Abstract
Background
Postoperative delirium is associated with an increased risk of morbidity and mortality, especially in the elderly. Delirium in the postanaesthesia care unit (PACU) could predict adverse clinical outcomes.
Methods
We investigated a potential link between intraoperative EEG patterns and PACU delirium as well as an association of PACU delirium with perioperative outcomes, readmission and length of hospital stay. The risk factors for PACU delirium were also explored. Data were collected from 626 patients receiving general anaesthesia for procedures that would not interfere with frontal EEG recording.
Results
Of the 626 subjects enrolled, 125 tested positive for PACU delirium. Whilst age, renal failure, and pre-existing neurological disease were associated with PACU delirium in the univariable analysis, the multivariable analysis revealed the importance of information derived from the EEG, anaesthetic technique, anaesthesia duration, and history of stroke or neurodegenerative disease. The occurrence of EEG burst suppression during maintenance [odds ratio (OR)=1.86 (1.13–3.05)] and the type of EEG emergence trajectory may be predictive of PACU delirium. Specifically, EEG emergence trajectories lacking significant spindle power were strongly associated with PACU delirium, especially in cases that involved ketamine or nitrous oxide [OR=6.51 (3.00–14.12)]. Additionally, subjects with PACU delirium were at an increased risk for readmission [OR=2.17 (1.13–4.17)] and twice as likely to stay >6 days in the hospital.
Conclusions
Specific EEG patterns were associated with PACU delirium. These findings provide valuable information regarding how the brain reacts to surgery and anaesthesia that may lead to strategies to predict PACU delirium and identify key areas of investigation for its prevention.
Keywords: EEG; delirium; general anaesthesia, complications; intraoperative monitoring; neurocognitive disorders; postoperative outcome; recovery room
Editor's key points.
-
•
Postoperative delirium is associated with adverse clinical outcomes, but methods of prediction, prevention, and treatment are insufficient.
-
•
Patients undergoing general anaesthesia at four institutions were assessed for preoperative risk factors for PACU delirium, monitored by frontal electroencephalography intraoperatively, and followed for postoperative outcomes for 30 days.
-
•
Multivariable analysis identified specific EEG patterns (intraoperative burst suppression and emergence trajectory), anaesthetic technique, anaesthesia duration, and history of stroke or neurodegenerative disease as predictive of PACU delirium.
-
•
Moreover, PACU delirium was associated with an increased risk of readmission and longer hospital length of stay.
-
•
Intraoperative EEG monitoring may provide a method to predict and prevent PACU delirium, and thus improve postoperative outcomes.
Patients undergoing surgery with general anaesthesia are at risk of developing adverse cognitive outcomes, such as delirium. Postoperative delirium (POD) has been studied at acute and sub-acute time courses ranging from postoperative Day 0–1 to as many as 5–30 days after surgery.1, 2 POD can be further subclassified by its clinical setting, such as delirium in the ICU (ICU delirium)3 or in the PACU [PACU delirium (PACU-D)].2, 4 Characteristic slow oscillations in EEG recordings are known to signal unconsciousness in deep sleep, general anaesthesia, and encephalopathies.5, 6 Aided by EEG, a clear distinction of different stages of sleep [N1–N3 and rapid eye movement (REM)] can be made. The wake-up from deep non-REM Stage 3 (N3) rather than lighter sleep stages (i.e. N1, N2, and REM) precipitates parasomnias (i.e. confusional arousal, sleepwalking, and sleep terrors).7,8 Although natural sleep and anaesthesia are inherently different, the overlap in their neurotransmitter receptor mechanisms and EEG findings prompted us to consider that the electroencephalographic state from which patients emerge at the end of surgery may correlate with the presence or absence of cognitive disturbance (i.e. PACU-D) immediately after surgery. Previously, we defined EEG patterns during emergence that have been compared with N3, N2, and REM sleep.9,10 These patterns are termed delta-dominant slow-wave anaesthesia (ddSWA) resembling N3, spindle-dominant SWA (sdSWA) with features similar to N2, and non-SWA (nSWA) resembling REM. Non-canonical patterns of transitions amongst these trajectories through anaesthesia emergence predicted agitation and pain in the recovery room.9
One EEG feature during (predominately stage N2) sleep, sleep spindles, may be of special interest. Spindles occur predominantly during the N2 stage of sleep from which brief arousals are not uncommon; however, multiple lines of evidence indicate that spindles are associated with an increased arousal threshold.11, 12, 13, 14 Because spindle density increases as the homeostatic drive for sleep decreases across the night, it has been hypothesised that the mechanism underlying spindle production is important for consolidating sleep in the early-morning hours and preventing premature awakening.11 We were motivated to explore the role of spindle-like EEG patterns during emergence in cognitive recovery after surgery with general anaesthesia, and hypothesised that patients with emergence trajectories that contain sdSWA will have lower odds of developing PACU-D.
Few studies have focused on PACU-D, and fewer still have used the most accepted delirium screening tool, the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)15,16,17 to screen for PACU-D. In the current study, we used the CAM-ICU to determine if an association between specific EEG trajectories and PACU-D exists. In addition, to provide clinical context, we also report on the associations between non-EEG factors and PACU-D, and investigate an association of PACU-D with negative post-surgical outcomes.
Methods
Data collection
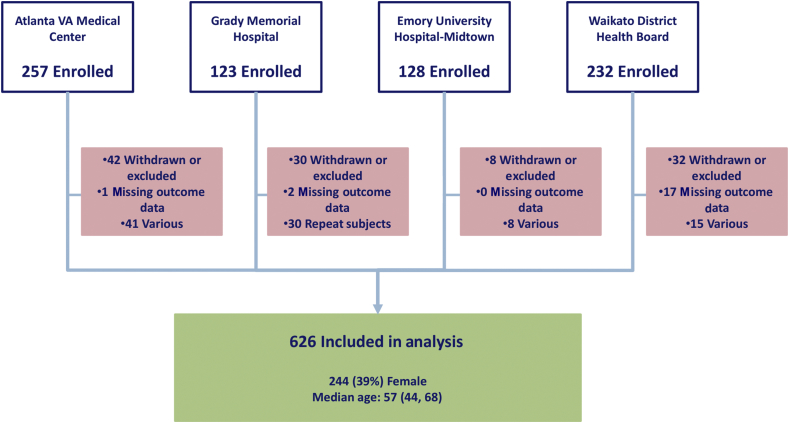
The study protocol was approved by local Ethics or Institutional Review Boards (both at Emory University and Waikato Hospital). A written informed consent was obtained from each patient. Four sites were involved in data collection: Emory University Hospital Midtown, Grady Memorial Hospital, and Atlanta VA Medical Center (all in Atlanta, GA, USA), and Waikato Hospital in Hamilton, New Zealand. Patients were eligible for enrolment in the study if they were expected to be admitted to the PACU after receiving general anaesthesia for non-emergency non-cardiac surgery. During the study design, we calculated needing at least 468 EEG recordings to distinguish between uncommon EEG patterns on emergence. We halted the enrolment after we were confident that we would have greater than this number of EEG recordings of sufficient quality for this analysis. The final number of subjects in the entire study was 626. Patient characteristics, co-morbidities, and social history were collected both by patient interview and medical chart review. In the operating room, trained study personnel applied the frontal EEG electrodes and monitored the signal quality throughout the case. The study staff collected intraoperative data at the bedside, occasionally confirming specific values (e.g. administered drug doses) with the anaesthetic record or anaesthesia staff. This purely observational study did not impose any restrictions on the anaesthetic plan, and the EEG data were collected by the research staff for research purposes. The anaesthesia staff were not given specific instructions on clinical decision-making regarding the EEG information; however, participants were subject to a standard emergence protocol after cessation of anaesthetics intended to minimise external stimuli (e.g. loud noises and oral suction) on the process of emergence. End emergence was defined as the first appearance of an observer's assessment of alertness/sedation [Observer's Assessment of Alertness/Sedation Scale (OAA/S)] score of 2 or greater.18 The OAA/S was performed by the study staff every minute from the time the end-tidal anaesthetic gas concentration reached minimum alveolar concentration-awake (sevoflurane 0.34 vol%), or in cases of total i.v. anaesthesia 5 min after the propofol dose was decreased to 25 μg kg−1 min−1 (or less). Further, we assessed the subject's accuracy in reporting the name and location by giving two commands: ‘tell me your name’ and ‘tell me where you are’, immediately after the end emergence. Using the CAM-ICU, we assessed for delirium 15 min after arrival in the PACU and 60 min after the end emergence was documented. There were approximately 30 min between these two assessments. All subjects assessed with the CAM-ICU were arousable to voice or non-painful stimulation (defined as end emergence) before PACU admission. Consequently, time to emergence was defined as the duration from turning off the delivery of the anaesthetic until the observation of end emergence. A Richmond agitation and sedation score (RASS) was measured with each CAM-ICU assessment. Pain management in the PACU was determined by the anaesthesia staff in collaboration with the PACU nurses. Typically, this involves the assessment of pain every 15 min, and the administration of i.v. fentanyl, hydromorphone, or morphine if the patient reports feeling uncomfortable (e.g. pain score >4/10). For subjects who had multiple surgeries after enrolment, only their first case was considered.
