Abstract
Background
Ovarian hyperstimulation syndrome (OHSS) is a potentially serious complication of ovarian stimulation in assisted reproduction technology (ART). It is characterised by enlarged ovaries and an acute fluid shift from the intravascular space to the third space, resulting in bloating, increased risk of venous thromboembolism and decreased organ perfusion. Most cases are mild, but forms of moderate or severe OHSS appear in 3% to 8% of in vitro fertilisation (IVF) cycles. The dopamine agonist cabergoline was introduced as a secondary prevention intervention for OHSS in women at high risk of OHSS undergoing ART treatment. As cabergoline seemed to be effective in preventing OHSS, other types of dopamine agonists, such as quinagolide and bromocriptine, have since been studied in ART to prevent OHSS.
Objectives
To assess the effectiveness and safety of dopamine agonists in preventing OHSS in high‐risk women undergoing ART treatment.
Search methods
We searched several databases from inception to August 2016 (Cochrane Gynaecology and Fertility Specialised Register of trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, PsycINFO, Clinicaltrials.gov and the World Health Organization International Trials Registry Platform (ICTRP)) for randomised controlled trials (RCTs) assessing the effect of dopamine agonist in preventing OHSS. We handsearched the reference lists of relevant studies.
Selection criteria
We considered RCTs which compared dopamine agonists with placebo/no intervention or another intervention for preventing OHSS in high‐risk women for inclusion. Primary outcome measures were incidence of moderate or severe OHSS and live birth rate. Secondary endpoints were clinical pregnancy rate, multiple pregnancy rate, miscarriage rate and any other adverse effects of the treatment.
Data collection and analysis
Two authors independently screened titles, abstracts and full texts of publications, selected studies, extracted data and assessed risk of bias. We resolved any disagreements by consensus. We reported pooled results as odds ratios (OR) and 95% confidence interval (95% CI) by the Mantel‐Haenszel method. In addition, we graded the overall quality of the evidence using GRADE criteria.
Main results
The search identified 14 new RCTs since the last published version of this review, resulting in 16 included RCTs involving 2091 high‐risk women for this updated review. They evaluated three types of dopamine agonists: cabergoline, quinagolide and bromocriptine.
When compared with placebo or no intervention, dopamine agonists seemed effective in the prevention of moderate or severe OHSS (OR 0.27, 95% CI 0.19 to 0.39; 1022 participants; 8 studies; I2 = 0%; moderate quality evidence). This suggests that if 29% of women undergoing ART experience moderate or severe OHSS, the use of dopamine agonists will lower this to 7% to 14% of women. There was no evidence of a difference in live birth rate, clinical pregnancy rate, multiple pregnancy rate or miscarriage rate (very low to moderate quality evidence). However, taking dopamine agonists (especially quinagolide) may increase the incidence of adverse events such as gastrointestinal adverse effects (OR 4.54, 95% CI 1.49 to 13.84; 264 participants; 2 studies; I2 = 49%, very low quality evidence).
When we compared dopamine agonist plus co‐intervention with co‐intervention, there was no evidence of a difference in the outcomes of moderate or severe OHSS, live birth rate, clinical pregnancy rate, miscarriage rate or adverse events. The co‐interventions were hydroxyethyl starch (two RCTs) and albumin (one RCT).
Cabergoline was associated with a lower risk of moderate or severe OHSS compared with human albumin (OR 0.21, 95% CI 0.12 to 0.38; 296 participants; 3 studies; I2 = 72%). However, there was no evidence of a difference between cabergoline and hydroxyethyl starch, coasting (withholding any more ovarian stimulation for a few days) or prednisolone. There was an increased clinical pregnancy rate in the cabergoline group when cabergoline was compared with coasting (OR 2.65, 95% CI 1.13 to 6.21; 120 participants; 2 studies; I2 = 0%). In other respects, there was no evidence of a difference in clinical pregnancy rate, multiple pregnancy rate or miscarriage rate between cabergoline and other active interventions.
The quality of the evidence between dopamine agonist and placebo or no intervention ranged from very low to moderate, mainly due to poor reporting of study methods (mostly a lack of details on randomisation or blinding) and serious imprecision for some comparisons.
Authors' conclusions
Dopamine agonists appear to reduce the incidence of moderate or severe OHSS in women at high risk of OHSS (moderate quality evidence). If a fresh embryo transfer is performed, the use of dopamine agonists does not affect the pregnancy outcome (live birth rate, clinical pregnancy rate and miscarriage rate) (very low to moderate quality evidence). However, dopamine agonists might increase the risk of adverse events, such as gastrointestinal symptoms. Further research should focus on dose‐finding, comparisons with other effective treatments and consideration of combination treatments. Therefore, large, well‐designed and well‐executed RCTs that involve more clinical endpoints (e.g., live birth rate) are necessary to further evaluate the role of dopamine agonists in OHSS prevention.
Keywords: Female; Humans; Pregnancy; Reproductive Techniques, Assisted; Abortion, Spontaneous; Abortion, Spontaneous/prevention & control; Administration, Oral; Aminoquinolines; Aminoquinolines/therapeutic use; Bromocriptine; Bromocriptine/therapeutic use; Cabergoline; Dopamine Agonists; Dopamine Agonists/administration & dosage; Dopamine Agonists/therapeutic use; Ergolines; Ergolines/therapeutic use; Ovarian Hyperstimulation Syndrome; Ovarian Hyperstimulation Syndrome/prevention & control; Pregnancy Rate; Randomized Controlled Trials as Topic
Dopamine agonists to prevent ovarian hyperstimulation syndrome in women undergoing assisted reproduction technology
Review question
Are dopamine agonists effective and safe for preventing ovarian hyperstimulation syndrome (OHSS) in women at high risk of OHSS (e.g. women with polycystic ovaries or a high oocyte yield following stimulation)? How effective are they compared to other active treatments (e.g. human albumin)?
Background
OHSS occurs because of overstimulation of the ovaries (female reproductive organs that produce eggs and sex hormones) in fertility treatment (called assisted reproductive technology). It is characterised by enlarged ovaries and movement of fluid from the blood vessels to other body cavities, resulting in abdominal (stomach) bloating, increased risk of blood clots and a reduction in the blood supply to important organs. In most cases, the condition is mild and resolves itself without treatment, but some women develop a moderate or severe form of OHSS, which requires hospitalisation. There is no cure for OHSS other than waiting for it to settle down and reducing symptoms while in hospital. Medicines called dopamine agonists have been introduced to try and prevent OHSS.
Study characteristics
This review included 16 randomised controlled trials involving 2091 women at high risk of OHSS, which evaluated three different dopamine agonists (cabergoline, bromocriptine and quinagolide). The main outcome measures were the number of new cases (incidence) of moderate or severe OHSS and live birth rate. The evidence is current to August 2016.
Key results
Dopamine agonists appear to reduce the incidence of moderate or severe OHSS in women at high risk of OHSS (moderate quality evidence) compared with placebo or no treatment. This suggests that if 29% of women taking placebo or no treatment have moderate or severe OHSS, between 7% and 14% of women taking dopamine agonists will have moderate or severe OHSS. For women who had a fresh embryo transferred as part of their treatment cycle, there was no evidence that dopamine agonists influenced pregnancy outcomes, but they might increase the risk of side effects, such as stomach upsets. There was no evidence of a difference between a dopamine agonist plus another active treatment versus another active treatment on incidence of moderate or severe OHSS and live birth rate.
There was no evidence of a difference in OHSS rates between cabergoline and placebo treatments (e.g. hydroxyethyl starch, prednisolone or 'coasting' (withholding any more ovarian stimulation for a few days)). Cabergoline was associated with an increased clinical pregnancy rate compared with coasting.
Quality of evidence
The quality of the evidence ranged from very low to moderate. Limitations included poor reporting of study methods and imprecision (too few events) for some comparisons.
Summary of findings
Summary of findings for the main comparison.
Dopamine agonist versus placebo/no intervention
| Dopamine agonist vs placebo/no intervention | ||||||
|
Patient or population: women of reproductive age undergoing any ART therapy Settings: ART unit Intervention: dopamine agonist Comparison: placebo/no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo/no intervention | Risk with dopamine agonist | |||||
| Incidence of moderate or severe OHSS | 286 per 1000 |
97 per 1000 (71 to 135) |
OR 0.27 (0.19 to 0.39) |
1022 (8 studies) |
⊕⊕⊕⊝ Moderate 1 | ‐ |
| Live birth rate | 509 per 1000 |
512 per 1000 (355 to 665) |
OR 1.01 (0.53 to 1.91) |
182 (1 studies) | ⊕⊕⊝⊝ Low 1,2 | |
| Clinical pregnancy rate | 401 per 1000 |
352 per 1000 (266 to 450) |
OR 0.81 (0.54 to 1.22) |
432 (4 studies) |
⊕⊕⊕⊝ Moderate 1 | |
| Multiple pregnancy | 50 per 1000 |
17 per 1000 (1 to 303) |
OR 0.32 (0.01 to 8.26) |
40 (1 study) | ⊕⊝⊝⊝ Very low 1,3 | |
| Miscarriage pregnancy rate | 72 per 1000 |
49 per 1000 (15 to 151) |
OR 0.66 (0.19 to 2.28) |
168 (2 studies) |
⊕⊕⊝⊝ Low 1,4 | |
| Adverse events | 43 per 1000 |
168 per 1000 (62 to 381) |
OR 4.54 (1.49 to 13.84) |
264 (2 studies) | ⊕⊝⊝⊝ Very low 1,5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ART: assisted reproductive technology; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level for serious risk of bias associated with poor reporting of study methods.
2 Downgraded one level for serious risk of imprecision: confidence interval compatible with benefit in either arm or with no difference between the groups.
3 Downgraded two levels for very serious risk of imprecision: only one event.
4 Downgraded one level for serious risk of imprecision: only 10 events.
5 Downgraded one level for serious risk of imprecision: only 29 events.
Background
Description of the condition
Ovarian hyperstimulation syndrome (OHSS) is a complication of assisted reproduction technology (ART) treatment. It can occur following exposure of the ovaries of susceptible women to human chorionic gonadotrophin (hCG) or luteinising hormone (LH) during controlled ovarian stimulation with follicle‐stimulating hormone (FSH). Women at risk of OHSS are generally young and have polycystic ovary syndrome (PCOS) (Costello 2012). OHSS is characterised by enlarged ovaries and an acute fluid shift from the intravascular space to the third space (mainly to the abdominal or thoracic cavity), which may result in an accumulation of fluid in the peritoneal cavity and pleura, an elevation of haematocrit and a decrease in organ perfusion (Aboulghar 2003; Soares 2008; Vloeberghs 2009). Its symptoms range from abdominal bloating and a feeling of fullness to shortness of breath (Vloeberghs 2009). OHSS was classified as mild, moderate or severe by Golan and colleagues (Golan 1989), modified from Rabau and colleagues (Rabau 1967) by incorporating ultrasonographic measurement of the stimulated ovaries. Despite measures adopted by physicians to prevent these sequelae, mild OHSS may affect up to 33% of in vitro fertilisation (IVF) cycles. Moderate or severe OHSS arises in 3% to 8% of IVF cycles (RCOG 2006). Young women with low body mass index and polycystic ovaries are at particular risk of OHSS and the only way to entirely avoid the condition for women with fallopian tube compromise or whose partner has impaired semen parameters is to undergo in vitro oocyte maturation which is an approach that is not available in most centres (Walls 2015).
The pathophysiology of OHSS is not yet completely elucidated. Increased vascular permeability causing the loss of fluid into the third space (abdominal and pleural cavity) is the central feature of clinically significant OHSS, which triggers events that result in the associated symptoms (such as abdominal pain and distension) (Ata 2009). Most cases of OHSS have been associated with the use of hCG to trigger oocyte maturation prior to oocyte retrieval, however it is recognised that hCG has no direct effect on the vascular system (Gómez 2002). Vasoactive substances are released by the ovaries in response to hCG administration. It is almost certain that vascular endothelial growth factor (VEGF) is a key substance that induces vascular hyperpermeability, leading to a shift of fluids from the intravascular system to the third space (Busso 2009; Soares 2008). Higher production of VEGF from the many follicles during stimulation by ovarian steroids and hCG appears to be the specific key process leading to the development of OHSS in high‐risk women.
Description of the intervention
Severe OHSS is a potentially life‐threatening condition that occurs in women undergoing ART cycles. Several measures have been introduced to prevent OHSS (Prakash 2009). These include cycle cancellation or 'coasting' (D'Angelo 2002; Delvigne 2002), use of intravenous fluids (Aboulghar 2002; Youssef 2010), cryopreservation of embryos rather than immediate fresh embryo transfer (D'Angelo 2007), and the use of progesterone as luteal phase support (van der Linden 2015). More recent treatments include 'minimal stimulation IVF' (using a combination of medications to gently stimulate the ovaries), in vitro maturation of oocytes (letting oocytes mature in vitro) (Walls 2012), the use of 'natural cycle' IVF (collecting and fertilising one egg released during the normal monthly cycle and without the use of fertility drugs) (Edwards 2007), the use of metformin in women with PCOS (Tso 2014), the use of gonadotropin‐releasing hormone (GnRH) antagonist, as opposed to GnRH agonist for ovarian downregulation (a prerequisite to assist in the timing of oocyte retrieval), adjusting stimulation protocols (Al‐Inany 2011), and the use of an agonist trigger prior to oocyte retrieval in an antagonist cycle (Casper 2015). Despite their availability, there is no consensus on what would be the most favourable strategy to prevent OHSS, and none of these strategies have led to the eradication of OHSS (Aboulghar 2009). Research suggests that the use of dopamine agonists may be a promising strategy for the prevention and treatment of OHSS (Busso 2009; Castelo‐Branco 2009).
How the intervention might work
With a better understanding of the pathophysiology of OHSS and recognition of the important role of VEGF in the development of OHSS, a series of blockers, such as SU5416 (a potent and selective inhibitor of the vascular endothelial growth factor receptor (VEGFR)), were introduced to reverse the hCG action on vascular permeability by targeting VEGFR‐2 expressed on human ovaries (Gómez 2002). However, these anti‐angiogenic drugs could not be used clinically to prevent or treat OHSS due to their adverse effect profile (such as thromboembolism) (Glade‐Bender 2003; Kuenen 2003), and the possibility of affecting embryo implantation (Alvarez 2007a). Another approach is to consider the use of a dopamine agonist, which show similar effects to anti‐angiogenic drugs on vascular permeability and appear not to exert undesirable adverse effects (Castelo‐Branco 2009; Soares 2012). Moreover, dopamine agonists have been used for many years in other fields of medicine, for example to treat elevated serum prolactin levels. However, since the dopamine agonist cabergoline has been associated with fibrotic valvular heart disease when used chronically, other types of dopamine agonists are now being examined for use in OHSS. Possible advantages are the different pharmacokinetic profiles (e.g. shorter half‐life of the drugs (about 17 hours for quinagolide versus about 65 hours for cabergoline)) thereby reducing exposure of embryos to possible teratogenic effects (Busso 2010), and in case of bromocriptine, lower costs and longer experience in use during pregnancy (Beltrame 2013).
Research findings in animal models of OHSS, as well as in humans, have shown that cabergoline can prevent the increase in vascular permeability (Gómez 2006). Several clinical trials have also evaluated the clinical value of cabergoline and showed that prophylactic use of cabergoline was associated with a decrease in the severity of OHSS (Manno 2005). Dopamine agonists may therefore provide a new, specific and non‐toxic approach to the prevention and treatment of OHSS (Alvarez 2007a; Knoepfelmacher 2006).
Why it is important to do this review
Though short‐term use of dopamine agonists for preventing OHSS represents no significant risk for women, long‐term data on its effectiveness and safety requires corroboration. An increased incidence of cardiac valve regurgitation is suggested when women took cabergoline or pergolide for treating Parkinson's disease or hyperprolactinaemia (Kars 2008; Martin 2009; Schade 2007; Zanettini 2007). Clinical studies have increasingly suggested that cabergoline can be safely administered in ART for preventing OHSS without influencing pregnancy outcomes. However, the role of other dopamine agonists (e.g. quinagolide and bromocriptine) for preventing OHSS remain uncertain due to lack of robust evidence for their efficacy and safety. This updated review broadened the scope from only cabergoline to include all other dopamine agonists. This review aimed to summarise the available evidence from randomised controlled trials (RCTs) to determine whether dopamine agonists can reduce the incidence of moderate or severe OHSS in high‐risk women undergoing ART and identify any safety concerns.
Objectives
To assess the effectiveness and safety of dopamine agonists in preventing OHSS in high‐risk women undergoing ART treatment.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished RCTs investigating the effectiveness and safety of dopamine agonists compared with placebo/no intervention or another intervention. We handled conference abstracts in the same way as full publications. We excluded quasi‐randomised trials and, in the case of cross‐over trials, included only pre‐crossover data.
Types of participants
High‐risk women of reproductive age undergoing any ART therapy (as defined by the separate studies).
Types of interventions
Trials were eligible for inclusion when they evaluated any dose of dopamine agonist alone or as an add‐on therapy versus placebo, no intervention or other active treatments.
Types of outcome measures
Primary outcomes
Incidence of moderate or severe OHSS (as determined by study authors) per woman randomised.
Live birth rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) defined as a live infant born after 20 weeks' gestation per woman randomised.
Secondary outcomes
Clinical pregnancy rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
Multiple pregnancy rate (as a result of an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
Miscarriage rate (following an embryo transferred in a fresh cycle using fertilised oocytes from the same menstrual cycle) per woman randomised.
Any other adverse events of the treatment per woman randomised.
