Abstract
Background
Disease‐related malnutrition has been reported in 10% to 55% of people in hospital and the community. Dietary advice encouraging the use of energy‐ and nutrient‐rich foods rather than oral nutritional supplements has been suggested as the initial approach for managing disease‐related malnutrition.
Objectives
To examine evidence that dietary advice in adults with disease‐related malnutrition improves survival, weight and anthropometry; to estimate the size of any additional effect of nutritional supplements combined with dietary advice and to compare the effects of dietary advice with oral nutritional supplements.
Search methods
Relevant publications were identified from comprehensive electronic database searches and handsearching.
Last search: 14 February 2010.
Selection criteria
Randomised controlled trials of dietary advice with or without oral nutritional supplements in people with disease‐related malnutrition in any health‐care setting compared with no advice, oral nutritional supplements or dietary advice given alone.
Data collection and analysis
Two authors independently assessed trial eligibility, risk of bias and extracted data.
Main results
Forty‐five studies (3186 participants) met the inclusion criteria; (dietary advice compared with: no advice (1053 participants); with oral nutritional supplements (332 participants); with dietary advice and oral nutritional supplements (731 participants); and dietary advice plus oral nutritional supplements compared with no additional intervention (1070 participants). Follow‐up ranged from 18 days to 24 months. No comparison showed a significant difference between groups for mortality or morbidity. There was a significant change in weight found between groups when comparing dietary advice to no advice for interventions lasting greater than 12 months, mean difference 3.75 kg (95% confidence interval 0.97 to 6.53), and when all studies were combined, mean difference 1.47 kg (95% confidence interval 0.32 to 2.61) although there was significant heterogeneity in the combined analysis (I2 = 90%). Similar improvements in weight were found for the comparison of dietary advice with nutritional supplements if required versus no advice, mean difference 2.20 kg (95% confidence interval 1.16 to 3.25). Dietary advice compared with no advice was also associated with significantly improved mid‐arm muscle circumference when all studies were combined, but with moderate heterogeneity, mean difference 0.81 mm (95% confidence interval 0.31 to 1.31). Dietary advice given with nutritional supplements compared with dietary advice alone resulted in improvements in: mid‐arm muscle circumference, mean difference ‐0.89 mm (95% confidence interval ‐1.35 to ‐0.43); triceps skinfold thickness, mean difference ‐1.22 mm (95% confidence interval ‐2.34 to ‐0.09); and grip strength, mean difference ‐1.67 kg (95% confidence interval ‐2.96 to ‐0.37), although the effects on triceps skinfold thickness and grip strength were heterogeneous. Dietary advice with supplements if required resulted in a significant increase in triceps skinfold thickness compared with no advice, mean difference 0.40 mm (95% confidence interval 0.10 to 0.70), although these results are from a single trial with only 29 participants.
Authors' conclusions
Evidence of variable quality suggests that dietary advice with or without oral nutritional supplements may improve weight, body composition and grip strength. We found no evidence of benefit of dietary advice or oral nutritional supplements given alone or in combination on survival. Studies addressing the impact of nutritional interventions on nutritional, functional and patient‐centred outcomes are needed.
Plain language summary
Advice on diet for malnutrition as a result of disease in adults
Ill people often have a poor appetite or feel sick due to treatments and eat less than usual. If this reduced food intake is prolonged, it can cause weight loss, malnutrition and death. Healthcare professionals may offer advice to encourage good eating habits of high‐protein and high‐energy foods so that weight can be gained and the person's nutritional status improved. Oral nutritional supplements are commonly offered with or without advice on increasing food intake. Forty‐five studies with a total of 3186 people are included in this review in four different comparisons: dietary advice to no advice; to oral nutritional supplements; to dietary advice plus oral nutritional supplements; and to dietary advice and nutritional supplements given together compared with no additional help. Follow‐up ranged from 18 days to 24 months. There are some significant results for change in weight, muscle bulk and strength suggesting that nutritional intervention is beneficial although for some comparisons there are big differences between the studies. The authors conclude that nutritional intervention appears to be more effective than no help at improving weight, muscle bulk and strength. More research is needed to work out the best ways to help people who are losing weight because of illness in order to improve their clinical outcomes and quality of life.
Background
Description of the condition
Disease is frequently associated with reduced food intake which, if prolonged, may result in weight loss and malnutrition. Malnutrition is a potentially serious complication of disease, which is associated with increased morbidity, mortality and increased length of stay in hospital (Kubrak 2007; McWhirter 1994; Naber 1997; Norman 2008a). Malnutrition may occur as a consequence of disease or result from a range of other physiological and social conditions and act as a co‐factor in the development of ill health. Clinically significant malnutrition consists of nutritional deficits that have serious adverse effects on the treatment and outcome of disease (Jensen 2010). In practice, disease‐related malnutrition varies along a spectrum from mild to severe. The difficulties in defining malnutrition are reflected to some extent in the variation in reported prevalence which has varied from 9% to 55% (Braunschweig 1999; Hanger 1999; Kubrak 2007; McWhirter 1994; Norman 2008a; Peake 1998a; Prieto 1996; Watson 1998; Weekes 1998).
It is likely that a substantial proportion of disease‐related malnutrition occurs and is managed in a community setting. Five to ten per cent of elderly people are malnourished (Guigoz 1997; McCormack 1997). In the UK, the prevalence of malnutrition in people with cancer, chronic diseases and after major surgery living in the community under the care of a General Practitioner has been reported to be around 10% (Edington 1996; Edington 1997). The Nutrition Screening Week carried out by the British Association for Enteral and Parenteral Nutrition (BAPEN) in 2008 demonstrated that malnutrition was present in almost one in three patients admitted to hospital, just over one in three patients admitted to care homes and one in five patients admitted to mental health units (Elia 2009). The majority of individuals admitted to healthcare facilities are admitted from their own home and it has been estimated that more than three million people in the UK are malnourished or at risk of malnutrition at any one time and that the majority of these (93%) are living at home (Elia 2009). Although malnutrition is present in patients from all disease backgrounds, all ages and in all healthcare settings, older patients are more likely to be malnourished than younger patients. Patients over the age of 80 have a five times higher prevalence of malnutrition than those under 50 years old (Age Concern 2006). Overall it has been estimated that malnutrition affects up to three million people in the UK and costs up to £13 billion a year (BAPEN 2009).
The management of disease‐related malnutrition in areas of food security is likely to be different from its management in poorer parts of the world. The focus of this review is the management of disease‐related malnutrition in 'Western' populations where food insecurity is much less likely to be an issue for sectors of the population. The term malnutrition is used throughout the review, it is intended to refer to undernutrition and not overnutrition or obesity.
Description of the intervention
In spite of the potentially adverse consequences of malnutrition it remains largely unrecognised (Lennard‐Jones 1992; McWhirter 1994). There are no internationally accepted protocols for nutritional intervention in the management of disease‐related malnutrition. People who are identified as malnourished in hospital and in the community may be considered for referral to a dietitian. In routine clinical practice the poor nutritional status of many patients is not recognised and many do not receive any advice (McWhirter 1994; Peake 1998a; Volkert 2010). Dietitians are uniquely qualified to provide nutritional intervention in the form of diet instruction and intensive nutritional support, but there is no theoretical reason to believe that other health professionals could not give effective dietary advice. The provision of dietary advice is a core dietetic skill, but it is not known whether it is effective at increasing nutrient intake and weight or influencing function and outcome. There are a range of dietetic strategies that may be used to increase weight in a malnourished individual including:
advice to increase food intake;
advice to modify food constituents to increase the energy density;
the provision of oral nutritional supplements without dietary advice;
a combination of advice to increase to food intake and provision of oral nutritional supplements.
Oral nutritional supplements are usually nutritionally complete, available on prescription and easy to use. However, compliance may be influenced by the fact that they are frequently sweet‐tasting drinks which may not be taken consistently due to monotony. A number of studies highlighted problems with the use of and the monitoring of people taking nutritional supplements (Bruce 2003; Gosney 2003; Keele 1997; Munro 1998; Peake 1998b).
Why it is important to do this review
In the UK, Department of Health expenditure on total oral nutrition is rising rapidly. In 2009/10 expenditure on oral nutrition accounted for approximately £98 million, an increase of £9 million from 2008/9 (London Procurement Programme 2010). Increased awareness of nutrition and active marketing by manufacturers may have contributed to the increased use of nutritional supplements. Additional or increased food intake resulting from targeted dietary advice to increase nutritional intake and weight has potential advantages in that it offers greater variety, can be tailored to individual eating habits and additional costs are not met by the health services, although people who are unwell may have some difficulties with shopping and the preparation of food. The increasing costs of oral nutritional supplements in London have resulted in enhanced scrutiny of prescribing practices and the encouragement of a "Food First" policy in some areas (London Procurement Programme 2010). There is limited evidence to support the hypothesis that food‐based interventions and oral nutritional supplements have equal efficacy in managing disease‐related malnutrition.
A systematic review of protein energy supplementation in adults which included studies of oral supplementation, modification of food constituents to increase energy density and studies of enteral feeding, concluded that weight and nutritional indices of adults may be improved by routine nutritional supplementation (Potter 1998). Nutritional supplementation was associated with a non‐significant trend towards reduction in mortality. The authors acknowledged that there remain uncertainties about whether supplements in routine care can improve outcomes. More recently, systematic reviews of oral nutritional supplements in the management of weight loss in adults across a range of clinical conditions have concluded that oral nutritional supplementation is associated with significant reductions in mortality and rates of complications in individual clinical conditions (Koretz 2007) and also when all clinical conditions are combined (Stratton 2003). This area has recently been scrutinized by the UK's National Institute for Clinical Excellence, who have updated the meta‐analysis of oral nutritional supplements in the management of weight loss and demonstrated that the use of nutritional supplements in the management of weight loss is associated with significant reductions in mortality, improvements in length of stay, reduced rates of complications and weight gain across a range of clinical conditions (NICE 2006). A cross‐over study in 36 malnourished elderly people over six weeks demonstrated that food enrichment to increase energy density resulted in significant increases in energy intake, but only small gains in weight (which were not sustained throughout the study) and no functional improvements (Olin 1996). The British Dietetic Association recommend that improving nutritional intake via ordinary foods and beverages is the first step in the process of providing nutritional support and that nutritional supplements are a second step in the process which may be used for some people (Manual of Dietetics). The evidence base for oral nutritional supplements has been extensively reviewed whereas that relating to dietary advice given with or without nutritional supplements has received relatively little attention. It may be possible to increase oral nutritional intake in a number of different ways and it is important to clarify the role and efficacy of each method as the service, staffing and financial implications differ.
Objectives
To examine the effects of dietary advice given by a dietitian or other healthcare professional to adults at nutritional risk or with disease‐related malnutrition compared with:
no advice;
the prescription of oral nutritional supplements;
dietary advice and oral nutritional supplements.
An additional objective was added during the 2004 version of this review, to examine the effects of dietary advice given with oral nutritional supplements if required compared with no advice and no prescription of oral nutritional supplements.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐randomised controlled trials.
Types of participants
Adults over 16 years of age with disease‐related malnutrition or described as at nutritional risk by the author or judged to be at nutritional risk by the review authors due to their clinical condition or clinical treatment or both. Studies conducted in all healthcare settings were considered.
Studies carried out in pregnant women or people with eating disorders and in conditions of food insufficiency were excluded.
Types of interventions
Dietary advice was defined as instruction in modification of food intake given with the aim of improving nutritional intake by a dietitian or other healthcare professional.
dietary advice compared with no advice (usual diet);
dietary advice compared with a prescription of an oral nutritional supplement, defined as a whole protein enteral food supplement which is marketed as a clinical product for the management of disease‐related malnutrition and taken for any period of time;
dietary advice compared with dietary advice plus an oral nutritional supplement;
dietary advice plus supplements if required compared with no advice and no supplements (usual diet);
The second comparison includes studies that examined the efficacy of the two different strategies.
The third comparison includes studies that aimed to explore whether there was additional benefit to giving nutritional supplements with dietary advice.
The fourth comparison was added post hoc as a result of an additional group of studies identified during searching and study identification for the 2004 update. These studies were considered relevant to this review as they examine dietary advice compared with no advice, but the dietary advice includes information on using oral calorie supplements if considered necessary. This style of providing dietary advice most closely reflects how dietary advice is given in practice.
Studies of elemental and semi‐elemental supplements, where the constituents are present in their simplest form, were excluded. These products are used primarily in the management of malabsorption.
Types of outcome measures
Primary outcomes
Mortality
Morbidity (assessed by risk of hospital admission or re‐admission and length of hospital stay)
Measures of nutritional status (such as change in weight, triceps skinfold thickness and mid‐arm muscle circumference)
Secondary outcomes
Nutritional intake before and after the intervention
Measures of clinical function (e.g. immune function, cardiac function, respiratory function and other indices of nutritional status)
Quality of life (QoL)
Cost
Search methods for identification of studies
Electronic searches
All publications describing RCTs of dietary advice versus no advice or oral nutritional supplements were identified from electronic searching of the following databases:
Cochrane Central Register of Controlled Trials (Issue 2, 2010);
Ovid MEDLINE 1950 to 14 February 2010;
Ovid EMBASE 1980 to 14 February 2010;
Ebsco CINAHL from 1981 to 14 February 2010;
National Cancer Institute CancerLit from 1999 to 30 June 2005;
Ovid AMED from 1999 to 30 June 2005;
ISI Web of Science 01 January 2005 to 14 February 2010;
Reed Elsevier SCOPUS 01 January 2005 to 14 February 2010.
Information from conference proceedings, dissertations and theses, reports and information leaflets were sought by searching ERIC 1992 to 1998 and Dissertation Abstracts 1861 to July 2000. Additional studies were identified from electronic searches carried out by the National Collaborating Centre for Acute Care undertaken in the production of a guideline on nutrition support in adults (NICE 2006). The searchs conducted for the previous version of the review (up to 2005) and the most recent searches are shown in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4). The Cochrane Central Register of Controlled Trials (Clinical Trials) was searched using the search strategy detailed in the appendices without restriction to the title field or search element C (Appendix 1).
In addition, relevant studies were identified from the Group's Trials Registers using the terms nutrition AND supplements OR diet.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (Clinical Trials) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journal ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Group's Trials Registers: 08 April 2010.
Searching other resources
The bibliographic references of all retrieved studies and reviews were assessed for additional reports of studies.
Unpublished work has been sought by contacting experts in clinical nutrition and the membership of the British Dietetic Association in 1999. The manufacturers of oral nutritional supplements were contacted for information on additional studies in 1999. The group of dietitians conducting handsearching of nutrition‐based journals to identify RCTs for inclusion in The Cochrane Library, were contacted in 1999 before undertaking any additional handsearching.
No additional handsearching has been undertaken for this update (August 2011).
Data collection and analysis
Selection of studies
One author (CB) reviewed the titles and abstracts from each search on screen. Until the update in 2007, two authors (CB, TP) obtained any potentially relevant studies identified and assessed these independently against the inclusion criteria. They resolved their differences by discussion and where necessary by consultation with a third author (SL). For the 2007 update and thereafter, two authors (CB, EW) carried out the study selection.
Data extraction and management
Both authors (CB, TP) independently extracted data from all papers obtained. They resolved their differences by discussion and where necessary by consultation with a third author (SL). For the 2007 update and thereafter, two authors (CB, EW) carried out the data extraction as described above.
We assessed data from inclusion to the end of intervention at the following time‐points: up to 3 months; 4 to 6 months; 7 to 12 months and over 12 months.
For data to be entered into a meta‐analysis it is necessary to have sufficient information for both the intervention and comparison groups to derive a mean change with standard deviation (SD) for continuous variables (weight, energy intake etc) or the numbers experiencing the event of interest and the total number in the group for dichotomous variables (death, hospital admissions). These data have either been available from the paper or the review authors have obtained these from the study investigators where possible. Unfortunately for a number of outcomes it has not been possible to obtain data in a format that can be entered into a meta‐analysis. The review authors performed the calculations outlined below to obtain the data they required.
They calculated the SD of the change for mean data from the paper by Rogers from the P values in order to allow analysis of the data in the review (Rogers 1992).
They obtained data on weight change from the paper by Arnold by reading mean changes off the graph, calculating the per cent weight change in pounds (baseline data is given in the text) and then converting to kilograms. They assumed that the error bars on the graph are standard errors (SE). The SD of the change has been derived from the data (Arnold 1989).
The authors derived the SD of the change in energy intake from the paper by Murphy and change in weight from the paper by Sharma from the data presented in the paper using the formula:
t = change in energy intake/SE (change in energy intake)
and calculated the SEs from the P values given in the paper (Murphy 1992; Sharma 2002).
Assessment of risk of bias in included studies
In the original review and updates up until 2007, the two authors (CB, TP) independently assessed the methodological quality of each study according to criteria described by Schulz (Schulz 1995). This assessment included an examination of the method of randomisation, whether the study was blinded and whether the number of participants lost to follow‐up or excluded from the study was recorded.
From the 2010 update and thereafter, two authors (CB, EW) carried out an assessment of the risk of bias as described in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); the authors recorded the results of this assessment in the risk of bias tables. The potential biases assessed were from:
sequence generation;
allocation;
blinding;
incomplete outcome data;
selective reporting;
other potential sources of bias.
Measures of treatment effect
For continuous outcomes, such as change in weight, the authors combined the data across studies using a mean difference (MD) and 95% confidence intervals (CIs) (Review Manager 2008). When different measurement scales were used, then they gave consideration as to whether a meaningful combined analysis was possible, for example, by using standardised mean difference (SMD).
For binary outcomes, such as mortality, the authors combined the data from the studies using risk ratios (RR) and 95% CIs.
Unit of analysis issues
Where studies with non‐standard designs such as cross‐over trials and cluster‐randomised trials are identified, the authors plan to seek advice from a specialist statistician on analysis.
Dealing with missing data
In order to allow an intention‐to‐treat analysis, the authors sought data on the number of participants, by allocated treatment group for each outcome, irrespective of compliance and whether or not the participant was later thought to be ineligible.
Where relevant data were not presented in the published trial reports, we contacted trial investigators for these data.
Where data were available on baseline and follow‐up measurements, mean change was calculated and SDs for mean change were imputed using a correlation coefficient of 0.8 assuming there was a strong correlation between baseline and follow‐up measurements (Higgins 2011).
Assessment of heterogeneity
The authors examined differences between the results of the studies for heterogeneity using the chi‐squared tests, by inspecting the results of the meta‐analysis and by using the I2 statistic (Higgins 2003). The authors used a P value of less than 0.1 rather than less than 0.05 as evidence of statistical heterogeneity. The I2 statistic describes the percentage of total variation across studies that are due to heterogeneity rather than by chance (Higgins 2003). The values of I2 lie between 0% and 100%, and a simplified categorization of heterogeneity that we plan to use is of low (I2 value of less than 33%), moderate (I2 value of 34 to 66%), and high (I2 value 67 % or more) (Higgins 2003).
Assessment of reporting biases
The authors examined studies to ensure that all the outcome variables stated in the 'Methods' section were presented in the 'Results' section of the published reports.
Data synthesis
Where studies addressed sufficiently similar participants, interventions or outcomes, and the authors did not consider heterogeneity between studies to be significant, then they performed a fixed‐effect analysis using the Mantel‐Haenszel method. Where moderate or high heterogeneity (I2 greater than 33%) existed between the studies, the authors investigated this and performed a random‐effects meta‐analysis.
Subgroup analysis and investigation of heterogeneity
In order to investigate any heterogeneity, when we are able to include sufficient studies in this review, we plan to conduct subgroup analyses based on clinical judgement on the factors likely to account for differences in outcome within and between groups as follows:
underlying clinical condition (e.g. cancer, lung disease, gastrointestinal disease);
age (under 65 years and over 65 years);
nutritional status at inclusion (percentage of malnourished participants versus participants at risk of malnutrition);
study setting (hospital versus community and mixed).
In the current version of this review, we did not conduct any subgroup analyses due to a lack of sufficient data.
Sensitivity analysis
When we are able to combine a sufficient number of studies (10 studies or more) (Higgins 2011), we plan to test the robustness of our results based on the risk of bias of the studies, e.g. according to rigour of randomisation method or randomised versus quasi‐randomised controlled trials. In this review, no sensitivity analyses were conducted due to a lack of data.
Results
Description of studies
Results of the search
The searches conducted to 2010 identified 141 studies, of which 126 were identified by electronic searches and 15 from other searching (mainly reference lists of reviews and included articles). Ninety‐three studies were excluded and at this update 45 studies (50 comparisons), including 3186 randomised participants, fulfilled the inclusion criteria for this review (of which 12 studies are new at this 2011 update).
Three studies are awaiting classification (Studies awaiting classification). The study by Penalva is in Spanish and is awaiting translation (Penalva 2009). The study by Shatenstein is a case report of outcomes for two participants in a larger randomised controlled trial, the authors have been contacted to determine whether data on all participants are available (Shatenstein 2008). The study by Magare is currently unavailable as the journal web site is not functioning (Margare 2002).
Additional data on outcomes of interest and on aspects of study quality have been sought from all 45 authors and replies obtained from 36 authors. For eight of the studies the authors were unable to provide the data and information requested (Berneis 2000; Evans 1987; Jensen 1997; Kendell 1982; Murphy 1992; Olejko 1984; Ovesen 1993; Sharma 2002). No replies were received from the authors of a further eight studies (Arnold 1989; Chandra 1985; Dixon 1984; Macia 1991; Moloney 1983; Rabeneck 1998; Rogers 1992; Wilson 2001).
Included studies
Please also see the additional tables which provide summaries of additional clinical outcomes (Table 5), additional functional outcomes (Table 6) and QoL assessments (Table 7) for all included studies across all interventions.
1. Summary of additional clinical outcomes reported in included studies.
| Study | Clinical measures (generic) | Clinical measures (disease specific) |
| Dietary advice versus no advice | ||
| Baldwin 2008 | ||
| Campbell 2008 | ||
| Dixon 1984 | ||
| Imes 1988 | Crohn's Disease Activity Index Need for medication Need for surgery Number of work days lost due to Crohn's |
|
| Macia 1991 | Clinical observation of symptoms Days of suspended treatment |
|
| Manguso 2005 | Disease severity (Childs Score) | |
| Ollenschlager 1992 | No. days with temperature >38.5 C | Number of complete remissions Clinical symptoms LAS |
| Ravasco 2005a | Symptom‐induced morbidity | |
| Ravasco 2005b | Symptom‐induced morbidity | |
| Rydwik 2008 | ||
| Weekes 2009 | Need for medication | |
| Wong 2004 | ||
| Dietary advice plus supplements versus no advice | ||
| Berneis 2000 | TNF R55, TNF R75, ILR2 | CD4 count |
| Chandra 1985 | ||
| Evans 1987 | Tumour response to chemotherapy | |
| Forli 2001 | ||
| Ganzoni 1994 | ||
| Hampson 2003 | ||
| Isenring 2004 | ||
| Jensen 1997 | ||
| Lovik 1996 | ||
| Moloney 1983 | ||
| Ovesen 1993 | Tumour response to chemotherapy | |
| Persson 2002 | ||
| Persson 2007 | ||
| Rogers 1992 | ||
| Dietary advice versus supplements | ||
| Baldwin 2008 | ||
| Gray‐Donald 1995 | Number of falls | |
| Kalnins 2005 | Faecal balance studies | |
| Ravasco 2005a (h&n) | Symptom‐induced morbidity | |
| Ravasco 2005b | Symptom‐induced morbidity | |
| Schwenk 1999 | ||
| Singh 2008 | Abdominal pain score (not validated) Faecal fat Endocrine and exocrine function |
|
| Dietary advice versus dietary advice and supplements | ||
| Arnold 1989 | Tumour response Treatment interruptions Radiation side effects |
|
| Baldwin 2008 | ||
| Beattie 2000 | Need for medication Number of wound and chest infections |
|
| de Luis 2003 | Viral load, CD4 | |
| Dixon 1984 | ||
| Fuenzalida 1990 | Skin antigen testing Lymphocyte count |
|
| Gonzalez‐Espinoza 2005 | Number of episodes of peritonitis | |
| Kendell 1982 | ||
| McCarthy 1999 | ||
| Murphy 1992 | ||
| Norman 2008b | Number of prescribed drugs on discharge | |
| Olejko 1984 | ||
| Paton 2004 | ||
| Rabeneck 1998 | ||
| Sharma 2002 | Self‐reported adverse effects | |
| Wilson 2001 | Time to nutritional repletion Number of days spent in hospital |
CD4: (cluster differentiation 4) cells of T‐mediated immune system h&n: head and neck ILR2: interlukin R2 LAS: lymphadenopathy syndrome TNF R55: Tumour necrosis factor R55 TNF R75: Tumour necrosis factor R75
2. Summary of additional functional outcomes reported in included studies.
| Study | Functional measures (physical) | Functional measures (status) | notes |
| Dietary advice versus no advice | |||
| Baldwin 2008 | |||
| Campbell 2008 | |||
| Dixon 1984 | Karnofsky scale | Pre‐ and post‐intervention (0 and 4 months) | |
| Imes 1988 | |||
| Macia 1991 | |||
| Manguso 2005 | |||
| Ollenschlager 1992 | |||
| Ravasco 2005a | |||
| Ravasco 2005b | |||
| Rydwik 2008 | Timed up and go Number of step‐ups in 30 seconds Walking speed over 10 m Modified figure of 8 test |
Functional independence measure Instrumental activities measure |
Between and within group differences in domain scores |
| Weekes 2009 | Respiratory muscle function (Pimax, Pe max) Respiratory function (FEV1 & FVC) |
ADL score Dyspnoea score |
|
| Wong 2004 | |||
| Dietary advice plus supplements versus no advice | |||
| Berneis 2000 | |||
| Chandra 1985 | |||
| Evans 1987 | |||
| Forli 2001 | |||
| Ganzoni 1994 | 6 minute walking distance | ||
| Hampson 2003 | |||
| Isenring 2004 | |||
| Jensen 1997 | Respiratory function (FEV1 & FVC) | Ordinal fatigue scale Lambert disability screening questionnaire |
Mean scores at baseline and 4 months |
| Lovik 1996 | |||
| Moloney 1983 | |||
| Ovesen 1993 | |||
| Persson 2002 | |||
| Persson 2007 | ADL (Katz) Cognitive function (MMSE) |
||
| Rogers 1992 | Respiratory muscle function (Pimax, Pe max) 12 minute walking distance |
Perceived dyspnoea (Borg) | |
| Dietary advice versus supplements | |||
| Baldwin 2008 | |||
| Gray‐Donald 1995 | |||
| Kalnins 2005 | Respiratory function (FEV1) | ||
| Ravasco 2005a (head & neck) | |||
| Ravasco 2005b | |||
| Schwenk 1999 | |||
| Singh 2008 | |||
| Dietary advice versus dietary advice and supplements | |||
| Arnold 1989 | |||
| Baldwin 2008 | |||
| Beattie 2000 | |||
| de Luis 2003 | |||
| Dixon 1984 | Karnofsky scale | Pre‐ and post‐intervention (0 and 4 months) | |
| Fuenzalida 1990 | Respiratory function (FEV1 & FVC) | ||
| Gonzalez‐Espinoza 2005 | |||
| Kendell 1982 | |||
| McCarthy 1999 | |||
| Murphy 1992 | |||
| Norman 2008b | Respiratory function (PEF) | ||
| Olejko 1984 | |||
| Paton 2004 | Sit to stand test | ||
| Rabeneck 1998 | Cognitive function (Buschke selective reminding test) | ||
| Sharma 2002 |
ADL: activities of daily living FEV1: forced expiratory volume at one second FVC: forced expiratory capacity MMSE: Mini mental state examination Pe max: maximal expiratory mouth pressure PEF: peak expiratory flow Pimax: maximal inspiratory mouth pressure
3. Summary of quality of life assessments made in included studies.
| Study | QOL instrument | notes |
| Dietary advice versus no advice | ||
| Baldwin 2008 | EORTC FAACT |
Mean change from baseline to 6 and 26 weeks |
| Campbell 2008 | ||
| Dixon 1984 | ||
| Imes 1988 | ||
| Macia 1991 | ||
| Manguso 2005 | ||
| Ollenschlager 1992 | ||
| Ravasco 2005a | EORTC | Mean change from baseline to 12 weeks |
| Ravasco 2005b | EORTC | Mean change from baseline to 12 weeks |
| Rydwik 2008 | ||
| Weekes 2009 | SF‐36 SGRQ |
Mean change from baseline to 6 and 12 months |
| Wong 2004 | ||
| Dietary advice plus supplements versus no advice | ||
| Berneis 2000 | Medical outcomes study instrument | Summary scores at baseline and 12 weeks |
| Chandra 1985 | ||
| Evans 1987 | ||
| Forli 2001 | ||
| Ganzoni 1994 | ||
| Hampson 2003 | ||
| Isenring 2004 | EORTC | Mean change from baseline to 12 weeks |
| Jensen 1997 | QOL index | Means values at baseline and 4 months |
| Lovik 1996 | ||
| Moloney 1983 | ||
| Ovesen 1993 | QOL index (modified) | Mean scores at baseline and 3 and 5 months |
| Persson 2002 | EORTC | Mean scores at baseline and 24 months |
| Persson 2007 | SF‐36 | |
| Rogers 1992 | Sickness impact profile | |
| Dietary advice versus supplements | ||
| Baldwin 2008 | EORTC FAACT |
Mean change from baseline to 6 and 26 weeks |
| Gray‐Donald 1995 | General self‐perceived health question General well‐being schedule |
Mean scores for both groups at baseline and 12 weeks |
| Kalnins 2005 | ||
| Ravasco 2005a (h&n) | EORTC | Mean change from baseline to 12 weeks |
| Ravasco 2005b | EORTC | Mean change from baseline to 12 weeks |
| Schwenk 1999 | ||
| Singh 2008 | ||
| Dietary advice versus dietary advice and supplements | ||
| Arnold 1989 | ||
| Baldwin 2008 | EORTC FAACT |
Mean change from baseline to 6 and 26 weeks |
| Beattie 2000 | SF‐36 | Summary and mean change scores physical and mental scores at baseline and final assessment |
| de Luis 2003 | ||
| Dixon 1984 | ||
| Fuenzalida 1990 | ||
| Gonzalez‐Espinoza 2005 | ||
| Kendell 1982 | ||
| McCarthy 1999 | ||
| Murphy 1992 | ||
| Norman 2008b | SF‐36 | Mean scores for all domains at baseline and 3 months |
| Olejko 1984 | ||
| Paton 2004 | SF‐36 (modified) | Summary and change scores for all domains |
| Rabeneck 1998 | 30 item QOL instrument (not validated) | |
| Sharma 2002 |
EORTC: European organisation for research and treatment of cancer FAACT: functional assessment anorexia‐cancer therapy QOL: quality of life SF‐36: short‐form SGRQ: St George respiratory questionnaire
Three studies included comparisons in two parts of the review (Dixon 1984; Ravasco 2005a; Ravasco 2005b) and one study included comparisons in three parts of the review (Baldwin 2008). The studies have been carried out in participants from a variety of clinical backgrounds. The length of intervention varied between studies; 28 (62%) of the 45 included studies presented interventions that were given for up to three months, 11 (24%) studies gave the intervention for up to six months and two (4%) of studies gave an active intervention for seven months or longer. In three of the studies the length of intervention was unclear (Dixon 1984; Macia 1991; Stratton 2007). The study by Persson appears to describe an intervention that lasts for up to two years (Persson 2002). Data at 3, 6, 12 and 24 months have been used in this review.
