Abstract
Background
This review replaces part of an earlier review that evaluated gabapentin for both neuropathic pain and fibromyalgia, now split into separate reviews for the two conditions. This review will consider pain in fibromyalgia only.
Fibromyalgia is associated with widespread pain lasting longer than three months, and is frequently associated with symptoms such as poor sleep, fatigue, depression, and reduced quality of life. Fibromyalgia is more common in women.
Gabapentin is an antiepileptic drug widely licensed for treatment of neuropathic pain. It is not licensed for the treatment of fibromyalgia, but is commonly used because fibromyalgia can respond to the same medicines as neuropathic pain.
Objectives
To assess the analgesic efficacy of gabapentin for fibromyalgia pain in adults and the adverse events associated with its use in clinical trials.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online, MEDLINE via Ovid and Embase via Ovid from inception to 24 May 2016. We also searched the reference lists of retrieved studies and reviews, and searched online clinical trial registries.
Selection criteria
Randomised, double‐blind trials of eight weeks' duration or longer for treating fibromyalgia pain in adults, comparing gabapentin with placebo or an active comparator.
Data collection and analysis
Two independent review authors extracted data and assessed trial quality and risk of bias. We planned to use dichotomous data to calculate risk ratio and number needed to treat for one additional event, using standard methods. We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created a 'Summary of findings' table.
Main results
Two studies tested gabapentin to treat fibromyalgia pain. One was identified in previous versions of the review and is included here. We identified another study as a conference abstract, with insufficient detail to determine eligibility for inclusion; it is awaiting assessment. The one included study of 150 participants was a 12‐week, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study using last‐observation‐carried‐forward imputation for withdrawals. The maximum dose was 2400 mg daily. The overall risk of bias was low, except for attrition bias.
At the end of the trial, the outcome of 50% reduction in pain over baseline was not reported. The outcome of 30% or greater reduction in pain over baseline was achieved by 38/75 participants (49%) with gabapentin compared with 23/75 (31%) with placebo (very low quality). A patient global impression of change any category of "better" was achieved by 68/75 (91%) with gabapentin and 35/75 (47%) with placebo (very low quality).
Nineteen participants discontinued the study because of adverse events: 12 in the gabapentin group (16%) and 7 in the placebo group (9%) (very low quality). The number of serious adverse events were not reported, and no deaths were reported (very low quality).
Authors' conclusions
We have only very low quality evidence and are very uncertain about estimates of benefit and harm because of a small amount of data from a single trial. There is insufficient evidence to support or refute the suggestion that gabapentin reduces pain in fibromyalgia.
Plain language summary
Gabapentin for pain in adults with fibromyalgia
Bottom Line
There is no good evidence to support or contradict the suggestion that gabapentin at daily doses of 1200 to 2400 mg reduces pain in fibromyalgia.
Background
Fibromyalgia is a complex disorder characterised by widespread pain, fatigue, poor sleep, low mood, and other bodily symptoms. Common pain‐relieving medicines such as paracetamol and ibuprofen are not usually considered effective. Antiepileptic drugs are commonly used to treat fibromyalgia, but there is uncertainty about how good they are.
Gabapentin is a medicine used to treat pain caused by nerves that are not working properly. Gabapentin changes the way that the nerves send messages to the brain. It can be taken in a tablet or a liquid, with or without food. Doses are usually 1200 mg to 2400 mg each day. At the start of treatment low doses are used to minimise side effects, but the dose is usually increased after a few weeks.
Study characteristics
In May 2016 we searched for clinical trials where gabapentin was used to treat pain due to fibromyalgia in adults. We found one study that met the requirements for this review. The study tested 1200 to 2400 mg/day of gabapentin compared with a placebo over 12 weeks, in 150 people.
Key results
The study did not report the number of people with pain reduced by half at the end of week 12. At that time 5 in 10 people taking gabapentin and 3 in 10 taking the placebo had their pain reduced by at least one third. A report of feeling better to any degree was reported by 9 in 10 taking gabapentin and 5 in 10 taking placebo.
About 2 in 10 people taking gabapentin stopped taking the medicine because of side effects, compared with 1 in 10 taking the placebo. The study did not report the number of people with serious side effects, but did report that there were no deaths.
Quality of the evidence
We rated the quality of the evidence as very low because there was only a single small study with important study limitations. Several factors reduced our confidence in the result. Very low quality evidence means that we are very uncertain about the results.
Summary of findings
Summary of findings for the main comparison. Gabapentin compared with placebo for fibromyalgia.
| Gabapentin compared with placebo for fibromyalgia | ||||||
|
Patient or population: adults with fibromyalgia Settings: community Intervention: gabapentin Comparison: placebo | ||||||
| Outcomes | Assumed risk ‐ probable outcome with intervention | Corresponding risk ‐ probable outcome with control | Relative effect (95% CI) | Number of studies, participants | Quality of the evidence (GRADE) | Comments |
| gabapentin | placebo | |||||
| 30% pain reduction at 12 weeks | 38/75 | 23/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation. Downgraded three levels because of small numbers and study limitations |
| 50% pain reduction at 12 weeks | No data | No data | ‐ | ‐ | very low | Outcome not reported |
|
PGIC ‐ any category of "better" at 12 weeks |
68/75 | 35/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation; non‐standard outcome ‐ usually top two categories of better, not top three, used Downgraded three levels because of small numbers and study limitations |
| Withdrawals due to adverse events | 12/75 | 7/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; few events Downgraded three levels because of small numbers |
| Serious adverse events | "No significant group differences" | ‐ | 1 study, 150 participants | very low | ‐ | |
| Deaths | None reported | ‐ | 1 study, 150 participants | very low | ‐ | |
| CI: Confidence interval; LOCF: last observation carried forward; PGIC: Patient Global Impression of Change | ||||||
| Descriptors for levels of evidence (EPOC 2015):
High quality: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
Moderate quality: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
Low quality: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
Very low quality: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. † Substantially different: a large enough difference that it might affect a decision. | ||||||
Background
This Cochrane review is based on a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
This Cochrane review has been split from an earlier review on fibromyalgia and neuropathic pain (Moore 2011a), and will consider only fibromyalgia, due to the pathogenesis of the pain being different from neuropathic pain. The most recent version of the review is being amended and updated to focus solely on neuropathic pain (Moore 2014a).
The history of earlier versions of this Cochrane review is available in Figure 1 and Appendix 2.
1.
