Abstract
Background
Electromechanical and robot‐assisted arm training devices are used in rehabilitation, and may help to improve arm function after stroke.
Objectives
To assess the effectiveness of electromechanical and robot‐assisted arm training for improving activities of daily living, arm function, and arm muscle strength in people after stroke. We also assessed the acceptability and safety of the therapy.
Search methods
We searched the Cochrane Stroke Group's Trials Register (last searched February 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2015, Issue 3), MEDLINE (1950 to March 2015), EMBASE (1980 to March 2015), CINAHL (1982 to March 2015), AMED (1985 to March 2015), SPORTDiscus (1949 to March 2015), PEDro (searched April 2015), Compendex (1972 to March 2015), and Inspec (1969 to March 2015). We also handsearched relevant conference proceedings, searched trials and research registers, checked reference lists, and contacted trialists, experts, and researchers in our field, as well as manufacturers of commercial devices.
Selection criteria
Randomised controlled trials comparing electromechanical and robot‐assisted arm training for recovery of arm function with other rehabilitation or placebo interventions, or no treatment, for people after stroke.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trial quality and risk of bias, and extracted data. We contacted trialists for additional information. We analysed the results as standardised mean differences (SMDs) for continuous variables and risk differences (RDs) for dichotomous variables.
Main results
We included 34 trials (involving 1160 participants) in this update of our review. Electromechanical and robot‐assisted arm training improved activities of daily living scores (SMD 0.37, 95% confidence interval (CI) 0.11 to 0.64, P = 0.005, I² = 62%), arm function (SMD 0.35, 95% CI 0.18 to 0.51, P < 0.0001, I² = 36%), and arm muscle strength (SMD 0.36, 95% CI 0.01 to 0.70, P = 0.04, I² = 72%), but the quality of the evidence was low to very low. Electromechanical and robot‐assisted arm training did not increase the risk of participant drop‐out (RD 0.00, 95% CI ‐0.02 to 0.03, P = 0.84, I² = 0%) with moderate‐quality evidence, and adverse events were rare.
Authors' conclusions
People who receive electromechanical and robot‐assisted arm and hand training after stroke might improve their activities of daily living, arm and hand function, and arm and hand muscle strength. However, the results must be interpreted with caution because the quality of the evidence was low to very low, and there were variations between the trials in the intensity, duration, and amount of training; type of treatment; and participant characteristics.
Keywords: Adult; Aged; Aged, 80 and over; Humans; Middle Aged; Young Adult; Activities of Daily Living; Artificial Limbs; Recovery of Function; Robotics; Stroke Rehabilitation; Upper Extremity; Exercise Therapy; Exercise Therapy/instrumentation; Exercise Therapy/methods; Muscle Strength; Muscle Strength/physiology; Randomized Controlled Trials as Topic; Stroke; Stroke/physiopathology
Electromechanical‐assisted training for improving arm function and disability after stroke
Review question
To assess the effects of electromechanical and robot‐assisted arm and hand training for improving arm function in people who have had a stroke.
Background
More than two‐thirds of people who have had a stroke have difficulties with reduced arm function, which can restrict a person's ability to perform everyday activities, reduce productivity, limit social activities, and lead to economic burden. Electromechanical and robot‐assisted arm training uses specialised machines to assist rehabilitation in supporting shoulder, elbow, or hand movements. However, the role of electromechanical and robot‐assisted arm training for improving arm function after stroke is unclear.
Study characteristics
We identified 34 trials (involving 1160 participants) up to March 2015 and included them in our review. Nineteen different electromechanical devices were described in the trials, which compared electromechanical and robot‐assisted arm training with a variety of other interventions. Participants were between 21 to 80 years of age, the duration of the trials ranged from two to 12 weeks, the size of the trials was between eight and 127 participants, and the primary outcome differed between the included trials. Most of the trials were done in rehabilitation facilities in the USA.
Key results
Electromechanical and robot‐assisted arm and hand training improved activities of daily living in people after stroke and function and muscle strength of the affected arm. As adverse events such as injuries and pain were seldom described, these devices can be applied as a rehabilitation tool, but we still do not know when or how often they should be used.
Quality of the evidence
The quality of the evidence was low to very low.
Summary of findings
Summary of findings for the main comparison.
Electromechanical and robotic‐assisted training versus all other interventions for improving activities of daily living, arm function, and arm muscle strength after stroke
| Electromechanical and robotic‐assisted training versus all other interventions for improving activities of daily living, arm function, and arm muscle strength after stroke | |||||
| Patient or population: people after stroke Settings: rehabilitation facilities Intervention: electromechanical and robotic‐assisted training versus all other interventions | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control | Electromechanical and robotic‐assisted training versus all other interventions | ||||
| Activities of daily living at the end of intervention phase ‐ all studies Measures of activities. Scale from: 0 to inf | The mean activities of daily living at the end of intervention phase ‐ all studies in the control groups was NA | The mean activities of daily living at the end of intervention phase ‐ all studies in the intervention groups was 0.37 SDs higher (0.11 to 0.64 higher) | 717 (18 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD 0.37 (0.11 to 0.64) |
| Activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase ‐ particpants treated in the acute and subacute phase of their stroke (within 3 months) Measures of activities. Scale from: 0 to inf | The mean activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase ‐ participants treated in the acute and subacute phase of their stroke (within 3 months) in the control groups was NA | The mean activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase ‐ participants treated in the acute and subacute phase of their stroke (within 3 month) in the intervention groups was 0.53 SDs higher (0.09 to 0.96 higher) | 320 (8 studies) | ⊕⊕⊝⊝ low2,3 | SMD 0.53 (0.09 to 0.96) |
| Activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase ‐ participants treated in the chronic phase (more than 3 months) Measures of activities. Scale from: 0 to inf | The mean activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase ‐ participants treated in the chronic phase (more than 3 months) in the control groups was NA | The mean activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase ‐ participants treated in the chronic phase (more than 3 months) in the intervention groups was 0.66 SDs higher (‐0.17 lower to 1.49 higher) | 397 (10 studies) | ⊕⊕⊝⊝ low1,2,3 | SMD 0.66 (‐0.08 to 1.41) |
| Arm function at the end of intervention phase Measures of arm function. Scale from: 0 to inf | The mean arm function at the end of intervention phase in the control groups was NA | The mean arm function at the end of intervention phase in the intervention groups was 0.35 SDs higher (0.18 to 0.51 higher) | 1078 (31 studies) | ⊕⊕⊝⊝ low1,3 | SMD 0.35 (0.18 to 0.51) |
| Arm muscle strength at the end of intervention phase Measures of arm muscle strength. Scale from: 0 to inf | The mean arm muscle strength at the end of intervention phase in the control groups was NA | The mean arm muscle strength at the end of intervention phase in the intervention groups was 0.36 SDs higher (0.01 to 0.7 higher) | 568 (16 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD 0.36 (0.01 to 0.7) |
| Acceptability: dropouts during intervention period Rate of dropouts and adverse events | Study population | 1160 (34 studies) | ⊕⊕⊕⊝ moderate1 | Risks were calculated from pooled risk differences | |
| 42 per 1000 | 45 per 1000 (22 to 72) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NA: Not applicable; RR: Risk ratio; SD: Standard deviation; SMD: Standardised mean difference | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded due to several ratings with 'high risk of bias'. 2 Downgraded due to considerable differences in effect sizes and unexplained heterogeneity. 3 Upper or lower confidence limit crosses an effect size of 0.5 in either direction.
Background
A stroke is a sudden, non‐convulsive loss of neurological function due to an ischaemic or haemorrhagic intracranial vascular event (WHO 2006). In general, strokes are classified by anatomic location in the brain, vascular distribution, aetiology, age of the affected individual, and haemorrhagic versus non‐haemorrhagic nature (Adams 1993). The prevalence of stroke depends on age and gender, and is estimated to be 1% of the population (Feigin 2009; Vos 2015). Stroke, taken together with ischaemic heart disease, is one of the largest sources of disease burden; in low‐ and middle‐income countries of Europe and Central Asia, these conditions account for more than a quarter of the total disease burden (Vos 2015).
Stroke is a major cause of chronic impaired arm function and may affect many activities of daily living. At hospital admission after stroke, more than two‐thirds of people have arm paresis, resulting in reduced upper extremity function (Jørgensen 1999; Nakayama 1994), and six months after stroke the affected arm of approximately half of all people remains without function (Kwakkel 2003). Therefore, to reduce this burden, many people receive multidisciplinary rehabilitation soon after stroke. However, despite intensive rehabilitation efforts, only approximately 5% to 20% of people reach complete functional recovery (Nakayama 1994); in other words, four out of five people leave rehabilitation with restricted arm function. Thus, there still exists an urgent need for new inpatient and outpatient rehabilitation and training strategies that match the specific needs of stroke survivors and their relatives (Barker 2005).
In recent years, new electromechanical‐assisted training strategies to improve arm function and activities of daily living have been developed for people after stroke. Examples of electromechanical and robot‐assisted arm training devices found in this review are:
Mirror Image Motion Enabler, MIME (Burgar 2000);
InMotion robot (Massachusetts Institute of Technology, MIT‐Manus) (Krebs 1998);
Assisted Rehabilitation and Measurement (ARM) Guide (Reinkensmeyer 2000b);
Robotic Rehabilitation System for upper limb motion therapy for the disabled, REHAROB (Fazekas 2007);
Neuro‐Rehabilitation‐Robot, NeReBot (Fazekas 2007);
Bi‐Manu‐Track (Hesse 2003);
Robot‐mediated therapy system, GENTLE/s (Coote 2003);
Arm robot, ARMin (Riener 2005); and
Amadeo (Hwang 2012).
Most of these devices provide passive movement of the person's arm. Other devices assist arm movements or provide resistance during training. Some devices may assist active movements of an isolated joint, like in continuous passive motion (Hesse 2003), while other devices are able to move multiple segments to perform reaching‐like movements (Burgar 2000). The progression of therapy with electromechanical devices is possible by, for example, varying the force, decreasing assistance, increasing resistance, and expanding the movement amplitude. Moreover, some devices, such as the Bi‐Manu‐Track and the MIME, may be used to provide bimanual exercise: the device simultaneously moves (mirrors) the affected limb passively, steered by the non‐paretic limb. Broadly considered, most robotic systems incorporate more than one modality into a single device.
Early studies and previous reviews suggested that an advantage of electromechanical and robotic devices, when compared with conventional therapies, may be an increase in repetitions during arm training due to an increase of motivation to train and also the opportunity for independent exercise (Kwakkel 2008; Prange 2006). Therefore, electromechanical‐assistive training devices allow a therapy paradigm that is intensive, frequent and repetitive, and accords to principles of motor learning.
However, contrary to the remarkable number of publications about electromechanical technologies, scientific evidence for the benefits of these technologies, which could justify costs and effort, is still lacking. We summarised the evidence in our first Cochrane review about this topic in 2008 and in our last update in 2012 (Mehrholz 2008; Mehrholz 2012), but many new studies have emerged in recent years. Therefore there is a need for an updated and systematic evaluation of the available literature to assess the effectiveness and acceptability of these electromechanical‐assisted training devices.
Objectives
To assess the effectiveness of electromechanical and robot‐assisted arm training for improving activities of daily living, arm function, and arm muscle strength in people after stroke. We also assessed the acceptability and safety of the therapy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and randomised controlled cross‐over trials (we only analysed the first study period as a parallel‐group trial).
Types of participants
We included studies with participants of either gender over 18 years of age after stroke (using the World Health Organization (WHO) definition of stroke, or a clinical definition of stroke when the WHO definition was not specifically stated) (WHO 2006), regardless of the duration of illness or level of initial impairment. If we found RCTs with mixed populations (such as traumatic brain injury and stroke), we included only those RCTs with more than 50% of participants with stroke in our analysis.
Although we initially included all studies regardless of the duration of illness in our analysis, we later compared participants in the acute and subacute phase of their stroke (within three months) with participants in the chronic phase (more than three months) in a subgroup analysis.
Types of interventions
We compared electromechanical and robot‐assisted arm training for recovery of arm function (such as robot‐aided technologies or any other newly developed electromechanical device) with any other intervention for:
improving activities of daily living (main analysis); and
improving impairments (secondary analysis).
An example of an eligible robot‐assisted intervention is the Mirror Image Motion Enabler, MIME (Burgar 2000). An example of an electromechanical‐assisted intervention is the Bi‐Manu‐Track (Hesse 2003). Other interventions could include other devices, other rehabilitation or placebo interventions, or no treatment.
Types of outcome measures
Primary outcomes
The primary outcome was activities of daily living. We preferred the Barthel Index, Wade 1987, and the Functional Independence Measure, Hamilton 1994, (scales were regarded as continuous scaled, higher scores indicate a good outcome) as primary outcome measures, if they were available. However, we accepted other scales that measured activities of daily living.
Secondary outcomes
The secondary outcomes were impairments, such as motor function and muscle strength. We measured arm and hand motor function with the Fugl‐Meyer score (regarded as continuous scaled, higher scores indicate a good outcome; Platz 2005) and measured arm and hand muscle strength with the Motricity Index Score (scales were regarded as continuous scaled, higher scores indicate a good outcome; Collin 1990; Demeurisse 1980). However, if these scales were not available we accepted other scales that measured arm and hand function and arm and hand muscle strength (in the following we will use the term 'arm function' instead of 'arm and hand function' and also 'arm muscle strength' instead of 'arm and hand muscle strength').
To measure the acceptance of electromechanical and robot‐assisted arm training we used withdrawal or dropouts from the study due to any reason (including deaths) during the study period. We investigated the safety of electromechanical and robot‐assisted arm training with the incidence of adverse outcomes, such as cardiovascular events, injuries and pain, and any other reported adverse events.
Depending on the aforementioned categories and the availability of variables used in the included trials, all review authors discussed and reached consensus on which outcome measures should be included in the analysis.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We did not restrict our searches by language, publication status, or date, and we arranged for the translation of articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched in February 2015) and the following bibliographic databases:
The Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2015, Issue 3) (Appendix 1);
MEDLINE (Ovid) (1950 to March 2015) (Appendix 2);
EMBASE (Ovid) (1980 to March 2015) (Appendix 3);
CINAHL (Ebsco) (1982 to March 2015) (Appendix 4);
AMED (Allied and Complementary Medicine) (Ovid) (1985 to March 2015) (Appendix 5);
SPORTDiscus (Ebsco) (1949 to March 2015) (Appendix 6);
Physiotherapy Evidence Database (PEDro, http://www.pedro.org.au/) (searched April 2015);
Compendex (1972 to March 2015) and Inspec (1969 to March 2015) (Engineering Village) (Appendix 7).
We developed the search strategy for MEDLINE with the help of the Cochrane Stroke Group Trials Search Co‐ordinator and modified it for the other databases.
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials not available in the major databases, we:
-
handsearched the following relevant conference proceedings:
World Congress for NeuroRehabilitation (WCNR, 1998, 2002, 2006, 2010, and 2014);
International Society of Physical and Rehabilitation Medicine World Congress (ISPRM 2001, 2003, 2005, 2007, 2009, and 2011);
World Confederation for Physical Therapy (2003, 2007, 2011, and 2015);
International Congress on Neurorehabilitation and Neural Repair (2015);
Deutsche Gesellschaft für Neurotraumatologie und Klinische Neurorehabilitation (2001 to 2015);
Deutsche Gesellschaft für Neurologie (2000 to 2014);
Deutsche Gesellschaft für Neurorehabilitation (1999 to 2014);
screened reference lists of all relevant articles;
-
identified and searched the following ongoing trials and research registers:
ISRCTN Registry (http://www.isrctn.com/) (searched June 2015);
ClinicalTrials.gov (http://clinicaltrials.gov/) (searched June 2015);
Stroke Trials Registry (www.strokecenter.org/trials) (searched June 2015);
contacted trialists, experts, and researchers in our field of study; and
-
contacted the following manufacturers of commercial devices:
Hocoma (last contact March 2015); and
Reha‐Stim (last contact May 2015).
Data collection and analysis
Selection of studies
Two review authors (JM and BE) independently read the titles and abstracts (if available) of identified publications and eliminated obviously irrelevant studies. We obtained the full text for the remaining studies, and the same two review authors independently examined potentially relevant studies using our predetermined criteria for including studies. Based on types of studies, participants, aims of interventions, and outcome measures, the review authors independently ranked these studies as relevant, irrelevant, or possibly relevant. We excluded all trials ranked initially as irrelevant, but included all other trials at that stage for further assessment. We excluded all trials of specific treatment components (such as electrical stimulation) as standalone treatment, and continuous passive motion treatment and continuous passive stretching. All review authors resolved disagreements through discussion. If further information was needed to reach consensus, we contacted the study authors.
Data extraction and management
Two review authors (JM and MP) independently extracted trial and outcome data from the selected trials. We used checklists to independently record details of the studies. If any review author was involved in any of the selected studies, another member of our review team not involved in the study was asked to handle the study information.
We established the characteristics of unpublished trials through correspondence with the trial co‐ordinator or principal investigator. We used checklists to independently record details of the:
methods of generating randomisation schedule;
method of concealment of allocation;
blinding of assessors;
use of an intention‐to‐treat analysis (all participants initially randomised were included in the analyses as allocated to groups);
adverse events and dropouts for all reasons;
important imbalance in prognostic factors;
participants (country, number of participants, age, gender, type of stroke, time from stroke onset to entry to the study, inclusion and exclusion criteria);
comparison (details of the intervention in treatment and control groups, details of co‐intervention(s) in both groups, duration of treatment); and
outcomes and time points of measures (number of participants in each group and outcome, regardless of compliance).
We checked all of the extracted data for agreement between review authors, with another review author (JK or BE) arbitrating any disagreements. We contacted study authors to request more information, clarification, or missing data if necessary.
Assessment of risk of bias in included studies
All review authors independently assessed the methodological quality of the included trials using the Cochrane 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We checked all methodological quality assessments for agreement between review authors, resolving any disagreements by discussion. Two review authors (MP and JM) were coauthors of one included trial (Hesse 2005); two other review authors (BE and JK) conducted the quality assessment for this trial.
Measures of treatment effect
The primary outcome variables of interest were treated as continuous data and entered as mean and standard deviations (SDs). We calculated a pooled estimate of the mean differences (MDs) with 95% confidence intervals (CIs). If studies used different scales for an outcome variable, or if we obtained only full data of all included studies regarding changes from baseline to study end, we entered data as mean changes and SDs of changes and used the standardised mean difference (SMD) with 95% CI instead of MDs. For all binary outcomes (such as the secondary outcome 'dropouts from all causes'), we calculated risk ratios (RRs) with 95% CIs. If studies reported no events, we calculated risk differences (RDs) with 95% CIs, instead of RRs.
Assessment of heterogeneity
We used the I² statistic to assess heterogeneity. We used a random‐effects model, regardless of the level of heterogeneity.
Data synthesis
We pooled the results of all eligible studies to present an overall estimate of the effect of electromechanical and robot‐assisted arm training (meta‐analysis). For all statistical analyses we used the latest version of the Cochrane Review Manager software, RevMan 5.3 (RevMan 2014). We calculated the overall effects using a random‐effects model, regardless of the level of heterogeneity. To test the robustness of the results, we did a sensitivity analysis by leaving out studies that we assessed to be of lower or ambiguous methodological quality (with respect to randomisation procedure, allocation concealment, and blinding of assessors). Clinical diversity and heterogeneity did not contribute to the decision about when to pool trials, but we described clinical diversity, variability in participants, interventions, and outcomes studied in Table 5.
Table 1.
Participant characteristics in studies
| Study ID | Age, mean (SD) EXP | Age, mean (SD) CON | Time poststroke EXP | Time poststroke CON | Gender EXP | Gender CON | Side‐paresis EXP | Side‐paresis CON | Stroke severity | Aetiology (ischaemic/haemorrhagic) |
| Abdullah 2011 | 76 (6) years | 70 (16) years | 4 (2) weeks | 4 (2) weeks | 3 F, 5 M | 8 F, 3 M | 3 L, 5 R | 6 L, 4 R, 1 both | Stage 1‐3 CMSA | Not stated |
| Amirabdollahian 2007 | 67 (7) years | 68 (9) years | 17 (12) months | 31 (22) months | 9 F, 7 M | 5 F, 10 M | 9 L, 7 R | 7 L, 8 R | Not stated | Not stated |
| Ang 2014 | 52 (7) years | 58 (19) years | 350 (131) days | 455 (110) days | 4 F, 10 M | 3 F, 4 M | Not stated | Not stated | Mean 27 points FMA upper extremity | 11/10 |
| Brokaw 2014 | 57 (12) years | 3 (2) years | 3 F, 9 M | 7 L, 5 R | Mean 22 points FMA upper extremity | Not stated | ||||
| Burgar 2011 | 60 (2) years* | 68 (3) years* | 17 (3) days* | 11 (1) days* | Not stated | Not stated | 18 L, 18 R | 5 L, 13 R | Mean 27 points FIM upper limb | Not stated |
| Conroy 2011 | 59 (13) years | 56 (6) years | 4 (5) years | 4 (6) years | 23 F, 18 M | 11 F, 10 M | Not stated | Not stated | Mean 72 points score on SIS, ADL | 51/6 |
| Daly 2005 | Not stated | Not stated | > 12 months | > 12 months | 0 F, 6 M | 3 F, 3 M | Not stated | Not stated | Not stated | 11/1 |
| Fazekas 2007 | 57 years | 56 years | 23 months | 10 months | 8 F, 7 M | 5 F, 10 M | 7 L, 8 R | 6 L, 9 R | Mean 30 points FIM self‐care | Not stated: also included people after head trauma |
| Hesse 2005 | 65 (12) years | 64 (12) years | 5 (1) weeks | 5 (1) weeks | 12 F, 10 M | 12 F, 10 M | 14 L, 8 R | 11 L, 11 R | Mean 42 of 100 Barthel points | 40/4 |
| Hesse 2014 | 71 (16) years | 70 (17) years | 5 (2) weeks | 5 (1) weeks | 12 F, 13 M | 10 F, 15 M | 14 L, 11 R | 13 L, 12 R | Mean 27 of 100 Barthel points | 41/9 |
| Hollenstein 2011 | 71 (8) years | 75 (11) years | 33 (14) days | 29 (10) days | 4 F, 3 M | 5 F, 1 M | 4 L, 3 R | 3 L, 3 R | Not stated | Not stated |
| Housman 2009 | 54 (12) years | 56 (11) years | > 12 months | > 12 months | 3 F, 11 M | 7 F, 7 M | 10 L, 4 R | 10 L, 4 R | Not stated | 17/9; 2 unknown |
| Hsieh 2011 | 54 (8) years | 54 (8) years | 17 (7) months | 28 (20) months | 2 F, 8 M | 1 F, 5 M | 6 L, 6 R | 4 L, 2 R | Not stated | 15/3 |
| Hsieh 2014 | 53 (10) years | 54 (10) years | 22 (14) months | 28 (19) months | 10 F, 22 M | 4 F, 12 M | 19 L, 13 R | 7 L, 9 R | Mean 34 points FMA upper extremity | 27/21 |
| Hwang 2012 | 50 (4) years | 51 (3) years | 7 (6) months | 5 (6) months | 4 F, 5 M | 2 F, 4 M | Not stated | Mean 43 (16) SIS activities | Not stated | |
| Kahn 2006 | 56 (12) years | 56 (12) years | 76 (46) months | 103 (48) months | 6 F, 4 M | 2 F, 7 M | 5 L, 5 R | 6 L, 3 R | Not stated | Not stated |
| Klamroth‐Marganska 2014 | 55 (13) years | 58 (14) years | 52 (44) months | 40 (45) months | 17 F, 21 M | 10 F, 25 M | Not stated | Mean SIS total score 63 (11) | Not stated | |
| Kutner 2010 | 62 (13) years | 51 (11) years | 270 (111) days | 184 (127) days | 5 F, 5 M | 2 F, 5 M | Not stated | Not stated | SIS ADL mean 59 and 68 for EXP and CTL groups, respectively | 12/5 |
| Liao 2011 | 55 (11) years | 54 (8) years | 23 (13) months | 22 (17) months | 4 F, 6 M | 3 F, 7 M | 4 L, 6 R | 3 L, 7 R | Mean 116 points FIM self‐care | Not stated |
| Lo 2010 | 66 (11) years | 64 (11) years | 4 (4) months | 5 (4) months | 2 F, 47 M | 3 F, 75 M | Not stated | Not stated | Mean 49 points score on SIS | 108/19 |
| Lum 2002 | 63 (4) years* | 66 (2) years* | 30 (6) months* | 29 (6) months* | 1 F, 12 M | 6 F, 8 M | 4 L, 9 R | 4 L, 10 R | Mean 87 of 100 Barthel points | Not stated |
| Lum 2006# | 67 years | 60 years | 11 weeks | 11 weeks | 8 F, 16 M | 2 F, 4 M | 11 L, 13 R | 2 L, 4 R | Not stated | Not stated |
| Masiero 2007 | 63 (13) years | 67 (12) years | Not stated | Not stated | 7 F, 10 M | 7 F, 11 M | 4 L, 11 R | 5 L, 10 R | Not stated | Not stated |
| Masiero 2011 | 72 (7) years | 76 (5) years | 10 (5) days | 13 (5) days | 2 F, 9 M | 3 F, 7 M | 9 L, 2 R | 8 L, 2 R | Mean total FIM 30 points | 18/3 |
| Mayr 2008 | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | 4 L | 4 L | Not stated | 6/2 |
| McCabe 2015 | 21‐49 years: n = 2; 50‐81 years: n = 10 | 21‐49 years: n = 5; 50‐81 years: n = 18 | 1‐3 years: n = 9; ≥4 years: n = 3 | 1‐3 years: n = 18; ≥4 years: n = 5 | 2 F, 10 M | 10 F, 13 M | Not stated | Not stated | 23 (6) FMA upper extremity points | Not stated |
| Rabadi 2008 | 80 (6) years | 69 (11) years | 10 (4) days | 14 (13) days | 5 F, 5 M | 6 F, 14 M | Not stated | Not stated | Mean FIM score 39 (11) | 3/0 |
| Sale 2014 | 68 (14) years | 68 (14) years | Not stated | Not stated | 11 F, 15 M | 11 F, 16 M | 16 L, 10 R | 13 L, 14 R | Mean CMSA 3 (1) | 53/0 |
| Susanto 2015 | 51 (9) years | 55 (11) years | 16 (6) months | 16 (5) months | 2 F, 7 M | 3 F, 7 M | 6 L, 3 R | 6 L, 4 R | Mean FMA 33 (9) | 8/11 |
| Timmermans 2014 | 62 (7) years | 57 (6) years | 3 (3) years | 4 (3) years | 3 F, 8 M | 3 F, 8 M | 7 L, 4 R | 8 L, 3 R | Mean FMA 52 | Not stated |
| Volpe 2000 | 62 (2) years* | 67 (2) years* | 23 (1) days* | 26 (1) days* | 14 F, 16 M | 12 F, 14 M | 17 L, 13 R | 14 L, 12 R | Not stated | 49/7 |
| Volpe 2008 | 62 (3) years* | 60 (3) years* | 35 (7) months* | 40 (11) months* | 3 F, 8 M | 3 F, 7 M | 5 L, 6 R | 5 L, 5 R | Mean 17 points NIHSS | 20/1 |
| Wu 2012 | 56 (11) years | 51 (6) years | 18 (11) months | 18 (10) months | 6 F, 22 M | 4 F, 22 M | 16 L, 12 R | 10 L, 4 R | Mean FMA 44 (10) points | Not stated |
| Yoo 2013 | 51 (11) years | 50 (9) years | 46 (42) months | 42 (33) months | 4 F, 7 M | 5 F, 6 M | 6 L, 5 R | 4 L, 7 R | Mean Barthel Index 76 (5) | 15/7 |
*SE instead of SD #EXP: all robot groups ADL: activities of daily living CMSA: Chedoke‐McMaster Stroke Assessment CON: control group EXP: experimental group F: female FIM: Functional Independence Measure FMA: Fugl‐Meyer AssessmentL: left M: male NIHSS: National Institutes of Health Stroke ScaleR: right SD: standard deviation SE: standard error SIS: Stroke Impact Scale
If studies had three or more intervention groups, for example two treatment groups and one control group, and the results of these intervention groups did not differ significantly, we combined the results of all intervention groups in one (collapsed) group and compared this with the results of the control group.
