Abstract
Background
This is an updated version of the original review published in The Cochrane Library in 1999 and updated in 2004 and 2010. Population‐based screening for lung cancer has not been adopted in the majority of countries. However it is not clear whether sputum examinations, chest radiography or newer methods such as computed tomography (CT) are effective in reducing mortality from lung cancer.
Objectives
To determine whether screening for lung cancer, using regular sputum examinations, chest radiography or CT scanning of the chest, reduces lung cancer mortality.
Search methods
We searched electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 5), MEDLINE (1966 to 2012), PREMEDLINE and EMBASE (to 2012) and bibliographies. We handsearched the journal Lung Cancer (to 2000) and contacted experts in the field to identify published and unpublished trials.
Selection criteria
Controlled trials of screening for lung cancer using sputum examinations, chest radiography or chest CT.
Data collection and analysis
We performed an intention‐to‐screen analysis. Where there was significant statistical heterogeneity, we reported risk ratios (RRs) using the random‐effects model. For other outcomes we used the fixed‐effect model.
Main results
We included nine trials in the review (eight randomised controlled studies and one controlled trial) with a total of 453,965 subjects. In one large study that included both smokers and non‐smokers comparing annual chest x‐ray screening with usual care there was no reduction in lung cancer mortality (RR 0.99, 95% CI 0.91 to 1.07). In a meta‐analysis of studies comparing different frequencies of chest x‐ray screening, frequent screening with chest x‐rays was associated with an 11% relative increase in mortality from lung cancer compared with less frequent screening (RR 1.11, 95% CI 1.00 to 1.23); however several of the trials included in this meta‐analysis had potential methodological weaknesses. We observed a non‐statistically significant trend to reduced mortality from lung cancer when screening with chest x‐ray and sputum cytology was compared with chest x‐ray alone (RR 0.88, 95% CI 0.74 to 1.03). There was one large methodologically rigorous trial in high‐risk smokers and ex‐smokers (those aged 55 to 74 years with ≥ 30 pack‐years of smoking and who quit ≤ 15 years prior to entry if ex‐smokers) comparing annual low‐dose CT screening with annual chest x‐ray screening; in this study the relative risk of death from lung cancer was significantly reduced in the low‐dose CT group (RR 0.80, 95% CI 0.70 to 0.92).
Authors' conclusions
The current evidence does not support screening for lung cancer with chest radiography or sputum cytology. Annual low‐dose CT screening is associated with a reduction in lung cancer mortality in high‐risk smokers but further data are required on the cost effectiveness of screening and the relative harms and benefits of screening across a range of different risk groups and settings.
Keywords: Adult; Female; Humans; Male; Controlled Clinical Trials as Topic; Lung Neoplasms; Lung Neoplasms/diagnostic imaging; Lung Neoplasms/mortality; Lung Neoplasms/pathology; Radiography, Thoracic; Randomized Controlled Trials as Topic; Smoking; Smoking/adverse effects; Sputum; Sputum/cytology; Tomography, X‐Ray Computed
Plain language summary
Screening for lung cancer
Lung cancer is the most common cause of cancer‐related death in the western world. It takes about 20 years to develop and cigarette smoking is a known cause. Most lung cancers are not found early in the development of the disease. Regular screening is offered to those considered to be at high risk of contracting the disease. Trials were made of early detection methods such as the testing of sputum, x‐ray and computed tomography (CT) scanning of the chest to see whether they made a difference to the number of people who were treated by surgery and the number of people who died as a result of the disease. This review examined the evidence from nine trials (with a total of 453,965 participants) and found that early screening with chest X‐ray or sputum testing does not reduce the number of people who die from lung cancer. Screening with low‐dose chest CT was found in one large trial to reduce the number of people who die from lung cancer but this trial only included very high‐risk smokers and ex‐smokers. CT screening however is associated with a high number of false positive results and there are also some people who have lung cancer detected and treated but in whom this cancer may not have progressed to cause death in their lifetime, even in the absence of treatment (referred to as overdiagnosis). More research is needed about the relative harms and benefits of CT screening in individuals at lower risk for lung cancer.
Summary of findings
Summary of findings for the main comparison. Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening for lung cancer.
| L ung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening for lung cancer | ||||||
| Patient or population: Patients with lung cancer Settings: Intervention: Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening | |||||
| Lung cancer mortality ‐ More frequent chest x‐ray screening versus less frequent screening | 7 per 1000 | 8 per 1000 (7 to 9) | RR 1.11 (0.95 to 1.31) | 81303 (4 studies) | ⊕⊕⊕⊝ moderate1 | |
| Lung cancer mortality ‐ Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 29 per 1000 | 25 per 1000 (21 to 29) | RR 0.88 (0.74 to 1.03) | 20427 (2 studies) | ⊕⊕⊕⊕ high | |
| All‐cause mortality ‐ More frequent chest x‐ray screening versus less frequent screening | 83 per 1000 | 84 per 1000 (78 to 90) | RR 1.01 (0.94 to 1.08) | 170149 (4 studies) | ⊕⊕⊝⊝ low2,3 | |
| All‐cause mortality ‐ Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 97 per 1000 | 100 per 1000 (88 to 111) | RR 1.03 (0.91 to 1.15) | 10040 (1 study) | ⊕⊕⊕⊕ high | |
| Lung cancer 5‐year survival ‐ More frequent chest x‐ray screening versus less frequent screening | 902 per 1000 | 820 per 1000 (784 to 857) | RR 0.91 (0.84 to 0.99) | 1775 (4 studies) | ⊕⊕⊝⊝ low4,5 | |
| Lung cancer 5‐year survival ‐ Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 700 per 1000 | 581 per 1000 (525 to 644) | RR 0.83 (0.75 to 0.92) | 837 (1 study) | ⊕⊕⊕⊝ moderate6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No trials had evidence of adequate allocation concealment and only half had adequate description of drop‐outs. 2 Only half of the trials had clearly reported randomisation and there was no evidence of allocation concealment; only half of the studies had descriptions of drop‐outs. 3 I² = 56% ‐ considerable heterogeneity. 4 No evidence of allocation concealment and only one study had clear evidence of blinding. 5 I² = 68% ‐ substantial heterogeneity. 6 Single study with unclear allocation concealment and unclear risk of bias from drop‐outs.
Summary of findings 2. Annual chest x‐ray screening versus usual care (no regular screening) for lung cancer.
| Annual chest x‐ray screening versus usual care (no regular screening) for lung cancer | ||||||
| Patient or population: Patients with lung cancer Settings: Intervention: Annual chest x‐ray screening versus usual care (no regular screening) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Annual chest x‐ray screening versus usual care (no regular screening) | |||||
| Lung cancer mortality at 6 years of follow‐up | 7 per 1000 | 6 per 1000 (6 to 7) | RR 0.91 (0.81 to 1.03) | 154901 (1 study) | ⊕⊕⊕⊕ high | |
| Lung cancer mortality at 13 years of follow‐up | 16 per 1000 | 16 per 1000 (14 to 17) | RR 0.99 (0.91 to 1.07) | 154901 (1 study) | ⊕⊕⊕⊕ high | |
| Deaths from all causes (excluding deaths from PLCO cancers) | 119 per 1000 | 117 per 1000 (115 to 121) | RR 0.98 (0.96 to 1.01) | 154901 (1 study) | ⊕⊕⊕⊕ high | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. Annual low‐dose CT screening versus annual chest x‐ray for lung cancer.
| Annual low dose CT screening versus annual chest x‐ray for lung cancer | ||||||
| Patient or population: Patients with lung cancer Settings: Intervention: Annual low‐dose CT screening versus annual chest x‐ray | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Annual low‐dose CT screening versus annual chest x‐ray | |||||
| Lung cancer mortality | 17 per 1000 | 13 per 1000 (12 to 15) | RR 0.8 (0.7 to 0.92) | 53454 (1 study) | ⊕⊕⊕⊕ high | |
| All‐cause mortality | 75 per 1000 | 70 per 1000 (66 to 75) | RR 0.94 (0.88 to 1) | 53454 (1 study) | ⊕⊕⊕⊕ high | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Lung cancer is the commonest form of cancer and causes the most cancer‐related death worldwide (Globocan 2008; Lopez 1995). Lung cancer currently accounts for approximately 5.9% of all deaths in high‐income countries and 2.4% of deaths globally, constituting a major public health problem (WHO 2008). The incidence of lung cancer amongst men in Canada, Ireland, Australia, the Netherlands, Sweeden, Denmark, Finland and Italy has fallen in the last few decades, but the rates in women have risen in these countries (Globocan 2008). The highest incidence rate remains in the United States of America (USA) where the incidence in men has been falling since the mid‐1980s and the epidemic in women reached a peak in the mid‐1990s (Bailar 1997; Globocan 2008). There has been only a minor improvement in five‐year survival from lung cancer in the last few decades, despite modern therapeutics. The current five‐year survival from lung cancer is 16% in the USA (Jemal 2009) and the case fatality rate is around 86% globally (Globocan 2008; Ries 1994).
The overwhelming majority of cases of lung cancer are attributable to cigarette‐smoking, and thus primary prevention should continue to be a major focus of public health campaigns. However, such measures are likely to have only a limited impact on mortality in the short term because of a lag phase in the order of 20 years. Most cases of lung cancer present at an advanced stage, and previous studies have therefore investigated the role of screening for the detection of pre‐clinical disease (Kubik 1986; Tockman 1986; Wilde 1989). Following a series of lung cancer screening trials conducted in the 1970s, it has generally been felt that early detection of lung cancer does not improve outcome, particularly disease‐specific mortality (ACS 1980; Eddy 1989). In these trials chest x‐ray screening was used, with or without sputum cytology, and the majority focused on high‐risk smokers only (Fontana 1984; Kubik 1986; Mayo Lung Project). The risk of lung cancer in smokers is dose‐dependent and while it attenuates following cessation of smoking, the risk still remains greater than that of a non‐smoker (Halpern 1993). In addition, other factors such as age at stopping smoking or coexisting airflow obstruction may affect the risk (Halpern 1993; Tockman 1987).
In more recent years low‐dose CT scanning has been demonstrated to be a more sensitive tool than chest radiography for the detection of early stage lung cancer (Henschke 1999; Kaneko 1996; Sone 1998). The ability of low‐dose CT scanning to detect lung cancer at an early and resectable stage, demonstrated in a large uncontrolled study (Henschke 2006), has led many experts to advocate widespread lung cancer screening with this technique. This study of over 30,000 at‐risk individuals screened by CT scanning reported that 85% of lung cancers detected from screening were stage 1, and those that underwent surgical resection had an estimated 10‐year survival rate of 92%. A number of other uncontrolled studies of low‐dose spiral CT scanning have also been published, supporting the sensitivity of CT scanning (Diederich 2000; Diederich 2002; Henschke 1999; Henschke 2001; Nawa 2002; Sobue 2002; Sone 1998; Sone 2001; Swensen 2002; Tiitola 2002). However promising these results appear to be, longer survival of a few is not necessarily equivalent to reduced mortality overall. Uncontrolled studies cannot establish the effectiveness of screening tools in improving survival because of screening biases such as lead‐time and overdiagnosis, which combine to inflate any assessment of survival, making it an unreliable statistic to evaluate progress against cancer over time (Welch 2007). A true evaluation of the value of screening must also determine the effects of the process on all participants. Overdiagnosis, in particular, a common adverse event of screening, leads to overtreatment, where people are being treated whose 'disease' may pose no threat (Black 2000; Patz 2000; Welch 2010). The harms associated with overtreatment include morbidity and mortality from lung cancer resection (and, to a small but measurable degree, from the screening process itself) and the anxiety and distress associated with a lung cancer diagnosis. Overdiagnosis is a well‐known adverse effect of screening, and was initially reported in early evaluations of the Mayo Lung Project. The potential for overdiagnosis in CT scanning may be greater than with chest x‐ray, as this screening tool has the ability to detect much smaller tumours than chest x‐ray (Welch 2007; Bach 2007a).
The purpose of this review is to assess the evidence regarding the ability of various screening methods to reduce lung cancer mortality and to evaluate the possible harms and costs associated with screening. Another purpose is to seek consumer participation since consumers may provide a different perspective on those outcomes of importance to them. For example, despite guidelines which do not support regular screening with chest x‐rays some consumers would choose to have an annual chest x‐ray (Woo 1985). The present systematic review is a major update of our original review first published in 1999 (Manser 1999) and updated in 2004 (Manser 2004) and 2010 (Manser 2010).
Objectives
To determine whether screening for lung cancer using chest x‐ray, computed tomography (CT) of the chest or sputum examination reduces lung cancer mortality.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) or controlled clinical trials (CCTs) for inclusion. Randomisation by groups, clusters or individuals was acceptable.
We excluded from the review non‐controlled clinical trials, trials which did not report disease‐specific mortality for lung cancer as an outcome and trials where the duration of follow‐up was less than five years.
Types of participants
Adults from all backgrounds including men and women, smokers, non‐smokers and ex‐smokers.
Types of interventions
Chest x‐ray, computed tomography (CT), sputum cytology or other sputum examinations, alone or in any possible combination or frequency.
Types of outcome measures
The results of screening studies may be influenced by lead‐time bias or overdiagnosis bias giving rise to an apparent improvement in survival in the intervention group. Disease‐specific mortality was therefore the primary outcome considered in the review.
Other outcomes considered include:
Compliance with screening and follow up;
Incidence of lung cancer;
Five‐year survival;
Stage at diagnosis;
Resection rate;
Postoperative deaths;
Harms of screening including adverse outcomes from further diagnostic testing in those who have a positive result on initial screening;
Costs;
All‐cause mortality;
Quality of life.
We considered any other outcomes presented in the primary studies, such as the impact of screening on smoking behaviour. We also collected information about the performance of the tests.
Search methods for identification of studies
Electronic databases
This is an updated version of the original review (Manser 1999). We systematically searched MEDLINE (1966 to May 2012), the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2012 Issue 5) and EMBASE (1974 to May 2012). The detailed search strategy for the 2012 update is outlined in Appendix 1. The search strategy for the 2004 and 2010 updates is outlined in Appendix 2 and the search strategy for the original review is provided in Appendix 3.
Other searches
We undertook handsearching of the journal Lung Cancer (1985 to December 2000), including abstracts from international lung cancer meetings. We searched the bibliographies of identified studies and narrative reviews for additional citations. We contacted authors of primary studies and experts in the field of lung cancer screening to determine whether they were aware of any additional relevant unpublished or published studies or works in progress.
Data collection and analysis
At least two independent authors (RM and either AL or DC) searched the titles and abstracts obtained from the initial electronic search for potentially relevant trials for full review. In the 2012 update the abstracts were searched by RM and AL. Initially we categorised studies into the following groups:
Included: RCT or CCT that met the described inclusion criteria (see 'Criteria for considering studies for this review') and those where it was impossible to tell from the abstract, title or MeSH headings;
Excluded: non‐RCT or CCT, or subject not screening for lung cancer.
Two authors (RM and CS in the original review and RM and AL in the 2012 update) then assessed the full text of the retrieved studies to determine whether the study met the inclusion criteria. We measured agreement using simple consensus and kappa statistics. We resolved disagreement by adjudication of a third party or by consensus.
Risk of Bias
Two authors (RM and LI in the original review and RM and AL in the 2012 update) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (Cochrane Handbook) to assess: allocation (random sequence generation and allocation concealment); blinding of participants and personnel, blinding of outcome assessors; incomplete outcome data; and other potential sources of bias. Each of these domains was scored separately as low risk of bias, unclear risk of bias (insufficient information to make a judgement) or high risk of bias as outlined below:
(1) Generation of allocation sequence For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as: • Low risk (any truly random process, e.g. random number table; computer random number generator); • High risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); • Unclear risk, if the trial was described as randomised, but the method used for the allocation sequence generation was not described or gave insufficient information.
(2) Allocation concealment For each included study we described the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the methods as:
• Low risk (e.g. telephone or central randomisation: consecutively numbered sealed opaque envelopes); • High risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); • Unclear risk, if the trial was described as randomised, but the method used to conceal the allocation was not described or gave insufficient information.
(3) Blinding or masking (checking for possible performance bias) For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the methods as:
• Low risk, high risk or unclear for participants; • Low risk, high risk or unclear for personnel; • Low risk, high risk or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs, protocol deviations) We assessed the methods as:
• Low risk (any one of the following): No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically‐relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically‐relevant impact on observed effect size; missing data have been imputed using appropriate methods.
• High risk (any one of the following): reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically‐relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically‐relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. • Unclear risk (any one of the following): insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number randomised not stated, no reasons for missing data provided); the study did not address this outcome.
