Abstract
Background
Community ambulation refers to the ability of a person to walk in their own community, outside of their home and also indoors in private or public locations. Some people choose to walk for exercise or leisure and may walk with others as an important aspect of social functioning. Community ambulation is therefore an important skill for stroke survivors living in the community whose walking ability has been affected.
Objectives
To determine: (1) whether interventions improve community ambulation for stroke survivors, and (2) if any specific intervention method improves community ambulation more than other interventions.
Search methods
We searched the Cochrane Stroke Group Trials Register (September 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (November 2013), PubMed (1946 to November 2013), EMBASE (1980 to November 2013), CINAHL (1982 to November 2013), PsycINFO (1887 to November 2013), Scopus (1960 to November 2013), Web of Science (1900 to November 2013), SPORTDiscus (1975 to November 2013), and PEDro, CIRRIE and REHABDATA (November 2013). We also searched ongoing trials registers (November 2013) and reference lists, and performed a cited reference search.
Selection criteria
Selection criteria included parallel‐group randomised controlled trials (RCTs) and cross‐over RCTs, studies in which participants are adult (aged 18 years or more) stroke survivors, and interventions that were aimed at improving community ambulation. We defined the primary outcome as participation; secondary outcomes included activity level outcomes related to gait and self‐efficacy.
Data collection and analysis
One review author independently screened titles. Two review authors screened abstracts and full text articles, with a third review author was available to resolve any disagreements. Two review authors extracted data and assessed risk of bias. All outcomes were continuous. The analysis for the primary outcome used the generic inverse variance methods for meta‐analysis, using the standardised mean difference (SMD) and standard error (SE) from the participation outcomes. Analyses for secondary outcomes all used SMD or mean difference (MD). We completed analyses for each outcome with all studies, and by type of community ambulation intervention (community or outdoor ambulation practice, virtual practice, and imagery practice). We considered trials for each outcome to be of low quality due to some trial design considerations, such as who knew what group the participants were in, and the number of people who dropped out of the studies.
Main results
We included five studies involving 266 participants (136 intervention; 130 control). All participants were adult stroke survivors, living in the community or a care home. Programmes to improve community ambulation consisted of walking practice in a variety of settings and environments in the community, or an indoor activity that mimicked community walking (including virtual reality or mental imagery). Three studies were funded by government agencies, and two had no funding.
From two studies of 198 people there was low quality evidence for the effect of intervention on participation compared with control (SMD, 0.08, 95% confidence interval (CI) ‐0.20 to 0.35 (using inverse variance). The CI for the effect of the intervention on gait speed was wide and does not exclude no difference (MD 0.12, 95% CI ‐0.01 to 0.24; four studies, 98 participants, low quality evidence). We considered the quality of the evidence to be low for all the remaining outcomes in our review: Community Walk Test (MD ‐6.35, 95% CI ‐21.59 to 8.88); Walking Ability Questionnaire (MD 0.53, 95% CI ‐5.59 to 6.66); Six‐Minute Walk Test (MD 39.62 metres, 95% CI ‐8.26 to 87.51) and self‐efficacy (SMD 0.32, 95% CI ‐0.09 to 0.72). We downgraded the quality of the evidence because of a high risk of bias and imprecision.
Authors' conclusions
There is currently insufficient evidence to establish the effect of community ambulation interventions or to support a change in clinical practice. More research is needed to determine if practicing outdoor or community walking will improve participation and community ambulation skills for stroke survivors living in the community.
Keywords: Adult, Humans, Stroke Rehabilitation, Activities of Daily Living, Environment Design, Gait, Gait/physiology, Randomized Controlled Trials as Topic, Residence Characteristics, Self Efficacy, Time Factors, Walking, Walking/physiology
Plain language summary
Interventions to help stroke survivors walk in their own community
Review question
We reviewed the evidence about the effect of interventions aimed at improving community ambulation in adult stroke survivors.
Background
We wanted to determine whether programmes aimed at improving community ambulation for stroke survivors were better or worse than usual treatment. Community ambulation refers to the ability of a person to walk in their own community, outside of their home and also indoors, in private or public locations. Some people choose to walk for exercise or leisure and may walk with others as an important aspect of social functioning. Community ambulation is therefore an important skill for many stroke survivors living in the community whose walking ability has been affected.
Study characteristics
The evidence in this review is current to November 2013. We included five studies with a total of 266 participants. All participants were adult stroke survivors who lived in the community or a care home. Programmes to improve community ambulation consisted of walking practice in a variety of settings and environments in the community (three studies), or an activity indoors that mimicked community walking (three studies). Three studies were funded by government agencies, and two had no funding.
Key results
The term 'participation' refers to the ability of a person to engage in activities that are meaningful to them, such as leisure activities, paid or volunteer work, or socialising with others. For the primary outcome of participation we could not be sure whether the intervention improved participation compared with control (two studies). When considering how fast a person walks, it is unclear if the speed of walking may increase with a community ambulation intervention ( four studies). Based on the included studies, the effect of the intervention on the ability to walk, how far people could walk in six minutes or their confidence in walking is unclear. There is currently insufficient evidence to establish the effect of community ambulation interventions or to support a change in clinical practice. No adverse effects of the interventions were reported in any of the included studies.
Quality of the evidence
We considered the quality of the evidence to be low across the studies for both the participation and walking speed outcomes. There were some study design considerations which led to the low score, such as who knew what group the participants were in, and the number of people who dropped out of the studies. Also, we included a small number of studies in this review, which limits how the results can be interpreted. More research is needed in this area.
Summary of findings
Summary of findings for the main comparison. Community ambulation intervention compared with other interventions for improving community ambulation in stroke survivors.
| Community ambulation intervention compared with other interventions for improving community ambulation in stroke survivors | ||||||
| Patient or population: adult stroke survivors Settings: community Intervention: community ambulation intervention Comparison: other | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other | Community ambulation intervention | |||||
| Participation Nottingham Leisure Questionnaire (0 to 60) and Subjective Index of Physical and Social Outcome (0 to 40) Follow‐up: mean 8 months | — | The mean participation in the intervention groups was 0.08 higher (0.2 lower to 0.35 higher) | — | 198 (2 studies) | ⊕⊕⊝⊝ low1,2 | SMD 0.08 (‐0.2 to 0.35) |
| Community Walk Test Community walk test (time multiplied by level of walking aid) Follow‐up: mean 0.5 months3 | — | The mean Community Walk Test in the intervention groups was 6.35 lower (21.59 lower to 8.88 higher) | — | 45 (2 studies) | ⊕⊕⊝⊝ low4,5 | — |
| Walking Ability Questionnaire Walking Ability Questionnaire (0 to 76) | — | The mean Walking Ability Questionnaire in the intervention groups was 0.53 higher (5.59 lower to 6.66 higher) | — | 45 (2 studies) | ⊕⊕⊝⊝ low6,7 | — |
| Gait speed m/s Follow‐up: mean 3.5 months8 | — | The mean gait speed in the intervention groups was 0.12 higher (0.01 lower to 0.24 higher) | — | 98 (4 studies) | ⊕⊕⊝⊝ low9,10 | — |
| Six‐Minute Walk Test Six‐Minute Walk Test (metres) Follow‐up: mean 3 months11 | — | The mean Six‐Minute Walk Test in the intervention groups was 39.62 higher (8.26 lower to 87.51 higher) | — | 55 (2 studies) | ⊕⊕⊝⊝ low12,13 | — |
| Self‐efficacy Activities Specific Balance Confidence Scale (0 to 100) and Falls Efficacy Scale (0 to 130) Follow‐up: mean 3.5 months14 | — | The mean self‐efficacy in the intervention groups was 0.32 SDs higher (0.09 lower to 0.72 higher) | — | 97 (4 studies) | ⊕⊕⊝⊝ low15,16 | SMD 0.32 (‐0.09 to 0.72) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The 95% CI included no effect and the upper and lower CI crosses an effect size of 0.5; total population is also < 400. 2Neither study had blinding of participants or personnel (although would be very difficult or impossible with the particular interventions); one study had a high attrition rate and ITT analysis was not used. 3Only one study had follow‐up. 4Neither study had blinding of participants or personnel (although would be very difficult or impossible with the particular interventions); ITT analysis was not used. 5The 95% CI included no effect ; total population is also < 400. 6Neither study had blinding of participants or personnel (although would be very difficult or impossible with the particular interventions); ITT analysis was not used. 7The 95% CI included no effect ; total population is also < 400. 8Two of four studies had follow‐up. 9No study had blinding of participants or personnel (although would be very difficult or impossible with the particular interventions); ITT analysis was not used. 10The 95% CI included no effect in 3 of 4 studies; total population is also < 400. 11Only 1 study had follow‐up. 12No study had blinding of participants or personnel (although would be very difficult or impossible with the particular interventions); ITT analysis was not used. 13The 95% CI included no effect in both studies; total population is also < 400. 14Follow‐up in two of four studies. 15No study had blinding of participants or personnel (although would be very difficult or impossible with the particular interventions); ITT analysis was not used. 16The 95% CI included no effect in all studies; total population is also < 400.
Background
Description of the condition
Stroke is defined by the World Health Organization (WHO) as "rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 hours or longer or leading to death, with no apparent cause other than of vascular origin" (WHO 2000). People who have had a stroke may experience a variety of symptoms, such as sudden and intense changes in perception, cognition, mood, and speech. Health‐related quality of life and activity (e.g. walking) are also affected (Mayo 1999). Health‐related quality of life is defined as "the value assigned to duration of life as modified by the impairments, functional states, perceptions and social opportunities influenced by disease, injury, treatment or policy" (Patrick 1993). It incorporates the constructs of physical, psychological, and social functioning or participation, as well as general well‐being (Sprangers 2002). Seventy‐five per cent of stroke survivors are left with some form of disability, which may range from minor to severe (Heart and Stroke 2014).
The estimated incidence of stroke ranges from 1.3 to 4.1 per 1000 person‐years, according to a review of population‐based epidemiological studies of stroke in 13 countries, published between 1992 and 2000; the prevalence of people living with the effects of stroke ranges from 46.1 to 73.3 per 1000 population (Feigin 2003). In 2004, there were an estimated 30.7 million stroke survivors across the world (WHO 2008). Worldwide each year, 15 million people have a stroke: five million die and five million are left with a permanent disability (WHO 2004). It is estimated that stroke costs the Canadian economy CAD 3.6 billion each year (Heart and Stroke 2014). The lifetime costs of stroke per patient have been estimated as ranging from USD 11,787 to USD 3,035,671 (Palmer 2005). With the increase in the ageing population, the number of people living with the effects of stroke and the cost to the economy will likely continue to increase.
Health‐related quality of life after a stroke can be affected by many factors, including a decrease in function and limitations in activities leading to participation restrictions, such as difficulties with community ambulation. Community ambulation incorporates both mobility (walking) and social aspects. It is defined as "independent mobility outside the home, which includes the ability to confidently negotiate uneven terrain, private venues, shopping centres and other public venues" (Lord 2004). Community ambulation, therefore, refers to walking outside of the person's home, either outdoors or indoors in public places, such as a park or a store; or private locations, such as a friend's home. People who walk in the community may do so for several reasons: for exercise, to get to public transportation, or to participate in social or leisure activities. The social aspect of community ambulation becomes apparent when one considers the importance of being with others and performing activities that interest a person. These activities may have walking as an integral component (e.g. playing golf, going for a walk in a park) or as a means of transportation to the activity (e.g. necessary for use of public transportation). Therefore, community ambulation is very closely linked to the participation of a person in the community. Survivors of stroke have described and supported the importance of walking outdoors, as well as the benefits and challenges of walking in the community (Barclay 2014).
