Abstract
The U.S. Army’s Standards of Medical Fitness, AR 40–501, state that “Prior burn injury (to include donor sites) involving a total body surface area of 40 percent or more does not meet the standard”. Inclusion of donor sites (sites harvested for skin grafts) in this Standard implies that thermoregulatory function is impaired within donor sites during exercise-heat stress; however, supporting evidence is currently lacking.
Purpose:
To test the hypothesis that well-healed donor and non-injured sites demonstrate similar elevations in skin blood flow and sweating during exercise-induced hyperthermia.
Methods:
Twenty burn survivors (>1 year post-injury; four females) cycled for 60 min in a 39.7 ± 0.3°C and 21.1 ± 3.3% relative humidity environment at ~50% of maximal aerobic capacity. Core and mean skin temperatures were recorded throughout exercise. Skin blood flow (laser-Doppler imaging) was measured at baseline and after exercise within donor (LDFDON) and adjacent non-injured control (LDFCON) sites. At 45 min of exercise, local sweat rates (Technical Absorbents) were measured within the same donor (LSRDON) and non-injured (LSRCON) areas.
Results:
After 60 min of exercise, core and skin temperatures reached 38.2 ± 0.4°C and 35.5 ± 1.2°C, respectively. The increase in skin blood flow from baseline to end-exercise (LDFDON: 91.6 ± 44.5 AU; LDFCON: 106.0 ± 61.6 AU; P = 0.17) and local sweat rates (LSRDON: 0.46 ± 0.26 mg cm−2 min−1; LSRCON: 0.53 ± 0.25 mg cm−2 min−1; P = 0.14) were not different between donor and non-injured control sites.
Conclusion:
Well-healed donor sites retain the ability to increase skin blood flow and sweating during exercise-heat stress, providing evidence against the inclusion of donor sites when determining whether a burn injury meets the Army’s Standards of Medical Fitness.
Keywords: sweat rate, skin blood flow, core temperature, burn injury, burn survivor
INTRODUCTION
Military personnel routinely perform strenuous physical activity in hot environmental conditions, making appropriate thermoregulatory function critical for successful duty performance. Soldiers with skin disorders that interfere with normal skin blood flow and sweating responses to exercise-heat stress, such as burn injuries, can impair physical performance (1) and increase the susceptibility to heat illness (2).
The U.S. Army’s Standards of Medical Fitness AR 40–501 (3) state that “Prior burn injury (to include donor sites) involving a total body surface area of 40% or more does not meet the standard.” The inclusion of donor sites in this standard implies that thermoregulatory function within donor sites is disrupted and should therefore be considered part of the burn injury when determining whether a burned soldier meets the Standard. Permanently suppressed or severely attenuated elevations in skin blood flow and local sweat rate within grafted skin areas are well-documented (4–10); however, there is presently no evidence indicating that donor sites exhibit impaired thermoregulatory function. In fact, two previous studies from our laboratory demonstrated similar cutaneous vasodilatory and local sweat rate responses within donor and an adjacent non-injured control sites (9, 10). Although the studies by Davis et al. (9, 10) challenge the inclusion of donor sites in the U.S. Army’s Standards of Medical Fitness, it should be noted that in those studies, whole-body passive heat stress was imposed via encapsulation in a water-perfused suit covering ~85% of body surface area while local cutaneous vascular and sweat rate responses were assessed from skin areas exposed to the surrounding thermoneutral environment. Whether donor and non-injured skin sites exhibit similar sweating and skin blood flow responses during combined exercise and environmental heat stress has not been investigated. The distinction between these sources of heat stress is of practical importance given that in training and operational settings, soldiers experience elevations in body temperature driven by both activity-related increases in metabolic heat production and high ambient temperatures.
The purpose of this investigation was to compare skin blood flow and sweating responses between a donor site and an adjacent non-injured control site in well-healed burn survivors during combined exercise and ambient heat stress. We hypothesized that the increase in skin blood flow and local sweat rate during exercise-induced hyperthermia and ambient heat stress would not be different between well-healed donor and non-injured control sites. This information will help determine if the donor sites of recruits and soldiers should be considered part of a burn injury in the Army’s Standards of Medical Fitness.
METHODS
Participants.
Twenty burn survivors with well-healed burn injuries (>1 y post-injury) and skin grafts covering at least 50% of those injuries were included in the study. Participant characteristics, including burn injury size, are presented in Table 1. Burn survivors were recruited from across North America, and testing was conducted throughout the year in Dallas, Texas. Prior to participation, a detailed medical history questionnaire was completed, and baseline heart rate/rhythm (via electrocardiogram) and blood pressure measurements were taken. Individuals taking medications known to negatively impact thermoregulatory and/or cardiovascular function were excluded. Participants were informed of the study procedures and potential risks before providing informed written consent. The study protocol was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center (STU 082015–030), Texas Health Presbyterian Hospital Dallas, and the Human Research Protections Office of the Defense Health Agency. All study procedures conformed to standards set forth in the Declaration of Helsinki.
