Abstract
Infections threaten successful outcomes after kidney transplantation. Our aim was to determine if the number, types of infections and the risk factors for common infections differed between older compared to younger kidney transplant (KT) recipients in the first year after surgery. We performed a single center retrospective cohort study. Between 2011–2015, 91 KTs were performed in patients ≥ 65 years of age; these were matched 1:1 (by year of transplantation, sex and race) to controls aged 40–60 years. Over 90% of both groups had an infectious complication. Urinary tract infections (UTIs) and CMV viremia were significantly more frequent in older recipients. Older adults had more late onset UTIs, including after stent removal. CMV viremia was more frequent in older adults in the 1–6 months post-transplant period. Due to our center-specific protocol utilizing pre-emptive monitoring in the CMV recipient-seropositive population, the higher CMV incidence in the aged recipient was driven by this subpopulation of older adults. No difference in pneumonias or bloodstream infections were found, nor in surgical complications, rejection or graft loss. Mortality was higher at one-year post transplant in the older recipients (9.9% vs 1.1%; p=0.018). Prophylactic and immunosuppressive strategies may need to be altered for older KT recipients.
Keywords: Kidney Transplantation, Aged, Infection
Introduction:
In the U.S., older adults represent 39% of the total population with ESRD1; over 250,000 persons 65 years and older are suffering from end-stage-renal-disease (ESRD). Transplantation is the treatment of choice for ESRD including in older adults for whom transplantation offers a survival benefit over dialysis, and outcomes such as mortality, graft loss and death-censored graft loss are favorable 2–4. Accordingly, age is no longer a contraindication for kidney transplantation5 and the number of older adults receiving kidney transplants has increased substantially6, representing for the first time in 2017 over 20% (3,666) of the total kidney transplants performed nationally [based on OPTN data as of August 28, 2018].
Despite acceptable outcomes2,7, infection has been associated with significant morbidity and mortality in older kidney transplant (KT) recipients8–10. Older adults are at high risk of infections11,12 due to immunosenescence, frailty, functional impairment and multiple comorbidities13. In the aged KT recipient, ESRD, the stress of surgery and immunosuppressive therapies further increase the risk of infections, and as such, increase the chance of a poor outcome10. To date, no change in immunosuppressive or prophylactic therapy is recommended based on the age of an adult KT recipient. The concept of individualized maintenance immunosuppression has been explored in KT recipients but has yet to be implemented in every center14–17.
There is a gap in the knowledge regarding infectious complications after kidney transplantation in older adults and in how infections differ from their younger counterparts. The primary objectives of this study were to determine if the number, type and risks for common infections differed among older compared to younger KT recipients. Secondary objectives were to determine if the number of rejection episodes, graft survival, patient survival, and hospital admission differed among older compared to younger KT recipients.
Materials and methods:
Study design and Study Center
This was a single center retrospective cohort study of kidney-only transplants performed between 2011–2015 at Duke University Medical Center in Durham, NC, a high volume transplant center; 551 kidney transplants were performed during the study period. Older KT recipients were defined as adults aged ≥65 years; younger KT recipient “controls” were defined as 40–60 years of age.
The study was approved by the Duke University Health System Institutional Review Board for Clinical Investigation (Pro00076804).
Patient cohorts
An institutional tool, the Duke Enterprise Data Unified Content Explorer (DEDUCE)18, was used to identify all KT recipients during the 5-year study period and their age. All 91 patients aged ≥65 that received a kidney-only transplant were included. Of the 257 potential controls aged 40–60 years, 91 patients were randomly matched 1:1 to the older KT recipients by year of transplantation, sex and, if possible, race.
Antimicrobial prophylaxis
The typical antimicrobial prophylaxis after KT included Pneumocystis jiroveci pneumonia (PJP) prophylaxis for twelve months and/or for three months after an acute rejection, whichever was longer. CMV prophylaxis depended on CMV serostatus. For donor and recipient negative CMV serostatus, valacyclovir or acyclovir was used for 90 days if the herpes simplex (HSV) serostatus was positive. For CMV seropositive recipients, preemptive monitoring was performed, which included weekly CMV monitoring for 12 weeks. High risk patients (CMV mismatch, or CMV seropositive recipients receiving anti-thymocyte globulin induction) received ganciclovir/valganciclovir for 180 days; following cessation of prophylaxis, CMV PCR monitoring was performed every 2 weeks for a minimum of 3 months. Additionally, viral prophylaxis was given after treatment with alemtuzumab or anti- thymocyte globulin for acute rejection, either until CD4>100 cells/mcl, or in the case of anti-thymocyte globulin, for 30 days. Standard perioperative antibacterial prophylaxis included cefazolin, or, if penicillin allergic, clindamycin +/− ciprofloxacin; this could be continued to up to 24 hours after surgery end time.
