Abstract
A 68-year-old man with type 2 diabetes mellitus and chronic hepatitis B infection was referred to the nephrology department before planned surgery for hepatocellular carcinoma. He had been receiving low-dose adefovir dipivoxil (ADV) for 11 years. Laboratory findings revealed impaired re-absorption in the proximal renal tubules. He had been diagnosed with diabetic kidney disease and osteomalacia due to vitamin D deficiency; thus, ADV was not discontinued until he was referred to us. In this case, concomitant diabetes mellitus and vitamin D deficiency might have prevented the early diagnosis of ADV-induced Fanconi syndrome.
Keywords: adefovir, diabetic kidney disease, Fanconi syndrome, osteomalacia, vitamin D deficiency
Introduction
Adefovir dipivoxil (ADV) is a nucleotide analog inhibitor of reverse transcriptase used for the treatment of chronic hepatitis B virus (HBV) infection. Initially, ADV therapy for HBV infection was reported to be safe (1, 2). However, it has become evident that even a low dose of ADV can cause proximal renal tubular injury that eventually leads to generalized re-absorptive dysfunction, known as Fanconi syndrome (3, 4). ADV-induced Fanconi syndrome, especially in cases manifesting with osteomalacia due to prolonged hypophosphatemia, has been drawing increasing attention in recent years. However, this condition is still rarely encountered by the majority of general practitioners.
We herein report a 68-year-old man with type 2 diabetes mellitus and chronic HBV infection with an 11-year history of low-dose ADV treatment. Three years after the initiation of ADV treatment, his serum creatinine level was 1.79 mg/dL, which was attributed to diabetic kidney disease (DKD). He had been suffering from treatment-resistant joint pain for the past two years. Four months before he presented to us, a diagnosis of osteomalacia due to vitamin D deficiency was made. His joint pain promptly responded to oral phosphate products and vitamin D supplementation; however, ADV was not discontinued. After referral to our nephrology department, a thorough medical history examination and careful analysis of his urine, electrolytes, and acid-base balance finally revealed the underlying ADV-induced Fanconi syndrome.
Case Report
A 68-year-old Japanese man with a history of type 2 diabetes mellitus and chronic HBV infection was referred to our hospital in January 2016 for the evaluation of a newly detected liver tumor. He was diagnosed with hepatocellular carcinoma (HCC) and referred to our nephrology department for the management of chronic kidney disease during the preoperative period (creatinine, 1.84 mg/dL; estimated glomerular filtration rate, 29.7 mL/min/1.73 m2). His type 2 diabetes mellitus and HBV infection were initially diagnosed in 1986. He started insulin therapy, and his HbA1c level was controlled at approximately 7.0%. He started receiving interferon for his HBV infection in 1993. In 2002, lamivudine, at a dose of 100 mg daily, was initiated. In June 2005, he experienced exacerbation of hepatitis, and the lamivudine resistance mutation YIDD was detected. Thus, low-dose ADV (10 mg daily) was added. Kidney dysfunction was initially noted in 2008 (creatinine, 1.79 mg/dL; estimated glomerular filtration rate, 31.7 mL/min/1.73 m2) but was attributed to DKD.
At the initial presentation to our nephrology department, he was 166 cm tall and weighed 51.8 kg (body mass index, 18.8 kg/m2). His blood pressure was 136/70 mmHg, heart rate 65 beats per minute with regular rhythm, and body temperature 36.7℃. A physical examination was unremarkable except for mild bilateral lower limb pain. Abdominal computed tomography showed slightly atrophic kidneys with no stones, tumors, or other obstructive lesions. Based on his long-term history of type 2 diabetes mellitus, DKD seemed the most likely cause of his kidney dysfunction. However, laboratory tests revealed hyperphosphatasemia (1,148 IU/L; normal range, 106-322 IU/L), hypophosphatemia (1.6 mg/dL; normal range, 2.7-4.6 mg/dL), and hypouricemia (1.6 mg/dL; normal range, 3.7-7.8 mg/dL) with increased fractional excretion of phosphate (79.3%) and uric acid (69.3%), normal anion gap metabolic acidosis (HCO3-, 14.6 mmol/L; anion gap, 13.3), highly elevated urinary β2-microglobulin (66,919 μg/L; normal range, 0-355 μg/L), and glycosuria (4+) with casual blood glucose of 184 mg/dL. Other laboratory data are summarized in Table. These findings indicated impaired re-absorption in the proximal renal tubules. Pan-aminoaciduria was later confirmed. He was subsequently diagnosed with Fanconi syndrome.
