Abstract
Until cytoplasmic recapping was discovered, decapping was thought to irreversibly destine an mRNA to degradation. Contradicting this idea, we readily observe mRNAs targeted by cytoplasmic capping in uncapped, yet stable forms. 5’ RACE shows that nearly all uncapped ends correspond to CAGE (capped analysis of gene expression) tags and that the recapping of ZNF207 mRNA may be restricted to a single splice isoform. Here, a modified RACE approach detected uncapped 5’ RNA ends mapping to 46 mRNAs in cells expressing a dominant negative cytoplasmic capping enzyme and in normal cells. 11 of 46 cloned mRNAs also contained splice isoform-limiting sequences. Collectively, these data reinforce earlier work and suggest that alternative splicing may play a role in targeting transcripts for– and/or determining the position of– cytoplasmic capping.
Keywords: cytoplasmic recapping, CAGE, cap homeostasis, alternative splicing, stable uncapped 5’ ends, epitranscriptomics
Introduction
The N7-methylguanosine (m7G) cap is a critical modification added to the 5’ ends of mRNAs during transcription. Nuclear capping occurs in three steps. First, the triphosphatase domain of RNA guanylyltransferase and 5’-phosphatase (RNGTT, capping enzyme hereafter) removes the gamma phosphate from the first transcribed nucleotide [1]. Capping enzyme then transfers an inverted guanosine residue onto the nascent RNA using its guanylyl transferase domain [1]. Finally, the cap is methylated by RNA guanine-7 methyltransferase (RNMT) [2]. The m7G cap is vital for proper mRNA splicing, processing, packaging, export, and translation [3, 4]. The m7G cap is also a focal point of different RNA surveillance pathways and helps protect the mRNA from cellular 5’ to 3’ exonucleases [4]. Although evidence for uncapped mRNA species with mono- and di-phosphate 5’ ends dates back to the 1970’s [5, 6], until recently, decapping was generally thought to irreversibly commit an mRNA to degradation [7, 8].
Certain nonsense-mediated decay (NMD) mRNA decay intermediates generated from premature termination codon containing β-globin mRNAs were found to violate this rule [9, 10]. These 5’-truncated mRNA decay intermediates were stable, accumulated in a capped form [9], and required NMD to form [11]. Since NMD occurs exclusively in the cytoplasm, these data alluded to a cytoplasmic RNA recapping mechanism [3, 9]. The capped β-globin decay intermediates remained an unexplained oddity for many years; however, the advent of reliable transcriptome-wide methods to assay different RNA populations uncovered the existence of different uncapped RNA species in plants [12, 13] and in mammals [14]. Finally, capped analysis of gene expression (CAGE), a technique designed to identify transcription start sites by tagging the position of m7G caps on mRNAs, showed that nearly 25% of mammalian m7G caps were not located at known transcription start sites [15]. Rather, they were found within the body of a transcript, and the authors hypothesized that mRNAs could be recapped after cleavage or truncation [15].
Cytoplasmic capping was first described around this time [16]. That work identified an RNA capping activity in cytoplasmic extracts and observed that preventing cytoplasmic capping by overexpressing a dominant negative cytoplasmic capping enzyme (DN-cCE) inhibited the cell’s ability to recover from stress (Figure 1A) [16]. The cytoplasmic protein Nck1 coordinates a monophosphate kinase and capping enzyme to facilitate the first two steps of cytoplasmic capping (Figure 1A) [17]. The newly added cap is then methylated by RNMT [18]. Caps added in the cytoplasm appear to be indistinguishable from nuclear-added caps. Roughly 2000 mRNAs are targeted by cytoplasmic capping and those mRNAs are less frequently associated with translating polysomes when DN-cCE is expressed [19]. Recent work has also shown that uncapped cytoplasmic capping targets retain translationally functional poly(A) tails [20] and that 5’ RACE can detect uncapped ends from 5’ truncated mRNAs in the vicinity of CAGE tags in cells expressing DN-cCE [21]. Collectively, these findings implied that novel 5’ ends from truncated mRNAs should be observable among the non-translating mRNAs in cells where cytoplasmic capping was unimpeded in addition to those where cytoplasmic capping had been blocked. Importantly, alluding to its biological significance, cytoplasmic capping also appears to have evolved independently in trypanosomes [22].
Figure 1. Utilizing a dominant negative capping enzyme (DN-cCE) to study cytoplasmic capping.

(A) A schematic demonstrating the organization and activities of the cytoplasmic capping complex. (B) Cytoplasmic extracts were harvested (see methods) from uninduced (-Dox) and DN-cCE-expressing U2OS cells (+Dox) and assayed via western blotting. A western blot probed with anti-Myc antibody shows inducible expression of DN-cCE from a representative pair of samples. The bottom portion of the same blot was probed with an anti-tubulin antibody and serves as a loading control. (C) A flow chart showing the workflow for the traditional 5’ RACE (blue) and dual adaptor RACE (green) arms of this two-armed study.
