Abstract
Background
Stroke and other adult‐acquired brain injury may impair perception leading to distress and increased dependence on others. Perceptual rehabilitation includes functional training, sensory stimulation, strategy training and task repetition.
Objectives
To examine the evidence for improvement in activities of daily living (ADL) six months post randomisation for active intervention versus placebo or no treatment.
Search methods
We searched the trials registers of the Cochrane Stroke Group and the Cochrane Infectious Diseases Group (May 2009) but not the Injuries Group, the Cochrane Central Register of Controlled Trials (The Cochrane Library 2009, Issue 3), MEDLINE (1950 to August 2009), EMBASE (1980 to August 2009), CINAHL (1982 to August 2009), PsycINFO (1974 to August 2009), REHABDATA and PsycBITE (May to June 2009). We also searched trials and research registers, handsearched journals, searched reference lists and contacted authors.
Selection criteria
Randomised controlled trials of adult stroke or acquired brain injury. Our definition of perception excluded visual field deficits, neglect/inattention and apraxia.
Data collection and analysis
One review author assessed titles, abstracts and keywords for eligibility. At least two review authors independently extracted data. We requested unclear or missing information from corresponding authors.
Main results
We included six single‐site trials in rehabilitation settings, involving 338 participants. Four trials included people with only stroke. All studies provided sensory stimulation, sometimes with another intervention. Sensory stimulation typically involved practising tasks that required visuo‐perceptual processing with occupational therapist assistance. Repetition was never used and only one study included functional training. No trials provided data on longer term improvement in ADL scores. Only three trials provided any data suitable for analysis. Two of these trials compared active to placebo intervention. There was no evidence of a difference in ADL scores at the scheduled end of intervention: mean difference (95% confidence interval (CI)) was 0.9 (‐1.6 to 3.5) points on a self‐care ADL scale in one study and odds ratio (95% CI) was 1.3 (0.56 to 3.1) for passing a driving test in the other, both in favour of active intervention. The trial that compared two active interventions did not find evidence of difference in any of the review outcomes.
Authors' conclusions
There is insufficient evidence to support or refute the view that perceptual interventions are effective. Future studies should be sufficiently large, include a standard care comparison and measure longer term functional outcomes. People with impaired perception problems should continue to receive neurorehabilitation according to clinical guidelines.
Keywords: Adult, Humans, Activities of Daily Living, Brain Injuries, Brain Injuries/complications, Brain Injuries/rehabilitation, Perceptual Disorders, Perceptual Disorders/rehabilitation, Randomized Controlled Trials as Topic, Stroke, Stroke/complications, Stroke Rehabilitation
Non‐pharmacological interventions for perceptual disorders following stroke and other adult‐acquired, non‐progressive brain injury
Healthy adult brains are capable of processing multiple and complex information from our senses. We can perceive colour, shape and size, recognise objects and people's faces, estimate location, depth and distance. We can also conduct higher level functions drawing on our memory and cultural experience, e.g. understand written symbols or emotional states conveyed by facial expressions. A stroke or other acquired brain injury, such as a head injury, can affect these simple and complex perceptual abilities. Occupational therapists and psychologists offer different types of therapy such as practising personal care tasks, practising perceptual activities and puzzles, teaching strategies or encouraging intensive repetition of tasks. We do not know if any approach is beneficial. We searched for all relevant research, found six studies and assessed the quality of each study. We pooled their results where possible to draw our overall conclusions. Some of the original researchers provided additional information beyond that in their published studies. However, most of the research was conducted more than 10 years ago and only the published work was available to us. We found that all six studies examined the therapy approach of practising perceptual activities (e.g. puzzles and tasks that involve processing sensory information) with stroke patients. No study examined whether the therapy provided benefits past six month in terms of the level of independence in undertaking everyday activities. On the basis of existing research evidence, the benefit or harm of therapy for adults who experience difficulty processing sensory information after stroke or brain injury remains unknown. People with perceptual problems should continue to be offered rehabilitation as recommended in guidelines intended for healthcare practitioners. Future studies should be large enough to be conclusive and should look at the longer‐term effects of therapy, including independence in doing everyday activities, emotions, outcome for family caregivers and potential harmful effects.
Summary of findings
Summary of findings for the main comparison.
| Non‐pharmacological interventions compared with standard care for perceptual disorders | ||||||
|
Patient or population: people with perceptual disorders following acquired brain injury (stroke and trauma) Settings: rehabilitation units Intervention: non‐pharmacological therapy Comparison: placebo or no treatment in addition to standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard | Perceptual intervention | |||||
| Independence in activities of daily living Effects lasting up to 6 months |
No data | No data | None | None | ||
| Independence in activities of daily living (ADL) Effects at scheduled end of intervention |
The mean Rivermead ADL scale was 10.0 in the only trial to report this outcome | The mean Rivermead ADL in the intervention groups was 10.9 | 33 (1) | ++OO low | Based on single small study with unclear methods: allocation and interim analysis processes | |
| Independence in activities of daily living (ADL): driving test pass rate Effects at scheduled end of intervention |
Medium risk population | OR 1.3 (0.56 to 3.1) | 97 (1) | +++O moderate | Based on single small study | |
| 28 per 100 | 34 per 100 (18 to 55) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Stroke is the second most frequent cause of mortality worldwide (Murray 1997; WHO 2008), a key cause of disability (Donnan 2008) and results in a greater range of disabilities than any other condition (Dept of Health 2000). Each year around 15 million people around the world have a stroke (Mackay 2004). Traumatic brain injury (TBI) is a leading cause of death and disability in adults worldwide (Perel 2006). Estimates from England suggest the annual incidence of adults admitted to hospital with traumatic brain injury is 113,000 out of a population of over 50 million (Dept of Health 2000).
Stroke, and other adult‐acquired brain injury, may impair a person's perceptual abilities. Psychologists use perception (and perceptual disorders) as an umbrella term for a wide range of abilities (and difficulties). One psychologist's detailed definition of perception is that it "involves active processing of the continuous torrent of sensations. This process comprises many successive and interactive stages. Those that deal with the simplest physical characteristics, such as colour, shape, or tone ... and ... more complex, 'higher' levels of semantic and visuoconceptual processing ..." (Lezak 1995).
There are several controversies within the topic of perception. The main argument is about what is and what is not a perceptual disorder. There is a difference of opinion as to whether perception is itself a subset of the broader area of cognition. Some health professionals distinguish between cognitive abilities (by which they mean attention, memory and thinking) and perceptual abilities. However, this review assumes that it is more useful to consider perception as a part of cognition when evaluating the adverse effects of brain injury and the interventions employed in rehabilitation.
The topic of perception is particularly difficult to delimit precisely and appears to overlap with other cognitive and sensory areas. Perceptual disorders may affect any or all of the sensory modalities. This is demonstrated in the wide range of perceptual disorders which include visual, object, visual object agnosia, prosopagnosia, spatial, visuospatial, tactile, body, sensation, location, motion, colour processing and auditory perceptual disorders. Visual perceptual disorders are the most commonly researched. In the latest version of Lezak's classic textbook of neuropsychological assessment, 30 of the 39 pages on perception are devoted to the assessment of visual perceptual disorders (Lezak 2004). In contrast, perceptual disorders of the auditory, tactile and olfactory modalities are briefly covered, particularly the latter two. Taste is not covered.
Assessment of perceptual disorders is one of the most difficult areas for neuropsychologists working in clinical settings (Beaumont 1992). Most perceptual assessment tools appear to draw on other cognitive functions as well, for example attention, spatial orientation, or memory. Lezak 1995 argues that such overlap in assessments is inevitable and in fact desirable given the overlap in the underlying mental functions and the ways they can be impaired.
There are several standardised assessment batteries. The oldest is the Rivermead Perceptual Assessment Battery (RPAB). The RPAB (Whiting 1985) contains 17 different subtests (e.g. object matching, figure ground, body image). The Visual Object and Space Perception (VOSP) battery (Warrington 1991) contains four visual object assessments (for example silhouettes) and four space perception tasks (for example position discrimination). The Birmingham Object Recognition Battery (BORB) contains 14 subtests (Riddoch 1993) including orientation match and foreshortened view. These test batteries tend to be used for identifying the underlying perceptual impairment rather than as measures of the impact on everyday functioning. What they illustrate is the number of ways in which visual perception can be affected.
The prevalence of visual perceptual problems after stroke or TBI varies depending on the timing and types of assessments. Reported rates range from 54% of hemiplegic patients experiencing visual perceptual disturbances up to two years post stroke (van Ravensberg 1984), to Edmans 1991, who identified perceptual problems in 69% of patients one month post stroke and in 74% of patients two years post stroke. When compared to normative samples, visual perceptual changes are evident in patients with severe TBI (McKenna 2006). Visual perceptual impairments at one year post injury have been reported for 31% of TBI patients, of which 18% had mild impairment and 12% severe impairment (Kersel 2001).
The impact of perceptual disorders on activities of daily living (ADL) is varied. It can range from difficulty crossing the road (due to an impairment of distance perception) to an inability to recognise a familiar object (for example a toothbrush ‐ object agnosia) or person's face (such as a spouse ‐ prosopagnosia). These disorders can cause distress for the person affected and their family, and increase their dependence on others. Perceptual disorders can also hinder a person's ability to participate fully in their rehabilitation programme, for example their sessions with the physiotherapist or occupational therapist. As such, the reduction of perceptual disorders, or their effects, is often an initial aim of therapists. Specialist rehabilitation resources, which could be used to focus on improving motor functioning, are often hampered by co‐existing perceptual disorders.
Description of the intervention
A critical review and synthesis of published research evidence (based on searching five electronic databases from 1995 to June 2002, and prior to this MEDLINE from 1970) for the effectiveness of treatments for visual perceptual disorders after stroke (Jutai 2003) concluded there was strong evidence for the treatment of perceptual disorders but not for any specific intervention type. There are several different intervention approaches likely to be used in clinical practice and these may be categorised as follows: functional training, sensory stimulation, strategy training and task repetition.
How the intervention might work
Functional training
Functional training involves the repetitive practice of activities of daily living, e.g. washing, dressing, and preparing meals. The emphasis is on treating the symptom rather than the cause of the problem (Edmans 2000). The rationale for the intervention is that patients will become more independent as their performance improves on the specific everyday tasks that are carried out in therapy.
Sensory stimulation
Sensory stimulation describes a mixed set of procedures that are designed to target visuo‐perceptual processes such as colour matching, shape recognition, judgement of line length. The rationale for the intervention is that when individuals carry out perceptual tasks their performance will improve on other (i.e. non‐trained) tasks that share similar perceptual elements. This is sometimes known as the transfer of training approach.
Strategy training
Strategy training involves learning a rule or technique that can be applied when the individual encounters activities requiring perceptual processing. Examples include verbalisation, self‐pacing and chunking. The rationale is that patients will process visual material more effectively if they have a strategy to help them overcome their acquired perceptual deficits.
Repetition
Repetition describes therapy in which the individual repeats a particular task(s) over and over until performance improves. It involves some components of the above interventions, but in straightforward repetition there is no practice of everyday activities (as in functioning training), no focus on basic sensory processing (as in sensory stimulation), and no explicit strategies are taught (as in strategy training).
Why it is important to do this review
This review was designed to evaluate the evidence for the rehabilitation of perceptual disorders. The working definition we adopted for this review excluded the rehabilitation of sensory impairments (for example visual field defects such as homonymous hemianopia) or attentional impairments (such as visual or spatial neglect or inattention). We also excluded the condition known as mild cognitive impairment (MCI) and the review focused on non‐progressive brain injury.
Objectives
This review examined the effectiveness of non‐pharmacological interventions aimed at the rehabilitation of perceptual disorders following stroke and other adult‐acquired brain injury. It considered three questions.
Did the intervention result in a persisting reduction in the level of disability when outcome was compared with those allocated to placebo or no intervention?
Was one specific targeted intervention more effective than another in terms of a persisting reduction in disability?
Were interventions effective for the subgroup of those with stroke?
The primary objective was to examine the evidence for an improvement in activities of daily living up to six months post randomisation for active intervention versus placebo or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of interventions aimed at the rehabilitation of perceptual disorders following stroke and other adult‐acquired brain injury. In the case of crossover trials we would have used only data from the first phase. The crossover design is inappropriate for this research question because first phase effects are expected to be maintained and would contaminate subsequent phases.
Types of participants
Participants were adults (18 years and older) with impaired perception following a stroke or other adult‐acquired brain injury (e.g. TBI, subarachnoid haemorrhage, meningitis, encephalitis). We considered all types of perceptual disorders for inclusion. We included visuo‐constructional impairments.
In clinical practice neglect is sometimes regarded as a perceptual disorder, but it is now accepted as an attentional disorder and therefore we excluded trials of unilateral neglect (e.g. visual or spatial or motor), which have been previously reviewed (Bowen 2007). Similarly we excluded apraxia (West 2008). We excluded the lay use of the term perception, such as perception of pain, numbness or weakness, from this review. We did not include children with stroke or acquired brain injury, adults with developmental brain injury, and adults with progressive dementia or malignant brain tumour. Children and adults with developmental pathology may not have developed the perceptual abilities expected of an unimpaired adult and the outcome measures may not be appropriate. Adults with progressive conditions may not respond to intervention in the way that those with stroke or other non‐progressive conditions would be expected to. Additionally, we excluded adults who have received a diagnosis of MCI based on the diagnostic criteria described in the ICD‐10 (WHO 1993) as "Objective evidence of decline in cognitive performance not attributable to other mental or behavioural disorders identified in ICD‐10. May be reversible". However, as cognitive problems post stroke are common, we included adults who were cognitively impaired at a mild level due to the effects of stroke or non‐progressive brain injury.
