Abstract
Purpose of review:
Prostate cancer is diagnosed in one out of every nine men and is the second leading cause of cancer death among men. While therapies targeting the androgen receptor are highly effective, development of resistance is universal and remains a major therapeutic challenge. Nonetheless, signaling via androgen receptor is frequently maintained despite standard androgen signaling inhibition. We review the current understanding of mechanisms of resistance as well as therapeutic approaches to improving treatment of prostate cancer via targeting of the androgen receptor.
Recent findings:
Resistance to androgen-receptor-targeting therapies may be mediated by several mechanisms, including amplification, mutation, and alternative splicing of androgen receptor; intratumoral androgen synthesis; activation of alternative signaling pathways; and in a minority of cases, emergence of androgen-receptor-independent phenotypes. Recent trials demonstrate that intensification of androgen blockade in metastatic castration-sensitive prostate cancer can significantly improve survival. Similar strategies are being explored in earlier disease states. In addition, several other cellular signaling pathways have been identified as mechanisms of resistance, offering opportunities for co-targeted therapy. Finally, immune-based approaches are in development to complement androgen-receptor-targeted therapies.
Summary:
Targeting the androgen receptor remains a critical focus in the treatment of prostate cancer.
Keywords: Prostate cancer, castration resistance, androgen receptor, androgen signaling inhibitors
Introduction
Prostate cancer (PCa) adapts to surgical or medical castration therapies (androgen deprivation therapy, ADT) that deplete testicular androgens by both increasing expression of androgen receptor (AR) and by increased intratumoral androgen synthesis. Therefore, although these tumors that relapse after castration are termed castration-resistant PCa (CRPC), they are still largely AR- and androgen-dependent. Hence, they generally respond to agents that further decrease androgen levels by suppressing the synthesis of precursor steroids (CYP17A1 inhibitor abiraterone, ABI) or to direct AR antagonists such as enzalutamide (ENZ) or apalutamide (APA). Unfortunately, although at least transient responses can be obtained in the majority of patients, most men recur within 1-2 years. A major focus of PCa research is now on the mechanisms that are driving resistance to these newer androgen-signaling inhibitors (ASIs), and on therapeutic approaches that may enhance the efficacy of AR-targeted therapies.
Mechanisms Driving Resistance to AR-Targeted Therapies
The use of ASIs may be increasing the number of PCa that become AR-independent (see below), but persistent AR expression and activity in the majority of ABI/ENZ-resistant tumors suggests that AR is still important for tumor growth (Figure 1). Many of the mechanisms that contribute to AR activity in CRPC are also likely operative in ABI/ENZ resistance. One such mechanism is increased AR expression. Recent genomic sequencing studies have confirmed that the AR gene is amplified in over half of cases (1-5), although it is not yet clear whether the extent of AR gene amplification is further increased in response to ASIs. Significantly, recent studies have identified a potent amplified upstream AR enhancer that may be the major driver of increased AR expression (3, 6, 7).
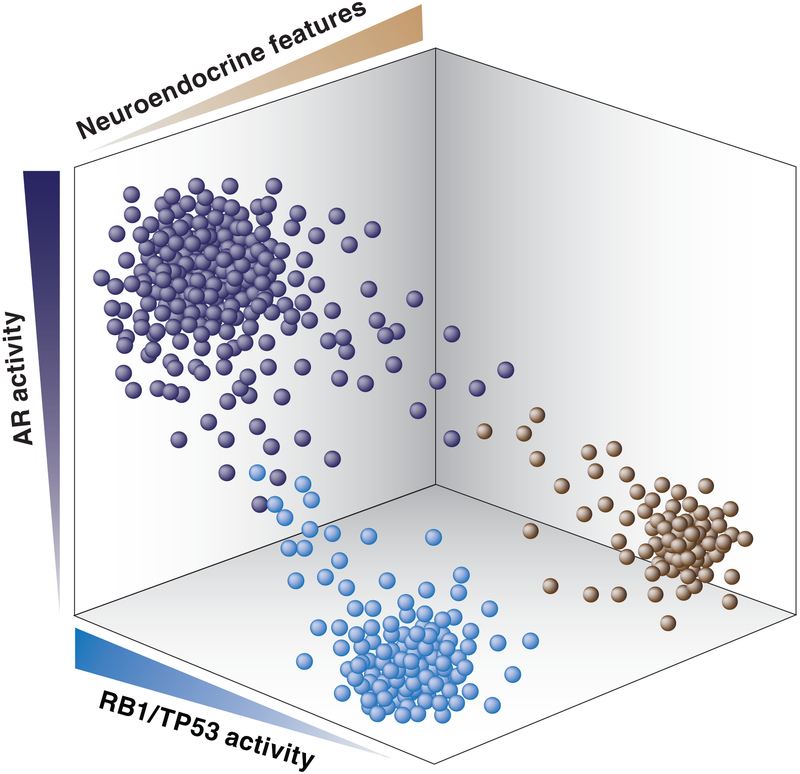
Figure 1. Landscape of AR activity, RB1/TP53 activity, and neuroendocrine features in advanced PCa.