Nine of the included studies provided data on additional follow‐up beyond the intervention for some outcomes for between six months and five years (Arnold 1989; Baldwin 2008; Evans 1987; Kalnins 2005; Moloney 1983; Paton 2004; Rydwik 2008; Weekes 2009; Wilson 2001).
Across the studies, it was not originally clear how grip strength had been measured as the units of measurement were described slightly differently. After consultation with a Professor of Applied Physiology, the authors have decided that the studies have all reported kg, with some calling it force and others kg force. We have therefore decided to present these data in the analysis with the unit of measurement denoted as kg force.
1. Dietary advice compared with no advice
Twelve studies were identified for this comparison (Baldwin 2008; Campbell 2008; Dixon 1984; Imes 1988; Macia 1991; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009; Wong 2004). Six studies were of people with cancer (Baldwin 2008; Dixon 1984; Macia 1991; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b), one was in the elderly (Rydwik 2008), one was of people with Crohn's disease (Imes 1988), one in people at risk of osteoporotic fractures (Wong 2004), one in people with chronic obstructive pulmonary disease (COPD) (Weekes 2009), one in people with liver cirrhosis (Manguso 2005) and one in people with chronic kidney disease (Campbell 2008). Data were available to enter into the analysis for all seven outcomes, although not all studies contribute data on all outcomes. Mortality data were reported in 10 studies (Baldwin 2008; Campbell 2008; Imes 1988; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009; Wong 2004). Data on the number of people admitted to hospital were available from two studies (Imes 1988; Weekes 2009), change in weight from nine studies (Baldwin 2008; Campbell 2008; Macia 1991; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009; Wong 2004) and change in energy intake from six studies (Campbell 2008; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Wong 2004). Data on mid‐arm muscle circumference (MAMC) were reported in two studies (Manguso 2005: Weekes 2009) and triceps skinfold thickness (TSF) were reported in three (Macia 1991; Manguso 2005: Weekes 2009) and grip strength from one study (Weekes 2009). For the remaining studies, the information for all outcomes was reported in a format that did not allow us to derive mean change with a SD (Dixon 1984; Imes 1988; Macia 1991; Ollenschlager 1992). Data have been obtained from authors for nine of the studies (Baldwin 2008; Campbell 2008; Imes 1988; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Weekes 2009: Wong 2004). The SDs of change in weight and TSF in one study were imputed using a correlation coefficient of 0.8 (Macia 1991).
2. Dietary advice compared with oral nutritional supplements
Eight studies were identified for this comparison (Baldwin 2008; Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stratton 2007). One study was in elderly participants (Gray‐Donald 1995), one in people with cystic fibrosis (Kalnins 2005), one in people with human immunodeficiency virus (HIV) (Schwenk 1999), three in people with cancer (Baldwin 2008; Ravasco 2005a; Ravasco 2005b), one in people with chronic pancreatitis (Singh 2008) and one in patients with fractured neck of femur (Stratton 2007). Data for mortality were available from two studies (Baldwin 2008; Gray‐Donald 1995); no deaths occurred in the other four studies (Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999). There was only one data point for numbers admitted to hospital (Schwenk 1999). Two studies provided data on MAMC and TSF (Gray‐Donald 1995; Singh 2008) and one study provided data on grip strength (Gray‐Donald 1995). Seven of eight studies contributed data on change in weight (Baldwin 2008; Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008) and six studies included data on energy intake (Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008). Additional data have been obtained from all authors. Clarification on the length of intervention and follow‐up and details of study design are awaited for the study by Stratton (Stratton 2007). The study by Kalnins includes 13 participants, of whom only five are older than 16 years of age; individual patient data have been obtained from the author for inclusion in this review (Kalnins 2005).
3. Dietary advice versus dietary advice plus oral nutritional supplements
Sixteen studies fulfilled the inclusion criteria for this comparison (Arnold 1989; Baldwin 2008; Beattie 2000; de Luis 2003; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kendell 1982; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Paton 2004; Rabeneck 1998; Sharma 2002; Wilson 2001). Four studies were in people with cancer (Arnold 1989; Baldwin 2008; Dixon 1984; McCarthy 1999), three were in surgical patients (Beattie 2000; Kendell 1982; Olejko 1984), three were in people with HIV (de Luis 2003; Murphy 1992; Rabeneck 1998), one study was in people with chronic obstructive pulmonary disease (COPD) (Fuenzalida 1990), one study in people with tuberculosis (Paton 2004), one in people with benign gastrointestinal disease (Norman 2008b) and three studies in people with renal failure (Gonzalez‐Espinoza 2005; Sharma 2002; Wilson 2001). Three studies presented data in a format that did not allow us to derive mean change with a SD (Dixon 1984; Kendell 1982; Olejko 1984). No deaths occurred in six studies (Beattie 2000; de Luis 2003; Fuenzalida 1990; McCarthy 1999; Murphy 1992; Norman 2008b). Data on weight change from the paper by Arnold have been obtained by reading mean changes off the graph, calculating the per cent weight change in pounds (baseline data is given in the text) and then converting to kg. We have assumed that the error bars on the graph are SEs. The SD of the change has been derived from the data (Arnold 1989). The SD of the change in energy intake from the paper by Murphy and change in weight from the paper by Sharma has been derived from the data presented in the paper using the formula:
t = change in energy intake/SE (change in energy intake)
and the SEs were calculated from the P values given in the paper (Murphy 1992; Sharma 2002).
The SDs of change in energy intake in one study (Murphy 1992) and TSF in another (Gonzalez‐Espinoza 2005) were imputed using a correlation coefficient of 0.8.
Data on weight and grip strength (Paton 2004), weight, mid‐upper arm circumference (MUAC), TSF and energy intake (de Luis 2003), weight, MAMC and energy intake (Gonzalez‐Espinoza 2005) and weight, MAMC and TSF (Norman 2008b) and energy intake (McCarthy 1999) have been obtained from the authors.
4. Dietary advice plus supplements, if required, compared with no advice and no supplements
Fourteen studies were identified for this comparison (Berneis 2000; Chandra 1985; Evans 1987; Forli 2001; Ganzoni 1994; Hampson 2003; Isenring 2004; Jensen 1997; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Persson 2007; Rogers 1992). Six studies were in people with cancer (Evans 1987; Isenring 2004; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002), two were in people with COPD (Ganzoni 1994; Rogers 1992), one in people undergoing lung transplantation (Forli 2001), one in people with HIV (Berneis 2000), one in elderly people with osteoporotic fractures (Hampson 2003), two in frail elderly patients (Chandra 1985; Persson 2007) and one study was in surgical patients (Jensen 1997). The studies by Evans and Foltz appear to describe the same group of participants; clarification has been sought from the authors (Evans 1987; Foltz 1987). The data in these studies and the studies by Berneis and Chandra were not in a format that allowed us to derive mean change with a SD for entry into analysis. Data were available for six outcomes, mortality (Forli 2001; Ganzoni 1994; Hampson 2003; Isenring 2004; Lovik 1996; Moloney 1983; Persson 2002; Persson 2007), weight change (Berneis 2000; Forli 2001; Hampson 2003; Isenring 2004; Lovik 1996; Persson 2002; Persson 2007; Rogers 1992), energy intake (Forli 2001; Hampson 2003; Moloney 1983), MAMC and TSF (Rogers 1992) and change in handgrip strength (Persson 2007; Rogers 1992). Additional data have been obtained from six authors (Forli 2001; Ganzoni 1994; Hampson 2003; Isenring 2004; Persson 2002; Persson 2007). The SD of the change for mean data from the paper by Rogers has been calculated from the P values (Rogers 1992).
The SDs of change in weight for one study (Berneis 2000) and energy intake in another (Moloney 1983) were imputed using a correlation coefficient of 0.8.
Additional information is awaited from one author (Jensen 1997).
Excluded studies
A total of 93 studies were excluded for the reasons detailed in the table 'Characteristics of excluded studies'. Forty‐one studies were excluded because after scrutiny the trial was not a randomised controlled trial and forty‐eight because the comparison did not meet the inclusion criteria. Four trials were excluded for other reasons such as the included patients being in normal nutritional status.
Risk of bias in included studies
In earlier versions of the review, the methodological quality of the included studies was assessed based on a method described by Schulz (Schulz 1995). In the current version of the review the risk of bias for each study has been assessed for each of the criteria below as high risk of bias, unclear risk of bias or low risk of bias as described in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Generation of the randomisation sequence and allocation concealment were assessed as low risk of bias, unclear risk of bias, or high risk of bias; blinding of outcome assessment was recorded as reported (low risk of bias), unclear (unclear risk of bias) or not reported (high risk of bias). Other sources of bias considered were the reporting of complete outcome data (accounting for all participants randomised in the study), avoidance of selective reporting of outcome variables and the inclusion of a comparison of baseline variables as well as recording information on any variables not similar at baseline. See 'Risk of bias tables' for details of individual studies (Characteristics of included studies).
Allocation
Generation of sequence
In 25 studies, the method of generation of randomisation sequence was assessed as having a low risk of bias (Baldwin 2008; Beattie 2000; Berneis 2000; Campbell 2008; de Luis 2003; Evans 1987; Forli 2001; Ganzoni 1994; Gonzalez‐Espinoza 2005; Gray‐Donald 1995; Isenring 2004; Lovik 1996; Macia 1991; Manguso 2005; McCarthy 1999; Norman 2008b; Ovesen 1993; Paton 2004; Persson 2002; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Weekes 2009; Wong 2004). Two of these studies used the coin toss as a method of randomisation (Macia 1991; McCarthy 1999).
Two studies used an inadequate method of randomisation, alternate allocation, which led to them being judged to have a high risk of bias (Kalnins 2005; Murphy 1992).
In one study the author could not recall how the sequence was generated, hence had an unclear risk of bias (Imes 1988). The remaining 16 studies did not report details of the randomisation and these studies have also been judged to have an unclear risk of bias (Arnold 1989; Chandra 1985; Dixon 1984; Fuenzalida 1990; Hampson 2003; Jensen 1997; Kendell 1982; Moloney 1983; Olejko 1984; Ollenschlager 1992; Persson 2007; Rabeneck 1998; Rogers 1992; Rydwik 2008; Sharma 2002; Wilson 2001). Details are awaited from the author for the study by Stratton and we currently judge this also to have an unclear risk of bias (Stratton 2007).
Allocation concealment
In 24 studies, allocation concealment was assessed as having a low risk of bias (Baldwin 2008; Beattie 2000; Campbell 2008; de Luis 2003; Evans 1987; Ganzoni 1994; Gonzalez‐Espinoza 2005; Gray‐Donald 1995; Hampson 2003; Imes 1988; Isenring 2004; Jensen 1997; Lovik 1996; Manguso 2005; Norman 2008b; Ovesen 1993; Paton 2004; Persson 2002; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Weekes 2009; Wong 2004).
Two studies used alternate allocation and so had a high risk of bias (Kalnins 2005; Murphy 1992).
Eighteen studies had an unclear risk of bias for allocation concealment (Arnold 1989; Berneis 2000; Chandra 1985; Dixon 1984; Forli 2001; Fuenzalida 1990; Kendell 1982; Macia 1991; McCarthy 1999; Moloney 1983; Olejko 1984; Ollenschlager 1992; Persson 2007; Rabeneck 1998; Rogers 1992; Rydwik 2008; Sharma 2002; Wilson 2001). Details are awaited from the author for the study by Stratton and we currently judge this also to have an unclear risk of bias (Stratton 2007).
Blinding
Blind assessment of all outcomes was reported in three studies or information was obtained on enquiry to the authors (Ganzoni 1994; Gonzalez‐Espinoza 2005; Singh 2008). These studies were judged to be at low risk of bias. The studies by Imes, Jensen and Macia reported blind assessment of clinical outcomes and were therefore graded as at low risk of bias for these assessments, but assessments of nutritional status were not blinded (high risk of bias) (Imes 1988; Jensen 1997; ). The studies by Forli and Gray‐Donald reported blind assessment of both clinical and functional outcomes but assessments of nutritional status were not blinded (Forli 2001; Gray‐Donald 1995). The study by Manguso reported blinded assessment of nutritional outcomes but not of clinical and functional outcomes (Manguso 2005). The studies where only some outcomes were assessed blinded to intervention groups were judged to be at low risk of bias for these outcomes but at high risk of bias for nutritional outcomes. Ten studies (Baldwin 2008; Beattie 2000; de Luis 2003; Kalnins 2005; McCarthy 1999; Murphy 1992; Persson 2007; Rabeneck 1998; Schwenk 1999; Weekes 2009) state in the text or on enquiry that outcomes were not assessed blinded to group allocation and these have been judged to be at high risk of bias. The remaining studies did not state whether outcomes were assessed blinded to assessment group and were therefore judged to have an unclear risk of bias.
Incomplete outcome data
The number of study exclusions together with reasons were reported or the information was obtained from authors in 22 out of 45 studies, which meant we judged these studies to have a low risk of bias. The amount of study exclusions varied from 7% to 56%. The number of study exclusions were not reported in three studies (Chandra 1985; Macia 1991; Moloney 1983), or were reported as a total for the study rather than for each group in three studies (Berneis 2000; Dixon 1984; Wilson 2001) and these studies have been judged to have a high risk of bias. In 16 of the remaining studies, study exclusions are reported but, with the exception of mortality, reasons for study exclusions are not given and these studies have been judged to have an unclear risk of bias (Baldwin 2008; Hampson 2003; Imes 1988; Jensen 1997; Manguso 2005; McCarthy 1999; Norman 2008b; Ovesen 1993; Paton 2004; Persson 2002; Rogers 1992; Rydwik 2008; Sharma 2002; Singh 2008; Weekes 2009; Wong 2004). Queries remain outstanding for the study by Stratton and we currently judge this to have an unclear risk of bias (Stratton 2007)
Selective reporting
Four out of 45 studies did not report all of the outcomes specified in the study methodology (Chandra 1985; Forli 2001; Rydwik 2008; Wilson 2001) and two studies make general statements about the results with no data provided (Kendell 1982; Olejko 1984). These six studies are judged to have a high risk of bias due to selective reporting. One study collected some outcome data for the intervention group only and so is judged to have a high risk of bias for these outcomes (Ollenschlager 1992). Nine studies have been judged to have an unclear risk of bias as some of the data are presented but cannot be extracted for direct entry into a meta‐analysis (Arnold 1989; Dixon 1984; Evans 1987; Ganzoni 1994; Hampson 2003; Imes 1988; Jensen 1997; Sharma 2002; Stratton 2007). In addition, in five studies some data were presented as mean (SD) at baseline and at end of intervention, therefore the mean change has been calculated and the SD imputed, these studies are judged to have an unclear risk of bias (Berneis 2000; Gonzalez‐Espinoza 2005; Macia 1991; Moloney 1983; Murphy 1992). The remaining studies have been judged to be free of selective reporting bias as the data are presented in the paper or have been obtained from the authors or have been derived (making assumptions) from data presented in the paper. Details for each paper are provided in the tables Characteristics of included studies.
Other potential sources of bias
Baseline variables were compared in 34 of 45 studies. In the original reports these may have been compared in a table or described in the text of the results or presented as characteristics of included studies. Baseline variables were similar between the groups in 21 of the 34 studies that compared data and these studies are considered to be at low risk of bias (Arnold 1989; Baldwin 2008; de Luis 2003; Dixon 1984; Evans 1987; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Isenring 2004; Lovik 1996; Manguso 2005; Ollenschlager 1992; Ovesen 1993; Paton 2004; Persson 2002; Rabeneck 1998; Rogers 1992; Rydwik 2008; Schwenk 1999; Singh 2008; Weekes 2009; Wong 2004). In the study by Norman the data on baseline characteristics are not shown but the parameters are described and there is a statement that there were no differences between groups (Norman 2008b).
In 10 studies no details of baseline characteristics were given (Berneis 2000; Chandra 1985; Ganzoni 1994; Kalnins 2005; Kendell 1982; Macia 1991; Olejko 1984; Persson 2007; Ravasco 2005a; Ravasco 2005b), these studies are judged to be at risk of bias. In the study by Sharma, the baseline characteristics are only compared for the participants who completed the study and five participants crossed over from the control group to the intervention group, there is therefore is a high risk of bias (Sharma 2002).
In 11 out of the 34 studies there were differences between some characteristics of the groups at baseline leading to a potential risk of bias (Beattie 2000; Campbell 2008; Forli 2001; Gray‐Donald 1995; Hampson 2003; Imes 1988; Jensen 1997; McCarthy 1999; Moloney 1983; Murphy 1992; Wilson 2001). In the study by Beattie the participants in the group who received advice plus supplements were significantly younger than those in the advice only group (Beattie 2000). In the study by Campbell the numbers of participants malnourished at baseline differed between groups (Campbell 2008). In the study by Forli some of the assessments of lung function differed significantly between groups (Forli 2001). In the Gray‐Donald study, reported appetite was better in the advice group than in the supplements group (Gray‐Donald 1995). In the study by Hampson there were differences in weight between the groups (Hampson 2003). In the study by Imes the participants in the group receiving no advice were younger and in better clinical condition than those in the group receiving dietary advice (Imes 1988). In the study by Jensen the participants in the no advice group were significantly older and heavier than those in the advice group (Jensen 1997). In the study by McCarthy the group receiving nutritional supplements were lighter and received a smaller amount of radiation (McCarthy 1999). In the study by Moloney the treatment group were older than the no treatment group (Moloney 1983). In the study by Murphy, the group receiving dietary advice plus nutritional supplements were 5 kg heavier at the start of the study than the group receiving dietary advice alone (Murphy 1992). In the study by Wilson the dietary counselling and supplement group were significantly older than the dietary group (Wilson 2001).
Queries remain outstanding for the study by Stratton and we currently judge this to have an unclear risk of bias (Stratton 2007).
Effects of interventions
All comparisons
Data on change in functional outcomes were limited to a small amount of data on grip strength. Data were collected on a variety of outcome measures encompassing clinical and functional status and QoL; however, because the authors used different measures to assess the outcomes or the data were reported in such a way that the data could not be analysed, it is not possible to pool the data within a meta‐analysis. The types of data collected and tools used are summarised in the Additional tables (Table 5; Table 6; Table 7).
Dietary advice compared with no advice
Twelve studies (1053 randomised participants) evaluated this comparison (Baldwin 2008; Campbell 2008; Dixon 1984; Imes 1988; Macia 1991; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009; Wong 2004), but there were no usable data from two of these studies (Dixon 1984; Macia 1991).
Primary Outcome
1. Mortality (Analysis 1.1)
Data were available from six studies where interventions lasted from zero to three months (Baldwin 2008; Campbell 2008; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008). There was no statistically significant difference in mortality between the participants who received dietary advice and those who received usual care; RR 1.35 (95% CI 0.59 to 3.08) (P = 0.47). Low heterogeneity was observed (I2 = 28%; P = 0.25).
Data were available from four studies for interventions that lasted from four to six months (Imes 1988; Ollenschlager 1992; Weekes 2009; Wong 2004). There was no statistically significant difference in mortality between the participants who received dietary advice and those who received usual care; RR 1.73 (95% CI 0.40 to 7.57) (P = 0.47). There was low heterogeneity (I2 = 1%; P = 0.32).
In the combined analysis there were data from 10 studies (Baldwin 2008; Campbell 2008; Imes 1988; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009; Wong 2004). In five studies there were no events (Imes 1988; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Wong 2004). There was no statistically significant difference in mortality between the participants who received dietary advice and those who received usual care; RR 1.43 (95% CI 0.70 to 2.94) (P = 0.32). There was no heterogeneity (I2 = 0%; P = 0.42) (Analysis 1.1).
1.1. Analysis.
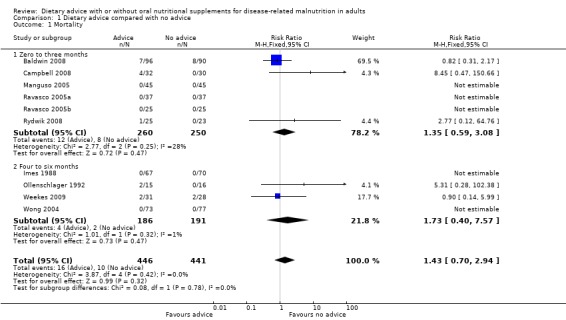
Comparison 1 Dietary advice compared with no advice, Outcome 1 Mortality.
2. Morbidity (Analysis 1.2)
Hospital admission data were available from two studies, both having interventions lasting between four and six months (Imes 1988; Weekes 2009). There was no statistically significant difference between the two groups, RR 0.89 (95% CI 0.52 to 1.50) (P = 0.65) and there was no heterogeneity (I2 = 0%; P = 0.85) (Analysis 1.2).
1.2. Analysis.
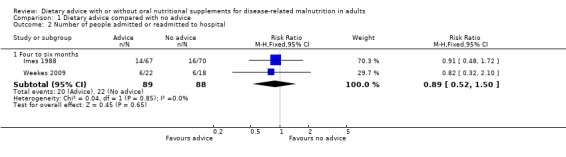
Comparison 1 Dietary advice compared with no advice, Outcome 2 Number of people admitted or readmitted to hospital.
3. Measures of nutritional status
a. Weight (Analysis 1.3)
Six studies reported data on weight change for interventions that lasted from zero to three months (Baldwin 2008; Campbell 2008; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008). Analysing these data with a random‐effects model, there was no statistically significant difference between the groups receiving dietary advice compared with routine care, MD 1.30 kg (95% CI ‐0.82 to 3.42) (P = 0.23). The heterogeneity was high (I2 = 93%; P < 0.00001). Removal of one study resulted in no significant effect on weight change between groups but reduced the heterogeneity to zero (MD) ‐0.11 kg (95% CI ‐0.66 to 0.44) (P = 0.7) (Ravasco 2005a). Two studies reported data on weight change for interventions that lasted from four to six months (Weekes 2009; Wong 2004). Again, using a random‐effects model to analyse the data, there were no statistically significant differences between groups receiving dietary advice compared with routine care MD 1.46 kg (95% CI ‐1.03 to 3.95) (P = 0.25). The heterogeneity was high, (I2 = 81%; P < 0.02).
One study reported data on weight change after 12 months of intervention in patients with cancer at three different sites, i.e. head and neck, breast and abdominal (Macia 1991). Analysing these data using a random‐effects model, there was a statistically significant benefit to receiving dietary advice compared with no advice MD 3.75 kg (95 % CI 0.97 to 6.53) (P = 0.008).
In the combined analysis nine studies contributed data to the analysis (Baldwin 2008; Campbell 2008; Macia 1991; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009; Wong 2004); although one study did not contribute estimable data in one arm (Ravasco 2005b). Participants receiving dietary advice gained more weight than participants receiving routine care, MD 1.47 kg (95% CI 0.32 to 2.61) (P = 0.01). The heterogeneity was high, (I2 = 90%; P < 0.00001) (Analysis 1.3).
1.3. Analysis.
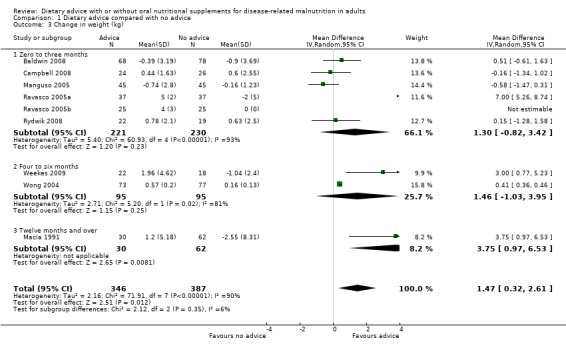
Comparison 1 Dietary advice compared with no advice, Outcome 3 Change in weight (kg).
b. Mid‐arm muscle circumference (Analysis 1.4)
Two studies contributed data on indices of body composition, i.e. mid‐arm muscle circumference (MAMC) and triceps skinfold thickness (TSF) (Manguso 2005; Weekes 2009). In the study by Manguso the intervention lasted for three months and in the study by Weekes the intervention lasted for six months. In the study by Manguso there was a significant improvement in MAMC in participants receiving dietary advice, MD 1.02 cm (95% CI 0.65 to 1.39) (P <0.00001). In the study by Weekes, there was no statistically significant difference between MAMC in groups receiving dietary advice or routine care, MD 0.50 cm (95% CI ‐0.09 to 1.09) (P = 0.09).
Combining the results of these two studies using a random‐effects analysis, there was a difference in MAMC favouring participants who received dietary advice, MD 0.81 cm (95% CI 0.31 to 1.31) (P = 0.001), moderate heterogeneity was observed (I2 = 54%; P = 0.14) (Analysis 1.4).
1.4. Analysis.
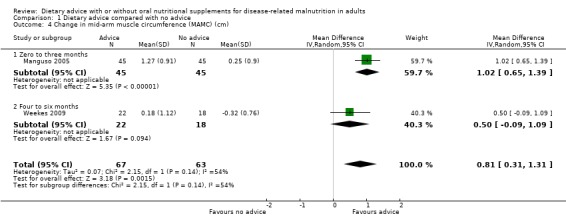
Comparison 1 Dietary advice compared with no advice, Outcome 4 Change in mid‐arm muscle circumference (MAMC) (cm).
c. Triceps skinfold thickness (TSF) (Analysis 1.5)
Three studies contributed data on TSF (Macia 1991; Manguso 2005; Weekes 2009). In the study by Manguso there was no statistically significant difference in TSF between participants receiving dietary advice or routine care at up to three months, MD ‐1.16 mm (95% CI ‐3.15 to 0.83) (P = 0.25). In the study by Weekes (four to six months), the group receiving dietary advice had a greater improvement in TSF compared with the group receiving routine care, MD 1.27 mm (95% CI ‐0.04 to 2.58) (P = 0.06). In the study by Macia, a 12‐month intervention resulted in no significant difference in TSF MD ‐0.14 mm (95 % CI ‐2.32 to 2.04) (P = 0.90).
Combining the results of these three studies using a random‐effects analysis, there was no statistically significant difference in TSF between the groups, MD 0.15 mm (95% CI ‐1.37 to 1.67) (P = 0.84), moderate heterogeneity was observed (I2 = 54%; P = 0.12) (Analysis 1.5).
1.5. Analysis.
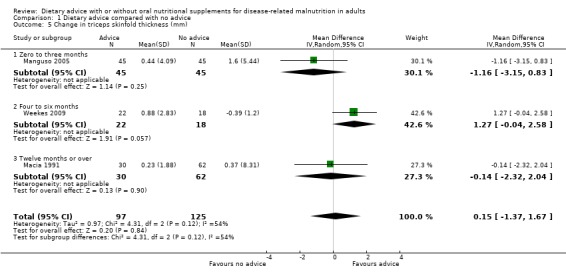
Comparison 1 Dietary advice compared with no advice, Outcome 5 Change in triceps skinfold thickness (mm).
Secondary Outcomes
1. Nutritional intake before and after the intervention (Analysis 1.6)
Seven studies reported changes in energy intake from the start to the end of intervention (Baldwin 2008; Campbell 2008; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Wong 2004). Six studies reported change in energy intake for interventions that lasted up to three months (Baldwin 2008; Campbell 2008; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008). Data were analysed using a random‐effects model; there was no statistically significant difference between those who received dietary advice and those who received routine care, MD 283.19 kcals (95% CI ‐107.18 to 673.56) (P = 0.16), the heterogeneity was high (I2 = 98%; P < 0.00001). One study measured energy intake in participants receiving dietary advice for four months and reported a significantly higher energy intake in those receiving dietary advice compared with routine care, MD 63.70 kcals (95% CI 55.29 to 72.11) (P <0.00001) (Wong 2004).
Analysis of data from all studies combined showed that participants who received dietary advice had a higher energy intake than those who received usual diet, MD 257.78 kcal/day (95% CI ‐0.74 to 516.30) (P = 0.05); however, the heterogeneity was high, (I2 = 98%; P < 0.00001) and removal of any one study or combination of studies did not reduce the heterogeneity (Analysis 1.6).
1.6. Analysis.
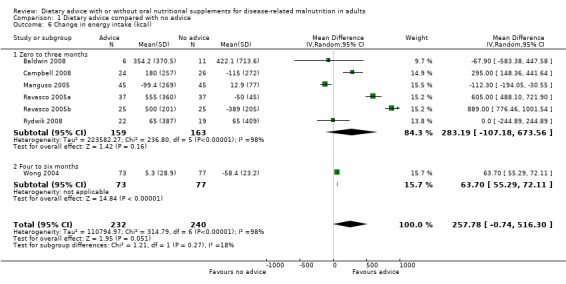
Comparison 1 Dietary advice compared with no advice, Outcome 6 Change in energy intake (kcal).
2. Measures of functional status (Analysis 1.7)
One study provided data on grip strength from baseline to the end of a six‐month intervention (Weekes 2009). No statistically significant difference was observed between the groups, MD 0.29 kg force (95% CI ‐1.58 to 2.16) (P = 0.76) (Analysis 1.7).
1.7. Analysis.

Comparison 1 Dietary advice compared with no advice, Outcome 7 Change in grip strength (kg force).
3. QoL
Four studies reported this outcome (Baldwin 2008; Ravasco 2005a; Ravasco 2005b; Weekes 2009). Data were not combined for analysis since four different QoL instruments were used and data were not reported in a way that allowed for meta‐analysis (Table 7). In three studies the European Organisation for Research and Treatment of Cancer (EORTC) questionnaire was used (Baldwin 2008; Ravasco 2005a; Ravasco 2005b). Median scores were reported for all domains at baseline, end of radiotherapy and at three months in two studies; however, data were not reported as a change from baseline (Ravasco 2005a; Ravasco 2005b). In the other study by Baldwin, score changes from baseline to 6 weeks and to 26 weeks are available for four domains (Baldwin 2008). In this study no significant differences were observed between the dietary advice and usual care groups at either 6 or 26 weeks (Baldwin 2008).