Subgroup analysis and investigation of heterogeneity
We conducted a formal subgroup analysis by splitting all participants into two subgroups: a subgroup of participants in the acute and subacute phase of their stroke (within three months) and a subgroup of participants treated in the chronic phase (more than three months after stroke). In this subgroup analysis, we did a formal comparison between the results of the primary outcome measure of participants treated in the acute and subacute phase of their stroke compared with the results of participants treated in the chronic phase (Deeks 2011).
We conducted another subgroup analysis by splitting all participants into two subgroups: a subgroup of participants who received mainly training for the distal arm and the hand (finger, hand, and radio‐ulnar joints) and a subgroup of participants who received training mainly of the proximal arm (shoulder and elbow joints) and a subgroup of participants treated in the chronic phase (more than three months after stroke). In this subgroup analysis, we did a formal comparison between the results of the subgroups for the primary outcome measure (activities of daily living) and the secondary outcome measure (arm function). To quantify heterogeneity we used the I² statistic implemented in RevMan 5.3 for all comparisons (RevMan 2014).
Sensitivity analysis
In accordance with the description in the Cochrane Handbook for Systematic Reviews of Interentions, we used the methodological features randomisation procedure, concealed allocation, and blinding of assessors to test the robustness of the main results in a sensitivity analysis (Higgins 2011).
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies, Table 5 and Table 6.
Table 2.
Details of study interventions
| Study ID | Duration of study | Frequency and intensity of treatment | Follow‐up | Device used |
| Abdullah 2011 | 8 to 11 weeks | 3 times a week (groups received the same time and frequency) | ‐ | Adapted 5 DOF industrial robot |
| Amirabdollahian 2007 | 3 weeks | 5 times a week (groups received the same time and frequency) | ‐ | GENTLE/s |
| Ang 2014 | 6 weeks | 3 times a week for 90 minutes (groups received the same time and frequency) | 6 weeks and 18 weeks | Haptic Knob and Haptic Knob with Brain‐Computer Interface |
| Brokaw 2014 | 3 months | 12 hours within a month (groups received the same time and frequency) | ‐ | ARMin III, HandSOME |
| Burgar 2011 | 3 weeks | 1 experimental group and the control group had 15 x 1‐hour therapy sessions over a 3‐week period (1 robot group received 30 1‐hour therapy sessions over a 3‐week period) | 6 months | MIME |
| Conroy 2011 | 6 weeks | 3 sessions per week for 1 hour (groups received the same time and frequency) | 3 months | InMotion 2.0 Shoulder/Arm Robot |
| Daly 2005 | 12 weeks | 5 hours a day, 5 days a week (groups received the same time and frequency) | 3 months | InMotion |
| Fazekas 2007 | 5 weeks | Control group received 30‐minute sessions on 20 consecutive workdays (Bobath, Kabat) Experimental group received same therapy as the control group, but also additional 30 minutes of robot therapy | ‐ | REHAROB |
| Hesse 2005 | 6 weeks | 30 minutes, 5 times a week (groups received the same time and frequency) | 3 months | Bi‐Manu‐Track |
| Hesse 2014 | 4 weeks | 30 minutes, 5 times a week (groups received the same time and frequency) | 3 months | Bi‐Manu‐Track, Reha‐Digit, Reha‐Slide, Reha‐Slide Duo |
| Hollenstein 2011 | 2 weeks | 5 times a week for 30 minutes (groups received the same time and frequency) | ‐ | Armeo |
| Housman 2009 | 8 to 9 weeks | 3 times a week for 1 hour (groups received the same time and frequency) | 6 months | T‐WREX |
| Hsieh 2011 | 4 weeks | Higher‐intensity robotic training group: 20 sessions for 90 to 105 minutes, 5 days per week Lower‐intensity robotic training group: same amount, but had only half of the repetitions by the device as in first group Conventional treatment group: same amount as in the other groups (groups received the same time and frequency) |
‐ | Bi‐Manu‐Track |
| Hsieh 2014 | 4 weeks | Participants in each group received 20 training sessions of 90 to 105 minutes/day, 5 days/week for 4 weeks. In addition to the intervention provided in the clinics, all participants were encouraged to use their affected upper limb during activities in their daily life situations (e.g. at home) RT + CIT group (received 2 weeks robot‐assisted arm therapy (Bi‐Manu‐Track 40 to 55 minutes plus 15 to 20 minutes conventional therapy without robot), afterwards 2 weeks constraint‐induced therapy 90 to 105 minutes therapy a day and 6 hours constraint daily) RT group (received robot‐assisted arm therapy (Bi‐Manu‐Track) as above) CT group (received a therapist‐mediated intervention using conventional occupational therapy techniques, including neurodevelopmental techniques, functional task practice, fine‐motor training, arm exercises or gross‐motor training, and muscle strengthening) |
‐ | Bi‐Manu‐Track |
| Hwang 2012 | 4 weeks | 4 weeks (20 sessions) of active robot‐assisted intervention versus 2 weeks (10 sessions) of early passive therapy followed by 2 weeks (10 sessions) of active robot‐assisted intervention (groups received the same time and frequency) | 4 weeks | Amadeo |
| Kahn 2006 | 8 weeks | 24 sessions for 45 minutes (groups received the same time and frequency) | ‐ | ARM Guide |
| Klamroth‐Marganska 2014 | 8 weeks | Robotic training or conventional therapy 3 times a week for at least 45 minutes (groups received the same time and frequency) | 26 weeks | ARMin |
| Kutner 2010 | 3 weeks | 1) 60 hours of repetitive‐task training over the course of 3 weeks 2) 30 hours of repetitive‐task training plus 30 hours of robotic‐assisted training with the Hand Mentor device over the course of 3 weeks (groups received the same time and frequency) |
2 months | Hand Mentor |
| Liao 2011 | 4 weeks | 5 days a week for 90 to 105 minutes per session (groups received the same time and frequency) | ‐ | Bi‐Manu‐Track |
| Lo 2010 | 12 weeks | Group A: a maximum of 36 sessions over a period of 12 weeks Group B: same time and frequency Group C: usual care at different time and frequency |
3, 6, 9 months | MIT‐Manus |
| Lum 2002 | 8 weeks | Control group received 55 minutes of physiotherapy for the arm and 5 minutes of robot training at each of the 24 sessions Experimental group received robot therapy for the same time and frequency | 8 months | MIME |
| Lum 2006 | 4 weeks | All groups received 15 1‐hour treatment sessions (all groups had same time and frequency) | 6 months | MIME |
| Masiero 2007 | 5 weeks | Experimental group received additional robotic training twice a day, 5 days a week Control group received similar exposure to the robot but with the unimpaired arm (both groups had same time and frequency) |
3 and 8 months | NeReBot |
| Masiero 2011 | 5 weeks | Experimental group received robotic training twice a day for 20 minutes, and 40 minutes conventional training, 5 days a week Control group received conventional functional rehabilitation for 80 minutes a day (groups received the same time and frequency) |
3 months | NeReBot |
| Mayr 2008 | 6 weeks | 5 times per week (both groups received the same time and frequency) | ‐ | ARMOR |
| McCabe 2015 | 5 weeks | 5 hours per day for 12 weeks (all groups received the same time and frequency) | ‐ | InMotion2 Shoulder/Elbow Robot |
| Rabadi 2008 | Not stated | Standard occupational and physical therapy for 3 hours per day + 12 additional sessions of 40 minutes of either occupational therapy, arm ergometry, or robotic‐assisted training for 5 days per week | ‐ | MIT‐Manus |
| Sale 2014 | 6 weeks | 30 sessions of robot‐assisted therapy (5 days a week for 6 weeks) versus 30 sessions (5 days a week for 6 weeks) of conventional rehabilitative treatment Experimental and control therapies were applied in addition to usual rehabilitation (groups received the same time and frequency) |
‐ | MIT‐Manus/InMotion2 |
| Susanto 2015 | 5 weeks | Hand exoskeleton robot‐assisted training for 20 1‐hour sessions versus control group (non‐assisted group) for 20 1‐hour sessions (groups received the same time and frequency) | 6 months | Self designed hand exoskeleton robot |
| Timmermans 2014 | 8 weeks | Robotic‐assisted training with the end‐effector robot HapticMaster versus arm‐hand training program during 8 weeks, 4 times/week, twice a day for 30 minutes (groups received the same time and frequency) | 6 month | HapticMaster |
| Volpe 2000 | 5 weeks | 1 hour per day, 5 days a week (for at least 25 sessions) (both groups received the same time and frequency) | ‐ | MIT‐Manus |
| Volpe 2008 | 6 weeks | 1 hour per session, 3 times a week (both groups received the same time and frequency) | 3 months | InMotion2 |
| Wu 2012 | 4 weeks | Therapist‐mediated bilateral arm training (TBAT group) versus robot‐assisted (Bi‐Manu‐Track) arm trainer (RBAT group) versus conventional therapy (involved weight bearing, stretching, strengthening of the paretic arms, co‐ordination, unilateral and bilateral fine‐motor tasks, balance, and compensatory practice on functional tasks; CT group). Each group received treatment for 90 to 105 minutes per session, 5 sessions on weekdays, for 4 weeks (groups received the same time and frequency) | ‐ | Bi‐Manu‐Track |
| Yoo 2013 | 6 weeks | 3‐dimensional robot‐assisted therapy (RAT) and conventional rehabilitation therapy (CRT) for a total of 90 minutes (RAT: 30 minutes, CRT: 60 minutes) a day with 10 minutes rest halfway through the session, received training 3 days a week for 6 weeks. The control group received only CRT for 60 minutes a day on the same days as the first group | ‐ | ReoGo |
DOF: Degrees of Freedom MIME: mirror image motion enabler
Results of the search
Our updated searches of the electronic bibliographic databases identified 5308 citations (Figure 1). One review author (BE) carried out additional searches of trials registers, commercial websites, conference proceedings, and reference lists, and from these and the search of the Cochrane Stroke Group's Trials Register, we identified three further studies for inclusion. Hence the number of records identified was 5311. After the elimination of duplicates, two review authors (BE and JM) assessed 4471 relevant abstracts and eliminated obviously irrelevant studies from the titles and abstracts alone. We obtained the full text of 49 possibly relevant papers. The same review authors (BE and JM) independently reviewed the full papers and selected 15 studies (30 full texts) that met our inclusion criteria. If necessary due to disagreements or uncertainties, we held consensus discussions involving additional review authors. We carefully considered and discussed a further 10 studies, but did not deem them eligible; we have detailed them in Characteristics of excluded studies.
Figure 1.
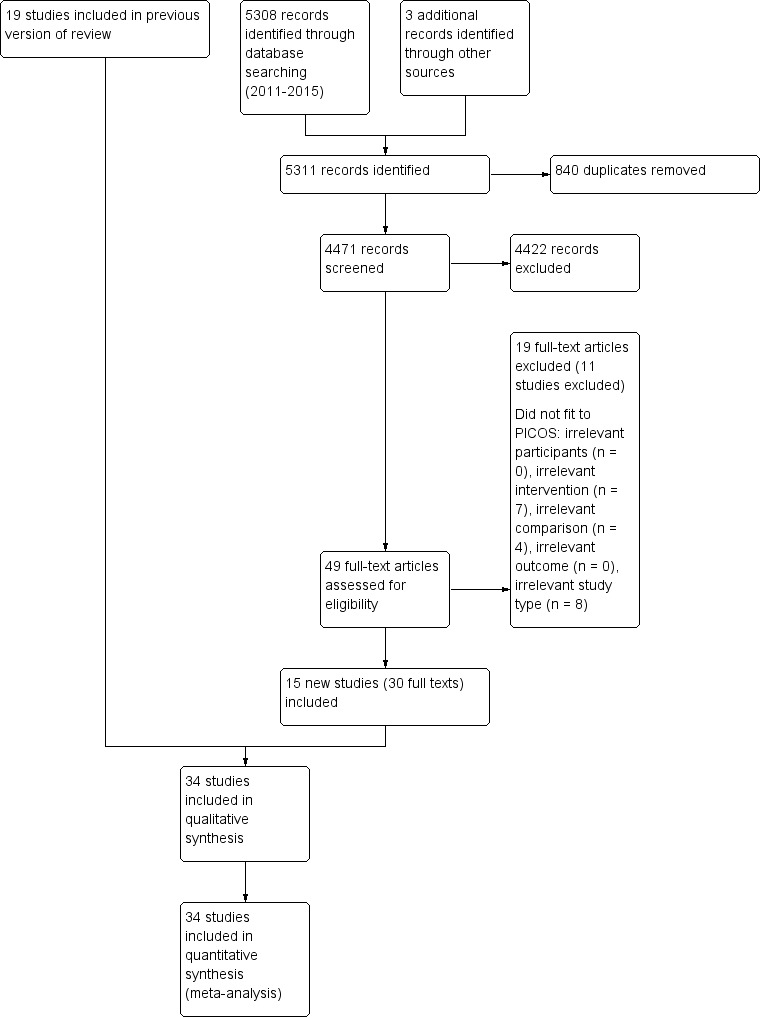
Study flow diagram. Please note that several studies have been published in multiple full‐text articles. Hence the number of assessed full‐text articles and the number of identified studies may differ.
We thus identified 15 new studies (30 full texts), and together with 19 studies included in the original review, we have included a total of 34 studies in this update. Five studies are still awaiting classification; we have described these studies in detail in Characteristics of studies awaiting classification. In addition, we identified 24 ongoing studies, which we have listed in Characteristics of ongoing studies.
Included studies
Thirty‐four trials, including a total of 1160 participants, met our inclusion criteria and have been included in the analysis (see Figure 1, Characteristics of included studies, Table 5, and Table 6).
Design
Two trials used a cross‐over design with random allocation to the order of treatment sequences (Amirabdollahian 2007; Hollenstein 2011). For Amirabdollahian 2007, we could not obtain outcome data from the trialists of this study, therefore we could not pool the data for this trial together with the data from other studies. In the study of Hollenstein 2011, we used the data of the first period before cross‐over. All other studies used a parallel‐group design with true randomisation‐to‐group allocation.
Sample sizes
The sample sizes in the trials ranged from eight participants, in Mayr 2008, to 127 participants, in Lo 2010 (sample size median = 30 interquartile range = 25). We have provided a more detailed description of trial characteristics in Characteristics of included studies and in Table 5 and Table 6.
Setting
Most of the trials were done in rehabilitation facilities in the USA. We have provided a more detailed description of trial characteristics in Characteristics of included studies.
Participants
The mean age of participants in the included studies ranged from 21 years, in McCabe 2015, to 80 years, in Rabadi 2008. We have provided a detailed description of participant characteristics in Table 5. There were significantly more males than females (66% males with 95% CI 63 to 69), and slightly more participants with left‐sided hemiparesis (53% left‐sided with 95% CI 49 to 57) included in the studies.
Twenty‐three studies provided information about baseline stroke severity (for example Functional Independence Measure, Barthel) or about the deficit of arm motor function (Fugl‐Meyer) (Table 5; Table 6).
For inclusion and exclusion criteria of every included study, see Characteristics of included studies.
Interventions
The duration of the studies (time frame where experimental interventions were applied) was heterogeneous, ranging from two weeks, in Hollenstein 2011, and three weeks, in Amirabdollahian 2007; Burgar 2011, to 12 weeks (Brokaw 2014; Daly 2005; Lo 2010). Most studies (eight out of 34) used a two‐, three‐, four‐, or six‐week study period (Table 6). The studies described and used 19 different electromechanical devices (see Table 6 for an overview); the devices used most often were the Bi‐Manu‐Track (Hesse 2005; Hesse 2014; Hsieh 2011; Hsieh 2014; Liao 2011; Wu 2012), the InMotion (Conroy 2011; Daly 2005; McCabe 2015; Volpe 2008), and the MIT‐Manus (Lo 2010; Rabadi 2008; Sale 2014; Volpe 2000).
Comparisons
The included trials compared electromechanical and robot‐assisted arm training with a variety of other interventions. We only did a formal meta‐analysis of studies that measured the same treatment effect. Thus we combined electromechanical and robot‐assisted arm training versus placebo (or no additional therapy) (two studies) with electromechanical and robot‐assisted arm training combined with physiotherapy versus physiotherapy alone (32 studies), as both estimate the effect of electromechanical and robot‐assisted arm training compared with a different treatment. However, we did not combine electromechanical and robot‐assisted arm training versus physiotherapy (or no treatment) with electromechanical and robot‐assisted arm training A versus electromechanical and robot‐assisted arm training B (Lum 2002), as these all measure entirely different treatment effects.
One study had four groups: three treatment (robot) groups and one control group (Lum 2006). Since the results of these experimental groups did not differ significantly, we combined the results of all experimental groups into one (collapsed, robot) group and compared this with the results of the control group. Nine other studies used three arms: two treatment (robot) groups and one control group or two control and one treatment group (Ang 2014; Burgar 2011; Conroy 2011; Hsieh 2011; Hsieh 2014; Lo 2010; McCabe 2015; Rabadi 2008; Wu 2012). As we were interested in the effects of robot therapy versus any other control intervention, we either combined the results of both experimental groups in one (collapsed) group and compared this with the results of the control group, or we combined the results of both control groups in one (collapsed) group and compared this with the results of the one treatment group.
For most trials the frequency of treatment was five times a week (see Table 6 for a detailed description of time and frequency for each single study).
The intensity of treatment (in terms of duration of experimental therapy provided) ranged from 20 minutes, in Masiero 2011, or 30 minutes, in Fazekas 2007, Hesse 2005, and Masiero 2007, to 90 minutes each working day, in Daly 2005 and Hsieh 2011, or even 90 to 105 minutes each day (Hsieh 2014). For some studies, the intensity of the experimental treatment is still unclear (Amirabdollahian 2007; Kahn 2006; Lo 2010). We have provided a detailed description for each single study in Table 6 and a more detailed description of the individual therapy in studies in Characteristics of included studies.
Outcomes
The primary outcomes of the included studies varied. See Characteristics of included studies for a detailed description of the primary outcomes for each trial.
In our pooled analysis for the primary outcome, activities of daily living, we used the Barthel Index score or the modified Barthel Index (Hesse 2005; Hesse 2014; Yoo 2013), the Functional Independence Measure (Burgar 2011; Fazekas 2007; Lum 2006; Masiero 2007; Volpe 2000), the ABILHAND (Hsieh 2011; Liao 2011), the Stroke Impact Scale 3.0 (motor function and social participation section) (Kutner 2010; Lo 2010; Wu 2012), the Stroke Impact Scale 2.0 (higher scores indicate a good outcome) (Volpe 2008), and the Frenchay Arm Test (Masiero 2011).
For our secondary outcome arm function, we used the Fugl‐Meyer score or the Chedoke‐McMaster Stroke Assessment (Abdullah 2011; Mayr 2008), and in one study the Wolf Motor Function Test for our pooled analysis and conducted a separate analysis for impaired arm function (Yoo 2013). For our secondary outcome arm strength, we accepted measures such as the Motricity Index score or Medical Research Council score (higher scores indicate a good outcome) or grip force.
Eighteen included studies assessed outcomes at the end of the study, but the follow‐up assessment varied between three months and nine months after study end (see Table 6 for a detailed description of time points of assessment for each single study) (Lo 2010). As reporting data of follow‐up measures were heterogeneous and limited mostly to our primary outcome, we did not conduct separate analyses for immediate data after study end and sustained data from follow‐up after study end. We therefore undertook just one analysis (immediately after the end of the intervention).
Excluded studies
We excluded 22 trials (see Excluded studies). We have excluded 11 trials from the previous version of the review and another 11 trials (19 full texts) from the current update. See Characteristics of excluded studies for details of reasons for excluding these trials. If there was any doubt about whether or not a study should be excluded, we retrieved the full text of the article. Where the two review authors (BE and JM) disagreed, a third review author (JK) decided on inclusion or exclusion of a study.
Ongoing studies
We identified 24 ongoing studies (see Ongoing studies), which we have described in Characteristics of ongoing studies. Nine of these studies were listed as ongoing studies in the previous version of the review. After we retrieved further information, four of these ongoing studies became included studies; one became a study awaiting classification; and three remained ongoing studies.
Risk of bias in included studies
We have provided all details about the methodological quality of each included study in Characteristics of included studies.
We wrote to the trialists of all the included studies requesting clarification of some design features or missing information in order to complete the quality ratings. The correspondence was via email or letter, and we wrote reminders every month if we did not receive an answer. Most trialists provided some or all of the requested data, but for four trials we did not receive all requested data.
We used the 'Risk of bias' tool to assess the methodological quality of all the included trials for random allocation, concealment of allocation, and blinding of assessors. (See Characteristics of included studies and Figure 2).
Figure 2.
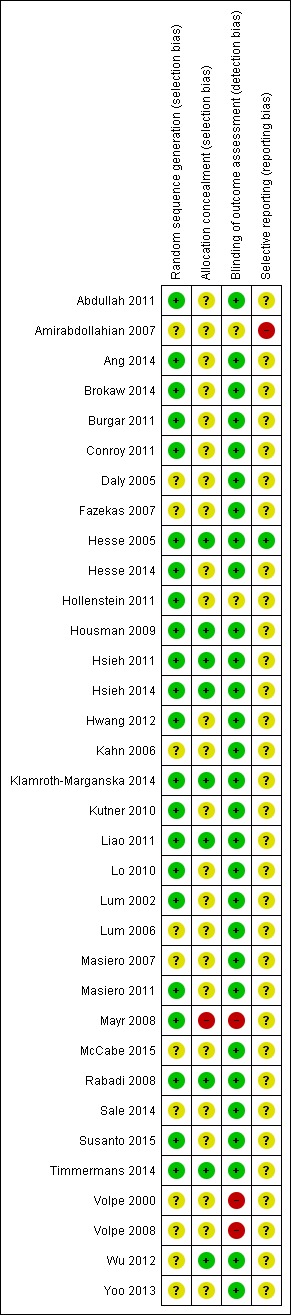
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Electromechanical and robot‐assisted arm training versus any other intervention
See Table 1.
Activities of daily living at the end of the intervention phase
Eighteen studies with a total of 717 participants compared electromechanical and robot‐assisted arm training versus any other intervention and measured activities of daily living. Electromechanical and robot‐assisted arm training improved activities of daily living scores. The pooled SMD (random‐effects model) for activities of daily living was 0.37 (95% CI 0.11 to 0.64, P = 0.005, level of heterogeneity I² = 62%; Analysis 1.1). We did not find graphical evidence in a funnel plot for publication bias (Figure 3).
Analysis 1.1.
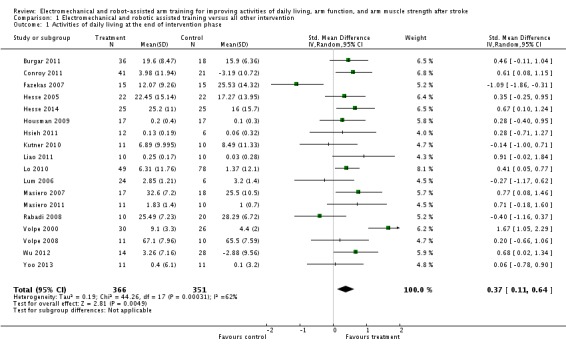
Comparison 1 Electromechanical and robotic assisted training versus all other intervention, Outcome 1 Activities of daily living at the end of intervention phase.
Figure 3.
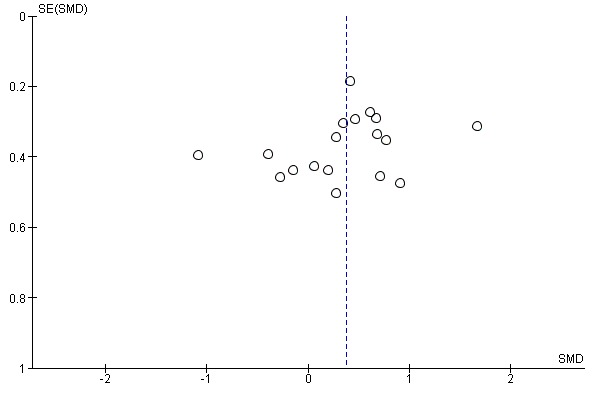
Funnel plot of comparison: 1 Electromechanical and robotic assisted training versus all other intervention, outcome: 1.1 Activities of daily living at the end of intervention phase.
