Abstract
Introduction:
Statins reduce cardiovascular disease risk and are generally well-tolerated, yet up to 0.5% of statin-treated patients develop incapacitating muscle symptoms including rhabdomyolysis. Our objective was to identify clinical factors related to statin-associated muscle symptoms (SAMS).
Methods:
Clinical and laboratory characteristics were evaluated in 748 statin-treated Caucasians (634 with SAMS and 114 statin-tolerant controls). Information was collected on statin type, concomitant drug therapies, muscle symptom history, comorbidities and family history. Logistic regression was used to identify associations.
Results:
Individuals with SAMS were 3.6 times (OR: 3.60, 95% CI: 2.08–6.22) more likely than statin-tolerant controls to have a family history of heart disease. Additional associations included obesity (OR=3.08, 95% CI: 1.18, 8.05), hypertension (OR=2.24, 95% CI: 1.33, 3.77), smoking (OR=2.08, 95% CI: 1.16, 3.74), and statin type.
Discussion:
Careful medical monitoring of statin-treated patients with the associated co-existing conditions may ultimately reduce muscle symptoms and lead to improved compliance.
Keywords: statins, statin-associated muscle symptoms, risk factors, rhabdomyolysis, muscle disease
Introduction
Statins selectively inhibit the rate-limiting enzyme in cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and constitute the main lipid-lowering agents prescribed to more than 40 million Americans. Statins have been the cornerstone in the reduction of risk for cardiovascular and cerebrovascular diseases1. While statins are generally well-tolerated, as many as 10–15% (4–6 million) of Americans develop muscle pain and/or weakness that may be reversible with modification of therapy; however, up to 0.5% of patients (as many as 200,000) with plasma creatine kinase greater than 10 times the upper limit of normal are at risk for severe, incapacitating muscle symptoms including debilitating pain and/or weakness that may be progressive leading to wheelchair dependence; some develop ruptured tendons2–4. The original 2013 American College of Cardiology/American Heart Association guidelines on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults were thought to potentially double the number of individuals taking statins in the U.S.5,6 and, therefore, potentially increase the number of Americans with muscle symptoms and those at risk for serious conditions. The guidelines were revised in 2016 and again in 2017 in which an appropriate target for LDLC concentration was reached and additional therapies were recommended if statins alone did not bring LDLC to target7.
Previously documented risk factors for the development of statin-associated muscle symptoms (SAMS) include but are not limited to a personal or family history of muscle disease or myopathy due to statin therapy, female sex, advanced age, certain concomitant medications, and hypothyroidism8–10.
The goal of our study was to identify clinical factors associated with statin-associated muscle symptoms (SAMS) in a retrospective analysis of statin-intolerant subjects compared to statin-tolerant subjects from actual clinical practice.
Methods
Subjects.
A total of 748 Caucasians (392 males, 356 females) with a history of statin therapy were enrolled in this retrospective study from five centers across the U.S. and Canada between 2004 and 2013. Collaborating institutions included Johns Hopkins University, Baltimore, MD; Cedars-Sinai Medical Center, Los Angeles, CA; the Medical College of Wisconsin, Milwaukee, WI; McMaster University Medical Center, Hamilton, Ontario, Canada; and the University of Oklahoma, College of Medicine, Tulsa, Oklahoma. Referring clinics included internal medicine (including cardiology patients and others), rheumatology (including the Johns Hopkins Myositis Center and other clinics), neuromuscular, and self-referrals in response to solicitations for participants. Caucasians represented more than 90% of referrals. Inadequate numbers from additional races were referred for meaningful statistical analyses.