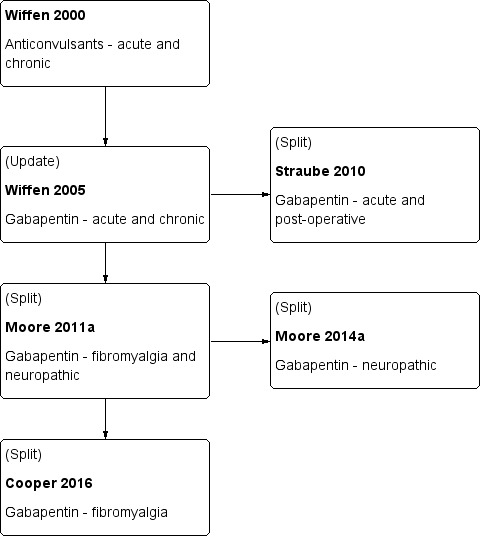
History of Earlier Reviews
Description of the condition
Fibromyalgia symptoms can be assessed by patient self‐report using the fibromyalgia criteria and severity scales for clinical and epidemiological studies, a modification of the American College of Rheumatology (ACR) Preliminary Diagnostic Criteria for Fibromyalgia (so‐called Fibromyalgia Symptom Questionnaire; Wolfe 2011). Fibromyalgia was previously defined by the ACR 1990 classification criteria as widespread pain lasting for longer than three months with tenderness on palpation at 11 or more of 18 specified tender points (Wolfe 1990). For a clinical diagnosis, the ACR 1990 classification criteria and the ACR 2010 preliminary diagnostic criteria can both be used (Wolfe 1990 and Wolfe 2010 respectively). As there is no specific laboratory test, diagnosis is established by obtaining a history of the key symptoms and the exclusion of somatic diseases sufficiently explaining the key symptoms (Wolfe 2010). The indexing of fibromyalgia within the international classification of diseases is under debate. While some rheumatologists have thought of it as a specific pain disorder and central sensitivity syndrome (Clauw 2014; Yunus 2008), recent research points at small fibre pathology in a subgroup of fibromyalgia patients that may be of pathophysiological importance (Oaklander 2013; Üçeyler 2013a). In psychiatry and psychosomatic medicine, fibromyalgia symptoms are categorised as a functional somatic syndrome, a bodily distress syndrome, a somatic physical symptom disorder, or a somatoform disorder (Häuser 2014).
Fibromyalgia is a heterogenous condition. The definite aetiology (causes) of this syndrome remains unknown. A model of interacting biological and psychosocial variables in the predisposition, triggering, and development of the chronicity of fibromyalgia symptoms has been suggested (Sommer 2012). Depression (Forseth 1999), genetics (Arnold 2013; Lee 2012), obesity combined with physical inactivity (Mork 2010), physical and sexual abuse in childhood (Häuser 2011), sleep problems (Mork 2010), and smoking (Choi 2010) are associated with future development of fibromyalgia. Psychosocial stress (working place and family conflicts) and physical stress (infections, surgery, accidents) might trigger the onset of chronic widespread pain and fatigue (Clauw 2014; Sommer 2012). Depression and post‐traumatic stress disorder worsen fibromyalgia symptoms (Häuser 2013a; Lange 2010).
Several factors are associated with the pathophysiology (functional changes associated with or resulting from disease) of fibromyalgia, but the relationship is unclear. The functional changes include alteration of sensory processing in the brain, reduced reactivity of the hypothalamus‐pituitary‐adrenal axis to stress, increased pro‐inflammatory and reduced anti‐inflammatory cytokine profiles (produced by cells involved in inflammation), and disturbances in neurotransmitters such as dopamine and serotonin (Oaklander 2013; Sommer 2012; Üçeyler 2013a). Prolonged exposure to stress, as outlined above, may contribute to these functional changes in predisposed individuals (Bradley 2009). There are similarities to, and differences from, neuropathic pain (Koroschetz 2011).
Patients often report high disability levels and poor quality of life along with extensive use of medical care (Häuser 2015). Many people with fibromyalgia are significantly disabled, and experience moderate or severe pain for many years (Bennett 2007). Chronic painful conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased health costs (Moore 2014a).
Fibromyalgia is common. One component of fibromyalgia, chronic widespread pain, is not only associated with other symptoms such as poor sleep, fatigue, and depression (Wolfe 2014), but is also estimated to affect 11% of the general population (Mansfield 2016). Numerous studies have investigated prevalence of fibromyalgia in different settings and countries. One review gives a global mean prevalence of potential cases of fibromyalgia of 2.7% (range 0.4% to 9.3%), with a mean of 3.1% in the Americas, 2.5% in Europe, and 1.7% in Asia (Queiroz 2013). Changes in diagnostic criteria do not appear to have significantly affected estimates of prevalence (Wolfe 2013). A large US survey using a modification of the 2010 ACR criteria found a prevalence of 1.8%, but 73% of these patients were given a different diagnosis by their physician (Walitt 2015). Estimates of prevalence in specific populations vary greatly, but have been reported to be as high as 9% in female textile workers in Turkey and 10% in metalworkers in Brazil (59% in those with repetitive strain injury; Queiroz 2013). When the 1990 ACR criteria are used for clinical surveys, women are more frequently diagnosed with the disorder. Using these criteria, the women to men ratio has ranged from 8:1 to 30:1 in patients who were studied in clinical institutions and surveys. However, with criteria that do not use tender point examination, the sex ratio can be close to equal. The sex ratio has ranged from 4:1 to 1:1 in studies that were conducted in the general population using the research criteria for fibromyalgia (Häuser 2015; Queiroz 2013).
Fibromyalgia pain is known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any intervention. A multidisciplinary approach is recommended by recent evidence‐based guidelines, with pharmacological treatment being combined with physical or cognitive training, or both. Interventions aim to reduce the key symptoms of fibromyalgia (pain, sleep problems, fatigue) and the associated symptoms (depression, disability) and to improve daily functioning (Eich 2012; Fitzcharles 2013). Conventional analgesics are usually not effective. Antidepressants such as serotonin and noradrenaline reuptake inhibitors (Häuser 2013b; Lunn 2014), tricyclic agents such as amitriptyline (Moore 2012a), or antiepileptics like gabapentin or pregabalin (Moore 2014b; Üçeyler 2013b; Wiffen 2013) are often offered as treatment. The proportion of patients who achieve worthwhile pain relief (typically at least 50% reduction in pain intensity) is small (Moore 2013b), and generally reaches only 10% to 25% more than with placebo, with number needed to treat for an additional beneficial outcome (NNT) between 9.8 and 14 in fibromyalgia (Wiffen 2013); somewhat higher (worse) than for neuropathic pain (Kalso 2013; Wiffen 2013). Those who do experience good levels of pain relief, however, also benefit from substantial reductions in other symptoms, such as fatigue, depression, and anxiety, with significant improvement in ability to function, sleep, work, and quality of life (Moore 2010c; Straube 2011). Fibromyalgia is not particularly different from other chronic pain with regard to a small proportion of trial participants having a good response to analgesic treatment (Moore 2013b).
Description of the intervention
Gabapentin, whilst licensed for the treatment of neuropathic pain in adults in many parts of the world, is not licensed for the treatment of fibromyalgia in any country. As fibromyalgia can respond to the same medicines as neuropathic pain, it has been used off‐license for the treatment of fibromyalgia. Pregabalin, which is closely related to gabapentin, is licensed for treatment of fibromyalgia in the USA.
Gabapentin is given orally, usually as tablets or capsules, but sometimes as an oral solution (50 mg/mL). Guidance suggests that gabapentin treatment can be started at a dose of 300 mg per day for treating neuropathic pain, which is replicated in its use for treating fibromyalgia pain. Based on individual patient response and tolerability, the dosage may be increased by 300 mg per day until the patient experiences satisfactory pain relief or adverse effects make taking the drug intolerable (EMC 2009). Gabapentin is marketed under various trade names, including NeurontinTM, and is also available as generic products in some parts of the world.
