Abstract
Background
To minimise the rate of recurrent prolapse after traditional native tissue repair (anterior colporrhaphy), clinicians have utilised a variety of surgical techniques.
Objectives
To determine the safety and effectiveness of surgery for anterior compartment prolapse.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In Process (23 August 2016), handsearched journals and conference proceedings (15 February 2016) and searched trial registers (1 August 2016).
Selection criteria
Randomised controlled trials (RCTs) that examined surgical operations for anterior compartment prolapse.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias and extracted data. Primary outcomes were awareness of prolapse, repeat surgery and recurrent prolapse on examination.
Main results
We included 33 trials (3332 women). The quality of evidence ranged from very low to moderate. Limitations were risk of bias and imprecision. We have summarised results for the main comparisons.
Native tissue versus biological graft
Awareness of prolapse: Evidence suggested few or no differences between groups (risk ratio (RR) 0.98, 95% confidence interval (CI) 0.52 to 1.82; five RCTs; 552 women; I2 = 39%; low‐quality evidence), indicating that if 12% of women were aware of prolapse after biological graft, 7% to 23% would be aware after native tissue repair.
Repeat surgery for prolapse: Results showed no probable differences between groups (RR 1.02, 95% CI 0.53 to 1.97; seven RCTs; 650 women; I2 = 0%; moderate‐quality evidence), indicating that if 4% of women required repeat surgery after biological graft, 2% to 9% would do so after native tissue repair.
Recurrent anterior compartment prolapse: Native tissue repair probably increased the risk of recurrence (RR 1.32, 95% CI 1.06 to 1.65; eight RCTs; 701 women; I2 = 26%; moderate‐quality evidence), indicating that if 26% of women had recurrent prolapse after biological graft, 27% to 42% would have recurrence after native tissue repair.
Stress urinary incontinence (SUI): Results showed no probable differences between groups (RR 1.44, 95% CI 0.79 to 2.64; two RCTs; 218 women; I2 = 0%; moderate‐quality evidence).
Dyspareunia: Evidence suggested few or no differences between groups (RR 0.87, 95% CI 0.39 to 1.93; two RCTs; 151 women; I2 = 0%; low‐quality evidence).
Native tissue versus polypropylene mesh
Awareness of prolapse: This was probably more likely after native tissue repair (RR 1.77, 95% CI 1.37 to 2.28; nine RCTs; 1133 women; I2 = 0%; moderate‐quality evidence), suggesting that if 13% of women were aware of prolapse after mesh repair, 18% to 30% would be aware of prolapse after native tissue repair.
Repeat surgery for prolapse: This was probably more likely after native tissue repair (RR 2.03, 95% CI 1.15 to 3.58; 12 RCTs; 1629 women; I2 = 39%; moderate‐quality evidence), suggesting that if 2% of women needed repeat surgery after mesh repair, 2% to 7% would do so after native tissue repair.
Recurrent anterior compartment prolapse: This was probably more likely after native tissue repair (RR 3.01, 95% CI 2.52 to 3.60; 16 RCTs; 1976 women; I2 = 39%; moderate‐quality evidence), suggesting that if recurrent prolapse occurred in 13% of women after mesh repair, 32% to 45% would have recurrence after native tissue repair.
Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome): This was probably less likely after native tissue repair (RR 0.59, 95% CI 0.41 to 0.83; 12 RCTs; 1527 women; I2 = 45%; moderate‐quality evidence), suggesting that if 10% of women require repeat surgery after polypropylene mesh repair, 4% to 8% would do so after native tissue repair.
De novo SUI: Evidence suggested few or no differences between groups (RR 0.67, 95% CI 0.44 to 1.01; six RCTs; 957 women; I2 = 26%; low‐quality evidence). No evidence suggested a difference in rates of repeat surgery for SUI.
Dyspareunia (de novo): Evidence suggested few or no differences between groups (RR 0.54, 95% CI 0.27 to 1.06; eight RCTs; n = 583; I2 = 0%; low‐quality evidence).
Native tissue versus absorbable mesh
Awareness of prolapse: It is unclear whether results showed any differences between groups (RR 0.95, 95% CI 0.70 to 1.31; one RCT; n = 54; very low‐quality evidence),
Repeat surgery for prolapse: It is unclear whether results showed any differences between groups (RR 2.13, 95% CI 0.42 to 10.82; one RCT; n = 66; very low‐quality evidence).
Recurrent anterior compartment prolapse: This is probably more likely after native tissue repair (RR 1.50, 95% CI 1.09 to 2.06; three RCTs; n = 268; I2 = 0%; moderate‐quality evidence), suggesting that if 27% have recurrent prolapse after mesh repair, 29% to 55% would have recurrent prolapse after native tissue repair.
SUI: It is unclear whether results showed any differences between groups (RR 0.72, 95% CI 0.50 to 1.05; one RCT; n = 49; very low‐quality evidence).
Dyspareunia: No data were reported.
Authors' conclusions
Biological graft repair or absorbable mesh provides minimal advantage compared with native tissue repair.
Native tissue repair was associated with increased awareness of prolapse and increased risk of repeat surgery for prolapse and recurrence of anterior compartment prolapse compared with polypropylene mesh repair. However, native tissue repair was associated with reduced risk of de novo SUI, reduced bladder injury, and reduced rates of repeat surgery for prolapse, stress urinary incontinence and mesh exposure (composite outcome).
Current evidence does not support the use of mesh repair compared with native tissue repair for anterior compartment prolapse owing to increased morbidity.
Many transvaginal polypropylene meshes have been voluntarily removed from the market, and newer light‐weight transvaginal meshes that are available have not been assessed by RCTs. Clinicans and women should be cautious when utilising these products, as their safety and efficacy have not been established.
Keywords: Female, Humans, Cystocele, Cystocele/surgery, Gynecologic Surgical Procedures, Gynecologic Surgical Procedures/methods, Pelvic Organ Prolapse, Pelvic Organ Prolapse/prevention & control, Pelvic Organ Prolapse/surgery, Randomized Controlled Trials as Topic, Rectal Prolapse, Rectal Prolapse/surgery, Secondary Prevention, Surgical Mesh, Suture Techniques, Urinary Incontinence, Urinary Incontinence/surgery, Uterine Prolapse, Uterine Prolapse/surgery
Plain language summary
Surgical management of pelvic organ prolapse in women
Review question
To determine the safety and effectiveness of surgery for anterior vaginal wall prolapse. Background
Pelvic organ prolapse occurs in up to 50% of women who have given birth. This can happen at different sites within the vagina; prolapse of the anterior compartment is most difficult to repair, and rates of recurrence are higher than at other vaginal sites. This challenge has resulted in the use of a variety of surgical techniques and grafts to improve outcomes of anterior compartment prolapse surgery. We aimed to evaluate surgical interventions for anterior compartment prolapse.
Study characteristics
Cochrane authors included in this review 33 randomised controlled trials (RCTs) evaluating 3332 surgeries to compare traditional native tissue anterior repair versus biological grafts (eight trials), absorbable mesh (three trials), permanent (polypropylene) mesh (16 trials) and abdominal paravaginal repair (two trials). Four trials compared a transvaginal graft versus another transvaginal graft, and four trials evaluated native tissue repair of anterior and/or posterior compartments of the vagina versus graft repair. Evidence is current to 23 August 2016.
Key results
Biological graft repair or absorbable mesh provides minimal advantage compared with native tissue repair. Results showed no evidence of differences between biological graft and native tissue repair in rates of awareness of prolapse or repeat surgery for prolapse. However, the recurrent anterior prolapse rate was higher after native tissue repair than after any biological graft. This suggests that if awareness of prolapse after biological graft occurs in 12% of women, 7% to 23% would be aware of prolapse after native tissue repair.
Permanent mesh resulted in lower rates of awareness of prolapse, recurrent anterior wall prolapse and repeat surgery for prolapse compared with native tissue repair. However, native tissue repair was associated with reduced risk of new stress urinary incontinence. Other benefits of native tissue repair included reduced bladder injury and reduced rates of repeat surgery for prolapse, stress urinary incontinence and mesh exposure (as a combined outcome).
Quality of the evidence
The quality of the data related to traditional native tissue anterior repair compared with both biological grafts and permanent mesh is generally low to moderate. The main limitations were incomplete reporting of study methods including allocation concealment and bias and imprecision in data outcomes. Data related to the efficacy of absorbable mesh are probably incomplete.
Summary of findings
Summary of findings for the main comparison. Anterior prolapse repair: native tissue versus biological graft in women with anterior compartment pelvic organ prolapse.
| Anterior prolapse repair: native tissue versus biological graft in women with anterior compartment pelvic organ prolapse | ||||||
|
Patient or population: women with anterior compartment pelvic organ prolapse
Setting: hospital departments of obstetrics and gynaecology
Intervention: native tissue (anterior repair, colporrhaphy) Comparison: biological graft | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Biological graft | Native tissue | |||||
| Awareness of prolapse (1‐2 years) | 124 per 1000 | 122 per 1000 (65 to 226) | RR 0.98 (0.52 to 1.82) | 552 (5 studies) | ⊕⊕⊕⊝ Lowa,b | |
| Repeat surgery for prolapse (1‐2 years) | 44 per 1000 | 45 per 1000 (23 to 86) | RR 1.02 (0.53 to 1.97) | 650 (7 studies) | ⊕⊕⊕⊝ Moderateb | |
| Recurrent anterior compartment prolapse (1‐2 years) | 257 per 1000 | 340 per 1000 (273 to 424) | RR 1.32 (1.06 to 1.65) | 701 (8 studies) | ⊕⊕⊝⊝ Moderatec | |
| Stress urinary incontinence (1‐2 years) | 130 per 1000 | 187 per 1000 (102 to 342) | RR 1.44 (0.79 to 2.64) | 218 (2 studies) | ⊕⊕⊕⊕ Moderateb | Repeat surgery for SUI was not reported by any studies |
| Dyspareunia (1‐2 years) | 149 per 1000 | 129 per 1000 (58 to 287) | RR 0.87 (0.39 to 1.93) | 151 (2 studies) | ⊕⊕⊕⊝ Lowb,d | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI = confidence interval; RR = risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aRisk of bias: allocation concealment not reported in 2/5, downgraded one level.
bSerious imprecision: wide confidence interval, greater than 25% increase in RR, downgraded one level. cDowngraded one level for serious risk of bias: five of eight trials did not report blinded outcome assessment, downgraded one level. dRisk of bias: blinded outcome assessment unreported in one of two trials, downgraded one level.
Summary of findings 2. Anterior prolapse repair: native tissue versus polypropylene mesh for women with anterior compartment pelvic organ prolapse.
| Anterior prolapse repair: native tissue versus polypropylene mesh for women with anterior compartment pelvic organ prolapse | ||||||
|
Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology Intervention: native tissue (anterior repair, colporrhaphy) Comparison: polypropylene mesh | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polypropylene mesh repair | Native tissue repair | |||||
| Awareness of prolapse (1‐3 years) | 130 per 1000 | 230 per 1000 (178 to 297) | RR 1.77 (1.37 to 2.28) | 1133 (9 studies) | ⊕⊕⊕⊝ Moderate1 | |
| Repeat surgery for prolapse (1‐3 years) | 18 per 1000 | 37 per 1000 (21 to 66) | RR 2.03 (1.15 to 3.58) | 1629 (12 studies) | ⊕⊕⊕⊝ Moderate2 | |
| Repeat surgery for stress urinary incontinence (1‐2 years) | 29 per 1000 |
35 per 1000 (17 to 69) |
RR 1.19 (0.60 to 2.36) |
881 (5 studies) |
Low3,4 | |
| Recurrent anterior compartment prolapse (1‐3 years) | 126 per 1000 | 379 per 1000 (317 to 453) | RR 3.01 (2.52 to 3.60) | 1976 (16 studies) | ⊕⊕⊝⊝ Low5,6 | |
| Stress urinary incontinence (de novo) (1‐3 years) | 102 per 1000 | 69 per 1000 (45 to 103) | RR 0.67 (0.44 to 1.01) | 957 (6 studies) | ⊕⊕⊝⊝ Low4,7 | |
| Dyspareunia (de novo) (1‐2 years) | 72 per 1000 |
39 per 1000 (19 to 76) |
RR 0.54 (0.27 to 1.06) |
583 (8 studies) |
⊕⊕⊕⊝ Low4,7 | |
| Repeat surgery for prolapse, SUI or mesh exposure (1‐3 years) | 97 per 1000 | 54 per 1000 |
RR 0.59 (0.41 to 0.83) |
1527 (12 studies) |
⊕⊕⊕⊝ Moderate2 | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI = confidence interval; RR = risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias: allocation concealment not reported in 4/9, downgraded one level. 2Risk of bias: allocation concealment not reported in 6/12, downgraded one level.
3Risk of bias: allocation concealment not reported in 2/5: downgraded one level.
4Serious imprecision: wide CI with lower RR (0.25), downgraded one level.
5Risk of bias: 11/15 trials did not report blinded outcome assessment, downgraded one level.
6Risk of bias: allocation concealment not reported in 7/15, downgraded one level.
7Risk of bias: poor methodological reporting of allocation concealment and/or blinding, downgraded one level.
Summary of findings 3. Anterior prolapse repair: native tissue versus absorbable mesh for women with anterior and/or posterior compartment pelvic organ prolapse.
| Anterior prolapse repair: native tissue repair versus absorbable mesh for women with anterior and/or posterior compartment pelvic organ prolapse | ||||||
|
Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology Intervention: native tissue repair (anterior colporrhaphy) Comparison: absorbable mesh |
||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Absorbable mesh | Native tissue repair | |||||
| Awareness of prolapse (2 years) | 760 per 1000 | 722 per 1000 (532 to 996) | RR 0.95 (0.70 to 1.31) | 54 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | |
|
Repeat surgery for prolapse (stage 2 or greater) at 2 years |
59 per 1000 | 125 per 1000 (25 to 636) | RR 2.13 (0.42 to 10.82) | 66 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Recurrent anterior compartment prolapse (3 months to 2 years) | 267 per 1000 | 401 per 1000 (291 to 550) | RR 1.50 (1.09 to 2.06) | 268 (3 studies) | ⊕⊕⊝⊝ Moderated | |
| De novo dyspareunia | Not reported in the included studies | |||||
| Stress urinary incontinence (2 years) | 818 per 1000 | 589 per 1000 (409 to 859) | RR 0.72 (0.50 to 1.05) | 49 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | Repeat surgery for SUI was not reported by any studies |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI = confidence interval; RR = risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aRisk of bias: at 2 years, 18% lost to review, downgraded one level. bSerious imprecision: single small trial with confidence interval compatible with benefit in either arm or no effect. Low event rate, downgraded one level. cPublication bias: evidence based on a single small trial, downgraded one level.
dRisk of bias: blinded outcome assessment not reported in 2/3 trials, and high attrition in one, downgraded one level.
Background
Description of the condition
Pelvic organ prolapse is common and is seen on examination in 40% to 60% of parous women (Handa 2004; Hendrix 2002). The annual aggregated rate of associated surgery in the United States is in the range of 10 to 30 per 10,000 women (Brubaker 2002). The anterior compartment of the vagina is the vaginal site most commonly affected by prolapse and is the most difficult to repair.
Pelvic organ prolapse is the descent of one or more of the pelvic organs (uterus, vagina, bladder or bowel). Different types of prolapse include:
upper vaginal prolapse (i.e. uterus, vaginal vault (after hysterectomy when the top of the vagina drops down));
anterior vaginal wall prolapse (i.e. cystocele (bladder descends), urethrocele (urethra descends), paravaginal defect (pelvic fascia defect)); and
posterior vaginal wall prolapse (i.e. enterocele (small bowel descends), rectocele (rectum descends), perineal deficiency).
A woman can present with prolapse of one or more of these sites.
The aetiology of pelvic organ prolapse is complex and multi‐factorial. Possible risk factors include pregnancy, childbirth, congenital or acquired connective tissue abnormalities, denervation or weakness of the pelvic floor, ageing, hysterectomy, menopause and factors associated with chronically raised intra‐abdominal pressure (Bump 1998; Gill 1998; MacLennan 2000).
Women with anterior compartment prolapse commonly have a variety of pelvic floor symptoms, only some of which are directly related to the prolapse. Generalised symptoms of prolapse include pelvic heaviness; a bulge, lump or protrusion coming down from the vagina; a dragging sensation in the vagina; and backache. Symptoms of bladder, bowel or sexual dysfunction are frequently present. For example, women may need to reduce the prolapse by using their fingers to push the prolapse up to aid urinary voiding or defecation. These symptoms may be directly related to the prolapsed organ, for example, poor urinary stream when a cystocele is present or obstructed defecation when a rectocele is present. Symptoms may also be independent of the prolapse, for example, symptoms of overactive bladder (urinary urgency) or urinary stress incontinence when a cystocele is present.
Description of the intervention
Treatment of a woman with prolapse depends on the severity of the prolapse, its symptoms, the woman's general health and surgeon preference and capabilities. Options for treatment include conservative, mechanical and surgical interventions.
Generally, conservative or mechanical treatments are considered for women with a mild degree of prolapse, those who wish to have more children, frail women and those unwilling to undergo surgery. Conservative and mechanical interventions have been considered in separate Cochrane reviews (Adams 2004; Hagen 2011). No good evidence was available to guide management in either of these reviews.
A wide variety of abdominal and vaginal surgical techniques are available for the treatment of patients with prolapse (Appendix 1). The most common and traditional procedure is the anterior repair (colporrhaphy) for anterior vaginal wall prolapse, which is frequently performed in conjunction with other interventions for prolapse and/or urinary stress incontinence. Together, anterior and posterior compartment surgeries account for more than 90% of all prolapse operations (Olsen 1997). Two main approaches can be used.
Vaginal approaches include vaginal hysterectomy, anterior or posterior vaginal wall repair (colporrhaphy), McCall culdoplasty, Manchester repair (amputation of the cervix with uterus suspension to the cardinal ligaments), prespinous and sacrospinous colpopexy, enterocele ligation, paravaginal repair, Le Fortes procedure and perineal reconstruction.
Abdominal approaches include hysterectomy, sacral colpopexy, paravaginal repair, vault suspending and uterosacral ligament plication, enterocele ligation and posterior vaginal wall repair. Abdominal surgery can be performed through an open incision or keyhole incisions via the laparoscope or robot.
The current review considers all surgical procedures for women with anterior compartment pelvic organ prolapse.
A combination of some of the above‐mentioned procedures may be employed concomitantly at the time of anterior compartment prolapse surgery. In addition to the variety of prolapse operations that can be performed, the surgeon must choose whether to use absorbable sutures such as polyglycolic acid‐based materials (e.g. polyglactin), delayed‐absorption sutures such as polydioxanone or non‐absorbable sutures such as polypropylene. Furthermore, to improve the anatomical outcomes of anterior compartment prolapse, some techniques employ grafts. Graft material can be synthetic (e.g. permanent polypropylene, absorbable polyglactin mesh) or biological. Biological grafts can be further divided into autologous (from a person's own tissue, such as fascial sheath), alloplastic (from animals, such as porcine dermis) and homologous (such as cadaveric fascia lata).
The choice of operation depends on several factors, which include the nature, site and severity of the prolapse; whether additional symptoms are affecting urinary, bowel or sexual function; the general health of the woman; and surgeon preference and capability. Concomitant procedures are often performed to treat or prevent urinary incontinence at the same time. Mid‐urethral slings and tapes that are utilised in continence surgery are not the topic of this review, and the reader is referred to Ford 2015 for a full review of continence surgery.
To aid in assessment of the surgery, clear preoperative and postoperative site‐specific vaginal grading, details of the operative intervention and impact of the surgery on functional aspects of bladder, bowel and sexual function should be recorded.
Over the past five years and following significant litigation regarding outcomes of prolapse surgery after transvaginal polypropylene mesh, many of the products evaluated in this review have been voluntarily removed from the market (Prolift ‐ Gynecare/Ethicon, Somerville, NJ, USA; Perigee American Medical Systems, Minnetonka, MN, USA; Avaulta® ‐ Bard, Covington, LA, USA), or companies have excluded transvaginal utilisation of the mesh product (Gynemesh PS, Gynecare/Ethicon). The reviewer needs to be mindful when reading this review that the data presented include some products that are no longer available for use.
How the intervention might work
The aim of surgery ‐ to improve quality of life ‐ is achieved by:
restoration of normal vaginal anatomy;
restoration or maintenance of normal bladder function;
restoration or maintenance of normal bowel function; or
restoration or maintenance of normal sexual function.
Why it is important to do this review
The wide variety of surgical treatments available for prolapse indicates lack of consensus as to the optimal treatment. No consensus guidelines have been published in the available literature, and treatment is based on evidence from studies of mixed type and quality (Carey 2001). Provided that a sufficient number of trials of adequate quality have been conducted, the most reliable evidence is likely to come from the findings of randomised controlled trials. We conducted this review to gather evidence that would help review authors identify optimal practice while highlighting areas in which further research is needed.
This review should be read as part of a series of six Cochrane reviews related to the surgical management of prolapse, including:
surgery for women with anterior compartment prolapse;
surgery for women with posterior compartment prolapse;
surgery for women with apical compartment prolapse;
continence outcomes in pelvic organ prolapse;
native tissue, biological grafts or mesh for transvaginal prolapse surgery (Maher 2016); and
perioperative interventions at prolapse surgery.
Objectives
To determine the safety and effectiveness of surgery for anterior compartment prolapse.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with 20 or more patients in each arm, in which at least one arm provided a surgical intervention for pelvic organ prolapse.
Types of participants
Adult women seeking treatment for symptomatic anterior compartment pelvic organ prolapse. Both primary prolapse and recurrent prolapse were considered.
Types of interventions
Anterior compartment pelvic organ prolapse includes cystocele, urethrocele and a paravaginal defect for which both vaginal and abdominal surgeries were the primary inclusion criteria. Included trials performed interventions solely for anterior compartment prolapse or for concomitant prolapse or incontinence. Comparison interventions included no treatment, conservative management and use of a mechanical device or an alternative approach to surgery.
Types of outcome measures
Primary outcomes
1. Awareness of prolapse: any affirmative response to questions related to awareness of prolapse or vaginal bulge, or any affirmative response to question three of the Pelvic Floor Distress Inventory (PFDI‐20): “Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?”
2. Repeat surgery
2.1 Repeat surgery for prolapse 2.2 Repeat surgery for stress urinary incontinence
2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome), if relevant
3. Recurrent anterior prolapse ‐ defined as any stage 2 or greater anterior vaginal prolapse (Pelvic Organ Prolapse Quantification (POPQ): prolapse 1 cm above or below the hymen)
Secondary outcomes
4. Adverse events
Outcomes to be reported include but are not limited to:
4.1 death (related to surgery);
4.2 mesh exposure;
4.3 injury to bladder (cystotomy);
4.4 injury to bowel (enterotomy); or
4.5 repeat surgery for mesh exposure.
5. Prolapse outcomes
5.1 Objective failure
5.1.1 Stage 2 or greater apical compartment prolapse (point C at or beyond 1 cm inside the introitus)
5.1.2 Stage 2 or greater posterior vaginal compartment prolapse (point Bp at or beyond 1 cm inside the introitus)
5.1.3 POPQ system scores: POPQ scores include nine measurements of the vagina performed to quantify and describe vaginal prolapse. For simplicity, we report four of these basic measurements
5.1.3.a Point Ba on POPQ (range ‐3 to +10 cm): Point Ba is approximately mid‐point on the anterior vaginal wall
5.1.3.b Point Bp on POPQ (range ‐3 to +10 cm): Point Bp is approximately mid‐point on the posterior vaginal wall
5.1.3.c Point C on POPQ (range ‐10 cm to non‐determined limit): Point C describes the vaginal apex (upper vagina)
5.1.3.d Total vaginal length (TVL) in cm (range 0 to 14 cm): TVL is the length from the vaginal entrance to the apex (cervix or vaginal cuff)
6. Bladder function
6.1 Stress urinary incontinence
6.2 De novo stress urinary incontinence
6.3 Surgery for stress urinary incontinence
6.4 De novo bladder overactivity or urge incontinence
6.5 Urinary voiding dysfunction
7. Bowel function
7.1 De novo faecal incontinence
7.2 De novo obstructed defecation
7.3 Constipation
8. Sexual function
8.1 Dyspareunia
8.2 De novo dyspareunia
8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ‐12; range 0 to 48): Higher score indicates better sexual function
9. Quality of life and satisfaction
Outcomes to be reported include but are not limited to:
9.1 Patient Global Impression of Improvement (PGI‐I) questionnaire (data presented on 7‐point Likert scale): Responses of "much" or "very much" better were considered affirmative and are presented as dichotomous outcomes;
9.2 Prolapse Quality of Life (PQOL) questionnaire (range 0 to 100): Higher score indicates greater dysfunction;
9.3 Pelvic Floor Distress Inventory (PFDI‐20; range 0 to 300): Higher score indicates greater dysfunction;
9.4 Pelvic Floor Impact Questionnaire (PFIQ‐7; range 0 to 300): Higher score indicates greater dysfunction; and
9.5 International Consultation on Incontinence Modular Questionnaires (ICIQ; variable range): Higher score indicates greater dysfunction.
10. Measures associated with surgery
10.1 Operating time (minutes)
10.2 Length of hospital stay (days)
10.3 Blood transfusion (%)
Search methods for identification of studies
We imposed no language limits and no other limits on any of the searches detailed below.
Electronic searches
This review has drawn on the search strategy developed in consultation with the Cochrane Incontinence Review Group Trials Search Co‐ordinator. We identified relevant trials from the Incontinence Group Specialised Register of controlled trials, which is described, along with the Review Group search strategy, under the Group module in the Cochrane Library. This Register contains trials identified by a search of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and by a handsearch of journals and conference proceedings. We searched the Incontinence Group Specialised Register using the keyword system of the Group (all searches were based on the keyword field of Reference Manager 12, Thomson Reuters). Search terms used were as follows: ({design.cct*} OR {design.rct*}) AND ({topic.prolapse*}) AND ({intvent.surg*}), and the search date was 23 August 2016.
Trials in the Incontinence Group Specialised Register are also included in CENTRAL.
Review authors also undertook searches of health care‐related bibliographic databases such as clinicaltrials.gov (most recent, 1 August 2016), as per Appendix 2.
Searching other resources
We handsearched conference proceedings for the International Urogynecological Association (IUGA) and the International Continence Society (ICS) for podium presentations from 2012 to 15 February 2016. We searched the reference lists of relevant articles and contacted researchers in the field (Moher 2009).
We constructed a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) chart (Moher 2009) to present search flow.
Data collection and analysis
Selection of studies
Two review authors assessed titles and, if available, abstracts of all possibly eligible studies for compliance with the inclusion criteria for this review. Two or more review authors independently assessed full reports of each study likely to be eligible for inclusion. We have listed excluded studies along with reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two or more review authors independently extracted and compared data to ensure accuracy. We resolved discrepancies by discussion or by consultation with a third party. When trial data were not reported adequately, we attempted to acquire the necessary information from the trialist.