Individuals were classified as having SAMS based on their responses to a questionnaire outlining statin type and dosage that first led to muscle symptoms, extent of symptoms during statin therapy, including date of onset, and duration of symptoms post statin cessation. The questionnaire was developed initially from clinical and laboratory information provided with specimens from SAMS patients referred to The Robert Guthrie Biochemical & Molecular Genetics Laboratory, Buffalo, NY. Every filled questionnaire was reviewed by one of the authors (GDV) for appropriateness of inclusion in the study. The clinical criteria used for a SAMS classification has been previously described by us11 and others12,13. Muscle symptoms are defined as the onset of muscle pain, weakness, or both muscle pain and weakness attributed to the initiation of statin therapy with no prior history of unusual muscle pain or weakness. The symptoms may begin immediately or within weeks or months after starting therapy. Symptoms may be disabling and life-threatening in severe cases. We relied on muscle symptom severity associated with the initiation of stain therapy as a main criterion for SAMS classification. More than 90% of cases were evaluated by a physician in a clinical setting and information from medical records was provided. Additional information was requested from self-referred subjects regarding their personal and family histories as well as contact information for their physicians for additional information. Participants were asked to provide information for the first statin they were prescribed that led to their symptoms since some were prescribed different statins over the course of their treatment.
The statin-tolerant control group consisted of those who continued to take statins post-enrollment in the study for at least 12 months without developing muscle symptoms. Statin-tolerant subjects completed follow-up questionnaires 6 and 12 months after the initial enrollment to assess whether statin tolerance continued during this period, and all remained free of muscle symptoms at the 12-month follow-up.
Data Collection.
At study enrollment, participants completed questionnaires providing demographic information, statin therapy type, family history of muscle and heart disease, lifestyle factors, comorbid conditions, and concomitant medications. Data from the questionnaires and additional clinical data were entered into a secure Microsoft Access database. An online secure interactive website was developed to query the database for analyses.
Each participant’s medication profile was compared to a key of concomitant medications with known statin drug-drug interactions or independently causative of myopathy without a statin interaction (Supplementary Table). The table key was based on drug-drug interactions listed for each statin plus medications contraindicated with statins and also the mechanism of drug-drug interactions derived from the Micromedex® Drug Reference (as of March 2014). A list of concomitant medications or supplements to potentially treat SAMS was included in the key. Finally, concomitant medications potentially causative of myopathy independent of a drug-drug interaction with a statin were added to the key (e.g., systemic corticosteroids and hydroxychloroquine)14. Four categories of concomitant medications were identified for each individual: those that can 1) treat symptoms of SAMS (e.g., analgesics, skeletal muscle relaxants, and coenzyme Q10); 2) independently cause myopathy (e.g., systemic corticosteroids and hydroxychloroquine); 3) increase statin blood levels (e.g., cytochrome P450 [CYP] 3A4 inhibitors in the participants treated with a CYP3A4-metabolized statin); and 4) increase statin metabolism and, therefore, potentially decrease statin blood levels (e.g., CYP3A4 inducers in the participants treated with a CYP3A4-metabolized statin); see Supplemental Table.
All participants provided informed consent approved by the New York State Department of Health, the University at Buffalo Health Sciences Institutional Review Board, and the respective institutional review boards of collaborating centers.
Statistical Analyses.
Descriptive analyses were performed to characterize differences between SAMS and statin-tolerant groups using t-tests and chi-square tests of independence, as appropriate. Similarly, frequencies of several clinical characteristics and family history information were compared. P-values less than 0.05 were considered statistically significant. We applied Bonferroni corrections to account for multiple testing of characteristics between SAMS and statin-tolerant subjects. The number of medications in each of the four categories of concomitant medications was compared between the two groups via the Wilcoxon rank-sum test. Logistic regression models were used to identify associations between SAMS (dependent variable) and age, sex, statin type, presence of coronary artery disease, family or personal history of heart disease, diabetes, family history of muscle disease, smoking, hypertension, hypothyroidism, obesity, previous heart attack, renal disease, heavy alcohol consumption (self-reported but not quantified), and liver disease (independent variables). The best fitting model was identified using stepwise fitting procedures and removal of variables at p>0.05. Adjusted odds ratios for the main effects of each respective factor and 95% confidence intervals were estimated, controlling for the other variables in the model. SAS was used for all analyses (SAS Institute, Inc., version 9.3, Cary, NC).
