Abstract
Background/Aims:
Anemia is common in patients with advanced chronic kidney disease (CKD). A proportion of patients present with macrocytic anemia, manifested by elevated mean corpuscular volume (MCV), which has been associated with worse outcomes in CKD patients. However, it is unknown whether elevated MCV is associated with higher mortality risk in incident hemodialysis (HD) patients.
Methods:
This retrospective observational cohort study examined all-cause, cardiovascular, and infectious mortality associations with both baseline and time-varying MCV in 109,501 incident HD patients using Cox proportional hazards models with three levels of hierarchical multivariable adjustment. Odds ratios of high versus low baseline MCV were evaluated using logistic regression.
Results:
The mean age of patients was 65±15 (SD) years and the cohort was 44% female, 58% diabetic, and 31% African American. Higher MCV was associated with older age, female sex, non-Hispanic white race-ethnicity, alcohol consumption, and having a decreased albumin or protein intake. Patients with higher MCV levels (>98 fl) had a higher all-cause, cardiovascular, and infectious mortality risk in both baseline and time varying models, and across all levels of adjustment. In the fully adjusted models, compared to a reference of MCV 92-<94 fl, patients with a baseline MCV>100+ fl had a 28% higher risk of all-cause mortality (HR: 1.28, 95%CI: 1.22, 1.34), 27% higher risk of cardiovascular mortality (HR: 1.27, 95%CI: 1.18, 1.36), and 18% higher risk of infectious mortality (HR: 1.18, 95%CI: 1.02, 1.38). These associations persisted across all examined subgroups of clinical characteristics.
Conclusions:
Higher MCV was associated with higher all-cause, cardiovascular, and infectious mortality in HD patients. Further investigation is necessary to understand the underlying nature of the observed association.
INTRODUCTION
Anemia is common in patients with advanced chronic kidney disease (CKD) with a prevalence of about 53% in CKD stage 5 [1]. Erythrocyte size in anemia can be differentially diagnosed into microcytic, normocytic, or macrocytic anemia by the index of mean corpuscular volume (MCV) [2]. Anemia in CKD patients is usually normocytic and normochromic [3], characterized by reduced erythropoietin production [4], and is associated with reduced red blood cell survival [5]. Some patients with advanced CKD present with macrocytosis [3]. Elevated MCV (generally 100+ fl) (macrocytic anemia) is often characteristic of underlying conditions such as nutritional deficiencies, drug use, or primary bone marrow disorders [2,6–8], and has been associated with worse outcomes in patients with acute decompensated heart failure [9] and patients undergoing coronary intervention [10].
In a recent study investigating a small cohort of 1,439 Taiwanese patients with stages 3–5 CKD, Hsieh et al., found that over a median of 1.9 years of follow up, patients with higher MCV (defined as greater than the median value of 90.8 fl) had a higher risk of all-cause, cardiovascular, and infection-related mortality, compared to patients with MCV levels below the median [11]. Additionally, an earlier prospective, single-center study including 150 prevalent Canadian dialysis patients found an association between higher MCV (defined as >102 fl) and mortality over nine months of follow up [12]. However, whether higher MCV is associated with mortality in incident hemodialysis patients is still unknown. We hypothesized that in a large nationally representative cohort of incident hemodialysis patients in the US, higher MCV levels would be associated with a higher risk of mortality.
METHODS
Study Population and Data Source
We examined the data from a total of 208,820 patients with end stage renal disease who initiated dialysis therapy from January 1, 2007 to December 31, 2011 within a large dialysis care organization in the United States. This cohort has been previously described [13]. We excluded 46,156 patients who had <60 days of total treatment during their total follow-up time, 29,502 for receiving treatment with a modality other than in-center hemodialysis, 21,145 for not having treatment data in the first patient quarter (91 days from patient first dialysis date), and 2,516 for not having MCV data in the first patient quarter. The final analytical cohort consisted of 109,501 incident hemodialysis patients (Figure S1).
Additionally, we created three subcohorts for sensitivity analysis where we further restricted the analytical cohort based on the availability of the covariate of interest: (I) we excluded 100,208 incident hemodialysis patients for missing folate in patient quarter 1, which led to a subcohort of 9,293 patients with averaged MCV and folate measurements in patient quarter 1, (II) for the second subcohort, 98,810 incident hemodialysis patients were excluded for missing vitamin B12 (B12) measurements in patient quarter 1, which resulted in a total of 12,691 patients with MCV and B12 measurements in the first patient quarter, and finally (III) 73,167 patients were excluded for missing residual renal urea clearance (KRU) values in patient quarter 1, and therefore the third subcohort consisted of 36,334 incident hemodialysis patients.
All data were obtained from electronic records of the dialysis organization. ICD-9 codes listed in patient electronic medical records were used to determine the following conditions: alcohol abuse, arteriosclerotic heart disease (ASHD), cerebrovascular disease (CBVD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), diabetes mellitus, dyslipidemia, history of cancer, hypertension, liver disease, human immunodeficiency virus (HIV), other cardiovascular disease and substance abuse. Blood samples were drawn using standardized techniques in all dialysis clinics and were transported to a central laboratory in Deland, Florida, typically within 24 hours. All laboratory values were measured using automated and standardized methods. Serum creatinine, phosphorus, calcium, albumin, bicarbonate, total iron-binding capacity, and red cell distribution width (RDW) were measured monthly. Serum intact parathyroid hormone (iPTH) and ferritin were measured at least quarterly. Hemoglobin was measured weekly to biweekly in most patients. Delivered dialysis dose was estimated by single-pool Kt/V (spKt/V) using the urea kinetic model [14]. Body mass index (BMI) was calculated as post-hemodialysis body weight in kilograms divided by height in meters squared. MCV was routinely reported in femtoliters (fl, or 10−15L) along with complete blood cell count and was calculated by dividing the Hematocrit (Hct) by the red blood cell count (RBC) as follows: MCV[fl] = (Hct [%] / RBC [in million per μL]).
To minimize variability, variables with repeated measures within each three-month period (91-day intervals) were averaged to obtain a single quarterly mean value. Measurements taken during the first 91 days on dialysis therapy, referred to as the “first patient quarter,” were used as baseline values.
The study was approved by the institutional review committees of the University of California, Irvine, and was exempt from informed written consent due to its nonintrusive nature and anonymity of patients.
Exposure and Outcome Ascertainment
MCV was the primary exposure of interest. All-cause, cardiovascular, and infectious mortality were examined as the primary and secondary outcomes, respectively. For the definition of cardiovascular and infectious mortality, please refer to Table S1A and S1B. Patients were divided into nine groups of MCV spaced into equal intervals of two fl: <86, 86−<88, 88−<90, 90− <92, 92−<94 (reference), 94−<96, 96−<98, 98−<100, and 100+. Associations of MCV with mortality outcomes were examined in both baseline and time-varying models. Patients were followed from the first date of hemodialysis (HD) treatment until the patients were censored through one of the following events: death, kidney transplantation, transfer to another dialysis provider, or end of the study period.
Statistical Analyses
Baseline patient characteristics were summarized across baseline MCV groups using proportions, mean (± standard deviation (SD)) for parametric variables, or median (inter-quartile range (IQR)) for non-parametric variables, and were compared using tests-for-trend. Odds ratios for high (MCV ≥93 fl) vs. low (MCV <93 fl) baseline MCV were calculated using logistic regression. Linear regression and correlation coefficients (Spearman or Pearson as appropriate) were also calculated to evaluate serum laboratory values and medication dosage associations with baseline MCV.
