Abstract
Background
Endoscopic ultrasound (EUS) is proposed as an accurate diagnostic device for the locoregional staging of gastric cancer, which is crucial to developing a correct therapeutic strategy and ultimately to providing patients with the best chance of cure. However, despite a number of studies addressing this issue, there is no consensus on the role of EUS in routine clinical practice.
Objectives
To provide both a comprehensive overview and a quantitative analysis of the published data regarding the ability of EUS to preoperatively define the locoregional disease spread (i.e., primary tumor depth (T‐stage) and regional lymph node status (N‐stage)) in people with primary gastric carcinoma.
Search methods
We performed a systematic search to identify articles that examined the diagnostic accuracy of EUS (the index test) in the evaluation of primary gastric cancer depth of invasion (T‐stage, according to the AJCC/UICC TNM staging system categories T1, T2, T3 and T4) and regional lymph node status (N‐stage, disease‐free (N0) versus metastatic (N+)) using histopathology as the reference standard. To this end, we searched the following databases: theCochrane Library (the Cochrane Central Register of Controlled Trials (CENTRAL)), MEDLINE, EMBASE, NIHR Prospero Register, MEDION, Aggressive Research Intelligence Facility (ARIF), ClinicalTrials.gov, Current Controlled Trials MetaRegister, and World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), from 1988 to January 2015.
Selection criteria
We included studies that met the following main inclusion criteria: 1) a minimum sample size of 10 patients with histologically‐proven primary carcinoma of the stomach (target condition); 2) comparison of EUS (index test) with pathology evaluation (reference standard) in terms of primary tumor (T‐stage) and regional lymph nodes (N‐stage). We excluded reports with possible overlap with the selected studies.
Data collection and analysis
For each study, two review authors extracted a standard set of data, using a dedicated data extraction form. We assessed data quality using a standard procedure according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) criteria. We performed diagnostic accuracy meta‐analysis using the hierarchical bivariate method.
Main results
We identified 66 articles (published between 1988 and 2012) that were eligible according to the inclusion criteria. We collected the data on 7747 patients with gastric cancer who were staged with EUS. Overall the quality of the included studies was good: in particular, only five studies presented a high risk of index test interpretation bias and two studies presented a high risk of selection bias.
For primary tumor (T) stage, results were stratified according to the depth of invasion of the gastric wall. The meta‐analysis of 50 studies (n = 4397) showed that the summary sensitivity and specificity of EUS in discriminating T1 to T2 (superficial) versus T3 to T4 (advanced) gastric carcinomas were 0.86 (95% confidence interval (CI) 0.81 to 0.90) and 0.90 (95% CI 0.87 to 0.93) respectively. For the diagnostic capacity of EUS to distinguish T1 (early gastric cancer, EGC) versus T2 (muscle‐infiltrating) tumors, the meta‐analysis of 46 studies (n = 2742) showed that the summary sensitivity and specificity were 0.85 (95% CI 0.78 to 0.91) and 0.90 (95% CI 0.85 to 0.93) respectively. When we addressed the capacity of EUS to distinguish between T1a (mucosal) versus T1b (submucosal) cancers the meta‐analysis of 20 studies (n = 3321) showed that the summary sensitivity and specificity were 0.87 (95% CI 0.81 to 0.92) and 0.75 (95% CI 0.62 to 0.84) respectively. Finally, for the metastatic involvement of lymph nodes (N‐stage), the meta‐analysis of 44 studies (n = 3573) showed that the summary sensitivity and specificity were 0.83 (95% CI 0.79 to 0.87) and 0.67 (95% CI 0.61 to 0.72), respectively.
Overall, as demonstrated also by the Bayesian nomograms, which enable readers to calculate post‐test probabilities for any target condition prevalence, the EUS accuracy can be considered clinically useful to guide physicians in the locoregional staging of people with gastric cancer. However, it should be noted that between‐study heterogeneity was not negligible: unfortunately, we could not identify any consistent source of the observed heterogeneity. Therefore, all accuracy measures reported in the present work and summarizing the available evidence should be interpreted cautiously. Moreover, we must emphasize that the analysis of positive and negative likelihood values revealed that EUS diagnostic performance cannot be considered optimal either for disease confirmation or for exclusion, especially for the ability of EUS to distinguish T1a (mucosal) versus T1b (submucosal) cancers and positive versus negative lymph node status.
Authors' conclusions
By analyzing the data from the largest series ever considered, we found that the diagnostic accuracy of EUS might be considered clinically useful to guide physicians in the locoregional staging of people with gastric carcinoma. However, the heterogeneity of the results warrants special caution, as well as further investigation for the identification of factors influencing the outcome of this diagnostic tool. Moreover, physicians should be warned that EUS performance is lower in diagnosing superficial tumors (T1a versus T1b) and lymph node status (positive versus negative). Overall, we observed large heterogeneity and its source needs to be understood before any definitive conclusion can be drawn about the use of EUS can be proposed in routine clinical settings.
Plain language summary
Ultrasound for determining the spread of stomach cancer
Review question
There is much debate on the diagnostic performance of endoscopic ultrasound (EUS) in the preoperative staging of gastric cancer. The aim of this review was to collect the available evidence and then to calculate how well EUS stages stomach cancer.
Background
EUS is a diagnostic test that can be used to determine how far (stage) cancer of the stomach reaches prior to surgery. It consists of an endoscope coupled with an ultrasound device capable of scanning the stomach wall, which shows the different layers of the stomach. Changes from the normal ultrasonographic patterns due to the tumor growth can be used to determine the extent of cancer in the stomach wall (T‐stage) and the lymph nodes related to the stomach (N‐stage). Since the correct staging of the tumor enables physicians to personalize cancer treatment, it is important to understand the reliability of staging devices.
Study characteristics
We conducted a meta‐analysis according to the most recent methods for diagnostic tests. The last literature search was performed in January 2015. We included 66 studies (of 7747 patients) in the review.
Key results
We found that EUS can distinguish between superficial (T1 ‐ T2) and advanced (T3 ‐ T4) primary tumors with a sensitivity and a specificity greater than 85%. This performance is maintained for the discrimination between T1 and T2 superficial tumors. However, EUS diagnostic accuracy is lower when it comes to distinguishing between the different types of early tumors (T1a versus T1b) and between tumors with versus those without lymph node disease.
Quality of the evidence
Overall, EUS provides physicians with some helpful information on the stage of gastric cancer. Nevertheless, in the light of the variability of the results reported in the international medical literature, its limitations in terms of performance must be kept in mind in order to make the most out of the diagnostic potential of this tool. Finally, more work is needed to assess whether some technical improvements and the combination with other staging instruments may increase our ability to correctly stage the disease and thus optimize patient treatment.
Summary of findings
Summary of findings'. 'Summary of findings Table.
| General information | ||
| General issue | What is the diagnostic performance of endoscopic ultrasound (EUS) in assessing disease stage in people with gastric carcinoma ? | |
| Specific questions | What is the diagnostic performance of EUS in assessing primary tumor depth ? | Superficial (T1 ‐ T2) versus advanced (T3 ‐ T4) tumors |
| Early (T1) versus muscular (T2) tumors | ||
| Mucosal (T1a) versus submucosal (T1b) tumors | ||
| What is the diagnostic performance of EUS in assessing regional lymph node status ? | Non‐metastatic (N0) versus metastatic (N+) lymph nodes | |
| Patients | Patients diagnosed with gastric carcinoma | |
| Settings | Pre‐treatment evaluation of disease stage | |
| Index tests | Endoscopic ultrasound (EUS) | |
| Reference standard | Histology of surgical or endoscopic specimen | |
| Importance | Choosing best treatment or treatment sequence of gastric carcinoma | |
| Studies | 66 studies enrolling 7747 patients | |
| Quality concerns | Overall judgement | Good quality |
| Applicability concerns | None | |
| Patient selection bias | None | |
| Index test interpretation bias | High risk: 5 studies | |
| Reference test interpretation bias | None | |
| Flow and timing selection bias | High risk: 2 studies Unclear risk: 2 studies |
|
| T1 ‐ T2 versus T3 ‐ T4 tumors | ||
| Studies | 50 (patients enrolled: 4397) | |
| Summary results | Sensitivity: 0.86 (95% CI: 0.81 to 0.90). Specificity: 0.90 (95% CI: 0.87 to 0.93) | |
| Consequences | In a hypothetical cohort of 1000 patients (T1 ‐ T2 prevalence: 50%) | Correctly classified: 880 |
| Overstaged: 70 | ||
| Understaged: 50 | ||
| T1 versus T2 tumors | ||
| Studies | 46 (patients enrolled: 2742) | |
| Summary results | Sensitivity: 0.85 (95% CI: 0.78 to 0.91). Specificity: 0.90 (95% CI: 0.85 to 0.93) | |
| Consequences | In a hypothetical cohort of 1000 patients (T1 prevalence: 70%) | Correctly classified: 865 |
| Overstaged: 105 | ||
| Understaged: 30 | ||
| T1a versus T1b tumors | ||
| Studies | 20 (patients enrolled: 3321) | |
| Summary results | Sensitivity: 0.87 (95% CI: 0.81 to 0.92). Specificity: 0.75 (95% CI: 0.62 to 0.84) | |
| Consequences | In a hypothetical cohort of 1000 patients (T1a prevalence: 70%) | Correctly classified: 834 |
| Overstaged: 91 | ||
| Understaged: 75 | ||
| N0 versus N+ tumors | ||
| Studies | 44 (patients enrolled: 3573) | |
| Summary results | Sensitivity: 0.83 (95% CI: 0.79 to 0.87). Specificity: 0.67 (95% CI: 0.61 to 0.72) | |
| Consequences | In a hypothetical cohort of 1000 patients (N+ prevalence: 50%) | Correctly classified: 750 |
| Overstaged: 85 | ||
| Understaged: 165 | ||
Background
Despite its declining incidence in Western countries, gastric cancer is still one of the most common cancers in the world (Ferlay 2010; Shah 2010), the fourth most commonly occurring cancer (9% of all cancers) after cancer of the lung, breast, and colorectum, and the second most common cancer‐related cause of death (10% of all cancer deaths) after lung cancer. In 2002, the incidence of gastric cancer was estimated at 934,000 cases, 56% of the new cases being derived from Eastern Asia, 41% from China, and 11% from Japan. On the whole, 65% to 70% of incident cases and deaths from gastric cancer are occurring in less developed countries. In the US, 21,000 new cases of this malignancy were estimated to occur in 2010, leading to 10,500 expected deaths (Jemal 2010).
Radical surgery still represents the mainstay of treatment with curative intent (Dicken 2005; Jackson 2009). However, new approaches are gaining importance in the therapeutic management of these patients. For instance, endoscopic mucosal resection (EMR) is proposed as an alternative to surgery for people with early gastric cancer (EGC) in the presence of favorable prognosis features (e.g. histologically well‐differentiated carcinoma limited to the mucosa, diameter less than 2 cm, absence of ulceration) (Bennett 2009; Hirasawa 2011; Kang 2011; Othman 2011). Moreover, different adjuvant and neoadjuvant chemotherapy regimens (combined or not with radiotherapy) have been shown to provide significant survival advantage to people with advanced gastric cancer (AGC) (House 2008; Jiang 2010; Paoletti 2010; Wagner 2010).
These strategies require reliable disease staging procedures in order to guarantee the most appropriate treatment (i.e. with the highest therapeutic index, the ratio between efficacy and toxicity) for each patient, according to the principles of personalized medicine. As for all solid tumors, the disease stage for gastric cancer is defined by the three categories of the TNM classification: T‐stage (indicating the primary tumor invasion through the layers of the gastric wall; T1: tumor invading mucosa‐submucosa layer; T2: muscolaris propria layer; T3: subserosa layer; T4: serosa layer or adjacent organs), N‐stage (indicating the regional lymph node involvement; N0: no metastasis; N1 ‐ 3: presence of increasing number of metastatic lymph nodes) and M‐stage (indicating the presence/absence of distant metastasis, such as hepatic or peritoneal metastasis; M0 ‐ 1) (Edge 2010).
Therefore, after the diagnosis of primary carcinoma of the stomach is made (usually by means of pathology evaluation of tumor biopsies obtained during a standard gastroscopy), staging is assessed both preoperatively (clinical staging) by means of imaging techniques, and postoperatively by pathology examination of the surgical specimen (pathological staging). Knowing the disease stage before surgery (clinical staging) can be extremely useful in providing patients with the best therapeutic option: for instance, AGC (i.e., T3 ‐ T4 tumors or tumors with lymph node metastasis (N+)) can be treated with neoadjuvant (preoperative) chemotherapy (or radiotherapy, or both) (House 2008; Jiang 2010; Paoletti 2010; Wagner 2010). On the other hand, early gastric cancer (T1 tumors) with no lymph node involvement (N0) can be treated with endoscopic rather than surgical resection (Bennett 2009; Hirasawa 2011; Kang 2011; Othman 2011).
Computed tomography (CT) is currently the most frequently used radiological tool for the preoperative staging of gastric cancer (Jensen 2007; Ly 2008); however, CT accuracy is high mainly for distant metastasis (M category, e.g., hepatic metastasis), whereas its accuracy for locoregional staging (i.e., definition of the T and N categories) is much lower, ranging in most series from 65% to 85% (Hur 2006; Kawaguchi 2011; Kim 2005; Kumano 2005; Stell 1996). For instance, a recent meta‐analysis shows that CT scan sensitivity and specificity for the identification of lymph node status are 77% and 78%, respectively (Seevaratnam 2012). No better results appear to be achievable with other techniques such as magnetic resonance imaging (MRI) or positron emission tomography (PET) (Ha 2011; Kim 2011; Seevaratnam 2012). Overall, only a limited proportion of people with locally‐advanced gastric cancer and an even smaller percentage of those with early gastric cancer can be identified preoperatively and can thus benefit from personalized treatments.
Endoscopic ultrasound (EUS) has been proposed as an accurate device for the locoregional staging of gastric cancer (Byrne 2002; Hargunani 2009; Polkowski 2009). Our aim is to systematically review and meta‐analyze the available evidence regarding the diagnostic accuracy of EUS in discriminating between different primary tumor depths of invasion (T‐stage), as well as in identifying metastasis within regional lymph nodes (N‐stage).
A glossary of terms is provided in Appendix 1.
Target condition being diagnosed
This review addresses the preoperative locoregional staging of primary gastric carcinoma to distinguish between EGC, which is suitable for endoscopic resection, and AGC, which is likely to benefit from neoadjuvant therapies. We have not considered other gastric malignancies (e.g., lymphomas, gastrointestinal stromal tumors (GIST)).
Index test(s)
In this review endoscopic ultrasonography (EUS) represents the index test. It consists of an endoscope equipped with an ultrasound probe that can scan the stomach wall in order to detect alterations in its normal layers caused by primary tumor growth, as well as the presence of metastatic lymph nodes. Usually EUS does not require patient sedation and is performed like a standard gastroscopy with the instrument being introduced into the stomach through the mouth, the only difference being the additional time required to scan the stomach wall. For this reason and because complications are virtually absent, it is usually performed on an outpatient basis. The ultrasound transducer, which is integrated in the distal end of the endoscope to allow its positioning close to the gastric wall, comes in two main types: the linear scanner gives a scanning range of 180°, whereas the radial scanner offers the advantage of a full panoramic view (360°).
Clinical pathway
People with suspected gastric cancer, based on history and clinical findings, generally undergo gastroscopy to make the disease diagnosis, usually defined by the pathology evaluation of the biopsy performed during the endoscopy. Then the malignant disease is staged to assess its spread through the gastric wall to adjacent organs/lymph nodes or to distant body sites; this step is crucial to setting up the best therapeutic strategy and thus to maximizing the likelihood of cure. False positive findings from a staging procedure (e.g., classifying an early disease as advanced ) might lead to over‐treatment (e.g., unnecessary neoadjuvant chemotherapy); false negative findings might lead to patient under‐treatment.
Prior test(s)
No test is usually performed before EUS.
Role of index test(s)
The index test (EUS) is currently utilized in clinical practice by many physicians to preoperatively stage gastric cancer. However there is no consensus on whether or not EUS should be routinely used for this as part of a standardized approach.
Alternative test(s)
Other diagnostic tools that can be used for gastric cancer staging are computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET). None of them is deemed sufficiently accurate to be considered as the optimal imaging technique for the preoperative evaluation of disease spread, although all are widely used in clinical practice. In particular, neither CT scan, nor MRI, nor PET are useful for the definition of early stages of gastric cancer, whereas they are commonly utilized to diagnose locally‐advanced gastric cancer (T3 ‐ T4 or N+ cases, or both). While the usefulness of these diagnostic tools in the locoregional staging of gastric carcinoma is debated, there is a general consensus about their use in defining the presence of distant metastatic disease, e.g., presence or absence of metastasis in the liver or lungs.
Rationale
With regard to preoperative assessment of disease spread, one of the most promising tools for the locoregional staging of gastric carcinoma is EUS (Byrne 2002; Hargunani 2009; Polkowski 2009). This endoscopy‐based diagnostic device can both distinguish the different layers that compose the gastric wall and visualize the perigastric lymph nodes by means of a miniaturized ultrasound probe. Based on numerous reports published over more than two decades, EUS is often reported as a highly accurate method for the locoregional staging of gastric cancer. However, findings are heterogeneous, e.g., sensitivity and specificity values can range from 50% to 100% (Hizawa 2002; Kelly 2001; Kwee 2007; Kwee 2008; Kwee 2009; Puli 2008; Reddy 2008; Shimoyama 2004; Weber 2004), and although thousands of people with gastric cancer have been enrolled in EUS‐based studies, no formal quantitative review of the available evidence has been published that comprehensively examines the staging performance of EUS using the most appropriate statistical tools for the meta‐analysis of diagnostic accuracy data (a hierarchical approach) (Harbord 2008; Leeflang 2008; Macaskill 2010; Reitsma 2005).
Our review aims to fill this gap in the medical literature by quantitatively summarizing the diagnostic role of EUS in the staging of primary gastric carcinoma.
Objectives
To provide both a comprehensive overview and a quantitative analysis of the published data regarding the ability of endoscopic ultrasonography (EUS) to preoperatively define the locoregional disease spread (i.e., primary tumor depth (T‐stage) and regional lymph node status (N‐stage)) in people with primary gastric carcinoma.
Secondary objectives
To provide the tools to calculate EUS diagnostic accuracy measures based on pre‐test information, such as gastric cancer T‐stage and N‐stage prevalence (Bayes nomograms).
To assess whether EUS performs differently in different subgroups of patients identified by the following parameters: year of publication, country (Western versus Eastern), EUS technical features (radial versus linear array; ultrasound frequency (MHz)), definition of target condition (for N‐stage: lymph node morphology versus size), gastric tumor site (any site versus cardia region only) and prevalence of target condition.
Methods
Criteria for considering studies for this review
Types of studies
We include studies that meet the following inclusion criteria:
A minimum sample size of 10 patients with histologically‐proven primary carcinoma of the stomach;
Evaluation of endoscopic ultrasonography (EUS) compared with histopathology of primary tumor (T‐stage) and regional lymph nodes (N‐stage);
Sufficient data to construct a two‐by‐two contingency table such that the cells in the table could be labeled as true positive, false positive, true negative, and false negative (see the Target conditions for more details).
This type of study typically include both retrospective and prospective series of patients. As long as the above information is available,we did not exclude any specific type of study design.
We excluded studies that had possible overlap with the selected studies (i.e. studies from the same study group, institution, and period of inclusion). We excluded studies reporting on EUS performed before preoperative chemotherapy and or radiotherapy (neoadjuvant therapy) in order to avoid the confounding effect of disease downstaging by neoadjuvant treatments.
Participants
For this review, patients were people with gastric carcinoma undergoing preoperative locoregional disease staging (T‐stage and N‐stage) by means of EUS and postoperative pathology evaluation of the surgical specimen, including those having early gastric cancer (EGC) or advanced gastric cancer (AGC). We imposed no restrictions by age, gender or any other category.
