blinding of randomisation ‐ yes 2. Blinding of intervention ‐ yes 3. Complete follow‐up ‐ yes 4. Blinding of outcome measure ‐ yes |
|
| Participants | Preterm infants from 24 to 32 weeks' gestational age at ≤ 72 hours' postnatal age who were ventilated for < 8 hours were eligible for inclusion. Exclusion criteria: major congenital anomalies, severe intrapartum asphyxia (5‐minute Apgar score ≤ 3), participation in other studies interfering with the NOPAIN trial procedures 67 infants were randomised. Demographic data: values presented as means (SDs) Midazolam group (n = 22) Gestational age: 28.6 weeks (2.5 weeks) Birth weight: 1245 grams (445 grams) Entry weight: 1224 grams (491 grams) Male: 54.5% Duration of infusion: 122.2 hours (122.1 hours) CRIB score: 5.7 (3.5) Morphine group (n = 24) Gestational age: 29.2 weeks (2.2 weeks) Birth weight: 1230 grams (475 grams) Entry weight: 1265 grams (501 grams) Male: 46.2% Duration of infusion: 81.0 hours (94.1 hours) CRIB score: 4.5 (3.1) Placebo (10% dextrose) group (n = 21) Gestational age: 28.1 weeks (2.2 weeks) Birth weight: 1049 grams (419 grams) Entry weight: 1188 grams (524 grams) Male: 57.1% Duration of infusion: 121.1 hours (120.8 hours) CRIB score: 6.6 (4.0) |
|
| Interventions | Midazolam was given as 200 microgram/kg loading dose followed by infusion of 20 microgram/kg/h, 40 microgram/kg/h or 60 microgram/kg/h for those whose gestational age was 24 to 26 weeks, 27 to 29 weeks or 30 to 33 weeks, respectively. Morphine was given as 100 microgram/kg loading dose, followed by infusion of 10 microgram/kg/h, 20 microgram/kg/h or 30 microgram/kg/h for those whose gestational age was 24 to 26 weeks, 27 to 29 weeks or 30 to 33 weeks, respectively. Additional analgesia was given, as needed, by intravenous morphine boluses at the discretion of the clinical team. The amount and frequency of additional morphine were recorded as an outcome measure. Infusions were weaned according to a set protocol. Maximum duration of study treatment was 14 days. |
|
| Outcomes | Severity of illness was measured by the CRIB score (The International Neonatal Network) and the NMI (Korner 1993). Primary outcome Incidence of adverse neurological events (neonatal death, grade III or IV IVH, PVL) Secondary outcomes Level of sedation, as measured by the COMFORT score (Ambuel 1992). Pain response to tracheal suctioning, as assessed by the PIPP (Stevens 1996). All of these scores were assessed before the start of study treatment, after 24 hours of infusion and at 10 to 12 hours after discontinuation of treatment. Incidence of pneumothorax, days of ventilatory support, continuous positive airway pressure and oxygen, length of intensive care unit and hospital stay and neurodevelopmental outcomes were measured by NAPI cluster scores (Korner 1991) at 36 weeks' corrected gestational age. | |
| Notes | Balanced randomisation by blocks stratified by each participating centre. Randomisation was performed by a 24‐hour automated telephone response system.
Reasons for non‐enrolment were provided.
Finnegan Neonatal Abstinence Scale (Finnegan 1975) was administered at 12 and 24 hours after discontinuation of study infusion, then daily. This study was supported by industry: Astra Pain Control, Södertälje, Sweden. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced randomisation was done in blocks, stratified by each participating centre via a 24‐hour automated telephone response system that was available 24 hours a day for authorised users at each site. |
| Allocation concealment (selection bias) | Low risk | Following enrolment, randomised group allocation was faxed to the participating NICU and hospital pharmacy. Only 1 pharmacist at each site had access to the codes regarding drug assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians, nurses and all NICU staff were masked to the identity of study drug. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians, nurses and all NICU staff were masked to the identity of study drug. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported on all infants enrolled in the trial. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot ascertain whether deviations from the protocol occurred. |
| Other bias | Low risk | Trial appears free of other bias. |
Arya 2001.
