Abstract
Rationale:
Observational studies suggest obesity is associated with sepsis survival, but these studies are small, fail to adjust for key confounders, measure body mass index (BMI) at inconsistent time points, and/or use administrative data to define sepsis.
Objective:
To estimate the relationship between BMI and sepsis mortality using detailed clinical data for case detection and risk-adjustment.
Design:
Retrospective cohort analysis of a large clinical data repository
Setting:
139 hospitals in the United States of America
Patients:
Adult inpatients with sepsis meeting Sepsis-3 criteria
Exposure:
BMI in six categories: underweight (BMI<18.5kg/m2), normal-weight (BMI=18.5–24.9kg/m2), overweight (BMI=25.0–29.9kg/m2), obese-class-I (BMI=30.0–34.9kg/m2), obese-class-II (BMI=35.0–39.9kg/m2), and obese-class-III (BMI≥40kg/m2).
Measurements:
Multivariate logistic regression with generalized estimating equations to estimate the effect of BMI category on short-term mortality (in-hospital death or discharge to hospice) adjusting for patient, infection, and hospital-level factors. Sensitivity analyses were conducted in subgroups of age, gender, Elixhauser comorbidity index, SOFA quartiles, bacteremic sepsis, and ICU admission.
Main results:
From 2009 to 2015, we identified 55,038 adults with sepsis and assessable BMI measurements: 6% underweight, 33% normal-weight, 28% overweight and 33% obese. Crude mortality was inversely proportional to BMI category: underweight (31%), normal-weight (24%), overweight (19%), obese-class-I (16%), obese-class-II (16%) and obese-class-III (14%). Compared to normal-weight, the adjusted odds ratio [95%CI] of mortality was 1.62[1.50–1.74] for underweight, 0.73[0.70–0.77] for overweight, 0.61[0.57–0.66] for obese-class-I, 0.61[0.55–0.67] for obese-class-II, and 0.65[0.59–0.71] for obese-class-III. Results were consistent in sensitivity analyses.
Conclusions:
In adults with clinically-defined sepsis, we demonstrate lower short-term mortality in patients with higher BMIs compared to those with normal BMIs (both unadjusted and adjusted analyses) and higher short-term mortality in those with low BMIs. Understanding how obesity improves survival in sepsis would inform prognostic and therapeutic strategies.
Keywords: Sepsis, Obesity, Body Mass Index, Survival, Mortality, Critical Illness
Introduction
Sepsis is a frequent and lethal syndrome. Multiple Phase 2 and Phase 3 clinical trials have failed to discover novel sepsis therapies that improve outcome.1 This failure has been attributed to the heterogeneity of study populations.2 Closer scrutiny of phenotypes and sub-phenotypes of patients that display strong survival signals in sepsis may enable us to understand novel mechanisms to improve treatment.
Body mass index (BMI) is one such phenotype.3–13 More than 25% of adults admitted to intensive care units (ICUs) in the United States are overweight or obese.14–16 Observational studies suggest an association between obesity (BMI ≥30kg/m2) and survival in sepsis, with an absolute mortality reduction ranging between 5% and 15% in obese compared to normal-weight patients.4–9 The biologic basis for this survival advantage is unclear, but theories include greater metabolic reserve17, renin-angiotensin system activation18, and secretion of immunomodulatory mediators such as leptin and soluble-tumor necrosis factor receptor-2 by adipose tissue.19
This obesity survival “paradox” has not been consistently reproduced10–13 and studies of sepsis and obesity have been limited by small numbers and failure to adjust for potential confounders such as illness severity11, co-morbidities10,11, site of infection6,7,10–13, recent weight-loss4–13, or geographic location. 4–6,10–12 Also, studies measured BMI at different time points relative to initiation of sepsis resuscitation.20–22 Obtaining weight measurements after fluid resuscitation can falsely elevate estimates. Likewise, measuring weights later in hospitalization may yield falsely low values if the patient has suffered weight loss due to prolonged hospitalization or sepsis catabolism. Both effects would confound meaningful interpretation of the impact of obesity on sepsis outcomes.21 Further, several studies used administrative codes to define sepsis4–6,12, which lack granularity around the timing of sepsis and are less accurate than sepsis identification using clinical data.23
Therefore, the true association between obesity and sepsis survival is unknown and compels further investigation with larger and more rigorous studies. Understanding whether obesity actually improves survival is important. If obesity is truly protective, the underlying mechanisms warrant additional research, which would yield insights into sepsis pathogenesis and novel sepsis therapies. If harmful, investigators may need to treat obese septic adults with different treatments. We, therefore, evaluated a large clinical data repository to rigorously measure associations between survival and BMI using detailed clinical data to identify sepsis and to adjust for potential confounders.
