Abstract
Background
Down's syndrome occurs when a person has three, rather than two copies of chromosome 21; or the specific area of chromosome 21 implicated in causing Down's syndrome. It is the commonest congenital cause of mental disability and also leads to numerous metabolic and structural problems. It can be life‐threatening, or lead to considerable ill health, although some individuals have only mild problems and can lead relatively normal lives. Having a baby with Down’s syndrome is likely to have a significant impact on family life.
Noninvasive screening based on biochemical analysis of maternal serum or urine, or fetal ultrasound measurements, allows estimates of the risk of a pregnancy being affected and provides information to guide decisions about definitive testing. However, no test can predict the severity of problems a person with Down’s syndrome will have.
Objectives
The aim of this review was to estimate and compare the accuracy of first trimester serum markers for the detection of Down’s syndrome in the antenatal period, both as individual markers and as combinations of markers. Accuracy is described by the proportion of fetuses with Down’s syndrome detected by screening before birth (sensitivity or detection rate) and the proportion of women with a low risk (normal) screening test result who subsequently had a baby unaffected by Down's syndrome (specificity).
Search methods
We conducted a sensitive and comprehensive literature search of MEDLINE (1980 to 25 August 2011), Embase (1980 to 25 August 2011), BIOSIS via EDINA (1985 to 25 August 2011), CINAHL via OVID (1982 to 25 August 2011), The Database of Abstracts of Reviews of Effectiveness (The Cochrane Library 25 August 2011), MEDION (25 August 2011), The Database of Systematic Reviews and Meta‐Analyses in Laboratory Medicine (25 August 2011), The National Research Register (Archived 2007), Health Services Research Projects in Progress database (25 August 2011). We did forward citation searching ISI citation indices, Google Scholar and PubMed ‘related articles’. We did not apply a diagnostic test search filter. We also searched reference lists and published review articles.
Selection criteria
We included studies in which all women from a given population had one or more index test(s) compared to a reference standard (either chromosomal verification or macroscopic postnatal inspection). Both consecutive series and diagnostic case‐control study designs were included. Randomised trials where individuals were randomised to different screening strategies and all verified using a reference standard were also eligible for inclusion. Studies in which test strategies were compared head‐to‐head either in the same women, or between randomised groups were identified for inclusion in separate comparisons of test strategies. We excluded studies if they included less than five Down's syndrome cases, or more than 20% of participants were not followed up.
Data collection and analysis
We extracted data as test positive or test negative results for Down's and non‐Down's pregnancies allowing estimation of detection rates (sensitivity) and false positive rates (1‐specificity). We performed quality assessment according to QUADAS (Quality Assessment of Diagnostic Accuracy Studies) criteria. We used hierarchical summary ROC meta‐analytical methods or random‐effects logistic regression methods to analyse test performance and compare test accuracy as appropriate. Analyses of studies allowing direct and indirect comparisons between tests were undertaken.
Main results
We included 56 studies (reported in 68 publications) involving 204,759 pregnancies (including 2113 with Down's syndrome). Studies were generally of good quality, although differential verification was common with invasive testing of only high‐risk pregnancies. We evaluated 78 test combinations formed from combinations of 18 different tests, with or without maternal age; ADAM12 (a disintegrin and metalloprotease), AFP (alpha‐fetoprotein), inhibin, PAPP‐A (pregnancy‐associated plasma protein A, ITA (invasive trophoblast antigen), free βhCG (beta human chorionic gonadotrophin), PlGF (placental growth factor), SP1 (Schwangerschafts protein 1), total hCG, progesterone, uE3 (unconjugated oestriol), GHBP (growth hormone binding protein), PGH (placental growth hormone), hyperglycosylated hCG, ProMBP (proform of eosinophil major basic protein), hPL (human placental lactogen), (free αhCG, and free ßhCG to AFP ratio. Direct comparisons between two or more tests were made in 27 studies.
Meta‐analysis of the nine best performing or frequently evaluated test combinations showed that a test strategy involving maternal age and a double marker combination of PAPP‐A and free ßhCG significantly outperformed the individual markers (with or without maternal age) detecting about seven out of every 10 Down's syndrome pregnancies at a 5% false positive rate (FPR). Limited evidence suggested that marker combinations involving PAPP‐A may be more sensitive than those without PAPP‐A.
Authors' conclusions
Tests involving two markers in combination with maternal age, specifically PAPP‐A, free βhCG and maternal age are significantly better than those involving single markers with and without age. They detect seven out of 10 Down's affected pregnancies for a fixed 5% FPR. The addition of further markers (triple tests) has not been shown to be statistically superior; the studies included are small with limited power to detect a difference.
The screening blood tests themselves have no adverse effects for the woman, over and above the risks of a routine blood test. However some women who have a ‘high risk’ screening test result, and are given amniocentesis or chorionic villus sampling (CVS) have a risk of miscarrying a baby unaffected by Down’s. Parents will need to weigh up this risk when deciding whether or not to have an amniocentesis or CVS following a ‘high risk’ screening test result.
Plain language summary
Screening tests for Down’s syndrome in first three months of pregnancy
Background Down's syndrome (also known as Down's or Trisomy 21) is an incurable genetic disorder that causes significant physical and mental health problems, and disabilities. However, there is wide variation in how Down's affects people. Some individuals are severely affected whilst others have mild problems and are able to lead relatively normal lives. There is no way of predicting how badly a baby might be affected. Expectant parents are given the choice to be tested for Down’s during pregnancy to assist them in making decisions. If a mother is carrying a baby with Down’s, then there is the decision about whether to terminate or continue with the pregnancy. The information offers parents the opportunity to plan for life with a Down’s child. The most accurate tests for Down’s involve testing fluid from around the baby (amniocentesis) or tissue from the placenta (chorionic villus sampling (CVS)) for the abnormal chromosomes associated with Down’s. Both these tests involve inserting needles through the mother's abdomen and are known to increase the risk of miscarriage. Thus, the tests are not suitable for offering to all pregnant women. Rather, tests that measure markers in the mother’s blood, urine or on ultrasound scans of the baby are used for screening. These screening tests are not perfect, they can miss cases of Down’s and also give a ‘high risk’ test result to a number of women whose babies are not affected by Down’s. Thus, pregnancies identified as ‘high risk’ using these screening tests require further testing using amniocentesis or CVS to confirm a diagnosis of Down’s.
What we did The aim of this review was to find out which of the blood screening tests done during the first three months of pregnancy are the most accurate at predicting the risk of a pregnancy being affected by Down's. We looked at 18 different blood markers that can be used alone or in combination, taken before 14 weeks gestation, thus creating 78 screening tests fro Down’s. We found 56 studies, involving 204,759 pregnancies of which 2113 had pregnancies affected by Down's.
What we found For the first 14 weeks of pregnancy, the evidence supports the use of the double test of two blood markers; pregnancy‐associated plasma protein A (PAPP‐A) and free beta human chorionic gonadotrophin (βhCG), in combination with the mother's age. This test detects around seven out of every 10 (68%) pregnancies affected by Down's. It is common practice to offer amniocentesis or CVS to women with a high risk test result. About one in 20 women (5%) having this test will have a ‘high risk’ result but most of these women will not be carrying a baby with Down’s. We found for tests in the first 14 weeks of pregnancy, there is little evidence to support the use of serum tests made up of more than two blood markers. Other important information to consider The blood tests themselves have no adverse effects for the woman, over and above the risks of a routine blood test. However some women who have a ‘high risk’ screening test result, and are given amniocentesis or CVS have a risk of miscarrying a baby unaffected by Down’s. Parents will need to weigh up this risk when deciding whether or not to have an amniocentesis or CVS following a ‘high risk’ screening test result.
Summary of findings
Background
This is one of a series of reviews on antenatal screening for Down's syndrome following a generic protocol (Alldred 2010) ‐ see Published notes for more details.
Target condition being diagnosed
Down’s syndrome
Down’s syndrome affects approximately one in 800 live‐born babies (Cuckle 1987a). It results from a person having three, rather than two, copies of chromosome 21, or the specific area of chromosome 21 implicated in causing Down's syndrome, as a result of trisomy or translocation. If not all cells are affected, the pattern is described as 'mosaic'. Down’s syndrome can cause a wide range of physical and mental problems. It is the commonest cause of mental disability, and is also associated with a number of congenital malformations, notably affecting the heart. There is also an increased risk of cancers such as leukaemia, and numerous metabolic problems including diabetes and thyroid disease. Some of these problems may be life‐threatening, or lead to considerable ill health, while some individuals with Down’s syndrome have only mild problems and can lead a relatively normal life.
There is no cure for Down’s syndrome, and antenatal diagnosis allows for preparation for the birth and subsequent care of a baby with Down’s syndrome, or for the offer of a termination of pregnancy. Having a baby with Down’s syndrome is likely to have a significant impact on family and social life, relationships and parents’ work. Special provisions may need to be made for education and care of the child, as well as accommodating the possibility of periods of hospitalisation.
Definitive invasive tests (amniocentesis and chorionic villus sampling (CVS)) exist that allow the diagnosis of Down's syndrome before birth, but carry a risk of miscarriage. No test can predict the severity of problems a person with Down’s syndrome will have. Noninvasive screening tests based on biochemical analysis of maternal serum or urine, or fetal ultrasound measurements, allow an estimate of the risk of a pregnancy being affected and provide parents with information to enable them to make choices about definitive testing. Such screening tests are used during the first and second trimester of pregnancy.
Screening tests for Down's syndrome
Initially, screening was determined solely by using maternal age to classify a pregnancy as high or low risk for trisomy 21, as it was known that older women had a higher chance of carrying a baby with Down’s syndrome (Penrose 1933).
Further advances in screening were made in the early 1980s, when Merkatz et al. investigated the possibility that low maternal serum alpha‐fetoprotein (AFP), obtained from maternal blood in the second trimester of pregnancy could be associated with chromosomal abnormalities in the fetus. Their retrospective case‐control study showed a statistically significant relationship between fetal trisomy, such as Down’s syndrome, and lowered maternal serum AFP (Merkatz 1984). This was further explored by Cuckle et al in a larger retrospective trial using data collected as part of a neural tube defect (NTD) screening project (Cuckle 1984). This work was followed by calculation of risk estimates using maternal serum AFP values and maternal age, which ultimately led to the introduction of the two screening parameters in combination (Alfirevic 2004).
In 1987, in a small case‐control study of women carrying fetuses with known chromosomal abnormalities, Bogart and colleagues investigated maternal serum levels of human chorionic gonadotrophin (hCG) as a possible screening tool for chromosomal abnormalities in the second trimester (Bogart 1987). This followed the observations that low hCG levels were associated with miscarriages, which are commonly associated with fetal chromosomal abnormalities. They concluded that high hCG levels were associated with Down’s syndrome and because hCG levels plateau at 18 to 24 weeks, that this would be the most appropriate time for screening. Later work suggested that the ß subunit of hCG was a more effective marker than total hCG (Macri 1990; Macri 1993).
Second trimester unconjugated oestriol (uE3), produced by the fetal adrenals and the placenta, was also evaluated as a potential screening marker. In another retrospective case‐control study, uE3 was shown to be lower in Down’s syndrome pregnancies compared with unaffected pregnancies. When used in combination with AFP and maternal age, it appeared to identify more pregnancies affected by Down’s syndrome than AFP and age alone (Canick 1988).
Further work suggested that all three serum markers (AFP, hCG and uE3) showed even higher detection rates when combined with maternal age (Wald 1988a; Wald 1988b) and appeared to be a cost‐effective screening strategy (Wald 1992a).
Two other serum markers, produced by the placenta, have been linked with Down’s syndrome, namely pregnancy associated plasma protein A or PAPP‐A, and first trimester Inhibin A. PAPP‐A has been shown to be reduced in the first trimester of Down’s syndrome pregnancies, with its most marked reduction in the early first trimester (Bersinger 1995). Inhibin A is high in the second trimester in pregnancies affected by Down’s syndrome (Cuckle 1995; Wallace 1995). There are some issues concerning the biological stability and hence reliability of this marker, and the effect this will have on individual risk.
Screening and parental choice
Antenatal screening is used for several reasons (Alfirevic 2004), but the most important is to enable parental choice regarding pregnancy management and outcome. Before a woman and her partner opt to have a screening test, they need to be fully informed about the risks, benefits and possible consequences of such a test. This includes the choices they may have to face should the result show that the woman has a high risk of carrying a baby with Down’s syndrome and implications of both false positive and false negative screening tests. They need to be informed of the risk of a miscarriage due to invasive diagnostic testing, and the possibility that a miscarried fetus may be chromosomally normal. If, following invasive diagnostic testing, the fetus is shown to have Down’s syndrome, further decisions need to be made about continuation or termination of the pregnancy, the possibility of adoption and finally, preparation for parenthood. Equally, if a woman has a test that shows she is at a low risk of carrying a fetus with Down’s syndrome, it does not necessarily mean that the baby will be born with a normal chromosomal make up. This possibility can only be excluded by an invasive diagnostic test (Alfirevic 2003).The decisions that may be faced by expectant parents inevitably engender a high level of anxiety at all stages of the screening process, and the outcomes of screening can be associated with considerable physical and psychological morbidity. No screening test can predict the severity of problems a person with Down's syndrome will have.
Index test(s)
This review examined serum screening tests used in the first trimester of pregnancy (up to 14 weeks' gestation) comprised of the following 18 individual markers; a disintegrin and metalloprotease 12 (ADAM12), AFP, inhibin, PAPP‐A, invasive trophoblast antigen (ITA), free βhCG, placental growth factor (PlGF), Schwangerschafts protein 1 (SP1), total hCG, progesterone, uE3, growth hormone binding protein (GHBP), placental growth hormone (PGH), hyperglycosylated hCG, proform of eosinophil major basic protein (ProMBP), human placental lactogen (hPL), free alpha human chorionic gonadotrophin (αhCG), and free ßhCG to AFP ratio. These markers can be used individually, in combination with age, and can also be used in combination with each other. The risks are calculated by comparing a woman's test result for each marker with values for an unaffected population, and multiplying this with her age‐related risk. Where several markers are combined, risks are computed using risk equations (often implemented in commercial software) that take into account the correlational relationships between the different markers and marker distributions in affected and unaffected populations.
Alternative test(s)
Down’s syndrome can be detected during pregnancy with invasive diagnostic tests such as amniocentesis or CVS, with or without prior screening. The ability to determine fetal chromosomal make up (also known as a karyotype) from amniotic fluid samples was demonstrated in 1966 by Steele and Breg (Steele 1966), and the first antenatal diagnosis of Down’s syndrome was made in 1968 (Vaklenti 1968). Amniocentesis is an invasive procedure that involves taking a small sample of the amniotic fluid (liquor) surrounding the baby, using a needle which goes through the abdominal wall into the uterus, and is usually performed after 15 weeks' gestation. CVS involves taking a sample of the placental tissue using a needle which goes through the abdominal wall and uterus or a cannula through the cervix. It is usually performed between 10 and 13 weeks' gestation. Amniocentesis and CVS are both methods of obtaining fetal chromosomal material, which are then used to diagnose Down’s syndrome. Both tests use ultrasound scans to guide placement of the needle. Amniocentesis carries a risk of miscarriage in the order of 1%; transabdominal CVS may carry a similar risk (Alfirevic 2003).
Rationale
This is one of a suite of Cochrane reviews, the aim of which is to identify all screening tests for Down's syndrome used in clinical practice, or evaluated in the research setting, in order to try to identify the most accurate test(s) available, and to provide clinicians, policy‐makers and women with robust and balanced evidence on which to base decisions about interpreting test results and implementing screening policies to triage the use of invasive diagnostic testing.
There are many different screening tests which are available and offered which will be the subject of additional Cochrane reviews (currently in preparation or published (Alldred 2012)), and there are other reviews looking at this area. Tests to be assessed in Cochrane reviews include second trimester serum tests; urine tests; first trimester ultrasound markers; tests that combine serum and ultrasound markers; and tests that combine markers from the first trimester with markers from the second trimester. Second trimester ultrasound markers have been assessed in a previous systematic review (Smith‐Bindman 2001).
The topic has been split into several different reviews to allow for greater ease of reading and greater accessibility of data, and also to allow the reader to focus on separate groups of tests, for example, first trimester serum tests alone, first trimester serum and ultrasound, second trimester serum alone, first and second trimester serum combinations, with or without ultrasound markers; and urine markers alone. An overview review will compare the best tests, focusing on commonly used strategies and the best tests from each of these categories. This review is written with the global perspective in mind, rather than to conform with any specific local or national policy, as not all tests will be available in all areas where screening for Down's syndrome is carried out.
A systematic review of second trimester ultrasound markers in the detection of Down’s syndrome fetuses was published in 2001 that concluded that nuchal fold thickening may be useful in detecting Down’s syndrome, but that it was not sensitive enough to use as a screening test. The review concluded that the other second trimester ultrasound markers did not usefully distinguish between Down’s syndrome and pregnancies without Down’s syndrome (Smith‐Bindman 2001). There has yet to be a systematic review and meta‐analysis of the observed data on serum, urine and first trimester ultrasound markers, in order to draw rigorous and robust conclusions about the diagnostic accuracy of available Down’s syndrome screening tests.
Objectives
The aim of this review was to estimate and compare the accuracy of first trimester serum markers for the detection of Down’s syndrome in the antenatal period, both as individual markers and as combinations of markers. Accuracy is described by the proportion of fetuses with Down’s syndrome detected by screening before birth (sensitivity or detection rate), and the proportion of women with a low risk (normal) screening test result who subsequently had a baby unaffected by Down's syndrome (specificity).
Investigation of sources of heterogeneity
We planned to investigate whether a uniform screening test is suitable for all women, or whether different screening methods are more applicable to different groups, defined by advanced maternal age, ethnic groups and aspects of the pregnancy and medical history such as multiple pregnancy, diabetes and family history of Down's syndrome. We also considered whether there existed evidence of overestimation of test accuracy in studies evaluating risk equations in the derivation sample rather than in a separate validation sample.
Methods
Criteria for considering studies for this review
Types of studies
We included studies in which all women from a given population had one or more index test(s) compared to a reference standard. Both consecutive series and diagnostic case‐control study designs were included. Randomised trials where individuals were randomised to different screening strategies and all verified using a reference standard were also eligible for inclusion. Studies in which test strategies were compared head‐to‐head, either in the same women, or between randomised groups were identified for inclusion in separate comparisons of test strategies. Studies were excluded if they included less than five Down's syndrome cases, or more than 20% of participants were not followed up.
Participants
Pregnant women at less than 14 weeks' gestation confirmed by ultrasound, who had not undergone previous testing for Down’s syndrome in their pregnancy were eligible. Studies were included if the pregnant women were unselected, or if they represented groups with increased risk of Down’s syndrome, or difficulty with conventional screening tests including maternal age greater than 35 years old, multiple pregnancy, diabetes mellitus and family history of Down’s syndrome.
Index tests
The following 18 index tests were examined; ADAM12, AFP, inhibin, PAPP‐A, ITA, free βhCG, PlGF, SP1, total hCG, progesterone, uE3, GHBP, PGH, hyperglycosylated hCG, ProMBP, hPL, free αhCG, and free ßhCG to AFP ratio, and combinations of these markers combined with maternal age.
We looked at comparisons of tests used in isolation and in 78 various combinations. These included single (one marker), double (two markers), triple (three markers), quadruple (four markers) and quintuple (five markers) tests, some of which were adjusted for maternal age.
Where tests were used in combination, we looked at the performance of test combinations according to predicted probabilities computed using risk equations and dichotomised into high risk and low risk.
Target conditions
Down's syndrome in the fetus due to trisomy, translocation or mosaicism.
Reference standards
We considered several reference standards, involving chromosomal verification and postnatal macroscopic inspection. Chromosomal verification is considered preferential but because of the risks involved, often not feasible. Where macroscopic inspection or examination raises a question about the possibility of an individual being affected by Down's syndrome, in clinical practice this is usually confirmed or refuted by formal karyotyping.
Amniocentesis and CVS are invasive chromosomal verification tests undertaken during pregnancy. They are highly accurate but the process carries a 1% miscarriage rate, and therefore they are only used in pregnancies considered to be high risk of Down's, or on the mother's request. All other types of testing (postnatal examination, postnatal karyotyping, birth registers and Down’s syndrome registers) are based on information available at the end of pregnancy. For the purposes of meta‐analysis they are considered equivalent. The greatest concern is not their accuracy, but the loss of the pregnancy to miscarriage between the timing of serum testing and the reference standard. Miscarriage with cytogenetic testing of the fetus is included in the reference standard where available.
We anticipated that older studies, and studies undertaken in older women were more likely to have used invasive chromosomal verification tests in all women. Studies undertaken in younger women and more recent studies were likely to use differential verification as they often only used prenatal karyotypic testing on fetuses considered screen positive or high risk according to the screening test; the reference standard for most unaffected infants is likely to be observation of a phenotypically normal baby. Although the accuracy of this combined reference standard is considered high, it is methodologically a weaker approach because pregnancies that miscarry between the index test and birth are likely to be lost from the analysis, and miscarriage is more likely to occur in Down’s than normal pregnancies.
Search methods for identification of studies
We used one generic search strategy to identify studies for all reviews in this series
Electronic searches
We applied a sensitive search strategy to search the following databases using the text words and MeSH terms detailed in Appendix 1, adapting the search strategy for each different database.
Databases searched included:
MEDLINE via OVID (1980 to 25 August 2011)
Embase via Dialog Datastar (1980 to 25 August 2011)
BIOSIS via EDINA (1985 to 25 August 2011)
CINAHL via OVID (1982 to 25 August 2011)
The Database of Abstracts of Reviews of Effectiveness (25 August 2011)
MEDION (25 August 2011)
The Database of Systematic Reviews and Meta‐Analyses in Laboratory Medicine (www.ifcc.org/) (25 August 2011)
The National Research Register (Archived 2007)
Health Services Research Projects in Progress database (HSRPROJ) (25 August 2011)
The search strategy combined three sets of search terms (see Appendix 1). The first set was made up of named tests, general terms used for screening/diagnostic tests and statistical terms. Note that the statistical terms were used to increase sensitivity and were not used as a methodological filter to increase specificity. The second set was made up of terms that encompass Down's syndrome and the third set made up of terms to limit the testing to pregnant women. All terms within each set were combined with the Boolean operator OR and then the three sets were combined using AND. The terms used were a combination of subject headings and free text terms. The search strategy was adapted to suit each database searched.
We attempted to identify cumulative papers which reported data from the same data set, and contacted authors to obtain clarification of the overlap between data presented in these papers, in order to prevent data from the same women being analysed more than once.
Searching other resources
In addition, we examined references cited in studies identified as being potentially relevant, and those cited by previous reviews. We contacted authors of studies where further information was required.
We carried out forward citation searching of relevant items, using the search strategy in ISI citation indices, Google scholar and Pubmed ‘related articles’.
We did not apply language restrictions to the search.
Data collection and analysis
Selection of studies
Two review authors screened the titles and abstracts (where available) of all studies identified by the search strategy. We obtained full‐text versions of studies identified as being potentially relevant and two review authors independently assessed these for inclusion, using a study eligibility screening pro forma according to the pre‐specified inclusion criteria. Any disagreement between the two review authors was settled by consensus, or where necessary, by a third party.
Data extraction and management
A data extraction form was developed and piloted using a subset of 20 identified studies. Two review authors independently extracted data, and where disagreement or uncertainty existed, a third review author validated the information extracted.
Data on each marker were extracted as binary test positive/test negative results for Down's and non‐Down's pregnancies, with a high‐risk result—as defined by each individual study—being regarded as test positive (suggestive or diagnostic of Down's syndrome), and a low‐risk result being regarded as test negative (suggestive of absence of Down's syndrome). Where results were reported at several thresholds, we extracted data at each threshold.
We made a note of those in special groups that posed either increased risk of Down’s syndrome or difficulty with conventional screening tests, including maternal age greater than 35 years old, multiple pregnancy, diabetes mellitus and family history of Down’s syndrome.
Assessment of methodological quality
We used a modified version of the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool (Whiting 2003), a quality assessment tool for use in systematic reviews of diagnostic accuracy studies, to assess the methodological quality of included studies. We anticipated that a key methodological issue would be the potential for bias arising from the differential use of invasive testing and follow‐up for the reference standard according to index test results, bias arising due to higher loss to miscarriage if false negatives than true negatives. We chose to code this issue as originating from differential verification in the QUADAS tool: we are aware that it could also be coded under delay in obtaining the reference standard, and reporting of withdrawals. We omitted the QUADAS item assessing quality according to length of time between index and reference tests, as Down's syndrome is either present or absent rather than a condition that evolves and resolves, and disregarding the differential reference standard issue, thus any length of delay is acceptable. Two review authors assessed each included study separately. Any disagreement between the two authors was settled by consensus, or where necessary, by a third party. Each item in the QUADAS tool was be marked as ‘yes’, ‘no’ or ‘unclear’, and scores are presented graphically and in tables. We did not use a summary quality score. See Appendix 3 for QUADAS questionnaire.
Statistical analysis and data synthesis
We initially examined each test or test strategy at each of the common risk thresholds used to define test positivity by plotting estimates of sensitivity and specificity from each study on forest plots and in receiver operating characteristic (ROC) space. Test strategies were selected for further investigation if they were evaluated in four or more studies or, if there were two or three studies, but the individual study results indicated performance likely to be superior to a sensitivity of 70% and specificity of 90%.
Estimation of average sensitivity and specificity
The analysis for each test strategy was undertaken first restricting to studies that reported a common threshold to estimate average sensitivity and specificity for each test at each threshold. Although data on all thresholds were extracted, we present only key common thresholds from the literature, close to risks of 1:384, 1:250 and the 5% false positive rate (FPR), unless other thresholds were more commonly reported. Where combinations of tests were used in a risk score we extracted the result for the test combination using the risk score and not the individual components that made up the test.
We undertook meta‐analyses using hierarchical summary ROC (HSROC) models, which included estimation of random‐effects in accuracy and threshold parameters when there were four or more studies. Where there were fewer than four studies and the studies reported test performance at a common threshold, we computed average sensitivity and specificity values by using univariate fixed‐effect or random‐effects logistic regression models to average logit sensitivity and logit specificity separately because of insufficient number of studies to reliably estimate all the parameters in the HSROC model. It is common in this field for studies to report sensitivity for a fixed specificity (usually a 5% FPR). This removes the requirement to account for the correlation between sensitivity and specificity across studies by using a bivariate meta‐analytical method since all specificities are the same value. Thus, at a fixed specificity value, logit sensitivities were pooled using a univariate random‐effects logistic regression model. This model was further simplified to a fixed‐effect model when there were only two or three studies and heterogeneity was not observed on the SROC plot. All analyses were undertaken using the NLMIXED procedure in SAS (version 9.2; SAS Institute, Cary, NC) and the xtmelogit command in Stata version 11.2 (Stata‐Corp, College Station, TX, USA).
Comparisons between tests
We made comparisons between tests, first by utilising all available studies, selecting one threshold from each study to estimate a summary ROC curve without restricting to a common threshold, and second, by making pair‐wise comparisons using studies that compared tests in the same mothers (direct head‐to‐head comparison). The threshold was chosen for each study according to the following order of preference a) the risk threshold closest to 1 in 250; b) a multiples of the median (MoM) or presence/absence threshold; c) the performance closest to a 5% FPR or 95th percentile. The 5% FPR was chosen as a cut‐off point as this is the cut‐off most commonly reported in the literature.
For the analysis that included data from all studies, we compared test strategies in a single HSROC model, including two indicator terms for each test to allow for differences in accuracy and threshold. There was no indication of differing SROC curve shape between tests and so a single SROC shape parameter was included in the model, such that the fitted SROC curves did not cross. The initial meta‐analyses of individual test strategies indicated there were differences in the variability of the accuracy parameter such that the assumption of equal variances may not be justifiable. We attempted to fit a model with separate variance terms for each test strategy for the accuracy parameter but the model did not converge. We therefore restricted the meta‐analysis that compared the accuracy of the different test strategies to only studies that used a 5% FPR threshold so that we could fit a univariate random effects logistic regression model that allowed for a separate variance term for the random‐effects of logit sensitivity for each test. Using nonlinear combinations of the parameter estimates from this model, we derived ratios of sensitivities for each pair of tests included in the model and obtained their corresponding 95% confidence interval (CI) by using the delta method. We used likelihood ratio tests to assess the statistical significance of differences in sensitivity between tests.
For direct comparisons between each pair of tests at the 5% FPR threshold, we used a separate model for each pair‐wise comparison and pooled logit sensitivities using a univariate random‐effects model. As studies rarely reported data cross‐classified by both tests for Down's and normal pregnancies, the analytical method did not take full account of the pairing of test results, but the restriction to direct head‐to‐head comparisons should have removed the potential confounding of test comparisons with other features of the studies. The strength of evidence for differences in performance of test strategies relied on evidence from both the direct and indirect comparisons.
Investigations of heterogeneity
We planned to undertake investigations of heterogeneity if there were 10 or more studies available for a test. We planned to investigate the effect of a covariate by adding covariate terms to the HSROC model to assess differences in accuracy and threshold.
Sensitivity analyses
Mothers with pregnancies identified as high risk for Down's syndrome by serum testing are often offered immediate definitive testing by amniocentesis, whereas those considered low risk are assessed for Down's syndrome by inspection at birth. Such delayed and differential verification will introduce bias most likely through there being greater loss to miscarriage in the Down's syndrome pregnancies that were not detected by the serum testing (the false negative diagnoses). Testing and detection of miscarriages is impractical in many situations, and no clear data are available on the magnitude of these miscarriage rates.
To account for the possible bias introduced by such a mechanism, we planned to perform sensitivity analyses by increasing the percentage of false negatives in studies where delayed verification in test negatives occurred (Mol 1999). We planned to incrementally increase the percentage from 10% to 50%, the final value representing a scenario where a third or more Down's pregnancies than normal pregnancies were likely to miscarry, thought to be higher than the likely value. We intended to conduct the sensitivity analyses on the analysis investigating the effect of maternal age on test sensitivity.
Results
Results of the search
The search for the whole suite of reviews identified a total of 15,394 papers, once the results from each bibliographic database were combined and duplicates were removed. After screening out obviously inappropriate papers based on their title and abstract, 1145 papers remained and we obtained full‐text copies for formal assessment of eligibility. From these, a total of 269 papers were deemed eligible and were included in the suite of reviews. We included a total of 56 studies (reported in 68 publications) in this review of first trimester serum screening, involving 204,759 pregnancies, of which 2113 were Down's syndrome pregnancies.
A total of 78 different test strategies or combinations, at one or more thresholds, were evaluated in the 56 studies. These tests were produced from combinations of 18 different serum tests with and without maternal age; ADAM12, AFP, inhibin, PAPP‐A, ITA, free βhCG, PlGF, SP1, total hCG, progesterone, uE3, GHBP, PGH, hyperglycosylated hCG, ProMBP, hPL, free αhCG, and free ßhCG to AFP ratio. Strategies evaluated included three quintuple tests, three quadruple tests, 12 triple tests, 27 double tests and 15 single tests in combination with maternal age, and three triple tests, five double tests and 10 single tests without maternal age.
The following combinations evaluated included four or more studies.
Double tests with maternal age
Free ßhCG, AFP and maternal age (five studies; 5160 women including 174 Down's syndrome pregnancies)
Free ßhCG, PAPP‐A and maternal age (31 studies; 158,878 women including 1430 Down's syndrome pregnancies)
Single tests with maternal age
Free ßhCG and maternal age (nine studies; 16,656 women including 549 Down's syndrome pregnancies)
PAPP‐A and maternal age(six studies; 13,742 women including 409 Down's syndrome pregnancies)
Single tests without maternal age
Free ßhCG (four studies; 4280 women including 390 Down's syndrome pregnancies)
PAPP‐A (six studies; 25,510 women including 430 Down's syndrome pregnancies)
Of the remaining test combinations, seven were evaluated in three studies, 17 were evaluated in two studies and the remainder were evaluated in single studies only.
Methodological quality of included studies
We judged the methodological quality of the studies to be high in most categories (Figure 1). Due to the nature of testing for Down's syndrome screening and the potential side effects of invasive testing, differential verification is almost universal in the general screening population, as most women whose screening test result is defined as low risk will have their screening test verified at birth, rather than by invasive diagnosis in the antenatal period. Additionally, it was not always possible to ascertain from the included studies whether or not the results of index tests and reference standards were blinded. It would be difficult to blind clinicians performing invasive diagnostic tests (reference standards) to the index test result, unless all women received the same reference standard, which would not be appropriate in most scenarios. Any biases secondary to a lack of clinician blinding are likely to be minimal.
1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Where details of completeness of follow‐up were poorly reported, most studies seemed to assume 100% follow‐up. However, there will inevitably be losses to follow‐up due to women moving out of area, for example. Studies sometimes accounted for these and it is unlikely that there were enough losses to follow‐up to have introduced significant bias. There was likely under‐ascertainment of miscarriage, and very few papers accounted for miscarriage, or performed tissue karyotyping in pregnancies resulting in miscarriage. Some studies attempted to adjust for predicted miscarriage rate and the incidence of Down's syndrome in this specific population, but most did not. We have not attempted to adjust for expected miscarriage rate in this review. There is a higher natural miscarriage rate in the first trimester, however this will be uniform across studies and therefore unlikely to introduce significant bias.
Some studies that provided estimates of risk using multivariable equations used the same data set to evaluate performance of the risk equation as was used to derive the equation. This is often thought to lead to over‐estimation of test performance.
Findings
The findings of the 21 most common and/or best performing test strategies are given in Table 1. The remaining 57 strategies are briefly summarised in Table 2. The test strategies evaluated by four or more studies are detailed below.
Summary of findings 1. Performance of first trimester serum test strategies with or without maternal age.
| Review Question | What is the accuracy of serum‐based markers for Down's syndrome screening in the first trimester? | ||||
| Population | Pregnant women at less than 14 weeks' gestation confirmed by ultrasound, who had not undergone previous testing for Down’s syndrome. Most studies were undertaken in women identified to be high risk based on maternal age | ||||
| Settings | All settings | ||||
| Numbers of studies, pregnancies and Down's syndrome cases | 56 studies (68 publications) involving 204,759 pregnancies of which 2113 were Down's syndrome pregnancies. | ||||
| Index tests | 18 serum markers (ADAM12, AFP, inhibin, PAPP‐A, ITA, free βhCG, PlGF, SP1, total hCG, progesterone, uE3, GHBP, PGH, hyperglycosylated hCG, ProMBP, hPL, free αhCG, and free ßhCG to AFP ratio) singly or in combination with or without maternal age. | ||||
| Reference standards | Chromosomal verification (amniocentesis and CVS undertaken during pregnancy, and postnatal karyotyping) and postnatal macroscopic inspection. | ||||
| Study limitations | 35 studies used selective chromosomal verification during pregnancy, and were at risk of under‐ascertainment of Down's syndrome cases due loss of the pregnancy to miscarriage between the serum test and the reference standard. | ||||
| Tests with at least 70% sensitivity and at least 95% specificity | |||||
| Test strategy | Studies | Women (cases) | Sensitivity (95% CI) | Specificity (95% CI) | Test* |
| Test strategies (with or without maternal age) evaluated by a single study | |||||
| Without maternal age | |||||
| Double tests | |||||
| PAPP‐A and AFP | 1 | 96 (16) | 81 (54 to 96) | 95 (88 to 99) | |
| PAPP‐A and ITA | 1 | 344 (24) | 71 (49 to 87) | 95 (92 to 97) | |
| Triple tests | |||||
| PAPP‐A, free ßhCG and ITA | 1 | 344 (24) | 75 (53 to 90) | 95 (92 to 97) | |
| PlGF, PAPP‐A and free ßhCG | 1 | 699 (90) | 72 (62 to 81) | 95 (93 to 97) | |
| With maternal age | |||||
| Double tests | |||||
| Free ßhCG and SP1 | 1 | 60 (14) | 71 (42 to 92) | 96 (85 to 99) | |
| PAPP‐A and Hyperglycosylated hCG | 1 | 10775 (23) | 74 (52 to 90) | 95 (95 to 95) | |
| Triple tests | |||||
| PAPP‐A, free ßhCG and Inhibin | 1 | 1110 (85) | 74 (63 to 83) | 95 (94 to 96) | |
| PAPP‐A, SP1 and ProMBP | 1 | 192 (15) | 73 (45 to 92) | 95 (91 to 98) | |
| hPL, PAPP‐A and free ßhCG (1:250 risk) | 1 | 183 (47) | 77 (62 to 88) | 95 (90 to 98) | |
| Quadruple tests | |||||
| GHBP, PGH, PAPP‐A and free ßhCG (1:250 risk) | 1 | 335 (74) | 76 (64 to 85) | 95 (91 to 97) | |
| Quintuple tests | |||||
| PAPP‐A, free ßhCG, AFP, uE3 and Inhibin | 1 | 1110 (85) | 78 (67 to 86) | 95 (94 to 96) | |
| PAPP‐A, total hCG, AFP, uE3 and Inhibin | 1 | 1110 (85) | 73 (62 to 82) | 95 (94 to 96) | |
| Test strategies (with or without maternal age) evaluated by at least two studies | |||||
| Free ßhCG | 4 | 4280 (390) | 25 (18 to 34) | 95 (94 to 96) | P < 0.001 |
| PAPP‐A | 4 | 2837 (325) | 52 (39 to 65) | 95 (94 to 96) | |
| Age, free ßhCG | 7 | 5893 (460) | 42 (36 to 48) | 95 (94 to 96) | |
| Age, PAPP‐A | 5 | 3491 (359) | 55 (46 to 63) | 95 (94 to 96) | |
| Age, free ßhCG and AFP | 3 | 2992 (157) | 49 (39 to 60) | 95 (94 to 96) | |
| Age, PAPP‐A and free ßhCG | 17 | 49827 (1037) | 68 (65 to 71) | 95 (95 to 95) | |
| Age, PAPP‐A, free ßhCG and AFP | 2 | 2705 (116) | 74 (65 to 81) | 95 (94 to 96) | |
| Age, ADAM 12, PAPP‐A and free ßhCG | 2 | 1222 (74) | 74 (63 to 83) | 95 (94 to 96) | |
| Age, PlGF, PAPP‐A and free ßhCG | 2 | 1144 (160) | 76 (69 to 82) | 95 (93 to 96) | |
*Likelihood ratio test for the difference in sensitivity between the nine test strategies that were formally compared in a single meta‐analytic model. ADAM12: a disintegrin and metalloprotease; AFP: alpha‐fetoprotein; αhCG: alpha human chorionic gonadotrophin; ßhCG: beta human chorionic gonadotrophin; CI: confidence interval; CVS: chorionic villus sampling; GHBP: growth hormone binding protein; hCG: human chorionic gonadotrophin; hPL: human placental lactogen; ITA: invasive trophoblast antigen; PAPP‐A: pregnancy‐associated plasma protein A; PGH: placental growth hormone; PIGF: placental growth factor; PROMBP: proform of eosinophil major basic protein; SPI: Schwangerschafts protein 1; uE3: unconjugated oestriol
Summary of findings 2. Performance of other first trimester serum test strategies with or without maternal age.
| Test strategy | Studies | Women (cases) | Sensitivity (95% CI) | Specificity (95% CI) | Threshold |
| Without maternal age | |||||
| Single tests | |||||
| AFP | 2 | 2248 (104) | 10 (4 to 21) | 95 | 5% FPR |
| ADAM 12 | 1 | 579 (17) | 41 (18 to 67) | 95 (93 to 97) | 5% FPR |
| Free ßhCG to AFP ratio | 1 | 476 (9) | 11 (0 to 48) | 98 (96 to 99) | 0.25 MoM |
| Inhibin | 3 | 2098 (184) | 19 (4 to 58) | 95 | 5% FPR |
| PlGF | 1 | 699 (90) | 28 (19 to 38) | 95 (93 to 97) | 5% FPR |
| Total hCG | 3 | 2098 (184) | 19 (4 to 58) | 95 | 5% FPR |
| SP1 | 3 | 1080 (53) | 32 (1 to 96) | 95 | 5% FPR |
| uE3 | 1 | 1110 (85) | 13 (7 to 22) | 95 (94 to 96) | 5% FPR |
| Double tests | |||||
| Free ßhCG and AFP | 1 | 1138 (19) | 16 (3 to 40) | 95 (94 to 96) | 5% FPR |
| Free ßhCG and Inhibin | 1 | 876 (76) | 30 (20 to 42) | 95 (93 to 96) | 5% FPR |
| PAPP‐A and free ßhCG | 2 | 795 (106) | 64 (50 to 76) | 95 | 5% FPR |
| Triple tests | |||||
| Total hCG, free αhCG and progesterone | 1 | 129 (17) | 53 (28 to 77) | 96 (90 to 99) | 0.34 MoM |
| With maternal age | |||||
| Single tests | |||||
| ADAM 12 | 2 | 703 (46) | 67 (46 to 83) | 91 (87 to 94) | 1:400 risk |
| AFP | 2 | 1397 (126) | 33 (23 to 46) | 95 | 5% FPR |
| Free αhCG | 1 | 512 (12) | 25 (5 to 57) | 89 (86 to 91) | 1:384 risk |
| GHBP | 1 | 335 (74) | 27 (17 to 39) | 95 (91 to 97) | 1:250 risk |
| hPL | 1 | 183 (47) | 45 (30 to 60) | 93 (88 to 97) | 1:250 risk |
| Inhibin | 1 | 1110 (85) | 32 (22 to 43) | 95 (94 to 96) | 5% FPR |
| ITA | 1 | 278 (54) | 48 (34 to 62) | 95 (91 to 98) | 5% FPR |
| PGH | 1 | 335 (74) | 41 (29 to 53) | 94 (91 to 97) | 1:250 risk |
| PlGF | 1 | 699 (90) | 43 (33 to 54) | 95 (93 to 97) | 5% FPR |
| ProMBP | 1 | 181 (25) | 36 (18 to 57) | 94 (89 to 97) | 1:250 risk |
| SP1 | 2 | 804 (29) | 38 (22 to 56) | 95 | 5% FPR |
| Total hCG | 1 | 512 (12) | 33 (10 to 65) | 94 (92 to 96) | 1:384 risk |
| uE3 | 1 | 512 (12) | 33 (10 to 65) | 86 (83 to 89) | 1:384 risk |
| Double tests | |||||
| ADAM 12 and PAPP‐A | 1 | 691 (46) | 61 (45 to 75) | 95 (93 to 97) | 5% FPR |
| AFP and free αhCG | 1 | 512 (12) | 33 (10 to 65) | 87 (83 to 89) | 1:384 risk |
| AFP and total hCG | 1 | 512 (12) | 33 (10 to 65) | 93 (90 to 95) | 1:384 risk |
| AFP and uE3 | 1 | 512 (12) | 42 (15 to 72) | 87 (84 to 90) | 1:384 risk |
| Free ßhCG and free αhCG | 1 | 512 (12) | 42 (15 to 72) | 94 (91 to 96) | 1:384 risk |
| Free ßhCG and Inhibin | 1 | 1110 (85) | 44 (33 to 55) | 95 (94 to 96) | 5% FPR |
| Free ßhCG and total hCG | 1 | 512 (12) | 25 (5 to 57) | 93 (90 to 95) | 1:384 risk |
| Free ßhCG and uE3 | 1 | 287 (41) | 61 (45 to 76) | 95 (92 to 97) | 5% FPR |
| GHBP and free ßhCG | 1 | 335 (74) | 61 (49 to 72) | 92 (88 to 95) | 1:250 risk |
| GHBP and PAPP‐A | 1 | 335 (74) | 66 (54 to 77) | 93 (89 to 96) | 1:250 risk |
| GHBP and PGH | 1 | 335 (74) | 47 (36 to 59) | 93 (90 to 96) | 1:250 risk |
| hPL and free ßhCG | 1 | 183 (47) | 68 (53 to 81) | 94 (89 to 97) | 1:250 risk |
| hPL and PAPP‐A | 1 | 183 (47) | 55 (40 to 70) | 94 (89 to 97) | 1:250 risk |
| PAPP‐A and AFP | 2 | 2705 (116) | 63 (50 to 74) | 95 | 5% FPR |
| PAPP‐A and Inhibin | 1 | 1110 (85) | 68 (57 to 78) | 95 (94 to 96) | 5% FPR |
| PAPP‐A and ITA | 2 | 622 (78) | 62 (46 to 75) | 95 | 5% FPR |
| PGH and free ßhCG | 1 | 335 (74) | 64 (52 to 74) | 93 (89 to 96) | 1:250 risk |
| PGH and PAPP‐A | 1 | 335 (74) | 65 (53 to 76) | 93 (89 to 96) | 1:250 risk |
| Total hCG and free αhCG | 1 | 512 (12) | 42 (15 to 72) | 92 (89 to 94) | 1:384 risk |
| Total hCG and Inhibin | 1 | 1110 (85) | 34 (24 to 45) | 95 (94 to 96) | 5% FPR |
| Total hCG and PAPP‐A | 2 | 4327 (133) | 66 (54 to 76) | 95 | 5% FPR |
| Total hCG and uE3 | 1 | 512 (12) | 42 (15 to 72) | 92 (89 to 94) | 1:384 risk |
| uE3 and free αhCG | 1 | 512 (12) | 33 (10 to 65) | 89 (86 to 91) | 1:384 risk |
| Triple tests | |||||
| AFP, free αhCG and uE3 | 1 | 512 (12) | 58 (28 to 85) | 82 (79 to 85) | 1:384 risk |
| Free ßhCG, AFP and uE3 | 1 | 287 (41) | 66 (49 to 80) | 95 (92 to 97) | 5% FPR |
| GHBP, PAPP‐A and free ßhCG | 1 | 335 (74) | 76 (64 to 85) | 94 (91 to 97) | 1:250 risk |
| PAPP‐A, total hCG and Inhibin | 1 | 1110 (85) | 69 (58 to 79) | 95 (94 to 96) | 5% FPR |
| PGH, PAPP‐A and free ßhCG | 1 | 335 (74) | 76 (64 to 85) | 94 (91 to 97) | 1:250 risk |
| Total hCG, AFP and uE3 | 1 | 512 (12) | 42 (15 to 72) | 91 (88 to 94) | 1:384 risk |
| Quadruple tests | |||||
| Free ßhCG, total hCG, AFP and uE3 | 1 | 512 (12) | 50 (21 to 79) | 92 (89 to 94) | 1:384 risk |
| Total hCG, AFP, uE3 and free αhCG | 1 | 512 (12) | 50 (21 to 79) | 90 (87 to 92) | 1:384 risk |
| Quintuple tests | |||||
| Free ßhCG, total hCG, AFP, uE3 and free αhCG | 1 | 512 (12) | 33 (10 to 65) | 90 (87 to 92) | 1:384 risk |
AFP: alpha‐fetoprotein; αhCG: alpha human chorionic gonadotrophin; ßhCG: beta human chorionic gonadotrophin; CI: confidence interval; FPR: false positive rate; GHBP: growth hormone binding protein; hCG: human chorionic gonadotrophin; hPL: human placental lactogen; ITA: invasive trophoblast antigen; PAPP‐A: pregnancy‐associated plasma protein A; PGH: placental growth hormone; PIGF: placental growth factor; PROMBP: proform of eosinophil major basic protein; SPI: Schwangerschafts protein 1; uE3: unconjugated oestriol
1) Free ßhCG, PAPP‐A and maternal age (double test)
Results for this double test were derived from 31 studies (Biagiotti 1998; Brambati 1994; Christiansen 2005; Christiansen 2007a; Christiansen 2009; Christiansen 2010; Cowans 2010; Crossley 2002a; De Graaf 1999a; Forest 1997; Gyselaers 2005; Haddow 1998; Kagan 2009; Kozlowski 2007 GC; Kozlowski 2007 PC; Krantz 2000; Muller 2003a; Niemimaa 2001a; O'Leary 2006; Orlandi 1997; Sahota 2010; Schaelike 2009; Scott 2004; Spencer 1999a; Torring 2010; Tsukerman 1999; Valinen 2007; Wald 2003a; Wapner 2003; Wojdemann 2005; Zaragoza 2009), and included 158,878 women in whom 1430 pregnancies were known to be affected by Down's syndrome. Seven studies contributed over 10,000 pregnancies each to the data (Crossley 2002a; Gyselaers 2005; Kagan 2009; Krantz 2000; O'Leary 2006; Sahota 2010; Schaelike 2009). Studies presented data for cut‐points of 5% FPR (Biagiotti 1998; Brambati 1994; Cowans 2010; De Graaf 1999a; Forest 1997; Haddow 1998; Kagan 2009; Sahota 2010; Spencer 1999a;Sahota 2010; Torring 2010; Tsukerman 1999; Wald 2003a; Wapner 2003; Zaragoza 2009), 1:250 risk (Christiansen 2005; Christiansen 2007a; Christiansen 2009; Christiansen 2010; Crossley 2002a; Kagan 2009; Muller 2003a; Niemimaa 2001a; Torring 2010; Valinen 2007; Wojdemann 2005), and 1:300 risk (Kozlowski 2007 GC; Kozlowski 2007 PC; Schaelike 2009). At a cut‐point of 5% FPR (17 studies), the sensitivity wa s estimated as 68% (95% confidence interval (CI) 65 to 71) and the specificity at 95% (95% CI 95 to 95). At a cut‐point of 1:250 FPR (11 studies), the sensitivity was estimated as 73% (95% CI 67 to 79) and the specificity as 93% (95% CI 91 to 94).
2) Free ßhCG, AFP and maternal age (double test)
Results for this double test were derived from five studies (Benattar 1999; Biagiotti 1995; Forest 1995; Tsukerman 1999; Wald 2003a), and included 5160 women in whom 174 pregnancies were known to be affected by Down's syndrome. Two contributed over 1000 pregnancies each to the data (Benattar 1999; Tsukerman 1999. Studies presented data for cut‐points of 5% FPR (Biagiotti 1995; Tsukerman 1999; Wald 2003a), 1:250 risk (Benattar 1999) and 1:384 risk (Forest 1995). At a cut‐point of 5% FPR (three studies), the sensitivity was estimated as 49% (95% CI 39 to 60) and the specificity as 95% (95% CI 94 to 96).
3) PAPP‐A and maternal age (single test)
Results for this single test were derived from six studies (Biagiotti 1998; Brambati 1993; Forest 1997; Krantz 2000; Spencer 1999a; Wald 2003a), and included 13,742 women in whom 409 pregnancies were known to be affected by Down's syndrome. Krantz 2000 was the largest study, contributing over 10,000 pregnancies to the data. Studies presented data for cut‐points of 5% FPR (Biagiotti 1998; Brambati 1993; Forest 1997; Spencer 1999a; Wald 2003a) and 1:105 risk (Krantz 2000). At a cut‐point of 5% FPR (five studies), the sensitivity was estimated as 55% (95% CI 46 to 63) and the specificity as 95% (95% CI 94 to 96).
4) Free ßhCG and maternal age (single test)
Results for this single test were derived from nine studies (Biagiotti 1995; Biagiotti 1998; Brambati 1994; Forest 1995; Forest 1997; Krantz 2000; Noble 1995; Spencer 1999a; Wald 2003a), and included 16,656 women in whom 549 pregnancies were known to be affected by Down's syndrome. Krantz 2000 contributed over 10,000 pregnancies to the data. Studies presented data for cut‐points of 5% FPR (Biagiotti 1995; Biagiotti 1998; Brambati 1994; Forest 1997, Noble 1995; Spencer 1999a; Wald 2003a), 1:384 risk (Forest 1995) and 1:105 risk (Krantz 2000). At a cut‐point of 5% FPR (seven studies), the sensitivity was estimated as 42% (95% CI 36 to 48) and the specificity as 95% (95% CI 94 to 96).
5) PAPP‐A alone (single test without maternal age)
Results for this single test were derived from six studies (Brambati 1993; Brameld 2008; Brizot 1994; Casals 1996; Spencer 1999a; Wald 2003a), and included 25,510 women in whom 430 pregnancies were known to be affected by Down's syndrome. Brameld 2008 was the largest study contributing over 20,000 pregnancies to the data. Studies presented data for cut‐points of 5% FPR (Brambati 1993; Brizot 1994; Casals 1996; Spencer 1999a; Wald 2003a) and ≤ 5th percentile (Brameld 2008). At a cut‐point of 5% FPR (four studies), the sensitivity was estimated as 52% (95% CI 39 to 65) and the specificity as 95% (95% CI 94 to 96).
6) Free ßhCG alone (single test without maternal age)
Results for this single test were derived from four studies (Casals 1996; Noble 1997; Spencer 1999a; Wald 2003a), and included 4280 women in whom 390 pregnancies were known to be affected by Down's syndrome. Studies were all of a similar size. Studies presented data at a 5% FPR. At this cut‐point, the sensitivity was estimated as 25% (95% CI 18 to 34) and the specificity as 95% (95% CI 94 to 96).
7) Other test combinations
Of the 73 test combinations evaluated in three or fewer studies, several test combinations demonstrated estimated sensitivities of more than 70% and estimated specificities of more than 90%. Twelve of these were evaluated in single studies (Table 2), however, three test combinations were evaluated in two or more studies.
A triple test ofPAPP‐A, free ßhCG, AFP and maternal age was evaluated in three studies (Muller 2003a; Tsukerman 1999; Wald 2003a), had an estimated sensitivity of 74% (95% CI 65 to 81) at a cut‐point of 5% FPR.
A triple test of ADAM 12, PAPP‐A, free ßhCG and maternal age was evaluated in three studies (Christiansen 2010; Torring 2010; Valinen 2009), had an estimated sensitivity of 74% (95% CI 63 to 83) at a cut‐point of 5% FPR.
A triple test of PlGF, PAPP‐A, free ßhCG and maternal age was evaluated in two studies (Cowans 2010; Zaragoza 2009), had an estimated sensitivity of 76% (95% CI 69 to 82) at a cut‐point of 5% FPR.
Comparative analysis of the nine selected test strategies
We chose to estimate detection rates at a 5% FPR, in common with much of the literature. Figure 2 shows point estimates of detection rates for a 5% FPR based on all available data for all nine test combinations described above, and the confidence intervals at a fixed 5% FPR. For example, the plot shows that for the double test with a marker combination of free βhCG, AFP and maternal age, the estimated detection rate at a 5% FPR was 49% (95% CI 39 to 60) based on data from three studies with 157 affected cases and 2992 total participants. The test combinations in Figure 2 are ordered according to decreasing detection rates. The single test strategies with and without maternal age (PAPP‐A alone; free βhCG alone, PAPP‐A and maternal age, and free βhCG and maternal age) have the worst performance, whereas, the triple test strategies (ADAM 12, PAPP‐A, free βhCG and maternal age; PAPP‐A, free βhCG, AFP and maternal age) have the highest performance. In between lie the double tests (free βhCG, PAPP‐A and maternal age; free βhCG, AFP and maternal age). However, it should be noted that the confidence intervals on these estimates are wide and overlap for the lower performing five strategies, suggesting that any of the differences observed may be explicable by chance.
2.