Activities of daily living at the end of the intervention phase: subgroup analysis comparing the acute and chronic phase
We included eight trials with a total of 320 participants in the acute and subacute phase after stroke. Electromechanical and robot‐assisted arm training improved activities of daily living scores in the acute phase after stroke; the SMD (random‐effects model) was 0.53 (95% CI 0.09 to 0.96, P = 0.02, level of heterogeneity I² = 69%). We included 10 trials with a total of 397 participants in the chronic phase (more than three months after stroke). Electromechanical and robot‐assisted arm training did not improve activities of daily living scores in the chronic phase after stroke; the SMD (random‐effects model) was 0.66 (95% CI ‐0.17 to 1.49, P = 0.12, level of heterogeneity I² = 92%; Analysis 1.2). The test for subgroup differences (between acute and subacute phase after stroke versus chronic phase after stroke) revealed no significant difference (P = 0.78, level of heterogeneity I² = 0%).
Analysis 1.2.
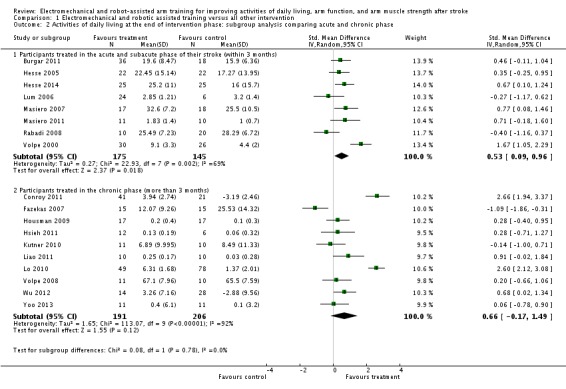
Comparison 1 Electromechanical and robotic assisted training versus all other intervention, Outcome 2 Activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase.
Arm function at the end of the intervention phase
Thirty‐one studies with a total of 1078 participants compared electromechanical and robot‐assisted arm training versus any other intervention and measured arm function. Electromechanical and robot‐assisted arm training improved arm function of the impaired arm. As we received the change data from baseline to study end for all trials that measured arm function, we used SMDs for this comparison. The pooled SMD (random‐effects model) for arm function was 0.35 (95% CI 0.18 to 0.51, P < 0.0001, level of heterogeneity I² = 36%; Analysis 1.3). We did not find graphical evidence in a funnel plot for publication bias (Figure 4).
Analysis 1.3.
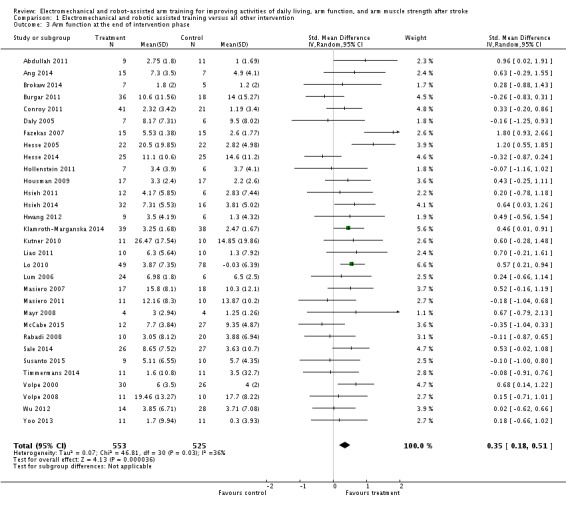
Comparison 1 Electromechanical and robotic assisted training versus all other intervention, Outcome 3 Arm function at the end of intervention phase.
Figure 4.
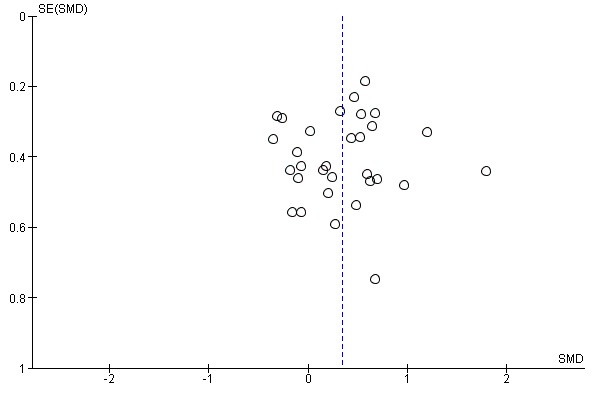
Funnel plot of comparison: 1 Electromechanical and robotic assisted training versus all other intervention, outcome: 1.3 Arm function at the end of intervention phase.
Arm muscle strength at the end of the intervention phase
Sixteen studies with a total of 568 participants compared electromechanical and robot‐assisted arm training versus another intervention and measured strength of arm. Electromechanical and robot‐assisted arm training improved arm muscle strength. The SMD (random‐effects model) for muscle strength was 0.36 (95% CI 0.01 to 0.70, P = 0.04, level of heterogeneity I² = 72%; Analysis 1.4). We did not find graphical evidence in a funnel plot for publication bias (Figure 5).
Analysis 1.4.
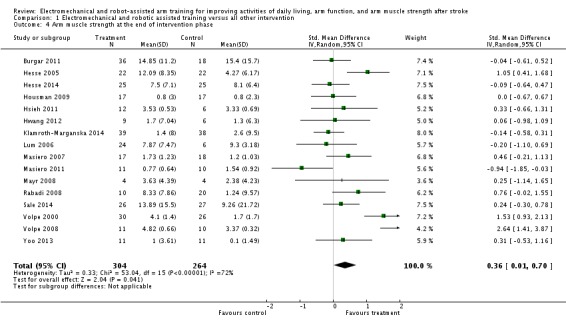
Comparison 1 Electromechanical and robotic assisted training versus all other intervention, Outcome 4 Arm muscle strength at the end of intervention phase.
Figure 5.
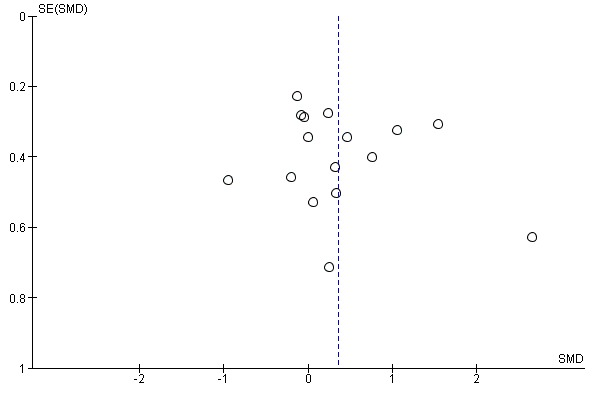
Funnel plot of comparison: 1 Electromechanical and robotic assisted training versus all other intervention, outcome: 1.4 Arm muscle strength at the end of intervention phase.
Acceptability: dropouts during the intervention period
We pooled all reported rates of participants who dropped out from all causes during the trial period. The use of electromechanical and robot‐assisted arm training in people after stroke did not increase the risk of participants dropping out. The RD (random‐effects model) for dropouts was 0.00 (95% CI ‐0.02 to 0.03, P = 0.84, level of heterogeneity I² = 0%; Analysis 1.5).
Analysis 1.5.
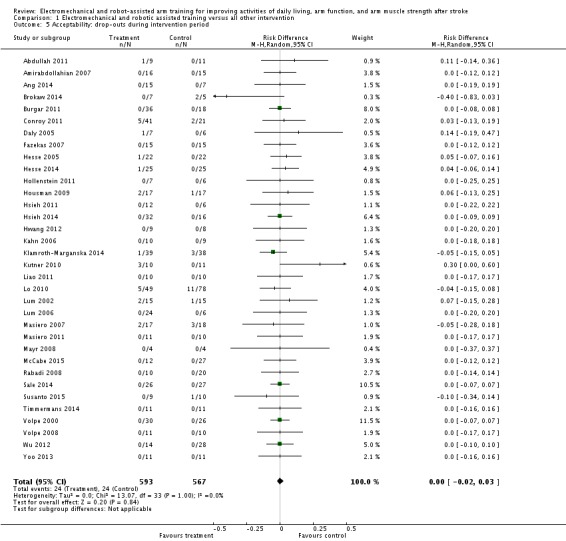
Comparison 1 Electromechanical and robotic assisted training versus all other intervention, Outcome 5 Acceptability: drop‐outs during intervention period.
The drop‐out rate for all reasons at the end of the treatment phase was relatively low (all included studies achieved a drop‐out rate of less than 16%), but for one study this is still unclear (Amirabdollahian 2007). Twenty‐one out of 34 included studies (62%) reported no dropouts at scheduled study end (Amirabdollahian 2007; Ang 2014; Burgar 2011; Fazekas 2007; Hollenstein 2011; Hsieh 2011; Hsieh 2014; Hwang 2012; Kahn 2006; Liao 2011; Lum 2006; Masiero 2011; Mayr 2008; McCabe 2015; Rabadi 2008; Sale 2014; Timmermans 2014; Volpe 2000; Volpe 2008; Wu 2012; Yoo 2013). The highest drop‐out rate in the treatment group was 12% (five dropouts out of 41 participants; Conroy 2011). The highest drop‐out rate in the control group was 14% (11 dropouts out of 78 participants; Lo 2010) and 16.7% (three dropouts out of 18 participants; Masiero 2011). Only one study in the early acute phase after stroke reported deaths during the treatment period (Masiero 2007). However, as explained by the authors via email correspondence, both deaths occurred in the control group. Other reasons for dropouts were:
personal reasons (treatment group) (Daly 2005);
personal reasons (control group) (Housman 2009);
withdrew (treatment group) (Abdullah 2011; Klamroth‐Marganska 2014);
withdrew (control group) (Klamroth‐Marganska 2014);
injured arm in daily life (treatment group) (Housman 2009);
depression (control group) (Housman 2009);
refusing therapy (treatment group) (Hesse 2005,Klamroth‐Marganska 2014);
medical complications (treatment group) (Conroy 2011; Lum 2002);
medical reasons (control group) (Klamroth‐Marganska 2014);
exclusion (control group) (Lum 2002);
lost to follow‐up (control group) (Susanto 2015);
unable to travel (Lo 2010) or transportation difficulties (treatment group) (Kutner 2010);
limited data (Conroy 2011; Hsieh 2014);
moved (Conroy 2011; Housman 2009);
did not met inclusion criteria after study commencement (Brokaw 2014).
Safety: adverse events during the intervention period
We did not carry out a pooled analysis because the reported rates of adverse events during the intervention period were rare and not related to the therapy (as described by the study authors). The reported adverse events were as described above: death in the control group, which was not related to the therapy (information as published by the study authors; Masiero 2007); and two participants experienced medical complications in the treatment group (information as published by the study authors; Lum 2002).
Sensitivity analysis: by trial methodology
Activities of daily living
To examine the robustness of the results, we specified variables in a sensitivity analysis that we believed could influence the size of effect observed (randomisation procedure, concealed allocation, and blinding of assessors) (Analysis 2.1). We did not investigate in this sensitivity analysis if selective reporting has an influence on the size of effect observed, because we did not find sufficient information to permit such a judgement.
Analysis 2.1.
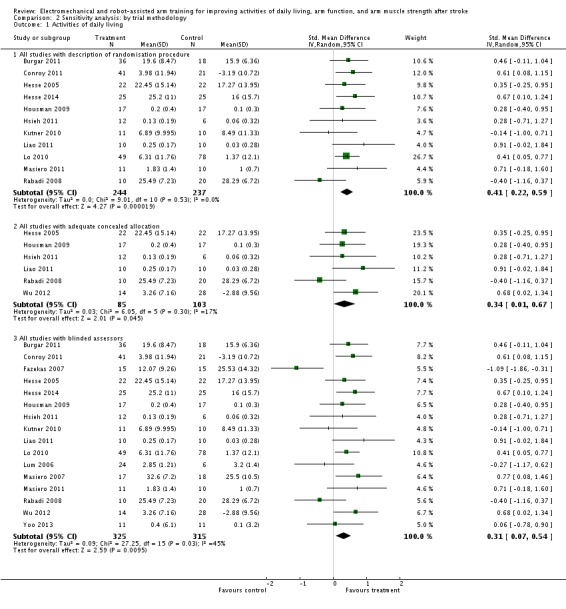
Comparison 2 Sensitivity analysis: by trial methodology, Outcome 1 Activities of daily living.
All studies with description of randomisation procedure
We included 11 trials with a total of 481 participants with an adequate description of the randomisation procedure. Electromechanical and robot‐assisted arm training improved activities of daily living. The SMD (random‐effects model) for activities of daily living was 0.41 (95% CI 0.22 to 0.59, P < 0.0001, level of heterogeneity I² = 0%).
All studies with adequately concealed allocation
We included six trials with a total of 188 participants with adequate concealment of allocation. Electromechanical and robot‐assisted arm training improved activities of daily living. The SMD (random‐effects model) for activities of daily living was 0.34 (95% CI 0.01 to 0.67, P = 0.04, level of heterogeneity I² = 17%).
All studies with blinded assessors
Sixteen trials with a total of 640 participants had blinded assessors for the primary outcome. Electromechanical and robot‐assisted arm training improved activities of daily living. The SMD (random‐effects model) for activities of daily living was 0.31 (95% CI 0.07 to 0.54, P = 0.009, level of heterogeneity I² = 45%).
Arm function
To examine the robustness of the results, we specified variables in a sensitivity analysis that we believed could influence the size of effect observed (randomisation procedure, concealed allocation, and blinding of assessors) (Analysis 2.2).
Analysis 2.2.
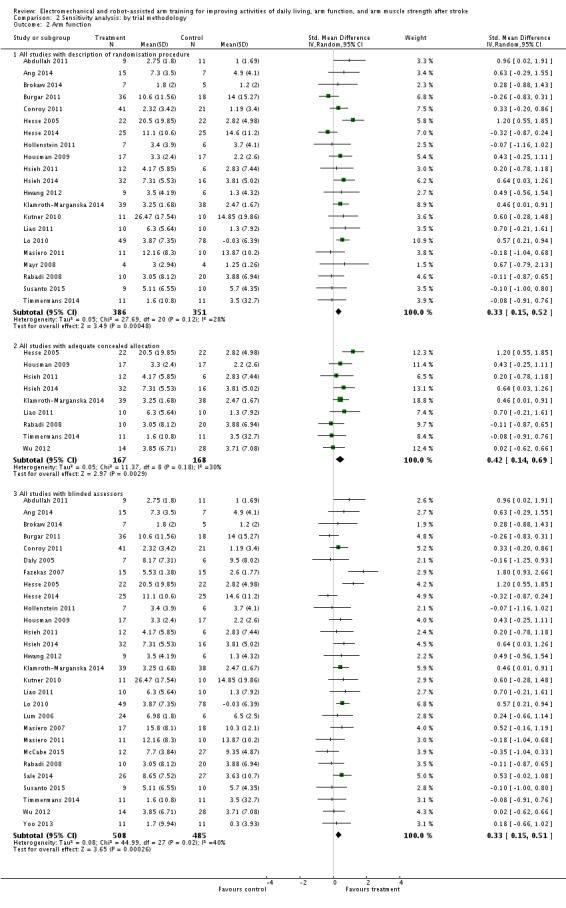
Comparison 2 Sensitivity analysis: by trial methodology, Outcome 2 Arm function.
All studies with description of randomisation procedure
We included 21 trials with a total of 737 participants with an adequate description of the randomisation procedure. Electromechanical and robot‐assisted arm training improved impaired arm function. The SMD (random‐effects model) for arm function was 0.33 (95% CI 0.15 to 0.52, P = 0.0005, level of heterogeneity I² = 28%).
All studies with adequately concealed allocation
We included nine trials with a total of 335 participants with adequate concealment of allocation. Electromechanical and robot‐assisted arm training improved impaired arm function. The SMD (random‐effects model) for arm function was 0.42 (95% CI 0.14 to 0.69, P = 0.003, level of heterogeneity I² = 30%).
All studies with blinded assessors
We included 28 trials with a total of 993 participants with blinded assessors. Electromechanical and robot‐assisted arm training improved impaired arm function. The SMD (random‐effects model) for arm function was 0.33 (95% CI 0.15 to 0.51, P = 0.0003, level of heterogeneity I² = 40%).
Subgroup analysis: by treatment approach
Activities of daily living at the end of intervention phase: subgroup analysis by treatment approach
The test for subgroup differences between a subgroup of participants who received mainly training for the distal arm and the hand (finger, hand, and radio‐ulnar joints) and a subgroup of participants who received training mainly of the proximal arm (shoulder and elbow joints) revealed no significant difference (P = 0.47, level of heterogeneity I² = 0%; Analysis 3.1).
Analysis 3.1.
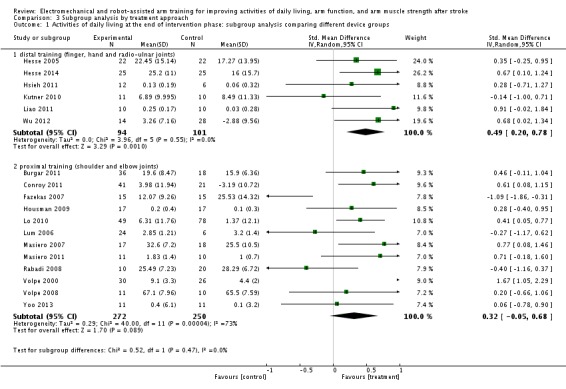
Comparison 3 Subgroup analysis by treatment approach, Outcome 1 Activities of daily living at the end of intervention phase: subgroup analysis comparing different device groups.
Arm function at the end of intervention phase: subgroup analysis by treatment approach
The test for subgroup differences between a subgroup of participants who received mainly training for the distal arm and the hand (finger, hand, and radio‐ulnar joints) and a subgroup of participants who received training mainly of the proximal arm (shoulder and elbow joints) revealed no significant difference (P = 0.75, level of heterogeneity I² = 0%; Analysis 3.2).
Analysis 3.2.
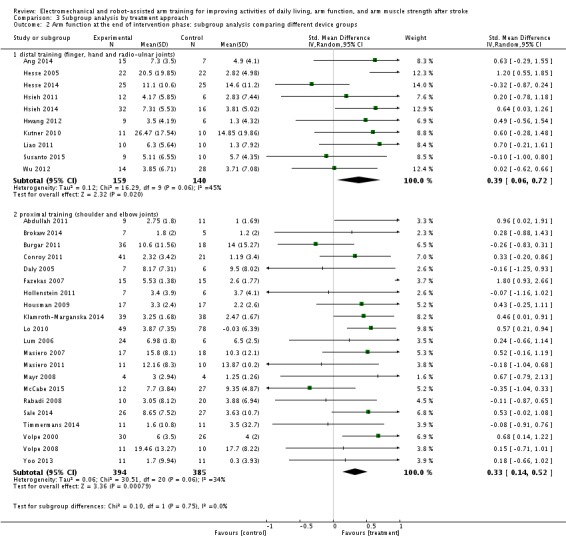
Comparison 3 Subgroup analysis by treatment approach, Outcome 2 Arm function at the end of intervention phase: subgroup analysis comparing different device groups.
Discussion
Summary of main results
We included 34 trials (involving 1160 participants) in this update of our systematic review of the effects of electromechanical and robot‐assisted therapy for improving activities of daily living, arm function, and arm muscle strength. We found that the use of electromechanical‐assistive devices in rehabilitation settings may improve activities of daily living, arm function, and arm strength, but the quality of evidence was rated as low to very low. Furthermore, adverse events and dropouts were uncommon and did not appear to be more frequent in those participants who received electromechanical and robot‐assisted arm training, graded with moderate‐quality evidence. This indicates that the use of electromechanical and robot‐assisted arm training devices could be safe and acceptable to most participants included in the trials that this review analysed.
Although the quality of evidence was very limited, there seems to be at least a potential benefit of electromechanical and robot‐assisted arm training.
When looking at certain groups of participants, we found significant improvements of activities of daily living in the subgroup of participants treated in the acute and subacute phase. We did not find such improvements for participants treated in the chronic phase. However, our statistical subgroup comparison does not indicate that people in the acute or subacute phase after stroke may improve more than people in the chronic phase with respect to activities of daily living. Participants who received mainly training for the distal arm and the hand (finger, hand, and radio‐ulnar joints) and participants who received training mainly of the proximal arm (shoulder and elbow joints) did not differ significantly with regard to activities of daily living and arm function.
Electromechanical and robot‐assisted therapy uses devices simply as 'vehicles' to apply an increased intensity in terms of many repetitions of arm training (Kwakkel 2008; Kwakkel 2015). It seems unlikely that motor therapy provided by robots will lead to better results than motor therapy provided by humans under the premise that intensity, amount, and frequency of therapy are exactly comparable. The potential advantage of electromechanical devices, when compared with conventional therapies, may be an increase in repetitions during arm training and an increase of motivation to train. Additionally, because people using electromechanical and robot‐assistance therapy are able to practise without a therapist, this type of training has the potential to increase the number of repetitions of practise. However, in our analysis of the included studies in this review update, we were not able to compare different amounts of repetitions of arm training. The amount of repetitions and also the exact intensity, time, dose, amount, and frequency of applied therapies were not described in detail in most of the studies included here. However, almost all of the included studies (but not Yoo 2013) had an active control group, and most studies matched the time for therapy between in‐treatment and control groups. One could therefore argue that robot‐assisted arm therapy after stroke is more effective in improving activities of daily living, arm function, and arm strength than other interventions if the same time of practise is offered. Then again, as mentioned above, it could just be that more repetitions in the same time were applied by robotic‐assisted arm training (higher dose). This appears to be an important issue that should be taken into account when discussing the effectiveness of electromechanical and robot‐assisted therapy for improving activities of daily living, arm function, and arm muscle strength.
Overall completeness and applicability of evidence
The results of this review seem to be quite generalisable for settings in industrialised countries and especially for rehabilitation centres with available electromechanical and robot‐assisted devices. However, the following factors produce uncertainty.
Most of the studies included participants with first‐ever stroke.
The majority of participants suffered from ischaemic stroke.
Nearly all of the participants were right‐handed.
The quality of evidence was rated as low to very low.
The exclusion of certain patient groups, such as people with unstable cardiovascular conditions, cognitive and communication deficits, or with a limited range of motion in the arm joints at the start of the intervention (it is well known that limited range of motion is common after stroke).
Hence, the results may be of limited applicability for people with recurrent stroke, haemorrhagic stroke, and people who are left‐handed. There is currently insufficient moderate‐ or high‐quality evidence to make conclusions about the benefits of robot therapy for improving activities of daily living, arm function, and arm muscle strength. However, as we found no evidence of side effects or harm, further research into this type of therapy appears to be justified.
The relatively tight selection criteria that have been applied to many studies should be considered. For example, the relatively younger age of people who were studied should be recognised, and also many of the people studied had no limitations of passive range of motion or were free of shoulder pain. It is well known in clinical practice that people are older and that the prevalence of comorbidities, such as pain, spasticity, or limitations to range of motion, is expected to be higher than described in the studies included here.
Additionally, electromechanical and robot‐assisted training could create additional costs of rehabilitation after stroke. The general applicability of robot therapy might therefore be limited simply due to lack of access of devices, for example in many low‐income countries, and there also appears to be fewer opportunities for therapists and patients to access robots in outpatient than in inpatient settings. All these points taken together might limit the applicability of this type of therapy in day‐to‐day clinical routine.
Quality of the evidence
We found heterogeneity regarding trial design (parallel‐group or cross‐over design, two or three or more intervention groups), therapy variables (type of device, bilateral or unilateral assistance, proximal or distal assistance, dosage of therapy), and participant characteristics (age, time poststroke, and severity of arm paresis).
There were enough studies to perform our planned sensitivity analysis examining the effects of methodological quality on the effectiveness of the intervention. We found that the effects of electromechanical‐assistive devices for improving activities of daily living and for improving arm function were quite stable and not affected by methodological quality (Analysis 2.1; Analysis 2.2).
Potential biases in the review process
The methodological rigour of Cochrane Reviews minimises bias in the process of conducting systematic reviews. A risk of publication bias, however, is present in all systematic reviews.
We searched extensively for relevant literature in databases and handsearched conference abstracts. Additionally, we contacted authors, trialists, and experts in the field for other unpublished and ongoing trials. We were unable to find graphical evidence for publication bias using funnel plots. There was heterogeneity between the trials in terms of trial design (two groups, four groups, parallel‐group or cross‐over trial, duration of study and follow‐up, and selection criteria for participants), characteristics of the therapy interventions (especially device used), and participant characteristics (length of time since stroke onset). There were also methodological differences in the mechanism of randomisation and allocation concealment methods used and blinding of primary outcomes.
After examination of the influence of methodological quality on the observed effect on activities of daily living and arm function, we did not find a change of benefit when we removed trials with unclear randomisation or allocation concealment procedures or unclear blinding.
While the methodological quality of the included trials was in general good to very good, although heterogeneous (Figure 2), trials investigating electromechanical and robot‐assisted arm training are subject to potential methodological limitations. These limitations include inability to blind the therapist and participants, so‐called contamination (provision of the intervention to the control group), and co‐intervention (when the same therapist unintentionally provides additional care to either treatment or comparison group). All these potential methodological limitations introduce the possibility of performance bias. However, as discussed above, our sensitivity analyses by methodological quality did not support this.
Some of the statistical analyses used in the review are based on parametric statistics. However, one could argue that it might not be appropriate to treat some scores for activities of daily living (for example Barthel Index score ranging from 0 to 100) and arm function (for example Fugl‐Meyer score ranging from 0 to 66) included in this review with this approach. Most of these scores were used in the included trials as continuous scales, and by others as ordinal scaled scores. However, it is unclear how this has led to an over‐ or underestimation of our described treatment effects.
