Abstract
Background
Surgical site infections (SSI) can delay wound healing, impair cosmetic outcome and increase healthcare costs. Topical antibiotics are sometimes used to reduce microbial contaminant exposure following surgical procedures, with the aim of reducing SSIs.
Objectives
The primary objective of this review was to determine whether the application of topical antibiotics to surgical wounds that are healing by primary intention reduces the incidence of SSI and whether it increases the incidence of adverse outcomes (allergic contact dermatitis, infections with patterns of antibiotic resistance and anaphylaxis).
Search methods
In May 2015 we searched: the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL. We also searched clinical trial registries for ongoing studies, and bibliographies of relevant publications to identify further eligible trials. There was no restriction of language, date of study or setting. The search was repeated in May 2016 to ensure currency of included studies.
Selection criteria
All randomized controlled trials (RCTs) and quasi‐randomised trials that assessed the effects of topical antibiotics (any formulation, including impregnated dressings) in people with surgical wounds healing by primary intention were eligible for inclusion.
Data collection and analysis
Two review authors independently selected studies and independently extracted data. Two authors then assessed the studies for risk of bias. Risk ratios were calculated for dichotomous variables, and when a sufficient number of comparable trials were available, trials were pooled in a meta‐analysis.
Main results
A total of 10 RCTs and four quasi‐randomised trials with 6466 participants met the inclusion criteria. Six studies involved minor procedures conducted in an outpatient or emergency department setting; eight studies involved major surgery conducted in theatre. Nine different topical antibiotics were included. We included two three‐arm trials, two four‐arm trials and 10 two‐arm trials. The control groups comprised; an alternative topical antibiotic (two studies), topical antiseptic (six studies) and no topical antibiotic (10 studies), which comprised inert ointment (five studies) no treatment (four studies) and one study with one arm of each.
The risk of bias of the 14 studies varied. Seven studies were at high risk of bias, five at unclear risk of bias and two at low risk of bias. Most risk of bias concerned risk of selection bias.
Twelve of the studies (6259 participants) reported infection rates, although we could not extract the data for this outcome from one study. Four studies (3334 participants) measured allergic contact dermatitis as an outcome. Four studies measured positive wound swabs for patterns of antimicrobial resistance, for which there were no outcomes reported. No episodes of anaphylaxis were reported.
Topical antibiotic versus no topical antibiotic
We pooled the results of eight trials (5427 participants) for the outcome of SSI. Topical antibiotics probably reduce the risk of SSI in people with surgical wounds healing by primary intention compared with no topical antibiotic (RR 0.61, 95% CI 0.42 to 0.87; moderate‐quality evidence downgraded once for risk of bias). This equates to 20 fewer SSIs per 1000 patients treated with topical antibiotics (95% CI 7 to 29) and a number needed to treat for one additional beneficial outcome (NNTB) (i.e. prevention of one SSI) of 50.
We pooled the results of three trials (3012 participants) for the outcome of allergic contact dermatitis, however this comparison was underpowered, and it is unclear whether topical antibiotics affect the risk of allergic contact dermatitis (RR 3.94, 95% CI 0.46 to 34.00; very low‐quality evidence, downgraded twice for risk of bias, once for imprecision).
Topical antibiotic versus antiseptic
We pooled the results of five trials (1299 participants) for the outcome of SSI. Topical antibiotics probably reduce the risk of SSI in people with surgical wounds healing by primary intention compared with using topical antiseptics (RR 0.49, 95% CI 0.30 to 0.80; moderate‐quality evidence downgraded once for risk of bias). This equates to 43 fewer SSIs per 1000 patients treated with topical antibiotics instead of antiseptics (95% CI 17 to 59) and an NNTB of 24.
We pooled the results of two trials (541 participants) for the outcome of allergic contact dermatitis; there was no clear difference in the risk of dermatitis between topical antibiotics and antiseptics, however this comparison was underpowered and a difference cannot be ruled out (RR 0.97, 95% CI 0.52 to 1.82; very low‐quality evidence, downgraded twice for risk of bias and once for imprecision).
Topical antibiotic versus topical antibiotic
One study (99 participants) compared mupirocin ointment with a combination ointment of neomycin/polymyxin B/bacitracin zinc for the outcome of SSI. There was no clear difference in the risk of SSI, however this comparison was underpowered (very low‐quality evidence downgraded twice for risk of bias, once for imprecision).
A four‐arm trial involved two antibiotic arms (neomycin sulfate/bacitracin zinc/polymyxin B sulphate combination ointment versus bacitracin zinc, 219 participants). There was no clear difference in risk of SSI between the combination ointment and the bacitracin zinc ointment. The quality of evidence for this outcome was low, downgraded once for risk of bias, and once for imprecision.
Authors' conclusions
Topical antibiotics applied to surgical wounds healing by primary intention probably reduce the risk of SSI relative to no antibiotic, and relative to topical antiseptics (moderate quality evidence). We are unable to draw conclusions regarding the effects of topical antibiotics on adverse outcomes such as allergic contact dermatitis due to lack of statistical power (small sample sizes). We are also unable to draw conclusions regarding the impact of increasing topical antibiotic use on antibiotic resistance. The relative effects of different topical antibiotics are unclear.
Keywords: Humans; Wound Healing; Administration, Topical; Anti‐Bacterial Agents; Anti‐Infective Agents, Local; Anti‐Infective Agents, Local/administration & dosage; Anti‐Infective Agents, Local/adverse effects; Dermatitis, Allergic Contact; Dermatitis, Allergic Contact/etiology; Drug Resistance, Bacterial; Randomized Controlled Trials as Topic; Surgical Wound Infection; Surgical Wound Infection/prevention & control
Plain language summary
Topical antibiotics (applied to the skin) for preventing surgical site infection in wounds that are stitched or held together another way
Background
The presence of micro‐organisms, such as bacteria, at wound sites following surgery can result in surgical site infections for patients. Surgical site infections can result in increased healthcare costs, delays in wound healing and pain. Antibiotics are medicines that kill bacteria or prevent them from developing. Antibiotics can be taken by mouth (orally), directly into veins (intravenously), or applied directly to the skin (topically). Topical antibiotics are often applied to wounds after surgery because it is thought that they prevent surgical site infection. There are thought to be benefits in using antibiotics topically rather than orally or intravenously. As topical antibiotics act only on the area of the body where they are applied, there is less likelihood of unwanted effects that affect the whole body, such as nausea and diarrhoea. Topical antibiotics are also thought to reduce the chances of bacterial resistance (bacteria changing to become resistant to medication). However topical antibiotics can also have unwanted effects, the most common being an allergic reaction on the skin (contact dermatitis), which can cause redness, itching and pain at the site where the topical antibiotic was applied.
Review question
We reviewed the evidence about how effective topical antibiotics are in preventing surgical site infection if applied directly to wounds after surgery. We focused on the effect of topical antibiotics on the type of surgical wound where the edges are held closely together so that the wound heals more easily (known as healing by primary intention). The edges of these wounds can be held together with stitches, staples, clips or glue.
What we found
In May 2016 we searched for as many relevant studies as we could find that investigated the use of topical antibiotics on surgical wounds healing by primary intention. We managed to identify 14 studies which compared topical antibiotics with no treatment, or with antiseptics (i.e. other treatments applied to the skin to prevent bacterial infection), and with other topical antibiotics. Eight of these trials involved general surgery and six involved dermatological surgery (surgery involving only the skin). Many of the studies were small, and of low quality or at risk of bias. After examining them all, the authors concluded that the risk of having a surgical site infection was probably reduced by the use of topical antibiotics applied to wounds after surgery, whether the antibiotics were compared with an antiseptic, or to no treatment. As infection is a relatively rare event after surgery, the actual reduction in the rate of infection was 4.3% on average when the use of topical antibiotic was compared with antiseptic, and 2% when use of the topical antibiotic was compared with no treatment. It would require 24 patients on average to be treated with topical antibiotics instead of antiseptic, and 50 patients to be treated with topical antibiotic compared to no treatment in order to prevent one wound infection. Four studies reported on allergic contact dermatitis, but there was insufficient evidence to determine whether allergic contact dermatitis occurred any more frequently with topical antibiotics than with antiseptics or no treatment, and this should also be considered before deciding to use them.
This plain language summary is up to date as of May 2016.
Summary of findings
Summary of findings for the main comparison. Topical antibiotics compared with no topical antibiotic.
| Topical antibiotics compared with no topical antibiotic for surgical wounds healing by primary intention | ||||||
| Patient or population: people presenting for surgery where healing of surgical wound(s) was planned to be by primary intention Setting: primary or secondary care Intervention: topical antibiotic Comparison: no topical antibiotic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment control | Risk with topical antibiotic | |||||
| Surgical site infection | Study population | RR 0.61 (0.42 to 0.87) | 5427 (7 RCTs and 1 Q‐RCT) | ⊕⊕⊕⊝ MODERATE 1 | Downgraded for risk of bias (‐1) | |
| 51 per 1000 | 31 per 1000 (21 to 44) | |||||
| Allergic contact dermatitis | Study population | RR 3.94 (0.46 to 34) | 3012 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2,3 | Downgraded for risk of bias (‐2) Downgraded for Imprecision (‐1) |
|
| There were 5 (out of 1255) cases of allergic contact dermatitis with topical antibiotics compared with none (out of 1787) in the control groups | ||||||
| Anaphylaxis | Not reported | N/A | N/A | |||
| Patterns of antibiotic resistance | Not reported | N/A | N/A | |||
| Wounds healed 5‐14 days | Study population | |||||
| 827 per 1000 | 827 per 1000 (794 to 854) | RR 1.00 (0.96 to 1.03) | 1034 (2 RCT and 2 Q‐RCTs) | ⊕⊕⊝⊝ LOW 4 | Downgraded for risk of bias (‐2) | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 The proportion of the information from studies at high risk of selection bias is sufficient to affect the interpretation of the results.
2 The confidence interval was wide and crossed no effect (0.46 to 34)
3 The majority of information was from a study at high risk of selection and performance bias, which also had unit of analysis issues.
4 The majority of information was from studies at high risk of selection, performance or detection bias.
Summary of findings 2. Topical antibiotics compared with antiseptic.
| Topical antibiotics compared with antiseptic for wounds healing by primary intention. | ||||||
| Patient or population: people presenting for surgery where healing of surgical wound planned to be by primary intention Setting: primary or secondary care Intervention: topical antibiotic Comparison: antiseptic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with topical antibiotic | |||||
| Surgical site infection | Study population | RR 0.49 (0.3 to 0.8) | 1299 (4 RCTs, 1 Q‐RCT) | ⊕⊕⊕⊝ MODERATE 1 | Downgraded for risk of bias (‐1) | |
| 84 per 1000 | 41 per 1000 (25 to 67) | |||||
| Allergic contact dermatitis | Study population | RR 0.97 (0.52 to 1.82) | 541 (1 RCT, 1 Q‐RCT) | ⊕⊝⊝⊝ VERY LOW 2,3 | Downgraded for risk of bias (‐2) Imprecision (‐1) |
|
| 77 per 1000 | 75 per 1000 (40 to 140) | |||||
| Anaphylaxis | Not reported | |||||
| Patterns of antibiotic resistance | Not reported | |||||
| Wounds healed 5‐14 days | Study population | |||||
| 574 per 1000 | 947 per 1000 (333 to 1000) | RR 1.65 (0.58 to 4.72) | 327 (2 Q‐RCT) | ⊕⊝⊝⊝ VERY LOW 4,5,6 | Downgraded for risk of bias (‐2) Imprecision (‐1) Inconsistency (‐2)7 |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The proportion of information from studies at high risk of selection, performance and attrition bias is sufficient to affect the interpretation of results.
2 The confidence interval was wide and crossed no effect (0.52 to 1.82)
3 The majority of information was from studies at high risk of selection, performance and attrition bias.
4 All of the information was from studies at high risk of selection, performance, detection or attrition bias.
5 The confidence intervals were broad and crossed no effect (0.58 to 4.72)
6 Heterogeneity 88%
7Downgraded maximum of three times to very low quality
Background
Description of the condition
Many surgical procedures are conducted each year. The majority of these procedures result in wounds that heal by primary intention, which means that the wound edges are brought together (approximated) using sutures, staples, clips or glue. Wounds can also heal by secondary intention, then the edges are not approximated and the wound heals by granulation, re‐epithelialisation and contraction. Most wounds heal without complications but surgical site infections (SSIs) can occur after surgery in the site where the surgery took place. Most wound infections are caused by contamination during surgery with the patient’s own micro‐organisms (Kulaylat 2007). They may be superficial and self‐limiting, involving the skin only, or they may be deeper and life‐threatening. SSIs are classified by the Centers for Disease Control and Prevention (CDC) as superficial incisional, deep incisional and organ/space infections (CDC 2014; Mangram 1999).
SSIs account for up to 20% of all of healthcare‐associated infections (Magill 2014). At least 5% of patients who have a surgical procedure will go on to develop an SSI, highlighting the importance of good prevention, detection and management (NICE 2008). SSIs can delay healing, impair cosmetic outcomes and potentially cause other morbidity, such as deeper infections, as well as potentially increasing costs, and the consumption of healthcare resources (Bratzler 2004).
In order to understand SSI, it is first important to understand the classification of surgical wounds. Surgical wounds are traditionally classified into different categories, and infection rates vary by category. This classification is important in order to predict postoperative infection rates and thus aid the decision to prescribe postoperative antibiotics, whether oral or topical (Table 3).
1. Wound classification.
| Preoperative classification | Wound type | Maximum expected postoperative infection rate | Example of wound |
| Class 1/clean | Non contaminated wound | 5% | Sterile minor skin excision |
| Class 2/clean contaminated | Operative wound in respiratory, alimentary, or genitourinary tract, or minor break in aseptic technique | 10% | Biliary tract, appendix, vagina, oropharynx |
| Class 3/contaminated | Open, fresh, accidental wound, acute nonpurulent inflammation, gross spillage from gastrointestinal tract,or major break in aseptic technique | 20% to 30% | Open cardiac massage, gross spillage from gastrointestinal tract |
| Class 4/dirty‐infected | Purulent inflammation, gross contamination with foreign bodies, penetrating trauma more than 4‐h old, devitalised tissue | 30% to 40% | Old traumatic wound, abscess |
None
Clean (class 1): Noninfective operative wounds in which no inflammation is encountered, with no involvement of respiratory, alimentary, genitourinary tract and oropharyngeal cavity. Additionally, these wounds must be the result of elective procedures, closed by primary intention and drained with closed drainage system if required.
Clean/contaminated (class 2): Operative wounds in which either the respiratory, alimentary, or genitourinary tract is entered under controlled conditions and with only minor contamination. This category specifically includes wounds as a result of operations involving the biliary tract, appendix, and oropharynx, provided no evidence of infection or a major break in sterile technique is encountered.
Contaminated (class 3): Fresh, accidental wounds, resulting from operations with major breaks in sterile technique or gross spillage from the gastrointestinal tract, and incisions in which acute, nonpurulent inflammation is encountered. This category includes traumatic lacerations.
Dirty (class 4): Old traumatic wounds with retained devitalised tissue and those that involve existing clinical infection or perforated viscera. Organisms causing postoperative infection are likely to be present in the operative field before the operation.
In a general surgical setting the acceptable rate of infection following clean surgery (class 1) is less than 5% (Cruse 1980; Culver 1991; Mangram 1999). In contrast, clean contaminated wounds (class 2) have a risk of infection of less than 10%. Therefore, in a general surgical setting, oral antibiotic prophylaxis of surgical wounds is usually considered optional for clean procedures, and reserved for certain at‐risk patients or high‐risk procedures (Bratzler 2004). If guidelines for prophylaxis after general surgery are extrapolated to a dermatological surgery setting, then most dermatological procedures, which are considered to be clean (class 1) surgery, should not require prophylaxis, and most guidelines reflect this (Maragh 2005; Messingham 2005; Wright 2008). However, as in general surgery, even within cohorts with a low overall risk of infection, some procedures may be at higher risk and infection rates may be greater than 5% in these high‐risk groups. Although limited guidelines exist for the use of oral antibiotics as infection prophylaxis, there are no guidelines for the use of topical antibiotics after general and dermatological surgery.
There is no universal agreement on the definition of SSI. A systematic review identified 41 different definitions, and 13 grading scales for SSI, the majority of which had not been validated (Bruce 2001). The most widely accepted description for surgical site infection, however, is based on the 1992 CDC classification, in which infection must occur within 30 days of surgery and involve skin or deep tissue at the incision site (Mangram 1999).
In addition, one of the following must apply:
purulent discharge from the incisional wound;
organisms are isolated on culture of aseptically obtained wound fluid or tissue;
one or more of the following is present: pain, tenderness, localised swelling, redness, heat, or the surgeon has deliberately re‐opened wound (unless culture of the incision is negative);
the treating doctor diagnoses a superficial incisional surgical site infection. Stitch abscesses are not defined as infection.
Although this definition has limitations, it is the most widely implemented standard definition of SSI, and is the closest to a gold standard available. Even when using guidelines, the diagnosis is still subjective and there may be inter‐ and intra‐observer variation.
Description of the intervention
The most common method of application of topical antibiotics is in the form of an ointment. Other possible delivery methods include cream, lotion, solution, gel, tincture, foam, paste, powder, and impregnated dressings. An ointment base classically contains 80% oil and 20% water, and therefore is more occlusive and will drive the medication into the skin more rapidly than a solution or cream base; thus ointments are an optimal delivery method for topical antibiotics. The only data available on the frequency of topical antibiotic use on wounds comes from a survey of plastic surgeons in the UK which revealed that 66% used chloramphenicol eye ointment in their practice, mainly as prophylaxis against infection (Erel 1999). Other uses for antibiotic ointment include the treatment of secondarily infected wounds (Leyden 1987), otitis externa, treatment of secondarily infected eczema and the treatment of impetigo (AEG 2010). Antibiotic ointments may also have a role in accelerating wound healing in both acute and chronic situations (Berger 2000; Eaglstein 1980; Geronemus 1979). Adverse effects may include allergic contact dermatitis (Blondeel 1978; Leyden 1979; Marks 1998), anaphylaxis (Saryan 1998), and the theoretical possibility of antibiotic resistance (Bradley 1995; Fukuda 2002; Miller 1996).
There are several different types of antibiotic ointments used in clinical practice, and the preferred choice varies by country (Table 4). Many of these topical antibiotic agents contain antibiotics that are not recommended for systemic use due to serious adverse effects. The risk of serious effects is considered low with topical use, thus they are safe for use in this form (Kasten 1999).
