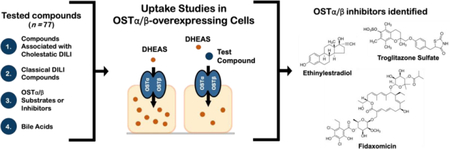
Abstract
Drug interactions with the organic solute transporter alpha/beta (OSTα/β) are understudied even though OSTα/β is an important transporter that is expressed in multiple human tissues including the intestine, kidneys and liver. In this study, an in vitro method to identify novel OSTα/β inhibitors was first developed using OSTα/β-overexpressing Flp-In 293 cells. Incubation conditions were optimized using previously reported OSTα/β inhibitors. A method including a 10-min preincubation step with the test compound was used to screen for OSTα/β inhibition by 77 structurally diverse compounds and fixed-dose combinations. Seven compounds and one fixed-dose combination (100 µM final concentration) inhibited OSTα/β-mediated DHEAS uptake by >25%. Concentration-dependent OSTα/β inhibition was evaluated for all putative inhibitors (atorvastatin, ethinylestradiol, fidaxomicin, glycochenodeoxycholate, norgestimate, troglitazone and troglitazone sulfate). Ethinylestradiol, fidaxomicin and troglitazone sulfate yielded a clear concentration-inhibition response with IC50 values <200 µM. Among all tested compounds, there was no clear association between physicochemical properties, the severity of hepatotoxicity, and the degree of OSTα/β inhibition. This study utilized a novel in vitro method to identify OSTα/β inhibitors, and for the first time, provided IC50 values for OSTα/β inhibition. These data provide evidence that several drugs, some of which are associated with cholestatic drug-induced liver injury, may impair the function of the OSTα/β transporter.
Keywords: basolateral efflux, bile acid, inhibition, cholestasis, drug-induced liver injury, SLC51A/B
Graphical Abstract
INTRODUCTION
Membrane-bound ATP-binding cassette (ABC) and solute carrier (SLC) transporters are major determinants of the disposition, efficacy and safety of many drugs. Therefore, several methods to assess transporter activity and inhibition have been developed to identify potential transporter-dependent drug interactions in drug discovery and development.1 Organic solute transporter alpha/beta (OSTα/β, SLC51A/B) is expressed in multiple human tissues with the highest levels in intestine, adrenal gland, testis and liver,2–4 where it mediates the transport of bile acids and sulfated steroid hormones, dehydroepiandrosterone sulfate (DHEAS), estrone sulfate (ES) and pregnenolone sulfate (PREGS).3, 5 OSTα/β also has been reported to facilitate the transport of a few xenobiotics including digoxin, rosuvastatin, atorvastatin and docetaxel.6, 7 However, substrates and inhibitors of OSTα/β are not routinely evaluated during drug development.
There is only limited data available regarding drugs that interact with OSTα/β. Initial studies in X. laevis oocytes suggested that steroidal compounds, such as spironolactone and taurolithocholic acid sulfate (TLCAS) dosed at a single concentration of 100–1000 μM, inhibited OSTα/β-mediated taurocholate (TCA) and ES uptake.2, 6 The largest study thus far examining OSTα/β inhibition evaluated 1,280 FDA-approved drugs as OSTα/β inhibitors in a double-transporter expression system [apical sodium bile acid transporter (ASBT) and OSTα/β] and identified clofazimine, an orphan antibiotic drug, as an OSTα/β inhibitor.8 Recently, we introduced a cell line ectopically expressing human OSTα/β and demonstrated that fidaxomicin, an antibiotic, and troglitazone sulfate, a metabolite of troglitazone, inhibited OSTα/β-mediated TCA transport.9
Given the limited information on OSTα/β substrates and inhibitors, it is understandable that there are no reports about OSTα/β-mediated clinical drug interactions. However, the role of OSTα/β in enterohepatic circulation of bile acids is well documented,10–13 and patients with OSTβ deficiency present with congenital diarrhea and features of cholestasis.10 OSTα/β seems to play a role in cholestasis based on the fact that hepatic expression of OSTα/β is strongly increased in patients with obstructive cholestasis, extrahepatic cholestasis,14 primary biliary cirrhosis,9, 15 and non-alcoholic steatohepatitis.9 All are cholestatic conditions with elevated bile acid concentrations in the plasma and liver.16 Hence, disrupted OSTα/β transport may impact the distribution of endogenous and exogenous OSTα/β substrates and cause clinically significant changes. The induction of OSTα/β in response to high bile acid concentrations, which has been replicated in cultured primary hepatocytes, emphasizes the importance of this protein as a “safety valve” for hepatic bile acid efflux.17–19 Thus, it is important to assess whether drugs interact and inhibit this transporter, potentially impairing the protective capacity of hepatocytes when bile acid exposure is high.
Currently, there are no standard methods to evaluate drug interactions with OSTα/β. Interpretation of the in vitro assay results is complicated by the bidirectional transport of OSTα/β and dependency of inhibition on the experimental set-up. For example, intracellular ES or TCA enhances OSTα/β-mediated TCA uptake in X. laevis oocytes,3 while inhibition of TCA uptake was observed when ES or TCA was added to the extracellular buffer.6 OSTα/β is not the only transporter displaying assay-dependent effects. A preincubation step with an inhibitor has enhanced the inhibitory potency determined against OATP1B1/SLCO1B1, OATP1B3/SLCO1B3, OAT3/SLC22A8, OCT1/SLC22A1, OCT2/SLC22A2, MATE2-K/SLC47A2 and P-glycoprotein/ABCB1.20–23 Furthermore, the U.S. Food and Drug Administration (FDA) has noted the potential impact of the experimental set-up on transporter inhibition results and the 2017 Draft Guidance for Industry recommends a preincubation step when assessing SLCO1B1 and SLCO1B3 inhibition.24
The first aim of this study was to select the optimal method to evaluate OSTα/β inhibition. Using the selected method, the second aim of this study was to identify novel inhibitors of OSTα/β from a chemically diverse set of compounds that are associated with cholestasis or drug-induced liver injury (DILI).
MATERIALS AND METHODS
Compounds.