Gabapentin has a half‐life of five to seven hours. It is absorbed through a saturable transport system, so that absorption is not linear, and the transporter is found only in the proximal small bowel. This means that the drug needs to be administered at least three times daily, and may result in plasma trough levels. Two new formulations have attempted to improve the availability of the drug. The first is an extended release, gastro‐retentive formulation, designed to provide continuous delivery at the optimal site of absorption over 8 to 10 hours (Sang 2013). The second uses an extended‐release prodrug (gabapentin encarbil) that is absorbed through a high capacity transport system found throughout the intestine, and then undergoes rapid hydrolysis to gabapentin. It is claimed to provide sustained, dose‐proportional gabapentin exposure (Backonja 2011), and can be administered twice daily.
Gabapentin can also be formulated as an aqueous solution for injection. This formulation is not available commercially.
How the intervention might work
Gabapentin's mechanism of action is primarily attributed to its effect on calcium channels located throughout the peripheral and central nervous systems, which modify the release of neurotransmitters and reduce excitability of nerve cells (Boyle 2014; Chang 2014). This mode of action confers antiepileptic, analgesic, and sedative effects. Research also indicates that gabapentin acts by blocking new synapse formation (Eroglu 2009).
Why it is important to do this review
Gabapentin is used off‐license to treat fibromyalgia. The earlier Cochrane review considered fibromyalgia alongside neuropathic pain (Moore 2011a), but an editorial decision was made to split the review by condition, which necessitated a new review and update of the evidence.
Like the earlier Cochrane review, this new review assesses evidence in ways that make both statistical and clinical sense, and uses developing criteria for what constitutes reliable evidence in chronic pain (Appendix 1; Moore 2010a). We have followed the standards of the Cochrane Pain, Palliative and Supportive Care (PaPaS) Group, as set out in the Cochrane PaPaS Group Author and Referee Guidance for pain studies (PaPaS 2012).
Objectives
To assess the analgesic efficacy of gabapentin for fibromyalgia pain in adults and the adverse events associated with its use in clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with double‐blind assessment of outcomes reporting outcomes after eight weeks of treatment or longer. We required full journal publication, with the exception of extended abstracts with sufficient data for analysis and online clinical trial results summaries of otherwise unpublished clinical trials. We excluded short abstracts (usually meeting reports), non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
Studies included participants aged 18 years and above, with fibromyalgia diagnosed using the 1990 or 2010 criteria (Wolfe 1990; Wolfe 2010), and with initial pain of at least moderate intensity.
Types of interventions
Gabapentin at any dose, by any route, administered for the relief of pain in fibromyalgia, and compared to placebo or any active comparator. We excluded studies that used gabapentin to treat pain resulting from the use of other drugs.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with most studies using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These were defined as:
at least 30% pain relief over baseline (moderate);
at least 50% pain relief over baseline (substantial);
much or very much improved on Patient Global Impression of Change (PGIC; moderate);
very much improved on PGIC (substantial).
These outcomes differ from those used in most earlier reviews (Wiffen 2005), as they concentrate on dichotomous outcomes where pain ratings do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50%, and ideally leaving them with no worse than mild pain (Moore 2013a; O'Brien 2010).
Primary outcomes
Participant‐reported pain relief of 30% or greater.
Participant‐reported pain relief of 50% or greater.
PGIC much or very much improved.
PGIC very much improved.
Secondary outcomes
Any pain‐related outcome indicating some improvement.
Withdrawals due to lack of efficacy or adverse events, or for any cause.
Any adverse event.
Any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or that may require an intervention to prevent one of the above characteristics or consequences.
Specific adverse events, particularly somnolence and dizziness.
These outcomes are not eligibility criteria for this review, but are outcomes of interest within the studies that meet the inclusion criteria of the review.
Search methods for identification of studies
Two review authors (TC and PW) independently performed literature searches for eligible studies. We resolved any disagreements or uncertainties by discussion with a third review author where necessary.
Electronic searches
We searched the following databases, without language restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL; via the Cochrane Register of Studies Online) to 24 May 2016.
MEDLINE (via Ovid) from 1946 to 24 May 2016.
Embase (via Ovid) from 1974 to 24 May 2016.
The individual search strategies for CENTRAL, MEDLINE, and Embase are shown in Appendix 4.
Searching other resources
We reviewed the bibliographies of all relevant RCTs and review articles, and searched the following clinical trial databases: ClinicalTrials.gov (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/), to identify additional published or unpublished data. We did not contact study investigators or study sponsors.
Data collection and analysis
Selection of studies
In order to determine study eligibility, two authors (TC and PW) independently read the abstract of each study identified by the search. We eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full‐text copies of the remaining study reports. TC and PW independently read these reports and reached agreement regarding inclusion by discussion. We did not anonymise the studies in any way before assessment. We have included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 2).
2.

Study flow diagram.
Data extraction and management
Two review authors (TC and PW) independently extracted the data using a standard form and confirmed agreement before entering the data into Review Manager 5 (RevMan 2014), or any other analysis tool. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event or serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion (Jadad 1996), limiting inclusion to studies that were randomised and double‐blind as a minimum.
Two review authors (TC and PW) independently assessed the risk of bias for each included study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group. We resolved any disagreements by discussion. We assessed the following for each included study.
Random sequence generation (checking for possible selection bias): We assessed the method used to generate the allocation sequence as being at either low risk of bias (any truly random process, for example random number table or computer random number generator) or unclear risk of bias (when the method used to generate the sequence is not clearly stated). We excluded studies at high risk of bias that use a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias): The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as being at either low risk of bias (for example, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes) or unclear risk of bias (when the method is not clearly stated). We excluded studies that did not conceal allocation and were therefore at high risk of bias (for example, an open list).
Blinding of participants, personnel, and outcome assessment (checking for possible performance and detection bias): We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as being at either low risk of bias (authors state that it was blinded and describe the method used to achieve blinding, for example, identical tablets, matched in appearance and smell) or unclear risk of bias (authors state that it was blinded but do not provide an adequate description of how blinding was achieved). We excluded studies at high risk of bias that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data): We assessed the methods used to deal with incomplete data as being at low risk of bias (less than 10% of participants did not complete the study, or authors used 'baseline observation carried forward' analysis, or both), unclear risk of bias (used 'last observation carried forward' (LOCF) analysis), or high risk of bias (used 'completer' analysis).
Size of study (checking for possible biases confounded by small size): We assessed studies as being at low risk of bias (200 participants or more per treatment arm), unclear risk of bias (50 to 199 participants per treatment arm), or high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We used dichotomous data to calculate the risk ratio (RR) with 95% confidence interval (CIs) using a fixed‐effect model unless we found evidence of significant statistical heterogeneity (see Assessment of heterogeneity).
We calculated NNTs as the reciprocal of the absolute risk reduction (McQuay 1998). For unwanted effects, the NNT becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. Where the unwanted effect is less common with treatment than control, we used the term Number Needed to Treat to Prevent (NNTp).
We did not use continuous data in analyses because it is inappropriate where there is an underlying skewed distribution, as is usually the case with analgesic response.