As is always the case in systematic reviews, a so‐called publication bias could have potentially affected our results. The visual inspection of funnel plots for our main outcomes did not show evidence of publication bias (Figure 3; Figure 4; Figure 5), however this does not mean the complete absence of publication bias. Publication bias could therefore potentially be an issue, but it is unclear if this has led to an overestimation of our described treatment effects.
Agreements and disagreements with other studies or reviews
As far as we know, no other systematic reviews of RCTs about electromechanical and robot‐assisted therapy for improving activities of daily living, arm function, and arm muscle strength have been conducted in the last three years. The most recent systematic review of this topic was done in 2008 (Kwakkel 2008). However, there are other systematic reviews within the context of our review.
A systematic review about the evidence of physiotherapy also searched for the effect of robotic training of different segments of the arm (Veerbeek 2014). The authors found 22 studies including a total of 648 participants investigating robotic arm training. Veerbeek and colleagues found small‐to‐moderate effect sizes for bilateral elbow‐wrist robotic training (four studies) compared with studies with robotic training for the shoulder, elbow, wrist, and hand. The study authors classified robotic devices on the basis of the joints they target: 1) shoulder‐elbow robots, 2) elbow‐wrist robots, and 3) shoulder‐elbow‐wrist‐hand robots, but they did not compare effect sizes (Veerbeek 2014). In our review, compared to Veerbeek 2014, the corresponding effect sizes and the differences in effect sizes between the different treatment approaches were lower. However, we were able to identify 34 trials including a total of 1160 participants. We therefore believe that our review has used a more sensitive search and hence shows a more comprehensive picture of the evidence.
Another up‐to‐date review also included trials using robotic training in combination with other interventions for people with stroke (Laver 2015). However, the authors specifically investigated the efficacy of virtual reality compared with an alternative intervention or no intervention on upper limb function and activity.
Authors' conclusions
We found that people after stroke who receive electromechanical or robot‐assisted arm training are more likely to show improvement in their activities of daily living, arm function, and muscle strength of the paretic arm, but we rated the quality of evidence as low to very low.
In practice, electromechanical or robot‐assisted arm training could increase the intensity of arm therapy. Perhaps more repetitions in the same time of therapy can be achieved if electromechanical and robot‐assisted therapy is given. Electromechanical devices could therefore be used as an adjunct to conventional therapies.
However, it is still not clear if the difference between electromechanical or robot‐assisted arm training and other interventions is clinically meaningful for most people. Perhaps one main difference between electromechanical or robot‐assisted arm training and other interventions could be an improvement in motivation due to the feedback of the device, or the novelty of a robotic device, or both. However, we can only speculate about this.
There is still a need for well‐designed, large‐scale, multicentre studies to evaluate benefits and harms of electromechanical‐assisted arm training after stroke. Further research should count the amount of repetitions in time and address specific questions about the type, timing, frequency, and duration of electromechanical and robot‐assisted arm training. Further research should also investigate whether or not there is any benefit over and above the amount of practice, for example if it would be useful or not if a robot prevents 'incorrect learning or movements'. Additionally, improved reporting of trial methods and the use of published reporting guidelines for trials are essential.
It may be useful if future studies could use arm function‐specific outcome measures and measures of repetitions during training to gain a better understanding of the explicit effects of this special form of training.
The heterogeneity of training dose could be seen as a limitation of this review. Future studies should therefore describe the total training time and dose and should also describe the personnel supervising participants during training.
Future studies should also investigate the most severely affected people and groups, who are not reflected so far in the existing trials.
We found a drop‐out rate of less than 5%. Future studies could determine their sample size calculations based on this drop‐out rate.
Acknowledgements
We thank Brenda Thomas for her help in developing the search strategy and providing us with relevant trials and systematic reviews from MEDLINE, EMBASE, CINAHL, AMED, SPORTDiscus, Compendex, and Inspec; Hazel Fraser for her helpful support in proofreading the manuscript and providing us with relevant trials and systematic reviews from the Cochrane Stroke Group Trials Register; Gabi Voigt for providing us with many useful studies. We thank all the authors and investigators who provided us with additional or unpublished information about their trials. We thank the German federal Competence Network Stroke (www.kompetenznetz‐schlaganfall.de).
We are grateful to the editors, the consumer reviewers (Kedar K Mate and Heather Goodare), and the peer reviewers (Professor Peter Langhorne, Dr Alex Pollock, Dr Valentina Assi, Brenda Thomas, Dr Gerald Fluet, and Dr Hanna Persson) for their comments on this updated review.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL) The Cochrane Library
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "cerebral small vessel diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] or [mh ^"brain injuries"] or [mh ^"brain injury, chronic"]
#2 (stroke* or poststroke or apoplex* or cerebral next vasc* or brain next vasc* or cerebrovasc* or cva* or SAH):ti,ab
#3 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or "middle cerebral artery" or MCA* or "anterior circulation" or "posterior circulation" or "basilar artery" or "vertebral artery" or "space‐occupying") near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal next gangli* or putaminal or putamen or "posterior fossa" or hemispher* or subarachnoid) near/5 (hemorrhag* or haemorrhage* or hematoma* or haematoma* or bleed*)):ti,ab
#5 [mh ^hemiplegia] or [mh paresis]
#6 (hemipleg* or hemipar* or paresis or paretic or brain next injur*):ti,ab
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 [mh "upper extremity"]
#9 (upper next limb* or upper next extremit* or arm or arms or shoulder or shoulders or hand or hands or axilla* or elbow* or forearm* or finger* or wrist*):ti,ab
#10 #8 or #9
#11 [mh robotics] or [mh automation] or [mh "orthotic devices"]
#12 [mh "equipment and supplies"] or [mh "self‐help devices"]
#13 [mh "physical therapy modalities"] or [mh "occupational therapy"]
#14 [mh "therapy, computer‐assisted"] or [mh "man‐machine systems"]
#15 [mh "exercise movement techniques"] or [mh exercise] or [mh "exercise therapy"] or [mh "muscle stretching techniques"] or [mh "motion therapy, continuous passive"]
#16 (robot* or orthos* or orthotic or automat* or computer next aided or computer next assisted or device*):ti,ab
#17 (electromechanical or "electro‐mechanical" or mechanical or mechanised or mechanized or driven):ti,ab
#18 ((continuous passive or cpm) near/3 therap*):ti,ab
#19 (MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin):ti,ab
#20 (assist* near/5 (train* or aid* or rehabilitat* or re‐educat*)):ti,ab
#21 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#22 #7 and #10 and #21
Number of hits: n=930
Appendix 2. MEDLINE (Ovid) search strategy
Medline (Ovid) Revised March 2015
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ or brain injuries/ or brain injury, chronic/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic or brain injur$).tw.
7. or/1‐6
8. exp upper extremity/
9. (upper limb$ or upper extremit$ or arm or arms or shoulder or shoulders or hand or hands or axilla$ or elbow$ or forearm$ or finger$ or wrist$).tw.
10. 8 or 9
11. robotics/ or automation/ or orthotic devices/
12. "equipment and supplies"/ or self‐help devices/
13. physical therapy modalities/ or occupational therapy/
14. therapy, computer‐assisted/ or man‐machine systems/
15. exercise movement techniques/ or exercise/ or exercise therapy/ or muscle stretching techniques/ or motion therapy, continuous passive/
16. (robot$ or orthos$ or orthotic or automat$ or computer aided or computer assisted or device$).tw.
17. (electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven).tw.
18. ((continuous passive or cpm) adj3 therap$).tw.
19. (MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin).tw.
20. (assist$ adj5 (train$ or aid$ or rehabilitat$ or re‐educat$)).tw.
21. or/11‐20
22. Randomized Controlled Trials as Topic/
23. random allocation/
24. Controlled Clinical Trials as Topic/
25. control groups/
26. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
27. double‐blind method/
28. single‐blind method/
29. Placebos/
30. placebo effect/
31. cross‐over studies/
32. randomized controlled trial.pt.
33. controlled clinical trial.pt.
34. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
35. (random$ or RCT or RCTs).tw.
36. (controlled adj5 (trial$ or stud$)).tw.
37. (clinical$ adj5 trial$).tw.
38. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
39. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
40. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
41. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
42. (cross‐over or cross over or crossover).tw.
43. (placebo$ or sham).tw.
44. trial.ti.
45. (assign$ or allocat$).tw.
46. controls.tw.
47. or/22‐46
48. 7 and 10 and 21 and 47
49. exp animals/ not humans.sh.
50. 48 not 49
Number of hits: n=552
Appendix 3. EMBASE (Ovid) search strategy
EMBASE (Ovid) Revised March 2015
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke unit/ or stroke patient/ or brain injury/ or acquired brain injury/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/ or paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic or brain injur$).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. exp arm/ or arm weakness/ or arm exercise/ or arm movement/
9. (upper limb$ or upper extremit$ or arm or arms or shoulder or shoulders or hand or hands or axilla$ or elbow$ or forearm$ or finger$ or wrist$).tw.
10. 8 or 9
11. robotics/ or automation/ or orthotics/
12. man machine interaction/ or biomedical engineering/ or device/ or machine/ or assistive technology/ or assistive technology device/ or computer assisted therapy/
13. passive movement/ or movement therapy/ or kinesiotherapy/ or exp exercise/ or muscle stretching/ or muscle training/
14. (robot$ or orthos$ or orthotic or automat$ or computer aided or computer assisted or computeri?ed or device$).tw.
15. (electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven).tw.
16. ((continuous passive or cpm) adj3 therap$).tw.
17. (MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin).tw.
18. (assist$ adj5 (train$ or aid$ or rehabilitat$ or re‐educat$)).tw.
19. or/11‐18
20. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
21. Randomization/
22. Controlled clinical trial/ or "controlled clinical trial (topic)"/
23. control group/ or controlled study/
24. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
25. Crossover Procedure/
26. Double Blind Procedure/
27. Single Blind Procedure/ or triple blind procedure/
28. placebo/ or placebo effect/
29. (random$ or RCT or RCTs).tw.
30. (controlled adj5 (trial$ or stud$)).tw.
31. (clinical$ adj5 trial$).tw.
32. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
33. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
34. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
35. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
36. (cross‐over or cross over or crossover).tw.
37. (placebo$ or sham).tw.
38. trial.ti.
39. (assign$ or allocat$).tw.
40. controls.tw.
41. or/20‐40
42. 7 and 10 and 19 and 41
43. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
44. 42 not 43
Number of hits: n=1265
Appendix 4. CINAHL (Ebsco) search strategy
CINAHL (Ebsco) Revised March 2015
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR .(MH "Brain Injuries")
S2 .(MH "Stroke Patients") OR (MH "Stroke Units")
S3 .TI ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) or AB ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH )
S4 .TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) or AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying )
S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypox* )
S6 .S4 and S5
S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid )
S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S9 .S7 and S8
S10 ..(MH "Hemiplegia")
S11 .TI ( hemipleg* or hemipar* or paresis or paretic or brain injur* ) or AB ( hemipleg* or hemipar* or paresis or paretic or brain injur*)
S12 .S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11
S13 .(MH "Upper Extremity+")
S14 .TI ( upper limb* or upper extremit* or arm or arms or shoulder or shoulders or hand or hands or axilla* or elbow* or forearm* or finger* or wrist* ) or AB ( upper limb* or upper extremit* or arm or arms or shoulder or shoulders or hand or hands or axilla* or elbow* or forearm* or finger* or wrist* )
S15 .S13 or S14
S16 ..(MH "Therapeutic Exercise") OR (MH "Motion Therapy, Continuous Passive") OR (MH "Muscle Strengthening+") OR (MH "Neuromuscular Facilitation") OR (MH "Upper Extremity Exercises+")
S17 .(MH "Exercise+")
S18 .(MH "Movement+")
S19 .(MH "Assistive Technology") OR (MH "Automation") OR (MH "Robotics")
S20 .(MH "Orthoses") OR (MH "Orthoses Design")
S21 .(MH "Biomedical Engineering") OR (MH "Assistive Technology Services")
S22 .(MH "Assistive Technology Devices") OR (MH "Equipment and Supplies")
S23 .(MH "Therapy, Computer Assisted
S24 .(MH "Biomechanics")
S25 .TI ( robot* or orthos* or orthotic or automat* or computer aided or computer assisted or device* ) OR AB ( robot* or orthos* or orthotic or automat* or computer aided or computer assisted or device* )
S26 .TI ( electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven ) OR AB ( electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven )
S27 .TI ( continuous passive or cpm ) OR AB ( continuous passive or cpm )
S28 .TI therap* OR AB therap*
S29 .S27 and S28
S30 .TI ( MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin) OR AB ( MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin)
S31 .TI assist* OR AB assist*
S32 .TI ( train* or aid* or rehabilitat* or re‐educat* ) OR AB ( train* or aid* or rehabilitat* or re‐educat* )
S33 .S31 AND S32
S34 .S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S29 OR S30 OR S33
S35 .(MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+")
S36 .(MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials")
S37 .(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies")
S38 .(MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect")
S39 .(MH "Crossover Design") OR (MH "Quasi‐Experimental Studies")
S40 .PT (clinical trial or randomized controlled trial)
S41 .TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S42 .TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*))
S43 .TI (clinical* N5 trial*) or AB (clinical* N5 trial*)
S44 .TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*))
S45 .TI ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*))
S46 .TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*))
S47 .TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover)
S48 .TI (placebo* or sham) or AB (placebo* or sham)
S49 .TI trial
S50 .TI (assign* or allocat*) or AB (assign* or allocat*)
S51 .TI controls or AB controls
S52 .TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
S53 .S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52
S54 .S12 AND S15 AND S34 AND S53
Number of hits: n=298
Appendix 5. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ or brain injuries/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic or brain injur$).tw.
7. or/1‐6
8. exp arm/
9. (upper limb$ or upper extremit$ or arm or arms or shoulder or shoulders or hand or hands or axilla$ or elbow$ or forearm$ or finger$ or wrist$).tw.
10. 8 or 9
11. robotics/ or orthotic devices/ or biomechanics/ or equipment design/ or equipment/ or biomechanics equipment/ or therapy computer assisted/
12. exercise/ or exercise movement techniques/ or exercise therapy/ or exp movement/ or continuous passive motion/
13. engineering/ or technology/ or technology medical/
14. (robot$ or orthos$ or orthotic or automat$ or computer aided or computer assisted or device$).tw.
15. (electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven).tw.
16. ((continuous passive or cpm) adj3 therap$).tw.
17. (MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin).tw.
18. (assist$ adj5 (train$ or aid$ or rehabilitat$ or re‐educat$)).tw.
19. or/11‐18
20. clinical trials/ or randomized controlled trials/ or random allocation/
21. research design/ or comparative study/
22. double blind method/ or single blind method/
23. placebos/
24. (random$ or RCT or RCTs).tw.
25. (controlled adj5 (trial$ or stud$)).tw.
26. (clinical$ adj5 trial$).tw.
27. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
28. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
29. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
30. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
31. (cross‐over or cross over or crossover).tw.
32. (placebo$ or sham).tw.
33. trial.ti.
34. (assign$ or allocat$).tw.
35. controls.tw.
36. or/20‐35
37. 7 and 10 and 19 and 36
Number of hits: n=117
Appendix 6. SPORTDiscus (Ebsco) search strategy
SportDISCUS (Ebsco) Revised march 2015
S1 .DE "CEREBROVASCULAR disease" OR DE "BRAIN ‐‐ Hemorrhage" OR DE "CEREBRAL embolism & thrombosis" OR DE "STROKE" OR DE "BRAIN ‐‐ Wounds & injuries" OR DE "BRAIN damage"
S2 .DE "CEREBROVASCULAR disease ‐‐ Patients"
S3 .TI ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) or AB ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH )
S4 .TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) or AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying )
S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypox* )
S6 .S4 AND S5
S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid )
S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S9 .S7 AND S8
S10 .DE "HEMIPLEGIA" OR DE "HEMIPLEGICS"
S11 .TI ( hemipleg* or hemipar* or paresis or paretic or brain injur* ) or AB ( hemipleg* or hemipar* or paresis or paretic or brain injur*)
S12 .S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11
S13 .DE "ARM" OR DE "BICEPS brachii" OR DE "ELBOW" OR DE "FOREARM" OR DE "HAND" OR DE "HUMERUS" OR DE "TRICEPS" OR DE "WRIST" OR DE "ARM exercises" OR DE "HAND exercises" OR DE "SHOULDER exercises"
S14 .TI ( upper limb* or upper extremit* or arm or arms or shoulder or shoulders or hand or hands or axilla* or elbow* or forearm* or finger* or wrist* ) or AB ( upper limb* or upper extremit* or arm or arms or shoulder or shoulders or hand or hands or axilla* or elbow* or forearm* or finger* or wrist* )
S15 .S13 OR S14
S16 .DE "EXERCISE" OR DE "EXERCISE therapy" OR DE "STRENGTH training" OR DE "MOVEMENT therapy” OR DE "SELF‐help devices for people with disabilities" OR DE "ROBOTICS in sports"
S17 .DE "ORTHOPEDIC apparatus" OR DE "EQUIPMENT & supplies" OR DE "ORTHOPEDIC braces" OR DE "ORTHOPEDIC slings"
S18 .DE "BIOMEDICAL engineering"
S19 .DE "ELECTRONIC games" OR DE "COMPUTER games" OR DE "INTERNET games" OR DE "VIDEO games"
S20 .DE "BIOMECHANICS"
S21 .TI ( robot* or orthos* or orthotic or automat* or computer aided or computer assisted or device* ) OR AB ( robot* or orthos* or orthotic or automat* or computer aided or computer assisted or device* )
S22 .TI ( electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven ) OR AB ( electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven )
S23 .TI ( continuous passive or cpm ) OR AB ( continuous passive or cpm )
S24 .TI therap* OR AB therap*
S25 .S23 AND S24
S26 .TI ( MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin) OR AB ( MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin)
S27 .TI assist* OR AB assist*
S28 .TI ( train* or aid* or rehabilitat* or re‐educat* ) OR AB ( train* or aid* or rehabilitat* or re‐educat* )
S29 .S27 AND S28
S30 .S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S25 OR S26 OR S29
S31 .S12 AND S15 AND S30
Number of hits: n=773
Appendix 7. Compendex and Inspec (Engineering Village) search strategy
Compendex and Inspec (Engineering Village) ((((robot* or orthos* or orthotic or automat* or computer aided or computer assisted or device* or electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven or MIT‐Manus or ARM guide or Bi‐Manu‐Track or ARMtrainer or MIME or Mirror‐Image Motion Enabler or InMOTION or REHAROB or NeReBot or "GENTLE/S" or ARMin) WN KY) AND ((stroke or cerebrovascular or poststroke or post‐stroke or hemipleg*) WN TI)) AND ((upper limb* or upper extremit* or arm* or shoulder* or hand* or axilla* or elbow* or forearm* or finger* or wrist*) WN KY))
Number of hits Compendex: n=718
Number of hits Inspec: n=655
Data and analyses
Comparison 1.
Electromechanical and robotic assisted training versus all other intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living at the end of intervention phase | 18 | 717 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.11, 0.64] |
| 2 Activities of daily living at the end of intervention phase: subgroup analysis comparing acute and chronic phase | 18 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Participants treated in the acute and subacute phase of their stroke (within 3 months) | 8 | 320 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.09, 0.96] |
| 2.2 Participants treated in the chronic phase (more than 3 months) | 10 | 397 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.17, 1.49] |
| 3 Arm function at the end of intervention phase | 31 | 1078 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.18, 0.51] |
| 4 Arm muscle strength at the end of intervention phase | 16 | 568 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.01, 0.70] |
| 5 Acceptability: drop‐outs during intervention period | 34 | 1160 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
Comparison 2.
Sensitivity analysis: by trial methodology
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies with description of randomisation procedure | 11 | 481 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.22, 0.59] |
| 1.2 All studies with adequate concealed allocation | 6 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.01, 0.67] |
| 1.3 All studies with blinded assessors | 16 | 640 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.07, 0.54] |
| 2 Arm function | 29 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies with description of randomisation procedure | 21 | 737 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.15, 0.52] |
| 2.2 All studies with adequate concealed allocation | 9 | 335 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.14, 0.69] |
| 2.3 All studies with blinded assessors | 28 | 993 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.15, 0.51] |
Comparison 3.
Subgroup analysis by treatment approach
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living at the end of intervention phase: subgroup analysis comparing different device groups | 18 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 distal training (finger, hand and radio‐ulnar joints) | 6 | 195 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.20, 0.78] |
| 1.2 proximal training (shoulder and elbow joints) | 12 | 522 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.05, 0.68] |
| 2 Arm function at the end of intervention phase: subgroup analysis comparing different device groups | 31 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 distal training (finger, hand and radio‐ulnar joints) | 10 | 299 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.06, 0.72] |
| 2.2 proximal training (shoulder and elbow joints) | 21 | 779 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.14, 0.52] |
What's new
Last assessed as up‐to‐date: 1 June 2015.
| Date | Event | Description |
|---|---|---|
| 2 June 2015 | New citation required and conclusions have changed | The conclusions of the review have changed. The previous version concluded that people who receive electromechanical and robot‐assisted arm training after stroke were more likely to improve their activities of daily living, paretic arm function may improve, but arm strength did not improve. This updated version concluded that people who receive electromechanical and robot‐assisted arm training after stroke are more likely to improve their activities of daily living, arm function, and arm muscle strength. |
| 2 June 2015 | New search has been performed | We have updated the searches to March 2015, and revised the text as appropriate. We have included 34 trials with 1160 participants in this update compared with 19 trials with 666 participants in the 2011 version of this review. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 27 October 2011 | New citation required and conclusions have changed | The conclusions of the review have changed. The previous version of this review concluded that people who receive electromechanical and robot‐assisted arm training after stroke are not more likely to improve their generic activities of daily living, but arm function and muscle strength of the paretic arm may improve. This updated version of the review concluded that people who receive electromechanical and robot‐assisted arm training after stroke are more likely to improve their activities of daily living, and paretic arm function may improve, but not arm strength. |
| 9 August 2011 | New search has been performed | We have updated the searches to July and August 2011, and revised the text as appropriate. We have included 19 trials with 666 participants in this update compared with 11 trials with 328 participants in the 2008 version of this review. |
| 31 March 2008 | Amended | Converted to new review format. |
Differences between protocol and review
In our protocol we stated that we would use the PEDro scale to assess the methodological quality of the included trials. However, in Chapter 8 of the latest edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), it is suggested that scales that yield a summary score should be avoided. We therefore have not used the PEDro scale to assess the methodological quality of the included trials, but used the Cochrane 'Risk of bias' tool instead.