Index tests
The index test is EUS. We compared the results of EUS to those of pathology evaluation (reference test) in terms of both T‐stage and N‐stage (see Target conditions for more details).
We did not consider any comparator test.
Target conditions
The target condition was gastric cancer locoregional staging, for both primary tumor depth and regional lymph node status.
For lymph node status (N‐stage), we considered a patient either negative if no lymph node was metastatic (N0) or positive if one or more lymph nodes were metastatic (N+), as assessed by pathology evaluation.
For the primary tumor invasion of the gastric wall (T‐stage), we considered two main conditions according to the clinical questions that EUS aims to answer:
In order to identify patients who would best benefit from surgery without preoperative radio‐chemotherapy, EUS was to be investigated for its ability to distinguish superficial tumors (T1 ‐ T2) versus advanced tumors (AGC, T3 ‐ T4, which are likely to benefit from neoadjuvant preoperative chemotherapy); in this case, a patient was considered either positive if his/her gastric cancer was classified as T1 ‐ T2 by pathology examination, or negative if his/her gastric cancer is classified as T3 ‐ T4.
Within the frame of superficial cancers (T1 ‐ T2), in order to identify patients with superficial tumors amenable to endoscopic resection (T1 tumors), EUS was investigated for its ability to distinguish T1 tumors (EGC) versus T2 tumors; in this case, a patient was considered either positive if his/her gastric cancer is classified as T1 by pathology evaluation, or negative if his/her gastric cancer is classified as T2.
Finally, where the data permitted and within the frame of EGC (T1 tumors), EUS was also tested for its ability to further discriminate between T1a and T1b tumors, since it is believed that the former type of cancers benefit the most from endoscopic mucosal resection (EMR). To this end, a patient was considered either positive if his/her gastric cancer was classified as T1a by pathology evaluation, or negative if his/her gastric cancer was classified as T1b.
Reference standards
The reference standard was routine histopathology evaluation (i.e., microscopic examination of hematoxylin‐eosin stained samples) of primary tumor and regional lymph nodes. Since pathological examination of the surgical specimen is the only way to know precisely the depth of invasion through the gastric wall as well as the status of regional lymph nodes, all eligible patients must have undergone surgery and all tumors must have undergone routine pathology evaluation. According to the pathology report, four T categories (T1 to T4) indicate the extent of gastric wall invasion by the primary tumor; the status of the regional lymph nodes (positive versus negative) was also taken into consideration.
Search methods for identification of studies
We performed a comprehensive search of the literature to identify articles that examined the diagnostic accuracy of EUS (the index test) in the evaluation of primary gastric cancer depth of invasion (T‐stage, according to the AJCC/UICC TNM staging system categories T1, T2, T3 and T4) and regional lymph node status (N‐stage, metastatic versus disease‐free) using histopathology as the reference standard.
Electronic searches
We grouped key words to combine four 'concepts' that must be included in a paper reporting on the subject under investigation in this review:
malignant neoplasm (cancer, carcinoma)
body site (gastric, stomach)
diagnostic method (endoscopic ultrasound, EUS)
disease staging
We systematically searched the following databases.
The Cochrane Library (the Cochrane Central Register of Controlled Trials (CENTRAL)) (2015, Issue 1) (Appendix 2)
MEDLINE (from 1988 to January 2015) (Appendix 3)
EMBASE (from 1988 to January 2015) (Appendix 4)
NIHR Prospero Register
MEDION (http://www.mediondatabase.nl/)
ClinicalTrials.gov (clinicaltrials.gov/)
Current Controlled Trials MetaRegister (www.controlled‐trials.com/mrct/)
WHO ICTRP (www.who.int/ictrp/en/)
Searching other resources
We searched for additional references by cross‐checking bibliographies of retrieved full‐text papers.
Data collection and analysis
Both review authors (SM and SP) conducted the literature search as well as data collection and management. Review author SM conducted the statistical analyses.
Selection of studies
Both review authors (SM and SP) independently selected the studies, resolving discrepancies by iteration, discussion and consensus. Where we retrieved articles in languages other than English, we were able to assess those in Italian, French and Spanish for eligibility.
Data extraction and management
We extracted relevant data from the articles selected for inclusion in the meta‐analysis. In addition to the accuracy data, we also recorded the following information for each study:
Overall study characteristics, including the first author, country, language, and date of publication;
Study patient characteristics;
Features of the index test, e.g. type of echoendoscope, ultrasound frequency, and EUS criteria for tumor depth and lymph node status.
In case of missing data, we contacted the authors of the study to obtain the missing information. None of the three authors we contacted (Caletti 1993; Dittler 1993; Murata 1988) was able to provide data.
When raw data were presented in three‐by‐three or four‐by‐four tables (e.g., when the tumor depth or lymph node stage are defined by more than two categories), we constructed two‐by‐two contingency tables by considering a given T, or any N‐positive category, as the 'positive' state to be distinguished from the other T categories, or from the N‐negative cases. For instance, if an article presented data in a table reporting the number of N0, N1, N2 and N3 cases, we collapsed the data into a table with N0 and N+ (sum of N1, N2 and N3) cases.
We extracted data separately on primary tumor depth (T‐stage) and regional lymph node status (N‐stage).
We assembled all data in a dedicated database built within an Excel spreadsheet, where each row corresponded to a single study and variables of interest were recorded in the columns.
Assessment of methodological quality
We assessed data quality using a standard procedure according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) criteria (Whiting 2011). When there was at least one 'no' or 'unclear' response to a signaling question for a given domain, we scored the risk of bias as high or unclear, respectively. See Appendix 5 for details of the findings.
Statistical analysis and data synthesis
We performed statistical analysis according to Cochrane guidelines for diagnostic test accuracy (DTA) reviews (Macaskill 2010).
We used coupled forest plots to display the number of true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN), as well as sensitivity and specificity, with their 95% confidence intervals (CI), for all included studies. Visual inspection of forest plots can provide a clue to heterogeneity within single studies. We also used summary receiver operating characteristic (SROC) plots to display the results of individual studies in a ROC space, each study being plotted as a single sensitivity‐specificity point.
As currently recommended for meta‐analysis of diagnostic accuracy studies (Harbord 2008; Leeflang 2008; Macaskill 2010; Reitsma 2005; Rutter 2001), we used hierarchical models to obtain summary estimates of EUS performance in terms of ability to discriminate primary gastric cancer depth of invasion (T‐stage) and regional lymph node status (N‐stage).
According to the bivariate method (Reitsma 2005), we calculated overall sensitivity and specificity and their 95% confidence intervals (CIs) and predictive intervals, based on the binomial distributions of the true positives and true negatives. Besides accounting for study size and between‐study heterogeneity, the bivariate model adjusts for the frequently observed negative correlation between the sensitivity and the specificity of the index test (threshold effect). An additional advantage of using the bivariate model is that the bivariate nature of the original data can be maintained throughout the analysis, allowing the generation of reliable summary estimates of sensitivity and specificity. For the bivariate model, the summary estimates of sensitivity and specificity represent an 'average' operating point across studies. We analyzed primary tumor depth (T‐stage) and regional lymph node status (N‐stage) separately.
We evaluated the clinical (or patient‐relevant) utility of EUS using likelihood ratios, which we computed directly from the summary estimates of sensitivity and specificity, to enable the calculation of post‐test probability (based on the Bayes' theorem) by means of the Fagan's nomogram (Deeks 2004). The Fagan’s nomogram is a graphical tool which in routine clinical practice allows one to use the results of a diagnostic test to estimate a patient’s probability of having a disease (post‐test probability) based on two pieces of information: the pre‐test probability (usually the incidence of the disease/condition) and the test result. In this nomogram, a straight line drawn from a patient’s pre‐test probability of disease (left axis) through the likelihood ratio of the test (middle axis) intersects with the post‐test probability of disease (right axis).
We conducted statistical analyses using both Review Manager 5 software (RevMan 2014) as well as the Metandi and Midas programs for the STATA software (Stata 2009).
Since currently available imaging tools are associated with accuracy sensitivity and specificity values around 80% (see Background section), we considered this as a 'desirable' value with which the EUS diagnostic performance can be compared.
Investigations of heterogeneity
As it is common in diagnostic accuracy studies, we anticipated that there would be substantial between‐study variation in reported pairs of sensitivity and specificity values.
Coupled forest plots, which display both sensitivity and specificity of all included studies, provide a visual clue to heterogeneity of the results on a single‐study basis.
In order to formally investigate potential sources of heterogeneity other than the threshold effect, we used subgroup analysis and meta‐regression by including covariates (study sample size, publication year, type of EUS array, study quality, country, stomach site) in the bivariate model, which enabled us to assess the effect of various factors on the diagnostic accuracy of EUS.
Sensitivity analyses
We conducted sensitivity analyses to assess the impact on the summary effects of low‐quality studies, as defined by the identification of a high risk of bias for one or more QUADAS‐2 items, as well as the presence of specific types of primary gastric cancer morphology (e.g., ulcerated tumors) or location (e.g., cardia region of the stomach).
We also used the 'leave‐one‐out' procedure to assess the impact of each study on the meta‐analysis results (leading study effect).
Assessment of reporting bias
We conducted formal testing for small‐study effects (which include publication bias) by a regression of diagnostic odds ratio (DOR), which describes the odds of positive test results in patients with disease compared with the odds of positive test results in those without disease, on a natural logarithm scale against 1/sqrt ESS (effective sample size), weighting by ESS. P < 0.10 for the slope coefficient indicates significant asymmetry of the funnel plot (Deeks 2005).
Results
Results of the search
The literature search identified 2168 potentially relevant studies (Figure 1). By reading abstracts we excluded 2044 articles, and by reading full‐text versions we eliminated another 54 articles. We identified four citations as potentially meeting the inclusion criteria but could not assess them by the time of publication, and will address them in a future update. Ultimately, 66 articles were eligible according to the inclusion criteria. The main characteristics of the eligible studies, which were published from 1988 through 2012, are reported in the Characteristics of included studies section. The main characteristics of the excluded studies are reported in the Characteristics of excluded studies section. Considering the included studies, overall 7747 patients were enrolled in 16 different countries, with a mean of 117 patients enrolled per study (range: 14 to 930). Most of these studies (41/66, 62%) were published after 1999. The available evidence came primarily from retrospective studies (50/66, 76%), which enrolled Asian patients in 39 series (59%). The target condition was gastric carcinoma arising from any site of the stomach in 60 out of 66 studies (91%), whereas in the remaining series the authors focused on the tumors arising in the cardia region, Finally, the radial type of endoscopic ultrasound (EUS) array was the more often utilized compared to the linear array (55/58 articles (95%) reporting the type of array adopted).
1.
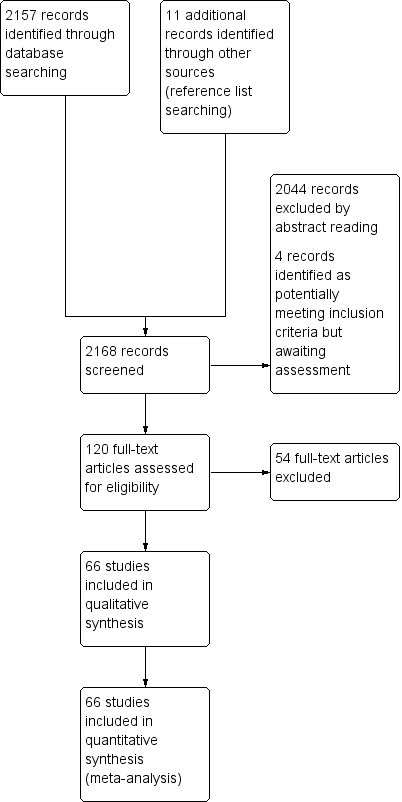
Study flow diagram.
Methodological quality of included studies
Overall, the quality of the included studies was good, as illustrated in the QUADAS‐2 results summary (Figure 2) and graph (Figure 3), and also summarized in Table 1. No concerns about applicability or patient selection bias or interpretation of reference test results were raised by the analysis of the available data. However, five studies (Bhandari 2004; Garlipp 2011; Heye 2009; Potrc 2006; Xi 2003) presented a high risk of index test interpretation bias due to the lack of threshold definition for the classification of T‐stage or N‐stage or both. Two other studies (Akashi 2006; Mouri 2009) presented a high risk of selection bias due to the lack of inclusion of all patients: in particular, 37 and 31 patients respectively were not included, due to uninterpretable EUS findings. In another two studies (Hizawa 2002; Yanai 1997) the same issue occurred for only seven and four patients respectively: accordingly we deemed the risk of bias in these cases as unclear. Overall, uninterpretable results were rarely reported, although this is not a guarantee that EUS findings are always easily interpretable; it might just reflect the attitude of the endoscopist to provide a classification 'at any cost'. In most studies the interval between the index test and the reference test was unreported, but we believe that this occurrence is unlikely to undermine the reliability of the results, since the diagnosis of a malignant disease such as gastric carcinoma is usually considered an indication for surgery and thus for pathological evaluation within a very short time (some days/a few weeks). This in turn is unlikely to be sufficient for the disease stage to change.
2.
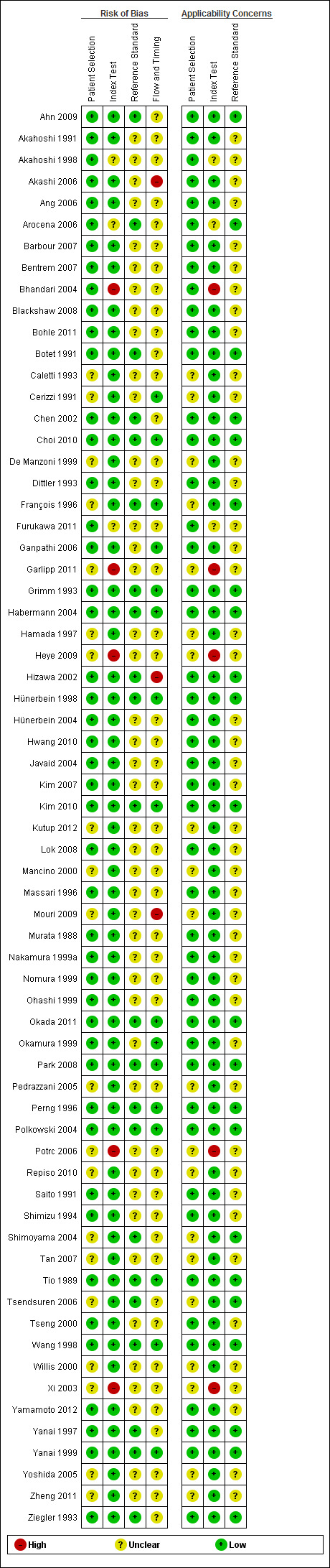
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
3.
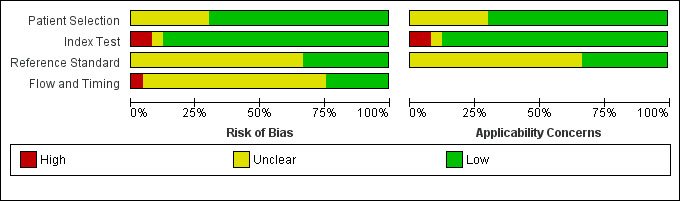
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
Findings
Primary tumor depth (T‐stage)
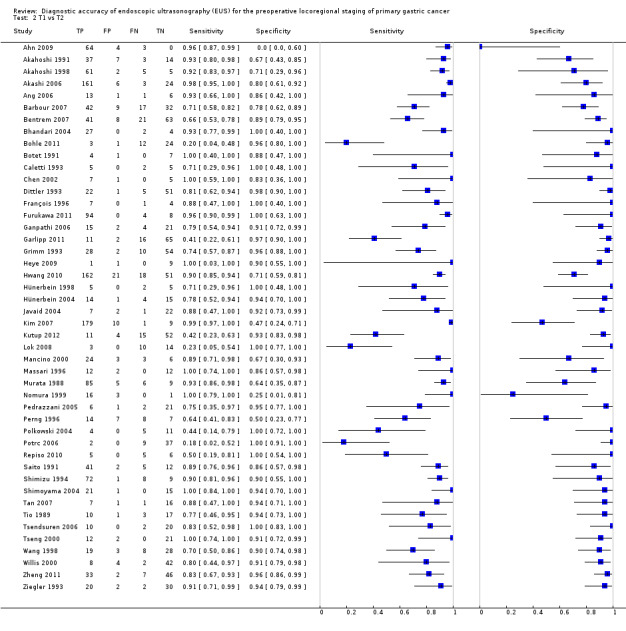
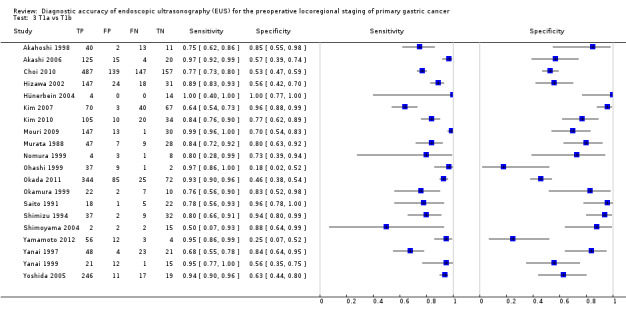
We first addressed the issue of EUS accuracy in discriminating T1 ‐ T2 (superficial) versus T3 ‐ T4 (advanced) gastric carcinomas. We therefore carried out a meta‐analysis of the eligible studies reporting relevant data. For this analysis (Data table 1), 50 studies were available, with a total of 4397 patients.
1. Test.
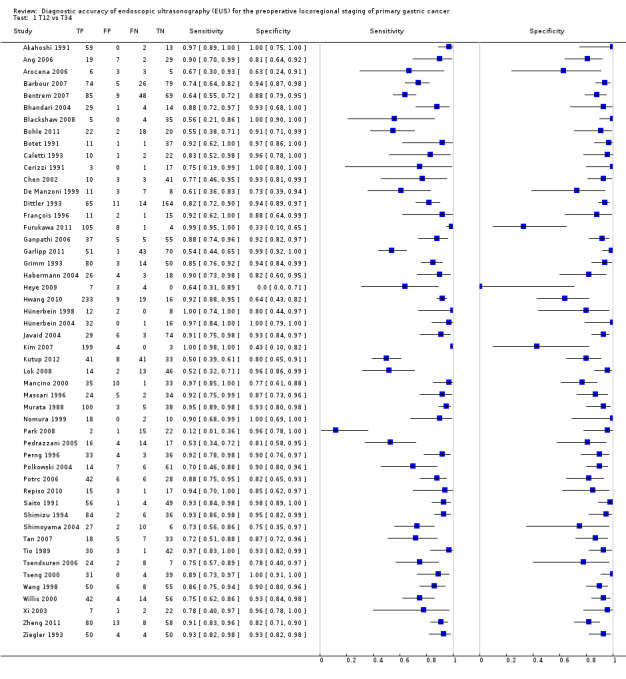
T12 vs T34.
The sensitivity and specificity of the single studies are shown in Data table 1. The summary receiver operating curve (SROC) curve along with the summary point and the 95% confidence and prediction regions are illustrated in Figure 4. The summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were 0.86 (95% confidence interval (CI): 0.81 to 0.90), 0.90 (95% CI: 0.87 to 0.93), 8.9 (95% CI: 6.8 to 11.6), 0.16 (95% CI: 0.12 to 0.22), and 56 (95% CI: 37 to 85), respectively.
4.