Unit of analysis issues
We accepted randomisation to the individual participant only. In the event of a study having more than one active treatment arm in which data were not combined for analysis, we planned to split the control treatment arm between active treatment arms.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis where the ITT population consists of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We assigned missing participants zero improvement wherever possible.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by combining studies that examine similar conditions, and to assess statistical heterogeneity visually (L'Abbé 1987), and with the I² statistic. When the I² statistic value was greater than 50%, we would consider the possible reasons for this.
Assessment of reporting biases
The aim of this Cochrane review was to use dichotomous outcomes of known utility and of value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review does not depend on what the authors of the original studies chose to report or not, though clearly difficulties would arise if included studies failed to report relevant dichotomous results.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a NNT of 10 or higher in this condition; Moore 2008). In the event, this was not possible.
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We would have used a random‐effects model if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
We planned to analyse data in three tiers, according to outcome and freedom from known sources of bias.
The first tier would use data meeting current best standards, where studies report the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of LOCF analysis or other imputation method for dropouts, report an ITT analysis, last eight or more weeks, have a parallel‐group design, and have at least 200 participants (preferably at least 400) in the comparison (Moore 1998; Moore 2010a; Moore 2012a; Moore 2012b). We planned to report these top‐tier results first.
The second tier would use data from at least 200 participants but where one or more of the first‐tier conditions above was not met (for example, reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
The third tier of evidence related to data from fewer than 200 participants, or where there were expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there was major heterogeneity between studies, or where there were shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable and may be misleading, but an indication of beneficial effects might be possible.
Quality of the evidence
We used the GRADE approach to assess the quality of evidence related to each of the key outcomes, and report our judgement on the quality of the evidence in the 'Summary of findings' table (Chapter 12, Appendix 6; Higgins 2011). Two review authors independently rated the quality of evidence for each outcome.
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there are so few data that the results are highly susceptible to the random play of chance (Moore 2008b), or if a studies use LOCF imputation in circumstances where there are substantial differences in adverse event withdrawals (Moore 2012b), one would have no confidence in the result, and would need to downgrade the quality of the evidence by 3 levels, to very low quality. In circumstances where there were no data reported for an outcome, we would report the level of evidence as very low quality (Guyatt 2013b).
Summary of findings table
We have included a 'Summary of findings' table as set out in the PaPaS author guide (PaPaS 2012), and recommended in the Cochrane Handbook (Chapter 11, Higgins 2011). The table includes, where possible, outcomes equivalent to moderate or substantial benefit of at least 30% and at least 50% pain intensity reduction, PGIC (possibly at least substantial improvement and at least moderate improvement) (Dworkin 2008), withdrawals due to lack of efficacy, withdrawals due to adverse events, serious adverse events, and death (a particular serious adverse event).
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015):
High: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
Moderate: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
Low: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
Very low: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high.
† Substantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
We did not plan to perform any subgroup analyses since the experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We did not plan to perform any sensitivity analyses.
Results
Description of studies
For this review we made no attempt to contact authors or manufacturers of gabapentin, as in previous versions of this review (Moore 2011a; Moore 2014a). Clinical trial reports or synopses from previously unpublished studies had became available as a result of legal proceedings in the USA for the previous update.
Results of the search
Searching identified two studies using gabapentin to treat pain in fibromyalgia. One was completed and could be included (Arnold 2007); this study was identified in a previous version of the review. Another was available only as a conference abstract, with insufficient detail to determine eligibility for inclusion (Mouzopoulos 2014); this study has been put into Studies awaiting classification. A flow diagram of the search results is shown in Figure 2.
Included studies
Arnold 2007 investigated 150 participants in a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants had diagnosis made using ACR (1990) criteria and had a pain score of 4/10 or greater on an NRS (moderate or severe pain) at randomisation; the mean initial pain score was 5.8/10. The median age was 48 years and 90% were women. The dose of gabapentin was titrated over the first six weeks of treatment, kept stable for a further six weeks, then tapered over one week. During the stable treatment phase, participants were required to take between 1200 and 2400 mg daily, administered in three doses. The median dosage was 1800 mg daily at endpoint.
Participants were excluded if they had previously been treated with gabapentin or pregabalin. Results were analysed using LOCF imputation for withdrawals.
See Characteristics of included studies table.
Excluded studies
We did not exclude any studies after reading the full article.
Risk of bias in included studies
We judged the included study to be at low or unclear risk of bias for all domains except for incomplete outcome reporting (attrition), for which we judged the risk of bias to be high. There was an unclear risk of bias for selection (both random sequence generation and allocation concealment). There was a low risk of bias for performance and detection bias (blinding), selective reporting, and size. See Figure 3 and the Characteristics of included studies table for our reasoning.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Efficacy
There was no first or second tier evidence.
Third tier evidence came from one study with fewer than 200 participants. We did not carry out any data synthesis because any results would potentially be misleading. See Table 1.
Participant‐reported pain relief of 30% or greater
This outcome was reported in 38/75 (49%) of participants with gabapentin and 23/75 (31%) with placebo, using LOCF.
Participant‐reported pain relief of 50% or greater
This outcome was not reported.
PGIC very much improved, or much or very much improved
These outcomes were not reported, but the study did report that 68% of participants described their condition as "better" at the end of treatment with gabapentin, as did 35% with placebo. These results are estimated from a graph, and use LOCF.
Any pain‐related outcomes
The Brief Pain Inventory average pain severity score assesses average pain over the previous 24 hours. After 12 weeks of treatment, the average score was 3.2 (standard deviation (SD) 2.0) with gabapentin and 4.6 (SD 2.6) with placebo, and the estimated difference (by longitudinal analysis) was ‐0.92 (95% CI ‐1.75 to ‐0.71).
Withdrawals
Lack of efficacy
One participant taking gabapentin and two taking placebo withdrew from the study due to lack of efficacy.
Adverse events
Twelve participants taking gabapentin and seven taking placebo withdrew from the study due to adverse events.
Any cause
Eighteen participants withdrew from treatment with gabapentin and 13 with placebo. Reasons for withdrawal other than lack of efficacy or adverse events were loss to follow up, withdrawal of consent, and patient decision (gabapentin 5, placebo 4).
Adverse events
Any adverse event
The number of participants experiencing any adverse event was not reported.
Any serious adverse event
The study reported that there were "no significant group differences in the percentage of serious treatment‐emergent adverse events", but not the number of participants experiencing any serious adverse event.
Specific adverse events
Treatment‐emergent adverse events occurring in at least 5% of participants in the gabapentin group were reported. Dizziness, lightheadedness, and sedation were reported significantly more often with gabapentin than placebo.
Discussion
Summary of main results
Limited evidence from a single trial over 12 weeks suggested that a small number of people with fibromyalgia may have a useful degree of pain relief with gabapentin, at a maximum dose of 2400 mg daily, compared with placebo. Other drugs ‐ pregabalin, duloxetine, milnacipran – have better evidence to support their use, but they also only provide benefits to around 10% or so of people with fibromyalgia (Cording 2015; Lunn 2014; Moore 2009). More and larger studies are obviously required to determine whether gabapentin is effective in fibromyalgia, and just what proportion of people would benefit.