Methods
Study design, data source, and population
We performed a retrospective cohort study using the CERNER™ HealthFacts electronic health record (EHR) database, which includes detailed clinical data collected during routine patient care from US hospitals, well distributed by region, volume, teaching status, and urban/rural status.24 This dataset has previously been used to evaluate incidence and trends in sepsis in the US.23 We included adults ≥20 years of age admitted as inpatients or under observation status or who died in the emergency department in calendar years 2009–2015. The NIH Office of Human Subject Research Protections (OHSRP) deemed that institutional review board approval was not required as the study used de-identified data alone. The STROBE guidelines for reporting cohort studies were followed.25
Sepsis definition
We defined sepsis as concurrent infection and organ dysfunction consistent with Sepsis-3 criteria.26 We defined presumed serious infections as a blood culture draw and administration of ≥4 days of new antibiotics, including at least one intravenous antibiotic day, as previously described.23 We defined organ dysfunction using the Sequential Organ Failure Assessment (SOFA) score. Sepsis was deemed present in a patient with suspected infection and a concurrent increase in SOFA by ≥2 points from baseline. Septic shock was defined as sepsis with a septic shock diagnosis code (ICD-9-CM 785.52) or use of ≥1 vasopressor(s) within two days of the culture draw. Further details about the case detection method are provided in eAppendices 1–3.
Body mass index definitions
We identified the subset of sepsis cases with body mass index (BMI), weight or height data on or preceding the day of sepsis diagnosis (defined as the index blood culture draw). For those without direct BMI measurements, we calculated BMI as weight in kilograms divided by height in meters squared (kg/m2). We categorized BMI as follows: underweight (<18.5kg/m2); normal-weight (18.5–24.9kg/m2); overweight (25.0–29.9kg/m2); obese-Class-I (30.0–34.9kg/m2); obese-Class-II (35.0–39.9kg/m2); and obese-Class-III (≥40.0kg/m2, morbidly obese).3 We excluded patients if their BMI values were <10kg/m2 or ≥100kg/m2, or if they had discordant BMI data (i.e., two or more recorded BMI values that fell into ≥2 BMI categories on the latest date on or before sepsis diagnosis). Sepsis cases without BMI data were excluded.
Implementation and statistical analyses
Using normal BMI as the reference BMI category, we reported the proportion of patients with short-term mortality (defined as in-hospital death or discharge to hospice), and the adjusted odds ratios of short-term mortality (aOR, 95% confidence interval). Variables evaluated in previous studies4–13,17–22 and others considered clinically relevant were included in our multivariate logistic regression models (eAppendix 4–6). We used generalized estimating equations (GEE) to account for clustering of patients from the same hospital.27 Because the normal-weight BMI category was compared with the five other BMI categories, we reported a p-value corrected for multiple testing (i.e., Bonferroni correction). We reported secondary outcomes of length of ICU and hospital stay across BMI categories. SAS version 9.4 was used for all statistical analyses. Power calculations were conducted using R software version 3.4.0 using the powerMediation package. Source code for analyses related to statistical computing [eg. R or SAS scripts] are provided (eAppendices 7–8). The underlying assumptions of logistic regression were tested (eAppendix9).
Sensitivity analyses
We conducted pre-specified sensitivity analyses in sepsis patients (eAppendix 7). Because malnutrition confounds the association between obesity and mortality in critical illness28, we used serum albumin data as a surrogate for malnutrition and performed a separate sensitivity analysis to determine whether outcomes across BMI categories varied by albumin level. To account for early versus late antibiotic administration, we performed a separate sensitivity analysis to assess whether outcomes varied across BMI categories by the timing of antibiotic initiation relative to the day of index blood culture draw (day −2, −1, 0, +1, +2). Interactions were tested in certain variables (age, gender, ethnicity, albumin quartile, SOFA quartile, Elixhauser quartile, site of infection and blood culture positivity) because mortality varied across BMI status and these variables. Lastly, we analyzed a separate cohort of patients with billing codes for gastrointestinal bleeding and at least one blood transfusion but without clinical markers of presumed infection to ascertain whether the association of BMI with short-term mortality was consistent in a select group of non-septic hospitalized patients, in whom no effect had previously been described. This cohort of with gastrointestinal bleeds was chosen as they were likely to have no infection, sepsis or antibiotic administration, compared to cohorts with exacerbations of congestive heart failure or chronic obstructive pulmonary disease.