Detection rates (sensitivity) at a 5% false positive rate for the nine selected test strategies. Each circle represents the summary sensitivity for a test strategy and the size of each circle is proportional to the number of Down's cases. The estimates are shown with 95% confidence intervals. The test strategies are ordered on the plot according to decreasing detection rate. The number of studies, cases and women included for each test strategy are shown on the horizontal axis.
A = Age, PlGF, PAPP‐A and free ßhCG; B = Age, PAPP‐A, free ßhCG and AFP; C = Age, ADAM 12, PAPP‐A and free ßhCG; D = Age, PAPP‐A and free ßhCG ; E = Age, PAPP‐A; F = PAPP‐A; G = Age, free ßhCG and AFP ; H = Age, free ßhCG; I = Free ßhCG
Table 3 shows pair‐wise direct comparisons (head‐to‐head) where studies were available. Such comparisons are regarded as providing the strongest evidence as they compare tests within pregnancies and are thus unconfounded. The table shows the ratios of sensitivities with 95% CIs and P values (P < 0.05 being considered a statistically significant difference) for each test comparison, the number of studies (K) for which data were available. The table shows that the sensitivity of the single test combinations (PAPP‐A alone, free βhCG alone, PAPP‐A and maternal age, and free βhCG and maternal age) tended to be significantly worse (P < 0.05) than the double and triple tests where data are available. The double test comprised of PAPP‐A, free βhCG and maternal age appears to have significantly better (P = 0.004) test accuracy than the double test comprised of free βhCG, AFP and maternal age. Otherwise, there was no strong evidence of significant improvements in sensitivity with the addition of a third marker. However, most comparisons in this table are based on only single studies and are unlikely to be powered to detect differences in detection rates.
1. Direct comparisons of the sensitivity of nine test strategies at the 5% false positive rate.
|
Ratio of sensitivity (95% CI), P value for comparison (studies) |
Free ßhCG | PAPP‐A | Age, free ßhCG | Age, PAPP‐A | Age, PAPP‐A , free ßhCG | Age, free ßhCG, AFP | Age, ADAM 12, PAPP‐A, free ßhCG | Age, PAPP‐A, free ßhCG, AFP |
| PAPP‐A | 1.78 (1.10 to 2.88), P = 0.02 (2) |
|||||||
| Age, free ßhCG | 1.67 (1.11 to 2.50). P = 0.013 (2) |
0.94 (0.68 to 1.29), P = 0.70 (2) |
||||||
| Age, PAPP‐A | 2.15 (1.37 to 3.38), P = 0.001 (2) |
1.20 (0.86 to 1.67), P = 0.29 (3) |
1.26 (1.02 to 1.57), P = 0.034 (4) |
|||||
| Age, PAPP‐A , free ßhCG | 2.62 (1.77 to 3.87), P < 0.001 (2) |
1.47 (1.09 to 2.00), P = 0.012 (2) |
1.61 (1.31 tp 1.98), P < 0.001 (5) |
1.26 (1.04 to 1.52), P = 0.02 (4) |
||||
| Age, free ßhCG, AFP | 2.19 (1.31 to 3.64), P = 0.002 (1) |
0.71 (0.52 to 0.98), P = 0.03 (1) |
1.08 (0.80 to 1.46), P = 0.62 (2) |
0.61 (0.46 to 0.82), P < 0.001 (1) |
0.63 (0.47 to 0.86), P = 0.004 (2) |
|||
| Age, ADAM 12, PAPP‐A, free ßhCG | — | — | — | — | 1.04 (0.85 to 1.26), P = 0.71 (2) |
— | ||
| Age, PAPP‐A, free ßhCG, AFP | 3.94 (2.49 to 6.23), P < 0.001 (1) |
1.29 (1.03 to 1.60), P = 0.024 (1) |
1.91 (1.42 to 2.56), P < 0.001 (1) |
1.11 (0.91 to 1.34), P = 0.31 (1) |
1.02 (0.88 to 1.20), P = 0.77 (2) |
1.62 (1.19 to 2.19), P = 0.002 (2) |
— | |
| Age, PlGF, PAPP‐A, free ßhCG | — | — | — | — | 1.03 (0.91 to 1.17), P = 0.61 (2) |
— | — | — |
— indicates that no comparative study was available for the pair of tests.
Direct comparisons were made only using data from studies which compared each pair of tests on the same women. Where there were at least two studies, meta‐analysis was performed to summarise and compare the sensitivities. The ratio of sensitivities was computed by division of the sensitivity for the column by the sensitivity for the row. If the ratio of sensitivity is greater than one then the sensitivity of the test for the column is higher than that for the row, if less than one the sensitivity of the test in the row is higher than in the column. All test comparisons that were evaluated by only one study were from Wald 2003. The ratio of the sensitivities for test comparisons from a single study were calculated as a ratio of two proportions.
ADAM12: a disintegrin and metalloprotease; AFP: alpha‐fetoprotein; ßhCG: beta human chorionic gonadotrophin; CI: confidence interval; PAPP‐A: pregnancy‐associated plasma protein A; PIGF: placental growth factor; PROMBP: proform of eosinophil major basic protein
Table 4 shows the same comparisons made using all available data (as used to create Figure 2). Results are in agreement with the direct comparisons, and in addition, showed that the triple test comprised of PlGF, PAPP‐A, free ßhCG and maternal age is significantly better (P = 0.024) than the double test comprised of PAPP‐A, free βhCG and maternal age. However, these comparisons are potentially confounded by differences between the studies, and are based on small numbers of studies.
2. Indirect comparisons of the sensitivity of nine test strategies at the 5% false positive rate.
|
Ratio of sensitivity (95% CI), P value for comparison |
Free ßhCG | PAPP‐A | Age, free ßhCG | Age, PAPP‐A | Age, PAPP‐A, free ßhCG | Age, free ßhCG, AFP | Age, ADAM 12, PAPP‐A, free ßhCG | Age, PAPP‐A, free ßhCG, AFP | ||
| Studies (cases/ women) | 4 (390/4280) |
4 (325/2837) |
7 (460/5893) |
5 (359/3491) |
17 (1037/49827) |
3 (157/2992) |
2 (74/1222) |
2 (116/2705) |
||
| Studies (cases/ women) | Sensitivity % (95% CI) | 25 (18 to 34) | 52 (39 to 65) | 42 (36 to 48) | 55 (46 to 63) | 68 (65 to 71) | 49 (39 to 60) | 74 (63 to 83) | 74 (65 to 81) | |
| PAPP‐A | 4 (325/2837) |
52 (39 to 65) | 2.05 (1.37 to 3.09), P = 0.001 |
|||||||
| Age, free ßhCG | 7 (460/5893) |
42 (36 to 48) | 1.66 (1.17 to 2.36), P = 0.004 |
0.81 (0.61 to 1.08), P = 0.15 |
||||||
| Age, PAPP‐A | 5 (359/3491) |
55 (46 to 63) | 2.16 (1.51 to 3.10), P < 0.001 |
1.05 (0.78 to 1.42), P = 0.73 |
1.30 (1.05 to 1.61), P = 0.015 |
|||||
| Age, PAPP‐A, free ßhCG | 17 (1037/49827) |
68 (65 to 71) | 2.70 (1.95 to 3.73), P < 0.001 |
1.31 (1.02 to 1.70), P = 0.037 |
1.62 (1.40 to 1.88), P < 0.001 |
1.25 (1.05 to 1.47), P = 0.01 |
||||
| Age, free ßhCG, AFP | 3 (157/2992) |
49 (39 to 60) | 1.95 (1.33 to 2.86), P = 0.001 |
0.95 (0.69 to 1.32), P = 0.76 |
1.18 (0.92 to 1.51), P = 0.20 |
0.90 (0.69 to 1.17), P = 0.45 |
0.72 (0.59 to 0.89), P = 0.003 |
|||
| Age, ADAM 12, PAPP‐A, free ßhCG | 2 (74/1222) |
74 (63 to 83) | 2.94 (2.07 to 4.16), P < 0.001 |
1.43 (1.07 to 1.90), P = 0.014 |
1.77 (1.46 to 2.14), P < 0.001 |
1.36 (1.10 to 1.67), P = 0.004 |
1.09 (0.95 to 1.25), P = 0.24 |
1.50 (1.17 to 1.92), P = 0.001 |
||
| Age, PAPP‐A, free ßhCG, AFP | 2 (116/2705) |
74 (65 to 81) | 2.93 (2.09 to 4.11), P < 0.001 |
1.43 (1.08 to 1.88), P = 0.011 |
1.76 (1.48 to 2.10), P < 0.001 |
1.35 (1.11 to 1.64), P = 0.002 |
1.09 (0.97 to 1.22), P = 0.16 |
1.50 (1.19, to 1.89). P = 0.001 |
1.00 (0.84 to 1.18), P = 0.98 |
|
| Age, PlGF, PAPP‐A, free ßhCG | 2 (160/1144) |
76 (69 to 82) | 3.01 (2.16 to 4.20), P < 0.001 |
1.47 (1.12 to 1.91), P = 0.005 |
1.81 (1.54 to 2.14), P < 0.001 |
1.39 (1.16 to 1.67), P < 0.001 |
1.12 (1.01 to 1.23), P = 0.024 |
1.54 (1.23 to 1.93), P < 0.001 |
1.03 (0.87 to 1.20), P = 0.75 |
1.03 (0.90 to 1.18), P = 0.7 |
Ratio of sensitivities were computed by division of the sensitivity for the column by the sensitivity for the row. If the ratio of sensitivity is greater than one then the sensitivity of the test for the column is higher than that for the row, if less than one the sensitivity of the test in the row is higher than in the column.
AFP: alpha‐fetoprotein; αhCG: alpha human chorionic gonadotrophin; ßhCG: beta human chorionic gonadotrophin; CI: confidence interval; PAPP‐A: Pregnancy‐associated plasma protein A
Investigation of heterogeneity and sensitivity analyses
The key characteristics of the 56 included studies is summarised in Table 5 with further details available in the Characteristics of included studies table. Only one test combination— PAPP‐A, free ßhCG and maternal age (17 studies) was evaluated by 10 or more studies but there were no data for investigation of the effect of maternal age or any other potential source of heterogeneity. The planned sensitivity analyses were also not possible.
3. Summary of study characteristics.
| Study | PAPP‐A, free ßhCG and age* | Maternal age (years) | Reference standard | Population | Study design | Study location |
| Baviera 2010 | Mean 35.3 for Down's cases, 30.4 for control | Amniocentesis or follow‐up to birth | Routine screening | Case‐control | Italy | |
| Benattar 1999 | Mean 32 (16‐46), 8.3% > 35 | Amniocentesis due to maternal age > 38 years (6.1% or women). Karyotyping encouraged for women with positive result on one or more index test. No details of reference standard for index test negative women | Routine screening | Prospective cohort | France | |
| Biagiotti 1995 | Not reported | Amniocentesis or CVS | High‐risk referral for invasive testing | Case‐control | Italy | |
| Biagiotti 1998 | X | Unclear (maybe all ≥ 38) | Amniocentesis or CVS | High‐risk referral for invasive testing | Retrospective case‐control | Italy |
| Brambati 1993 | Median 38 (20‐47) | CVS | High‐risk referral for invasive testing | Retrospective cohort | Italy | |
| Brambati 1994 | X | Not reported | CVS | High‐risk referral for invasive testing | Case‐control | Italy |
| Brameld 2008 | Median 31 (14‐47), 20% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Australia | |
| Brizot 1994 | Median 38 (22‐45) | Fetal karyotyping | High‐risk referral for invasive testing | Retrospective case‐control | UK | |
| Casals 1996 | 94.4% > 35 | CVS | High‐risk referral for invasive testing | Retrospective case‐control | Spain | |
| Christiansen 1999 | Not reported | Karyotyping | High‐risk referral for invasive testing | Case‐control | Denmark | |
| Christiansen 2004 | Not reported | CVS (for 120 of cases of Down's) or follow‐up to birth (for 36 of cases of Down's) | Routine screening | Case‐control | Denmark | |
| Christiansen 2005 | Not reported | Karyotyping | Screening programmes for syphilis and Down's syndrome | Case‐control | Denmark | |
| Christiansen 2007a | X | Median 37.7 (24‐48) for Down's cases, 36.4 (22‐44) for controls | Karyotyping or follow‐up to birth | Routine screening | Case‐control | Denmark |
| Christiansen 2009 | X | Median 37.5 for Down's cases, 36.4 for controls | Karyotyping or follow‐up to birth | Routine screening | Case‐control | Denmark |
| Christiansen 2010 | X | Median 36 (25‐44) for Down's cases, 29 (17‐45) for controls | Karyotyping or follow‐up to birth | Routine screening | Case‐control | Denmark |
| Cowans 2010 | X | Mean 37.0 (IQR 32.9‐40.5) for Down's cases, 32.4 (IQR 29.0‐35.9) for controls | Karyotyping or follow‐up to birth | Routine screening | Case‐control | UK |
| Crandall 1993 | 90% > 35 | Amniocentesis | High‐risk referral for invasive testing | Retrospective cohort | USA | |
| Crossley 2002a | Median 29.9, 15.4% ≥ 35 | CVS offered where women had high NT measurements. Also amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | UK | |
| De Graaf 1999a | X | Not reported | Amniocentesis or CVS | High‐risk referral for invasive testing | Case‐control | The Netherlands |
| Forest 1995 | Mean 29.1 (SD 4.7), 10.7% ≥ 35 | Follow‐up to birth | Routine screening | Case‐control | Canada | |
| Forest 1997 | X | Mean 27.9, 10.7% ≥ 35 | Follow‐up to birth | Routine screening | Case‐control | Canada |
| Gyselaers 2005 | Not reported | Amniocentesis, CVS and postnatal karyotyping | Routine screening | Prospective cohort | Belgium | |
| Haddow 1998 | X | Median 37 (15‐51) | Amniocentesis or CVS | High‐risk referral for invasive testing | Prospective cohort | USA |
| Kagan 2009 | X | Mean 35.4 (14.1‐52.2) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | UK |
| Kornman 1998 | Not reported | CVS | High‐risk referral for invasive testing | Case‐control | The Netherlands | |
| Kozlowski 2007 GC | Median 32 (15‐48), 26.4% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | |
| Kozlowski 2007 PC | Median 34 (14‐46), 43.2% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | |
| Krantz 2000 | 34.7% ≥ 35 | Not reported | Routine screening | Prospective cohort | USA | |
| Kratzer 1991 | Missing | CVS | High‐risk referral for invasive testing | Case‐control | USA | |
| Laigaard 2003 | Not reported | Karyotyping, unclear reference standard for controls | Routine screening | Case‐control | Denmark | |
| Macintosh 1993 | Median 38 (27‐40) | CVS | High‐risk referral for invasive testing | Retrospective cohort | UK and Italy | |
| Muller 2003a | Not reported | Invasive testing (offered to women with high NT measurement) or follow‐up to birth | Routine screening | Retrospective cohort | France | |
| Nebiolo 1990 | Approximately 75% ≥ 35 | CVS | High‐risk referral for invasive testing | Retrospective cohort | Italy | |
| Niemimaa 2001a | 17.5% ≥35 | Invasive testing (patients considered high‐risk based on NT screening) or follow‐up to birth | Routine screening | Prospective cohort | Finland | |
| Noble 1995 | Median 34 (15‐47), 47% ≥ 35 | Karyotyping performed (27%), ultrasound examination at 20 weeks (65%), or follow‐up to birth (9%). | Routine screening in a high‐risk population | Prospective cohort | UK | |
| Noble 1997 | Median 34 (15‐47) | CVS, follow‐up to birth not reported | Routine screening | Case‐control | UK | |
| O'Leary 2006 | Median 31 (14‐47), 20% ≥ 35 years | CVS or amniocentesis (women assessed to be high risk on screening) or follow‐up to birth | Routine screening | Prospective cohort | Australia | |
| Orlandi 1997 | Range 15‐46, 35% ≥ 35 | Not reported | Routine screening | Prospective cohort | Italy | |
| Palomaki 2007 | Mean maternal age 32.3 years (SD 4.6 years) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Canada | |
| Qin 1997 | Not reported | CVS, amniocentesis, karyotyping at birth, unclear reference standard for control | Routine screening | Case‐control | Denmark | |
| Sahota 2010 | X | Median 33.1, 30.1% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | China |
| Schaelike 2009 | 31.0% ≥35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Germany | |
| Scott 2004 | Median 32 (15‐44), 29% ≥ 35 | Invasive testing or follow‐up to birth | Routine screening | Prospective cohort | Australia | |
| Spencer 1999a | X | Median cases 38 (19‐46), controls 36 (15‐47) | Invasive testing (high‐risk women) or follow‐up to birth | Referred for invasive testing or self‐referred for screening | Case‐control | UK |
| Spencer 2002a | Median cases 36 (20‐44), controls 30 (16‐41) | Not reported | Routine screening | Case‐control | UK | |
| Torring 2010 | X | Mean 35 for Down's, 31 for controls | Karyotyping or follow‐up to birth | Routine screening | Case‐control | Denmark |
| Tsukerman 1999 | X | Not reported | Karyotyping, karyotyping at birth, follow‐up to birth not reported | Routine screening | Case‐control | Belarus |
| Valinen 2007 | Mean 29.6, 18.6% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Finland | |
| Valinen 2009 | Not reported | Karyotyping or follow‐up to birth | Routine screening | Case‐control | Finland | |
| Van Lith 1992 | Not reported | CVS | High‐risk referral for invasive testing | Case‐control | The Netherlands | |
| Wald 2003a | X | Missing | Invasive testing (following second trimester screening) or follow‐up to birth | Routine screening | Case‐control | UK and Austria |
| Wallace 1995 | Mean 32 (22‐44) for Down's cases, 28 (19‐38) for controls | Not reported | Routine screening | Case‐control | UK | |
| Wapner 2003 | X | Mean 35 (SD 4.6), 50% ≥ 35 | Invasive testing, miscarriage with cytogenetic testing, follow‐up to birth | Routine screening | Prospective cohort | USA |
| Weinans 2005 | Mean 38 (SD 2.7) for Down's cases, 37 (SD 3.0) for controls | CVS | High‐risk referral for invasive testing | Case‐control | The Netherlands | |
| Wojdemann 2005 | Mean 29, 10.8% ≥ 35 | Invasive testing (in cases of increased risk) or follow‐up to birth | Routine screening | Prospective cohort | Denmark | |
| Zaragoza 2009 | X | Median 37.9 (19.1‐46.5) for Down's cases, 32.7 (16.1‐45.2) for controls | Karyotyping or follow‐up to birth | Routine screening | Case‐control | UK |
*The PAPP‐A, free ßhCG and age test combination was the only test evaluated by at least 10 studies. X indicates that the test was evaluated in the study.
CVS: chorionic villus sampling; IQR: interquartile range; SD: standard deviation.
Discussion
Summary of main results
The systematic review found a large number of studies evaluating first trimester Down's syndrome serum screening tests, including studies evaluating the commonly used double test. Few studies were available to evaluate the performance of test strategies involving newer markers, such as ADAM 12, and few studies provided unconfounded comparisons of test strategies by applying and comparing several strategies using the same serum sample, the majority of studies only evaluating a single test combination. A summary of results for the nine most common and best performing strategies is given in Table 1, briefer details for the remaining strategies are given in Table 2.
Three key findings were noted.
The double test comprised of PAPP‐A, free βhCG and maternal age appears to have significantly better (P < 0.05) test accuracy than the double test comprised of free βhCG, AFP and maternal age, and the single tests (both the markers alone and in combination with maternal age). This test detects around seven out of every 10 Down's affected pregnancies for a fixed 5% FPR. By comparison, the double test comprised of free βhCG, AFP and maternal age, and single tests alone and in combination with maternal age detects between two and five out of every 10 Down's affected pregnancies for a fixed 5% FPR.
Whilst the triple test combinations show the highest detection rates, they were not shown to be statistically superior to the double test comprised of PAPP‐A, free βhCG and maternal age. Whilst some significant differences between these categories of tests were noted in the indirect comparisons, the potential for confounding is of concern. Estimates suggest that triple test combinations may detect between seven and eight out of every 10 Down's syndrome pregnancies at a 5% FPR, however these estimates are based on data from two or three studies evaluating small numbers of women. It is difficult to make strong recommendations on the use of triple tests, as we cannot rule out possible differences due to the limited power there is to detect a difference.
The evidence for higher numbers of markers shows similar detection rates to double and triple markers, but are based on data from one study only, therefore further evaluation of these tests is required. Furthermore, there are other combinations of double markers that show similar detection rates to standard double markers commonly used in clinical practice, which may warrant further study.
Strengths and weaknesses of the review
This review is the first comprehensive review of first trimester serum screening. We examined papers from around the world, covering a wide cross‐section of women in varying populations. We contacted authors to verify data where necessary to give as complete a picture as possible, while trying to avoid replication of data.
There were a number of factors that made meta‐analysis of the data difficult, which we tried to adapt for, in order to allow for comparability of data presented in different studies.
There were many different cut‐points used to define pregnancies as high or low risk for Down's syndrome. This means that direct comparison is more difficult than if all studies used the same cut‐point to dichotomise their populations. This is less of an issue for first trimester serum screening, compared to second trimester serum screening, as the majority of authors chose a cut‐point of 5% FPR.
There were many different risk equations and software applications in use for combination of multiple markers, which were often not described in the papers. This means that risks may be calculated by different formulae and they may not be directly comparable for this reason. It is possible that this is responsible for unexplained heterogeneity in results.
Different laboratories and clinics run different assays and use different machines and methods. This may influence raw results and subsequent risk calculations. Many laboratories have a quality assessment or audit trail, however, this may not necessarily be standard across the board. For example, how many assays are run, how often medians are calculated and adjusted for a given population and how quickly samples are tested from initially being taken.
Few papers made direct comparisons between tests, making it difficult to detect if a real difference exists between tests (i.e. how different tests perform in the same population). There were differences in populations, with assay medians being affected, for example, by race. It is not certain whether it is appropriate to make comparisons between populations which are inherently different.
We were unable to perform the investigations of heterogeneity that we had originally intended to because the data simply were not available. The vast majority of papers looking at pregnancies conceived by IVF, affected by diabetes, multiple gestation or a family history of Down's syndrome involved unaffected pregnancies only.
In addition, the search for this review was last updated in August 2011, and it is possible that new studies may have been published which have not been included. Since the search was completed we have kept a watching brief on outputs and are not aware of any studies with substantial sample sizes which could substantially affect the findings.
Applicability of findings to the review question
Potentially, when planning screening policy or a clinical screening programme, clinicians and policy makers need to make decisions about a finite number of tests or type of tests that can be offered. These policies are often driven by both the needs of a specific population and by financial resources. Economic analysis was considered to be outside of the scope of this review. Many of the tests examined as part of this review are already commercially available and in use in the clinical setting. The studies were carried out on populations of typical pregnant women and therefore, the results should be considered comparable with most pregnant populations encountered in every day clinical practice.
We were unable to extract information about harms of testing, information about miscarriage rates and uptake of definitive testing as the data were not available the majority of the time. Whilst it is unlikely that major differences between the tests evaluated here exist in terms of direct harms of testing, as they are all based on a single blood sample, differences in accuracy may lead to differences in the use of definitive testing and its consequent adverse outcomes.
In some countries with a defined screening policy (i.e. the UK), first trimester screening plays a major role, although usually in combination with first trimester ultrasound scanning. In others however, there may only be a limited range of tests or markers available, often second trimester markers, rather than first trimester markers. The results of this review should be interpreted and applied in the context of test availability and local restrictions, populations or policies.
Authors' conclusions
Implications for practice.
The evidence supports the use of the first trimester double test comprised of PAPP‐A, free βhCG and maternal age, there is little evidence to recommend the use of first trimester serum tests with three or more markers, however the data available on these tests are limited, and based on generally small populations of women. We would not recommend that these tests be introduced into wider clinical practice without careful consideration of cost.
The review has shown that tests involving two or three markers in combination with maternal age are significantly better than those involving one marker. We would therefore recommend that one marker tests are not used for Down's syndrome screening. The choice of multiple markers will depend on the availability of certain assays in local laboratories. On the basis of this review we would recommend the combination of PAPP‐A, free βhCG and maternal age, as it significantly outperforms free βhCG, AFP and maternal age, and is widely available. The data for other test combinations limits our ability to make any other recommendations about specific test combinations. Alternative screening methods should also be considered (i.e. use of ultrasound markers in the first trimester) when making policy decisions, and are the subject of other reviews in this suite.
The screening blood tests themselves have no adverse effects for the woman, over and above the risks of a routine blood test. However some women who have a ‘high risk’ screening test result, and are given amniocentesis or CVS have a risk of miscarrying a baby unaffected by Down’s. Parents will need to weigh up this risk when deciding whether or not to have an amniocentesis or CVS following a ‘high risk’ screening test result.
Implications for research.
Further evaluations of test combinations involving three or more markers are required to determine whether their apparent advantages are not chance findings.
Future studies should ensure that adequate sample sizes are recruited, and take opportunities to make comparisons of test performance testing several alternative test combinations on the same serum samples. Such direct comparison removes issues of confounding when making test comparisons, and allows a clear focus on testing the incremental benefit of increasingly complex and expensive testing strategies. The reporting of studies of test accuracy can be improved and more closely adhere to the standards for the reporting of diagnostic accuracy studies (STARD) guideline. Three key aspects of this are 1) formally testing the statistical significance of differences in test performance in direct comparisons and estimating incremental changes in detection rates (together with confidence intervals), 2) clearly reporting the number of mothers studied and their results, and 3) reporting the numbers of women who are lost to follow‐up. Many authors reported results of extrapolating findings to age‐standardised national cohorts to demonstrate the performance of the test, and failed to report the actual numbers studied and evaluated.
For the purposes of meta‐analysis and to allow for comparisons to be made between different tests and combinations, we would recommend the publication of consensus standard algorithms for estimating risk, and reporting of test performance at a standard set of thresholds. This would be difficult to achieve and implement, but an attempt at consensus should be made.
Notes
This is one of a suite of planned systematic diagnostic test reviews planned for prenatal testing for fetal Down’s syndrome. The plans for these reviews were described in a generic protocol (Alldred 2010) published in the Cochrane Library in 2010. The five reviews were to be of: first trimester serum tests only; first trimester ultrasound tests alone, and in combination with first trimester serum tests; second trimester serum tests only; first and second trimester serum tests with and without first trimester ultrasound tests; and urine tests. One of these reviews has been published already (Alldred 2012). Diagnostic test reviews are relatively new, and this project has proven much larger, more complex and difficult to complete than had been anticipated. Whilst not fulfilling the usual Cochrane up‐to‐date criteria (the electronic search was done in 2011), this review is published because it provides historical context in what is a rapidly‐changing field, and because it is unlikely to ever be repeated.
Acknowledgements
We acknowledge the assistance of the Pregnancy and Childirth Cochrane Review Group Editorial base with writing the searches (detailed in the generic protocol Alldred 2010) and other aspects of this review.
Appendices
Appendix 1. Search Strategy
Database: Ovid MEDLINE
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Prenatal Diagnosis/
2 nuchal translucency.mp.
3 exp Pregnancy‐Associated Plasma Protein‐A/
4 pregnancy associated plasma protein a.mp.
5 papp‐a.mp.
6 exp Chorionic Gonadotropin, beta Subunit, Human/
7 (b‐hcg or bhcg).mp.
8 human chorionic gonadotropin.mp.
9 exp alpha‐Fetoproteins/
10 alphafetoprotein$.mp.
11 alpha‐fetoprotein$.mp.
12 afp.mp.
13 (unconjugated estriol or unconjugated oestriol).mp.
14 ue3.mp.
15 exp INHIBINS/
16 inhibin a.mp.
17 ultrasound.mp.
18 amniocentesis/
19 chorion$ vill$ sampling.mp.
20 Chorionic Villi‐Sampling/
21 nasal bone.mp.
22 tricuspid regurgitation.mp.
23 ductus venosus.mp
24 marker$.mp.
25 screen$.mp.
26 detect$.mp.
27 accura$.mp.
28 predict$.mp.
29 ROC.mp.
30 ROC curve/
31 AUC.mp.
32 Area under curve/
33 exp false negative reactions/ or exp false positive reactions/
34 (false positive$ or false negative$).mp.
35 likelihood ratio$.mp.
36 sensitiv$.mp.
37 specific$.mp.
38 diagnos$.ti,ab.
39 "reproducibility of results".mp.
40 reference value$.mp.
41 reference standard$.mp.
42 exp Down Syndrome/
43 downs syndrome.mp.
44 down syndrome.mp.
45 trisomy 21.mp.
46 Aneuploidy/
47 aneuploidy.mp.
48 Mosaicism/
49 mosaicism.mp.
50 or/1‐41
51 or/42‐49
52 50 and 51
53 (antenatal$ or prenatal$ or trimester$ or pregnan$ or fetus or foetus or fetal or foetal).mp.
54 52 and 53
55 animal/ not (humans/ and animal/)
56 54 not 55
*******************************************************
Embase via Dialog Datastar
1. PRENATAL‐DIAGNOSIS#.DE.
2. FETUS‐ECHOGRAPHY#.DE.
3. PREGNANCY‐ASSOCIATED‐PLASMA‐PROTEIN‐A#.DE.
4. CHORIONIC‐GONADOTROPIN‐BETA‐SUBUNIT#.DE.
5. HCG.AB.
6. PAPP.AB.
7. ALPHA‐FETOPROTEIN#.DE.
8. AFP.AB.
9. ALPHA ADJ FETOPROTEIN$
10. ALPHAFETOPROTEIN$
11. BETA ADJ HUMAN ADJ CHORIONIC ADJ GONADOTROPIN
12. PREGNANCY ADJ ASSOCIATED ADJ PLASMA ADJ PROTEIN
13. (UNCONJUGATED ADJ ESTRIOL OR UNCONJUGATED ADJ OESTRIOL).TI.
14. (UNCONJUGATED ADJ ESTRIOL OR UNCONJUGATED ADJ OESTRIOL).AB.
15. UE3
16. INHIBIN‐A#.DE.
17. INHIBIN ADJ A
18. ULTRASOUND
19. AMNIOCENTESIS
20. CHORION‐VILLUS‐SAMPLING.DE.
21. NASAL ADJ BONE
22. TRICUSPID ADJ REGURGITATION
23. DUCTUS ADJ VENOSUS
24. MARKER OR MARKERS
25. SCREEN OR SCREENING
26. DETECT OR DETECTING OR DETECTION
27. FALSE ADJ POSITIVE$
28. FALSE ADJ NEGATIVE$
29. SENSITIVITY OR SENSITIVE OR SENSITIVITIES
30. SPECIFICITY OR SPECIFICITIES
31. (DIAGNOSE OR DIAGNOSIS OR DIAGNOSTIC OR DIAGNOSTICS OR DIAGNOSES
OR DIAGNOSED).TI.
32. (DIAGNOSE OR DIAGNOSIS OR DIAGNOSTIC OR DIAGNOSTICS OR DIAGNOSES
OR DIAGNOSED).AB.
33. ROC.AB.
34. AUC.AB.
35. AREA‐UNDER‐THE‐CURVE.DE.
36. ROC‐CURVE.DE.
37. ACCURA$
38. PREDICT$
39. REPRODUCIBILITY.DE.
40. REFERENCE ADJ VALUE$
41. REFERENCE‐VALUE.DE.
42. REFERENCE ADJ STANDARD$
43. DOWN‐SYNDROME#.DE.
44. DOWN ADJ SYNDROME OR DOWNS ADJ SYNDROME
45. TRISOMY ADJ '21'
46. MOSAICISM
47. ANEUPLOIDY
48. ANTENATAL$ OR PRENATAL$ OR PREGNANCY OR PREGNANT OR TRIMESTER$ OR MATERNAL OR FETUS OR FOETUS OR FOETAL OR FETAL
49. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42
50. 43 OR 44 OR 45 OR 46 OR 47
51. 48 AND 49 AND 50
52. HUMAN=YES
53. 51 AND 52
ADJ = adjacent AB = abstract
TI = title $ = truncation symbol DE = descriptor (similar to MeSH)
*******************************************************
CINAHL via OVID
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Prenatal Diagnosis/
2 nuchal translucency.mp.
3 pregnancy associated plasma protein.mp.
4 papp$.ti,ab.
5 exp Gonadotropins, chorionic/
6 (b‐hcg or bhcg).mp.
7 human chorionic gonadotropin.mp.
8 exp alpha‐Fetoproteins/
9 alphafetoprotein$.mp.
10 alpha‐fetoprotein$.mp.
11 afp.mp.
12 (unconjugated estriol or unconjugated oestriol).mp.
13 ue3.mp.
14 inhibin$.mp.
15 ultrasound.mp.
16 amniocentesis/
17 chorion$ vill$ sampling.mp.
18 Chorionic Villi‐Sampling/
19 nasal bone.mp.
20 tricuspid regurgitation.mp.
21 ductus venosus.mp.
22 marker$.mp.
23 screen$.mp.
24 detect$.mp.
25 accura$.mp.
26 predict$.mp.
27 ROC.mp.
28 ROC curve/
29 AUC.mp.
30 "area under curve".mp.
31 exp false negative reactions/ or exp false positive reactions/
32 (false positive$ or false negative$).mp.
33 likelihood ratio$.mp.
34 sensitiv$.mp.
35 specific$.mp.
36 diagnos$.ti,ab.
37 "reproducibility of results".mp.
38 reference value$.mp.
39 reference standard$.mp.
40 exp Down Syndrome/
41 downs syndrome.mp.
42 down syndrome.mp.
43 trisomy 21.mp.
44 aneuploidy.mp.
45 mosaicism.mp.
46 (antenatal$ or prenatal$ or trimester$ or pregnan$ or fetus or foetus or fetal or foetal).mp.
47 or/1‐39
48 or/40‐45
49 47 and 48 and 46
*******************************************************
Search terms and instructions for Biosis
The following search terms were entered separately in standard search box (select ‘Titles/subject/abstract’ from the drop‐down box on the right of the search box).
“reference standard*”
“reference value*”
“reproducibility of results”
diagnos*
sensitiv*
specific*
“likelihood ratio*”
“false negative*
“false positive”
“area under curve”
ROC
AUC
predict*
detect*
marker*
screen*
accura*
“ductus venosus”
“nasal bone”
“tricuspid regurgitation”
“chorion* vill* sampling”
amniocentesis
ultrasound
inhibin*
“unconjugaed oestriol”
“unconjugated estriol”
afp
“alpha fetoprotein*”
alphafetoprotein*
“ bhcg”
“human chorionic gonadotrophin”
“papp a”
“pregnancy associated plasma protein”
“nuchal translucency”
foetal
fetal
foetus
foetal
prenatal*
antenatal*
pregnan*
maternal*
“trisomy 21”
mosaicism
“down* syndrome”
The search then used the history function to combine terms:
1‐34 – combine using OR
35 – 42 – combine using OR
43 – 45 – combine using OR
The three sets were combined using AND
The combined search strategy had the form
(((((((al: "trisomy 21") or (al: (mosaicism))) or (al: "down* syndrome"))) and (((((((((((((((((((((((((((((((((((al: "reference standard*") or (al: "reference value*")) or (al: "reproducibility of results")) or (al: (diagnos*))) or (al: (specific*))) or (al: (sensitiv*))) or (al: "likelihood ratio*")) or (al: "false negative*")) or (al: "false positive*")) or (al: "area under curve")) or (al: (auc))) or (al: (roc))) or (al: (predict*))) or (al: (accura*))) or (al: (detect*))) or (al: (screen*))) or (al: (marker*))) or (al: "ductus venosus")) or (al: "tricuspid regurgitation")) or (al: "nasal bone")) or (al: "chorion* vill* sampling")) or (al: (amniocentesis))) or (al: (ultrasound))) or (al: (inhibin*))) or (al: "unconjugated oestriol")) or (al: "unconjugated estriol")) or (al: (afp))) or (al: "alpha feto protein*")) or (al: "alpha fetoprotein*")) or (al: "b hcg")) or (al: "human chorionic gonadotropin")) or (al: "papp a")) or (al: "pregnancy associated plasma protein")) or (al: "nuchal translucency")))) and (((((((((al: (foetal)) or (al: (fetal))) or (al: (foetus))) or (al: (fetus))) or (al: (pregnan*))) or (al: (trimester*))) or (al: (prenatal*))) or (al: (antenatal*))))))
*******************************************************
The Database of Abstracts of Reviews of Effectiveness (DARE), National Research Register and Health Services Research Projects in Progress database
:
Down syndrome (MeSH)
down* next syndrome
trisomy
aneuploidy
mosaicism
OR/ 1‐5
*******************************************************
MEDION (http://www.mediondatabase.nl/)
ICPC code for pregnancy – ‘W’.
*******************************************************
The Database of Systematic Reviews and Meta‐Analyses in Laboratory Medicine – download the database to a .pdf file and search for the following terms separately:
Down
Trisomy
Aneuploidy
Pregnant
Pregnancy
Pregnancies
Mosaicism
*******************************************************
Appendix 2. Glossary of terms (adapted in part from the UK National Screening Committee Glossary)
| Abnormal ductus venosus flow velocity | The ductus venosus is a vessel in the fetus which allows oxygenated blood from the placenta to bypass the fetal liver and flow straight to the heart. In conditions such as Down’s syndrome the pressure in this vessel can be abnormally high. |
| Absent nasal bone | Absence of the bone that forms the bridge of the nose, which may be detected at ultrasound scan during early pregnancy. |
| Affected individuals | Those individuals who are affected by the disorder for which they are being screened. |
| Amniocentesis | Amniocentesis is an invasive procedure which involves taking a small sample of the amniotic fluid (liquor) surrounding the baby, using a needle which goes through the abdominal wall into the uterus, and is usually performed after 15 weeks' gestation. |
| Chorionic villus sampling (CVS) | Chorionic villus sampling involves taking a sample of the placental tissue using a needle which goes through the abdominal wall and uterus or a cannula through the cervix. It is usually performed between 10 and 13 weeks' gestation. |
| Combined test | First trimester test (up to 13 + 6 weeks of pregnancy) based on combining nuchal translucency (NT) measurement with free beta‐hCG, pregnancy‐associated plasma protein A (PAPP‐A) and the woman’s age. |
| Diagnostic accuracy | The amount of agreement between the information from the index test and the reference standard (see below). |
| Diagnostic test | A definitive test, performed after a positive screening test result that gives a diagnosis (i.e. yes or no)? |
| Double test | Second trimester test (from 13 + 6 up to 24 weeks of pregnancy) based on the measurement of alpha‐fetoprotein (AFP), human chorionic gonadotrophin (hCG ß either free beta‐hCG or total hCG), together with the woman’s age. |
| First trimester | Pregnancy from conception up to 13 weeks and 6 days. |
| Iatrogenic | A disease or condition in a patient occurring as a result of treatment. |
| Index test | A test or group of tests being evaluated in a systematic review. |
| Integrated test | Measurements performed at different times of pregnancy combined into a single test result. Unless otherwise specified, 'integrated test' refers to the combination of nuchal translucency measurement and PAPP‐A in the first trimester, with the quadruple test (see below) in the second. |
| Mosaicism | This is a condition in which person has some cells containing a normal number of chromosomes, and some containing an abnormal number. The more abnormal cells there are, the greater the effect. |
| Multiple of the median (MOM) | The serum test concentration for a pregnant woman divided by the average (median) for unaffected pregnancies in a defined population at the same stage of pregnancy. |
| Quadruple test | Second trimester test (from 13 + 6 up to 24 weeks of pregnancy) based on the measurement of AFP, uE3, free beta‐hCG (or total hCG), and inhibin‐A together with the woman’s age. |
| Reference Standard | The best available method for establishing the presence or absence of the target disease or condition. |
| Second trimester | Pregnancy from 14 weeks to 28 weeks' gestation. Note that for the purposes of this Cochrane review, second trimester testing refers to the period of 14 to 24 weeks' gestation. |
| Tricuspid regurgitation | Leakiness of or backflow of blood through the tricuspid valve of the heart. The tricuspid valve separates the upper and lower chambers of the right side of the heart. |
| Triple test | Second trimester test (from 14 up to 24 weeks of pregnancy) based on the measurement of AFP, unconjugated oestriol (uE3), and hCG (either total hCG or free beta‐hCG) together with the woman's age. |
| Trisomy | The presence of an extra chromosome resulting in three copies of a particular chromosome instead of the normal two. |
| Translocation | Part of one chromosome is broken off and attached to another chromosome. This does not usually cause the individual any problems as they have a normal amount of chromosomes, but in an abnormal arrangement. It can be passed on as an extra chromosome to offspring, resulting in conditions such as Down's syndrome. |
Appendix 3. QUADAS questionnaire
QUADAS criteria included the following 10 questions.
Was the spectrum of women representative of the women who will receive the test in practice? (criteria met if the sample was selected from a wide range of childbearing ages, or selected from a specified ‘high risk’ group such as over 35s, family history of Down’s Syndrome, multiple pregnancy or diabetes mellitus, provided all affected and unaffected fetuses included that could be tested at the time point when the screening test would be applied; criteria not met if the sample taken from a select or unrepresentative group of women (i.e. private practice), was an atypical screening population or recruited at a later time point when selection could be affected by selective fetal loss)
Is the reference standard likely to correctly classify the target condition? (amniocentesis, chorionic villus sampling, postnatal karyotyping, miscarriage with cytogenetic testing of the fetus, a phenotypically normal baby or birth registers are all regarded as meeting this criteria)
Did the whole sample or a random selection of the sample receive verification using a reference standard of diagnosis?
Did women receive the same reference standard regardless of the index test result?
Was the reference standard independent of the index test result (i.e. the index test did not form part of the reference standard)?
Were the index test results interpreted without knowledge of the results of the reference standard?
Were the reference standard results interpreted without knowledge of the results of the index test?
Were the same clinical data (i.e. maternal age and weight, ethnic origin, gestational age) available when test results were interpreted as would be available when the test is used in practice?
Were uninterpretable/intermediate test results reported?
Were withdrawals from the study explained?
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
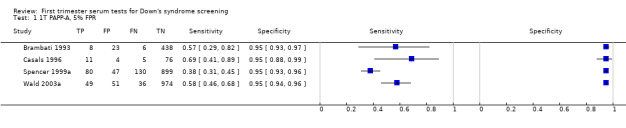
1T PAPP‐A, 5% FPR.
2. Test.

1T PAPP‐A, ≤5th percentile.
3. Test.

1T PAPP‐A, mixed cut‐points.
4. Test.

1T free ßhCG, 5% FPR.
5. Test.
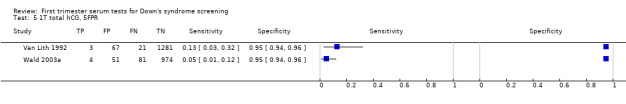
1T total hCG, 5FPR.
6. Test.
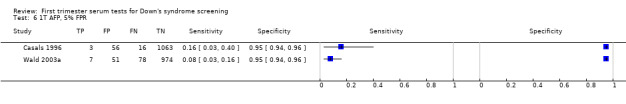
1T AFP, 5% FPR.
10. Test.
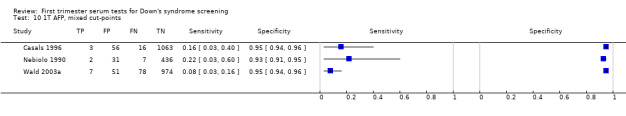
1T AFP, mixed cut‐points.
11. Test.

1T Inhibin, 5FPR.
12. Test.

1T ADAM 12, 5FPR.
13. Test.

1T SP1, 5% FPR.
17. Test.
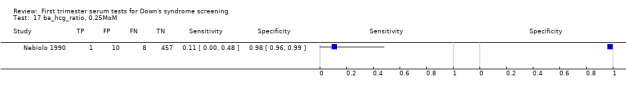
ba_hcg_ratio, 0.25MoM.
18. Test.
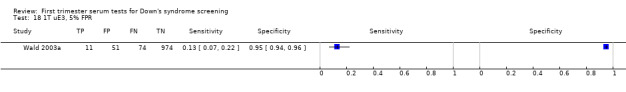
1T uE3, 5% FPR.
19. Test.
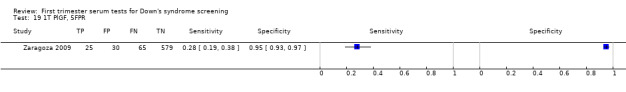
1T PlGF, 5FPR.
20. Test.
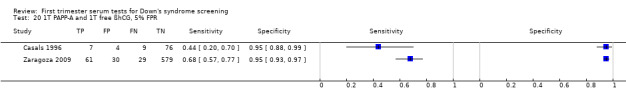
1T PAPP‐A and 1T free ßhCG, 5% FPR.
21. Test.
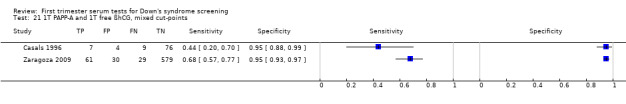
1T PAPP‐A and 1T free ßhCG, mixed cut‐points.
22. Test.

1T PAPP‐A and 1T AFP, 5% FPR.
23. Test.

1T PAPP‐A and 1T ITA, 3% FPR.
24. Test.

1T PAPP‐A and 1T ITA, 5% FPR.
25. Test.

1T free ßhCG and 1T Inhibin, 5% FPR.
26. Test.
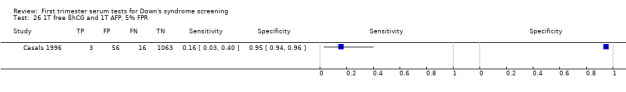
1T free ßhCG and 1T AFP, 5% FPR.
27. Test.

1T PAPP‐A and 1T ITA, 10% FPR.
28. Test.

1T PAPP‐A, 1T free ßhCG and 1T ITA, 5% FPR.
29. Test.

1T PAPP‐A, 1T free ßhCG and 1T ITA,3% FPR.
30. Test.

1T PAPP‐A, 1T free ßhCG and 1T ITA, 10% FPR.
31. Test.

1T total hCG, 1T free αhCG and 1T progesterone, 0.34 MoM.
32. Test.

Age, 1T Inhibin, risk 1:100.
33. Test.

Age, 1T Inhibin, risk 1:250.
34. Test.
Age, 1T Inhibin, risk 1:400.
35. Test.
Age, 1T Inhibin, 5FPR.
36. Test.
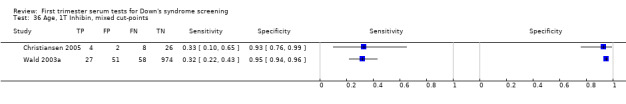
Age, 1T Inhibin, mixed cut‐points.
37. Test.
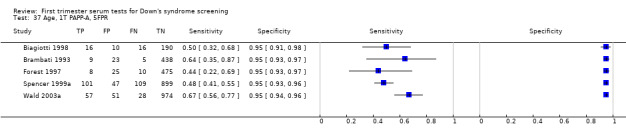
Age, 1T PAPP‐A, 5FPR.
38. Test.

Age, 1T PAPP‐A, mixed cut‐points.
39. Test.

Age, 1T free ßhCG, 5FPR.
40. Test.
Age, 1T free ßhCG, risk 1:384.
41. Test.
Age, 1T free ßhCG, mixed cut‐points.
42. Test.
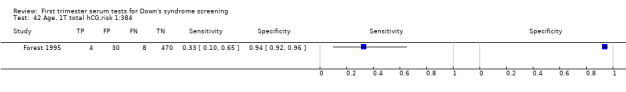
Age, 1T total hCG,risk 1:384.
43. Test.
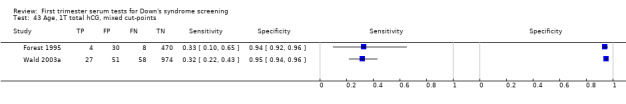
Age, 1T total hCG, mixed cut‐points.
44. Test.
Age, 1T AFP, 5FPR.
45. Test.
Age, 1T AFP, risk1:384.
46. Test.

Age, 1T AFP,mixed cut‐points.
47. Test.
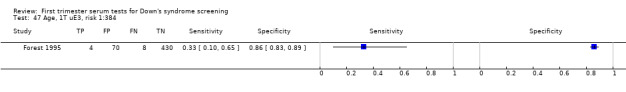
Age, 1T uE3, risk 1:384.
48. Test.
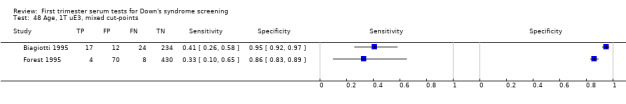
Age, 1T uE3, mixed cut‐points.
49. Test.

Age, 1T free αhCG, risk 1:384.
50. Test.
Age, 1T SP1, 5FPR.
51. Test.

Age, 1T ProMBP, risk 1:250.
52. Test.

Age, 1T ITA, 5FPR.
53. Test.
Age, 1T ADAM 12, risk 1:400.
54. Test.

Age, 1T PAPP‐A and 1T free ßhCG, risk 1:250.
55. Test.
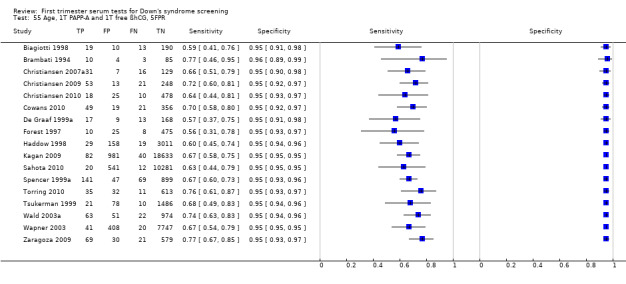
Age, 1T PAPP‐A and 1T free ßhCG, 5FPR.
56. Test.

Age, 1T PAPP‐A and 1T free ßhCG, mixed cut‐points.
57. Test.
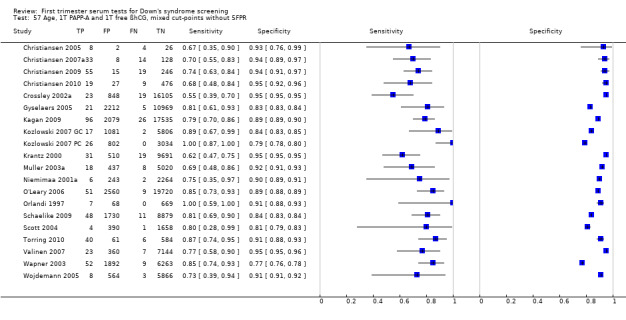
Age, 1T PAPP‐A and 1T free ßhCG, mixed cut‐points without 5FPR.
58. Test.
Age, 1T total hCG and 1T PAPP‐A, 5FPR.
59. Test.

Age, 1T PAPP‐A and 1T Inhibin, risk 1:100.
60. Test.

Age, 1T PAPP‐A and 1T Inhibin, risk 1:250.
61. Test.

Age, 1T PAPP‐A and 1T Inhibin, risk 1:400.
62. Test.

Age, 1T PAPP‐A and 1T Inhibin, 5FPR.
63. Test.
Age, 1T PAPP‐A and 1T Inhibin, mixed cut‐points.
64. Test.
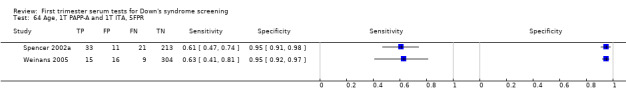
Age, 1T PAPP‐A and 1T ITA, 5FPR.
65. Test.
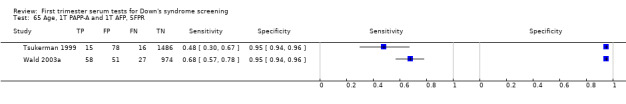
Age, 1T PAPP‐A and 1T AFP, 5FPR.
66. Test.
Age, 1T free ßhCG and 1T Inhibin, risk 1:100.
67. Test.

Age, 1T free ßhCG and 1T Inhibin, risk 1:250.
68. Test.

Age, 1T free ßhCG and 1T Inhibin, risk 1:400.
69. Test.

Age, 1T free ßhCG and 1T Inhibin, 5FPR.
70. Test.
Age, 1T free ßhCG and 1T Inhibin, mixed cut‐points.
71. Test.
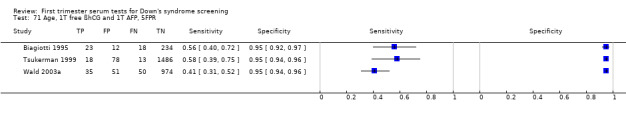
Age, 1T free ßhCG and 1T AFP, 5FPR.
72. Test.

