Abstract
Purpose of review
Radical treatments for prostate cancer are associated with significant morbidity, including incontinence and erectile dysfunction. Advances in the field of prostate MRI and desire to reduce treatment morbidities have led to a rapid growth in focal treatments for prostate cancer. Here, we review novel focal prostate cancer treatments and their associated recent clinical data, with a particular focus on data reported within the last 24 months.
Recent findings
High-intensity focal ultrasound, focal laser ablation, irreversible electroporation, focal cryotherapy, and photodynamic therapy have been used as treatment modalities for localized prostate cancer treatment. Despite the great variety of treatment techniques, each of these modalities is characterized by a significant rate of prostate cancer persistence within treatment zones (6–50%) and the presence of residual cancer within the prostate on rebiopsy (24–49%). These treatments, however, are associated with very low rates of high-grade complications, rare incontinence, and only mild or transient reductions in erectile function. The most common adverse events are urinary tract infections, hematuria, and urinary retention.
Summary
Prostate cancer focal therapy is an attractive option for well-selected patients because of its low complication profile; however, long-term oncologic outcome is still lacking and early recurrence rates are high, limiting the ability of most urologic associations from endorsing its routine use.
Keywords: focal laser ablation, focal therapy, high-intensity focused ultrasound, photodynamic therapy, prostate cancer
INTRODUCTION
Our failures as healthcare providers frequently highlight the opportunities before us. Since the early 1990s, we have been able to increase the detection of prostate cancer while simultaneously reducing the numbers of prostate cancer deaths [1]. However, in the process we have also increased disease treatment-associated morbidity, namely, erectile dysfunction and incontinence, despite rather substantial treatment advances [2–4]. Additionally, mortality from prostate cancer remains common as 29 430 men are estimated to die from prostate cancer in 2018 in the United States [1]. With the US Preventive Service Task Force's temporary stance against prostate specific antigen (PSA) testing (which has now been partially retracted) attention to the morbidity of prostate cancer treatment has been a focus of critique, as well as spurred technological development [5].
In the 1990s, the general ethos among urologists was that with refinements of surgical techniques, erectile dysfunction and incontinence from prostate surgery could be largely reduced [6]. In the 2000s, with improvements of instrumentation and visualization associated with adoption of robotic surgery, further improvements were expected. In fact, some improvements were made as 12 month continence rates in some series have reached as high as 96% (69–96%) and 12 month potency rates have increased from 26–50% with open retropubic radical prostatectomy to 55–81% with robotic-assisted laparoscopic prostatectomy [3,7–12]. Prostate radiotherapy demonstrates similar incontinence and impotence rates, with the added sequelae of increased bladder cancer risk, rectal bleeding, bowel dysfunction, and urethral stricture disease [13]. Predictability of these complications is far too uncertain and is still too frequent for the individual patient [11]. Understandably, with the improvements in prostate cancer imaging and the treatment morbidity associated with whole gland treatment, the medical community began to explore focal treatment options with hopes of avoiding injury to the neurovascular bundle and urinary continence mechanisms.
Although one of the earliest reports of focal therapy for prostate cancer was published in 2002 [14], mainstream popularization of focal treatment of prostate cancer was dependent on the development of multiparametric MRI, which allowed for tumor localization and biopsy guidance [15–17]. Once a lesion could be reproducibly visualized on imaging, targeting for destruction of localized lesions in the prostate became increasingly feasible. Multiparametric MRI, however, can be unreliable in detecting prostate cancer [17,18], particularly lower Gleason score disease [19], and underestimates the size of prostate cancer lesions [20]. These imaging limitations can translate into incomplete ablation of identified lesions and cancers missed by imaging and/or systematic biopsy [17]. Furthermore, prostate cancer is a largely multifocal disease creating questions of how many prostate lesions can be safely ablated [21]. Similarly at this time, there is no consensus on whether Gleason ≥8 lesions are even candidates for focal therapy. Based on these limitations, one study espoused that only 38.5% of patients are possible candidates for focal therapy [22].
Box 1.