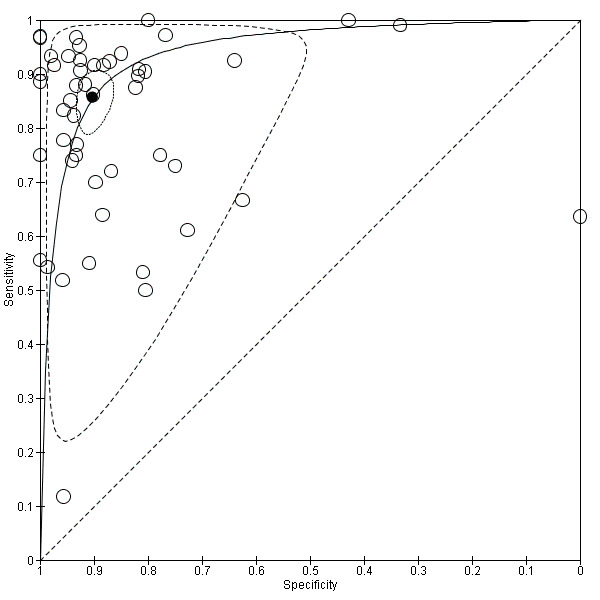
Summary ROC Plot of studies assessing the accuracy of EUS in discriminating T1 ‐ T2 versus T3 ‐ T4 gastric carcinomas. Each study sensitivity/specificity value is represented by an empty circle. The summary point for sensitivity/specificity is represented by a black filled circle. Dotted closed line: 95% confidence region of the summary point. Dashed closed line: 95% prediction region.
As shown in Figure 4 by both confidence and prediction regions, the results indicate a lower variability for specificity as compared to sensitivity, suggesting that EUS might be more reliable in correctly identifying T3 ‐ T4 cases compared to T1 ‐ T2 cases.
Although both summary sensitivity and specificity values were relatively satisfactory, between‐study heterogeneity was substantial, as visually assessable through both the forest plot (Data table 1) and predictive ellipse (Figure 4).
The Fagan plot (Figure 5) illustrates that EUS may be clinically useful because it increases the previous probability of being classified as T1 ‐ T2 from 50% (average prevalence of T1 ‐ T2 cases) to 90% when positive, and it lowers the same probability to 14% when negative. However, the likelihood ratio (LR) scattergram (Figure 6) shows that the summary point of positive and negative LR is located in the lower right quadrant, suggesting that EUS accuracy ‐ although close to values desirable for a diagnostic tool ‐ is not optimal either for tumor depth confirmation or exclusion.
5.
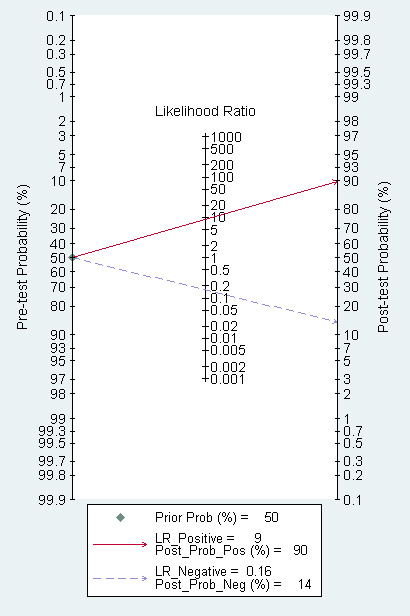
Fagan plot estimating how much the result of EUS changes the probability that a patient has a T1 ‐ T2 (rather than T3 ‐ T4) gastric cancer, considering a given pre‐test probability (here the mean pre‐test probability found in eligible studies is shown as an example).
6.
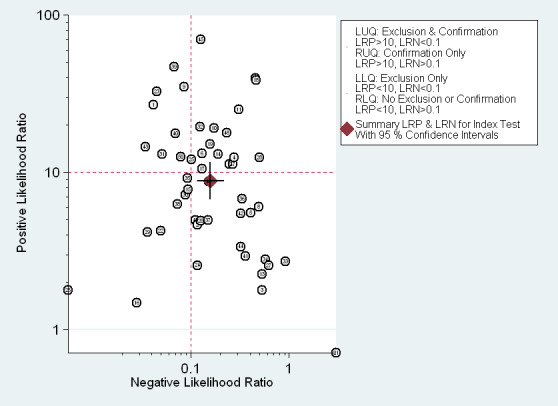
EUS ability to discriminate between T1‐T2 and T3‐T4 gastric carcinomas. Likelihood ratio (LR) scattergram defining quadrants of informativeness based on desirable thresholds (positive LR>10, negative LR<0.1): left upper quadrant (test suitable both for diagnosis exclusion and confirmation), right upper (confirmation only), left lower (exclusion only), right lower (neither confirmation nor exclusion).
These findings imply that in a hypothetical cohort of 1000 people with gastric carcinoma, EUS would correctly classify 880 of them, but would also over‐stage 70 patients by classifying them as T3 ‐ T4 instead of T1 ‐ T2, and under‐stage 50 patients by classifying them as T1 ‐ T2 instead of T3 ‐ T4 (see Table 1).
Since the proportion of heterogeneity likely caused by the threshold effect was low (12%), we looked for other sources of heterogeneity. In this regard subgroup analysis (Table 2) demonstrated that publication year has a significant impact on EUS diagnostic performance, since studies conducted before the year 2000 reported on average significantly higher sensitivity (0.91 (95% CI 0.87 to 0.96) versus 0.81 (95% CI 0.75 to 0.88)) and specificity (0.94 (95% CI 0.91 to 0.96) versus 0.88 (95% CI 0.84 to 0.9)). Also the type of EUS array appeared to be correlated with diagnostic performance, with radial array being more accurate than linear array (Table 2); however, only four studies used the latter type of array, which makes it unwise to draw any definitive conclusion on this topic. The other subgroup and sensitivity analyses were not informative.
1. Subgroup and sensitivity analysis for T1 ‐ T2 versus T3 ‐ T4 gastric tumors.
| Variable | Category | Studies | Sensitivity (95% CI) | Specificity (95% CI) | P value* |
| Sample size | >100 | 18 | 0.89 (0.83 to 0.95) | 0.90 (0.85 to 0.94) | 0.46 |
| <100 | 32 | 0.83 (0.77 to 0.90) | 0.91 (0.87 to 0.94) | ||
| Year of publication | 2000 or later | 32 | 0.81 (0.75 to 0.88) | 0.88 (0.84 to 0.91) | <0.01 |
| before 2000 | 18 | 0.91 (0.87 to 0.96) | 0.94 (0.91 to 0.96) | ||
| Country | Western | 27 | 0.82 (0.75 to 0.89) | 0.91 (0.87 to 0.94) | 0.27 |
| Eastern | 23 | 0.89 (0.84 to 0.94) | 0.90 (0.86 to 0.94) | ||
| EUS array | Radial | 39 | 0.88 (0.84 to 0.93) | 0.90 (0.87 to 0.93) | <0.01 |
| Linear | 4 | 0.68 (0.40 to 0.96) | 0.86 (0.73 to 1.00) | ||
| Tumor site | Cardia only | 6 | 0.70 (0.49 to 0.91) | 0.90 (0.82 to 0.98) | 0.12 |
| Any site | 44 | 0.87 (0.83 to 0.91) | 0.90 (0.88 to 0.93) | ||
| Quality | High | 45 | 0.86 (0.82 to 0.91) | 0.90 (0.88 to 0.93) | 0.59 |
| Low | 5 | 0.78 (0.72 to 1.00) | 0.90 (0.81 to 0.99) |
CI: confidence interval EUS: endoscopic ultrasonography *P values are from likelihood ratio test for model with and without the covariate, to identify diagnostic performance differences across variable categories.
Regression testing for funnel plot asymmetry (Deeks 2005) showed no evidence of statistically significant small‐study effect bias (P = 0.48).
Exclusion of studies with a high risk of bias did not significantly change the above findings (Table 2).
For EUS diagnostic ability to distinguish T1 (early gastric cancer, EGC) versus T2 (muscle‐infiltrating) tumors, the meta‐analysis of 46 studies (n = 2742; Data table 2) (see forest plot and SROC curve in Data table 2 and Figure 7, respectively) showed that the summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were 0.85 (95% CI 0.78 to 0.91), 0.90 (95% CI 0.85 to 0.93), 8.5 (95% CI 5.9 to 12.3), 0.17 (95% CI 0.12 to 0.24), and 50 (95% CI 32 to 79), respectively.
2. Test.
T1 vs T2.
7.
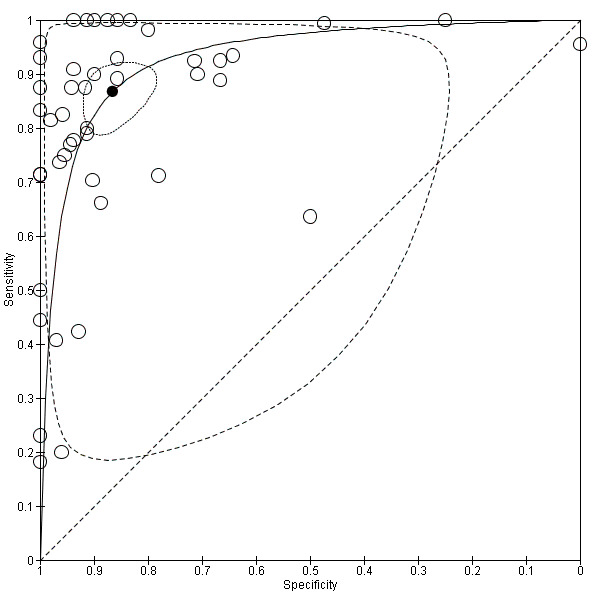
Summary ROC Plot of 46 studies investigating the EUS ability to discriminate between T1 versus T2 gastric carcinomas.
Although both summary sensitivity and specificity values were relatively satisfactory, between‐study heterogeneity was substantial, as visually assessable through both the forest plot (Data table 2) and predictive ellipse (Figure 7).
The Fagan plot (Figure 8) illustrates that EUS may be clinically useful because it increases the previous probability of being classified as T1 from 70% (average prevalence of T1 cases) to 94% when positive, and it lowers the same probability to 26% when negative. However, the likelihood ratio (LR) scattergram (Figure 9) shows that the summary point of positive and negative LR is located in the lower right quadrant, suggesting that EUS accuracy, although close to ideal values, is not optimal either for disease depth confirmation or exclusion.
8.
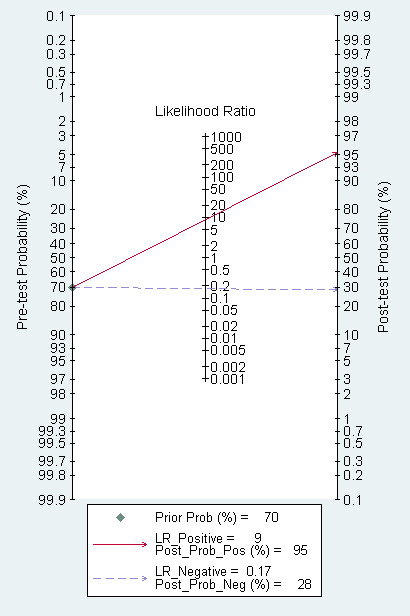
Fagan plot estimating how much the result of EUS changes the probability that a patient has a T1 (rather than T2) gastric cancer, considering a given pre‐test probability (here the mean pre‐test probability found in eligible studies is shown as an example).
9.
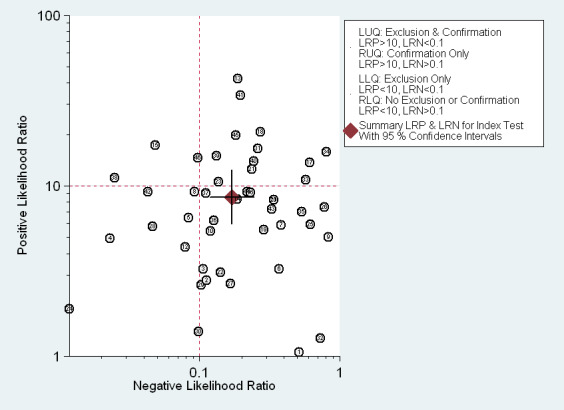
EUS ability to discriminate between T1 and T2 gastric carcinomas. Likelihood ratio (LR) scattergram defining quadrants of informativeness based on desirable thresholds (positive LR>10, negative LR<0.1): left upper quadrant (test suitable both for diagnosis exclusion and confirmation), right upper (confirmation only), left lower (exclusion only), right lower (neither confirmation nor exclusion).
These findings imply that in a hypothetical cohort of 1000 people with gastric carcinoma, EUS would correctly classify 865 of them, but would also over‐stage 105 patients by classifying them as T2 instead of T1, and under‐stage 30 patients by classifying them as T1 instead of T2 (see Table 1).
Since the proportion of heterogeneity likely caused by the threshold effect was moderate (30%), we looked for further sources of heterogeneity. Subgroup analysis suggested that sample size, country of origin and type of EUS array might have a (limited) impact on EUS diagnostic performance (Table 3). However, these results are to be interpreted cautiously because of the low number of studies in some comparison groups. The other subgroup and sensitivity analyses were not informative.
2. Subgroup and sensitivity analysis for T1 versus T2 gastric tumors.
| Variable | Category | Studies | Sensitivity (95% CI) | Specificity (95% CI) | P value* |
| Sample size | >100 | 5 | 0.97 (0.93 to 1.00) | 0.73 (0.53 to 0.93) | 0.01 |
| <100 | 41 | 0.81 (0.74 to 0.87) | 0.91 (0.88 to 0.95) | ||
| Year of publication | 2000 or later | 29 | 0.82 (0.74 to 0.90) | 0.91 (0.87 to 0.96) | 0.51 |
| before 2000 | 17 | 0.88 (0.80 to 0.96) | 0.87 (0.80 to 0.95) | ||
| Country | Western | 22 | 0.71 (0.59 to 0.82) | 0.94 (0.91 to 0.97) | <0.01 |
| Eastern | 24 | 0.92 (0.88 to 0.96) | 0.84 (0.77 to 0.91) | ||
| EUS array | Radial | 37 | 0.85 (0.79 to 0.91) | 0.90 (0.85 to 0.94) | <0.01 |
| Linear | 3 | 0.92 (0.77 to 1.00) | 0.98 (0.93 to 1.00) | ||
| Tumor site | Cardia only | 5 | 0.91 (0.80 to 1.00) | 0.89 (0.77 to 1.00) | 0.59 |
| Any site | 41 | 0.84 (0.77 to 0.90) | 0.90 (0.86 to 0.94) | ||
| Quality | High | 41 | 0.85 (0.79 to 0.91) | 0.89 (0.85 to 0.93) | 0.39 |
| Low | 5 | 0.79 (0.57 to 1.00) | 0.96 (0.90 to 1.00) |
CI: confidence interval EUS: endoscopic ultrasonography *P values are from likelihood ratio test for model with and without the covariate, to identify diagnostic performance differences across variable categories.
Regression testing for funnel plot asymmetry showed no evidence of statistically significant small‐study effect bias (P = 0.58).
Exclusion of studies with a high risk of bias did not significantly change the above findings (Table 3).
We then focused on the EUS ability to distinguish between T1a (mucosal) versus T1b (submucosal) cancers: the meta‐analysis of 20 studies (n = 3321; Data table 3) (see forest plot and SROC curve in Data table 3 and Figure 10, respectively) showed that the summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio DOR were 0.87 (95% CI 0.81 to 0.92), 0.75 (95% CI 0.62 to 0.84), 3.4 (95% CI 2.3 to 5.0), 0.17 (95% CI 0.12 to 0.24), and 20 (95% CI 12 to 33), respectively.
3. Test.
T1a vs T1b.
10.
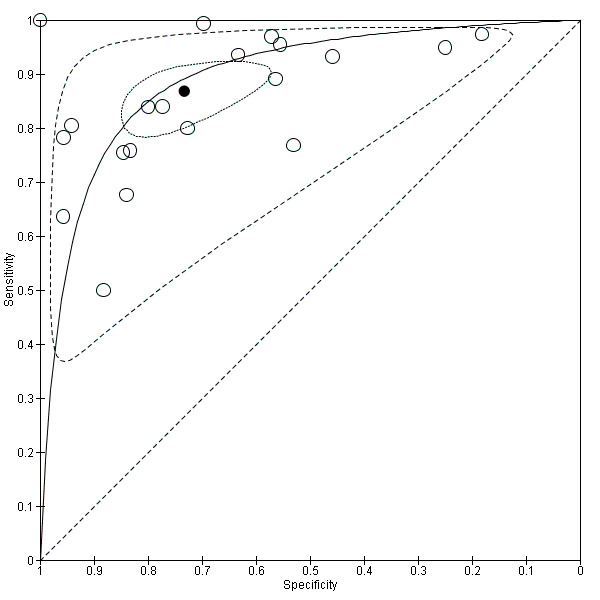
Summary ROC Plot of 20 studies investigating the diagnostic ability of EUS to discriminate between T1a versus T1b tumors.
Summary sensitivity (but not specificity) value was relatively high, but between‐study heterogeneity was substantial as visually assessable through both the forest plot (Data table 3) and predictive ellipse (Figure 10).
The Fagan plot (Figure 11) illustrates that EUS may be clinically useful because it increases the previous probability of being classified as T1 from 70% (average prevalence of T1a cases) to 88% when positive, and it lowers the same probability to 30% when negative. However, the likelihood ratio (LR) scattergram (Figure 12) shows that the summary point of positive and negative LR is located in the lower right quadrant, suggesting that EUS accuracy is not optimal either for disease depth confirmation or exclusion.
11.
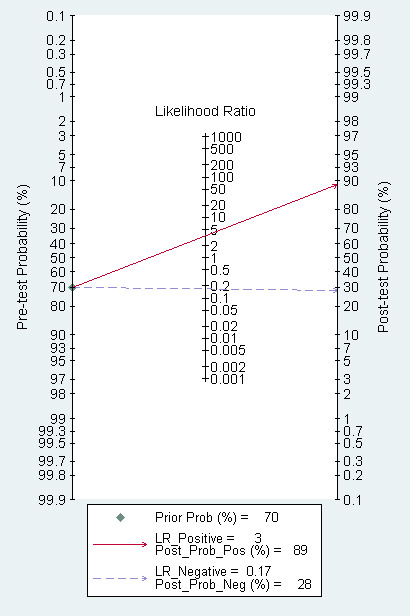
Fagan plot estimating how much the result of EUS changes the probability that a patient has a T1a (rather than T1b) gastric cancer, considering a given pre‐test probability (here the mean pre‐test probability found in eligible studies is shown as an example).
12.
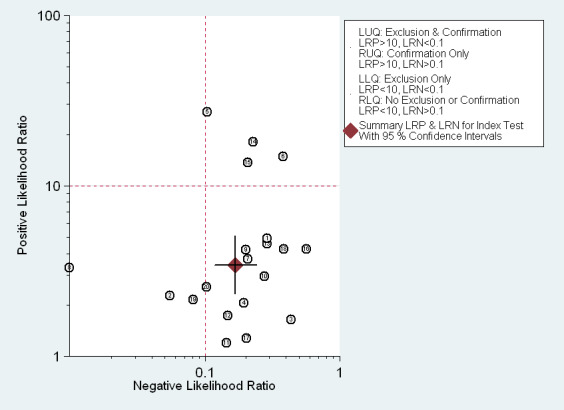
EUS ability to discriminate between T1a and T1b gastric carcinomas. Likelihood ratio (LR) scattergram defining quadrants of informativeness based on desirable thresholds (positive LR>10, negative LR<0.1): left upper quadrant (test suitable both for diagnosis exclusion and confirmation), right upper (confirmation only), left lower (exclusion only), right lower (neither confirmation nor exclusion).
These findings imply that in a hypothetical cohort of 1000 people with gastric carcinoma, EUS would correctly classify 834 of them, but would also over‐stage 91 patients by classifying them as T1b instead of T1a, and under‐stage 75 patients by classifying them as T1a instead of T1b (see Table 1).
