Abstract
Background
Experiencing anxiety and depression is very common in people with dementia and mild cognitive impairment (MCI). Psychological interventions have been suggested as a potential treatment for these populations. Current research suggests that people with dementia and MCI have limited opportunities for psychological treatments aimed at improving their well‐being. A systematic review of the evidence on their effectiveness is likely to be useful in terms of improving outcomes for patients and for future recommendations for practice.
Objectives
The main objective of this review was to assess the effectiveness of psychological interventions in reducing anxiety and depression in people with dementia or mild cognitive impairment (MCI).
Search methods
We searched the Cochrane Dementia and Cognitive Improvement Group Specialized Register and additional sources for both published and unpublished data.
Selection criteria
We included randomised controlled trials (RCTs) comparing a psychological intervention with usual care or a placebo intervention (social contact control) in people with dementia or MCI.
Data collection and analysis
Two review authors worked independently to select trials, extract data and assess studies for risk of bias, using a data extraction form. We contacted authors when further information was not available from the published articles.
Main results
Six RCTs involving 439 participants with dementia were included in the review, but no studies of participants with MCI were identified. The studies included people with dementia living in the community or in nursing home care and were carried out in several countries. Only one of the studies was classified as low risk of bias. Five studies were at unclear or high risk of bias due to uncertainties around randomisation, blinding and selective reporting of results. The studies used the different psychological approaches of cognitive behavioural therapy (CBT), interpersonal therapy and counselling. Two studies were of multimodal interventions including a specific psychological therapy. The comparison groups received either usual care, attention‐control educational programs, diagnostic feedback or services slightly above usual care.
Meta‐analysis showed a positive effect of psychological treatments on depression (6 trials, 439 participants, standardised mean difference (SMD) ‐0.22; 95% confidence interval (CI) ‐0.41 to ‐0.03, moderate quality evidence) and on clinician‐rated anxiety (2 trials, 65 participants, mean difference (MD) ‐4.57; 95% CI ‐7.81 to ‐1.32, low quality evidence), but not on self‐rated anxiety (2 trials, SMD 0.05; 95% CI ‐0.44 to 0.54) or carer‐rated anxiety (1 trial, MD ‐2.40; 95% CI ‐4.96 to 0.16). Results were compatible with both benefit and harm on the secondary outcomes of patient quality of life, activities of daily living (ADLs), neuropsychiatric symptoms and cognition, or on carers' self‐rated depressive symptoms, but most of the studies did not measure these outcomes. There were no reports of adverse events.
Authors' conclusions
We found evidence that psychological interventions added to usual care can reduce symptoms of depression and clinician‐rated anxiety for people with dementia. We conclude that psychological interventions have the potential to improve patient well‐being. Further high quality studies are needed to investigate which treatments are most effective and to evaluate the effect of psychological interventions in people with MCI.
Keywords: Humans, Anxiety, Anxiety/therapy, Cognitive Dysfunction, Cognitive Dysfunction/psychology, Cognitive Therapy, Cognitive Therapy/methods, Counseling, Counseling/methods, Dementia, Dementia/psychology, Depression, Depression/therapy, Psychotherapy, Psychotherapy/methods, Randomized Controlled Trials as Topic
Psychological treatments for depression and anxiety in dementia and mild cognitive impairment
Symptoms of depression and anxiety are common in people with dementia and mild cognitive impairment (MCI). Although treatment of these symptoms is widely recommended in guidelines, the best way to do this is not clear. Drugs are thought to have limited effectiveness in this context and carry the risk of significant side effects. Psychological treatments can be adapted for use with people with cognitive impairment and may offer an alternative treatment. This review identified six randomised controlled trials, including 439 participants, in which a psychological treatment for people with dementia was compared to usual care. Most participants had mild dementia, but one trial was conducted with nursing home residents who had more severe dementia. We found no trials of participants with MCI. The psychological interventions used were based on established psychological models such as cognitive behavioural therapy (CBT), counselling, and interpersonal psychodynamic therapy. In two trials, the psychological treatment was combined with other interventions. We found evidence that psychological treatments can reduce depressive symptoms in people with dementia. There was also some evidence from two trials that CBT may reduce clinician‐rated anxiety symptoms in people with mild dementia. Due to the imprecision of our results, we could not tell whether psychological treatments had an effect on patients' quality of life, ability to perform daily activities, overall psychiatric symptoms, or cognition, or on carers' self‐rated depressive symptoms, but most studies did not measure these outcomes. Although these results are promising, the small number of studies and the variation between them in the type and duration of treatment make it difficult to draw conclusions about the best way to provide psychological treatment for people with dementia who have symptoms of depression or anxiety. More high quality trials in this area would be beneficial, including trials involving participants with MCI.
Summary of findings
Summary of findings for the main comparison.
Psychological treatment compared to treatment as usual for depression and anxiety in dementia and mild cognitive impairment
| Psychological treatment compared to treatment as usual for depression and anxiety in dementia and mild cognitive impairment | ||||||
| Patient or population: Patients with depression and anxiety in dementia and mild cognitive impairment Settings: Community, residential care Intervention: Psychological treatment Comparison: Treatment as usual | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Treatment as usual | Psychological treatment | |||||
| Depression Geriatric Depression Scale (range of scores 0‐15, 0‐30), Cornell Scale for Depression in Dementia (range of scores 0‐38), Montgomery‐Asberg Depression Rating Scale (range of scores 0‐50) Follow‐up: 6 ‐ 48 weeks | The mean depression in the intervention groups was 0.22 standard deviations lower (0.41 to 0.03 lower) | 439 (6 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.22 (‐0.41 to ‐0.03) | ||
| Anxiety RAID Rating Anxiety in Dementia. Scale from: 0 to 54. Follow‐up: 15 ‐ 48 weeks | The mean anxiety RAID ranged across control groups from 17.89 to 17.2 points2 | The mean anxiety RAID in the intervention groups was 4.57 lower (7.81 to 1.32 lower) | 65 (2 studies) | ⊕⊕⊝⊝ low3,4 | Higher scores indicate higher levels of anxiety symptoms | |
| Anxiety Self ratings Hospital Anxiety and Depression Scale (range of scores 0 ‐ 21), Geriatric Anxiety Inventory (range of scores 0‐20) Follow‐up: 15 ‐ 48 weeks | The mean anxiety self ratings in the intervention groups was 0.05 standard deviations higher (0.44 lower to 0.54 higher) | 65 (2 studies) | ⊕⊕⊝⊝ low5,6 | SMD 0.05 (‐0.44 to 0.54) | ||
| Anxiety NPI‐A Follow‐up: 6 months | The mean anxiety NPI‐A in the intervention groups was 2.4 lower (4.96 lower to 0.16 higher) | 26 (1 study) | See comment | Higher scores indicate higher levels of anxiety symptoms | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In two of the studies blinding of outcome assessment is not sufficiently reported. There is evidence of selective reporting at least in 1 study. Only 1 study describes blinding of personnel. 2 These values are based on scores in the control group across studies in the systematic review 3 Potential selection bias in one of the studies included (allocation concealment was not specified). 4 Small number of studies 5 Potential selection bias in one of the studies included (allocation concealment was not specified). 6 Small number of studies
Background
Description of the condition
There are particular difficulties defining and diagnosing depression in the context of dementia. It is only relatively recently that attempts have been made to produce inclusion and exclusion criteria for the diagnosis of depression specifically in Alzheimer's disease (AD), based on affective and behavioural features (Olin 2002). Prevalence rates of depressive symptoms in people with dementia range from 10% to 62% (Enache 2011), with lower rates (0.9% to 4.8%) reported when employing strict criteria for major depression (Weiner 2002). A recent review shows that approximately 20% to 30% of patients with AD have depression, with higher rates in vascular dementia (VaD) and dementia with Lewy bodies (DLB) (Enache 2011). Being of younger age, experiencing significant medical conditions or having a family history of depression increases the risk of experiencing depressive symptoms (Gellis 2009). In MCI, rates of depressive symptoms are reported as moderate to high, ranging from 36% (Palmer 2007) to 63% (Solfrizzi 2007).
Although anxiety symptoms are common both in dementia and MCI (Absher 1994; Hwang 2004), there is a lack of consensus about how to define and conceptualise anxiety in dementia. Studies have employed diagnostic criteria (for example the Diagnostic and Statistical Manual of Mental Disorders‐IV (DSM‐IV)) or measures of anxiety symptomatology (Ferreti 2001). There are contrasting views in the literature as to whether anxiety and agitation are the same or different constructs (Ballard 1996; Hersen 1992). Recently Starkstein 2007 proposed a revision of diagnostic criteria for generalized anxiety disorder (GAD) in individuals with dementia consisting of excessive anxiety and worry (criteria A and B of the DSM‐IV) and any three of the symptoms of restlessness, irritability, muscle tension, fears and respiratory symptoms. Prevalence estimates in dementia range from 8% to 71% for symptoms of anxiety (Ballard 2000) and 5% to 21% for anxiety disorders (Ferreti 2001). In people with MCI, anxiety symptoms range from 10% to 74% (Monastero 2009). Relatively less is known about the prevalence of anxiety disorders in MCI, with rates up to 49% (Lopez 2005) when using the Consortium to Establish a Registry for Alzheimer’s Disease Behavioral Rating Scale for Dementia (CERAD‐BRSD) criteria. It is notable that anxiety and depression can coexist in individuals with dementia and MCI with common symptoms across both affective states, which can add to the difficulties of definition (Seignourel 2008).
Previous studies indicate that the frequency of depressive and anxiety symptoms varies as a function of the severity of dementia. For example, experiencing depression is more common in patients with mild to moderate dementia, whereas elevated levels of anxiety may be more frequent in later stages of the disease (Lopez 2003). Different types of dementia (for example vascular versus AD) have also been associated with different prevalence rates of depression and anxiety (Groves 2000; Sultzer 1993).
Regarding the impact of the conditions, consistent evidence suggests that the severity of depression increases the severity of neurological impairments, institutionalisation (Lyketsos 1997; Stern 1997), and levels of caregiver burden (Gonzalez‐Salvador 1999) in people with dementia. Anxiety is associated with decreased independence (Schultz 2004), increased risk of nursing home placement (Gibbons 2002), and can lead to an overestimation of dementia severity (Yesavage 1984). Early symptoms of depression in MCI can be persistent and refractory to antidepressant medication (Devanand 2003), and increase the risk of developing dementia at follow‐up (Li 2001). In fact both depressive (Gabryelewicz 2004) and anxiety symptoms (Palmer 2007) in people with MCI predict higher rates of progression to AD (Lu 2009) and are often implicated in greater impairments in activities of daily living (Rozzini 2009).
Description of the intervention
The main psychotherapeutic approaches in treating depression and anxiety in adults, according to the World Health Organization (WHO), are cognitive behavioural therapy (CBT), psychodynamic therapy, interpersonal therapy (IPT), and supportive counselling (Rogerian person‐centred therapy) (WHO 2007). Previous work has concluded that offering psychotherapy to older people is worthwhile as it promotes improvements in depression and increases general psychological well‐being as well as reducing depression in seniors with mental disorders (Pinquart 2001). However, several systematic reviews of psychological treatments in older adults experiencing depression (Gould 2012; Wilson 2008) find that there is currently no strong support for psychotherapeutic treatments in the management of depression in older adults due to the paucity of high quality research (Gould 2012; Wilson 2008). Recent studies indicate that specific biological and psychological aspects of aging such as executive dysfunction may impact on the effectiveness of treatments for late life depression and anxiety disorders (Areán 2010; Mohlman 2005). Several studies have investigated the benefits of adapting psychological therapies in order to accommodate age‐related clinical diversity (Yost 1986). Amongst the adaptations are the emphasis on behavioural techniques, use of different sensory modalities and repeating information in order to compensate for age‐related losses in attention and declining sensory function (Grant 1995).
A wide variety of psychological interventions have been studied within the context of improving affective function for patients with cognitive impairment. Behavioural management therapy techniques include specific caregiver training to reduce the occurrence of behavioural symptoms and modify precipitants of patient distress that impair daily functioning Teri 2003. Relaxation therapy or training has often been used as an attention placebo control comparison in psychological therapy trials, but recent reviews (Borkovec 2001; Gould 1997) have considered this approach as an active behavioural therapeutic intervention. Other psychotherapies (that is psychodynamic, interpersonal therapy, supportive psychotherapy), where the primary focus is to increase patient well‐being and psychological function (Rosenthal 1999), have been less frequently evaluated in people with dementia. It should be noted that there are several areas of overlap between these therapies and current research suggests that the various approaches are not used in isolation (Ballard 2001).
Although there are a number of other types of interventions which incorporate some psychological elements and which are used to target anxiety and depression in dementia, for example reminiscence (Woods 2012) and interventions focusing on environmental changes (Gitlin 2003) or exercise (Rolland 2007), the present re
view focuses on psychological interventions, that is interventions primarily based on psychological models as defined by WHO 2007. Interventions that involve reminiscence therapy are the subject of another Cochrane review (Woods 2012).
How the intervention might work
Most psychotherapies emphasize dysfunctional beliefs as well as incorporating components of behavioural therapy aiming to challenge negative cognitions that maintain depressive symptomatology and anxiety symptoms (WHO 2007). An important component of CBT is monitoring and identifying thoughts and behaviours that contribute to depression or anxiety, or both (Beck 1979). Treatment components of CBT for anxiety in older adults often include additional techniques such as teaching older adults relaxation skills (Stanley 2004). Modifications of CBT target cognitive strategies in early‐stage dementia and behavioural strategies in later stages, by aiming to reduce the cognitive load on the person by increasing repetition of information and utilizing concrete examples (Gellis 2009). Amongst the modifications to the therapeutic process for people with dementia are slowing the pace of therapy and simplifying patient‐therapist communications (Robie 1999). Implementing CBT in people with cognitive impairment requires providing the therapy in a highly structured format as well as monitoring that participants understand the therapeutic material presented (Gellis 2009).
Behavioural therapies for people with dementia involve both patients and caregivers in the therapeutic approach Teri 2003. In these training programs caregivers are encouraged to identify pleasant activities for patients, which encourage positive interactions and increase the involvement of physical and social activity Teri 2003. Other psychological therapies (that is supportive psychotherapy) encourage focusing on past successes and limiting social contacts to those that are reinforcing and emotionally rewarding (Novalis 1993).
Why it is important to do this review
Recent recommendations stress that the treatment of anxiety and depressive symptoms should be an essential part of the treatment of AD and other dementias (Alexopoulos 2005; Azermai 2012). Currently, pharmacological approaches are commonly used for anxiety and depression in dementia, despite recent research suggesting poor efficacy and side effects of antidepressants (Banerjee 2011). Symptoms of anxiety and depression may contribute to the overuse of antipsychotics, which are associated with substantial adverse effects such as an increased risk of sedation, falls and death (van der Hooft 2008). Evidence that more than 40% of people with dementia are being prescribed antipsychotic drugs indicates inappropriate and unnecessary prescribing (Margallo‐Lana 2001).
Although most current clinical practice guidelines (Salzman 2008) recommend the use of non‐pharmacological interventions as the first line of approach in treating both anxiety and depression in dementia (Hogan 2008), empirical evidence supporting the efficacy of psychological interventions is rather sparse (Doody 2001). Previous reviews have concluded that there is a lack of evidence that psychological treatments improve patients' affective function (Douglas 2004; Lyketsos 2002a), however these reviews often include a broad family of interventions that are termed 'psychological'. The evidence base, therefore, for psychological treatment in dementia is based on reviews of studies that include a variety of behavioural and cognitive treatments that are not based on well‐defined theory‐driven psychological interventions or are not primarily aimed at people with dementia.
The results of the present review will be useful for establishing guidelines and recommendations for reducing depression and anxiety in older adults with cognitive impairment, given that current evidence is inconclusive.
Objectives
Primary objective
To assess the effectiveness of (1) psychological interventions in reducing anxiety and depression in people with dementia or mild cognitive impairment (MCI).
Secondary objectives
To determine whether (2) psychological interventions improve patient quality of life, cognition, activities of daily living, and reduce behavioural and psychological symptoms of dementia other than anxiety and depression compared to usual care, and (3) whether psychological treatments improve caregiver quality of life or reduce carer burden.
Methods
Criteria for considering studies for this review
Types of studies
We included studies in this review if they fulfilled the following criteria:
they were randomised controlled trials (RCTs), including cluster randomised trials;
included a control group (usual care) or a comparison group receiving no specific psychological intervention;
provided adequate information about study design and results (including means, standard deviations (SDs), and numbers of participants (n));
provided separate data on participants with dementia and MCI if the study was of a mixed population (e.g. also including older adults with normal cognition).