In this report, we readily detected uncapped and 5’ truncated mRNAs in cytoplasmic extracts from uninduced U2OS cells, where cytoplasmic capping was normal, in addition to those expressing DN-cCE. Nearly all of the uncapped ends mapped to CAGE tags. While most of the putative recapping sites yielded sequences that could not discriminate between different splice isoforms, surprisingly, one cluster of 5’ ends was specific to a single isoform of ZNF207 mRNA. To better evaluate this intriguing possibility, we used a dual adaptor RACE approach to identify additional uncapped mRNA ends. Upon closer examination, almost one in four of the cloned mRNA ends contained sequences specific to a limited number of splice isoforms. These findings offer the first hints that sequence cues may help determine the identity of– and/or the position of– cytoplasmic mRNA recapping.
Materials and Methods
Cell culture and preparation of cytoplasmic extracts.
DN-cCE cells generated in [16] were cultured in McCoy’s medium (Gibco) supplemented with 10% tetracycline-tested fetal bovine serum. 150 mm tissue culture dishes were seeded with 3 × 106 log-phase cells. 24h later, expression of DN-cCE was induced by adding doxycycline (Dox) (1 μg/ml). 24h later, cells were rinsed twice with phosphate buffered saline, scraped from plates with cell lifters, and pelleted by centrifugation at 2500 ×g for 5m. Cytoplasmic extracts were prepared as in earlier works [16–21]. Breifly, pellets were lysed in 5 volumes of ice cold cytoplasmic lysis buffer (50 mM Tris-HCl pH 7.5, 10 mM KCl, 10 mM MgCl2, 150 mM NaCl, 0.2% NP-40, 2 mM DTT, 0.5 mM PMSF, 1 mM sodium orthovanadate, supplemented with 5 μl/ml RNAseOUT (Life Technologies), 25 μl/ml protease inhibitor cocktail (Sigma), 10 μl/ml each phosphatase inhibitor cocktails 2 and 3 (Sigma)) followed by incubation on ice for 10m with gentle agitation every 2m. Nuclei were removed by centrifuging at 16100 ×g for 10m at 4°C and supernatants were used for western blotting and cytoplasmic RNA isolation.
Western blotting
Western blots were performed as in [20]. Cytoplasmic extracts were heated to 95°C in 1x Laemmli sample buffer for 5m, separated on 10% Mini-PROTEAN® TGX™ gels (Bio-Rad) and transferred onto Immobilon-FL PVDF membranes (Millipore). Membranes were blocked with 1% milk in Tris-buffered saline containing 0.05% Tween-20 (TBS-T), and incubated with rabbit anti-MYC or anti-Tubulin polyclonal antibodies (1:2500 dilution) in 0.1% nonfat milk overnight at 4°C. Blots were washed with TBS-T, incubated with LI-COR IR-680 anti-rabbit secondary antibody (1:10000 dilution) for 2h, washed with TBS-T and visualized using Odyssey V.3.0.30 software (LI-COR).
Oligonucleotides used in this study
All oligonucleotides used in this study are shown in Table S1.
Isolation and preparation of cytoplasmic RNA
RNA was harvested from cytoplasmic extracts using Direct-Zol kits (Zymo) according to the manufacturer’s reaction clean-up protocol (including DNase I digestion). 5 μg of cytoplasmic RNA was treated with Ribo-Zero Gold (Illumina), and all RACE experiments used the rRNA-depleted equivalent of 1 μg of cytoplasmic RNA.
5’ rapid amplification of cDNA ends (RACE)
Standard 5’ RACE was performed exactly as described in [21]. 25% of the ligation reaction was reverse transcribed (Superscript III, Life Technologies) using the manufacturer’s gene specific primer protocol (Table S1). After RNase H treatment, cDNAs were amplified using nested PCR with the indicated (Table S1) primers. Primary nested PCR products were purified using DNA Clean and Concentrator-5 columns (Zymo). Secondary PCRs were performed using CAGE site targeting primers (Table S1) as in [21], column purified, cloned into the pGEM-T-easy vector system (Promega), transformed, plated onto plates containing ampicillin, Isopropyl β-D-1-thiogalactopyranoside (IPTG), and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal), and incubated at 37°C overnight. White colonies were assayed by colony PCR using primers complimentary to T7 and SP6 promoter sequences (Table S1). Products were separated using 2% agarose gels and ~300+ bp inserts were Sanger sequenced at the OSU Genomics Shared Resource. Sequences lacking RACE adaptors or those mapping to ribosomal RNA were discarded, and adaptor-containing sequences were identified using NCBI’s Basic Local Alignment Search Tool (BLAST) and splice variants were assigned using NCBI databases.
CAGE databases
CAGE tags mapping to the RefSeq annotated transcription start site and cloned 5’ RACE ends of ITGB1, SARS, and ZNF207 mRNAs were mined from the following predefined track: [FANTOM CAGE TSS] All FANTOM5 CAGE libraries (n=1897 pooled) the week of Oct 15, 2018 using the ZENBU genome browser [23]. Only CAGE tags mapping to the sense strand were retained.
