Abstract
Abemaciclib, an inhibitor of cyclin dependent kinases 4 and 6, is indicated for metastatic breast cancer treatment. Reversible increases in serum creatinine levels of ~15–40% over baseline have been observed following abemaciclib dosing. This study assessed the in vitro and clinical inhibition of renal transporters by abemaciclib and its metabolites using metformin (a clinically relevant transporter substrate), in a clinical study that quantified glomerular filtration and iohexol clearance. In vitro, abemaciclib inhibited metformin uptake by organic cation transporter 2, multidrug and toxin extrusion (MATE)1, and MATE2‐K transporters with a half‐maximal inhibitory concentration of 0.4–3.8 μM. Clinically, abemaciclib significantly increased metformin exposure but did not significantly affect measured glomerular filtration rate, serum neutrophil gelatinase‐associated lipocalin (NGAL), serum cystatin‐C, or the urinary markers of kidney tubular injury, NGAL and kidney injury molecule‐1.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Multiple drugs decrease estimated glomerular filtration rate (eGFR) via inhibition of renal transporters without directly affecting renal function. Clinical studies of abemaciclib revealed treatment‐associated increases in serum creatinine (SCr) and decreases in eGFR.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Does abemaciclib cause increases in SCr due to inhibition of renal transporters or because of alterations to GFR?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Abemaciclib inhibits organic cation transporter 2, multidrug and toxin extrusion (MATE) 1, and MATE2‐K transporters in vitro and significantly decreases renal clearance of metformin, a transporter substrate. Abemaciclib did not significantly increase measured GFR or acute renal damage biomarkers, suggesting that observed increases in SCr are due to inhibition of proximal tubule secretory transporters.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ These findings indicate that abemaciclib does not seem to affect renal function: alternative renal function assessment methods may be required in patients taking abemaciclib when increases in creatinine are reported and clinicians are concerned about impairment of renal function. The design allows transporter inhibition and effects upon GFR to be evaluated in the same study.
Abemaciclib is an orally administered small molecule that is a potent and selective inhibitor of cyclin‐dependent kinases (CDKs) 4 and 6.1 Inhibition of CDK4 and CDK6 prevents cell cycle progression through the G1 restriction point, which controls entry into S phase, thus arresting tumor growth.2 Abemaciclib is indicated for the treatment of metastatic breast cancer.
In clinical studies of patients with cancer and healthy subjects, reversible increases in serum creatinine (SCr) levels of ~15–40% over baseline were measured following dosing with abemaciclib.2, 3 Creatinine, an endogenous product of creatine phosphate metabolism in skeletal muscle, is the most widely used marker to estimate glomerular filtration rate (eGFR) in the clinical setting. Although creatinine is primarily filtered in the kidneys,4, 5 active tubular secretion accounts for ~10–40% of creatinine clearance.6, 7, 8, 9 Thus, the glomerular filtration rate (GFR) calculated using SCr levels could be an overestimate.
Active tubular secretion of creatinine is mediated by multiple solute carrier (SLC) transporters in the kidney, including organic cation transporter 2 (OCT2: SLC22A2), multidrug and toxin extrusion protein (MATE) 1 (SLC47A1), and MATE2‐K (SLC47A2).5, 10, 11 OCT2 is expressed on the basolateral membrane of proximal tubule cells and mediates uptake of organic cations, such as creatinine and metformin, from blood into the cells by facilitated diffusion;12 however, MATE1 and MATE2‐K are expressed on the apical membrane of proximal tubule cells and are responsible for proton‐coupled efflux of drugs and endogenous compounds from cells to urine.13 Inhibition of OCT2 and/or MATEs can, therefore, lead to a decrease in creatinine clearance, and a corresponding increase in SCr due to inhibition of its tubular secretion. These changes can occur without clinically meaningful alterations in renal function or impact on measured GFR (mGFR).9, 10, 13
The in vitro assessment of OCT2 and/or MATE inhibition is important in evaluating potential for drug–drug interactions (DDIs) at these transporters and for many drugs recognized as having inhibitory potential.14 A number of marketed drugs cause elevations in SCr by inhibition of these renal transporters.15, 16 Early assessment of the clinical inhibition of renal transporters may be prudent if transporter inhibition has been identified in vitro or if increases in SCr concentrations have been identified during preclinical development, or first‐in‐human clinical trials.
A widely used drug, metformin, is excreted unchanged in urine, principally via active tubular secretion in the kidneys17, 18 by OCT2‐mediated uptake followed by efflux via MATEs. It is well documented that concomitant administration of metformin with MATE and/or OCT2 inhibitors, such as pyrimethamine, cimetidine, or dolutegravir, changes the pharmacokinetics (PKs) of metformin.18, 19, 20, 21
In the past decade, alternative markers for assessing GFR and renal function have been identified, including other endogenous substances, such as cystatin‐C, which unlike SCr, is not subject to active secretion and not affected by changes in diet or muscle mass.22 Techniques using clearance rates of freely filtered and nonmetabolized exogenous compounds, such as iohexol, are also used to calculate absolute GFR and better estimate the actual rate of glomerular filtration.23 A range of biomarkers, including kidney injury molecule 1 (KIM‐1) and neutrophil gelatinase‐associated lipocalin (NGAL), have been shown to be promising markers of acute kidney injury compared to SCr.16, 24, 25, 26
This study aimed to assess the in vitro and clinical inhibition of renal transporters by abemaciclib and its active metabolites, M2 and M20, using metformin as a clinically relevant competing transporter substrate. The effect of abemaciclib administration on metformin PK was examined using a clinical study design incorporating monitoring of renal filtration, by measuring iohexol clearance, to demonstrate that observed increases in creatinine concentrations and subsequent decreases in calculated creatinine clearance and eGFR were due to transporter inhibition and not changes in renal function.