Types of interventions
We included trials in which a comparison was made between an active treatment group that received one of the various perceptual interventions versus a control group that received either placebo or no treatment (Objectives 1) or an alternative perceptual intervention (Objectives 2). We included interventions aimed specifically at reducing the resulting perceptual impairments or the disabilities. We categorised the studies according to therapeutic approach (see descriptions above). These approaches included:
functional training (practicing activities of daily living, e.g. washing, dressing, preparing meals, household tasks);
sensory stimulation (which may include cueing or visual scanning);
strategy training;
repetition (of a task).
As the focus of the review is on non‐pharmacological interventions, we excluded trials including only drug therapies.
Types of outcome measures
Primary outcomes
We measured the primary outcome at the disability (activity) level, for example the average level of independence in activities of daily living. We used data from standardised measures, for example the Barthel Index (BI) (Mahoney 1965), the Functional Independence Measure (FIM) (Keith 1987) and the Assessment of Motor and Process Skills (AMPS) (Fisher 1994). We also used data from structured observational instruments and considered subjective measures of improvement. If a trial provided data on more than one of these, we extracted the BI data above those from the FIM and then the AMPS. If a trial provided outcome data at several time points we extracted the data from the last time point within the six‐month follow‐up.
Secondary outcomes
Secondary outcomes included:
independence in ADL at the scheduled end of the intervention (ordinal);
performance on standardised impairment level measures of perception e.g. RPAB, BORB, VOSP at end of intervention and at six months (ordinal);
quality of life measures at six months (ordinal);
effects on carer at six months (ordinal);
destination on discharge: institutional care setting or not (binary);
adverse events, such as death, fatigue, falls, accident rates (binary).
Search methods for identification of studies
Electronic searches
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the trials registers of the Cochrane Stroke Group and the Cochrane Infectious Diseases Group (last searched May 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 3), MEDLINE (1950 to August 2009) (Appendix 1), EMBASE (1980 to August 2009) (Appendix 2), CINAHL (1982 to August 2009), PsycINFO (1974 to August 2009) (Appendix 3), REHABDATA (http://www.naric.com/research) and PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy: http://www.psycbite.com/) (May to June 2009).
Searching other resources
In an effort to identify further published, ongoing and unpublished studies:
-
we searched the following trials and research registers in May and June 2009:
UK National Research Register Archive (http://www.nrr.nhs.uk/search.htm) (records up to September 2007);
UK Clinical Research Network Study Portfolio (http://public.ukcrn.org.uk/search/);
Current Controlled Trials Register (http://www.controlled‐trials.com);
we handsearched the Journal of Clinical and Experimental Neuropsychology (1979 to June 2009) and Psychology and Aging (1986 to June 2009). To avoid duplication of effort, we searched only relevant journals that had not been handsearched by The Cochrane Collaboration (see Master List of Journals at http://apps1.jhsph.edu/cochrane/masterlist.asp). At the time of publishing our protocol we had planned to handsearch five journals but, when it came to carrying out the review, expansion of the Master List reduced our workload;
we searched reference lists of included articles;
we contacted authors of included articles and other researchers in the field.
We contacted the Cochrane Injuries Group to request a search of their trials register but they confirmed there was no need to search their register as all trials were sent regularly to CENTRAL. We searched for trials in all languages and planned to arrange translation of trial reports published in languages other than English: we found no relevant non‐English language trials.
Data collection and analysis
Selection of studies
One review author searched titles, abstracts and keywords of both published and unpublished papers to assess their eligibility for inclusion using a systematic approach. We discarded only those papers that obviously did not meet the eligibility criteria. We obtained and screened articles that possibly met the inclusion criteria. All review authors read the remaining studies and formed a consensus on the final inclusion and data extraction.
Data extraction and management
At least two review authors independently extracted data. In addition to outcome data, we documented the following:
setting (e.g. hospital, community, nursing home);
type of intervention;
length of rehabilitation;
profession(s) involved;
co‐interventions implemented;
length of disease;
level of severity;
presence of other symptoms that may affect the level of disability (e.g. hemiplegia, unilateral spatial neglect);
tools the authors used to identify perceptual disorders.
We contacted the corresponding authors to request additional information that was unclear or missing from the reports. We recorded duration and frequency of intervention and also service delivery issues (for example by which professional and in what setting).
We recorded a number of design features and quality criteria in addition to risk of bias indicators below, including:
randomisation method (whether stratified or unclear);
type of design (e.g. parallel, factorial, crossover);
prospective power calculation (whether reported, correct and realistic);
definition of terms (e.g. of stroke, apraxia, outcome, and intervention);
outcome measures (e.g. total number and whether a primary outcome was stated);
intention‐to‐treat analysis (whether undertaken, possible from report, impossible or unclear);
selection of patients clearly described;
groups of patients comparable at baseline;
interventions clearly described;
concordance to treatment comparable in groups.
Assessment of risk of bias in included studies
At least two review authors independently performed assessment. We described the risk of bias in the included studies for the following aspects: allocation process; blinding of outcome assessment; incomplete outcome data; selective reporting; and others.
Measures of treatment effect
We treated activities of daily living and other ordinal scales for the secondary outcomes as continuous outcomes as accepted meta‐analytic techniques for ordinal outcome data are not yet available. We abstracted, calculated or requested means and standard deviations. For all binary outcomes, we incorporated deaths in the worse outcome category and calculated Peto odds ratios. We excluded deaths from outcomes that were treated as continuous. We envisaged that the death rates between the two groups would be low and similar because studies would only have included patients who were well enough to undergo rehabilitation for perceptual disorders. We discuss any imbalance in death rates between the groups, including descriptive consideration of whether analyses of raw data from individual trials could alter conclusions.
Our intention was to analyse the mean (and standard deviation) for the primary outcome. However, where activities of daily living were reported as a binary outcome this was not appropriate. Instead we abstracted and compared binary data for the primary outcome as an additional secondary analysis.
Unit of analysis issues
We did not anticipate any specific unit of analysis issues. Crossover trials would not be appropriate in this setting, and we would have only included data from the first phase of such trials.
Dealing with missing data
Where missing outcome data remained unavailable following correspondence with study authors, their potential to alter the review conclusions was considered via sensitivity analyses.
Assessment of heterogeneity
We noted and discussed statistical heterogeneity guided by the I2 statistic.
Assessment of reporting biases
We assessed the scope for reporting bias by absence of anticipated outcomes, less detailed reporting of non‐significant outcomes, and control for multiple hypothesis testing (via either statistical adjustment or pre‐specification of a primary outcome).
We would have examined a funnel plot for suggestion of possible publication bias if 10 or more studies had been identified reporting a single outcome.
Data synthesis
Our primary analysis pooled all therapeutic studies of active intervention versus placebo or no treatment to answer question 1 (seeObjectives). We stratified this analysis according to therapeutic approach, as outlined under Types of interventions, to answer question 2 (seeObjectives). This included a comparison of each approach versus placebo or no treatment, as well as direct comparisons of different approaches. To answer question 3 (seeObjectives) we repeated the analyses planned for questions 1 and 2 but restricted these to the subgroup of stroke patients. This subgroup was operationally defined as deriving from studies that only included stroke patients, and mixed aetiology studies where stroke patient data could be separately analysed. If the stroke data could not be separated we included the study if at least 80% of the sample had stroke.
Where possible, we combined results for continuous outcomes using mean difference (MD) by a fixed‐effect model. However, we anticipated that studies would use different scales to measure the same underlying constructs. Where this occurred, we used the standardised mean difference (SMD) and translated the results back into one of the original scales for reporting purposes. We combined results for binary outcomes using the Peto odds ratio, and translated to risk differences across the observed range of control group rates for reporting purposes.
Subgroup analysis and investigation of heterogeneity
We prospectively planned the subgroup analysis of stroke patients to address this review's third objective. We would consider further post‐hoc subgroups defined by methodological characteristics in the exploration of heterogeneity on the primary outcome.
Sensitivity analysis
We carried out sensitivity analyses on the primary outcome. These included use of a random‐effects analysis, omission of studies that did not describe an adequate method of allocation concealment, and imputing values for missing data where appropriate.
Results
Description of studies
Results of the search
The searches were initially run in 2008 and updated between May and August 2009. For the period 1950 (MEDLINE searches) to August 2009 we identified 25 potentially eligible studies: six studies that met our inclusion criteria, 16 that we excluded and three that are awaiting assessment. Searching on the topic of 'perception' was problematic due to the lack of clinical agreement on terminology. Our search strategies resulted in thousands of unrelated hits. The search of the Cochrane Stroke Group's trials register identified 274 publications including all six included trials.
Included studies
We included six studies with a total of 338 participants from three countries (Canada, UK, and USA). The number initially randomised per study ranged from 10 (Hajek 1993) to 97 (Mazer 2003). These six studies are described in the Characteristics of included studies table and in a summary table comparing them (Table 4).
Table 1.
Variability in participants, interventions and outcomes
| Taylor 1971 | Lincoln 1985 | Hajek 1993 | Dirette 1999 | Edmans 2000 | Mazer 2003 | |
| Participants | 78 people from USA randomised: entered 65, analysed 47 Mean age whole sample 58.5 years Groups "comparable" in terms of sex distribution, but no numbers stated Mean 55.2 days since stroke |
33 people from UK were analysed: 21 (64%) had stroke, and 6 (18%) each had head injury and SAH Mean age 50 years (17 to 69 years) 17 (52%) of all participants male Mean 2.7 months (SD 1.8) since stroke |
20 participants from Canada Age and sex not reported separately for randomised participants 1 to 5 months since stroke |
30 people from USA 2 (7%) participants post‐CVA, others were trauma‐related Mean age 38 years (21 to 56 years) 22 (73%) of all participants were male Mean 5 months (2 to 12 months) since injury |
80 people from UK: analysed 79, 1 died before completion Mean age 69 years Overall 40 (50%) male Mean 34 days (14 to 84 days) since stroke |
97 people from Canada randomised; analysed 86 Overall mean age 66 years Overall 70 (72%) male Mean 78 days since stroke |
| Interventions |
Intervention: sensory stimulation Experimental group: individually tailored programme directed to patients' perceptual and cognitive deficits Control group: treatment directed at patients' motor deficits. Motor skill tasks were completed until functional skills were achieved 20 treatment days Separate teams of therapists worked with control and experimental groups in physical therapy and occupational Therapy Co‐interventions not reported |
Intervention: sensory stimulation Experimental group: practiced various perceptual tasks of the type commonly used in occupational therapy departments Control group: the same amount of therapy time, but carried out activities that were designed to improve physical rather than perceptual abilities 4 hours per week over 4 weeks |
Intervention: sensory stimulation Experimental group: Bracy's computerised visuospatial training package, which comprised 7 different visuospatial exercises Control group: routine rehabilitation therapies provided by the hospital 3 x 30‐minute sessions per week over 4 weeks 2 trained research assistants (professional background unknown) All participants received routine rehabilitation therapies provided by the hospital, including physiotherapy and occupational therapy treatment |
Intervention: sensory stimulation coupled with strategy training Experimental group: received 4 sessions of an 'IQ Builder' computer programme together with instruction in the use of 3 compensatory strategies Control group: given the same computer programme for the same length of time, but did not receive instruction in the use of compensatory strategies Frequency: 1 hour per week over 6 weeks (only 4 weeks involved active therapy) Profession of 'therapist': occupational therapist Co‐interventions: regular attendance at outpatient "cognitive rehabilitation program" |
Intervention: sensory stimulation and functional training Sensory group: practiced perceptual tasks to produce improvement on tasks with similar perceptual elements (to treat the impairment) Functional group: patients repeatedly practicing everyday tasks (to treat the symptom) 2.5 hours per week (5 x 30 minute sessions) over 6 weeks Occupational therapists (research OT and ward‐based OT) Co‐interventions reported: additional general occupational therapy treatment |
Intervention: 2 types ‐ sensory stimulation coupled with strategy training Experimental group: comprised 4 commercially available computerised software programmes (Tetris, Othello, Mastermind, Jigs@w Puzzle) commonly used by occupational therapists to retrain perceptual and cognitive functions Control group: computerised treatment using the 'Useful Field of View' (UFOV) that targeted visual processing speed, visual divided attention and visual selective attention 2 to 4 sessions per week (each 30 to 60 minutes) for 20 sessions, thrice weekly Occupational therapist Co‐interventions: all participants received 4 sessions of physical retraining on the Baltimore Therapeutic Equipment work simulator |
| Outcomes | End of intervention ADL, but not in analysable format End of intervention perception impairment, but not in analysable format |
End of intervention ADL End of intervention perception impairment Specified primary outcome(s): no 16 tests of RPAB and ADL presented with no apparent hierarchy Time points for outcomes: end of intervention Total number of outcomes: 17 ‐ includes 16 measures of perception in RPAB Assessed by third party "unaware of the treatment group" |
End of intervention ADL End of intervention perception impairment Specified primary outcome(s): no ‐ impairment and activity level measures with no explicit hierarchy Time points for outcomes: end of intervention Total number of outcomes: > 40 in battery Assessed for neuropsychological battery, functional indices and mobility |
End of intervention perception impairment Specified primary outcome(s): no ‐ multiple outcomes with no specified hierarchy Time points for outcomes: 1 week after end of intervention Total number of outcomes: 9 ‐ computer‐based tasks with measures of speed and accuracy, the Paced Auditory Serial Addition Task and the Matching Accuracy Test Assessed by third party: computer‐based assessments with data collected by staff member (speech pathologist) blinded to allocation |
End of intervention ADL End of intervention perception impairment: yes Adverse events: death 5 "main outcomes": RPAB, Barthel ADL and Edmans ADL Time points for outcomes: end of intervention (6 weeks) Total number of outcomes: 25 reported Assessed by third party, independent assessor |
End of intervention ADL: no scale reported or measured, but driving evaluation End of intervention perception impairment: yes Specified primary outcome(s): pass/fail of on‐road driving evaluation Time points for outcomes: end of intervention Total number of outcomes: 31 Assessed by third party, independent occupational therapist for impairment, plus driving instructor for on‐road evaluation |
ADL: activities of daily living CVA: cerebrovascular accident RPAB: Rivermead Perceptual Assessment Battery SAH: subarachnoid haemorrhage SD: standard deviation
Authors of four of the studies provided helpful personal communication, clarifying methods and clinical issues (for details seeCharacteristics of included studies table). We were unable to contact the authors of one study (Hajek 1993), and a co‐author of the sixth and oldest study (Taylor 1971) confirmed that the first author had died and no unpublished data or details were available.