2. Topical antibiotics.
| Ointment | Trade name, availability | Mode of activity | Range of activity | Main use | Side effects/additional considerations |
| Mupirocin | Bactroban | Inhibitor of bacterial protein synthesis | Gram‐positive organisms, especially Staphylococcus aureus | Impetigo, elimination of S aureus from anterior nares | Anaphylaxis reported |
| Bacitracin | Ingredient of triple antibiotic ointment | Interferes with bacterial cell wall synthesis | Gram‐positive organisms | Impetigo, furunculosis, pyodermas | Cross‐sensitisation with neomycin |
| Polymixin B | Available singly, combined with bacitracin or in triple antibiotic ointment | Disrupts bacterial cell membrane and increases cell permeability | Gram‐negative organisms, including Pseudomonas aeruginosa, Enterbacter species and Escherichia coli | Bacterial conjunctivitis | Limited spectrum of activity |
| Neomycin | Available alone, or as ingredient of triple antibiotic ointment | Interferes with bacterial cell wall synthesis | Aerobic Gram‐positive and Gram‐negative bacilli | Prevention of infection in superficial abrasions, cuts or burns | Allergic contact dermatitis |
| Polymixin B, neomycin and bacitracin | Triple antibiotic ointment | Combination of mechanisms | Range of Gram‐positive and Gram‐negative organisms | Prevention of infection in superficial abrasions, cuts or burns | Allergic contact dermatitis |
| Erythromycin | Eryacne | Inhibitor of bacterial protein synthesis | Gram‐positive cocci | Acne | Low incidence of sensitisation |
| Chloramphenicol | Chlormycetin or Chlorsig | Disrupts bacterial cell membrane | Wide range of Gram‐positive and Gram‐negative organisms | Bacterial conjunctivitis | Aplastic anaemia |
How the intervention might work
The role of topical antibiotics is to reduce the microbial contaminant exposure following the surgical procedure. A surgeon may choose to use a topical antibiotic on a wound after considering the likelihood of infection and weighing up the risks and benefits of treatment. There is a lack of evidence in the literature regarding the effects of antibiotic ointment in preventing wound infection.
Topical antibiotics have a number of mechanisms of action. Chloramphenicol is a bacteriostatic broad‐spectrum antibiotic that exerts an effect by inhibiting protein synthesis of the bacteria and interfering with transfer of activated amino acids to ribosomes. Neomycin has moderate Gram‐negative action through inhibition of protein synthesis. Mupirocin is active against Gram‐positive aerobic bacteria by inhibiting bacterial protein synthesis (HCN 2014). Antibiotics differ from antiseptics as they target specific organisms selectively, whereas antiseptics destroy or inhibit the growth of organisms non selectively (McDonnell 1999).
Why it is important to do this review
Rationalising the use of antibiotics is important in order to reduce the risk of antibiotic resistance. The evidence for use of topical antibiotics is conflicting, and therefore a systematic review of trials is important to guide clinical practice. In some countries, such as the USA, topical antibiotics are available over‐the‐counter, whereas in others they are only available when prescribed by a doctor. The effectiveness of this treatment is therefore important to consumers, as well as health practitioners. Better information about effectiveness could assist in rationalising use and contribute to controlling development of antibiotic resistance in the community.
Objectives
The primary objective of this review was to determine whether the application of topical antibiotics to surgical wounds that are healing by primary intention reduces the incidence of SSI and whether it increases the incidence of adverse outcomes (allergic contact dermatitis, infections with patterns of antibiotic resistance and anaphylaxis).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized controlled trials (quasi‐RCTs) with a parallel group design. Quasi‐RCTs are trials which use a quasi‐random allocation strategy, such as alternate days, date of birth, or hospital number. We included trials published as abstracts if sufficient data were available. We also included unpublished RCTs if sufficient data were available. We accepted trials with paired designs (one wound treated with topical antibiotic, and the other treated without topical antibiotic, at different sites in the same patient).
Types of participants
We included:
people of any age, gender or country of origin who had undergone surgical procedures where healing of the surgical wound was planned by primary intention, i.e. where wounds had edges approximated with sutures, staples, clips or glue;
any surgical setting, including dermatology outpatients or inpatients, emergency department, general surgery and primary care;
all types of surgery (i.e. by risk of contamination); and
studies involving mixed populations (if the data allowed the results from the relevant population to be extracted). Our definition of mixed populations for the purpose of this review was a trial in which some of the participants fulfilled the inclusion criteria and others did not.
We excluded:
studies involving people with wounds that were already infected (secondarily infected wounds), i.e. we did not include antibiotics for treating ‐ rather than preventing ‐ wound infection;
wounds healing by secondary intention; and
instances where there had been antibiotic irrigation or washout of wounds, subcutaneous infiltration of the antibiotic, or any topical treatment applied only prior to wound closure (not after).
Types of interventions
The intervention was topical antibiotics in the form of ointments, creams, lotions, solutions, gels, tinctures, foams, pastes, powders and impregnated dressings. We excluded silver and antiseptics from our definition of topical antibiotics. We required the topical antibiotic to have been applied after the wound was closed by primary intention, therefore we excluded antibiotic irrigation and washouts, subcutaneous infiltration of antibiotics and any topical treatment applied only prior to closure of the wound. We also excluded studies of antibiotic‐coated sutures. We originally planned to exclude studies where patients received concomitant systemic antibiotics, however these studies were included. We included single application postoperatively, or multiple applications in the postoperative period. We recorded dosage of antibiotic if this information was available. The topical antibiotic may have been applied with or without a dressing. The comparison group was placebo ‐ which could have contained the vehicle of the topical antibiotic ‐ oral antibiotic, alternative topical antibiotic, topical antiseptic or no treatment. We did not consider the comparator groups to be homogenous for the purposes of data synthesis.
Types of outcome measures
We did not consider outcomes to be eligibility criteria. We considered secondary outcomes with and without validated scales.
Primary outcomes
SSI, as defined by the CDC definition of SSI. In this definition infection must occur within 30 days of the procedure, therefore this time point was used as a cut‐off for this primary outcome measure. We also accepted the trial authors' definitions of infection.
Proportion of patients with any relevant adverse effect within 30 days of the procedure, i.e. allergic contact dermatitis, anaphylaxis, or infections with patterns of antibiotic resistance.
Secondary outcomes
Wound healing: time‐to‐healing or proportion of wounds healed at the end of the trial.
Patient satisfaction measured within six months of the procedure.
Health‐related quality of life at 30 days and three months.
Financial cost for each infection prevented (number needed to treat for an additional beneficial outcome (NNTB)). We planned to make this calculation by using the NNTB to calculate the financial cost of prescribing topical prophylactic antibiotics to a number of patients in order to prevent a single wound infection.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant RCTs or quasi‐RCTs:
Cochrane Wounds Specialised Register (searched 31 May 2016);
The Cochrane Central Registrar of controlled trials (CENTRAL; the Cochrane Library 2015, Issue 4);
Ovid MEDLINE (1946 to 31 May 2016);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 31 May 2016);
Ovid Embase (1974 to 31 May 2016);
EBSCO CINAHL (1982 to 31 May 2016).
The search was first conducted in May 2015. The search was repeated in May 2016 to ensure currency of included studies.
The search strategies used for CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). We did not restrict studies with respect to language, date of publication or study setting.
We searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov);
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/Default.aspx);
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
We searched the bibliographies of all retrieved and relevant publications identified by the database searches for additional eligible trials. We contacted manufacturers and pharmaceutical companies regarding studies for inclusion.
Data collection and analysis
We followed guidelines given by the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), and Cochrane Wounds.
Selection of studies
Two review authors (CH and JB) independently screened the studies identified by the literature search. These review authors analyzed the titles and abstracts of all citations found through the search strategy described above. They obtained a copy of the full article for each citation reporting a potentially eligible trial. Independently, the two review authors applied the eligibility criteria; any discrepancies were resolved by consensus discussion with the third review author (MVD). Where necessary and possible, additional information was sought from the principal investigator of the trial concerned. We justified, in the final report, any exclusion of a potentially eligible trial from the review. We completed a PRISMA flowchart to summarize this process (Figure 1) (Liberati 2009).
1.
Study flow diagram
Data extraction and management
Two review authors (CH and PL) independently extracted data. We summarised data using a pre designed data extraction form. We piloted the data extraction tool before use. Data from trials published in duplicate were included only once. Any discrepancy was resolved by discussion or in consultation with a third review author (MVD).
We extracted the following data:
source (study ID);
eligibility (confirm eligibility for review);
characteristics of the trial (date of study, setting, location of care, country, source of funding);
methods (study design, sequence generation, allocation sequence concealment, blinding, other concerns about bias);
participants (number, diagnostic criteria, age, sex, comorbidities, class of wound);
intervention (type of topical antibiotic, delivery vehicle, dose, frequency of application, co interventions);
comparative intervention (placebo ointment, alternative antibiotic ointment, no treatment control);
for each outcome of interest: outcome definition, unit of measurement, upper and lower limits for scales;
primary outcomes (definition of SSI, unit of measurement);
secondary outcomes (outcome definition and unit of measurement);
results (number of participants allocated to each intervention group, sample size, missing participants, summary data ‐ e.g. 2x2 data for dichotomous data, means and standard deviations for continuous data, estimate of effect with confidence intervals and P value, subgroup analysis).
key conclusions of study authors.
Assessment of risk of bias in included studies
Two review authors (CH and PL) independently assessed each included study. Assessment was undertaken using the Cochrane tool for assessing risk of bias (Higgins 2011). The 'Risk of bias' tool considers the domains of:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
freedom from selective reporting; and
other potential bias.
We acknowledge that there is no accepted definition of what constitutes a trial at high risk of bias, therefore we set a threshold so that trials that we assessed as being at risk for any one of the following essential elements of risk of bias ‐ sequence generation, allocation concealment and assessor blinding ‐ we considered to be at high risk of bias. Also, if missing outcome data were unequally distributed over the intervention arms, we discussed this, considered the study at high risk of attrition bias, and considered performing intention‐to‐treat (ITT) analysis.
We completed a 'Risk of bias' table for each eligible study. We combined these data into a 'Risk of bias' summary figure.
Measures of treatment effect
The primary outcome was dichotomous (SSI or no SSI) and was measured using risk ratio as the effect measure, with 95% confidence interval. We planned to use mean difference with standard deviation and 95% confidence interval to analyse continuous variables (patient satisfaction) using the same scales. Where different scales were used to assess continuous outcomes, we planned to use standardised mean difference with standard deviation in the analysis (Deeks 2011). Time‐to‐healing is a form of time‐to‐event data, more correctly analyzed using survival methods which can account for censoring (i.e. just for the time that people were observed, so it takes account of when they dropped out); it would have been inappropriate to report and analyse time‐to‐wound healing as if it were a continuous variable unless everyone healed and there was no loss to follow‐up. In practice there were no continuous variables in our review, and time‐to‐event data were not available for analysis in a usable format.
Unit of analysis issues
The unit of analysis in trials was most likely to be the patient recruited into the trial. It was possible that cluster‐randomized trial designs would be encountered, for example randomisation by surgeon, or by operating list, or by general practice surgery or hospital. We planned to analyse such trials based on allocation, using summary values for each cluster, allowing the clusters to become the individuals and analyse them as such. We planned to use analysis from the trials that adjusted for clustering. Where trials did not adjust for clustering, we planned to attempt adjust the analysis for correlation. This can be done through a number of methods, ideally based on a direct estimate of the required effect measure as stated in Deeks 2011. We planned to use the generic inverse variance method in Review Manager 5 (RevMan 2014) to pool data from cluster randomized trials (Deeks 2011). In practice, there were no cluster‐randomized trials encountered in our review.
If there were three arms in a study, where two of the arms were clinically similar, for the purposes of the review, we combined them to create a single pair‐wise comparison. Where we could not combine arms and we included multiple arms in the same analysis, we planned to divide the control group(s) between the two arms for the purpose of comparison.
In order to avoid unit of analysis error when measurement occurred at multiple time points, we planned only to pool data from one time point that was closest to that of the other included studies.
Including multiple wounds
We considered adjusting for clustering when multiple wounds were included in the same patient. We could not find a published standard value for the inter‐cluster correlation (ICC) that should be used to adjust for clustering for this scenario. Therefore we explored three potential situations with different values used for ICC, and then performed a sensitivity analysis on the overall effect of the two most extreme scenarios on the overall results.
Dealing with missing data
If the results of a trial were published, but information on the outcome of interest was not reported, we attempted, whenever possible, to contact the trial authors for the missing information. If continuous data were not presented as mean and standard deviation, we planned, whenever possible, to contact the trial authors to request the information in this format. If the data were not available, we planned to impute the missing standard deviation by borrowing from similar studies, or we calculated the standard deviation from P values, t values, confidence intervals or standard errors, whichever was available. We followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). In the completed review, we report all efforts made to obtain additional information.
Excluding participants from the analysis after randomisation, or ignoring participants lost to follow‐up can, in effect, undo the process of randomisation, and thus potentially introduce bias into the trial. Therefore, where possible, all analyses were to be by intention‐to‐treat (Hollis 1999). If participants were allocated to one intervention (for example, antibiotic ointment), but after randomisation underwent a different intervention (for example, placebo ointment), they were to be analyzed according to their randomisation allocation.
If the results for dichotomous variables were not reported in some participants, we planned originally to base our analysis on both a worst possible outcome (for example, wound infection occurred in all non reported cases), and a best possible outcome (for example, wound infection did not occur in any non reported cases). Where participants were excluded from analysis without good cause we planned to conduct a sensitivity analysis to determine any effect of attrition bias.
Assessment of heterogeneity
We explored the presence or absence of heterogeneity using visual inspection of forest plots. If there was no apparent face value heterogeneity (e.g. clearly different populations or types of wounds, different category of control group) we performed a Chi2 test with significance set at P value 0.10. We also calculated the I2 statistic (Deeks 2011). This explores the proportion of variability caused by heterogeneity rather than by chance. Thresholds for the interpretation of the I2 statistic can be misleading. A rough guide to interpretation of the I2 statistic is:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: represents considerable heterogeneity.
When interpreting and exploring the I2 statistic, we took factors such as clinical and methodological heterogeneity ‐ in particular the placebo treatment used ‐ along with whether the heterogeneity was in the magnitude of effect or in the direction of effect, into account, particularly where ranges overlapped (Deeks 2011). We explored this further in subgroup analyses. We planned that if heterogeneity was very high (> 75%), we would not pool these studies; we explored the impact of heterogeneity on the overall outcome with a sensitivity analysis (see Sensitivity analysis).
Assessment of reporting biases
We compared the reported outcomes with those stated in the published protocol of the studies, if available, or in the methods section of the published report, and also those listed in clinical trials registries as both primary and secondary outcomes (for example www.clinicaltrials.gov). If sufficient studies were identified (a minimum of 10), we planned to assess the risk of publication bias by creating a funnel plot using software within Review Manager 5 (RevMan 2014), using visual inspection and statistical tests for asymmetry.
Data synthesis
One review author (CH) entered quantitative data into Review Manager 5 (RevMan 2014), and a second (PL) checked the data. We calculated summary estimates of treatment effect (with 95% confidence interval) for each outcome and every comparison. For continuous outcomes, we presented the pooled mean difference with the standard deviation as a measure of the spread. For dichotomous outcomes, we calculated the risk ratio as the effect measure, with 95% confidence interval. We also calculated the absolute risk difference, that would allow us to calculate the NNTB. We meta‐analysed the results of clinically homogenous studies using Review Manager 5 (RevMan 2014). We conducted meta‐analyses using a random‐effects model. If insufficient data were available for meta‐analyses, we presented a narrative synthesis of the outcome across the included studies. We presented all results in 'Summary of findings' tables, and rated the quality of evidence using the GRADE system (see below) (Schünemann 2011a).
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b).
We presented the following primary outcomes in the `Summary of findings´ tables:
superficial surgical site infection;
adverse events;
the proportion of wounds healed during the time period.
Subgroup analysis and investigation of heterogeneity
Where there were sufficient trials of adequate size and it was possible to conduct subgroup analyses, we planned to conduct subgroup analyses for:
clean versus clean/contaminated versus contaminated wounds;
dermatological versus general surgery;
class of antibiotic used;
single application versus multiple applications; and
no treatment control versus placebo ointment control.
Sensitivity analysis
We performed a sensitivity analysis to assess the impact of heterogeneity on the overall estimate of effect by first pooling all studies, and subsequently removing the outlying studies that seemed to be contributing to the statistical heterogeneity. We also performed sensitivity analysis to assess the impact of risk of bias on the overall effect measure. We compared the outcomes of these analyses and described the implications for the conclusion of the review. We removed studies at high risk of bias in order to assess the effect of this on the result.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables for full details of the studies identified. We did not identify any studies which were about to start. We are not aware of any relevant ongoing studies (we checked ISRCTN register on 31st May 2016).
Results of the search
The results of our search are documented in a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart (Figure 1)
The search identified 763 studies of potential relevance. After the first screening 66 citations were considered potentially relevant. Full text articles of these abstracts were obtained and screened by two review authors independently against the inclusion criteria. No ongoing trials were identified. We are awaiting a reply from the study authors of one study in order to allocated it to a classification.
Included studies
A total of 10 RCTs and four quasi‐RCTs with 6466 participants met the inclusion criteria. One manuscript reported two trials which were conducted consecutively (Gough 1990a; Gough 1990b); this was treated as two separate trials for the purpose of the review.
Five of the included trials were published since 2006 (Dixon 2006; Heal 2009; Khalighi 2014; Neri 2008; Pradhan 2009); the earliest study was published in 1967.
Two trials were conducted in Australia (Dixon 2006; Heal 2009), seven in Europe (Caro 1967; Gilmore 1973a; Gough 1990a; Gough 1990b; Iselin 1990; Kamath 2005; Neri 2008), four in the USA (Dire 1995; Hood 2004; Khalighi 2014; Smack 1996), and one in Asia (Pradhan 2009). One European trial was conducted in France, and translation was required (Iselin 1990).
The types of surgical procedures were varied, and included skin cancer surgery (three trials) (Dixon 2006; Heal 2009; Smack 1996); repair of lacerations and soft tissue injuries (three trials) (Caro 1967; Dire 1995; Hood 2004); circumcision (two trials) (Gough 1990a; Gough 1990b); caesarian section (one trial) (Pradhan 2009); appendicectomy (one trial) (Gilmore 1973a); hip replacement (one trial) (Kamath 2005); hand surgery (one trial) (Iselin 1990); umbilical laparoscopic port (one trial) (Neri 2008); and cardiac device implantation (one trial) (Khalighi 2014).
Six studies involved minor procedures which were all conducted in an outpatient or emergency department setting (Caro 1967; Dire 1995; Dixon 2006; Heal 2009; Hood 2004; Smack 1996). Eight studies involved major surgery which were conducted in theatre (Gilmore 1973a; Gough 1990a; Gough 1990b; Iselin 1990; Kamath 2005; Khalighi 2014; Neri 2008; Pradhan 2009).
The surgical procedures in each trial were classified as being clean (three trials) (Dixon 2006; Heal 2009; Smack 1996); clean contaminated (seven trials) (Gilmore 1973a; Gough 1990a; Gough 1990b; Kamath 2005; Khalighi 2014; Neri 2008; Pradhan 2009); contaminated (four trials) (Caro 1967; Dire 1995; Hood 2004; Iselin 1990). There were no dirty procedures.
The type of topical antibiotic applied varied, and included neomycin/bacitracin/polymixin B (four trials) (Caro 1967; Dire 1995; Gilmore 1973a; Hood 2004); chloramphenicol (two trials) (Heal 2009; Kamath 2005); neomycin (one trial) (Khalighi 2014); bacitracin (two trials) (Dire 1995; Smack 1996); rifamycin (two trials) (Iselin 1990; Neri 2008); mupirocin (two trials) (Dixon 2006; Hood 2004); soframycin (two trials) (Gough 1990a; Gough 1990b); and fusidic acid (one trial) (Pradhan 2009).