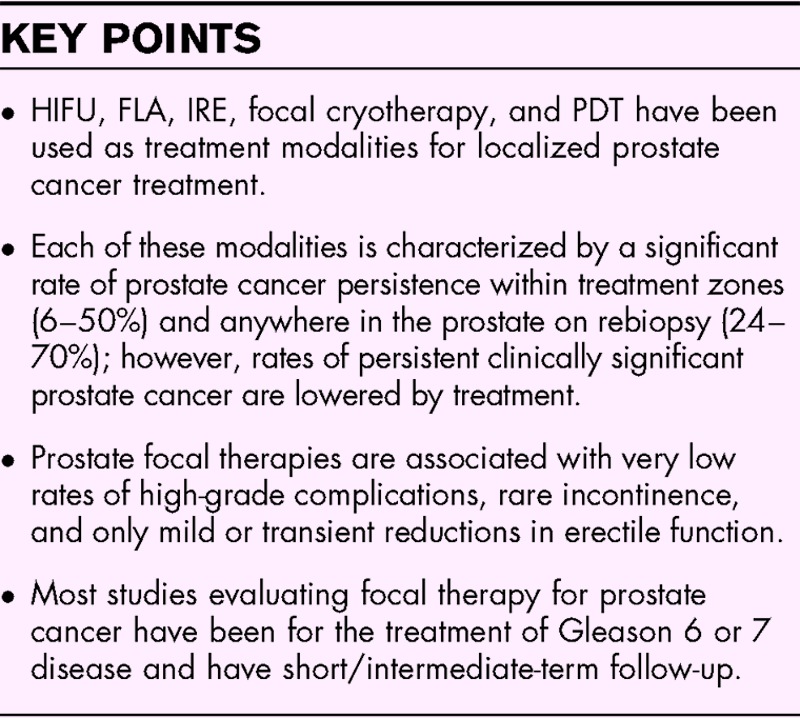
no caption available
HIGH-INTENSITY FOCUSED ULTRASOUND
Despite the limitations noted above, the enthusiasm to explore focal therapy has been strong. High-intensity focused ultrasound (HIFU) is among the most popularly adopted focal therapies. Prostate HIFU uses transrectally delivered focal ultrasound to the prostate to induce coagulative necrosis [23]. As energy is delivered transrectally, the rectal mucosa is often actively cooled to prevent rectal injury and urinary fistula. Given no portion of the device penetrates tissues, HIFU is the only truly noninvasive commercially available focal therapy modality for prostate cancer as other modalities require transrectal or percutaneous probe placement. Most commercially available HIFU platforms are limited to ∼4 cm depth of tissue ablation making treatment of anterior lesions more complicated or impossible [24]. In addition to focused ultrasound, there is also a transurethral nonfocused ablative ultrasound with MRI thermometry under experimental evaluation for focal therapy. Given the paucity of clinical data for the treatment of focal prostate cancer, this review will focus on HIFU only.
The results of the largest focal HIFU trial to date were published in June 2018 [25▪▪]. Guillaumier et al. treated 625 prostate cancer patients with HIFU and reported their 5-year follow-up data [25▪▪]. Eighty three percent of patients had low risk (Gleason 3 + 3 = 6 and 3 + 4 = 7), 14% Gleason 4 + 3 = 7, and 2% Gleason ≥8 disease, respectively. All 625 patients received primary HIFU treatment and 19% (n = 121) required at least two retreatments. Of these only 35% (n = 29) of patients underwent posttreatment rebiopsy, which demonstrated a 14% rate of recurrence within the treatment field, also known as infield recurrence. Failure-free survival, defined as avoidance of the need for radical or systemic therapy, was 88% at 5-year follow-up and metastasis-free survival was 95% at 5 years. In total, 97% of patients did not require urinary absorptive pads at 1–2 year posttreatment, urinary tract infections occurred in 8.5% (53/625), and rectourethral fistulas were rare (n = 2/625) [25▪▪]. These results have been largely mirrored by smaller prior studies [26▪,27,28]. Among five studies, 171 patients were treated as part of published study protocols. In-field recurrences were detected in 0–21% of patients [29]. Incontinence rates were less than 1%, erectile dysfunction rates ranged from 0–25%, and urinary retention occurred in up to 5% of patients [29].
Advances in HIFU technology have led to technical improvements in prostate cancer ablation [30]; however even with state-of the art systems, significant shortcomings remain. This was seen in a 2018 report by Hardenberg et al.[28] of 24 patients treated with a recently updated HIFU platform. Nineteen patients had MRI-visible lesions (Prostate Imaging Reporting and Data System 3–5) and five had lesions undetectable by MRI. Patients underwent focal or regional HIFU, with 12 month postoperative biopsy demonstrating persistence of cancer in 8 of 20 patients (40%) [28]. Although many of these treatment failures represent Gleason 6 disease, ultimately 20% of the patients in this study proceeded to prostatectomy at 1-year follow-up. No patient developed urinary complications, however, there was a mild 2 point reduction in average erectile function as measured by the International Index of Erectile Function in preoperatively potent patients at 12 months (confidence interval 15.79–22.21; P = 0.044) [28]. Only one patient experienced greater than grade II Clavien-Dindo complication [28].