Results
In vitro analysis of transporter interactions
The inhibitory effects of abemaciclib and its two active metabolites, M2 and M20, on renal transport of metformin were assessed in vitro using transfected human embryonic kidney (HEK) cells expressing OCT2, MATE1, and MATE2‐K. Abemaciclib, M2, and M20 inhibited OCT2, MATE1, or MATE2‐K–mediated metformin uptake with half‐maximal inhibitory concentration (IC50) values ranging from 0.4−3.8 μM (Table 1 and Figure 1). When the unbound peak plasma concentration (Cmax) to in vitro IC50 ratio (DDI index) for abemaciclib was combined with DDI indices of M2 and M20, the DDI indices of OCT2, MATE1, and MATE2‐K were 0.03, 0.13, 0.06, respectively (Figure 1). The DDI index for MATE1 and MATE2‐K exceeded the regulatory guidance threshold of 0.02 indicating the potential for a DDI at these transporters at clinically relevant concentrations.27, 28
Table 1.
Subject demographics and baseline characteristics
| Overall (N = 40) | ||
|---|---|---|
| Age (years) | Mean (SD) | 50.4 (10.6) |
| Range | 23–69 | |
| Age group (%) | 21–30 years | 1 (2.5) |
| 31–40 years | 6 (15.0) | |
| 41–50 years | 13 (32.5) | |
| 51–60 years | 12 (30.0) | |
| 61–70 years | 8 (20.0) | |
| Sex (%) | Male | 4 (10.0) |
| Female | 36 (90.0) | |
| Ethnicity (%) | Hispanic or Latino | 8 (20.0) |
| Not Hispanic or Latino | 32 (80.0) | |
| Race (%) | Asian | 3 (7.5) |
| Black or African American | 18 (45.0) | |
| White | 19 (47.5) | |
| Weight (kg) | Mean (SD) | 73.56 (11.22) |
| Range | 54.7–99.2 | |
| BMI (kg/m2) | Mean (SD) | 27.25 (3.06) |
| Range | 20.4–31.8 | |
| eGFR (mL/minute/1.73 m2) | Mean (SD) | 99.84 (15.63) |
| Range | 62.6–128.4 |
BMI, body mass index; eGFR, estimated glomerular filtration rate, as calculated with the Chronic Kidney Disease Epidemiology Collaboration equation38; N, number of subjects.
Figure 1.
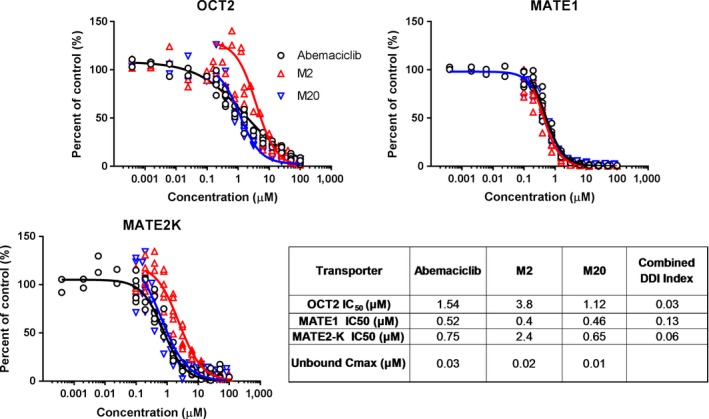
Inhibition of organic cation transporter (OCT2), multidrug and toxin extrusion (MATE1), and MATE2‐K–dependent transport of metformin by abemaciclib and metabolites M2 and M20. Combined drug–drug interaction index (DDI) = (Iu abemaciclib/half‐maximal inhibitory concentration (IC50) abemaciclib) + (Iu M2/IC50 M2) + (Iu M20/IC50 M20) where Iu = unbound peak plasma concentration (Cmax).
Clinical study
Demographics.
Forty healthy subjects, 4 men and 36 women, between the ages of 23 and 69 years were enrolled in the study. The demographic and baseline characteristics of the subjects are shown in Table 1.
All subjects were randomly assigned to a dosing sequence and received at least one dose of study drug. Twenty‐five subjects completed the study through to the follow‐up visit (Figure S1 ).
One subject discontinued due to adverse events of elevated alkaline phosphatase, elevated alanine aminotransferase, elevated aspartate aminotransferase, and elevated gamma glutamyltransferase enzymes, considered to be related to study treatment beginning on day 15 of period 2 (14 days after receiving abemaciclib and metformin). Five subjects were lost to follow‐up, and five subjects withdrew their consent for personal reasons. For the PK analysis, data were available from 30 subjects who received placebo and metformin, 32 subjects who received abemaciclib and metformin, 32 subjects who received placebo and iohexol, and 33 subjects who received abemaciclib and iohexol. Figure S1 summarizes subject disposition and data evaluability.
Effect of abemaciclib coadministration on metformin PKs.
Coadministration of metformin with abemaciclib increased exposure to metformin (Figure 2), causing a statistically significant increase in metformin geometric least squares (LS) mean area under the concentration‐time curve from zero to infinity (AUC0–∞) and Cmax, and a nonsignificant delay in time of maximum plasma concentration (Tmax; Table 2).
Figure 2.