Assessment of risk of bias in included studies
Two review authors used the Cochrane risk of bias assessment tool (Higgins 2011) to independently examine studies for risk of bias by assessing selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. We planned to resolve disagreements by discussion or by consultation with a third review author. We described all judgements fully and presented our conclusions in the Risk of bias table, which we incorporated into our interpretation of review findings via sensitivity analyses (see below).
Measures of treatment effect
For dichotomous data, we used numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs). For continuous data, if all studies reported exactly the same outcomes, we calculated mean differences (MDs) between treatment groups. If similar outcomes were reported on different scales, we calculated the standardised mean difference (SMD). We presented 95% confidence intervals (CIs) for all outcomes, and we compared the magnitude and direction of effect reported by studies with how they are presented in the review, while taking account of legitimate differences.
Unit of analysis issues
All analyses were performed per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (once randomised to an intervention, participants were analysed because intervention and analysis include all randomised participants) and attempted to obtain missing data from the original trialist. When these could not be obtained, we analysed only available data.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measuring I2. We regarded an I2 measurement greater than 50% as indicating substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If we included 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (i.e. a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If studies were sufficiently similar, we combined the data using RevMan software (RevMan 2014) and a fixed‐effect model for the following comparisons.
Native tissue versus biological graft.
Native tissue versus polypropylene mesh.
Native tissue versus absorbable mesh.
One graft versus another graft for anterior compartment prolapse.
Vaginal repair versus abdominal repair.
Native tissue repair versus graft repair for anterior and/or posterior prolapse.
Subgroup analysis and investigation of heterogeneity
When data were available, we conducted subgroup analyses to identify separate evidence for primary outcomes within the following subgroups.
Native tissue versus biological graft in studies using porcine dermis graft (compared with studies using other types of biological graft).
Native tissue versus polypropylene mesh in studies of mesh currently available on the market.
If we detected substantial heterogeneity, we explored possible explanations in sensitivity analyses. We took statistical heterogeneity into account when interpreting the results, especially if we noted variation in the direction of effect. When concern regarding heterogeneity persisted, we used a random‐effects model.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes, provided data were sufficient (five or more studies), to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether review conclusions would have differed if:
a random effects model had been adopted; or
the summary effect measure had been odds ratio rather than risk ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEPRO software (GRADEpro GDT 2014), in accordance with Cochrane methods (Higgins 2011). In this table, we evaluated the overall quality of the body of evidence for main review outcomes (awareness of prolapse, repeat surgery for prolapse or stress urinary incontinence, recurrent anterior compartment prolapse, dyspareunia) with regard to the main review comparisons (native tissue vs biological graft, native tissue vs polypropylene mesh, native tissue vs absorbable mesh) by using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias) (Atkins 2004). We justified, documented and incorporated judgements about evidence quality (high, moderate, low) into reporting of results for each outcome. Two review authors working independently assessed GRADE ratings and resolved disagreements by discussion.
Results
Description of studies
Results of the search
We included in this report full reports on 33 studies (Ali 2006 abstract;Allahdin 2008;Altman 2011; Carey 2009;Colombo 2000;Dahlgren 2011; Delroy 2013; De Ridder 2004 abstract;De Tayrac 2013;El‐Nazer 2007;Farthmann 2012;Feldner 2010; Gandhi 2005; Guerette 2009;Gupta 2014;Hviid 2010; Lamblin 2014;Menefee 2011; Meschia 2007; Minassian 2010 abstract; Natale 2009;Nguyen 2008;Nieminen 2008; Robert 2014;Rudnicki 2014;Sand 2001; Sivaslioglu 2008; Tamanini 2015;Thijs 2010 abstract; Turgal 2013;Vollebregt 2011; Weber 2001;Withagen 2011). Two studies (Ek 2010; Ek 2011) are ancillary reports to Altman 2011, and three trials (Allahdin 2008; Dahlgren 2011; Menefee 2011) contributed to multiple comparisons.
The flow of literature through the assessment process is shown in the PRISMA flow chart (Figure 1).
1.
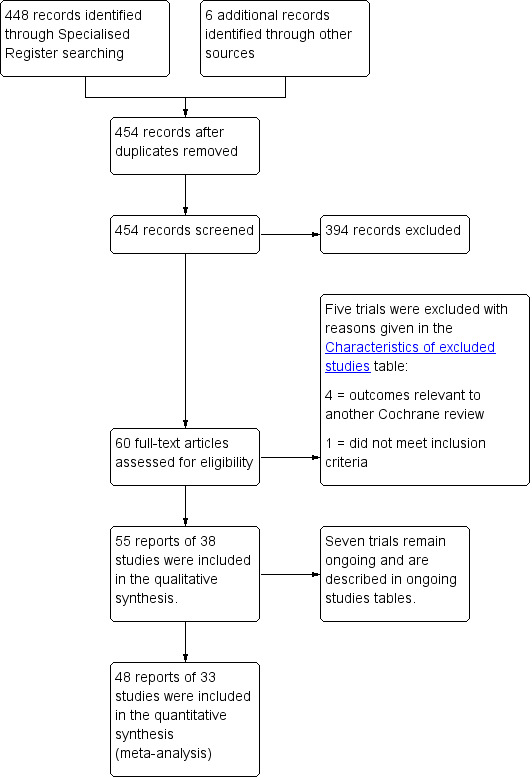
PRISMA study flow diagram for 2016 review.
No study authors reported median follow‐up of less than one year, and the following study authors reported extended reviews: 2 years ‐ Delroy 2013; De Ridder 2004 abstract;Guerette 2009; Meschia 2007;Minassian 2010 abstract;Tamanini 2015; 3 years ‐ Farthmann 2012; Natale 2009; Nieminen 2008; and 5 years ‐ Colombo 2000.
Included studies
Study design and setting
In total, 33 randomised controlled trials were conducted in 15 countries (Italy, USA, Argentina, Australia, Belgium, the Netherlands, Finland, India, Germany, Chile, France, Norway, Denmark, Sweden and Turkey). Fourteen trials were multi‐centre randomised trials (Altman 2011; Dahlgren 2011: De Tayrac 2013; Farthmann 2012; Guerette 2009; Lamblin 2014; Menefee 2011; Meschia 2007; Natale 2009; Nieminen 2008; Rudnicki 2014; Thijs 2010 abstract; Vollebregt 2011; Withagen 2011). All studies used a parallel design.
Participants
We evaluated in this review 33 randomised controlled trials evaluating 3332 women in the surgical management of anterior compartment prolapse.
Interventions
Eight articles (Dahlgren 2011; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Menefee 2011; Meschia 2007; Robert 2014) compared native tissue repair anterior colporrhaphy (n = 413) versus various biological grafts (n = 450) and assessed the following interventions. Porcine dermis (Pelvicol) was utilised in four trials (Dahlgren 2011; Hviid 2010; Menefee 2011; Meschia 2007), small intestine submucosa in two (Feldner 2010; Robert 2014), cadaveric fascia lata patch in one (Gandhi 2005) and bovine pericardium collagen in one (Guerette 2009). Meschia 2007 evaluated only primary anterior compartment prolapse, and Dahlgren 2011 included only those who had undergone at least one failed prior surgical intervention in the treated compartment. Hviid 2010 included only those with anterior compartment prolapse and excluded those undergoing concomitant surgery.
-
Sixteen trials (Ali 2006 abstract; Altman 2011; Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Gupta 2014; Lamblin 2014; Menefee 2011; Nieminen 2008; Nguyen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Thijs 2010 abstract; Turgal 2013; Vollebregt 2011) assessed anterior colporrhaphy (n = 926) versus permanent polypropylene mesh (n = 959). Each study evaluated a relatively similar cohort of women; however, the following exclusions were introduced in individual trials: prior graft surgery (Lamblin 2014; Nguyen 2008; Tamanini 2015), concomitant surgery (Altman 2011; Nieminen 2008; Rudnicki 2014), prior prolapse surgery (Rudnicki 2014; Turgal 2013; Vollebregt 2011), urinary incontinence (Turgal 2013) and bladder injury at surgery (De Tayrac 2013).
Many of the mesh products that we evaluated have been voluntarily removed from the market, including Prolift (Gynecare/Ethicon, Somerville, NJ, USA) (Altman 2011), Perigee (American Medical Systems, Minnetonka, MN, USA) (Lamblin 2014; Nguyen 2008; Thijs 2010 abstract) and Avaulta (Bard, Covington, LA, USA) (Rudnicki 2014; Vollebregt 2011). In addition, some companies have excluded transvaginal utilisation of mesh products, including Gynemesh PS and Gynecare/Ethicon (Ali 2006 abstract; Carey 2009; El‐Nazer 2007; Gupta 2014).
Three trials (Allahdin 2008; Sand 2001; Weber 2001) evaluated effects of absorbable polyglactin (Vicryl) mesh inlay used to augment prolapse repair. Data from two trials were aggregated in a meta‐analysis, as they included follow‐up of at least 12 months (Sand 2001; Weber 2001), and the non‐mesh arms from one trial (traditional anterior vaginal wall repair and ultra‐lateral anterior vaginal wall repair) were aggregated for comparison with the mesh arm in one trial (Weber 2001).
Four trials compared one type of graft versus another for management of anterior compartment prolapse. Biological graft (Pelvicol) was compared with polypropylene mesh by Menefee 2011 and Natale 2009, and with absorbable mesh (polyglactin) by De Ridder 2004 abstract. Natale included only those with recurrent prolapse and reported three‐year outcomes. Farthmann 2012 compared a conventional polypropylene mesh with lighter‐weight polypropylene mesh with an absorbable coating.
Two studies (Colombo 2000; Minassian 2010 abstract) are included in this subgroup. Both compared anterior colporrhaphy and abdominal paravaginal repair/Burch as the interventions. In Colombo 2000, vaginal interventions included vaginal hysterectomy and uterosacral colpopexy as compared with abdominal group abdominal hysterectomy and uterosacral suspension. In Minassian 2010 abstract, the non‐randomised surgery in both groups was a sacral colpopexy.
Four trials (Allahdin 2008; Carey 2009; Dahlgren 2011; Withagen 2011) were included in another subgroup. Prior prolapse surgery was an inclusion criterion for Withagen 2011, and prior prolapse surgery in the treated compartment was an inclusion criterion for Dahlgren 2011. Native tissue repair in the anterior or posterior compartment was compared with absorbable mesh in Allahdin 2008, permanent polypropylene mesh in Carey 2009 and Withagen 2011 and porcine dermis in Dahlgren 2011.
Outcomes
Trialists reported a wide variety of prolapse outcomes that broadly followed the outcomes listed under Methods. No studies reported all outcomes, and no studies reported no outcomes.
Fourteen trials (Altman 2011; Dahlgren 2011; Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Gandhi 2005; Gupta 2014; Hviid 2010; Lamblin 2014; Meschia 2007; Nieminen 2008; Robert 2014; Turgal 2013; Vollebregt 2011) reported on awareness of prolapse.
Twelve trials (Altman 2011; Delroy 2013; De Tayrac 2013; Lamblin 2014, Menefee 2011; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Thijs 2010 abstract; Vollebregt 2011) reported on repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome).
Seven trials (Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Feldner 2010; Lamblin 2014; Rudnicki 2014; Tamanini 2015) reported on repeat anterior compartment prolapse on objective examination.
Full details of the included trials are given in the Characteristics of included studies table.
Excluded studies
Overall, we excluded five studies from the review (Heinonen 2011; Kringel 2010; Tincello 2009; Van Der Steen 2011; Weemhoff 2011); details are given in the Characteristics of excluded studies table.
Risk of bias in included studies
Allocation
Fifteen of the RCTs (Allahdin 2008; Altman 2011; Dahlgren 2011; Delroy 2013; El‐Nazer 2007; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Meschia 2007; Minassian 2010 abstract; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Weber 2001) provided sufficient detail by adequately describing the randomisation process and by confirming that secure concealment of the randomisation process was used, for example, allocation by a remote person or by sealed envelopes.
Of the remainder, 11 trials (Carey 2009; De Ridder 2004 abstract; Farthmann 2012; Gupta 2014; Lamblin 2014; Menefee 2011; Sand 2001; Sivaslioglu 2008; Tamanini 2015; Vollebregt 2011; Withagen 2011) utilised computer‐generated number lists, but it was unclear whether allocation was concealed before assignment. Randomisation was performed by drawing lots in De Tayrac 2013, and Tamanini 2015 described a raffle used in the randomisation process. In Colombo 2000, randomisation was appropriate; however, the open randomisation list ensured high risk of allocation bias. Neither randomisation nor allocation concealment was reported in the final two reports presented as abstracts El‐Nazer 2007; Minassian 2010 abstract.
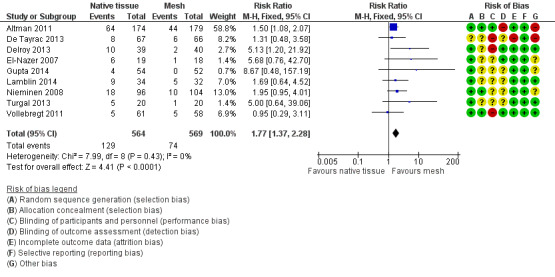
Review authors rated 28 RCTs as having low risk of bias related to sequence generation, four as unclear risk and one as high risk. We rated 14 trials as having low risk of bias related to allocation concealment, 15 as unclear risk and four as high risk.
Blinding
Women and surgeons could not be blinded to the procedure when different surgical routes were compared (Colombo 2000; Delroy 2013; Minassian 2010 abstract; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Tamanini 2015). Three trials (Allahdin 2008; Menefee 2011; Nguyen 2008) blinded participants and the postoperative reviewer. Non‐surgeons conducted outcome assessments in five trials (Allahdin 2008; El‐Nazer 2007; Feldner 2010; Natale 2009; Weber 2001). These findings, which are summarised in Figure 2 show that five trials were at low risk of performance bias, 17 at unclear risk and 11 at high risk. We rated eight trials as having low risk of detection bias, 16 unclear risk and nine high risk.
2.
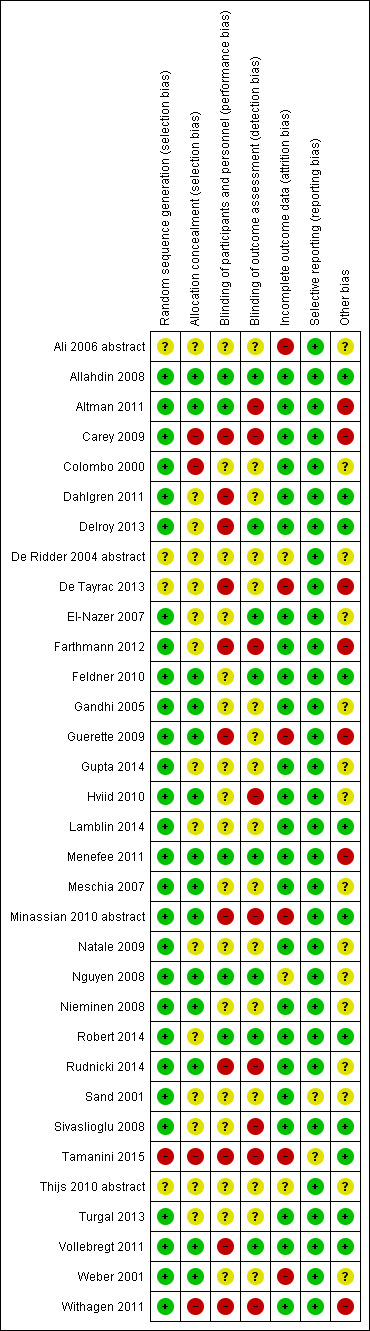
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Incomplete outcome data
Loss to follow‐up was a variable problem, ranging from zero (Allahdin 2008) to 53% in Guerette 2009 (49/93) at two years. Weber 2001 reported a statistically significantly greater loss to follow‐up in one arm of the trial (ultra‐lateral anterior vaginal wall repair). Twenty‐four trials were at low risk of attrition bias, three at uncertain risk and six at high risk.
Selective reporting
Generally, the level of reporting was adequate for one trial (Altman 2011), which did not report the rate of mesh exposure. However, data were supplied as personal communication. We rated all trials as having low risk of bias in this domain.
Other potential sources of bias
Thirteen trials (Allahdin 2008; Altman 2011; Dahlgren 2011; Delroy 2013; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Lamblin 2014; Meschia 2007; Nguyen 2008; Nieminen 2008; Rudnicki 2014) provided a CONSORT flow diagram, randomisation techniques, allocation statements and sample size calculations. These findings are summarised in Figure 3.
3.
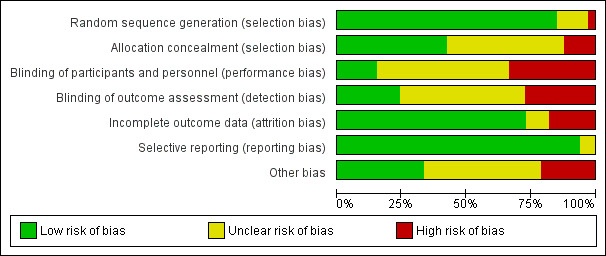
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
All trials except three reported baseline descriptive characteristics that were equally distributed. Sand 2001 reported that previous hysterectomy was more common in the mesh overlay group. Withagen 2011 reported that those in the native tissue group had a greater degree of prolapse than those in the mesh group at point A posterior (Ap), point B posterior (Bp) and genital hiatus (GH), and that prior sacral colpopexy was three times more frequent in the mesh group than in the native tissue group. Lamblin 2014 reported that the rate of hysterectomy performed as concomitant surgery was 77% in the native tissue group versus 33% in the transvaginal polypropylene mesh group (P < 0.001).
All trials but one (De Ridder 2004 abstract) reported preoperative prolapse status, and two trials (Ali 2006 abstract; Sand 2001) did not specifically report equal distribution and severity of prolapse between groups. In one trial (Weber 2001), 7% of women had stage 1 anterior vaginal wall prolapse preoperatively (at the time of inclusion), which would have been classified as a postoperative success.
Effects of interventions
See: Table 1; Table 2; Table 3
1 Native tissue versus biological graft
Eight trials (Dahlgren 2011; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Menefee 2011; Meschia 2007; Robert 2014) compared native tissue repair (anterior colporrhaphy) (n = 384) versus biological grafts (n = 422).
Primary outcomes
1.1 Awareness of prolapse (1 to 2 years)
1.1.1 Native tissue repair (anterior colporrhaphy) versus any biological graft
Women showed no difference in awareness of prolapse between native tissue repair (anterior colporrhaphy) and any biological graft (average RR 0.98, 95% CI 0.52 to 1.82; five RCTs; n = 552; I2 = 39%; low‐quality evidence; Analysis 1.1; Figure 4). These data suggest that if awareness of prolapse after biological graft occurs in 12% of women, then 7% to 23% would be aware of prolapse after native tissue repair (anterior colporrhaphy).
1.1. Analysis.
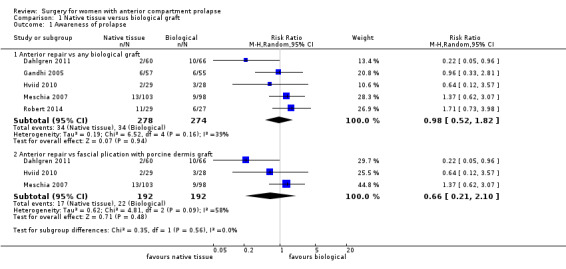
Comparison 1 Native tissue versus biological graft, Outcome 1 Awareness of prolapse.
4.
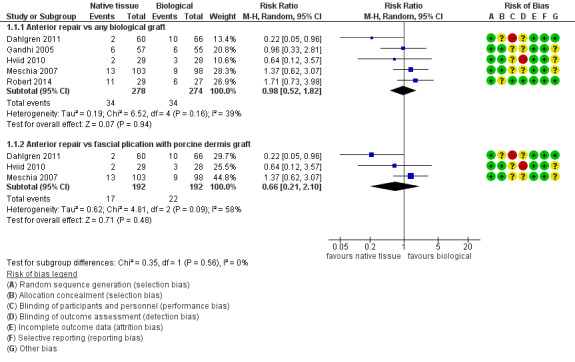
Forest plot of comparison: 1 Native tissue versus biological graft, outcome: 1.1 Awareness of prolapse.
1.1.2 Subgroup analysis by type of graft
Results showed no difference in awareness of prolapse between native tissue repair (anterior colporrhaphy) and biological graft with porcine dermis (average RR 0.66, 95% CI 0.21 to 2.10; three RCTs; n = 384; I2 = 58%; Analysis 1.1). The test for subgroup differences showed no evidence of differences between studies that used porcine dermis and those using other types of biological graft (test for subgroup differences: Chi² = 1.16; df = 1 (P = 0.28); I² = 13.6%).
1.2 Repeat surgery (1 to 2 years)
1.2.1 Repeat surgery for prolapse
We found no evidence of a difference between native tissue repair (anterior colporrhaphy) and biological graft (RR 1.02, 95% CI 0.53 to 1.97; seven RCTs; n = 650; I2 = 0%; moderate‐quality evidence; Analysis 1.2). Data suggest that if repeat prolapse surgery after biological graft was required in 4% of women, then 2% to 9% would undergo repeat prolapse surgery after native tissue repair (anterior colporrhaphy).
1.2. Analysis.
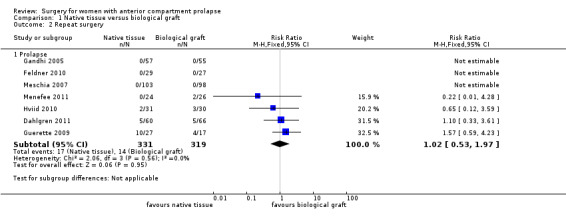
Comparison 1 Native tissue versus biological graft, Outcome 2 Repeat surgery.
1.2.2 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
1.2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
1.3 Recurrent anterior wall prolapse (1 to 2 years)
1.3.1 Anterior compartment
Native tissue repair (anterior colporrhaphy) was associated with increased risk of recurrent anterior wall prolapse compared with biological graft (RR 1.32, 95% CI 1.06 to 1.65; eight RCTs; n = 701; I2 = 26%; moderate‐quality evidence; Analysis 1.3). These data suggest that if recurrent anterior wall prolapse after biological graft occurs in 26% of women, then 27% to 42% would have recurrence after native tissue repair (anterior colporrhaphy).
1.3. Analysis.
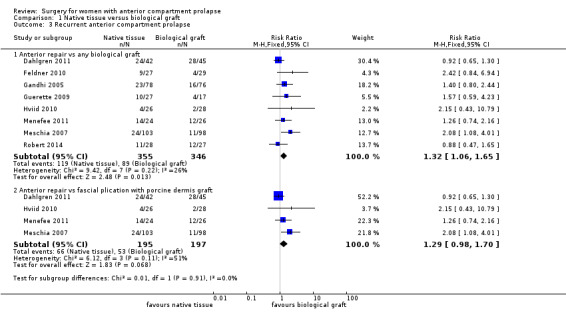
Comparison 1 Native tissue versus biological graft, Outcome 3 Recurrent anterior compartment prolapse.
1.3.2 Subgroup analysis by type of graft
Studies provided no evidence of a difference in recurrent anterior wall prolapse between native tissue repair and porcine dermis repair (RR 1.29, 95% CI 0.98 to 1.70; four RCTs; n = 392; I2 = 51%; Analysis 1.3). The test for subgroup differences showed no evidence of a difference between studies that used porcine dermis and those using other types of biological graft (test for subgroup differences: Chi² = 0.05, df = 1 (P = 0.83); I² = 0%).
Secondary outcomes
1.4 Adverse events
1.4.1 Death (related to surgery)
Studies provided no data for this outcome.
1.4.2 Mesh exposure
Studies provided no data for this outcome.
1.4.3 Injury to bladder (cystotomy)
Studies provided no data for this outcome.
1.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
1.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
1.5 Objective failure
1.5.1 Stage 2 or greater apical compartment prolapse (point C at or beyond 1 cm inside the introitus)
Studies provided no data for this outcome.
1.5.2 Stage 2 or greater posterior vaginal compartment prolapse (point Bp at or beyond 1 cm inside the introitus)
Studies provided no data for this outcome.
1.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
1.5.3.1 Point Ba ‐ Native tissue repair was associated with less pronounced Ba score compared with biological graft as reported in a single trial (MD 0.50, 95% CI 0.02 to 0.98; one RCT; n = 56; Analysis 1.5)
1.5. Analysis.
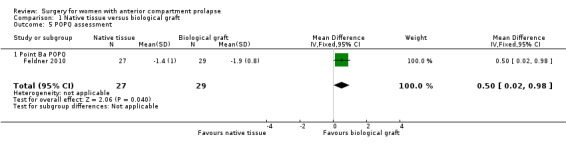
Comparison 1 Native tissue versus biological graft, Outcome 5 POPQ assessment.
1.5.3.2 Point Bp ‐ Studies provided no data for this outcome
1.5.3.3 Point C ‐ Studies reported no data for this outcome
1.5.3.4 Total vaginal length ‐ Studies provided no data for this outcome
1.6 Bladder function
1.6.1 Stress urinary incontinence (one to two years)
Studies provided no evidence of a postoperative difference in stress urinary incontinence between native tissue repair (anterior colporrhaphy) and biological graft (RR 1.44, 95% CI 0.79 to 2.64; two RCTs; n = 218; I2 = 0%; moderate‐quality evidence; Analysis 1.4). These data suggest that if the rate of stress urinary incontinence is 13% in patients receiving a biological graft, then 10% to 34% have stress urinary incontinence after a native tissue repair.
1.4. Analysis.
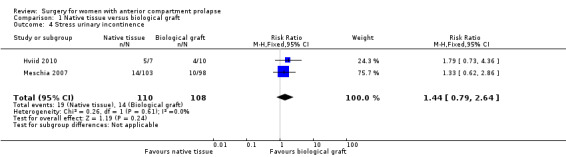
Comparison 1 Native tissue versus biological graft, Outcome 4 Stress urinary incontinence.
1.6.2 De novo stress urinary incontinence
Studies provided no data for this outcome.
1.6.3 De novo urge incontinence (one year)
Studies provided no evidence of a postoperative difference in urge incontinence between native tissue repair and biological graft (RR 1.14, 95% CI 0.61 to 2.14; one RCT; n = 201; Analysis 1.6).
1.6. Analysis.
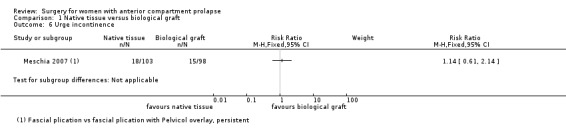
Comparison 1 Native tissue versus biological graft, Outcome 6 Urge incontinence.
1.6.4 Urinary voiding dysfunction (one year)
Studies provided no evidence of a postoperative difference between native tissue repair and biological graft in voiding dysfunction (RR 1.13, 95% CI 0.71 to 1.80; two RCTs; n = 155; I2 = 0%; Analysis 1.7).
1.7. Analysis.
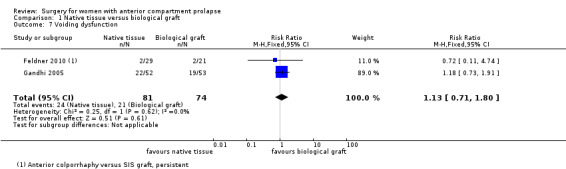
Comparison 1 Native tissue versus biological graft, Outcome 7 Voiding dysfunction.