In a study of patients with COPD, two QoL instruments were used; a generic questionnaire (Short Form‐36) and the St George's Respiratory Questionnaire (SGRQ) which is a disease‐specific questionnaire (Weekes 2009). Using the SF‐36, significant differences were observed in the health change score, with the group receiving dietary advice reporting improved QoL compared with the previous year and the control group reporting poorer QoL (P = 0.003; controlling for baseline scores using ANCOVA). Significant differences between the groups were also reported in the vitality (P = 0.04), pain (P = 0.05) and general health (P = 0.05) domains at six months (controlling for baseline scores using ANCOVA). Disease‐specific QoL using the SGRQ, significant differences between the groups were reported in the activity (P = 0.01), impacts (P = 0.04) and total scores (P = 0.01), but not in the symptoms score (P = 0.50) controlling for baseline scores using ANCOVA.
4. Cost
One study has collected data on cost (Weekes 2009). The data are currently undergoing re‐analysis and will be included in the next update of the review.
Dietary advice compared with oral nutritional supplements
Eight studies (332 randomised participants) evaluated this comparison (Baldwin 2008; Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stratton 2007).
Primary outcome
1. Mortality (Analysis 2.1)
Data on mortality were available from six of the eight studies (Baldwin 2008; Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999). The interventions in all of these studies lasted from zero to three months. Only two studies reported any deaths and data were analysed using a fixed‐effect model (Baldwin 2008; Gray‐Donald 1995). There was no statistically significant difference in mortality between the two groups, RR 0.56 (95% CI 0.24 to 1.31) (P = 0.18), there was no heterogeneity (I2 0%; P = 0.60) (Analysis 2.1).
2.1. Analysis.
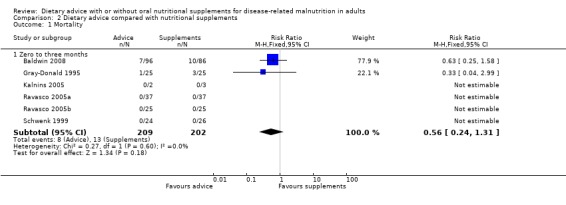
Comparison 2 Dietary advice compared with nutritional supplements, Outcome 1 Mortality.
2. Morbidity (Analysis 2.2)
Data were available from only one study (50 participants) on numbers admitted to hospital (Schwenk 1999). The difference between groups was not significant, RR 0.36 (95% CI 0.04 to 3.24) (P = 0.36) (Analysis 2.2).
2.2. Analysis.
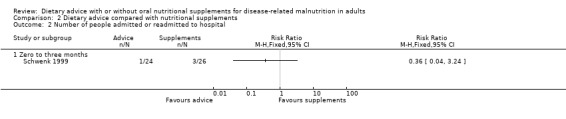
Comparison 2 Dietary advice compared with nutritional supplements, Outcome 2 Number of people admitted or readmitted to hospital.
3. Measures of nutritional status
a. Weight (Analysis 2.3)
Data on change in weight were available from seven of eight studies (Baldwin 2008; Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008); but one of the studies did not contribute estimable data in one arm (Ravasco 2005b). The duration of intervention in all studies was up to three months.
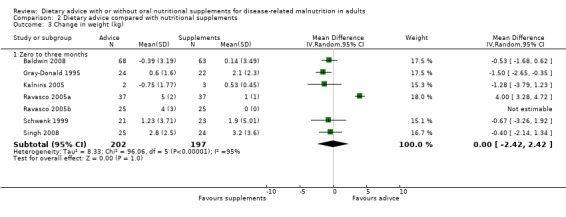
Data were analysed using a random‐effects model; weight change did not differ statistically significantly between the groups, MD ‐0.00 kg (95% CI ‐2.42 to 2.42) (P = 1.00), heterogeneity was high (I2 = 95%; P < 0.00001) (Analysis 2.3). Removal of one study (Ravasco 2005a) reduced the heterogeneity to zero and there was significantly greater weight gain in the groups receiving oral nutritional supplements, MD ‐0.91 kg (95% CI ‐1.60 to ‐0.23) (P = 0.009).
2.3. Analysis.
Comparison 2 Dietary advice compared with nutritional supplements, Outcome 3 Change in weight (kg).
b. Mid‐arm muscle circumference (Analysis 2.4)
One study contributed data on MAMC (Gray‐Donald 1995) and there were no statistically significant differences between groups, MD ‐0.80 cm (95% CI ‐5.29 to 3.69) (P = 0.73) (Analysis 2.4).
2.4. Analysis.

Comparison 2 Dietary advice compared with nutritional supplements, Outcome 4 Change in mid‐arm muscle circumference (MAMC) (cm).
c. TSF (Analysis 2.5)
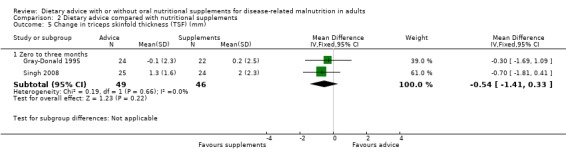
Two studies contributed data on TSF (Gray‐Donald 1995; Singh 2008). There was no statistically significant difference between the groups MD ‐0.54 mm (95% CI ‐1.41 to 0.33) (P = 0.22), there was no heterogeneity (I2 = 0%; P = 0.66) (Analysis 2.5).
2.5. Analysis.
Comparison 2 Dietary advice compared with nutritional supplements, Outcome 5 Change in triceps skinfold thickness (TSF) (mm).
Secondary outcomes
1. Nutritional intake before and after the intervention (Analysis 2.6)
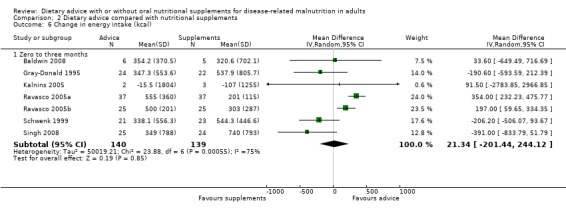
Information on change in energy intake was available from seven of eight studies (Baldwin 2008; Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008). Analysing data using a random‐effects model, energy intake did not differ statistically significantly between the two groups up to three months of intervention, MD 21.34 kcals/day (95% CI ‐201.44 to 244.12) (P = 0.85), high heterogeneity was observed (I2 = 75%; P = 0.0005) (Analysis 2.6).
2.6. Analysis.
Comparison 2 Dietary advice compared with nutritional supplements, Outcome 6 Change in energy intake (kcal).
2. Measures of functional status (Analysis 2.7)
Only one study contributed data on handgrip strength and there were no statistically significant differences between the groups, MD 0.16 kg force (95% CI ‐1.54 to 1.86) (P = 0.85) (Gray‐Donald 1995) (Analysis 2.7).
2.7. Analysis.

Comparison 2 Dietary advice compared with nutritional supplements, Outcome 7 Change in grip strength (kg force).
3. QoL
Four studies reported this outcome (Baldwin 2008; Gray‐Donald 1995; Ravasco 2005a; Ravasco 2005b). Data were not combined for analysis since four different QoL instruments were used and data were not reported in a way that allowed for meta‐analysis (Table 7). In three studies the European Organisation for Research and Treatment of Cancer (EORTC) questionnaire was used (Baldwin 2008; Ravasco 2005a; Ravasco 2005b). Median scores were reported for all domains at baseline, end of radiotherapy and at three months in two studies; however data were not reported as a change from baseline (Ravasco 2005a; Ravasco 2005b). In the third study, score changes from baseline to 6 weeks and 26 weeks are available for four domains and no significant differences were observed between the groups at either 6 or 26 weeks (Baldwin 2008).
In one study of frail elderly patients, two instruments were used to measure the effects of the interventions on QoL, the General Well‐being Schedule (GWS) (Dupuy 1978) and a general self‐perceived health question (GSHQ) (Rodin 1993) (Gray‐Donald 1995). Data for the GWS were reported as means (SD) for scores at baseline and 12 weeks for the two groups. No significant differences were observed between the groups at baseline or 12 weeks. Data obtained using the GSHQ were presented as per cent of participants in each category (i.e. excellent, very good, good, fair or poor quality of life) for both groups at baseline and 12 weeks. No significant differences were observed between the groups at baseline or 12 weeks (Gray‐Donald 1995).
4. Cost
There were no data reported in any of the studies for this outcome.
Dietary advice compared with dietary advice plus oral nutritional supplements
Sixteen studies (731 randomised participants) evaluated this comparison (Arnold 1989; Baldwin 2008; Beattie 2000; de Luis 2003; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kendell 1982; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Paton 2004; Rabeneck 1998; Sharma 2002; Wilson 2001). There were no usable data for three of these studies (Dixon 1984; Kendell 1982; Olejko 1984).
Primary outcome
1. Mortality (Analysis 3.1)
Data on mortality were available from seven studies (Arnold 1989; Baldwin 2008; Beattie 2000; de Luis 2003; Fuenzalida 1990; Murphy 1992; Norman 2008b), of which only two reported any deaths and therefore contributed to the analysis (Arnold 1989; Baldwin 2008). Six studies assessed mortality for interventions lasting from zero to three months (Arnold 1989; Baldwin 2008; Beattie 2000; de Luis 2003; Fuenzalida 1990; Norman 2008b). Data were analysed using a random‐effects analysis and there was no statistically significant difference between groups receiving dietary advice with nutritional supplements and groups receiving dietary advice alone, RR 0.55 (95% CI 0.08 to 3.95) (P = 0.55), there was moderate heterogeneity (I2 = 48%; P = 0.16). One study assessed mortality for interventions lasting from four to six months and there were no deaths in either group (Murphy 1992) (Analysis 3.1).
3.1. Analysis.
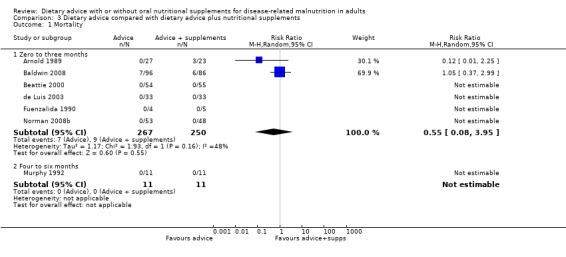
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 1 Mortality.
No combined analysis was conducted on mortality as, although there were data from seven studies for this outcome, only two of the seven studies reported events (Arnold 1989; Baldwin 2008). Both of these studies were of interventions lasting up to three months. The remaining studies all reported no deaths
2. Morbidity (Analysis 3.2)
Two studies reported data on hospital admissions; one having an intervention lasting from zero to three months (Norman 2008b) and the other lasting from four to six months (Gonzalez‐Espinoza 2005). In the study with an intervention lasting from zero to three months there was no statistically significant difference in number of hospital readmissions between participants receiving dietary advice and dietary advice and supplements, RR 1.81 (95% CI 0.97 to 3.36) (P = 0.06). Similarily, in the study with an intervention lasting from four to six months there was no statistically significant difference in the number of hospital readmissions between the groups, RR 1.19 (95%CI 0.70 to 2.02) (P = 0.51).
In the combined analysis, participants who received dietary advice and supplements were less likely to be admitted to hospital than those who received dietary advice alone, RR 1.53 (95 % CI 1.00 to 2.34) (P = 0.05), low heterogeneity was observed in this combined analysis (I2 = 13%; P = 0.28) (Analysis 3.2).
3.2. Analysis.
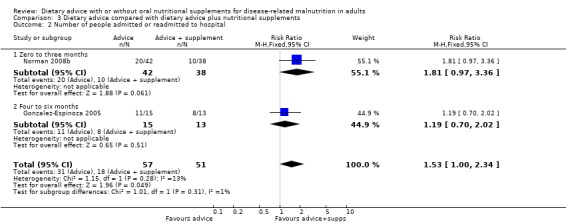
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 2 Number of people admitted or readmitted to hospital.
3. Measures of nutritional status
a. Weight (Analysis 3.3)
Data on weight change were available from nine studies with interventions lasting up to three months (Arnold 1989; Baldwin 2008; Beattie 2000; de Luis 2003; Fuenzalida 1990; Norman 2008b; Paton 2004; Rabeneck 1998; Sharma 2002) and two studies with interventions lasting from four to six months (Gonzalez‐Espinoza 2005; Murphy 1992). Using a random‐effects model, there was no statistically significant difference in weight change between groups receiving dietary advice with nutritional supplements for up to three months compared with dietary advice alone, MD 0.97 kg (95% CI ‐0.12 to 2.06) (P = 0.08), there was high heterogeneity in this analysis (I2 = 74%; P = 0.0002). In the studies comparing an intervention lasting for up to six months, there was no statistically significant difference between the groups, MD 0.50 kg (95 % CI ‐1.52 to 2.53) (P = 0.63), there was low heterogeneity (I2 = 1 %; P = 0.31).
In the combined analysis of all studies, there was no statistically significant difference between participants who received dietary advice and supplements and dietary advice alone, MD 0.95 kg (95 % CI ‐0.03 to 1.93) (P = 0.06), there was high heterogeneity in this analysis (I2 = 69%; P = 0.0004) (Analysis 3.3).
3.3. Analysis.
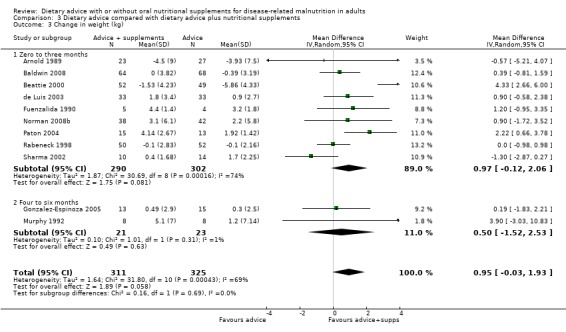
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 3 Change in weight (kg).
Removal of two studies reduced heterogeneity to zero (Beattie 2000; Paton 2004); however, there was still no significant difference in weight change between groups, MD 0.20 (95% CI ‐0.36 to 0.75) (P = 0.48) (Analysis 3.4).
3.4. Analysis.
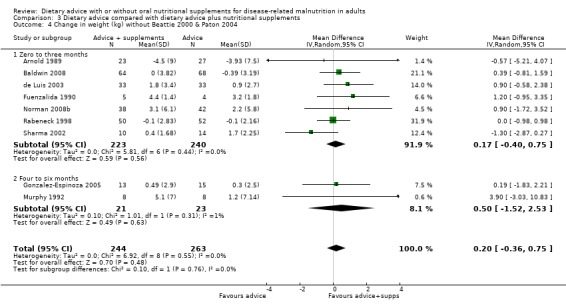
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 4 Change in weight (kg) without Beattie 2000 & Paton 2004.
b. MAMC (Analysis 3.5)
Data on MAMC were available from three studies, two with interventions lasting from zero to three months (Beattie 2000; de Luis 2003) and one with an intervention lasting from four to six months (Gonzalez‐Espinoza 2005). These data were analysed using a fixed‐effect analysis. In the analysis of studies with interventions lasting from zero to three months, participants receiving dietary advice with supplements had greater improvements in MAMC than participants receiving dietary advice alone, MD ‐0.85 cm (95 % CI ‐1.34 to ‐0.36) (P = 0.0007), with no heterogeneity (I2 = 0%; P = 0.95). In the study comparing MAMC for interventions lasting up to six months, there was no statistically significant difference between the groups, MD ‐1.23 cm (95 % CI ‐2.65 to 0.19) (P = 0.09).
In the combined analysis a significant beneficial effect in favour of dietary advice plus supplements was observed, MD ‐0.89 cm (95 % CI ‐1.35 to ‐0.43) (P = 0.0002), with no heterogeneity (I2 = 0%; P = 0.88) (Analysis 3.5).
3.5. Analysis.
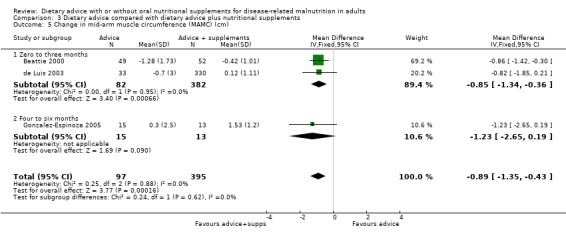
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 5 Change in mid‐arm muscle circumference (MAMC) (cm).
c. TSF (Analysis 3.6)
Six studies reported data on TSF: in five of these interventions lasted between zero and three months (Beattie 2000; de Luis 2003; Fuenzalida 1990; Norman 2008b; Rabeneck 1998); and in one study the intervention lasted for four to six months (Gonzalez‐Espinoza 2005). At zero to three months, using a random‐effects model, a beneficial effect was observed in favour of dietary advice and supplements, MD ‐1.32 (95% CI ‐2.51 to ‐0.12) (P = 0.03), heterogeneity was high (I2 = 83%; P = 0.0001) and could not be reduced by removal or any one study or combination of studies (Analysis 3.6). In the study that reported data at four to six months, there was no statistically significant difference in TSF between groups MD 0.10 mm (95 % CI ‐3.99 to 4.19) (P = 0.96) (Gonzalez‐Espinoza 2005).
3.6. Analysis.
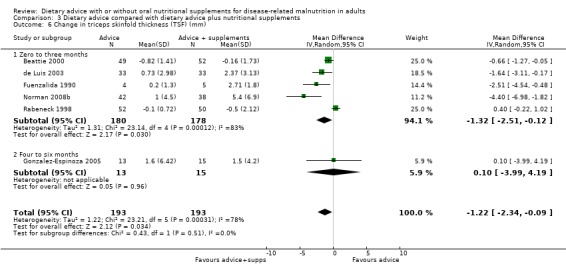
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 6 Change in triceps skinfold thickness (TSF) (mm).
In the combined analysis a statistically significant difference was observed in TSF favouring dietary advice plus supplements MD ‐1.22 mm (95 % CI ‐2.34 to ‐0.09) (P = 0.03), high heterogeneity was observed (I2 = 78 %; P = 0.0003).
Secondary Outcomes
1. Nutritional intake before and after the intervention (Analysis 3.7)
Six studies provided data on changes in energy intake, four with interventions lasting between zero and three months (Baldwin 2008; de Luis 2003; McCarthy 1999; Paton 2004) and two with interventions lasting between four and six months (Gonzalez‐Espinoza 2005; Murphy 1992). Data were analysed using a random‐effects model. There were greater improvements in energy intake in groups receiving dietary advice with supplements compared with groups receiving dietary advice alone for up to three months, MD ‐344.88 kcal/day (95% CI ‐600.28 to ‐89.47) (P = 0.008), this analysis had moderate heterogeneity (I2 = 58%; P = 0.07). Groups receiving dietary advice with or without supplements for up to six months had no statistically significant difference between their mean change in energy intake, MD 226.84 kcal/day (95 % CI ‐223.19 to 676.87) (P = 0.32). This analysis had low heterogeneity (I2 = 27%; P = 0.24).
In the combined analysis, changes in energy intake were similar in the two groups, MD ‐192.80 kcal/day (95% CI ‐481.92 to 96.31) (P = 0.19), this analysis had high heterogeneity (I2 = 71%; P = 0.004) (Analysis 3.7).
3.7. Analysis.
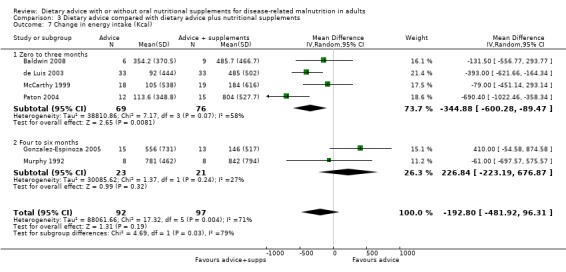
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 7 Change in energy intake (Kcal).
2. Measures of functional status (Analysis 3.8)
Four studies reported data on handgrip strength (Beattie 2000; Norman 2008b; Paton 2004; Rabeneck 1998), all interventions lasted between zero and three months and data were analysed using a random‐effects model. Handgrip strength was greater in those who received dietary advice and supplements compared with those who received dietary advice alone, MD ‐1.67 kg (95 % CI ‐2.96 to ‐0.37) (P = 0.01) although the effect was moderately heterogeneous (I2 = 50%; P = 0.11) (Analysis 3.8).
3.8. Analysis.
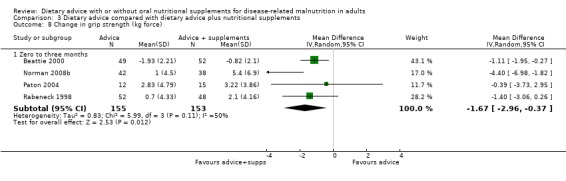
Comparison 3 Dietary advice compared with dietary advice plus nutritional supplements, Outcome 8 Change in grip strength (kg force).
3. QoL
Five studies reported this outcome (Baldwin 2008; Beattie 2000; Norman 2008b; Paton 2004; Rabeneck 1998). Data were not combined for analysis since four different QoL instruments were used and data were not reported in a way that allowed for meta‐analysis (Table 7). In one study in cancer patients the EORTC was used (Baldwin 2008). In this study score changes from baseline to 6 weeks and 26 weeks are available for four domains and no significant differences were observed between the groups at either 6 or 26 weeks (Baldwin 2008). Two studies used the SF‐36 instrument (Beattie 2000: Norman 2008b) and one other used a modified version of the SF‐36 (Paton 2004). Data could not however be combined since one study reported changes in scores for all domains from baseline to three months (Norman 2008b), while the other reported changes only in the summary physical and mental scores from baseline to 10 weeks (Beattie 2000). The study using the modified SF‐36 reported changes in scores from baseline to 6, 12 and 24 weeks and these were analysed using ANCOVA tests controlling for baseline scores (Paton 2004). These data were therefore, not in a format that allowed for inclusion in a meta‐analysis. In one study, a 30‐item QoL instrument was designed specifically for use in the study (Rabeneck 1998). Change scores were reported from baseline to six weeks and no significant difference was observed between the groups.
4. Cost
No data were reported in any of the studies for this outcome.
Dietary advice plus supplements if required compared with no advice and no supplements
Fourteen studies (1070 randomised participants) were identified for this comparison (Berneis 2000; Chandra 1985; Evans 1987; Forli 2001; Ganzoni 1994; Hampson 2003; Isenring 2004; Jensen 1997; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Persson 2007; Rogers 1992). There were no usable data for four of these studies (Berneis 2000; Chandra 1985; Evans 1987; Jensen 1997). Data from the study by Persson were available at 3, 6, 12 and 24 months (Persson 2002).
Primary outcome
1. Mortality (Analysis 4.1)
Data were available on mortality from eight separate studies (the 2002 Persson study contributed data at multiple time points); four assessing interventions that lasted for up to three months (Forli 2001; Isenring 2004; Lovik 1996; Persson 2002), three assessing interventions lasting between four and six months (Ovesen 1993; Persson 2002; Persson 2007), three of interventions lasting greater than seven months and up to 12 months (Ganzoni 1994; Hampson 2003; Persson 2002) and one at 12 months or over (Persson 2002). Data were analysed using a fixed‐effect model. There were no statistically significant differences in mortality between groups in the studies comparing nutritional intervention with usual care at: zero to three months RR 0.95 (95% CI 0.47 to 1.93) (P = 0.89), I2 = 0% (P = 0.54); four to six months RR 1.04 (95% CI 0.54 to 2.00) (P = 0.91), I2 = 45% (P = 0.16); interventions lasting 7 to 12 months RR 1.26 (95% CI 0.76 to 2.10) (P = 0.37), I2 = 0% (P = 0.68) and interventions lasting for more than twelve months RR 0.94 (95% CI 0.84 to 1.05) (P = 0.28), I2 = 0% (P = 0.92) (Analysis 4.1).
4.1. Analysis.
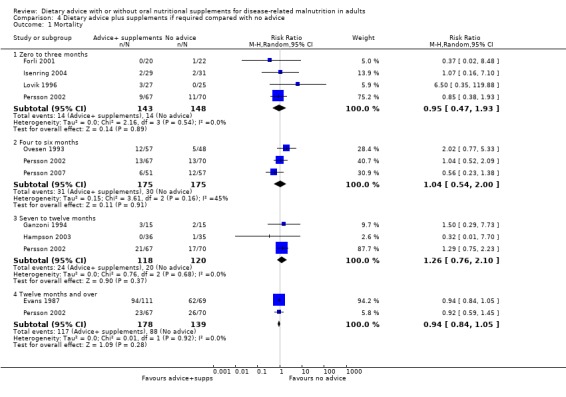
Comparison 4 Dietary advice plus supplements if required compared with no advice, Outcome 1 Mortality.
An analysis was conducted for all studies combined to end of intervention (removing data for interim time points for the 2002 study by Persson and there was no significant differences between groups with no heterogeneity, RR 0.95 (95% CI 0.85 to 1.00) (P = 0.31), I2 =0% (P = 0.57) (Analysis 4.2).
4.2. Analysis.
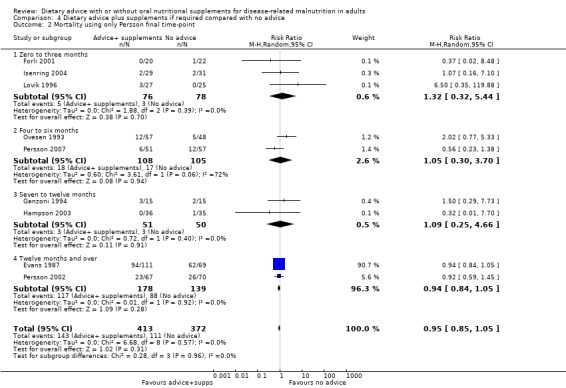
Comparison 4 Dietary advice plus supplements if required compared with no advice, Outcome 2 Mortality using only Persson final time‐point.
2. Morbidity (assessed by risk of hospital admission or re‐admission and length of hospital stay)
No data were reported in any of the studies for this outcome.
3. Measures of nutritional status
a. Weight (Analysis 4.3)
Nine studies contributed data on weight change, five with interventions lasting up to 3 months (Berneis 2000; Forli 2001; Isenring 2004; Lovik 1996; Persson 2002) and four with interventions lasting from 4 to 6 months (Ovesen 1993; Persson 2002; Persson 2007; Rogers 1992). Only one study reported data at between 7 and 12 months (Persson 2002) and two studies at 12 months and over (Ganzoni 1994; Persson 2002). There was high heterogeneity between studies and so a random‐effects model was used for the analysis. Groups receiving dietary advice and supplements for up to 3 months and for between 4 and 6 months gained more weight than groups receiving no advice or supplements, MD 1.74 kg (95% CI 0.53 to 2.95) (P = 0.005), with moderate heterogeneity (I2 51% (P = 0.08)) and MD 1.87 kg (95% CI ‐0.07 to 3.81) (P = 0.06), with high heterogeneity (I2 81% (P = 0.001)) respectively. There were no statistically significant differences in weight change between groups at between 7 and 12 months, MD 0.70 kg (95% CI ‐0.84 to 2.24) (P = 0.37). At 12 months and over the result was likewise not statistically significant, MD 2.17 kg (95% CI ‐1.20 to 5.54) (P = 0.21) with moderate heterogeneity (I2 44% (P = 0.18)) (Analysis 4.3).
4.3. Analysis.
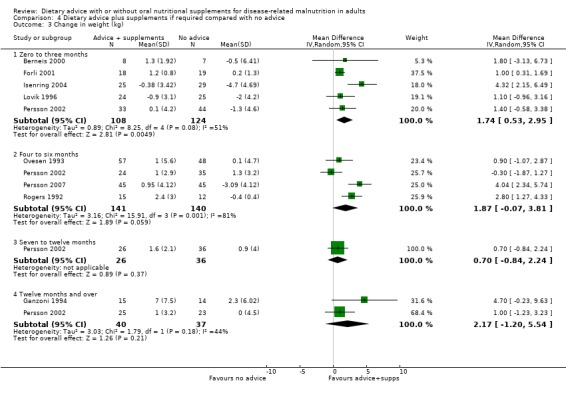
Comparison 4 Dietary advice plus supplements if required compared with no advice, Outcome 3 Change in weight (kg).
A combined analysis was conducted using data from the 2002 Persson study for the final time‐point (Persson 2002). Groups receiving dietary advice and nutritional supplements had a significantly greater weight gain than those receiving dietary advice alone, MD 2.20 kg (95% CI 1.16 to 3.25) (P <0.0001), heterogeneity was moderate, I2 63% (P = 0.006) (Analysis 4.4).
4.4. Analysis.
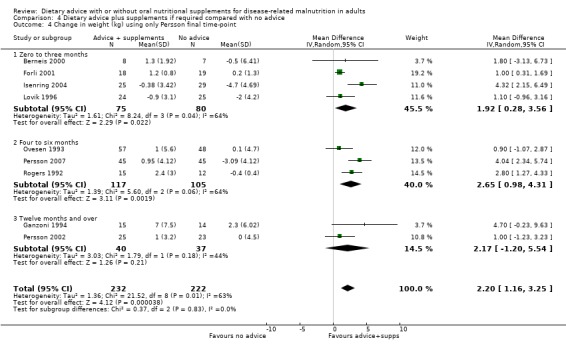
Comparison 4 Dietary advice plus supplements if required compared with no advice, Outcome 4 Change in weight (kg) using only Persson final time‐point.
b. MAMC
No study reported data on MAMC.
c. TSF (Analysis 4.5)
Only one study contributed data on change in TSF following an intervention lasting for 4 months (Rogers 1992). The participants receiving dietary advice and supplements showed greater improvements in than those receiving routine care, MD 0.40 mm (95% CI 0.10 to 0.70) (P = 0.01) (Analysis 4.5).
4.5. Analysis.

Comparison 4 Dietary advice plus supplements if required compared with no advice, Outcome 5 Change in triceps skinfold thickness (TSF) (mm).
Secondary outcomes
1. Nutritional intake before and after the intervention (Analysis 4.6)
Six studies contributed data on change in energy intake, four for interventions lasting from 0 to 3 months (Berneis 2000; Forli 2001; Isenring 2004; Moloney 1983), one for interventions lasting from 4 to 6 months (Ovesen 1993) and one for an intervention lasting for 12 months (Hampson 2003). Data were analysed using a random‐effects model. In groups receiving dietary advice and oral supplements for up to 3 months there was no significant difference in energy intake MD 184.40 kcal/day (95% CI ‐109.01 to 477.81) (P = 0.22), heterogeneity was high (I2 = 83% (P = 0.0005)). One study compared an intervention lasting from 4 to 6 months and there were no differences between groups, MD 71.00 kcal/day (95% CI ‐125.65 to 267.65) (P = 0.48). In the study where intervention lasted for more than 7 months groups receiving dietary advice and supplements had a higher energy intake than those receiving no advice and no supplements, MD 464.00 kcal/day (95% CI 270.07 to 657.93) (P < 0.00001).