Ongoing studies were identified but were not included in the meta‐analysis.
Types of participants
The inclusion criteria for participants were:
older adults diagnosed with dementia, Alzheimer's disease, organic brain syndrome, etc. according to the DSM‐IV, International Classification of Diseases‐10 (ICD‐10), or comparable, and participants with a diagnosis of mild cognitive impairment (MCI). Any definition of MCI was acceptable as long as the criteria used were published and included evidence of objective cognitive impairment but no dementia (e.g. Petersen 1999; Petersen 2003; Visser 2005);
any setting (e.g. home, community, institution).
Types of interventions
For the purposes of this review, we defined a psychological intervention as an intervention that: (a) was designed to reduce anxiety and depression or improve adaptive functioning, or both, b) was based on a psychological theory (for example learning theory), and c) involved a structured interaction between a facilitator and a participant which incorporated psychological methods (for example behavioural, cognitive behavioural, family systems), in line with previous meta‐analytic studies (Pai 2006). We included interventions facilitated by psychologists, therapists in training, and other trained professionals. We grouped eligible interventions, where possible, into the following.
Cognitive behavioural therapies (which include cognitive behavioural therapy (CBT), cognitive analytic therapy (CAT), behavioural therapy, behaviour management therapy, brief rational insight and problem‐solving therapy).
Relaxation training therapies (i.e. progressive muscle relaxation).
Psychodynamic therapies (including brief psychotherapy and insight orientated psychotherapy).
Interpersonal therapies (ITP).
Supportive or counselling therapies.
We excluded treatments identified as medication, exercise, reminiscence therapy, music therapy, art and drama therapy, befriending or bibliotherapy. If an intervention could not be grouped into any of the preceding categories, we classified it as psychological and included it in the review if there was some attempt to teach participants skills to reduce psychological distress such as depression and anxiety. We included both individual and group psychotherapies. The psychotherapy could be of any intensity, duration or frequency. Control conditions included no treatment (usual care) or a comparison group engaging in non‐specific psychosocial activity (for example attention‐control, by controlling for effects of staff attention or social contact). We did not consider comparisons with other therapeutic interventions in this review. We included studies that used combinations of different psychological treatments, or combinations of pharmacological and psychological interventions. In the case of combinations of pharmacological and psychological treatments, a comparison group of the pharmacological intervention alone or with the above control treatments was required.
Types of outcome measures
We included studies if they reported an outcome measure of depression or anxiety, measured by a standardised test.
Primary outcomes
Measures of depression (e.g. Cornell Scale for Depression in Dementia, Hamilton Depression Scale, Beck Depression Inventory, Geriatric Depression Scale) and anxiety (e.g. The Worry Scale, Rating of Anxiety in Dementia Scale). We included clinician, carer and patient ratings.
Secondary outcomes
Measures of patient quality of life, cognition, daily activity level (e.g. ADLs), and frequency of neuropsychiatric symptoms (e.g. Neuropsychiatric Inventory (NPI))
Caregivers' quality of life, and experience of caregiver burden
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group (CDCIG) Specialized Register. We searched all "Treatment MCI" and "Treatment Dementia" studies in combination with the following terms or phrases: Depression or Dysthymi* or "Adjustment Disorder/s" or "Mood Disorder/s" or "Affective Disorder/s" or "Affective Symptoms", Anxiety or Anxious or phobia/s or "Panic Disorder", psychotherapy, "cognitive therapy", "behaviour therapy", "cognitive behaviour therapy". ALOIS is maintained by the Trials Search Co‐ordinator for CDCIG and contains dementia and cognitive impairment studies identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINHAL, PsycINFO and LILACS.
Monthly searches of a number of trial registers: meta Register of Controlled Trials (mRCT); Umin Japan Trial Register; World Health Organization International Clinical Trials Registry Platform (ICTRP/WHO) portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical Trials Register; German Clinical Trials Register; Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others).
Quarterly searches of the Central Register of Controlled Trials (CENTRAL) in The Cochrane Library.
Six‐monthly searches of a number of grey literature sources: ISI Web of knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
We ran additional separate searches in many of the above sources to ensure that the most up‐to‐date results were retrieved. The search strategy that was used for the retrieval of reports of trials from MEDLINE (via the OvidSP platform) can be seen in Appendix 1. These searches were completed in January 2013.
Searching other resources
We searched identified citations for additional trials and contacted the corresponding authors of identified trials for additional references and unpublished data. We scanned the reference lists of identified publications and all review papers that were related to depression and anxiety in dementia.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Selection of studies
Two review authors (VO, AQ) worked independently to identify RCTs that met the inclusion criteria. We discussed any disagreements with the fourth author (MO) and third author (AS). We excluded those studies that clearly did not meet the inclusion criteria and obtained copies of the full texts of potentially relevant references. We documented reasons for exclusion of studies. Where necessary, we requested additional information from study authors.
Data extraction and management
Two review authors (VO, AQ) extracted data independently using a standardized data extraction form, which was piloted before use. We contacted the authors of the primary trials if there were doubts regarding missing data or methodological details of the trial. The extracted information included data on methods, participants, interventions, outcomes and results.
Methods: in relation to the methods used, data were extracted on methodologies used for randomisation.
Participants: items related to participant characteristics were number, gender and age of participants, diagnostic criteria, and other items of importance (exclusion criteria for patients).
Interventions: data relevant to the interventions used were specifics of duration, intensity, type, and frequency of the psychological intervention.
Outcomes: primary outcomes included ratings of anxiety and depression. Secondary outcomes included measures of quality of life, daily activity level (for example ADLs), and frequency of neuropsychiatric symptoms (e.g. NPI) for the patient. Secondary outcome measures for the caregiver were quality of life and experience of caregiver burden.
Results: summary statistics for each RCT were the mean value of the outcome measurements in each intervention group, standard deviations of the outcome measurements in each intervention group, and the number of participants for whom the outcome was measured in each intervention group (in the case of continuous data).
Assessment of risk of bias in included studies
We employed the recommended approach by the Cochrane Handbook for Systematic Reviews of Interventions for assessing risk of bias. This addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other issues. In each domain, the review authors worked independently in relation to input of entries in a risk of bias table and we resolved differences by discussion or by appeal to a third author (MO or AS). In cases where no information was available in order to make a judgment, this was explicitly stated. We summarised results in both a 'Risk of bias' graph and a 'Risk of bias' summary. Results of the meta‐analysis were interpreted by considering the findings with respect to risk of bias.
Measures of treatment effect
For five of the analyses conducted, the studies that were pooled used different scales, therefore the absolute mean difference divided by the standard deviation (the standardised mean difference) was used. We did not identify any binary outcomes.
Unit of analysis issues
We did not include any cluster randomised trials or trials with multiple treatment groups.
Dealing with missing data
We recorded missing data and dropout rates for each individual RCT. We reported the number of participants included in the final analysis as a proportion of all participants in the study.
Assessment of heterogeneity
We assessed heterogeneity between the included studies by using a formal statistical test of the significance of heterogeneity (Chi2 test) (Deeks 2001). We considered P values < 0.10 to be statistically significant. We quantified heterogeneity by using the I2 statistic.
Assessment of reporting biases
See sections below.
Data synthesis
We used a fixed‐effect model to present overall estimated effects. We used Review Manager 5 in order to conduct the meta‐analysis. We used standardised mean differences in some of the analyses as not all studies used the same outcome scale.
Subgroup analysis and investigation of heterogeneity
There was little heterogeneity between studies. We did not conduct any subgroup analyses.
Sensitivity analysis
We did not conduct any sensitivity analyses.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
A total of 349 references were identified through database searching, which was conducted from December 2011 to January 2013. Three additional references were identified by the authors via other sources. After removal of duplicates and clearly irrelevant articles, 62 full text records were retrieved. Of these 62 references, 22 could be excluded at this stage as not being relevant, leaving 40 full text references to be fully assessed for eligibility. Of these, a total of 32 studies were excluded as they did not meet the review criteria (see Characteristics of excluded studies), one study is ongoing (see Characteristics of ongoing studies) and one study is awaiting classification (see Characteristics of studies awaiting classification). Thus six studies were found to be eligible for inclusion. See Figure 1 for a PRISMA flow diagram detailing the search process.
Figure 1.
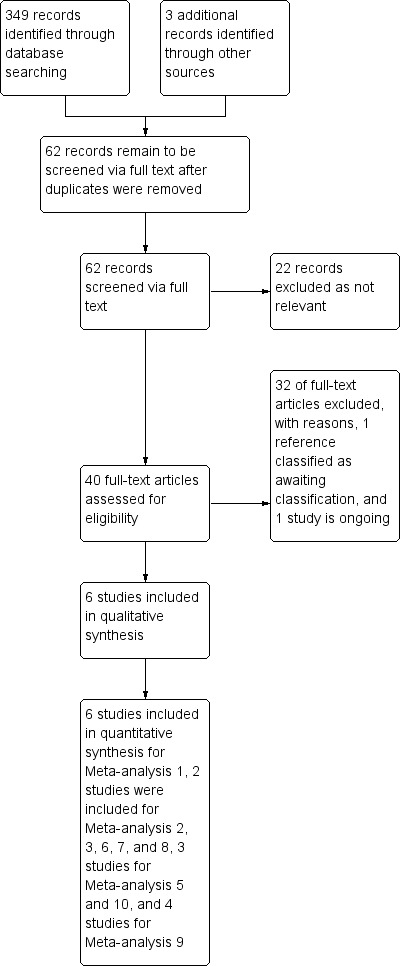
Study flow diagram.
Included studies
Six studies met the inclusion criteria for this review: Burgener 2008; Burns 2005; Spector 2012; Stanley 2012; Tappen 2009 and Waldorff 2012 (see Characteristics of included studies). All six trials were included in the first analysis on effects of psychological treatment on depression. For the second set of analyses two studies (Spector 2012; Stanley 2012) contributed to evaluating the effects of psychological treatment on anxiety. Three of the studies (Spector 2012; Stanley 2012; Waldorff 2012) were included in the third analysis on effects of self‐rated quality of life. Two studies (Spector 2012; Waldorff 2012) were included in the analysis on carer‐rated quality of life, and ADLs (Burns 2005; Waldorff 2012). Data from Spector 2012 and Waldorff 2012 were pooled in the analyses on effects of treatment on neuropsychiatric symptoms. A total of four studies contributed to the analysis for effects of treatment on cognition (Burgener 2008; Burns 2005; Spector 2012; Waldorff 2012). Data from Spector 2012, Stanley 2012 and Waldorff 2012 were pooled for the final analysis on effects of carer depression. All the included studies were published in English. Authors were contacted for clarification of the procedures of randomisation, blinding and additional data. The included studies varied in the following: (1) participant characteristics such as setting, severity of dementia or cognitive function; (2) number of treatment sessions and duration of the psychological intervention; (3) type of psychological treatment evaluated; (4) control group characteristics; and (5) outcome measures. We contacted five authors for additional data and information in relation to the included studies. Three authors responded to the call for provision of additional data or information. An overview of 'Characteristics of included studies' can be seen in Table 5.
Table 1.
Overview of characteristics of included studies
| Study | Sample | Method | Measures | Intervention | Outcome Data Timepoints |
| Burgener 2008 | n = 43 Inclusion criteria (1) confirmed diagnosis of dementia (AD, Lewy body, vascular, frontal lobe, or mixed dementia) (2) CDR < 2.0 |
RCT |
Patient Outcomes Depression Geriatric Depression Scale (GDS‐15) Other behavioral outcomes Rosenberg’s Self‐Esteem Scale (SES) |
Type Multimodal CBT including Tai Chi, CBT, and support group Duration 20 weeks (note that intervention lasted 40 weeks) |
Outcome data included in the Review 20 weeks |
|
Baseline CDR and MMSE Treatment group CDR 1.15 MMSE 24.8 Control group CDR 1.22 MMSE 22.9 |
Control group Attention‐control educational program |
Cognition Mini‐Mental State Examination (MMSE) Physical function Single leg stance (SLS) Berg Balance Scale (BBS) Cumulative Illness Rating Scale (CIRS) |
Intensity/Frequency Tai Chi 3 times a week (60 minutes) CBT 2 times a week (90 minutes) Support group 2 times a week (90 minutes, alternating with CBT) |
Outcome data reported (for both groups) at 20 weeks control group enters treatment at 20 weeks, scores at 40 weeks (representing no treatment) not available |
|
| Burns 2005 | n = 40 Inclusion criteria 1) diagnosis of AD ‐ NINCDS–ADRDA criteria 2) CDR of 1 3) MMSE ≥ 15 4) living in own home with carer in regular contact 5) ability to communicate verbally |
RCT |
Patient Outcomes Depression Cornell Scale for Depression in Dementia (CSDD) Function Activities of Daily Living (BADLS) Other patient outcomes Cognition Mini‐Mental State Examination (MMSE) Clinician’s Interview‐Based Global Impression of Change (CIBI) |
Type Psychodynamic interpersonal therapy based on interpersonal theory Duration 6 weeks |
Outcome data included in the Review 6 weeks |
|
Medication 20% on antidepressants 68% on cholinesterase inhibitors Baseline MMSE Treatment group MMSE 24.4 Control group MMSE 21.5 |
Control group Standard treatment in AD, (general advice on diagnosis and treatment of dementia, with out‐patient review) |
Carer outcomes Revised Memory and Behavior Problems Checklist (RMBPC) ‐ Carer's reaction General Health Questionnaire (GHQ‐12) Beck Depression Inventory (BDI‐II) (scores not reported) Ways of Coping Checklist (WCCL) |
Intensity/Frequency Once a week (50 minutes ‐ of which 10 minutes are spent with carer) |
Outcome data reported at 6 weeks (end of the intervention) and 3 months |
|
| Spector 2012 | n = 50 Inclusion criteria 1) Meet DSM‐IV criteria for mild to moderate dementia, CDR of 0.5, 1 or 2 2) Clinical anxiety, ≥ 11 on the RAID 3) Living in the community 4) Presence of carer willing to participate in the therapy 5) Ability to understand and communicate in English 6) Willing to engage in therapy Exclusion criteria 1) Co‐morbid psychiatric disorder or challenging behaviour 2) Learning disability or severe physical illness |
RCT |
Patient outcomes Rating Anxiety in Dementia (RAID) Clinical Services Receipt Inventory (CSRI) Secondary outcomes Hospital Anxiety and Depression Scale (HADS) Quality of Life‐Alzheimer’s Disease (QOL‐AD) (self and proxy ratings) Neuropsychiatric Inventory (NPI) Mini‐Mental State Examination (MMSE) Cornell Scale for Depression in Dementia (CSDD) Quality of Caregiver and Patient Relationship (QCPR) |
Type CBT identifying strategies for feeling safe, calming thoughts and behavioural experiments, with telephone contact offered between sessions. Carers involved Duration 15 weeks |
Outcome data included in the Review 15 weeks |
|
Control group Standard treatment including medication or no treatment |
Carer outcomes Hospital Anxiety and Depression Scale (HADS) Quality of Caregiver and Patient Relationship (QCPR) |
Intensity/Frequency 10 sessions (60 minutes) for 15 weeks, and telephone contact |
Outcome data reported at 15 weeks (end of intervention) and 6 months | ||
| Stanley 2012 | n = 32 Inclusion criteria 1) diagnosis of dementia (confirmed by the patient’s medical provider) 2) an NPI‐A ≥ 4 3) CDR score of 0.5 ‐ 2.0 Exclusion criteria ‐primary psychiatric diagnosis of major depression ‐active psychosis, bipolar disorder, active suicidal intent ‐recent verbal or physical aggression |
RCT |
Patient outcomes Depression Geriatric Depression Scale (GDS) (self report) Quality of Life Quality of Life in Alzheimer disease (QOL‐AD) (self report) Other outcomes Anxiety Neuropsychiatric Inventory–Anxiety (NPI‐A) subscale (collateral report) Rating of Anxiety in Dementia (RAID) (administered as a clinical interview to patient and carer) Geriatric Anxiety Inventory (GAI) (self report) Worry Penn State Worry Questionnaire–Abbreviated (PSWQ‐A) (self report) |
Type CBT targeting anxiety (self‐monitoring/deep breathing), written materials on dementia education, collateral stress and communication. Carers involved as coaches Duration 6 months |
Outcome data included in the Review 6 months |
|
Dementia diagnosis AD (62.5%), DLB (3.1%), VaD (9.4%) and 25% dementia not otherwise specified (NOS) Principal DSM‐IV Diagnoses 43.8% GDA, 18.8% other anxiety disorder, 43.8% comorbid anxiety and depression, 34.4% no diagnosis, 3.1% a depression diagnosis Dementia Severity 46.9% CDR of 0.5 or 1 Medication 75% on at least one psychotropic medication |
Control group Receives diagnostic feedback |
Carer Outcomes Depression Patient Health Questionnaire (PHQ‐9) Distress Distress item from the NPI‐A |
Intensity/Frequency 12 weekly sessions (30 to 60 minutes) for 3 months, 8 telephone booster appointments during months 3–6 |
Outcome data reported at 3 and 6 months (end of intervention) | |
| Tappen 2009 | n = 36 Inclusion criteria 1) diagnosis of probable AD, NINCDS‐ADRDA criteria 2) MMSE ≤ 25 3) ability to speak English Exclusion criteria 1) Individuals entirely mute |
RCT |
Patient Outcomes Depression The Montgomery‐Asberg Depression Rating Scale (MADRS) |
Type Modified counselling offered individually consisting of therapeutic conversation Duration 16 weeks |
Outcome data included in the Review 16 weeks |
|
Mean stay in nursing care Treatment group 561 days Control group 595 days Baseline MMSE Treatment group 10.60 Control group 12.26 |
Control group Usual care (not an attention‐control group) |
Other Patient Outcomes Mood Dementia Mood Assessment Scale (DMAS) The Alzheimer’s Disease and Related Disorders (AD‐RD) Mood Scale |
Intensity/Frequency 3 times a week (30 minutes) |
Outcome data reported at 16 weeks (end of intervention) |
|
| Waldroff 2012 | n = 330 Inclusion criteria 1) community‐dwelling 2) age ⋝ 50 years 3) MMSE ⋝ 20 4) diagnosis of probable AD, mixed AD with vascular components or DLB, established in the last 12 months, meeting DSM‐IV, NINCDS‐ADRDA or the McKeith 1996 criteria for DLB Exclusion criteria 1) severe somatic or psychiatric comorbidity 2) participating in other intervention studies 3) residing in a nursing home at baseline 5) diagnosis of frontotemporal dementia |
RCT |
Patient Outcomes Depression Cornell Scale for Depression in Dementia (CSDD) Function Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale (ADSC‐ADL) Quality of Life Quality of Life in Alzheimer's Disease (patient and proxy ratings) (QoL‐AD) Other outcomes EuroQoL VAS (patient and proxy ratings Quality of Life) (EQ‐VAS) Neuropsychiatric Inventory Questionnaire (NPI‐Q) Mini Mental State Examination (MMSE) |
Type Mutlifaceted and semi‐tailored intervention consisting of counselling sessions, teaching, education and outreach telephone support to patients and carers. Provided individually and as a group intervention Duration 8 to 12 months |
Outcome data included in the Review 12 months |
|
Dementia diagnosis AD 72.4%, Mixed or VaD 24.8%, DLB 2.7% Medication 93.3% on cholinesterase inhibitors 1% memantine Baseline MMSE Treatment group 24.0 Control group 24.1 |
Control group Provided with overall information and guidance, directed towards local support programmes (provided to both the control and treatment group) |
Carer outcomes Depression Geriatric Depression Scale (GDS) Other outcomes Quality of Life EuroQoL VAS (EQ‐VAS) |
Intensity/Frequency 6 counselling sessions (plus one optional session) 5 educational courses (2 hours) 5‐8 telephone support calls within 3‐4 week intervals |
Outcome data reported at 6 and 12 months (end of intervention) |
Design
All six studies were randomised controlled trials (RCTs) and evaluated the effectiveness of a psychological intervention aimed to reduce depression or anxiety, or both, or to improve mood and well‐being in people with dementia.