The proportion of heterogeneity likely caused by the threshold effect was 49%. Subgroup and sensitivity analyses suggested that none of the covariates we considered was associated with between‐study heterogeneity (Table 4).
3. Subgroup and sensitivity analysis for T1a versus T1b gastric tumors.
| Variable | Category | Studies | Sensitivity (95% CI) | Specificity (95% CI) | P value* |
| Sample size | >100 | 8 | 0.90 (0.84 to 0.96) | 0.67 (0.50 to 0.85) | 0.49 |
| <100 | 12 | 0.85 (0.76 to 0.93) | 0.79 (0.67 to 0.91) | ||
| Year of publication | 2000 or later | 11 | 0.90 (0.84 to 0.95) | 0.70 (0.55 to 0.85) | 0.59 |
| before 2000 | 9 | 0.84 (0.75 to 0.94) | 0.79 (0.66 to 0.93) | ||
| Country | Western | 1 | 1.00 (0.40 to 1.00) | 1.00 (0.69 to 1.00) | N/A |
| Eastern | 19 | 0.88 (0.81 to 0.92) | 0.73 (0.61 to 0.82) | ||
| EUS array | Radial | 18 | 0.89 (0.82 to 0.93) | 0.72 (0.59 to 0.82) | N/A |
| Linear | 1 | 0.50 (0.07 to 0.93) | 0.88 (0.64 to 0.99) | ||
| Tumor site | Cardia region | 1 | 0.50 (0.07 to 0.93) | 0.88 (0.64 to 0.99) | N/A |
| Any site | 19 | 0.88 (0.82 to 0.92) | 0.74 (0.61 to 0.83) | ||
| Quality | High | 18 | 0.84 (0.79 to 0.89) | 0.75 (0.64 to 0.86) | 0.05 |
| Low | 2 | 0.98 (0.96 to 1.00) | 0.64 (0.26 to 1.00) |
CI: confidence interval EUS: endoscopic ultrasonography *P values are from likelihood ratio test for model with and without the covariate, to identify diagnostic performance differences across variable categories.
Regression testing for funnel plot asymmetry showed evidence of statistically significant small‐study effect bias (P = 0.04).
Exclusion of studies with a high risk of bias did not significantly change the above findings (Table 4).
Lymph node status (N‐stage)
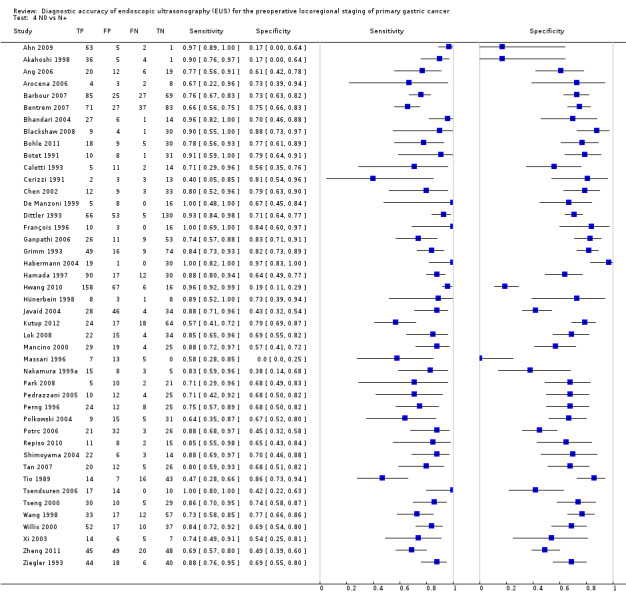
We then carried out a meta‐analysis of the eligible studies reporting data on N‐stage (positive versus negative) to evaluate the diagnostic ability of EUS to assess the status of regional lymph nodes in people with gastric carcinoma. Forty‐four studies were available, with a total of 3573 patients (Data table 4).
4. Test.
N0 vs N+.
The sensitivity and specificity of each single study are shown in Data table 4. The SROC curve along with the summary point and the 95% confidence and prediction regions are illustrated in Figure 13.
13.
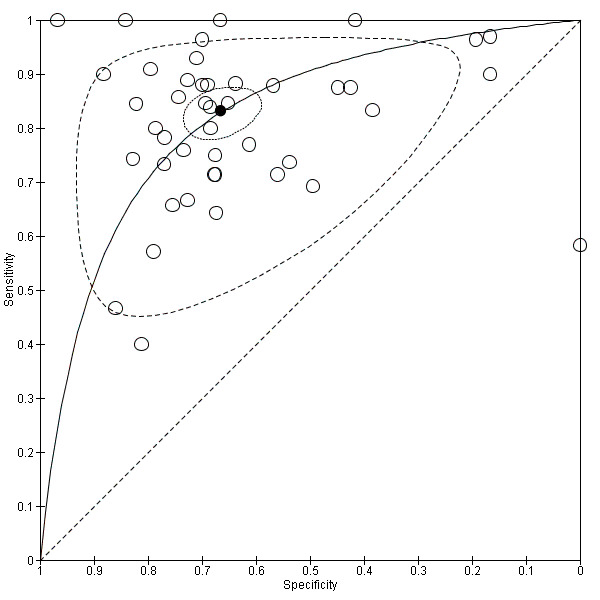
Summary ROC Plot of 44 studies addressing the issue of EUS ability to discriminate between lymph node negative (N0) and positive (N+) cases.
Summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were 0.83 (95% CI 0.79 to 0.87), 0.67 (95% CI 0.61 to 0.72), 2.5 (95% CI 2.1 to 2.9), 0.25 (95% CI 0.20 to 0.31), and 10 (95% CI 7 to 13), respectively.
Summary sensitivity (but not specificity) value was relatively high, but between‐study heterogeneity was substantial as visually assessable through both the forest plot (Data table 4) and predictive ellipse (Figure 13).
The Fagan plot (Figure 14) shows that EUS may be clinically informative because it increases the previous probability of being classified as N+ from 50% (average prevalence of N+ cases) to 62% when positive, and it lowers the same probability to 14% when negative. However, the likelihood ratio (LR) scattergram (Figure 15) shows that the summary point of positive and negative LR is located in the lower right quadrant, suggesting that EUS accuracy is not optimal either for lymph node metastatic involvement confirmation or exclusion.
14.
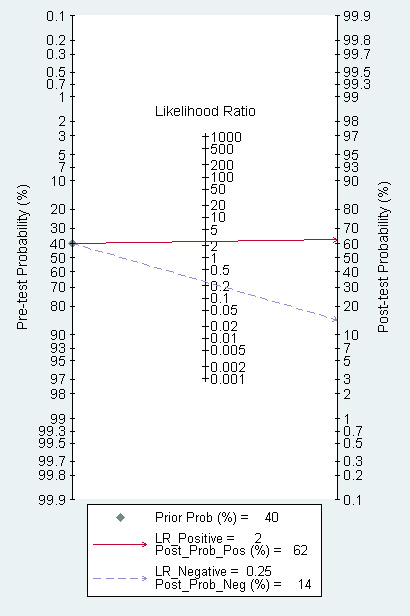
Fagan plot estimating how much the result of EUS changes the probability that a patient has a N+ (metastatic lymph nodes) (rather than a N0, disease free lymph nodes) gastric cancer, considering a given pre‐test probability (here the mean pre‐test probability found in eligible studies is shown as an example).
15.
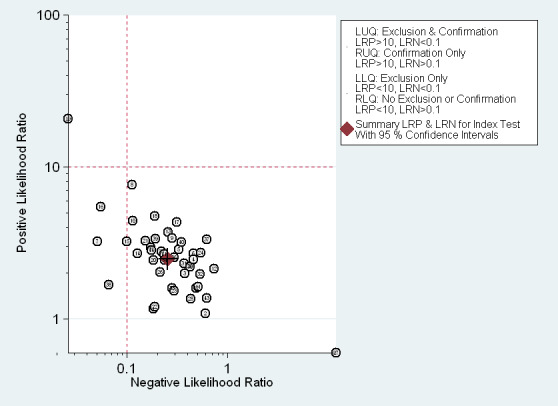
EUS ability to discriminate between N+ (metastatic lymph nodes) and N0 (disease free lymph nodes) gastric carcinomas. Likelihood ratio (LR) scattergram defining quadrants of informativeness based on desirable thresholds (positive LR>10, negative LR<0.1): left upper quadrant (test suitable both for diagnosis exclusion and confirmation), right upper (confirmation only), left lower (exclusion only), right lower (neither confirmation nor exclusion).
These findings imply that in a hypothetical cohort of 1000 people with gastric carcinoma, EUS would correctly classify 750 of them, but would also over‐stage 85 patients by classifying them as T1b instead of T1a, and under‐stage 165 patients by classifying them as T1a instead of T1b (see Table 1).
The proportion of heterogeneity likely caused by the threshold effect was moderate (17%). Subgroup and sensitivity analyses (Table 5) suggested that none of the covariates we considered were associated with between‐study heterogeneity, although analysis by country of origin was of borderline significance.
4. Subgroup and sensitivity analysis for N0 versus N+ gastric tumors.
| Variable | Category | Studies | Sensitivity (95% CI) | Specificity (95% CI) | P value* |
| Sample size | >100 | 12 | 0.83 (0.77 to 0.89) | 0.65 (0.55 to 0.75) | 0.90 |
| <100 | 32 | 0.84 (0.79 to 0.88) | 0.67 (0.61 to 0.74) | ||
| Year of publication | 2000 or later | 28 | 0.83 (0.79 to 0.88) | 0.66 (0.59 to 0.73) | 0.95 |
| before 2000 | 16 | 0.83 (0.76 to 0.89) | 0.68 (0.58 to 0.77) | ||
| Country | Western | 24 | 0.80 (0.75 to 0.86) | 0.72 (0.66 to 0.79) | 0.04 |
| Eastern | 20 | 0.86 (0.81 to 0.90) | 0.59 (0.51 to 0.68) | ||
| EUS array | Radial | 35 | 0.84 (0.80 to 0.88) | 0.66 (0.60 to 0.73) | 0.11 |
| Linear | 4 | 0.83 (0.69 to 0.98) | 0.66 (0.46 to 0.86) | ||
| Tumor site | Cardia region | 6 | 0.86 (0.76 to 0.96) | 0.76 (0.63 to 0.88) | 0.27 |
| Any site | 38 | 0.83 (0.79 to 0.87) | 0.65 (0.59 to 0.71) | ||
| Quality | High | 41 | 0.83 (0.79 to 0.87) | 0.67 (0.62 to 0.73) | 0.57 |
| Low | 3 | 0.88 (0.77 to 0.99) | 0.56 (0.32 to 0.80) |
CI: confidence interval EUS: endoscopic ultrasonography *P values are from likelihood ratio test for model with and without the covariate, to identify diagnostic performance differences across variable categories.
Regression testing for funnel plot asymmetry showed no evidence of statistically significant small‐study effect bias (P = 0.96).
Exclusion of studies with a high risk of bias did not significantly change the above findings (Table 5).
Discussion
In this systematic review of the diagnostic performance of endoscopic ultrasound (EUS) for the locoregional staging of gastric cancer we collected the data from the largest series of patients ever considered in the international medical literature (n = 7747). Using modern statistical methods specifically dedicated to diagnostic meta‐analysis, i.e., the hierarchical bivariate model, we quantitatively summarized the available evidence and found that overall EUS provides clinically useful information regarding gastric cancer locoregional spread. EUS summary sensitivities and specificities ranged from 0.83 to 0.87 and from 0.90 to 0.67 respectively, all significantly higher than the 0.50 'null' value. This means that EUS performs better than the prediction made with a flip of a coin: the 95% confidence intervals of those summary estimates do not in fact cross the 0.50 value, which is the probability value of being 'diseased' (e.g. the probability of having metastatic lymph nodes) assigned to each patient by the flip of a coin (i.e., 50%). This finding is strengthened by the results of the Bayesian analysis (see Fagan plots), which demonstrate that EUS also performs better than a 'smart observer', that is, one who knows the prevalence of the condition (e.g. percentage of T1 gastric cancers as a proportion of all gastric cancers) and thus would assign this probability value to patients, which would ultimately increase the predictive accuracy compared to the more simplistic flip‐of‐a‐coin approach. Consider the T1 versus T2 setting as an example: if the proportion of T1 tumors is 0.70 (based on previous epidemiological studies), one could use this value to classify patients by assigning to each patient the probability of being T1 equal to the prevalence of the condition (0.70): this would lead to an accuracy of 70% based on the fact that 70% of patients would be correctly identified by randomly classifying 70% of them as T1. This approach would yield a better diagnostic performance compared to the flip‐of‐a‐coin approach, which would assign a 0.50 probability to all patients, and thus would achieve 50% accuracy. Compared to these two approaches, EUS can better discriminate between T1 and T2 cases, since it changes the likelihood of being T1 from 70% to 94% when the test is positive and it lowers the same probability to 26% when tests are negative (overall accuracy: 93%). This could be very helpful for clinicians during the decision‐making process of patient therapeutic management.
Critical issues
Despite these favorable findings, some critical aspects must be emphasized to correctly appreciate the limitations of this diagnostic tool.
First, the remarkable heterogeneity of results we found across eligible studies, most of which (50/66, 76%) are retrospective in design, casts some doubts on the reliability and reproducibility of EUS in the locoregional staging of gastric carcinoma. Unfortunately, we did not identify any technical (e.g. EUS probe frequency) or tumor‐related (e.g. stomach site) feature that might explain such variability in results reported in the relevant literature, which does not allow us to suggest any strategy that might improve the performance of EUS. Notably, we could not explore the experience of the endoscopist as a source of heterogeneity, as no such information is available in the literature. However, we failed to detect an association between heterogeneity and the sample size (a potential surrogate for the experience of the endoscopy center). The only suspected source of heterogeneity we could identify was the year of publication (better and more homogeneous results in earlier studies), which was especially evident for distinguishing T1 ‐ T2 from T3 ‐ T4 tumors; this finding might be due to a 'first study' effect, i.e. more enthusiasm surrounding the procedure during the first years after its implementation in the clinical setting. However, we cannot rule out the possibility that the diffusion of EUS in clinical practice following the initial encouraging results might have led to the use of this tool by less experienced endoscopists, with an increased probability of less accurate interpretations of the test. Due to the lack of data, we could not explore the effect of other potential sources of heterogeneity, such as primary tumor characteristics, e.g., diameter, morphology (flat versus ulcerated versus vegetant), and experience of the endoscopist; these and other factors therefore remain to be investigated to better define the limits of EUS in the locoregional staging of gastric carcinoma.
Second, not all parameters of diagnostic performance reached ideal values, i.e., values believed to be desirable for the implementation of a diagnostic tool in clinical practice. In particular, the EUS summary specificity for the diagnosis of mucosal (T1a) versus submucosal (T1b) tumors and for the diagnosis of lymph node metastasis (N0 versus N+) was 0.75 and 0.67 respectively, which are below the desirable value of 0.8. Moreover, since the diagnostic ability of a test depends not only on its discriminatory value but also on the prevalence of the disease, we considered the likelihood ratios (LRs) associated with EUS performance, as illustrated in the LR matrices (see Figure 6; Figure 9; Figure 12; Figure 15). EUS showed an acceptable performance for the differentiation of T1 ‐ T2 from T3 ‐ T4 tumors and T1 from T2 tumors, but not for distinguishing T1a from T1b tumors or for diagnosis of lymph node status (N0 versus N+).
Comparison with existing literature
Two systematic reviews (without meta‐analysis) and three meta‐analyses have been published on this topic between 2007 and 2011. The two reviews, one dedicated to primary tumor depth and the other to lymph node status, concluded that EUS is a reliable imaging modality in staging tumor depth but not for the definition of lymph node status (Kwee 2007; Kwee 2009). These conclusions are similar to those we present here, although our work provides formal evidence to sustain these hypotheses as well as a quantification of the average performance of this endoscopic tool. This information, along with the Bayesian nomograms, enables clinicians to get a precise sense of the risk of making errors, both in terms of false‐positive and false‐negative predictions, while using EUS, which ultimately can help them optimize the therapeutic management of patients based on statistically‐estimated diagnostic accuracy parameters and not on dichotomous personal opinions (i.e. 'works' versus 'does not work') on EUS performance, such as those deriving from qualitative reviews. Between 2001 and 2011, three meta‐analyses were also published on both primary tumor depth staging and regional lymph node staging of gastric cancer with EUS (Kelly 2001; Mocellin 2011; Puli 2008). The reliability of the first two articles (Kelly 2001; Puli 2008) is undermined by the use of a statistical method, based on the Moses‐Littenberg model, that is no longer considered scientifically sound for the meta‐analysis of diagnostic accuracy studies (Harbord 2008; Leeflang 2008; Macaskill 2010; Reitsma 2005; Rutter 2001). Furthermore, the number of included studies (13 and 22 respectively) was much lower than our retrieval rate and analysis (n = 66). The third meta‐analysis (Mocellin 2011), which was conducted with modern statistics on 54 studies, reported results slightly better than those described here: this difference might be due to the lower number of studies included in that meta‐analysis and is in line with the above‐mentioned trend towards better results in earlier series.
In conclusion, by analyzing the data from the largest series ever considered, we found that the diagnostic accuracy of EUS can be considered clinically useful, although not optimal, to guide physicians in the locoregional staging of patients with gastric carcinoma. However, the heterogeneity of the results warrants some caution, as well as further investigation for the identification of factors influencing the outcome of this diagnostic tool. Physicians should also be warned that EUS performance is slightly lower in diagnosing superficial tumors (T1a versus T1b) and lymph node status (positive versus negative).
Summary of main results
The main results of our review, summarized in Table 1, are the following:
By analyzing the data from 66 articles published from 1988 through 2012, we collected data on 7747 people with gastric cancer who were staged with endoscopic ultrasonography (EUS): this represents the largest series ever reported on this topic.
The meta‐analysis of 50 studies (n = 4397) showed that the summary sensitivity and specificity of EUS in discriminating T1 ‐ T2 (superficial) versus T3 ‐ T4 (advanced) gastric carcinomas were 0.86 (95% CI 0.81 to 0.90) and 0.90 (95% CI 0.87 to 0.93), respectively.
For the diagnostic capacity of EUS to distinguish T1 (early gastric cancer, EGC) versus T2 (muscle‐infiltrating) tumors, the meta‐analysis of 46 studies (n = 2742) showed that the summary sensitivity and specificity were 0.85 (95% CI 0.78 to 0.91) and 0.90 (95% CI 0.85 to 0.93) respectively. When we addressed the capacity to distinguish between T1a (mucosal) versus T1b (submucosal) cancers the meta‐analysis of 20 studies (n = 3321) showed that the summary sensitivity and specificity were 0.87 (95% CI 0.81 to 0.92) and 0.75 (95% CI 0.62 to 0.84) respectively.
For the metastatic involvement of lymph nodes (N‐stage), the meta‐analysis of 44 studies (n = 3573) showed that the summary sensitivity and specificity were 0.83 (95% CI 0.79 to 0.87) and 0.67 (95% CI 0.61 to 0.72) respectively.
Overall, EUS accuracy can be considered clinically useful to guide physicians in the locoregional staging of patients with gastric cancer.
However, between‐study heterogeneity was not negligible: unfortunately, we could not identify any consistent source of the observed heterogeneity, and thus all the results presented here must be interpreted cautiously.
Moreover, the analysis of positive and negative likelihood values revealed that EUS diagnostic performance cannot be considered optimal either for disease confirmation or for exclusion, especially for distinguishing T1a (mucosal) from T1b (submucosal) cancers and positive from negative lymph node status.