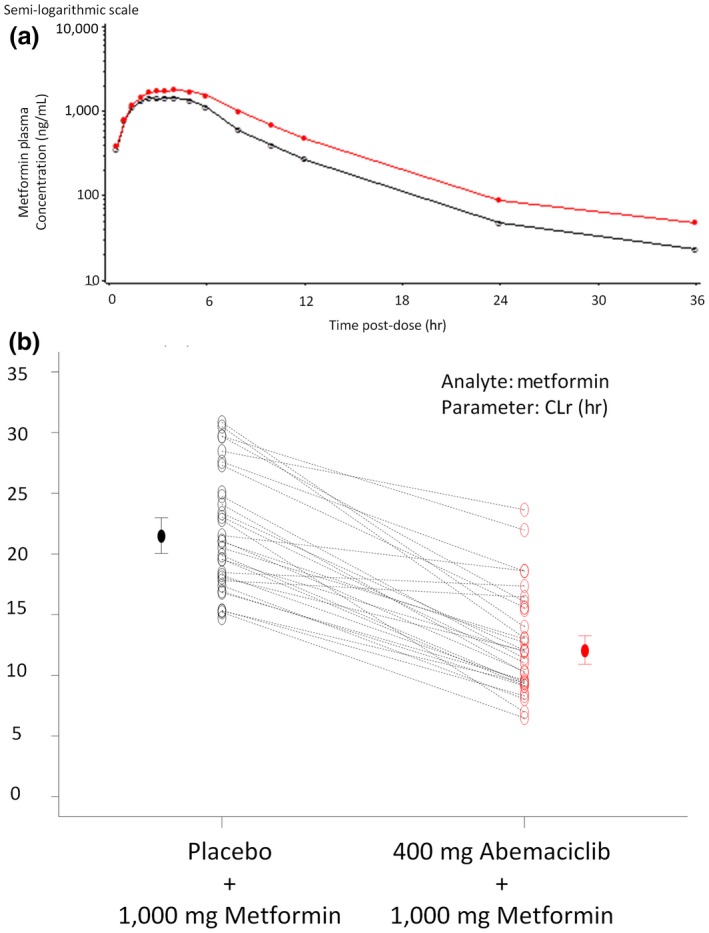
Metformin pharmacokinetics following placebo or abemaciclib administration. (a) Mean metformin plasma concentration‐time curves after administration of metformin with placebo (open circle) or abemaciclib (red circle) to 30 or 28 healthy subjects, respectively. (b) Individual (open circles) and geometric mean values (± 90% confidence interval, closed circles) of metformin renal clearance (CLR) following a single oral dose of 1,000 mg metformin with a single oral dose of placebo (black circle) or 400 mg abemaciclib (red circle).
Table 2.
Summary of metformin pharmacokinetics
| Parameter | Treatment | N | Geometric LS means | Ratio of means | 90% CI | P value |
|---|---|---|---|---|---|---|
| AUC0–∞ (ng.hour/mL) | Placebo + 1,000 mg metformin (reference) | 30 | 12,245 | 1.37 | 1.28−1.46 | NA |
| 400 mg abemaciclib + 1,000 mg metformin (test) | 28 | 16,718 | ||||
| Cmax (ng/mL) | Placebo + 1,000 mg metformin (reference) | 30 | 1,586 | 1.22 | 1.13−1.30 | NA |
| 400 mg abemaciclib + 1,000 mg metformin (test) | 28 | 1,930 | ||||
| CLR (L/hour) | Placebo + 1,000 mg metformin (reference) | 30 | 21.5 | 0.550 | 0.504−0.600 | NA |
| 400 mg abemaciclib + 1,000 mg metformin (test) | 28 | 11.8 | ||||
| CLRS (L/hour) | Placebo + 1,000 mg metformin (reference) | 29 | 16.0 | 0.381 | 0.323−0.450 | NA |
| 400 mg abemaciclib + 1,000 mg metformin (test) | 25 | 6.08 | ||||
| Tmax (hour) | Placebo + 1,000 mg metformin (reference) | 24 | 3.03a | 0.500b | 0−0.983c | 0.070 |
| 400 mg abemaciclib + 1,000 mg metformin (test) | 24 | 3.76a |
CLRS was calculated using Eq. 3 and assumes no renal tubular reabsorption.AUC0–∞, area under the concentration‐time curve from zero to infinity; CI, confidence interval; CLR, renal clearance; CLRS, clearance of renal secretion; Cmax, peak plasma concentration; LS, least squares; NA, not applicable; Tmax, time of maximum plasma concentration.
aMedian. bMedian of differences. cApproximate 90% CI.
Statistically significant decreases in metformin renal clearance (CLR) and clearance of renal secretion (CLRS) were observed in the presence of abemaciclib (Figure 2 and Table 2).
Effect of abemaciclib on iohexol clearance and GFR.
Coadministration of abemaciclib with iohexol did not affect iohexol PK, and there was notable overlap of the iohexol plasma concentration vs. time profiles in the presence of placebo or 400 mg abemaciclib (Figure 3). Iohexol clearance (mGFR) was equivalent following coadministration of iohexol with placebo or abemaciclib, with a ratio of geometric LS means of 0.982 (90% confidence interval (CI) 0.958−1.01, mean mGFR of 89.0 and 85.6 mL/minute for placebo and abemaciclib periods, respectively; Figure 3 b).
Figure 3.