Search methods for identification of studies
See: Cochrane Gynaecology and Fertility (formerly Menstrual Disorders and Subfertility Group, MDSG) methods used in reviews (CGF).
We searched for published and unpublished articles in any language, that described or might describe RCTs of dopamine agonists (and more specifically cabergoline, quinagolide or bromocriptine) for preventing OHSS, in consultation with the Cochrane Gynaecology and Fertility Information Specialist.
Electronic searches
We searched:
the Cochrane Gynaecology and Fertility Group's (formerly Menstrual Disorders and Subfertility Group) Specialised Register using key terms on a Procite platform (from inception to 15 August 2016, see Appendix 1). This register also contains unpublished trial abstracts;
-
the following databases were also searched:
Cochrane CENTRAL Register of studies Online (CRSO), Web platform (from inception to 15 Aug 2016), see Appendix 2;
MEDLINE, Ovid platform (from 1946 to 15 August 2016), see Appendix 3;
Embase, Ovid platform (from 1974 to 15 August 2016), see Appendix 4;
PsycINFO, Ovid platform (from 1806 to 15 August 2016), see Appendix 5.
CINAHL through the EBSCO platform (from 1982 to 15 August 2016) see Appendix 6;
the World Health Organization (WHO) International Trials Registry Platform (ICTRP), Web platform (from inception up to 15 August 2016), see Appendix 7;
Clinicaltrials.gov, Web platform (from inception up to 15 August 2016), see Appendix 8;
The OpenSIGLE database, for European grey literature, Web platform (from inception up to 15 August 2016); opensigle.inist.fr/).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Section 6.4.11) (Higgins 2011).
We combined the Embase searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random).
Searching other resources
We searched the citation lists of relevant publications and included studies, review articles and abstracts of conferences, and asked manufacturers, experts and specialists in the field for any trials that they were aware of.
We conducted handsearching in the appropriate journals of gynaecology and reproductive medicine; the conference proceedings (for abstracts) of the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM), as well as related textbooks.
We searched for conference abstracts on the Web of Science (wokinfo.com/).
Data collection and analysis
Selection of studies
Two review authors (SM and HT) independently reviewed the titles and abstracts of the trials, in accordance with the search protocol. We review full‐text articles and considered them for inclusion. If the published study was judged to contain insufficient information, we contacted trial authors. Two review authors (SM and HT) independently critically appraised the trials against the inclusion criteria. We resolved any disagreements by consensus or referral to a third review author (RH).
Data extraction and management
Two review authors (SM and HT) independently extracted data using a piloted data extraction form (Appendix 10). We compared the two sets of extracted data and resolved discrepancies by discussion. The data extraction forms included methodological quality and allocation scores. We included this information in the review and presented it in the Characteristics of included studies and Characteristics of excluded studies tables following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
Two review authors (SM and HT) independently critically assessed risk of bias in all studies included in this review, including the following domains: sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; and selective outcome reporting (described in Cochrane's tool for assessing risk of bias) (Higgins 2011). We judged each domain as being at low risk of bias, high risk of bias or unclear risk of bias for either a lack of information or uncertainty regarding the potential for bias, with any disagreements resolved by consensus or by discussion with a third author (RH).
Measures of treatment effect
We anticipated that all data would be dichotomous. We used the numbers of events in the control and intervention groups of each study to calculate odds ratios (OR) with 95% confidence intervals (CI).
Unit of analysis issues
The primary analysis unit was per woman randomised.
Dealing with missing data
Our meta‐analysis used an intention‐to‐treat (ITT) approach, meaning that we included all women randomised in the analysis, in the groups to which they were randomised. In case of missing data, we contacted the trial authors by email. We assumed that events did not occur in the women for whom data were unobtainable. The imputation undertaken was subjected to sensitivity analysis.
Assessment of heterogeneity
We carried out a test for statistical heterogeneity for each meta‐analysis and assessed heterogeneity by the I2 statistic. This quantifies inconsistency, describing the impact of heterogeneity on the meta‐analysis and measuring the degree of inconsistency across studies. We considered an I2 statistic less than 25% as low level heterogeneity, 25% to 50% as moderate level heterogeneity and higher than 50% as high level heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to use a funnel plot to assess the potential for reporting bias where 10 or more trials per comparison reported data.
Data synthesis
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We pooled data where appropriate, using the Mantel‐Haenszel method. Before pooling data from more than one primary study, we considered heterogeneity. If heterogeneity was low or moderate, we used a fixed‐effect model, otherwise we used a random‐effects model, with further investigation (subgroup analysis) to explore the possible causes of the heterogeneity. We combined data to calculate pooled ORs and 95% CIs.
We stratified the primary analysis by type of intervention (cabergoline, quinagolide or bromocriptine)
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses where there were sufficient data (at least five studies per comparison in the analysis). We performed analyses to determine effects within the following subgroups:
severity of OHSS (severe OHSS versus moderate OHSS);
dose of dopamine agonist (high dose versus low dose).
Sensitivity analysis
We planned a sensitivity analysis for the primary review outcomes by excluding the studies with high risk of bias for any domain. In addition, we tested the effect by using a random‐effects model and evaluated the impact of bias from assumptions made about missing data.
Overall quality of the body of evidence: 'Summary of findings' table
We generated a 'Summary of findings' table using GRADEpro software (GRADEpro GDT 2015). This table evaluated the overall quality of the body of evidence for the main review comparison (dopamine agonists versus placebo or no intervention) for the main review outcomes (i.e. incidence of moderate or severe OHSS, live birth rate, multiple pregnancy rate, clinical pregnancy rate miscarriage rate and any other adverse effect), using GRADE criteria. We assessed the following factors that might decrease the quality level of a body of evidence: study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias. We incorporated judgements about evidence quality (high, moderate, low and very low) into reporting of results for each outcome. Two review authors independently conducted evidence grading, and resolved disagreements by consensus.
Results
Description of studies
Results of the search
As the scope of this review was broadened for the update and we added new key terms, the literature searches were first run without a date restriction, up to 15 September 2015; an updated date‐restricted search was performed from September 2015 up to 15 August 2016. After excluding duplicate abstracts, we retrieved 212 citations using the search strategy). After independent evaluation by two review authors, we excluded 171 articles (non‐RCT, quasi‐RCT, animal experiment). Two review authors (SM and HT) independently reviewed the remaining 41 articles for possible inclusion. Finally, we included 14 new RCTs for meta‐analysis in this update, and categorised one study as 'awaiting classification' (Ahmadi 2010) (Figure 1).
Figure 1.
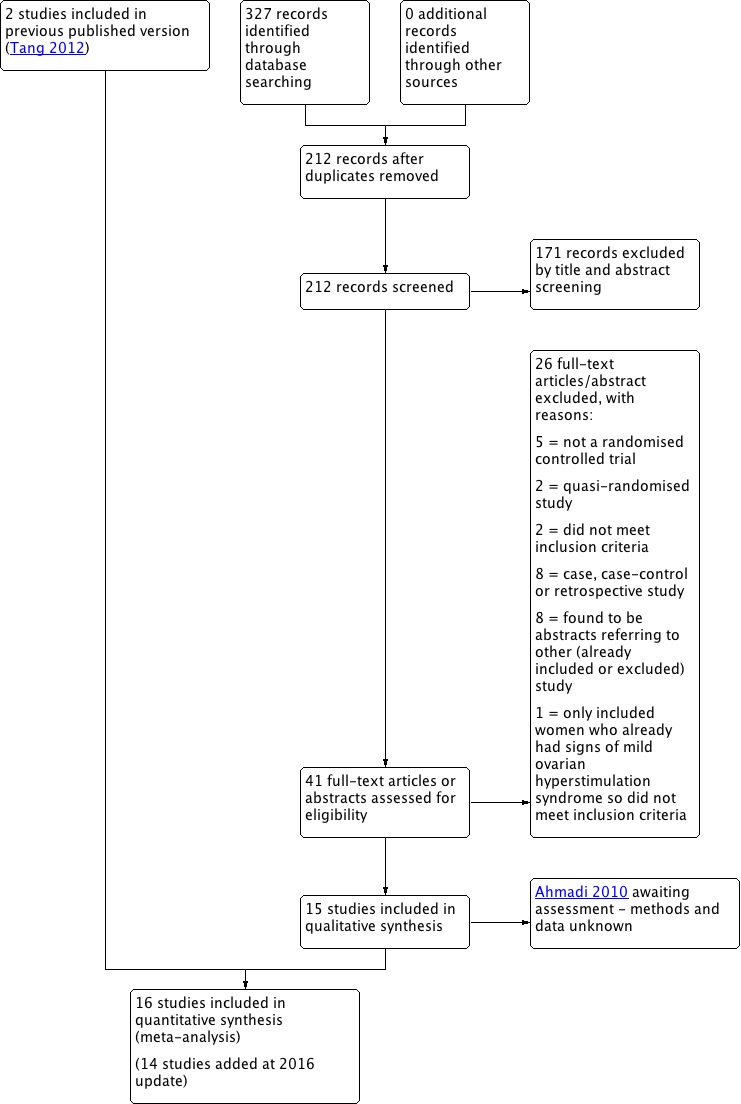
Study flow diagram search August 2016.
See the inclusion and exclusion criteria for the studies in the Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification tables.
Included studies
In total, we included 16 studies (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Ghahiri 2015; Jellad 2016; Matorras 2013; Salah 2012; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013) (see Characteristics of included studies table). We contacted some trial authors for more detailed information (Dalal 2014; Fetisova 2014; Ghahiri 2015; Jellad 2016; Salah 2012; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). In addition, we classified one meeting abstract as 'awaiting classification' due to lack of information for assessment despite attempts to contact the authors (Ahmadi 2010). From the trial registries, six ongoing or recently finished trials had potential to be included in this review, but were not published yet as abstracts or full‐text papers (Bassiouny 2015; El Khattan 2015; Hendricks 2015; Kamel 2015; Khaled 2014; NCT01530490). We attempted to contact the authors to inquire about the trials' status (e.g. recruiting phase, analysis phase, finished but unpublished or publication pending), but only one trial author replied (Bassiouny 2015), who confirmed that the trial was in the analysis phase. See Characteristics of studies awaiting classification and Characteristics of ongoing studies table.
Participants
The 16 studies enrolled 2091 high‐risk women. One study included only oocyte donors (Alvarez 2007a).
The studies were performed in ten different countries: four studies came from Iran (Ghahiri 2015; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013); three from Spain (Alvarez 2007a; Busso 2010; Matorras 2013);two from Brazil ( Beltrame 2013; Carizza 2008); and one each from Syria (Alhalabi 2011), Israel (Amir 2015), United Arab Emirates (Salah 2012), Russia (Fetisova 2014), Egypt (Shaltout 2012), Tunisia (Jellad 2016), and India (Dalal 2014).
One study included women with PCOS only (Salah 2012), without additional risk factors for OHSS (such as a minimum oestradiol (E2 or number of follicles/oocytes retrieved), whereas other studies either excluded women with PCOS (Beltrame 2013), or included women with and without PCOS (Alhalabi 2011; Alvarez 2007a; Amir 2015; Busso 2010; Carizza 2008; Fetisova 2014; Ghahiri 2015; Jellad 2016; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
Most studies selected women aged under 37 years or under 40 years, but Salah 2012 selected women aged 25 to 35 years at high risk for OHSS. The definition of 'high risk of OHSS' varied widely between studies; some used a minimum number of follicles of a certain diameter (18 or more over 12 mm at day of hCG (Jellad 2016); 20 or more over 12 mm at day of hCG (Alhalabi 2011; Amir 2015; Matorras 2013; Shaltout 2012), with or without a minimum E2 level at day of hCG (greater than 2500 pg/mL (Torabizadeh 2013 and Dalal 2014 (the latter mentioned only number of 20 or more follicles without mentioning size of follicles)); greater than 3000 pg/mL (Ghahiri 2015; Jellad 2016; Matorras 2013; Sohrabvand 2009); greater than 3500 pg/mL (Shaltout 2012); greater than 4000 pg/mL (Alhalabi 2011; Amir 2015; Carizza 2008)). Five studies also incorporated the retrieval of 20 or more oocytes as a criterion (Alvarez 2007a; Ghahiri 2015; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013), whereas one study used transvaginal aspiration of 15 or more follicles (Fetisova 2014). One study also considered women with previous history of OHSS as high risk (Ghahiri 2015). One study included only oocyte donors who consequently did not proceed to have an embryo transferred (Alvarez 2007a).
Some studies excluded women with very high E2 levels (greater than 5000 pg/mL (Matorras 2013; Shaltout 2012); greater than 6000 pg/mL (Busso 2010)) because of their very high risk to develop OHSS, and assigned those women to cycle cancellation. One study excluded coasting cases, without stating when a woman was eligible for coasting (Jellad 2016).
Interventions
Comparisons with cabergoline
Five studies involving 521 women compared cabergoline in the treatment group with placebo or no intervention in the control group (Alvarez 2007a; Amir 2015; Fetisova 2014; Jellad 2016; Salah 2012). Amir 2015 also used coasting in almost half of the women in both the intervention and control group. We tried to contact the authors to retrieve more information about which women received coasting and whether these women developed OHSS, but received no reply. Other studies excluded women that were received coasting.
Three studies gave oral cabergoline 0.5 mg daily for eight days from the day of hCG injection (Alvarez 2007a; Amir 2015; Jellad 2016), one study gave oral cabergoline 0.5 mg daily from the day after oocyte retrieval for five days before embryo transfer day (Fetisova 2014), and one study gave oral cabergoline 0.5 mg on two successive days, starting from the day of hCG injection and repeated one week later (Salah 2012). The Salah 2012 study also had a third treatment arm of oral prednisolone 10 mg daily from the day of hCG injection to the day of the pregnancy test (Salah 2012).
Two studies involving 382 women compared cabergoline plus hydroxyethyl starch (HES) versus HES alone (500 mL of HES by intravenous infusion during follicle aspiration plus oral cabergoline 0.5 mg daily for eight days starting on the day of hCG administration for Matorras 2013; 500 mL of HES by intravenous infusion on day of follicle aspiration and oral cabergoline 0.25 mg daily by mouth for eight days starting on the day of hCG administration for Shaltout 2012).
Two studies involving 235 women compared oral cabergoline 0.5 mg daily with human albumin (albumin 20 g 20% on day of oocyte retrieval and cabergoline for seven days beginning on the day of oocyte retrieval in Tehraninejad 2012; albumin 10 units 20% on day of oocyte retrieval and cabergoline for eight days beginning on the day of hCG injection in Torabizadeh 2013).
One study with 91 women involved three arms (oral cabergoline 0.5 mg daily for seven days after oocyte retrieval versus albumin (100 mL intravenous 30 minutes after retrieval within four hours) versus 6% HES 1000 mL intravenous 30 minutes after oocyte retrieval within four hours) (Ghahiri 2015).
One study involving 166 women compared cabergoline 0.5 mg daily for three weeks beginning the day after oocyte retrieval plus albumin 20 g on day of oocyte retrieval versus albumin 20 g alone (Carizza 2008).
Two studies involving 120 women compared cabergoline with coasting (cabergoline group received cabergoline 0.5 mg daily for seven or eight days after hCG administration and coasting group had gonadotropin administration withheld until serum E2 level was below 3000 pg/mL or serum E2 level started to decline before hCG administration) (Dalal 2014; Sohrabvand 2009). However, a fluid of 6% HES was also given to 58 women in the study by Dalal 2014, and the remaining included woman received an ascites tap instead of HES.
Comparisons with quinagolide
Two studies involving 454 women compared quinagolide versus placebo (quinagolide 150 µg daily for 15 days beginning on the day of hCG administration for Alhalabi 2011; three subgroups with doses of quinagolide 50 μg daily, 100 μg daily and 200 µg daily from the day of hCG administration until the day of serum hCG test (which was 17 ± 2 days after oocyte retrieval) for Busso 2010).
Comparisons with bromocriptine
One trial involving 47 women compared bromocriptine 2.5 mg daily versus folic acid 2.0 mg daily (as a placebo), both for 14 days, beginning the day of hCG administration (Beltrame 2013).
Outcomes
All 16 included studies reported the incidence of severe or moderate OHSS but only two studies reported on live birth rate (Busso 2010; Shaltout 2012). Ten studies reported the clinical pregnancy rate (Alvarez 2007a; Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). Torabizadeh 2013 only reported pregnancy rates of the women who developed moderate or severe OHSS (no significant difference between groups) and Alhalabi 2011 only mentioned that pregnancy rates were 'equal' between groups, without providing data on this outcome. Eight studies reported miscarriage rate (Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Tehraninejad 2012), four studies reported multiple pregnancy rate (Amir 2015; Carizza 2008; Dalal 2014; Tehraninejad 2012), and four studies reported any other adverse events of the treatment (Alvarez 2007a; Busso 2010; Carizza 2008; Shaltout 2012).
Excluded studies
We excluded 26 studies in the 2015 and 2016 searches together. The reasons for exclusion are explained in the Characteristics of excluded studies table.
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risk of bias. We contacted the original authors by e‐mail to clarify any information on methodological quality and study characteristics that were unclear (see 'Risk of bias' table in the Characteristics of included studies table).
Figure 2.