Each dot reflects hypothetical level of AR activity, RB1/TP53 activity, and neuroendocrine differentiation in an individual advanced tumor that is resistant to AR targeted therapies.
Recurrent AR mutations that allow the AR to be activated by alternative ligands are also found in a subset of ABI-ENZ-resistant tumors. These include mutations in codons 875 and 878 that allow AR to be strongly activated by progesterone (which is upstream of CYP17A1 and markedly increased by ABI treatment), and a codon 702 mutation that allows activation by cortisol (1-5, 8). Codon 877mutation can allow activation by ENZ, but this has only rarely been detected in clinical samples, perhaps because a second mutation in codon 878 is required in order to obtain robust activation by ENZ (9).
One basis of increased intratumoral androgen synthesis is a common polymorphism that increases the stability of HSD3B1, which converts DHEA to androstenedione (10-12). This HSD3B1 variant can also increase the metabolism of ABI to an AR agonist, which may contribute to ABI resistance (13). More generally, persistent/increased intratumoral androgens could contribute to ENZ resistance as it is a competitive antagonist, and there is no clear evidence that ENZ gains partial agonist activity. Similarly, persistent androgen synthesis may contribute to ABI resistance (14, 15). However, several studies that have attempted to further decrease androgen levels, including ABI dose escalation (16), adding dutasteride to ABI (17), adding ABI to ENZ at time of ENZ resistance (18), or treatment with an AKR1C3 inhibitor (19), have not yet yielded evidence of efficacy. Nonetheless, it remains unclear whether AR activity in ABI/ENZ-resistant tumors is mediated by an unliganded or ENZ-liganded AR, by alternative ligands, or by low levels of androgen-liganded AR (which may be hyperactive due to posttranslational modifications, alterations in coactivator proteins, or in chromatin structure).
A further mechanism that can drive resistance to current AR-directed therapies, which all target the AR ligand binding domain (LBD), is expression of AR splice variants that contain the transcriptionally active N-terminal domain (NTD) and DNA binding domain (DBD), but delete the LBD (20). Low levels of transcripts predicted to encode multiple such isoforms have been identified, but the major transcripts (which have been confirmed to encode proteins) are AR-V7 (which is most common) and ARv567es. Previous studies have shown that AR-V7 expression increases with progression to CRPC, and a series of recent studies found that AR-V7 expression in circulating tumor cells (CTCs) is associated with ABI/ENZ resistance (21-23). However, increased AR-V7 is associated with increased full length AR (AR-FL), and it remains unclear whether AR-V7 is a biomarker or also a driver (dependent or independent of AR-FL) of resistance. A recent study using a validated AR-V7-specific antibody has established that AR-V7 is very rarely expressed in primary PCa, but is expressed in ~75% of CRPC prior to ABI/ENZ therapy, and is further increased in ABI/ENZ resistance (24). Although all tumors that were AR-V7 negative responded to ABI or ENZ, 54% of the AR-V7 positive tumors also responded. Therefore, while AR-V7 detection in CTCs may be more predictive than in tissue, AR-V7 expression clearly does not preclude responses to ASIs, and its role as a biomarker remains to be clarified (25, 26).
The extent to which AR-V7 (or AR-FL) is driving ABI/ENZ resistance will not be clear until effective inhibitors are available. In addition to APA, there are several other new agents in clinical trials that target CYP17A1 and/or AR (including orteronel, seviteronel, and darolutamide), but there is no clear mechanistic basis by which they might be substantially better that ABI or ENZ. In contrast, several groups are developing novel agents that bind the AR LBD and target it to a ubiquitin ligase to drive AR degradation (27), with one such agent poised to enter clinical trials (ARV-110, Arvinas). To the extent that ABI/ENZ-resistant tumors are being driven by AR-FL (either as homodimers or possibly heterodimers with AR-V7), these agents may be very effective. There are also many efforts to develop agents targeting the AR NTD or DBD, which should be effective against AR-V7 and other splice variants, but this has proven very challenging (28-31).
While AR activity persists in most ABI/ENZ-resistant tumors, it is generally decreased, and additional mechanisms are likely decreasing AR dependence. One such mechanism is increased expression of glucocorticoid receptor, which can drive the expression of multiple pro-growth/survival genes, with some also being AR targets (32-35). Recent sequencing efforts have expanded the number of identified recurrently mutated genes in PCa, and shown that most (with the striking exception of SPOP) are more frequently mutated in metastatic CRPC (mCRPC) versus primary PCa (1-5). Significantly, with the exception of APC, all were enriched in mCRPC relative to metastatic castration-sensitive PCa (mCSPC), suggesting a role in progression to castration resistance. In addition to AR, the genes more frequently altered in mCRPC include RB1, BRCA2, CCND1, KMT2D, KMT2C, FOXA1, and MYC. These alterations may have direct or indirect effects on AR, and enhance tumor growth by AR-independent mechanisms.
