Abstract
Background
Peripheral nerve blocks can be performed using ultrasound guidance. It is not yet clear whether this method of nerve location has benefits over other existing methods. This review was originally published in 2009 and was updated in 2014.
Objectives
The objective of this review was to assess whether the use of ultrasound to guide peripheral nerve blockade has any advantages over other methods of peripheral nerve location. Specifically, we have asked whether the use of ultrasound guidance:
1. improves success rates and effectiveness of regional anaesthetic blocks, by increasing the number of blocks that are assessed as adequate
2. reduces the complications, such as cardiorespiratory arrest, pneumothorax or vascular puncture, associated with the performance of regional anaesthetic blocks
Search methods
In the 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 8); MEDLINE (July 2008 to August 2014); EMBASE (July 2008 to August 2014); ISI Web of Science (2008 to April 2013); CINAHL (July 2014); and LILACS (July 2008 to August 2014). We completed forward and backward citation and clinical trials register searches.The original search was to July 2008. We reran the search in May 2015. We have added 11 potential new studies of interest to the list of 'Studies awaiting classification' and will incorporate them into the formal review findings during future review updates.
Selection criteria
We included randomized controlled trials (RCTs) comparing ultrasound‐guided peripheral nerve block of the upper and lower limbs, alone or combined, with at least one other method of nerve location. In the 2014 update, we excluded studies that had given general anaesthetic, spinal, epidural or other nerve blocks to all participants, as well as those measuring the minimum effective dose of anaesthetic drug. This resulted in the exclusion of five studies from the original review.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We used standard Cochrane methodological procedures, including an assessment of risk of bias and degree of practitioner experience for all studies.
Main results
We included 32 RCTs with 2844 adult participants. Twenty‐six assessed upper‐limb and six assessed lower‐limb blocks. Seventeen compared ultrasound with peripheral nerve stimulation (PNS), and nine compared ultrasound combined with nerve stimulation (US + NS) against PNS alone. Two studies compared ultrasound with anatomical landmark technique, one with a transarterial approach, and three were three‐arm designs that included US, US + PNS and PNS.
There were variations in the quality of evidence, with a lack of detail in many of the studies to judge whether randomization, allocation concealment and blinding of outcome assessors was sufficient. It was not possible to blind practitioners and there was therefore a high risk of performance bias across all studies, leading us to downgrade the evidence for study limitations using GRADE. There was insufficient detail on the experience and expertise of practitioners and whether experience was equivalent between intervention and control.
We performed meta‐analysis for our main outcomes. We found that ultrasound guidance produces superior peripheral nerve block success rates, with more blocks being assessed as sufficient for surgery following sensory or motor testing (Mantel‐Haenszel (M‐H) odds ratio (OR), fixed‐effect 2.94 (95% confidence interval (CI) 2.14 to 4.04); 1346 participants), and fewer blocks requiring supplementation or conversion to general anaesthetic (M‐H OR, fixed‐effect 0.28 (95% CI 0.20 to 0.39); 1807 participants) compared with the use of PNS, anatomical landmark techniques or a transarterial approach. We were not concerned by risks of indirectness, imprecision or inconsistency for these outcomes and used GRADE to assess these outcomes as being of moderate quality. Results were similarly advantageous for studies comparing US + PNS with NS alone for the above outcomes (M‐H OR, fixed‐effect 3.33 (95% CI 2.13 to 5.20); 719 participants, and M‐H OR, fixed‐effect 0.34 (95% CI 0.21 to 0.56); 712 participants respectively). There were lower incidences of paraesthesia in both the ultrasound comparison groups (M‐H OR, fixed‐effect 0.42 (95% CI 0.23 to 0.76); 471 participants, and M‐H OR, fixed‐effect 0.97 (95% CI 0.30 to 3.12); 178 participants respectively) and lower incidences of vascular puncture in both groups (M‐H OR, fixed‐effect 0.19 (95% CI 0.07 to 0.57); 387 participants, and M‐H OR, fixed‐effect 0.22 (95% CI 0.05 to 0.90); 143 participants). There were fewer studies for these outcomes and we therefore downgraded both for imprecision and paraesthesia for potential publication bias. This gave an overall GRADE assessment of very low and low for these two outcomes respectively. Our analysis showed that it took less time to perform nerve blocks in the ultrasound group (mean difference (MD), IV, fixed‐effect ‐1.06 (95% CI ‐1.41 to ‐0.72); 690 participants) but more time to perform the block when ultrasound was combined with a PNS technique (MD, IV, fixed‐effect 0.76 (95% CI 0.55 to 0.98); 587 participants). With high levels of unexplained statistical heterogeneity, we graded this outcome as very low quality. We did not combine data for other outcomes as study results had been reported using differing scales or with a combination of mean and median data, but our interpretation of individual study data favoured ultrasound for a reduction in other minor complications and reduction in onset time of block and number of attempts to perform block.
Authors' conclusions
There is evidence that peripheral nerve blocks performed by ultrasound guidance alone, or in combination with PNS, are superior in terms of improved sensory and motor block, reduced need for supplementation and fewer minor complications reported. Using ultrasound alone shortens performance time when compared with nerve stimulation, but when used in combination with PNS it increases performance time.
We were unable to determine whether these findings reflect the use of ultrasound in experienced hands and it was beyond the scope of this review to consider the learning curve associated with peripheral nerve blocks by ultrasound technique compared with other methods.
Plain language summary
Ultrasound guidance for upper and lower limb blocks
Background
Nerve blocks are used to numb all or part of the arms or legs (peripheral blockade) for surgery, or to provide pain relief after the operation, or both. Using ultrasound, anaesthetists can 'see' vital structures below the skin, which should allow them to place the local anaesthetic injection accurately and avoid damaging other tissues or organs. We aimed to assess whether ultrasound has any advantages over other nerve‐locating techniques for nerve blocks of the arms or legs in adults.
Study characteristics
The evidence is current up to 27 August 2014. We found 32 studies with 2844 participants. Most studies compared ultrasound with electrical nerve stimulators or compared ultrasound combined with nerve stimulators against nerve stimulators alone. We reran the search in May 2015. We will deal with the 11 studies of interest when we next update the review.
Key results
We combined the results of studies using statistical tests and found that nerve blocks were more likely to be assessed as adequate for surgery and were less likely to need additional anaesthetic when performed using ultrasound guidance or ultrasound guidance combined with other techniques. We also found that there were fewer complications such as 'pins and needles' or accidental punctures of blood vessels. It also took less time to perform the nerve block when ultrasound alone was used.
Quality of the evidence
There was variation in the quality of the studies and authors had not always made sufficient attempts to ensure that the outcome assessors were unaware of what technique had been used for the nerve block. Studies had also often not clearly explained how experienced the people giving the nerve block were. This is particularly important, as ultrasound is still a relatively new technique and some anaesthetists may have limited experience. We rated our evidence for whether the nerve blocks were sufficient and adequate for surgery as of moderate quality, but evidence for our other outcomes was either low or very low.
Conclusions
Our evidence suggests that ultrasound is superior to other techniques for peripheral nerve blocks. However, we are unable to say whether this result depends on the experience of the practitioner in the technique being used.
Summary of findings
Summary of findings for the main comparison. Ultrasound guidance for upper and lower limb blocks.
| Ultrasound guidance for upper and lower limb blocks | ||||||
| Patient or population: People undergoing upper and lower limb blocks Settings: hospital Intervention: ultrasound guidance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Ultrasound guidance | |||||
| Block success (predicted adequacy of block) | Study population | OR 2.49 (2.14 to 4.04) | 1346 (17 studies) | ⊕⊕⊕⊝ moderate1,2,3 | ||
| 791 per 1000 | 902 per 1000 (894 to 942) | |||||
| Moderate | ||||||
| Block success (supplementation or conversion to general anaesthesia | Study population | OR 0.28 (0.20 to 0.39) | 1807 (18 studies) | ⊕⊕⊕⊝ moderate1,3 | ||
| 185 per 1000 | 73 per 1000 (54 to 95) | |||||
| Moderate | ||||||
| Paraesthesia | Study population | OR 0.42 (0.23 to 0.76) | 471 (6 studies) | ⊕⊝⊝⊝ very low4,5,6 | ||
| 171 per 1000 | 80 per 1000 (44 to 135) | |||||
| Moderate | ||||||
| Vascular puncture | Study population | OR 0.19 (0.07 to 0.57) | 387 (5 studies) | ⊕⊕⊝⊝ low4,6 | ||
| 93 per 1000 | 20 per 1000 (7 to 55) | |||||
| Moderate | ||||||
| Time to perform block | The mean time to perform block in the intervention groups was 1.06 lower (1.41 to 0.72 lower) | 690 (10 studies) | ⊕⊝⊝⊝ very low4,7,8 | |||
| Number of attempts9 | See comment | See comment | Not estimable9 | 0 (7 studies) | ⊕⊕⊝⊝ low4,6 | |
| Patient discomfort9 | See comment | See comment | Not estimable9 | 0 (7 studies) | ⊕⊕⊝⊝ low4,6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Unavoidable performance bias due to lack of blinding but does not appear to affect results. Not possible to fully explore potential for operator bias according to preference and/or experience of devices 2Participants given different blocks, but low level of statistical heterogeneity in results I² = 15% 3Narrow confidence interval, suggesting lack of imprecision in effect estimate 4Unavoidable performance bias due to lack of blinding, also potential for operator bias. The effect of this on results is unclear 5High level of unexplained statistical heterogeneity, I² at 75%. One study is heavily weighted with large number of events in the control group 6There are few event data for this outcome and we have therefore downgraded it for imprecision 7High level of unexplained statistical heterogeneity, I² at 88% 8This analysis did not include several studies that reported on time to perform block with different calculations. We have downgraded it for imprecision 9Different methods used by each study to report data for this outcome, so not possible to pool
Background
Description of the condition
Regional anaesthesia (using an injection of local anaesthetic to produce a 'nerve block' to numb a part of the body) has a well‐established role in anaesthetic practice. Nerve blocks may be used as the sole form of anaesthesia or to provide postoperative analgesia. For the block to work effectively, the local anaesthetic has to be injected in the correct place, near the nerve, so locating the nerve is vital (Denny 2005).
Various techniques have been used for finding nerves. Easily identifiable landmarks, usually bones or arteries, may be used to guide the point of needle insertion. Low‐current electrical nerve stimulators linked to the injection needle have also been used to locate the nerve. As the needle nears the nerve, muscles supplied by the nerve can be seen to twitch in time with the pulses of current. Latterly, ultrasound has been used to guide nerve block insertion and a number of approaches to nerves and plexuses (groups of nerves) have been reported (Chan 2003; Chan 2006; Kapral 1994; Kirchmair 2001; Sandhu 2002).
How the intervention might work
Proponents of ultrasound‐guided blocks suggest many benefits over other methods of nerve location (for instance, Marhofer 2005). 'Seeing' the nerve, needle, and spread of local anaesthetic as it is injected is said to be an advantage over the other techniques outlined above. It is possible to use the ultrasound image to position the needle more precisely, which should lead to a higher success rate and allow smaller volumes of drug to be used whilst still producing the desired effect. As the severity of the life‐threatening complications of local anaesthetic injection is proportional to the dose of drug injected, this should make blocks safer. Other important structures, for instance blood vessels, tendons, and pleura, can be more easily avoided. Some reports have also suggested faster onset times (Marhofer 1997; Marhofer 2004; Sandhu 2002), longer duration of block (Marhofer 2004), and improved quality of anaesthesia (Marhofer 1997; Marhofer 2004; Williams 2003).
Why it is important to do this review
Complications of regional anaesthesia are rare but can be serious. In a prospective study of 21,278 patients receiving peripheral nerve blocks, there were three episodes of cardiac arrest (1.4/10,000); 16 seizures (7.5/10,000); and four cases of neurological damage (radiculopathy) (1.9/10,000) (Auroy 1997). A follow‐up study in 2002 collected serious complications self‐reported by anaesthetists over a 10‐month period. Out of 50,223 peripheral nerve blocks, patients showed one cardiac arrest (0.2/10,000); two episodes of acute respiratory failure (0.3/10 000); six seizures (1.2/10,000); and 12 episodes of peripheral neuropathy (2.3/10,000) (Auroy 2002). However, due to underreporting, actual complication rates may be higher than is stated in the literature. It has been suggested that the use of ultrasound may reduce complication rates by allowing more accurate needle placement and avoidance of other structures.
Whilst it is clear that peripheral nerve blocks can be successfully performed using ultrasound guidance, it is important to systematically review the evidence supporting its use.
Objectives
The objective of this review was to assess whether the use of ultrasound to guide peripheral nerve blockade has any advantages over other methods of peripheral nerve location. Specifically, we have asked whether the use of ultrasound guidance:
improves success rates and effectiveness of regional anaesthetic blocks, by increasing the number of blocks that are assessed as adequate
reduces the complications, such as cardiorespiratory arrest, pneumothorax or vascular puncture, associated with the performance of regional anaesthetic blocks
Methods
Criteria for considering studies for this review
Types of studies
We included all identified randomized controlled trials (RCTs) comparing ultrasound‐guided peripheral nerve block with at least one other method of nerve location (anatomical landmark, paraesthesia, or use of an electrical peripheral nerve stimulator).
We excluded the use of ultrasound to guide epidural and spinal anaesthetic injections. We considered blocks performed for treatment of chronic pain to be beyond the scope of this review, as techniques and assessed outcomes are likely to be different. In this updated review we also excluded studies which had been designed to test the minimum effective volume of anaesthetic (MEAV), as it was not possible to effectively measure our primary outcome with this design. We also excluded studies that had given general anaesthetic, spinal, epidural or additional nerve blocks to all participants in addition to the nerve block under investigation, and therefore also excluded studies that described the purpose of the nerve block as 'postoperative analgesic' only. Also see Differences between protocol and review.
Types of participants
We aimed to include studies of adults undergoing surgery where peripheral nerve blocks were used as the primary anaesthetic technique. We only included studies where blocks were formally assessed with sensory testing. We included studies in which participants were given a nerve block for tourniquet pain in both groups in addition to the nerve block under investigation.
We excluded studies in children (aged less than 16 years) as there may be differences in the technique of nerve block in this group.
Types of interventions
The use of ultrasound to guide needle or catheter placement for peripheral nerve blockade compared with any other method of peripheral nerve location. As ultrasound may be used in addition to other localization techniques, we have examined the use of ultrasound alone or in combination with other practised techniques, including peripheral nerve stimulation and landmark approaches. We considered blocks performed by anaesthesiologists and other staff but have noted the level of experience in use of ultrasound and in block insertion in the description of studies included in the review. We took an a priori decision to only include limb blocks in the 2014 update.
Types of outcome measures
We reconsidered the outcome definitions for the 2014 update and made alterations to improve clarity. We divided the primary outcome of block success into two outcomes to distinguish between the assessments used to define block success, i.e. predicted adequacy of the block with the use of motor or sensory testing, and the assessment of whether surgical anaesthesia had been achieved without the need for supplementary anaesthesia or conversion to general anaesthesia. We adapted the complications outcome to include all complications. We did not include studies that specifically assessed the volume of anaesthetic given during nerve blocks, as outcome data from these studies could not adequately measure our primary outcome. Also see Differences between protocol and review
Primary outcomes
Block success defined as predicted adequacy of block (using sensory and motor testing)
Block success defined as participants given supplementation of block/conversion to general anaesthetic
Block complications
Secondary outcomes
Time to perform block; onset time; block duration time
Number of attempts to perform block (attempts defined as documented change in technique or in person attempting block)
Participant discomfort during block placement (pain on needle insertion)
Search methods for identification of studies
Electronic searches
We searched the following databases for relevant published trials: the Cochrane Central Register of Controlled Trials (CENTRAL;2014, Issue 8); MEDLINE (July 2008 to August 2014); EMBASE (July 2008 to August 2014); ISI Web of Science (2008 to April 2013); CINAHL (July 2014); and LILACS (July 2008 to August 2014). The original search (Walker 2009) was to July 2008.
We used the search strategy found in Appendix 1 to search MEDLINE (Ovid SP). We adapted this search for EMBASE (OvidSP) (see Appendix 2), CINAHL (EBSCO host) (see Appendix 3), ISI Web of Science (see Appendix 4) and LILACS (see Appendix 5). This search included the Cochrane Highly Sensitive Search Strategy to identify randomized controlled trials and controlled clinical trials. We used the search strategy found in Appendix 6 to search CENTRAL.
We checked registers of ongoing trials (www.clinicaltrials.gov; www.controlled‐trials.com) in August 2014 for relevant completed trials.
We reran the search in May 2015. We have added 11 potential new studies of interest to the list of Studies awaiting classification and will incorporate them during the next review update.
Searching other resources
We performed backward and forward citation searching of studies published in the last five years. In the original review (Walker 2009) the authors had made attempts to contact known authors in the research field as well as handsearching of journals. We did not complete this level of searching for the 2014 update.
We did not impose any language restriction.
Data collection and analysis
Selection of studies
For the 2014 updated review two authors from a team of four (Sharon Lewis (SL), Andrew Smith (AS), Kevin Walker (KJW) and Ken McGrattan (KMcG)) independently selected relevant trials by reviewing titles and abstracts from the searches. We obtained full copies of potentially relevant trials using the Criteria for considering studies for this review outlined above. We then assessed the methodological quality of the trials meeting these criteria. We included abstracts identified during the electronic searches that had been published without a full report if they presented sufficient information. Reasons for excluding trials are detailed in Characteristics of excluded studies.
Data extraction and management
Two authors from a team of four (SL, KJW, KMcG, and Ana Price (AP)) independently extracted data using a data extraction form. We attempted to contact primary authors for missing data. One author (SL or AP) entered the data into Review Manager 5 (RevMan 5.3) and the other author validated them.
Assessment of risk of bias in included studies
Due to changes to the ’Risk of bias’ tool in RevMan 5.3 since the original review (Walker 2009), we reconsidered the risk of bias for all included studies. These changes included separation of blinding of participants and personnel from blinding of outcome assessors. We considered each individual outcome for performance and detection bias. We assessed methodological quality using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed individual studies for adequacy of sequence generation, allocation concealment, blinding, handling of incomplete outcome data, selective outcome reporting, and other potential forms of bias. We made judgements of 'low' or 'high' risk of bias, and 'unclear', meaning that there was insufficient information to make a judgement. We made these judgements based on the information presented in the published papers only.
Measures of treatment effect
Given the changes to our eligibility criteria we reconsidered the decision not to pool the results, and felt that it was now appropriate to use meta‐analysis for each outcome where sufficient and appropriate data were available.
For dichotomous outcomes we used a Mantel‐Haenszel odds ratio with a fixed‐effect model. We combined continuous outcomes using the mean difference, inverse variance method.
We described a P value of less than 0.05 as statistically significant.
Unit of analysis issues
We included studies with three arms in this review. For each of these studies, we thought it reasonable and appropriate to combine dichotomous data for either the two similar intervention arms or two comparison arms and compare these data against the alternative group. For continuous data, i.e. time to perform block, we took data from one of the intervention arms and from the comparison arm only.
Dealing with missing data
In the event that a study did not include denominator figures, and we were unable to acquire the relevant data from the authors, we did not include these studies in meta‐analysis.
Assessment of heterogeneity
We expected the findings for any given outcome to differ between studies in this review. This heterogeneity may be due to:
different comparisons (peripheral nerve stimulation, anatomical landmark technique or transarterial approach)
different types of nerve block
catheter placement versus no catheter placement
differing experience of practitioners
We assessed heterogeneity using Chi² and I² statistics (Higgins 2003). We considered heterogeneity to be important at a Chi² P value less than 0.1 or I² greater than 50%, and carried out subgroup analyses to explore these differences.
Assessment of reporting biases
As we had a sufficient number of studies, we considered a visual analysis of a funnel plot, generated in RevMan 5.3, to consider the potential of publication bias in our included studies.
Data synthesis
For outcomes where there were sufficient studies, we combined data in meta‐analysis. For outcome data with insufficient studies or with results that were reported differently (for example with a P value only), we present these results individually in a narrative form.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analysis for those outcomes with a Chi² P value less than 0.1 or I² greater than 50%, in this case for the outcomes 'Time to perform block' and 'Paraesthesia', considering the groups above (in Assessment of heterogeneity).
Sensitivity analysis
We carried out sensitivity analysis on our results, stratified by risk of bias.
Results
Description of studies
We include summary descriptions of each study in Characteristics of included studies tables.
Results of the search
We identified 2804 studies assessed from electronic searches and a further 240 studies from backward and forward citation searching in the 2014 search. We found three ongoing studies from clinical trial databases. We considered a total of 2831 unique titles and abstracts, and from these we assessed a further 139 full texts for eligibility, alongside the included studies in Walker 2009. See Figure 1.
1.
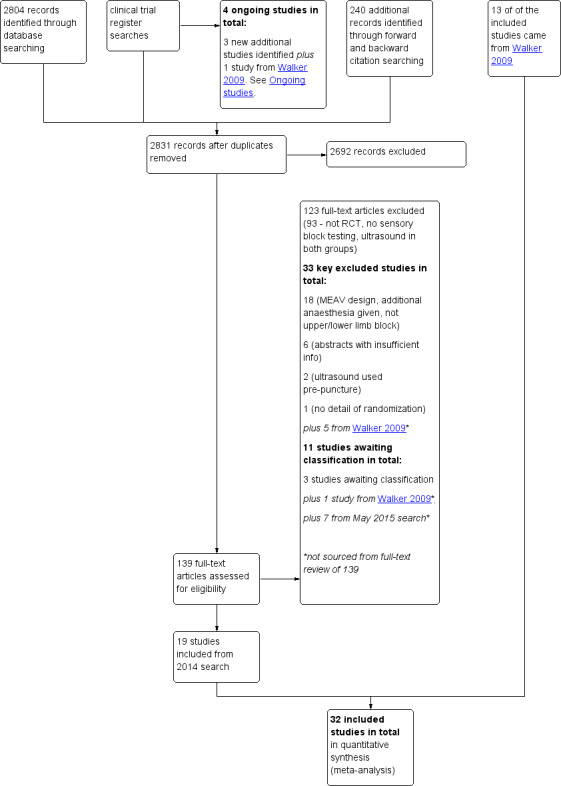
Study flow diagram.
We reran the search in May 2015. We found seven studies of interest which we will incorporate when we next update the review. See also Studies awaiting classification.