| Methods | Randomised, double‐blind, placebo‐controlled trial 1. Blinding of randomisation ‐ yes 2. Blinding of intervention ‐ yes 3. Complete follow‐up ‐ yes 4. Blinding of outcome measure ‐ yes Study location: neonatal unit of a tertiary hospital, New Dehli, India Study period: not stated |
|
| Participants | Newborn infants < 2000 grams needing mechanical ventilation during first week of life were eligible for inclusion. Exclusion criteria: encephalopathy, birth asphyxia, major malformation, maternal benzodiazepine use before delivery 33 infants were randomised. 3 in each group did not complete the first 24 hours of the study; 4 in each group did not complete the first 48 hours of the study Reasons for withdrawal: death (13) and extubation (1) Demographic data: values presented as means (SDs), unless indicated Midazolam group (n = 17) Gestational age: 31.5 weeks (2.4 weeks) Birth weight: 1263 grams (326 grams) Male: 58.8% PIP at baseline: 19.9 cmH2O (5.5 cmH2O) MAP at baseline: 8.7 cmH2O (3.2 cmH2O) Median (range) OI at baseline: 5 (1 to 22) Median (range) AaDO2 at baseline: 205 (13 to 619) Placebo group (n = 16) Gestational age: 32.3 weeks (2.2 weeks) Birth weight: 1337 grams (297 grams) Male: 75.0% PIP at baseline: 21.2 cmH2O (7.1 cmH2O) MAP at baseline: 9.8 cmH2O (4.3 cmH2O) Median (range) OI at baseline: 5 (2 to 55) Median (range) AaDO2 at baseline: 234.5 (59 to 553) |
|
| Interventions | Midazolam was given as 200 microgram/kg loading dose followed by infusion of 60 microgram/kg/h. Duration of infusion and method of weaning were not specified. Infants in both groups received morphine infusion at 10 microgram/kg/h during the study. Study consisted of 48 hours of infusion. | |
| Outcomes |
Primary outcome Adequacy of sedation was measured every 6 hours by a 5‐item behavioural scale (facial expression, sucking, continuous motor activity, excitability and response to stimulation, excessive flexing) (Barrier 1989); physiological measures of sedation level included mean daily values of heart rate and blood pressure. Secondary outcomes Intracranial haemorrhage and epileptiform movement, haemodynamic instability (hypotension, tachycardia, oliguria) with the need for volume expansion or vasoactive drugs, or both, ventilation requirement (peak inspiratory and PEEP, MAP and rate), days of ventilation, incidence of pulmonary air leak |
|
| Notes | Randomisation was performed with opaque envelopes containing computer‐generated random numbers. Ranbaxy Laboratoried Ltd provided drugs and placebo for the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were placed in opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes contained computer‐generated random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was manufactured with colour and vial volume similar to those of study drug. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sedation score was determined by an investigator who was blinded to allocation of infants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients each in midazolam and placebo groups did not complete 24 hours of follow‐up after enrolment. Additionally, 4 patients in the midazolam group and 4 in the placebo group did not complete 48 hours of follow‐up. Reasons for withdrawal included death (6 in midazolam group and 7 in placebo group) and cessation of mechanical ventilation (1 in midazolam group). |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot ascertain whether deviations from the protocol occurred. |
| Other bias | Low risk | Trial appears free of other bias. |
Jacqz‐Aigrain 1994.