A subset of PCa that recurs after castration, and particularly after treatment with ASIs, has low or absent AR expression and activity, may have neuroendocrine features including small cell histology and expression of genes such as SYP and CHGA, and is associated with genomic losses of RB1 and TP53 (36, 37) (Figure 1). A recent analysis of metastatic PCa biopsies, primarily from men progressing on ABI/ENZ, supports an association between small cell histology, decreased AR expression/activity, neuroendocrine markers, and RB1 loss, but show that these subsets are only partially overlapping (38). A second recent study examined tumors obtained through rapid autopsies from 2012-2016, versus a cohort prior to the introduction of ASIs (from 1998-2011), and found a doubling (6.3% to 13.3%) in the fraction of patients with neuroendocrine PCa (negative for AR/PSA and positive for SYP/CHGA) in the 2012-2016 cohort, and in addition these tumors were enriched for RB1 loss (39). However, this study also found an increase in tumors that were AR- and SYP/CHGA-negative (from 5.4% to 23.3% in the latter period). Together these and other studies indicate that up to ~30% of late-stage ASI-resistant tumors may have minimal or no AR dependence, that ~15% may have neuroendocrine features, and that this latter group is enriched for RB1 loss. However, the precise role of RB1 or TP53 loss, and of additional transcription modulators, in neuroendocrine differentiation is not yet clear (40-47). Unfortunately, therapies that can effectively target RB1 and/or TP53 loss are lacking, and it is not clear whether there are exploitable vulnerabilities conferred by neuroendocrine differentiation (48).
Therapeutic Approaches to Enhance Efficacy of AR-Targeted Therapies
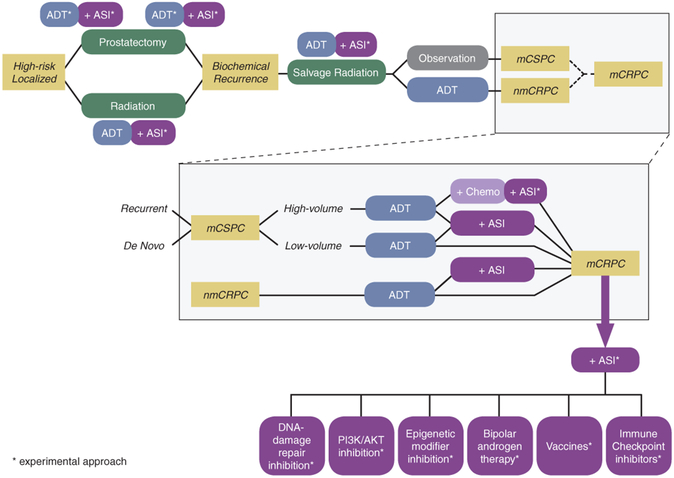
Novel strategies for targeting AR include intensified androgen blockade in earlier disease settings, co-targeting other pathways, bipolar androgen therapy, and immuno-oncology approaches (Figure 2).
Figure 2.
Standard and experimental approaches to target AR across the spectrum of PCa.
Intensified Androgen Blockade Earlier in the Disease
The addition of ABI to ADT for mCSPC improves overall survival (OS) (49, 50). An ongoing trial (NCT01957436) will test whether this strategy should be further intensified with the addition of docetaxel to ADT and ABI. Another trial (NCT01809691) will evaluate the effectiveness of orteronel in mCSPC. The addition of ABI even earlier may also be effective, such as in the pre-metastatic setting (50), although longer-term follow-up is needed.
The addition of AR antagonists for mCSPC (with or without docetaxel) is also under study in ongoing trials, including ENZ (NCT02446405, NCT02677896), APA (NCT02489318), and darolutamide (NCT02799602). Of note, a trial of combined docetaxel and APA in mCRPC (NCT03093272) is on hold due to concerns regarding pneumonitis, raising the possibility of unexpected toxicity. Finally, although adding ABI to ENZ at time of ENZ-resistance was not effective in mCRPC (18), the up-front combination of ABI and ENZ is being tested in mCSPC (NCT00268476).
Although the use and timing of ADT for “pre-metastatic” biochemically recurrent PCa (BCR) is controversial, intensified AR blockade is effective in nonmetastatic CRPC (nmCRPC) patients experiencing biochemical progression, particularly those with shorter PSA doubling times, as the addition of APA or ENZ to ADT improved metastasis-free survival versus ongoing ADT alone (51, 52). Metastasis-free survival was used as a surrogate for OS in these nmCRPC trials, but OS data are not yet mature. It is not certain that this strategy will be superior to starting these agents after development of metastatic disease. In addition, up-front ABI with ADT for localized or BCR CSPC may be an alternative strategy (50), but longer follow-up is needed to assess effects on OS.
Recent studies have examined neoadjuvant intensive androgen blockade in men with high-risk localized PCa (53-56). While this reduces disease in the primary site, further follow-up will be needed to determine whether this translates into improved disease-free survival or OS, and whether recurrent disease is more aggressive. A similar strategy is being tested in very high-risk localized and oligometastatic PCa (NCT03436654). Molecular analysis of residual tumor in these cases may reveal mechanisms of resistance that can be used to guide adjuvant therapies or strategies for overcoming resistance in more advanced disease (57). Intensified androgen blockade is also under investigation in combination with primary radiation therapy for localized PCa (NCT02446444).