To illustrate trajectories of quarterly averaged MCV across 20 patient quarters, we used a mixed-effects regression model and stratified trajectories by the baseline MCV groups, baseline median weekly erythropoiesis stimulating agent (ESA) dose groups, or baseline cumulative monthly intravenous (IV) iron dose groups. The cut points for the median weekly ESA dose groups and cumulative monthly IV iron dose groups were data derived. We further performed time-varying adjustment for weekly median ESA dose and cumulative monthly IV iron dose in the MCV baseline stratified model, weekly median ESA dose in the baseline ESA stratified model, and monthly cumulative IV iron dose in the baseline IV iron stratified model.
Associations of MCV with all-cause, cardiovascular, and infectious mortality outcomes were estimated using Cox proportional hazards models for both baseline and time-varying models. The proportionality assumption was checked using plots of log (−log(survival rate)) against log(survival time). We additionally explored potentially non-linear relationships between MCV and mortality outcomes using restricted cubic spline models with four knots placed at the 5th, 35th, 65th, and 95th percentile values of MCV. Mortality associations of high vs. low MCV were also examined across a priori selected subgroups in fully adjusted models, and were tested for interactions using the Wald test. In sensitivity analyses, we used competing risk regression to further explore the association between MCV and cardiovascular and infectious mortality, with cardiovascular or infectious death as the event of interest and non-cardiovascular or non-infectious mortality as the competing event, respectively.
All models were examined across three levels of hierarchical multivariable adjustment. The first level was unadjusted and included only MCV as the exposure. The second level was case-mix adjusted, which additionally included demographic data (age and sex), race-ethnicity (non-Hispanic white, African American, Hispanic, Asian, and other), comorbid conditions (alcoholism, ASHD, CBVD, CHF, COPD, diabetes mellitus, dyslipidemia, history of cancer, hypertension, liver disease, other cardiovascular disease, and substance abuse), insurance type (Medicare, Medicaid, private/other), hemodialysis access type (central venous catheter, arteriovenous fistula, arteriovenous graft, other), and spKt/V. The third level (fully adjusted model) added markers of the malnutrition-inflammation complex syndrome (MICS), for which we used 17 surrogates of nutritional and inflammatory status: hemoglobin, serum albumin, calcium, phosphorus, intact PTH (iPTH), iron saturation, total iron-binding capacity, ferritin, bicarbonate, white blood cell count, lymphocyte percentage, creatinine, alkaline phosphatase (ALP), BMI, normalized protein catabolic rate (nPCR), cumulative monthly IV iron dose per quarter, and ESA quarterly median dose per week.
In logistic regression analyses, models were restricted to patients that had the risk factor of interest, and were adjusted for all other components in the fully adjusted model minus the exposure. In time-varying models MCV, access type, laboratory values, and medication use were time updated. In additional analyses, we adjusted for (I) folate, (II) B12 and (III) KRU individually, to evaluate potential confounding by these covariates in models restricted to patients with available data on these variables in the first patient quarter.
For most covariates including laboratory markers, baseline data were <1% missing for the total cohort. Folate, B12, and KRU were missing at 92%, 88%, and 67%, respectively, and therefore were only included in subgroup analyses. In time varying analyses, MCV values were carried forward until subsequent updated values were reported. Missing values were handled by imputation by median for quantitative variables, or creation of a missing category for categorical data. All analyses were implemented using Stata, version 13.1 (Stata Corporation, College Station, TX).
RESULTS
Study Population
The analytical cohort included 109,501 HD patients and total cohort median (IQR) follow-up time was 494 (230, 922) days. Baseline characteristics stratified by the nine MCV categories are shown in Table 1. Mean±SD age of the cohort was 63±15 years. The cohort was comprised of 44% females, 58% diabetic patients, and 31% African American patients.
Table 1.
Baseline characteristics of 109,501 patients by baseline mean corpuscular volume (MCV) level
| MCV [fl] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <86 | 86–<88 | 88–<90 | 90–<92 | 92–<94 | 94–<96 | 96–<98 | 98–<100 | 100+ | P | ||
| N (%) | 109,501 | 12,029 (11) | 8,441 (8) | 12,032 (11) | 14,884 (14) | 15,983 (15) | 14,490 (13) | 11,366 (10) | 8,081 (7) | 12,195 (11) | |
| Age, y (mean ± SD) | 63±15 | 57±15 | 58±15 | 59±15 | 61±15 | 63±15 | 64±15 | 66±14 | 67±14 | 69±14 | <0.001 |
| Female sex (%) | 44 | 38 | 39 | 40 | 42 | 43 | 46 | 47 | 49 | 49 | <0.001 |
| Diabetes (%) | 58 | 65 | 64 | 63 | 61 | 59 | 57 | 55 | 53 | 48 | <0.001 |
| BMI (kg/m2) | 28.±7.3 | 29.3±7.5 | 28.9±7.4 | 28.8±7.3 | 28.3±7.3 | 27.9±7.1 | 27.8±7.2 | 27.6±7.3 | 27.2±7.2 | 27.0±7.3 | <0.001 |
| Race (%) | |||||||||||
| Asian | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 4 | 0.973 |
| African American | 31 | 55 | 41 | 35 | 30 | 28 | 26 | 25 | 24 | 22 | <0.001 |
| Non-Hispanic white | 47 | 26 | 36 | 41 | 45 | 48 | 51 | 54 | 57 | 62 | <0.001 |
| Hispanic | 15 | 11 | 16 | 17 | 18 | 17 | 16 | 14 | 13 | 10 | <0.001 |
| Native American, Other | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | <0.001 |