| Methods | Randomised, double‐blind, placebo‐controlled trial
Randomisation was stratified by 2 gestational age groups (< 33 weeks and ≥ 33 weeks). 1. Blinding of randomisation ‐ cannot determine 2. Blinding of intervention ‐ yes 3. Complete follow‐up ‐ yes 4. Blinding of outcome measure ‐ yes |
|
| Participants | Newborn infants ≤ 48 hours of age who required intubation and ventilation for respiratory distress syndrome were eligible for inclusion. Exclusion criteria: previous exposure to benzodiazepines (maternal/infant), congenital anomalies, major neurological abnormalities, low Apgar score at 5 minutes (score not defined by study authors) 48 preterm infants were enrolled. 1 who received midazolam previously and 1 with 5‐minute Apgar score of 0 were excluded. 46 infants (25 at ≤ 33 weeks', 21 at > 33 weeks' gestational age) were included in the analysis. Demographic data: values presented as means (SDs) Midazolam group (n = 24) Gestational age: 32.1 weeks (2.8 weeks) Birth weight: 1820 grams (647 grams) Male: 58.3% 5‐Minute Apgar score: 9.0 (1.2) MAP at enrolment: 12 mmHg (2 mmHg) FiO2 at enrolment: 49% (13%) Duration of infusion: 78.7 hours (30.9 hours) Placebo group (n = 22) Gestational age: 32.8 weeks (2.6 weeks) Birth weight: 2000 grams (548 grams) Male: 59.1% 5‐Minute Apgar score: 8.1 (2.3) MAP at enrolment: 13 mmHg (2 mmHg) FiO2 at enrolment: 51% (16%) |
|
| Interventions | 24 infants received midazolam infusion. For infants ≥ 33 weeks: 60 microgram/kg/h for up to 5 days For infants < 33 weeks: 60 microgram/kg/h for 1 day, then 30 microgram/kg/h for up to 5 days Infusion could have been stopped after 48 hours if no longer required. 22 infants received a manufactured placebo. Additional sedation with fentanyl or use of muscle relaxant was permitted; the study protocol was interrupted in such cases, but data from these infants were used in the analysis. | |
| Outcomes |
Primary outcome Adequacy of sedation was measured 4 times per day (twice by nurses and twice by physicians) on a 5‐item behavioural scale (facial expression, sucking, spontaneous motor activity, excitability and response to stimulation, excessive flexing); physiological measures of sedation level included mean daily values of hourly heart rate, systolic and diastolic blood pressures. Secondary outcomes Incidence of intracranial haemorrhage and epileptiform movement; haemodynamic instability (need for fluid, albumin, vasoactive drugs); ventilation requirement (PIP, PEEP, MAP); days of ventilation; days of supplemental oxygen; incidence of pneumothorax and pulmonary interstitial emphysema; total days of NICU stay Serum concentrations of midazolam were monitored before and 24 and 48 hours after the start of infusion and at the end of treatment. |
|
| Notes | Randomisation was performed by selecting the next envelope in 2 boxes, 1 for each gestational age stratum.
Protocol for weaning of study drug was not described. Midazolam and placebo were supplied by Laboratories Roche, Paris, France. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided. |
| Allocation concealment (selection bias) | Low risk | Randomisation to treatment or placebo was stratified by gestational age at birth (< 33 weeks and ≥ 33 weeks). The intensive care unit had 2 boxes containing individual treatments for 5 days. When an infant met all entry criteria, investigators used the next envelope in the appropriate box. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medical staff, radiologist and pharmacologist interpreting the study were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medical staff, radiologist and pharmacologist interpreting the study were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all enrolled infants except 1; 1 infant was immediately excluded because he had previously received midazolam, and 1 was excluded because of an Apgar score of 0 at 5 minutes of age. Data for the remaining 46 infants were analysed. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot ascertain whether deviations from the protocol occurred. |
| Other bias | Low risk | Trial appears free of other bias. |
AaDO2: alveolar‐arterial oxygen gradient; CRIB: Clinical Risk Index for Babies; FiO2: fraction of inspired oxygen concentration; IVH: intraventricular haemorrhage; MAP: mean airway pressure; NAPI: Neurobehavioral Assessment of the Premature Infant; NICU: neonatal intensive care unit; NMI: Neonatal Medical Index; NOPAIN: Neonatal Outcome and Prolonged Analgesia In Neonates; OI: oxygenation index; PEEP: positive end‐expiratory pressures; PIP: peak inspiratory pressure; PIPP: Premature Infant Pain Profile; PVL: periventricular leukomalacia; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kawakami 1998 | A randomised, controlled trial comparing intravenous lidocaine (1.5 mg/kg) vs intravenous midazolam (0.1 mg/kg) in addition to lidocaine for anaesthetic induction in 27 neonates undergoing surgery. We excluded this study because midazolam was given as a single bolus dose and was not used as a sedative. |
| McCarver‐May 1996 | A randomised cross‐over trial comparing intravenous midazolam (0.2 mg/kg) vs oral chloral hydrate (75 mg/kg) for sedation during neuroimaging studies in 7 full‐term neonates. We excluded this study because midazolam was given as a single bolus dose. |
| Parkinson 1997 | A randomised, controlled trial comparing oral chloral hydrate (25 mg/kg to 50 mg/kg) vs promethazine (0.5 mg/kg to 1.0 mg/kg) vs an intravenous midazolam infusion (50 microgram/kg/h to 300 microgram/kg/h) for sedation in critically ill children. We excluded this study because the population studied included children from 1 day to 15 years of age, and because we could not extract from the study data for neonates. |
Differences between protocol and review
We added the method and the plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol nor in the last published review.