Statistical analyses and model construction
All statistics were conducted using native toolboxes and custom scripts in MATLAB R2015b (MathWorks, Natick, MA, USA) with the exception of the final multivariable logistic model, which was constructed in Stata 14 (StataCorp, College Station, TX, USA). The final number of subjects was 626 (see Fig. 1), but only 477 were used in the multivariable analysis because of technical issues with raw EEG collection, and the fact that we did not impute any missing data points. All effect sizes are presented as odds ratio (OR) [95% confidence interval (CI)] unless otherwise noted. The summary data for each variable are presented as either the median value and inter-quartile range, or as the number and percentage of individuals with negative and positive CAM-ICU results, respectively. All percentages are based on the full data set [n(CAM–)=501 and n(CAM+)=125], irrespective of missing data. P-values reported from the univariable logistic regression are based on the Wald statistic. The ORs for developing PACU-D are reported in square brackets for P-values <0.05, and represent a one-unit increase of the independent variable for continuous and discrete variables, unless otherwise noted by a value in the OR scaling factor column in Table 1 and Supplementary Table S1.19,20
Fig 1.
Summary of subject enrolment. Subjects were recruited at four sites. The number of excluded subjects is displayed in the red boxes. Median age is shown with 25th and 75th percentiles.
Table 1.
Relevant results from univariable logistic regression of preoperative patient factors, interventions, and intra-/postoperative patient characteristics. CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CI, confidence interval, ddSWA, delta-dominant slow-wave anaesthesia; MAC, minimum alveolar concentration; nSWA, non-slow-wave anaesthesia; OR, odds ratio; sdSWA, spindle-dominant slow-wave anaesthesia. ∗Data are represented as median (25th percentile, 75th percentile) or number (percentage of non-delirious and delirious patients, respectively). †P-values are based on the Wald statistic from univariate logistic regression. ORs are shown when P-values are <0.05. ORs for discrete and continuous variables represent a one-unit increase except where indicated otherwise by the OR scaling factor. ‡Neurodegenerative disease includes dementia, mild cognitive impairment, and Parkinson's. Parkinson's results are not displayed because n=1. ¶Variables considered a priori. §Additional variables included in multivariable model before pruning
| Variable | PACU delirium |
P-value† | OR (95% CI)† | OR scaling factor† | |
|---|---|---|---|---|---|
| Absent (n=501) | Present (n=125) | ||||
| Preoperative patient factors | |||||
| Patient characteristics | |||||
| Age (yr)¶ | 56 (44, 67)∗ | 63 (50, 71) | 0.004 | 1.21 (1.06–1.37) | 10 |
| Female | 193 (39)∗ | 51 (41) | 0.64 | ||
| Weight (kg) | 87.2 (75.6, 103.2) | 86.5 (71.2, 100.8) | 0.18 | ||
| BMI | 29.2 (25.2, 33.5) | 29 (24, 33.7) | 0.64 | ||
| ASA physical status¶ | 0.007 | 1.48 (1.11–1.96) | |||
| 1 | 53 (11) | 13 (10) | |||
| 2 | 221 (44) | 37 (30) | |||
| 3 | 215 (43) | 66 (53) | |||
| 4 | 12 (2) | 9 (7) | |||
| Relevant co-morbid conditions | |||||
| Respiratory disease | 85 (17) | 28 (22) | 0.16 | ||
| Asthma | 55 (11) | 12 (10) | 0.66 | ||
| Chronic obstructive pulmonary disease | 35 (7) | 17 (14) | 0.02 | 2.10 (1.13–3.88) | |
| Cardiovascular disease | 249 (50) | 77 (62) | 0.02 | 1.62 (1.09–2.42) | |
| Congestive heart failure | 14 (3) | 4 (3) | 0.81 | ||
| Hypertension | 239 (48) | 74 (59) | 0.02 | 1.59 (1.07–2.37) | |
| History of myocardial infarction | 24 (5) | 12 (10) | 0.04 | 2.11 (1.02–4.35) | |
| Stroke or neurodegenerative disease‡¶ | 19 (4) | 19 (15) | <0.001 | 4.55 (2.33–8.88) | |
| History of cerebrovascular accident | 15 (3) | 15 (12) | <0.001 | 4.42 (2.10–9.31) | |
| Dementia | 1 (0) | 4 (3) | 0.01 | 16.53 (1.83–149.22) | |
| Mild cognitive impairment | 3 (1) | 3 (2) | 0.09 | ||
| Chronic renal insufficiency¶ | 35 (7) | 20 (16) | 0.002 | 2.54 (1.41–4.57) | |
| Alcohol use | 152 (30) | 28 (22) | 0.08 | ||
| Alcohol or other substance disuse, current or previous | 58 (12) | 22 (18) | 0.07 | ||
| Previous heavy alcohol use | 24 (5) | 12 (10) | 0.04 | 2.11 (1.02–4.35) | |
| Heavy alcohol use (>14 drinks per week) | 18 (4) | 6 (5) | 0.53 | ||
| Interventions | |||||
| Procedure characteristics | |||||
| Surgical discipline§ | |||||
| Orthopaedics, not spine | 110 (22) | 16 (13) | 0.02 | 0.52 (0.30–0.92) | |
| Spine surgery | 7 (1) | 7 (6) | 0.009 | 4.19 (1.44–12.17) | |
| Ear/nose/throat | 8 (2) | 4 (3) | 0.25 | ||
| General | 162 (32) | 42 (34) | 0.79 | ||
| Gynaecology | 57 (11) | 17 (14) | 0.49 | ||
| Plastic | 17 (3) | 4 (3) | 0.91 | ||
| Thoracic | 6 (1) | 2 (2) | 0.72 | ||
| Urology | 62 (12) | 13 (10) | 0.54 | ||
| Vascular | 57 (11) | 15 (12) | 0.85 | ||
| Other | 15 (3) | 5 (4) | 0.57 | ||
| Relevant pre- and intraoperative medications | |||||
| Pre-/intraoperative opioids (fentanyl equivalents) | |||||
| Absolute dose (μg)¶ | 250 (150, 350) | 300 (200, 475) | 0.005 | 2.48 (1.32–4.65) | 750 |
| Relative dose (μg kg−1) | 2.8 (1.8, 4.3) | 3.8 (2.2, 5.7) | 0.001 | 2.50 (1.43–4.36) | 8 |
| Maintenance anaesthetics | |||||
| Isoflurane | 5 (1) | 3 (2) | 0.22 | ||
| Sevoflurane | 414 (83) | 95 (76) | 0.1 | ||
| Desflurane | 81 (16) | 21 (17) | 0.82 | ||
| Propofol bolus(es) | 47 (9) | 11 (9) | 0.87 | ||
| Propofol infusion, not TIVA§ | 17 (3) | 13 (10) | 0.002 | 3.34 (1.58–7.09) | |
| TIVA | 8 (2) | 4 (3) | 0.24 | ||
| Adjunct anaesthetic(s)§ | 14 (3) | 12 (10) | 0.001 | 3.74 (1.68–8.30) | |
| Ketamine | 5 (1) | 5 (4) | 0.03 | 4.18 (1.19–14.67) | |
| Nitrous oxide | 9 (2) | 7 (6) | 0.02 | 3.28 (1.20–8.99) | |
| Neuromuscular block | |||||
| Succinylcholine | 85 (17) | 38 (30) | 0.001 | 2.10 (1.34–3.29) | |
| Anaesthesia duration (min)¶ | 104 (60, 159) | 149 (93, 209) | <0.001 | 2.74 (1.81–4.15) | 180 |