Tritium-labeled OSTα/β model substrates, [3H]-dehydroepiandrosterone 3-sulfate (DHEAS) (60 Ci/mmol, radiochemical purity > 97%), and [3H]-taurocholate (TCA) (9.7–15.4 Ci/mmol, radiochemical purity >97%) were purchased from PerkinElmer Life Sciences (Boston, MA). Other compounds were obtained from Sigma-Aldrich (St. Louis, MO), Fisher Scientific (Fair Lawn, NJ), Toronto Research Chemicals (North York, ON, Canada), and Cayman Chemical Company (Ann Arbor, MI) and were dissolved in dimethyl sulfoxide (DMSO), except amoxicillin, which was dissolved in distilled water and ciprofloxacin, which was prepared in 0.1 M hydrochloric acid.
Cell Cultures.
The Flp-In 293 cell line overexpressing human OSTα and human OSTβ proteins (OSTab), and the control Flp-In 293 cell line transfected with empty vector (Mock) were established using the HEK-293 cell line and cultured as previously described.9 The OSTab cell line expresses OSTα and OSTβ mRNAs and proteins at very high levels, similar to those in bile acid exposed primary human hepatocytes, and the uptake of TCA is typically at least 10-fold higher in OSTab cells than in Mock cells. 9 Briefly, the cells were cultured in 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/mL streptomycin, and 1% GlutaMAX™ supplemented Dulbecco’s modified Eagle’s high glucose medium (D1190–044), all from Thermo Fisher Scientific. The medium was renewed every 2 to 3 days, and the cells were subcultured once every week. Cell viability was determined by Trypan blue exclusion.
Cellular Transport Studies.
The transport experiments were carried out as previously described with a few modifications9. Briefly, OSTab and Mock cells were seeded in 24-well plates at a density of 5 × 105 cells per well. After two days, the cells were washed and conditioned at 37°C for 10 min in modified extracellular fluid (ECF, pH 7.4), where 122 mM NaCl was replaced with 122 mM potassium chloride (KCl). Uptake was initiated by addition of warm modified ECF (37°C) containing DHEAS, TCA or digoxin as an OSTα/β probe substrate (20, 4 or 1 μM final concentration, with 200–300 nCi/mL of [3H]-labeled compound). After a designated uptake time, the uptake buffer was aspirated, and the cells were washed twice with ice-cold ECF. Each cell monolayer was solubilized, and the radioactivity was analyzed with Bio-Safe II counting cocktail (RPI Corp, Mt Prospect, IL, USA) using a Tri-Carb 3100TR liquid scintillation analyzer (PerkinElmer Inc.). The amount of the substrate transported into the cells was normalized to the total protein of the cell culture (Pierce BCA Protein assay kit, Thermo Fisher Scientific).
Characterization of OSTα/β-mediated Transport.
The absolute transport rate may be crucial for the sensitivity of the uptake assay. Thus, the effect of time on OSTα/β-mediated DHEAS transport was determined by performing cellular uptake studies over a range of time points from 5 s to 30 min. The uptake of DHEAS over a wide concentration range of 0.003–1000 µM was reported to be linear in OSTab cells and therefore, Km was not calculated.9 For the inhibition studies, a DHEAS concentration of 4 µM was selected, which is within the range of physiological DHEAS plasma concentrations of 1–5 µM.25 The uptake kinetics of the other OSTα/β model substrates, TCA and digoxin, was previously tested and reported.9 The concentrations of TCA (4 µM and 20 µM) and digoxin (1 µM) as well as the uptake time (30 s) used in the present experiments were within the linear range of uptake.
Evaluation of OSTα/β Inhibition Methods.
No standard methods have been established to study OSTα/β inhibition. Some compounds have been reported to either inhibit or stimulate OSTα/β, depending on whether the compound was added to the buffer immediately before measuring substrate uptake, whether the compound was dosed into the cells, or whether longer exposure times were used to allow some of the compound administered extracellularly to accumulate within the cells.3, 6, 9 Therefore, three different experimental methods were evaluated by comparing the inhibitory effect of four previously reported OSTα/β inhibitors (fidaxomicin, indomethacin, spironolactone, TLCAS)2, 6, 9 on OSTα/β-mediated DHEAS uptake. The inhibitor (100 µM) was applied to the cells using one of the following three approaches: Method 1) only during the preincubation phase; Method 2) only simultaneously with the substrate during the uptake phase; Method 3) during both the preincubation and the uptake phases.
Test Compound Selection.
In total, seventy-seven compounds or fixed-dose combinations were studied to identify novel OSTα/β inhibitors (Supporting Table 1). Fifty-five compounds and five fixed-dose combinations associated with cholestatic DILI based on a dataset of 190 cholestatic patients in the Drug-Induced Liver Injury Network (DILIN) were selected. All the compounds and fixed-dose combinations that were reported in more than one DILI case were included in the study (25 compounds; Supporting Table 1). In addition, 28 compounds involved only in one DILI case were tested. The compounds in the fixed-dose combinations were tested as a combination and separately. Of the additional test compounds, seven test compounds were previously reported to be OSTα/β substrates or inhibitors,3, 6, 9 four compounds were bile acids that are elevated in cholestasis,26, 27 and five compounds were associated with DILI,28 but were not present in the DILIN dataset.
Calculation of molecular descriptors and principal component analysis.
Molecular descriptors for the test compounds were calculated from the Simplified Molecular Input Line Entry Specification (SMILES) of the compounds using the rcdk package in R version 3.4.329 with RStudio.30 The rcdk is an interface to the modular Java libraries of the Chemistry development kit (CDK) for chemoinformatics. To generate a reference set of the FDA-approved drugs, descriptors were calculated in the same way for the integrated database of ADMET and adverse effects of predictive modeling (IDA2PM) compound library31 based on the SMILES available on the database website (http://idaapm.helsinki.fi/). Principal component analysis (PCA) was performed with R on the joint dataset of the test compounds and the IDA2PM library descriptors (total of 1,583 unique compounds). The following descriptors were selected for the PCA: MW, molecular weight; nRotB, number of rotating bonds; MLogP, logP determined by Moriguchi’s method; nAromRings, number of aromatic rings; nAcid, acidic group count; nBase, basic group count; nSmallRings, number of small rings; TopoPSA, topological polar surface area; nHBDonor, number of hydrogen bond donors; and nHBAcc, number of hydrogen bond acceptors (Supporting Fig. 1, Supporting Table 2). The distribution of the test compounds in the chemical space of FDA-approved drugs was visualized by plotting the first two components of the PCA (Fig. 1).