Results
Sepsis cases
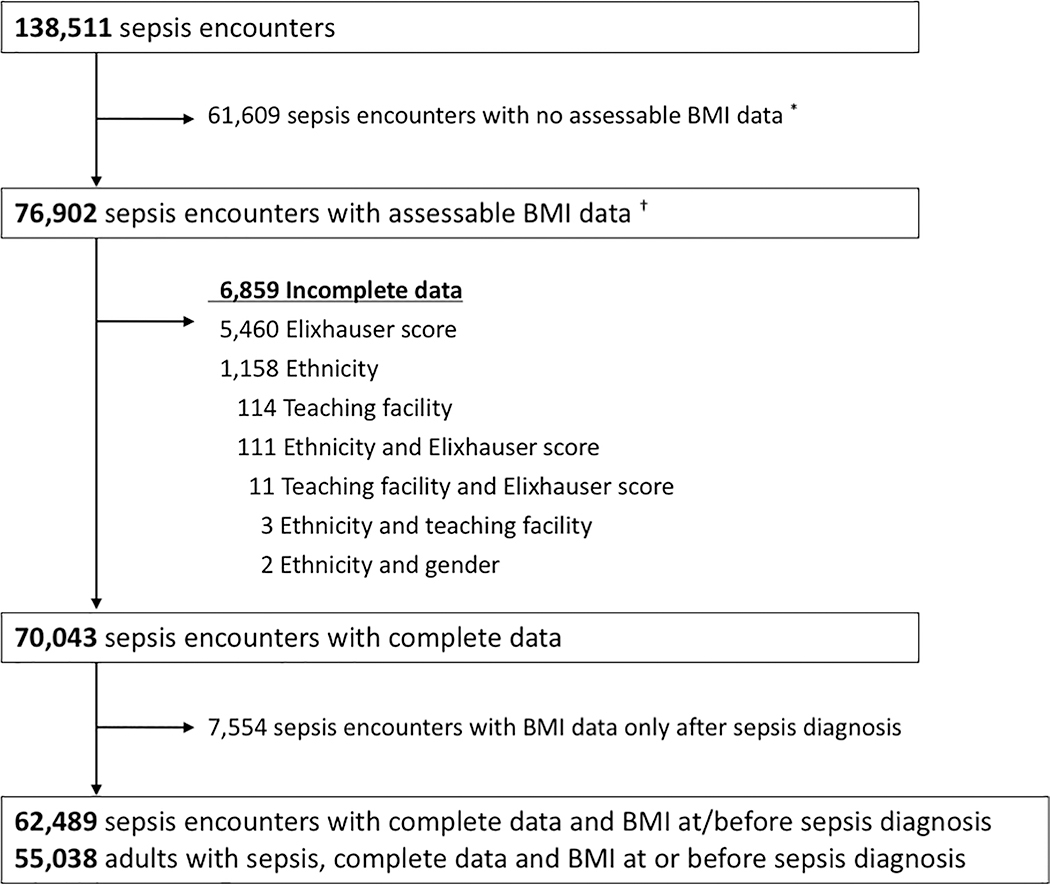
Among 2,529,158 adult encounters admitted to 139 hospitals between 2009 and 2015, we identified 138,511 sepsis encounters. Of these, 61,609 had no assessable BMI data, 6,859 had incomplete data, and 7,554 had BMI data only after sepsis diagnosis (Figure 1). Complete data for 62,489 sepsis encounters and 55,038 adult inpatients with sepsis were available for analysis.
Figure 1: Study flow diagram.
* Includes BMI data after hospital discharge
† BMI data prior to hospital admission and until hospital discharge
Baseline characteristics of sepsis cases
Table E1–2 summarizes baseline characteristics for 55,038 adults with sepsis according to BMI categories. The BMI proportions were underweight (6%; BMI<18.5kg/m2), normal-weight (33%; BMI=18.5–24.9kg/m2), overweight (28%; BMI=25.0–29.9kg/m2), obese-Class-I (16%; BMI=30.0–34.9kg/m2), obese-Class-II (8%; BMI=35.0–39.9kg/m2) and obese-Class-III (9%; BMI≥40kg/m2, morbidly obese). Proportions of patients initiating antibiotics on day −2, −1, 0, +1, and +2 relative to the index blood culture day were similar across BMI categories. Procedures performed following sepsis onset were also similar across BMI categories, except central venous catheter insertion which was more frequent in the morbidly obese than those of normal-weight BMI (29% [1,415/4,881] versus 22% [3,930/18,164]). Adults with BMI data had similar baseline characteristics to those without BMI data, except for admission year and urban-rural setting (Table E1).
Outcomes
Unadjusted mortality (death in hospital or discharge to hospice) was inversely proportional to BMI category: underweight (31%; 1,080/3,520), normal-weight (24%; 4,300/18,164), overweight (19%; 2,897/15,193), obese-Class-I (16%; 1,458/8,916), obese-Class-II (16%; 678/4,364), and obese-Class-III (14%; 704/4,881)(Table E3). Median ICU length of stay (9 days [IQR 6–15]) was similar across BMI categories for the 14,511 adults admitted to the ICU. Median hospital length of stay (8 days [IQR 6–13]) was similar across BMI categories for the 55,038 adults.