Table.
Laboratory Data on Admission.
| Blood cell count | Biohemical data | ||||
| WBC (/μL) | 4,300 | (3,300-8,600) | Aspartate aminotransferase (IU/L) | 23 | (13-30) |
| RBC (×104/μL) | 341* | (435-555) | Alanine aminotransferase (IU/L) | 21 | (10-42) |
| Hemoglobin (g/dL) | 12.0* | (13.7-16.8) | Alkaline phosphatase (IU/L) | 1,148* | (106-322) |
| Platelet (×104/μL) | 16.8 | (15.8-34.8) | γ-glutamyltransferase (IU/L) | 22 | (13-64) |
| Urinalysis | Blood urea nitrogen (mg/dL) | 14.9 | (8-18.4) | ||
| pH | 6.0 | Creatinine (mg/dL) | 1.84* | (0.65-1.07) | |
| Glucose | (4+)* | Uric acid (mg/dL) | 1.6* | (3.7-7.8) | |
| Ketones | (-) | Sodium (mEq/L) | 140 | (138-145) | |
| Blood | (1+)* | Potassium (mEq/L) | 4.1 | (3.6-4.8) | |
| Protein | (2+)* | Chloride (mEq/L) | 112* | (101-108) | |
| RBCs (/HPF) | 1-4 | Calcium (mg/dL) | 9.1 | (8.8-10.1) | |
| WBCs (/HPF) | 1-4 | Inorganic phosphate (mg/dL) | 2.6* | (2.7-4.6) | |
| β2-microglobulin (μg/L) | 66,919* | (13-287) | HCO3- | 14.6* | (24-26) |
| NAG (U/L) | 8.0 | (<11.3) | M-protein | (-) | |
| Sodium (mEq/L) | 78 | Blood sugar (mg/dL) | 148 | ||
| Potassium (mEq/L) | 31.1 | HbA1c (%) | 7.1* | (4.6-6.2) | |
| Phosphate (mg/dL) | 58.9 | 1, 25-(OH)2 vitamin D (pg/mL) | 9* | (20-60) | |
| Calcium (mg/dL) | 7.2 | 25-OH vitamin D (ng/mL) | 10.1 | (7-41) | |
| Creatinine (mg/dL) | 52.6 | intact-PTH (pg/mL) | 39 | (10-65) | |
| Uric acid (mg/dL) | 31.7 | PTH-related protein | <1.1 | ||
| Protein (mg/dL) | 96 | FGF-23 (pg/mL) | <10 | ||
| Bence Jones protein | (-) | C-reactive protein (mg/dL) | 0.27* | (0-0.14) | |
| Others | Anti-nuclear antibody | <40 | |||
| FEP (%) | 79.3* | (10-20) | Anti-SS-A antibody | <0.5 | |
| FEUA (%) | 69.3* | (5.5-11) | Anti-SS-B antibody | <0.5 | |
| HBs-Ag (C.O.I) | 1,513.1* | (<0.9) | Rheumatoid factor (IU/mL) | 3 | (0-18) |
| HBe-Ag (C.O.I) | 0.4 | (<0.9) | Immunoglobulin G (mg/dL) | 860* | (861-1,747) |
| HBe-Ab (C.O.I) | 33.5 | (<44.9) | Immunoglobulin A (mg/dL) | 187 | (93-393) |
| HBV-DNA (log.C/mL) | (-) | Immunoglobulin M (mg/dL) | 220* | (33-183) | |
| CH50 (U/mL) | 32 | (30-45) |
Abnormal values are indicated by asterisks (*).