Overall completeness and applicability of evidence
The evidence is very weak. A single study with small numbers of participants and events, and with other possible quality issues, means that no reliance can be placed in the results we have for both efficacy and harm.
Quality of the evidence
We downgraded the evidence to very low because of the sparseness of evidence from this single trial (Guyatt 2013a), and because the use of LOCF imputation in the face of greater adverse event withdrawals for active than placebo can lead to overestimation of treatment effects (Moore 2012b). Very low quality evidence means that we are very uncertain about the results.
Potential biases in the review process
We carried out extensive searches of major databases using broad search criteria, and also searched two large clinical trial registries. We feel that it is unlikely that we have missed significant studies.
Agreements and disagreements with other studies or reviews
Two previous systematic reviews found the same single study for gabapentin in fibromyalgia (Häuser 2009; Tzellos 2010), as did previous versions of this review (Moore 2011a; Moore 2014a). Guidelines for treatment of fibromyalgia recommend a multidisciplinary approach with an emphasis on development of coping strategies and exercise, but pharmacological interventions may be useful for some people; these may include anticonvulsants, but most of the evidence supporting their use relates to pregabalin (Fitzcharles 2013; Macfarlane 2016).
Authors' conclusions
Implications for practice.
For people with fibromyalgia
The evidence that gabapentin improves pain or other symptoms of fibromyalgia is weak and of very low quality. At best it may benefit a few people with the condition.
For clinicians
The evidence that gabapentin improves pain or other symptoms of fibromyalgia is weak and of very low quality. At best it may benefit a few people with the condition.
For policy‐makers
Because gabapentin is used widely for neuropathic pain, therapeutic trials may be considered, particularly if it is possible to switch to another drug in place in the event of failure.
For funders
Since no single treatment is effective in a majority of individuals with fibromyalgia, the relatively small number who benefit from gabapentin may be considered worthwhile, particularly if switching rules are in place. The magnitude of benefit in those people who do respond makes studying potential treatments worthwhile, as the benefit can extend to major improvements in quality of life, function, and ability to work (Moore 2010b; Moore 2014b). This probably makes successful treatment of fibromyalgia cost‐effective, as people with moderate or severe chronic pain consume much more health service and non‐health service resources than those with well treated pain (Moore 2014b)
Implications for research.
General
Because the classic trial design of the study included in this review used the LOCFimputation method for study withdrawals, post‐hoc individual participant level analyses using baseline observation carried forward would be regarded as appropriate to strengthen the findings, especially if the pain reduction was linked to improved quality of life and function.
There is, however, a gap between the dosing regimens used in clinical trials, where fairly rapid dose elevations are made over a few weeks, and clinical practice, where dosing increases can be quite slow. Practical research about the most effective use of medicines known to be effective in only a small proportion of patients might be important. Indeed, situations could be envisaged where the degree of recruitment to successful treatment might have a major impact on treating a very difficult, debilitating, and costly condition.
Design
The trial design is generally adequate, but reporting of clinically relevant outcomes using appropriate imputation for withdrawal would improve the relevance of the findings for clinical practice. The comparison of enriched enrolment, randomised withdrawal (EERW) design to classic trial design indicates that good quality EERW designs of long duration may be appropriate for fibromyalgia (Moore 2015).
We know of no obvious design for testing whether differences in initial dosing regimens might produce better overall results, especially flexible dosing regimens over a longer period of time compared with the typically fixed dosing regimens over a shorter period of time as typically used in clinical trials.
Measurement (endpoints)
Assessment of fibromyalgia symptoms should be based on dichotomous participant‐reported outcomes of proven clinical utility. For EERW trials, the end point of maintenance of therapeutic response without withdrawal might be more clearly stated in trial reports, and used as a primary outcome in future trials, including pragmatic trials of dosing regimens.
Comparison between active treatments
Studies involving other treatments, including non‐pharmacological interventions, may be valuable in this context. A multi‐component approach reflects current practice.
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2018 | Review declared as stable | See Published notes |
History
Protocol first published: Issue 5, 2016 Review first published: Issue 1, 2017
| Date | Event | Description |
|---|---|---|
| 18 July 2017 | Amended | Updated contact details. |
Notes
A restricted search in March 2018 did not identify any potentially relevant studies likely to change the conclusions. The authors and editors are confident that further research will not change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The Oxford Pain Relief Trust provided institutional support.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group. Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
The protocol followed the agreed template for fibromyalgia, which we developed in collaboration with the Cochrane Musculoskeletal Group and Cochrane Neuromuscular Diseases (NMD) Group. The Cochrane PaPaS Group managed the editorial process.
We thank the peer reviewer Mike Lunn, Joint Co‐ordinating Editor of the Cochrane NMD Group, for his useful comments on the protocol.
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, and avoid problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. Below we summarise some of the recent insights that we must consider in this new Cochrane review.
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011b; Moore 2011c), back pain (Moore 2010d), and arthritis (Moore 2010c), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
Consequently, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In people with arthritis, trials of less than 12 weeks duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010c); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010c; Moore 2013b; Moore 2014c; Straube 2008; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia, and lower in central pain and fibromyalgia; Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are grounds for doing so.
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, which affects the quality of life in a significant way (Moore 2010b; Moore 2014b).
Imputation methods, such as LOCF, used when participants withdraw from clinical trials, can overstate drug efficacy, especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012b).
Appendix 2. History of earlier versions of this review
A flow diagram of this history is available in Figure 1.
This is an update of a Cochrane review published in 2011 (Moore 2011a). That review was an update of a previous Cochrane review titled 'Gabapentin for acute and chronic pain' (Wiffen 2005), which itself was an extension to a review previously published in the Cochrane Library on 'Anticonvulsant drugs for acute and chronic pain' (Wiffen 2000). The effects of gabapentin in established acute postoperative pain have been published as a separate review in 2010 (Straube 2010).
The decision to split the review in 2011 was undertaken after discussions with the Editor‐in‐Chief of Cochrane at a meeting in Oxford in early 2009. That meeting was in response to controversy in the USA over the effectiveness of gabapentin as an analgesic (Landefeld 2009), together with calls for the 2005 Cochrane review to be updated with the inclusion of unpublished information made available through litigation (Vedula 2009). It was agreed to update the review by splitting the earlier one into two components: this review which looks at the role of gabapentin in chronic neuropathic pain (including neuropathic pain of any cause, and fibromyalgia), and a second one to determine the effects of gabapentin in acute postoperative pain (Straube 2010). Other reviews may examine gabapentin in chronic musculoskeletal pain. After the Cochrane review on gabapentin for acute and chronic pain was published in 2005 (Wiffen 2005), the licence holders of the first gabapentin product to be marketed released unpublished data, and the Moore 2011a review included these data. The latest update (Cooper 2017, in press) has an expanded background, in line with other reviews of antiepileptic drugs used to treat neuropathic pain and fibromyalgia, and includes three new studies for oral gabapentin plus additional information on an already included study. We have also identified a number of ongoing studies.