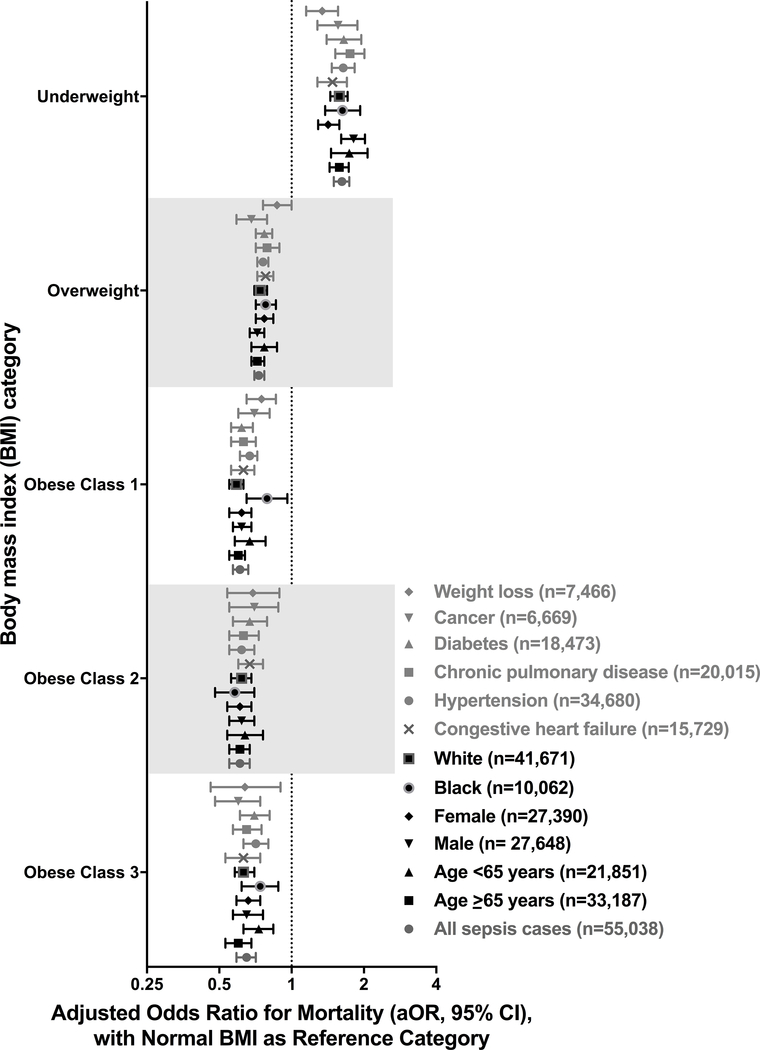
On multivariate analysis, the adjusted odds ratio [95%CI] of short-term mortality (death or hospice) was 1.62 [1.50–1.74, p<0.0001] for underweight BMI; 0.73 [0.70–0.77, p<0.0001] for overweight BMI; 0.61 [0.57–0.66, p<0.0001] for obese-Class-I BMI; 0.61 [0.55–0.67, p<0.0001] for obese-Class-II BMI; and 0.65 [0.59–0.71, p<0.0001] for obese-Class-III BMI relative to normal-weight BMI (Table E3). These results were similar when patients with BMI data were divided into BMI quintiles and quintile3 (including median BMI=26.67) was used as the reference group in logistic regression.
Sensitivity Analyses
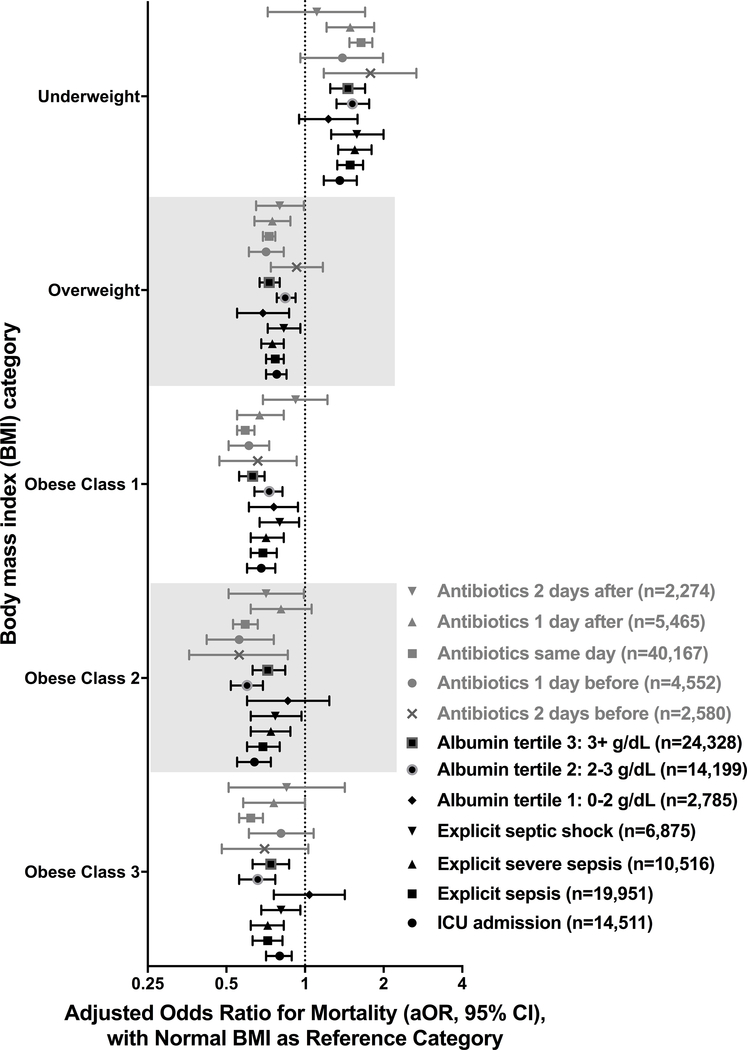
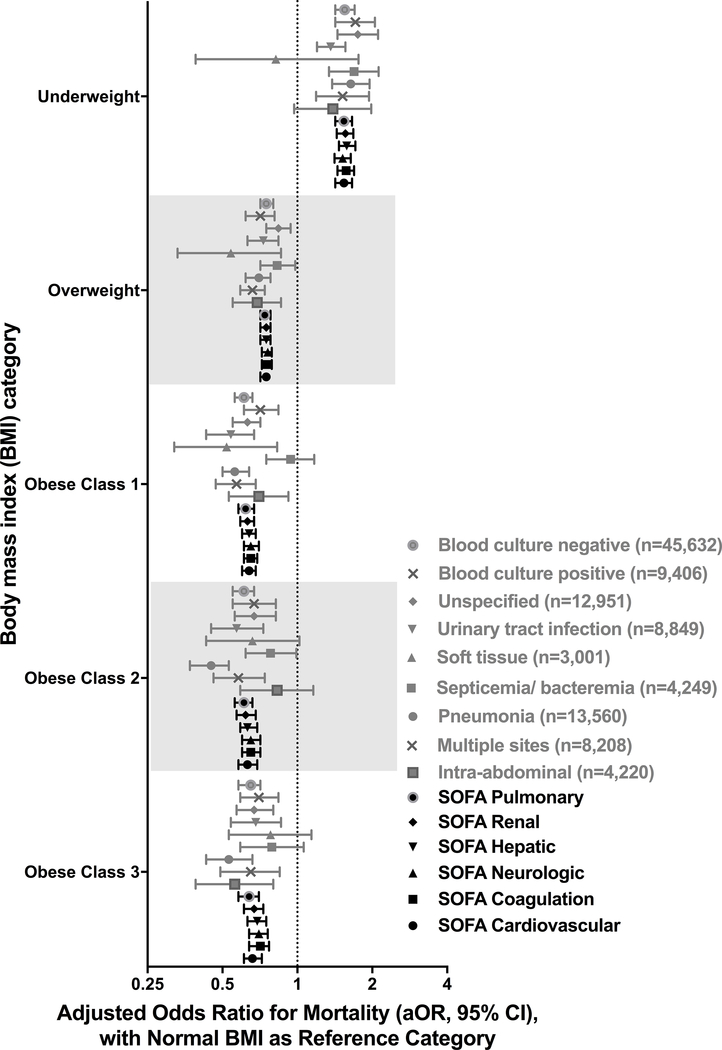
Results were consistent in sensitivity analyses (Figures 2–4; Table E5; Figures E1–E15). Statistically significant interactions were demonstrated between BMI and gender (p=0.035), and BMI and albumin quartile (p=0.001); but not with age, ethnicity, SOFA quartile, Elixhauser quartile, site of infection or blood culture positivity. In adults with albumin levels <2g/dL, those with overweight and obese-Class-I BMIs had improved survival but not those with obese-Class-II or Class-III BMIs. Analysis of a separate cohort of patients with gastrointestinal bleeds and without infection also showed a similar trend of lower mortality with increasing BMI (Table E6, Figure E16).
Figure 2: Adjusted hospital mortality according to body mass index category (all sepsis cases, demographics and co-morbid illnesses).
See online data supplement (Table E5 and Figures E1 – E6) for details
Figure 4: Adjusted hospital mortality according to body mass index category (ICU admission, explicit sepsis definitions, albumin tertile and antibiotic timing).
See online data supplement (Table E5 and Figures E11 – E15) for details
Discussion
In a cohort of 55,038 adults hospitalized with sepsis, short-term mortality (death or hospice) was lower in overweight and obese patients compared to those with normal-weight BMIs. Underweight patients had increased mortality compared to normal-weight BMIs. This relationship persisted after adjusting for multiple potential confounders including demographic factors, admission year, hospital-level factors, infection factors, and severity of illness. These results were consistent across several sensitivity analyses including co-morbidities, site of infection, and timing of antibiotics.