Contributions of authors
E Ng.
Development and writing of the protocol.
Literature search and identification of trials for inclusion.
Evaluation of methodological quality of included trials.
Abstraction of data independent of co‐review author.
Entry of data into RevMan.
Writing of Results section.
Writing of Discussion section.
Writing of all updates.
A Taddio.
Development of the protocol.
Literature search and identification of trials for inclusion.
Evaluation of methodological quality of included trials.
Abstraction of data independent of co‐review author.
Verification of data entered into RevMan.
Revision of the 2016 update.
A Ohlsson.
Development of the protocol.
Literature search and identification of trials for inclusion.
Verification of data entered into RevMan.
Writing the text of the update of the review.
Development of 'Summary of findings' tables.
Revision of risk of bias tables in 2016.
Revision of the final review and of 2009, 2012 and 2016 updates.
All review authors participated in completion of the 2009 and 2016 updates. However, only two review authors (E Ng, A Ohlsson) participated in completion of the 2012 update.
Sources of support
Internal sources
Sunnybrook Health Sciences Centre, Toronto, Canada.
The Hospital for Sick Children, Toronto, Canada.
Mount Sinai Hospital, Toronto, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
E Ng ‐ no conflicts of interest to declare.
A Taddio ‐ no conflicts of interest to declare.
A Ohlsson ‐ no conflicts of interest to declare.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Anand 1999 {published data only}
- Anand KJS, McIntosh N, Lagercrantz H, Pelausa E, Young TE, Vasa R. Analgesia and sedation in preterm neonates who require ventilatory support ‐ results from the NOPAIN trial. Archives of Pediatrics and Adolescent Medicine 1999;153:331‐8. [DOI] [PubMed] [Google Scholar]
Arya 2001 {published data only}
- Arya V, Ramji S. Midazolam sedation in mechanically ventilated newborns: a double blind randomized placebo controlled trial. Indian Pediatrics 2001;38:967‐72. [PubMed] [Google Scholar]
Jacqz‐Aigrain 1994 {published data only}
- Jacqz‐Aigrain E, Daoud P, Burtin P, Desplanques L, Beaufils F. Placebo‐controlled trial of midazolam sedation in mechanically ventilated newborn babies. The Lancet 1994;344:646‐50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kawakami 1998 {published data only}
- Kawakami K, Ohata J, Kadosaki M, Saito I, Iwasawa K, Mitono H. Midazolam for anesthetic induction in neonates. Masui‐Japanese Journal of Anesthesiology 1998;47:570‐5. [PubMed] [Google Scholar]
McCarver‐May 1996 {published data only}
- McCarver‐May DG, Kang J, Aouthmany M, Elton R, Mowery JL, Slovis TL, et al. Comparison of chloral hydrate and midazolam for sedation of neonates for neuroimaging studies. Journal of Pediatrics 1996;128:573‐6. [DOI] [PubMed] [Google Scholar]
Parkinson 1997 {published data only}
- Parkinson L, Hughes J, Gill A, Billingham I, Ratcliffe J, Choonara I. A randomized controlled trial of sedation in the critically ill. Paediatric Anaesthesia 1997;7:405‐10. [DOI] [PubMed] [Google Scholar]
Additional references
AAP/CPS 2000
- American Academy of Pediatrics, Committee on Fetus and Newborn, Committee on Drugs, Sections on Anesthesiology, Section on Surgery, Canadian Paediatric Society, Fetus and Newborn Committee. Prevention and management of pain and stress in the neonate. Pediatrics 2000;105:454‐61. [PubMed] [Google Scholar]
Adams 1997
- Adams MM, Hahn JS, Benitz WE. A series of neonatal patients with paradoxical seizure‐like reactions to bolus intravenous injections of midazolam. Pediatric Research 1997;41:134A. [Google Scholar]
Ambuel 1992
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. Journal of Pediatric Psychology 1992;17:95‐109. [DOI] [PubMed] [Google Scholar]
Anand 1987