The 2011 update (Moore 2011a) included 29 studies in 29 reports with 3571 participants with neuropathic pain and fibromyalgia. The Moore 2014a review included 36 studies in neuropathic pain (5483 participants) and one study in fibromyalgia (150 participants).
Appendix 3. CENTRAL search strategy
MESH DESCRIPTOR Pain EXPLODE ALL TREES (30244)
MESH DESCRIPTOR Peripheral Nervous System Diseases EXPLODE ALL TREES (2585)
MESH DESCRIPTOR Somatosensory Disorders EXPLODE ALL TREES (709)
MESH DESCRIPTOR Fibromyalgia EXPLODE ALL TREES (533)
MESH DESCRIPTOR Myofascial Pain Syndromes EXPLODE ALL TREES (339)
MESH DESCRIPTOR Polymyalgia Rheumatica EXPLODE ALL TREES (44)
((pain* or discomfort*) and (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)): TI,AB,KY (19856)
(fibromyalgi* or fibrosti* or FM or FMS): TI,AB,KY (1953)
((neur* or nerv*) and (compress* or damag*)): TI,AB,KY (2135)
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 (48230)
(gabapentin* or neurontin* or neurotonin*):TI,AB,KY (1018)
10 AND 11 (371)
Appendix 4. MEDLINE (via Ovid) search strategy
exp PAIN/ (328984)
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (123022)
exp SOMATOSENSORY DISORDERS/ (17645)
FIBROMYALGIA/ (6749)
exp MYOFASCIAL PAIN SYNDROMES/ (5920)
POLYMYALGIA RHEUMATICA/ (2248)
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (74639)
(fibromyalgi* or fibrosti* or FM or FMS).mp. (24358)
((neur* or nerv*) adj6 (compress* or damag*)).mp. (55239)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (528383)
(gabapentin* or neurontin* or neurotonin*).mp. (5166)
randomized controlled trial.pt. (411494)
controlled clinical trial.pt. (91674)
randomized.ab. (333337)
placebo.ab. (168066)
drug therapy.fs. (1836743)
randomly.ab. trial.ab. (240444)
trial.ab. (347248)
groups.ab. (1499758)
12 or 13 or 14 or 15 or 16 or 17 or 19 (3657357)
10 and 11 and 20 (1655)
Appendix 5. Embase (via OVID) search strategy
exp PAIN/ (928121)
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (54585)
exp SOMATOSENSORY DISORDERS/ (71019)
FIBROMYALGIA/ (14722)
exp MYOFASCIAL PAIN SYNDROMES/ (6839)
POLYMYALGIA RHEUMATICA/ (3640)
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (128563)
(fibromyalgi* or fibrosti* or FM or FMS).mp. (33777)
((neur* or nerv*) adj6 (compress* or damag*)).mp. (72816)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (1095698)
Gabapentin/ (22468)
(gabapentin* or neurontin* or neurotonin*).mp (23231)
11 or 12 (23231)
crossover‐procedure/ (44583)
double‐blind procedure/ (126205)
randomized controlled trial/ (387066)
(random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or (doubl* adj blind*) or assign* or allocat*).tw. (1380427)
14 or 15 or 16 or 17 (1463359)
10 and 13 and 18 (1912)
Appendix 6. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
High: randomised trials; or double‐upgraded observational studies.
Moderate: downgraded randomised trials; or upgraded observational studies.
Low: double‐downgraded randomised trials; or observational studies.
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide confidence intervals).
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
large magnitude of effect;
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
dose‐response gradient.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arnold 2007.
| Methods |
Randomised: yes Controlled: placebo controlled Blinding: double‐blind Design: multi centre, parallel groups, LOCF analysis Study dates and duration: September 2003 to January 2006 Participants were seen weekly for the first 2 weeks of treatment; thereafter, study visits were scheduled at 2‐week intervals. Participants then entered a 1‐week tapering phase. |
|
| Participants |
Inclusion criteria: female and male, 18 years and over, FM patients meeting ACR criteria for FM (1990), score ≥ 4 on BPI scale at screening and randomisation Exclusion Criteria: Other rheumatic or medical disorders that contributed to the symptoms of FM; pain from traumatic injury or structural or regional rheumatic disease; rheumatoid arthritis, inflammatory arthritis, or autoimmune disease; unstable medical or psychiatric illness; lifetime history of psychosis, hypomania or mania, epilepsy, or dementia; substance abuse in the last 6 months; serious risk of suicide; pregnancy or breastfeeding; unacceptable contraception in those of childbearing potential; patients who, in the opinion of the investigator, were treatment refractory; previous treatment with gabapentin or pregabalin; and treatment with an investigational drug within 30 days of screening. Concomitant medication exclusions consisted of medications or herbal agents with CNS effects, with the exception of episodic use of sedating antihistamines (antidepressants required a 14‐day washout period, or 30 days for fluoxetine); analgesics, with the exception of paracetamol or OTC NSAIDs; and unconventional or alternative therapies. N = 150 Gender: F (135) 90%, M (15) 10% Age: intervention: 49.2 ± 10.6 years; control: 47.3 ± 11.8 years Number randomised: 75 intervention, 75 control Number completed: 57 intervention, 62 control Setting: 3 outpatient centres, USA |
|
| Interventions |
Duration of treatment: 12 weeks + 1 week taper Follow‐up period: unstated Treatment group (n = 75): gabapentin 1200 to 2400 mg/day in 3 doses; titration to limit of tolerability or maximum 2400 mg daily over 6 weeks, then 6 weeks at stable dose (12 weeks in total) “300 mg once a day at bedtime for 1 week, 300 mg twice a day for 1 week, 300 mg twice a day and 600 mg once a day at bedtime for 2 weeks, 600 mg 3 times a day for 2 weeks, and 600 mg twice a day and 1,200 mg once a day at bedtime (2,400 mg/day) for the remainder of the study beginning at week 6. If a patient could not tolerate 2,400 mg/day, the dosage was reduced to a minimum of 1,200 mg/day, administered 3 times a day. The study medication dose was stable for at least the last 4 weeks of the therapy phase. During the tapering phase, the dosage was decreased by 300 mg/day until discontinuation.” Control group (n = 75): placebo Standard treatments to all groups: paracetamol and OTC NSAIDs allowed (no dose limit stated) Co‐interventions: none mentioned |
|
| Outcomes |
Primary Outcomes
Secondary Outcomes
|
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Method of randomisation not given |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of allocation concealment not given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “matching placebo” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: “Self‐reported or self‐administered” Comment: No clear mention of who provides and prepares drugs or who monitors the weekly visits, and whether they are blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: LOCF imputation; more adverse event withdrawals with active |
| Selective reporting (reporting bias) | Low risk | Comment: All primary and secondary outcomes were reported upon, with clear data provided. |
| Size | Unclear risk | Comment: 75 participants per treatment arm |
| Other bias | Low risk | None known |
ACR: American College of Rheumatology; BPI: Brief Pain Inventory; CNS: central nervous system; DB: double‐blind; FM: fibromyalgia; LOCF: last observation carried forward; MOS: Medical Outcomes Study; N: number of participants in study; n: number of participants in treatment arm; NSAID: nonsteroidal anti‐inflammatory drug; OTC: over‐the‐counter; R: randomised; W: withdrawals.