Description of the intervention
Intervention studies that address ambulation among community dwelling stroke survivors have included walking activities in various settings, such as shopping centre walking and road crossing (Lord 2008), treadmill training (Ada 2003; Ada 2009; Langhammer 2010), walking training in a variety of community settings with increasing difficulty of tasks and environments (Park 2011), and community walking using virtual reality technology (Yang 2008). Interventions have also included circuit training or programmes addressing aspects of walking, such as strength, flexibility, and balance. Although some studies have focused on improving walking capacity after stroke with circuit training, community ambulation was not a specific outcome of treatment (Pang 2005; Salbach 2004).
How the intervention might work
A qualitative study of 11 physiotherapists identified important skills and abilities that allow a person living with the effects of stroke to walk in the community. The skills were organised into six themes and included: "ability to walk at speed and physical fitness, ability to negotiate different terrains, ambient conditions, ability to reason and monitor the environment, support of a person or aid, and drive (internal or external) to walk in the community" (Corrigan 2012). These skills are similar to the eight dimensions previously described as important for community mobility (Patla 1999). Specific physical interventions have been proposed to address many of these skills. Different modes of exercising to improve cardiovascular respiratory capacity have been proposed (MacKay‐Lyons 2005). Interventions that emphasise motor learning theory are common, whereby practice conditions are manipulated to maximise learning of motor tasks, such as walking over uneven terrain, negotiating curbs, or varying gait speed (Shumway‐Cook 2012). It is possible that existing intervention strategies, alone or in combination, may affect improvement in the foundation competencies necessary for outdoor ambulation thus increasing the ability to participate in community ambulation.
Why it is important to do this review
Community ambulation is related to participation in the community and to health‐related quality of life for stroke survivors. The ability to walk is of primary concern for people who have had a stroke, and community ambulation is considered an important outcome of rehabilitation (Mayo 1999). In a recent study of 'winter walkability', people identified reasons for walking outside that included "transportation, health benefits, and recreation" (Ripat 2010). Barriers to walking outdoors included "advancing age, physical limitation, difficulties using assistive devices, fear of falling and injury, and poor conditions" (Ripat 2010). At three months after sustaining a stroke, people have been shown to be somewhat dissatisfied with components of participation, such as their ability to engage in socialising, outings, and travel, each of which can have significant walking components (Mayo 1999). In the first year after stroke, endurance (the ability to walk a distance in six minutes) had a direct influence on participation in the community (Mayo 1999). Participation is defined as "involvement in a life situation" (WHO 2001) and includes aspects such as work, recreation, and socialising. It seems likely, therefore, that community ambulation and participation are closely linked, and it could be argued that there is evidence consistent with community ambulation being a strong contributor to participation for many people. A model of participation at 12 months post stroke includes the constructs of accomplishment (including aspects of social function, recreation, work or activity, driving, and usual activities), restricted roles (reflecting role limitations due to emotional or physical health problems), and health efficacy (including perception of recovery after a stroke and perception of health) (Barclay‐Goddard 2012a). In verifying the model, focus groups of people who have had a stroke described aspects of community ambulation that were aligned with the constructs of accomplishments and restricted roles (Barclay‐Goddard 2012a). Walking has been associated with self‐perceived difficulties in participation (Danielsson 2011).
Stroke survivors living in the community report fewer walking‐related activities and trips in the community, as well as less satisfaction with community ambulation, than people who have not had a stroke (Robinson 2011a). In a survey of 130 stroke survivors, 75% indicated that community ambulation was essential or very important (Lord 2004). It is estimated that between 20% and 66% of stroke survivors walk independently in the community (Lord 2008). In one study, approximately one‐third of stroke survivors who were living in the community were unable to walk outdoors independently, and of those who had independent community ambulation, most were hesitant to use public transportation, thereby relying on others for transportation when leaving the house (Lord 2004).
Objectives
To determine: (1) whether interventions improve community ambulation for stroke survivors, and (2) if any specific intervention method improves community ambulation more than other interventions.
Methods
Criteria for considering studies for this review
Types of studies
We sought parallel‐group randomised controlled trials (RCTs) and cross‐over RCTs.
Types of participants
We included studies in which participants were adult (aged 18 years or older) stroke survivors.
Types of interventions
We searched for trials specifically designed to investigate the impact of an intervention on community ambulation as indicated by either the title or the purpose statement. Interventions of interest were those that involved work by the participants in the form of practice, rehearsal, or exercise. Comparison interventions that we considered included: no treatment, 'usual' treatment, 'other' treatment, or placebo treatment.
It is important to note that there is potential overlap between this Cochrane review and various other published Cochrane reviews of stroke that report outcomes related to walking, such as treadmill training (Mehrholz 2014), physical fitness training (Saunders 2013), overground gait training (States 2009), circuit class training (English 2010), repetitive task training (French 2007), and virtual reality (Laver 2011). However, we focused on interventions specifically intended to improve ambulation in the community.
Types of outcome measures
We categorised outcomes using the WHO International Classification of Functioning, Disability, and Health (ICF). The ICF defines participation as "involvement in a life situation", activity as "the execution of a task or action by an individual", and body functions as "the physiological functions of body systems (including psychological function)" (WHO 2001). There is some controversy as to the best way to measure community ambulation (Lord 2005). Many outcomes have been used, such as gait speed, endurance, observation of outdoor walking, or the number or type of activities in which the person participates (Lord 2004; Lord 2005; Robinson 2011a; Robinson 2011b). There is no single accepted measure of community ambulation as it encompasses not only walking but aspects of participation.
We did not pre‐specify the timing of the outcome assessments.
Primary outcomes
The ability to walk in the community may increase opportunities for socialisation, leisure, and work activities (all aspects of participation). People may also choose to use community ambulation as exercise and as a leisure activity in itself. Therefore, we chose the primary outcome to focus on participation. If community ambulation is improved, participation may also be improved. Participation can be measured with generic or stroke‐specific measures of participation. Many health‐related quality of life measures also include subscales of participation.
Measures of participation that could have been used are described as follows.
In the Community Health Activities Model Program for Seniors (CHAMPS) (Stewart 2001), individuals are asked if they participate in a certain activity and, if they do, the number of hours per week is recorded.
The Reintegration to Normal Living Index evaluates aspects of participation, including moving around indoors, in the community, work, recreation, and social activities. It is scored on a three or four‐point scale with 11 items in total. Inter‐rater reliability, construct validity, and responsiveness to change of the scores has been demonstrated in people with various diagnoses (Wood‐Dauphinee 1988).
The London Handicap Scale (Harwood 1994a) consists of six dimensions (questions) related to participation with six response options each. Responses are weighted, leading to a score from 0 to 1, with 1 representing no difficulties with participation. Validity and reliability of the scale has been evaluated in stroke survivors (Harwood 1994b).
Participation subscales of health‐related quality of life measures that could have been utilised to describe participation are described as follows.
The Stroke Impact Scale was developed specifically for stroke survivors and has undergone testing for reliability, validity, and sensitivity to change. There are eight domains, one of which is the participation domain. Each domain results in a score from 0 to 100, with 100 being the better score (Duncan 1999).
SF‐36 is a generic measure of health‐related quality of life with eight subscales, one of which is the social function subscale (Ware 1992). Validity for use with stroke survivors has been evaluated. However, the social function subscale (0 to 100, with 100 representing a better score) did not correlate well with other measures of social function (Anderson 1996).
Secondary outcomes
Secondary outcomes included activity level outcomes such as gait speed, walking endurance, and ability to walk in different environments. We had looked for measures to ideally be measured outdoors, but we accepted an indoor 'proxy'.
Gait speed is often measured with a 10 metre walk. Gait speed considered necessary for independent community walking is 0.8 metres/second (m/s) (Perry 1995). Another cut‐off point suggested is 0.66 m/s (van de Port 2008). There is some discussion as to whether gait speed can be used to explain or predict community ambulation (Lord 2005; Taylor 2006).
Walking endurance after stroke is often measured with the Six‐Minute Walk Test (6MWT) (Liu 2008). The 6MWT is typically done indoors, but has also been adapted to outdoor use (Donovan 2008; Wevers 2011).
Walking in different environments has been evaluated with the Emory Functional Ambulation Profile, which measures the time that it takes to walk in different environments, and incorporates the use of various assistive devices (Wolf 1999).
Other secondary outcomes included balance self efficacy (e.g. the Activities‐Specific Balance Confidence Scale) (Powell 1995; Robinson 2011b), health‐related quality of life, depression, economic outcomes, and adverse effects (such as falls or cardiac events) of the interventions.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (September 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (November 2013) (Appendix 1), PubMed (1946 to November 2013) (Appendix 2), EMBASE (1980 to November 2013) (Appendix 3), CINAHL (1982 to November 2013) (Appendix 4), PsycINFO (1887 to November 2013) (Appendix 5), Scopus (1960 to November 2013) (Appendix 6), Web of Science (1900 to November 2013) (Appendix 7), SPORTDiscus (1975 to November 2013) (Appendix 8), and PEDro, CIRRIE and REHABDATA (November 2013). We also searched ongoing trials registers (November 2013) and reference lists, and performed a cited reference search.
Searching other resources
To enable identification of other published, unpublished, and ongoing trials we:
scanned the reference lists of all relevant publications; and
carried out citation searches of important articles in Web of Science and Scopus.
Data collection and analysis
Selection of studies
One review author (CN) independently screened titles produced by the literature search and excluded trials that were obviously unrelated to the objectives and inclusion criteria. Two review authors (CN and JR, or RB and TS) reviewed the abstracts of the remaining studies, and excluded clearly irrelevant studies. We retrieved the full text articles of all remaining citations, which two review authors reviewed in detail. We selected studies that met the inclusion criteria. Review authors resolved any disagreements through negotiation, with a third review author serving as arbiter as necessary. A review author independent of the selection process managed the lists of titles, abstracts, and full‐text manuscripts.
Data extraction and management
Two review authors (TS and RB) independently extracted data from the included trials. Extracted data included the number of participants, mean age, sex, information on stroke severity, time since stroke, functional level, living location, description of the intervention and control treatments, outcomes used, dosage (number of minutes per week multiplied by the number of weeks for total number of minutes), and the mean and standard deviation (SD) of the post‐treatment evaluation on all outcomes. One review author (RB) completed meta‐analysis when there were at least two trials that employed a similar intervention strategy and examined a common outcome. We used RevMan 2014 for all data analyses.
Assessment of risk of bias in included studies
Two review authors (TS and RB) examined the methodological quality of each included trial; no disagreements arose. We used Cochrane's 'Risk of bias' tool (Higgins 2011a). It includes an evaluation of random sequence allocation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. We provided information on study characteristics, quality, and biases as well as deaths and drop outs in the 'Characteristics of included studies' table, which includes the risk of bias for each study. Based on the 'Risk of bias' assessment, one review author assessed GRADE for all the review outcomes (GRADEpro 2014).
Measures of treatment effect
We anticipated that outcomes would all be continuous. When the same outcome measures were combined, we calculated the mean difference (MD) and 95% confidence interval (CI). When different outcome measures were used to reflect a common construct, we used the standardised mean difference (SMD) and 95% CI. If trials reported dichotomous outcomes (e.g. proportion of participants who achieve community ambulation versus those who do not), we would have used the odds ratio (OR).
We anticipated the following comparison groups: treatment to improve community ambulation versus usual treatment; treatment to improve community ambulation plus usual treatment versus usual treatment; treatment to improve community ambulation versus placebo treatment; treatment to improve community ambulation versus no treatment; and treatment to improve community ambulation versus other treatment. If interventions were categorised as indoor only, walking in the community, or a combination of indoor and community, we would have analysed these groups separately.