CH50: hemolytic complement activity, C.O.I: cut off index, FEUA: fractional excretion of uric acid, FEP: fractional excretion of phosphate, FGF: fibroblast growth factor, NAG: N-acetyl-β-D-glucosaminidase, PTH: parathyroid hormone
After attempting to obtain a more detailed medical history, he recounted that he had received some medications for bone disease in the past two years. We contacted the relevant medical facilities and confirmed his entire history before the nephrology referral. He had felt muscle weakness and experienced difficulty walking because of pain in the bilateral knee joints and left plantar lesion in January 2014. Non-traumatic fracture of the left first metatarsal bone was found, and weekly oral alendronate sodium hydrate 35 mg was initiated at a local hospital under a diagnosis of osteoporosis. In March 2015, treatment was switched from alendronate to teriparatide injections of 20 μg daily. However, his pain did not respond to these medications. In September 2015 (four months before nephrology referral), he visited another local orthopedic clinic, and multiple pathological fractures in his bilateral metatarsal bones were discovered. He was referred to the department of orthopedic surgery at another general hospital for a further evaluation. At that time, blood tests showed hyperphosphatasemia (684 IU/L), hypophosphatemia (1.4 mg/dL), and decreased serum 25-hydroxy vitamin D (18 ng/mL; normal range, 7-41). X-ray of the metatarsal bones and proximal tibias showed multiple radiolucent zones (Looser zones). He was diagnosed with hypophosphatemic osteomalacia caused by vitamin D deficiency due to chronic liver disease. Supplementation with oral phosphate products and vitamin D (calcitriol) promptly relieved his pain.
We performed whole-body bone scintigraphy with 99mTc hydroxymethylene. The findings showed multiple lesions with an increased uptake in the bilateral ribs, lumbar spine, knee joints, ankle joints, and metatarsal bones, indicating insidious pathological fractures caused by hypophosphatemic osteomalacia. Since other possible diseases were unlikely (Table) and interferon, insulin, and lamivudine monotherapy have not been reported as causes of Fanconi syndrome, the 11-year history of ADV treatment was considered to be the main cause of his Fanconi syndrome and hypophosphatemic osteomalacia.
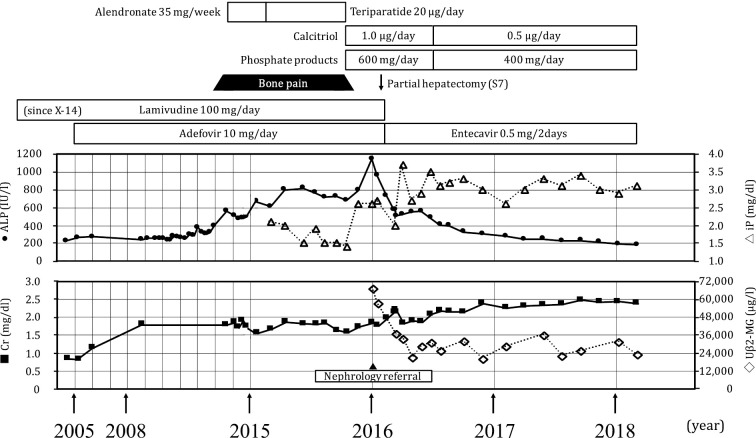
After undergoing partial hepatic resection for HCC and recovering uneventfully, he was discharged and continued with medical follow up at our hospital. ADV was switched to entecavir at a dose of 1.0 mg every other day. Supplementation with oral phosphate products and vitamin D was continued for his hypophosphatemic osteomalacia. Oral sodium bicarbonate was also added for the treatment of normal anion gap metabolic acidosis. The patient's hyperphosphatasemia, hypophosphatemia, and metabolic acidosis gradually improved, and his lower limb pain did not recur. His kidney function was almost unchanged, but the level of urinary β2-microglobulin remained high over the following two years (Figure). The pan-aminoaciduria, glycosuria, and hypouricemia were not completely ameliorated.
Figure.
