Abstract
Background
Radioactive iodine treatment for differentiated thyroid cancer possibly results in xerostomia. Amifostine has been used to prevent the effects of irradiation to salivary glands. To date, the effects of amifostine on salivary glands in radioactive iodine treated differentiated thyroid cancer remain uncertain.
Objectives
To assess the effects of amifostine on salivary glands in high‐dose radioactive iodine treated differentiated thyroid cancer.
Search methods
Studies were obtained from computerized searches of MEDLINE, EMBASE, The Cochrane Library and paper collections of conferences held in Chinese.
Selection criteria
Randomised controlled clinical trials and quasi‐randomised controlled clinical trials comparing the effects of amifostine on salivary glands after radioactive iodine treatment for differentiated thyroid cancer with placebo and a duration of follow up of at least three months.
Data collection and analysis
Two authors independently assessed risk of bias and extracted data.
Main results
Two trials with 130 patients (67 and 63 patients randomised to intervention versus control) were included. Both studies had a low risk of bias. Amifostine versus placebo showed no statistically significant differences in the incidence of xerostomia (130 patients, two studies), the decrease of scintigraphically measured uptake of technetium‐99m by salivary or submandibular glands at twelve months (80 patients, one study), and the reduction of blood pressure (130 patients, two studies). Two patients in one study collapsed after initiation of amifostine therapy and had to be treated by withdrawing the infusion and volume substitution. Both patients recovered without sequelae. Meta‐analysis was not performed on the function of salivary glands measured by technetium‐99m scintigraphy at three months after high dose radioactive iodine treatment due to the highly inconsistent findings across studies (I2 statistic 99%). None of the included trials investigated death from any cause, morbidity, health‐related quality of life or costs.
Authors' conclusions
Results from two randomised controlled clinical trials suggest that the amifostine has no significant radioprotective effects on salivary glands in high‐dose radioactive iodine treated differentiated thyroid cancer patients. Moreover, no health‐related quality of life and other patient‐oriented outcomes were evaluated in the two included trials. Randomised controlled clinical trials with low risk of bias investigating patient‐oriented outcomes are needed to guide treatment choice.
Keywords: Humans; Adenocarcinoma, Follicular; Amifostine; Amifostine/therapeutic use; Carcinoma, Papillary; Carcinoma, Papillary/radiotherapy; Iodine Radioisotopes; Iodine Radioisotopes/adverse effects; Radiation Injuries; Radiation Injuries/diagnostic imaging; Radiation Injuries/prevention & control; Radiation‐Protective Agents; Radiation‐Protective Agents/therapeutic use; Radionuclide Imaging; Randomized Controlled Trials as Topic; Salivary Glands; Salivary Glands/diagnostic imaging; Salivary Glands/drug effects; Salivary Glands/radiation effects; Thyroid Neoplasms; Thyroid Neoplasms/radiotherapy; Xerostomia; Xerostomia/prevention & control
Plain language summary
Amifostine for salivary glands in high‐dose radioactive iodine treated differentiated thyroid cancer
Thyroid cancer is the most common malignancy of the endocrine system consisting of several subtypes like papillary carcinoma (accounting for 80% of cases) and follicular carcinoma (accounting for 11% of cases). These are collectively referred to as 'differentiated thyroid cancer'. Treatment with radioactive iodine after surgery (ablation of the thyroid gland or 'thyroidectomy') is important for the detection of metastatic disease and for the destruction of the remaining thyroid tissue with microscopic cancer. After radioactive iodine treatment, adverse effects may happen in the salivary glands and cause salivary gland swelling and pain, usually involving the parotid. The symptoms may develop immediately after a therapeutic dose of radioactive iodine or months later and progress in intensity with time. Secondary complications reported include dry mouth ('xerostomia') and taste alterations.
Amifostine is thought to be a radioprotector of salivary glands used in conjunction with radioiodine therapy. This medication is administered intravenously and was reported to ameliorate the damage of salivary glands caused by radioactive iodine therapy.
We found only two randomised controlled trials in which the effects of amifostine were compared with placebo. The two randomised clinical trials investigated 130 patients treated with high dose radioactive iodine for thyroid cancer. Altogether data from the two trials suggest that amifostine has no obvious protective effects on the salivary glands in these patients. Two patients in one study collapsed after initiation of amifostine therapy and had to be treated by withdrawing the infusion and volume substitution. Both patients recovered without sequelae.
Until better data become available, the use of sour candy or lemon juice to increase salivation might be more appropriate during radioactive iodine treatment for patients with differentiated thyroid cancer. Patients should be well informed of the importance of hydration, acid stimulation and glandular massage after radioactive iodine treatment. In addition, early recognition and treatment of xerostomia may improve outcomes.
Background
Description of the condition
Radioactive iodine for thyroid remnants and metastatic disease of differentiated thyroid carcinoma
Thyroid cancer is the most common endocrine malignancy (Edwards 2002; Wartofsky 2002) with the most frequent histologic subtype of thyroid carcinoma being papillary (accounting for 80% of cases) followed by follicular carcinoma (accounting for 11% of cases). These are commonly collectively referred to as differentiated thyroid cancer (Edwards 2002; Sawka 2004).
Radioactive iodine ablation of remnant thyroid tissue in patients with differentiated thyroid cancer who have undergone total or subtotal thyroidectomy is important for the detection of metastatic disease, and for the destruction of the remaining thyroid tissue with residual microscopic cancer (Fraker 1997; Zidan 2004). In many centres, the use of radioactive iodine has been established for the ablation of remnant thyroid tissue (Haugen 2006; Sawka 2004) and metastatic disease of differentiated thyroid carcinoma (Bohuslavizki 1999a; Schlumberger 1998) after patients had a thyroidectomy. A systematic review suggested that radioactive iodine ablation may be beneficial in decreasing recurrence of well‐differentiated thyroid cancer (Sawka 2004). However, results are inconsistent both for some outcomes and the incremental benefit of remnant ablation in low‐risk patients treated with bilateral thyroidectomy.
Irradiation effects of high dose radioactive iodine on salivary glands
In the management of thyroid remnants and metastatic disease of differentiated thyroid carcinoma, the sodium iodide symporter (NIS) plays an important role in radioactive iodine uptake. NIS is an integral plasma membrane glycoprotein which (as the crucial first step for thyroid hormone synthesis) mediates the active transport of the iodide ion into the thyroid follicular cells (Dai 1996;Levy 1998; Smanik 1996; Spitzweg 1998). NIS expression has also been demonstrated in many non thyroidal tissues, including salivary glands, lacrimal glands, gastric mucosa, lactating mammary glands, placenta and thymus (Chung 2002; Spitzweg 1999).
After radioactive iodine treatment, common side effects may include salivary gland swelling and pain, usually involving the parotid. The symptoms may develop immediately after a therapeutic dose of radioactive iodine or months later and progress in intensity with time. In conjunction with the radiation associated sialadenitis, secondary complications reported include xerostomia and taste alterations (Mandel 2003).
Apart from specific uptake by thyroid tissue, the ß‐emitting radioactive iodine is accumulated actively by an adenosine triphosphate (ATP)‐dependent sodium/potassium/chloride cotransport, i.e. the sodium iodide symporter in salivary glands due to its similar atomic diameter and its comparable electric charge (Albrecht 1976; Baum 1993; Helman 1987). Therefore, the salivary glands also have the capacity to concentrate iodide selectively. The principal site of the iodide transport into saliva is the epithelium of the parotid salivary glands' intralobular ducts (Gates 1967; Mishkin 1981). Radioiodine is extracted from periductal capillaries and concentrated by the ductal epithelium whereupon it is secreted into the duct lumen and transported into the oral cavity. The salivary concentration of radioiodine has been reported to vary from 20 to 100 times that found in the serum (Levenson 1994; Maier 1987; Myant 1960; Rigler 1955,). It has been calculated that up to 24% of the administered radioactive iodine dose for thyroid cancer therapy is lost in the saliva (Mandel 2003). In the process of concentrating the radioactive iodine, the salivary glands are exposed to the damaging effects of irradiation.