Results
Table 1 shows characteristics for the SAMS group versus the statin-tolerant group. No statistically significant differences in age or sex were observed at the start of statin therapy between the two groups. Atorvastatin was the most common statin used. Changing therapy between at least two different statins occurred more frequently in the SAMS group than in the statin-tolerant group. Reasons why individuals with SAMS terminated statin therapy were primarily for unrelenting and intolerable symptoms of muscle pain and/or weakness even with dosage adjustments or changing to a different statin; up to 6% independently reported “foggy memory” or memory loss as their reason for terminating therapy. The length of time under statin therapy before cessation of treatment varied, however, the study design only allowed for a 6-month tracking period for this characteristic.
Table 1:
Statin use in SAMS group versus statin-tolerant group
| Variable, N(%) or Mean ± SD |
SAMS Group (n=634) |
Statin-Tolerant Group (n=114) |
P-value |
|---|---|---|---|
| Age at starting statin, yr | 58.0 ± 10.9 (n=573)a | 58.1 ± 11.9 (n=79)a | 0.90 |
| Sex, Male | 340 (53.6) | 52 (46.4) | 0.16 |
| Statin used | |||
| Atorvastatin | 254 (40.1) | 69 (60.5) | 0.0001b |
| Simvastatin | 113 (17.8) | 11 (9.6) | |
| Rosuvastatin | 68 (10.7) | 5 (4.4) | |
| Lovastatin | 20 (3.2) | 6 (5.3) | |
| Pravastatin | 38 (6.0) | 5 (4.4) | |
| Two statins (switched from one to another) | 129 (20.4) | 10 (8.8) | |
| Other (pitavastatin, fluvastatin or cerivastatin) | 12 (1.8) | 8 (7.0) | |
| Statin dose | |||
| 5 mg | 29 (4.6) | 4 (3.5) | 0.049 |
| 10 mg | 169 (26.7) | 41 (36.0) | |
| 20 mg | 183 (28.9) | 25 (21.9) | |
| 40 mg | 138 (21.8) | 19 (16.7) | |
| 80 mg | 36 (5.6) | 12 (10.5) | |
| Other | 79 (12.4) | 13 (11.4) | |
| Stopped statin therapy | 538 (84.9) | 5 (4.4) | 0.0001 |
| Age stopped statin, yr | 59.9 ±11.0 (n=262) | 59.5±5 (n=6) | |
| Those who stopped statins with known interval (n=424) | |||
| ≤30 days | 47 (11.1) | 0 (0.0) | |
| 30–180 days | 98 (23.1) | 0 (0.0) | |
| ≥180 days | 279 (65.8) | 5 (4.4) | |
Age at start of statin therapy was missing for some participants.
P-value calculated excluding ‘other’ or missing statin type.
SAMS: statin-associated muscle symptoms; mg: milligram
Table 2 shows seven characteristics that differed between the two groups; six factors remained statistically significant after Bonferroni correction for multiple testing (0.05/14=cutoff of p<0.004). The patients with inflammatory muscle disease represented 9% of cases. SAMS cases had myalgias, muscle tenderness, cramping, tendonitis, weakness, fatigue and elevations in plasma CK with most having a combination of two or more symptoms all attributed to statin therapy.
Table 2:
Distribution of clinical characteristics in SAMS group and statin-tolerant group
| Characteristic N (%) |
SAMS Group (n=634) |
Statin-Tolerant Group (n=114) |
P-value* |
|---|---|---|---|
| Family history of heart disease | 341 (53.8) | 22 (19.3) | 0.0001 |
| Hypertension | 322 (50.8) | 27 (23.7) | 0.0001 |
| History of smoking | 239 (37.7) | 18 (15.8) | 0.0001 |
| Obesity | 127 (20.0) | 5 (4.4) | 0.0001 |
| Coronary artery disease | 136 (21.5) | 8 (7.0) | 0.0003 |
| Inflammatory muscle disease | 57 (9.0) | 0 (0.0) | 0.001 |
| Cognitive impairment | 33 (5) | NA | |
| Persistent symptoms ≥6 months | 226 (36) | NA | |
| Progressive course | 234 (37) | NA | |
| CK >4XULN (only 40% had CK measured in test group; 253/634) | 144/253 (57) | 1 | |
| Hypothyroidism | 89 (14.0) | 6 (5.3) | 0.01 |
| Metabolic muscle disease | 19 (3.0) | 0 (0.0) | 0.06 |
| Previous heart attack | 91 (14.4) | 9 (7.9) | 0.06 |
| Heavy alcohol consumption | 17 (2.7) | 0 (0.0) | 0.08 |
| Liver disease | 17 (2.7) | 0 (0.0) | 0.08 |
| Diabetes | 103 (16.3) | 12 (10.5) | 0.12 |
| Family history of muscle disease | 39 (6.2) | 4 (3.5) | 0.26 |
| Renal disease | 15 (2.4) | 1 (0.9) | 0.31 |
P-values denote results chi-square tests.