Age, 1T free ßhCG and 1T AFP, risk 1:250.
73. Test.
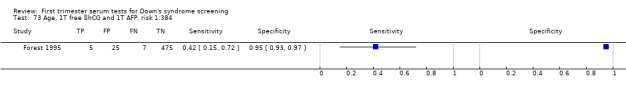
Age, 1T free ßhCG and 1T AFP, risk 1:384.
74. Test.

Age, 1T free ßhCG and 1T AFP, mixed cut‐points.
75. Test.
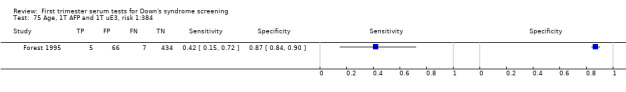
Age, 1T AFP and 1T uE3, risk 1:384.
76. Test.

Age, 1T AFP and 1T free αhCG, risk 1:384.
77. Test.
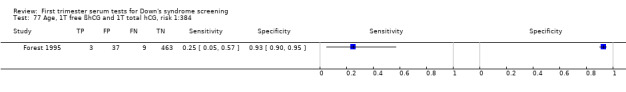
Age, 1T free ßhCG and 1T total hCG, risk 1:384.
78. Test.
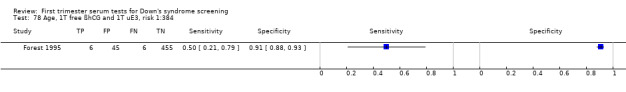
Age, 1T free ßhCG and 1T uE3, risk 1:384.
79. Test.

Age, 1T free ßhCG and 1T uE3, 5FPR.
80. Test.
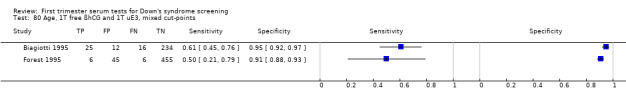
Age, 1T free ßhCG and 1T uE3, mixed cut‐points.
81. Test.

Age, 1T free ßhCG and 1T SP1, 5FPR.
82. Test.

Age, 1T free ßhCG and 1T SP1 risk 1:250.
83. Test.

Age, 1T AFP and 1T total hCG, 1:384.
84. Test.
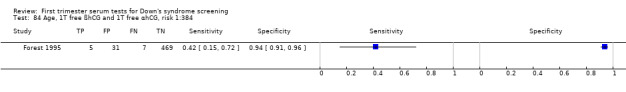
Age, 1T free ßhCG and 1T free αhCG, risk 1:384.
85. Test.

Age, 1T total hCG and 1T uE3, risk 1:384.
86. Test.

Age, 1T total hCG and 1T Inhibin, 5FPR.
87. Test.
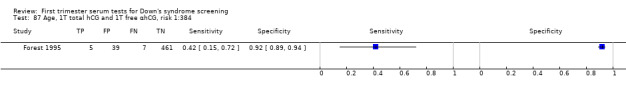
Age, 1T total hCG and 1T free αhCG, risk 1:384.
88. Test.
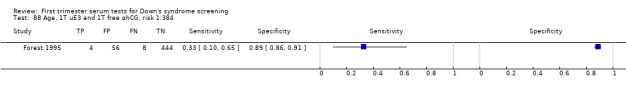
Age, 1T uE3 and 1T free αhCG, risk 1:384.
89. Test.
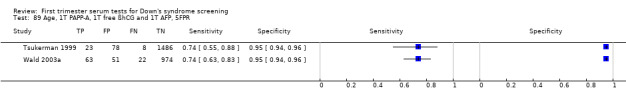
Age, 1T PAPP‐A, 1T free ßhCG and 1T AFP, 5FPR.
90. Test.
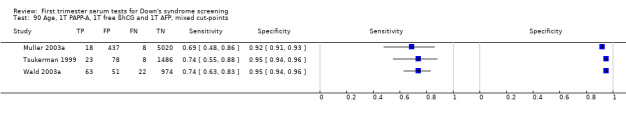
Age, 1T PAPP‐A, 1T free ßhCG and 1T AFP, mixed cut‐points.
91. Test.

Age, 1T free ßhCG, 1T AFP and 1T uE3, 5FPR.
92. Test.
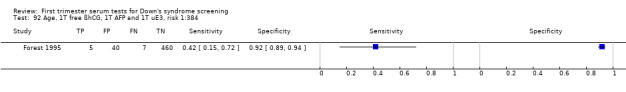
Age, 1T free ßhCG, 1T AFP and 1T uE3, risk 1:384.
93. Test.

Age, 1T free ßhCG, 1T AFP and 1T uE3, mixed cut‐points.
94. Test.
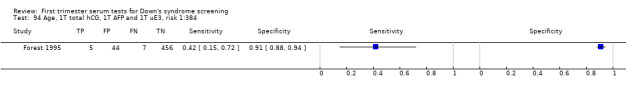
Age, 1T total hCG, 1T AFP and 1T uE3, risk 1:384.
95. Test.
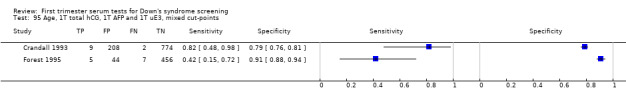
Age, 1T total hCG, 1T AFP and 1T uE3, mixed cut‐points.
96. Test.

Age, 1T AFP, free αhCG and 1T uE3, risk 1:384.
97. Test.

Age, 1T PAPP‐A, 1T free ßhCG and 1T Inhibin, 5FPR.
98. Test.

Age, 1T PAPP‐A, 1T total hCG and 1T Inhibin, 5FPR.
99. Test.

Age, 1T PAPP‐A, sp1 and 1T ProMBP, 5FPR.
100. Test.

Age, 1T PAPP‐A, sp1 and 1T ProMBP, risk 1:250.
101. Test.
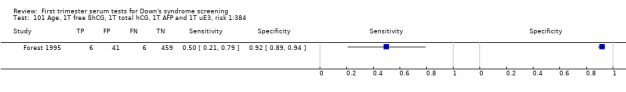
Age, 1T free ßhCG, 1T total hCG, 1T AFP and 1T uE3, risk 1:384.
102. Test.

Age, 1T total hCG, 1T AFP, 1T uE3 and 1T free αhCG, risk 1:384.
103. Test.

Age, 1T PAPP‐A, 1T free ßhCG, 1T AFP, 1T uE3 and 1T Inhibin, 5FPR.
104. Test.

Age, 1T PAPP‐A, 1T total hCG, 1T AFP, 1T uE3 and 1T Inhibin, 5FPR.
105. Test.

Age, 1T free ßhCG, 1T total hCG, 1T AFP, 1T uE3 and 1T free αhCG, risk 1:384.
106. Test.

Age, 1T hPL, risk 1:250.
107. Test.

Age, 1T hPL, 1T PAPP‐A, risk 1:250.
108. Test.

Age, 1T hPL, 1T free ßhCG, risk 1:250.
109. Test.

Age, 1T hPL, 1T PAPP‐A, 1T free ßhCG, risk 1:250.
110. Test.

Age, 1T PGH, risk 1:250.
111. Test.

Age, 1T PGH, 1T PAPP‐A , risk 1:250.
112. Test.

Age, 1T PGH, 1T free ßhCG , risk 1:250.
113. Test.

Age, 1T PGH, 1T PAPP‐A, 1T free ßhCG , risk 1:250.
114. Test.
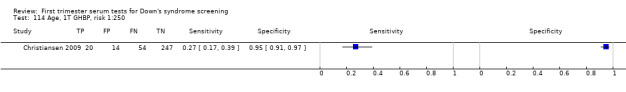
Age, 1T GHBP, risk 1:250.
115. Test.

Age, 1T GHBP, 1T PAPP‐A, risk 1:250.
116. Test.

Age, 1T GHBP, 1T free ßhCG, risk 1:250.
117. Test.

Age, 1T GHBP, 1T PGH, risk 1:250.
118. Test.

Age, 1T GHBP, 1T PAPP‐A, 1T free ßhCG , risk 1:250.
119. Test.

Age, 1T GHBP, 1T PGH, 1T PAPP‐A, 1T free ßhCG , risk 1:250.
120. Test.

Age, 1T ADAM 12, risk 1:250.
121. Test.

Age, 1T ADAM 12, 1T PAPP‐A, 1T free ßhCG, risk 1:250.
122. Test.
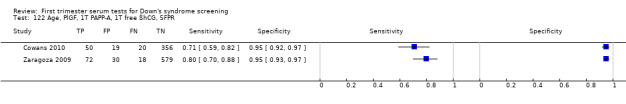
Age, PlGF, 1T PAPP‐A, 1T free ßhCG, 5FPR.
123. Test.

Age, 1T PAPP‐A and 1T free ßhCG, risk 1:300.
124. Test.

Age, 1T PAPP‐A, 1T Hyperglycosylated hCG, 5FPR.
128. Test.

Age, ADAM 12, 1T PAPP‐A, 5FPR.
129. Test.
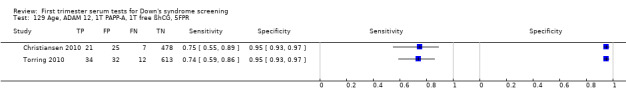
Age, ADAM 12, 1T PAPP‐A, 1T free ßhCG, 5FPR.
130. Test.

Age, 1T PlGF, 5FPR.
131. Test.

1T PlGF, 1T PAPP‐A, 1T free ßhCG, 5FPR.
132. Test.

Age, 1T ADAM 12, 1T PAPP‐A, 1T free ßhCG, mixed cut‐points.
133. Test.