The antibiotic formulations varied and included ointment (eight trials) (Dire 1995; Dixon 2006; Heal 2009; Hood 2004; Kamath 2005; Khalighi 2014; Neri 2008; Smack 1996); cream (one trial) (Pradhan 2009); spray (two trials) (Caro 1967; Gilmore 1973a); impregnated dressing (two trials) (Gough 1990a; Gough 1990b); and solution (one trial) (Iselin 1990). Two studies did not specify if the antibiotic was delivered as cream or an ointment (Iselin 1990; Pradhan 2009). The topical antibiotic was either compared with no treatment control (six trials) (Caro 1967; Dixon 2006; Gilmore 1973a; Kamath 2005; Khalighi 2014; Neri 2008); an alternative topical antibiotic (two trials) (Dire 1995; Hood 2004); an inert topical control (five trials) (Dire 1995; Dixon 2006; Gough 1990b; Heal 2009; Smack 1996); or an antiseptic (six trials) (Dire 1995; Gilmore 1973a; Gough 1990a; Iselin 1990; Khalighi 2014; Pradhan 2009). One study compared one topical antibiotic with another topical antibiotic (Hood 2004). None of the included studies compared topical antibiotics with systemic antibiotics. Four trials involved participants who were all given concurrent systemic antibiotics in addition to the topical antibiotic or control (Kamath 2005; Khalighi 2014; Neri 2008; Pradhan 2009), and in one trial, only some of the participants were given systemic antibiotics, but it was not specified which (Gilmore 1973a).
Seven studies used multiple applications of the study agent (Caro 1967; Gilmore 1973a; Hood 2004; Iselin 1990; Kamath 2005; Neri 2008; Smack 1996). Three studies applied the study agent before and after suturing (Caro 1967, Gilmore 1973a; Iselin 1990), and four studies used multiple applications of the study agent postoperatively. One study applied the ointment three times per day until the wound check appointment at approximately one week (Hood 2004). Another study applied ointment at the time of suturing and at three days postoperatively (Kamath 2005), while another applied ointment at the time of suturing and 12, 24, 36, 48 and 72 hours postoperatively (Neri 2008). A final study applied the study agent after suturing and then daily for four weeks (Smack 1996).
There were two three‐arm studies (Dixon 2006; Gilmore 1973a), and two four‐arm studies (Dire 1995; Khalighi 2014), included in the review. One of the three‐arm studies compared one topical antibiotic, one antiseptic (povidone‐iodine) and one no treatment control (Gilmore 1973a). We compared the antibiotic arm with the antiseptic arm in a single pair‐wise comparison in one analysis, and with the no treatment arm in another analysis.The other three‐arm study compared one topical antibiotic, one paraffin ointment and one no treatment arm (Dixon 2006). We combined the two no antibiotic arms , and compared with the antibiotic arm in a simple pair‐wise comparison. One of the four‐arm studies had two antibiotic arms (bacitracin and neomycin/polymixin B/bacitracin zinc), an antiseptic arm (silver), and an inert ointment control arm (petroleum) (Dire 1995). We compared the combined two antibiotic arms with the antiseptic arm in one comparison, and with the no antibiotic arm in another comparison. We compared the bacitracin arm with the neomycin/polymixin B/bacitracin arm in another analysis. In the other four‐arm trial there was one topical antibiotic group (neomycin) and three control groups (antiseptic ointment, non‐adherent dressing and standard dressing) (Khalighi 2014). We combined the antibiotic arm with the two combined dressing arms in one comparison and the antiseptic arm in another comparison. We were not required to divide the control group between the two arms for the purpose of comparison in any analysis.
One study identified in the most recent search (May 2016) is awaiting classification (Ruiz 2015).
Primary outcome measures
Surgical site infection
Twelve of the trials reported SSI rates (Caro 1967; Dire 1995; Dixon 2006; Gilmore 1973a; Heal 2009; Hood 2004; Iselin 1990; Kamath 2005; Khalighi 2014; Neri 2008; Pradhan 2009; Smack 1996), although in one trial these data were not extractable for the pooled data analysis for this outcome (Neri 2008). The definition of infection varied, and six trials included more than one definition of infection. One trial defined infection according to the CDC criteria for SSI, (Heal 2009), which is considered to be the gold‐standard definition for wound infection (Mangram 1999). One trial used another validated scale (SIGN 2015 ; Kamath 2005). Seven trials used a self‐devised set of clinical criteria (Dire 1995; Gilmore 1973a; Hood 2004; Iselin 1990; Khalighi 2014; Neri 2008; Smack 1996), and six trials used a self‐devised wound scale to define infection (Dire 1995; Dixon 2006; Heal 2009; Hood 2004; Khalighi 2014; Smack 1996). Two trials required positive wound swabs to define infection (Gilmore 1973a; Smack 1996). A third trial included it as part of their definition of infection, but it was not mandatory (Khalighi 2014). One trial did not record the definition of infection used (Pradhan 2009), while another used the term 'non‐healing' as its definition of wound infection (Caro 1967).
Adverse effects
We specified in our protocol three adverse effects of interest as primary outcomes: allergic contact dermatitis, patterns of antibiotic resistance and anaphylaxis.
Allergic contact dermatitis
Four trials measured allergic contact dermatitis as an outcome (Dire 1995; Dixon 2006; Iselin 1990; Smack 1996), and two studies reported at least one event of allergic contact dermatitis (Dire 1995; Smack 1996). One trial reported 'cutaneous intolerance' which was classified as allergic contact dermatitis for the purpose of this review (Iselin 1990). One trial reported that there had been no episodes of allergic contact dermatitis (Dixon 2006).
Patterns of antibiotic resistance
Four studies undertook wound swabs to assess patterns of antimicrobial resistance (Heal 2009; Kamath 2005; Khalighi 2014; Smack 1996). Two studies reported infections with methicillin‐resistant Staphylococcus aureus (MRSA) (Kamath 2005; Khalighi 2014). The Kamath 2005 study reported two positive cultures of MRSA in the control group while Khalighi 2014 reported four positive MRSA cultures and two positive methicillin‐resistant Staphylococcus epidermidis cultures, but did not report to which intervention they belonged. Another trial reported a culture of S aureus which showed resistance to erythromycin (Heal 2009), and a culture of Pseudomonas aeruginosa. Both of these swabs were taken from participants in the control group. A final study reported nine cultures of S aureus and one culture of Proteus mirabilis in the control group, and two cultures of P aeruginosa, one of an Enterobacter species and one of P mirabilis in the intervention group (Smack 1996). There were no patterns of resistance in either group. Overall there were no patterns of antibiotic resistance in any of these studies that was related to antibiotic use.
One study reported that pus culture from all infected wounds showed Staphylococcus but did not specify how many participants had a positive swab or if there were patterns of resistance (Pradhan 2009). One study stated that swabs of serous discharge were performed, but provided no results (Gilmore 1973a). Another study stated that wounds with abscess formation or involvement beyond local site would be swabbed (Dixon 2006), but provided no results.
Anaphylaxis
No trials reported anaphylaxis.
Other adverse outcomes
Four studies measured or reported other adverse effects (Dixon 2006; Hood 2004; Iselin 1990; Khalighi 2014). In one study there was a specified list of adverse events stated in the methods section (adverse scar outcomes, postoperative bleeding, allergy to dressing, allergy to skin preparation, postoperative pain, contact dermatitis, local recurrence, subcutaneous fibrosis, granuloma, dehiscence, pruritus, persistent pain, nerve damage, ectropion, nodal involvement and distant metastases) (Dixon 2006), however, from this list, only scar complications were reported in the results as having occurred. One study listed 'any adverse event' which occurred during the study period in the methods section (Hood 2004), but did not define these adverse events ‐ this study reported an episode of paraesthesia around the wound site. One study measured and reported further surgery as an adverse outcome (Iselin 1990). One other study reported an episode of 'pocket infection' which required removal of a pacemaker device and prolonged systemic antibiotics which occurred in a control group (Khalighi 2014).
Secondary outcome measures
Wound healing
Six trials reported healing (Caro 1967; Gough 1990a; Gough 1990b; Iselin 1990; Neri 2008; Smack 1996). Three of the 14 included trials had extractable data for the outcome of wound healing, and no data for SSI (Gough 1990a; Gough 1990b; Neri 2008). All of the six trials which reported wound healing reported the proportion of wounds healed between five days and two weeks, rather than time to healing, or the proportion of wounds healed at the end of the trial, and this time point differed significantly between the studies. We changed the definition of healing in the review, from proportion of wounds healed at the end of the trial or time to healing, as stated in the protocol, to proportion of wounds healed in 5 to 14 days.
Patient satisfaction
One trial reported patient satisfaction measured between six and nine months of the procedure, which was not within the time period of six months stated in our protocol (Dixon 2006).
Quality of life
No trials reported health‐related quality of life at 30 days or three months.
Financial cost per infection prevented
No trials reported the financial cost for each infection prevented (NNTB). One study reported NNTB, but did not report a financial cost (Heal 2009). Another study planned to conduct a cost‐effectiveness analysis comparing Bactroban to Neosporin antibiotic ointment (Hood 2004). As there was clear difference in effectiveness of the two ointments in the trial, a basic comparison of cost of each antibiotic was made.
Other outcomes
Although we did not pre specify pain as an outcome in our protocol, it was reported in five studies, (Dixon 2006; Hood 2004; Iselin 1990; Kamath 2005; Neri 2008), and tenderness was reported in two studies (Dire 1995; Smack 1996). In one study pain was treated as a separate primary outcome (Neri 2008), while in five it was included in the definition of SSI (Dire 1995; Dixon 2006; Iselin 1990; Kamath 2005; Smack 1996). In three studies pain was classified as an adverse effect (Dixon 2006; Hood 2004; Neri 2008), while in one study it was a component of patient satisfaction scales (Dixon 2006).
Excluded studies
The Characteristics of excluded studies table provides details of the trials that did not meet the inclusion criteria. In total, 52 studies were excluded after screening of the full text. There were a number of reasons for the exclusions including two studies that were not RCTs (Erel 1999, Thakur 1997); 15 studies that included wounds healing by secondary intention (Andrew 2012; Bayerl 2004; Blobel 1970; Bos 2007; Campbell 2005; Draelos 2011; Johnson 2005;Kircik 2013; Livingston 1990; Mann 2001; Mayer 1973; Motta 2005; Ruschulte 2009; Taylor 2011; Wright 1980), and 26 studies where the antibiotic was applied only prior to suturing (Andersen 1970; Andersen 1972; Bates 1974; Battista 2001; Bencini 1991; Bird 1971; Charalambous 2003; Czarnecki 1992; Evans 1974; Fielding 1965; Finch 1979; Gilmore 1973b; Hildred 1977; Jackson 1971; Jensen 1975; Kenning 1980; Merrild 1985; Mountain 1970; Ostergaard 1981; Pollock 1975; Praveen 2009; Saik 1971; Tanphiphat 1976; Theophilus 2011; Vander Salm 1989; Varga 2009). One study was excluded due to healing by secondary intention and the surgery being on mucosal surfaces (Nicholson 2004). A further two studies were excluded where the intervention antibiotic was delivered systemically (Bluhm 1986; Eason 2004), five where delivery was by irrigation (Juul 1985; Olthuis 1968; Sarr 1988; Stoller 1965;Tanphiphat 1978), and one study where the wound was already infected (Leyden 1985). Full details are given in the Characteristics of excluded studies table.
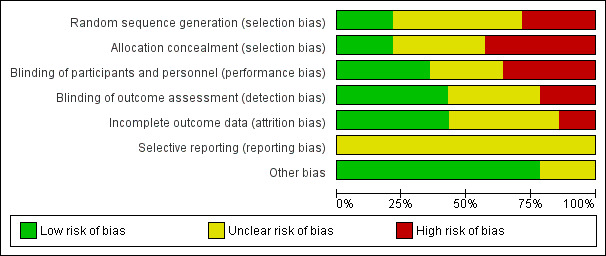
Risk of bias in included studies
A summary of the assessment of risk of bias based on the criteria outlined in Higgins 2011 is given in Figure 2 and Figure 3. Additionally a brief descriptive analysis of the studies is provided below. One of the authors had a conflict of interest regarding assessment of a study (Heal 2009), and an alternative author (MVD) rated this study for risk of bias. In general, the overall methodological quality of the included studies was relatively poor. We classified studies as being at high risk of bias if they were rated as 'high risk' for any one of the three risk of bias criteria which we had specified in the protocol. A total of seven studies were deemed to be at high risk of bias (Caro 1967; Dixon 2006; Gilmore 1973a; Gough 1990a; Gough 1990b; Iselin 1990; Neri 2008), five were at unclear risk of bias (Dire 1995; Hood 2004; Kamath 2005; Khalighi 2014; Pradhan 2009), and two at low risk of bias (Heal 2009; Smack 1996).
2.
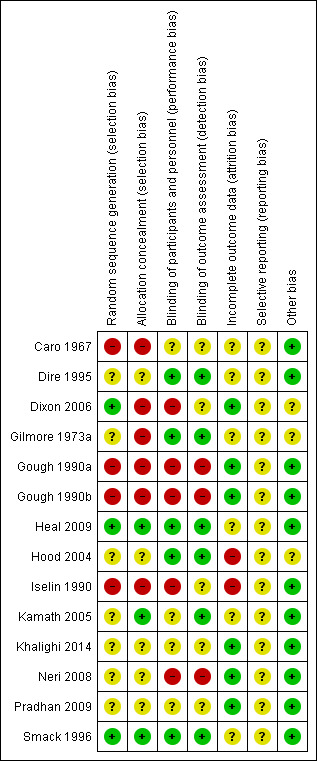
3.
Three trials reported a sample size calculation (Dixon 2006; Heal 2009; Smack 1996). It was not clear whether informed consent was obtained in seven trials (Caro 1967; Gilmore 1973a; Gough 1990a; Gough 1990b; Iselin 1990; Neri 2008; Pradhan 2009). Ethics approval was reported in five studies (Dire 1995; Dixon 2006; Heal 2009; Hood 2004; Smack 1996).
Allocation
Sequence generation
Ten of the included studies were described as being 'randomized'. Two provided information that confirmed that adequate sequence generation had taken place (Heal 2009; Smack 1996), one study selected discs from a barrel until empty and then repeated (Dixon 2006). One study used three coded lettering recurring seven times every 21 cases (Gilmore 1973a). Four studies were quasi‐randomized using alternate patients (Caro 1967; Gough 1990a; Gough 1990b; Iselin 1990), and in the remaining six studies the method of random sequence generation was unclear.
Allocation concealment
In five trials it was unclear whether the randomisation sequence was concealed at the point of participant contact (Dire 1995; Hood 2004; Khalighi 2014; Neri 2008; Pradhan 2009). In six of the trials, the method of sequence generation meant that allocation concealment was at high risk of bias (Caro 1967; Dixon 2006; Gilmore 1973a; Gough 1990a; Gough 1990b; Iselin 1990). In three studies, the method of allocation concealment was well described and at low risk of bias (Heal 2009; Kamath 2005; Smack 1996).
Blinding
Blinding of participants and personnel
In four studies both the participants and personnel were reported to be blinded (Dire 1995; Heal 2009; Hood 2004; Smack 1996), and this could be verified in three studies (Heal 2009; Hood 2004; Smack 1996). In three studies either just the participants (Gilmore 1973a), or just the personnel (Dixon 2006; Kamath 2005), were blinded.
Blinding of outcome assessment
In five studies the outcome assessor was blinded to treatment allocation (Gilmore 1973a; Heal 2009; Hood 2004; Kamath 2005; Smack 1996), while in the remaining nine studies it was unclear if the outcome assessor was blinded.
Blinding of participants, personnel and outcome assessor
Three trials blinded participants, personnel and outcome assessors, (Heal 2009; Hood 2004; Smack 1996).
Incomplete outcome data
Drop out rate described and acceptable?
There were no trials where participants were excluded from the analysis in sufficient numbers to increase risk of bias. The drop out rate was not greater than 20% in any trial, and numbers of dropouts were balanced between the intervention and control groups when group allocation was recorded. In seven trials there were no dropouts and all participants were analyzed (Dixon 2006; Gilmore 1973a; Gough 1990a; Gough 1990b; Khalighi 2014; Neri 2008; Pradhan 2009). All of these studies were rated at low risk for attrition bias. In one trial the number of dropouts was unclear (Caro 1967), and this study was rated unclear for attrition bias. In the remaining six studies, the proportion of drop outs was recorded, however in three of these it was not clear whether these were allocated to the intervention or the control group (Dire 1995; Hood 2004; Kamath 2005). In another study, 39/465 (8.4%) participants who received the allocated treatment were lost to follow‐up, but the allocation group was unrecorded, so it was unclear if this was balanced between the intervention and control groups (Dire 1995). In one study 21/120 (17.5%) participants who received their allocated treatment were lost to follow‐up, but again the allocation group was unrecorded (Hood 2004). One study reported 8/100 (8%) participants were lost to follow‐up because of death or severe disability (Kamath 2005), but it was not specified whether these participants were in the intervention or the control group. In another trial 45/268 (16.7%) (20 control, 25 intervention) participants who had received their allocated intervention were lost to follow‐up (Iselin 1990). One trial reported 28/912 (3%) (13 versus 15) participants who had received their allocated intervention were lost to follow‐up (Smack 1996). In this study another 10 participants were randomized, but did not receive their allocated intervention ‐ it was not specified whether these participants were in the intervention or the control group. In one study 42/1014 (4.1%) (21 intervention, 21 control) randomized participants were lost to follow‐up (Heal 2009). We rated studies at high risk of attrition bias if more than 10% of participants were lost to follow‐up and it was unclear if this was balanced between intervention and control.
Intention‐to‐treat (ITT) analysis
Intention‐to‐treat (ITT) analysis involves the analysis of the results for all study participants according to the treatment groups to which they were originally randomized, irrespective of what happened subsequently (Hollis 1999). There is no consensus regarding the optimal way of dealing with missing data in meta‐analysis. For the purposes of this review, we defined ITT analysis as occurring when all randomized participants were reported or analyzed in the group to which they were allocated for the outcome measurement of SSI, irrespective of non‐compliance and co interventions. In studies of wound infection, the outcome of SSI cannot be measured in participants lost to follow‐up. Seven trials with no missing outcome data conducted an ITT analysis (Dixon 2006; Gilmore 1973a; Gough 1990a; Gough 1990b; Khalighi 2014; Neri 2008; Pradhan 2009). One trial used an alternative definition of ITT which included protocol violators in the analysis, but not participants lost to follow‐up (Heal 2009). In one trial it was unclear if there were missing outcome data (Caro 1967). In the remaining five trials, there were no outcomes recorded for the missing participants, so imputation would be required for them (Iselin 1990; Dire 1995; Hood 2004; Smack 1996;Kamath 2005 ). In three of these studies it was also not specified to which group the missing participants had been allocated, so data imputation for the group allocation would be required (e.g. assuming that dropouts had been evenly distributed between the intervention and the control group) (Dire 1995; Hood 2004; Kamath 2005).
An SSI rate of between 1% and 10% is typical and this was reflected in the included trials. If a sensitivity analysis was conducted on a best/worst case scenario basis, with the worst‐case scenario assuming all missing participants were treatment failures (i.e. had developed an infection) then the rate of SSI would be falsely elevated in the intervention group to a rate greater than an expected baseline infection rate. This scenario would also be extremely unlikely, and does not reflect clinical reality. If we calculate the ITT analysis on the best‐case scenario that missing participants did not develop an SSI, we do not think it would affect our results as the maximum missing data were 17.5%. An alternative approach would be to impute data based on the event rate observed in the control group, however as rates of missing data were less than 20% we do not think this is necessary. In summary we decided to perform a complete case analysis for all trials in the review, and we recognise this issue in the assessment of attrition bias in the 'Risk of bias' assessment and then in the GRADE assessment.