NA, not reported in statin-tolerant group.
SAMS: statin-associated muscle symptoms; CK: creatine kinase
An abnormal elevation in plasma CK was a main characteristic found in the SAMS group that was essentially absent from the statin-tolerant group. Only 40% of the SAMS group even reported the measurement of CK and of these, 57% stopped taking statins due to a plasma CK elevated to >4XULN. Approximately 37% of the SAMS group had progressively worsening symptoms post-therapy; some had permanent disability.
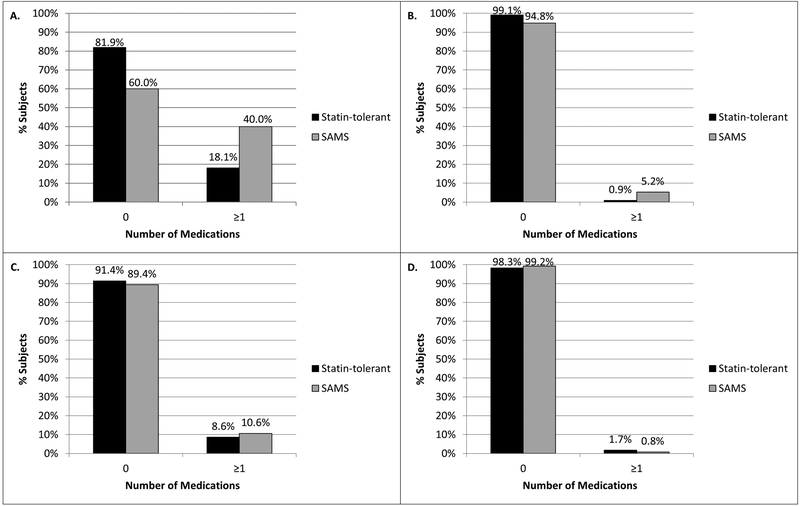
Individuals with SAMS took more medications for the treatment of muscle symptoms than the statin-tolerant group (Figure 1A). SAMS subjects also took more medications causing myopathy independent of a drug-drug interaction with a statin (Figure 1B). However, the SAMS subjects were taking similar numbers of concomitant medications that could increase or decrease statin exposure as in the statin-tolerant group (Figures 1C and 1D, respectively).
Figures 1A-D.
Figure 1A) number of concomitant medications that can treat statin-associated muscle symptoms (SAMS, e.g., analgesics, skeletal muscle relaxants, and coenzyme Q10) in SAMS group vs. statin-tolerant (p<0.0001); Figure 1B) number of concomitant medications that can independently cause myopathy (e.g., systemic corticosteroids and hydroxychloroquine) in SAMS group vs. statin-tolerant group (p=0.038); Figure 1C) number of concomitant medications that can increase statin exposure (e.g. cytochrome P450 [CYP] 3A4 inhibitors in the participants treated with a CYP3A4-metabolized statin) in SAMS group vs. statin-tolerant group (p=0.524); and Figure 1D) number of concomitant medications that can decrease statin exposure (e.g. CYP3A4 inducers in the participants treated with a CYP3A4-metabolized statin) in SAMS group vs. statin-tolerant group (p=0.341). The number of medications in each of these four categories was compared between the SAMS and statin-tolerant groups via the Wilcoxon rank sum test.