Sample size
There were 43 participants in Burgener 2008, 40 in Burns 2005, 50 in Spector 2012, 32 in Stanley 2012, 36 in Tappen 2009, and 330 in Waldorff 2012.
Settings
Burns 2005 and Spector 2012 were conducted in the UK and recruited patients living in the community. Stanley 2012 recruited participants from primary care outpatient clinics and community day centres specializing in dementia care, in Houston, Texas. Tappen 2009 was based at a large long‐term care facility in Miami, Florida. Waldorff 2012 was conducted in community settings in the Frederiksberg and Copenhagen municipalities in Denmark. Burgener 2008 was conducted in the USA and did not specify the setting.
Participants
In Burgener 2008 all patients had a confirmed diagnosis of dementia (Alzheimer's, Lewy body, vascular, frontal lobe, or mixed dementia) and a score of < 2.0 on the Clinical Dementia Rating Scale (Hughes 1982) indicative of mild disease stage. Diagnostic criteria used for the presence of dementia were not specified. Authors mentioned that all participants were screened using the Mini‐Mental State Examination (MMSE) and the Clinical Dementia Rating (CDR). Participants were on average 77 years of age and 53% were male. There were no differences in the two groups at baseline in terms of demographic characteristics, cognitive functioning, physical and behavioral measures, or baseline morbidity. The mean MMSE (Folstein 1975) scores were 24.8 and 22.9 for the treatment and control groups respectively.
In Burns 2005, all patients had a diagnosis of Alzheimer's Disease according to the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS‐ADRDA) criteria McKhann 1984, a CDR of 1 (Morris 1993) indicating mild dementia and a score of 15 or above on the MMSE (Folstein 1975). Participants lived in their own home, they had a mean age of 75 years and 52% were male. A total of 40% of the sample were on antidepressants and approximately two thirds of each group (37% of total sample) were on cholinesterase inhibitor drugs. The mean MMSE (Folstein 1975) scores were 24.4 and 21.5 for the treatment and control groups respectively.
Spector 2012 recruited a total of 50 people with dementia who were living in the community (60% females). All participants had a diagnosis of dementia in the mild to moderate range according to DSM‐IV criteria. The mean age was 78 years, and the MMSE mean score was 20.9. Approximately half of the sample had a rating of 1 on the CDR, with the remaining participants scoring 0.5 or 2. Clinical anxiety was determined by a score of 11 or above on the Rating Anxiety in Dementia scale (RAID). The mean anxiety rating (RAID) at baseline was 19.7, and the mean depression rating (Cornell Scale for Depression in Dementia (CSDD) was 15.9. Patients with a comorbid psychiatric disorder, challenging behaviour, learning disability or severe physical illness that could impact on participation were excluded.
In Stanley 2012, anxiety was indicated by a positive response to at least one of three screening questions from the NPI–Anxiety (NPI‐A) subscale (Cummings 1994). Participants had a diagnosis of dementia (confirmed by the patient’s medical provider), an NPI‐A ≥ 4, and a CDR score of 0.5 to 2.0. Patients with a primary psychiatric diagnosis of major depression, psychosis, bipolar disorder, suicidal intent, or verbal or physical aggression were excluded. Participants had a mean age of 78 years and 59% were female. Most of the sample had AD, with the remaining participants having a diagnosis of dementia with Lewy bodies (DLB), vascular dementia (VaD), or dementia not otherwise specified. Nearly half of the sample scored a CDR of 0.5 or 1, and 75% were prescribed at least one psychotropic medication. A total of 43.8% had a diagnosis of GDA, 18.8% a diagnosis of other anxiety disorder, 43.8% comorbid anxiety and depression, 34.4% no diagnosis and 3.1% a depression diagnosis.
For the Tappen 2009 study, all patients were nursing home residents with probable AD using the NINCDS‐ADRDA criteria (McKhann 1984) and a MMSE score (Folstein 1975) of 25 or less (range 0 to 25). The length of stay in the facility ranged from 160 to 1750 days, with a mean of 561 and 595 days for the treatment and control groups respectively (this difference was not significant). Mean age was 87 years, with the treatment group being younger compared to the control group. The mean MMSE of the treatment group was 10.60, and 12.26 for the control group (not significant).
Waldorff 2012 included community‐dwelling patients (age ≥ 50 years, mild dementia with MMSE ≥ 20) with mild recently diagnosed dementia. All participants had a diagnosis of probable AD, mixed AD or DLB established in the last 12 months, meeting the DSM‐IV criteria, NINCDS‐ADRDA criteria (McKhann 1984) for probable AD or the McKeith 1996 criteria. The mean age for participants was 76 years and 45.8% were male. Of the 330 patients, 72% had AD, 25% had mixed AD and VaD, and 3% DLB. Patients received antidementia treatment which was reported as either cholinesterase inhibitors (93%) or memantine (1%). Patients with severe somatic or psychiatric comorbidity, those residing in a nursing home at baseline, or those with a diagnosis of frontotemporal dementia were excluded. The mean MMSE of the treatment group was 24.0, and 24.1 for the control group.
Psychological interventions
The type of psychological intervention varied from therapeutic counselling (therapeutic conversation, counselling with education and support) to CBT (combined with social support and Tai Chi exercises, CBT aimed at reducing anxiety) to interpersonal psychodynamic therapy. Burgener 2008 tested the effectiveness of a multimodal intervention consisting of Tai Chi exercises, CBT and support group participation compared to an attention‐control educational program. Tai Chi exercises consisted of strength and balance training adapted for people with dementia, with components from traditional Taiji training (that is form choreography, dynamic Qigong, and standing and sitting meditation). Instructors had received extensive training in Tai Chi, with a minimum of five years of practice. The CBT intervention included both small groups and individual therapy sessions (based on individual needs). Each session lasted for 90 minutes and used standard CBT treatments for mood following guidelines by Teri 1991. Cognitive strategies were challenging dysfunctional cognitions, developing positive coping skills to manage the effects of the disease, and enhancing personal control. Behavioural strategies included participating in pleasant activities thereby increasing success. Social workers certified in individual and family therapy conducted the CBT intervention. The support group focused on coping and problem‐solving in relation to the effects of dementia and developing relationships with others sharing similar experiences, using the group structure for support groups outlined by Yale 1995.
Burns 2005 evaluated the effects of a brief psychodynamic interpersonal therapy program versus treatment as usual (consisting of general advice about diagnosis and treatment of dementia with out‐patient review). Interpersonal psychodynamic therapy was provided (six sessions in total) by an experienced psychotherapist and was based on the conversational model of psychotherapy (Hobson 1985) adapted for people with AD (Brierley 2003). The principal aim of the therapy was identifying interpersonal conflicts or difficulties causing emotional distress. Emphasis was placed on improving interpersonal relationships. Carers were also involved in the treatment, which included the therapist listening to their needs and informing them of therapeutic progress (10 minutes of the 50‐minute sessions). The intervention confirmed adherence to the model (Shapiro 1993), where adherence was evident by high scores on psychodynamic interpersonal therapy and generic subscales and low scores on the CBT scale.
Spector 2012 evaluated a CBT intervention for people with anxiety and dementia (10 sessions in total). The intervention included identifying and practicing strategies for feeling safe, challenging negative thoughts, and incorporating calming thoughts and behavioural experiments with the use of cue cards. The degree to which cognitive and behavioural elements were used varied between the people with dementia and clinicians were provided with specific guidelines on how to adapt the themes of each session to each individual. In between the CBT sessions telephone support was offered, which included answering questions and encouraging ongoing work. Other components included building a collaborative relationship with the therapist and socialisation to the CBT model. Carers were encouraged to be involved by supporting the person with dementia in implementing CBT strategies, such as applying the therapy content to everyday life and consolidating learned skills. The intervention was delivered by clinical or counselling psychologists with experience working with people with dementia. Participants in the control group received standard treatment available to people with anxiety and dementia, which included either medication or no treatment.
Stanley 2012 evaluated the Peaceful Mind program, a CBT–based intervention targeting anxiety in dementia, involving self‐monitoring for anxiety, deep breathing and optional skills (coping self‐statements, behavioural activation, and sleep management). Patients' learned skills and the collaterals served as coaches (in weekly skill learning, serving as a coach for the patients’ practice between sessions). The intervention was provided by two masters‐level graduate student clinicians and a predoctoral intern over six months. The program included up to 12 weekly in‐home sessions (lasting 30 to 60 minutes) over the initial three months and up to eight brief telephone booster appointments during months three to six. Modification from traditional CBT included repeated instructions, more in‐session practice, spaced retrieval, reminder cues, and simplified session summaries. Materials were provided to patients and their collaterals (Paukert 2010), including handouts to address communication, dementia education and stress reduction for the collaterals (available for use as needed). Participants assigned to usual care received diagnostic feedback but did not receive any additional study contact other than the scheduled assessments. A random sample of 20% of the sessions were reviewed by an independent treatment integrity rater indicating adequate adherence and competency.
In Tappen 2009 the intervention consisted of modified counselling sessions (therapeutic conversation), a psychotherapeutic approach modified for AD (Tappen 1997; Tappen 2001). The goals of therapeutic conversation were reducing anxiety, forming and maintaining supportive relationships, and providing an opportunity for the individual to express his or her feelings, in line with the theory of Interpersonal relations by Peplau 1991. The intervener was a graduate nursing student trained to provide the intervention. Treatment implementation was monitored by the investigator meeting with the intervener for supervision. The control group received usual care provided by the staff of the long‐term care facility.
In Waldorff 2012 the intervention comprised a multifaceted and semi‐tailored intervention which included a counselling programme (including telephone counselling and support), educational course, written information supporting patients and caregivers during the initial months after a diagnosis had been established in order to support the counselling sessions, and logbooks. The objective of the intervention was to prevent depression and impairment of quality of life in patients and caregivers, by focusing on positive resources, intact functions and retaining skills (for patients). Counselling sessions aimed towards preventing or reducing depressive symptoms, and were based on constructivist principles (Peavey 1997). The teaching course provided information about living and coping with dementia, and served as a reference guide after completion of the intervention. Outreach telephone counselling was provided to ensure regular contact and to follow up on issues discussed during the individual counselling sessions, or issues relevant to individual participants. Patients and caregivers were each supplied with a logbook encouraging them to make notes about their daily life and to prepare for the counselling sessions (optional). The control group did not receive the intervention, however, as both groups of participants were interviewed about symptoms and were informed about available support programmes it is likely that the control group received services above the level of usual care, but no structured intervention was offered.
Length, number and duration of psychological interventions
The duration of the psychological intervention varied from six weeks to 12 months, with the length of the sessions varying from 30 to 90 minutes.
In Burgener 2008, Tai Chi exercises consisted of one‐hour classes offered three times weekly for 20 weeks (although the duration of the intervention was 40 weeks, comparison data between the treatment and control groups were available for 20 weeks only). Qigong relaxation exercises encompassed approximately 30 minutes of the 60‐minute class. The CBT intervention was conducted bi‐weekly for 20 weeks, lasting 90 minutes. The support group met bi‐weekly, alternating with the CBT group, lasting also for 90 minutes.
In Burns 2005 patients received six sessions of psychodynamic interpersonal therapy, with the duration of the intervention being six weeks. The length of the sessions was 50 minutes each, of which 10 minutes were spent with the carer.
In Spector 2012 participants received a total of 10 sessions of CBT, with the duration of the treatment being 15 weeks. The length of each CBT session was 60 minutes. Participants were offered telephone support in between the sessions.
In Stanley 2012 participants received 12 weekly in‐home sessions during the initial three months and up to eight brief telephone sessions during months three to six, with the duration of the intervention being six months. Each session lasted 30 to 60 minutes and was provided at the participant's home (Paukert 2010).
In Tappen 2009 therapeutic conversation was provided three times a week, with a total duration for the intervention of 16 weeks, with each session lasting 30 minutes.
In Waldorff 2012, the intervention was provided for a duration of eight to 12 months. Counselling sessions comprised six sessions in total with an additional optional network session (that is family). The educational course was composed of a total of five sessions (two hours each). Telephone counselling was provided approximately five to eight times within three‐ to four‐week intervals. Compliance was defined as patients who had participated with their caregivers in at least three counselling sessions and three educational (teaching) sessions.
Adherence to psychological treatment
Burgener 2008 reported attendance at exercise, CBT, and support sessions. For the Tai Chi classes, 75% of participants attended all three sessions weekly, with 90% attending at least two of the three weekly sessions. The authors stated that attendance rates for the CBT and support groups were similar. In the study by Burns 2005 no adherence data were reported, but the authors reported that one session from each individual therapy was rated for adherence Shapiro 1993, with the intervention showing high scores on the model used (that is psychodynamic interpersonal therapy). In Spector 2012 a total of 56% of those in the treatment group completed all sessions, and 28% participated in more than half of the sessions. Stanley 2012 reported that the average number of in‐person sessions was eight (average duration of completed sessions was 47 minutes). Participants completed an average of 3.5 homework exercises per week and spent an average of 81 hours per week with the collateral. Between months three and six, dyads received an average of five of a possible eight telephone booster calls (66%). Participants reported using breathing most often (58%), followed by behavioural activation (50%) and calming thoughts (41%). In Tappen 2009 treatment implementation was monitored. Waldorff 2012 defined compliance as the rate of adherence to the major parts of the intervention (that is participating in at least half of the counselling and educational teaching sessions). A total of 72% in the intervention group completed the intervention.