All six were single‐site trials that recruited patients from rehabilitation settings. Stroke was the most common aetiology with the exception of one study in which it was exclusively head trauma (Dirette 1999). A second study included people with TBI or subarachnoid haemorrhage but the majority of their participants had a stroke (Lincoln 1985).
Time since onset varied from approximately two weeks to five months. Collectively the studies represent a wide age range, from 17 to 86 years, although the oldest person in Dirette 1999 was 56 years old, and an upper age limit was sometimes set e.g. 70 years (Taylor 1971). Examples of other exclusion criteria employed by the studies were: previous stroke/injury (Taylor 1971; Hajek 1993; Dirette 1999), bilateral lesions (Mazer 2003), and unable to transfer with two nurses (Edmans 2000).
The tools used to confirm the presence of a perceptual problem varied but were always a battery of neuropsychological tests, sometimes including other cognitive abilities such as memory and attention. In two studies (Hajek 1993; Dirette 1999) the original authors did not select their participants on the basis of a perceptual impairment (e.g. "visual processing" in Dirette 1999) but further details from personal communication informed our decision that inclusion in this review was warranted.
The severity and nature of the perceptual disorders was difficult to determine in most studies. In some cases (Lincoln 1985) a cut‐off was pre‐set e.g. > 2 SDs below norms on at least three subtests of the RPAB, which has eight subtests that further subdivide into 14 tests (four subtests for Edmans 2000). Mazer 2003 described the severity within each treatment arm as: 28% mild, 51% moderate, 21% severe (experimental group), 28% mild, 54% moderate, and 18% severe (control group).
As commonly experienced in rehabilitation trials of other cognitive conditions, the interventions were rarely described with sufficient detail to allow replication or implementation into practice. This is most likely due to word limit restrictions set by journals and the clinical heterogeneity of the samples studied. Some studies published separate reports on the interventions.
Three of the four expected interventions were used in the included studies: functional training, sensory stimulation and strategy training, with no studies providing repetition as a sole strategy. See the Characteristics of included studies table for further details on the intervention provided in each study, but in summary all six studies provided sensory stimulation. Two studies (Dirette 1999; Mazer 2003) coupled this with strategy training but the latter was never provided in isolation. Sensory stimulation typically involved one‐to‐one time with an occupational therapist practising tasks that required visuo‐perceptual processing. In one study (Hajek 1993) it was not known whether the research assistants providing the intervention were occupational therapists. Tasks included shape recognition games, stick length sorting and cube copying. Three studies included computerised tasks (Hajek 1993; Dirette 1999; Mazer 2003). Sessions lasted for 30 to 60 minutes, usually several days per week for a total duration ranging from four sessions (Dirette 1999) to 30 sessions (Edmans 2000). Only one study (Edmans 2000) included a functional training intervention.
Control interventions included activities aimed at reducing motor or physical disabilities or what was termed as 'routine rehabilitation' (Taylor 1971; Lincoln 1985; Hajek 1993). Two studies (Dirette 1999; Mazer 2003) provided computerised tasks such as those addressing visual attention and speed. Edmans 2000 compared two interventions: functional training (e.g. practising everyday tasks such as dressing) and transfer of training (categorised in this review under our definition of sensory stimulation).
Excluded studies
We excluded 16 studies and these are individually described in the Characteristics of excluded studies table. We excluded five studies on the basis of design: not a randomised controlled trial (Gordon 1985; Towle 1990; Flynn 2000; Connor 2002; Beschin 2005). Another 10 studies were not evaluating interventions for perceptual problems, e.g. some recruited people with unilateral neglect or other cognitive problems. The sixteenth study (Wagenaar 1992) was neither a randomised controlled trial nor a perceptual study, but we list it here as excluded because it was included in the Jutai 2003 review.
Risk of bias in included studies
SeeFigure 1 'Risk of bias graph' and Figure 2 'Risk of bias summary'.
Figure 1.
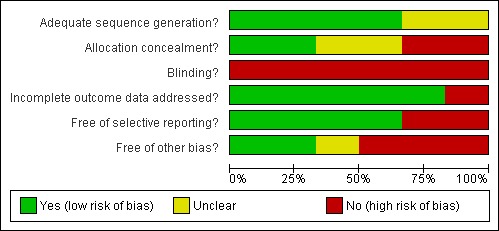
Risk of Bias graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 2.
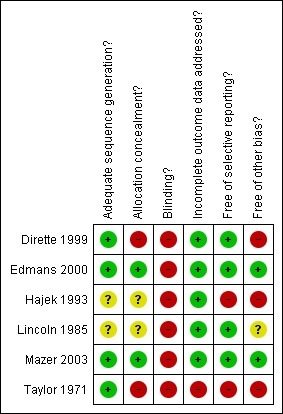
Risk of Bias summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All included studies reported using random allocation. It was not possible to confirm the methods of allocation used in two of the earlier studies (Lincoln 1985; Hajek 1993). Only Mazer 2003 clearly used adequate allocation concealment. In Edmans 2000 the investigator herself prepared the sealed randomisation envelopes. Although not strictly 'adequate' we have rated this as low risk of bias following discussion with the investigator. The earlier studies were unclear or inadequate on the issue of concealment.
Blinding
Blinding of interventions to clinician and patient is not possible in this setting. All included studies are therefore at high risk of bias in this regard. Attempted blinding of at least some assessment was attempted by all studies except the earliest (Taylor 1971). In Dirette 1999 assessments were computer‐based, but supervised by a blinded therapist. In the only study to assess success of intended blinding (Mazer 2003) allocation was correctly identified in nearly 80% of participants.
Incomplete outcome data
Losses to follow‐up were unlikely to bias results in all except Taylor 1971. The remaining studies experienced very low loss to follow‐up except Mazer 2003, but losses were clearly described and unlikely to be related to intervention.
Selective reporting
Selective reporting was clearly an issue in Hajek 1993, where results were tabulated only for statistically significant items within the battery of assessments. However, the data from this study are not included in meta‐analyses for other reasons. Similarly, Taylor 1971 reported tables of F‐statistics and P‐values for all assessments, but only reported means for each group within the text for those differences that were statistically significant. There was no evidence of selective reporting in the other studies.
Other potential sources of bias
In Taylor 1971 separate teams of therapists worked within each treatment group. Any differences observed could be due to the particular skill sets of the study therapists rather than to differences between the therapies themselves.
In Lincoln 1985 there was a change to eligibility criteria part‐way through the recruitment phase of the study. This was due to slow recruitment, but it is unclear what interim analyses were undertaken and exactly what the decision‐making process was for continuation, adaptation and eventual stopping of the trial.
In Hajek 1993 the first 10 patients were randomised, but the second 10 were allocated systematically. We have not been able to contact the authors to seek data for the randomised participants. Risk of bias would remain high even if raw data were available, as it is unclear what interim analyses were undertaken on the accruing data.
The study by Dirette 1999 was a matched pairs design, in which participants were allocated to the alternative therapy if their baseline characteristics matched a previously randomised participant. No account of this design was taken in the analysis.
Effects of interventions
See: Table 1
Perceptual intervention versus control
From a total of five studies that randomised 248 participants, only two studies with 130 participants (Lincoln 1985; Mazer 2003) provided data suitable for analysis. We were not able to obtain separate data for the randomised participants in Hajek 1993, and the authors confirmed the loss of original data or summary statistics by group for both Taylor 1971 and Dirette 1999. The sixth study (Edmans 2000) compared two interventions rather than a control condition and so is described instead under 'functional training versus sensory stimulation' below.
Primary outcome
No trials reported on sustained differences in activity level up to six months following the end of intervention.
Secondary outcomes
Only outcomes at end of scheduled intervention were recorded (seeAnalysis 1.2). For activity level Lincoln 1985 reported a difference (95% confidence interval (CI)) of 0.94 (‐1.6 to 3.48) on a scale of ADL self‐care following sensory stimulation, favouring perceptual intervention. Mazer 2003 reported pass rates for a driving test assessment of 16/47 (34%) following mixed strategy training and sensory stimulation versus 14/50 (28%) using control intervention. This translates to OR (95% CI) of 1.3 (0.56 to 3.1) in favour of perceptual intervention. Taylor 1971 and Dirette 1999 each reported that differences were not statistically significant.
Analysis 1.2.
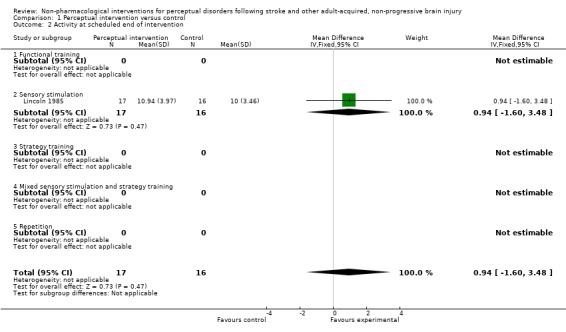
Comparison 1 Perceptual intervention versus control, Outcome 2 Activity at scheduled end of intervention.
We combined impairment level data for Lincoln 1985 and Mazer 2003 to give a SMD (95% CI) of 0.07 (‐0.29 to 0.43) standard deviations with no evidence of statistical heterogeneity: I2 = 0% (seeAnalysis 1.4). Using the standard deviations observed in these two trials this confidence interval rules out a difference of more than 8.5 points on the shape copy task from the RPAB or two points on the Motor‐free Visual Perception Test. Taylor 1971 and Dirette 1999 each reported that differences were not statistically significant.
Analysis 1.4.
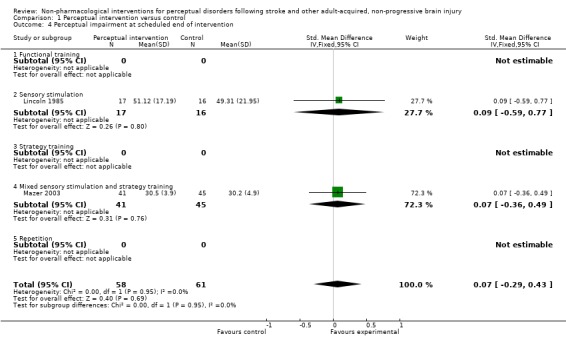
Comparison 1 Perceptual intervention versus control, Outcome 4 Perceptual impairment at scheduled end of intervention.
None of the remaining secondary outcomes was reported by any trial.
Functional training versus sensory stimulation
This comparison was assessed by a single trial that randomised 80 participants (Edmans 2000). Original (raw) data were provided by the author who also confirmed that all participants were eligible for inclusion in this review. The analyses we present here are not available from the report.
Primary outcome
There was no data collection regarding differences in activity level up to six months following the end of intervention.
Secondary outcomes
For activity level outcome, mean improvement in Barthel Score at scheduled end of intervention was 3.9 in the functional training group and 3.0 in the sensory stimulation group. This gave a mean difference (95% CI) of 1.0 (‐0.4 to 2.4) points favouring functional training (seeAnalysis 2.2). This translates to a SMD (95% CI) of 0.31 (‐0.13 to 0.75) standard deviations.
Analysis 2.2.
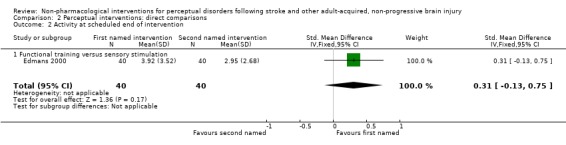
Comparison 2 Perceptual interventions: direct comparisons, Outcome 2 Activity at scheduled end of intervention.