(5) Free of other bias (bias due to problems not covered elsewhere in the table) For each included study we described any important concerns we have about other possible sources of bias (e.g. baseline imbalance, bias of the presentation data, etc.) • Low risk of bias, the trial appears to be free of other components that could put it at risk of bias; • Unclear risk, the trial may or may not be free of other components that could put it at risk of bias; • High risk of bias, there are other factors in the trial that could put it at risk of bias, e.g. no sample size calculation made, academic fraud, industry involvement, or extreme baseline imbalance.
We measured inter‐author reliability using kappa and weighted kappa statistics. We resolved disagreement by adjudication of a third party or by consensus. Where possible we asked the authors of included studies to verify the assessments of study methodology.
One of the authors (RM) extracted data and entered these into the Review Manager 5 (RevMan) software (RevMan 2008). We asked authors of included studies to confirm the data extracted. However, authors from only one of the studies have confirmed our data extraction to date (Mayo Lung Project). Where original data were not available it was necessary to extrapolate from graphs. A second study member (CS) extracted data for the main study results.
We combined outcomes from included trials using Review Manager 5. For dichotomous outcomes, we reported risk ratios (RRs) with 95% confidence intervals (CIs). We tested homogeneity of effects sizes between studies being pooled using a cut‐off level of P < 0.10 for statistical significance. For pooled analyses we also calculated the I² statistic, which describes the percentage of total variation across studies caused by heterogeneity rather than sampling error; less than 25% was considered as low‐level heterogeneity; 25% to 50% as moderate‐level, and higher than 50% as high‐level heterogeneity (Higgins 2003). For those outcomes where there was significant statistical heterogeneity, we reported risk ratios using the random‐effects model. For other outcomes we used the fixed‐effect model. We analysed data on an intention‐to‐screen basis.
We assessed the statistical significance of differences in the proportion of participants with particular baseline prognostic factors (between intervention and control groups) using Fisher's Exact test. For many of the studies, data were presented on multiple baseline variables and we used the conservative Bonferroni correction to adjust for multiple comparisons (Bland 1995). This defines significance as occurring when P is less than 0.05 divided by the number of comparisons.
In August 2003, this review was updated (search updated up to January 2003) (Manser 2004), with no new eligible studies identified. In May 2008, we updated this review again (search updated to November 2007) (Manser 2010), identifying no new trials, but one publication that reported on extended follow‐up of the Mayo Lung Project (Marcus 2006). In May 2012, we updated this review again (search to May 2012). We identified two new trials for inclusion in the review (North American NLST; PLCO Trial).
Results
Description of studies
In the original review we found 1869 citations with the MEDLINE search, of which we selected 119 for full text‐review (kappa = 0.54; moderate agreement). Following the full‐text review, we selected six studies (all with multiple citations) for inclusion in the review (kappa = 0.9; very good agreement). We selected a further study for inclusion after searching bibliographies of review articles. Searches of EMBASE, PREMEDLINE, handsearching of Lung Cancer and contact with primary authors and experts in the field did not reveal any further relevant studies that had not been identified by the MEDLINE search. Of the 119 citations selected for full‐text review, 39 citations were relevant for inclusion, 52 citations described uncontrolled or non‐experimental studies, four were case‐control studies and 24 were narrative reviews or commentaries. We identified no additional eligible studies by the updated search of MEDLINE in January 2003. We identified one study which published extended follow‐up of the Mayo Lung Project (16 additional years) in the updated search in 2007 (Marcus 2006), but found no other new randomised controlled trials.
For the 2012 update we identified two new trials for inclusion in the review (North American NLST; PLCO Trial). An additional three randomised controlled trials of CT screening were excluded at this stage in keeping with our review protocol, because the duration of follow‐up was less than five years (DANTE; DLCST; MILD). These trials are described in more detail in the Characteristics of ongoing studies table. In the DANTE trial only 6.5% of participants had five years or more of follow‐up, while in the MILD study the median follow‐up was 4.4 years and in the DLCST 4.81 years. A further two randomised controlled trials of CT screening were excluded because they were feasibility studies that did not include mortality as an outcome (Depiscan Group; Yang 2008). Three ongoing trials which are yet to publish mortality data were identified and are described in the ongoing trial section (ITALUNG; LUSI; NELSON 2003). A further publication identified was a combined mortality analysis of two studies (Johns Hopkins Study; Mem Sloan‐Kettering) that were included in the original review (Doria‐Rose 2009). Details of the excluded studies are outlined in the Characteristics of excluded studies table.
In the original review we identified seven controlled trials of screening strategies for lung cancer. Six of these studies were randomised (Czech Study; Johns Hopkins Study; Kaiser Foundation Study; Mayo Lung Project; Mem Sloan‐Kettering; North London Study ). One study was a controlled trial (Erfurt County Study). In all these studies, participants in the control groups underwent variable degrees of screening. Further details are outlined in the Characteristics of included studies tables.
In the Erfurt County Study all men (aged 40 to 65) in 10 districts of Erfurt County were offered screening with chest x‐ray at intervals of one to two years (control group), and the intervention group consisted of all men in four additional districts of Erfurt County who were offered screening with chest x‐ray at six‐monthly intervals. Screening took place between 1972 and 1977.
In the North London Study, industrial firms (mainly factories) were stratified by type of work and area and randomised into two groups. There were 75 firms in the intervention group and 44 in the control group. Male volunteers (aged 40 years or older) from firms in the intervention group were offered screening with chest x‐ray at six‐monthly intervals for three years. The control group were offered chest x‐ray screening only at the beginning and end of the study. Screening took place between 1960 and 1964. There were no apparent occupational hazards in the industrial firms where the subjects were employed that might be expected to increase the risk of lung cancer, but none of the reports included the details of occupational exposures.
The Czech Study had an unusual design. Male smokers (aged 40 to 64 years) were selected from the general population and initially all underwent a prevalence screen. Those without evidence of lung cancer at baseline were then randomised into two groups. The intervention group received semi‐annual chest x‐ray (postero‐anterior view only) and sputum cytology for three years, while the control group were only offered screening at the end of the first three years with chest x‐ray and sputum cytology. Subsequently, both the control and intervention groups underwent screening with annual chest x‐ray (but not cytology) for a further three years (years four to six). The study began in 1976.
In the Kaiser Permanente Multiphasic Evaluation Study (Kaiser Foundation Study), members (men and women aged 35 to 54) of the Kaiser Permanente medical care programme were randomised into two groups. The intervention group were urged to undergo an annual multiphasic health check‐up (MHC). Members of the control group were not urged to undergo MHCs, but were free to arrange their own MHCs as part of the care provided by the Kaiser Permanente medical care programme. Spirometry and chest radiography were among the screening tests offered as part of the multiphasic health check‐up. The study took place between 1964 and 1980.
Three other studies were carried out by the National Cancer Institute (NCI) (USA) (Johns Hopkins Study; Mayo Lung Project; Mem Sloan‐Kettering). In the Mayo Lung Project, 10,933 Mayo Clinic outpatients (male smokers aged 45 years and over) underwent a prevalence screen for lung cancer with chest x‐ray and sputum cytology. The chest x‐ray was postero‐anterior initially, then both postero‐anterior and lateral views were undertaken. Individuals who tested negative for lung cancer, with an adequate life expectancy (at least five years) and respiratory reserve, were invited to take part in a randomised study. A total of 9211 men took part in the randomised study. The intervention group were offered screening with chest x‐ray and sputum cytology at four‐monthly intervals for six years. The control group, on enrolment into the trial, received the Mayo Clinic standard 1970 recommendation to undergo an annual chest x‐ray and sputum cytology test, but were not offered active screening or reminded to undergo screening during the study. Recruitment took place between 1971 and 1976. The Johns Hopkins Lung Project (Johns Hopkins Study) and the Memorial Sloan‐Kettering Study (Mem Sloan‐Kettering) were both designed to assess whether sputum cytology at four‐monthly intervals would improve lung cancer mortality when added to screening with annual chest x‐ray. Male smokers (aged 45 years and over) were recruited through various publicity techniques and direct mail to health insurance prescribers and motor vehicle license holders. There were approximately 10,000 participants in each study. The intervention and control groups were both offered annual screening with chest x‐ray (postero‐anterior and lateral views); the intervention group were also offered sputum cytology three times a year.
In all these studies, lung cancer mortality was reported as an outcome. Many of them reported multiple outcomes, including lung cancer survival and resection rates.
In 2012 two more randomised controlled trials were included in the review after we conducted an updated search. The Prostate, Lung, Colorectal, Ovarian (PLCO) Cancer Screening Trial was a large NCI‐sponsored randomised controlled trial conducted across ten sites in the USA (PLCO Trial). In this two‐arm randomised trial participants were randomised to a screening group which were referred for multiple screening tests for lung and colon cancer and either ovarian or prostate cancer (depending on gender). The control group were not offered screening. The lung cancer screening component consisted of an annual chest x‐ray for four years (including the baseline test). There were a total of 154,934 participants, aged 55 to 74 years including both smokers and non‐smokers. The National Lung Screening Trial was also funded by the National Cancer Institute in the USA (North American NLST). In this multicentre randomised controlled study 53,454 current or former smokers aged 55 to 74 years were randomised to receive three annual screenings with either low‐dose CT or single‐view postero‐anterior chest x‐ray.
Risk of bias in included studies
We assessed risk of bias in all included studies according to the 'Risk of bias' tool described in the Cochrane Handbook. 'Risk of bias' tables are provided for each study in the 'Characteristics of included studies' table. Judgements about the risk of bias are also summarised in Figure 1 (risk of bias graph) and Figure 2 (risk of bias summary).
1.
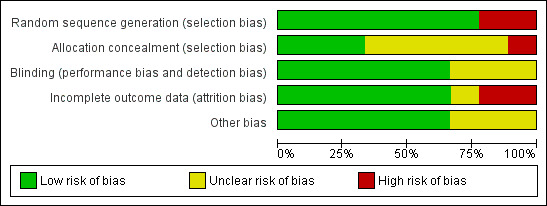
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
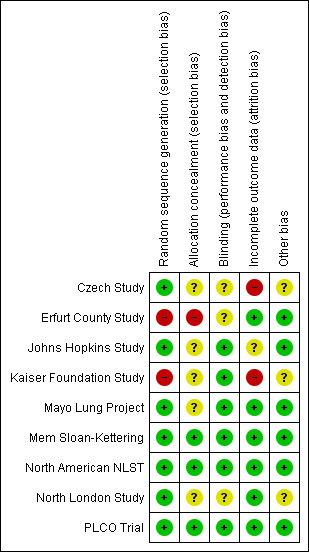
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Randomisation
Allocation concealment was judged as adequate in three out of nine studies, suggesting a low risk of bias (Mem Sloan‐Kettering; North American NLST; PLCO Trial). Concealment of allocation was inadequate in the Kaiser Foundation Study. Allocation concealment was unclear in four of the randomised studies (Czech Study; Johns Hopkins Study; Mayo Lung Project; North London Study). However, this is likely to be less important in a cluster‐randomised study such as the North London Study. One study was a non‐randomised controlled trial (Erfurt County Study).
Sequence generation was judged as adequate in seven out of nine studies, suggesting a low risk of bias (Czech Study; Johns Hopkins Study; Mayo Lung Project; Mem Sloan‐Kettering; North American NLST; North London Study; PLCO Trial). Patient record numbers (with a concealed code) were used to randomise participants in the Kaiser Foundation Study, and this method is generally considered inadequate (Schulz 1995). One study was non‐randomised (Erfurt County Study).
Given the potential for inadequate concealment of allocation to bias the results of randomised studies, we further examined the comparability of baseline data between randomised groups for each of the studies (Chalmers 1983; Schulz 1995). In general, these data were poorly reported. In fact, continuous variables such as age, means and standard deviations were not reported (only the proportion of participants within various age strata was reported). The details of smoking history by screening allocation were poorly reported in the Erfurt County Study, Kaiser Foundation Study, Mem Sloan‐Kettering study and North London Study. After adjusting for multiple comparisons, there were statistically significant differences in some baseline variables in two of the studies (Kaiser Foundation Study; North London Study). While these differences may occur due to chance, they are more likely to occur if the randomisation process is inadequate (Chalmers 1983). In the North London Study, the proportion of ex‐smokers was greater in the control group (18.8% versus 19.6%, P = 0.02) and the proportion of participants aged 60 to 64 years was greater in the intervention group (8.8% versus 8.2%, P = 0.01). The proportion of people over the age of 70 was also greater in the intervention group (0.5% versus 0.2%, P = 0.0003). Using a significance level of P < 0.005 to allow for the multiple comparisons, the difference in the proportion of those aged 70 or more remains significant. Although these differences would not appear to be clinically significant, they raise the possibility that the randomisation process was inadequate. Differences in baseline variables might be expected to occur more often in cluster‐randomised studies. In the Kaiser Foundation Study a chart review of clinical records was undertaken in a large subset of study and control group participants and this highlighted statistically significant differences in multiple baseline variables. For example, the racial composition differed significantly between the groups (29.3% versus 21.7%, P = 0.001), there were more participants in the intervention group with respiratory disease other than pneumonia, acute bronchitis or chronic lung disease (38% versus 33%, P = 0.03) and the proportion of participants with hypertension and hyperlipidaemia was significantly greater in the control group (18.5% versus 24.5%, P = 0.006; 3.8% versus 6.9%, P = 0.01 respectively). Allowing for multiple comparisons and using a P < 0.0025, the differences in racial composition remain significant. These results are consistent with the inadequate randomisation process used in this study. In the Czech Study randomisation was stratified by age, smoking status, socioeconomic status, place of residence and occupational exposure. The number of strata used was not specified. However, where a large number of strata are used imbalances for prognostic variables can occur between study groups as a result of incomplete filling of permuted blocks within strata (Kernan 1999). The intervention and control groups appear to have been well matched for the stratified variables at baseline, but details were not provided for all variables in the published reports. For example, average lifetime cigarette consumption was greater in the intervention group than in the control group (266,334 versus 263,046). This difference was apparently not significant, but standard deviations and statistical tests were not reported (Kubik 1986). Of note, all‐cause mortality was greater in the intervention group (P = 0.04) and the number of smoking‐related deaths was also greater in the intervention group (P = 0.02).
Intervention and control groups were well matched on reported baseline characteristics in the Johns Hopkins Study, Mayo Lung Project, Mem Sloan‐Kettering, North American NLST and PLCO Trial.
Blinding of outcome assessment
Cause of death was assessed by investigators blinded to the screening allocation in six of the studies (Johns Hopkins Study; Kaiser Foundation Study; Mayo Lung Project; Mem Sloan‐Kettering; North American NLST; PLCO Trial). Blinding of the outcome assessment was not described in the remaining three studies (Czech Study; Erfurt County Study; North London Study).
Description of withdrawals and drop‐outs
Withdrawals and drop‐outs were adequately described in six studies (Erfurt County Study; Mayo Lung Project; Mem Sloan‐Kettering; North American NLST; North London Study; PLCO Trial ). In the Erfurt County Study, however, losses to follow‐up were significantly greater in the control group (4.9% versus 3.6%, P = 0.0001); drop‐outs were described as people who had moved from the area or refused 'medical control'. Follow‐up was poor in the Kaiser Foundation Study. In 1980 only 64% of participants were still health plan members and the response rate to follow‐up surveys was only 75%. Follow‐up was not adequately reported in the Czech Study. In the Johns Hopkins Study, 1.3% of participants were lost to follow‐up, however no further details were provided (Tockman 1986). Prolonged follow‐up was reported for the Czech Study and Mayo Lung Project. For years 7 to 15 of the Czech Study, lung cancer mortality was recorded but the methods and details were not described. The authors did note that eight of the participants diagnosed with lung cancer during the initial six years of the study were lost to follow‐up. Losses to follow‐up by screening allocation were not described. At the end of 1996 the vital status of participants in the Mayo Lung Project was assessed. For those in whom it was not known from the Mayo Clinic records, vital status was ascertained by searching the National Death Index. Follow‐up was similar in both groups.
Analysis
This was not a prespecified quality criterion. However, in several of the studies the statistical analysis was inappropriate. For example, stratification was not taken into account in the analysis of the Czech Study. The North London Study was not analysed using methods recommended for the analysis of cluster‐randomised studies (Kerry 1998; Peto 1976). It is unlikely, however, that the findings of these studies would be altered substantially by such analyses.
Agreement between authors on methodological quality
Allocation concealment (kappa = 0.53; moderate agreement);
Method of randomisation (kappa = 0.4; fair agreement);
Blinding of outcome assessment (kappa = 0.70; good agreement);
Description of withdrawals and drop‐outs (kappa = 0.42; fair agreement).