1.7 Bowel function
1.7.1 De novo faecal incontinence
Studies provided no data for this outcome.
1.7.2 De novo obstructed defecation
Studies provided no data for this outcome.
1.7.3 Constipation
Studies provided no data for this outcome.
1.8 Sexual function
1.8.1 Dyspareunia (one to two years)
Studies provided no evidence of a difference between native tissue repair and biological graft (RR 0.87, 95% CI 0.39 to 1.93; two RCTs; n= 151; I2 = 0%; low‐quality evidence; Analysis 1.8). Data suggest that if the rate of dyspareunia is 15% after biological graft, then 6% to 29% would have dyspareunia after native tissue repair.
1.8. Analysis.
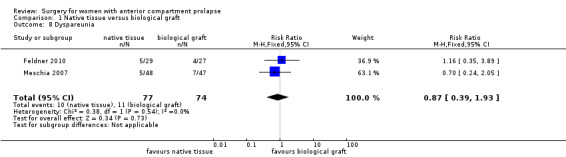
Comparison 1 Native tissue versus biological graft, Outcome 8 Dyspareunia.
1.8.2 De novo dyspareunia
Studies provided no data for this outcome.
1.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ)
Studies provided no data for this outcome.
1.9 Quality of life and satisfaction
1.9.1 Patient Global Impression of Improvement (PG1‐1) ‐ Studies provided no data for this outcome
1.9.2 Prolapse Quality of Life Questionnaire (PQOL): Studies provided no evidence of a difference in quality of life outcomes between native tissue repair and biological graft when the PQOL was used in a single study (MD ‐1.00, 95% CI ‐6.01 to 4.01; one RCT; n = 56; Analysis 1.9)
1.9.3 Pelvic Floor Distress Inventory (PFDI‐20) ‐ Studies provided no data for this outcome
1.9.4 Pelvic Floor Impact Questionnaire (PFIQ‐7) ‐ Studies provided no data for this outcome
1.10. Measures associated with surgery
1.10.1 Operating time (minutes)
Native tissue repair was associated with reduced operating time compared with use of a biological graft (MD ‐10.35, 95% CI ‐14.45 to 6.24; two RCTs; n = 113; I2 = 42%; Analysis 1.10).
1.10. Analysis.
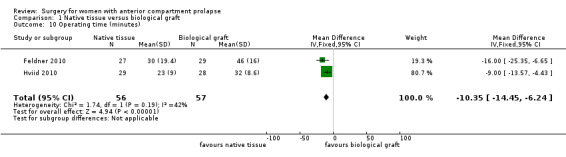
Comparison 1 Native tissue versus biological graft, Outcome 10 Operating time (minutes).
1.10.2 Length of hospital stay
Studies provided no evidence of a difference in length of hospital stay between native tissue repair and biological graft (MD 0.30 days, 95% CI ‐0.09 to 0.69; one RCT; n = 201; Analysis 1.11).
1.11. Analysis.
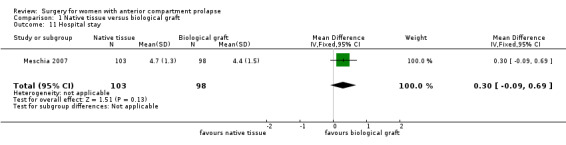
Comparison 1 Native tissue versus biological graft, Outcome 11 Hospital stay.
1.10.3 Blood transfusion
Studies provided no data for this outcome.
We have summarised data outcomes in Table 1.
2 Native tissue versus polypropylene mesh
Fifteen trials (Ali 2006 abstract; Altman 2011; Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Gupta 2014;Lamblin 2014;Menefee 2011; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Thijs 2010 abstract; Vollebregt 2011) assessed native tissue repair (anterior colporrhaphy) (n = 906) versus use of permanent polypropylene mesh (n = 949). Three studies (Allahdin 2008;Carey 2009;Withagen 2011) included both anterior and posterior prolapse.
Primary outcomes
2.1 Awareness of prolapse (one to three years)
Awareness of prolapse was more likely after native tissue repair (anterior colporrhaphy) than after mesh repair (RR 1.77, 95% CI 1.37 to 2.28; nine RCTs; n = 1133; I2 = 0%; moderate‐quality evidence; Analysis 2.1). This suggests that if awareness of prolapse after polypropylene mesh repair occurs in 13% of women, then 18% to 30% would develop awareness of prolapse after native tissue repair (anterior colporrhaphy) (Figure 5).
2.1. Analysis.
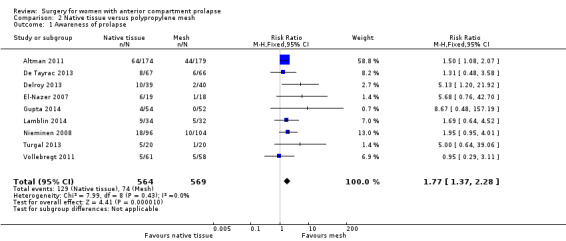
Comparison 2 Native tissue versus polypropylene mesh, Outcome 1 Awareness of prolapse.
5.
Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.1 Awareness of prolapse.
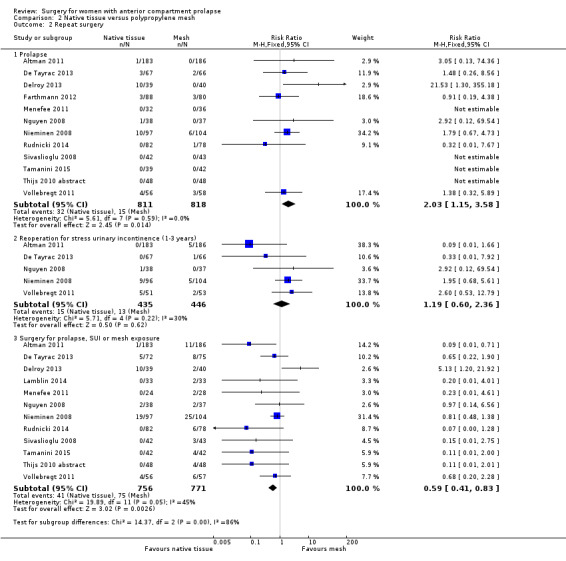
2.2 Repeat surgery (one to three years)
2.2.1 Repeat surgery for prolapse
Repeat surgery for prolapse was more likely after native tissue repair than after mesh repair (RR 2.03, 95% CI 1.15 to 3.58; 11 RCTs; n = 1461; I2 = 39%; moderate‐quality evidence; Analysis 2.2). This suggests that if 2% underwent repeat surgery for prolapse after polypropylene mesh repair, then 2% to 7% would require surgery after native tissue repair (anterior colporrhaphy).
2.2. Analysis.
Comparison 2 Native tissue versus polypropylene mesh, Outcome 2 Repeat surgery.
2.2.2 Repeat surgery for stress urinary incontinence (one to three years)
Studies provided no evidence of a difference in the rate of repeat surgery for urinary stress urinary incontinence between native tissue repair (anterior colporrhaphy) and polypropylene mesh repair (RR 1.19, 95% CI 0.60 to 2.36; five RCTs; n = 881; I2 = 30%; Analysis 2.2).
2.2.3 Reoperation rate for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome) was less likely after native tissue repair than after polypropylene mesh repair (RR 0.59, 95% CI 0.41 to 0.83; 12 RCTs; n = 1527; I2 = 45%; Analysis 2.2). This suggests that if 10% underwent subsequent surgery after polypropylene mesh repair, then 4% to 8% would require subsequent surgery after native tissue repair.
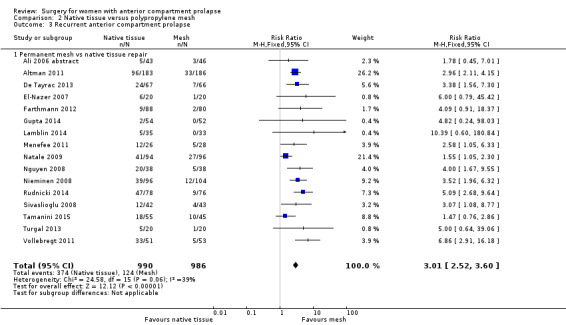
2.3 Recurrent anterior wall prolapse (one to three years)
Recurrence of anterior wall prolapse was more likely after native tissue repair than after polypropylene mesh repair (RR 3.01, 95% CI 2.52 to 3.60; 16 RCTs; n = 1976; I2 = 39%; moderate‐quality evidence; Analysis 2.3;Figure 6). This suggests that if recurrent anterior wall prolapse occurred in 13% of women after polypropylene mesh repair, then 32% to 45% would have recurrence after native tissue repair.
2.3. Analysis.
Comparison 2 Native tissue versus polypropylene mesh, Outcome 3 Recurrent anterior compartment prolapse.
6.
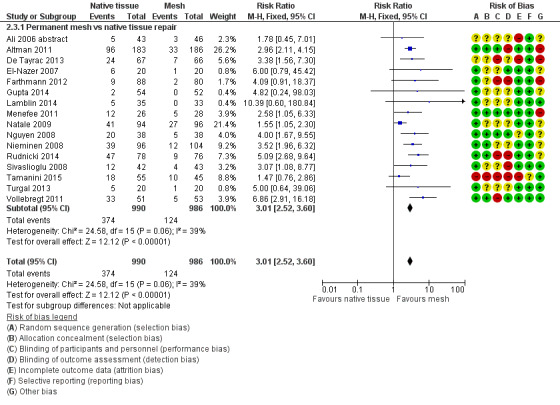
Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.
Secondary outcomes
2.4 Adverse events
2.4.1 Death (related to surgery)
Studies provided no data for this outcome.
2.4.2 Mesh exposure
The mesh exposure rate after transvaginal polypropylene mesh was 11.3% (101/896) at one to three years (Table 4).
1. Anterior transvaginal mesh exposure rate.
| Study ID | Mesh exposure | Mesh repairs |
| Al‐Nazer 2007 | 1 | 21 |
| Ali 2006 abstract | 3 | 46 |
| Altman 2011 | 21 | 183 |
| De Tayrac 2013 | 7 | 76 |
| Delroy 2013 | 2 | 40 |
| Gupta 2014 | 4 | 44 |
| Lamblin 2014 | 2 | 33 |
| Menefee 2011 | 5 | 28 |
| Nguyen 2008 | 2 | 37 |
| Nieminen 2008 | 18 | 104 |
| Rudnick 2014 | 12 | 78 |
| Sivaslioglu 2008 | 3 | 43 |
| Tamanini 2014 | 7 | 42 |
| Turgal 2014 | 3 | 20 |
| Thijs 2010 abstract | 9 | 48 |
| Vollebregt 2011 | 2 | 53 |
| Total | 101 | 896 |
| Anterior repair vs absorbable mesh | ||
| Sand 2001 | 0 | 73 |
| Weber 2001 | 1 | 26 |
2.4.3 Injury to bladder (cystotomy)
Intraoperative cystotomy was less likely after native tissue repair (1/416) than after polypropylene mesh repair (11/455) (RR 0.21, 95% CI 0.06 to 0.82; six RCTs; n = 871; I2 = 0%; Analysis 2.4). Wide confidence intervals reflect the low event rates for this outcome.
2.4. Analysis.
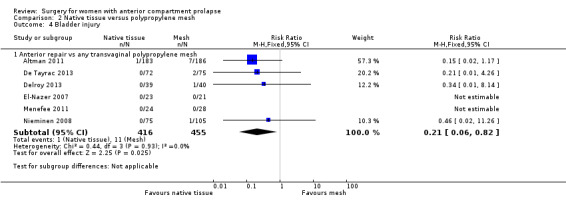
Comparison 2 Native tissue versus polypropylene mesh, Outcome 4 Bladder injury.
2.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
2.4.5 Repeat surgery for mesh exposure
The repeat surgery rate for mesh exposure was 7.3% (56/768) at one to three years (Table 5).
2. Reoperation for mesh exposure.
| Study ID | Surgery mesh exposure | Mesh repairs |
| Altman 2011 (1) | 6 | 183 |
| De Tayrac 2013 (2) | 4 | 76 |
| Delroy 2013 (3) | 2 | 40 |
| Gupta 2014 (4) | 2 | 44 |
| Nguyen 2008 (5) | 2 | 37 |
| Nieminen 2008 (6) | 14 | 104 |
| Rudnick 2014 (7) | 5 | 78 |
| Sivaslioglu 2008 (8) | 3 | 43 |
| Tamanini 2014 (9) | 7 | 42 |
| Turgal 2014 | 5 | 20 |
| Thijs 2010 abstract (10) | 4 | 48 |
| Vollebregt 2011 (11) | 2 | 53 |
| Total | 56 | 768 |
2.5 Objective failure
2.5.1 Stage 2 or greater apical compartment prolapse
Studies provided no data for this outcome.
2.5.2 Stage 2 or greater posterior vaginal compartment prolapse
Studies provided no data for this outcome. Subsequent prolapse in the posterior or apical compartment was less likely after native tissue repair than after polypropylene mesh repair (RR 0.54, 95% CI 0.30 to 0.99; two RCTs; n = 300; I2 = 0%; Analysis 2.5). This suggests that if 18% of women developed prolapse in the apical or posterior compartment on examination after polypropylene mesh repair, then 5% to 18% would develop apical or posterior compartment prolapse after native tissue repair.
2.5. Analysis.
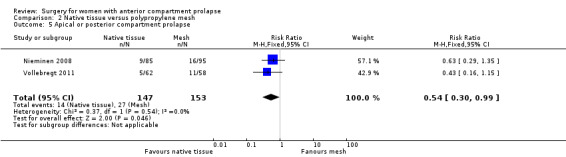
Comparison 2 Native tissue versus polypropylene mesh, Outcome 5 Apical or posterior compartment prolapse.
2.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
2.5.3.1 Point Ba (one to two years) ‐ Anatomical assessment based on POPQ revealed less support at point Ba (mid‐anterior vaginal wall) after native tissue repair than after polypropylene mesh (average MD 0.55 cm, 95% CI 0.30 to 0.80; six RCTs; n = 568; I2 = 56%; Analysis 2.6).
2.6. Analysis.
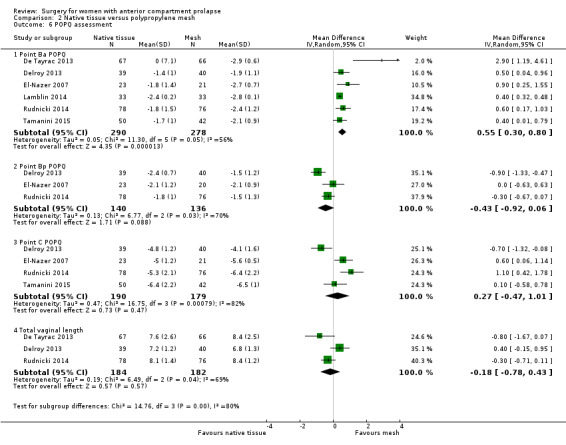
Comparison 2 Native tissue versus polypropylene mesh, Outcome 6 POPQ assessment.
2.5.3.2 Point Bp (one to two years) ‐ Studies provided no evidence of a difference between native tissue repair and mesh repair for anatomical assessment based on POPQ at point Bp (average MD ‐0.43 cm, 95% CI ‐0.92 to 0.06; four RCTs; n = 355; I2 = 70%; Analysis 2.6).
2.5.3.3 Point C (one to two years) ‐ Studies provided no evidence of a difference between native tissue repair and mesh repair for anatomical assessment based on POPQ at point C (vaginal apex) (MD 0.27 cm, 95% CI ‐0.47 to 1.01; four RCTs; n = 369; I2 = 82%; Analysis 2.6).
2.5.3.4 Total vaginal length (one to two years) ‐ Studies reported no difference between native tissue repair and mesh repair for anatomical assessment based on total vaginal length (MD ‐0.18, 95% CI ‐0.78 to 0.43; three RCTs; n = 366; I2 = 69%; Analysis 2.6).
2.6 Bladder function
2.6.1 Stress urinary incontinence (one to three years)
Studies provided no data for this outcome.
2.6.2 De novo stress urinary incontinence
Native tissue repair was associated with a reduction in de novo urinary stress incontinence compared with mesh repair (RR 0.67, 95% CI 0.44 to 1.01; six RCTs; n = 957; I2 = 26%; moderate‐quality evidence; Analysis 2.7). This suggests that if after mesh repair 10% developed de novo stress urinary incontinence, then 5% to 10% would develop de novo stress urinary incontinence after native tissue repair.
2.7. Analysis.
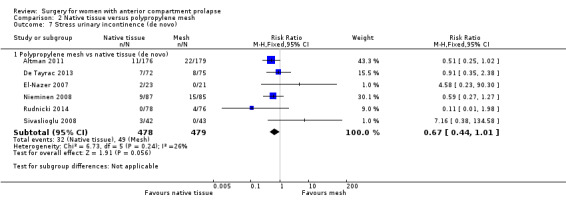
Comparison 2 Native tissue versus polypropylene mesh, Outcome 7 Stress urinary incontinence (de novo).
2.6.3 Urge incontinence (one year)
Studies provided no evidence of postoperative differences between groups in rate of urge incontinence (RR 2.20, 95% CI 0.33 to 14.68; two RCTs; n = 198; I2 = 0%; Analysis 2.10). Caution is advised in interpreting these results owing to low event rates and wide confidence intervals crossing the line of no effect, suggesting imprecision.
2.10. Analysis.
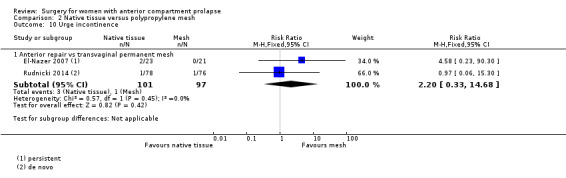
Comparison 2 Native tissue versus polypropylene mesh, Outcome 10 Urge incontinence.
2.6.4 Urinary voiding dysfunction (one to two years)
Studies provided no evidence of postoperative differences between groups in rate of voiding dysfunction (RR 1.22, 95% CI 0.33 to 4.47; three RCTs; n = 277; I2 = 15%; Analysis 2.9). Caution is advised in interpreting these results owing to low event rates and wide confidence intervals crossing the line of no effect, suggesting imprecision.
2.9. Analysis.
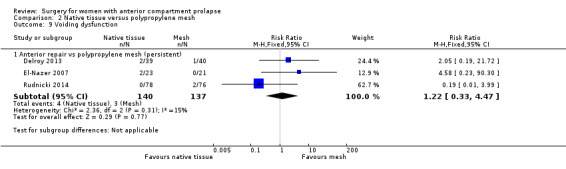
Comparison 2 Native tissue versus polypropylene mesh, Outcome 9 Voiding dysfunction.
2.7 Bowel function
Studies provided no data for this outcome.
2.8 Sexual function
2.8.1 De novo dyspareunia (one to three years)
Studies provided no evidence of differences between groups in rate of de novo dyspareunia between native tissue repair and mesh repair (RR 0.54, 95% CI 0.27 to 1.06; eight RCTs; n = 583; I2 = 0%; moderate‐quality evidence; Analysis 2.8). This suggests that if 7% developed de novo dyspareunia after mesh repair, then 2% to 8% would experience de novo dyspareunia after native tissue repair.
2.8. Analysis.
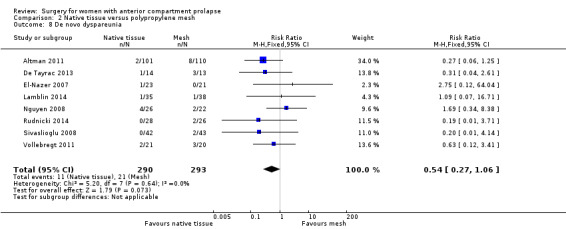
Comparison 2 Native tissue versus polypropylene mesh, Outcome 8 De novo dyspareunia.
2.8.2 Dyspareunia (one to two years)
Studies provided no evidence of a postoperative difference in rate of dyspareunia between native tissue repair and mesh repair (RR 1.06, 95% CI 0.59 to 1.90; eight RCTs; n = 1096; I2 = 5%; Analysis 2.11).
2.11. Analysis.
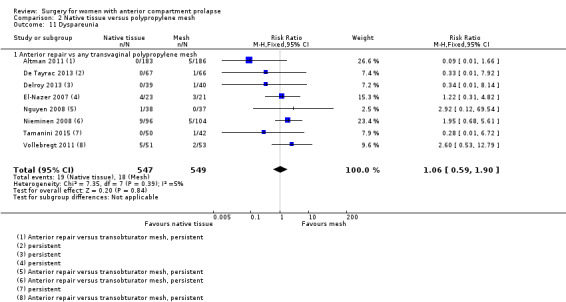
Comparison 2 Native tissue versus polypropylene mesh, Outcome 11 Dyspareunia.
2.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ) (one year)
Studies provided no evidence of differences between groups on the PISQ (MD ‐0.06, 95% CI ‐0.76 to 0.64; four RCTs; n = 741; I2 = 25%; Analysis 2.12.4).
2.12. Analysis.
Comparison 2 Native tissue versus polypropylene mesh, Outcome 12 Quality of life PROLAPSE.
2.9 Quality of life and satisfaction
Quality of life questionnaires: Only four of the 15 studies (Ali 2006 abstract; El‐Nazer 2007; Gupta 2014; Vollebregt 2011) did not report a validated quality of life outcome.
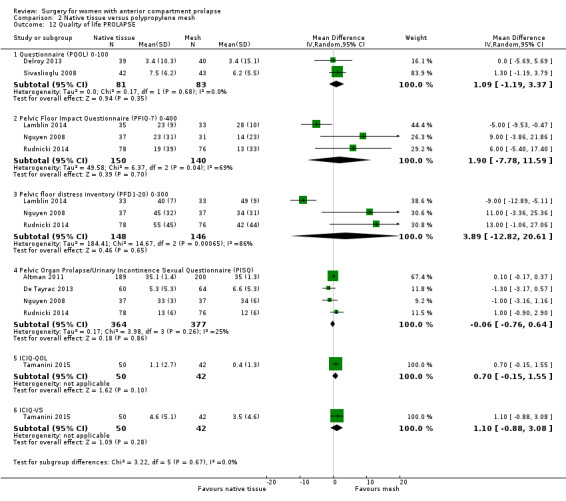
2.9.1 Prolapse Quality of Life questionnaire (PQOL) (one to two years)
Studies provided no evidence of differences in PQOL scores between groups (MD 1.09, 95% CI ‐1.19 to 3.37; two RCTs; n = 164; I2 = 0%; Analysis 2.12).
2.9.2 Pelvic Floor Impact Questionnaire (PFIQ‐7) (one to two years)
Studies provided no evidence of differences between groups on the PFIQ‐7 (average MD 1.90, 95% CI ‐7.78 to 11.59; three RCTs; n = 290; I2 = 69%; Analysis 2.12).
2.9.3 Pelvic Floor Distress Inventory (PFDI‐20) (one to two years)
Studies provided no evidence of differences between groups on the PFDI‐20 (average MD 3.89, 95% CI ‐12.82 to 20.61; three RCTs; n = 294; I2 = 86%; Analysis 2.12.3).
2.9.4 International Consultation on Incontinence Modular Questionnaire (ICIQ) (one to two years)
Studies provided no evidence of differences between groups on the ICIQ (MD 0.70, 95% CI ‐0.15 to 1.55; one RCT; n = 92; Analysis 2.12) or in ICIQ vaginal symptoms (MD 1.10, 95% CI ‐0.88 to 3.08; one RCT; n = 92; Analysis 2.12).
2.10 Perioperative outcomes
2.10.1 Operating time
Operating time was shorter after native tissue repair (anterior colporrhaphy) than after polypropylene mesh repair (MD ‐17.89 minutes, 95% CI ‐25.81 to ‐9.98; seven RCTs; n = 1099; I2 = 91%). Because of heterogeneity, we have not reported the data in a meta‐analysis (Analysis 2.14).
2.14. Analysis.
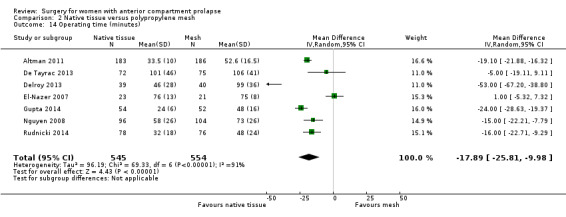
Comparison 2 Native tissue versus polypropylene mesh, Outcome 14 Operating time (minutes).
2.10.2 Hospital stay
Studies provided no evidence of differences between groups in hospital stay (MD 0.08 days, 95% CI ‐0.17 to 0.33; five RCTs; n = 707; I2 = 67%; Analysis 2.13).
2.13. Analysis.
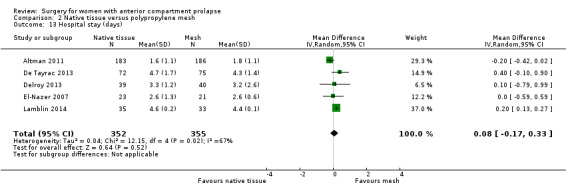
Comparison 2 Native tissue versus polypropylene mesh, Outcome 13 Hospital stay (days).
2.10.3 Transfusion
Blood transfusion was less likely after native tissue repair than after mesh repair (RR 0.42, 95% CI 0.24 to 0.76; four RCTs; n = 486; I2 = 42%; Analysis 2.15).
2.15. Analysis.
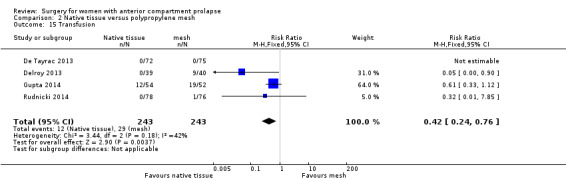
Comparison 2 Native tissue versus polypropylene mesh, Outcome 15 Transfusion.
We have summarised study findings in Table 2.
Subgroup analysis by market availability
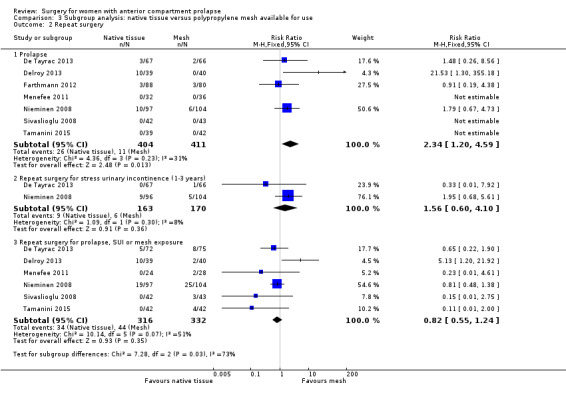
We conducted a post hoc subgroup analysis for our primary outcomes that was restricted to studies of meshes currently on the market. This did not change our main findings with respect to awareness of prolapse (RR 2.12, 95% CI 1.35 to 3.35; nine RCTs; n = 518; I2 = 0%; Analysis 3.1), repeat surgery for prolapse (RR 2.34, 95% CI 1.20 to 4.59; 12 RCTs; n = 815; I2 = 31%; Analysis 3.2.1) or prolapse on examination (RR 2.35, 95% CI 1.83 to 3.01; 16 RCTs; n = 970; I2 = 31%; Analysis 3.3). However, rates of repeat surgery for prolapse, stress urinary incontinence or mesh exposure were no longer significantly different between groups (RR 0.82, 95% CI 0.55 to 1.24; 12 RCTs; n = 648; I2 = 51%; Analysis 3.2.3).