Gene Ontology (GO) analysis
Gene IDs from Table S3 were loaded into the Gene Ontology Consortium’s website (http://geneontology.org/page/go-enrichment-analysis) and GO searches (performed on 1/17/2019) used the consortium’s default parameters. LYSMD1 mRNA was listed as unclassified and was excluded from the results.
Reverse transcription, qPCR and standard RT-PCR
1 μg of cytoplasmic RNA harvested from induced and uninduced DN-cCE cells was spiked with 0.1 ng of luciferase control mRNA (Promega) and reverse transcribed with Superscript III (Life Technologies) using the manufacturer’s random priming protocol. Traditional RT-PCRs used gene specific primers with 2X Hot Start Master Mix (Apex) and were separated on agarose gels and visualized with a Gel Doc (Bio-Rad). Triplicate qPCR reactions used SsoAdvanced Universal SYBR Green Supermix as instructed by the supplier (Bio-Rad) and data were analyzed via CFX Maestro software (Bio-Rad). All amplicons for qPCR and RT-PCR were validated by purifying bands from agarose gels and Sanger sequencing.
Dephosphorylation and phosphorylation of RNA ends
5 μg of mRNA harvested from the mRNP fractions of polysome gradients harvested during [20] was Ribo-Zero Gold-depleted and 50% of the resulting RNA was heated to 65°C for 5m and flash cooled on ice. Samples were treated with recombinant shrimp alkaline phosphatase (rSAP) (NEB). To minimize precipitation steps, rSAP treatment used 1x polynucleotide kinase (PNK) buffer, where rSAP activity is reduced. Therefore, the reaction time was increased to 3h at 37°C. rSAP was inactivated by adding EDTA and heating to 65°C for 5m. Mixtures were then supplemented with additional MgCl2, 10x PNK buffer, ATP, and PNK. Samples were incubated for 1h at 37°C and reactions were stopped with EDTA and heat denaturation. Kinased RNAs were ethanol precipitated and resuspended in water.
Dual adaptor RACE
The dual adaptor RACE protocol utilizes rSAP and PNK treated RNA (above) and was performed as standard 5’ RACE until the ligation step was completed. 25% of the ligation reaction was primed with the poly(A) adjacent adaptor (Table S1) and reverse transcribed with Superscript III (Life Technologies) according to the manufacturer’s instructions for oligo dT priming. cDNA was RNase H digested and PCR amplified (25 cycles) using primers targeting the 5’ and 3’ adaptor sequences (Table S1). PCR products were purified, ligated, transformed, plated, and colonies were screened and sequenced as above.
Results and Discussion
Most uncapped 5’ ends for ITGB1, SARS, and ZNF207 mRNAs support earlier observations and map to CAGE tags
Previous works have shown that uncapped forms of mRNAs targeted by cytoplasmic recapping were enriched in non-translating RNA pools [19], retain translationally functional poly(A) tails [20], and had uncapped 5’ mRNA ends in the vicinity of CAGE tags [21] when cytoplasmic capping was blocked. A large population of uncapped yet stable mRNAs, called natively uncapped mRNAs, were also present when cytoplasmic capping was normal [19]. We reasoned that these novel 5’ mRNA ends should be observable among non-translating mRNAs in cells where cytoplasmic capping was unimpeded as well as those expressing DN-cCE (Figure 1B). Three mRNA targets, a cell membrane receptor (ITGB1), a seryl tRNA synthetase (SARS), and a cell cycle- linked kinetochore- and microtubule-binding protein (ZNF207), were revisited with more detailed 5’ RACE experiments using cytoplasmic RNA harvested from U2OS cells to evaluate this possibility [20].
In total, 98 positive clones were obtained, 51 from uninduced and 47 from DN-cCE- expressing cells, which mapped to 28 different truncated 5’ ends (Figure 2, Table S2). The recovery of uncapped mRNA ends in uninduced cells, where cytoplasmic capping is normal, in addition to DN-cCE-expressing cells confirms earlier published results and shows that a pool of uncapped mRNAs are present in normal cells [19]. When examined closely, 62 of the cloned 5’ RACE ends (Figure 2A, Table S2) exactly matched (41), or were within 7nt (21) of previously reported uncapped 5’ ends, meaning that nearly two-thirds of these 5’ RACE clones agree with previously published data [21]. Of the assayed mRNAs, ITGB1 showed the largest difference when detecting downstream uncapped 5’ ends in DN-cCE-expressing or uninduced cells (Figure 2B, Table S2). In contrast, downstream 5’ ends for SARS and ZNF207 mRNAs were more readily detectable in cells where cytoplasmic capping was blocked (Figure 2B, Table S2). As expected, these results show that different uncapped mRNA species behave differently in vivo although these data are insufficient to suggest a possible regulatory mechanism to account for the observed differences.
Figure 2. The uncapped 5’-ends of truncated cytoplasmic capping-targeted mRNAs correspond to CAGE tags.