In our protocol we planned to quantify heterogeneity with the I² statistic and to use a cutoff of I² = 50% for all comparisons. Additonally, we planned to calculate the overall effects using a random‐effects model instead of a fixed‐effect model when we found substantial heterogeneity. However, in this update we calculated the overall effects using a random‐effects model regardless of the level of heterogeneity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdullah 2011
| Methods | RCT | |
| Participants | Country: Canada Sample size: 20 participants (9 in treatment group, 11 in control group) Inclusion criteria: first single, unilateral stroke; informed consent; age between 16 and 90 years; 2 to 8 weeks after stroke; motor arm impairment between stages 1 and 4 measured by CMSA Exclusion criteria: shoulder pain between 1 and 3 as measured by CMSA pain inventory scale; presence of other condition in the affected shoulder or elbow |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcome measures were assessed at baseline and at the end of intervention period Primary outcome measure: Chedoke Arm and Hand Activity Inventory (CAHAI‐7) Secondary outcome measures: CMSA, client satisfaction using a 10‐point Likert scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A physiotherapist unrelated to the study randomized the participants into one of two groups using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An occupational therapist blinded to patient allocation administered the CAHAI‐7 and the CMSA at admission and discharge." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Amirabdollahian 2007
| Methods | Cross‐over RCT Method of randomisation: selecting a sealed envelope | |
| Participants | Countries: UK and Republic of Ireland Sample size: 31 participants (16 in treatment group, 15 in control group) Inclusion criteria: medically stable; first stroke; over 60 years of age; able to give informed consent; a score higher than 24 in the Short Orientation‐Memory‐Concentration Test Exclusion criteria: people with pacemakers | |
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded before and after baseline, after 3 weeks of therapy and again 3 weeks later (after each cross‐over) Fugl‐Meyer scale (0 to 66) |
|
| Notes | We planned to use Phase B data for group 1 (experimental) and Phase C data for group 2 (control) in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not exactly stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding procedure not exactly stated |
| Selective reporting (reporting bias) | High risk | One or more outcomes are reported incompletely |
Ang 2014
| Methods | RCT | |
| Participants | Country: Singapore Sample size: 21 participants (7 in treatment group brain computer interface with haptic knob device (BCI‐HK); 8 in treatment group HK; 7 in control group) Inclusion criteria: first‐ever stroke, confirmed by neuroimaging; age 21 to 80 years; time since stroke > 4 months; FMA‐score 10 to 50 points (moderate to severe arm impairment); motor power grade 2 to 5 MRC shoulder abduction, grade 2 to 5 MRC elbow flexion, and grade 1 to 3 MRC in wrist dorsiflexion and finger flexion Exclusion criteria: medical instability; postural hypotension; terminal illness; severe aphasia; inattention; hemispatial neglect; severe visual impairment; epilepsy; severe depression; psychiatric disorders; recurrent stroke; skull defect; severe spasticity; fixed joint contractures; skin lesions |
|
| Interventions | 3 groups:
|
|
| Outcomes | Outcomes were measured at baseline (week 0), at mid‐intervention (week 3), at the end of intervention period (week 6), 6 weeks' follow‐up (week 12), and 18 weeks' follow‐up (week 24) Primary outcome: total FMA score |
|
| Notes | We combined the results of both HK groups in 1 (collapsed) group and compared this collapsed group with the results of the standard arm therapy group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization block size was 3 and the allocation sequence was 1:1:1 generated using STATA software" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "As subject blinding was not feasible, all outcome assessments for this study were performed by occupational therapist DXD who was blinded to allocation." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Brokaw 2014
| Methods | Randomised cross‐over trial | |
| Participants | Country: USA Sample size: 12 participants Inclusion criteria: adult with ischaemic/haemorrhagic stroke at least 6 months before; persistent hemiparesis (score 1 to 2 on the National Institutes of Health Stroke Scale); voluntary wrist and finger extension; shoulder elevation Exclusion criteria: a score of less than 24 on the Mini‐Mental State Examination; hemispatial neglect; severe sensory loss; excessive pain in any joint of the affected hemisphere or upper extremity injury |
|
| Interventions | 2 groups:
|
|
| Outcomes | FMA ARAT BBT |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using a random number generator function in Matlab (MathWorks Inc, Natick, MA) that generated a list of numbers (1‐10) randomly ordered" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The first 5 listed subject numbers received conventional therapy first and the second set received robot therapy first." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The OT performing recruitment and clinical evaluations was not aware of the randomization order, so was blinded to group assignment." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Burgar 2011
| Methods | Prospective, single‐blinded RCT Method of randomisation: stratified random number table | |
| Participants | Country: USA Sample size: 54 participants (19 in the first treatment group, 17 in the second treatment group, and 18 in the control group) Inclusion criteria: primary diagnosis of stroke Exclusion criteria: people were excluded if they exhibited upper limb joint pain that restricted normal movement, had absent proprioception at the elbow or shoulder joints, or scored less than 22 on the Mini Mental State Examination. People with cardiovascular, orthopaedic, or neurological conditions that would have precluded exercise in short‐duration, moderate‐workload trials were also excluded |
|
| Interventions | 3 groups:
|
|
| Outcomes | Outcomes were recorded at baseline, just after completion of training (after 3 weeks), and 6 months later (follow‐up)
*The strength of 14 shoulder and elbow muscle groups was assessed by performing manual muscle testing of isolated joint actions and applying the MRC Motor Power grading scale (0 to 5) with a maximum possible score of 70 (scapular abduction/upward rotation, scapular elevation, adduction, adduction/depression, adduction/downward rotation, flexion, extension, abduction, horizontal adduction, horizontal abduction, external rotation, internal rotation, elbow flexion, elbow extension) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear, not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A second therapist at each site, blinded to group assignment, performed a clinical assessment battery just before study initiation, just after completion of training, and again at the 6‐month follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Conroy 2011
| Methods | Prospective, single‐blinded RCT Method of randomisation: choosing a sealed envelope | |
| Participants | Country: USA Sample size: 62 participants (41 in the treatment group and 21 in the control group) Inclusion criteria: diagnosis of clinically defined, unilateral hemiparetic stroke with radiologic exclusion of other possible diagnoses; onset of stroke 6 months before randomisation for ischaemic stroke, 12 months for haemorrhagic stroke; manual muscle testing of grade 3 or lower for at least 1 muscle of the affected arm; > 18 years of age Exclusion criteria: serious complicating medical illness or stroke occurring within the previous 6 months (or both); contractures or orthopaedic problems limiting the range of joint movement in the potential study arm; visual loss limiting the ability to see the test patterns on the robot monitor; Botox injection of the affected arm 3 months before study onset or during the study |
|
| Interventions | 3 groups:
|
|
| Outcomes | Outcomes were recorded 3 times at baseline and after 6 weeks and 3 months later (follow‐up)
|
|
| Notes | We combined the results of both the planar group and the planar and vertical group in 1 (collapsed) group and compared this collapsed group with the results of the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation by a computer scheme |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment was performed by a single experienced evaluator blinded to group assignment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Daly 2005
| Methods | RCT Method of randomisation: drawing of tickets from envelopes by a person not involved in or aware of the allocation process | |
| Participants | Country: USA Sample size: 13 participants (7 in treatment group, 6 in control group) Inclusion criteria: > 12 months after stroke, at least grade 1 muscle contraction in wrist extensors, and a score of > 10 on the Fugl‐Meyer upper‐limb score (0 to 66) Exclusion criteria: not stated | |
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks and 3 months later
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The investigators describe a stratified randomisation, but there is insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Author stated that a blinded examiner scored the primary outcome measure from a videotape |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Fazekas 2007
| Methods | RCT Method of randomisation: by a person not involved in the study | |
| Participants | Country: Hungary Sample size: 30 participants (15 in treatment group, 15 in control group; 22 after stroke and 8 after traumatic brain injury) Inclusion criteria: hemiparesis after stroke or traumatic brain injury Exclusion criteria: not stated | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after the 10th session and at the end of the training
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Author stated that assessment was performed by a blinded physiotherapist |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Hesse 2005
| Methods | RCT Method of randomisation: participant drew a lot out of the sealed envelope presented by an independent person | |
| Participants | Country: Germany Sample size: 44 participants (22 in treatment group, 22 in control group) Inclusion criteria: first‐time supratentorial stroke; stroke interval before study onset 4 to 8 weeks; severe arm paresis with no or only a palpable volitional activity of the wrist and finger extensors (i.e. MRC 0 or 1); an initial Fugl‐Meyer arm motor score (0 to 66) of less then 18; absent or moderate elbow, wrist, and finger spasticity; able to understand the meaning of the study; and written informed consent to participate in the approved study Exclusion criteria: apraxia (i.e. 1 fault in the tasks waving goodbye, saluting, and making a fist with the non‐affected hand after verbal instruction and demonstration, and using an eraser, comb, and screwdriver with the objects handed to the person and verbally instructed); shoulder pain insensitive to standard therapy; hand swelling sufficient to prevent fist formation; painful arthritis of the wrist or finger joints; and forearm skin ulcers | |
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and after 6 weeks and 3 months later
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was done by shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Using sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded therapist rated the videos of all participants |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
Hesse 2014
| Methods | RCT | |
| Participants | Country: Germany Sample size: 50 (25 in the experimental group and 25 in the control group) Inclusion criteria: first‐time supratentorial stroke; time since stroke more than 8 weeks; aged between 18 and 90 years; being able to get out of bed and mobilised in a wheelchair or being able to walk; Fugl‐Meyer score < 35 Exlusion criteria: severe arm spasticity; hemiparetic shoulder pain; swollen hand impeding closing the fist |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline, after 4 weeks at the end of intervention period, and at 3 months' follow‐up Primary outcome: FMA Secondary outcomes: ARAT, BBT, MRC (upper limb muscles), MAS (upper limbs), Barthel Index |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation of patients to the two groups (robot‐assisted group therapy or individual arm therapy) was conducted online by using a web‐based randomization tool (http://www.randomizer.at)." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation of patients to the two groups (robot‐assisted group therapy or individual arm therapy) was conducted online by using a web‐based randomization tool (http://www.randomizer.at)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The test was videographed with a mirror placed behind the patient to ensure later blind rating by an external experienced therapist." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Hollenstein 2011
| Methods | Cross‐over RCT A cross‐over design was used (only the first period before cross‐over was used for data analysis) Methods of randomisation: described as follows: "subjects were randomly assigned by lottery of the supervising therapist" |
|
| Participants | Country: Germany Sample size: 13 participants (7 in treatment group, 6 in control group) Inclusion criteria: first‐time stroke, affected arm and first rehabilitation Exclusion criteria: none described |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded before and after 10 treatment sessions
|
|
| Notes | This study was published in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by lottery of the supervising therapist |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information about the concealment of allocation |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Housman 2009
| Methods | RCT Methods of randomisation: participants were randomly assigned by a supervising therapist |
|
| Participants | Country: USA Sample size: 34 participants (17 in treatment group, 17 in control group) Inclusion criteria: single ischaemic or haemorrhagic stroke at least 6 months prior to participation, moderate to severe upper extremity hemiparesis (characterised by arm motor Fugl‐Meyer scores > 10 and < 30) Exclusion criteria: significant pain or shoulder instability, current enrolment in ongoing upper extremity therapy, severe cognitive dysfunction, aphasia, hemispatial neglect, or apraxia |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded before and after every treatment session and 6 months after treatment completion
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by lottery of the supervising therapist |
| Allocation concealment (selection bias) | Low risk | The treating therapist and participants were blinded to assignment until each participant had consented and was enrolled in the project |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A single‐blinded rater performed the clinical assessments |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Hsieh 2011
| Methods | Pilot RCT Methods of randomisation: by using a random‐number table, a sealed envelope was given to the therapists after a new eligible participant was registered, to deliver therapy accordingly |
|
| Participants | Country: Taiwan Sample size: 18 participants (6 in higher‐intensity robot‐assisted group, 6 in lower‐intensity robot‐assisted group, 6 in conventional rehabilitation group) Inclusion criteria: unilateral stroke onset at least 6 months prior to study; an initial upper extremity subsection of the Fugl‐Meyer Assessment score of 30 to 56, indicating moderate to mild motor impairment; no excessive spasticity in elbow and wrist finger joints of the affected upper extremity (MAS < 3); ability to follow study instructions and perform study tasks (Mini‐Mental State Examination > 24); no upper limb fracture within 3 months; no participation in any experimental rehabilitation or drug studies during the study period; and written informed consent Exclusion criteria: painful arthritis of the elbow, wrist, or finger joints; severe neuropsychologic impairments; physician‐determined major medical problems or poor physical condition that would interfere with participation; and cerebellar or brain stem lesions to limit potential interference of other symptoms or signs with task accomplishment |
|
| Interventions | 3 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment
|
|
| Notes | We combined the results of both the planar and the planar + vertical in 1 (collapsed) group and compared this collapsed group with the results of the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By random‐number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes to accordingly deliver the intervention to the registered participant |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinical measures were administered to the participants by the same blinded rater |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Hsieh 2014
| Methods | RCT Methods of randomisation: random‐number table |
|
| Participants | Country: Taiwan Sample size: 48 Inclusion criteria: at least 6 months after onset of a unilateral stroke, an initial score of the FMA arm assessment of 20 to 50 (SD 25), minimal hand function (i.e. extension of the wrist ≥ 10°, extension of at least 2 fingers > 0° and > 10°, and abduction of thumb ≥ 10°, no excessive spasticity in any of the joints of the affected arm (MAS ≥ 4), no arm fracture within 3 months or painful arthritis of the joints, and able to follow study instructions and perform study tasks (Mini‐Mental State Examination score ≥ 22) Exlusion criteria: none described |
|
| Interventions | 3 groups:
Participants in each group received 20 training sessions of 90 to 105 min/day, 5 days/week for 4 weeks. In addition to the intervention provided in the clinics, all participants were encouraged to use their affected upper limb during activities in their daily life situations (e.g. at home) |
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment after 4 weeks
|
|
| Notes | We combined the results of both the RT + CIT group and the RT group (collapsed) group and compared this collapsed group with the results of the CT group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation assignments were generated from a random‐number table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, and opaque envelopes and a blinded investigator assigned each participant to a treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Hwang 2012
| Methods | RCT Methods of randomisation: random allocation of participants to 2 groups was performed using a random‐assignments generator (Wichmann–Hill random‐number generator) |
|
| Participants | Country: Republic of Korea Sample size: 15 Inclusion criteria: ≥ 18 years old, more than 3 months after stroke, > 10° voluntary range of motion of the second metacarpophalangeal joint, a FMA arm motor scale of 2 to 20 for the wrist and hand subportion and requiring a > 25% longer time to finish the 9‐hole pegboard test with the affected arm compared with the contralateral arm. Exlusion criteria: apraxia (≤ 2 on the Alexander scale), impaired consciousness (≥ 1 for the NIH Stroke Scale question Ia–c), sensory impairment (< 75% of the contralateral score on the Nottingham Sensory Scale), increased spasticity (4 on the Ashworth scale), aphasia (≥ 2 for the NIH Stroke Scale question IX) or depression (≥ 8 on the Geriatric Depression Scale), with a combined disabling disease on the hemiparetic hand, or who refused to participate |
|
| Interventions | 2 groups:
The robot‐assisted therapy included individual finger synchronisation (Amadeo, Tyromotion, Austria) |
|
| Outcomes | Outcomes were recorded at baseline and at 2, 4, and 8 weeks after starting therapy
|
|
| Notes | We used the data from the first 2 weeks of intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation of participants into 2 groups was performed using a random‐assignments generator (Wichmann–Hill random‐number generator) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors quote: "assessor‐blinded" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Kahn 2006
| Methods | RCT Method of randomisation: not stated | |
| Participants | Country: USA Sample size: 19 participants (10 in treatment group, 9 in control group) Inclusion criteria: unilateral stroke at least 1 year previously, CMSA 3 to 5 points scale Exclusion criteria: not stated | |
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and after end of training
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | By a blinded evaluator |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Klamroth‐Marganska 2014
| Methods | RCT Methods of randomisation: computer‐generated list of random numbers was used that paired a unique sequential number with a treatment type (robotic or conventional). Pairs were sealed in tamper‐evident envelopes by the study co‐ordinator |
|
| Participants | Country: Switzerland Sample size: 77 Inclusion criteria: diagnosis of 1, first‐ever cerebrovascular accident verified by brain imaging (magnetic resonance imaging or computed tomography); chronic impairment after stroke (minimum 6 months); moderate to severe arm paresis as indicated by a score of 8 to 38 on arm section of FMA (which has a maximum of 66 points); aged ≥ 18 years; stable recovery stage; able to sit in a chair without any additional support and without leaning on the back rest; passive range of motion in the shoulder as assessed with the neutral zero method: anteversion/retroversion 80°/0°/20°, abduction/adduction 60°/0°/10°, inner and outer rotation 20°/0°/20°; passive range of motion in the elbow as assessed with the neutral zero method: flexion/extension 100°/40°/40° Exlusion criteria: excessive spasticity of the affected arm (MAS ≤ 3); serious medical or psychiatric disorder as assessed by their physician; participation in any clinical investigation within previous 4 weeks; participation in any therapeutic treatment (apart from assigned therapy) done with the paretic arm during the therapy phase of the study; anticipated need for any major surgery during the study; pregnancy or breastfeeding; orthopaedic, rheumatological, or other disease restricting movements of therapeutic arm; shoulder subluxation (palpation < 2 fingers); skin ulcerations at the paretic arm; not able to communicate effectively with the examiner such that the validity of the participant’s data could not be compromised; cyber sickness (e.g. nausea when looking at a screen or playing computer games); pacemaker or other implanted electric devices; bodyweight above 120 kg; serious cognitive defects or aphasia preventing effective use of ARMin |
|
| Interventions | 2 groups:
Therapy was given 3 times a week for a period of 8 weeks (sum of 24 sessions). Minimum session time (excluding time for preparation, diagnostics, and documentation) was 45 minutes |
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment every 2 weeks
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated list of random numbers was used, which paired a unique sequential number with a treatment type (robotic or conventional)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Pairs were sealed in tamper‐evident envelopes by the study co‐ordinator." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessors were masked to treatment allocation" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Kutner 2010
| Methods | RCT Methods of randomisation: sealed envelope method |
|
| Participants | Country: USA Sample size: 21 participants (11 in experimental group and 10 in combined therapy group) Inclusion criteria: first clinical stroke diagnosis; time since stroke between 3 and 9 months; Mini‐Mental State Examination score of > 24; being able to stand for 2 minutes; passive range of motion ≥ 45° for shoulder abduction, flexion, or external rotation and pronation of the forearm; active wrist extension ≥ 10°; active thumb extension and ≥ 10° of extension in at least 2 additional digits Exlusion criteria: not described |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline, at the end of intervention, and at 2 months postintervention Primary outcome measure: health‐related quality of life (SIS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned by the sealed envelope method" |
| Allocation concealment (selection bias) | Unclear risk | It was not described whether the sealed envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Research staff blinded to treatment assignment conducted interview‐based outcome assessments." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Liao 2011
| Methods | Prospective RCT Methods of randomisation: participants were randomly assigned to either the treatment or control group in accordance with a random‐number table, then a sealed envelope was given to the therapists to deliver therapy accordingly |
|
| Participants | Country: Taiwan Sample size: 20 participants (10 in treatment group and 10 in control group) Inclusion criteria: clinical diagnosis of the first cortical or subcortical stroke, more than 6 months poststroke, initial upper limb FMA score of 28 to 56 (0 to 66), Mini‐Mental State examination > 22, no excessive spasticity in elbow or wrist joints of the affected arm (MAS < 3) Exclusion criteria: stroke lesions in other than brain areas (cerebellum or brainstem), comorbidity with other severe neurological diseases (epilepsy), severe shoulder pain or painful arthritis of the elbow, wrist, or finger joints, unable to follow treatment instructions |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and immediately after the 4 weeks of intervention
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment in accordance with a number table to either treatment or control group |
| Allocation concealment (selection bias) | Low risk | By sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Lo 2010
| Methods | Multicentre RCT Method of randomisation: a permuted‐block design that was stratified according to site |
|
| Participants | Country: USA Sample size: 127 participants (49 in intensive robot‐assisted group, 50 in intensive comparison group, and 28 in usual‐care group) Inclusion criteria: age 18 years and older; stroke that occurred at least 6 months prior to enrolment to the study; long‐term, moderate to severe motor impairment of the upper limb (described as a score between 7 and 38 of the Fugl‐Meyer score); and written informed consent from all participants Exclusion criteria: all patients with a baseline Fugl‐Meyer score outside the required range of 7 to 38 |
|
| Interventions | 3 groups:
|
|
| Outcomes | Outcomes were recorded at baseline, then 6 and 12 weeks after randomisation, then again 6 months and 9 months after treatment completion
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment with a permuted‐block design that was according to participants stratified to 1 or the other site of intervention |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained blinded raters |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Lum 2002
| Methods | RCT Method of randomisation: list of random numbers | |
| Participants | Country: USA Sample size: 30 participants (15 in treatment group, 15 in control group) Inclusion criteria: diagnosis of a single stroke, more than 6 months post‐stroke, obvious deficit in upper‐limb motor function as a result of the stroke, had completed all formal outpatient therapy but continued with any home‐based exercise regimen or community‐based stroke programmes they were enrolled in at the time of intake into the study Exclusion criteria: upper‐extremity joint pain or range‐of‐motion limitations that would affect their ability to complete the protocols; any unstable cardiovascular, orthopaedic, or neurologic conditions; cognitive impairments if people were unable to co‐operate with the study tasks | |
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and after 4 and 8 weeks (end of training) and 8 months after baseline
|
|
| Notes | Incorporates results of Burgar 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to either group based on a list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An occupational therapist blinded to group assignment tested all participants with a battery of clinical evaluations |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Lum 2006
| Methods | RCT Method of randomisation: list of random numbers | |
| Participants | Country: USA Sample size: 30 participants (9 in the robot‐unilateral group, 10 in the robot‐bilateral group, 5 in the robot‐combined group, and 6 in the control group) Inclusion criteria: diagnosis of stroke, 1 to 5 months poststroke Exclusion criteria: any upper‐limb joint pain or range‐of‐motion limitations that would affect their ability to complete the protocols; any unstable cardiovascular, orthopaedic, or neurological conditions; cognitive impairments (scored < 21 of the Folstein Mini‐Mental State Examination) | |
| Interventions | 4 groups:
Groups 1 to 3 were collapsed to 1 robot treatment group (pooled as 1 group) in our analysis |
|
| Outcomes | Outcomes were recorded immediately before treatment started, immediately post‐treatment, and 6 months after treatment ended
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An occupational therapist blinded to group assignment tested all participants with a battery of clinical evaluations |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Masiero 2007
| Methods | RCT Method of randomisation: not stated | |
| Participants | Country: Italy Sample size: 35 participants (17 in treatment group, 18 in control group) Inclusion criteria: first, single unilateral ischaemic stroke using the World Health Organization definition of stroke Exclusion criteria: neurologic or cardiovascular instability contraindicating exercise (e.g. uncontrolled hypertension), early severe spasticity, multiple cerebrovascular lesions, severe neuropsychologic impairment (global aphasia, severe attention deficit or neglect), not able to follow instructions | |
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and after 1.5, 3, and 8 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were performed for all participants by the same blinded clinician |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Masiero 2011
| Methods | RCT Method of randomisation: sequence of computer‐generated random numbers |
|