3.1. Analysis.
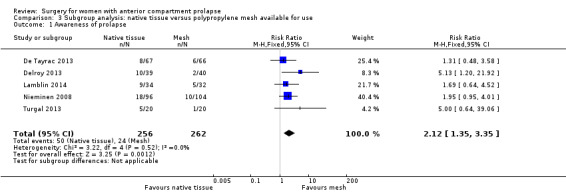
Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 1 Awareness of prolapse.
3.2. Analysis.
Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 2 Repeat surgery.
3.3. Analysis.
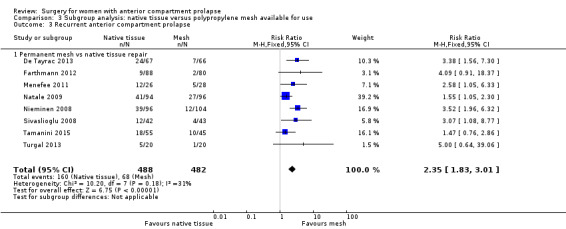
Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 3 Recurrent anterior compartment prolapse.
3 Native tissue compared with absorbable mesh
Three trials (Allahdin 2008; Sand 2001; Weber 2001) evaluated the effects of using absorbable polyglactin (Vicryl) mesh inlay to augment prolapse repairs.
Primary outcomes
3.1 Awareness of prolapse (two years)
Studies provided no evidence of a difference in awareness of prolapse at the two‐year review between native tissue repair and absorbable mesh (RR 0.95, 95% CI 0.70 to 1.31; one RCT; n = 54; very low‐quality evidence). We downgraded evidence for attrition bias, imprecision and publication bias. Evidence suggests that if awareness of prolapse after absorbable mesh repair occurred in 76% of women, then 53.2% to 99.6% would develop awareness of prolapse after native tissue repair (anterior colporrhaphy). See Table 3.
3.2 Repeat surgery (two years)
3.2.1 Repeat surgery for prolapse
Studies provided no evidence of a difference in the need for repeat surgery for prolapse at the two‐year review between native tissue repair and absorbable mesh (RR 2.13, 95% CI 0.42 to 10.82; one RCT; n = 66; very low‐quality evidence). We downgraded evidence for attrition bias, imprecision and publication bias. The evidence suggests that if repeat surgery for prolapse were required after absorbable mesh repair in 5.9% of women, then 2.5% to 63.6% would require repeat surgery after native tissue repair (anterior colporrhaphy). See Table 3.
3.2.2 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
3.2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
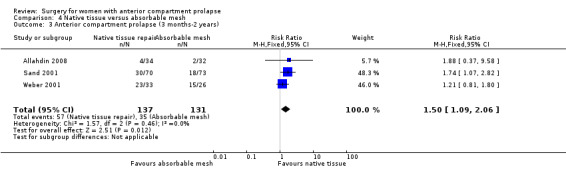
3.3 Recurrent anterior prolapse (one to three years)
Recurrent anterior wall prolapse was more likely after native tissue repair than after absorbable mesh repair (RR 1.50, 95% CI 1.09 to 2.06; three RCTs; n = 268; I2 = 0%; moderate‐quality evidence; Analysis 4.3). We downgraded evidence for attrition bias. The evidence suggests that if the rate of anterior wall prolapse after absorbable mesh repair were 26.7%, then 29.1% to 55% would have recurrent anterior wall prolapse after native tissue repair. See Table 3.
4.3. Analysis.
Comparison 4 Native tissue versus absorbable mesh, Outcome 3 Anterior compartment prolapse (3 months‐2 years).
Secondary outcomes
3.4. Adverse events
3.4.1 Death (related to surgery)
Two trials (Allahdin 2008; Weber 2001) reported no events of death in native tissue or absorbable mesh groups (Analysis 4.4).
4.4. Analysis.
Comparison 4 Native tissue versus absorbable mesh, Outcome 4 Death.
3.4.2 Mesh exposure
Two trials (Sand 2001; Weber 2001) reported one vaginal polyglactin mesh erosion (1/99; 1%; Table 4), and in Allahdin 2008, two women needed partial removal of mesh (2/32; 6.1%) for undisclosed reasons.
3.4.3 Injury to bladder (cystotomy)
Studies provided no data for this outcome.
3.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
3.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
3.5 Objective failure
3.5.1 Stage 2 or greater apical compartment prolapse
Studies provided no data for this outcome.
3.5.2 Stage 2 or greater posterior vaginal compartment prolapse
Studies provided no evidence of a difference between native tissue repair and absorbable mesh repair for posterior compartment prolapse (RR 0.88, 95% CI 0.31 to 2.49; one RCT; n = 132).
3.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
Studies provided no data for this outcome.
3.6 Bladder function
3.6.1 Stress urinary incontinence
Studies provided no evidence of a difference between native tissue repair and absorbable mesh for stress urinary incontinence (RR 0.72, 95% CI 0.50 to 1.05; one RCT; n = 49; very low‐quality evidence). We downgraded evidence for attrition bias, imprecision and publication bias. The evidence suggests that if stress urinary incontinence after absorbable mesh repair occurred in 81.8% of women, then 40.9% to 85.9% would have stress urinary incontinence after native tissue repair (anterior colporrhaphy). See Table 3.
3.6.2 De novo stress urinary incontinence
Studies provided no data for this outcome.
3.6.3 De novo bladder overactivity or urge incontinence
Studies provided no data for this outcome.
3.6.4 Urinary voiding dysfunction
Studies provided no data for this outcome.
3.7 Bowel function
Studies provided no data for this outcome.
3.8 Sexual function
Studies provided no data for this outcome.
3.9 Quality of life and satisfaction
3.9.1 Non‐validated quality of life visual analogue scale (0 to 10)
Studies provided no evidence of a difference in quality of life visual analogue score between native tissue and absorbable mesh groups (MD 0.00, 95% CI ‐2.82 to 2.82; one RCT; n = 54; very low‐quality evidence). We downgraded the evidence for attrition bias, imprecision and publication bias. See Table 3. We noted no difference in quality of life scores between the two groups.
3.10 Measures associated with surgery
Studies provided no data for this outcome.
4 One graft versus another graft for anterior compartment prolapse
Four trials compared one type of graft versus another for management of anterior compartment prolapse. Menefee 2011 and Natale 2009 compared biological graft (Pelvicol) versus polypropylene mesh, and De Ridder 2004 abstract compared biological graft versus absorbable mesh (polyglactin). Natale 2009 included only those with recurrent prolapse and reported three‐year outcomes. Farthmann 2012 compared a conventional polypropylene mesh versus a lighter‐weight polypropylene mesh with an absorbable coating.
Primary outcomes
4.1 Awareness of prolapse
Studies provided no evidence of a difference in awareness of prolapse after polypropylene mesh and biological graft in a single study (RR 0.98, 95% CI 0.20 to 4.73; one RCT; n = 190; very low‐quality evidence; Analysis 5.1). We downgraded the evidence for inadequate methodological reporting, imprecision and publication bias. Evidence suggests that if after a biological graft 3.2% of women were aware of prolapse, then 0.6% to 15.1% would be aware of prolapse after use of polypropylene mesh.
5.1. Analysis.
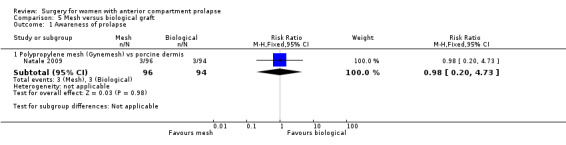
Comparison 5 Mesh versus biological graft, Outcome 1 Awareness of prolapse.
4.2 Repeat surgery (two years)
4.2.1 Repeat surgery for prolapse
Studies provided no evidence of differences in the rate of repeat surgery for prolapse between groups (RR 3.05, 95% CI 0.87 to 10.73; two RCTs; n = 315; I2 = 0%; very low‐quality evidence; Analysis 5.2). We downgraded the evidence for inadequate methodological reporting and imprecision. Evidence suggests that if repeat surgery for prolapse was required in 1.9% of women after a biological graft, then 1.7% to 20.5% would require repeat surgery after use of polypropylene mesh.
5.2. Analysis.
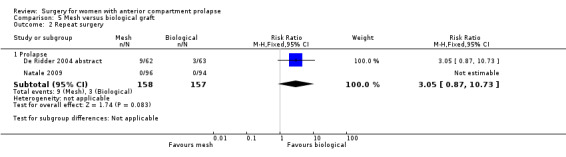
Comparison 5 Mesh versus biological graft, Outcome 2 Repeat surgery.
4.2.2 Surgery for stress urinary incontinence
Studies provided no data for this outcome.
4.2.3 Surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
4.3 Recurrent anterior prolapse
4.3.1 Recurrent anterior wall compartment prolapse
Overall, studies provided no evidence of a difference between mesh (permanent or absorbable) and a biological graft (RR 1.38, 95% CI 0.28 to 6.85; two RCTs; n = 315; very low‐quality evidence; Analysis 5.3). We downgraded the evidence for inadequate methodological reporting, imprecision and inconsistency. Evidence suggests that if recurrent anterior compartment prolapse occurred in 29.9% of women after biological graft, then 8.4% to 100% would have recurrent anterior compartment prolapse after use of polypropylene mesh.
5.3. Analysis.
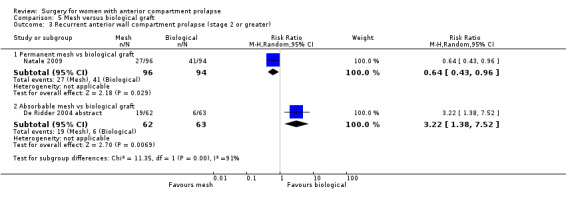
Comparison 5 Mesh versus biological graft, Outcome 3 Recurrent anterior wall compartment prolapse (stage 2 or greater).
The test for subgroup differences was significant (Chi² = 11.35, df = 1 (P = 0.0008), I² = 91.2%). Recurrent anterior wall prolapse after polypropylene mesh (32/124; 26%) was less than after porcine dermis (53/120; 44%) (RR 0.64, 95% CI 0.43 to 0.96; one RCT; n = 190; Analysis 5.3). The rate of recurrent anterior wall prolapse after absorbable mesh was higher than after a biological graft (RR 3.22, 95%CI 1.38 to 7,52; one RCT; n = 125; Analysis 5.3).
Secondary outcomes
4.4 Adverse events
4.4.1 Death (related to surgery)
Studies provided no data for this outcome.
4.4.2 Mesh exposure
Mesh exposure was less in the polypropylene mesh group (no events) than in the biological graft group (RR 0.09, 95% CI 0.01 to 0.69; two RCTs; n = 241; I2 = 0%; Analysis 5.4).
5.4. Analysis.
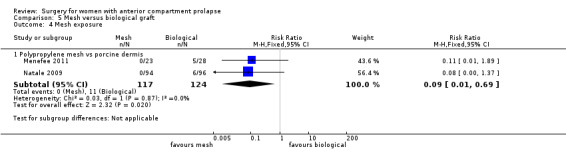
Comparison 5 Mesh versus biological graft, Outcome 4 Mesh exposure.
4.4.3 Injury to bladder (cystotomy)
Studies provided no data for this outcome.
4.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
4.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
4.5 Objective failure
Studies provided no data for this outcome.
4.6 Bladder function
4.6.1 Stress urinary incontinence
Studies provided no evidence of a difference between polypropylene mesh and biological graft groups for stress urinary incontinence (RR 1.96, 95% CI 0.18 to 21.23; one RCT; n = 190; Analysis 4.5; very low‐quality evidence). We downgraded the evidence for inadequate methodological reporting, imprecision and publication bias. If stress urinary incontinence occurred in 1.1% of women after biological graft, then 0.2% to 22.6% would experience stress urinary incontinence after use of polypropylene mesh.
4.5. Analysis.
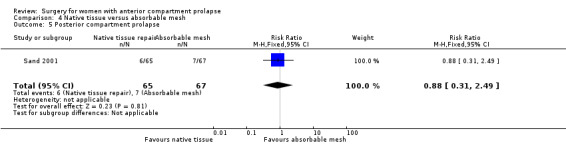
Comparison 4 Native tissue versus absorbable mesh, Outcome 5 Posterior compartment prolapse.
4.6.2 De novo stress urinary incontinence
Studies provided no data for this outcome.
4.6.3 De novo bladder overactivity or urge incontinence
Studies provided no evidence of a difference between polypropylene mesh and biological graft groups for this outcome (RR 0.47, 95% CI 0.05 to 4.78; one RCT; n = 37; Analysis 4.6).
4.6. Analysis.
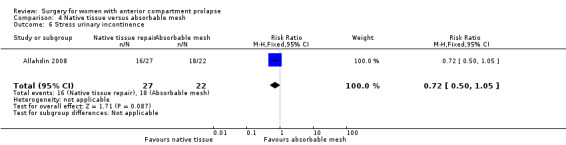
Comparison 4 Native tissue versus absorbable mesh, Outcome 6 Stress urinary incontinence.
4.6.4 Urinary voiding dysfunction
Studies provided no data for this outcome.
4.7 Bowel function
4.7.1 De novo faecal incontinence ‐ Studies provided no data for this outcome
4.7.2 De novo obstructed defecation ‐ Studies provided no data for this outcome
4.7.3 Constipation ‐ Studies provided no data for this outcome
4.8 Sexual function
4.8.1 Dyspareunia
Studies provided no evidence of a difference between polypropylene mesh and biological graft groups for this outcome (RR 0.82, 95% CI 0.37 to 1.80; one RCT; n = 190).
4.8.2 De novo dyspareunia
Studies provided no data for this outcome.
4.8.3 Prolapse and Incontinence Sexual Questionaire (PISQ)
Studies provided no data for this outcome.
4.9 Quality of life and satisfaction
Studies provided no data for this outcome.
4.10. Measures associated with surgery
Studies provided no data for this outcome.
5 Vaginal repair versus abdominal repair for anterior compartment prolapse
Two studies (Colombo 2000; Minassian 2010 abstract) were included in this subgroup. Both compared anterior colporrhaphy and abdominal paravaginal repair/Burch as the interventions. In Colombo 2000, vaginal interventions included vaginal hysterectomy and uterosacral colpopexy as compared with abdominal group abdominal hysterectomy and uterosacral suspension. In Minassian 2010 abstract, the non‐randomised surgery performed in both groups was a sacral colpopexy.
Primary outcomes
5.1. Awareness of prolapse
Studies provided no data for this outcome.
5.2. Repeat surgery
Studies provided no data for this outcome.
5.3 Recurrent anterior prolapse
Studies provided no evidence of a difference in recurrent anterior compartment prolapse between vaginal and abdominal surgery groups (average RR 0.31, 95% CI 0.03 to 3.46; two RCTs; n = 118; I2 = 81%; Analysis 6.1; very low‐quality evidence). We downgraded the evidence for high risk of bias, imprecision and inconsistency. Evidence suggests that if recurrent anterior compartment prolapse occurred in 36.7% of women in the abdominal repair group, then 1.1% to 100% of women in the vaginal repair group would have recurrent anterior compartment prolapse.
6.1. Analysis.
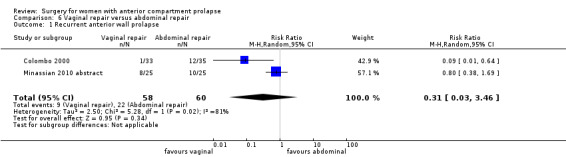
Comparison 6 Vaginal repair versus abdominal repair, Outcome 1 Recurrent anterior wall prolapse.
Secondary outcomes
5.4 Adverse events
5.4.1 Death (related to surgery)
Studies provided no data for this outcome.
5.4.2 Mesh exposure
Studies provided no data for this outcome.
5.4.3 Injury to bladder
Studies provided no evidence of a difference between vaginal and abdominal repair groups for bladder injury (RR 0.97, 95% CI 0.06 to 14.88; one RCT; n = 67; Analysis 6.2; very low‐quality evidence). We downgraded the evidence for high risk of bias, imprecision and publication bias. Evidence suggests that if bladder injury occurred in 3% of women after abdominal repair, then 0.2% to 45.1% of women in the vaginal repair group would have a bladder injury.
6.2. Analysis.
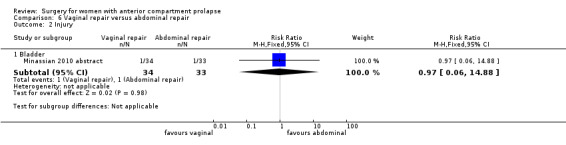
Comparison 6 Vaginal repair versus abdominal repair, Outcome 2 Injury.
5.4.4 Injury to bowel
Studies provided no data for this outcome.
5.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
5.5 Objective failure
5.5.1 Stage 2 or greater apical compartment prolapse (point C at or beyond 1 cm inside the introitus)
Studies provided no data for this outcome.
5.5.2 Stage 2 or greater posterior vaginal compartment prolapse (point Bp at or beyond 1 cm inside the introitus)
Studies provided no evidence of a difference in the rate of posterior compartment prolapse between groups (RR 1.81, 95% CI 0.17 to 19.65; two RCTs; n = 118; I2 = 65%; Analysis 6.3).
6.3. Analysis.
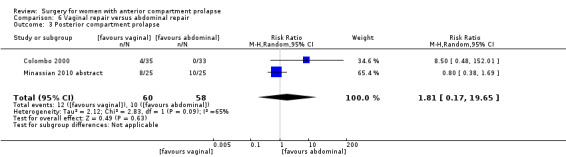
Comparison 6 Vaginal repair versus abdominal repair, Outcome 3 Posterior compartment prolapse.
5.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
5.5.3.1 Point Ba on POPQ ‐ Studies provided no evidence of a difference between vaginal and abdominal repair groups for point Ba (MD 0.90, 95% CI ‐0.15 to 1.95; one RCT; n = 50; Analysis 6.4)
6.4. Analysis.
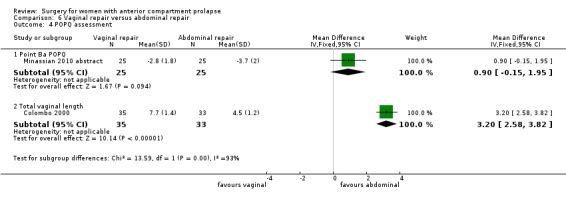
Comparison 6 Vaginal repair versus abdominal repair, Outcome 4 POPQ assessment.
5.5.3.2 Point Bp on POPQ ‐ Studies provided no data for this outcome
5.5.3.3 Point C on POPQ ‐ Studies provided no data for this outcome
5.5.3.4 Total vaginal length (TVL) in cm (range 0 to 14 cm): Total vaginal length was longer after vaginal repair than after abdominal repair (MD 3.20 cm, 95% CI 2.58 to 3.82; one RCT; n = 68; Analysis 6.4).
5.6 Bladder function
Studies provided no data for this outcome.
5.7. Bowel function
Studies provided no data for this outcome.
5.8 Sexual function
5.8.1 Dyspareunia
Dyspareunia was more likely after vaginal anterior colporrhaphy than after paravaginal/Burch (3/49; 6%) (RR 5.17, 95% CI 1.63 to 16.35; two RCTs; n = 97; I2 = 0%; Analysis 6.5).
6.5. Analysis.
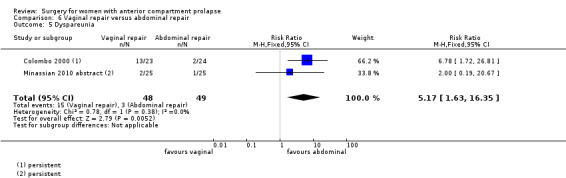
Comparison 6 Vaginal repair versus abdominal repair, Outcome 5 Dyspareunia.
5.8.2 De novo dyspareunia
Studies provided no data for this outcome.
5.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ)
Studies provided no evidence of a difference between vaginal and abdominal repair in PISQ score (MD ‐2.00, 95% CI ‐6.24 to 2.24; one RCT; n = 50; Analysis 6.6).
6.6. Analysis.
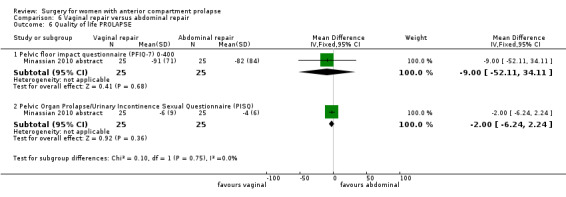
Comparison 6 Vaginal repair versus abdominal repair, Outcome 6 Quality of life PROLAPSE.
5.9 Quality of life and satisfaction
5.9.1 Pelvic Floor Impact Questionnaire (PFIQ‐7)
Studies provided no evidence of a difference between vaginal and abdominal repair in PFIQ‐7 scores (MD ‐9.00, 95% CI ‐52.11 to 34.11; one RCT; n = 50; Analysis 6.6).
5.10 Measures associated with surgery
5.10.1 Operating time (minutes)
Studies provided no evidence of a difference in operating time between vaginal and abdominal repair groups (MD 16.00 minutes, 95% CI ‐24.48 to 56.48; one RCT; n = 67; Analysis 6.7).
6.7. Analysis.
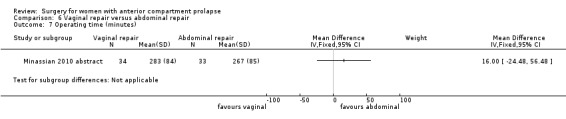
Comparison 6 Vaginal repair versus abdominal repair, Outcome 7 Operating time (minutes).
5.10.2 Length of hospital stay
Studies provided no data for this outcome.
5.10.3 Blood transfusion
Studies provided no evidence of differences in risk of needing a blood transfusion between vaginal and abdominal repair groups (RR 0.97, 95% CI 0.06 to 14.88; one RCT; n = 67; Analysis 6.8).
6.8. Analysis.
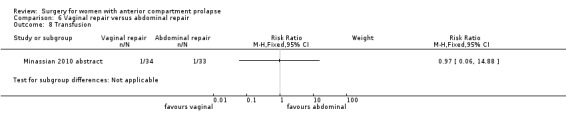
Comparison 6 Vaginal repair versus abdominal repair, Outcome 8 Transfusion.
6 Native tissue repair versus graft repair for anterior and/or posterior compartment prolapse
Four trials (Allahdin 2008; Carey 2009; Dahlgren 2011; Withagen 2011) evaluated these interventions. Prior prolapse surgery was an inclusion criterion for Withagen 2011, and prior prolapse surgery in the treated compartment was an inclusion criterion for Dahlgren 2011. Allahdin 2008 compared native tissue repair in the anterior or posterior compartment versus absorbable mesh, Carey 2009 and Withagen 2011 versus permanent polypropylene mesh and Dahlgren 2011 versus porcine dermis.
Carey 2009 and Withagen 2011 were similar enough in inclusion criteria and interventions undertaken to be included in a meta‐analysis comparing native tissue repair in the anterior and/or posterior vagina versus use of transvaginal polypropylene mesh.
Primary outcomes
6.1 Awareness of prolapse
When anterior and/or posterior repair was compared with polypropylene mesh, studies provided no evidence of a difference between groups in awareness of prolapse (average RR 0.85, 95% CI 0.36 to 1.99; three RCTs; n = 406; I2 = 59%; very low‐quality evidence; Analysis 7.1). We downgraded the evidence for high risk of bias and inconsistency. Evidence suggests that if 15.5% of women had awareness of prolapse after mesh repair, then 5.6% to 30.9% would have awareness of prolapse after native tissue repair.
7.1. Analysis.
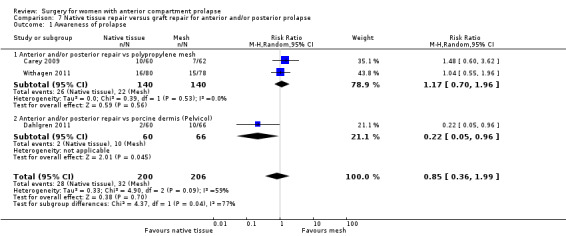
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 1 Awareness of prolapse.
6.2 Repeat surgery
6.2.1 Repeat surgery for prolapse
When anterior and/or posterior repair was compared with polypropylene mesh, investigators reported no difference in repeat surgery rate for prolapse between groups (RR 2.09, 95% CI 0.06 to 5.48; three RCTs; n = 416; I2 = 18%; very low‐quality evidence; Analysis 7.2). We downgraded the evidence for high risk of bias and imprecision. Evidence suggests that if 2.4% of women had repeat surgery for prolapse after mesh repair, then 1.9% to 13.1% would require repeat surgery after native tissue repair.
7.2. Analysis.
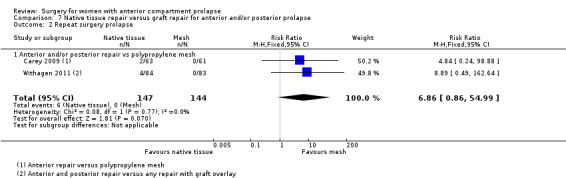
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 2 Repeat surgery prolapse.
6.2.2 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
6.2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
6.3 Recurrent anterior wall prolapse (stage 2 or higher)
When anterior colporrhaphy was compared with polypropylene mesh, studies provided no evidence of a difference between groups in rates of recurrent anterior wall prolapse on examination (RR 1.03, 95% CI 0.60 to 1.40; three RCTs; n = 367; I2 = 0%; low‐quality evidence; Analysis 7.3). We downgraded the evidence for high risk of bias. Evidence suggests that if 27% of women had recurrent anterior wall prolapse after mesh repair, then 20.5% to 37.8% would have recurrent anterior wall prolapse after native tissue repair.
7.3. Analysis.
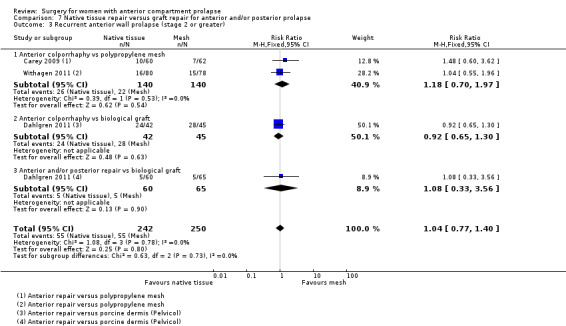
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 3 Recurrent anterior wall prolapse (stage 2 or greater).
Secondary outcomes
6.4 Adverse events
6.4.1 Death (related to surgery)
Studies provided no data for this outcome.
6.4.2 Mesh exposure
The mesh exposure rate was 12.3% (18/146; Table 6).
3. Anterior and/or posterior mesh exposure.
| Study ID | Mesh exposure | Mesh repairs |
| Carey 2009 | 4 | 63 |
| Withagen 2011 | 14 | 83 |
| Total | 18 | 146 |
6.4.3 Injury to bladder
Studies provided no evidence of a difference between groups in rate of cystotomy (bladder injury) (RR 0.20, 95% CI 0.01 to 4.01; one RCT; n = 166; Analysis 6.4).