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. New England Journal of Medicine 1987;317:1321‐9. [DOI] [PubMed] [Google Scholar]
Anand 1992
- Anand KJ, Hickey PR. Halothane‐morphine compared with high‐dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. New England Journal of Medicine 1992;326:1‐9. [DOI] [PubMed] [Google Scholar]
Barrier 1989
- Barrier G, Attia J, Mayer MN, Amiel‐Tison C, Shnider SM. Measurement of post‐operative pain and narcotic administration in infants using a new clinical scoring system. Intensive Care Medicine 1989;15(Suppl 1):S37‐9. [DOI] [PubMed] [Google Scholar]
Bergman 1991
- Bergman I, Steeves M, Burckart G, Thompson A. Reversible neurologic abnormalities associated with prolonged intravenous midazolam and fentanyl administration. Journal of Pediatrics 1991;119:644‐9. [DOI] [PubMed] [Google Scholar]
Burtin 1991
- Burtin P, Daoud P, Jacqz‐Aigrain E, Mussat P, Moriette G. Hypotension with midazolam and fentanyl in the newborn. The Lancet 1991;337:1545‐6. [DOI] [PubMed] [Google Scholar]
Carbajal 2015
- Carbajal R, Eriksson M, Courtois E, Boyle E, Avila‐Alvarez A, Andersen RD, et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. The Lancet Respiriratory Medicine 2015;3(10):796–812. [DOI] [PubMed] [Google Scholar]
Collins 1991
- Collins S, Carter JA. Resedation after bolus administration of midazolam to an infant and its reversal by flumazenil. Anaesthesia 1991;46:471‐2. [DOI] [PubMed] [Google Scholar]
Craig 1984
- Craig KD, McMahon RJ, Morison JD, Zaskow C. Developmental changes in infant pain expression during immunization injections. Social Science and Medicine 1984;19(12):1331‐7. [DOI] [PubMed] [Google Scholar]
Finnegan 1975
- Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addictive Diseases 1975;2(1‐2):141‐58. [PubMed] [Google Scholar]
Greenough 1983
- Greenough A, Morley C, Davis J. Interaction of spontaneous respiration with artificial ventilation in preterm babies. Journal of Pediatrics 1983;103:769‐73. [DOI] [PubMed] [Google Scholar]
Harte 1997
- Harte GJ, Gray PH, Lee TC, Steer PA, Charles BG. Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. Journal of Paediatrics and Child Health 1997;33:335‐8. [DOI] [PubMed] [Google Scholar]
Hartwig 1991
- Hartwig S, Roth B, Theisohn M. Clinical experience with continuous intravenous sedation using midazolam and fentanyl in the paediatric intensive care unit. European Journal of Pediatrics 1991;150:784‐8. [DOI] [PubMed] [Google Scholar]
Hebebrand 1988
- Hebebrand J, Hofmann D, Reichelt R, Schnarr S, Knapp M, Propping P, et al. Early ontogeny of the central benzodiazepine receptor in human embryos and fetuses. Life Sciences 1988;43:2127‐36. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jacqz‐Aigrain 1992
- Jacqz‐Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. European Journal of Clinical Pharmacology 1992;42:329‐32. [DOI] [PubMed] [Google Scholar]
Jacqz‐Aigrain 1996
- Jacqz‐Aigrain E, Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clinical Pharmacokinetics 1996;31:423‐43. [DOI] [PubMed] [Google Scholar]
Koch 2008
- Koch SC, Fitzgerald M, Hathway GJ. Midazolam potentiates nociceptive behavior, sensitizes cutaneous reflexes, and is devoid of sedative action in neonatal rats. Anesthesiology 2008;108(1):122–9. [DOI] [PubMed] [Google Scholar]
Korner 1991
- Korner AF, Constantinou J, Dimiceli S, Brown BW, Thom VA. Establishing the reliability and developmental validity of a neurobehavioural assessment for preterm infants: a methodological process. Child Development 1991;62:1200‐8. [PubMed] [Google Scholar]
Korner 1993
- Korner AF, Stevenson DK, Kraemer HC, Spiker D, Scott DT, Constantinou J, et al. Prediction of the development of low birth weight preterm infants by a new neonatal medical index. Journal of Developmental and Behavioral Pediatrics 1993;14:106‐11. [PubMed] [Google Scholar]
Lee 1999