When all studies are combined in one analysis, groups receiving dietary advice and supplements had a higher energy intake than groups receiving routine care, MD 212.71 kcal/day (95% CI ‐0.91 to 426.32) (P = 0.05), however high heterogeneity was observed I2 = 82% (P = 0.0001) (Analysis 4.6).
4.6. Analysis.
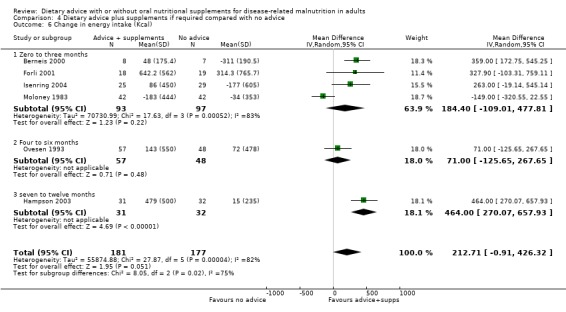
Comparison 4 Dietary advice plus supplements if required compared with no advice, Outcome 6 Change in energy intake (Kcal).
2. Measures of functional status (Analysis 4.7)
Two studies contributed data on handgrip strength for interventions lasting 4 months (Persson 2007; Rogers 1992). Data analysed using a random‐effects model show handgrip strength was not significantly higher in those who received dietary advice and supplements if required compared with those who received no advice and no supplements, MD 6.44 (95% CI ‐3.15 to 16.03) (P = 0.19), the effect had high heterogeneity (I2 = 94%; P < 0.0001). Heterogeneity could not be explored because of insufficient data in this analysis (Analysis 4.7).
4.7. Analysis.
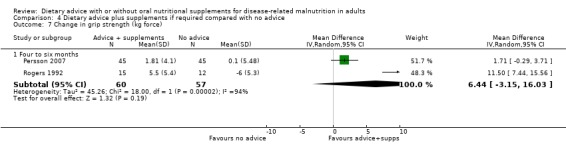
Comparison 4 Dietary advice plus supplements if required compared with no advice, Outcome 7 Change in grip strength (kg force).
3. QoL
Seven studies reported this outcome (Berneis 2000; Isenring 2004; Jensen 1997; Ovesen 1993; Persson 2002; Persson 2007; Rogers 1992). Data were not combined for analysis since five different QoL instruments were used and data were not reported in a way that allowed for meta‐analysis (Table 7). Two studies used the EORTC (Isenring 2004; Persson 2002). In one study mean scores at baseline, 4, 8 and 12 weeks were reported for the global health and the physical function domains (Isenring 2004); while in the other data comparing the dietary advice and supplements group with the no advice and no supplements group were not presented (Persson 2002). In a study of HIV‐infected patients, the Medical Outcomes Study instrument was used and data were presented as mean summary scores at baseline and 12 weeks (Berneis 2000). No significant differences were observed between the two groups in any of the summary scores. Two studies used the QoL Index (Jensen 1997; Ovesen 1993). Data could not be combined for meta‐analysis since one study did not report data comparing the group receiving dietary advice and supplements with the group receiving no dietary advice and no supplements (Jensen 1997). In a study conducted on patients receiving chemotherapy for cancer, mean (standard deviation) scores at baseline, three and five months were reported and no significant differences were observed between the groups (Ovesen 1993). In one study the SF‐36 was used and mean scores at baseline and four months were reported for the physical and mental health summary scores (Persson 2007). No significant differences were observed between the groups over time. In a study of patients with COPD, the Sickness Impact Profile (SIP) was used (Rogers 1992). The authors state there were no statistically significant differences between the groups in SIP scores at enrolment or four months; however, no data were reported to support this statement.
4. Cost
No studies reported data on costs.
Discussion
Summary of main results
The aim of this review was to look for an effect of dietary advice given with or without supplements to malnourished or nutritionally at risk individuals on nutritional and clinical outcomes. In all four comparisons the effect on mortality was not significant and there was no heterogeneity. Dietary advice alone was associated with significant benefits to weight after twelve months of intervention and significantly increased mid‐arm muscle circumference when all studies were combined although there was moderate heterogeneity. The findings on weight change should be interpreted with caution as they are derived from one quasi‐randomised study with a high risk of bias (Macia 1991). In addition, the data were not available from the paper and SDs were therefore imputed. There were no differences between the groups when dietary advice was compared with oral nutritional supplements. Dietary advice given with nutritional supplements compared with dietary advice alone was associated with improvements in weight, MAMC and grip strength although the effects on weight and grip strength were heterogeneous. Dietary advice given with oral nutritional supplements if required when compared with no intervention was associated with a significant increase in energy intake, although there was moderate heterogeneity. It was not possible to draw conclusions about clinical outcomes and cost. Data on QoL and functional outcomes have not been analysed for this update. There was statistical and clinical heterogeneity across all of the studies contributing to the findings of this review, apart from the effects on mortality. Studies for each intervention have been combined and therefore the findings of this review must be interpreted with caution. The possibility that the effects of interventions vary according to factors which it has not been possible to identify must be bourne in mind, since we cannot assume that the effects of different interventions will be the same in all clinical groups, care settings and patients of different age. Until there are more homogenous studies in different patient groups the effects of dietary advice given with or without oral nutritional supplements in individual patients and groups of patients cannot be fully evaluated.
Overall completeness and applicability of evidence
There was statistical and clinical heterogeneity across all groups of studies in this review. This has been addressed statistically by using a random‐effects model where heterogeneity was moderate or high and by removing studies to allow examination of the effect of the intervention with and without heterogeneity present. It must be appreciated that decisions to remove studies have been made on statistical grounds and these may not necessarily be clinically justified. The heterogeneity could be explained by a number of factors including background clinical condition, stage and disease severity of the patient, healthcare setting, frequency and intensity of intervention and other yet to be identified factors. The identified studies represented a wide range of clinical conditions, but the numbers of participants with any one condition were small with the possible exception of studies conducted in patients with cancer. Even amongst studies in cancer patients, there was wide variation in site, stage and treatment. In the majority of included studies the mean age of patients was over 65 years, but a wide age range was represented overall. By far the majority of studies were conducted in outpatients, with only seven studies including patients who spent some time in hospital. Whilst it was possible to obtain information on the duration of intervention in most of the studies, there was almost no information on the nature, intensity and content of the dietary intervention. It is important to note that in the majority of studies, the intervention lasted for three months. To date there is insufficient information to determine whether this is the optimal length of intervention of this kind and indeed whether it represents a realistic goal in clinical practice. Furthermore, in clinical practice, interventions might consist of only one or two visits by a dietitian to an inpatient through to regular repeated dietetic outpatient visits in patients with long‐term chronic disease, e.g. renal failure. In addition details of the experience and training of the dietitian giving the advice was not reported. However, in all studies there was a consistent aim of improving nutritional intake with the goal of minimising weight loss or promoting weight gain. Dietitians receive referrals to provide nutritional support to patients from a variety of clinical backgrounds and different healthcare settings. It is not possible from the findings of this review to be specific about the effect size that can be achieved in any one patient group and indeed it is likely that the effect size will vary according to all of the above variables. But this review suggests that it is possible to achieve an increase in energy intake and weight gain with dietary advice with or without supplements and in some cases the increase in weight gain may be accompanied by beneficial changes in body composition.
Although this review has summarised the findings of 45 separate studies of dietary advice there remains a lack of good quality evidence for all reported outcomes and in particular there is a need for more evidence of the effects of dietary advice on patient‐centred outcomes and costs.
Quality of the evidence
The quality of evidence in this review is at best low to moderate. The main issue is that whilst sequence generation and allocation concealment were often adequate, very few studies were adequately blinded and most were small and inadequately powered. It is important to bear in mind that it is difficult to conceive of an adequate placebo for dietary advice. It is impossible to prevent some patients in the control arm from seeking other sources of dietary advice which act as confounders. Whilst dietary advice can be compared with usual care, this has varied enormously in terms of quality and duration between studies. Whilst in theory it might be possible to design a study where outcome assessment may be blinded, this was not the case in many of the included studies and a possible reason for this is inadequate funding for this type of research.
Potential biases in the review process
The protocol for this review specified three comparison groups. An additional group was added after the first searches conducted in 1999 when a comparison was identified that had not been anticipated and most closely represented actual dietetic practice. Since this comparison was included in the first version of the review the potential for bias at this stage is minimal. The original search strategy for this review and the current update was comprehensive in that six databases were used including databases other than the most commonly used (Avenell 2001); there was no language restriction on papers retrieved and two reviewers have selected studies independently. However, no handsearching has been undertaken and searching of the grey literature has not been possible because of time constraints. Furthermore the results of funnel plots undertaken on some of the larger analyses may be explained by the heterogeneity of the results. It may also be explained by the number of small studies identified, there is a lack of large studies in this review (Figure 1; Figure 2).
1.
Funnel plot of comparison: 1 Dietary advice compared with no advice, outcome: 1.3 Change in weight (kg).
2.
Funnel plot of comparison: 4 Dietary advice plus supplements if required compared with no advice, outcome: 4.3 Change in weight (kg).
Agreements and disagreements with other studies or reviews
The authors are unaware of any other systematic reviews that have addressed the potential benefits of dietary advice given with or without oral nutritional supplements. National policy in the UK on the management of disease‐related malnutrition is based on analysis of data from patients receiving oral nutritional supplements compared with usual care. The analysis combines the data from all clinical backgrounds and suggests that overall improvements in weight and complication rates can be achieved with the use of oral nutritional supplements when compared with usual care (NICE 2006). European guidance from The European Society for Clinical Nutrition and Metabolism (ESPEN) is broken down according to clinical background of the patient and so guidance varies with intervention being associated with benefits to weight in elderly patients and patients with liver disease (Plauth 2006; Volkert 2006). The guidance from ESPEN does not show the results of meta‐analysis and so direct comparison of the effect size and heterogeneity cannot be made. This review shows that dietary advice with or without nutritional supplements can result in similar improvements in weight gain to the analysis by NICE; however, both the NICE review and this one exhibit considerable heterogeneity and so the effect size for either oral nutritional supplements or dietary advice cannot yet be determined.
Guidance from ESPEN and a recent systematic review of the effects of enteral nutrition on clinical outcome have examined the effects of oral nutritional supplements on mortality in patients from a range of clinical backgrounds (Koretz 2007; Plauth 2006; Volkert 2006 ). The ESPEN guidelines report a survival benefit associated with nutritional supplements in elderly patients and patients with liver cirrhosis. The review by Koretz shows that in 16 RCTs of oral nutritional supplements in 1733 elderly patients there was a significant reduction in mortality associated with receiving nutritional supplements, absolute risk reduction ‐4% (95% CI ‐7% to ‐1%) (Koretz 2007). The review by NICE of 25 studies with nearly 3000 randomised participants also demonstrates a significant reduction in mortality associated with receiving nutritional supplements across a range of clinical conditions RR 0.82 (95% CI 0.69 to 0.98) with no significant heterogeneity (P = 0.63). This current review included 1600 participants in 19 studies of which five studies reported no deaths; this finding suggests that dietary advice may have no effect on mortality, but this requires further investigation.
Furthermore, it is reasonable to presume that the benefits derived from nutritional supplements result from their ability to increase nutrient intake (or balance of nutrients). It then follows that if a similar increase in nutrient intake can be achieved by dietary means rather than using supplements, similar clinical benefits would be expected to occur. A caveat to this is that we do not know which nutrient or combination of nutrients is responsible for the benefit (protein, energy, vitamins, trace elements) and it may not be possible to reproduce the exact changes induced by supplements using ordinary food. Studies of dietary advice rarely report the details of specific foods and combinations of foods used to increase nutrient intake.
Authors' conclusions
Implications for practice.
There is reasonable evidence of variable quality to suggest that dietary advice given with or without nutritional supplements improves weight, body composition and grip strength in people with disease‐related malnutrition or at nutritional risk.
This review found no evidence for a beneficial effect of dietary advice on survival and a complete lack of evidence for the effects on patient‐centred outcomes such as QoL and functional outcomes which are difficult to interpret and will be included in the next update. Furthermore, there is a complete lack of evidence regarding cost benefits. All interventions in this review were given by a dietitian and, until there is further evidence in this area, there is no reason to suggest that people with weight loss secondary to disease should not continue to be managed by referral to a dietitian.
Implications for research.
Evidence
Dietary advice given with or without nutritional supplements is associated with improvements to mid‐arm muscle and in some cases energy intake in patients with malnutrition or at risk of malnutrition. The potential benefits to other measures of body composition, functional and clinical outcomes is less clear. There are limited data on patient‐centred outcomes and cost savings.
Population
Research is needed in populations of patients:
homogeneous for amount of malnutrition at study inclusion, defined using standard assessment tools;
with a range of clinical conditions where the stage and treatment intent of the condition is clearly described;
in the hospital setting and in a variety of community settings.
Intervention
More evidence is needed for food‐based interventions used in standard dietetic practice:
dietary advice;
dietary advice with supplements if required.
Studies need to include details of the type, intensity and duration of the intervention given as well as recording the nutrient content of any improvements achieved. The level of expertise of the person giving the advice should be reported.
Comparison
Two types of comparison are relevant:
no dietary advice or usual care. Ideally this would be standardised across studies and would not include access to any specific advice from a dietitian. The precise details of this type of comparison must be recorded together with any measures of change in nutrient intake associated with usual care;
nutritional supplements.
Outcomes
Mortality
Measures of morbidity e.g. length of hospital stay, complications
Weight and change in body composition
Nutrient intake
Functional changes which are relevant to the patient group under consideration
QoL
Patient satisfaction
What's new
| Date | Event | Description |
|---|---|---|
| 22 May 2012 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 19 July 2011 | New citation required but conclusions have not changed | The title of the review has been changed from 'Dietary advice for illness‐related malnutrition in adults' to 'Dietary advice with or without oral nutritional supplements for disease‐related malnutrition in adults' following advice from the peer reviewers and the editor. |
| 8 June 2011 | New search has been performed | In total 12 new studies have been included in the review (Baldwin 2008; Campbell 2008; Chandra 1985; Gonzalez‐Espinoza 2005; Manguso 2005; Norman 2008b; Persson 2007; Ravasco 2005b; Rydwik 2008; Sharma 2002; Singh 2008; Stratton 2007). Whilst updating this review, a separate group of studies of supportive interventions to enhance nutritional intake have been identified. These studies will be included in a new review. Two studies originally included in this review meet the inclusion criteria of the new review and therefore have been removed from this review at this update and will be included in the new review (Hickson 2004; Turic 1998). After consideration by both authors, 30 new studies have been excluded from the review (Arutiunov 2009; Beck 2008; Botella‐Carretero 2008; Carlsson 2005; Duncan 2006; Forli 2006; Idilman 2009; Jie 2009; Krasnoff 2006; Kruizenga 2005; Lejeune 2005; Manders 2009; Nijs 2006; Olofsson 2007; Parrott 2006; Pedersen 2005; Planas 2005; Plank 2008; Rabinovitch 2006; Rassmussen 2006; Rüfenacht 2010; Salas‐Salvado 2005; Simmons 2008; Smoliner 2008; Solerte 2008; Swanenburg 2007; Tatsumi 2009; Taylor 2006; Watson 2008; Wouters‐Wessling 2005). There are three studies 'Awaiting classification' (Margare 2002; Penalva 2009; Shatenstein 2008). |
| 12 November 2008 | Amended | Converted to new review format. |
| 14 November 2007 | New citation required and conclusions have changed | Substantive amendment. Tessa Parsons and Stuart Logan have stepped down as authors on this review. A new co‐author, Elizabeth Weekes, has been recruited. |
| 14 November 2007 | New search has been performed | The latest search did not identify any studies eligible for inclusion in the review.
Two papers previously listed as 'Awaiting Assessment' have now been moved to 'Included studies' (Kalnins 2005; Weekes 2006). The Kalnins 2005 paper is the primary paper for the previously included study (abstracts) ‐ Kalnins 1996.
In the previous version of the review it was unclear how the different studies measured grip strength and so we removed the graphs showing these data and presented the reported means and standard deviations in an additional table. We have now been able to clarify this issue and the data for this outcome is again presented in the analysis. The plain language summary has been updated in light of the current guidance from The Cochrane Collaboration. |
| 15 November 2006 | New search has been performed | Eleven studies have been added to the 'Included studies' section and there are now two studies listed as 'Awaiting Assessment'. It is unclear how the different studies have measured grip strength. Until this has been clarified, we have removed the graphs showing these data and presented the reported means and standard deviations in an additional table. The previous version of this review suggested that nutritional supplements were associated with significantly greater short‐term weight gains. The addition of data at this update has challenged this finding, although it has not been possible to combine the new data in a meta‐analysis. Additionally, this review demonstrates significant improvements in weight in people receiving dietary advice with nutritional supplements rather than dietary advice alone or no intervention. This review has still failed to find any evidence for clinical benefits, such as improved survival, rate of complications and reductions in numbers of hospital admissions and length of stay, of dietary advice. |
| 19 February 2004 | New search has been performed | Two studies (McCarthy 1999; Persson 2002) have been added to the 'Included studies' section. Data are not currently available from these studies, but are being sought from the authors. The reviewers aim to incorporate these data into the next update of the review. Data from a study previously included in 'Studies awaiting assessment' has been obtained from the author and this study is now incorporated into the review (Hickson 2002). |
| 27 February 2002 | New search has been performed | This includes the addition of one study into the "Studies Awaiting Assessment" section of the review. The Hickson 2002 study has not been published in full, but has been submitted for publication and will be incorporated into a future update of this review. |
Acknowledgements
We acknowledge the valuable input from Professor Stuart Logan and Dr Tessa Parsons in the development of the protocol and the full review and also with updating the review until January 2007, when they stepped down from the review team.
We are grateful to the following authors who have provided additional data and information for the included studies:
Alison Beattie, Ninewells Hospital, Dundee, UK Dr Brandli, Surcher Hohenklinik Wald, Lausanne, Switzerland Dr Katrina Campbell, King's College London Professor T Cederholm, Uppsala University Hospital, Sweden Dr Daniel de Luis, Hospital Universitario Rio Hortega, Spain Dr R Fonseca, Dean of School of Dental Medicine, PA, USA Dr Liv Forli, Rikshospitalet, Norway Dr Liliana Gonzalez‐Espinoza, Hospital de Especialidades, Mexico Dr Geeta Hampson, St Thomas' Hospital, London Dr Mary Hickson, Hammersmith Hospital, UK Sharleen Imes, University of Alberta, Canada Dr Elizabeth Isenring, Flinders University, Adelaide, Australia Dr Martin Jensen, Denmark Professor Ulrich Keller, University Hospital, Basel, Switzerland Daina Kalnins, The Hospital for Sick Children, Toronto, Canada Astrid Lovik, Norway Dr Francesco Manguso, Fredico II University of Naples, Italy Kathleen Mayer, Ross Laboratories, Colombus, OH, USA Professor Donna McCarthy, University of Wisconsin Hospitals, USA Joseph Murphy, Ottawa Hospital, Ottawa, Canada Dr Kristina Norman, Universitatsmedizin Berlin, Germany Dr Guenter Ollenschlager, Cologne, Germany Dr Nick Paton, St Georges Hospital, London Prof Helene Payette, University of Sherbrooke, Canada Dr Christina Persson, Akademiska Sjukhuset, Uppsala, Sweden Dr Margareta Persson, Uppsala University Hospital, Sweden Dr Thomas Petty, Presbyterian St. Luke's Hospitals, CO, USA Dr Paula Ravasco, Santa Maria University Hospital, Portugal Dr Robert Rogers, University of Pittsburgh, PA, USA Dr Elizabeth Rydwik, Karolinska Institutet, Sweden Dr Achim Schwenk, St Georges Hospital, London, UK Dr Siddharth Singh, All India Institute of Medical Sciences, India Dr Elizabeth Weekes, Guys & St Thomas' NHS Foundation Trust, London Dr Samuel Y Wong, Prince of Wales Hospital, Hong Kong Additionally we are grateful to Professor Marinos Elia, Institute of Human Nutrition, Southampton General Hospital, UK for his expert advice and guidance on clinical nutrition for this review. Also to Reinhard Wentz, Dipl. Bibl., Campus Library Manager, Imperial College London, UK and Nick Woolley, Information Specialist King's College, University of London, UK for their help with developing the search strategy.
Many thanks to The Systematic Review Training Unit who provided support, funded by the London Regional Health Authority.
Appendices
Appendix 1. Search strategy used from 2002‐2005 on MEDLINE, EMBASE, CINAHL, CancerLit and AMED, using OVID notation with slight variations
| Search terms |
| A. nutrition$ or nutritive or diet or diet therapy or (energy and intake) or dietary service$ or dietary or eating or food or feeding or feeding behaviour or feeding behavior or food habit$ or diet advice or dietary advice or dietetics or dietician$ or caloric intake or calorie intake or (dietary and supplement$) or (formula$ and food) or food supplements or elemental).af or dh.fs |
| B. weight gain or (weight adj5 gain) or nutrition$ status or (nutrition$ adj5 status) or ((improv$ or gain$ or increase$) adj5 (weight or intake)).af |
| C. (random$ or rct$ or double blind or single blind or treble blind or triple blind or (control$ and trual$) or (clinical adj5 trial$) or trial or trials or systematic$ review$ or metaanal$ or meta‐analys$).af |
| ((A.ti and B) or (A and B.ti)) and C |
Appendix 2. Search strategy for searches undertaken on Medline and Embase from 2005 to February 2010
| Search terms |
| 1 nutrit*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (197693) 2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/ (167285) 3 diet*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (374221) 4 Diet/ or exp *Diet Therapy/ (96580) 5 eat*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (70476) 6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/(35764) 7 food.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (269212) 8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/ (37268) 9 feed*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (231101) 10 exp *Eating/ (17444) 11 calori*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (47662) 12 exp *Energy Intake/ (7820) 13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/ (13550) 14 energy.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (250080) 15 oral nutritional supplement.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (25) 16 exp *Dietary Supplements/ (10700) 17 exp *Nutritional Support/ (21933) 18 sip feed.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (17) 19 suppl*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (287335) 20 exp *Adult/ or exp *Dietary Supplements/ (35396) 21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5 (1362303) 22 weight.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (626210) 23 weight.mp. (626210) 24 weight gain.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (34990) 25 exp *Weight Gain/ (4196) 26 nutrit* status.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (24171) 27 exp *Nutritional Status/ (6871) 28 27 or 25 or 22 or 24 or 26 or 23 (642887) 29 28 and 21 (180107) 30 limit 29 to yr="2005 ‐ 2008" (34527) 31 limit 30 to humans (22734) 32 limit 31 to controlled clinical trial (200) 33 from 32 keep 1‐200 (200) 34 from 33 keep 1‐200 (200) 35 from 33 keep 1‐200 (200) |
Appendix 3. Search strategy for searches undertaken on Cinahl from 2005 to February 2010
| Search terms |
| (TX+(weight)+OR+(TX+(nutrit*) +AND+ (TX+(nutrit*) +OR+(TX+(diet*) +OR+(TX+(eat) +OR+(TX+(food) +OR+(TX+(feed*) +OR+(TX+(calori*) +OR+(TX+(energy) +OR+(TX+(oral+nutritional+supplement) +OR+(TX+(sip+feed) +OR+(TX+(suppl*) +OR+(TX+(educat*) +OR+(TX+(behav*) +OR+(TX+(snack) |
TX= full text
Appendix 4. Search strategy for searches undertaken on Web of Science from 2005 to February 2010
| Search terms |
| (nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav*) AND (nutrit* OR weight gain) AND (random* OR RCT OR control* OR clinical) NOT (child* OR infant OR paediatric) NOT (animal OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog) |
Appendix 5. Searches undertaken on SCOPUS from 2005 to February 2010
| Search terms |
| (nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav*) AND (nutrit* OR weight gain) AND (random* OR RCT OR control* OR clinical) NOT (child* OR infant OR paediatric) NOT (animal OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog) |
Data and analyses
Comparison 1. Dietary advice compared with no advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 10 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.70, 2.94] |
| 1.1 Zero to three months | 6 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.59, 3.08] |
| 1.2 Four to six months | 4 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.40, 7.57] |
| 2 Number of people admitted or readmitted to hospital | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Four to six months | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.50] |
| 3 Change in weight (kg) | 9 | 733 | Mean Difference (IV, Random, 95% CI) | 1.47 [0.32, 2.61] |
| 3.1 Zero to three months | 6 | 451 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐0.82, 3.42] |
| 3.2 Four to six months | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 1.46 [‐1.03, 3.95] |
| 3.3 Twelve months and over | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 3.75 [0.97, 6.53] |
| 4 Change in mid‐arm muscle circumference (MAMC) (cm) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.81 [0.31, 1.31] |
| 4.1 Zero to three months | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.02 [0.65, 1.39] |
| 4.2 Four to six months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.09, 1.09] |
| 5 Change in triceps skinfold thickness (mm) | 3 | 222 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐1.37, 1.67] |
| 5.1 Zero to three months | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐3.15, 0.83] |
| 5.2 Four to six months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 1.27 [‐0.04, 2.58] |
| 5.3 Twelve months or over | 1 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐2.32, 2.04] |
| 6 Change in energy intake (kcal) | 7 | 472 | Mean Difference (IV, Random, 95% CI) | 257.78 [‐0.74, 516.30] |
| 6.1 Zero to three months | 6 | 322 | Mean Difference (IV, Random, 95% CI) | 283.19 [‐107.18, 673.56] |
| 6.2 Four to six months | 1 | 150 | Mean Difference (IV, Random, 95% CI) | 63.70 [55.29, 72.11] |
| 7 Change in grip strength (kg force) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Four to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Dietary advice compared with nutritional supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Zero to three months | 6 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.31] |
| 2 Number of people admitted or readmitted to hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Zero to three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in weight (kg) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Zero to three months | 7 | 399 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐2.42, 2.42] |
| 4 Change in mid‐arm muscle circumference (MAMC) (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Zero to three months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in triceps skinfold thickness (TSF) (mm) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Zero to three months | 2 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.41, 0.33] |
| 6 Change in energy intake (kcal) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Zero to three months | 7 | 279 | Mean Difference (IV, Random, 95% CI) | 21.34 [‐201.44, 244.12] |
| 7 Change in grip strength (kg force) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Zero to three months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Dietary advice compared with dietary advice plus nutritional supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Zero to three months | 6 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.08, 3.95] |
| 1.2 Four to six months | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of people admitted or readmitted to hospital | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.00, 2.34] |
| 2.1 Zero to three months | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.97, 3.36] |
| 2.2 Four to six months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.70, 2.02] |
| 3 Change in weight (kg) | 11 | 636 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.03, 1.93] |
| 3.1 Zero to three months | 9 | 592 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐0.12, 2.06] |
| 3.2 Four to six months | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐1.52, 2.53] |
| 4 Change in weight (kg) without Beattie 2000 & Paton 2004 | 9 | 507 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.36, 0.75] |
| 4.1 Zero to three months | 7 | 463 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.40, 0.75] |
| 4.2 Four to six months | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐1.52, 2.53] |
| 5 Change in mid‐arm muscle circumference (MAMC) (cm) | 3 | 492 | Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.35, ‐0.43] |
| 5.1 Zero to three months | 2 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.34, ‐0.36] |
| 5.2 Four to six months | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1.23 [‐2.65, 0.19] |
| 6 Change in triceps skinfold thickness (TSF) (mm) | 6 | 386 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.34, ‐0.09] |
| 6.1 Zero to three months | 5 | 358 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐2.51, ‐0.12] |
| 6.2 Four to six months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐3.99, 4.19] |
| 7 Change in energy intake (Kcal) | 6 | 189 | Mean Difference (IV, Random, 95% CI) | ‐192.80 [‐481.92, 96.31] |
| 7.1 Zero to three months | 4 | 145 | Mean Difference (IV, Random, 95% CI) | ‐344.88 [‐600.28, ‐89.47] |
| 7.2 Four to six months | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 226.84 [‐223.19, 676.87] |
| 8 Change in grip strength (kg force) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Zero to three months | 4 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.67 [‐2.96, ‐0.37] |
Comparison 4. Dietary advice plus supplements if required compared with no advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Zero to three months | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.47, 1.93] |
| 1.2 Four to six months | 3 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 2.00] |
| 1.3 Seven to twelve months | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.76, 2.10] |
| 1.4 Twelve months and over | 2 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 2 Mortality using only Persson final time‐point | 9 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.05] |
| 2.1 Zero to three months | 3 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.32, 5.44] |
| 2.2 Four to six months | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.30, 3.70] |
| 2.3 Seven to twelve months | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.25, 4.66] |
| 2.4 Twelve months and over | 2 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 3 Change in weight (kg) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Zero to three months | 5 | 232 | Mean Difference (IV, Random, 95% CI) | 1.74 [0.53, 2.95] |
| 3.2 Four to six months | 4 | 281 | Mean Difference (IV, Random, 95% CI) | 1.87 [‐0.07, 3.81] |
| 3.3 Seven to twelve months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.7 [‐0.84, 2.24] |
| 3.4 Twelve months and over | 2 | 77 | Mean Difference (IV, Random, 95% CI) | 2.17 [‐1.20, 5.54] |
| 4 Change in weight (kg) using only Persson final time‐point | 9 | 454 | Mean Difference (IV, Random, 95% CI) | 2.20 [1.16, 3.25] |
| 4.1 Zero to three months | 4 | 155 | Mean Difference (IV, Random, 95% CI) | 1.92 [0.28, 3.56] |
| 4.2 Four to six months | 3 | 222 | Mean Difference (IV, Random, 95% CI) | 2.65 [0.98, 4.31] |
| 4.3 Twelve months and over | 2 | 77 | Mean Difference (IV, Random, 95% CI) | 2.17 [‐1.20, 5.54] |
| 5 Change in triceps skinfold thickness (TSF) (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Four to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in energy intake (Kcal) | 6 | 358 | Mean Difference (IV, Random, 95% CI) | 212.71 [‐0.91, 426.32] |
| 6.1 Zero to three months | 4 | 190 | Mean Difference (IV, Random, 95% CI) | 184.40 [‐109.01, 477.81] |
| 6.2 Four to six months | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 71.0 [‐125.65, 267.65] |
| 6.3 seven to twelve months | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 464.0 [270.07, 657.93] |
| 7 Change in grip strength (kg force) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Four to six months | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 6.44 [‐3.15, 16.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arnold 1989.