Table 1.
Participant characteristics
| Characteristic | Mean ± SD | Range |
|---|---|---|
| Age (years) | 39 ± 11 | 22 – 55 |
| VO2max (L min−1) | 2.59 ± 0.63 | 1.57 – 3.89 |
| Mass (kg) | 80.7 ± 12.1 | 64.0 – 105.9 |
| Height (m) | 1.73 ± 0.09 | 1.46 – 1.85 |
| BSA (m2) | 1.94 ± 0.17 | 1.59 – 2.18 |
| Injury size | ||
| Total injury (%BSA) | 36.3 ± 16.2 | 12.6 – 65.9 |
| Grafted area (%BSA) | 29.8 ± 15.8 | 6.1 – 63.9 |
| Donor area (%BSA) | 16.9 ± 9.5 | 2.9 – 40.9 |
Values are means ± standard deviations for 20 subjects (16 male, 4 female). VO2max, maximal rate of oxygen uptake; BSA, total body surface area.
Measurements.
Body mass and standing height were measured using a precision balance with ± 10 g accuracy (Mettler Toledo, OH) and stadiometer (Detecto, Webb City, MO), respectively. BSA was then calculated using the DuBois equation (11). The sizes of donor and grafted skin sites were determined with the tablet-based Burn Medical Education application (BurnMed, Johns Hopkins University). Urine specific gravity (USG) was assessed via refractometer (Atago Inc., Bellevue, WA). Core temperature was recorded from the gastrointestinal tract using a telemetric pill ingested 2 h prior to testing (HQ Inc., Palmetto, FL) in all but one participant, for whom telemetric pill ingestion was contraindicated by prior gastrointestinal surgery. For this participant, core temperature was measured in the esophagus using a general-purpose thermocouple probe inserted ~40 cm beyond the external naris (Mon-a-therm, Mallinckrodt Medical, St. Louis, MO). Mean skin temperature was taken as a weighted average of local temperatures on the chest, upper back, lower back, abdomen, anterior thigh, and calf (12). Expired gases were analyzed using a metabolic cart (Parvo Medics TrueOne 2400, Sandy, UT), and indirect calorimetry was used to estimate metabolic rate (M) from the rate of oxygen uptake (VO2), the respiratory exchange ratio (RER), and the caloric equivalents for the oxidation of carbohydrate (ec, 21.12 kJ L−1 O2) and lipids (ef, 19.61 kJ L−1 O2) (13):
The external work rate was controlled using a semi-recumbent cycle ergometer (Lode Corival, Groningen, Netherlands). Metabolic heat production was calculated as the difference between metabolic rate and the external work rate and expressed per kilogram of total body mass (W/kg).
Skin blood flow and local sweat rates were measured within donor and non-injured skin sites immediately-adjacent to one another (Table 2). Measurements were performed in different body regions due to unavoidable between-subject heterogeneity in the location of donor sites (Table 2). Skin blood flow was indexed by laser-Doppler flux values measured simultaneously at donor (LDFDON) and non-injured (LDFCON) skin sites using a laser-Doppler imager (Perimed, Sweden) in 19/20 participants, and reported as the elevation from baseline to end-exercise. The LDF measurement area averaged 3.5 ± 2.8 cm2 and 3.7 ± 2.5 cm2 in non-injured and donor sites, respectively. Within the same skin areas used to assess skin blood flow, local sweat rates were assessed in donor (LSRDON) and non-injured (LSRCON) sites using absorbent sweat patches (Technical Absorbents, Grimsby, UK) according to established protocol (14) in 16/20 participants. The surface area of each absorbent patch was customized to the size of the donor and non-injured skin sites, averaging 13.7 ± 3.8 cm2 and 15.5 ± 5.3 cm2 in non-injured and donor sites, respectively. All patches included a 1.0 cm border to prevent contamination of the sample by the translocation of sweat from neighboring areas. Following instrumentation, patches (without borders) were placed in a sealed Ziploc bag and weighed using a precision scale to the nearest 0.1 mg (Mettler Toledo PM200, Toledo, OH). The patches and borders were then reassembled and placed back in the sealed bag. Approximately 1 min prior to measurement, the patches and borders were removed and assembled. The participant momentarily ceased pedaling, the application site was dried with paper towel, and then the absorbent patches were placed onto the target skin sites and secured with a tubular net bandage (Owens & Minor MediChoice, Mechanicsville, VA). After a 5-min sampling period, during which time the subjects had resumed exercise, the patches were removed from the skin surface, placed in their respective sealed bags without the borders, and reweighed. Local sweat rate at each site was calculated as the change in mass of the patch per square centimeter of absorbent patch surface area per minute of sampling time.