Outcomes
All studies reported outcomes immediately after the intervention was finished, with the exception of the study by Burgener 2008 in which outcome data for the control group at the end of the intervention were not available. The following time points were included in the present review: for Burgener 2008 depression and cognition were measured at 20 weeks; for Burns 2005 depression, ADLs and cognition were measured at six weeks (end of treatment); for Spector 2012 depression, anxiety, self‐ and carer‐rated quality of life, neuropsychiatric symptoms, cognition and carer depression were measured at 15 weeks (end of intervention). We extracted data from Stanley 2012 for depression, anxiety, self‐rated quality of life, and carer depression at six months (end of intervention); for Tappen 2009 depression at 16 weeks (end of treatment), and for Waldorff 2012 depression, self‐ and carer‐rated quality of life, ADLs, neuropsychiatric symptoms, cognition and carer depression measured at 12 months (end of treatment).
Depression
Depression was measured in all studies using a standardised instrument. Burgener 2008 measured depression using the Geriatric Depression Scale (GDS) (Yesavage 1983), at baseline and at 20 weeks. Scores in this scale (15‐item GDS) range from 0 to 15, with higher scores indicating greater depression. Data from the control group at 40 weeks were not available, therefore outcome data at 20 weeks were included in the analysis. Stanley 2012 used the 30‐item GDS scale (Yesavage 1983) (range of scores 0 to 30, higher scores indicating greater depression) to measure depression at baseline, three and six months. Burns 2005, Spector 2012 and Waldorff 2012 used the Cornell Scale for Depression in Dementia (Alexopoulos 1988). Burns 2005 measured depression at baseline, six weeks and three months; Spector 2012 at baseline, 15 weeks and six months; whereas Waldorff 2012 measured depression levels at baseline, six and 12 months. For this scale scores range from 0 to 38, with higher scores indicating higher levels of depression. Tappen 2009 measured depressive symptoms with the Montgomery‐Asberg Depression Rating Scale (Montgomery 1979), which is used less often in people with dementia, measured at baseline and at 16 weeks (rated by a psychiatrist, a general practitioner and a nurse). In this scale, higher scores indicate increasing depressive symptoms (range of scores 0 to 50). Note that Spector 2012 also measured self‐reported depression for people with dementia with the Hospital Anxiety and Depressions Score (HADS), however these data were not included in the analyses.
Tappen 2009 used the Dementia Mood Assessment Scale (DMAS) (Sunderland 1988), which measures general mood as opposed to depression, including only items 1 to 17 for the analysis, and the Alzheimer’s Disease and Related Disorders (AD‐RD) Mood Scale (Tappen 2008), which represents both positive and negative moods, and specifically feelings of spirituality, content, hostility, apathy and sadness. These outcome measures were not included in the analysis on the basis that they do not specifically measure feelings of depression or anxiety but negative or positive mood in general.
Anxiety
Both Spector 2012 and Stanley 2012 measured anxiety with the Rating Anxiety in Dementia (RAID) (Shankar 1999). Scores on this 18‐item, clinician‐rated scale range from 0 to 54, with higher scores indicating higher levels of anxiety. Spector 2012 measured anxiety at baseline, 15 weeks and six months; whereas Stanley 2012 at baseline, three and six months. In Spector 2012 self‐rated anxiety by people with dementia was measured with the HADS (Zigmond 1983) at baseline, 15 weeks and six months. The HADS is a 14‐item self‐report questionnaire designed to assess generalized anxiety (a seven‐item subscale), with scores ranging from 0 to 21 (higher scores indicating greater symptoms). Stanley 2012 measured self‐ratings of anxiety by people with dementia with the Geriatric Anxiety Inventory (GAI) (Pachana 2007) at baseline, three and six months. Scores on the GAI range from 0 to 20, with higher scores indicating greater anxiety. Stanley 2012 measured anxiety based on collateral report using the Neuropsychiatric Inventory–Anxiety (NPI‐A) subscale (Cummings 1994) at baseline, three and six months. Scores on this seven‐item scale range from 0 to 12, with higher scores indicating higher levels of anxiety.
Quality of life
Spector 2012, Stanley 2012 and Waldorff 2012 measured patient quality of life (self‐ratings) using the Quality of Life in Alzheimer's Disease (QOL‐AD) (Logsdon 1999) in which scores range from 13 to 52, with higher scores indicating better quality of life. Spector 2012 measured quality of life at baseline, 15 weeks and six months; Stanley 2012 at baseline, three and six months; and Waldorff 2012 at baseline, six and 12 months. Both Spector 2012 and Waldorff 2012 used the same scale to measure carer‐rated quality of life using the QOL‐AD.
Activities of daily living (ADL)
Burns 2005 measured ADL using the Bristol Activities of Daily Living Scale (Bucks 1996), where higher scores indicate lower levels of ADL (scores range from 0 to 60). Waldorff 2012 used the Alzheimer’s Disease Cooperative Study ‐ Activities of Daily Living scale (ADSC‐ADL) (Galasko 1997). The ADSC‐ADL Inventory total score ranges from 0 (lower functioning status) to 78 (higher functioning status).
Neuropsychiatric symptoms
Both Spector 2012 and Waldorff 2012 measured neuropsychiatric symptoms. Spector 2012 used the Neuropsychiatric Inventory (NPI) (Cummings 1994) at baseline, 15 weeks and six months (12‐item NPI). Scores on the NPI range from 0 to 144 with higher scores indicating greater behavioural disturbance. Waldorff 2012 used the NPI‐Questionnaire (NPI‐Q) (Cummings 1994) with scores ranging from 0 to 28, with higher scores indicating higher levels of symptoms. Waldorff 2012 measured neuropsychiatric symptoms at baseline, six and 12 months.
Cognition
Burgener 2008, Burns 2005, Spector 2012 and Waldorff 2012 measured cognition before and after the intervention. All four trials used the MMSE (Folstein 1975), with range of scores from 0 to 30, with lower scores indicative of greater impairments in cognitive function.
Other patient outcomes
Burns 2005 provided data (detransformed log means) on behavioural problems (carer's reaction) measured by the Revised Memory and Behaviour Problems Checklist (Teri 1992). Stanley 2012 measured self‐reported experience of worry using the Penn State Worry Questionnaire–Abbreviated (PSWQ‐A) (Crittendon 2006). Waldorff 2012 provided data on proxy (carer)‐rated quality of life using the EQ‐5D (EuroQoL 1990). Spector 2012 used the Quality of the Caregiver Patient Relationship (QCPR) (Spruytte 2002) to measure patient ratings of the quality of the caregiving relationship.
Caregiver outcomes
Spector 2012 measured carer depression at baseline, 15 weeks and six months, using the HADS. Stanley 2012 measured depression in carers using the Patient Health Questionnaire (PHQ‐9) at baseline, three and six months. Scores on the PHQ‐9 range from 0 to 24 with higher scores indicating higher levels of depression. Waldorff 2012 measured carers' depressive symptoms using the GDS (Yesavage 1983). Although Burns 2005 used the Beck Depression Inventory (BDI) (Beck 1961) to measure depression in carers, data were not provided.
Other caregiver outcomes
Waldorff 2012 measured health‐related quality of life in carers using the EQ‐5D (EuroQoL 1990). Spector 2012 measured carer ratings of the quality of the caregiving relationship using the QCPR (Spruytte 2002). Spector 2012 measured caregiver distress using the NPI (Cummings 1994), whereas Stanley 2012 measured distress specifically related to anxiety symptoms using the NPI‐A subscale (Cummings 1994).
Excluded studies
We excluded a total of 32 studies. A total of 24 were excluded because the intervention did not meet the criteria for a psychological intervention. In six studies participants did not fulfil the criteria for a diagnosis of dementia or MCI. Two studies were excluded as they were not conducted as RCTs.
Studies not meeting 'Types of participants' criteria
In the studies by Abraham 1992, Hyer 2008, Kiosses 2010, Kiosses 2011, Konnert 2009 and Yesavage 1981 participants did not fulfil the criteria for a diagnosis of dementia.
Studies not meeting 'Types of interventions' criteria
In the following studies the intervention did not meet the criteria for a psychological intervention: Beck 2002; Kolanowski 2011; Lichtenberg 2005; Vespa 2002 (psychosocial activities); Brodaty 2003; Hinchliffe 1997: McSweeney 2012 (case management); Finnema 2005 (emotion‐oriented care); Fischer‐Terworth 2011 (CBT‐based environmental intervention); Fossey 2006; Proctor 1999; Selbaek 2010 (staff training); Garland 2007 (simulated family presence); Lam 2010 (CBT‐based occupational therapy), Logsdon 2010 (social support), Mittelman 1996 (individual or family counselling for carers), Onor 2007; Dartigues 2008 (cognitive intervention); Teri 2003; Prick 2011 (exercise and caregiver training); Rovner 2012 (behavioural activation for cognitive decline); Helcer 2012; Kurz 2012 (cognitive training with CBT); Laakkonen 2012 (self management).
Studies not meeting 'Types of studies' criteria
Two studies were excluded as they were not conducted as RCTs (Cheston 2003; Joosten‐Weyn Banningh2011).
Several of those studies excluded on the basis of the criteria above additionally were not conducted as an RCT: Fischer‐Terworth 2011; Garland 2007; Selbaek 2010; Yesavage 1981; or had a control or comparison group receiving an active intervention: Dartigues 2008 (psychoeducative group for carers); Fischer‐Terworth 2011 (occupational therapy); Helcer 2012 (memory training); Kiosses 2010; Kiosses 2011 (supportive therapy); Kurz 2012 (occupational therapy or carer counselling); Lam 2010 (occupational therapy); Rovner 2012 (supportive therapy); Yesavage 1981 (cognitive training).
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph which shows assessments about each risk of bias item presented as percentages across all included studies (Characteristics of included studies), and Figure 3 for a summary of our judgments about each risk of bias item for each included study.
Figure 2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
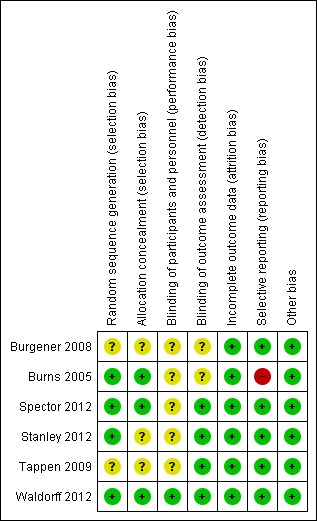
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies reported use of randomisation, however detailed descriptions varied between the studies. Burns 2005, Spector 2012 and Waldorff 2012 provided an adequate description of allocation concealment and random sequence generation and were classified as having low risk of bias in this domain. For Stanley 2012 random sequence generation was specified but there was no information about allocation concealment, therefore the study was classified as having unclear risk in this domain. Burgener 2008 and Tappen 2009 did not describe how allocation concealment and random sequence generation were performed and were therefore classified in this domain as having unclear risk of bias.
Blinding
In Waldorff 2012 raters were blind to treatment allocation and the authors stated that the personnel co‐ordinating and offering the treatment were not employed by the same institution as the raters (low risk of bias). Burgener 2008 communicated that the assessors were blind in some but not all assessments and this study was therefore classified as unclear in this domain. Burns 2005 did not report details of blinding and was also classified as unclear (no further information from the authors was provided). In Spector 2012, Stanley 2012 and Tappen 2009 raters were blind to allocation so the studies were classified as at low risk of detection bias. No information was provided for blinding of personnel, so in the domain of performance bias Burgener 2008, Burns 2005, Spector 2012, Stanley 2012 and Tappen 2009 were classified as having unclear risk of bias in this domain.
Incomplete outcome data
Burgener 2008 and Tappen 2009 reported attrition for both the treatment and the control groups, and Burns 2005, Spector 2012 and Stanley 2012 reported attrition and specified the use of intention‐to‐treat analyses, so all five studies were classified as low risk. Waldorff 2012 also specified use of intention‐to‐treat analyses and reported details of attrition, so we classified the study as low risk. Considering the characteristics of the population, attrition in the studies was relatively small, with zero attrition reported in one study (Burns 2005).
Selective reporting
Burgener 2008, Spector 2012, Stanley 2012, Tappen 2009 and Waldorff 2012 reported all pre‐specified outcomes and were classified as at low risk of bias. Burns 2005 reported all outcomes for patients but did not report depression scores for family carers (BDI) (Beck 1961). This study was classified as at high risk of bias in this domain.
Other potential sources of bias
No other apparent bias was identified in each of the studies.
Effects of interventions
See: Table 1
We were able to pool data from all six studies (Burgener 2008; Burns 2005; Spector 2012; Stanley 2012; Tappen 2009; Waldorff 2012) for depression. When testing the effects of psychological treatment on anxiety only, Spector 2012 and Stanley 2012 contributed data. Data from three (Spector 2012; Stanley 2012; Waldorff 2012) and two studies (Spector 2012; Waldorff 2012) were pooled for self‐rated and carer‐rated patient quality of life respectively. Data from Burns 2005 and Waldorff 2012 were pooled for testing the effects of the psychological treatment on ADL. Spector 2012 and Waldorff 2012 contributed data on effects on neuropsychiatric symptoms. Data from Burgener 2008, Burns 2005, Spector 2012 and Waldorff 2012 were pooled for analyses on effects on cognition. No evidence of heterogeneity was detected in the pooled studies, using the Chi² test. The final analysis included pooled data from Spector 2012, Stanley 2012 and Waldorff 2012 on effects of carer depression. In this analysis heterogeneity was evident. A summary of the findings can be seen in the 'Summary of findings' table 1.
Primary outcomes
Depression
The first meta‐analysis on effects of psychological treatment on patient depression included 439 participants (Analysis 1.1); results favoured the psychological treatment (standardised mean difference (SMD) ‐0.22; 95% confidence interval (CI) ‐0.41 to ‐0.03) in reducing depressive symptoms for people with dementia (Figure 4). This analysis was strongly influenced by Waldorff 2012, which was the largest study, but there was little heterogeneity between studies (I2 = 21%) (Figure 4).
Analysis 1.1.
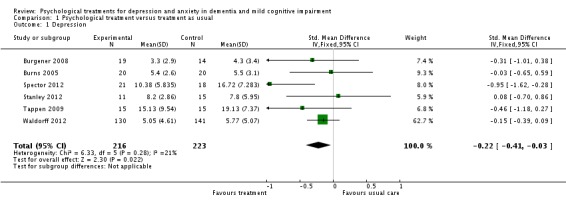
Comparison 1 Psychological treatment versus treatment as usual, Outcome 1 Depression.
Figure 4.
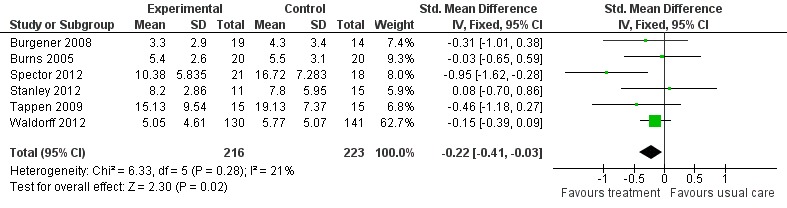
Forest plot of comparison: 1 Psychological treatment versus treatment as usual, outcome: 1.1 Depression.
Anxiety
Psychological treatment reduced clinician‐rated anxiety measured with the RAID (2 studies, 65 participants, MD ‐4.57; 95% CI ‐7.81 to ‐1.32) (Analysis 1.2, Figure 5). However, there was no effect on self‐rated anxiety (2 studies, 65 participants, SMD 0.05; 95% CI ‐0.44 to 0.54) (Analysis 1.3, Figure 6) or carer‐rated anxiety measured with the NPI‐A (1 study, 26 participants, MD ‐2.40; 95% CI ‐4.96 to 0.16) (Analysis 1.4, Figure 7).
Analysis 1.2.