Included studies
Thirty‐two studies with a total of 2844 participants met our inclusion criteria (see Characteristics of included studies). All included studies were randomized controlled trials. All 32 studies were from single centres, involved only adult participants, and included people of both sexes. Of these, 19 were new studies identified from the updated search in June 2014 and 13 were from the original review.
Twelve studies assessed axillary brachial plexus block (Bloc 2010; Casati 2007a; Chan 2007; Conceição 2009; Geiser 2011; Gurkan 2008; Liu 2005; Meierhofer 2014; Morros 2009; Shrestha 2011; Sites 2006; Strub 2011), four studies assessed infraclavicular brachial plexus block (Brull 2009; Dhir 2008; Sauter 2008; Trabelsi 2013) and three assessed supraclavicular brachial plexus block (Renes 2009; Williams 2003; Zaragoza‐Lemus 2012), all for surgery of the hand, wrist or forearm. One study assessed a wrist block (Macaire 2008) for carpal tunnel release. There were four studies assessing interscalene brachial plexus block (Danelli 2012; Kapral 2008; Liu 2009a; Salem 2012) and one coracoid infraclavicular brachial plexus block (Taboada 2009), all for surgery of the shoulder or upper arm. Soeding 2005 assessed both an interscalene brachial plexus block and an axillary brachial plexus block within the same study. Seidel 2013 assessed a sciatic nerve block for foot or ankle, Domingo‐Triado 2007 assessed a mid‐femoral sciatic block and three studies a popliteal block for foot or ankle surgery (Dufour 2008; Perlas 2008; Van Geffen 2009). One popliteal fossa block was for hallux vagus correction (Cataldo 2012).
Seventeen studies compared the use of ultrasound alone with peripheral nerve stimulation (Brull 2009; Casati 2007a; Chan 2007; Conceição 2009; Danelli 2012; Geiser 2011; Kapral 2008; Macaire 2008; Meierhofer 2014; Perlas 2008; Renes 2009; Sauter 2008; Seidel 2013; Taboada 2009; Trabelsi 2013; Van Geffen 2009; Zaragoza‐Lemus 2012). Nine studies compared the use of ultrasound combined with nerve stimulation (ultrasound + nerve stimulation) with nerve stimulation alone (Cataldo 2012; Chan 2007;Domingo‐Triado 2007;Dufour 2008;Gurkan 2008; Morros 2009; Salem 2012; Shrestha 2011; Williams 2003). Remaining studies compared ultrasound with landmark technique (Soeding 2005; Strub 2011) or transarterial approach (Sites 2006) or were three‐arm studies comparing ultrasound (in‐plane approach) with ultrasound (out‐of‐plane approach) and with peripheral nerve stimulation (Bloc 2010), ultrasound + nerve stimulation with nerve stimulation (with or without a stimulating catheter) (Dhir 2008), or ultrasound (single injection) with ultrasound (double injection) and with nerve stimulation (double injection) (Liu 2005).
There were three studies that included placement of a catheter in the nerve block procedure (Danelli 2012; Dhir 2008; Salem 2012). In Danelli 2012, the catheter was placed after the injection of local anaesthetic and was therefore comparable with non‐catheter‐placement studies. For Dhir 2008 and Salem 2012, however, the local anaesthetic was administered through the catheter. We included six studies that gave details of additional nerve blocks for tourniquet pain (Domingo‐Triado 2007; Dufour 2008; Meierhofer 2014; Perlas 2008; Seidel 2013; Van Geffen 2009).
Primary outcomes
Twenty‐five studies evaluated our primary outcome of predicted adequacy of the block using methods of sensory and motor testing to describe the block as complete or adequate (Bloc 2010; Brull 2009; Cataldo 2012; Chan 2007; Dhir 2008; Domingo‐Triado 2007; Dufour 2008; Geiser 2011; Gurkan 2008; Kapral 2008; Liu 2005; Macaire 2008; Meierhofer 2014; Morros 2009; Perlas 2008; Renes 2009; Salem 2012; Sauter 2008; Sites 2006; Soeding 2005; Taboada 2009; Trabelsi 2013; Van Geffen 2009; Williams 2003; Zaragoza‐Lemus 2012). Twenty‐six studies also assessed whether participants required either supplementation of the block or conversion to a general anaesthetic (Brull 2009; Casati 2007a; Chan 2007; Conceição 2009; Dhir 2008; Domingo‐Triado 2007; Dufour 2008; Geiser 2011; Gurkan 2008; Kapral 2008; Liu 2005; Macaire 2008; Meierhofer 2014; Morros 2009; Perlas 2008; Renes 2009; Salem 2012; Sauter 2008; Seidel 2013; Shrestha 2011; Sites 2006; Soeding 2005; Strub 2011; Taboada 2009; Williams 2003; Zaragoza‐Lemus 2012)
Twenty‐one studies evaluated and reported a variety of complications (Bloc 2010; Brull 2009; Conceição 2009; Danelli 2012; Dhir 2008; Domingo‐Triado 2007; Dufour 2008; Gurkan 2008; Kapral 2008; Liu 2005; Meierhofer 2014; Morros 2009; Perlas 2008; Salem 2012; Sauter 2008; Shrestha 2011; Sites 2006; Soeding 2005; Strub 2011; Taboada 2009; Williams 2003).
Secondary outcomes
Twenty‐five studies measured time to perform block (Bloc 2010; Brull 2009; Cataldo 2012; Chan 2007; Conceição 2009; Danelli 2012; Dhir 2008; Domingo‐Triado 2007; Dufour 2008; Geiser 2011; Gurkan 2008; Liu 2005; Macaire 2008; Meierhofer 2014; Morros 2009; Perlas 2008; Salem 2012; Sauter 2008; Shrestha 2011; Sites 2006; Strub 2011; Taboada 2009; Trabelsi 2013; Van Geffen 2009; Williams 2003). Fifteen studies measured onset time of block (Casati 2007a; Cataldo 2012; Danelli 2012; Domingo‐Triado 2007; Gurkan 2008; Kapral 2008; Macaire 2008; Meierhofer 2014; Salem 2012; Sauter 2008; Seidel 2013; Shrestha 2011; Strub 2011; Taboada 2009; Trabelsi 2013). Four studies measured duration of block ( Dhir 2008; Domingo‐Triado 2007; Kapral 2008; Soeding 2005).
There were seven studies which reported the number of attempts to perform the block (Casati 2007a; Cataldo 2012; Danelli 2012; Dufour 2008; Sauter 2008; Shrestha 2011; Van Geffen 2009).
Seven studies reported participant discomfort during block placement (Bloc 2010; Casati 2007a; Dufour 2008; Macaire 2008; Meierhofer 2014; Sauter 2008; Van Geffen 2009). Two further studies reported participants' level of satisfaction with the procedure (Cataldo 2012; Soeding 2005).
Excluded studies
There were 18 studies in the original review (Walker 2009). Following the changes made to the review inclusion criteria in the 2014 update, we excluded five of these previously included studies. Four of them gave additional anaesthesia to all participants following the nerve block (Danelli 2009a; Dolan 2008; Marhofer 1997; Marhofer 1998) and one had used a MEAV study design (Casati 2007b).
During the updated search we identified a further 18 studies that were excluded either as MEAV study designs, studies in which additional anaesthesia was given to all participants or studies in which participants were scheduled for surgery other than for lower/upper extremity procedures.
We excluded six abstracts which were potentially eligible but provided insufficient detail. We excluded two studies as ultrasound was used pre‐puncture in both groups, and one study due to lack of randomization details in the full text. Redborg 2009 had previously been in Characteristics of studies awaiting classification but was assessed for this update as not eligible due to the use of volunteer non‐surgical participants.
In total, we excluded 33 studies from the updated review. Details are in Characteristics of excluded studies.
Ongoing studies
In the original review (Walker 2009) there were six potentially relevant studies listed as 'ongoing'. Two of these studies are now published; Liu 2009a is included in this update, and McCartney 2008 is an abstract only and has insufficient information to include. A third study (Dhir 2013) is complete but the results are not yet available, and has been included in Characteristics of studies awaiting classification. Two were no longer available in online clinical trials registers (www.clinicaltrials.gov; www.controlled‐trials.com) and we were unable to find any details for these in our searches. These studies (previously referenced as Freitas 2007 and Schwemmer 2006) were therefore removed from the list of ongoing studies.
One study was still ongoing (NCT 00213954), along with a further three studies (NCT 009956683; NCT 01010412; NCT02020096) identified from an up‐to‐date search of the above clinical trials registers. Details for these are given in Characteristics of ongoing studies
There are now four ongoing studies in the updated review.
Studies awaiting assessment
There are four studies awaiting assessment in the updated review. We have been unable to access the full text of one study (González 1993), and we await full texts for two studies (Dhir 2013; NCT 01579747), while one Chinese study (Li 2013) requires translation. Details are given in Characteristics of studies awaiting classification.
We identified a further seven studies during a rerun of the search in May 2015 (Aytac 2015; Eren 2014; Kumar 2014; Lam 2014; Martinez Navas 2014; Smith 2014; Stavrati 2014). We will assess these and incorporate them into the next review update. There are now 11 studies awaiting assessment.
Risk of bias in included studies
We conducted a 'Risk of bias' assessment for each study and give details in the Characteristics of included studies tables. Summaries of our assessment are included in Figure 2 and Figure 3.
2.
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All 32 studies were described as randomized but only 20 provided sufficient detail of the methods used to be judged as being at low risk of bias (Brull 2009; Casati 2007a; Chan 2007; Conceição 2009; Danelli 2012; Domingo‐Triado 2007; Dufour 2008; Geiser 2011; Kapral 2008; Liu 2005; Liu 2009a; Meierhofer 2014; Morros 2009; Perlas 2008; Renes 2009; Sauter 2008; Seidel 2013; Shrestha 2011; Sites 2006; Strub 2011). There were only two studies that provided an adequate description of the methods used to conceal group allocation to participants and personnel (Kapral 2008; Perlas 2008).
Blinding
Due to the nature of the intervention, blinding of the practitioner was never going to be possible and it is therefore an unavoidable source of bias. It was necessary for us to judge performance bias across all outcomes in all studies as being at high risk of bias due to this lack of blinding. It was, however, possible for detection bias to be reduced by ensuring that observers/investigators collecting data for some of the outcomes were blinded to group allocation. Seventeen studies had reported sufficient information on whether outcome assessors were blinded to group allocation on at least one of the outcomes (Brull 2009; Casati 2007a; Cataldo 2012; Domingo‐Triado 2007; Dufour 2008; Gurkan 2008; Liu 2005; Liu 2009a;Meierhofer 2014; Perlas 2008; Sauter 2008; Seidel 2013; Shrestha 2011; Sites 2006; Taboada 2009; Trabelsi 2013; Van Geffen 2009). Only two trials made an attempt to blind the participants to the technique being used through the use of a sham ultrasound device (Chan 2007; Perlas 2008).
Incomplete outcome data
There were few losses of study participants overall and all but four of the studies (Brull 2009; Chan 2007; Dufour 2008; Sites 2006) were assessed as being at a low risk of attrition bias.
Brull 2009 had few losses for all outcomes except complications, for which only 49% of participants were contacted for follow‐up at one week postoperatively. We assessed this as being at unclear risk of bias. Chan 2007 lost 14% of its intended participants who were required to go to surgery before the end of 30 minutes post‐block. We judged that some outcome data could still have been collected from these participants and we therefore felt that this study was at high risk of attrition bias, along with Dufour 2008 which also had several losses. We judged Sites 2006 as being at high risk of bias, as this study stopped early due to a high number of failed blocks in the transarterial approach group.
Selective reporting
We were able to source the protocols for five of the studies from clinicaltrials.gov and compare the reported outcomes with protocol outcomes (Chan 2007; Danelli 2012; Perlas 2008; Sauter 2008; Seidel 2013). We judged these as being at low risk of selective outcome reporting bias, as all outcomes were reported as planned. However, we were unable to make a judgement on the remaining studies for high or low risks of bias.
Other potential sources of bias
There was one study that failed to report any baseline characteristics (Soeding 2005) and we were therefore unable to make a judgement on whether any bias could have been introduced. A further four studies reported baseline characteristics for which there were some discrepancies between groups: in Dhir 2008 there were more older participants in the nerve stimulation (with stimulating catheter) group; in Domingo‐Triado 2007 there were differences between groups in the types of surgery; in Geiser 2011 there were more women than men reported in the table, although the text stated that there was no difference; and in Dufour 2008 there was a difference in the ASA status between groups. We were unsure whether these differences could potentially introduce any bias into the results, and assessed them as being at unclear risk. The remaining 27 studies all had comparable baseline characteristics between participants.
We were interested in whether study authors had been provided with any funding for their research and therefore considered this in our assessment of risk of bias. There were five studies that declared that the ultrasound or nerve stimulator equipment had been provided by the manufacturer for the purpose of the study (Brull 2009; Chan 2007; Gurkan 2008; Sites 2006; Van Geffen 2009). We judged these studies to be at a higher risk of bias. All other studies either declared funding from departmental sources only, or did not make any funding declarations, and we assessed them as being at low risk of bias.
The experience of practitioners in both ultrasound and control techniques, as well as the number of practitioners involved, varied across studies. There were 12 studies that described the person giving the block as experienced (Bloc 2010; Brull 2009; Casati 2007a; Danelli 2012; Kapral 2008; Meierhofer 2014; Morros 2009; Perlas 2008; Salem 2012; Sauter 2008; Soeding 2005; Taboada 2009). However, even for those that stated that the practitioners had experience in both techniques, it was not clear whether this experience was equivalent for each technique. For this reason we were unable to judge whether any bias had been introduced by the practitioners in these studies. Some procedures were performed by anaesthesia residents under supervision (Chan 2007; Williams 2003), and we felt that could be likely to introduce bias, particularly for block performance time, and therefore assessed them as being at high risk. We also rated studies at high risk of bias if it was clear that the practitioner had more experience in one technique than the other, or that different procedures were intentionally performed by different practitioners.
Effects of interventions
See: Table 1
Primary outcomes
1. Block success ‐ predicted adequacy of block
There were 17 studies with 1346 participants comparing ultrasound guidance with either nerve stimulation (15 studies: Bloc 2010; Brull 2009; Chan 2007; Geiser 2011; Kapral 2008; Liu 2009a; Macaire 2008; Meierhofer 2014; Perlas 2008; Renes 2009; Sauter 2008; Taboada 2009; Trabelsi 2013; Van Geffen 2009; Zaragoza‐Lemus 2012), anatomical landmark technique (one study: Soeding 2005) or a transarterial approach (one study: Sites 2006) and reporting on predicted adequacy of the block. This outcome was often described by the authors as "block success" and was evaluated using appropriate sensory and motor testing at intervals following the procedure, using a scale to determine the degree of block. We combined data described as "complete", "successful" of "sufficient" block. If studies separated results for adequacy of sensory and motor success, we used data from the sensory block.
For the purpose of this analysis we combined the two ultrasound groups in both Bloc 2010 and Liu 2005. We also included data for Chan 2007 for the ultrasound alone versus nerve stimulation group. The analysis demonstrated a statistically significant difference between the ultrasound versus nerve stimulation groups, with a higher rate of predicted adequacy of the block in the ultrasound group than the comparison group (Mantel‐Haenszel (M‐H) odds ratio (OR) 3.01 (95% confidence interval (CI) 2.11 to 4.31), 1250 participants, P value < 0.00001). When data for Soeding 2005 (anatomical landmark technique) and Sites 2006 (transarterial approach) were also included the result remained statistically significant in favour of ultrasound (M‐H OR 3.06 (95% CI 2.18 to 4.30), 1346 participants, P value < 0.00001). This result shows a low level of heterogeneity (I² = 13%). Considering the potential effect of bias on this result we graded this to be moderate‐quality evidence of an effect in the 'Summary of findings' table. A funnel plot did not suggest publication bias for this outcome. See Analysis 1.1, Table 1 and Figure 4.
1.1. Analysis.
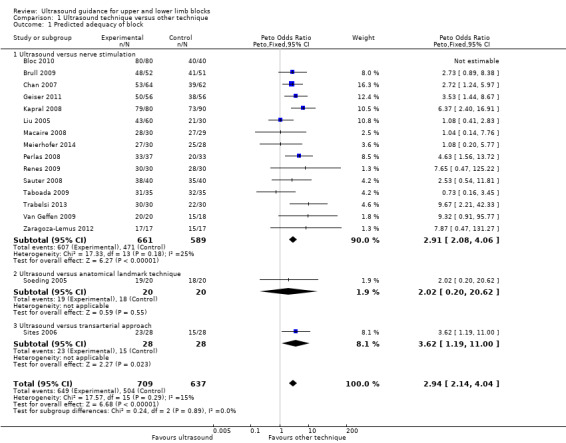
Comparison 1 Ultrasound technique versus other technique, Outcome 1 Predicted adequacy of block.
4.
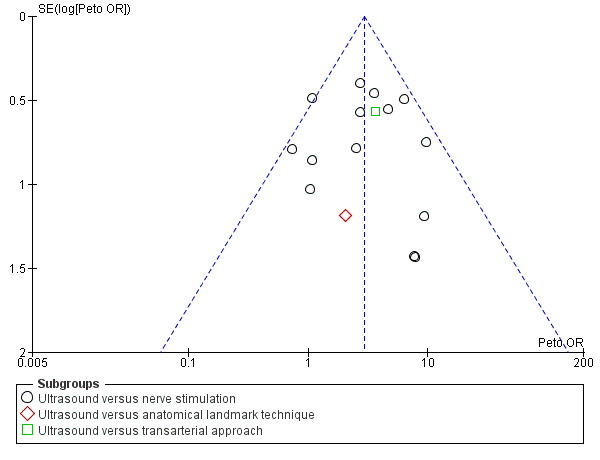
Funnel plot of comparison: 1 Ultrasound technique versus other technique, outcome: 1.1 Predicted adequacy of block.
There were nine studies with 719 participants comparing ultrasound + nerve stimulation versus nerve stimulation technique and reporting on predicted adequacy of the block (Cataldo 2012; Chan 2007; Dhir 2008; Domingo‐Triado 2007; Dufour 2008; Gurkan 2008; Morros 2009; Salem 2012; Williams 2003). For the purpose of this analysis, we combined data in Dhir 2008 for the two nerve stimulation groups. The analysis demonstrated a statistically significant difference between the two groups, again with a higher rate of predicted adequacy of the block in the ultrasound group, than the comparison group (M‐H OR 3.33 (95% CI 2.13 to 5.20), P value < 0.00001). This result shows a low level of heterogeneity (I² = 26%). See Analysis 2.1.
2.1. Analysis.
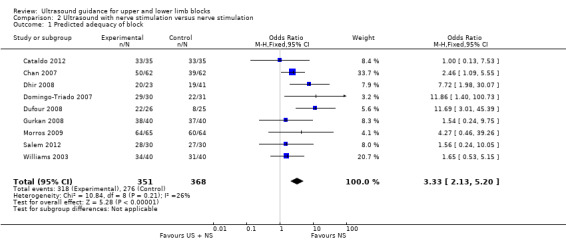
Comparison 2 Ultrasound with nerve stimulation versus nerve stimulation, Outcome 1 Predicted adequacy of block.
2. Block success ‐ supplementation requirement
There were 18 studies with 1807 participants comparing ultrasound guidance with either nerve stimulation (15 studies: Bloc 2010; Casati 2007a; Chan 2007; Conceição 2009; Geiser 2011; Kapral 2008; Liu 2005; Liu 2009a; Macaire 2008; Meierhofer 2014; Perlas 2008; Renes 2009; Sauter 2008; Seidel 2013; Taboada 2009), anatomical landmark technique (two studies: Soeding 2005; Strub 2011) or a transarterial approach (one study: Sites 2006) and reporting on supplementation rates. Authors sometimes described this outcome as "block failure" and defined it as the need for participants to be given either supplementary block, local anaesthetic, supplementary intraoperative analgesics or conversion to general anaesthesia. For the purpose of this outcome, we combined all supplementary anaesthesia/analgesia together. As above, we combined the two ultrasound groups in Liu 2005. There was a statistically significant difference between groups with fewer participants in the ultrasound group requiring additional supplementation (Mantel‐Haenszel (M‐H) OR 0.31 (95% CI 0.21 to 0.46),1570 participants, P value < 0.00001). When we included data for Soeding 2005 and Strub 2011 (anatomical landmark technique) and Sites 2006 (transarterial approach), the results remained statistically significant (M‐H OR 0.28 (95% CI 0.20 to 0.39), P value < 0.00001). Again, there was a low level of heterogeneity in this result (I² = 16%), and we graded it as moderate‐quality evidence of an effect in the 'Summary of findings' table. See Analysis 1.2 and Table 1.
1.2. Analysis.
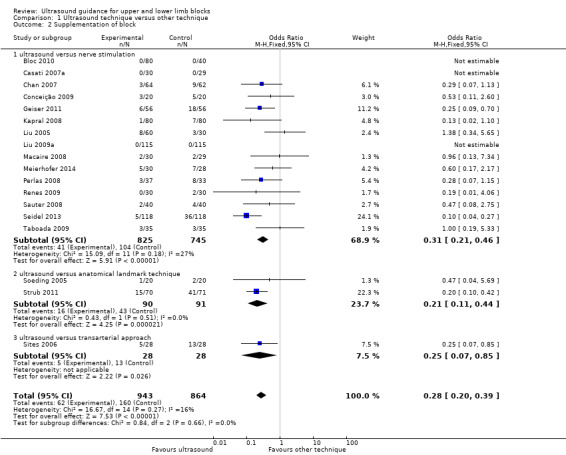
Comparison 1 Ultrasound technique versus other technique, Outcome 2 Supplementation of block.
There were nine studies (Chan 2007; Dhir 2008; Domingo‐Triado 2007; Dufour 2008; Gurkan 2008; Morros 2009; Salem 2012; Shrestha 2011; Williams 2003) with 712 participants comparing ultrasound guidance + nerve stimulation versus nerve stimulation technique and reporting on supplementation rates, as above. We combined data for the two nerve stimulation groups in Dhir 2008. The results were statistically significantly different, again with a lesser need for supplementation in the ultrasound group (M‐H OR 0.34 (95% CI 0.21 to 0.56), 1807 participants, P value < 0.00001, I² = 0%. See Analysis 2.2.