Table 2.
Locations of skin blood flow and local sweat rate measurements.
| Subject # | Sex | Measurement Location | Years Post-injury |
|---|---|---|---|
| 1 | M | Left thigh, anterior surface* | 12 |
| 2 | M | Lower left leg, medial surface* | 14 |
| 3 | M | Left thigh, anterior surface | 8 |
| 4 | M | Below axilla, left side | 14 |
| 5 | M | Lower right leg, medial surface | 7 |
| 6 | M | Left thigh, anterior surface | 13 |
| 7 | M | Right thigh, anterior surface | 7 |
| 8 | F | Left forearm, ventral surface | 20 |
| 9 | M | Left thigh, medial surface | 8 |
| 10 | F | Right thigh, anterior surface | 34 |
| 11 | M | Right thigh, anterior surface | 2 |
| 12 | M | Right scapula† | 1 |
| 13 | M | Torso, right side | 17 |
| 14 | M | Left thigh, anterior surface* | 7 |
| 15 | M | Left thigh, anterior surface* | 9 |
| 16 | F | Lower left leg, medial surface | 17 |
| 17 | F | Lower left leg, anterior surface | 20 |
| 18 | M | Lower left leg, medial surface | 45 |
| 19 | M | Lower left leg, lateral surface | 9 |
| 20 | F | Left thigh, lateral surface | 17 |
M, male; F, female
Indicates only skin blood flow values were measured.
Indicates only local sweat rates were measured.
Experimental Protocol.
On separate occasions, participants completed (i) an incremental test to exhaustion for the determination of their maximum rate of oxygen uptake (VO2max) using methods previously described (15), and (ii) an exercise test in the heat to assess thermoregulatory function within non-injured and donor skin sites. The two tests were separated by 24–72 hours. Prior to each experimental visit, participants were asked to avoid alcohol and strenuous exercise, and to consume a light meal and ~500 ml of water 2 h prior to arrival at the laboratory. A urine sample was collected upon arrival to determine USG, and euhydration was accepted if USG ≤ 1.025 (16). After a nude body mass measurement was taken, participants donned a standard clothing ensemble of cotton athletic shorts, socks, athletic footwear, and a sports bra for female participants. Following instrumentation, participants entered the climate chamber having conditions of 39.7 ± 0.3 °C and 21.1 ± 3.3% RH. The subjects remained seated on the ergometer for 30 min to equilibrate with the environment, after which baseline skin blood flow measurements were obtained. The participants then performed 60 min of cycling exercise at a target rate of metabolic heat production of 4.5 watts per kilogram of total body mass (W kg−1) approximating 50% VO2max, which is consistent with moderate-intensity military activities such as foot patrol (17). Drinking water, maintained at core body temperature, was provided ad libitum. Expired gases were analyzed for 10-min time periods beginning at 0, 25, and 50 min to confirm maintenance of the target rate of metabolic heat production. Local sweat rates were measured between 45 and 50 min of exercise. Immediately upon completion of exercise, final skin blood flow measurements were taken.
Data and Statistical Analyses.
Data were acquired at a sampling frequency of 25 Hz (Biopac MP150, Santa Barbara, CA). Core and mean skin temperature values represent 2-min average values at the relevant time points. A Shapiro-Wilk test was first used to screen data for normality. Paired-samples Student’s t-tests were then employed to compare thermoregulatory responses between donor and adjacent non-injured sites. Statistical analyses were performed using commercial software (GraphPad Prism 7.0, La Jolla, CA). Alpha was set at the 0.05 level. All data are reported as means ± standard deviation.
RESULTS
During exercise, the external work rate averaged 72 ± 12 W, yielding an average metabolic heat production of 4.6 ± 0.2 W kg−1 and a relative exercise intensity of 51.0 ± 10.1% of VO2max. Water intake averaged 351 ± 275 ml throughout the exercise bout. Core temperature increased by 0.9 ± 0.4°C from a baseline value of 37.3 ± 0.3°C to 38.2 ± 0.4°C by 60 min of exercise. Mean skin temperature increased slightly from 35.3 ± 0.9°C to 35.5 ± 1.2°C, averaging 35.4 ± 0.8°C during the 60-min exercise bout.