Three mRNAs known to be cytoplasmic capping targets were assayed using 5’ RACE. (A) The total number of novel 5’ ends detected, their overlap with, or proximity to, previously published 5’ RACE data are indicated. (B) The number of 5’ truncated mRNA ends mapping to each assayed cytoplasmic capping target are shown. Whether they were detected in the presence or absence of DN-cCE is also indicated. (C) The overlap between the 5’ ends identified in this work and previously published CAGE tags, plus the positions of the 5’ RACE ends in regards to the coding sequences of their mRNAs, are indicated. (D) Schematics showing the positions of the truncated 5’ ends cloned by 5’ RACE relative to the full-length ZNF207 mRNA. The green box on each line represents the coding sequence of the mRNA. The full-length mRNA (top) is indicated by m7G, and the nucleotide positions (for isoform 2) of the novel, 5’-truncated ends are shown to the left of each schematic. The number of times each 5’ end was cloned (blue box) and the number of CAGE tags mapping to that base (grey box) are indicated to the right of each schematic. @ denotes an exact 5’ RACE match to uncapped ends published in [21].
The previous work also mined 18 CAGE libraries to determine if these uncapped 5’ ends correlated with published CAGE tags. This analysis was revisited by using the Zenbu interface for Phantom5 to mine (see methods) nearly 1900 CAGE libraries (Figures 2C, S1) for CAGE tags mapping to these three mRNAs [23]. In total, 26 of the 28 downstream, uncapped 5’ ends for ZNF207, SARS and ITGB1 mRNAs detected by our 5’ RACE experiments map to documented CAGE tags (Figures 2C, S1). Meaning that nearly all of these 5’ ends correspond to cap positions distinct from the annotated transcription start site and several lie within the coding sequences of their respective mRNAs (Figures 2D, S1). While downstream transcription initiation events can’t be ruled out as the source of the observed CAGE tags, such events are unlikely to be the direct source of the novel 5’ ends observed here. This is because mRNAs are capped co-transcriptionally, meaning that they would need to be decapped prior to being identified in our 5’ RACE protocol. Importantly, recapping and translation from the majority of these truncated mRNAs would yield truncated polypeptides.
An alternatively spliced isoform of ZNF207 mRNA is preferentially recovered by 5’ RACE
A closer examination of the 5’ RACE products showed that seventeen of twenty 5’ RACE clones near the middle of ZNF207 mRNA included exon 8/10 junction sequences (Table S2). The three predominant isoforms of ZNF207 mRNA have been shown to differ by their inclusion of exons 6 and 9 (Figure 3A) with exon 8/10 junctions exclusively present in isoform 2 [24]. Importantly, the three outliers were too short and lacked that isoform-defining junction (Figure 3A, Table S2). One possible explanation of this result was that only isoform 2 was expressed in U2OS cells. Traditional RT-PCR experiments show that only isoforms 2 and 3 of ZNF207 mRNA are expressed in U2OS cells (Figure 3B). These RT-PCR experiments also show that isoform 2 is more common than isoform 3 and that the total levels of ZNF207 mRNA decreased slightly in response to DN-cCE expression (Figure 3B). The latter result was validated with qPCR experiments which show a ~20% reduction in total ZNF207 mRNA levels upon DN-cCE treatment. Further, both expressed isoforms of ZNF207 decrease similarly upon DN-cCE induction (Figure 3C). The functional relevance of this isoform-specific recapping is still unclear, but expression isoform 2 of ZNF207 is enriched in differentiated cells, with isoform 3 being more prevalent in cells that retain stem cell-like characteristics [25].
Figure 3. ZNF207 isoform 2 is a target of cytoplasmic mRNA recapping.