Effects of interventions
See: Table 1; Table 2; Table 3
Lung cancer mortality
For the purposes of analysis we grouped the studies into three categories. Firstly, those that compared more intense chest x‐ray screening and/or sputum cytology with less intense screening with chest x‐ray and/or sputum cytology; secondly, those that compared chest x‐ray screening with usual care (no screening); and finally, studies of CT screening.
More intense chest x‐ray screening (and/or sputum cytology) compared with less intense chest x‐ray screening (and/or sputum cytology)
There were five studies that effectively compared more frequent chest x‐ray screening (plus or minus sputum cytology) with less frequent chest x‐ray screening, and four provided sufficient data for meta‐analysis. We included the Kaiser Foundation Study in this category because the majority of participants in the control group underwent some screening during the study period. In the pooled analysis the risk ratio (RR) of death from lung cancer was 1.11 (95% confidence interval (CI) 0.95 to 1.31, fixed‐effect model) and there was no significant statistical heterogeneity between the results of the different studies (P = 0.67) (Analysis 1.1.1). When data from the prolonged follow‐up reported in the Mayo Lung Project and Czech Study were included in the analysis, mortality from lung cancer was actually significantly greater in the group undergoing more frequent chest x‐ray screening compared with the group receiving less frequent screening (RR 1.11, 95% CI 1.00 to 1.23, P = 0.05) (Analysis 1.2.1). In the Erfurt County Study, (which could not be included in the meta‐analysis due to insufficient raw data) lung cancer mortality was 0.6% per year in the control group and 0.8% per year in the intervention group during the six years of the study and there was reportedly no statistical difference.
1.1. Analysis.
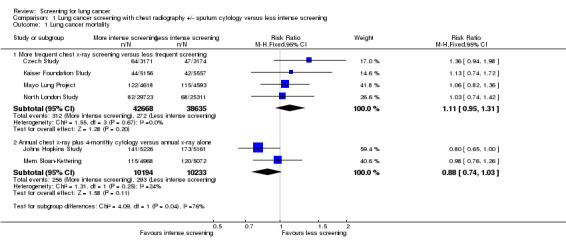
Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 1 Lung cancer mortality.
1.2. Analysis.
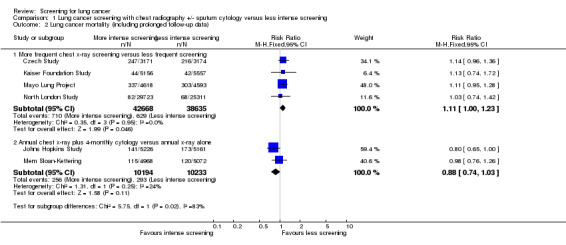
Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 2 Lung cancer mortality (including prolonged follow‐up data).
Two studies compared annual chest x‐ray screening with annual chest x‐ray screening plus four‐monthly sputum cytology, and these were pooled separately (Johns Hopkins Study; Mem Sloan‐Kettering). In this pooled analysis there was a trend to a reduction in deaths from lung cancer in the intervention group, but this was not statistically significant (RR 0.88, 95% CI 0.74 to 1.03) (Analysis 1.1.2).
The key findings for this category are further summarised in the Table 1.
We proposed other subgroup analyses prior to undertaking the review. However, there were insufficient studies to examine the influence of age, sex and smoking history on the outcome. We had also planned to undertake sensitivity analyses based on trial quality. However, except for the Mem Sloan‐Kettering study, all of the studies included in the original review had potential methodological weaknesses. Using different methods of meta‐analysis did not substantially alter the results. For all outcomes the random‐effects and fixed‐effect models produced results which were not significantly different.
Annual chest x‐ray screening versus usual care (no screening)
The PLCO Trial is the only chest x‐ray screening study that included a control group that were not offered any screening. After six years of follow‐up the risk ratio of death from lung cancer in the screened group was 0.91 (95% CI 0.81 to 1.03); after 13 years of follow‐up the risk ratio of death from lung cancer in the screened group was 0.99 (95% CI 0.91 to 1.07). The key findings for this category are presented in the Table 2.
Annual low‐dose CT screening versus annual chest x‐ray screening
In the North American NLST (the only trial included in the review that compared annual low‐dose CT screening with chest radiography) the risk ratio of death from lung cancer in the group screened with low‐dose CT was 0.80 (95% CI 0.70 to 0.92) after six years of follow‐up post‐randomisation.
All‐cause mortality
More intense chest x‐ray screening (and/or sputum cytology) compared with less intense chest x‐ray screening (and/or sputum cytology)
Four out of the five studies that effectively compared more frequent chest x‐ray screening (plus or minus sputum cytology) with less frequent chest x‐ray screening provided sufficient data for on all‐cause mortality for meta‐analysis (Czech Study; Erfurt County Study; Kaiser Foundation Study; Mayo Lung Project) and one study did not report all‐cause mortality (North London Study). In the pooled analysis the risk ratio of death from all causes was 1.01 (95% CI 0.94 to 1.08, random‐effects model) (Analysis 1.3.1). There was significant statistical heterogeneity in the results P = 0.08 (using a threshold value for significance of P < 0.10). Visual inspection of the graph for overlap of the 95% confidence intervals suggests that the results of the Czech Study differ from the other studies and, after removal of this study from the analysis, the risk ratio was 0.97 (95% CI 0.94 to 1.01, fixed‐effect model) and there was no significant statistical heterogeneity (P = 0.47, I² drops from 56% to 0%). Of the two studies comparing annual chest x‐ray screening plus four‐monthly sputum cytology with annual chest x‐ray screening alone, the Johns Hopkins Study did not report all‐cause mortality, and in the Mem Sloan‐Kettering study there was no difference in all‐cause mortality between the screening groups (RR 1.03, 95% CI 0.91 to 1.15).
1.3. Analysis.
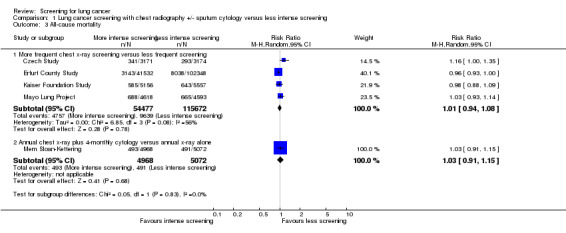
Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 3 All‐cause mortality.
Annual chest x‐ray screening versus usual care (no screening)
In the PLCO Trial all‐cause mortality was not reported, but an analysis of deaths from causes other than prostate, lung, colorectal or ovarian cancer was presented with no significant difference between the intervention and control groups (RR 0.98, 95% CI 0.96 to 1.01).
Annual low‐dose CT screening versus annual chest x‐ray screening
In the North American NLST all‐cause mortality was reduced in the low‐dose CT screening group compared with the chest x‐ray group (RR 0.94, 95% CI 0.88 to 1.00, P = 0.04).
Compliance with screening and contamination in the control group
In the Mayo Lung Project, compliance with scheduled screening averaged 75% in the intervention group. Of the control group, 73% received chest x‐rays during the final two years of the study. In the North London Study, both intervention and control groups were screened at the end of the third year. In the intervention group, 63.2% of workers attended for a final chest x‐ray and 62.7% in the control group. None of the firms in the control group underwent mass radiography examinations during the three‐year period.
In the Erfurt County Study, compliance with scheduled screening was not described in detail. In the Czech Study, both the intervention and control group underwent screening at the end of the third year, with 92% of the intervention group and 95% of the control group attending. The proportion of participants in the control group who underwent screening outside the study was not reported but only one asymptomatic case was detected by a non‐study x‐ray in the control group.
In the Mem Sloan‐Kettering study, participants were considered compliant if they had their last x‐ray in 1982, more than five years after enrolment or within one year of death (Melamed 1984). Of the intervention (dual screen) group, 63% were compliant, as were 65% of the control group. There was no comment about the proportion of participants in the control group who underwent screening with sputum cytology. In the Johns Hopkins Study 19% of participants withdrew from active screening but the proportion by screening group was not described (Tockman 1986). In the Kaiser Foundation Study the mean number of multiphasic health check‐ups (MHCs) per person during the study period was 6.8 (maximum 18, median 6) in the intervention group and 2.8 (maximum 17, median 1) in the control group. In the intervention group, 15.7% received no MHCs, compared with 36.2% of the control group.
In the North American NLST adherence to screening across the three rounds was 95% in the low‐dose CT group and 93% in the chest x‐ray group. Amongst participants in the chest x‐ray group the average annual rate of helical CT screening during the screening phase of the trial was estimated to be 4.3%.
In the PLCO Trial adherence to screening was 86.6% at the baseline screen, falling to 79% by year three, with an overall adherence rate of 83.5%. Ninety‐one point two per cent of participants underwent at least one radiographic screening. In the usual care group (not offered screening) the rate of chest radiograph screening was estimated at 11% during the screening phase of the trial. Of those with a positive screen for cancer at baseline, 82% were known to have had diagnostic follow‐up, while between 93% and 95% of those who had a positive screening test on subsequent screening rounds were known to have had diagnostic follow‐up.
Number of cases of lung cancer detected
Prevalence and incidence data were not reported in the Kaiser Foundation Study. In the Mayo Lung Project and Czech Study, prevalent cases were not included in the study populations. In the Erfurt County Study, Johns Hopkins Study, Mem Sloan‐Kettering study and North London Study, both prevalent and incident cases were included.
In those studies examining chest x‐ray screening at different frequencies, there was a tendency for increased incidence of cancer in the intervention group. In the Czech Study, the risk ratio of lung cancer was 1.33 (95% CI 0.99 to 1.75) in the intervention group. In the Mayo Lung Project, the risk ratio was 1.28 (95% CI 1.05 to 1.57). After 16 additional years of follow‐up, the risk ratio was similar (1.16, 95% CI 1.04 to 1.30). In the North London Study, the risk ratio was 1.16 (95% CI 0.89 to 1.51). In the Erfurt County Study, the risk ratio was 1.38 (95% CI 1.22 to 1.57). The increased incidence of lung cancer in the intervention group is consistent with overdiagnosis bias, but it might also occur if the groups were not comparable at baseline with respect to important risk factors. It is generally believed that the radiation exposure associated with chest radiography in these studies would be insufficient to substantially increase the incidence of lung cancer (Diederich 2000b). In the Mem Sloan‐Kettering study and Johns Hopkins Study, there were no significant differences in the number of cases of lung cancer diagnosed in the intervention and control groups (RR 1.01, 95% CI 0.82 to 1.24; RR 0.95, 95% CI 0.8 to 1.14, respectively). In the PLCO Trial there was no significant difference between the number of cases of lung cancer diagnosed between the intervention and control groups (RR 1.05, 95% CI 0.98 to 1.12) after 13 years of follow‐up post‐randomisation. In the North American NLST the number of cases of lung cancer diagnosed was significantly greater in the group offered low‐dose CT screening compared with those offered screening with chest x‐ray (RR 1.13, 95% CI 1.03 to 1.23) The results are summarised in Table 4.
1. Number of lung cancer cases diagnosed by screening group.
| Study | Intervention n (%) | Intervention N | Control n(%) | Control N | Relative risk |
| Czech Study | 108 (3.4%) | 3171 | 82 (2.6%) | 3174 | 1.33 (0.99,1.75) |
| Erfurt County Study | 374 (0.9%) | 41532 | 667 (0.7%) | 102348 | 1.38 (1.22,1.57) |
| Mayo Lung Project* | 585 (12.7%) | 4618 | 500 (10.9%) | 4593 | 1.16 (1.04,1.3) |
| North London Study | 132 (0.44%) | 29723 | 97 (0.38%) | 25311 | 1.16 (0.89,1.51) |
| Johns Hopkins Study | 238 (4.6%) | 5226 | 246 (4.8%) | 5161 | 0.95 (0.8,1.14) |
| Mem Sloan‐Kettering | 176 (3.5%) | 4968 | 178 (3.5%) | 5072 | 1.01 (0.82,1.24) |
| PLCO Trial | 1696 (2.2%) | 77445 | 1620 (2.1%) | 77456 | 1.05 (0.98, 1.12) |
| North American NLST | 1060 (4.0%) | 26722 | 941 (3.5%) | 26732 | 1.13 (1.03, 1.23) |
*Data from prolonged period of follow‐up reported post‐study.
Survival
We examined survival by comparing the proportion of participants alive five years after diagnosis. There was a trend to improved survival in the intervention group in all the studies apart from the Czech Study (survival for all participants diagnosed with lung cancer during the entire six years of the study). In the North London Study, the trend to improved survival did not reach statistical significance but in the remaining studies the differences in survival were statistically significant. In the pooled analysis of studies comparing more frequent chest x‐ray screening with less frequent chest x‐ray screening the risk ratio was 0.91 (95% CI 0.84 to 0.99, random effects model). There was significant statistical heterogeneity in the results (P=0.02, I2= 68%). (Analysis 1.4). Visual inspection of the graph for overlap of the 95% confidence intervals suggests that the Mayo Lung Project differs from the other results and, after removal of this study from the analysis, the risk ratio was 0.94 (95% CI 0.90 to 0.98, fixed effects model) and there was no significant statistical heterogeneity (P=0.47 and I2 drops from 68% to 0%). Five‐year survival data were presented for the Johns Hopkins Study and Mem Sloan‐Kettering study as a combined analysis already in the literature (Fleihinger 1994). In the graph, the data presented under the heading of the Johns Hopkins Study includes the data for both the Mem Sloan‐Kettering and Johns Hopkins Study.
1.4. Analysis.
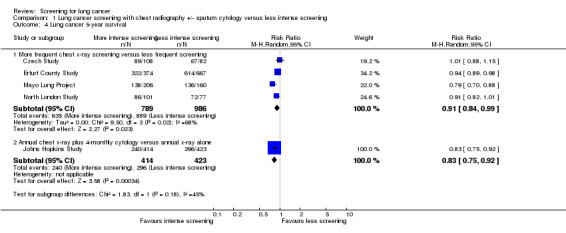
Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 4 Lung cancer 5‐year survival.
Survi val was not reported in three trials (Kaiser Foundation Study; North American NLST; PLCO Trial).
Stage distribution
In the Erfurt County Study, stage distribution was reported for the resected patients only. Information about stage at diagnosis was not provided in the North London Study and Kaiser Foundation Study.
Because of the potential for overdiagnosis bias in the screened group, there may be an increase in the number of early stage cancers detected by screening, regardless of the efficacy of the intervention. If a screening programme is effective, then there should also be a corresponding reduction in the number of people presenting with advanced disease in the screened group. During the first three years of the Czech Study, 54% of lung cancers in the intervention group were diagnosed stage I or II, compared with 21% of cancers in the control group. In the Mayo Lung Project, the proportion of early‐stage cancers (stages 0, I or II) was greater in the intervention group: 99/206 compared with 51/160 (P = 0.002). However, there was no reduction in the absolute number presenting with advanced disease (stages III and IV): 107 cases in the intervention group and 109 in the control group. In the Mem Sloan‐Kettering study and Johns Hopkins Study, the proportion of early‐stage cancers detected was similar in the intervention and control groups: 58/143 versus 68/154; 83/194 versus 93/202 respectively. When assessing the stage distribution it is important to evaluate both the proportion of early‐ and late‐stage cancers.
In the PLCO Trial, 39.5% (574/1454) of cancers in the intervention group were stage I or II compared with 35% (479/1378) in the control group (P = 0.01). However the absolute number of stage III and IV cancers were similar between the groups with 359 stage III and 514 stage IV in the intervention group and 365 stage III and 530 stage IV in the control group. In the North American NLST 57% (593/1040) of cancers were stage I or II in the low‐dose CT (intervention) group compared with 39% (363/929) in the plain chest x‐ray (control) group (P = 0.0001). In addition there was a significant reduction in the absolute number of cancers presenting as stage III and IV in the low‐dose CT group compared with the chest x‐ray group (447 vs 566, P = 0.0002; the denominator for this calculation was the total number of participants in each group).
Resection rates
In the Czech Study (prior to screening at the end of the 3rd year), the resection rate was 25% in the intervention group and 16% in the control group (P = 0.33). By the end of the six years, the resection rates were the same in both groups (23%). In the North London Study, the resection rate for all cases diagnosed during the study was 46% in the intervention group and 38% in the control group (P = 0.27). In the Mayo Lung Project, the resection rate was 46% in the intervention group and 32% in the control group (P < 0.01). In the Erfurt County Study, the resection rate was 28% in the intervention group and 18.7% in the control group (P < 0.001). In the Mem Sloan‐Kettering study, the resection rates were similar in the intervention and control groups: 51% and 53% respectively. In the Johns Hopkins Study, the resection rate was 53% in the intervention group and 44% in the control group (P = 0.12). Resection rates were not reported in the Kaiser Foundation Study.