Characteristics of studies awaiting assessment [ordered by study ID]
Mouzopoulos 2014.
| Methods |
Randomised: yes Controlled: active comparator Blinding: not stated Design: multicentre, parallel groups Study dates and duration: January 2008 to May 2011 |
| Participants |
Inclusion criteria: patients meeting ACR criteria for the diagnosis of fibromyalgia and presenting to the department Exclusion criteria: patients with other painful disorders N = 68 Age: unknown Gender: unknown Number randomised: unknown Number completed: unknown Setting: Orthepaedic Kythira General Hospital, Potamos and Chios General Hospital, Chios, Greece |
| Interventions |
Duration of treatment: 2 months Follow‐up period: 2 years Treatment group (n = unknown): 1200 mg oral gabapentin, daily for 2 months Control group (n = unknown): 60 mg oral duloxetine, daily for 2 months Additional analgesia/standard treatments to all groups: unknown Co‐interventions: unknown |
| Outcomes |
Primary Outcomes
Secondary Outcomes
|
| Notes |
ACR: American College of Rheumatology; MOS: Medical Outcomes Study; N: number of participants in study; n: number of participants in treatment arm; VAS: visual analogue scale
Differences between protocol and review
We have extended the description of the GRADE assessment for exceptional circumstances to explain possible decisions. We have also removed one secondary outcome (any disability‐related or mental health‐related outcome) because, on reflection, this is not usually reported in trials.
Contributions of authors
PW registered the title.
TC, RAM, SD, and PW wrote the protocol.
TC and PW performed screening and data extraction.
All authors were involved in writing the full review.
PW will be responsible for updates in the future.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
TC: none known.
SD: none known.
PW: none known.
RAM has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Arnold 2007 {published data only}
- Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE Jr, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double‐blind, placebo‐controlled, multicenter trial. Arthritis and Rheumatism 2007;56(4):1336‐44. [DOI: 10.1002/art.22457] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mouzopoulos 2014 {published data only}
- Mouzopoulos G, Tsembeli A, Skevofilax I, Nomikos G, Vasiliadis V. Duloxetine is superior to gabapentin in the treatment of the fibromyalgia. Pain Practice. Blackwell Publishing Inc, 2014; Vol. 14:45.
Additional references
Arnold 2013
- Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, Kushner I, et al. The fibromyalgia family study: a genome‐wide linkage scan study. Arthritis and Rheumatism 2013;65(4):1122‐8. [DOI: 10.1002/art.37842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Backonja 2011
- Backonja MM, Canafax DM, Cundy KC. Efficacy of gabapentin enacarbil vs placebo in patients with postherpetic neuralgia and a pharmacokinetic comparison with oral gabapentin. Pain Medicine 2011;12(7):1098‐108. [DOI: 10.1111/j.1526-4637.2011.01139.x] [DOI] [PubMed] [Google Scholar]
Bennett 2007
- Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskeletal Disorders 2007;8:27. [DOI: 10.1186/1471-2474-8-27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2014
- Boyle Y, Fernando D, Kurz H, Miller SR, Zucchetto M, Storey J. The effect of a combination of gabapentin and donepezil in an experimental pain model in health volunteers: Results of a randomised controlled trial. Pain 2014;155(12):2510‐6. [DOI: 10.1016/j.pain.2014.09.003] [DOI] [PubMed] [Google Scholar]
Bradley 2009
- Bradley LA. Pathophysiology of fibromyalgia. American Journal of Medicine 2009;122:S22‐30. [DOI: 10.1016/j.amjmed.2009.09.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2014
- Chang CY, Challa CK, Shah J, Eloy JD. Gabapentin in acute postoperative pain management. Biomed Research International 2014;2014:631756. [DOI: 10.1155/2014/631756] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2010
- Choi CJ, Knutsen R, Oda K, Fraser GE, Knutsen SF. The association between incident self‐reported fibromyalgia and nonpsychiatric factors: 25‐years follow‐up of the Adventist Health Study. Journal of Pain 2010;11:994‐1003. [DOI: 10.1016/j.jpain.2010.01.267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clauw 2014
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547‐55. [DOI: 10.1001/jama.2014.3266] [DOI] [PubMed] [Google Scholar]
Cording 2015
- Cording C, Derry S, Phillips T, Moore RA, Wiffen PJ. Milnacipran for pain in fibromyalgia in adults. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD008244.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Eich 2012
- Eich W, Häuser W, Arnold B, Bernardy K, Brückle W, Eidmann U, et al. Fibromyalgia syndrome. General principles and coordination of clinical care and patient education. Schmerz 2012;26:268‐75. [DOI: 10.1007/s00482-012-1167-z] [DOI] [PubMed] [Google Scholar]
EMC 2009
- Electronic Medicines Compendium. http://emc.medicines.org.uk/ (accessed 1 May 2009).
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). 23. Worksheets for preparing a Summary of Findings using GRADE. Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 30 November 2016) 2015.
Eroglu 2009
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, et al. Gabapentin receptor alpha2delta‐1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009;139(2):380‐92. [DOI: 10.1016/j.cell.2009.09.025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzcharles 2013
- Fitzcharles MA, Ste‐Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Research Management 2013;18:119‐26. [PUBMED: 23748251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forseth 1999
- Forseth KO, Husby G, Gran JT, Førre O. Prognostic factors for the development of fibromyalgia in women with self‐reported musculoskeletal pain. A prospective study. Journal of Rheumatology 1999;26:2458‐67. [PUBMED: 10555910] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
Hoffman 2010
- Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy?. Pain 2010;149(2):194‐201. [DOI: 10.1016/j.pain.2009.09.017] [DOI] [PubMed] [Google Scholar]
Häuser 2009
- Häuser W, Bernardy K, Uçeyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin‐‐a meta‐analysis of randomized controlled trials. Pain 2009;145(1‐2):69‐81. [DOI: 10.1016/j.pain.2009.05.014] [DOI] [PubMed] [Google Scholar]
Häuser 2011
- Häuser W, Kosseva M, Üceyler N, Klose P, Sommer C. Emotional, physical, and sexual abuse in fibromyalgia syndrome: a systematic review with meta‐analysis. Arthritis Care & Research 2011;63:808‐20. [DOI: 10.1002/acr.20328] [DOI] [PubMed] [Google Scholar]
Häuser 2013a
- Häuser W, Galek A, Erbslöh‐Möller B, Köllner V, Kühn‐Becker H, Langhorst J, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154:1216‐23. [DOI: 10.1016/j.pain.2013.03.034] [DOI] [PubMed] [Google Scholar]
Häuser 2013b
- Häuser W, Urrútia G, Tort S, Uçeyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010292] [DOI] [PubMed] [Google Scholar]
Häuser 2014
- Häuser W, Henningsen P. Fibronyalgia syndrome ‐ a somatoform disorder?. European Journal of Pain 2014;18:1052‐9. [DOI: 10.1002/j.1532-2149.2014.00453.x] [DOI] [PubMed] [Google Scholar]
Häuser 2015
- Häuser W, Ablin J, Fitzcharles MA, Littlejohn J, Luciano JV, Usui C, et al. Fibromyalgia. Nature Reviews Disease Primers 2015;1:15022. [DOI: 10.1038/nrdp.2015.22] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: 10.1016/0197-2456(95)00134-4] [DOI] [PubMed] [Google Scholar]
Kalso 2013
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. [DOI: 10.1136/bmj.f7339] [DOI] [PubMed] [Google Scholar]
Koroschetz 2011