Table 3 shows the results from the logistic regression model with the most statistically significant factors associated with SAMS. A total of 549 SAMS subjects and 99 statin-tolerant subjects with complete information were used for the regression analysis. Sex and statin dose were not significantly associated with muscle symptoms and therefore were not included in the final model. Family history of heart disease was the most significant factor; individuals who reported having a family history of heart disease were 3.6 times more likely to be in the SAMS group. Even after consideration of family history of heart disease, several other variables were also identified as having statistically significant independent associations with SAMS; these included obesity, hypertension, history of smoking, and statin type. Compared to individuals who took atorvastatin, those taking rosuvastatin or simvastatin were more likely to be in the SAMS group (Table 3).
Table 3.
Logistic regression model showing the associations between clinical factors and SAMS.
(SAMS group: n=549; statin-tolerant group: n=99)
| Variables | Odds Ratio (95% CI) |
|---|---|
| Family history of heart disease* | 3.60 (2.08, 6.22) |
| Hypertension* | 2.24 (1.33, 3.77) |
| Obesity* | 3.08 (1.18, 8.05) |
| History of smoking* | 2.08 (1.16, 3.74) |
| Rosuvastatin vs Atorvastatin | 2.69 (1.00, 7.24) |
| Simvastatin vs Atorvastatin | 2.42 (1.18, 4.97) |
| Rosuvastatin vs Simvastatin | 1.11 (0.35, 3.49) |
compared to no family history, no hypertension, no obesity, or no smoking history.
SAMS: statin-associated muscle symptoms
In a sensitivity analysis, we evaluated the possibility that hypertension may have developed after individuals stopped statin therapy and before entering our study, reasoning that hypertension could have arisen as a result of statin therapy cessation rather than being independently associated with muscle symptoms. Here, we estimated the interval in years from when hypertensive or normotensive patients with SAMS stopped taking a statin and when they joined the study. The interval was 2.29 years in hypertensive participants and 2.44 years in normotensive participants; this difference was not statistically significant, indicating that termination of statin therapy prior to joining our study was likely not a factor in the development of hypertension.
Discussion
An array of factors was identified that are significantly associated with SAMS, including family history of heart disease, obesity, hypertension, smoking history, and statin type. Previously described risk factors8,12,15 were also found in the SAMS group; heavy alcohol consumption and diabetes were not significantly associated with SAMS, as similarly reported16. While our study could not attribute direct causality, evidence suggests that certain factors deserve further study toward developing a preventive strategy for SAMS.
More than 40 million people take statins in the U.S. and up to 20% (~8 million) have experienced associated muscle symptoms in clinical practice while, in contrast, only 3–6% have been reported in randomized controlled trials17,18; meta-analysis including 42 clinical trials supports these findings19. Clinical trial participants, unlike those from clinical practice, were eliminated if muscle symptoms appeared during the run-in period or if they had comorbidities. Our study derived participants entirely from clinical practice. The vast majority of participants were referred by physicians at collaborating centers with the remainder being self-reported. There are limitations to using self-reported medical histories attributed to drug therapies20; yet there is precedence for self-reporting where up to 85% with perceived SAMS adhered to the Naranjo adverse drug reaction probability scale causality criteria21,22.
As reported by others23, we found no age- or sex-associated risk for developing muscle symptoms. Tendonitis and ruptured tendons, in particular, have been reported in up to 25% of reported cases20,23–25. The most significant predictor of SAMS, family history of heart disease, has several possible explanations. Statins are the cornerstone of treatment for dyslipidemia and are essential for the primary and secondary prevention of cardiovascular disease26. Subjects with a personal or family history of heart disease are at higher risk for cardiovascular disease than the general population and more likely to be encouraged by their physicians to continue statin therapy while tolerating muscle symptoms. Anecdotal evidence was provided to us by a number of subjects in the SAMS group stating that their physicians had directed them to continue statin therapy in spite of significant muscle symptoms. There were also significantly more subjects with a family history of heart disease in the SAMS group than in the statin-tolerant group as was true with other negative health features. It is possible that heart disease singly provides a higher degree of vulnerability for SAMS than other factors.