Of note in the Czech Study was that a number of participants with potentially resectable lung cancer did not undergo surgery because they either declined it or were otherwise medically unfit. In fact, the proportion of participants with potentially resectable cancers who underwent surgery during the first three years of the study was significantly greater in the control group compared with the intervention group; 91% versus 50% (P = 0.04).
In the North American NLST, 60.6% of those diagnosed with cancer in the low‐dose CT group underwent surgery (either alone or in combination with chemotherapy and/or radiotherapy) compared with 44.1% in the chest x‐ray group. In the low‐dose CT group 44.6% of participants diagnosed with cancer were treated with surgery alone, compared with 26.8% in the chest x‐ray screening group. In the low‐dose CT group there were 12 participants with stage I or II cancer who did not receive any treatment, compared with 13 participants in the chest x‐ray group. In the PLCO Trial 34.3% of participants diagnosed with lung cancer in the chest x‐ray screening group underwent surgical resection (with or without chemotherapy) compared with 30.1% in the usual care group.
Postoperative deaths and harm associated with screening
In general, harms associated with screening were poorly reported. In the Mem Sloan‐Kettering study, postoperative deaths were reported in the participants diagnosed with lung cancer. There were four such deaths in the intervention group and five in the control group, which were counted as lung cancer deaths (Melamed 1984). In the Mayo Lung Project, there were seven postoperative deaths in the intervention group and six in the control group. These were included in the lung cancer mortality data. In the Czech Study, postoperative mortality within the first 30 days was three out of 33 resected cases of screen‐detected cancer and one out of 11 resected cases of non‐screen‐detected cancer. In the Erfurt County Study, the postoperative mortality was 2.9% (three people) in the intervention group and 4.0% (five people) in the control group (P = 0.7). Postoperative deaths were not reported in the Johns Hopkins Study, Kaiser Foundation Study or North London Study.
For some of the studies, information was provided on the number of participants with abnormal screen results (refer to section on test performance). For example, in the Johns Hopkins Study of the 10,362 prevalence screens, there were 1574 participants with initially abnormal results. A large proportion of these were resolved by further radiographic evaluation. In general, the proportion of people requiring invasive diagnostic work‐ups, including procedures such as bronchoscopy or biopsies, was not systematically reported. Of note, from the prevalence screens in all three National Cancer Institute (NCI) studies (Johns Hopkins Study; Mayo Lung Project; Mem Sloan‐Kettering) of over 20,000 men, there were six postoperative deaths in people with lung cancer and two in people without lung cancer(Bailar 1984).
In the North American NLST there were a total of 18,146 positive test results in the low‐dose CT group over the three annual screening rounds. Complete diagnostic follow‐up information was available for 17,702, and amongst this subgroup there were 245 reports of at least one complication and 1075 invasive procedures performed (including 457 for investigation of abnormalities that did not turn out to be lung cancer). There were 84 major complications in individuals who underwent an invasive procedure (including 11 in individuals who did not have lung cancer). Invasive procedures included thoracotomy or thoracoscopy or mediastinoscopy or bronchoscopy or needle biopsy. In the low‐dose CT group there were 16 participants who died within 60 days of an invasive procedure (10 of whom had lung cancer); it was not known if the procedures caused the deaths.
In the chest radiography group of the North American NLST there were a total of 5043 positive test results and complete diagnostic information was available for 4953 of these. Amongst this subgroup there were 81 reports of at least one complication and 334 invasive procedures were performed (including 70 for investigation of abnormalities that did not turn out to be lung cancer). There were 24 major complications in individuals who underwent an invasive procedure (including one individual who did not turn out to have lung cancer). There were 10 deaths reported within 60 days of an invasive procedure (all in individuals with lung cancer).
In the PLCO Trial data on postoperative deaths were not reported and information about diagnostic procedures was not available for the usual care group. In the group offered screening the authors reported a complication rate of 0.4% amongst participants having a diagnostic follow‐up procedure (the total number of participants with complications was 54). The most common complications were pneumothorax (29%), atelectasis (15%) and infection (10%).
Costs
None of the studies reported costs. A cost‐effectiveness analysis is planned by the investigators involved in the North American NLST but this has not been published at the time of the most recent update of this review.
Quality of life
None of the studies included in the review assessed the impact of screening on quality of life.
Test performance
None of the studies was able to assess sensitivity and specificity accurately since there is no 'gold standard' which can be applied to all participants at the time of testing.
Some of the studies provided data on the proportion of total lung cancer cases detected by screening. In the North London Study, 73% of cancers in the intervention group were detected by screening compared with 38% in the control group (P < 0.001). In the Czech Study (prior to screening in both groups at the end of the third year) none of the cases in the control group were diagnosed by screening, while 72% of those in the screened group were detected by screening (P < 0.001). By the end of six years, however, the proportion of lung cancers detected by screening was not significantly different; 56% in the intervention group and 46% in the control group (P = 0.17), consistent with the fact that screening took place in both groups during years three to six of the study.
In the Mayo Lung Project, 75% of cancers in the intervention group were detected by screening, including some non‐study x‐rays, and 26% of cancers in the control group were detected by non‐study x‐rays (P < 0.001). By the end of the Mem Sloan‐Kettering study, 69% of cancers were detected by screening in the intervention group and 61% by screening in the control group (P = 0.14). In the intervention (dual screen) group, cytology detected 29% of lung cancer cases and x‐ray detected 51% of cancers. Both screening methods detected 10%. In the Johns Hopkins Study 67% of cancers were detected by screening in the intervention group and 62% by screening in the control group (P = 0.3). Of note, there were significantly more squamous cell carcinomas detected in the intervention group (37% versus 25%, P = 0.026). Sputum cytology was good at detecting squamous cell carcinoma but relatively poor at detecting adenocarcinoma and undifferentiated large‐cell carcinoma (Johns Hopkins Study).
In the Erfurt County Study, 47% of cancers were detected by screening in the intervention group compared with 27% in the control group (P < 0.001). The proportion detected by screening was not presented in the Kaiser Foundation Study.
Data were published on the positive predictive value of prevalence screens only for the three NCI studies and the Czech Study. In the NCI studies chest x‐rays were initially classified as negative for cancer, indeterminate, or suspicious for cancer. For x‐rays classified as suspicious or indeterminate, the positive predictive values were 16%, 4.4% and 3.8% in the Mayo Lung Project, Johns Hopkins Study and Mem Sloan‐Kettering study respectively. Considering only those classified as suspicious for cancer, the positive predictive values were 41%, 60% and 53% respectively. Sputum cytology results were classified as carcinoma cells, marked atypia, moderate atypia, slight atypia or normal. For cytology results classified as carcinoma cells, marked atypia and moderate atypia, the positive predictive values were 60%, 11% and 34% for the Mayo Lung Project, Johns Hopkins Study and Mem Sloan‐Kettering study respectively. For cytology results classified as carcinoma cells or marked atypia, the positive predictive values were 75%, 64% and 68% respectively.
In the Czech Study, from the prevalence screen of 6364 there were 232 abnormal x‐rays and 17 cases of lung cancer (positive predictive value = 7.3%). During subsequent screening in the control group there were 166 abnormal chest x‐rays and 25 lung cancer cases diagnosed (positive predictive value = 15%) (Kubik 1986).
In the Mayo Lung Project, of the 109 cancers detected (up to 1982) in the dual screen group, 64% were visible on earlier x‐rays after retrospective review. Some of these were visible on x‐rays taken up to 53 months prior to the diagnosis (Muhm 1983). In a similar review of cases diagnosed during the Mem Sloan‐Kettering study, 23% of interval cases of non‐small cell lung cancer were visible in retrospect and 65% of screen‐detected cases were also visible on previous x‐rays (mean size 1.3 cm for retrospective films).
False positive rates were high in the low‐dose CT group in the North American NLST, across the three screening rounds False positive rates were 96.4% of the positive results in the low‐dose CT group and 94.5% of those in the radiography group. False positives were more common during the first two rounds of screening. Over the three rounds of screening the total number of low‐dose CT screenings tests classified as positive was 24.2%, and 23.3% had false positive results. In the radiography group, 6.9% of screening tests were classified as positive (over the three screening rounds) and 6.5% had false positive results.
In the North American NLST lung cancer was diagnosed in 1060 participants in the group offered low‐dose CT screening. In 649 participants it was diagnosed after a positive screening test and in 44 it was diagnosed after a negative screening test. In 367 it was diagnosed either after the screening phase had finished or in those who were due for a screening test or were never screened. In the chest radiography screening group of the North American NLST 279 lung cancers were diagnosed after a positive screening test, 137 were diagnosed after a negative screening test and 525 were diagnosed either during the post‐screening phase of the trial or in those who were never screened or were due for their next screening test.
Smoking behaviour
Most studies reported that participants were advised to stop smoking but there were no specific smoking cessation strategies employed. In the Czech Study smoking histories recorded during the study period showed no differences in terms of the proportions who stopped smoking or switched to pipes or cigars. In the Mayo Lung Project at the end of one year of follow‐up 90% of participants were still smoking or had resumed smoking, but figures were not reported by screening allocation.
Data were not available on the impact of screening on smoking behaviour for the North American NLST and PLCO Trial at the time this review was last updated, but may be published in the future.
Consumer perspectives
Those consumers most likely to be affected by the results of the review are healthy current or ex‐smokers. Other high‐risk groups include those with chronic obstructive airways disease and those who have previously been treated for lung cancer. We asked consumers or consumer advocates to participate in the review process, and consulted a consumer at the time of writing of the review protocol. This consumer was interviewed about the language of the review and the outcomes being considered. He identified some additional outcomes of relevance to consumers, including what the impact of screening would be on smoking behaviour and quality of life, and we incorporated these outcomes into the review. Another consumer (consumer advocate) was asked to evaluate the full review. After reading the review she was interviewed using a semi‐structured format to identify issues of concern to consumers. We sought comments on the process of screening, the importance of various outcomes and the language of the review. From the point of view of consumers of screening services, she suggested that it would be important to be able to evaluate the burden of involvement in regular screening. She felt that many consumers "would not want to be living their lives around it". The studies in this review did not comprehensively outline the potential time commitments or inconveniences to consumers, nor did they evaluate the potential psychosocial effects of screening. It is not clear, for example, what proportion of people with abnormal results required more frequent monitoring or invasive procedures. She also suggested that there is likely to be a diversity of attitudes to screening in the community. If the natural history of lung cancer is not altered by screening then some people may think there is still merit in undergoing screening, while others would not want early diagnosis just for the sake of knowing.
Discussion
This is a major update of the systematic review of lung cancer screening first published in The Cochrane Library in 1999 (Manser 1999) and updated in 2004 (Manser 2004) and 2010 (Manser 2010). The conclusions of the current review have been changed by the inclusion of two recently published substantial trials (North American NLST; PLCO Trial). In the original review, a meta‐analysis of chest x‐ray screening studies found that overall more frequent chest x‐ray screening does not result in reduced lung cancer mortality compared with less frequent screening. In fact, when data from the prolonged periods of follow‐up reported for the Mayo Lung Project and the Czech Study were included in the analysis, more frequent chest x‐ray screening was associated with an 11% relative increase in lung cancer mortality compared with less frequent screening. Survival as an outcome in screening studies will be influenced by screening biases, including overdiagnosis, lead‐time and length‐time (for prevalent cases). The finding in this review of a significant increase in survival from lung cancer in association with an increase in disease‐specific mortality emphasises the unreliability of survival as an outcome measure in screening trials. Screening which includes four‐monthly sputum cytology in addition to an annual chest x‐ray was not associated with an improvement in lung cancer mortality compared with annual chest x‐ray screening alone. However the 95% confidence intervals are relatively wide and include a range of potentially clinically significant values (for example, the true effect might lie between a 26% relative reduction in lung cancer mortality and a 3% relative increase in lung cancer mortality). The PLCO Trial included in the present review is the only chest x‐ray screening trial that included women and non‐smokers in addition to smokers. It is also the only trial that had a control group that was not offered any form of screening. The finding in the PLCO Trial of a lack of any reduction in mortality from lung cancer associated with chest x‐ray screening is consistent with the results of earlier chest x‐ray screening trials included in our original review. Importantly however, this was a methodologically rigorous trial and they did not find any increase in mortality associated with chest x‐ray screening. We identified methodological weaknesses in most of the trials included in the original review. In particular the adequacy of allocation concealment was unclear in many of the studies. As has been reported by others recently, it is probable that a volunteer effect in the setting of a compromised randomisation process may account for the increased mortality from lung cancer noted in the Mayo Lung Project intervention group (Dominion 2011).
The findings of the North American NLST comparing low‐dose CT screening with chest x‐ray screening support the finding of earlier uncontrolled CT trials which have consistently demonstrated that CT screening is more sensitive than chest x‐ray screening. This methodologically rigorous trial shows that in current and ex‐smokers (at least 30 pack‐years) who have quit within the last 15 years and are aged between 55 and 74 years, CT screening is associated with a relative reduction of 20% in lung cancer mortality compared with chest x‐ray screening. The lack of any harm or benefit from annual chest x‐ray screening found in the PLCO Trial suggests that we can be more confident about the relative benefit of CT screening in high‐risk smokers compared with current usual care. It is not clear, however, whether the results of the North American NLST will be generalisable to other populations. There are several smaller randomised trials that are currently ongoing in Europe, and these may add to our understanding of the applicability of CT screening in different settings (DANTE; DLCST; ITALUNG; LUSI; MILD; NELSON 2003). Unlike the North American NLST the majority of European studies have a control group that was not offered any screening and have offered a longer duration of screening in the intervention group. In addition they have included participants with a lower total tobacco exposure, and the LUSI and NELSON 2003 trials have used population‐based methods to recruit participants. These studies are also likely to add to our knowledge of nodule management (NELSON 2003). Several of the European studies have published preliminary mortality data; however, we have excluded these studies from the present review because the duration of follow‐up was not sufficient. We specified in our exclusion criteria that studies with follow‐up of less than five years would be excluded, and we have therefore excluded DANTE, DLCST, and MILD. Our review will be updated in future to include such studies once more follow‐up data become available.
Although there is now some evidence to support the effectiveness of low‐dose CT screening in individuals at high risk for lung cancer, there are several issues that need to be addressed in deciding the role of low‐dose CT screening in current clinical practice and how these findings should influence current public health policy. The false positive rates of CT screening are high (approximately three to four times that seen with chest radiography screening in the North American NLST) and while the number of invasive tests performed for investigation of benign lesions was relatively low in the North American NLST, the majority of false positives results will lead to more frequent follow‐up CT scans and hence additional costs and radiation exposure. The most important radiation‐related hazard from low‐dose CT screening in the population likely to be screened is radiation‐induced lung cancer (Brenner 2004). The estimated risk from a single CT screening examination is relatively small (< 0.06%) but for annual screening in a current male smoker aged 50 to 75 it is estimated that screening could lead to a 1.5% increase in the risk of lung cancer, and for a female smoker it is estimated that screening could lead to a 5% increase in risk if screening were offered between the ages of 50 and 75 (Brenner 2004). It is also possible that screening conducted outside the trial setting may be associated with higher rates of invasive testing or complications if conducted by institutions or radiologists and clinicians with less experience in managing pulmonary nodules and lung cancers than those involved in the North American NLST. None of the studies included in this review have reported on costs or cost effectiveness to date. The North American NLST investigators are planning to report on quality of life effects, costs and cost effectiveness of screening in their trial, and to use modelling (in collaboration with the Cancer Intervention and Surveillance Modeling Network) to examine the impact that different variables may have on cost effectiveness and outcomes. Based on previous cost‐effectiveness analyses however, even if screening is effective in groups at lower risk for lung cancer than those in the North American NLST it is unlikely to be cost‐effective in this population (Mahadevia 2003). Many smokers and ex‐smokers at risk for lung cancer are likely to fall into a lower‐risk group for lung cancer than those in the North American NLST. If public health agencies recommend screening only in heavy smokers then consumers may well perceive this as inequitable. Ma 2013 recently estimated that 8.6 million Americans met the North American NLST criteria for lung cancer screening in 2010 and that if screening were offered to this eligible population then 12,000 deaths could be averted each year; however this represents just 7.6% of the total number of lung cancer deaths per year in the United States. The authors did not comment about the number of former smokers or current smokers who would be ineligible for screening, but in the 2008 to 2010 National Health Interview Survey 15.8% of the population in the United States were daily current smokers, 4.4% were non‐daily current smokers and 21% were former smokers (Schoenborn 2013). In a recent report on a surgical cohort of people with lung cancer it was shown that approximately 60% had stopped smoking more than 10 years ago and approximately 39% had stopped more than 20 years ago (Mong 2011). Offering screening to lower‐risk groups such as those who quit more than 15 years ago or those in a younger age group might be seen as a way to increase the impact of screening at a population level, but as previously indicated this is not likely to be a cost‐effective strategy within the current CT screening paradigm (Mahadevia 2003).