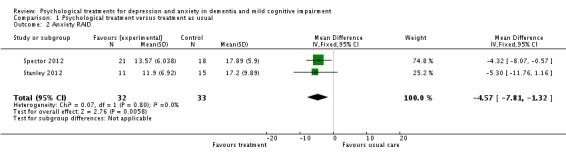
Comparison 1 Psychological treatment versus treatment as usual, Outcome 2 Anxiety RAID.
Figure 5.

Forest plot of comparison: 1 Psychological treatment versus treatment as usual, outcome: 1.2 Anxiety RAID.
Analysis 1.3.
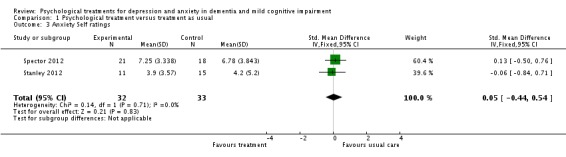
Comparison 1 Psychological treatment versus treatment as usual, Outcome 3 Anxiety Self ratings.
Figure 6.
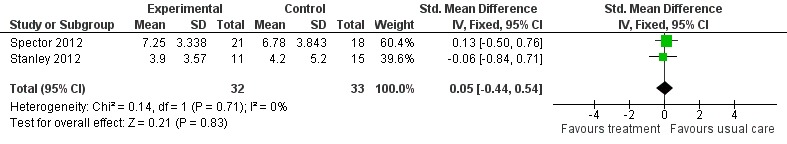
Forest plot of comparison: 1 Psychological treatment versus treatment as usual, outcome: 1.3 Anxiety (self ratings).
Analysis 1.4.
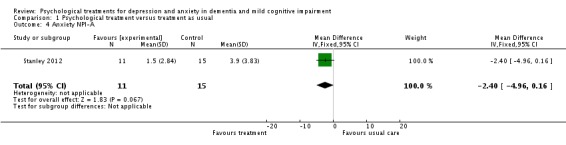
Comparison 1 Psychological treatment versus treatment as usual, Outcome 4 Anxiety NPI‐A.
Figure 7.

Forest plot of comparison: 1 Psychological treatment versus treatment as usual, outcome: 1.4 Anxiety NPI‐A.
Secondary outcomes
Patient quality of life
Psychological treatment had no effect on patient self‐rated quality of life (3 studies, 334 participants, MD 0.37; 95% CI ‐1.01 to 1.75) (Analysis 2.1, Figure 8) or on carer‐rated patient quality of life (2 studies, 313 participants, MD 0.66; 95% CI ‐0.77 to 2.09) (Analysis 2.2, Figure 9).
Analysis 2.1.

Comparison 2 Psychological intervention versus treatment as usual, Outcome 1 Quality of Life (Self ratings).
Figure 8.
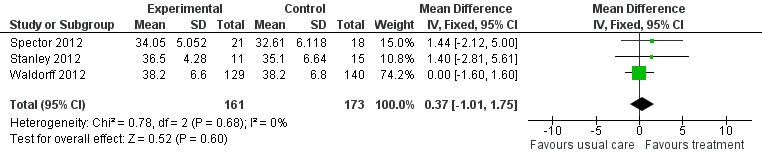
Forest plot of comparison: 2 Psychological intervention versus treatment as usual, outcome: 2.1 Quality of life (self ratings).
Analysis 2.2.
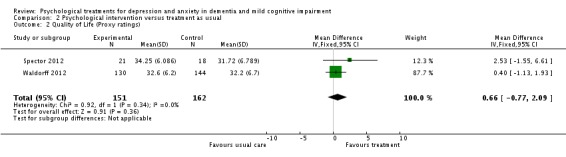
Comparison 2 Psychological intervention versus treatment as usual, Outcome 2 Quality of Life (Proxy ratings).
Figure 9.
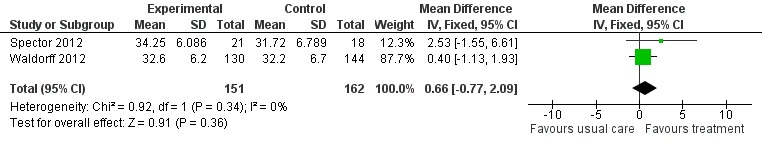
Forest plot of comparison: 2 Psychological intervention versus treatment as usual, outcome: 2.2 Quality of life (proxy ratings).
Activities of daily living (ADL)
Psychological treatment had no effect on ADL for people with dementia (2 studies, 313 participants, SMD ‐0.13; 95% CI ‐0.35 to 0.09) (Analysis 2.3, Figure 10).
Analysis 2.3.
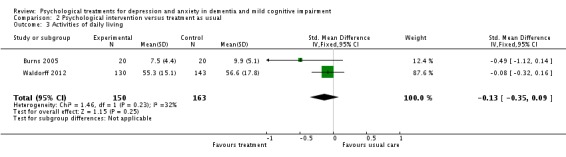
Comparison 2 Psychological intervention versus treatment as usual, Outcome 3 Activities of daily living.
Figure 10.
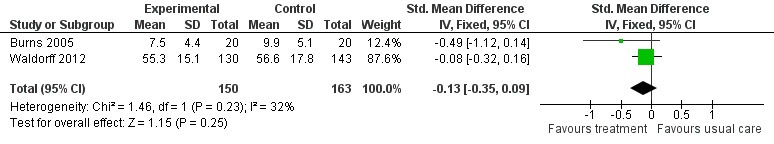
Forest plot of comparison: 2 Psychological intervention versus treatment as usual, outcome: 2.3 Activities of daily living.
Neuropsychiatric symptoms
Psychological treatment had no effect on neuropsychiatric symptoms (2 studies, 311 participants, SMD 0.06; 95% CI ‐0.16 to 0.28) (Analysis 2.4, Figure 11).
Analysis 2.4.
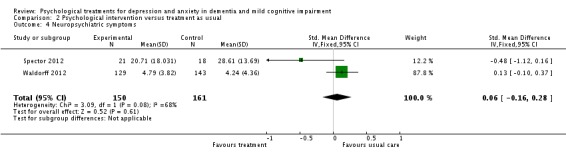
Comparison 2 Psychological intervention versus treatment as usual, Outcome 4 Neuropsychiatric symptoms.
Figure 11.
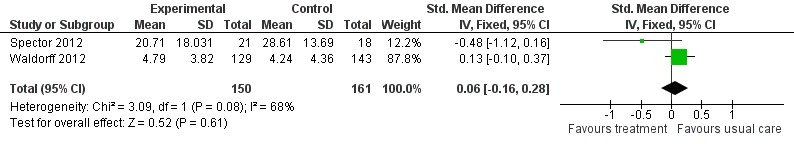
Forest plot of comparison: 2 Psychological intervention versus treatment as usual, outcome: 2.4 Neuropsychiatric symptoms.
Cognition
Psychogical treatment had no effect on cognition (4 studies, 381 participants, MD ‐0.80; 95% CI ‐1.70 to 0.11) (Analysis 2.5, Figure 12).
Analysis 2.5.
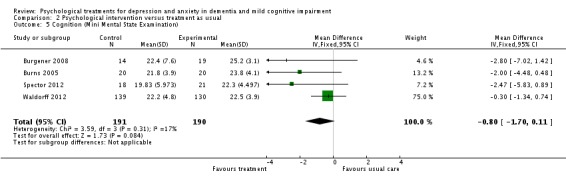
Comparison 2 Psychological intervention versus treatment as usual, Outcome 5 Cognition (Mini Mental State Examination).
Figure 12.

Forest plot of comparison: 2 Psychological intervention versus treatment as usual, outcome: 2.5 Cognition (Mini‐Mental State Examination).
Carer's depression
The final analysis showed that psychological treatment for people with dementia had no effect on self‐rated depressive symptoms for carers (3 studies, 337 participants, SMD 0.07; 95% CI ‐0.14 to 0.29 Analysis 3.1, Figure 13). There was moderate heterogeneity between studies in this analysis.
Analysis 3.1.
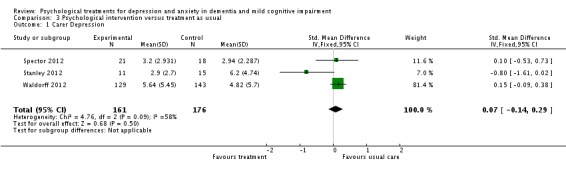
Comparison 3 Psychological intervention versus treatment as usual, Outcome 1 Carer Depression.
Figure 13.
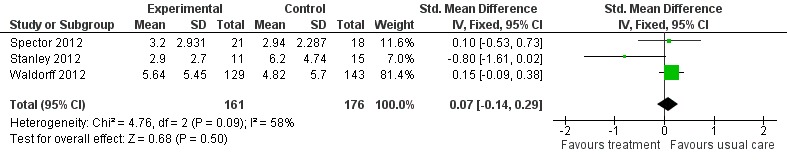
Forest plot of comparison: 3 Psychological intervention versus treatment as usual, outcome: 3.1 Carer depression.
Other outcomes
Behavioral problems
Burns 2005 used the Revised Memory and Behavior Problems Checklist (Teri 1992), to measure carer’s reaction to behavioural problems. We were not able to extract data for this outcome as Burns 2005 did not provide sufficient data to be entered in the meta‐analysis (detransformed log means).
Adverse effects
None of the studies reported or described adverse events.
Discussion
Summary of main results
Six RCTs with a total of 439 participants (216 receiving psychological treatment, 223 receiving usual care or an attention and social contact control treatment) evaluated the efficacy of psychological treatment in reducing depression in people with dementia and met the inclusion criteria of the present review. The majority of participants had mild dementia. Results pooled from the six RCTs showed that psychological treatments reduce depressive symptoms in people with dementia. Data from two studies showed that psychological treatments benefit people with dementia by reducing anxiety symptoms measured with the RAID, a validated clinician‐rated tool. These results compare favourably with minimal or no benefits of pharmacological interventions in treating depression in dementia (Bains 2009) and highlight the need for continuing research in the area. The results may be particularly noteworthy as in some studies the control group was enriched beyond usual care, as opposed to standard care. Although both anxiety and depression were primary outcomes for this review, only two studies were identified that provided data for anxiety.
We found no effect of psychological treatment on any of the secondary outcomes, activities of daily living, self‐ and carer‐rated patient quality of life (QOL), neuropsychiatric symptoms, cognition, or carers' self‐reported depressive symptoms. We did not identify any trials of psychological treatment aimed at people with MCI that met our inclusion criteria. The three studies identified either did not employ an RCT design, participants had cognitive impairment that was not specified according to established criteria of MCI, or the intervention that was evaluated was psychologically based but specifically targeted cognitive decline.
The psychological interventions we included all targeted symptoms of anxiety and depression through a structured psychological approach involving therapist and patient communication, which included directly teaching people with dementia skills to reduce anxiety and depression. Nevertheless, the included trials evaluated a range of different psychological interventions and some used a combination of treatments.
The length and duration of intervention also varied in the studies, leading to differences in intensity and 'dosage' of the psychological treatments. A limitation of this review, therefore, is the substantial variation between studies in terms of the nature, duration and intensity of the psychological therapy evaluated, which may contribute to difficulties interpreting the data. Adherence to treatment appears to have been good in all studies, but adherence in randomised controlled trials may not translate into similar levels of adherence in normal practice. The current review showed that psychological interventions are of benefit for people with dementia as they reduce both anxiety and depressive symptoms.
Overall completeness and applicability of evidence
The studies that were included in the present review only partially answer the research questions posed. We did not identify any studies of people with MCI. We identified six studies of people with dementia and were able to pool the results for depression, anxiety, self‐ and carer‐rated quality of life, ADL, neuropsychiatric symptoms, cognition and carer self‐reported depression. Results for anxiety, QOL, ADL and neuropsychiatric symptoms were each based on only two trials. We were not able to perform any subgroup analyses.
Quality of the evidence
Only one of the studies was classified as low risk in all domains of the Cochrane Collaboration 'Risk of bias' tool (Waldorff 2012). We classified the remaining five studies as being at unclear risk of bias in certain domains, due to limitations such as uncertainties about random sequence generation (Burgener 2008; Tappen 2009), allocation concealment (Burgener 2008; Stanley 2012; Tappen 2009), blinding of participants and personnel (Burgener 2008; Burns 2005; Spector 2012; Stanley 2012; Tappen 2009) and outcome assessment (Burgener 2008; Burns 2005). There was also evidence of selective reporting in one trial (Burns 2005). Based on the GRADE system, we have classified the quality of the evidence as moderate for depression and low for clinician‐rated anxiety, due to the methodological limitations of the identified studies and the limited number of trials.
Potential biases in the review process
We used a comprehensive and sensitive strategy to identify studies that could potentially meet the inclusion criteria for this review. We also contacted first authors of included studies as well as authors of relevant references in order to identify further studies. Selection of studies, data extraction, and assessments of risk of bias were independently conducted by the first and second authors, and any disagreement was resolved by contacting the authors of the studies or the remaining two authors of this review. The present review presents and discusses all outcomes described in the protocol that were available for analysis, regardless of whether or not there was statistical significance.
Agreements and disagreements with other studies or reviews
The current review is distinctive in systematically analysing psychological interventions to reduce anxiety or depression that are conducted primarily with people with dementia or MCI, rather than focusing on environmental changes or skills building for family carers (Brodaty 2012). Previous reviews have concentrated on the effectiveness of other interventions of a psychosocial nature (including cognitive stimulation, cognitive rehabilitation, reminiscence, activities‐based interventions etc.), which are not aimed specifically at anxiety or depression (Livingston 2005; Olazarán 2010). These reviews do suggest that non‐pharmacological interventions can be useful, and potentially cost‐effective, in terms of improving psychological outcomes (Livingston 2005; Olazarán 2010).
Authors' conclusions
Evidence from six RCTs shows that psychological treatments based on a psychological model may benefit people with dementia by reducing depressive symptoms. Evidence from two studies shows that psychological treatments reduce symptoms of clinician‐rated anxiety for people with dementia. Psychological treatments for people with dementia appear to be safe, with no adverse events reported in the literature. This review concludes that psychological treatments that primarily target depression and anxiety have the potential to improve psychological well‐being for people with dementia.
Although many studies have been published examining the effectiveness or feasibility of psychological treatments in reducing anxiety and depression in dementia, there are few well‐designed randomised controlled trials. The present review suggests that psychological treatments are of benefit to people with dementia as they reduce depression and anxiety symptoms. Future RCTs should adhere to the current highest standards of methodology and reporting, following the Consolidated Standards of Reporting Trials (CONSORT) statement. They will be most helpful if they focus on well‐defined psychological approaches, rather than on multimodal approaches which combine a variety of treatments. Based on the literature and the evidence from this review, psychological treatments in dementia, and particularly CBT techniques, merit further research. There is a need to study additional outcomes, particularly quality of life. Research is also needed into psychological treatments for people with depression and MCI, and to define the effect of the severity of dementia on treatment efficacy. Future RCTs should be conducted alongside cost‐effectiveness studies.
Acknowledgements
We would like to thank the Cochrane Dementia and Cognitive Improvement Review Group and Sue Marcus, Managing Editor.
Appendices
Appendix 1. MEDLINE search strategy
| MEDLINE (Ovid SP) search strategy |
| 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. "cognit* impair*".mp. 22. exp *Cognition Disorders/ 23. MCI.ti,ab. 24. ACMI.ti,ab. 25. ARCD.ti,ab. 26. SMC.ti,ab. 27. CIND.ti,ab. 28. BSF.ti,ab. 29. AAMI.ti,ab. 30. MD.ti,ab. 31. LCD.ti,ab. 32. QD.ti,ab. 33. AACD.ti,ab. 34. MNCD.ti,ab. 35. MCD.ti,ab. 36. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 37. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 38. "preclinical AD".mp. 39. "pre‐clinical AD".mp. 40. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 41. (aMCI or MCIa).ti,ab. 42. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 43. ("GDS 3" or "stage 3 GDS").ti,ab. 44. ("global deterioration scale" and "stage 3").mp. 45. "Benign senescent forgetfulness".ti,ab. 46. "mild neurocognit* disorder*".ti,ab. 47. (prodrom* adj2 dement*).ti,ab. 48. or/21‐47 49. 20 or 48 50. depress*.ti,ab. 51. dysthymi*.ti,ab. 52. "adjustment disorder*".mp. 53. "mood disorder*".mp. 54. "affective disorder*".mp. 55. "affective symptom*".mp. 56. anxiety.mp. 57. anxious.mp. 58. ?phobi*.mp. 59. "panic disorder".mp. 60. BPSD.ti,ab. 61. "behavioural and psychological symptoms of dementia".mp. 62. ("neuropsychiatric symptom*" or NPS).mp. 63. exp Behavioral Symptoms/ or Psychomotor Agitation/ 64. Depression/ 65. Anxiety/ or Anxiety Disorders/ 66. or/50‐65 67. 49 and 66 68. (psychotherapy or "cognitive therap*").mp. 69. "behaviour*".mp. 70. counselling.ti,ab. 71. "cognitive analytic therapy".mp. 72. "interpersonal therap*".mp. 73. relaxation.mp. 74. ("non‐pharmacological intervention*" or "non‐pharmacological treatment*").mp. 75. "psychodynamic therap*".mp. 76. (behavi* adj2 therap*).ti,ab. 77. "rational insight therap*".mp. 78. "problem‐solving therap*".mp. 79. CBT.ti,ab. 80. psychosocial.ti,ab. 81. psycho‐social.ti,ab. 82. exp Psychotherapy/ or Psychotherapy, Multiple/ or Psychotherapy, Group/ or Psychotherapy, Brief/ or Psychotherapy, Rational‐Emotive/ 83. or/68‐82 84. 67 and 83 85. randomized controlled trial.pt. 86. controlled clinical trial.pt. 87. random*.ab. 88. trial.ab. 89. groups.ab. 90. or/85‐89 91. (animals not (humans and animals)).sh. 92. 90 not 91 93. 84 and 92 |
Data and analyses
Comparison 1.