Clinical course. After the discontinuation of ADV therapy and supplementation with an oral phosphate product and vitamin D, the hyperphosphatasemia and hypophosphatemia gradually improved. His kidney function was almost unchanged, but the level of urinary β2-microglobulin remained high. ADV: adefovir dipivoxil, ALP: alkaline phosphatase, Cr: creatinine, iP: inorganic phosphate, Uβ2-MG: urinary beta2-microglobulin
Discussion
Low-dose ADV therapy for HBV infection was initially reported to be safe (1, 2). However, subsequent studies have demonstrated a high prevalence of renal toxicity associated with prolonged usage (3, 4). The exact pathophysiology of ADV-induced renal injury remains unknown. The human organic anion transporter 1, which is located in the basolateral membrane of the proximal renal tubules and regulates the uptake of drugs from the blood (5), and multidrug resistance-associated proteins, which are found in the apical membrane and regulate drug excretion (6), have been suggested to be involved in the excessive drug accumulation in tubular epithelial cells. As a result, mitochondrial DNA replication is inhibited, leading to the generalized impairment of re-absorption in the proximal renal tubules (Fanconi syndrome) (7). Massive loss of filtered solutes, especially the sustained loss of calcium and phosphate, disturbs normal bone mineralization and eventually results in osteomalacia. Park et al. recently confirmed histologically that the mitochondrial damage of the proximal tubules was severe, while that of the distal tubules was slight on electron microscopy, as observed in their case of ADV-induced Fanconi syndrome. They also showed that FGF-23, the crucial regulatory hormone for the renal handling of phosphate, was lower than that of a normal control, suggesting that FGF-23 was not the cause of hypophosphatemia (8). Since our patient did not undergo a kidney biopsy, the histological features of his kidney injury remain unknown. However, his serum level of FGF-23 was below the normal value (<10 pg/mL); thus, we can plausibly say that FGF-23 was not involved in the development of hypophosphatemia-associated osteomalacia in our patient.
The true incidence of ADV-induced Fanconi syndrome has not been clarified in any prospective or retrospective studies to date. An increasing number of case reports have described the development of Fanconi syndrome among patients receiving long-term low dose ADV therapy (9-19). However, awareness of this condition seems unsatisfactory in daily clinical practice, since some patients with ADV-induced Fanconi syndrome have been undiagnosed or even misdiagnosed. Li et al. (20) reported a 41-year-old man with an 8-year history of low-dose ADV therapy for HBV. He had suffered from chest wall pain and lower limb weakness over a six-month period. Positron emission tomography-computed tomography (PET-CT) showed multiple hypermetabolic and osteogenic spots. Initially, he was referred to the hematology department because multiple myeloma or metastatic carcinoma was suspected. A bone marrow biopsy denied the possibility of hematological diseases. In another case report, Wei et al. (21) reported a 65-year-old man with HBV infection who had suffered from bone pain in both femurs and the chest wall after surgery for hepatocellular carcinoma. Initially, metastatic hepatic carcinoma was suspected, but fluorodeoxyglucose-PET denied that possibility. ADV was used over the last two years in this patient. In both cases, a thorough evaluation of the acid-base and electrolyte balances and a careful medical history interview identified ADV as the cause of Fanconi syndrome. A retrospective study reported that 14 out of 28 patients (50%) continued ADV treatment after the development of joint symptoms (22). These patients had to visit several medical facilities, undergo repeated examinations, and receive ineffective treatments before the correct diagnosis was made. Recently, Chen et al. reported that a misdiagnosis of ADV-induced Fanconi syndrome was observed in 27 out of 120 patients. The misdiagnoses included osteoporosis (18.0%), ankylosing spondylitis (16.0%), vertebral disc herniation (12.0%), osteoarthropathy (10.0%), and bone tumors (10.0%) (23).