| Intraoperative patient characteristics | |||||
| Intraoperative EEG | |||||
| Burst suppression | |||||
| Any | 187 (37) | 55 (44) | 0.02 | 1.72 (1.09–2.71) | |
| Any during peri-induction | 155 (31) | 46 (37) | 0.15 | ||
| Any during maintenance¶ | 80 (16) | 30 (24) | 0.01 | 1.86 (1.13–3.05) | |
| Emergence trajectory¶ | |||||
| 1: ddSWA to sdSWA to nSWA to wake | 63 (13) | 4 (3) | 0.01 | 0.25 (0.09–0.76) | |
| 2: ddSWA to sdSWA to wake, with <120 s nSWA | 49 (10) | 6 (5) | 0.14 | ||
| 3: ddSWA+nSWA to wake | 112 (22) | 28 (22) | Reference | ||
| 4: ddSWA to wake | 74 (15) | 19 (15) | 0.94 | ||
| 5: nSWA to wake | 46 (9) | 23 (18) | 0.04 | 2.00 (1.04–3.83) | |
| 6: sdSWA to wake | 34 (7) | 5 (4) | 0.31 | ||
| 7: Other | 30 (6) | 8 (6) | 0.89 | ||
| Emergence latency | |||||
| From MAC-awake to emerged (min) | 3 (1, 6) | 4 (2, 9) | 0.002 | 1.28 (1.09–1.51) | 5 |
| Postoperative patient characteristics (CAM-ICU, pain) | |||||
| Accuracy to name and location | <0.001 | 0.32 (0.25–0.40) | |||
| 0: Inaccurate, or not verbalised | 76 (15) | 68 (54) | |||
| 1: One accurate response | 56 (11) | 24 (19) | |||
| 2: Accurately verbalised | 354 (71) | 31 (25) | |||
| Any pain score >4 | 287 (57) | 68 (54) | 0.91 | ||
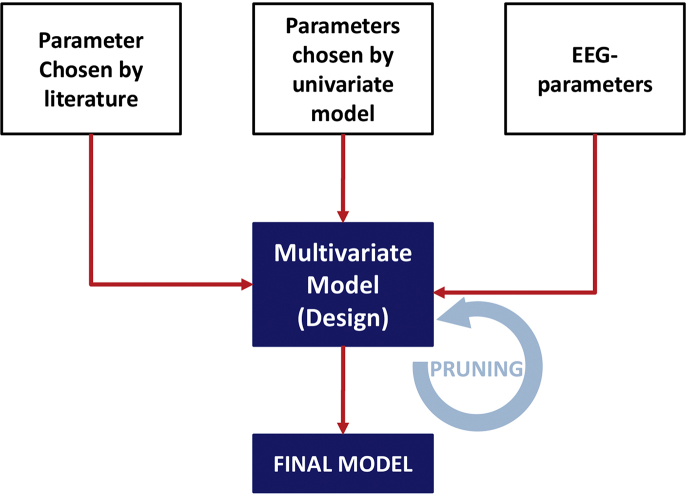
We undertook multivariable model building (Supplementary Fig. S1) to understand if the associations between EEG during emergence and PACU-D were explained by confounding factors. A subset of variables was considered a priori for inclusion in the multivariable logistic model as permitted by the method of purposeful selection.21, 22, 23 These variables (age,4, 24, 25, 26 stroke,27 case length,24,25,28 ASA physical status,4,27 opioids,4,24 renal insufficiency,26 and EEG features29,30) were chosen based on the literature and are designated with a superscript ‘¶’ in Table 1 and Supplementary Table S1. As very little has been published on PACU-D, we used a univariable analysis to help identify potential contributions of other perioperative indicators, for example, surgery type and anaesthetic technique. This led to the inclusion of specific surgical cases (i.e. orthopaedic surgery and spine surgery) and use of specific anaesthetic agents (i.e. adjunctive use of ketamine or nitrous oxide) as separate variables in the model. These are designated with a superscript ‘§’ in Table 1 and Supplementary Table S1. Several factors went into choosing which parameters from the univariable analysis were ultimately included in the multivariable model.For example, variables with demonstrated significance by univariable analysis would only be considered if they were not already accounted for by an a priori variable (chosen from the literature) and not assumed (through clinical judgement) to be better accounted for by another variable. Lastly, we attempted to keep the number of variables low to avoid overfitting.31 Supplementary Table S1 contains the results from the univariate logistic regression for all tested variables.
After an initial variable selection was completed, to provide insight into the weighted influence, potential interactions, and possible confounding factors, we proceeded to construct the multivariable model as follows. We used a population-averaged generalised estimating equation (pa-GEE) multivariable model because it allowed us to make inferences about the study population as a whole (rather than site by site), whilst still adjusting the estimated variances for lack of independence between subjects clustered at the same hospital. As prescribed by purposeful selection, insignificant variables were pruned from the model one by one, and model estimates were recalculated with each removal until all remaining variables were statistically significant. Despite our interest in developing a smaller model, we were still vigilant about confounding. Variables that adjusted the coefficient of another variable by 20% or more, but were not statistically significant themselves in the context of the multivariable model, were noted and are reported in Results. Only one term in the final model was a continuous variable (anaesthesia duration). Because of the limited tools available to compare between pa-GEE models, we used a standard maximum-likelihood-estimate version of the model to assess the linearity of this variable. We confirmed the linearity for this variable both graphically, by locally weighted scatterplot smoothing curves, and parametrically using fractional polynomials (as described22). We report the OR, for this variable (interaction term between stroke or neurodegenerative disease and anaesthesia duration), for three different estimated case lengths: the 25th percentile shortest case length, the median (50th percentile) case length, and the 75th percentile case length. Next, we used P-values from the pa-GEE model Wald statistic to test the significance of interaction terms. When interaction terms were significant, we reported ORs for discrete levels of the interacting variables. Finally, we conducted a Hosmer–Lemeshow (HL) test for goodness of fit of the multivariable model (Supplementary Fig. S2).
The univariable analyses of the outcomes after PACU evaluation for delirium (i.e. length of stay, 30 days readmission rate, and ICU admissions) included Wilcoxon rank sum for lengths of stay and Fisher's exact test for the binary variables; effect sizes are shown when P-values from these tests were <0.05. The effect sizes for discrete variables were measured as right-tail ratios (RTRs) using version 1.4 of Hentschke's and Stüttgen's Measures of Effect Size Toolbox for MATLAB.32
Each variable was captured in more than 99% of patients with the exceptions listed in Supplementary Table S2. The most common reason for missing EEG data was the file was of insufficient quality for quantitative analysis. The EEG-derived variables were the only variables included in our multivariable analysis with >1% of their values missing; therefore, we did not impute missing data.