Strengths and weaknesses of the review
The main strength of this review is the number of patients enrolled (n = 7747), which is the highest ever reported and guarantees a good representation of the results obtained with this diagnostic tool worldwide. Moreover, we provide not only conventional meta‐analysis results, such as summary estimates of diagnostic performance measures, but also findings from additional analyses such as Bayesian analysis, which add further information of clinical use, including Fagan plots and likelihood ratio matrices. The main limitation of this review is that, despite the high number of patients enrolled, heterogeneity is remarkably high, which may partially undermine the reliability and reproducibility of most reported results. Furthermore, the data available in the literature did not allow identification of possible sources of heterogeneity.
Applicability of findings to the review question
The number of studies identified (66) and the number of patients enrolled (7747) were sufficient to address the review question, i.e., quantification of EUS diagnostic performance in the locoregional staging of gastric carcinoma. Patients enrolled, technical features of both index test and reference standard, and clinical settings were homogeneously suitable for our analysis across all studies. As expected in diagnostic test accuracy meta‐analysis, heterogeneity was a problem: unfortunately, we could not identify consistent sources of heterogeneity, which did not allow us to suggest factors potentially influencing the performance of this diagnostic tool.
Authors' conclusions
Implications for practice.
Our findings partly support the use of endoscopic ultrasonography (EUS) for the locoregional staging of people with gastric carcinoma. EUS diagnostic performance, although not optimal, may be considered clinically useful to guide physicians in disease staging and thus in the development of the most appropriate therapeutic strategy on an individual‐patient basis, according to personalized medicine principles. However, physicians should be warned that EUS performance is lower in diagnosing superficial tumors (T1a versus T1b) and lymph node status (positive versus negative). The remarkable heterogeneity of the evidence currently available warrants some caution in interpreting the present results. Overall, we observed considerable heterogeneity and its sources need to be understood before any definitive conclusion can be drawn about the use of EUS can be proposed in a routine clinical setting.
Implications for research.
The valid but suboptimal diagnostic accuracy of EUS for the locoregional staging of gastric cancer, with special regard to the diagnosis of superficial T1 tumors and lymph node status, prompts further investigation to improve the performance of this tool, especially for the diagnosis of superficial tumors (T1a versus T1b) and lymph node status (positive versus negative). Technological improvements, such as the combination of EUS with fine needle aspiration of suspicious lymph nodes (Dumonceau 2011), may lead to the optimization of gastric cancer staging, which should ultimately ameliorate the therapeutic management of these patients. It will also be important to compare the diagnostic performance of different tools (e.g., EUS, CT, MRI) and to investigate the diagnostic potential of combining these tools in order to optimize disease staging and ultimately to personalize patient treatment.
Acknowledgements
We thank Dr. Marta Briarava (data manager, Dept. Surgery, Oncology and Gastroenterology, University of Padova, Italy) for setting up a dedicated database for the collection of information from the included studies, which greatly facilitated data management and analysis.
We also thank the Trials Search Co‐ordinator of the Cochrane Upper Gastrointestinal & Pancreatic Diseases Group, Racquel Simpson, for her expertise in designing the search strategies for each database
Appendices
Appendix 1. Glossary
Clinical staging: the instrumental assessment of the extent of the primary tumor growth (T‐stage), the status of the lymph nodes close to the primary tumor (N‐stage) and the presence or absence of metastasis to distant organs (M‐stage).
Computed tomography (CT) scan: a radiology diagnostic device that exploits the different ability of X‐rays to go through body tissues (normal and pathologic) characterized by different density. The resulting image depicts the human body anatomy in the form of virtual transversal "slices" that enable the user to easily identify the relationship between organs (normal and diseased).
Distant staging: the definition of M‐stage.
Endoscopy: medical procedure performed with a tube‐like device called endoscope, which enables the operator to see the lumen of an hollowed organ (such as the stomach) by means of an optical channel.
Hepatic: adjective of the noun "liver".
Locoregional staging: the definition of T‐stage and N‐stage.
Magnetic resonance imaging (MRI): a radiology diagnostic device that exploits the different content of hydrogen proper of different human tissues and detected by means of strong magnetic fields. The resulting image depicts the human body anatomy in the form of virtual transversal "slices" that enable the user to easily identify the relationship between organs (normal and diseased).
Mucosa: the inner layer of hollowed organs such as the stomach, small bowel and large bowel.
Muscolaris propria: the intermediate layer of hollowed organs such as the stomach, small bowel and large bowel; contains the muscle fibers responsible for gastrointestinal movements.
Negative predictive value (NPV): a diagnostic accuracy measure that indicates the proportion of actually negative cases (e.g., healthy, or non‐metastatic) among those classified as negative by a given diagnostic test. For instance, a 90% NPV means that among 100 cases classified as negative by a given test, 90 are actually negative.
Pathological staging: definition of T‐stage, N‐stage and M‐stage by pathological examination of primary tumor, lymph nodes and distant metastasis, respectively.
Peritoneal: adjective of the noun "peritoneum".
Positive predictive value (PPV): a diagnostic accuracy measure that indicates the proportion of actually positive cases (e.g., diseased, or metastatic) among those classified as positive by a given diagnostic test. For instance, a 90% PPV means that among 100 cases classified as positive by a given test, 90 are actually positive.
Positron emission tomography (PET): a nuclear medicine diagnostic device that exploits the ability of some tissues (such as cancer and inflammatory tissues) to avidly uptake glucose. When the glucose is labeled with a positron‐emitting tracer, PET can scan the human body to find areas that concentrate the tracer and thus can be considered suspicious.
Sensitivity: a diagnostic accuracy measure that indicates the proportion of positive (e.g., diseased, or metastatic) cases correctly classified by a given diagnostic test (it is also known as true positive rate). For instance, a 90% sensitivity means that the test correctly classifies 90 out of 100 cases known to be positive.
Serosa: the outer layer of hollowed organs such as the stomach, small bowel and large bowel. It is made of a membrane called peritoneum.
Specificity: a diagnostic accuracy measure that indicates the proportion of negative (e.g., healthy, or non‐metastatic) cases correctly classified by a given diagnostic test (it is also known as true negative rate). For instance, a 90% specificity means that the test correctly classifies 90 out of 100 cases known to be negative.
Appendix 2. CENTRAL search strategy
EBM Reviews ‐ Cochrane Central Register of Controlled Trials in OvidSP
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or adenocarcin$ or malig$).mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
(Digest$ or Gastr$ or gut or stomach$).mp.
1 and 2
Neoplasm Staging/
Neoplasm Invasiveness/
Lymphatic Metastasis/
(lymph adj2 node adj2 metastasis).tw.
disease progression/
t‐stag*.tw.
Stomach Neoplasms/
(gastric adj2 staging).tw.
or/4‐11
Endosonography/
(endoscop* adj3 (ultrasound or ultrasonograph* or ultrasonic)).mp.
endosonograph*.mp.
EUS.ti,ab.
Diagnostic Imaging/
or/13‐17
3 and 12 and 18
Appendix 3. MEDLINE search strategy
Ovid MEDLINE(R)
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or adenocarcin$ or malig$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
(Digest$ or Gastr$ or gut or stomach$).mp.
1 and 2
Neoplasm Staging/
*Lymphatic Metastasis/
(lymph adj2 node adj2 metastasis).tw.
disease progression/
t‐stag*.tw.
Stomach Neoplasms/pa [Pathology]
(gastric adj2 staging).tw.
or/4‐10
Endosonography/
(endoscop* adj2 (ultrasound or ultrasonograph* or ultrasonic)).tw.
EUS.ti,ab.
*Diagnostic Imaging/
Neoplasm Invasiveness/us [Ultrasonography]
Peritoneal Neoplasms/us [Ultrasonography]
Abdominal Neoplasms/us [Ultrasonography]
Stomach Neoplasms/us [Ultrasonography]
or/12‐19
3 and 11 and 20
Appendix 4. EMBASE search strategy
Embase in OvidSP
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or adenocarcin$ or malig$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
(Digest$ or Gastr$ or gut or stomach$).mp.
1 and 2
*cancer staging/
*lymph node metastasis/
(lymph adj2 node adj2 metastasis).tw.
*disease course/
t‐stag*.tw.
stomach tumor/co [Complication]
(gastric adj2 staging).tw.
or/4‐10
endoscopic echography/
(endoscop* adj3 (ultrasound or ultrasonograph* or ultrasonic)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
EUS.ti,ab.
*diagnostic imaging/
cancer infiltration/di, dm, th [Diagnosis, Disease Management, Therapy]
*cancer invasion/di, dm, th [Diagnosis, Disease Management, Therapy]
exp abdominal tumor/di [Diagnosis]
stomach tumor/di [Diagnosis]
or/12‐19
3 and 11 and 20
Appendix 5. QUADAS‐2
Questions (in bold) were used to score studies as at high or low or unclear risk of bias or with high or low or unclear applicability concerns.
Domain 1: Patient Selection
Risk of Bias: Could the selection of patients have introduced bias?
Signaling question 1: Was a consecutive or random sample of patients enrolled?
Signaling question 2: Was a case–control design avoided? (yes versus no)
Signaling question 3: Did the study avoid inappropriate exclusions? (For example, large primary tumors that do not allow to technically perform EUS, ulcerated primary tumors, or doubtful findings)
Applicability concern: Are there concerns that the included patients and setting do not match the review question?
Domain 2: Index Test
Risk of Bias: Could the conduct or interpretation of the index test have introduced bias?
Signaling question 1: Were the index test results interpreted without knowledge of the results of the reference standard? (yes versus no)
Signaling question 2: If a threshold was used, was it prespecified? (For example, definition of T and N categories.)
Applicability concern: Are there concerns that the index test, its conduct, or its interpretation differ from the review question?
Domain 3: Reference Standard
Risk of Bias: Could the reference standard, its conduct, or its interpretation have introduced bias?
Signaling question 1: Is the reference standard likely to correctly classify the target condition? (That is, is the pathology examination performed according to the worldwide accepted standards, so to guarantee that the target condition can be correctly classified? For instance, the description of the pathology methods were used to assess whether or not a risk of bias exists for this item.)
Signaling question 2: Were the reference standard results interpreted without knowledge of the results of the index test? (yes versus no)
Applicability concern: Are there concerns that the target condition as defined by the reference standard does not match the question?
Domain 4: Flow and Timing
Risk of Bias: Could the patient flow have introduced bias ?
Signaling question 1: Was there an appropriate interval between the index test and reference standard? (Since the disease can progress since the index test is performed, a time interval between EUS and the pathology evaluation longer than two months was considered a potential source of bias.)
Signaling question 2: Did all patients receive the same reference standard? (yes versus no)
Signaling question 3: Were all patients included in the analysis? (high risk: 5 or more patients)
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 T12 vs T34 | 50 | 4397 |
| 2 T1 vs T2 | 46 | 2742 |
| 3 T1a vs T1b | 20 | 3321 |
| 4 N0 vs N+ | 44 | 3573 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 2009.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 71. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T3 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 5 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 (67/71) vs T2, 2) N0 (65/71) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No uninterpretable findings reported | ||
| Comparative | |||
| Notes | Country: Korea Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Akahoshi 1991.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 74. Age: 17 ‐ 85 yrs. Gender: 49 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (61/74) vs T3 ‐ T4, 2) T1 (40/59) vs T2 Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Akahoshi 1998.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 73. Age: 36 ‐ 84 yrs. Gender: 55 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 ‐ T2, N0/N+ and T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 15; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 (66/73) vs T2, 2) N0 (40/46) vs N+, 3) T1a (53/61) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection for T1 cases) Reference and index test completely independent |
||
| Flow and timing | Information on all target conditions was not available for all cases No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Akashi 2006.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 267. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 ‐ T4 and T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 (237/267) vs T2, 2) T1a (164/237) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection for T1 cases) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported 37 undefined cases reported (as defined by EUS) All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Ang 2006.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 57. Age: 23 ‐ 85 yrs. Gender: 54 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Representative spectrum? YesT1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: unreported; criterion for N‐stage definition: lymph node size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (21/57) vs T3 ‐ T4, 2) T1 (14/19) vs T2, 3) N0 (26/57) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Singapore Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Arocena 2006.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 17. Age: 56 ‐ 81 yrs. Gender: 14 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): 12.5; criterion for T‐stage definition: unreported; criterion for N‐stage definition: lymph node morphology and size (> 5 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (9/17) vs T3 ‐ T4, 2) N0 (6/17) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Spain Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Barbour 2007.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 206. Age: 25 ‐ 85 yrs. Gender: 173 men Patients diagnosed with gastric cancer (cardia region) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled (cardia region of the stomach) |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (100/184) vs T3 ‐ T4, 2) T1 (55/74) vs T2, 3) N0 (112/206) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: USA Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Bentrem 2007.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 218. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (133/211) vs T3 ‐ T4, 2) T1 (54/85) vs T2, 3) N0 (108/218) vs N+ Reference standard: pathology evaluation of surgical specimen Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: USA Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Bhandari 2004.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 48. Age: 27 ‐ 81 yrs. Gender: 40 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: unreported; criterion for N‐stage definition: unreported | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (33/48) vs T3 ‐ T4, 2) T1 (28/29) vs T2, 3) N0 (28/48) vs N+ Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection for T1 cases) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Korea Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Blackshaw 2008.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 44. Age: 48 ‐ 79 yrs. Gender: 38 men Patients diagnosed with gastric cancer (cardia region) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled (cardia region of the stomach) |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 6 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (9/44) vs T3 ‐ T4, 2) N0 (10/44) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: UK Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Bohle 2011.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 62. Age: 63 yrs. Gender: 48 men Patients diagnosed with gastric cancer and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 10 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (40/62) vs T3 ‐ T4, 2) T1 (15/40) vs T2, 3) N0 (23/62) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Botet 1991.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 50. Age: 33 ‐ 81 yrs. Gender: 26 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Representative spectrum? YesT1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (12/50) vs T3 ‐ T4, 2) T1 (4/11) vs T2; 3) N0 (11/50) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: USA Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Caletti 1993.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 35. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (12/35) vs T3 ‐ T4, 2) T1 (5/10) vs T2; 3) N0 (7/32) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Italy Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Cerizzi 1991.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 21. Age (mean): 63. Gender: 12 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: linear; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (4/21) vs T3 ‐ T4, 2) N0 (5/21) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Italy Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Chen 2002.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 57. Age: 32 ‐ 82 yrs. Gender: 36 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (13/57) vs T3 ‐ T4, 2) T1 (7/10) vs T2, 3) N0 (15/57) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Taiwan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Choi 2010.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 930. Age (mean): 60 yrs. Gender: 658 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (487/930) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Korea Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
De Manzoni 1999.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 29. Age (mean): 65 yrs. Gender: unreported Patients diagnosed with gastric cancer (cardia region) and undergoing surgery Spectrum: T1 ‐ 4 and N0/N+ cases only enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (18/29) vs T3 ‐ T4, 2) N0 (5/29) vs N+ Reference standard: pathology evaluation of surgical specimen or endoscopic mucosal resection Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Italy Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Dittler 1993.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 254. Age: 28 ‐ 79. Gender: 165 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): unreported; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (79/254) vs T3 ‐ T4, 2) T1 (27/65) vs T2, 3) N0 (71/254) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