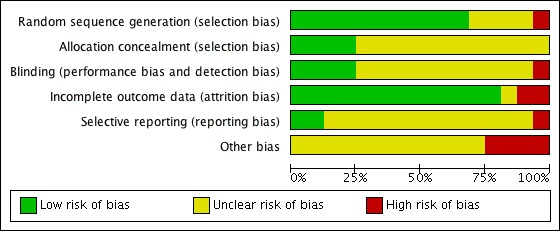
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
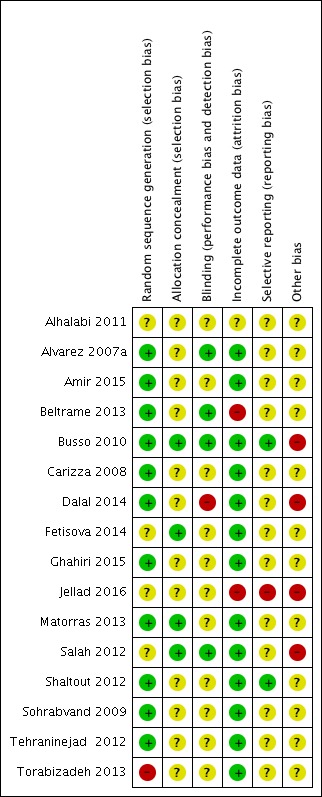
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Sequence generation (selection bias)
Eleven trials used computer‐generated randomisation (Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Carizza 2008; Dalal 2014; Ghahiri 2015; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). The randomisation process of three trials remained unclear from the publications (Alhalabi 2011; Fetisova 2014; Salah 2012). One trial mentioned that the already randomised participants were subsequently also included 'every other person', which we judged as high risk of bias (Torabizadeh 2013).
Allocation
Of the 16 included trials, four trials reported they allocated with sealed or closed envelopes (Busso 2010; Fetisova 2014; Matorras 2013; Salah 2012). The other 12 trials were unclear due to lack of detailed allocation information (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Carizza 2008; Dalal 2014; Ghahiri 2015; Jellad 2016; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013).
Blinding
Three studies were blinded to both assessors and participants (Alvarez 2007a; Beltrame 2013; Busso 2010), and one study was only blinded to participants (Salah 2012), while in five other studies used no blinding (Carizza 2008; Dalal 2014; Ghahiri 2015; Shaltout 2012; Tehraninejad 2012). One study blinded neither the women nor the lead physicians but did blind the ultrasound reporters (Amir 2015). Two studies blinded the lead physicians, but not the participants (Matorras 2013; Torabizadeh 2013). Seven studies reported no or limited information on blinding (Alhalabi 2011; Carizza 2008; Fetisova 2014; Jellad 2016; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012). For objective outcomes (e.g. pregnancy outcomes or live birth rate), blinding is not as important as for subjective outcomes, so we rated the unblinded studies as unclear rather than high risk of bias.
Incomplete outcome data
Six studies reported the information on dropouts and described the exact reasons (Alvarez 2007a; Busso 2010; Dalal 2014; Ghahiri 2015; Shaltout 2012; Tehraninejad 2012). Two other studies only stated that women withdrew from the study, without exact reasons (Carizza 2008; Salah 2012). However, only a small proportion of women (less than 5%) were lost to follow‐up, which does not have a clinically relevant impact on observed effect size, and hence we rated the studies at low risk of bias (Amir 2015; Fetisova 2014; Matorras 2013; Sohrabvand 2009; Torabizadeh 2013). The study of Beltrame 2013 had a high dropout number (40%) without mentioning reasons for dropout, and was therefore at high risk of bias. Jellad 2016 only reported on the subgroups of women within each arm of the study that actually went on to develop OHSS. Data from the non‐OHSS participants were lacking.
Selective reporting
Only two studies reported on the primary outcome of live birth rate (Busso 2010; Shaltout 2012). Fourteen studies reported on the primary outcome of incidence of moderate or severe OHSS.
Ten studies fully reported pregnancy rates (Alvarez 2007a; Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012), and two studies mentioned pregnancy rates without complete data (Alhalabi 2011; Torabizadeh 2013). Four studies reported multiple pregnancy (Amir 2015; Carizza 2008; Dalal 2014; Tehraninejad 2012). Eight studies reported miscarriage rate (Amir 2015; Busso 2010; Carizza 2008; Dalal 2014; Fetisova 2014; Matorras 2013; Shaltout 2012; Tehraninejad 2012). Jellad 2016 only reported pregnancy and miscarriage rates of the women in each arm that actually developed OHSS. Four studies reported adverse events (Alvarez 2007a; Busso 2010; Carizza 2008; Shaltout 2012).
Because of limited (fewer than 10) trials included per comparison, we were unable to make this assessment for the primary outcomes in this version of the review. In future updates of the review, where 10 or more trials are included, we will use a visual inspection of the funnel plot to look at reporting biases.
Other potential sources of bias
One study was sponsored by Ferring Pharmaceuticals (Busso 2010). One trial included young women with PCOS without other high risk factors identified (e.g. based on E2 or ultrasound) (Salah 2012).
Effects of interventions
See: Table 1
1 Dopamine agonist versus placebo/no intervention
Primary outcomes
1.1 Incidence of moderate or severe ovarian hyperstimulation syndrome per woman randomised
Eight studies reported the incidence of moderate or severe OHSS (Alhalabi 2011; Alvarez 2007a; Amir 2015; Beltrame 2013; Busso 2010; Fetisova 2014; Jellad 2016; Salah 2012). Dopamine agonists were associated with a lower risk of moderate or severe OHSS as compared with placebo/no intervention (OR 0.27, 95% CI 0.19 to 0.39; 1022 participants; 8 studies; I2 = 0%; moderate quality evidence) (Analysis 1.1; Figure 4). This suggests that if 28.6% of women taking placebo or no intervention experience moderate or severe OHSS, between 7.1% and 13.5% of women taking dopamine agonists will do so. When compared with placebo/no intervention, cabergoline (OR 0.26, 95% CI 0.16 to 0.42; 521 participants; 5 studies I2 = 0%), and quinagolide (OR 0.28, 95% CI 0.15 to 0.51; 454 participants; 2 studies; I2 = 30%) were associated with a lower risk of moderate or severe OHSS (Analysis 1.1; Figure 4). However, there was no evidence of a difference between bromocriptine and placebo (OR 0.29, 95% CI 0.08 to 1.14; 47 participants; 1 study) (Analysis 1.1; Figure 4).
Analysis 1.1.
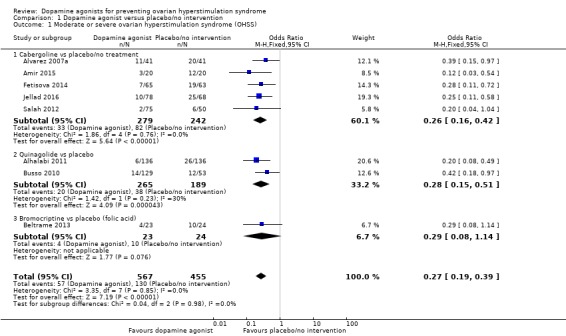
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).
Figure 4.
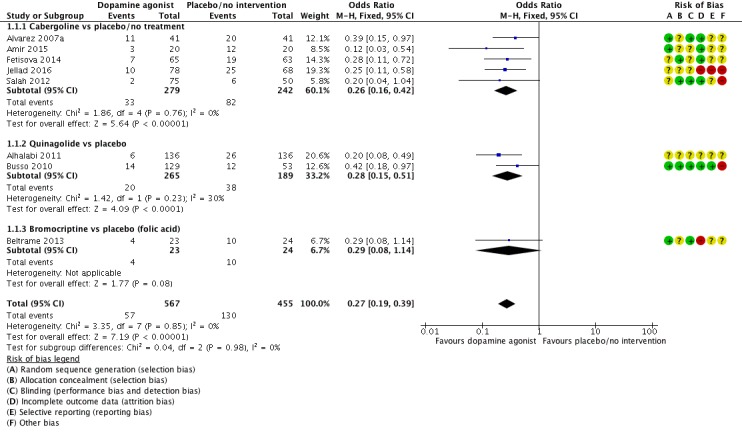
Forest plot of comparison 1: Dopamine agonist (without co‐intervention) versus placebo/no intervention, outcome: 1.1 moderate or severe ovarian hyperstimulation syndrome.
Subgroup analyses
1.1.1 severity of ovarian hyperstimulation syndrome The effect estimates were similar in the two subgroups and the test for subgroup differences showed no evidence of a difference between them (test for subgroup differences: Chi2 = 0.40, degrees of freedom (df) = 1 (P = 0.53), I2 = 0%) (Analysis 1.2).
Analysis 1.2.
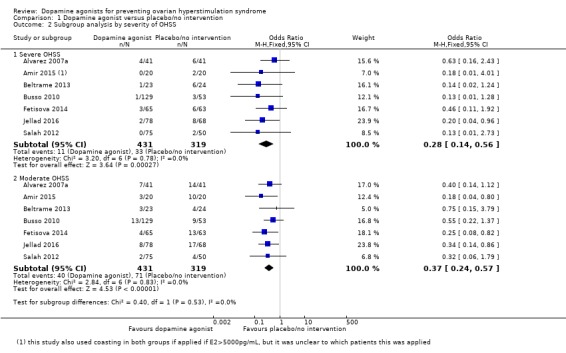
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 2 Subgroup analysis by severity of OHSS.
1.1.2 Dose of dopamine agonist All studies with cabergoline used a dose of 0.5 mg daily so subgroup analysis for dose was not possible.
1.2 Live birth rate per woman randomised
One study reported data on live birth rate (Busso 2010). There was no evidence of a difference between dopamine agonist (only including quinagolide) and placebo/no intervention (OR 1.01, 95% CI 0.53 to 1.91; 182 participants; 1 study; low quality evidence) (Analysis 1.3). This suggests that if 51% of women taking placebo or no intervention experience live birth, between 36% and 67% of women taking dopamine agonists will do so. In addition, there was no evidence of a difference between quinagolide and placebo/no intervention in the subgroup analysis for dose.
Analysis 1.3.
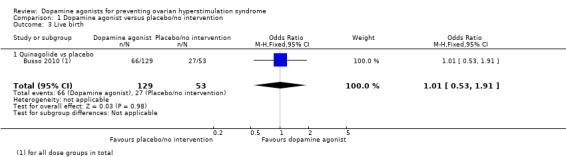
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 3 Live birth.
Secondary outcomes
1.3 Clinical pregnancy rate
Four trials reported the clinical pregnancy rate (Alvarez 2007a; Amir 2015; Busso 2010; Fetisova 2014). There was no evidence of a difference between dopamine agonist and placebo/no intervention (OR 0.81, 95% CI 0.54 to 1.22; 432 participants; 4 studies; I2 = 0%; moderate quality evidence) (Analysis 1.4). This suggests that if 40% of women taking placebo or no intervention experience clinical pregnancy, between 27% and 45% of women taking dopamine agonists will do so. There was no evidence of a difference between cabergoline and placebo/no intervention (OR 0.81, 95% CI 0.48 to 1.38; 250 participants; 3 studies; I2 = 0%), and between quinagolide and placebo (OR 0.81, 95% CI 0.43 to 1.54; 182 participants; 1 study).
Analysis 1.4.
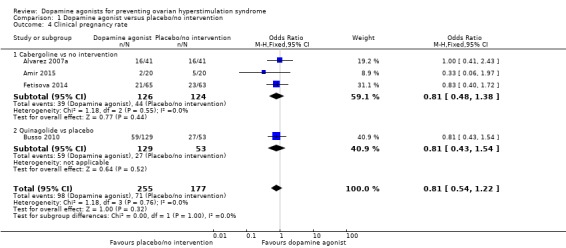
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 4 Clinical pregnancy rate.
1.4 Multiple pregnancy rate
Only one study reported multiple pregnancy rate (Amir 2015), and there was no evidence of difference between cabergoline and placebo (OR 0.32, 95% CI 0.01 to 8.26; 40 participants; 1 study; very low quality evidence) (Analysis 1.5). This suggests that if 5% of women taking placebo or no intervention experience multiple pregnancy, between 1% and 30% of women taking dopamine agonists will do so.
Analysis 1.5.
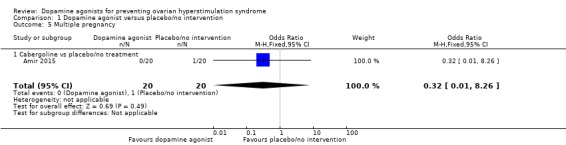
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 5 Multiple pregnancy.
1.5 Miscarriage rate
Two studies reported miscarriage rate (Amir 2015; Fetisova 2014). There was no conclusive evidence of a difference between dopamine agonist and placebo/no intervention (OR 0.66, 95% CI 0.19 to 2.28; 168 participants; 2 studies; I2 = 0%; low quality evidence) (Analysis 1.6). This suggests that if 7% of women taking placebo or no intervention experience a miscarriage, between 2% and 15% of women taking dopamine agonists will do so.
Analysis 1.6.
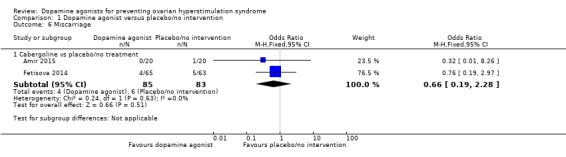
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 6 Miscarriage.
1.6 Any other adverse events of the treatment
Two trials reported adverse events (Alvarez 2007a; Busso 2010). Dopamine agonists were associated with an increased risk of adverse events (OR 4.54, 95% CI 1.49 to 13.84; 264 participants; 2 studies; I2 = 49%; very low quality evidence) (Analysis 1.7). This suggests that if 4% of women taking placebo or no intervention experience adverse events, between 6% and 38% of women taking dopamine agonists will do so. However, there was no conclusive evidence of a difference between cabergoline and placebo/no intervention (OR 2.24, 95% CI 0.62 to 8.14; 82 participants; 1 study) (Analysis 1.7). One trial reported that 17 women in the quinagolide group discontinued because of adverse events and no women in the placebo group (OR 16.64, 95% CI 0.98 to 282.02; 182 participants; 1 study) (Analysis 1.7) (Busso 2010).
Analysis 1.7.
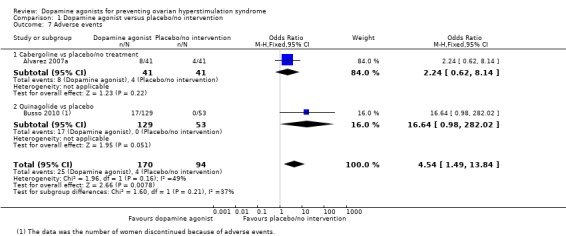
Comparison 1 Dopamine agonist versus placebo/no intervention, Outcome 7 Adverse events.
2 Dopamine agonist plus co‐intervention versus co‐intervention
Three studies compared dopamine agonist plus co‐intervention versus co‐intervention. The co‐interventions were HES (two RCTs) and albumin (one RCT).
Primary outcomes
2.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
Three studies reported the incidence of moderate or severe OHSS (Carizza 2008; Matorras 2013; Shaltout 2012). Dopamine agonists plus co‐intervention were not significantly associated with a lower risk of moderate or severe OHSS as compared with co‐intervention alone (OR 0.57, 95% CI 0.31 to 1.03; 548 participants; 3 studies; I2 = 44%) (Analysis 2.1; Figure 5). There was no evidence of a difference between the cabergoline plus albumin group and the albumin group (OR 0.55, 95% CI 0.23 to 1.34; 166 participants; 1 study), or between the cabergoline plus HES group versus the HES group (OR 0.58, 95% CI 0.26 to 1.30; 382 participants; 2 studies; I2 = 72%) (Analysis 2.1; Figure 5). As we included only three studies, we did not perform a subgroup analysis.
Analysis 2.1.
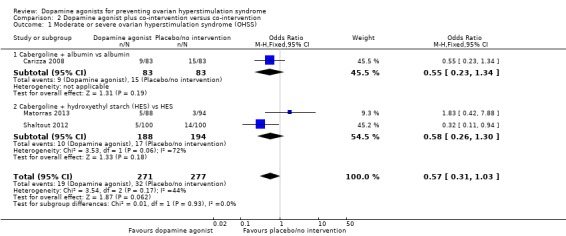
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).
Figure 5.
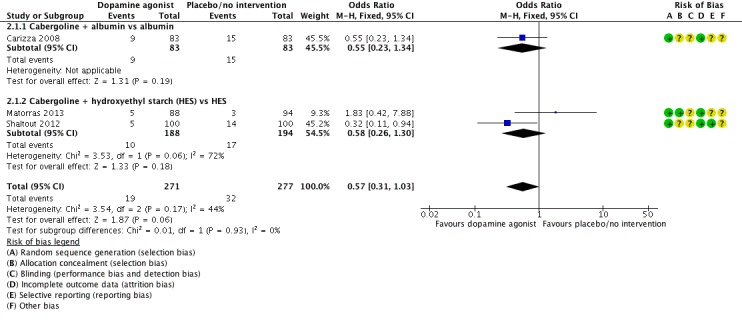
Forest plot of comparison: 2 Dopamine agonist plus co‐intervention versus co‐intervention, outcome: 2.1 Moderate or severe ovarian hyperstimulation syndrome.
2.2 Live birth rate per woman randomised
One study reported data on live birth rate (Shaltout 2012). There was no evidence of a difference between cabergoline plus HES and HES (OR 1.04, 95% CI 0.59 to 1.86; 200 participants; 1 study) (Analysis 2.2).
Analysis 2.2.
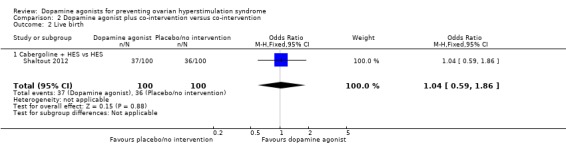
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 2 Live birth.