2.2. Analysis.
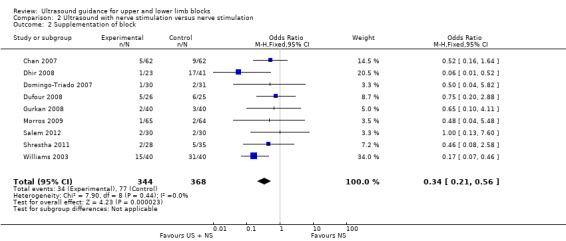
Comparison 2 Ultrasound with nerve stimulation versus nerve stimulation, Outcome 2 Supplementation of block.
3. Complications
Complication rates were recorded in 23 trials There were no reported incidences of major complications (cardiorespiratory arrest, seizures, pneumothorax, nerve injury) in any included study. Paraesthesia and vascular puncture were the most frequently reported complications and we included data for these in meta‐analysis.
There were six studies (Bloc 2010; Brull 2009; Conceição 2009; Liu 2005; Sauter 2008; Soeding 2005) with 471 participants that reported data for paraesthesia for the groups ultrasound versus nerve stimulation (five studies) and anatomical landmark technique (one study). This analysis showed more incidences of paraesthesia in the nerve stimulation group (M‐H OR, 0.42 (95% CI 0.23 to 0.76)). However there was a high level of heterogeneity in this analysis (I² = 75%) and it is clear that the result is influenced by Brull 2009 with a very large number of events (22 events, 45%) in the nerve stimulation group. We downgraded the quality of this evidence to very low on account of the relatively few events reported in studies and the high level of heterogeneity. See Analysis 1.4 and Table 1. There were only three studies (Dhir 2008; Dufour 2008; Shrestha 2011) with 178 participants that reported data for paraesthesia for the groups ultrasound + nerve stimulation versus nerve stimulation alone. There was no significant difference in this analysis (M‐H OR, 0.97 (95% CI 0.30 to 3.12), P value = 0.95). See Analysis 2.4.
1.4. Analysis.
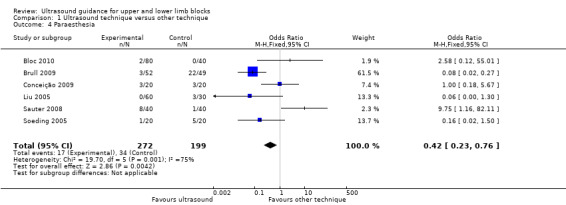
Comparison 1 Ultrasound technique versus other technique, Outcome 4 Paraesthesia.
2.4. Analysis.
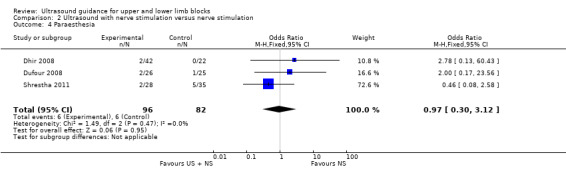
Comparison 2 Ultrasound with nerve stimulation versus nerve stimulation, Outcome 4 Paraesthesia.
There were five studies with 387 participants that reported data for vascular puncture for the groups ultrasound versus nerve stimulation (four studies: Bloc 2010; Brull 2009; Conceição 2009; Taboada 2009) and transarterial approach (one study: Sites 2006). The result (M‐H OR 0.19 (95% CI 0.07 to 0.57)) showed that there were fewer incidences of vascular puncture in the ultrasound groups and we graded this as low level of evidence in the 'Summary of findings' table. See Analysis 1.5 and Table 1. There were only two studies (Gurkan 2008; Shrestha 2011; 143 participants) that reported data for vascular puncture from our comparison groups of ultrasound + nerve stimulation versus nerve stimulation alone, and again this analysis showed a statistically significant effect of fewer incidences of vascular puncture in the ultrasound + nerve stimulation group (M‐H OR 0.22 (95% CI 0.05 to 0.90)). See Analysis 2.5.
1.5. Analysis.
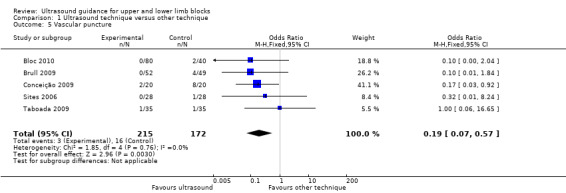
Comparison 1 Ultrasound technique versus other technique, Outcome 5 Vascular puncture.
2.5. Analysis.

Comparison 2 Ultrasound with nerve stimulation versus nerve stimulation, Outcome 5 Vascular puncture.
One study reported data for axillary vessels puncture (Liu 2005, three incidences in the nerve stimulation group). Meierhofer 2014 gave data for venous puncture (one incidence in the nerve stimulation group) and for arterial puncture (two incidences in both groups) and Morros 2009 also for arterial puncture (one incidence in the ultrasound group and two in the nerve stimulation group).
Studies gave differing terms for nerve damage, but it was not always clear whether these effects were immediate, medium‐ or long‐term and whether they were equivalent between studies. It was therefore not possible to combine them in meta‐analysis. Studies that reported on such complications were Domingo‐Triado 2007; Kapral 2008; Perlas 2008; Salem 2012; Sites 2006; and Strub 2011. Of these, Kapral 2008 and Sites 2006 reported no events and Salem 2012 reported that such effects were equivalent between groups. Perlas 2008 reported more numbness at 24 hours postoperatively in the ultrasound group (eight versus four events) and more weakness at this time point in the ultrasound group (10 versus two events). Strub 2011 reported two events of neuralgia in the hand in the traditional nerve block technique and no events in the ultrasound group. Domingo‐Triado 2007 reported that one participant had neuropathic pain at one week in the nerve stimulation group which resolved within 10 days.
Other effects reported were tachycardia (Brull 2009, one event in the nerve stimulation group), subcutaneous haematoma (Liu 2005, one event in the nerve stimulation group), haematoma requiring additional manual compression (Sites 2006, two events in the transarterial approach group), axillary haematoma (Strub 2011, five events in the traditional nerve block technique and two in the ultrasound group), prolonged pain in axilla (Strub 2011, three in traditional group and one in ultrasound group) and respiratory discomfort (Williams 2003, one participant in each group).
Secondary outcomes
1a.Time to perform block
There were 25 studies that reported data for time to perform block (Bloc 2010; Brull 2009; Cataldo 2012; Chan 2007; Conceição 2009; Danelli 2012; Dhir 2008; Domingo‐Triado 2007; Dufour 2008; Geiser 2011; Gurkan 2008; Liu 2005; Macaire 2008; Meierhofer 2014; Morros 2009; Perlas 2008; Salem 2012; Sauter 2008; Shrestha 2011; Sites 2006; Strub 2011; Taboada 2009; Trabelsi 2013; Van Geffen 2009; Williams 2003). However, some of these data were presented as median and interquartile range, or presented means without standard deviations, and it was therefore not possible to include them in the meta‐analysis (Bloc 2010; Brull 2009; Conceição 2009; Danelli 2012; Domingo‐Triado 2007; Macaire 2008; Sauter 2008; Strub 2011). We did not combine data for Cataldo 2012 in our analysis which had given time data by each blocked nerve. We also decided that those studies which included catheter placement should not be included in this analysis, due to the increased length of time involved in this procedure. We therefore excluded Dhir 2008 and Salem 2012. Whilst there was some variation in definition of performance time, we felt that these were similar enough to warrant meta‐analysis. We present definitions, where available, in Characteristics of included studies.
Our first meta‐analysis was conducted using only 10 studies of ultrasound versus nerve stimulation (nine studies: Chan 2007; Conceição 2009; Geiser 2011; Liu 2005; Meierhofer 2014; Perlas 2008; Taboada 2009; Trabelsi 2013; Van Geffen 2009) and transarterial approach (one study: Sites 2006) with a total of 690 participants. For Liu 2005, it was not possible to combine the data for the two ultrasound groups and we therefore only compared the ultrasound (double‐injection) group against the nerve stimulation (double‐injection) group.
The analysis showed a statistically significant difference, with performance time being less in the ultrasound group (mean difference (MD), IV fixed‐effect, ‐1.06 (95% CI ‐1.41 to ‐0.72), P value < 0.00001). See Analysis 1.3.
1.3. Analysis.
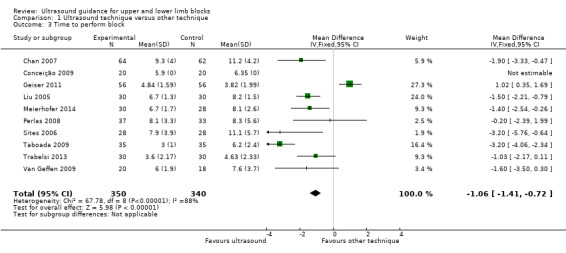
Comparison 1 Ultrasound technique versus other technique, Outcome 3 Time to perform block.
For performance time, seven studies with a total of 587 participants (Chan 2007; Dufour 2008; Gurkan 2008; Morros 2009; Salem 2012; Shrestha 2011; Williams 2003) compared ultrasound guidance + nerve stimulation with nerve stimulation technique. There was a statistically significant difference between groups, with performance time being less in the nerve stimulation group (MD, IV, fixed‐effect, 0.76 (95% CI 0.55 to 0.98)). See Analysis 2.3.
2.3. Analysis.
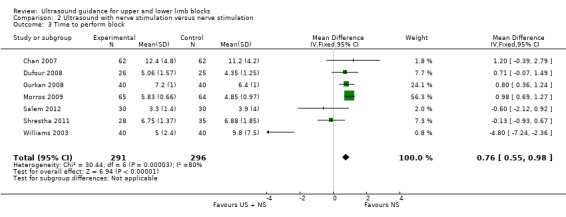
Comparison 2 Ultrasound with nerve stimulation versus nerve stimulation, Outcome 3 Time to perform block.
There was a high level of heterogeneity for both these analyses (I² = 88% in Analysis 1.3 and I² = 80% in Analysis 2.3). We subsequently downgraded the quality of evidence for this result to very low, taking into account the high level of heterogeneity and potential differences in experience of personnel which could affect this result. See Table 1.
We also considered those studies for which we did not conduct meta‐analysis for this outcome. Of those studies comparing ultrasound with nerve stimulation, five reported a statistically significantly shorter time to perform block in the ultrasound groups (Bloc 2010: P value < 0.05; Brull 2009: P value < 0.001; Danelli 2012: P value = 0.01; Macaire 2008: P value = 0.02; Sauter 2008: P value = 0.003). Conceição 2009 reported a shorter time in the ultrasound group but this was not statistically significant. Cataldo 2012 reported a statistically significantly shorter time in the ultrasound + nerve stimulation group (P value = 0.02). Dhir 2008 reported a statistically significantly shorter time in the nerve stimulation compared to other groups (P value < 0.0001). Strub 2011 reported no significant differences between groups in time to perform block. We were unable to extract data for Salem 2012, due to their methods of presentation of results.
1b. Onset time of block
There were 15 studies (Casati 2007a; Cataldo 2012; Danelli 2012; Domingo‐Triado 2007; Gurkan 2008; Kapral 2008; Macaire 2008; Meierhofer 2014; Salem 2012; Sauter 2008; Seidel 2013; Shrestha 2011; Strub 2011; Taboada 2009; Trabelsi 2013) that evaluated onset time of block. However, the studies reported data in different ways, sometimes reporting median (range or interquartile range) and sometimes mean and standard deviation. There were also differences in whether results were presented for each nerve separately or combined, and whether or not sensory or motor block onset time was reported separately. We therefore did not combine these data in meta‐analysis. Six studies reported a statistically significant difference between groups, favouring a shorter onset time in the ultrasound group with P value less than 0.05 (Casati 2007a (for sensory block only); Kapral 2008; Seidel 2013; Shrestha 2011; Strub 2011; Trabelsi 2013 (for the sensory block only)). Nine studies reported no significant differences between groups in onset time (Casati 2007a (for motor block only); Cataldo 2012; Danelli 2012; Domingo‐Triado 2007; Meierhofer 2014; Salem 2012; Sauter 2008; Taboada 2009; Trabelsi 2013 (for motor block only)). Macaire 2008 reported a significantly shorter onset time in the nerve stimulation group (P value < 0.02).
1c. Duration of block
There were four studies that evaluated duration of block. Kapral 2008 reported a statistically significant difference between groups, with blocks in the ultrasound group having a longer duration than the nerve stimulation group (P value < 0.05). The remaining studies reported that there was no significant difference between groups for block duration time (Dhir 2008; Domingo‐Triado 2007; Soeding 2005).
2. Number of attempts
There were seven studies (Casati 2007a; Cataldo 2012; Danelli 2012; Dufour 2008; Sauter 2008; Shrestha 2011; Van Geffen 2009) that reported on the number of attempts, defined as needle/skin punctures or needle passes (forward movement preceded by retraction of needle). Individual study definitions are given in Characteristics of included studies. Results were reported as mean and standard deviation or median and range, and it was not possible to pool data. Three studies reported that there were significantly fewer needle passes or skin punctures in the ultrasound group (Danelli 2012: P value = 0.01; Sauter 2008: P value < 0.001; Van Geffen 2009: P value = 0.029). Shrestha 2011 reported fewer attempts in the ultrasound + nerve stimulation group than the nerve stimulation group. Cataldo 2012 reported significantly more needle punctures in the intervention group (P value = 0.004). Dufour 2008 , whilst reporting significantly more needle passes to locate the first nerve in the comparison, reported with Casati 2007a that the difference in the number of skin punctures was not significant. We graded this evidence as being of low quality in the Table 1.
3. Participant discomfort
There were seven studies (Bloc 2010; Casati 2007a; Dufour 2008; Macaire 2008; Meierhofer 2014; Sauter 2008; Van Geffen 2009) in which participants reported discomfort. Five of these reported responses on a visual analogue scale or numerical rating score for satisfaction with the procedure or discomfort/pain during procedure (Dufour 2008; Macaire 2008; Meierhofer 2014; Sauter 2008; Van Geffen 2009). All five studies reported that there was no statistically significant difference between groups. Casati 2007a asked if participants would accept the same procedure again and there were no statistically significant differences between groups. Only Bloc 2010 reported a statistically significant difference for this outcome, with fewer participants describing the procedure as unpleasant in the ultrasound (out‐plane approach) than the ultrasound (in‐plane approach) or nerve stimulation group. We graded evidence for this outcome as being of low quality in the Table 1.
Subgroup analysis
The outcomes 'Time to perform block' and 'Paraesthesia' both had a high level of statistical heterogeneity (I² = 88% in Analysis 1.3; I² = 80% in Analysis 2.3; I²= 75% in Analysis 1.4).
1.Different types of comparisons
We performed subgroup analysis according to the comparison group, i.e. nerve stimulation, anatomical landmark or transarterial approach. For 'Time to perform block', all but one study compared with nerve stimulation and there remained a high level of heterogeneity in this group, I² = 89%. See Analysis 3.1. Similarly for 'Paraesthesia', we were not able to explain heterogeneity by subgroup analysis, again with only one study not comparing against nerve stimulation and statistical heterogeneity remaining high for this group (I² = 79%). See Analysis 5.1.
3.1. Analysis.

Comparison 3 Time to perform block by subgroups US vs other, Outcome 1 By type of intervention/comparison.
5.1. Analysis.
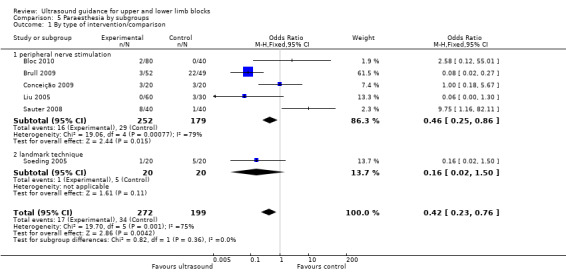
Comparison 5 Paraesthesia by subgroups, Outcome 1 By type of intervention/comparison.
2. Different types of nerve blocks
We performed subgroup analysis according to the type of nerve block and block approach. For 'Time to perform block' results remained statistically significant and with a high level of heterogeneity in the axillary and infraclavicular brachial plexus block, but for those studies which used the popliteal fossa sciatic block there was no difference in the time to perform the block between ultrasound and nerve stimulation use (MD, IV, fixed‐effect ‐1.00 (95% CI ‐2.43 to 0.44), I² = 0%). However there were only two studies using this block in this analysis (see Analysis 3.2). For those studies combining ultrasound with peripheral nerve stimulation, there were only single studies in the nerve block approaches other than for axillary plexus block, and for this there was no difference in the result and I² remained similarly high (see Analysis 4.1).
3.2. Analysis.
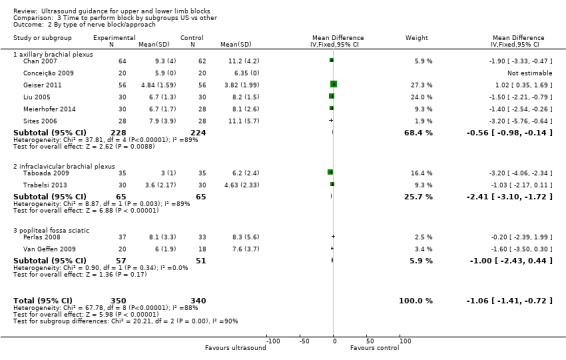
Comparison 3 Time to perform block by subgroups US vs other, Outcome 2 By type of nerve block/approach.
4.1. Analysis.
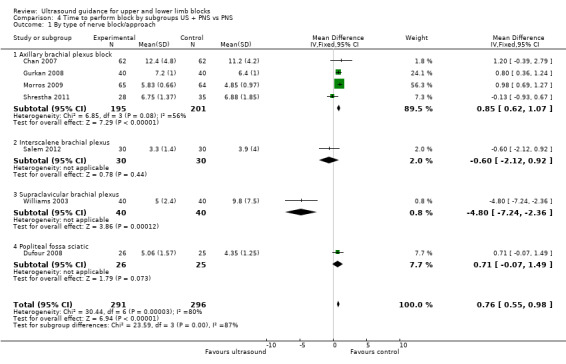
Comparison 4 Time to perform block by subgroups US + PNS vs PNS, Outcome 1 By type of nerve block/approach.
For the outcome 'Paraesthesia', subgroup analysis showed that for the infraclavicular brachial plexus block there were still significantly fewer events of paraesthesia with ultrasound use, although statistical heterogeneity remained high (I² = 87%). But for the axillary brachial plexus block there was no longer any statistical difference between block technique, with only moderate heterogeneity (I² = 40%). See Analysis 5.2.
5.2. Analysis.
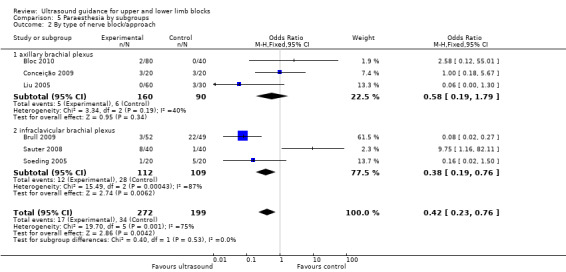
Comparison 5 Paraesthesia by subgroups, Outcome 2 By type of nerve block/approach.
3. Studies with catheter placement
Three of our studies (Danelli 2012; Dhir 2008; Salem 2012) had included catheter placement as part of the nerve block procedure and whilst we had included Dhir 2008 and Salem 2012 in the main analysis which had a low I² value, we separated Salem 2012 from the 'Time to perform block' outcome. This outcome remained statistically significantly in favour of the control. See Analysis 4.2.
4.2. Analysis.

Comparison 4 Time to perform block by subgroups US + PNS vs PNS, Outcome 2 By catheter/no catheter placement.
4. Experience of practitioners
Although experience of practitioners is an important consideration for this review, we did not perform subgroup analysis. Several studies did not provide details on experience, and for those that did it was often unclear whether the experience was equivalent between techniques. Subgroup analysis would not have provided a reliable result.
5. Other heterogeneity
It is likely that heterogeneity for the outcome of 'Time to perform block' may be as a result of the variety of definitions used by study authors for this outcome measure. However, these outcomes varied such that it was not feasible to perform subgroup analysis and provide a reliable result.
Sensitivity analysis
In sensitivity analysis, we considered the effect of bias on our primary outcome only.
We removed those studies that had not reported clearly on their methods of sequence generation (Macaire 2008; Taboada 2009; Trabelsi 2013; Van Geffen 2009; Zaragoza‐Lemus 2012) and this did not affect the results. As it was feasible for outcomes to be assessed by blinded observers, we removed those studies that we had judged as being at either unclear or high risk of bias for this domain (Chan 2007; Geiser 2011; Kapral 2008; Macaire 2008; Renes 2009; Taboada 2009; Van Geffen 2009; Zaragoza‐Lemus 2012) and again this did not affect the results.
We similarly removed studies at high or unclear risk of attrition bias (Brull 2009; Chan 2007), with no difference to meta‐analysis results.
We had reported on whether studies had received any funding assistance and for sensitivity analysis removed those studies that we had assessed as being at high risk of bias due to the supplying of study equipment (Brull 2009; Chan 2007; Gurkan 2008; Sites 2006; Van Geffen 2009). For Analysis 1.1, we removed Brull 2009, Chan 2007, Sites 2006 and Van Geffen 2009, and for Analysis 2.1, we removed Gurkan 2008. This did not make any difference to our statistically significant result in favour of ultrasound.
In sensitivity analysis, we chose to remove those studies which we had judged as having a high risk of bias for practitioner experience (Cataldo 2012; Chan 2007; Dufour 2008; Sites 2006; Strub 2011; Van Geffen 2009; Williams 2003).This did not make any difference to our results in favour of ultrasound guidance for success of the block.
Discussion
Summary of main results
As we had changed the eligibility criteria in the 2014 update, resulting in the exclusion of several studies, we felt that the included studies were now more homogeneous and that it was appropriate to combine the results of our data with meta‐analysis.
This review, based on 32 studies in 2844 adult participants, has found that ultrasound guidance produces superior peripheral nerve block success rates, with more blocks being assessed as complete or sufficient for surgery following sensory or motor testing, and fewer blocks requiring supplementation or conversion to general anaesthetic compared with the use of nerve stimulation, anatomical landmark techniques or transarterial approach. This result was similarly advantageous for studies that compared ultrasound, either alone or combined with nerve stimulation, against nerve stimulation. Results suggest that there are fewer incidences of paraesthesia and vascular puncture when using ultrasound approaches, and the review authors' interpretation of other results also suggests a reduction in complications such as nerve damage.