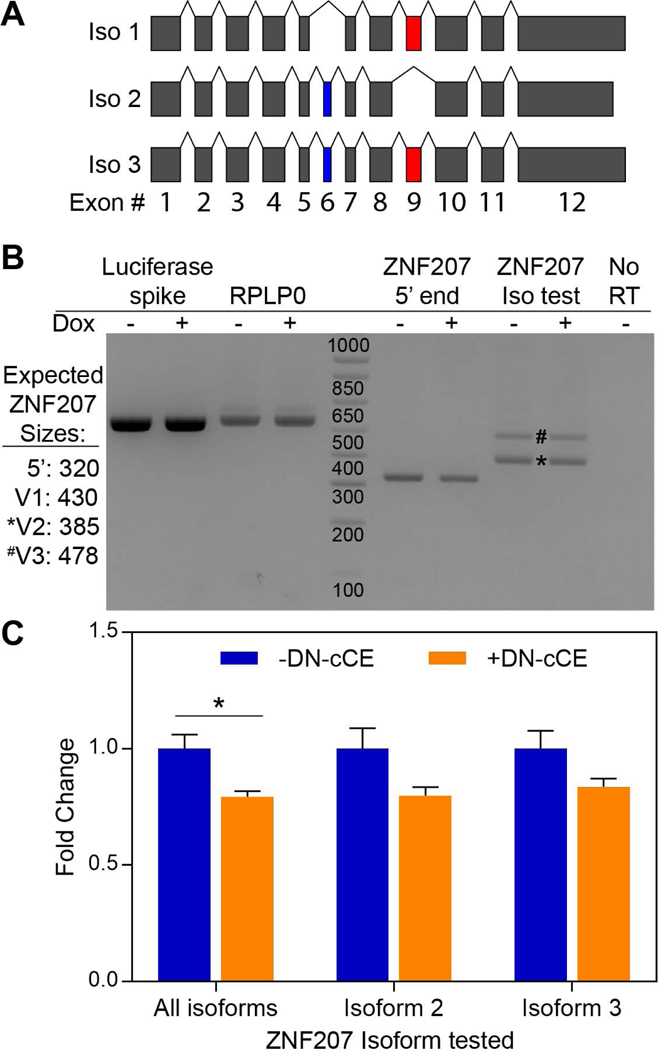
(A) An exon map (exons drawn to scale) of the three most common ZNF207 splice isoforms. The isoform-distinguishing alternatively spliced exons, 6 and 9 are shaded (blue) and (red) respectively. (B) RNA was purified from cytoplasmic extracts of normal and DN-cCE-expressing cells, spiked with 0.1 ng of Luciferase RNA, reverse transcribed, and ZNF207 isoforms were assayed by standard RT-PCR. The predicted sizes of RT-PCR products for each isoform are shown to the left of the gel. Reactions using cDNA from a minus reverse transcriptase (No RT) sample (ZNF207 iso test PCR primers) and RT-PCR reactions targeting spiked luciferase and endogenous RPLP0 serve as controls. A representative agarose gel (three independent experiments) is shown. The positions of isoform 2- and 3-specific bands are marked by an * and # respectively. (C) cDNA remaining from above was assayed for expressed ZNF207 isoforms by qPCR (see methods). All data were normalized to spiked Luciferase mRNA and an endogenous control mRNA (RPLP0). The data shown are the means (± SEM) of three independent experiments each of which was analyzed in triplicate. The asterisk indicates a T-test p-value of <0.05.
Every 5’ RACE read targeting ITGB1 and SARS mRNAs spanned multiple exons; however, none of them could be conclusively mapped to an individual splice isoform. While this was the case, nearly all (29/35) of the SARS clones mapped either to isoform 1 and 3, suggesting that the non-coding isoform 2 of SARS, NR_034072.1, is unlikely to be cytoplasmic capping target (Table S2).
Identification of novel mRNA targets and uncapped 5’ ends for cytoplasmic mRNA recapping
Since uncapped ZNF207 mRNA was enriched in a single splice isoform, we reasoned that alternative splicing may help define which mRNAs were targeted by cytoplasmic capping. This hypothesis was tested by implementing a dual adaptor RACE (Figure 1C) strategy to identify novel 5’ RNA ends. As the guanylyl transferase activity of cytoplasmic capping enzyme is the penultimate step of cytoplasmic recapping, expressing DN-cCE may create a population of uncapped RNAs with 5’ diphosphate ends (Figure 1A) [5, 17]. Since 5’ RACE requires a 5’ monophosphate end, rSAP was used to remove all phosphate groups from the 5’ end of the RNA, and PNK treatment returned a single phosphate to any uncapped RNAs. Importantly, rSAP and PNK treatments have no effect on capped RNAs. In total, 75 mRNA ends mapping to 46 different mRNAs (Figure 4A, Table S3) were cloned and sequenced. A gene ontology search showed that these 46 genes were enriched in genes involved in RNA binding and catabolism, translation initiation, and ribosome components (Table S4). Eleven mRNAs were cloned multiple times with RPL31 (14 clones) being the most common. A total of 60 new 5’ ends were identified, of which 39 were cloned only once (Figure 4, Table S3). As before, recovering uncapped mRNA ends in cells where cytoplasmic capping is unimpeded concurs with earlier results that showed stable uncapped mRNAs in normal cells [19]. Further, even with a limited number of clones, 6 mRNAs previously identified as cytoplasmic capping targets were recovered and validated (Table S3) [19].
Figure 4. Dual adaptor RACE finds novel 5’ ends from truncated mRNAs.

(A) A numerical summary of the novel 5’ mRNA ends discovered using the dual adaptor RACE approach. (B) A table listing the eleven mRNAs which included splice isoform-specific sequences. Also included are the number of 5’ ends observed, the number of possible and excluded isoforms and the whether the clones were observed in DN-cCE-expressing (+) or uninduced (−) cells. (C) RNA was purified from cytoplasmic extracts of uninduced and DN-cCE-expressing cells, reverse transcribed, and RPL31 isoforms were assayed by qPCR (see methods). All data were normalized to an external spike mRNA (Luciferase) and an endogenous control mRNA (RPLP0). The data shown are the means (± SEM) of three independent experiments each of which was analyzed in triplicate. *** indicates a T-test p-value of <0.0001.