| Participants | Country: Italy Sample size: 21 participants (11 in treatment group, 10 in control group) Inclusion criteria: (1) diagnosis of recent single‐sided stroke (ischaemic or haemorrhagic) demonstrated by brain computerised axial tomography or nuclear magnetic resonance, (2) sufficient cognitive and language capacities to understand the operator’s instructions (Modified Mini‐Mental State Examination score > 18), (3) paralysis or paresis (Motor Power score between 8 and 12) with no ability for active movement against gravity or weak resistance Exclusion criteria: (1) cardiovascular instability (severe uncontrolled hypertension, severe coronary artery disease, etc.) or orthopaedic or neurological conditions, (2) multiple cerebrovascular lesions, (3) > 3 points Ashworth Scale, (4) upper‐limb joint pain or limitations to range of motion that would have affected the participant’s ability to complete the protocols, (5) severe neuropsychological impairment (global aphasia, severe attention deficit, or severe space inattention), (6) age > 85 years or < 18 years |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline, 5 weeks after treatment onset, and after 3‐month follow‐up:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved with use of a sequence of computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participant assessments were performed by the same blinded clinician, who had previously attended a training course qualifying him or her to use the scales, was not directly involved in the delivery of either robot‐aided or standard rehabilitation therapy within the study, and did not know which participants had been enrolled in the EG and the CG |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Mayr 2008
| Methods | Cross‐over RCT Method of randomisation: not stated |
|
| Participants | Country: Austria Sample size: 8 (4 in treatment group, 4 in control group) Inclusion criteria: < 3 months poststroke with severe to moderate upper‐limb paresis, sufficient communication abilities to complete the study, and written informed consent Exclusion criteria: painful arthritis of the wrist and finger or physician‐determined major medical problems |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and then after each cross‐over (after 2, 4, and 6 weeks since baseline)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | High risk | No concealment of allocation was provided (information provided by the investigator) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded (information provided by the investigator) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
McCabe 2015
| Methods | RCT Method of randomisation: not described |
|
| Participants | Country: USA Sample size: 39 participants (12 in the experimental and 27 in the control group) Inclusion criteria: single unilateral stroke; > 1 year upper extremity impairment; a trace muscle contraction in the wrist extensors; mobility and function sufficient for independent performance of activities; stable medical condition; not other neurologic condition; ability to follow 2‐step commands; informed consent Exlusion criteria: not explicitly stated |
|
| Interventions | 3 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment every 2 weeks Primary outcome: AMAT Secondary outcomes: AMAT subscale wrist/hand; AMAT subscale shoulder/elbow; FMA (shoulder/elbow and wrist/hand subscales); AMAT (function scale) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "There was 1 assessor, who was blinded to the group assignment of the subject." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Rabadi 2008
| Methods | RCT Method of randomisation: sealed, opaque envelopes |
|
| Participants | Country: USA Sample size: 30 participants (10 in experimental group and 20 in control groups) Inclusion criteria: First acute stroke; time since stroke < 4 weeks; admission to an inpatient rehabilitation facility; arm weakness as defined by Medical Research Council grade < 2 in the shoulder joint; informed consent Exlusion criteria: Anterior or severe inferior shoulder subluxation (≥ 3 cm) of the affected arm; shoulder pain on passive range of 60° forward flexion and 60° abduction of the weak arm; trophic skin changes and significant oedema (shoulder‐hand syndrome); prior rotator cuff surgery; bursitis or biceps tendonitis; recent cardiac event; medications enhancing motor recovery such as Botox or d‐amphetamine |
|
| Interventions | 3 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and at discharge Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients who consented were randomized by sealed, opaque envelopes in blocks of six (two patients in each group) at a time." |
| Allocation concealment (selection bias) | Low risk | Quote: "Envelopes were identical for the three groups of patients. These sealed envelopes were kept in a locked place. Participants were assigned to one of the three groups by a designated nurse on the unit not associated with the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome measures were recorded at baseline and on discharge by an evaluator (LD) blinded to treatment allocation." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Sale 2014
| Methods | RCT Method of randomisation: Lehmer's algorithm |
|
| Participants | Country: Italy Sample size: 53 Inclusion criteria: subacute first‐ever stroke, unilateral paresis, ability to understand and follow simple instructions, ability to remain in a sitting posture Exlusion criteria: bilateral impairment, severe sensory deficits in the paretic upper limb, cognitive impairment or behavioural dysfunction that would influence the ability to comprehend or perform the experiment, refusal or inability to provide informed consent, other current severe medical problems |
|
| Interventions | 2 groups:
Experimental and control therapies were applied in addition to usual rehabilitation |
|
| Outcomes | Outcomes were recorded at baseline, after 3 weeks and post‐treatment after 6 weeks
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A Lehemer algorithm was applied to achieve a balanced allocation in the EG and CG groups. Therapists were randomly assigned to patients within each group using the same algorithm." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The random allocation to treatment was concealed and based upon dedicated software." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinical assessments were carried out by blinded assessors..." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Susanto 2015
| Methods | RCT Method of randomisation: random‐number generator |
|
| Participants | Country: China Sample size: 19 Inclusion criteria: primary stroke 6 to 24 months prior to the beginning of the intervention, moderate stroke condition (50 > FMA score > 20), ability to understand simple commands (Mini‐Mental State Examination score > 21), and ability to differentiate sensation on 1 finger from the other fingers Exclusion criteria: recurrent stroke; other neurological, neuromuscular, or orthopaedic disease; or shoulder or arm contracture/pain |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline (within 2 weeks from the first training session, within 1 week from the first session), within 3 days after the last session, and at 6 months' follow‐up
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "single‐blinded so the assessors were of no knowledge of the grouping." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Timmermans 2014
| Methods | RCT Method of randomisation: blocked randomisation, using opaque envelopes |
|
| Participants | Country: the Netherlands Sample size: 22 Inclusion criteria: first‐ever stroke, age between 18 and 85 years, clinically diagnosed with a central paresis of the arm/hand (strength: MRC grade 2 to 4 at entry into study), poststroke time ≥ 12 months, fair to good cognitive level (Mini‐Mental State Examination score ≥ 26), able to read and understand the Dutch language, unable to fully perform at least 2 of the following skills: drinking from a cup, eating with knife and fork, taking money from a purse and using a tray, motivated to train at least 2 of the above‐mentioned skills. (At the start of the last 6 months of the inclusion period, inclusion criteria were adjusted to poststroke time ≥ 8 months, to facilitate participant inclusion) Exclusion criteria: severe neglect (Bell Test, Letter Cancellation Test: minimum omission score of 15%), hemianopsia, severe spasticity (MAS total arm > 3, severe additional neurological, orthopaedic, or rheumatoid impairments prior to stroke that could interfere with task performance, Broca's aphasia, Wernicke's aphasia, global aphasia (determined by the Akense Afasie Test), apraxia (apraxia test of Van Heugten), and attending another study or therapy to improve arm‐hand function |
|
| Interventions | 2 groups:
Training was provided during 8 weeks, 4 times/week, twice a day for 30 minutes (separated by 0.5 hour to 1 hour of rest) |
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment every 2 weeks
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly allocated to ... using blocked randomization (block size = 2). The randomization procedure was performed by an independent researcher using 2 opaque envelopes with in each envelope a training condition code." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization procedure was performed by an independent researcher using 2 opaque envelopes within each envelope a training condition code." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Persons involved in data collection were blinded for group allocation." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Volpe 2000
| Methods | RCT Method of randomisation: not stated | |
| Participants | Country: USA Sample size: 56 participants (30 in treatment group, 26 in control group) Ambulatory at study onset Inclusion criteria: first, single stroke, hemiparesis or hemiplegia of the upper and lower extremity, to be able to follow simple instructions, written informed consent Exclusion criteria: not stated | |
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded before and after end of treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants and the medical and rehabilitation team providing the clinical care were "masked" to the group assignment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Volpe 2008
| Methods | RCT Method of randomisation: not stated | |
| Participants | Country: USA Sample size: 21 participants (11 in treatment group, 10 in control group) Inclusion criteria: people after stroke with impaired arm and hand mobility for at least 6 months Exclusion criteria: not able to follow simple instructions, minimally impaired (Fugl‐Meyer shoulder‐elbow section > 33 points), neurosurgical procedure, second stroke, fixed contracture | |
| Interventions | 2 groups:
All participants had an identical number of treatment sessions, and the sessions were of the same duration (1 hour per session, 3 times a week for 6 weeks) |
|
| Outcomes | Outcomes were recorded at 3 preliminary evaluations (Pre1, Pre2, Pre3), at midpoint, at discharge, and at 3‐month follow‐up
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the concealment of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment was done |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Wu 2012
| Methods | RCT Method of randomisation: not described |
|
| Participants | Country: Taiwan Sample size: 42 Inclusion criteria: unilateral stroke at least 6 months previously, mild to moderate motor impairment (total score of 26 to 66 on the upper extremity part of the FMA, no severe spasticity in the paretic arm (MAS score of 2 in any joint), no serious cognitive deficits (Mini‐Mental State Examination score of 22), no other neurologic, neuromuscular, or orthopaedic disease and no participation within the previous 3 months in any experimental rehabilitation or drug studies Exlusion criteria: none described |
|
| Interventions | 3 groups:
Each group received treatment for 90 to 105 minutes per session, 5 sessions on weekdays, for 4 weeks |
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment after 4 weeks
|
|
| Notes | We combined the results of both the TBAT and the CT Group (collapsed) group and compared this collapsed group with the results of the RBAT group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Exact method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation to group was concealed from the investigators" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the participants were blinded to the study hypotheses." and "Clinical outcome measures were administered ... by ... therapists blinded to the participant group." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
Yoo 2013
| Methods | RCT Method of randomisation: not clearly described |
|
| Participants | Country: South Korea Sample size: 22 Inclusion criteria: no visual neglect or impaired cognitive function (Mini‐Mental State Examination score > 24 points), written informed consent Exlusion criteria: none |
|
| Interventions | 2 groups:
|
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment after 6 weeks
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to..." The method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients and investigators were blind to the test results and intervention grouping because this study used a double‐blinded design." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
ADL: activities of daily living AMAT: Arm Motor Ability Test ARAT: Action Research Arm Test BBT: Box and Block Test CG: control group CMSA: Chedoke‐McMaster Stroke Assessment EG: experimental group EMG: electromyography FIM: Functional Independence Measure FMA: Fugl‐Meyer Assessment MAL: Motor Activity Log MAS: Modified Ashworth Scale MIME: mirror image motion enabler MRC: Medical Research Council NIH: National Institutes of Health RCT: randomised controlled trial RT: robot training SD: standard deviation SIS: Stroke Impact Scale WMFT: Wolf Motor Function Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdollahi 2014 | Compared 2 different approaches of robotic training |
| Aisen 1997 | Not an RCT; participants were allocated by stratification not by randomisation; inclusion criteria not fulfilled |
| Dodakian 2013 | Not an RCT; participants were allocated using an approach that kept age and baseline motor deficits matched across the 2 groups |
| Fasoli 2003 | All included participants received a kind of robotic therapy |
| Fluet 2012 | Irrelevant comparison: 2 different approaches of robotic training tested |
| Hesse 2007 | All participants received the same robotic therapy |
| Hill 2011 | Not an electromechanical‐assistive device; used functional electrical stimulation only |
| Hu 2015 | Irrelevant comparison: 2 different approaches of robotic training tested |
| Jackson 2013 | Not an RCT; description of a project/plan for RCT |
| Krebs 2000 | Not an RCT |
| Luft 2004 | Inclusion criteria of robot‐aided or electromechanical‐assisted technology not fulfilled; device used is a mechanical device without robot aid and without an electromechanical‐assisted technology |
| Lum 2004a | Not an RCT |
| Lum 2004b | This trial was excluded after correspondence with the study authors because it overlaps with another trial included in the analysis |
| Page 2012 | Not an electromechanical‐assistive device; used functional electrical stimulation only |
| Prange 2015 | Not an electromechanical‐assistive device; used sling support and feedback only |
| Reinkensmeyer 2000a | Not an RCT |
| Takahashi 2008 | No strict randomisation process; inclusion criteria not fulfilled |
| Takebayashi 2013 | Not a genuine RCT |
| Thorsen 2013 | Investigated myoelectrically controlled functional electrical stimulation |
| Tropea 2013 | Irrelevant comparison: 2 different approaches of robotic training were tested |
| Volpe 1999 | Not an RCT |
| Whitall 2000 | Inclusion criteria of robot‐aided or electromechanical‐assisted technology not fulfilled; device used is a mechanical device without robot aid and without an electromechanical‐assisted technology |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
AIAS
| Methods | RCT |
| Participants | Country: UK Participants: estimated enrolment n = 18 Inclusion criteria: age above 18 years; clinical diagnosis of stroke; Medical Research Council Scale for arm impairment between 1 and 4; medically stable; informed consent; ability to understand and follow simple instructions; sitting balance sufficient to use Armeo arm orthosis safely Exclusion criteria: orthosis cannot be fitted to affected limb; bone instability of hemiparetic upper limb; pre‐existing upper limb deficits; pronounced, fixed contractures of hemiparetic upper limb; shoulder instability or excessive pain; severe spasticity; severe spontaneous movements; confused; non‐co‐operative; requiring isolation due to infection; severe visual, perceptual, or cognitive problems precluding participation in study protocol; involved in any other intervention study |
| Interventions | Experimental group 1: standard care + ArmeoSpring arm orthosis intervention for 60 minutes per day, 5 days a week for 2 weeks or discharge (whichever is sooner) Experimental group 2: standard care + ArmeoSpring arm orthosis intervention for 40 minutes per day, 3 days a week for 2 weeks or discharge (whichever is sooner) Control group (active): standard care, provided by physiotherapists or occupational therapists |
| Outcomes | Outcomes will be collected at the end of intervention period and at 3‐month follow‐up Primary outcome measure: feasibility of experimental intervention (number of per‐protocol interventions recorded at the end of intervention period; reasons for non‐compliance recorded at end of intervention period); acceptability/satisfaction of experimental intervention: informal interviews with participants completed at the end of intervention period Secondary outcome measures: arm pain (5‐point scale); shoulder subluxation (clinical report); fatigue (Borg Scale of Perceived Exertion); all adverse events; ARAT; FMA; disability (Barthel Index) |
| Notes |
Chisari 2014
| Methods | RCT |
| Participants | Country: Italy Sample size: 18 Inclusion criteria: chronic stroke Exclusion criteria: not described |
| Interventions | 2 groups:
The treatments were matched in terms of intensity, duration, and tasks |
| Outcomes |
|
| Notes |
Faran 2008
| Methods | RCT |
| Participants | Countries: USA, Germany 20 participants between 3 weeks and 3 months poststroke |
| Interventions | 2 groups, 20 sessions of either Reo‐Therapy system (Motorika USA Inc, NJ) or air splint therapy |
| Outcomes |
|
| Notes |
| Methods | RCT |
| Participants | Country: USA Inclusion criteria: 3 to 12 months post‐stroke; able to extend wrist and fingers at least 10°; functional hearing and vision; able to follow instructions; lives at home, not institution; stable medications for 3 months Exclusion criteria: excessive cognitive impairments; taking/receiving medicines/shots to make arm/hand less stiff; severe pain in the impaired arm; stroke was more than 12 months ago |
| Interventions | Experimental group: electromechanical‐assisted hand therapy at home for 6 weeks (device: Hand Mentor) Control group: not described |
| Outcomes | Primary outcome: WMFT Secondary outcomes: compliance with recommended use, FMA, SIS |
| Notes | Estimated enrolment: 70 participants |
Reinkensmeyer 2012
| Methods | RCT |
| Participants | Country: USA Sample size: 27 Inclusion criteria: single ischaemic or haemorrhagic stroke; time since stroke at least 3 months; upper extremity FMA between 10 to 35 out of 66; written informed consent Exclusion criteria: significant pain; instability or subluxation of the affected shoulder; cognitive dysfunction interfering with the study tasks; visual deficits; severe neglect or apraxia; current other upper extremity therapy |
| Interventions | Experimental group: 24 x 1‐hour treatment sessions with the Pneu‐WREX device, 3 times a week for 8 to 9 weeks Control group (active): conventional exercises typical of home exercise programs, including self range‐of‐motion stretches, active range‐of‐motion strengthening exercises, and ADL tasks plus 30 minutes training on the Pneu‐WREX per week |
| Outcomes | Outcomes were collected at baseline, at the end of intervention phase, and at 3‐month follow‐up Primary outcome measures: FMA Other outcome measures: Rancho Functional Test for the Hemiplegic/Paretic Upper Extremity; MAL; BBT; Nottingham Sensory Assessment |
| Notes |
ADL: activities of daily living ARAT: Action Research Arm Test BBT: Box and Block Test FMA: Fugl‐Meyer Assessment MAL: Motor Activity Log RCT: randomised controlled trial SIS: Stroke Impact Scale WMFT: Wolf Motor Function Test
Characteristics of ongoing studies [ordered by study ID]
Krebs 2007
| Trial name or title | Robot‐aided neurorehabilitation: a robot for wrist rehabilitation |
| Methods | RCT |
| Participants | Country: USA Inclusion criteria: first, single focal unilateral lesion with diagnosis verified by brain imaging (MRI or CT scans) that occurred at least 6 months prior; cognitive function sufficient to understand the experiments and follow instructions (Mini‐Mental State score of 22 or higher or interview for aphasic participants), Motor Power Score 1/5 and 4/5 (neither hemiplegic nor fully recovered motor function in the muscles of the shoulder and elbow and wrist), never experienced robot‐assisted therapy, given informed written consent to participate in the study Exclusion criteria: fixed contraction deformity in the affected limb |
| Interventions | 4 groups:
|
| Outcomes | Primary outcomes: FMA (shoulder/elbow and wrist/hand subsections); motor power |
| Starting date | Not described |
| Contact information | Principal Investigator: Hermano Igo Krebs, PhD, Principal Research Scientist & Lecturer, Massachusetts Institute of Technology, Mechanical Engineering Department, 77 Massachusetts Ave, 3‐137 Cambridge, MA 02139 USA, Tel: +1 617 253 8112, Fax: +1 617 258 7018, hikrebs@mit.edu |
| Notes | Estimated enrolment: 160 participants |
Linder 2013
| Trial name or title | The home stroke rehabilitation and monitoring system trial |
| Methods | RCT Method of randomisation: adaptive, stratified, computer‐driven minimisation procedure |
| Participants | Country: USA Sample size: 96 Inclusion criteria: age ≥ 18, unilateral ischaemic or haemorrhagic stroke within 6 months confirmed with neuroimaging, persistent hemiparesis with UE voluntary activity as indicated by a score of 11–55 on the FMA, limited access to an organised stroke rehabilitation programme, preserved cognitive function as indicated by a score of ≤ 3 on the Short Portable Mental Status Questionnaire, and no UE injury or condition that limited function of the more‐affected side before the stroke Exlusion criteria: inability to provide informed consent, not independent before the stroke as determined by a score of > 1 on the Modified Rankin Scale, sensory loss ≥ 2 on the sensory item of the NIHSS, hemispatial neglect as determined by asymmetry > 3 errors on the Star Cancellation Test, spastic hypertonus of hemiparetic hand or wrist musculature ≥ 3 on MAS, Botox injection in hemiparetic upper extremity within 6 months of enrolment, and life expectancy ≤ 1 year |
| Interventions | 2 groups:
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment every 2 weeks
|
| Starting date | Not described |
| Contact information | Jay L Alberts: albertj@ccf.org |
| Notes |
| Trial name or title | Robots for stroke survivors |
| Methods | RCT |
| Participants | Country: USA Inclusion criteria: 1 year poststroke and difficulties with picking up small objects Exclusion criteria: not described |
| Interventions | Not described |
| Outcomes | Primary outcomes: not described |
| Starting date | Not described |
| Contact information | Bambi Brewer, Carnegie Mellon University, Pittsburgh, Pennsylvania, USA, Tel: +1 412‐241‐9423, bambi@andrew.cmu.edu Study chairs or principal investigators:
|
| Notes | Assessed on 27 May 2015 |
| Trial name or title | Pilot study ‐ Comparison of upper body ergometer versus robot in upper extremity motor recovery post‐stroke |
| Methods | RCT |
| Participants | Country: USA Participants: estimated enrolment n = 30 Inclusion criteria: age between 19 and 90 years; stroke in the last 4 weeks; UE plegia (MRC grade ≤ 2 at the shoulder joint); written informed consent; being able to follow simple directions Exclusion criteria: anterior or severe inferior shoulder subluxation (≥ 3 cms) of the plegic arm; no shoulder pain on passive range of 75° forward flexion and 75° abduction of the plegic arm; trophic skin changes and significant oedema; prior rotator cuff surgery; people with bursitis or biceps tendonitis, or both; recent cardiac events |
| Interventions | Experimental group: unilateral arm training with a robot Control group: bilateral arm training with upper body ergometer |
| Outcomes | Not described |
| Starting date | |
| Contact information | |
| Notes | This study has been completed. No study results posted |
| Trial name or title | The effect of proximal and distal training on stroke recovery |
| Methods | RCT |
| Participants | Country: USA Estimated enrolment: 160 participants Inclusion criteria: never experienced robot‐assisted therapy; first, single focal unilateral lesion with diagnosis verified by brain imaging (MRI or CT scans) that occurred at least 6 months prior; cognitive function sufficient to understand the experiments and follow instructions (Mini‐Mental State score of 22 and higher or interview for aphasic participants); average Motor Power score ≥ 1/5 or ≤ 3/5 (neither hemiplegic nor fully recovered motor function in 6 muscles of the shoulder, elbow, and wrist); informed written consent to participate in the study Exclusion criteria: fixed contraction deformity in the affected limb |
| Interventions | Robotic arm training; no further description |
| Outcomes | Primary outcome measures: FMA, Motor Power Secondary outcomes: WMFT, SIS |
| Starting date | June 2004 |
| Contact information | Principal Investigator: Hermano Igo Krebs, PhD, Principal Research Scientist & Lecturer, Massachusetts Institute of Technology, Mechanical Engineering Department, 77 Massachusetts Ave, 3‐137 Cambridge, MA 02139, USA, Tel: +1 617 253 8112, Fax: +1 617 258 7018, hikrebs@mit.edu |
| Notes |
| Trial name or title | Effectiveness of adding robotic therapy to conventional therapy for acute stroke patients with upper extremity paresis |
| Methods | RCT |
| Participants | Country: USA Participants: estimated enrolment n = 40 Inclusion criteria: age between 65 and 84 years; right‐hemispheric unilateral ischaemic stroke; time since stroke < 15 days; arm weakness; right‐handedness; MRC grade ≥ 2; being able to follow 2‐3 step commands; head, neck, and trunk control; maintain upright posture for at least 45 minutes; some synergistic movements at shoulder flexion or abduction > 30°; ≥ 45° elbow flexion Exclusion criteria: previous stroke; haemorrhagic, cerebellar stroke or subarachnoid haemorrhage; contractures in the involved upper extremity; moderate to severe muscle tone in the involved upper extremity; full, active isolated movement of the involved upper extremity; corrected visual acuity worse than 20/50 for distance; cognitive or other deficits that would negatively affect their ability to follow directions or track visual targets; unstable cardiovascular, orthopaedic, or neurological conditions that would preclude exercise in short‐duration, high‐workload trials |
| Interventions | Experimental group: ReoGo robotic arm trainer additional to conventional therapy Control group: conventional therapy |
| Outcomes | Outcomes will be collected at baseline and at study end Primary outcomes: FMA Secondary outcomes: EMG ‐ muscle activation and co‐contraction index |
| Starting date | September 2008 |
| Contact information | Lauren McDonagh, PT; lmcdonagh@KESSLER‐REHAB.com Christine Post, OT; CHPost@selectmedicalcorp.com |
| Notes |
| Trial name or title | fMRI and robot‐assisted practice of activities of daily living |
| Methods | RCT |
| Participants | Country: USA Participants: estimated enrolment n = 61 Inclusion criteria: age between 30 to 85 years; right‐handedness; unilateral ischaemic stroke in the motor control area with resulting hemiparesis in the arm; time since stroke at least 6 months; residual movement of at least 15° shoulder flexion or adduction and 15° active elbow flexion and extension; no claustrophobia; not depressed; passes the fMRI scanner; being able to understand the instructions and complete the tracking tasks; no history of neurological disorders Exclusion criteria: brainstem stroke; spasticity > 3 at elbow or fingers on Ashworth Scale; visuospatial, language, or attention deficits of a severity that prevents understanding of the task; shoulder pain or joint pain during movements; decline to participate; will not comply with full protocol; pregnant; allergic to Gore‐Tex and conductivity gel |
| Interventions | Experimental group: robot therapy with ADLs 3 times a week for 4 weeks Control group: occupational therapy 3 times a week for 4 weeks |
| Outcomes | Outcomes will be collected at baseline, at the end of study, and at follow‐up Primary outcomes:
|
| Starting date | November 2008 |
| Contact information | Michel Torbey, MD; Medical College of Wisconsin |
| Notes | This study has been completed. No study results posted |
| Trial name or title | Rehabilitation robot for upper limbs, component project 5: effect on shoulder training using rehabilitation robot for stroke patients |
| Methods | RCT |
| Participants | Country: Taiwan Participants: estimated enrolment n = 12 Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | 2 groups:
|
| Outcomes | Outcomes will be measured at 1‐year follow‐up Primary outcomes:
|
| Starting date | January 2010 |
| Contact information | Wen‐Shiang Chen, MD, PhD; wenshiang@gmail.com |
| Notes |
| Trial name or title | Evaluation of robot assisted neuro‐rehabilitation (SRT3) |
| Methods | RCT |
| Participants | Country: USA Participants: estimated enrolment n = 75 Inclusion criteria: age over 21 years; clinically defined unilateral hemiparetic stroke (radiologically confirmed); adequate language and cognitive function to participate in training, testing, and informed consent; FMA score with a range of 7 to 38 in the study arm; stroke onset at least 6 months for ischaemic and at least 1 year for haemorrhagic stroke Exclusion criteria: seizures or treatment with anticonvulsants in the past 10 years (for transcranial magnetic stimulation testing); any medication known to interfere with brain stimulation; serious complicating medical conditions, contractures, or orthopaedic problems in the study arm limiting the range of motion for study positions; serious visual loss; Botox injection 3 months prior to enrolment; any change in the exercise regimen involving the study arm |
| Interventions | Experimental group: 12 weeks of robot therapy consisting of a progression through 3 robot modules: wrist, planar, and alternating wrist and planar robot. The progression will be sequential, with 4 weeks of training on each robotic device Control group: 12 weeks of task‐specific practise of functional activities using the hemiparetic arm |
| Outcomes | Outcomes will be collected at baseline and at the end of study Primary outcomes: FMA Secondary outcomes: motor cortex excitability via transcranial magnetic stimulation |
| Starting date | April 2011 |
| Contact information | Christopher Bever, MD; Baltimore VA Medical Center VA Maryland Health Care System, Baltimore, MD |
| Notes |
| Trial name or title | Robotic therapy early after stroke events |
| Methods | RCT |