François 1996.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 29. Age: 38 ‐ 84 yrs. Gender: 24 men Patients diagnosed with gastric cancer (cardia region) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled (cardia region of the stomach) |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (12/29) vs T3 ‐ T4; 2) T1 (8/11) vs T2; 3) N0 (10/29) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: France Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Furukawa 2011.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 175. Age (mean): 66 yrs. Gender: 133 men Patients diagnosed with gastric cancer and undergoing surgery or endoscopic mucosal resection Patients with T1 ‐ T4 tumors enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (143/175) vs T3 ‐ T4; 2) T1 (126/143) vs T2 Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Ganpathi 2006.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 102. Age (mean): 63 yrs. Gender: 72 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (42/102) vs T3 ‐ T4, 2) T1 (18/37) vs T2, 3) N0 (35/99) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawals reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Singapore Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Garlipp 2011.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 165. Age (mean): 65 yrs. Gender: 123 men Patients diagnosed with gastric cancer (any site) and undergoing surgery T1 ‐ T4 cases enrolled |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): unreported; criterion for T‐stage definition: unreported | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (51/165) vs T3 ‐ T4, 2) T1 (23/51) vs T2 Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Quality: many missing data Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Grimm 1993.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 148. Age (mean): 61 yrs. Gender: 122 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (94/147) vs T3 ‐ T4, 2) T1 (37/80) vs T2, 3) N0 (58/148) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Habermann 2004.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 51. Age: 47 ‐ 76 yrs. Gender: 34 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T2 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 8 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (29/51) vs T3 ‐ T4, 2) N0 (19/50) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hamada 1997.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 149. Age: 17 ‐ 84 yrs. Gender: 102 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Sprectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) N0 (102/149) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Heye 2009.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 14. Age: 47 ‐ 87 yrs. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery T1 ‐ T4 cases enrolled (no data on lymph node status reported) |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): unreported; criterion for T‐stage definition: unreported | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (11/14) vs T3 ‐ T4, 2) T1 (1/7) vs T2 Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Quality: many data unreported Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Hizawa 2002.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 227. Age: 17 ‐ 84 yrs. Gender: 102 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (165/220) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported 7 uninterpretable cases reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Hwang 2010.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 277. Age (mean): 53 yrs. Gender: 171 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 5 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node size (> 8 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (252/277) vs T3 ‐ T4, 2) T1 (180/233) vs T2, 3) N0 (164/247) vs N+ Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection for T1 cases) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Korea Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Hünerbein 1998.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 22. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (12/22) vs T3 ‐ T4, 2) T1 (7/12) vs T2, 3) N0 (9/20) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hünerbein 2004.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 49. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 ‐ T4 cases enrolled (no data on lymph node status) |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (33/49) vs T3 ‐ T4, 2) T1 (18/33) vs T2, 3) T1a (4/14) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Javaid 2004.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 112. Age: 35 ‐ 75 yrs. Gender: 60 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (32/112) vs T3 ‐ T4, 2) T1 (8/29) vs T2, 3) N0 (32/112) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: India Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Kim 2007.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 206. Age (mean): 57 yrs. Gender: 79 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 ‐ T4 cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (199/206) vs T3 ‐ T4, 2) T1 (180/199) vs T2, 3) T1a (110/180) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Korea Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Kim 2010.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 169. Age: 32 ‐ 82 yrs. Gender: 122 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (125/169) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Korea Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kutup 2012.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 123. Age (mean): 61 yrs. Gender: 78 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 5 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (82/123) vs T3 ‐ T4, 2) T1 (26/82) vs T2, 3) N0 (42/123) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Lok 2008.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 75. Age (mean): 67 yrs. Gender (M:F): 3:1 Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (27/75) vs T3 ‐ T4, 2) T1 (8/14) vs T2, 3) N0 (26/75) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Hong Kong Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Mancino 2000.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 79. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (36/79) vs T3 ‐ T4, 2) T1 (27/35) vs T2, 3) N0 (33/77) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Italy Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Massari 1996.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 65. Age: 24 ‐ 79 yrs. Gender: 53 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (26/65) vs T3 ‐ T4, 2) T1 (12/26) vs T2, 3) N0 (12/65) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Italy Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Mouri 2009.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 222. Age (mean): 66 yrs. Gender: 174 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (148/191) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported 31 uninterpretable cases reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Murata 1988.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 146. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 10; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (105/146) vs T3 ‐ T4; 2) T1 (88/100) vs T2; 3) T1a (55/85) vs T1b Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Nakamura 1999a.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 31. Age (mean): 61 yrs. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T2 cases enrolled |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): unreported; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) N0 (18/31) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Nomura 1999.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 30. Age (mean): 58 yrs. Gender: 24 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 ‐ T4 cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (20/30) vs T3 ‐ T4; 2) T1 (16/20) vs T2; 3) T1a (5/16) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Ohashi 1999.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 30. Age (mean): 58 yrs. Gender: 24 men Patients diagnosed with gastric cancer (any site) and undergoing endoscopic mucosal resection Spectrum: T1 cases only enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a vs T1b Reference standard: pathology evaluation of endoscopic mucosal resection Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Okada 2011.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 526. Age (mean): 67 yrs. Gender: 385 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (369/526) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Okamura 1999.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 41. Age: unreported. Gender: 34 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (29/41) vs T1b Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Park 2008.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 40. Age: 36 ‐ 70 yrs. Gender: 30 men Patients diagnosed with gastric cancer (any site) and undergoing preoperative neoadjuvant chemotherapy followed by surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (17/40) vs T3 ‐ T4, 2) N0 (7/38) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No no withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Korea All patients underwent preoperative neoadjuvant chemotherapy Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Pedrazzani 2005.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 51. Age: 27 ‐ 84 yrs. Gender (M:F): 6:1 Patients diagnosed with gastric cancer (cardia only) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled (cardia region of the stomach) |
||
| Index tests | Index test: EUS; array: linear; frequency (MHz): 7.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (30/51) vs T3 ‐ T4, 2) T1 (8/16) vs T2, 3) N0 (14/51) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Italy Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Perng 1996.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 76. Age: 28 ‐ 72 yrs. Gender: 40 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (36/76) vs T3 ‐ T4, 2) T1 (21/33) vs T2, 3) N0 (32/69) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Taiwan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Polkowski 2004.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 88. Age (mean): 63 yrs. Gender: 56 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node size (> 8 mm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (20/88) vs T3 ‐ T4, 2) T1 (9/14) vs T2, 3) N0 (14/60) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Poland Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Potrc 2006.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 82. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: unreported; criterion for N‐stage definition: unreported | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (48/82) vs T3 ‐ T4, 2) T1 (11/42) vs T2, 3) N0 (24/82) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Slovenia Quality: Many data unreported Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Repiso 2010.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 36. Age: 36 ‐ 81. Gender: 32 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology and size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (16/36) vs T3 ‐ T4, 2) T1 (10/15) vs T2, 3) N0 (13/36) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Spain Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Saito 1991.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 110. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (60/110) vs T3 ‐ T4, 2) T1 (45/56) vs T2, 3) T1a (22/41) vs T1b Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Shimizu 1994.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 128. Age: unreported. Gender: unreported Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 cases enrolled |
||
| Index tests | Index test: EUS; array: unreported; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (90/128) vs T3 ‐ T4, 2) T1 (77/84) vs T2, 3) T1a (45/71) vs T1b Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Shimoyama 2004.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 45. Age: 37 ‐ 89. Gender: 37 men Patients diagnosed with gastric cancer (cardia only) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled (cardia region of the stomach) |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage positivity: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage positivity: lymph node morphology and size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (37/45) vs T3 ‐ T4, 2) T1 (21/27) vs T2, 3) N0 (25/45) vs N+, 4) T1a (4/17) vs T1b Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Tan 2007.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 63. Age: 29 ‐ 75 yrs. Gender: 37 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (25/63) vs T3 ‐ T4, 2) T1 (7/18) vs T2, 3) N0 (25/63) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: China Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Tio 1989.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 80. Age: 13 ‐ 87 yrs. Gender: 51 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (31/76) vs T3 ‐ T4, 2) T1 (13/30) vs T2, 3) N0 (30/80) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Netherlands Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Tsendsuren 2006.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 41. Age: 28 ‐ 80 yrs. Gender: 29 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: linear; frequency (MHz): 5 ‐ 7.5; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node shape | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (32/41) vs T3 ‐ T4, 2) T1 (12/24) vs T2, 3) N0 (17/41) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: China Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Tseng 2000.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 74. Age: 26 ‐ 83 yrs. Gender: 40 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (35/74) vs T3 ‐ T4, 2) T1 (12/31) vs T2, 3) N0 (35/74) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Taiwan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Wang 1998.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 119. Age: 26 ‐ 82 yrs. Gender: 75 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node size (> 1 cm) | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (58/119) vs T3 ‐ T4, 2) T1 (27/50) vs T2, 3) N0 (45/119) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Taiwan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Willis 2000.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 116. Age: 33 ‐ 86 yrs. Gender: 72 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (56/116) vs T3 ‐ T4, 2) T1 (10/42) vs T2, 3) N0 (62/116) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Xi 2003.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 32. Age: 28 ‐ 78 yrs. Gender: 25 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: unreported; criterion for N‐stage definition: unreported | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (9/32) vs T3 ‐ T4, 2) N0 (19/32) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: China Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Yamamoto 2012.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 75. Age: 41 ‐ 86 yrs. Gender: 62 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1 cases only enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 12 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (59/75) vs T1b Reference standard: pathology evaluation of surgical specimen or endoscopic mucosal resection Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Yanai 1997.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 100. Age: 31 ‐ 87 yrs. Gender: 76 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (71/96) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported 4 uninterpretable cases reported (as defined by EUS) All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Yanai 1999.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 49. Age: 32 ‐ 81 yrs. Gender: 40 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (22/49) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Yoshida 2005.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 293. Age: 38 ‐ 91 yrs. Gender: 222 men Patients diagnosed with gastric cancer (any site) and undergoing surgery or endoscopic mucosal resection Spectrum: T1a ‐ T1b cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 20; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1a (263/293) vs T1b Reference standard: pathology evaluation of surgical specimen (or endoscopic mucosal resection) Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Japan Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Zheng 2011.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 165. Age (mean): 58 yrs. Gender: 127 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage definition: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage definition: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (91/162) vs T3 ‐ T4, 2) T1 (42/91) vs T2, 3) N0 (65/162) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: China Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Ziegler 1993.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 108. Age: 29 ‐ 82 yrs. Gender: 58 men Patients diagnosed with gastric cancer (any site) and undergoing surgery Spectrum: T1 ‐ T4 and N0/N+ cases enrolled |
||
| Index tests | Index test: EUS; array: radial; frequency (MHz): 7.5 ‐ 12; criterion for T‐stage positivity: EUS‐based 5‐layer structure of gastric wall; criterion for N‐stage positivity: lymph node morphology | ||
| Target condition and reference standard(s) | Target conditions: gastric carcinoma 1) T1 ‐ T2 (54/108) vs T3 ‐ T4, 2) T1 (22/50) vs T2, 3) N0 (50/108) vs N+ Reference standard: pathology evaluation of surgical specimen Reference and index test completely independent |
||
| Flow and timing | No withdrawal reported No uninterpretable findings reported All cases verified by reference standard test | ||
| Comparative | |||
| Notes | Country: Germany Relevant clinical information: same clinical data available for test results interpretation as those available when test used in practice |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
EUS: endoscopic ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 1993 | Review article (no original data) |
| Aibe 1986 | Article in Japanese |
| Aibe 1992 | No raw data reported (no data necessary for building 2x2 classification tables) |
| Ajani 2004 | EUS performed before neoadjuvant chemotherapy (EUS used to assess response to therapy, not to stage gastric cancer) |
| Akahoshi 1992 | Overlapping with Akahoshi 1991 |
| Akahoshi 1997 | Overlapping with Akahoshi 1998 |
| Asaki 1989 | The article reports on EUS combined with submucosography (no data on EUS alone) |
| Bösing 2003 | Article in German |
| Chen 2004 | No stomach specific data (miscellany of esophageal and gastric cancer) |
| Chen 2010 | Article in Chinese on contrast enhanced EUS (no data on EUS alone) |
| Davies 2006 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Dewitt 2005 | No separate data (mixed esophageal and gastric cancer data) |
| Fiore 2006 | No stomach specific data (miscellany of gastric and esophageal data) |
| Futawatari 2008 | Article on tumor volume (not on tumor infiltration of the gastric wall) |
| Ghiţă 2011 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Giovannini 1993 | No separate data for gastric carcinoma (miscellany of carcinoma, lymphoma and carcinoid cases) |
| Gorshkov 2001 | Article in Russian |
| Greenberg 1994 | Fewer than 10 patients with gastric cancer are analyzed |
| Grimm 1992 | Data overlapping with Grimm 1993 |
| Grotenhuis 2013 | No gastric cancer data (only distal esophageal cancer) |
| Heeren 2004 | No raw data reported (no data necessary for building 2x2 classification tables) |
| Heintz 1991a | Article in German |
| Heintz 1991b | Article in German |
| Heyer 1998 | Article in German |
| Hirata 1989 | Article in Japanese |
| Holden 1996 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Hünerbein 1995 | No data on EUS (only data on laparoscopic ultrasonography) |
| Hünerbein 1996 | Data overlapping with Hunerbein 1998 |
| Kang 2010 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Kida 1998 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Kienle 2002 | No separate data (mixed esophageal and gastric cancer data) |
| Kroep 2003 | Data on tumor response to treatment (no data on tumor staging) |
| Lavonius 2002 | Data on laparoscopic ultrasound only (no EUS data) |
| Li 2012 | No data on EUS reported: only data on DCEUS (double contrast enhanced ultrasound) were reported |
| Matthes 2006 | No separate data for gastric carcinoma (miscellany of carcinoma, lymphoma and carcinoid cases) |
| Meining 2002 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Mortensen 2007 | No separate data for gastric carcinoma (miscellany of carcinoma, lymphoma and carcinoid cases) |
| Nagler 2011 | Review article (no original data) |
| Nakamura 1999b | Overlapping with Nakamura 1999 |
| Nakamura 2000 | Overlapping with Nakamura 1999 |
| Ohashi 1989 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Okai 1991 | Data on type of tumor growth (not on tumor staging) |
| Park 2011 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Patel 2007 | EUS performed before neoadjuvant chemotherapy (EUS used to assess response to therapy, not to stage gastric cancer) |
| Pedrazzani 2007 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Power 2009 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Rau 1995 | Article in German |
| Richards 2000 | No separate data (miscellany of esophageal and gastric cancer data) |
| Rösch 1992 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Songür 1996 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Tsuzuki 2011 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
| Venkataraman 2010 | Hydrogastric sonography data only (no EUS data) |
| Wakelin 2002 | No separate data (miscellany of esophageal and gastric cancer data) |
| Yoshinaga 2012 | No raw data reported (no data necessary for building 2 x 2 classification tables) |
EUS: endoscopic ultrasonography
Characteristics of studies awaiting classification [ordered by study ID]
Feng 2013.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 610 | ||
| Index tests | EUS, MSCT | ||
| Target condition and reference standard(s) | Target condition: gastric cancer Reference standard: pathology evaluation of surgical specimen | ||
| Flow and timing | EUS staging followed by surgery and then pathology evaluation | ||
| Comparative | Results demonstrated that the overall accuracies of EUS and MSCT for preoperative staging were not significantly different | ||
| Notes | Country: China | ||
Lei 2013.
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 38 | ||
| Index tests | EUS, MRI, MRI + EUS |
||
| Target condition and reference standard(s) | Target condition: gastric cancer Reference standard: pathology evaluation of surgical specimen | ||
| Flow and timing | EUS staging followed by surgery and then pathology evaluation | ||
| Comparative | The accuracy was similar and improved significantly when the 2 procedures were combined | ||
| Notes | Country: China | ||
Mehmedović 2014.
| Study characteristics | |||
| Patient sampling | Prospective study (unclear) | ||
| Patient characteristics and setting | Sample size: 277. Gender: 171 men, 106 women | ||
| Index tests | EUS MDCT | ||
| Target condition and reference standard(s) | Target condition: gastric cancer Reference standard: pathology evaluation of surgical specimen | ||
| Flow and timing | EUS staging followed by surgery and then pathology evaluation | ||
| Comparative | Unclear | ||
| Notes | Country: Bosnia and Herzegovina | ||
Spolverato 2015.
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 223 | ||