Most, but not all, recapping substrates could yield translatable ORFs when recapped
Most (59/75) of the novel 5’ ends reside within the 5’ UTR or the coding sequence of their host RNAs, suggesting that a truncated mRNA recapped at that position could possibly be translated into an N-terminally truncated polypeptide. As 5’ UTR elements such as RNA secondary structures and upstream open reading frames are known to influence translation, truncations within 5’ UTR sequences can also affect the translational efficiency or regulation of their mRNAs [26, 27]. Of the fourteen 5’ ends that mapped within 5’ UTRs, four (eIF3D, HMCES, LRP5L-2, and LYSMD1) would truncate more than 150 bases from the mRNA’s traditional 5’ UTR (Table S3). As the number of putative cytoplasmic recapping sites continues to expand, it will be interesting to elucidate how different 5’ UTR sequence elements affect cytoplasmic recapping.
At the other extreme, five novel 5’ mRNA ends (DNAJB5, EEF1D, EMC6, NDUFA6, and RPL18A) were between 4 and 110 bases away from their stop codons, and four additional mRNAs (ATP6AP1, DAPK3, MTX3, and SPARC) had novel 5’ ends that were located entirely within the 3’UTR of the mRNA (Table S3). Since these nine truncated mRNAs begin close to- or after the stop codon, and do not contain obvious open reading frames, their translation into proteins or polypeptides is unlikely. It is possible that such recapping targets could function as competing endogenous RNAs (ceRNAs). ceRNAs are becoming better characterized, and some have been shown to act as miRNA sponges to modulate the regulation of miRNA-targeted genes in certain contexts [28–31]. CD44, VCAN, and ESR1 are just a few mRNAs whose 3’ UTRs have been shown to serve as ceRNAs that regulate the expression of other mRNAs [32–34]. Further work is required to test this possibility.
The truncated 5’ mRNA ends detected by dual adaptor RACE may be determined by alternative splicing
As above, nearly every cloned sequence spanned one or more splice junctions; although, in most cases, they could not differentiate between splice isoforms. However, in eleven cases, our clones contained splice junctions that excluded one or more isoforms (Figure 4B, Table S3). The result was most pronounced for RPL31 mRNA, where 11 of 14 newly cloned ends contained sequences that were restricted to isoform 1 (Table S3). As with the isoform-specific reads for ZNF207 mRNA above, the three ambiguous clones were too short to contain splice isoform defining sequences. qPCR experiments determined that the three best characterized protein coding RPL31isoforms were all expressed in U2OS cells, although at substantially different levels, with isoform 1 being the most common and 2 being the rarest. The levels of RPL31 mRNA isoforms were differentially affected by expressing DN-cCE. While the total pool of RPL31mRNA and isoform 1 did not change significantly in response to DN-cCE expression (Figure 4C), the two more poorly-expressed isoforms decreased roughly two-fold.
Significant progress has been made in characterizing the proteins involved in, and the mRNAs targeted by, mammalian cytoplasmic mRNA recapping [35]. Nck1 nucleates the assembly of the cytoplasmic capping complex via interactions between its SH3 domains and capping enzyme [17]. RNMT is also part of the complex and methylates the newly capped transcripts making them identical to a nuclear-capped mRNAs [18]. A newly published paper has shown that RNA binding proteins can interact directly with capping enzyme and may recruit RNAs to the cytoplasmic capping complex [36]. As for the targets of cytoplasmic capping, a preliminary list of mRNAs has been identified [19], CAGE tags were shown to approximate the positions of uncapped 5’ mRNA ends [21], and uncapped cytoplasmic capping targets were shown to retain translatable poly(A) tails [20]. The work presented here reinforces and extends upon these earlier observations [19, 21].
Until now, comparatively little progress has been made in determining the rules that designated an mRNA for, or marked the position of, cytoplasmic mRNA recapping. The observation that a single splice isoform of ZNF207, and possibly other mRNAs, is targeted by recapping is a provocative one. It suggests that recapping may be determined by the sequences of the mRNAs themselves. This implies that cytoplasmic capping is a regulated process and that sequence elements included, excluded, or generated by alternative splicing may determine the occurrence and/or position of cytoplasmic recapping. While a possible link between alternative splicing and cytoplasmic capping is exciting, since only a small number of transcripts were cloned and each splice isoform-defining transcript appeared only a handful of times, a more in-depth analysis is still required. Importantly, this work also confirms that a population of mRNAs, the natively uncapped pool, exists in an uncapped, yet stable, form even when cytoplasmic capping is normal [19–21]. Collectively, these data offer the first insights into identifying an mRNA recapping signature sequence that not only selects mRNAs for recapping but may also mark the actual recapping sites.