| Insurance (%) | |||||||||||
| Medicare | 54 | 48 | 48 | 49 | 51 | 53 | 55 | 57 | 60 | 62 | <0.001 |
| Medicaid | 7 | 8 | 8 | 8 | 8 | 7 | 7 | 6 | 5 | 5 | <0.001 |
| Other | 39 | 43 | 43 | 42 | 41 | 40 | 38 | 38 | 35 | 33 | <0.001 |
| Comorbid Conditions (%) | |||||||||||
| Alcohol | 0.24 | 0.17 | 0.18 | 0.2 | 0.24 | 0.23 | 0.19 | 0.33 | 0.26 | 0.37 | <0.001 |
| ASHD | 14 | 15 | 14 | 15 | 14 | 14 | 14 | 14 | 15 | 15 | 0.732 |
| CBVD | 1.8 | 1.8 | 1.7 | 1.6 | 1.8 | 1.8 | 1.8 | 1.9 | 2 | 1.8 | 0.063 |
| CHF | 37 | 39 | 38 | 38 | 36 | 36 | 36 | 36 | 37 | 36 | <0.001 |
| COPD | 5.1 | 4.4 | 4.3 | 4.6 | 4.8 | 5 | 5.1 | 5.4 | 6.3 | 6.3 | <0.001 |
| Dyslipidemia | 25 | 26 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 0.358 |
| History of Cancer | 2.32 | 1.65 | 1.97 | 1.77 | 2.18 | 2.53 | 2.51 | 2.54 | 2.82 | 2.85 | <0.001 |
| HIV | 0.5 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.7 | 0.9 | <0.001 |
| Hypertension | 51 | 55 | 52 | 52 | 50 | 51 | 51 | 50 | 51 | 50 | <0.001 |
| Liver Disease | 1.49 | 1.36 | 1.07 | 1.16 | 1.39 | 1.36 | 1.46 | 1.55 | 1.91 | 2.19 | <0.001 |
| Other Cardiac Disease | 15 | 15 | 14 | 15 | 14 | 15 | 15 | 15 | 17 | 16 | <0.001 |
| Access Type (%) | |||||||||||
| AVF | 15 | 16 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 0.350 |
| AVG | 4 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 0.582 |
| CVC | 75 | 73 | 75 | 75 | 76 | 76 | 75 | 74 | 75 | 73 | 0.103 |
| Others | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.341 |
| Unknown | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 7 | <0.001 |
| Serum Laboratory Values | |||||||||||
| Albumin (g/dL) | 3.51±0.48 | 3.54±0.48 | 3.52±0.48 | 3.52±0.48 | 3.52±0.48 | 3.51±0.47 | 3.51±0.47 | 3.49±0.48 | 3.48±0.48 | 3.46±0.49 | <0.001 |
| ALP(units/L) | 104±74 | 102±64 | 103±79 | 102±65 | 103±72 | 103±69 | 103±73 | 104±75 | 107±81 | 110±89 | <0.001 |
| Bicarbonate (mEq/L) | 23.6±2.7 | 23.7±2.6 | 23.6±2.6 | 23.6±2.6 | 23.5±2.7 | 23.6±2.7 | 23.5±2.7 | 23.6±2.8 | 23.6±2.8 | 23.6±2.9 | 0.9375 |
| Calcium (mg/dL) | 9.10±0.56 | 9.08±0.56 | 9.08±0.55 | 9.07±0.56 | 9.08±0.55 | 9.08±0.56 | 9.09±0.57 | 9.11±0.57 | 9.13±0.57 | 9.15±0.57 | <0.001 |
| Creatinine (mg/dL) | 5.9±2.4 | 6.4±2.5 | 6.2±2.5 | 6.1±2.5 | 6.0±2.4 | 5.9±2.4 | 5.7±2.3 | 5.6±2.2 | 5.5±2.1 | 5.3±2.0 | <0.001 |
| Ferritin (ng/mL) | 283 (164, 485) | 230 (126, 409) | 250 (142, 430) | 258 (149, 442) | 273 (161, 464) | 283 (166, 476) | 298 (176, 508) | 306 (183, 516) | 321 (189, 542) | 341 (202, 585) | <0.001 |
| Folate (ng/mL) | 10.2±4.3 | 9.9±4.2 | 9.9±4.2 | 9.6±4.2 | 10.2±4.2 | 10.2±4.3 | 10.4±4.3 | 10.4±4.3 | 10.7±4.5 | 10.5±4.5 | <0.001 |
| Hemoglobin (g/dL) | 11.1±1.2 | 10.9±1.2 | 11.1±1.2 | 11.1±1.2 | 11.2±1.2 | 11.2±1.2 | 11.1±1.2 | 11.2±1.2 | 11.2±1.2 | 11.1±1.2 | <0.001 |
| iPTH (pg/mL) | 313 (197, 486) | 354 (228, 535) | 332 (214, 506) | 326 (209, 503) | 318 (204, 486) | 315 (198, 484) | 307 (190, 478) | 301 (186, 476) | 293 (182, 460) | 273 (168, 435) | <0.001 |
| Iron (ug/dL) | 50.5±20.1 | 44.9±18.4 | 46.6±18.8 | 47.6±18.3 | 49.3±19.1 | 51.1±19.0 | 51.6±20.2 | 52.7±20.0 | 54.0±20.8 | 58.0±23.3 | <0.001 |
| Iron Saturation (%) | 23.1±9.1 | 20.0±8.1 | 20.9±8.1 | 21.5±8.1 | 22.3±8.2 | 22.8±8.2 | 23.6±8.9 | 24.4±9.1 | 25.1±9.4 | 27.5±11.6 | <0.001 |
| KRU (ml/min) | 3.27 (1.69, 5.49) | 2.88 (1.4, 4.9) | 2.99 (1.54, 5) | 3.11 (1.59, 5.18) | 3.18 (1.66, 5.33) | 3.2 (1.69, 5.42) | 3.4 (1.77, 5.67) | 3.47 (1.83, 5.77) | 3.52 (1.91, 5.88) | 3.62(1.92, 6) | <0.001 |
| LDH (U/L) | 199 (71, 235) | 200 (86, 234) | 198 (83, 234) | 200 (89, 235) | 199 (79, 234) | 199 (79, 234) | 198 (78, 235) | 199 (89, 236) | 201 (71, 236) | 202 (74, 241) | <0.001 |
| Lymphocyte (% of WBC) | 20.7±7.5 | 21.6±7.5 | 21.0±7.3 | 20.9±7.3 | 20.6±7.2 | 20.5±7.3 | 20.5±7.5 | 20.4±7.6 | 20.3±7.6 | 20.4±8.4 | <0.001 |
| nPCR (g/kg/day) | 0.79±0.22 | 0.79±0.21 | 0.79±0.21 | 0.79±0.22 | 0.80±0.22 | 0.79±0.22 | 0.80±0.22 | 0.78±0.22 | 0.79±0.22 | 0.78±0.22 | <0.001 |
| Phosphorus (mg/dL) | 4.92±1.15 | 5.01±1.12 | 5.03±1.16 | 5.00±1.14 | 4.98±1.16 | 4.94±1.16 | 4.90±1.16 | 4.86±1.15 | 4.81±1.13 | 4.73±1.13 | <0.001 |
| RDW (%) | 16.3±1.7 | 17.2±2.0 | 16.3±1.7 | 16.2±1.6 | 16.1±1.6 | 16.1±1.6 | 16.1±1.6 | 16.2±1.6 | 16.3±1.7 | 16.8±1.9 | <0.001 |
| Reticulocyte (%) | 2.7 (2.1, 3.4) | 2.7 (2.1, 3.4) | 2.8 (2.2, 3.4) | 2.8 (2.2, 3.4) | 2.8 (2.2, 3.4) | 2.7 (2.1, 3.4) | 2.7 (2.1, 3.4) | 2.6 (2.1, 3.4) | 2.7 (2.1, 3.5) | 2.6 (2.0, 3.4) | 0.006 |
| spKt/V | 1.46±0.33 | 1.41±0.33 | 1.43±0.32 | 1.45±0.33 | 1.46±0.33 | 1.47±0.31 | 1.49±0.33 | 1.49±0.32 | 1.51±0.33 | 1.51±0.32 | <0.001 |
| TIBC (mg/dL) | 225±49 | 231±49 | 228±48 | 227±48 | 226±48 | 225±48 | 224±49 | 221±49 | 221±49 | 221±50 | <0.001 |
| Vitamin B12 (pg/mL) | 620 (447, 880) | 627 (460, 892) | 596 (448, 859) | 587 (432, 829) | 601 (435, 867) | 599 (436, 858) | 626 (449, 859) | 636 (461, 921) | 651 (447, 917) | 653 (459, 936) | <0.001 |
| WBC (x103/uL) | 7.8±2.7 | 7.7±2.6 | 7.8±2.5 | 7.9±2.5 | 7.8±2.5 | 7.9±2.6 | 7.9±2.7 | 7.8±2.7 | 7.8±2.6 | 7.7±3.2 | <0.001 |
| Medications | |||||||||||
| ESA Use (U/week) | 4708 (1500, 11957) | 4872 (1540, 12168) | 4600 (1500, 11896) | 4618 (1466, 11880) | 4677 (1500, 11661) | 4694 (1500, 11917) | 4652 (1467, 11818) | 4793 (1513, 12168) | 4767 (1523, 11822) | 4734 (1508, 12100) | 0.832 |
| IV Iron (mg/month) | 1000 (400, 1400) | 1000 (500, 1525) | 1000 (500, 1500) | 1000 (500, 1500) | 1000 (425, 1450) | 1000 (450, 1400) | 1000 (400, 1400) | 1000 (400, 1400) | 900 (350, 1400) | 800 (250, 1300) | <0.001 |
Note: Data are presented as means ± standard deviations, medians (interquartile ranges), or percentages, and compared across groups with tests for trend.