Age, 1T PAPP‐A, 1T free ßhCG and 1T Inhibin, risk 1:250.
134. Test.
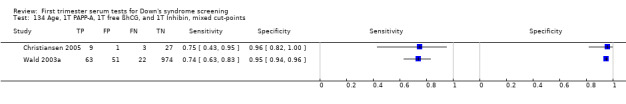
Age, 1T PAPP‐A, 1T free ßhCG, and 1T Inhibin, mixed cut‐points.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baviera 2010.
| Clinical features and settings | Routine screening. | |
| Participants | 579 participants: 17 cases and 562 controls matched for gestational age. Italy ‐ single centre. December 2006‐May 2009. Pregnant women. Mean maternal age 35.3 years (cases) and 30.4 years (controls). Singleton pregnancies. 7‐10 and 14‐17 weeks' gestation. |
|
| Study design | Case‐ control study. | |
| Target condition and reference standard(s) | Down's syndrome: 17 cases (14 identified by amniocentesis, 3 from follow‐up to birth). Reference standards: amniocentesis or follow‐up to birth. |
|
| Index and comparator tests | Frozen serum samples tested for: First trimester and second trimester ADAM12s (time resolved fluorescence immunoassay, DELFIA assay kit, Perkin Elmer Life and Analytical Sciences). First trimester PAPP‐A (details not reported). Second trimester AFP, uE3 and hCG (details not reported). |
|
| Follow‐up | Details of follow‐up not reported. | |
| Aim of study | To demonstrate the potential value of repeated measures of ADAM12s for the screening of Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Benattar 1999.
| Clinical features and settings | Routine screening. | |
| Participants | 1656 participants. France ‐ single centre. January to December 1995. Singleton pregnancies. Pregnant women. Mean age 32 years (16‐46 years). Enrolled before 13 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 5 cases. Reference standards: amniocentesis due to maternal age > 38 years (6.1% or women). Karyotyping encouraged for women with positive result on 1 or more index test. No details of reference standard for index test negative women. |
|
| Index and comparator tests | Maternal age. NT at 12‐14 weeks (Toshiba SSA 270), cut‐point 1/250. First trimester (12‐14 weeks) serum AFP and free ßhCG (Elsa AFP and Elsa free ßhCG; Cis‐Bio International). Second trimester (15‐18 weeks) serum AFP and total hCG (AFP‐2T and hCG‐60; Ortho‐Clinical Diagnostics). |
|
| Follow‐up | Details of follow‐up not stated. Unclear whether women were followed up to birth. | |
| Aim of study | To evaluate the sequential combination of ultrasound screening for fetal aneuploidy at 11‐14 weeks with maternal biochemistry at 12‐14 and 15‐18 weeks of gestation. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | 12 patients were lost to follow‐up due to miscarriages. |
Biagiotti 1995.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 287 participants: 41 cases and 246 controls matched for maternal and gestational age, and duration of sample storage (from cohort of 4452 participants undergoing invasive testing). Italy ‐ single centre. Dates not specified. Pregnant women. Singleton pregnancies. 8‐12 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 41 cases. Reference standards: amniocentesis or CVS. |
|
| Index and comparator tests | Maternal age. Frozen samples tested for: First trimester AFP ‐ AFP‐M‐K S Kit. First trimester uE3 ‐ Amersham Amerlex M. First trimester Intact hCG ‐ Hybritech tandem. First trimester free ßhCG ‐ ELSA Free beta hCG CIS. |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | Evaluate first trimester maternal serum AFP, uE3 and hCG to assess the efficacy of different combinations of these markers on a screening test for Down's syndrome in the first trimester. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Karyotyping. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had invasive testing. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results (lab analysis blinded). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Biagiotti 1998.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 232 participants: 32 cases and 200 randomly selected controls (selected from series of 3731 women). Italy ‐ single centre. July 1993 to December 1996. Pregnant women. Singleton pregnancies. 10 to 13 weeks' gestation. |
|
| Study design | Retrospective case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases. Reference standards: amniocentesis or CVS. |
|
| Index and comparator tests | Maternal age. First trimester NT (in longitudinal section of the fetus with caliper measurements to the nearest 0.1 mm). First trimester serum PAPP‐A (Amerlex‐M PAPP‐A IRMA, Ortho‐Clinical Diagnostics). First trimester serum free ßhCG (Elsa9free B‐hCG CIS). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate the potential effectiveness of maternal serum PAPP‐A and free beta hCG in combination with NT measurement in the first trimester of pregnancy. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Karyotyping. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Unclear | No details of withdrawals given. |
Brambati 1993.
| Clinical features and settings | HIgh‐risk referral for invasive testing. | |
| Participants | 522 participants. Italy. Dates not specified. Pregnant women. Median age 38 years (20‐47 years). Singleton pregnancies. 6‐11 weeks' gestation. |
|
| Study design | Retrospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 14 cases. Reference standard: CVS. |
|
| Index and comparator tests | Maternal age. Frozen blood sample tested for: FIrst trimester serum PAPP‐A (radio‐immunoassay). |
|
| Follow‐up | 100% karyotyping. 47 women who miscarried prior to CVS were excluded from the study. |
|
| Aim of study | To assess the relationship between maternal serum PAPP‐A in the first trimester and the outcome of pregnancy by karyotype. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Brambati 1994.
| Clinical features and settings | HIgh‐risk referral for invasive testing. | |
| Participants | 102 participants: 13 case and 89 randomly selected controls matched for gestational age. Italy. Dates not specified. Pregnant women. 8‐12 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases. Reference standard: CVS. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: FIrst trimester PAPP‐A (radioimmunoassay, as described in Sinosich 1982). First trimester free ßhCG (radioimmunoassay, CIS, UK). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To report the results for the combined measurement of serum PAPP‐A and free‐ßhCG in women attending for prenatal diagnosis. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results (biochemical testing conducted blind to karyotype results). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Brameld 2008.
| Clinical features and settings | Routine screening. | |
| Participants | 22,280 participants with complete screening results and outcome data. August 2001‐October 2003. Australia ‐ State‐wide screening programme evaluation. Pregnant women. Median maternal age 31 years (range 14‐47 years), 20% ≥ 35 years. Singleton pregnancies. 10‐14 weeks' gestation. |
|
| Study design | Retrospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 60 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester PAPP‐A, free ßhCG and NT (details not reported). Risk cut‐point 1:300. |
|
| Follow‐up | Data on outcome from the Western Australia Midwives data collection, Birth Defects Registry and hospital morbidity and mortality data. | |
| Aim of study | To identify first trimester indicators of adverse pregnancy outcomes. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Brizot 1994.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 393 participants: 45 cases of Down’s syndrome and 348 controls matched for crown rump length, maternal age and storage time. UK. Dates not specified. Pregnant women. Median age 38 years (22‐45 years). Singleton pregnancies. 10‐13 weeks' gestation. |
|
| Study design | Retrospective case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 45 cases. Reference standard: fetal karyotyping. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First Trimester PAPP‐A (Double sandwich time resolved immunofluorometric assay with chelated europium as a label. Antibody to PAPPA binding Ig was polyclonal rabbit IgG in stabilised solution). |
|
| Follow‐up | 100% karyotyping | |
| Aim of study | To determine if the risk for fetal trisomies during the first trimester of pregnancy can be derived by combining data from maternal serum PAPP‐A and fetal NT thickness. | |
| Notes | Cases were pre‐selected for increased NT thickness. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Karyotyping. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results (biochemical testing conducted blind to karyotype results). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Casals 1996.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 1138 participants. Spain. 1990‐1993. Pregnant women. 94.4% of women aged > 35 years. Singleton pregnancies. 10‐13 weeks' gestation. |
|
| Study design | Retrospective case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases. Reference standard: CVS. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First trimester serum free ßhCG (IMx microparticle enzyme immunoassay technology) (19 case and > 80 control samples). First trimester serum AFP (Stratus fluorometric enzyme immunoassay) (19 case and > 80 control samples). First trimester serum PAPP‐A (radioimmunoassay) (only for 16 case and 80 control samples). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To examine the value of ßhCG, AFP and PAPP‐A in biochemical screening for Down's syndrome in 19 women carrying Down's syndrome versus normal pregnancies. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index test interpreted without knowledge of reference standard results (PAPP‐A testing conducted blind to CVS results but presence of blinding is not stated for free ßhCG and AFP). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Christiansen 1999.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 181 participants (for first trimester serum samples): 25 cases and 156 controls matched for length of storage. Denmark. Dates not specified. Pregnant women. 5‐20 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases. Reference standard: karyotyping. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First and second trimester ProMBP (2 site immunoradiometric assay samples reduced and alkylated and added to microtitre wells coated with monoclonal antibody J13 6B6). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To examine whether the maternal serum concentration of ProMBP was influenced by the presence of a Down's syndrome fetus. To evaluate its potential as a screening marker for Down's syndrome in the first and second trimester of pregnancy. To examine the performance characteristics of a serum screening programme using ProMBP in combination with age as risk markers. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Karyotyping. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Christiansen 2004.
| Clinical features and settings | Routine screening. | |
| Participants | 334 participants: 156 cases, 546 control samples (348 control women, 198 of these were sampled from the same women in first and second trimesters). Denmark. Dates not specified. Pregnant women. Singleton pregnancies. 4‐20 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 156 cases. Reference standard: CVS (for 120 of cases of Down's) or follow‐up to birth (for 36 of cases of Down's). |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First/second trimester hCG and AFP (AutoDELFIA analytical system). First/second trimester PAPP‐A and SP1 (in‐house sandwich immunoassays). First/second trimester ProMBP (2 site immunoradiometric assay (IRMA)). First/second trimester free ßhCG and some AFP (dual label kit). |
|
| Follow‐up | The Danish Cytogenetic Central Registry was routinely used to ascertain that none of the controls were pregnancies with a chromosomally diseased fetus. | |
| Aim of study | To evaluate 6 markers of fetal Down's syndrome pregnancies (includes first trimester markers). | |
| Notes | Unclear criteria for the selection of controls. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Christiansen 2005.
| Clinical features and settings | Screening programmes for syphilis and Down's syndrome. | |
| Participants | 108 participants: 27 cases of Down's syndrome and 81 controls. Denmark ‐ Statens Serum Institute. Dates not specified. 5‐11 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 27 cases (18 diagnosed in 2nd trimester, 9 at birth). Reference standard: karyotyping. |
|
| Index and comparator tests | Maternal age. First trimester (week 11‐14) NT. Frozen samples tested for: First trimester (week 5‐11) 1T Inhibin A (dimer assay kit MCA 950KZZ, Serotec). First trimester (week 5‐11) ßhCG (available for some samples). First trimester (week 5‐11) PAPP‐A (available for some samples) (combined PAPP‐A and B‐hCG TrIFMA assay). Risk cut‐points of 1:100, 1:250 and 1:400. Performance assessed with SPlus algorithm. |
|
| Follow‐up | All diagnosis were verified by karyotyping. | |
| Aim of study | To investigate whether first trimester Inhibin A can be used in the first trimester for Down's syndrome screening. | |
| Notes | Identified through the Danish central cytogenetic registry as part of quality assurance programme. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping. |
| Partial verification avoided? All tests | Yes | All women had a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of NT results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Christiansen 2007a.
| Clinical features and settings | Routine screening. | |
| Participants | 183 participants: 47 cases and 136 controls matched for gestational age. Dates not reported. Denmark – Statens Serum Institute. Pregnant women. Singleton pregnancies. Median age cases 37.7 years (24‐48 years) and controls 36.4 years (22‐44 years). 8‐14 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 47 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. Fresh serum samples tested for: First trimester PAPP‐A and free βhCG (AutoDelfia, PerkinElmer, Turku or Kryptor, Brahms). Frozen serum samples tested for: First trimester human placental lactogen (hPL) (hPL ELISA, enzyme immunoassay (EIA)‐1283, DRG Instruments GmBH). |
|
| Follow‐up | Cross‐referencing with the Danish Cytogenetic Central Registry. | |
| Aim of study | To examine the potential of human placental lactogen as a first trimester maternal serum screening marker for fetal Down’s syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Christiansen 2009.
| Clinical features and settings | Routine screening. | |
| Participants | 335 participants: 74 cases and 261 controls matched for length of sample storage and maternal age. Denmark ‐ screening programme. Dates not reported. Pregnant women. Singleton pregnancies. Median maternal age cases 37.5 years and controls 36.4 years. 8‐13 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 74 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (details not reported). Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (AutoDelfia, PerkinElmer, Turku or Kryptor, Brahms). Frozen serum samples tested for: First trimester placental growth hormone (PGH) (double monoclonal ELISA, DSL‐10‐19 200, Diagnostic Systems Laboratory Inc). First trimester growth hormone binding protein (GHBP) (Enzyme‐amplified ELISA, DSL‐10‐48 100, Diagnostic Systems Laboratory Inc). |
|
| Follow‐up | Cross‐referencing with the Danish Cytogenetic Central Registry. | |
| Aim of study | To examine the potential of placental growth hormone and growth hormone binding protein as maternal serum screening markers for Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Christiansen 2010.
| Clinical features and settings | Routine screening. | |
| Participants | 531 participants: 28 cases and 503 controls. Denmark ‐ screening programme. Dates not specified. Pregnant women. Singleton pregnancies. Median age cases 36 years (range 25‐44 years) and controls 29 years (range 17‐45 years). 8‐14 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 28 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (details not reported). First trimester PAPP‐A and free ßhCG (details not reported). First trimester ADAM12s (AutoDELFIA/Delfia ADAM12 Research kit 4025‐0010, PerkinElmer Life and Analytical Sciences, on the 1235 AutoDELFIA automatic immunoassay system). |
|
| Follow‐up | Cross‐referencing with the Danish Cytogenetic Central Registry. | |
| Aim of study | To examine the efficiency of a second generation assay for ADAM12. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Cowans 2010.
| Clinical features and settings | Routine screening. | |
| Participants | 445 participants: 70 cases and 375 controls matched for storage time and gestational age. January 2007‐October 2008. UK. Pregnant women. Singleton pregnancies. Mean maternal age cases 37.0 years (IQR 32.9‐40.5 years) and controls 32.4 years (IQR 29.0‐35.9 years). 11‐13 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 70 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF certified sonographers). Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms). Frozen serum samples tested for: First trimester placental growth factor (PlGF) (Solid‐phase, two‐site fluoroimmunometric research assay (4083‐0010) on 6000 DELFIA Xpress random access platform, PerkinElmer). Modelled on UK 2002 population data. |
|
| Follow‐up | Karyotype and results for pregnancy outcome were received from cytogenetics laboratories and maternity units where deliveries took place. | |
| Aim of study | To examine placental growth factor levels in first trimester maternal serum in trisomy 21 pregnancies and to investigate the potential value of PlGF in a first trimester screening test. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Crandall 1993.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 893 participants. USA ‐ 3 centres. Dates not specified. Pregnant women, 90% > 35 years. 11‐15 weeks' gestation. |
|
| Study design | Retrospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 11 cases. Reference standard: amniocentesis. |
|
| Index and comparator tests | Maternal age. Frozen samples tested for: First trimester serum AFP (Tandem E kit). First trimester serum uE3 (Amerlex M). First trimester serum hCG (Hybritech tandem E kit). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To investigate whether hCG is a useful predictor of Down's syndrome between 11 and 15 weeks' gestation. | |
| Notes | Unclear criteria for the selection of controls. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Amniocentesis. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | Yes | 33 samples excluded because out of the date range or insufficient sample volume or information. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Crossley 2002a.
| Clinical features and settings | Routine screening. | |
| Participants | 17,229 participants. UK ‐ 15 centres. Dates not specified. Pregnant women with median age 29.9 years, 15.4% ≥ 35 years. 10‐14 weeks' gestation. |
|
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 45 cases. Reference standards: CVS offered where women had high NT measurements, amniocentesis or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. NT (FMF method) in 73% of patients. Clotted blood samples tested for: Free ßhCG and PAPP‐A (Kryptor analyser) in 98.4% of patients. |
|
| Follow‐up | Reported that the outcome of all pregnancies was followed up. | |
| Aim of study | To evaluate the use of ultrasound measurements of fetal NT obtained in a routine antenatal clinic setting in combination with appropriate biochemical markers as a first trimester screening test for Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population . |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | Yes | Report average success rate of NT (72.9%). |
| Withdrawals explained? All tests | Yes | Numbers of patients not undergoing NT and biochemical testing given. |
De Graaf 1999a.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 292 participants (207 participants before 14 weeks' gestation): 37 cases and 255 controls matched for maternal age (within 2 years), gestational age (within 2 weeks) and duration of sample storage (within 2 months) The Netherlands ‐ single centre. 1994‐1997. Pregnant women. 9‐15 weeks' gestation (in a few cases, blood samples for serum testing taken at 15‐19 weeks). |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 37 cases (30 affected pregnancies in women with serum testing enrolled before 14 weeks' gestation). Reference standards: CVS or amniocentesis. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF methods) with cut off > 3 mm. Frozen serum samples tested for: First trimester free ßhCG and AFP (DELFIA dual labelled time resolved fluorescent assay). First trimester serum PAPP‐A (DELFIA research assay (CR61‐105)). First trimester serum AFP. |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To determine the expected detection rate and false positive rate for Down's syndrome achievable by early pregnancy screening with combined measurements of serum PAPP‐A, free beta hCG and fetal NT, with the addition of AFP. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Karyotyping. |
| Partial verification avoided? All tests | Yes | All women had a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | Yes | Failed to measure NT in 11 control women. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Forest 1995.
| Clinical features and settings | Routine screening. | |
| Participants | 1023 participants (512 first trimester participants). Canada ‐ 6 centres. June 1989‐October 1993. Pregnant women. 23 cases of Down’s syndrome (12 in of women recruited first trimester and 11 in second trimester). 1000 control samples (100 for each gestational week from 9‐18) matched to the age of the original cohort in which Down’s cases were detected (n = 14,612). Mean maternal age 29.1 (SD 4.7) years, 10.7% aged ≥ 35 years. Singleton pregnancies. 9‐13 (first trimester) and 14‐18 (second trimester) weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases (first trimester). Reference standards: follow‐up to birth. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First trimester AFP and total hCG (Enzymum‐Test enzyme immunoassay analyser, ES‐300 analyser). First trimester uE3 (ultra sensitive radioimmunometric assay). First trimester free αhCG and free ßhCG (radioimmunometric assays). 3 different models used for risk calculation (Wald, Spencer and Ryall). |
|
| Follow‐up | Review of maternal and neonatal charts in each centre. | |
| Aim of study | Evaluate the impact of risk estimation parameters for screening for Down's syndrome during the first and second trimesters of pregnancy. | |
| Notes | 3 different models used for risk calculation (Wald, Spencer and Ryall). | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | Unclear | Unclear if women received different reference standards. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Forest 1997.
| Clinical features and settings | Routine screening. | |
| Participants | 518 participants. Canada ‐ 6 centres. June 1989‐January 1995. Pregnant women. 18 cases of Down’s syndrome. 500 control samples (representative of the cohort from which they were taken: n = 10,160). 100 for each gestational week from 9‐13 weeks. Mean maternal age 27.9 years, 10.7% aged ≥ 35 years. Singleton pregnancies. 9‐13 weeks' gestation at enrolment. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 18 cases. Reference standards: follow‐up to birth. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First trimester PAPP‐A (fluorescence‐linked immunosorbent assay). First trimester free ßhCG (radioimmunometric assay, Bioclone Australia). Risk cut‐point 1:384. |
|
| Follow‐up | Review of maternal and neonatal charts in each centre and consulting the database of the local cytogenetic laboratories. | |
| Aim of study | To confirm the usefulness of free ßhCG and AFP as first trimester screening markers in a prospective study. | |
| Notes | Exclusion of cases of babies that died before 20 weeks' gestation. Unclear criteria (apart from age) for the selection of controls. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | Unclear | Unclear if women received different reference standards. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Gyselaers 2005.
| Clinical features and settings | Routine screening. | |
| Participants | 13,267 participants (13,207 participant received both NT test and serum testing). Belgium ‐ multicentre study (35 centres). First Jan 2004‐First April 2004 (data added to previous database from before 2003). Pregnant women. Singleton pregnancies. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases. Reference standards: amniocentesis, CVS and postnatal karyotyping. |
|
| Index and comparator tests | Maternal age. First trimester serum PAPP‐A (ELISA 2397, DRG International Inc). First trimester serum free ßhCG (free ßhCG IRMA K1P1001, BioSource Europe SA). Second trimester PAPP‐A and free ßhCG. First trimester NT. Risk cut‐points of 1:200 and 1:300. |
|
| Follow‐up | Follow‐up to birth reported by mail by obstetricians. Non‐responding obstetricians contacted personally to obtain missing data. Cases of miscarriages (n = 49) and other fetal chromosomal abnormalities excluded from the study. Unclear if other patients were lost to follow‐up. | |
| Aim of study | To evaluate the performance of a first trimester fetal aneuploidy screening programme. | |
| Notes | Women with miscarriages or cases of other chromosomal defects were excluded from the study. 9 live births of babies with Down's syndrome. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | Numbers of women excluded due to miscarriage or other chromosomal defects and numbers not undergoing NT and biochemical testing reported. |
Haddow 1998.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 3217 participants. USA ‐ 16 prenatal diagnostic centres. June 1994 to November 1996. Pregnant women aged 15‐51 years (median 37 years). Singleton pregnancies. 9‐14 weeks' gestation. |
|
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 48 cases. Reference standards: amniocentesis or CVS. |
|
| Index and comparator tests | Maternal age. Fresh serum sample tested for: First trimester serum hCG (hCG MAIA clone assay). First trimester serum PAPP‐A (enzyme‐linked immunosorbent assay, Dako). First trimester free ßhCG and AFP ‐ Fluoroimmunoassay (DELFIA hAFP/Free beta hCG dual kit). First trimester uE3 (Ultrasensitive uE3 kit). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To further examine the efficacy of serum and ultrasound screening for Down's syndrome in the first trimester and the possible advantages and disadvantages of screening at this time rather than in the second trimester. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Karyotyping. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women had the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Kagan 2009.
| Clinical features and settings | Routine screening. | |
| Participants | 56,954 participants with available outcome data. UK ‐ multicentre study. July 1999 ‐ April 2007. Pregnant women. Singleton pregnancies. Mean maternal age 35.4 years (14.1‐52.2 years). 11‐13 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 395 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT. First trimester fetal heart rate (pulsed‐wave Doppler). First trimester nasal bone (FMF certified sonographers). First trimester ductus venous flow (FMF certified sonographers). First trimester flow across tricuspid valve (FMF certified sonographers). First trimester PAPP‐A and free ßhCG (Kryptor, Brahms AG or Delfia Express, Perkin Elmer). Multiple publications with different test evaluations. |
|
| Follow‐up | Karyotype results and details of pregnancy outcome were added to databases as they became available. Women without complete screening and outcome data (n = 3053, 5.1%) were excluded from the study. | |
| Aim of study | To examine the performance of first‐trimester screening for trisomies 21, 18 and 13 by maternal age, fetal NT thickness, fetal heart rate and maternal serum free ß‐hCG and PAPP‐A. Other objectives in related publications. |
|
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Kornman 1998.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | The Netherlands ‐ antenatal diagnosis unit. October 1990‐February 1994. Pregnant women. 15 cases of Down’s syndrome. 97 control samples (matched on gestational age, sample storage time and maternal age). Singleton pregnancies. 8‐12 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases. Reference standard: CVS. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First trimester serum SP1 (modified commercial radioimmunoassay, RIA‐gnost SP1)). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To compare SP1 levels in Down's syndrome versus normal pregnancies. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Kozlowski 2007 GC.
| Clinical features and settings | Routine referral. | |
| Participants | 6906 participants with complete outcome data. Germany ‐ gynaecologists practices. January 2000‐December 2003. Pregnant women. Median maternal age 32 years (15‐48 years), 26.4% ≥ 35 years. 11‐14 weeks' gestation. |
|
| Study design | Cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases. Reference standard: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF certified gynaecologists). First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms). Risk cut‐point 1:300. |
|
| Follow‐up | Data on pregnancy outcome were obtained by contacting the patient or their general gynaecologist. Women without complete outcome data (36%) were excluded from the study. | |
| Aim of study | To evaluate and compare the screening performance for fetal trisomy 21 in the first trimester of pregnancy in general gynaecologists practices and specialised centres for prenatal care in Germany. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | 146 women (including 11 with Down's syndrome) excluded as results could not be assigned to gynaecologists or prenatal centre group. |
Kozlowski 2007 PC.
| Clinical features and settings | Routine referral. | |
| Participants | 3862 participants with complete outcome data. Germany ‐ tertiary level prenatal centres. January 2000‐December 2003. Pregnant women. Median maternal age 34 years (14‐46 years), 43.2% ≥ 35 years. 11‐14 weeks' gestation. |
|
| Study design | Cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases. Reference standard: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF certified sonographers). First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms). Risk cut‐point 1:300. |
|
| Follow‐up | Data on pregnancy outcome were obtained by contacting the patient or their general gynaecologist. Women without complete outcome data (8%) were excluded from the study. | |
| Aim of study | To evaluate and compare the screening performance for fetal trisomy 21 in the first trimester of pregnancy in general gynaecologists practices and specialised centres for prenatal care in Germany. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | 146 women (including 11 with Down's syndrome) excluded as results could not be assigned to gynaecologists or prenatal centre group. |
Krantz 2000.
| Clinical features and settings | Routine screening. | |
| Participants | 10,251 participants. USA. September 1995 to June 1998. Pregnant women. Singleton pregnancies. Maternal age 34.7% ≥ 35 years. No diabetes. 9‐13 weeks' gestation. |
|
| Study design | Prospective cohort.. | |
| Target condition and reference standard(s) | Down's syndrome: 50 cases (33 had undergone biochemical testing). Reference standard: not reported. |
|
| Index and comparator tests | Maternal age. Dried blood samples tested for: First trimester NT in 5809 patients (FMF methods). First trimester free ßhCG and PAPP‐A in 10,251 patients (enzyme‐linked immunosorbent assay procedures). |
|
| Follow‐up | No details of follow‐up reported. | |
| Aim of study | To assess the effectiveness of free ßhCG, PAPP‐A and NT for first‐trimester screening for Down's syndrome and trisomy 18. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Unclear | Unclear reference standard. |
| Partial verification avoided? All tests | Unclear | Unclear if all patients had a reference standard. |
| Differential verification avoided? All tests | Unclear | Unclear if choice of reference depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Kratzer 1991.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 141 participants. USA. Dates not stated. Pregnant women. Controls matched for maternal age. Singleton pregnancies. 9‐12 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 17 cases Reference standard: CVS |
|
| Index and comparator tests | Frozen serum samples tested for: First trimester hCG and free ßhCG (double antibody radio‐immunoassay). First trimester free αhCG (radio‐immunoassay, monoclonal antibody, Biomerica Inc). First trimester progesterone (radio‐immunoassay). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To present evidence on the value of first trimester serum assays as an early, non‐invasive screen for aneuploidy. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index tests interpreted without knowledge of reference standard results (index tests conducted blind to outcome). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Laigaard 2003.
| Clinical features and settings | Routine screening. | |
| Participants | 172 participants (18 cases of Down’s syndrome and 154 controls). Denmark ‐ University hosp1T ITAl. Dates not specified. Pregnant women. Singleton pregnancies. 8‐13 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 18 cases. Reference standards: karyotyping, unclear reference standard for controls. |
|
| Index and comparator tests | Frozen serum tested for: First trimester ADAM12 (ELISA, 6E6 and 8F8 antibodies). |
|
| Follow‐up | No details of follow‐up reported. | |
| Aim of study | To determine whether ADAM12 concentration is a useful indicator of fetal health. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Unclear | Unclear reference standard in controls. |
| Partial verification avoided? All tests | Unclear | Unclear if all women had a reference standard. |
| Differential verification avoided? All tests | No | Different reference standards used. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Macintosh 1993.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 692 participants. UK and Italy. Dates not specified. Pregnant women. Median maternal age 38 years (27‐40 years). Singleton pregnancies. 6‐12 weeks' gestation. |
|
| Study design | Retrospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 14 cases. Reference standard: CVS. |
|
| Index and comparator tests | Maternal age. Frozen serum tested for: First trimester serum SP1 (Radioimmunoassay). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To examine the relationship between first trimester maternal serum SP1 and the karyotype of the pregnancy and to quantify its potential use as a screening test. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Muller 2003a.
| Clinical features and settings | Routine screening. | |
| Participants | 5694 pregnant women who had first trimester NT and biochemical testing. France ‐ 9 centres serving 12 maternity units. January 1998‐June 2001. Singleton pregnancies. Maternal age not reported. 11‐13 weeks' gestation. |
|
| Study design | Retrospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases. Reference standards: invasive testing (offered to women with high NT measurement) or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT in 98% of patients (methods not specified. 60 sonographers ‐ 2 trained by FMF, who trained 30 in turn. 8 externally trained in France. 20 were self‐taught. Machines not specified). Frozen serum tested for: First trimester PAPP‐A (99% of patients), free ßhCG 99% of patients and AFP (93% of patients) (time‐resolved fluorescent assay, Perkin‐Elmer Life sciences). Risk cut‐point 1:250. |
|
| Follow‐up | Data from the French national screening programme used for follow‐up at birth. 211 women (3.7%) who did not return after NT or were found to be > 14 weeks were excluded. It is unclear how many patients had follow‐up to birth. | |
| Aim of study | Prospective study of NT and retrospective evaluation of serum (in same patient population) to evaluate whether or not to move the national French Down's screening programme to a first trimester programme. | |
| Notes | FMF methods ‐ some self‐taught sonographers. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice . |
| Uninterpretable results reported? All tests | Yes | Women with NT too small to measure assumed to have NT of < 0.5 mm. |
| Withdrawals explained? All tests | Yes | Women failing to return or who more than 14 weeks' pregnant were excluded (214). |
Nebiolo 1990.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 492 participants. Italy. Dates not specified. Pregnant women, approximately 75% were aged ≥ 35 years. Singleton pregnancies. 8‐12 weeks' gestation. |
|
| Study design | Retrospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 9 cases. Reference standard: CVS. |
|
| Index and comparator tests | Frozen serum tested for: First trimester serum AFP and beta/alpha hCG ratio (simultaneous sandwich monoclonal based radioimmunoassay). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To determine the efficacy of combined maternal serum AFP and hCG screening in detecting chromosome defects in the first trimester of pregnancy. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results (biochemical tests conducted blind to pregnancy outcome). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | Yes | Samples from 48 patients were either no longer available or had been stored at 4oC and were discarded. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Niemimaa 2001a.
| Clinical features and settings | Routine screening. | |
| Participants | 2515 participants. Finland ‐ primary care centres and maternity clinics of hospitals. During 1999. Pregnant women, 17.5% ≥ 35 years. 10‐13 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases. Reference standards: invasive testing (patients considered high risk based on NT screening) or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (≥ 3 mm) (64% of women) (method not described). Fresh serum tested for: First trimester free ßhCG and PAPP‐A (Wallac analytes and 1st trimester risk calculation programme Maternal weight correction). Risk cut‐point 1:250. |
|
| Follow‐up | Follow‐up data from maternity clinics and the National Research and Development Centre for Welfare and Health. Test negative patients followed up by contacting all maternity clinics and the National Research and Development Centre for Welfare and Health. Unclear if follow‐up information was obtained in all cases. | |
| Aim of study | To evaluate efficacy of combining first trimester maternal serum and fetal NT measurement in screening for Down's syndrome in Finland. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Noble 1995.
| Clinical features and settings | Routine screening in a high‐risk population. | |
| Participants | 2529 participants. UK. October 1994 to April 1995. Singleton pregnancies. Pregnant women. Median maternal age 34 years (15‐47 years), 47% ≥ 35 years. 10‐14 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 61 cases. Reference standards: karyotyping performed (27% of women) due to increased NT (14%), advanced maternal age (10%), previous chromosomally abnormal child (0.5%) or parental anxiety (2%). Ultrasound examination at 20 weeks (65% of patients). Follow‐up to birth (9% of women). |
|
| Index and comparator tests | Maternal age. First trimester NT (methods not stated). Fresh serum (or serum frozen over a weekend) tested for: First trimester free ßhCG (immunoradiometric assay, CIS). |
|
| Follow‐up | Pregnancy outcome obtained from maternity units or the patients themselves. Follow‐up information only appears to have been obtained in 9% of cases (second trimester ultrasound used as reference standard for other women). | |
| Aim of study | To measure the contribution of maternal serum free ßhCG in a screening programme for fetal trisomy 21 based on fetal NT in the first trimester of pregnancy. | |
| Notes | No proper results, data are presented for this study. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | No | Invasive testing, ultrasound at 20 weeks or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Noble 1997.
| Clinical features and settings | Routine screening, women self‐referred for first trimester NT. | |
| Participants | 876 participants. UK ‐ Research Centre for Fetal Medicine. Dates not stated. 76 cases of Down’s syndrome. 800 controls matched for maternal and gestational age. Pregnant women. Median maternal age 34 years (15‐47 years). Singleton pregnancies. 10‐14 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 76 cases. Reference standards: CVS, follow‐up to birth not reported. |
|
| Index and comparator tests | Maternal age. Frozem serum tested for: First trimester serum Inhibin A (ELISA). Fresh serum (or serum stored over weekend) tested for: First trimester serum free ßhCG (immunoradiometric assay, CIS France). |
|
| Follow‐up | Details of methods of follow‐up not reported. | |
| Aim of study | To determine the relationship between maternal serum first trimester Inhibin A and free ßhCG concentrations in chromosomally normal pregnancies and to compare 2 biochemical markers for their sensitivity in identifying trisomy 21 pregnancies. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Unclear | Unclear what the reference standard was. |
| Partial verification avoided? All tests | Unclear | Unclear if all women had a reference standard. |
| Differential verification avoided? All tests | Unclear | Unclear if the reference standard differed between women. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results (biochemical tests conducted blind to pregnancy outcome). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
O'Leary 2006.
| Clinical features and settings | Routine screening. | |
| Participants | 22,340 participants. Australia ‐ 13 ultrasound practices. August 2001 to October 2003. Singleton pregnancies. Pregnant women aged 14‐47 years (median 31 years), 20% ≥ 35 years. 11‐13 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 60 cases. Reference standards: CVS or amniocentesis (women assessed to be high risk on screening), or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF methods). First trimester free ßhCG and PAPP‐A (machine not stated). All study participants underwent all tests. Risk cut‐point 1:300. |
|
| Follow‐up | Follow‐up data obtained by review of the Midwives Notification System and the Birth Defects Registry. 415 patients (1.8%) excluded due to no follow‐up data. Patients with multiple pregnancies or incomplete screens (n = 3946) were also excluded from the study. | |
| Aim of study | To assess fetal outcomes for pregnancies identified at increase risk for Down's syndrome by first trimester combined ultrasound examination and maternal serum biochemistry. | |
| Notes | Appears likely that patients with miscarriages and terminations excluded. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Unclear | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | Details given of patients excluded due to incomplete screening data or loss to follow‐up. |
Orlandi 1997.
| Clinical features and settings | Routine screening of general‐ and high‐risk women. | |
| Participants | 2,010 participants (744 in subgroup undergoing NT testing). Italy. Dates not reported. Recruited through private physician or genetic counselling program for women of advanced maternal age. Pregnant women aged 15‐46 years, 35% > 35 years. Singleton pregnancies. 9‐13 weeks' gestation. |
|
| Study design | Cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 11 cases (7 in subgroup with NT testing). Reference standards: not reported. |
|
| Index and comparator tests | Maternal age. First trimester NT (37% of patients) (FMF methods, Toshiba SSA 250A or Acuson XP 10). First trimester free ßhCG and PAPP‐A (all patients) (dried blood samples, enzyme‐linked immunosorbent assays). Risk cut‐point 1:380. |
|
| Follow‐up | Not reported. | |
| Aim of study | To evaluate first trimester combined screening for Down's syndrome. | |
| Notes | Unclear as to what reference standard (if any) was used. All cases of Down's syndrome identified had been picked up by screening. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Unclear | Reference standard not reported. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | Unclear | Unclear if the choice of reference standard depended on screening results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | Details given of women undergoing NT but not biochemical testing. |
Palomaki 2007.
| Clinical features and settings | Routine screening. | |
| Participants | 10,775 participants. Canada – General Hospital. October 2003–November 2004. Pregnant women. Mean maternal age 32.3 years (SD 4.6 years). 10‐13 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 23 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (encouraged to only accept measurements from sonographers with FMF certification). First trimester PAPP‐A (AutoDELFIA, PerkinElmer). First trimester hyperglycosylated‐hCG (Nichols Advantage Specialty system, Nochols Institute Diagnosics). |
|
| Follow‐up | From electronic record searches of local patient and cytogenetic records and case finding of local and regional birth records. | |
| Aim of study | To validate Down’s syndrome screening protocols that include hyperglycosylated‐hCG measurements. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Qin 1997.
| Clinical features and settings | Routine screening. | |
| Participants | 702 participants. Copenhagen. Dates not specified. Pregnant women. 156 cases of Down’s syndrome (25 in weeks 3‐9 and 131 in weeks 10‐20 gestation). 546 controls (260 in weeks 3‐9 and 286 in weeks 10‐20 gestation). 5‐20 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases (3‐9 weeks' gestation). Reference standards: CVS, amniocentesis, karyotyping at birth, unclear reference standard for controls. |
|
| Index and comparator tests | Frozen serum tested for: First trimester schwangerschaftsprotein 1 (SP1) (non‐competitive time‐resolved immunofluorometric assay, A131, DAKO A/S)). |
|
| Follow‐up | No details of follow‐up reported. | |
| Aim of study | To assess the potential of the maternal concentration of schwangerschaftsprotein 1 as a marker for Down’s syndrome pregnancies. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results . |
| Index test results blinded? All tests | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Sahota 2010.
| Clinical features and settings | Routine screening. | |
| Participants | 10,854 pregnancies with complete outcome data. China ‐ University Hospital. January 2005‐May 2008. Pregnant women. Singleton pregnancies. Median maternal age 33.1 years, 30.1% of women aged ≥ 35 years. 10‐13 weeks' gestation. |
|
| Study design | Retrospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF accredited sonographers, HDI 5000, Philips Medical System). First trimester PAPP‐A and free ßhCG (kryptor analyser, Brahms Diagnostica GmbH). |
|
| Follow‐up | Fetal karyotypes were entered into a database when information was available. Data on pregnancy outcomes were obtained from either a local maternity database (for those who delivered in the unit) or via telephone calls to patients. | |
| Aim of study | To assess the relative performance of a multi‐stage first trimester screening protocol for fetal Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Schaelike 2009.
| Clinical features and settings | Routine screening. | |
| Participants | 10,668 participants with complete outcome data. Germany ‐ Private centre. Pregnant women. November 2000‐December 2006. Singleton pregnancies. Maternal age ≥ 35 years in 31.0% of women. 11‐13 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 59 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF certified physicians). First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms GmbH). Cut‐point 1:300. |
|
| Follow‐up | Information provided by either obstetric departments or obstetricians. Results obtained from CVS and amniocentesis, as well as karyotypes from aborted fetal tissue or postnatal investigations. 3.9% of women were lost to follow‐up and were excluded from the study. | |
| Aim of study | To assess the performance of a combined first trimester screening concept for trisomies 21, 18 and 13 applied to a low‐ and high‐risk patient sample in a specialised private centre for prenatal medicine. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Scott 2004.
| Clinical features and settings | Routine screening. | |
| Participants | 2053 participants. Australia ‐ Private practice (Sydney Ultrasound for Women). July 2000 to May 2002. Pregnant women 15‐44 years (median 32 years). Singleton pregnancies. 11‐14 weeks' gestation. |
|
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down’s syndrome: 5 cases. Reference standards: invasive testing or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF methods, sagittal plane, ATL 5000; Philips). First trimester free ßhCG and PAPP‐A (kryptor analyser, Brahms Diagnostics). All participants had all tests. Risk cut‐point 1:300. |
|
| Follow‐up | Data obtained from referring doctors or patients via letter, phone or completed feedback form given at the time of consultation. Only cases of known outcome included in the study. 68 (1.3%) lost to follow‐up, largely due to miscarriage (n = 20) and loss to follow‐up (n = 40). | |
| Aim of study | To report the sensitivity of combined first trimester biochemistry and ultrasound screening for Down's syndrome in an Australian private practice specialising in obstetric ultrasound. | |
| Notes | Only women having biochemical testing before NT were included in the study. This was done to avoid bias from women declining biochemical testing following negative NT. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | No details of withdrawals given. |
Spencer 1999a.
| Clinical features and settings | Women referred for invasive testing or self‐referred for screening. | |
| Participants | 1156 participants. UK ‐ Fetal medicine research centre. Dates not specified. 210 cases of Down's syndrome, maternal age 19‐46 (median 38 years). 946 controls matched for gestational and maternal age, maternal age 15‐47 years (median age 36 years) 10‐14 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 210 cases. Reference standards: invasive testing (high‐risk women) or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (methods not reported). First trimester free ßhCG and PAPP‐A (Kryptor analyser, time resolved amplified cryptate emission (TRACE)). |
|
| Follow‐up | Details of methods for follow‐up to birth not reported. | |
| Aim of study | To examine the potential impact of combining maternal age with fetal NT thickness and maternal serum free ßhCG and PAPP‐A in screening for trisomy 21 at 10‐14 weeks of gestation. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Spencer 2002a.
| Clinical features and settings | Routine screening. | |
| Participants | 278 participants. UK ‐ Single hosp1T ITAl study (OSCAR screening program). Samples collected since 1998. 54 cases of Down's syndrome, maternal age 20‐44 years, median 36 years. 224 controls (no details of selection), maternal age16‐41 years, median 30 years. 11‐13 weeks' gestation.. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 54 cases. Reference standards: no description given. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF methods). First trimester free ßhCG, PAPP‐A and ThCG (Kryptor Analyser (TRACE) and automated immunofluorescent assays). All women underwent all tests. |
|
| Follow‐up | Methods for follow‐up to birth not reported. | |
| Aim of study | To assess serum hyperglycosylated hCG for use in the first trimester of pregnancy as a marker of Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth (Nicolaides ref (OSCAR)). |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear of all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Torring 2010.
| Clinical features and settings | Routine screening. | |
| Participants | 691 participants: 46 cases and 645 controls. Denmark ‐ nationwide screening programme. Dates not reported. Pregnant women. Singleton pregnancies. Mean maternal age cases 35 years, controls 31 years. 8‐11 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 46 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (11‐13 weeks' gestation) (FMF certified sonographers). Fresh serum tested for: First trimester PAPP‐A and free ßhCG (8‐11 weeks' gestation) (Kryptor analyser, Brahms). Frozen serum tested for: First trimester ADAM12s (8‐11 weeks' gestation) (Kyptor analyser, assay by Cezanne SAS, TRACE technology). |
|
| Follow‐up | Details not reported. | |
| Aim of study | To determine whether ADAM12s is a useful serum marker for fetal trisomy 21 using the mixture model. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Tsukerman 1999.
| Clinical features and settings | Routine screening. | |
| Participants | 1595 participants. Belarus. Started January 1996. Pregnant women. 1,564 controls matched for gestational age and duration of storage. 8‐13 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 31 cases. Reference standards: karyotyping, karyotyping at birth, follow‐up to birth not reported. |
|
| Index and comparator tests | Frozen or fresh serum tested for: First trimester free ßhCG, AFP and PAPP‐A (DELFIA, EG&G Wallac Oy). |
|
| Follow‐up | No details of follow‐up reported. | |
| Aim of study | To report results of a large population study looking at AFP, free ßhCG and PAPP‐A in the first trimester of pregnancy among women having routine ultrasound dating as part of NT assessment. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Women received different reference standards. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of some index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Valinen 2007.
| Clinical features and settings | Routine screening | |
| Participants | 7534 participants. Finland ‐ screening programme. 2002‐2004. Pregnant women. Singleton pregnancies. Mean maternal age 29.6 years, 18.6% ≥ 35 years. 10‐12 weeks' gestation. |
|
| Study design | Retrospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 30 cases (24 underwent NT as well as biochemical testing). Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (trained nurses, midwives and doctors) (4765 women). First trimester PAPP‐A and free ßhCG (details not reported) (7534 women). Cut‐point 1:250. |
|
| Follow‐up | Contacted chromosome laboratory at the department of clinical genetics in the Oulu university clinic and the Finish Register of Congenital Malformation and the National Research and Development Centre for Welfare and Health. | |
| Aim of study | To compare the efficacy of both separate and combined maternal serum testing and fetal NT measurement in the first trimester screening for Down's syndrome in northern Finland. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Valinen 2009.
| Clinical features and settings | Routine screening. | |
| Participants | 279 participants: 53 cases and 226 controls matched for maternal and gestational age and sample storage time. Finland ‐ screening programme. May 2002‐December 2007. Pregnant women. Maternal age not reported. 9‐13 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 53 cases (in 5 cases, NT not measured). Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. Fresh serum tested for: First trimester PAPP‐A and free ßhCG (details not reported). Frozen serum tested for: First trimester ADAM12 (DELFIA/AutoDELFIA ADAM12 research kit, PerkinElmer Wallac). Cut‐point 1:250. |
|
| Follow‐up | Details not reported. | |
| Aim of study | To investigate whether incorporating the measurement of ADAM12 in the risk calculation program LifeCycle can improve Down's syndrome screening in the first trimester. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Van Lith 1992.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 1372 participants (24 cases and 1348 controls, criteria for matching not reported). The Netherlands – 6 prenatal diagnostic centres. Dates not stated. Pregnant women. Less than 13 weeks' gestation. |
|
| Study design | Case‐control study (changed from cohort). | |
| Target condition and reference standard(s) | Down’s syndrome: 24 cases. Reference standard: CVS. |
|
| Index and comparator tests | Frozen serum rested for: Total hCG (IMx hCG assay, Abbott). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To assess the value of MS‐hCG in the first trimester of pregnancy in screening for Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results (biochemical measurement conducted blind to pregnancy outcome). |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Wald 2003a.
| Clinical features and settings | Routine screening. | |
| Participants | 606 participants. UK and Austria ‐ multicentre trial. September 1996 to April 2000. Pregnant women: 101 cases, 505 controls matched for gestation, duration of storage and centre. 9‐13 and 14‐20 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 101 cases. Reference standards: invasive testing (following second trimester screening) or follow‐up to birth. |
|
| Index and comparator tests | First trimester NT (midsagittal section, optimal magnification of thickness of translucent space between inner skin surface and fascia covering cervical spine (white black interface (outer) ‐ black white interface (inner), 41 models of ultrasound machine, 20 minutes allotted scanning time). First and second trimester serum AFP, hCG, uE3, PAPP‐A, free ßhCG (time resolved fluoroimmunoassay, AutoDELFIA). First and second trimester inhibin A (Sandwich enzyme linked immunosorbent assay, Oxford bioinnovation). First and second trimester urinary beta core fragment, total hCG, ITA and free ßhCG (ITA and beta core fragment, Quest diagnostics USA). |
|
| Follow‐up | Follow‐up by: 1) Staff at local hospitals completed a study outcome form at, or just after delivery, 2) Study records of CVS, amniocentesis or karyotype at birth linked to information from cytogenic laboratories, 3) Study records linked to records of cases of Down's syndrome from the National Down's Syndrome Cytogenetic Register, 4) Information obtained from local obstetrical outcome records, 5) Forms sent to all women with a request to return details of the outcome of their pregnancy, 6) individual searches in respect of women whose outcomes of pregnancy had not been obtained by any of the previous methods. 96% birth/karyotype full outcome documentation obtained. | |
| Aim of study | To identify the most effective, safe and cost‐effective strategy for antenatal screening for Down's syndrome using NT, maternal serum and urine markers in the first and second trimesters of pregnancy and maternal age in various combinations. | |