Although all salivary glands are involved in the transport of the radioactive iodine into the saliva, the parotid glands are most active and their serous cells are more susceptible than mucous acini to the deleterious effects of ionizing radiation. Therefore, the serous parotid gland will demonstrate a more intense radiation sialadenitis than the mixed mucous and serous cell‐containing submandibular and sublingual salivary glands (Abramson 1969; Rigler 1955). Serous cells are particularly concerned with secretion of salts and zymogen, the precursor of amylase. The mucous cells secrete mucin, a lubricant that eases swallowing and acts as a protective oral mucosal barrier. The radioactive iodine irradiation of the salivary glands also causes endothelial damage to the glandular vasculature (Maier 1987). An increase in capillary permeability results in the leakage of plasma proteins and electrolytes into the surrounding interstitial tissues. The simultaneously injured irradiated intralobular ducts lose their ability to filter and prevent plasma proteins from entering the saliva. As a result of these two mechanisms, elevated protein levels are evident in parotid saliva (Deeg 1988; Maier 1987). Elevated sodium and chloride levels are also found in parotid saliva because a radiation‐damaged duct does not have the normal duct's ability to resorb these electrolytes secreted by the terminal acinar cells as saliva progresses through the duct system. Furthermore, salivary phosphate levels are decreased when the damaged epithelium of the intralobular duct's wall fails in its normal function to transport phosphate into the saliva. Biochemical changes in saliva can be expected in all patients receiving therapeutic radioactive iodine (Levenson 1994; Maier 1987), the extent of which is dose dependent.
Another side effect of radioiodine treatment is damage of normal tissues in the radiation port, in particular the salivary glands (Mossman 1994), resulting in transient or long‐lasting xerostomia (Bohuslavizk 1995; Bohuslavizki 1996; Bohuslavizki 1997). Therefore, radioiodine treatment is performed under salivary gland stimulation to decrease impairment of salivary gland function (Becker 1995; Clarke 1994; Spiegel 1986). However, even under salivary gland‐stimulating conditions, parenchymal damage could be demonstrated after radioactive iodine treatment using quantitative salivary gland scintigraphy (Allweiss 1984; Bohuslavizki 1996; Bohuslavizki 1997; Bohuslavizki 1998a; Bohuslavizki 1998b).
Salivary gland scintigraphy and effects of amifostine on salivary glands
Salivary gland scintigraphy is a diagnostic test with high sensitivity, specificity, accuracy and positive and negative predictive values in detecting Sjögren's Syndrome (Dugonji 2008). The imaging technique is widely accepted as an objective assessment of salivary gland function and a diagnostic criterion of primary Sjögren's Syndrome (Vinagre 2008). Quantitative and semi‐qualitative salivary gland scintigraphy performed in a standardized method as described (Bohuslavizk 1995) enables the quantitative evaluation of salivary glands' parenchymal function after radioactive iodine treatment in the management of differentiated thyroid cancer. It is characterized by an excellent intraindividual observer variability, as well as by a good reproducibility allowing the detection of changes in parenchymal function in the range of as little as 5% to 10% (Bohuslavizki 1998a; Bohuslavizki 1998b). This enables both the early detection of the Sjögren's Syndrome (Bohuslavizk 1995) and parenchyma impairment of salivary glands following radioactive iodine therapy (Bohuslavizki 1998a; Bohuslavizki 1998b). Therefore, salivary gland scintigraphy proves to be a suitable imaging modality for quantitative evaluation of salivary gland parenchymal function.
Description of the intervention
Amifostine is a radioprotector of salivary glands used in conjunction with radioiodine therapy. After being administered intravenously, amifostine is rapidly cleared from plasma with an alpha half‐life of less than one minute and a beta half‐life of less than 10 minutes (Shaw 1988). Before radioactive iodine administration, doses of amifostine given ranged from 300 mg/m2 (Kim 2008) to 500 mg/m2 over 15 minutes (Bohuslavizki 1998b). It was reported that amifostine lessened the parenchymal damage of salivary glands induced by radioactive iodine therapy (Bohuslavizki 1998a; Bohuslavizki 1998b). This may improve quality of life of patients with differentiated thyroid cancer treated with radioactive iodine. However, a recent study did not show cytoprotective effects of amifostine for differentiated thyroid cancer patients treated with radioactive iodine (Kim 2008). As a result of contradictory evidence, it is necessary to establish the effect of amifostine for salivary glands in radioactive iodine treated differentiated thyroid cancer.
Adverse effects of the intervention
The most common adverse effects of amifostine are emesis, hypotension, somnolence, and sneezing (Boehme 2004; Bohuslavizki 1999b; Rades 2004). Less common side effects include a metallic taste, flushing, malaise, hypocalcaemia, chills, and idiosyncratic reactions such as fever and rash (Boehme 2004; Rades 2004). Consequently, careful inspections should be undertaken while intravenous administration of amifostine: First, blood pressure should be checked prior to the amifostine infusion and every minute during the infusion and up to 15 min afterwards. Secondly, amifostine infusion should be stopped if acute toxicity such as allergic reactions equal or greater than grade 2 (according to the common toxicity criteria) or vomiting and hypotension equal or greater than grade 3 occur (Rades 2004; Trotti 2000). Thirdly, due the emetic potency of amifostine, treatment with 40 mg dexamethasone and 5 mg tropisetron (Bohuslavizki 1998a; Bohuslavizki 1999b) or 8 mg ondansetron are administered before amifostine treatment ( Kim 2008; Rades 2004). In order to reduce the risk of amifostine induced hypotension, patients irradiated for head and neck cancer receive an intravenous infusion of 1000 ml fluid (Rades 2004). Hypotension may be overcome by withdrawal of amifostine and volume substitution (Bohuslavizki 1999b). However, results from patients, irradiated for head and neck cancer, suggested an association with a high rate of serious adverse effects which resulted in the discontinuation of amifostine. The investigators concluded that the intravenous application of amifostine must be regarded as a treatment with a high toxicity potential, which has been underestimated until recently (Rades 2004). Alternatives of intravenous amifostine are considered. Subcutaneous application is reported to be less toxic than intravenous application. However, the rate of severe adverse effects was greater than 10% (Anne 2002; Koukourakis 2000).
How the intervention might work
Amifostine is an organic thiophosphate that is dephosphorylated in normal tissues to its active metabolite, WR‐1065 (Cappizi 1999). Amifostine was originally developed as a radioprotective agent within the Anti‐Radiation Drug Development Program initiated by the United States Army at the Walter Reed Army Institute of Research (Washington D. C) in the early 1950s (Patt 1949). Since numerous preclinical studies in cell cultures and animal models showed that, following dephosphorylation to its active metabolite WR‐1065, amifostine selectively protected normal tissue from damaging effects of irradiation (Menard 1984; Phillips 1973; Pratt 1980; Sodicoff 1978a; Sodicoff 1978b; Washburn 1978; Yuhas 1980), several clinical studies were initiated, confirming its radioprotective potency (McDonald 1994; Niibe 1985; Takahashi 1986). This resulted in an approval of amifostine in Germany, in 1995, for the supportive therapy of patients with ovarian cancer being treated with cisplatin derivatives in order to minimize the myelotoxic side effects of cisplatin.
The mechanism of radioprotective effects on normal tissue as compared to tumour tissue by amifostine, is suggested to be caused by two effects. First, amifostine is accumulated much more in normal tissue as compared to malignant transformed cells (Rasey 1985; Rasey 1986b; Utley 1976a; Yuhas 1980). Secondly, the alkaline phosphatase necessary for dephosphorylation of amifostine to its active compound WR‐1065 is more effective in the alkaline environment of normal tissue than in the acidic tumour tissues (Utley 1976b). Once activated inside the cell, WR‐1065 acts as a scavenger of oxygen free radicals. Since amifostine is known to be accumulated extensively in salivary glands (Phillips 1973; Rasey 1985; Rasey 1986a; Rasey 1988; Utley 1976b; Utley 1981; Washburn 1974; Yuhas 1980), it seemed reasonable to use amifostine as a radioprotector in patients with head and neck tumours receiving external radiation therapy. Some studies have been undertaken in head and neck tumours yielding promising results concerning the reduction of radiation‐induced salivary gland damage (McDonald 1994; Niibe 1985; Takahashi 1986). Since radiation effects of external radiation and radioiodine therapy are in general caused by the same mechanisms, i.e. the production of free radicals, it seemed promising to use this radioprotection of salivary glands by amifostine in high‐dose radioiodine therapy.
Why it is important to do this review
The most frequent non‐thyroidal complication of radioiodine therapy for differentiated thyroid cancer is salivary gland dysfunction, which may be transient or permanent. Since differentiated thyroid cancer has a good prognosis, reduction of long‐term side effects is important. Even after using salivary gland acid stimulating agents, parenchymal damage could be demonstrated, following high‐dose radioiodine treatment, using quantitative salivary gland scintigraphy (Allweiss 1984; Bohuslavizki 1996; Bohuslavizki 1997; Bohuslavizki 1998a; Bohuslavizki 1998b). Amifostine is a potent radioprotector of salivary glands in radioiodine therapy. As a result, it has been reported that the parenchymal damage in salivary glands induced by high‐dose radioiodine therapy can be reduced significantly by amifostine. This may improve quality of life of patients with differentiated thyroid cancer treated with radioactive iodine. Up to now, the impact of amifostine for salivary glands in radioactive iodine treated differentiated thyroid cancer is controversial as no cytoprotective effect of amifostine has been found for these patients.