Unit of analysis issues
When we identified a cross‐over trial, we used the first time period for analysis. Occasionally, data are presented in a format that cannot be used for meta‐analysis. If this was the case, we contacted trial authors to request clarification and provision of mean and SD values. If we were unable to contact a trial author, we had a statistician calculate the mean and SD, where possible.
Dealing with missing data
We noted missing data for each included trial. If there were missing data, we contacted the trial authors to request the data where possible. If data presented in an abstract or article were incomplete and we were unable to get the information from a trial author, we asked a statistician for assistance in dealing with the missing data.
Assessment of heterogeneity
We examined the statistical heterogeneity for each comparison using the I² statistic, which describes the percentage of variability in the effect estimates due to heterogeneity. For those comparisons where I² > 50%, we planned to perform sensitivity analyses to attempt to explain clinical and methodological reasons for the observed heterogeneity.
Assessment of reporting biases
We evaluated reporting biases along with other potential biases as part of the 'Risk of bias' assessment.
Data synthesis
We used information about the type of treatment intervention to describe three types of treatment: interventions involving walking in the community, indoor interventions only, and a combination of both indoor and community interventions.
Subgroup analysis and investigation of heterogeneity
We anticipated being able to perform subgroup analyses on the basis of factors related to: (1) participant characteristics (age and time since stroke onset); and (2) experimental intervention, such as type of intervention and dosage (minutes spent in intervention in each trial).
Sensitivity analysis
We planned a sensitivity analysis to determine whether overall results of the study might be affected if different decisions about how to combine studies were made. Sensitivity analysis was related to the effect of methodological factors, such as the use of blinded assessors, presence of allocation concealment, and methodological issues related to how the intervention and control groups were defined.
Results
Description of studies
Results of the search
The search produced 1690 unique titles available for screening. Of these, we excluded 1277, leaving 413 abstracts for screening. We excluded 250 abstracts and 20 publications were unavailable through our medical library, resulting in 143 full text articles for review. We then excluded 137 articles, leaving six articles (Dickstein 2013; Logan 2004; Lord 2008; Mirelman 2009; Park 2011; Yang 2008). We excluded Mirelman 2009 at the point of using the data collection form ‐ we chose not to combine the Mirelman 2009 data, because the virtual reality (and robotic training) involved manipulating a boat or airplane with ankle movement, but not walking in a community setting. We identified three non‐English papers (two in Mandarin and one in German) which we arranged to have translated and eventually excluded them. See Figure 1 for details. We also identified two ongoing studies (Logan 2012; Mansfield 2013).
1.

Study flow diagram.
Included studies
The Characteristics of included studies tables contain detailed information about each included study. All studies were RCTs. Dickstein 2013 was a half‐crossover study, for which we used the data from the first time period before the crossover ‐ only the control group crossed over in the second part of the study. All other included studies were parallel RCTs. The five studies came from a variety of countries: Taiwan (Yang 2008); Israel (Dickstein 2013); United Kingdom (Logan 2004); New Zealand (Lord 2008); and Korea (Park 2011). We contacted the authors of three included trials as some data were missing. Reasons included: presenting median and interquartile range instead of mean and SD or visual representations of outcomes (Logan 2004) or no SD available (Lord 2008; Dickstein 2013). We received data from the authors of Lord 2008 and Dickstein 2013, and a statistician was able to determine required data from Logan 2004 .
The five included studies included a total of 266 participants (136 intervention; 130 control). Most participants (168) were from the Logan 2004 study. Participants lived in the community (Dickstein 2013; Lord 2008; Yang 2008), community or care home (Logan 2004), or were undergoing inpatient rehabilitation (Park 2011). Chronicity of stroke was 'post‐acute' (Lord 2008), and chronic (Dickstein 2013; Logan 2004; Park 2011; Yang 2008).
The experimental interventions fell into two main categories: walking practice in a variety of settings and environments in the community (Logan 2004; Lord 2008; Park 2011), or an activity that mimicked community walking (Dickstein 2013; Yang 2008). Two of the community walking interventions were in addition to usual practice (Logan 2004; Park 2011). The interventions that included mimicking community walking included treadmill walking in a virtual reality community environment with hazards and obstacles (Yang 2008); and motor imagery of home walking as well as indoor and outdoor community walking (Dickstein 2013).
The dosage of the experimental intervention varied across studies. We could not calculate dosage in minutes for two studies (Logan 2004; Lord 2008), as there was no information provided regarding the length of each session. The dosages of the experimental intervention in other studies were: 720 minutes (Park 2011), 180 minutes (Yang 2008), and 108 minutes (Dickstein 2013).
The comparison groups varied across studies, including a generic or 'usual treatment' control such as: two physiotherapy treatments per week based on motor learning (Lord 2008), a single occupational therapy session that included advice, encouragement, and provision of a leaflet describing local mobility services ‐ a 'routine' occupational therapy session (Logan 2004), and one hour per day of "functional training based on the Bobath concept" (Park 2011). Other comparisons included treadmill training only (Yang 2008) and upper extremity exercises (Dickstein 2013).
Three studies were funded by government agencies (Dickstein 2013; Logan 2004; Yang 2008), and two studies had no funding (Lord 2008; Park 2011).
Only two studies had participation as an outcome (Logan 2004; Lord 2008), using the Nottingham Leisure Questionnaire and the Subjective Index of Physical and Social Outcomes, respectively. Park 2011 and Yang 2008 evaluated community ambulation with the Community Walk Test and the Walking Ability Questionnaire. Four studies determined gait speed (Dickstein 2013; Lord 2008; Park 2011; Yang 2008); and two studies evaluated 6MWT (Lord 2008; Park 2011). Self efficacy was evaluated with the Activities‐specific Balance Confidence scale (Lord 2008; Park 2011; Yang 2008) and the Falls Efficacy Scale (Dickstein 2013).
The authors of the included trials did not report the other secondary outcomes of health‐related quality of life, depression, and economic outcomes.
The timing of assessments of outcome were as follows: baseline, three‐week, seven‐week follow‐up (Yang 2008); baseline and four‐week (Park 2011); baseline, four‐month, 10‐month follow‐up (Logan 2004); baseline, seven‐week, six‐month follow‐up (Lord 2008); and baseline and four‐week, with a two‐week follow‐up (Dickstein 2013). Dickstein 2013 had a follow‐up period but, due to the study design, it did not present experimental and control groups separately.
No adverse effects of the interventions were reported in any of the included studies.
Excluded studies
We excluded Mirelman 2009 (described in the Characteristics of excluded studies table).
Risk of bias in included studies
See 'Risk of bias graph' (Figure 2) and 'Risk of bias summary' (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We determined that the quality of evidence across the studies for each outcome was low (see Table 1).
Allocation
Two studies were unclear regarding allocation concealment (Dickstein 2013; Logan 2004), with a low risk of bias in the other included trials. It is a concern that these studies may not have used allocation concealment.
Blinding
None of the included studies blinded participants and treatment providers, leading to high risk of performance bias. However, given the type of study, it would be very difficult to blind participants and treatment providers. All studies were at low risk of detection bias as all assessors were blinded.
Incomplete outcome data
Risk of attrition bias varied across studies. One study had no loss to follow‐up and included all participants in the analysis (Logan 2004) (low risk of attrition bias). The remaining studies did account for dropouts but only analysed those who completed the study, leading to a designation of high risk of attrition bias.
Selective reporting
All studies appears to be at low risk of reporting bias as all outcomes appear to have been reported.
Other potential sources of bias
We noted no other potential sources of bias.
Effects of interventions
See: Table 1
All studies evaluated an intervention to improve community ambulation, with some form of community walking: community or outdoor ambulation practice (Logan 2004; Lord 2008; Park 2011), mimicking community ambulation with virtual reality (Yang 2008), or imagery of community ambulation (Dickstein 2013). We chose to analyse the comparison of 'community ambulation intervention versus other intervention'. We created subgroups of: community or outdoor ambulation practice, virtual community ambulation, and imagery of community ambulation.
The number of included studies was insufficient to enable evaluation of subgroups with dosage (time spent in intervention) or time post stroke.
Results of meta‐analyses
1.1 Participation
For the primary outcome of participation, both Lord 2008 and Logan 2004 had participation outcomes with the Subjective Index of Physical and Social Outcomes and the Nottingham Leisure Questionnaire respectively, requiring the use of the SMD. Both studies used an intervention that involved community or outdoor ambulation practice. We calculated the participation data for Logan 2004. The Nottingham Leisure Questionnaire was presented as mean difference with 95% CI in a figure, so we calculated the standard error (SE) and SD from the CIs and N (Higgins 2011b). We calculated the SMD and SE of SMD using a formula from Borenstein 2009. We calculated the SMD and CI for the Lord 2008 participation data from RevMan 2014 and SE and SD from CIs and N (Higgins 2011b). The analysis for this outcome used the generic inverse variance methods for meta‐analysis, using SMD and SE from the participation outcomes in both measures (Higgins 2011c). There was no statistically significant difference between the intervention and control groups in the participation outcome (SMD 0.08, 95% CI ‐0.20 to 0.35; two trials, 198 participants; Analysis 1.1) using inverse variance. Both random‐effects and fixed‐effect analyses had the same results. Heterogeneity was insignificant with an I² statistic value of 0% in this and all following analyses.
1.1. Analysis.
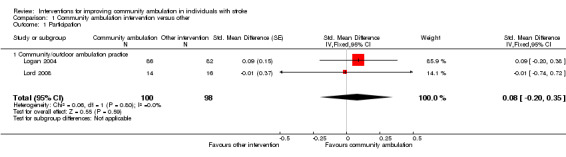
Comparison 1 Community ambulation intervention versus other, Outcome 1 Participation.
1.2 Community Walk Test and 1.3 Walking Ability Questionnaire
Park 2011 and Yang 2008 evaluated community ambulation with the Community Walk Test and the Walking Ability Questionnaire. Neither study showed significant results. When pooled for meta‐analysis, neither the Community Walk Test (MD ‐6.35, 95% CI ‐21.59 to 8.88; two trials, 45 participants; Analysis 1.2) nor the Walking Ability Questionnaire outcome results (MD 0.53, 95%CI ‐5.59 to 6.66; two trials, 45 participants; Analysis 1.3) demonstrated a difference between the experimental and control groups. Both random‐effects and fixed‐effect analyses had the same results.
1.2. Analysis.

Comparison 1 Community ambulation intervention versus other, Outcome 2 Community Walk Test.
1.3. Analysis.

Comparison 1 Community ambulation intervention versus other, Outcome 3 Walking Ability Questionnaire.
1.4 Gait speed
Four studies (50 experimental participants, 48 control participants) used a gait speed outcome: Lord 2008 and Park 2011 (community or outdoor ambulation practice); Yang 2008 (virtual); and Dickstein 2013 (imagery). The pooled analysis marginally failed to demonstrate a statistically significant difference between groups (MD 0.12, 95% CI ‐0.01 to 0.24; four trials, 98 participants; Analysis 1.4). There was no statistically significant difference between the subgroups (P = 0.96). Both random‐effects and fixed‐effect analyses had the same results for the overall meta‐analysis. However, for the community or outdoor ambulation practice subgroup (Lord 2008; Park 2011), the fixed‐effect analysis was MD 0.13 (95% CI ‐0.01 to 0.27) and the random‐effects analysis was MD 0.12 (95% CI ‐0.09 to 0.33).