For impairment level outcome, mean improvement in the RPAB at scheduled end of intervention was 22.0 in the functional training group and 21.6 in the sensory stimulation group. This gave a mean difference (95% CI) of 0.4 (‐11.8 to 12.6) points favouring functional training (seeAnalysis 2.4). This translates to a SMD (95% CI) of 0.02 (‐0.42 to 0.45) standard deviations.
Analysis 2.4.
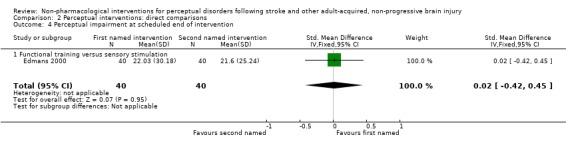
Comparison 2 Perceptual interventions: direct comparisons, Outcome 4 Perceptual impairment at scheduled end of intervention.
None of our remaining secondary outcomes were reported by this trial.
Effectiveness for the subgroup with stroke
Five studies recruited people with stroke and three of them (Lincoln 1985; Edmans 2000; Mazer 2003) provided data suitable for this review. One of these (Lincoln 1985) also included people with TBI or subarachnoid haemorrhage although the majority (64%) of participants had stroke. The above results are therefore predominately based on data from the clinical population of people with stroke and will not be repeated here.
Discussion
Summary of main results
There is currently insufficient evidence to support or refute the view that interventions for perceptual problems are effective. Only six studies were eligible for inclusion, three of which provided data suitable for analysis. In these three, sample sizes were small (ranging from 33 to 89 participants) and smaller studies provide less precise estimates of effect. We are aware of three further studies (seeCharacteristics of studies awaiting classification) but these have even smaller sample sizes.
As shown in the Table 1, there were no available data with which to answer the primary research question, nor several of the secondary questions. Where data were available, the difference in the effects of the interventions overlaps the null value, therefore the results of the analysis are compatible with both benefit and harm.
It is important to consider the nature of the comparator when examining the results of any study. In our review, one study (Edmans 2000) usefully compared two potentially active interventions to see if one was better than the other. Dirette 1999 and Mazer 2003 explored the benefits of coupling sensory stimulation with strategy training. Both studies provided the control group with computerised sensory stimulation without the strategy training. Interpretation of evidence from these three studies needs to bear in mind that they do not provide evidence on whether perceptual intervention is better than no treatment or usual care or placebo. Hajek 1993 included a 'routine rehabilitation' control group to investigate any added benefit of the sensory stimulation intervention. However, such 'usual care' requires careful definition and measurement as it varies between services and from country to country. Taylor 1971 and Lincoln 1985 included a control condition where tasks focused on motor or physical skills. This model is designed to isolate any specific effect of working on perceptual skills over and above any general 'dose' effect and is becoming more popular as seen in the emergent use of 'attention control' in rehabilitation research.
There are many remaining uncertainties. In fact, on completion of this review the only certainty is that the question of effectiveness has not yet been answered by existing research. It remains uncertain whether any intervention is more effective than no intervention, or one intervention is better than any other, or whether combining interventions is beneficial. Similarly, we have no evidence from the studies to guide us on the amount or duration of intervention, nor information about service delivery and organisational issues. These issues include when and where intervention should be provided (e.g. early in‐patient versus post‐acute community rehabilitation), and whether it should be delivered by a qualified occupational therapist or psychologist, or an assistant working under their supervision. Only adequately sized trials, using relevant outcome measures, could provide answers to these clinically important questions.
Overall completeness and applicability of evidence
This review aimed to synthesise evidence relevant to adults with stroke and other non‐progressive brain injuries. The included studies covered a good age range from 17 to 86 years. It was encouraging to note that, with one exception (Taylor 1971), studies did not impose artificial cut‐offs, such as retirement age.
Most of the participants in the included trials had a stroke. A small proportion had TBI or subarachnoid haemorrhage. However, we argue that this does not restrict the review’s relevance. In designing the review’s protocol we argued that interventions for perceptual impairments would be similar for people with stroke and non‐progressive injuries. This remains our view.
As outlined in the Background to this review one of the greyest areas concerns the boundaries and exact nature of the set of impairments known as disorders of perception. Disorders of perception include a diverse range of restricted abilities which can include failure to recognise common objects or familiar people, difficulty judging distance to safely cross a road or spatial disorientation affecting ability to find one’s way around the hospital or when out shopping. Current studies used standardised diagnostic assessments to select participants and aimed for broad inclusion either by type of perceptual disorder or breadth of severity. However, the detail provided in the published reports is not sufficient to provide a clear picture of the clinical population to whom the results might be generalised. Journal restrictions on word length may have prevented more detailed reporting. Future trials should consider publishing separate papers on demographic and clinical characteristics to inform the external validity of the trial.
The crucial limitation in fully meeting this review’s objectives is the absence in the included studies of longer‐term functional outcome measures, which we defined as measurements taken up to six months post‐randomisation. Although data were reported on functional outcome at the scheduled end of intervention, a key aim of rehabilitation research is to evaluate maintenance of benefits. Similarly the identified trials did not conduct longer‐term follow‐up on the impairment level measures used. A lack of data on quality of life, destination on discharge, and effects on carers limit the scope of the evidence resulting from this review.
In terms of fit to current practice, the included studies were conducted in Canada, UK, and USA. We cannot say with certainty that interventions represented standard occupational therapy practice in those countries, but an interesting observation is that all six studies included an intervention approach categorised as sensory stimulation. All participants were assisted by a therapy worker and this was confirmed as an occupational therapist in all but one study (Hajek 1993). Although different materials were used, including three which involved computerised tasks (Hajek 1993; Dirette 1999; Mazer 2003) all studies used a common approach of practising activities that draw on visual perceptual abilities. Sessions lasted for 30 to 60 minutes, usually several days per week for several weeks (from four to 30 sessions). Less typical of clinical practice (certainly in the UK) was the absence of functional task training: it was used in only one (UK‐based) study in the review.
Quality of the evidence
The evidence summarised in this review comes from six small studies conducted in three countries spanning more than 30 years. The total number of participants was 338 although we could extract outcome data from only three studies, the largest of which randomised 97 people (all with stroke). A robust conclusion cannot be drawn about the effectiveness of non‐pharmacological interventions for people with perceptual disorders following stroke and other adult, acquired, non‐progressive brain injury. Key methodological limitations of the included studies and recommendations for future trials are detailed below (seeImplications for research).
Potential biases in the review process
In our opinion this review identified all relevant studies. We successfully contacted several of the original authors, who provided clarification and data that were not available in the published reports.
Agreements and disagreements with other studies or reviews
Jutai 2003 conducted a "critical review and synthesis" of published research evidence of visual perceptual disorders following stroke (based on searching five electronic databases from 1995 to June 2002 and MEDLINE from 1970 to 1994). Their definition of perception was broader than our own, including both spatial neglect and apraxia. Although only brief details of the search terms used are provided the reviewers did assess the methodological quality of each published study that they included (using PEDro) and two reviewers independently assessed each article. They included eight studies (classifying six as RCTs) concluding there was "strong evidence" that specific treatment of perceptual disorders improves perceptual functioning based on summarising the original studies’ findings as three positive, one negative and one mixed. They also concluded that one study which included head injury patients (Lincoln 1985) did not show a significant difference for perceptual training and that there was "moderate evidence" that one approach was no more effective than another, based on the Edmans 2000 study.
Our systematic review differed in methods and conclusions. We searched for unpublished as well as published studies. We included adult, acquired, non‐progressive brain injury such as TBI and subarachnoid haemorrhage in addition to stroke. We excluded neglect and apraxia, which were separately systematically reviewed (Bowen 2007; West 2008). We considered and excluded six of the eight studies included by Jutai 2003 either because the participants had neglect or because the study was not a randomised controlled trial (Weinberg 1977; Weinberg 1979; Weinberg 1982; Carter 1983, Gordon 1985, Wagenaar 1992). Our review and Jutai 2003 included only two studies in common (Lincoln 1985; Edmans 2000). We share Jutai’s conclusion that no one intervention approach has proven efficacy over any other. We disagree with their conclusion that there is evidence for the effectiveness of specific treatments for perceptual disorders.
Cicerone 2005 conducted an updated systematic review (as far as 2002) of the effectiveness of cognitive rehabilitation for people with TBI or stroke. In addition to examining the evidence for comprehensive holistic cognitive rehabilitation the authors searched for studies categorised under six other categories, one of which was visual perception as distinct from the category of apraxia but including visual neglect. All of the studies identified were for neglect rehabilitation or interventions for visual field loss. They concluded that the rehabilitation of "more complex visuospatial abilities required for functional activities (e.g. meal preparation, driving)" requires randomised controlled trials.
Four other Cochrane Reviews have been conducted in related areas or interventions (Legg 2006; French 2007; Doyle 2010; Hoffmann 2010). However, none of these examined non‐pharmacological interventions for perceptual problems and so there is no overlap. French 2007 did not look at the use of repetition to rehabilitate perceptual problems and no study in our review used repetition exclusively as a rehabilitation intervention. The intervention labelled in our review as 'sensory stimulation' involved one‐to‐one time with an occupational therapist practising tasks that required visuo‐perceptual processing such as shape recognition games or computerised tasks, whereas Doyle 2010 reviewed interventions for sensory impairment of the upper limb. As expected, we found that occupational therapists were the professionals most likely to deliver the interventions for perceptual problems. The reader may be interested in two other reviews of occupational therapy, Legg 2006 for patients with problems in activities of daily living after stroke, and Hoffmann 2010 for occupational therapy for cognitive impairment in stroke patients.
Authors' conclusions
There is insufficient evidence to support or refute the view that any specific intervention is effective at reducing the impact of impaired perceptual functioning, and so more conclusive evidence is required before decisions are made on the provision of these services. Absence of evidence is not evidence of absence. The four main intervention approaches may be categorised as: functional training, sensory stimulation, strategy training and task repetition. Although research interest has focused on sensory stimulation to the exclusion of repetition, at present the possible merits of any one treatment approach over any other are unknown. Anecdotal evidence suggests that all four approaches are currently used in clinical practice, often in combination with each other. As we did not review whether individuals with perceptual problems benefit from general rehabilitation (e.g. physiotherapy, occupational therapy, nursing, etc), they should continue to receive standard neurorehabilitation services in accordance with available clinical guidelines.
Future studies should:
provide a sufficiently detailed theoretical rationale for, and description of, the interventions including type and amount to allow implementation into clinical practice and research replication;
provide a standard care control group, carefully documenting the content and amount of standard care, which can be highly variable;
include detailed diagnostic information on individuals' perceptual problems given the heterogeneity in perceptual problems in terms of type, severity and likely impact on everyday function;
ensure low risk of study bias through rigorous methodological development and reporting, e.g. ensure allocation concealment, attempt to blind outcome assessors and report the success or failure, report all loss to follow‐up, report results from all outcome measures, control for other possible sources of bias;
be of sufficient size to have adequate statistical power to answer clinically important questions about long‐term functional outcomes;
specify a primary endpoint and include analysis of other key outcomes such as adverse events, psychosocial benefits and other outcomes deemed important by service users;
adopt an intention‐to‐treat approach to measurement of outcomes in all individuals as well as to analysis of measured outcomes by treatment group;
include a health economic assessment.
Acknowledgements
We gratefully acknowledge the contributions of Michelle Fairnie and Sarah Gilpin to the original protocol, and to Brenda Thomas and Hazel Fraser (Cochrane Stroke Review Group), Val Haigh (librarian, Salford Royal Hospitals NHS Foundation Trust) and Emma Patchick (University of Manchester) for invaluable help and advice with the searches.
Appendices
Appendix 1. MEDLINE search strategy
The search strategy for MEDLINE (Ovid) is given below and we adapted this for the other databases.