Data Extraction
Demographic, clinical, microbiological and outcome data were extracted manually from the medical charts. Data collected were managed using REDCap™ electronic data capture tool hosted at Duke19. Infection data collection included information about infectious syndromes, microbiological data (sample collection with culture type, serologies and polymerase chain reaction (PCR) and immunohistochemistry if applicable). Standard definitions and definitions per CDC/NSHN as described elsewhere were used20,21. Corticosteroid dose was calculated as the mean daily dose of prednisone equivalent in the seven days prior to infection. Functional status data were available for mobility and were classified as “independent”, “needs assistance” (e.g. cane, walker) or “dependent” for ambulation. Levels of calcineurin inhibitors were not collected due to their unreliability of being true troughs in a retrospective review. The standard approach is to maintain tacrolimus levels within 5–10 ng/ml during the first year, roughly 8–10 ng/ml in the first month, 6–8 ng/ml up to month 3 and lower thereafter, depending on the individual patients’ sensitization and infection history.
Statistical Analysis
Descriptive results are shown as total numbers/percentages, mean/standard deviations and medians/ interquartile range (IQR). Several analyses were performed, including the total number of infections. Several types of infections were measured: pneumonia, urinary tract infection (UTI), surgical site infection (SSI), intraabdominal infection (other than SSI), blood stream infection (BSI), infective endocarditis (IE), skin and soft tissue infection (SSTI), Clostridium difficile colitis, meningitis, osteomyelitis, prosthetic joint infection (PJI), hepatitis, sepsis, central line-associated bloodstream infection (CLABSI), candidemia, other (not listed previously), as well as cytomegalovirus (CMV) and BK polyomavirus (BKV) viremia and disease. Along with standard definitions of uncomplicated, complicated and catheter-associated UTI consistent with CDC/NHSN guidelines20, asymptomatic bacteriuria was also included in the definition of UTI for the first year after kidney transplantation. Concerning coagulase-negative Staphylococcus BSI, only cases not deemed to be contaminants were included in the analysis.
Analysis methods:
The primary aim was to assess the predictors of infections within groups, and differences between groups. Patients were followed until death, or the one-year mark after transplant, whichever occurred first. Thus, the rates of infections controlled for the ‘time on study’. Several versions of infections were assessed. First, the total number of all types of infections was analyzed by Poisson regression to incorporate differing time on study due to death. Second, the total number of unique types and the number of infections within types was analyzed by Poisson regression. Third, since infections often co-occur on the same date, we calculated the number of unique dates (episodes) when an infection occurred. These were analyzed by Proportional Hazards to assess the (1) risk of infection, (2) differences between age groups in that risk, and, (3) the change in risk of subsequent infection following an infection. In an exploratory analysis, a stepwise logistic regression model was employed to determine risk factors for infections and UTI. The candidate variables for the prediction model were: diabetes mellitus, cardiovascular disease (CVD), history of pre-transplant genitourinary conditions, prior transplant, donor age, deceased donation, extended criteria donor (ECD), donor after cardiac death (DCD), anti-thymocyte globulin induction and ureteral stent use. Those variables found to be significant (p<0.05) were carried to the final analysis. Statistical analysis was performed using SAS software, version 9.4. Copyright© SAS Institute Inc., Cary, NC, USA.
Results:
Baseline characteristics
Ninety-one kidney-only transplants were performed in adults aged ≥65 years at our institution between January 1, 2011 and December 31, 2015. Ninety-one younger KT controls aged 40–60 years were matched randomly to these older KT recipients. Baseline characteristics are shown in Table 1. Four older Asian KT recipients did not have controls matched for race. Diabetes mellitus and cardiovascular disease were significantly more frequent in the older adults. Regarding donor characteristics, there was a significant difference in age with a median of 46 years (range 2–69) for the older adults, versus 40 (range 3–65) for the younger adults, but no difference in sex, type of donation (deceased/living) or race. Older adults were more likely than younger adults to receive extended criteria donors, 22.7% (n=15) versus 3.3 (n=2), p=0.002, and organs classified as donation after cardiac death (DCD), 33.3% (n=22) versus 9.84% (n=6), p=0.002. There was no statistically significant difference in the kidney donor profile index (KDPI), a median of 66 (range 10–93) in older adults versus 51 (range 5–92) in the younger group, or the presence of positive donor urine or blood cultures.
Table 1.