At the turn of the 21st century, Fleischmann and colleagues first described improved survival with increasing BMI in a cohort of patients with end-stage renal disease.29 This association has since been reported in acute and chronic illnesses such as congestive heart failure30, acute coronary syndrome31, chronic obstructive pulmonary disease32, and critical illness.33–35 Prior systematic reviews21,22 have suggested improved sepsis survival with increasing BMI and worse survival with decreasing BMI, but their interpretations were limited by weaknesses of their source studies including low power due to small sample sizes, inconsistent timing of BMI measurements, and incomplete adjustment for potential confounders.21 Our study used detailed clinical, laboratory and physiologic data to strengthen the finding that obesity is independently associated with improved short-term survival in adults with sepsis.
We found better short-term survival in those with higher BMI in sepsis. Several large well-conducted studies have reported poorer long-term survival with higher BMI in healthy adults and those with diabetes.36–38 We consider these investigations to be complementary since our study evaluated short-term outcomes39 and does not preclude the possibility of long-term adverse consequences of obesity in sepsis. Notably, two studies with both short- and long-term outcomes in surgical sepsis and pneumonia reported improved 30-day survival in the obese but no difference in 5-year survival.35,40 We observed improved survival in obese (versus normal-weight) patients in a separate cohort of non-septic adults with gastrointestinal bleeds, a population in which, to the best of our knowledge, this phenomenon has not been previously described. This observation along with evidence from previous studies on other non-septic populations30–32 suggests that the survival advantage associated with higher BMI may not be sepsis-specific but a general pattern in acute illness.
We observed lower mortality rates in patients with higher BMIs and caution against labeling this as “the obesity paradox”. BMI is a poor proxy for adiposity and may not accurately represent fat content, the proportions of muscle and fat, or overall body composition.41 Future prospective studies should quantify adipose tissue at or before sepsis diagnosis by either performing anthropometric indices of increased adiposity (e.g., waist circumference, waist-to-hip ratio, percentage visceral versus total body fat42); or assessing body composition with computerized tomography or dual-energy X-ray absorptiometry. Abdominal computerized tomography to measure visceral adipose tissue-to-subcutaneous adipose tissue (VAT/SAT) ratios may be informative, but its utility is diminished if performed after fluid resuscitation in sepsis.43 Prior studies reported that the absolute quantity of muscle tissue is increased in those with obese BMIs compared to those with normal-weight BMIs44, and that muscle wasting occurs early and rapidly during the first week of critical illness and is more severe among those with multi-organ failure. 45 Finally, low skeletal muscle area as assessed by CT scan during the early stage of critical illness is an independent risk factor for mortality.46 Future studies should therefore also explore the contribution of muscle mass on sepsis survival.
We detected a significant interaction between BMI and albumin levels, a surrogate marker for malnutrition. This finding echoes that of a prior study where obese critically ill patients had similar survival to non-obese ones after adjusting for objective measures of malnutrition.28 However, sample sizes in these groups were relatively small. Also, albumin is a negative acute-phase protein, so it is unclear whether these low albumin levels represent malnutrition or immune response differences. Investigators should determine whether better biomarkers of malnutrition can be used than albumin in large multicenter studies 47,48
Reports from animal models of obesity and sepsis are inconsistent with our findings. Obese mice and rats are at higher risk of death than non-obese ones.49 These models use young animals in contrast to elderly human patients who develop sepsis; survival is not risk-adjusted for illness severity or baseline imbalances at the time of sepsis onset; and animals do not receive antimicrobial therapy or other supportive measures. These differences in baseline factors and interventions make it difficult to analyze and extrapolate the effect of obesity in animal models to human disease.
We highlight several strengths of our study. Although retrospective in nature and investigating a known phenomenon among patients with sepsis, this is the largest analysis conducted to date assessing the association between BMI and sepsis mortality. Our large cohort included sepsis patients admitted to over 130 US hospitals that were well distributed by region. Thus, our findings are unlikely to be biased by regional hospital practices. Furthermore, our results are representative of more recent patterns of sepsis care compared to another large database study from 1996–2008 that predates our study period.4 We used detailed electronic clinical data to apply SOFA/ Sepsis 3 definitions rather than using administrative codes for sepsis.23 We used BMI data on or before the day of sepsis onset (i.e., dry body weight) to minimize potential weight gain due to fluid resuscitation9 or potential weight loss following the catabolic effects of sepsis.45 We adjusted for several known confounders such as illness severity, co-morbidities, site of infection, recent weight loss or geographic location. We performed several sensitivity analyses, which showed a consistent association between BMI and sepsis survival. We specifically did not adjust for other interventions, such as mechanical ventilation or dialysis following sepsis onset, as these could mediate the effect of BMI on sepsis survival. Our findings are unlikely to have a “selective-survivor” effect as our cohort included all adults 20 years of age or older. For example, if we had limited our cohort to only those adults 65 years or older, it is possible that healthier obese patients would survive to age 65, but less healthy adults may die before age 65. Despite several strengths of study design, our analysis is not without limitations.