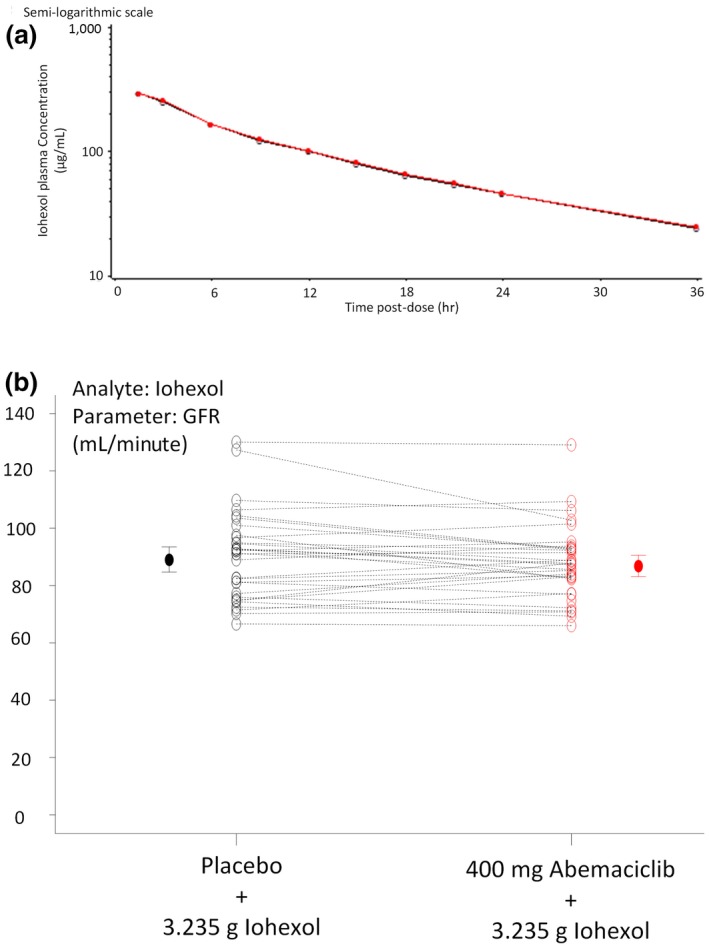
Iohexol pharmacokinetics following placebo or abemaciclib administration. (a) Mean iohexol plasma concentration‐time curves after administration of iohexol with placebo (open circle) or abemaciclib (red circle) to 32 or 30 healthy subjects, respectively. (b) Individual (open circles) and geometric mean values (± 90% confidence interval, solid circles) of growth factor receptor (GFR) following a single infusion of 3.235 g iohexol with a single oral dose of placebo (black circles) or 400 mg abemaciclib (red circles).
Following both placebo and abemaciclib administration, SCr concentrations increased between 10 and 12 hours postdose (mean increases of 25–29 and 35.7–44.6 μmol/L, following placebo and abemaciclib administration, respectively), then decreased over time, with the maximum increase being higher following abemaciclib administration. Based on the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation using SCr, eGFR decreased in the presence of abemaciclib compared to placebo for the combination with metformin (65.4–57.2 mL/minute/1.73 m2) and iohexol (62.2 and 53.6 mL/minute/1.73 m2), respectively.
EGFR calculated using serum cystatin‐C concentrations did not change between abemaciclib and placebo periods. At the time of maximal postdose creatinine concentrations, eGFR calculated using CKD‐EPI showed decreases corresponding to higher creatinine values, whereas the eGFR calculated using cystatin‐C at the same time point did not change. There were no trends in serum concentrations of cystatin‐C following administration of placebo or the study drugs.
There were no trends in serum cystatin‐C or NGAL indicative of renal injury following administration of placebo or abemaciclib with either metformin or iohexol (Figure 4). When the ratio of urine concentrations of KIM‐1 and NGAL to urine creatinine concentrations were calculated, there seemed to be higher variability following dosing of abemaciclib with metformin but no other notable changes following dosing in any of the groups (Figure 5).
Figure 4.
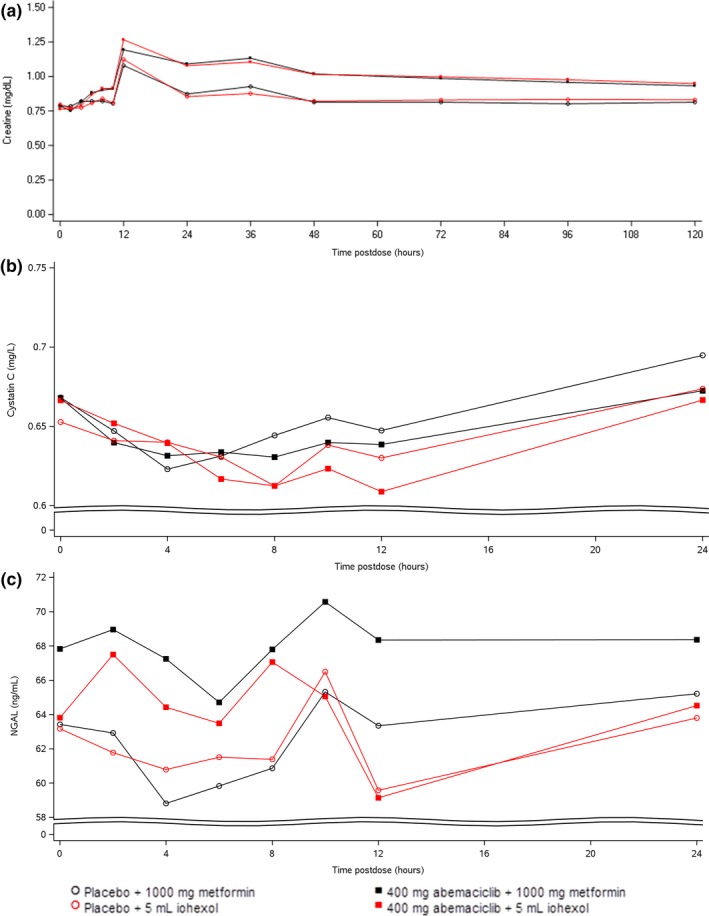
Mean serum creatinine (a), cystatin‐C (b), and neutrophil gelatinase‐associated lipocalin (NGAL) (c) concentrations following a single oral dose of placebo or 400 mg abemaciclib with a single oral dose of 1,000 mg metformin or a single infusion of 3.235 g iohexol in healthy subjects up to 24 hours postdose. Data is presented ± SD.
Figure 5.