Secondary outcomes
2.3 Clinical pregnancy rate
Three trials reported the clinical pregnancy rate (Carizza 2008; Matorras 2013; Shaltout 2012). There was no evidence of a difference between dopamine agonist plus co‐intervention and co‐intervention alone (OR 1.00, 95% CI 0.71 to 1.40; 548 participants; 3 studies; I2 = 0%) (Analysis 2.3). There was no evidence of a difference between cabergoline plus albumin and albumin (OR 1.05, 95% CI 0.56 to 1.96; 166 participants; a study), and between cabergoline plus HES and HES (OR 0.98, 95% CI 0.65 to 1.47; 382 participants; 2 studies; I2 = 0%) (Analysis 2.3).
Analysis 2.3.
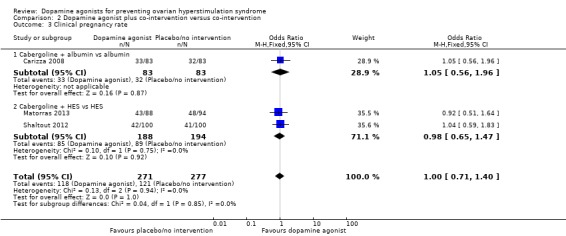
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 3 Clinical pregnancy rate.
2.4 Multiple pregnancy rate
Only one study reported multiple pregnancy rate (Carizza 2008). There was no evidence of a difference between cabergoline plus albumin and albumin (OR 2.02, 95% CI 0.18 to 22.77; 166 participants; 1 study) (Analysis 2.4).
Analysis 2.4.
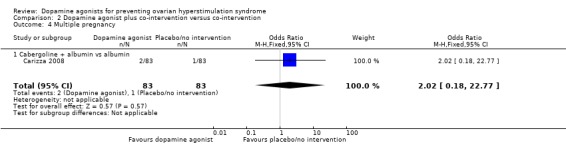
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 4 Multiple pregnancy.
2.5 Miscarriage rate
Three studies reported miscarriage rate (Carizza 2008; Matorras 2013; Shaltout 2012). There was no conclusive evidence of a difference between dopamine agonist plus co‐intervention and co‐intervention (OR 0.65, 95% CI 0.30 to 1.42; 548 participants; 3 studies; I2 = 0%) (Analysis 2.5). There was no evidence of a difference between cabergoline plus albumin and albumin (OR 0.33, 95% CI 0.03 to 3.19; 166 participants; 1 study), and between cabergoline plus HES and HES (OR 0.73, 95% CI 0.31 to 1.68; 382 participants; 2 studies; I2 = 0%) (Analysis 2.5).
Analysis 2.5.
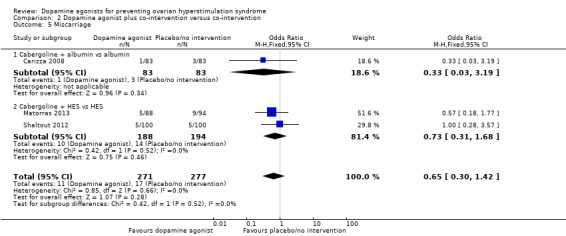
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 5 Miscarriage.
2.6 Any other adverse events of the treatment
Two trials reported adverse events (Carizza 2008; Shaltout 2012). Dopamine agonists plus co‐intervention were associated with an increased risk of adverse events (OR 3.03, 95% CI 0.12 to 75.28; 366 participants; 2 studies; I2 = 0%) (Analysis 2.6). However, there was no inclusive evidence of a difference between cabergoline plus HES and HES (OR 3.03, 95% CI 0.12 to 75.28; 200 participants; 1 study). One trial detected no adverse events (Carizza 2008).
Analysis 2.6.
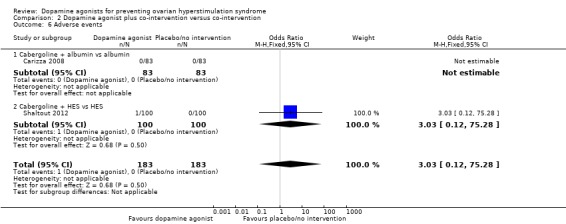
Comparison 2 Dopamine agonist plus co‐intervention versus co‐intervention, Outcome 6 Adverse events.
3 Dopamine agonist versus other active intervention
3.1 Cabergoline versus human albumin
Primary outcomes
3.1.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
Three studies reported the incidence of moderate or severe OHSS for the comparison of cabergoline versus human albumin (Ghahiri 2015; Tehraninejad 2012; Torabizadeh 2013). Cabergoline was associated with a lower incidence of severe or moderate OHSS than human albumin (OR 0.21, 95% CI 0.12 to 0.38; 296 participants; 3 studies; I2 = 72%) (Analysis 3.1; Figure 6).
Analysis 3.1.
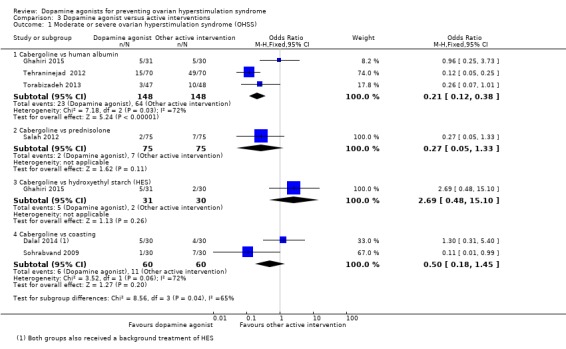
Comparison 3 Dopamine agonist versus active interventions, Outcome 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS).
Figure 6.
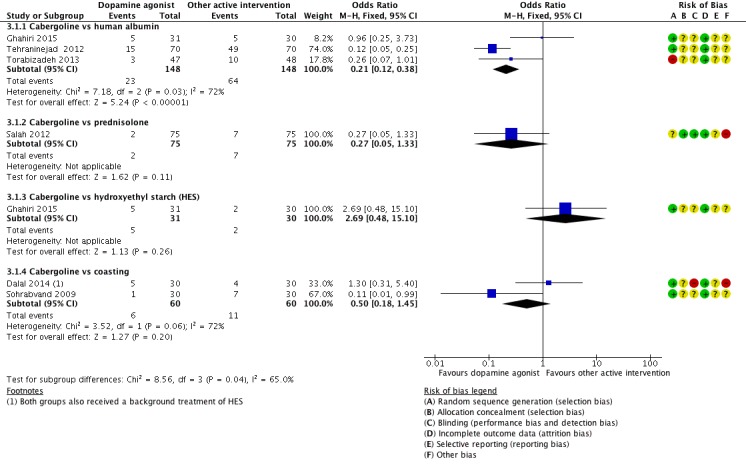
Forest plot of comparison 3: Cabergoline versus active interventions, outcome: 3.1 moderate or severe ovarian hyperstimulation syndrome.
3.1.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus human albumin on live birth rate.
Secondary outcomes
3.1.3 Clinical pregnancy rate
There was no evidence of any difference between the cabergoline and human albumin groups in the study of Tehraninejad 2012 (OR 0.68, 95% CI 0.33 to 1.38; 140 participants; 1 study) (Analysis 3.2). The study of Torabizadeh 2013 only reported pregnancy rates in women who developed moderate or severe OHSS.
Analysis 3.2.
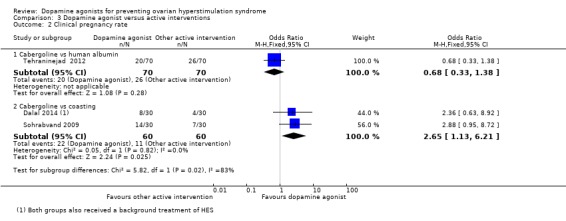
Comparison 3 Dopamine agonist versus active interventions, Outcome 2 Clinical pregnancy rate.
3.1.4 Multiple pregnancy rate
There was no evidence of a difference between the cabergoline and human albumin groups (OR 0.58, 95% CI 0.13 to 2.54; 140 participants; 1 study) (Analysis 3.3).
Analysis 3.3.
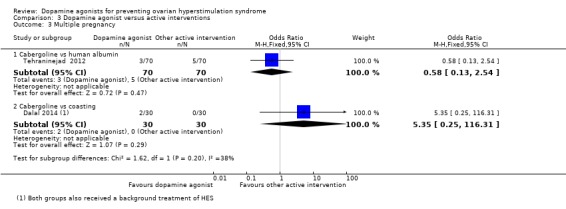
Comparison 3 Dopamine agonist versus active interventions, Outcome 3 Multiple pregnancy.
3.1.5 Miscarriage rate
There was no evidence of any difference between the cabergoline and human albumin groups (OR 0.32, 95% CI 0.03 to 3.19; 140 participants; 1 study) (Analysis 3.4).
Analysis 3.4.
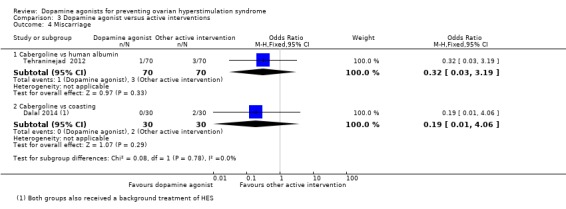
Comparison 3 Dopamine agonist versus active interventions, Outcome 4 Miscarriage.
3.1.6 Any other adverse effects of the treatment
There trials reported no data comparing cabergoline versus prednisolone on any other adverse effects.
3.2 Cabergoline versus prednisolone
3.2.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
Only one trial reported on the comparison of cabergoline versus prednisolone in the incidence of severe or moderate OHSS (Salah 2012). There was no evidence of a difference between the groups (OR 0.27, 95% CI 0.05 to 1.33; 150 participants; 1 study) (Analysis 3.1; Figure 6).
3.2.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus prednisolone on live birth rate.
Secondary outcomes
3.2.3 Clinical pregnancy rate
We found no trials comparing cabergoline versus prednisolone on clinical pregnancy rate.
3.2.4 Multiple pregnancy rate
We found no trials comparing cabergoline versus prednisolone on multiple pregnancy rate.
3.2.5 Miscarriage rate
We found no trials comparing cabergoline versus prednisolone on miscarriage rate.
3.2.6 Any other adverse effects of the treatment
We found no trials comparing cabergoline versus prednisolone on adverse effects.
3.3 Cabergoline versus hydroxyethyl starch
Primary outcomes
3.3.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
There was no evidence of a difference between the cabergoline and HES group in incidence of severe or moderate OHSS (OR 2.69, 95% CI 0.48 to 15.10; 61 participants; 1 study) (Analysis 3.1; Figure 6).
3.3.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus HES on live birth rate.
Secondary outcomes
3.3.3 Clinical pregnancy rate
We found no trials comparing cabergoline versus HES on clinical pregnancy rate.
3.3.4 Multiple pregnancy rate
We found no trials comparing cabergoline versus HES on multiple pregnancy rate.
3.3.5 Miscarriage rate
We found no trials comparing cabergoline versus HES on miscarriage rate.
3.3.6 Any other adverse effects of the treatment
We found no trials comparing cabergoline versus HES on adverse effects.
3.4 Cabergoline versus coasting
Primary outcomes
3.4.1 Incidence of severe or moderate ovarian hyperstimulation syndrome per woman randomised
Two trials provided data on the incidence of severe or moderate OHSS (Dalal 2014; Sohrabvand 2009). There was no evidence of a difference between the groups (OR 0.50, 95% CI 0.18 to 1.45; 120 participants; 2 studies; I2 = 72%) (Analysis 3.1; Figure 6).
3.4.2 Live birth rate per woman randomised
We found no trials comparing cabergoline versus coasting on live birth rate.
Secondary outcomes
3.4.3 Clinical pregnancy rate
There was a higher clinical pregnancy rate with cabergoline compared with coasting clinical pregnancy rate (OR 2.65, 95% CI 1.13 to 6.21; 120 participants; 2 studies; I2 = 0%) (Analysis 3.2).
3.4.4 Multiple pregnancy rate
There was no evidence of a difference between the cabergoline and coasting on multiple pregnancy rate (OR 5.35, 95% CI 0.25 to 116.31; 60 participants; 1 study) (Analysis 3.3).
3.4.5 Miscarriage rate
There was no evidence of a difference between the cabergoline and coasting on miscarriage rate (OR 0.19, 95% CI 0.01 to 4.06; 60 participants; 1 study) (Analysis 3.4).
Publication bias
A funnel plot was not necessary as we included fewer than 10 trials in the analyses. This will be assessed in future updates if there are 10 or more trials.
Sensitivity analysis
We conducted a prespecified sensitivity analysis. When we excluded four studies with high risk of bias from Analysis 1.1 (Beltrame 2013; Busso 2010; Jellad 2016; Salah 2012), the lower incidence of moderate or severe OHSS with dopamine agonists compared with placebo/no intervention remained unchanged (OR 0.25, 95% CI 0.15 to 0.41; 522 participants; 4 studies; I2 = 0%). The results were similar for moderate or severe OHSS between cabergoline and human albumin (OR 0.20, 95% CI 0.11 to 0.38; 201 participants; 2 studies; I2 = 86%) when we excluded Torabizadeh 2013 from Analysis 3.1. However, cabergoline became associated with a lower risk of moderate or severe OHSS than coasting (OR 0.11. 95% CI 0.01 to 0.99; 60 participants; 1 study) when Dalal 2014 was excluded from Analysis 3.1. In addition, use of a random‐effects model or the assumptions made about missing data did not affect the results.
Discussion
Summary of main results
This systematic review evaluated the effectiveness and safety of dopamine agonists for preventing OHSS in high‐risk women during ART treatment and performed a meta‐analysis. Eight trials compared dopamine agonist with placebo or no intervention, three trials compared dopamine agonist in combination with co‐intervention with co‐intervention and five trials compared dopamine agonists with other active interventions. Overall, when compared with placebo or no intervention, dopamine agonists had a lower risk of developing moderate or severe OHSS without influencing pregnancy outcomes such as live birth rate for those women who proceeded to have a fresh embryo transfer, clinical pregnancy rate, multiple pregnancy rate and miscarriage rate. However, data on the live birth rate were scarce in the included trials. In general in OHSS trials, it will be considered unethical to withhold women who are at risk of OHSS of having all their embryos frozen for replacement in a subsequent cycle, as current embryo survival rates after freezing are generally excellent and the transfer of a frozen embryo in an unstimulated cycle avoids the risk of OHSS in that cycle.
There was an increased risk of adverse events, which occurred rarely, associated with dopamine agonists particularly when using quinagolide. The use of cabergoline was associated with a lower risk of moderate or severe OHSS, without influencing pregnancy outcomes when compared with placebo or no intervention. Quinagolide appeared to reduce the risk of moderate or severe OHSS, but might increase the incidence of adverse events. With the limited data available, bromocriptine did not influence the incidence of moderate or severe OHSS. There was no evidence of a difference between dopamine agonist plus co‐intervention and co‐intervention in the outcomes of interest. For dopamine agonists compared with other active interventions, we found only a comparison with cabergoline. Compared with human albumin, cabergoline might reduce the incidence of moderate or severe OHSS, but there was no evidence of a difference for comparisons between cabergoline and prednisolone, HES or coasting. Cabergoline was associated with a higher clinical pregnancy rate than coasting. In other respects, there was no evidence of a difference between cabergoline and other active interventions with respect to the other studied outcomes.
The quality of the evidence for the comparison of dopamine agonist with placebo/no intervention was generally moderate; the main limitations were poor reporting of study methods (mostly lack of details on randomisation and blinding), heterogeneity across trials and risk of imprecision (low events or small sample sizes).
Overall completeness and applicability of evidence
Compared with previous review (Tang 2012), we included 14 additional trials. In total, this updated Cochrane Review included 16 trials involving 2091 high‐risk women. The study populations varied among these trials regarding the definition of 'high‐risk' of OHSS. This may influence the incidence of OHSS and limits the applicability of study results in practice. However, as some trials even excluded the truly high‐risk women from participating, it can be postulated that the effect of dopamine agonists could be even larger when these women would have been included. Most of the trials defined moderate or severe OHSS according to Golan's classification (Golan 1989), but three trials used other definitions, which may induce bias when pooling the data of the various studies. Only a few studies reported pregnancy outcomes such as live birth. The influence of dopamine agonists on pregnancy outcomes requires further study; however, many units will practice an embryo 'freeze‐all' approach for women at risk of OHSS and therefore data for pregnancy outcomes may not be forthcoming. Most of the trials evaluated the dopamine agonist cabergoline, whereas two trials evaluated quinagolide and one trial evaluated bromocriptine. In addition, our evidence was applicable in low‐ to middle‐income countries as most trials were performed in these countries. Finally, due to the lack of studies comparing a dopamine agonist with another dopamine agonist, we are unable to determine which dopamine agonist is most effective in preventing OHSS.
Quality of the evidence
The methodological quality of the 16 included trials varied. Eleven trials used correct random sequence generation, and only four trials had a low risk of bias in the domain of allocation concealment. Four trials were either single or double blind. One trial was at high risk of bias due to a high percentage of dropouts without reported reasons (Beltrame 2013). All trials reported the outcomes of OHSS, but only two studies provided the primary outcome of 'live birth rate'. See Figure 2 and Figure 3 for the 'Risk of bias' assessments of the included studies.
Moreover, the overall body of evidence for primary outcomes between dopamine agonist and placebo or no intervention was moderate. The main reasons for downgrading the quality of the evidence were: poor reporting of study methods (e.g. 25% of RCTs did not report the methods of allocation concealment or blinding) and risk of imprecision (e.g. low events). See Table 1 for more details.