Unit of analysis issues
Including multiple wounds
We did not encounter any cluster‐randomized controlled trials which randomized by surgeon, operating list or hospital, despite anticipating that we would and describing how we would deal with these in our in our protocol. However we did need to consider adjusting for clustering when multiple wounds were included.
All studies except two included one wound per patient.
One study included multiple wounds per patient; randomisation was at the level of the patient and the unit of analysis was also the patient rather than the wound (Smack 1996). We did not adjust for clustering in this study as this was considered to be an aggregation issue (losing results by combining wounds) rather than a clustering issue. Only 10% of patients had multiple wounds.
Another study included multiple wounds per patient and randomisation was at the level of the patient, but the unit of analysis was the wound (Dixon 2006).
We could not find a published standard value for the inter‐cluster correlation (ICC) which should be used to adjust for clustering for this scenario. Therefore we explored three potential situations using different values for the ICC, and then performed a sensitivity analysis on the effect of the two most extreme scenarios on the overall results. If the ICC was 1.0 (assuming all results within a cluster are identical), as opposed to 0 (no correlation of results within a cluster), we found that the risk ratio (RR) changed from 0.59 (95% confidence interval (95% CI) 0.43 to 0.81) to 0.57 (95% CI 0.43 to 0.75). As a result of these calculations we decided to perform no additional adjustment for clustering as it seemed to have a negligible effect on overall results.
Selective reporting
We attempted to compare the outcomes reported in the results sections of trial reports with those listed in published protocols of the studies, in clinical trials registries as both primary and secondary outcomes, or in the methods section of the published report. We were not able to find any separately published protocols, therefore all studies were considered as being at unclear risk of publication bias. One study was registered in a clinical trials registry (Heal 2009), and did not show selective reporting. None of the other studies showed selective reporting when we compared the outcomes listed in the methods section of the published paper with the published results.
Other potential sources of bias
Publication bias
We did not have sufficient studies (> 10) for the primary outcome measure of SSI to assess for publication bias in any of our comparator groups.
Financial support
Three trial groups reported that they had received financial support; in two cases from pharmaceutical companies. One study was supplied with the intervention and control agents by the manufacturer, Alvex Limited (Gilmore 1973a). Another study was supported by a grant from Pfizer Consumer Healthcare who manufactured one of the study ointments used in this trial (Hood 2004). A third study received funding through the Chris Silagy scholarship from the Royal Australia College of General Practitioners, and the study was reported as being independent of this funding (Heal 2009). The remaining 12 trials did not report financial support.
Baseline comparability
Six trials reported data that confirmed baseline comparability for patient and wound characteristics (Dire 1995; Dixon 2006; Hood 2004; Iselin 1990; Khalighi 2014; Smack 1996). In seven trials, baseline comparison was not discussed (Caro 1967; Gilmore 1973a; Gough 1990a; Gough 1990b; Kamath 2005; Neri 2008; Pradhan 2009), while in one trial it was reported that treatment groups were not comparable at baseline and adjustments were made in the analysis to compensate for this imbalance (Heal 2009).
Effects of interventions
Comparison 1: topical antibiotic compared with no topical antibiotic
Primary outcome 1: surgical site infection (SSI)
We pooled the results of eight trials (5427 participants) using a random‐effects model to compare the effects of topical antibiotics with no topical antibiotics on SSI rates (all comparator groups combined). The 'no topical antibiotic' comparator group included inert ointment (Dire 1995; Dixon 2006; Heal 2009; Smack 1996) and no treatment (Caro 1967; Dixon 2006; Gilmore 1973a; Kamath 2005; Khalighi 2014). Three studies were at high risk of bias, three were at unclear risk of bias and two were at low risk of bias. There were fewer SSIs with topical antibiotics than without (RR 0.61; 95% CI 0.42 to 0.87; Analysis 1.1). There was an absolute risk difference of 20 fewer SSIs per 1000 patients (95% CI 7 fewer to 31 fewer) and a NNTB with topical antibiotics to avoid one additional SSI (NNT) of 50. There was moderate inter study heterogeneity (I² = 44%).The quality of evidence for this outcome was graded as moderate, downgraded once for the proportion of the information from studies at high risk of selection bias, as this was sufficient to affect the interpretation of the results.
1.1. Analysis.
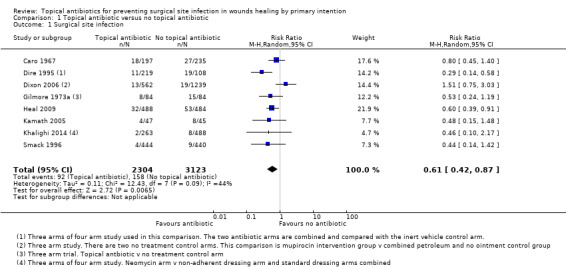
Comparison 1 Topical antibiotic versus no topical antibiotic, Outcome 1 Surgical site infection.
A further study for this comparison did not provide usable data for this outcome (Neri 2008).
We performed a sensitivity analysis to examine the effect of removing the studies at high risk of bias on I2 and RR (Caro 1967; Dixon 2006; Gilmore 1973a). The effect estimate was robust to removal of high risk of bias studies (RR 0.49, 95% CI 0.35 to 0.67; 3026 participants; 5 studies; I2 = 0%).
Primary outcome 2a: allergic contact dermatitis
Three trials (3012 participants) examined the effect of topical antibiotics on the rate of allergic contact dermatitis when compared with no topical antibiotic (Analysis 1.2). One study was at high risk of bias, one was unclear and one was at low risk of bias. One of these trials did not report any events (Dixon 2006). We found no clear difference between groups for risk of allergic contact dermatitis (RR 3.94, 95% CI 0.46 to 34.00; 3012 participants; 3 studies; I2 = 0%; P = 0.5). The estimate was highly imprecise. The quality of evidence for this outcome was assessed as being very low (downgraded twice for the majority of information being from a study at high risk of selection and performance bias, and unit of analysis issues, once for imprecision).
1.2. Analysis.
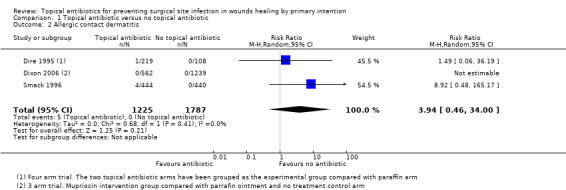
Comparison 1 Topical antibiotic versus no topical antibiotic, Outcome 2 Allergic contact dermatitis.
Primary outcome 2b: anaphylaxis
No study reported anaphylaxis.
Primary outcome 2c: patterns of antibiotic resistance
No study reported patterns of antibiotic resistance.
Secondary outcome 1: wounds healed by 5 to 14 days
Four trials for this comparison reported the proportions of wounds that were healed at a defined point in time, rather than time to healing.Three studies were at a high risk of bias, the remaining study was at a low risk of bias. The four studies (1034 participants) were pooled (Analysis 1.3); the time point at which healing was assessed varied between 5 and 14 days. There was no clear difference in wounds healed (RR 1.00, 95% CI 0.96 to 1.03; 1034 participants; 4 studies; I2 = 0%).The quality of evidence for this outcome was rated as low (downgraded twice for the majority of information being from studies at a high risk of selection, performance or detection bias).
1.3. Analysis.
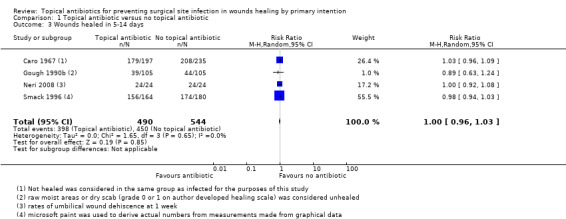
Comparison 1 Topical antibiotic versus no topical antibiotic, Outcome 3 Wounds healed in 5‐14 days.
Secondary outcome 2: patient satisfaction measured within six months of the procedure
No study reported patient satisfaction measured within six months of the procedure.
Secondary outcome 3: health‐related quality of life at 30 days and three months
No study reported health‐related quality of life at 30 days or three months.
Secondary outcome 4: financial cost for each infection prevented
No study reported the financial cost for each infection prevented.
Comparison 2: topical antibiotic compared with topical antiseptic
Primary outcome 1: SSI
We pooled five trials (1299 participants) that compared topical antibiotics with antiseptics using a random‐effects model to examine effects on risk of SSI (Analysis 2.1). There were fewer SSIs in those treated with topical antibiotics than with antiseptics (RR 0.49, 95% CI 0.30 to 0.80). This difference reflected an absolute difference in risk of 43 fewer cases of SSI per 1000 people treated with topical antibiotics instead of antiseptics (95% CI 17 fewer to 59 fewer per 1000; NNTB of 24). There was minor inter study heterogeneity ( I2 = 12%). The quality of evidence for this outcome was rated as moderate and was downgraded once because the proportion of the information from studies at high risk of selection, performance and attrition bias was sufficient to affect the interpretation of the results.
2.1. Analysis.
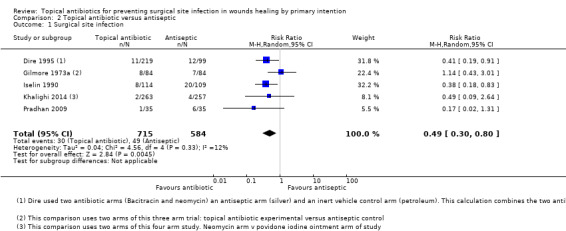
Comparison 2 Topical antibiotic versus antiseptic, Outcome 1 Surgical site infection.
Two studies were at high risk of bias and three were at unclear risk of bias. We performed a sensitivity analysis to examine the effect of removing the studies at high risk of bias on I2 and RR (Gilmore 1973a; Iselin 1990). The overall effect was robust to removal of the high risk of bias studies (RR 0.39, 95% CI 0.20 to 0.76; 908 participants; 3 studies) and heterogeneity was reduced (I² = 0%).
Primary outcome 2a: allergic contact dermatitis
Two trials (541 participants) compared the effects of topical antibiotics and antiseptics on the rates of allergic contact dermatitis (Analysis 2.2). Pooled analysis indicated no clear evidence of a difference (RR 0.97, 95% CI 0.52 to 1.82; I2 = 0%, P = 0.92).
2.2. Analysis.
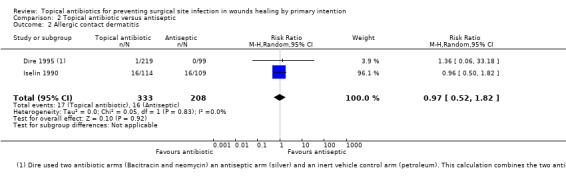
Comparison 2 Topical antibiotic versus antiseptic, Outcome 2 Allergic contact dermatitis.
One of the two studies was at high risk of bias and the other was at unclear risk of bias.The overall quality of the evidence was rated as very low (downgraded twice for the majority of information being from a study at high risk of selection, performance and attrition bias, and once for imprecision).
Primary outcome 2b: anaphylaxis
No trials reported anaphylaxis.
Primary outcome 2c: patterns of antibiotic resistance
No study reported patterns of antibiotic resistance.
Secondary outcomes 1: wounds healed at 5 to 14 days
Both trials reported the proportions of wounds healed at a defined point in time, rather than time to healing and these were pooled (Analysis 2.3) (time points varied between 5 and 14 days). There was no clear evidence of a difference in wound healing (RR 1.65, 95% CI 0.58 to 4.72; 327 participants; 2 studies; I2 = 88%). Both studies were at a high risk of bias.The quality of evidence for this outcome was graded as very low; downgraded twice due to all of the information being from studies at high risk of selection, performance, detection or attrition bias, once for imprecision and once for inconsistency.
2.3. Analysis.
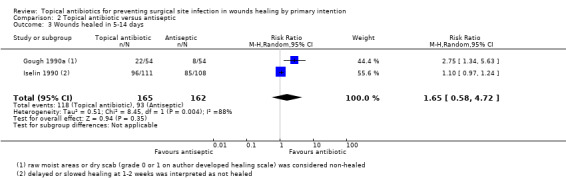
Comparison 2 Topical antibiotic versus antiseptic, Outcome 3 Wounds healed in 5‐14 days.
Secondary outcome 2: patient satisfaction measured within six months of the procedure
No study reported patient satisfaction measured within six months of the procedure.
Secondary outcome 3: health related quality of life at 30 days and three months
No study reported health‐related quality of life at 30 days or three months.
Secondary outcome 4: financial cost for each infection prevented
No study reported the financial cost for each infection prevented.
Comparison 3: topical antibiotics compared with alternative topical antibiotic
Primary outcome 1: SSI
One study (99 participants) was a head‐to‐head comparison of mupirocin and neomycin (Hood 2004). There was no clear evidence of a difference between mupirocin and neomycin in risk of SSI (RR 0.20; 95% CI 0.01 to 4.14; P= 0.3). The quality of evidence for this outcome was rated as very low, downgraded twice for this single trial being at a high risk of attrition bias, and once for imprecision.
Another study was a four‐arm trial; two arms of which were antibiotic arms (combination ointment (neomycin sulfate, bacitracin zinc, and polymyxin B sulphate) versus bacitracin zinc) (Dire 1995). Comparison of the results for these two arms showed no clear evidence of a difference in risk of SSI (RR 0.83; 95% CI 0.26 to 2.63; 219 participants, P= 0.75).
These two trials did not compare similar topical antibiotics and so were not pooled. The quality of evidence of this outcome was rated as very low, and was downgraded twice for risk of bias and once for imprecision.
There was no information available for the outcomes of allergic contact dermatitis, anaphylaxis and patterns of antibiotic resistance, or the secondary outcome measures of wound healing, patient satisfaction, quality of life or financial cost for either study.
Comparison 4: topical antibiotics compared with oral antibiotics
There were no trials that compared topical antibiotics with oral antibiotics.
Discussion
Summary of main results
The primary aim of this systematic review was to summarize and interpret all the existing evidence for the effects of topical antibiotics on rates of surgical site infection (SSI) in surgical wounds healing by primary intention. Ten randomized controlled trials (RCTs) and four quasi‐randomised controlled trials met the eligibility criteria for inclusion in the review.
Surgical site infection
Topical antibiotics applied to surgical wounds healing by primary intention probably reduce the risk of SSI relative to no antibiotic and relative to topical antiseptics (moderate quality evidence).
Adverse effects
We are unable to draw conclusions regarding the effects of topical antibiotics on allergic contact dermatitis due to lack of statistical power (small sample sizes). Any use of a topical antibiotic needs to be tempered by consideration of side effects such as allergic contact dermatitis. The evidence for this outcome, while critical in the decision making for the use of topical antibiotics, was found to be of low quality. There were no data regarding patterns of antibiotic resistance or risk of anaphylaxis reported in any of the studies identified.
Wound healing
There was no clear evidence of an effect of topical antibiotics or antiseptics on wound healing, however this comparison is underpowered and the evidence is of very low quality, so a difference cannot be ruled out.
Patient satisfaction
No data could be extracted from the included studies for the outcome of patient satisfaction, according to our definition.
Patient quality of life
No data could be extracted from the included studies for the outcome of quality of life, according to our definition.
Financial cost
Financial cost was not reported in any of the studies.
Overall completeness and applicability of evidence
The trials were all conducted in clinical practice and the evidence is clinically applicable. The settings that were used varied, but were across a range of general surgical and dermatological surgery settings, and involved a range of wound classes.
We were unable to complete any of our planned subgroup analyses because of lack of a sufficient number of studies.
Some studies had very low baseline rates of infection of around 2% (Dixon 2006; Khalighi 2014; Smack 1996), for all other trials the baseline rates were 10% to 20%. In several of the studies (Heal 2009; Gilmore 1973a; Pradhan 2009), this baseline infection rate was higher than is considered to be acceptable and than would be expected in normal clinical practice (Cruse 1980), and this may limit the applicability of the evidence. This clinical heterogeneity is worthy of additional attention. The mean absolute risk reduction was 4.3% when compared with antiseptics and 2% when compared with no treatment, but this result was heterogenous in both comparisons and was much lower in the individual studies with low baseline infection rates. Two of the three studies with low baseline infection rate were trials of patients with clean (class 1) wounds – the baseline results in these studies raises a question about whether prophylaxis should be attempted in populations with clean (class 1) wounds. The number needed to treat for an additional beneficial outcome was 24 in the antiseptic comparison group and 50 in the no treatment comparator group, but again this is based on a mean absolute risk reduction result which was heterogenous, and it would be much higher in situations where the baseline infection rate is low.
The decision to use topical antibiotics is complex, and any benefit must be weighed against adverse effects and healthcare costs, and therefore there are limitations to applying the findings of this review clinically.
Quality of the evidence
The quality of the individual studies varied. There was a tendency for older studies to have higher risk of bias. Year as an indicator of bias and quality has been reported in analysis of Cochrane reviews (Kicinski 2015). The majority of studies were more than 10 years old and did not follow CONSORT reporting guidelines (Schulz 2010). Most bias in individual studies, and thus most effect on quality, came from methods that were at high risk of bias for random sequence generation and allocation concealment, and also lack of blinding of the outcome assessor. Two of the studies were at a high risk and six studies at an unclear risk of attrition bias, as data from all participants was not included in the analysis and intention‐to‐treat (ITT) analysis had not been performed. Although we considered imputation methods and ITT analysis for these studies, we decided to perform complete case analysis for all studies. We then reflected this in our assessment of attrition bias in these individual studies, and also in our overall GRADE criteria.
Using the GRADE criteria, there were no outcomes where we were required to downgrade the quality of the overall evidence because the removal of high‐risk studies impacted the overall result. We downgraded quality where there was moderate to severe heterogeneity, as defined in our protocol (inconsistency of results). We defined imprecision where the confidence interval of the overall effect of an outcome crossed the line of 'no effect' (or 1), in addition to the confidence intervals crossing either 0.75 or 1.25. We rated all included studies as being direct in their relevance to the review question. There was not a sufficient number of studies to enable us to assess for publication bias for any of the outcomes.
The quality of the evidence varied by outcome. The quality of evidence, and therefore our confidence in the effect size of our first primary outcome measure, SSI, was moderate in both comparator groups. However the quality of evidence for our second primary outcome, allergic contact dermatitis, was rated as very low, and this diminished our confidence in the effect size for this outcome.
RCTs need to be adequately powered so that they are able to detect treatment effects of a specified size, should these exist. As the incidence of SSI is often low, an adequate number of participants needs to be recruited to detect a clinically significant difference. Only three of the trials included in this review had sample size calculations, and several of the trials which had reported no effect were likely to have been inadequately powered for the effect size to have reached statistical significance. In one study the baseline incidence of SSI was lower than expected when the sample size was calculated, which resulted in the study being underpowered (Dixon 2006). Therefore we feel that some of the studies had reported topical antibiotics to be ineffective inappropriately, rather than acknowledging the limitations of their sample size.
Potential biases in the review process
There is some potential for bias in the review process, however strict attention was paid to Cochrane review methods to avoid bias where possible.
One of the review authors led a study which was included in the review (Heal 2009). The data extraction and risk of bias were completed and checked by review authors who had no connection with the particular study.
Secondly, even with exhaustive searches, it is possible that we could have missed trials. No published protocols were identified for any of the included trials. Only one trial was registered in a clinical trials registry so that the outcomes listed could be compared with the reported results (Heal 2009).