Models that examine the cost effectiveness of low‐dose CT screening need to take into account the impact of overdiagnosis by screening, but this may be difficult to estimate. The proportion of cancers 'overdiagnosed' by screening can be estimated by examining the difference in incidence between the intervention and control group in screening studies. At the end of screening it is expected that the incidence of cancer will be higher in the screened group as screening advances the time of diagnosis; however, with a period of follow‐up after the cessation of screening the magnitude of this difference is likely to fall as some previously undiagnosed but clinically significant cancers are diagnosed in the control group due to the presence of symptoms or signs. If there is a persistent excess of cancers in the intervention group years after screening has ceased this is likely to represent the proportion of overdiagnosed cases. The optimal duration of follow‐up post‐screening is not clear, but ideally would include the lead‐time of the slowest growing tumours, although competing mortality risk is also an important factor (Welch 2007). Some experts have estimated overdiagnosis with chest x‐ray screening to be as high as 50% using data from the Mayo Lung Project. However, given that there are doubts about the adequacy of the randomisation process in that study, this may not be a reliable estimate (Dominion 2011; Welch 2007). The only other randomised trial included in this review that showed a statistically significant difference in incidence between intervention and control groups was the North American NLST (see Table 4). In this trial there was at least five years of follow‐up after the end of screening, at which time there had been 1060 lung cancers diagnosed in the CT screening group compared with 941 in the chest x‐ray group (an excess of 119 cases). Of note, there were 95 screen‐detected cases of bronchioalveolar cell carcinoma in the CT screening group compared with only 13 in the chest x‐ray group (North American NLST). The term bronchioalveolar cell carcinoma is no longer recommended and it is likely that most of these tumours would fall into the category of either adenocarcinoma in situ, minimally invasive or lepidic predominant invasive adenocarcinoma according to current pathological criteria (North American NLST; Travis 2011). Adenocarcinoma in situ refers to lesions that are 3 cm in size or less and have a purely lepidic growth pattern. Observational studies have shown that these lesions are associated with 100% disease‐free survival after complete resection and therefore could potentially be overdiagnosed by screening (Travis 2011). However, these pre‐invasive lesions may also progress over time to become invasive and therefore longer periods of follow‐up will be needed in randomised controlled trial of screening to determine the magnitude of overdiagnosis and the potential impact of screening on these more indolent tumours. The true rate of overdiagnosis by CT screening may not be accurately assessed in the North American NLST because the control group were offered chest x‐ray screening which is also likely to overdiagnose some cases of lung cancer, albeit to a lesser extent. Of the randomised controlled trials of low‐dose CT scanning currently underway in Europe, the NELSON 2003 trial will provide valuable data about overdiagnosis, as the investigators are planning 10 years of follow‐up and the control group have not been offered any screening. Screening studies have provided valuable insights into the clinical, radiological and pathological features of adenocarcinoma in situ and other pre‐invasive lesions and these data has been used to develop new recommendations for the management of such lesions that aim to minimise the extent of invasive procedures and may reduce the impact of overdiagnosis in screening trials (Godoy 2012).
Authors' conclusions
Implications for practice.
The current evidence suggests that screening with annual plain chest radiography in smokers and non‐smokers, and more frequent chest radiography screening in smokers and ex‐smokers, is not effective at reducing lung cancer mortality and cannot be recommended for clinical practice. Annual screening with low‐dose CT scanning was associated with a significant reduction in lung cancer mortality in one large study of high‐risk individuals (aged 55 to 74 years with 30 pack‐years or more of smoking, or who quit 15 years or less prior to entry if ex‐smokers); however, more data are needed on the cost effectiveness of screening that takes into account the frequency of screening and both the benefits and harms (such as false positives and overdiagnosis) before recommendations can be made for large‐scale screening programmes. In the interim, physicians should discuss the relative risks and benefits of screening with people at high risk for lung cancer before recommending screening, and should ensure that any screening and subsequent management of abnormal results takes place in a healthcare setting with adequate experience in managing pulmonary nodules and lung cancer. Further studies are needed before recommendations can be made about screening individuals at lower risk for lung cancer, such as non‐smokers or those with less than 30 pack‐years of smoking exposure.
Implications for research.
The current low‐dose CT screening trials underway in Europe will provide further data on the generalisability of the results of the North American NLST conducted in the USA (DANTE; DLCST; ITALUNG; LUSI; MILD; NELSON 2003). These studies will also provide further insights into factors such as the frequency of screening, nodule management strategies and the rates of overdiagnosis. However, it is likely that the poor specificity of CT screening will remain a major barrier to the implementation of screening in clinical practice and public health screening programmes. Future research should focus on developing strategies to target CT screening at very high‐risk populations by taking into account not only smoking status but the presence of chronic obstructive pulmonary disease, emphysema and other clinical factors (Raji 2012; Wilson 2008). Models incorporating age, smoking status, genetic factors and spirometric data are currently being developed (Young 2012). In addition the development of biomarkers may further help to refine populations at highest risk using samples such as blood, exhaled human breath or sputum (Boshuizen 2012; Carpagnano 2005; Machado 2005; Phillips 1999; Phillips 2003; Rahman 2005; Zhong 2005).
What's new
| Date | Event | Description |
|---|---|---|
| 7 June 2013 | New search has been performed | New search conducted May 2012 |
| 2 November 2012 | New citation required and conclusions have changed | Two new studies have been included. Conclusions of the review have changed. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 11 January 2009 | New search has been performed | Conclusions not changed |
| 25 March 2008 | Amended | Converted to new review format |
Acknowledgements
We wish to thank the Iberoamerican Cochrane Centre, Carol Roberts, and Ivan Solà, Trials Search Co‐ordinator of the Cochrane Lung Cancer Group for assistance with database searches. We would also like to thank Dr Consol Serra and Jordi Pardo (previous Managing Editors of the Cochrane Lung Cancer Group) for assistance with protocol development, general advice and editing of the review. We would like to acknowledge the help provided by authors of primary studies who have responded to our correspondence and provided additional information (Dr Robert Fontana, Dr Myron Melamed). We are also grateful to Russell McGowan, who reviewed and commented on the protocol from the consumer perspective. We would like to acknowledge the valuable comments provided by Frances Hanks, who reviewed the final original review from the consumer perspective. We are grateful to Dr Sera Tort, the current Joint Co‐ordinating Editor for the Cochrane Lung Cancer Review Group, for editorial input and general advice.
Appendices
Appendix 1. Search strategy for the 2012 update
MEDLINE (PubMed; 23.05.2012)
#1 "Mass Screening"[Mesh] 89670
#2 screen*[tiab] 395893
#3 #1 OR #2 427494
#4 "Lung Neoplasms"[Mesh] 155750
#5 lung neoplasm*[tiab] 803
#6 lung cancer[tiab] 76090
#7 lung[ti] 168525
#8 #4 OR #5 OR #6 OR #7 265010
#9 #3 AND #8 6552
#10 (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT (humans[mh] AND animals[mh])) 2551469
#11 #9 AND #10 1458
#12 #9 AND #10 Filters: Publication date from 2007/12/01 601
CENTRAL (The Cochrane Library 2012 issue 5; 24.05.2012)
#1 MeSH descriptor Mass Screening explode all trees 4194
#2 screen*:ti,ab,kw 15543
#3 (#1 OR #2) 15802
#4 MeSH descriptor Lung Neoplasms explode all trees 4104
#5 lung neoplasm*:ti,ab,kw 4854
#6 lung cancer:ti,ab,kw 6860
#7 lung:ti 8947
#8 (#4 OR #5 OR #6 OR #7) 11447
#9 (#3 AND #8) 393
#10 (#3 AND #8), from 2007 to 2012 180 (122 in Clinical Trials)
EMBASE (Ovid 1974 to 2012 May 23, 24.05.2012)
1 exp screening/ (390837)
2 screen*.ti,ab. (493764)
3 1 or 2 (687613)
4 exp lung cancer/ (168835)
5 lung neoplasm*.ti,ab. (876)
6 lung cancer.ti,ab. (99518)
7 lung.ti. (205866)
8 4 or 5 or 6 or 7 (311507)
9 3 and 8 (12036)
10 random:.tw. or placebo:.mp. or double‐blind:.mp. (945378)
11 9 and 10 (994)
12 limit 11 to yr="2007 ‐Current" (545)
Appendix 2. Search strategy for 2004 and 2010 updates
Searches for the review updates
MEDLINE
In 2003, 2007 we updated the MEDLINE search. We searched MEDLINE (PubMed) from 1995 to November 2007 using a search strategy to identify controlled and uncontrolled trials with the following:
1 explode lung neoplasms (all headings) 2 explode mass screening 3 screen*.ti,ab 4 sputum*.ti,ab 5 explode radiography, thoracic 6 explode tomography, x‐ray 7 explode tomography, x‐ray computed 8 "explode tomography, Spiral computed" 9 Helical computed tomography 10 Helical CT screening 11 Positron emission tomographic 12 PET 13 Molecular marker 14 or/2‐13 15 14 AND 1
CENTRAL
We searched the Lung Cancer Group Trials Register.
We undertook an advanced search of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 4) in November 2007 using the following strategy:
1 LUNG AND CANCER 2 BRONCHOGENIC AND CARCINOMA 3 LUNG AND NEOPLASM* 4 1 OR 2 OR 3 5 SCREEN* 6 4 OR 5
EMBASE
We searched EMBASE from 1980 to November 2007 using the following strategy:
1 Clinical trial/ or Phase 1 clinical trial/ or Phase 2 clinical trial/ or Phase 3 clinical trial/ or Phase 4 clinical trial/ or Randomized controlled trial/ 2 Randomization/ 3 Double blind procedure/ or Meta analysis/ or Single blind procedure/ 4 exp controlled study/ 5 Placebo/ 6 "150".tg. 7 "197".tg. 8 (clinic$ adj10 trial).ti, ab. 9 (clinic$ adj10 trial$).ti, ab. 10 (controlled adj trial$).ti, ab. 11 ((singl$ or doubl$ or trebl$ or tripl$) adj10 (blind$ or mask$)).ti, ab. 12 (placebo$ or random$).ti, ab. 13 1 or 2 or 3 or 4 or 5 or 6 or 7 or 9 or 10 or 11 or 12 14 limit 13 to human 15 Cancer screening/ 16 mass screening/ 17 screening/ 18 screen$.ti, ab. 19 exp lung tumor/ 20 ((lung or pulmon$) adj10 (tumor$ or tumour$ or cancer or neoplas$ or carcinoma$ or adenocarcinoma$ or squamous or oat cell or small cell)).ti, ab. 21 exp thorax radiography/ 22 exp computer assisted tomography/ 23 exp sputum examination/ 24 (radiogra$ adj10 (thora$ or chest)).ti, ab. 25 (x rays adj10 (thora$ or chest)).ti, ab. 26 (CT adj10 (thora$ or chest)).ti, ab. 27 (computed tomography adj10 (thora$ or chest)).ti, ab. 28 (sputum examination or sputum analysis or sputum cytology or sputum screening).ti, ab. 29 15 or 16 or 17 or 18 30 19 or 20 31 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 32 cancer mortality/ 33 exp prognosis/ or exp survival/ 34 mortality/ 35 (benef$ or effectiv$ or surviv$ or mortality or prognos$).ti, ab. 36 32 or 33 or 34 or 35 37 29 and 30 and 31 and 36 38 14 and 37
Appendix 3. Search strategy for the original review
MEDLINE
We searched MEDLINE (OVID) from 1966 to July 2000 using the recommended Cochrane search strategy for randomised controlled trials in addition to the following:
1 explode lung neoplasms (all headings) 2 explode mass screening 3 screen$.ti,ab 4 sputum$.ti,ab 5 explode radiography, thoracic 6 explode tomography, x‐ray 7 explode tomography, x‐ray computed 8 or/2‐7 9 8 AND 1
We also searched PREMEDLINE using the following strategy:
1 lung carcinoma.mp 2 lung neoplasm.mp 3 lung cancer.mp 4 1 or 2 or 3 5 screening.mp 6 screen$.ti,ab 7 sputum$.ti,ab 8 x‐ray.mp 9 radiography.mp 10 computed tomography.mp 11 or/5‐10 12 4 and 11
An advanced search of the Cochrane Central Register of Controlled Trials (CENTRAL) was also undertaken using the following strategy: 1 LUNG AND CANCER 2 BRONCHOGENIC AND CARCINOMA 3 LUNG AND NEOPLASM* 4 1 OR 2 OR 3 5 SCREEN* 6 4 OR 5 EMBASE was also searched using the following strategy: 1 Clinical trial/ or Phase 1 clinical trial/ or Phase 2 clinical trial/ or Phase 3 clinical trial/ or Phase 4 clinical trial/ or Randomized controlled trial/ 2 Randomization/ 3 Double blind procedure/ or Meta analysis/ or Single blind procedure/ 4 exp controlled study/ 5 Placebo/ 6 "150".tg. 7 "197".tg. 8 (clinic$ adj10 trial).ti, ab. 9 (clinic$ adj10 trial$).ti, ab. 10 (controlled adj trial$).ti, ab. 11 ((singl$ or doubl$ or trebl$ or tripl$) adj10 (blind$ or mask$)).ti, ab. 12 (placebo$ or random$).ti, ab. 13 1 or 2 or 3 or 4 or 5 or 6 or 7 or 9 or 10 or 11 or 12 14 limit 13 to human 15 Cancer screening/ 16 mass screening/ 17 screening/ 18 screen$.ti, ab. 19 exp lung tumor/ 20 ((lung or pulmon$) adj10 (tumor$ or tumour$ or cancer or neoplas$ or carcinoma$ or adenocarcinoma$ or squamous or oat cell or small cell)).ti, ab. 21 exp thorax radiography/ 22 exp computer assisted tomography/ 23 exp sputum examination/ 24 (radiogra$ adj10 (thora$ or chest)).ti, ab. 25 (x rays adj10 (thora$ or chest)).ti, ab. 26 (CT adj10 (thora$ or chest)).ti, ab. 27 (computed tomography adj10 (thora$ or chest)).ti, ab. 28 (sputum examination or sputum analysis or sputum cytology or sputum screening).ti, ab. 29 15 or 16 or 17 or 18 30 19 or 20 31 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 32 cancer mortality/ 33 exp prognosis/ or exp survival/ 34 mortality/ 35 (benef$ or effectiv$ or surviv$ or mortality or prognos$).ti, ab. 36 32 or 33 or 34 or 35 37 29 and 30 and 31 and 36 38 14 and 37
Data and analyses
Comparison 1. Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung cancer mortality | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 81303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.31] |
| 1.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 2 | 20427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.03] |
| 2 Lung cancer mortality (including prolonged follow‐up data) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 81303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.00, 1.23] |
| 2.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 2 | 20427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.03] |
| 3 All‐cause mortality | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 170149 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.08] |
| 3.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 1 | 10040 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.15] |
| 4 Lung cancer 5‐year survival | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 4.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 1 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
Comparison 2. Annual chest x‐ray screening versus usual care (no regular screening).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung cancer mortality at 6 years of follow up | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.03] |
| 2 Lung cancer mortality at 13 years of follow up | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 3 Deaths from all causes (excluding deaths from PLCO cancers) | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.96, 1.01] |
2.1. Analysis.
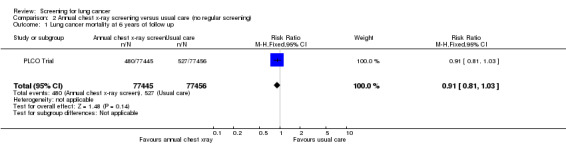
Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 1 Lung cancer mortality at 6 years of follow up.
2.2. Analysis.
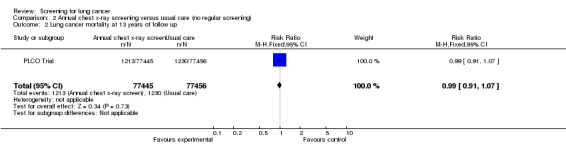
Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 2 Lung cancer mortality at 13 years of follow up.