Additional details were not available as to the type of inflammatory disorder that was present. While these cases could have been excluded from the study, it was not clear whether SAMS or their inflammatory symptoms occurred first; it remains that this feature was significantly more prevalent in SAMS cases vs statin-tolerant.
Individuals with a family history of heart disease may be genetically predisposed for the development of skeletal muscle symptoms since certain genes causative for cardiac disease also have variants implicated in skeletal muscle disorders, e.g., the titin gene (TTN)27. Titin plays a critical role in sarcomere structure and organization during development and in mature muscles. Pathogenic variants in TTN have been associated with a number of muscle disorders, e.g., tibial muscular dystrophy, limb girdle muscular dystrophy, centronuclear myopathy and others27. We have found an association between pathogenic variants in TTN and SAMS (unpublished data).
Washout periods (2 to 6 weeks) prior to changing treatment regimens are used to render patients statin-tolerant12; however, identification of an alternative dosage or drug has only been successful in about 60% of patients with SAMS16. Approximately 20% of the SAMS group in our study attempted a regimen change without success in alleviating symptoms. Bias may exist in that symptomatic individuals may have successfully changed their regimens and not joined our study. A recent survey on the management of patients with SAMS showed that improved management to mitigate CVD risk and CVD-associated mortality is needed; 52% of SAMS cases were prescribed a lower statin dose while leaving approximately 48% with no statin therapy28.
Elevated plasma CK was strongly associated with SAMS; 57% of those tested had elevations greater than 4 times the upper limit of normal, the level at which cessation of statin therapy is recommended12. Unfortunately, only 40% of test group subjects even reported CK measurement suggesting that up to 60% of cases did not have CK measured. Presently, plasma CK measurement is only recommended for symptomatic patients and screening is not performed routinely in patients taking statins29,30. However, clinicians are increasingly aware of the value in measuring CK prior to prescribing statins for baseline determinations and increased awareness that asymptomatic elevation of plasma CK is relatively common in the general population30.
Persistent muscle symptoms for 6 months or longer following cessation of statin therapy is not uncommon and was found in more than one-third of our SAMS group. Increased genetic risk for underlying metabolic myopathies in SAMS, e.g., McArdle disease, carnitine palmitoyltransferase II deficiency, and malignant hyperthermia, suggests that statins likely trigger these underlying disorders11,31. Histochemical and biochemical evidence for an association with myotonic dystrophy, Kennedy disease32 and mitochondrial disease33 also exists. Statin-induced necrotizing autoimmune myopathy with associated anti HMG-CoA reductase antibodies has been identified in SAMS cases and may also account for prolonged symptoms post-therapy34. Only members of the SAMS group reported evidence for inflammatory myopathy with none in the statin-tolerant group; the HMG-CoA reductase autoantibody was not determined.
As expected, SAMS subjects were taking significantly more medications and supplements used to treat SAMS. However, the lack of a significant difference between the two groups in the number of medications used that interact with statins by increasing or decreasing statin blood levels was unexpected since statin exposure correlates with risk of muscle symptoms.35,36 Therefore patients taking a CYP3A4 inhibitor along with a CYP3A4-metabolized statin would be expected to have increased risk of SAMS. Alternatively, patients taking a CYP3A4 inducer would be expected to have decreased risk of SAMS; we did not find either of those associations to be statistically significant. A possible explanation could be that although known inhibitors and inducers of statin metabolism affect blood levels of the statins, the effects do not always translate into a patient-reported outcome such as SAMS. A limitation is that the dose of the co-medication was not provided in all cases and the dose-dependence of drug-drug interactions with statins may explain our inability to detect a significant difference. Moreover, physicians may have empirically adjusted the statin dose based on known pharmacokinetic interactions present in the subject’s medication profile. Also, the distinctions between the categories of the concomitant medications is not always clear and may depend on the indication. For example, systemic corticosteroids can cause myopathy independent of a drug-drug interaction with a statin14, however, because they are anti-inflammatory medications, they could also be used to treat SAMS. The study questionnaire did not capture the level of detail needed to distinguish such ambiguities. Another limitation is that our co-medication scheme did not account for the magnitude of drug-drug interaction; i.e., each interacting co-medication was given equal weight. Future directions include a more in-depth analysis of co-medication interactions, a closer evaluation of the specific culprit medications in each of the four categories, discovery of novel drug-drug interactions, and adjustment for clinical covariates.