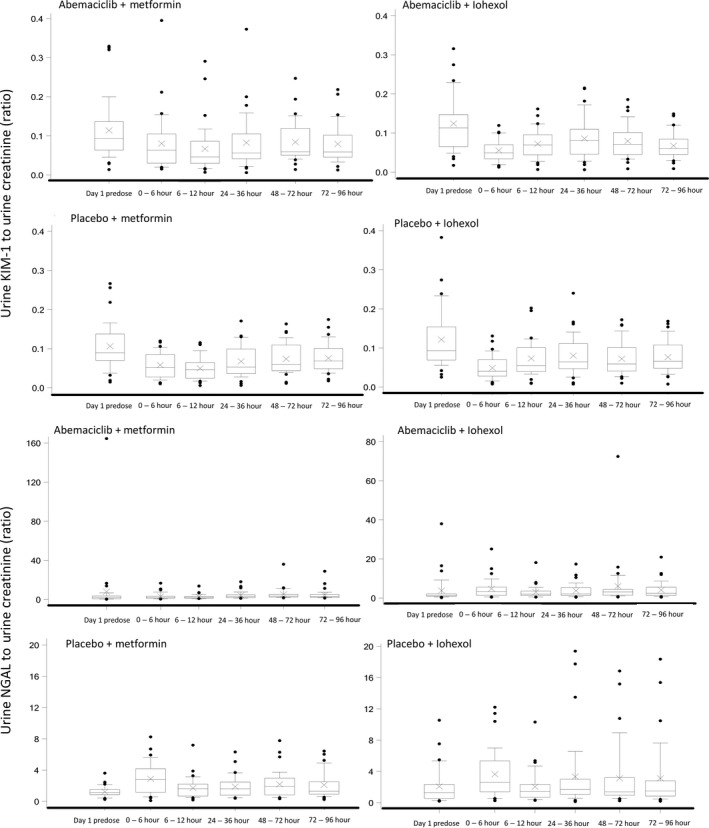
Creatinine‐corrected urine kidney injury molecule (KIM)‐1 and neutrophil gelatinase‐associated lipocalin (NGAL) concentrations following a single oral dose of placebo or 400 mg abemaciclib with a single oral dose of 1,000 mg metformin or a single infusion of 3.235 g iohexol in healthy subjects up to 96 hours postdose. The middle line in each box plot represents the median, the top and bottom margins of the box represent the 75th and 25th percentiles. The whiskers extend to the 90th and 10th percentiles. Data extending beyond the 90th and 10th percentiles are plotted individually.
Discussion
Abemaciclib administration has been associated with mild and variable but consistent elevations in SCr.14, 15, 29 Increased SCr can arise from a reduction in glomerular filtration, indicating damage to the nephron and associated reduction in renal function, or from inhibition of the active tubular secretion of creatinine. The active tubular secretion of creatinine, and other organic cations, is mediated at least in part by OCT2 at the basolateral membrane and MATE1/2‐K at the apical membrane of the proximal tubule.6
Inhibition constants (IC50) determined in vitro predicted that at therapeutic concentrations, abemaciclib and its major circulating metabolites would inhibit OCT2 and MATE1/2‐K clinically.
This study was designed to clinically evaluate the hypothesis that mild, abemaciclib‐induced elevations in SCr are a result of inhibition of active tubular secretion of creatinine and not a reduction in kidney function (as determined by mGFR).
In this study, healthy subjects were included from a broad age range and multiple races. The majority (90%) of subjects were women. Abemaciclib is approved for the treatment of breast cancer, which primarily affects women. Limited evidence exists regarding the influence of sex upon renal transporter expression in humans; however, evidence from rodent studies suggests that sex has an indeterminate effect on transporter expression, with evidence for increased and decreased expression of renal transporter genes in female rodents compared to males.30, 31
A single oral dose of 400 mg abemaciclib, which approximated the steady‐state concentration following dosing with 200 mg abemaciclib twice‐daily, caused a statistically significant increase in metformin exposure of ~30% (a 37% and 22% increase in metformin AUC0–∞ and Cmax, respectively) compared to placebo. A statistically significant decrease in metformin clearance was also observed. However, despite this reduction, metformin terminal half‐life did not change, which may be due to a concurrent reduction in apparent volume of distribution mediated through inhibition of transporters that move metformin into tissues, such as the liver. Metformin clearance is primarily due to active secretion (approximately three times GFR) via secretory transporters, and the secretion clearance is reduced by 62% in the presence of abemaciclib. Metformin is not metabolized in vivo and is excreted unchanged in urine. The transport of metformin from the circulation into the renal epithelia is mediated by OCT2, and the renal excretion is mediated primarily by MATE1 and MATE2‐K. In contrast, the overall CLR of metformin is reduced by only 45%, which is consistent with the observation with iohexol that abemaciclib does not affect GFR. Furthermore, the 62% reduction of metformin secretion clearance suggests that abemaciclib may not completely inhibit the transporters that mediate metformin secretion. These results are consistent with the in vitro findings that abemaciclib and its major metabolites inhibit the transporters OCT2, MATE1, and MATE2‐K, which are involved in the active secretion of substrates, such as metformin and endogenous creatinine. This analysis assumes that tubular reabsorption of metformin is negligible (Eq. (3)). However, if the CLRS were to approximate renal plasma flow (~38 L/hour in a 70‐kg human32), then a fraction reabsorbed of ~50% would be estimated from the metformin CLR we observed (Eq. (2)), and the estimated reduction in metformin CLRS would be modestly lower at ~50%.
Elevations in SCr post abemaciclib dose generally followed the expected course: Notable rises occurred with a peak at 12 hours postdose, and concentrations gradually declined over the 120‐hour collection period, with return to baseline or near‐baseline levels by the end of the washout period or follow‐up. This peak in SCr occurred ~3–5 hours following abemaciclib Tmax. Both placebo groups seemed also to have transient elevations in SCr at 12 hours postdose; however, the SCr elevations were not as great as those observed following abemaciclib dosing and may be related to the meal administered at ~10 hours postdose. The statistical significance of this change was, however, not calculated.