| Methods | Randomised controlled trial. Duration 6 months. Intervention to 10 weeks and follow‐up to 6 months for some outcomes. | |
| Participants | Adults (n = 50: 29 men and 21 women aged 34 ‐ 88 years) living at home, planned to receive radiotherapy for cancers of head and neck. Mean weight in treatment and comparison groups was 1 ‐ 2 kg below usual weight at study entry. 3 deaths in the dietary counselling and supplement group. |
|
| Interventions | Intensive dietary counselling (n = 27) versus intensive dietary counselling and the prescription of nutritional supplements to provide an additional 960 ‐ 1080 kcal/day (n = 23). | |
| Outcomes | Survival*, number having a complete response to therapy, radiation side‐effects, tumour status, body weight*, serum albumin, transferrin and change in dietary energy*, protein intake. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details of method. |
| Allocation concealment (selection bias) | Unclear risk | No details of method of allocation concealment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. 3 deaths in the dietary counselling and supplement group. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. Data on mortality obtained from the paper. Data on weight change obtained by extrapolation from Figure 3. Energy intake data presented in a figure with no standard deviations or standard error, therefore risk of bias. No response received from author to request for data. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
Baldwin 2008.
| Methods | Randomised controlled trial. Duration 12 months. Intervention 6 weeks and follow‐up to 12 months. | |
| Participants | Adults (n = 358: 256 men, 102 women; median age 66, range 24 ‐ 88 years) with locally advanced or metastatic cancers of the gastrointestinal tract (n = 277) or non‐small‐cell lung cancer or mesothelioma. All patients had lost weight at the start of the trial (mean 9.8% (SD 6%) in lung patients and 11.2% (SD 6.4%) GI patients). 153 participants were alive at 12 months: No intervention group: 47 deaths and 2 withdrawals. Dietary advice group: 52 deaths and 2 withdrawals. Nutritional supplements group: 55 deaths and 2 withdrawals. Dietary advice and supplements group: 44 deaths and 1 withdrawal. |
|
| Interventions | This was a 2 x 2 factorial trial. Group 1 received no additional intervention (n = 96). Group 2 received dietary advice to increase intake by 600 kcals per day) (n = 90). Group 3 received an oral nutritional supplement providing 588 kcals per day (n = 86). Group 4 received dietary advice to increase intake by 600 kcals per day and an oral nutritional supplement (n = 86). |
|
| Outcomes | Survival, QoL, weight, handgrip strength, energy intake. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an independent trials centre using a computer‐generated list. Participants were stratified for performance status and site of disease. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed until participants had signed consent to participate. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | The outcome assessors were not blinded. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The outcome assessors were not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 153 participants were alive at 12 months: No intervention group: 47 deaths and 2 withdrawals. Dietary advice group: 52 deaths and 2 withdrawals. Nutritional supplements group: 55 deaths and 2 withdrawals. Dietary advice and supplements group: 44 deaths and 1 withdrawal. Reasons for withdrawal not known for all patients and therefore risk of bias. |
| Selective reporting (reporting bias) | Low risk | The study is published as an abstract at present. The data on survival, change in weight and change in energy intake are presented in the abstract but not in a format that would enable entry into a meta‐analysis, therefore original data have been provided by the authors for this review. The numbers of participants completing assessment of energy intake was only 31 of 358 and so these data should be interpreted with caution. |
| Other bias | Low risk | Baseline characteristics for the 4 groups were similar. |
Beattie 2000.
| Methods | Randomised controlled trial. Duration 10 weeks. | |
| Participants | Adults (n = 109, men and women) resuming oral food intake after surgery. BMI < 20 kg/m2, TSF or MAMC <15th percentile or > 5% weight loss. Mean (SD) age dietary advice group 62.4 years (10.9 years) and in dietary advice and supplement group 54.4 years (19.4 years). 101 completed study, 5 dropouts in routine nutritional management group and 3 in supplement group. | |
| Interventions | Routine nutritional management (n = 54) or routine nutritional management and 400 ml of a 1.5 kcal/ml nutritional supplement (n = 55). | |
| Outcomes | Survival*, weight*, BMI*, MAMC*, TSF*, handgrip strength*, complication rate, wound infection, chest infection, antibiotic use, QOL. | |
| Notes | Routine nutritional management provided by more than one dietitian and not described in the paper. Information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | The allocation was not concealed physically but the list of numbers was not consulted until the participant was recruited. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | The paper states that assessments were not blinded to treatment. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The paper states that assessments were not blinded to treatment. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The paper states that assessments were not blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 dropouts (2 lost to follow‐up, 3 required artificial nutritional support) in routine nutritional management group and 3 (1 transferred to intensive care unit, 2 required artificial nutritional support) in supplement group. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported and data for analysis were extracted from the paper. Additional information on study quality obtained from authors. |
| Other bias | Unclear risk | Baseline variables provided, but groups not similar ‐ group receiving advice plus supplements was younger than the advice only group. Routine nutritional management provided by more than one dietitian and not described in the paper. |
Berneis 2000.
| Methods | Randomised controlled trial. Duration 12 weeks. | |
| Participants | Adults (n = 15, 14 men and 1 woman, age not reported) with HIV infection and weight loss 5% or more in 6 months or BMI < 21 or CD4 cell count < 500/mm‐3. Original group consisted of 18 participants, but 3 not included because of non‐adherence and severe disease complications. | |
| Interventions | Dietary advice and nutritional supplements (target unspecified, n = 8) versus no nutritional therapy (n = 7). | |
| Outcomes | Weight*, lean and fat mass, macronutrient intake, energy intake*, immune function, QOL. | |
| Notes | Additional data and information on quality requested from authors. Received a reply to say information no longer available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author states that randomisation performed by pharmacy using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | The author could not supply details about allocation concealment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Original group consisted of 18 participants, but 3 not included because of non‐adherence and severe disease complications. Author unable to provide details of which groups the drop‐outs were in. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported, as mean change at baseline and end of follow‐up therefore change scores were calculated and SDs imputed. |
| Other bias | High risk | Baseline variables not stated, don't know if groups similar at baseline. |
Campbell 2008.
| Methods | Randomised controlled trial. Duration 12 weeks. | |
| Participants | Adults (n = 62) with stage 4 chronic kidney disease, 56 started study, male n = 34, female n = 22, intervention group mean age 69.5 (SD 11.7) years; control mean age 70.9 (SD 11.6) years. Nutritional status assessed using SGA, intervention group 24% malnourished (SGA B), control group 11% malnourished (SGA B). 50 patients completed the study. |
|
| Interventions | Dietary counselling to increase energy intake, maintain protein intake and information on appropriate nutritional choices for renal patients (n = 24) versus usual care (generic information on nutrition, n = 26). | |
| Outcomes | Dietary intake*, body weight*, nutritional status (SGA), body composition (total body potassium), survival * biochemistry. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number sequence. |
| Allocation concealment (selection bias) | Low risk | Described as concealed to recruiting officer until after baseline assessment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 62 participants randomised in the study: 6 participants did not receive the intervention; 6 were lost to follow; 4 deaths ( all in the intervention group); 2 participants received dialysis. |
| Selective reporting (reporting bias) | Low risk | Data on mortality, change in weight and energy intake were used in this review. The data are reported in the paper but the weight data is presented as a mean (SD) at baseline and at 12 weeks and therefore the mean change with SD has been obtained from the authors. The energy intake data reported in kJ/kg, mean change (SD) in kcals obtained from the author. |
| Other bias | Unclear risk | Baseline variables stated, groups similar at baseline apart from amounts of malnutrition (see above). |
Chandra 1985.
| Methods | Randomised controlled trial. Duration 4 weeks. | |
| Participants | Elderly adults (n = 30, aged 70 ‐ 84 years) with clinical and biochemical parameters suggesting malnutrition. No information on dropouts. | |
| Interventions | Dietary advice and supplements (n = 15) versus no intervention (n = 15). | |
| Outcomes | Weight*, TSF*, biochemistry. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details. |
| Selective reporting (reporting bias) | High risk | Not all outcome data reported. Data presented on change in weight and TSF and prealbumin for the intervention group only. No data extracted and no response to request for data from the author. |
| Other bias | High risk | Baseline characteristics not stated. |
de Luis 2003.
| Methods | Randomised controlled trial. Duration 3 months. | |
| Participants | Adults (n = 70, dietary advice + supplement group 71.4% men, mean age (SD) 37.5 years (11), dietary advice group 82.8 % men, mean age (SD) 39.9 years (9 years)) with HIV infection and 5% or more weight loss in previous 6 months. | |
| Interventions | Dietary advice to increase energy and protein intake and 3 x 250 ml supplement (Ensure) (n = 33) versus dietary advice to increase energy and protein intake (n = 33). | |
| Outcomes | Survival*, weight*, BMI*, TSF*, MUAC*, energy intake*, immune function, cardiac function. | |
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from the author indicated that a random number series was used to generate a sequence. |
| Allocation concealment (selection bias) | Low risk | Information from the author indicated that sealed envelopes were used to conceal allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Information from the author indicated that outcome assessment was not blinded. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Information from the author indicated that outcome assessment was not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Information from the author indicated that outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants withdrew between randomisation and baseline: Intervention group: 4 deaths, 1 loss to follow‐up; Control group: 1 loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but not all in a format usable for meta‐analysis. Change in weight, TSF, MAMC and energy intake are reported as mean (SD) at baseline and end of intervention. Mean change (SD) obtained from authors. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
Dixon 1984.
| Methods | Randomised controlled trial. Duration 4 months. | |
| Participants | Adults (n = 88, 50 men and 38 women, mean (SD) age 59.6 years (13.7 years) with >5% weight loss in previous 2 months or persistent change to eating habits or problems interfering with eating, undergoing palliative treatment or chemotherapy for cancer affecting a variety of sites (main sites colorectal (27%) lymphoma (16%)). 63% of participants completed the study. 23 deaths and 10 dropouts, groups not specified. |
|
| Interventions | Nutritional counselling (n = 9), nutritional counselling and a range of nutritional supplements (n = 9), structured relaxation training, relaxation training and a nutritional supplement and a control group receiving home visits but no nutritional advice (n = 10). | |
| Outcomes | Survival*, body weight*, TSF*, MAMC*, performance status (Karnofsky scale), subjective evaluation of helpfulness. | |
| Notes | Data from two interventions will be used: 1. nutritional counselling versus no dietary advice; and 2. nutritional counselling versus nutritional counselling and nutritional supplements. Nutritional counselling provided by nurses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 63% of participants completed the study. 23 deaths and 10 dropouts, groups not specified therefore risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. No data usable for analysis because data are presented as mean change from desirable weight and change from standard for TSF and analysed using ANOVA. No response received from author. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
Evans 1987.
| Methods | Randomised controlled trial. Duration 12 weeks (all outcomes) & 3 ‐ 5 years (survival). | |
| Participants | Adults (n = 180, 109 men and 71 women; intervention group aged 23 ‐ 79 years, control group aged 33 ‐ 78 years) receiving chemotherapy for advanced colorectal and non‐small‐cell lung cancer. 46% of patients were malnourished at study entry defined as >5% weight loss. 156 deaths in the 3 study groups. |
|
| Interventions | Nutritional counselling to achieve a target caloric intake (using supplements if required, n = 51) versus nutritional counselling to achieve target caloric intake but including 25% of calories as protein (using food and protein supplements) plus a supplement of zinc and magnesium versus ad lib food intake (n = 69). | |
| Outcomes | Body weight*, energy intake*, survival*, tumour response to chemotherapy. | |
| Notes | Data from two interventions will be used: 1. nutritional counselling to achieve target caloric intake 2. ad lib food intake (111 participants). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a central office. Participants were stratified and randomisation blocked. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by using a central office. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 156 (88 lung cancer; 68 colorectal, 94 in the two intervention groups combined and 62 in the control group) deaths in the 3 study groups. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but data presented as median % change and therefore not in a usable format and author unable to supply data. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
Forli 2001.
| Methods | Randomised controlled trial. Duration 10‐18 days. | |
| Participants | Adults (n = 37, 18 men and 19 women, dietary advice and supplement group, mean age 49 years (range 44 ‐ 53); and no advice group, mean age 48 years range (44 ‐ 52)) with end‐stage lung disease awaiting transplantation. All patients malnourished defined as BMI <18.7 kg/m2. 2 participants withdrew from each group. |
|
| Interventions | Dietary advice to take an energy‐rich diet and supplements if wanted (n = 20) versus normal hospital diet (n = 22). | |
| Outcomes | Survival*, weight*, BMI, TSF*, MAMC*, MUAC*, respiratory function*. | |
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described in the paper as using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Stated in the paper as assessed blind to intervention group. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Stated in the paper as assessed blind to intervention group. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | All assessments of nutritional status performed by the study dietitian who was not blinded to intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew from each group, the reasons for withdrawals are clearly stated. |
| Selective reporting (reporting bias) | High risk | Data not reported for clinical and functional outcomes but stated as not significantly different. Data on weight reported as median change with no SD, therefore mean change (SD) obtained from author. Data on energy intake reported as median intake kj/kg therefore obtained from authors. |
| Other bias | Unclear risk | Baseline variables given, but one assessment of lung function was significantly different. |
Fuenzalida 1990.
| Methods | Randomised controlled trial. Duration 42 days (21 days in hospital and 21 days at home). | |
| Participants | Adults (n = 9, all men, mean age (SD) 62.4 years (5.6 years)) with COPD with FEV1 30 ‐ 50% of predicted and >5% weight loss, mean per cent IBW at study entry 78.5% (SD 9.6%). | |
| Interventions | Individualised diet planned by dietitian to provide 100% of recommended daily intake (n = 4) versus individually planned diet and 1080 kcal of a nutritional supplement (n = 5). | |
| Outcomes | Survival*, weight*, BMI*, TSF*, MAMC*, MUAC*, energy intake*, measures of pulmonary function (FEV1*), measures of immune function. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses occurred during the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported but not in a format suitable for direct entry into a meta‐analysis. Data in the paper on weight have been used to derive mean change (SD). Information on study quality obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Ganzoni 1994.
| Methods | Randomised controlled trial. Duration 12 months. | |
| Participants | Adults (n = 30, average age 66 years) with COPD, average FEV1 0.81 (range 0.4 ‐ 1.51), average body weight 52 kg (range 38 ‐ 68 kg), comment on use of cut‐off <90% IBW in introduction. 20 participants completed the study, there were 5 deaths. | |
| Interventions | Nutritional counselling to use a high calorie diet using a variety of methods including nutritional supplements if required (n = 15) versus no individual nutritional counselling (n = 14). Participants may have attended a group session where diet was discussed. | |
| Outcomes | Body weight*, 4‐site skinfolds (summed), survival*, energy intake*, respiratory function (FEV1* and 6‐minute walking distance). | |
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information obtained from author, randomisation performed using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Information from the author, a person not involved in the study administered the random allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Information obtained from author confirmed blind assessment of outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Information obtained from author confirmed blind assessment of outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Information obtained from author confirmed blind assessment of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 participants completed the study, there were 5 deaths, 3 in the intervention group and 2 in the control group. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but not in a format suitable for entry into meta‐analysis. Data on mortality obtained from the author as the detail in the paper was unclear. Data on mean change for weight are reported without a SD and therefore the original data have been obtained from the authors. Data on energy intake are reported as mean and range with no SD at baseline and end of follow‐up. Data requested from authors but no detail available. |
| Other bias | High risk | Baseline variables not given, don't know if groups similar at baseline. |
Gonzalez‐Espinoza 2005.
| Methods | Randomised controlled trial. Duration 6 months. | |
| Participants | Adults receiving continuous peritoneal dialysis (n = 30) (19 males and 9 females in final study group). Mean (SD) age dietary advice group 47.6 (17.4) years and mean (SD) age dietary advice and supplements group 45.7 (14.4) years. All patients malnourished according to SGA. 2 patients not included in the analysis from the dietary advice and supplements group because of deterioration in health. |
|
| Interventions | Nutrtional counselling plus a dried egg‐albumin‐based supplement added to milk or sprinkled on food (n = 13) versus nutritional counselling (n = 15). | |
| Outcomes | Survival, weight*, energy intake* BMI, MAMC*, TSF* hospital admission | |
| Notes | information on nutritional supplement from www.inovaalimentos.com/#Ultrashock | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | Low risk | Performed by a person external to the study once the patient had provided informed consent. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Information from author, outcomes assessed blinded to intervention. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Information from author, outcomes assessed blinded to intervention. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Information from author, outcomes assessed blinded to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients not included in the analysis from the dietary advice and supplements group because of deterioration in health. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported but not in a format suitable for entry into meta‐analysis. Data on change in weight, energy intake, TSF and MAMC are presented as mean (SD) at baseline and at end of intervention. Data on mean change (SD) from baseline were obtained from the authors for weight, energy and MAMC. SDs for change in TSF were imputed. Data on hospital admissions obtained from the authors. |
| Other bias | Low risk | Baseline characteristics reported. |
Gray‐Donald 1995.
| Methods | Randomised controlled trial.
Duration 12 weeks. Stratified randomisation according to gender and nutritional risk. |
|
| Participants | Elderly people living at home (n = 50, mean age 78 years) with involuntary weight loss of >5% in last month, >7.5% in last 3 months, >10% in last 6 months and BMI <27 or BMI <24. 4 deaths, 3 in the supplement group and 1 in the dietary counselling group. | |
| Interventions | Weekly visits from a dietitian with dietary counselling (n = 25) versus weekly visits from a dietitian and 2 x 235 ml of a nutritional supplement (n = 25). | |
| Outcomes | Survival*, body weight*, MAMC*, MUAC skinfold (triceps*, subscapular, suprailliac), energy intake*, handgrip strength*, perception of health, general well‐being score, number of falls. | |
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation according to gender and nutritional risk. |
| Allocation concealment (selection bias) | Low risk | Information from author indicates that sealed envelopes were used. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Clinical outcomes assessed blind. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Functional outcomes assessed blind. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Nutritional outcomes not assessed blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 deaths, 3 in the supplement group and 1 in the dietary counselling group. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported and data on mortality, change in weight, TSF, MAMC were extracted from the paper. Data on energy intake are presented as mean change in daily intake averaged over 3 months, therefore mean change (SD) from baseline to 3 months has been obtained from the authors. Data on grip strength are presented as a mean (SD) at baseline and at end of intervention, therefore mean change (SD) obtained from the author. |
| Other bias | Unclear risk | Baseline variables given, appetite was better in advice group than supplement group. |
Hampson 2003.
| Methods | Randomised controlled trial. Duration 12 months. | |
| Participants | Adult women (n = 71, dietary advice and supplement group, mean (SD) age 76 years (4.2), no advice group mean (SD) age 76.7 years (5.7)) with osteoporosis. All patients malnourished with BMI <21. 6 participants withdrew from the study, 5 in the dietary advice and supplement group. |
|
| Interventions | Dietary advice to increase intake and 2x 200ml supplement (Nutricia) +1 g calcium and 800 units cholecalciferol (n = 36) versus no dietary advice +1 g calcium and 800 units cholecalciferol (n = 35). | |
| Outcomes | Survival*, weight*, bone mineral density, fat mass, lean mass, energy intake*. | |
| Notes | Additional data on outcomes requested from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generated in a department external to the study (Department of Public Health). |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants withdrew from the study, 5 in the dietary advice and supplement group. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but not in a format suitable for entry into meta‐analysis. Data on energy intake reported as mean (SD) for groups at baseline and end of intervention, therefore mean change data obtained from the authors. Data on weight change reported as % change. Data requested from authors but not received. |
| Other bias | Unclear risk | Baseline variables given, treatment group were significantly lighter and had lower fat mass than the no treatment group. |
Imes 1988.
| Methods | Randomised controlled trial. Duration 6 months. | |
| Participants | Adults (n = 137, 62 men and 75 women, aged 17.5 ‐ 71.0 years) with Crohn's disease. CDAI (mean (SD) 110 (96) range 0 ‐ 463). People taking medication (42% taking prednisolone, 45% salazopyrin) or vitamin supplements (50%) and with active and inactive disease included. |
|
| Interventions | Monthly dietary counselling sessions aiming to achieve the 'Canadian Recommended Dietary Allowances' (n = 67). Control group no dietary intervention (n = 70). |
|
| Outcomes | Energy* and protein intake, vitamin and mineral intake, assessments of clinical condition, survival*, MAMC*, MUAC*, TSF*, hospital admissions*. | |
| Notes | Additional outcomes and longer follow up reported in separate papers. Additional data and information on quality obtained from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author cannot recall how the sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blind assessments of clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional assessments made. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Assessment of nutritional outcomes not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information obtained from the author. 137 participants randomised, 125 completed 6 months of study, 8 drop‐outs in the dietary advice group and 4 in the no dietary advice group. There were no deaths. Reasons for drop‐outs not reported. |
| Selective reporting (reporting bias) | Unclear risk | Data on mortality and hospital admissions could not be extracted from the papers and have been obtained from author. Additional outcomes and longer follow‐up reported in separate papers. |
| Other bias | Unclear risk | Baseline variables given, advice group were younger and had a lower CDAI that the no advice group. |
Isenring 2004.
| Methods | Randomised controlled trial. Duration 12 weeks. | |
| Participants | Adults (n = 60, 51 men and 9 women, mean age (SD) 61.9 years (14)) receiving radiotherapy for cancers of head & neck (88%) or abdomen (12%). At baseline 65% of participants were well‐nourished and 35 % malnourished (PG‐SGA). 6 participants were lost to follow up, 4 from the dietary advice and supplement group, 5 participants from no advice group requested referral to a dietitian. |
|
| Interventions | Individualised intensive nutritional counselling and nutritional supplements if appropriate (n = 29) versus standard nutrition booklet and participants could request referral to a dietitian (n = 31). | |
| Outcomes | Survival*, weight*, grip strength*, fat free mass (BIA), QOL, change in PG‐SGA score, energy intake*. | |
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details provided by the author, a random number table. |
| Allocation concealment (selection bias) | Low risk | Details provided by the author, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants were lost to follow up, details not given in the paper but provided by the author on request. 4 deaths (2 in the intervention group and 2 in the control group) and 2 others lost to follow up in the intervention group (1 as a result of deterioration in condition and 1 because patient discontinued treatment and withdrew from the study). |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported in text and figures but not in a format usable for meta‐analysis. Data on mortality and mean change (SD) for weight and energy intake obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Jensen 1997.
| Methods | Randomised controlled trial. Duration 110 days. | |
| Participants | Adults (n = 87, 42 men and 45 women, separated into >75 years and <75 years) post‐surgery (operable cancer colon/rectum (50), diverticulitis (15), ulcer (5) and other (17)). Nutritional status at inclusion was unclear. 28 dropouts (20 dietary advice group and 8 in no advice group). |
|
| Interventions | Dietary counselling to improve nutritional intake and aiming for a protein intake of 1.5 g/kg using oral nutritional supplements if required (n = 40) versus no nutritional advice (n = 47). | |
| Outcomes | Weight *, body composition (DEXA), energy intake*, appetite, fatigue assessments, handgrip strength*, work capacity, respiratory function*, QoL. | |
| Notes | Additional data awaited from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of sequence generation reported. Randomisation was stratified. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The paper states that the surgeon was blinded to intervention group. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Insufficient information in the paper. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Assessment not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 28 dropouts (20 dietary advice group and 8 in no advice group). Reasons for withdrawals not given in paper and not provided by authors on request. |
| Selective reporting (reporting bias) | Unclear risk | All specified outcomes reported but not in a format usable for meta analysis, energy intake data presented as kcal/kg and weight change data described in text as mean at baseline and end of follow‐up. Additional data on mean change (SD) for weight and energy intake requested from authors but not provided. |
| Other bias | Unclear risk | Baseline variables given, control group (no advice) were significantly older and heavier than the treatment (advice plus supplements if required) group. |
Kalnins 2005.
| Methods | Quasi‐randomised controlled trial. Duration 6 months. Intervention for 3 months and follow‐up to 6 months. | |
| Participants | Adults and children (n = 13, mean age (SD) 27.4 years (8.4 years)) with CF. <90% weight for height or 5% reduction in weight for height over 3 months. 2 dropouts, 1 in each group. |
|
| Interventions | Dietary counselling to increase food intake by 20% of predicted requirements (n = 2) versus a nutritional supplement to increase energy intake by 20% of predicted requirements (n = 3). | |
| Outcomes | Survival*, z scores for weight* and height, weight for height, anthropometric measures, pulmonary function*, energy* and nutrient intake, faecal balance studies. | |
| Notes | Data on participants >16 years of age (n = 5) obtained from author. Data on children not used. No dropouts amongst adults. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised using cards with advice or supplement written on them. The patient selected a card blind. Then the next patient randomised received the other intervention group. |
| Allocation concealment (selection bias) | High risk | Investigators used alternate allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | No blind assessment. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | No blind assessment. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | No blind assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported 2 dropouts, 1 in each group. Trial was of mixed ages, obtained information from authors that drop outs were children and not adults. |
| Selective reporting (reporting bias) | Low risk | This paper reports outcomes for adults and children combined. Details of mean change (SD) weight and energy intake for the 5 adults have been obtained from the authors. |
| Other bias | High risk | Baseline variables not given, unsure if groups similar at baseline. |
Kendell 1982.
| Methods | Randomised controlled trial. Duration 6 weeks. | |
| Participants | Adults (n = 24, 5 men and 19 women, mean age (SD) 25 years (8.1)), awaiting elective orthognathic surgery. 12 of 24 patients had a weight below IBW at inclusion. 100% follow up. |
|
| Interventions | Dietary instruction given verbally and in writing (n = 12) versus dietary instruction and an oral nutritional supplement (1.5 kcal/ml) to provide 50% of calculated energy requirements (n = 12). | |
| Outcomes | Survival*, body weight*, MAUC, MAMC*, TSF*, serum chemistry and creatinine height index, macro and micronutrient intake, length of hospital stay. | |
| Notes | Data not available from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow up. |
| Selective reporting (reporting bias) | High risk | All outcomes reported but using general statements e.g. 'at each time interval, there no statistically significant differences in body weight, MAC, TSF and creatinine height index between the experimental and control groups'. Data presented in table as % deficit and data not available from the authors, therefore risk of bias due to partial reporting. |
| Other bias | High risk | Baseline variables not given, no information available from authors, not sure if groups similar at baseline. |
Lovik 1996.
| Methods | Randomised controlled trial. Duration 6 weeks. | |
| Participants | Adults (n = 52, men and women, age range 34 ‐ 86 years) who had received radiotherapy for cancers of head and neck. Nutritional status at study entry unclear, 10% reported weight loss and BMI ranged from 18 ‐ 37kg/m2. 3 deaths (group not reported). |
|
| Interventions | Intensive dietary instruction from a dietitian including advice to use nutritional supplements if required (n = 28) versus a standard information sheet providing information on all aspects of treatment and including advice to eat a nutritious diet (n = 24). | |
| Outcomes | Body weight*, BMI*, TSF*, MAMC*, MUAC, energy intake*, survival*, serum chemistry, albumin and transferrin. | |
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details from author, sequence generation using a random number list. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 deaths all in the intervention arm. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported. Data on change in weight extracted from the paper, but clarification needed for mortality data. Data on TSF, MAMC presented as number of patients with values below 85% of the normal limit and so not included. Data on energy intake is expressed according to expected intake therefore not usable. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Macia 1991.
| Methods | Randomised controlled trial. Duration of follow up not reported. | |
| Participants | Adults (n = 92, age and sex not reported) receiving radiotherapy for cancers of head and neck, breast and abdominopelvic area. Nutritional status of participants unclear. Numbers of withdrawals and deaths not reported. |
|
| Interventions | Dietary instructions on appropriate alimentation during radiotherapy given verbally and in writing (n = 30) versus ad lib food intake and no dietary instruction (n = 62). | |
| Outcomes | Weight*, TSF*, MAUC*, MAMC*, BMI*, total protein, albumin, transferrin, total lymphocyte count, iron, cholesterol, triglycerides, clinical observations, overall food* intake. | |
| Notes | Dietary advice given by doctors from nutrition and dietetic unit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used coin toss to randomise participants. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Paper states that clinical variables were assessed by doctors unaware of group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers of withdrawals and deaths not reported. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but as mean change at baseline and end of follow‐up according to site of tumour therefore change scores were calculated and SDs imputed. No response received from author to requests for data. |
| Other bias | High risk | Baseline variables not given, not sure if groups similar at baseline. |
Manguso 2005.
| Methods | Randomised controlled cross‐over trial. Total duration of trial 6 months, only results from the first 3 months will be considered. | |
| Participants | Adults (n = 90, mean age 60 years (IQR 9), 52 males, 38 females) admitted to a specialist unit for the management of liver cirrhosis. Nutritional status not categorised. 3 withdrawals, but number of deaths not reported. |
|
| Interventions | Controlled diet consisting of specific prescription for macronutrients and calcium (n = 45) versus spontaneous diet (n = 45). | |
| Outcomes | weight*, MAMC*, TSF*, energy intake*, Childs Score, biochemistry profile | |
| Notes | Weight may be inappropriate in analysis due to possible presence of ascites. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | None measured. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Information from author, the assessor was blinded to intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information from author: no deaths occurred in the study. 1 patient withdrew from the dietary advice group and 2 from the no intervention group. |
| Selective reporting (reporting bias) | Low risk | Results on all specified outcome measures reported but as mean (SD) at baseline and end of intervention. Mean change (SD) for weight, energy intake, TSF and MAMC and additional information obtained from authors. |
| Other bias | Low risk | Baseline characteristics comparable between groups. |
McCarthy 1999.
| Methods | Randomised controlled trial. Duration 4 weeks. | |
| Participants | 40 adults (mean (SD) age treatment group 59.6 years (9.6 years), mean (SD) age control group 55.6 years (14 years) beginning a course of curative radiotherapy for stage 1 or 2 cancer. Nutritional status of participants unclear. 8 participants lost to follow up, 6 in the experimental group and 2 in the control group. |
|
| Interventions | Weekly nutritional counselling to maintain recommended dietary intake of calories and protein plus 8 oz of 1.0 kcal/ml nutritional supplement (n = 19) versus weekly nutritional counselling (n = 18). | |
| Outcomes | Energy intake*. | |
| Notes | Data obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss used to randomise participants. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | None measured. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | None measured. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Paper states that assessments were made by the nurse and dietitian that implemented the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 participants lost to follow up, 6 in the experimental group and 2 in the control group. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but presented in a figure and so not in a format usable for meta‐analysis. Mean change (SD) in energy intake obtained from authors. |
| Other bias | Unclear risk | Baseline variables given, the supplement group weighed less and received less radiotherapy. |
Moloney 1983.
| Methods | Randomised controlled trial. Duration unclear, outcomes reported at different time points. Intervention given for 3‐5 weeks, survival reported to 1 year. | |
| Participants | Adults (n = 84, 50 men, 34 women, dietary advice and supplement group mean age 63.26 years, no advice group mean age 55.2 years) with cancer (various sites) undergoing radiotherapy. No information on nutritional status given. No information on attrition. | |
| Interventions | Dietary counselling and supplements versus (n = 42) no advice (n = 42). | |
| Outcomes | Survival*, energy intake*, macro and micronutrient intake. | |
| Notes | Data for survival given at 9 months for dietary advice and supplement group and at 11 months for no advice group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on attrition. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. Mortality data obtained from the paper. Data on change in energy intake is expressed as mean (SD) at baseline and end of intervention therefore change scores were calculated and SDs imputed. No response received from author. |
| Other bias | Unclear risk | Baseline variables given, treatment group were older. |
Murphy 1992.
| Methods | Randomised controlled trial.