Quantitative EEG analysis
EEG was recorded with a sample rate of 250 Hz in Atlanta using a SedLine monitor (Masimo, Irvine, CA, USA), and with 128 Hz in Waikato using a BIS XP monitor (Covidien–Medtronic, Dublin, Ireland). Raw EEG was converted to micro-volts during preprocessing. The highest possible cut-off frequency was 43 Hz. The SedLine EEG was down sampled to 125 Hz after low-pass filtering to 47 Hz using MATLAB. All parameters were calculated over 10 s EEG episodes, and we shifted the analysis window by 1 s. We used the eegfilt function from the EEGLAB toolbox33 and a simple artifact algorithm to detect and exclude possible artifacts from analysis based on EEG absolute amplitude thresholds, maximum amplitude difference between two sampling points, and zero-line detection. We calculated the power spectral density (PSD) using the pwelch function with default settings (Hamming window, 50% overlap, seven or eight windows). Based on this PSD matrix, we were able to plot the spectrograms over the entire recording duration and derive the parameters for specified frequency ranges. The EEG spectrograms were used to categorise heuristically the emergence into one of seven distinct sequences (Fig. 2; Supplementary Table S3). These categories were modified from previously published work9 and statistically analysed for an association with PACU-D. Briefly, peak power in the alpha (7–17 Hz) and delta (0.5–4 Hz) ranges were compared. If the oscillatory component of the alpha power was greater than the delta power, the EEG section was considered ‘spindle’ dominant (sdSWA), and if the delta power is larger, delta dominant (ddSWA). If neither value was above a 7 dB threshold, that section was classified as nSWA, an ‘REM-like’ state.9 Hypnograms, depicting these state changes over time, were assessed to ascertain the emergence trajectory. The spectrograms with corresponding hypnograms (Fig. 3) were visually assessed by two observers blinded to patient outcomes. Patients needed to remain in a particular state (e.g. sdSWA or nSWA) for a minimum of 60 s to be included in the appropriate trajectory category. Each EEG recording was inspected by two blinded independent observers for the occurrence of burst suppression. An EEG episode was defined as burst suppression if at least one bursting period could be detected between two suppression-like EEG episodes.
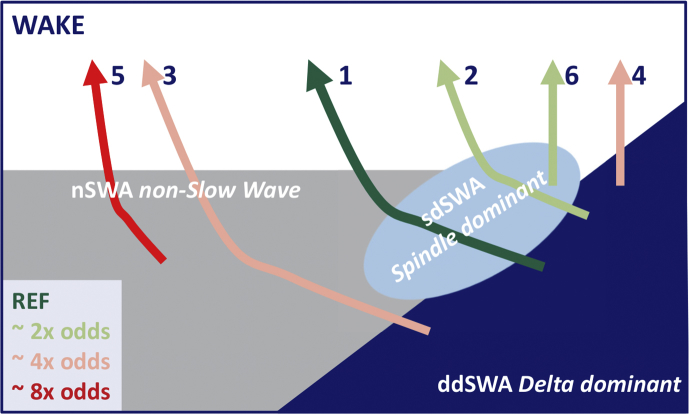
Fig 2.
Different emergence trajectories. Emergence from anaesthesia can take different trajectories. The trajectory used as reference (1, dark green) was the most favourable (lowest odds of PACU delirium). It started with a delta-dominant slow-wave anaesthesia (ddSWA) EEG pattern, followed by an episode of spindle-dominant slow-wave anaesthesia (sdSWA) EEG and non-slow-wave anaesthesia (nSWA) EEG, before returning to the awake state. Trajectories 2 and 6 that also contained episodes of sdSWA EEG had an odds ratio around 2 (light green) of being at risk for PACU delirium. The trajectories with higher risk (i.e. an odds ratio of around 4 or 8 are depicted in red colours). Trajectory 7 is not presented in this plot, because this category pools all ‘other’ trajectories that could not be clearly assigned to Trajectories 1–6.
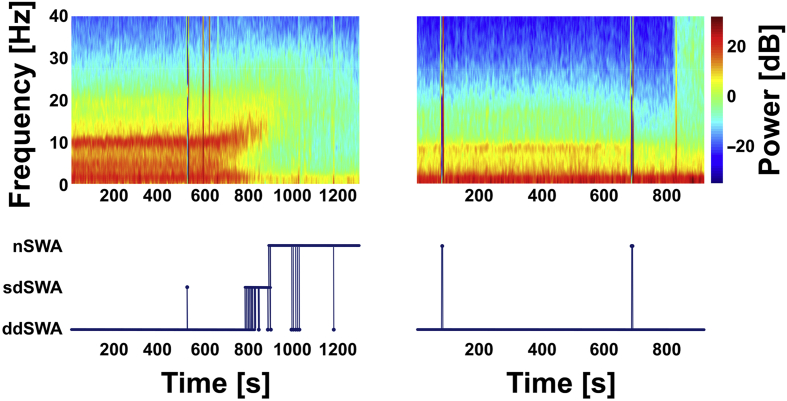
Fig 3.
Spectrograms and assigned stages for two exemplary patients. Spectrograms of two representative patients are in the top two panels. Start of emergence begins at 600 s in both examples, and return of responsiveness to verbal stimulation is at the end of the traces. The bottom two panels are hypnograms showing the corresponding progression of the EEG through various stages during emergence (nSWA, non-slow-wave anaesthesia; sdSWA, spindle-dominant slow-wave anaesthesia; ddSWA, delta-dominant slow-wave anaesthesia), as described.9 The patient on the left (29 yr of age) transitions from a delta-dominant state to a period of spindle dominance before entering a non-slow-wave state before waking (i.e. Trajectory 1). In contrast, the patient depicted on the right (75 yr of age) remains in a delta-dominant state right until return of responsiveness (i.e. Trajectory 4).
Postoperative outcomes
Major postoperative outcomes at 30 days after surgery were determined by chart review and statistically analysed using native toolboxes and custom scripts in MATLAB R2015b (MathWorks, Natick, MA, USA).
Results
Each hospital represents a unique perioperative environment, in which to capture heterogeneity in the data set (Fig. 1). Twenty enrolled patients were missing data on the primary outcome, and their information was excluded from the analysis. Because of the high proportion of veterans in the study, the number of men (61%) exceeded the number of women (39%). The overall incidence of PACU-D was 20% (125 out of 626 total subjects). All subjects were arousable (i.e. eye opening) to voice (RASS ≥–3) in the PACU.
Univariable analysis
In the univariable analysis of 626 subjects, numerous preoperative factors, medical interventions, and intra-/postoperative patient characteristics were associated with increased odds of having PACU-D (Table 1 and Supplementary Table S1).
Patient characteristics and co-morbidities
Notable patient characteristic factors with ORs >1 were age [1.21 (1.06–1.37), for every 10 yr increase in age] and ASA physical status [1.48 (1.11–1.96), for every integer increase]. Non-neurological preoperative co-morbidities associated with increased odds of PACU-D included chronic obstructive pulmonary disease (COPD) [2.10 (1.13–3.88)], cardiovascular disease [1.62 (1.09–2.42)], and chronic renal insufficiency [2.54 (1.41–4.57)]. From the social history, only previous heavy alcohol use increased the odds of PACU-D [2.11 (1.02–4.35)]. Whilst previous stroke and history of neurodegenerative disease were associated with increased odds [4.42 (2.10–9.31)], mild cognitive impairment was not (P=0.09).
Surgery and anaesthesia
A 3 h increase in duration of anaesthesia caused a marked increase in PACU-D, OR of 2.74 (1.81–4.15). When the time to emerge from anaesthesia was measured, a 5 min prolongation of emergence led to a >25% increase in odds of PACU-D [1.28 (1.09–1.51)]. When compared with other surgeries, spine cases had increased odds of delirium [4.19 (1.44–12.17)]. Notably, the univariable analysis revealed decreased odds of delirium [0.52 (0.30–0.92)] in patients having orthopaedic surgery not involving the spine. Pain score (>4) in the recovery room was not associated with PACU-D (P=0.91). Other terms associated with decreased odds of PACU-D were orientation to the name and location at end emergence [0.32 (0.25–0.40)] and EEG trajectory. Two EEG metrics were of particular note. Any incidence of burst suppression on the intraoperative EEG during maintenance anaesthesia (between surgery start and surgery end) was associated with a 75% increase in odds [1.75 (1.10–2.78)], but burst suppression occurring near induction (initiation of anaesthesia) was not. Further examination of EEG emergence trajectories reveals a relationship between the transitions between anaesthesia states during emergence and PACU-D (see Chander and colleagues9 and Hight and colleagues,10 and Supplementary material). Subjects who did not transition through periods of spindle dominance (sdSWA, with pronounced alpha oscillations) had an increased risk of PACU-D (Table 2). But, the overlap between burst suppression and EEG trajectory was minimal, as depicted in Fig 4, and no significant interaction was determined (P=0.591).