The evidence in this review, both using meta‐analysis and interpretation of individual authors' results, also suggests that it takes less time to perform the block when using ultrasound techniques alone rather than nerve stimulation. As expected, it takes longer to perform the block when nerve stimulation is used as an additional technique combined with ultrasound than when nerve stimulation is used alone. The results for analysis of this outcome had moderate to high levels of heterogeneity.
The performance of peripheral nerve blocks is clearly dependent on experience and expertise of the practitioner and we were concerned about the influence of this bias on the results. Our subgroup analysis, removing studies which we had judged as being at high level of risk of bias for this outcome, remained consistent with the main analysis that ultrasound‐guided techniques require less time to perform.
Overall completeness and applicability of evidence
We carried out a thorough search, both in the original review (Walker 2009) and in the 2014 update, using appropriate electronic databases. We also included backward and forward citation tracking and details of studies posted on clinical trials registers. Where necessary, we made attempts to contact authors for additional study details. Despite narrowing our eligibility criteria for this update, we were still able to identify 32 relevant studies that met our eligibility criteria for participant and interventions.
Studies included a variety of nerve blocks for procedures of the upper and lower limbs, including different approaches to the brachial plexus block and the sciatic and popliteal fossa nerves. Whilst we did not restrict the comparison to a particular nerve block technique, the majority of included studies compared ultrasound against nerve stimulation, with only two studies comparing against anatomical landmark technique and one comparing against a transarterial approach. With so few studies comparing against anatomical landmark technique or transarterial approach, we are not able to reliably rate the applicability of these findings against these two comparisons.
Results of our review are applicable to peripheral nerve blocks of the upper and lower limbs for which peripheral nerve block is the intended sole anaesthetic, as we had excluded studies of other blocks.
Quality of the evidence
The methodological quality of the trials was moderate, at best. Details of methods of randomization, allocation concealment and blinding of outcome assessors were inconsistent across studies and it is unclear whether this was due to a failure by authors to report study details or to a lack of methodological rigour.
We accepted that it was not possible to blind the anaesthetist and this inevitably skewed our 'Risk of bias' assessment as all studies were at an increased risk of performance bias. Another important aspect of performance bias for this review was the likelihood of varying experience of the practitioners and their attitude towards ultrasound or alternative techniques. It is possible that those 12 studies (Bloc 2010; Brull 2009; Casati 2007a; Danelli 2012; Kapral 2008; Meierhofer 2014; Morros 2009; Perlas 2008; Salem 2012; Sauter 2008; Soeding 2005; Taboada 2009) which described their practitioners as 'experienced' had used personnel who were ultrasound enthusiasts, with considerable experience in ultrasound. Equally, studies may have used personnel with considerably less experience in ultrasound. Unfortunately without this information we were unable to explore this further and do not know whether our results could be applicable to experienced ultrasound users only.
Whilst our results were consistent during sensitivity analysis, we did not feel able to grade the quality of evidence as high for any outcomes, and subsequently graded our results as moderate, low or very low quality.
Potential biases in the review process
Our decision to restrict the eligibility criteria in the 2014 update meant that we excluded several of the original studies (Casati 2007a; Danelli 2009a; Dolan 2008; Marhofer 1997; Marhofer 1998), as well as additional studies that would have been included in our latest search. Whilst this restriction could have introduced bias into the results, we felt that it reduced the heterogeneity between studies and allowed meta‐analysis that previously had not been possible.
In the original review (Walker 2009) the authors had made attempts to contact known authors in the research field, as well as conducting handsearches of journals. We did not replicate this level of searching in the 2014 update and, whilst our searches ultimately identified 32 included studies, we do not know whether we would have identified further eligible studies had we searched to this extent.
We reran the search in May 2015 and found seven studies of interest. We added them to the list of 'Studies awaiting classification' and will incorporate them into the next review update.
Agreements and disagreements with other studies or reviews
In the original review, we had concluded that there was limited evidence to support the routine use of ultrasound for peripheral nerve blocks. However, our evidence as a result of further included studies and meta‐analyses in this update demonstrate the benefits in the use of ultrasound guidance over other techniques. This is consistent with other reviews in the field (Gelfand 2010; Liu 2009b) which support the use of ultrasound for peripheral nerve blocks with improved block success and fewer adverse events.
Authors' conclusions
Implications for practice.
We have presented evidence in this review to support the routine use of ultrasound guidance techniques for upper and lower limb blocks. Our analysis demonstrates that ultrasound improves the quality of the sensory blockade, reduces the need for supplementation, with fewer minor complications reported and shorter performance time.
We were unable to confirm whether or not these findings reflect the use of ultrasound in experienced hands.
Implications for research.
Future research should specify the experience of practitioners and assess if ultrasound use improves the success of nerve blocks with less experienced personnel.
Our results are only applicable to nerve blocks of the upper and lower limbs. Further systematic reviews would be required to assess whether these findings are consistent with other nerve blocks.
What's new
| Date | Event | Description |
|---|---|---|
| 17 August 2015 | New citation required and conclusions have changed | This review is an update of the previous Cochrane systematic review (Walker 2009) that included 18 RCTs. One previous author (Kristine Aas‐Eng) decided not to update the review. Two new authors: Sharon R Lewis and Anastasia Price have joined the review team We altered the review eligibility criteria. We included 32 studies that met our new eligibility criteria; 19 of these from our 2014 search 13 from Walker 2009. There are four studies awaiting classification and four ongoing studies. We excluded a total of 33 key studies; five from Walker 2009 due to the change in criteria and 27 from the 2014 search. We reran the search in 2015 and found a further seven studies which are awaiting classification. There are now 11 studies awaiting classification. We updated the methods to take into account Revman 5.3 and carried out 'Risk of bias' assessment to include the 13 studies from Walker 2009. We extracted data from eligible studies and completed meta‐analysis, leading to a different conclusion. |
| 17 August 2015 | New search has been performed | The original search was to July 2008 (Walker 2009). In this updated review we reran the searches until August 2014. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 1 February 2013 | Amended | Contact details updated. |
| 27 August 2009 | Amended | Kristine Aas‐Eng's department added to affiliation |
Notes
We have altered the title from the original review (see Walker 2009) to reflect the more specific peripheral blocks included in this 2014 update.
Acknowledgements
Anastasia Price died before publication of this review and we would like to acknowledge the significant contribution she made to this update. Her work was always comprehensive, incisive and much appreciated.
We would also like to acknowledge the contribution of Kristine Aas‐Eng to the original review (Walker 2007; Walker 2009).
We would like to thank Dr Mathew Zacharias (content editor), Dr Marialena Trivella (statistical editor), Dr Giorgio Danelli, Dr Vincent Chan (peer reviewers) and Sandra Oliveira (Cochrane Consumer Network) for their help and editorial advice during the preparation of the review (Walker 2009). We also thank Dr McCartney, Dr Dhir, Dr Marhofer and Dr Danelli for additional information provided in the original review (Walker 2009).
We would also like to thank Dr Mathew Zacharias, Dr Geert Jan van Geffen, Dr Andrea Casati and Kathie Godfrey for their help and editorial advice during the preparation of the protocol for the review (Walker 2007).
Appendices
Appendix 1. Search strategy for MEDLINE (Ovid SP)
1. exp Anesthesia, Local/ or exp Nerve Block/ or exp Brachial Plexus/ or exp Cervical Plexus/ or exp Lumbosacral Plexus/ or exp Thoracic Nerves/ or Femoral‐Nerve/ or Intercostal‐Nerves/ or Median‐Nerve/ or Obturator‐Nerve/ or Peroneal‐Nerve/ or Tibial‐Nerve/ or Radial‐Nerve/ or Sciatic‐Nerve/ or Superior‐Cervical‐Ganglion/ or Sural‐Nerve/ or Ulnar‐Nerve/ or ((nerve or plexus) adj3 block*).mp. or (local adj3 an?esth*).mp. or ((brachial or cervical or lumbosacral) adj3 plexus).mp. or ((femoral or intercostal or median or obturator or peroneus or tibial or radial or saphenous or sciatic or sural or ulnar) adj3 nerv*).mp. or (cervical adj3 ganglion).mp. 2. exp Ultrasonography/ or (ultrasound or ultrason* or echograph*).mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 2. Search strategy for EMBASE on OvidSP
1 local anesthesia/ or nerve block/ or brachial plexus/ or cervical plexus/ or lumbosacral plexus/ or femoral nerve/ or intercostal nerve/ or median nerve/ or obturator nerve/ or peroneus nerve/ or tibial nerve/ or radial nerve/ or sciatic nerve/ or superior cervical ganglion/ or sural nerve/ or ulnar nerve/ or ((nerve or plexus) adj3 block*).ti,ab. or (local adj3 an?esth*).ti,ab. or ((brachial or cervical or lumbosacral) adj3 plexus).ti,ab. or ((femoral or intercostal or median or obturator or peroneus or tibial or radial or saphenous or sciatic or sural or ulnar) adj3 nerv*).ti,ab. or (cervical adj3 ganglion).ti,ab. 2 exp echography/ or (ultrasound or ultrason* or echograph*).ti,ab. 3 1 and 2 4 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 5 3 and 4
Appendix 3. Search strategy for CINAHL (EBSCO host)
S1. ( (MM "Anesthesia, Local") OR (MM "Nerve Block") OR (MM "Brachial Plexus") OR (MM "Cervical Plexus") OR (MM "Lumbosacral Plexus") OR (MM "Thoracic Nerves") OR (MM "Femoral Nerve") OR (MM "Intercostal Nerves") OR (MM "Median Nerve") OR (MM "Peroneal Nerve") OR (MM "Tibial Nerve") OR (MM "Sciatic Nerve") OR (MM "Radial Nerve") OR (MM "Ulnar Nerve") ) OR ( ((nerve or plexus) and block*) ) OR (local N3 an?esth*) OR ( ((brachial or cervical or lumbosacral) N3 plexus) ) OR ( ((femoral or intercostal or median or obturator or peroneus or tibial or radial or saphenous or sciatic or sural or ulnar) N3 nerv*) ) OR (cervical N3 ganglion) S2. (MH "Ultrasonography+") OR ( ultrasound or ultrason* or echograph* ) S3. S1 AND S2 S4. ( (MM "Randomized Controlled Trials") OR (MM "Random Assignment") OR (MH "Clinical Trials") OR (MH "Placebos") ) OR ( random* or (trial* and (clinical or controlled)) or multicenter or prospective ) S5. S3 AND S4
Appendix 4. Search strategy for ISI Web of Science
TS=(nerve block*) or TS=(plexus block*)
TS=(local SAME (anaesth* or anesth*))
TS=(Brachial Plexus)
TS=(Cervical Plexus)
TS=(Lumbosacral Plexus)
TS=(Thoracic Nerve*)
TS=((Brachial or Cervical or Lumbosacral) SAME Plexus)
TS=((Median or Intercostal or Femoral or Thoracic or Obturator or Peroneal or Tibial or Radial or Sciatic or Sural or Ulnar) SAME nerv*)
TS=Superior Cervical Ganglion
TS=(Cervical SAME Ganglion)
#10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
TS=ultrasound or TS=ultrason*
#13 #12 AND #11
TS=random*
TS=(clin* SAME trial*)
TS=((singl* or doubl* or trebl* or tripl*) SAME (blind* or mask*))
#17 #16 OR #15 OR #14
#17 AND #13
Appendix 5. Search strategy for LILACS via BIREME
"local anaesth$" or "local anesth$" or "NERVE BLOCK/" or "BRACHIAL PLEXUS" or "CERVICAL PLEXUS/" or "LUMBOSACRAL PLEXUS/" or "THORACIC NERVES/" or "MEDIAN NERVE/" or "INTERCOSTAL NERVES/" or "FEMORAL NERVE/" or "OBTURATOR NERVE/" or "PERONEAL NERVE/" or "TIBIAL NERVE/" or "RADIAL NERVE/" or "SCIATIC NERVE/" or "SUPERIOR CERVICAL GANGLION/" [Words]
Appendix 6. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor: [Anesthesia, Local] explode all trees #2 MeSH descriptor: [Nerve Block] explode all trees #3 MeSH descriptor: [Brachial Plexus] explode all trees #4 MeSH descriptor: [Cervical Plexus] explode all trees #5 MeSH descriptor: [Lumbosacral Plexus] explode all trees #6 MeSH descriptor: [Thoracic Nerves] explode all trees #7 MeSH descriptor: [Femoral Nerve] explode all trees #8 MeSH descriptor: [Intercostal Nerves] explode all trees #9 MeSH descriptor: [Median Nerve] explode all trees #10 MeSH descriptor: [Obturator Nerve] explode all trees #11 MeSH descriptor: [Peroneal Nerve] explode all trees #12 MeSH descriptor: [Radial Nerve] explode all trees #13 MeSH descriptor: [Sciatic Nerve] explode all trees #14 MeSH descriptor: [Superior Cervical Ganglion] explode all trees #15 MeSH descriptor: [Sural Nerve] explode all trees #16 ((nerve or plexus) near block*) or (local near an?esth*) or ((brachial or cervical or lumbosacral) near plexus) or ((femoral or Intercostal or Median or Obturator or Peroneus or Tibial or Radial or Saphenous or Sciatic or Sural or Ulnar) near Nerv*) or (cervical near ganglion) #17 MeSH descriptor: [Ulnar Nerve] explode all trees #18 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 MeSH descriptor: [Ultrasonography] explode all trees #20 ultrason* or echograph* or sonograph* #21 #19 or #20 #22 #18 and #21
Data and analyses
Comparison 1. Ultrasound technique versus other technique.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Predicted adequacy of block | 17 | 1346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.94 [2.14, 4.04] |
| 1.1 Ultrasound versus nerve stimulation | 15 | 1250 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.91 [2.08, 4.06] |
| 1.2 Ultrasound versus anatomical landmark technique | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [0.20, 20.62] |
| 1.3 Ultrasound versus transarterial approach | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.62 [1.19, 11.00] |
| 2 Supplementation of block | 18 | 1807 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.20, 0.39] |
| 2.1 ultrasound versus nerve stimulation | 15 | 1570 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.21, 0.46] |
| 2.2 ultrasound versus anatomical landmark technique | 2 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.44] |
| 2.3 ultrasound versus transarterial approach | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.85] |
| 3 Time to perform block | 10 | 690 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.41, ‐0.72] |
| 4 Paraesthesia | 6 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.23, 0.76] |
| 5 Vascular puncture | 5 | 387 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.57] |
Comparison 2. Ultrasound with nerve stimulation versus nerve stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Predicted adequacy of block | 9 | 719 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [2.13, 5.20] |
| 2 Supplementation of block | 9 | 712 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.21, 0.56] |
| 3 Time to perform block | 7 | 587 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.55, 0.98] |
| 4 Paraesthesia | 3 | 178 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.30, 3.12] |
| 5 Vascular puncture | 2 | 143 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.90] |
Comparison 3. Time to perform block by subgroups US vs other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 By type of intervention/comparison | 10 | 690 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.41, ‐0.72] |
| 1.1 nerve stimulation | 9 | 634 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.37, ‐0.67] |
| 1.2 transarterial approach | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐5.76, ‐0.64] |
| 2 By type of nerve block/approach | 10 | 690 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.41, ‐0.72] |
| 2.1 axillary brachial plexus | 6 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.98, ‐0.14] |
| 2.2 infraclavicular brachial plexus | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐3.10, ‐1.72] |
| 2.3 popliteal fossa sciatic | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐2.43, 0.44] |
Comparison 4. Time to perform block by subgroups US + PNS vs PNS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 By type of nerve block/approach | 7 | 587 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.55, 0.98] |
| 1.1 Axillary brachial plexus block | 4 | 396 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.62, 1.07] |
| 1.2 Interscalene brachial plexus | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐2.12, 0.92] |
| 1.3 Supraclavicular brachial plexus | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐4.80 [‐7.24, ‐2.36] |
| 1.4 Popliteal fossa sciatic | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐0.07, 1.49] |
| 2 By catheter/no catheter placement | 7 | 587 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.55, 0.98] |
| 2.1 No catheter use | 6 | 527 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.57, 1.01] |
| 2.2 Catheter placement | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐2.12, 0.92] |
Comparison 5. Paraesthesia by subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 By type of intervention/comparison | 6 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.23, 0.76] |
| 1.1 peripheral nerve stimulation | 5 | 431 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.86] |
| 1.2 landmark technique | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.50] |
| 2 By type of nerve block/approach | 6 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.23, 0.76] |
| 2.1 axillary brachial plexus | 3 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.19, 1.79] |
| 2.2 infraclavicular brachial plexus | 3 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.19, 0.76] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bloc 2010.
| Methods | RCT, parallel design | |
| Participants | 120 (ASA I ‐ III) participants scheduled for elbow, forearm, wrist, hand surgery Excluded: Pregnant women, patients < 18 yrs, those with contraindication to RA, such as known allergic reaction to LA, local infection at site of puncture and treatment or disease that severely affects coagulation |
|
| Interventions | Ultrasound in‐plane (n = 40) versus ultrasound out‐of‐plane (n = 40) versus nerve stimulation (n = 40) Axillary brachial plexus block of 4 nerves (median, ulnar, radial, musculocutaneous); each blocked separately with no more than 40 ml in total of 1.5% mepivacaine Ultrasound: 8 ‐ 13 MHz probe (LOGIQe); endpoint ‐ visualisation of proper spread of the local anaesthetic around the targeted nerves. All 4 nerves blocked with 5‐7ml LA each. Neurostimulation: pulse duration of 100 µsec, frequency 1 Hz, initial current 1.5 mA. Nerves were located according to specific motor‐evoked muscular contractions. Current reduced to 0.5 mA. 15 ml of LA for median and radial nerves, and 5 ml for the musculocutaneous nerve. |
|
| Outcomes |
|
|
| Notes | 4 practitioners described as being experienced in both techniques For the purpose of analysis, we combined the data from the 2 groups ultrasound with in‐plane and ultrasound with out‐plane technique |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelopes used, but no further details |
| Allocation concealment (selection bias) | Unclear risk | As above |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias):Complications | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Anaesthetist not blinded. |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | Assume assessed by anaesthetist but no details reported |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | Assume assessed by anaesthetist but no details reported |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Assessed by independent observer |
| Blinding of outcome assessment (detection bias): Patient discomfort | Unclear risk | Assessed by independent observer. No details of whether participant is blinded but assume not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought. Some expected outcomes not effectively reported – success of block, sensory testing |
| Baseline characteristics | Low risk | Largely equivalent. |
| Funding sources | Low risk | None, other departmental funding |
| Operator expertise | Unclear risk | “Four senior anaesthesiologists experienced in both neurostimulation and ultrasound techniques performed the block”. Unknown if experience was equivalent and whether participants stratified by anaesthetist. |
Brull 2009.
| Methods | RCT, parallel design | |
| Participants | 106 ASA I ‐ III participants scheduled for elbow, forearm, wrist or hand surgery Excluded: age < 18 or > 70 yr, language barrier, contraindication(s) to regional anaesthesia, weight > 100 kg, pre‐existing neurological deficit in the distribution to be anaesthetized, local infection, coagulopathy, chest or shoulder deformities, severe respiratory disease, or clavicle fracture. |
|
| Interventions | Ultrasound (n = 53) versus nerve stimulation (n = 53) Intraclavicular brachial plexus block of radial, ulnar, median and musculocutaneous nerves. Total volume 30 ml local anaesthetic (2% lidocaine 15 mL and 0.5% bupivacaine 15 mL with epinephrine 1:200,000) Ultrasound: either linear probe 7 – 13 MHz (Philips/ATL HDI 5000) a 5 – 12 MHz (Philips HD11); endpoint ‐ visualization of lateral and posterior cord, LA injected incrementally to total volume of 30 ml. Nerve stimulation (Stimuplex): motor endpoints sought (elbow/ finger flexion, thumb opposition, wrist extension) at stimulating current of 0.3 ‐ 0.5 mA. 15 mL of LA injected incrementally at each position for a total of 30 mL. All participants given midazolam 2 – 4 mg iv as premedication. For nerve stimulation group, If 2 motor responses were not elicited within 20 min of needle insertion, procedure abandoned in favour of a different approach to brachial plexus blockade, and participant excluded from analysis. |
|
| Outcomes |
|
|
| Notes | One of 4 experienced regional anaesthesiologists ‐ no further details of whether experience is balanced between techniques Supported by grant funding, equipment received from manufacturers for purpose of study ‐ no interests declared. Time to perform block reported as median (interquartile range) and therefore not possible to combine in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias):Complications | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Anaesthetist not blinded |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Evaluated by blinded observer |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Evaluated by blinded observer |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Evaluated by blinded observer |
| Blinding of outcome assessment (detection bias): Patient discomfort | Low risk | Participants blinded with use of 'sham' equipment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Small number of exclusions (5 participants) with clear reasons given. However, only 49% participants available for assessment of complications at postoperative day 7 |
| Selective reporting (reporting bias) | Unclear risk | No data available for pain on injection. Also published protocol not sought |
| Baseline characteristics | Low risk | Largely comparable, although some differences in types of surgery between groups |
| Funding sources | High risk | Funding from grants, equipment supplied by named manufacturers. Unclear whether any bias has been introduced |
| Operator expertise | Unclear risk | Anaesthetists described as experienced but no detail of whether experience is equivalent for both techniques |
Casati 2007a.