Currently, there are no systematic reviews of studies of amifostine for salivary glands in radioactive iodine treated differentiated thyroid cancer.
Objectives
To assess the effects of amifostine on salivary glands in high‐dose radioactive iodine treated differentiated thyroid cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials and quasi‐randomised controlled clinical trials.
Types of participants
Patients of any age or sex who have differentiated thyroid carcinoma after total or near ‐total thyroidectomy followed by radioactive iodine and amifostine treatment were included.
Patients with initial salivary gland dysfunction, connective tissue disease, or other conditions causing salivary gland dysfunction were excluded.
The diagnosis of differentiated thyroid carcinoma had to be established according to the definite pathohistological evaluation of the surgical specimen. Salivary gland dysfunction had to be established by means of questionnaires for symptoms or through salivary gland scintigraphy.
Types of interventions
Intervention
Amifostine treatment.
Control
Placebo or gland acid stimulating agents.
Types of outcome measures
Primary outcomes
xerostomia (dry mouth);
health‐related quality of life.
Secondary outcomes
death from any cause;
morbidity;
adverse effects;
costs;
salivary functions, measured by 99mTc‐pertechnetate scintigraphy.
Covariates, effect modifiers and confounders
age;
gender;
dose of radioactive iodine.
Timing of outcome measurement
The follow up had to be at least three months after administration of amifostine and radioactive iodine.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library (issue 2, 2009);
MEDLINE (until April 2009);
EMBASE (until 2009).
We also searched databases of ongoing trials: 'Current Controlled Trials' (www.controlled‐trials.com ‐ with links to other databases of ongoing trials). For detailed search strategies please see Appendix 1.
Additional key words of relevance could have been detected during any of the electronic or other searches. If this was the case, electronic search strategies would have been modified to incorporate these terms. Studies published in any language were included.
Searching other resources
We tried to identify additional studies by searching the reference lists of included trials and (systematic) reviews, meta‐analyses and health technology assessment reports noticed.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two authors (MC and XJW) independently scanned the abstract, title or both sections of every record retrieved. All potentially relevant articles were investigated as full text. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). Differences were marked and if these studies were included, the influence of the primary choice was planned to be subjected to a sensitivity analysis. Where differences in opinion existed, they were resolved by a third party. If resolving disagreement was not possible, the article was added to those 'awaiting assessment' and authors were contacted for clarification. An adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection is attached (Moher 1999).
Data extraction and management
For studies that fulfilled inclusion criteria, two authors (MC and XJW) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details see 'Characteristics of included studies' and Table 1, Appendix 2 with any disagreements to be resolved by discussion, or if required by a third party. Any relevant missing information on the trial was planned to be sought from the original author(s) of the article, if required.
1. Study populations data.
| study ID | intervention | [n] screened | [n] randomised | [n] safety | [n] ITT | [n] finishing study | comments |
| Bohuslavizki 1998b | amifostine (I) placebo (C) |
I: 25 C: 25 Total: 50 |
I: 25 C: 25 Total: 50 |
I: 25 C: 25 Total: 50 |
I: not mentioned C: not mentioned |
I: 25 C: 25 Total: 50 |
none |
| Kim 2008 | amifostine (I) placebo (C) |
I: 42 C: 38 Total: 80 |
I: 42 C: 38 Total: 80 |
I: 42 C: 38 Total: 80 |
I: not mentioned C: not mentioned |
I: 42 C: 38 Total: 80 |
none |
ITT: intention‐to‐treat
Assessment of risk of bias in included studies
Two authors (MC and XJW) assessed each trial independently. Possible disagreement were resolved by consensus, or with consultation of a third party in case of disagreement. We explored the influence of individual bias criteria in a sensitivity analysis (see under 'sensitivity analyses') using the Cochrane Collaboration's risk of bias tool. Interrater agreement for key bias indicators (e.g. allocation concealment, incomplete outcome data) were calculated using the kappa statistic (Cohen 1960). In cases of disagreement, the rest of the group was consulted and a judgement was made based on consensus.
Measures of treatment effect
Dichotomous data
For dichotomous data (for example, patients experiencing sialoadenitis, hypogeusia or taste loss, dry mouth with or without repeated sialadenitis, the incidence of radiation induced salivary gland neoplasms and other adverse effects), the number of participants experiencing the event and the total number of participants in each arm of the trial were extracted. Relative risks were used.
Continuous data
For continuous data (for example, maximal secretion, uptake and uptake time of 99mTc pertechnetate in parotid and submandibular glands), we extracted the arithmetic means and standard deviations for each group. Mean differences were used.
Unit of analysis issues
Special issues in the analysis of studies with non‐standard designs, such as cluster‐randomised trials, were planned to be described.
Dealing with missing data
Relevant missing data were planned to obtain from authors, if feasible. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat (ITT) and per‐protocol (PP) population was carefully performed. Attrition rates, for example drop‐outs, losses to follow up and withdrawals were investigated. Issues of missing data and techniques to handle these (for example, last‐observation‐carried‐forward (LOCF)) were critically appraised.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we tried to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) obtained priority.
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, study results were not combined by means of meta‐analysis. Heterogeneity was identified by visual inspection of the forest plots, by using a standard χ2‐test and a significance level of α = 0.1, in view of the low power of such tests. Heterogeneity was specifically examined with I2 (Higgins 2002), where I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
Funnel plots were planned to be used to assess for the potential existence of small study bias. There are a number of explanations for the asymmetry of a funnel plot (Sterne 2001). Therefore, we planned to carefully interpret results (Lau 2006).
Data synthesis
Data were summarised statistically if they were available, sufficiently similar and of sufficient quality. Statistical analysis was performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to be mainly performed if one of the primary outcome parameters demonstrated statistically significant differences between intervention and control groups. In any other case subgroup analyses would have been clearly marked as a hypothesis generating exercise.
The following subgroup analyses were planned:
age;
gender;
dose of radioactive iodine.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of risk of bias, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results was also planned to be tested by repeating the analysis using different measures of effects size (relative risk, odds ratio etc.) and different statistical models (fixed‐ and random‐effects models).
Results
Description of studies
Results of the search
The electronic searches and hand searches revealed 92 studies. Of these references, we excluded 66 citations based on their title, abstract or both because they were not relevant to the question under study. After reading the full text of 26 potentially relevant publications, 21studies were excluded because they did not fulfil the inclusion criteria, five potential controlled clinical trials were retrieved for further assessment. Two randomised controlled clinical trials (Bohuslavizki 1998b; Kim 2008) were included. Trial durations were from 2004 to 2006 in one trial (Kim 2008), but not mentioned in the other (Bohuslavizki 1998b). Summary details of these trials are given in the 'Characteristics of included studies' section. Three seemingly duplicate studies were excluded (Bohuslavizki 1998a; Bohuslavizki 1999a; Bohuslavizki 1999b). For an overview (Figure 1), please see the adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection (Moher 1999).
1.
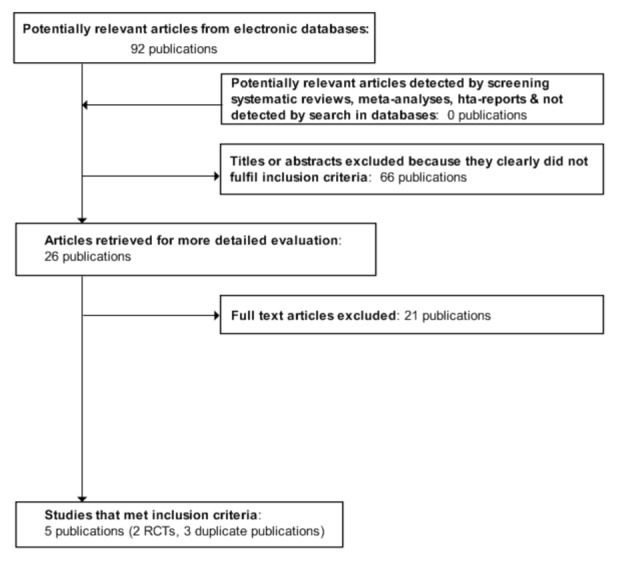
Adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection
Dealing with duplicate publications
Four controlled clinical trials by the same author were published in 1998 and 1999. We included the original publication (the oldest version).