Table 2.
Univariable logistic regression of EEG emergence trajectories. CI, confidence interval; ddSWA, delta-dominant slow-wave anaesthesia; nSWA, non-slow-wave anaesthesia; OR, odds ratio; sdSWA, spindle-dominant slow-wave anaesthesia. ∗Data are presented as number (percentage of non-delirious or delirious patients).
| Emergence trajectory | PACU delirium |
P-value | OR (95% CI) | Alternative P-value | Alternative OR (95% CI) | |
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| 1: ddSWA to sdSWA to nSWA to wake | 63 (13)∗ | 4 (3) | 0.01 | 0.25 (0.09–0.76) | Reference | — |
| 2: ddSWA to sdSWA to wake, with <120 s nSWA | 49 (10) | 6 (5) | 0.14 | 0.49 (0.19–1.26) | 0.33 | 1.93 (0.52–7.21) |
| 3: ddSWA+nSWA to wake | 112 (22) | 28 (22) | Reference | — | 0.01 | 3.94 (1.32–11.74) |
| 4: ddSWA to wake | 74 (15) | 19 (15) | 0.94 | 1.03 (0.53–1.97) | 0.02 | 4.04 (1.31–12.51) |
| 5: nSWA to wake | 46 (9) | 23 (18) | 0.04 | 2.00 (1.04–3.83) | <0.001 | 7.88 (2.55–24.32) |
| 6: sdSWA to wake | 34 (7) | 5 (4) | 0.31 | 0.59 (0.21–1.64) | 0.23 | 2.32 (0.58–9.20) |
| 7: Other | 30 (6) | 8 (6) | 0.89 | 1.07 (0.44–2.58) | 0.03 | 4.20 (1.17–15.05) |
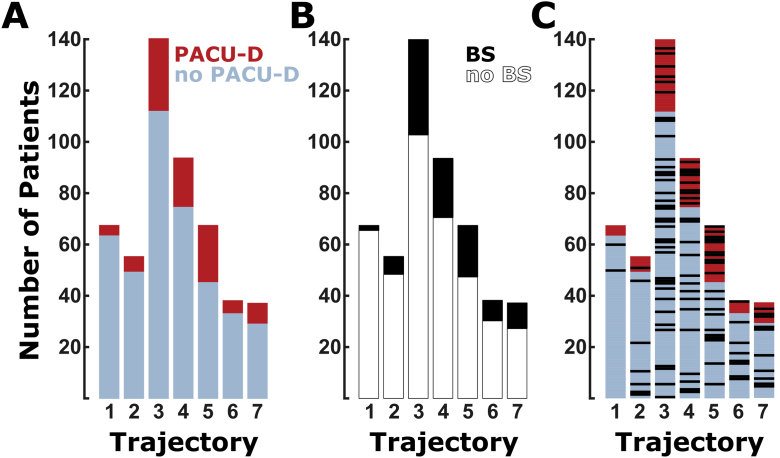
Fig 4.
Bar plots of the EEG trajectory (1–7) compared with (A) presence and absence of PACU delirium (PACU-D), (B) presence and absence of burst suppression, and (C) PACU-D and burst suppression relationship. (A) Patients with emergence trajectories 1, 2, and 6 have the lowest PACU-D (red) to no PACU-D (blue) ratio, and those with trajectories 3, 4, and 5 have the highest PACU-D to no PACU-D ratio. (B) Black indicates the number of patients with burst suppression during maintenance; white indicates the number of patients without burst suppression. (C) This plot is a combination of data presented in (A) and (B). Blue and red indicate the number of patients without PACU-D (blue) and with PACU-D (red). Individual patients who exhibited burst suppression during maintenance are indicated with black bars. No significant interaction between maintenance burst suppression and emergence trajectory (P=0.591) was observed.
Multivariable analysis
Details on constructing the model from 477 subjects are in the Methods and summarised in Supplementary Figure S2.
Patient characteristics and co-morbidities
With the exception of previous stroke and neurodegenerative disease, common patient characteristic factors and co-morbidities (i.e. age, ASA physical status, and renal disease) were insignificant contributors to PACU-D and non-confounding in multivariable analysis (see Supplementary Table S1 for all inaugural co-variates). Although use of propofol infusion and opioid dose were not independently associated with PACU-D in multivariable analysis, these variables confound the association of PACU-D with spine surgery. It is not possible to separate these variables from the association of PACU-D with spine surgery. The significant predictors of PACU-D remaining after model construction were stroke or neurodegenerative disease, type of orthopaedic surgery (spine and non-spine), anaesthesia duration, burst suppression, anaesthetic adjuncts, and EEG trajectory (see Supplementary material).
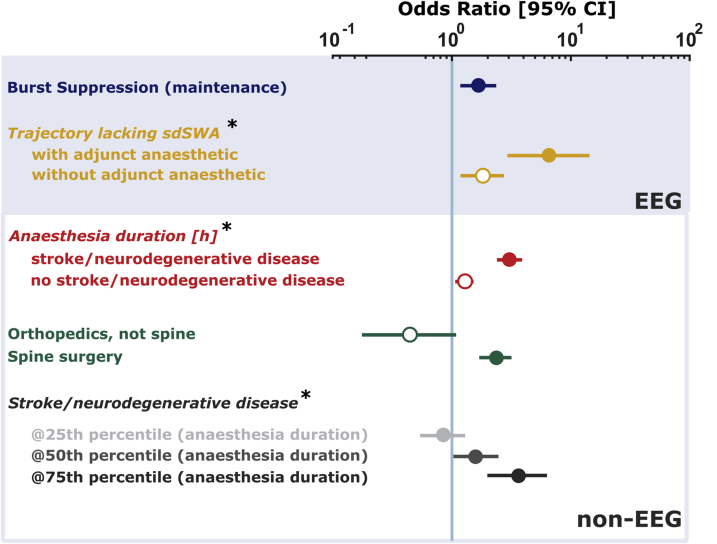
Only 477 (n=90 CAM-ICU positive) subjects had EEG records of sufficient quality for inclusion in the multivariable model. Figure 5 shows the adjusted ORs for PACU-D from the final statistical model, including interaction between variables. Burst suppression during the anaesthetic maintenance phase remained a significant factor associated with PACU-D [1.66 (1.20–2.29)]. No statistically significant interactions were noted for burst suppression with any other variables in the model (Wald statistic P>0.05). Subjects undergoing spine surgery were more likely to develop PACU-D [2.35 (1.84–3.00)]. EEG trajectories remained strongly associated with PACU-D. Subjects lacking spindle-dominant trajectories during emergence had over six times the odds [6.51 (3.00–14.12)] of experiencing PACU-D if their anaesthetic regimens involved specific adjunct anaesthetics (ketamine or nitrous oxide). In the absence of these medications, the odds of PACU-D remained elevated in subjects lacking sdSWA [1.81 (1.23–2.67)]. Anaesthesia duration and history of stroke or neurodegenerative disease were associated with PACU-D. For every 1 h increase in anaesthetic duration, subjects without a history of stroke or neurodegenerative disease had an increased odds of PACU-D [1.28 (1.19–1.37)], whereas those with a history of stroke or neurodegenerative disease had a three-fold increase in the odds [3.02 (2.55–3.57)]. Interestingly, subjects with a history of stroke or neurodegenerative disease having short procedures (<25th percentile for case length) did not have an increased OR of PACU-D [0.84 (0.58–1.21)]. Patients with a diagnosis of stroke or neurodegenerative disease that had the longest procedures (>75th percentile for case length) had >3.5 times the odds of developing PACU-D [3.61 (2.13–6.13)].
Fig 5.
Adjusted odds ratios from multivariable logistic regression. Odds ratios and [95% confidence intervals (CIs)] calculated from the model described in Methods and Supplementary information of the relevant EEG and non-EEG parameters. *Odds ratios adjusted for interaction with another co-variate. Legend quantities are odds ratios. See text for an explanation of confounding regarding spine surgery.