| Notes | Performance of screening assessed at 17 weeks' gestation. Study tried to be non‐interventional in the first trimester ‐ second trimester testing was aimed to be used as the basis for any referral for invasive testing. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | Yes | Rates of NT failure on average 9%. pre‐10 weeks' gestation, > 33% failure rate, declined to 7% at 12 weeks. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Wallace 1995.
| Clinical features and settings | Routine screening. | |
| Participants | 112 participants. UK. Dates not stated. Pregnant women. 23 cases (maternal age 22‐44 years, mean 32 years). 89 controls matched for gestational age and duration of sample storage (maternal age 19‐38 years, mean 28 years). 11‐13 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down’s syndrome: 23 cases. Reference standard: not reported. |
|
| Index and comparator tests | Frozen serum tested for dimeric first trimester Inhibin A (enzyme‐linked two‐site immunoassay). | |
| Follow‐up | Methods of follow‐up not reported. | |
| Aim of study | To evaluate dimeric first trimester inhibin A as a possible first trimester screening marker for Down’s syndrome screening. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Unclear | Unclear reference standard. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | Unclear | Unclear if the reference standard differed between women. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Wapner 2003.
| Clinical features and settings | Routine screening. | |
| Participants | 8216 participants. USA ‐ multicentre study (12 prenatal diagnostic centres). Dates not specified. Singleton pregnancies. Pregnant women with mean age 35 years (SD 4.6), 50% ≥ 35 years. 11 to 14 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 61 cases. Reference standards: invasive testing, miscarriage with cytogenetic testing, follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (FMF methods). Dried blood samples tested for: First trimester free ßhCG and PAPP‐A (dried blood samples, enzyme‐linked immunoadsorbent assay as previously described). Risk cut‐point 1:270. |
|
| Follow‐up | Follow‐up to birth by directly following up women and reviewing delivery records. An effort was also made to obtain information on terminated or miscarried pregnancies. 196 (2.3%) of patients without follow‐up information were excluded. | |
| Aim of study | To evaluate the use of combined first trimester markers for aneuploidy in clinical practice. | |
| Notes | 16 live Down’s syndrome births. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | Yes | No details of withdrawals given. |
Weinans 2005.
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 344 participants. The Netherlands ‐ antenatal diagnosis unit. 1999‐2002. Pregnant women with mean age 38 years (SD 2.7 years) for cases and 37 years (SD 3.0) for controls 24 cases, 320 controls matched for maternal and gestational age and length of storage. Singleton pregnancies. 9 to 11 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 24 cases. Reference standard; CVS. |
|
| Index and comparator tests | Maternal age. Frozen serum samples tested for: First trimester serum free ßhCG and PAPP‐A (fluoroimmunoassay, Auto Delfia, Perkin Elmer). First trimester maternal serum ITA (immunochemiluminometric assay, Nichols Advantage platform). |
|
| Follow‐up | 100% karyotyping. | |
| Aim of study | To investigate Down's syndrome screening performance of serum ITA before 12 weeks' gestation and compare it with performance of PAPP‐A and free ßhCG in the same sample set. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? All tests | Yes | CVS. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same reference standard. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Wojdemann 2005.
| Clinical features and settings | Referrals for screening. | |
| Participants | 8622 participants (6441 with serum screening). Denmark ‐ 3 obstetrics departments. March 1998 to June 2001 Pregnant women with mean age 29 years, 10.8% ≥ 35 years. Singleton pregnancies. 11 to 14 weeks' gestation. |
|
| Study design | Prospective cohort. | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases. Reference standards: invasive testing (in cases of increased risk) or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. First trimester NT (all patients) (FMF methods, Logic 700 MR machine). First trimester free ßhCG (AFP/ßhCG Auto Delfia kit) and PAPP‐A (In‐house ELISA (Sandwich)) in 6,441 patients (75%). Risk cut‐point 1:250. |
|
| Follow‐up | Cross‐checking with all the chromosome laboratories in Denmark. Follow‐up in 96.2% of pregnancies through patients records. | |
| Aim of study | To determine the performance of screening for Down's syndrome and other major chromosomal abnormalities using NT, free ßhCG and PAPP‐A in a prospective study of a non‐selected population. | |
| Notes | Uptake of screening was 73% (9941 accepted out of 13,621 offered screening). Women with miscarriages excluded from the study. 3 live Down’s syndrome births. |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | Yes | NT could not be measured in 2.5% of cases. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
Zaragoza 2009.
| Clinical features and settings | Routine screening. | |
| Participants | 699 participants: 90 cases and 609 controls matched for length of storage. UK ‐ single centre. Dates not reported. Pregnant women. Singleton pregnancies. Median maternal age cases 37.9 years (19.1‐46.5 years), controls 32.7 years (16.1‐45.2 years). 11‐13 weeks' gestation. |
|
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 90 cases. Reference standards: karyotyping or follow‐up to birth. |
|
| Index and comparator tests | Maternal age. Fresh samples tested for: First trimester PAPP‐A and free ßhCG (Delfia Express system, PerkinElmer, Waltham). Frozen samples tested for: First trimester PlGF (ELISA, Quantikine human PlGF immunoassay, R&D systems Europe Ltd). |
|
| Follow‐up | Karyotype results and details on pregnancy outcome were added to database as soon as they became available. | |
| Aim of study | To investigate the potential value of maternal serum placental growth factor (PlGF) in first trimester screening from trisomy 21 and other major chromosomal abnormalities. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? All tests | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? All tests | Yes | All women received a reference standard. |
| Differential verification avoided? All tests | No | Choice of reference standard depended on index test results. |
| Incorporation avoided? All tests | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? All tests | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? All tests | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results. |
| Relevant clinical information? All tests | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? All tests | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? All tests | No | No details of withdrawals given. |
AFP: alpha‐fetoprotein αhCG: alpha human chorionic gonadotrophin ßhCG: beta human chorionic gonadotrophin CVS: chorionic villus sampling ELISA: enxyme‐linked immunosorbent assay FMF: Fetal Medicine Foundation GHBP: growth hormone binding protein hCG: human chorionic gonadotrophin ITA: invasive trophoblast antigen IQR: interquartile range NT: nuchal translucency PAPP‐A: Pregnancy‐associated plasma protein A PGH: placental growth hormone PIGF: placental growth factor PROMBP: proform of eosinophil major basic protein SD: standard deviation SP 1: Schwangerschafts protein 1 uE3: unconjugated oestriol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbas 1995 | Unable to extract useful data. |
| Abdul‐Hamid 2004 | No Down's syndrome pregnancies. |
| Abraha 1999 | Unable to extract useful data. |
| Abu‐Rustum 2010 | Not Down’s syndrome specific. |
| Achiron 2010 | Study only includes cases of Down’s syndrome. |
| Adekunle 1999 | Unable to extract useful information. |
| Agaard‐Tillery 2010 | Results presented in another study. |
| Aitken 1993 | Unable to extract useful data. |
| Aitken 1996a | Fewer than 80% of pregnancies had gestational age confirmed by USS. |
| Aitken 1996b | Fewer than 80% of pregnancies had gestational age confirmed by USS. |
| Ajayi 2011 | No diagnostic data. |
| Akbas 2001 | Less than 5 Down's syndrome pregnancies. |
| Alexioy 2009 | Study only includes test positives. |
| Allingham‐Hawkins 2011 | Quantitative fluorescent polymerase chain reaction study. |
| American College 2009 | Discussion article. |
| Antona 1998 | Likely fewer than 80% of pregnancies dated by USS. |
| Antsaklis 1999 | Women screened at greater than 24 weeks' gestation. |
| Anuwutnavin 2009 | Second trimester ultrasound. |
| Ashwood 1987 | Unable to extract useful data. |
| Asrani 2005 | Review article. |
| Audibert 2001 | Unable to ascertain whether part of screening population in Rozenberg et al. No response from authors, therefore excluded to reduce risk of data replication. |
| Axt‐Fleidner 2006 | Unable to extract useful data. |
| Azuma 2002 | Unable to extract useful data. |
| Baghagho 2004 | Unable to obtain paper. |
| Bahado‐Singh 1995 | USS markers greater than 14 weeks' gestation. |
| Bahado‐Singh 1996 | USS markers greater than 14 weeks' gestation. |
| Bahado‐Singh 1999 | USS markers greater than 14 weeks' gestation. |
| Bahado‐Singh 2002 | USS markers greater than 14 weeks' gestation. |
| Bahado‐Singh 2003 | Review article. |
| Ball 2007 | Data from the FASTER trial. |
| Bar‐Hava 2001 | No Down's pregnancies in study population. |
| Barkai 1996 | No Down's pregnancies in study population. |
| Barnabei 1995 | No Down's pregnancies in study population. |
| Bartels 1988 | Unable to extract useful data. |
| Bartels 1993 | No Down's pregnancies in study population. |
| Barth 1991 | Second trimester ultrasound study. |
| Bas‐Budecka 2007 | No diagnostic data. |
| Baviera 2004 | Unclear method of confirmation of gestational age. |
| Bazzett 1998 | Male versus female fetuses. |
| Beke 2008 | Results are not specific to Down’s syndrome. |
| Bellver 2005 | No Down's syndrome pregnancies in study. |
| Benn 1995 | Less than 80% follow‐up. |
| Benn 1996 | Less than 80% follow‐up. |
| Benn 1997 | No Down's pregnancies in study population. |
| Benn 1998 | Less than 80% follow‐up. |
| Benn 2001 | Statistical modelling (computer simulation). |
| Benn 2002 | Modelled data. |
| Benn 2003a | Less than 80% of pregnancies dated by USS. |
| Benn 2003b | Editorial. |
| Benn 2005a | No Down's pregnancies included. |
| Benn 2005b | Mathematical model. |
| Benn 2007 | No follow‐up information. |
| Berry 1995 | Less than 80% of pregnancies USS dated. |
| Berry 1997 | Less than 80% of pregnancies USS dated. |
| Bersinger 1994 | Gestational age not USS estimated. |
| Bersinger 2000 | Unable to extract useful data. |
| Bersinger 2001 | No Down's syndrome pregnancies in study population. |
| Bersinger 2003 | Unable to extract useful data. |
| Bersinger 2004 | No Down's syndrome pregnancies in study population. |
| Bersinger 2005 | No Down's syndrome pregnancies in study population. |
| Bestwick 2008 | All healthy pregnancies. |
| Biggio 2004 | Cost‐effectiveness analysis. |
| Bilardo 2011 | Not a proper sample ‐ most had elevated NT. |
| Bindra 2002 | Review article. |
| Blundell 1999 | Unable to extract useful data. |
| Boormans 2010 | Study of testing on amniocentesis samples. |
| Boots 1989 | Population risk factor calculations. |
| Bornstein 2009a | No diagnostic data. |
| Bornstein 2009b | No diagnostic data. |
| Bornstein 2010 | No diagnostic data. |
| Borowski 2007 | No diagnostic data. |
| Borrell 2007 | No follow‐up data. |
| Borruto 2002 | Unable to extract useful data. |
| Bottalico 2009 | Second trimester ultrasound. |
| Boue 1990 | Review article. |
| Bradley 1994 | Screen negative population gestations not confirmed by ultrasound. |
| Braithwaite 1996 | Review article. |
| Brambati 1995 | USS screening inclusive of women greater than 14 weeks' gestation. |
| Brambati 1996 | Review article. |
| Brizot 1995a | Unable to extract useful data. |
| Brizot 1995b | Unable to extract useful data. |
| Brizzi 1989 | Second trimester ultrasound. |
| Brock 1990 | Unable to extract useful data. |
| Calda 2010 | No data for false positive rates. |
| Campogrande 2001 | Unable to extract useful data. |
| Canick 1988 | Unable to extract useful data. |
| Canick 1995 | Unable to extract useful data. |
| Canini 2002 | No Down's syndrome pregnancies in study population. |
| Cans 1998 | Second trimester ultrasound. |
| Carreras 1991 | Second trimester ultrasound. |
| Caughey 2007 | No diagnostic data. |
| Cebesoy 2008 | No diagnostic data. |
| Chelli 2008 | No follow‐up for false negatives. |
| Chen 1999 | Review article. |
| Chen 2002 | No Down's syndrome pregnancies in study population. |
| Chen 2004 | Less than 5 Down's cases in study population. |
| Chen 2005 | Unable to extract useful data. |
| Chen 2008 | No diagnostic data. |
| Cheng 1993 | Likely that fewer than 80% of gestational age confirmed by USS. |
| Cheng 1999 | Case series. No Down's syndrome pregnancies in study population. |
| Cheng 2004a | No Down's syndrome pregnancies in study population. |
| Cheng 2004b | No Down's syndrome pregnancies in study population. |
| Chitayat 2002 | Less than 5 Down's cases in study population. |
| Chiu 2011 | Study of maternal DNA testing. |
| Cho 2009 | Study of testing amniotic fluid. |
| Chou 2009 | Not possible to calculate specificity. |
| Christiansen 2002 | Unable to extract useful data. |
| Christiansen 2007b | Unable to extract useful data. |
| Christiansen 2008 | No diagnostic data. |
| Chung 2000 | Less than 5 Down's syndrome pregnancies in study population. |
| CNGOF 1996 | Unable to obtain translation. |
| Cole 1996 | Review article. |
| Comas 2001 | USS at greater than 14 weeks. |
| Comas 2002a | USS at greater than 14 weeks. |
| Comas 2002b | USS at greater than 14 weeks. |
| Comstock 2006 | Unable to extract useful data. |
| Conde 1998 | Review article. |
| Cowans 2011 | No diagnostic data. |
| Crossley 1991 | Less than 80% of pregnancies had gestational age confirmation by ultrasound. |
| Crossley 1993 | Less than 80% of pregnancies had gestational age confirmation by ultrasound. |
| Crossley 1996 | No Down's syndrome pregnancies in study population. |
| Crossley 2002b | Adjustment factors for smokers. |
| Cuckle 1984 | Gestational age not confirmed by USS. |
| Cuckle 1987a | Gestational age not confirmed by USS. |
| Cuckle 1987b | No gestational age limits given. |
| Cuckle 1990 | Paper presenting adjustment factors. |
| Cuckle 1996 | Data modelled on 4 meta‐analysed studies. |
| Cuckle 1999a | Unable to extract useful data. |
| Cuckle 1999b | Review article. |
| Cullen 1990 | Abnormal scans only in study population. |
| Cusick 2004 | Less than 5 Down's syndrome pregnancies in study population. |
| Cusick 2007 | Second trimester ultrasound. |
| D'Ottavio 1997 | Second trimester USS. |
| Dancoine 2001 | No Down's syndrome pregnancies in study population. |
| Dane 2008 | Not specific to Down’s syndrome. |
| De Biasio 2000 | Unable to extract useful information. |
| De Biasio, 1999 | Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response. |
| De Biasio, 2001 | Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response. |
| De Graaf 1991 | Unable to extract useful data. |
| De Graaf 1999b | Modelled data. |
| Del Carmen Saucedo 2009 | No follow‐up information. |
| DeVore 2001 | Second trimester ultrasound. |
| Dhaifalah 2007a | Unable to obtain translation. |
| Dhaifalah 2007b | Unable to obtain translation. |
| Dhallan 2007 | DNA testing of blood samples from parents. |
| Dickerson 1994 | Comment. |
| Dimaio 1987 | Gestational age by USS only in screen‐positive population. |
| Doran 1986 | Ultrasound confirmation of gestational age performed in screen positive women only. |
| Dreux 2008 | No information for specificity. |
| Drugan 1996a | Second trimester ultrasound. |
| Drugan 1996b | Unable to extract useful data. |
| Drysdale 2002 | Fewer than 5 Down's syndrome pregnancies in population. |
| Dugoff 2008 | Not specific to Down’s syndrome. |
| Ebell 1999 | Review article. |
| Economides 1998 | Unable to extract useful data. |
| Erickson 2004 | No Down's syndrome pregnancies in population. |
| Evans 1996 | No Down's syndrome pregnancies in population. |
| Evans 2007 | Data previously presented in another study. |
| Falcon 2005 | Unable to extract useful data. |
| Falcon 2006 | Unable to extract useful data. |
| Ford 1998 | Audit. |
| Frishman 1997 | No Down's syndrome pregnancies in population. |
| Fukada 2000 | Unable to extract useful data. |
| Gaudry 2009 | Study of karyotyping. |
| Gebb 2009 | Study only examines screen positives. |
| Geerts 2008 | Study only examines abnormal fetuses. |
| Geipel 2010 | Second trimester ultrasound. |
| Gekas 2009 | Diagnostic data from other studies. |
| Gekas 2011a | Diagnostic data from other studies. |
| Gekas 2011b | Diagnostic parameters from other studies. |
| Gerovassili 2007 | No diagnostic data. |
| Ghidini 1998 | Comparison of male versus female fetuses. |
| Goetzinger 2010 | Second trimester ultrasound. |
| Goldie 1995 | Fewer than 80% of study population and gestational age confirmed by USS. |
| Gollo 2008 | Only one case of Down’s syndrome. |
| Gonçalves 2004 | Greater than 14 weeks USS screening. |
| Goodburn 1994 | Likely that fewer than 80% of pregnancies had gestational age estimated by USS. |
| Gorduza 2007 | Study of FISH technique. |
| Grace 2010 | Second trimester ultrasound. |
| Grati 2010 | No diagnostic data. |
| Gray 2009 | Second trimester ultrasound. |
| Gregor 2007 | Unable to obtain translation. |
| Gregor 2009 | Unable to obtain translation. |
| Grether 2009 | Systematic review and guidelines. |
| Grozdea 2002 | Unable to extract useful data. |
| Guo 2010 | Study of fetal samples. |
| Gyselaers 2004a | Less than 80% follow‐up. |
| Gyselaers 2004b | Less than 80% follow‐up. |
| Gyselaers 2006a | Unaffected pregnancies only. |
| Gyselaers 2006b | Unable to extract useful data. |
| Hackshaw 1995 | No Down's syndrome pregnancies in population. |
| Hackshaw 2001 | No Down's syndrome pregnancies in population |
| Haddow 1992 | Less than 80% of pregnancies had gestational age confirmed by ultrasound scan. |
| Hadzsiev 2007 | Study of FISH technique. |
| Hafner 1995 | Less than 5 Down's pregnancies in study population. |
| Hallahan 1998 | Gestational age greater than 24 weeks. |
| Han 2008 | Study of findings on amniocentesis. |
| Harper 2010 | Second trimester ultrasound. |
| Harrison 2006 | Less than 80% of pregnancies had gestational age confirmed by ultrasound scan. |
| Harry 2006 | Editorial. |
| Hayashi 1995 | Unable to extract useful data. |
| Hayashi 1996 | Less than 5 Down's pregnancies in study population. |
| Heikkila 1997 | Fewer than 80% of pregnancies had gestational age confirmed by USS. |
| Heinig 2007 | No Down’s syndrome data. |
| Heinonen 1996 | No Down's syndrome pregnancies in population. |
| Herman 2000 | No Down's syndrome pregnancies in study population. |
| Herman 2003 | Correlation between markers, not evaluation of screening tests. |
| Herrou 1992 | Unable to extract useful data. |
| Hershey 1985 | Gestation unclear. |
| Hershey 1986 | Gestation based on LMP. |
| Hewitt 1993 | Unable to extract useful data. |
| Hills 2010 | Study of testing on CVS and amniocentesis samples. |
| Ho 2010 | Study of FISH diagnosis. |
| Hogdall 1992 | Unclear method of determination of gestational age. Unable to extract useful data. |
| Hong Kong Practitioner | CME. |
| Hoogendoorn 2008 | Diagnostic data from other studies used. |
| Howe 2000 | Second trimester ultrasound scans. |
| Hsiao 1991 | Unable to obtain translation. |
| Hsieh 1999 | No Down's syndrome pregnancies in study population. |
| Hsu 1997 | Adjustment factors. |
| Hsu 1998 | No Down's syndrome pregnancies in study population. |
| Hsu 1999 | No Down's pregnancies. |
| Hu 2007 | Same data as Liu 2010. |
| Huang 2003 | No Down's syndrome pregnancies in study population. |
| Huang 2007a | Not possible to obtain detection rate. |
| Huang 2007b | No diagnostic data. |
| Huggon 2004 | Study of cardiac function in pregnancies with normal and abnormal NT results. |
| Hui 2003 | No Down's syndrome pregnancies in population. |
| Hui 2005 | No Down's syndrome pregnancies in population. |
| Hultén 2004 | Editorial/commentary. |
| Hung 2003 | Modelling. |
| Hung 2008 | Second trimester ultrasound. |
| Hurley 1993 | Unable to extract useful data. |
| Huttly 2004 | No Down's syndrome pregnancies in population. |
| Hwa 2004 | Less than 5 Down's pregnancies in population. |
| Iles 1996 | Review. |
| Ind 1994 | Unable to extract useful data. |
| Ivorra‐Deleuze 2010 | No diagnostic data. |
| Jakobsen 2011 | Not Down’s syndrome specific. |
| Jean‐Pierre 2005 | Review article. |
| Johnson 1991 | Gestational age estimated by USS in fewer than 80% of cases. |
| Johnson 1993 | Normal pregnancies only. |
| Jorgensen 1999 | Gestation greater than 14 weeks for USS. |
| Jorgez 2007 | Study of DNA testing on maternal blood. |
| Josefsson 1998 | No Down's syndrome pregnancies in study population. |
| Jou 2001 | Less than 5 Down's syndrome pregnancies in study population. |
| Jung 2007 | Second trimester ultrasound. |
| Kagan 2006 | Screen positive pregnancies only. |
| Kagan 2007 | No diagnostic data. |
| Kagan 2008 | Not Down’s syndrome detection. |
| Kalelioglu 2007 | Second trimester ultrasound. |
| Kautzmann 1995 | Fewer than 80% pregnancies had gestational age estimated by USS. |
| Kazerouni 2009 | Not possible to obtain complete diagnostic data. |
| Keith 1992 | Summary article. |
| Kelekci 2004 | Less than 5 Down's syndrome pregnancies in population. |
| Kellner 1995a | Less than 5 Down's syndrome pregnancies in population. |
| Kellner 1995b | Less than 80% follow‐up. Unable to ascertain proportion of population with gestational age confirmed by USS. |
| Kellner 1997 | Assumption of normal karyotype without reference standard in significant proportion of control pregnancies. |
| Kirkegaard 2008 | False positive rate only calculated for subset of the cohort. |
| Kjaergaard 2008 | Unable to obtain translation. |
| Knight 1990 | Review article. |
| Knight 2001 | Validation of a specific assay. |
| Knight 2005 | Less than 80% of pregnancies had gestational age confirmed by ultrasound scan. |
| Koos 2006 | Review article. |
| Kornman 1996 | Less than 5 Down's syndrome pregnancies in population. |
| Kornman 1997 | Unable to extract useful information. |
| Kotaska 2007 | No new data. |
| Kramer 1998 | No Down's syndrome pregnancies in study population. |
| Krantz 1996 | Modelled data. |
| Krantz 2005 | Adjustment factor. |
| Krantz 2007 | Uses data from other published studies. |
| Kulch 1993 | No Down's cases in population. |
| Lai 1998 | Modelled population. |
| Lai 2003 | No Down's syndrome pregnancies in study population. |
| Laigaard 2006a | Unable to extract useful data. |
| Laigaard 2006b | Simulation. |
| Lam 1997 | Unable to extract useful data. |
| Lam 1998 | Fewer than 80% pregnancies had gestational age estimated by USS. |
| Lam 1999a | No Down's syndrome pregnancies in population. |
| Lam 1999b | Unable to extract useful data. |
| Lam 2000 | Study of women's decisions about screening. |
| Lam 2001 | Male versus female fetuses. |
| Lambert‐Messerlian 1996 | Fewer than 80% of pregnancies USS dated. |
| Lambert‐Messerlian 1998 | Unable to extract useful data. |
| Lauria 2007 | No diagnostic data. |
| Lehavi 2005 | Down's syndrome pregnancies only. |
| Leung 2006 | Unable to separate twins from singletons therefore unable to extract useful data. |
| Leymarie 1993 | Appears to be a review article (French). |
| Li 1998 | Unable to obtain translation. |
| Li 1999 | Unable to obtain translation. |
| Li 2010 | No diagnostic data. |
| Liao 1997 | Unable to obtain translation. |
| Liao 2001 | Unable to extract useful data. |
| Lim 2002 | Second trimester ultrasound. |
| Lippman 1987 | Editorial. |
| Liu 2003 | Unable to obtain translation. |
| Liu 2010 | Not possible to separate out data for cases of Down’s syndrome. |
| Lo 2010 | Pooled test results. |
| Lustig 1988 | Gestational age by LMP only. |
| Luthgens 2008 | False positive rate and detection rate obtained from different cohorts. |
| MacDonald 1991 | Fewer than 80% of gestational ages estimated by USS. |
| Macintosh 1994 | Unable to extract useful data. |
| Macintosh 1997 | Unable to extract useful data. |
| MacRae 2010 | Pooled test results. |
| Macri 1994 | Likely fewer than 80% evaluated for gestational age by ultrasound examination. |
| Macri 1996 | Likely fewer than 80% evaluated for gestational age by ultrasound examination. |
| Malone 1998 | Review article. |
| Malone 2003 | Review article. |
| Mandryka‐Stankewycz 2009 | No diagnostic data. |
| Mangione 2001 | Abnormal screening results only. |
| Markov 2008 | Unable to obtain paper. |
| Maymon 2001a | No Down's syndrome pregnancies in study population. |
| Maymon 2001b | No normal test results included therefore unable to extract meaningful data. |
| Maymon 2002 | No Down's syndrome pregnancies in study population. |
| Maymon 2004 | No Down's syndrome pregnancies in study population. |
| Maymon 2005 | Modelled data. |
| McDuffie 1996 | USS dating on screen positive women only. |
| Meier 2002 | Observed versus expected cases of Down's syndrome in a population. |
| Merkatz 1984 | Gestational age not confirmed by ultrasound scan. |
| Merz 2005 | Editorial. |
| Merz 2008 | Data available for only combined ultrasound marker (nuchal translucency) and serum tests |
| Metzenbauer 2001 | Normal pregnancies only. |
| Metzenbauer 2002 | Unable to extract useful data. |
| Mikic 1999 | No Down's syndrome pregnancies in study population. |
| Miller 1991 | Unable to extract useful data. |
| Milunsky 1989 | Fewer than 80% gestational age estimated by USS. |
| Milunsky 1996 | Fewer than 80% gestational age estimated by USS. |
| Minobe 2002 | Gestational age greater than specified limits. |
| Miron 2008 | No diagnostic data. |
| Miron 2009 | No diagnostic data. |
| Miron 2010 | No diagnostic data. |
| Miyamura 1999 | Unable to extract useful data. |
| Moghadam 1998 | Unable to extract useful data. |
| Monni 2000 | Less than 5 Down's syndrome pregnancies. |
| Monni 2002 | Review article. |
| Mooney 1994 | Greater than 24 weeks' gestation. |
| Muhcu 2008 | No diagnostic data. |
| Muller 1994 | No Down's syndrome pregnancies in study population. |
| Muller 1996 | Unable to extract useful data. |
| Muller 1999 | Unable to extract useful data. |
| Muller 2002a | Gestational age greater than 24 weeks. |
| Muller 2002b | Unable to extract meaningful data ‐ unable to separate double‐ and triple‐test data. |
| Muller 2003b | No Down's syndrome pregnancies in study population. |
| Murta 2002 | Unable to extract useful data. |
| Musone 2000 | Unable to extract useful data. |
| Musto 1986 | Fewer than 80% USS dated. |
| Myrick 1990 | Unable to extract useful data. |
| Naidoo 2008 | Not specific Down’s syndrome results. |
| Nau 2009a | No diagnostic data. |
| Nau 2009b | No diagnostic data. |
| Neveux 1996a | No Down's syndrome pregnancies in population. |
| Neveux 1996b | Unable to extract useful data. |
| Ng 2004 | Unable to extract useful data. |
| Nicolaides 1992 | Study of outcomes of abnormal NT results. |
| Nicolaides 2000 | Review article. |
| Nicolaides 2004 | Review article. |
| Nicolaides 2005a | Unable to obtain translation ‐ appears to be a review article. |
| Nicolaides 2005b | Unable to obtain translation ‐ appears to be a review article. |
| Nicolaides 2005c | Unable to obtain translation ‐ appears to be a review article. |
| Nicolaides 2005d | Unable to obtain translation ‐ appears to be a review article. |
| Nicolaides 2005e | Unable to obtain translation ‐ appears to be a review article. |
| Nicolaides 2005f | Review article. |
| Niemimaa 2001b | No Down's pregnancies in study population. |
| Niemimaa 2002 | No Down's syndrome pregnancies in population. |
| Niemimaa 2003 | No Down's syndrome pregnancies in population. |
| Noble 1997a | Unable to extract useful data. |
| Norgaard 1990 | Less than 80% of gestational ages confirmed by USS. |
| Norton 1992 | Unable to extract useful data. |
| Novakov‐Mikic 2007 | Out of first trimester screening time frame. |
| O'Brien 1997a | No Down's syndrome pregnancies in population. |
| O'Brien 1997b | No Down's syndrome pregnancies in population. |
| Odibo 2004 | Gestational age of greater than 14 weeks in USS population. |
| Odibo 2007 | Second trimester ultrasound. |
| Odibo 2008 | Second trimester ultrasound. |
| Odibo 2009 | No results presented. |
| Offerdal 2008 | Second trimester ultrasound. |
| Ognibene 1999 | Unable to extract useful data. |
| Oh 2007 | No diagnostic data. |
| Olajide 1989 | Unable to extract useful data. |
| Onda 1996 | Unable to extract useful data. |
| Onda 1998 | Unable to extract useful data. |
| Onda 2000 | Less than 80% follow‐up. |
| Orlandi 2002 | No Down's syndrome pregnancies in study population. |
| Ozkaya 2010 | Only healthy pregnancies. |
| Paladini 2007 | No diagnostic data. |
| Palka 1998 | Twin data used in calculation of the median. |
| Palomaki 1989 | Fewer than 80% USS dated. |
| Palomaki 1993 | No Down's syndrome pregnancies in population. |
| Palomaki 1994 | No Down's syndrome pregnancies in population. |
| Palomaki 1996 | Meta‐analysis. |
| Palomaki 2005 | Unable to extract meaningful data. |
| Panburana 2001 | Less than 5 Down's syndrome pregnancies in population. |
| Pandya 1994 | Study of outcomes of abnormal nuchal translucency results. |
| Pandya 1995 | Review article. |
| Papadopoulou 2008 | No diagnostic data. |
| Parra‐Cordero 2007 | Second trimester ultrasound. |
| Paterlini‐Brechot 2007 | Editorial, no new data. |
| Paul 2001 | Unable to extract useful data. |
| Peralta 2005 | Unable to extract useful data. |
| Perenc 1998 | No Down's syndrome pregnancies in study population. |
| Perheentupa 2002 | No Down's syndrome pregnancies in population. |
| Perona 1998 | Smokers versus non smokers. |
| Persico 2008 | Second trimester ultrasound. |
| Petervari 2000 | Unable to extract useful data. |
| Petrocik 1989 | Likely fewer than 80% USS dated. |
| Phillips 1992 | Gestational age confirmed by USS in less than 80% of population. |
| Phillips 1993 | Gestational age confirmed by USS in less than 80% of population. |
| Pihl 2008 | Only 2 cases of Down’s syndrome. |
| Pinette 2003 | Women screened prior to recruitment. |
| Platt 2004 | Unable to extract useful data. |
| Podobnik 1995 | Abnormal results only. |
| Poon 2009 | No diagnostic data. |
| Prefumo 2002 | Comparison of prevalence and prediction. |
| Prefumo 2004 | Comparison of a marker in women of different ethnic origins. |
| Price 1998 | Unable to extract useful data. |
| Páez 2004 | Unable to obtain translation. |
| Raty 2000 | No Down's syndrome pregnancies in population. |
| Rembouskos 2004 | Unable to extract useful data. |
| Ren 1992 | Review article. |
| Renier 1998 | Method of ascertainment of gestational age unclear. Twin gestations included in general population. |
| Resta 1990 | Second trimester USS. |
| Reynders 1997 | Fewer than 5 Down's cases. |
| Reynolds 1989 | Explanation of mathematical techniques. |
| Reynolds 1999 | Unable to extract useful data. |
| Reynolds 2008 | Not full diagnostic data. |
| Ribbert 1996 | No Down's syndrome pregnancies in study population. |
| Rice 2005 | Down's syndrome pregnancies excluded from study. |
| Rich 1991 | Unable to extract useful data. |
| Roberts 1995 | No Down's syndrome pregnancies in study population. |
| Robertson 1991 | Editorial. |
| Rode 2003 | No Down's pregnancies. |
| Ronge 2006 | Editorial ‐ summary of FASTER results. |
| Rose 1995 | Review article. |
| Ross 1997 | Review article. |
| Rotmensch 1996 | Unable to extract useful data. |
| Rotmensch 1999 | No Down's syndrome pregnancies in study population. |
| Rozenberg 2006 | USS greater than 14 weeks' gestation. |
| Rudnicka 2002 | No Down's syndrome pregnancies in population. |
| Ryall 1992 | Unable to determine method of confirmation of gestational age. |
| Ryall 2001 | High‐risk results only included (i.e. no screen‐negative group for comparison). |
| Räty 2002 | No Down's pregnancies in population. |
| Sabriá 2002 | Unable to ascertain how numbers calculated and from which populations. |
| Sacchini 2003 | Unable to extract useful data. |
| Sahota 2009 | No diagnostic data. |
| Sahota 2010a | Included in Sahota 2010. |
| Salazar 2007 | Unable to obtain paper. |
| Salazar 2008 | Only one case of Down’s syndrome. |
| Saller 1997 | Down's syndrome secondary to Robertsonian translocation only. No controls. |
| Salomon 2001 | No Down's syndrome pregnancies in population. |
| Salonen 1997 | Fewer than 80% had gestational age estimated by USS. |
| Saltvedt 2005 | Gestation greater than 14 weeks for nuchal scanning. |
| Saridogan 1996 | Down's syndrome and Edward's syndrome affected pregnancies only. |
| Savoldelli 1993 | Unable to extract useful data. |
| Schielen 2009 | Full study information not given. |
| Schiott 2006 | Unable to extract useful data. |
| Schmidt 2007a | Not specific to Down’s syndrome. |
| Schmidt 2007b | No separate Down’s syndrome data. |
| Schmidt 2007c | No diagnostic data. |
| Schmidt 2008a | Not specific to Down’s syndrome. |
| Schmidt 2008b | Not specific to Down’s syndrome. |
| Schmidt 2008c | Not specific to Down’s syndrome. |
| Schmidt 2010 | No follow‐up data for test negatives. |
| Schuchter 1998 | No Down's pregnancies in study population. |
| Scott 1995 | Less than 5 Down's syndrome pregnancies in study population. |
| Seeds 1990 | Review article. |
| Seki 1995 | No Down's syndrome pregnancies in study population. |
| Shenhav 2003 | No Down's syndrome pregnancies. |
| Shintaku 1989 | Unable to extract useful data. |
| Shulman 2003 | No Down's syndrome pregnancies in population. |
| Sieroszewski 2008 | No Down’s syndrome specific information for specificity. |
| Simon‐Bouy 1999 | Review article. |
| Simpson 1986 | Gestational age confirmed by USS in less than 80% of population. |
| Smith 1990 | Analysis of screen‐positive results. |
| Smith 1996 | Review/meta‐analysis. |
| Smith 1999 | Unable to extract useful data. |
| Smith‐Bindman 2001 | Meta‐analysis of second trimester ultrasound markers. |
| Smith‐Bindman 2003 | Population study, not examining DTA. |
| Snijders 1995 | Study of prevalence, not screening. |
| Snijders 1999 | Study of prevalence, not screening. |
| Soergel 2006 | Less than 80% follow‐up. |
| Sokol 1998 | Observation of Down's prevalence stratified by age. |
| Sonek 2003 | Editorial. |
| Sonek 2007 | Second trimester ultrasound. |
| Sood 2010 | No diagnostic data. |
| Sooklim 2010 | Second trimester ultrasound. |
| Spencer 1985 | Fewer than 80% USS dated. |
| Spencer 1991a | Likely fewer than 80% USS dated. |
| Spencer 1991b | Unable to extract useful data. |
| Spencer 1992 | Unable to extract useful data. |
| Spencer 1993a | Fewer than 80% USS dated. |
| Spencer 1993b | No Down's pregnancies in study population. |
| Spencer 1993c | Unable to extract useful data. |
| Spencer 1993d | Fewer than 80% of pregnancies had gestational age confirmed by USS. |
| Spencer 1993e | Unable to extract useful data. |
| Spencer 1995 | No Down's pregnancies in population. |
| Spencer 1996 | Fewer than 80% of pregnancies had gestational age confirmed by USS. |
| Spencer 1997 | Statistical modelling, aneuploid pregnancies only in study population. |
| Spencer 1998a | No Down's pregnancies in population. |
| Spencer 1998b | Unable to extract useful data. |
| Spencer 1999b | Review. |
| Spencer 1999c | Statistical methods paper. |
| Spencer 2000a | Examination of median shifts rather than an evaluation of screening. |
| Spencer 2000b | No Down's syndrome pregnancies in population. |
| Spencer 2000c | No Down's syndrome pregnancies in population. |
| Spencer 2000d | No Down's cases. |
| Spencer 2000e | Male versus female fetuses. |
| Spencer 2000f | No Down's cases in population. |
| Spencer 2000g | No Down's pregnancies in population. |
| Spencer 2000h | No Down's pregnancies in population. |
| Spencer 2000i | Comparison of fetal sex. |
| Spencer 2001a | No Down's syndrome pregnancies in population. |
| Spencer 2001b | Unable to extract useful data. |
| Spencer 2001c | Unable to extract useful data. |
| Spencer 2001d | Unable to extract useful data. |
| Spencer 2001e | No Down's syndrome pregnancies in population. |
| Spencer 2002b | No Down's pregnancies. |
| Spencer 2002c | Risk validation study. |
| Spencer 2002d | No Down's syndrome pregnancies in population. |
| Spencer 2002e | Demonstration of median changes with time, rather than evaluation of screening. |
| Spencer 2003a | No Down's pregnancies in population. |
| Spencer 2003b | No Down's pregnancies in population. |
| Spencer 2003c | Calculation of weight correction factor. |
| Spencer 2003d | Fewer than 5 Down's syndrome pregnancies. |
| Spencer 2004 | Calculation of smoking correction factor. |
| Spencer 2005a | No Down's pregnancies. |
| Spencer 2005b | No Down's pregnancies. |
| Spencer 2005c | Comparison of 2 different assays ‐ not actual screening evaluation. |
| Spencer 2008 | Unable to extract appropriate data for unaffected pregnancies. |
| Spong 1999 | Comparison of male and female fetuses. |
| Staboulidou 2009 | No diagnostic data. |
| Stevens 1998 | Literature review. |
| Stoll 1992 | Review article. |
| Stressig 2011 | Second trimester ultrasound. |
| Su 2002 | Unable to extract useful data. |
| Suchet 1995 | Review article. |
| Suchy 1990 | Unable to ascertain method of confirmation of gestational age. |
| Summers 2003a | Only 55% gestational ages estimated by USS. |
| Summers 2003b | No Down's syndrome pregnancies in study population. |
| Suntharasaj 2005 | Examination of inter‐observer variation in NT scanning. |
| Susman 2010 | No diagnostic data. |
| Sutton 2004 | Unable to extract useful data. |
| Suzuki 1998 | Unable to extract useful data. |
| Tabor 1987 | Gestational age not confirmed by USS. |
| Tanski 1999 | Information on screen positive pregnancies only. |
| Thilaganathan 1998 | No Down's syndrome pregnancies in study population. |
| Thilaganathan 1999 | Editorial. |
| Tislaric 2002 | No Down's syndrome pregnancies in population. |
| Torok 1997 | Unable to extract useful data. |
| Torring 2009 | Not possible to obtain full diagnostic data. |
| Trninic‐Pjevic 2007 | Unable to obtain translation. |
| Tsai 2001 | Less than 5 Down's syndrome pregnancies in study population. |
| Valerio 1996 | Fewer than 80% pregnancies had gestational age estimated by USS. |
| Van Blerk 1992 | Unable to extract useful data. |
| Van Dyke 2007 | Not possible to obtain full diagnostic data. |
| Van Heesch, 2006 | No Down's syndrome pregnancies in study population. Software comparison study. |
| Van Lith 1991 | Unable to extract useful data. |
| Van Lith 1993 | Unable to extract useful data. |
| Van Lith 1994 | Unable to extract useful data. |
| Veress 1986 | Unable to extract useful data. |
| Veress 1988 | Unable to extract useful data. |
| Vergani 2008 | Second trimester ultrasound. |
| Vintzileos 2003 | Second trimester USS. |
| Wald 1988a | Less than 80% had gestational age confirmed by ultrasound. |
| Wald 1988b | Gestational age not confirmed by USS. |
| Wald 1991 | No Down's pregnancies in study. |
| Wald 1992a | Less than 80% had gestational age confirmed by ultrasound. |
| Wald 1992b | No Down's pregnancies in study. |
| Wald 1992c | No Down's pregnancies in study. |
| Wald 1993 | No USS dating. |
| Wald 1994 | No Down's syndrome pregnancies in population. |
| Wald 1994a | Review article. |
| Wald 1996a | No Down's pregnancies. |
| Wald 1996b | Dated by LMP. |
| Wald 1996c | No Down's syndrome pregnancies in population. |
| Wald 1996d | Gestational age greater than 24 weeks. |
| Wald 1997 | Data modelled on 3 separate populations of women. |
| Wald 1998 | Unable to extract useful data. |
| Wald 1999a | Unable to extract useful data. |
| Wald 1999b | Gestational age not confirmed by USS. |
| Wald 1999c | No Down's syndrome pregnancies. |
| Wald 1999d | Modelled on several studies, some of which have no USS dating. |
| Wald 2003b | No cases. |
| Wald 2003c | Less than 80% had gestational age confirmed by USS. |
| Wald 2006 | Modelled on SURRUS data. |
| Wallace 1994 | Unable to extract useful data. |
| Wallace 1997 | No Down's syndrome pregnancies in study population. |
| Wang 2010 | Second trimester ultrasound. |
| Ward 2005 | Review article. |
| Watt 1996a | No Down's syndrome pregnancies in study population. |
| Watt 1996b | No Down's syndrome pregnancies in study population. |
| Wax 2007 | No diagnostic data. |
| Weinans 2001 | Unable to extract useful data. |
| Weinans 2004 | Study of women's views on screening. |
| Weisz 2007 | Cohort split into people having different tests and non‐representative samples of women assessed for each test. |
| Welborn 1994 | Abnormal results only (cystic hygroma). |
| Wenstrom 1993 | Less than 80% of pregnancies had gestational age confirmed by USS. |
| Wenstrom 1995a | Adjustment factors. |
| Wenstrom 1995b | Less than 80% of pregnancies had gestational age confirmed by USS. |
| Wetta 2011 | No diagnostic data. |
| Whitlow 1998a | Unable to extract useful data. |
| Whitlow 1998b | Unable to extract useful data. |
| Whitlow 1999 | Unable to extract useful data. |
| Williamson 1994 | Likely fewer than 80% USS dated. |
| Wilson 2000 | Review. |
| Wojdemann 2001 | No Down's syndrome pregnancies in study population. |
| Wong 2003 | Less than 5 Down's syndrome pregnancies in population. |
| Wright 2006 | Mathematical model. |
| Wright 2007 | Simulation study, no new data. |
| Xie 2010 | Only cases of false negatives and true negatives included. |
| Yagel 1998 | Second trimester USS. |
| Yamamoto 2001a | Unable to extract useful data. |
| Yamamoto 2001b | Method of determination of gestational age unclear. |
| Yamamoto 2001c | Unable to extract useful data. |
| Yaron 2001 | Male versus female fetuses. |
| Ye 1995 | Unable to obtain translation. |
| Yoshida 2000 | Fewer than 80% pregnancies had gestational age estimated by USS. |
| Zalel 2008 | No diagnostic data. |
| Zeitune 1991 | Only aneuploid pregnancies included in study. |
| Zelop 2005 | No Down's cases in population. |
| Zhang 2011 | No diagnostic data. |
| Zhao 1998 | Unable to obtain translation. |
| Zhong 2011 | Second trimester ultrasound. |
| Zoppi 2003 | Inappropriate study design. |
CME: continuing medical education CVS: chorionic villus sampling DTA: diagnostic test accuracy FISH technique: fluorescence in situhybridization LMP: last menstrual period NT: nuchal translucency USS: ultrasound scan
Differences between protocol and review
The protocol intended to investigate several additional outcomes downstream from test accuracy, should they be reported in the test accuracy studies. When we attempted to extract this information however, it was found to be available in very few studies, and where such information was found it was difficult to extract meaningful data to allow for comparison between studies, as data were not reported in a universal manner. In several studies such outcomes were estimated rather than measured. Often they were not reported at all. The outcomes stated in the protocol which have not been included are: harms of testing; need for further testing; side effects of test; interventions and side effects; other abnormalities detected by testing; spontaneous miscarriage; miscarriage subsequent to invasive procedure, with or without normal karyotype; fetal karyotype; termination of pregnancy (prior to definitive testing or in a karyotypically normal pregnancy and following confirmation of Down’s syndrome or following detection of other chromosomal abnormalities); stillbirth; livebirth of affected and unaffected fetus; uptake of definitive testing by women.
The following refinements to the eligibility criteria were imposed to ensure that the quality of the included literature remained high. We excluded studies that identified fewer than five Down's syndrome pregnancies in their study population. We excluded studies that had less than 80% follow‐up of participants.
In addition, the analytical strategy was informed by the volume of tests and studies included, and developed so that we focused on key tests and test combinations by a) only meta‐analysed tests that were included in four or more studies or b) showed more than 70% sensitivity for more than 95% specificity. In addition, a requirement that a minimum of 10 studies for a single test was required before subgroup analysis was undertaken. Consequently several possible sources of heterogeneity were not investigated due to lack of data.
Contributions of authors
KA undertook the searches, applied eligibility criteria, extracted and entered data and wrote the first and second draft of the review.
ZA applied eligibility criteria, provided senior clinical input, oversaw the review process, and approved the final draft of the review.
JD supervised and planned the review, checked data extraction, supervised statistical analyses and wrote the second draft of the review.
JN applied eligibility criteria, provided senior clinical input, oversaw the review process, and approved the final draft of the review.
BG checked data extraction and undertook statistical analyses.
MP applied eligibility criteria, extracted and entered data for the updated literature search, and entered characteristics of studies information.
YT checked data extraction, undertook statistical analyses, contributed to the first draft of the review, and approved the final draft of the review.
Sources of support
Internal sources
-
University of Birmingham, UK.
Funding of the research time of JD and BG
External sources
-
NIHR Health Technology Assessment Programme, UK.
Project grant ‐ need to have reference number etc. Jim/Zarko can you add please?
-
NIHR Health Technology Assessment Programme, UK.
Funding for the Cochrane Reviews of Diagnostic Test Accuracy Support Unit, based at the University of Birmingham (JD).
Declarations of interest
KA: None known.
ZA: None known.
JD: None known.
JN: None known.
BG: None known.
MP: None known.
YT: None known.
New
References
References to studies included in this review
Baviera 2010 {published data only}
- Baviera G, Chimicata S, Domenico R, Granese R, Carbone C, Dugo N, et al. First‐ and second‐trimester ADAM12s in Down syndrome screening. Clinical Chemistry 2010;56(8):1355‐7. [DOI] [PubMed] [Google Scholar]
Benattar 1999 {published data only}
- Benattar C, Audibert F, Taieb J, Ville Y, Roberto A, Lindenbaum A, et al. Efficiency of ultrasound and biochemical markers for Down's syndrome risk screening. A prospective study. Fetal Diagnosis and Therapy 1999;14(2):112‐7. [DOI] [PubMed] [Google Scholar]
Biagiotti 1995 {published data only}
- Biagiotti R, Cariati E, Brizzi L, d'Agata A. Maternal serum screening for Down's syndrome in the first trimester of pregnancy. British Journal of Obstetrics and Gynaecology 1995;102(8):660‐2. [DOI] [PubMed] [Google Scholar]
Biagiotti 1998 {published data only}
- Biagiotti R, Brizzi L, Periti E, d'Agata A, Vanzi E, Cariati E. First trimester screening for Down's syndrome using maternal serum PAPP‐A and free beta‐hCG in combination with fetal nuchal translucency thickness. British Journal of Obstetrics and Gynaecology 1998;105(8):917‐20. [DOI] [PubMed] [Google Scholar]
Brambati 1993 {published data only}
- Brambati B, Macintosh MC, Teisner B, Maguiness S, Shrimanker K, Lanzani A, et al. Low maternal serum levels of pregnancy associated plasma protein A (PAPP‐A) in the first trimester in association with abnormal fetal karyotype. British Journal of Obstetrics and Gynaecology 1993;100(4):324‐6. [DOI] [PubMed] [Google Scholar]
Brambati 1994 {published data only}
- Brambati B, Tului L, Bonacchi I, Shrimanker K, Suzuki Y, Grudzinskas JG. Serum PAPP‐A and free beta‐hCG are first‐trimester screening markers for Down syndrome. Prenatal Diagnosis 1994;14(11):1043‐7. [DOI] [PubMed] [Google Scholar]
Brameld 2008 {published data only}
- Brameld KJ, Dickinson JE, O'Leary P, Bower C, Goldblatt J, Hewitt B, et al. First trimester predictors of adverse pregnancy outcomes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2008;48(6):529‐35. [DOI] [PubMed] [Google Scholar]
Brizot 1994 {published data only}
- Brizot ML, Snijders RJ, Bersinger NA, Kuhn P, Nicolaides KH. Maternal serum pregnancy‐associated plasma protein A and fetal nuchal translucency thickness for the prediction of fetal trisomies in early pregnancy. Obstetrics and Gynecology 1994;84(6):918‐22. [PubMed] [Google Scholar]
Casals 1996 {published data only}
- Casals E, Fortuny A, Grudzinskas JG, Suzuki Y, Teisner B, Comas C, et al. First‐trimester biochemical screening for Down syndrome with the use of PAPP‐A, AFP, and beta‐hCG. Prenatal Diagnosis 1996;16(5):405‐10. [DOI] [PubMed] [Google Scholar]
Christiansen 1999 {published data only}
- Christiansen M, Oxvig C, Wagner JM, Qin QP, Nguyen TH, Overgaard MT, et al. The proform of eosinophil major basic protein: a new maternal serum marker for Down syndrome. Prenatal Diagnosis 1999;19(10):905‐10. [DOI] [PubMed] [Google Scholar]
Christiansen 2004 {published data only}
- Christiansen M, Larsen SO, Oxvig C, Qin QP, Wagner JM, Overgaard MT, et al. Screening for Down's syndrome in early and late first and second trimester using six maternal serum markers. Clinical Genetics 2004;65(1):11‐6. [DOI] [PubMed] [Google Scholar]
Christiansen 2005 {published data only}
- Christiansen M, Norgaard‐Pedersen B. Inhibin A is a maternal serum marker for Down's syndrome early in the first trimester. Clinical Genetics 2005;68(1):35‐9. [DOI] [PubMed] [Google Scholar]
Christiansen 2007a {published data only}
- Christiansen M, Sorensen TL, Norgaard‐Pedersen B. Human placental lactogen is a first‐trimester maternal serum marker of Down syndrome. Prenatal Diagnosis 2007;27(1):1‐5. [DOI] [PubMed] [Google Scholar]
Christiansen 2009 {published data only}
- Christiansen M. Placental growth hormone and growth hormone binding protein are first trimester maternal serum markers of Down syndrome. Prenatal Diagnosis 2009;29(13):1249‐55. [DOI] [PubMed] [Google Scholar]
Christiansen 2010 {published data only}
- Christiansen M, Pihl K, Hedley PL, Gjerris AC, Lind PO, Larsen SO, et al. ADAM 12 may be used to reduce the false positive rate of first trimester combined screening for Down syndrome. Prenatal Diagnosis 2010;30(2):110‐4. [DOI] [PubMed] [Google Scholar]
Cowans 2010 {published data only}
- Cowans NJ, Stamatopoulou A, Spencer K. First trimester maternal serum placental growth factor in trisomy 21 pregnancies. Prenatal Diagnosis 2010;30(5):449‐53. [DOI] [PubMed] [Google Scholar]
Crandall 1993 {published data only}
- Crandall BF, Hanson FW, Keener S, Matsumoto M, Miller W. Maternal serum screening for alpha‐fetoprotein, unconjugated estriol, and human chorionic gonadotropin between 11 and 15 weeks of pregnancy to detect fetal chromosome abnormalities.[see comment]. American Journal of Obstetrics and Gynecology 1993;168(6 Pt 1):1864‐7; discussion 1867‐9. [DOI] [PubMed] [Google Scholar]
Crossley 2002a {published data only}
- Crossley JA, Aitken DA, Cameron AD, McBride E, Connor JM. Combined ultrasound and biochemical screening for Down's syndrome in the first trimester: a Scottish multicentre study. BJOG: an international journal of obstetrics and gynaecology 2002;109(6):667‐76. [DOI] [PubMed] [Google Scholar]
De Graaf 1999a {published data only}
- Graaf I, Pajkrt E, Bilardo CM, Leschot NJ, Cuckle HS, Lith JM. Early pregnancy screening for fetal aneuploidy with serum markers and nuchal translucency. Prenatal Diagnosis 1999;19(5):458‐62. [DOI] [PubMed] [Google Scholar]
Forest 1995 {published data only}
- Forest JC, Masse J, Rousseau F, Moutquin JM, Brideau NA, Belanger M. Screening for Down syndrome during the first and second trimesters: impact of risk estimation parameters. Clinical Biochemistry 1995;28(4):443‐9. [DOI] [PubMed] [Google Scholar]
Forest 1997 {published data only}
- Forest JC, Masse J, Moutquin JM. Screening for Down syndrome during first trimester: a prospective study using free beta‐human chorionic gonadotropin and pregnancy‐associated plasma protein A. Clinical Biochemistry 1997;30(4):333‐8. [DOI] [PubMed] [Google Scholar]
Gyselaers 2005 {published data only}
- Gyselaers WJ, Vereecken AJ, Herck EJ, Straetmans DP, Jonge ET, Ombelet WU, et al. Population screening for fetal trisomy 21: easy access to screening should be balanced against a uniform ultrasound protocol. Prenatal Diagnosis 2005;25(11):984‐90. [DOI] [PubMed] [Google Scholar]
Haddow 1998 {published data only}
- Haddow JE, Palomaki GE, Knight GJ, Williams J, Miller WA, Johnson A. Screening of maternal serum for fetal Down's syndrome in the first trimester. New England Journal of Medicine 1998;338(14):955‐61. [DOI] [PubMed] [Google Scholar]
Kagan 2009 {published data only}
- Kagan KO, Cicero S, Staboulidou I, Wright D, Nicolaides KH. Fetal nasal bone in screening for trisomies 21, 18 and 13 and Turner syndrome at 11‐13 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2009;33(3):259‐64. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Etchegaray A, Zhou Y, Wright D, Nicolaides KH. Prospective validation of first‐trimester combined screening for trisomy 21. Ultrasound in Obstetrics & Gynecology 2009;34(1):14‐8. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Staboulidou I, Cruz J, Wright D, Nicolaides KH. Two‐stage first‐trimester screening for trisomy 21 by ultrasound assessment and biochemical testing. Ultrasound in Obstetrics & Gynecology 2010;36(5):542‐7. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Valencia C, Livanos P, Wright D, Nicolaides KH. Tricuspid regurgitation in screening for trisomies 21, 18 and 13 and Turner syndrome at 11+0 to 13+6 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2009;33(1):18‐22. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Wright D, Baker A, Sahota D, Nicolaides KH. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta‐human chorionic gonadotropin and pregnancy‐associated plasma protein‐A. Ultrasound in Obstetrics & Gynecology 2008;31(6):618‐24. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Wright D, Spencer K, Molina FS, Nicolaides KH. First‐trimester screening for trisomy 21 by free beta‐human chorionic gonadotropin and pregnancy‐associated plasma protein‐A: impact of maternal and pregnancy characteristics. Ultrasound in Obstetrics & Gynecology 2008;31(5):493‐502. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Wright D, Valencia C, Maiz N, Nicolaides KH. Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free beta‐hCG and pregnancy‐associated plasma protein‐A. Human Reproduction 2008;23(9):1968‐75. [DOI] [PubMed] [Google Scholar]
Kornman 1998 {published data only}
- Kornman LH, Morssink LP, Hoor KA, Wolf BT, Kloosterman MD, Beekhuis JR, et al. Schwangerschafts protein 1 (SP1) adds little to the Age‐related detection of fetal Down syndrome in the first trimester of pregnancy. Prenatal Diagnosis 1998;18(10):1086‐90. [PubMed] [Google Scholar]
Kozlowski 2007 GC {published data only}
- Kozlowski P, Knippel AJ, Stressig R. Comparing first trimester screening performance: routine care gynaecologists' practices vs. prenatal centre. Ultraschall in der Medizin 2007;28(3):291‐5. [DOI] [PubMed] [Google Scholar]
Kozlowski 2007 PC {published data only}
- Kozlowski P, Knippel AJ, Stressig R. Comparing first trimester screening performance: routine care gynaecologists' practices vs. prenatal centre. Ultraschall in der Medizin 2007;28(3):291‐5. [DOI] [PubMed] [Google Scholar]
Krantz 2000 {published data only}
- Krantz DA, Hallahan TW, Orlandi F, Buchanan P, Larsen JW Jr, Macri JN. First‐trimester Down syndrome screening using dried blood biochemistry and nuchal translucency. Obstetrics and Gynecology 2000;96(2):207‐13. [DOI] [PubMed] [Google Scholar]
Kratzer 1991 {published data only}
- Kratzer PG, Golbus MS, Monroe SE, Finkelstein DE, Taylor RN. First‐trimester uneuploidy screening using serum human chorionic gonadotropin (hCG), free alphahCG, and progesterone. Prenatal Diagnosis 1991;11(10):751‐63. [DOI] [PubMed] [Google Scholar]
Laigaard 2003 {published data only}
- Laigaard J, Sorensen T, Frohlich C, Pedersen BN, Christiansen M, Schiott K, et al. ADAM12: a novel first‐trimester maternal serum marker for Down syndrome. Prenatal Diagnosis 2003;23(13):1086‐91. [DOI] [PubMed] [Google Scholar]
Macintosh 1993 {published data only}
- Macintosh MC, Brambati B, Chard T, Grudzinskas JG. First‐trimester maternal serum Schwangerschafts protein 1 (SP1) in pregnancies associated with chromosomal anomalies. Prenatal Diagnosis 1993;13(7):563‐8. [DOI] [PubMed] [Google Scholar]
Muller 2003a {published data only}
- Muller F, Benattar C, Audibert F, Roussel N, Dreux S, Cuckle H. First‐trimester screening for Down syndrome in France combining fetal nuchal translucency measurement and biochemical markers. Prenatal Diagnosis 2003;23(10):833‐6. [DOI] [PubMed] [Google Scholar]
Nebiolo 1990 {published data only}
- Milunsky A, Wands J, Brambati B, Bonacchi I, Currie K. First‐trimester maternal serum alpha‐fetoprotein screening for chromosome defects. American Journal of Obstetrics and Gynecology 1988;159(5):1209‐13. [DOI] [PubMed] [Google Scholar]
- Nebiolo L, Ozturk M, Brambati B, Miller S, Wands J, Milunsky A. First‐trimester maternal serum alpha‐fetoprotein and human chorionic gonadotropin screening for chromosome defects. Prenatal Diagnosis 1990;10(9):575‐81. [DOI] [PubMed] [Google Scholar]
Niemimaa 2001a {published data only}
- Niemimaa M, Suonpaa M, Perheentupa A, Seppala M, Heinonen S, Laitinen P, et al. Evaluation of first trimester maternal serum and ultrasound screening for Down's syndrome in Eastern and Northern Finland. European Journal of Human Genetics 2001;9(6):404‐8. [DOI] [PubMed] [Google Scholar]
Noble 1995 {published data only}
- Brizot ML, Snijders RJM, Butler J, Bersinger NA, Nicolaides KH. Maternal serum hCG and fetal nuchal translucency thickness for the prediction of fetal trisomies in the first trimester of pregnancy. British Journal of Obstetrics and Gynaecology 1995;102:127‐32. [DOI] [PubMed] [Google Scholar]
- Noble PL, Abraha HD, Snijders RJ, Sherwood R, Nicolaides KH. Screening for fetal trisomy 21 in the first trimester of pregnancy: maternal serum free beta‐hCG and fetal nuchal translucency thickness. Ultrasound in Obstetrics & Gynecology 1995;6(6):390‐5. [DOI] [PubMed] [Google Scholar]
Noble 1997 {published data only}
- Noble PL, Wallace EM, Snijders RJ, Groome NP, Nicolaides KH. Maternal serum 1T Inhibin‐A and free beta‐hCG concentrations in trisomy 21 pregnancies at 10 to 14 weeks of gestation. British Journal of Obstetrics and Gynaecology 1997;104(3):367‐71. [DOI] [PubMed] [Google Scholar]
O'Leary 2006 {published data only}
- O'Leary P, Breheny N, Dickinson JE, Bower C, Goldblatt J, Hewitt B, et al. First‐trimester combined screening for Down syndrome and other fetal anomalies. Obstetrics and Gynecology 2006;107(4):869‐76. [DOI] [PubMed] [Google Scholar]
Orlandi 1997 {published data only}
- Orlandi F, Damiani G, Hallahan TW, Krantz DA, Macri JN. First‐trimester screening for fetal aneuploidy: biochemistry and nuchal translucency. Ultrasound in Obstetrics & Gynecology 1997;10(6):381‐6. [DOI] [PubMed] [Google Scholar]