6.4.4 Injury to bowel
Studies provided no data for this outcome.
6.4.5 Repeat surgery for mesh exposure
Repeat surgery for mesh exposure was undertaken in 5.6% (8/146; Table 7).
4. Reoperation for mesh exposure: anterior and/or posterior mesh repair.
| Study ID | Reoperation mesh exposure | Mesh repairs |
| Carey 2009 | 3 | 63 |
| Withagen 2011 | 5 | 83 |
| Total | 8 | 146 |
6.5 Objective failure
A single study (Withagen 2011) demonstrated significantly improved outcomes for POPQ points Ba and Bp as compared with native tissue repair; however, these data were reported as median values and could not be included in the meta‐analysis.
6.6 Bladder function
6.6.1 Stress urinary incontinence
Studies provided no data for this outcome.
6.6.2 De novo stress urinary incontinence
Studies provided no evidence of a difference between groups for de novo stress urinary incontinence as reported in one study (RR 0.98, 95% CI 0.34 to 2.85; one RCT; n = 105; Analysis 7.5).
7.5. Analysis.
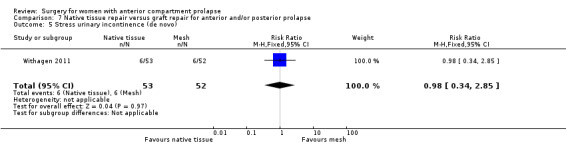
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 5 Stress urinary incontinence (de novo).
6.6.3 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
6.6.4 De novo bladder overactivity or urge incontinence
Studies provided no data for this outcome.
6.6.5 Urinary voiding dysfunction
Studies provided no data for this outcome.
6.7 Bowel function
Studies provided no data for this outcome.
6.8 Sexual function
6.8.1 Dyspareunia
Studies provided no evidence of a difference between groups in rate of persistent dyspareunia (RR 1.03, 95% CI 0.70 to 1.52; one RCT; n = 122; Analysis 7.6).
7.6. Analysis.
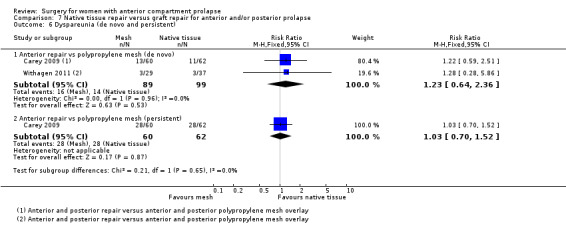
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 6 Dyspareunia (de novo and persistent).
6.8.2 De novo dyspareunia
Studies reported no difference between groups in rate of de novo dyspareunia (RR 1.23, 95% CI 0.64 to 2.36; two RCTs; n = 188; I2 = 0%; low‐quality evidence; Analysis 7.6). We downgraded the evidence for high risk of bias. Evidence shows that if 14.1% of women experienced de novo dyspareunia after mesh repair, then 9.1% to 33.4% would experience de novo dyspareunia after native tissue repair.
6.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ)
Studies provided no evidence of a difference between groups in PISQ scores (MD 0.40, 95% CI ‐2.74 to 3.54; one RCT; n = 60; Analysis 7.7).
7.7. Analysis.
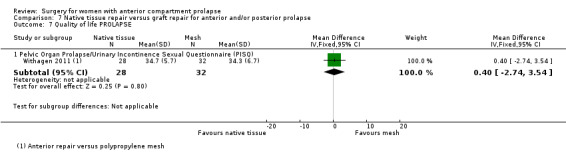
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 7 Quality of life PROLAPSE.
6.9 Quality of life and satisfaction
Studies provided no data for this outcome.
6.10 Measures associated with surgery
Studies provided no data for this outcome.
Additional analyses
We conducted our planned sensitivity analyses for the primary outcomes. These did not substantially differ from our main findings.
We constructed a funnel plot for analysis of data from more than 10 studies. We found no strong suggestion of publication bias. See Figure 7 for a funnel plot of Analysis 2.3.
7.
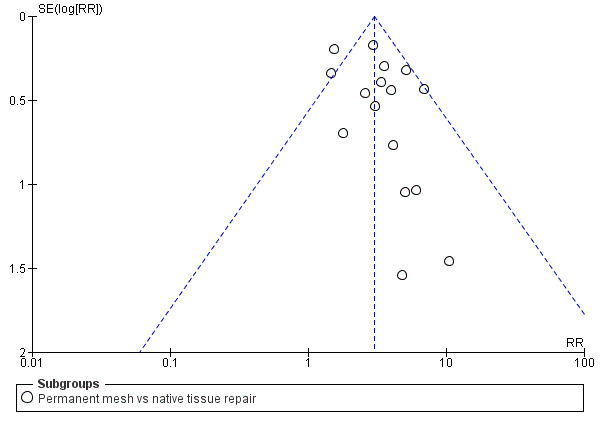
Funnel plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.
Discussion
Summary of main results
Available information on the repair of anterior compartment prolapse is increasing. Evidence generally is not supportive of the use of absorbable mesh or biological grafts as compared with native tissue for repair (anterior colporrhaphy) of anterior compartment prolapse. Two trials (Colombo 2000; Minassian 2010 abstract) compared anterior colporrhaphy and retropubic suspensions (paravaginal/Burch colposuspension) for anterior compartment prolapse and demonstrated similar outcomes for recurrent prolapse; however, the rate of dyspareunia was lower after the abdominal approach was used. These results should be viewed with caution, as the baseline non‐randomised intervention was different in each trial.
Sixteen trials demonstrated advantages of using a polypropylene mesh as compared with native tissue for repair, including lower rates of awareness of prolapse, of anterior wall prolapse on examination and of rate of repeat surgery for prolapse. Disadvantages of mesh utilisation include longer operating time, higher rates of cystotomy, the need for transfusion, de novo stress urinary incontinence and subsequent prolapse of apical or posterior vaginal compartments. The rate of mesh erosion was 11.3%, and 7% required surgery to manage the mesh exposure. The total rate of repeat surgery for prolapse, stress urinary incontinence, mesh exposure or pain was significantly higher after use of transvaginal permanent mesh (10.0%) than after native tissue repair (5.7%). The rate of postoperative dyspareunia was similar in the two groups. Investigators noted no differences between groups on validated pelvic floor dysfunction questionnaires, including the Prolapse and Incontinence Sexual Questionnaire (PISQ), the Prolapse Quality of Life questionnaire (PQOL), the Pelvic Floor Distress Inventory (PFDI‐20), the Urinary Distress Inventory (UDI) and the Pelvic Floor Impact Questionnaire (PFIQ). The quality of evidence supporting these findings ranged from low to moderate.
In conclusion, data demonstrate a clear risk/benefit analysis that can help to guide clinicians and patients in deciding whether to utilise transvaginal polypropylene mesh as compared with native tissue for anterior compartment prolapse repair. Since 2011, some of the mesh products evaluated in this section, including Avaulta (Bard), the Gynemesh overlay, Prolift (Ethicon) and the AMS mesh kit (Perigee), have been removed from the market. However, separate evaluation of primary outcomes with products that remain available for use reveals little change in outcome measures except that rates of repeat surgery for prolapse, stress urinary incontinence and mesh exposure have been evaluated under the auspices of randomised controlled trials. Newer, lighter‐weight mesh implants available for the management of anterior wall prolapse have not been evaluated under the auspices of a randomised controlled trial.
Overall completeness and applicability of evidence
The quality of the 33 trials that addressed the surgical management of anterior vaginal compartment prolapse was variable but is improving. All trials published over the past four years included a CONSORT flow statement, and all reported some form of objective evaluation of anterior vaginal support for the specific pelvic floor defect that was repaired, but full vaginal site‐specific outcomes were available for only 12 trials (Altman 2011; Colombo 2000; Delroy 2013; De Tayrac 2013; Farthmann 2012; Menefee 2011; Nguyen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Vollebregt 2011; Weber 2001). No trials reported outcomes for less than 12 months. Delroy 2013; De Ridder 2004 abstract;Guerette 2009; Lamblin 2014;Meschia 2007;Minassian 2010 abstract and Tamanini 2015 reported two‐year data; Farthmann 2012; Natale 2009 and Nieminen 2008 three‐year data; and Colombo 2000 five‐year data.
Although investigators generally performed randomisation by using a computer‐generated randomisation list, recent trials have reported drawing lots (De Tayrac 2013) and conducting a raffle (Tamanini 2015). Nineteen trials (Allahdin 2008; Altman 2011; Dahlgren 2011; Delroy 2013; El‐Nazer 2007; Feldner 2010; Gandhi 2005; Guerette 2009; Gupta 2014; Hviid 2010; Lamblin 2014; Meschia 2007; Minassian 2010 abstract; Natale 2009; Nguyen 2008; Nieminen 2008; Rudnicki 2014;Sivaslioglu 2008; Weber 2001) reported allocation concealment. Few trials have reported clearly on whether participants and/or reviewers were blinded.
Generally, reporting on the impact of surgery on bladder and sexual function is improving; however, great variation is evident in trialists' choice of outcomes measures. Recent studies (Altman 2011; Delroy 2013; Farthmann 2012;Feldner 2010; Lamblin 2014;Nguyen 2008;Rudnicki 2014;Sivaslioglu 2008; Tamanini 2015;Thijs 2010 abstract; Vollebregt 2011; Withagen 2011) usually include a validated pelvic floor quality of life outcome and report data suitable for meta‐analysis (mean and standard deviation). No trialists have provided a cost analysis.
To minimise risk of bias, it is preferable if surgeons who design studies do not have a financial relationship with the company whose product is being evaluated. Unfortunately, for several studies in this review (Altman 2011; Carey 2009; De Tayrac 2013; Farthmann 2012; Guerette 2009; Withagen 2011), this conflict was feasible and was exacerbated by lack of assurance that reviewers were blinded, resulting in possibly heightened risk of bias for reported outcomes.
Although the rate of mesh exposure reported in this review is consistent with previous reports, adverse events of vaginal pain and/or dyspareunia were commonly reported to the Food and Drug Administration (FDA) in America, and this ultimately triggered the FDA 2011 transvaginal mesh alert that resulted in voluntary removal of many permanent meshes from the market. Among the 15 included trials that evaluated permanent transvaginal mesh only De Tayrac 2013 reported the outcome of vaginal pain on examination at one year, at a rate of 18% in the mesh group as compared with 9% in the native tissue repair group. No reports among the nearly 1000 cases of transvaginal permanent anterior mesh repair described in this review described subsequent surgery performed for vaginal pain and/or dyspareunia.
Quality of the evidence
The quality of evidence in comparisons of native tissue repair versus use of biological grafts is low to moderate owing to risk of bias and imprecision, with lack of reporting of study methods, especially blinding status of reviewers and participants, and unclear risk of data attrition. Low event rate was problematic for both stress urinary incontinence and dyspareunia.
The quality of evidence in comparisons of native tissue repair versus use of polypropylene mesh is generally low to moderate owing to serious risk of bias and imprecision. Bias was related to lack of reporting of allocation concealment and reports that reviewers were unblinded. Findings for stress urinary incontinence and dyspareunia were imprecise.
The quality of evidence in comparisons of native tissue repair versus absorbable mesh is generally low to very low, reflecting smaller, older studies with lack of reporting of study methods, risk of bias due to lack of blinding and high rates of attrition and imprecision.
The quality of evidence related to native tissue repair and use of polypropylene mesh for anterior and/or posterior compartment prolapse is generally low owing to risk of bias (with neither trial reporting methods of allocation concealment and reports that reviewers were unblinded) and imprecision.
Potential biases in the review process
We are sure that we have avoided bias during the review process. We have systematically searched multiple electronic databases for published and unpublished evidence, regardless of language or date of publication. We have adhered to Cochrane methods for selection of studies and extraction of data for inclusion in this review.
Agreements and disagreements with other studies or reviews
The Medicines and Healthcare Products Regulatory Agency (MHRA) presented a report in late 2014 (MHRA 2014) after reviewing literature described in the York report in 2012, commissioning another literature review in 2012, taking submissions from support groups and reviewing adverse event reports submitted to MHRA, engaging with professional organisations and regulatory bodies in the European Union and the USA and participating in the European Commission (EC) Task Force Group on vaginal mesh implants. The full report was extensive and on the basis of reported data concluded that:
for most women, the use of vaginal mesh implants is safe and effective; and
when these products are used correctly, they can help alleviate the very distressing symptoms of stress urinary incontinence (SUI) and pelvic organ prolapse (POP), thus resulting in benefit outweighing risk.
We have demonstrated advantages associated with transvaginal mesh related to utilisation in the anterior compartment, where permanent mesh resulted in decreased awareness of prolapse, prolapse on examination and reoperation for prolapse; however, transvaginal permanent mesh was associated with increased morbidity, including increased operating time, blood loss, transfusion and cystotomy, and higher rates of de novo stress urinary incontinence and de novo dyspareunia, as compared with native tissue. The rate of mesh exposure was 11.3%, and surgery for mesh exposure was required in 7%.
We have concluded, in contrast to the MHRA 2014 report, that although mesh utilisation may be warranted in individual cases of anterior compartment prolapse, it cannot be considered a first‐line treatment option for women with anterior compartment prolapse because of the not insignificant morbidity surrounding transvaginal mesh usage.
These differences in findings and conclusions between the MHRA 2014 report and our report can be explained by the fact that many new randomised controlled trials were not included in the MHRA 2014 review, which was informed by literature reviews published in 2012.
It is interesting to note that in our review of nearly 1000 cases of anterior transvaginal mesh, we were unable to identify a single case in which further surgical intervention was undertaken for pain and/or dyspareunia, although these were among the leading adverse events reported to the FDA in the USA in 2011. In the MHRA 2014 report, adverse events were reported separately by healthcare professionals and members of the public. In reports from healthcare professionals, mesh exposure accounted for 42% of complaints, and pain accounted for 13%. In contrast, in reports made by the public, pain was the leading complaint in 15%, and mesh exposure was described in 12% of reports. The disparity between the facts that pain accounted for many complaints to regulatory authorities in the United Kingdom and the USA but it did not account for a single surgical intervention in nearly 1000 transvaginal mesh surgeries included in this review remains difficult to explain.
Furthermore, and in contrast to the MHRA 2014 report, we have highlighted that most of the data informing our report were derived from transvaginal mesh products that were voluntarily removed from the market in 2012, and that transvaginal mesh products currently available for use have not been evaluated under the auspices of randomised controlled trials. We believe it is prudent that until data on currently available transvaginal mesh products become available, these products should be utilised under the discretion of the local ethics committee.
Authors' conclusions
Implications for practice.
The first implication for practice is that little evidence supports the use of absorbable mesh or biological grafts in anterior compartment prolapse surgery. Distinct advantages and disadvantages of utilising transvaginal polypropylene mesh as compared with native tissue for repair have necessitated detailed consultation and consent before permanent mesh is used. Given the significant morbidity associated with anterior transvaginal polypropylene mesh, current evidence does not support its use as a first‐line intervention for anterior compartment prolapse. Furthermore, as many of the mesh products evaluated have been voluntarily removed from the market, and given that none of the newer, lighter‐weight, single‐incision mesh products have been evaluated under the rigours of a well‐designed randomised controlled trial, clinicians must be cautious in using these newer products and would be best served by doing so only after ethics committee review. Careful detailed and transparent consultation with individual patients is required before these procedures are performed. Reporting of longer‐term results is encouraged.
Implications for research.
Urgent evaluation of newer, lighter‐weight polypropylene meshes is needed to determine whether they are effective, and if morbidity is less than with earlier products. Trialists should include validated quality of life data in their methods. Newer materials, possibly produced via tissue engineering and bio‐design, are worthy of further research to assist in the development of products that will supplement and deliver excellent anatomical and functional outcomes of reconstructive gynaecological surgery.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2017 | Amended | Acknowledgements section edited to recognise the contribution of the Cochrane Incontinence Group's Information Specialist Sheila Wallace; detail added to External sources of support by NIHR, UK |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 20 October 2016 | New citation required but conclusions have not changed | Review updated with 10 new trials incorporated (Dahlgren 2011; Delroy 2013; De Tayrac 2013; Farthmann 2012; Gupta 2014; Lamblin 2014; Robert 2014; Rudnicki 2014; Tamanini 2015; Turgal 2013) |
| 20 October 2016 | New search has been performed | Review updated with 10 new trials incorporated |
| 14 April 2010 | Amended | Changed citation, added conflicts |
| 17 November 2009 | New citation required but conclusions have not changed | Full reports of 59 potentially eligible studies were assessed; for this update, 23 new eligible studies were assessed (Al‐Nazer 2007a; Ali 2006a; Allahdin 2008; Barber 2006; Biller 2008; Borstad 2008; Braun 2007a; Carramao 2008a; Constantini 2008; de Tayrac 2008; Dietz 2008a; Glavind 2007; Guerette 2006a; Lim 2007a; Meschia 2007a; Natale 2007; Natale 2009; Nguyen 2008; Nieminen 2008; Pantazis 2008a; Schierlitz 2007a; Segal 2007; Sivaslioglu 2008). Overall, 17 studies were excluded from the review, six during this update (Barber 2006; Biller 2008; Carramao 2008a; Glavind 2007; Meschia 2007a; Segal 2007): full details are given under 'Characteristics of excluded studies'. In this second update, 18 new trials were added (Al‐Nazer 2007; Ali 2006; Allahdin 2008; Borstad 2008; Braun 2007a; Constantini 2007; Constantini 2008; de Tayrac 2008; Dietz 2008a; Guerette 2006; Lim 2007; Natale 2007; Natale 2009; Nguyen 2008; Nieminen 2008; Pantazis 2008; Schierlitz 2007; Sivaslioglu 2008), and three previously included studies were updated (Brubaker 2008; Meschia 2007; Roovers 2004). |
| 9 February 2009 | New search has been performed | New search February 2009 |
| 10 October 2008 | Amended | Converted to new review format |
| 17 April 2007 | New citation required and conclusions have changed | Substantive update (2007, Issue 3). 22 RCTs (8 new included trials). Findings are insufficient to provide robust evidence to support current and new practice (such as whether to perform a concurrent continence operation, or to use mesh or grafts). |
Acknowledgements
We would like to thank Sheila Wallace, Information Specialist of the Cochrane Incontinence Review Group, for designing the search strategy and running the searches for this review. We gratefully acknowledge the work and support of the Cochrane Gynaecology and Fertility Group, specifically, Helen Nagels, Jane Marjoribanks and Professor Cindy Farquhar, in completing this review.
Appendices
Appendix 1. Types of operations
Sacral colpopexy
Aim To correct upper genital tract prolapse
Indication Usually reserved for recurrent prolapse of the upper vagina (recurrent cystocele, vault or enterocele) or massive vaginal eversion
Surgical technique
Usually performed under general anaesthesia
Performed through an incision on the lower abdomen or keyhole
Bladder and rectum freed from the vagina and permanent mesh supporting front and back wall of the vagina
Mesh secured to the sacrum (upper tailbone)
Peritoneum (lining of the abdominal cavity) closed over the mesh
Other repairs performed as required at the same time, including paravaginal repair, perineoplasty, colposuspension or rectopexy
Bowel preparation required before surgery
McCall culdoplasty
Indications
Vault prolapse or an enterocele
Often performed at the time of vaginal hysterectomy to prevent future prolapse
Surgical technique
After removal of the uterus at the time of hysterectomy, uterosacral ligaments identified and incorporated into closure of the peritoneum and upper vagina with one to two sutures
Anterior or posterior vaginal repair often performed at the same time
Sacrospinous fixation
Aim To offer support to the upper vagina, minimising risk of recurrent prolapse at this site. The advantage of this surgery is that vaginal length is maintained
Indication Upper vaginal prolapse (uterine or vault prolapse, enteroceles)
Procedure can be used in reconstructive vaginal surgery when increased vaginal length is required
Procedure
Procedure can be performed under regional or general anaesthesia
Routine posterior vaginal incision is made and is extended to the top of the vagina
Using sharp dissection, the vagina is freed from the underlying rectovaginal fascia and rectum until the pelvic floor (puborectalis) muscle is seen
Via sharp and blunt dissection, the sacrospinous ligament running from the ischial spine to the sacral bone is palpated and identified
Two sutures are placed through the strong ligament and are secured to the top of the vagina. This results in increased support to the upper vagina with no shortening of the vagina
Other fascial defects in the vagina are repaired, and the vaginal skin is closed
Anterior vaginal repair (colporrhaphy)
Indication
Prolapse of the bladder or urethra
Sometimes used to treat urinary stress incontinence
Surgical technique
Procedure can be performed under regional or general anaesthesia
Vagina overlying the bladder and urethra is incised at the midline
Dissection in a plane directly below the vagina allows exposure of the damaged fascia supporting the bladder and urethra
Fascia is plicated at the midline via delayed absorbable or permanent sutures
Sometimes excessive vaginal skin is removed
Vaginal skin is then closed
Other sites of prolapse are repaired as required
Posterior vaginal repair and perineoplasty
Indications Treatment of rectocele (rectum bulges or herniates forward into the vagina) and defects of the perineum (area separating entrance of the vagina and anus)
Aim To correct defects in the rectovaginal fascia separating rectum and vagina while allowing bowel function to be maintained or corrected without interfering with sexual function
Surgical technique
Incision is made on the posterior wall of the vagina starting at the entrance and finishing at the top of the vagina
Vagina and rectovaginal fascia are dissected from the vagina until the pelvic floor muscles (puborectalis) are located
Defects in the fascia are corrected by central plication of the fascia with delayed absorption sutures
Perineal defects are repaired by placing deep sutures into the perineal muscles to build up the perineal body
Overlying vaginal and vulval skin is then closed
A pack is usually placed into the vagina and a catheter into the bladder at the end of surgery
Anterior or posterior vaginal repair, or both (colporrhaphy)
Indications
Anterior repair: treatment for prolapse of bladder (bladder bulges forward into the vagina; cystocele) or urethra
Posterior repair: correction of bowel prolapse (rectum bulges forward into the vagina; rectocele)
Vault repair: treatment for prolapse of upper vagina
Depending on the side of the defect, the repair can be anterior, posterior, vault or total. Repair is achieved by placement of permanent mesh, which may result in a stronger repair
Surgical technique
Procedure can be performed under regional or general anaesthesia Anterior vaginal repair
Midline incision to the vagina overlying the bladder and urethra
Dissection in a plane directly below the vagina and lateral to the bladder allows exposure of damaged fascia supporting the bladder
Fascia is plicated at the midline with sutures
Mesh can be used to reinforce the repair and can be used as an inlay or anchored through the obturator foramen, exiting through small incisions at both sides of the upper inner thigh
Vaginal skin is closed
Posterior and vault repair
Incision is made to the posterior wall of the vagina
Dissection below the vagina identifies the rectovaginal fascia and opens the space between the rectum and the pelvic floor muscle to the sacrospinous ligaments
Defects in the fascia are corrected by central plication of the fascia with sutures
Mesh can be used to reinforce the repair and can be used as an inlay or anchored bilaterally to the pelvic side wall, exiting through a small incision approximately 3 cm lateral and down from the anus
Vaginal skin is closed
Vaginal paravaginal repair
Aim To reattach detached lateral vaginal fascia to its normal point of insertion on the lateral side wall. This firm area of attachment is termed the white line or arcus tendineus fascia pelvis
Indication Repair of anterior wall prolapse due to defects in lateral supporting tissues
Procedure Procedure can be performed under regional or general anaesthesia
Routine anterior repair Sharp dissection of the vagina from the bladder fascia continues laterally until the pelvic side wall can be identified
Permanent or delayed absorbable sutures are placed from the lateral vagina to the firm pelvic side wall tissue (white line or arcus tendineus fascia pelvis). Three to four sutures are placed on each side
Routine anterior repair with midline plication of the fascia, trimming of excess vaginal skin as required, and closure of the vaginal skin
Appendix 2. Searches
Search strategy
The Incontinence Group Specialised Register was searched using the Group's own keyword system (all searches were of the keyword field of Reference Manager 2012). The search terms used were:
({design.cct*} OR {design.rct*}) AND ({topic.prolapse*}) AND ({intvent.surg*})
Date of the most recent search of the register for this review: 23 August 2016
Ongoing studies:
Search registered trials: clinicaltrials.gov: date 1 August 2016. Terms: "Vaginal prolapse", "Surgery for prolapse" with 176 trials identified
Data and analyses
Comparison 1. Native tissue versus biological graft.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Anterior repair vs any biological graft | 5 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.82] |
| 1.2 Anterior repair vs fascial plication with porcine dermis graft | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.21, 2.10] |
| 2 Repeat surgery | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 7 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.53, 1.97] |
| 3 Recurrent anterior compartment prolapse | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Anterior repair vs any biological graft | 8 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |
| 3.2 Anterior repair vs fascial plication with porcine dermis graft | 4 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.98, 1.70] |
| 4 Stress urinary incontinence | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.79, 2.64] |
| 5 POPQ assessment | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| 5.1 Point Ba POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| 6 Urge incontinence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Voiding dysfunction | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.71, 1.80] |
| 8 Dyspareunia | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.39, 1.93] |
| 9 Quality of life PROLAPSE | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Questionnaire (P‐QOL) 0‐100 | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.01, 4.01] |
| 10 Operating time (minutes) | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐10.35 [‐14.45, ‐6.24] |
| 11 Hospital stay | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.09, 0.69] |
1.9. Analysis.
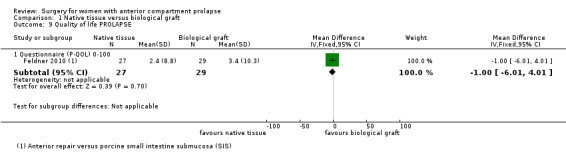
Comparison 1 Native tissue versus biological graft, Outcome 9 Quality of life PROLAPSE.