The 30 day follow-up revealed an association of PACU-D with adverse outcomes. PACU-D was associated with longer length of hospital stay [RTR (95% CI): 2.07 (1.38–3.16)]; (Table 3). Subjects who were delirious in the PACU also had twice the odds of being readmitted to the hospital within 30 days than subjects who had a negative screen by CAM-ICU in the PACU [2.17 (1.13–4.17)].
Table 3.
Outcomes after evaluation for delirium in the PACU. ∗Data are presented as median (25th percentile, 75th percentile) or number (percentage of non-delirious and delirious, respectively). †Wilcoxon rank sum. ‡Right-tail ratio (95% confidence interval), threshold=overall mean+0.5*standard deviation. ¶Fisher's exact. §Odds ratio (95% confidence interval).
| PACU delirium |
P-value | Effect size | ||
|---|---|---|---|---|
| Absent (n=501) | Present (n=125) | |||
| Postoperative length of stay (day) | 1 (0, 2)∗ | 2 (1, 6) | <0.001† | 2.07 (1.38–3.16)‡ |
| Total length of stay (day) | 1 (0, 2) | 2 (1, 7) | 0.001† | 1.82 (1.12–2.63)‡ |
| 30 day hospital readmission (yes/no) | 30 (6) | 15 (12) | 0.03¶ | 2.17 (1.13–4.17)§ |
| 30 day ICU admission (yes/no) | 3 (1) | 2 (2) | 0.26¶ | |
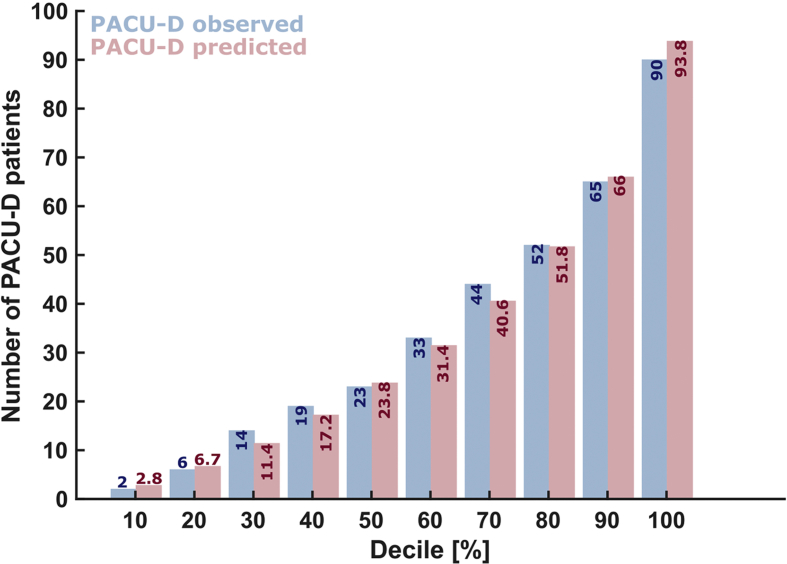
Whilst any model would first need to be validated with an independent data set before use as a predictive tool, the performance of our model did not show significant differences between observed and predicted frequencies of PACU-D (goodness-of-fit test, HL statistic=7.44; degrees of freedom=8; P=0.491; Supplementary Fig. S2).
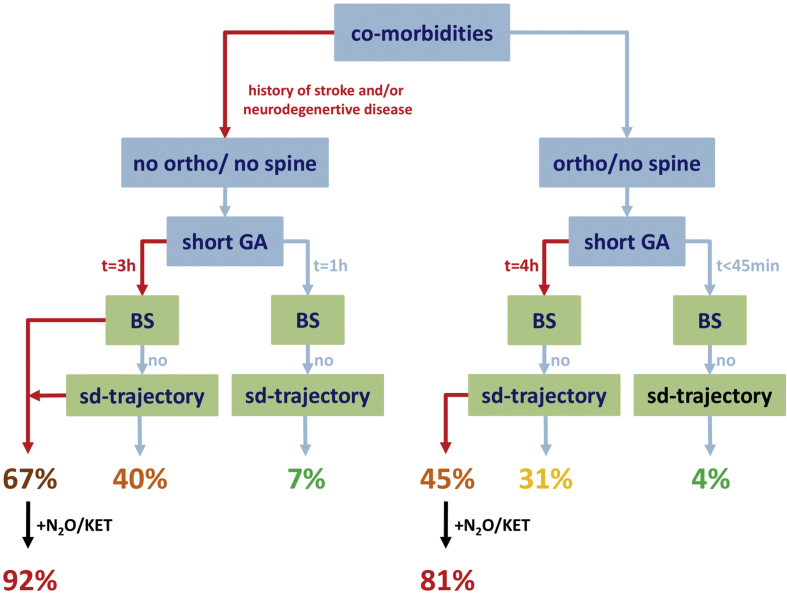
We also used our model to estimate the incidence of developing delirium given specific clinical scenarios in our data set (Supplementary Fig. S3).
Discussion
This prospective multi-institutional investigation detected an association of EEG features with delirium in the immediate post-surgical period. To provide clinical context, we also link PACU-D to increased hospital stay and readmission, and relate the importance of intraoperative neurophysiology data in the context of other perioperative risk factors to the development of PACU-D.
In relation to PACU-D outcomes, our EEG emergence trajectory data suggest a natural division between trajectories with and without spindle dominance. It is tempting to postulate that patients who progress through a spindle-dominant state experience ‘wake-ups’ that are more impervious to external stimuli and proceed more like natural sleep-to-wake transitions than those who do not pass through a spindle-dominant state.34 It should be noted that the spindle oscillations observed during anaesthesia are longer in duration and occur over a broader frequency range than the (11–15 Hz) 0.5–3 s spindles observed in sleep. Still, use of power density as a proxy for spindle activity is well supported in the sleep literature for human studies12,13 and in rodents that lack canonical sleep spindles.35, 36, 37 To what extent our trajectories mechanistically overlap with the archetypal N3 to N2/N1 to REM to wake transition remains to be determined. However, the association of non-spindle-dominant emergence trajectories with poor PACU-D outcomes encourages further interventional investigations.
There is an interaction between anaesthetic regimens that involve nitrous oxide or ketamine and the EEG trajectories most associated with PACU-D. This means that patients without a favourable (spindle dominant) emergence trajectory have even greater odds of PACU-D when ketamine or nitrous is used. Our current study is limited in power to delve into these questions in greater detail. Whilst maintenance with nitrous oxide is associated with deep delta waves38 and maintenance with ketamine is typically associated with increased activity in the high EEG frequencies,39 it is unclear how these EEG features relate to our EEG trajectories.
Age is a known risk factor for delirium of any type. Although age was pruned from our multivariable model, it would be inaccurate to conclude that age does not influence PACU-D. Age decreases the alpha frequency in a linear fashion,40 and spectral power and spectral coherence in the alpha range linearly decrease with age in patients undergoing general anaesthesia.41 Although older patients may be at greater risk of not developing alpha spindles (see Fig. 3), we suspect that our inclusion of neurophysiological parameters resulted in the pruning of age as a variable. A formal comparison of models would need to be completed to better characterise this supposition.
We suggest that the ‘cognitive age’ of the patient may be captured in the EEG (see Lindeboom and Weinstein42 for a review of cognitive age). Assuming that alpha spindles are representative of adequate anaesthesia, we interpret our findings in the following context. We know that EEG alpha power decreases with age.43,41 Further, in a patient population >60 yr old, intraoperative alpha power negatively correlated with cognitive performance before operation.44 Our study supports a possible link between alpha power and the ‘cognitive state’ of the brain. A published case report describes a patient who repeatedly developed PACU-D and did not have any alpha spindle containing trajectory during emergence, and had alpha-spindle characteristics that resembled a much older patient.45 Another patient with prolonged POD also did not have any alpha-spindle activity.46 Hence, the lack of alpha spindles may reflect a ‘cognitively older’ brain, more susceptible to adverse outcomes.