The eGFR (as assessed by CKD‐EPI) showed decreases following abemaciclib dosing, which were not reflected in mGFR (as determined by iohexol clearance), or GFR calculated from serum cystatin‐C concentrations. No notable change in creatinine‐normalized urinary concentrations of NGAL and KIM‐1 (both biomarkers of renal injury24, 33) were observed. These data, combined with the observed changes in metformin PK, indicate that the changes in SCr observed in clinical studies of abemaciclib are due to reversible inhibition of renal tubular secretion of creatinine, and are not the result of acute kidney injury.
The majority of commonly used estimates of GFR, such as those recommended by the Modification of Diet in Renal Disease study34 and CKD‐EPI, rely on accurate steady‐state measurement of SCr, which can introduce errors in estimates of renal function. With a crossover design, this study was able to combine a measurement of “true” GFR (as assessed by iohexol clearance) alongside renal transporter inhibition (as assessed by metformin PK) to both quantify the inhibitory effect of an investigational drug and its impact on renal function in vivo.
The results described confirm that increases in SCr following abemaciclib administration are likely caused by inhibition of renal transporters and that abemaciclib does not affect renal function as assessed by mGFR, or increase in concentrations of urinary biomarkers of renal injury. The findings suggest that patients dosed with abemaciclib will experience a mild (~10–40%) reversible increase in SCr due to renal transport inhibition. However, it is possible that the observed increases in SCr following abemaciclib administration may be further elevated in subjects with already reduced renal function due to reduced renal reserve. When clinically indicated, alternative measurements of renal function, aside from creatinine‐derived eGFR, should be used in patients taking abemaciclib to accurately assess renal function.
Methods
In vitro analysis of transporter interactions
Materials.
Abemaciclib and its metabolites M2 (LSN2839567) and M20 (LSN3106726) were synthesized by Eli Lilly (Indianapolis, IN). Radiolabeled (14C) metformin was purchased from American Radiolabeled Chemicals (St. Louis, MO). All other chemicals were of analytical grade and purchased from commercial sources.
Stably transfected HEK cells expressing OCT2 or vector control (VC) were generated using previously described methods.35 HEK cells transiently transfected with MATE1, MATE2‐K, or VC were purchased from Corning Life Sciences (Transporto cells, Bedford, MA).
In vitro inhibition studies.
All inhibition studies were performed with (14C) metformin as a substrate for 2 minutes at 37°C in VC, OCT2, MATE1, and MATE2‐K cells in 24‐well BioCoat Poly‐D‐Lysine plates (Becton Dickinson, Franklin Lakes, NJ).
OCT2 and VC cells were washed and preincubated for 10 minutes in varying concentrations of inhibitor (0.00038−100 μM abemaciclib, M2, or M20, along with a positive control inhibitor, 100 μM imipramine) in duplicate wells per concentration. Experiments were initiated by the addition of 200 μL (14C) metformin (10 μM, 0.1 μCi/mL) in the presence of 0.00038−100 μM abemaciclib, M2, M20, imipramine, or absence of the inhibitor.
MATE1, MATE2‐K, and VC cells were washed and preincubated in 40 mM ammonium chloride for 20 minutes. Inhibition studies were performed in experiment solution containing 200 μL (14C) metformin (2 μM, 0.2 μCi/mL) in the presence of 0.00038−100 μM abemaciclib, M2, M20, 100 μM pyrimethamine, or absence of inhibitor in duplicate wells per concentration.
The reaction was stopped by addition of ice cold Hanks balanced salt solution. Each well was then aspirated and washed once with ice‐cold phosphate‐buffered saline. Following the final aspiration, 400 μL 1% Triton X‐100 in phosphate‐buffered saline (v/v) was added per well for radiochemical detection and protein quantification.
Analysis of data for in vitro inhibition studies
For all inhibition studies, values were corrected for passive diffusion by subtracting the average velocity of control cells from the velocity at each concentration in transfected cells.
The inhibitor concentration resulting in 50% inhibition (IC50 value) was determined by nonlinear regression analysis using WinNonLin Professional, version 6.4 (Certara L.P., Princeton, NJ), using the following equation:
| (1) |
where [I] is inhibitor concentration, Y min is minimum percentage activity in relation to control, Y max is percentage activity when there is no inhibitor present, and slope is the slope of the curve. Four‐parameter fitting was used for the IC50 estimation for all fittings.
Combined DDI index for each transporter was calculated by adding unbound Cmax (I u) divided by IC50 of abemaciclib, M2, and M20, as described by Lutz and Isoherranen36:
| (2) |
Bioanalytical methods
Abemaciclib and metformin concentrations were measured in plasma and/or urine (as applicable), at Q2 Solutions (Ithaca, NY) and Covance Laboratories (Madison, WI), and iohexol concentrations were measured in plasma at Covance Bioanalytical Services (Indianapolis, IN) using validated liquid chromatograph tandem mass spectrometric methods.
Renal biomarkers were measured in serum and/or urine (as applicable) at Covance Central Laboratory Services (Indianapolis, IN).
Clinical study design
A randomized, single‐center, single‐blind, four‐period, placebo‐controlled crossover study was conducted in healthy subjects (n = 40) at Covance Clinical Research Unit, Dallas, TX. An independent ethical review board (Midlands Institutional Review Board, Kansas) reviewed and approved the trial protocol. The trial was registered at ClinicalTrials.gov (NCT02884089). Enrolled subjects received each of four dosing regimens (400 mg oral abemaciclib or placebo followed 5 hours later by 1,000 mg oral metformin and 400 mg abemaciclib or placebo followed 8 hours later by 3,235 mg iohexol administered as a 15‐minute i.v. infusion) according to a randomized schedule. A 400‐mg dose of abemaciclib was administered, as this was expected to achieve plasma concentrations approximating steady state following twice‐daily dosing with 200 mg, and doses were timed such that the peak plasma concentrations of the coadministered drugs coincided. Subjects underwent a meal stabilization period of 3 days prior to dosing in order to minimize variation in SCr from diet.