Abbreviations: BMI, body mass index; ASHD, arteriosclerotic heart disease; CBVD, cerebrovascular disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheters; ALP, alkaline phosphatase; iPTH, intact parathyroid hormone; KRU, residual renal urea clearance; LDH, lactic acid dehydrogenase; nPCR, normalized protein catabolic rate; RDW, red cell distribution width; spKt/V, single-pooled Kt/V; TIBC, total iron binding capacity; WBC, white blood cell count, ESA, erythropoiesis stimulating agent; IV, intravenous
At baseline, patients with higher MCV were more likely to be female, older, non-diabetic, non-Hispanic White, on Medicare, and have comorbidities of alcoholism, COPD, history of cancer, liver disease, and other cardiac disease. Patients with lower MCV were more likely to have comorbidities of congestive heart failure (CHF) and hypertension. At higher levels of MCV, ALP, calcium, ferritin, folate, serum iron, iron saturation, and spKt/V increased while albumin, creatinine, iPTH, KRU, lymphocyte, phosphorous, and total iron binding capacity decreased. Patients with lower levels of MCV were also more likely to receive higher doses of IV iron.
Predictors of Higher MCV
Odds ratios of having MCV levels above the median (≥93 fl) in 109,501 HD patients are shown in Table 2 for unadjusted, case-mix adjusted, and fully adjusted (case-mix plus MICS adjusted) models. In fully adjusted models, odds of higher baseline MCV were associated with older age, female sex, alcohol consumption, COPD, and higher ALP. Conversely, being diabetic, being not of non-Hispanic White race-ethnicity (in particular African American race-ethnicity), having higher albumin, or having higher nPCR, was associated with lower likelihood of having a baseline MCV above the median.
Table 2.
Likelihood of having baseline MCV levels (≥93 fl) in 109,501 HD patients in the unadjusted, case-mix, and fully adjusted models
| Unadjusted | Case-Mix | Case-Mix + MICS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P | OR | (95% CI) | P | OR | (95% CI) | P | |
| Age (per 10 years) | 1.36 | (1.35, 1.37) | <0.001 | 1.30 | (1.29, 1.31) | <0.001 | 1.34 | (1.33, 1.36) | <0.001 |
| Female sex (%) | 1.30 | (1.27, 1.34) | <0.001 | 1.30 | (1.27, 1.34) | <0.001 | 1.32 | (1.28, 1.35) | <0.001 |
| Diabetes (%) | 0.71 | (0.70, 0.73) | <0.001 | 0.69 | (0.67, 0.70) | <0.001 | 0.72 | (0.70, 0.74) | <0.001 |
| BMI (kg/m2) | 0.98 | (0.98, 0.98) | <0.001 | 0.99 | (0.98, 0.99) | <0.001 | 0.99 | (0.99, 0.99) | <0.001 |
| Race (%) | |||||||||
| Non-Hispanic white | 1-referent | 1-referent | 1-referent | ||||||
| Asian | 0.73 | (0.69, 0.79) | <0.001 | 0.78 | (0.73, 0.84) | <0.001 | 0.70 | (0.65, 0.75) | <0.001 |
| African American | 0.46 | (0.44, 0.47) | <0.001 | 0.55 | (0.54, 0.57) | <0.001 | 0.53 | (0.51, 0.55) | <0.001 |
| Hispanic | 0.61 | (0.59, 0.63) | <0.001 | 0.77 | (0.74, 0.80) | <0.001 | 0.73 | (0.71, 0.76) | <0.001 |
| Other | 0.63 | (0.59, 0.67) | <0.001 | 0.75 | (0.70, 0.81) | <0.001 | 0.72 | (0.67, 0.77) | <0.001 |
| Insurance (%) | |||||||||
| Medicare | 1-referent | 1-referent | 1-referent | ||||||
| Medicaid | 0.65 | (0.62, 0.68) | <0.001 | 0.99 | (0.95, 1.05) | 0.840 | 0.97 | (0.92, 1.02) | 0.221 |
| Other | 0.76 | (0.74, 0.78) | <0.001 | 0.93 | (0.91, 0.96) | <0.001 | 0.95 | (0.92, 0.97) | <0.001 |
| Comorbid Conditions (%) | |||||||||
| Alcohol | 1.22 | (0.95, 1.55) | 0.114 | 1.48 | (1.14, 1.91) | 0.003 | 1.31 | (1.01, 1.71) | 0.045 |
| ASHD | 1.01 | (0.98, 1.05) | 0.425 | 0.96 | (0.93, 1.00) | 0.055 | 0.96 | (0.92, 1.00) | 0.047 |
| CBVD | 1.05 | (0.96, 1.15) | 0.253 | 0.97 | (0.88, 1.07) | 0.527 | 0.98 | (0.89, 1.08) | 0.671 |
| CHF | 0.93 | (0.91, 0.95) | <0.001 | 1.00 | (0.98, 1.03) | 0.829 | 1.04 | (1.01, 1.07) | 0.008 |
| COPD | 1.22 | (1.15, 1.28) | <0.001 | 1.03 | (0.97, 1.10) | 0.281 | 1.10 | (1.04, 1.17) | 0.002 |
| Dyslipidemia | 1.00 | (0.97, 1.03) | 0.995 | 1.03 | (1.00, 1.06) | 0.091 | 1.02 | (0.99, 1.06) | 0.111 |
| History of Cancer | 1.36 | (1.25, 1.47) | <0.001 | 1.08 | (0.99, 1.17) | 0.069 | 1.00 | (0.91, 1.08) | 0.917 |
| Hypertension | 0.95 | (0.93, 0.97) | <0.001 | 0.94 | (0.92, 0.97) | <0.001 | 0.95 | (0.93, 0.98) | <0.001 |
| Liver Disease | 1.29 | (1.17, 1.42) | <0.001 | 1.34 | (1.21, 1.50) | <0.001 | 1.07 | (0.96, 1.20) | 0.247 |
| Other CD | 1.08 | (1.04, 1.11) | <0.001 | 1.01 | (0.97, 1.05) | 0.654 | 1.02 | (0.98, 1.06) | 0.440 |
| Access Type (%) | |||||||||
| CVC | 1-referent | 1-referent | 1-referent | ||||||
| AVF | 1.01 | (0.98, 1.04) | 0.616 | 0.96 | (0.92, 0.99) | 0.012 | 0.93 | (0.90, 0.97) | <0.001 |