Our findings are hypothesis-generating and do not imply causality. Residual confounding remains possible because quantitative data on smoking status50 and weight changes were not included in our multivariate regression model. However, we adjusted our findings using the Elixhauser co-morbidity index, which includes illnesses caused by smoking such as cardiac disease, chronic pulmonary disease, and cancer. Also, our multivariate model adjusted for qualitative weight loss using the Elixhauser co-morbidity index. We did not determine the appropriateness of antibiotics because pathogens were not always identified and susceptibility data were not always available. Our findings may not be generalizable to other countries or those younger than 20 years of age. Despite a comprehensive search algorithm, BMI was available or could be indirectly calculated in only 49% of sepsis encounters, which exceeded the BMI availability (32%) of the largest prior retrospective study that explored this question.4 Finally, the sizable proportion of missing BMI data was unlikely to have introduced systematic bias as the populations with and without BMI availability were similar.
Future prospective studies that aim to assess the effect of BMI on sepsis outcome will need to: i) uniformly record weight and height measurements; ii) quantify body composition, smoking consumption and weight loss; iii) adjust for potential confounders and iv) report the effects of interventions used in sepsis, and v) assess long-term mortality Based on data from our study, we estimate that such a cohort will need to enroll over 8,500 sepsis cases (Table E7) to have sufficient statistical power to detect true mortality differences across all BMI groups using normal-weight BMI as the reference group.
In conclusion, our analysis of a large data repository shows that higher BMIs are associated with higher sepsis short-term survival after controlling for potential confounders. These findings emphasize the need for well-conducted prospective clinical trials that assess obesity’s impact on survival in sepsis and that aim to understand the exact underlying mechanisms. These studies could enhance our understanding of sepsis pathogenesis and inform therapeutic strategies.
Supplementary Material
Figure 3: Adjusted hospital mortality according to body mass index category (SOFA category, site of infection, blood culture positivity).
See online data supplement (Table E5 and Figures E7 – E10) for details
Acknowledgments
Grant support: This study was supported by the National Institutes of Health Intramural Research Program, Clinical Center. Dr. Rhee was supported by the Agency for Healthcare Research and Quality (grant number K08HS025008).
Copyright form disclosure: Dr. Pepper received other support from intramural funding from the National Institutes of Health (NIH). Drs. Pepper, Demirkale, Sun, Fram, Eichacker, Suffredini, and Kadri received support for article research from the NIH. Drs. Pepper, Demirkale, Sun, Suffredini, and Kadri disclosed government. Dr. Rhee’s institution received support for article research from AHRQ, and he received support for article research from AHRQ. Dr. Fram’s institution received funding from the NIH; he received funding from Commonwealth Informatics (stockholder); and he disclosed work for hire. Dr. Klompas’ institution received funding from the CDC.
Footnotes
Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Competing interests: The authors declare that they have no competing interests.
References
- 1.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014. April;20(4):195–203. [DOI] [PubMed] [Google Scholar]
- 2.Scicluna BP, van Vught LA, Zwinderman AH, et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med. 2017. October;5(10):816–826 [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health. National Heart, Lung, and Blood Institute. Managing Overweight and Obesity in Adults: Systematic Evidence Review from the Obesity Expert Panel. Published November 2013 Accessed March 20, 2018 https://www.nhlbi.nih.gov/sites/default/files/media/docs/obesity-evidence-review.pdf
- 4.Arabi YM, Dara SI, Tamim HM, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit Care. 2013;17(2):R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaulton TG, Marshall MacNabb C, Mikkelsen ME, et al. A retrospective cohort study examining the association between body mass index and mortality in severe sepsis. Intern Emerg Med. 2015;10:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott HC, Chang VW, O’Brien JM Jr, et al. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med. 2014;42:1766–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakr Y, Madl C, Filipescu D, et al. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008;34(11):1999–2009 [DOI] [PubMed] [Google Scholar]
- 8.Wacharasint P, Boyd JH, Russell JA, et al. One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Crit Care. 2013;17(3):R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurzinger B, Dünser MW, Wohlmuth C, et al. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Win Klin Wochenschr. 2010;122(1–2):31–6. [DOI] [PubMed] [Google Scholar]
- 10.Adamzik M, Frey UH, Möhlenkamp S, et al. Aquaporin 5 gene promoter−−1364A/C polymorphism associated with 30-day survival in severe sepsis. Anesthesiology. 2011;114(4):912–7 [DOI] [PubMed] [Google Scholar]
- 11.Chalkias A, Nitsotolis T, Papalexandrou A, et al. Sagittal abdominal diameter may effectively predict future complications and increased mortality in intensive care unit patients with severe sepsis. J Crit Care. 2013;28:964–9 [DOI] [PubMed] [Google Scholar]