| Methods | RCT, parallel design | |
| Participants | 59 patients, ASA I ‐ III, for scheduled forearm, wrist, or hand surgery Excluded: coagulopathy, local infection, allergy to local anaesthetics, severe cardiac or respiratory disease, diabetes, known neuropathies, chronic opioid use | |
| Interventions | Ultrasound (n = 30) versus nerve stimulation (n = 29) Axillary brachial plexus block; 4 nerves located individually in both groups (ulnar, radial, median, musculocutaneous); each nerve blocked with 5 ml 0.75% bupivacaine in both groups Ultrasound: 10 MHz linear probe (GE LOGIQ book XP); endpoint ‐ visualized spread of local anaesthetic around each nerve, needle position moved to allow optimal spread Nerve stimulation: pulse duration 0.15 msec, initial current density 1 mA, frequency 2 Hz; endpoint ‐ stimulation at < 0.5 mA |
|
| Outcomes |
|
|
| Notes | 2 practitioners with "substantial" experience
1 participant excluded due to failure to locate nerves with nerve stimulator Results for onset time presented in a graph, not possible to extract data for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelope technique" ‐ no further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias):Complications | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Anaesthetist not blinded |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Assessed by blinded observer |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Assessed by blinded observer |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Assessed by blinded observer |
| Blinding of outcome assessment (detection bias): Patient discomfort | High risk | Assessed by blinded observer, but participant aware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Largely equivalent |
| Funding sources | Low risk | From departmental sources only |
| Operator expertise | Unclear risk | Anaesthetists reported as having substantial experience in regional anaesthesia ‐ however, does not specify if experience is equivalent for both techniques. No details of which anaesthetist worked with which group |
Cataldo 2012.
| Methods | RCT, parallel design | |
| Participants | 70 participants, ASA I ‐ III scheduled for hallux vagus correction Excluded: patient refusal to procedure, neurologic or neuromuscular disease, acquired or congenital coagulopathy, skin infection at needle insertion site. |
|
| Interventions | Ultrasound + nerve stimulation (n = 35) versus nerve stimulation (n = 35) Popliteal block of tibial and peroneal nerve; 20 ml LA (10 mL 0.75% ropivacaine and 10 mL 2% lidocaine without epinephrine) ‐ 12 ml close to tibial nerve, 8 ml close to peroneal nerve Ultrasound: 7.5 ‐ 12 Mhz linear probe; nerve stimulator turned on to confirm correct identification of nerves, then switched off for remaining procedure; endpoint ‐ stimulating current increased to obtain motor response Nerve stimulation (Stimuplex): pulse duration 100 µsec, initial current density 1 mA, frequency 2 Hz; endpoint ‐ stimulation at < 0.4 mA |
|
| Outcomes |
|
|
| Notes | All participants first given metatarsal osteotomy with ankle tourniquet. Blocks performed by 2 resident anaesthetists with prior experience of regional anaesthesia using nerve stimulator, but novices to ultrasound and to popliteal block |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized using sealed envelopes. No further details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetists |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetists |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetists |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetists |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Sensory block assessed by a blinded investigator |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Block performance time registered by senior staff not blinded to procedure, but blinded observer monitored onset and progression of sensory block |
| Blinding of outcome assessment (detection bias): Patient discomfort | Unclear risk | Blinded observer collected participant satisfaction levels in post‐op period, but unclear if participant blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Largely comparable. More women than men, but expected for this surgery |
| Funding sources | Low risk | No funding |
| Operator expertise | High risk | Anaesthetists had more experience in nerve stimulation use than ultrasound but none had experience of popliteal block |
Chan 2007.
| Methods | RCT, parallel design | |
| Participants | 188 patients, ASA I ‐ III, scheduled for hand surgery Exclusion criteria: local anaesthetic allergy, local infection, coagulopathy, neurological upper limb disorder, psychiatric or cognitive disorder, history of substance abuse or opiate use | |
| Interventions | Ultrasound (n = 64) versus ultrasound + nerve stimulation (n = 62) versus nerve stimulation (n = 62) Axillary brachial plexus block; 3 nerves targeted individually in each group (radial, ulnar, median); all groups received 21 ml 2% lidocaine with 1:200,000 epinephrine and 21 ml 0.5% bupivacaine (14 ml around each nerve) Ultrasound: linear 5 ‐ 12 MHz probe (Philips HDI 5000); endpoint ‐ local anaesthetic spread around each nerve Nerve stimulation (Stimuplex): pulse duration 0.1 msec, frequency 2 MHz; endpoint ‐ stimulation at < 0.5 mA Ultrasound + nerve stimulation: needle positioned with ultrasound, further needle positioning to obtain stimulation at < 0.5 mA; endpoint ‐ circumferential spread of local anaesthetic |
|
| Outcomes |
|
|
| Notes | Multiple practitioners ‐ experience in technique not given, although some anaesthetists and some fellow/resident anaesthetists who were supervised Registered in clinicaltrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, but no further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist, although use of "sham" equipment in order to ensure participant blinding |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | High risk | Need for rescue block assessed by anaesthetist who was not blinded |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed this, possibly anaesthetist |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | Assessed by an independent observer, although unclear whether blinded |
| Blinding of outcome assessment (detection bias): Patient discomfort | Low risk | Assessed during follow‐up telephone conversation ‐ participant blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 participants (14%) not included as surgery commenced before 30‐minute assessment. Still possible to collect some outcome data for these participants |
| Selective reporting (reporting bias) | Low risk | Protocol details published in clinicaltrials.gov. NCT 00221884. Outcomes appear to be reported |
| Baseline characteristics | Low risk | Age, gender, weight, height, BMI, surgical time. All comparable |
| Funding sources | High risk | Funding sources reported, to include supply of ultrasound equipment by manufacturers |
| Operator expertise | High risk | Some blocks performed by supervised residents, which would have increased time to perform block and would introduce bias for this outcome |
Conceição 2009.
| Methods | RCT, parallel design | |
| Participants | 40 participants, ASA I ‐ II scheduled for elective hand surgery under brachial plexus block. Excluded: Absolute contraindication of regional block, diabetes mellitus, or any other neurological disorder of the upper extremity |
|
| Interventions | Ultrasound (n = 20) versus nerve stimulation (n = 20) Axillary brachial plexus block of radial, ulnar and median nerves; blocked with 40 ml 0.5% ropivacaine Ultrasound: 5 – 10 MHz linear probe (SonoAce 8000 SE); endpoint ‐ LA solution injected around each of the terminal branches of brachial plexus (median, ulnar and radial), 20 ml 0.5% ropivacaine in region of radial nerve, 10 ml ulnar, 10 ml median Nerve stimulation (Stimuplex): motor response to a current < 0.5 mA and > 0.2 mA Participants sedated with propofol TCI 1 ‐ 1.5 ng/ml |
|
| Outcomes | 1. Supplementation rate (when 50 ‐ 100 µg fentanyl necessary to guarantee analgesia; or when conversion to GA required) 2. Complications (vascular puncture, paraesthesia) 3. Time to perform block (from palpation of axillary artery/ from transducer placed on the skin) 4. Participant discomfort |
|
| Notes | No details given of number of practitioners and their experience | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronically‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed outcomes |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed outcomes |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details of who assessed outcomes |
| Blinding of outcome assessment (detection bias): Patient discomfort | Unclear risk | No details of who assessed outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Comparable |
| Funding sources | Low risk | No funding sources reported |
| Operator expertise | Unclear risk | No details given of practitioners and levels of experience |
Danelli 2012.
| Methods | RCT, parallel design | |
| Participants | 50 participants, ASA I ‐ III, scheduled for elective coracoacromial ligament repair Excluded: < 18 yrs, > 85 yrs, unable to express informed consent, with known allergy to study medications, chronic opioid use, ipsilateral upper limb neurological deficits, or contraindications to continuous block placement |
|
| Interventions | Ultrasound versus nerve stimulation. Interscalene brachial plexus block for nerve roots of brachial plexus; blocked with 20 ml 1% lidocaine Ultrasound: 10 – 12 MHz linear probe (LOGIQ E), in‐plane approach; endpoint ‐ direct visualization of LA spread around nerve roots. Catheter inserted after injection of LA Nerve stimulation: pulse duration 0.2 ms, initial current 1 mA, frequency 2 Hz; endpoint ‐ stimulation of deltoid muscle motor responses at 0.5 mA. Stimulating perineural catheter was then inserted through the needle and advanced to maintain the adequate motor response at ≤ 0.4 mA. LA injected in increments |
|
| Outcomes |
|
|
| Notes | 2 practitioners experienced in both techniques Registered in clinicaltrials.gov No numerical data presented for supplementation rate ‐ "There were no differences in the...requirements for GA" No denominator figures provided. Email request sent to authors, but as yet no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence of random numbers |
| Allocation concealment (selection bias) | Unclear risk | “sealed envelope technique” but no further details given |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed this outcome |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed this outcome |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | Some outcomes recorded by a nurse, but no details as to whether nurse was blinded |
| Blinding of outcome assessment (detection bias): Patient discomfort | Unclear risk | No details of whether participant blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Low risk | Protocol details published in clinicaltrials.gov. NCT 00702416. Outcomes appear to be reported |
| Baseline characteristics | Low risk | Comparable |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | Only 2 practitioners, both experienced in both techniques |
Dhir 2008.
| Methods | RCT, parallel design | |
| Participants | 67 participants, ASA I ‐ III, for elective hand surgery Exclusion criteria: allergy to local anaesthetic; coagulopathy; known neurological deficits; pregnancy; congestive heart failure; scarring or infection at injection site | |
| Interventions | Continous infraclavicular block (catheter) with ultrasound guidance + nerve stimulation (n = 23) versus nerve stimulation (catheter not stimulated) (n = 22) versus nerve stimulation with stimulation of catheter (n = 22) Ultrasound: 5 ‐ 10 MHz linear probe (Sonosite titan); position confirmed with nerve stimulation and agitated dextrose; catheter position confirmed visually with agitated dextrose Nerve stimulation (Pajunk): pulse width 0.1 msec; frequency 2 Hz; starting current 0.5 mA; endpoint 0.2 mA; catheter position not confirmed Nerve stimulation + stimulating catheter: as for nerve stimulation; catheter position confirmed with nerve stimulation aiming for posterior cord stimulation |
|
| Outcomes |
|
|
| Notes | For analysis, we combined the 2 nerve stimulation groups and compared them against the ultrasound group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | Closed envelopes used; no further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | High risk | Outcome assessment not blinded “the same anesthesiologist performed and evaluated the block” |
| Blinding of outcome assessment (detection bias): Complications | High risk | Outcome assessment not blinded “the same anesthesiologist performed and evaluated the block” |
| Blinding of outcome assessment (detection bias): time outcomes | High risk | Outcome assessment not blinded “the same anesthesiologist performed and evaluated the block” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants excluded from analysis and details given. Low attrition rate |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Unclear risk | Age, height, weight, gender, ASA status. Significantly older participants in group with nerve stimulator with stimulating catheter. |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | No details |
Domingo‐Triado 2007.
| Methods | RCT, parallel design | |
| Participants | 61 patients, ASA I ‐ III scheduled foot or ankle surgery Exclusions: contra‐indication to regional anaesthesia, pre‐existing neuropathy | |
| Interventions | Ultrasound + nerve stimulation (n = 30) versus nerve stimulation (n = 31) Midfemoral sciatic nerve block with 35 ml 0.5% ropivacaine in both groups Ultrasound: 7.5 ‐ 11 MHz linear probe (Toshiba Aplio); neurostimulation commenced when needle near nerve; endpoint ‐ neurostimulation at 0.5 mA Nerve stimulation(Stimuplex): pulse duration 300 msec, frequency 2 Hz; endpoint ‐ stimulation at 0.5 mA |
|
| Outcomes |
|
|
| Notes | 1 radiologist and one anaesthetist for all blocks. Experience in technique not given Additional saphenous nerve block given as required for tourniquet pain Time to perform block and onset time presented as median (range) and therefore not possible to combine in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed this outcome |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed this outcome |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Quality of nerve block assessed by anaesthetist unaware of group allocation, but no details for other outcomes |
| Blinding of outcome assessment (detection bias): Patient discomfort | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Unclear risk | Some differences in types of surgery between groups |
| Funding sources | Low risk | No apparent external funding |
| Operator expertise | Unclear risk | Same anaesthetist performed both blocks |
Dufour 2008.
| Methods | RCT, parallel design | |
| Participants | 51 patients, ASA I ‐ II, scheduled for foot surgery Exclusion criteria: type I and type II diabetes; history of abnormal bleeding; laboratory evidence of abnormal coagulation; infection at injection site; central or peripheral neurological disease; muscular disease |
|
| Interventions | Ultrasound + nerve stimulation (n = 26) versus nerve stimulation (n = 25) Popliteal sciatic nerve block of tibial and common popliteal nerve with 20 ml 0.5% bupivacaine in both groups (10 ml tibial nerve; 10 ml common popliteal nerve) Ultrasound: 5 ‐ 10 MHz linear probe (GE LOGIQ book); endpoint ‐ neurostimulation < 0.5 mA; spread of local anaesthetic observed but no repositioning of needle to aid spread permitted Neurostimulation: pulse duration 0.1 msec; frequency 1 Hz; starting current 1.5 mA; endpoint‐ stimulation < 0.5 mA |
|
| Outcomes |
|
|
| Notes | Participants withdrawn from study if block not completed within 7 min Additional saphenous nerve block performed to prevent tourniquet pain 1 practitioner performed all US blocks while another performed all control blocks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Evaluation of block by independent blinded observer |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed complications at consultation 2 ‐ 4 weeks post‐surgery |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Evaluation of sensory nerve block done by independent observer |
| Blinding of outcome assessment (detection bias): Patient discomfort | High risk | Participants not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of exclusions, although details provided |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Unclear risk | Some differences in ASA status, with twice as many ASAII participants in ultrasound group |
| Funding sources | Low risk | None apparent |
| Operator expertise | High risk | Different anaesthetists performed each block |
Geiser 2011.
| Methods | RCT, parallel design | |
| Participants | 112 ASA I ‐ II participants scheduled for surgery on distal upper limb Exclusion criteria: poor general health, contraindications to regional anaesthesia |
|
| Interventions | Ultrasound (n = 56) versus nerve stimulation (n = 56) Axillary brachial plexus block with single injection 50 ml 1% mepivacaine in both groups Ultrasound: 8 ‐ 13 MHz linear probe (Vivid i, Fa. GE); out‐of‐plane approach; Nerve stimulation (Stimuplex): pulse duration ≥ 0.1 msec; frequency 1 Hz; starting current not stated |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelope. No further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist to procedure technique |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist to procedure technique |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Unclear risk | More women than men in the US group, but described as no difference by authors |
| Funding sources | Low risk | Assume none |
| Operator expertise | Unclear risk | No details |
Gurkan 2008.
| Methods | RCT, parallel design | |
| Participants | 80 ASA I or II patients, scheduled for hand, wrist and forearm surgery Excluded: Patients who could not co‐operate, those with disease that could prevent sensory block assessment in upper extremity, patients with coagulopathy, allergy to study drugs, known pregnancy or patients whose pervious surgery or trauma prevented anatomic localization of injection point |
|
| Interventions | Ultrasound + nerve stimulation (n = 40) versus nerve stimulation (n = 40) Infraclavicular brachial plexus block of median, ulnar and radial nerves with 20 ml levobupivacaine, 5 mg/ml and 20 ml of lidocaine and 20 mg/ml with 5 µg/ml epinephrine (total vol 40 ml) Ultrasound: US probe (GE Logic) (8 ‐ 13 MHz) placed below clavicle about 0.5 ‐ 1 cm inferior to site of needle entry. After identification of axillary artery and cords, stimulating needle was positioned posterior to axillary artery. Electrical stimulator used to obtain motor response distal to elbow (ulnar, median or radial nerve responses). Needle repositioned if necessary to get motor response, then LA injected dorsal to axillary artery. LA distribution around cords and axillary artery observed with US Nerve stimulation (Stimuplex): 22 G, 100 mm insulated needle connected to negative pole of the nerve stimulator and set to deliver 1.5 mA current impulses of 0.1 ms duration at a frequency of 2 Hz. |
|
| Outcomes |
|
|
| Notes | Specialist anaesthesiologist and single senior resident. Experienced in block procedure. Does not indicate if experienced in US technique Results for onset time presented as median (range), therefore not possible to combine for this review. No difference between the 2 groups for this outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details given |
| Allocation concealment (selection bias) | Unclear risk | “sealed envelope technique” – no further details. |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetists |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetists |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetists |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Assessed by a blinded anaesthetist. |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Assessed by a blinded anaesthetist. |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | No details given as to who measured time to perform block. Possibly blinded anaesthetist? |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Published protocol sought |
| Baseline characteristics | Low risk | Mostly comparable |
| Funding sources | High risk | US device provided by General Electrics Company |
| Operator expertise | Unclear risk | Not clear if anaesthetists experienced in ultrasound techniques |
Kapral 2008.
| Methods | RCT, parallel design | |
| Participants | 160 patients, ASA I ‐ II for scheduled trauma surgery of shoulder or upper arm Exclusion criteria: allergy to local anaesthetic; pre‐existing respiratory, metabolic or neurological diseases; history of cardiac, hepatic or renal failure; pregnancy |
|
| Interventions | Ultrasound guidance (n = 80) versus nerve stimulation (n = 80) Interscalene brachial plexus block with 20 ml 0.75% bupivacaine in both groups Ultrasound: 5 ‐ 10 MHz linear probe (Sonosite); endpoint ‐ visualized spread local anaesthetic around all nerve roots (C5 ‐ T1), multiple injections permitted Nerve stimulation: pulse duration 0.1 msec, frequency 2 Hz; endpoint ‐ stimulation hand or forearm < 0.5 mA; single injection |
|
| Outcomes |
|
|
| Notes | All blocks performed by same 3 anaesthetists with experience in both guidance techniques Results for onset time given as median (range) therefore not possible to combine for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | External preparation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially‐numbered envelopes |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | Evaluation of nerve block by independent anaesthetist; no details if assessor is blinded |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed this outcome |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | Evaluation of nerve block by independent anaesthetist; no details if assessor is blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | baseline characteristics comparable |
| Funding sources | Low risk | No apparent external funding |
| Operator expertise | Unclear risk | All blocks by same experienced anaesthetists |
Liu 2005.
| Methods | RCT, parallel design | |
| Participants | 90 patients, ASA I ‐ II, scheduled for forearm, wrist or hand surgery Exclusions: patient refusal, allergy to local anaesthetics, pre‐existing neuropathy, dementia | |
| Interventions | Ultrasound (double injection) (n = 30) versus ultrasound (single injection) (n = 30) versus nerve stimulation (double injection) (n = 30) Axillary brachial plexus block with 0.5 ml/kg 1.5% lignocaine with epinephrine (5 µg/kg) in nerve stimulation group and 30 ml 1.5% lignocaine with epinephrine (5 µg/kg) in ultrasound groups Ultrasound: 12 MHz probe (Hawk model 2102, B‐K medical); endpoint ‐ visualization of local anaesthetic spread around axillary artery. Either 1 injection (superior to artery) or 2 injections (superior and inferior to artery) Nerve stimulation (Stimuplex): 2 Hz; endpoint ‐ stimulation of median nerve and radial nerve. No current thresholds given |
|
| Outcomes |
|
|
| Notes | 1 practitioner performed all blocks, experience not given For the purpose of analysis of Adequacy of block and Supplementation rate, we combined the data for the double and single injection ultrasound groups For the purpose of analysis of Time to perform block, we took data only from ultrasound (double injection) versus nerve stimulation (double injection) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Assessment done by anaesthetist unaware of group allocation |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Assessment done by anaesthetist unaware of group allocation |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Assessment done by anaesthetist unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or exclusions |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Baseline characteristics comparable |
| Funding sources | Low risk | No apparent external funding |
| Operator expertise | Unclear risk | Same anaesthetist for all blocks |
Liu 2009a.
| Methods | RCT, parallel design | |
| Participants | 230 patients, scheduled to undergo outpatient shoulder arthroscopy under interscalene block Excluded: < 18 yrs, > 75 yrs, patient refusal, pregnancy, dementia, severe pulmonary disease, and known pre‐existing neurological disorders involving the operative limb |
|
| Interventions | Ultrasound (n = 115) vs nerve stimulation group (n = 115) Interscalene block of axillary, musculocutaneous, ulnar, radial and median nerves with mepivacaine 1.5% with 1:300,000 epinephrine and NaCO3 (1 meq/10mL); for participants < 50 kg, total dose of 45 ‐ 55 ml was used for patients ≥ 50 kg, total dose of 55 ‐ 65 mL was used Ultrasound: A linear 10 ‐ 13 MHz US probe was used to visualize the brachial plexus. Initial US visualization was at interscalene area. 5 cm 22 G needle placed through middle scalene muscle, into interscalene groove, in‐plane US guidance to visualize the entire needle. LA injected in divided doses with frequent aspiration under ultrasound visualization. Nerve stimulation (Stimuplex): 5 cm 22 G insulated needle placed into interscalene groove. Current decreased to range between 0.2 mA and 0.5 mA. If not still present then needle adjusted. LA injected in divided doses with frequent aspiration |
|
| Outcomes |
|
|
| Notes | No details of experience of anaesthetists giving nerve blocks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Used a “sealed envelope sequence”. No further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Adequacy of block assessed by an investigator who was unaware of group allocation |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Neurological complications assessed and analyzed by blinded investigator |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | Time to perform block assessed by an investigator not performing block but no details as to whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some losses to follow‐up but relatively small number and details given |
| Selective reporting (reporting bias) | Unclear risk | Pre‐published protocol not sought |
| Baseline characteristics | Low risk | All comparable |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | No details of who, how many and experience of anaesthetists giving blocks |
Macaire 2008.
| Methods | RCT, parallel design | |
| Participants | 60 patients undergoing elective endoscopic carpal tunnel release Exclusions: none stated |
|
| Interventions | Ultrasound (n = 30) versus nerve stimulation (n = 29) Median and ulnar nerve block (at wrist) with 4 ml 1.5% mepivacaine injected around each nerve in both groups Ultrasound: 13 MHz linear probe (GE LOGIQ e); endpoint ‐ local anaesthetic spread around each nerve. Needle repositioning to allow adequate spread Nerve stimulation (HNS 12, B Braun): pulse duration 0.1 msec; Freq 2 Hz; endpoint ‐ stimulation < 0.5 mA motor or sensory response |
|
| Outcomes |
|
|
| Notes | Palmaris radius blocked blindly in both groups Author contact attempted in original review for additional information for risk of bias table No details of experience of anaesthetist giving block Data for Block Performance time and Onset time presented as median (interquartile range), so not possible to combine in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | Sensory loss assessed by investigator not involved in block performance, but no details of whether blinded |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details of who assessed this outcome |
| Blinding of outcome assessment (detection bias): Patient discomfort | High risk | No details of whether participants blinded to group allocation, assume not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 withdrawal due to protocol violation ‐ low attrition rate |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Baseline characteristics largely equivalent |
| Funding sources | Low risk | No apparent funding sources |
| Operator expertise | Unclear risk | No details |
Meierhofer 2014.