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or cerebrovascular accident/ or exp brain infarction/ or exp cerebrovascular trauma/ or exp hypoxia‐ischemia, brain/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial haemorrhages/ or exp vertebral artery dissection/ 2. (stroke$ or post stroke$ or post‐stroke$ or cerebral vascular or cerebrovascular or cva$).tw. 3. (cerebral or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or apoplexy or emboli$).tw. 4. (cerebral or brain or subarachnoid) adj5 (haemorrhage or haemorrhage or haematoma or hematoma or bleed).tw. 5. (trauma$ or acquired) adj5 brain injur$).tw. 6. exp brain damage, chronic/ or brain injuries/ or exp brain concussion/ or exp brain haemorrhage, traumatic/ or brain injury, chronic/ or diffuse axonal injury/ 7. craniocerebral trauma/ or exp head injuries, closed/ or exp intracranial haemorrhage, traumatic/ 8. exp brain abscess/ or exp central nervous system infections/ or exp encephalitis/ or exp meningitis, viral/ 9. (encephalitis or meningitis).tw. 10. exp brain neoplasms/ 11. (brain or cerebr$) adj5 (neoplasm$ or lesion$ or tumor$ or tumour$)).tw. 12. or/1‐11 13. exp perceptual disorders/ or exp perception/ 14. (perception or visuo?perception or visual?perception or agnosia or prosopagnosia or stereognosis).tw. 15. (percept$ or visuo?percept$ or visual?percept$ or visuo?spatial or visual?spatial or visuo?construct$ or visual?construct$) adj5 (disorder$ or impairment$ or problem$ or abilit$ or difficult$ or deficit$ or training or re?training or remediation or rehabilitation or intervention or therapy)).tw. 16. or/13‐15 17. Randomized Controlled Trials/ 18. random allocation/ 19. Controlled Clinical Trials/ 20. control groups/ 21. clinical trials/ 22. double‐blind method/ 23. single‐blind method/ 24. Placebos/ 25. placebo effect/ 26. cross‐over studies/ 27. Multicenter Studies/ 28. Therapies, Investigational/ 29. Research Design/ 30. Program Evaluation/ 31. evaluation studies/ 32. randomized controlled trial.pt. 33. controlled clinical trial.pt. 34. clinical trial.pt. 35. multicenter study.pt. 36. evaluation studies.pt. 37. random$.tw. 38. (controlled adj5 (trial$ or stud$)).tw. 39. (clinical$ adj5 trial$).tw. 40. (control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$).tw. 41. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 42. (multicenter or multicentre or therapeutic) adj5 (trial$ or stud$).tw. 43. (control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$).tw. 44. (singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$).tw. 45. (coin adj5 (flip or flipped or toss$).tw. 46. latin square.tw. 47. versus.tw. 48. (cross‐over or cross over or crossover).tw. 49. placebo$.tw. 50. sham.tw. 51. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 52. controls.tw. 53. or/17‐52 54. 12 and 16 and 53 55. limit 54 to humans
Appendix 2. EMBASE search strategy
1 Cerebrovascular Disorders/ (16422) 2 exp basal ganglia cerebrovascular disease/ (113) 3 exp brain ischemia/ (45292) 4 exp carotid artery diseases/ (21630) 5 Stroke/ (68071) 6 exp brain infarction/ (26669) 7 exp cerebrovascular trauma/ (24594) 8 exp hypoxia‐ischemia, brain/ (45292) 9 exp intracranial arterial diseases/ (874) 10 exp "intracranial embolism"/ and "thrombosis "/ (80) 11 exp intracranial hemorrhages/ (38079) 12 exp vertebral artery dissection/ (3817) 13 (stroke$ or poststroke$ or post‐stroke$ or cerebral vascular or cerebrovascular or cva$).tw. (109262) 14 ((cerebral or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or apoplexy or emboli$)).tw. (43959) 15 ((cerebral or brain$ or subarachnoid) adj5 (haemorrhage or hemorrhage or haematoma or hematoma or bleed)).tw. (16392) 16 ((trauma$ or acquired) adj5 brain injur$).tw. (10356) 17 exp brain damage, chronic/ (261) 18 Brain Injuries/ (45966) 19 exp brain concussion/ (898) 20 exp brain hemorrhage, traumatic/ (38079) 21 Brain Injury, Chronic/ (45966) 22 Diffuse Axonal Injury/ (331) 23 Craniocerebral Trauma/ (19791) 24 exp head injuries, closed/ (101463) 25 exp intracranial hemorrhage, traumatic/ (38079) 26 exp brain abscess/ (4216) 27 exp central nervous system infections/ (65815) 28 exp encephalitis/ (32942) 29 exp meningitis, viral/ (1423) 30 (encephalitis or meningitis).tw. (34571) 31 exp brain neoplasms/ (56761) 32 ((brain or cerebr$) adj5 (neoplasm$ or lesion$ or tumor$ or tumour$)).tw. (36761) 33 exp perceptual disorders/ (8058) 34 exp perception/ (94573) 35 33 or 34 (100645) 36 (perception or visuo?perception or visual?perception or agnosia or prosopagnosia or stereognosis).tw. (47235) 37 ((percept$ or visuo?percept$ or visual?percept$ or visuo?spatial or visual?spatial or visuo?construct$ or visual?construct$) adj5 (disorder$ or impairment$ or problem$ or abilit$ or difficult$ or deficit$ or training or re?training or remediation or rehabilitation or intervention therapy)).tw. (6904) 38 35 or 37 or 36 (130920) 39 Randomized Controlled Trial/ (171725) 40 Random Allocation/ (26967) 41 Controlled Clinical Trial/ (64098) 42 Control Groups/ (4194) 43 Clinical Trial/ (549766) 44 Double‐Blind Method/ (73417) 45 Single‐Blind Method/ (8388) 46 Placebos/ (129417) 47 Placebo Effect/ (271) 48 Cross‐Over Studies/ (21585) 49 Multicenter Study/ (46769) 50 Therapies, Investigational/ (382) 51 Research Design/ (414056) 52 Program Evaluation/ (55867) 53 Evaluation Studies/ (54946) 54 random.tw. (88168) 55 (controlled adj5 (trial$ or stud$)).tw. (130272) 56 (clinical adj5 trial).tw. (46741) 57 ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. (607058) 58 (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. (1024) 59 ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. (58882) 60 ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. (85295) 61 ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. (95822) 62 (coin adj5 (flip or flipped or toss)).tw. (56) 63 latin square.tw. (1124) 64 versus.tw. (245008) 65 (cross‐over or cross over or crossover).tw. (39465) 66 placebo$.tw. (112155) 67 sham.tw. (37685) 68 (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. (167922) 69 contols.tw. (45) 70 62 or 58 or 48 or 66 or 65 or 63 or 43 or 67 or 41 or 60 or 39 or 50 or 69 or 45 or 59 or 52 or 56 or 46 or 53 or 42 or 64 or 47 or 54 or 55 or 44 or 51 or 68 or 61 or 40 or 57 or 49 (2069902) 71 32 or 21 or 7 or 26 or 17 or 2 or 1 or 18 or 30 or 16 or 27 or 25 or 28 or 20 or 14 or 24 or 10 or 31 or 11 or 22 or 13 or 23 or 29 or 6 or 3 or 9 or 12 or 15 or 8 or 4 or 19 or 5 (465477) 72 38 and 71 and 70 (2021) 73 limit 72 to human (1692) 74 limit 72 to yr="2007‐current" (355) 75 from 74 keep 1‐355 (355) 76 from 75 keep 1‐355 (355)
Appendix 3. PsycINFO search strategy
1 exp Cerebrovascular Disorders/ (9239) 2 exp basal ganglia/ (12036) 3 exp cerebral ischemia/ (1219) 4 exp carotid arteries/ (361) 5 Stroke/ (6947) 6 exp vertebral artery dissection/ (0) 7 (stroke$ or poststroke$ or post‐stroke$ or cerebral vascular or cerebrovascular or cva$).tw. (13149) 8 ((cerebral or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or apoplexy or emboli$)).tw. (2480) 9 ((cerebral or brain$ or subarachnoid) adj5 (haemorrhage or hemorrhage or haematoma or hematoma or bleed)).tw. (687) 10 ((trauma$ or acquired) adj5 brain injur$).tw. (6499) 11 exp brain damage/ (20526) 12 Traumatic Brain Injury/ (5669) 13 exp brain concussion/ (427) 14 exp head injuries/ (3939) 15 exp encephalitis/ (1000) 16 exp meningitis/ (252) 17 (encephalitis or meningitis).tw. (2401) 18 exp brain neoplasms/ (899) 19 ((brain or cerebr$) adj5 (neoplasm$ or lesion$ or tumor$ or tumour$)).tw. (8314) 20 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (57647) 21 exp perceptual distubances/ (0) 22 exp perception/ (222011) 23 (perception or visuo?perception or visual?perception or agnosia or prosopagnosia or stereognosis).tw. (110968) 24 ((percept$ or visuo?percept$ or visual?percept$ or visuo?spatial or visual?spatial or visuo?construct$ or visual?construct$) adj5 (disorder$ or impairment$ or problem$ or abilit$ or difficult$ or deficit$ or training or re?training or remediation or rehabilitation or intervention therapy)).tw. (15629) 25 21 or 22 or 23 or 24 (286572) 26 exp sampling/ (1968) 27 best practices/ (244) 28 treatment effectiveness evaluation/ (10973) 29 Control Groups/ (586) 30 Clinical Trial/ (3120) 31 clinical trials/ (3120) 32 exp Placebo/ (2384) 33 cultural differences/ (29215) 34 Research Design/ (7427) 35 program evaluation/ (8022) 36 evaluation/ (11057) 37 random.tw. (28273) 38 (controlled adj5 (trial$ or stud$)).tw. (22707) 39 (clinical adj5 trial).tw. (5224) 40 ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. (133153) 41 (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. (271) 42 ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. (5558) 43 ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. (16996) 44 ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. (14175) 45 (coin adj5 (flip or flipped or toss)).tw. (65) 46 latin square.tw. (384) 47 versus.tw. (42047) 48 (cross‐over or cross over or crossover).tw. (4655) 49 placebo$.tw. (22867) 50 sham.tw. (5390) 51 (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. (74075) 52 contols.tw. (4) 53 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 (354273) 54 53 and 20 and 25 (1090) 55 limit 54 to yr="2007‐current" (165) 56 limit 55 to human (147) 57 from 56 keep 1‐147 (147) 58 from 57 keep 1‐147 (147)
Appendix 4. CINAHL (EBSCO)
Search strategy
S109.S107 and S108 S108. Limiters ‐ Published Date from: 200701‐200912 S107.S29 and S61 and S106 S106.S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 or S73 or S74 or S75 or S76 or S77 or S78 or S79 or S80 or S81 or S82 or S83 or S84 or S85 or S86 or S87 or S88 or S89 or S90 or S91 or S92 or S93 or S94 or S95 or S96 or S97 or S98 or S99 or S100 or S101 or S102 or S103 or S104 or S105 S105.controls S104.assign* or alternate or allocat* or counterbalance* or multiple baseline S103.sham S102.placebo* S101.cross‐over or cross over or crossover S100.versus S99.latin square S98.coin N5 toss S97.coin N5 flipped S96.coin N5 flip S95.trebl* N5 blind* S94.trebl* N5 mask* S93.tripl* N5 mask* S92.tripl* N5 blind* S91.doubl* N5 blind* S90.doubl* N5 mask* S89.singl* N5 mask* S88.singl* N5 blind* S87.control N5 manage* or experiment* N5 manage* or conservative N5 manage* S86.control N5 procedure or experiment* N5 procedure or conservative N5 procedure S85.control N5 treatment or experiment* N5 treatment or conservative N5 treatment S84.control N5 therapy or experiment* N5 therapy or conservative N5 therapy S83.multicenter N5 stud* or multicentre N5 stud* or therapeutic N5 stud* S82.multicenter N5 trial* or multicentre N5 trial* or therapeutic N5 trial* S81.quasi‐random* or quasi random or pseudo‐random* or pseudo random S80.intervention N5 group* or intervention N5 subject* or intervention N5 patient* S79.experiment* N5 group* or experiment* N5 subject* or experiment N5 patient* S78.control N5 group* or control N5 subject* or control N5 patient* S77.treatment N5 group* or treatment N5 subject* or treatment N5 patient* S76.(ZT "clinical trial") or (ZT "research") or (ZT "systematic review") S75.controlled n5 stud* S74.controlled n5 trial* S73.clinical n5 trial S72.random S71.(MH "Formative Evaluation Research") or (MH "Evaluation Research") or (MH "Summative Evaluation Research") or (MH "Concurrent Prospective Studies") S70.(MH "Program Evaluation") S69.(MH "Study Design") or (MH "Cross Sectional Studies") S68.(MH "Multicenter Studies") S67.(MH "Crossover Design") S66.(MH "Placebos") or (MH "Placebo Effect") S65.(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies") S64.(MH "Control Group") S63.(MH "Resource Allocation") or (MH "Random Sample") S62.(MH "Clinical Trials") S61.S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 S60.abilit* N5 Visual?percept* or difficult* N5 visual?percept* and deficit* N5 visual?percept* S59.rehabilitation N5 percept* or intervention therapy N5 percept* S58.rehabilitation N5 visuo?percept* or intervention therapy N5 visuo?percept* S57.rehabilitation N5 visual?percept* or intervention therapy N5 visual?percept* S56.rehabilitation N5 visual?spatial or intervention therapy N5 visual?spatial S55.rehabilitation N5 visuo?spatial or intervention therapy N5 visuo?spatial S54.rehabilitation N5 visuo?construct* or intervention therapy N5 visuo?construct* S53.rehabilitation N5 visual?construct* or intervention therapy N5 visual?construct* S52.training N5 visual?construct* or re?training N5 visual?construct* or remediation N5 visual?construct* S51.training N5 visuo?construct* or re?training N5 visuo?construct* or remediation N5 visuo?construct* S50.training N5 visuo?spatial or re?training N5 visuo?spatial or remediation N5 visuo?spatial S49.training N5 visual?spatial or re?training N5 visual?spatial or remediation N5 visual?spatial S48.training N5 visual?percept* or re?training N5 visual?percept* or remediation N5 visual?percept* S47.training N5 visuo?percept* or re?training N5 visuo?percept* or remediation N5 visuo?percept* S46.training N5 percept* or re?training N5 percept* or remediation N5 percept* S45.abilit* N5 percept* or difficult* N5 percept* or deficit* N5 percept* S44.abilit* N5 visual?construct* or difficult* N5 visual?construct* or deficit* N5 Visual?construct* S43.abilit* N5 visuo?construct* or difficult* N5 visuo?construct* or deficit* N5 Visuo?construct* S42.abilit* N5 visuo?percept* or difficult* N5 visuo?percept* or deficit* N5 Visuo?percept* S41.abilit* N5 visual?spatial or difficult* N5 visual?spatial or deficit* N5 Visual?spatial S40.abilit* N5 visuo?spatial or difficult* N5 visuo?spatial or deficit* N5 Visuo?spatial S39.disorder* N5 visuo?spatial or impairment* N5 visuo?spatial or problem* N5 Visuo?spatial S38.disorder* N5 visual?construct* or impairment* N5 visual?construct* or problem* N5 Visual?construct* S37.disorder* N5 visuo?construct* or impairment* N5 visuo?construct* or problem* N5 Visuo?construct* S36.disorder* N5 visual?spatial or impairment* N5 visual?spatial or problem* N5 Visual?spatial S35.disorder* N5 visual?percept* or impairment* N5 visual?percept* or problem* N5 Visual?percept* S34.disorder* N5 visuo?percept* or impairment* N5 visuo?percept* or problem* N5 Visuo?percept* S33.disorder* N5 percept* or impairment* N5 percept* or problem* N5 percept* S32.perception or visuo?perception or visual?perception or agnosia or prosopagnosia or stereognosis S31.(MH "Perception+") S30.(MH "Perceptual Disorders+") S29.S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 S28.brain N5 tumour* or cerebr* N5 tumour* S27.brain N5 tumor* or cerebr* N5 tumor* S26.brain N5 lesion* or cerebr* N5 lesion* S25.brain N5 neoplasm* or cerebr* N5 neoplasm* S24.cerebral N5 bleed or brain* N5 bleed or subarachnoid N5 bleed S23.cerebral N5 haematoma or brain* N5 haematoma or subarachnoid N5 haematoma S22.cerebral N5 hematoma or brain* N5 hematoma or subarachnoid N5 hematoma S21.cerebral N5 hemorrhage or brain* N5 hemorrhage or subarachnoid N5 hemorrhage S20.cerebral N5 haemorrhage or brain* N5 haemorrhage or subarachnoid N5 haemorrhage S19.(MH "Brain Neoplasms+") S18.encephalitis or meningitis S17.(MH "Meningitis, Viral") S16.(MH "Encephalitis+") S15.(MH "Central Nervous System Infections+") S14.acquired n5 brain injur* S13.trauma* n5 brain injur* S12.stroke* or poststroke* or post‐stroke* or cerebral vascular or cerebrovascular or cva* S11.(MH "Head Injuries+") S10.(MH "Brain Damage, Chronic") S9.(MH "Vertebral Artery Dissections") S8.(MH "Intracranial Hemorrhage+") S7.(MH "Cerebral Embolism and Thrombosis") S6.(MH "Intracranial Arterial Diseases+") S5.(MH "Anoxia") S4.(MH "Stroke") S3.(MH "Carotid Artery Diseases+") S2.(MH "Cerebral Ischemia+") or (MH "Brain Abscess+") or (MH "Brain Concussion+") or (MH "Brain Injuries") or (MH "Brain Damage, Chronic") S1.(MH "Cerebrovascular Disorders") or (MH "Basal Ganglia Cerebrovascular Disease+")
Data and analyses
Comparison 1.