1.4. Analysis.

Comparison 1 Community ambulation intervention versus other, Outcome 4 Gait speed.
We carried out a sensitivity analysis for the gait speed outcome to investigate the effect of the intervention when it also included the control treatment. In Park 2011, the intervention included the control physiotherapy treatment based on the Bobath concept plus the outdoor walking community ambulation intervention. In Yang 2008, the intervention included treadmill walking (control) with the addition of virtual reality of community ambulation situations. Combining Park 2011 and Yang 2008 only led to a statistically significant result in favour of the intervention (MD 0.20, 95% CI 0.03 to 0.37). Combining only Lord 2008 and Dickstein 2013, where the intervention did not also include the control, resulted in MD 0.03 (95% CI ‐0.15 to 0.20).
1.5 Six‐Minute Walk Test (6MWT)
Two studies used the 6MWT with community or outdoor ambulation practice interventions (Lord 2008; Park 2011) (MD 39.62, 95% CI ‐8.26 to 87.51; two trials, 55 participants; Analysis 1.5). These results did not demonstrate a statistically significant difference between groups, with 27 participants in the experimental group and 28 participants in the control group. Again, random‐effects and fixed‐effect analyses results were the same.
1.5. Analysis.

Comparison 1 Community ambulation intervention versus other, Outcome 5 Six‐Minute Walk Test.
1.6 Self‐efficacy
Four studies evaluated self‐efficacy (47 experimental participants, 50 control participants). Three trials (Lord 2008, Park 2011: community or outdoor ambulation practice; and Yang 2008 : virtual community ambulation) used the Activities‐specific Balance Confidence scale, while Dickstein 2013 (imagery of community ambulation) used the Falls‐related Efficacy Scale. When pooled for meta‐analysis, we found no significant difference between groups (SMD 0.32, 95% CI ‐0.09 to 0.72; four trials; 97 participants; Analysis 1.6). There was no statistically significant difference between the subgroups (P = 0.97) and no difference between random‐effects and fixed‐effect analyses.
1.6. Analysis.

Comparison 1 Community ambulation intervention versus other, Outcome 6 Self‐efficacy.
We did not complete the subgroup analyses of dosage as we could not calculate dosage in minutes for each included study.
Discussion
Summary of main results
The five studies that met the inclusion criteria included 266 participants: 136 in the experimental group, and 130 in a control group. Logan 2004 was the largest study, with a total of 168 participants; however, the only outcome we could include in the meta‐analysis was participation. Due to the small number of studies, varying interventions, and varying outcomes measured across the studies, a robust conclusion is not possible. None of the comparisons and outcomes showed a statistically significant difference between the experimental community ambulation intervention and control. This appears to be an immature body of literature; lack of a standard way to measure participation and community ambulation and a wide variation in the type and content of the interventions was evident. Motor learning theory and task‐oriented or goal‐oriented treatment suggests that an intervention practiced in the same or similar environment will be beneficial in improving that particular task (Shumway‐Cook 2012). Practice in the same environment (i.e. walking in the community) was evident in Logan 2004, Lord 2008, and Park 2011. Practice in a similar environment was evident in Dickstein 2013 and Yang 2008. We were unable to determine if any specific intervention method has a greater effect than others.
Only two studies measured our main outcome of interest, participation (Logan 2004; Lord 2008). We suggest that participation be measured in future studies of community ambulation, as improved participation is often the ultimate goal when striving to improve community ambulation. Sensitivity analysis of the gait speed outcome suggests that the intervention may be likely to show a statistically significant result when it is combined with the control treatment; however, this should be interpreted with great care as the number of included studies is small and the specific interventions and controls varied across studies.
Overall completeness and applicability of evidence
This Cochrane review found insufficient evidence to confirm the effectiveness of interventions to improve community ambulation in stroke survivors. Like the existing reviews of interventions for ambulatory capacity (English 2010; French 2007; Laver 2011; Mehrholz 2014; Saunders 2013; States 2009), community ambulation as a distinct competency was not consistently included as an outcome. Therefore, a robust examination of this issue waits for further research. Further research is also required into interventions that are specifically designed to improve community ambulation. We have noted two ongoing studies which will increase data available for future reviews (see Characteristics of ongoing studies tables). There is a large ongoing study of community ambulation (Logan 2012) which has an anticipated enrolment of 676 participants; results of the study will likely impact on the results of this review.
It is known that walking outdoors and in the community is complicated. Components of community ambulation include: walking distance required for certain walking tasks, such as the distance required to walk from a parking lot into a store; time limitations, such as crossing a street; weather and light conditions; unevenness of terrain; external loads, such as carrying objects or opening doors; demands on attention such as concentrating or talking while walking; changes in posture, such as standing up to start walking or changing directions while walking; and the amount of traffic in the area (Patla 1999). Which aspects should be the focus of community ambulation interventions? There is likely no single answer; many personal factors also need to be addressed (Robinson 2011b).
Quality of the evidence
We determined that the quality of evidence according to GRADE assessments across the included studies for each outcome was low (see Table 1). The primary reason for the low score was from two areas: risk of bias and imprecision. For risk of bias, blinding was not possible in these studies for the participants or those providing the intervention and only one of the studies appeared to use intention‐to‐treat analysis. For imprecision, the 95% CI included no effect in all outcomes, and the total study population was less than 400 participants.
Potential biases in the review process
We did an exhaustive search in multiple databases and are confident that we have identified all relevant studies. We were unable to obtain all relevant data due to missing data in some studies. Some data were estimated statistically; this may have introduced some bias. We were unable to obtain 20 conference abstracts through our medical library. We do not know if any of these may be relevant trials.
Agreements and disagreements with other studies or reviews
There are multiple gait‐related Cochrane reviews on stroke, which we had expected might have minimal overlap with this review. The reviews are as follows: treadmill training (Mehrholz 2014), physical fitness training (Saunders 2013), overground gait training (States 2009), circuit class training (English 2010), repetitive task training (French 2007), and virtual reality (Laver 2011).
Laver 2011 included one study on virtual reality (Yang 2008) that we included for a gait speed outcome, resulting in no significant difference between groups. No community ambulation outcome was included in the review. The physical fitness training review (Saunders 2013) did include some community ambulation outcomes including the Community Walk Test and the Walking Ability Questionnaire, with the Park 2011 study, which we also included; there were no other data to combine with the Park 2011 study. Community ambulation speed (> 0.8 m/sec or < 0.8 m/sec) was also evaluated with data from three studies; none of those included studies had community ambulation as a purpose, and no significant effect was noted. Mehrholz 2014, English 2010, French 2007 and States 2009 did not include a community ambulation outcome in their studies, with no overlapping or duplication of studies in this review.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to establish the effect of community ambulation interventions or to support a change in clinical practice. More research is needed to determine whether or not practicing outdoor or community walking will improve participation and community ambulation skills for stroke survivors living in the community.
Implications for research.
More research is needed in this area; interventions of the included studies were quite varied. Feasibility studies which lead to RCTs of interventions developed specifically to improve community ambulation are important steps in being able to determine the effectiveness of community ambulation interventions. Studies evaluating community or outdoor walking practice should incorporate components of community ambulation in the intervention, as described by Patla 1999. Careful consideration of task and goal‐oriented treatment and personal factors are also important in the design of the intervention. Control groups should be developed thoughtfully. Attention to important outcomes and the reporting of these outcomes, such as participation and community ambulation, would benefit the field.
Acknowledgements