Palomaki 2007 {published data only}
- Palomaki GE, Neveux LM, Haddow JE, Wyatt P. Hyperglycosylated‐hCG (h‐hCG) and Down syndrome screening in the first and second trimesters of pregnancy. Prenatal Diagnosis 2007;27(9):808‐13. [DOI] [PubMed] [Google Scholar]
Qin 1997 {published data only}
- Qin QP, Christiansen M, Nguyen TH, Sorensen S, Larsen SO, Norgaard‐Pedersen B. Schwangerschaftsprotein 1 (SP1) as a maternal serum marker for Down syndrome in the first and second trimesters. Prenatal Diagnosis 1997;17(2):101‐8. [DOI] [PubMed] [Google Scholar]
Sahota 2010 {published data only}
- Sahota DS, Leung TY, Chan LW, Law LW, Fung TY, Chen M, et al. Comparison of first‐trimester contingent screening strategies for Down syndrome. Ultrasound in Obstetrics & Gynecology 2010;35(3):286‐91. [DOI] [PubMed] [Google Scholar]
- Sahota DS, Leung TY, Chen M, Chan LW, Fung TY, Lau TK. Comparison of likelihood ratios of first‐trimester nuchal translucency measurements: multiples of median, delta or mixture. Ultrasound in Obstetrics & Gynecology 2010;36(1):15‐9. [DOI] [PubMed] [Google Scholar]
Schaelike 2009 {published data only}
- Schaelike M, Kossakiewicz M, Kossakiewicz A, Schild RL. Examination of a first‐trimester Down syndrome screening concept on a mix of 11,107 high‐ and low‐risk patients at a private center for prenatal medicine in Germany. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;144(2):140‐5. [DOI] [PubMed] [Google Scholar]
Scott 2004 {published data only}
- Scott F, Peters H, Bonifacio M, McLennan A, Boogert A, Kesby G, et al. Prospective evaluation of a first trimester screening program for Down syndrome and other chromosomal abnormalities using maternal age, nuchal translucency and biochemistry in an Australian population. Australian & New Zealand Journal of Obstetrics & Gynaecology 2004;44(3):205‐9. [DOI] [PubMed] [Google Scholar]
Spencer 1999a {published data only}
- Spencer K, Souter V, Tul N, Snijders R, Nicolaides KH. A screening program for trisomy 21 at 10‐14 weeks using fetal nuchal translucency, maternal serum free beta‐human chorionic gonadotropin and pregnancy‐associated plasma protein‐A.[see comment]. Ultrasound in Obstetrics & Gynecology 1999;13(4):231‐7. [DOI] [PubMed] [Google Scholar]
Spencer 2002a {published data only}
- Spencer K, Talbot JA, Abushoufa RA. Maternal serum hyperglycosylated human chorionic gonadotrophin (HhCG) in the first trimester of pregnancies affected by Down syndrome, using a sialic acid‐specific lectin immunoassay.[see comment]. Prenatal Diagnosis 2002;22(8):656‐62. [DOI] [PubMed] [Google Scholar]
Torring 2010 {published data only}
- Torring N, Ball S, Wright D, Sarkissian G, Guitton M, Darbouret B. First trimester screening for trisomy 21 in gestational week 8‐10 by ADAM12‐S as a maternal serum marker. Reproductive Biology & Endocrinology 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsukerman 1999 {published data only}
- Tsukerman GL, Gusina NB, Cuckle HS. Maternal serum screening for Down syndrome in the first trimester: experience from Belarus. Prenatal Diagnosis 1999;19(6):499‐504. [DOI] [PubMed] [Google Scholar]
Valinen 2007 {published data only}
- Valinen Y, Rapakko K, Kokkonen H, Laitinen P, Tekay A, Ahola T, et al. Clinical first‐trimester routine screening for Down syndrome in singleton pregnancies in northern Finland. American Journal of Obstetrics and Gynecology 2007;196(3):278‐5. [DOI] [PubMed] [Google Scholar]
Valinen 2009 {published data only}
- Valinen Y, Laitinen P, Ranta J, Ignatius J, Jarvela I, Ryynanen M. Effect of a new marker, ADAM12, on Down risk figures in first trimester screening. Journal of Maternal‐fetal & Neonatal Medicine 2009;22(7):602‐7. [DOI] [PubMed] [Google Scholar]
Van Lith 1992 {published data only}
- Lith JM, Mantingh A, Kloosterman MD, Kanhai HHH, Wolf H, Everhardt E, et al. First‐trimester maternal serum human chorionic gonadotrophin as a marker for fetal chromosomal disorders. Prenatal Diagnosis 1992;12(6):495‐504. [DOI] [PubMed] [Google Scholar]
Wald 2003a {published data only}
- Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Seminars in Perinatology 2005;29(4):225‐35. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective.[see comment]. BJOG: an international journal of obstetrics and gynaecology 2004;111(6):521‐31. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Journal of Medical Screening 2003;10(2):56‐104. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM, et al. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Health Technology Assessment (Winchester, England) 2003;7(11):1‐77. [DOI] [PubMed] [Google Scholar]
Wallace 1995 {published data only}
- Wallace EM, Grant VE, Swanston IA, Groome NP. Evaluation of maternal serum dimeric 1T Inhibin A as a first‐trimester marker of Down's syndrome. Prenatal Diagnosis 1995;15(4):359‐62. [DOI] [PubMed] [Google Scholar]
Wapner 2003 {published data only}
- Wapner R, Thom E, Simpson JL, Pergament E, Silver R, Filkins K, et al. First‐trimester screening for trisomies 21 and 18.[see comment]. New England Journal of Medicine 2003;349(15):1405‐13. [DOI] [PubMed] [Google Scholar]
- Wapner RJ. First trimester screening: the BUN study. Seminars in Perinatology 2005;29(4):236‐9. [DOI] [PubMed] [Google Scholar]
Weinans 2005 {published data only}
- Weinans MJ, Sancken U, Pandian R, Ouweland JM, Bruijn HW, Holm JP, et al. Invasive trophoblast antigen (hyperglycosylated human chorionic gonadotropin) as a first‐trimester serum marker for Down syndrome. Clinical Chemistry 2005;51(7):1276‐9. [DOI] [PubMed] [Google Scholar]
Wojdemann 2005 {published data only}
- Wojdemann KR, Shalmi AC, Christiansen M, Larsen SO, Sundberg K, Brocks V, et al. Improved first‐trimester Down syndrome screening performance by lowering the false‐positive rate: a prospective study of 9941 low‐risk women. Ultrasound in Obstetrics & Gynecology 2005;25(3):227‐33. [DOI] [PubMed] [Google Scholar]
Zaragoza 2009 {published data only}
- Zaragoza E, Akolekar R, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11‐13 weeks in chromosomally abnormal pregnancies. Ultrasound in Obstetrics & Gynecology 2009;33(4):382‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbas 1995 {published data only}
- Abbas A, Chard T, Nicolaides K. Fetal and maternal hCG concentration in aneuploid pregnancies. British Journal of Obstetrics and Gynaecology 1995;102(7):561‐3. [DOI] [PubMed] [Google Scholar]
Abdul‐Hamid 2004 {published data only}
- Abdul‐Hamid S, Fox R, Martin I. Maternal serum screening for trisomy 21 in women with a false positive result in last pregnancy. Journal of Obstetrics and Gynaecology 2004;24(4):374‐6. [DOI] [PubMed] [Google Scholar]
Abraha 1999 {published data only}
- Abraha HD, Noble PL, Nicolaides KH, Sherwood RA. Maternal serum S100 protein in normal and Down syndrome pregnancies. Prenatal Diagnosis 1999;19(4):334‐6. [PubMed] [Google Scholar]
Abu‐Rustum 2010 {published data only}
- Abu‐Rustum RS, Daou L, Abu‐Rustum SE. Role of first‐trimester sonography in the diagnosis of aneuploidy and structural fetal anomalies. Journal of Ultrasound in Medicine 2010;29(10):1445‐52. [DOI] [PubMed] [Google Scholar]
Achiron 2010 {published data only}
- Achiron R, Gindes L, Gilboa Y, Weissmann‐Brenner A, Berkenstadt M. Umbilical vein anomaly in fetuses with Down syndrome. Ultrasound in Obstetrics & Gynecology 2010;35(3):297‐301. [DOI] [PubMed] [Google Scholar]
Adekunle 1999 {published data only}
- Adekunle O, Gopee A, el‐Sayed M, Thilaganathan B. Increased first trimester nuchal translucency: pregnancy and infant outcomes after routine screening for Down's syndrome in an unselected antenatal population. British Journal of Radiology 1999;72(857):457‐60. [DOI] [PubMed] [Google Scholar]
Agaard‐Tillery 2010 {published data only}
- Agaard‐Tillery KM, Flint Porter T, Malone FD, Nyberg DA, Collins J, Comstock CH, et al. Influence of maternal BMI on genetic sonography in the FaSTER trial. Prenatal Diagnosis 2010;30(1):14‐22. [DOI] [PubMed] [Google Scholar]
Aitken 1993 {published data only}
- Aitken DA, McCaw G, Crossley JA, Berry E, Connor JM, Spencer K, et al. First‐trimester biochemical screening for fetal chromosome abnormalities and neural tube defects. Prenatal Diagnosis 1993;13(8):681‐9. [DOI] [PubMed] [Google Scholar]
Aitken 1996a {published data only}
- Aitken DA, Syvertsen BS, Crossley JA, Berry E, Connor JM. Heat‐stable and immunoreactive placental alkaline phosphatase in maternal serum from Down's syndrome and trisomy 18 pregnancies.[see comment]. Prenatal Diagnosis 1996;16(11):1051‐4. [DOI] [PubMed] [Google Scholar]
Aitken 1996b {published data only}
- Aitken DA, Wallace EM, Crossley JA, Swanston IA, Pareren Y, Maarle M, et al. Dimeric Inhibin A as a marker for Down's syndrome in early pregnancy. New England Journal of Medicine 1996;334(19):1231‐6. [DOI] [PubMed] [Google Scholar]
Ajayi 2011 {published data only}
- Ajayi GO. Is there any effect of fetal gender on the markers of first trimester Down's syndrome screening?. Clinical and Experimental Obstetrics & Gynecology 2011;38(2):162‐4. [PubMed] [Google Scholar]
Akbas 2001 {published data only}
- Akbas SH, Ozben T, Alper O, Ugur A, Yucel G, Luleci G. Maternal serum screening for Down's syndrome, open neural tube defects and trisomy 18. Clinical Chemistry and Laboratory Medicine 2001;39(6):487‐90. [DOI] [PubMed] [Google Scholar]
Alexioy 2009 {published data only}
- Alexioy E, Alexioy E, Trakakis E, Kassanos D, Farmakidis G, Kondylios A, et al. Predictive value of increased nuchal translucency as a screening test for the detection of fetal chromosomal abnormalities. Journal of Maternal‐fetal & Neonatal Medicine 2009;22(10):857‐62. [DOI] [PubMed] [Google Scholar]
Allingham‐Hawkins 2011 {published data only}
- Allingham‐Hawkins DJ, Chitayat D, Cirigliano V, Summers A, Tokunaga J, Winsor E, et al. Prospective validation of quantitative fluorescent polymerase chain reaction for rapid detection of common aneuploidies. Genetics in Medicine 2011;13(2):140‐7. [DOI] [PubMed] [Google Scholar]
American College 2009 {published data only}
- American CofN‐M. [Share with women. Prenatal tests for Down syndrome]. [Spanish]. Journal of Midwifery & Women's Health 2009;54(6):527‐8. [PubMed] [Google Scholar]
Antona 1998 {published data only}
- Antona D, Wallace EM, Shearing C, Ashby JP, Groome NP. Inhibin A and pro‐alphaC Inhibin Ain Down syndrome and normal pregnancies. Prenatal Diagnosis 1998;18(11):1122‐6. [DOI] [PubMed] [Google Scholar]
Antsaklis 1999 {published data only}
- Antsaklis A, Papantoniou N, Mesogitis S, Michalas S, Aravantinos D. Pregnant women of 35 years of age or more: Maternal serum markers or amniocentesis?. Journal of Obstetrics and Gynaecology 1999;19(3):253‐6. [DOI] [PubMed] [Google Scholar]
Anuwutnavin 2009 {published data only}
- Anuwutnavin S, Wanitpongpan P, Chanprapaph P. Specificity of fetal tricuspid regurgitation in prediction of Down syndrome in Thai fetuses at 17‐23 weeks of gestation. Journal of the Medical Association of Thailand 2009;92(9):1123‐30. [PubMed] [Google Scholar]
Ashwood 1987 {published data only}
- Ashwood ER, Cheng E, Luthy DA. Maternal serum alpha‐fetoprotein and fetal trisomy‐21 in women 35 years and older: implications for alpha‐fetoprotein screening programs. American Journal of Medical Genetics 1987;26(3):531‐9. [DOI] [PubMed] [Google Scholar]
Asrani 2005 {published data only}
- Asrani CH. Triple marker. National Journal of Homoeopathy 2005;7(3):174. [Google Scholar]
Audibert 2001 {published data only}
- Audibert F, Dommergues M, Benattar C, Taieb J, Thalabard JC, Frydman R. Screening for Down syndrome using first‐trimester ultrasound and second‐trimester maternal serum markers in a low‐risk population: a prospective longitudinal study. Ultrasound in Obstetrics & Gynecology 2001;18(1):26‐31. [DOI] [PubMed] [Google Scholar]
Axt‐Fleidner 2006 {published data only}
- Axt Fliedner, Schwarze A, Kreiselmaier P, Krapp M, Smrcek J, Diedrich K. Umbilical cord diameter at 11‐14 weeks of gestation: Relationship to nuchal translucency, ductus venous blood flow and chromosomal defects. Fetal Diagnosis and Therapy 2006;21(4):390‐5. [DOI] [PubMed] [Google Scholar]
Azuma 2002 {published data only}
- Azuma M, Yamamoto R, Wakui Y, Minobe S, Satomura S, Fujimoto S. A novel method for the detection of Down syndrome with the use of four serum markers. American Journal of Obstetrics and Gynecology 2002;187(1):197‐201. [DOI] [PubMed] [Google Scholar]
Baghagho 2004 {published data only}
- Baghagho EE, Kharboush IF, El‐Kaffash DM, KarKour TA, Ismail SR, Mortada MM. Maternal serum alpha fetoprotein among pregnant females in Alexandria. Journal of the Egyptian Public Health Association 2004;79(1‐2):59‐81. [PubMed] [Google Scholar]
Bahado‐Singh 1995 {published data only}
- Bahado‐Singh RO, Goldstein I, Uerpairojkit B, Copel JA, Mahoney MJ, Baumgarten A. Normal nuchal thickness in the midtrimester indicates reduced risk of Down syndrome in pregnancies with abnormal triple‐screen results. American Journal of Obstetrics and Gynecology 1995;173(4):1106‐10. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 1996 {published data only}
- Bahado‐Singh RO, Tan A, Deren O, Hunter D, Copel J, Mahoney MJ. Risk of Down syndrome and any clinically significant chromosome defect in pregnancies with abnormal triple‐screen and normal targeted ultrasonographic results. American Journal of Obstetrics and Gynecology 1996;175(4 I):824‐9. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 1999 {published data only}
- Bahado‐Singh RO, Oz AU, Flores D, Cermik D, Acuna E, Mahoney MJ, et al. Nuchal thickness, urine ß‐core fragment level, and maternal age for down syndrome screening. American Journal of Obstetrics and Gynecology 1999;180(2 I):491‐5. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 2002 {published data only}
- Bahado‐Singh R, Shahabi S, Karaca M, Mahoney MJ, Cole L, Oz UA. The comprehensive midtrimester test: high‐sensitivity Down syndrome test. American Journal of Obstetrics and Gynecology 2002;186(4):803‐8. [DOI] [PubMed] [Google Scholar]
Bahado‐Singh 2003 {published data only}
- Bahado‐Singh R, Cheng CC, Matta P, Small M, Mahoney MJ. Combined serum and ultrasound screening for detection of fetal aneuploidy. Seminars in Perinatology 2003;27(2):145‐51. [DOI] [PubMed] [Google Scholar]
Ball 2007 {published data only}
- Ball RH, Caughey AB, Malone FD, Nyberg DA, Comstock CH, Saade GR, et al. First‐ and second‐trimester evaluation of risk for Down syndrome. Obstetrics and Gynecology 2007;110(1):10‐7. [DOI] [PubMed] [Google Scholar]
Bar‐Hava 2001 {published data only}
- Bar‐Hava I, Yitzhak M, Krissi H, Shohat M, Shalev J, Czitron B, et al. Triple‐test screening in in vitro fertilization pregnancies. Journal of Assisted Reproduction and Genetics 2001;18(4):226‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkai 1996 {published data only}
- Barkai G, Goldman B, Ries L, Chaki R, Dor J, Cuckle H. Down's syndrome screening marker levels following assisted reproduction. Prenatal Diagnosis 1996;16(12):1111‐4. [DOI] [PubMed] [Google Scholar]
Barnabei 1995 {published data only}
- Barnabei VM, Krantz DA, Macri JN, Larsen JW Jr. Enhanced twin pregnancy detection within an open neural tube defect and Down syndrome screening protocol using free‐ß hCG and AFP. Prenatal Diagnosis 1995;15(12):1131‐4. [DOI] [PubMed] [Google Scholar]
Bartels 1988 {published data only}
- Bartels I, Lindemann A. Maternal levels of pregnancy‐specific ß 1‐glycoprotein (SP‐1) are elevated in pregnancies affected by Down's syndrome. Human Genetics 1988;80(1):46‐8. [DOI] [PubMed] [Google Scholar]
Bartels 1993 {published data only}
- Bartels I, Hoppe‐Sievert B, Bockel B, Herold S, Caesar J. Adjustment formulae for maternal serum alpha‐fetoprotein, human chorionic gonadotropin, and unconjugated oestriol to maternal weight and smoking. Prenatal Diagnosis 1993;13(2):123‐30. [DOI] [PubMed] [Google Scholar]
Barth 1991 {published data only}
- Barth WH Jr, Frigoletto FD Jr, Krauss CM, MacMillin MD, Stryker JM, Benacerraf BR. Ultrasound detection of fetal aneuploidy in women with elevated maternal serum alpha‐fetoprotein. Obstetrics and Gynecology 1991;77(6):897‐900. [PubMed] [Google Scholar]
Bas‐Budecka 2007 {published data only}
- Bas‐Budecka E, Perenc M, Sieroszewski P. [Abnormal second trimester screening for fetal chromosomal abnormalities as a predictor of adverse pregnancy outcome]. [Polish]. Ginekologia Polska 2007;78(11):877‐80. [PubMed] [Google Scholar]
Baviera 2004 {published data only}
- Baviera G, Carbone C, Corrado F, Mastrantonio P. Placental growth hormone in Down's syndrome screening. Journal of Maternal‐fetal & Neonatal Medicine 2004;16(4):241‐3. [DOI] [PubMed] [Google Scholar]
Bazzett 1998 {published data only}
- Bazzett LB, Yaron Y, O'Brien JE, Critchfield G, Kramer RL, Ayoub M, et al. Fetal gender impact on multiple‐marker screening results. American Journal of Medical Genetics 1998;76(5):369‐71. [PubMed] [Google Scholar]
Beke 2008 {published data only}
- Beke A, Barakonyi E, Belics Z, Joo JG, Csaba A, Papp C, et al. Risk of chromosome abnormalities in the presence of bilateral or unilateral choroid plexus cysts. Fetal Diagnosis and Therapy 2008;23(3):185‐91. [DOI] [PubMed] [Google Scholar]
Bellver 2005 {published data only}
- Bellver J, Lara C, Soares SR, Ramirez A, Pellicer A, Remohi J, et al. First trimester biochemical screening for Down's syndrome in singleton pregnancies conceived by assisted reproduction. Human Reproduction 2005;20(9):2623‐7. [DOI] [PubMed] [Google Scholar]
Benn 1995 {published data only}
- Benn PA, Horne D, Briganti S, Greenstein RM. Prenatal diagnosis of diverse chromosome abnormalities in a population of women identified by triple‐marker testing as screen positive for Down syndrome. American Journal of Obstetrics and Gynecology 1995;173(2):496‐501. [DOI] [PubMed] [Google Scholar]
Benn 1996 {published data only}
- Benn PA, Horne D, Craffey A, Collins R, Ramsdell L, Greenstein R. Maternal serum screening for birth defects: results of a Connecticut regional program. Connecticut Medicine 1996;60(6):323‐7. [PubMed] [Google Scholar]
Benn 1997 {published data only}
- Benn PA, Clive JM, Collins R. Medians for second‐trimester maternal serum alpha‐fetoprotein, human chorionic gonadotropin, and unconjugated estriol; differences between races or ethnic groups. Clinical Chemistry 1997;43(2):333‐7. [PubMed] [Google Scholar]
Benn 1998 {published data only}
- Benn PA. Preliminary evidence for associations between second‐trimester human chorionic gonadotropin and unconjugated oestriol levels with pregnancy outcome in Down syndrome pregnancies. Prenatal Diagnosis 1998;18(4):319‐24. [PubMed] [Google Scholar]
Benn 2001 {published data only}
- Benn PA, Ying J, Beazoglou T, Egan JF. Estimates for the sensitivity and false‐positive rates for second trimester serum screening for Down syndrome and trisomy 18 with adjustment for cross‐identification and double‐positive results. Prenatal Diagnosis 2001;21(1):46‐51. [PubMed] [Google Scholar]
Benn 2002 {published data only}
- Benn PA, Kaminsky LM, Ying J, Borgida AF, Egan JF. Combined second‐trimester biochemical and ultrasound screening for Down syndrome. Obstetrics and Gynecology 2002;100(6):1168‐76. [DOI] [PubMed] [Google Scholar]
Benn 2003a {published data only}
- Benn PA, Fang M, Egan JFX, Horne D, Collins R. Incorporation of inhibin‐A in second‐trimester screening for Down syndrome. Obstetrics and Gynecology 2003;101(3):451‐4. [DOI] [PubMed] [Google Scholar]
Benn 2003b {published data only}
- Benn P. Improved antenatal screening for Down's syndrome. Lancet 2003;361(9360):794‐5. [DOI] [PubMed] [Google Scholar]
Benn 2005a {published data only}
- Benn P, Wright D, Cuckle H. Practical strategies in contingent sequential screening for Down syndrome. Prenatal Diagnosis 2005;25(8):645‐52. [DOI] [PubMed] [Google Scholar]
Benn 2005b {published data only}
- Benn P, Donnenfeld AE. Sequential Down syndrome screening: the importance of first and second trimester test correlations when calculating risk. Journal of Genetic Counseling 2005;14(6):409‐13. [DOI] [PubMed] [Google Scholar]
Benn 2007 {published data only}
- Benn PA, Campbell WA, Zelop CM, Ingardia C, Egan JF. Stepwise sequential screening for fetal aneuploidy. American Journal of Obstetrics and Gynecology 2007;197(3):312‐5. [DOI] [PubMed] [Google Scholar]
Berry 1995 {published data only}
- Berry E, Aitken DA, Crossley JA, Macri JN, Connor JM. Analysis of maternal serum alpha‐fetoprotein and free ß human chorionic gonadotrophin in the first trimester: implications for Down's syndrome screening. Prenatal Diagnosis 1995;15(6):555‐65. [DOI] [PubMed] [Google Scholar]
Berry 1997 {published data only}
- Berry E, Aitken DA, Crossley JA, Macri JN, Connor JM. Screening for Down's syndrome: changes in marker levels and detection rates between first and second trimesters. British Journal of Obstetrics and Gynaecology 1997;104(7):811‐7. [DOI] [PubMed] [Google Scholar]
Bersinger 1994 {published data only}
- Bersinger NA, Brizot ML, Johnson A, Snijders RJ, Abbott J, Schneider H, et al. First trimester maternal serum pregnancy‐associated plasma protein A and pregnancy‐specific ß 1‐glycoprotein in fetal trisomies. British Journal of Obstetrics and Gynaecology 1994;101(11):970‐4. [DOI] [PubMed] [Google Scholar]
Bersinger 2000 {published data only}
- Bersinger NA, Xin WZ. Glycosylation of pregnancy‐associated plasma protein a (PAPP‐A) and pregnancy‐specific (ß)(1)‐glycoprotein (SP1): Relevance for fetal down syndrome screening and for placental function studies. Immuno‐Analyse et Biologie Specialisee 2000;15(6):402‐8. [Google Scholar]
Bersinger 2001 {published data only}
- Bersinger NA, Chanson A, Crazzolara S, Hänggi W, Pescia G, Scheier M, et al. Serum levels of placenta protein markers: The relevance of differences between spontaneous and after in vitro fertilization pregnancies for fetal trisomy screening. Journal fur Fertilitat und Reproduktion 2001;11(3):7‐13. [Google Scholar]
Bersinger 2003 {published data only}
- Bersinger NA, Noble P, Nicolaides KH. First‐trimester maternal serum PAPP‐A, SP1 and M‐CSF levels in normal and trisomic twin pregnancies. Prenatal Diagnosis 2003;23(2):157‐62. [DOI] [PubMed] [Google Scholar]
Bersinger 2004 {published data only}
- Bersinger NA, Wunder D, Vanderlick F, Chanson A, Pescia G, Janecek P, et al. Maternal serum levels of placental proteins after in vitro fertilisation and their implications for prenatal screening. Prenatal Diagnosis 2004;24(6):471‐7. [DOI] [PubMed] [Google Scholar]
Bersinger 2005 {published data only}
- Bersinger NA, Vanderlick F, Birkhäuser MH, Janecek P, Wunder D. First trimester serum concentrations of placental proteins in singleton and multiple IVF pregnancies: Implications for Down syndrome screening. Immuno‐Analyse et Biologie Specialisee 2005;20(1):21‐7. [Google Scholar]
Bestwick 2008 {published data only}
- Bestwick JP, Huttly WJ, Wald NJ. First trimester Down's syndrome screening marker values and cigarette smoking: new data and a meta‐analysis on free beta human chorionic gonadotophin, pregnancy‐associated plasma protein‐A and nuchal translucency. Journal of Medical Screening 2008;15(4):204‐6. [DOI] [PubMed] [Google Scholar]
Biggio 2004 {published data only}
- Biggio Jr, Morris TC, Owen J, Stringer JSA. An outcomes analysis of five prenatal screening strategies for trisomy 21 in women younger than 35 years. American Journal of Obstetrics and Gynecology 2004;190(3):721‐9. [DOI] [PubMed] [Google Scholar]
Bilardo 2011 {published data only}
- Bilardo CM, Timmerman E, Medina PG, Clur SA. Low‐resistance hepatic artery flow in first‐trimester fetuses: an ominous sign. Ultrasound in Obstetrics & Gynecology 2011;37(4):438‐43. [DOI] [PubMed] [Google Scholar]
Bindra 2002 {published data only}
- Bindra R, Heath V, Nicolaides KH. Screening for chromosomal defects by fetal nuchal translucency at 11 to 14 weeks. Clinical Obstetrics and Gynecology 2002;45(3):661‐70. [DOI] [PubMed] [Google Scholar]
Blundell 1999 {published data only}
- Blundell G, Ashby JP, Martin C, Shearing CH, Langdale‐Brown B, Keeling J, et al. Clinical follow‐up of high mid‐trimester maternal serum intact human chorionic gonadotrophin concentrations in singleton pregnancies. Prenatal Diagnosis 1999;19(3):219‐23. [DOI] [PubMed] [Google Scholar]
Boormans 2010 {published data only}
- Boormans EM, Birnie E, Oepkes D, Galjaard RJ, Schuring‐Blom GH, Lith JM, et al. Comparison of multiplex ligation‐dependent probe amplification and karyotyping in prenatal diagnosis. Obstetrics and Gynecology 2010;115(2 Pt 1):297‐303. [DOI] [PubMed] [Google Scholar]
Boots 1989 {published data only}
- Boots LR, Davis RO, Foster JM, Goldenberg RL. Maternal serum alpha‐fetoprotein prenatal screening for Down syndrome. Alabama Medicine 1989;59(1):25‐7. [PubMed] [Google Scholar]
Bornstein 2009a {published data only}
- Bornstein E, Lenchner E, Donnenfeld A, Barnhard Y, Seubert D, Divon MY. Advanced maternal age as a sole indication for genetic amniocentesis; risk‐benefit analysis based on a large database reflecting the current common practice. Journal of Perinatal Medicine 2009;37(2):99‐102. [DOI] [PubMed] [Google Scholar]
Bornstein 2009b {published data only}
- Bornstein E, Lenchner E, Donnenfeld A, Kapp S, Keeler SM, Divon MY. Comparison of modes of ascertainment for mosaic vs complete trisomy 21. American Journal of Obstetrics and Gynecology 2009;200(4):440‐5. [DOI] [PubMed] [Google Scholar]
Bornstein 2010 {published data only}
- Bornstein E, Lenchner E, Donnenfeld A, Jodicke C, Keeler SM, Kapp S, et al. Complete trisomy 21 vs translocation Down syndrome: a comparison of modes of ascertainment. American Journal of Obstetrics and Gynecology 2010;203(4):391‐5. [DOI] [PubMed] [Google Scholar]
Borowski 2007 {published data only}
- Borowski D, Czuba B, Cnota W, Hincz P, Czekierdowski A, Gajewska J, et al. [Evaluation of pregnancy‐associated plasma protein A (PAPP‐A) and free beta subunit of human chorionic gonadotropin (beta hCG) levels and sonographic assesement of fetal nuchal translucency (NT) in singleton pregnancies between 11 and 14 weeks of gestation‐‐Polish multi‐centre research]. [Polish]. Ginekologia Polska 2007;78(5):384‐7. [PubMed] [Google Scholar]
Borrell 2007 {published data only}
- Borrell A, Mercade I, Casals E, Borobio V, Seres A, Soler A, et al. Combining fetal nuchal fold thickness with second‐trimester biochemistry to screen for trisomy 21. Ultrasound in Obstetrics & Gynecology 2007;30(7):941‐5. [DOI] [PubMed] [Google Scholar]
Borruto 2002 {published data only}
- Borruto F, Comparetto C, Acanfora L, Bertini G, Rubaltelli FF. Role of ultrasound evaluation of nuchal translucency in prenatal diagnosis. Clinical and Experimental Obstetrics & Gynecology 2002;29(4):235‐41. [PubMed] [Google Scholar]
Bottalico 2009 {published data only}
- Bottalico JN, Chen X, Tartaglia M, Rosario B, Yarabothu D, Nelson L. Second‐trimester genetic sonogram for detection of fetal chromosomal abnormalities in a community‐based antenatal testing unit. Ultrasound in Obstetrics & Gynecology 2009;33(2):161‐8. [DOI] [PubMed] [Google Scholar]
Boue 1990 {published data only}
- Boue A, Muller F. Screening for Down's syndrome with maternal serum human chorionic gonadotropin at midtrimester. Current Opinion in Pediatrics 1990;2(6):1157‐60. [Google Scholar]
Bradley 1994 {published data only}
- Bradley LA, Horwitz JA, Dowman AC, Ponting NR, Peterson LM. Triple marker screening for fetal Down syndrome. International Pediatrics 1994;9(3):168‐74. [Google Scholar]
Braithwaite 1996 {published data only}
- Braithwaite JM, Economides DL. Nuchal translucency and screening for Down's syndrome. Contemporary Reviews in Obstetrics and Gynaecology 1996;8(2):75‐81. [Google Scholar]
Brambati 1995 {published data only}
- Brambati B, Cislaghi C, Tului L, Alberti E, Amidani M, Colombo U, et al. First‐trimester Down's syndrome screening using nuchal translucency: a prospective study in women undergoing chorionic villus sampling. Ultrasound in Obstetrics & Gynecology 1995;5(1):9‐14. [DOI] [PubMed] [Google Scholar]
Brambati 1996 {published data only}
- Brambati B, Tului L, Alberti E. Sonography in the first trimester screening of trisomy 21 and other fetal aneuploidies. Early Pregnancy 1996;2(3):155‐67. [PubMed] [Google Scholar]
Brizot 1995a {published data only}
- Brizot ML, Bersinger NA, Xydias G, Snijders RJ, Nicolaides KH. Maternal serum Schwangerschafts protein‐1 (SP1) and fetal chromosomal abnormalities at 10‐13 weeks' gestation. Early Human Development. 1995;43(1):31‐6. [DOI] [PubMed] [Google Scholar]
Brizot 1995b {published data only}
- Brizot ML, Kuhn P, Bersinger NA, Snijders RJ, Nicolaides KH. First trimester maternal serum alpha‐fetoprotein in fetal trisomies. British Journal of Obstetrics and Gynaecology 1995;102(1):31‐4. [DOI] [PubMed] [Google Scholar]
Brizzi 1989 {published data only}
- Brizzi L, Cariati E, Periti E, Nannini R, Torricelli F, Cappelli G, et al. Evaluation of maternal serum alpha‐fetoprotein and ultrasound examination to screen fetal chromosomal abnormalities. Journal of Nuclear Medicine and Allied Sciences 1989;33(3 Suppl):85‐8. [PubMed] [Google Scholar]
Brock 1990 {published data only}
- Brock DJ, Barron L, Holloway S, Liston WA, Hillier SG, Seppala M. First‐trimester maternal serum biochemical indicators in Down syndrome. Prenatal Diagnosis 1990;10(4):245‐51. [DOI] [PubMed] [Google Scholar]
Calda 2010 {published data only}
- Calda P, Sipek A, Gregor V. Gradual implementation of first trimester screening in a population with a prior screening strategy: population based cohort study. Acta Obstetricia et Gynecologica Scandinavica 2010;89(8):1029‐33. [DOI] [PubMed] [Google Scholar]
Campogrande 2001 {published data only}
- Campogrande M, Viora E, Errante G, Bastonero S, Sciarrone A, Grassi Pirrone P, et al. Correlations between first and second trimester markers for Down's syndrome screening. Journal of Medical Screening 2001;8(3):163‐4. [DOI] [PubMed] [Google Scholar]
Canick 1988 {published data only}
- Canick JA, Knight GJ, Palomaki GE, Haddow JE, Cuckle HS, Wald NJ. Low second trimester maternal serum unconjugated oestriol in pregnancies with Down's syndrome. British Journal of Obstetrics and Gynaecology 1988;95(4):330‐3. [DOI] [PubMed] [Google Scholar]
Canick 1995 {published data only}
- Canick JA, Kellner LH, Saller DN Jr, Palomaki GE, Walker RP, Osathanondh R. Second‐trimester levels of maternal urinary gonadotropin peptide in down syndrome pregnancy. Prenatal Diagnosis 1995;15(8):739‐44. [DOI] [PubMed] [Google Scholar]
Canini 2002 {published data only}
- Canini S, Prefumo F, Famularo L, Venturini PL, Palazzese V, Biasio P. Comparison of first trimester, second trimester and integrated Down's syndrome screening results in unaffected pregnancies. Clinical Chemistry and Laboratory Medicine 2002;40(6):600‐3. [DOI] [PubMed] [Google Scholar]
Cans 1998 {published data only}
- Cans C, Amblard F, Devillard F, Pison H, Jalbert P, Jouk PS. Population screening for aneuploidy using maternal age and ultrasound. Prenatal Diagnosis 1998;18(7):683‐92. [PubMed] [Google Scholar]
Carreras 1991 {published data only}
- Carreras de Paz JJ, Silva Mendoza JM, Violante Diaz M, Cerrillo Hinojosa M, Ahued Ahued JR. [Proposed normal values for alpha fetoprotein in maternal serum for the detection of neural tube closure defects and Down syndrome. Preliminary study]. [Spanish]. Ginecologia y Obstetricia de Mexico 1991;59:261‐4. [PubMed] [Google Scholar]
Caughey 2007 {published data only}
- Caughey AB, Musci TJ, Belluomini J, Main D, Otto C, Goldberg J. Nuchal translucency screening: how do women actually utilize the results?. Prenatal Diagnosis 2007;27(2):119‐23. [DOI] [PubMed] [Google Scholar]
Cebesoy 2008 {published data only}
- Cebesoy FB. Combining 'nasal bone length assessment as MoM' with other markers for trisomy 21 screening: could it be more effective?. American Journal of Obstetrics and Gynecology 2008;198(6):726‐7. [DOI] [PubMed] [Google Scholar]
Chelli 2008 {published data only}
- Chelli D, Dimassi K, Chaabouni M, Ben Saad M, Mssaed H, Bchir F, et al. [Prenatal diagnosis of trisomy 21: the Tunisian experience]. [French]. Sante 2008;18(4):199‐203. [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen FM. Integrated screening for Down's syndrome. Journal of Family Practice 1999;48(11):846‐7. [PubMed] [Google Scholar]
Chen 2002 {published data only}
- Chen M, Lam YH, Tang MH, Lee CP, Sin SY, Tang R, et al. The effect of ethnic origin on nuchal translucency at 10‐14 weeks of gestation. Prenatal Diagnosis 2002;22(7):576‐8. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen M, Lam YH, Lee CP, Tang MHY. Ultrasound screening of fetal structural abnormalities at 12 to 14 weeks in Hong Kong. Prenatal Diagnosis 2004;24(2):92‐7. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen CP, Lin CJ, Wang W. Impact of second‐trimester maternal serum screening on prenatal diagnosis of Down syndrome and the use of amniocentesis in the Taiwanese population. Taiwanese Journal of Obstetrics and Gynecology 2005;44(1):31‐5. [Google Scholar]
Chen 2008 {published data only}
- Chen M, Lee CP, Lam YH, Tang RY, Chan BC, Wong SF, et al. Comparison of nuchal and detailed morphology ultrasound examinations in early pregnancy for fetal structural abnormality screening: a randomized controlled trial. Ultrasound in Obstetrics & Gynecology 2008;31(2):136‐46. [DOI] [PubMed] [Google Scholar]
Cheng 1993 {published data only}
- Cheng EY, Luthy DA, Zebelman AM, Williams MA, Lieppman RE, Hickok DE. A prospective evaluation of a second‐trimester screening test for fetal Down syndrome using maternal serum alpha‐fetoprotein, hCG, and unconjugated estriol. Obstetrics and Gynecology 1993;81(1):72‐7. [PubMed] [Google Scholar]
Cheng 1999 {published data only}
- Cheng PJ, Liu CM, Chang SD, Lin YT, Soong YK. Elevated second‐trimester maternal serum hCG in women undergoing haemodialysis. Prenatal Diagnosis 1999;19(10):955‐8. [DOI] [PubMed] [Google Scholar]
Cheng 2004a {published data only}
- Cheng CC, Bahado‐Singh RO, Chen SC, Tsai MS. Pregnancy outcomes with increased nuchal translucency after routine Down syndrome screening. International Journal of Gynaecology and Obstetrics 2004;84(1):5‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2004b {published data only}
- Cheng PJ, Chu DC, Chueh HY, See LC, Chang HC, Weng DR. Elevated maternal midtrimester serum free ß‐human chorionic gonadotropin levels in vegetarian pregnancies that cause increased false‐positive Down syndrome screening results. American Journal of Obstetrics and Gynecology 2004;190(2):442‐7. [DOI] [PubMed] [Google Scholar]
Chitayat 2002 {published data only}
- Chitayat D, Farrell SA, Huang T, Meier C, Wyatt PR, Summers AM. Double‐positive maternal serum screening results for down syndrome and open neural tube defects: An indicator for fetal structural or chromosomal abnormalities and adverse obstetric outcomes. American Journal of Obstetrics and Gynecology 2002;187(3):758‐63. [DOI] [PubMed] [Google Scholar]
Chiu 2011 {published data only}
- Chiu RW, Akolekar R, Zheng YW, Leung TY, Sun H, Chan KC, et al. Non‐invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ 2011;342:c7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2009 {published data only}
- Cho EH, Park BY, Kang YS, Lee EH. Validation of QF‐PCR in a Korean population. Prenatal Diagnosis 2009;29(3):213‐6. [DOI] [PubMed] [Google Scholar]
Chou 2009 {published data only}
- Chou CY, Hsieh FJ, Cheong ML, Lee FK, She BQ, Tsai MS. First‐trimester Down syndrome screening in women younger than 35 years old and cost‐effectiveness analysis in Taiwan population. Journal of Evaluation in Clinical Practice 2009;15(5):789‐96. [DOI] [PubMed] [Google Scholar]
Christiansen 2002 {published data only}
- Christiansen M, Hogdall EV, Larsen SO, Hogdall C. The variation of risk estimates through pregnancy in second trimester maternal serum screening for Down syndrome. Prenatal Diagnosis 2002;22(5):385‐7. [DOI] [PubMed] [Google Scholar]
Christiansen 2007b {published data only}
- Christiansen M, Spencer K, Laigaard J, Cowans NJ, Larsen SO, Wewer UM. ADAM 12 as a second‐trimester maternal serum marker in screening for Down syndrome. Prenatal Diagnosis 2007;27(7):611‐5. [DOI] [PubMed] [Google Scholar]
Christiansen 2008 {published data only}
- Christiansen M, Sorensen TL, Larsen SO, Norgaard‐Pedersen B. First‐trimester maternal serum progesterone in aneuploid pregnancies. Prenatal Diagnosis 2008;28(4):319‐22. [DOI] [PubMed] [Google Scholar]
Chung 2000 {published data only}
- Chung BL, Kim YP, Nam MH. The application of three‐dimensional ultrasound to nuchal translucency thickness measurement at 10‐14 weeks of gestation. Prenatal and Neonatal Medicine 2000;5(1):17‐21. [Google Scholar]
CNGOF 1996 {published data only}
- Anon. Blood screening of Down's syndrome (Trisomy 21) and reimbursement of karyotype for women under 38. Revue Francaise de Gynecologie et d'Obstetrique 1996;91(11):575‐7. [Google Scholar]
Cole 1996 {published data only}
- Cole L, Isozaki T, Palomaki G, Canick J, Iles R, Kellner L, et al. Detection of ß‐core fragment in second trimester Down's syndrome pregnancies. Early Human Development 1996;47 Suppl:S47‐S8. [DOI] [PubMed] [Google Scholar]
Comas 2001 {published data only}
- Comas C, Antolín E, Torrents M, Muñoz A, Figueras F, Echevarría M, et al. Early screening for chromosomal abnormalities: New strategies combining biochemical, sonographic and doppler parameters. Prenatal and Neonatal Medicine 2001;6(2):95‐102. [Google Scholar]
Comas 2002a {published data only}
- Comas C, Torrents M, Munoz A, Antolin E, Figueras F, Echevarria M. Measurement of nuchal translucency as a single strategy in trisomy 21 screening: should we use any other marker?. Obstetrics and Gynecology 2002;100(4):648‐54. [DOI] [PubMed] [Google Scholar]
Comas 2002b {published data only}
- Comas C, Carrera JM. Early sonographic screening for chromosomal abnormalities. Ultrasound Review of Obstetrics and Gynecology 2002;2(2):88‐91. [Google Scholar]
Comstock 2006 {published data only}
- Comstock CH, Malone FD, Ball RH, Nyberg DA, Saade GR, Berkowitz RL, et al. Is there a nuchal translucency millimeter measurement above which there is no added benefit from first trimester serum screening?. American Journal of Obstetrics and Gynecology 2006;195(3):843‐7. [DOI] [PubMed] [Google Scholar]
Conde 1998 {published data only}
- Conde Agudelo A, Kafury‐Goeta AC. Triple‐marker test as screening for down syndrome: a meta‐analysis. Obstetrical and Gynecological Survey 1998;53(6):369‐76. [DOI] [PubMed] [Google Scholar]
Cowans 2011 {published data only}
- Cowans NJ, Stamatopoulou A, Torring N, Spencer K. Early first‐trimester maternal serum placental growth factor in trisomy 21 pregnancies. Ultrasound in Obstetrics & Gynecology 2011;37(5):515‐9. [DOI] [PubMed] [Google Scholar]
Crossley 1991 {published data only}
- Crossley JA, Aitken DA, Connor JM. Prenatal screening for chromosome abnormalities using maternal serum chorionic gonadotrophin, alpha‐fetoprotein, and age. Prenatal Diagnosis 1991;11(2):83‐101. [DOI] [PubMed] [Google Scholar]
Crossley 1993 {published data only}
- Crossley JA, Aitken DA, Connor JM. Second‐trimester unconjugated oestriol levels in maternal serum from chromosomally abnormal pregnancies using an optimized assay.[see comment]. Prenatal Diagnosis 1993;13(4):271‐80. [DOI] [PubMed] [Google Scholar]
Crossley 1996 {published data only}
- Crossley JA, Berry E, Aitken DA, Connor JM. Insulin‐dependent diabetes mellitus and prenatal screening results: current experience from a regional screening programme. Prenatal Diagnosis 1996;16(11):1039‐42. [DOI] [PubMed] [Google Scholar]
Crossley 2002b {published data only}
- Crossley JA, Aitken DA, Waugh SM, Kelly T, Connor JM. Maternal smoking: age distribution, levels of alpha‐fetoprotein and human chorionic gonadotrophin, and effect on detection of Down syndrome pregnancies in second‐trimester screening. Prenatal Diagnosis 2002;22(3):247‐55. [DOI] [PubMed] [Google Scholar]
Cuckle 1984 {published data only}
- Cuckle HS, Wald NJ, Lindenbaum RH. Maternal serum alpha‐fetoprotein measurement: a screening test for Down syndrome. Lancet 1984;i(8383):926‐9. [DOI] [PubMed] [Google Scholar]
Cuckle 1987a {published data only}
- Cuckle HS, Wald NJ, Thompson SG. Estimating a woman's risk of having a pregnancy associated with Down's syndrome using her age and serum alpha‐fetoprotein level. British Journal of Obstetrics and Gynaecology 1987;94(5):387‐402. [DOI] [PubMed] [Google Scholar]
Cuckle 1987b {published data only}
- Cuckle HS, Nanchahal K, Wald NJ. Maternal serum alpha‐fetoprotein and ethnic origin. British Journal of Obstetrics and Gynaecology 1987;94(11):1111‐2. [DOI] [PubMed] [Google Scholar]
Cuckle 1990 {published data only}
- Cuckle HS, Wald NJ, Densem JW, Royston P, Knight GJ, Haddow JE, et al. The effect of smoking in pregnancy on maternal serum alpha‐fetoprotein, unconjugated oestriol, human chorionic gonadotrophin, progesterone and dehydroepiandrosterone sulphate levels. British Journal of Obstetrics and Gynaecology 1990;97(3):272‐4. [DOI] [PubMed] [Google Scholar]
Cuckle 1996 {published data only}
- Cuckle HS, Holding S, Jones R, Groome NP, Wallace EM. Combining Inhibin A with existing second‐trimester markers in maternal serum screening for Down's syndrome. Prenatal Diagnosis 1996;16(12):1095‐100. [DOI] [PubMed] [Google Scholar]
Cuckle 1999a {published data only}
- Cuckle HS, Sehmi I, Jones R, Evans LW. Maternal serum activin A and follistatin levels in pregnancies with Down syndrome. Prenatal Diagnosis 1999;19(6):513‐6. [PubMed] [Google Scholar]
Cuckle 1999b {published data only}
- Cuckle HS, Lith JM. Appropriate biochemical parameters in first‐trimester screening for Down syndrome.[see comment]. Prenatal Diagnosis 1999;19(6):505‐12. [DOI] [PubMed] [Google Scholar]
Cullen 1990 {published data only}
- Cullen MT, Gabrielli S, Green JJ, Rizzo N, Mahoney MJ, Salafia C, et al. Diagnosis and significance of cystic hygroma in the first trimester. Prenatal Diagnosis 1990;10(10):643‐51. [DOI] [PubMed] [Google Scholar]
Cusick 2004 {published data only}
- Cusick W, Provenzano J, Sullivan CA, Gallousis FM, Rodis JF. Fetal nasal bone length in euploid and aneuploid fetuses between 11 and 20 weeks' gestation: a prospective study. Journal of Ultrasound in Medicine 2004;23(10):1327‐33. [DOI] [PubMed] [Google Scholar]
Cusick 2007 {published data only}
- Cusick W, Shevell T, Duchan LS, Lupinacci CA, Terranova J, Crombleholme WR. Likelihood ratios for fetal trisomy 21 based on nasal bone length in the second trimester: how best to define hypoplasia?. Ultrasound in Obstetrics & Gynecology 2007;30(3):271‐4. [DOI] [PubMed] [Google Scholar]
D'Ottavio 1997 {published data only}
- D'Ottavio G, Meir YJ, Rustico MA, Pecile V, Fischer‐Tamaro L, Conoscenti G, et al. Screening for fetal anomalies by ultrasound at 14 and 21 weeks. Ultrasound in Obstetrics & Gynecology 1997;10(6):375‐80. [DOI] [PubMed] [Google Scholar]
Dancoine 2001 {published data only}
- Dancoine F, Couplet G, Mainardi A, Sukno F, Jaumain P, Nowak E, et al. Antenatal screening for Dawn's syndrome with serum markers: Influence of maternal weight, smoking habits and diabetes. Immuno‐Analyse et Biologie Specialisee 2001;16(6):381‐9. [Google Scholar]
Dane 2008 {published data only}
- Dane B, Dane C, Cetin A, Kiray M, Sivri D, Yayla M. Pregnancy outcome in fetuses with increased nuchal translucency. Journal of Perinatology 2008;28(6):400‐4. [DOI] [PubMed] [Google Scholar]
De Biasio, 1999 {published data only}
- De Biasio, Siccardi M, Volpe G, Famularo L, Santi F, Canini S. First‐trimester screening for down syndrome using nuchal translucency measurement with free ß‐hCG and PAPP‐A between 10 and 13 weeks of pregnancy ‐ The combined test. Prenatal Diagnosis 1999;19(4):360‐3. [DOI] [PubMed] [Google Scholar]
De Biasio, 2001 {published data only}
- De Biasio, Ferrero S, Prefumo F, Canini S, Marchini P, Bruzzone I, et al. Down's syndrome: First trimester approach. Italian Journal of Gynaecology and Obstetrics 2001;13(1):22‐6. [Google Scholar]
De Biasio 2000 {published data only}
- Biasio P, Canini S, Prefumo F, Famularo L, Venturini PL. Extent of correlation between first and second trimester markers for Down's syndrome screening. Journal of Medical Screening 2000;7(3):163. [DOI] [PubMed] [Google Scholar]
De Graaf 1991 {published data only}
- Graaf I, Cuckle HS, Pajkrt E, Leschot NJ, Bleker OP, Lith JM. Co‐variables in first trimester maternal serum screening. Prenatal Diagnosis 1991;20(3):186‐9. [PubMed] [Google Scholar]
De Graaf 1999b {published data only}
- Graaf I, Pajkrt E, Bilardo CM, Leschot NJ, Cuckle HS, Lith JM. Early pregnancy screening for fetal aneuploidy with serum markers and nuchal translucency. Prenatal Diagnosis. 1999;19(5):458‐62. [DOI] [PubMed] [Google Scholar]
Del Carmen Saucedo 2009 {published data only}
- Carmen Saucedo M, DeVigan C, Vodovar V, Lelong N, Goffinet F, Khoshnood B. Measurement of nuchal translucency and the prenatal diagnosis of Down syndrome. Obstetrics and Gynecology 2009;114(4):829‐38. [DOI] [PubMed] [Google Scholar]
DeVore 2001 {published data only}
- DeVore GR, Romero R. Combined use of genetic sonography and maternal serum triple‐marker screening: an effective method for increasing the detection of trisomy 21 in women younger than 35 years.[see comment]. Journal of Ultrasound in Medicine 2001;20(6):645‐54. [DOI] [PubMed] [Google Scholar]
Dhaifalah 2007a {published data only}
- Dhaifalah I, Mickova I, Vrbicka D, Santavy J, Curtisova V. [Advanced maternal age as an indication for invasive prenatal diagnostics?]. [Czech]. Ceska Gynekologie 2007;72(3):181‐4. [PubMed] [Google Scholar]
Dhaifalah 2007b {published data only}
- Dhaifalah I, Mickova I, Santavy J, Vrbicka D, Zapletalova D, Curtisova V. [Efficiency of measuring nasal bone as an ultrasound marker of Down syndrome in 11th to 13th+6 week of pregnancy]. [Czech]. Ceska Gynekologie 2007;72(1):19‐23. [PubMed] [Google Scholar]
Dhallan 2007 {published data only}
- Dhallan R, Guo X, Emche S, Damewood M, Bayliss P, Cronin M, et al. A non‐invasive test for prenatal diagnosis based on fetal DNA present in maternal blood: a preliminary study. Lancet 2007;369(9560):474‐81. [DOI] [PubMed] [Google Scholar]
Dickerson 1994 {published data only}
- Dickerson VM. Multiple marker screening. Western Journal of Medicine 1994;161(2):161. [PMC free article] [PubMed] [Google Scholar]
Dimaio 1987 {published data only}
- Dimaio MS, Baumgarten A, Greenstein RM, Saal HM, Mahoney MJ. Screening for fetal Down's syndrome in pregnancy by measuring maternal serum alpha‐fetoprotein levels. New England Journal of Medicine 1987;317(6):342‐6. [DOI] [PubMed] [Google Scholar]
Doran 1986 {published data only}
- Doran TA, Cadesky K, Wong PY, Mastrogiacomo C, Capello T. Maternal serum alpha‐fetoprotein and fetal autosomal trisomies. American Journal of Obstetrics and Gynecology 1986;154(2):277‐81. [DOI] [PubMed] [Google Scholar]
Dreux 2008 {published data only}
- Dreux S, Olivier C, Dupont JM, Leporrier N, Study G, Oury JF, et al. Maternal serum screening in cases of mosaic and translocation Down syndrome. Prenatal Diagnosis 2008;28(8):699‐703. [DOI] [PubMed] [Google Scholar]
Drugan 1996a {published data only}
- Drugan A, Reichler A, Bronstein M, Johnson MP, Sokol RJ, Evans MI. Abnormal biochemical serum screening versus 2nd‐trimester ultrasound‐detected minor anomalies as predictors of aneuploidy in low‐risk women. Fetal Diagnosis and Therapy 1996;11(5):301‐5. [DOI] [PubMed] [Google Scholar]
Drugan 1996b {published data only}
- Drugan A, O'Brien JE, Dvorin E, Krivchenia EL, Johnson MP, Sokol RJ, et al. Multiple marker screening in multifetal gestations: failure to predict adverse pregnancy outcomes. Fetal Diagnosis and Therapy 1996;11(1):16‐9. [DOI] [PubMed] [Google Scholar]
Drysdale 2002 {published data only}
- Drysdale K, Ridley D, Walker K, Higgins B, Dean T. First‐trimester pregnancy scanning as a screening tool for high‐risk and abnormal pregnancies in a district general hospital setting. Journal of Obstetrics and Gynaecology 2002;22(2):159‐65. [DOI] [PubMed] [Google Scholar]
Dugoff 2008 {published data only}
- Dugoff L, Cuckle HS, Hobbins JC, Malone FD, Belfort MA, Nyberg DA, et al. Prediction of patient‐specific risk for fetal loss using maternal characteristics and first‐ and second‐trimester maternal serum Down syndrome markers. American Journal of Obstetrics and Gynecology 2008;199(3):290‐6. [DOI] [PubMed] [Google Scholar]
Ebell 1999 {published data only}
- Ebell M. Is the integrated test better for screening for Down's syndrome than the traditional triple test?. Evidence‐Based Practice 1999;2(11):4‐5. [Google Scholar]
Economides 1998 {published data only}
- Economides DL, Whitlow BJ, Kadir R, Lazanakis M, Verdin SM. First trimester sonographic detection of chromosomal abnormalities in an unselected population. British Journal of Obstetrics and Gynaecology 1998;105(1):58‐62. [DOI] [PubMed] [Google Scholar]
Erickson 2004 {published data only}
- Erickson JA, Ashwood ER, Gin CA. Evaluation of a dimeric inhibin‐A assay for assessing fetal Down syndrome: establishment, comparison, and monitoring of median concentrations for normal pregnancies. Archives of Pathology & Laboratory Medicine 2004;128(4):415‐20. [DOI] [PubMed] [Google Scholar]
Evans 1996 {published data only}
- Evans MI, O'Brien JE, Dvorin E, Krivchenia EL, Drugan A, Hume RF Jr, et al. Similarity of insulin‐dependent diabetics' and non‐insulin‐dependent diabetics' levels of ß‐hCG and unconjugated estriol with controls: no need to adjust as with alpha‐fetoprotein. Journal of the Society for Gynecologic Investigation 1996;3(1):20‐2. [PubMed] [Google Scholar]
Evans 2007 {published data only}
- Evans MI, Galen RS. Comparison of serum markers in first‐trimester down syndrome screening. Obstetrics and Gynecology 2007;109(3):782. [DOI] [PubMed] [Google Scholar]
Falcon 2005 {published data only}
- Falcon O, Cavoretto P, Peralta CF, Csapo B, Nicolaides KH. Fetal head‐to‐trunk volume ratio in chromosomally abnormal fetuses at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2005;26(7):755‐60. [DOI] [PubMed] [Google Scholar]
Falcon 2006 {published data only}
- Falcon O, Faiola S, Huggon I, Allan L, Nicolaides KH. Fetal tricuspid regurgitation at the 11 + 0 to 13 + 6‐week scan: association with chromosomal defects and reproducibility of the method. Ultrasound in Obstetrics & Gynecology 2006;27(6):609‐12. [DOI] [PubMed] [Google Scholar]
Ford 1998 {published data only}
- Ford C, Moore AJ, Jordan PA, Bartlett WA, Wyldes MP, Jones AF, et al. The value of screening for Down's syndrome in a socioeconomically deprived area with a high ethnic population.[see comment]. British Journal of Obstetrics and Gynaecology 1998;105(8):855‐9. [DOI] [PubMed] [Google Scholar]
Frishman 1997 {published data only}
- Frishman GN, Canick JA, Hogan JW, Hackett RJ, Kellner LH, Saller DN Jr. Serum triple‐marker screening in in vitro fertilization and naturally conceived pregnancies. Obstetrics and Gynecology 1997;90(1):98‐101. [DOI] [PubMed] [Google Scholar]
Fukada 2000 {published data only}
- Fukada Y, Takizawa M, Amemiya A, Yoda H, Kohno K, Hoshi K. Detection of aneuploidy with fetal nuchal translucency and maternal serum markers in Japanese women. Acta Obstetricia et Gynecologica Scandinavica 2000;79(12):1124‐5. [PubMed] [Google Scholar]
Gaudry 2009 {published data only}
- Gaudry P, Lebbar A, Choiset A, Girard S, Lewin F, Tsatsaris V, et al. Is rapid aneuploidy screening used alone acceptable in prenatal diagnosis? An evaluation of the possible role of ultrasound examination. Fetal Diagnosis and Therapy 2009;25(2):285‐90. [DOI] [PubMed] [Google Scholar]
Gebb 2009 {published data only}
- Gebb J, Dar P. Should the first‐trimester aneuploidy screen be maternal age adjusted? Screening by absolute risk versus risk adjusted to maternal age. Prenatal Diagnosis 2009;29(3):245‐7. [DOI] [PubMed] [Google Scholar]
Geerts 2008 {published data only}
- Geerts L. Prenatal diagnosis of chromosomal abnormalities in a resource‐poor setting. International Journal of Gynaecology and Obstetrics 2008;103(1):16‐21. [DOI] [PubMed] [Google Scholar]
Geipel 2010 {published data only}
- Geipel A, Willruth A, Vieten J, Gembruch U, Berg C. Nuchal fold thickness, nasal bone absence or hypoplasia, ductus venosus reversed flow and tricuspid valve regurgitation in screening for trisomies 21, 18 and 13 in the early second trimester. Ultrasound in Obstetrics & Gynecology 2010;35(5):535‐9. [DOI] [PubMed] [Google Scholar]
Gekas 2009 {published data only}
- Gekas J, Gagne G, Bujold E, Douillard D, Forest JC, Reinharz D, et al. Comparison of different strategies in prenatal screening for Down's syndrome: cost effectiveness analysis of computer simulation. BMJ 2009;338:b138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gekas 2011a {published data only}
- Gekas J, Berg DG, Durand A, Vallee M, Wildschut HI, Bujold E, et al. Rapid testing versus karyotyping in Down's syndrome screening: cost‐effectiveness and detection of clinically significant chromosome abnormalities. European Journal of Human Genetics 2011;19(1):3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gekas 2011b {published data only}
- Gekas J, Durand A, Bujold E, Vallee M, Forest JC, Rousseau F, et al. Cost‐effectiveness and accuracy of prenatal Down syndrome screening strategies: should the combined test continue to be widely used?. American Journal of Obstetrics and Gynecology 2011;204(2):175‐8. [DOI] [PubMed] [Google Scholar]
Gerovassili 2007 {published data only}
- Gerovassili A, Garner C, Nicolaides KH, Thein SL, Rees DC. Free fetal DNA in maternal circulation: a potential prognostic marker for chromosomal abnormalities?. Prenatal Diagnosis 2007;27(2):104‐10. [DOI] [PubMed] [Google Scholar]
Ghidini 1998 {published data only}
- Ghidini A, Spong CY, Grier RE, Walker CN, Pezzullo JC. Is maternal serum triple screening a better predictor of Down syndrome in female than in male fetuses?. Prenatal Diagnosis 1998;18(2):123‐6. [DOI] [PubMed] [Google Scholar]
Goetzinger 2010 {published data only}
- Goetzinger KR, Dicke JM, Gray DL, Stamilio DM, Macones GA, Odibo AO. The effect of fetal gender in predicting Down syndrome using long bone ultrasonographic measurements. Prenatal Diagnosis 2010;30(10):950‐5. [DOI] [PubMed] [Google Scholar]
Goldie 1995 {published data only}
- Goldie DJ, Astley JP, Beaman JM, Bickley DA, Gunneberg A, Jones SR. Screening for Down's syndrome: the first two years experience in Bristol. Journal of Medical Screening 1995;2(4):207‐10. [DOI] [PubMed] [Google Scholar]
Gollo 2008 {published data only}
- Gollo CA, Murta CG, Bussamra LC, Santana RM, Moron AF. [Predictive value for fetal outcome of Doppler velocimetry of the ductus venosus between the 11th and the 14th gestation week]. [Portuguese]. Revista Brasileira de Ginecologia e Obstetricia 2008;30(1):5‐11. [DOI] [PubMed] [Google Scholar]
Gonçalves 2004 {published data only}
- Gonçalves LF, Espinoza J, Lee W, Schoen ML, Devers P, Mazor M, et al. Phenotypic characteristics of absent and hypoplastic nasal bones in fetuses with down syndrome: Description by 3‐dimensional ultrasonography and clinical significance. Journal of Ultrasound in Medicine 2004;23(12):1619‐27. [DOI] [PubMed] [Google Scholar]
Goodburn 1994 {published data only}
- Goodburn SF, Yates JR, Raggatt PR, Carr C, Ferguson‐Smith ME, Kershaw AJ, et al. Second‐trimester maternal serum screening using alpha‐fetoprotein, human chorionic gonadotrophin, and unconjugated oestriol: experience of a regional programme. Prenatal Diagnosis 1994;14(5):391‐402. [DOI] [PubMed] [Google Scholar]
Gorduza 2007 {published data only}
- Gorduza EV, Onofriescu M, Martiniuc V, Grigore M, Mihalceanu E, Iliev G. [FISH technique in aneuplodies prenatal diagnosis]. [Romanian]. Revista Medico‐Chirurgicala a Societatii de Medici Si Naturalisti Din Iasi 2007;111(4):990‐5. [PubMed] [Google Scholar]
Grace 2010 {published data only}
- Grace D, Eggers P, Glantz JC, Ozcan T. Mitral valve‐tricuspid valve distance as a sonographic marker of trisomy 21. Ultrasound in Obstetrics & Gynecology 2010;35(2):172‐7. [DOI] [PubMed] [Google Scholar]
Grati 2010 {published data only}
- Grati FR, Barlocco A, Grimi B, Milani S, Frascoli G, Meco AM, et al. Chromosome abnormalities investigated by non‐invasive prenatal testing account for approximately 50% of fetal unbalances associated with relevant clinical phenotypes. American Journal of Medical Genetics 2010;Part A. 152A(6):1434‐42. [DOI] [PubMed] [Google Scholar]
Gray 2009 {published data only}
- Gray DL, Dicke JM, Dickerson R, McCourt C, Odibo AO. Reevaluating humeral length for the detection of fetal trisomy 21. Journal of Ultrasound in Medicine 2009;28(10):1325‐30. [DOI] [PubMed] [Google Scholar]
Gregor 2007 {published data only}
- Gregor V, Sipek A, Horacek J. [Birth defects in the Czech Republic‐‐the prenatal diagnostic]. [Czech]. Ceska Gynekologie 2007;72(4):262‐8. [PubMed] [Google Scholar]
Gregor 2009 {published data only}
- Gregor V, Sipek A, Sipek AJ, Horacek J, Langhammer P, Petrzilkova L, et al. [Prenatal diagnostics of chromosomal aberrations Czech Republic: 1994‐2007]. [Czech]. Ceska Gynekologie 2009;74(1):44‐54. [PubMed] [Google Scholar]
Grether 2009 {published data only}
- Grether González P, Aguinaga Ríos M, Colegio Mexicano de Especialistas en Ginecología y Obstetricia. [Prenatal genetic screening: biochemical markers of the first and second quarter]. [Spanish]. Ginecologia y Obstetricia de Mexico 2009;77(2):S27‐46. [PubMed] [Google Scholar]
Grozdea 2002 {published data only}
- Grozdea J, Farge F, Bourrouillou G, Calot M, Cambus JP, Valdiguie P. Maternal serum urea resistant alkaline phosphatase in Down syndrome pregnancy. Early Human Development 2002;67(1‐2):55‐9. [DOI] [PubMed] [Google Scholar]
Guo 2010 {published data only}
- Guo Q, Zhou Y, Wang X, Li Q. Simultaneous detection of trisomies 13, 18, and 21 with multiplex ligation‐dependent probe amplification‐based real‐time PCR. Clinical Chemistry 2010;56(9):1451‐9. [DOI] [PubMed] [Google Scholar]
Gyselaers 2004a {published data only}
- Gyselaers WJ, Vereecken AJ, Herck EJ, Straetmans DP, Martens GE, Jonge ET, et al. Screening for trisomy 21 in Flanders: a 10 years review of 40.490 pregnancies screened by maternal serum. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2004;115(2):185‐9. [DOI] [PubMed] [Google Scholar]
Gyselaers 2004b {published data only}
- Gyselaers WJA, Vereecken AJ, Van Herck, Straetmans DPL, De Jonge, Ombelet WUA, et al. Single‐step maternal serum screening for trisomy 21 in the era of combined or integrated screening. Gynecologic and Obstetric Investigation 2004;58(4):221‐4. [DOI] [PubMed] [Google Scholar]
Gyselaers 2006a {published data only}
- Gyselaers WJ, Vereecken AJ, Herck EJ, Straetmans DP, Ombelet WU, Nijhuis JG. Nuchal translucency thickness measurements for fetal aneuploidy screening: Log NT‐MoM or Delta‐NT, performer‐specific medians and ultrasound training. Journal of Medical Screening 2006;13(1):4‐7. [DOI] [PubMed] [Google Scholar]
Gyselaers 2006b {published data only}
- Gyselaers WJ, Roets ER, Holsbeke CD, Vereecken AJ, Herck EJ, Straetmans DP, et al. Sequential triage in the first trimester may enhance advanced ultrasound scanning in population screening for trisomy 21. Ultrasound in Obstetrics & Gynecology 2006;27(6):622‐7. [DOI] [PubMed] [Google Scholar]