| Methods | RCT, parallel design | |
| Participants | 60 patients, ASA I ‐ II, scheduled for hand, forearm and elbow surgery Exlusion criteria: ASA > III, < 18 years and > 85 years, general contraindications for plexus block, known muscular or neurological deficits, pregnant or breastfeeding women, comprehension difficulties |
|
| Interventions | Ultrasound (n = 30) versus nerve stimulation (n = 30) Axillary plexus block of median, radial, musculocutaneous, and ulnar nerve with 40 ‐ 50 ml 1.5% mepivacaine (plexus) and 5 ‐ 10 ml mepivacaine 0.5% (subcutaneous in medial skin of arm) Ultrasound: no details in paper Nerve stimulation (Braun): pulse duration 0.1 msec. Endpoint ‐ visible muscle contractions at current of 0.3 ‐ 0.5 mA |
|
| Outcomes |
|
|
| Notes | 5 practitioners with more than 5 years experience Participants given tourniquet block in both groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Assessor blinded from group allocation |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Assessor blinded from group allocation |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Assessor blinded from group allocation |
| Blinding of outcome assessment (detection bias): Patient discomfort | Low risk | Assessor blinded from group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 losses, clearly explained |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Age, gender, BMI, type of surgery, time length of surgery. All comparable |
| Funding sources | Low risk | None |
| Operator expertise | Unclear risk | 5 practitioners with more than 5 years experience |
Morros 2009.
| Methods | RCT, parallel design | |
| Participants | 129 ASA I ‐ II patients scheduled for elective orthopaedic surgery of elbow, forearm, wrist or finger Exclusion criteria: patient refusal, neurological disturbance, infection/inflammation of the upper extremity, coagulopathy, inability to communicate, those expected to have an axillary catheter |
|
| Interventions | Ultrasound with nerve stimulation (n = 65) versus nerve stimulation only (n = 62) Axillary brachial plexus block of median, musculocutaneous, cubital and radial nerve Each nerve blocked with 10 ml 1% mepivacaine (40 ml in total) in each group Ultrasound: 5 ‐ 10 MHz linear probe (Titan, Sonosite) out‐of‐plane approach, once brachial plexus structures seen nerve stimulator used Nerve stimulation (Stimuplex): pulse duration 0.3 msec, Freq 2 Hz; endpoint ‐ stimulation of motor response at 0.4 mA |
|
| Outcomes |
|
|
| Notes | 2 anaesthesiologists with extensive experience in both techniques | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of random number table |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed outcomes |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details of who assessed outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Age, gender, weight, height, ASA status reported. All comparable |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | Two anaesthesiologists with extensive experience in both techniques |
Perlas 2008.
| Methods | RCT, parallel design | |
| Participants | 70 patients, ASA I ‐ II, scheduled for elective foot and ankle surgery Exclusions: contraindication to nerve block; significant peripheral neuropathy or neurological disease affecting lower extremity; pregnancy; history of alcohol or drug abuse; long‐standing opiate intake; significant psychiatric conditions |
|
| Interventions | Ultrasound guidance (n = 37) versus nerve stimulation (n = 33) Popliteal fossa sciatic nerve block with 30 ml local anaesthetic mixture in both groups (15 ml 2% lignocaine and 15 ml 0.5% bupivacaine) Ultrasound: 4 ‐ 7 MHz or 4 ‐ 8 MHz linear probe (Philips Ultrasound); endpoint ‐ local anaesthetic spread around nerve; needle position adjustment permitted (Note: current required to elicit motor stimulation recorded once needle positioned with ultrasound but no further needle adjustment) Nerve stimulation (Stimuplex): duration 0.1 msec; frequency 2 Hz; endpoint ‐ stimulation of foot or toes at < 0.5 mA |
|
| Outcomes |
|
|
| Notes | Saphenous nerve block also given if required, for tourniquet pain Registered in clinicaltrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number |
| Allocation concealment (selection bias) | Low risk | "group allocation not disclosed to patients" ‐ but no details given |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | Low risk | Sham ultrasound used, although relevant participant outcomes not reported |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Investigator blinded to group allocation assessed progress of block |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Blinded anaesthetist responsible for all intraoperative care including induction of GA |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Investigator blinded to group allocation assessed progress of block |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants excluded but low attrition rate |
| Selective reporting (reporting bias) | Low risk | Protocol details published in clinicaltrials.gov. NCT 00221920. Outcomes appear to be reported |
| Baseline characteristics | Low risk | Baseline characteristics largely comparable, although some differences in gender ratios |
| Funding sources | Low risk | Funding from university research award |
| Operator expertise | Unclear risk | Experienced anaesthetists in both techniques |
Renes 2009.
| Methods | RCT, parallel design | |
| Participants | 60 patients, ASA I ‐ III, scheduled for elbow, forearm, wrist and hand surgery under supraclavicular brachial plexus block without sedation Excluded: Patients refusing supraclavicular block, inability to obtain informed consent, hemidiaphragmatic dysfunction, coagulation disorders, neuropathy, pulmonary and cardiac disorders, BMI 35 kg/m² or higher, pregnancy, allergy to LA |
|
| Interventions | Ultrasound (n = 30) vs neurostimulation (n = 30) Supraclavicular brachial plexus block of ulnar, median, radial and musculocutaneous nerves with 20 ml 0.75% ropivacaine Ultrasound: 38 mm 6 ‐ 13 MHz broadband linear array US probe to identify brachial plexus in short‐axis view located lateral to the subclavian artery. In‐plane approach Nerve stimulation (HNS 11, B. Braun):pulse duration 0.1 msec, frequency 2 Hz. Endpoint: Flexion of both fingers and wrist or extension of fingers at a stimulation current between 0.20 and 0.50 mA |
|
| Outcomes |
|
|
| Notes | For Supplementation rate we took data for intravenous fentanyl administration only. There were no participants requiring local anaesthetic or GA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed this and whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses after randomization |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocol not sought |
| Baseline characteristics | Low risk | Largely comparable |
| Funding sources | Low risk | None; department funding only |
| Operator expertise | Unclear risk | No details given of who gave anaesthetic and level of expertise |
Salem 2012.
| Methods | RCT, parallel design | |
| Participants | 60 patients scheduled for shoulder surgery Excluded: Hypersensitivity to local anaesthetics, neurologic deficits, bleeding tendency, respiratory failure, local infection, refusal to participate in the study or request for GA |
|
| Interventions | Ultrasound + nerve stimulation (n = 30) vs nerve stimulation alone (n = 30) Interscalene brachial plexus block; In both cases, 30 ml of prilocaine was used followed by catheter insertion Ultrasound: Roots of brachial plexus sought between anterior and middle scalene muscles in an axial oblique plane. After sonographic plexus identification, injected glucose 5% to scan fluid around the plexus, then fixed needle. Nerve stimulation then switched on looking for muscle contractions as below Nerve stimulation (Stimuplex): pulse duration 0.1 msec, frequency 2 Hz, initial current mA. 5 ml sytrine with NaCl 0.9% through injection line inserted in a caudal slightly lateral and discrete dorsal orientation After 3 ‐ 4 cm, biceps contractions and then current reduced incrementally until 0.2 ‐ 0.3 mA was reached. Then needle retracted slightly and LA slowly injected |
|
| Outcomes |
|
|
| Notes | Study published in journal's 'short communication', therefore limited details Results for onset time presented as median and range, not possible to combine in this review. No statistically significant difference reported for this outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed this outcome and whether blinded |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details of who assessed this outcome and whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Comparable |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | 2 anaesthetic consultants with "over 10 years experience" |
Sauter 2008.
| Methods | RCT, parallel design | |
| Participants | 80 patients, ASA I ‐ II, scheduled for elective forearm or hand surgery Exclusions: not detailed (inclusion criteria: ASA I or II; weight 50 ‐ 100 kg; normal neurological status; co‐operative) |
|
| Interventions | Ultrasound (n = 40) versus peripheral nerve stimulation (n = 40) Interscalene brachial plexus block of musculocutaneous, radial, median, ulnar and antebrachial cutaneous nerves with 0.6 ml/kg mepivacaine with epinephrine in both groups Ultrasound: 5 ‐ 8 MHz curved probe (Sonosite); endpoint ‐ spread local anaesthetic around all cords or spread from 3 o'clock to 11 o'clock around artery. Multiple injections permitted Nerve stimulation (Stimuplex): 0.1 msec duration, frequency 2 Hz; starting current 1.5 mA; endpoint ‐ motor response in finger or hand from posterior or middle cord at 0.2 ‐ 0.5 mA. Single injection |
|
| Outcomes |
|
|
| Notes | Registered in clinicaltrials.gov Data for block performance time not presented as mean (SD) and therefore not possible to combine in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers; permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes opened immediately before block performed. No additional details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Assessed by blinded observer |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | Unclear who assessed this outcome and whether blinded |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details of who assessed these outcomes and whether blinded |
| Blinding of outcome assessment (detection bias): Patient discomfort | High risk | Participant not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 loss, low attrition rate |
| Selective reporting (reporting bias) | Low risk | Protocol details published in clinicaltrials.gov. NCT 00321425. Outcomes appear to be reported |
| Baseline characteristics | Low risk | Baseline characteristics comparable |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | 2 anaesthetists experienced in both techniques |
Seidel 2013.
| Methods | Randomized, parallel design | |
| Participants | 250 ASA I ‐ III adult patients scheduled for orthopaedic foot surgery under tourniquet Exclusion criteria not given |
|
| Interventions | Ultrasound (n = 125) versus nerve stimulation (n = 125); Distal sciatic block of sciatic, tibial and common peroneal nerves; each nerve blocked with 20 ml 1% prilocaine and 10 ml 0.75% ropivacaine Ultrasound: 6 ‐ 13 MHz linear probe (Sonosite); intraepineural needle position Nerve stimulation (Stimuplex): 0.1 msec duration, frequency 2 Hz; starting current 1.0 mA |
|
| Outcomes |
|
|
| Notes | Saphenous nerve block for tourniquet pain given to all participants Registered in clinicaltrials.gov Data for block onset time presented as log‐rank test results and therefore not possible to combine for this review Reported as significantly shorter onset time in US group, P value < 0.01 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Observer blinded from group allocation |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Observer blinded from group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants in each group excluded from analysis, but low attrition rate |
| Selective reporting (reporting bias) | Low risk | Protocol details published in clinicaltrials.gov. NCT 01643616. Outcomes appear to be reported |
| Baseline characteristics | Low risk | Age, weight, height, gender, surgery time, diabetes mellitis. Some difference in gender balance but otherwise all comparable |
| Funding sources | Low risk | No conflicts of interest or funding declared |
| Operator expertise | Unclear risk | No details |
Shrestha 2011.
| Methods | RCT, parallel design | |
| Participants | 70 adult patients ASA I and II requiring upper arm surgery (wrist, forearm and hand) under axillary block Exclusion criteria: History of coagulopathy, allergy to drug, diabetes, local infection at site of block, patients requiring bilateral hand surgery, patients’ denial, cases that needed conversion to GA, surgery lasting more than 3.5 hours and patients beyond age of 20 ‐ 65 yrs, and body weight of 45 ‐ 65 kg |
|
| Interventions | Ultrasound + nerve stimulation (n = 35) versus nerve stimulation (n = 35) Axillary brachial plexus block for ulnar, radial, median and musculocutaneous nerves Nerves blocked with a total of 24 ml of bupivacaine 0.5% with injection dexamethasone 4 mg, each individual nerve blocked with 6 ml Ultrasound: 8 MHz linear probe (Toshiba); endpoint ‐ nerve location confirmed by nerve stimulation and then local anaesthetic deposited under ultrasound guidance Nerve stimulation (Stimuplex): 0.2 msec duration, frequency 2 Hz; starting current 1.0 mA. Current reduced to 0.6 mA then 1 ml of LA injected to see if twitches disappeared. Then remaining 5 ml given |
|
| Outcomes |
|
|
| Notes | 1 anaesthetist performed all blocks, but no details of their experience in the 2 techniques Results for onset time reported as statistically significant difference between groups with shorter onset time in US group, P value < 0.01 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a “lottery method” |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Data recorded by blinded observer |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Data recorded by blinded observer |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Data recorded by blinded observer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | All comparable |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | Only 1 person performing all blocks |
Sites 2006.
| Methods | RCT, parallel design | |
| Participants | 56 patients, ASA I ‐ III, scheduled for hand surgery Exclusions: < 18 years, pregnant, allergy to study drug | |
| Interventions | Ultrasound perivascular approach (n = 28) versus transarterial approach (n = 28) Axillary brachial plexus block with 30 ml 1.5% lidocaine in both groups Ultrasound: 3 ‐ 12 MHz probe (Philips EnVisor); endpoint ‐ circumferential spread of local anaesthetic around artery Transarterial: local anaesthetic injected when no blood aspirated on either side of artery Separate musculocutaneous nerve block could be done at discretion of operator prior to study protocol (uncontrolled) |
|
| Outcomes |
|
|
| Notes | 7 practitioners performing blocks (3 trainees and 4 experienced) Musculocutaneous nerve block not controlled for and used at anaesthetist's preference 5 ml 1.5% lidocaine with 5 µg/ml epinephrine given to all participants for analgesia for tourniquet pain Study terminated early due to high level of block failure in transarterial group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of block randomization |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but no further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist/personnel |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Block assessed by blinded observer. Upgrade to GA etc. made by anaesthetist or surgeon not aware of group allocation |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed this outcome |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Blinded research nurse |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial stopped early due to high failure rate in transarterial group |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported despite early stopping. Published protocol not sought |
| Baseline characteristics | Low risk | Baseline characteristics comparable |
| Funding sources | High risk | Ultrasound equipment provided by manufacturer during trial |
| Operator expertise | High risk | Some blocks performed by a supervised resident, some by an experienced anaesthetist |
Soeding 2005.
| Methods | RCT, parallel design | |
| Participants | 40 patients for elective upper limb surgery
Interscalene block for shoulder surgery (24 participants) and axillary plexus block for hand surgery (16 participants) Exclusion criteria: pre‐existing neurological deficit, local sepsis, respiratory failure, patient refusal |
|
| Interventions | Ultrasound (n = 20) versus landmark technique (n = 20) Interscalene or axillary brachial plexus block with 3 mg/kg ropivacaine (0.75% for interscalene; 0.6% for axillary) Ultrasound: 13 MHz probe (Siemens sonoline); endpoint ‐ local anaesthetic spread around individual nerves/trunks. Needle position altered during injection to optimize local anaesthetic spread Landmark: needle positioned by landmark palpation, paraesthesia not purposely sought. |
|
| Outcomes |
|
|
| Notes | 1 practitioner performed all blocks. Previous experience in ultrasound blocks and tuition from radiologist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not given |
| Allocation concealment (selection bias) | Unclear risk | Details not given |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | Assessments done by independent investigator but no details of whether they were blinded. |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details of who assessed this and whether blinded to group allocation |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details of who assessed this and whether blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Unclear risk | No standard baseline characteristics reported |
| Funding sources | Low risk | No apparent additional funding sources |
| Operator expertise | Unclear risk | Same operator performed all blocks |
Strub 2011.
| Methods | RCT, parallel design | |
| Participants | 141 patients scheduled for hand surgery distal to the elbow with and estimated duration < 2 hrs Excluded: Those that declined to give informed consent, had known allergy to any anaesthetic substance, an infection in region of injection site, severe coagulopathy, pathological enlargement of axillary lymph nodes, those who had had previous surgery on the axilla |
|
| Interventions | Ultrasound (n = 70) vs landmark technique (n = 71) Axillary brachial plexus block; dose of 20 ml LA deposited behind artery next to radial nerve; 10 ml LA then injected around mediocranial median nerve and mediocaudal ulnar nerve Ultrasound: cross‐section of axillary artery is imaged. Individual nerves are then identified. Cannula introduced under US. 20 G 105” bevelled needle. LA injected around all 4 nerves individually starting with radial nerve. Aim of infiltration is to see a circular perineural spread of the fluid in the ultrasound image. Bupivacaine hydrochloride (5 mg/ml) with 0.5% adrenaline and mepivacaine hydrochloride (10 mg/ml) in a ratio of 1:1 Neurostimulation: Anatomic landmarks used for orientation. Needle inserted in space between axillary artery and coracobrachial muscle, near to axillary fold. Needle at an angle to skin of 50 ‐ 90°, inserted past facial click and advanced cranially, caudally or transarterially past axillary artery |
|
| Outcomes |
|
|
| Notes | Training had been given to those conducting the ultrasound technique; baseline of 10 procedures with US before study, or 300 in conventional technique Not possible to combine data for Time to perform block or Onset time as no standard deviation reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):Complications | High risk | Surgeon not blinded |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Surgeon not blinded |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | High risk | “No blinding” reported at end of discussion |
| Blinding of outcome assessment (detection bias): Complications | High risk | “No blinding” reported at end of discussion |
| Blinding of outcome assessment (detection bias): time outcomes | High risk | “No blinding” reported at end of discussion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | All comparable |
| Funding sources | Low risk | None |
| Operator expertise | High risk | Surgeon with limited experience (10 previous attempts) using ultrasound technique |
Taboada 2009.
| Methods | RCT, parallel design | |
| Participants | 70 ASA I ‐ III patients scheduled for hand/wrist surgery Exclusion criteria: Patient refusal, neurologic or neuromuscular disease, anticoagulation, skin infection at site of needle insertion |
|
| Interventions | Ultrasound (n = 35) versus nerve stimulation (n = 35) Coracoid infraclavicular brachial plexus block with single injection of 40 ml mepivacaine 1.5% Ultrasound: probe 6 ‐ 13 MHz (MicroMaxx); endpoint ‐ visualization under US of spread of local anaesthetic posterior to axillary artery Nerve stimulation (Pajunk Medizintechnologic): 0.1 msec duration, frequency 2 Hz; starting current 1.5 mA Current decreased to 0.5 mA or less when radial nerve stimulation could still be elicited. Then LA injected slowly |
|
| Outcomes |
|
|
| Notes | 2 practitioners performing blocks with long‐standing experience in performance of both nerve stimulator and US coracoid infraclavicular brachial plexus blocks Block considered a failure if taken more than 15 minutes to perform |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias):Complications | High risk | Anaesthetist not blinded |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Anaesthetist not blinded |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed this outcome – assume anaesthetists? |
| Blinding of outcome assessment (detection bias): Complications | Low risk | Assessed by an independent observer not aware of technique |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Assessed by an independent observer not aware of technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Prepublished protocol not sought |
| Baseline characteristics | Low risk | Some differences in type of surgery but unlikely to introduce bias, otherwise all comparable |
| Funding sources | Low risk | None |
| Operator expertise | Unclear risk | 2 experienced anaesthetists |
Trabelsi 2013.
| Methods | RCT, parallel design | |
| Participants | 60 ASA I ‐ II patients scheduled for surgery of the upper limb (wrist, hand, elbow or distal arm) Exclusion criteria: none |
|
| Interventions | Ultrasound (n = 30) versus nerve stimulation (n = 30) Infraclavicular brachial plexus block; nerves blocked with 15 ml 0.5% bupivacaine Ultrasound: 10 ‐ 12 MHz linear probe (Logiq 7) in‐plane approach; endpoint ‐ visualized LA injection around each brachial plexus cord with approximately 5 ml Nerve stimulation (Stimuplex): Initial stimulating current at 1 ‐ 1.5 mA; endpoint ‐ current gradually decreased until responses still present at 0.3 mA or less |
|
| Outcomes |
|
|
| Notes | Surgical tourniquet used in all participants Note: supplementary GA was used at discretion of attending anaesthetist and was not reported as an outcome. However, all participants were described as being awake during surgery Data for onset time of block reported as median (IQR), therefore not possible to combine in analysis. Onset time for motor block in US group 20 min (15 ‐ 26) vs 23 min (16 ‐ 32) in NS group; onset time for sensory block in US group 10 min (10 ‐ 15) vs 14 min (12 ‐ 25) in NS group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by "distributing sealed, opaque envelopes". No further details |
| Allocation concealment (selection bias) | Unclear risk | As above. No further details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Low risk | Assessed by anaesthetist blinded to technique used |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | Assessed by anaesthetist blinded to technique used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Age, gender, ASA status, weight, height, BMI, surgical duration. All comparable. |
| Funding sources | Low risk | None apparent |
| Operator expertise | Unclear risk | No details given |
Van Geffen 2009.
| Methods | RCT, parallel design | |
| Participants | 40 ASA I ‐ III patients undergoing surgery of foot or ankle with distal sciatic nerve block in popliteal fossa Exclusion criteria: Patient refusal, pre‐existing neuropathy, kidney or liver disease, pregnancy, skin infection at site of needle insertion and inability to communicate |
|
| Interventions | Ultrasound (n = 20) versus nerve stimulation (n = 20) Distal sciatic nerve block in the popliteal fossa with lignocaine 1.5% with adrenaline 5 µg/ml – at discretion of anaesthetist, min of 25 ml and max of 40 ml Ultrasound: with 7 ‐ 13 MHz 38 mm linear probe (Sonosite); endpoint ‐ visualization of LA distribution around nerve Nerve stimulation (HNS 11, Braun): 0.1 msec duration, frequency 2 Hz; starting current 1.0 mA. Initial current reduced until responses maintained with minimum of 0.2 mA and max 0.5 mA |
|
| Outcomes |
|
|
| Notes | Anaesthetists had extensive clinical experience with nerve stimulation guided popliteal sciatic nerve blocks but no experience with ultrasound guidance block Some participants also given saphenous nerve block if tourniquet required for surgery; no details in results of those that were given additional block |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized using sealed envelopes. No further detail |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Anaesthestists not blinded |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Anaesthestists not blinded |
| Blinding of participants and personnel (performance bias): Patient discomfort | High risk | Anaesthestists not blinded |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details of who assessed this. Assume anaesthetist? |
| Blinding of outcome assessment (detection bias): time outcomes | Low risk | Assessor blinded to block technique |
| Blinding of outcome assessment (detection bias): Patient discomfort | Low risk | Assessor blinded to block technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Age, weight, height, ASAI‐III, gender, type of surgery. All comparable |
| Funding sources | High risk | Funding from dept sources. Study equipment supported was provided from Sonosite Inc, Bothell, WA, USA |
| Operator expertise | High risk | Anaesthetists had extensive clinical experience with nerve stimulation guided popliteal sciatic nerve blocks but no experience with ultrasound guidance block |
Williams 2003.