Supplementary Material
Schematics showing the positions of the novel 5’ ends cloned by 5’ RACE relative to full-length (A) ITGB1 and (B) SARS mRNAs. The green boxes in each schematic represent the coding sequence of the mRNA. The full-length transcript for each mRNA is indicated by m7G, and the nucleotide positions of the novel, 5’-truncated ends are shown to the left of each diagram. The number of times each 5’ end was cloned (blue box) and the number of CAGE tags mapping to that nucleotide position in the mRNA (grey box) are indicated to the right. The @ denotes an exact 5’ RACE match to uncapped ends published in [21]
Acknowledgements
DLK conceived, designed, performed (or supervised) the experiments, and wrote the paper. MRB cloned, screened, and identified the novel 5’ ends for the dual adaptor RACE experiments and RA performed the RT-PCR and RT-qPCR e1xperiments. MRB, RA, and DLK edited the manuscript. This work was supported by grant GM084177 from the National Institute of General Medical Sciences to Daniel R. Schoenberg and by startup funds provided by the Houston Methodist Research Institute (to DLK). Startup funds from the Houston Methodist Research Institute (to Daniel Kiss) were also used for this research. The authors would like to thank Dr. Daniel Schoenberg his support of this work and his suggestions on the manuscript. MRB (undergraduate researcher) was supported by a Research Scholar award and a Mayer’s Summer Research Scholarship from The Ohio State University’s Office of Undergraduate Research and Creative Inquiry and College of Arts and Sciences respectively. The OSU Genomics Shared Resource receives support from the National Cancer Institute (P30CA016058). The content is solely the responsibility of the authors and does not represent the official views of The Ohio State University, the Houston Methodist Research Institute, or the National Institutes of Health.
Abbreviations:
- m7G
N7-methylguanosine cap
- RNMT
RNA guanine-7 methyltransferase
- NMD
nonsense-mediated decay
- CAGE
capped analysis of gene expression
- RACE
rapid amplification of cDNA ends
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- X-Gal
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
- DN-cCE
dominant negative cytoplasmic capping enzyme
- Dox
doxycyclin
References
- 1.Ramanathan A, Robb GB & Chan SH (2016) mRNA capping: biological functions and applications, Nucleic Acids Res. 44, 7511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ensinger MJ & Moss B (1976) Modification of the 5’ terminus of mRNA by an RNA (guanine-7-)-methyltransferase from HeLa cells, J Biol Chem. 251, 5283–91. [PubMed] [Google Scholar]
- 3.Schoenberg DR & Maquat LE (2009) Re-capping the message, Trends Biochem Sci. 34, 435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao X, Chang JH, Kilic T, Tong L & Kiledjian M (2013) A mammalian pre-mRNA 5’ end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing, Mol Cell. 50, 104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schibler U & Perry RP (1977) The 5’-termini of heterogeneous nuclear RNA: a comparison among molecules of different sizes and ages, Nucleic Acids Res. 4, 4133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schibler U & Perry RP (1976) Characterization of the 5’ termini of hn RNA in mouse L cells: implications for processing and cap formation, Cell. 9, 121–30. [DOI] [PubMed] [Google Scholar]
- 7.Decker CJ & Parker R (1993) A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation, Genes Dev. 7, 1632–43. [DOI] [PubMed] [Google Scholar]
- 8.Grudzien-Nogalska E & Kiledjian M (2017) New insights into decapping enzymes and selective mRNA decay, Wiley Interdiscip Rev RNA. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SK & Maquat LE (1992) Human beta-globin mRNAs that harbor a nonsense codon are degraded in murine erythroid tissues to intermediates lacking regions of exon I or exons I and II that have a cap-like structure at the 5’ termini, EMBO J. 11, 3271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim SK, Sigmund CD, Gross KW & Maquat LE (1992) Nonsense codons in human beta-globin mRNA result in the production of mRNA degradation products, Mol Cell Biol. 12, 1149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mascarenhas R, Dougherty JA & Schoenberg DR (2013) SMG6 cleavage generates metastable decay intermediates from nonsense-containing beta-globin mRNA, PLoS One. 8, e74791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory BD, O’Malley RC, Lister R, Urich MA, Tonti-Filippini J, Chen H, Millar AH & Ecker JR (2008) A link between RNA metabolism and silencing affecting Arabidopsis development, Dev Cell. 14, 854–66. [DOI] [PubMed] [Google Scholar]