Although the treatment outcomes may demonstrate suboptimal efficacy, the low rates of adverse effects and noninvasive nature of HIFU make it an attractive option among those patients who may have low-volume disease and who want treatment but not definitive whole gland therapy. However, the absence of long-term oncologic follow-up remains a major limitation to satisfactory patient counseling regarding this approach.
FOCAL LASER ABLATION
Although HIFU has garnered favor in clinical use because of its noninvasive marketing label, relative ease of use, and availability of commercial platforms, there are some advantages to the competing therapy of focal laser ablation (FLA) in the treatment of prostate cancer. FLA requires placement of a laser fiber directly into the cancer lesion through a perineal or transrectal puncture. Energy is then transmitted into the lesion creating a volume of thermal necrosis [31]. Although HIFU renders sequential rice-kernel-sized ablations across columns or spheres, FLA creates larger, homogenous circular or elliptical ablation zones around the laser tip. Repeated ellipses of ablation are manually overlaid, creating a sharply demarcated treatment zone [32]. This can be done under local anesthesia, whereas HIFU requires general anesthesia and a transrectal approach. Additionally, unlike HIFU which is limited in its depth of penetration to 4 cm, FLA can conceivably be used to ablate any region of the prostate. FLA provides the added benefits of MRI compatibility, allowing for use of real-time in-bore MRI guidance and thermometry, and the ability to perform it without need for general anesthesia [33,34].
Given the technical difficulties of FLA and the lack of prostate-specific commercially developed FLA targeting, monitoring, or navigation platforms, there are limited data for FLA in treating prostate cancer. In a 2016 published phase II trial, Eggener et al. treated 27 men with a mean PSA of 4.4 and Gleason 6 or 7 disease [35]. At 3 and 12 months, 96% and 89% of patients, respectively, were absent of disease recurrence in the ablation zone (in-field recurrence); however, 37% of patients were found to have some residual cancer at any location within the prostate gland on 12 month systematic 12-core rebiopsy (out-of-field recurrence) [35]. There were no significant changes in urinary symptoms (measured with I-PSS scores) or erectile function [measured by sexual health inventory for men (SHIM) score] at 12-month follow-up. The most common adverse events were hematuria (15%) and urinary retention (8%) [35]. Unfortunately, the majority of the published FLA data is limited to cohorts of less than 30 patients, limiting the conclusions that can be drawn about this treatment modality [31,34–39].
The largest study assessing transrectal FLA is currently ongoing. Interim results were recently reported in 2018 (Feller et al.) on the treatment of 98 patients and 138 tumor foci using real time MRI guidance [40]. They reported 23% rate of in-field cancer recurrence, with no serious adverse events and no statistically significant changes in International Prostate Symptom Score or SHIM scores at 12 months [40]. Currently, there is no long-term oncologic follow-up on the efficacy of FLA, limiting its use to largely clinical trial settings. The limited side-effect profile, excellent precision of ablation obtainable with laser energy delivery and the absence of documented rectal fistula are potential advantages of FLA. Additional navigation and verification software need to be developed to ensure better overlapping treatments and refine the precision and accuracy of this approach for larger planned treatment volumes. MRI–transrectal ultrasound (TRUS) fusion guidance platforms have been developed (at the National Institutes of Health (NIH) and University of California, Los Angeles) for TRUS MRI fusion guidance, but broadly available FLA platforms are not yet available [34,41].
FOCAL CRYOTHERAPY
In contrast to heating energy modalities, cryotherapy, initially used for whole gland ablation, has been repurposed for treatment of focal prostate lesions. Through repeating freeze and thaw cycles, cryotherapy induces irreversible cell rupture and apoptosis [42]. As with the other treatment modalities, efficacy of cryotherapy has been quite variable with 2–25% of posttreatment biopsies demonstrating in-field recurrences, 0–31% rates of erectile dysfunction, 1–17% rates of urinary retention and less than 5% rates of urinary incontinence [29,43,44]. Although 12-month erectile function is generally poor [45], there is some evidence that at long-term follow-up of 4 years erectile dysfunction can match levels of active surveillance controls [46]. Rare urinary fistulas have been reported [43]. Given the historically high rates of erectile dysfunction and fistula associated with whole gland cryoablation, this modality seems to have been associated with a certain stigma which may limit its adoption.