| AVG | 1.04 | (0.98, 1.11) | 0.156 | 0.98 | (0.92, 1.04) | 0.496 | 0.96 | (0.90, 1.03) | 0.262 |
| Others | 0.90 | (0.61, 1.32) | 0.589 | 0.97 | (0.65, 1.44) | 0.874 | 0.95 | (0.63, 1.42) | 0.785 |
| Unknown | 1.09 | (1.04, 1.15) | <0.001 | 1.11 | (1.05, 1.17) | <0.001 | 1.08 | (1.02, 1.14) | 0.006 |
| Serum Laboratory Values | |||||||||
| Albumin (per 0.5 g/dL) | 0.75 | (0.72, 0.79) | <0.001 | 0.79 | (0.75, 0.83) | <0.001 | 0.89 | (0.83, 0.96) | 0.001 |
| ALP(per l00 IU/L) | 1.05 | (1.03, 1.07) | <0.001 | 1.11 | (1.09, 1.13) | <0.001 | 1.08 | (1.06, 1.10) | <0.001 |
| B12 (per 100 pg/mL) | 1.02 | (1.01, 1.03) | <0.001 | 1.02 | (1.01, 1.03) | <0.001 | 1.02 | (1.00, 1.03) | 0.005 |
| Bicarbonate (mEq/L) | 1.00 | (1.00, 1.00) | 0.942 | 0.97 | (0.97, 0.98) | <0.001 | 0.97 | (0.97, 0.98) | <0.001 |
| Calcium (mg/dL) | 1.12 | (1.10, 1.14) | <0.001 | 1.04 | (1.02, 1.06) | 0.001 | 1.06 | (1.03, 1.09) | <0.001 |
| Creatinine (mg/dL) | 0.91 | (0.90, 0.91) | <0.001 | 1.01 | (1.00, 1.01) | 0.075 | 1.00 | (1.00, 1.01) | 0.337 |
| Ferritin (per 500 ng/mL) | 1.26 | (1.24, 1.28) | <0.001 | 1.22 | (1.20, 1.24) | <0.001 | 1.03 | (1.01, 1.05) | 0.003 |
| Folate (per 5 ng/mL) | 1.14 | (1.09, 1.19) | <0.001 | 1.05 | (1.00, 1.10) | 0.057 | 1.06 | (1.00, 1.11) | 0.036 |
| Hemoglobin (g/dL) | 1.05 | (1.04, 1.06) | <0.001 | 1.03 | (1.02, 1.04) | <0.001 | 1.00 | (0.99, 1.02) | 0.489 |
| iPTH (per 250 pg/mL) | 0.93 | (0.92, 0.94) | <0.001 | 1.02 | (1.01, 1.03) | <0.001 | 1.02 | (1.01, 1.04) | <0.001 |
| Iron (μg/dL) | 1.02 | (1.01, 1.02) | <0.001 | 1.02 | (1.02, 1.02) | <0.001 | 1.01 | (1.01, 1.02) | <0.001 |
| Iron Saturation (%) | 1.05 | (1.04, 1.05) | <0.001 | 1.05 | (1.05, 1.05) | <0.001 | 1.05 | (1.05, 1.05) | <0.001 |
| KRU (ml/min) | 0.96 | (0.95, 0.97) | <0.001 | 0.96 | (0.95, 0.97) | <0.001 | 0.97 | (0.96, 0.97) | <0.001 |
| LDH (U/L) | 1.00 | (1.00, 1.00) | <0.001 | 1.00 | (1.00, 1.00) | <0.001 | 1.00 | (1.00, 1.00) | <0.001 |
| Lymphocyte (per 10 %) | 0.91 | (0.90, 0.93) | <0.001 | 1.04 | (1.02, 1.06) | <0.001 | 0.98 | (0.96, 0.99) | 0.009 |
| nPCR (g/kg/d) | 0.90 | (0.85, 0.95) | <0.001 | 0.89 | (0.84, 0.95) | <0.001 | 0.85 | (0.79, 0.92) | <0.001 |
| Phosphorus (mg/dL) | 0.89 | (0.88, 0.90) | <0.001 | 1.00 | (0.99, 1.01) | 0.732 | 1.00 | (0.99, 1.02) | 0.748 |
| RDW (%) | 0.99 | (0.98, 0.99) | <0.001 | 0.96 | (0.95, 0.96) | <0.001 | 0.94 | (0.93, 0.94) | <0.001 |
| Reticulocyte (%) | 1.00 | (0.97, 1.04) | 0.751 | 1.02 | (0.99, 1.06) | 0.177 | 1.01 | (0.98, 1.05) | 0.471 |
| spKt/V (per 1 unit) | 1.59 | (1.53, 1.65) | <0.001 | 1.15 | (1.10, 1.20) | <0.001 | 1.05 | (1.01, 1.10) | 0.026 |
| TIBC (per 100 μg/dL) | 0.79 | (0.77, 0.81) | <0.001 | 0.82 | (0.80, 0.84) | <0.001 | 1.09 | (1.05, 1.13) | <0.001 |
| WBC count, x103/μL | 1.00 | (1.00, 1.00) | 0.991 | 0.99 | (0.98, 0.99) | <0.001 | 1.00 | (1.00, 1.01) | 0.816 |
| Medications | |||||||||
| ESA Use (per 1,000 units) | 1.00 | (0.999, 1.001) | 0.543 | 1.00 | (0.999, 1.001) | 0.919 | 1.00 | (0.999, 1.001) | 0.625 |
| IV Iron (per 100 mg) | 0.98 | (0.98, 0.98) | <0.001 | 0.98 | (0.98, 0.99) | <0.001 | 1.00 | (1.00, 1.00) | 0.616 |
*In logistic regression analyses, models were restricted to patients that had the risk factor of interest, and were adjusted for all other components in the multilevel adjustment minus the exposure
Abbreviations: BMI, body mass index; ASHD, arteriosclerotic heart disease; CBVD, cerebrovascular disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; Other CD, other cardiac disease; CVC, central venous catheters; AVF, arteriovenous fistula; AVG, arteriovenous graft; ALP, alkaline phosphatase; B12, vitamin B12; iPTH, intact parathyroid hormone; KRU, residual renal urea clearance; LDH, lactic acid dehydrogenase; nPCR, normalized protein catabolic rate; RDW, red cell distribution width; spKt/V, single-pooled Kt/V; TIBC, total iron binding capacity; WBC, white blood cell count, ESA, erythropoiesis stimulating agent; IV, intravenous
Linear regression analysis and correlations showed similar results (Table S2). MCV had positive correlations with values for ALP, B12, calcium, ferritin, folate, hemoglobin, serum iron, iron saturation, and spKt/V. It had negative correlations with albumin, creatinine, iPTH, KRU, lymphocyte, nPCR, phosphorous, RDW, total iron binding capacity, white blood cell count, and IV iron. However, the correlations between MCV and various laboratory variables were very weak. The strongest correlation was found between MCV and iron saturation. With every percent increase in iron saturation, MCV increased by 0.15 fl (correlation coefficient and p-value, 0.23 and <0.001).