Baseline characteristics of older and younger KT recipients.
| Older adults (65+) n=91 | Younger adults (40–60 years of age) n=91 | p value | |
|---|---|---|---|
| Age, median [range] | 68 [65, 75] | 49 [40, 60] | N/A |
| Male n(%) | 55 (60.4) | 55 (60.4) | N/A |
| Race n(%) | N/A | ||
| African-American | 29 (31.9) | 29 (31.9) | |
| Caucasian | 56 (61.5) | 60 (65.9) | |
| Asian | 6 (6.6) | 2 (2.2) | |
| Prior dialysis n(%) | 70 (77) | 72 (79) | 0.86 |
| Comorbidities n(%) | 91 (100) | 91 (100) | 1 |
| Diabetes mellitus | 43 (47.3) | 16 (17.6) | <0.001 |
| Hypertension | 85 (93.4) | 78 (85.7) | 0.15 |
| CV disease | 45 (49.5) | 16 (17.6) | <0.001 |
| GU conditions | 14(15.4) | 6 (6.6) | 0.10 |
| Recurrent UTIs | 1 (1.1) | 4 (4.4) | 0.36 |
| Prior abdominal surgery | 45 (49.5) | 42 (46.15) | 0.77 |
| Prior transplant | 8 (8.8) | 18 (19.8) | 0.06 |
| Prior KT | 4 (50) | 15 (83.3) | 0.08 |
| Data on immunization n(%) | 56 (61.5) | 69 (75.8) | 0.05 |
N/A: not applicable. CV: cardiovascular. UTI: urinary tract infection. GU: genitourinary. KT: kidney transplant. NS: not significant.
CMV serostatus was defined as high risk (donor seropositive, recipient seronegative), intermediate risk (recipient seropositive) and low risk (donor and recipient seronegative) for CMV infection. There was no statistically significant difference between old and young KT recipients in high (18.7% vs 18/7%), intermediate (69.2% vs. 59.4%) and low (12.1% vs. 22.0%) CMV serostatus risk category. Table 2 outlines selected peritransplant characteristics. Only length of stay was different between older adults (median 6 days) and younger adults (median 5 days).
Table 2.
Other peritransplant characteristics.
| Older adults (65+) n=91 | Younger adults (40–60 years of age) n=91 | p value | |
|---|---|---|---|
| Time from dialysis to transplant in days, median (range) | 1308 [38, 4971] | 1372 [14, 7037] | 0.83 |
| Time on the waiting list in days, median [range] | 589 [0,2336] | 467 [0,4598] | 0.83 |
| Ischemia & surgical times in minutes, median [range] | |||
| Cold | 996 [10,2505] | 858 [32,2197] | 0.30 |
| Warm | 28 [5,63] | 34 [18,60] | 0.06 |
| Surgery | 234 [132, 628] | 236 [148, 439] | 0.95 |
| PRA (%), median [range] | 0 [0,99] | 0 [0,100] | NS |
| Induction regimen, n(%) | |||
| Basiliximab | 44 (48.4) | 45 (49.5) | 0.88 |
| ATG | 27 (29.7) | 31 (34.1) | 0.53 |
| None† | 18 (19.8) | 15 (16.5) | 0.56 |
| Ureteral stent used, n(%) | 71 (78.0) | 69 (75.8) | 0.86 |
| Maintenance immunosuppression, n(%) | |||
| Prednisone | 89 (97.8) | 88 (96.7) | 0.61 |
| MMF | 88 (96.7) | 88 (96.7) | 1 |
| Tacrolimus | 90 (98.9) | 88 (96.7) | 0.62 |
| Sirolimus | 0 (0.0) | 1 (1.1) | NS |
| Everolimus | 2 (2.2) | 0 (0.0) | NS |
| Belatacept | 0 (0.0) | 1 (1.1) | NS |
| Total length of mechanical ventilation in days, median (IQR)‡ | 0(0,0) | 0(0,0) | NS |
| Any versus none n (%) | 10 (11.0) | 7(7.69) | 0.62 |
| Transfusions (PRBCs) during transplant surgery, n(%) | 7 (7.69) | 9 (9.89) | 0.79 |
| Length of hospital stay (days), median (IQR) | 6 (5,9) | 5 (4,8) | 0.04 |
| Delayed graft function, n(%) | 24 (26.4) | 15 (16.7) | 0.15 |
| Days on dialysis after transplant, median (IQR) | 9 (5, 16) | 7 (1, 11) | 0.26 |
| Discharge location | |||
| Home | 87(95.6) | 91(100.00) | NS |
| Nursing Home | 1(1.10) | 0.0(0.00) | |
| Hospice | 1(1.10) | 0.0(0.00) | |
| Death during transplant admission | 2(2.20) | 0.0(0.00) | |
PRA: panel reactive antibodies. ATG: Anti-thymocyte globulin. MMF: mycophenolate mofetil. PRBCs: packed red blood cells NS: not significant.
No induction immunosuppression would still involve methylprednisolone as per protocol.
Total days of mechanical ventilation during transplant admission.