Potential biases in the review process
We tried to identify all eligible trials by conducting a systematic review of the literature without restrictions of publication type or language. Moreover, we contacted the authors of trials for more information about any unpublished data. In addition, we made assumptions about missing data, but they seemed to be robust in the sensitivity analysis.
Agreements and disagreements with other studies or reviews
Our results are in agreement with most of the systematic reviews and meta‐analyses on dopamine agonists for the prevention for OHSS (Baumgarten 2013; Guo 2016; Kalampokas 2013; Kasum 2014; Leitao 2014; Youssef 2010). The first systematic review published in 2010 included only four RCTs with 570 women, and showed that cabergoline might reduce the incidence of OHSS. However, it did not show evidence of a reduction in severe OHSS (Youssef 2010), which is consistent with our previous Cochrane Review (Tang 2012). This might be caused by a small sample size or low event rate of severe OHSS. In 2014, another systematic review included eight trials involving 858 women and showed that cabergoline could reduce the risk of moderate or severe OHSS, as well as severe OHSS (Leitao 2014). In 2016, one systematic review and network meta‐analysis of 31 RCTs involving 7181 women showed that cabergoline was superior to placebo or human albumin, or glucocorticoid in decreasing OHSS incidence, and there was no evidence of any difference between cabergoline and other active interventions (e.g. aspirin, HES, calcium infusion or metformin). However, until 2016, few systematic reviews included types of dopamine agonist other than cabergoline. One systematic review showed that a dopamine agonist appeared to be effective for the prevention of OHSS (Baumgarten 2013). Moreover, no evidence of adverse effects on pregnancy outcomes was detected (Baumgarten 2013; Leitao 2014; Youssef 2010). Compared with previous systematic reviews, our review includes more trials and women, and can therefore draw a more robust conclusion that the use of dopamine agonists could reduce the incidence of moderate or severe OHSS.
Authors' conclusions
In high‐risk women, dopamine agonists seem to reduce the incidence of moderate or severe ovarian hyperstimulation syndrome (OHSS) when compared to placebo/no intervention, based on moderate quality evidence. The dopamine agonists cabergoline and quinagolide reduce the incidence of moderate or severe OHSS. There is very minimal evidence from one trial that bromocriptine does not reduce the incidence of moderate or severe OHSS. There is no evidence that cabergoline or quinagolide influence pregnancy outcomes such as live birth rate, clinical pregnancy rate, multiple pregnancy rate and miscarriage rate. However, quinagolide might increase the incidence of adverse events, and we should therefore weigh the benefits and harms of this medication before starting treatment. In addition, some evidence suggests that a dopamine agonist plus other active intervention might not offer an additive benefit in the incidence of moderate or severe OHSS, as well as other outcomes of interest when compared with other active intervention.
Further research should consider the risks of dopamine agonists, compare different types of dopamine agonists with regard to clinical outcomes and safety profiles, compare different doses (lowest possible dose while safe‐guarding the preventive effect) and investigate the potential role of bromocriptine in OHSS prevention. Moreover, comparisons with other treatments that have been proven effective (such as the use of gonadotropin‐releasing hormone (GnRH) antagonist protocols or metformin in women with polycystic ovary syndrome (PCOS)) and the consideration of combination treatments should be studied to find the most effective strategy to prevent OHSS. Special attention should be paid to the definition of high‐risk women. Thus, large, well‐designed and well‐executed randomised controlled trials (RCTs) that involve all clinical endpoints (i.e. moderate and severe OHSS, and if women were to proceed to a fresh embryo transfer; clinical pregnancy rate, miscarriage rate, ongoing pregnancy rate, live birth rate and adverse events) are necessary to evaluate the promising role of dopamine agonists in OHSS prevention further.
Acknowledgements
We acknowledge the Cochrane Gynaecology and Fertility Group (formerly MDSG), especially the Information Specialist, Marian Showell, and Managing Editors, Helen Nagels and Editor Jane Marjoribanks. Thanks to Dr Amr Salah, Dr Farnaz Sohrabvand, Dr Wellington Martins, Dr Irina Fetisova and Dr Rutvij Dalal for providing more detailed information on several trials.
The authors of the 2016 update thank Dr Tamara Hunter, Dr Yongfang Hu and Dr Xiaoyan Sheng for their contributions to previous versions of this review.
Appendices
Appendix 1. Gynaecology and Fertility (formerly MDSG) search string
PROCITE Platform
From inception until 15 August 2016
Keywords CONTAINS "ovarian hyperstimulation syndrome " or "ovarian hyperstimulation" or "OHSS" or Title CONTAINS "ovarian hyperstimulation syndrome " or "ovarian hyperstimulation" or "OHSS"
AND
Keywords CONTAINS "cabergoline" or "Dopamine agonists" or "Dopamine" or "bromocriptine" or "quinagolide" or Title CONTAINS "cabergoline" or "Dopamine agonists" or "Dopamine" or "bromocriptine" or "quinagolide" (36 hits)
Appendix 2. CENTRAL search strategy
CRSO Web platform
from inception until 15 August 2016
#1 MESH DESCRIPTOR Ovarian Hyperstimulation Syndrome EXPLODE ALL TREES 163
#2 OHSS:TI,AB,KY 274
#3 (Ovar* adj2 Hyperstimulation):TI,AB,KY 902
#4 #1 OR #2 OR #3 968
#5 MESH DESCRIPTOR Ergolines EXPLODE ALL TREES 925
#6 Ergoline*:TI,AB,KY 237
#7 cabergoline:TI,AB,KY 171
#8 (Dostinex or Cabaser*):TI,AB,KY 4
#9 (Dopamine Agonist*):TI,AB,KY 937
#10 MESH DESCRIPTOR Dopamine Agonists EXPLODE ALL TREES 1449
#11 MESH DESCRIPTOR Bromocriptine EXPLODE ALL TREES 455
#12 Bromocriptine:TI,AB,KY 844
#13 quinagolide*:TI,AB,KY 17
#14 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 2461
#15 #4 AND #14 38
Appendix 3. MEDLINE search strategy
OVID platform
From 1946 to 15 August 2016
1 exp Ovarian Hyperstimulation Syndrome/ (1962) 2 OHSS.tw. (1420) 3 (Ovar$ adj2 Hyperstimulation).tw. (4387) 4 or/1‐3 (4799) 5 exp Ergolines/ (20838) 6 cabergoline.tw. (1215) 7 Ergoline$.tw. (546) 8 (Dostinex or Cabaser$).tw. (13) 9 Dopamine Agonist$.tw. (6908) 10 exp Dopamine Agonists/ (27910) 11 exp Bromocriptine/ (6867) 12 Bromocriptine.tw. (6547) 13 quinagolide$.tw. (117) 14 or/5‐13 (43020) 15 4 and 14 (105) 16 randomized controlled trial.pt. (428367) 17 controlled clinical trial.pt. (91556) 18 randomized.ab. (366942) 19 placebo.tw. (182638) 20 clinical trials as topic.sh. (178949) 21 randomly.ab. (261578) 22 trial.ti. (160440) 23 (crossover or cross‐over or cross over).tw. (70771) 24 or/16‐23 (1085156) 25 (animals not (humans and animals)).sh. (4266646) 26 24 not 25 (999370) 27 26 and 15 (41)
Appendix 4. Embase search strategy
OVID platform
From 1974 to 15 August 2016
1 exp ovary hyperstimulation/ (7481) 2 (ovar$ adj2 hyperstimulation).tw. (6125) 3 OHSS.tw. (2299) 4 or/1‐3 (9268) 5 cabergoline.tw. (1762) 6 exp ergoline derivative/ (990) 7 ergoline$.tw. (638) 8 (Dostinex or Cabaser$).tw. (347) 9 exp dopamine receptor stimulating agent/ or exp cabergoline/ (172347) 10 (dopamine adj2 agent$).tw. (509) 11 (dopamine adj2 agonist$).tw. (12113) 12 quinagolide$.tw. (170) 13 exp bromocriptine/ (17941) 14 bromocriptine.tw. (7195) 15 or/5‐14 (174581) 16 Clinical Trial/ (862238) 17 Randomized Controlled Trial/ (413467) 18 exp randomization/ (71619) 19 Single Blind Procedure/ (22711) 20 Double Blind Procedure/ (130713) 21 Crossover Procedure/ (48263) 22 Placebo/ (279471) 23 Randomi?ed controlled trial$.tw. (141716) 24 Rct.tw. (21228) 25 random allocation.tw. (1552) 26 randomly allocated.tw. (25411) 27 allocated randomly.tw. (2146) 28 (allocated adj2 random).tw. (762) 29 Single blind$.tw. (17830) 30 Double blind$.tw. (164731) 31 ((treble or triple) adj blind$).tw. (580) 32 placebo$.tw. (237405) 33 prospective study/ (346790) 34 or/16‐33 (1604891) 35 case study/ (39627) 36 case report.tw. (312069) 37 abstract report/ or letter/ (969653) 38 or/35‐37 (1314128) 39 34 not 38 (1563365) 40 4 and 15 and 39 (97)
Appendix 5. PsycINFO search strategy
OVID platform
From 1806 to 15 August 2016
1 Ovarian Hyperstimulation Syndrome.tw. (4) 2 OHSS.tw. (6) 3 (Ovar$ adj2 Hyperstimulation).tw. (10) 4 or/1‐3 (14) 5 exp dopamine agonists/ (19735) 6 cabergoline.tw. (114) 7 Ergoline$.tw. (45) 8 (Dostinex or Cabaser$).tw. (2) 9 Dopamine Agonist$.tw. (2260) 10 Bromocriptine.tw. (660) 11 exp bromocriptine/ (292) 12 quinagolide$.tw. (5) 13 or/5‐12 (21063) 14 4 and 13 (0)
Appendix 6. CINAHL
EBSCO platform
From inception until 15 August 2016
| # | Query | Results |
| S14 | S4 AND S13 | 11 |
| S13 | S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 | 1,805 |
| S12 | TX quinagolide* | 4 |
| S11 | TX Bromocriptine | 303 |
| S10 | (MM "Bromocriptine") | 97 |
| S9 | TX (Dostinex or Cabaser*) | 1 |
| S8 | TX cabergoline | 110 |
| S7 | TX Dopamine Agonist* | 1,487 |
| S6 | (MM "Dopamine Agonists+") | 822 |
| S5 | TX Ergoline* | 14 |
| S4 | S1 OR S2 OR S3 | 362 |
| S3 | TX (Ovar* N2 Hyperstimulation) | 352 |
| S2 | TX OHSS | 79 |
| S1 | (MM "Ovarian Hyperstimulation Syndrome") | 140 |
Appendix 7. ICTRP
Web platform
15 August 2016
OHSS AND dopamine OR cabergoline OR quinagolide OR bromocriptine (75 hits)
Appendix 8. Clinicaltrials.gov
Web platform
15 August 2016
OHSS AND dopamine OR cabergoline OR quinagolide OR bromocriptine (46 hits)
Appendix 9. PubMed
Web platform
15 August 2016
OHSS[Title/Abstract] AND (dopamine[Title/Abstract] OR cabergoline[Title/Abstract] OR quinagolide[Title/Abstract]) AND Clinical Trial[ptyp] (12 hits)
Appendix 10. Data extraction form
| General trial characteristics |
| First author Publish year Citation: Contact author detail: |
| Eligibility |
| 1. Is the study an RCT? 2. High‐risk Women? 3. How OHSS defined? 4. Administration of cabergoline? Decision: If all replies yes means include, otherwise exclude |
| Characteristics of the included studies |
| Risk of bias |
|
| Methods |
| Inclusion criteria: Exclusion criteria: |
| Participants |
| Total number: Diagnosis criteria: Age (mean ± SD): treat group vs control group: BMI (mean ± SD): treat group vs control group: Duration of infertility: Causes of infertility: |
| Interventions |
| Treat group:(dose, administration of drug, duration of treatment) Control group (placebo or no intervention): |
| Outcomes |
|
| Results |
For each outcome of interest:
|
| Miscellaneous |
BMI: body mass index; OHSS: ovarian hyperstimulation syndrome; RCT: randomised controlled trial; SD: standard deviation. |
Data and analyses
Comparison 1.
Dopamine agonist versus placebo/no intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) | 8 | 1022 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.19, 0.39] |
| 1.1 Cabergoline vs placebo/no treatment | 5 | 521 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.42] |
| 1.2 Quinagolide vs placebo | 2 | 454 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.51] |
| 1.3 Bromocriptine vs placebo (folic acid) | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.14] |
| 2 Subgroup analysis by severity of OHSS | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Severe OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.14, 0.56] |
| 2.2 Moderate OHSS | 7 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.24, 0.57] |
| 3 Live birth | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 3.1 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.91] |
| 4 Clinical pregnancy rate | 4 | 432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.22] |
| 4.1 Cabergoline vs no intervention | 3 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.38] |
| 4.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.43, 1.54] |
| 5 Multiple pregnancy | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 5.1 Cabergoline vs placebo/no treatment | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 6 Miscarriage | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cabergoline vs placebo/no treatment | 2 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.28] |
| 7 Adverse events | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.54 [1.49, 13.84] |
| 7.1 Cabergoline vs placebo/no treatment | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.62, 8.14] |
| 7.2 Quinagolide vs placebo | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.64 [0.98, 282.02] |
Comparison 2.
Dopamine agonist plus co‐intervention versus co‐intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.03] |
| 1.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.34] |
| 1.2 Cabergoline + hydroxyethyl starch (HES) vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.30] |
| 2 Live birth | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 2.1 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.86] |
| 3 Clinical pregnancy rate | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.40] |
| 3.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.96] |
| 3.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 4 Multiple pregnancy | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 4.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.77] |
| 5 Miscarriage | 3 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.42] |
| 5.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.19] |
| 5.2 Cabergoline + HES vs HES | 2 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.68] |
| 6 Adverse events | 2 | 366 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
| 6.1 Cabergoline + albumin vs albumin | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Cabergoline + HES vs HES | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.28] |
Comparison 3.
Dopamine agonist versus active interventions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Moderate or severe ovarian hyperstimulation syndrome (OHSS) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Cabergoline vs human albumin | 3 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.12, 0.38] |
| 1.2 Cabergoline vs prednisolone | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.33] |
| 1.3 Cabergoline vs hydroxyethyl starch (HES) | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.48, 15.10] |
| 1.4 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.18, 1.45] |
| 2 Clinical pregnancy rate | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.38] |
| 2.2 Cabergoline vs coasting | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.13, 6.21] |
| 3 Multiple pregnancy | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.54] |
| 3.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.25, 116.31] |
| 4 Miscarriage | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cabergoline vs human albumin | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.19] |
| 4.2 Cabergoline vs coasting | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.06] |
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2016 | New search has been performed | Amended title and methods to include all kinds of dopamine agonist, new searches, included 14 studies (Alhalabi 2011; Amir 2015; Beltrame 2013; Busso 2010; Dalal 2014; Fetisova 2014; Ghahiri 2015; Jellad 2016, Matorras 2013; Salah 2012; Shaltout 2012; Sohrabvand 2009; Tehraninejad 2012; Torabizadeh 2013). |
| 21 August 2016 | New citation required and conclusions have changed | The extended scope and addition of 14 studies have led to a change in the conclusions of this review. |
History
Protocol first published: Issue 7, 2010 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 24 April 2013 | New search has been performed | Review Update, more data extracted from Shaltout 2012 |
| 17 December 2012 | New search has been performed | Review updated, three trials added: Salah 2012; Shaltout 2012; Tehraninejad 2012 |
| 17 December 2012 | New citation required but conclusions have not changed | Three new trials added, but no change to conclusions |
| 4 September 2011 | New search has been performed | Search updated to 2 September 2011; substantive amendment |
| 10 January 2010 | Amended | Converted to new review format. |
| 2 January 2010 | New citation required and major changes | Substantive amendment |
Differences between protocol and review
2016 update: we amended the protocol to broaden the scope of the review from "cabergoline" to "dopamine agonists" as the studied intervention. We changed the search strategies, inclusion criteria and title of the review accordingly.
Methods: changed Review authors for selection of studies or data extraction and management.
Subgroups: added subgroups by type of dopamine agonist.
Sensitivity analysis: added sensitivity analyses by excluding trials with high risk of bias and by using a random‐effects model.
Subgroup analysis on route of administration of drugs could not be performed as all dopamine agonists were administered orally.
Subgroup analysis on number of embryos transferred could not be performed as the RCTs did not provide these data.