We acknowledge Dr Rasheda Rabbani, biostatistician, for assistance with determining values for missing data. We also thank Dr S Lord and Dr R Dickstein for supplying data to enable meta‐analysis.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor Cerebrovascular Disorders explode all trees #2 MeSH descriptor Brain Injuries explode all trees #3 MeSH descriptor Hemiplegia explode all trees #4 MeSH descriptor Paresis explode all trees #5 MeSH descriptor Dystonia explode all trees #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 stroke or poststroke or post‐stroke or cva or cerebrovascular or "cerebral vascular" or "brain injury" or "brain injured" or "brain injuries" or hemipleg* or paresis or pareses or hemipares* or parapares* or paretic or hemiparetic or paraparetic or dystoni* #8 brain OR cerebro* OR cerebra* OR cerebell* OR intracran* OR intracerebral OR vertebrobasilar #9 ischemi* OR ischaemi* OR infarct* OR thrombo* OR emboli* OR occlus* #10 (#8 AND #9) #11 brain OR cerebro* OR cerebra* OR cerebell* OR intracerebral OR intracranial OR subarachnoid #12 haemorrhag* OR hemorrhag* OR haematoma* OR hematoma* OR bleed* #13 (#11 AND #12) #14 (#7 OR #10 OR #13) #15 (#6 OR #14) #16 MeSH descriptor Walking explode all trees #17 MeSH descriptor Locomotion explode all trees #18 MeSH descriptor Gait explode all trees #19 MeSH descriptor Exercise Movement Techniques explode all trees #20 MeSH descriptor Gait Disorders, Neurologic explode all trees #21 MeSH descriptor Mobility Limitation explode all trees #22 (#16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 ambulat* OR ambulant OR walk* OR locomot* OR gait* OR mobil* OR participat* #24 (#22 OR #23) #25 MeSH descriptor Social Participation, this term only #26 social* OR communit* OR outdoor* OR outside OR house OR home OR dwell* OR neighbourhood* OR neighborhood* #27 (#25 OR #26) #28 (#15 AND #24 AND #27)
Appendix 2. PubMed
1. cerebrovascular disorders [mh] OR brain injuries [mh] OR hemiplegia [mh] OR paresis [mh] OR dystonia [mh] 2. stroke [tw] OR poststroke [tw] OR post‐stroke [tw] OR cerebrovasc* [tw] OR brain vasc* [tw] OR cerebral vasc* [tw] OR cva [tw] OR apoplex* [tw] 3. brain [tw] OR cerebro* [tw] OR cerebra* [tw] OR cerebell* [tw] OR intracran* [tw] OR intracerebral [tw] OR vertebrobasilar [tw] 4. ischemi* [tw] OR ischaemi* [tw] OR infarct*[tw] OR thromboa* [tw] OR thrombob* [tw] OR thromboc* [tw] OR thromboe* [tw] OR thrombof* [tw] OR thrombog* [tw] OR thromboh* [tw] OR thromboi* [tw] OR thrombok* [tw] OR thrombol* [tw] OR thrombom* [tw] OR thrombon* [tw] OR thromboo* [tw] OR thrombop* [tw] OR thromboq* [tw] OR thrombor* [tw] OR thrombos* [tw] OR thrombot* [tw] OR thrombou* [tw] OR thrombov* [tw] OR thrombox* [tw] OR thromboy* [tw] OR thromboz* [tw] OR emboli* [tw] OR occlus* [tw] 5. #3 AND #4 6. brain [tw] OR cerebro* [tw] OR cerebra* [tw] OR cerebell* [tw] OR intracerebral [tw] OR intracranial [tw] OR subarachnoid [tw] 7. haemorrhag* [tw] OR hemorrhag* [tw] OR haematoma* [tw] OR hematoma* [tw] OR bleed* [tw] 8. #6 AND #7 9. brain injury [tw] OR brain injuries [tw] OR brain injured [tw] 10. hemipleg* [tw] OR hemipar* [tw] OR paresis [tw] OR paretic [tw] OR dystoni* [tw] 11. #1 OR #2 OR #5 OR #8 OR #9 OR #10 12. "Walking"[Mesh] OR "Locomotion"[Mesh:NoExp] OR "Gait"[Mesh] OR "Exercise Movement Techniques"[Mesh] OR "Gait Disorders, Neurologic"[Mesh] OR “Mobility Limitation” [Mesh] 13. ambulat* [tw] OR ambulant [tw] OR walk* [tw] OR locomot* [tw] OR gait* [tw] OR mobil* [tw] OR participat* [tw] 14. #12 OR #13 15. "Social Participation"[Mesh] 16. social* [tw] OR communit* [tw] OR outdoor* [tw] OR outside [tw] OR house [tw] OR home [tw] OR dwell* [tw] OR neighbourhood* [tw] OR neighborhood* [tw] 17. #15 OR #16 18. #11 AND #14 AND #17 19. "Randomized Controlled Trials as Topic"[Mesh] OR "Random Allocation"[Mesh] OR "Controlled Clinical Trials as Topic"[Mesh:NoExp] OR "Control Groups"[Mesh] OR "Clinical Trials as Topic"[Mesh] OR "Single‐Blind Method"[Mesh] OR Double‐Blind Method"[Mesh] OR "Placebos"[Mesh] OR "Placebo Effect"[Mesh] OR "Cross‐Over Studies"[Mesh] OR "Multicenter Studies as Topic"[Mesh] OR "Research Design"[Mesh] OR "Program Evaluation"[Mesh] OR "Evaluation Studies as Topic"[Mesh] 20. "Randomized Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] OR "Clinical Trial" [Publication Type] OR "Multicenter Study" [Publication Type] OR "Evaluation Studies" [Publication Type] 21. random* [tiab] 22. controlled [tiab] AND (trial* [tiab] or stud* [tiab]) 23. (control [tiab] OR treatment [tiab] OR experiment* [tiab] OR intervention [tiab]) AND (group [tiab] OR groups [tiab] OR subject* [tiab] OR patient [tiab] OR patients [tiab]) 24. quasi‐random* [tiab] OR quasi random* [tiab] OR pseudo‐random* [tiab] OR pseudo random* [tiab] 25. (multicenter [tiab] OR multicentre [tiab] OR therapeutic [tiab]) AND (trial* [tiab] OR study [tiab] OR studies [tiab]) 26. (control [tiab] OR experiment* [tiab] OR conservative [tiab]) AND (treatment* [tiab] OR therapy [tiab] OR therapies [tiab] OR procedure* [tiab] OR manag* [tiab]) 27. coin [tiab] AND (flip [tiab] OR flipped [tiab] OR toss* [tiab]) 28. versus [tiab] 29. cross‐over [tiab] OR cross over [tiab] OR crossover [tiab] 30. placebo* [tiab] 31. assign* [tiab] OR alternate [tiab] OR allocat* [tiab] OR counterbalance* [tiab] OR multiple baseline [tiab] 32. controls [tiab] 33. treatment* [tiab] AND order [tiab] 34. #19 OR #20 OR # 21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 35. #18 AND #34
Appendix 3. EMBASE OvidSP
1. exp cerebrovascular‐disease/ or exp neurologic‐disease/ or paresis/ or exp hemiplegia/ or hemiparesis/ or dystonia/ 2. brain injuries/ 3. (stroke$ or poststroke or post‐stroke or cva or cerebrovascular or cerebral vascular or brain injur$ or hemipleg$ or paresis or pareses or hemipares$ or parapares$ or paretic or hemiparetic or paraparetic or dystoni$).tw. 4. ((cerebral or cerebellar or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy)).tw. 5. ((cerebral or brain$ or subarachnoid) adj5 (haemorrhage or hemorrhage or haematoma or hematoma or bleed$)).tw. 6. 1 or 2 or 3 or 4 or 5 7. exp walking/ or locomotion/ or gait/ or kinesiotherapy/ or patient mobility/ 8. (ambulat$ or ambulant or walk$ or locomot$ or gait$ or mobil$ or participat$).tw. 9. 7 or 8 10. (social$ or communit$ or outdoor$ or outside or house or home or dwell$ or neighbourhood$ or neighborhood$).tw. 11. (social$ adj5 (participat$ or interact$)).tw. 12. 10 or 11 13. 6 and 9 and 12 14. randomized controlled trial/ 15. controlled clinical trial/ 16. randomization/ 17. clinical trial/ 18. control group/ 19. single blind procedure/ 20. placebo/ 21. placebo effect/ 22. crossover procedure/ 23. "multicenter study (topic)"/ 24. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. random$.tw. 26. (controlled adj5 (trial$ or stud$)).tw. 27. (clinical$ adj5 trial$).tw. 28. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 29. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 30. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 31. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 32. (cross‐over or cross over or crossover).tw. 33. placebo$.tw. 34. ((doubl$ or singl$) adj blind$).tw. 35. (allocat$ or volunteer$ or assign$).tw. 36. (coin adj5 (flip or flipped or toss$)).tw. 37. versus.tw. 38. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 39. controls.tw. 40. 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 41. double blind procedure/ 42. 40 or 41 43. 24 or 42 44. case report/ 45. case report.tw. 46. abstract report/ 47. letter/ 48. 44 or 45 or 46 or 47 49. 43 not 48 50. 13 and 49
Appendix 4. CINAHL EbscoHost
S1 (MH "Cerebrovascular Disorders+") or (MH "Stroke Patients") or (MH "Brain Injuries") or (MH "Hemiplegia") or (MH "Dystonia") S2 TI ( stroke or poststroke or post‐stroke or cva or cerebrovascular or cerebral vascular or brain injur* or hemipleg* or paresis or pareses or hemipares* or parapares* or paretic or hemiparetic or paraparetic or dystoni* ) OR AB ( stroke or poststroke or post‐stroke or cva or cerebrovascular or cerebral vascular or brain injur* or hemipleg* or paresis or pareses or hemipares* or parapares* or paretic or hemiparetic or paraparetic or dystoni* ) S3. TI ( cerebral n5 infarct* OR cerebellar n5 infarct* OR brain n5 infarct* OR vertebrobasilar n5 infarct* ) OR AB ( cerebral n5 infarct* OR cerebellar n5 infarct* OR brain n5 infarct* OR vertebrobasilar n5 infarct* ) S4 TI ( cerebral n5 thrombo* OR cerebellar n5 thrombo* OR brain n5 thrombo* OR vertebrobasilar n5 thrombo* ) OR AB ( cerebral n5 thrombo* OR cerebellar n5 thrombo* OR brain n5 thrombo* OR vertebrobasilar n5 thrombo* ) S5 TI ( cerebral n5 emboli* OR cerebellar n5 emboli* OR brain n5 emboli* OR vertebrobasilar n5 emboli* ) OR AB ( cerebral n5 emboli* OR cerebellar n5 emboli* OR brain n5 emboli* OR vertebrobasilar n5 emboli* ) S6 TI ( cerebral n5 apoplexy OR cerebellar n5 apoplexy OR brain n5 apoplexy OR vertebrobasilar n5 apoplexy ) OR AB (cerebral n5 apoplexy OR cerebellar n5 apoplexy OR brain n5 apoplexy OR vertebrobasilar n5 apoplexy) S7 TI ( cerebral n5 ischaemi* OR cerebellar n5 ischaemi* OR brain n5 ischaemi* OR vertebrobasilar n5 ischaemi* ) OR AB ( cerebral n5 ischaemi* OR cerebellar n5 ischaemi* OR brain n5 ischaemi* OR vertebrobasilar n5 ischaemi* ) S8 TI ( cerebral n5 ischemi* OR cerebellar n5 ischemi* OR brain n5 ischemi* OR vertebrobasilar n5 ischemi* ) OR AB ( cerebral n5 ischemi* OR cerebellar n5 ischemi* OR brain n5 ischemi* OR vertebrobasilar n5 ischemi* S9 TI ( cerebral n5 haemorrhage OR brain n5 haemorrhage OR subarachnoid n5 haemorrhage ) OR AB ( cerebral n5 haemorrhage OR brain n5 haemorrhage OR subarachnoid n5 haemorrhage ) S10 TI ( cerebral n5 hemorrhage OR brain n5 hemorrhage OR subarachnoid n5 hemorrhage ) OR AB ( cerebral n5 hemorrhage OR brain n5 hemorrhage OR subarachnoid n5 hemorrhage ) S11 TI ( cerebral n5 haematoma OR brain n5 haematoma OR subarachnoid n5 haematoma ) OR AB ( cerebral n5 haematoma OR brain n5 haematoma OR subarachnoid n5 haematoma ) S12 TI ( cerebral n5 hematoma OR brain n5 hematoma OR subarachnoid n5 hematoma ) OR AB ( cerebral n5 hematoma OR brain n5 hematoma OR subarachnoid n5 hematoma ) S13 TI ( cerebral n5 bleed* OR brain n5 bleed* OR subarachnoid n5 bleed* ) OR AB ( cerebral n5 bleed* OR brain n5 bleed* OR subarachnoid n5 bleed* ) S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15 (MH "Walking+") OR (MH "Gait+") OR (MH "Locomotion") OR (MH "Therapeutic Exercise") OR (MH "Gait Disorders, Neurologic+") S16 TI ( ambulat* or ambulant or walk* or locomot* or gait* or mobil* or participat* ) AND AB ( ambulat* or ambulant or walk* or locomot* or gait* or mobil* or participat* ) S17 S15 OR S16 S18 (MH "Social Participation") S19 TI ( social* or communit* or outdoor* or outside or house* or home* or dwell* or neighbourhood* or neighborhood* ) AND AB ( social* or communit* or outdoor* or outside or house* or home* or dwell* or neighbourhood* or neighborhood* ) S20 S18 OR S19 S21 S14 AND S17 AND S20
Appendix 5. PsycINFO ProQuest