The results suggest that individuals with certain characteristics in their personal and family histories, some of which are modifiable, may be at higher risk for SAMS. Our findings point to the potential for modification or prevention of SAMS using personalized medicine to mitigate the risk of rare adverse side effects without compromising cardiovascular benefit. Further study is needed to determine whether clinicians should alter their treatment regimens for individuals with a constellation of pre-existing adverse health indicators.
Supplementary Material
Acknowledgements.
This work was supported by grants from the John R. Oishei Foundation; an Interdisciplinary Research and Creative Activities Award from the University at Buffalo’s Office of the Vice President for Research (GDV); and National Institutes of Health grants R01 HL085800 (GDV) & R21 AR055704 (PJI); NIH Loan Repayment Program L30 HL110279 (JAL); NIH K23 GM100372 (JPK), & R01 MD011307 (JPK). The People’s Pharmacy (Joe & Terry Graedon) for assistance in the recruitment of self-referrals.
List of acronyms or abbreviations
- ACC
American College of Cardiology
- AHA
American Heart Association
- CI
confidence interval
- CK
creatine kinase
- CVD
cardiovascular disease
- HMG-CoA reductase
3-hydroxy-3-methylglutaryl coenzyme A reductase
- LDLC
LDL-cholesterol
- OR
odds ratio
- RCT
randomized controlled trials
- SAMS
statin-associated muscle symptoms
- ULN
upper limits of normal
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest: Dr. Lisa Christopher-Stine has received royalties from Inova Diagnostics for intellectual property interests related to the anti-HMG-CoA reductase assay. All other authors have no conflicts of interest.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63: 2889–2934. [DOI] [PubMed] [Google Scholar]
- 2.Vladutiu G Genetic predisposition to statin myopathy. Curr Opin Rheumatol 2008;20: 648–655. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003;289: 1681–1690. [DOI] [PubMed] [Google Scholar]
- 4.Bays H Statin safety: an overview and assessment of the data−−2005. Am J Cardiol 2006;97: 6C–26C. [DOI] [PubMed] [Google Scholar]
- 5.Yeboah J, Sillau S, Delaney JC, Blaha MJ, Michos ED, Young R, et al. Implications of the new American College of Cardiology/American Heart Association cholesterol guidelines for primary atherosclerotic cardiovascular disease event prevention in a multi ethnic cohort: Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2015;169: 387–395 e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagidipati NJ, Navar AM, Mulder H, Sniderman AD, Peterson ED, Pencina MJ. Comparison of Recommended Eligibility for Primary Prevention Statin Therapy Based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA 2017;317: 1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr., DePalma SM, et al. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2017;70: 1785–1822. [DOI] [PubMed] [Google Scholar]
- 8.Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009;150: 858–868. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad Z Statin intolerance. Am J Cardiol 2014;113: 1765–1771. [DOI] [PubMed] [Google Scholar]
- 10.Needham M, Mastaglia FL. Statin myotoxicity: a review of genetic susceptibility factors. Neuromuscul Disord 2014;24: 4–15. [DOI] [PubMed] [Google Scholar]
- 11.Vladutiu GD, Isackson PJ, Kaufman K, Harley JB, Cobb B, Christopher-Stine L, et al. Genetic risk for malignant hyperthermia in non-anesthesia-induced myopathies. Mol Genet Metab 2011;104: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36(17):1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosshammer D, Schaeffeler E, Schwab M, Morike K. Mechanisms and assessment of statin-related muscular adverse effects. Br J Clin Pharmacol 2014;78: 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones JD, Kirsch HL, Wortmann RL, Pillinger MH. The causes of drug-induced muscle toxicity. Curr Opin Rheumatol 2014;26: 697–703. [DOI] [PubMed] [Google Scholar]