| Methods | RCT, parallel design | |
| Participants | 80 patients undergoing distal arm, forearm or hand surgery Exclusions: coagulopathy, infection at injection site, allergy to local anaesthetics, severe pulmonary pathology, age < 18, language barriers, mental incapacity, BMI > 35, pre‐existing weakness or loss of sensation in operative limb | |
| Interventions | Ultrasound + nerve stimulation (n = 40) versus nerve stimulation (n = 40) Supraclavicular brachial plexus block with 0.5 ml/kg of 0.5% bupivacaine/2% lidocaine and 1:200,000 epinephrine to maximum of 40 ml Ultrasound: 7.5 MHz probe (Aloka); endpoint ‐ neurostimulation at < 0.6 mA Neurostimulation: endpoint ‐ neurostimulation at < 0.6 mA |
|
| Outcomes |
|
|
| Notes | All blocks attempted by 1 anesthesiology resident (limited experience of both techniques) Help obtained by senior anaesthetist if block not performed within 20 min | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias): time outcomes | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details given of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details given of who assessed outcomes and whether blinded |
| Blinding of outcome assessment (detection bias): time outcomes | Unclear risk | No details given of who assessed outcomes and whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not sought |
| Baseline characteristics | Low risk | Baseline characteristics comparable |
| Funding sources | Low risk | No apparent funding |
| Operator expertise | High risk | Performed by anesthesiology resident who had experience of blocks whilst under supervision by staff anaesthesiologist. This may have increased time to perform block and would introduce bias for this outcome |
Zaragoza‐Lemus 2012.
| Methods | RCT, parallel design | |
| Participants | 34 adult ASA 1 ‐ III patients scheduled for surgery of upper limb Exclusions: Allergy to local anaesthetic, coagulopathy, infection at site of injection, pre‐existing neurological lesion |
|
| Interventions | Ultrasound (n = 17) versus nerve stimulation (n = 17) Posterior approach to brachial plexus block of axillary, musculocutaneous, radial and median nerves; single injection of 30 ml 0.325% ropivacaine Ultrasound: 6 ‐ 13 mHz., cervical nerve root identified as a hypoechoic, circular image. Endpoint ‐ visualization of distribution of LA around nerve Nerve stimulation (Stimuplex): 0.1 msec duration, frequency 2 Hz; starting current 0.8 mA. Endpoint ‐ not stated |
|
| Outcomes |
|
|
| Notes | No details of experience of practitioners given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias):adequacy of block, need for supplementation | High risk | Not possible to blind anaesthetist |
| Blinding of participants and personnel (performance bias):Complications | High risk | Not possible to blind anaesthetist |
| Blinding of outcome assessment (detection bias): adequacy of block, supplementation of block | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias): Complications | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Postoperative pain outcomes not reported. Published protocol not sought |
| Baseline characteristics | Low risk | Some differences in gender between groups, otherwise comparable |
| Funding sources | Low risk | No details. Assume no funding from external sources. |
| Operator expertise | Unclear risk | No details |
RCT = randomized controlled trials; RA = regional anaesthetic; LA = local anaesthetic; GA = general anaesthetic; ASA = American Society of Anesthesiologists; yr = year(s); min(s) = minute(s); BMI = body mass index; TCI = target controlled infusion; US = ultrasound; NS = nerve stimulation; SD = standard deviation; IQR = interquartile range
Note: The blank cells in the risk of bias tables are domains which are not applicable to the particular study.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aveline 2010 | Participants in both groups given GA after nerve block |
| Casati 2007b | Included in 2009 version of review. Excluded in this update due to inclusion criteria changes (see Differences between review and current update). MEAV study design |
| Danelli 2009a | Included in 2009 version of review. Excluded in this update due to inclusion criteria changes (see Differences between review and current update). Aim to give femoral block to all participants following popliteal sciatic block |
| Danelli 2009b | MEAV study design |
| Demirci 2013 | Trunk block ‐ not upper or lower limb block |
| Dolan 2008 | Included in 2009 version of the review. Excluded in this update due to inclusion criteria changes (see Differences between review and current update). Assume participants given additional anaesthetic for knee/hip replacement surgery |
| Ko 2013 | Trunk block ‐ not upper or lower limb block |
| Li 2011 | Participants in both groups given GA after nerve block |
| Maalouf 2012 | Participants in both groups given a spinal‐epidural block |
| Maldini 2010 | Abstract published in 2010. No contact details available in abstract |
| Marhofer 1997 | Included in 2009 version of review. Excluded in this update due to inclusion criteria changes (see Differences between review and current update). Aim to give spinal anaesthetic to all participants following nerve block |
| Marhofer 1998 | Included in 2009 version of review. Excluded in this update due to inclusion criteria changes (see Differences between review and current update). Aim to give spinal anaesthetic to all participants following nerve block |
| Mariano 2009a | Nerve block given for postoperative analgesic. Assume further anaesthetic given |
| Mariano 2009b | Nerve block given for postoperative analgesic. Assume further anaesthetic given |
| Mariano 2009c | Nerve block given for postoperative analgesic. Assume further anaesthetic given |
| Mariano 2010a | Nerve block given for postoperative analgesic. Assume further anaesthetic given |
| Mariano 2010b | Nerve block given for postoperative analgesic. Assume further anaesthetic given |
| Martinez Navas 2011 | Abstract only. Insufficient detail to include |
| McCartney 2008 | Abstract only. Results of study presented for some outcomes but no denominator figures and no details for risk of bias. No contact details available in abstract |
| McCartney 2009 | MEAV study design |
| McNaught 2011 | MEAV study design |
| Nassar 2010 | Abstract published in 2010. No contact details available in abstract |
| Ponrouch 2010 | MEAV study design |
| Redborg 2009 | Included in Characteristics of studies awaiting classification in 2009 version of review. Excluded in this update due to study enrolment of volunteers, not surgical patients |
| Sala‐Blanch 2012 | Ultrasound used in all participants to initially identify needle entry point prior to group allocation to ultrasound or nerve stimulation technique |
| Thomas 2011a | Participants in both groups given GA after nerve block |
| Thomas 2011b | Abstract only. Insufficient detail to include |
| Tognu 2010 | Ultrasound is used pre‐puncture only |
| Tran 2010 | Nerve block not used for sole operative anaesthesia |
| Villeneuve 2009 | Participants in both groups given GA after nerve block |
| Wildy 2009 | Abstract published in 2009. No contact details available in abstract |
| Yi 2012 | MEAV study design |
| Zencirci 2011 | Not described as randomized in full text |
MEAV = minimum effective anaesthetic volume; GA = general anaesthetic
Characteristics of studies awaiting assessment [ordered by study ID]
Aytac 2015.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in search run in May 2015. Full‐text review not yet completed |
Dhir 2013.
| Methods | Randomized, parallel design |
| Participants | 210 patients undergoing elective hand or elbow surgery requiring infraclavicular plexus block |
| Interventions | US versus stimulating needle and catheter placement |
| Outcomes | Motor and sensory block success Complications Intraoperative analgesia requirement Time for catheter insertion |
| Notes | Study registered on clinicaltrials.gov (identifier: NCT 01136447). Completed but results not yet posted or study published |
Eren 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in search run in May 2015. Full‐text review not yet completed |
González 1993.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to access study from British Library |
Kumar 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in search run in May 2015. Full‐text review not yet completed |
Lam 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in search run in May 2015. Full‐text review not yet completed |
Li 2013.
| Methods | Randomized, parallel design |
| Participants | 60 ASA I ‐ II patients scheduled for upper extremity procedures requiring supraclavicular brachial plexus block |
| Interventions | US versus NS |
| Outcomes | Haemodynamic responses Rate of complete block |
| Notes | Full text requires translation from Chinese |
Martinez Navas 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in search run in May 2015. Full‐text review not yet completed |
NCT 01579747.
| Methods | Randomized, parallel design |
| Participants | Surgical patients aged over 18 years requiring lateral popliteal approach to the sciatic nerve block |
| Interventions | US versus NS |
| Outcomes | Procedural Time Number of Redirections |
| Notes | Clinical trials identifier: NCT 01579747. Results available on clinicaltrials.gov. Email sent to author to enquire about publications (awaiting reply at time of publication) |
Smith 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in search run in May 2015. Full‐text review not yet completed |
Stavrati 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Identified in search run in May 2015. Full‐text review not yet completed |
US = ultrasound; NS = nerve stimulation
Characteristics of ongoing studies [ordered by study ID]
NCT 00213954.
| Trial name or title | Ultrasound guidance in nerve block anaesthesia |
| Methods | Randomized, parallel design |
| Participants | Patients aged over 18 years, scheduled for one of these blocks: interscalene, humeral, parasacral or lumbar blocks, for trauma or orthopaedic surgeries |
| Interventions | US + PNS versus PNS |
| Outcomes | Sensory and motor block quality Complications |
| Starting date | May 2005 |
| Contact information | Principal Investigator: Laurence Le Gourrier, MD., Unité d'Anesthésiologie et de Réanimation Chirurgicale, Hôpital de Hautepierre, Strasborg, France |
| Notes | Completion date: February 2009 |
NCT 009956683.
| Trial name or title | Dual endpoint nerve stimulation versus ultrasound in infraclavicular block for hand surgery |
| Methods | Randomized, parallel design |
| Participants | Patients aged over 18 years, scheduled for upper limb surgery at or below the elbow |
| Interventions | US verus NS |
| Outcomes | Block success Ease of nerve location Speed of onset Duration of block Complications |
| Starting date | July 2009 |
| Contact information | Prinical investigator: Dr C McCartney, University Health Network, Toronto, Canada |
| Notes | Clinical trials identifier: NCT 009956683 |
NCT 01010412.
| Trial name or title | Ultrasound visualization versus electrical nerve stimulation |
| Methods | Randomized design |
| Participants | Patients aged over 18 years undergoing unilateral elective surgical procedure requiring interscalene block |
| Interventions | US versus NS |
| Outcomes | Incidence of successful block Amount of sedation required for block |
| Starting date | February 2009 |
| Contact information | Principal Investigator: Nanette Schwann, M.D., Allentown Anesthesia Associates, Pensylvannia, US |
| Notes | Clinical trials identifier: NCT 01010412 |
NCT02020096.
| Trial name or title | Ultrasound plus nerve stimulator versus nerve stimulator guided lumbar plexus block |
| Methods | Randomized, parallel design |
| Participants | Patients 18 ‐ 70 years scheduled to undergo knee arthroscopy surgery |
| Interventions | US + NS versus NS |
| Outcomes | Time required to complete lumbar plexus block Total ultrasound visibility score Hymnody changes after skin incision Performance time of block |
| Starting date | 28 November 2013 |
| Contact information | Principal Investigator: Wei Mei, MD., PhD., Huazhong University of Science and Technology, China |
| Notes | Clinical trials identifier: NCT 02020096 |
US = ultrasound; (P)NS = (peripheral) nerve stimulation
Differences between protocol and review
Differences between review (Walker 2009) and current update.
New authors, Sharon R Lewis and Anastasia Price, contributed to the 2014 update whilst Kristine Aas‐Eng decided not to contribute and was therefore removed from the author list.
In the 2014 update we excluded studies that had given general, spinal or epidural anaesthetic, or additional nerve blocks as part of standard care in addition to the peripheral nerve block under investigation. We also excluded studies that were designed to assess anaesthetic drug volume. As a result of these changes, we excluded some studies which had been included in the original review.
In the 2014 update we altered the review outcomes. The primary outcome of block success was divided into two outcomes to distinguish between the assessments used to define block success, i.e. predicted adequacy of the block with the use of motor or sensory testing, and the assessment of whether surgical anaesthesia had been achieved without the need for supplementary anaesthesia or conversion to general anaesthesia. We adapted the complications outcome to include all complications. We did not include studies that specifically assessed the volume of anaesthetic given during nerve blocks, as outcome data from these studies could not adequately measure our primary outcome. We took an a priori decision to only include limb blocks in the review.
In the 2014 update we expanded the Methods section to include headings: Unit of analysis issues; Dealing with missing data; Assessment of heterogeneity; Assessment of reporting biases; Data synthesis; Subgroup analysis and investigation of heterogeneity; and Sensitivity analysis. We also included a 'Summary of findings' table and incorporated this into the results.
Contributions of authors
2014 updated review authors: Sharon R Lewis (SL), Anastasia Price (AP)a, Kevin J Walker (KJW), Ken McGrattan (KMcG), Andrew F Smith (AFS).
Conceiving the review: AFS Co‐ordinating the review: SL Undertaking manual searches: SL Screening search results: SL, KMcG, KJW, AFS Organizing retrieval of papers: SL Screening retrieved papers against inclusion criteria: SL, KMcG, KJW, AFS Appraising quality of papers: SL, KJW, AP, AFS Abstracting data from papers: SL,KJW, AP, AFS Data management for the review: SL Entering data into Review Manager (RevMan 5.3): SL, AP Analysis of data: SL, AFS Interpretation of data: SL, AFS Writing the review: SL, AP Guarantor for the review (one author): AFS
aAnastasia Price died before publication of the review (December 2014). Her contribution was complete as listed above.
Sources of support
Internal sources
No internal sources of support., UK.
External sources
-
NIHR Cochrane Collaboration Programme Grant: Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04, UK., UK.
This grant funds the work of SRL and AFS for this review
Declarations of interest
Sharon R Lewis: none known
Anastasia Price: deceased; no declarations of interest available
Kevin J Walker: none known
Ken McGrattan: none known
Andrew F Smith: none known
Deceased
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bloc 2010 {published data only}
- Bloc S, Mercadal L, Garnier T, Komly B, Leclerc P, Morel B, et al. Comfort of the patient during axillary blocks placement: A randomized comparison of the neurostimulation and the ultrasound guidance techniques. European Journal of Anaesthesiology 2010;27(7):628‐33. [EMBASE: 2010346101] [DOI] [PubMed] [Google Scholar]
Brull 2009 {published data only}
- Brull R, Lupu M, Perlas A, Chan VW, McCartney CJ. Compared with dual nerve stimulation, ultrasound guidance shortens the time for infraclavicular block performance. Canadian Journal of Anaesthesia 2009;56(11):812‐8. [CENTRAL: CN‐00727909] [DOI] [PubMed] [Google Scholar]
Casati 2007a {published data only}
- Casati A, Danelli G, Baciarello M, Corradi M, Leone S, Cianni S, et al. A prospective, randomized comparison between ultrasound and nerve stimulation guidance for multiple injection axillary brachial plexus block. Anesthesiology 2007;106(5):992‐6. [PUBMED: 17457131] [DOI] [PubMed] [Google Scholar]
Cataldo 2012 {published data only}
- Cataldo R, Carassiti M, Costa F, Martuscelli M, Benedetto M, Cancilleri F, et al. Starting with ultrasonography decreases popliteal block performance time in inexperienced hands: A prospective randomized study. BMC Anesthesiology 2012;12:33. [EMBASE: 2013011260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Chan V, Perlas A, McCartney C, Brull R, Xu D, Abbas S. Ultrasound guidance improves success rate of axillary brachial plexus block. Canadian Journal of Anaesthesia 2007;54(3):176‐82. [PUBMED: 17331928] [DOI] [PubMed] [Google Scholar]
Conceição 2009 {published data only}
- Conceição DB, Helayel PE, Oliveira Filho GR. [A comparative study between ultrasound and neurostimulation guided axillary brachial plexus block]. Revista Brasileira de Anestesiologia 2009;59(5):585‐91. [CENTRAL: CN‐00731129] [PubMed] [Google Scholar]
Danelli 2012 {published data only}
- Danelli G, Bonarelli S, Togn A, Ghisi D, Fanelli A, Biondini S, et al. Prospective randomized comparison of ultrasound‐guided and neurostimulation techniques for continuous interscalene brachial plexus block in patients undergoing coracoacromial ligament repair. British Journal of Anaesthesia 2012;108(6):1006‐10. [EMBASE: 2012297232] [DOI] [PubMed] [Google Scholar]
Dhir 2008 {published data only (unpublished sought but not used)}
- Dhir S, Ganapathy S. Comparative evaluation of ultrasound‐guided continuous infraclavicular brachial plexus block with stimulating catheter and traditional technique: a prospective randomized trial. Acta Anaesthesiologica Scandinavica 2008;52(8):1158‐66. [PUBMED: 18840118] [DOI] [PubMed] [Google Scholar]
Domingo‐Triado 2007 {published data only}
- Domingo‐Triado V, Selfa S, Martinez F, Sanchez‐Contreras D, Reche M, Tecles J, et al. Ultrasound guidance for lateral midfemoral sciatic nerve block: a prospective comparative randomized study. Anesthesia and Analgesia 2007;104(5):1270‐4. [PUBMED: 17456685] [DOI] [PubMed] [Google Scholar]
Dufour 2008 {published data only}
- Dufour E, Quennesson P, Robais A L, Ledon F, Laloe P A, Liu N, et al. Combined ultrasound and neurostimulation guidance for popliteal sciatic nerve block: a prospective, randomized comparison with neurostimulation alone. Anesthesia and Analgesia 2008;106(5):1553‐8. [PUBMED: 18420875] [DOI] [PubMed] [Google Scholar]
Geiser 2011 {published data only}
- Geiser T, Lang D, Neuburger M, Ott B, Augat P, Buttner J. Perivascular brachial plexus block: Ultrasound versus nerve stimulator. [German]. Der Anaesthesist 2011;60(7):617‐24. [EMBASE: 2011416604] [DOI] [PubMed] [Google Scholar]
Gurkan 2008 {published data only}
- Gurkan Y, Acar S, Solak M, Toker K. Comparison of nerve stimulation vs. ultrasound‐guided lateral sagittal infraclavicular block. Acta Anaesthesiologica Scandinavica 2008;52(6):851‐5. [EMBASE: 2008287554] [DOI] [PubMed] [Google Scholar]
Kapral 2008 {published data only}
- Kapral S, Greher M, Huber G, Willschke H, Kettner S, Kdolsky R, et al. Ultrasonographic guidance improves the success rate of interscalene brachial plexus blockade. Regional Anesthesia and Pain Medicine 2008;33(3):253‐8. [PUBMED: 18433677 ] [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu F, Liou J, Tsai, Li A, Day Y, Hui Y, et al. Efficacy of ultrasound‐guided axillary brachial plexus block: a comparative study with nerve stimulator‐guided method. Chang Gung Medical Journal 2005;28:396‐402. [MEDLINE: ] [PubMed] [Google Scholar]
Liu 2009a {published data only}
- Liu SS, Zayas VM, Gordon MA, Beathe JC, Maalouf DB, Paroli L, et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesthesia and Analgesia 2009;109(1):265‐71. [PUBMED: 19535720] [DOI] [PubMed] [Google Scholar]
Macaire 2008 {published data only}
- Macaire P, Singelyn F, Narchi P, Paqueron X. Ultrasound‐ or nerve stimulation‐ guided wrist blocks for carpal tunnel release: a randomized prospective comparative study. Regional Anesthesia and Pain Medicine 2008;33(4):363‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meierhofer 2014 {published data only}
- Meierhofer JT, Anetseder M, Roewer N, Wunder C, Schwemmer U. Guidance of axillary multiple injection technique for plexus anesthesia. Ultrasound versus nerve stimulation. [German]. Der Anaesthesist 2014;63(7):568‐73. [EMBASE: 2014499331] [DOI] [PubMed] [Google Scholar]
Morros 2009 {published data only}
- Morros C, Perez‐Cuenca MD, Sala‐Blanch X, Cedo F. Contribution of ultrasound guidance to the performance of the axillary brachial plexus block with multiple nerve stimulation. [Spanish]. Revista Espanola de Anestesiologia y Reanimacion 2009;56(2):69‐74. [PUBMED: 19334654] [DOI] [PubMed] [Google Scholar]
Perlas 2008 {published data only}
- Perlas A, Brull R, Chan V W, McCartney C J, Nuica A, Abbas S. Ultrasound guidance improves the success of sciatic nerve block at the popliteal fossa. Regional Anesthesia and Pain Medicine 2008;33(3):259‐65. [PUBMED: 18433678 ] [DOI] [PubMed] [Google Scholar]
Renes 2009 {published data only}
- Renes SH, Spoormans HH, Gielen MJ, Rettig HC, Geffen GJ. Hemidiaphragmatic paresis can be avoided in ultrasound‐guided supraclavicular brachial plexus block. Regional Anesthesia and Pain Medicine 2009;34(6):595‐9. [CENTRAL: CN‐00732118] [DOI] [PubMed] [Google Scholar]
Salem 2012 {published data only}
- Salem MH, Winckelmann J, Geiger P, Mehrkens HH, Salem KH. Electrostimulation with or without ultrasound‐guidance in interscalene brachial plexus block for shoulder surgery. Journal of Anesthesia 2012;26(4):610‐3. [EMBASE: 2012629376] [DOI] [PubMed] [Google Scholar]