S1 ((su(EXACT.EXACT.EXPLODE("Cerebrovascular Disorders")) OR su(EXACT.EXACT.EXPLODE("Brain Damage")) OR su(EXACT.EXACT.EXPLODE("Nervous System Disorders")) OR su.EXACT("Hemiplegia") OR su(EXACT.EXACT.EXPLODE("Neuromuscular Disorders")) OR su.EXACT("General Paresis")) S2 (ti(stroke* OR poststroke OR post‐stroke OR cva OR cerebrovascular OR cerebral vascular OR brain injur* OR hemipleg* OR paresis OR pareses OR hemipares* OR parapares* OR paretic OR hemiparetic OR paraparetic OR dystoni*) OR ab(stroke* OR poststroke OR post‐stroke OR cva OR cerebrovascular OR cerebral vascular OR brain injur* OR hemipleg* OR paresis OR pareses OR hemipares* OR parapares* OR paretic OR hemiparetic OR paraparetic OR dystoni*)) S3 (ti(((cerebral OR cerebellar OR brain* OR vertebrobasilar) NEAR/5 (infarct* OR isch?emi* OR thrombo* OR emboli* OR apoplexy))) OR ab(((cerebral OR cerebellar OR brain* OR vertebrobasilar) NEAR/5 (infarct* OR isch?emi* OR thrombo* OR emboli* OR apoplexy)))) S4 (ti((cerebral OR brain* OR subarachnoid) NEAR/5 (h?emorrhage OR h?ematoma OR bleed*)) OR ab((cerebral OR brain* OR subarachnoid) NEAR/5 (h?emorrhage OR h?ematoma OR bleed*)))) S5 1 OR 2 OR 3 OR 4 S6 ((su(EXACT.EXACT.EXPLODE("Walking")) OR su.EXACT("Locomotion" OR "Physical Mobility") OR su.EXACT("Movement Therapy" OR "Exercise")) S7 (ti(ambulat* OR ambulant OR walk* OR locomot* OR gait* OR mobil* OR participat*) OR ab(ambulat* OR ambulant OR walk* OR locomot* OR gait* OR mobil* OR participat*))) S8 6 OR 7 S9 ((su.EXACT("Interpersonal Interaction" OR "Social Interaction") OR su.EXACT("Community Involvement" OR "Involvement")) S10 (ti(social* OR communit* OR outdoor* OR outside OR house* OR home* OR dwell* OR neighbourhood* OR neighborhood*) OR ab(social* OR communit* OR outdoor* OR outside OR house* OR home* OR dwell* OR neighbourhood* OR neighborhood*))) S11 9 OR 10 S12 5 AND 8 AND 11
Appendix 6. Scopus
1. TITLE‐ABS‐KEY(stroke OR poststroke OR post‐stroke OR cva OR cerebrovascular OR "cerebral vascular" OR "brain injury" OR "brain injured" OR "brain injuries" OR hemipleg* OR paresis OR pareses OR hemipares* OR parapares*) 2. TITLE‐ABS‐KEY(paretic OR hemiparetic OR paraparetic OR dystoni*) 3. TITLE‐ABS‐KEY((cerebral OR cerebellar OR brain* OR vertebrobasilar) W/5 (infarct* OR isch?emi* OR thrombo* OR emboli* OR apoplexy)) 4. TITLE‐ABS‐KEY((cerebral OR brain* OR subarachnoid) W/5 (haemorrhage OR hemorrhage OR haematoma OR hematoma OR bleed*)) 5. #1 OR #2 OR #3 OR #4 6. TITLE‐ABS‐KEY(ambulat* OR ambulant OR walk* OR locomot* OR gait* OR mobil* OR participat*) 7. TITLE‐ABS‐KEY(social* OR communit* OR outdoor* OR outside OR house* OR home* OR dwell* OR neighbourhood* OR neighborhood*) 8. #5 AND #6 AND #7 9. TITLE‐ABS‐KEY(human* AND adult*) 10. #8 AND #9 11. TITLE‐ABS‐KEY(random*) 12. TITLE‐ABS‐KEY(controlled W/5 (trial* OR stud*)) 13. TITLE‐ABS‐KEY(clinical* W/5 trial*) 14. TITLE‐ABS‐KEY((control OR treatment OR experiment* OR intervention) W/5 (group* OR subject* OR patient*)) 15. TITLE‐ABS‐KEY(quasi‐random* OR quasi random* OR pseudo‐random* OR pseudo random*) 16. TITLE‐ABS‐KEY((multicenter OR multicentre OR therapeutic) W/5 (trial* OR stud*)) 17. TITLE‐ABS‐KEY((control OR experiment* OR conservative) W/5 (treatment OR therapy OR procedure OR manage*)) 18. TITLE‐ABS‐KEY("cross‐over" OR "cross over" OR crossover) 19. TITLE‐ABS‐KEY(placebo*) 20. TITLE‐ABS‐KEY((doubl* OR singl*) W/1 blind* 21. TITLE‐ABS‐KEY(allocat* OR volunteer* OR assign*) 22. TITLE‐ABS‐KEY(coin W/5 (flip OR flipped OR toss*)) 23. TITLE‐ABS‐KEY(versus) 24. TITLE‐ABS‐KEY(alternate OR allocat* OR counterbalance* OR "multiple baseline") 25. TITLE‐ABS‐KEY(controls) 26. #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 27. #10 AND #26
Appendix 7. Web of Science
1. Topic=(stroke OR poststroke OR post‐stroke OR cva OR cerebrovascular OR "cerebral vascular" OR "brain injury" OR "brain injured" OR "brain injuries" OR hemipleg* OR paresis OR pareses OR hemipares* OR parapares*) 2. Topic=(paretic OR hemiparetic OR paraparetic OR dystoni*) 3. Topic=((cerebral OR cerebellar OR brain* OR vertebrobasilar) NEAR/5 (infarct* OR isch$emi* OR thrombo* OR emboli* OR apoplexy)) 4. Topic=((cerebral OR brain* OR subarachnoid) NEAR/5 (haemorrhage OR hemorrhage OR haematoma OR hematoma OR bleed*)) 5. #4 OR #3 OR #2 OR #1 6. Topic=(ambulat* OR ambulant OR walk* OR locomot* OR gait* OR mobil* OR participat*) 7. Topic=(social* OR communit* OR outdoor* OR outside OR house* OR home* OR dwell* OR neighbourhood* OR neighborhood*) 8. #7 AND #6 9. #8 AND #5 10. Topic=(random*) 11. Topic=(controlled NEAR/5 (trial* OR stud*)) 12. Topic=(clinical* NEAR/5 trial*) 13. Topic=((control OR treatment OR experiment* OR intervention) NEAR/5 (group* OR subject* OR patient*)) 14. Topic=(quasi‐random* OR (quasi NEAR/1 random*) OR pseudo‐random* OR (pseudo NEAR/1 random*)) 15. Topic=((multicenter OR multicentre OR therapeutic) NEAR/5 (trial* OR stud*)) 16. Topic=((control OR experiment* OR conservative) NEAR/5 (treatment OR therapy OR procedure OR manage*)) 17. Topic=("cross‐over" OR cross NEAR/1 over OR crossover) 18. Topic=(placebo*) 19. Topic=((doubl* OR singl*) NEAR/1 blind*) 20. Topic=(coin NEAR/5 (flip OR flipped OR toss*)) 21. Topic=(versus) 22. Topic=(allocat* OR volunteer* OR assign*) 23. Topic=(controls) 24. #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 25. #24 AND #9
Appendix 8. SPORTDiscus EbscoHost
S1 DE "STROKE" OR DE "BRAIN ‐‐ Wounds & injuries" OR DE "HEMIPLEGIA" OR DE "HEMIPLEGICS" OR DE "DYSTONIA" S2 TI ( stroke or poststroke or post‐stroke or cva or cerebrovascular or cerebral vascular or brain injur* or hemipleg* or paresis or pareses or hemipares* or parapares* or paretic or hemiparetic or paraparetic or dystoni* ) OR AB ( stroke or poststroke or post‐stroke or cva or cerebrovascular or cerebral vascular or brain injur* or hemipleg* or paresis or pareses or hemipares* or parapares* or paretic or hemiparetic or paraparetic or dystoni* ) S3 TI ( cerebral n5 infarct* OR cerebellar n5 infarct* OR brain n5 infarct* OR vertebrobasilar n5 infarct* ) OR AB ( cerebral n5 infarct* OR cerebellar n5 infarct* OR brain n5 infarct* OR vertebrobasilar n5 infarct* ) S4 TI ( cerebral n5 thrombo* OR cerebellar n5 thrombo* OR brain n5 thrombo* OR vertebrobasilar n5 thrombo* ) OR AB ( cerebral n5 thrombo* OR cerebellar n5 thrombo* OR brain n5 thrombo* OR vertebrobasilar n5 thrombo* ) S5 TI ( cerebral n5 emboli* OR cerebellar n5 emboli* OR brain n5 emboli* OR vertebrobasilar n5 emboli* ) OR AB ( cerebral n5 emboli* OR cerebellar n5 emboli* OR brain n5 emboli* OR vertebrobasilar n5 emboli* ) S6 TI ( cerebral n5 apoplexy OR cerebellar n5 apoplexy OR brain n5 apoplexy OR vertebrobasilar n5 apoplexy ) OR AB ( cerebral n5 apoplexy OR cerebellar n5 apoplexy OR brain n5 apoplexy OR vertebrobasilar n5 apoplexy ) S7 TI ( cerebral n5 ischaemi* OR cerebellar n5 ischaemi* OR brain n5 ischaemi* OR vertebrobasilar n5 ischaemi* ) OR AB ( cerebral n5 ischaemi* OR cerebellar n5 ischaemi* OR brain n5 ischaemi* OR vertebrobasilar n5 ischaemi* ) S8 TI ( cerebral n5 ischemi* OR cerebellar n5 ischemi* OR brain n5 ischemi* OR vertebrobasilar n5 ischemi* ) OR AB ( cerebral n5 ischemi* OR cerebellar n5 ischemi* OR brain n5 ischemi* OR vertebrobasilar n5 ischemi* ) S9 TI ( cerebral n5 haemorrhage OR brain n5 haemorrhage OR subarachnoid n5 haemorrhage ) OR AB ( cerebral n5 haemorrhage OR brain n5 haemorrhage OR subarachnoid n5 haemorrhage ) S10 TI ( cerebral n5 hemorrhage OR brain n5 hemorrhage OR subarachnoid n5 hemorrhage ) OR AB ( cerebral n5 hemorrhage OR brain n5 hemorrhage OR subarachnoid n5 hemorrhage ) S11 TI ( cerebral n5 haematoma OR brain n5 haematoma OR subarachnoid n5 haematoma ) OR AB ( cerebral n5 haematoma OR brain n5 haematoma OR subarachnoid n5 haematoma ) S12 TI ( cerebral n5 hematoma OR brain n5 hematoma OR subarachnoid n5 hematoma ) OR AB ( cerebral n5 hematoma OR brain n5 hematoma OR subarachnoid n5 hematoma ) S13 TI ( cerebral n5 bleed* OR brain n5 bleed* OR subarachnoid n5 bleed* ) OR AB ( cerebral n5 bleed* OR brain n5 bleed* OR subarachnoid n5 bleed* ) S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15 DE "WALKING" OR DE "HUMAN locomotion" OR DE "GAIT in humans" S16 TI ( ambulat* or ambulant or walk* or locomot* or gait* or mobil* or participat* ) AND AB ( ambulat* or ambulant or walk* or locomot* or gait* or mobil* or participat* ) S17 S15 OR S16 S18 TI ( social* or communit* or outdoor* or outside or house* or home* or dwell* or neighbourhood* or neighborhood* ) OR AB ( social* or communit* or outdoor* or outside or house* or home* or dwell* or neighbourhood* or neighborhood* ) S19 S14 AND S17 AND S18
Data and analyses
Comparison 1. Community ambulation intervention versus other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participation | 2 | 198 | Std. Mean Difference (Fixed, 95% CI) | 0.08 [‐0.20, 0.35] |
| 1.1 Community/outdoor ambulation practice | 2 | 198 | Std. Mean Difference (Fixed, 95% CI) | 0.08 [‐0.20, 0.35] |
| 2 Community Walk Test | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐6.35 [‐21.59, 8.88] |
| 2.1 Community/outdoor ambulation practice | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐10.68 [‐35.22, 13.86] |
| 2.2 Virtual community ambulation | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.64 [‐23.07, 15.79] |
| 3 Walking Ability Questionnaire | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐5.59, 6.66] |
| 3.1 Community/outdoor ambulation practice | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐6.71, 8.79] |
| 3.2 Virtual community ambulation | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐10.31, 9.69] |
| 4 Gait speed | 4 | 98 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.01, 0.24] |
| 4.1 Community/outdoor ambulation practice | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.01, 0.27] |
| 4.2 Virtual community ambulation | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.36, 0.54] |
| 4.3 Imagery of community ambulation | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.21, 0.35] |
| 5 Six‐Minute Walk Test | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | 39.62 [‐8.26, 87.51] |
| 5.1 Community/outdoor ambulation practice | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | 39.62 [‐8.26, 87.51] |
| 6 Self‐efficacy | 4 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.09, 0.72] |
| 6.1 Community/outdoor ambulation practice | 2 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.25, 0.84] |
| 6.2 Virtual community ambulation | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.48, 1.31] |
| 6.3 Imagery of community ambulation | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.54, 1.10] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Lord 2008.