MCV trajectories over 20 Patient Quarters
Overall, patients showed a gradual increasing trend towards higher MCV levels over 20 patient quarters. The hierarchical order of the baseline MCV groups were maintained, and the differences between groups remained relatively constant (Figure S2A). Adjustment for time-varying cumulative IV iron dose and median ESA dose did not substantially change the course of the trajectory (Figure S2B). Additionally, after stratifying patients according to their baseline ESA dose into four groups, MCV increased during 20 patient quarters and showed very little variance across ESA strata (Figure S2C). The trajectory remained consistent after adjusting for patient quarter median weekly ESA dose (Figure S2D). Likewise, we stratified patients into four strata for baseline cumulative monthly IV iron dose, and found no significant difference in mean MCV level across iron strata over the course of 20 patient quarters (Figure S2E), which was not altered after adjustment for time varying monthly cumulative IV iron dose (Figure S2F).
All-Cause Mortality
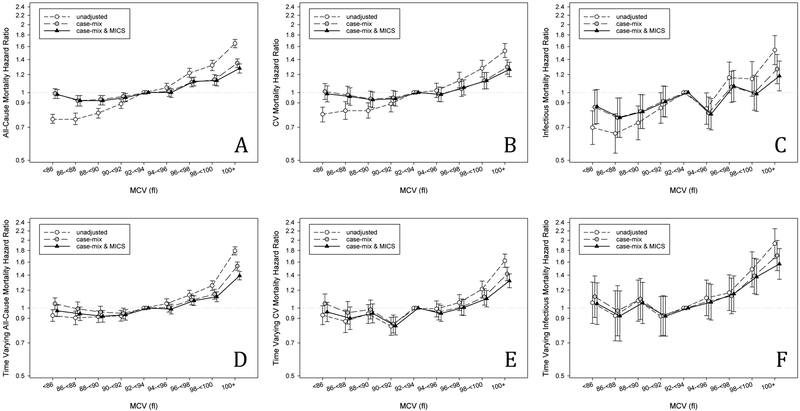
All-cause mortality rates increased linearly across the nine groups of MCV and more than doubled from the group with the lowest MCV (<86 fl) to the group with the highest MCV (≥100 fl) (117 deaths versus 250 deaths per 1,000 person-years, Table S3). In baseline unadjusted hazard ratio models, MCV was linearly associated with mortality risk (Figure 1A), where patients with MCV ≥100 fl had a 65% higher risk of mortality compared to the referent (hazard ratio (HR): 1.65, 95%CI: 1.58, 1.72). After case-mix or case-mix plus MICS covariate adjustments, the protective benefit of lower MCV was attenuated, where patients with MCV below the referent had a similar mortality risk to the referent. In fully adjusted models, patients with high MCV maintained a higher mortality risk, with the highest risk for patients having MCV 100+ fl (HR: 1.28, 95%CI: 1.22, 1.34).
Figure 1.
Baseline all-cause (A) and cardiovascular (B) mortality and time-varying all-cause (C) and cardiovascular (D) mortality hazard ratios (and 95%CI error bars) by MCV levels across three levels of multivariable adjustment.
In the three subcohorts including (I) 9,293 incident hemodialysis patients with baseline folate and MCV measurements, (II) 12,691 incident hemodialysis patients with baseline B12 and MCV measurements, and (III) 36,334 incident hemodialysis patients with baseline KRU and MCV measurements, the MCV-mortality association was similar to the main cohort in the case-mix plus MICS adjusted model. Further adjustment, separately for (I) folate, (II) B12 and (III) KRU, as shown in Figure S3A–C, did not attenuate the association of higher MCV with mortality.
Time-varying models followed a similar pattern as the baseline MCV-mortality association. Higher MCV was associated with higher short term risk of all-cause mortality across all levels of adjustment; however, lower MCV had no protective benefit compared to the referent (Figure 1D, Table S4). Restricted cubic splines modeling hazard ratios for baseline (Figure S4A–C) and time-varying (Figure S5A–C) associations showed similar results and did not suggest a presence of non-linear relationships between MCV and all-cause mortality.
Cardiovascular Mortality
Associations of MCV with cardiovascular mortality showed similar patterns to those in all-cause mortality models. In both baseline and time-varying models, the protective effect of lower MCV was attenuated by covariate adjustments (Figure 1B, 1E, Table S5, S6). Patients with higher MCV maintained a higher mortality risk across all levels of adjustment. In baseline fully adjusted models, patients with MCV 100+ fl had a 27% higher cardiovascular mortality risk compared to the referent (HR: 1.27, 95%CI: 1.18, 1.36). Competing risk regression subhazard ratios (Table S7) showed that the same patterns hold after accounting for non-cardiovascular mortality as a competing event.
Restricted cubic splines for baseline (Figure S4D–F) and time-varying (Figure S5D–F) cardiovascular mortality hazard ratios did not suggest the presence of non-linear relationships between MCV and cardiovascular mortality.
Infectious Mortality
Patterns for infectious mortality were less consistent than those for all-cause or cardiovascular mortality. The highest group of baseline MCV was associated with a higher risk of infectious mortality across all levels of adjustment (Figure 1C, Table S8), with an 18% increased risk of infectious mortality for MCV 100+ fl compared to the referent (HR: 1.18, 95%CI: 1.02, 1.38) in the fully adjusted model. Associations of lower baseline MCV with lower infectious mortality risk were found, but only persisted after all levels of adjustment for MCV groups 86−<88 and 88−<90. One group above the referent, MCV 94−<96, also had reduced risk of infectious mortality. Competing risk regression subhazard ratios (Table S10) showed that after accounting for non-infectious mortality as a competing event, the same three MCV groups maintained reduced infectious mortality risk, and the highest group maintained increased risk.
In time varying analysis (Figure 1F, Table S9), no association was observed between lower levels of MCV and infectious mortality, but the two highest MCV levels were associated with higher mortality.
Restricted cubic splines for baseline (Figure S4G–I) and time-varying (Figure S5G–I) infectious mortality hazard ratios did not suggest the presence of non-linear relationships between MCV and infectious mortality.
Subgroup Analyses
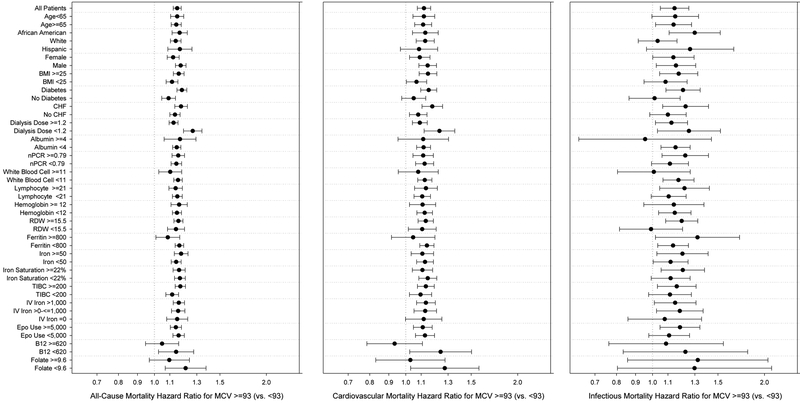
MCV above or equal to the median (≥93 fl) versus below the median (<93 fl) was consistently associated with higher all-cause, cardiovascular, and infectious mortality across substrata (Figure 2, Table S11). Associations of higher MCV with all-cause mortality were stronger for patients who had diabetes or who were receiving a lower dialysis dose. Dialysis dose also similarly modified the association of higher MCV with cardiovascular mortality.