Hackshaw 1995 {published data only}
- Hackshaw AK, Densem J, Wald NJ. Repeat maternal serum testing for Down's syndrome screening using multiple markers with special reference to free alpha and free ß‐hCG. Prenatal Diagnosis 1995;15(12):1125‐30. [DOI] [PubMed] [Google Scholar]
Hackshaw 2001 {published data only}
- Hackshaw AK, Wald NJ. Repeat testing in antenatal screening for Down syndrome using dimeric inhibin‐A in combination with other maternal serum markers. Prenatal Diagnosis 2001;21(1):58‐61. [PubMed] [Google Scholar]
Haddow 1992 {published data only}
- Haddow JE, Palomaki GE, Knight GJ, Williams J, Pulkkinen A, Canick J, et al. Prenatal screening for Down's syndrome with use of maternal serum markers. New England Journal of Medicine 1992;327(9):588‐93. [DOI] [PubMed] [Google Scholar]
Hadzsiev 2007 {published data only}
- Hadzsiev K, Czako M, Veszpremi B, Kosztolanyi G. [Rapid diagnosis of fetal chromosomal abnormalities by fluorescence in situ hybridization]. [Hungarian]. Orvosi Hetilap 2007;148(30):1401‐4. [DOI] [PubMed] [Google Scholar]
Hafner 1995 {published data only}
- Hafner E, Schuchter K, Philipp K. Screening for chromosomal abnormalities in an unselected population by fetal nuchal translucency. Ultrasound in Obstetrics & Gynecology 1995;6(5):330‐3. [DOI] [PubMed] [Google Scholar]
Hallahan 1998 {published data only}
- Hallahan TW, Krantz DA, Tului L, Alberti E, Buchanan PD, Orlandi F, et al. Comparison of urinary free ß (hCG) and ß‐core (hCG) in prenatal screening for chromosomal abnormalities. Prenatal Diagnosis 1998;18(9):893‐900. [DOI] [PubMed] [Google Scholar]
Han 2008 {published data only}
- Han SH, An JW, Jeong GY, Yoon HR, Lee A, Yang YH, et al. Clinical and cytogenetic findings on 31,615 mid‐trimester amniocenteses. Korean Journal of Laboratory Medicine 2008;28(5):378‐85. [DOI] [PubMed] [Google Scholar]
Harper 2010 {published data only}
- Harper LM, Gray D, Dicke J, Stamilio DM, Macones GA, Odibo AO. Do race‐specific definitions of short long bones improve the detection of down syndrome on second‐trimester genetic sonograms?. Journal of Ultrasound in Medicine 2010;29(2):231‐5. [DOI] [PubMed] [Google Scholar]
Harrison 2006 {published data only}
- Harrison G, Goldie D. Second‐trimester Down's syndrome serum screening: double, triple or quadruple marker testing?. Annals of Clinical Biochemistry 2006;43(1):67‐72. [DOI] [PubMed] [Google Scholar]
Harry 2006 {published data only}
- Harry WG, Reed KL. Nuchal translucency and first‐trimester screening. Journal of the Society for Gynecologic Investigation 2006;13(3):153‐4. [DOI] [PubMed] [Google Scholar]
Hayashi 1995 {published data only}
- Hayashi M, Kozu H. Maternal urinary ß‐core fragment of hCG/creatinine ratios and fetal chromosomal abnormalities in the second trimester of pregnancy. Prenatal Diagnosis 1995;15(1):11‐6. [DOI] [PubMed] [Google Scholar]
Hayashi 1996 {published data only}
- Hayashi M, Kozu H, Takei H. Maternal urinary free ß‐subunit of human chorionic gonadotrophin: Creatinine ratios and fetal chromosomal abnormalities in the second trimester of pregnancy. British Journal of Obstetrics and Gynaecology 1996;103(6):577‐80. [DOI] [PubMed] [Google Scholar]
Heikkila 1997 {published data only}
- Heikkila A, Ryynanen M, Kirkinen P, Saarikoski S. Results and views of women in population‐wide pregnancy screening for trisomy 21 in east Finland. Fetal Diagnosis and Therapy 1997;12(2):93‐6. [DOI] [PubMed] [Google Scholar]
Heinig 2007 {published data only}
- Heinig J, Steinhard J, Schmitz R, Nofer JR, Kiesel L, Klockenbusch W. Maternal serum free beta‐hCG and PAPP‐A in patients with habitual abortion‐influence on first‐trimester screening for chromosomal abnormalities. Prenatal Diagnosis 2007;27(9):814‐6. [DOI] [PubMed] [Google Scholar]
Heinonen 1996 {published data only}
- Heinonen S, Ryynanen M, Kirkinen P, Hippelainen M, Saarikoski S. Effect of in vitro fertilization on human chorionic gonadotropin serum concentrations and Down's syndrome screening. Fertility and Sterility 1996;66(3):398‐403. [DOI] [PubMed] [Google Scholar]
Herman 2000 {published data only}
- Herman A, Weinraub Z, Dreazen E, Arieli S, Rozansky S, Bukovsky I, et al. Combined first trimester nuchal translucency and second trimester biochemical screening tests among normal pregnancies. Prenatal Diagnosis 2000;20(10):781‐4. [DOI] [PubMed] [Google Scholar]
Herman 2003 {published data only}
- Herman A, Dreazen E, Tovbin Y, Reish O, Bukovsky I, Maymon R. Correlation and overlapping between nuchal translucency and triple test among Down syndrome‐affected pregnancies. Fetal Diagnosis and Therapy 2003;18(3):196‐200. [DOI] [PubMed] [Google Scholar]
Herrou 1992 {published data only}
- Herrou M, Leporrier N, Leymarie P. Screening for fetal Down syndrome with maternal serum hCG and oestriol: a prospective study. Prenatal Diagnosis 1992;12(11):887‐92. [DOI] [PubMed] [Google Scholar]
Hershey 1985 {published data only}
- Hershey DW, Crandall BF, Schroth PS. Maternal serum alpha‐fetoprotein screening of fetal trisomies. American Journal of Obstetrics and Gynecology 1985;153(2):224‐5. [DOI] [PubMed] [Google Scholar]
Hershey 1986 {published data only}
- Hershey DW, Crandall BF, Perdue S. Combining maternal age and serum alpha‐fetoprotein to predict the risk of Down syndrome. Obstetrics and Gynecology 1986;68(2):177‐80. [PubMed] [Google Scholar]
Hewitt 1993 {published data only}
- Hewitt B. Nuchal translucency in the first trimester. Australian & New Zealand Journal of Obstetrics & Gynaecology 1993;33(4):389‐91. [DOI] [PubMed] [Google Scholar]
Hills 2010 {published data only}
- Hills A, Donaghue C, Waters J, Waters K, Sullivan C, Kulkarni A, et al. QF‐PCR as a stand‐alone test for prenatal samples: the first 2 years' experience in the London region. Prenatal Diagnosis 2010;30(6):509‐17. [DOI] [PubMed] [Google Scholar]
Ho 2010 {published data only}
- Ho SS, Choolani MA. FlashFISH: "same day" prenatal diagnosis of common chromosomal aneuploidies. Methods in Molecular Biology 2010;659:261‐8. [DOI] [PubMed] [Google Scholar]
Hogdall 1992 {published data only}
- Hogdall CK, Hogdall EV, Arends J, Norgaard‐Pedersen B, Smidt‐Jensen S, Larsen SO. CA‐125 as a maternal serum marker for Down's syndrome in the first and second trimesters. Prenatal Diagnosis 1992;12(3):223‐7. [DOI] [PubMed] [Google Scholar]
Hong Kong Practitioner {published data only}
- Anon. Screening tests in pregnancy. Hong Kong Practitioner 2001;23(10):461‐5. [Google Scholar]
Hoogendoorn 2008 {published data only}
- Hoogendoorn M, Evers SM, Schielen PC, Genugten ML, Wit GA, Ament AJ. Costs and effects of prenatal screening methods for Down syndrome and neural tube defects. Community Genetics 2008;11(6):359‐67. [DOI] [PubMed] [Google Scholar]
Howe 2000 {published data only}
- Howe DT, Gornall R, Wellesley D, Boyle T, Barber J. Six year survey of screening for Down's syndrome by maternal age and mid‐trimester ultrasound scans. BMJ 2000;320(7235):606‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsiao 1991 {published data only}
- Hsiao KJ, Lee SY, Chuang HC. [Antenatal screening of maternal alpha‐fetoprotein with dried‐blood spot samples on filter paper]. [Chinese]. Journal of the Formosan Medical Association 1991;90(6):598‐604. [PubMed] [Google Scholar]
Hsieh 1999 {published data only}
- Hsieh TT, Hsu JJ, Lo LM, Liou JD, Soong YK. Maternal urine alpha‐fetoprotein concentrations between 14 and 21 weeks of gestation. Changgeng Yi Xue Za Zhi 1999;22(2):234‐9. [PubMed] [Google Scholar]
Hsu 1997 {published data only}
- Hsu JJ, Hsieh TT, Soong YK. Influence of maternal age and weight on second‐trimester serum alpha‐fetoprotein, total and free ß human chorionic gonadotropin levels. Changgeng Yi Xue Za Zhi 1997;20(3):181‐6. [PubMed] [Google Scholar]
Hsu 1998 {published data only}
- Hsu JJ, Hsieh TT, Hung TH, Chiang CH. Midtrimester maternal serum free ß‐human chorionic gonadotropin levels: normal reference values for Taiwanese women. Changgeng Yi Xue Za Zhi 1998;21(3):277‐82. [PubMed] [Google Scholar]
Hsu 1999 {published data only}
- Hsu JJ, Hsieh TT, Hung TH, Chen KC, Soong YK. Urine free ß‐human chorionic gonadotropin levels between 14 and 21 weeks of gestation in Taiwanese pregnancies. Changgeng Yi Xue Za Zhi 1999;22(1):11‐6. [PubMed] [Google Scholar]
Hu 2007 {published data only}
- Hu YL, Birth Defect Intervention Group of Jiangsu Province. [Serum screening of fetal chromosome abnormality during second pregnancy trimester: results of 26,803 pregnant women in Jiangsu Province]. [Chinese]. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2007;87(35):2476‐80. [PubMed] [Google Scholar]
Huang 2003 {published data only}
- Huang T, Summers AM, Wyatt PR, Meier C, Cote GB. Maternal serum marker medians in Aboriginal Canadian women. Prenatal Diagnosis 2003;23(2):98‐100. [DOI] [PubMed] [Google Scholar]
Huang 2007a {published data only}
- Huang T, Boucher K, Summers AM. Second trimester prenatal screening for Down syndrome: the associations between the levels of serum markers in successive pregnancies. Prenatal Diagnosis 2007;27(12):1138‐42. [DOI] [PubMed] [Google Scholar]
Huang 2007b {published data only}
- Huang T, Wang FL, Boucher K, O'Donnell A, Rashid S, Summers AM. Racial differences in first trimester nuchal translucency. Prenatal Diagnosis 2007;27(12):1174‐6. [DOI] [PubMed] [Google Scholar]
Huggon 2004 {published data only}
- Huggon IC, Turan O, Allan LD. Doppler assessment of cardiac function at 11‐14 weeks' gestation in fetuses with normal and increased nuchal translucency. Ultrasound in Obstetrics & Gynecology 2004;24(4):390‐8. [DOI] [PubMed] [Google Scholar]
Hui 2003 {published data only}
- Hui PW, Tang MH, Lam YH, Ng EH, Yeung WS, Ho PC. Maternal serum hCG and alpha‐fetoprotein levels in pregnancies conceived after IVF or ICSI with fresh and frozen‐thawed embryos. Human Reproduction 2003;18(3):572‐5. [DOI] [PubMed] [Google Scholar]
Hui 2005 {published data only}
- Hui PW, Tang MH, Lam YH, Yeung WS, Ng EH, Ho PC. Nuchal translucency in pregnancies conceived after assisted reproduction technology. Ultrasound in Obstetrics & Gynecology 2005;25(3):234‐8. [DOI] [PubMed] [Google Scholar]
Hultén 2004 {published data only}
- Hultén M. Combined serum and nuchal translucency screening in the first trimester achieves 85% to 90% detection rate for Down and Edward syndromes. Evidence‐Based Healthcare 2004;8(2):82‐4. [Google Scholar]
Hung 2003 {published data only}
- Hung JH, Fu CY, Yuan CC, Chen CL, Yang ML, Shu LP, et al. Nuchal translucence incorporated into a one‐stage multifactorial screening model for Down syndrome prediction at second‐trimester pregnancy. Ultrasound in Medicine & Biology 2003;29(12):1667‐74. [DOI] [PubMed] [Google Scholar]
Hung 2008 {published data only}
- Hung JH, Fu CY, Chen CY, Chao KC, Hung J. Fetal nasal bone length and Down syndrome during the second trimester in a Chinese population. Journal of Obstetrics and Gynaecology Research 2008;34(4):518‐23. [DOI] [PubMed] [Google Scholar]
Hurley 1993 {published data only}
- Hurley PA, Ward RH, Teisner B, Iles RK, Lucas M, Grudzinskas JG. Serum PAPP‐A measurements in first‐trimester screening for Down syndrome. Prenatal Diagnosis 1993;13(10):903‐8. [DOI] [PubMed] [Google Scholar]
Huttly 2004 {published data only}
- Huttly W, Rudnicka A, Wald NJ. Second‐trimester prenatal screening markers for Down syndrome in women with insulin‐dependent diabetes mellitus. Prenatal Diagnosis 2004;24(10):804‐7. [DOI] [PubMed] [Google Scholar]
Hwa 2004 {published data only}
- Hwa HL, Yen MF, Hsieh FJ, Ko TM, Chen TH. Evaluation of second trimester maternal serum screening for Down's Syndrome using the Spiegelhalter‐Knill‐Jones (S‐KJ) approach. Journal of Perinatal Medicine 2004;32(5):407‐12. [DOI] [PubMed] [Google Scholar]
Iles 1996 {published data only}
- Iles RK. Urinary analysis for Down's syndrome: Is the measurement of urinary ß‐core the future of biochemical screening for Down's syndrome. Early Human Development 1996;47(Suppl):S41‐S45. [DOI] [PubMed] [Google Scholar]
Ind 1994 {published data only}
- Ind TEJ, Iles RK, Cuckle HS, Chard T. Second trimester maternal serum placental alkaline phosphatase concentrations in Down's syndrome. Journal of Obstetrics and Gynaecology 1994;14(5):305‐8. [Google Scholar]
Ivorra‐Deleuze 2010 {published data only}
- Ivorra‐Deleuze D, Bretelle F, Heinemann M, Levy A, Toga C, Philip N, et al. [Combined screening for Down syndrome in Marseille multidisciplinary prenatal centers]. [French]. Gynecologie, Obstetrique & Fertilite 2010;38(12):786‐8. [DOI] [PubMed] [Google Scholar]
Jakobsen 2011 {published data only}
- Jakobsen TR, Sogaard K, Tabor A. Implications of a first trimester Down syndrome screening program on timing of malformation detection. Acta Obstetricia et Gynecologica Scandinavica 2011;90(7):728‐36. [DOI] [PubMed] [Google Scholar]
Jean‐Pierre 2005 {published data only}
- Jean‐Pierre C. Fetal nasal bone: review of first trimester findings. Ultrasound Review of Obstetrics and Gynecology 2005;5(2):102‐4. [Google Scholar]
Johnson 1991 {published data only}
- Johnson A, Cowchock FS, Darby M, Wapner R, Jackson LG. First‐trimester maternal serum alpha‐fetoprotein and chorionic gonadotropin in aneuploid pregnancies. Prenatal Diagnosis 1991;11(7):443‐50. [DOI] [PubMed] [Google Scholar]
Johnson 1993 {published data only}
- Johnson MP, Johnson A, Holzgreve W, Isada NB, Wapner RJ, Treadwell MC, et al. First‐trimester simple hygroma: cause and outcome. American Journal of Obstetrics and Gynecology 1993;168(1):156‐61. [DOI] [PubMed] [Google Scholar]
Jorgensen 1999 {published data only}
- Jorgensen FS, Valentin L, Salvesen KA, Jorgensen C, Jensen FR, Bang J, et al. MULTISCAN‐‐a Scandinavian multicenter second trimester obstetric ultrasound and serum screening study. Acta Obstetricia et Gynecologica Scandinavica 1999;78(6):501‐10. [PubMed] [Google Scholar]
Jorgez 2007 {published data only}
- Jorgez CJ, Dang DD, Wapner R, Farina A, Simpson JL, Bischoff FZ. Elevated levels of total (maternal and fetal) beta‐globin DNA in maternal blood from first trimester pregnancies with trisomy 21. Human Reproduction 2007;22(8):2267‐72. [DOI] [PubMed] [Google Scholar]
Josefsson 1998 {published data only}
- Josefsson A, Molander E, Selbing A. Nuchal translucency as a screening test for chromosomal abnormalities in a routine first trimester ultrasound examination. Acta Obstetricia et Gynecologica Scandinavica 1998;77(5):497‐9. [PubMed] [Google Scholar]
Jou 2001 {published data only}
- Jou HJ, Shih JC, Wu SC, Li TC, Tzeng CY, Hsieh FJ. First‐trimester Down's syndrome screening by fetal nuchal translucency measurement in Taiwan. Journal of the Formosan Medical Association 2001;100(4):257‐61. [PubMed] [Google Scholar]
Jung 2007 {published data only}
- Jung E, Won HS, Lee PR, Kim A. Ultrasonographic measurement of fetal nasal bone length in the second trimester in Korean population. Prenatal Diagnosis 2007;27(2):154‐7. [DOI] [PubMed] [Google Scholar]
Kagan 2006 {published data only}
- Kagan KO, Avgidou K, Molina FS, Gajewska K, Nicolaides KH. Relation between increased fetal nuchal translucency thickness and chromosomal defects.[see comment]. Obstetrics and Gynecology 2006;107(1):6‐10. [DOI] [PubMed] [Google Scholar]
Kagan 2007 {published data only}
- Kagan KO, Frisova V, Nicolaides KH, Spencer K. Dose dependency between cigarette consumption and reduced maternal serum PAPP‐A levels at 11‐13+6 weeks of gestation. Prenatal Diagnosis 2007;27(9):849‐53. [DOI] [PubMed] [Google Scholar]
Kagan 2008 {published data only}
- Kagan KO, Anderson JM, Anwandter G, Neksasova K, Nicolaides KH. Screening for triploidy by the risk algorithms for trisomies 21, 18 and 13 at 11 weeks to 13 weeks and 6 days of gestation. Prenatal Diagnosis 2008;28(13):1209‐13. [DOI] [PubMed] [Google Scholar]
Kalelioglu 2007 {published data only}
- Kalelioglu IH. Humerus length measurement in Down syndrome screening. Clinical and Experimental Obstetrics & Gynecology 2007;34(2):93‐5. [PubMed] [Google Scholar]
Kautzmann 1995 {published data only}
- Kautzmann M, Solis RL, Luberta A, Fernandez JL, Navarro J, Rodriguez L, et al. Study of the efficiency of screening for trisomy 21 based on maternal serum levels of AFP and hCG combined with maternal age. Journal of Clinical Ligand Assay 1995;18(3):181‐5. [Google Scholar]
Kazerouni 2009 {published data only}
- Kazerouni NN, Currier B, Malm L, Riggle S, Hodgkinson C, Smith S, et al. Triple‐marker prenatal screening program for chromosomal defects. Obstetrics and Gynecology 2009;114(1):50‐8. [DOI] [PubMed] [Google Scholar]
Keith 1992 {published data only}
- Keith D. Maternal serum screening for neural tube defects and Down syndrome. Clinical Laboratory Science 1992;5(5):274‐6. [PubMed] [Google Scholar]
Kelekci 2004 {published data only}
- Kelekci S, Yazicioglu HF, Oguz S, Inan I, Yilmaz B, Sonmez S. Nasal bone measurement during the 1st trimester: is it useful?. Gynecologic and Obstetric Investigation 2004;58(2):91‐5. [DOI] [PubMed] [Google Scholar]
Kellner 1995a {published data only}
- Kellner LH, Weiner Z, Weiss RR, Neuer M, Martin GM, Mueenuddin M, et al. Triple marker (alpha‐fetoprotein, unconjugated estriol, human chorionic gonadotropin) versus alpha‐fetoprotein plus free‐ß subunit in second‐trimester maternal serum screening for fetal Down syndrome: a prospective comparison study.[see comment]. American Journal of Obstetrics and Gynecology 1995;173(4):1306‐9. [DOI] [PubMed] [Google Scholar]
Kellner 1995b {published data only}
- Kellner LH, Weiss RR, Weiner Z, Neuer M, Martin GM, Schulman H, et al. The advantages of using triple‐marker screening for chromosomal abnormalities. American Journal of Obstetrics and Gynecology 1995;172(3):831‐6. [DOI] [PubMed] [Google Scholar]
Kellner 1997 {published data only}
- Kellner LH, Canick JA, Palomaki GE, Neveux LM, Saller DN Jr, Walker RP, et al. Levels of urinary ß‐core fragment, total oestriol, and the ratio of the two in second‐trimester screening for Down syndrome. Prenatal Diagnosis 1997;17(12):1135‐41. [DOI] [PubMed] [Google Scholar]
Kirkegaard 2008 {published data only}
- Kirkegaard I, Petersen OB, Uldbjerg N, Torring N. Improved performance of first‐trimester combined screening for trisomy 21 with the double test taken before a gestational age of 10 weeks. Prenatal Diagnosis 2008;28(9):839‐44. [DOI] [PubMed] [Google Scholar]
Kjaergaard 2008 {published data only}
- Kjaergaard S, Hahnemann JM, Skibsted L, Jensen LN, Sperling L, Zingenberg H, et al. [Prenatal diagnosis of chromosome aberrations after implementation of screening for Down's syndrome]. [Danish]. Ugeskrift for Laeger 2008;170(14):1152‐6. [PubMed] [Google Scholar]
Knight 1990 {published data only}
- Knight GJ, Palomaki GE. Maternal serum alpha fetoprotein screening for fetal down syndrome. Journal of Clinical Immunoassay 1990;13(1):23‐9. [Google Scholar]
Knight 2001 {published data only}
- Knight GJ, Palomaki GE, Neveux LM, Haddow JE, Lambert‐Messerlian GM. Clinical validation of a new dimeric inhibin‐A assay suitable for second trimester Down's syndrome screening. Journal of Medical Screening 2001;8(1):2‐7. [DOI] [PubMed] [Google Scholar]
Knight 2005 {published data only}
- Knight GJ, Palomaki GE, Neveux LM, Smith DE, Kloza EM, Pulkkinen A, et al. Integrated serum screening for Down syndrome in primary obstetric practice. Prenatal Diagnosis 2005;25(12):1162‐7. [DOI] [PubMed] [Google Scholar]
Koos 2006 {published data only}
- Koos BJ. First‐trimester screening: Lessons from clinical trials and implementation. Current Opinion in Obstetrics and Gynecology 2006;18(2):152‐5. [DOI] [PubMed] [Google Scholar]
Kornman 1996 {published data only}
- Kornman LH, Morssink LP, Beekhuis JR, Wolf BT, Heringa MP, Mantingh A. Nuchal translucency cannot be used as a screening test for chromosomal abnormalities in the first trimester of pregnancy in a routine ultrasound practice.[see comment]. Prenatal Diagnosis 1996;16(9):797‐805. [DOI] [PubMed] [Google Scholar]
Kornman 1997 {published data only}
- Kornman LH, Morssink LP, Wortelboer MJ, Beekhuis JR, Wolf BT, Pratt JJ, et al. Maternal urinary ß‐core hCG in chromosomally abnormal pregnancies in the first trimester. Prenatal Diagnosis 1997;17(2):135‐9. [DOI] [PubMed] [Google Scholar]
Kotaska 2007 {published data only}
- Kotaska A. Prenatal screening for fetal aneuploidy. Journal of Obstetrics & Gynaecology Canada: JOGC 2007;29(6):499‐500. [DOI] [PubMed] [Google Scholar]
Kramer 1998 {published data only}
- Kramer RL, Yaron Y, O'Brien JE, Critchfield G, Ayoub M, Johnson MP, et al. Effect of adjustment of maternal serum alpha‐fetoprotein levels in insulin‐dependent diabetes mellitus. American Journal of Medical Genetics 1998;75(2):176‐8. [PubMed] [Google Scholar]
Krantz 1996 {published data only}
- Krantz DA, Larsen JW, Buchanan PD, Macri JN. First‐trimester Down syndrome screening: free ß‐human chorionic gonadotropin and pregnancy‐associated plasma protein A. American Journal of Obstetrics and Gynecology 1996;174(2):612‐6. [DOI] [PubMed] [Google Scholar]
Krantz 2005 {published data only}
- Krantz DA, Hallahan TW, Macri VJ, Macri JN. Maternal weight and ethnic adjustment within a first‐trimester Down syndrome and trisomy 18 screening program. Prenatal Diagnosis 2005;25(8):635‐40. [DOI] [PubMed] [Google Scholar]
Krantz 2007 {published data only}
- Krantz DA, Hallahan TW, Macri VJ, Macri JN. Genetic sonography after first‐trimester Down syndrome screening. Ultrasound in Obstetrics & Gynecology 2007;29(6):666‐70. [DOI] [PubMed] [Google Scholar]
Kulch 1993 {published data only}
- Kulch P, Keener S, Matsumoto M, Crandall BF. Racial differences in maternal serum human chorionic gonadotropin and unconjugated oestriol levels. Prenatal Diagnosis 1993;13(3):191‐5. [DOI] [PubMed] [Google Scholar]
Lai 1998 {published data only}
- Lai FM, Yeo GS. Down syndrome screening in Singapore‐‐the effectiveness of a second trimester serum screening policy modelled on 29,360 pregnancies in KK Women's and Children's Hospital. Singapore Medical Journal 1998;39(2):69‐75. [PubMed] [Google Scholar]
Lai 2003 {published data only}
- Lai TH, Chen SC, Tsai MS, Lee FK, Wei CF. First‐trimester screening for Down syndrome in singleton pregnancies achieved by intrauterine insemination. Journal of Assisted Reproduction and Genetics 2003;20(8):327‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laigaard 2006a {published data only}
- Laigaard J, Cuckle H, Wewer UM, Christiansen M. Maternal serum ADAM12 levels in Down and Edwards' syndrome pregnancies at 9‐12 weeks' gestation. Prenatal Diagnosis 2006;26(8):689‐91. [DOI] [PubMed] [Google Scholar]
Laigaard 2006b {published data only}
- Laigaard J, Spencer K, Christiansen M, Cowans NJ, Larsen SO, Pedersen BN, et al. ADAM 12 as a first‐trimester maternal serum marker in screening for Down syndrome. Prenatal Diagnosis 2006;26(10):973‐9. [DOI] [PubMed] [Google Scholar]
Lam 1997 {published data only}
- Lam YH, Tang MH, Tang LC, Lee CP, Ho PK. Second‐trimester maternal urinary gonadotrophin peptide screening for fetal Down syndrome in Asian women. Prenatal Diagnosis 1997;17(12):1101‐6. [PubMed] [Google Scholar]
Lam 1998 {published data only}
- Lam YH, Ghosh A, Tang MH, Tang LC, Lee CP, Sin SY, et al. Second‐trimester maternal serum alpha‐fetoprotein and human chorionic gonadotrophin screening for Down's syndrome in Hong Kong. Prenatal Diagnosis 1998;18(6):585‐9. [PubMed] [Google Scholar]
Lam 1999a {published data only}
- Lam YH, Yeung WS, Tang MH, Ng EH, So WW, Ho PC. Maternal serum alpha‐fetoprotein and human chorionic gonadotrophin in pregnancies conceived after intracytoplasmic sperm injection and conventional in‐vitro fertilization. Human Reproduction 1999;14(8):2120‐3. [DOI] [PubMed] [Google Scholar]
Lam 1999b {published data only}
- Lam YH, Tang MH. Second‐trimester maternal serum inhibin‐A screening for fetal Down syndrome in Asian women. Prenatal Diagnosis 1999;19(5):463‐7. [DOI] [PubMed] [Google Scholar]
Lam 2000 {published data only}
- Lam YH, Tang MH, Lee CP, Sin SY, Tang R, Wong HS, et al. Acceptability of serum screening as an alternative to cytogenetic diagnosis of down syndrome among women 35 years or older in Hong Kong. Prenatal Diagnosis 2000;20(6):487‐90. [DOI] [PubMed] [Google Scholar]
Lam 2001 {published data only}
- Lam YH, Tang MH. The effect of fetal gender on second‐trimester maternal serum inhibin‐A concentration. Prenatal Diagnosis 2001;21(8):662‐4. [PubMed] [Google Scholar]
Lambert‐Messerlian 1996 {published data only}
- Lambert‐Messerlian GM, Canick JA, Palomaki GE, Schneyer AL. Second trimester levels of maternal serum inhibin A, total inhibin, alpha inhibin precursor, and activin in Down's syndrome pregnancy. Journal of Medical Screening 1996;3(2):58‐62. [DOI] [PubMed] [Google Scholar]
Lambert‐Messerlian 1998 {published data only}
- Lambert‐Messerlian GM, Luisi S, Florio P, Mazza V, Canick JA, Petraglia F. Second trimester levels of maternal serum total activin A and placental inhibin/activin alpha and ßA subunit messenger ribonucleic acids in Down syndrome pregnancy. European Journal of Endocrinology 1998;138(4):425‐9. [DOI] [PubMed] [Google Scholar]
Lauria 2007 {published data only}
- Lauria MR, Branch MD, LaCroix VH, Harris RD, Baker ER. Clinical impact of systematic genetic sonogram screening in a low‐risk population. Journal of Reproductive Medicine 2007;52(5):359‐64. [PubMed] [Google Scholar]
Lehavi 2005 {published data only}
- Lehavi O, Aizenstein O, Evans MI, Yaron Y. 2nd‐trimester maternal serum human chorionic gonadotropin and alpha‐fetoprotein levels in male and female fetuses with Down syndrome. Fetal Diagnosis and Therapy 2005;20(3):235‐8. [DOI] [PubMed] [Google Scholar]
Leung 2006 {published data only}
- Leung TY, Spencer K, Leung TN, Fung TY, Lau TK. Higher median levels of free ß‐hCG and PAPP‐A in the first trimester of pregnancy in a Chinese ethnic group. Implication for first trimester combined screening for Down's syndrome in the Chinese population. Fetal Diagnosis and Therapy 2006;21(1):140‐3. [DOI] [PubMed] [Google Scholar]
Leymarie 1993 {published data only}
- Leymarie P, Leporrier N. Maternal serum markers and prenatal screening for Down syndrome. Archives Francaises de Pediatrie 1993;50(5):455‐7. [PubMed] [Google Scholar]
Li 1998 {published data only}
- Li G, Huang X. [Clinical uses of maternal serum markers in the prenatal diagnosis] [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih 1998;33(4):252‐4. [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li W, Zhou Y. [Measurement of pregnancy‐associated plasma protein A in maternal peripheral blood and Down syndrome] [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih 1999;34(10):631‐3. [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li HW, Hui PW, Tang MH, Lau ET, Yeung WS, Ho PC, et al. Maternal serum anti‐Mullerian hormone level is not superior to chronological age in predicting Down syndrome pregnancies. Prenatal Diagnosis 2010;30(4):320‐4. [DOI] [PubMed] [Google Scholar]
Liao 1997 {published data only}
- Liao S, Wang Y, Ye G. [AFP, uE3, ß‐hCG levels applied for prenatal diagnosis of Down's syndrome]. [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih 1997;32(11):655‐8. [PubMed] [Google Scholar]
Liao 2001 {published data only}
- Liao AW, Heath V, Kametas N, Spencer K, Nicolaides KH. First‐trimester screening for trisomy 21 in singleton pregnancies achieved by assisted reproduction. Human Reproduction 2001;16(7):1501‐4. [DOI] [PubMed] [Google Scholar]
Lim 2002 {published data only}
- Lim KI, Pugash D, Dansereau J, Wilson RD. Nuchal index: a gestational age independent ultrasound marker for the detection of Down syndrome. Prenatal Diagnosis 2002;22(13):1233‐7. [DOI] [PubMed] [Google Scholar]
Lippman 1987 {published data only}
- Lippman A, Evans JA. Screening for maternal serum alpha‐fetoprotein: what about the low side?. Canadian Medical Association Journal 1987;136(8):801‐4. [PMC free article] [PubMed] [Google Scholar]
Liu 2003 {published data only}
- Liu JT, Hao N, Sun NH, Wang FY, Xu YH, Gai MY, et al. [Screening by maternal serum markers for Down's syndrome]. [Chinese]. Chung‐Kuo i Hsueh Ko Hsueh Yuan Hsueh Pao Acta Academiae Medicinae Sinicae 2003;25(2):156‐9. [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu YH, Li LF, Wu YM. [Analysis of Down syndrome screening by maternal serum detection in mid‐pregnancy]. [Chinese]. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University 2010;30(3):532‐4. [PubMed] [Google Scholar]
Lo 2010 {published data only}
- Lo TK, Lai FK, Leung WC, Lau WL, Tang LC, Chin RK. A new policy for prenatal screening and diagnosis of Down syndrome for pregnant women with advanced maternal age in a public hospital. Journal of Maternal‐fetal & Neonatal Medicine 2010;23(8):914‐9. [DOI] [PubMed] [Google Scholar]
Lustig 1988 {published data only}
- Lustig L, Clarke S, Cunningham G, Schonberg R, Tompkinson G. California's experience with low MS‐AFP results. American Journal of Medical Genetics 1988;31(1):211‐22. [DOI] [PubMed] [Google Scholar]
Luthgens 2008 {published data only}
- Luthgens K. Comparison of the new PRC software with the established algorithm of the FMF UK for the detection of trisomy 21 and 18/13. Fetal Diagnosis and Therapy 2008;24(4):376‐84. [DOI] [PubMed] [Google Scholar]
MacDonald 1991 {published data only}
- MacDonald ML, Wagner RM, Slotnick RN. Sensitivity and specificity of screening for Down syndrome with alpha‐fetoprotein, hCG, unconjugated estriol, and maternal age.[see comment]. Obstetrics and Gynecology 1991;77(1):63‐8. [PubMed] [Google Scholar]
Macintosh 1994 {published data only}
- Macintosh MCM, Iles R, Teisner B, Sharma K, Chard T, Grudzinskas J, et al. Maternal serum human chorionic gonadotrophin and pregnancy‐associated plasma protein A, markers for fetal Down syndrome at 8‐14 weeks. Prenatal Diagnosis 1994;14(3):203‐8. [DOI] [PubMed] [Google Scholar]
Macintosh 1997 {published data only}
- Macintosh MCM, Nicolaides KH, Noble P, Chard T, Gunn L, Iles R. Urinary ß‐core hCG: Screening for aneuploidies in early pregnancy (11‐14 weeks' gestation). Prenatal Diagnosis 1997;17(5):401‐5. [PubMed] [Google Scholar]
MacRae 2010 {published data only}
- MacRae AR, Chodirker BN, Davies GA, Palomaki GE, Knight GJ, Minett J, et al. Second and first trimester estimation of risk for Down syndrome: implementation and performance in the SAFER study. Prenatal Diagnosis 2010;30(5):459‐66. [DOI] [PubMed] [Google Scholar]
Macri 1994 {published data only}
- Macri JN, Kasturi RV, Krantz DA, Cook EJ, Moore ND, Young JA, et al. Maternal serum Down syndrome screening: free ß‐protein is a more effective marker than human chorionic gonadotropin.[see comment]. American Journal of Obstetrics and Gynecology 1990;163(4):1248‐53. [DOI] [PubMed] [Google Scholar]
- Macri JN, Spencer K, Garver K, Buchanan PD, Say B, Carpenter NJ, et al. Maternal serum free ß hCG screening: results of studies including 480 cases of Down syndrome.[see comment]. Prenatal Diagnosis 1994;14(2):97‐103. [DOI] [PubMed] [Google Scholar]
- Spencer K, Macri JN. Early detection of Down's syndrome using free ß human choriogonadotropin. Annals of Clinical Biochemistry 1992;19(3):349‐50. [DOI] [PubMed] [Google Scholar]
Macri 1996 {published data only}
- Macri JN, Anderson RW, Krantz DA, Larsen JW, Buchanan PD. Prenatal maternal dried blood screening with alpha‐fetoprotein and free ß‐human chorionic gonadotropin for open neural tube defect and Down syndrome. American Journal of Obstetrics and Gynecology 1996;174(2):566‐72. [DOI] [PubMed] [Google Scholar]
Malone 1998 {published data only}
- Malone FD, D'Alton ME. Ultrasound clinics. Fetal nuchal fold translucency screening. Contemporary OB/GYN 1998;43(3):117‐8. [Google Scholar]
Malone 2003 {published data only}
- Malone FD, D'Alton ME. First‐trimester sonographic screening for Down syndrome. Obstetrics and Gynecology 2003;102(5):1066‐79. [DOI] [PubMed] [Google Scholar]
Mandryka‐Stankewycz 2009 {published data only}
- Mandryka‐Stankewycz S, Perenc M, Dec G, Sieroszewski P. [Noninvasive prenatal test in the first trimester of pregnancy (NT and estimation of beta‐hCG and PAPP‐A) in the diagnosis of fetal abnormalities in Polish population‐‐comparison of the biochemistry own normal ranges and literature reported data]. [Polish]. Ginekologia Polska 2009;80(11):851‐5. [PubMed] [Google Scholar]
Mangione 2001 {published data only}
- Mangione R, Guyon F, Taine L, Wen ZQ, Roux D, Vergnaud A, et al. Pregnancy outcome and prognosis in fetuses with increased first‐trimester nuchal translucency. Fetal Diagnosis and Therapy 2001;16(6):360‐3. [DOI] [PubMed] [Google Scholar]
Markov 2008 {published data only}
- Markov D, Dimitrova V. [Ultrasound screening for chromosomal anomalies by assessment of the fetal nasal bone during 11‐14 weeks of gestation‐‐a pilot study]. [Bulgarian]. Akusherstvo i Ginekologiia 2008;47(1):3‐9. [PubMed] [Google Scholar]
Maymon 2001a {published data only}
- Maymon R, Shulman A. Comparison of triple serum screening and pregnancy outcome in oocyte donation versus IVF pregnancies. Human Reproduction 2001;16(4):691‐5. [DOI] [PubMed] [Google Scholar]
Maymon 2001b {published data only}
- Maymon R, Dreazen E, Buckovsky I, Weinraub Z, Herman A. Does a 'notched' nuchal translucency indicate Down syndrome fetuses or other adverse pregnancy outcome?. Prenatal Diagnosis 2001;21(5):403‐8. [DOI] [PubMed] [Google Scholar]
Maymon 2002 {published data only}
- Maymon R, Shulman A. Serial first‐ and second‐trimester Down's syndrome screening tests among IVF‐versus naturally‐conceived singletons. Human Reproduction 2002;17(4):1081‐5. [DOI] [PubMed] [Google Scholar]
Maymon 2004 {published data only}
- Maymon R, Shulman A. Integrated first‐ and second‐trimester Down syndrome screening test among unaffected IVF pregnancies. Prenatal Diagnosis 2004;24(2):125‐9. [DOI] [PubMed] [Google Scholar]
Maymon 2005 {published data only}
- Maymon R, Cuckle H, Jones R, Reish O, Sharony R, Herman A. Predicting the result of additional second‐trimester markers from a woman's first‐trimester marker profile: a new concept in Down syndrome screening. Prenatal Diagnosis 2005;25(12):1102‐6. [DOI] [PubMed] [Google Scholar]
McDuffie 1996 {published data only}
- McDuffie RS Jr, Haverkamp AD, Stark CF, Haverkamp C, Barth CK. Prenatal screening using maternal serum alpha‐fetoprotein, human chorionic gonadotropin, and unconjugated estriol: two‐year experience in a health maintenance organization. Journal of Maternal‐fetal Medicine 1996;5(2):70‐3. [DOI] [PubMed] [Google Scholar]
Meier 2002 {published data only}
- Meier C, Huang T, Wyatt PR, Summers AM. Accuracy of expected risk of Down syndrome using the second‐trimester triple test. Clinical Chemistry 2002;48(4):653‐5. [PubMed] [Google Scholar]
Merkatz 1984 {published data only}
- Merkatz IR, Nitowsky HM, Macri JN, Johnson WE. An association between low maternal serum alpha‐fetoprotein and fetal chromosomal abnormalities. American Journal of Obstetrics and Gynecology 1984;148(7):886‐94. [DOI] [PubMed] [Google Scholar]
Merz 2005 {published data only}
- Merz E. The fetal nasal bone in the first trimester ‐ Precise assessment using 3D sonography. Ultraschall in der Medizin 2005;26(5):365‐6. [DOI] [PubMed] [Google Scholar]
Merz 2008 {published data only}
- Merz E, Thode C, Alkier A, Eiben B, Hackeloer BJ, Hansmann M, et al. A new approach to calculating the risk of chromosomal abnormalities with first‐trimester screening data. Ultraschall in der Medizin 2008;29(6):639‐45. [DOI] [PubMed] [Google Scholar]
Metzenbauer 2001 {published data only}
- Metzenbauer M, Hafner E, Hoefinger D, Schuchter K, Stangl G, Ogris E, et al. Three‐dimensional ultrasound measurement of the placental volume in early pregnancy: method and correlation with biochemical placenta parameters. Placenta 2001;22(6):602‐5. [DOI] [PubMed] [Google Scholar]
Metzenbauer 2002 {published data only}
- Metzenbauer M, Hafner E, Schuchter K, Philipp K. First‐trimester placental volume as a marker for chromosomal anomalies: preliminary results from an unselected population. Ultrasound in Obstetrics & Gynecology 2002;19(3):240‐2. [DOI] [PubMed] [Google Scholar]
Mikic 1999 {published data only}
- Mikic TS, Johnson P. Second trimester maternal serum ß human chorionic gonadotrophin and pregnancy outcome. British Journal of Obstetrics and Gynaecology 1999;106(6):598‐600. [DOI] [PubMed] [Google Scholar]
Miller 1991 {published data only}
- Miller CH, O'Brien TJ, Chatelain S, Butler BB, Quirk JG. Alteration in age‐specific risks for chromosomal trisomy by maternal serum alpha‐fetoprotein and human chorionic gonadotropin screening. Prenatal Diagnosis 1991;11(3):153‐8. [DOI] [PubMed] [Google Scholar]
Milunsky 1989 {published data only}
- Milunsky A, Jick SS, Bruell CL, Maclaughlin DS, Tsung Y‐K, Jick H, et al. Predictive values relative risks and overall benefits of high and low maternal serum alpha fetoprotein screening in singleton pregnancies ‐ new epidemiological data. American Journal of Obstetrics and Gynecology 1989;161(2):291‐7. [DOI] [PubMed] [Google Scholar]
Milunsky 1996 {published data only}
- Milunsky A, Nebiolo L. Maternal serum triple analyte screening and adverse pregnancy outcome. Fetal Diagnosis and Therapy 1996;11(4):249‐53. [DOI] [PubMed] [Google Scholar]
Minobe 2002 {published data only}
- Minobe S. [A study on the screening of prenatal trisomy 21 using the fucosylated alpha‐fetoprotein ratio measured by a liquid‐phase binding assay]. [Japanese]. Hokkaido Igaku Zasshi ‐ Hokkaido Journal of Medical Science 2002;77(6):527‐32. [PubMed] [Google Scholar]
Miron 2008 {published data only}
- Miron P, Cote YP, Lambert J. Effect of maternal smoking on prenatal screening for Down syndrome and trisomy 18 in the first trimester of pregnancy. Prenatal Diagnosis 2008;28(3):180‐5. [DOI] [PubMed] [Google Scholar]
Miron 2009 {published data only}
- Miron P, Cote YP, Lambert J. Nuchal translucency thresholds in prenatal screening for Down syndrome and trisomy 18. Journal of Obstetrics & Gynaecology Canada: JOGC 2009;31(3):227‐35. [DOI] [PubMed] [Google Scholar]
Miron 2010 {published data only}
- Miron P, Lambert J, Marcil A, Cowans NJ, Stamatopoulou A, Spencer K. Maternal plasma levels of follistatin‐related gene protein in the first trimester of pregnancies with Down syndrome. Prenatal Diagnosis 2010;30(3):224‐8. [DOI] [PubMed] [Google Scholar]
Miyamura 1999 {published data only}
- Miyamura T, Saito N, Touno A, Nagata S, Hidaki T, Ishimaru T, et al. Multicenter study for maternal serum triple markers to establish Japanese standards: Maternal serum marker study group, Japan Association of Prenatal Diagnostics. Acta Obstetrica et Gynaecologica Japonica 1999;51(11):1042‐8. [Google Scholar]
Moghadam 1998 {published data only}
- Moghadam S, Engel W, Bougoussa M, Hennen G, Igout A, Sancken U. Maternal serum placental growth hormone and insulinlike growth factor binding proteins 1 and 3 in pregnancies affected by fetal aneuploidy and other abnormalities: implications for prenatal diagnosis of trisomy 21. Fetal Diagnosis and Therapy 1998;13(5):291‐7. [DOI] [PubMed] [Google Scholar]
Monni 2000 {published data only}
- Monni G, Zoppi MA, Ibba RM, Putzolu M, Floris M. Nuchal translucency in multiple pregnancies. Croatian Medical Journal 2000;41(3):266‐9. [PubMed] [Google Scholar]
Monni 2002 {published data only}
- Monni G, Zoppi MA. New ultrasonographic markers of aneuploidies: nasal bones. Ultrasound Review of Obstetrics and Gynecology 2002;2(4):229‐34. [Google Scholar]
Mooney 1994 {published data only}
- Mooney RA, Peterson CJ, French CA, Saller DN Jr, Arvan DA. Effectiveness of combining maternal serum alpha‐fetoprotein and hCG in a second‐trimester screening program for Down syndrome. Obstetrics and Gynecology 1994;84(2):298‐303. [PubMed] [Google Scholar]
Muhcu 2008 {published data only}
- Muhcu M, Mungen E, Atay V, Ipcioglu OM, Dundar O, Ergur R, et al. First trimester screening for Down syndrome in rhesus negative women. Prenatal Diagnosis 2008;28(5):404‐7. [DOI] [PubMed] [Google Scholar]
Muller 1994 {published data only}
- Muller F, Bussieres L, Pelissier MC, Oury JF, Boue C, Uzan S, et al. Do racial differences exist in second‐trimester maternal hCG levels? A study of 23,369 women. Prenatal Diagnosis 1994;14(7):633‐6. [DOI] [PubMed] [Google Scholar]
Muller 1996 {published data only}
- Muller F, Dommergues M, Bussieres L, Aegerter P, Fiblec B, Uzan S, et al. Prenatal screening for Down syndrome: should first trimester ultrasound replace maternal serum screening?. Early Human Development 1996;47 Suppl:S37‐S39. [DOI] [PubMed] [Google Scholar]
Muller 1999 {published data only}
- Muller F, Ngo S, Rebiffe M, Oury JF, Uzan S, Satge D. Maternal serum s100b protein is ineffective for Down syndrome screening. Prenatal Diagnosis 1999;19(11):1086. [DOI] [PubMed] [Google Scholar]
Muller 2002a {published data only}
- Muller F, Dreux S, Oury JF, Luton D, Uzan S, Uzan M, et al. Down syndrome maternal serum marker screening after 18 weeks' gestation. Prenatal Diagnosis 2002;22(11):1001‐4. [DOI] [PubMed] [Google Scholar]
Muller 2002b {published data only}
- Muller F, Forestier F, Dingeon B, for the ABA Study Group. Second trimester trisomy 21 maternal serum marker screening. Results of a countrywide study of 854,902 women. Prenatal Diagnosis 2002;22(10):925‐9. [DOI] [PubMed] [Google Scholar]
Muller 2003b {published data only}
- Muller F, Dreux S, Lemeur A, Sault C, Desgres J, Bernard MA, et al. Medically assisted reproduction and second‐trimester maternal serum marker screening for Down syndrome. Prenatal Diagnosis 2003;23(13):1073‐6. [DOI] [PubMed] [Google Scholar]
Murta 2002 {published data only}
- Murta CG, Moron AF, Avila MA, Weiner CP. Application of ductus venosus Doppler velocimetry for the detection of fetal aneuploidy in the first trimester of pregnancy. Fetal Diagnosis and Therapy 2002;17(5):308‐14. [DOI] [PubMed] [Google Scholar]
Musone 2000 {published data only}
- Musone R, Bonafiglia R, Menditto A, Paccone M, Cassese E, Russo G, et al. Fetuses with cystic hygroma. A retrospective study. Panminerva Medica 2000;42(1):39‐43. [PubMed] [Google Scholar]
Musto 1986 {published data only}
- Musto JD, Pizzolante JM, Chesarone VP, Sassi AM, Sane R. Alpha‐fetoprotein: an enhanced‐sensitivity assay for neural tube defect and Down syndrome evaluation. Clinical Chemistry 1986;32(7):1412. [PubMed] [Google Scholar]
Myrick 1990 {published data only}
- Myrick JE, Caudill SP, Hubert IL, Robinson MK, Adams MJ Jr, Pueschel SM. Identification of haptoglobin alpha‐2FF variants in mid‐trimester maternal serum as potential markers for Down syndrome. Applied and Theoretical Electrophoresis 1990;1(5):233‐41. [PubMed] [Google Scholar]
Naidoo 2008 {published data only}
- Naidoo P, Erasmus I, Jeebodh J, Nicolaou E, Gelderen CJ. Nuchal translucency as a method of first‐trimester screening for aneuploidy. South African Medical Journal 2008;Suid‐Afrikaanse Tydskrif Vir Geneeskunde. 98(4):295‐9. [PubMed] [Google Scholar]
Nau 2009a {published data only}
- Nau JY. [Screening for trisomy 21 in France]. [French]. Revue Medicale Suisse 2009;5(211):1531. [PubMed] [Google Scholar]
Nau 2009b {published data only}
- Nau JY. [Trisomy 21, after a half century]. [French]. Revue Medicale Suisse 2009;5(190):380. [PubMed] [Google Scholar]
Neveux 1996a {published data only}
- Neveux LM, Palomaki GE, Larrivee DA, Knight GJ, Haddow JE. Refinements in managing maternal weight adjustment for interpreting prenatal screening results. Prenatal Diagnosis 1996;16(12):1115‐9. [DOI] [PubMed] [Google Scholar]
Neveux 1996b {published data only}
- Neveux LM, Palomaki GE, Knight GJ, Haddow JE. Multiple marker screening for Down syndrome in twin pregnancies. Prenatal Diagnosis 1996;16(1):29‐34. [DOI] [PubMed] [Google Scholar]
Ng 2004 {published data only}
- Ng EK, El‐Sheikhah A, Chiu RW, Chan KC, Hogg M, Bindra R, et al. Evaluation of human chorionic gonadotropin ß‐subunit mRNA concentrations in maternal serum in aneuploid pregnancies: a feasibility study. Clinical Chemistry 2004;50(6):1055‐7. [DOI] [PubMed] [Google Scholar]
Nicolaides 1992 {published data only}
- Nicolaides KH, ZAR G, Snijders RJM, Gosden CM. Fetal nuchal oedema associated malformations and chromosomal defects. Fetal Diagnosis and Therapy 1992;7(2):123‐31. [DOI] [PubMed] [Google Scholar]
Nicolaides 2000 {published data only}
- Nicolaides KH, Cicero S, Liao AW. One‐stop clinic for assessment of risk of chromosomal defects at 12 weeks of gestation. Prenatal and Neonatal Medicine 2000;5(3):145‐54. [DOI] [PubMed] [Google Scholar]
Nicolaides 2004 {published data only}
- Nicolaides KH. Nuchal translucency and other first‐trimester sonographic markers of chromosomal abnormalities. American Journal of Obstetrics and Gynecology 2004;191(1):45‐67. [DOI] [PubMed] [Google Scholar]
Nicolaides 2005a {published data only}
- Nicolaides KH, Wegrzyn P. [First trimester diagnosis of chromosomal defects][Polish]. Ginekologia Polska 2005;76(1):1‐8. [PubMed] [Google Scholar]
Nicolaides 2005b {published data only}
- Nicolaides KH, Wegrzyn P. [Sonographic features of chromosomal defects at 11(+0) to 13(+6) weeks of gestation] [Polish]. Ginekologia Polska 2005;76(6):423‐30. [PubMed] [Google Scholar]
Nicolaides 2005c {published data only}
- Nicolaides KH, Wegrzyn P. [Increased nuchal translucency with normal karyotype]. [Polish]. Ginekologia Polska 2005;76(8):593‐601. [PubMed] [Google Scholar]
Nicolaides 2005d {published data only}
- Nicolaides KH, Wegrzyn P. [Fetal nuchal translucency]. [Polish]. Ginekologia Polska 2005;76(3):179‐86. [PubMed] [Google Scholar]
Nicolaides 2005e {published data only}
- Nicolaides KH, Wegrzyn P. [Fetal nuchal translucency thickness and risk for chromosomal defects]. [Polish]. Ginekologia Polska 2005;76(4):257‐63. [PubMed] [Google Scholar]
Nicolaides 2005f {published data only}
- Nicolaides Kypros H. First‐trimester screening for chromosomal abnormalities. Seminars in Perinatology (Philadelphia) 2005;29(4):190‐4. [DOI] [PubMed] [Google Scholar]
Niemimaa 2001b {published data only}
- Niemimaa M, Heinonen S, Seppala M, Hippelainen M, Martikainen H, Ryynanen M. First‐trimester screening for Down's syndrome in in vitro fertilization pregnancies. Fertility and Sterility 2001;76(6):1282‐3. [DOI] [PubMed] [Google Scholar]
Niemimaa 2002 {published data only}
- Niemimaa M, Suonpaa M, Heinonen S, Seppala M, Bloigu R, Ryynanen M. Maternal serum human chorionic gonadotrophin and pregnancy‐associated plasma protein A in twin pregnancies in the first trimester. Prenatal Diagnosis 2002;22(3):183‐5. [PubMed] [Google Scholar]
Niemimaa 2003 {published data only}
- Niemimaa M, Heinonen S, Seppala M, Ryynanen M. The influence of smoking on the pregnancy‐associated plasma protein A, free ß human chorionic gonadotrophin and nuchal translucency. BJOG: an international journal of obstetrics and gynaecology 2003;110(7):664‐7. [PubMed] [Google Scholar]
Noble 1997a {published data only}
- Noble PL, Snijders RJ, Abraha HD, Sherwood RA, Nicolaides KH. Maternal serum free ß‐hCG at 10 to 14 weeks of gestation in trisomic twin pregnancies. British Journal of Obstetrics and Gynaecology 1997;104(6):741‐3. [DOI] [PubMed] [Google Scholar]
Norgaard 1990 {published data only}
- Norgaard Pedersen B, Larsen SO, Arends J, Svenstrup B, Tabor A. Maternal serum markers in screening for Down syndrome. Clinical Genetics 1990;37(1):35‐43. [DOI] [PubMed] [Google Scholar]
Norton 1992 {published data only}
- Norton ME, Golbus MS. Maternal serum CA 125 for aneuploidy detection in early pregnancy. Prenatal Diagnosis 1992;12(9):779‐81. [DOI] [PubMed] [Google Scholar]
Novakov‐Mikic 2007 {published data only}
- Novakov‐Mikic A, Potic Z, Pjevic A. [Ultrasound screening program for chromosomal abnormalities‐‐the first 2000 women]. [Serbian]. Medicinski Pregled 2007;60(1‐2):66‐70. [DOI] [PubMed] [Google Scholar]
O'Brien 1997a {published data only}
- O'Brien JE, Dvorin E, Yaron Y, Ayoub M, Johnson MP, Hume RF Jr, et al. Differential increases in AFP, hCG, and uE3 in twin pregnancies: Impact on attempts to quantify Down syndrome screening calculations. American Journal of Medical Genetics 1997;73(2):109‐12. [DOI] [PubMed] [Google Scholar]
O'Brien 1997b {published data only}
- O'Brien JE, Dvorin E, Drugan A, Johnson MP, Yaron Y, Evans MI. Race‐ethnicity‐specific variation in multiple‐marker biochemical screening: Alpha‐fetoprotein, hCG, and estriol. Obstetrics and Gynecology 1997;89(3):355‐8. [DOI] [PubMed] [Google Scholar]
Odibo 2004 {published data only}
- Odibo AO, Sehdev HM, Dunn L, McDonald R, Macones GA. The association between fetal nasal bone hypoplasia and aneuploidy. Obstetrics and Gynecology 2004;104(6):1229‐33. [DOI] [PubMed] [Google Scholar]
Odibo 2007 {published data only}
- Odibo AO, Sehdev HM, Stamilio DM, Cahill A, Dunn L, Macones GA. Defining nasal bone hypoplasia in second‐trimester Down syndrome screening: does the use of multiples of the median improve screening efficacy?. American Journal of Obstetrics and Gynecology 2007;197(4):361‐4. [DOI] [PubMed] [Google Scholar]
Odibo 2008 {published data only}
- Odibo AO, Sehdev HM, Gerkowicz S, Stamilio DM, Macones GA. Comparison of the efficiency of second‐trimester nasal bone hypoplasia and increased nuchal fold in Down syndrome screening. American Journal of Obstetrics and Gynecology 2008;199(3):281‐5. [DOI] [PubMed] [Google Scholar]
Odibo 2009 {published data only}
- Odibo AO, Schoenborn JA, Haas K, Macones GA. Does the combination of fronto‐maxillary facial angle and nasal bone evaluation improve the detection of Down syndrome in the second trimester?. Prenatal Diagnosis 2009;29(10):947‐51. [DOI] [PubMed] [Google Scholar]
Offerdal 2008 {published data only}
- Offerdal K, Blaas HG, Eik‐Nes SH. Prenatal detection of trisomy 21 by second‐trimester ultrasound examination and maternal age in a non‐selected population of 49 314 births in Norway. Ultrasound in Obstetrics & Gynecology 2008;32(4):493‐500. [DOI] [PubMed] [Google Scholar]
Ognibene 1999 {published data only}
- Ognibene A, Ciuti R, Tozzi P, Messeri G. Maternal serum superoxide dismutase (SOD): a possible marker for screening Down syndrome affected pregnancies.[see comment]. Prenatal Diagnosis 1999;19(11):1058‐60. [PubMed] [Google Scholar]
Oh 2007 {published data only}
- Oh C, Harman C, Baschat AA. Abnormal first‐trimester ductus venosus blood flow: a risk factor for adverse outcome in fetuses with normal nuchal translucency. Ultrasound in Obstetrics & Gynecology 2007;30(2):192‐6. [DOI] [PubMed] [Google Scholar]
Olajide 1989 {published data only}
- Olajide F, Kitau MJ, Chard T. Maternal serum AFP levels in the first trimester of pregnancy. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1989;30(2):123‐8. [DOI] [PubMed] [Google Scholar]
Onda 1996 {published data only}
- Onda T, Kitagawa M, Takeda O, Sago H, Kubonoya K, Iinuma K, et al. Triple marker screening in native Japanese women. Prenatal Diagnosis 1996;16(8):713‐7. [DOI] [PubMed] [Google Scholar]
Onda 1998 {published data only}
- Onda T, Tanaka T, Takeda O, Kitagawa M, Kuwabara Y, Yamamoto H, et al. Agreement between predicted risk and prevalence of Down syndrome in second‐trimester triple‐marker screening in Japan. Prenatal Diagnosis 1998;18(9):956‐8. [PubMed] [Google Scholar]
Onda 2000 {published data only}
- Onda T, Tanaka T, Yoshida K, Nakamura Y, Kudo R, Yamamoto H, et al. Triple marker screening for trisomy 21, trisomy 18 and open neural tube defects in singleton pregnancies of native Japanese pregnant women. Journal of Obstetrics and Gynaecology Research 2000;26(6):441‐7. [DOI] [PubMed] [Google Scholar]
Orlandi 2002 {published data only}
- Orlandi F, Rossi C, Allegra A, Krantz D, Hallahan T, Orlandi E, et al. First trimester screening with free ß‐hCG, PAPP‐A and nuchal translucency in pregnancies conceived with assisted reproduction. Prenatal Diagnosis 2002;22(8):718‐21. [DOI] [PubMed] [Google Scholar]
Ozkaya 2010 {published data only}
- Ozkaya O, Sezik M, Ozbasar D, Kaya H. Abnormal ductus venosus flow and tricuspid regurgitation at 11‐14 weeks' gestation have high positive predictive values for increased risk in first‐trimester combined screening test: results of a pilot study. Taiwanese Journal of Obstetrics & Gynecology 2010;49(2):145‐50. [DOI] [PubMed] [Google Scholar]
Páez 2004 {published data only}
- Páez L, Peña E, González F, Bello F, Bellorín J, Espinoza F, et al. Plasma protein "A" and chorionic gonadotropin at first trimester pregnancy. Informe Medico 2004;6(2):99‐109. [Google Scholar]
Paladini 2007 {published data only}
- Paladini D, Sglavo G, Penner I, Pastore G, Nappi C. Fetuses with Down syndrome have an enlarged anterior fontanelle in the second trimester of pregnancy. Ultrasound in Obstetrics & Gynecology 2007;30(6):824‐9. [DOI] [PubMed] [Google Scholar]
Palka 1998 {published data only}
- Palka G, Guanciali Franchi P, Papponetti M, Marcuccitti J, Morizio E, Calabrese G, et al. Prenatal diagnosis using the triple test. Minerva Ginecologica 1998;50(10):411‐5. [PubMed] [Google Scholar]
Palomaki 1989 {published data only}
- Palomaki GE, Williams J, Haddow JE. Combining maternal serum alpha‐fetoprotein measurements and age to screen for Down syndrome in pregnant women under age 35. American Journal of Obstetrics and Gynecology 1989;160(3):575‐81. [DOI] [PubMed] [Google Scholar]
Palomaki 1993 {published data only}
- Palomaki GE, Knight GJ, Haddow JE, Canick JA, Wald NJ, Kennard A. Cigarette smoking and levels of maternal serum alpha‐fetoprotein, unconjugated estriol, and hCG: Impact on Down syndrome screening. Obstetrics and Gynecology 1993;81(5):675‐8. [PubMed] [Google Scholar]
Palomaki 1994 {published data only}
- Palomaki GE, Knight GJ, Haddow JE. Human chorionic gonadotropin and unconjugated oestriol measurements in insulin‐dependent diabetic pregnant women being screened for fetal Down syndrome. Prenatal Diagnosis 1994;14(1):65‐8. [DOI] [PubMed] [Google Scholar]
Palomaki 1996 {published data only}
- Palomaki GE, Neveux LM, Haddow JE. Can reliable Down's syndrome detection rates be determined from prenatal screening intervention trials?. Journal of Medical Screening 1996;3(1):12‐7. [DOI] [PubMed] [Google Scholar]
Palomaki 2005 {published data only}
- Palomaki GE, Knight GJ, Neveux LM, Pandian R, Haddow JE. Maternal serum invasive trophoblast antigen and first‐trimester Down syndrome screening. Clinical Chemistry 2005;51(8):1499‐504. [DOI] [PubMed] [Google Scholar]
Panburana 2001 {published data only}
- Panburana P, Ajjimakorn S, Tungkajiwangoon P. First trimester Down Syndrome screening by nuchal translucency in a Thai population. International Journal of Gynaecology and Obstetrics 2001;75(3):311‐2. [DOI] [PubMed] [Google Scholar]
Pandya 1994 {published data only}
- Pandya PP, Brizot ML, Kuhn P, Snijders RJ, Nicolaides KH. First‐trimester fetal nuchal translucency thickness and risk for trisomies. Obstetrics and Gynecology 1994;84(3):420‐3. [PubMed] [Google Scholar]
Pandya 1995 {published data only}
- Pandya PP, Santiago C, Snijders RJM, Nicolaides KH. First trimester fetal nuchal translucency. Current Opinion in Obstetrics and Gynecology 1995;7(2):95‐102. [DOI] [PubMed] [Google Scholar]
Papadopoulou 2008 {published data only}
- Papadopoulou E, Sifakis S, Giahnakis E, Fragouli Y, Karkavitsas N, Koumantakis E, et al. Human placental growth hormone is increased in maternal serum in pregnancies affected by Down syndrome. Fetal Diagnosis and Therapy 2008;23(3):211‐6. [DOI] [PubMed] [Google Scholar]
Parra‐Cordero 2007 {published data only}
- Parra‐Cordero M, Quiroz L, Rencoret G, Pedraza D, Munoz H, Soto‐Chacon E, et al. Screening for trisomy 21 during the routine second‐trimester ultrasound examination in an unselected Chilean population. Ultrasound in Obstetrics & Gynecology 2007;30(7):946‐51. [DOI] [PubMed] [Google Scholar]
Paterlini‐Brechot 2007 {published data only}
- Paterlini‐Brechot P. [Non invasive prenatal diagnosis of trisomy 21: dream or reality?]. [French]. Medecine Sciences : M/S 2007;23(6‐7):592‐4. [DOI] [PubMed] [Google Scholar]
Paul 2001 {published data only}
- Paul C, Krampl E, Skentou C, Jurkovic D, Nicolaides KH. Measurement of fetal nuchal translucency thickness by three‐dimensional ultrasound. Ultrasound in Obstetrics & Gynecology 2001;18(5):481‐4. [DOI] [PubMed] [Google Scholar]
Peralta 2005 {published data only}
- Peralta CF, Falcon O, Wegrzyn P, Faro C, Nicolaides KH. Assessment of the gap between the fetal nasal bones at 11 to 13 + 6 weeks of gestation by three‐dimensional ultrasound. Ultrasound in Obstetrics & Gynecology 2005;25(5):464‐7. [DOI] [PubMed] [Google Scholar]
Perenc 1998 {published data only}
- Perenc M, Dudarewicz L, Kaluzewski B. Analysis of triple test results in 27 cases of twin pregnancies. Acta Geneticae Medicae et Gemellologiae 1998;47(3‐4):249‐54. [DOI] [PubMed] [Google Scholar]
Perheentupa 2002 {published data only}
- Perheentupa A, Ruokonen A, Tuomivaara L, Ryynänen M, Martikainen H. Maternal serum (ß)‐HCG and (alpha)‐fetoprotein concentrations in singleton pregnancies following assisted reproduction. Human Reproduction 2002;17(3):794‐7. [DOI] [PubMed] [Google Scholar]
Perona 1998 {published data only}
- Perona M, Mancini G, Dall'Amico D, Guaraldo V, Carbonara A. Influence of smoking habits on Down's syndrome risk evaluation at mid‐trimester through biochemical screening. International Journal of Clinical & Laboratory Research 1998;28(3):179‐82. [DOI] [PubMed] [Google Scholar]
Persico 2008 {published data only}
- Persico N, Borenstein M, Molina F, Azumendi G, Nicolaides KH. Prenasal thickness in trisomy‐21 fetuses at 16‐24 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2008;32(6):751‐4. [DOI] [PubMed] [Google Scholar]
Petervari 2000 {published data only}
- Petervari L, Varga A, Tanko A, Szabo L, Godo G. [Significance of nuchal edema in fetuses of pregnant women under 35 years of age]. [Hungarian]. Orvosi Hetilap 2000;141(8):399‐402. [PubMed] [Google Scholar]
Petrocik 1989 {published data only}
- Petrocik E, Wassman ER, Kelly JC. Prenatal screening for Down syndrome with maternal serum human chorionic gonadotropin levels.[see comment]. American Journal of Obstetrics and Gynecology 1989;161(5):1168‐73. [DOI] [PubMed] [Google Scholar]
Phillips 1992 {published data only}
- Phillips OP, Elias S, Shulman LP, Andersen RN, Morgan CD, Simpson JL. Maternal serum screening for fetal Down syndrome in women less than 35 years of age using alpha‐fetoprotein, hCG, and unconjugated estriol: a prospective 2‐year study. Obstetrics and Gynecology 1992;80(3):353‐8. [PubMed] [Google Scholar]
Phillips 1993 {published data only}
- Phillips OP, Shulman LP, Elias S, Simpson JL. Maternal serum screening for fetal Down syndrome using alpha‐ fetoprotein, human chorionic gonadotrophin, and unconjugated estriol in adolescents. Adolescent and Pediatric Gynecology 1993;6(2):91‐4. [Google Scholar]
Pihl 2008 {published data only}
- Pihl K, Larsen T, Jonsson L, Hougaard D, Krebs L, Norgaard‐Pedersen B, et al. [Quality control of prenatal screening]. [Danish]. Ugeskrift for Laeger 2008;170(35):2691‐5. [PubMed] [Google Scholar]
Pinette 2003 {published data only}
- Pinette MG, Egan JF, Wax JR, Blackstone J, Cartin A, Benn PA. Combined sonographic and biochemical markers for Down syndrome screening. Journal of Ultrasound in Medicine 2003;22(11):1185‐90. [DOI] [PubMed] [Google Scholar]
Platt 2004 {published data only}