Functional status: Mobility
Functional status assessed as independent for ambulation, dependent (needs cane or walker), or dependent was significantly better in the younger group than in older adults. In the pre-transplant setting, 75.8% of the older adults were independent for ambulation, 20.9% needed assistance and 1.1% were dependent, vs 95.5%, 5.5% and 0%, respectively in the younger group, p=0.005. At one-year after KT, 47.6% of the mobility of older adults remained independent, 23.8% needed assistance and 2.4% were dependent, vs 85.7%, 11.1% and 1.1% of the patients in the younger group; p<0.001. This information was missing at the one-year mark in 26% of the older group.
Infectious complications
The majority of the patients experienced an infectious complication in the first year after kidney transplantation; 92.3% (n=84) of the older adults and 90.1% (n=82) of the younger group, p=0.79 experienced an infectious complication. Per table 3, the most frequent infections were UTIs which were significantly more frequent in older adults. In those patients with a UTI, the mean number of UTIs in did not differ significantly, 2.11 (SD 1.0) in the older group vs 1.83 (SD 1.34) in the younger group, p=0.31. Pathogens isolated from available blood and urine cultures are shown in table 4.
Table 3.
Infectious complications in the first year after KT at a patient level.
| Older adults (65+) n=84 n (%) | Younger adults (40–60 years of age) n=82 n (%) | p value | |
|---|---|---|---|
| UTI | 44 (52.4) | 30 (36.6) | 0.049 |
| Pneumonia | 9 (10.7) | 7 (8.5) | 0.79 |
| Surgical site infections | 11 (13.1) | 17 (20.7) | 0.30 |
| BSI † | 17 (20.2) | 10 (12.2) | 0.28 |
| Sepsis | 8 (9.5) | 5 (6.1) | 0.57 |
| SSTI | 4 (4.7) | 9 (11.0) | 0.25 |
| C. difficile colitis | 7 (8.3) | 6 (7.3) | 1 |
| CMV viremia | 51 (60.7) | 34 (41.5) | 0.0131 |
| BK viremia | 28 (33.3) | 23 (28.0) | 0.51 |
| BKV nephropathy | 1 (3.6) | 2 (8.7) | |
84/91 (92.3%) of the older adults had an infection, versus 82/81 (90.1%) of the younger group. UTI: urinary tract infection; BSI: bloodstream infections; SSTI: skin and soft tissue infection; C. difficile: Clostridium difficile. CMV: cytomegalovirus; BK: BK polyomavirus.
The bloodstream infections were all bacteremias; there were no catheter-associated BSIs. There were no episodes of infective endocarditis (IE) or prosthetic joint infection (PJI). NS: not significant.
Table 4.
Most frequent pathogens isolated from urine and blood cultures in older and younger adults in the first year after KT.
| Pathogens | Older adults (65+) n (%) | Younger adults (40–60 years of age) n (%) |
|---|---|---|
| Blood cultures | n=23 † | n=12 |
| Gram negatives | ||
| Klebsiella spp | 8 (34.8) | 2 (11.1) |
| E. coli | 5 (21.7) | 4 (22.2) |
| Serratia spp | 1 (4.3) | 2 (11.1) |
| Pseudomonas spp | 3 (13.0) | 0 (0) |
| Citrobacter spp | 1 (4.3) | 0 (0) |
| Gram positives | ||
| Enterococcus | 2 (8.7) | 2 (11.1) |
| CoNS | 5 (21.7) | 1 (5.5) |
| Other | ||
| Candida | 0 (0) | 1 (5.5) |
| M. abscessus | 1 (4.3) | 0 (0) |
| Urine cultures | n=79 | n=50 |
| Gram negatives | ||
| Klebsiella spp | 25 (31.6) | 22 (44.0) |
| E. coli | 14 (17.7) | 6 (12.0) |
| Enterobacter spp | 11 (13.9) | 3 (6.0) |
| Pseudomonas spp | 9 (11.4) | 0 (0) |
| Serratia spp | 1 (1.3) | 2 (4.0) |
| Citrobacter spp | 3 (3.8) | 2 (4.0) |
| Proteus spp | 0 (0) | 1 (2.0) |
| Morganella morganii | 2 (2.5) | 0 (0) |
| Gram positives | ||
| Enterococcus spp | 10 (12.7) | 10 (20) |
| Streptococcus spp | 3 (3.8) | 0 (0) |
| Other | ||
| Yeast | 2 (2.5) | 3 (6.0) |
Three bacteremias in the older group were polymicrobial. CoNS: coagulase-negative staphylococci.
As only 16 patients in both age groups had no infection, the model to predict risk, including diabetes mellitus, cardiovascular disease, history of pre-transplant genitourinary conditions, prior transplant, donor age, deceased donation, ECD donation, DCD donation, anti-thymocyte globulin induction and ureteral stent use as infection risks, was not reliable. There was no difference in the mean prednisone dose at the time of first infection (median 20mg (IQR 2–25mg) in the older group vs 20mg (IQR 5–28.93mg) in the younger group, p=0.576).