Comparison 2. Native tissue versus polypropylene mesh.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse | 9 | 1133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.37, 2.28] |
| 2 Repeat surgery | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 12 | 1629 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.15, 3.58] |
| 2.2 Reoperation for stress urinary incontinence (1‐3 years) | 5 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.36] |
| 2.3 Surgery for prolapse, SUI or mesh exposure | 12 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.83] |
| 3 Recurrent anterior compartment prolapse | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| 3.1 Permanent mesh vs native tissue repair | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| 4 Bladder injury | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Anterior repair vs any transvaginal polypropylene mesh | 6 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.82] |
| 5 Apical or posterior compartment prolapse | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.99] |
| 6 POPQ assessment | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Point Ba POPQ | 6 | 568 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.30, 0.80] |
| 6.2 Point Bp POPQ | 3 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 6.3 Point C POPQ | 4 | 369 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.47, 1.01] |
| 6.4 Total vaginal length | 3 | 366 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.78, 0.43] |
| 7 Stress urinary incontinence (de novo) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Polypropylene mesh vs native tissue (de novo) | 6 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.01] |
| 8 De novo dyspareunia | 8 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.06] |
| 9 Voiding dysfunction | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Anterior repair vs polypropylene mesh (persistent) | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.33, 4.47] |
| 10 Urge incontinence | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Anterior repair vs transvaginal permanent mesh | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.33, 14.68] |
| 11 Dyspareunia | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 11.1 Anterior repair vs any transvaginal polypropylene mesh | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 12 Quality of life PROLAPSE | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Questionnaire (PQOL) 0‐100 | 2 | 164 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐1.19, 3.37] |
| 12.2 Pelvic Floor Impact Questionnaire (PFIQ‐7) 0‐400 | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐7.78, 11.59] |
| 12.3 Pelvic floor distress inventory (PFD1‐20) 0‐300 | 3 | 294 | Mean Difference (IV, Random, 95% CI) | 3.89 [‐12.82, 20.61] |
| 12.4 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 4 | 741 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.76, 0.64] |
| 12.5 ICIQ‐QOL | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.15, 1.55] |
| 12.6 ICIQ‐VS | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.88, 3.08] |
| 13 Hospital stay (days) | 5 | 707 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.17, 0.33] |
| 14 Operating time (minutes) | 7 | 1099 | Mean Difference (IV, Random, 95% CI) | ‐17.89 [‐25.81, ‐9.98] |
| 15 Transfusion | 4 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.76] |
Comparison 3. Subgroup analysis: native tissue versus polypropylene mesh available for use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse | 5 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.35, 3.35] |
| 2 Repeat surgery | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.20, 4.59] |
| 2.2 Repeat surgery for stress urinary incontinence (1‐3 years) | 2 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.60, 4.10] |
| 2.3 Repeat surgery for prolapse, SUI or mesh exposure | 6 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.24] |
| 3 Recurrent anterior compartment prolapse | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
| 3.1 Permanent mesh vs native tissue repair | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
Comparison 4. Native tissue versus absorbable mesh.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse (2‐year review) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.31] |
| 2 Repeat surgery for prolapse (2 years) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.42, 10.82] |
| 3 Anterior compartment prolapse (3 months‐2 years) | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.09, 2.06] |
| 4 Death | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Posterior compartment prolapse | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.31, 2.49] |
| 6 Stress urinary incontinence | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 7 Quality of life | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| 7.1 VA QOL | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
4.1. Analysis.
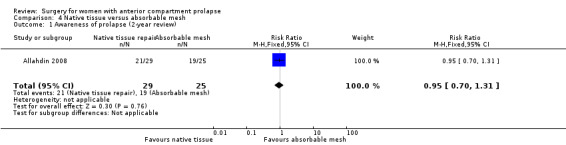
Comparison 4 Native tissue versus absorbable mesh, Outcome 1 Awareness of prolapse (2‐year review).
4.2. Analysis.
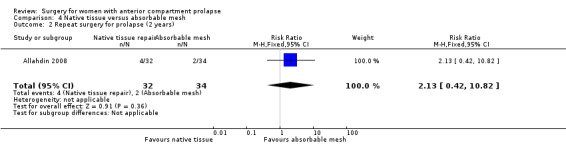
Comparison 4 Native tissue versus absorbable mesh, Outcome 2 Repeat surgery for prolapse (2 years).
4.7. Analysis.
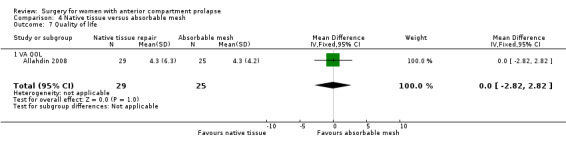
Comparison 4 Native tissue versus absorbable mesh, Outcome 7 Quality of life.
Comparison 5. Mesh versus biological graft.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Polypropylene mesh (Gynemesh) vs porcine dermis | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.73] |
| 2 Repeat surgery | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.87, 10.73] |
| 3 Recurrent anterior wall compartment prolapse (stage 2 or greater) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Permanent mesh vs biological graft | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 3.2 Absorbable mesh vs biological graft | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 3.22 [1.38, 7.52] |
| 4 Mesh exposure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Polypropylene mesh vs porcine dermis | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 5 Stress urinary incontinence (de novo) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.18, 21.23] |
| 6 Urgency, detrusor overactivity or overactive bladder (de novo) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.78] |
| 7 Dyspareunia (persistent) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.80] |
5.5. Analysis.
Comparison 5 Mesh versus biological graft, Outcome 5 Stress urinary incontinence (de novo).
5.6. Analysis.
Comparison 5 Mesh versus biological graft, Outcome 6 Urgency, detrusor overactivity or overactive bladder (de novo).
5.7. Analysis.
Comparison 5 Mesh versus biological graft, Outcome 7 Dyspareunia (persistent).
Comparison 6. Vaginal repair versus abdominal repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent anterior wall prolapse | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.46] |
| 2 Injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bladder | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 14.88] |
| 3 Posterior compartment prolapse | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.17, 19.65] |
| 4 POPQ assessment | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Point Ba POPQ | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.15, 1.95] |
| 4.2 Total vaginal length | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [2.58, 3.82] |
| 5 Dyspareunia | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [1.63, 16.35] |
| 6 Quality of life PROLAPSE | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Pelvic floor impact questionnaire (PFIQ‐7) 0‐400 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐52.11, 34.11] |
| 6.2 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐6.24, 2.24] |
| 7 Operating time (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 7. Native tissue repair versus graft repair for anterior and/or posterior prolapse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse | 3 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.36, 1.99] |
| 1.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.70, 1.96] |
| 1.2 Anterior and/or posterior repair vs porcine dermis (Pelvicol) | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.96] |
| 2 Repeat surgery prolapse | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| 2.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| 3 Recurrent anterior wall prolapse (stage 2 or greater) | 3 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| 3.1 Anterior colporrhaphy vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.70, 1.97] |
| 3.2 Anterior colporrhaphy vs biological graft | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.30] |
| 3.3 Anterior and/or posterior repair vs biological graft | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.56] |
| 4 Bladder injury | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.01] |
| 5 Stress urinary incontinence (de novo) | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.34, 2.85] |
| 6 Dyspareunia (de novo and persistent) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Anterior repair vs polypropylene mesh (de novo) | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.64, 2.36] |
| 6.2 Anterior repair vs polypropylene mesh (persistent) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 7 Quality of life PROLAPSE | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.74, 3.54] |
7.4. Analysis.
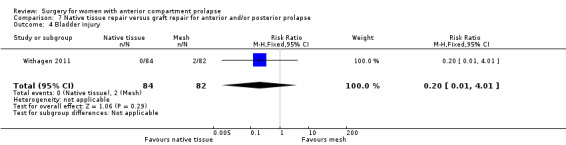
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 4 Bladder injury.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 2006 abstract.
| Methods | Single‐centre RCT Inclusion grade 3 or 4 cysto‐urethrocele (BW halfway system) No exclusion No power Randomisation and concealment, blinding NS 6/12 follow‐up |
|
| Participants | No CONSORT N = 108 Inclusion: women with grade 3 or 4 cysto‐urethrocele (BW halfway system) No significant differences between groups regarding preoperative storage symptoms, urodynamics and degree of prolapse |
|
| Interventions | A (54): anterior colporrhaphy alone B (54): anterior colporrhaphy with tension‐free polypropylene (Gynemesh PS) overlay |
|
| Outcomes | Assessed at 6 months postop Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant attrition: group AC: 46/54: mesh 43/54 completed 6/12‐month review |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Unclear risk | Not stated |
Allahdin 2008.
| Methods | Single‐centre RCT comparing vaginal fascial repair with or without polyglactin mesh and with polydioxanone or polyglactin sutures; 2 × 2 factorial design PC randomisation, "secure" remote concealment Blinded participants, ward staff and follow‐up assessor Follow‐up at 3 months with exam, at 6 months with non‐validated questionnaire, at 2 years with validated questionnaire |
|
| Participants | 73 randomised, 7 ineligible after randomisation, 66 included in trial Lost to follow‐up: 8 at 3 months, 4 at 6 months, 12 at 2 years Inclusion: grade 2 or greater prolapse (unclear examination technique), anterior and/or posterior prolapse Concomitant procedures: vaginal hysterectomy 14, cervical amputation (Manchester) 18, TVT 13 |
|
| Interventions | A (32): repair with polyglactin mesh overlay B (34): repair without mesh C (33): repair of fascia with polydioxanone sutures D (33): repair of fascia with polyglactin sutures |
|
| Outcomes | Assessed at 3 months, 6 months and 2 years postop Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Secure method of concealment of randomisation (remote computer allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Allocation concealed from women |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reviewers blinded; participant‐completed questionnaires; data entry blinded to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal non‐response between groups, medical records seen for all non‐responders: 1 year ‐ Vicryl mesh 29/32, no mesh 32/34, PDS 29/33, Vicryl suture 33/33 |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | Unfunded study |
Altman 2011.
| Methods | Multi‐centre RCT: 53 centres, 58 surgeons 90% powered to detect 20% differences between groups with 1% type 1 error, central randomisation PC Participants blinded Reviews conducted for 2 and 12 months by surgeon 1/3, non‐surgeon 2/3 Completed before and at 1 year: Urogenital Distress Inventory (UDI) and Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ‐12) |
|
| Participants | 1685 screened, 389 randomised Underwent surgery: A 182, B 191 Lost to follow‐up: A 7, B 14 (1 year: A 182, B 186) Inclusion: > 18 years, ≥ stage 2 symptomatic cystocele POPQ Exclusion: previous cancer of any pelvic organ, systemic glucocorticoid treatment, insulin‐treated diabetes, inability to participate or provide consent, need for concomitant surgery |
|
| Interventions | A (182): anterior colporrhaphy, slow absorption monofilament thread, sham skin markings, excessive trimming of vagina discouraged B (191): Gynecare transvaginal anterior mesh (Prolift), absorbable sutures, excessive vaginal trimming discouraged, catheter care at discretion of surgeon |
|
| Outcomes | Assessed at 1 year postop Reported the following review outcomes at 1 year
Sexual function: dyspareunia, PISQ (end scores with 95% CI) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Secure concealment with remote computer |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded (sham skin markings) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reviewers: surgeon 1/3, non‐surgeon 2/3 Participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant flow accounted for completely in both groups: at 1 year ‐ 186/206 AC, 182/204 mesh |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported with exception of mesh exposure (personal communication) |
| Other bias | High risk | Funded by Karolinska Institute and Ethicon: Conflict of interest statements from members of Nordic transvaginal mesh group who were reviewers of surgery were not provided |
Carey 2009.
| Methods | Single‐centre RCT CONSORT: no Randomisation computer generated Allocation concealment NS Participants, surgeons and reviewers not blinded 12‐Month follow‐up |
|
| Participants | Inclusion criteria: women recommended for vaginal surgery for anterior and posterior compartment with ≥ grade 2 prolapse Exclusion criteria: requiring only anterior or posterior compartment surgery with apical prolapse beyond the hymen, those requiring abdominal mesh surgery Randomised: 139 (A 70, B 69); 10 women breached study protocol and 11 more were recruited. All were analysed. Lost to follow‐up: A 6, B 9 Analysed at 12 months: A 63, B 61 |
|
| Interventions | A (70): traditional anterior and posterior fascial plication with polydioxanone sutures B (69): anterior and posterior repair with Gynemesh PS augmentation |
|
| Outcomes | Assessed at 6 months and 1 year postop Reported the following review outcomes at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | No information on allocation concealment. Significant preoperative data missing, as above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear follow‐up of participants in both groups: 1 year ‐ no mesh 62/78 (89%), mesh 61/69 (88%) |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | High risk | |
Colombo 2000.
| Methods | Single‐centre RCT (computer‐generated open number list ) Burch or anterior repair for pelvic organ prolapse and stress urinary incontinence PC open list Follow‐up: A 14.2, B 13.9 years | |
| Participants | 71 randomised Lost to follow‐up: 3 (A 2, B 1) 68 analysed Inclusion: USI, cystocele > 2 or 3, swab test > 30% Exclusion: detrusor overactivity, previous pelvic floor surgery, high risk for abdominal operation | |
| Interventions | A (35): Burch group: total abdominal hysterectomy and vault to uterosacral ligament, Moschcowitz, Burch with 3‐4 Ethibond B (33): anterior colporrhaphy: vaginal hysterectomy, pouch of Douglas obliteration, anchoring of vaginal cuff to uterosacral ligament, catgut plication | |
| Outcomes | Definition of cure: no subjective stress urinary incontinence, no positive stress test Objective cure ‐ cystocele: A 23/35, B 32/33 Subjective cure ‐ stress urinary incontinence: A 30/35, B 17/32 Objective cure ‐ stress urinary incontinence: A 26/35, B 14/32 Overactive bladder symptoms, voiding, dyspareunia Total vaginal length: A 7.9 cm, B 4.7 cm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Inadequate: computer‐generated randomisation by an open list |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | AC group 33/34 (97%), Burch colposuspension 35/37 (95%) |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Unclear risk | Not stated |
Dahlgren 2011.
| Methods | Multi‐centre (8), Swedish open RCT Computer‐generated block randomisation stratified for each centre Allocation concealment by opaque sealed envelopes SS 160 would allow 90% power to detect 15% difference between groups with 5% alpha error and dropout rate of 10% 3‐Year review Intention to treat and CONSORT not stated |
|
| Participants | Inclusion: recurrent (prior surgery on prolapsing site) POP in anterior and/or posterior compartment No exclusion criteria 135 randomised Gp A native tissue repair 66, 3 years 60/66 Gp B porcine dermis repair 65, 3 years 65/68 |
|
| Interventions | Standardised surgery with 2 meeting workshops before the study Native tissue repair; midline fascial plication with interrupted polydioxanone suture, vagina closed with polyglactin absorbable suture Porcine: porcine dermal implant (Pelvicol, Bard, Sweden) as inlay with no fascial plication: inlay anchored to vaginal wall and fascia with 6‐8 polydioxanone suture, vagina closed with polyglactin suture Concomitant MUS, apical support and levator plication performed as required |
|
| Outcomes | Assessed at 3 months and 3 years Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomisation list stratified for each centre |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes (unclear if consecutive) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nil |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Gp A 60/68, Gp B 65/68 completed 3‐year review |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | Low risk | No COI; funded by local research institutes |
De Ridder 2004 abstract.
| Methods | RCT (unclear randomisation and concealment) Pelvicol vs Vicryl for stage 3 cystocele repair Follow‐up: 25/26 months | |
| Participants | 134 included A 65, B 69 Inclusion: stage 3 cystocele | |
| Interventions | A (65): Raz 4 defect cystocele repair reinforced with porcine dermis overlay (Pelvicol) B (69): as above, reinforced with Vicryl Concomitant surgery: vaginal hysterectomy and rectocele repair | |
| Outcomes | Primary outcome: recurrence of cystocele stage 2: A 6/63, B 19/62 (P = 0.002) Number having repeat prolapse surgery: A 3/63, B 9/62 No differences in questionnaires | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Outcome data reported |
| Other bias | Unclear risk | Not stated |
De Tayrac 2013.
| Methods | Multi‐centre (12 French hospitals) RCT 12‐Month review Randomisation by drawing lots, stratified by centre Allocation concealment not discussed Intention to treat stated yes, but participants already randomised were removed if cystotomy occurred during surgery CONSORT guidelines Sample size of 194 provided 80% power to detect 20% difference with 5% alpha error and dropout rate of 10% Assessors not clear |
|
| Participants | Inclusion criteria: symptomatic stage 2 anterior wall prolapse, 60 years of age or older Exclusion criteria: steroids, poorly controlled diabetes, prior pelvic radiation, untreated vaginal or urinary infection, ascites, bladder injury during procedure All used preoperative oestrogen therapy 163 included, 162 randomised Gp A (82): 1 year 67/82 Gp B (80): 1 year 66/60 Preop demographics and potential confounders similar in both groups, except colorectal impact greater in AC group |
|
| Interventions | Gp A anterior colporrhaphy (AC): no mesh (plication of fascia with 2.0 polyglactin absorbable suture), uterosacral colpopexy and hysterectomy as required Gp B anterior polypropylene: macroporous mesh (Ugtex, Sofradim, Covidien), 4‐armed transobturator mesh, fixed with 2 × 2.0 permanent polypropylene sutures to uterine isthmus or uterosacral ligaments and 2 × 2.0 polyglactin sutures to inferior edge of pubic rami, vaginal trimming minimised Concomitant surgery: MUS, hysterectomy and any native tissue repair, but no other transvaginal mesh intervention included |
|
| Outcomes | Assessed at 1‐year follow‐up Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by drawing lots? |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Gp A 82, 1 year 67/82 Gp B 80, 1 year 66/80, 20% attrition |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | High risk | Study author COI with Sofradim, which provided partial funding and whose product was being evaluated |
Delroy 2013.
| Methods | Single‐centre non‐inferiority RCT Computer‐generated random number list Allocation at inclusion with surgeon aware only in OT Envelope allocation Sample size; 35 in each group, 80% power to detect 5% significant change with 10% dropout Intention‐to‐treat analysis Assessors blinded Participants unblinded |
|
| Participants | Any anterior POP point Ba ≥ +1 on POPQ Excluded malignant urogenital disease, prior radiation, acute genitourinary infection, connective tissue disorders, steroid treatments, insulin‐dependent diabetes |
|
| Interventions | All procedures performed under spinal by 3 experienced surgeons AC: Plicate fascia pursestring Vicryl 0, vaginal trimming, transvaginal Trocar‐guided polypropylene mesh (kits donated by Promedon), Nazca TC (Promedon, Corboda, Argentina), prepubic and 2 transobturator macroporous monofilaments, vagina closed in overlapping fashion 355 accessed, 79 randomised AC: 39 completed 1‐year review Anterior mesh: 40 randomised, 40 completed 1‐year review Concomitant surgery as required |
|
| Outcomes | Assessed at 1 year Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation tables |
| Allocation concealment (selection bias) | Unclear risk | Envelopes (opaque?, sealed?) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 79 randomised; all completed 1‐year review |
| Selective reporting (reporting bias) | Low risk | Most outcome data reported |
| Other bias | Low risk | Funded by Federal university of Sao Paulo, Brazil; Promedon contributed product free of charge No author COI reported |
El‐Nazer 2007.
| Methods | Single‐centre RCT for stage 2 POPQ prolapse PC‐generated randomisation 2‐Year follow‐up No CONSORT statement Blinding not stated Power of 80%, need sample size of 20 in each arm if subsequent prolapse surgery in 1 group 11% and 44% in mesh group |
|
| Participants | 40 randomised Inclusion criteria: stage 2 POPQ cystocele with no plans for pregnancy in 12 months Exclusion criteria: contemplating pregnancy, patients with paravaginal defects, need for continence surgery, prior colposuspension or vaginal surgery, immunocompromised, diabetic |
|
| Interventions | A (23): anterior colporrhaphy AC 0 polyglactin Vicryl suture B (21): self‐styled armless soft polypropylene (Gynemesh) mesh without AC |
|
| Outcomes | Assessed at 6 weeks, 3 months, then every 6 months to 2 years postop Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | PC‐generated randomised number tables |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes to ensure allocation concealment: as not consecutive, rated as unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reviewers blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 1 year, completed review; no mesh 20/23, mesh 20/21 |
| Selective reporting (reporting bias) | Low risk | Main outcome reported |
| Other bias | Unclear risk | Funding not stated; study authors reported no COI |
Farthmann 2012.
| Methods | Prospective open‐label RCT, multi‐centre (n = 6) 3 years Randomisation and allocation concealment not stated CONSORT and intention to treat, no |
|
| Participants | Inclusion: cystocele greater than or equal to stage 2, with risk factors of recurrent prolapse, overweight, COPD, chronic obstipation Exclusion: younger than 18 years, not completed family, allergy to polypropylene, prior mesh; prior cancer of lower urinary tract, genital organs, rectosigmoid 200 randomised, 177/200 at 3 years |
|
| Interventions | GP A: partially absorbable polypropylene mesh (Seratom, Germany), coated in polyglycolic acid and caprolactone, which is absorbed at 120 days, leaving light weight of 17 g/m2 GP B: polypropylene mesh, 6 arms, 29 g/m2 Concomitant surgery performed |
|
| Outcomes | Review outcomes at 3 years
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, computer generated, stratified for each centre |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 175/200 at 3 years, groups Gp A: partially absorbable polypropylene mesh 89/97 (92%); Gp B: polypropylene mesh 86/102 (84%) |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | High risk | Study sponsored by company whose product was evaluated; unblinded reviewers had COI with the company whose product was being evaluated (Serag‐Wiessner, Naila, Germany) |
Feldner 2010.
| Methods | Single‐centre RCT Randomisation and allocation concealment described Evaluated 1 year after anterior colporrhaphy (AC) as compared with small intestine submocosa graft Blinded reviewers |
|
| Participants | Inclusion criteria: women with point Ba ≥ ‐1 Exclusion criteria: those with hypertension, prior radiation, pelvic sepsis, diabetes and chronic illness Concomitant surgery allowed, including vaginal hysterectomy, if greater than stage 2 uterine prolapse |
|
| Interventions | Gp A (27): anterior colporrhaphy with interrupted 0 Vicryl sutures Gp B (29): non‐cross‐linked xenograft porcine small intestine submucosa 7 × 10 cm, with dissection to suprapubic arch, fixed with 0 prolene ×3 each side |
|
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Allocation concealment appropriate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded reviewers and participant‐completed validated questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data well described: 1 year AC 27/27, SIS 29/29 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data reported |
| Other bias | Low risk | No COI and no external funding |
Gandhi 2005.
| Methods | Single‐centre RCT (computer generated, opaque envelopes, adequate concealment) Anterior colporrhaphy with and without fascia lata for primary or recurrent anterior vaginal wall prolapse | |
| Participants | 162 signed consent form 154 randomised A 76, B 78 Loss to follow‐up: 2 in B, but in results, 78 and 77 analysed Inclusion: anterior vaginal wall prolapse to hymen or beyond on straining, > 18 years of age, willing to comply with return visits Concomitant surgery: vaginal hysterectomy in 49%/47%, sacrospinous fixation in 43%/42% (all cases with vaginal vault prolapse to mid‐vagina or beyond), posterior repair in 99%/94%, Coopers' ligament sling in 67%/55%, mid‐urethral sling in 13%/10% Enterocele: A 75%, B 73% Baseline voiding dysfunction (slow stream): A 48/68, B 42/65 | |
| Interventions | A (76): "ultra‐lateral" midline plication of anterior endopelvic connective tissue using Vicryl buttress sutures (as described by Weber 2001), plus additional cadaveric fascia lata patch (Tutoplast) anchored at the lateral limits of the colporrhaphy B (78): as above without allograft | |
| Outcomes | Assessed at 1 year Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete at 1 year: AC 76/78 (97%), biological 76/76 |
| Selective reporting (reporting bias) | Low risk | Main outcome reported |
| Other bias | Unclear risk | No COI and no funding statement |
Guerette 2009.
| Methods | Multi‐centre RCT 24‐Month follow‐up Randomisation computer generated Allocation concealment without blinding of participants or surgeon Not according to CONSORT |
|
| Participants | Randomised: Gp A 47, Gp B 47 2 years: Gp A 33, Gp B 26 Examination: A 27, B 17 Inclusion criteria: point Ba ≥ ‐1, Exclusion criteria: TVL < 6 cm, severe atrophy, isolated paravaginal defect, allergic bovine material prior vaginal implant surgery, those with ulceration |
|
| Interventions | A (46): anterior colporrhaphy B (44): anterior colporrhaphy with bovine pericardium collagen matrix graft reinforcement |
|
| Outcomes | Assessed at 6 months, 1 year and 2 years Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether assessors were blinded, participant‐completed questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Equal losses in both groups, only 50% at 2‐year review: AC 33/47: biological 26/47 |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | High risk | Extensive COI reported: study funded in part by Synovis Life Technology, whose product was being evaluated ‐ bovine pericardium |
Gupta 2014.
| Methods | Single‐centre RCT, India; computer‐generated randomisation Allocation concealment ‐ not stated Blinding of participants and reviewers ‐ not stated Sample size 106, with 80% power to detect 21% difference between groups, with 5% type 1 error |
|
| Participants | Inclusion: stage 2 or greater anterior compartment prolapse Exclusion: SUI, dominant post vaginal prolapse; suspected malignancy; vaginal infection |
|
| Interventions | Group A: anterior colporrhaphy, 2.0 Vicryl (n = 54), 1 year (n = 41) Group B: self‐styled, 4 arms, monofilament polypropylene mesh (Vypro mesh, J&J) (n = 52), 1 year (n = 44) |
|
| Outcomes | Assessed at 6 months, 1 year Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Gp A 41/54 (76%), Gp B 44/52 (87%), at 1 year |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
Hviid 2010.
| Methods | Single‐centre RCT Computer‐generated randomisation and allocation concealment were appropriate, with sealed envelopes opened in operating room Reviews by non‐blinded surgeon No concomitant surgery 80% power to detect 20% difference, 5% type 1 error |
|
| Participants | Inclusion criteria: symptomatic prolapse, point Ba ≥ ‐1; defects in posterior or apical compartment; prior pelvic surgery; Exclusion: history of collagen or endocrine disorders Allocated: Gp A 31, Gp B 30 1 year: A 26, B 28 |
|
| Interventions | A (31): 2.0 interrupted Vicryl plication B (30): no plication, Pelvicol porcine dermis 4 × 7 cm anchored with 2.0 Vicryl sutures No concomitant surgery |
|
| Outcomes | Assessed at 1 year Reported the following review outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Sealed non‐transparent sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reviewers non‐blinded, participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 1 year: AC 26/31 (84%), biological 28/30 (93%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No COI declared, no statement on funding |
Lamblin 2014.
| Methods | Single‐centre RCT, France Computer‐generated 6‐block randomisation Allocation concealment not stated Blinding ‐ no participants or reviewers Intention to treat not stated |
|
| Participants | Inclusion: stage 3 or greater anterior compartment prolapse Exclusion: pregnancy, family not completed, prior cancer or radiation, poorly controlled DM, polypropylene sensitivity, immunocompromised Concomitant surgery performed |
|
| Interventions | Gp A: AC with bilateral vaginal colposuspension (Ethibond suture) (n = 35), at 2 years (n = 32) Gp B: polypropylene transobturator mesh (Perigee AMS) (n = 33), at 2 years (n = 31) More women underwent hysterectomy in the colposuspension group (77%) than in the mesh group (33%), P < 0.001 |
|
| Outcomes | Assessed at 3 months, 1 year and 2 years Reported the following review outcomes at 2 years
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 2 years: Gp A 32/35 (91%), Gp B 31/33 (94%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | Funded by the Claude Bernard University. Study authors reported no COI. |
Menefee 2011.
| Methods | Double‐blinded triple‐arm RCT Randomisation, allocation concealment, NS power, 33 in each group, 80% power to detect 35% difference with 5% type 2 error 2‐Year review |
|
| Participants | Inclusion: women ≥ 18 years of age with a POPQ point Ba ≥ 0 Exclusion: NS Concomitant surgery: hysterectomy, colpopexy, posterior repair, continence at surgeon's discretion |
|
| Interventions | 99 randomised A (32): standard anterior colporrhaphy using midline plication with delayed absorbable suture B (31): vaginal paravaginal repair using free‐hand formed porcine dermis graft (Pelvicol) C (36): vaginal/paravaginal repair using free‐formed polypropylene mesh (M). All graft material was secured to the arcus tendineus fascia pelvis by a Capio device with permanent monofilament suture |
|
| Outcomes | Assessed at 2 years Reported the following review outcomes at 2 years
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate attrition 1 year: AC 24/32 (75%) ‐ porcine 26/31 (84%), mesh 28/36 (77%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | High risk | Study authors reported COI with companies producing product evaluated; funding by Boston Scientific, whose product Capio was being evaluated |
Meschia 2007.