| Methods | RCT | |
| Participants | Age: experimental: 60.7 (17.6) years, control: 64.2 (14.8) years Sex: men: 18 (9 experimental, 9 control), women: 2 (5 experimental, 7 control) Chronicity: 'post‐acute': experimental: 80.3 (33.0) days post stroke, control: 83.1 (29.8) days post stroke Minimal cognitive level: MMSE > 24 Minimal functional requirement: able to 'walk to their letterbox and no further' and have independent community ambulation as a primary rehabilitation goal |
|
| Interventions | Experimental (n = 14): walking in the participant's community twice a week for 7 weeks; whole task practice of functional gait activities in a community environment related to each participant, i.e. mall, bowling, park, etc. Activities such as endurance, walking in crowds, crossing the street, and dual tasks are described in an appendix Control (n = 16): 14 physiotherapy sessions (2 per week for 7 weeks) based on motor relearning programme under supervision of physical therapist |
|
| Outcomes | Participant‐level instrument: Subjective Index of Physical and Social Outcome (SIPSO) (10 item) Community ambulation‐specific measure: considered specific items from Activities‐specific Balance Confidence (ABC) Scale and SIPSO as indicators of community ambulation Other: gait speed with 10‐Metre Walk Test (m/min); 6MWT; activities‐specific Balance Confidence scale Timing of assessments of outcome: baseline, 7 weeks, 6‐month follow‐up |
|
| Setting from which participants were recruited | Acute medical wards and Inpatient‐rehabilitation. All participants lived in the community at time of study | |
| Notes | Dosage of experimental intervention: twice a week for 7 weeks, unable to calculate total minutes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes by third party |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total attrition rate = 25% (9/36); early withdrawal not included in analysis, not ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes addressed |
Logan 2004.
| Methods | RCT | |
| Participants | Age: experimental: 74 (8.4) years, control: 74 (8.6) years Sex: men: 91 (40 experimental, 51 control), women: 77 (46 experimental, 31 control) Chronicity: chronic: experimental: 11 (8.4) months post‐stroke; control: 10 (9) months post‐stroke Minimal cognitive level: not specified Minimum functional requirement: none specified Minimum motor requirement: none specified |
|
| Interventions | Experimental (n = 86): control intervention + individualized OT assessment and treatment that included provision of transportation information, use of aids, strategies to overcome fear or apprehension and increase confidence, including accompanying participants out of doors. "...up to 7 treatment sessions at home for up to 3 months" Control (n = 82): single OT session that included advice, encouragement, and provision of leaflet describing local mobility services ‐ 'routine' OT session |
|
| Outcomes | Participation‐level instrument: Nottingham Leisure Questionnaire Community ambulation‐specific measure: responses to specific questions:
Other: general health questionnaire Timing of assessments of outcome: baseline, 4‐month, 10‐month follow‐up |
|
| Setting from which participants were recruited | Community living including people in care homes | |
| Notes | Dosage of experimental intervention: "up to 7 treatment sessions at home for up to 3 months", with a mean of 4.7 visits; unable to calculate total minutes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a telephone randomisation service, computer‐generated, random sequence stratified by age and amount of travel out of the house |
| Allocation concealment (selection bias) | Unclear risk | Used a telephone randomisation service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Park 2011.
| Methods | RCT | |
| Participants | Sex: men: 12; women: 13 Chronicity: 6 months to 5 years post‐CVA: experimental: 28.08 (12.59) months post‐stroke; control: 28.67 (17.96) months post‐stroke Minimal cognitive level: > 25 on MMSE Minimum functional requirement: walking speed < 0.7 m/sec |
|
| Interventions | Experimental (n = 13): control intervention (below) plus 12 hours (1 hour/day, 3 days/week for 4 weeks) walking in progressively more complicated environment (surface, attention demands, distance) – hospital foyer, walking path, outdoor route, shopping centre Control (n = 12): 1 hour/day of "functional training based on the Bobath concept" |
|
| Outcomes | Participation‐level instrument: none Community ambulation‐specific measure:
Timing of assessments of outcome: baseline and at 4 weeks |
|
| Setting from which participants were recruited | Rehabilitation unit | |
| Notes | Dosage of experimental intervention: 12 hours (720 minutes) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant blindly drew a card from an envelope with 2 cards in it |
| Allocation concealment (selection bias) | Low risk | Each participant blindly drew a card from an envelope with 2 cards in it |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, therapists not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop outs not included, not ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described |
Yang 2008.
| Methods | RCT | |
| Participants | Age: > 30 years Sex: men: 10; women: 10 Chronicity: ≥ 6 months: experimental: 5.93 (4.17) years post‐stroke; control: 6.10 (10.32) years post stroke Minimal cognitive level: able to understand instructions and follow commands Minimum functional requirement: limited household walker, unlimited household walker, or most‐limited community walker by functional walking category |
|
| Interventions | Experimental (n = 11): motorised treadmill training in a virtual reality environment for a total of 3 hours (20 min/day, 3 days/week for 3 weeks). The virtual environment required the participant to walk through typical environments in Taipei. The training programme was progressed Control (n = 9): treadmill training with instructions to execute various tasks that simulated stepping over obstacles, uphill/downhill walking and fast walking for a total of 3 hours (20 minutes/day, 3 days/week for 3 weeks) |
|
| Outcomes | Participation‐level instrument: none Community ambulation‐specific measure:
The timing of assessments of outcome: baseline, 3‐week and 7‐week follow‐up |
|
| Setting from which participants were recruited | Community‐living | |
| Notes | Dosage of experimental intervention: 3 hours (180 minutes) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Envelopes used, with independent person |
| Allocation concealment (selection bias) | Low risk | Envelopes used, with independent person |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop outs not included in analysis, not ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Dickstein 2013.
| Methods | Half‐crossover RCT | |
| Participants | Age: experimental: 71.3 years; control: 72.2 years Chronicity: chronic: experimental: 66.3 (39.3) weeks post‐stroke; control: 83.4 (55.4) weeks post stroke |
|
| Interventions | Experimental (n = 12): 'Integrated imagery practice': used individual goals to develop 'imagined walking tasks'. Used the same script for each of 3 weekly sessions over 4 weeks. The script was revised each week. Participants sat on a couch with eyes closed for the imagery. 15 minutes for each session: 3 minutes relaxation exercises to start, 3 minutes relaxation exercises to finish, 3 minutes imagery for walking in participant's home, 3 minutes imagery for indoor community walking, and 3 minutes imagery for outdoor community walking (approximately 9 minutes/session of imagining walking) Control (n = 11): upper extremity exercises: 3 minutes reach with transport of an object, 3 minutes bilateral exercises, 3 minutes manipulation activity for affected arm. Exercises the same for each of 3 weekly session, and revised each week (approximately 9 minutes/session) |
|
| Outcomes | Participation‐level instrument: none Community ambulation‐specific measure: number of steps with activity monitor Other: gait speed with 10 metre walk test (m/sec); Falls Efficacy Scale Timing of assessments of outcome: baseline and 4 weeks, follow‐up 2 weeks after intervention cross over (with both groups combined) |
|
| Setting from which participants were recruited | Community dwelling, treatment in participant's home | |
| Notes | Dosage of experimental intervention: 108 minutes of imagery of walking (not including relaxation exercises) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Minimization scheme' ‐ balance in gait speed, age, sex. However, no details |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and physical therapists could not be blinded in this type of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two physical therapists were blinded to group assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop outs accounted for, only analysed completed, not ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes discussed |
6MWT: Six‐Minute Walk Test CVA: cerebrovascular accident ITT: intention‐to‐treat MMSE: Mini Mental State Examination m/s: metres per second OT: occupational therapy RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mirelman 2009 | The virtual reality and robotic training intervention involved manipulating a boat or airplane with ankle movement, but not walking in a community setting. |
Characteristics of ongoing studies [ordered by study ID]
Logan 2012.
| Trial name or title | A multicentre randomised controlled trial of rehabilitation aimed at improving outdoor mobility for people who have had a stroke (TOMAS) |
| Methods | Two‐arm, parallel groups, multicentre RCT |
| Participants | Individuals 18 years of age or older, who had a stroke at least 6 months previously, wished to get out of the house more often and had completed their post‐stroke intermediate or active rehabilitation. Anticipated enrolment = 676 |
| Interventions | Intervention group: specifically designed training/practice programme that emphasises attempts to try outdoor mobility (e.g. use of taxis, buses, voluntary drivers and mobility scooters). Intervention group participants will receive up to 7 rehabilitation outdoor mobility sessions of about an hour each over 4 months Control group: routine intervention for outdoor mobility limitations, that is, verbal advice and provision of leaflets provided over one 1‐hour session |
| Outcomes |
|
| Starting date | 1 August 2009 |
| Contact information | Pip Logan (pip.logan.nottingham.ac.uk) |
| Notes | ISRCTN58683841 |
Mansfield 2013.
| Trial name or title | Using wireless technology in clinical practice: does feedback of daily walking activity improve walking outcomes of individuals receiving rehabilitation post‐stroke? |
| Methods | Two‐arm, parallel groups, observer blinded, RCT |
| Participants | Individuals with subacute stroke attending inpatient rehabilitation who have identified improving walking function as a rehabilitation goal and can walk without supervision at the time of recruitment into the study |
| Interventions | Experimental group: treating physical therapists will receive regular reports of the participants' walking activity from accelerometers worn between sessions. This data will be used to help with goal setting Control group: accelerometer‐based indicators of walking ability will not be available to physical therapists for goal setting |
| Outcomes |
|
| Starting date | October 2012 |
| Contact information | Avril Mansfield (avril.mansfield@uhn.ca) |
| Notes | NCT01521234 |
RCT: randomised controlled trial
Differences between protocol and review
In the protocol, we stated that we would use random‐effects modelling for the analysis (Barclay‐Goddard 2012b). We completed both random‐effects and fixed‐effect analyses and have described any similarities or differences in the Results section. However, due to the low number of included studies, we have presented fixed‐effect analyses in the figures and text, unless otherwise stated.
Contributions of authors
William Poluha developed the search strategies for all databases, performed, and updated the searches. Ruth Barclay wrote the initial draft of the protocol with substantial input from Ted Stevenson. Jacquie Ripat, Cristabel Nett, and William Poluha. Jacquie Ripat, Cristabel Nett, Ruth Barclay, and Ted Stevenson reviewed titles, abstracts, and papers; Cynthia Srikesavan managed the lists of titles, abstracts, and full‐text manuscripts; and Ruth Barclay and Ted Stevenson completed data extraction and data analysis. Ruth Barclay completed the review draft with substantial input from Ted Stevenson, as well as review and input from all other review authors.
Declarations of interest
Ruth E Barclay: none known. Ted J Stevenson: none known. William Poluha: none known. Jacquie Ripat: none known. Cristabel Nett: receives payment for providing physiotherapy advice and treatment to clients with brain injury including CVA to improve their walking and mobility, apart from her employment as an instructor at the University of Manitoba. Cynthia S Srikesavan: none known.