Figure 2.
Subgroup analysis of all-cause (A and B) and cardiovascular (C and D) mortality hazard ratios (and 95%CI error bars) of high MCV (MCV≥93 fl) vs low MCV (MCV <93 fl) across three levels of multivariable adjustment.
Abbreviations: BMI, body mass index; CHF, congestive heart failure; nPCR, normalized protein catabolic rate; RDW, red cell distribution width; TIBC, total iron binding capacity; iv, intravenous; B12, vitamin B12
For all-cause mortality, significant interactions between MCV ≥93 fl and subgroups were observed for race, BMI, diabetes, and dialysis dose (Table S12). For cardiovascular mortality, interactions were observed for CHF and dialysis dose subgroups. No significant interactions were observed for infectious mortality.
DISCUSSION
In a large nationally representative cohort of 109,501 adult incident HD patients, our primary finding was a robust and consistent relationship between higher MCV with higher risk of all-cause, cardiovascular, and infectious mortality in both baseline and time-varying models, independent of malnutrition and inflammatory status. Patients with higher MCV tended to be older, female, alcoholic, non-diabetic, of non-Hispanic white race, and had elevated markers of poor nutrition, namely lower baseline albumin, nPCR, phosphorus concentrations, serum creatinine, and BMI. Furthermore, KRU was lower in patients with higher MCV levels.
Macrocytosis may be a surrogate for an end-stage malnutrition-related poor health condition. While hypoalbuminemia is associated with malnutrition and inflammation [15], lower nPCR and phosphorus may be indicative of low protein intake [16,17]. Lower serum creatinine correlates with a lower muscle mass [18,19] in dialysis patients and declining residual kidney function may be a factor in malnutrition and inflammation[20]. In fact, protein-energy wasting and inflammation are commonly found in dialysis patients [21,22]. It has been postulated that malnutrition could lead to a change in osmotic pressure contributing to erythrocyte swelling and a higher MCV [11]. Malnutrition may partially confound the relationship between MCV and mortality, but the association of higher MCV with mortality persisted after MICS adjustment. Additionally, adjusting for residual kidney function in a subset of patients did not remove the association. Therefore we conclude that malnutrition alone cannot fully explain the association between high MCV and mortality.
Associations between high MCV and B12 and folic acid deficiencies have been well established [6,23,24]. Soohoo et al.[25] studied B12- and folate-mortality associations using the same database as was used in this study. They concluded that higher B12 (B12≥550 pg/ml) and lower folate (folate<6.2 ng/ml) were associated with higher all-cause mortality. However, in additional analysis including only a subset of our analytical cohort, we found that high MCV was associated with higher all-cause mortality even after adjusting for folate and B12.
Another potential source of residual confounding is the impact of ESA therapy. ESA can lead to an increase in the reticulocyte population[12], which can thereby lead to increased MCV. A previous study described an association between MCV >102 fl and higher darbepoetin to hemoglobin ratio in maintenance hemodialysis patients [12], while in another study the MCV-mortality association was not significantly altered after adjustment for medication therapy including ESA [11]. Even though we adjusted for ESA in our baseline and time-varying models, one can argue that this may not account for the time to effect of ESA, since the ESA dose and MCV levels were measured in the same patient quarter. However, the trajectory model of mean MCV over 20 patient quarters stratified by baseline MCV group was not altered by ESA and IV iron adjustment.
Another potential explanation for the association between elevated MCV and mortality may be linked to the molecular aging process. In our study, older age was associated with higher MCV, which is consistent with other studies [26]. Relationships of leukocyte telomere length with heart disease, cancer, infections, and overall mortality have been previously described [27–29]. With every division, DNA polymerase cannot fully replicate the 3” end of a DNA strand; thus the telomere length becomes shorter and eventually reaches a critical length that can lead to cell death [27]. Kozlitina and Garcia measured the length of genomic DNA isolated from circulating leukocytes in 3,157 subjects that were enrolled in the Dallas Heart Study 2. Using a multiple regression model they found that shorter telomeres were significantly associated with larger MCV [30]. Furthermore, shorter telomeres may be related to infectious diseases [31,32]. In our analysis, associations of higher MCV with mortality were only slightly attenuated after adjustment for age; however, we were unable to account for differing telomere length.
Furthermore, red blood cell metabolism and homeostasis strongly affect the antioxidant properties of the whole body, and alteration of the erythrocytes membrane may result in a reduction of its antioxidant capacity [9,33,34]. Solak et al. showed that MCV was associated with endothelial dysfunction, independent of inflammation, suggesting that the harmful impact of higher MCV level on endothelial function may be a consequence of the compromised antioxidant potential of macrocytic erythrocytes [34]. Interestingly, overproduction of reactive oxygen species may play a causative role in the development and progression of cardiovascular disease [9].
We were unable to account for these factors in our cohort analysis, and thus recommend future studies with the ability to capture data on oxidative stress explore this possibility. The precise mechanisms by which MCV and mortality are related are yet to be determined.
Strengths of this study include the large sample size, thorough adjustment for common markers of malnutrition and inflammation, and refined categories of MCV that allowed us to examine non-linear relationships. Limitations include the possibility of residual confounding and our inability to infer causality due to the retrospective observational study design. We also lacked data on certain confounders, namely measures of C-reactive protein, reactive oxygen species, other inflammatory markers, the number of blood transfusions, and medications known to induce macrocytosis. Moreover, we cannot provide any information on pre-ESRD treatment such as ESA dose or IV iron, which might confound the baseline MCV-mortality association. For our baseline models, monthly cumulative IV iron and weekly median ESA use were documented after dialysis initiation over a 90-day period, during the same time frame as the baseline MCV measurement.
In conclusion, in a large national cohort of incident HD patients in the US, higher MCV levels were associated with higher risk of all-cause, cardiovascular, and infectious mortality. Further studies are needed to understand the pathophysiology underlying this relationship, and the potential advanced utility of evaluating MCV level in clinical practice.
Supplementary Material
Acknowledgements:
We thank DaVita Clinical Research® (DCR) for providing statistically deidentified data used in this study.
Funding Source: KKZ is supported by NIH (NIDDK) grants K24-DK091419, and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Joseph Lee, and AVEO, Inc.
Footnotes
Potential Conflict of Interest: KKZ has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, ZS-Pharma. CPK has received honoraria from Sanofi-Aventis, Relypsa and ZS Pharma.