We did not generate a funnel plot to investigate for publication bias, because of the small number of studies involved. Therefore our ability to assess risk of overall publication bias was limited because of the small number of studies available and the fact that not all studies assessed the same outcomes. We did not perform a statistical test for publication bias, as these tests are only valid if there are more than 10 studies, as they are otherwise underpowered to detect much and tend to lead to conclusions that are not justified (Sterne 2000).
Finally, some studies did not provide data which could be extracted for all outcomes for use in the meta‐analysis. When possible we reported these studies descriptively.
Agreements and disagreements with other studies or reviews
We identified one relevant editorial (Grey 2009a), two literature reviews (Diehr 2007; Rosengren 2010a), and three previous systematic reviews (Huiras 2012; McHugh 2011; Saco 2015), which, in all but one case (Saco 2015), concluded that there is little evidence for the effects of topical antibiotics to prevent SSI, particularly in dermatological surgery. Only one existing study used meta‐analysis of pooled data (Saco 2015), and this was limited to only four studies.
A systematic review and meta‐analysis of topical antibiotics versus petroleum/paraffin for prevention of SSI for dermatological procedures (including wounds healing by primary or secondary intention) favoured topical antibiotics, with a pooled odds ratio from four studies of 0.71 (95% CI 0.42 to 0.1.19) (Saco 2015). A literature review, Diehr 2007, focused on topical antibiotics and wound healing and used commentary from three of the studies in our review to conclude that there was Level A evidence that antibiotic ointments reduce infection rates (Dire 1995; Hood 2004; Smack 1996). A systematic review which, in contrast to our review, included non‐randomised studies and antibiotic implants and washouts, concluded that topical antibiotics reduce SSI rates in some surgical procedures, namely joint arthroplasty, cataract surgery, breast augmentation and abdominal surgery in obese patients, although they also concluded that evidence for topical antibiotics is lacking outside these indications (McHugh 2011). Another literature review with similar inclusion criteria concluded a lack of evidence for the use of local antibiotics (Huiras 2012). An evidence‐based review of topical antibiotic prophylaxis for dermatological surgery concluded that topical antibacterial ointments make no difference to healing or the incidence of SSIs (Rosengren 2010a). An editorial drew on three of the studies included in this review ‐ Dixon 2006, Heal 2009, and Smack 1996 ‐ to conclude that topical antibiotics do not prevent SSI in class 1/clean minor surgical procedures where appropriate preoperative preparation and aseptic technique have been applied (Grey 2009a). This latter conclusion is consistent with two international guidelines (NICE 2008; SIGN 2015), which recommend that antibiotic prophylaxis, not limited to topical antibiotics, is not required for clean minor surgical procedures. NICE guidelines also state "do not use topical antibiotics in wounds healing by primary intention to reduce the risk of surgical site infection" (NICE 2008). Our review has contributed additional evidence, though practice must be guided by clinical judgement of risks and benefits.
Authors' conclusions
Implications for practice.
Topical antibiotics probably prevent surgical site infection (SSI) whether compared with antiseptic or to no topical antibiotic (moderate quality evidence). Our review only identified studies involving clean (class1), clean contaminated (class 2), and contaminated (class 3) surgery, and we cannot draw any conclusions regarding dirty (class 4) surgery. In clean (class 1) surgery, where the baseline infection rate is already low, and the absolute risk reduction in SSI is probably smaller, the case for the use of topical antibiotics is weaker. We are unable to draw conclusions regarding the effects of topical antibiotics on adverse outcomes such as allergic contact dermatitis due to lack of statistical power (small sample sizes; very low‐quality evidence). We are also unable to draw conclusions regarding the impact of increasing topical antibiotic use on antibiotic resistance. Any risk of adverse events is important when evaluating the use of topical antibiotics, and there is insufficient evidence in this review to inform this judgement.
Implications for research.
There are only a few high quality randomized controlled trials on this topic, and also a lack of studies which measured the adverse outcomes of allergic contact dermatitis and patterns of antibiotic resistance. Other outcomes which were not assessed by our included studies in the time frames defined by our protocol included patient satisfaction, quality of life and cost. Further research of these outcomes would be of benefit to provide a holistic understanding of the role of topical antibiotics in SSI prophylaxis, as well as establishing stronger evidence for the safety of topical antibiotic use.
Quantifying the additive benefit of topical to systemic antibiotics is as yet unaddressed, and there is a case for future research to evaluate the effects of topical antibiotics alone versus systemic antibiotics alone versus a combination of systemic and topical antibiotics in preventing SSIs.
It would be of interest to conduct studies assessing the effect of topical antibiotics infection prophylaxis in subgroups which result in a higher risk of SSI. This could be high‐risk patients (for instance patients with diabetes or immunosuppression), body sites or types of surgery. This would help to confirm if higher‐risk wounds receive more benefit from topical antibiotics, and establish groups where they may be of more benefit.
Acknowledgements
The authors would like to acknowledge the contributions of the following people to this review:
James Cook University College of Medicine and Dentistry: Mr Stephen Anderson, Librarian, James Cook University, Townsville.
Cochrane Wounds Group peer reviewers: Kurinchi Gurusamy, Duncan Chambers, Zippy Iheozor‐Ejiofor, Mark Corbett, Elmer Villanueva, Zubir Ahmed, Roy Buffery and Janet Yarrow.
Copy‐editors: Clare Dooley and Elizabeth Royle.
Ms Alex Pinchon.
Professor Petra Buttner.
Associate Professor Herwig Drobetz, Dr Nils Wagner.
Appendices
Appendix 1. Search strategies
The Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Antibiotic Prophylaxis] this term only #2 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees #3 MeSH descriptor: [Ointments] this term only #4 MeSH descriptor: [Skin Cream] this term only #5 MeSH descriptor: [Administration, Topical] explode all trees #6 #1 or #2 #7 #5 and #6 #8 (topical near/5 antibiotic*):ti,ab,kw #9 (mupirocin or bactroban or bacitracin or "polymixin B" or neomycin or erythromycin or chloramphenicol or chlormycetin or chlorsig or neosporin):ti,ab,kw #10 (antibiotic* near/5 (foam* or tincture* or gel or gels or solution* or lotion* or cream*)):ti,ab,kw #11 (antibiotic* near/5 (powder* or liquid* or drop* or spray* or paste* or ointment*)):ti,ab,kw #12 ((antibiotic* or impregnat*) near/5 dressing*):ti,ab,kw #13 #3 or #4 or #7 or #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Surgical Wound Infection] explode all trees #15 MeSH descriptor: [Surgical Wound Dehiscence] this term only #16 (surg* near/5 infect*):ti,ab,kw #17 (surg* near/5 wound*):ti,ab,kw #18 (surg* near/5 site*):ti,ab,kw #19 (surg* near/5 incision*):ti,ab,kw #20 (surg* near/5 dehisc*):ti,ab,kw #21 (wound* near/5 dehisc*):ti,ab,kw #22 (wound* near/5 infect*):ti,ab,kw #23 (wound* near/5 disrupt*):ti,ab,kw #24 wound next complication*:ti,ab,kw #25 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 #26 #13 and #25
Ovid MEDLINE
1 Antibiotic Prophylaxis/ 2 exp Anti‐Bacterial Agents/ 3 Ointments/ 4 Skin Cream/ 5 exp Administration, Topical/ 6 1 or 2 7 5 and 6 8 (topical adj5 antibiotic*).tw. 9 (mupirocin or bactroban or bacitracin or polymixin B or neomycin or erythromycin or chloramphenicol or chlormycetin or chlorsig or neosporin).tw. 10 (antibiotic* adj5 (foam* or tincture* or gel or gels or solution* or lotion* or cream*)).tw. 11 (antibiotic* adj5 (powder* or liquid* or drop* or spray* or paste* or ointment*)).tw. 12 ((antibiotic* or impregnat*) adj5 dressing*).tw. 13 3 or 4 or 7 or 8 or 9 or 10 or 11 or 12 14 Surgical Wound Infection/ 15 Surgical Wound Dehiscence/ 16 (surg* adj5 infect*).tw. 17 (surg* adj5 wound*).tw. 18 (surg* adj5 site*).tw. 19 (surg* adj5 incision*).tw. 20 (surg* adj5 dehisc*).tw. 21 (wound* adj5 dehisc*).tw. 22 (wound* adj5 infect*).tw. 23 (wound* adj5 disrupt*).tw. 24 wound complication*.tw. 25 or/14‐24 26 13 and 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomi?ed.ab. 30 placebo.ab. 31 clinical trials as topic.sh. 32 randomly.ab. 33 trial.ti. 34 or/27‐33 35 exp animals/ not humans.sh. 36 34 not 35 37 26 and 36
Ovid Embase
1 antibiotic prophylaxis/ 2 exp antibiotic agent/ 3 exp ointment/ 4 skin cream/ 5 exp topical drug administration/ 6 1 or 2 7 5 and 6 8 (topical adj5 antibiotic*).tw. 9 (mupirocin or bactroban or bacitracin or polymixin B or neomycin or erythromycin or chloramphenicol or chlormycetin or chlorsig or neosporin).tw. 10 (antibiotic* adj5 (foam* or tincture* or gel or gels or solution* or lotion* or cream*)).tw. 11 (antibiotic* adj5 (powder* or liquid* or drop* or spray* or paste* or ointment*)).tw. 12 ((antibiotic* or impregnat*) adj5 dressing*).tw. 13 3 or 4 or 7 or 8 or 9 or 10 or 11 or 12 14 surgical infection/ 15 wound dehiscence/ 16 (surg* adj5 infect*).tw. 17 (surg* adj5 wound*).tw. 18 (surg* adj5 site*).tw. 19 (surg* adj5 incision*).tw. 20 (surg* adj5 dehisc*).tw. 21 (wound* adj5 dehisc*).tw. 22 (wound* adj5 infect*).tw. 23 (wound* adj5 disrupt*).tw. 24 wound complication*.tw. 25 or/14‐24 26 13 and 25 27 Randomized controlled trials/ 28 Single‐Blind Method/ 29 Double‐Blind Method/ 30 Crossover Procedure/ 31 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. 32 (doubl$ adj blind$).ti,ab. 33 (singl$ adj blind$).ti,ab. 34 or/27‐33 35 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 36 human/ or human cell/ 37 and/35‐36 38 35 not 37 39 34 not 38 40 26 and 39
EBSCO CINAHL
S39 S26 AND S38 S38 S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 S37 MH "Quantitative Studies" S36 TI placebo* or AB placebo* S35 MH "Placebos" S34 TI random* allocat* or AB random* allocat* S33 MH "Random Assignment" S32 TI randomi?ed control* trial* or AB randomi?ed control* trial* S31 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S30 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S29 TI clinic* N1 trial* or AB clinic* N1 trial* S28 PT Clinical trial S27 MH "Clinical Trials+" S26 S13 AND S25 S25 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 S24 TI wound N1 complication* OR AB wound N1 complication* S23 TI wound* N5 disrupt* OR AB wound* N5 disrupt* S22 TI wound* N5 infect* OR AB wound* N5 infect* S21 TI wound* N5 dehisc* OR AB wound* N5 dehisc* S20 TI surg* N5 dehisc* OR AB surg* N5 dehisc* S19 TI surg* N5 incision* OR AB surg* N5 incision* S18 TI surg* N5 site* OR AB surg* N5 site* S17 TI surg* N5 wound* OR AB surg* N5 wound* S16 TI surg* N5 infect* OR AB surg* N5 infect* S15 (MH "Surgical Wound Dehiscence") S14 (MH "Surgical Wound Infection") S13 S3 OR S4 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S12 TI ( (antibiotic* or impregnat*) N5 dressing* ) OR AB ( (antibiotic* or impregnat*) N5 dressing* ) S11 TI ( antibiotic* N5 (powder* or liquid* or drop* or spray* or paste* or ointment*) ) OR AB ( antibiotic* N5 (powder* or liquid* or drop* or spray* or paste* or ointment*) ) S10 TI ( antibiotic* N5 (foam* or tincture* or gel or gels or solution* or lotion* or cream*) ) OR AB ( antibiotic* N5 (foam* or tincture* or gel or gels or solution* or lotion* or cream*) ) S9 TI ( mupirocin or bactroban or bacitracin or "polymixin B" or neomycin or erythromycin or chloramphenicol or chlormycetin or chlorsig or neosporin ) OR AB ( mupirocin or bactroban or bacitracin or "polymixin B" or neomycin or erythromycin or chloramphenicol or chlormycetin or chlorsig or neosporin ) S8 TI topical N5 antibiotic* OR AB topical N5 antibiotic* S7 S5 AND S6 S6 S1 OR S2 S5 (MH "Administration, Topical+") S4 (MH "Creams") OR (MH "Powders") S3 (MH "Ointments") S2 (MH "Antibiotics+") S1 (MH "Antibiotic Prophylaxis")
Appendix 2. Risk of bias assessment criteria
Random sequence generation
Low risk of bias: the method used was either adequate (e.g. computer‐generated random numbers, table of random numbers) or unlikely to introduce confounding (coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.)
Uncertain risk of bias: there was insufficient information to assess whether the method used was likely to introduce confounding.
High risk of bias: the method used was likely to introduce confounding with a non‐random component in the sequence generation process. For example: sequence generated by odd or even date of birth; sequence generation based on date (or day) of admission; sequence based on hospital or clinic record number.
Allocation concealment
Low risk of bias: the method used was unlikely to induce bias on the final observed effect. Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
Uncertain risk of bias: there was insufficient information to assess whether the method used was likely to induce bias on the estimate of effect. For example the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
High risk of bias: the method used was likely to induce selection bias on the final observed effect. Participants or investigators enrolling participants could possibly foresee assignments such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Blinding of participants and personnel
Low risk of bias: blinding was performed adequately, or the outcome or outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of outcome effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome or outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data (attrition bias)
Low risk of bias: either no missing data, or the underlying reasons for missing data were unlikely to make treatment effects depart from plausible values, or proper methods were employed to handle missing data. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.The proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
Uncertain risk of bias: there was insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data was likely to induce bias on the estimate of effect.
High risk of bias: the crude estimate of effects would clearly be biased due to the underlying reasons for missing data, and the methods used to handle missing data were unsatisfactory (e.g. complete case estimate).
Selective outcome reporting
Low risk of bias: the trial protocol was available and all of the trial's prespecified outcomes that are of interest in the review have been reported; or, if the trial protocol was not available, all the primary outcomes in this review were reported.
Uncertain risk of bias: there was insufficient information to assess whether the study was high or low risk of bias for selective outcome reporting.
High risk of bias: not all of the trial's prespecified primary outcomes were reported.
Other sources of potential bias
Low risk of bias: the study appears to be free of other sources of bias.
Uncertain risk of bias: there may be a risk of bias but there is insufficient information to assess whether an important risk of bias exists, or whether the identified problem will introduce bias.
High risk of bias: has a potential source of bias due to the specific study design used, or has some other problem.
Data and analyses
Comparison 1. Topical antibiotic versus no topical antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 8 | 5427 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.87] |
| 2 Allergic contact dermatitis | 3 | 3012 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.46, 34.00] |
| 3 Wounds healed in 5‐14 days | 4 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.96, 1.03] |
Comparison 2. Topical antibiotic versus antiseptic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 5 | 1299 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.80] |
| 2 Allergic contact dermatitis | 2 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.82] |
| 3 Wounds healed in 5‐14 days | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.58, 4.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Caro 1967.
| Methods | 2‐arm prospective quasi‐randomised trial. Contaminated (class 3) surgery | |
| Participants | The study aimed to enrol 500 consecutive participants with superficial lacerations who presented at St James' Hospital, Balham; results from 432 participants were reported and it is not clear whether the further 68 participants were enrolled. Exclusion criteria: people currently receiving antibiotics or requiring deep haemostatic sutures Setting: emergency department in residential London |
|
| Interventions | Intervention (n = 197): neomycin/polymyxin B/bacitracin aerosol was applied to the surface of the wound before and after suturing. Strength probably 3.5 mg:400[iU]:5000 [iU] in 1g Control (n = 235): no intervention |
|
| Outcomes | Wound healing (dichotomous) was assessed at 5 days for lacerations of head/neck and at 10 days for other sites. | |
| Notes | An attempt was made to collect 500 cases but, in fact, 432 were recorded. It is not clear whether the further 68 participants were enrolled or to which group they may have been allocated. Wound healing definition: unhealed wound evidenced by erythema or purulent discharge Concurrent illness: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Alternate cases were allotted to the antibiotic and no antibiotic group." |
| Allocation concealment (selection bias) | High risk | Comment: Allocation by alternate participants would allow prediction of the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding of participants and personnel is not described. It is likely they were not blinded as both groups would be aware if the antibiotic was or was not sprayed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: It is not reported who conducted the outcome assessment and whether they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "An attempt was made to collect 500 cases but, in fact, 432 were finally completely documented." Comment: It is unclear if 68 participants were not included or not recorded for unknown reasons. Thus it is unclear if intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes listed in the methods section of the study were reported in the results. Study protocol was not available to identify any unreported outcomes. |
| Other bias | Low risk | Comment: None was identified. |
Dire 1995.
| Methods | 4‐arm double‐blind, placebo‐controlled RCT. Contaminated (class 3) surgery | |
| Participants | 465 participants with minor, uncomplicated soft‐tissue wounds < 12 h old for suturing were enrolled. 426 participants were analyzed, 28 participants were lost to follow‐up, and 11 participants were excluded after study commenced for protocol violations. Exclusion criteria: puncture wounds; immunocompromise/co‐morbidities; underlying fracture/neurovascular compromise; allergy to agent; pregnancy; use of antibiotics within previous 7 days Setting: emergency department of Army Community Hospital in Texas |
|
| Interventions | Intervention 1 (n = 109): bacitracin zinc ointment 500 U/g applied 3 times/day until return for suture removal. Intervention 2 (n = 110): neomycin sulfate, bacitracin zinc, polymyxin B sulphate combination ointment (probably 3.5 mg/5000 U/400 U per gram) applied 3 times/day until return for suture removal. Intervention 3 (n = 99): silversulfadiazine cream 1% applied 3 times/day until return for suture removal. Control (n = 108): petrolatum ointment applied 3 times/day until return for suture removal. |
|
| Outcomes | SSI (dichotomous); SSI (0‐4 wound scale, 4 = most severe infection); adverse effects (dichotomous). All outcomes assessed at time of suture removal (time differed for site of wound and was not specified). | |
| Notes | 28 participants were lost to follow‐up, 11 participants were excluded for protocol violations. Definition of infection 1: presence of clinical criteria for wound infection. Definition of infection 2: severity of infection by self‐developed wound scale. Definition of adverse effect: cutaneous hypersensitivity reaction reported. Concurrent illness: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants blinded using pre‐filled, identical vials. Blinding of personnel was unclear, however the study was reported as double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: wounds were examined by one of the study authors. It is not clear which author assessed outcomes and if any author may have been aware of allocation, however the study ws reported as double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 39 participants were missing from analysis, this accounted for 8% of participants. 28 randomized participants were lost to follow‐up and were not included in the analysis. 11 participants were excluded after randomisation for protocol violations. It is not clear to which groups the missing participants belonged, and therefore whether missing outcome data was balanced across interventions. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: although not listed in the methods section, an episode of `cutaneous hypersensitivity´ was reported in the results. Study protocol was not available to identify any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified |
Dixon 2006.