IRREVERSIBLE ELECTROPORATION
Perhaps the most novel of the contemporary focal therapy techniques is irreversible electroporation (IRE). In IRE, electroneedle probes are placed through the perineum around the ablative target under ultrasound or MRI guidance. High-voltage bursts of electric current are then passed through the probes causing pores to form in prostate cell walls which ultimately result in cell death [47]. Results from one of the largest IRE studies was recently published by van den Bos et al.[48▪] in which 63 patients with Gleason 6–7 disease were treated with IRE. Sixteen percent of patients had an in-treatment field recurrence and 24% were found to have persistent cancer anywhere within the prostate. No high-grade adverse events occurred and physical, mental, bowel, and urinary quality of life measures remained unchanged at 6 months postoperatively. Despite the theoretical claim that IRE might be less damaging to nerve tissue, mild declines in sexual quality of life median score from 66 to 54 at 6 months (P < 0.001) were seen [48▪]. This novel method has yet to be investigated further in larger scaled studies.
PHOTODYNAMIC THERAPY
Photodynamic therapy (PDT) is among the most well-studied focal therapy modalities in the short/intermediate term. PDT is accomplished through systemic administration of relatively biologically inert drug which can be activated to release a cytotoxic substance when exposed to light. These drugs are commonly referred to as photosensitizers. A phase III randomized controlled trial comparing PDT vs. active surveillance was recently published [49▪▪,50▪▪]. Anesthetized patients in the PDT arm were given a systemic photosensitizer (padeliporfin) intravenously, which was then activated by infrared light, typically introduced into the prostate via probes placed through the perineum. Activation of the photosensitizer leads to generation of superoxide and hydroxyl free radicals in the infrared-exposed areas, resulting in vascular thrombosis and coagulative necrosis [51].
In October 2018, Gill et al.[49▪▪] reported a 4-year update of the results from the phase III randomized trial comparing PDT to active surveillance. In total, 413 men with low-risk prostate cancer (Gleason 6 and 7) were randomized to PDT (n = 207) vs. active surveillance (n = 206). At 2 year follow-up, 50% of patients in the treatment arm were still found to have cancer anywhere in the prostate, with 25% having residual cancer within the treatment field [49▪▪]. These rates of in-field recurrences are comparable to those seen in the FLA and HIFU trials [28,35,49▪▪]. Metastasis-free survival was the same in both groups (99% vs. 99%) at four years [49▪▪]. Ultimately, the authors argue that the utility of this treatment is in the reduction of patient progression to radical therapy. The conversion rate to radical therapy was 24% in the PDT group vs. 53% in the active surveillance group (hazard ratio 0.31, 95% confidence interval 0.21–0.46) [49▪▪]. This result, however, was partially confounded by the fact that more patients in the active surveillance arm chose to undergo radical therapy without a clinical indication. Earlier results from the same trial, including complications, were reported by Azzouzi et al.[50▪▪]. Adverse events were more common in the treatment arm with erectile dysfunction rates reaching 38 vs. 11% in the control arm, respectively. Urinary complications of retention or incontinence affected 27 vs. 7%, respectively. Given that all of these patients fell into a low-risk category preoperatively, some authors have questioned if PDT treatment was worth these adverse events [52]. Although PDT is well studied, the outcomes are comparable to other focal therapies. Newer photosensitizers targeting prostate-specific membrane antigen (PSMA) are also being developed, allowing for more targeted and specific release of reactive species in areas expressing PSMA which may theoretically improve disease targeting and treatment outcomes [53].
CONCLUSION
Each mode of focal therapy demonstrates some early promise. However, they are all limited by poor navigation, inadequacy of imaging to delineate tumor boundaries, and variable precision of tissue destruction. These shortcomings may explain the high rates of in-treatment field recurrence and the high frequency of cancer detection globally within the prostate gland after treatment. Among well-selected patients, focal therapies demonstrate short-term local disease control (Table 1) with minimal adverse side-effects (Table 2). For certain well-selected patients, the prospect of focal therapy in place of radical surgery or active surveillance may be an attractive alternative, or may be an adjunct to active surveillance [41]. At present, the lack of long-term oncologic outcomes, imaging and navigation shortcomings, and uncertainties about the clinical efficacy of focal treatment for high-grade lesions has led organizations such as the American Urologic Association/American Society for Radiation Oncology/Society of Urologic Oncology, and European Association of Urology to recommend against focal therapy as part of standard of care treatment at this time [29,54]. New guidelines and advancements require evidence-based medicine to better study the long-term results of focal therapy in the setting of clinical trials or registries.
Table 1.