| Methods | Multi‐centre RCT (computer generated) on primary surgery, anterior vaginal wall prolapse
Allocation concealed Power calculation: 90 in each arm required Follow‐up: 2 years Intention‐to‐treat analysis: yes, including women with missing data at 2 years but with 1‐year follow up completed |
|
| Participants | 206 randomised
Lost to follow‐up: 5 ‐ A 2, B 3
Inclusion: primary anterior prolapse, POPQ point Ba ‐1 (≥ stage 2)
Exclusion: none
Baseline stress urinary incontinence: A 22/100, B 18/106
Baseline overactive bladder: A 44/100, B 35/106
Baseline sexually active: A 65/100, B 74/106; with dyspareunia: A 12/65, B 11/74 No differences between the 2 groups with respect to demographic and clinical characteristics At 2 years, number available for analysis: 176 (A 91, B 85) Intention‐to‐treat analysis: 201 analysed (A 103, B 98) |
|
| Interventions | A (100): interrupted fascial plication Vicryl 00 WITH Pelvicol overlay, fixed with PDS suburethrally and uterosacral cardinal ligament distally B (106): surgery as above WITHOUT Pelvicol overlay Concomitant surgery standardised Vaginal hysterectomy, McCall culdoplasty, posterior compartment defect, fascial plication | |
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 year: AC 101/106, 98/100 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
Minassian 2010 abstract.
| Methods | Single‐centre 2‐surgeon RCT Randomisation list PC generated and sealed opaque envelopes 32 in each group, 80% power to to detect 25% difference with 5% type 1 error Participants and surgeons unblinded, along with who reviewed NS 2‐Year review Intention‐to‐treat analysis |
|
| Participants | Inclusion criteria: women over the age of 18 with symptomatic cystoceles scheduled for reconstructive surgery Exclusion criteria: pregnant or planning to have a future pregnancy, 2 previous failed anterior vaginal wall repairs 90 screened, 70 randomised AC (34): 2 years (n = 25) Paravaginal (33): 2 years (n = 25) |
|
| Interventions | A (34): AC ‐ plication of the cystocele in the midline was performed with 0‐polydioxanone interrupted mattress sutures over a polyglactin 910 (Vicryl) mesh within the imbricated fold of vaginal muscularis and adventitia B (33): paravaginal defect repair, 0‐polydioxanone sutures were used to attach the pubovesical fascia to that of the obturator and pubococcygeus muscle, also over a Vicryl mesh 2 surgeons Concomitant POP and continence surgery allowed Most undergoing sacral colpopexy, 74% both groups, MUS 76% hysterectomy, AC group 25/34 (74%), paravaginal group 14/34 (42%); P = 0.01 |
|
| Outcomes | 2‐Year review
Perioperative outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | AC group: 34 randomised, 25 at 2 years (73%) Paravaginal repair: 33 randomised, 25 at 2 years (76%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | No COI |
Natale 2009.
| Methods | CONSORT statement: no Power calculation: 100 in each arm Type of randomisation: computer generated Blinding strategy: not specified Allocation concealment: not specified Definition of cure: point Ba < ‐1 (i.e. stage 0 or 1 according to the POPQ system) Follow‐up: 24 months Prolapse assessment: POPQ Update of Cervigni 2005 abstract |
|
| Participants | Inclusion: recurrent symptomatic stage 2 or greater anterior vaginal wall prolapse (point Ba ≥ ‐1), planning to undergo secondary pelvic reconstructive surgery Exclusion: need for concomitant anti‐incontinence procedure, diabetes mellitus or collagen disease Randomised: 190 Analysed: 190 Women were comparable at baseline in terms of demographic data, degree of POP and clinical or urodynamic findings. Previous hysterectomy: A 60/96, B 54/94 |
|
| Interventions | A (96): cystocele repair with armed monofilament polypropylene mesh (Gynemesh) B (94): cystocele repair with armed porcine dermis graft (Pelvicol) Concomitant surgery: not specified. Prophylactic antibiotic cover All underwent tension‐free cystocele repair (TCR) and levator myorrhaphy and vaginal hysterectomy, if required Sheets of both Pelvicol graft and synthetic mesh were trimmed to an identical rounded shape, with 2 lateral wings/arms. In each operation, the central, rounded portion of the graft was positioned under the urinary bladder in a tension‐free fashion, while its arms were inserted deep into the periurethral tissue on both sides towards the pubic bone. A single fixating Monocryl 2/0 suture was performed at the base of 1 wing of mesh, at the periurethral level |
|
| Outcomes | 2‐Year outcomes (3‐year abstract for objective failure rate)
|
|
| Notes | Trialists concluded that Gynemesh was not statistically significantly superior to porcine graft in the management of anterior compartment prolapse at 2 years. Sexuality and PQOL were superior in the porcine graft group as compared with the Gynemesh PS group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2‐Year mesh 96/96, Pelvicol 94/94 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
Nguyen 2008.
| Methods | Single‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 38 in each arm Type of randomisation: computer generated Blinding strategy: primary surgeon ‐ until the surgery day; patients, research nurse and medical assistant remained blinded Allocation concealment: sealed opaque envelopes Definition of cure
Follow‐up: 12 months (full publication) and 24 months (abstract only) Prolapse assessment: POPQ |
|
| Participants | Inclusion: 21 years old and older with POPQ stage 2 or greater anterior prolapse requiring surgical correction Exclusion: pregnancy (present or contemplated), prior repair with graft, systemic infection, compromised immune system, uncontrolled diabetes mellitus, previous pelvic irradiation/cancer, polypropylene allergy, scheduled for concomitant Burch or pubovaginal sling Randomised: 76 Withdrawal: 1 Lost to follow‐up: 1 Analysed: 76 |
|
| Interventions | A (38): anterior colporrhaphy (AC) with delayed absorbable (PDS) sutures B (38): AC + polypropylene 4‐armed mesh kit repair (Perigee, American Medical Systems) Concomitant surgery: vaginal hysterectomy, bilateral salpingo‐oophorectomy, uterosacral suspension, mid‐urethral tape, site‐specific rectocele repair, perineoplasty, Apogee mesh kit repair Concomitant prolapse and suburethral tape surgeries were performed in both groups |
|
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
|
|
| Notes | Data regarding study methods were obtained from the full published article, with follow‐up at 12 months PFDI ‐ Pelvic Floor Distress Inventory (quality of life measure) PFIQ ‐ Pelvic Floor Incontinence Questionnaire (quality of life measure) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data set complete: AC 37/38, mesh 37/38 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
Nieminen 2008.
| Methods | Muti‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 101 in each arm Type of randomisation: computer generated Allocation concealment: opaque envelopes Blinding strategy: not specified, but lack of a non‐surgical blinded outcome reviewer Definition of cure: less than stage 2 prolapse at Aa or Ba Follow‐up: 24 months Prolapse assessment: POPQ |
|
| Participants | Inclusion: postmenopausal women with symptomatic anterior vaginal wall prolapse to the hymen or beyond Exclusion: apical defect indicating vaginal fixation or stress urinary incontinence necessitating surgery, or main symptomatic prolapse component in the posterior vaginal wall. Also, gynaecological tumour or malignancy calling for laparotomy or laparoscopy and untreated vaginal infection Randomised: 202 Withdrawal: 1 Lost to follow‐up: 1 Analysed: 200 No significant differences in baseline demographics, prior hysterectomy or prolapse surgeries between the 2 groups |
|
| Interventions | A (96): anterior colporrhaphy (AC) with a 0 or 2/0 multi‐filament suture B (104): AC + self‐tailored (from a 6 × 11 cm mesh patch), 4‐armed low‐weight polypropylene mesh Type of mesh: non‐absorbable monofilament polypropylene (Parietene Light, Sofradim, France) Sutures for AC: absorbable 0 or 2/0 multi‐filament suture Concomitant surgery: vaginal hysterectomy, posterior repair, culdoplasty as required, no concomitant continence surgeries |
|
| Outcomes | Assessed at 2 months and 1, 2 and 3 years Reported the following review outcomes at 3 years
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2‐Year AC 85/96 (88%), mesh 97/104 (93%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | Not clear |
Robert 2014.
| Methods | Parallel‐group RCT | |
| Participants | Included: women with a cystocele requiring surgical management Excluded: allergy to graft material, immunocompromised, non‐English speaking, unavailable for follow‐up Concomitant surgery and previous non‐anterior prolapse surgery were not exclusion criteria |
|
| Interventions | Small intestine mesh‐augmented procedure vs same anterior repair without mesh | |
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation through data manager |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up performed by blinded clinician blinded to allocation, with no involvement in participant care |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 55/57 women randomised (96%) and included in analysis for objective cure, 57/57 (100%) for subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Reported expected outcomes |
| Other bias | Low risk | Supplier of product (Cook) partially funded the study, but double‐blinding overcame potential biases |
Rudnicki 2014.
| Methods | Multi‐centre (6) international RCT, Nordic countries ‐ Norway, Sweden, Denmark and Finland Block computer‐generated randomisation list Allocation concealment, opaque sealed envelopes Intention‐to‐treat analysis Sample size: 130 participants allowed, 80% power to detect 20% difference with an alpha error of 5% and a dropout rate of 15% Assessors: surgeons Participants: unblinded Surgeons trained (?) to ensure that uniform surgery was performed |
|
| Participants | Inclusion criteria: ≥ 55 years, anterior wall prolapse stage 2, POPQ Aa or Ba ≥ ‐1 Exclusion criteria: previous major pelvic surgery, with the exception of a hysterectomy for reasons other than genital prolapse; previous vaginal surgery or hysterectomy for POP; concomitant prolapse of the uterus or an enterocele of stage 1 or greater; previous incontinence sling surgery performed through the obturator membrane; current treatment with corticosteroids; history of genital or abdominal cancer All surgery covered with intraoperative antibiotics and presurgical and postsurgical local oestrogens Concomitant surgery allowed posterior repair |
|
| Interventions | AC group: interrupted absorbable suture fascial plication, vaginal trimming and closure with running unlocked absorbable suture Mesh: biosynthetic system monofilament polypropylene mesh with central portion coated in absorbable hydrophilic porcine collagen film (Bard, Avulta Plus anterior) 169 available for randomisation, with 161 randomised AC (79): randomised, 1 year ‐ 76 Mesh (82): randomised, 1 year ‐ 78 |
|
| Outcomes | Assessed at 3 months, 1 year and 3 years Reported the following review outcomes at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked computer‐generated randomisation list for each of 4 countries |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgeons evaluated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1‐Year evaluation, randomised AC 76/79 (96%), mesh 78/82 (95%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No COI |
Sand 2001.
| Methods | Single‐centre RCT (computer‐generated number table) Vaginal repair with or without Vicryl mesh overlay for cystocele and rectocele Follow‐up: A 12, B 12 months | |
| Participants | 143 women Inclusion: cystocele to or beyond hymenal ring on standing Exclusion: younger than 18 years of age, pregnancy, contemplating pregnancy within 1 year, paravaginal defect only, anterior enterocele 161 randomised 1 excluded (anterior enterocele) 17 lost to follow‐up | |
| Interventions | A (70): no mesh ‐ Vicryl plication of anterior endopelvic fascia B (73): mesh ‐ as above with Vicryl mesh folded underneath trigone and cuff and secured Vicryl to fascia; also added to posterior wall if posterior repair performed Posterior repair performed: A 67/70, B 65/73 | |
| Outcomes | Assessed at 2, 6 and 12 weeks and at 1 year Reported the following review outcomes at 1 year
|
|
| Notes | No subjective success No urinary, bowel or sexual function data No perioperative data No intention‐to‐treat analysis No CONSORT No blinding Standardised concomitant surgery Review by surgeon | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 143/170 (84%) completed 1‐year review |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Unclear risk | No conflict of interest statement |
Sivaslioglu 2008.
| Methods | Single‐centre RCT comparing polypropylene mesh surgery with site‐specific surgery in the treatment of patients with cystocoele CONSORT statement: yes Power calculation: 45 in each arm Type of randomisation: computer generated Blinding strategy: no (assessment was performed by non‐blinded reviewers) Allocation concealment: not specified Definition of cure/failure: 'Acceptable cure' defined as cystocele less than ‐1 cm (stage 1 POPQ) Follow‐up: mean 12 months (range 8 to 16) Prolapse assessment: POPQ |
|
| Participants | Inclusion: primary cystocele Exclusion: stress urinary incontinence, concomitant rectocele or enterocoele or recurrent cystocoele Randomised: 90 (45 to each arm) Analysed: 85 Lost to follow‐up: 5 |
|
| Interventions | A (42): site‐specific polyglactin 910 anterior repair B (43): self‐styled 4‐armed polypropylene (Parietene, Sofradim, France) mesh, no anterior repair Concomitant surgery not standardised, management of concomitant apical prolapse not specified in either group |
|
| Outcomes | Assessed at 6 weeks, 6 months and annually Reported the following review outcomes at mean follow‐up of 1 year (range 8 to 16 months)
|
|
| Notes | Sivaslioglu and colleagues evaluated a site‐specific polyglactin 910 repair and self‐styled 4‐armed polypropylene (Parietene, Sofradim) mesh Management of concomitant apical prolapse was not specified in either group, and assessment was performed by non‐blinded reviewers. Three patients in the AC group developed de novo SUI, and 2 in the mesh group developed de novo dyspareunia. Operating time and blood loss were not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded reviewers' objective assessment, participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | AC 42/45, mesh 43/45 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | No funding and no COI |
Tamanini 2015.
| Methods | Single‐unit raffle randomisation before surgery No allocation concealment described Surgeons and participants unblinded Unclear who performed assessments (blinded?) Sample size: 100 women, 80% power to detect 26% difference between groups with alpha error of 5% and 20% loss to follow‐up at 2 years |
|
| Participants | 122 reviewed, 100 randomised AC (55): 1 year 54, 2 year 50 Mesh (45): 1 year 43, 2 year 42 Inclusion criteria: 45 years old or older, AVWP ≥ 2 (POPQ stage) (7) without previous surgical correction or with previous surgical treatment of AVWP without the use of PM Exclusion criteria: previous treatment (due to AVWP or SUI) with PM, receiving oncological treatment, altered Papanicolau smear exam or uterine bleeding, genital or acute urinary infection, lack of commitment to ambulatory follow‐up, refusal to sign informed consent All preop urodynamics |
|
| Interventions | Spinal anaesthesia with antibiotics NAZCA TC Kit (Promedon, Cordoba, Argentina), monofilament macroporous, 4 arms (1 prepubic and 1 transobturator each side), concomitant surgery as required: hysterectomy, apical or posterior repair AC group: 2.0 Vicryl fascial plication mid‐urethral sling if SUI on preop UDS (14/55) |
|
| Outcomes | Assessed at 1 year and 2 years Reported the following review outcomes at 2 years
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Raffle randomisation: 55 in AC, 45 in mesh |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | AC group: 42/55 at 2 years (76%) Mesh group: 42/45 completed (93%), high risk due to disparity between groups |
| Selective reporting (reporting bias) | Unclear risk | Significant outcome data |
| Other bias | Low risk | No COI |
Thijs 2010 abstract.
| Methods | Multi‐centre and multi‐national RCT Randomisation and allocation concealment NS 90% power to detect 20% difference, urinary distress inventory prolapse domain at 1 year with 5% type 1 error, with 38 in each group |
|
| Participants | A (48): anterior colporrhaphy B (48): Perigee transobturator polypropylene mesh A (35): AC only, 5 SSF, 5 hysterectomy, 6 mid‐urethral sling B (34): Perigee only, 4 SSF, 8 hysterectomy, 1 mid‐urethral sling |
|
| Interventions | Inclusion: stage 2 or greater cystocele Exclusion: anterior not the leading prolapse Concomitant surgery allowed Stage 2 or greater uterine prolapse hysterectomy or sacrospinous ligament fixation (SSF) SUI mid‐urethral sling |
|
| Outcomes | A, median 50; B, median 100 Blood loss > 500 mL: A 1, B 1 UDI: A vs B at baseline Discomfort: 27 (24), 27 (23) Overactive bladder: 34 (30), 41 (33) Obstructive micturition: 28 (32), 19 (20) Prolapse: 56 (30), 58 (35) Incontinence: 23 (24), 19 (20) UDI: A vs B at 1 year Discomfort: 13 (19), 8 (12) Overactive bladder: 16 (25), 15 (23) Obstructive micturition: 15 (23), 11 (19) Prolapse: 12 (22), 1 (4) Incontinence: 18 (29), 16 (23) B mesh erosion: 9/48 B surgery mesh exposure: 4/48 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
Turgal 2013.
| Methods | Parallel‐group RCT | |
| Participants | Inclusion criteria: grade 2 or greater cystocele Exclusion: urinary incontinence, prior gynaecological surgery, concomitant rectocele or enterocele, recurrent cystocele |
|
| Interventions | Polypropylene mesh (00000.3, Sofradim, Parieten) (20 women) vs AC (20 women) | |
| Outcomes | Assessed at 6 weeks, 6 months, 1 year Reported the following review outcomes at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocated by computer programme" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 40/40 randomised women were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Reports "no conflict of interest". No other potential bias identified |
Vollebregt 2011.
| Methods | Mutli‐centre RCT Randomisation was computerised, and stratification was performed for the presence of uterine descent ≥ 2. No blinding to group assignment was performed. Allocation concealment NS Power 80 to detect 25% difference between groups with 5% type 1 error from sample size of 50 in each group |
|
| Participants | Inclusion: ≥ stage 2 cystocele Exclusions: history of urogynaecological surgery for pelvic organ prolapse or incontinence, cancer or COPD, concomitant urinary stress incontinence with an indication for surgical correction, recurrent lower urinary tract infection (> 3 culture‐proven infections/y), maximum bladder capacity < 300 mL, indication for hysterectomy, childbearing potential and inadequate birth control measures Randomised: A 64, B 61 Withdrawals before surgery: A 2, B 2 12 months: A 51, B 53 |
|
| Interventions | A: AC; B: trocar‐guided transobturator synthetic mesh (AVULTA) | |
| Outcomes | Assessed at 6 months and 1 year Reported the following review outcomes at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Research nurse from online list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reviewers blinded by strapping of thighs before review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | AC 55/56, mesh 55/58 ‐ 1 year |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | No funding and no COI |
Weber 2001.
| Methods | RCT with computer‐generated random number tables. Sealed envelopes concealed assignment. Investigators compared 3 surgical techniques 3 arms, 1 centre Length of follow‐up: A + B + C, 23.3 months | |
| Participants | 83 women Inclusion: all women undergoing cystocele repair Exclusion: continence surgery (i.e. colposuspension or sling) 114 randomised 5 withdrawals 26 lost to follow‐up (A 2, B 15, C 9), leaving 83 in the trial | |
| Interventions | A (33): anterior repair: midline plication without tension, 0 PDS B (24): ultra‐lateral: dissection to pubic rami laterally, plication paravaginal with tension, 0 PDS interrupted C (26): anterior repair plus mesh: standard plication midline Vicryl mesh overlay, Vicryl sutures | |
| Outcomes | Assessed at 6 months, 1 year and 2 years Reported the following review outcomes at median follow‐up 23 months (range 4.5 to 44.4 months)
|
|
| Notes | Number and level of surgeons unknown Adequate power Non‐standardised concomitant surgery Intention to treat: yes No CONSORT No stratification Significant disparity in total numbers in Table 1, and actual numbers with prolapse reported Except for point Aa POPQ, no individual outcome data reported for the 3 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2‐Year review: AC 33/37 (89%); ultra‐lateral AC 24/39 (62%); Vicryl mesh 28/36 (78%) ‐ high risk due to disparity between groups |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | NS |
Withagen 2011.
| Methods | Multi‐centre randomised controlled trial 13 centres, 22 surgeons Randomisation list computer generated for each of 13 centres. Allocation concealment was not discussed, and participants, surgeon and assessor (surgeon) were not blinded. Surgeons underwent specific Prolift mesh training. Full‐power calculation was completed. |
|
| Participants | Randomised: GP A 99, Gp B 95 1‐Year examination: A 84, B 83 Inclusion criteria: recurrent stage 2 or higher anterior and/or posterior wall prolapse Exclusion criteria: pregnancy, future pregnancy, prior vaginal mesh repair, compromised immune system or any other condition that would compromise healing, previous pelvic irradiation or cancer, blood coagulation disorders, renal failure, upper urinary tract obstruction, renal failure and upper urinary tract obstruction, presence of large ovarian cysts or myomas |
|
| Interventions | Gp A: Conventional surgery was performed at the discretion of the surgeon, although absorbable sutures were specified and hysterectomies permitted Gp B: standardised and structured in the tension‐free vaginal mesh; performed as described by Fatton (Fatton 2007) previously, and no hysterectomies performed nor T incisions allowed |
|
| Outcomes | Assessed at 6 months and at 1 year Reported the following review outcomes at 1 year
Duration of surgery: reported median and range Definition of success is unorthodox and different in Methods (≥ grade 2 prolapse in the treated site) and Results sections (≥ grade 2 POP in treated compartment or subsequent prolapse surgery). Furthermore, definition of treated compartment varies in each group. A includes all surgical sites; B excludes sites at which mesh was not utilised. |
|
| Notes | Study authors concluded that at 12 months, anatomical failure was less in Gp B (Prolift mesh) as compared with Gp A. These findings were overshadowed by the fact that the 2 groups were significantly different before intervention in terms of important findings. Lack of allocation concealment in the randomisation process, variability in and unorthodox definitions of success, non‐blinded surgeons reviewing their own surgery ‐ significant limitations of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not described. Preoperatively, group A was significantly different from the mesh group B, as demonstrated by a greater degree of prolapse at Ap, Bp and GH in Table 4, and a significantly greater number with ≥ stage 2 apical compartment prolapse among those in Table 1 undergoing prior apical surgery: 36% (16/45) in the non‐mesh group vs 18% (10/56) in the mesh group (P = 0.04, OR 2.54); finally, prior sacral colpopexy was 3 times as frequent in the mesh group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded reviewers; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 year ‐ AC 90/95, mesh 96/99 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | High risk | Funded university research: All authors reported financial support from Ethicon, the company manufacturing the product being evaluated by non‐blinded reviewers |
AC = anterior colporrhaphy
AVWP = anterior vaginal wall prolapse
BW = Baden‐Walker
CI = confidence interval
COI = conflict of interest
COPD = chronic obstructive pulmonary disease
DM = diabetes mellitus
GH = genital hiatus
ICS = International Continence Society
IVS = intravaginal slingplasty
MUCP = maximum urethral catheter pressure
MUS = Mid‐urethral sling
NS = Not stated
OAB = overactive bladder
OR = odds ratio
OT = Operating time
PC = personal computer
PDS = absorbable polydioxanone surgical suture (PDS)
PFDI = Pelvic Floor Distress Inventory
PFIQ = Pelvic Floor Impact Questionnaire
PGI‐I = Patient Global Impression of Improvement
PISQ = Prolapse and Incontinence Sexual Questionnaire
POP = pelvic organ prolapse
POPQ = Pelvic Organ Prolapse Quantification (according to ICS)
PQOL = Prolapse Quality of Life Questionnaire
RCT = randomised controlled trial
SD = standard deviation
SIS = Small intestine submucosa
SS = statistically significant
SSF = sacrospinous (ligament) fixation
SUI = stress urinary incontinence (symptom diagnosis)
TCR = tension‐free cystocele repair
TVT = tension‐free vaginal tape
UDI = Urogenital Distress Inventory
UI = urinary incontinence
USI = urinary stress incontinence
UTI = urinary tract infection
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Heinonen 2011 | Heinonen and Nieminen evaluated outcomes of anterior vaginal wall mesh augmentation with concomitant sacrospinous ligament fixation (SSLF) (n = 14) or with concomitant posterior intravaginal slingplasty (IVS) (n = 8) for uterovaginal or vaginal vault prolapse. On the basis of a predetermined decision that papers with fewer than 20 individuals in each treatment group would not be included in the review, we excluded the manuscript. |
| Kringel 2010 | Kringel and colleagues compared interventions in a 3‐arm RCT (indwelling urinary catheter for 24 hours or 96 hours or suprapubic catheter for 96 hours) after an anterior colporrhaphy. Study authors concluded that optimal removal of an indwelling urinary catheter took place after 24 hours. We excluded this study from this review and will review catheter issues only at the time of prolapse surgery as a separate subgroup analysis within the surgical management of pelvic organ prolapse. |
| Tincello 2009 | Tincello and associates reported a pilot randomised patient preference study that compared colposuspension or TVT for urinary incontinence at the time of anterior repair for prolapse. Although 31 women were recruited, only 4 (2 in each arm) were randomised. On the basis of a predetermined decision that papers with fewer than 20 individuals in each treatment group would not be included in the review, we excluded this manuscript. |
| Van Der Steen 2011 | In a prospective randomised controlled trial, Van Der Steen compared 1‐day and 3‐day suprapubic catheters in women undergoing anterior colporrhaphy to determine the optimal duration of catheterisation. A total of 179 participants were randomly allocated to the 2 groups. We excluded this study from this review and will review catheter issues only at the time of prolapse surgery as a separate subgroup analysis within the surgical management of pelvic organ prolapse. |
| Weemhoff 2011 | Weemhoff and colleagues compared the numbers of temporary catheter replacements and urinary tract infections after indwelling catheterisation for 2 vs 5 days after an anterior colporrhaphy. A total of 246 participants were randomly assigned to 2 or 5 days of indwelling catheterisation. We excluded this study from this review and will review catheter issues only at the time of prolapse surgery as a separate subgroup analysis within the surgical management of pelvic organ prolapse. |
IVS = intravaginal slingplasty.
RCT = randomised controlled trial.
SSLF = sacrospinous ligament fixation.