- Platt LD, Greene N, Johnson A, Zachary J, Thom E, Krantz D, et al. Sequential pathways of testing after first‐trimester screening for trisomy 21. Obstetrics and Gynecology 2004;104(4):661‐6. [DOI] [PubMed] [Google Scholar]
Podobnik 1995 {published data only}
- Podobnik M, Singer Z, Podobnik‐Sarkanji S, Bulic M. First trimester diagnosis of cystic hygromata using transvaginal ultrasound and cytogenetic evaluation. Journal of Perinatal Medicine 1995;23(4):283‐91. [DOI] [PubMed] [Google Scholar]
Poon 2009 {published data only}
- Poon LC, Chelemen T, Minekawa R, Frisova V, Nicolaides KH. Maternal serum ADAM12 (A disintegrin and metalloprotease) in chromosomally abnormal pregnancy at 11‐13 weeks. American Journal of Obstetrics and Gynecology 2009;200(5):508‐6. [DOI] [PubMed] [Google Scholar]
Prefumo 2002 {published data only}
- Prefumo F, Thilaganathan B. Agreement between predicted risk and prevalence of Down syndrome in first trimester nuchal translucency screening. Prenatal Diagnosis 2002;22(10):917‐8. [DOI] [PubMed] [Google Scholar]
Prefumo 2004 {published data only}
- Prefumo F, Sairam S, Bhide A, Penna L, Hollis B, Thilaganathan B. Maternal ethnic origin and fetal nasal bones at 11‐14 weeks of gestation. BJOG: an international journal of obstetrics and gynaecology. 2004;111(2):109‐12. [DOI] [PubMed] [Google Scholar]
Price 1998 {published data only}
- Price KM, Lith JM, Silman R, Mantingh A, Grudzinskas JG. First trimester maternal serum concentrations of fetal antigen 2 in normal pregnancies and those affected by trisomy 21. Human Reproduction 1998;13(6):1706‐8. [DOI] [PubMed] [Google Scholar]
Raty 2000 {published data only}
- Raty R, Virtanen A, Koskinen P, Laitinen P, Forsstrom J, Salonen R, et al. Maternal midtrimester serum AFP and free ß‐hCG levels in in vitro fertilization twin pregnancies. Prenatal Diagnosis 2000;20(3):221‐3. [PubMed] [Google Scholar]
Räty 2002 {published data only}
- Räty R, Virtanen A, Koskinen P, Anttila L, Forsström J, Laitinen P, et al. Serum free (ß)‐HCG and alpha‐fetoprotein levels in IVF, ICSI and frozen embryo transfer pregnancies in maternal mid‐trimester serum screening for Down's syndrome. Human Reproduction 2002;17(2):481‐4. [DOI] [PubMed] [Google Scholar]
Rembouskos 2004 {published data only}
- Rembouskos G, Cicero S, Longo D, Vandecruys H, Nicolaides KH. Assessment of the fetal nasal bone at 11‐14 weeks of gestation by three‐dimensional ultrasound. Ultrasound in Obstetrics & Gynecology 2004;23(3):232‐6. [DOI] [PubMed] [Google Scholar]
Ren 1992 {published data only}
- Ren S‐G, Braunstein GD. Human chorionic gonadotropin. Seminars in Reproductive Endocrinology 1992;10(2):95‐105. [Google Scholar]
Renier 1998 {published data only}
- Renier MA, Vereecken A, Herck E, Straetmans D, Ramaekers P, Buytaert P. Second trimester maternal dimeric inhibin‐A in the multiple‐marker screening test for Down's syndrome. Human Reproduction 1998;13(3):744‐8. [DOI] [PubMed] [Google Scholar]
Resta 1990 {published data only}
- Resta RG, Nyberg D. The role of ultrasound in screening for Down syndrome. Birth Defects: Original Article Series 1990;26(3):104. [PubMed] [Google Scholar]
Reynders 1997 {published data only}
- Reynders CS, Pauker SP, Benacerraf BR. First trimester isolated fetal nuchal lucency: significance and outcome. Journal of Ultrasound in Medicine 1997;16(2):101‐5. [DOI] [PubMed] [Google Scholar]
Reynolds 1989 {published data only}
- Reynolds TM, Penney MD. The mathematical basis of multivariate risk screening: with special reference to screening for Down's syndrome associated pregnancy. Annals of Clinical Biochemistry 1989;27(5):452‐8. [DOI] [PubMed] [Google Scholar]
Reynolds 1999 {published data only}
- Reynolds TM, Schaeffer HJ, Schlensker S. Estimation of Down's syndrome risks in the first trimester of pregnancy: Experience of testing with PAPP‐A, total hCG and free ß‐ hCG levels in maternal blood samples in a German population. Clinical Laboratory 1999;45(1‐2):49‐53. [Google Scholar]
Reynolds 2008 {published data only}
- Reynolds TM, Aldis J. Median parameters for Down's syndrome screening should be calculated using a moving time‐window method. Annals of Clinical Biochemistry 2008;45(Pt 6):567‐70. [DOI] [PubMed] [Google Scholar]
Ribbert 1996 {published data only}
- Ribbert LS, Kornman LH, Wolf BT, Simons AH, Jansen CA, Beekhuis JR, et al. Maternal serum screening for fetal Down syndrome in IVF pregnancies. Prenatal Diagnosis 1996;16(1):35‐8. [DOI] [PubMed] [Google Scholar]
Rice 2005 {published data only}
- Rice JD, McIntosh SF, Halstead AC. Second‐trimester maternal serum screening for Down syndrome in in vitro fertilization pregnancies. Prenatal Diagnosis 2005;25(3):234‐8. [DOI] [PubMed] [Google Scholar]
Rich 1991 {published data only}
- Rich N, Boots L, Davis R, Finley S. Efficiency of maternal serum hCG AFP and free estriol in the identification of trisomy 21 and other complications of pregnancy. Journal of the Alabama Academy of Science 1991;62(2‐3):135. [Google Scholar]
Roberts 1995 {published data only}
- Roberts LJ, Bewley S, Mackinson AM, Rodeck CH. First trimester fetal nuchal translucency: problems with screening the general population. 1. British Journal of Obstetrics and Gynaecology 1995;102(5):381‐5. [DOI] [PubMed] [Google Scholar]
Robertson 1991 {published data only}
- Robertson EF. Maternal serum screening for neural tube defects and Down's syndrome.[see comment]. Medical Journal of Australia 1991;155(2):67‐8. [PubMed] [Google Scholar]
Rode 2003 {published data only}
- Rode L, Wojdemann KR, Shalmi AC, Larsen SO, Sundberg K, Norgaard‐Pedersen B, et al. Combined first‐ and second‐trimester screening for Down syndrome: an evaluation of proMBP as a marker. Prenatal Diagnosis 2003;23(7):593‐8. [DOI] [PubMed] [Google Scholar]
Ronge 2006 {published data only}
- Ronge R. Combined first trimester screening for Down's syndrome is superior to quadruple test. Geburtshilfe und Frauenheilkunde 2006;66(4):332. [Google Scholar]
Rose 1995 {published data only}
- Rose NC, Mennuti MT. Multiple marker screening for women 35 and older. Contemporary OB/GYN 1995;40(9):55‐6. [Google Scholar]
Ross 1997 {published data only}
- Ross HL, Elias S. Maternal serum screening for fetal genetic disorders. Obstetrics & Gynecology Clinics of North America 1997;24(1):33‐47. [DOI] [PubMed] [Google Scholar]
Rotmensch 1996 {published data only}
- Rotmensch S, Liberati M, Kardana A, Copel JA, Ben‐Rafael Z, Cole LA. Nicked free ß‐subunit of human chorionic gonadotropin: A potential new marker for Down syndrome screening. American Journal of Obstetrics and Gynecology 1996;174(2):609‐11. [DOI] [PubMed] [Google Scholar]
Rotmensch 1999 {published data only}
- Rotmensch S, Celentano C, Shalev J, Vishne TH, Lipitz S, Ben‐Rafael Z, et al. Midtrimester maternal serum screening after multifetal pregnancy reduction in pregnancies conceived by in vitro fertilization. Journal of Assisted Reproduction and Genetics 1999;16(1):8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rozenberg 2006 {published data only}
- Rozenberg P, Bussieres L, Chevret S, Bernard JP, Malagrida L, Cuckle H, et al. Screening for Down syndrome using first‐trimester combined screening followed by second‐trimester ultrasound examination in an unselected population. American Journal of Obstetrics and Gynecology 2006;195(5):1379‐87. [DOI] [PubMed] [Google Scholar]
Rudnicka 2002 {published data only}
- Rudnicka AR, Wald NJ, Huttly W, Hackshaw AK. Influence of maternal smoking on the birth prevalence of Down syndrome and on second trimester screening performance. Prenatal Diagnosis 2002;22(10):893‐7. [DOI] [PubMed] [Google Scholar]
Ryall 1992 {published data only}
- Ryall RG, Staples AJ, Robertson EF, Pollard AC. Improved performance in a prenatal screening programme for Down's syndrome incorporating serum‐free hCG subunit analyses. Prenatal Diagnosis 1992;12(4):251‐61. [DOI] [PubMed] [Google Scholar]
Ryall 2001 {published data only}
- Ryall RG, Callen D, Cocciolone R, Duvnjak A, Esca R, Frantzis N, et al. Karyotypes found in the population declared at increased risk of Down syndrome following maternal serum screening. Prenatal Diagnosis 2001;21(7):553‐7. [DOI] [PubMed] [Google Scholar]
Sabriá 2002 {published data only}
- Sabriá J, Cabrero D, Bach C. Aneuploidy screening: ultrasound versus biochemistry. Ultrasound Review of Obstetrics and Gynecology 2002;2(4):221‐8. [Google Scholar]
Sacchini 2003 {published data only}
- Sacchini C, El‐Sheikhah A, Cicero S, Rembouskos G, Nicolaides KH. Ear length in trisomy 21 fetuses at 11‐14 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2003;22(5):460‐3. [DOI] [PubMed] [Google Scholar]
Sahota 2009 {published data only}
- Sahota DS, Leung TY, Chan LW, Law LW, Fung TY, Chan OK, et al. First‐trimester fetal nasal bone length in an ethnic Chinese population. Ultrasound in Obstetrics & Gynecology 2009;34(1):33‐7. [DOI] [PubMed] [Google Scholar]
Sahota 2010a {published data only}
- Sahota DS, Leung TY, Chen M, Chan LW, Fung TY, Lau TK. Comparison of likelihood ratios of first‐trimester nuchal translucency measurements: multiples of median, delta or mixture. Ultrasound in Obstetrics & Gynecology 2010;36(1):15‐9. [DOI] [PubMed] [Google Scholar]
Salazar 2007 {published data only}
- Salazar López R, Ibarra Gallardo AL, Iduma Meléndrez M, Leyva Bojórquez R. [Specificity of biochemical markers of pregnancy second trimester]. [Spanish]. Ginecologia y Obstetricia de Mexico 2007;75(10):608‐14. [PubMed] [Google Scholar]
Salazar 2008 {published data only}
- Salazar López R, Ibarra Gallardo AL, Iduma Meléndrez M, Leyva R. [Evaluation of plasmatic A protein as only marker during first trimester of pregnancy]. [Spanish]. Ginecologia y Obstetricia de Mexico 2008;76(10):576‐81. [PubMed] [Google Scholar]
Saller 1997 {published data only}
- Saller DN Jr, Canick JA, Kellner LH, Rose NC, Garza J, French CA, et al. Maternal serum analyte levels in pregnancies with fetal Down syndrome resulting from translocations. American Journal of Obstetrics and Gynecology 1997;177(4):879‐81. [DOI] [PubMed] [Google Scholar]
Salomon 2001 {published data only}
- Salomon LJ, Bernard JP, Taupin P, Benard C, Ville Y. Relationship between nuchal translucency at 11‐14 weeks and nuchal fold at 20‐24 weeks of gestation. Ultrasound in Obstetrics & Gynecology 2001;18(6):636‐7. [DOI] [PubMed] [Google Scholar]
Salonen 1997 {published data only}
- Salonen R, Turpeinen U, Kurki L, Lappalainen M, Ammala P, Hiilesmaa V, et al. Maternal serum screening for Down's syndrome on population basis. Acta Obstetricia et Gynecologica Scandinavica 1997;76(9):817‐21. [DOI] [PubMed] [Google Scholar]
Saltvedt 2005 {published data only}
- Saltvedt S, Almstrom H, Kublickas M, Valentin L, Bottinga R, Bui TH, et al. Screening for Down syndrome based on maternal age or fetal nuchal translucency: a randomized controlled trial in 39,572 pregnancies. Ultrasound in Obstetrics & Gynecology 2005;25(6):537‐45. [DOI] [PubMed] [Google Scholar]
Saridogan 1996 {published data only}
- Saridogan E, Djahanbakhch O, Naftalin AA. Screening for Down's syndrome: experience in an inner city health district. British Journal of Obstetrics and Gynaecology 1996;103(12):1205‐11. [DOI] [PubMed] [Google Scholar]
Savoldelli 1993 {published data only}
- Savoldelli G, Binkert F, Achermann J, Schmid W. Ultrasound screening for chromosomal anomalies in the first trimester of pregnancy. Prenatal Diagnosis 1993;13(6):513‐8. [DOI] [PubMed] [Google Scholar]
Schielen 2009 {published data only}
- Schielen PC, Wildschut HI, Loeber JG. Down syndrome screening: determining the cutoff level of risk for invasive testing. Prenatal Diagnosis 2009;29(2):190‐2. [DOI] [PubMed] [Google Scholar]
Schiott 2006 {published data only}
- Schiott KM, Christiansen M, Petersen OB, Sorensen TL, Uldbjerg N. The "Consecutive Combined Test"‐‐using double test from week 8 + 0 and nuchal translucency scan, for first trimester screening for Down syndrome. Prenatal Diagnosis 2006;26(12):1105‐9. [DOI] [PubMed] [Google Scholar]
Schmidt 2007a {published data only}
- Schmidt P, Rom J, Maul H, Vaske B, Hillemanns P, Scharf A. Advanced first trimester screening (AFS): an improved test strategy for the individual risk assessment of fetal aneuploidies and malformations. Archives of Gynecology and Obstetrics 2007;276(2):159‐66. [DOI] [PubMed] [Google Scholar]
Schmidt 2007b {published data only}
- Schmidt P, Staboulidou I, Soergel P, Wustemann M, Hillemanns P, Scharf A. Comparison of Nicolaides' risk evaluation for Down's syndrome with a novel software: an analysis of 1,463 cases. Archives of Gynecology and Obstetrics 2007;275(6):469‐74. [DOI] [PubMed] [Google Scholar]
Schmidt 2007c {published data only}
- Schmidt P, Pruggmayer M, Steinborn A, Schippert C, Staboulidou I, Hillemanns P, et al. Are nuchal translucency, pregnancy associated plasma protein‐A or free‐beta‐human chorionic gonadotropin depending on maternal age? A multicenter study of 8,116 pregnancies. Archives of Gynecology and Obstetrics 2007;276(3):259‐62. [DOI] [PubMed] [Google Scholar]
Schmidt 2008a {published data only}
- Schmidt P, Hormansdorfer C, Pruggmayer M, Schutte C, Neumann A, Gerritzen A, et al. Improved prenatal aneuploidy screening using the novel advanced first‐trimester screening algorithm: a multicenter study of 10,017 pregnancies. Journal of Clinical Ultrasound 2008;36(7):397‐402. [DOI] [PubMed] [Google Scholar]
Schmidt 2008b {published data only}
- Schmidt P, Staboulidou I, Elsasser M, Vaske B, Hillemanns P, Scharf A. How imprecise may the measurement of fetal nuchal translucency be without worsening first‐trimester screening?. Fetal Diagnosis and Therapy 2008;24(3):291‐5. [DOI] [PubMed] [Google Scholar]
Schmidt 2008c {published data only}
- Schmidt P, Hormansdorfer C, Oehler K, Hartel H, Hillemanns P, Scharf A. [Three‐dimensional scatter plot analysis to estimate the risk of foetal aneuloidy]. [German]. Zeitschrift fur Geburtshilfe und Neonatologie 2008;212(4):127‐35. [DOI] [PubMed] [Google Scholar]
Schmidt 2010 {published data only}
- Schmidt P, Hormansdorfer C, Golatta M, Scharf A. Analysis of the distribution shift of detected aneuploidies by age independent first trimester screening. Archives of Gynecology and Obstetrics 2010;281(3):393‐9. [DOI] [PubMed] [Google Scholar]
Schuchter 1998 {published data only}
- Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10‐13 weeks of pregnancy. Prenatal Diagnosis 1998;18(3):281‐6. [PubMed] [Google Scholar]
Scott 1995 {published data only}
- Scott F, Boogert A, Smart S, Anderson J. Maternal serum screening and routine 18‐week ultrasound in the detection of all chromosomal abnormalities. Australian & New Zealand Journal of Obstetrics & Gynaecology 1995;35(2):165‐8. [DOI] [PubMed] [Google Scholar]
Seeds 1990 {published data only}
- Seeds JW, Watson WJ. Ultrasound and maternal serum alpha‐fetoprotein screening: a complementary relationship. Ultrasound Quarterly 1990;8(2):145‐66. [Google Scholar]
Seki 1995 {published data only}
- Seki K, Mitsui C, Nagata I. Measurement of urinary free ß‐human chorionic gonadotropin by immunoradiometric assay. Gynecologic and Obstetric Investigation 1995;40(3):162‐7. [DOI] [PubMed] [Google Scholar]
Shenhav 2003 {published data only}
- Shenhav S, Gemer O, Sherman DJ, Peled R, Segal S. Midtrimester triple‐test levels in women with chronic hypertension and altered renal function. Prenatal Diagnosis 2003;23(2):166‐7. [DOI] [PubMed] [Google Scholar]
Shintaku 1989 {published data only}
- Shintaku Y, Takabayashi T, Sasaki H, Ozawa N, Shinkawa O, Hamazaki Y, et al. [Screening for chromosomal anomalies with maternal serum alpha‐fetoprotein]. [Japanese]. Nippon Sanka Fujinka Gakkai Zasshi ‐ Acta Obstetrica et Gynaecologica Japonica 1989;41(2):185‐90. [PubMed] [Google Scholar]
Shulman 2003 {published data only}
- Shulman A, Maymon R. Mid‐gestation Down syndrome screening test and pregnancy outcome among unstimulated assisted‐conception pregnancies. Prenatal Diagnosis 2003;23(8):625‐8. [DOI] [PubMed] [Google Scholar]
Sieroszewski 2008 {published data only}
- Sieroszewski P, Perenc M, Budecka EB, Sobala W, Deutinger J. Sonographical integrated test for detection of chromosomal aberrations. Ultraschall in der Medizin 2008;29(2):190‐6. [DOI] [PubMed] [Google Scholar]
Simon‐Bouy 1999 {published data only}
- Simon‐Bouy B. [Markers for trisomy 21][French]. Fertilite Contraception Sexualite 1999;27(9):289‐91. [PubMed] [Google Scholar]
Simpson 1986 {published data only}
- Simpson JL, Baum LD, Marder R, Elias S, Ober C, Martin AO. Maternal serum alpha‐fetoprotein screening: low and high values for detection of genetic abnormalities. American Journal of Obstetrics and Gynecology 1986;155(3):593‐7. [DOI] [PubMed] [Google Scholar]
Smith 1990 {published data only}
- Smith C, Grube GL, Wilson S. Maternal serum alpha‐fetoprotein screening and the role of ultrasound. Journal of Diagnostic Medical Sonography 1990;6(6):312‐6. [Google Scholar]
Smith 1996 {published data only}
- Smith ER, Petersen J, Okorodudu AO, Bissell MG. Does the addition of unconjugated estriol in maternal serum screening improve the detection of trisomy 21? A meta‐analysis. Clinical Laboratory Management Review 1996;10(2):176‐81. [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith NC, Hau C. A six year study of the antenatal detection of fetal abnormality in six Scottish health boards. British Journal of Obstetrics and Gynaecology 1999;106(3):206‐12. [DOI] [PubMed] [Google Scholar]
Smith‐Bindman 2001 {published data only}
- Smith‐Bindman R, Hosmer W, Feldstein VA, Deeks JJ, Goldberg JD. Second‐trimester ultrasound to detect fetuses with Down syndrome: a meta‐analysis.[see comment]. JAMA 2001;285(8):1044‐55. [DOI] [PubMed] [Google Scholar]
Smith‐Bindman 2003 {published data only}
- Smith‐Bindman R, Chu P, Bacchetti P, Waters JJ, Mutton D, Alberman E. Prenatal screening for Down syndrome in England and Wales and population‐based birth outcomes. American Journal of Obstetrics and Gynecology 2003;187(4):980‐5. [DOI] [PubMed] [Google Scholar]
Snijders 1995 {published data only}
- Snijders RJM, Sebire NJ, Nicolaides KH. Maternal age and gestational age‐specific risk for chromosomal defects. Fetal Diagnosis and Therapy 1995;10(6):356‐67. [DOI] [PubMed] [Google Scholar]
Snijders 1999 {published data only}
- Snijders RJM, Sundberg K, Holzgreve W, Henry G, Nicolaides KH. Maternal age‐ and gestation‐specific risk for trisomy 21. Ultrasound in Obstetrics and Gynecology 1999;13(3):167‐70. [DOI] [PubMed] [Google Scholar]
Soergel 2006 {published data only}
- Soergel P, Pruggmayer M, Schwerdtfeger R, Muhlhaus K, Scharf A. Screening for trisomy 21 with maternal age, fetal nuchal translucency and maternal serum biochemistry at 11‐14 weeks: a regional experience from Germany. Fetal Diagnosis and Therapy 2006;21(3):264‐8. [DOI] [PubMed] [Google Scholar]
Sokol 1998 {published data only}
- Sokol AI, Kramer RL, Yaron Y, O'Brien JE, Muller F, Johnson MP, et al. Age‐specific variation in aneuploidy incidence among biochemical screening programs. American Journal of Obstetrics and Gynecology 1998;179(4):971‐3. [DOI] [PubMed] [Google Scholar]
Sonek 2003 {published data only}
- Sonek JD. Nasal bone evaluation with ultrasonography: a marker for fetal aneuploidy. Ultrasound in Obstetrics and Gynecology 2003;22(1):11‐5. [DOI] [PubMed] [Google Scholar]
Sonek 2007 {published data only}
- Sonek J, Borenstein M, Downing C, McKenna D, Neiger R, Croom C, et al. Frontomaxillary facial angles in screening for trisomy 21 at 14‐23 weeks' gestation. American Journal of Obstetrics and Gynecology 2007;197(2):160‐5. [DOI] [PubMed] [Google Scholar]
Sood 2010 {published data only}
- Sood M, Rochelson B, Krantz D, Ravens R, Tam Tam H, Vohra N, et al. Are second‐trimester minor sonographic markers for Down syndrome useful in patients who have undergone first‐trimester combined screening?. American Journal of Obstetrics and Gynecology 2010;203(4):408‐4. [DOI] [PubMed] [Google Scholar]
Sooklim 2010 {published data only}
- Sooklim R, Manotaya S. Fetal facial sonographic markers for second trimester Down syndrome screening in a Thai population. International Journal of Gynaecology and Obstetrics 2010;111(2):144‐7. [DOI] [PubMed] [Google Scholar]
Spencer 1985 {published data only}
- Spencer K, Carpenter P. Screening for Down's syndrome using serum alpha fetoprotein: a retrospective study indicating caution. BMJ (Clinical Research Ed.) 1985;290(6486):1940‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 1991a {published data only}
- Spencer K. Evaluation of an assay of the free ß‐subunit of choriogonadotropin and its potential value in screening for Down's syndrome. Clinical Chemistry 1991;37(6):809‐14. [PubMed] [Google Scholar]
Spencer 1991b {published data only}
- Spencer K. Maternal serum CA125 is not a second trimester marker for Down's syndrome. Annals of Clinical Biochemistry 1991;28(3):299‐300. [DOI] [PubMed] [Google Scholar]
Spencer 1992 {published data only}
- Spencer K, Coombes EJ, Mallard AS, Ward AM. Free ß human choriogonadotropin in Down's syndrome screening: a multicentre study of its role compared with other biochemical markers.[see comment]. Annals of Clinical Biochemistry 1992;29(5):506‐18. [DOI] [PubMed] [Google Scholar]
Spencer 1993a {published data only}
- Spencer K, Carpenter P. Prospective study of prenatal screening for Down's syndrome with free ß human chorionic gonadotrophin.[see comment]. BMJ 1993;307(6907):764‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 1993b {published data only}
- Spencer K, Macri JN, Carpenter P, Anderson R, Krantz DA. Stability of intact chorionic gonadotropin (hCG) in serum, liquid whole blood, and dried whole‐blood filter‐paper spots: impact on screening for Down syndrome by measurement of free ß‐hCG subunit. Clinical Chemistry 1993;39(6):1064‐8. [PubMed] [Google Scholar]
Spencer 1993c {published data only}
- Spencer K, Wood PJ, Anthony FW. Elevated levels of maternal serum inhibin immunoreactivity in second trimester pregnancies affected by Down's syndrome. Annals of Clinical Biochemistry 1993;30(Pt 2):219‐20. [DOI] [PubMed] [Google Scholar]
Spencer 1993d {published data only}
- Spencer K, Macri JN, Anderson RW, Aitken DA, Berry E, Crossley JA, et al. Dual analyte immunoassay in neural tube defect and Down's syndrome screening: results of a multicentre clinical trial. Annals of Clinical Biochemistry 1993;30(4):394‐401. [DOI] [PubMed] [Google Scholar]
Spencer 1993e {published data only}
- Spencer K. Free alpha‐subunit of human chorionic gonadotropin in Down syndrome. American Journal of Obstetrics and Gynecology 1993;168(1):132‐5. [DOI] [PubMed] [Google Scholar]
Spencer 1995 {published data only}
- Spencer K. The influence of gravidity on Down's syndrome screening with free ß hCG. Prenatal Diagnosis 1995;15(1):87‐9. [DOI] [PubMed] [Google Scholar]
Spencer 1996 {published data only}
- Spencer K, Wallace EM, Ritoe S. Second‐trimester dimeric inhibin‐A in Down's syndrome screening. Prenatal Diagnosis 1996;16(12):1101‐10. [DOI] [PubMed] [Google Scholar]
Spencer 1997 {published data only}
- Spencer K, Noble P, Snijders RJ, Nicolaides KH. First‐trimester urine free ß hCG, ß core, and total oestriol in pregnancies affected by Down's syndrome: implications for first‐trimester screening with nuchal translucency and serum free ß hCG. Prenatal Diagnosis 1997;17(6):525‐38. [DOI] [PubMed] [Google Scholar]
Spencer 1998a {published data only}
- Spencer K. The influence of smoking on maternal serum AFP and free ß hCG levels and the impact on screening for Down syndrome. Prenatal Diagnosis 1998;18(3):225‐34. [DOI] [PubMed] [Google Scholar]
Spencer 1998b {published data only}
- Spencer K, Carpenter P. Is prostate‐specific antigen a marker for pregnancies affected by Down syndrome?. Clinical Chemistry 1998;44(11):2362‐5. [PubMed] [Google Scholar]
Spencer 1999b {published data only}
- Spencer K. Second trimester prenatal screening for Down's syndrome using alpha‐fetoprotein and free ß hCG: a seven year review. British Journal of Obstetrics and Gynaecology 1999;106(12):1287‐93. [DOI] [PubMed] [Google Scholar]
Spencer 1999c {published data only}
- Spencer K. Accuracy of Down's syndrome risks produced in a prenatal screening program. Annals of Clinical Biochemistry 1999;36(1):101‐3. [DOI] [PubMed] [Google Scholar]
Spencer 2000a {published data only}
- Spencer K, Berry E, Crossley JA, Aitken DA, Nicolaides KH. Is maternal serum total hCG a marker of trisomy 21 in the first trimester of pregnancy?. Prenatal Diagnosis 2000;20(4):311‐7. [DOI] [PubMed] [Google Scholar]
Spencer 2000b {published data only}
- Spencer K. Screening for trisomy 21 in twin pregnancies in the first trimester using free ß‐hCG and PAPP‐A, combined with fetal nuchal translucency thickness. Prenatal Diagnosis 2000;20(2):91‐5. [DOI] [PubMed] [Google Scholar]
Spencer 2000c {published data only}
- Spencer K. The influence of smoking on maternal serum PAPP‐A and free ß hCG levels in the first trimester of pregnancy. Prenatal Diagnosis 1999;19(11):1065‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2000d {published data only}
- Spencer K, Ong CY, Liao AW, Nicolaides KH. The influence of parity and gravidity on first trimester markers of chromosomal abnormality. Prenatal Diagnosis 2000;20(10):792‐4. [PubMed] [Google Scholar]
Spencer 2000e {published data only}
- Spencer K. The influence of fetal sex in screening for Down syndrome in the second trimester using AFP and free ß‐hCG. Prenatal Diagnosis 2000;20(8):648‐51. [DOI] [PubMed] [Google Scholar]
Spencer 2000f {published data only}
- Spencer K, Ong CY, Liao AW, Nicolaides KH. The influence of ethnic origin on first trimester biochemical markers of chromosomal abnormalities. Prenatal Diagnosis 2000;20(6):491‐4. [PubMed] [Google Scholar]
Spencer 2000g {published data only}
- Spencer K, Tul N, Nicolaides KH. Maternal serum free ß‐hCG and PAPP‐A in fetal sex chromosome defects in the first trimester. Prenatal Diagnosis 2000;20(5):390‐4. [DOI] [PubMed] [Google Scholar]
Spencer 2000h {published data only}
- Spencer K. Second‐trimester prenatal screening for Down syndrome and the relationship of maternal serum biochemical markers to pregnancy complications with adverse outcome. Prenatal Diagnosis 2000;20(8):652‐6. [PubMed] [Google Scholar]
Spencer 2000i {published data only}
- Spencer K, Ong CY, Liao AW, Papademetriou D, Nicolaides KH. The influence of fetal sex in screening for trisomy 21 by fetal nuchal translucency, maternal serum free ß‐hCG and PAPP‐A at 10‐14 weeks of gestation. Prenatal Diagnosis 2000;20(8):673‐5. [PubMed] [Google Scholar]
Spencer 2001a {published data only}
- Spencer K. Age related detection and false positive rates when screening for Down's syndrome in the first trimester using fetal nuchal translucency and maternal serum free ßhCG and PAPP‐A. BJOG: an international journal of obstetrics and gynaecology 2001;108(10):1043‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2001b {published data only}
- Spencer K, Liao AW, Ong CY, Geerts L, Nicolaides KH. First trimester maternal serum placenta growth factor (PIGF)concentrations in pregnancies with fetal trisomy 21 or trisomy 18. Prenatal Diagnosis 2001;21(9):718‐22. [DOI] [PubMed] [Google Scholar]
Spencer 2001c {published data only}
- Spencer K, Liao AW, Ong CY, Geerts L, Nicolaides KH. Maternal serum levels of dimeric Inhibin A in pregnancies affected by trisomy 21 in the first trimester. Prenatal Diagnosis 2001;21(6):441‐4. [DOI] [PubMed] [Google Scholar]
Spencer 2001d {published data only}
- Spencer K, Liao AW, Skentou H, Ong CY, Nicolaides KH. Maternal serum levels of total activin‐A in first‐trimester trisomy 21 pregnancies. Prenatal Diagnosis 2001;21(4):270‐3. [DOI] [PubMed] [Google Scholar]
Spencer 2001e {published data only}
- Spencer K. Screening for trisomy 21 in twin pregnancies in the first trimester: does chorionicity impact on maternal serum free ß‐hCG or PAPP‐A levels?. Prenatal Diagnosis 2001;21(9):715‐7. [DOI] [PubMed] [Google Scholar]
Spencer 2002b {published data only}
- Spencer K, Nicolaides KH. A first trimester trisomy 13/trisomy 18 risk algorithm combining fetal nuchal translucency thickness, maternal serum free ß‐hCG and PAPP‐A. Prenatal Diagnosis 2002;22(10):877‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2002c {published data only}
- Spencer K. Accuracy of Down syndrome risks produced in a first‐trimester screening programme incorporating fetal nuchal translucency thickness and maternal serum biochemistry. Prenatal Diagnosis 2002;22(3):244‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2002d {published data only}
- Spencer K, Cuckle HS. Screening for chromosomal anomalies in the first trimester: does repeat maternal serum screening improve detection rates?. Prenatal Diagnosis 2002;22(10):903‐6. [DOI] [PubMed] [Google Scholar]
Spencer 2002e {published data only}
- Spencer K, Crossley JA, Aitken DA, Nix AB, Dunstan FD, Williams K. Temporal changes in maternal serum biochemical markers of trisomy 21 across the first and second trimester of pregnancy. Annals of Clinical Biochemistry 2002;39(6):567‐76. [DOI] [PubMed] [Google Scholar]
Spencer 2003a {published data only}
- Spencer K, Crossley JA, Aitken DA, Nix AB, Dunstan FD, Williams K. The effect of temporal variation in biochemical markers of trisomy 21 across the first and second trimesters of pregnancy on the estimation of individual patient‐specific risks and detection rates for Down's syndrome. Annals of Clinical Biochemistry 2003;40(3):219‐31. [DOI] [PubMed] [Google Scholar]
Spencer 2003b {published data only}
- Spencer K. The influence of different sample collection types on the levels of markers used for Down's syndrome screening as measured by the Kryptor Immunosassay system. Annals of Clinical Biochemistry 2003;40(2):166‐8. [DOI] [PubMed] [Google Scholar]
Spencer 2003c {published data only}
- Spencer K, Bindra R, Nicolaides KH. Maternal weight correction of maternal serum PAPP‐A and free ß‐hCG MoM when screening for trisomy 21 in the first trimester of pregnancy. Prenatal Diagnosis 2003;23(10):851‐5. [DOI] [PubMed] [Google Scholar]
Spencer 2003d {published data only}
- Spencer K, Nicolaides KH. Screening for trisomy 21 in twins using first trimester ultrasound and maternal serum biochemistry in a one‐stop clinic: a review of three years experience. BJOG: an international journal of obstetrics and gynaecology 2003;110(3):279‐80. [PubMed] [Google Scholar]
Spencer 2004 {published data only}
- Spencer K, Bindra R, Cacho AM, Nicolaides KH. The impact of correcting for smoking status when screening for chromosomal anomalies using maternal serum biochemistry and fetal nuchal translucency thickness in the first trimester of pregnancy. Prenatal Diagnosis 2004;24(3):169‐73. [DOI] [PubMed] [Google Scholar]
Spencer 2005a {published data only}
- Spencer K, Cicero S, Atzei A, Otigbah C, Nicolaides KH. The influence of maternal insulin‐dependent diabetes on fetal nuchal translucency thickness and first‐trimester maternal serum biochemical markers of aneuploidy. Prenatal Diagnosis 2005;25(10):927‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2005b {published data only}
- Spencer K, Heath V, El‐Sheikhah A, Ong CY, Nicolaides KH. Ethnicity and the need for correction of biochemical and ultrasound markers of chromosomal anomalies in the first trimester: a study of Oriental, Asian and Afro‐Caribbean populations. Prenatal Diagnosis 2005;25(5):365‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2005c {published data only}
- Spencer K. First trimester maternal serum screening for Down's syndrome: an evaluation of the DPC Immulite 2000 free ß‐hCG and pregnancy‐associated plasma protein‐A assays.[see comment]. Annals of Clinical Biochemistry 2005;42(1):30‐40. [DOI] [PubMed] [Google Scholar]
Spencer 2008 {published data only}
- Spencer K, Cowans NJ, Uldbjerg N, Vereecken A, Torring N. First trimester intact hCG as an early marker of trisomy 21: a promise unrecognised?. Prenatal Diagnosis 2008;28(12):1156‐9. [DOI] [PubMed] [Google Scholar]
Spong 1999 {published data only}
- Spong CY, Ghidini A, Stanley‐Christian H, Meck JM, Seydel FD, Pezzullo JC. Risk of abnormal triple screen for Down syndrome is significantly higher in women with female fetuses. Prenatal Diagnosis 1999;19(4):337‐9. [DOI] [PubMed] [Google Scholar]
Staboulidou 2009 {published data only}
- Staboulidou I, Galindo A, Maiz N, Karagiannis G, Nicolaides KH. First‐trimester uterine artery Doppler and serum pregnancy‐associated plasma protein‐a in preeclampsia and chromosomal defects. Fetal Diagnosis and Therapy 2009;25(3):336‐9. [DOI] [PubMed] [Google Scholar]
Stevens 1998 {published data only}
- Stevens SL. The use of nuchal lucency as a screening tool in first trimester sonography. Journal of Diagnostic Medical Sonography 1998;14(6):251‐4. [Google Scholar]
Stoll 1992 {published data only}
- Stoll C. A new approach of prenatal prevention of constitutional disabilities ‐ the study of markers of maternal serum. Journal de Medecine de Strasbourg 1992;23(1):25‐7. [Google Scholar]
Stressig 2011 {published data only}
- Stressig R, Kozlowski P, Froehlich S, Siegmann HJ, Hammer R, Blumenstock G, et al. Assessment of the ductus venosus, tricuspid blood flow and the nasal bone in second‐trimester screening for trisomy 21. Ultrasound in Obstetrics & Gynecology 2011;37(4):444‐9. [DOI] [PubMed] [Google Scholar]
Su 2002 {published data only}
- Su YN, Hsu JJ, Lee CN, Cheng WF, Kung CC, Hsieh FJ. Raised maternal serum placenta growth factor concentration during the second trimester is associated with Down syndrome. Prenatal Diagnosis 2002;22(1):8‐12. [DOI] [PubMed] [Google Scholar]
Suchet 1995 {published data only}
- Suchet IB. Ultrasonography of the fetal neck in the first and second trimesters. Part 2. Anomalies of the posterior nuchal region. Canadian Association of Radiologists Journal 1995;46(5):344‐52. [PubMed] [Google Scholar]
Suchy 1990 {published data only}
- Suchy SF, Yeager MT. Down syndrome screening in women under 35 with maternal serum hCG. Obstetrics and Gynecology 1990;76(1):20‐4. [PubMed] [Google Scholar]
Summers 2003a {published data only}
- Summers AM, Farrell SA, Huang T, Meier C, Wyatt PR. Maternal serum screening in Ontario using the triple marker test. Journal of Medical Screening 2003;10(3):107‐11. [DOI] [PubMed] [Google Scholar]
Summers 2003b {published data only}
- Summers AM, Huang T, Meier C, Wyatt PR. The implications of a false positive second‐trimester serum screen for Down syndrome. Obstetrics and Gynecology 2003;101(6):1301‐6. [DOI] [PubMed] [Google Scholar]
Suntharasaj 2005 {published data only}
- Suntharasaj T, Ratanasiri T, Chanprapaph P, Kengpol C, Kor‐anantakul O, Leetanaporn R, et al. Variability of nuchal translucency measurement: a multicenter study in Thailand. Gynecologic and Obstetric Investigation 2005;60(4):201‐5. [DOI] [PubMed] [Google Scholar]
Susman 2010 {published data only}
- Susman MR, Amor DJ, Muggli E, Jaques AM, Halliday J. Using population‐based data to predict the impact of introducing noninvasive prenatal diagnosis for Down syndrome. Genetics in Medicine 2010;12(5):298‐303. [DOI] [PubMed] [Google Scholar]
Sutton 2004 {published data only}
- Sutton JM, Cole LA. Sialic acid‐deficient invasive trophoblast antigen (sd‐ITA): a new urinary variant for gestational Down syndrome screening. Prenatal Diagnosis 2004;24(3):194‐7. [DOI] [PubMed] [Google Scholar]
Suzuki 1998 {published data only}
- Suzuki Y, Takada J, Iwaki T, Isaka K, Takayama M. Screening for trisomy 21 in the first trimester by measurement of serum PAPP‐A and free ß‐hCG. Acta Obstetrica et Gynaecologica Japonica 1998;50(1):37‐40. [Google Scholar]
Tabor 1987 {published data only}
- Tabor A, Larsen SO, Nielsen J, Nielsen J, Philip J, Pilgaard B, et al. Screening for Down's syndrome using an iso‐risk curve based on maternal age and serum alpha‐fetoprotein level. British Journal of Obstetrics and Gynaecology 1987;94(7):636‐42. [DOI] [PubMed] [Google Scholar]
Tanski 1999 {published data only}
- Tanski S, Rosengren SS, Benn PA. Predictive value of the triple screening test for the phenotype of Down syndrome. American Journal of Medical Genetics 1999;85(2):123‐6. [DOI] [PubMed] [Google Scholar]
Thilaganathan 1998 {published data only}
- Thilaganathan B, Khare M, Williams B, Wathen NC. Influence of ethnic origin on nuchal translucency screening for Down's syndrome. Ultrasound in Obstetrics & Gynecology 1998;12(2):112‐4. [DOI] [PubMed] [Google Scholar]
Thilaganathan 1999 {published data only}
- Thilaganathan B. First‐trimester nuchal translucency and maternal serum biochemical screening for Down's syndrome: a happy union?. Ultrasound in Obstetrics and Gynecology 1999;13(4):229‐30. [DOI] [PubMed] [Google Scholar]
Tislaric 2002 {published data only}
- Tislaric D, Brajenovic‐Milic B, Ristic S, Latin V, Zuvic‐Butorac M, Bacic J, et al. The influence of smoking and parity on serum markers for Down's syndrome screening. Fetal Diagnosis and Therapy 2002;17(1):17‐21. [DOI] [PubMed] [Google Scholar]
Torok 1997 {published data only}
- Torok O, Veress L, Szabo M, Zsupan I, Buczko Z, Bolodar A, et al. [Biochemical and ultrasonic screening of chromosomal aneuploidies in the second trimester of pregnancy]. [Hungarian]. Orvosi Hetilap 1997;138(3):123‐7. [PubMed] [Google Scholar]
Torring 2009 {published data only}
- Torring N. Performance of first‐trimester screening between gestational weeks 7 and 13. Clinical Chemistry 2009;55(8):1564‐7. [DOI] [PubMed] [Google Scholar]
Trninic‐Pjevic 2007 {published data only}
- Trninic‐Pjevic A, Novakov‐Mikic A. [First trimester ultrasound screening of chromosomal abnormalities]. [Serbian]. Srpski Arhiv Za Celokupno Lekarstvo 2007;135(3‐4):153‐6. [DOI] [PubMed] [Google Scholar]
Tsai 2001 {published data only}
- Tsai MS, Huang YY, Hwa KY, Cheng CC, Lee FK. Combined measurement of fetal nuchal translucency, maternal serum free ß‐hCG, and pregnancy‐associated plasma protein A for first‐trimester Down's syndrome screening. Journal of the Formosan Medical Association 2001;100(5):319‐25. [PubMed] [Google Scholar]
Valerio 1996 {published data only}
- Valerio D, Aiello R, Altieri V, Fagnoni P. Maternal serum screening of fetal chromosomal abnormalities by AFP, UE3, hCG and free‐ß hCG. Prospective and retrospective results. Minerva Ginecologica 1996;48(5):169‐73. [PubMed] [Google Scholar]
Van Blerk 1992 {published data only}
- Blerk M, Smitz J, Catte L, Kumps C, Elst J, Steirteghem AC. Second‐trimester cancer antigen 125 and Down's syndrome.[see comment]. Prenatal Diagnosis 1992;12(12):1062‐6. [DOI] [PubMed] [Google Scholar]
Van Dyke 2007 {published data only}
- Dyke DL, Ebrahim SA, Al Saadi AA, Powell SA, Zenger‐Hain JL, Micale MA, et al. The impact of maternal serum screening programs for Down syndrome in southeast Michigan, 1988‐2003. Prenatal Diagnosis 2007;27(6):583‐4. [DOI] [PubMed] [Google Scholar]
Van Heesch, 2006 {published data only}
- Heesch PN, Schielen PC, Wildhagen MF, Hollander K, Steegers EA, Wildschut HI. Combined first trimester screening for trisomy 21: Lack of agreement between risk calculation methods. Journal of Perinatal Medicine 2006;34(2):162‐5. [DOI] [PubMed] [Google Scholar]
Van Lith 1991 {published data only}
- Lith JM, Mantingh A, Beekhuis JR, Bruijn HW, Breed AS. First trimester CA 125 and Down's syndrome. British Journal of Obstetrics and Gynaecology 1991;98(5):493‐4. [DOI] [PubMed] [Google Scholar]
Van Lith 1993 {published data only}
- Lith JM, Mantingh A, Bruijn HW. Maternal serum CA 125 levels in pregnancies with chromosomally‐normal and ‐abnormal fetuses. Dutch Working Party on Prenatal Diagnosis. Prenatal Diagnosis 1993;13(12):1123‐31. [DOI] [PubMed] [Google Scholar]
Van Lith 1994 {published data only}
- Lith JM, Mantingh A, Pratt JJ. First‐trimester maternal serum immunoreactive inhibin in chromosomally normal and abnormal pregnancies. Dutch Working Party on Prenatal Diagnosis. Obstetrics and Gynecology 1994;83(5 Pt 1):661‐4. [PubMed] [Google Scholar]
Veress 1986 {published data only}
- Veress L, Szabo M, Horvath K, Polgar K, Papp Z. [Low maternal serum alpha‐fetoprotein concentration and Down syndrome]. [Hungarian]. Orvosi Hetilap 1986;127(20):1232‐3. [PubMed] [Google Scholar]
Veress 1988 {published data only}
- Veress L, Szabo M, Polgar K, Takacs L, Papp Z. [Prenatal screening for Down's syndrome by measuring the AFP concentration in the maternal serum]. [Hungarian]. Orvosi Hetilap 1988;129(31):1677. [PubMed] [Google Scholar]
Vergani 2008 {published data only}
- Vergani P, Ghidini A, Weiner S, Locatelli A, Pozzi E, Biffi A. Risk assessment for Down syndrome with genetic sonogram in women at risk. Prenatal Diagnosis 2008;28(12):1144‐8. [DOI] [PubMed] [Google Scholar]
Vintzileos 2003 {published data only}
- Vintzileos A, Walters C, Yeo L. Absent nasal bone in the prenatal detection of fetuses with trisomy 21 in a high‐risk population. Obstetrics and Gynecology 2003;101(5):905‐8. [DOI] [PubMed] [Google Scholar]
Wald 1988a {published data only}
- Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ 1988;297(6653):883‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 1988b {published data only}
- Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Canick JA, Haddow JE, et al. Maternal serum unconjugated oestriol as an antenatal screening test for Down's syndrome. British Journal of Obstetrics and Gynaecology 1988;95(4):334‐41. [DOI] [PubMed] [Google Scholar]
Wald 1991 {published data only}
- Wald N, Cuckle H, Wu TS, George L. Maternal serum unconjugated oestriol and human chorionic gonadotrophin levels in twin pregnancies: implications for screening for Down's syndrome. British Journal of Obstetrics and Gynaecology 1991;98(9):905‐8. [DOI] [PubMed] [Google Scholar]
Wald 1992a {published data only}
- Wald NJ, Kennard A, Densem JW, Cuckle HS, Chard T, Butler L. Antenatal maternal serum screening for Down's syndrome: results of a demonstration project.[see comment]. BMJ 1992;305(6850):391‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 1992b {published data only}
- Wald NJ, Cuckle HS, Densem JW, Stone RB. Maternal serum unconjugated oestriol and human chorionic gonadotrophin levels in pregnancies with insulin‐dependent diabetes: implications for screening for Down's syndrome. British Journal of Obstetrics and Gynaecology 1992;99(1):51‐3. [DOI] [PubMed] [Google Scholar]
Wald 1992c {published data only}
- Wald NJ, Cuckle HS, Densem JW, Kennard A, Smith D. Maternal serum screening for Down's syndrome: the effect of routine ultrasound scan determination of gestational age and adjustment for maternal weight.[see comment]. British Journal of Obstetrics and Gynaecology 1992;99(2):144‐9. [DOI] [PubMed] [Google Scholar]
Wald 1993 {published data only}
- Wald N, Densem J, Stone R, Cheng R. The use of free ß‐hCG in antenatal screening for Down's syndrome.[see comment]. British Journal of Obstetrics and Gynaecology 1993;100(6):550‐7. [DOI] [PubMed] [Google Scholar]
Wald 1994 {published data only}
- Wald NJ, Densem JW. Maternal serum free alpha‐human chorionic gonadotrophin levels in twin pregnancies: implications for screening for Down's syndrome. Prenatal Diagnosis 1994;14(8):717‐9. [DOI] [PubMed] [Google Scholar]
Wald 1994a {published data only}
- Wald NJ, Watt HC. Choice of serum markers in antenatal screening for Down's syndrome. Journal of Medical Screening 1994;1(2):117‐20. [DOI] [PubMed] [Google Scholar]
Wald 1996a {published data only}
- Wald NJ, Watt HC. Serum markers for Down's syndrome in relation to number of previous births and maternal age. Prenatal Diagnosis 1996;16(8):699‐703. [DOI] [PubMed] [Google Scholar]
Wald 1996b {published data only}
- Wald NJ, George L, Smith D, Densem JW, Petterson K. Serum screening for Down's syndrome between 8 and 14 weeks of pregnancy. International Prenatal Screening Research Group.[see comment]. British Journal of Obstetrics and Gynaecology. 1996;103(5):407‐12. [DOI] [PubMed] [Google Scholar]
Wald 1996c {published data only}
- Wald NJ, Watt HC, George L. Maternal serum inhibin‐A in pregnancies with insulin‐dependent diabetes mellitus: implications for screening for Down's syndrome. Prenatal Diagnosis 1996;16(10):923‐6. [DOI] [PubMed] [Google Scholar]
Wald 1996d {published data only}
- Wald NJ, Densem JW, George L, Muttukrishna S, Knight PG. Prenatal screening for Down's syndrome using inhibin‐A as a serum marker. Prenatal Diagnosis 1996;16(2):143‐53. [DOI] [PubMed] [Google Scholar]
Wald 1997 {published data only}
- Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first‐trimester screening for Down's syndrome.[see comment]. Prenatal Diagnosis 1997;17(9):821‐9. [PubMed] [Google Scholar]
Wald 1998 {published data only}
- Wald NJ, Watt HC, Haddow JE, Knight GJ. Screening for Down syndrome at 14 weeks of pregnancy. Prenatal Diagnosis 1998;18(3):291‐3. [DOI] [PubMed] [Google Scholar]
Wald 1999a {published data only}
- Wald NJ, Hackshaw AK, Diamandis EP, Melegos DN. Maternal serum prostate‐specific antigen and Down syndrome in the first and second trimesters of pregnancy. Prenatal Diagnosis 1999;19(7):674‐6. [PubMed] [Google Scholar]
Wald 1999b {published data only}
- Wald NJ, Watt HC, Norgaard‐Pederson B, Christiansen M. SP1 in pregnancies with Down syndrome in the first trimester of pregnancy. Prenatal Diagnosis 1999;19(6):517‐20. [PubMed] [Google Scholar]
Wald 1999c {published data only}
- Wald NJ, White N, Morris JK, Huttly WJ, Canick JA. Serum markers for Down's syndrome in women who have had in vitro fertilisation: implications for antenatal screening. British Journal of Obstetrics and Gynaecology 1999;106(12):1304‐6. [DOI] [PubMed] [Google Scholar]
Wald 1999d {published data only}
- Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down's syndrome on the basis of tests performed during the first and second trimesters.[see comment]. New England Journal of Medicine 1999;341(7):461‐7. [DOI] [PubMed] [Google Scholar]
Wald 2003b {published data only}
- Wald NJ, Rish S, Hackshaw AK. Combining nuchal translucency and serum markers in prenatal screening for Down syndrome in twin pregnancies. Prenatal Diagnosis 2003;23(7):588‐92. [DOI] [PubMed] [Google Scholar]
Wald 2003c {published data only}
- Wald NJ, Huttly WJ, Hackshaw AK. Antenatal screening for Down's syndrome with the quadruple test.[see comment]. Lancet 2003;361(9360):835‐6. [DOI] [PubMed] [Google Scholar]
Wald 2006 {published data only}
- Wald NJ, Rudnicka AR, Bestwick JP. Sequential and contingent prenatal screening for Down syndrome. Prenatal Diagnosis 2006;26(9):769‐77. [DOI] [PubMed] [Google Scholar]
Wallace 1994 {published data only}
- Wallace EM, Harkness LM, Burns S, Liston WA. Evaluation of maternal serum immunoreactive Inhibin Aas a first trimester marker of Down's syndrome. Clinical Endocrinology 1994;41(4):483‐6. [DOI] [PubMed] [Google Scholar]
Wallace 1997 {published data only}
- Wallace EM, Crossley JA, Ritoe SC, Groome NP, Aitken DA. Maternal serum inhibin‐A in pregnancies complicated by insulin dependent diabetes mellitus. British Journal of Obstetrics and Gynaecology 1997;104(8):946‐8. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang E, Chen C, Glimco E, Grobman W. The performance of second trimester long bone ratios for Down syndrome screening is influenced by gestational age. Journal of Maternal‐fetal & Neonatal Medicine 2010;23(7):642‐5. [DOI] [PubMed] [Google Scholar]
Ward 2005 {published data only}
- Ward A. Nuchal translucency measurement. Synergy (http://www.highbeam.com/doc/1P3‐866108421.html) (accessed 2007) 2005.
Watt 1996a {published data only}
- Watt HC, Wald NJ, Smith D, Kennard A, Densem J. Effect of allowing for ethnic group in prenatal screening for Down's syndrome. Prenatal Diagnosis 1996;16(8):691‐8. [DOI] [PubMed] [Google Scholar]
Watt 1996b {published data only}
- Watt HC, Wald NJ, George L. Maternal serum inhibin‐A levels in twin pregnancies: implications for screening for Down's syndrome. Prenatal Diagnosis 1996;16(10):927‐9. [DOI] [PubMed] [Google Scholar]
Wax 2007 {published data only}
- Wax JR, Pinette MG, Cartin A, Blackstone J. Optimal crown‐rump length for measuring the nuchal translucency. Journal of Clinical Ultrasound 2007;35(6):302‐4. [DOI] [PubMed] [Google Scholar]
Weinans 2001 {published data only}
- Weinans MJN, Pratt JJ, De Wolf, Mantingh A. First‐trimester maternal serum human thyroid‐stimulating hormone in chromosomally normal and Down syndrome pregnancies. Prenatal Diagnosis 2001;21(9):723‐5. [PubMed] [Google Scholar]
Weinans 2004 {published data only}
- Weinans MJ, Kooij L, Müller MA, Bilardo KM, Lith JM, Tymstra T. A comparison of the impact of screen‐positive results obtained from ultrasound and biochemical screening for Down syndrome in the first trimester: a pilot study. Prenatal Diagnosis 2004;24(5):347‐51. [DOI] [PubMed] [Google Scholar]
Weisz 2007 {published data only}
- Weisz B, Pandya P, Chitty L, Jones P, Huttly W, Rodeck C. Practical issues drawn from the implementation of the integrated test for Down syndrome screening into routine clinical practice. BJOG: an international journal of obstetrics and gynaecology 2007;114(4):493‐7. [DOI] [PubMed] [Google Scholar]
Welborn 1994 {published data only}
- Welborn JL, Timm NS. Trisomy 21 and cystic hygromas in early gestational age fetuses. American Journal of Perinatology 1994;11(1):19‐20. [DOI] [PubMed] [Google Scholar]
Wenstrom 1993 {published data only}
- Wenstrom KD, Williamson RA, Grant SS, Hudson JD, Getchell JP. Evaluation of multiple‐marker screening for Down syndrome in a statewide population. American Journal of Obstetrics and Gynecology 1993;169(4):793‐7. [DOI] [PubMed] [Google Scholar]
Wenstrom 1995a {published data only}
- Wenstrom KD, Owen J, Boots L, Ethier M. The influence of maternal weight on human chorionic gonadotropin in the multiple‐marker screening test for fetal Down syndrome. American Journal of Obstetrics and Gynecology 1995;173(4):1297‐300. [DOI] [PubMed] [Google Scholar]
Wenstrom 1995b {published data only}
- Wenstrom KD, Desai R, Owen J, Dubard MB, Boots L. Comparison of multiple‐marker screening with amniocentesis for the detection of fetal aneuploidy in women greater than or equal35 years old. American Journal of Obstetrics and Gynecology 1995;173(4):1287‐92. [DOI] [PubMed] [Google Scholar]
Wetta 2011 {published data only}
- Wetta L, Biggio JJ, Owen J. Use of ethnic‐specific medians for Hispanic patients reduces ethnic disparities in multiple marker screening. Prenatal Diagnosis 2011;31(4):331‐3. [DOI] [PubMed] [Google Scholar]
Whitlow 1998a {published data only}
- Whitlow BJ, Lazanakis ML, Kadir RA, Chatzipapas I, Economides DL. The significance of choroid plexus cysts, echogenic heart foci and renal pyelectasis in the first trimester. Ultrasound in Obstetrics & Gynecology 1998;12(6):385‐90. [DOI] [PubMed] [Google Scholar]
Whitlow 1998b {published data only}
- Whitlow BJ, Economides DL. First trimester detection of fetal abnormalities in an unselected population. Contemporary Reviews in Obstetrics and Gynaecology 1998;10(4):245‐53. [DOI] [PubMed] [Google Scholar]
Whitlow 1999 {published data only}
- Whitlow BJ, Chatzipapas IK, Lazanakis ML, Kadir RA, Economides DL. The value of sonography in early pregnancy for the detection of fetal abnormalities in an unselected population. British Journal of Obstetrics and Gynaecology 1999;106(9):929‐36. [DOI] [PubMed] [Google Scholar]
Williamson 1994 {published data only}
- Williamson R. Expanded maternal serum alpha fetoprotein screening. Iowa Medicine 1994;84(9):397‐400. [PubMed] [Google Scholar]
Wilson 2000 {published data only}
- Wilson K. New first‐trimester prenatal screening for down syndrome. Laboratory Medicine 2000;31(11):591. [Google Scholar]
Wojdemann 2001 {published data only}
- Wojdemann KR, Larsen SO, Shalmi A, Sundberg K, Christiansen M, Tabor A. First trimester screening for Down syndrome and assisted reproduction: no basis for concern. Prenatal Diagnosis 2001;21(7):563‐5. [DOI] [PubMed] [Google Scholar]
Wong 2003 {published data only}
- Wong SF, Choi H, Ho LC. Nasal bone hypoplasia: is it a common finding amongst chromosomally normal fetuses of southern Chinese women?. Gynecologic and Obstetric Investigation 2003;56(2):99‐101. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright D, Bradbury I, Cuckle H, Gardosi J, Tonks A, Standing S, et al. Three‐stage contingent screening for Down syndrome. Prenatal Diagnosis 2006;26(6):528‐34. [DOI] [PubMed] [Google Scholar]
Wright 2007 {published data only}
- Wright D, Spencer K, Nix B. First trimester screening for Down syndrome using free beta hCG, total hCG and PAPP‐A: an exploratory study. Prenatal Diagnosis 2007;27(12):1118‐22. [DOI] [PubMed] [Google Scholar]
Xie 2010 {published data only}
- Xie Z, Lu S, Li H. Contingent triple‐screening for Down syndrome in the second trimester: a feasibility study in Mainland Chinese population. Prenatal Diagnosis 2010;30(1):74‐6. [DOI] [PubMed] [Google Scholar]
Yagel 1998 {published data only}
- Yagel S, Anteby EY, Hochner‐Celnikier D, Ariel I, Chaap T, Ben Neriah Z. The role of midtrimester targeted fetal organ screening combined with the "triple test" and maternal age in the diagnosis of trisomy 21: a retrospective study. American Journal of Obstetrics and Gynecology 1998;178(1):40‐4. [DOI] [PubMed] [Google Scholar]
Yamamoto 2001a {published data only}
- Yamamoto R, Azuma M, Kishida T, Yamada H, Satomura S, Fujimoto S. Total alpha‐fetoprotein and Lens culinaris agglutinin‐reactive alpha‐fetoprotein in fetal chromosomal abnormalities. BJOG: an international journal of obstetrics and gynaecology 2001;108(11):1154‐8. [DOI] [PubMed] [Google Scholar]
Yamamoto 2001b {published data only}
- Yamamoto R, Azuma M, Hoshi N, Kishida T, Satomura S, Fujimoto S. Lens culinaris agglutinin‐reactive alpha‐fetoprotein, an alternative variant to alpha‐fetoprotein in prenatal screening for Down's syndrome. Human Reproduction 2001;16(11):2438‐44. [DOI] [PubMed] [Google Scholar]
Yamamoto 2001c {published data only}
- Yamamoto R, Azuma M, Wakui Y, Kishida T, Yamada H, Okuyama K, et al. Alpha‐fetoprotein microheterogeneity: a potential biochemical marker for Down's syndrome. Clinica Chimica Acta 2001;304(1‐2):137‐41. [DOI] [PubMed] [Google Scholar]
Yaron 2001 {published data only}
- Yaron Y, Wolman I, Kupferminc MJ, Ochshorn Y, Many A, Orr‐Urtreger A. Effect of fetal gender on first trimester markers and on Down syndrome screening. Prenatal Diagnosis 2001;21(12):1027‐30. [DOI] [PubMed] [Google Scholar]
Ye 1995 {published data only}
- Ye G, Liao S, Zhao X. The possibility of prenatal screening for fetal abnormalities in second‐trimester pregnancies by measuring AFP, ß‐HCG and uE‐3 levels. Xi'an Yike Daxue Xuebao 1995;16(4):408‐11. [Google Scholar]
Yoshida 2000 {published data only}
- Yoshida K, Kuwabara Y, Tanaka T, Onda T, Kudo R, Yamamoto H, et al. Dimeric Inhibin A as a fourth marker for Down's syndrome maternal serum screening in native Japanese women. Journal of Obstetrics and Gynaecology Research 2000;26(3):171‐4. [DOI] [PubMed] [Google Scholar]
Zalel 2008 {published data only}
- Zalel Y, Achiron R, Yagel S, Kivilevitch Z. Fetal aberrant right subclavian artery in normal and Down syndrome fetuses. Ultrasound in Obstetrics & Gynecology 2008;31(1):25‐9. [DOI] [PubMed] [Google Scholar]
Zeitune 1991 {published data only}
- Zeitune M, Aitken DA, Crossley JA, Yates JR, Cooke A, Ferguson‐Smith MA. Estimating the risk of a fetal autosomal trisomy at mid‐trimester using maternal serum alpha‐fetoprotein and age: A retrospective study of 142 pregnancies. Prenatal Diagnosis 1991;11(11):847‐57. [DOI] [PubMed] [Google Scholar]
Zelop 2005 {published data only}
- Zelop CM, Milewski E, Brault K, Benn P, Borgida AF, Egan JFX. Variation of fetal nasal bone length in second‐trimester fetuses according to race and ethnicity. Journal of Ultrasound in Medicine 2005;24(11):1487‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang J, Lambert‐Messerlian G, Palomaki GE, Canick JA. Impact of smoking on maternal serum markers and prenatal screening in the first and second trimesters. Prenatal Diagnosis 2011;31(6):583‐8. [DOI] [PubMed] [Google Scholar]
Zhao 1998 {published data only}
- Zhao Xiaolan, Ye Guoling, Liu Qi. Using maternal serum PAPP‐A and other pregnancy‐associated proteins in screening for fetal abnormalities. Xi'an Yike Daxue Xuebao 1998;19(1):94‐6, 110. [Google Scholar]
Zhong 2011 {published data only}
- Zhong Y, Longman R, Bradshaw R, Odibo AO. The genetic sonogram: comparing the use of likelihood ratios versus logistic regression coefficients for Down syndrome screening. Journal of Ultrasound in Medicine 2011;30(4):463‐9. [DOI] [PubMed] [Google Scholar]
Zoppi 2003 {published data only}
- Zoppi MA, Ibba RM, Floris M, Manca F, Axiana C, Monni G. Changes in nuchal translucency thickness in normal and abnormal karyotype fetuses. BJOG: an international journal of obstetrics and gynaecology. 2003;110(6):584‐8. [PubMed] [Google Scholar]
Additional references
Alfirevic 2003
- Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2004
- Alfirevic Z, Neilson JP. Antenatal screening for Down's syndrome. BMJ 2004;9(329(7470)):811‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alldred 2010
- Alldred SK, Deeks JJ, Neilson JP, Alfirevic Z. Antenatal screening for Down's syndrome: generic protocol. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD007384.pub2] [DOI] [Google Scholar]
Alldred 2012
- Alldred SK, Deeks JJ, Guo B, Neilson JP, Alfirevic Z. Second trimester serum tests for Down's Syndrome screening. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009925] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bersinger 1995
- Bersinger NA, Zakher A, Huber U, Pescia G, Schneider H. A sensitive enzyme immunoassay for pregnancy‐associated plasma protein A (PAPP‐A): a possible first trimester method of screening for Down syndrome and other trisomies. Archives of Gynecology and Obstetrics 1995;256(4):185‐92. [DOI] [PubMed] [Google Scholar]
Bogart 1987
- Bogart MH, Pandian MR, Jones OW. Abnormal maternal serum chorionic gonadotropin levels in pregnancies with fetal chromosome abnormalities. Prenatal Diagnosis 1987;7(9):623‐30. [DOI] [PubMed] [Google Scholar]
Cuckle 1995
Macri 1990
- Macri JN, Kasturi RV, Krantz DA, Cook EJ, Moore ND, Young JA, et al. Maternal serum Down syndrome screening: free beta‐protein is a more effective marker than human chorionic gonadotropin. American Journal of Obstetrics and Gynecology 1990;163(4 Pt 1):1248‐53. [DOI] [PubMed] [Google Scholar]
Macri 1993
- Macri JN, Spencer K, Aitken D, Garver K, Buchanan PD, Muller F, et al. First‐trimester free beta (hCG) screening for Down syndrome. Prenatal Diagnosis 1993;13(7):557‐62. [DOI] [PubMed] [Google Scholar]
Mol 1999
- Mol BW, Lijmer JG, Meulen J, Pajkrt E, Bilardo CM, Bossuyt PM. Effect of study design on the association between nuchal translucency measurement and Down syndrome. Obstetrics and Gynecology 1999;94(5 Pt 2):864‐9. [DOI] [PubMed] [Google Scholar]
Penrose 1933
- Penrose LS. The relative effects of parental and maternal age in mongolism. Journal of Genetics 1933;27:219‐24. [DOI] [PubMed] [Google Scholar]
Steele 1966
- Steele MW, Breg WR. Chromosome analysis of human amniotic‐fluid cells . Lancet 1966;i:383‐5. [DOI] [PubMed] [Google Scholar]
Vaklenti 1968
- Vaklenti C, Schutta EJ, Kehaty T. Prenatal diagnosis of Down's syndrome. Lancet 1968;ii:220. [DOI] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]