Psychological treatment versus treatment as usual
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 6 | 439 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.41, ‐0.03] |
| 2 Anxiety RAID | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐4.57 [‐7.81, ‐1.32] |
| 3 Anxiety Self ratings | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.44, 0.54] |
| 4 Anxiety NPI‐A | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐4.96, 0.16] |
Comparison 2.
Psychological intervention versus treatment as usual
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of Life (Self ratings) | 3 | 334 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐1.01, 1.75] |
| 2 Quality of Life (Proxy ratings) | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [‐0.77, 2.09] |
| 3 Activities of daily living | 2 | 313 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.35, 0.09] |
| 4 Neuropsychiatric symptoms | 2 | 311 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.16, 0.28] |
| 5 Cognition (Mini Mental State Examination) | 4 | 381 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.70, 0.11] |
Comparison 3.
Psychological intervention versus treatment as usual
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Carer Depression | 3 | 337 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.14, 0.29] |
Differences between protocol and review
A new author has been included in this review. Cognition has been included as an additional secondary outcome.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burgener 2008
| Methods | RCT Repeated‐measures randomised design Outcomes at 20 weeks are included in this review Control group: outcomes at 20 weeks only, delayed treatment group (Control group was offered the intervention following the 20‐week assessment) |
|
| Participants | N = 43 (23M, 20F) Confirmed diagnosis of dementia (Alzheimer’s, Lewy body, vascular, frontal lobe, or mixed dementia) A score of < 2.0 on the Clinical Dementia Rating Scale (early to early‐middle disease) Mean age = 76.95 |
|
| Interventions | Multimodal intervention consisting of Tai Chi exercises, cognitive‐behavioral therapy and support group Tai Chi: strength and balance training and relaxation CBT: challenging dysfunctional cognitions and developing positive coping skills Support group: coping with dementia and positive problem solving Duration of intervention: 20 weeks (however complete intervention offered for 40 weeks) Control group received attention‐control educational program |
|
| Outcomes | Patient Outcomes Cognitive function: Mini‐Mental State Examination (MMSE) Physical functioning: Single leg stance (SLS), Berg Balance Scale (BBS), and Cumulative Illness Rating Scale (CIRS) Depression: Geriatric Depression Scale (GDS) Other behavioral outcomes: Rosenberg’s Self‐Esteem Scale (SES) Outcomes measured at 20 weeks and 40 weeks for the intervention group and at 20 weeks only for the control group |
|
| Notes | Treatment duration 40 weeks Tai Chi exercises: 1 hour classes offered weekly Cognitive‐behavioral therapy: 90 minute sessions offered bi‐weekly Support group: 90 minute sessions offered bi‐weekly alternating with the CBT group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation described in the study, insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details of method of concealment described to allow judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of blinding in the study, all assessments and the intervention were conducted at a neutral location |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors communicated that assessors were blind in some but not all outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% attrition for the treatment group and 24% for the control group, with reasons reported |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other apparent bias |
Burns 2005
| Methods | RCT | |
| Participants | N = 40 (21M, 19F) Dementia diagnosis according to NINCDS‐ADRDA criteria, a Clinical Dementia Rating (CDR) of 1 indicating mild dementia, MMSE score > 15 Living in own home with a carer with regular contact Clinically stable (for the last 2 months) if on anti‐dementia or psychotropic medication Mean age = 75.8 |
|
| Interventions | Psychodynamic interpersonal therapy (improving social and interpersonal relationships, identification of conflicts that cause or maintain emotional distress). Therapist also spends time with carer (listening to their needs and informing them of therapeutic progress) Duration of intervention: 6 weeks Control group described as standard treatment in AD, consisted of general advice regarding the diagnosis and treatment of dementia, with out‐patient review |
|
| Outcomes | Patient Outcomes Cognitive function: Mini‐Mental State Examination (MMSE) Depression: Cornell Scale for Depression in Dementia (CSDD) Other patient outcomes: Bristol Activities of Daily Living (BADLS) Clinician’s Interview‐Based Global Impression of Change (CIBI) Caregiver outcomes Revised Memory and Behavior Problems Checklist (RMBPC) ‐ Carers reaction General Health Questionnaire (GHQ‐12) Beck Depression Inventory ((BDI‐II) (scores on this measure not available) Ways of Coping Checklist (WCCL) Outcomes reported at 6 weeks and 3 months (only 6 week data included in the review) |
|
| Notes | 6 sessions of psychodynamic interpersonal therapy of 50 minutes each over 6 weeks NINCDS‐ADRDA ‐ National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to one of the two groups using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Computer generated random numbers were organised independently |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information is provided about assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses conducted No attrition |
| Selective reporting (reporting bias) | High risk | One pre‐specified outcome (Beck Depression Inventory for carers) not reported |
| Other bias | Low risk | No other apparent bias |
Spector 2012
| Methods | RCT not published but data are available single‐blind, multicentre, pilot RCT of CBT versus TAU for people with dementia |
|
| Participants | N = 50 (20M, 30F) Inclusion criteria: 1. Meet DSM‐IV criteria for dementia in mild to moderate range, Clinical Dementia Rating (CDR) score of 0.5, 1 or 2. 2. Clinical anxiety, as determined by a score of 11 or above on the Rating Anxiety in Dementia scale (RAID) 3. Living in the community 4. Presence of a carer who is willing to participate in the therapy 5. Ability to understand and communicate in English 6. Willing to engage in therapy involving discussion of thoughts and feelings Exclusion criteria: 1. Co‐morbid psychiatric disorder (e.g. psychosis) or challenging behaviour (e.g. severe agitation) likely to prevent engagement in therapy 2. Presence of a learning disability or severe physical illness that could impact on participation Mean age was 78.4 (SD = 7.0), and MMSE mean score was 20.9 (SD = 5.2). A total of 26.3% scored .5 on the CDR scale, 47.4% had a rating of 1, and 14.0% had a rating of 2. Mean anxiety rating (RAID) at baseline was 19.7 (SD = 6.0), and the mean depression rating (CSDD) was 15.9 (SD = 6.2). |
|
| Interventions | CBT for people with anxiety and dementia 10 weekly sessions (60 minutes each) comprising of identifying strategies for feeling safe, calming thoughts and behavioural experiments, with telephone contact offered between sessions. The intervention was delivered by clinical or counselling psychologists, with experience of working with people with dementia Control group: standard treatment available to people with anxiety and dementia, including medication or no treatment |
|
| Outcomes | Primary outcomes Rating Anxiety in Dementia (RAID) Clinical Services Receipt Inventory (CSRI) Secondary outcomes Hospital Anxiety and Depression Scale (HADS) Quality of Life‐Alzheimer’s Disease (QOL‐AD) (self and proxy ratings) Neuropsychiatric Inventory (NPI) Mini‐Mental State Examination (MMSE) Cornell Scale for Depression in Dementia (CSDD) Quality of Caregiver and Patient Relationship (QCPR) Caregiver outcomes Hospital Anxiety and Depression Scale (HADS) Outcomes reported at 15 weeks and 6 months (only 15 week data included in the review) |
|
| Notes | 10 sessions of CBT for anxiety of 60 minutes each for 15 weeks ISRCTN46521766 Data were made available by the lead investigator but are not currently published CBT ‐ Cognitive Behavioural Therapy TAU ‐ Treatment as Usual DSM‐IV ‐ Diagnostic and Statistical Manual of Mental Disorders, 4th edition |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The author communicated that random allocation was performed with Stata's random allocation command with varying block sizes of four and six |
| Allocation concealment (selection bias) | Low risk | Central allocation, randomisation conducted by telephoning an independent administrator within the Clinical Trials Unit |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome measures administered by a researcher blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and reasons reported by the author. Use of intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other apparent bias |
Stanley 2012
| Methods | RCT (randomisation in blocks of 4 or 6 stratified by CDR scores ‐ 0.5, 1., or 2., completed in SAS) |
|
| Participants | n = 32 (13M, 19F) Inclusion criteria 1) diagnosis of dementia (confirmed by the patient’s medical provider) 2) an NPI‐A ≥ 4 3) CDR score of 0.5 to 2.0 Exclusion criteria Ps with a primary psychiatric diagnosis of major depression, active psychosis, bipolar disorder, active suicidal intent, recent verbal or physical aggression Dementia diagnosis AD (62.5%), DLB (3.1%), VaD (9.4%) and 25.0% dementia not otherwise specified (NOS) Principal DSM‐IV Diagnoses 43.8% had a diagnosis of GAD, 18.8% a diagnosis of other anxiety disorder, 43.8% comorbid anxiety and depression, 34.4% no diagnosis and 3.1% a depression diagnosis. A total of 46.9% of the sample scored a CDR of 0.5 or 1, and 75% were on at least one psychotropic medication |
|
| Interventions | A cognitive–behavioral therapy–based intervention for anxiety in dementia, of 12 weekly in‐home based sessions and brief telephone sessions, involving self‐monitoring for anxiety, deep breathing, and optional skills (coping self‐statements, behavioral activation, and sleep management). Carers were involved as coaches. Additional materials were provided on communication, education of dementia and stress reduction for collaterals Control group receives usual care incorporating diagnostic feedback |
|
| Outcomes | Patient outcomes Depression: Geriatric Depression Scale (GDS) self report Anxiety: Neuropsychiatric Inventory–Anxiety (NPI‐A) subscale (collateral anxiety report) Rating Anxiety in Dementia (RAID) (administered as a clinical interview to the patient and the caregiver) Geriatric Anxiety Inventory (GAI) self report Worry: Penn State Worry Questionnaire–Abbreviated (PSWQ‐A) self report Quality of Life: Quality of Life in Alzheimer disease (QOL‐AD) Carer Outcomes Depression: Patient Health Questionnaire (PHQ‐9) Distress: distress item from the NPI‐A |
|
| Notes | Peaceful Mind was provided by two master’s‐level graduate‐student clinicians and a predoctoral intern supervised by clinical psychologists and a geriatric social worker. A total of 12 weekly in‐home sessions were provided and up to 8 brief telephone sessions, for 6 months, with each session lasting 30 to 60 minutes. AD ‐ Alzheimer's disease DLB ‐ Dementia with Lewy bodies VaD ‐ Vascular Dementia GAD ‐ General Anxiety Disorder |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The author communicated that randomisation was completed in blocks of four or six stratified by CDR (Clinical Dementia Rating scores), in SAS |
| Allocation concealment (selection bias) | Unclear risk | No details of method of concealment described to allow judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome measures were administered in‐person by independent evaluators who were unaware of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and reasons reported, intention‐to treat analyses |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other apparent bias |
Tappen 2009
| Methods | RCT Two‐groups repeated measures design |
|
| Participants | N = 36 (3M, 33F) Dementia diagnosis according to NINCDS‐ADRDA criteria MMSE < 25 or less Residents of long‐term care facility Mean age = 87.03 Age range 73‐100 Range of length of stay in long‐term care facility ranged from 160 to 1750 days Ps that were mute were excluded |
|
| Interventions | Individual therapeutic conversation based on modified counselling provided individually Usual care control group (usual care by staff provided in the long‐term care facility), described as a non‐attention control group Duration of intervention: 16 weeks |
|
| Outcomes | Patient Outcomes Depression: Montgomery‐Asberg Depression Rating Scale (MADRS) (rated by clinicians) Other behavioral outcomes: Mood Dementia Mood Assessment Scale (DMAS) Alzheimer’s Disease and Related Disorders Mood Scale (AD‐RD) Outcomes measured at 16 weeks |
|
| Notes | Therapeutic conversation of 30 minutes provided three times a week for 16 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation described in the study, insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details of method of concealment described to allow judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and reasons reported |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other apparent bias |
Waldorff 2012
| Methods | RCT Multicentre rater‐blinded trial 1:1 randomisation Treatment as usual beyond the level of usual care |
|
| Participants | N = 330 (151M, 179F) Clinical diagnosis of dementia using DSM‐IV and NINCDS‐ADRDA or McKeith criteria Patients with mild recently diagnosed dementia Mean age = 76.2 Living in the community, age ≥ 50 years with MMSE ≥ 20 Recruited only patients receiving a conventional diagnostic evaluation by specialists Excluded: Participants with severe somatic or psychiatric comorbidity, participating in other interventions, living in nursing home at baseline, or having frontotemporal dementia |
|
| Interventions | Mutlifaceted and semi‐tailored intervention consisting of counselling sessions, teaching, education and outreach telephone support to patients and carers Provided individually and as a group intervention Duration of intervention 8 to 12 months Control group was provided with overall information and guidance, and was directed towards local support programmes (provided to both the control and treatment group) |
|
| Outcomes | Patient Outcomes Depression: Cornell Scale for Depression in Dementia (CSDD) Other behavioral outcomes: EuroQoL VAS (patient and proxy rated) (EQ‐VAS) Quality of Life in Alzheimer's Disease (patient and proxy rated) (QoL‐AD) Neuropsychiatric Inventory Questionnaire (NPI‐Q) Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale (ADSC‐ADL) Cognitive function: Mini Mental State Examination (MMSE) Caregiver outcomes: Geriatric Depression Scale (GDS) EuroQoL VAS (Quality of Life) (EQ‐VAS) Outcomes measured at 6 months and at 12 months |
|
| Notes | Intervention consisted of 6 or 7 counselling sessions, 5 educational courses, 5‐8 telephone calls within a 3‐ to 4‐week intervals approximately DSM‐IV ‐ Diagnostic and Statistical Manual of Mental Disorders, 4th edition NINCDS‐ADRDA ‐ National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer programme |
| Allocation concealment (selection bias) | Low risk | Central allocation process was used to randomise participants by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Raters were not employed by the same institution as study coordinators and counsellors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on attrition and reasons provided, authors mention use of intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes specified were reported |
| Other bias | Low risk | No other apparent bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1992 | Participants did not fulfil criteria for a diagnosis of dementia Cognitive impairment was measured and defined by scores < 80 on the Modified Mini‐Mental State Examination (3MS) (Teng 1987) |
| Beck 2002 | Intervention does not meet criteria for a psychological intervention Intervention based on psychosocial activities (i.e. drawings, life review). Outcomes based on direct observations of PwD |
| Brodaty 2003 | Intervention does not meet criteria for a psychological intervention Multi‐component case management approach |
| Cheston 2003 | This study was not conducted as a RCT Feasibility study of a 10 week psychotherapy group aimed at reducing depression in people with dementia |
| Dartigues 2008 | Intervention does not meet criteria for a psychological intervention RCT of cognitive training, and reminiscence therapy. Control group received psychoeducation for carers |
| Finnema 2005 | Intervention does not meet criteria for a psychological intervention RCT evaluating an emotion oriented care program acknowledging residents' experiences |
| Fischer‐Terworth 2011 | Intervention does not meet criteria for a psychological intervention A TEACCH (Treatment and Education of Autistic and related Communication handicapped Children)‐based cognitive‐behavioural and environmental intervention. This study was not conducted as an RCT and the control group received occupational therapy |