Included studies
Altogether 130 patients with differentiated thyroid cancer participated in two trials. Fifty participants in Germany (Bohuslavizki 1998b) and 80 participants in the Republic of Korea were recruited ranging in age from 19 to 81 years. The diagnosis of xerostomia was established according to the World Health Organization (WHO) criteria in one trial (Bohuslavizki 1998b), and by a xerostomia questionnaire for symptoms assessment in another trial (Kim 2008). No inclusion or exclusion criteria were described in the two trials (Bohuslavizki 1998b; Kim 2008). Neither trial described disease duration. According to the results mentioned in the two included trials, there were no losses to follow up. Co‐medications such as antiemetic treatment with 40 mg dexamethasone and 5 mg tropisetron were used in one trial (Bohuslavizki 1998b), and 8 mg ondansetron in the other trial (Kim 2008). Co‐morbidities were not mentioned.
Excluded studies
Three potential trials being duplicate publications by the same author were excluded (Bohuslavizki 1998a; Bohuslavizki 1999a; Bohuslavizki 1999b).
Risk of bias in included studies
Two randomised controlled clinical trials were included. The risk of bias of the included trials was considered as low. No patients were lost to follow up. Details of the studies are listed in the Characteristics of included studies and Risk of bias in included studies tables.
Allocation
Allocation concealment was not mentioned in the included studies.
Blinding
One trial reported blinding (Bohuslavizki 1998b).
Incomplete outcome data
Outcome data were available for all randomised patients.
Selective reporting
None detected.
Other potential sources of bias
Summary data on age, gender, tumour pathology and staging were reported for all participants. No significant differences were found between comparison groups at baseline.
Effects of interventions
Primary outcomes
Amifostine versus placebo showed no significant differences in the incidence of xerostomia ((dry mouth and hypogeusia): 130 patients, two trials, Analysis 1.1.
1.1. Analysis.
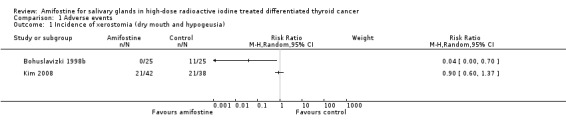
Comparison 1 Adverse events, Outcome 1 Incidence of xerostomia (dry mouth and hypogeusia).
No study analysed health‐related quality of life.
Secondary outcomes
No study investigated death from any cause, morbidity or economic outcomes.
There were no significant differences in salivary functions, measured by 99mTc‐pertechnetate scintigraphy (80 patients, one trial, Analysis 2.1, Analysis 2.2).
2.1. Analysis.
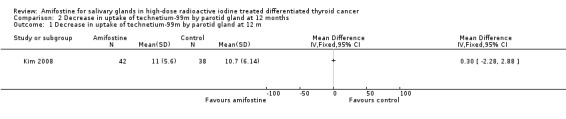
Comparison 2 Decrease in uptake of technetium‐99m by parotid gland at 12 months, Outcome 1 Decrease in uptake of technetium‐99m by parotid gland at 12 m.
2.2. Analysis.
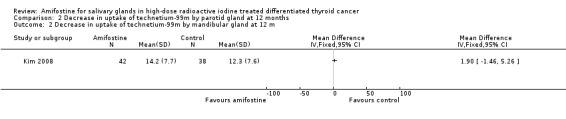
Comparison 2 Decrease in uptake of technetium‐99m by parotid gland at 12 months, Outcome 2 Decrease in uptake of technetium‐99m by mandibular gland at 12 m.
Meta‐analysis was not performed on the decreased uptake of 99mTc‐pertechnetate in scintigraphy by parotid and submandibular glands at three months due to the highly inconsistent findings across studies (I2 = 99%).
Adverse effects other than incidence of xerostomia
There were no significant differences in reduction of blood pressure (130 patients, two trials, Analysis 1.2). However, two patients in one trial collapsed during amifostine infusion due to a drop in systolic/diastolic blood pressure from 170/80 mm Hg to 130/60 mm Hg and 180/82 mm Hg to 145/75 mm Hg, respectively. This adverse effect was treated by withdrawing the infusion and volume substitution. Both patients recovered without sequelae (Bohuslavizki 1998b). Fur full details of adverse events, see Appendix 2.
1.2. Analysis.
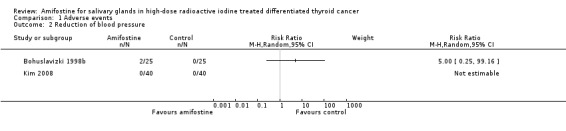
Comparison 1 Adverse events, Outcome 2 Reduction of blood pressure.
Compliance
Amifostine treatment was taken for a short term only. Compliance appeared satisfactorily in the two trials.
Subgroup analyses
Not possible for age and gender due to low number of studies.
In one trial, radioactive doses of 3 GBq versus 6 GBq (25 patients, one trial, Analysis 3.1, Analysis 3.2) showed no significant differences of salivary glands' function at three months in patients treated with amifostine (Bohuslavizki 1998b).
3.1. Analysis.
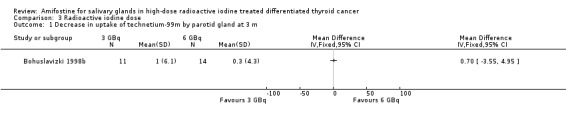
Comparison 3 Radioactive iodine dose, Outcome 1 Decrease in uptake of technetium‐99m by parotid gland at 3 m.
3.2. Analysis.
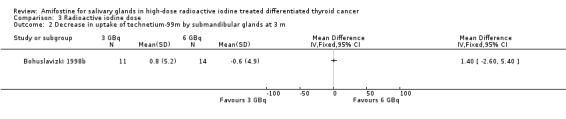
Comparison 3 Radioactive iodine dose, Outcome 2 Decrease in uptake of technetium‐99m by submandibular glands at 3 m.
Sensitivity analyses
Not possible due to low number of studies.
Discussion
Summary of main results
Two randomised controlled clinical trials involving 130 patients were included. All of them had a low risk of bias.
No significant benefits of amifostine therapy were found for the salivary glands in high‐dose radioactive iodine treated differentiated thyroid cancer patients in terms of incidence of xerostomia (dry mouth and hypogeusia). Amifostine versus placebo showed no significant differences in adverse effects like reduction of systolic blood pressure and salivary functions as measured by 99mTc‐pertechnetate scintigraphy.
The very wide confidence intervals for both xerostomia and adverse effects may be due to the different durations of follow up, three months (Bohuslavizki 1998b), and 12 months (Kim 2008), respectively. Furthermore, no inclusion or exclusion criteria were reported in the two included studies, patients with salivary gland dysfunction, connective tissue disease, or other conditions causing salivary gland dysfunction should be excluded in these trials. Different doses of amifostine were used with 500 mg/m2 in one trial (Bohuslavizki 1998b) and 300 mg/m2 amifostine in the other study (Kim 2008), which may also lead to different therapeutic effects. None of the included trials evaluated death from any cause, morbidity, health‐related quality of life or costs.
Overall completeness and applicability of evidence
The two included randomised controlled clinical trials, in which age, gender, tumour pathology and tumour staging were similar at baseline, had similar overall low risk of bias. There were no withdrawals nor losses to follow up in the two trials. The diagnosis of xerostomia was established according to the World Health Organization (WHO) criteria in one trial (Bohuslavizki 1998b), and a xerostomia questionnaire for symptoms assessment in another trial (Kim 2008). No inclusion or exclusion criteria were reported in the two included studies. Dry mouth is a common disorder in elderly individuals (Narhi 1994) and xerostomia related symptoms should be assessed before radioactive iodine treatment for patients with differentiated thyroid cancer. Patients with dry month or a systemic autoimmune disorder should be excluded in these trials. Therefore, amifostine cannot currently be recommended for patients with radioactive iodine treated differentiated thyroid cancer.
Radioactive iodine doses of equal or less than 3 or greater than 6 GBq (Bohuslavizki 1998b) and 3.75 to 7.5 GBq (Kim 2008) were used in the included trials. In a subgroup analysis on the effects of radioactive iodine doses on salivary glands function, Bohuslavizki et al reported no significant differences of 3 GBq versus 6 GBq doses (Bohuslavizki 1998b). The other included study did not show an association between higher radioactive iodine doses and the development of xerostomia (Kim 2008).
Quality of the evidence
Only two trials with low risk of bias but generally inadequately reported investigated 130 participants. There was no information about patient‐oriented endpoints like death from any cause, morbidity, health‐related quality of life or economic outcomes. Calculation of sample size was not reported in any trial. No inclusion or exclusion criteria were reported in both included studies. Different doses of amifostine were used with 500 mg/m2 in one trial (Bohuslavizki 1998b) and 300 mg/m2 amifostine in the other trial (Kim 2008), which may lead to different therapeutic effects. A follow up at three and 12 months in the two trials does not appear to be long enough to evaluate potential irradiation induced damage on salivary function. Different calculations of scintigraphically detected technetium‐99m (99mTc‐pertechnetate) uptake by salivary glands were used in the two trials (Bohuslavizki 1998b; Kim 2008). Because of the described heterogeneity, we did not calculate pooled effect sizes in meta‐analyses.