References
- 1.Stauffer ME, Fan T: Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014;9:e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarma PR: Red Cell Indices; in Walker HK, Hall WD, Hurst JW (eds): Clinical Methods: The History, Physical, and Laboratory Examinations; Boston, 1990 [PubMed] [Google Scholar]
- 3.Suega K, Bakta M, Dharmayudha TG, Lukman JS, Suwitra K: Profile of anemia in chronic renal failure patients: comparison between predialyzed and dialyzed patients at the Division of Nephrology, Department of Internal Medicine, Sanglah Hospital, Denpasar, Bali, Indonesia. Acta Med Indones 2005;37:190–194. [PubMed] [Google Scholar]
- 4.Eschbach JW, Adamson JW: Recombinant human erythropoietin: implications for nephrology. Am J Kidney Dis 1988;11:203–209. [DOI] [PubMed] [Google Scholar]
- 5.Ly J, Marticorena R, Donnelly S: Red blood cell survival in chronic renal failure. AAm J Kidney Dis 2004;44:715–719. [PubMed] [Google Scholar]
- 6.Aslinia F, Mazza JJ, Yale SH: Megaloblastic anemia and other causes of macrocytosis. Clin Med Res 2006;4:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oosterhuis WP, Niessen RW, Bossuyt PM, Sanders GT, Sturk A: Diagnostic value of the mean corpuscular volume in the detection of vitamin B12 deficiency. Scand J Clin Lab Invest 2000;60:9–18. [DOI] [PubMed] [Google Scholar]
- 8.Savage DG, Ogundipe A, Allen RH, Stabler SP, Lindenbaum J: Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci 2000;319:343–352. [DOI] [PubMed] [Google Scholar]
- 9.Ueda T, Kawakami R, Horii M, Sugawara Y, Matsumoto T, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y: High mean corpuscular volume is a new indicator of prognosis in acute decompensated heart failure. Circ J 2013;77:2766–2771. [DOI] [PubMed] [Google Scholar]
- 10.Myojo M, Iwata H, Kohro T, Sato H, Kiyosue A, Ando J, Sawaki D, Takahashi M, Fujita H, Hirata Y, Nagai R: Prognostic implication of macrocytosis on adverse outcomes after coronary intervention. Atherosclerosis 2012;221:148–153. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh YP, Chang CC, Kor CT, Yang Y, Wen YK, Chiu PF: Mean Corpuscular Volume and Mortality in Patients with CKD. Clin J Am Soc Nephrol 2017;12:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tennankore KK, Soroka SD, West KA, Kiberd BA: Macrocytosis may be associated with mortality in chronic hemodialysis patients: a prospective study. BMC Nephrol 2011;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R: Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 2015;30:1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vashistha T, Streja E, Molnar MZ, Rhee CM, Moradi H, Soohoo M, Kovesdy CP, Kalantar-Zadeh K: Red Cell Distribution Width and Mortality in Hemodialysis Patients. Am J Kidney Dis 2016;68:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Molnar MZ, Rattanasompattikul M, Hatamizadeh P, Benner D, Kopple JD, Kovesdy CP, Kalantar-Zadeh K: Relative contributions of inflammation and inadequate protein intake to hypoalbuminemia in patients on maintenance hemodialysis. Int Urol Nephrol 2013;45:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriguchi R, Obi Y, Streja E, Tortorici AR, Rhee CM, Soohoo M, Kim T, Kovesdy CP, Kalantar-Zadeh K: Longitudinal Associations among Renal Urea Clearance-Corrected Normalized Protein Catabolic Rate, Serum Albumin, and Mortality in Patients on Hemodialysis. Clin J Am Soc Nephrol 2017;12:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 2008;88:1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noori N, Kovesdy CP, Bross R, Lee M, Oreopoulos A, Benner D, Mehrotra R, Kopple JD, Kalantar-Zadeh K: Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis 2011;57:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, Heymsfield S, Kopple JD, Kovesdy CP, Kalantar-Zadeh K: Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle 2013;4:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang AY, Lai KN: The importance of residual renal function in dialysis patients. Kidney Int 2006;69:1726–1732. [DOI] [PubMed] [Google Scholar]
- 21.Kaizu Y, Ohkawa S, Odamaki M, Ikegaya N, Hibi I, Miyaji K, Kumagai H: Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis 2003;42:295–302. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2001;38:1251–1263. [DOI] [PubMed] [Google Scholar]
- 23.Haltmayer M, Mueller T, Poelz W: Erythrocyte mean cellular volume and its relation to serum homocysteine, vitamin B12 and folate. Acta Med Austriaca 2002;29:57–60. [DOI] [PubMed] [Google Scholar]
- 24.Toprak B, Yalcin HZ, Colak A: Vitamin B12 and folate deficiency: should we use a different cutoff value for hematologic disorders? Int J Lab Hematol 2014;36:409–414. [DOI] [PubMed] [Google Scholar]
- 25.Soohoo M, Ahmadi SF, Qader H, Streja E, Obi Y, Moradi H, Rhee CM, Kim TH, Kovesdy CP, Kalantar-Zadeh K: Association of serum vitamin B12 and folate with mortality in incident hemodialysis patients. Nephrol Dial Transplant 2017;32:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann JJ, Nabbe KC, van den Broek NM: Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med 2015;53:2015–2019. [DOI] [PubMed] [Google Scholar]
- 27.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, Broer L, Nyholt DR, Mateo Leach I, Salo P, Hagg S, Matthews MK, Palmen J, Norata GD, O’Reilly PF, Saleheen D, Amin N, Balmforth AJ, Beekman M, de Boer RA, Bohringer S, Braund PS, Burton PR, de Craen AJ, Denniff M, Dong Y, Douroudis K, Dubinina E, Eriksson JG, Garlaschelli K, Guo D, Hartikainen AL, Henders AK, Houwing-Duistermaat JJ, Kananen L, Karssen LC, Kettunen J, Klopp N, Lagou V, van Leeuwen EM, Madden PA, Magi R, Magnusson PK, Mannisto S, McCarthy MI, Medland SE, Mihailov E, Montgomery GW, Oostra BA, Palotie A, Peters A, Pollard H, Pouta A, Prokopenko I, Ripatti S, Salomaa V, Suchiman HE, Valdes AM, Verweij N, Vinuela A, Wang X, Wichmann HE, Widen E, Willemsen G, Wright MJ, Xia K, Xiao X, van Veldhuisen DJ, Catapano AL, Tobin MD, Hall AS, Blakemore AI, van Gilst WH, Zhu H, Erdmann J, Reilly MP, Kathiresan S, Schunkert H, Talmud PJ, Pedersen NL, Perola M, Ouwehand W, Kaprio J, Martin NG, van Duijn CM, Hovatta I, Gieger C, Metspalu A, Boomsma DI, Jarvelin MR, Slagboom PE, Thompson JR, Spector TD, van der Harst P, Samani NJ: Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet 2013;45:422–427, 427e421–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G: Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86–105. [DOI] [PubMed] [Google Scholar]
- 29.Rode L, Nordestgaard BG, Bojesen SE: Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Nat Cancer Inst 2015;107:djv074. [DOI] [PubMed] [Google Scholar]
- 30.Kozlitina J, Garcia CK: Red blood cell size is inversely associated with leukocyte telomere length in a large multi-ethnic population. PLoS One 2012;7:e51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, Henson SM, ten Berge IJ, Akbar AN, van Lier RA: Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol 2010;184:3417–3423. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ: Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 2013;309:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsantes AE, Bonovas S, Travlou A, Sitaras NM: Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid Redox Signal 2006;8:1205–1216. [DOI] [PubMed] [Google Scholar]
- 34.Solak Y, Yilmaz MI, Saglam M, Demirbas S, Verim S, Unal HU, Gaipov A, Oguz Y, Kayrak M, Caglar K, Vural A, Turk S, Covic A, Kanbay M: Mean corpuscular volume is associated with endothelial dysfunction and predicts composite cardiovascular events in patients with chronic kidney disease. Nephrology (Carlton) 2013;18:728–735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.