Oncologic outcomes of focal prostate ablation
| Study (references) | Ablation modality | Design | Participants (n) | Preoperative Gleason grade = 6 | Preoperative Gleason grade = 7 | Preoperative Gleason grade ≥ 8 | Time to oncologic follow-up (months) | In-Field recurrence | Out-of-field recurrence | Absence of clinically significant cancer | Absence of any prostate cancer |
| Ahmed et al. 2015 [26▪] | HIFU | Prospective cohort | 56 | 67% | 28% | 6% | 6 | 50% | 12% | 81% | 58% |
| Guillaumier et al. 2018 [25▪▪] | HIFU | Prospective cohort | 625 | 28% | 69% | 2% | 12 | 6%c | 4%a | – | 89%a |
| von Hardenberg et al. 2018 [28] | HIFU | Prospective cohort | 24 | – | – | 0% | 12 | 40% | – | – | 50% |
| Eggener et al. 2016 [35] | FLA | Prospective cohort | 27 | 85% | 15% | 0% | 12 | 11% | 30% | – | 63% |
| Natarajan et al. 2016 [37] | FLA | Prospective cohort | 10 | 18% | 82% | 0% | 6 | 30% | 40% | 60% | 30% |
| Lindner et al. 2009 [39] | FLA | Prospective cohort | 12 | 100% | 0% | 0% | 6 | 33% | 17% | – | 50% |
| Mendez et al. 2015 [43] | Cryotherapy | Prospective cohort | 317 | 100% | 0% | 0% | 12 | – | – | – | 86%b |
| Valerio et al. 2017 [44] | Cryotherapy | Prospective cohort | 18 | 28% | 72% | 0% | 12 | – | – | – | – |
| Tay et al. et al. 2017 [45] | Cryotherapy | Propensity matched case controlled | 166 | 36% | 65% | 0% | 24–36 | – | – | – | 96%c |
| Van den Bos et al. 2018 [48▪] | IRE | Prospective cohort | 63 | 14% | 86% | 0% | 6 | 16% | 9% | – | 76% |
| Azzouzi et al. 2018 [50▪▪]/Gill et al. 2018 [49▪▪] | PDT | Prospective Randomized Controlled | 413 | 100% | 0% | 0% | 24 | 25% | 19% | – | 50% |
Key.
Unavailable data is represented by a dash (–).
FLA, focal laser ablation; HIFU, high-intensity focal ultrasound, IRE, irreversible electroporation; PDT, photodynamic therapy.
aOnly 222 patients underwent postoperative biopsy. Half of these were done for cause.
bOnly 17% of patients underwent posttreatment biopsy.
cOnly 28.9% of patients underwent posttreatment biopsy. Two of 48 patients biopsied demonstrated cancer.
Table 2.
Complications of focal prostate ablation
| Study (references) | Ablation modality | Design | Participants (n) | Urinary tract infection rate | No pad continence (6 month) | Erectile dysfunction (%) | Fistula rate | Urinary retention rate | Hematuria |
| Ahmed et al. 2015 [26▪] | HIFU | Prospective cohort | 56 | 18% | 96% | 25% | 0% | – | 64% |
| Guillaumier et al. 2018 [25▪▪] | HIFU | Prospective cohort | 625 | 9% | 97% | – | 40% | – | – |
| von Hardenberg et al. 2018 [28] | HIFU | Prospective cohort | 24 | 17% | 88% | 20% | – | 13% | 8% |
| Eggener et al. 2016 [35] | FLA | Prospective cohort | 27 | – | – | 0% | – | 8% | 15% |
| Natarajan et al. 2016 [37] | FLA | Prospective cohort | 10 | – | 100% | 0% | – | – | – |
| Lindner et al. 2009 [39] | FLA | Prospective cohort | 12 | – | – | 0% | 0% | 0% | 17% |
| Mendez et al. 2015 [43] | Cryotherapy | Prospective cohort | 317 | – | 100% | 31% | 2% | 1% | – |
| Valerio et al. 2017 [44] | Cryotherapy | prospective cohort | 18 | 17% | – | 0% | – | 17% | – |
| Tay et al. et al. 2017 [45] | Cryotherapy | Propensity matched case controlled | 166 | – | 95% | 30% | 0% | 7% | – |
| Van den Bos et al. 2018 [48▪] | IRE | Prospective cohort | 63 | – | 98% | 23% | – | 2% | – |
| Azzouzi et al. 2018 [50▪▪]/Gill et al. 2018 [49▪▪] | PDT | Prospective randomized controlled | 413 | 21% | 99% | 38% | – | 16% | 28% |
Key.
Unavailable data is represented by a dash (–).
FLA, focal laser ablation; HIFU, high-intensity focal ultrasound, IRE, irreversible electroporation; PDT, photodynamic therapy.