Duration 16 weeks. Outcomes not assessed blind. |
|
| Participants | Adults (n = 22, all men, mean age (SD) 37.3 years (6.7 years) with HIV infection who had involuntary weight loss of >5%. 6 dropouts, 5 in the dietary counselling group and in the dietary counselling and supplement group. |
|
| Interventions | Dietary counselling verbally and in writing to consume a calculated amount of energy and protein per day (n = 11) versus dietary counselling (as above) and 2x 235 ml of a supplement (1.5 kcal/ml) (n = 11). | |
| Outcomes | Survival*, body weight*, BMI*, MUAC*, serum albumin, energy* and protein intake. | |
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were selected consecutively as they presented with weight loss. No details of the group for the first participant. |
| Allocation concealment (selection bias) | High risk | Investigators used alternate allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Paper states outcomes not assessed blind. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Paper states outcomes not assessed blind. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Paper states outcomes not assessed blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 dropouts, 5 in the dietary counselling group and 1 in the dietary counselling and supplement group. 5 dropouts because of subsequent GI disease, and 1 due to self exclusion. |
| Selective reporting (reporting bias) | Unclear risk | All specified outcomes reported but continuous variables presented as mean (SD) at baseline and end of intervention. Mean change with SDs for weight has been imputed. Data on change in energy intake are presented with precise P values and so mean change (SD) obtained by calculation. Additional data and information on quality obtained from authors. |
| Other bias | High risk | Baseline variables given, dietary counselling group and weighed 5 kg less than the dietary counselling group and supplement group. |
Norman 2008b.
| Methods | Randomised controlled trial. Duration 3 months. | |
| Participants | Adults (n = 101) with benign gastrointestinal disorders (gender not stated). Intervention group mean age 52.2 (SD 16.5), control group mean age 53.6 (SD 16.8). All malnourished according to SGA (grade B or C). 21 dropouts, 10 intervention group (withdrew before baseline) and 11 lost to follow‐up in the control group. |
|
| Interventions | Dietary counselling from a dietitian to increase energy and protein intake from food and up to 3 x 200 ml Fresubin protein energy drinks (n = 48) versus dietary counselling to increase energy and protein intake from food (n = 48). | |
| Outcomes | Energy intake*, weight*, height, BMI*, TSF*, MUAC*, body composition (BIA), handgrip strength*, length of stay, number of readmissions*, number of prescribed drugs on discharge, peak expiratory flow. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Operated by co‐worker not involved in the study. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21 dropouts:
Dietary counselling and supplement group: 10 withdrew before baseline;
Dietary counselling alone: 11 lost to follow‐up. Also, in the dietary counselling and supplement group 8 known to not take the supplement, but included in the ITT analysis. In the dietary counselling group, 4 reported consuming nutritional supplements during the study period. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but not all in a format usable for meta‐analysis. Data on mean change (SD) for weight and grip strength were extracted from the paper. Data on TSF and MUAC were not presented but were assessed and so have been obtained from author. Details of hospital admissions are not reported clearly and therefore have been clarified with the authors. |
| Other bias | Low risk | Baseline characteristics described in text as not different and data given for some variables. |
Olejko 1984.
| Methods | Randomised controlled trial. Duration 6 weeks. | |
| Participants | Adults (n = 24, 12 women and 12 men, mean (SD) age 22.8 years (6.1) awaiting elective orthognathic surgery. 12 of 24 patients had a weight below IBW at study inclusion. 100% follow up. |
|
| Interventions | Dietary instruction given verbally and in writing versus dietary instruction and an oral nutritional supplement (1.5 kcal/ml) to provide 50% of energy requirements (n = 16 )versus dietary instruction, an oral nutritional supplement to provide 50% of energy requirements and a nutritional supplement to take preoperatively (n = 8). | |
| Outcomes | Survival*, body weight*, MUAC*, MAMC*, TSF*, serum chemistry and creatinine height index, macro and micronutrient intake. | |
| Notes | No additional data available for this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow up. |
| Selective reporting (reporting bias) | High risk | All outcomes reported, but using general statements about change rather than numerical presentation e.g. 'the pre‐load group reported an average weight gain of 3.1% during the one month pre‐operative period, which was significantly greater (P < 0.05) than that of the other two groups'. No data are available from the authors therefore unclear risk of bias due to partial reporting. |
| Other bias | High risk | Baseline variables not given, not sure if groups similar at baseline. |
Ollenschlager 1992.
| Methods | Randomised controlled trial. Mean duration 25.5 weeks. | |
| Participants | Adults (n = 29, aged 17 ‐ 60 years), undergoing chemotherapy for acute leukaemia who had undesired weight loss >5% or weight 90% below ideal body weight. 2 deaths in the dietary advice group. |
|
| Interventions | Daily dietary instruction and modification of diet (n = 15) versus ad libitum intake (n = 16). | |
| Outcomes | Weight*, survival*, number of complete remissions and days temperature >38.5 C, nutrient intake*, subjective well‐being. | |
| Notes | Data given for mean study period. Data on nutrient intake and subjective well‐being only collected for intervention group so not used. Additional data and information obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 deaths in the dietary advice group. |
| Selective reporting (reporting bias) | High risk | Data given for mean study period.
Data on nutrient intake and subjective well‐being only collected for intervention group so not used. Data on weight change presented as % of ideal body weight, mean change (SD) not available from authors and so not included in the review. Additional data on mortality and information on some outcomes obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Ovesen 1993.
| Methods | Randomised controlled trial. Duration 5 months. | |
| Participants | Adults (n = 137, 75% men and 25% women age range for groups combined 22 ‐ 80 years) receiving chemotherapy for small‐cell‐lung cancer, ovarian cancer or breast cancer. 30 deaths: 20 in the dietary advice group and 10 in the no advice group. 50% of patients malnourished at study entry defined as >5% weight loss in the previous 3 months. 19 withdrawals: 9 in the dietary advice group and 10 in the no advice group. |
|
| Interventions | Dietary instruction given twice monthly to exceed the Nordic recommended allowances using supplements if indicated (n = 74) versus no dietary advice (n = 63). | |
| Outcomes | Survival*, weight*, TSF*, MAMC*, MUAC*, energy intake*, QoL, tumour response. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was reported as using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was reported as using sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 deaths: 20 in the dietary advice group and 10 in the no advice group. 19 withdrawals: 9 in the dietary advice group and 10 in the no advice group. No reasons given. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported at baseline and monthly to five months after the intervention. The data on mortality at interim time‐points is unclear and it has not been possible to clarify with authors, therefore only data from baseline to 5 months have been used in meta‐analysis. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Paton 2004.
| Methods | Randomised controlled trial. Duration 24 weeks. Intervention for 12 weeks and follow‐up to 24 weeks. | |
| Participants | Adults (n = 36, dietary advice and supplement group 8 men and 11 women mean (SD) age 39.5 years (14.3); dietary advice group 8 men and 9 women, mean (SD) age 38.4 years (19.3)) with tuberculosis and BMI <20. 10 participants lost to follow up, 4 in dietary advice and supplement group and 6 in dietary advice group. |
|
| Interventions | Dietary advice to achieve a calculated target intake and 2 ‐ 3x 200 ml supplement (n = 15) versus instruction to increase food intake (n = 13). | |
| Outcomes | Weight*, BMI, body composition (DEXA), energy intake*, grip strength*, QoL, stand/sit test. | |
| Notes | Additional data requested from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by a member of staff not otherwise involved in the study. |
| Allocation concealment (selection bias) | Low risk | Described as shuffling of sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 participants lost to follow up, 4 in dietary advice and supplement group and 6 in dietary advice group. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but some in unusable format. Data on change in energy intake was presented in the text and not suitable for entry into meta‐analysis therefore obtained from author. Other data were extracted from the paper although 'n' for weight and grip strength were clarified with the authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Persson 2002.
| Methods | Randomised controlled trial. Duration 24 months. | |
| Participants | Adults (n = 142, age range 42 ‐ 89 years) with <5% weight loss who were newly diagnosed with colorectal or gastric cancer. | |
| Interventions | Nutritional counselling to increase food intake to Nordic Nutrition Recommendations and a prescription for nutritional supplements if wanted (n = 67) versus standard care (n = 70). | |
| Outcomes | Survival*, weight*, BMI*, energy intake*. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from the author, random sequence generated on computer by independent centre. |
| Allocation concealment (selection bias) | Low risk | Information from the author, allocation performed by independent centre, allocation concealed until patient recruited. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information from author. 137 patients randomised in the study, at 24 months there were 25 deaths, 5 withdrawals and 3 patients excluded in the intervention group and 26 deaths, 14 patients withdrawn and 1 exclusion in the control group. |
| Selective reporting (reporting bias) | Low risk | The data on mortality is unclear in the paper. The data on weight change is presented partly in text and partly in figures and not suitable for direct entry into meta‐analysis. The data on energy intake is presented as % recommendations. All data included in the review has been obtained from the author including data at 4 time‐points. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Persson 2007.
| Methods | Randomised controlled trial. Duration median 4.3 months, range 3.6 to 6.9 months. | |
| Participants | Elderly patients (n = 108) admitted for trauma or acute illness, mean age intervention group 85 years (SD 5.9), control group 85 years (SD 6.1). All at risk of malnutrition defined by MNA score <10. 54 dropouts. |
|
| Interventions | Individualised counselling to increase food intake, plus a nutritional supplement and a multivitamin supplement (n = 51) versus brief written dietary advice (n = 57). | |
| Outcomes | Weight *, BMI*, handgrip strength*, energy intake*, activities of daily living, cognitive function, peak expiratory flow, QoL. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Further information needed. |
| Allocation concealment (selection bias) | Unclear risk | Further information needed. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Not blinded. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis using data obtained at inclusion carried forward and used at follow‐up for those who were still alive but not examined. Treated as protocol analysis also included. 54 participants dropped out of the study; there were 6 deaths in the intervention group and 12 deaths in the control group. The remaining patients withdrew consent or declined follow‐up. 8 patients in the control group had the intervention prescribed during the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported but not in a format suitable for meta‐analysis. Data on change in weight and handgrip strength are presented as mean (SD) at the start and end of the intervention and have therefore been obtained from authors. Data on mortality extracted from the paper. |
| Other bias | High risk | Stated in text that baseline characteristics not different but data not shown. |
Rabeneck 1998.
| Methods | Randomised controlled trial. Duration 6 weeks. | |
| Participants | Adults (n = 118, all men, mean age (SD) dietary counselling group 41.1 years (9.7 years); and dietary counselling and supplement group 39.3 years (8.8 years)) with HIV infection who were <90% ideal weight or who had >10% weight loss in previous 6 months. 12 dropouts in dietary counselling group and 16 dropouts in dietary counselling and supplement group. |
|
| Interventions | Nutritional counselling to achieve specific energy target (n = 52) versus nutritional counselling to achieve target and an oral nutritional supplement (n = 50). | |
| Outcomes | Weight*, MUAC*, skinfold measurements at all sites*, body composition (BIA), grip strength*, cognitive function, quality of life, energy intake*. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Assessment not blinded. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Assessment not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Assessment not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 dropouts in dietary counselling group and 16 dropouts in dietary counselling and supplement group. Reasons for dropouts reported for the 19 patients who failed to complete at least 4 of the 6 week treatment period. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported and extracted from paper. No data obtained from the author. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Ravasco 2005a.
| Methods | Randomised controlled trial. Duration 42 days intervention plus 3 months. | |
| Participants | Adults (n = 111, 66 men and 45 women, mean (SD) age 58 years (15)) with colorectal cancer undergoing radiotherapy. At baseline 42/111 participants were 'malnourished' (identified by PG‐SGA); 15 in Group 1, 14 in Group 2, 13 in Group 3). No participants lost to follow‐up. |
|
| Interventions | Individualised dietary counselling to achieve calculated energy and protein requirements (n = 37) versus 2x 200 ml cans of nutritional supplement (n = 37) versus ad libitum food intake (n = 37). | |
| Outcomes | Survival*, weight*, BMI*, energy intake*, protein intake, symptom‐induced morbidity, QoL. | |
| Notes | Data will be used in 2 parts of the review dietary advice versus no advice and dietary advice versus nutritional supplements. Additional data and information obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow up. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but in text and figures but not in a format or sufficient detail to make them usable for meta‐analysis. Additional data on mean change (SD) for weight and energy intake obtained from author. Author confirmed that no deaths occurred in the 3‐month study. |
| Other bias | High risk | Baseline variables not given, not sure if groups similar at baseline. |
Ravasco 2005b.
| Methods | Randomised controlled trial. Duration 42 days intervention plus 3 months. | |
| Participants | Adults (n = 75) receiving radiotherapy for head and neck cancer, mean age 60 years (range 36 to 79 years). At baseline 45/75 participants were 'malnourished' (identified by PG‐SGA); 16 in Group 1, 14 in Group 2, 15 in Group 3). No participants lost to follow‐up. |
|
| Interventions | Individualised dietary counselling to achieve calculated energy and protein requirements (n = 25) versus 2x 200 ml cans of nutritional supplement (n = 25) versus ad libitum food intake (n = 25). | |
| Outcomes | Survival*, weight*, energy intake*, nutritional status (PG‐SGA), symptom‐induced morbidity, QoL. | |
| Notes | Data will be used in 2 parts of the review dietary advice versus no advice and dietary advice versus nutritional supplements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using computer‐generated random assignments. |
| Allocation concealment (selection bias) | Low risk | Concealed in numbered opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow up. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but in text and figures but not in a format or sufficient detail to make them usable for meta‐analysis. Additional data on mean change (SD) for weight and energy intake obtained from author. Author confirmed that no deaths occurred in the 3‐month study. |
| Other bias | High risk | Baseline variables not given, not sure if groups similar at baseline. |
Rogers 1992.
| Methods | Randomised controlled trial. Duration 4 months. | |
| Participants | Adults (n = 28, mean age 62 years (SE 2.0 years)) with COPD and weight <90% of IBW and FEV1/FVC <0.6. 1 withdrawal in the no advice group. |
|
| Interventions | Nutritional counselling to achieve a balanced meal plan plus supplements as needed (n = 15) versus no dietary advice (n = 12). Advice provided during 4‐week inpatient phase and then at each outpatient visit. | |
| Outcomes | Weight*, TSF*, MUAC*, grip strength*, respiratory function*, QoL. | |
| Notes | Additional data and information awaited from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 withdrawal in the no advice group. Reason not given. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but not in a format for entry into meta‐analysis. Change in weight, TSF, MAMC and handgrip strength are reported as mean (SD) at the start and end of intervention with a P value. No data obtained from authors therefore mean change (SD) derived using data in the paper. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Rydwik 2008.
| Methods | Randomised controlled trial. Duration 12 weeks intervention, and a further 6 months follow‐up. | |
| Participants | Frail elderly (n = 96), aged over 75 years with unintentional weight loss >5% and/or BMI <20 kg/m2 and low physical activity level. 32 dropouts. |
|
| Interventions | Dietary counselling to increase energy intake (n = 22) versus dietary counselling plus exercise training versus exercise training alone, versus control (n = 19). | |
| Outcomes | weight*, BMI*, TSF*, energy intake*, muscle strength, balance, time‐to‐up‐and‐go, walking speed. | |
| Notes | Data on dietary counselling group and control group will be used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Further details needed. |
| Allocation concealment (selection bias) | Unclear risk | Further details needed. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 32 dropouts, 7 in the dietary counselling group, 11 in the dietary counselling plus exercise, 4 in the exercise alone group and 10 in the control group. |
| Selective reporting (reporting bias) | High risk | All specified outcomes reported apart from TSF. The data was requested from the authors but unavailable. Data on mean change in weight and energy intake were reported without a standard deviation and so have been obtained from the author. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Schwenk 1999.
| Methods | Randomised controlled trial (block randomisation). Duration 8 weeks. | |
| Participants | HIV positive adults (n = 50, 47 men, 3 women; dietary counselling group mean (SD) age 39.5 years (10.2 years); supplement group mean (SD) age 39.4 years (9.2 years)) who had lost >5% of usual weight or who were actively losing weight, >3% in last month. 3 dropouts in dietary counselling group and 2 dropouts in supplement group. |
|
| Interventions | Dietary counselling to increase food intake by 600 kcal using household food items (n = 24) versus oral nutritional supplements (0.6 ‐ 1.5 kcal/ml) to increase energy intake by 600 kcal (n = 26). | |
| Outcomes | Survival*, change in body cell mass and change in weight*, change in energy intake*, hospital admissions*. | |
| Notes | Additional data and information obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author, block randomisation derived using random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared by a person not involved in the study. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Information from author indicates that the study was not blinded. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Information from author indicates that the study was not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Information from author indicates that the study was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts in dietary counselling group and 2 dropouts in supplement group. Reasons for drop out were opportunistic infections in 4 cases and change of residence in one. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but data was not in a form usable for meta analysis. Data on weight change were reported as % change in area under the curve and data on energy intake was reported as mean calories per kg, therefore mean change (SD) obtained from authors. Data on number of hospital admissions confirmed with the author. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Sharma 2002.
| Methods | Randomised controlled trial. Duration 1 month. | |
| Participants | Adults with renal disease receiving maintenance dialysis (n= 47). All patients had a BMI <20 kg/m2. 7 dropouts (5 in the intervention group and 2 in the control group). |
|
| Interventions | Dietary counselling to increase intake but in line with current recommendations for renal disease plus 300 ml of supplement (500 kcals, 15g protein) or home produced blend providing similar kcals and protein (n = 10) versus dietary counselling to increase intake but in line with current recommendations for renal disease (n = 14). | |
| Outcomes | Weight*, biochemistry, energy intake*, supplementation acceptability questionnaire. | |
| Notes | Study is eligible for inclusion on the basis of the intervention however due to the high number of control participants that crossed over to the intervention, data cannot be included without further analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 dropouts (5 in the intervention group and 2 in the control group). Reasons not given. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures are reported. Data not all in usable format and not available from author, therefore mean change (SD) has been derived by calculation from the data in Table 2. |
| Other bias | High risk | Baseline data only presented on participants that completed the study (n = 40). 5 patients crossed over from the control to the supplement group. |
Singh 2008.
| Methods | Randomised controlled trial. Duration 3 months. | |
| Participants | Adults (n = 60) with chronic pancreatitis, (male n = 50 and females n = 10), dietary advice group mean age 32 (SD 10) years, nutritional supplement group mean age 28 (SD 10) years. All patients undernourished, BMI <18.5 kg/m2 or >10% weight loss in previous 6 months. 6 dropouts. |
|
| Interventions | Dietary advice from a dietitian to meet predicted energy requirements (n = 25) versus a MCT‐enriched nutritional supplement to meet predicted energy requirements (n = 24). | |
| Outcomes | BMI*, TSF*, MAMC* nitrogen balance, faecal fat, pain score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Carried out by individual not otherwise involved in the study. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Outcome assessment blinded to treatment. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Outcome assessment blinded to treatment. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Outcome assessment blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dietary counselling group: 2 lost to follow‐up at 1 and a half months. Nutritional supplement group: 4 lost to follow‐up at 1 and a half months. But all included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported but not in a format usable for meta analysis. Data on change in weight, TSF, MAC and energy intake were reported as mean (SD) at baseline and mean (SD) at end of intervention, therefore mean change (SD) obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline |
Stratton 2007.
| Methods | Randomised controlled trial. Duration to be confirmed | |
| Participants | Adults (n = 50, 42 females, 8 males) with hip fractures. Mean age 82 years (range 46‐97 years). All patients at risk of malnutrition assessed by Malnutrition Universal Screening Tool (MUST). |
|
| Interventions | Readily available snacks (300 kcal/portion) (n = 24) versus oral nutritional supplements (300 kcal/carton) (n = 26). | |
| Outcomes | ||
| Notes | Data awaited from author to clarify aspects of study design and data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not possible to assess from abstract. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to assess from abstract no data in abstract suitable for entry into meta‐analysis. Authors unwilling to provide the data prior to full publication. |
| Other bias | Unclear risk | Not possible to assess from abstract. |
Weekes 2009.
| Methods | Randomised controlled trial. Duration 12 months Six months intervention and follow‐up to 12 months. | |
| Participants | Adults (n = 66; 35 males and 31 females) outpatients with severe COPD. Mean age 69 years. 66 randomised; 37 completed; 5 deaths in each group; all patients malnourished defined as unintentional weight loss and poor intake or BMI <18.5 kg/m2. 11% dropped out before baseline assessment. |
|
| Interventions | Dietary counselling to increase intake and advice on food fortification (n = 31) versus usual care (n = 28). | |
| Outcomes | Survival*, weight*, BMI*, triceps skinfold*, MAC*, MAMC*, grip strength*, energy intake*, cost*, respiratory function, respiratory muscle function, QoL. | |
| Notes | Usual care consisted of leaflet. Data have been obtained from the author. Paper submitted, awaiting decision re acceptance. Post hoc analysis of cost data will be available at the next update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | All assessments made by the lead investigator who was not blinded to group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | All assessments made by the lead investigator who was not blinded to group allocation. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | All assessments made by the lead investigator who was not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 66 randomised; 37 completed; 5 deaths in each group; 11% dropped out before baseline assessment. |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported but not as mean change (SD) at 6 months (end of intervention) for the outcomes of interest therefore not usable for meta‐analysis. Additional data and information obtained from the author. |
| Other bias | Low risk | Baseline characteristics reported and no differences between groups. |
Wilson 2001.
| Methods | Randomised controlled trial. Duration 9 months (6 month treatment and 3 months follow‐up). | |
| Participants | Adults (n = 32, dietary advice and supplement group 39% men, 61% women, mean (SD) age 64 years (10 years); and dietary advice group 14% men and 86 % women, mean (SD) age 58 years (8.6 years)) with hypoalbuminaemia (serum albumin 3.5 ‐ 3.7 g/dL) receiving hemodialysis. An additional group is included with severe hypoalbuminaemia (serum albumin 2.5 to 3.4 g/dL who received intervention according to current practice. 5 participants were not included in the analysis but details of the group allocation is unclear. |
|
| Interventions | Dietary counselling to increase energy and protein intakes and 1‐2 cans of supplement (250 calories per serving) (n = 16) versus dietary counselling to increase energy and protein intake (n = 16). | |
| Outcomes | Time to nutritional repletion, number of days spent in hospital*. | |
| Notes | No usable data from this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants were not included in the analysis but details of the group allocation is unclear therefore risk of bias. |
| Selective reporting (reporting bias) | High risk | The methods section of the paper states that height, weight and weight history and serum albumin are collected at baseline, The results section reports % achieving nutritional repletion defined by improvement in serum albumin and length of hospital stay but no data on weight change. No response received from authors. |
| Other bias | High risk | Baseline variables given, the dietary counselling and supplement group were significantly older than the dietary group, therefore risk of bias. |
Wong 2004.