Our work should not be considered as proof that EEG features or other perioperative risk factors are causative for PACU-D. Extensions of the EEG montage (i.e. parietal and occipital electrodes) may reveal important attributes not captured by our study. It will be necessary to test our model on independently derived data sets to determine its ability to predict accurately PACU-D in other populations. Like other work on the prevalence of delirium using CAM-ICU as a clinical screening tool, sophisticated cognitive testing is not performed before operation. This limitation prevents us from attributing PACU-D directly to intraoperative events. Further, our study is not large enough to determine specific factors that could increase the odds of hyperactive vs hypoactive delirium. A recent publication highlights that subdivisions of hypoactive delirium may exist.47 Other studies will be necessary to determine the best prediction model for the development of PACU-D. One of the strongest predictors (in univariable analysis) of a PACU stay without delirium was the subject's accurate response to the checks of orientation (person and place) given immediately after extubation. The clinical significance of this will need to be corroborated with other studies. Another limitation of our study is the use of a screening tool for delirium originally designed for use in ICU patients receiving sedation; other tools could be developed that better capture the clinical features essential for anaesthesiologists. A standard screening tool for PACU-D does not exist, and a comparison of available tools has not yet been performed. Further, the definition of the trajectories was not fully automated, leaving some room for future refinements. But, the coarse classification based on very distinct EEG features should have minimised possible bias.
Previous work on delirium after surgery with general anaesthesia has shown an association of hypoactivity in the recovery room with POD.25 However, several risk factors previously associated with post-anaesthesia cognitive impairment (e.g. age, premedication, and ASA physical status) were pruned from our multivariable model because of lack of significance. We suspect that this is because our study is unique in its inclusion of neurophysiological data in the construction of the model. This suggests that EEG indicators are potential predictors of cognitive impairment in the immediate post-surgical period. This is supported by existing evidence showing specific EEG changes with advanced age and administered drugs.41,48,49 However, it remains possible that the causes of PACU-D and POD are different and could be further examined. Additionally, our results cannot inform anaesthesiologists about agitation during emergence, as all subjects in our study were evaluated after achieving return of consciousness. Nomenclature that incorporates POD with more persistent cognitive problems coincident with surgery and general anaesthesia has been proposed.50
Although our study is not large enough to draw definitive conclusions regarding pharmacological choices for particular surgeries that might influence PACU-D, some interesting insight can be developed. The univariable analysis revealed that propofol infusion was associated with increased odds of developing PACU-D. Interestingly, TIVA alone did not exhibit the same association. Both TIVA and infusions of propofol are common in spine surgery cases. A larger study focused on PACU-D in association with spine surgery should be undertaken to determine if a protective strategy exists. Additionally, the univariable analysis revealed non-spine orthopaedic surgery as potentially protective against PACU-D [0.52 (0.30–0.92); P=0.02]; however, the adjusted OR calculated using the multivariable model did not reach statistical significance [–0.82 (–1.70, 0.06); P=0.07]. Given that POD has been extensively studied after hip fracture,51 it is fair to say our orthopaedic (non-spine) procedures were not dominated by these surgeries.
A key finding from the current study was that patients with PACU-D had significant correlations with long-term negative outcomes, such as 30 day readmissions and length of hospital stay. Although other studies have demonstrated an association between PACU-D and longer hospital stays, those studies did not have EEG data nor use the CAM-ICU to screen for delirium.2,24,25 We found two new, potentially modifiable perioperative indicators of ensuing PACU-D: (i) EEG burst suppression during maintenance and (ii) EEG emergence trajectory. The former has been previously implicated as a predictor of POD,30 whilst the latter is novel and may show promise as a monitored EEG parameter.
First approaches in evaluating our model as a predictive model in an independent data set might follow an approach like Supplementary Figure S3. In the current prospective observational study, we chose PACU-D as our primary outcome measure because of its temporal proximity to emergence and its implication as an early marker of POD.1,52 POD (occurring on or after the first postoperative day) has revealed associations with adverse outcomes, such as increased functional decline up to 1 month after surgery,53 cognitive decline up to 6 months after surgery,54 length of ICU26,55 and hospital stay,26, 55, 56, 57 hospital costs,26, 55, 57, 58 post-discharge institutionalisation,26,56 30 day hospital readmission,56 and mortality.26,59 Our intention was to evaluate whether perioperative EEG features are associated with the development of PACU-D. It remains to be seen if PACU-D has any association with POD.
Here, we established an association of EEG features with PACU-D, and demonstrated that PACU-D might be predicted intraoperatively. Our data suggest that PACU-D (like regular delirium) can and should be recognised by anaesthesiologists, as it may herald an increase in worse outcomes. Recent interest in anaesthesia reversal agents represents a potentially promising clinical research strategy.37,60
Authors' contributions
Study design: PSG, JWS
Data collection: PSG, JWS, SH, DH, AG, PD, DS, NBT, MKW, SL.
Data analysis: all authors.
Manuscript preparation: all authors.
Acknowledgements
The authors gratefully acknowledge helpful discussions from Anesthesiologists Concerned with Cognition, Emergence, Sleep, and Sedation research conferences (www.accesshq.org). This is the primary analysis of this collaborative group. Portions of this database have been published as case reports, and presented in abstract form at national and international meetings. Portions of this database are freely available for secondary analysis. The authors would also like to acknowledge the administrative and clinical research coordination efforts from D. Beschen, S. Eisenberg, and N. Pitre.
Editorial decision: 11 September 2018
Handling editor: H.C. Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.09.016.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
Career Development Award #BX00167 from the US Department of Veteran Affairs, Biomedical Laboratory Research and Development Service, and the James S. McDonnell Foundation (Grant number 220023046) to P.S.G.; National Institutes of Health (T32 grant 5T32NS007480-15) to Emory University for Translational Research in Neurology and a Burroughs Wellcome Fund Collaborative Research Travel Grant (1015183) to S.H.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig S1.
Flow chart of the multivariate model design. The parameters chosen for the multivariate model were selected according to previous findings from the literature, significant results in the univariate analysis, and the EEG parameter burst suppression during maintenance and emergence trajectories. Some parameters could fit in multiple categories (e.g. age: literature and univariate analysis, or burst suppression during maintenance: EEG and univariate analysis).
Supplementary Fig S2.
Observed and predicted frequencies of PACU-D were not significantly different based on the results of the Hosmer–Lemeshow goodness-of-fit test (HL statistic=7.44; degrees of freedom=8; P=0.491). PACU-D, PACU delirium.
Supplementary Fig S3.
Using the multivariate model to estimate likelihood of PACU-D in our patient sample.