In our case, there were two major pitfalls that likely led to the diagnostic delay. First, our patient had concurrent type 2 diabetes mellitus. Three years after the initiation of low-dose ADV therapy, his creatinine level was found to have increased to 1.79 mg/dL. DKD was suspected at that time, and no further evaluation was conducted. If a more detailed evaluation, such as an investigation of urinary β2-microglobulin, serum phosphate, or uric acid, had been performed, drug-induced proximal renal tubular injury might have been suspected at an earlier time. After referral to our nephrology department, his long-term history of diabetes mellitus as well as urinary findings (glycosuria and proteinuria without hematuria) initially suggested the possibility of DKD. However, his exceptionally high levels of urinary β2-microglobulin suggested additional tubulointerstitial damage. His serum levels of phosphate and uric acid, which are typically elevated in patients with ordinary chronic kidney disease, were low with an increased fractional excretion. In addition, his metabolic acidosis was relatively severe compared to his serum creatinine level, and pan-aminoaciduria was later confirmed. Taken together, these findings indicated underlying generalized re-absorptive dysfunction in the proximal tubules. Since other possible causes were excluded (Table), we identified ADV as the principal cause of his Fanconi syndrome. The urinary β2-microglobulin level has been suggested to be a biomarker of kidney injury among chronic HBV patients treated with low-dose ADV (24). Takagi et al. reported the sensitive change in the urinary β2-microglobulin level after dose reduction of ADV (25). Thus, a careful evaluation of the urinary β2-microglobulin level is mandatory for the early recognition of ADV-induced tubular damage.
Second, vitamin D deficiency is well known to be involved in liver disease. Vitamin D deficiency (defined as 25-hydroxy vitamin D <10 ng/mL) and vitamin D insufficiency (25-hydroxy vitamin D ≥10 and <20 ng/mL) were noted in 34% and 47% of patients with chronic HBV infection, respectively (26). In our patient, the serum level of 25-hydroxy vitamin D measured at the other hospital met the criteria for vitamin D insufficiency (18 ng/mL). Thus, the diagnosis of vitamin D deficiency due to liver disease was correct, and the supplementation of oral phosphate products and vitamin D was, as expected, effective for his joint pain. However, his prompt response to phosphate and vitamin D therapy might have precluded the need for further evaluations at the former hospital, including investigations into the possibility of drug-induced phosphate loss.
The majority of ADV-induced Fanconi syndrome cases are diagnosed by hepatologists. However, other specialists, such as orthopedists (13, 27) or nephrologists, may be required to diagnose this rare condition. Our case indicates that the careful evaluation of kidney dysfunction can reveal the concomitant drug-induced proximal tubular injury. ADV-induced Fanconi syndrome should be properly diagnosed and managed in order to prevent the progression of kidney dysfunction and the incidence of pathological fractures due to hypophosphatemic osteomalacia, which inevitably impair patients' quality of life.
Conclusion
ADV-induced Fanconi syndrome and osteomalacia remain uncommon in daily clinical practice. Concomitant diabetes and vitamin D deficiency can hamper the early diagnosis and management. A careful evaluation of concurrent electrolyte and acid base disorders and the measurement of the urinary β2-microglobulin level is necessary for the early recognition of ADV-induced tubular damage.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 348: 800-807, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 348: 808-816, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Suzuki F, Seko Y, et al. Renal dysfunction and hypophosphatemia during long-term lamivudine plus adefovir dipivoxil therapy in patients with chronic hepatitis B. J Gastroenterol 49: 470-480, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu M, Furusyo N, Ikezaki H, et al. Predictors of kidney tubular dysfunction induced by adefovir treatment for chronic hepatitis B. World J Gastroenterol 21: 2116-2123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20: 641-648, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Miller DS. Nucleoside phosphonate interactions with multiple organic anion transporters in renal proximal tubule. J Pharmacol Exp Ther 299: 567-574, 2001. [PubMed] [Google Scholar]