UTIs
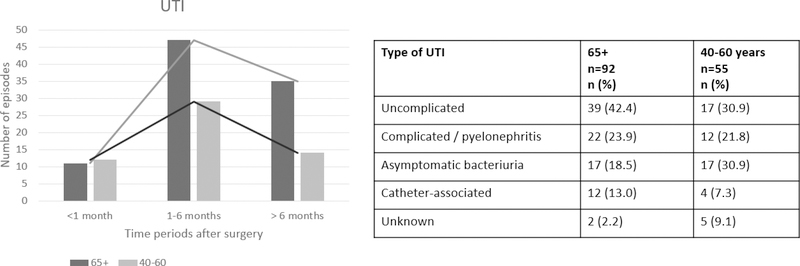
UTIs were analyzed separately as UTIs were significantly more frequent in older adults compared to younger adults (52.4% vs 36.6%, p<0.05) even after exclusion of asymptomatic bacteriuria. Figure 1 shows the timing and type of UTIs after transplantation. Table 4 shows the most frequently isolated pathogens in the available urine cultures.
Figure 1.
Timeline and types of urinary tract infections (UTIs).
In an exploratory analysis, a stepwise regression logistic model was employed to evaluate additional factors associated with an increased risk for UTI. The same variables mentioned above were used in addition to recipients’ gender. Three variables entered the final model: recipients’ sex (female), genitourinary conditions and donor risk classification “donation after cardiac death” (DCD). The DCD classification was driven by the older group, whereas a history of pre-transplant genitourinary condition and sex were driven by the younger group. All three variables were significantly associated with an increased risk of UTI after KT.
CMV
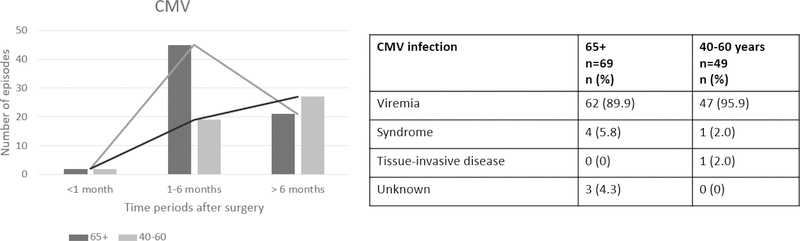
CMV viremia was significantly more frequent in older compared with the younger group: 69 episodes in 51 patients vs 47 episodes in 34 patients, respectively. Figure 2 shows the timeline and type of CMV infections. Older patients had later CMV reactivation compared to the younger group. As shown in figure 2, most of these were episodes of viremia and only a few met definitions for CMV syndrome and tissue-invasive disease.
Figure 2.
Timeline and total number of CMV episodes in the first year after KT. 56% of older KT recipients and 37.4% of the younger group had at least one episode of CMV viremia.
Hospital admission
There were a total of 116 hospital admissions among the older (65 admissions) and younger (51 admissions) groups in the first year after KT. The number one reason for hospital admission during the first year after KT in both groups were infectious complications (65.5%). Admissions for infections were significantly more frequent in older adults, 53.9% (n=49) vs 29.7% (n=27), p=0.0015. Other frequent reason for admissions were surgical complications (24.2% vs16.5%, p=0.27), and lab abnormalities (34.1% vs 20.9%, p=0.067). Only one older KT recipient (1.1%) was admitted for a cardiovascular event, vs 5 (5.5%) (p=0.21) younger patients.
Patient and graft survival, rejection
There was no significant difference in the number of rejections in older KT recipients compared to younger KT recipients: 7.7% (n=7) vs 14.3% (n=13) (p=0.24). There were three graft losses in each group (3.3%). Mortality was higher in older KT vs younger KT recipients, 9.9% (n=9) vs 1.1% (n=1), p=0.018. All but one death in the older group occurred with a functioning graft. The primary causes of death in older adults were malignancies (33.3%), followed by infections (22.2%); the only death in the control group was secondary to an infection.
Discussion:
In this study we describe differences in infectious complications during the first year after KT in older compared with younger adult KT recipients. In fact, infections were the number one reason for hospital admission in the first year after surgery, and as such were more frequent than admissions secondary to cardiovascular disease (CVD). Given the higher prevalence of CVD and diabetes mellitus in the older age group, this was an unexpected finding22. Notably, pneumonia, which is a common infection and cause of mortality in older adults11, was not more frequent in older adults in the post-kidney transplant setting whereas UTIs and CMV viremia were. In addition, while there was not more rejection in the older recipients, there was significantly higher mortality, longer transplant hospitalizations and decrease in functional status during the first year post transplant.