Subgroup analyses on duration of treatment were not performed due to varied duration among the trials, which might result in only one included trial.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled prospective study No details on randomisation Cabergoline vs no drugs Setting: Syria |
|
| Participants | 272 high‐risk women undergoing ICSI with long protocol using GnRHa, E2 level on day of hCG ≥ 4000 pg/mL, ≥ 20 follicles ≥ 10 mm in diameter Quinagolide group: 136 women Control group: 136 women June 2007 to January 2010 |
|
| Interventions | Quinagolide group: quinagolide (Norprolac) 150 mg/day from the day of hCG administration for 15 days (6/136 (4.41%) women developed OHSS) Control group: no drugs (126/136 (9.12%) women developed OHSS) |
|
| Outcomes | OHSS symptoms assessed according to Gola's classification system, 4, 8 and 12 days after hCG administration Incidence of OHSS (quinagolide group vs control group): 6/136 vs 26/136 Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated, numbers reported as "similar rates" Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | 2 different abstracts: in the Human Reproduction abstract: control group = 98 women, in the Fertility and Sterility abstract: control group = 136 women. This difference made it at risk for improper randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement; only abstract available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of sufficient information to permit judgement; only abstract available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lack of sufficient information to permit judgement; only abstract available |
| Selective reporting (reporting bias) | Unclear risk | Lack of sufficient information to permit judgement; only abstract available. No reporting on adverse effects or tolerability |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement; only abstract available. 2 different abstracts with different control group size, suggesting improper randomisation |
| Methods | Parallel design, single‐centre randomised controlled trial Computer‐based randomisation Cabergoline vs placebo Setting: Spain |
|
| Participants | 82 oocytes donors, high‐risk women with development of 20 to 30 follicles > 12 mm in diameter and retrieval of > 20 oocytes Exclusion criterion: coasting Cabergoline group: 41 women, only 35 women remained, because 6 women were discarded for < 20 oocytes retrieved Control group: 41 women, only 32 women remained, because 7 women were discarded for < 20 oocytes retrieved and 2 donors decided to withdraw No differences between groups in age or BMI; did not report the duration of infertility and causes of infertility |
|
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day for 8 days from the day of hCG injection Control group: placebo tablet daily for 8 days |
|
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate (cabergoline group vs control group): 16/41 vs 16/41 Multiple pregnancy rate: not stated Any other adverse effects of the treatment (cabergoline group vs control group): 8/41 vs 4/41 (adverse effects) |
|
| Notes | Supported by Grant SAF2004‐06028 from Spanish Government | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated into two groups based on a computer randomization" |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessor and participants blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "thirteen patients discarded for not meeting the inclusion criteria and two donors decided to withdraw" |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Parallel design, single‐centre, randomised controlled trial Computer‐based randomisation Cabergoline vs no intervention Setting: Israel |
|
| Participants | 40 high‐risk women undergoing IVF/ET or IVF‐PGD, aged 18 to 40 years, serum E2 > 4000 pg/mL or the development of > 20 follicles > 12 mm in diameter Exclusion criteria: systemic disease and participating in other research studies Cabergoline group: 20 women Control group: 20 women |
|
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day for 8 days from the day of hCG injection Control group: no cabergoline |
|
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989) assessed at day of ET, ET+7, ET+12
Live birth rate: not reported Miscarriage rate (cabergoline group vs control group): 0/20 vs 1/20 Clinical pregnancy rate (live heart beat) (cabergoline group vs control group): 2/20 vs 5/20 Multiple pregnancy rate (cabergoline group vs control group): 0/20 vs 1/20 Any other adverse effects of the treatment: not stated |
|
| Notes | Did apply coasting to both groups in about 50% of women if serum E2 level > 5000 pg/mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient data to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Neither participants nor physicians blinded, only ultrasound experts were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient data to permit judgement |
| Methods | Multicentre, prospective, randomised, double‐blind, placebo‐controlled study 3 clinics Bromocriptine vs folic acid Setting: Brazil |
|
| Participants | 47 women aged < 38 years undergoing IVF with ≥ 20 follicles as assessed by transvaginal ultrasound and E2 > 3000 pg/mL on the day prior to hCG administration Exclusion criteria: hyperprolactinaemia; use of dopaminergic agents or other medications for the treatment of hyperprolactinaemia or pituitary tumours; systemic diseases, such as arterial hypertension, hypotension, orthostatic hypotension, cardiovascular disease and diabetes mellitus; polycystic ovaries Bromocriptine group: 23 women, 12/23 dropped out Folic acid group: 24 women, 7/24 dropped out |
|
| Interventions | Bromocriptine group: bromocriptine 2.5 mg/day continued for 14 days Folic acid group (placebo): folic acid 2.0 mg/day continued for 14 days Capsules same appearance and form |
|
| Outcomes | Incidence of OHSS (subgroups mild, moderate, severe), VEGF levels, urinary function Moderate and severe OHSS according to its OHSS criteria
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using a random number generation algorithm |
| Allocation concealment (selection bias) | Unclear risk | Lack of information to permit a judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind; medication and folic acid as a placebo in same appearance capsules |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout numbers without dropout analysis |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of information to permit a judgement |
| Methods | Randomised, parallel, double‐blind randomised controlled trial Quinagolide vs placebo Setting: Spain |
|
| Participants | 182 women undergoing IVF and ICSI treatment and at risk of developing OHSS with ≥ 20 follicles of ≥ 10 mm on the day of hCG administration Exclusion criteria: > 30 follicles or serum E2 6000 pg/mL (or both) had cycle cancellation, previous coasting in this cycle, any clinically significant systemic disease, endocrine or metabolic abnormalities (pituitary, adrenal, pancreas, liver or kidney), history of recurrent miscarriage, undiagnosed vaginal bleeding Quinagolide 50 μg group: 51 women Quinagolide 100 μg group: 52 women Quinagolide 200 μg group: 26 women Control group: 53 women |
|
| Interventions | 4 tablets for every woman (combination of placebo/quinagolide 50 μg) Quinagolide 50 μg group: quinagolide 50 μg + 3 placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval Quinagolide 100 μg group: quinagolide 100 μg + 2 placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval Quinagolide 200 μg group: quinagolide 200 μg + no placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval Control group: 4 placebo tablets once daily, continuing until the day before the serum hCG test which took place 17+2 days after oocyte retrieval |
|
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate (quinagolide 50 μg group vs quinagolide 100 μg group vs quinagolide 200 μg group vs placebo group): 23/51 vs 29/52 vs 14/26 vs 27/53 Miscarriage rate: not stated Clinical pregnancy rate (quinagolide 50 μg group vs quinagolide 100 μg group vs quinagolide 200 μg group vs placebo group): 22/51 vs 26/52 vs 11/26 vs 27/53 Multiple pregnancy rate: not stated Discontinued because of adverse events (quinagolide 50 μg group vs quinagolide 100 μg group vs quinagolide 200 μg group vs placebo group): 3/51 vs 7/52 vs 7/26 vs 0/53 Any other adverse effects of the treatment: nausea, dizziness, somnolence, diarrhoea, vomiting, lower abdominal pain, headache, abdominal distension, flatulence, upper abdominal pain, syncope |
|
| Notes | Sponsored by Ferring Pharmaceuticals WHO registry reference: EUCTR2006‐000415‐15‐ES |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list prepared for each centre by a statistician not involved in the trial, and based on this the clinics were provided with individual code envelopes that were sealed to conceal the treatment group allocation |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation list provided to the clinics with individual code envelopes that were sealed to conceal the treatment group allocation. Block size was not disclosed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind (participants, staff and trial sponsor). All participants received 4 tablets (medication or placebo, or both) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Systematic OHSS evaluation performed; high‐dose arm stopped after poor tolerability of high‐dose medication |
| Selective reporting (reporting bias) | Low risk | Most of outcomes were evaluated |
| Other bias | High risk | Poor tolerability of high dose could have revealed allocated group Sponsored by Ferring Pharmaceuticals Very high‐risk women (> 30 follicles or serum E2 6000 pg/mL, or both) excluded and underwent cycle cancellation |
| Methods | Parallel, single‐centre randomised controlled trial Computer‐based randomisation Cabergoline vs no intervention Setting: Brazil |
|
| Participants | 166 women undergoing IVF and ICSI treatment and at risk of developing OHSS, defined as serum E2 > 4000 pg/mL on the day of hCG administration Exclusion criteria: not stated Cabergoline group: 83 women Control group: 83 women, 3 women were withdrawn for not completing the follow‐up tests No differences between groups in age or BMI Did not report the duration of infertility and causes of infertility |
|
| Interventions | All participants received routine preventive IV HA 20 g on the day of oocyte retrieval Cabergoline group: cabergoline 0.5 mg/day for 3 weeks from the day after oocyte retrieval Control group: no intervention |
|
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate (cabergoline group vs control group): 1/83 vs 3/83 Clinical pregnancy rate (cabergoline group vs control group): 33/83 vs 32/83 Multiple pregnancy rate (cabergoline group vs control group): multiple pregnancies were documented in all the severe cases of OHSS in both groups (2/83 vs 1/83) Any other adverse effects of the treatment: not stated |
|
| Notes | Authors reported no financial or commercial conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/200 women in control group could not complete their follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Single‐centre randomised controlled trial Computer‐based randomisation by independent research assistant Cabergoline vs coasting Setting: India |
|
| Participants | 60 women undergoing IVF or ICSI cycles and at risk of developing OHSS, defined as the presence of preovulatory follicles ≥ 20 in both ovaries and the E2 level ≥ 2500 pg/mL Exclusion criteria: not stated Cabergoline group: 30 women Coasting group: 30 women |
|
| Interventions | Cabergoline group: cabergoline 0.5 mg/day orally from the day of hCG for 8 days Coasting group: gonadotropins were withheld (while GnRHa was maintained), until the serum level of E2 started to decline in each group. 1 woman needed ascites tapped, and the remaining 29 women received 6% HES infusion |
|
| Outcomes | Moderate and severe OHSS: classification not described but according to Golan (Golan 1989) criteria (from private correspondence with author)
Live birth rate: not stated Miscarriage rate (cabergoline group vs coasting group): 0/30 vs 2/30 Clinical pregnancy rate (defined as presence of gestational sac or cardiac activity 3 weeks after transfer) (cabergoline group vs coasting group): 8/30 vs 4/30 Multiple pregnancy rate (cabergoline group vs coasting group): 2/30 vs 0/30 Any other adverse effects of the treatment: cancelling of ET due to poor embryo quality (cabergoline group vs coasting group): 1/30 vs 1/30. Other adverse events not stated |
|
| Notes | Received draft of full‐text article in peer review currently per private email; additional information per private correspondence with first author. 58 women received fluid of 6% HES and the remaining included woman received an ascites tap instead of HES. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of randomisation software (www.randomizer.org/) |
| Allocation concealment (selection bias) | Unclear risk | Independent research assistant allocated; concealment unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding involved. The participants and clinicians were aware in which arm of the study they were |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/loss of follow‐up in the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | High risk | OHSS identified/classification not described. 29 participants in both groups also received HES infusion, 1 participant from each group had ascites tap, unclear which participant was involved |
| Methods | Randomised trial based on blinded envelopes Cabergoline vs no intervention Setting: Russia |
|
| Participants | 168 women included, but only 128 high‐risk women defined as transvaginal aspiration of ≥ 15 follicles Cabergoline group: 65 women Control group (no intervention): 63 women No significant difference between groups in somatic and obstetric anamnesis |
|
| Interventions | Cabergoline group: cabergoline 0.5 mg/day from the day after oocyte retrieval for 5 days before ET day Control group: no intervention |
|
| Outcomes | Moderate and severe OHSS, diagnosis OHSS not stated
Live birth rate: not stated Miscarriage rate (cabergoline group vs control group): 4/65 vs 6/63 Clinical pregnancy rate (cabergoline group vs control group): 21/65 vs 23/63 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Lack of information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Blinded envelopes method |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Randomised controlled trial based on random number table Cabergoline vs albumin vs HES Setting: Iran |
|
| Participants | 91 high‐risk women with E2 > 3000 pg/mL or > 20 follicles on the day of hCG administration or previous history of OHSS, or a combination Cabergoline group: 31 women Albumin group: 30 women HES group: 30 women No significant difference between groups regarding gravidity, parity, death, ectopic pregnancy, abortion and mean age |
|
| Interventions | Cabergoline group: cabergoline 0.5 mg daily for 7 days after oocyte retrieval Albumin group: 2 vials (2 × 50 mL) HAs IV 30 minutes after oocyte retrieval within 4 hours HES group: 1000 mL of 6% HES IV 30 minutes after oocyte retrieval within 4 hours |
|
| Outcomes | Moderate and severe OHSS identified by the classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Single‐centre, prospective randomised study ("randomly divided in two groups") Cabergoline vs no medication Setting: Tunisia |
|
| Participants | 146 women undergoing IVF or ICSI and receiving GnRHa. OHSS risk defined as a plasma E2 level > 3000 pg/mL on the day of hCG administration or the development of ≥ 18 follicles > 12 mm in diameter, or both Exclusion criteria: coasting cases, aged > 40 years, history of uterine surgery, and submucosal and intramural fibromas > 5 cm Cabergoline group: 78 women Control group: 68 women |
|
| Interventions | Cabergoline group: cabergoline 0.5 mg/day for 8 days starting on the day of hCG injection Control group (no intervention): no medication treatment |
|
| Outcomes | Moderate and severe OHSS identified according to the criteria of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: only reported for women who developed OHSS (cabergoline group vs control group): 3/25 vs 6/25 Clinical pregnancy rate only reported for women who developed OHSS (cabergoline group vs control group): 20/25 vs 14/25 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Lack of information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Lack of information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No follow‐up data from the non‐OHSS women in both groups, no data on possible loss to follow‐up or dropout |
| Selective reporting (reporting bias) | High risk | Pregnancy data from the non‐OHSS women in both groups not reported |
| Other bias | High risk | Coasting cases (women at highest risk for severe OHSS) were excluded, unclear based on what criteria coasting was opted for |
| Methods | Blinded randomised controlled trial Randomisation based on computer‐generated numbers in sequentially numbered sealed envelopes Cabergoline + 6% HES vs 6% HES Setting: Spain |
|
| Participants | 182 women undergoing IVF using their own oocytes and receiving GnRHa treatment and considered at risk of OHSS (all aged < 40 years). OHSS risk defined as a plasma E2 level > 3000 pg/mL on the day of hCG administration or development of 20 follicles >12 mm, or both Exclusion criteria: E2 levels > 5000 pg/mL where cycles were cancelled Cabergoline group: 88 women Control group: 94 women |
|
| Interventions | Cabergoline group: slow IV infusion of 500 mL of 6% HES during follicle aspiration plus cabergoline 0.5 mg orally for 8 days starting on day of hCG administration Control group: slow IV infusion of 500 mL of 6% HES during follicle aspiration |
|
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate (cabergoline + HES group vs control group): 5/88 vs 9/94 Clinical pregnancy rate (cabergoline + HES group vs control group): 43/88 vs 48/94 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Both the embryologists and the gynaecologists performing oocyte aspiration, ET and post‐transfer follow‐up, were blinded to the co‐administration of cabergoline. Participants were not blinded; however, low risk of causing bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | High‐risk cycles were cancelled (E2 > 5000 pg/mL), which might have excluded severe OHSS cases |
| Methods | Blinded randomised controlled trial Cabergoline vs prednisolone vs no intervention Setting: United Arab Emirates |
|
| Participants | 200 women with polycystic ovarian syndrome undergoing IVF treatment and possibility of developing OHSS Exclusion criteria: previous oophorectomy, immune diseases that affect the permeability of blood vessels, such as systemic lupus, disseminated sclerosis and rheumatoid arthritis Cabergoline group: 75 women, 2 women lost to follow‐up Prednisolone group: 75 women, 3 women lost to follow‐up Control group (no intervention): 50 women, 2 women lost to follow‐up |
|