2.3. Analysis.
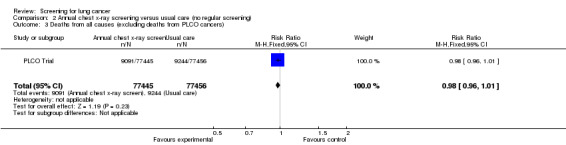
Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 3 Deaths from all causes (excluding deaths from PLCO cancers).
Comparison 3. Annual low dose CT screening versus annual chest x‐ray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung cancer mortality | 1 | 53454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.70, 0.92] |
| 2 All‐cause mortality | 1 | 53454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |
3.1. Analysis.
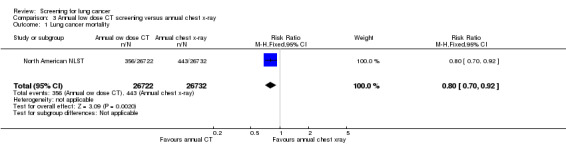
Comparison 3 Annual low dose CT screening versus annual chest x‐ray, Outcome 1 Lung cancer mortality.
3.2. Analysis.
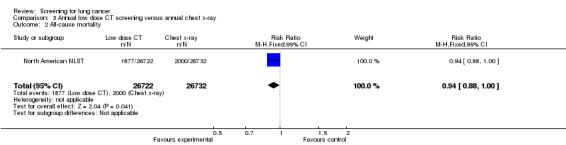
Comparison 3 Annual low dose CT screening versus annual chest x‐ray, Outcome 2 All‐cause mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Czech Study.
| Methods | Randomised controlled trial. Czechoslovakia 1976 to 1982. | |
| Participants | Men aged 40 to 64 years. Current smokers with a lifetime cigarette consumption of greater than 150,000. Participants were included in the study if their initial prevalence screen was negative. They were excluded if they were not likely to participate for at least five years in periodic screening due to serious disease or other reasons. | |
| Interventions | Intervention group: semi‐annual chest x‐rays and sputum cytology. Control group: one chest x‐ray and sputum cytology at the end of the study. Screening duration: three years. Afterwards, both groups had annual chest x‐rays (no sputum cytology) for a further three years. |
|
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Assessment of cause of death | Unclear risk | Blinding of the assessment of cause of death not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported. |
| Other bias | Unclear risk | Randomisation was stratified by age, smoking status, socioeconomic status, place of residence and occupational exposure, but number of strata used was not specified. Details not provided for all variables at baseline in the published reports. |
Erfurt County Study.
| Methods | Controlled (non‐randomised) trial. Germany 1972 to 1977. | |
| Participants | Men aged 40 to 65 years. All men living in the Erfurt county in Germany at the time of the study were included (smokers and non‐smokers); 41,532 men in the intervention group and 102,348 in the control group. | |
| Interventions | Intervention group: chest x‐ray at six‐monthly intervals. Control group: chest x‐ray at 18‐monthly intervals. Screening duration: five years. |
|
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not used. |
| Allocation concealment (selection bias) | High risk | Not used. |
| Blinding (performance bias and detection bias) Assessment of cause of death | Unclear risk | Blinding of the assessment of cause of death not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and drop‐outs adequately described, but losses to follow‐up significantly greater in the control group. |
| Other bias | Low risk | |
Johns Hopkins Study.
| Methods | Randomised controlled trial. USA 1973 to 1978. | |
| Participants | Men over 45 years of age. 5161 men in the x‐ray‐only group and 5226 in the dual‐screen group. Smokers (at least 1 pack per day). Recruited from the Baltimore metropolitan area using mail‐outs (motor vehicle drivers' licenses) and local industrial and occupational groups. | |
| Interventions | Intervention group: annual chest x‐rays and four‐monthly sputum cytology.
Control group: annual chest x‐rays (chest x‐rays included postero‐anterior and lateral views). Screening duration: five years. |
|
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Individuals volunteered for the study by telephoning the Johns Hopkins Lung Project at which time they were randomised into intervention and control groups and given an appointment for screening. A total of 10,828 men were initially randomised, but 441 were automatically disqualified for failing to meet the age or cigarette‐smoking criteria of the study. |
| Blinding (performance bias and detection bias) Assessment of cause of death | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1.3% of participants lost to follow‐up but no further details provided. |
| Other bias | Low risk | The investigators reported on 36 baseline variables including multiple age strata, occupational exposures and smoking history. There were significantly more black participants in the control group (621 versus 701, P = 0.009) but this difference was not statistically significant after adjusting for multiple comparisons (P < 0.0014). |
Kaiser Foundation Study.
| Methods | Randomised controlled trial. USA 1964 to 1980. | |
| Participants | Men and women aged 35 to 54 at entry. 5156 people in study group and 5557 in control group. Both smokers and non‐smokers were included (about 17% of participants were smokers in both groups). All were members of Kaiser Permanente Medical Care Progam. | |
| Interventions | Intervention group: encouraged to undergo an annual Multiphasic Health Checkup (MHC) which included a chest x‐ray. Control group: participants not urged to take MHCs but could voluntarily do so as part of the care they received. |
|
| Outcomes | All‐cause mortality and mortality from 'potentially postponable' causes including lung cancer mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patient record numbers (with a concealed code). |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Assessment of cause of death | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up was poor; in 1980 only 64% of participants were still health plan members and the response rate to follow‐up surveys was only 75%. |
| Other bias | Unclear risk | Statistically significant differences in some baseline variables. |
Mayo Lung Project.
| Methods | Randomised controlled trial. USA 1971 to 1976. | |
| Participants | Men over 45 years of age recruited from Mayo Clinic outpatients. Current smokers. 4618 men in the intervention group and 4593 in the control group. Participants were included in the study if their initial prevalence screen x‐ray was normal. | |
| Interventions | Intervention group: four‐monthly chest x‐rays and sputum cytology. Control group: standard Mayo Clinic recommendations to have an annual chest x‐ray and sputum cytology test with their local medical officer. Screening duration: six years. |
|
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | There were initially 10,933 men interviewed and entered in the prevalence phase of the study. Randomisation for the incidence phase of the study took place on entry to the prevalence study but 16% of men were excluded after randomisation. The exclusions included 91 prevalent lung cancers, six upper respiratory tract cancers, 971 who were ineligible because their life expectancy was less than five years or were thought unable to tolerate lobectomy, and 653 participants who did not complete the prevalence screening. Clinical judgements about eligibility were made by clinicians independent of the study, but the screening group allocation was marked on the participant's record on enrolment and therefore clinicians would have been aware of the allocation at the time of assessing eligibility. Randomisation was undertaken by staff interviewers (not primary investigators) on site, using a random number table, but it is unclear whether or not this was concealed or open (personal communication with Dr Fontana). |
| Blinding (performance bias and detection bias) Assessment of cause of death | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate. |
| Other bias | Low risk | The intervention and control groups were well matched for measured known confounders at baseline. Adjusting for these confounders (including smoking history, exposure to non‐tobacco carcinogens and history of other pulmonary diseases) did not significantly alter the results of the study. |
Mem Sloan‐Kettering.
| Methods | Randomised controlled trial. USA 1974 to 1978. | |
| Participants | Men (current smokers) over 45 years of age. 5072 men in the x‐ray‐only group and 4968 men in the dual‐screen group. | |
| Interventions | Intervention group: annual chest x‐rays and four‐monthly sputum cytology. Control group: annual chest x‐rays (chest x‐rays included postero‐anterior and lateral views). Screening duration: five years. |
|
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Adequate. Although randomisation took place on site, participants were randomised only after baseline data were entered and they were accepted into the study. Randomisation was co‐ordinated by clerical staff independent of the study investigators. Investigators met with participants only after they were randomised (this information was confirmed by contacting one of the study authors, M. Melamed). |
| Blinding (performance bias and detection bias) Assessment of cause of death | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate. |
| Other bias | Low risk | In this study, we examined 13 baseline variables reported in published reports and there was a greater proportion of participants with a history of exposure to asbestos (6% versus 5%, P = 0.03) and nickel (P = 0.03) in the intervention group, but these differences were no longer statistically significant after adjusting for multiple comparisons (P < 0.0039) (Berlin 1984). |
North American NLST.
| Methods | Randomised controlled multicentre study. 33 centres in the USA. 2002 and 2004. | |
| Participants | Men (59%) and women aged between 55 and 74, with a history of cigarette‐smoking of at least 30 pack‐years and if former smokers had quit within the previous 15 years. Individuals were excluded with a previous diagnosis of lung cancer, or who had undergone CT chest within 18 months before enrolment, or with a history of haemoptysis or unexplained weight loss of more than 6.8 kg in the preceding year. 53,454 persons were enrolled, 26,722 were assigned to screening with low‐dose CT and 26,732 to screening with chest radiography. Participants were recruited by the 33 screening centres. At each screening centre participants were made aware of the trial through direct mailing and use of local radio, newspaper advertisements, outreach including health fairs and presentations to unions an community groups, National Cancer Institute and institutional websites, Internet‐based advertising and public service television and radio announcements. |
|
| Interventions | The intervention group were offered a total of three screenings with low‐dose CT at yearly intervals. The control group were offered a total of three screenings with chest radiography (postero‐anterior projection) at yearly intervals. All low‐dose CT scans were acquired using multidetector scanners with a minimum of four channels. The acquisition variables were chosen to reduce exposure to an average effective dose of 1.5 mSv. Low‐dose CT scans that revealed any non‐calcified nodule measuring at least 4 mm in any diameter and radiographic images that revealed any noncalcified nodule or mass were classified as positive "suspicious for" lung cancer. Other abnormalities such as adenopathy or effusion could also be classified as positive. At the third screening round abnormalities suspicious for lung cancer that were stable across the three rounds could be classified as minor abnormalities rather than positive results. No specific nodule‐evaluation approach was mandated by the trial protocol and the recommendations of the interpreting radiologist were reported in writing to the participant and his or her healthcare provider within four weeks of the examination. |
|
| Outcomes | The primary outcome was lung cancer mortality; secondary outcomes included all‐cause mortality, incidence of lung cancer, lung cancer case survival (as measured from date of diagnosis), and lung cancer stage distribution. | |
| Notes | The number of lung cancer screening tests conducted outside the NSLT was estimated by self‐administered questionnaires that were mailed to a random sample of approximately 500 participants annually. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised after consent was obtained using a central process (ACRIN website). Randomisation was stratified by age, gender and screening centre, and blocked such that at each centre, each arm had equal numbers of participants within each gender and age category. |
| Allocation concealment (selection bias) | Low risk | As above, participants randomised after consent using a centralised process. |
| Blinding (performance bias and detection bias) Assessment of cause of death | Low risk | Death certificates were obtained for participants who were known to have died. An end point verification team determined whether the cause of death was lung cancer. Deaths selected for review included those with a notation of lung cancer on the death certificate and those occurring among participants ever diagnosed with lung cancer, as well as deaths within six months of a screen suspicious for lung cancer and deaths within 60 days of certain diagnostic evaluation procedures associated with a screen suspicious for lung cancer or a lung cancer diagnosis. Members of the end point verification team were not aware of group assignments. A distinction was made between a death due to lung cancer and a death that resulted from the diagnostic evaluation for or treatment of lung cancer; however the deaths in the latter category were counted as lung cancer deaths in the primary end point analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The vital status was known for 97% of participants in the low‐dose CT group and 96% of the chest radiography group. The median duration of follow‐up was 6.5 years and maximum duration was 7.4 years. According to Consort statement no individuals were excluded from the analysis of the primary outcomes and it is likely that losses to follow‐up were censored. |
| Other bias | Low risk | All prespecified primary outcomes were reported and the intervention and control groups were comparable at baseline. |
North London Study.
| Methods | Cluster‐randomised controlled trial (industrial firms randomised). UK 1960 to 1964. | |
| Participants | Men aged 40 and over. 29,723 in intervention group and 25,311 in control group. Both smokers and non‐smokers included. | |
| Interventions | Intervention group: six‐monthly chest x‐rays. Control group: chest x‐ray on entry and at the end of the study period. Mobile x‐ray unit used for x‐rays. Screening duration: three years. |
|
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Assessment of cause of death | Unclear risk | Blinding of the assessment of cause of death not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate. |
| Other bias | Unclear risk | Statistically significant differences in some baseline variables. |
PLCO Trial.
| Methods | Randomised controlled multiple cancer (prostate, lung, ovarian and colon cancers) screening study. Multicentre ‐10 screening centres. USA 1993 to 2001. | |
| Participants | Men and women between the ages of 55 and 74 years. Included smokers and non‐smokers. 77,470 participants in the control group and 77,464 in the intervention group. Exclusions included anyone participating in another cancer screening trial or primary prevention trial. Men who had taken finasteride in the six months before entry or who had had more than one prostate‐specific antigen blood test in the past three years, and individuals who had had colonoscopy, sigmoidoscopy or a barium enema examination in the past three years. Individuals with previous surgical removal of the entire prostate gland, one lung, or the entire colon were also excluded. Women with prior removal of both ovaries were initially excluded, but were allowed to enrol from 1996 onwards. Participants were also excluded if they had a history of any prostate, lung, ovarian or colon cancer or were currently receiving treatment for cancer. Recruitment was targeted to healthy volunteers primarily through direct mail. Enhanced recruitment methods were used to target minority populations. |
|
| Interventions | The intervention group were offered a single‐view posterioranterior chest x‐ray at baseline and then annually for three years (a total of four screens including the baseline x‐ray). A chest x‐ray was considered positive when a radiologist identified a mass (> 3 cm), nodule(< 3 cm), infiltrate, or any other abnormality considered suspicious for cancer. Never‐smokers randomised after April 1995 were not offered the final screen. The control group were assigned to usual care (no formalised screening). Participants who received a positive screening result were referred to their primary healthcare provider for further evaluation. The trial protocol did not specify a diagnostic algorithm. Chest radiographic screening in the usual‐care group was assessed by surveying a random sample of just more than 1% of participants using biennial and later annual health status questionnaires. | |
| Outcomes | The primary outcome was lung cancer mortality; secondary outcomes included lung cancer incidence, cancer stage, survival, harms from screening and all‐cause mortality. | |
| Notes | 49.5% of participants in both the intervention and usual‐care groups were men and approximately 52% were former or current smokers in both groups. Adherence to screening was 86.6% for the baseline screen, decreasing to 79% by year three. 91.2% of participants underwent at least one radiographic screening. In the usual‐care group the contamination rate was estimated at 11% during the screening phase of the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation scheme used blocks of random permutations of varying lengths and was stratified by screening centre, gender, and age. |
| Allocation concealment (selection bias) | Low risk | Random assignment was implemented using compiled software and encrypted files loaded on to microcomputers at each of the screening centres. |
| Blinding (performance bias and detection bias) Assessment of cause of death | Low risk | Deaths were ascertained by annual follow‐up questionnaire and where necessary repeat mailings or telephone follow‐up in addition to periodic linkage to the National Death Index. An end point adjudication process was used to assign cause of death. All deaths with causes potentially related to a prostate, ovarian, colorectal or lung cancer were reviewed. Death reviewers were blinded to the trial group of the deceased participant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of losses to follow‐up were not described. For the primary outcome person‐time was measured from randomisation to the earliest death date or date of last follow‐up (censoring date). All individuals randomised were included in the primary analysis. The median follow‐up time in each group was 11.2 years (interquartile range 10.0 to 13 years in each group). |
| Other bias | Low risk | Groups were comparable at baseline and all prespecified outcomes were reported. |
CT: computed tomography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bach 2007 | Screening with low‐dose CT; uncontrolled study. |
| Depiscan Group | Pilot randomised controlled trial of low ‐ose CT versus chest x‐ray, small study and no mortality data provided. |
| Diederich 2000 | Uncontrolled study (screening with low‐dose CT). |
| Diederich 2002 | Uncontrolled study (screening with low‐dose spiral CT). |
| Ebeling 1987 | Observational, case‐control study. |
| Garg 2002 | Small randomised controlled trial of low‐dose spiral CT screening. Feasibility study with no outcome data. |
| Gohagan 2005 | Small randomised controlled trial of low‐dose CT screening. Feasibility study with no mortality data. |
| Henschke 1999 | Screening with low‐dose CT; uncontrolled study. |
| Henschke 2001 | Screening with low‐dose CT; uncontrolled study. |
| Henschke 2006 | Screening with low‐dose CT: uncontrolled study. |
| Kakinuma 1999 | Uncontrolled study (screening with low‐dose spiral CT), report on false negative results. |
| Kaneko 1996 | Uncontrolled study (screening with low‐dose CT). |
| Matsumoto 1995 | Uncontrolled study. |
| Nawa 2002 | Uncontrolled study (screening with low‐dose spiral CT). |
| Sobue 1992 | Observational, case‐control study. |
| Sobue 2002 | Uncontrolled study (screening with low‐dose spiral CT). |
| Sone 1998 | Uncontrolled study (screening with spiral CT). |
| Sone 2001 | Uncontrolled study (screening with spiral CT). |
| Swensen 2002 | Uncontrolled study (screening with low‐dose spiral CT). |
| Tiitola 2002 | Uncontrolled study (screening with CT in asbestos‐exposed workers). |
| Yang 2008 | Randomised controlled trial with a total of 523 participants, comparing low‐dose CT screening plus p16 gene methylation detection with chest x‐ray screening. This was a feasibility study and did not include mortality data. |
CT: computed tomography
Characteristics of ongoing studies [ordered by study ID]
DANTE.