Fig. 1.
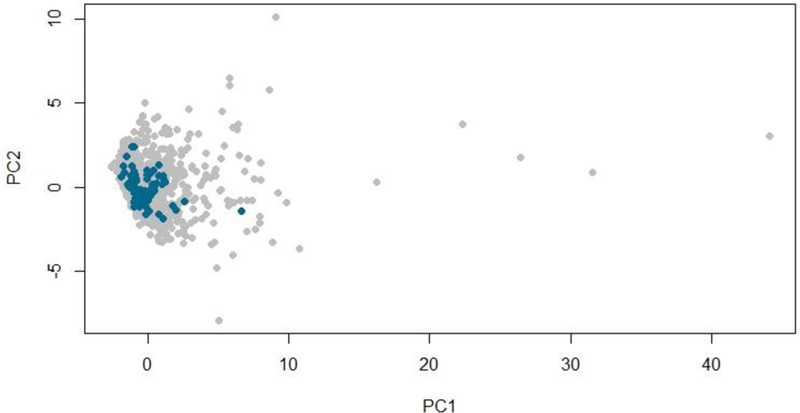
Chemical diversity of the compounds tested for OSTα/β inhibition. The chemical space of test compounds (blue) and FDA-approved drugs (grey) was described using a principal component analysis (PCA) of ten selected molecular descriptors. The first two principal components (PCs), PC1 and PC2, which together account for 69% of the variability in the dataset, are plotted in this graph.
Evaluation for Inhibition of OSTα/β-mediated Transport.
First, selected compounds were screened at 100 μM final concentration using Method 1 as described above. The OSTα/β inhibition by ethinylestradiol and fidaxomicin (100 µM) was evaluated using digoxin (1 µM) as an OSTα/β substrate. In addition, Method 1 was used for ethinylestradiol and Method 2 was used for fidaxomicin. Concentration-dependent inhibition of OSTα/β-mediated DHEAS uptake was determined by exposing the OSTab and Mock cells to 0.125–200 μM of the most efficient putative OSTα/β inhibitors using Method 1.
Data analysis.
OSTα/β-mediated uptake was calculated by subtracting the substrate uptake in the Mock cells from the uptake in OSTab cells. Inhibition of uptake was presented as a percentage of control (DHEAS uptake in the presence of vehicle = 100%). Results are presented as mean and standard deviation (SD) or standard error of the mean (SEM). The half-maximal inhibitory concentration (IC50) values were generated by fitting concentration-response curves to the data by nonlinear regression analysis using Prism 7 (GraphPad Software Inc., La Jolla, CA). The Hill coefficient was set at 1 and the minimum and maximum values at 0% and 100%, respectively. Statistical analysis of the data was conducted either by 1-way or 2-way ANOVA followed by Dunnett’s multiple comparisons test using Prism 7.
RESULTS
Molecular Characteristics of the Test Compounds.
According to the PCA, the chemical space of the tested compounds (n = 77) resembled that of the FDA-approved drugs (Fig. 1). The majority were small molecules (MW <900) (Supporting Fig. 1). The first principal component (PC1), which is driven by multiple parameters (Supporting Fig. 2), including MW and nRot, explained 53% of the variability of the chemical space. The MW of the compounds ranged from 114.0 to 1447.4 with a mean value of 395.3 g/mol, which was close to the mean for the FDA-approved drugs (417.5 g/mol). The second principal component (PC2) was governed by MlogP, nAromRings, nAcid and nSmallRings (Supporting Fig. 2). Overall, the tested compounds did not differ substantially from clinically used drugs in their physicochemical properties.
DHEAS Uptake Kinetics.
The effect of time on DHEAS transport in OSTab cells is shown in Fig. 2. Uptake in OSTab cells was rapid, particularly within the first minute. After 5–10 min, the uptake started to plateau, suggesting that intracellular and extracellular substrate concentrations were approaching equilibrium. DHEAS uptake resembles the previously reported OSTα/β-mediated TCA uptake in OSTab cells as a function of time.9 Based on these observations, the uptake experiments were performed over 30 s, which was within the linear range of initial DHEAS uptake. Using the optimized uptake time and concentration of DHEAS (4 µM), the uptake in OSTab cells was at least 10-fold larger than that in Mock cells in all further experiments.
Fig. 2.
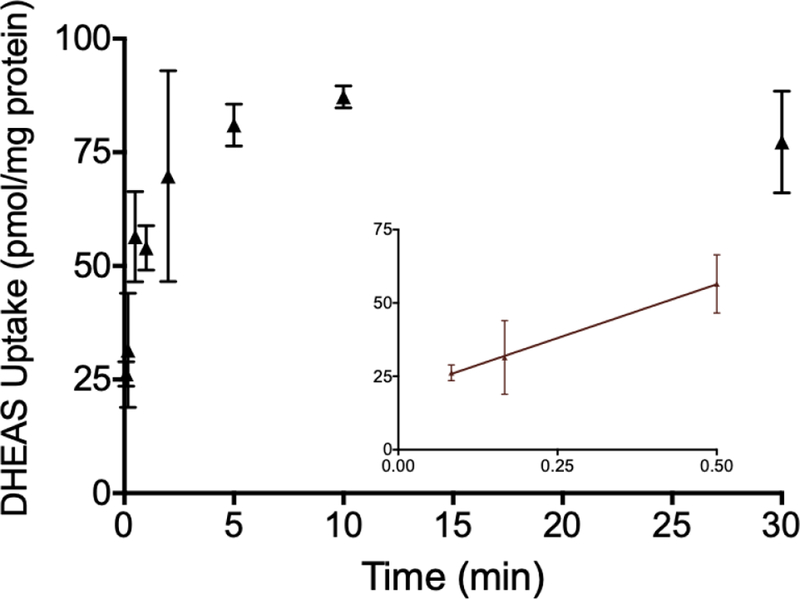
Time dependence of [³H]-dehydroepiandrosterone sulfate (DHEAS) transport in OSTα/β-overexpressing cells (OSTab). OSTab and Mock cells were incubated with DHEAS (300 nCi/ml; 4 µM final concentration) in extracellular fluid (pH 7.4) at 37°C for designated times. Background levels derived from Mock cells were subtracted, and uptake values were normalized to total cell protein. Each value represents the mean ± SD from two independent experiments, performed in triplicate. The inset shows DHEAS uptake during the early time points (5 s, 10 s and 0.5 min).