| Interventions | Cabergoline group: cabergoline 0.5 mg tablets, 1 tablet on 2 successive days, starting from the day of hCG injection, and repeated 1 week later Prednisolone group: prednisolone 10 mg tablets twice a day to day of pregnancy test Control group: no intervention |
|
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | No high‐risk women identified (e.g. based on E2 or ultrasound) except that this population was young women with PCOS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Lack of information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blind to the participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/200 women after randomisation could not complete their follow‐up, no reasons stated |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | High risk | No high‐risk women identified (e.g. based on E2 or ultrasound) except this population was young women with PCOS |
| Methods | Randomised controlled trial Computer‐based randomisation Cabergoline vs no intervention Setting: Egypt |
|
| Participants | 200 women undergoing ICSI treatment and at risk of developing OHSS, defined by E2 level on day of hCG > 3500 pg/mL with ≥ 20 follicles > 12 mm diameter Cabergoline group: 100 women; 2 had empty follicles, 2 had failure of fertilisation and 1 discontinued Control group: 100 women; 3 had empty follicles and 1 had failure of fertilisation Exclusion criterion: E2 ≥ 5000 pg/mL No differences between the groups in age, BMI and causes of infertility |
|
| Interventions | Cabergoline group: cabergoline tablet 0.25 mg/day for 8 days from the day of hCG injection Control group: no intervention |
|
| Outcomes | Moderate and severe OHSS identified according to Golan and colleagues (Golan 1989)
Live birth rate (cabergoline group vs control group): 37/100 vs 36/100 Miscarriage rate (cabergoline group vs control group): 5/100 vs 5/100 Clinical pregnancy rate (cabergoline group vs control group): 42/100 vs 41/100 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | Number of women excluded for dropout (no ET because no oocytes found, no embryos yielded, etc., 1 adverse event) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 women could not complete their follow‐up but exact reasons not stated |
| Selective reporting (reporting bias) | Low risk | Most outcomes were included |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Parallel design, randomised controlled trial Block randomisation Cabergoline vs coasting Setting: Iran |
|
| Participants | 60 women at risk of OHSS defined by ≥ 20 follicles in both ovaries, most being ≤ 14 mm in diameter and serum E2 level 3000 pg/mL Cabergoline group: 30 women Coasting group: 30 women Exclusion criterion: contraindication to dopamine agonists No significant differences between groups in age, BMI, menstrual cycle pattern, duration of infertility and causes of infertility |
|
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day for 7 days after hCG administration Coasting group: gonadotropin administration was ceased until serum E2 levels reached < 3000 pg/mL before hCG administration |
|
| Outcomes | Moderate and severe OHSS identified by the classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate (cabergoline group vs coasting group): 14/30 vs 7/30 Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table blocks according to Biostatistics in Health Systems |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Lack of sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Parallel, single‐centre randomised controlled trial Not blinded Computer‐based randomisation Cabergoline vs HA Setting: Iran |
|
| Participants | 140 women aged 15 to 37 years Inclusion criteria: risk of developing OHSS, defined by the development of 20 to 30 follicles > 12 mm in diameter on the day of hCG administration and retrieval of > 20 oocytes, ovarian stimulation with long protocol Exclusion criteria: coasting cases, aged > 37 years, previous uterine surgery, intramural or submucosal myoma sizes > 5 cm Cabergoline group: 70 women, 1 woman lost to follow‐up Albumin group: 70 women, 1 woman lost to follow‐up No differences between groups in age, BMI, duration of infertility, type of infertility, basal FSH, LH levels and E2 levels on the day of hCG administration but there was a difference in cause of infertility. |
|
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day 7 days beginning on day of oocyte retrieval Control group: HA 20% IV infusion |
|
| Outcomes | Moderate and severe OHSS identified by the modified classification of Golan and colleagues (Golan 1989)
Live birth rate: not stated Miscarriage rate (cabergoline group vs control group): 1/70 vs 3/70 Clinical pregnancy rate (cabergoline group vs control group): 20/70 vs 26/70 Multiple pregnancy rate (cabergoline group vs control group): 3/70 vs 5/70 Any other adverse effects of the treatment: not stated |
|
| Notes | 1 dropout in each group. Not reported when they dropped out or if they had even started. Excluded from analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Midwife open the sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/140 women lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
| Methods | Single‐centre, randomised controlled trial Blinded for sampling. No statement on blinding for allocation Randomisation not described Cabergoline vs HA Setting: Iran |
|
| Participants | 95 women, every other participant sampled. > 20 oocytes during oocyte retrieval, ovary size > 10 cm, serum E2 > 2500 pg/mL, considered eligible if high risk with > 20 follicles; randomisation when confirmed > 20 follicles retrieved in both ovaries at day of hCG injection Exclusion criterion: < 20 oocytes retrieved Cabergoline group: 47 women Albumin group: 48 women |
|
| Interventions | Cabergoline group: cabergoline 0.5 mg/day oral from day of hCG injection to 8 days Control group: 10 units IV HA at the start of oocyte retrieval |
|
| Outcomes | Moderate and severe OHSS; identified/classification not described other than "classified according to related criteria"
Live birth rate: not stated Miscarriage rate: not stated Clinical pregnancy rate: not stated Multiple pregnancy rate: not stated Any other adverse effects of the treatment: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The method of sampling was randomized sampling as we selected every other person. Randomization was used to allocate the patients to two groups immediately after confirmation of retrieval of >20 oocytes. but intervention started already on day 2 before retrieval (hCG administration)"! |
| Allocation concealment (selection bias) | Unclear risk | Lack of sufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "physician who controlled the patients was blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No exclusions (no live birth rated mentioned) |
| Other bias | Unclear risk | Lack of sufficient information to permit judgement |
BMI: body mass index; E2: oestradiol; ET: embryo transfer; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; GnRHa: gonadotropin‐releasing hormone agonist; HA: human albumin; hCG: human chorionic gonadotrophin; HES: hydroxyethyl starch; ICSI: intracytoplasmic sperm injection; IV: intravenous; IVF: in vitro fertilisation; LH: luteinising hormone; OHSS: ovarian hyperstimulation syndrome; PGD: preimplantation genetic diagnosis; VEGF: vascular endothelial growth factor; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aflatoonian 2008 | Not randomised, "divided into two groups according to patients convenience" |
| Agha Hosseini 2010 | Not an RCT; historic control group |
| Alvarez 2007b | A pilot study, not an RCT |
| Ata 2009 | Case report |
| Fouda 2016 | Studied co‐intervention on top of cabergoline rather than cabergoline |
| Ghaebi 2016 | Only women who were already developed signs of (mild) OHSS included |
| Gualtieri 2011 | Retrospective analysis, not an RCT |
| Guvendag 2010 | Case control study, not an RCT |
| Hatton 2012 | A retrospective study, not an RCT |
| Hosseini 2011 | Not an RCT |
| Khan 2010 | Not an RCT |
| Naredi 2013 | Quasi‐randomised, odd/even participants appointed to intervention groups |
| Rollene 2009a | Case series |
| Rollene 2009b | Retrospective cohort study |
| Saad 2016 | Quasi‐randomised (odd and even numbers) |
| Seow 2013 | 2 differently timed cabergoline regimens, no control group |
| Sherwal 2010 | Historical matched control group |
| Soliman 2011 | Not an RCT |
| Spitzer 2011 | Retrospective study |
| Zargar 2011 | Evaluated 2 different cabergoline regimens on prevention of OHSS |
OHSS: ovarian hyperstimulation syndrome; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective randomised controlled trial Cabergoline vs human albumin |
| Participants | 112 high‐risk women undergoing ART Cabergoline group: 56 women Albumin group: 56 women No statistically significant differences in age, BMI, number of follicles and oocyte retrieved, and serum E2 on the day of hCG injection |
| Interventions | Cabergoline group: cabergoline tablet 0.5 mg/day until 12 days from oocytes retrieval Albumin group: 20 g IV human albumin on the day of oocyte retrieval |
| Outcomes | The OHSS frequency was significantly lower in the cabergoline group (P < 0.001). There were no significant differences in pregnancy rate, implantation and miscarriages between groups |
| Notes | Meeting abstract, no numbers mentioned, no response from authors yet |
ART: assisted reproduction technology; BMI: body mass index; E2: oestradiol; hCG: human chorionic gonadotrophin; IV: intravenous; OHSS: ovarian hyperstimulation syndrome.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Cabergoline and Coasting to Prevent OHSS; Combining Cabergoline and Coasting in Gonadotropin Releasing Hormone(GnRH)Agonist Protocol in Intracytoplasmic Sperm Injection (ICSI) to Prevent Ovarian Hyperstimulation Syndrome (OHSS): a Randomized Clinical Trial |
| Methods | RCT To randomly compare 3 study groups involving high‐risk women to 1 of 3 arms of management, either coasting for 1 to 3 days or receiving cabergoline for 8 days or coasting for 1 day plus receiving cabergoline for 8 days in women undergoing ICSI following the long luteal GnRHa protocol |
| Participants | Women undergoing ICSI Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: active comparator: coasting. In their ICSI cycle, participants will continue their agonist treatment while stopping the hMG injections for 1 to 3 days until drop of E2 to a safe level to prevent OHSS. Early OHSS assessed at day of ET and 7 days after this date. Late OHSS assessed 14 days after ET Group 2: active comparator: cabergoline. In their ICSI cycle, participants will take cabergoline 0.25 mg/day for 8 days from hCG triggering day to prevent OHSS. Early OHSS assessed at day of ET and 7 days after this date. Late OHSS assessed 14 days after ET Group 3: active comparator: coasting + cabergoline. In their ICSI cycle, participants will continue their agonist treatment while stopping the hMG injections for 1 day plus receiving cabergoline 0.25 mg/day for 8 days from hCG triggering day to prevent OHSS. Early OHSS assessed at day of ET and 7 days after this date. Late OHSS assessed 14 days after ET |
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcomes:
|
| Starting date | |
| Contact information | yasminbassiouny@gmail.com |
| Notes |
| Trial name or title | Comparative Study Between Cabergoline and Intravenous Calcium in the Prevention of Ovarian Hyperstimulation in Women with Polycystic Ovarian Disease Undergoing Intracytoplasmic Sperm Injection (ICSI) |
| Methods | |
| Participants | |
| Interventions | Cabergoline group: cabergoline (Dostinex) 0.5 mg/day oral tablets for 8 days from the day of hCG injection. Once in the trial Calcium gluconate group: intravenous infusion of 10% calcium gluconate 10 mL in 200 mL of physiological saline on the day of ovum pickup. Once in the treatment cycle and each participant will undergo 1 treatment cycle during the trial To monitor the adherence to the medication, we ask the participant for the drug tablet return |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | July 2013 |
| Contact information | emyelkattan@gmail.com |
| Notes |
| Trial name or title | Study of Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (OHSS) in In Vito Fertilization Cycles and Derivation of OHSS Biomarkers |
| Methods | Randomised controlled trial Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | Inclusion criterion:
Exclusion criteria:
|
| Interventions | Cabergoline group: cabergoline 0.5 mg tablet, 1 tablet daily for 8 days Control group: placebo 1 tablet daily for 8 days |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 15 February 2012 |
| Contact information | mariannehendricksemail@gmail.com |
| Notes | NCT01535859 |
| Trial name or title | Effect of Cabergoline on Endometrial Vascularity During Intracytoplasmic Sperm Injection |
| Methods | Allocation: non‐randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: diagnostic |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Cabergoline group: women AT RISK of OHSS receiving cabergoline 0.5 mg/day for 8 days from the day of oocyte pickup for prevention of hyperstimulation Control group: women AT RISK of OHSS not receiving cabergoline Control group 2: will serve as a control group and will include age‐ and BMI‐matched women NOT AT RISK of OHSS, and not receiving cabergoline |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | December 2014 |
| Contact information | Dr.ahmed.m.kamel@gmail.com |
| Notes | NCT02306564 |
| Trial name or title | Diosmin versus Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (Infertility) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: single group assignment Masking: single blind (participant) Primary purpose: prevention |
| Participants | 200 women at risk of OHSS during ICSI cycles will be randomly scheduled into 2 equal groups Inclusion criteria: infertile women undergoing ICSI or polycystic ovarian syndrome, aged 23 to 48 years with 1 of the following:
Exclusion criteria: none |
| Interventions | Diosmin group: diosmin 2 × 500 mg tablets every 8 hours will be given from day of hCG injection for 14 days Cabergoline group: cabergoline 1 × 0.5 mg tablet/day will be given from day of hCG injection for 8 days |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | May 2014 |
| Contact information | dr.khalidkhader77@yahoo.com |
| Notes | NCT02134249 |
| Trial name or title | Cabergoline and Hydroxyethyl Starch in Ovarian Hyperstimulation Syndrome Prevention |
| Methods | Randomised open, parallel trial |
| Participants | Women aged 18 to 40 years Inclusion criterion:
Exclusion criterion:
|
| Interventions | Cabergoline group: slow infusion of 500 mL of 6% HES during follicular aspiration alone or combined with cabergoline 0.5 mg administration for 8 days, starting on the day of hCG administration Control group: slow infusion of 500 mL of 6% HES during follicular aspiration |
| Outcomes | Primary outcome: risk of OHSS Secondary outcome: pregnancy rate |
| Starting date | August 2007 |
| Contact information | None |
| Notes | NCT01530490; this is the Matorras 2013 paper |
3D: 3‐dimensional; β‐hCG: β‐human chorionic gonadotrophin; BMI: body mass index; COH: controlled ovarian hyperstimulation; E2: oestradiol; ET: embryo transfer; GnRH: gonadotropin‐releasing hormone; GnRHa: gonadotropin‐releasing hormone agonist; hCG: human chorionic gonadotrophin; HES: hydroxyethyl starch; hMG: human menopausal gonadotropin; ICSI: intracytoplasmic sperm injection; OHSS: ovarian hyperstimulation syndrome; RCT: randomised controlled trial.
Contributions of authors
HT: proposed the original title, drafted the protocol and review, selected studies, extracted data, assessed studies, analysed and interpreted data, and updated the review.
SM: proposed the 2016 title change and update, drafted the updated review, selected studies, extracted data, assessed studies, and analysed and interpreted data.
SZ: co‐drafted the protocol and review.
RH: assisted in drafting the protocol and original review, and drafting of the updated review.
Sources of support
Internal sources
-
Peking University Third Hospital, China.
Peking University Third Hospital
-
King Edward Memorial Hospital, Australia, Australia.
King Edward Memorial Hospital
-
The University of Western Australia, King Edward Memorial Hospital and Fertility Specialists of Western Australia, Australia.
The University of Western Australia, King Edward Memorial Hospital and Fertility Specialists of Western Australia
External sources
No sources of support supplied
Declarations of interest
RH is part owner and shareholder of an in vitro fertilisation (IVF) company; he has received travel grants and honoraria from pharmaceutical manufacturers of gonadotrophins and is on the medical advisory board of pharmaceutical companies that manufacture gonadotrophins.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Alhalabi M, Samawi S, Kafri N, Sharif J, Saker A, Othman A. Role of quinagolide (Norprolac) in preventing ovarian hyperstimulation syndrome (OHSS) in high risk intracytoplasmic sperm injection (ICSI) patients. Fertility and Sterility 2010;98(3 Suppl 1):S103. [Google Scholar]; Alhalabi M, Samawi S, Taha A, Kafri N, Modi S, Khatib A. Quinagolide reduces OHSS in high risk ICSI patients. Human Reproduction 2011;26(Suppl 1):P‐508. [Google Scholar]
- Alvarez C, Bosch E, Melo MAB, Fernandez‐Sanches M, Munoz E A, Remohi J, et al. The dopamine agonist cabergoline prevents moderate‐severe early ovarian hyperstimulation syndrome (OHSS) in high‐risk ART patients. Human Reproduction 2006;21:i96. [Google Scholar]; Alvarez C, Martí‐Bonmatí L, Novella‐Maestre E, Sanz R, Gómez R, Fernández‐Sánchez M, et al. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. Journal of Clinical Endocrinology and Metabolism 2007;92:2931‐7. [DOI] [PubMed] [Google Scholar]
- Amir H, Yaniv D, Hasson J, Amit A, Gordon D, Azem F. Cabergoline for reducing ovarian hyperstimulation syndrome in assisted reproductive technology treatment cycles. A prospective randomized controlled trial. Journal of Reproductive Medicine 2015;60(1‐2):48‐54. [PubMed] [Google Scholar]; Amir H, Yaniv Kovalski D, Amit A, Azem F. Can dopamine agonist cabergoline reduce ovarian hyperstimulation syndrome in ART treatment cycles? A prospective randomized study. Fertility and Sterility 2011;95(4):S84. [Google Scholar]
- Beltrame AL, Serafini P, Motta ELA, Soares JM Jr, Baracat. The effects of bromocriptine on VEGF, kidney function and ovarian hyperstimulation syndrome in in vitro fertilization patients: a pilot study. Gynecological Endocrinology 2013;29(3):201‐4. [DOI] [PubMed] [Google Scholar]
- Busso C, Fernandez‐Sanchez M, Garcia‐Velasco JA, Landeras J, Ballesteros A, Munoz E, et al. The non‐ergot derived dopamine agonist quinagolide in prevention of early ovarian hyperstimulation syndrome in IVF patients: a randomized, double‐blind, placebo‐controlled trial. Human Reproduction 2010;25(4):995‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carizza C, Abdelmassih V, Abdelmassih S, Ravizzini P, Salgueiro L, Salgueiro PT, et al. Cabergoline reduces the early onset of ovarian hyperstimulation syndrome: a prospective randomized study. Reproductive Biomedicine Online 2008;17:751‐5. [DOI] [PubMed] [Google Scholar]; Carizza C, Abdelmassih V, Ravizzini PC, Salgueiro LL. Ongoing clinical pregnancy is not affected by cabergoline use in women undergoing ICSI with estradiol levels beyond 4000 pg/ml on the day of the hCG. Human Reproduction 2008;23(suppl 1):i141. [Google Scholar]