Moreover, outcome assessment could have been blinded but the trials did not report this blinding method. This could have resulted in exaggerated or minimized effects of the intervention.
Potential biases in the review process
None known.
Agreements and disagreements with other studies or reviews
Xerostomia characterized by hypogeusia and dry mouth is one of the adverse effects of radioactive iodine treatment for differentiated thyroid cancer. Xerostomia causes significant oropharyngeal disorders, pain and an impaired quality of life (Loesche 1995). It seems possible that the greater the dose of radioactive iodine, the higher the possibility of xerostomia (Bohuslavizki 1998b; Kim 2008). A higher radioactive iodine (greater than 6 GBq) dose showed significantly decreased technetium‐99m uptake in the parotid and submandibular glands in patients without amifostine treatment (Bohuslavizki 1998b). This was confirmed by another study (Kim 2008), so the dose of radioactive iodine had significant effects on the salivary gland function in patients with differentiated thyroid cancer. Similar to the results in this review, sixty per cent of distorted taste in patients treated with a dose of 4 GBq radioactive iodine has been reported. Twenty‐seven per cent hypogeusia were found in patents treated with a dose of 5.5 GBq radioactive iodine (Brown 1984). However, 3 GBq vs 6 GBq radioactive iodine (subgroup analysis) doses had no significant effects on salivary glands in 25 patients treated with amifostine, which indicates that amifostine may have protective effects on salivary glands in patients treated with higher dose radioactive iodine (Bohuslavizki 1998b).
It has not been proven whether or not amifostine influences the therapeutic efficacy of radioactive iodine in the treatment of differentiated thyroid cancer (Mandel 2003). Due to the limited results from the two included trials (Bohuslavizki 1998b; Kim 2008), the use of sour candy (Freitas 1985; Mazzaferri 2000) or lemon juice (Spiegel 1985) to increase salivation might be more appropriate during radioactive iodine treatment for patients with differentiated thyroid cancer. Patients should be well informed of the importance of hydration, acid stimulation and glandular massage after radioactive iodine treatment. In addition, early recognition and treatment of xerostomia serves to lessen patient morbidity (Mandel 2003).
Authors' conclusions
Implications for practice.
There is no good evidence from well‐designed trials for or against amifostine therapy for salivary glands in high‐dose radioactive iodine treated differentiated thyroid cancer. However, our findings suggest that amifostine has no significant radioprotective effects on salivary glands in this patient cohort.
Implications for research.
Randomised controlled clinical trials with low risk of bias are required to evaluate amifostine for salivary glands in high dose radioactive iodine treated differentiated thyroid cancer. The effects of amifostine should be evaluated and compared in the same dose for these patients. Patients with systemic autoimmune disorders should be excluded. Outcome measures should include health‐related quality of life, morbidity, death from any cause and costs, adverse events and the effects of radioactive iodine doses on salivary glands. The salivary function should be dynamically surveyed in the longer term and in the same way. The diagnosis of xerostomia should be based on the same diagnostic criteria.
Acknowledgements
None.
Appendices
Appendix 1. Search strategies
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. MEDLINE (Ovid interface): 1.exp Amifostine/ 2.(amifostin$ or ethyol$).ab,ti,ot. 3.exp Cytoprotection/ 4.((cyto or radio or chemo) adj6 protect$).ab,ti,ot. 5. or/1‐4 6.exp Thyroid Neoplasms/ 7.(thyroid$ and (cancer or tumo?r$ or neoplasm$)).ab,ti,ot. 8.exp Salivary glands/ 9. (salivary adj6 gland$).ab,ti,ot. 10.or/6‐9 11.randomized controlled trial.pt. 12.controlled clinical trial.pt. 13. randomized.ab. 14.placebo.ab. 15.drug therapy.fs. 16. randomly.ab. 17.trial.ab. 18.groups.ab. 19.or/11‐18 20.Meta‐analysis.pt. 21.exp Review/ 22.exp Technology Assessment, Biomedical/ 23.exp Meta‐analysis/ 24.exp Meta‐analysis as topic/ 25.hta.tw,ot. 26. (health technology adj6 assessment$).tw,ot. 27.(meta analy$ or metaanaly$ or meta?analy$).tw,ot. 28. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or chochrane or cinhal or psychinfo or psychlit or healthstar or biosis or current content$ or systemat$)).tw,ot. 29.or/20‐28 30.19 or 29 31.5 and 10 and 30 32.limit 31 to animals 33.limit 31 to humans 34.32 not 33 35.31 not 34 EMBASE: 1. exp Amifostine/ 2. (amifostin$ or ethyol$).ab,ti. 3. exp Cell protection/ 4.((cyto or radio or chemo) adj6 protect$).ab,ti. 5.or/1‐4 6.exp Thyroid Tumor/ 7.(thyroid$ and (cancer or tumo?r$ or neoplasm$ or carcinom$)).ab,ti. 8. exp Salivary Gland/ 9.(salivary adj6 gland$).ab,ti. 10.or/6‐9 11.random$.tw. 12.(crossover$ or cross over$).tw. 13.placebo$.tw. 14.(double adj blind$).tw. 15.(single adj blind$).tw. 16.(assign$ or allocat$ or volunteer$).tw. 17.Crossover Procedure/ 18.Double Blind Procedure/ 19.Randomized Controlled Trial/ 20.Controlled Clinical Trial/ 21.Single Blind Procedure/ 22.Randomization/ 23.or/11‐22 24.exp meta analysis/ 25.exp Review/ 26.(metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 27.((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinhal or psychinfo or psychlit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 28.exp Literature/ 29.exp Biomedical Technology Assessment/ 30.hta.tw,ot. 31.(health technology adj6 assessment$).tw,ot. 32.or/24‐31 33.23 or 32 34.5 and 10 and 33 35.limit 34 to animal 36.limit 34 to human 37.35 not 36 38.34 not 37 The Cochrane Library: #1 MeSH descriptor Amifostine explode all trees #2 (amifostin* or ethyol*) #3 MeSH descriptor Cytoprotection explode all trees #4 ((cyto or radio or chemo) near6 protect*) #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Thyroid Neoplasms explode all trees #7 MeSH descriptor Salivary Glands explode all trees #8 (thyroid* and (cancer or carcinom* or tumo?r* or neoplasm*)) #9 (salivary near6 gland*) #10 (#6 OR #7 OR #8 OR #9) #11 (#5 AND #10) |
Appendix 2. Adverse events
| Characteristic | Bohuslavizki 1998b | Kim 2008 |
| Intervention (I) Control (C) |
amifostine (I) placebo (C) |
amifostine (I) placebo (C) |
| Deceased participants [n/N] | I: 0 / 25 C: 0 / 25 Total: 0 / 50 |
I: 0 / 42 C: 0 / 38 Total: 0 / 80 |
| Adverse events [n / %] | I: 2 / 8 C: 0 / 0 Total: 2 / 4 |
I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
| Serious adverse events [n / %] | I: 2 / 8 C: 0 / 0 Total: 2 / 4 |
I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
| Drop‐outs due to adverse events [n / %] | I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
| Hospitalisation [n / %] | I: 2 / 8 C: 0 / 0 Total: 2 / 4 |
I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
| Out‐patient treatment [n / %] | I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
| Symptoms [n / %] | I: 2 / 8 C: 0 / 0 Total: 2 / 4 |
I: 0 / 0 C: 0 / 0 Total: 0 / 0 |
| Footnotes: | ||
Data and analyses
Comparison 1. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of xerostomia (dry mouth and hypogeusia) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Reduction of blood pressure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 2. Decrease in uptake of technetium‐99m by parotid gland at 12 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decrease in uptake of technetium‐99m by parotid gland at 12 m | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Decrease in uptake of technetium‐99m by mandibular gland at 12 m | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Radioactive iodine dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decrease in uptake of technetium‐99m by parotid gland at 3 m | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Decrease in uptake of technetium‐99m by submandibular glands at 3 m | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bohuslavizki 1998b.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL (RCT): yes RANDOMISATION RATIO: 1:1 NON‐INFERIORITY DESIGN: no EQUIVALENCE DESIGN: no PARALLEL / CROSSOVER / FACTORIAL RCT: PARALLEL RCT CONTROLLED CLINICAL TRIAL (CCT): no. |
|
| Participants | WHO PARTCIPATED:
Patients (M/F = 19/31) aged between 19 and 81 years, with differentiated thyroid cancer. 25 participants were on intervention, 25 participants were on control. INCLUSION CRITERIA: not reported. EXCLUSION CRITERIA: not reported. DIAGNOSTIC CRITERIA: Pathologically established differentiated thyroid cancer, World Health Organization (WHO) criteria for xerostomia. CO‐MORBIDITIES: not reported. CO‐MEDICATIONS: not reported. |
|
| Interventions | NUMBER OF STUDY CENTRES: Two centres. COUNTRY/ LOCATION: Germany. SETTING: In‐patients. INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Intervention: intravenous, 500 mg/m2 amifostine. Control: physiologic saline solution. TREATMENT BEFORE STUDY: 40 mg dexamethasone and 5 mg tropisetron as an antiemetic treatment. TITRATION PERIOD: not applicable. |
|
| Outcomes | PRIMARY OUTCOME(S):
The outcomes were evaluated by salivary function, on the basis of principal symptoms, signs and salivary gland imaging after three months an one year, respectively.