- 15.Mancini GB, Tashakkor AY, Baker S, Bergeron J, Fitchett D, Frohlich J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013;29: 1553–1568. [DOI] [PubMed] [Google Scholar]
- 16.Harris LJ, Thapa R, Brown M, Pabbathi S, Childress RD, Heimberg M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011;5: 299–307. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011;78: 393–403. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med 2013;158: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J 2014;168: 6–15. [DOI] [PubMed] [Google Scholar]
- 20.Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA, The National Lipid Association’s Muscle Safety Expert Panel. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol 2014;8: S58–71. [DOI] [PubMed] [Google Scholar]
- 21.Cham S, Evans MA, Denenberg JO, Golomb BA. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy 2010;30: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30: 239–245. [DOI] [PubMed] [Google Scholar]
- 23.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005;19: 403–414. [DOI] [PubMed] [Google Scholar]
- 24.Marie I, Delafenetre H, Massy N, Thuillez C, Noblet C, Network of the French Pharmacovigilance Centers. Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990–2005 and review of the literature. Arthritis Rheum 2008;59: 367–372. [DOI] [PubMed] [Google Scholar]
- 25.Beri A, Dwamena FC, Dwamena BA. Association between statin therapy and tendon rupture: a case-control study. J Cardiovasc Pharmacol 2009;53: 401–404. [DOI] [PubMed] [Google Scholar]
- 26.Abt TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf 2011;10: 373–387. [DOI] [PubMed] [Google Scholar]
- 27.Chauveau C, Rowell J, Ferreiro A. A rising titan: TTN review and mutation update. Hum Mutat 2014;35: 1046–1059. [DOI] [PubMed] [Google Scholar]
- 28.Hovingh GK, Gandra SR, McKendrick J, Dent R, Wieffer H, Catapano AL, et al. Identification and management of patients with statin-associated symptoms in clinical practice: A clinician survey. Atherosclerosis 2016;245: 111–117. [DOI] [PubMed] [Google Scholar]
- 29.Elhayany A, Mishaal RA, Vinker S. Is there clinical benefit to routine enzyme testing of patients on statins? Expert Opin Drug Saf 2012;11: 185–190. [DOI] [PubMed] [Google Scholar]
- 30.Rallidis LS, Fountoulaki K, Anastasiou-Nana M. Managing the underestimated risk of statin-associated myopathy. Int J Cardiol 2012;159: 169–176. [DOI] [PubMed] [Google Scholar]
- 31.Vladutiu GD, Simmons Z, Isackson PI, Tarnopolsky M, Peltier WL, Barboi AC, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006;34: 153–162. [DOI] [PubMed] [Google Scholar]
- 32.Tsivgoulis G, Spengos K, Karandreas N, Panas M, Kladi A, Manta P. Presymptomatic neuromuscular disorders disclosed following statin treatment. Arch Intern Med 2006;166: 1519–1524. [DOI] [PubMed] [Google Scholar]
- 33.Hou T, Li Y, Chen W, Heffner RR, Vladutiu GD. Histopathologic and Biochemical Evidence for Mitochondrial Disease Among 279 Patients with Severe Statin Myopathy. J Neuromuscul Dis 2017;4: 77–87. [DOI] [PubMed] [Google Scholar]
- 34.Albayda J, Mammen AL. Is statin-induced myositis part of the polymyositis disease spectrum? Curr Rheumatol Rep 2014;16: 433. [DOI] [PubMed] [Google Scholar]
- 35.Hermann M, Bogsrud MP, Molden E, Asberg A, Mohebi BU, Ose L, et al. Exposure of atorvastatin is unchanged but lactone and acid metabolites are increased several-fold in patients with atorvastatin-induced myopathy. Clin Pharmacol Ther 2006;79: 532–539. [DOI] [PubMed] [Google Scholar]
- 36.Talameh JA, Kitzmiller JP. Pharmacogenetics of Statin-Induced Myopathy: A Focused Review of the Clinical Translation of Pharmacokinetic Genetic Variants. J Pharmacogenomics Pharmacoproteomics 2014;5(2):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.