| Index tests | EUS | ||
| Target condition and reference standard(s) | Target condition: gastric cancer Reference standard: pathology evaluation of surgical specimen | ||
| Flow and timing | EUS staging followed by surgery and then pathology evaluation | ||
| Comparative | N/A | ||
| Notes | Country: USA | ||
EUS: endoscopic ultrasonography MDCT: multi‐detector computed tomography MRI: magnetic resonance imaging MSCT: multi slice computed tomography
Contributions of authors
Simone Mocellin: study design and coordination, literature search, data collection and management, statistical analysis, manuscript writing.
Sandro Pasquali: literature search, data collection and management, manuscript writing.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
The review authors declare no conflict of interest.
New
References
References to studies included in this review
Ahn 2009 {published data only}
- Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, Kim SH, et al. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. Journal of Surgical Oncology 2009;99(1):20‐7. [PUBMED: 18937292] [DOI] [PubMed] [Google Scholar]
Akahoshi 1991 {published data only}
- Akahoshi K, Misawa T, Fujishima H, Chijiiwa Y, Maruoka A, Ohkubo A, et al. Preoperative evaluation of gastric cancer by endoscopic ultrasound. Gut 1991;32(5):479‐82. [PUBMED: 2040468] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akahoshi 1998 {published data only}
- Akahoshi K, Chijiwa Y, Hamada S, Sasaki I, Nawata H, Kabemura T, et al. Pretreatment staging of endoscopically early gastric cancer with a 15 MHz ultrasound catheter probe. Gastrointestinal Endoscopy 1998;48(5):470‐6. [PUBMED: 9831834] [DOI] [PubMed] [Google Scholar]
Akashi 2006 {published data only}
- Akashi K, Yanai H, Nishikawa J, Satake M, Fukagawa Y, Okamoto T, et al. Ulcerous change decreases the accuracy of endoscopic ultrasonography diagnosis for the invasive depth of early gastric cancer. International Journal of Gastrointestinal Cancer 2006;37(4):133‐8. [PUBMED: 18080789] [DOI] [PubMed] [Google Scholar]
Ang 2006 {published data only}
- Ang TL, Ng TM, Fock KM, Teo EK. Accuracy of endoscopic ultrasound staging of gastric cancer in routine clinical practice in Singapore. Chinese Journal of Digestive Diseases 2006;7(4):191‐6. [PUBMED: 17054580] [DOI] [PubMed] [Google Scholar]
Arocena 2006 {published data only}
- Arocena MG, Barturen A, Bujanda L, Casado O, Ramírez MM, Oleagoitia JM, et al. MRI and endoscopic ultrasonography in the staging of gastric cancer. Revista Espanola de Enfermedades Digestivas 2006;98(8):582‐90. [PUBMED: 17048994] [DOI] [PubMed] [Google Scholar]
Barbour 2007 {published data only}
- Barbour AP, Rizk NP, Gerdes H, Bains MS, Rusch VW, Brennan MF, et al. Endoscopic ultrasound predicts outcomes for patients with adenocarcinoma of the gastroesophageal junction. Journal of the American College of Surgeons 2007;205(4):593‐601. [PUBMED: 17903735] [DOI] [PubMed] [Google Scholar]
Bentrem 2007 {published data only}
- Bentrem D, Gerdes H, Tang L, Brennan M, Coit D. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Annals of Surgical Oncology 2007;14(6):1853‐9. [PUBMED: 17357856] [DOI] [PubMed] [Google Scholar]
Bhandari 2004 {published data only}
- Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, et al. Usefulness of three‐dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy,EUS, and histopathology. Gastrointestinal Endoscopy 2004;59(6):619‐26. [PUBMED: 15114303] [DOI] [PubMed] [Google Scholar]
Blackshaw 2008 {published data only}
- Blackshaw G, Lewis WG, Hopper AN, Morgan MA, Al‐Khyatt W, Edwards P, et al. Prospective comparison of endosonography, computed tomography, and histopathological stage of junctional oesophagogastric cancer. Clinical Radiology 2008;63(10):1092‐8. [PUBMED: 18774355] [DOI] [PubMed] [Google Scholar]
Bohle 2011 {published data only}
- Bohle W, Scheidig A, Zoller WG. Endosonographic tumor staging for treatment decision in resectable gastric cancer. Journal of Gastrointestinal and Liver Diseases 2011;20(2):135‐9. [PUBMED: 21725509] [PubMed] [Google Scholar]
Botet 1991 {published data only}
- Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, et al. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology 1991;181(2):426‐32. [PUBMED: 1924784] [DOI] [PubMed] [Google Scholar]
Caletti 1993 {published data only}
- Caletti G, Ferrari A, Brocchi E, Barbara L. Accuracy of endoscopic ultrasonography in the diagnosis and staging of gastric cancer and lymphoma. Surgery 1993;113(1):14‐27. [PUBMED: 8417483] [PubMed] [Google Scholar]
Cerizzi 1991 {published data only}
- Cerizzi A, Crosta C, Botti F, Carrara A, Alloni R, Taschieri AM. Preoperative staging of gastric carcinoma using endosonography (EUS) [Stadiazione pre‐operatoria del carcinoma gastrico mediante endosonografia (EUS)]. Annali Italiani di Chirurgia 1992;63(4):465‐9. [PUBMED: 1463259] [PubMed] [Google Scholar]
Chen 2002 {published data only}
- Chen CH, Yang CC, Yeh YH. Preoperative staging of gastric cancer by endoscopic ultrasound: the prognostic usefulness of ascites detected by endoscopic ultrasound. Journal of Clinical Gastroenterology 2002;35(4):321‐7. [PUBMED: 12352295] [DOI] [PubMed] [Google Scholar]
Choi 2010 {published data only}
- Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy 2010;42(9):705‐13. [PUBMED: 20652857] [DOI] [PubMed] [Google Scholar]
De Manzoni 1999 {published data only}
- Manzoni G, Pedrazzani C, Leo A, Bonfiglio M, Tedesco P, Tasselli S, et al. Experience of endoscopic ultrasound in staging adenocarcinoma of the cardia. European Journal of Surgical Oncology 1999;25(6):595‐8. [PUBMED: 10556006] [DOI] [PubMed] [Google Scholar]
Dittler 1993 {published data only}
- Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in gastric carcinoma. Endoscopy 1993;25(2):162‐6. [PUBMED: 8491133] [DOI] [PubMed] [Google Scholar]
François 1996 {published data only}
- François E, Peroux J, Mouroux J, Chazalle M, Hastier P, Ferrero J, et al. Preoperative endosonographic staging of cancer of the cardia. Abdominal Imaging 1996;21(6):483‐7. [PUBMED: 8875868] [DOI] [PubMed] [Google Scholar]
Furukawa 2011 {published data only}
- Furukawa K, Miyahara R, Itoh A, Ohmiya N, Hirooka Y, Mori K, et al. Diagnosis of the invasion depth of gastric cancer using MDCT with virtual gastroscopy: comparison with staging with endoscopic ultrasound. American Journal of Roentgenology 2011;197(4):867‐75. [PUBMED: 21940574] [DOI] [PubMed] [Google Scholar]
Ganpathi 2006 {published data only}
- Ganpathi IS, So JB, Ho KY. Endoscopic ultrasonography for gastric cancer: does it influence treatment?. Surgical Endoscopy 2006;20(4):559‐62. [PUBMED: 16446988] [DOI] [PubMed] [Google Scholar]
Garlipp 2011 {published data only}
- Garlipp B, Schwalenberg J, Adolf D, Lippert H, Meyer F. Epidemiology, surgical management and early postoperative outcome in a cohort of gastric cancer patients of a tertiary referral center in relation to multi‐center quality assurance studies. Polski Przeglad Chirurgiczny 2011;83(3):123‐34. [PUBMED: 22166314] [DOI] [PubMed] [Google Scholar]
Grimm 1993 {published data only}
- Grimm H, Binmoeller KF, Hamper K, Koch J, Henne‐Bruns D, Soehendra N. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy 1993;25(3):224‐30. [PUBMED: 8519241] [DOI] [PubMed] [Google Scholar]
Habermann 2004 {published data only}
- Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology 2004;230(2):465‐71. [PUBMED: 14752188] [DOI] [PubMed] [Google Scholar]
Hamada 1997 {published data only}
- Hamada S, Akahoshi K, Chijiiwa Y, Nawata H, Sasaki I. Relationship between histological type and endosonographic detection of regional lymph node metastases in gastric cancer. British Journal of Radiology 1997;70(835):697‐702. [PUBMED: 9245881] [DOI] [PubMed] [Google Scholar]
Heye 2009 {published data only}
- Heye T, Kuntz C, Düx M, Encke J, Palmowski M, Autschbach F, et al. CT and endoscopic ultrasound in comparison to endoluminal MRI: preliminary results in staging gastric carcinoma. European Journal of Radiology 2009;70(2):336‐41. [PUBMED: 18337043] [DOI] [PubMed] [Google Scholar]
Hizawa 2002 {published data only}
- Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer?. Endoscopy 2002;34(12):973‐8. [PUBMED: 12471541] [DOI] [PubMed] [Google Scholar]
Hünerbein 1998 {published data only}
- Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointestinal Endoscopy 1998;48(4):371‐5. [PUBMED: 9786108] [DOI] [PubMed] [Google Scholar]
Hünerbein 2004 {published data only}
- Hünerbein M, Handke T, Ulmer C, Schlag PM. Impact of miniprobe ultrasonography on planning of minimally invasive surgery for gastric and colonic tumors. Surgical Endoscopy 2004;18(4):601‐5. [PUBMED: 14752658] [DOI] [PubMed] [Google Scholar]
Hwang 2010 {published data only}
- Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector‐row computed tomography. Journal of Gastroenterology and Hepatology 2010;25(3):512‐8. [PUBMED: 20370729] [DOI] [PubMed] [Google Scholar]
Javaid 2004 {published data only}
- Javaid G, Shah OJ, Dar MA, Shah P, Wani NA, Zargar SA. Role of endoscopic ultrasonography in preoperative staging of gastric carcinoma. Australian and New Zealand Journal of Surgery 2004;74(3):108‐11. [PUBMED: 14996154] [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim JH, Song KS, Youn YH, Lee YC, Cheon JH, Song SY, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointestinal Endoscopy 2007;66(5):901‐8. [PUBMED: 17963876] [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim GH, Park do Y, Kida M, Kim DH, Jeon TY, Kang HJ, et al. Accuracy of high‐frequency catheter‐based endoscopic ultrasonography according to the indications for endoscopic treatment of early gastric cancer. Journal of Gastroenterology and Hepatology 2010;25(3):506‐11. [PUBMED: 20074167] [DOI] [PubMed] [Google Scholar]
Kutup 2012 {published data only}
- Kutup A, Vashist YK, Groth S, Vettorazzi E, Yekebas EF, Soehendra N, et al. Endoscopic ultrasound staging in gastric cancer: Does it help management decisions in the era of neoadjuvant treatment?. Endoscopy 2012;44(6):572‐6. [PUBMED: 22528672] [DOI] [PubMed] [Google Scholar]
Lok 2008 {published data only}
- Lok KH, Lee CK, Yiu HL, Lai L, Szeto ML, Leung SK. Current utilization and performance status of endoscopic ultrasound in a community hospital. Journal of Digestive Diseases 2008;9(1):41‐7. [PUBMED: 18251793] [DOI] [PubMed] [Google Scholar]
Mancino 2000 {published data only}
- Mancino G, Bozzetti F, Schicchi A, Schiavo M, Spinelli P, Andreola S. Preoperative endoscopic ultrasonography in patients with gastric cancer. Tumori 2000;86(2):139‐41. [PUBMED: 10855851] [DOI] [PubMed] [Google Scholar]
Massari 1996 {published data only}
- Massari M, Cioffi U, De Simone M, Bonavina L, D'elia A, Rosso L, et al. Endoscopic ultrasonography for preoperative staging of gastric carcinoma. Hepatogastroenterology 1996;43(9):542‐6. [PUBMED: 8799392] [PubMed] [Google Scholar]
Mouri 2009 {published data only}
- Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. Journal of Clinical Gastroenterology 2009;43(4):318‐22. [PUBMED: 19077733] [DOI] [PubMed] [Google Scholar]
Murata 1988 {published data only}
- Murata Y, Suzuki S, Hashimoto H. Endoscopic ultrasonography of the upper gastrointestinal tract. Surgical Endoscopy 1988;2(3):180‐3. [PUBMED: 3070801] [DOI] [PubMed] [Google Scholar]
Nakamura 1999a {published data only}
- Nakamura K, Kamei T, Ohtomo N, Kinukawa N, Tanaka M. Gastric carcinoma confined to the muscularis propria: how can we detect, evaluate, and cure intermediate‐stage carcinoma of the stomach?. American Journal of Gastroenterology 1999;94(8):2251‐5. [PUBMED: 10445558] [DOI] [PubMed] [Google Scholar]
Nomura 1999 {published data only}
- Nomura N, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Usefulness of contrast‐enhanced EUS in the diagnosis of upper GI tract diseases. Gastrointestinal Endoscopy 1999;50(4):555‐60. [PUBMED: 10502181] [DOI] [PubMed] [Google Scholar]
Ohashi 1999 {published data only}
- Ohashi S, Segawa K, Okamura S, Mitake M, Urano H, Shimodaira M, et al. The utility of endoscopic ultrasonography and endoscopy in the endoscopic mucosal resection of early gastric cancer. Gut 1999;45(4):599‐604. [PUBMED: 10486372] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okada 2011 {published data only}
- Okada K, Fujisaki J, Kasuga A, Omae M, Yoshimoto K, Hirasawa T, et al. Endoscopic ultrasonography is valuable for identifying early gastric cancers meeting expanded‐indication criteria for endoscopic submucosal dissection. Surgical Endoscopy 2011;25(3):841‐8. [PUBMED: 20734082] [DOI] [PubMed] [Google Scholar]
Okamura 1999 {published data only}
- Okamura S, Tsutsui A, Muguruma N, Ichikawa S, Sogabe M, Okita Y, et al. The utility and limitations of an ultrasonic miniprobe in the staging of gastric cancer. Journal of Medical Investigation 1999;46(1‐2):49‐53. [PUBMED: 10408157] [PubMed] [Google Scholar]
Park 2008 {published data only}
- Park SR, Lee JS, Kim CG, Kim HK, Kook MC, Kim YW, et al. Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Cancer 2008;112(11):2368‐76. [PUBMED: 18404697] [DOI] [PubMed] [Google Scholar]
Pedrazzani 2005 {published data only}
- Pedrazzani C, Bernini M, Giacopuzzi S, Pugliese R, Catalano F, Festini M, et al. Evaluation of Siewert classification in gastro‐esophageal junction adenocarcinoma: what is the role of endoscopic ultrasonography?. Journal of Surgical Oncology 2005;91(4):226‐31. [PUBMED: 16121346] [DOI] [PubMed] [Google Scholar]
Perng 1996 {published data only}
- Perng DS, Jan CM, Wang WM, Chen LT, Su YC, Liu GC, et al. Computed tomography, endoscopic ultrasonography and intraoperative assessment in TN staging of gastric carcinoma. Journal of the Formosan Medical Association 1996;95(5):378‐85. [PUBMED: 8688702] [PubMed] [Google Scholar]
Polkowski 2004 {published data only}
- Polkowski M, Palucki J, Wronska E, Szawlowski A, Nasierowska‐Guttmejer A, Butruk E. Endosonography versus helical computed tomography for locoregional staging of gastric cancer. Endoscopy 2004;36(7):617‐23. [PUBMED: 15243885] [DOI] [PubMed] [Google Scholar]
Potrc 2006 {published data only}
- Potrc S, Skalicky M, Ivanecz A. Does endoscopic ultrasound staging already allow individual treatment regimens in gastric cancer. Wien Klin Wochenschr 2006;118(Suppl 2):48‐51. [PUBMED: 16817044] [DOI] [PubMed] [Google Scholar]
Repiso 2010 {published data only}
- Repiso A, Gómez‐Rodríguez R, López‐Pardo R, Lombera MM, Romero M, Aranzana A, et al. Usefulness of endoscopic ultrasonography in preoperative gastric cancer staging: diagnostic yield and therapeutic impact. Revista Espanola de Enfermedades Digestivas 2010;102(7):413‐20. [PUBMED: 20617861] [DOI] [PubMed] [Google Scholar]
Saito 1991 {published data only}
- Saito N, Takeshita K, Habu H, Endo M. The use of endoscopic ultrasound in determining the depth of cancer invasion in patients with gastric cancer. Surgical Endoscopy 1991;5(1):14‐9. [PUBMED: 1871669] [DOI] [PubMed] [Google Scholar]
Shimizu 1994 {published data only}
- Shimizu S, Tada M, Kawai K. Endoscopic ultrasonography for early gastric cancer. Endoscopy 1994;26(9):767‐8. [PUBMED: 7712983] [DOI] [PubMed] [Google Scholar]
Shimoyama 2004 {published data only}
- Shimoyama S, Yasuda H, Hashimoto M, Tatsutomi Y, Aoki F, Mafune K, et al. Accuracy of linear‐array EUS for preoperative staging of gastric cardia cancer. Gastrointestinal Endoscopy 2004;60(1):50‐5. [PUBMED: 15229425] [DOI] [PubMed] [Google Scholar]
Tan 2007 {published data only}
- Tan SY, Wang JY, Shen L, Luo HS, Shen ZX. Relationship between preoperative staging by endoscopic ultrasonography and MMP‐9 expression in gastric carcinoma. World Journal of Gastroenterology 2007;13(14):2108‐12. [PUBMED: 17465457] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tio 1989 {published data only}
- Tio TL, Coene PP, Schouwink MH, Tytgat GN. Esophagogastric carcinoma: preoperative TNM classification with endosonography. Radiology 1989;173(2):411‐7. [PUBMED: 2678255] [DOI] [PubMed] [Google Scholar]
Tsendsuren 2006 {published data only}
- Tsendsuren T, Jun SM, Mian XH. Usefulness of endoscopic ultrasonography in preoperative TNM staging of gastric cancer. World Journal of Gastroenterology 2006;12(1):43‐7. [PUBMED: 16440415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tseng 2000 {published data only}
- Tseng LJ, Mo LR, Tio TL, Fresner YT, Jao N, Lin RC, et al. Video‐endoscopic ultrasonography in staging gastric carcinoma. Hepatogastroenterology 2000;47(33):897‐900. [PUBMED: 10919057] [PubMed] [Google Scholar]
Wang 1998 {published data only}
- Wang JY, Hsieh JS, Huang YS, Huang CJ, Hou MF, Huang TJ. Endoscopic ultrasonography for preoperative locoregional staging and assessment of resectability in gastric cancer. Clinical Imaging 1998;22(5):355‐9. [PUBMED: 9755399] [DOI] [PubMed] [Google Scholar]
Willis 2000 {published data only}
- Willis S, Truong S, Gribnitz S, Fass J, Schumpelick V. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surgical Endoscopy 2000;14(10):951‐4. [PUBMED: 11080410] [DOI] [PubMed] [Google Scholar]
Xi 2003 {published data only}
- Xi WD, Zhao C, Ren GS. Endoscopic ultrasonography in preoperative staging of gastric cancer: determination of tumor invasion depth, nodal involvement and surgical resectability. World Journal of Gastroenterology 2003;9(2):254‐7. [PUBMED: 12532442] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2012 {published data only}
- Yamamoto S, Nishida T, Kato M, Inoue T, Hayashi Y, Kondo J, et al. Evaluation of endoscopic ultrasound image quality is necessary in endosonographic assessment of early gastric cancer invasion depth. Gastroenterology Research and Practice 2012;2012(Sept 16):1‐5. [DOI: 10.1155/2012/194530; PUBMED: 23024651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yanai 1997 {published data only}
- Yanai H, Matsumoto Y, Harada T, Nishiaki M, Tokiyama H, Shigemitsu T, et al. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointestinal Endoscopy 1997;46(3):212‐6. [PUBMED: 9378206] [DOI] [PubMed] [Google Scholar]
Yanai 1999 {published data only}
- Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, et al. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut 1999;44(3):361‐5. [PUBMED: 10026321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshida 2005 {published data only}
- Yoshida S, Tanaka S, Kunihiro K, Mitsuoka Y, Hara M, Kitadai Y, et al. Diagnostic ability of high‐frequency ultrasound probe sonography in staging early gastric cancer, especially for submucosal invasion. Abdominal Imaging 2005;30(5):518‐23. [PUBMED: 15688103] [DOI] [PubMed] [Google Scholar]
Zheng 2011 {published data only}
- Zheng Z, Yu Y, Lu M, Sun W, Wang F, Li P, et al. Double contrast‐enhanced ultrasonography for the preoperative evaluation of gastric cancer: a comparison to endoscopic ultrasonography with respect to histopathology. American Journal of Surgery 2011;202(5):605‐11. [PUBMED: 21824594] [DOI] [PubMed] [Google Scholar]
Ziegler 1993 {published data only}
- Ziegler K, Sanft C, Zimmer T, Zeitz M, Felsenberg D, Stein H, et al. Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut 1993;34(5):604‐10. [PUBMED: 8504959] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abe 1993 {published data only}
- Abe S, Lightdale CJ, Brennan MF. The Japanese experience with endoscopic ultrasonography in the staging of gastric cancer. Gastrointestinal Endoscopy 1993;39(4):586‐91. [PUBMED: 8365619] [DOI] [PubMed] [Google Scholar]
Aibe 1986 {published data only}
- Aibe T, Takemoto T. [Diagnosis of the infiltrating depth of gastric cancer by endoscopic ultrasonography]. Gan No Rinsho 1986;32(10):1173‐5. [PUBMED: 3537361] [PubMed] [Google Scholar]
Aibe 1992 {published data only}
- Aibe T, Fujimura H, Yanai H, Okita K, Takemoto T. Endosonographic diagnosis of metastatic lymph nodes in gastric carcinoma. Endoscopy 1992;24(Suppl 1):315‐9. [PUBMED: 1633772] [DOI] [PubMed] [Google Scholar]
Ajani 2004 {published data only}
- Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, et al. Multi‐institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. Journal of Clinical Oncology 2004;22(14):2774‐80. [PUBMED: 15254045] [DOI] [PubMed] [Google Scholar]
Akahoshi 1992 {published data only}
- Akahoshi K, Misawa T, Fujishima H, Chijiiwa Y, Nawata H. Regional lymph node metastasis in gastric cancer: evaluation with endoscopic US. Radiology 1992;182(2):559‐64. [PUBMED: 1732981] [DOI] [PubMed] [Google Scholar]
Akahoshi 1997 {published data only}
- Akahoshi K, Chijiiwa Y, Sasaki I, Hamada S, Iwakiri Y, Nawata H, et al. Pre‐operative TN staging of gastric cancer using a 15 MHz ultrasound miniprobe. British Journal of Radiology 1997;70(835):703‐7. [PUBMED: 9245882] [DOI] [PubMed] [Google Scholar]
Asaki 1989 {published data only}
- Asaki S, Nakayama Y, Ohara M, Hirasawa Y, Kanazawa N, Ota K. Comparison of the efficacy of endoscopic ultrasonography and submucosography in diagnosing the depth of gastric cancer invasion. Tohoku Journal of Experimental Medicine 1989;159(3):227‐35. [PUBMED: 2696138] [DOI] [PubMed] [Google Scholar]
Bösing 2003 {published data only}
- Bösing N, Schumacher B, Frieling T, Ohmann C, Jungblut R, Lübke H, et al. Endoscopic ultrasound in routine clinical practice for staging adenocarcinomas of the stomach and distal esophagus. Chirurg 2003;74(3):214‐21. [PUBMED: 12647078] [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen VK, Eloubeidi MA. Endoscopic ultrasound‐guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri‐intestinal lymphadenopathy. American Journal of Gastroenterology 2004;99(4):628‐33. [PUBMED: 15089893] [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen RJ, Huang PT, Li YP, Zheng ZQ, Zhao YP, Huang FG, et al. Comparison of preoperative T staging by oral contrast enhanced ultrasonography and double contrast enhanced ultrasonography in advanced gastric carcinoma. Zhonghua Zhong Liu Za Zhi 2010;32(7):551‐4. [PUBMED: 21029703] [PubMed] [Google Scholar]
Davies 2006 {published data only}