| Methods | Blinded, 3‐arm, RCT. Clean (class 1) surgery | |
| Participants | 778 participants, 18 years and older who were able to consent and comply with treatment, with 1801 skin lesions requiring excision. Exclusion criteria: skin contamination before surgery; surgical site not amenable to a moist, occlusive dressing; known allergy to the dressing or study agent. Partial‐thickness skin graft donor sites were not included. Participants, not wounds, were randomized to receive the intervention, but results were reported by wound. Setting: metropolitan skin cancer clinic Geelong, Australia |
|
| Interventions | Intervention: (n = 262 participants; 562 wounds) mupirocin ointment 20 mg/g Comparative intervention: (n = 269 participants; 729 wounds) paraffin ointment Control: (n = 247 participants; 510 wounds) no ointment |
|
| Outcomes | SSI rates (dichotomous, using clinical criteria); postoperative pain (self‐devised 6‐point scale, higher = greater pain); adverse outcomes/complications (dichotomous), clinically assessed until healing was complete, sutures were removed, or if a complication developed. | |
| Notes | Definition of SSI: presence of clinical criteria for wound infection. Definition of adverse effects 1: presence of clinical criteria for complications and adverse outcomes. This included allergic contact dermatitis. Definition of adverse effects 2: postoperative pain on a 6‐point scale. Concurrent illness: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using coloured discs in a barrel, quote: "Patients (not wounds) were randomized prospectively to one of 3 groups by an independent person drawing one of 150 discs (50 for each group) from a barrel; upon completing the barrel the process was repeated." Comment: this method of random sequence generation, although a little unconventional, is acceptable in principle. |
| Allocation concealment (selection bias) | High risk | Comment: the randomisation was performed by an 'independent person' drawing coloured discs from a barrel, but actual allocation concealment was not described. If the 'independent person' was the practice nurse, then this was not concealed from the researcher who applied the treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither surgeon nor patient was aware of the randomisation, although patients could not be completely blinded to the application of an ointment by the nursing staff." The nurse (personnel) and participants were aware whether they applied/received an ointment or not. It was unclear whether the active component could be differentiated by colour or smell from the paraffin ointment, and no attempt was made to see if the patients were blinded successfully by asking them which group they thought they were in. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: it is not clear who assessed wound outcomes. Only the surgeon was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: complete outcome data were reported for all participants for infection and adverse effect outcomes. ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: there were no incidences of allergic contact dermatitis. Several of the adverse event outcomes that were listed in the methods section of the study were not reported in the results, however it is likely that this is because they did not occur. Study protocol was not available to identify any unreported outcome. |
| Other bias | Unclear risk | Comment: wounds instead of participants were the basis for the main analysis. |
Gilmore 1973a.
| Methods | 3‐arm, double‐blinded RCT. Clean/contaminated (class 2) surgery | |
| Participants | 253 participants undergoing elective or emergency open appendicectomy, 1 participant was excluded postoperatively for meeting exclusion criterion. We have assumed this was prior to randomisation. Exclusion criteria: drain removal through a separate incision Setting: a busy district general hospital in Reading, London |
|
| Interventions | Intervention (n = 84): Dispray ‐ neomycin sulphate/bacitracin zinc/polymyxin B sulphate powder in a pressurised aerosol (3.5 mg:500 U:5000 U/g) was applied for 8 seconds from 25 cm away into the open wound following peritoneal closure and after closure of wound. Comparative intervention (n = 84): Disdine ‐ 5% povidone iodine in a pressurised aerosol was applied for 8 seconds from 25 cm away into the open wound following peritoneal closure and after closure of wound. Control (n = 84): no aerosol applied. |
|
| Outcomes | SSI (dichotomous) was assessed on day 6 postoperatively. | |
| Notes | 1 participant was excluded postoperatively for meeting exclusion criterion. Defintion of SSI: any purulent discharge from the wound or serous discharge with a positive wound culture in a 4‐week period. Concurrent illness: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random allocation of patients to treatment was achieved using the three‐lettered coding (L, N, Q) recurring 7 times in each 21 cases." Comment: it is unclear how the sequence was generated. |
| Allocation concealment (selection bias) | High risk | Comment: allocation concealment was not reported, however the randomisation method may have allowed prediction of the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The code was kept outside the theatre, and the surgeon was only told which code was applicable after the appendix was removed and the peritoneum closed." Comment: the surgeon conducting the operation was not blinded. The surgeon was not the outcome assessor. The patient was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcomes were assessed by a registrar from another team or an infection control nurse who were not aware of allocation. It is unlikely blinding was broken. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: outcome data were recorded for all participants; 1 participant was excluded after enrolment, we have assumed this was prior to randomisation and receiving intervention. It was not clear to which group this participant had been allocated. ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes listed in methods section, although no wound swab results were reported. Study protocol was not available to identify any unreported outcomes. |
| Other bias | Unclear risk | Comment: materials were supplied by Allvex Limited who manufactured the sprays that were used. |
Gough 1990a.
| Methods | 2‐arm quasi‐randomised trial. Clean/contaminated (class 2) surgery | |
| Participants | 108 boys with medical indications for circumcision including persistent painful micturition with phimosis, > 2 episodes of balanoposthitis, true phimosis after 10 years of age and balanitis xerotica obliterans. Exclusion criterion: non‐retracting prepuce under the age of 10 without symptoms Setting: boys with medical indication for circumcision at a children's hospital in Manchester, UK |
|
| Interventions | Intervention (n = 54): wrapping with soframycin‐impregnated tulle gras folded into a bandage and applied to wound after closure until it came away spontaneously. Comparative intervention (n = 54): 0.5 inch ribbon gauze soaked in tincture of benzoin compound and applied to the wound after closure until it came away spontaneously. |
|
| Outcomes | Wound healing (0‐3 scale, 3 = healed) assessed at 1 week | |
| Notes | The study was abandoned after 108 cases, as dressing was adversely affecting healing in 1 group. Definition of wound healing: self‐devised 4‐point clinical scale Concurrent illness: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were allocated to each group alternatively from the start of the study irrespective of age or reason for circumcision." |
| Allocation concealment (selection bias) | High risk | Comment: allocation concealment was not reported. Alternate allocation would allow prediction of sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of patient and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcomes assessor was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome data were presented for enrolled participants. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes intended to be measured. Study protocol was not available for identification of any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified. |
Gough 1990b.
| Methods | 2‐arm quasi‐randomised controlled trial. Clean/contaminated (class 2) surgery | |
| Participants | 210 boys with medical indications for circumcision including persistent painful micturition with phimosis, > 2 episodes of balanoposthitis, true phimosis after 10 years of age and balanitis xerotica obliterans. Exclusion criterion: non‐retracting prepuce under the age of 10 without symptoms Setting: boys with medical indication for circumcision at a children's hospital in Manchester, UK |
|
| Interventions | Intervention: sofretulle dressing tied in place with sutures after wound closure. Sutures were released at 24 h to allow dressing to fall away spontaneously. Comparative intervention: paraffin tulle dressing tied in place with sutures after wound closure. Sutures were released at 24 h to allow dressing to fall away spontaneously. |
|
| Outcomes | Wound healing (0‐3 scale, 3 = healed) assessed at 1 week | |
| Notes | Definition of wound healing: self‐devised 4‐point clinical scale Concurrent illness: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were allocated alternatively to paraffin tulle or sofratulle dressing." |
| Allocation concealment (selection bias) | High risk | Comment: allocation concealment was not reported. Alternate allocation would allow prediction of sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessor was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome data were presented for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes listed in methods section. Study protocol was not available for identification of any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified. |
Heal 2009.
| Methods | 2‐arm prospective, double blind RCT. Clean (class 1) surgery | |
| Participants | 1014 participants presenting for minor skin excisions were enrolled, 42 participants were lost to follow‐up and so 972 participants were analyzed. Exclusion criteria: people: taking oral antibiotics or where antibiotics were clinically indicated; on immunosuppressive therapy; requiring excision of sebaceous cyst; with an allergy to study agent; personal or family history of aplastic anaemia Setting: minor skin excisions conducted in general practice in a regional centre, Mackay, Australia |
|
| Interventions | Intervention (n = 509): Chloromycetin ointment 1% applied once by sterile forceps immediately after suturing. Control (n = 505): paraffin ointment applied once by sterile forceps immediately after suturing. |
|
| Outcomes | SSI: incidence (dichotomous); SSI severity (self‐devised 0‐4 scale, 4 = most severe infection); adverse effects (dichotomous); NNTB (mathematical equation) assessed at the time of removal of sutures. | |
| Notes | 42 participants were lost to follow‐up. Definition of SSI ‐ incidence: presence of criteria from CDC National Nosocomial Infection Surveillance System definition of SSI (Mangram 1999) Definition of SSI ‐ severity: self‐developed 5‐point clinical scale Definition of adverse effect: antimicrobial resistance confirmed by wound culture Definition of NNTB: The number of wounds treated for each infection prevented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used computer generated random numbers and opaque sealed envelopes to randomise patients." |
| Allocation concealment (selection bias) | Low risk | Comment: allocation was concealed in opaque sealed envelopes so the recruiting nurses did not know the allocation of the next patient to be included in the study (additional information from authors). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the principal investigator was aware of the identity of the coded ointments." Comment: participants and personnel were blinded to the identity of the ointment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: nurses assessing patient for SSI at the time of removal of sutures were blinded to the identity of the ointment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: outcome data were not reported for all randomized participants. 1014 participants were randomized and 972 were analyzed. Missing participants accounted for 4% of the enrolled population and this was balanced in both arms with 21 participants missing from the intervention group and 25 from the control group. An alternative definition for ITT was used by the study and a sensitivity analysis was performed to assess this effect. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes intended to be measured. When we compared the reported outcomes to those listed in the trial registry (ISRCTN73223053), there was no selective reporting. There was no study protocol. |
| Other bias | Low risk | Comment: none were identified. |
Hood 2004.
| Methods | 2‐arm RCT. Contaminated (class 3) surgery | |
| Participants | 120 participants who sustained uncomplicated soft tissue wounds within the previous 24 h were enrolled; 99 participants were analyzed; 21 participants were lost to follow‐up. Exclusion criteria: puncture wound, underlying fracture, use of antibiotics within the last 7 days, known allergy to the study agents, wounds closed with Dermabond, wounds which required use of oral/parenteral antibiotics or wounds infected at the time of presentation Setting: participants with uncomplicated soft tissue wounds in an emergency department, Ohio |
|
| Interventions | Intervention: Bactroban 2% was applied to wound immediately after closure and 3 times/day until return for wound check. Comparative intervention: Neosporin (neomycin/polymyxin B/bacitracin zinc) 3.5 mg/10000 U/400 U/g was applied to wound immediately after closure and 3 times/day until return for wound check. |
|
| Outcomes | Superficial SSI ‐ incidence (dichotomous); superficial SSI ‐ severity(1‐4 scale, 4 = most severe); adverse effects ‐ pre and post operative pain (visual analogue scale, not described); adverse effects ‐ incidence (dichotomous) | |
| Notes | 21 participants were lost to follow‐up, group not specified. Definition of SSI ‐ incidence: presence of clinical criteria for wound infection Definition of SSI ‐ severity: self‐developed 4‐point scale Definition of adverse effects ‐ pre and postoperative pain: self‐reported pain using visual analogue scale Definition of adverse effects ‐ incidence: safety assessment for any adverse event associated with medication use during study period Concurrent illness: diabetes mellitus |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: reported as randomized, however method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patient, treating physician and study investigators were blinded to the identity of the study medication". Comment: the study ointment was dispensed in identical containers. The study was reported as blinded and it is unlikely blinding was broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: treating physician, patient and study investigators were blinded to identity of medication. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 21 participants were lost to follow‐up and not included in analysis. This accounted for 17.5% of the enrolled population. It was not specified why these patients were lost to follow‐up or to which group they were allocated. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: it was stated in the methods section that a cost‐effectiveness analysis was planned, however this was abandoned as there were no difference between intervention and control arms. Otherwise all outcomes listed in methods were reported in results. Study protocol not available for identification of any unreported outcomes. |
| Other bias | Unclear risk | Comment: sponsored by Pfizer who manufactured topical antibiotics. |
Iselin 1990.
| Methods | 2‐arm quasi‐randomised controlled trial. Contaminated (class 3) surgery | |
| Participants | 268 consecutive patients presenting for urgent hand surgery were enrolled. 45 participants were excluded after randomisation; 5 were included by mistake, 33 were lost to follow‐up, 5 were excluded for protocol violation, 2 for unknown cause. Exclusion criteria: patients currently receiving immunosuppressants including steroids, renal or hepatic disease, allergy to iodine or rifampicin, burns, cutaneous laceration only, iodising radiation, current malnutrition Setting: hospital inpatients and outpatients |
|
| Interventions | Intervention: before closure wound was rinsed with rifamycin solution and after closure wound was covered with soaked pad of rifamycin. Control: before closure wound was rinsed with povidone iodine dermal solution and after closure wound was covered with soaked pad of povidone iodine dermal solution. |
|
| Outcomes | SSI (dichotomous); adverse effect (dichotomous); wound healing (dichotomous) | |
| Notes | 45 participants were excluded after randomisation; 5 were included by mistake, 33 were lost to follow‐up, 5 were excluded for protocol violation, 2 for unknown cause. Definition of SSI: presence of clinical criteria for wound infection Definition of adverse effects: cutaneous intolerance, need for further surgery, unspecified general complications Definition of wound healing ‐ proportion of wounds healed: clinical criteria for healing at 1 week and 2 weeks Concurrent Illness: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: this study was quasi randomized, alternate patients were randomized into 2 parallel groups. |
| Allocation concealment (selection bias) | High risk | Comment: allocation concealment was not described, however alternate allocation would allow prediction of allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel were unable to be blinded, as the 2 interventions were different in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcome assessor was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 45 participants were excluded after randomisation, this accounts for 17% of enrolled participants. 20 missing participants were from the intervention group and 25 were from the control group. 5 were included by mistake, 33 were lost to follow‐up, 5 were excluded for protocol violation, 2 for unknown cause.The analysis was made per protocol rather than ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes listed in methods. Study protocol was not available to identify any unreported outcomes. Patients excluded from the trial were well recorded, as were patients who dropped out of the trial. |
| Other bias | Low risk | Comment: none were identified |
Kamath 2005.
| Methods | 2‐arm, blinded RCT. Clean/contaminated (class 2) surgery | |
| Participants | 100 participants with a fractured neck of femur enrolled. 1 participant was excluded after randomisation due to confirmed metastatic disease and 7 participants died before completion of the trial. Exclusion criteria: pathological fractures, undisplaced intracapsular neck of femur fractures requiring internal fixation Setting: orthopaedic hospital inpatients at a District General Hospital, UK |
|
| Interventions | Intervention: Chloramphenicol 1% ointment applied immediately after wound closure and on day 3 Control: no ointment applied. |
|
| Outcomes | SSI (dichotomous); adverse effects (dichotomous); wound healing (dichotomous) | |
| Notes | 1 participant excluded for confirmed metastatic disease, 7 participants died before completion of trial. Definition of SSI: presence of Scottish Centre for Infection and Enrinomental Health guidelines for SSI surveillance Definition of adverse effects: not defined ‐ participants who developed chest infections, urinary tract infections and positive wound swab results were included. Definition of wound healing: number of wounds not healed at 30 days Concurrent illness: rheumatoid arthritis, diabetes mellitus, systemic medical condition unspecified, smoker |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study was reported as randomized but method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "All the cases once included in the study were randomized in an opaque sealed envelope, which was opened at the end of the surgical procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "All observations were done by one tissue viability nurse, who was blinded as to the treatment group of patients." Comment: participants were likely aware of allocation (ointment vs no ointment). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessor was blinded; participants were not. Outcomes measured were unlikely to be affected by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: data not reported for all enrolled participants; 7 died during study and 1 participant was excluded due to morbidity. Outcome data were not recorded in these 8 subjects, who constituted 8/100 (8%) of the participants, but their allocated groups were not specified. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes listed in methods. Study protocol was not available for identification of any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified. |
Khalighi 2014.
| Methods | 4‐arm RCT. Clean/contaminated (class 2) surgery | |
| Participants | 1008 participants who underwent transvenous cardiac electronic implantable device insertions. Exclusion criteria: none reported Setting: hospital in Eason, Pennsylvania |
|
| Interventions | Intervention 1: povidone iodine ointment 10%, single dose applied immediately after closure. Intervention 2: neomycin ointment 3.5 mg/g, single dose applied immediately after closure. Control 1: non‐antibiotic, non‐antiseptic placebo (see authors additional notes below). Control 2: sterile non‐adherent pad applied immediately after closure (see authors additional notes below). |
|
| Outcomes | Surgical site inflammation/infection 4‐point grading system ( A‐B = inflammation, C‐D = infection); incidences of wound abscess or erosion of pacing system was part of grade D infection. Wounds with discharge were swabbed. Infection could occur up to 12 months after procedure. | |
| Notes | Adverse effects: positive wound swab cultures Concurrent illness: diabetes mellitus, chronic kidney disease, malignancy, steroids (not specified), anticoagulation therapy (not specified) Quotes from direct correspondence with author: "In the ‘non‐antibiotic, non‐antiseptic arm of the trial’, these patients actually did not have an inert ointment applied to their wound, we used regular sterile gauze (4x4 gauze folded in half) as the dressing. The neomycin and iodine ointment arms received the same dressing,. The ‘sterile adherent pad’ arm only used "Telfa" (sterile non adherent pad) available commercially in most/all hospitals. "Patients classified as having grade 1 and grade 2 superficial inflammations (echemosis, oozing) were not considered as infection, since they ruled out to have infections (no discharge/no positive cultures/no 'ill looking' erythema)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study described as randomized, but method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome data were reported for all participants. Analysis was per ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes listed in the methods section. Study protocol was not available for identification of any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified. |
Neri 2008.
| Methods | 2‐arm, prospective RCT. Clean/contaminated (class 2) surgery | |
| Participants | 48 participants with uncomplicated gallstones undergoing video‐laparoscopic cholecystectomy during study period. Exclusion criteria: acute cholecystitis with localised peritonitis, umbilical hernia, immunodepressed patients, uncompensated diabetes, perforation of the gallbladder in the peritoneal cavity during procedure, perforation of the gallbladder during its removal through the umbilicus with bile leakage Setting: uncomplicated laparoscopic cholecystectomy patients in a metropolitan Italian surgical ward |
|
| Interventions | Intervention: 3 mL rifamycin ointment (250 mg) applied to umbilical wound immediately after closure and at 12, 24, 36, 48 and 72 h postoperatively. Control: no ointment |
|
| Outcomes | SSI ‐ inflammation (dichotomous); SSI ‐ purulent leakage (dichotomous); adverse effects ‐ postoperative pain (0‐5 scale, 5 = worst pain); wound healing ‐ dehiscence (dichotomous); wounds healed at end of trial ‐ incisional hernia (dichotomous) | |
| Notes | Definition of SSI ‐ inflammation: presence of clinical criteria for infection Definition of SSI ‐ purulent leakage: presence of purulent leakage through umbilical wound Definition of adverse effects: postoperative pain at umbilical site rated by self‐developed 6‐point scale Definition of wound healing: dehiscence: dehiscence of umbilical skin sutures during study period Definition of wound healing ‐ incisional hernia: presence of incisional umbilical hernia at 60 days postoperatively Concurrent illness: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: reported as randomized, method of random sequence generation was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was likely participants would be aware of allocation ‐ administration of ointment or no ointment. Blinding of personnel not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: self‐reported postoperative pain may be influenced by absence of blinding. Measurement of other clinical outcomes was unlikely to be affected by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome data were reported for all participants, ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Comments: although it was stated that infection would be measured, these data were not extractable from the results section. Study protocol was not available for identification of any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified. |
Pradhan 2009.