Following study drug administration, timed blood samples were collected up to 120 hours post abemaciclib dose, 36 hours post metformin dose and 6 hours post iohexol dose for assessment of the respective analyte concentrations in plasma. Urine metformin concentrations were also assessed. Blood and urine were also collected up to 120 hours postdose for analysis of renal biomarkers serum cystatin‐C, NGAL, and creatinine and urine NGAL, KIM‐1, and creatinine, respectively. Study periods were separated by at least 16 days, except the placebo/metformin dosing period after which there was at least 5 days of separation.
Statistical analysis
PK parameter estimates for metformin and abemaciclib were calculated using standard noncompartmental methods using Phoenix WinNonlin software version 6.4 (Certara USA, Princeton, NJ). Iohexol PK parameters were calculated using a two‐compartment i.v. infusion model.23 Metformin CLR was calculated by dividing the cumulative amount excreted in urine collected for 36 hours by the AUC0–36. 37 In general, CLR is given by:
| (3) |
where fu,p is the fraction unbound in plasma for metformin, which was assumed to be unity (reference), CLRS is the clearance of renal secretion, and F is the fraction of metformin entering the renal tubule that is reabsorbed. In this analysis, we assumed that renal tubular reabsorption was negligible and F was set to zero. Metformin CLRS was calculated for each subject as follows (Eq. (4)):
| (4) |
PK parameter estimates for plasma and urine were evaluated to delineate effects of abemaciclib on metformin PK. Log‐transformed Cmax, AUC0–∞, CLR, and CLRS estimates were evaluated in a linear mixed‐effects analysis of variance model with fixed effects for period, sequence, and treatment (metformin with abemaciclib (test) and metformin with placebo (reference)), and a random effect for subject. The differences were back‐transformed to present the ratios of geometric LS means of metformin with abemaciclib vs. metformin with placebo and the corresponding 90% CIs. Metformin Tmax was analyzed using a Wilcoxon signed rank test. Medians of differences between the abemaciclib and placebo dosing periods, the 90% CI for the medians of differences, and P values were calculated.
The GFR was analyzed using a similar method to that described for metformin PK, in which iohexol with abemaciclib was the test and iohexol with placebo was the reference. The eGFR was calculated using SCr or cystatin‐C using the CKD‐EPI creatinine or cystatin‐C equations.
Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Analysis of exploratory biomarkers
End points for biomarker and safety analyses included serum concentrations of cystatin‐C, NGAL, and creatinine, creatinine CL, and urinary concentrations of NGAL, KIM‐1, and creatinine. Renal biomarker concentrations at the time of maximal creatinine concentration were calculated for each dosing period. Ratios of urine renal biomarkers to urine creatinine were calculated.
Funding
The research described in this manuscript was funded by Eli Lilly and Company. Covance conducted the clinical and bioanalytical studies described in this manuscript. J.C.C., P.K.T., Y.A.P., J.B., A.Y.C., S.D.H., and P.K. are current or former employees of Eli Lilly. J.R. is an employee of Covance. J.V.B. is supported in part by National Institutes of Health (NIH) grants R37D039773 and R01DK072381. J.V.B. is coinventor on kidney injury molecule 1 patents assigned to Partners Healthcare.
Conflict of Interest
J.C.C., P.K.T., Y.A.P., J.B., A.Y.C., S.D.H., and P.K. are current or former employees of Eli Lilly.
Author Contributions
J.C.C., P.K.T., Y.A.P., J.B., A.Y.C., J.R., S.D.H., P.K., and J.V.B. wrote the manuscript. J.C.C., P.K.T., Y.A.P., J.B., A.Y.C., J.R., S.D.H., P.K., and J.V.B designed the research. J.C.C., Y.A.P., J.B., and A.Y.C. performed the research. J.C.C., P.K.T., Y.A.P., J.B., A.Y.C., J.R., S.D.H., P.K., and J.V.B. analyzed the data.
Supporting information
Figure S1. Clinical study subject disposition.
Acknowledgments
The authors thank Carwyn Edwards and Becky Jubb for providing medical writing services on behalf of Covance.