Comparison of Methods to Assess OSTα/β Inhibition.
The objective of these studies was to develop a practical method to screen multiple compounds for potential OSTα/β inhibition. Studies with reported OSTα/β inhibitors indicated that indomethacin and spironolactone displayed modest inhibition of DHEAS uptake (~20%) only after a preincubation phase (Method 1), whereas no inhibition was observed with co-incubation (Method 2) or with the combination of preincubation and co-incubation (Method 3) (Fig. 3). Fidaxomicin, a large macrocyclic antibiotic, inhibited DHEAS uptake with all methods, but the extent of inhibition was strongest (>80%) with Methods 2 and 3, which included a co-incubation step. In contrast, the sulfate-conjugated bile acid TLCAS inhibited DHEAS uptake by ~20% only with Method 3. When TCA was used as the OSTα/β substrate, the inhibitory profile was similar; fidaxomicin inhibited >70% of the uptake with all three Methods, spironolactone inhibited uptake ~20% with Method 1 only, while inhibition by indomethacin and TLCAS did not reach statistical significance. TLCAS appeared to stimulate OSTα/β-mediated TCA uptake by ~25% with Method 2. The differences in the extent of inhibition between the methods were not marked. Because the inhibition of OSTα/β-mediated DHEAS or TCA uptake was most often detected with Method 1, this approach was used for subsequent screening to capture the largest possible number of putative inhibitors. This method was also practical and economical, because the same solution with the labeled substrate could be added to all wells preincubated with diverse inhibitors.
Fig. 3.
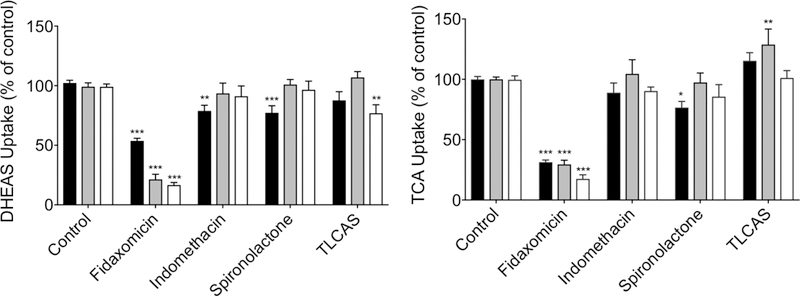
The effect of preincubation on the inhibition of OSTα/β-mediated transport of probe substrates. OSTab and Mock cells were preincubated with inhibitor (100 µM) for 10 min (Method 1, black), co-incubated with inhibitor and substrate during the uptake phase (Method 2, grey), or both preincubated and co-incubated, as described for Methods 1 and 2 (Method 3, white); the probe substrate, [³H]-dehydroepiandrosterone sulfate (DHEAS) or [³H]-taurocholate (TCA) (300 nCi/ml; 20 µM final concentration; 30 s uptake), was added in extracellular fluid (pH 7.4) at 37°C. Background levels derived from Mock cells were subtracted, and uptake measurements were normalized to total cell protein and uptake in vehicle treated cells. Each value represents the mean ± SEM from three independent experiments. ***, p< 0.001; **, p<0.005; *, p<0.05, significantly different than substrate uptake in control group. TLCAS, taurolithocholic acid sulfate.
Interaction of Test Compounds with OSTα/β.
In the screening studies, seven compounds and one fixed-dose combination of the 77 investigated (10.4%) inhibited OSTα/β-mediated DHEAS transport significantly at 100 µM (Fig. 4A). The compounds inhibiting OSTα/β >50% were denoted as strong inhibitors (n = 3), and those that inhibited between 25% and 50% of the DHEAS transport were defined as moderate inhibitors (n = 5). Based on these criteria, novel OSTα/β inhibitors were identified (Fig. 4B): ethinylestradiol, norgestimate and their fixed-dose combination, atorvastatin and troglitazone. Inhibition of OSTα/β was confirmed for ethinylestradiol and fidaxomicin using digoxin as the substrate (Supporting Fig. 3.). Six previously reported OSTα/β inhibitors2, 6 were not identified as strong/moderate inhibitors of DHEAS uptake in our assay: digoxin, estrone sulfate, indomethacin, probenecid, spironolactone and sulfobromophthalein.
Fig. 4.
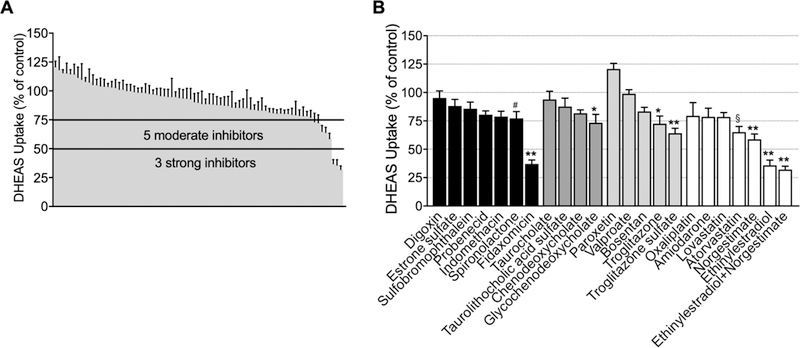
The inhibitory effect of test compounds or fixed-dose combinations on OSTα/β-mediated dehydroepiandrosterone sulfate (DHEAS) uptake in OSTα/β-overexpressing (OSTab) cells. A) Compounds or fixed-dose combinations inhibiting DHEAS transport by >50% were denoted as strong inhibitors (n = 3), and those that inhibited between 25% and 50% of the DHEAS transport were designated as moderate inhibitors (n = 5). B) The compounds, illustrated in groups, were previously reported OSTα/β substrates or inhibitors (black), bile acids elevated in cholestasis (dark grey), classical hepatotoxic compounds (light grey), and compounds associated with cholestatic DILI from the Drug-Induced Liver Injury Network (DILIN) database (only compounds inhibiting DHEAS transport by >20% (white) are shown). The inhibition was studied using Method 1. OSTab and Mock cells were preincubated with putative inhibitor at a concentration of 100 μM for 10 min; the probe substrate, [³H]-DHEAS, was added in extracellular fluid (300 nCi/ml; 4 µM final concentration; pH 7.4) and 30-s uptake was measured at 37°C; inhibition was calculated as described in Materials and Methods. Each value represents the mean ± SEM from three independent experiments. **p< 0.0001; *p<0.0005; §p<0.05; #p<0.01 significantly different than substrate uptake in control group.