Therapies Targeting AR in Combination with other Pathways
The addition of docetaxel to ADT improved OS in mCSPC (58, 59), although the benefit is limited to patients with greater disease volume (60). Since intensified AR blockade appears to be effective even in low-volume mCSPC, an important question is whether patients who present with high-volume mCSPC are a molecularly distinct subset (rather than just patients who present later in their disease course), and whether there are biomarkers that might identify this subset. Disease volume may correlate with loss of tumor suppressor genes (61), but precise biomarkers and mechanisms of taxane sensitization are unclear. Currently, clinicians may offer ADT plus docetaxel or ABI to patients with high-volume mCSPC, and ADT plus ABI to patients with low-volume disease, understanding that both agents are likely to be used at some point in the disease course.
There is substantial cross-talk between AR signaling and multiple other pathways that might be exploited by combination therapies. DNA-damage repair (DDR) defects in ~20% of mCRPC can sensitize patients to platinum or PARP inhibitor therapy (62, 63). Monotherapy with PARP inhibitors (plus ongoing ADT) is increasingly becoming standard-of-care in DDR-deficient tumors, although better predictive biomarkers are imperative. ADT may also directly or indirectly (through G0/G1 arrest) impair DDR and/or impart PARP-dependence (64-69). One study tested the hypothesis that AR/PARP co-targeting with ABI and veliparib would improve outcomes (70). Surprisingly, adding veliparib did not improve responses over ABI alone, even in the DDR-deficient subset. In contrast, DDR alterations seemed to predict for better response to ABI with/without PARP inhibition, although this is not a consistent finding (71). Moreover, adding olaparib to ABI did improve responses in another phase 2 study (72).
PTEN loss and PI3K/AKT pathway activation are common in mCRPC and (depending on the model) can enhance or repress AR expression/activity. Unfortunately, PI3K inhibition alone has had little clinical efficacy, possibly due to dose-limiting on-target toxicity that might be addressed with isoform-specific inhibitors. Promising results were seen in the first-line mCRPC setting with ABI plus an AKT inhibitor (ipatasertib), suggesting earlier use of combination therapies in selected patients may be effective (73). There are a series of CDK4/6 inhibitor trials in mCRPC, including one of abemaciclib combined with ABI (NCT03706365), although by analogy with results in breast cancer, these agents might be most effective if used earlier in CSPC together with aggressive androgen blockade. Additional compelling therapeutic targets in mCRPC that may interact with AR include BRD4 (74-79) and EZH2 (80, 81). The latter may also contribute to neuroendocrine differentiation (40, 82, 83), and EZH2 inhibition is being tested in ASI-exposed patients in combination with a second ASI (NCT03480646).
Bipolar Androgen Therapy (BAT)
The adaptive overexpression of AR in mCRPC may present a therapeutic vulnerability, as supraphysiological testosterone can cause growth arrest and death in PCa models with AR overexpression through transient DNA damage, downregulation of MYC, or enhanced expression of genes driving differentiation. Thus, alternating high and low testosterone (“BAT”) may offer a therapeutic strategy in mCRPC, and PSA responses to BAT and ENZ re-challenge have been demonstrated (84). DDR alterations may especially sensitize PCa to BAT (85).
AR-Targeted Therapy and Immuno-Oncology
The impact of AR-targeted therapy on the immune milieu of PCa is complex and may include both pro- and anti-inflammatory effects. The dendritic cell vaccine sipuleucel-T conferred a survival benefit in the pre-ASI era, and trials are looking at combining ASIs with sipuleucel-T (NCT01981122, NCT01487863), and ADT with novel vaccines (NCT02649855, NCT02107391, NCT01696877, NCT01436968, NCT01496131) or ASI with novel vaccines (NCT01875250). Inhibition of CTLA-4 and PD-1/PD-L1 had disappointing results in advanced mCRPC, but more recent data suggest that there may be a subset of PCa that is responsive to this strategy (86, 87). Responses were seen with the addition of PD-1 inhibition in the setting of ENZ resistance (88), although one response was likely explained by microsatellite instability (89). PD-L1 expression appears to be higher in mCRPC versus primary PCa and more prevalent than previously appreciated (90, 91), but this may not predict responses. Moreover, castration may increase immune-suppressive cells in the tumor microenvironment (92), prompting trials of vaccines and checkpoint inhibitors in pre-castration/pre-ASI BCR (NCT02649439, NCT03637543). Of note, myeloid-derived suppressor cells may also drive development of CRPC, potentially targetable with IL-23 blockade (93).
Conclusion
The earlier use of intensive androgen blockade appears promising. AR appears to remain a therapeutic target in many men who progress to ABI/ENZ resistance, but the basis for its persistent activity is unclear and novel agents that directly or indirectly ablate AR activity are needed. ASIs are increasing the fraction of AR-independent tumors, a subset of which have neuroendocrine features, but loss of RB1 and TP53 in many of these tumors presents a major therapeutic challenge.
Key Points.
Androgen receptor-mediated signaling remains a key driver of prostate cancer even after resistance to androgen receptor-targeted therapies.
Several recently identified mechanisms may preserve androgen receptor signaling in the treatment-resistant setting.