| Methods | 2‐arm RCT. Clean/contaminated (class 2) surgery | |
| Participants | 70 women undergoing emergency lower segment caesarean section, both primigravida and multigravida were included. Exclusion criteria: not reported Setting: emergency caesarean patients in a Nepalese hospital |
|
| Interventions | Intervention: single dose of topical fusidic acid 2% was applied to wound immediately after closure. Control: no ointment was applied after closure. |
|
| Outcomes | SSI (dichotomous) | |
| Notes | Definition of SSI: presence of infection within 5 postoperative days. Criteria for infection not reported. Concurrent illness: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcome assessment was not reported. Measurement of these outcomes was unlikely to be affected by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome data were reported for all participants. Analysis was per ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented for all outcomes listed in methods section measured. Study protocol was not available for identification of any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified. |
Smack 1996.
| Methods | 2‐arm prospective, double‐blind, RCT. Clean (class 1) surgery | |
| Participants | 922 participants with 1249 wounds who underwent dermatological surgical procedures were enrolled in the trial, 884 participants with 1207 wounds were studied and analyzed. Exclusion criteria: pregnancy; age < 18 years; known allergy to bacitracin ointment; documented/suspected infection prior to procedure; documented HIV positivity; requirement of systemic antibiotic prophylaxis prior to surgical procedures Setting: a general outpatient dermatology clinic and a tertiary referral advanced surgical procedure clinic in Washington, DC |
|
| Interventions | Intervention (n = 444 participants, 597 wounds): bacitracin ointment (500 U/g) applied after procedure, daily for 7‐10 days by participants and at follow‐up appointments by staff. Control (440 participants, 610 wounds): petrolatum ointment applied after procedure, daily for 7‐10 days by patients and at follow‐up appointments by staff. |
|
| Outcomes | SSI ‐ severity (0‐2 scale, 2 = severe); SSI ‐ confirmed infection (dichotomous); proportion of adverse effects ‐ allergy to ointment (dichotomous); wound healing (1‐4 scale, 4 = mature scar present) | |
| Notes | 38 participants with 42 wounds were lost to follow‐up, 13 participants were allocated to intervention group and 15 were allocated to control group. It is unclear to which group the remaining 10 participants were allocated. Definition of SSI ‐ severity: presence of pus, erythema, tenderness or itch – graded by severity Definition of SSI ‐ confirmed infection: positive wound culture plus pus, erythema or tenderness Definition of adverse event: patch testing with bacitracin, neomycin and petrolatum for participants who had a score of 2 for itch (see SSI ‐ severity) Definition of wound healing: clinical healing scale Concurrent illness: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to a treatment group using an ointment dispensing list generated by a computer program based on random number generation." |
| Allocation concealment (selection bias) | Low risk | Comment: each participant was randomized at pharmacy at time of collecting agent. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: personnel and participants were adequately blinded and it is unlikely this blinding was broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: enrolled participants were lost to follow‐up; 13 missing participants had been allocated to the intervention group and 15 to the control group. It was unclear to which group the remaining 10 missing participants were allocated. Missing participants accounted for 4% of the enrolled population. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data were presented in results for all outcomes listed in methods. Study protocol was not available for identification of any unreported outcomes. |
| Other bias | Low risk | Comment: none were identified. |
Abbreviations
ITT: intention to treat NNTB: number needed to treat for an additional beneficial outcome RCT: randomized controlled trial SSI: surgical site infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 1970 | Pre‐operative application of antibiotic, prior to closure. |
| Andersen 1972 | Antibiotic applied prior to closure in this study. |
| Andrew 2012 | Wound did not heal by primary intention. |
| Bates 1974 | Antibiotic applied prior to closure in this study. |
| Battista 2001 | Antibiotic applied prior to closure in this study. |
| Bayerl 2004 | Wound did not heal by primary intention. |
| Bencini 1991 | Antibiotic applied prior to closure in this study. |
| Bird 1971 | Antibiotic applied prior to closure in this study. |
| Blobel 1970 | Topical antibiotics to catheter to prevent UTIs, not applied to wound site after closure. |
| Bluhm 1986 | Study used systemic not topical antibiotics |
| Bos 2007 | Wound did not heal by primary intention. |
| Campbell 2005 | Wound did not heal by primary intention. |
| Charalambous 2003 | Antibiotics applied prior to closure in this study. |
| Czarnecki 1992 | Antibiotics applied prior to surgery in this study. |
| Draelos 2011 | Wound did not heal by primary intention. |
| Eason 2004 | Oral not topical antibiotics used in this study. |
| Evans 1974 | Antibiotics applied prior to closure in this study. |
| Fielding 1965 | Antibiotics applied prior to closure in this study. |
| Finch 1979 | Antibiotics applied prior to closure in this study. |
| Gilmore 1973b | Antibiotics applied prior to closure in this study. |
| Grandis 1994 | Antibiotic not applied directly to wound in this study (parenteral antibiotic/antibiotic mouthwash used). |
| Hildred 1977 | Antibiotics applied prior to closure in this study. Study was not randomised or pseudorandomised. |
| Jackson 1971 | Antibiotics applied prior to closure in this study. |
| Jensen 1975 | Antibiotics applied prior to closure in this study. |
| Johnson 2005 | Wound not sutured in this study. Not by primary intention. |
| Juul 1985 | Study used antibiotic washout, not topical application after closure. |
| Kenning 1980 | Antibiotics applied prior to closure in this study. |
| Kircik 2013 | Not by primary intention. |
| Leyden 1985 | Wounds already infected in this study. |
| Livingston 1990 | Wound did not heal by primary intention. |
| Mann 2001 | Wound did not heal by primary intention. |
| Mayer 1973 | Topical antibiotics to catheter to prevent infection, not applied directly to sutured wound site after closure. |
| Merrild 1985 | Antibiotics applied prior to closure in this study. |
| Motta 2005 | Wound did not heal by primary intention. |
| Mountain 1970 | Antibiotics applied prior to closure in this study. |
| Nicholson 2004 | Healing by secondary intention; mucosal surface. |
| Olthuis 1968 | Study did not utilise topical antibiotic. |
| Ostergaard 1981 | Antibiotics applied prior to closure in this study. |
| Pollock 1975 | Antibiotics applied prior to closure in this study. |
| Praveen 2009 | Antibiotics applied prior to closure in this study. |
| Ruschulte 2009 | Wound did not heal by primary intention. |
| Saik 1971 | Antibiotics applied prior to closure in this study. Pre‐operative skin preparation used. |
| Sarr 1988 | Study used antibiotic washout, not topical application after closure. |
| Stoller 1965 | Study used antibiotic washout, not topical application after closure. |
| Tanphiphat 1976 | Antibiotics applied prior to closure in this study. |
| Tanphiphat 1978 | Study used antibiotic irrigation, not topical application after closure. |
| Taylor 2011 | Wound did not heal by primary intention. |
| Thakur 1997 | Journal club report of another clinical trial. |
| Theophilus 2011 | Antibiotics applied prior to closure in this study. |
| Vander Salm 1989 | Antibiotics applied prior to closure in this study. |
| Varga 2009 | Antibiotics applied prior to closure (antibiotic impregnated sponge). |
| Wright 1980 | Wound did not heal by primary intention. |
Characteristics of studies awaiting assessment [ordered by study ID]
Ruiz 2015.
| Methods | RCT of topical mupirocin vs silver dressing vs no treatment |
| Participants | Bowel surgery |
| Interventions | Mupirocin, silver‐impregnated dressing, no treatment |
| Outcomes | SSI, antibiotic resistance |
| Notes | Awaiting information from study authors |
Abbreviations
RCT: randomized controlled trial SSI: surgical site infection
Differences between protocol and review
The role of the authors changed between the protocol and the review. Two authors (CH and JB) independently screened the studies identified by the literature search and analyzed studies for inclusion. PL extracted the data and CH checked the data for accuracy.
In one study (Heal 2009), an author (CH) had a conflict of interest and MVD conducted the risk of bias assessment for this study together with PL.
Some of the trials involve spraying the wound with topical antibiotics both before and after suturing. We had not anticipated this, and our protocol excluded studies with antibiotics used before closure, but included studies with antibiotics applied after closure. We decided to include studies where topical antibiotics were applied both before and after closure.
In our protocol, we had excluded cases where the patient was concurrently on a systemic antibiotic. In practice, in many studies involving major surgery it is routine to use systemic antibiotics perioperatively, or both perioperatively and postoperatively. If we exclude these studies, we basically exclude any major surgery. We decided therefore to include these studies.
The definition of healing was changed in the review, from proportion of wounds healed at the end of the trial in the protocol to proportion of wounds healed by 5 to 14 days. This was because all studies reported their healing in this time frame rather than at the end of the trial. No trials reported time to healing, so this was not reported as an outcome measure. This is described in the results section of the review.
We did not perform intention to treat (ITT) analysis, and the justification for this is described in the methods section.
Contributions of authors
Clare Heal:
conceived, designed and co‐ordinated the review;
extracted and checked data and performed the statistical analysis;
undertook and checked the quality assessment;
analysed and interpreted the data;
led the writing and editing of the review.
Jennifer Banks:
checked data extraction;
led the screening of the literature search;
undertook and checked the quality assessment;
analysed and interpreted the data;
contributed to the writing and editing of the review.
Phoebe Lepper:
extracted and checked data;
analysed and interpreted the data;
contributed to the writing and editing of the review;
wrote to study authors, experts, and companies.
Evan Kontopantelis:
contributed to the analysis and interpretation of data.
Mieke Van Driel:
checked data extraction and quality assessment;
analysed and interpreted the data;
contributed to the writing and editing of the review.
Contributions of editorial base
Kurinchi Gurusamy (Editor): advised on methodology, interpretation and protocol content, approved the final protocol prior to submission.
Nicky Cullum (Editor): advised on methodology, interpretation and review content, approved the final review prior to submission.
Sally Bell‐Syer: (Managing Editor) co‐ordinated the editorial process, advised on methodology, interpretation and content and edited the protocol.
Gill Rizzello: (Managing Editor) co‐ordinated the editorial process, advised on interpretation and content and edited the review.
Amanda Briant (Information Specialist): designed the search strategy.
Reetu Child: (Information Specialist): edited the search methods section and ran the searches.
Sources of support
Internal sources
James Cook University College of Medicine and Dentistry, Australia.
Mr Stephen Anderson, Librarian, James Cook University, Townsville, Australia.
External sources
-
The National Institute of Health Research (NIHR), UK.
This project was supported by the National Institute for Research via Cochrane Infrastructure funding. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health UK
Declarations of interest
Clare Heal – none known. Mieke van Driel – none known. Phoebe Lepper – none known. Evan Kotopantelis ‐ none known. Jennifer Banks – none known.
New
References
References to studies included in this review
Caro 1967 {published data only}
- Caro D, Reynolds KW, DeSmith J. An investigation to evaluate a topical antibiotic in the prevention of wound sepsis in a casualty department. The British Journal of Clinical Practice 1967;21(12):605‐7. [PubMed] [Google Scholar]
Dire 1995 {published data only}
- Dire DJ, Coppola M, Dwyer DA, Lorette JJ, Karr JL. Prospective evaluation of topical antibiotics for preventing Infections in uncomplicated soft‐tissue wounds repaired in the ED. Academic Emergency Medicine 1995;2(1):4‐10. [DOI] [PubMed] [Google Scholar]
Dixon 2006 {published data only}
- Dixon AJ, Dixon MP, Dixon JB. Randomized clinical trial of the effect of applying ointment to surgical wounds before occlusive dressing. British Journal of Surgery 2006;93(8):937‐43. [DOI] [PubMed] [Google Scholar]
Gilmore 1973a {published data only}
- Gilmore OJA, Martin TDM, Fletcher BN. Prevention of wound infection after appendicectomy. The Lancet 1973;1(7797):220‐2. [DOI] [PubMed] [Google Scholar]
Gough 1990a {published data only}
- Gough DCS, Lawton N. Circumcision – which dressing?. British Journal of Urology 1990;65(4):418‐9. [DOI] [PubMed] [Google Scholar]
Gough 1990b {published data only}
- Gough DCS, Lawton N. Circumcision – which dressing?. British Journal of Urology 1990;65(4):481‐9. [DOI] [PubMed] [Google Scholar]
Heal 2009 {published data only}
- Heal CF, Buettner PG, Cruickshank R, Graham D, Browning S, Pendergast J, et al. Does single application of topical chloramphenicol to high risk sutured wounds reduce incidence of wound infection after minor surgery? Prospective randomised placebo controlled double blind trial. BMJ 2009;338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hood 2004 {published data only}
- Hood R, Shermock KM, Emerman C. A prospective, randomized pilot evaluation of topical triple antibiotic versus mupirocin for the prevention of uncomplicated soft tissue wound infection. American Journal of Emergency Medicine 2004;22(1):1‐3. [DOI] [PubMed] [Google Scholar]
Iselin 1990 {published data only}
- Iselin F, Audren JL, Gouet O, Hautefort E, Peze W, Pradet G. Comparative study of the effects of a local antibiotic and a local antiseptic in emergency hand surgery [Étude comparée des effets d'un antibiotique et d'un antiseptique locaux en chirurgie d'urgence de la main]. Annales de Chirurgie de la Main et du Membre Superieur: Organe Officiel des Societes de Chirurgie de la Main 1990;9(1):65‐71. [DOI] [PubMed] [Google Scholar]
Kamath 2005 {published data only}
- Kamath S, Sinha S, Shaari E, Young D, Campbell AC. Role of topical antibiotics in hip surgery. A prospective randomised study. International Journal of the Care of the Injured 2005;36(6):783‐7. [DOI] [PubMed] [Google Scholar]
Khalighi 2014 {published data only}
- Khalighi K, Aung TT, Elmi F. The role of prophylaxis topical antibiotics in cardiac device implantation. Pacing and Clinical Electrophysiology 2014;37(3):304‐11. [DOI] [PubMed] [Google Scholar]
Neri 2008 {published data only}
- Neri V, Fersini A, Ambrosi A, Tartaglia N, Valentino TP. Umbilical port‐site complications in laparoscopic cholecystectomy: role of topical antibiotic therapy. Journal of the Society of Laparoendoscopic Surgeons 2008;12(2):126‐32. [PMC free article] [PubMed] [Google Scholar]
Pradhan 2009 {published data only}
- Pradhan GBN, Agrawal J. Comparative study of post operative wound infection following lower segment caesarean section with and without the topical use of fusidic acid. Nepal Medical College Journal 2009;11(3):189‐91. [PubMed] [Google Scholar]
Smack 1996 {published data only}
- Smack DP, Harrington AC, Dunn C, Howard RS, Szkutnik AJ, Krivda SJ, et al. Infection and allergy Incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment: a randomized controlled trial. JAMA 1996;276(12):972‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Andersen 1970 {published data only}
- Andersen B, Ostergaard H. Ampicillin in the prophylaxis of wound infection in elective operation for cancer of the colon. A controlled study of the value of local application to the wound [Ampicillinprofylake mod sarinfektion ved elektiv operation for coloncancer]. Ugeskrift for Laeger 1970;132(24):1133‐5. [PubMed] [Google Scholar]
Andersen 1972 {published data only}
- Andersen B, Bendtsen L, Schantz A. Wound infections after appendicectomy: I A controlled trial on the prophylactic efficacy of topical ampicillin in non‐perforated appendix. II A controlled trial on the prophylactic efficacy of delayed primary suture and topical ampicillin in perforated appendix. Acta Chirurgica Scandinavica 1972;138(5):531‐6. [PubMed] [Google Scholar]
Andrew 2012 {published data only}
- Andrew R, Luecke G, Dozier S, Diven D. A pilot study to investigate the efficacy of tobramycin‐dexamethasone ointment in promoting wound healing. Dermatology and Therapy 2012;2(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
Bates 1974 {published data only}
- Bates T, Down RHL, Houghton MCV, Lloyd GJ. Topical ampicillin in the prevention of wound infection after appendicectomy. British Journal of Surgery 1974;61:489‐92. [DOI] [PubMed] [Google Scholar]
Battista 2001 {published data only}
- Battista F, Zund S, Giordano H, Soto F, Cascarelli L. Use of antibiotics in topical form for prevention of surgical site infection [Utilizacion de antibioticos en forma topica en la prevencion de infeccion de sitio quirurgico]. Revista Argentina de Residentes de Cirugia 2001;6(2):44‐6. [Google Scholar]
Bayerl 2004 {published data only}
- Bayerl C, Volp A. [Tyrothicin‐puder bei hautverletzungen: Reduktion des wundradius bei infizierten und infektionsgefahrdeten wunden ‐ eine randomisierte, placebo kontrollierte doppelblind‐studie]. Pharmazie 2004;59(11):864‐8. [PubMed] [Google Scholar]
Bencini 1991 {published data only}
- Bencini P, Galimberti M, Signorini M, Crosti C. Antibiotic prophylaxis of wound infections in skin surgery. Archives of Dermatology 1991;127:1357‐60. [PubMed] [Google Scholar]
Bird 1971 {published data only}
- Bird G, Bunch G, Croft C, Hoffmann D, Humphrey C, Rhind J, et al. Topical noxythiolin antisepsis. British Journal of Surgery 1971;58(6):447‐8. [DOI] [PubMed] [Google Scholar]
Blobel 1970 {published data only}
- Blobel R, Polack J. Attempt to 'locally' prevent postoperative urinary tract infections following gynecologic surgery [Versuch einer 'lokalen' prophylaxe der postoperativen harnwegsinfektionen nach vaginalen gynakologischen operationen]. Die Medizinische Welt 1970;36:1572‐4. [PubMed] [Google Scholar]
Bluhm 1986 {published data only}
- Bluhm G, Nordlander R, Ransjo U. Antibiotic prophylaxis in pacemaker surgery: a prospective double blind trial with systemic administration of antiobiotic versus placebo at implantation of cardiac pacemakers. Pacing and Clinical Electrophysiology 1986;9(5):720‐6. [DOI] [PubMed] [Google Scholar]
Bos 2007 {published data only}
- Bos A, Tilburg M, Sorge A, Klinkenbijl. Randomized clinical trial of surgical technique and local antibiotics for ingrowing toenail. British Journal of Surgery 2007;94:292‐6. [DOI] [PubMed] [Google Scholar]
Campbell 2005 {published data only}
- Campbell R, Perlis C, Fisher E, Gloster H. Gentamicin ointment versus petrolatum for management of auricular wounds. Dermatologic Surgery 2005;31:664‐9. [DOI] [PubMed] [Google Scholar]
Charalambous 2003 {published data only}
- Charalambous C, Tryfonidis M, Swindell R, Lipsett AP. When should old therapies be abandoned? A modern look at old studies on topical ampicillin. Journal of Infection 2003;47:203‐9. [DOI] [PubMed] [Google Scholar]
Czarnecki 1992 {published data only}
- Czarnecki D, Meehan C, Nash C. Prevention of post‐excisional wound infections: a comparison of oral cephalexin with topical mupirocin and topical cetrimide‐chlorhexidine cream. International Journal of Dermatology 1992;31(5):359‐60. [DOI] [PubMed] [Google Scholar]
Draelos 2011 {published data only}
- Draelos Z, Trookman N, Rizer, R. Postprocedural wound healing efficacy and safety in a clinical setting: a comparison of topical antibiotic ointment versus skin protectant ointment. Journal of the American Academy of Dermatology 2011;64(2 SUPP):S23‐9. [DOI] [PubMed] [Google Scholar]
Eason 2004 {published data only}
- Eason A, Wells G, Garber G, Hopkins M. Prophylactic antibiotics for abdominal hysterectomy: indication for low‐risk Canadian women. Journal of Obstetrics and Gynaecology Canada 2004;26(12):1067‐71. [DOI] [PubMed] [Google Scholar]
Evans 1974 {published data only}
- Evans C, Pollock A, Rosenberg I. The reduction of surgical wound infections by topical cephaloridine: a controlled clinical trial. British Journal of Surgery 1974;61:133‐5. [DOI] [PubMed] [Google Scholar]
Fielding 1965 {published data only}
- Fielding G, Rao A, Davis N, Wernigk N. Prophylactic topical use of antibiotics in surgical wounds: a controlled clinical trial using 'Polybactrin'. The Medical Journal of Australia 1965;2(4):159‐61. [PubMed] [Google Scholar]
Finch 1979 {published data only}
- Finch D, Taylor L, Morris P. Wound sepsis following gastrointestinal surgery: a comparison of topical and two‐dose systemic cephradine. British Journal of Surgery 1979;66(8):580‐2. [DOI] [PubMed] [Google Scholar]
Gilmore 1973b {published data only}
- Gilmore O, Welbourn R. Controlled trial of wound spraying with poly‐antibiotic or povidone iodine aerosols. British Journal of Surgery 1973;60(11):910‐1. [PubMed] [Google Scholar]
Grandis 1994 {published data only}
- Grandis J, Vickers R, Rihs J, Yu V, Johnson J. Efficacy of topical amoxicillin plus clavulanate/ticarcillin plus clavulanate and clindamycin in contaminated head and neck surgery: effect of antibiotic spectra and duration of therapy. The Journal of Infectious Diseases 1994;170(3):729‐32. [DOI] [PubMed] [Google Scholar]
Hildred 1977 {published data only}
- Hildred S, Henderson C. Does antibiotic spray reduce wound infection?. British Medical Journal 1977;2:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 1971 {published data only}
- Jackson D, Pollock A, Tindal D. The effect of an antibiotic spray in the prevention of wound infection. A controlled trial. British Journal of Surgery 1971;58(8):565‐6. [DOI] [PubMed] [Google Scholar]
Jensen 1975 {published data only}
- Jensen M, McKenzie R, Hugh T, Lake B. Topical ampicillin‐cloxacillin in the prevention of abdominal wound sepsis. The British Journal of Clinical Practice 1975;29(5):115‐8. [PubMed] [Google Scholar]
Johnson 2005 {published data only}
- Johnson D, Eps C, Mudge D, Wiggins K, Armstrong K, Hawley C, et al. Randomized, controlled trial of topical exit‐site application of honey (Medihoney) versus mupirocin for the prevention of catheter‐associated infections in hemodialysis patients. Journal of the American Society of Nephrology 2005;16:1456‐62. [DOI] [PubMed] [Google Scholar]
Juul 1985 {published data only}
- Juul P, Merrild M, Kronborg M. Topical ampicillin in addition to a systemic antibiotic prophylaxis in elective colorectal surgery. A prospective randomized study. Diseases of the Colon and Rectum 1985;28:804‐6. [DOI] [PubMed] [Google Scholar]
Kenning 1980 {published data only}
- Kenning BR, McIntosh I, Nagesh A, Rosenberg P. A comparative trial of cephaloridine with polybactrin, povidone‐iodine and control for prophylaxis of wound infection in abdominal surgery. British Journal of Clinical Practice 1980;34(2):45‐6,54. [PubMed] [Google Scholar]
Kircik 2013 {published data only}
- Kircik L, Dickerson J, Kitten C, Weedon K, Slade H. Allogeneic growth arrested keratinocytes and fibroblasts delivered in a fibrin spray accelerate healing in Mohs micrographic surgery wounds. Journal of Drugs in Dermatology 2013;12(5):558‐61. [PubMed] [Google Scholar]
Leyden 1985 {published data only}
- Leyden J. Effect of bactroban ointment: neosporin ointment and garamycin ointment in the prophylaxis of infection of cutaneous wounds in human volunteers. Bactroban (mupirocin): Proceedings of an international symposium; 1984 May 21‐22; Nassau, Bahama Islands. New York: Excerpta Medica, 1985:84‐90.