| Fossey 2006 | Intervention does not meet criteria for a psychological intervention Cluster RCT of training and support to nursing home staff for managing agitation |
| Garland 2007 | Intervention does not meet criteria for a psychological intervention Intervention primarily targeted agitated behaviour. This study was not conducted as an RCT |
| Helcer 2012 | Intervention does not meet criteria for a psychological intervention RCT of memory training (MT), or CBT in conjunction with MT targeting hopelessness in PwD |
| Hinchliffe 1997 | Intervention does not meet criteria for a psychological intervention RCT of an intervention helping carers to cope with specific problem behaviours |
| Hyer 2008 | Participants did not fulfil criteria for a diagnosis of dementia Cognitive decline measured and defined by the MMSE (Folstein 1975). RCT of CBT in residents with depression in long‐term care |
| Joosten‐Weyn Banningh2011 | This study was not conducted as an RCT Intervention was psychoeducation, cognitive rehabilitation in conjunction with CBT in people with MCI |
| Kiosses 2010 | Participants did not fulfil criteria for a diagnosis of dementia Older adults with depression, cognitive decline, and disability. 23.3% had probable or definite dementia. RCT comparing PATH (problem‐solving therapy) to supportive therapy. No eligible control or comparison group |
| Kiosses 2011 | Participants did not fulfil criteria for a diagnosis of dementia Cognitive impairment measured and defined by scores 90 ‐ 133 inclusive on the Dementia Rating Scale (DRS). RCT comparing PATH (problem‐solving therapy) to supportive therapy. No eligible control or comparison group |
| Kolanowski 2011 | Intervention does not meet criteria for a psychological intervention RCT of an activities‐based intervention in reducing agitation in nursing home residents with dementia |
| Konnert 2009 | Participants did not fulfil criteria for a diagnosis of dementia Cognitive decline measured and defined by scores < 21 on the MMSE. RCT of a CBT intervention in nursing home residents at risk of depression |
| Kurz 2012 | Intervention does not meet criteria for a psychological intervention RCT of cognitive rehabilitation with CBT elements |
| Laakkonen 2012 | Intervention does not meet criteria for a psychological intervention RCT of self‐management for PwD based on a rehabilitation model |
| Lam 2010 | Intervention does not meet criteria for a psychological intervention RCT of skills training occupational therapy with CBT elements |
| Lichtenberg 2005 | Intervention does not meet criteria for a psychological intervention Intervention of behavioural activities delivered by nursing home assistants in dementia care units |
| Logsdon 2010 | Intervention does not meet criteria for a psychological intervention RCT of support groups for PwD that did not follow a psychological model (can not be described as a supportive therapy intervention) |
| McSweeney 2012 | Intervention does not meet criteria for a psychological intervention Cluster RCT of a multidisciplinary specialist mental health consultation for depression in dementia provided to care staff |
| Mittelman 1996 | Intervention does not meet criteria for a psychological intervention RCT of counselling and support for caregivers to delay nursing home placement in PwD |
| Onor 2007 | Intervention does not meet criteria for a psychological intervention RCT of a multimodal rehabilitation program of cognitive stimulation therapy and psychoeducation for carers |
| Prick 2011 | Intervention does not meet criteria for a psychological intervention RCT of home‐based physical exercise for people with dementia and their carers |
| Proctor 1999 | Intervention does not meet criteria for a psychological intervention RCT of a staff training and educational programme in residential care |
| Rovner 2012 | Intervention does not meet criteria for a psychological intervention RCT of a Behavioural Activation (BA) program to prevent cognitive decline in MCI. No eligible control or comparison group |
| Selbaek 2010 | Intervention does not meet criteria for a psychological intervention Staff nursing training program based on CBT. This study was not conducted as an RCT |
| Teri 2003 | Intervention does not meet criteria for a psychological intervention RCT of home‐based exercise combined with caregiver training for delaying institutionalisation in PwD |
| Vespa 2002 | Intervention does not meet criteria for a psychological intervention Evaluation of a creative activities‐based therapy in reducing anti‐social behaviour in PwD |
| Yesavage 1981 | Participants did not fulfil criteria for a diagnosis of dementia. This study was not conducted as a RCT. No eligible control or comparison group |
Characteristics of studies awaiting assessment [ordered by study ID]
Carreel 1990
| Methods | Unknown whether this study was an RCT |
| Participants | Elderly depressed older adults with dementia |
| Interventions | Group sessions with therapeutic aim described as conversation groups |
| Outcomes | Linguistic skills |
| Notes | Article in French |
Characteristics of ongoing studies [ordered by study ID]
Forstmeier 2011
| Trial name or title | Cognitive‐Behavioral Treatment for Mild Alzheimer's Patients and Their Caregivers (CBTA) |
| Methods | RCT |
| Participants | Inclusion criteria: 1) 50 to 95 years 2) meeting NINCDS‐ADRDA criteria for probable or possible AD McKhann 1984 3) only AD cases with a mild dementia severity will be included, determined by the Clinical Dementia Rating (CDR) scale, with scores 0.5 or 1, and by MMSE scores ≥20 4) Patient must suffer any non‐cognitive symptom that motivates him/her to accept psychotherapeutic help 5) A caregiver must be available to take part in most of the therapy sessions Exclusion Criteria: 1) concomitant alcohol/drug addiction, history of malignant disease, severe organ failure, metabolic or hematologic disorders, or other neurological conditions. |
| Interventions | CBT‐based multi‐component programme of 8 modules (diagnosis & goal setting; psycho‐education; engagement in pleasant activities; cognitive restructuring; live review; training caregiver in behavior management techniques; interventions for the caregiver; and couples counselling). 20 weekly sessions (and 5 single sessions with caregiver). The control group receives standard medical and psychosocial care (each patient/caregiver will receive at least three out of six interventions: (1) psychoeducation on dementia and treatment of dementia (oral and written); (2) appropriate medical treatment; (3) social counseling by specialized staff; (4) memory training in group setting; (5) self‐help group for the patient; (6) self‐help group for the caregiver) |
| Outcomes | Primary patient outcomes Geriatric Depression Scale (GDS) Secondary patient outcomes Neuropsychiatric Inventory (NPI), Bayer‐Activities of Daily Living (B‐ADL), Stress Coping Inventory (SCI), Apathy Evaluation Scale (AES) Caregiver outcomes Center for Epidemiologic Studies Depression Scale (CES‐D), State Trait Anxiety Inventory (STAI), Anger‐in and anger‐out scales of the State Trait Anger Expression Inventory (STAXI), Short‐Form Health Survey (SF‐12), Zarit Burden Interview (ZBI), and Stress Coping Inventory (SCI) Outcomes are measured at 15 weeks and 12 months |
| Starting date | January 2011 |
| Contact information | Dr Simon Forstmeier, University of Zurich, s.forstmeier@psychologie.uzh.ch |
| Notes | ClinicalTrials.gov Identifier: NCT01273272 |
Contributions of authors
VO ‐ correspondence; drafting review versions; search for trials; selection of RCTs; extraction of data; entry of data; data analysis; interpretation of statistical analyses; updating review.
AQ ‐ selection of RCTs; extraction of data; entry of data; data analysis; interpretation of statistical analyses; updating review.
AS ‐ selection of RCTs; interpretation of statistical analyses; updating review.
MO ‐ selection of RCTs; interpretation of statistical analyses; updating review.
Declarations of interest
The second, third and fourth author of this review are investigators in one of the included studies. There are no other known conflicts of interest.
New
References
References to studies included in this review
- Burgener SC, Yang Y, Gilbert R, Marsh‐Yant S. The effects of a multimodal intervention on outcomes of persons with early‐stage dementia. American Journal of Alzheimer’s Disease and Other Dementias 2008;23:382‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A, Guthrie E, Marino‐Francis F, Busby C, Morris J, Russell E, et al. Brief psychotherapy in Alzheimer's disease: randomised controlled trial. The British Journal of Psychiatry 2005;187:143‐7. [DOI] [PubMed] [Google Scholar]
- Spector A, Orrell M, Lattimer M, Hoe J, King M, Harwood K, et al. Cognitive behavioural therapy (CBT) for anxiety in people with dementia: study protocol for a randomised controlled trial. Trials 2012;13:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley MA, Calleo J, Bush AL, Wilson N, Snow AL, Kraus‐Schuman C, et al. The Peaceful Mind Program: A pilot test of a cognitive–behavioral therapy–based intervention for anxious patients with dementia. American Journal of Geriatric Psychiatry 2012;21(7):696‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappen RM, Williams CL. Therapeutic conversation to improve mood in nursing home residents with Alzheimer’s disease. Research in Gerontological Nursing 2009;2:267‐75. [DOI] [PubMed] [Google Scholar]
- Waldorff FB, Buss DV, Eckermann A, Rasmussen MLH, Keiding N, Rishøj S, et al. Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: the multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ 2012;345:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abraham IL, Neundorfer MM, Currie LJ. Effects of group interventions on cognition and depression in nursing home residents. Nursing Research 1992;41:196‐202. [PubMed] [Google Scholar]
- Beck, C, Vogelpohl, T, Rasin, J, Topps, J, O’Sullivan, P, Walls, R, et al. Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nursing Research 2002;51(4):219‐28. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Draper BM, Millar J, Low L, Lie D, Sharah S, et al. Randomized controlled trial of different models of care for nursing home residents with dementia complicated by depression or psychosis. Journal of Clinical Psychiatry 2003;64:63‐72. [DOI] [PubMed] [Google Scholar]
- Cheston R, Jones K, Gilliard J. Group psychotherapy and people with dementia. Aging and Mental Health2003; Vol. 7:452‐61. [DOI] [PubMed]
- Dartigues JF. Efficacy Assessment of Three Non Pharmacological Therapies in Alzheimer's Disease (ETNA3). Trials Register.
- Finnema E, Droës R, Ettema T, Ooms M, Adèr H, Miel Ribbe M, et al. The effect of integrated emotion‐oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: a randomised clinical trial. International Journal of Geriatric Psychiatry 2005;20:330‐43. [DOI] [PubMed] [Google Scholar]
- Fischer‐Terworth C, Probst P. Evaluation of a TEACCH‐ and music therapy‐based psychological intervention in mild to moderate dementia: a controlled trial. Geropsychology 2011;24:93‐101. [Google Scholar]
- Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006;332:756‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland K, Beer E, Eppingstall B, O'Connor DW. A comparison of two treatments of agitated behavior in nursing home residents with dementia: Simulated family presence and preferred music. American Journal of Geriatric Psychiatry 2007;15:514‐21. [DOI] [PubMed] [Google Scholar]
- Helcer J, Santorelli G, Choi J. Cognitive behavioral therapy to combat hopelessness and low self efficacy in Alzheimer's disease. Alzheimer's Association International Conference. 2012. [Google Scholar]
- Hinchliffe AC, Katone C, Livingston G. The assessment and management of behavioural manifestations of dementia: a review and results of a controlled trial. International Journal of Psychiatry in Clinical Practice 1997;1:157‐68. [DOI] [PubMed] [Google Scholar]
- Hyer L, Yeager CA, Hilton N, Sacks A. Group, individual, and staff therapy: an efficient and effective cognitive behavioral therapy in long‐term care. American Journal of Alzheimer’s Disease and Other Dementias 2008;23:528‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten‐Weyn Banningh LW, Prins JB, Vernooij‐Dassen MJ, Wijnen HH, Olde Rikkert MG, Kessels RP. Group therapy for patients with mild cognitive impairment and their significant others: results of a waiting‐list controlled trial. Gerontology 2011;57:444‐54. [DOI] [PubMed] [Google Scholar]
- Kiosses DN, Arean PA, Teri L, Alexopoulos GS. Home‐delivered problem adaptation therapy (PATH) for depressed, cognitively impaired, disabled elders: A preliminary study. American Journal of Geriatric Psychiatry 2010;18:988‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses DN. Home‐delivered intervention for depressed, cognitively impaired elders. Trials Register2011. [DOI] [PMC free article] [PubMed]
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT. A randomized clinical trial of theory‐based activities for the behavioral symptoms of dementia in nursing home residents. American Journal of Geriatric Psychiatry 2011;59:1032‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnert C, Dobson K, Stelmach L. The prevention of depression in nursing home residents: A randomised clinical trial of cognitive–behavioral therapy. Aging and Mental Health 2009;13:288‐99. [DOI] [PubMed] [Google Scholar]
- Kurz A, Thone‐Otto A, Cramer B, Egert S, Frolich L, Gertz HJ, et al. Cordial: cognitive rehabilitation and cognitive‐behavioral treatment for early dementia in Alzheimer disease. Alzheimer Disease and Associated Disorders 2012;26:246‐53. [DOI] [PubMed] [Google Scholar]
- Laakkonen M‐L, Holtta EH, Savikko N, Strandberg TE, Suominen M, Pitkala KH. Psychosocial group intervention to enhance self‐management skills of people with dementia and their caregivers: study protocol for a randomized controlled trial. Trials 2012;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LCW, Lui VWC, Luk DNY, Chau R, So C, Poon V, et al. Effectiveness of an individualized functional training program on affective disturbances and functional skills in mild and moderate dementia—a randomised control trial. International Journal of Geriatric Psychiatry 2010;25:133–41. [DOI] [PubMed] [Google Scholar]
- Lichtenberg PA, Kemp‐Havican J, MacNeill SE, Schafer Johnson A. Pilot study of behavioral treatment in dementia care units. The Gerontologist 2005;45:406–10. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, Pike KC, McCurry SM, Hunter P, Maher J, Snyder L, et al. Early‐stage memory loss support groups: outcomes from a randomized controlled clinical trial. Journal of Gerontology: Psychological Sciences 2010;65B:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney K, Jeffreys A, Griffith J, Plakiotis C, Kharsas R, O’Connor DW. Specialist mental health consultation for depression in Australian aged care residents with dementia: a cluster randomized trial. International Journal of Geriatric Psychiatry 2012;27:1163‐71. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA 1996;276:1725‐31. [PubMed] [Google Scholar]
- Onor M L, Trevisiol M, Negro C, Signorini A, Saina M, Aguglia E. Impact of a multimodal rehabilitative intervention on demented patients and their caregivers. American Journal of Alzheimer's Disease and Other Dementias 2007;22:261‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prick AE, Lange J, Scherder E, Pot AM. Home‐based exercise and support programme for people with dementia and their caregivers: study protocol of a randomised controlled trial. BMC Public Health 2011;11:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R, Burns A, Powell HS, Tarrier N, Faragher B, Richardson G, et al. Behavioural management in nursing and residential homes: a randomised controlled trial. Lancet 1999;354:26–9. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Casten RJ, Hegel MT, Leiby BE. Preventing cognitive decline in older African Americans with mild cognitive impairment: design and methods of a randomized clinical trial. Contemporary Clinical Trials 2012;33:712‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbaek, G. Multidisciplinary intervention for challenging behaviour (agitation) in patients with dementia. Trial Register2010.
- Terri L, Gibbons, LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 2003;290:2015‐22. [DOI] [PubMed] [Google Scholar]
- Vespa A, Gori G, Spazzafumo L. Evaluation of non‐pharmacological intervention on antisocial behavior in patients suffering from Alzheimer's disease in a day care center. Archives of Gerontology and Geriatrics 2002;34:1‐8. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Westphal J, Rush L. Senile dementia: combined pharmacologic and psychologic treatment. Journal of the American Geriatrics Society 1981;29:164‐71. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Carreel C. Value of conversation groups in institutions for the elderly. Revue de Laryngologie 1990;111:319‐23. [PubMed] [Google Scholar]
References to ongoing studies
- Forstmeier S. Cognitive‐behavioral treatment for mild Alzheimer's patients and their caregivers. Clinical Trials Register2011.