| Methods | Randomised controlled trial. Duration 4 months. | |
| Participants | Adults (n = 189, dietary counselling group 18% men and 82% women, mean (SD) age 75.8 years (9.5); no advice group 15% men and 85% women, mean (SD) age 73.8 years (11.6)) presenting with osteoporotic fractures. Mean (SD) BMI at baseline 22.6 (3.9), no details of numbers malnourished. 39 participants lost to follow‐up. |
|
| Interventions | Tailored dietary advice with recipes and specific goals for energy, protein and calcium +500 mg calcium & anti‐resorptive agent (n = 73) versus no advice +500 mg calcium & anti‐resorptive agent (n = 77). | |
| Outcomes | Weight*, BMI*, energy intake* | |
| Notes | Additional data and information requested from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author indicated that a computer‐generated list of random numbers was used. |
| Allocation concealment (selection bias) | Low risk | Information from author indicated that allocation concealment was achieved by an independent person managing this aspect of the trial. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 39 participants lost to follow‐up. 18 in the intervention group and 21 in the control group but reasons not given, therefore risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. Mortality data confirmed with authors. Additional information on study quality obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
* outcomes included in this review if data usable BASDEC: brief assessment schedule depression cards BIA: bioelectric impedence analysis BMI: body mass index CDAI: Crohn's disease activity index CF: cystic fibrosis COPD: chronic obstructive pulmonary disease DEXA: dual energy X‐ray absorptiometry FEV1: forced expiratory volume at one second FVC: forced vital capacity GI: gastro‐intestinal HIV: human immunodeficiency virus IBW: ideal body weight IQR: interquartile range ITT: intention‐to‐treat MAC: mid‐arm circumference MAMA: mid‐arm muscle area MAMC: mid‐arm muscle circumference MCT: medium chain triglycerides MNA: mini nutritional assessment MUAC: mid‐upper arm circumference PG‐SGA: patient generated subjective global assessment QoL: quality of life REE: resting energy expenditure SD: standard deviation SE: standard error SGA: subjective global assessment TSF: triceps skinfold thickness
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antila 1993 | The included patients are well‐nourished and advice is given to maintain normal nutritional status. |
| Arutiunov 2009 | This was an observational study and therefore did not meet the inclusion criteria of randomised controlled trial design. |
| Bachmann 1998 | Not a randomised controlled trial. |
| Bauer 1994 | Not disease‐related malnutrition and not adults, participants are adolescent weight lifters. |
| Beange 1995 | Not a randomised controlled trial, no control group, the 88 participants were "chosen" from 550 residents. |
| Beck 2008 | Intervention was nutrition plus exercise compared with a control group receiving neither. |
| Bills 1993 | Not a randomised controlled trial, a questionnaire survey of nutritional practices in a nursing home. |
| Bolton 1990 | Not a randomised controlled trial, but a palatability study of nutritional supplements. |
| Bories 1994 | Not a randomised controlled trial. |
| Botella‐Carretero 2008 | Comparison does not meet inclusion criteria, this is a 3‐arm trial which compares 2 different oral nutritional supplements with routine care. |
| Bozzetti 1998 | Not a randomised controlled trial, a letter with no data. |
| Bugge 1997 | Not a randomised controlled trial. |
| Bunout 1989 | Comparison does not meet inclusion criteria, this trial compares an enhanced calorie and protein based diet plus a specialized nutritional supplement with a standard hospital diet. |
| Burger 1993 | Not a randomised controlled trial, a 6‐month prospective follow‐up of nutritional counselling in malnourished patients with HIV infection. |
| Carlsson 2005 | Comparison does not meet inclusion criteria, this is a 3‐arm trial comparing an oral nutritional supplement with an oral nutritional supplement plus nandrolone (appetite stimulant) with routine care. |
| Duncan 2006 | Comparison does not meet inclusion criteria, the intervention is help with eating from a dietetic assistant compared with routine care. |
| Efthimiou 1988 | Comparison does not meet inclusion criteria, this is a 3‐arm trial which compares an oral nutritional supplement with routine care. The 3rd group are patients that are normally nourished and receiving usual diet. |
| Elbanna 1996 | Not a randomised controlled trial, this study examines preoperative nutritional support in 2 groups of patients but the control group are purposively recruited before the intervention group. |
| Elkort 1981 | Comparison does not meet inclusion criteria, this trial compared an oral nutritional supplement with routine care, both groups are given encouragement to eat a balanced diet which was considered not to constitute dietary advice. |
| Elmstaahl 1987 | Comparison does not meet inclusion criteria, this study has 3 arms and compares 3 different oral nutritional supplements. |
| Eneroth 1997 | Not a randomised controlled trial and comparison does not meet inclusion criteria. This study compares supplementary nutrition, which can consist of an oral nutritional supplement, enteral or parenteral feeding with hospital food. |
| Engel 1995 | Not a randomised controlled trial. |
| Flynn 1987 | Comparison does not meet inclusion criteria, this study compares individualised nutritional counselling to an oral nutritional supplement with standard nutritional. |
| Forli 2006 | Not a randomised controlled trial. |
| Franzoni 1996 | Not a randomised controlled trial. |
| Glimelius 1992 | Not a randomised controlled trial, an historical control group was used. |
| Heberer 1984 | Incorrect comparisons, study of parenteral nutrition. |
| Henquin 1989 | Not a randomised controlled trial. |
| Hickson 2004 | Comparison does not meet inclusion criteria, the intervention is help with eating from a dietetic assistant compared with routine care. |
| Hogan 1997 | Not a randomised controlled trial. |
| Holder 2003 | Not a randomised controlled trial, a review article. |
| Hulsewe 1997 | Not a randomised controlled trial, a discussion of perioperative nutritional interventions. |
| Idilman 2009 | Comparison does not meet inclusion criteria, this is a retrospective review of nutrition intervention and outcomes in patients with alcoholic liver disease. |
| Ireton 1995 | Not a randomised controlled trial, an observational study. |
| Jamieson 1997 | Not a randomised controlled trial, a retrospective audit. |
| Jie 2009 | Comparison does not meet inclusion criteria, this study compares enteral and parenteral feeding. |
| Johnson 1993 | Not a randomised controlled trial and comparison does not meet inclusion criteria, this is a comparison of oral nutritional supplements with no nutritional supplement. Both groups follow their usual diet, therefore there is no counselling component. |
| Keller 1995 | Not a randomised controlled trial a retrospective survey of outcomes in malnourished and normally nourished patients. |
| Knowles 1988 | Comparison does not meet inclusion criteria, this is a study of an oral nutritional supplement with no nutritional supplement. |
| Kondrup 1998 | Not a randomised controlled trial, a retrospective survey of outcomes in malnourished patients |
| Krasnoff 2006 | Comparison does not meet inclusion criteria, this is a study of nutritional counselling plus exercise compared with routine care. |
| Kruizenga 2005 | Not a randomised controlled trial, a controlled study using historical controls. |
| Lejeune 2005 | Comparison does not meet inclusion criteria, this is a study of dietary advice to achieve weight loss in moderately overweight patients. |
| Levine 1982 | Comparison does not meet inclusion criteria, this is a study of standard diet compared with parenteral nutrition. |
| Lipschitz 1985 | Not a randomised controlled trial. |
| Lynch 1983 | Not a randomised controlled trial, a prospective study. |
| Manders 2009 | Comparison does not meet inclusion criteria, this is a study of oral nutritional supplements compared with no nutritional supplement. |
| McWhirter 1996 | Incorrect comparison (oral versus NG supplementation). |
| Mendenhall 1993 | Comparison does not meet inclusion criteria, this study compares a nutritional supplement with a placebo nutritional supplement. |
| Mendenhall 1995 | Comparison does not meet inclusion criteria, this study compares hospital diet plus an oral nutritional supplement and a vitamin and mineral supplement with hospital diet plus a vitamin and mineral supplement plus a placebo nutritional supplement. |
| Monnin 1993 | Not a randomised controlled trial, a report of the findings from a questionnaire on nutritional counselling in breast cancer. |
| Munck 1998 | Not a randomised controlled trial, a review of dietary counselling. |
| Neidich 1985 | Not a randomised controlled trial, the intervention is a high nitrogen food supplement and the participants are mainly children. |
| Newmark 1981 | Not a randomised controlled trial. |
| Neyman 1996 | Not a randomised controlled trial and comparison does not meet inclusion criteria. This study compares outcomes in participants in a congregate‐site meals programme with people not participating in the programme. |
| Nijs 2006 | The comparison does not meet the inclusion criteria, this study compares family‐style dining versus traditional dining. |
| Olofsson 2007 | The intervention included many aspects of medical care in addition to a nutritional intervention that may have accounted for any reported benefits. |
| Openbrier 1984 | Not a randomised controlled trial, this is a prospective evaluation of nutritional intervention in malnourished patients with emphysema. |
| Ottery 1996 | Not a randomised controlled trial, a description of improvements following nutritional intervention. |
| Parrott 2006 | The comparison does not meet the inclusion criteria, this study compares a snack‐type supplement provided to people with Alzheimers disease in a nursing home which is not the same as dietary advice. |
| Patel 1998 | Not a randomised controlled trial and comparison does not meet inclusion criteria. This study examines the efficacy of dietary advice to avoid weight gain. |
| Payette 2002 | Comparison does not meet inclusion criteria, this study compares an oral nutritional supplement plus encouragement to improve food intake with routine care. |
| Pedersen 2005 | Not a randomised controlled trial, this is a quasi‐experimental study of nurse‐facilitated patient involvement in care. |
| Pietersma 2003 | Comparison does not meet inclusion criteria, this study compares patient selection of one meal a day from the food cart compared with receiving the usual plated meal. |
| Planas 2005 | Comparison does not meet inclusion criteria, this study compares two groups both receiving dietary advice and supplements but the target energy intake varied between the groups. |
| Plank 2008 | Comparison does not meet the inclusion criteria, this study compares oral nutritional supplements with no nutritional supplements. |
| Rabinovitch 2006 | Not a randomised controlled trial, a re‐analysis of data. |
| Rassmussen 2006 | Not a randomised controlled trial and comparison does not meet inclusion criteria, as the intervention does not aim to increase nutritional intake. |
| Rüfenacht 2010 | Comparison does not meet inclusion criteria. This study compares hospital diet plus ONS with dietary counselling plus ONS as required. |
| Salas‐Salvado 2005 | The comparison is unclear but appears to be dietary advice plus provision of puree diet and inclusion of a snack‐type supplement based on natural lypolysed food compared with dietary advice plus provision of a puree diet. |
| Saudny 1997 | Comparison does not meet inclusion criteria, this study compares hospital diet and a supplement or extra food with hospital diet only. |
| Simmons 2008 | Potential for bias in patient selection because to be eligible for inclusion, the nursing home residents had to demonstrate that they were responsive to one of the feeding assistance interventions. |
| Smoliner 2008 | Comparison does not meet the inclusion criteria, this study compares the provision of fortified food with routine care in a nursing home which does not meet the definition of dietary advice. |
| Solerte 2008 | Comparison does not meet inclusion criteria, this study compares an amino acid mixture with a placebo. |
| Solomon 1978 | Comparison does not meet inclusion criteria, this study compares a combination of pre‐operative and post‐operative diets including a hypo‐caloric, carbohydrate‐free, protein‐containing diet with normal diet. |
| Sridar 1994 | Not a randomised controlled trial, a prospective study of 12 patients with COPD after nutritional intervention. |
| Stack 1996 | Not a randomised controlled trial, a prospective, descriptive trial with no control group. |
| Stark 1990 | Comparison does not meet inclusion criteria, all participants were children. |
| Swanenburg 2007 | Comparison does not meet inclusion criteria, this study compares exercise plus an oral nutritional supplement with no exercise and no supplement. |
| Tatsumi 2009 | Comparison does not meet the inclusion criteria, the intervention in this study is "Hochuekkito" which is a herbal medicine. |
| Taylor 2006 | Comparison does not meet the inclusion criteria, this study compares meal frequency (5 meals versus 3 meals) on nutritional outcomes. |
| Turic 1998 | Comparison does not meet the inclusion criteria, this study involves the provision of a nutritional supplement or snacks to nursing home residents which does not meet the definition of dietary advice. |
| Unosson 1992 | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| Vargas 1995 | Comparison does not meet inclusion criteria, this study has 4 arms comparing different combination of nutritional supplements and training. |
| Volkert 1996 | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| Watson 2008 | The intervention consists of both dietetic and educational and psychological motivation. It would be difficult to attribute any reported benefits to nutrition alone. |
| Williams 1989a | Study mainly in children with some adults. No participants over 16 years of age in control group therefore no comparison group. |
| Williams 1989b | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| Woo 1994 | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| Wouters‐Wessling 2005 | Comparison does not meet the inclusion criteria, this study compares a nutritional supplement with routine care. |
| Wright 2008 | Not a randomised trial, a prospective observational study with retrospective control group examining feeding assistance to increase intake. |
| Yoneda 1992a | Comparison does not meet inclusion criteria, this study reports on the clinical course of patients with asthma. |
| Yoneda 1992b | Comparison does not meet inclusion criteria, study is in Japanese but appears to be an intervention to reduce psychological stress in patients with respiratory disease. |
COPD: chronic obstructive pulmonary disease NG: naso‐gastric ONS: oral nutritional supplements
Characteristics of studies awaiting assessment [ordered by study ID]
Margare 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not possible to obtain this paper. Journal website currently down and due to be available later in the year. |
Penalva 2009.
| Methods | Prospective open randomised trial. |
| Participants | 125 patients admitted to a haematology ward. |
| Interventions | Additional food items to increase intake vs nutritional supplements (flavoured or neutral). |
| Outcomes | Weight, SGA score, energy intake. |
| Notes | This paper needs full translation in order to determine eligibility. |
Shatenstein 2008.
| Methods | Quasi‐experimental (3 clinics intervention, the remainder control). |
| Participants | Elderly with early stage dementia. |
| Interventions | Tailored nutritional intervention vs usual care. |
| Outcomes | Core questionnaire (socio‐demographic, general health information, medication use, health perception and physical activity), VAS for hunger and appetite, functional status (ADL and IADL), weight, height, grip strength, nutrient intake. |
| Notes | The identified study is a case report on 2 patients included in the study. The authors have been contacted to ask whether data are available on all participants. |
ADL: activities of daily living IADL: instrumental activities of daily living SGA: subjective global assessment VAS: visual analogue scale
Differences between protocol and review
The intervention 'dietary advice plus supplements if required compared with no advice and no supplements' was added post hoc as a result of an additional group of studies identified during searching and study identification. These studies were considered relevant to this review as they examine dietary advice compared with no advice, but the dietary advice includes information on using oral calorie supplements if considered necessary.
Contributions of authors
Christine Baldwin and Tessa Parsons conducted the searches, selected studies for inclusion, entered data and prepared the analyses.
Christine Baldwin, Tessa Parsons and Stuart Logan contributed to the development of the protocol and preparation of the review.
Until January 2007, all updates were prepared by Christine Baldwin and Tessa Parsons. After this date, both Tessa Parsons and Stuart Logan stepped down as authors on this review. A new co‐author, Elizabeth Weekes was recruited at this time and has since contributed to the updates.
Christine Baldwin acts as guarantor of the review.
Sources of support
Internal sources
Systematic Reviews Training Unit (funded by the London Regional Health Authority), UK.
External sources
British Dietetic Association, UK.
Declarations of interest
The first year of work on the protocol for this review was funded by the British Dietetic Association.
The authors of this review are both first authors of studies included in the review.
Edited (no change to conclusions)
References
References to studies included in this review
Arnold 1989 {published data only}
- Arnold C, Richter MP. The effect of oral nutritional supplements on head and neck cancer. International Journal Of Radiation Oncology, Biology, Physics 1989;16(6):1595‐9. [DOI] [PubMed] [Google Scholar]
Baldwin 2008 {published and unpublished data}
- Baldwin C, Spiro A, McGough C, Norman AR, O'Brien M, Cunningham DC, Andreyev HJN. The NUT study: the effect of dietetic and oral nutritional interventions on survival and quality of life in patients with weight loss undergoing palliative chemotherapy for gastrointestinal or lung malignancy, a randomised controlled trial. Proceedings of the Nutrition Society 2008;67(OCE4):E136. [Google Scholar]
- Bonney AHM, Baldwin C, Whelan K, Andreyev HJN. The NUT study: what effect does nutritional intervention have on energy intake in patients with gastrointestinal cancer receiving chemotherapy. Proceedings of the Nutrition Society 2008;67(OCE4):E141. [Google Scholar]
Beattie 2000 {published data only}
- Beattie A, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000;46(6):813‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berneis 2000 {published data only}
- Berneis K, Battegay M, Bassetti S, Nuesch R, Leisibach A, Bilz S, et al. Nutritional supplements combined with dietary counselling diminish whole body protein catabolism in HIV‐infected patients. European Journal of Clinical Investigation 2000;30(1):87‐94. [DOI] [PubMed] [Google Scholar]
Campbell 2008 {published data only}
- Campbell KL, Ash S, Davies PSW, Bauer JD. Randomized controlled trial of nutritional counselling on body composition and dietary intake in severe CKD. American Journal of Kidney Diseases 2008;51(5):748‐58. [DOI] [PubMed] [Google Scholar]
Chandra 1985 {published data only}
- Chandra RK, Puri S. Nutritional support improves antibody response to influenza virus vaccine in the elderly. BMJ 1985;291(6497):705‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Luis 2003 {published and unpublished data}
- Luis D, Aller R, Bachiller P, Gonzalez‐Sagrado M, deLuis J, Izaola O, et al. Isolated dietary counselling program versus supplement and dietary counselling in patients with human immunodeficiency virus infection [Consejo nutricional aislado frente a suplemento y consejo nutricionales en pacientes con infeccion por el virus do la inmunodeficiencia humana]. Medicina clinica 2003;120(15):565‐7. [DOI] [PubMed] [Google Scholar]
Dixon 1984 {published data only}
- Dixon J. Effect of nursing interventions on nutritional and performance status in cancer patients. Nursing Research 1984;33(6):330‐5. [PubMed] [Google Scholar]
Evans 1987 {published data only}
- Evans WK, Nixon DW, Daly JM, Ellenberg SS, Gardner L, Wolfe E, et al. A randomized study of oral nutritional support versus ad lib nutritional intake during chemotherapy for advanced colorectal and non‐small‐cell lung cancer. Journal of Clinical Oncology 1987;5(1):113‐24. [DOI] [PubMed] [Google Scholar]
- Foltz A, Besser P, Ellenberg S, Carty C, Mitchell S, Paul K, et al. Effectiveness of nutritional counselling on caloric intake, weight change, and percent protein intake in patients with advanced colorectal and lung cancer. Nutrition 1987;3(4):263‐71. [Google Scholar]
Forli 2001 {published and unpublished data}
- Forli L, Bjortuft O, Vatn M, Kofstad J, Boe J. A study of intensified dietary support in underweight candidates for lung transplantation. Annals of nutrition and metabolism 2001;45(4):159‐68. [DOI] [PubMed] [Google Scholar]
Fuenzalida 1990 {published data only}
- Fuenzalida CE, Petty TL, Jones ML, Jarrett S, Harbeck RJ, Terry RW, et al. The immune response to short‐term nutritional intervention in advanced chronic obstructive pulmonary disease. American Review of Respiratory Disease 1990;142(1):49‐56. [DOI] [PubMed] [Google Scholar]
Ganzoni 1994 {published and unpublished data}
- Ganzoni A, Heilig P, Schonenberger K, Hugli O, Fitting JW, Brandli O. High‐caloric nutrition in chronic obstructive lung disease [Hochkalorische Ernahrung bei chronischer obstruktiver Lungenkrankheit]. Schweizerische Rundschau Fuer Medizin Praxis 1994;83(1):13‐6. [PubMed] [Google Scholar]
Gonzalez‐Espinoza 2005 {published data only}
- Gonzalez‐Espinoza L, Gutierrez‐Chavez J, Campo FM, Martinez‐Ramirez HR, Cortez‐Sanabria L, Rojas‐Campos E, et al. Randomized, open‐label, controlled clinical trial of oral administration of an egg albumen‐based protein supplement to patients on continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 2005;25(2):173‐80. [PubMed] [Google Scholar]
Gray‐Donald 1995 {published and unpublished data}
- Gray‐Donald K, Payette H, Boutier V. Randomized clinical trial of nutritional supplementation shows little effect on functional status among free‐living frail elderly. Journal of Nutrition 1995;125(12):2965‐71. [DOI] [PubMed] [Google Scholar]
Hampson 2003 {published and unpublished data}
- Hampson G, Martin FC, Moffat K, Vaja S, Sankaralingam S, Cheung J, et al. Effects of dietary improvement on bone metabolism in elderly underweight women with osteoporosis: a randomised controlled trial. Osteoporosis international 2003;14(9):750‐6. [DOI] [PubMed] [Google Scholar]
Imes 1988 {published and unpublished data}
- Imes S, Pinchbeck B, Dinwoodie A, Walker K, Thomson ABR. Effect of Ensure, a defined formula diet, in patients with Crohn's disease. Digestion 1986;35(3):158‐69. [DOI] [PubMed] [Google Scholar]
- Imes S, Pinchbeck B, Thompson ABR. Diet counselling improves the clinical course of patients with Crohn's disease. Digestion 1988;39(1):7‐19. [DOI] [PubMed] [Google Scholar]
- Imes S, Pinchbeck BR, Thomson ABR. Diet counselling modifies nutrient intake of patients with Crohn's disease. Journal of the American Dietetic Association 1987;87(4):457‐62. [PubMed] [Google Scholar]
Isenring 2004 {published and unpublished data}
- Isenring EA, Bauer JD, Capra S. Nutrition support using the American Dietetic Association medical nutrition therapy protocol for radiation oncology patients improves dietary intake compared with standard practice. Journal of American Dietetic Association 2007;107(3):404‐12. [DOI] [PubMed] [Google Scholar]
- Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. British Journal of Cancer 2004;91(3):447‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1997 {published data only}
- Jensen MB, Hessov I. Dietary supplementation at home improves the regain of lean body mass after surgery. Nutrition 1997;13(5):422‐30. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Hessov I. Randomization to nutritional intervention at home did not improve postoperative function, fatigue or well‐being. British Journal of Surgery 1997;84(1):113‐8. [PubMed] [Google Scholar]
Kalnins 2005 {published and unpublished data}
- Kalnins D, Corey M, Ellis L, Pencharz PB, Tullis E, Durie PR. Failure of conventional strategies to improve nutritional status in malnourished adolescents and adults with cystic fibrosis. Journal of Pediatrics 2005;147(3):399‐401. [DOI] [PubMed] [Google Scholar]
- Kalnins D, Durie P. Oral supplements vs normal food intake in children and adults [abstract]. Israel Journal of Medical Sciences 1996;32 Suppl:S120‐1. [Google Scholar]
- Kalnins D, Durie PR, Corey M, Ellis L, Pencharz P, Tullis E. Are oral dietary supplements effective in the nutritional management of adolescents and adults with CF? [abstract]. Pediatric Pulmonology 1996;381(Suppl 13):314‐5. [Google Scholar]
Kendell 1982 {published data only}
- Kendell BD, Fonseca RJ, Lee M. Postoperative nutritional supplementation for the orthognathic surgery patient. Journal of Oral and Maxillofacial Surgery 1982;40(4):205‐13. [DOI] [PubMed] [Google Scholar]
Lovik 1996 {published data only}
- Lovik A, Almendingen K, Dotterud M, Forli L, Boysen M, Omarhus M, et al. Dietary information after radiotherapy of head and neck cancer [Kostveiledning etter stralebehandling for kreft i hode‐ og halsregionen]. Tidsskrift For Den Norske Laegeforening 1996;116(19):2303‐6. [PubMed] [Google Scholar]
Macia 1991 {published data only}
- Macia E, Moran J, Santos J, Blanco M, Mahedero G, Salas J. Nutritional evaluation and dietetic care in cancer patients treated with radiotherapy: prospective study. Nutrition 1991;7(3):205‐9. [PubMed] [Google Scholar]
Manguso 2005 {published data only}
- Manguso F, D'Ambra G, Menchise A, Sollazzo R, D'Agostino L. Effects of an appropriate oral diet on the nutritional status of patients with HCV‐related liver cirrhosis: a prospective study. Clinical Nutrition 2005;24(5):751‐9. [DOI] [PubMed] [Google Scholar]
McCarthy 1999 {published and unpublished data}
- McCarthy D, Weihofen D. The effect of nutritional supplements on food intake in patients undergoing radiotherapy. Oncology Nursing Forum 1999;26(5):897‐900. [PubMed] [Google Scholar]
Moloney 1983 {published data only}
- Moloney M, Moriarty M, Daly L. Controlled studies of nutritional intake in patients with malignant disease undergoing treatment. Human Nutrition: Applied Nutrition 1983;37A:30‐5. [PubMed] [Google Scholar]
Murphy 1992 {published data only}
- Murphy J, Cameron DW, Garber G, Conway B, Denomme N. Dietary counselling and nutritional supplementation in HIV infection. Journal of the Canadian Dietetic Association 1992;53(3):205‐8. [Google Scholar]
Norman 2008b {published data only}
- Norman K, Kirchner H, Freudenreich M, Ockenga J, Lochs H, Pirlich M. Three‐month intervention with protein and energy rich supplements improved muscle function and quality of life in malnourished patients with none neoplastic gastrointestinal disease ‐ a randomized controlled trial. Clinical Nutrition 2008;27:48‐56. [DOI] [PubMed] [Google Scholar]
Olejko 1984 {published data only}
- Olejko TD, Fonseca RJ. Preoperative nutritional supplementation for the orthognathic surgery patient. Journal of Oral and Maxillofacial Surgery 1984;42(9):573‐7. [DOI] [PubMed] [Google Scholar]
Ollenschlager 1992 {published data only}
- Ollenschlager G, Thomas W, Konkol K, Diehl V, Roth E. Nutritional behaviour and quality of life during oncological polychemotherapy: results of a prospective study on the efficacy of oral nutrition therapy in patients with acute leukaemia. European Journal Of Clinical Investigation 1992;22(8):546‐53. [DOI] [PubMed] [Google Scholar]
Ovesen 1993 {published data only}
- Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. Journal of Clinical Oncology 1993;11(10):2043‐9. [DOI] [PubMed] [Google Scholar]
Paton 2004 {published and unpublished data}
- Paton NI, Chua YK, Earnest A, Chee CBE. Randomised controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. American Journal of Clinical Nutrition 2004;80(2):460‐5. [DOI] [PubMed] [Google Scholar]
Persson 2002 {published and unpublished data}
- Persson CR, Johansson BB, Sjoden PO, Glimelius BL. A randomized study of nutritional support in patients with colorectal and gastric cancer. Nutrition and Cancer 2002;42(1):48‐58. [DOI] [PubMed] [Google Scholar]
Persson 2007 {published data only}
- Persson M, Hytter‐Landahl A, Brismar K, Cederholm T. Nutritional supplementation and dietary advice in geriatric patients at risk of malnutrition. Clinical Nutrition 2007;26(2):216‐24. [DOI] [PubMed] [Google Scholar]
Rabeneck 1998 {published data only}
- Rabeneck L, Palmer A, Knowles JB, Seidehamel RJ, Harris CL, Merkel KL, et al. A randomized controlled trial evaluating nutrition counselling with or without oral supplementation in malnourished HIV‐infected patients. Journal of the American Dietetic Association 1998;98(4):434‐8. [DOI] [PubMed] [Google Scholar]
Ravasco 2005a {published and unpublished data}
- Ravasco P, Monteiro‐Grillo I, Marques Vidal P, Camilo ME. Dietary counselling improves patient outcomes: A prospective, randomised, controlled trial in colorectal cancer patients undergoing radiotherapy. Journal of Clinical Oncology 2005;23(7):1431‐8. [DOI] [PubMed] [Google Scholar]
Ravasco 2005b {published data only}
- Ravasco P, Monteiro‐Grillo I, Vidal PM, Camillo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head and Neck 2005;27(8):659‐68. [DOI] [PubMed] [Google Scholar]
Rogers 1992 {published data only}
- Rogers RM, Donahoe M, Costantino J. Physiologic effects of oral supplemental feeding in malnourished patients with chronic obstructive pulmonary disease. A randomized control study. American Review of Respiratory Disease 1992;146(6):1511‐7. [DOI] [PubMed] [Google Scholar]
Rydwik 2008 {published data only}
- Rydwik E, Lammes E, Frandin K, Ackner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomised controlled pilot treatment trial. Aging Clinical and Experimental Research 2008;20(2):159‐70. [DOI] [PubMed] [Google Scholar]
Schwenk 1999 {published and unpublished data}
- Schwenk A, Steuck H, Kremer G. Oral supplements as adjunctive treatment to nutritional counseling in malnourished HIV‐infected patients: randomized controlled trial. Clinical Nutrition 1999;18(6):371‐4. [DOI] [PubMed] [Google Scholar]
Sharma 2002 {published data only}
- Sharma M, Rao M, Jacob S, Jacob CK. A controlled trial of intermittent enteral nutrient supplementation in maintenance haemodialysis patients. Journal of Renal Nutrition 2002;12(4):229‐37. [DOI] [PubMed] [Google Scholar]
Singh 2008 {published data only}
- Singh S, Midha S, Singh N, Joshi YK, Garg PK. Dietary counselling versus dietary supplements for malnutrition in chronic pancreatitis: a randomised controlled trial. Clinical Gastroenterology and Hepatology 2008;6(3):353‐9. [DOI] [PubMed] [Google Scholar]
Stratton 2007 {published data only}
- Stratton RJ, Bowyer G, Elia M. Fewer complications with liquid supplements that food snacks in fracture patients at risk of malnutrition [abstract]. Clinical Nutrition 2007;9(Suppl 2):9 (0014). [Google Scholar]
- Stratton RJ, Bowyer G, Elia M. Food snacks or liquid oral nutritional supplements as a first line treatment for malnutrition in post operative patients [abstract]. Proceedings of Nutrition Society 2006;65:Abstract 4a. [Google Scholar]
- Stratton RJ, Bowyer G, Elia M. Greater total energy and protein intakes with liquid supplements than food snacks in patients at risk of malnutrition [abstract]. Proceedings of ESPEN XXVIIIth Congress, 19 ‐ 22 October, Istanbul, Turkey. 2006:158.
Weekes 2009 {published and unpublished data}
- Weekes CE. Nutritional intervention in COPD. Personal Communication 2006.