References
- 1.Sharma P.T., Sieber F.E., Zakriya K.J. Recovery room delirium predicts postoperative delirium after hip-fracture repair. Anesth Analg. 2005;101:1215–1220. doi: 10.1213/01.ane.0000167383.44984.e5. [table of contents] [DOI] [PubMed] [Google Scholar]
- 2.Hernandez B.A., Lindroth H., Rowley P. Post-anaesthesia care unit delirium: incidence, risk factors and associated adverse outcomes. Br J Anaesth. 2017;119:288–290. doi: 10.1093/bja/aex197. [DOI] [PubMed] [Google Scholar]
- 3.Veiga D., Luis C., Parente D. Postoperative delirium in intensive care patients: risk factors and outcome. Rev Bras Anestesiol. 2012;62:469–483. doi: 10.1016/S0034-7094(12)70146-0. [DOI] [PubMed] [Google Scholar]
- 4.Card E., Pandharipande P., Tomes C. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br J Anaesth. 2015;115:411–417. doi: 10.1093/bja/aeu442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown E.N., Lydic R., Schiff N.D. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rampil I.J. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Espa F., Ondze B., Deglise P., Billiard M., Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;111:929–939. doi: 10.1016/s1388-2457(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 8.Howell M.J. Parasomnias: an updated review. Neurotherapeutics. 2012;9:753–775. doi: 10.1007/s13311-012-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chander D., Garcia P.S., MacColl J.N., Illing S., Sleigh J.W. Electroencephalographic variation during end maintenance and emergence from surgical anesthesia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hight D.F., Dadok V.M., Szeri A.J., Garcia P.S., Voss L., Sleigh J.W. Emergence from general anesthesia and the sleep-manifold. Front Syst Neurosci. 2014;8:146. doi: 10.3389/fnsys.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang-Vu T.T., McKinney S.M., Buxton O.M., Solet J.M., Ellenbogen J.M. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010;20:R626–R627. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Dijk D.J. EEG slow waves and sleep spindles: windows on the sleeping brain. Behav Brain Res. 1995;69:109–116. doi: 10.1016/0166-4328(95)00007-g. [DOI] [PubMed] [Google Scholar]
- 13.Dijk D.J., Hayes B., Czeisler C.A. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–199. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 14.Ehrhart J., Ehrhart M., Muzet A., Schieber J.P., Naitoh P. K-complexes and sleep spindles before transient activation during sleep. Sleep. 1981;4:400–407. [PubMed] [Google Scholar]
- 15.Wei L.A., Fearing M.A., Sternberg E.J., Inouye S.K. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNicoll L., Pisani M.A., Ely E.W., Gifford D., Inouye S.K. Detection of delirium in the intensive care unit: comparison of confusion assessment method for the intensive care unit with confusion assessment method ratings. J Am Geriatr Soc. 2005;53:495–500. doi: 10.1111/j.1532-5415.2005.53171.x. [DOI] [PubMed] [Google Scholar]
- 17.Ely E.W., Inouye S.K., Bernard G.R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 18.Chernik D.A., Gillings D., Laine H. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 19.Fleisher L.A., Beckman J.A., Brown K.A. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169–e276. doi: 10.1161/CIRCULATIONAHA.109.192690. [DOI] [PubMed] [Google Scholar]
- 20.National Collaborating Centre for Acute Care (UK) 2003. Preoperative tests: the use of routine preoperative tests for elective surgery. London. [PubMed] [Google Scholar]
- 21.Katz M.H. Multivariable analysis: a primer for readers of medical research. Ann Intern Med. 2003;138:644–650. doi: 10.7326/0003-4819-138-8-200304150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hosmer D.W., Jr., Lemeshow S., Sturdivant R.X. 3rd Edn. John Wiley & Sons; Hoboken, NJ: 2013. Applied logistic regression. [Google Scholar]
- 23.Bursac Z., Gauss C.H., Williams D.K., Hosmer D.W. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radtke F.M., Franck M., Hagemann L., Seeling M., Wernecke K.D., Spies C.D. Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence. Minerva Anestesiol. 2010;76:394–403. [PubMed] [Google Scholar]
- 25.Xara D., Silva A., Mendonca J., Abelha F. Inadequate emergence after anesthesia: emergence delirium and hypoactive emergence in the postanesthesia care unit. J Clin Anesth. 2013;25:439–446. doi: 10.1016/j.jclinane.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Robinson T.N., Raeburn C.D., Tran Z.V., Angles E.M., Brenner L.A., Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 27.Monk T.G., Weldon B.C., Garvan C.W. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 28.Lepouse C., Lautner C.A., Liu L., Gomis P., Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96:747–753. doi: 10.1093/bja/ael094. [DOI] [PubMed] [Google Scholar]
- 29.Martin J.C., Liley D.T., Harvey A.S., Kuhlmann L., Sleigh J.W., Davidson A.J. Alterations in the functional connectivity of frontal lobe networks preceding emergence delirium in children. Anesthesiology. 2014;121:740–752. doi: 10.1097/ALN.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 30.Fritz B.A., Kalarickal P.L., Maybrier H.R. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 2016;122:234–242. doi: 10.1213/ANE.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 32.Hentschke H., Stuttgen M.C. Computation of measures of effect size for neuroscience data sets. Eur J Neurosci. 2011;34:1887–1894. doi: 10.1111/j.1460-9568.2011.07902.x. [DOI] [PubMed] [Google Scholar]
- 33.Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Sanders R.D., Tononi G., Laureys S., Sleigh J.W. Unresponsiveness not equal unconsciousness. Anesthesiology. 2012;116:946–959. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Astori S., Wimmer R.D., Prosser H.M. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wimmer R.D., Astori S., Bond C.T. Sustaining sleep spindles through enhanced SK2-channel activity consolidates sleep and elevates arousal threshold. J Neurosci. 2012;32:13917–13928. doi: 10.1523/JNEUROSCI.2313-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safavynia S.A., Keating G., Speigel I. Effects of gamma-aminobutyric acid type A receptor modulation by flumazenil on emergence from general anesthesia. Anesthesiology. 2016;125:147–158. doi: 10.1097/ALN.0000000000001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavone K.J., Akeju O., Sampson A.L., Ling K., Purdon P.L., Brown E.N. Nitrous oxide-induced slow and delta oscillations. Clin Neurophysiol. 2016;127:556–564. doi: 10.1016/j.clinph.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maksimow A., Särkelä M., Långsjö J. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin Neurophysiol. 2006;117:1660–1668. doi: 10.1016/j.clinph.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- 41.Purdon P.L., Pavone K.J., Akeju O. The ageing brain: age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015;115:i46–i57. doi: 10.1093/bja/aev213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindeboom J., Weinstein H. Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease, and vascular cognitive impairment. Eur J Pharmacol. 2004;490:83–86. doi: 10.1016/j.ejphar.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 43.Schultz A., Grouven U., Zander I., Beger F.A., Siedenberg M., Schultz B. Age-related effects in the EEG during propofol anaesthesia. Acta Anaesthesiol Scand. 2004;48:27–34. doi: 10.1111/j.1399-6576.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- 44.Giattino C.M., Gardner J.E., Sbahi F.M. Intraoperative frontal alpha-band power correlates with preoperative neurocognitive function in older adults. Front Syst Neurosci. 2017;11:24. doi: 10.3389/fnsys.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreuzer M., Whalin M.K., Hesse S.D.W., Riso M.A., Garcia P.S. Anesthetic management of a patient with multiple previous episodes of postanesthesia care unit delirium: a case report. A A Case Rep. 2017;8:311–315. doi: 10.1213/XAA.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 46.Whalin M.K., Kreuzer M., Halenda K.M., Garcia P.S. Missed opportunities for intervention in a patient with prolonged postoperative delirium. Clin Ther. 2015;37:2706–2710. doi: 10.1016/j.clinthera.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Hight D.F., Sleigh J., Winders J. Inattentive delirium versus disorganized thinking: a new axis to subcategorize PACU delirium. Front Syst Neurosci. 2018;12:22. doi: 10.3389/fnsys.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akeju O., Westover M.B., Pavone K.J. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014;121:990–998. doi: 10.1097/ALN.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akeju O., Pavone K.J., Westover M.B. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121:978–989. doi: 10.1097/ALN.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evered L., Silbert B., Knopman D.S. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery. Br J Anaesth. 2018;121:1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sieber F.E., Zakriya K.J., Gottschalk A. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neufeld K.J., Leoutsakos J.M., Sieber F.E. Outcomes of early delirium diagnosis after general anesthesia in the elderly. Anesth Analg. 2013;117:471–478. doi: 10.1213/ANE.0b013e3182973650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudolph J.L., Inouye S.K., Jones R.N. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58:643–649. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saczynski J.S., Marcantonio E.R., Quach L. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown C.H., 4th, Laflam A., Max L. The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann Thorac Surg. 2016;101:1663–1669. doi: 10.1016/j.athoracsur.2015.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gleason L.J., Schmitt E.M., Kosar C.M. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg. 2015;150:1134–1140. doi: 10.1001/jamasurg.2015.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zywiel M.G., Hurley R.T., Perruccio A.V., Hancock-Howard R.L., Coyte P.C., Rampersaud Y.R. Health economic implications of perioperative delirium in older patients after surgery for a fragility hip fracture. J Bone Jt Surg Am. 2015;97:829–836. doi: 10.2106/JBJS.N.00724. [DOI] [PubMed] [Google Scholar]
- 58.Franco K., Litaker D., Locala J., Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42:68–73. doi: 10.1176/appi.psy.42.1.68. [DOI] [PubMed] [Google Scholar]
- 59.Gottesman R.F., Grega M.A., Bailey M.M. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solt K., Cotten J.F., Cimenser A., Wong K.F., Chemali J.J., Brown E.N. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.