New
References
References to studies included in this review
Dickstein 2013 {published data only}
- Dickstein R, Deutsch JE, Yoeli Y, Kafri M, Falash F, Dunsky A, et al. Effects of integrated motor imagery practice on gait of individuals with chronic stroke: a half‐crossover randomized study. Archives of Physical Medicine and Rehabilitation 2013;94(11):2119‐25. [DOI] [PubMed] [Google Scholar]
Logan 2004 {published data only}
- Logan PA, Gladman JRF, Avery A, Walker MF, Dyas J, Groom L. Randomised controlled trial of an occupational therapy intervention to increase outdoor mobility after stroke. BMJ 2004;329(7479):1372‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lord 2008 {published and unpublished data}
- Lord SE, McPherson KM, McNaughton HK, Rochester L, Weatherall M. How feasible is the attainment of community ambulation after stroke? A pilot randomized controlled trial to evaluate community‐based physiotherapy in subacute stroke. Clinical Rehabilitation 2008;22(3):215‐25. [DOI] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park HJ, Oh DW, Kim SY, Choi JD. Effectiveness of community‐based ambulation training for walking function of post‐stroke hemiparesis: a randomized controlled pilot trial. Clinical Rehabilitation 2011;25(5):451‐9. [DOI] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang YR, Tsai MP, Chuang TY, Sung WH, Wang RY. Virtual reality‐based training improved community ambulation in individuals with stroke: a randomized controlled trial. Gait & Posture 2008;28(2):201‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mirelman 2009 {published data only}
- Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot‐virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke 2009;40(1):169‐74. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Logan 2012 {published data only}
- Logan PA, Leighton MP, Walker MF, Armstrong S, Gladman JRF, Sach TH, et al. A multi‐centre randomised controlled trial of rehabilitation aimed at improving outdoor mobility for people after stroke: study protocol for a randomised controlled trial. Trials 2012;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansfield 2013 {published data only}
- Mansfield A, Wong JS, Bayley M, Biasin L, Brooks D, Brunton K, et al. Using wireless technology in clinical practice: does feedback of daily walking activity improve walking outcomes of individuals receiving rehabilitation post‐stroke? Study protocol for a randomized controlled trial. BMC Neurology 2013;13:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ada 2003
- Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo‐controlled, randomized trial. Archives of Physical Medicine and Rehabilitation 2003;84(10):1486‐91. [DOI] [PubMed] [Google Scholar]
Ada 2009
- Ada L, Dean CM, Lindley R, Lloyd G. Improving community ambulation after stroke: the AMBULATE trial. BMC Neurology 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1996
- Anderson C, Laubscher S, Burns R. Validation of the Short Form 36 (SF‐36) health survey questionnaire among stroke patients. Stroke 1996;27(10):1812‐6. [DOI] [PubMed] [Google Scholar]
Barclay 2014
Barclay‐Goddard 2012a
- Barclay‐Goddard R, Ripat J, Mayo NE. Developing a model of participation post‐stroke: a mixed‐methods approach. Quality of Life Research 2012;21(3):417‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M. Introduction to Meta‐Analysis. Chichester: John Wiley & Sons, 2009. [Google Scholar]
Corrigan 2012
- Corrigan R, McBurney H. Community ambulation: perceptions of rehabilitation physiotherapists in rural and regional communities. Physiotherapy Theory and Practice 2012;28(1):10‐7. [DOI] [PubMed] [Google Scholar]
Danielsson 2011
- Danielsson A, Willén C, Sunnerhagen KS. Is walking endurance associated with activity and participation later after stroke?. Disability and Rehabilitation 2011;33(21‐2):2053‐7. [DOI] [PubMed] [Google Scholar]
Donovan 2008
- Donovan K, Lord SE, McNaughton HK, Weatherall M. Mobility beyond the clinic: the effect of environment on gait and its measurement in community‐ambulant stroke survivors. Clinical Rehabilitation 2008;22(6):556‐63. [DOI] [PubMed] [Google Scholar]
Duncan 1999
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30(10):2131‐40. [DOI] [PubMed] [Google Scholar]
English 2010
- English C, Hillier SL. Circuit class therapy for improving mobility after stroke. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007513.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feigin 2003
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population‐based studies of incidence, prevalence, and case‐fatality in the late 20th century. Lancet Neurology 2003;2(1):43‐53. [DOI] [PubMed] [Google Scholar]
French 2007
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006073.pub2] [DOI] [PubMed] [Google Scholar]
GRADEpro 2014
- GRADEpro. [Computer program on www.gradepro.org]. McMaster University 2014.
Harwood 1994a
- Harwood RH, Rogers A, Dickinson E, Ebrahim S. Measuring handicap: the London Handicap Scale, a new outcome measure for chronic disease. Quality in Health Care 1994;3(1):11‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harwood 1994b
- Harwood RH, Gompertz P, Ebrahim S. Handicap one year after a stroke: validity of a new scale. Journal of Neurology, Neurosurgery and Psychiatry 1994;57(7):825‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heart and Stroke 2014
- Heart and Stroke Foundation Canada. Stroke statistics. http://www.heartandstroke.com/site/c.ikIQLcMWJtE/b.3483991/k.34A8/Statistics.htm#stroke (accessed 17 July 2014).
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Section 7.7.3: Data extraction for continuous outcomes. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Altman DG, Sterne JAC. 7.7.7.1 Effect estimates and generic inverse variance meta‐analysis. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Langhammer 2010
- Langhammer B, Stanghelle JK. Exercise on a treadmill of walking outdoors? A randomized controlled trial comparing effectiveness of two walking exercise programmes late after stroke. Clinical Rehabilitation 2010;24(1):46‐54. [DOI] [PubMed] [Google Scholar]
Laver 2011
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008349.pub2] [DOI] [PubMed] [Google Scholar]
Liu 2008
- Liu J, Drutz C, Kumar R, McVicar L, Weinberger R, Brooks D, et al. Use of the six‐minute walk test poststroke: is there a practice effect?. Archives of Physical Medicine and Rehabilitation 2008;89(9):1686‐92. [DOI] [PubMed] [Google Scholar]
Lord 2004
- Lord S, McPherson K, McNaughton H, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive?. Archives of Physical Medicine and Rehabilitation 2004;85(2):234‐9. [DOI] [PubMed] [Google Scholar]
Lord 2005
- Lord SE, Rochester L. Measurement of community ambulation after stroke ‐ current status and future developments. Stroke 2005;36(7):1457‐61. [DOI] [PubMed] [Google Scholar]
MacKay‐Lyons 2005
- MacKay‐Lyons MJ, Howlett J. Exercise capacity and cardiovascular adaptations to aerobic training early after stroke. Topics in Stroke Rehabiliation 2005;12(1):31‐44. [DOI] [PubMed] [Google Scholar]
Mayo 1999
- Mayo N, Wood‐Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, et al. Disablement following stroke. Disability and Rehabilitation 1999;21(5/6):258‐68. [DOI] [PubMed] [Google Scholar]
Mehrholz 2014
- Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD002840.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2005
- Palmer AJ, Valentine WJ, Roze S, Lammert M, Spiesser J, Gabriel S. Overview of costs of stroke from published, incidence‐based studies spanning 16 industrialized countries. Current Medical Research and Opinion 2005;21(1):19‐26. [DOI] [PubMed] [Google Scholar]
Pang 2005
- Pang MY, Eng JJ, Dawson AS, McKay HA, Harris JE. A community‐based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. Journal of the American Geriatrics Society 2005;53(10):1667‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patla 1999
- Patla AE, Shumway‐Cook A. Dimensions of mobility: defining the complexity and difficulty associated with community mobility. Journal of Aging and Physical Activity 1999;7:7‐19. [Google Scholar]
Patrick 1993
- Patrick D, Erickson P. Health Status and Health Policy ‐ Quality of Life in Health Care Evaluation and Resource Allocation. Oxford: Oxford University Press, 1993. [Google Scholar]
Perry 1995
- Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995;26(6):982‐9. [DOI] [PubMed] [Google Scholar]
Powell 1995
- Powell LE, Myers AM. The Activities‐specific Balance Confidence (ABC) Scale. Journal of Gerontology 1995;50A(1):M28‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripat 2010
- Ripat JD, Redmond JD, Grabowecky BR. The Winter Walkability Project: occupational therapists' role in promoting citizen engagement. Canadian Journal of Occupational Therapy 2010;77(1):7‐14. [DOI] [PubMed] [Google Scholar]
Robinson 2011a
- Robinson CA, Shumway‐Cook A, Matsuda PN, Ciol MA. Understanding physical factors associated with participation in community ambulation following stroke. Disability and Rehabilitation 2011;33(12):1033‐42. [DOI] [PubMed] [Google Scholar]
Robinson 2011b
- Robinson CA, Shumway‐Cook A, Ciol MA, Kartin D. Participation in community walking following stroke: subjective versus objective measures and the impact of personal factors. Physical Therapy 2011;91(12):1865‐76. [DOI] [PubMed] [Google Scholar]
Salbach 2004
- Salbach NM, Mayo NE, Wood‐Dauphinee S, Henley JA, Richards CL, Côté R. A task‐oriented intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clinical Rehabilitation 2004;18(5):509‐19. [DOI] [PubMed] [Google Scholar]
Saunders 2013
- Saunders DH, Sanderson M, Brazzelli M, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD003316.pub5] [DOI] [PubMed] [Google Scholar]
Shumway‐Cook 2012
- Shumway‐Cook A, Woollacott MH. Motor Control – Translating Research into Clinical Practice. 4th Edition. Baltimore, MD: Lippincott, Williams, and Wilkins, 2012. [Google Scholar]
Sprangers 2002
- Sprangers MA. Quality‐of‐life assessment in oncology. Achievements and challenges. Acta Oncologica 2002;41(3):229‐37. [DOI] [PubMed] [Google Scholar]
States 2009
- States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006075.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2001
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and Science in Sports and Exercise 2001;33(7):1126‐41. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor D, Stretton CM, Mudge S, Garrett N. Does clinic‐measured gait speed differ from gait speed measured in the community in people with stroke?. Clinical Rehabilitation 2006;20(5):438‐44. [DOI] [PubMed] [Google Scholar]
van de Port 2008
- Port IG, Kwakkel G, Lindeman E. Community ambulation in patients with chronic stroke: how is it related to gait speed?. Journal of Rehabilitation Medicine 2008;40(1):23‐7. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐Item Short‐Form Health Survey (SF‐36) I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wevers 2011
- Wevers LEG, Kwakkel G, Port IGL. Is outdoor use of the six‐minute walk test with a global positioning system in stroke patients' own neighbourhoods reproducible and valid?. Journal of Rehabilitation Medicine 2011;43(11):1027‐31. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization, Truelson T, Begg S, Mathers C. The Global Burden of Cerebrovascular Disease 2000. www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf (accessed 5 July 2012):1‐2.
WHO 2001
- World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization, 2001. [Google Scholar]
WHO 2004
- World Health Organization, Mackay J, Mensch G (editors). The Atlas of Heart Disease and Stroke 2004. www.who.int/cardiovascular_diseases/resources/atlas/en (accessed 5 July 2012).
WHO 2008
- World Health Organization. The Global Burden of Disease 2004 Update. www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_part3.pdf (accessed 5 July 2012).
Wolf 1999
- Wolf SL, Catlin PA, Gage K, Gurucharri K, Robertson R, Stephen K. Establishing the reliability and validity of measurements of walking time using the Emory Functional Ambulation Profile. Physical Therapy 1999;79(12):1122‐33. [PubMed] [Google Scholar]
Wood‐Dauphinee 1988
- Wood‐Dauphinee SL, Opzoomer MA, Williams JI, Marchand B, Spitzer WO. Assessment of global function: the Reintegration to Normal Living Index. Archives of Physical Medicine and Rehabiliation 1988;69(8):583‐90. [PubMed] [Google Scholar]
References to other published versions of this review
Barclay‐Goddard 2012b
- Barclay‐Goddard RE, Stevenson TJ, Poluha W, Ripat J, Nett C. Interventions for improving community ambulation in individuals with stroke. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010200] [DOI] [PMC free article] [PubMed] [Google Scholar]