One potential approach is the use of intensive androgen blockade using androgen-signaling inhibitors in addition to conventional androgen deprivation therapy in earlier disease settings.
Potential methods of treating castration-resistant disease include co-targeting of alternative pathways including DNA damage repair, PI3K/AKT, and epigenetic modifiers; bipolar androgen therapy; and a variety of immunomodulatory approaches in combination with androgen receptor-targeted therapy.
Acknowledgments
Financial Support and Sponsorship
DJE:
Congressionally Directed Medical Research Programs (CDMRP) Prostate Cancer Research Program (PCRP) Physician Research Award #W81XWH-17-1-0350
Koch/DFHCC Bridge Grant (partnership between the Koch Institute for Integrative Cancer Research at MIT and the Dana-Farber/Harvard Cancer Center)
Prostate Cancer Foundation Challenge Award
Bristol-Myers Squibb (research funding to institution)
Trovagene (research funding to institution)
SA:
Research Fellowship Award from Gunma University Hospital, Japan
SPB:
Koch/DFHCC Bridge Grant Koch/DFHCC Bridge Grant (partnership between the Koch Institute for Integrative Cancer Research at MIT and the Dana-Farber/Harvard Cancer Center)
Prostate Cancer Foundation Challenge Award
DOD Prostate Cancer Research Program Impact Award (W81XWH-16-1-043)
DOD Prostate Cancer Research Program Idea Development Award (PC170715)
NIH R01 CA168393
NIH P01 CA163227
NIH P50 CA090381
Footnotes
Conflicts of Interest
Dr. Einstein is the PI of NCT03637543 and receives institutional research support from Bristol-Myers Squibb. Dr. Balk has consulted for Sanofi, Janssen, and Astellas.
References
- 1.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. *.Armenia J, Wankowicz SAM, Liu D, Gao J, Kundra R, Reznik E, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50(5):645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified 70 new significantly mutated genes found at low prevalence in PCa and compared genomic markers in primary versus metastatic disease.
- 3. **.Quigley DA, Dang HX, Zhao SG, Lloyd P, Aggarwal R, Alumkal JJ, et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell. 2018;174(3):758–69 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified amplification of an AR enhancer as a major mechanism driving increased AR expression in CRPC, and identifed an associatiin between CD12 loss and tandem duplications.
- 4.Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. *.Wedge DC, Gundem G, Mitchell T, Woodcock DJ, Martincorena I, Ghori M, et al. Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat Genet. 2018;50(5):682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a major sequencing effort that identified 22 novel putative driver genes harboring coding mutations, and it examined temporal genomic events in the progression of prostate cancer.
- 6. **.Takeda DY, Spisak S, Seo JH, Bell C, O’Connor E, Korthauer K, et al. A Somatically Acquired Enhancer of the Androgen Receptor Is a Noncoding Driver in Advanced Prostate Cancer. Cell. 2018;174(2):422–32 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified the major enhancer of the AR and showed that it is frequently amplified in CRPC.
- 7. **.Viswanathan SR, Ha G, Hoff AM, Wala JA, Carrot-Zhang J, Whelan CW, et al. Structural Alterations Driving Castration-Resistant Prostate Cancer Revealed by Linked-Read Genome Sequencing. Cell. 2018;174(2):433–47 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified amplification of an AR enhancer in CRPC, and a tandem duplication phenotype with CDK12 loss.
- 8.McKay RR, Werner L, Mostaghel EA, Lis R, Voznesensky O, Zhang Z, et al. A Phase II Trial of Abiraterone Combined with Dutasteride for Men with Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prekovic S, van Royen ME, Voet AR, Geverts B, Houtman R, Melchers D, et al. The Effect of F877L and T878A Mutations on Androgen Receptor Response to Enzalutamide. Mol Cancer Ther. 2016;15(7):1702–12. [DOI] [PubMed] [Google Scholar]
- 10.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almassi N, Reichard C, Li J, Russell C, Perry J, Ryan CJ, et al. HSD3B1 and Response to a Nonsteroidal CYP17A1 Inhibitor in Castration-Resistant Prostate Cancer. JAMA Oncol. 2018;4(4):554–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hearn JWD, Xie W, Nakabayashi M, Almassi N, Reichard CA, Pomerantz M, et al. Association of HSD3B1 Genotype With Response to Androgen-Deprivation Therapy for Biochemical Recurrence After Radiotherapy for Localized Prostate Cancer. JAMA Oncol. 2018;4(4):558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alyamani M, Emamekhoo H, Park S, Taylor J, Almassi N, Upadhyay S, et al. HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J Clin Invest. 2018;128(8):3333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamae D, Mostaghel E, Montgomery B, Nelson PS, Balk SP, Kantoff PW, et al. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chemico-biological interactions. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. *.Mostaghel EA, Zhang A, Hernandez S, Marck BT, Zhang X, Tamae D, et al. Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer. Clin Cancer Res. 2019;25(1):426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study suggested that additional blockade of adrenal androgens, beyond the effect of abiraterone, could have clinical benefit.