1. Dry mouth hypogeusia
2. Total effectiveness rate SECONDARY OUTCOMES: 1. Adverse effects 2. Uptake decrease of Tc‐99m‐pertechnetate in parotid and submandibular glands (three months). ADDITIONAL OUTCOMES: Subgroup analysis: radioactive doses 3 GBq vs 6 GBq uptake on salivary glands function. |
|
| Study details | DURATION OF INTERVENTION: Intervention: 25 participants received 500 mg/m2 amifostine intravenously. Control: 25 participants received physiologic saline solution before high‐dose radioactive iodine treatment. DURATION OF FOLLOW‐UP: 3,12 months. RUN‐IN PERIOD: not reported. | |
| Publication details | LANGUAGE OF PUBLICATION: English. COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: yes PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): no |
|
| Stated aim of study | "to examine prospectively the effects of amifostine in differentiated thyroid cancer patients following high‐dose radioiodine treatment" | |
| Notes | ./. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Patients were assigned randomly either to the placebo group or to the treatment group, which received 500 mg/m2 amifostine". |
| Allocation concealment? | Unclear risk | Not mentioned. |
| Blinding? All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data are available for all included patients. |
| Free of selective reporting? | Low risk | All included patients were reported. |
| Free of other bias? | Low risk | None detected. |
Kim 2008.
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL (RCT): yes RANDOMISATION RATIO: 1:1 NON‐INFERIORITY DESIGN: no EQUIVALENCE DESIGN: no PARALLEL / CROSSOVER / FACTORIAL RCT: PARALLEL RCT CONTROLLED CLINICAL TRIAL (CCT): no |
|
| Participants | WHO PARTCIPATED:
Patients(M/F = 9/71) aged between 21 and 58 years, with differentiated thyroid cancer. 42 participants were on intervention, 38 participants were on control. INCLUSION CRITERIA: not reported. EXCLUSION CRITERIA: not reported. DIAGNOSTIC CRITERIA: Pathologically established well differentiated thyroid cancer, xerostomia symptoms were self‐assessed by means of a detailed questionnaire covering the symptoms of dry mouth (dryness, dental deterioration, gingivitis, infections, parotitis, lack of taste, and difficulty swallowing dry foods) and dry eyes (dryness, photosensitivity, sticking, burning, heaviness, redness, and eye infection). CO‐MORBIDITIES: not reported. CO‐MEDICATIONS: not reported. |
|
| Interventions | NUMBER OF STUDY CENTRES:
Single centres. COUNTRY/ LOCATION: Republic of Korea. SETTING: In‐patients. INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Intervention: intravenous, 300 mg/m2 amifostine. Control: placebo. TREATMENT BEFORE STUDY: 8mg ondansetron one hour before radioactive iodine administration and every 8 hours after radioactive iodine application as an antiemetic treatment. TITRATION PERIOD: not applicable. |
|
| Outcomes | PRIMARY OUTCOME(S): The outcomes were evaluated by salivary function, on the basis of principal symptoms, signs and salivary gland imaging after 3,12 months, respectively. 1. Dry mouth and hypogeusia 2. Total effectiveness rate. SECONDARY OUTCOMES: 1. Adverse effects 2. Uptake decrease of Tc‐99m‐pertechnetate in parotid and submandibular glands (3 months) | |
| Study details | DURATION OF INTERVENTION: Intervention: 42 patients received 300 mg/m2 amifostine intravenously. Control: 38 patients received placebo. DURATION OF FOLLOW‐UP: 3,12 months. RUN‐IN PERIOD: not reported. | |
| Publication details | LANGUAGE OF PUBLICATION: English. COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: yes PUBLICATION STATUS (PEER REVIEW JOURNAL): yes PUBLICATION STATUS (JOURNAL SUPPLEMENT): no PUBLICATION STATUS (ABSTRACT): no |
|
| Stated aim of study | "to investigate with serial quantitative analysis of salivary gland scans the cytoprotective effect of amifostine on salivary glands in 131I‐treated DTC patients" | |
| Notes | ./. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "forty‐two patients were assigned randomly to the amifostine treatment group". |
| Allocation concealment? | Unclear risk | Not mentioned. |
| Blinding? All outcomes | Low risk | Quote: "double‐blind". |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome data were available for all included patients. |
| Free of selective reporting? | Low risk | All included patients were reported. |
| Free of other bias? | Low risk | None detected. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bohuslavizki 1998a | duplicate publication |
| Bohuslavizki 1999a | duplicate publication |
| Bohuslavizki 1999b | duplicate publication |
Differences between protocol and review
No.
Contributions of authors
MA CHAO: Drafting and co‐drafting of the protocol/review, data selection and analysis.
XIE JIAWEI: Drafting and co‐drafting of the protocol/review.
CHEN QINGFENG: Searching, selection of studies.
WANG GUOMING: Assistance with data selection.
ZUO SHUYAO: Searching, selection of studies.
Sources of support
Internal sources
West China Hospital Evidence Based Center, China.
External sources
West China Hospital Evidence Based Center, China.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Bohuslavizki 1998b {published data only}
- Bohuslavizki KH, Klutmann S, Brenner W, Mester J, Henze E, Clausen M. Salivary gland protection in high‐dose radioiodine treatment: Results of a double blind placebo controlled study. Journal of Clinical Oncology 1998;16:3542‐9. [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim SJ, Choi HY, Kim IJ, Kim YK, Jun S, Nam HY, et al. Limited cytoprotective effects of amifostine in high‐dose radioactive iodine 131‐treated well‐differentiated thyroid cancer patients: analysis of quantitative salivary scan. Thyroid 2008;18:325‐31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bohuslavizki 1998a {published data only}
- Bohuslavizki KH, Brenner W, Klutmann S, Hubner RH, Lassmann S, Feyerabend B, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine therapy. Journal of Nuclear Medicine 1998;39:1237‐42. [PubMed] [Google Scholar]
Bohuslavizki 1999a {published data only}
- Bohuslavizki KH, Klutmann S, Brenner W, Kroger S, Buchert R, Bleckmann C, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine treatment. Strahlentherapie und Onkologie 1999;175(Suppl 4):6‐12. [PubMed] [Google Scholar]
Bohuslavizki 1999b {published data only}