- 13.Jiao Y, Riechmann JL & Meyerowitz EM (2008) Transcriptome-wide analysis of uncapped mRNAs in Arabidopsis reveals regulation of mRNA degradation, Plant Cell. 20, 2571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karginov FV, Cheloufi S, Chong MM, Stark A, Smith AD & Hannon GJ (2010) Diverse endonucleolytic cleavage sites in the mammalian transcriptome depend upon microRNAs, Drosha, and additional nucleases, Mol Cell. 38, 781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Affymetrix, E. T. P. & Cold Spring Harbor Laboratory, E. T. P. (2009) Post-transcriptional processing generates a diversity of 5’-modified long and short RNAs, Nature. 457, 1028–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka Y, Kedersha NL & Schoenberg DR (2009) Identification of a cytoplasmic complex that adds a cap onto 5’-monophosphate RNA, Mol Cell Biol. 29, 2155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee C, Bakthavachalu B & Schoenberg DR (2014) The cytoplasmic capping complex assembles on adapter protein nck1 bound to the proline-rich C-terminus of Mammalian capping enzyme, PLoS Biol. 12, e1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trotman JB, Giltmier AJ, Mukherjee C & Schoenberg DR (2017) RNA guanine-7 methyltransferase catalyzes the methylation of cytoplasmically recapped RNAs, Nucleic Acids Res. 45, 10726–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee C, Patil DP, Kennedy BA, Bakthavachalu B, Bundschuh R & Schoenberg DR (2012) Identification of cytoplasmic capping targets reveals a role for cap homeostasis in translation and mRNA stability, Cell Rep. 2, 674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiss DL, Oman KM, Dougherty JA, Mukherjee C, Bundschuh R & Schoenberg DR (2016) Cap homeostasis is independent of poly(A) tail length, Nucleic Acids Res. 44, 304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiss DL, Oman K, Bundschuh R & Schoenberg DR (2015) Uncapped 5’ ends of mRNAs targeted by cytoplasmic capping map to the vicinity of downstream CAGE tags, FEBS Lett. 589, 279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignatochkina AV, Takagi Y, Liu Y, Nagata K & Ho CK (2015) The messenger RNA decapping and recapping pathway in Trypanosoma, Proc Natl Acad Sci U S A. 112, 6967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Severin J, Lizio M, Harshbarger J, Kawaji H, Daub CO, Hayashizaki Y, Consortium F, Bertin N & Forrest AR (2014) Interactive visualization and analysis of large-scale sequencing datasets using ZENBU, Nat Biotechnol. 32, 217–9. [DOI] [PubMed] [Google Scholar]
- 24.Toh CX, Chan JW, Chong ZS, Wang HF, Guo HC, Satapathy S, Ma D, Goh GY, Khattar E, Yang L, Tergaonkar V, Chang YT, Collins JJ, Daley GQ, Wee KB, Farran CA, Li H, Lim YP, Bard FA & Loh YH (2016) RNAi Reveals Phase-Specific Global Regulators of Human Somatic Cell Reprogramming, Cell Rep. 15, 2597–607. [DOI] [PubMed] [Google Scholar]
- 25.Fang F, Xia N, Angulo B, Carey J, Cady Z, Durruthy-Durruthy J, Bennett T, Sebastiano V & Reijo Pera RA (2018) A distinct isoform of ZNF207 controls self-renewal and pluripotency of human embryonic stem cells, Nat Commun. 9, 4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppek K, Das R & Barna M (2018) Functional 5’ UTR mRNA structures in eukaryotic translation regulation and how to find them, Nat Rev Mol Cell Biol. 19, 158–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritsch C, Herrmann A, Nothnagel M, Szafranski K, Huse K, Schumann F, Schreiber S, Platzer M, Krawczak M, Hampe J & Brosch M (2012) Genome-wide search for novel human uORFs and N-terminal protein extensions using ribosomal footprinting, Genome Res. 22, 2208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ & Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology, Nature. 465, 1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A & Bozzoni I (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA, Cell. 147, 358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, Lieberman J, Rigoutsos I & Pandolfi PP (2011) Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs, Cell. 147, 344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, Krauthammer M, Halaban R, Provero P, Adams DJ, Tuveson DA & Pandolfi PP (2011) In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma, Cell. 147, 382–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeyapalan Z, Deng Z, Shatseva T, Fang L, He C & Yang BB (2011) Expression of CD44 3’-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis, Nucleic Acids Res. 39, 3026–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DY, Jeyapalan Z, Fang L, Yang J, Zhang Y, Yee AY, Li M, Du WW, Shatseva T & Yang BB (2010) Expression of versican 3’-untranslated region modulates endogenous microRNA functions, PLoS One. 5, e13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu HS, Llobet-Navas D, Yang X, Chung WJ, Ambesi-Impiombato A, Iyer A, Kim HR, Seviour EG, Luo Z, Sehgal V, Moss T, Lu Y, Ram P, Silva J, Mills GB, Califano A & Sumazin P (2015) Cupid: simultaneous reconstruction of microRNA-target and ceRNA networks, Genome Res. 25, 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trotman JB & Schoenberg DR (2018) A recap of RNA recapping, Wiley Interdiscip Rev RNA, e1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trotman JB, Agana BA, Giltmier AJ, Wysocki VH & Schoenberg DR (2018) RNA-binding proteins and heat-shock protein 90 are constituents of the cytoplasmic capping enzyme interactome, J Biol Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematics showing the positions of the novel 5’ ends cloned by 5’ RACE relative to full-length (A) ITGB1 and (B) SARS mRNAs. The green boxes in each schematic represent the coding sequence of the mRNA. The full-length transcript for each mRNA is indicated by m7G, and the nucleotide positions of the novel, 5’-truncated ends are shown to the left of each diagram. The number of times each 5’ end was cloned (blue box) and the number of CAGE tags mapping to that nucleotide position in the mRNA (grey box) are indicated to the right. The @ denotes an exact 5’ RACE match to uncapped ends published in [21]