Perceptual intervention versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Functional training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Mixed sensory stimulation and strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Repetition | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Activity at scheduled end of intervention | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [‐1.60, 3.48] |
| 2.1 Functional training | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Sensory stimulation | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [‐1.60, 3.48] |
| 2.3 Strategy training | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Mixed sensory stimulation and strategy training | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Repetition | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Perceptual impairment up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Functional training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Mixed sensory stimulation and strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Repetition | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Perceptual impairment at scheduled end of intervention | 2 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.29, 0.43] |
| 4.1 Functional training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Sensory stimulation | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.59, 0.77] |
| 4.3 Strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Mixed sensory stimulation and strategy training | 1 | 86 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.36, 0.49] |
| 4.5 Repetition | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Quality of life up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Functional training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Mixed sensory stimulation and strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Repetition | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Effects on carer up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Functional training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Mixed sensory stimulation and strategy training | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Repetition | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Discharged to institutional care | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Functional training | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Sensory stimulation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Strategy training | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed sensory stimulation and strategy training | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 Repetition | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Falls or accidents | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Functional training | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Sensory stimulation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Strategy training | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Mixed sensory stimulation and strategy training | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Repetition | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Perceptual interventions: direct comparisons
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Functional training versus sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Activity at scheduled end of intervention | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.13, 0.75] |
| 2.1 Functional training versus sensory stimulation | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.13, 0.75] |
| 3 Perceptual impairment up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Functional training versus sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Perceptual impairment at scheduled end of intervention | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.42, 0.45] |
| 4.1 Functional training versus sensory stimulation | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.42, 0.45] |
| 5 Quality of life up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Functional training versus sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Effects on carer up to 6 months of follow‐up | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Functional training versus sensory stimulation | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Discharged to institutional care | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Functional training versus sensory stimulation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Falls or accidents | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Functional training versus sensory stimulation | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 4, 2011
| Date | Event | Description |
|---|---|---|
| 9 May 2008 | Amended | Converted to new review format. |
Differences between protocol and review
Since publication of the protocol we have re‐structured the text and reporting of the Results to be compatible with the Review Manager software (RevMan 2008).
We could not analyse certain outcomes as no data were available. As another review (Jutai 2003) included apraxia within their definition of perception we added a comment that we excluded apraxia from ours and added a reference to the Cochrane Review of apraxia (West 2008).
At the time of publishing our protocol we had planned to handsearch five journals but, when it came to carrying out the review, expansion of the Master List of journals meant this was reduced to two.
Characteristics of studies
Characteristics of included studies [ordered by year of study]
| Methods | Setting: Detroit Rehabilitation Institute Sites: 1 Countries: USA Trial design: parallel Recruitment dates: not specified | |
| Participants | Numbers: 78 randomised, 65 entered programme, 47 analysed Definition of stroke: this is probably only clinical, given the age of the study, though this is not explicitly stated. It does state a clear history of stroke involving the right cerebral hemisphere Time since stroke: eligible if 14 to 180 days at admission, "average" 55.2 days Time since start of rehabilitation: not specified Comorbidities affecting disability reported: no Excluded: yes – excluded if unable to co‐operate, vision too poor to read, inadequate cardiovascular reserve, amputee, history of psychiatric care, previous stroke, neurologic disorder Tools used to define perceptual disorders: PCMF test battery Severity of perceptual disorders: not specified Included visuospatial neglect: yes; frequency/severity not stated Age: average for whole sample 58.5 years (eligibility limits: 40 to 70 years) Sex: Groups "comparable" (but numbers not stated) Race/ethnicity: not specified | |
| Interventions | Described adequately to replicate: no, but treatment procedures were outlined in a manual (apparently unpublished) and a final report (also apparently unpublished) Broad class: sensory stimulation Specific nature: the intervention group received an individually tailored programme directed to patients' perceptual and cognitive deficits. This included gait training, visual tracking, object identification, and assembly tasks. When motor function was primarily involved, patients' attention was drawn to their sensory deficits The control group received treatment directed at patients' motor deficits according to "orders written by the attending physician". Motor skill tasks were completed until functional skills were achieved Frequency: 20 treatment days (where less than 3 consecutive treatment days could be lost to acute illness, and less than 10 scheduled hours lost for any reason) Duration (from first to last treatment): 20 treatment days (presumably consecutive) Profession of 'therapist': separate teams of therapists worked with control and experimental groups in physical therapy and occupational therapy Co‐interventions reported: not stated | |
| Outcomes | Reported:
Adverse events:
|
|
| Notes | No additional information available from authors, confirmed by contact with co‐author Dr Blumenthal who also confirmed that the first author has since died | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Not fully described, but referenced development of "a random system" developed by Richard Remington PhD, a pioneer of randomised trials in the USA |
| Allocation concealment? | High risk | Even if sequence concealed, randomisation occurred before pre‐treatment evaluation, which 13/78 (17%) eligible patients failed to complete. Allocation is likely to have been clear before final assessment of eligibility |
| Blinding? All outcomes | High risk | No reference to blinding |
| Incomplete outcome data addressed? All outcomes | High risk | Loss to follow‐up at least 28% (18/65), arguably 40% (31/78) |
| Free of selective reporting? | High risk | Tables of F‐ratio and whether P < 0.01 for all outcomes. Only reported mean values (in text) for statistically significant outcomes |
| Free of other bias? | High risk | "Separate teams of therapists worked with control and experimental procedures". Differential effects of therapy are not identifiable in the sense that they could be equally attributed to effects of the teams |
| Methods | Setting: Rivermead Rehabilitation Centre Sites: 1 Countries: UK Trial design: parallel Recruitment dates: June 1981 to end November 1982 | |
| Participants | Number: 33 analysed Definition of stroke: unspecified; included 21 (64%) with stroke, 6 (18%) each with head injury and subarachnoid haemorrhage Time since stroke: 2.7 months, SD ≈ 1.8 (Table 4), mean (SD) in experimental group 2.4 (1.0) months, control group 3.1 (2.4) months Comorbidities affecting disability: not reported Excluded: no Tools used to define perceptual disorders: Rivermead Perceptual Assessment Battery (RPAB) Severity of perceptual disorders: 3 or more subtests on which performance was > 2 SDs from mean for normal non‐brain damaged adult score Included visuospatial neglect: yes probably: frequency/severity: not stated, but they did not exclude people with neglect Age: mean 50 years, range 17 to 69 years; mean (SD) in experimental group 49 (14.6) years, control group 51 (16.0) years Sex: 17 (52%) male: experimental group 9 (53%), control group 8 (50%) | |
| Interventions | Described adequately to replicate: no Broad class: sensory stimulation Specific nature: the experimental group practiced various perceptual tasks (e.g. stick length sorting, picture lotto, colour matching squares, shape recognition games) of the type commonly used in occupational therapy departments The control group received the same amount of therapy time but carried out activities that were designed to improve physical rather than perceptual abilities (e.g. games, craft work, gardening) Frequency: 4 hours per week Duration (from first to last treatment): 4 weeks Profession of 'therapist': not explicitly stated but 'Acknowledgements' section suggests that occupational therapists carried out the experimental intervention and also provided activities for the control group Co‐interventions reported: any occupational therapy provided in addition to the 4 study hours per week was aimed at gross motor performance and provided to both groups Excluded: no | |
| Outcomes | Reported:
Adverse events:
Specified primary outcome(s): no, 16 tests of RPAB and ADL presented with no apparent hierarchy Time points for outcomes: end of intervention Total number of outcomes: 17, includes 16 measures of perception in RPAB but no overall score Possible ceiling/floor effects: evident from presented means and SD for some subtests of the RPAB Assessed by: third party "unaware of the treatment group" |
|
| Notes | Personal communication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No detail beyond "patients were randomly allocated" |
| Allocation concealment? | Unclear risk | No information on process |
| Blinding? All outcomes | High risk | Intended blinded outcome assessment, but not reported success |
| Incomplete outcome data addressed? All outcomes | Low risk | No suggestion of loss to follow‐up |
| Free of selective reporting? | Low risk | No suggestion of unreported outcomes |
| Free of other bias? | Unclear risk | Original eligibility criteria restricted entry to right‐hemisphere stroke patients. Later extended to head injury, subarachnoid haemorrhage and left hemisphere stroke "to obtain reasonable numbers within the time". Not clear what interim analyses were undertaken, and possible consequences for interpretation of the final data |
| Methods | Setting: rehabilitation hospital Sites: 1 Countries: Canada Trial design: parallel (first 10 recruited participants only) Recruitment dates: not reported Duration: 30 months | |
| Participants | Number: 10 randomised, further 10 systematically allocated. Report pools data Definition of stroke: included radiology (all had CT scan) Time since stroke: not reported separately for randomised participants; range within 1 to 5 months Time since start of rehabilitation: not reported separately for randomised participants Comorbidities affecting disability: not reported Excluded: previous stroke, pre‐existing visual impairment, cataracts, glaucoma, diabetes, psychological distress Tools used to define perceptual disorders: participants not chosen on basis of perceptual impairment. Authors state 5 measures of visuospatial functioning were taken: Rey‐Osterreith (copy and recall), WAIS block design, Raven’s Coloured Matrices, Benton’s Line Orientation. These may test memory and executive functioning as well as perception Severity of perceptual disorders: not reported separately for randomised participants Included visuospatial neglect: unclear; if ‘yes’, frequency/severity Age: not reported separately for randomised participants Sex: not reported separately for randomised participants Race/ethnicity: not stated | |
| Interventions | Described adequately to replicate: no Broad class: sensory stimulation Specific nature: the experimental intervention consisted of Bracy's computerised visuospatial training package, which comprised 7 different visuospatial exercises (paddle ball, fine motor, maze, cube in the box, line orientation I, line orientation II, visual perception test) The control group received routine rehabilitation therapies provided by the hospital Frequency: 3 x 30‐minute sessions per week Duration (from first to last treatment): 4 weeks Profession of 'therapist': 2 trained research assistants (professional background unknown) Co‐interventions: all participants received routine rehabilitation therapies provided by the hospital, including physiotherapy and occupational therapy treatment Excluded: no | |
| Outcomes | Reported:
Specified primary outcome(s): no, impairment and activity level measures with no explicit hierarchy. Addressed "large number of test variables" using Dunn‐Sidak procedure to adjust nominal significance level rather than through hierarchy of outcome measures Time points for outcomes: end of intervention Total number of outcomes: > 40 in battery (detailed in appendix) Possible ceiling/floor effects ‐ identified by trialists: no; identified by reviewer: yes (e.g. Table 2 Barthel) Assessed by: third party for neuropsychological battery, nurses for functional indices and physiotherapists for mobility |
|
| Notes | Only 10 randomised participants. All results given for 19 completers or 20 allocated participants. Would need data from authors for inclusion in meta‐analyses. Unsuccessful attempts to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not specified beyond "the first 10 patients were randomly assigned" |
| Allocation concealment? | Unclear risk | No information on process |
| Blinding? All outcomes | High risk | Partial: intended blinded assessment of ADL but not of perceptual impairments. Not reported success of blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | 1 discharged prior to outcome assessment. Not clear whether this was one of the first 10 randomised participants |
| Free of selective reporting? | High risk | Results tabulated only for statistically significant results within test battery |
| Free of other bias? | High risk | Participants 11 to 20 allocated systematically "so as to match the already assigned patients" |
| Methods | Setting: rehabilitation hospital Sites: 1 Countries: USA Trial design: matched pairs allocated to parallel treatment arms. 'Match' determined by severity of injury (mild, moderate, severe), sex, age (within 10 years) and time since injury (up to 6 months, 6 to 18 months, more than 18 months) (details obtained from personal communication) Recruitment dates: 1995 to 1996 (personal communication) Duration: a little over 12 months (personal communication) | |