- 7.Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D'agati VD. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum Pathol 32: 734-740, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Kim WI, Cho DH, et al. Adefovir-induced Fanconi syndrome associated with osteomalacia. Clin Mol Hepatol 24: 339-344, 2017(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 9.Jung YK, Yeon JE, Choi JH, et al. Fanconi's syndrome associated with prolonged adefovir dipivoxil therapy in a hepatitis b virus patient. Gut Liver 4: 389-393, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girgis CM, Wong T, Ngu MC, et al. Hypophosphataemic osteomalacia in patients on adefovir dipivoxil. J Clin Gastroenterol 45: 468-473, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Law ST, Li KK, Ho YY. Acquired Fanconi syndrome associated with prolonged adefovir dipivoxil therapy in a chronic hepatitis B patient. Am J Ther 20: e713-e716, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Law ST, Li KK, Ho YY. Nephrotoxicity, including acquired Fanconi's syndrome, caused by adefovir dipivoxil - is there a safe dose? J Clin Pharm Ther 37: 128-131, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Setoguchi T, Ishidou Y, et al. Pathological femoral fractures due to osteomalacia associated with adefovir dipivoxil treatment for hepatitis B: a case report. Diagn Pathol 7: 108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimohata H, Sakai S, Ogawa Y, Hirayama K, Kobayashi M. Osteomalacia due to Fanconi's syndrome and renal failure caused by long-term low-dose adefovir dipivoxil. Clin Exp Nephrol 17: 147-148, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Wu C, Zhang H, Qian Y, Wang L, Gu X, Dai Z. Hypophosphatemic osteomalacia and renal Fanconi syndrome induced by low-dose adefovir dipivoxil: a case report and literature review suggesting ethnic predisposition. J Clin Pharm Ther 38: 321-326, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi H, Tsuruta M, Tani J, Kuwahara R, Hiromatsu Y. Hypophosphatemic osteomalacia due to drug-induced Fanconi's syndrome associated with adefovir dipivoxil treatment for hepatitis B. Intern Med 53: 233-237, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Palermo A, Strollo R, Papalia R, et al. Severe hypophosphatemic osteomalacia secondary to fanconi syndrome due to adefovir: a case report. Endocr Pract 20: e246-e249, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Lee SSM, Quek TPL, Seow CJ, Leow MKS. Adefovir-induced Fanconi syndrome: diagnostic pearls and perils of late or missed diagnosis. CEN Case Rep 3: 183-187, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Zhuo Y, Zhang D. Nephrolithiasis and osteomalacia associated with adefovir-induced Fanconi syndrome in a patient with hepatitis B. BMC Nephrol 18: 275, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Shen M, Sun WJ, Huang ZX, An N, Zhang JJ. Misdiagnosis of bone metastasis cancer after using adefovir dipivoxi in a hepatitis B patient with Fanconi syndrome. Indian J Hematol Blood Transfus 32: 329-331, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei WJ, Sun ZK, Shen CT, Qiu ZL, Luo QY. Adefovir-induced hypophosphatemic osteomalacia mimicking bone metastases from primary hepatocarcinoma. Clin Nucl Med 42: e405-e406, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Pan F, Wang Y, et al. Adefovir dipivoxil-induced Fanconi syndrome and its predictive factors: a study of 28 cases. Oncol Lett 13: 307-314, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N, Zhang JB, Zhang Q, et al. Adefovir dipivoxil induced hypophosphatemic osteomalacia in chronic hepatitis B: a comparative study of Chinese and foreign case series. BMC Pharmacol Toxicol 19: 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia HY, Ding F, Chen JY, et al. Early kidney injury during long-term adefovir dipivoxil therapy for chronic hepatitis B. World J Gastroenterol 21: 3657-3662, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi J, Morita H, Ito K, et al. Urinary β-2 microglobulin levels sensitively altered in an osteomalacia patient receiving add-on adefovir dipivoxil therapy for hepatitis B virus infection. Intern Med 55: 1599-1603, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Farnik H, Bojunga J, Berger A, et al. Low vitamin D serum concentration is associated with high levels of hepatitis B virus replication in chronically infected patients. Hepatology 58: 1270-1276, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Kim BK, Lee HJ, Dan J. Pathologic femoral neck fracture due to Fanconi syndrome induced by adefovir dipivoxil therapy for hepatitis B. Clin Orthop Surg 8: 232-236, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]