| Trial name or title | DANTE |
| Methods | Randomised controlled trial. |
| Participants | 2,811 men aged 60 to 75 years, smokers of 20 or more pack‐years. |
| Interventions | Low‐dose CT versus control (no active screening) at baseline and every year for four years. |
| Outcomes | Lung cancer mortality, resectability, stage distribution. |
| Starting date | March 2001. |
| Contact information | |
| Notes | Three‐year preliminary results published in 2009. |
DLCST.
| Trial name or title | DLCST (Danish Lung Cancer Screening Trial). |
| Methods | Randomised controlled trial. |
| Participants | 4104 men and women 50 to 70 years, current or former smokers (at least 20 pack years). |
| Interventions | Five annual low‐dose CT screenings versus no screening. |
| Outcomes | Lung cancer mortality and all‐cause mortality. |
| Starting date | October 2004. |
| Contact information | |
| Notes | Preliminary results published in 2012 but median follow‐up was < 5 years (median 4.81 years), further follow‐up is planned. |
ITALUNG.
| Trial name or title | ITALUNG |
| Methods | Randomised controlled trial. |
| Participants | 3206 men and women aged 55 to 69 years, smokers and former smokers with at least a 20 pack‐year history of smoking. |
| Interventions | Low‐dose CT screening for four years versus no screening. |
| Outcomes | Lung cancer mortality. |
| Starting date | |
| Contact information | |
| Notes |
LUSI.
| Trial name or title | LUSI (Lung Cancer Screening Intervention trial). |
| Methods | Randomised controlled trial. |
| Participants | 4052 men and women, heavy smokers, aged 50 to 69 years. |
| Interventions | Five annual low‐dose, multislice CT versus no screening. |
| Outcomes | Lung cancer mortality. |
| Starting date | October 2007. |
| Contact information | |
| Notes |
MILD.
| Trial name or title | MILD (Multicentric Italian Lung Detection project). |
| Methods | Randomised controlled trial. |
| Participants | Men and women aged 49 years and above, current or former smokers (at least 20 pack‐years of smoking) and having quit within 10 years of recruitment. |
| Interventions | Annual low‐dose CT versus biennial low‐dose CT versus control (no active screening). |
| Outcomes | Lung cancer mortality, lung cancer incidence, all‐cause mortality. |
| Starting date | September 2005. |
| Contact information | |
| Notes | The trial is ongoing, preliminary results published in 2012, but median duration of follow‐up was 4.4 years (< 5 years). |
NELSON 2003.
| Trial name or title | Dutch‐Belgian randomised lung cancer screening trial (Nederlands Leuvens Longkanker Screenings Onderzoek). |
| Methods | Multicentre trial, randomised, parallel group, no blinding. |
| Participants | Target number, n = 15,600. Born between 1928 and 1956; current long‐term smokers or quit smoking < 10 years prior. |
| Interventions | 16 detector multislice computed tomography of the chest in year four; pulmonary function test; blood sampling; questionnaires; smoking cessation advice for current smokers, versus smoking cessation advice for current smokers. |
| Outcomes | Primary: reduction in lung cancer mortality. Secondary: cost effectiveness; quality of life. |
| Starting date | August 2003. |
| Contact information | Klaveren RJ van; http://www.nelsonproject.nl. |
| Notes |
CT: computed tomography
Contributions of authors
2001 and 2004 versions of the review
Renée Manser initiated the review and helped to write the protocol. She also carried out the literature search, reviewed abstracts and full text studies for inclusion, participated in the quality assessments, data extraction, analysis and writing of the review.
Christine Stone helped with the assessment of studies (full text) for inclusion in the review, undertook data extraction of the main results and helped with writing of the review.
Graham Byrnes provided statistical advice regarding the analysis and statistical issues relevant to the quality assessment of included studies. He also helped to revise and write the final version.
Lou Irving helped with the writing of the protocol, assessment of methodological quality of included studies and revisions of the final version.
Michael Abramson participated in the protocol development, quality assessments and revision and writing of the final version.
Donald Campbell reviewed abstracts from the initial search for inclusion in the review and assisted with revisions and writing of the final version.
2008 update
Anne Lethaby undertook the update of the review in 2008. She selected studies for inclusion, extracted data (only one publication identified: longer follow‐up of a study already included) and assessed all the included studies for risk of bias according to the new 'Risk of bias' tool. She also added to the Discussion section of the review.
Renée Manser selected studies for inclusion and commented on the updated Discussion section of the review.
2012 update
Renée Manser updated the review in 2012. Anne Lethaby and Renée Manser searched the abstracts and selected studies for inclusion in the review after reviewing the full text of the relevant studies. Anne Lethaby and Renée Manser extracted the data for inclusion in the review and assessed the quality of included studies. Renée Manser rewrote the abstract, introduction, results and discussion and Anne Lethaby wrote the summary of findings tables and assisted with editing and revision of the results and discussion and approved the final version of the review. The final version of the review was approved by Don Campbell, Louis Irving, Michael Abramson and Graham Byrnes.
Declarations of interest
None known. The authors of this review were not involved in any of the primary studies included in the review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Czech Study {published data only}
- Kubik A. Risk groups and effective frequency of X‐ray miniature screening of bronchial carcinoma [Risikogruppen und effektive schirmbildscreeningfrequenz des bronchialkrebses]. Zeitchrift fu Erankungen de Atmungsovgane 1983;160(1):18‐25. [PubMed] [Google Scholar]
- Kubik A, Haerting J. Survival and mortality in a randomised study of lung cancer detection. Neoplasma 1990;37(4):467‐75. [PubMed] [Google Scholar]
- Kubik A, Parkin DM, Khlat M, Erban J, Polak J. Adamec M. Lack of benefit from semi‐annual screening for cancer of the lung: follow‐up report of a randomised controlled trial on a population of high‐risk males in Czechoslavakia. International Journal of Cancer 1990;45(1):26‐33. [DOI] [PubMed] [Google Scholar]
- Kubik A, Polak J. Lung cancer detection. Results of a randomized prospective study in Czechoslovakia. Cancer 1986;57:2427‐37. [DOI] [PubMed] [Google Scholar]
- Kubik, AK, Parkin DM, Zatioukal P. Czech study on lung cancer screening: Post‐trial follow up of lung cancer deaths up to year 15 since enrolment. Cancer 2000;89(Suppl):2363‐8. [DOI] [PubMed] [Google Scholar]
- Walter SD, Kubik A, Parkin DM, Reissigova J, Adamec M, Khlat M. The natural history of lung cancer estimated from the results of a randomised trial of screening. Cancer Causes and Control 1992;3(2):115‐23. [DOI] [PubMed] [Google Scholar]
Erfurt County Study {published data only}
- Wilde J. A 10 year follow‐up of semi‐annual screening for early detection of lung cancer in the Erfurt County,GDR. European Respiratory Journal 1989;2(7):656‐62. [PubMed] [Google Scholar]
- Wilde J, Durschmied H, Lorenz H, Niegsch G, Schmidt H, Simer HM. Effectiveness of photography screening studies in the early detection of bronchial carcinoma [Effektivitat der schirmbildreihenuntersuchung zur fuhen erfassung des brondhialkarzinoms]. Zeitschrift fur Erkrankigender Atmungsorgane 1983;160:128‐41. [PubMed] [Google Scholar]
Johns Hopkins Study {published data only}
- Baker R, Tockman MS, Marsh BR, Stitik FP, Ball WC, Eggleston JC, et al. Screening for bronchogenic carcinoma: the surgical experience. Journal of Thoracic Cardiovascular Surgery 1979;78:876‐82. [PubMed] [Google Scholar]
- Frost NK, Ball WC, Levin ML, Tockman MS, Baker RR, Carter D, et al. Early lung cancer detection: results in the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins Study. American Review of Respiratory Disease 1984;130:549‐54. [DOI] [PubMed] [Google Scholar]
- Levin ML, Tockman MS, Frost JK, Ball WC. Lung cancer mortality in males screened by chest x‐ray and cytologic sputum examination: a preliminary report. Recent Results in Cancer Research 1982;82:138‐46. [DOI] [PubMed] [Google Scholar]
- Stitik FP, Tockman MS. Radiographic screening in the early detection of lung cancer. Radiologic Clinics of North America. 1978;16(3):347‐66. [PubMed] [Google Scholar]
- Tockman MS. Survival and mortality from lung cancer in a screened population. Chest 1986;89(Suppl):324‐5. [Google Scholar]
- Tockman MS, Frost JK, Stitik FP, Levin ML, Ball WC, Marsh BR. Screening and detection of lung cancer. In: Joseph Aisner editor(s). Lung Cancer. New York: Churchill Livingstone, 1985:25‐36. [Google Scholar]
Kaiser Foundation Study {published data only}
- Dales L, Friedman GD, Collen MF. Evaluating periodic multiphasic health checkups: a controlled trial. Journal of Chronic Diseases 1979;32:385‐404. [DOI] [PubMed] [Google Scholar]
- Friedman G, Collen MF, Fireman BH. Multiphasic health checkup evaluation: a 16 year follow up. Journal of Chronic Diseases 1986;39:453‐63. [DOI] [PubMed] [Google Scholar]
Mayo Lung Project {published data only}
- Flehinger BJ, Kimmel M, Polyak T, Melamed MR. Screening for lung cancer. The Mayo Lung Project revisited. Cancer 1993;72(5):1573‐80. [DOI] [PubMed] [Google Scholar]
- Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo clinic study. American Review of Respiratory Diseases 1984;130:561‐5. [DOI] [PubMed] [Google Scholar]
- Fontana RS, Sanderson DR, Woolner LB, Miller WE, Bernatz PE, Payne WS, et al. The Mayo Lung Project for early detection and localization of bronchogenic carcinoma: a status report. Chest 1975;67(5):511‐22. [DOI] [PubMed] [Google Scholar]
- Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR. Lung cancer screening: the Mayo program. Journal of Occupational Medicine 1986;28(8):746‐50. [DOI] [PubMed] [Google Scholar]
- Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR, et al. Screening for lung cancer: a critique of the Mayo Lung Project. Cancer 1991;67(4 Suppl):1155‐64. [DOI] [PubMed] [Google Scholar]
- Marcus PM, Bergstrahl EJ, Fagerstrom RM, Williams DE, Fontana RS, Taylor WF, et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow up. Journal of the National Cancer Institute 2000;92(16):1308‐16. [DOI] [PubMed] [Google Scholar]
- Marcus PM, Bergstralh EJ, Zweig MH, Harris A, Offord KP, Fontana RS. Extended lung cancer incidence follow‐up in the Mayo Lung Project and overdiagnosis. Journal of the National Cancer Institute 2006;98(11):748‐56. [DOI] [PubMed] [Google Scholar]
- Marcus PM, Prorok PC. Reanalysis of the Mayo Lung Project data: the impact of confounding and effect modification. Journal of Medical Screening 1999;6(1):47‐9. [DOI] [PubMed] [Google Scholar]
- Muhm JR, Miller WE, Fontana RS, Sanderson DR, Uhlenhopp MA. Lung cancer detected during a screening program using four month chest radiographs. Radiology 1983;148(3):609‐15. [DOI] [PubMed] [Google Scholar]
- Sanderson D, Fontana R. Results of the Mayo Lung Project: an interim report. Recent Results in Cancer Research 1982;82:179‐86. [DOI] [PubMed] [Google Scholar]
- Sanderson DR. Lung cancer screening. The Mayo study. Chest 1986;89(4 Suppl):324. [Google Scholar]
- Taylor WF, Fontana RS. Biometric design of the Mayo Lung project for early detection and localization of bronchogenic carcinoma. Cancer 1972;30(5):1344‐7. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Fontana RS, Uhlenhopp MA, Davis CS. Some results of screening for early lung cancer. Cancer 1981;47:1114‐20. [DOI] [PubMed] [Google Scholar]
- Woolner L, Fontana RS, Cortese DA, Sanderson DR, Bernatz PE, Payne WS, et al. Roentgenographically occult lung cancer: pathologic findings and frequency of multicentricity during a 10 year period. Mayo Clinic Proceedings 1984;59:453‐66. [DOI] [PubMed] [Google Scholar]
- Woolner LB, Fontana RS, Sanderson DR, Miller WE, Muhm JR, Taylor WF, et al. Mayo Lung Project. Evaluation of lung cancer screening through December 1979. Mayo Clinic Proceedings 1981;56(9):544‐55. [PubMed] [Google Scholar]
Mem Sloan‐Kettering {published data only}
- Flehinger BJ, Kimmel M. The natural history of lung cancer in a periodically screened population. Biometrics 1987;43:127‐44. [PubMed] [Google Scholar]
- Flehinger BJ, Melamed MR. Current status of screening for lung cancer. Chest Surgery Clinics of North America 1994;4(1):1‐15. [PubMed] [Google Scholar]
- Flehinger BJ, Melamed MR, Zaman MB, Heelan RT, Perchick WB, Martini N. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Memorial Sloan‐Kettering study. American Review of Respiratory Diseases 1984;130(4):555‐60. [DOI] [PubMed] [Google Scholar]
- Heelan RT, Flehinger BJ, Melamed MR, Zaman MB, Perchick WB, Caravelli JF, et al. Non‐small‐cell lung cancer: results of the New York screening program. Radiology 1984;151:289‐93. [DOI] [PubMed] [Google Scholar]
- Martini N. Results of the Memorial Sloan‐Kettering study in screening for early lung cancer. Chest 1986;89(4 Suppl):325. [DOI] [PubMed] [Google Scholar]
- Melamed M, Flehinger BJ, Miller D, Osborne R, Zaman M, McGinnis C, et al. Preliminary report of the Lung Cancer Detection Program in New York. Cancer 1977;39:369‐82. [DOI] [PubMed] [Google Scholar]
- Melamed MR, Flehinger BJ, Zaman MB. Impact of early detection on the clinical course of lung cancer. Surgical Clinics of North America 1987;67(5):909‐24. [DOI] [PubMed] [Google Scholar]
- Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer. Results of the Memorial Sloan‐Kettering study in New York. Chest 1984;86(1):44‐53. [DOI] [PubMed] [Google Scholar]
North American NLST {published data only}
- National Lung Screening Trial Research Team. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. New England Journal of Medicine 2011;365(5):395‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Lung Screening Trial Research Team. The National Lung Screening Trial: overview and study design. Radiology 2011;258(1):243‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
North London Study {published data only}
- Anon. Lung cancer detection by chest x‐rays at 6 monthly intervals. Nova Scotia Medical Bulletin 1970;49(1):14‐5. [PubMed] [Google Scholar]
- Brett G. The presymptomatic diagnosis of lung cancer. Proceedings of the Royal Society of Medicine 1966;59:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett G. The value of lung cancer detection by 6 monthly chest radiographs. Thorax 1968;23:414‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett GZ. Earlier diagnosis and survival in lung cancer. British Medical Journal 1969;4(678):260‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
PLCO Trial {published data only}
- Hocking WG, Hu P, Oken MM, Winslow SD, Kvale PA, Prorok PC, et al. Lung cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Journal of the National Cancer Institute 2010;102(10):722‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer BS, Gohagan J, Prorok PC. A randomised study of chest x‐ray screening for lung cancer as part of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Trial.. Lung Cancer 1994;11(Suppl 2):82‐3. [Google Scholar]
- Oken MM, Marcus PM, Hu P, Beck TM, Hocking Wm Kvale PA, et al. Baseline chest radiograph for lung cancer detection in the randomized Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Journal of the National Cancer Institute 2005;97(24):1832‐9. [DOI] [PubMed] [Google Scholar]
- Oken, MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality. The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306(17):1865‐73. [DOI] [PubMed] [Google Scholar]
- Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials 2000;21:273S‐309S. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bach 2007 {published data only}
Depiscan Group {published data only}
- Blanchon T, Bréchot J‐M, Grenier PA, Ferretti GR, Lemarié E, Milleron B, et al. Baseline results of the Depiscan study: A French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest x‐ray (CXR). Lung Cancer 2007;58:50‐8. [DOI] [PubMed] [Google Scholar]
Diederich 2000 {published data only}
- Diederich S, Wormanns D, Lenzen H, Semik M, Thomas M, Peters PE. Screening for asymptomatic early bronchogenic carcinoma with low dose CT of the Chest. Cancer 2000;89(Suppl):2483‐4. [DOI] [PubMed] [Google Scholar]