- Bohuslavizki KH, Klutmann S, Bleckmann C, Brenner W, Lassmann S, Mester J, et al. Salivary gland protection by amifostine in high‐dose radioiodine therapy of differentiated thyroid cancer. Strahlentherapie und Onkologie 1999;175:57‐61. [DOI] [PubMed] [Google Scholar]
Additional references
Abramson 1969
- Abramson AL, Levy LM, Goodman M, Attie JN. Salivary gland scinti‐scanning with technetium 99m pertechnetate. Laryngoscope 1969;79:1105‐17. [DOI] [PubMed] [Google Scholar]
Albrecht 1976
- Albrecht HH, Creutzig H. Salivary gland scintigraphy after radio‐iodine therapy. Functional scintigraphy of the salivary gland after high dose radio‐iodine therapy. Nuklearmedizin 1976;125:546‐53. [DOI] [PubMed] [Google Scholar]
Allweiss 1984
- Allweiss P, Braunstein GD, Katz A, Waxman A. Sialadenitis following I‐131 therapy for thyroid carcinoma: Concise communication. Journal of Nuclear Medicine 1984;25:755‐8. [PubMed] [Google Scholar]
Anne 2002
- Anne PR. Phase II trial of subcutaneous amifostine in patients undergoing radiation therapy for head and neck cancer. Seminars in Oncology 2002;29:80‐3. [DOI] [PubMed] [Google Scholar]
Baum 1993
- Baum BJ. Principles of saliva secretion. Annals of the New York Aacademy of Sciences 1993;694:17‐23. [DOI] [PubMed] [Google Scholar]
Becker 1995
- Becker DV, Hurley JR. Treatment of thyroid cancer with radioiodine. In: SandIer MP, PattonJA, Coleman RE, Gottschalk A, Wackers FiT, Hoffer PB editor(s). Diagnostic nuclear medicine. Baltimore: Williams & Wilkins, 1995:959‐89. [Google Scholar]
Boehme 2004
- Boehme S, Wilson DB. Amifostine‐induced fever: case report and review of the literature. Pharmacotherapy 2004;24:155‐8. [DOI] [PubMed] [Google Scholar]
Bohuslavizk 1995
- Bohuslavizki KH, Brenner W, Wolf H, Sippel C, Toenshoff G, Tinnemeyer S, et al. Value of quantitative salivary gland scintigraphy in the early stage of Sjgren's syndrome. Nuclear Medicine Communications 1995;16:917‐22. [DOI] [PubMed] [Google Scholar]
Bohuslavizki 1996
- Bohuslavizki KH, Brenner W, Lassmann S, Tinnemeyer S, Toenshoff G, Sippel C, Wolf H, Clausen M, Henze E. Quantitative salivary gland scintigraphy in the diagnosis of parenchymal damage after treatment with radioiodine. Nuclear Medicine Communications 1996;17:681‐6. [DOI] [PubMed] [Google Scholar]
Bohuslavizki 1997
- Bohuslavizki KH, Brenner W, Lassmann S, Tinnemeyer S, Kalina S, Clausen M, et al. Quantitative salivary gland scintigraphy ‐ a recommended examination prior to and after radioiodine therapy. Nuklearmedizin 1997;36:103‐9. [PubMed] [Google Scholar]
Bohuslavizki 1998a
- Bohuslavizki KH, Brenner W, Klutmann S, Hubner RH, Lassmann S, Feyerabend B, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine therapy. Journal of Nuclear Medicine 1998;39:1237‐42. [PubMed] [Google Scholar]
Bohuslavizki 1998b
- Bohuslavizki KH, Klutmann S, Brenner W, Mester J, Henze E, Clausen M. Salivary gland protection in high‐dose radioiodine treatment: Results of a double blind placebo‐controlled study. Journal of Clinical Oncology 1998;16:3542‐9. [DOI] [PubMed] [Google Scholar]
Bohuslavizki 1999a
- Bohuslavizki KH, Klutmann S, Brenner W, Kroger S, Buchert R, Bleckmann C, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine treatment. Strahlentherapie und Onkologie 1999;175(Suppl 4):6‐12. [PubMed] [Google Scholar]
Bohuslavizki 1999b
- Bohuslavizki KH, Klutmann S, Bleckmann C, Brenner W, Lassmann S, Mester J, et al. Salivary gland protection by amifostine in high‐dose radioiodine therapy of differentiated thyroid cancer. Strahlentherapie und Onkologie 1999;175:57‐61. [DOI] [PubMed] [Google Scholar]
Brown 1984
- Brown AP, Greening WP, McCready VR, Shaw HJ, Harmer CL. Radioiodine treatment of metastatic thyroid carcinoma: the Royal Marsden Hospital experience. British Journal of Radiology 1984;57:323‐7. [DOI] [PubMed] [Google Scholar]
Cappizi 1999
- Cappizi RL. The preclinical basis for broad‐spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine. Seminars in Oncology 1999;26:3‐21. [PubMed] [Google Scholar]
Chung 2002
- Chung JK. Sodium iodide symporter: its role in nuclear medicine. Journal of Nuclear Medicine 2002;43:1188‐1200. [PubMed] [Google Scholar]
Clarke 1994
- Clarke SEM. Radiojodine therapy of the thyroid. In: MurrayI PC, ElI PJ editor(s). Nuclear Medicine in Clinical Diagnosis and Therapy. Edinburgh: Churchill Livingstone, 1994:833‐45. [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Dai 1996
- Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature 1996;379:458‐60. [DOI] [PubMed] [Google Scholar]
Deeg 1988
- Deeg M, Maier H, Bihl H, Adler D. Clinical picture and possible causes of functional disorders of the parotid gland in radioiodine therapy of differentiated thyroid cancer. Laryngologie, Rhinologie, Otologie 1988;67:362‐6. [PubMed] [Google Scholar]
Dugonji 2008
- Dugonji S, Ajdinovi B, Stefanovi D, Jaukovi L. Diagnostic validity of dynamic salivary gland scintigraphy with ascorbic acid stimulation in patients with Sjoegren's syndrome: comparation with unstimulated whole sialometry. Vojnosanitetski pregled 2008;65:41‐6. [DOI] [PubMed] [Google Scholar]
Edwards 2002
- Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, FeigalEG. Annual report to the nation on the status of cancer, 1973‐1999, featuring implications of age and aging on the U.S. cancer burden. Cancer 2002;94:2766‐92. [DOI] [PubMed] [Google Scholar]
Fraker 1997
- Fraker DL, Skarulis M, Livolsi V. Thyroid tumors, Cancer. Principles and practice of oncology. Lippincott, 1997. [Google Scholar]
Freitas 1985
- Freitas JE, Gross MD, Ripley S, Shapiro B. Radionuclide diagnosis and therapy of thyroid cancer: current status report. Seminars in nuclear medicine 1985;5:106‐131. [DOI] [PubMed] [Google Scholar]
Gates 1967
- Gates GA, Work WP. Radioisotope scanning of the salivary glands. Laryngoscope 1967;77:861‐75. [DOI] [PubMed] [Google Scholar]
Haugen 2006
- Haugen BR. Patients with differentiated thyroid carcinoma benefit from radioiodine remnant ablation. Journal of Clinical Endocrinology and Metabolism 2004;89:3665‐7. [DOI] [PubMed] [Google Scholar]
Helman 1987
- Helman J, Turner RJ, Fox PC, Baum BJ. 99mTc‐pertechnetate uptake in parotid acinar cells by the Na+/K+/Cl‐ co‐transport system. The Journal of Clinical Investigation 1987;79:1310‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration, available from www.cochrane‐handbook.org. 2008.