Livingston 1990 {published data only}
- Livingston D, Cryer H, Miller F, Malangoni M, Polk H, Weiner L. A randomized prospective study of topical antimicrobial agents on skin grafts after thermal injury. Plastic and Reconstructive Surgery 1990;86(6):1059‐64. [PubMed] [Google Scholar]
Mann 2001 {published data only}
- Mann T, Orlikowski C, Gurrin L, Keils A. The effect of the biopatch, a chlorhexidine impregnated dressing, on bacterial colonization of epidural catheter exit sites. Anasthaesia and Intensive Care 2001;29:600‐3. [DOI] [PubMed] [Google Scholar]
Mayer 1973 {published data only}
- Mayer HG. Prevention of urinary tract infections following gynecologic surgery [Zur verhutung von harnwegsinfektionen nach gynäkologischen operationen]. Wiener medizinische Wochenschrift 1973;123(12):188‐90. [PubMed] [Google Scholar]
Merrild 1985 {published data only}
- Merrild U, Juul P, Kronburg O. Local ampicillin prevention of wound infection after elective colorectal surgery [Lokal ampicillinprofylakse mod sårinfektion efter elektiv kolorektal kirugi]. Ugeskrift for Laeger 1985;147(10):876‐8. [PubMed] [Google Scholar]
Motta 2005 {published data only}
- Motta G, Trigilia D. The effect of an antimicrobial drain sponge dressing on specific bacterial isolates at tracheostomy sites. Ostomy/Wound Management 2005;51(1):60‐2, 64‐6. [PubMed] [Google Scholar]
Mountain 1970 {published data only}
- Mountain J, Seal P. Topical ampicillin in grid‐iron appendicectomy wounds. The British Journal of Clinical Practice 1970;24(3):111‐5. [PubMed] [Google Scholar]
Nicholson 2004 {published data only}
- Nicholson TJ, Armstrong D. Toical metronidazole (10 per cent) decreases posthemorrhoidectomy pain and improved healing. Diseases of the Colon and Rectum 2004;47(5):711‐6. [DOI] [PubMed] [Google Scholar]
Olthuis 1968 {published data only}
- Olthuis G, Oosterwijk W, Oomen H. The value of chloramphenicol in the prevention of wound infection in abdominal surgery [De waarde van chlooramfenicol voor de profylaxe van wondinfecties bij de buikchirurgie]. Nederlands Tijdschrift voor Geneeskunde 1968;112(17):786‐8. [PubMed] [Google Scholar]
Ostergaard 1981 {published data only}
- Østergaard A, Wamberg P. Topical or oral antibiotics against wound infection in colorectal surgery. Aktuelle Probleme in Chirurgie und Orthopadie 1981;19:105‐7. [PubMed] [Google Scholar]
Pollock 1975 {published data only}
- Pollock A, Evans M. Povidone‐iodine for the control of surgical wound infection: a controlled clinical trial against topical cephaloridine. British Journal of Surgery 1975;62(4):292‐4. [DOI] [PubMed] [Google Scholar]
Praveen 2009 {published data only}
- Praveen S, Rohaizak M. Local antibiotics are equivalent to intravenous antibiotics in the prevention of superficial wound infection in inguinal hernioplasty. Asian Journal of Surgery 2005;32(1):59‐63. [DOI] [PubMed] [Google Scholar]
Ruschulte 2009 {published data only}
- Ruschulte H, Franke M, Gastmeier P, Zenz S, Mahr K, Bucholz S, et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Annals of Hematology 2009;88(3):267‐72. [DOI] [PubMed] [Google Scholar]
Saik 1971 {published data only}
- Saik R, Walz C, Rhoads J. Evaluation of a bacitracin‐neomycin surgical skin preparation. The American Journal of Surgery 1971;121(5):557‐60. [DOI] [PubMed] [Google Scholar]
Sarr 1988 {published data only}
- Sarr M, Parikh K, Sanfey H, Minken S, Cameron J. Topical antibiotics in high‐risk biliary surgical patient. The American Journal of Surgery 1988;155(2):337‐42. [DOI] [PubMed] [Google Scholar]
Stoller 1965 {published data only}
- Stoller J. Topical antibiotic therapy in acute appendicitis. The British Journal of Clinical Practice 1965;12(19):687‐8. [PubMed] [Google Scholar]
Tanphiphat 1976 {published data only}
- Tanphiphat C, Udomchaya A, Wacharasin T, Suwanpen T, Panichabhongse V, Vajrabukka C. Wound infection in emergency appendectomy: a prospective trial with topical ampicillin and savlon. Journal of the Medical Association of Thailand 1976;59(8):355‐8. [PubMed] [Google Scholar]
Tanphiphat 1978 {published data only}
- Tanphiphat C, Udomchanya S, Wacharasin T, Suwanpen T, Panichabhongse V, Vajrabukka C. Wound infection in emergency appendectomy: a prospective trial with topical ampicillin and savlon. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 1978;65(2):89‐91. [PubMed] [Google Scholar]
Taylor 2011 {published data only}
- Taylor S, Averyhart A, Heath C. Postprocedural wound‐healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. Journal of the American Academy of Dermatology 2011;64(3 (Supp)):S30‐5. [DOI] [PubMed] [Google Scholar]
Thakur 1997 {published data only}
- Thakur N. Topical ointments and wound healing. Journal of Family Practice 1997;44(1):26‐7. [PubMed] [Google Scholar]
Theophilus 2011 {published data only}
- Theophilus S, Adnan J. A randomised control trial on the use of topical methicillin in reducing post‐operative ventriculoperitoneal shunt infection. Malaysian Journal of Medical Science 2011;18(1):30‐7. [PMC free article] [PubMed] [Google Scholar]
Vander Salm 1989 {published data only}
- Vander Salm T, Okike O, Pasque M, Pezzella A, Lew R, Traina V, et al. Reduction of sternal infection by application of topical vancomycin. Journal of Thoracic and Cardiovascular Surgery 1989;98(4):618‐22. [PubMed] [Google Scholar]
Varga 2009 {published data only}
- Varga M, Sixta B, Bern R, Adamec M, Jirkovska A. Influence of gentamicincollagen sponge on wound healing after metatarsophalangeal joint resection in diabetic patients. European Wound Management Association Journal 2009;2:Abstract 125. [Google Scholar]
Wright 1980 {published data only}
- Wright V, Hatch L, Lanning N. Use of a topical triple‐antibiotic spray to reduce morbidity from pelvic infection after gynecologic operations. The Canadian Journal of Surgery 1980;23(4):366‐72. [PubMed] [Google Scholar]
References to studies awaiting assessment
Ruiz 2015 {published data only}
- Ruiz‐Tovar J, Llavero C, Morales V, Gamallo C. Total occlusive ionic silver containing dressing vs. mupirocin ointment application vs. conventional dressing in elective colorectal surgery: Effect on incisional surgical site infection. Journal of American College of Surgeons 2015;221(2):424‐9. [DOI] [PubMed] [Google Scholar]
Additional references
AEG 2010
- Antibiotic Expert Group (AEG). Therapeutic Guidelines: antibiotic. Vol. 14, North Melbourne, Victoria: Therapeutic Guidelines Limited, 2010. [Google Scholar]
Berger 2000
- Berger RS, Pappert AS, Zile PS, Cetnarowski WE. A newly formulated topical triple‐antibiotic ointment minimizes scarring. Cutis 2000;65(6):401‐4. [PubMed] [Google Scholar]
Blondeel 1978
- Blondeel A, Oleffe J, Achten G. Contact allergy in 330 dermatological patients. Contact Dermatitis 1978;4(5):270‐6. [DOI] [PubMed] [Google Scholar]
Bradley 1995
- Bradley SF, Ramsey MA, Morton TM, Kauffman CA. Mupirocin resistance: clinical and molecular epidemiology. Infection Control and Hospital Epidemiology 1995;16(6):354‐8. [DOI] [PubMed] [Google Scholar]
Bratzler 2004
- Bratzler DW, Houck PM, Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clinical Infectious Diseases 2004;38(12):1706–15. [DOI] [PubMed] [Google Scholar]
Bruce 2001
- Bruce J, Russell EM, Mollison J, Krukowski ZH. The quality of measurement of surgical wound infection as the basis for monitoring: a systematic review. The Journal of Hospital Infection 2001;49(2):99‐108. [DOI] [PubMed] [Google Scholar]
CDC 2014
- Centers for Disease Control and Prevention (CDC). Surgical Site Infection (SSI) Event. www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf (accessed 25 February 2014).
Cruse 1980
- Cruse PJ, Foord R. The epidemiology of wound infection: a 10‐year prospective study of 62,939 wounds. Surgical Clinics of North America 1980;60(1):27‐40. [DOI] [PubMed] [Google Scholar]
Culver 1991
- Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. The American Journal of Medicine 1991;91(3):152‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Diehr 2007
- Diehr S, Hamp A, Jamieson B. Do topical antibiotics improve wound healing?. The Journal of Family Practice 2007;56(2):140‐4. [PubMed] [Google Scholar]
Eaglstein 1980
- Eaglstein WH, Mertz PM. 'Inert' vehicles do affect wound healing. Journal of Investigative Dermatology 1980;74(2):90‐1. [DOI] [PubMed] [Google Scholar]
Erel 1999
- Erel E, Platt AJ, Ramakrishnan V. Chloramphenicol use in plastic surgery. British Journal of Plastic Surgery 1999;52(4):326‐7. [PubMed] [Google Scholar]
Fukuda 2002
- Fukuda M, Ohashi H, Matsumoto C, Mishima S, Shimomura Y. Methicillin‐resistant Staphylococcus aureus and methicillin‐resistant coagulase‐negative Staphylococcus ocular surface infection efficacy of chloramphenicol eye drops. Cornea 2002;21(7):86‐9. [DOI] [PubMed] [Google Scholar]
Geronemus 1979
- Geronemus RG, Mertz PM, Eaglstein WH. Wound healing: The effects of topical antimicrobial agents. Archives of Dermatology 1979;115(11):1311‐4. [DOI] [PubMed] [Google Scholar]
Grey 2009a
- Grey J, Healy B, Harding K. Antibiotic prophylaxis for minor dermatological surgery in primary care. BMJ 2009;338:(a2749). [DOI] [PubMed] [Google Scholar]
HCN 2014
- Health Communication Network (HCN), Phoenix Medical Publishing. AusDI ‐ Evidence based medicines information resource. http://www.phoenixmedical.com.au/ausdi.php (accessed 25 February 2014).
Higgins 2011
- Higgins JP, Altman DG, Sterne AC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huiras 2012
- Huiras P, Logan J, Papadopoulos S, Whitney D. Local antimicrobial administration for prophylaxis of surgical site infections. Review of Therapeutics 2012;32(11):1006‐19. [DOI] [PubMed] [Google Scholar]
Kasten 1999
- Kasten MJ. Clindamycin, metronidazole, and chloramphenicol. Mayo Clinic Proceedings 1999;74:825‐33. [DOI] [PubMed] [Google Scholar]
Kicinski 2015
- Kicinski M, Springate DA, Kontopantelis E. Publication bias in meta‐analyses from the Cochrane Database of Systematic Reviews. Statistics in Medicine 2015;34(20):2781‐93. [DOI] [PubMed] [Google Scholar]
Kulaylat 2007
- Kulaylat M, Dayton M. Surgical Wound Complications. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL editor(s). Sabiston Textbook of Surgery. 18th Edition. Philadephia: Saunders Elsevier, 2007:331‐4. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, editor(s). Chapter 6: Searching for studies In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leyden 1979
- Leyden JJ, Kligman AM. Contact dermatitis to neomycin sulfate. JAMA 1979;242(12):1276‐8. [PubMed] [Google Scholar]
Leyden 1987
- Leyden JJ, Bartelt NM. Comparison of topical antibiotic ointments, a wound protectant, and antiseptics for the treatment of human blister wounds contaminated with Staphylococcus aureus. The Journal of Family Practice 1987;24(6):601‐4. [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care Interventions: Explanation and elaboration. PLOS Med (2009);6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magill 2014
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point‐prevelance survey of health care‐associated infections. The New England Journal of Medicine 2014;370(13):1198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangram 1999
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, Centres for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. American Journal of Infection Control 1999;27(2):97‐132. [PubMed] [Google Scholar]
Maragh 2005
- Maragh SL, Otley CC, Roenigk RK, Phillips PK. Antibiotic prophylaxis in dermatologic surgery: updated guidelines. Dermatologic Surgery 2005;31(1):83‐91. [DOI] [PubMed] [Google Scholar]
Marks 1998
- Marks JG, Belsito DV, DeLeo VA, Fowler JF, Fransway AF, Maibach HI, et al. North American Contact Dermatitis Group patch test results for the detection of delayed‐type hypersensitivity to topical allergens. Journal of the American Academy of Dermatology 1998;38(6):911‐8. [DOI] [PubMed] [Google Scholar]
McDonnell 1999
- McDonnell G, Denver Russell A. Antiseptics and disinfectants: activity, action and resistance. Clinical Microbiology Reviews 1999;12(1):147‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
McHugh 2011
- McHugh S, Collins C, Corrigan M, Hill A, Humphreys H. The role of topical antibiotics used as prophylaxis in surgical site infection prevention. Journal of Antimicrobial Chemotherapy 2011;66(4):693‐701. [DOI] [PubMed] [Google Scholar]
Messingham 2005
- Messingham MJ, Arpey CJ. Update on the use of antibiotics in cutaneous surgery. Dermatologic Surgery 2005;31(8):1068‐78. [DOI] [PubMed] [Google Scholar]
Miller 1996
- Miller MA, Dascal A, Portnoy J, Mendelson J. Development of mupirocin resistance among methicillin‐resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infection Control and Hospital Epidemiology 1996;17(12):811‐3. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Care Excellence (NICE). Surgical site infection (Clinical guideline 74). www.nice.org.uk/CG74 (accessed 25 February 2014).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosengren 2010a
- Rosengren H, Dixon A. Antibacterial prophylaxis in dermatologic surgery: an evidence based review. American Journal of Clinical Dermatology 2010;11(1):35‐44. [DOI] [PubMed] [Google Scholar]
Saco 2015
- Saco M, Howe N, Nathoo R, Cherpelis B. Topical antibiotic prophylaxis for prevention of surgical wound infections from dermatologic procedures: a systematic review and meta‐analysis. Journal of Dermatolocial Treatment 2015;26(2):151‐8. [DOI] [PubMed] [Google Scholar]
Saryan 1998
- Saryan JA, Dammin TC, Bouras AE. Anaphylaxis to topical bacitracin zinc ointment. The American Journal of Emergency Medicine 1998;16(5):512‐3. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement. http://www.consort‐statement.org/consort‐2010 (accessed 25 February 2014).
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Flasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 22 March 2016).
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [DOI] [PubMed] [Google Scholar]
Wright 2008
- Wright TI, Baddour LM, Berbari EF, Roenigk RK, Phillips PK, Jacobs MA, et al. Antibiotic prophylaxis in dermatologic surgery: advisory statement 2008. Journal of the American Academy of Dermatology 2008;59(3):464‐73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Heal 2014
- Heal C, Driel M, Lepper PD, Banks J. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011426] [DOI] [PMC free article] [PubMed] [Google Scholar]