The increased rate of UTIs in older KT recipients mirrors results of those reported in a case-control study in the literature assessing infection in younger and older KT recipients9. With advancing age, we expect functional and dynamic outflow changes that predispose to UTIs, and a higher frequency of bacteria in the urine11. Given this increased vulnerability of older adults to UTIs and given the effect of immunosuppression is typically highest during the first year after KT, the high incidence of UTIs in the older KT population is not unexpected9. We found that UTIs most commonly occurred more than one-month post-transplant in both groups but more so in older adults. The most frequent pathogens were enteric organisms, specifically, gram negatives among which Klebsiella spp. were the most common, closely followed by Enterococus spp. The predominance of enteric pathogens would support a local source for the infection with pathophysiology linked to low flow state or other permissive urodynamic changes.
Traditional risk factors for UTI in KT recipients include female sex, prolonged use of indwelling urinary catheter, ureteral stent use, age and delayed graft function23–26. We found that history of genitourinary conditions and female sex were risk factors for UTIs in younger KT recipients whereas DCD donor status was predictive of UTI in older KT recipients. Renal stents had already been removed by the one month post-transplant and were not found to be a risk for UTIs in our study. While prolonged ischemia-reperfusion injury or delayed graft function might predispose to low flow states, neither of these factors were more common in our older adult population. How DCD may influence risk for UTI in the absence of delayed graft function requires further study. DCD has been related to increased rejection rates and mortality in older recipients27 but not infectious complications specifically.
Use of antibiotics may have impacted the timeline of UTIs post-transplant. Asymptomatic bacteriuria is generally treated during the first three months after KT in this population23,28 which potentially impacted timing of UTIs. Interestingly, the UTIs occurred despite trimethoprim-sulfamethoxazole used for PJP prophylaxis, although this could certainly affect the incidence and potentially shift the prevalence of certain pathogens.
CMV was not only the most common infectious complication in adults29 after KT but also significantly more frequent in older recipients. Consistent with current guidelines30, pre-emptive monitoring is performed in intermediate risk seropositive recipients at our institution. Thus, CMV reactivation/asymptomatic viremia during months 2–6 post-transplant is not unexpected. However, the overall percentage of patients with CMV viremia (47.8%) is higher than anticipated in most seropositive recipients (15–25%), and even higher for older KT recipients (60.7%). Further analysis is needed to understand if these were self-limited episodes, required treatment and what their clinical impact was. Historically, risk factors for CMV have included age, positive donor serostatus, T-cell depleting induction, rejection and other co-infections31,32. Although the increased incidence of CMV reactivation could be interpreted as a marker of over-immunosuppression, we would have expected a difference in episodes of BKV viremia and rejections, but these were not found. Given the importance of CMV, both through direct as well as indirect effects33 on the graft, we believe further research into its clinical impact on older recipient is needed. Furthermore, CMV has been linked to the process of “inflammaging34“. The concept of inflammaging relates to a continuous pro-inflammatory state that is involved in immunesenescence. The role of CMV in the maintenance of this pro-inflammatory state has been a topic of controversy35–37 and is beyond the scope of this article. That said, current prophylactic guidelines don’t take into account age or immunesenescence when defining CMV risk. This is an area where immune profiling could help decide who is at highest risk of reactivation.
There were no differences in rejections and graft-losses between groups, which might be a result of the older recipients in this series receiving kidneys from older donors. Although there’s still controversy about incidence of rejection in aged recipients, it is generally thought that acute rejections are less frequent38–41 secondary to changes in the humoral and cellular immune system. As expected, our older group had an increased mortality42 at the one year-mark but the majority of older recipients that died, did so with a functioning graft. Although not the main cause of death, infections were important in both groups, with malignancies constituting the number one cause of death in older adults. Age and immunosuppression increases the risk for malignancy43–45. Considering these issues as a whole, older adults may indeed require less immunosuppression compared with younger KT recipients.
Our data on functional was limited to mobility, and unfortunately there was some missing data, especially at the one-year mark for older KT recipients. However, we found a significant difference in functional status between older and younger KT recipients at baseline, that is, before surgery. This finding raises two important questions: 1. “Would the improvement of functional status in the pre-transplant setting decrease infectious complications?”; and 2. “If we are able to decrease the number of infections, could we improve functional status and survival?”. Functional status, including mobility, has been studied in KT recipients46–48. Even patients with low function seem to have a survival benefit over dialysis49 but its impact on non-traditional outcomes needs to be examined in prospective studies46,50. Finally, it might be prudent to address the patients “biological” rather than “chronological” age51 upon pre-transplant assessment in order to carefully select the most appropriate candidates for KT. Biological aging denotes the heterogeneity of different biomarkers, genomic predictors, epigenetic clocks and biological processes in individuals. While biological aging is seen in many chronic diseases, we have little understanding on how it could be utilized as a true biomarker52.