| Participants | Numbers: 30 allocated Definition of stroke: not stated; 2 (7%) participants post‐CVA, others trauma‐related Time since injury: mean 5 months (range 2 to 12 months) Time since start of rehabilitation: recruited at start of cognitive rehabilitation programme (personal communication) Comorbidities affecting disability: not recorded (personal communication) Excluded: previous injury, previous cognitive rehabilitation, visual impairment not corrected by spectacles, language impairment, neglect, major physical limitations of upper limb (personal communication) Tools used to define perceptual disorders: condition considered as 'visual processing' rather than perception. Assessed by neuropsychological battery to enter specialist cognitive rehabilitation programme (personal communication) Severity of perceptual disorders: pre‐ and post‐tests were computer‐based visual processing tasks Included visuospatial neglect: excluded (personal communication) Age: mean 38 years (range 21 to 56 years) Sex: 22 (73%) male Race/ethnicity: not recorded (personal communication) | |
| Interventions | Described adequately to replicate: no Broad class: sensory stimulation coupled with strategy training Specific nature: the intervention group received 4 sessions of an 'IQ Builder' computer programme (specifically the sections 'Memory for numbers' and 'Memory for letters') together with instruction in the use of 3 compensatory strategies (verbalisation, chunking, pacing) The control group was given the same computer programme for the same length of time, but did not receive instruction in the use of compensatory strategies Frequency: 1 hour per week Duration (from first to last treatment): 4 weeks (study duration was 6 weeks, but only 4 weeks involved active therapy) Profession of 'therapist': occupational therapist (personal communication) Co‐interventions: regular attendance at outpatient "cognitive rehabilitation program" Excluded: no | |
| Outcomes | Reported:
Specified primary outcome(s): no; multiple outcomes with no specified hierarchy Time points for outcomes: 1 week after end of intervention Total number of outcomes: 9; computer‐based tasks (address copying, data entry and reading) with measures of speed and accuracy, the Paced Auditory Serial Addition Task and the Matching Accuracy Test (one words, one numerical) (personal communication) Possible ceiling/floor effects: unclear Assessed by: third party; computer‐based assessments with data collected by staff member (speech pathologist) blinded to allocation |
|
| Notes | Matched pairs design not accounted for in analysis Reported comparisons of outcome using repeated measures ANOVA with Greenhouse‐Geisser correction cite F statistics and P‐values only. Not possible to determine group differences from reported data Personal communication with author confirmed raw data have been lost and no record of group means or differences reported in other publications including PhD thesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Cases screened for eligibility by, and gave consent to, neuropsychologist making referral. Cases allocated according to coin‐toss by treating therapist, or to reverse of previous allocation if earlier match had been enrolled. Effectively stratified randomisation in blocks of size two (personal communication) |
| Allocation concealment? | High risk | Coin toss not verifiable and allocation apparent for every second participant |
| Blinding? All outcomes | High risk | Computer‐based assessment supervised by blinded speech pathologist. All participants received same contact time from single therapist (Dirette 1999) and were unaware as to which intervention was 'experimental' (personal communication) |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow‐up. Small number of cases allocated but no subsequent match identified. These were omitted from analyses (personal communication) |
| Free of selective reporting? | Low risk | All collected outcomes analysed, and group comparison reported in consistent manner (personal communication). Selective reporting of 'auxiliary analyses' |
| Free of other bias? | High risk | Analysis does not account for matched pairs design |
| Methods | Setting: hospital (Nottingham Stroke Unit) Sites: 1 Countries: UK Trial design: parallel Recruitment dates: May 1992 to July 1994 (excluding February and March 1994 when occupational therapist was unwell) | |
| Participants | Numbers: 80 randomised, 79 analysed (1 died before completion) Definition of stroke: patients on stroke unit after randomisation in trial of stroke unit versus non‐stroke unit care Time since stroke: mean 34 days (range 14 to 84 days) Transfer of training approach (ToT) group: mean 37.7 days (SD 16.6), range 16 to 84, post‐stroke Functional approach group: mean 31.2 days (SD 10.1), range 14 to 56, post‐stroke Since start of rehabilitation: 2 weeks Comorbidities affecting disability ‐ Reported: no Excluded: patients unable to be assessed on the RPAB; participants with insufficient use of one hand to complete RPAB and complete the test tasks; patients unable to transfer with 2 nurses or fewer; patients with a planned discharge date; patients unable to do 2 or more activity of daily living tasks Tools used to define perceptual disorders: RPAB Severity of perceptual disorders: 4 or more low scores (> 2 SDs below mean) from 16 tests Included visuospatial neglect: yes If ‘yes’, frequency/severity: 42 participants (53%) Mean age: overall 69 years; ToT group mean (SD) 70 years (9.1), range 47 to 84 years; FA group mean (SD) 68 years (11.4), range 26 to 86 years Sex: overall 40 (50%) male; ToT group 18 (45%) male; FA group 22 (55%) male Race/ethnicity: not specified | |
| Interventions | Described adequately to replicate: no; the published paper reports that further details are to be published in a later article, but no such article has been identified Broad class: 2 types compared with each other: sensory stimulation (termed by the authors "Transfer of training") and functional training (termed "Functional approach") Specific nature: Transfer of training involved patients practicing particular perceptual tasks in order to produce improvement on tasks with similar perceptual elements, i.e. the cause is treated. For example, patients with difficulty dressing due to spatial relations problems are allowed to practise a spatial task such as cube copying with the expectation that this will produce functional improvement in dressing The Functional approach involved patients repeatedly practicing everyday tasks (usually activities of daily living, such as dressing), i.e. the symptom is treated. So, by practicing dressing the patient will learn to dress, but the expectation is that the underlying perceptual problems remain The authors acknowledge that the basic strategies used in treatment were similar for both groups Frequency: 2.5 hours per week (5 x 30 minute sessions) Duration (from first to last treatment): 6 weeks Profession of 'therapist': occupational therapists (research OT and ward‐based OT) Co‐interventions reported: additional general occupational therapy treatment Excluded: no | |
| Outcomes | Reported:
Adverse events:
Specified primary outcome(s): 5 'main outcomes': RPAB, Barthel ADL and Edmans ADL, with each ADL scale assessed by both nurses and therapists Time points for outcomes: end of intervention (6 weeks) Total number of outcomes: 25 reported; length of stay, number of appointments and total duration of treatment, total scores on RPAB, Barthel (2 assessments), Edmans ADL (2 assessments), RMA gross function score, 16 subtests of RPAB Possible ceiling/floor effects: yes (e.g. ceiling in Barthel, floor in RMA) Assessed by: third party, independent assessor (in discussion with nurses, and separate assessment by ward therapist for ADL measures) |
|
| Notes | Personal communication and primary data provided by Dr Edmans | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number tables |
| Allocation concealment? | Low risk | Personal communication. Dr Edmans prepared sequentially numbered, sealed envelopes, opened at recruitment with witness. Not adequate in that researcher prepared list, but assessed as low risk of bias from assurance of inability to remember sequence |
| Blinding? All outcomes | High risk | Intended independent assessor for outcomes covered by this review, but not reported success |
| Incomplete outcome data addressed? All outcomes | Low risk | No withdrawals and only one (1%) death |
| Free of selective reporting? | Low risk | Outcomes described at both impairment and disability levels, and reported in equal detail regardless of statistical significance |
| Free of other bias? | Low risk | |
| Methods | Setting: rehabilitation hospital Sites: 1 Countries: Canada Trial design: parallel Recruitment dates: start and end not reported | |
| Participants | Numbers: 97 randomised, 86 analysed Definition of stroke: medical (hospital) records (personal communication) Time since stroke: mean 78 days: experimental group mean (SD) 91 days (52); controls 67 days (28) Time since rehabilitation: mean ≈ 52 days Comorbidities affecting disability reported: yes Excluded: yes: standard contraindications to driving, plus bilateral lesion, cerebellar or brainstem stroke, and severe cognitive, perceptual, comprehension or motor deficit Tools used to define perceptual disorders: this test battery, which has been described elsewhere, included the Complex Reaction Timer, Motor‐Free Visual Perception Test33 (MVPT), Single and Double Letter Cancellation Test, 34 Money Road Map Test of Direction Sense, 35 Trail Making Test (TMT) Parts A and B, 36 Bells test, 37 and Charron test 38. Collectively, these tests provide information on overall visuoperceptual skills, including visual scanning ability, reaction time to visual stimuli, figure ground discrimination, spatial relations, visual memory, visual processing time, and direction sense Severity of perceptual disorders: exclusion criteria included "severe perceptual deficit", yet "severe visual processing dysfunction" was one of the randomisation strata Experimental group: 28% mild, 51% moderate, 21% severe Control group: 28% mild, 54% moderate, 18% severe Included visuospatial neglect: not reason for exclusion. Only excluded if of such severity that driving licence revoked (personal communication) Age: mean 66 years: experimental group mean (SD) 65 years (11.4), controls 66 years (8.9) Sex: 70 (72%) male: experimental group 35 (74%), controls 35 (70%) Race/ethnicity: 67 (69%) French speakers, 30 (31%) English speakers | |
| Interventions | Described adequately to replicate: no Broad class: 2 types ‐ sensory stimulation coupled with strategy training Specific nature: the experimental intervention comprised 4 commercially available computerised software programmes (Tetris, Othello, Mastermind, Jigs@w Puzzle) commonly used by occupational therapists to retrain perceptual and cognitive functions. The OT selected the simplest level then increased complexity, provided verbal suggestions and taught appropriate problem‐solving strategies The control intervention comprised a computerised treatment using the 'Useful Field of View' (UFOV) that targeted visual processing speed, visual divided attention and visual selective attention Frequency: 2 to 4 sessions per week (each 30 to 60 minutes) Duration (from first to last treatment): scheduled 20 sessions at 3 per week Profession of 'therapist': occupational therapist Co‐interventions reported: in addition to the interventions described above, all participants received 4 sessions of physical retraining on the Baltimore Therapeutic Equipment work simulator Excluded: no | |
| Outcomes | Reported:
Adverse events:
Specified primary outcome(s): pass/fail of on‐road driving evaluation Time points for outcomes: end of intervention Total number of outcomes: 31 ‐ Driving evaluation, 21 visuoperception scores, 9 tests of everyday attention (TEA) Possible ceiling/floor effects: possible in visuoperception scores Assessed by: third party, independent occupational therapist for impairment, plus driving instructor for on‐road evaluation |
|
| Notes | Barbara Mazer provided additional information and clarification (September 2009). Has another study prepared for submission, see 'Characteristics of ongoing studies'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random number tables in stratified blocks |
| Allocation concealment? | Low risk | No description of process. From personal communication, statistician (third party) prepared sequential envelopes independently |
| Blinding? All outcomes | High risk | Intended blinded assessment, but correctly identified for 79% of participants |
| Incomplete outcome data addressed? All outcomes | Low risk | Explicit reasons given for missing outcome evaluation in 13 (13%) participants. None clearly related to intervention: decided not to return to driving (5); became medically unfit (3); died (3); moved (1); not legally able to drive (1) |
| Free of selective reporting? | Low risk | Does not appear selective |
| Free of other bias? | Low risk | |
ADL: activities of daily living CT: computerised tomography CVA: cerebrovascular accident PCMF: Percept‐concept‐motor function QoL: quality of life RMA: Rivermead Motor Assessment RPAB: Rivermead Perceptual Assessment Battery SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beschin 2005 | Not a randomised controlled trial |
| Carter 1983 | No evidence from the paper that the participants had perceptual deficits |
| Connor 2002 | Not a randomised controlled trial |
| Flynn 2000 | Not a randomised controlled trial |
| Gordon 1985 | Not a randomised controlled trial |
| Lincoln 1997 | Not a perceptual intervention |
| Morioka 2008 | The participants had hemiplegia and received "sensory perception" exercises but did not appear to have perceptual problems |
| Perez 1997 | Participants did not have perceptual deficits |
| Rossi 1990 | Participants did not have perceptual problems |
| Shapovalenko 2008 | Not perceptual intervention for patients with perceptual deficits but a broad multifaceted intervention for movement, proprioception and cognitive functions |
| Shi 1994 | No clear evidence that the participants had perceptual deficits nor that the intervention was aimed at perceptual rehabilitation |
| Towle 1990 | Not a randomised controlled trial |
| Wagenaar 1992 | Participants had inattention not perceptual problems and the study design was single case rather than a randomised controlled trial. |
| Weinberg 1977 | Participants had neglect not perceptual problems and were included in Bowen 2007 |
| Weinberg 1979 | Unclear whether the participants had neglect or perceptual problems as defined by this review. Previously (for Bowen 2007) unable to obtain clarification from authors on eligibility and to confirm randomisation was used |
| Weinberg 1982 | Unclear whether the participants had neglect or perceptual problems as defined by this review. Previously (for Bowen 2007) unable to obtain clarification from authors on eligibility and to confirm randomisation was used |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Pilot randomised trial |
| Participants | 16 left hemiplegic stroke patients from an in‐patient rehabilitation unit, with visuoperceptual impairment. Awaiting reply from authors regarding whether all or some of these were in fact neglect patients |
| Interventions | Computerised visual perception rehabilitation with interactive patient‐computer interface applying motion tracking technology versus PSS CogRehab programme for 12 sessions (3 times a week, 30 minutes per session for 4 weeks) under the supervision of an occupational therapist |
| Outcomes | MMSE, MFVPT, Korean version of the modified Barthel Index, survey of patients' interest in the interventions |
| Notes | October 2009 ‐ second unsuccessful attempt to obtain clarification from authors on whether the participants were eligible for inclusion in this review |
| Methods | Not yet known |
| Participants | Stroke patients with visual perceptual problems |
| Interventions | Not yet known |
| Outcomes | Not yet known |
| Notes | Student thesis from 26 years ago difficult to obtain |
| Methods | Pilot randomised trial |
| Participants | 32 people with first acute (within 2 weeks) lacunar stroke and various types of cognitive problems possibly including some with perceptual problems |
| Interventions | 3 months of regular cognitive training by a neuropsychologist versus standard care without cognitive training |
| Outcomes | An extensive neuropsychological test battery was administered 3 months after baseline assessments, including assessment of visuospatial functions. Physiological measures were also taken but are not relevant to this review |
| Notes | Unable to obtain confirmation from authors on whether any of the 32 participants met our eligibility criteria |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Personal communication with Mazer in 2009 for her included study (Mazer 2003) revealed she has a relevant ongoing study |
Contributions of authors
Bowen and Knapp are research psychologists, Gillespie is a practising clinical psychologist, Nicolson is a health informatics researcher, and Vail is a statistician.