Molecular Characteristics of the OSTα/β inhibitors.
Ten evaluated molecular descriptors describing size, lipophilicity and polarity of the compounds did not show remarkable differences between the inhibitors (>25% OSTα/β inhibition) and the non-inhibitors (<25% OSTα/β inhibition) (Supporting Table 2). However, OSTα/β inhibitors exhibited significantly higher mean MlogP values (3.7) than the non-inhibitors (2.5). In addition, the MW of OSTα/β inhibitors tended to be higher with a mean of 531 g/mol, whereas the mean of the non-inhibitors was 381 g/mol. The MW of the inhibitors was slightly distorted by the size of fidaxomicin (1058 g/mol); when fidaxomicin was excluded, the mean MW of OSTα/β inhibitors was still 443 g/mol. More than half of the putative inhibitors (atorvastatin, fidaxomicin, troglitazone and troglitazone sulfate) carried a negative charge at pH 7.4, whereas the rest of the inhibitors (ethinylestradiol, norgestimate) exhibited a steroidal structure. Glycochenodeoxycholic acid (GCDCA) is negatively charged and has a steroidal structure.
Concentration-Dependent Inhibition of OSTα/β Transport.
For all seven putative OSTα/β inhibitors, concentration-dependent inhibition was studied using DHEAS as the substrate and IC50 values were estimated (Fig. 5). Three compounds exhibited a clear concentration-inhibition relationship exceeding 50% inhibition, and they were ranked from the lowest to the highest IC50 value: ethinylestradiol > fidaxomicin > troglitazone sulfate. The IC50 values of norgestimate, atorvastatin and glycochenodeoxycholate were >200 μM (the highest concentration tested) and their maximal inhibition was less than 50%, while troglitazone did not yield a consistent inhibition curve.
Fig. 5.
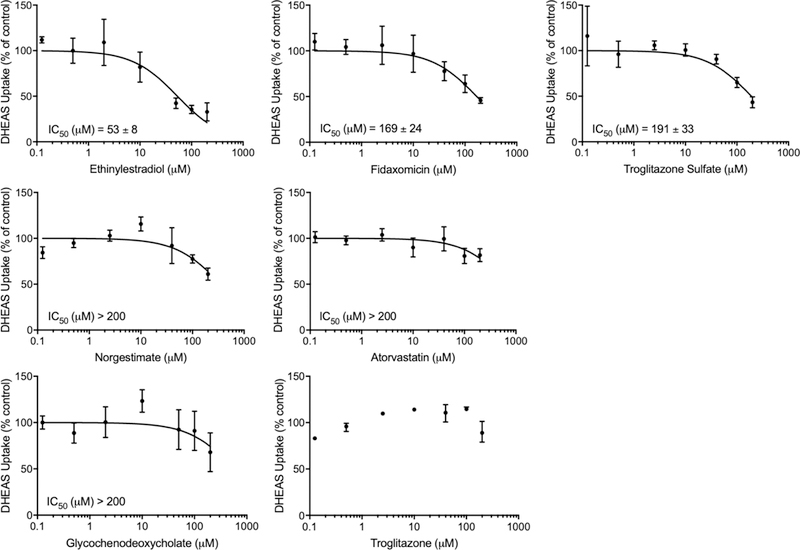
Concentration-dependent inhibitory effect of ethinylestradiol, fidaxomicin, troglitazone sulfate, norgestimate, atorvastatin, glycochenodeoxycholate and troglitazone on OSTα/β-mediated DHEAS transport. OSTab and Mock cells were preincubated with inhibitors for 10 min (Method 1; 0–200 μM) prior to the 30-s uptake with [3H]-dehydroepiandrosterone sulfate (DHEAS; 200 nCi/ml; 4 μM) at 37°C. Data are expressed as percentage of vehicle control; each value represents the mean ± SD of two independent experiments, each performed in triplicate. For troglitazone, only one experiment is shown (mean ± range).
DISCUSSION
OSTα/β is an important transporter involved in the enterohepatic circulation of bile acids in the intestine and liver. The expression of OSTα/β in the liver is increased in cholestatic conditions, suggesting that it has a role in protecting hepatocytes from bile acid-mediated toxicity. Inhibition of OSTα/β by drugs could impair this protective role, but there is currently little information on this interaction. In earlier studies, inhibitors were usually assessed at single concentrations2, 6, and to our knowledge, IC50 values for OSTα/β inhibitors have not been reported previously. Based on published time course data generated at three different inhibitor concentrations8, the IC50 value of clofazimine is estimated to be 30–50 μM. In the present studies, new OSTα/β inhibitors were identified from a dataset of 77 compounds or fixed-dose combinations known to be associated with cholestatic DILI or considered likely to interact with OSTα/β, and their inhibitory potential was evaluated. These data support the hypothesis that OSTα/β may be subject to drug interactions.