References
- 1. Eli Lilly and Company Verzenio (Abemaciclib) [package insert] (Eli Lilly and Company, Indianapolis, IN, 2017). [Google Scholar]
- 2. Patnaik, A. et al Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non–small cell lung cancer, and other solid tumors. Cancer Discov. 6, 740–753 (2016). [DOI] [PubMed] [Google Scholar]
- 3. Sledge, G.W. et al MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884 (2017). [DOI] [PubMed] [Google Scholar]
- 4. Perrone, R.D. , Madias, N.E. & Levey, A.S. Serum creatinine as an index of renal function: new insights into old concepts. Clin. Chem. 38, 1933–1953 (1992). [PubMed] [Google Scholar]
- 5. Levey, A.S. , Perrone, R.D. & Madias, N.E. Serum creatinine and renal function. Annu. Rev. Med. 39, 465–490 (1988). [DOI] [PubMed] [Google Scholar]
- 6. Lepist, E.‐I. et al Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 86, 350–357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inker, L.A. et al Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367, 20–29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levey, A.S. , Becker, C. & Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 313, 837–846 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schützer, K.‐M. , Svensson, M.K. , Zetterstrand, S. , Eriksson, U.G. & Wåhlander, K. Reversible elevations of serum creatinine levels but no effect on glomerular filtration during treatment with the direct thrombin inhibitor AZD0837. Eur. J. Clin. Pharmacol. 66, 903–910 (2010). [DOI] [PubMed] [Google Scholar]
- 10. Lepist, E.‐I. & Ray, A.S. Renal drug‐drug interactions: what we have learned and where we are going. Expert Opin. Drug Metab. Toxicol. 8, 433–448 (2012). [DOI] [PubMed] [Google Scholar]
- 11. Zhang, Y. et al Impact on creatinine renal clearance by the interplay of multiple renal transporters: a case study with INCB039110. Drug Metab. Dispos. 43, 485–489 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Yonezawa, A. & Inui, K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 164, 1817–1825 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Busch, A.E. et al Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J. Biol. Chem. 271, 32599–32604 (1996). [DOI] [PubMed] [Google Scholar]
- 14. Arya, V. et al Creatinine as an endogenous marker for renal function‐emerging role of transporters in the overall assessment of renal toxicity: PII‐012. Clin. Pharmacol. Ther. 95, S65 (2014). [Google Scholar]
- 15. Chu, X. , Bleasby, K. , Chan, G.H. , Nunes, I. & Evers, R. The complexities of interpreting reversible elevated serum creatinine levels in drug development: does a correlation with inhibition of renal transporters exist? Drug Metab. Dispos. 44, 1498–1509 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Koyner, J.L. et al Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin. J. Am. Soc. Nephrol. 5, 2154–2165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gong, L. , Goswami, S. , Giacomini, K.M. , Altman, R.B. & Klein, T.E. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet. Genomics 22, 820–827 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stage, T.B. , Brøsen, K. & Christensen, M.M.H. A comprehensive review of drug‐drug interactions with metformin. Clin. Pharmacokinet. 54, 811–824 (2015). [DOI] [PubMed] [Google Scholar]
- 19. Somogyi, A. , McLean, A. & Heinzow, B. Cimetidine‐procainamide pharmacokinetic interaction in man: evidence of competition for tubular secretion of basic drugs. Eur. J. Clin. Pharmacol. 25, 339–345 (1983). [DOI] [PubMed] [Google Scholar]
- 20. Kusuhara, H. et al Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects. Clin. Pharmacol. Ther. 89, 837–844 (2011). [DOI] [PubMed] [Google Scholar]
- 21. Song, I.H. et al The effect of dolutegravir on the pharmacokinetics of metformin in healthy subjects. J. Acquir. Immune Defic. Syndr. 72, 400 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dharnidharka, V.R. , Kwon, C. & Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta‐analysis. Am. J. Kidney Dis. 40, 221–226 (2002). [DOI] [PubMed] [Google Scholar]
- 23. Krutzén, E. , Bäck, S.E. , Nilsson‐Ehle, I. & Nilsson‐Ehle, P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J. Lab. Clin. Med. 104, 955–961 (1984). [PubMed] [Google Scholar]
- 24. Bonventre, J.V. Kidney injury molecule‐1 (KIM‐1): a specific and sensitive biomarker of kidney injury. Scand. J. Clin. Lab. Invest. Suppl. 241, 78–83 (2008). [DOI] [PubMed] [Google Scholar]
- 25. Waikar, S.S. et al Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule‐1 in five cohort studies. Nephrol. Dial. Transplant. 31, 1460–1470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wasung, M.E. , Chawla, L.S. & Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 438, 350–357 (2015). [DOI] [PubMed] [Google Scholar]
- 27. European Medicines Agency. Guideline on the investigation of drug interactions (CPMP/EWP/560/95/Rev. 1 Corr. 2). <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf> (2012).
- 28. Food and Drug Administration. In vitro metabolism and transporter mediated drug‐drug interaction studies: guidance for industry. <https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm(2017).
- 29. Mathialagan, S. , Rodrigues, A.D. & Feng, B. Evaluation of renal transporter inhibition using creatinine as a substrate in vitro to assess the clinical risk of elevated serum creatinine. J. Pharm. Sci. 106, 2535–2541 (2017). [DOI] [PubMed] [Google Scholar]
- 30. Morris, M.E. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol. Rev. 55, 229–240 (2003). [DOI] [PubMed] [Google Scholar]
- 31. Babelova, A. , Burckhardt, B.C. , Wegner, W. , Burckhardt, G. & Henjakovic, M. Sex‐differences in renal expression of selected transporters and transcription factors in lean and obese Zucker spontaneously hypertensive fatty rats. J. Diabetes Res. 2015, 483238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie, F. et al Metformin's intrinsic blood‐to‐plasma partition ratio (b/p): reconciling the perceived high in vivo B/P >10 with the in vitro equilibrium value of unity. J. Pharmacol. Exp. Ther. 354, 225–229 (2015). [DOI] [PubMed] [Google Scholar]
- 33. Devarajan, P. Neutrophil gelatinase‐associated lipocalin: a promising biomarker for human acute kidney injury. Biomark. Med. 4, 265–280 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Kidney Foundation . Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 39, S1–S266 (2002). [PubMed] [Google Scholar]
- 35. Posada Maria, M. et al Prediction of transporter‐mediated drug‐drug interactions for baricitinib. Clin. Transl. Sci. 10, 509–519 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lutz, J.D. & Isoherranen, N. In vitro‐to‐in vivo predictions of drug‐drug interactions involving multiple reversible inhibitors. Expert Opin. Drug Metab. Toxicol. 8, 449–466 (2012). [DOI] [PubMed] [Google Scholar]
- 37. Idkaidek, N. , Arafat, T. , Melhim, M. , Alawneh, J. & Hakooz, N. Metformin IR versus XR pharmacokinetics in humans. J. Bioequiv. Availab. 3, 233–235 (2011). [Google Scholar]
- 38. Levey, A.S. et al A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Clinical study subject disposition.