Sauter 2008 {published data only}
- Sauter AR, Dodgson MS, Stubhaug A, Halstensen AM, Klaastad O. Electrical nerve stimulation or ultrasound guidance for lateral sagittal infraclavicular blocks: a randomized, controlled, observer‐blinded, comparative study. Anesthesia and Analgesia 2008;106(6):1910‐5. [PUBMED: 18499631 ] [DOI] [PubMed] [Google Scholar]
Seidel 2013 {published data only}
- Seidel R, Natge U, Schulz J. [Distal sciatic nerve blocks : Randomized comparison of nerve stimulation and ultrasound guided intraepineural block]. Der Anaesthesist 2013;62(3):183‐92. [PUBMED: 23494021] [DOI] [PubMed] [Google Scholar]
Shrestha 2011 {published data only}
- Shrestha BR. Nerve stimulation under ultrasound guidance expedites onset of axillary brachial plexus block. Journal of Nepal Health Research Council 2011;9(2):145‐9. [PUBMED: 22929843] [PubMed] [Google Scholar]
Sites 2006 {published data only}
- Sites B, Beach M, Spence B, Wiley C, Shiffrin J, Hartman G, et al. Ultrasound guidance improves the success rate of a perivascular axillary plexus block. Acta Anaesthesiologica Scandinavica 2006;50(6):678‐84. [PUBMED: 16987361] [DOI] [PubMed] [Google Scholar]
Soeding 2005 {published data only}
- Soeding P, Sha S, Royse C, Marks P, Hoy G, Royse A. A randomized trial of ultrasound‐guided brachial plexus anaesthesia in upper limb surgery. Anaesthesia and Intensive Care 2005;33(6):719‐25. [PUBMED: 16398375] [DOI] [PubMed] [Google Scholar]
Strub 2011 {published data only}
- Strub B, Sonderegger J, Campe A, Grunert J, Osterwalder JJ. What benefits does ultrasound‐guided axillary block for brachial plexus anaesthesia offer over the conventional blind approach in hand surgery?. Journal of Hand Surgery: European Volume 2011;36(9):778‐86. [21750097] [DOI] [PubMed] [Google Scholar]
Taboada 2009 {published data only}
- Taboada M, Rodriguez J, Amor M, Sabate S, Alvarez J, Cortes J, et al. Ultrasound guidance superior to conventional nerve stimulation for coracoid infraclavicular brachial plexus block?. Regional Anesthesia and Pain Medicine 2009;34(4):357‐60. [EMBASE: 2009549811] [DOI] [PubMed] [Google Scholar]
Trabelsi 2013 {published data only}
- Trabelsi W, Amor MB, Lebbi MA, Romdhani C, Dhahri S, Ferjani M. Ultrasound does not shorten the duration of procedure but provides a faster sensory and motor block onset in comparison to nerve stimulator in infraclavicular brachial plexus block. Korean Journal of Anesthesiology 2013;64(4):327‐33. [EMBASE: 2013286698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Geffen 2009 {published data only}
- Geffen GJ, Broek E, Braak GJ, Giele JL, Gielen MJ, Scheffer GJ. A prospective randomised controlled trial of ultrasound guided versus nerve stimulation guided distal sciatic nerve block at the popliteal fossa. Anaesthesia and Intensive Care 2009;37(1):32‐7. [EMBASE: 2009069819] [DOI] [PubMed] [Google Scholar]
Williams 2003 {published data only}
- Williams S, Chouinard P, Arcand G, Harris P, Ruel M, Boudreault D, et al. Ultrasound guidance speeds execution and improves the quality of supraclavicular block. Anesthesia and Analgesia 2003;97(5):1518‐23. [PUBMED: 14570678] [DOI] [PubMed] [Google Scholar]
Zaragoza‐Lemus 2012 {published data only}
- Zaragoza‐Lemus G, Leal‐Gudino L, Chavez‐Heres T, Pena‐Riveron A, Torres‐Maldonado AS. Interscalene cervical block or extremidad superior subsequent surgery: comparative study of ultrasound vs. neurostimulation. Revista Mexicana de Anestesiologia 2012;35(2):107‐14. [EMBASE: 2012497884] [Google Scholar]
References to studies excluded from this review
Aveline 2010 {published data only}
- Aveline C, Roux A, Hetet H, Vautier P, Cognet F, Bonnet F. Postoperative efficacies of femoral nerve catheters sited using ultrasound combined with neurostimulation compared with neurostimulation alone for total knee arthroplasty. European Journal of Anaesthesiology 2010;27(11):978‐84. [EMBASE: 2010624841] [DOI] [PubMed] [Google Scholar]
Casati 2007b {published data only}
- Casati A, Baciarello M, Cianni S, Danelli G, Marco G, Leone S, et al. Effects of ultrasound guidance on the minimum effective anaesthetic volume required to block the femoral nerve. British Journal of Anaesthesia 2007;98(6):823‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Danelli 2009a {published data only}
- Danelli G, Fanelli A, Ghisi D, Moschini E, Rossi M, Ortu A, et al. Ultrasound vs nerve stimulation multiple injection technique for posterior popliteal sciatic nerve block. Anaesthesia 2009;64(6):638‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Danelli 2009b {published data only}
- Danelli G, Ghisi D, Fanelli A, Ortu A, Moschini E, Berti M, et al. The effects of ultrasound guidance and neurostimulation on the minimum effective anesthetic volume of mepivacaine 1.5% required to block the sciatic nerve using the subgluteal approach. Anesthesia and Analgesia 2009;109(5):1674‐8. [PUBMED: 19843807] [DOI] [PubMed] [Google Scholar]
Demirci 2013 {published data only}
- Demirci A, Efe EM, Türker G, Gurbet A, Kaya FN, Anil A, et al. Iliohypogastric/ilioinguinal nerve block in the inguinal hernia repair for postoperative pain management: Comparison of the anatomical landmark and ultrasound guided techniques. Regional Anesthesia and Pain Medicine 2013;38(Suppl 1):E150. [71366546] [Google Scholar]
Dolan 2008 {published data only}
- Dolan J, Williams A, Murney E, Smith M, Kenny G. Ultrasound guided fascia iliaca block: a comparison with the loss of resistance technique. Regional Anaesthesia and Pain Medicine 2008;33(6):526‐31. [EMBASE: 2008499403] [DOI] [PubMed] [Google Scholar]
Ko 2013 {published data only}
- Ko SH, Kang BS, Hwang CH. Ultrasonography‐ or electrophysiology‐guided suprascapular nerve block in arthroscopic acromioplasty: a prospective, double‐blind, parallel‐group, randomized controlled study of efficacy. Arthroscopy ‐ Journal of Arthroscopic and Related Surgery 2013;29(5):794‐801. [EMBASE: 2013275908] [DOI] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li M, Xu T, Han WY, Wang XD, Jia DL, Guo XY. Use of ultrasound to facilitate femoral nerve block with stimulating catheter. Chinese Medical Journal 2011;124(4):519‐24. [EMBASE: 2011104114] [PubMed] [Google Scholar]
Maalouf 2012 {published data only}
- Maalouf D, Liu SS, Movahedi R, Goytizolo E, Memstoudis SG, Yadeau JT, et al. Nerve stimulator versus ultrasound guidance for placement of popliteal catheters for foot and ankle surgery. Journal of Clinical Anesthesia 2012;24(1):44‐50. [EMBASE: 2012061455] [DOI] [PubMed] [Google Scholar]
Maldini 2010 {published data only}
- Maldini B, Zdravcevic KS, Adam VN, Goranovic T, Zdencar D, Baranovic S. A comparative study between ultrasound versus neurostimulation guided sciatic nerve block in the popliteal fossa. Regional Anesthesia and Pain Medicine 2010;35(5):E128. [70287489] [Google Scholar]
Marhofer 1997 {published and unpublished data}
- Marhofer P, Schrögendorfer K, Koinig H, Kapral S, Weinstabl C, Mayer N. Ultrasonographic guidance improves sensory block and onset time of three‐in‐one blocks. Anesthesia and Analgesia 1997;85(4):854‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marhofer 1998 {published and unpublished data}
- Marhofer P, Schrögendorfer K, Wallner T, Koinig H, Mayer N, Kapral S. Ultrasonic guidance reduces the amount of local anesthetic for 3‐in‐1 blocks. Regional Anesthesia and Pain Medicine 1998;23(6):584‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mariano 2009a {published data only}
- Mariano ER, Cheng GS, Choy LP, Loland VJ, Bellars RH, Sandhu NS, et al. Electrical stimulation versus ultrasound guidance for popliteal‐sciatic perineural catheter insertion a randomized controlled trial. Regional Anesthesia and Pain Medicine 2009;34(5):480‐5. [EMBASE: 2010049165] [DOI] [PubMed] [Google Scholar]
Mariano 2009b {published data only}
- Mariano ER, Loland VJ, Bellars RH, Sandhu NS, Bishop ML, Abrams RA, et al. Ultrasound guidance versus electrical stimulation for infraclavicular brachial plexus perineural catheter insertion. Journal of Ultrasound in Medicine 2009;28(9):1211‐8. [EMBASE: 2009503137] [DOI] [PubMed] [Google Scholar]
Mariano 2009c {published data only}
- Mariano ER, Loland VJ, Sandhu NS, Bellars RH, Bishop ML, Afra R, et al. Ultrasound guidance versus electrical stimulation for femoral perineural catheter insertion. Journal of Ultrasound in Medicine 2009;28(11):1453‐60. [PUBMED: 19854959] [DOI] [PubMed] [Google Scholar]
Mariano 2010a {published data only}
- Mariano ER, Loland VJ, Sandhu NS, Bishop ML, Lee DK, Schwartz AK, et al. Comparative efficacy of ultrasound‐guided and stimulating popliteal‐sciatic perineural catheters for postoperative analgesia. Canadian Journal of Anesthesia 2010;57(10):919‐26. [2010544877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariano 2010b {published data only}
- Mariano ER, Loland VJ, Sandhu NS, Bishop ML, Meunier MJ, Afra R, et al. A trainee‐based randomized comparison of stimulating interscalene perineural catheters with a new technique using ultrasound guidance alone. Journal of Ultrasound in Medicine 2010;29(3):329‐36. [20194929] [DOI] [PubMed] [Google Scholar]
Martinez Navas 2011 {published data only}
- Martinez Navas A, Ortiz de la Tabla Gonzalez R, Rodriguez de la Torre R, Davila Arias M, Medina Madrid E, Almeida Gonzalez C, et al. Has ultrasonography contribute with more efficacy and security than nerve stimulation in posterior sciatic nerve block?. Regional Anesthesia and Pain Medicine 2011;36(Suppl 2):E161‐2. [70735638] [Google Scholar]
McCartney 2008 {published data only}
- McCartney CJ, Chan VW, Brull R, Perlas A. A randomized, double‐blind study comparing dual endpoint nerve stimulation with ultrasound‐guided infraclavicular block for hand surgery. British Journal of Anaesthesia 2008;100(4):575P. [Google Scholar]
McCartney 2009 {published data only}
- McCartney CJL, Shastri U, McNaught AF, Carmichael NM, Holtby RM. Does ultrasound reduce the minimum effective anaesthetic volume required for Interscalene brachial plexus block (ISBPB)?. British Journal of Anaesthesia 2009;103(2):323P. [70009412] [Google Scholar]
McNaught 2011 {published data only}
- McNaught A, Shastri U, Carmichael N, Awad IT, Columb M, Cheung J, et al. Ultrasound reduces the minimum effective local anaesthetic volume compared with peripheral nerve stimulation for interscalene block. British Journal of Anaesthesia 2011;106(1):124‐30. [PUBMED: 21059701] [DOI] [PubMed] [Google Scholar]
Nassar 2010 {published data only}
- Nassar T, Seet M, Subeh Z, Elqestawy B, Elgendy H. Comparison of ultrasound guided technique with nerve stimulator guided technique for multiple injection axillary block: a prospective randomized trial. Regional Anesthesia and Pain Medicine 2010;35(5):E52. [70287216] [Google Scholar]
Ponrouch 2010 {published data only}
- Ponrouch M, Bouic N, Bringuier S, Biboulet P, Choquet O, Kassim M, et al. Estimation and pharmacodynamic consequences of the minimum effective anesthetic volumes for median and ulnar nerve blocks: a randomized, double‐blind, controlled comparison between ultrasound and nerve stimulation guidance. Anesthesia and Analgesia 2010;111(4):1059‐64. [PUBMED: 20705778] [DOI] [PubMed] [Google Scholar]
Redborg 2009 {published data only}
- Redborg KE, Sites BD, Chinn CD, Gallagher JD, Ball PA, Antonakakis JG, et al. Ultrasound improves the success rate of a sural nerve block at the ankle. Regional Anesthesia and Pain Medicine 2009;34(1):24‐8. [PUBMED: 19258984] [DOI] [PubMed] [Google Scholar]
Sala‐Blanch 2012 {published data only}
- Sala‐Blanch X, Riva N, Carrera A, Lopez AM, Prats A, Hadzic A. Ultrasound‐guided popliteal sciatic block with a single injection at the sciatic division results in faster block onset than the classical nerve stimulator technique. Anesthesia and Analgesia 2012;114(5):1121‐7. [EMBASE: 2012239380] [DOI] [PubMed] [Google Scholar]
Thomas 2011a {published data only}
- Thomas LC, Graham SK, Osteen KD, Porter HS, Nossaman BD. Comparison of ultrasound and nerve stimulation techniques for interscalene brachial plexus block for shoulder surgery in a residency training environment: a randomized, controlled, observer‐blinded trial.[Erratum appears in Ochsner J. 2012 Spring;12(1):86]. Ochsner Journal 2011;11(3):246‐52. [PUBMED: 21960758] [PMC free article] [PubMed] [Google Scholar]
Thomas 2011b {published data only}
- Thomas L, Forth NE, Nossaman B. Randomized study of ultrasound guidance vs. nerve stimulation for popliteal fossa block of the sciatic nerve. Regional Anesthesia and Pain Medicine 2011; Vol. 36, issue 5:Conference abstract.. [CENTRAL: CN‐01033609; DOI: 10.1097/AAP.0b013e318228df0c; EMBASE: 70728202] [DOI]
Tognu 2010 {published data only}
- Tognu A, Gullotta S, Danelli G, Borghi B, Niebel T, Bonarelli S, et al. Nerve guidance with versus without prepuncture ultrasound visualization for psoas compartment block and perineural catheter insertion: A randomized, prospective, blinded study. European Journal of Pain Supplements 2010; Vol. 4, issue 4:313‐7. [EMBASE: 2010613988]
Tran 2010 {published data only}
Villeneuve 2009 {published data only}
- Villeneuve A, Levesque S, Nadeau MJ, Dion NN, Cote D, Nicole PC, et al. Continuous femoral block: nerve stimulation vs US‐guidance. Canadian Journal of Anesthesia 2009;56:S74. [70078748] [Google Scholar]
Wildy 2009 {published data only}
- Wildy J, Cooper P, Gambala G, Osborne L, Spence D, Pellegrini J. Comparison of ultrasound‐guided and peripheral nerve stimulation techniques for axillary brachial plexus anesthesia on success, duration, and complications. AANA Journal 2009;77(5):403. [EMBASE: 2010449890.] [Google Scholar]
Yi 2012 {published data only}
- Yi J, Lin HH, Li SZ. Ultrasound guided sciatic nerve block, in comparison of the neurostimulation during the popliteal sciatic nerve block: a randomized clinical trail. European Journal of Anaesthesiology 2012;29:120. [EMBASE: 71084382] [Google Scholar]
Zencirci 2011 {published data only}
- Zencirci B. Comparision of nerve stimulator and ultrasonography as the techniques applied for brachial plexus anesthesia. International Archives of Medicine 2011;4(1):4. [EMBASE: 2011084628] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aytac 2015 {published data only}
- Aytac S, Atalan G, Gulen G, Yilmaz H. Comparison of femoral nerve block by neurostimulator accompanied with ultrasound and without ultrasound in knee artroplasty. Journal of Clinical and Analytical Medicine 2015;6:208‐11. [Google Scholar]
Dhir 2013 {published data only}
- Dhir S, Ganapathy S. Success rate of perineural infra‐clavicular brachial plexus catheters: Randomized controlled single blind trial comparing peripheral nerve stimulation with ultrasound guided catheter placement. Regional Anesthesia and Pain Medicine 2013;38(4):Conference Abstract. [CENTRAL: CN‐01061661; EMBASE: 71378197] [Google Scholar]
Eren 2014 {published data only}
- Eren G, Altun E, Pektas Y, Polat Y, Cetingok H, Demir G, et al. To what extent can local anesthetics be reduced for infraclavicular block with ultrasound guidance?. Der Anaesthesist 2014;63(10):760‐5. [PUBMED: 25098777] [DOI] [PubMed] [Google Scholar]
González 1993 {published data only}
- González González F, Salas Colín S, Parra Ortíz A, Castañeda Gaxiola R, Mancer Reyes V, Interiano Portillo F, et al. Bloqueo del nervio ciático poplíteo interno mediante su localización con ultrasonido doppler. Revista de Sanidad Militar 1993;47(5):165‐8. [Google Scholar]
Kumar 2014 {published data only}
- Kumar A, Sharma D, Sibi ME, Datta B, Gogoi B. Comparison of peripheral nerve stimulator versus ultrasonography guided axillary block using multiple injection technique. Indian Journal of Anaesthesia 2014;58(6):700‐4. [PUBMED: 25624532] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2014 {published data only}
- Lam NC, Petersen TR, Gerstein NS, Yen T, Starr B, Mariano ER. A randomized clinical trial comparing the effectiveness of ultrasound guidance versus nerve stimulation for lateral popliteal‐sciatic nerve blocks in obese patients. Journal of Ultrasound in Medicine 2014;33(6):1057‐63. [PUBMED: 24866613] [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li T, Ye XH, Nan Y, Shi T, Ye QG, Ma JF, et al. Comparison of ultrasound and nerve stimulation techniques for brachial plexus block for regional hemodynamic changes of upper extremity. [Chinese]. National Medical Journal of China 2013;93(3):187‐90. [EMBASE: 2013127999] [PubMed] [Google Scholar]
Martinez Navas 2014 {published data only}
- Martinez Navas A, Echevarria Moreno M, Rodriguez de la Torre R, Davila Arias ML, Cuellar Obispo E. Sciatic nerve block: Is ultrasonography better than nerve stimulation?. 33rd Annual European Society of Regional Anaesthesia and Pain Therapy, ESRA Congress 2014 Seville Spain. Conference Start: 20140903 Conference End: 20140906. 2014; Vol. 39:e241.
NCT 01579747 {published data only}
Smith 2014 {published data only}
- Smith A, Kumara P, Ismail K, Elland E. A comparison of the duration of brachial plexus block between ultrasound guided and nerve stimulator techniques in elective shoulder surgery. 33rd Annual European Society of Regional Anaesthesia and Pain Therapy, ESRA Congress 2014 Seville Spain. Conference Start: 20140903 Conference End: 20140906. 2014; Vol. 39:e259.
Stavrati 2014 {published data only}
- Stavrati M, Vogiatzaki T, Chloropoulou P, Terleme E, Christofis C, Iatrou C. Comparing onset times and vascular punctures in axillary blocks with nerve stimulation or ultrasound: a randomised controlled trial. European Anaesthesiology Congress, EUROANAESTHESIA 2014 Stockholm Sweden. Conference Start: 20140531 Conference End: 20140603. 2014; Vol. 31:131.
References to ongoing studies
NCT 00213954 {published data only}
- Ultrasound guidance in nerve block anaesthesia. Ongoing study May 2005.
NCT 009956683 {published data only}
- Dual endpoint nerve stimulation versus ultrasound in infraclavicular block for hand surgery. Ongoing study July 2009.
NCT 01010412 {published data only}
- Ultrasound visualization versus electrical nerve stimulation. Ongoing study February 2009.
NCT02020096 {published data only}
- Ultrasound plus nerve stimulator versus nerve stimulator guided lumbar plexus block. Ongoing study 28 November 2013.
Additional references
Auroy 1997
- Auroy Y, Narchi P, Messiah A, Litt L, Rouvier B, Samii K. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology 1997;87(3):479‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Auroy 2002
- Auroy Y, Benhamou D, Bargues L, Ecoffey C, Falissard B, Mercier F, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002;97(5):1274‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2003
- Chan VW, Perlas A, Rawson R, Odukoya O. Ultrasound‐guided supraclavicular brachial plexus block. Anesthesia and Analgesia 2003;97(5):1514‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2006
- Chan VS, Nova H, Abbas S, McCartney CL, Perlas A, Xu D. Ultrasound examination and localization of the sciatic nerve: a volunteer study. Anesthesiology 2006;104(2):309‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Denny 2005
- Denny NM, Harrop‐Griffiths W. Location, location, location! Ultrasound imaging in regional anaesthesia. British Journal of Anaesthesia 2005;94(1):1‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Freitas 2007
- Freitas NM, Semedo E, Marques A, Orfao R, Nunes JM. Patients preferences concerning ultrasound guided regional anesthesia. Regional Anesthesia and Pain Medicine 2007;32:99. [Google Scholar]
Gelfand 2010
- Gelfand H, Lesley M, Ko P, Eng C, Wu C. An updated meta‐analysis of ultrasound‐guided nerve blocks. Regional Anesthesia and Pain Medicine. 35th Annual Regional Anesthesia Meeting and Workshops, ASRA 2010 Toronto, ON Canada. Conference Start: 20100422 Conference End: 20100425. 2010; Vol. 35 (5):var. pagings. [70736136]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011..
Kapral 1994
- Kapral S, Krafft P, Eibenberger K, Fitzgerald R, Gosch M, Weinstabl C. Ultrasound‐guided supraclavicular approachfor regional anesthesia of the brachial plexus. Anesthesia and Analgesia 1994;78(3):507‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirchmair 2001
- Kirchmair L, Entner T, Kapral S, Mitterschiffthaler G. Ultrasound guidance for the psoas compartment block: an imaging study. Anesthesia and Analgesia 2002;94(3):706‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2009b
- Liu XZ, Zhou L, Zhang YJ, Liu B. Efficacy and safety of nerve‐stimulator‐guide needle placement in peripheral nerve block: a systematic review. [Chinese]. Chinese Journal of Evidence‐Based Medicine 2009; Vol. 9, issue 5:542‐51. [2009448109]
Marhofer 2004
- Marhofer P, Sitzwohl C, Greher M, Kapral S. Ultrasound guidance for infraclavicular brachial plexus anaesthesia in children. Anaesthesia 2004;59(7):642‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marhofer 2005
- Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anaesthesia. British Journal of Anaesthesia 2005;94(1):7‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandhu 2002
- Sandhu NS, Capan LM. Ultrasound‐guided infraclavicular brachial plexus block. British Journal of Anaesthesia 2002;89(2):254‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schwemmer 2006
- Schwemmer U, Anetseder M, Meierhofer J, Obach S, Roewer N. Axillary plexus block: faster onset by ultrasound guidance compared to stimulation of “fascia click". Anesthesiology 2006;105:A1140. [Google Scholar]
References to other published versions of this review
Walker 2007
- Walker KJ, McGrattan K, Aas‐Eng K, Smith AF. Ultrasound guidance for peripheral nerve blockade. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006459] [DOI] [PubMed] [Google Scholar]
Walker 2009
- Walker KJ, McGrattan K, Aas‐Eng K, Smith AF. Ultrasound guidance for peripheral nerve blockade. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006459.pub2] [DOI] [PubMed] [Google Scholar]