The effects of four previously reported OSTα/β inhibitors (fidaxomicin, indomethacin, spironolactone, and TLCAS)2, 6, 9 were different depending on whether cells were incubated with the compound prior to substrate addition (Method 1), or dosed together with the substrate without (Method 2) or with the preincubation step (Method 3). More inhibitors were detected using Method 1 (preincubation) than the other methods. The importance of preincubation suggests that intracellular accumulation of indomethacin, spironolactone and TLCAS, and perhaps other compounds, may be needed for OSTα/β inhibition. A similar phenomenon with preincubation has been reported for other SLC transporters.20–22 For example, preincubation of cyclosporine results in 3.6–5.9-fold lower IC50 values of SLCO1B1 than co-administration with the SLCO substrate, estradiol 17β-glucuronide.23, 32
The identified inhibitors, ethinylestradiol, fidaxomicin and troglitazone sulfate, represent multiple drug classes. This is in agreement with a previous OSTα/β inhibition study,8 where steroids, azoles, benzenoids, statins and antibiotics were found to interact with OSTα/β.8 The majority of the compounds tested herein (26%) were antibacterials, but fidaxomicin was the only compound in this class to show inhibition. It was not possible to identify specific physicochemical properties that distinguished inhibitors from non-inhibitors due to the small number of OSTα/β inhibitors identified to date. However, with the exception of fidaxomicin, the OSTα/β inhibitors identified in the present study were either steroidal (ethinylestradiol) or anionic compounds (troglitazone sulfate), in agreement with results from the initial OSTα/β experiments with X. laevis oocytes.2, 6
The current test compounds included several statins (atorvastatin, lovastatin, rosuvastatin, and simvastatin), which are known to interact with numerous transport proteins in the intestine and liver and can cause transporter-mediated drug interactions.33Atorvastatin, which previously has been suggested to be an OSTα/β substrate,7 was the only statin that showed any signs of OSTα/β inhibition in this study. Lovastatin decreased DHEAS uptake modestly (~21%), but the effect was not statistically significant, and rosuvastatin did not inhibit OSTα/β, in agreement with an earlier finding.7
Some contradictory results on the inhibition of OSTα/β between the current and previous studies were observed for a few other compounds. For example, Ballatori et al.2 reported that digoxin (500 μM), estrone sulfate (200 μM), sulfobromophthalein (100 μM), probenecid (1000 μM) and indomethacin (200 μM) modestly affected OSTα/β-mediated transport (25–38% inhibition). In the present study, these compounds (100 μM) did not markedly inhibit OSTα/β (5–23% inhibition). The previously reported OSTα/β inhibitory effect of 25 μM ezetimibe7 could not be reproduced, despite the use of a preincubation phase in both studies. These contradictory findings might be explained, in part, by the use of different test compound concentrations. Also, different substrates were used in previous (TCA or ES),2, 7, 8 and the present (DHEAS) studies. Indeed, substrate-dependent inhibition was seen for indomethacin and TLCAS in the method optimization experiments. Similar substrate-dependency of inhibition also has been reported for other transporters such as SLCO1B1.34–36
Exogenous compounds may have a significantly lower affinity towards OSTα/β than endogenous compounds. Using an exogenous compound, such as digoxin or rosuvastatin, as an OSTα/β substrate may result in stronger inhibitory effects and give a more realistic evaluation of involvement of OSTα/β in drug interactions. In fact, when we applied digoxin as an OSTα/β substrate, the inhibitory effect of fidaxomicin and ethinylestradiol (both 90% inhibition) was higher than when DHEAS was used as a substrate (71% and 63% inhibition, respectively). Therefore, future work should focus on using drugs as victim substrates.
The majority of the compounds tested here for OSTα/β inhibition were derived from a database of compounds associated with cholestatic DILI. However, OSTα/β inhibition did not correlate clearly with the DILI causality of the test compounds (Supporting Table 1, Supporting Fig. 4). Interestingly, amoxicillin, clavulanic acid, amiodarone, atorvastatin, ethinylestradiol and norgestimate, which are associated with more than 100 cases of DILI,37 decreased OSTα/β-mediated DHEAS uptake during screening, although the decrease was substantial (35–64% inhibition) and statistically significant only for the latter three drugs. In addition, doxycycline, which belongs to the top ten most common drugs leading to DILI,37 decreased OSTα/β activity only by ~19% (Supporting Table 1).
Based on the in vitro IC50 values determined for ethinylestradiol (53 µM), fidaxomicin (169 µM) and troglitazone sulfate (191 µM), the concentration in hepatocytes or in plasma must be fairly high for these drugs to cause clinically important OSTα/β-mediated interactions. At clinical doses, these concentrations are not achieved in plasma.38, 39 However, the concentration of troglitazone sulfate, the primary metabolite of troglitazone, is ~700 ng/mg protein in hepatocyte cultures,40 which is equivalent to ~190 µM, and is very close to the IC50 value determined in the current study. The derived IC50 values may however underestimate the potency of inhibition due to the high expression of OSTα/β in the cell line used, because apparent IC50 values can be influenced by transporter expression.41 In addition, IC50 values are also highly dependent on the substrate concentration used and the mechanism of inhibition.
It should be noted that the novel OSTα/β inhibitors identified in this study can also inhibit other transporters. For example, ethinylestradiol is reported to interact with ABCB11 and ABCC3 (Supporting Table 1), whereas troglitazone sulfate inhibits ABCB11 and ABCC4.42, 43 Fidaxomicin, the second most potent OSTα/β inhibitor tested, has been reported to inhibit ABCC3.44 The simultaneous inhibition of multiple bile acid transporters by these novel OSTα/β inhibitors may alter bile acid disposition and lead to cholestatic DILI. The contribution of several pathways to drug injury may also explain the lack of correlation found here between OSTα/β inhibition and DILI causality.
In conclusion, OSTα/β inhibition depends on the method used to introduce the inhibitor. By using our novel in vitro method, ethinylestradiol, fidaxomicin and troglitazone sulfate were identified as concentration-dependent OSTα/β inhibitors. This investigation provides insights into the nature of OSTα/β inhibitors, provides OSTα/β IC50 values for the first time, and suggests that OSTα/β inhibition may have an impact on the disposition of endogenous and exogenous OSTα/β substrates.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Elizabeth (Liz) Cirulli from Duke University, Durham, NC, United States is acknowledged for providing the dataset of causal drugs in 190 cholestatic DILI cases. We thank Lilly Wong, Brandon Phares and Melanie Stewart for assistance with the uptake assays, buffer preparation, total protein measurement and molecular analysis. This research was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Numbers R01 GM041935 and R35 GM122576 (K.L.R.B.) and in part by the Erasmus+ Programme Global Mobility funds (A.K., P.H.). M.M. was supported in part by the Finnish Cultural Foundation and Orion Research Foundation, and N.S. by the Sigrid Juselius Foundation.