Additional references
- Absher JR, Cummings JL. Cognitive and noncognitive aspects of dementia syndromes: An overview. In: Burns A, Levy R editor(s). Dementia. London: Chapman & Hall Medical, 1994. [Google Scholar]
- Alexopoulos GS, Abrams RC, Young RC. Cornell Scale for Depression in Dementia. Biological Psychiatry 1988;23:271‐84. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Jeste DV, Chung H, Carpenter D, Ross R, Docherty JP. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgraduate Medicine 2005;Spec No:6‐22. [PubMed] [Google Scholar]
- Areán P A, Raue P, Mackin S, Kanellopoulos D, McCulloch C, Alexopoulos G S. Problem‐solving therapy and supportive therapy in older adults with major depression and executive dysfunction. American Journal of Psychiatry 2010;167:1391‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azermai M, Petrovic M, Elseviers MM, Bourgeois J, Bortel LM, Vander Stichele RH. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Research Reviews 2012;11:78‐86. [DOI] [PubMed] [Google Scholar]
- Bains J, Birks J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database of Systematic Reviews 2009, Issue Issue 4. Art. No.: CD003944. DOI: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- Ballard C, Boyle A, Bowler C, Lindesay J. Anxiety disorders in dementia sufferers. International Journal of Geriatric Psychiatry 1990;11:987‐90. [Google Scholar]
- Ballard C, Neill D, O'Brien J, McKeith IG, Ince P, Perry R. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. Journal of Affective Disorders 2000;59(2):97‐106. [DOI] [PubMed] [Google Scholar]
- Ballard C, O'Brien J, James I. Dementia: Management of Behavioural and Psychological Symptoms. Oxford: Oxford University Press, 2001. [Google Scholar]
- Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, et al. Sertraline or mirtazapine for depression in dementia (HTA‐SADD): a randomised, multicentre, double‐blind, placebo‐controlled trial. Lancet 2011;378:403‐11. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M. An inventory for measuring depression. Archives of General Psychiatry 1962;4:561‐71. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press, 1979. [Google Scholar]
- Borkovec TD, Ruscio AM. Psychotherapy for generalized anxiety disorder. Journal of Clinical Psychiatry 2001;62:15‐9. [PubMed] [Google Scholar]
- Brierley E, Guthrie E, Busby C. Psychodynamic interpersonal therapy for early Alzheimer’s disease. British Journal of Psychotherapy 2003;19:435‐46. [Google Scholar]
- Brodaty H, Arasaratnam C. Meta‐analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. The American Journal of Psychiatry 2012;169:946‐53. [DOI] [PubMed] [Google Scholar]
- Bucks RS, Ashworth DL, Wilcock GK. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age and Ageing 1996;25:113‐20. [DOI] [PubMed] [Google Scholar]
- Crittendon J, Hopko DR. Assessing worry in older and younger adults: psychometric properties of an Abbreviated Penn StateWorry Questionnaire (PSWQ‐A). Journal of Anxiety Disorders 2006;20:1036–54. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐14. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London UK: BMJ Pubication Group, 2001. [Google Scholar]
- Devanand DP, Pelton GH, Marston K. Sertraline treatment of elderly patients with depression and cognitive impairment. International Journal of Geriatric Psychiatry 2003;18:123–30. [DOI] [PubMed] [Google Scholar]
- Doody R S, Stevens J C, Beck C, Dubinsky R M, Kaye J A, Gwyther L, et al. Practice parameter: management of dementia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56(9):1154‐66. [DOI] [PubMed] [Google Scholar]
- Douglas S, James I, Ballard C. Non‐pharmacological interventions in dementia. Advances in Psychiatric Treatment 2004;10:171‐7. [Google Scholar]
- Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms,and treatment. Current Opinion in Psychiatry 2011;24:461‐72. [DOI] [PubMed] [Google Scholar]
- EuroQoL. EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy 1990;1:199‐208. [DOI] [PubMed] [Google Scholar]
- Ferreti SM, McCurry R, Logsdon L, Gibbons L, Teri L. Anxiety and Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology 2001;14:52‐8. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘‘Mini‐Mental State’’: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
- Gabryelewicz T, Styczynska M, Pfeffer A, Wasiak B, Barczak A, Luczywek E, et al. Prevalence of major and minor depression in elderly persons with mild cognitive impairment‐‐MADRS factor analysis. International Journal of Geriatric Psychiatry 2004;19(12):1168‐72. [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11:33‐9. [PubMed] [Google Scholar]
- Gellis ZD, McClive‐Reed KP, Brown EL. Treatments for depression in older persons with dementia. Clinical Care and Aging 2009;17:29‐36. [PMC free article] [PubMed] [Google Scholar]
- Gibbons LE, Teri L, Logsdon R. Anxiety symptoms as predictors of nursing home placement in patients with Alzheimer's disease. Journal of Clinical Geropsychology 2002;8:335‐42. [Google Scholar]
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill‐building program on the caregiver‐care recipient dyad: 6‐month outcomes from the Philadelphia REACH Initiative. The Gerontologist 2003;43:532–546. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Salvador MT, Arango C, Lyketsos CG, Barba AC. The stress and psychological morbidity of the Alzheimer patient caregiver. International Journal of Geriatric Psychiatry 1999;14(9):701‐10. [DOI] [PubMed] [Google Scholar]
- Gould RA, Otto MW, Pollack MH, Yap L. Cognitive behavioural and pharmacological treatment of generalized anxiety disorders: a preliminary meta‐analysis. Behaviour Therapy 1997;28:285‐305. [Google Scholar]
- Gould RL, Coulson MC, Howard RJ. Cognitive behavioral therapy for depression in older people: a meta‐analysis and meta‐regression of randomized controlled trials. Journal of the American Geriatrics Society 2012;60:1817‐30. [DOI] [PubMed] [Google Scholar]
- Grant RW, Casey DA. Adapting cognitive behavioural therapy for the frail elderly. International Psychogeriatrics 1995;7(4):561‐71. [DOI] [PubMed] [Google Scholar]
- Groves WC, Brandt J, Steinberg M, Warren A, Rosenblatt A, Baker A, et al. Vascular dementia and Alzheimer's disease: Is there a difference?. The Journal of Neuropsychiatry and Clinical Neurosciences 2000;12:305‐15. [DOI] [PubMed] [Google Scholar]
- Hersen M, Hasselt VB. Behavioural assessment and treatment of anxiety in the elderly. Clinical Psychology Review 1992;12:619‐40. [Google Scholar]
- Hobson RF. Forms of Feeling:The Heart of Psychotherapy. London: Tavistock Publications, 1985. [Google Scholar]
- Hogan DB, Bailey P, Black S, Carswell A, Chertkow H, Clarke B, et al. Diagnosis and treatment of dementia: Nonpharmacologic and pharmacologic therapy for mild to moderate dementia. Canadian Medical Association Journal 2008;179(10):1019‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Berg I, Danzinger W, Coben L, Martin R. A new clinical scale for the staging of dementia. The British Journal of Psychiatry 1982;140:566‐72. [DOI] [PubMed] [Google Scholar]
- Hwang TJ, Masterman DL, Ortiz F, Fairbanks LA, Cummings JL. Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Disease and Associated Disorders 2004;18(1):17‐21. [DOI] [PubMed] [Google Scholar]
- Li YS, Meyer JS, Thornby J. Longitudinal follow‐up of depressive symptoms among normal versus cognitively impaired elderly. International Journal of Geriatric Psychiatry 2001;16:718‐27. [DOI] [PubMed] [Google Scholar]
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG, Old Age Task Force of the World Federation of Biological Psychiatry. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. The American Journal of Psychiatry 2005;162:1996‐2021. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, Gibbons LE, McCurry S, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver report (QoL‐AD). Journal of Mental Health and Aging 1999;5:21–32. [Google Scholar]
- Lopez O L, Becker J T, Sweet R A, Klunk, W, Kaufer D I, Saxton J, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences 2003;15:346‐53. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA. Non‐cognitive symptoms in mild cognitive impairment subjects. Neurocase 2005;11:65‐71. [DOI] [PubMed] [Google Scholar]
- Lu PH, Edland SD, Teng E, Tingus K, Petersen RC, Cummings JL, et al. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 2009;72(24):2115‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Steele C, Baker L, Galik E, Kopunek S, Steinberg M, et al. Major and minor depression in Alzheimer's disease: prevalence and impact. Journal of Neuropsychiatry and Clinical Neurosciences 1997;9(4):556‐61. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Olin J. Depression in Alzheimer's disease: overview and treatment. Biological Psychiatry 2002;52(3):243‐52. [DOI] [PubMed] [Google Scholar]
- Margallo‐Lana M, Swann A, O’Brien J. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. International Journal of Geriatric Psychiatry 2001;16:39‐44. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113‐24. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS & ADRDA Work Group under the auspices of Department of Health and Human ServicesTask Force on Alzheimer’s Disease. Neurology 1984;34:939‐44. [DOI] [PubMed] [Google Scholar]
- Mohlman J. Does executive dysfunction affect treatment outcome in late‐life mood and anxiety disorders?. Journal of Geriatric Psychiatry and Neurology 2005;18:97‐108. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Chilovi BV, Peli M, Conti M, Rozzini R, Trabucchi M, et al. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. Journal of Alzheimers Disorders 2009;18:11‐30. [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry 1979;134:382‐89. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412‐4. [DOI] [PubMed] [Google Scholar]
- Novalis P, Rojcewicz S, Peele R. Clinical manual of supportive psychotherapy. American Psychiatric Publishing, 1993. [Google Scholar]
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña‐Casanova J, Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: A systematic review of efficacy. Dementia and Geriatric Cognitive Disorders 2010;30:161‐78. [DOI] [PubMed] [Google Scholar]
- Olin JT, Schneider LS, Katz IR, Meyers BS, Alexopoulos GS, Breitner JC, et al. Provisional diagnostic criteria for depression of Alzheimer disease. American Journal of Geriatric Psychiatry 2002;10:125‐8. [PubMed] [Google Scholar]
- Pachana NA, Byrne GJA, Siddle H. Development and validation of the Geriatric Anxiety Inventory. International Psychogeriatrics 2007;19:103–14. [DOI] [PubMed] [Google Scholar]
- Pai ALH, Drotar D, Zebracki K, Moore M, Youngstrom E. A meta‐analysis of the effects of psychological interventions in pediatric oncology on outcomes of psychological distress and adjustment. Journal of Pediatric Psychology 2006;31:978‐88. [DOI] [PubMed] [Google Scholar]
- Palmer K, Berger A K, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology 2007;68(19):1596‐602. [DOI] [PubMed] [Google Scholar]
- Paukert AL, Calleo J, Kraus‐Schuman C, Snow L, Wilson N, Petersen NJ, et al. Peaceful Mind: an open trial of cognitive‐behavioral therapy for anxiety in persons with dementia. International Psychogeriatrics 2010;22:1012‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey VR. Sociodynamic Counselling: A Constructivist Perspective for the Practice of Counselling in the 21st Century. Victoria: Trafford Publishing, 1997. [Google Scholar]
- Peplau HE. Interpersonal relations in nursing: A conceptual frame of reference for psychodynamic nursing. New York: Springer, 1991. [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999;56:303‐8. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Conceptual Overview. In: Petersen R C editor(s). Cognitive Impairment: Aging to Alzheimer's Disease. New York: Oxford University Press, 2003:1‐14. [Google Scholar]
- Pinquart M, Soerensen S. How effective are psychotherapeutic and other psychosocial interventions with older adults? A meta‐analysis. Journal of Mental Health and Aging 2001;7:207–43. [Google Scholar]
- Robie KJ. An exploration of psychologists' perceptions of the process of psychotherapy for the treatment of psychological concerns and mental disorders in elders who have concomitant dementia. Dissertation Abstracts International1999; Vol. 59:5108.
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1‐year randomized, controlled trial. Journal of the American Geriatrics Society 2007;55:158‐65. [DOI] [PubMed] [Google Scholar]
- Rosenthal RN, Muran JC, Pinsker H, Hellerstein D, Winston A. Interpersonal change in brief supportive psychotherapy. Journal of Psychotherapy Practice and Research 1999;8(1):55‐63. [PMC free article] [PubMed] [Google Scholar]
- Rozzini L, Chilovi BV, Peli M, Conti M, Rozzini R, Trabucchi M, et al. Anxiety symptoms in mild cognitive impairment. International Journal of Geriatric Psychiatry 2009;24:300‐5. [DOI] [PubMed] [Google Scholar]
- Salzman C, Jeste DV, Meyer RE, Cohen‐Mansfield J, Cummings J, Grossberg GT, et al. Elderly patients with dementia‐related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. Journal of Clinical Psychiatry 2008;69:889‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, Hoth A, Buckwalter K. Anxiety and impaired social function in the elderly. Annals of Clinical Psychiatry 2004;16(1):47‐51. [DOI] [PubMed] [Google Scholar]
- Seignourel PJ, Kunik ME, Snow L, Wilson N, Stanley M. Anxiety in dementia: a critical review. Clinical Psychology Review 2008;28(7):1071‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar KK, Walker M, Frost D. The development of a valid and reliable scale for Rating Anxiety in Dementia (RAID). Aging and Mental Health 1999;3:39–49. [Google Scholar]
- Shapiro DA, Startup M. Measuring therapist adherence in exploratory therapy. Journal of Psychotherapy Practice and Research 1993;2:193‐203. 22700145 [Google Scholar]
- Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Parigi A, Caselli RJ, et al. Incident occurrence of depressive symptoms among patients with mild cognitive impairment ‐ the Italian longitudinal study on aging. Dementia and Geriatric Cognitive Disorders 2007;24(1):55‐64. [DOI] [PubMed] [Google Scholar]
- Spruytte N, Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychology and Psychotherapy: Theory, Research and Practice 2002;75:295‐311. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Diefenbach GJ, Hopko DR. Cognitive behavioral treatment for older adults with generalized anxiety disorder. A therapist manual for primary care settings. Behaviour Modification 2004;28:73‐117. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Petracca G, Robinson RG. The construct of generalized anxiety disorder in Alzheimer disease. American Journal of Geriatric Psychiatry 2007;15:42‐9. [DOI] [PubMed] [Google Scholar]
- Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA 1997;277(10):806‐12. [PubMed] [Google Scholar]
- Sultzer DL, Levin HS, Mahler ME, High WH, Cummings JL. A comparison of psychiatric symptoms in vascular dementia and Alzheimer's disease. The American Journal of Psychiatry 1993;150:1806‐12. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Alterman IS, Yount D, Hill JL, Tariot PN, Newhouse PA. A new scale for the assessment of depressed mood in demented patients. The American Journal of Psychiatry 1988;145:955‐9. [DOI] [PubMed] [Google Scholar]
- Tappen RM, Williams‐Burgess C, Edelstein J, Touhy T, Fishman S. Communicating with individuals with Alzheimer’s disease: Examination of recommended strategies. Archives of Psychiatric Nursing 1997;11:249‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappen RM, Williams CL, Barry C, DiSesa D. Conversation intervention with Alzheimer’s patients: Increasing the relevance of communication. Clinical Gerontologist 2001;24:63‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappen RM, Williams CL. Development and testing of the Alzheimer’s Disease and Related Dementias Mood Scale. Nursing Research 2008;57:426‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The modified Mini‐Mental State (3MS) Examination. Journal of Clinical Psychiatry 1987;48:314‐7. [PubMed] [Google Scholar]
- Teri L, Gallagher‐Thompson D. Cognitive‐behavioural interventions for treatment of depression in Alzheimer's patients. The Gerontologist 1991;31(3):413‐6. [DOI] [PubMed] [Google Scholar]
- Teri L, Truax R, Logsdon R. Assessment of behavioural problems in dementia: the Revised Memory and Behavior Problems Checklist. Psychology and Aging 1992;4:622‐31. [DOI] [PubMed] [Google Scholar]
- Hooft CS, Schoofs MWCJ, Ziere G, Hofman A, Pols HAP, Sturkenboom MCJM, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. British Journal of Clinical Pharmacology 2008;66:276‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Verhey FR. Do MCI criteria in drug trials accurately identify subjects with predementia Alzheimer's disease?. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76:1348‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MF, Doody RS, Sairam R. Prevalence and incidence of major depressive disorder in Alzheimer’s disease: findings from two databases. Dementia and Geriatric Cognitive Disorders 2002;13:8‐12. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organisation, Regional Office for Europe, Health Evidence Network. Evidence for decision makers. http://www.euro.who.int/HEN/ 20072007.
- Wilson KC, Mottram PG, Vassilas CA. Psychotherapeutic treatments for older depressed people. Cochrane Database Systematic Reviews 2008;23:doi: 10.1002/14651858.CD004853.pub2. [DOI] [PubMed] [Google Scholar]
- Woods RT, Spector AE, Jones CA, Orrell M, Davies SP. Reminiscence therapy for dementia. Cochrane Database of Systematic Reviews 2009, Issue 2 Art. No.: CD001120. DOI: 10.1002/14651858. [Google Scholar]
- Yale R. Developing Support Groups for Individuals With Early‐Stage Alzheimer’s Disease: Planning, Implementation, and Evaluation. Baltimore, MD: Health Professions Press, 1995. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatry Research 1983;17:37‐49. [DOI] [PubMed] [Google Scholar]
- Yesavage JA. Relaxation and memory training in 39 elderly patients. The American Journal of Psychiatry 1984;141(6):778‐81. [DOI] [PubMed] [Google Scholar]
- Yost E, Beutler L, Corbishley A, Allender J. Group Cognitive therapy: A treatment approach for depressed older adults. New York: Pergamon Press, 1986. [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychologica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]