Perhaps with further improvements of imaging modalities and improved targeting of ablation zones, the efficacy of focal treatments will improve. For example, in recent years, novel PET agents that more effectively detect prostate cancer have reached the US commercial market. Platforms such as PSMA PET in combination with MRI fusion provide improved cancer detection, localization, and characterization [55,56]. Additionally, contrast enhanced and superhigh-frequency ultrasound have shown some promise for improved cancer detection [57,58]. It has yet to be seen how integration of these new technologies will affect focal therapy outcomes, but optimism abounds about our future ability to treat well-selected patients with localized prostate cancer with less invasive approaches and less morbidity.
Acknowledgements
None.
Financial support and sponsorship
NIH and Philips have a Cooperative Research and Development Agreement. NIH has intellectual property in the field, including among other patents and patent applications, Patent: ‘System, methods, and instrumentation for image-guided prostate treatment’ US Patent number: 8948845, with inventors/authors B.W. and P.P. NIH and Philips (InVivo Inc) have a licensing agreement. NIH and authors B.W. and P.P. receive royalties for a licensing agreement with Philips/InVivo Inc. NIH does not endorse or recommend any commercial products, processes, or services. The views and personal opinions of authors expressed herein do not necessarily reflect those of the US Government, nor reflect any official recommendation nor opinion of the NIH nor National Cancer Institute.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a “work of the United States Government” for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
REFERENCES
- 1. [[Accessed 7 January 2019]]. SEER Cancer Statistics Review. U.S. Department of Health and Human Services 2018; https://seer.cancer.gov/statfacts/html/prost.html. [Google Scholar]
- 2.Pinsky PF, Parnes HL, Andriole G. Mortality and complications after prostate biopsy in the Prostate, Lung, Colorectal and Ovarian Cancer Screening (PLCO) trial. BJU Int 2014; 113:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012; 62:405–417. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V, Sooriakumaran P, Novara G, et al. Systematic review of methods for reporting combined outcomes after radical prostatectomy and proposal of a novel system: the survival, continence, and potency (SCP) classification. Eur Urol 2012; 61:541–548. [DOI] [PubMed] [Google Scholar]
- 5.Kearns JT, Holt SK, Wright JL, et al. PSA screening, prostate biopsy, and treatment of prostate cancer in the years surrounding the USPSTF recommendation against prostate cancer screening. Cancer 2018; 124:2733–2739. [DOI] [PubMed] [Google Scholar]
- 6.Walsh PC. Radical retropubic prostatectomy with reduced morbidity: an anatomic approach. NCI Monogr 1988; 133–137. [PubMed] [Google Scholar]
- 7.Novara G, Ficarra V, Rosen RC, et al. Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol 2012; 62:431–452. [DOI] [PubMed] [Google Scholar]
- 8.Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 2012; 62:382–404. [DOI] [PubMed] [Google Scholar]
- 9.Novara G, Ficarra V. Reply to Stefano C.M. Picozzi, Cristian Ricci and Luca Carmignani's letter to the editor re: Giacomo Novara, Vincenzo Ficarra, Simone Mocellin, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 62:382–404. Eur Urol. 2013;63(2):e29-31. [DOI] [PubMed] [Google Scholar]
- 10.Zaorsky NG, Shaikh T, Murphy CT, et al. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer. Cancer Treat Rev 2016; 48:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schauer I, Keller E, Muller A, Madersbacher S. Have rates of erectile dysfunction improved within the past 17 years after radical prostatectomy? A systematic analysis of the control arms of prospective randomized trials on penile rehabilitation. Andrology 2015; 3:661–665. [DOI] [PubMed] [Google Scholar]
- 12.Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 2012; 62:418–430. [DOI] [PubMed] [Google Scholar]
- 13.Wallis CJ, Glaser A, Hu JC, et al. Survival and complications following surgery and radiation for localized prostate cancer: an international collaborative review. Eur Urol 2018; 73:11–20. [DOI] [PubMed] [Google Scholar]
- 14.Onik G, Narayan P, Vaughan D, et al. Focal ‘nerve-sparing’ cryosurgery for treatment of primary prostate cancer: a new approach to preserving potency. Urology 2002; 60:109–114. [DOI] [PubMed] [Google Scholar]