- Koroschetz J, Rehm SE, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Fibromyalgia and neuropathic pain‐‐differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurology 2011;11:55. [DOI: 10.1186/1471-2377-11-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI] [PubMed] [Google Scholar]
Landefeld 2009
- Landefeld CS, Steinman MA. The Neurontin legacy ‐ marketing through misinformation and manipulation. The New England Journal of Medicine 2009;360(2):103‐6. [DOI: 10.1056/NEJMp0808659] [DOI] [PubMed] [Google Scholar]
Lange 2010
- Lange M, Petermann F. Influence of depression on fibromyalgia: A systematic review. Schmerz 2010;24:326‐33. [DOI: 10.1007/s00482-010-0937-8] [DOI] [PubMed] [Google Scholar]
Lee 2012
- Lee YH, Choi SJ, Ji JD, Song GG. Candidate gene studies of fibromyalgia: a systematic review and meta‐analysis. Rheumatology International 2012;32:417‐26. [DOI: 10.1007/s00296-010-1678-9] [DOI] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfarlane 2016
- Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, et al. EULAR revised recommendations for the management of fibromyalgia. Annals of the Rheumatic Diseases 2016;July:Epub ahead of print. [DOI: 10.1136/annrheumdis-2016-209724] [DOI] [PubMed] [Google Scholar]
Mansfield 2016
- Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta‐analysis of the prevalence of chronic widespread pain in the general population. Pain 2016;157(1):55‐64. [DOI: 10.1097/j.pain.0000000000000314] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5] [Google Scholar]
Moore 2008b
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything‐‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010d
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses‐‐do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: 10.1016/j.pain.2010.07.013] [DOI] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] [DOI] [PubMed] [Google Scholar]
Moore 2011c
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [DOI: 10.1097/EJA.0b013e328343c569] [DOI] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI: 10.1016/j.pain.2011.10.004] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI] [PubMed] [Google Scholar]
Moore 2014c
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions‐‐individual patient data responder analysis. European Journal of Pain 2014;18(1):67‐75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Wiffen PJ, Eccleston C, Derry S, Baron R, Bell RF, et al. Systematic review of enriched enrolment, randomised withdrawal trial designs in chronic pain: a new framework for design and reporting. Pain 2015;156(8):1382‐95. [DOI: 10.1097/j.pain.0000000000000088] [DOI] [PubMed] [Google Scholar]
Mork 2010
- Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: longitudinal data from the Norwegian Nord‐Trøndelag Health Study. Arthritis Care & Research 2010;62:611‐7. [DOI: 10.1002/acr.20118] [DOI] [PubMed] [Google Scholar]
O'Brien 2010
- O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, et al. Patient‐centered perspective on treatment outcomes in chronic pain. Pain Medicine 2010;11(1):6‐15. [DOI: 10.1111/j.1526-4637.2009.00685.x] [DOI] [PubMed] [Google Scholar]
Oaklander 2013
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small‐fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013;154:2310‐6. [DOI: 10.1016/j.pain.2013.06.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
PaPaS 2012
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. http://papas.cochrane.org/papas‐documents (accessed 17 August 2015).
Queiroz 2013
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Current Pain and Headache Reports 2013;17(8):356. [DOI: 10.1007/s11916-013-0356-5] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sang 2013
- Sang CN, Sathyanarayana R, Sweeney M, DM‐1796 Study Investigators. Gastroretentive gabapentin (G‐GR) formulation reduces intensity of pain associated with postherpetic neuralgia (PHN). Clinical Journal of Pain 2013;29(4):281‐8. [DOI: 10.1097/AJP.0b013e318258993e] [DOI] [PubMed] [Google Scholar]
Sommer 2012
- Sommer C, Häuser W, Burgmer M, Engelhardt R, Gerhold K, Petzke F, et al. Etiology and pathophysiology of fibromyalgia syndrome. Schmerz 2012;26:259‐67. [DOI: 10.1007/s00482-012-1174-0] [DOI] [PubMed] [Google Scholar]
Straube 2008
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2010
- Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia‐‐responder analysis from individual patient data. BMC Musculoskeletal Disorders 2010;11:150. [DOI: 10.1186/1471-2474-11-150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2011
- Straube S, Moore RA, Paine J, Derry S, Phillips CJ, Hallier E, et al. Interference with work in fibromyalgia: effect of treatment with pregabalin and relation to pain response. BMC Musculoskeletal Disorders 2011;12:125. [DOI: 10.1186/1471-2474-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: 10.1186/1471-2377-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzellos 2010
- Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, et al. Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta‐analysis. Journal of Clinical Pharmacology and Therapeutics 2010;35(6):239‐56. [DOI: 10.1111/j.1365-2710.2009.01144.x] [DOI] [PubMed] [Google Scholar]
Vedula 2009
- Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry‐sponsored trials of gabapentin for off‐label use. The New England Journal of Medicine 2009;361(20):1963‐71. [DOI: 10.1056/NEJMsa0906126] [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walitt 2015
- Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One 2015;10(9):e0138024. [DOI: 10.1371/journal.pone.0138024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2000
- Wiffen PJ, Collins S, McQuay H, Carroll D, Jadad A, Moore A. Anticonvulsant drugs for acute and chronic pain. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD001133.pub2] [DOI] [PubMed] [Google Scholar]
Wiffen 2005
- Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005452] [DOI] [PubMed] [Google Scholar]
Wiffen 2013
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice ASC, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1990
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33(2):160‐72. [DOI] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care and Research 2010;62(5):600‐10. [DOI: 10.1002/acr.20140] [DOI] [PubMed] [Google Scholar]
Wolfe 2011
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology 2011;38:1113‐22. [DOI: 10.3899/jrheum.100594] [DOI] [PubMed] [Google Scholar]
Wolfe 2013
- Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care and Research 2013;65(5):777‐85. [DOI: 10.1002/acr.21931] [DOI] [PubMed] [Google Scholar]
Wolfe 2014
- Wolfe F, Walitt BT, Häuser W. What is fibromyalgia, how is it diagnosed and what does it really mean?. Arthritis Care and Research 2014;66(7):969‐71. [DOI: 10.1002/acr.22207] [DOI] [PubMed] [Google Scholar]
Yunus 2008
- Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Seminars in Arthritis and Rheumatism 2008;37:339‐52. [DOI: 10.1016/j.semarthrit.2007.09.003] [DOI] [PubMed] [Google Scholar]
Üçeyler 2013a
- Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel‐Schneider S, Schmid A, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136:e247. [DOI: 10.1093/brain/awt053] [DOI] [PubMed] [Google Scholar]
Üçeyler 2013b
- Üçeyler N, Sommer C, Walitt B, Häuser W. Anticonvulsants for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010782] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Moore 2011a
- Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007938.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Wiffen PJ, Derry S, Toelle T, Rice ASC. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007938.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