- Dalal R, Mishra A. Comparison of coasting with cabergoline administration for prevention of early severe OHSS. BJOG 2014;121:78. [Google Scholar]
- Fetisova S, Korneeva I, Saroyan T, Krechetova L. Ivanets T. Effects of cabergoline administration for the prevention of OHSS upon the levels of VEGF, VEGFR‐1 and VEGFR‐2 in high risk IVF/ICSI patients. Human Reproduction 2014;29(Suppl 1):P‐482. [Google Scholar]
- Ghahiri A, Mogharehabed N, Hosseini N. Evaluation of intravenous hydroxyethyl starch, intravenous albumin 20% and oral cabergoline for prevention of ovarian hyperstimulation syndrome in patients undergoing ovulation induction. Journal of Research in Medical Sciences 2015;20(7):693‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ghahiri A, Mogharehabed N, Hosseini N. Evaluation of three different strategies (intravenous hydroxyl ethyl starch, intravenous albumin 20%, and oral cabergoline) for prevention of ovarian hyperstimulation syndrome in patients undergoing ovulation induction. Iranian Journal of Reproductive Medicine 2014;12(6):4. [Google Scholar]
- Jellad S, Haj Hassine A, Basly M, Mrabet A, Chibani M, Rachdi R. Vascular endothelial growth factor antagonist reduces the early onset and the severity of ovarian hyperstimulation syndrome. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction 2016 May 27 [Epub ahead of print]. [DOI] [PubMed]
- Matorras R, Andres M, Mendoza R, Prieto B, Pijoan JI, Exposito A. Prevention of ovarian hyperstimulation syndrome in GnRH agonist IVF cycles in moderate risk patients: randomized study comparing hydroxyethyl starch versus cabergoline and hydroxyethyl starch. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013;170(2):439‐43. [DOI] [PubMed] [Google Scholar]
- Salah AMR, EI‐Helew Y. Can cabergoline prevent ovarian hyperstimulation syndrome in polycystic ovarian patients undergoing gonadotropin stimulation?. Evidence Based Women's Health Journal 2012;2(2):56‐9. [Google Scholar]; Salah Edeen AMR, Alhelou YM. Can cabergoline prevent ovarian hyperstimulation syndrome in PCO patients undergoing gonadotropin stimulation? Comparative study with prednisolone. Human Reproduction 2009;24(1):i60. [Google Scholar]
- Shaltout A, Shohayeb A, Eid M. Role of cabergoline in preventing ovarian hyperstimulation syndrome in high risk intracytoplasmic sperm injection (ICSI) patients and effect on outcome. Human Reproduction 2009;24(Suppl 1):O‐154. [Google Scholar]; Shaltout A, Shohayeb A, Youssef MA. Can dopamine agonist at a low dose reduce ovarian hyperstimulation syndrome in women at risk undergoing ICSI treatment cycles? A randomized controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2012;165(2):254‐8. [DOI] [PubMed] [Google Scholar]
- Sohrabvand F, Ansaripour S, Bagheri M, Shariat M, Jafarabadi M. Cabergoline versus coasting in the prevention of ovarian hyperstimulation syndrome and assisted reproductive technologies outcome in high risk patients. International Journal of Fertility and Sterility 2009;3(1):35‐40. [Google Scholar]
- Tehraninejad ES, Hafezi M, Arabipoor A, Aziminekoo E, Chehrazi M, Bahmanabadi A. Comparison of cabergoline and intravenous albumin in the prevention of ovarian hyperstimulation syndrome: a randomized clinical trial. Journal of Assisted Reproduction and Genetics 2012;29(3):259‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabizadeh A, Vahidroodsari F, Ghorbanpour Z. Comparison of albumin and cabergoline in the prevention of ovarian hyperstimulation syndrome: a clinical trial study. Iranian Journal of Reproductive Medicine 2013;11(10):837‐42. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Aflatoonian A, Ghandi S, Tabibnejad N. Comparison of coasting with cabergoline administration for prevention of early severe OHSS in ART cycles. Iranian Journal of Reproductive Medicine 2008;6(2):51‐5. [Google Scholar]
- Agha Hosseini M, Aleyasin A, MAhdavi A, Nezami R, Safdarian L, Fallahi P. Cabergoline for prevention of ovarian hyperstimulation syndrome in high risk patients. Iranian Journal of reproductive Medicine 2010;8:70. [PMC free article] [PubMed] [Google Scholar]
- Alvarez C, Alonso‐Muriel I, Garcia G, Crespo J, Bellver J, Simon C, et al. Implantation is apparently unaffected by the dopamine agonist cabergoline when administered to prevent ovarian hyperstimulation syndrome in women undergoing assisted reproduction treatment: a pilot study. Journal of Clinical Endocrinology and Metabolism 2007;22:3210‐4. [DOI] [PubMed] [Google Scholar]
- Ata B, Seyhan A, Orhaner S, Urman B. High dose cabergoline in management of ovarian hyperstimulation syndrome. Fertility and Sterility 2009;92(3):1168.e1‐4. [DOI] [PubMed] [Google Scholar]
- Fouda UM, Sayed AM, Elshaer HS, Hammad BEM, Shaban MM, Elsetohy KA, et al. GnRH antagonist rescue protocol combined with cabergoline versus cabergoline alone in the prevention of ovarian hyperstimulation syndrome: a randomized controlled trial. Journal of Ovarian Research 2016;9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaebi NK, Amirian M, Zarmehri B, Zabihi H. Comparison of the effect of letrozole versus cabergoline for prevention of ovarian hyperstimulation syndrome (OHSS) in patients under ovulation induction treatments and IVF cycles. Iranian Journal of Obstetrics, Gynecology and Infertility 2016;18(184):1‐8. [Google Scholar]
- Gualtieri M, Hoffman D, Barrionuevo M, Christie D, Mouhayar Y, Ory SJ. The effects of cabergoline on ovarian hyperstimulation syndrome (OHSS) and pregnancy outcomes on in vitro fertilization (IVF) patients. Fertility and Sterility 2011;95(4):S259‐60. [Google Scholar]
- Guvendag GES, Dilbaz S, Cinar O, Ozdegirmenci O, Aydin S. The effect of cabergoline on follicular microenvironment profile in patients with high risk of OHSS. Fertility and Sterility 2010;94(4):S180‐1. [Google Scholar]
- Hatton A, Parry S, Hughes G, Wallbutton S, Binnersley S, Lavender A, et al. Cabergoline does not appear to affect pregnancy rates in patients undergoing assisted reproduction. Human Fertility 2012;15(s1):16. [Google Scholar]
- Hosseini MA, Aleyasin A, Mahdavi A, Nezami R, Safdarian L, Fallahi P. The effectiveness of cabergoline for the prevention of ovarian hyperstimulation syndrome. Iranian Journal of Medical Science 2011;36(3):207‐12. [PMC free article] [PubMed] [Google Scholar]
- Khan Z, Rollene N, Amols M, Gada R, Coddington C. Cabergoline and ganirelix therapy for early moderate to severe ovarian hyperstimulation syndrome (OHSS) results in faster recovery than in early untreated OHSS. Fertility and Sterility 2010;93(5):S14. [Google Scholar]
- Naredi N, Karunakaran S. Calcium gluconate infusion is as effective as the vascular endothelial growth factor antagonist cabergoline for the prevention of ovarian hyperstimulation syndrome. Journal of Human Reproductive Sciences 2013;6(4):248‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollene NL, Amols MH, Hudson SBA, Coddington CC. Treatment of ovarian hyperstimulation syndrome using a dopamine agonist and gonadotropin releasing hormone antagonist: a case series. Fertility and Sterility 2009;92(3):1169.e15‐7. [DOI] [PubMed] [Google Scholar]
- Rollene NL, Amols MH, Hudson SBA, Jensen JR, Morbeck DE, Coddington CC. Cabergoline and ganirelix treatment of ovarian hyperstimulation syndrome (OHSS) results in rapid clinical improvement. Fertility and Sterility 2009;92(3):S240‐1. [DOI] [PubMed] [Google Scholar]
- Saad AS, Mohamed KA, Saad SAA. Calcium dobesilate versus cabergoline for prevention of ovarian hyperstimulation syndrome. Human Reproduction 2016;31(Supp1):P‐580. [Google Scholar]
- Seow KM, Lin YH, Bai CH, Chen HJ, Hsieh BC, Huang LW, et al. Clinical outcome according to timing of cabergoline initiation for prevention of OHSS: a randomized controlled trial. Reproductive Biomedicine Online 2013;26(6):562‐8. [DOI] [PubMed] [Google Scholar]
- Sherwal V, Malik S, Bhatia V. Effect of bromocriptine on the severity of ovarian hyperstimulation syndrome and outcome in high responders undergoing assisted reproduction. Journal of Human Reproductive Sciences 2010;3(2):85‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman BS. Cabergoline vs intravenous albumin or combination of both for prevention of the early onset ovarian hyperstimulation syndrome. Middle East Fertility Society Journal 2011;16(1):56‐60. [Google Scholar]
- Spitzer D, Wogatzky J, Murtinger M, Zech MH, Haidbauer R, Zech NH. Dopamine agonist bromocriptine for the prevention of ovarian hyperstimulation syndrome. Fertility and Sterility 2011;95(8):2742‐4.e1. [DOI] [PubMed] [Google Scholar]
- Zargar M, Nikbakht R, Pourmatroud E, Ghasemi K, Hemadi M. Comparison of the clinical efficacy of two different cabergoline regimens on prevention of ovarian hyperstimulation syndrome (OHSS). Research Journal of Obstetrics and Gynecology 2011;4(2):51‐8. [Google Scholar]
References to studies awaiting assessment
- Ahmadi S, Rahmani E, Oskouian H. Cabergoline versus human albumin in prophylaxis of ovarian hyperstimulation syndrome. Reproductive Biomedicine Online 2010;20:S41. [Google Scholar]; Ahmadi SH, Rahmani E, Oskouian H. Cabergoline versus human albumin in prophylaxis of ovarian hyperstimulation syndrome. Iranian Journal of Reproductive Medicine 2011;9(Suppl 1):6 Abstract O‐13. [Google Scholar]
References to ongoing studies
- Cabergoline and Coasting to Prevent OHSS; Combining Cabergoline and Coasting in Gonadotropin Releasing Hormone(GnRH)Agonist Protocol in Intracytoplasmic Sperm Injection (ICSI) to Prevent Ovarian Hyperstimulation Syndrome (OHSS): a Randomized Clinical Trial. Ongoing study Starting date of trial not provided. Contact author for more information.
- Comparative Study Between Cabergoline and Intravenous Calcium in the Prevention of Ovarian Hyperstimulation in Women with Polycystic Ovarian Disease Undergoing Intracytoplasmic Sperm Injection (ICSI). Ongoing studyJuly 2013.
- Study of Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (OHSS) in In Vito Fertilization Cycles and Derivation of OHSS Biomarkers. Ongoing study 15 February 2012.
- Effect of Cabergoline on Endometrial Vascularity During Intracytoplasmic Sperm Injection. Ongoing studyDecember 2014.
- Diosmin versus Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome (Infertility). Ongoing studyMay 2014.
- NCT01530490. Cabergoline and hydroxyethyl starch in ovarian hyperstimulation syndrome prevention. clinicaltrials.gov/ct2/show/NCT01530490 Date first received: 26 January 2012.
Additional references
- Aboulghar M, Evers JLH, Al‐Inany HG. Intravenous albumin for preventing severe ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001302] [DOI] [PubMed] [Google Scholar]
- Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Human Reproduction Update 2003;9(3):275‐89. [DOI] [PubMed] [Google Scholar]
- Aboulghar M. Symposium: update on prediction and management of OHSS ‐ prevention of OHSS. Reproductive Biomedicine Online 2009;19(1):33‐42. [DOI] [PubMed] [Google Scholar]
- Al‐Inany H, Youssef M, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD001750.pub3] [DOI] [PubMed] [Google Scholar]
- Baumgarten M, Polanski L, Campbell B, Raine‐Fenning N. Do dopamine agonists prevent or reduce the severity of ovarian hyperstimulation syndrome in women undergoing assisted reproduction? A systematic review and meta‐analysis. Human Fertility (Cambridge, England) 2013;16(3):168‐74. [DOI] [PubMed] [Google Scholar]
- Busso CE, Garcia‐Velasco J, Gomez R, Alvarez C, Simon C, Pellicer A. Symposium: update on prediction and management of OHSS: prevention of OHSS ‐ dopamine agonists. Reproductive Biomedicine Online 2009;19(1):43‐51. [DOI] [PubMed] [Google Scholar]
- Casper RF. Reducing the risk of OHSS by GnRH agonist triggering. Journal of Clinical Endocrinology and Metabolism 2015;100(12):4396‐8. [DOI] [PubMed] [Google Scholar]
- Castelo‐Branco C, Pino M, Valladares E. Ovarian hyperstimulation, hyperprolactinaemia and LH gonadotroph adenoma. Reproductive Biomedicine Online 2009;19(2):153‐5. [DOI] [PubMed] [Google Scholar]
- Cochrane Gynaecology and Fertility Group. Module. http://www.cochranelibrary.com/review‐group/Gynaecology%20and%20Fertility%20Group/.
- Costello MF, Misso ML, Wong J, Hart R, Rombauts L, Melder A, et al. Treatment of infertility in polycystic ovary syndrome: a brief update. Australian & New Zealand Journal of Obstetrics & Gynaecology 2012;52(4):400‐3. [DOI] [PubMed] [Google Scholar]
- D'Angelo A, Amso NN. Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002811] [DOI] [PubMed] [Google Scholar]
- D'Angelo A, Amso NN. Embryo freezing for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002806.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvigne A, Rozenberg S. A qualitative systematic review of coasting, a procedure to avoid ovarian hyperstimulation syndrome in IVF patients. Human Reproduction Update 2002;18(3):291‐6. [DOI] [PubMed] [Google Scholar]
- Edwards RG. IVF, IVM, natural cycle IVF, minimal stimulation IVF ‐ time for a rethink. Reproductive Biomedicine Online 2007;15(1):106‐19. [DOI] [PubMed] [Google Scholar]
- Glade‐Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opinion on Biological Therapy 2003;3:263‐76. [DOI] [PubMed] [Google Scholar]
- Golan A, Ron‐el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstetrical & Gynecological Survey 1989;44(6):430‐40. [DOI] [PubMed] [Google Scholar]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
- Guo JL, Zhang DD, Zhao Y, Zhang D, Zhang XM, Zhou CQ, et al. Pharmacologic interventions in preventing ovarian hyperstimulation syndrome: a systematic review and network meta‐analysis. Scientific Reports 2016;6:19093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez R, Simon C, Remohi J, Pellicer A. Vascular endothelial growth factor receptor‐2 activation induces vascular permeability in hyperstimulated rats, and this effect is prevented by receptor blockade. Endocrinology 2002;143:4339‐48. [DOI] [PubMed] [Google Scholar]
- Gómez R, González‐Izquierdo M, Zimmermann RC, Novella‐Maestre E, Alonso‐Muriel I, Sanchez‐Criado J, et al. Low‐dose dopamine agonist administration blocks vascular endothelial growth factor (VEGF)‐mediated vascular hyperpermeability without altering VEGF receptor 2‐dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology 2006;147:5400‐11. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Kalampokas T, Creatsas G, Kalampokas E. Cabergoline as treatment of ovarian hyperstimulation syndrome: a review. Gynecological Endocrinology 2013;29(2):98‐100. [DOI] [PubMed] [Google Scholar]
- Kars M, Delgade V, Holman ER, Feelders RA, Smit JWA, Romijn JA, et al. Aortic valve calcification and mild tricuspid regurgitation but no clinical heart disease after 8 years of dopamine agonist therapy for prolactinoma. Journal of Clinical Endocrinology and Metabolism 2008;93(9):3348‐56. [DOI] [PubMed] [Google Scholar]
- Kasum M, Vrčić H, Stanić P, Ježek D, Orešković S, Beketić‐Orešković L, et al. Dopamine agonists in prevention of ovarian hyperstimulation syndrome. Gynecological Endocrinology 2014;30(12):845‐9. [DOI] [PubMed] [Google Scholar]
- Knoepfelmacher M, Danilovic DL, Rosa Nasser RH, Mendonca BB. Effectiveness of treating ovarian hyperstimulation syndrome with cabergoline in two patients with gonadotropin‐producing pituitary adenomas. Fertility and Sterility 2006;86(3):719 e15‐8. [DOI] [PubMed] [Google Scholar]
- Kuenen BC, Tabernero J, Baselga J, Cavalli F, Pfanner E, Conte PF, et al. Efficacy and toxicity of the angiogenesis inhibitor SU5416 as a single agent in patients with advanced renal cell carcinoma, melanoma, and soft tissue sarcoma. Clinical Cancer Research 2003;9:1648‐55. [PubMed] [Google Scholar]
- Leitao V, Moroni R, Seko L, Nastri C, Martins W. Cabergoline for the prevention of ovarian hyperstimulation syndrome: a systematic review and meta‐analysis of randomized controlled trials. Fertility and Sterility 2014;101(3):664‐75. [DOI] [PubMed] [Google Scholar]
- Manno M, Tomei F, Marchesan E, Adamo V. Cabergoline: a safe, easy, cheap, and effective drug for prevention/treatment of ovarian hyperstimulation syndrome?. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;122(1):127‐8. [DOI] [PubMed] [Google Scholar]
- Martin NM, Tan T, Meeran K. Dopamine agonists and hyperprolactinaemia. BMJ 2009;338:b381. [DOI] [PubMed] [Google Scholar]
- Prakash A, Karasu T, Mathur R. Ovarian hyperstimulation syndrome: pathophysiology, prevention and management. Obstetrics, Gynaecology and Reproductive Medicine 2009;19(9):274‐52. [Google Scholar]
- Rabau E, David A, Serr DM, Mashiach S, Lunenfeld B. Human menopausal gonadotropins for anovulation and sterility. Results of 7 years of treatment. American Journal of Obstetrics and Gynecology 1967;98(1):92‐8. [DOI] [PubMed] [Google Scholar]
- Royal College of Obstetricians and Gynaecologists (RCOG). The management of ovarian hyperstimulation syndrome. London (UK): Royal College of Obstetricians and Gynaecologists (RCOG) 2006;Sept:11. [Google Scholar]
- Schade R, Andershon S, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac‐valve regurgitation. New England Journal of Medicine 2007;356:29‐38. [DOI] [PubMed] [Google Scholar]
- Soares SR, Gomez R, Simon C, Garcia‐Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Human Reproduction Update 2008;14(4):321‐3. [DOI] [PubMed] [Google Scholar]
- Soares SR. Etiology of OHSS and use of dopamine agonists. Fertility and Sterility 2012;97(3):517‐22. [DOI] [PubMed] [Google Scholar]
- Tang H, Hunter T, Hu Y, Zhai SD, Sheng X, Hart RJ. Cabergoline for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008605.pub2] [DOI] [PubMed] [Google Scholar]
- Tso L, Costello M, Albuquerque L, Andriolo R, Macedo C. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD006105.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD009154.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vloeberghs V, Peeraer K, Pexsters A, D'Hooghe T. Ovarian hyperstimulation syndrome and complications of ART. Best Practice & Research. Clinical Obstetrics & Gynaecology 2009;23(5):691‐709. [DOI] [PubMed] [Google Scholar]
- Walls M, Junk S, Ryan JP, Hart R. IVF versus ICSI for the fertilization of in‐vitro matured human oocytes. Reproductive Biomedicine Online 2012;25(6):603‐7. [DOI] [PubMed] [Google Scholar]
- Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Human Reproduction 2015;30(1):88‐96. [DOI] [PubMed] [Google Scholar]
- Youssef MAFM, Wely M, Hassan MA, Al‐Inany HG, Mochtar M, Khattab S, et al. Can dopamine agonists reduce the incidence and severity of OHSS in IVF/ICSI treatment cycles? A systematic review and meta‐analysis. Human Reproduction Update 2010;16(5):459‐66. [DOI] [PubMed] [Google Scholar]
- Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. New England Journal of Medicine 2007;356:39‐46. [DOI] [PubMed] [Google Scholar]