This study has several limitations. First, it is a single-center retrospective study, which limits the generalizability of our findings to other populations with different antimicrobial strategies or resources. Second, the sample size is too small for accurate modeling due to the high prevalence and incidence of infections and therefore, as mentioned above, some of the analyses were underpowered. However, the study presents new data on different infection dynamics between older and younger KT recipients and ultimately addresses a knowledge gap regarding infectious complications in this growing subpopulation of older KT recipients.
In conclusion, adult KT recipients have a high incidence of infectious complications during the first year. Infections were the most frequent reason for hospital admission and older KT recipients were at higher risk than younger KT recipients for this event. Older recipients have a very high incidence of UTIs and CMV reactivations. Older adults also have more late onset UTIs when compared to the younger group. Risk factor for UTIs in older recipients was the receipt of a DCD graft, whilst in the younger group a history of pre-transplant genitourinary condition and sex played a significant role. CMV reactivation is significantly more frequent in the older group, with the majority being delayed onset. Further analysis is needed to elucidate the risk factors, impact and patterns of CMV reactivation in this subpopulation, and clarify if a different antimicrobial prophylactic and/or immunosuppressive approach is needed.
Future projects should include prospective multicenter studies to evaluate post-transplant complications in renal transplant populations aged 65 years and greater. It is likely that risks for infections in the new era of contemporary immunosuppression14 and antimicrobial prophylaxis need to be revised. Immune profiling could help understand who is at highest risk of certain infections. Immunosuppression regimens might need adjustment for this growing population of KT recipients. Additionally, the absence of standardized measurements regarding non-classic transplant outcomes that older adults rate as critically important (e.g. quality of life, independence) is a major gap and opportunity for research in this population.
Acknowledgements:
This work was supported the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [grant number 5T32AI100851 to MHM and BDA]; and the National Institute on Aging, Duke Pepper Older Americans Independence Center [grant number P30AG028716 to KES]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.National Kidney Foundation. End Stage Renal Disease in the United States. 2016; https://www.kidney.org/news/newsroom/factsheets/End-Stage-Renal-Disease-in-the-US. Accessed 08/29/2018.
- 2.McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL. Trends in kidney transplant outcomes in older adults. Journal of the American Geriatrics Society. 2014;62(12):2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999;341(23):1725–1730. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2093–2109. [DOI] [PubMed] [Google Scholar]
- 5.Knoll GA. Kidney transplantation in the older adult. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(5):790–797. [DOI] [PubMed] [Google Scholar]
- 6.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: Kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018;18 Suppl 1:18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai X, Chen G, Qiu J, Wang C, Chen L. Recipient-related risk factors for graft failure and death in elderly kidney transplant recipients. PloS one. 2014;9(11):e112938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Semin Nephrol. 2009;29(6):621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trouillhet I, Benito N, Cervera C, et al. Influence of age in renal transplant infections: cases and controls study. Transplantation. 2005;80(7):989–992. [DOI] [PubMed] [Google Scholar]
- 10.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney international. 2001;59(4):1539–1543. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa TT, Norman DC. Geriatric Infectious Diseases: Current Concepts on Diagnosis and Management. Journal of the American Geriatrics Society. 2017;65(3):631–641. [DOI] [PubMed] [Google Scholar]
- 12.Mouton CP, Bazaldua OV, Pierce B, Espino DV. Common infections in older adults. American family physician. 2001;63(2):257–268. [PubMed] [Google Scholar]
- 13.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on Frailty in Solid Organ Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018. doi: 10.1111/ajt.15198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peeters LEJ, Andrews LM, Hesselink DA, de Winter BCM, van Gelder T. Personalized immunosuppression in elderly renal transplant recipients. Pharmacological research. 2018;130:303–307. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson PA, Schladt D, Oetting WS, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(12):3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinbokel T, Elkhal A, Liu G, Edtinger K, Tullius SG. Immunosenescence and organ transplantation. Transplantation reviews (Orlando, Fla). 2013;27(3):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier-Kriesche H, Ojo AO, Arndorfer JA, et al. Need for individualized immunosuppression in elderly renal transplant recipients. Transplantation proceedings. 2001;33(1–2):1190–1191. [DOI] [PubMed] [Google Scholar]
- 18.Horvath MM, Rusincovitch SA, Brinson S, Shang HC, Evans S, Ferranti JM. Modular design, application architecture, and usage of a self-service model for enterprise data delivery: the Duke Enterprise Data Unified Content Explorer (DEDUCE). Journal of biomedical informatics. 2014;52:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2017; https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 21.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;64(1):87–91. [DOI] [PubMed] [Google Scholar]
- 22.AHA. Percentage of adults in the U.S. who had coronary heart disease in 2011–2014, by age and gender. 2018; https://www.statista.com/statistics/671371/coronary-heart-disease-prevalence-us-adults-by-age-and-gender/. Accessed 8/22/18.