- Weekes CE, Emery PW, Elia M. Dietary counselling and food fortification in stable COPD: a randomised controlled trial. Thorax 2009;64(4):326‐31. [DOI] [PubMed] [Google Scholar]
Wilson 2001 {published data only}
- Wilson B, Fernandez‐Madrid A, Hayes A, Hermann K, Smith J, Wassell A. Comparison of the effects of two early intervention strategies on the health outcomes of malnourished hemodialysis patients. Journal of Renal Nutrition 2001;11(9):166‐71. [DOI] [PubMed] [Google Scholar]
Wong 2004 {published and unpublished data}
- Wong SY, Lau EM, Lau WW, Lynn HS. Is dietary counselling effective in increasing dietary calcium, protein and energy intakes in patients with osteoporotic fractures? A randomised controlled clinical trial. Journal of Human Nutrition & Dietietics 2004;17(4):359‐64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Antila 1993 {published data only}
- Antila H, Salo M, Nanto V, Forssell D, Salonen M, Kirvela O. The effect of intermaxillary fixation on leucocyte zinc, serum trace elements and nutritional status of patients undergoing maxillofacial surgery. Clinical Nutrition 1993;12(4):223‐9. [DOI] [PubMed] [Google Scholar]
Arutiunov 2009 {published data only}
- Arutiunov GP, Kostiukevich OI, Bylova NA. Prevalence and clinical significance of malnutrition and effectiveness of nutritional support for patients suffering from chronic heart failure. Eksperimental' Naia i Klinicheskaia Gastroenterologiia 2009;2:22‐33. [PubMed] [Google Scholar]
Bachmann 1998 {published data only}
- Bachmann P, Gordiani B, Ranchere JY, Lallemand Y, Combret D, Latour JF, et al. Assessment of indication and quality of nutritional support in medical oncology. Nutrition Clinique et Metabolisme 1998;12(1):3‐12. [Google Scholar]
Bauer 1994 {published data only}
- Bauer S, Jakob E, Berg A, Keul J. Energy and nutrient intake in adolescent weight‐lifters before and after nutritional counselling. Schweizerische Zeitschrift fur Medizin und Traumatologie 1994;3:35‐42. [PubMed] [Google Scholar]
Beange 1995 {published data only}
- Beange H, Gale L, Stewart L. Project re nourish: A dietary intervention to improve nutritional status in people with multiple disabilities. Australian and New Zealand Journal of Developmental Disability 1995;20(3):165‐74. [Google Scholar]
Beck 2008 {published data only}
- Beck AM, Damkjaer K, Beyer N. Multifacted nutritional intervention among nursing‐home residents has a positive influence on nutrition and function. Nutrition 2008;24(11‐12):1073‐80. [DOI] [PubMed] [Google Scholar]
Bills 1993 {published data only}
- Bills DJ, Spangler AA. Impact of dietitians on quality of care in 16 nursing homes in east central Indiana. Journal Of The American Dietetic Association 1993;93(1):73‐5. [DOI] [PubMed] [Google Scholar]
Bolton 1990 {published data only}
- Bolton J, Shannon L, Smith V, Abbott R, Bell SJ, Stubbs L, et al. Comparison of short‐term and long‐term palatability of six commercially available oral supplements. Journal of Human Nutrition and Dietetics 1990;3(5):317‐21. [Google Scholar]
Bories 1994 {published data only}
- Bories PN, Campillo B. One‐month regular oral nutrition in alcoholic cirrhotic patients. Changes of nutritional status, hepatic function and serum lipid pattern. British Journal of Nutrition 1994;72(6):937‐46. [DOI] [PubMed] [Google Scholar]
Botella‐Carretero 2008 {published data only}
- Botella‐Carretero JI, Iglesias B, Balsa JA, Zamarron I, Arrieta F, Vazquez C. Effects of oral nutritional supplements in normally nourished or mildly undernourished geriatric patients after surgery for hip fracture: a randomised clinical trial. Journal of Parenteral and Enteral Nutrition 2008;32(2):120‐8. [DOI] [PubMed] [Google Scholar]
Bozzetti 1998 {published data only}
- Bozzetti F. Comments on: Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies, [comment on: Andreyev et al., European Journal of Cancer 1998, 34, pp. 503‐509] (multiple letters). European Journal of Cancer 1998;34(13):2132‐3. [DOI] [PubMed] [Google Scholar]
Bugge 1997 {published data only}
- Bugge KE, Tschudi MC. Developing a research‐based innovation protocol on nutritional support to patients with cancer in the gastrointestinal tract [Norwegian]. Vard I Norden Nursing Science and Research in the Nordic Countries 1997;17:24‐7. [Google Scholar]
Bunout 1989 {published data only}
- Bunout D, Aicardi V, Hirsch S, Petermann M, Kelly M, Silva G, et al. Nutritional support in hospitalized patients with alcoholic liver disease. European Journal of Clinical Nutrition 1989;43(9):615‐21. [PubMed] [Google Scholar]
Burger 1993 {published data only}
- Burger B, Ollenschlager G, Schrappe M, Stute A, Fischer M, Wessel D, et al. Nutrition behavior of malnourished HIV‐infected patients and intensified oral nutritional intervention. Nutrition 1993;9(1):43‐4. [PubMed] [Google Scholar]
Carlsson 2005 {published data only}
- Carlsson P, Tidermark J, Ponzer S, Soderqvist A, Cederholm T. Food habits and appetite of elderly women at the time of femoral neck fracture and after nutritional and anabolic support. Journal of Human Nutrition and Dietetics 2005;18:117‐120. [DOI] [PubMed] [Google Scholar]
Duncan 2006 {published data only}
- Duncan DG, Beck SJ, Hood K, Johansen A. Using dietetic assistants to improve the outcome of hip fracture: a randomised controlled trial of nutritional support in an acute trauma ward. Age and Ageing 2006;35(2):148‐53. [DOI] [PubMed] [Google Scholar]
Efthimiou 1988 {published data only}
- Efthimiou J, Fleming J, Gomes C, Spiro SG. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1988;137(5):1075‐82. [DOI] [PubMed] [Google Scholar]
Elbanna 1996 {published data only}
- Elbanna HM, Tolba KG, Darwish OA. Dietary management of surgical patients: effects on incisional wound healing. Eastern Mediterranean Health Journal 1996;2:243‐54. [Google Scholar]
Elkort 1981 {published data only}
- Elkort RJ, Baker FL, Vitale JJ, Cordano A. Long‐term nutritional support as an adjunct to chemotherapy for breast cancer. Journal of Parenteral and Enteral Nutrition 1981;5(5):385‐90. [DOI] [PubMed] [Google Scholar]
Elmstaahl 1987 {published data only}
- Elmstahl S, Steen B. Hospital nutrition in geriatric long‐term care medicine: II. Effects of dietary supplements. Age and Ageing 1987;16(2):73‐80. [DOI] [PubMed] [Google Scholar]
Eneroth 1997 {published data only}
- Eneroth M, Apelqvist J, Larsson J, Persson BM. Improved wound healing in transtibial amputees receiving supplementary nutrition. International Orthopaedics 1997;21(2):104‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engel 1995 {published data only}
- Engel B, Kon P, Raftery MJ. Strategies to identify and correct malnutrition in hemodialysis patients. Journal of Renal Nutrition 1995;5:62‐6. [Google Scholar]
Flynn 1987 {published data only}
- Flynn MB, Leightty FF. Preoperative outpatient nutritional support of patients with squamous cancer of the upper aerodigestive tract. American Journal of Surgery 1987;154(4):359‐62. [DOI] [PubMed] [Google Scholar]
Forli 2006 {published data only}
- Forli L, Moum T, Bjortuft O, Vatn M, Boe J. The influence of underweight and dietary support on well‐being in lung transplant candidates. Respiratory Medicine 2006;100(7):1239‐46. [DOI] [PubMed] [Google Scholar]
Franzoni 1996 {published data only}
- Franzoni S, Frisoni GB, Boffelli S, Rozzini R, Trabucchi M. Good nutritional oral intake is associated with equal survival in demented and non‐demented very old patients. Journal of the American Geriatrics Society 1996;44(11):1366‐70. [DOI] [PubMed] [Google Scholar]
Glimelius 1992 {published data only}
- Glimelius B, Birgegard G, Hoffman K, Hagnebo C, Hogman G, Kvale G, et al. Improved care of patients with small cell lung cancer. Nutritional and quality of life aspects.. Acta Oncologica 1992;31(8):823‐31. [DOI] [PubMed] [Google Scholar]
Heberer 1984 {published data only}
- Heberer M, Durig M, Harder F. Oropharyngoesophageal carcinoma ‐ aspects of the nutritional status and immunoresistance [Die oro‐pharyngo‐osophagealen Karzinome ‐ Aspekt des Ernahrungszustandes und der Immunabwehr]. Helvetica Chirurgica Acta 1984;50(5):493‐503. [PubMed] [Google Scholar]
Henquin 1989 {published data only}
- Henquin N, Havivi E, Reshef A, Barak F, Horn Y. Nutritional monitoring and counselling for cancer patients during chemotherapy. Oncology 1989;46(3):173‐7. [DOI] [PubMed] [Google Scholar]
Hickson 2004 {published data only}
- Hickson M, Bulpitt C, Nunes M, Peters R, Cooke J, Nicholl C, Frost G. Does additional feeding support provided by health care assistants improve nutritional status and outcome in acutely ill older in‐patients?‐a randomised control trial. Clinical Nutrition 2004;23(1):69‐77. [DOI] [PubMed] [Google Scholar]
Hogan 1997 {published data only}
- Hogan SE, Evers SE. A nutritional rehabilitation program for persons with severe physical and developmental disabilities. Journal of the American Dietetic Association 1997;97(2):162‐6. [DOI] [PubMed] [Google Scholar]
Holder 2003 {published data only}
- Holder H. Nursing management of nutrition in cancer and palliative care. British Journal of Nursing 2003;12(11):667‐8. [DOI] [PubMed] [Google Scholar]
Hulsewe 1997 {published data only}
- Hulsewe KWE, Meijerink WJHJ, Soeters PB, Meyenfeldt MF. Assessment of outcome of perioperative nutritional interventions. Nutrition 1997;13(11‐12):996‐8. [DOI] [PubMed] [Google Scholar]
Idilman 2009 {published data only}
- Idilman R, Seval G, Cinar K, Mizrak D, Bozkus Y, Arsaln M, Bektas M, Erden E, Ozden A. The effect of dietary intervention on the long‐term clinical outcome of non‐alcoholic fatty liver disease. Journal of Hepatology 2009;50:1027. [Google Scholar]
Ireton 1995 {published data only}
- Ireton JC, Garritson B, Kitchens L. Nutrition intervention in cancer patients: does the registered dietitian make a difference?. Topics in Clinical Nutrition 1995;10:42‐8. [Google Scholar]
Jamieson 1997 {published data only}
- Jamieson CP, Norton B, Day T, Lakeman M, Powell TJ. The quantitative effect of nutrition support on quality of life in outpatients. Clinical Nutrition 1997;16(1):25‐8. [Google Scholar]
Jie 2009 {published data only}
- Jie B. The impact of nutritional support on clinical outcome in patients at nutritional risk: multicenter study in Baltimore and Beijing teaching hospitals. Annals of Nutrition and Metabolism 2009;55:131‐2. [DOI] [PubMed] [Google Scholar]
Johnson 1993 {published data only}
- Johnson LE, Dooley PA, Gleick JB. Oral nutritional supplement use in elderly nursing home patients. Journal of the American Geriatrics Society 1993;41(9):947‐52. [DOI] [PubMed] [Google Scholar]
Keller 1995 {published data only}
- Keller HH. Weight gain impacts morbidity and mortality in institutionalized older persons. Journal of the American Geriatrics Society 1995;43(2):165‐9. [DOI] [PubMed] [Google Scholar]
Knowles 1988 {published data only}
- Knowles JB, Fairbarn MS, Wiggs BJ, Chan‐Yan C, Pardy RL. Dietary supplementation and respiratory muscle performance in patients with COPD. Chest 1988;93(5):977‐83. [DOI] [PubMed] [Google Scholar]
Kondrup 1998 {published data only}
- Kondrup J, Bak L, Hansen BS, Ipsen B, Ronneby H. Outcome from nutritional support using hospital food. Nutrition 1998;14(3):319‐21. [DOI] [PubMed] [Google Scholar]
Krasnoff 2006 {published data only}
- Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, Painter PL. A randomised trial of exercise and dietary counselling after liver transplantation. American Journal of Transplantation 2006;6(8):1896‐905. [DOI] [PubMed] [Google Scholar]
Kruizenga 2005 {published data only}
- Kruizenga HM, Tulder MW, Seidell JC, Thijs A, Ader HJ, Bokhorst‐de van de Scheuren MAE. Effectiveness and cost‐effectiveness of early screening and treatment of malnourished patients. American Journal of Clinical Nutrition 2005;82(5):1082‐9. [DOI] [PubMed] [Google Scholar]
Lejeune 2005 {published data only}
- Lejeune MPGM, Kovacs EMR, Westerterp‐Plantenga MS. Additional protein intake limits weight regain after weight loss in humans. British Journal of Nutrition 2005;93(2):281‐9. [DOI] [PubMed] [Google Scholar]
Levine 1982 {published data only}
- Levine AS, Brennan MF, Ramu A, Fisher RI, Pizzo PA, Glaubiger DL. Controlled clinical trials of nutritional intervention as an adjunct to chemotherapy, with a comment on nutrition and drug resistance. Cancer Research 1982;42(Suppl 2):774s‐781s. [PubMed] [Google Scholar]
Lipschitz 1985 {published data only}
- Lipschitz DA, Mitchell CO, Steele RW, Milton KY. Nutritional evaluation and supplementation of elderly subjects participating in a 'meals on wheels' program. Journal of Parenteral Enteral Nutrition 1985;9(3):343‐7. [DOI] [PubMed] [Google Scholar]
Lynch 1983 {published data only}
- Lynch K, Henry DA, Roberts C, Coburn JW. Clinical trial with oral carbohydrate supplement in hemodialysis patients: A nutritional evaluation. Dialysis and Transplantation 1983;12(8):566‐8. [Google Scholar]
Manders 2009 {published data only}
- Manders M, Groot LC, Hoefnagels WH, Dhonukshe‐Rutten RA, Wouters‐Wessling W, Mulders AJ, et al. The effect of a nutrient dense drink on mental and physical function in institutionalized elderly people. Journal of Nutrition, Health and Aging 2009;13(9):760‐7. [DOI] [PubMed] [Google Scholar]
McWhirter 1996 {published data only}
- McWhirter JP, Pennington CR. A comparison between oral and nasogastric nutritional supplements in malnourished patients. Nutrition 1996;12(7‐8):502‐6. [DOI] [PubMed] [Google Scholar]
Mendenhall 1993 {published data only}
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: Results of a Department of Veterans Affairs cooperative study. Hepatology 1993;17(4):564‐76. [DOI] [PubMed] [Google Scholar]
Mendenhall 1995 {published data only}
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. Protein energy malnutrition in severe alcoholic hepatitis: Diagnosis and response to treatment. Journal of Parenteral and Enteral Nutrition 1995;19(4):258‐65. [DOI] [PubMed] [Google Scholar]
Monnin 1993 {published data only}
- Monnin S, Schiller MR. Nutrition counselling for breast cancer patients. Journal of the American Dietetic Association 1993;93(1):72‐3. [DOI] [PubMed] [Google Scholar]
Munck 1998 {published data only}
- Munck A, Le‐Bourgeois M, Gerardin M, Navarro J. Cystic fibrosis: Dietary counselling and nutritional support. Nutrition Clinique et Metabolisme 1998;12(4):283‐8. [Google Scholar]
Neidich 1985 {published data only}
- Neidich G, Schissel K, Sharp HL. Noninvasive outpatient nutritional therapy in inflammatory bowel disease. Journal of Parenteral and Enteral Nutrition 1985;9(3):350‐2. [DOI] [PubMed] [Google Scholar]
Newmark 1981 {published data only}
- Newmark SR, Simpson S, Daniel P, Sublett D, Black J, Geller R. Nutritional support in an inpatient rehabilitation unit. Archives of Physical Medicine and Rehabilitation 1981;62(12):634‐7. [PubMed] [Google Scholar]
Neyman 1996 {published data only}
- Neyman MR, Zidenberg CS, McDonald RB. Effect of participation in congregate‐site meal programs on nutritional status of the healthy elderly. Journal of the American Dietetic Association 1996;96:475‐82. [DOI] [PubMed] [Google Scholar]
Nijs 2006 {published data only}
- Nijs KAN, Graaf C, Kok FJ, Staveren WA. Effect of family style mealtimes on quality of life, physical performance, and body weight of nursing home residents: cluster randomised controlled trial. BMJ 2006;332(7551):1180‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olofsson 2007 {published data only}
- Olofsson B, Stenvall M, Svensson O, Gustafson Y. Malnutrition in hip fracture patients: an intervention study. The Journal of Clinical Nursing 2007;16(11):2027‐38. [DOI] [PubMed] [Google Scholar]
Openbrier 1984 {published data only}
- Openbrier DR, Irwin MM, Dauber JH, et al. Factors affecting nutritional status and the impact of nutritional support in patients with emphysema. Chest 1984;85(6 Suppl):67S‐69S. [Google Scholar]
Ottery 1996 {published data only}
- Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12(1 Suppl):S15‐S19. [DOI] [PubMed] [Google Scholar]
Parrott 2006 {published data only}
- Parrott MD, Young KW, Greenwood CE. Energy‐containing nutritional supplements can affect usual energy intake post‐supplementation in institutionalised seniors with probable Alzheirmer's disease. Journal of American Geriatrics Society 2006;54(9):1382‐7. [DOI] [PubMed] [Google Scholar]
Patel 1998 {published data only}
- Patel MG. The effect of dietary intervention on weight gains after renal transplantation. Journal of Renal Nutrition 1998;8(3):137‐41. [DOI] [PubMed] [Google Scholar]
Payette 2002 {published data only}
- Payette H, Boutier V, Coulombe C, Gray‐Donald K. Benefits of nutritional supplementation in free‐living, frail, undernourished elderly people: A prospective randomised community trial. Journal of American Dietetic Association 2002;102(8):1088‐95. [PubMed] [Google Scholar]
Pedersen 2005 {published data only}
- Pedersen PU. Nutritional care: the effectiveness of actively involving older patients. Journal of Clinical Nursing 2005;14(2):247‐55. [DOI] [PubMed] [Google Scholar]
Pietersma 2003 {published data only}
- Pietersma P, Follett‐Bick S, Wilkinson B, Guebert N, Fisher K, Pereira J. A bedside food cart as an alternate food service for acute and palliative oncological patients. Supportive Care in Cancer 2003;11(9):611‐4. [DOI] [PubMed] [Google Scholar]
Planas 2005 {published data only}
- Planas M, Alvarez J, Garcia‐Peris PA, Cuerda C, Lucas P, Castella M, et al. Nutritional support and quality of life in stable chronic obstructive pulmonary disease (COPD) patients. Clinical Nutrition 2005;24(3):433‐41. [DOI] [PubMed] [Google Scholar]
Plank 2008 {published data only}
- Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, et al. Noctural nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12 month trial. Hepatology 2008;48(2):557‐66. [DOI] [PubMed] [Google Scholar]
Rabinovitch 2006 {published data only}
- Rabinovitch R, Grant B, Berkey BA, Raben D, Ang KK, Fu KK, et al. Impact of nutrition support on treatment outcome in patients with a locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: a secondary analysis of RTOG trial 90‐03. Head and Neck 2006;28(4):287‐96. [DOI] [PubMed] [Google Scholar]
Rassmussen 2006 {published data only}
- Rasmussen HH, Kondrup J, Staun M, Ladefoged K, Lindorff K, Jorgensen LM, et al. A method for implementation of nutrition therapy in hospitals. Clinical Nutrition 2006;25(3):515‐23. [DOI] [PubMed] [Google Scholar]
Rüfenacht 2010 {published data only}
- Rüfenacht U, Ruhlin M, Wegmann M, Imoberdorf R, Ballmer PE. Nutritional counselling improves quality of life and nutrient intake in hospitalized undernourished patients. Nutrition 2010;26(1):53‐60. [DOI] [PubMed] [Google Scholar]
Salas‐Salvado 2005 {published data only}
- Salas‐Salvado J, Torres M, Planas M, Altimir S, Pagan C, Gonzalez ME, et al. Effect of oral administration of a whole formula diet on nutritional and cognitive status in patients with Alzheirmer's disease. Clinical Nutrition 2005;24(3):390‐7. [DOI] [PubMed] [Google Scholar]
Saudny 1997 {published data only}
- Saudny UH, Martin JG, Gray DK. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):794‐9. [DOI] [PubMed] [Google Scholar]
Simmons 2008 {published data only}
- Simmons SF, Keeler E, Zhuo X, Hickey KA, Sato H, Schnelle JF. Prevention of unintentional weight loss in nursing home residents: a controlled trial of feeding assistance. Journal of the American Geriatric Society 2008;56(8):1466‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smoliner 2008 {published data only}
- Smoliner C, Norman K, Scheufele R, Hartig W, Pirlich M, Lochs H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition 2008;24(11‐12):1139‐44. [DOI] [PubMed] [Google Scholar]
Solerte 2008 {published data only}
- Solerte SE, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, et al. Nutritional supplements with oral amino acid mixtures increases whole‐body lean mass and insulin sensitivity in elderly subjects with sarcopenia. American Journal of Cardiology 2008;101(Suppl):69E‐77E. [DOI] [PubMed] [Google Scholar]
Solomon 1978 {published data only}
- Solomon MJ, Smith MF, Dowd JB, Bistrian BR, Blackburn GL. Optimal nutritional support in surgery for bladder cancer: preservation of visceral protein by amino acid infusions. Journal of Urology 1978;119(3):350‐4. [DOI] [PubMed] [Google Scholar]
Sridar 1994 {published data only}
- Sridhar MK, Galloway A, Lean MEJ, Banham SW. An out‐patient nutritional supplementation programme in COPD patients. European Respiratory Journal 1994;7(4):720‐4. [DOI] [PubMed] [Google Scholar]
Stack 1996 {published data only}
- Stack JA, Bell SJ, Burke PA, Forse A. High‐energy, high‐protein, oral, liquid, nutrition supplementation in patients with HIV infection: effect on weight status in relation to incidence of secondary infection. Journal of the American Dietetic Association 1996;96:337‐41. [DOI] [PubMed] [Google Scholar]
Stark 1990 {published data only}
- Stark LJ, Bowen AM, Tyc VL, Evans S, Passero MA. A behavioral approach to increasing calorie consumption in children with cystic fibrosis. Journal of Pediatric Psychology 1990;15(3):309‐26. [DOI] [PubMed] [Google Scholar]
Swanenburg 2007 {published data only}
- Swanenburg J, Stauffacher M, Mulder T, Uebelhart D. Effects of exercise and nutrition on postural balance and risk of falling in elderly people with decreased bone mineral density: randomised controlled trial pilot study. Clinical Rehabilitation 2007;21(6):523‐34. [DOI] [PubMed] [Google Scholar]
Tatsumi 2009 {published data only}
- Tatsumi K, Shinozuka N, Nakayama K, Sekiya N, Kuriyama T, Fukuchi Y. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary disease. Journal of the American Geriatrics Society 2009;57(1):169‐72. [DOI] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor KA, Barr SI. Provision of small frequent meals does not improve energy intake of elderly residents with dysphagia who live in an extended‐care facility. Journal of American Dietetic Association 2006;106(7):1115‐8. [DOI] [PubMed] [Google Scholar]
Turic 1998 {published data only}
- Turic A, Gordon KL, Craig LD, Ataya DG, Voss AC. Nutrition supplementation enables elderly residents of long‐term‐care facilities to meet or exceed RDAs without displacing energy or nutrient intakes from meals. Journal of American Dietetic Association 1998;98(12):1457‐9. [DOI] [PubMed] [Google Scholar]
Unosson 1992 {published data only}
- Unosson M, Larsson J, Ek AC, Bjurulf P. Effects of dietary supplement on functional condition and clinical outcome measured with a modified Norton scale. Clinical Nutrition 1992;11(3):134‐9. [DOI] [PubMed] [Google Scholar]
Vargas 1995 {published data only}
- Vargas M, Puig A, Maza M, Morales P, Vargas D, Bunout B, et al. Patients with chronic airflow limitation: effects of the inspiratory muscle training with threshold load valve, built with appropriate technology, associated to nutritional support. Revista Medica De Chile 1995;123(10):1225‐34. [PubMed] [Google Scholar]
Volkert 1996 {published data only}
- Volkert D, Hubsch S, Oster P, Schlierf G. Nutrition support and functional status in undernourished geriatric patients during hospitalization and 6‐month follow‐up. Aging: Clinical and Experimental Research 1996;8(6):386‐95. [DOI] [PubMed] [Google Scholar]
Watson 2008 {published data only}
- Walton H, Bilton D, Truby H. A randomised controlled trial of a behavioural nutrition education programme "Eat Well with CF" for adults with CF [abstract}. Journal of Cystic Fibrosis 2006;5(Suppl 1):S73. [Google Scholar]
- Watson H, Bilton D, Truby H. A randomized controlled trial of a new behavioral home‐based nutrition education program, "Eat Well with CF" in adults with cystic fibrosis. The Journal of the American Dietetic Association 2008;108(5):847‐52. [DOI] [PubMed] [Google Scholar]
- Watson H, Bilton D, Truby H. Evaluating the process of a behavioural nutrition education programme "Eat Well with CF" in adults with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl 1):S73. [Google Scholar]
- Watson H, Truby H, Haworth CS, Bilton D. Pilot study to evaluate a home based behavioural nutrition education programme for adults [abstract]. Journal of Cystic Fibrosis 2004;3(Suppl 1):S75. [Google Scholar]
Williams 1989a {published data only}
- Williams J, Handy DJ, Weller PH. Improving oral energy intake in older children with cystic fibrosis. Journal of Human Nutrition and Dietetics 1989;2(4):279‐85. [Google Scholar]
Williams 1989b {published data only}
- Williams CM, Driver LT, Older J, Dickerson JW. A controlled trial of sip‐feed supplements in elderly orthopaedic patients. European Journal of Clinical Nutrition 1989;43(4):267‐74. [PubMed] [Google Scholar]
Woo 1994 {published data only}
Wouters‐Wessling 2005 {published data only}
- Wouters‐Wesseling W, Slump E, Kleijer CN, Groot LCPGM, Staveren WA. Early nutritional supplementation immediately after diagnosis of infectious disease improves body weight in psychogeriatric nursing home residents. Aging and Clinical Experimental Research 2006;18(1):70‐4. [DOI] [PubMed] [Google Scholar]
Wright 2008 {published data only}
- Wright L, Cotter D, Hickson M. The effectiveness of targeted feeding assistance to improve the nutritional intake of elderly dysphagic patients in hospital. Journal of Human Nutrition and Dietetics 2008;21(6):555‐62. [DOI] [PubMed] [Google Scholar]
Yoneda 1992a {published data only}
- Yoneda T, Yoshikawa M, Tsukaguchi K, Fu A, Tokuyama T, Cho S, et al. Supplementary nutrition therapy is effective in patients with chronic obstructive pulmonary disease. Japanese Journal of Thoracic Diseases 1992;30(10):1807‐13. [PubMed] [Google Scholar]
Yoneda 1992b {published data only}
- Yoneda T, Yoshikawa M, Tsukaguchi K, Fu A, Tomoda K, Egawa S, et al. Nutritional assessment and the effect of supplementary oral nutrition in patients with pulmonary emphysema. Japanese Journal of Thoracic Diseases 1992;30(8):1483‐87. [PubMed] [Google Scholar]
References to studies awaiting assessment
Margare 2002 {published data only}
- Margare N, Lymperopoulos N, Iordanou P, Noula M, Gesoule‐Voltyrake E, Bellow‐Milona P. The role of dietary counselling on food intake in lung cancer patients undergoing chemotherapy. ICUs & Nursing Web Journal 2002; Vol. 11.
Penalva 2009 {published data only}
- Penalva A, San Martin A, Rossello J, Perez‐Portabella C, Palacios A, Planas JYM. Oral nutritional supplementation in hematologic patients [Suplementacion oral nutricional en pacientes hemotologicos]. Nutricion Hospitalaria 2009;24(1):10‐6. [PubMed] [Google Scholar]
Shatenstein 2008 {published data only}
- Shatenstein B, Kergoat MJ, Reid I, Chicoine ME. Dietary intervention in older adults with early stage alzheimer dementia: early lessons learned. The Journal of Nutrition, Health and Aging 2008;12(7):461‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Age Concern 2006
- Age Concern. Hungry to be heard. www.ageconcern.org.uk/ 2006.
Avenell 2001
- Avenell A, Handoll HH, Grant AM. Lessons for search strategies from a systematic review, in The Cochrane Library, of nutritional supplementation trials in patients after hip fracture.. American Journal of Clinical Nutrition 2001;73(3):505‐510. [DOI] [PubMed] [Google Scholar]
BAPEN 2009
- British Association for Enteral and Parenteral Nutrition (BAPEN). Combating malnutrition: recommendations for action. www.bapen.org.uk/ (accessed 2009).
Braunschweig 1999
- Braunschweig CA. Creating a clinical nutrition registry: prospects, problems and preliminary results. Journal American Dietetic Association 1999;99(4):467‐70. [DOI] [PubMed] [Google Scholar]
Bruce 2003
- Bruce D, Laurance I, McGuiness M, Ridley M. Nutritional supplements after hip fracture: poor compliance limits effectiveness. Clinical Nutrition 2003;22(5):497‐500. [DOI] [PubMed] [Google Scholar]
Dupuy 1978
- Dupuy HJ. Self‐representations of general psychological well being of American adults. Proceedings of American Public Health Association, Los Angeles, CA. 1978.
Edington 1996
- Edington J, Kon P, Martyn CN. Prevalence of malnutrition in patients in general practice. Clinical Nutrition 1996;15(2):60‐3. [DOI] [PubMed] [Google Scholar]
Edington 1997
- Edington J, Kon P, Martyn CN. Prevalence of malnutrition after major surgery. Journal of Human Nutrition and Dietetics 1997;10:111‐6. [Google Scholar]
Elia 2009
- Elia M, Russell CA. Nutrition screening survey in the UK in 2008. Hospitals, care homes and mental health units.. www.bapen.org.uk/ (accessed 2009).
Foltz 1987
- Foltz A, Besser P, Ellenberg S, Carty C, Mitchell S, Paul K, et al. Effectiveness of nutritional counselling on caloric intake, weight change, and percent protein intake in patients with advanced colorectal and lung cancer. Nutrition 1987;3(4):263‐71. [Google Scholar]
Gosney 2003
- Gosney M. Are we wasting our money on food supplements in elder care wards?. Journal of Advanced Nursing 2003;43(3):275‐80. [DOI] [PubMed] [Google Scholar]
Guigoz 1997
- Guigoz Y, Vellas BJ. Malnutrition in the elderly: the Mini Nutritional Assessment (MNA). Therapeutische Umschau. Revue therapeutique 1997;54(6):345‐50. [PubMed] [Google Scholar]
Hanger 1999
- Hanger HC, Smart EJ, Merrilees MJ, Frampton CM. The prevalence of malnutrition in elderly hip fracture patients. New Zealand Medical Journal 1999;112(1084):88‐90. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 2010
- Jensen GL, Mirtallo J, Compher C, Dhailval R. Adult starvation and disease‐related malnutrition: a proposal for etiology‐based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clinical Nutrition 2010;29(2):151‐3. [DOI] [PubMed] [Google Scholar]
Keele 1997
- Keele AM, Bray MJ, Emery PW, Duncan HD, Silk DBA. Two phase randomised controlled clinical trial of postoperative oral dietary supplements in surgical patients. Gut 1997;40(3):393‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koretz 2007
- Koretz RL, Avenell A, Lipman TO, Braunschwieg CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials.. Amerian Journal of Gastroenterology 2007;102:412‐29. [DOI] [PubMed] [Google Scholar]
Kubrak 2007
- Kubrak C, Jensen L. Malnutrition in acute care patients: a narrative review. International Journal of Nursing Studies 2007;44(6):1036‐54. [DOI] [PubMed] [Google Scholar]
Lennard‐Jones 1992
- Lennard‐Jones JE. A positive approach to nutrition as treatment. Kings Fund Centre, London. London, 1992.
London Procurement Programme 2010
- London Procurement Programme. Clinical Oral Nutrition Support Project (Adults). www.lpp.nhs.uk/ (accessed 2010).
Manual of Dietetics
- Thomas B, editor. Manual of Dietetic Practice. 3rd Edition. London: Blackwell Publishing Ltd, 2001. [ISBN 0632055243] [Google Scholar]
McCormack 1997
- McCormack P. Undernutrition in the elderly population living at home in the community: a review of the literature. Journal of Advanced Nursing 1997;26(5):856‐63. [DOI] [PubMed] [Google Scholar]
McWhirter 1994
- McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ 1994;308(6934):945‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munro 1998
- Munro J, Passey C, Brosman S, Margetts B, Rivers H (published in association with BAPEN 1998). Value‐added benefits. Are nutritional supplements used effectively. Nursing Times 1998;94(Dec 4):59‐60. [Google Scholar]
Naber 1997
- Naber TH, Schermer T, de‐Bree A, Nusteling K, Eggink L, Kruimel JW, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. American Journal of Clinical Nutrition 1997;66(5):1232‐9. [DOI] [PubMed] [Google Scholar]
NICE 2006
- National Collaborating Centre for Acute Care. Nutrition support in adults: oral supplements, enteral and parenteral feeding. www.nice.org.uk (accessed 2006). [PubMed]
Norman 2008a
- Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease‐related malnutrition. Clinical Nutrition 2008;27(1):5‐15. [DOI] [PubMed] [Google Scholar]
Olin 1996
- Olin AO, Osterberg P, Hadell K, Armyr I, Jerstrom S, Ljungquist O. Energy‐enriched hospital food to improve energy intake in elderly patients. Journal of Parenteral and Enteral Nutrition 1996;20(2):93‐7. [DOI] [PubMed] [Google Scholar]
Peake 1998a
- Peake HJ, Evans WC, Maltby AA, Bartram J, Frost G. Determining the incidence of malnutrition to plan and target nutritional support strategies [Abstract OC28]. British Association of Parenteral and Enteral Nutrition (BAPEN) 1998.
Peake 1998b
- Peake HJ, Evans S, Chambers A, Riches C, Frost CG. Nutritional supplementation: how much do people drink [abstract]. Proceedings of the Nutrition Society. 1998; Vol. 57:94a.
Plauth 2006
- Plauth M, Cabre E, Riggio O, Assis‐Camilo M, Pirlich M, Kondrup J. ESPEN Guidelines on enteral nutrition: Liver disease. Clinical Nutrition 2006;25(2):285‐94. [DOI] [PubMed] [Google Scholar]
Potter 1998
- Potter J, Langhorne P, Roberts M. Routine protein energy supplementation adults: a systematic review. BMJ 1998;317(7157):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prieto 1996
- Prieto RM, Garcia YC, Gordon‐del RA, Redel‐del PJ, Arevalo JE. Incidence of malnutrition in surgical departments of the Reina Sofia hospital of Cordoba. Nutrition Hospital 1996;11:286‐90. [PubMed] [Google Scholar]
Review Manager 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rodin 1993
- Rodin J, McAvay G. Determinants of change in perceived health in a longitudinal study of older adults. Journal Gerontology 1993;47(6):373‐84. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Stratton 2003
- Stratton RJ, Green CJ, Elia M. Disease‐related malnutrition: an evidence‐based approach. Oxford: CABI, 2003. [ISBN 0851996485] [Google Scholar]
Volkert 2006
- Volkert D, Berner YN, Berry E, Cederholm T, Coti‐Bertrand P, Milne A, et al. ESPEN Guidelines on enteral nutrition: Geriatrics. Clinical Nutrition 2006;25:330‐60. [DOI] [PubMed] [Google Scholar]
Volkert 2010
- Volkert D, Saeglitz C, Gueldenzoph H, Sieber CC, Stehle P. Undiagnosed malnutrition and nutrition‐related problems in geriatric patients. Journal of Nutrition Health and Aging 2010;14(5):387‐92. [DOI] [PubMed] [Google Scholar]
Watson 1998
- Watson JL. The prevalence of malnutrition in patients admitted to Care of the Elderly wards [abstract]. British Association of Parenteral and Enteral Nutrition (BAPEN). 1998:PC50.
Weekes 1998
- Weekes E. The incidence of malnutrition in medical patients admitted to a hospital in south London [abstract]. British Association of Parenteral and Enteral Nutrition (BAPEN). 1998:PC2.
References to other published versions of this review
Baldwin 2001
- Baldwin C, Parsons T, Logan S. Dietary advice for illness‐related malnutrition in adults. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002008] [DOI] [PubMed] [Google Scholar]
Baldwin 2008
- Baldwin C, Weekes CE. Dietary advice for illness‐related malnutrition in adults. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002008.pub3] [DOI] [PubMed] [Google Scholar]