Bowen, Vail and Knapp wrote the protocol. All review authors formed a consensus on study inclusion and extracted the data from the included studies. At least two of the review authors independently performed quality assessment. Vail oversaw the analysis. Bowen and Gillespie led on interpretation of clinical and psychological issues. Bowen wrote the first draft of the review. All review authors contributed to the final draft of the review.
Declarations of interest
None known
New
References
References to studies included in this review
- Dirette DK, Hinojosa J, Carnevale GJ. Comparison of remedial and compensatory interventions for adults with acquired brain injuries. Journal of Head Trauma Rehabilitation 1999;14(6):595‐601. [DOI] [PubMed] [Google Scholar]
- Edmans JA, Webster J, Lincoln N. A comparison of two approaches in the treatment of perceptual problems after stroke. Clinical Rehabilitation 2000;14:230‐43. [DOI] [PubMed] [Google Scholar]
- Hajek VE, Kates MH, Donnelly R, McGree S. The effect of visuo‐spatial training in patients with right hemisphere stroke. Canadian Journal of Rehabilitation 1993;6(3):175‐86. [Google Scholar]
- Lincoln NB, Whiting SE, Cockburn J, Bhavnani G. An evaluation of perceptual retraining. International Rehabilitation Medicine 1985;7:99‐101. [DOI] [PubMed] [Google Scholar]
- Mazer B, Sofer S, Korner‐Bitensky N, Gelinas I, Hanley J, Wood‐Dauphinee S. Effectiveness of a visual attention retraining program on the driving performance of clients with stroke. Archives of Physical Medicine and Rehabilitation 2003;84:541‐50. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Schaeffer JN, Blumenthal FS, Grisell JL. Perceptual training in patients with left hemiplegia. Archives of Physical Medicine Rehabilitation 1971;52:163‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
- Beschin N, Cocchini G, Della Sala S. Effect of optokinetic stimulation, prism adaptation and tens on anosognosia for motor deficits. In: Proceedings of the The Stroke Association 10th Scientific Conference. 2005. [Google Scholar]
- Carter LT, Howard BE, O'Neil WA. Effectiveness of cognitive skill remediation in acute stroke patients. American Journal of Occupational Therapy 1983;37:320‐6. [DOI] [PubMed] [Google Scholar]
- Connor BB, Wing AM, Humphreys GW, Bracewell RM, Harvey DA. Errorless learning using haptic guidance: research in cognitive rehabilitation following stroke. Proceedings of the 4th International Conference on Disability, Virtual Reality and Associated Technologies. 2002:77‐83. [Google Scholar]
- Flynn A. Investigation into whether psychology led cognitive rehabilitation of attentional and visio‐spatial skills improves performance in these areas beyond spontaneous recovery. National Research Register2001, issue 1:https://portal.nihr.ac.uk/Profiles/NRR.aspx?Publication_ID=N0280012930 (accessed 21 January 2011).
- Gordon WA, Hibbard MR, Egelko S, Diller L, Shaver MS, Lieberman A, et al. Perceptual remediation in patients with right brain damage: a comprehensive program. Archives of Physical Medicine and Rehabilitation 1985;66:353‐9. [PubMed] [Google Scholar]
- Lincoln N, Drummond A, Berman P. Perceptual impairment and its impact on rehabilitation outcome. Disability and Rehabilitation 1997;19(6):231‐4. [DOI] [PubMed] [Google Scholar]
- Morioka S, Fujita H, Kataoka Y. Effects of hardness discrimination training on sitting postural balance in hemiplegic patients following stroke. International Journal of Stroke 2008;3 Suppl 1:350. [Google Scholar]
- Perez L, Wittink M, Kalra L. Visuospatial dysfunction and stroke rehabilitation. Cerebrovascular Diseases 1997;7 Suppl 4:83. [Google Scholar]
- Rossi PW, Kheyfets S, Reading MJ. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect . Neurology 1990;40:1597‐9. [DOI] [PubMed] [Google Scholar]
- Shapovalenko T, Baidova T, Isaeva T. Influence on proprioception and cognitive disorders in the early period of acute disturbances of cerebral blood flow. International Journal of Stroke. 2008;3 Suppl 1:348. [Google Scholar]
- Shi J, Wang SY, Huan SS, Huan QM, Wang CL. Activity of daily living (ADL) of patients with stroke after rehabilitation training. Chinese Journal of Physical Therapy 1994;17(3):160‐1. [Google Scholar]
- Towle D, Edmans J, Lincoln N. An evaluation of a group treatment programme for stroke patients with perceptual deficits. International Journal of Rehabilitation Research 1990;13:328‐35. [DOI] [PubMed] [Google Scholar]
- Wagenaar RC, Wieringen PC, Netelenbos JC, Meijer OG, Kuik DJ. The transfer of scanning training effects in visual inattention after stroke: five single case studies. Disability and Rehabilitation 1992;14:51‐60. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Diller L, Gordon WA, Gerstman LJ, Lieberman A, Lakin P, et al. Visual scanning training effect on reading related tasks in acquired right brain damage. Archives of Physical Medicine and Rehabilitation 1977;58:479‐86. [PubMed] [Google Scholar]
- Weinberg J, Diller L, Gordon WA, Gerstman LJ, Lieberman A, Lakin P, et al. Training sensory awareness and spatial organisation in people with right brain damage. Archives of Physical Medicine and Rehabilitation 1979;60:491‐6. [PubMed] [Google Scholar]
- Weinberg J, Piasetsky E, Diller L, Gordon W. Treating perceptual organization deficits in non‐neglecting RBD stroke patients. Journal of Clinical Neuropsychology 1982;4(1):59‐75. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Kang SH, Kim D‐K, Seo KM, Choi KM, Yoo JY, Sung SY, et al. A computerised visual perception rehabilitation programme with interactive computer interface using motion tracking technology ‐ a randomised controlled, single blinded, pilot clinical trial study. Clinical Rehabilitation 2009;23:434‐44. [DOI] [PubMed] [Google Scholar]
- Leer WB. . . Block Design Training with Stroke Patients: A Study on the Effects of Cognitive Retraining on Improving Certain Activities of Daily Living Skills. Michigan, USA: Michigan State University, 1984. [Google Scholar]
- Matz K, Teuschl Y, Eckhardt R, Herbst A, Dachenhausen A, Brainin M. Cognitive training in patients with first lacunar stroke ‐ a randomized pilot trial for the prevention of post‐stroke cognitive decline. Cerebrovascular Diseases 2007;23 Suppl 2:42. [Google Scholar]
References to ongoing studies
- Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
- Beaumont J, Davidoff J. In: Crawford JR, Parker DM, McKinlay WW editor(s). A handbook of neuropsychological assessment. Hove: Lawrence Earlbaum Associates, 1992. [Google Scholar]
- Bowen A, Lincoln NB. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD003586.pub2] [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence‐based cognitive rehabilitation:updated review of the literature from 1998‐2002. Archives of Physical Medicine and Rehabilitation 2005;86:1681‐92. [DOI] [PubMed] [Google Scholar]
- Hospital Episode Statistics. Department of Health, UK 2000‐2001.
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008;371:1612‐23. [DOI] [PubMed] [Google Scholar]
- Doyle S, Bennett S, Fasoli SE, McKenna KT. Interventions for sensory impairment in the upper limb after stroke. Cochrane Database of Systematic Reviews 2010, Issue 6. [Art. No.: CD006331. DOI: 10.1002/14651858.CD006331.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmans JA, Lincoln N. The recovery of perceptual problems after stroke and the impact on daily life. Clinical Rehabilitation 1991;5:301‐9. [DOI] [PubMed] [Google Scholar]
- Fisher AG. Development of a functional assessment that adjusts ability measures for task simplicity and rater leniency. In: Wilson M editor(s). Objective Measurement: Theory into Practice. Norwood: Ablex, 1994. [Google Scholar]
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [Art. No.: CD006073. DOI: 10.1002/14651858.CD006073.pub2] [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Bennett S, Koh CL, McKenna KT. Occupational therapy for cognitive impairment in stroke patients. Cochrane Database of Systematic Reviews 2010, Issue 9. [Art. No.: CD006430. DOI: 10.1002/14651858.CD006430.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutai JW, Bhogal SK, Foley NC, Teasell RW, Speechley MR. Treatment of visual perceptual disorders post stroke. Topics in Stroke Rehabilitation 2003;10(2):77‐107. [DOI] [PubMed] [Google Scholar]
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. In: Eisenberg NG, Grzesiak RC editor(s). Advances in Clinical Rehabilitation. New York: Springer, 1987. [PubMed] [Google Scholar]
- Kersel D, Marsh N, Havill D, Sleigh J. Neuropsychological functioning during the year following severe traumatic brain injury. Brain Injury 2001;15(4):283‐96. [DOI] [PubMed] [Google Scholar]
- Legg L, Drummond A, Langhorne P. Occupational therapy for patients with problems in activities of daily living after stroke. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003585.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3rd Edition. New York: Oxford University Press, 1995. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th Edition. Oxford: Oxford University Press, 2004. [Google Scholar]
- Mackay J, Mensah G, editors. The Atlas of Heart Disease and Stroke. Geneva: World Health Organization, 2004. [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
- McKenna K, Cooke D, Fleming J, Jefferson A, Ogden S. The incidence of visual perceptual impairments in patients with severe traumatic brain injury. Brain Injury 2006;20(5):507‐18. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269‐76. [DOI] [PubMed] [Google Scholar]
- Perel P, Edwards P, Wentz R, Roberts I. Systematic review of prognostic models in traumatic brain injury. BMC Medical Informatics and Decision Making 2006;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Riddoch J, Humphreys G. Birmingham Object Recognition Battery. Psychology Press, 1993. [Google Scholar]
- Ravensberg C, Tyldesley D, Rozendal R, Whiting H. Visual perception in hemiplegic patients. Archives of Physical Medicine and Rehabilitation 1984;65:304‐9. [PubMed] [Google Scholar]
- Warrington EK, James M. Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company, 1991. [Google Scholar]
- West C, Bowen A, Hesketh A, Vail A. Interventions for motor apraxia following stroke. Cochrane Database of Systematic Reviews 2008, Issue 1. [10.1002/14651858. CD001432.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting S, Lincoln NB, Cockburn J, Bhavnani G. The Rivermead Perceptual Assessment Battery. Windsor: NFER‐Nelson, 1985. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The ICD‐10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization, 1993. [Google Scholar]
- The World Health Report 2008 ‐ Primary Health Care (Now More Than Ever). World Health Organization2008:http://www.who.int/whr/2008/whr08_en.pdf (accessed 21 January 2011).