- Davies AR, Deans DA, Penman I, Plevris JN, Fletcher J, Wall L, et al. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro‐esophageal cancer. Diseases of the Esophagus 2006;19(6):496‐503. [PUBMED: 17069595] [DOI] [PubMed] [Google Scholar]
Dewitt 2005 {published data only}
- DeWitt J, Kesler K, Brooks JA, LeBlanc J, McHenry L, McGreevy K, et al. Endoscopic ultrasound for esophageal and gastroesophageal junction cancer: Impact of increased use of primary neoadjuvant therapy on preoperative locoregional staging accuracy. Diseases of the Esophagus 2005;18(1):21‐7. [PUBMED: 15773837] [DOI] [PubMed] [Google Scholar]
Fiore 2006 {published data only}
- Fiore D, Baggio V, Ruol A, Bocus P, Casara D, Corti L, et al. Multimodal imaging of esophagus and cardia cancer before and after treatment. Radiologia Medica 2006;111(6):804‐17. [PUBMED: 16896560] [DOI] [PubMed] [Google Scholar]
Futawatari 2008 {published data only}
- Futawatari N, Kikuchi S, Sakuramoto S, Kida M, Watanabe M. A new diagnostic method for early gastric cancer: volume measurement by 3‐dimensional endoscopic ultrasonography in early gastric cancer and its clinical significance. Anticancer Research 2008;28(5B):2907‐12. [PUBMED: 19031933] [PubMed] [Google Scholar]
Ghiţă 2011 {published data only}
- Ghiţă D, Glavici A, Pleşea IE, Săftoiu A, Dumitrescu D, Ciurea T. Invasion assessment in gastric carcinoma ‐ imagistic and histopathologic combined study. Romanian Journal of Morphology and Embryology 2011;52(Suppl 1):349‐61. [PUBMED: 21424074] [PubMed] [Google Scholar]
Giovannini 1993 {published data only}
- Giovannini M, Seitz JF, Thomas P, Houvenaeghel G, Delpero JR, Giudicelli R, et al. Electronic sectorial ultrasound endoscopy in benign and malignant tumoral pathology of the stomach. Results in 30 patients [Echoendoscopie sectorielle electronique en pathologie tumorale benigne et maligne de l'estomac. Resultats chez 30 patients]. Gastroenterologie Clinique et Biologique 1993;17(1):26‐32. [PUBMED: 8467967] [PubMed] [Google Scholar]
Gorshkov 2001 {published data only}
- Gorshkov AN, Meshkov VM, Gracheva NI, Zaritskaia VA. Possibilities of radiologic methods (ultrasonography, computed tomography) in the preoperative evaluation of intramural invasion of gastric cancer [Vozmozhnosti luchevykh metodov issledovaniia (UZI, KT) v predoperatsionnoi otsenke vnutristenochnoi invazii raka zheludka]. Vestnik Rentgenologii i Radiologii 2001;2(Mar‐Apr):27‐34. [PUBMED: 11503175] [PubMed] [Google Scholar]
Greenberg 1994 {published data only}
- Greenberg J, Durkin M, Drunen M, Aranha GV. Computed tomography or endoscopic ultrasonography in preoperative staging of gastric and esophageal tumors. Surgery 1994;116(4):696‐701. [PUBMED: 7940168] [PubMed] [Google Scholar]
Grimm 1992 {published data only}
- Grimm H, Hamper K, Binmoeller KF, Soehendra N. Enlarged lymph nodes: malignant or not?. Endoscopy 1992;24(Suppl 1):320‐3. [PUBMED: 1633773] [DOI] [PubMed] [Google Scholar]
Grotenhuis 2013 {published data only}
- Grotenhuis BA, Wijnhoven BP, Poley JW, Hermans JJ, Biermann K, Spaander MC, et al. Preoperative assessment of tumor location and station‐specific lymph node status in patients with adenocarcinoma of the gastroesophageal junction. World Journal of Surgery 2013;37(1):147‐55. [PUBMED: 23015224] [DOI] [PubMed] [Google Scholar]
Heeren 2004 {published data only}
- Heeren PA, Westreenen HL, Geersing GJ, Dullemen HM, Plukker JT. Influence of tumor characteristics on the accuracy of endoscopic ultrasonography in staging cancer of the esophagus and esophagogastric junction. Endoscopy 2004;36(11):966‐71. [PUBMED: 15520913] [DOI] [PubMed] [Google Scholar]
Heintz 1991a {published data only}
- Heintz A, Junginger T. Endosonographic staging of cancers of the esophagus and stomach. Comparison with surgical and histopathologic staging [Endosonographisches staging von karzinomen in spreiserohre und magen. Vergleich mit der chirurgischen und histopathologischen stadieneinteilung]. Bildgebung 1991;58(1):4‐8. [PUBMED: 2070101] [PubMed] [Google Scholar]
Heintz 1991b {published data only}
- Heintz A, Junginger T. Endosonography in preoperative staging of gastrointestinal tumors [Die endosonographie zur praoperativen stadienbeurteilung gastrointestinaler tumoren]. Langenbecks Archiv fur Chirurgie 1991;376(1):3‐8. [PUBMED: 2034002] [DOI] [PubMed] [Google Scholar]
Heyer 1998 {published data only}
- Heyer T, Frieling T, Häussinger D. How accurate is preoperative staging as a basis for treatment decisions in gastric carcinoma? [Wie sicher ist das praoperative staging als grundlage der therapieentscheidung beim magenkarzinom?]. Praxis (Bern 1994) 1998;87(13):443‐6. [PUBMED: 9584569] [PubMed] [Google Scholar]
Hirata 1989 {published data only}
- Hirata K, Fukuda M. Progress in image diagnosis: endoscopic ultrasonography. Hokkaido Igaku Zasshi 1989;64(5):537‐41. [PUBMED: 2687144] [PubMed] [Google Scholar]
Holden 1996 {published data only}
- Holden A, Mendelson R, Edmunds S. Pre‐operative staging of gastro‐oesophageal junction carcinoma: comparison of endoscopic ultrasound and computed tomography. Australasian Radiology 1996;40(3):206‐12. [PUBMED: 8826718] [DOI] [PubMed] [Google Scholar]
Hünerbein 1995 {published data only}
- Hünerbein M, Rau B, Schlag PM. Laparoscopy and laparoscopic ultrasound for staging of upper gastrointestinal tumours. European Journal of Surgical Oncology 1995;21(1):50‐5. [PUBMED: 7851554] [DOI] [PubMed] [Google Scholar]
Hünerbein 1996 {published data only}
- Hünerbein M, Dohmoto M, Rau B, Schlag PM. Endosonography and endosonography‐guided biopsy of upper‐GI‐tract tumors using a curved‐array echoendoscope. Surgical Endoscopy 1996;10(12):1205‐9. [PUBMED: 8939844] [DOI] [PubMed] [Google Scholar]
Kang 2010 {published data only}
- Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surgical Endoscopy 2010;24(3):509‐16. [PUBMED: 19585066] [DOI] [PubMed] [Google Scholar]
Kida 1998 {published data only}
- Kida M, Tanabe S, Watanabe M, Kokutou M, Kondou I, Yamada Y, et al. Staging of gastric cancer with endoscopic ultrasonography and endoscopic mucosal resection. Endoscopy 1998;30(Suppl 1):A64‐8. [PUBMED: 9765088] [DOI] [PubMed] [Google Scholar]
Kienle 2002 {published data only}
- Kienle P, Buhl K, Kuntz C, Düx M, Hartmann C, Axel B, et al. Prospective comparison of endoscopy, endosonography and computed tomography for staging of tumours of the oesophagus and gastric cardia. Digestion 2002;66(4):230‐6. [PUBMED: 12592099] [DOI] [PubMed] [Google Scholar]
Kroep 2003 {published data only}
- Kroep JR, Groeningen CJ, Cuesta MA, Craanen ME, Hoekstra OS, Comans EF, et al. Positron emission tomography using 2‐deoxy‐2‐[18F]‐fluoro‐D‐glucose for response monitoring in locally advanced gastroesophageal cancer; a comparison of different analytical methods. Molecular Imaging and Biology 2003;5(5):337‐46. [PUBMED: 14630513] [DOI] [PubMed] [Google Scholar]
Lavonius 2002 {published data only}
- Lavonius MI, Gullichsen R, Salo S, Sonninen P, Ovaska J. Staging of gastric cancer: a study with spiral computed tomography, ultrasonography, laparoscopy, and laparoscopic ultrasonography. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 2002;12(2):77‐81. [PUBMED: 119428291] [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li S, Huang P, Wang Z, Chen J, Xu H, Wang L, et al. Preoperative T staging of advanced gastric cancer using double contrast‐enhanced ultrasound. Ultraschall in der Medizin 2012;33(7):E218‐24. [PUBMED: 22744445] [DOI] [PubMed] [Google Scholar]
Matthes 2006 {published data only}
- Matthes K, Bounds BC, Collier K, Gutierrez A, Brugge WR. EUS staging of upper GI malignancies: results of a prospective randomized trial. Gastrointestinal Endoscopy 2006;64(4):496‐502. [PUBMED: 16996338] [DOI] [PubMed] [Google Scholar]
Meining 2002 {published data only}
- Meining A, Dittler HJ, Wolf A, Lorenz R, Schusdziarra V, Siewert JR, et al. You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut 2002;50(5):599‐603. [PUBMED: 11950802] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortensen 2007 {published data only}
- Mortensen MB, Edwin B, Hünerbein M, Liedman B, Nielsen HO, Hovendal C. Impact of endoscopic ultrasonography (EUS) on surgical decision‐making in upper gastrointestinal tract cancer: an international multicenter study. Surgical Endoscopy 2007;21(3):431‐8. [PUBMED: 17180286] [DOI] [PubMed] [Google Scholar]
Nagler 2011 {published data only}
- Nagler AK, Aslanian HR, Siddiqui UD. Endoscopic ultrasound and gastric lesions. Journal of Clinical Gastroenterology 2011;45(3):215‐21. [PUBMED: 21278579] [DOI] [PubMed] [Google Scholar]
Nakamura 1999b {published data only}
- Nakamura K, Morisaki T, Sugitani A, Ogawa T, Uchiyama A, Kinukawa N, et al. An early gastric carcinoma treatment strategy based on analysis of lymph node metastasis. Cancer 1999;85(7):1500‐5. [PUBMED: 10193939] [PubMed] [Google Scholar]
Nakamura 2000 {published data only}
- Nakamura K, Morisaki T, Noshiro H, Torata N, Kinukawa N, Tanaka M. Morphometric analysis of regional lymph nodes with and without metastasis from early gastric carcinoma. Cancer 2000;88(11):2438‐42. [PUBMED: 10861417] [PubMed] [Google Scholar]
Ohashi 1989 {published data only}
- Ohashi S, Nakazawa S, Yoshino J. Endoscopic ultrasonography in the assessment of invasive gastric cancer. Scandinavian Journal of Gastroenterology 1989;24(9):1039‐48. [PUBMED: 2688069] [DOI] [PubMed] [Google Scholar]
Okai 1991 {published data only}
- Okai T, Yamakawa O, Matsuda N, Kawakami H, Watanabe H, Satomura Y, et al. Analysis of gastric carcinoma growth by endoscopic ultrasonography. Endoscopy 1991;23(3):121‐5. [PUBMED: 1860438] [DOI] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park JM, Ahn CW, Yi X, Hur H, Lee KM, Cho YK, et al. Efficacy of endoscopic ultrasonography for prediction of tumor depth in gastric cancer. Journal of Gastric Cancer 2011;11(2):109‐15. [PUBMED: 22076211] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2007 {published data only}
- Patel PR, Mansfield PF, Crane CH, Wu TT, Lee JH, Lynch PM, et al. Clinical stage after preoperative chemoradiation is a better predictor of patient outcome than the baseline stage for localized gastric cancer. Cancer 2007;110(5):989‐95. [PUBMED: 17636525] [DOI] [PubMed] [Google Scholar]
Pedrazzani 2007 {published data only}
- Pedrazzani C, Manzoni G, Marrelli D, Giacopuzzi S, Corso G, Minicozzi AM, et al. Lymph node involvement in advanced gastroesophageal junction adenocarcinoma. Journal of Thoracic and Cardiovascular Surgery 2007;134(2):378‐85. [PUBMED: 17662776] [DOI] [PubMed] [Google Scholar]
Power 2009 {published data only}
- Power DG, Schattner MA, Gerdes H, Brenner B, Markowitz AJ, Capanu M, et al. Endoscopic ultrasound can improve the selection for laparoscopy in patients with localized gastric cancer. Journal of the American College of Surgeons 2009;208(2):173‐8. [PUBMED: 19228527] [DOI] [PubMed] [Google Scholar]
Rau 1995 {published data only}
- Rau B, Hünerbein M, Schlag PM. Laparoscopy and laparoscopic endosonography as staging examination of tumors of the upper gastrointestinal tract. Zentralblatt fur Chirurgie 1995;120(5):346‐9. [PUBMED: 7541926] [PubMed] [Google Scholar]
Richards 2000 {published data only}
- Richards DG, Brown TH, Manson JM. Endoscopic ultrasound in the staging of tumours of the oesophagus and gastro‐oesophageal junction. Annals of the Royal College of Surgeons of England 2000;82(5):311‐7. [PUBMED: 11041028] [PMC free article] [PubMed] [Google Scholar]
Rösch 1992 {published data only}
- Rösch T, Lorenz R, Zenker K, Wichert A, Dancygier H, Höfler H, et al. Local staging and assessment of resectability in carcinoma of the esophagus, stomach, and duodenum by endoscopic ultrasonography. Gastrointestinal Endoscopy 1992;38(4):460‐7. [PUBMED: 1511822] [DOI] [PubMed] [Google Scholar]
Songür 1996 {published data only}
- Songür Y, Okai T, Fujii T. Endosonographic staging of gastric carcinoma. Digestive Endoscopy 1996;8:5‐13. [Google Scholar]
Tsuzuki 2011 {published data only}
- Tsuzuki T, Okada H, Kawahara Y, Nasu J, Takenaka R, Inoue M, et al. Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer. Acta Medica Okayama 2011;65(2):105‐12. [PUBMED: 21519368] [DOI] [PubMed] [Google Scholar]
Venkataraman 2010 {published data only}
- Venkataraman I, Rao HK, Singh P, Elangovan S, Kate V. Efficacy of hydrogastric sonography and spiral computed tomography in staging of gastric carcinoma‐‐a comparative study. Journal of Clinical Ultrasound 2010;38(9):480‐5. [PUBMED: 20848570] [DOI] [PubMed] [Google Scholar]
Wakelin 2002 {published data only}
- Wakelin SJ, Deans C, Crofts TJ, Allan PL, Plevris JN, Paterson‐Brown S. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago‐gastric carcinoma. European Journal of Radiology 2002;41(2):161‐7. [PUBMED: 11809546] [DOI] [PubMed] [Google Scholar]
Yoshinaga 2012 {published data only}
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World Journal of Gastrointestinal Endoscopy 2012;4(6):218‐26. [PUBMED: 22720122] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Feng 2013 {published data only}
- Feng XY, Wang W, Luo GY, Wu J, Zhou ZW, Li W, et al. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer ‐ results of a single institution study of 610 Chinese patients. PLoSOne 2013;8(11):e78846. [PUBMED: 24223855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lei 2013 {published data only}
- Lei C, Huang L, Wang Y, Huang Y, Huang Y. Comparison of MRI and endoscope ultrasound detection in preoperative T/N staging of gastric cancer. Molecular and Clinical Oncology 2013;1(4):699‐702. [DOI: 10.3892/mco.2013.103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehmedović 2014 {published data only}
- Mehmedović A, Mesihović R, Saray A, Vanis N. Gastric cancer staging: EUS and CT. Medical Archives 2014;68(1):34‐6. [DOI: 10.5455/medarh.2014.68.34-36; PUBMED: 24783909] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spolverato 2015 {published data only}
- Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi‐institutional study of the US gastric cancer collaborative. Journal of the American College of Surgeons 2015;220(1):48‐56. [DOI: 10.1016/j.jamcollsurg.2014.06.023; PUBMED: 25283742] [DOI] [PubMed] [Google Scholar]
Additional references
Bennett 2009
- Bennett C, Wang Y, Pan T. Endoscopic mucosal resection for early gastric cancer. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004276.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byrne 2002
- Byrne MF, Jowell PS. Gastrointestinal imaging: endoscopic ultrasound. Gastroenterology 2002;122(6):1631‐48. [PUBMED: 12016428] [DOI] [PubMed] [Google Scholar]
Deeks 2004
- Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329(7458):168‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882‐93. [DOI] [PubMed] [Google Scholar]
Dicken 2005
- Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Annals of Surgery 2005;241(1):27‐39. [PUBMED: 15621988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumonceau 2011
- Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)‐guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2011;43(10):897‐912. [DOI] [PubMed] [Google Scholar]
Edge 2010
- Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th Edition. New York: Springer, 2010. [Google Scholar]
Ferlay 2010
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. [DOI] [PubMed] [Google Scholar]
Ha 2011
- Ha TK, Choi YY, Song SY, Kwon SJ. F18‐fluorodeoxyglucose‐positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer. Journal of the Korean Surgical Society 2011;81(2):104‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2008
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta‐analysis of diagnostic accuracy showed hierarchical models are necessary. Journal of Clinical Epidemiology 2008;61(11):1095‐103. [PUBMED: 19208372] [DOI] [PubMed] [Google Scholar]
Hargunani 2009
- Hargunani R, Maclachlan J, Kaniyur S, Power N, Pereira SP, Malhotra A. Cross‐sectional imaging of gastric neoplasia. Clinical Radiology 2009;64(4):420‐9. [DOI] [PubMed] [Google Scholar]
Hirasawa 2011
- Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, et al. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointestinal Endoscopy 2011;74(6):1268‐75. [DOI] [PubMed] [Google Scholar]
House 2008
- House MG, Brennan MF. Neoadjuvant therapy for gastric cancer. Advances in Surgery 2008;42:151‐68. [DOI] [PubMed] [Google Scholar]
Hur 2006
- Hur J, Park MS, Lee JH, Lim JS, Yu JS, Hong YJ, et al. Diagnostic accuracy of multidetector row computed tomography in T‐ and N staging of gastric cancer with histopathologic correlation. Journal of Computer Assisted Tomography 2006;30(3):372‐7. [DOI] [PubMed] [Google Scholar]
Jackson 2009
- Jackson C, Cunningham D, Oliveira J. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow‐up. Annals of Oncology 2009;20(Suppl 4):34‐6. [PUBMED: 19454457] [DOI] [PubMed] [Google Scholar]
Jemal 2010
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians 2010;60(5):277‐300. [PUBMED: 20610543] [DOI] [PubMed] [Google Scholar]
Jensen 2007
- Jensen EH, Tuttle TM. Preoperative staging and postoperative surveillance for gastric cancer. Surgical Oncology Clinics of North America 2007;16(2):329‐42. [DOI] [PubMed] [Google Scholar]
Jiang 2010
- Jiang Y, Ajani JA. Multidisciplinary management of gastric cancer. Current Opinion in Gastroenterology 2010;26(6):640‐6. [DOI] [PubMed] [Google Scholar]
Kang 2011
- Kang KJ, Kim KM, Min BH, Lee JH, Kim JJ. Endoscopic submucosal dissection of early gastric cancer. Gut and Liver 2011;5(4):418‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawaguchi 2011
- Kawaguchi T, Ichikawa D, Komatsu S, Okamoto K, Murayama Y, Shiozaki A, et al. Clinical evaluation of JCGC and TNM staging on multidetector‐row computed tomography in preoperative nodal staging of gastric cancer. Hepatogastroenterology 2011;58(3):838‐41. [PubMed] [Google Scholar]
Kelly 2001
- Kelly S, Harris KM, Berry E, Hutton J, Roderick P, Cullingworth J, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro‐oesophageal carcinoma. Gut 2001;49(4):534‐9. [PUBMED: 11559651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2005
- Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdominal Imaging 2005;30(4):465‐72. [DOI] [PubMed] [Google Scholar]
Kim 2011
- Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast‐enhanced CT. European Journal of Radiology 2011;79(2):183‐8. [DOI] [PubMed] [Google Scholar]
Kumano 2005
- Kumano S, Murakami T, Kim T, Hori M, Iannaccone R, Nakata S, et al. T staging of gastric cancer: role of multi‐detector row CT. Radiology 2005;237(3):961‐6. [DOI] [PubMed] [Google Scholar]
Kwee 2007
- Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. Journal of Clinical Oncology 2007;25(15):2107‐16. [PUBMED: 17513817] [DOI] [PubMed] [Google Scholar]
Kwee 2008
- Kwee RM, Kwee TC. The accuracy of endoscopic ultrasonography in differentiating mucosal from deeper gastric cancer. American Journal of Gastroenterology 2008;103(7):1801‐9. [PUBMED: 18564110] [DOI] [PubMed] [Google Scholar]
Kwee 2009
- Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009;12(1):6‐22. [PUBMED: 19390927] [DOI] [PubMed] [Google Scholar]
Leeflang 2008
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Annals of Internal Medicine 2008;149(12):889‐97. [PUBMED: 19075208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ly 2008
- Ly QP, Sasson AR. Modern surgical considerations for gastric cancer. Journal of the National Comprehensive Cancer Network 2008;6(9):885‐94. [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors); Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0. The Cochrane Collaboration, 2010. Available from http://srdta.cochrane.org/handbook‐dta‐reviews.
Mocellin 2011
- Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta‐analysis. Gastrointestinal Endoscopy 2011;73(6):1122‐34. [DOI] [PubMed] [Google Scholar]
Othman 2011
- Othman MO, Wallace MB. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) in 2011, a Western perspective. Clinics and Research in Hepatology and Gastroenterology 2011;35(4):288‐94. [DOI] [PubMed] [Google Scholar]
Paoletti 2010
- Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta‐analysis. JAMA 2010;303(17):1729‐37. [DOI] [PubMed] [Google Scholar]
Polkowski 2009
- Polkowski M. Endosonographic staging of upper intestinal malignancy. Best Practice and Research. Clinical Gastroenterology 2009;23(5):649‐61. [PUBMED: 19744630] [DOI] [PubMed] [Google Scholar]
Puli 2008
- Puli SR, Batapati Krishna Reddy J, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta‐analysis and systematic review. World Journal of Gastroenterology 2008;14(25):4011‐9. [PUBMED: 18609685] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2008
- Reddy NK, Markowitz AB, Abbruzzese JL, Bhutani MS. Knowledge of indications and utilization of EUS: a survey of oncologists in the United States. Journal of Clinical Gastroenterology 2008;42(8):892‐6. [PUBMED: 18645535] [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [PUBMED: 16168343] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [DOI] [PubMed] [Google Scholar]
Seevaratnam 2012
- Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta‐analysis. Gastric Cancer 2012;15 Suppl 1:S3‐18. [DOI] [PubMed] [Google Scholar]
Shah 2010
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. Journal of the National Comprehensive Cancer Network 2010;8(4):437‐47. [PUBMED: 20410336] [DOI] [PubMed] [Google Scholar]
Stata 2009 [Computer program]
- StatCorp LP. Stata Statistical Software. Version Release 11. College Station, TX: StatCorp LP, 2009.
Stell 1996
- Stell DA, Carter CR, Stewart I, Anderson JR. Prospective comparison of laparoscopy, ultrasonography and computed tomography in the staging of gastric cancer. British Journal of Surgery 1996;83(9):1260‐2. [PubMed] [Google Scholar]
Wagner 2010
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004064.pub3] [DOI] [PubMed] [Google Scholar]
Weber 2004
- Weber WA, Ott K. Imaging of esophageal and gastric cancer. Seminars in Oncology 2004;31(4):530‐41. [PUBMED: 15297944] [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]