- Lee TC, Charles BG, Harte GJ, Gray PH, Steer PA, Flenady VJ. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation. Anesthesiology 1999;90:451‐7. [DOI] [PubMed] [Google Scholar]
Magny 1994
- Magny JF, d'Allest AM, Nedelcoux H, Zupan V, Dehan M. Midazolam and myoclonus in neonate. European Journal of Pediatrics 1994;153:389‐92. [DOI] [PubMed] [Google Scholar]
Marx 1994
- Marx CM, Smith PG, Lowrie LH, Hamlett KW, Ambuel B, Yamashita TS, et al. Optimal sedation of mechanically ventilated pediatric critical care patients. Critical Care Medicine 1994;22(1):163‐70. [DOI] [PubMed] [Google Scholar]
Modanlou 1997
- Modanlou HD, Beharry K. Mechanism of midazolam‐induced hypotension: possible role of prostanoids and calcium. Pediatric Research 1997;41:57A. [Google Scholar]
Ng 2002
- Ng E, Klinger G, Shah V, Taddio A. Safety of benzodiazepines in newborns. Annals of Pharmacotherapy 2002;36:1150‐5. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with weights less than 1500 grams. Journal of Pediatrics 1978;92:529‐34. [DOI] [PubMed] [Google Scholar]
Pellier 1999
- Pellier I, Monrigal JP, Moine P, Rod B, Rialland X, Granry JC. Use of intravenous ketamine‐midazolam association for pain procedure in children with cancer. A prospective study. Paediatric Anaesthesia 1999;9:61‐8. [PubMed] [Google Scholar]
Perlman 1985
- Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood‐flow velocity in preterm infants with respiratory distress syndrome. New England Journal of Medicine 1985;312:1353‐7. [DOI] [PubMed] [Google Scholar]
Quinn 1993
- Quinn MW, Wild J, Dean HG, Rushforth JA, Puntis JW, Levene MI. Randomised double‐blind controlled trial of effect of morphine on catecholamine concentrations in ventilated pre‐term babies. The Lancet 1993;342:324‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robieux 1991
- Robieaux I, Kumar R, Radhakrishnan S, Koren G. Assessing pain and analgesia with a lidocaine‐prilocaine emulsion in infants and toddlers during venipuncture. Journal of Pediatrics 1991;118(6):971‐3. [DOI] [PubMed] [Google Scholar]
Rosen 1991
- Rosen DA, Rosen KR. Midazolam for sedation in the paediatric intensive care unit. Intensive Care Medicine 1991;17:S15‐9. [DOI] [PubMed] [Google Scholar]
Schunemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Grade Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook. Updated 2013.
Snider 2005
- Snider L, Tremblay S, Limperopoulos C, Majnemer A, Filion F, Johnston C. Construct validity of the neurobehavioral assessment of preterm infants. Physical & Occupational Therapy in Pediatrics 2005;25(3):81‐95. [PubMed] [Google Scholar]
Stenhammar 1994
- Stenhammar L, Högberg L, Lewander P, Nordvall M, Tjellström B. Intravenous midazolam in small bowel biopsy. Archives of Disease in Childhood 1994;71:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 1996
- Stevens BJ, Johnson CC, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clinical Journal of Pain 1996;12:13‐22. [DOI] [PubMed] [Google Scholar]
The International Neonatal Network
- The International Neonatal Network. The CRIB (Clinical Risk Index for Babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The Lancet 1993;342:193‐8. [PubMed] [Google Scholar]
van den Anker 1992
- Anker JN, Sauer PJJ. The use of midazolam in the preterm neonate. European Journal of Pediatrics 1992;151:152. [DOI] [PubMed] [Google Scholar]
van Straaten 1992
- Straaten HLM, Rademaker CMA, Vries LS. Comparison of the effect of midazolam or vecuronium on blood pressure and cerebral blood flow velocity in the premature newborn. Developmental Pharmacology and Therapeutics 1992;19:191‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ng 2000
- Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002052] [DOI] [PubMed] [Google Scholar]
Ng 2003
- Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002052] [DOI] [PubMed] [Google Scholar]