- 15.Kurhanewicz J, Vigneron D, Carroll P, Coakley F. Multiparametric magnetic resonance imaging in prostate cancer: present and future. Curr Opin Urol 2008; 18:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Visschere PJ, Briganti A, Futterer JJ, et al. Role of multiparametric magnetic resonance imaging in early detection of prostate cancer. Insights Imaging 2016; 7:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futterer JJ, Briganti A, De Visschere P, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 2015; 68:1045–1053. [DOI] [PubMed] [Google Scholar]
- 18.Borofsky S, George AK, Gaur S, et al. What are we missing? False-negative cancers at multiparametric MR imaging of the prostate. Radiology 2018; 286:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Visschere PJ, Naesens L, Libbrecht L, et al. What kind of prostate cancers do we miss on multiparametric magnetic resonance imaging? Eur Radiol 2016; 26:1098–1107. [DOI] [PubMed] [Google Scholar]
- 20.Kamrava M, Chung M, Mesko S, et al. Correlation of quantitative diffusion-weighted and dynamic contrast-enhanced MRI parameters with NCCN risk group, Gleason score, and maximum tumor diameter in prostate cancer. Pract Radiat Oncol 2013; 3 2 Suppl 1:S4. [DOI] [PubMed] [Google Scholar]
- 21.Le JD, Tan N, Shkolyar E, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol 2015; 67:569–576. [DOI] [PubMed] [Google Scholar]
- 22.Nassiri N, Chang E, Lieu P, et al. Focal therapy eligibility determined by magnetic resonance imaging/ultrasound fusion biopsy. J Urol 2018; 199:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindner U, Trachtenberg J, Lawrentschuk N. Focal therapy in prostate cancer: modalities, findings and future considerations. Nat Rev Urol 2010; 7:562–571. [DOI] [PubMed] [Google Scholar]
- 24.Barkin J. High intensity focused ultrasound (HIFU). Can J Urol 2011; 18:5634–5643. [PubMed] [Google Scholar]
- 25▪▪.Guillaumier S, Peters M, Arya M, et al. A Multicentre study of 5-year Outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol 2018; 74:422–421. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospective cohort trial reporting the results of HIFU for the treatment of Gleason 6–8 cancers for 625 patients. This study reports low cancer recurrence rates, however, posttreatment biopsies were not performed in all patients.
- 26▪.Ahmed HU, Dickinson L, Charman S, et al. Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. Eur Urol 2015; 68:927–936. [DOI] [PubMed] [Google Scholar]; Prospective cohort trial reporting the results of HIFU for the treatment of Gleason 6–7 cancers for 56 patients.
- 27.Ghai S, Perlis N, Lindner U, et al. Magnetic resonance guided focused high frequency ultrasound ablation for focal therapy in prostate cancer: phase 1 trial. Eur Radiol 2018; 28:4281–4287. [DOI] [PubMed] [Google Scholar]
- 28.von Hardenberg J, Westhoff N, Baumunk D, et al. Prostate cancer treatment by the latest focal HIFU device with MRI/TRUS-fusion control biopsies: A prospective evaluation. Urol Oncol 2018; 36:401.e1–401.e9. [DOI] [PubMed] [Google Scholar]
- 29.van der Poel HG, van den Bergh RC, Briers E, et al. Focal therapy in primary localised prostate cancer: The European Association of Urology position in 2018. Eur Urol 2018; 74:84–91. [DOI] [PubMed] [Google Scholar]
- 30.Uchida T, Tomonaga T, Kim H, et al. Improved outcomes with advancements in high intensity focused ultrasound devices for the treatment of localized prostate cancer. J Urol 2015; 193:103–110. [DOI] [PubMed] [Google Scholar]
- 31.Colin P, Mordon S, Nevoux P, et al. Focal laser ablation of prostate cancer: definition, needs, and future. Adv Urol 2012; 2012:589160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafford RJ, Shetty A, Elliott AM, et al. Magnetic resonance guided, focal laser induced interstitial thermal therapy in a canine prostate model. J Urol 2010; 184:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordeiro ER, Cathelineau X, Thuroff S, et al. High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int 2012; 110:1228–1242. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan S, Jones TA, Priester AM, et al. Focal laser ablation of prostate cancer: feasibility of magnetic resonance imaging-ultrasound fusion for guidance. J Urol 2017; 198:839–847. [DOI] [PubMed] [Google Scholar]
- 35.Eggener SE, Yousuf A, Watson S, et al. Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer. J Urol 2016; 196:1670–1675. [DOI] [PubMed] [Google Scholar]
- 36.Oto A, Sethi I, Karczmar G, et al. MR Imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology 2013; 267:932–940. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan S, Raman S, Priester AM, et al. Focal laser ablation of prostate cancer: phase I clinical trial. J Urol 2016; 196:68–75. [DOI] [PubMed] [Google Scholar]