TVT = tension‐free vaginal tape.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000159459.
| Trial name or title | Anterior Pelvic Organ Prolapse Surgery: A randomised controlled trial of Xenform anterior repair versus anterior colporrhaphy |
| Methods | Patients will be recruited directly by participating surgeons. Randomisation will occur prior to surgery, with a central office co‐ordinating block randomisation with sealed envelopes |
| Participants | Women of age greater than 40 years, who are symptomatic anterior POP at or beyond hymen (point Ba greater than or equal to 0) AND have a desire for surgery |
| Interventions | Xenform anterior repair versus anterior colporrhaphy. |
| Outcomes | Primary
Secondary
|
| Starting date | Anticipated date of first participant enrolment: 11 February 2016 |
| Contact information | PI: Dr Todd Ladanchuk, King Edward Memorial Hospital 374 Bagot Road, Subiaco, Western Australia 6008 +61(8)93402222; todd.ladanchuk@health.wa.gov.au |
| Notes |
Cortesse 2010.
| Trial name or title | ATHENA |
| Methods | RCT |
| Participants | Women with occult UI |
| Interventions | POP + SUI surgery vs POP surgery alone |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Glazener 2009.
| Trial name or title | PROSPECT (PROlapse Surgery: Pragmatic Evaluaiton and Randomised Controlled Trials) |
| Methods | RCT |
| Participants | Women having prolapse surgery |
| Interventions | Anterior and posterior repair (colporrhaphy) with or without non‐absorbable or biological mesh inlay, or mesh kit |
| Outcomes | Prolapse symptoms (POP‐SS), prolapse stage (POP‐Q), economic outcomes |
| Starting date | 01‐09‐2009 |
| Contact information | c.glazener@abdn.ac.uk |
| Notes | HTA‐funded study in UK |
Lucot 2015.
| Trial name or title | Prosthetic Pelvic Organ Prolapse Repair (Prospere) |
| Methods | RCT |
| Participants | Cystocele |
| Interventions | Lap sacral colpopexy vs vaginal mesh procedure unspecified |
| Outcomes | |
| Starting date | 2012 |
| Contact information | http://clinicaltrials.gov/show/NCT01637441 |
| Notes | Study has been completed but outcomes relevant to this review have not yet been reported |
NCT00955448.
| Trial name or title | Trial of Small Intestine Submucosa (SIS) Mesh for Anterior Repair |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair vs SIS biograft (Cook) |
| Outcomes | |
| Starting date | 2009 |
| Contact information | http://clinicaltrials.gov/show/NCT00955448 |
| Notes | Study completed, but unable to identify publication |
NCT01497171.
| Trial name or title | The Elegant Trial: Elevate Transvaginal Mesh Versus Anterior Colporrhaphy |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair vs elevate (AMS) anterior repair |
| Outcomes | |
| Starting date | 2011 |
| Contact information | http://clinicaltrials.gov/show/NCT01497171 |
| Notes | Study terminated owing to funding termination |
Verleyen 2004.
| Trial name or title | Porcine Dermis vs Vicryl Plug in Raz Cystocele Repair |
| Methods | |
| Participants | 79 women (76 with concomitant prolapse) |
| Interventions | RCT, porcine dermis vs Vicryl |
| Outcomes | UDI, IIQ, urinary urgency, recurrent cystocele |
| Starting date | 2003? |
| Contact information | Dr P Verleyen, University Hospitals, Gassthuisberg |
| Notes |
AMS = American Medical Systems
HTA = Health Technology Assessment
IIQ = impact of urinary incontinence on activities, roles, and emotional states
POP = pelvic organ prolapse
POP‐Q = prolapse stage
POP‐SS = prolapse symptoms
RCT = randomised controlled trial
SIS = small intestine submucosa
SUI = stress urinary incontinence
TVT = tension‐free vaginal tape
UDI = Urinary Distress Inventory
UI = urinary infection
Differences between protocol and review
We conducted a post hoc subgroup analysis for the second comparison, which limited analysis to the study of meshes currently available on the market. This step was added at the request of a peer reviewer.
Contributions of authors
All review authors contributed to writing of the protocol. Four review authors (C Maher, C Schmid, B Feiner, K Baessler) assessed the relevance and eligibility of studies for inclusion in the review. They then assessed the quality of included studies; five (C Maher, N Haya, C Schmid, K Baessler, B Feiner) independently extracted data from trial reports, interpreted results and contributed to the writing of the draft version of this review. Julie Brown assisted with preparation of the review for publication.
Sources of support
Internal sources
-
Cochrane, UK.
Cochrane Review Support Programme: Pelvic organ prolapse reviews
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Incontinence Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
Declarations of interest
Nil.
Edited (no change to conclusions)
References
References to studies included in this review
Ali 2006 abstract {published data only}
- Ali S, Han HC, Lee LC. A prospective randomized trial using Gynemesh PS (trademark) for the repair of anterior vaginal wall prolapse (Abstract number 292). International Urogynecology Journal 2006;17 Suppl 2:221. [Google Scholar]
Allahdin 2008 {published data only}
- Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. Journal of Obstetrics and Gynaecology 2008;28(4):427‐31. [DOI] [PubMed] [Google Scholar]
- Madhuvrata P, Glazener C, Boachie C, Allahdin S, Bain C. A randomised controlled trial evaluating the use of polyglactin (Vicryl) mesh, polydioxanone (PDS) or polyglactin (Vicryl) sutures for pelvic organ prolapse surgery: outcomes at 2 years. Journal of Obstetrics and Gynaecology 2011;31(5):429‐35. [DOI] [PubMed] [Google Scholar]
Altman 2011 {published data only}
- Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C, for the Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic‐organ prolapse. New England Journal of Medicine 2011;364(19):1826‐36. [41463] [DOI] [PubMed] [Google Scholar]
- Ek M, Altman D, Elmér C, Gunnarsson J, Falconer C, Tegerstedt G. Clinical efficacy of a trocar guided mesh kit for the repair of anterior lateral defects (Abstract number 556). Proceedings of the 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland. 2011.
- Ek M, Tegerstedt G, Falconer C, Kjaeldgaard A, Rezapour M, Rudnicki M, et al. Urodynamic assessment of anterior vaginal wall surgery: a randomized comparison between colporrhaphy and transvaginal mesh. Neurourology and Urodynamics 2010;29:527‐31. [39589] [DOI] [PubMed] [Google Scholar]
Carey 2009 {published data only}
- Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG 2009;116(10):1380‐6. [32066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2000 {published data only}
- Colombo M, Vitobello D, Proietti F, Milani R. Randomised comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. BJOG 2000;107(4):544‐51. [DOI] [PubMed] [Google Scholar]
Dahlgren 2011 {published data only}
- Dahlgren E, Kjolhede P, on behalf of the RPOP‐PELVICOL Study Group∗. Long‐term outcome of porcine skin graft in surgical treatment of recurrent pelvic organ prolapse. An open randomized controlled multicenter study. Acta Obstetricia et Gynecologica Scandinavica 2011;90:1393‐401. [DOI] [PubMed] [Google Scholar]
Delroy 2013 {published data only}
- Delroy CA, Castro Rde A, Dias MM, Feldner PC, Bortolini MA, Girao MS. The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial. International Urogynecology Journal 2013;24(11):1899‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Ridder 2004 abstract {published data only}
- Ridder D, Claehout F, Verleyen P, Boulanger S, Deprest J. Porcine dermis xenograft as reinforcement for cystocele stage III repair: a prospective randomized controlled trial (Abstract). Neurourology and Urodynamics 2004;23:435‐6. [Google Scholar]
De Tayrac 2013 {published data only}
- Tayrac R, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso A, et al. Comparison between trans‐obturator trans‐vaginal mesh and traditional anterior colporrhaphy in the treatment of anterior vaginal wall prolapse: results of a French RCT. International Urogynecology Journal 2013;24:1651‐61. [DOI] [PubMed] [Google Scholar]
El‐Nazer 2007 {published data only}
- Al‐Nazer MA, Ismail WA, Gomaa IA. Comparative study between anterior colporrhaphy versus vaginal wall repair with mesh for management of anterior vaginal wall prolapse (Abstract number 84). International Urogynecology Journal 2007;18 Suppl 1:49‐50. [Google Scholar]
- El‐Nazer M, Gomaa I, Ismail Madkour W, Swidan K, El‐Etriby M. Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study. Archives of Gynecology and Obstetrics 2012;286:965‐72. [DOI] [PubMed] [Google Scholar]
Farthmann 2012 {published data only}
- Farthmann J, Niesel A, Fuenfgeld C, Kraus A, Lenz F, Augenstein H, et al. PARETO trial: three‐year follow‐up of a prospective randomized study on mesh exposure rates, recurrences and quality of life after mesh implantation for pelvic organ prolapse. International Urogynecology Journal 2013;24(083):S63. [DOI] [PubMed] [Google Scholar]
Feldner 2010 {published data only}
- Feldner PC Jr, Castro RA, Cipolotti LA, Delroy CA, Sartori MG, Girao MJ. Anterior vaginal wall prolapse: a randomized controlled trial of SIS graft versus traditional colporrhaphy. International Urogynecology Journal 2010;21(9):1057‐63. [40053] [DOI] [PubMed] [Google Scholar]
- Feldner PC Jr, Castro RA, Delroy CA, Dias MM, Sartori MG, Girao MJ. Surgical treatment of anterior vaginal wall prolapse: comparison of small intestine submucosa (SIS) graft and traditional repair (Abstract number 160). International Urogynecology Journal 2009;20 Suppl 2:S208‐9. [39890] [Google Scholar]
Gandhi 2005 {published data only}
- Gandhi S, Goldberg RP, Kwon C, Koduri S, Beaumont JL, Abramov Y, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. American Journal of Obstetrics and Gynecology 2005;192:1649‐54. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Kwon C, Goldberg RP, Abramov Y, Beaumont JL, Koduri S, et al. A randomized controlled trial of fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Neurourology and Urodynamics 2004;23(5/6):558. [Google Scholar]
- Kwon C, Goldberg R, Evaston IL, Koduri S, Franklin WI, Gandhi S, et al. Preliminary results of a prospective randomized trial of tutoplast processed fascia lata to prevent recurrent cystoceles and rectoceles. Journal of Urology 2002;167:203. [Google Scholar]
Guerette 2009 {published data only}
- Guerette NL, Aguirre O, VanDrie DM, Biller DH, Davila GW. Multi‐center, randomized, prospective trial comparing anterior colporrhaphy alone to bovine pericardium collagen matrix graft reinforced anterior colporrhaphy: 12‐month analysis (Abstract number 11). International Urogynecology Journal 2006;17 Suppl 2:63‐4. [Google Scholar]
- Guerette NL, Peterson TV, Aguirre OA, VanDrie DM, Biller DH, Davila GW. Anterior repair with or without collagen. Obstetrics & Gynecology 2009;114:59‐65. [DOI] [PubMed] [Google Scholar]
Gupta 2014 {published data only}
- Gupta B, Vaid NB, Suneja A, Guleria K, Jain S. Anterior vaginal prolapse repair: a randomised trial of traditional anterior colporrhaphy and self‐tailored mesh repair. South African Journal of Obstetrics and Gynaecology 2014;20(2):47‐50. [Google Scholar]
Hviid 2010 {published data only}
- Hviid U, Hviid TV, Rudnicki M. Porcine skin collagen implants for anterior vaginal wall prolapse: a randomised prospective controlled study. International Urogynecology Journal 2010;21(5):529‐34. [39449] [DOI] [PubMed] [Google Scholar]
Lamblin 2014 {published data only}
- Lamblin G, Van‐Nieuwenhuyse A, Chabert P, Lebail‐Carval K, Moret S, Mellier G. A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh. International Urogynecology Journal 2014;25:961–70. [DOI: 10.1007/s00192-014-2344-7] [DOI] [PubMed] [Google Scholar]
Menefee 2011 {published data only}
- Dyer K, Nguyen J, Lukacz E, Simsiman A, Luber K, Menefee S. The Optimal Anterior Repair Study (OARS): a triple arm randomized double blinded clinical trial of standard colporrhaphy, porcine dermis or polypropylene mesh augmented anterior vaginal wall repair (Abstract number 252). Neurourology and Urodynamics 2009;28(7):894‐5. [39346] [Google Scholar]
- Dyer K, Nguyen J, Simsiman A, Lukacz E, Luber K, Menefee S. The Optimal Anterior Repair Study (OARS): a triple arm randomized double blinded clinical trial of standard colporrhaphy versus vaginal paravaginal repair with porcine dermis graft or polypropylene mesh (Abstract number 281). Neurourology and Urodynamics 2010;29(6):1207‐8. [40164] [Google Scholar]
- Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft‐reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstetrics and Gynecology 2011;118(6):1337‐44. [42866] [DOI] [PubMed] [Google Scholar]
Meschia 2007 {published and unpublished data}
- Kocjancic E, Crivellaro S, Bernasconi F, Magatti F, Frea B, Meschia M. A two years follow‐up, prospective randomized study on cystocele repair with or without Pelvicol (trademark) implant (Abstract number 1374). Proceedings of the Annual Meeting of the American Urological Association, 19‐24 May 2007, Anaheim (CA). 2007.
- Meschia M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kojancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicentre, randomized study. Journal of Urology 2007;177:192‐5. [DOI] [PubMed] [Google Scholar]
- Meschia M, Pifarotti P, Magatti F, Bernasconi F, Riva D, Kojancic E. Porcine skin collagen implants (Pelvicol) (trademark) to prevent anterior vaginal wall prolapse recurrence: a randomized study (Abstract). Neurourology and Urodynamics 2005;24(5/6):587‐8. [Google Scholar]
Minassian 2010 abstract {published data only}
- Minassian V, Parekh M, Poplawsky D, Litzy L. Randomized controlled trial comparing anterior colporrhaphy to abdominal paravaginal defect repair for anterior vaginal wall prolapse (Abstract number 54). Neurourology and Urodynamics 2010;29(6):885‐6. [40126] [Google Scholar]
- Minassian VA, Parekh M, Poplawsky D, Gorman J, Litzy L. Randomized controlled trial comparing two procedures for anterior vaginal wall prolapse. Neurourology and Urodynamics 2014;33:72‐7. [DOI] [PubMed] [Google Scholar]
Natale 2009 {published data only}
- Cervigni M, Natale F, Weir J, Galante L, Panei M, Agostini M, et al. Prospective randomized trial of two new materials for the correction of anterior compartment prolapse: Pelvicol and Prolene Soft (Abstract). Neurourology and Urodynamics 2005;24(5/6):585‐6. [Google Scholar]
- Natale F, Penna C, Padoa A, Agostini M, Simone E, Cervigni M. A prospective, randomized, controlled study comparing Gynemesh(R), a synthetic mesh, and Pelvicol(R), a biologic graft, in the surgical treatment of recurrent cystocele. International Urogynecology Journal 2009;20(1):75‐81. [DOI] [PubMed] [Google Scholar]
Nguyen 2008 {published and unpublished data}
- Nguyen JN, Burchette RJ. Anatomy and visceral function after anterior vaginal prolapse repair: a randomized controlled trial (Abstract number 42). Proceedings of the 29th Annual Meeting of the American Urogynecologic Society (AUGS), Sept 4‐6, Chicago. 2008.
- Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair. Randomized controlled trial. Obstetrics and Gynecology 2008;111(4):891‐8. [DOI] [PubMed] [Google Scholar]
Nieminen 2008 {published data only}
- Hiltunen R, Nieminen K, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Low‐weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstetrics and Gynecology 2007;110(2 Pt 2):455‐62. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Hiltunen R, Heiskanen E, Takala T, Niemi K, Merikari M, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. International Urogynecology Journal 2008;19(12):1611‐6. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow‐up. American Journal of Obstetrics and Gynecology 2010;203(3):235.e1‐8. [40020] [DOI] [PubMed] [Google Scholar]
Robert 2014 {published data only}
- Robert M, Girard I, Brennand E, Tang S, Birch C, Murphy M, et al. Absorbable mesh augmentation compared with no mesh for anterior prolapse: a randomised controlled trial. Obstetrics and Gynecology 2014;123(2 Part 1):288‐94. [DOI] [PubMed] [Google Scholar]
Rudnicki 2014 {published data only}
- Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen‐coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. BJOG 2014;121:102‐11. [DOI] [PubMed] [Google Scholar]
Sand 2001 {published data only}
- Goldberg RP, Koduri S, Lobel RW, Culligan PJ, Tomezsko JE, Winkler HA, et al. Long‐term effects of three different anti‐incontinence procedures on the posterior compartment (Abstract). Proceedings of the International Continence Society (ICS) 31st Annual Meeting; 2001 Sept 18‐21; Seoul, Korea. 2001.
- Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. American Journal of Obstetrics and Gynecology 2001;184(7):1357‐64. [DOI] [PubMed] [Google Scholar]
Sivaslioglu 2008 {published data only}
- Sivaslioglu AA, Unlubilgin E, Dolen I. A randomized comparison of polypropylene mesh surgery with site‐specific surgery in the treatment of cystocoele. International Urogynecology Journal 2008;19(4):467‐71. [DOI] [PubMed] [Google Scholar]
Tamanini 2015 {published data only}
- Tamanini JT, Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, Girão MJ. A prospective, randomized, controlled trial for the treatment of anterior vaginal wall prolapse: medium term followup. Journal of Urology 2015;193(4):1298‐304. [DOI] [PubMed] [Google Scholar]
- Tamanini JT, Oliveira Souza Castro RC, Tamanini JM, Castro RA, Sartori MG, João M. Treatment of anterior vaginal wall prolapse with and without polypropylene mesh: a prospective, randomized and controlled trial ‐ Part I. International Brazilian Journal of Urology 2013;39(4):519‐30. [DOI] [PubMed] [Google Scholar]
Thijs 2010 abstract {published data only}
- Thijs S, Deprest J, Ridder D, Claerhout F, Roovers J. A randomized controlled trial of anterior colporrhaphy and Perigee™ as a primary surgical correction of symptomatic cystocele (Abstract number 96). International Urogynecology Journal 2010;21 Suppl 1:S142‐3. [40133] [Google Scholar]
Turgal 2013 {published data only}
- Turgal M, Sivaslioglu A, Yildiz A, Dolen I. Anatomical and functional assessment of anterior colporrhaphy versus polypropylene mesh surgery in cystocele treatment. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013;170(2):555‐8. [DOI] [PubMed] [Google Scholar]
Vollebregt 2011 {published data only}
- Vollebregt A, Fischer K, Gietelink D, Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar‐guided transobturator anterior mesh. BJOG 2011;118(12):1518‐27. [42606] [DOI] [PubMed] [Google Scholar]
- Vollebregt A, Gietelink D, Fischer K, Vaart H. One year results of colporrhaphy anterior versus a trocar guided transobturator synthetic mesh in primary cystocele repair: a randomized controlled trial (Abstract number 51). Neurourology and Urodynamics 2010;29(6):880‐2. [40124] [Google Scholar]
Weber 2001 {published data only}
- Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. American Journal of Obstetrics and Gynecology 2001;185(6 Pt 1):1299‐306. [DOI] [PubMed] [Google Scholar]
Withagen 2011 {published and unpublished data}
- Milani AL, Withagen MI, The HS, Nedelcu‐Van der Wijk I, Vierhout ME. Sexual function following trocar‐guided mesh or vaginal native tissue repair in recurrent prolapse: a randomized controlled trial. Journal of Sexual Medicine 2011;8(10):2944‐53. [42064] [DOI] [PubMed] [Google Scholar]
- Withagen MI, Milani AL, Boon Den J, Vervest HA, Vierhout ME. Tension free vaginal mesh compared to conventional vaginal prolapse surgery in recurrent prolapse: a randomized controlled trial (Abstract number 090). International Urogynecology Journal 2009;20 Suppl 2:S153‐4. [39885] [Google Scholar]
- Withagen MI, Milani AL, Boon J, Vervest HA, Vierhout ME. Trocar‐guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstetrics and Gynecology 2011;117(2 Pt 1):242‐50. [40881] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Heinonen 2011 {published data only}
- Heinonen PK, Nieminen K. Combined anterior vaginal wall mesh with sacrospinous ligament fixation or with posterior intravaginal slingplasty for uterovaginal or vaginal vault prolapse. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;157(2):230‐3. [DOI] [PubMed] [Google Scholar]
Kringel 2010 {published data only}
- Kringel U, Reimer T, Tomczak S, Green S, Kundt G, Gerber B. Postoperative infections due to bladder catheters after anterior colporrhaphy: a prospective, randomized three‐arm study. International Urogynecology Journal 2010;21(12):1499‐504. [DOI] [PubMed] [Google Scholar]
Tincello 2009 {published data only}
- Tincello DG, Kenyon S, Slack M, Toozs‐Hobson P, Mayne C, Jones D, et al. Colposuspension or TVT with anterior repair for urinary incontinence and prolapse: results of and lessons from a pilot randomised patient‐preference study (CARPET 1). BJOG 2009;116(13):1809‐14. [DOI] [PubMed] [Google Scholar]
- Tincello DG, Mayne CJ, Toozs‐Hobson P, Slack M. Randomised controlled trial of colposuspension versus anterior repair plus TVT for urodynamic stress incontinence with anterior vaginal prolapse: proposal (Abstract). Proceedings of the International Continence Society, 11th Annual Scientific Meeting; 2004 Mar 18‐19; Bournemouth, United Kingdom. 2004:46. [17170]
Van Der Steen 2011 {published data only}
- Steen A, Detollenaere R, Boon J, Eijndhoven H. One‐day versus 3‐day suprapubic catheterization after vaginal prolapse surgery: a prospective randomized trial. International Urogynecology Journal 2011;22(5):563‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weemhoff 2011 {published data only}
- Weemhoff M, Wassen MM, Korsten L, Serroyen J, Kampschöer PH, Roumen FJ. Postoperative catheterization after anterior colporrhaphy: 2 versus 5 days. A multicentre randomized controlled trial. International Urogynecology Journal 2011;22(4):477‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000159459 {unpublished data only}
- ACTRN12616000159459. Anterior Pelvic Organ Prolapse Surgery: A randomised controlled trial of Xenform anterior repair versus anterior colporrhaphy [Anterior Pelvic Organ Prolapse Surgery: A randomised controlled trial of Xenform anterior repair versus anterior colporrhaphy evaluating at one‐year: recurrence, quality of life and need for re‐operation on anterior pelvic organ prolapse]. http://www.anzctr.org.au/ACTRN12616000159459.aspx 13 November 2015.
Cortesse 2010 {published data only}
- Cortesse A. Evaluating the necessity of TOT implantation in women with pelvic organ prolapse and occult stress urinary incontinence (ATHENA). http://clinicaltrials.gov/ct2/show/NCT01095692 (accessed 19 April 2011) 2011. [41350]
Glazener 2009 {published data only}
- Glazener CMA. Clinical and cost‐effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomised controlled trials within a comprehensive cohort study (PROSPECT). www.controlled‐trials.com/ISRCTN60695184 (accessed 13 April 2010) 2009.
Lucot 2015 {published data only}
- Lucot JP, Cosson M, Debodinance P, Bader G, Youssef A, Akladios C, et al. PROSPERE randomized controlled trial: Laparoscopic sacropexy versus vaginal mesh for cystocele pop repair. International Urogynecology Journal and Pelvic Floor Dysfunction 2015;26(1 Suppl 1):Abstract number PP 03. [Google Scholar]
NCT00955448 {published data only}
- NCT00955448. Randomized Controlled Trial of SIS Mesh for Anterior Repair: A Pilot Study [Trial of Small Intestine Submucosa (SIS) Mesh for Anterior Repair: A Pilot Study (Anterior SIS)]. http://clinicaltrials.gov/show/NCT00955448 5 August 2009.
NCT01497171 {published data only}
- NCT01497171. The Elegant Trial: Elevate Transvaginal Mesh Versus Anterior Colporrhaphy [Safety and Efficacy of Transvaginal Mesh Colposuspension for Anterior Vaginal Prolapse: the Elevate vs. Anterior Colporrhaphy Trial]. http://clinicaltrials.gov/show/NCT01497171 20 December 2011.
Verleyen 2004 {published data only}
- Verleyen P, Filip C, Bart K, Frank VDA, Jan D, Dirk DR. A prospective randomised trial comparing Pelvicol (trademark) and Vicryl (trademark) for cystocoele repair in the Raz‐colposuspension (Abstract number 613). Proceedings of the International Continence Society (34th Annual Meeting) and the International Urogynecological Association; 2004 Aug 23‐27; Paris. 2004.
Additional references
Adams 2004
- Adams E, Thomson A, Maher C, Hagen S. Mechanical devices for pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004010.pub2] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brubaker 2002
- Brubaker L, Bump R, Jacquetin B, Schuessler B, Weidner A, Zimmern P, et al. Pelvic organ prolapse. Incontinence: 2nd International Consultation on Incontinence. 2nd Edition. Plymouth: Health Publication Ltd, 2002:243‐65. [Google Scholar]
Bump 1998
- Bump R, Norton P. Epidemiology and natural history of pelvic floor dysfunction. Obstetrics and Gynecology Clinics of North America 1998;25(4):723‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carey 2001
- Carey MP, Dwyer PL. Genital prolapse: vaginal versus abdominal route of repair. Current Opinion in Obstetrics and Gynecology 2001;13(5):499‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ek 2010
- Ek M, Tegerstedt G, Falconer C, Kjaeldgaard A, Rezapour M, Rudnicki M, et al. Urodynamic assessment of anterior vaginal wall surgery: a randomized comparison between colporrhaphy and transvaginal mesh. Neurourology and Urodynamics 2010;29:527‐31. [39589] [DOI] [PubMed] [Google Scholar]
Ek 2011
- Ek M, Altman D, Elmér C, Gunnarsson J, Falconer C, Tegerstedt G. Clinical efficacy of a trocar guided mesh kit for the repair of anterior lateral defects (Abstract number 556). Proceedings of the 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland. 2011.
Fatton 2007
- Fatton B, Amblard J, Debodinance P, Cosson M, Jacqutin B. Transvaginal repair of genital prolapse: preliminary results of anew tension‐free vaginal mesh (Prolift technique) ‐ a case series multicentric study. International Urogynecology Journal and Pelvic Floor Dysfunction 2007;18:743–52. [DOI] [PubMed] [Google Scholar]
FDA 2011
- Food, Drug Administration (FDA). Surgical mesh for POP and SUI repair: FDA executive summary. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ObstetricsandGynecologyDevices/UCM270402.pdf. Published 23 August 2011 Accessed 19th September 2015.
Ford 2015
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid‐urethral sling operations for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD006375.pub3] [DOI] [PubMed] [Google Scholar]
Gill 1998
- Gill EJ, Hurt WG. Pathophysiology of pelvic organ prolapse. Obstetrics and Gynecology Clinics of North America 1998;25(4):759‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- McMaster University. GRADEpro GDT 2014. Version Hamilton (ON) GRADE Working Group. McMaster University, 2014 [Version accessed prior to November 2016].
Hagen 2011
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003882.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handa 2004
- Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. American Journal of Obstetrics and Gynecology 2004;190(1):27‐32. [DOI] [PubMed] [Google Scholar]
Hendrix 2002
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. American Journal of Obstetrics and Gynecology 2002;186(6):1160‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. handbook.cochrane.org. [Other: www.cochrane‐handbook.org.] 2011.
MacLennan 2000
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. British Journal Obstetrics and Gynaecology 2000;107(12):1460‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maher 2016
- Maher C, Feiner B, Baessler K, Christmann‐Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012079] [DOI] [PMC free article] [PubMed] [Google Scholar]
MHRA 2014
- Medicines and Healthcare Products Regulatory Agency (MHRA). A summary of the evidence on the benefits and risks of vaginal mesh implants. https://www.gov.uk/government/publications/vaginal‐mesh‐implants‐summary‐of‐benefits‐and‐risks. October 2014.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Olsen 1997
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstetrics and Gynecology 1997;89(4):501‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager 5 (RevMan 5) Version 5.3. Copenhagen: Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
References to other published versions of this review
Maher 2004
- Maher C, Baessler K, Glazener CMA, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004014.pub2] [DOI] [PubMed] [Google Scholar]