- 16.Friedlander TW, Graff JN, Zejnullahu K, Anantharaman A, Zhang L, Paz R, et al. High-Dose Abiraterone Acetate in Men With Castration Resistant Prostate Cancer. Clin Genitourin Cancer. 2017;15(6):733–41 e1. [DOI] [PubMed] [Google Scholar]
- 17.McKay RR, Werner L, Mostaghel EA, Lis R, Voznesensky O, Zhang Z, et al. A Phase II Trial of Abiraterone Combined with Dutasteride for Men with Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2017;23(4):935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. *.Attard G, Borre M, Gurney H, Loriot Y, Andresen-Daniil C, Kalleda R, et al. Abiraterone Alone or in Combination With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer With Rising Prostate-Specific Antigen During Enzalutamide Treatment. J Clin Oncol. 2018;36(25):2639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]; This negative trial tested whether additional ligand depletion with the addition of abiraterone could overcome resistance to enzalutamide.
- 19.Loriot Y, Fizazi K, Jones RJ, Van den Brande J, Molife RL, Omlin A, et al. Safety, tolerability and anti-tumour activity of the androgen biosynthesis inhibitor ASP9521 in patients with metastatic castration-resistant prostate cancer: multi-centre phase I/II study. Invest New Drugs. 2014;32(5):995–1004. [DOI] [PubMed] [Google Scholar]
- 20.Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. 2016;19(3):231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2015;1(5):582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016;2(11):1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. *.Sharp A, Coleman I, Yuan W, Sprenger C, Dolling D, Rodrigues DN, et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129(1):192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]; This comprehensive assessment of AR-V7 with a validated antibody established its pattern of expression in tumor tissue and association with response to AR targeted therapy.
- 25.Bubley GJ, Balk SP. Association Between Androgen Receptor Splice Variants and Prostate Cancer Resistance to Abiraterone and Enzalutamide. J Clin Oncol. 2017;35(19):2103–5. [DOI] [PubMed] [Google Scholar]
- 26.Dehm SM, Montgomery B, Plymate SR. AR Variant Positive CTC: A Surrogate for a Surrogate for Taxane Therapy Outcome? Clin Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]
- 27.Salami J, Alabi S, Willard RR, Vitale NJ, Wang J, Dong H, et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol. 2018;1:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalal K, Roshan-Moniri M, Sharma A, Li H, Ban F, Hassona MD, et al. Selectively targeting the DNA-binding domain of the androgen receptor as a prospective therapy for prostate cancer. J Biol Chem. 2014;289(38):26417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalal K, Che M, Que NS, Sharma A, Yang R, Lallous N, et al. Bypassing Drug Resistance Mechanisms of Prostate Cancer with Small Molecules that Target Androgen Receptor-Chromatin Interactions. Mol Cancer Ther. 2017;16(10):2281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalal K, Morin H, Ban F, Shepherd A, Fernandez M, Tam KJ, et al. Small molecule-induced degradation of the full length and V7 truncated variant forms of human androgen receptor. Eur J Med Chem. 2018;157:1164–73. [DOI] [PubMed] [Google Scholar]
- 31.Dalal K, Ban F, Li H, Morin H, Roshan-Moniri M, Tam KJ, et al. Selectively targeting the dimerization interface of human androgen receptor with small-molecules to treat castration-resistant prostate cancer. Cancer Lett. 2018;437:35–43. [DOI] [PubMed] [Google Scholar]
- 32.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puhr M, Hoefer J, Eigentler A, Ploner C, Handle F, Schaefer G, et al. The Glucocorticoid Receptor Is a Key Player for Prostate Cancer Cell Survival and a Target for Improved Antiandrogen Therapy. Clin Cancer Res. 2018;24(4):927–38. [DOI] [PubMed] [Google Scholar]
- 34.Shah N, Wang P, Wongvipat J, Karthaus WR, Abida W, Armenia J, et al. Regulation of the glucocorticoid receptor via a BET-dependent enhancer drives antiandrogen resistance in prostate cancer. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kach J, Long TM, Selman P, Tonsing-Carter EY, Bacalao MA, Lastra RR, et al. Selective Glucocorticoid Receptor Modulators (SGRMs) Delay Castrate-Resistant Prostate Cancer Growth. Mol Cancer Ther. 2017;16(8):1680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22(3):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol. 2018;15(5):271–86. [DOI] [PubMed] [Google Scholar]
- 38. **.Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol. 2018;36(24):2492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]; This large study of transcriptomes shows a complex relationship between AR signaling, RB1 status, and neuroendocrine features in tumors that become resistant to AR targeted therapies.
- 39. **.Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell. 2017;32(4):474–89 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that tumors that are negative for AR and neuroendocrine features are becoming more frequent since the introduction of new AR targeted therapies.