Abbreviations
- ABC
ATP-binding cassette
- ASBT
apical sodium bile acid transporter
- BSEP
bile salt export pump
- DHEAS
dehydroepiandrosterone sulfate
- DILI
drug-induced liver injury
- ES
estrone sulfate
- FDA U.S.
Food and Drug Administration
- GCDCA
glycochenodeoxycholic acid
- MATE
multidrug and toxin extrusion
- MRP
multidrug resistance-associated protein
- NTCP
sodium taurocholate co-transporting polypeptide
- OATP
organic anion transporting polypeptide
- OCT
organic cation transporter
- OSTα/β
organic solute transporter alpha/beta
- PC
principal component
- PCA
principal component analysis
- SLC
solute carrier
- SLCO
solute carrier organic anion
- SMILES
simplified molecular input line entry specification
- TCA
taurocholate
- TLCAS
taurolithocholic acid sulfate
Footnotes
ASSOCIATED CONTENT
Supporting Information
OSTα/β inhibition by 77 test compounds/fixed dose combinations, registered drug-induced liver injury (DILI) cases, reported transporter inhibition, and molecular characteristics of the test compounds, as well as the inhibitory effect of ethinylestradiol and fidaxomicin on digoxin uptake and the correlation between OSTα/β inhibition and DILI causality are shown in Supporting Tables 1 and 2, and Supporting Figures 1–4, respectively.
REFERENCES
- 1.Brouwer KL; Keppler D; Hoffmaster KA; Bow DA; Cheng Y; Lai Y; Palm JE; Stieger B; Evers R In vitro methods to support transporter evaluation in drug discovery and development. Clin Pharmacol Ther 2013, 94, (1), 95–112. [DOI] [PubMed] [Google Scholar]
- 2.Seward DJ; Koh AS; Boyer JL; Ballatori N Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem 2003, 278, (30), 27473–82. [DOI] [PubMed] [Google Scholar]
- 3.Ballatori N; Christian WV; Lee JY; Dawson PA; Soroka CJ; Boyer JL; Madejczyk MS; Li N OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 2005, 42, (6), 1270–9. [DOI] [PubMed] [Google Scholar]
- 4.Uhlen M; Fagerberg L; Hallstrom BM; Lindskog C; Oksvold P; Mardinoglu A; Sivertsson A; Kampf C; Sjostedt E; Asplund A; Olsson I; Edlund K; Lundberg E; Navani S; Szigyarto CA; Odeberg J; Djureinovic D; Takanen JO; Hober S; Alm T; Edqvist PH; Berling H; Tegel H; Mulder J; Rockberg J; Nilsson P; Schwenk JM; Hamsten M; von Feilitzen K; Forsberg M; Persson L; Johansson F; Zwahlen M; von Heijne G; Nielsen J; Ponten F Proteomics. Tissue-based map of the human proteome. Science 2015, 347, (6220), 1260419. [DOI] [PubMed] [Google Scholar]
- 5.Fang F; Christian WV; Gorman SG; Cui M; Huang J; Tieu K; Ballatori N Neurosteroid transport by the organic solute transporter OSTalpha-OSTbeta. J Neurochem 2010, 115, (1), 220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W; Seward DJ; Li L; Boyer JL; Ballatori N Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci U S A 2001, 98, (16), 9431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz UI Intestinal and hepatic drug transporters and their role in the disposition of lipid-lowering drugs The University of Western Ontario, 2012. [Google Scholar]
- 8.van de Wiel SMW; de Waart DR; Oude Elferink RPJ; van de Graaf SFJ Intestinal Farnesoid X Receptor Activation by Pharmacologic Inhibition of the Organic Solute Transporter α-β. Cell Mol Gastroenterol Hepatol 2018, 5, (3), 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malinen MM; Ali I; Bezencon J; Beaudoin JJ; Brouwer KLR Organic solute transporter OSTalpha/beta is overexpressed in nonalcoholic steatohepatitis and modulated by drugs associated with liver injury. Am J Physiol Gastrointest Liver Physiol 2018, 314, (5), G597–G609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sultan M; Rao A; Elpeleg O; Vaz FM; Abu-Libdeh B; Karpen SJ; Dawson PA Organic solute transporter-beta (SLC51B) deficiency in two brothers with congenital diarrhea and features of cholestasis. Hepatology 2017. [DOI] [PMC free article] [PubMed]
- 11.Rao A; Haywood J; Craddock AL; Belinsky MG; Kruh GD; Dawson PA The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A 2008, 105, (10), 3891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrebee CB; Li J; Haywood J; Pachura K; Robinson BS; Hinrichs BH; Jones RM; Rao A; Dawson PA Organic Solute Transporter alpha-beta Protects Ileal Enterocytes From Bile Acid-Induced Injury. Cell Mol Gastroenterol Hepatol 2018, 5, (4), 499–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballatori N; Fang F; Christian WV; Li N; Hammond CL Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol 2008, 295, (1), G179–G186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaap FG; van der Gaag NA; Gouma DJ; Jansen PL High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2009, 49, (4), 1228–35. [DOI] [PubMed] [Google Scholar]
- 15.Boyer JL; Trauner M; Mennone A; Soroka CJ; Cai SY; Moustafa T; Zollner G; Lee JY; Ballatori N Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 2006, 290, (6), G1124–30. [DOI] [PubMed] [Google Scholar]
- 16.Ferslew BC; Johnston CK; Tsakalozou E; Bridges AS; Paine MF; Jia W; Stewart PW; Barritt A. S. t.; Brouwer KL Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther 2015, 97, (4), 419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaffner CA; Mwinyi J; Gai Z; Thasler WE; Eloranta JJ; Kullak-Ublick GA The organic solute transporters alpha and beta are induced by hypoxia in human hepatocytes. Liver Int 2015, 35, (4), 1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo C; LaCerte C; Edwards JE; Brouwer KR; Brouwer KLR Farnesoid X Receptor Agonists Obeticholic Acid and Chenodeoxycholic Acid Increase Bile Acid Efflux in Sandwich-Cultured Human Hepatocytes: Functional Evidence and Mechanisms. J Pharmacol Exp Ther 2018, 365, (2), 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J; Lu H; Lu YF; Lei X; Cui JY; Ellis E; Strom SC; Klaassen CD Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci 2014, 141, (2), 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsby R; Chidlaw S; Outteridge S; Sullivan R; Pickering S, Further investigation of the impact of inhibitor pre-incubation on human OATP1B1, OAT3, OCT2 and MATE1 transporter in vitro inhibitory potencies. In AAPS, American Association of Pharmaceutical Scientists; 2016; p M1022. [Google Scholar]
- 21.Shitara Y; Sugiyama Y Preincubation-dependent and long-lasting inhibition of organic anion transporting polypeptide (OATP) and its impact on drug-drug interactions. Pharmacol Ther 2017, 177, 67–80. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson H; Clements V; Meadows J; Outteridge S; Pickering S; Radcliffe A; Elsby R, Investigation of inhibitor preincubation condition on human OATP1B1, P-gp and BCRP transporter in vitro inhibitory potencies. In The 9th annual AAPS Joint Workshop on Drug Transporters in ADME: From the Bench to the Bedside, American Association of Pharmaceutical Scientists: Herndon, Virginia, 2018. [Google Scholar]
- 23.Tátrai P; Schweigler P; Poller B; Hanna I; Gáborik Z; Huth F, Time-dependent inhibition demonstrated across multiple classes of uptake transporter. In The 9th annual AAPS Joint Workshop on Drug Transporters in ADME: From the Bench to the Bedside, American Association of Pharmaceutical Scientists: Herndon, Virginia, 2018. [Google Scholar]
- 24.FDA, Draft Guidance for Industry - In vitro metabolism and transporter mediated drug-drug interaction studies 2017.