| Participants | Country: UK Participants: estimated enrolment n = 80 Inclusion criteria: age above 18 years; confirmed diagnosis of stroke; randomisation by 7 days; upper limb impairment (FMA score < 50 at randomisation); being able to comply with requirements of the protocol Exclusion criteria: other significant upper limb impairment; diagnosis likely to interfere with rehabilitation or outcome assessments; participation in other stroke rehabilitation trial |
| Interventions | Experimental group: robotic therapy using InMotion device plus standard care for up to 12 1‐hourly sessions Control group: rehabilitation therapy according to local guidelines |
| Outcomes | Primary outcomes will be collected at 1‐month follow‐up, and secondary outcomes will be collected at 3‐month follow‐up Primary outcomes:
Secondary outcomes:
|
| Starting date | March 2012 |
| Contact information | Jesse Dawson, MD; jesse.dawson@glasgow.ac.uk |
| Notes |
| Trial name or title | Randomized trial of robotic rehabilitation, mirror therapy, and dose‐matched control intervention for upper‐limb rehabilitation in patients with chronic stroke: comparative efficacy and clinimetric study |
| Methods | RCT with factorial assignment |
| Participants | Country: Taiwan Participants: estimated enrolment n = 100 Inclusion criteria: unilateral stroke; onset more than 6 months; written informed consent; initial scores on the upper extremity FMA score of 25 to 56 or 18 to 50; Mini‐Mental State Examination ≥ 24 points; no upper limb fracture in the last 3 months Exclusion criteria: recurrent stroke or seizures during the intervention; serious or continuous pain on affected upper extremity; history of other neurological disease or severe orthopaedic condition |
| Interventions | Experimental group 1: robotic rehabilitation combined functional electrical stimulation (5 to 10 minutes of warm‐up, 1 hour of robotic rehabilitation with combined functional electrical stimulation, and 15 to 20 minutes of functional‐activities training 5 days a week for 4 weeks) Experimental group 2: mirror therapy (1 hour mirror therapy and 0.5 hour functional training per day, 5 days a week for 4 weeks); focuses on symmetrical bimanual movements and simultaneously observing the mirror visual feedback reflected by the unaffected upper extremity Experimental group 3: robotic rehabilitation (5 to 10 minutes of warm‐up, 1 hour of robotic rehabilitation, and 15 to 20 minutes of functional‐activities training 5 days a week for 4 weeks) Control group 1 (active): conventional rehabilitation (participants in this group received a structured protocol based on occupational therapy such as neurodevelopmental techniques and task‐oriented approach for 1.5 hours per day, 5 days a week for 4 weeks) Control group 2 (placebo): like experimental group 1 but without any electrical current applied for 1.5 hours per day, 5 days a week for 4 weeks |
| Outcomes | Outcomes will be collected at baseline and at 4, 8, 16, and 28 weeks Primary outcomes:
Secondary outcomes:
|
| Starting date | August 2011 |
| Contact information | Keh‐chung Lin, ScD; kehchunglin@ntu.edu.tw |
| Notes |
| Trial name or title | Effects and mechanisms of intensive robot‐assisted therapy in patients with subacute stroke: outcomes in brain/movement reorganization, sensorimotor and daily functions, and physiological markers |
| Methods | RCT |
| Participants | Country: Taiwan Participants: estimated enrolment n = 90 Inclusion criteria: age between 20 and 75 years; first‐ever unilateral stroke; time since stroke < 3 months; initial motor part of upper limb FMA score ranging from 10 to 40; Mini‐Mental State Examination score > 23) Exclusion criteria: pregnant or breastfeeding; aphasia interfering with understanding of instructions; major health problems or poor physical condition; current participation in other research; contraindications to fMRI |
| Interventions | Experimental group 1 (higher‐intensity robotic training group; 1200 to 1800 repetitions during robot‐assisted functional rehabilitation with the Bi‐Manu‐Track device): 90 to 120 minutes per day for 5 days a week for 4 consecutive weeks Experimental group 2 (lower‐intensity robotic training group; 600 to 900 repetitions during robot‐assisted functional rehabilitation with the Bi‐Manu‐Track device): 90 to 120 minutes per day for 5 days a week for 4 consecutive weeks Control group (active): Neurodevelopmental techniques with emphasis on functional tasks |
| Outcomes | Outcomes will be measured at baseline and at the end of study Primary outcomes:
|
| Starting date | January 2013 |
| Contact information | Ching‐Yi Wu, ScD; cywu@mail.cgu.edu.tw |
| Notes |
| Trial name or title | Comparative efficacy research of robot‐assisted therapy with and without constraint‐induced therapy in stroke rehabilitation: does the combined therapy improve outcomes compared with monotherapy? |
| Methods | RCT with factorial assignment |
| Participants | Country: Taiwan Participants: estimated enrolment n = 80 Inclusion criteria: aged between 20 to 80 years; unilateral first‐ever stroke; 6 months from onset; initial upper extremity FMA score of 20 to 56; minimal motor criteria to receive constraint‐induced therapy (i.e. ≥ 100 wrist extension and ≥ 100 extension at the thumb and any other 2 digits); MAS ≤ 3 of the affected upper extremity; no upper limb fracture within the last 3 months; Mini‐Mental State Examination ≥ 24 points; written informed consent Exclusion criteria: major medical problems or poor physical condition that would interfere with participation; excessive pain in any joint that might limit participation |
| Interventions | Experimental group 1: distributed constraint‐induced therapy (placement of the hand in a mitt for 6 hours/day and intensive training of the affected upper limb in functional tasks for 1.5 hours/weekday over 4 weeks) Control group (active): dose‐matched control therapy for 1.5 hours/weekday over 4 weeks Experimental group 2: robot‐assisted therapy (ArmeoSpring) for 1.5 hours/weekday over 4 weeks Experimental group 3: robot‐assisted therapy (ArmeoSpring) for 1.5 hours/weekday over 2 weeks plus distributed constraint‐induced therapy for 1.5 hours/weekday over 2 weeks |
| Outcomes | Outcomes will be collected at baseline and at 2 and 4 weeks Primary outcomes:
Secondary outcomes:
|
| Starting date | August 2013 |
| Contact information | Keh‐chung Lin, ScD; School of Occupational Therapy, College of Medicine, National Taiwan University, Taiwan Yi‐shiung Horng, PhD; Buddhist Tzu Chi General Hospital Taipei Branch |
| Notes |
| Trial name or title | Efficacy of unilateral versus bilateral approach to robot‐assisted rehabilitation on motor control/performance, daily functions, and physiological responses in patients with subacute stroke |
| Methods | RCT |
| Participants | Country: Taiwan Participants: estimated enrolment n = 84 Inclusion criteria: first stroke; time since stroke less than 6 months and more than 2 weeks; initial motor impairment between 24 to 52 points on the upper extremity FMA; Mini‐Mental State Examination ≥ 24 points Exclusion criteria: aphasia that might limit ability to understand instructions; chronic inflammatory, autoimmune, or haematological disorders; intake of anti‐inflammatory drugs; major health problems or poor physical condition that might interfere with participation; current enrolment in other research |
| Interventions | Experimental group 1: robot‐assisted therapy with InMotion3 for 90 minutes per day, 5 days a week for 4 weeks Experimental group 2: robot‐assisted therapy with Bi‐Manu‐Track for 90 minutes per day, 5 days a week for 4 weeks Control group (active): control intervention for 90 minutes per day, 5 days a week for 4 weeks |
| Outcomes | Outcomes will be collected at baseline, at study end, and at 6‐month follow‐up Primary outcomes:
Secondary outcomes:
|
| Starting date | August 2013 |
| Contact information | Ching‐Yi Wu, ScD; cywu@mail.cgu.edu.tw Chia‐Ling Chen, PhD, MD; clingchen@gmail.com |
| Notes |
| Trial name or title | Interactive intention‐driven upper‐limb training robotic system |
| Methods | RCT |
| Participants | Country: China Participants: estimated enrolment n = 70 Inclusion criteria: age above 18 years; pure unilateral motor paresis after ischaemic or haemorrhagic stroke; sufficient cognition to understand instructions; being able to sit upright for 1 hour Exclusion criteria: excessive spasticity of the affected arm; involvement in any other therapy |
| Interventions | Experimental group 1: hand robotic training for 20 1‐hourly sessions, 3 to 5 times per week Experimental group 2: hand and arm robotic training for 20 1‐hourly sessions, 3 to 5 times per week Control therapy (active): conventional therapy for 20 1‐hourly sessions, 3 to 5 times per week |
| Outcomes | Outcomes will be measured at baseline, at the end of study, and at 3‐ and 6‐months follow‐up Primary outcomes:
Secondary outcomes:
|
| Starting date | January 2014 |
| Contact information | Raymond KY Tong, PhD; k.y.tong@polyu.edu.hk |
| Notes |
| Trial name or title | Efficacy study of an interactive robot for the rehabilitation of the upper limb in acute stroke patients |
| Methods | RCT |
| Participants | Country: Belgium Inclusion criteria:
Exclusion criteria:
|
| Interventions | 2 groups:
|
| Outcomes | Outcomes were recorded at baseline and post‐treatment every 2 weeks
|
| Starting date | Not described |
| Contact information | Thierry Lejeune, Professor: thierry.lejeune@uclouvain.be |
| Notes |
| Trial name or title | Neurocognitive robot‐assisted rehabilitation of hand function after stroke |
| Methods | RCT |
| Participants | Country: Switzerland Participants: estimated enrolment n = 20 Inclusion criteria: aged between 18 and 90 years; first stroke with resulting hemiparesis; time since stroke less than 6 weeks Exclusion criteria: insufficient state of consciousness; severe aphasia; severe cognitive deficits; severe pathologies of the upper extremity of traumatic or rheumatic nature; severe pain in the affected arm; people with metal implants |
| Interventions | Experimental group: robot‐assisted neurocognitive therapy (ReHapticKnob) for 45 minutes 4 times per week Control group: conventional neurocognitive therapy (Perfetti) for 45 minutes 4 times per week |
| Outcomes | Outcomes will be collected at baseline, at 4 and 8 weeks, and at 6 months Primary outcomes:
Secondary outcomes:
|
| Starting date | April 2013 |
| Contact information | Daria Dinacci, MD; d.dinacci@clinica‐hildebrand.ch |
| Notes |
| Trial name or title | Refinement and clinical evaluation of the H‐Man: a novel, portable, inexpensive planar robot for arm rehabilitation after stroke |
| Methods | RCT |
| Participants | Country: Singapore Participants: estimated enrolment n = 60 Inclusion criteria: age between 21 and 85 years; first‐ever clinical stroke confirmed by imaging; time since stroke between 3 and 24 months; hemiplegic pattern of motor impairment with MRC motor power of shoulder and elbow flexion grade ≥ 3; FMA score of the affected upper limb between 20 and 50 points; motor inco‐ordination or motor ataxia Exclusion criteria: other causes of arm motor impairment; severe medical conditions; palliative care; severe arm pain; inability to sit for 90 minutes; local fractures; spasticity of MAS grades 3 to 4; skin wounds; shoulder pain > 5/10 visual analogue scale; severe sensory impairment of affected limb; severe visual impairment; hemispatial neglect or homonymous hemianopia; cognitive impairments or uncontrolled behaviour; Mini‐Mental State Examination < 26/28 |
| Interventions | Experimental group: H‐Man (end‐effector upper limb robot; dosage not stated) Control group: additional conventional therapy (repetitive goals‐based arm therapy; dosage not stated) |
| Outcomes | Outcomes will be collected at baseline and at 3, 6, 12, and 24 weeks after start of the intervention Primary outcomes:
Secondary outcomes:
|
| Starting date | July 2014 |
| Contact information | Chua SG Sui Geok; karen_chua@ttsh.com.sg |
| Notes |
| Trial name or title | Upper extremity rehabilitation using robot and botulinum toxin |
| Methods | RCT |
| Participants | Country: Republic of Korea Participants: estimated enrolment n = 348 Inclusion criteria: first‐ever stroke; shoulder or elbow flexor spasticity ≥ MAS 1+; being able to follow instructions from the investigator Exclusion criteria: history of surgery or fracture of affected upper limb; Botox injection within the last 6 months |
| Interventions | Experimental group: early InMotion and Botox (robotic rehabilitation with the InMotion device and Botox for 8 weeks; dosage not stated) Control group 1: Botox, then InMotion (robotic rehabilitation 4 weeks after botulinum toxin injection; dosage not stated) Control group 2: InMotion, then Botox (robotic rehabilitation from the baseline, then Botox injection at 4 weeks after baseline; dosage not stated) Control group 3: late Inmotion and Botox (no intervention, then robotic rehabilitation and Botox injection at 4 weeks after baseline; dosage not stated) |
| Outcomes | Outcomes will be collected at baseline and 4, 8, and 12 weeks from baseline Primary outcomes:
Secondary outcomes:
|
| Starting date | March 2014 |
| Contact information | Joon‐Ho Shin, MS; asfreelyas@gmail.com |
| Notes |
| Trial name or title | Effects of proximal and distal robot‐assisted therapy combined with functional training on stroke rehabilitation |
| Methods | RCT |
| Participants | Country: Taiwan Participants: estimated enrolment n = 92 Inclusion criteria: unilateral stroke, radiologically confirmed; time since onset more than 6 months; upper extremity FMA score between 10 and 50; Mini‐Mental State Examination > 24 points; being able to follow commands Exclusion criteria: serious visual or visual perception problems; orthopaedic or other neurological problems in the last 6 months prior to enrolment; participation in other studies in the last 3 months |
| Interventions | Experimental group 1: proximal robot‐assisted therapy (InMotion2 device); dosage not described Experimental group 2: distal robot‐assisted therapy (InMotion3 device); dosage not described Experimental group 3: combined robot‐assisted therapy (InMotion2 and InMotion3 devices); dosage not described Control group (active): dose‐matched, individualised intensive therapy; dosage not described |
| Outcomes | Outcomes will be collected at baseline, 2 weeks, and 4 weeks Primary outcomes:
Secondary outcomes:
|
| Starting date | September 2014 |
| Contact information | Ching‐Yi Wu, ScD; cywu@mail.cgu.edu.tw |
| Notes |
| Trial name or title | Effects of robot‐assisted combined therapy in upper limb rehabilitation in stroke patients |
| Methods | Randomised cross‐over trial |
| Participants | Country: Taiwan Participants: estimated enrolment n = 120 Inclusion criteria: age 18 to 80 years; first‐ever unilateral stroke > 3 months after onset; upper extremity FMA score between 18 to 56 points; no excessive spasticity in the affected upper extremity; being able to follow study instructions and to perform study tasks; written informed consent Exclusion criteria: neural or psychological problems that may interfere with study; severe joint pain; upper limb fracture within the last 3 months; participation in any other research |
| Interventions | Experimental group 1: robot‐assisted therapy and neuromuscular electrical stimulation for 1.5 hours per day, 5 days a week for 4 weeks Experimental group 2: robot‐assisted therapy and mirror therapy for 1.5 hours per day, 5 days a week for 4 weeks Experimental group 3: mirror therapy for 1.5 hours per day, 5 days a week for 4 weeks Experimental group 4: unilateral robot‐assisted therapy (InMotion device) for 1.5 hours per day, 5 days a week for 4 weeks Experimental group 5: bilateral robot‐assisted therapy (Bi‐Manu‐Track) for 1.5 hours per day, 5 days a week for 4 weeks Control group (active): conventional rehabilitation for 1.5 hours per day, 5 days a week for 4 weeks |
| Outcomes | Outcomes will be collected at baseline and at the end of study at 4 weeks Primary outcomes:
Secondary outcomes:
Other outcome measures:
|
| Starting date | August 2014 |
| Contact information | Keh‐Chung Lin; kehchunglin@ntu.edu.tw Chung‐Shan Hung; f00429003@ntu.edu.tw |
| Notes |
| Trial name or title | Brain Computer Interface (BCI) System for stroke rehabilitation |
| Methods | RCT |
| Participants | Country: China Participants: estimated enrolment n = 60 Inclusion criteria: age above 18 years; hemiparesis resulting from a single unilateral lesion of the brain; at least 6 months after onset; subcortical ischaemic lesion within the territory of the middle cerebral artery; being able to follow simple instructions; understand purpose and content of the experiment; moderate to severe motor disability in the paretic upper limb Exclusion criteria: severe hand spasticity; open hand wound or hand deformity; visual‐field defects; aphasia; neglect; apraxia; participation in any therapeutic treatment outside the study; history of substance abuse; bilateral infarctions; uncontrolled medical problems; serious cognitive deficits; other MRI contraindications |
| Interventions | Experimental group 1: EEG‐guided robotic training based on ipsilesional EEG signals for 30 sessions Experimental group 2: EEG‐guided training based on both ipsilesional and contralesional EEG signals for 30 sessions Control group: placebo comparator robot for 30 sessions |
| Outcomes | Outcomes will be collected at 3‐month follow‐up Primary outcomes:
Secondary outcomes:
|
| Starting date | May 2015 |
| Contact information | Raymond Tong, PhD; +852 3943 8454 |
| Notes |
NTR3669
| Trial name or title | Feasibility of supervised care and rehabilitation involving personal telerobotics for arm/hand function of chronic stroke patients |
| Methods | RCT |
| Participants | Country: the Netherlands Participants: estimated enrolment n = 20 Inclusion criteria: age between 18 and 80 years; unilateral and ischaemic or haemorrhagic stroke; time since stroke between 6 and 12 months; clinical diagnosis of central paresis of arm or hand with 15° active elbow flexion; 1/4 range of active finger flexion; ability to complete measurements and training sessions; discharged from medical centre; living at home and having Internet access; having a carer who is co‐resident or closely involved in care; ability to read, understand, and follow instructions; device fits to the person; written informed consent Exclusion criteria: receiving additional therapy to the affected upper extremity during the study; not eligible to join normal rehabilitation; other severe comorbidities; severe sensory impairments; severe neglect; visual impairments; cognitive impairment |
| Interventions | Experimental group: 60 minutes of technology‐assisted arm/hand training for 18 sessions during 6 weeks (consisting of computerised gaming wearing the SCRIPT hand device to support hand opening and the SaeboMAS for gravity compensation) Control group: 60 minutes of technology‐assisted arm/hand training for 18 sessions during 6 weeks of conventional home training (standard arm and hand exercises) |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | January 2013 |
| Contact information | Sharon Nijenhuis, MSc; s.nijenhuis@rrd.nl |
| Notes |
RATULS
| Trial name or title | Robot Assisted Training for the Upper Limb after Stroke (RATULS) |
| Methods | Multicentre RCT |
| Participants | Country: UK Inclusion criteria: adults with acute or chronic stroke causing moderate to severe upper limb functional limitation |
| Interventions | 3 groups:
|
| Outcomes | Primary outcome: upper limb function measured by ARAT at 3 months' postrandomisation Secondary outcomes: upper limb impairment, activities of daily living, quality of life, resource use, and adverse events measured at 3 and 6 months' postrandomisation |
| Starting date | April 2014 |
| Contact information | https://research.ncl.ac.uk/ratuls/contact%20us/ |
| Notes | Sample size: 720 participants Study duration: 57 months |
ADL: activities of daily living ARAT: Action Research Arm Test BBT: Box and Block Test BOLD: blood oxygenation level dependent BI: Barthel Index CT: computerised tomography EEG: electroencephalogram EMG: electromyography FIM: Functional Independence Measure FMA: Fugl‐Meyer Assessment fMRI: functional magnetic resonance imaging MAL: Motor Activity Log MAS: Modified Ashworth Scale MRC: Medical Research Council MRI: magnetic resonance imaging NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial SIS: Stroke Impact Scale UE: upper extremity WMFT: Wolf Motor Function Test
Contributions of authors
Jan Mehrholz contributed to the conception and design of the protocol and approved the final manuscript. He searched electronic databases and conference proceedings, screened titles and abstracts of publications identified by the search, selected and assessed trials, extracted trial and outcome data, guided the analysis and interpretation of the data, and contributed to and approved the final manuscript of the review.
Marcus Pohl extracted trial and outcome data, contributed to the conception and design of the review, and drafted the protocol. Together with Jan Mehrholz, he contacted trialists about unpublished data and also entered the data, carried out statistical analysis, helped with the interpretation of the data, drafted the review, and approved the final manuscript of the review.
Thomas Platz contributed to the interpretation of the data and approved the final manuscript of the review.
Joachim Kugler assessed and extracted trial and outcome data, assessed the methodological quality of selected trials, contributed to the interpretation of the data, and contributed to and approved the final manuscript of the review.
Bernhard Elsner searched electronic databases and conference proceedings, screened titles and abstracts of publications identified by the search, selected and assessed trials, extracted trial data, guided the analysis and the interpretation of the data, and contributed to and approved the final manuscript of the review.
Sources of support
Internal sources
Wissenschaftliches Institut, Klinik Bavaria Kreischa, Germany.
Department of Public Health, TU Dresden, Germany.
SRH Fachhochschule für Gesundheit Gera gGmbH, Germany.
External sources
No sources of support supplied
Declarations of interest
Jan Mehrholz: was a co‐author of one included trial (Hesse 2005). He did not participant in the quality assessment or data extraction of this study. Marcus Pohl: was a co‐author of one included trial (Hesse 2005). He did not participant in the quality assessment or data extraction of this study. Thomas Platz: none known. Joachim Kugler: none known. Bernhard Elsner: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Abdullah HA, Tarry C, Lambert C, Barreca S, Allen BO. Results of clinicians using a therapeutic robotic system in an inpatient stroke rehabilitation unit. Journal of NeuroEngineering and Rehabilitation 2011;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tarry C, Lambert C, Barreca S, Allen BO. Results of clinicians using a therapeutic robotic system in an inpatient stroke rehabilitation unit. Journal of NeuroEngineering and Rehabilitation2011; Vol. 8:50. [MEDLINE: ; 1743‐0003] [DOI] [PMC free article] [PubMed]
- Amirabdollahian F, Loureiro R, Gradwell E, Collin C, Harwin W, Johnson G. Multivariate analysis of the Fugl‐Meyer outcome measures assessing the effectiveness of GENTLE/S robot‐mediated stroke therapy. Journal of NeuroEngineering and Rehabilitation 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Coote S, Murphy BT, Stokes EK. The effect of robot mediated therapy on upper extremity function post stroke. 14th International Congress of the World Confederation for Physical Therapy, Barcelona, Spain. World Confederation for Physical Therapy, 2003. [Google Scholar]; Coote S, Stokes EK. The effect of robot mediated therapy on upper extremity function following stroke ‐ initial results. Irish Journal of Medical Science 2003;172(2):26‐7. [Google Scholar]
- Ang KK, Guan C, Phua KS, Wang C, Zhou L, Tang KY, et al. Brain‐computer interface‐based robotic end effector system for wrist and hand rehabilitation: Results of a three‐armed randomized controlled trial for chronic stroke. Frontiers in Neuroengineering.2014; Vol. 7:30. [DOI] [PMC free article] [PubMed]; NCT01287975. Brain computer interface (BCI) technology for stroke hand rehabilitation. https://clinicaltrials.gov/ct2/show/NCT01287975 (accessed 3 June 2015).
- Brokaw EB, Nichols D, Holley RJ, Lum PS. Robotic therapy provides a stimulus for upper limb motor recovery after stroke that is complementary to and distinct from conventional therapy. Neurorehabilitation and Neural Repair 2014;28(4):367‐76. [DOI] [PubMed] [Google Scholar]; NCT00995774. Extension of the MIME robotic system for stroke rehabilitation. https://clinicaltrials.gov/ct2/show/NCT00995774 (accessed 3 June 2015).
- Burgar CG, Lum PS, Scremin AM, Garber SL, Loos HF, Kenney D, et al. Robot‐assisted upper‐limb therapy in acute rehabilitation setting following stroke: Department of Veterans Affairs multisite clinical trial. Journal of Rehabilitation Research and Development 2011;48(4):445‐58. [DOI] [PubMed] [Google Scholar]; NCT00011583. Robot assisted upper limb neuro‐rehabilitation. https://clinicaltrials.gov/ct2/show/NCT00011583 (accessed 3 June 2015).
- Conroy SS, Whitall J, Dipietro L, Jones‐Lush LM, Zhan M, Finley MA, et al. Effect of gravity on robot‐assisted motor training after chronic stroke: a randomized trial. Archives of Physical Medicine and Rehabilitation 2011;92(11):1754‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00333983. Evaluation of robotic arm rehabilitation in stroke patients. http://clinicaltrials.gov/ct2/show/NCT00333983 (accessed 1 August 2011).
- Daly JJ, Hogan N, Perepezko EM, Krebs HI, Rogers JM, Goyal KS, et al. Response to upper‐limb robotics and functional neuromuscular stimulation following stroke. Journal of Rehabilitation Research and Development 2005;42(6):723‐36. [DOI] [PubMed] [Google Scholar]
- Fazekas G, Horvath M, Troznai T, Toth A. Robot‐mediated upper limb physiotherapy for patients with spastic hemiparesis: a preliminary study. Journal of Rehabilitation Medicine 2007;39(7):580‐2. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML. Computerized arm training improves the motor control of the severely affected arm after stroke: a single‐blinded randomized trial in two centers. Stroke 2005;36(9):1960‐6. [DOI] [PubMed] [Google Scholar]
- Hesse S, Buschfort R, Hess A, Kabbert N, Werner C. Effect on arm function and cost of robot‐assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: A randomized controlled trial [German]. Neurologie und Rehabilitation2014; Vol. 20, issue 2:67‐73. [MEDLINE: ] [DOI] [PubMed]; Hesse S, Heß A, Werner C, Kabbert N, Buschfort RÇ. Effect on arm function and cost of robot‐assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: a randomized controlled trial. Clinical Rehabilitation2014; Vol. 28, issue 7:637‐47. [MEDLINE: ; 02692155] [DOI] [PubMed]
- Hollenstein C, Cabri C. [Zusatztherapie mit computerunterstütztem Trainingssystem im Vergleich zu ergotherapeutischer Armgruppentherapie]. Neuroreha 2011;3(1):40‐2. [Google Scholar]
- Housman SJ, Scott KM, Reinkensmeyer DJ. A randomized controlled trial of gravity‐supported, computer‐enhanced arm exercise for individuals with severe hemiparesis. Neurorehabilitation and Neural Repair 2009;23(5):505‐14. [DOI] [PubMed] [Google Scholar]
- Hsieh YW, Wu CY, Liao WW, Lin KC, Wu KY, Lee CY. Effects of treatment intensity in upper limb robot‐assisted therapy for chronic stroke: a pilot randomized controlled trial. Neurorehabilitation and Neural Repair 2011;25(6):503‐11. [DOI] [PubMed] [Google Scholar]
- Hsieh YW, Lin KC, Horng YS, Wu CY, Wu TC, Ku FL. Sequential combination of robot‐assisted therapy and constraint‐induced therapy in stroke rehabilitation: a randomized controlled trial. Journal of Neurology 2014;261(5):1037‐45. [DOI] [PubMed] [Google Scholar]
- Hwang CH. Individual‐finger‐synchronized‐robot‐assisted hand rehabilitation in sub‐acute to chronic stroke; a prospective randomized clinical trial of efficacy. Brain Injury2012; Vol. 26, issue 4‐5:444‐5. [MEDLINE: ] [DOI] [PubMed]; Hwang CH, Seong JW, Son DS. Individual finger synchronized robot‐assisted hand rehabilitation in subacute to chronic stroke: a prospective randomized clinical trial of efficacy. Clinical Rehabilitation2012; Vol. 26, issue 8:696‐704. [MEDLINE: ] [DOI] [PubMed]
- Kahn L, Averbuch M, Rymer W, Reinkensmeyer J. Comparison of robot‐assisted reaching to free reaching in promoting recovery from chronic stroke. In: Mokhtari M editor(s). Integration of Assistive Technology in the Information Age. Amsterdam: IOS Press, 2001:39‐44. [Google Scholar]; Kahn L, Zygman M, Rymer W, Reinkensmeyer D. Robot‐assisted reaching exercise promotes arm recovery in chronic hemiparetic stroke: a randomized controlled pilot study. Journal of NeuroEngineering and Rehabilitation 2006;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamroth‐Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T, et al. Three‐dimensional, task‐specific robot therapy of the arm after stroke: a multicentre, parallel‐group randomised trial. The Lancet Neurology 2014;13(2):159‐66. [DOI] [PubMed] [Google Scholar]; NCT00719433. Functional recovery in stroke patients with task‐specific robot‐aided arm therapy. https://clinicaltrials.gov/ct2/show/NCT00719433 (accessed 3 June 2015). ; Riener R. Exoskeletal arm robotics for stroke rehabilitation: what are the chances?. 1st European NeuroRehabilitation Congress, 20‐22 October, 2011. Merano, Italy: ENCR, 2011:32. [NCT00719433] [Google Scholar]
- Kutner NG, Zhang R, Butler AJ, Wolf SL, Alberts JL. Quality‐of‐life change associated with robotic‐assisted therapy to improve hand motor function in patients with subacute stroke: a randomized clinical trial. Physical Therapy 2010;90(4):493‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00729625. Air muscle and task practice in upper limb stroke rehab. clinicaltrials.gov/show/NCT00729625 (accessed 1 August 2011). ; Stein J. Air muscle and task practice in upper limb stroke rehab. Study of neurological recovery with robotic aids. http://www.strokecenter.org/trials/clinicalstudies/air‐muscle‐and‐task‐practice‐in‐upper‐limb‐stroke‐rehab (accessed 1 August 2011).
- Hsieh YW, Wu CY, Liao WW, Lin KC, Wu KY, Lee CY. Effects of treatment intensity in upper limb robot‐assisted therapy for chronic stroke: a pilot randomized controlled trial. Neurorehabilitation and Neural Repair2011; Vol. 25, issue 6:503‐11. [MEDLINE: ] [DOI] [PubMed]; Liao WW, Wu CY, Hsieh YW, Lin KC, Chang WY. Effects of robot‐assisted upper limb rehabilitation on daily function and real‐world arm activity in patients with chronic stroke: a randomized controlled trial. Clinical Rehabilitation2012; Vol. 26, issue 2:111‐20. [MEDLINE: ; 1477‐0873] [DOI] [PubMed]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot‐assisted therapy for long‐term upper‐limb impairment after stroke. The New England Journal of Medicine 2010;362(19):1772‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00372411. Robotic assisted upper‐limb neurorehabilitation in stroke patients. http://clinicaltrials.gov/ct2/show/NCT00372411 (accessed 1 August 2011).