- 40.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355(6320):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355(6320):84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop JL, Thaper D, Vahid S, Davies A, Ketola K, Kuruma H, et al. The Master Neural Transcription Factor BRN2 Is an Androgen Receptor-Suppressed Driver of Neuroendocrine Differentiation in Prostate Cancer. Cancer Discov. 2017;7(1):54–71. [DOI] [PubMed] [Google Scholar]
- 43.Rotinen M, You S, Yang J, Coetzee SG, Reis-Sobreiro M, Huang WC, et al. ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat Med. 2018;24(12):1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akamatsu S, Wyatt AW, Lin D, Lysakowski S, Zhang F, Kim S, et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell reports. 2015;12(6):922–36. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Coleman IM, Brown LG, True LD, Kollath L, Lucas JM, et al. SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer. Clin Cancer Res. 2015;21(20):4698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flores-Morales A, Bergmann TB, Lavallee C, Batth TS, Lin D, Lerdrup M, et al. Proteogenomic Characterization of Patient-Derived Xenografts Highlights the Role of REST in Neuroendocrine Differentiation of Castration-Resistant Prostate Cancer. Clin Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Donmez N, Sahinalp C, Xie N, Wang Y, Xue H, et al. SRRM4 Drives Neuroendocrine Transdifferentiation of Prostate Adenocarcinoma Under Androgen Receptor Pathway Inhibition. Eur Urol. 2017;71(1):68–78. [DOI] [PubMed] [Google Scholar]
- 48. *.Beltran H, Oromendia C, Danila DC, Montgomery B, Hoimes C, Szmulewitz RZ, et al. A Phase II Trial of the Aurora Kinase A Inhibitor Alisertib for Patients with Castration-resistant and Neuroendocrine Prostate Cancer: Efficacy and Biomarkers. Clin Cancer Res. 2019;25(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a trial that tested aurora kinase inhibition for neuroendocrine prostate cancer. It was negative for its primary endpoint, but exceptional responders were identified.
- 49.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. New England Journal of Medicine. 2017;377(4):352–60. [DOI] [PubMed] [Google Scholar]
- 50.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. New England Journal of Medicine. 2017;377(4):338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. *.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. New England Journal of Medicine. 2018;378(15):1408–18. [DOI] [PubMed] [Google Scholar]; This trial demonstrated improved metastasis-free survival with the addition of apalutamide to ADT for non-metastatic castration-resistant prostate cancer.
- 52. *.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore ND, Demirhan E, et al. PROSPER: A phase 3, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). American Society of Clinical Oncology; 2018. [Google Scholar]; This trial demonstrated improved metastasis-free survival with the addition of enzalutamide to ADT for non-metastatic castration-resistant prostate cancer.
- 53.Montgomery B, Tretiakova MS, Joshua AM, Gleave ME, Fleshner N, Bubley GJ, et al. Neoadjuvant Enzalutamide Prior to Prostatectomy. Clin Cancer Res. 2017;23(9):2169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mostaghel EA, Nelson PS, Lange P, Lin DW, Taplin ME, Balk S, et al. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J Clin Oncol. 2014;32(3):229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense Androgen-Deprivation Therapy With Abiraterone Acetate Plus Leuprolide Acetate in Patients With Localized High-Risk Prostate Cancer: Results of a Randomized Phase II Neoadjuvant Study. J Clin Oncol. 2014;32(33):3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKay RR, Montgomery B, Xie W, Zhang Z, Bubley GJ, Lin DW, et al. Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis. 2018;21(3):364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. *.Sowalsky AG, Ye H, Bhasin M, Van Allen EM, Loda M, Lis RT, et al. Neoadjuvant-intensive androgen deprivation therapy selects for prostate tumor foci with diverse subclonal oncogenic alterations. Cancer Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found subclonal oncogenic genomic alterations in tumor foci that were resistant to intensive neoadjuvant androgen blockade.
- 58.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. The Lancet. 2016;387(10024):1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. New England Journal of Medicine. 2015;373(8):737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. *.Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. Journal of Clinical Oncology. 2018;36(11):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]; This long-term follow-up study confirmed lack of benefit from the addition of docetaxel to castration-sensitive low-volume prostate cancer.
- 61.Hamid A, Gray KP, Shaw G, MacConaill LE, Evan C, Bernard BD, et al. Tumor suppressor aberrations and outcomes in localized and metastatic hormone sensitive prostate cancer (PrCa). American Society of Clinical Oncology; 2018. [Google Scholar]
- 62.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. New England Journal of Medicine. 2015;373(18):1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pomerantz MM, Spisák S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The association between germline BRCA2 variants and sensitivity to platinum‐based chemotherapy among men with metastatic prostate cancer. Cancer. 2017;123(18):3532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao S, Gao Y, He HH, Han D, Han W, Avery A, et al. Androgen Receptor Tumor Suppressor Function Is Mediated by Recruitment of Retinoblastoma Protein. Cell reports. 2016;17(4):966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asim M, Tarish F, Zecchini HI, Sanjiv K, Gelali E, Massie CE, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017;8(1):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Min A, Jang H, Kim S, Lee KH, Kim DK, Suh KJ, et al. Androgen Receptor Inhibitor Enhances the Antitumor Effect of PARP Inhibitor in Breast Cancer Cells by Modulating DNA Damage Response. Mol Cancer Ther. 2018;17(12):2507–18. [DOI] [PubMed] [Google Scholar]
- 69.Jividen K, Kedzierska KZ, Yang CS, Szlachta K, Ratan A, Paschal BM. Genomic analysis of DNA repair genes and androgen signaling in prostate cancer. BMC Cancer. 2018;18(1):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. *.Hussain M, Daignault-Newton S, Twardowski PW, Albany C, Stein MN, Kunju LP, et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. Journal of Clinical Oncology. 2018;36(10):991. [DOI] [PMC free article] [PubMed] [Google Scholar]; This trial showed no improvement in responses to abiraterone with the addition of veliparib, regardless of DNA-damage-repair status, but improved responses to abiraterone regardless of veliparib in the DNA-damage-repair-deficient subgroup.