Diederich 2002 {published data only}
- Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, et al. Screening for early lung cancer with low‐dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222(3):773‐81. [DOI] [PubMed] [Google Scholar]
Ebeling 1987 {published data only}
- Ebeling K, Nischan P. Screening for lung cancer ‐ results from a case‐control study. International Journal of Cancer 1987;40(2):141‐4. [DOI] [PubMed] [Google Scholar]
Garg 2002 {published data only}
- Garg K, Keith RL, Byers T, Kelly K, Kerzner AL, Lynch DA, et al. Randomized controlled trial with low‐dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 2002;225(2):506‐10. [DOI] [PubMed] [Google Scholar]
Gohagan 2005 {published data only}
- Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, Kramer BS, Prorok PC, et al. Final results of the Lung Screening Study, a randomised feasibility study of spiral CT versus chest X‐ray screening for lung cancer. Lung Cancer 2005;47:9‐15. [DOI] [PubMed] [Google Scholar]
Henschke 1999 {published data only}
- Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early lung cancer action project: overall design and findings from baseline screening. Lancet 1999;354:99‐105. [DOI] [PubMed] [Google Scholar]
Henschke 2001 {published data only}
- Henschke C, Naidich DP, Yankelevitz DF, McGuinness G, McCauley DI, Smith JP, et al. Early Lung Cancer Action Project: initial findings on repeat screening. Cancer 2001;92:153‐9. [DOI] [PubMed] [Google Scholar]
Henschke 2006 {published data only}
- Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage 1 lung cancer detected on CT screening. New England Journal of Medicine 2006;355(17):1763‐71. [DOI] [PubMed] [Google Scholar]
Kakinuma 1999 {published data only}
- Kakinuma R, Ohmatsu H, Kaneko M, Eguchi K, Naruke T, Nagai K, et al. Detection failures in spiral CT screening for lung cancer: analysis of CT findings. Radiology 1999;212:61‐6. [DOI] [PubMed] [Google Scholar]
Kaneko 1996 {published data only}
- Kaneko M, Eguchi K, Ohmatsu H, Kakinuma R, Naruke T, Suemasa K, et al. Peripheral lung cancer: screening and detection with low‐dose spiral CT versus radiography. Radiology 1996;201(3):798‐802. [DOI] [PubMed] [Google Scholar]
Matsumoto 1995 {published data only}
- Matsumoto M, Horikoshi H, Moteki T, Hatori N, Tateno Y, Iinuma T, et al. A pilot study with lung‐cancer screening CT (LSCT) at the secondary screening for lung cancer detection. Nippon Igaku Hoshasen Gakkai Zasshi [Nippon Acta Radiologica] 1995;55(3):172‐9. [PubMed] [Google Scholar]
Nawa 2002 {published data only}
- Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H. Lung cancer screening using low‐dose spiral CT: results of baseline and 1‐year follow‐up studies. Chest 2002;122:15‐20. [DOI] [PubMed] [Google Scholar]
Sobue 1992 {published data only}
- Sobue T, Suzuki T, Naruke T, Japanese lung‐cancer‐screening research group. A case control study for evaluating lung cancer screening in Japan. International Journal of Cancer 1992;50(2):230‐7. [DOI] [PubMed] [Google Scholar]
Sobue 2002 {published data only}
- Sobue T, Moriyama N, Kaneko M, Kusumoto M, Kobayashi T, Tsuchiya R, et al. Screening for lung cancer with low‐dose helical computed tomography: Anti‐Lung Cancer Association project. Journal of Clinical Oncology 2002;20(4):911‐20. [DOI] [PubMed] [Google Scholar]
Sone 1998 {published data only}
- Sone S, Takashima S, Li F, Yang Z, Honda T, Maruyama Y, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 1998;351(9111):1242‐5. [DOI] [PubMed] [Google Scholar]
Sone 2001 {published data only}
- Sone S, Li F, Yang Z‐G, Honda T, Maruyama Y, Takashima S, et al. Results of three‐year mass screening programme for lung cancer using mobile low‐dose spiral computed tomography scanner. British Journal of Cancer 2001;84(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swensen 2002 {published data only}
- Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, et al. Screening for lung cancer with low‐dose spiral computed tomography. American Journal of Respiratory and Critical Care Medicine 2002;165:508‐13. [DOI] [PubMed] [Google Scholar]
Tiitola 2002 {published data only}
- Tiitola M, Kivisaari L, Huuskonen MS, Mattson K, Koskinen H, Lehtola H, et al. Computed tomography screening for lung cancer in asbestos‐exposed workers. Lung Cancer 2002;35:17‐22. [DOI] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang M, Wang J, Meng L‐J, Wang Y, Xu L, Liu F‐Y, et al. Analysis of feasibility of lung cancer screening with low‐radiation‐dose spiral CT scan plus detection of p16 gene methylation in serum. Chinese Journal of Cancer Prevention and Treatment 2008;15(1):8‐10. [Google Scholar]
References to ongoing studies
DANTE {published data only}
- Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G Ceresoli G, et al. A randomized study of lung cancer screening with spiral computed tomography: three‐year results from the DANTE trial. American Journal of Respiratory and Critical Care Medicine 2009;180:445‐53. [DOI] [PubMed] [Google Scholar]
DLCST {published data only}
- Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clemensten PF, et al. CT screening for lung cancer brings forward early disease. The Randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low‐dose CT. Thorax 2012;67:296‐301. [DOI] [PubMed] [Google Scholar]
ITALUNG {published data only}
- Pegna AL, Picozzi G, Mascalchi M, Carrozzi FM, Carozzi L, Comin C, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low‐dose CT. Lung Cancer 2009;64:34‐40. [DOI] [PubMed] [Google Scholar]
LUSI {published data only}
- Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. Journal of Cancer Research and Clinical Oncology 2012;138(9):1475‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
MILD {published data only}
- Pastorino U, Rossi M, Rosato V, Marchianò A, Sverzellati N, Morosi C, et al. Annual or biennial CT screening versus observation in heavy smokers: 5 year results of the MILD trial. European Journal of Cancer Prevention 2012;21:308‐15. [DOI] [PubMed] [Google Scholar]
NELSON 2003 {published data only}
- Lersel CA, Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, et al. Risk‐based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch‐Belgian Randomised Lung Cancer Multi‐slice CT Screening Trial (NELSON). International Journal of Cancer 2006;120:868‐74. [DOI] [PubMed] [Google Scholar]
- Zhao, YR, Xie X, Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging 2011;11:S79‐S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACS 1980
- American Cancer Society. Report on the cancer related health checkup: cancer of the lung. CA: A Cancer Journal for Clinicians 1980;30:199‐207. [Google Scholar]
Bach 2007a
- Bach PB, Silvestri GA, Hauger M, Jett JR. Screening for lung cancer. ACCP evidence‐based clinical practice guidelines. Chest 2007;132(2 Suppl):69S‐77S. [DOI] [PubMed] [Google Scholar]
Bailar 1984
- Bailar JC. Screening for lung cancer ‐ where are we now?. Am Rev Respir Dis 1984;130(4):541‐2. [DOI] [PubMed] [Google Scholar]
Bailar 1997
- Bailar J, Gornick HL. Cancer undefeated. New England Journal of Medicine 1997;336:1569‐74. [DOI] [PubMed] [Google Scholar]
Berlin 1984
- Berlin I, Buncher CR, Fontana RS, Frost JK, Melamed MR. The National Cancer Institute cooperative early lung cancer detection program. Results of the initial screen (prevalence). Early lung cancer detection: introduction. American Review of Respiratory Disease 1984;130(4):545‐9. [DOI] [PubMed] [Google Scholar]
Black 2000
- Black WC. Overdiagnosis: an under recognised cause of confusion and harm in cancer screening. Journal of the National Cancer Institute 2000;92(16):1280‐2. [DOI] [PubMed] [Google Scholar]
Bland 1995
- Bland J, Altman DG. Multiple significance tests: the Bonferroni method. BMJ 1995;310:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boshuizen 2012
- Boshuizen R, Kuhn P, Heuvel M. Circulating tumor cells in non‐small cell lung carcinoma. Journal of Thoracic Disease 2012;4(5):456‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brenner 2004
- Brenner DJ. Radiation risks potentially associated with low‐dose CT screening in adult smokers for lung cancer. Radiology 2004;231:440‐5. [DOI] [PubMed] [Google Scholar]
Carpagnano 2005
- Carpagnano GE, Foschino‐Barbaro MP, Spanevello A, Resta O, Carpagnano F, Mulé G, et al. 3p microsatellite alterations in exhaled breath condensate from patients with non‐small cell lung cancer. American Journal of Respiratory Critical Care Medicine 2005;172:738‐44. [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H Jr. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Diederich 2000b
- Diederich S, Lenzen H. Radiation exposure associated with imaging of the chest. Cancer 2000;89(Supplement):2457‐60. [DOI] [PubMed] [Google Scholar]
Dominion 2011
- Dominion L, Poli A, Montovani W, Rotolol N, Imperatori A. Volunteer effect and compromised randomisation in the Mayo Project of screening for lung cancer. European Journal of Epidemiology 2011;26:79‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doria‐Rose 2009
- Doria‐Rose VP, Marcus PM, Szabo E, Tockman MS, Melamed MR, Prorok PC. Randomized controlled trials of the efficacy of lung cancer screening by sputum cytology revisited: a combined mortality analysis from the Johns Hopkins Lung Project and the Memorial Sloan‐Kettering Lung Study. Cancer 2009;115:5007‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eddy 1989
- Eddy DM. Screening for lung cancer. Annals of Internal Medicine 1989;111(3):232‐7. [DOI] [PubMed] [Google Scholar]
Fontana 1984
- Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo clinic study. American Review of Respiratory Disease 1984;130(4):561‐5. [DOI] [PubMed] [Google Scholar]
Globocan 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr (accessed 29th December 2012).
Godoy 2012
- Godoy MC, Sabloff B, Naidich DP. Subsolid pulmonary nodules: imaging evaluation and strategic management. Current Opinion in Pulmonary Medicine 2012;18(4):304‐12. [DOI] [PubMed] [Google Scholar]
Halpern 1993
- Halpern M, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. Journal of the National Cancer Institute 1993;85:457‐63. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SJ, Deeks JJ, Altman DJ. Measuring inconsistencies in meta‐analyses. BMJ 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jemal 2009
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA: A Cancer Journal for Clinicians 2009;59:225‐49. [DOI] [PubMed] [Google Scholar]
Kernan 1999
- Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomisation for clinical trials. Journal of Clinical Epidemiology 1999;52:19‐26. [DOI] [PubMed] [Google Scholar]
Kerry 1998
- Kerry SM, Bland JM. Analysis of a trial randomised in clusters. BMJ 1998;316:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubik 1986
- Kubik AP, Polak J. Lung cancer detection. Results of a randomised prospective study in Czechoslovakia. Cancer 1986;57:2427‐37. [DOI] [PubMed] [Google Scholar]
Lopez 1995
- Lopez A. The lung cancer epidemic in developed countries. Adult mortality in developed countries: from description to explanation. Oxford: Clarendon Press, 1995:111‐34. [Google Scholar]
Ma 2013
- Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer 2013;119:1381‐5. [DOI] [PubMed] [Google Scholar]
Machado 2005
- Machado RF, Laskowski D, Deffenderfer O, Burch T, Zheng S, Mazzone PJ, et al. Detection of lung cancer by sensor array analyses of exhaled breath. American Journal of Respiratory Critical Care Medicine 2005;171:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahadevia 2003
- Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost‐effectiveness analysis. JAMA 2003;289(3):313‐22. [DOI] [PubMed] [Google Scholar]
Mong 2011
- Mong C, Garon EB, Fuller C, Mahtabifard A, Mirocha J, Mosenifar Z, et al. High prevalence of lung cancer in a surgical cohort of lung cancer patients a decade after smoking cessation.. Journal of Cardiothoracic Surgery 2011;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patz 2000
- Patz E, Goodman PC, Bepler G. Screening for lung cancer. New England Journal of Medicine 2000;343:1627‐33. [DOI] [PubMed] [Google Scholar]
Peto 1976
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomised clinical trials requiring prolonged observation of each patient. British Journal of Cancer 1976;34:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 1999
- Phillips M, Gleeson K, Hughes JM, Greenberg J, Cataneo RN, Baker L, et al. Volatile organic compounds in breath as markers for lung cancer: a cross sectional study. Lancet 1999;353:1930‐3. [DOI] [PubMed] [Google Scholar]
Phillips 2003
- Phillips M, Cataneo RN, Cummin AR, Gagliardi AJ, Gleeson K, Greenberg J, et al. Detection of lung cancer with volatile markers in the breath. Chest 2003;123:2115‐23. [DOI] [PubMed] [Google Scholar]
Rahman 2005
- Rahman SM, Shyr Y, Yildiz PB, Gonzalez AL, Li H, Zhang X, et al. Proteomic patterns of preinvasive bronchial lesions. American Journal of Respiratory Critical Care Medicine 2005;172(12):1556‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raji 2012
- Raji OY, Duffy SW, Agbaje OF, Baker SG, Christiani DC, Cassidy A, et al. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case‐control and cohort validation study. Annals of Internal Medicine 2012;157(4):242‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Ries 1994
- Ries LA. Influence of extent of disease, histology and demographic factors on lung cancer survival in the seer population‐based data. Seminars in Surgical Oncology 1994;10(1):21‐30. [DOI] [PubMed] [Google Scholar]
Schoenborn 2013
- Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: United States, 2008–2010. National Center for Health Statistics. Vital Health Statistics 2013;10(257):21‐4. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Tockman 1986
- Tockman MS. Survival and mortality from lung cancer in a screened population. Chest 1986;89:S324‐S325. [Google Scholar]
Tockman 1987
- Tockman M, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Annals of Internal Medicine 1987;106:512‐8. [DOI] [PubMed] [Google Scholar]
Travis 2011
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. Journal of Thoracic Oncology 2011;6(2):244‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2007
- Welch HG, Woloshin S, Schwartz LM, Gordis L, Gotzsche PC, Harris R, et al. Overstating the evidence for lung cancer screening. Archives of Internal Medicine 2007;167(21):2289‐95. [DOI] [PubMed] [Google Scholar]
Welch 2010
- Welch HG, Black WC. Overdiagnosis in cancer. Journal of the National Cancer Institute 2010;102:605‐13. [DOI] [PubMed] [Google Scholar]
WHO 2008
- World Health Organization. The top 10 causes of death. World Health Organization , Media Centre, Fact Sheet 310 (updated June 2011). Available at www.who.int/mediacentre/factsheets/fs310/en/index.html (accessed 26th January 2013).
Wilde 1989
- Wilde J. A 10 year follow‐up of semi annual screening for for early detection of lung cancer in the Efurt County, GDR. European Respiratory Journal 1989;2(7):656‐62. [PubMed] [Google Scholar]
Wilson 2008
- Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. American Journal of Respiratory and Critical Care Medicine 2008;178(7):738‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woo 1985
- Woo B, Woo B, Cook EF, Weisberg M, Goldman L. Screening procedures in the asymptomatic adult: comparison of physicians' recommendations, patients' desires, published guidelines and actual practice. JAMA 1985;254:1480‐4. [DOI] [PubMed] [Google Scholar]
Young 2012
- Young RP, Hopkins RJ. Diagnosing COPD and targeted lung cancer screening (correspondence). European Respiratory Journal 2012;40(4):1063‐4. [DOI] [PubMed] [Google Scholar]
Zhong 2005
- Zhong L, Hidalgo GE, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Using protein microarray as a diagnostic assay for non‐small cell lung cancer. American Journal of Respiratory Critical Care Medicine 2005;172:1308‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Manser 1999
- Manser RL, Irving LB, Stone C, Byrnes G, Abramson M, Campbell D. Screening for lung cancer. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001991] [DOI] [PubMed] [Google Scholar]
Manser 2004
- Manser R, Irving LB, Stone C, Byrnes G, Abramson MJ, Campbell D. Screening for lung cancer. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001991.pub2] [DOI] [PubMed] [Google Scholar]
Manser 2010
- Manser R, Irving LB, Stone C, Byrnes G, Abramson MJ, Campbell D. Screening for lung cancer. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001991.pub2] [DOI] [PubMed] [Google Scholar]