Koukourakis 2000
- Koukourakis MI, Kyrias G, Kakolyris S, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. Journal of Clinical Oncology 2000;18:2226‐33. [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levenson 1994
- Levenson D, Coulec S, Sonnenberg M, Lai E, Goldsmith SJ, Larson SM. Peripheral facial nerve palsy after high‐dose radioiodine therapy in patients with papillary thyroid carcinoma. Annals of Internal Medicine 1994;120:576‐8. [DOI] [PubMed] [Google Scholar]
Levy 1998
- Levy O, Vieja A, Carrasco N. The Na+I‐ symporter (NIS): recent advances. Journal of Bioenergetics and Biomembranes 1998;30:195‐206. [DOI] [PubMed] [Google Scholar]
Loesche 1995
- Loesche WJ, Abrams J, Terpenning MS, Bretz WA, Dominguez BL, Grossman NS, Hildebrandt GH, Langmore SE, Lopatin DE. Dental findings in geriatric populations with diverse medical background. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 1995;80:43‐54. [DOI] [PubMed] [Google Scholar]
Maier 1987
- Maier H, Bihl H. Effect of radioactive iodine therapy on parotid gland function. Acta Oto‐laryngologica 1987;103:318‐24. [PubMed] [Google Scholar]
Mandel 2003
- Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid 2003;13:265‐71. [DOI] [PubMed] [Google Scholar]
Mazzaferri 2000
- Mazzaferri 2000. Carcinoma of the follicular epithelium. The Thyroid. Lippincott, Philadelphi, 2000. [Google Scholar]
McDonald 1994
- McDonald S, Meyerowitz C, Smudzin T, et al. Preliminary results of a pilot study using WR‐272t before fractionated irradiation of the head and neck to reduce salivary gland dysfunction. International Journal of Radiation Oncology, Biology, Physics 1994;29:747‐54. [DOI] [PubMed] [Google Scholar]
Menard 1984
- Menard TW, Izutsu KT, Ensign WY, et al. Radioprotection by WR‐2721 of gamma‐irradiated rat parotid gland:effect on gland weight and secretion at 8 ‐10 days post irradiation. International Journal of Radiation Oncology, Biology, Physics 1984;10:1555‐9. [DOI] [PubMed] [Google Scholar]
Mishkin 1981
- Mishkin FS. Radionuclide salivary gland imaging. Seminars in Nuclear Medicine 1981;11:258‐65. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐1900. [DOI] [PubMed] [Google Scholar]
Mossman 1994
- Mossman KL. Frequent short‐term oral complications of head and neck radiotherapy. Ear, Nose, and Throat Journal 1994;73:316‐20. [PubMed] [Google Scholar]
Myant 1960
- Myant NB. Iodine metabolism of salivary glands. Annals of the New York Academy of Sciences 1960;85:208‐14. [DOI] [PubMed] [Google Scholar]
Narhi 1994
- Narhi TO. Prevalence of subjective feelings of dry mouth in the elderly. Journal of Dental Research 1994;73:20‐5. [DOI] [PubMed] [Google Scholar]
Niibe 1985
- Niibe H, Takahashi I, Mitsuhashi N, et al. An evaluation of the clinical usefulness of amifostine (YM‐08310) radioprotective agent. A double‐blind placebo‐controlled study. 1. Head and neck tumor. Nippon Gan Chiryo Gakkai Shi 1985;20:984‐93. [PubMed] [Google Scholar]
Patt 1949
- Patt HM, Tyree EB, Straube RL, et al. Cystein protection against X irradiation. Science 1994;110:213‐4. [DOI] [PubMed] [Google Scholar]
Phillips 1973
- Phillips TL, Kane LJ, Utley JF. Radioprotection of tumor and normal tissues by thiophosphonate compounds. Cancer 1973;32:528‐35. [DOI] [PubMed] [Google Scholar]
Pratt 1980
- Pratt NE, Sodicoff M, Liss J, et al. Radioprotection of the rat parotid gland by WR‐2721 S‐2‐3 aminopropylaminoethyl phosphorothioate morphology at 60 days post irradiation. International Journal of Radiation Oncology, Biology, Physics 1980;6:431‐5. [DOI] [PubMed] [Google Scholar]
Rades 2004
- Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiotherapy and Oncology 2004;70:261‐4. [DOI] [PubMed] [Google Scholar]
Rasey 1985
- Rasey JS, Grunbaum Z, Krohn KA, Menard TW, Spence AM. Biodistribution of the radioprotective drug S‐labeled 3‐amino‐2‐hydroxypropyl phosphorothioate (WR‐77913). Radiation Research 1885;102:130‐7. [PubMed] [Google Scholar]
Rasey 1986a
- Rasey JS, Krohn KA, Grunbaum Z, et al. Synthesis, biodistribution, and autoradiography of radiolabeled S‐2‐(3‐methylaminopropylamino)‐ethylphosphorothioie acid (WR‐3689). Radiation Research 1986;106:366‐79. [PubMed] [Google Scholar]
Rasey 1986b
- Rasey JS, Grunbaum Z, Krohn KA, et al. Biodistribution of the radioprotectivedrug S‐labeled 3‐amino‐2‐hydroxypropyl phosphorothioate (WR‐77913). Radiation Research 1986;102:130‐7. [PubMed] [Google Scholar]
Rasey 1988
- Rasey JS, Spence AM, Badger CC, et al. Specific protection of different normal tissues. Pharmacology & Therapeutics 1988;39:3343. [DOI] [PubMed] [Google Scholar]
Rigler 1955
- Rigle RG, Scanlon PW. Radiation parotitis from radioactive iodine therapy. Proceedings of the staff meetings. Mayo Clinic 1955;30:149‐53. [PubMed] [Google Scholar]
Sawka 2004
- Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. A systematic review and meta‐analysis of the effectiveness of radioactive iodine remnant ablation for well‐differentiated thyroid cancer. Journal of Clinical Endocrinology and Metabolism 2004;89:3668‐76. [DOI] [PubMed] [Google Scholar]
Schlumberger 1998
- Schlumberger M, Baudin E, Travagli JP. Papillary and follicular cancers of the thyroid. Presse Medicale 1998;27:1479‐81. [PubMed] [Google Scholar]
Shaw 1988
- Shaw LM, Glover DJ, Turrisi A, et al. Pharmacokinetics of WR‐2721. Pharmacology & Therapeutics 1988;39:195‐201. [DOI] [PubMed] [Google Scholar]
Smanik 1996
- Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, et al. Cloning of the human sodium iodide symporter. Biochemical and Biophysical Research Communications 1996;226:339‐45. [DOI] [PubMed] [Google Scholar]
Sodicoff 1978a
- Sodicoff M, Conger AD, Trepper P, et al. Short‐term radioprotective effectsof WR‐2721 on the rat parotid glands. Radiation Research 1978;76:317‐26. [PubMed] [Google Scholar]
Sodicoff 1978b
- Sodicoff M, Conger AD, Pratt NE, et al. Radioprotection by WR‐2721 against long‐term chronic damage to the rat parotid gland. Radiation Research 1978;76:172‐9. [PubMed] [Google Scholar]
Spiegel 1985
- Spiegel W, Reiners C, Borner W. Sialadenitis following iodine‐131 therapy for thyroid carcinoma. Journal of nuclear medicine 1985;26:816‐817. [PubMed] [Google Scholar]
Spiegel 1986
- Spitzweg C, Joba W, Schriever K, Goellner JR, Morris JC, Heufelder AE. Analysis of human sodium iodide symporter immunoreactivity in human exocrine glands. Journal of Clinical Endocrinology and Metabolism 1999;84:4178‐84. [DOI] [PubMed] [Google Scholar]
Spitzweg 1998
- Spitzweg C, Heufelder AE. The sodium iodide symporter: its emerging relevance to clinical endocrinology. European Journal of Endocrinology 1998;138:374‐5. [DOI] [PubMed] [Google Scholar]
Spitzweg 1999
- Spitzweg C, Joba W, Schriever K, Goellner JR, Morris JC, Heufelder AE. Analysis of human sodium iodide symporter immunoreactivity in human exocrine glands. Journal of Clinical Endocrinology and Metabolism 1999;84:4178‐84. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Takahashi 1986
- Takahashi I, Nagai T, Miyaishi K, et al. Clinical study of the radioprotective effects of amifostine (YM‐083 10. WR‐272I ) on chronic radiation injury. International Journal of Radiation Oncology, Biology, Physics 1986;12:935‐8. [DOI] [PubMed] [Google Scholar]
Trotti 2000
- Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. International Journal of Radiation Oncology, Biology, Physics 2000;47:13‐47. [DOI] [PubMed] [Google Scholar]
Utley 1976a
- Utley JF, Marlowe C, Waddell WJ. Distribution of 3SS‐labeled WR‐2721 in normal and malignant tissues of the mouse. Radiation Research 1976;68:284‐91. [PubMed] [Google Scholar]
Utley 1976b
- Utley JF, Phillips TL, Kane LJ. Protection of normal tissues by WR‐2721 during fractionated irradiation. AACN Clinical Issues 1976;1:699‐703. [DOI] [PubMed] [Google Scholar]
Utley 1981
- Utley JF, Quinn CA, White FC, Seaver NA, Bloor CM. Protection of normal tissue against late radiation injury by WR‐2721. Radiation Research 1981;85:408‐15. [PubMed] [Google Scholar]
Vinagre 2008
- Vinagre F, Santos A, Santos M, Prata A, Oliveira A, Silva JC. Salivary gland scintigraphy in the evaluation of patients with sicca complaints. Acta Reumatológica Portuguesa 2008;33:422‐8. [PubMed] [Google Scholar]
Wartofsky 2002
- Wartofsky L. rhTSH‐Stimulated Thyroglobulin Study Group Techniques in thyroidology: management of low‐risk well‐differentiated thyroid cancer based only on thyroglobulin measurement after recombinant human thyrotropin. Thyroid 2002;12:583‐9. [DOI] [PubMed] [Google Scholar]
Washburn 1974
- Washburn LC, Carlton JE, Hayes RL, et al. Distribution of WR‐2721 in normal and malignant tissues of mice and rats bearing solid tumors: dependence on tumor type, drug dose aud species. Radiation Research 1974;59:475‐83. [PubMed] [Google Scholar]
Washburn 1978
- Washburn LC, Sun TT, Anon JB, Hayes RL. Effect of structure on tumor specificity of alicyclic alpha‐amino acids. Cancer Research 1978;38:2271‐3. [PubMed] [Google Scholar]
Yuhas 1980
- Yuhas JM, Spellman JM, Culo F. The role of WR‐2721 in radiotherapy and/or chemotherapy. Cancer Clinical Trials 1980;3:211‐216. [PubMed] [Google Scholar]
Zidan 2004
- Zidan J, Hefer E, Iosilevski G, Drumea K, Stein ME, Kuten A, et al. Efficacy of I131 ablation therapy using different doses as determined by postoperative thyroid scan uptake in patients with differentiated thyroid cancer. International Journal of Radiation Oncology, Biology, Physics 2004;59:1330‐6. [DOI] [PubMed] [Google Scholar]