- Burgar C, Lum P, Shor P, Loos H. Development of robots for rehabilitation therapy: the Palo Alto VA/Stanford experience. Journal of Rehabilitation Research and Development 2000;37(6):663‐73. [PubMed] [Google Scholar]; Lum PS, Burgar CG, Shor PC, Majmundar M, Loos M. Robot‐assisted movement training compared with conventional therapy techniques for the rehabilitation of upper‐limb motor function after stroke. Archives of Physical Medicine and Rehabilitation 2002;83(7):952‐9. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Loos M, Shor PC, Majmundar M, Yap R. MIME robotic device for upper‐limb neurorehabilitation in subacute stroke subjects: a follow‐up study. Journal of Rehabilitation Research and Development 2006;43(5):631‐42. [DOI] [PubMed] [Google Scholar]
- Masiero S, Celia A, Rosati G, Armani M. Robotic‐assisted rehabilitation of the upper limb after acute stroke. Archives of Physical Medicine and Rehabilitation 2007;88(2):142‐9. [DOI] [PubMed] [Google Scholar]
- Masiero S, Armani M, Ferlini G, Chiasera A, Rosati G, Rossi A, et al. A novel robot‐assisted upper‐limb rehabilitation program in acute management of post‐stroke patients: a randomized controlled trial. Neurorehabilitation and Neural Repair2012; Vol. 26, issue 4:401. [MEDLINE: ] ; Masiero S, Armani M, Ferlini G, Rosati G, Rossi A. Randomized trial of a robotic assistive device for the upper extremity during early inpatient stroke rehabilitation. Neurorehabilitation and Neural Repair2014; Vol. 28, issue 4:377‐86. [MEDLINE: ; 1552‐6844] [DOI] [PubMed]; Masiero S, Armani M, Rosati G. Upper‐limb robot‐assisted therapy in rehabilitation of acute stroke patients: focused review and results of new randomized controlled trial. Journal of Rehabilitation Research and Development 2011;48(4):355‐66. [DOI] [PubMed] [Google Scholar]; NCT01102309. Robot‐assisted rehabilitation of the upper limb in acute and subacute post‐stroke patients. https://clinicaltrials.gov/ct2/show/NCT01102309 (accessed 3 June 2015).
- Mayr A, Kofler M, Saltuari L. ARMOR: an electromechanical robot for upper limb training following stroke. A prospective randomised controlled pilot study. Handchirurgie, Mikrochirurgie, Plastische Chirurgie 2008;40(1):66‐73. [DOI] [PubMed] [Google Scholar]
- Daly JJ, Rogers J, McCabe J, Monkiewicz M, Burdsall R, Pundik S. Recovery of actual functional tasks in response to motor learning, robotics, and functional electrical stimulation. Stroke 2010;41(4):e355‐6. [Google Scholar]; McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2015;96(6):981‐90. [DOI] [PubMed] [Google Scholar]
- NCT00343304. Pilot study ‐ comparison of upper body ergometer vs. robot in upper extremity motor recovery post‐stroke. https://clinicaltrials.gov/ct2/show/NCT00343304 (accessed 3 June 2015). ; Rabadi M, Galgano M, Lynch D, Akerman M, Lesser M, Volpe B. A pilot study of activity‐based therapy in the arm motor recovery post stroke: a randomized controlled trial. Clinical Rehabilitation 2008;22:1071‐82. [DOI] [PubMed] [Google Scholar]
- Franceschini M. Robot‐aided therapy for upper limb in sub‐acute stroke patients: a randomized control trial. 1st European NeuroRehabilitation Congress, 20‐22 October, 2011. Merano, Italy: ENCR, 2011; Vol. 1:32. [Google Scholar]; Franceschini M, Mazzoleni S, Palma E, Agosti M, Posteraro F. Effects of upper limb robot‐assisted therapy on motor recovery in subacute stroke patients. Journal of NeuroEngineering and Rehabilitation2014; Vol. 11:104. [MEDLINE: ; 1743‐0003] [DOI] [PMC free article] [PubMed]; Mazzoleni S, Buono L, Dario P, Posteraro F. Upper limb robot‐assisted therapy in subacute and chronic stroke patients: preliminary results on initial exposure based on kinematic measures. 5th IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics, BioRob, 12‐15 August2014:265‐9. [MEDLINE: ; 21551774] ; NCT01658111. Robot‐aided therapy in stroke patients for upper limb rehabilitation. https://clinicaltrials.gov/ct2/show/NCT01658111 (accessed 3 June 2015). [DOI] [PubMed]; Sale P, Franceschini M, Mazzoleni S, Palma E, Agosti M, Posteraro F. Effects of upper limb robot‐assisted therapy on motor recovery in subacute stroke patients. Journal of NeuroEngineering and Rehabilitation 2014;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sale P, Mazzoleni S, Lombardi V, Galafate D, Massimiani MP, Posteraro F, et al. Recovery of hand function with robot‐assisted therapy in acute stroke patients: a randomized‐controlled trial. International Journal of Rehabilitation Research2014; Vol. 37, issue 3:236‐42. [MEDLINE: ; 03425282] [DOI] [PubMed]
- Susanto EA, Tong RK, Ockenfeld C, Ho NS. Efficacy of robot‐assisted fingers training in chronic stroke survivors: a pilot randomized‐controlled trial. Journal of NeuroEngineering and Rehabilitation 2015;12(1):42. [DOI: 10.1186/s12984-015-0033-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN82787126. Technology‐supported task‐oriented training of arm‐hand function in persons with chronic stroke. http://www.isrctn.com/ISRCTN82787126 (accessed 3 June 2015). ; Lemmens RJM, Timmermans AAA, Janssen‐Potten YJM, Pulles SANT, Geers RPJ, Bakx WGM, et al. Accelerometry measuring the outcome of robot‐supported upper limb training in chronic stroke: a randomized controlled trial. PLoS One 2014;9(5):e96414. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Timmermans AA, Lemmens RJM, Monfrance M, Geers RPJ, Bakx W, Smeets RJEM, et al. Effects of task‐oriented robot training on arm function, activity, and quality of life in chronic stroke patients: a randomized controlled trial. Journal of NeuroEngineering and Rehabilitation2014; Vol. 11:45. [MEDLINE: ; 1743‐0003] [DOI] [PMC free article] [PubMed]
- Fasoli SE, Krebs HI, Ferraro M, Hogan N, Volpe BT. Does shorter rehabilitation limit potential recovery poststroke?. Neurorehabilitation and Neural Repair 2004;18:88‐94. [DOI] [PubMed] [Google Scholar]; Volpe BT, Krebs HI, Hogan N, Edelstein OL, Diels C, Aisen M. A novel approach to stroke rehabilitation: robot‐aided sensorimotor stimulation. Neurology 2000;54(10):1938‐44. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Lynch D, Rykman‐Berland A, Ferraro M, Galgano M, Hogan N, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabilitation and Neural Repair 2008;22(3):305‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01525979. Comparative efficacy research of uni‐ vs bi‐lateral arm training poststroke. https://clinicaltrials.gov/ct2/show/NCT01525979 (accessed 3 June 2015). ; Wu C‐Y, Chen M‐D, Chen Y‐T, Wu L‐L, Lin K‐C. Unilateral and bilateral robot‐assisted arm training had differential effects on upper limb function in chronic stroke survivors. Brain Injury2012; Vol. 26, issue 4‐5:362‐3. [MEDLINE: ; 0269‐9052] ; Wu C‐Y, Chuang L‐L, Chen M‐D, Chen Y‐T, Lin K‐C. Therapist‐based and robot‐assisted physical training have differential effects on motor control of upper limb and quality of life after chronic stroke. Circulation2012; Vol. 125, issue 10 Suppl 1:Abst. P289. [MEDLINE: ] ; Wu CY, Yang CL, Chuang LL, Lin KC, Chen HC, Chen MD, et al. Effect of therapist‐based versus robot‐assisted bilateral arm training on motor control, functional performance, and quality of life after chronic stroke: a clinical trial. Physical Therapy 2012;92(8):1006‐16. [MEDLINE: ; 00319023] [DOI] [PubMed] [Google Scholar]
- Yoo DH, Cha YJ, Kim SK, Lee JS. Effect of three‐dimensional robot‐assisted therapy on upper limb function of patients with stroke. Journal of Physical Therapy Science2013; Vol. 25, issue 4:407‐9. [MEDLINE: ; 0915‐5287]
References to studies excluded from this review
- Abdollahi F, Case Lazarro ED, Listenberger M, Kenyon RV, Kovic M, Bogey RA, et al. Error augmentation enhancing arm recovery in individuals with chronic stroke: a randomized crossover design. Neurorehabilitation and Neural Repair2014; Vol. 28, issue 2:120‐8. [1552‐6844] [DOI] [PMC free article] [PubMed]
- Aisen ML, Krebs HI, Hogan N, McDowell F, Volpe BT. The effect of robot‐assisted therapy and rehabilitative training on motor recovery following stroke. Archives of Neurology 1997;54(4):443‐6. [DOI] [PubMed] [Google Scholar]
- Der‐Yeghiaian L, Sharp KG, See J, Abidi NS, Mai K, Le VH, et al. Robotic therapy after stroke and the influence of baseline motor status. Stroke 2009;40(4):e169. [Google Scholar]; Dodakian L, Sharp KG, See J, Abidi NS, Mai K, Fling BW, et al. Targeted engagement of a dorsal premotor circuit in the treatment of post‐stroke paresis. NeuroRehabilitation 2013;33(1):13‐24. [DOI] [PubMed] [Google Scholar]
- Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Archives of Physical Medicine and Rehabilitation 2003;84(4):477‐82. [DOI] [PubMed] [Google Scholar]; Stein J, Krebs HI, Frontera WR, Fasoli SE, Hughes R, Hogan N. Comparison of two techniques of robot‐aided upper limb exercise training after stroke. American Journal of Physical Medicine and Rehabilitation 2004;83(9):720‐8. [DOI] [PubMed] [Google Scholar]
- Fluet G. Robotically Facilitated Virtual Rehabilitation of Arm Transport Integrated With Finger Movement Versus Isolated Training of the Arm and Hand in Persons With Hemiparesis [Dissertation]. University of Medicine and Dentistry of New Jersey, 2012. [MEDLINE: ; 978‐1‐267‐42769‐4] [Google Scholar]
- Hesse S. Transcranial direct current stimulation (tDCS) of the lesioned or non‐lesioned hemisphere plus computer‐assisted arm trainer: a randomized, placebo‐controlled double‐blind multi‐centre study in patients with severe arm paresis early after stroke. https://clinicaltrials.gov/show/NCT00407667 (accessed 1 December 2007).
- Hill V, Page S. The efficacy of using a combined regimen of portable robotics and a repetitive task specific practice to increase motor function in the upper arm. Archives of Physical Medicine and Rehabilitation2011; Vol. 92, issue 10:1718. [MEDLINE: ; 0003‐9993] ; Page S, Hill V, White S. Portable upper extremity robotics is as efficacious as upper extremity rehabilitative therapy. Archives of Physical Medicine and Rehabilitation2012; Vol. 93, issue 10:E21. [MEDLINE: ] [DOI] [PMC free article] [PubMed]; Page S, Hill V, White S. Portable upper extremity robotics is as efficacious as upper extremity rehabilitative therapy: a randomized controlled pilot trial. Clinical Rehabilitation2013; Vol. 27, issue 6:494‐503. [MEDLINE: ; 1477‐0873] [DOI] [PMC free article] [PubMed]
- Hu XL, Tong RK, Ho NS, Xue JJ, Rong W, Li LS. Wrist rehabilitation assisted by an electromyography‐driven neuromuscular electrical stimulation robot after stroke. Neurorehabilitation and Neural Repair 2015;29(8):767‐76. [DOI] [PubMed] [Google Scholar]
- ISRCTN16472838. iPAM (robot) Stroke Intervention Trial (iSIT) for arm weakness. http://www.isrctn.com/ISRCTN16472838 (accessed 3 June 2015). [DOI: 10.1186/ISRCTN16472838] [DOI]; Jackson AE, Levesley MC, Makower SG, Cozens JA, Bhakta BB. Development of the iPAM MkII system and description of a randomized control trial with acute stroke patients. 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR 2013), 24‐26 June 2013, Seattle, Washington, USA. [MEDLINE: ] [DOI] [PubMed]
- Krebs HI, Volpe BT, Aisen ML, Hogan N. Increasing productivity and quality of care: robot‐aided neuro‐rehabilitation. Journal of Rehabilitation Research and Development 2000;37(6):639‐52. [PubMed] [Google Scholar]
- Luft AR, McCombe‐Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA 2004;292(15):1853‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum PS, Taub E, Schwandt D, Postman M, Hardin P, Uswatte G. Automated constraint‐induced therapy extension (AutoCITE) for movement deficits after stroke. Journal of Rehabilitation Research and Development 2004;41(3A):249‐58. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for improved muscle activation patterns after retraining of reaching movements with the MIME robotic system in subjects with post‐stroke hemiparesis. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2004;12(2):186‐94. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levin L, Hermann V, Dunning K, Levine P. Longer versus shorter daily durations of electrical stimulation during task‐specific practice in moderately impaired stroke. Archives of Physical Medicine and Rehabilitation2012; Vol. 93, issue 2:200‐6. [MEDLINE: ] [DOI] [PubMed]
- Prange GB, Kottink AIR, Buurke JH, Eckhardt MMEM, Keulen‐Rouweler BJ, Ribbers GM, et al. The effect of arm support combined with rehabilitation games on upper‐extremity function in subacute stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2015;29:174‐82. [DOI] [PubMed] [Google Scholar]; Rietman JS, Prange G, Kottink A, Ribbers G, Buurke J. The effect of an arm supporting training device in sub‐acute stroke patients: randomized clinical trial. Archives of Physical Medicine and Rehabilitation2014; Vol. 95, issue 10:e8. [MEDLINE: ]
- Reinkensmeyer DJ, Kahn LE, Averbuch M, McKenna‐Cole A, Schmit BD, Rymer WZ. Understanding and treating arm movement impairment after chronic brain injury: progress with the ARM guide. Journal of Rehabilitation Research and Development 2000;37(6):653‐62. [PubMed] [Google Scholar]
- Takahashi CD, Der‐Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot‐based handmotor therapy after stroke. Brain 2008;131:425‐37. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Amano S, Domen K, Hachisuka K, Kimura T, Takebayashi T. Which stroke patients benefit from robotic therapy for the upper extremities?. Cerebrovascular Diseases2013:145. [MEDLINE: ; 1015‐9770] ; Takahashi K, Domen K, Hachisuka K, Sakamoto T, Toshima M, Otaka Y, et al. Upper extremity robotic therapy is effective in post‐stroke hemiplegia: a randomized controlled trial. In: Proceedings of the International Stroke Conference 2011. 8‐11 February 2011; Los Angeles, USA. [MEDLINE: ] [DOI] [PubMed]; Takebayashi T, Koyama T, Amano S, Hanada K, Tabusadani M, Hosomi M, et al. A 6‐month follow‐up after constraint‐induced movement therapy with and without transfer package for patients with hemiparesis after stroke: a pilot quasi‐randomized controlled trial. Clinical Rehabilitation2013; Vol. 27, issue 5:418‐26. [MEDLINE: ] [DOI] [PubMed]; Takebayashi T, Takahashi K, Amano S, Domen K, Hachisuka K, Kimura T. Which stroke patients benefit from robotic therapy for the upper extremities?. Cerebrovascular Diseases2013; Vol. 35 Suppl 3:145. [MEDLINE: ]
- Thorsen R, Cortesi M, Jonsdottir J, Carpinella I, Morelli D, Casiraghi A, et al. Myoelectrically driven functional electrical stimulation may increase motor recovery of upper limb in poststroke subjects: a randomized controlled pilot study. Journal of Rehabilitation Research and Development 2013;50(6):785‐94. [MEDLINE: ; 0748‐7711] [DOI] [PubMed] [Google Scholar]
- Tropea P, Cesqui B, Monaco V, Aliboni S, Posteraro F, Micera S. Effects of the alternate combination of error‐enhancing and active assistive robot‐mediated treatments on stroke patients. IEEE Journal of Translational Engineering in Health and Medicine 2013;1:2100‐9. [MEDLINE: ; 2168‐2372] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N, Edelsteinn L, Diels CM, Aisen ML. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology 1999;53(8):1874‐6. [DOI] [PubMed] [Google Scholar]
- Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke 2000;31(10):2390‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- ISRCTN33421390. Arm Intervention After Stroke (AIAS): a feasibility study. http://www.isrctn.com/ISRCTN33421390 (accessed 12 June 2015).
- Chisari C, Frisoli A, Sotgiu E, Procopio C, Bertolucci F, Bergamasco M, et al. Training and assessment of upper limb motor function with a robotic exoskeleton in chronic stroke patients. Gait and Posture 2014;40:S27‐8. [MEDLINE: ; 0966‐6362] [Google Scholar]
- Faran S, Kaick S, Eickhoff C, Mahoney R, Mauritz KH. An active and repetitive robot assisted training improves functional motor recovery of the arm in sub‐acute stroke patients. Stroke 2008;39(2):617. [Google Scholar]
- NCT00435617. Study of hand therapy 3 to 24 months after stroke. Clinical assessment of a massed practice therapy device ‐ study of hand therapy 3 to 24 months after stroke. http://clinicaltrials.gov/ct2/show/NCT00435617 (accessed 27 May 2015).
- Reinkensmeyer DJ, Wolbrecht ET, Chan V, Chou C, Cramer SC, Bobrow JE. Comparison of three‐dimensional, assist‐as‐needed robotic arm/hand movement training provided with Pneu‐WREX to conventional tabletop therapy after chronic stroke. American Journal of Physical Medicine and Rehabilitation 2012;91(11 Suppl):s232‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Krebs HI, Volpe BT, Williams D, Celestino J, Charles SK, Lynch D, et al. Robot‐aided neurorehabilitation: a robot for wrist rehabilitation. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2007;15(3):327‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder SM, Rosenfeldt AB, Reiss A, Buchanan S, Sahu K, Bay CR, et al. The home stroke rehabilitation and monitoring system trial: a randomized controlled trial. International Journal of Stroke2013; Vol. 8, issue 1:46‐53. [MEDLINE: ; 1747‐4949] [DOI] [PMC free article] [PubMed]
- NCT00272259. Robots for stroke survivors. http://clinicaltrials.gov/ct2/show/NCT00272259 (accessed 29 May 2015).
- NCT00343304. Pilot study ‐ comparison of upper body ergometer vs. robot in upper extremity motor recovery post‐stroke. https://clinicaltrials.gov/ct2/show/NCT00343304 (accessed 3 June 2015).
- NCT00453843. Shoulder, or elbow, or wrist: what should we train first after a stroke? The effect of proximal and distal training on stroke recovery. http://clinicaltrials.gov/ct2/show/NCT00453843 (accessed 29 May 2015 ).
- NCT00785343. Study of robot‐assisted arm therapy for acute stroke patients. https://clinicaltrials.gov/ct2/show/NCT00785343 (accessed 3 June 2015).
- NCT00878085. Functional magnetic resonance imaging (fMRI) and robot‐assisted practice of activities of daily living. https://clinicaltrials.gov/ct2/show/NCT00878085 (accessed 3 June 2015).
- NCT01117194. Rehabilitation robot for upper limbs, component project 5: effect on shoulder training using rehabilitation robot for stroke patients. https://clinicaltrials.gov/show/NCT01117194 (accessed 3 June 2015).
- NCT01253018. Evaluation of robot assisted neuro‐rehabilitation. https://clinicaltrials.gov/ct2/show/NCT01253018 (accessed 3 June 2015).
- NCT01552733. Robotic therapy early after stroke events. https://clinicaltrials.gov/ct2/show/NCT01552733 (accessed 3 June 2015).
- NCT01655446. Effects of RR and MT on patients with stroke. https://clinicaltrials.gov/ct2/show/NCT01655446 (accessed 3 June 2015). ; NCT01724164. Robot‐ versus mirror‐assisted rehabilitation in stroke patients. https://clinicaltrials.gov/ct2/show/NCT01724164 (accessed 3 June 2015).
- NCT01767480. Effects and mechanisms of intensive robot‐assisted therapy in patients with subacute stroke: outcomes in brain/movement reorganization, sensorimotor and daily functions, and physiological markers. https://clinicaltrials.gov/show/NCT01767480 (accessed 3 June 2015).
- NCT01907139. Comparative efficacy research of robot‐assisted therapy with and without constraint‐induced therapy in stroke rehabilitation. https://clinicaltrials.gov/ct2/show/NCT01907139 (accessed 3 June 2015).
- NCT01939041. Efficacy of unilateral versus bilateral approach to robot‐assisted rehabilitation in patients with subacute stroke. https://clinicaltrials.gov/ct2/show/NCT01939041 (accessed 3 June 2015).
- NCT02077439. Trial: Interactive intention‐driven upper‐limb training robotic system. https://clinicaltrials.gov/show/NCT02077439 (accessed 3 June 2015).
- NCT0279779. Efficacy study of an interactive robot for the rehabilitation of the upper limb in acute stroke patients. https://clinicaltrials.gov/ct2/show/NCT02079779 (accessed 28 May 2015).
- NCT02096445. Neurocognitive robot‐assisted rehabilitation of hand function after stroke. https://clinicaltrials.gov/ct2/show/NCT02096445 (accessed 3 June 2015).
- NCT02188628. Refinement and clinical evaluation of the H‐Man for arm rehabilitation after stroke. https://clinicaltrials.gov/ct2/show/NCT02188628 (accessed 3 June 2015).
- NCT02228863. Upper extremity rehabilitation using robot and botulinum toxin. https://clinicaltrials.gov/ct2/show/NCT02228863 (accessed 3 June 2015).
- NCT02254343. Effects of proximal and distal robot‐assisted therapy combined with functional training. https://clinicaltrials.gov/ct2/show/NCT02254343 (accessed 3 June 2015).
- NCT02319785. Effects of robot‐assisted combined therapy in upper limb rehabilitation in stroke patients. https://clinicaltrials.gov/ct2/show/NCT02319785 (accessed 3 June 2015).
- NCT02323061. Brain Computer Interface (BCI) system for stroke rehabilitation. https://clinicaltrials.gov/ct2/show/NCT02323061 (accessed 3 June 2015). ; NCT02323074. Brain training system using electroencephalography (EEG) for neurorehabilitation of hand function after stroke. https://clinicaltrials.gov/ct2/show/NCT02323074 (accessed 3 June 2015).
- NTR3669. Feasibility of supervised care & rehabilitation involving personal tele‐robotics for arm/hand function of chronic stroke patients. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3669 (accessed 12 June 2015).
- Stroke Research Group. RATULS: Robot Assisted Training for the Upper Limb after Stroke. https://research.ncl.ac.uk/ratuls/ (accessed 28 May 2015). [MEDLINE: ]
Additional references
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24(1):35‐41. [DOI] [PubMed] [Google Scholar]
- Barker RN, Brauer SG. Upper limb recovery after stroke: the stroke survivors' perspective. Disability and Rehabilitation 2005;30(20):1213‐23. [DOI] [PubMed] [Google Scholar]
- Burgar CG, Lum PS, Shor PC, Machiel Van der Loos HF. Development of robots for rehabilitation therapy: the Palo Alto VA/Stanford experience. Journal of Rehabilitation Research and Development 2000;37(6):663‐73. [PubMed] [Google Scholar]
- Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. Journal of Neurology, Neurosurgery and Psychiatry 1990;53(7):576‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote S, Stokes EK. A Gentle Robot – attitudes to the first European prototype of a robot mediated therapy system. Proceedings of the World Congress of Physical Therapy; 2003 June 7‐12 Barcelona, Spain. Barcelona, Spain: WCPT, 2003:RR‐PL‐1940. [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. European Neurology 1980;19(6):382‐9. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: a systematic review. The Lancet Neurology 2009;8(4):355‐69. [DOI] [PubMed] [Google Scholar]
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7‐level functional independence measure (FIM). Scandinavian Journal of Rehabilitation Medicine 1994;26(3):115‐9. [PubMed] [Google Scholar]
- Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Current Opinion in Neurology 2003;16(6):705‐10. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery. The Copenhagen Stroke Study. Physical Medicine and Rehabilitation Clinics of North America 1999;10(4):887‐906. [PubMed] [Google Scholar]
- Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot‐aided neurorehabilitation. IEEE Transactions on Rehabilitation Engineering 1998;6(1):75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34(9):2181‐6. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, Krebs HI. Effects of robot‐assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabilitation and Neural Repair 2008;22(2):111‐21. [DOI: 10.1177/1545968307305457] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Wegen EE, Meskers CM. Invited commentary on comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2015;96:991–3. [DOI] [PubMed] [Google Scholar]
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD008349.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation 1994;75(4):394‐8. [DOI] [PubMed] [Google Scholar]
- Platz T, Pinkowski C, Wijck F, Kim IH, Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl‐Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clinical Rehabilitation 2005;19(4):404‐11. [DOI] [PubMed] [Google Scholar]
- Prange GB, Jannink MJ, Groothuis‐Oudshoorn CG, Hermens HJ, Ijzerman MJ. Systematic review of the effect of robot‐aided therapy on recovery of the hemiparetic arm after stroke. Journal of Rehabilitation Research and Development 2006;43(2):171‐84. [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Kahn LE, Averbuch M, McKenna‐Cole A, Schmit BD, Rymer WZ. Understanding and treating arm movement impairment after chronic brain injury: progress with the ARM Guide. Journal of Rehabilitation Research and Development 2000;37(6):653‐62. [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Riener R, Nef T, Colombo G. Robot‐aided neurorehabilitation of the upper extremities. Medical and Biological Engineering and Computing 2005;43(1):2‐10. [DOI] [PubMed] [Google Scholar]
- Veerbeek JM, Wegen E, Peppen R, Wees PJ, Hendriks E, Rietberg M. What is the evidence for physical therapy poststroke? A systematic review and meta‐analysis. PLoS One 2014;9(2):e87987. [DOI: 10.1371/journal.pone.0087987] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Barber RM, Ryan M, Bell B, Bertozzi‐Villa A, Amelia IR, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DT, Hewer RL. Functional abilities after stroke: measurement, natural history and prognosis. Journal of Neurology, Neurosurgery and Psychiatry 1987;50(2):177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Cerebrovascular accident, stroke. http://www.who.int/topics/cerebrovascular_accident/en/ (accessed 1 July 2007).
References to other published versions of this review
- Mehrholz J, Platz T, Kugler J, Pohl M. Electromechanical and robot‐assisted arm training for improving arm function and activities of daily living after stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006876.pub2] [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Hädrich A, Platz T, Kugler J, Pohl M. Electromechanical and robot‐assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD006876.pub3] [DOI] [PubMed] [Google Scholar]