- 71.Annala M, Struss WJ, Warner EW, Beja K, Vandekerkhove G, Wong A, et al. Treatment Outcomes and Tumor Loss of Heterozygosity in Germline DNA Repair-deficient Prostate Cancer. Eur Urol. 2017;72(1):34–42. [DOI] [PubMed] [Google Scholar]
- 72. *.Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975–86. [DOI] [PubMed] [Google Scholar]; This trial demonstrated improved responses to abiraterone with the addition of olaparib, unselected by DNA-damage-repair status.
- 73. *.de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]; This trial demonstrated improved responses to abiraterone with the addition of ipatasertib, especially in the PTEN-deficient subset.
- 74.Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AM, et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2016;113(26):7124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. *.Welti J, Sharp A, Yuan W, Dolling D, Nava Rodrigues D, Figueiredo I, et al. Targeting Bromodomain and Extra-Terminal (BET) Family Proteins in Castration-Resistant Prostate Cancer (CRPC). Clin Cancer Res. 2018;24(13):3149–62. [DOI] [PubMed] [Google Scholar]; This study showed correlation of BRD4 expression with treatment resistance, and BET inhibition demonstrated anti-tumor effects in preclinical models.
- 76.Chan SC, Selth LA, Li Y, Nyquist MD, Miao L, Bradner JE, et al. Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015;43(12):5880–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urbanucci A, Barfeld SJ, Kytola V, Itkonen HM, Coleman IM, Vodak D, et al. Androgen Receptor Deregulation Drives Bromodomain-Mediated Chromatin Alterations in Prostate Cancer. Cell reports. 2017;19(10):2045–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510(7504):278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asangani IA, Wilder-Romans K, Dommeti VL, Krishnamurthy PM, Apel IJ, Escara-Wilke J, et al. BET Bromodomain Inhibitors Enhance Efficacy and Disrupt Resistance to AR Antagonists in the Treatment of Prostate Cancer. Mol Cancer Res. 2016;14(4):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338(6113):1465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J, Lee Y, Lu X, Song B, Fong KW, Cao Q, et al. Polycomb- and Methylation-Independent Roles of EZH2 as a Transcription Activator. Cell reports. 2018;25(10):2808–20 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dardenne E, Beltran H, Benelli M, Gayvert K, Berger A, Puca L, et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell. 2016;30(4):563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Zheng D, Zhou T, Song H, Hulsurkar M, Su N, et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun. 2018;9(1):4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. *.Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, Bruns A, et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol. 2018;19(1):76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]; This trial demonstrated the effectiveness of bipolar androgen therapy in targeting androgen receptor and reversing enzalutamide resistance.
- 85.Teply BA, Kachhap S, Eisenberger MA, Denmeade SR. Extreme response to high-dose testosterone in BRCA2-and ATM-mutated prostate cancer. European Urology. 2017;71(3):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Bono JS, Goh JC, Ojamaa K, Piulats Rodriguez JM, Drake CG, Hoimes CJ, et al. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology; 2018. [Google Scholar]
- 87. *.Hansen A, Massard C, Ott P, Haas N, Lopez J, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Annals of Oncology. 2018;29(8):1807–13. [DOI] [PubMed] [Google Scholar]; This trial demonstrated a subset of responses to PD-1 inhibition in metastatic castration-resistant prostate cancer.
- 88.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33):52810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New England Journal of Medicine. 2015;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calagua C, Russo J, Sun Y, Schaefer R, Lis R, Zhang Z, et al. Expression of PD-L1 in Hormone-naïve and Treated Prostate Cancer Patients Receiving Neoadjuvant Abiraterone Acetate plus Prednisone and Leuprolide. Clinical Cancer Research. 2017. [DOI] [PubMed] [Google Scholar]
- 91. *.Haffner MC, Guner G, Taheri D, Netto GJ, Palsgrove DN, Zheng Q, et al. Comprehensive Evaluation of Programmed Death-Ligand 1 Expression in Primary and Metastatic Prostate Cancer. Am J Pathol. 2018;188(6):1478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was a large-scale analysis of PD-L1 expression across disease states in prostate cancer.
- 92.Mehra N, Seed G, Lambros M, Sharp A, Fontes MS, Crespo M, et al. Myeloid-derived suppressor cells (MDSCs) in metastatic castration-resistant prostate cancer (CRPC) patients (PTS). Annals of Oncology. 2016;27(suppl_6). [Google Scholar]
- 93.Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363. [DOI] [PMC free article] [PubMed] [Google Scholar]