- 23.Lee JR, Bang H, Dadhania D, et al. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation. 2013;96(8):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clinical transplantation. 2006;20(4):401–409. [DOI] [PubMed] [Google Scholar]
- 25.Kotagiri P, Chembolli D, Ryan J, Hughes PD, Toussaint ND. Urinary Tract Infections in the First Year Post-Kidney Transplantation: Potential Benefits of Treating Asymptomatic Bacteriuria. Transplantation proceedings. 2017;49(9):2070–2075. [DOI] [PubMed] [Google Scholar]
- 26.Khoury JA, Brennan DC. Infectious complications in kidney transplant recipients: review of the literature. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2005;16(4):453–497. [PubMed] [Google Scholar]
- 27.Peters-Sengers H, Berger SP, Heemskerk MB, et al. Stretching the Limits of Renal Transplantation in Elderly Recipients of Grafts from Elderly Deceased Donors. Journal of the American Society of Nephrology : JASN. 2017;28(2):621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes-Penfield NW, Trautner BW, Jump RLP. Urinary Tract Infection and Asymptomatic Bacteriuria in Older Adults. Infectious disease clinics of North America. 2017;31(4):673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park WY, Kang SS, Jin K, Park SB, Han S. Is the Clinical Outcome Good or Bad in Patients Hospitalized Within 1 Year After Kidney Transplantation? Transplantation proceedings. 2018;50(4):1001–1004. [DOI] [PubMed] [Google Scholar]
- 30.Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102(6):900–931. [DOI] [PubMed] [Google Scholar]
- 31.De Keyzer K, Van Laecke S, Peeters P, Vanholder R. Human cytomegalovirus and kidney transplantation: a clinician’s update. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58(1):118–126. [DOI] [PubMed] [Google Scholar]
- 32.Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(12):2058–2070. [DOI] [PubMed] [Google Scholar]
- 33.Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation - challenging the status quo. Clinical transplantation. 2007;21(2):149–158. [DOI] [PubMed] [Google Scholar]
- 34.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 35.Sadighi Akha AA. Aging and the immune system: An overview. Journal of immunological methods. 2018;463:21–26. [DOI] [PubMed] [Google Scholar]
- 36.Bartlett DB, Firth CM, Phillips AC, et al. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging cell. 2012;11(5):912–915. [DOI] [PubMed] [Google Scholar]
- 37.Sansoni P, Vescovini R, Fagnoni FF, et al. New advances in CMV and immunosenescence. Experimental gerontology. 2014;55:54–62. [DOI] [PubMed] [Google Scholar]
- 38.Mendonca HM, Dos Reis MA, de Castro de Cintra Sesso R, Camara NO, Pacheco-Silva A. Renal transplantation outcomes: a comparative analysis between elderly and younger recipients. Clinical transplantation. 2007;21(6):755–760. [DOI] [PubMed] [Google Scholar]
- 39.Hod T, Goldfarb-Rumyantzev AS. Clinical issues in renal transplantation in the elderly. Clinical transplantation. 2015;29(2):167–175. [DOI] [PubMed] [Google Scholar]
- 40.McKay D, Jameson J. Kidney transplantation and the ageing immune system. Nature reviews Nephrology. 2012;8(12):700–708. [DOI] [PubMed] [Google Scholar]
- 41.Rana A, Murthy B, Pallister Z, et al. Profiling risk for acute rejection in kidney transplantation: recipient age is a robust risk factor. Journal of nephrology. 2017;30(6):859–868. [DOI] [PubMed] [Google Scholar]
- 42.Abecassis M, Bridges ND, Clancy CJ, et al. Solid-organ transplantation in older adults: current status and future research. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(10):2608–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80(2 Suppl):S254–264. [DOI] [PubMed] [Google Scholar]
- 44.Kim C, Cheng J, Colegio OR. Cutaneous squamous cell carcinomas in solid organ transplant recipients: emerging strategies for surveillance, staging, and treatment. Seminars in oncology. 2016;43(3):390–394. [DOI] [PubMed] [Google Scholar]
- 45.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397(6719):530–534. [DOI] [PubMed] [Google Scholar]
- 46.Hartmann EL, Kitzman D, Rocco M, et al. Physical Function in Older Candidates for Renal Transplantation: An Impaired Population. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(3):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bui K, Kilambi V, Rodrigue JR, Mehrotra S. Patient Functional Status at Transplant and Its Impact on Posttransplant Survival of Adult Deceased-Donor Kidney Recipients. Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clinical transplantation. 2018:e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reese PP, Shults J, Bloom RD, et al. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(5):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation. 2018;102(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danovitch G, Savransky E. Challenges in the counseling and management of older kidney transplant candidates. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;47(4 Suppl 2):S86–97. [DOI] [PubMed] [Google Scholar]
- 52.Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors. EBioMedicine. 2017;21:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]