- 12.Kuperman EF, Showalter JW, Lehman EB, et al. The impact of obesity on sepsis mortality: a retrospective review. BMC Infect Dis. 2013;13:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakr Y, Alhussami I, Nanchal R, et al. Being overweight is associated with greater survival in ICU patients: results from the intensive care over nations audit. Crit Care Med. 2015;43(12):2623–32. [DOI] [PubMed] [Google Scholar]
- 14.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008. January;36(1):151–8 [DOI] [PubMed] [Google Scholar]
- 15.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring). 2008; 16: 515–521 [DOI] [PubMed] [Google Scholar]
- 16.Hogue CW Jr., Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009; 35: 1152–1170 [DOI] [PubMed] [Google Scholar]
- 17.Ng PY, Eikermann M. The obesity conundrum in sepsis. BMC Anesthesiol. 2017. October 25;17(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004. June;89(6):2548–56 [DOI] [PubMed] [Google Scholar]
- 19.Winkler G, Kiss S, Keszthelyi L, et al. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol. 2003. August;149(2):129–35. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: a systematic review. J Crit Care. 2015;30:518–24 [DOI] [PubMed] [Google Scholar]
- 21.Pepper DJ, Sun J, Welsh J, et al. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care. 2016;20:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Liu X, Chen Q, et al. The role of increased body mass index in outcomes of sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2017. August 31;17(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee C, Dantes R, Epstein L, et al. CDC Prevention Epicenter Program. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA. 2017. October 3;318(13):1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeShazo JP, Hoffman MA. A comparison of a multistate inpatient EHR database to the HCUP Nationwide Inpatient Sample. BMC Health Serv Res. 2015. September 15;15:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. STROBE The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007. October 20;370(9596):1453–7. [DOI] [PubMed] [Google Scholar]
- 26.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang KY, Zeger SL. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika. 1986;73:13–22. [Google Scholar]
- 28.Robinson MK, Mogensen KM, Casey JD, et al. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. 2015. January;43(1):87–100 [DOI] [PubMed] [Google Scholar]
- 29.Fleischmann E, Teal N, Dudley J, et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999. April;55(4):1560–7. [DOI] [PubMed] [Google Scholar]
- 30.Oreopoulos A, Padwal R, Kalantar-Zadeh K, et al. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008. July;156(1):13–22. [DOI] [PubMed] [Google Scholar]
- 31.Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol. 2014. November;29(11):801–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013. April;10(2):81–9. [DOI] [PubMed] [Google Scholar]
- 33.Hutagalung R, Marques J, Kobylka K, et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011. November;37(11):1793–9. [DOI] [PubMed] [Google Scholar]
- 34.Pickkers P, de Keizer N, Dusseljee J, et al. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013. August;41(8):1878–83 [DOI] [PubMed] [Google Scholar]
- 35.Utzolino S, Ditzel CM, Baier PK, et al. The obesity paradox in surgical intensive care patients with peritonitis. J Crit Care. 2014. October;29(5):887.e1–5 [DOI] [PubMed] [Google Scholar]
- 36.Di Angelantonio E, Bhupathiraju ShN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016. August 20;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014. January 16;370(3):233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan SS, Ning H, Wilkins JT, et al. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018. February 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepper DJ, Sun J, Suffredini AF, Kadri S. Body-mass index and all-cause mortality. Lancet. 2017. June 10;389(10086):2284. [DOI] [PubMed] [Google Scholar]
- 40.Prescott HC, Chang VW. Overweight or obese BMI is associated with earlier, but not later survival after common acute illnesses. BMC Geriatr. 2018. February 6;18(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christopher KB. The Body Mass Index Paradox. Crit Care Med. 2015. December;43(12):2693–4 [DOI] [PubMed] [Google Scholar]
- 42.Pichard C, Kyle UG, Morabia A, et al. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004. April;79(4):613–8 [DOI] [PubMed] [Google Scholar]
- 43.Pepper DJ. Adipose Tissue Measurements in Patients With Sepsis. Crit Care Med. 2017. March;45(3):e331. [DOI] [PubMed] [Google Scholar]
- 44.Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity (Silver Spring). 2007. July;15(7):1886–94 [DOI] [PubMed] [Google Scholar]
- 45.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013. October 16;310(15):1591–600 [DOI] [PubMed] [Google Scholar]
- 46.Weijs PJ, Looijaard WG, Dekker IM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Critical care (London, England). 2014;18(2):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elia M, Lunn PG. Biological markers of protein-energy malnutrition. Clin Nutr. 1997. March;16 Suppl 1:11–7. [DOI] [PubMed] [Google Scholar]
- 48.Combs GF Jr, Trumbo PR, McKinley MC, et al. Biomarkers in nutrition: new frontiers in research and application. Ann N Y Acad Sci. 2013. March;1278:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittwede PN, Clemmer JS, Bergin PF, et al. OBESITY AND CRITICAL ILLNESS: INSIGHTS FROM ANIMAL MODELS. Shock. 2016. April;45(4):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huttunen R, Laine J, Lumio J, et al. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC Infect Dis. 2007. March 9;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.