- 25.Dharia S; Parker CR Jr. Adrenal androgens and aging. Semin Reprod Med 2004, 22, (4), 361–8. [DOI] [PubMed] [Google Scholar]
- 26.Masubuchi N; Sugihara M; Sugita T; Amano K; Nakano M; Matsuura T Oxidative stress markers, secondary bile acids and sulfated bile acids classify the clinical liver injury type: Promising diagnostic biomarkers for cholestasis. Chem Biol Interact 2016, 255, 83–91. [DOI] [PubMed] [Google Scholar]
- 27.Trottier J; Bialek A; Caron P; Straka RJ; Heathcote J; Milkiewicz P; Barbier O Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: a pilot study. Dig Liver Dis 2012, 44, (4), 303–10. [DOI] [PubMed] [Google Scholar]
- 28.Chen M; Suzuki A; Thakkar S; Yu K; Hu C; Tong W DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21, (4), 648–53. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team, R: A language and environment for statistical computing www.R-project.org (1.3.2018),
- 30.RStudio Team, RStudio: Integrated Development for R www.rstudio.com (1.3.2018),
- 31.Legehar A; Xhaard H; Ghemtio L IDAAPM: integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data. J Cheminform 2016, 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett L; Burton I; Chidlaw S; Foley S; Elsby R, Impact of inhibitor pre-incubation on human OATP1B1, OAT3, OCT2 and MATE1 transporter in vitro inhibitory potencies. In AAPS/ITC Joint Workshop on Drug Transporters in ADME, American Association of Pharmaceutical Scientists: Baltimore, Maryland, 2015. [Google Scholar]
- 33.Elsby R; Hilgendorf C; Fenner K Understanding the critical disposition pathways of statins to assess drug-drug interaction risk during drug development: it’s not just about OATP1B1. Clin Pharmacol Ther 2012, 92, (5), 584–98. [DOI] [PubMed] [Google Scholar]
- 34.Izumi S; Nozaki Y; Komori T; Maeda K; Takenaka O; Kusano K; Yoshimura T; Kusuhara H; Sugiyama Y Substrate-dependent inhibition of organic anion transporting polypeptide 1B1: comparative analysis with prototypical probe substrates estradiol-17beta-glucuronide, estrone-3-sulfate, and sulfobromophthalein. Drug Metab Dispos 2013, 41, (10), 1859–66. [DOI] [PubMed] [Google Scholar]
- 35.Izumi S; Nozaki Y; Maeda K; Komori T; Takenaka O; Kusuhara H; Sugiyama Y Investigation of the impact of substrate selection on in vitro organic anion transporting polypeptide 1B1 inhibition profiles for the prediction of drug-drug interactions. Drug Metab Dispos 2015, 43, (2), 235–47. [DOI] [PubMed] [Google Scholar]
- 36.Noe J; Portmann R; Brun ME; Funk C Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos 2007, 35, (8), 1308–14. [DOI] [PubMed] [Google Scholar]
- 37.Björnsson ES Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol 2015, 89, (3), 327–34. [DOI] [PubMed] [Google Scholar]
- 38.Lennernas H Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet 2003, 42, (13), 1141–60. [DOI] [PubMed] [Google Scholar]
- 39.Sears P; Crook DW; Louie TJ; Miller MA; Weiss K Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with Clostridium difficile infection. Clin Infect Dis 2012, 55 Suppl 2, S116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostrubsky VE; Sinclair JF; Ramachandran V; Venkataramanan R; Wen YH; Kindt E; Galchev V; Rose K; Sinz M; Strom SC The role of conjugation in hepatotoxicity of troglitazone in human and porcine hepatocyte cultures. Drug Metab Dispos 2000, 28, (10), 1192–7. [PubMed] [Google Scholar]
- 41.Kalvass JC; Pollack GM Kinetic considerations for the quantitative assessment of efflux activity and inhibition: implications for understanding and predicting the effects of efflux inhibition. Pharm Res 2007, 24, (2), 265–76. [DOI] [PubMed] [Google Scholar]
- 42.Funk C; Pantze M; Jehle L; Ponelle C; Scheuermann G; Lazendic M; Gasser R Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology 2001, 167, (1), 83–98. [DOI] [PubMed] [Google Scholar]
- 43.Yang K; Pfeifer ND; Kock K; Brouwer KL Species differences in hepatobiliary disposition of taurocholic acid in human and rat sandwich-cultured hepatocytes: implications for drug-induced liver injury. J Pharmacol Exp Ther 2015, 353, (2), 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali I; Welch MA; Lu Y; Swaan PW; Brouwer KLR Identification of novel MRP3 inhibitors based on computational models and validation using an in vitro membrane vesicle assay. Eur J Pharm Sci 2017, 103, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