- 38.Lindner U, Weersink RA, Haider MA, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol 2009; 182:1371–1377. [DOI] [PubMed] [Google Scholar]
- 39.Lindner U, Lawrentschuk N, Trachtenberg J. Focal laser ablation for localized prostate cancer. J Endourol 2010; 24:791–797. [DOI] [PubMed] [Google Scholar]
- 40.Feller J, Greenwood B, Jones W, Toth R. Mp30-02 transrectally delivered, outpatient MRI-guided laser focal therapy of prostate cancer: seven year interim results of NCT #02243033. The Journal of Urology 2018; 199:e374–e375. [Google Scholar]
- 41.Bloom JB, Gold SA, Hale GR, et al. Super-active surveillance’: MRI ultrasound fusion biopsy and ablation for less invasive management of prostate cancer. Gland Surg 2018; 7:166–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998; 37:171–186. [DOI] [PubMed] [Google Scholar]
- 43.Mendez MH, Passoni NM, Pow-Sang J, et al. Comparison of outcomes between preoperatively potent men treated with focal versus whole gland cryotherapy in a matched population. J Endourol 2015; 29:1193–1198. [DOI] [PubMed] [Google Scholar]
- 44.Valerio M, Shah TT, Shah P, et al. Magnetic resonance imaging-transrectal ultrasound fusion focal cryotherapy of the prostate: A prospective development study. Urol Oncol 2017; 35:150.e1–150.e7. [DOI] [PubMed] [Google Scholar]
- 45.Tay KJ, Polascik TJ, Elshafei A, et al. Propensity score-matched comparison of partial to whole-gland cryotherapy for intermediate-risk prostate cancer: an analysis of the cryo on-line data registry data. J Endourol 2017; 31:564–571. [DOI] [PubMed] [Google Scholar]
- 46.Werneburg GT, Kongnyuy M, Halpern DM, et al. Effects of focal vs total cryotherapy and minimum tumor temperature on patient-reported quality of life compared with active surveillance in patients with prostate cancer. Urology 2018; 113:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005; 33:223–231. [DOI] [PubMed] [Google Scholar]
- 48▪.van den Bos W, Scheltema MJ, Siriwardana AR, et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int 2018; 121:716–724. [DOI] [PubMed] [Google Scholar]; Prospective cohort trial reporting the results of IRE for the treatment of Gleason 6-8 cancers for 63 patients.
- 49▪▪.Gill IS, Azzouzi AR, Emberton M, et al. Randomized trial of partial gland ablation with vascular targeted phototherapy versus active surveillance for low risk prostate cancer: extended followup and analyses of effectiveness. J Urol 2018; 200:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospective randomized controlled trial reporting the results of PDT for the treatment of Gleason 6 cancers as an alternative to active surveillance to prevent the need for radical therapy. This study is a follow-up to the results of Azzouzi et al. 2017.
- 50▪▪.Azzouzi AR, Vincendeau S, Barret E, et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 2017; 18:181–191. [DOI] [PubMed] [Google Scholar]; Prospective randomized controlled trial reporting the results of PDT for the treatment of Gleason 6 cancers as an alternative to active surveillance to prevent the need for radical therapy. In total, 50% of patients had no detectable cancer following treatment.
- 51.Kimm SY, Tarin TV, Monette S, et al. Nonthermal ablation by using intravascular oxygen radical generation with WST11: dynamic tissue effects and implications for focal therapy. Radiology 2016; 281:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritch CR, Punnen S. Photodynamic therapy for low risk prostate cancer. BMJ 2017; 356:j575. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Chatterjee S, Lisok A, et al. A PSMA-targeted theranostic agent for photodynamic therapy. J Photochem Photobiol B 2017; 167:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. Part I: risk stratification, shared decision making, and care options. J Urol 2018; 199:683–690. [DOI] [PubMed] [Google Scholar]
- 55.Piert M, Shankar PR, Montgomery J, et al. Accuracy of tumor segmentation from multiparametric prostate MRI and (18)F-choline PET/CT for focal prostate cancer therapy applications. EJNMMI Res 2018; 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eiber M, Weirich G, Holzapfel K, et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol 2016; 70:829–836. [DOI] [PubMed] [Google Scholar]
- 57.Postema AW, Frinking PJ, Smeenge M, et al. Dynamic contrast-enhanced ultrasound parametric imaging for the detection of prostate cancer. BJU Int 2016; 117:598–603. [DOI] [PubMed] [Google Scholar]
- 58.Ghai S, Eure G, Fradet V, et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: creation of the micro-ultrasound protocol for prostate risk identification. J Urol 2016; 196:562–569. [DOI] [PubMed] [Google Scholar]