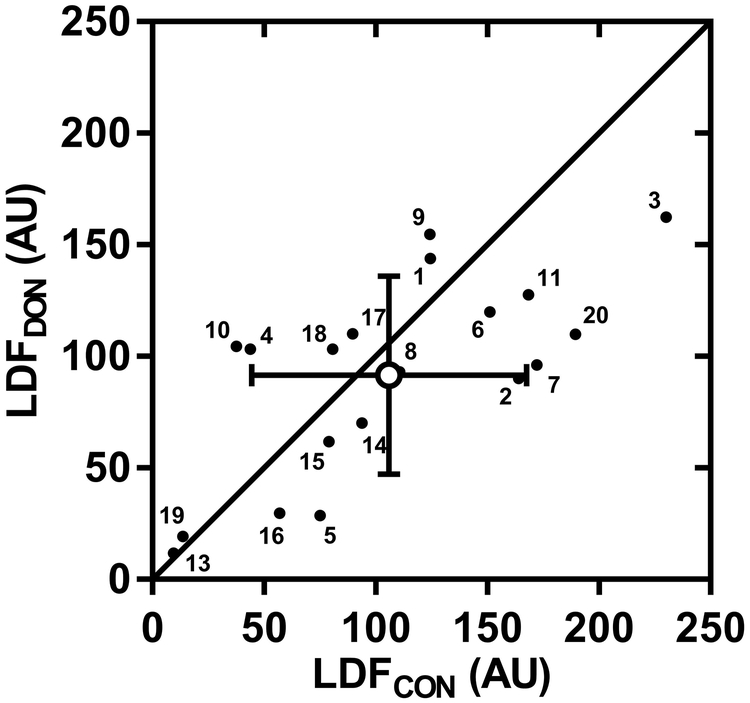
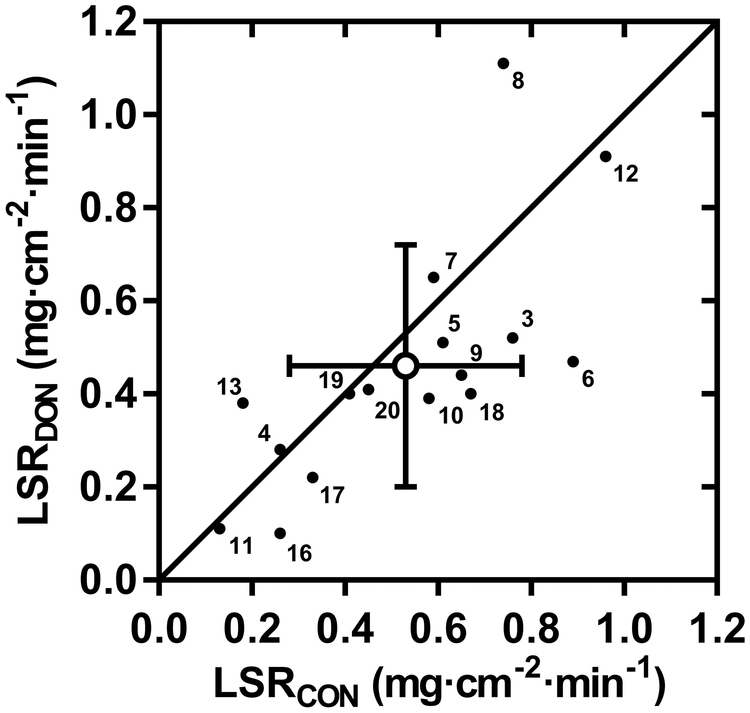
Individual and mean skin blood flow responses are presented in Fig. 1. Baseline LDFCON and LDFDON values were not different, averaging 62 ± 27 AU and 62 ± 31 AU, respectively (P = 0.99). The elevation in LDFCON and LDFDON from baseline was 106 ± 62 and 92 ± 45 AU, respectively, with no difference evident between sites (P = 0.17). Fig. 2 shows the individual and mean local sweat rate values within non-injured control and donor sites. Similar to the skin blood flow response, no difference in local sweat rate was observed between skin sites, with mean values for LSRCON and LSRDON of 0.53 ± 0.25 and 0.46 ± 0.26 mg cm−2 min−1, respectively (P = 0.14).
FIGURE 1—
Comparison of the elevation in skin blood flow within non-injured (LDFCON) and donor (LDFDON) skin sites from baseline to the end of a 60-min bout of exercise in the heat. Individual (n=19; small closed circles with participant number) and mean (± SD; large open circle) skin blood flow values are expressed as laser-Doppler flux in arbitrary perfusion units (AU).
FIGURE 2—
Comparison of local sweat rates within non-injured (LSRCON) and donor (LSRDON) skin sites during minutes 45 to 50 of exercise in the heat. Individual (n=16; small closed circles with participant number) and mean (± SD; large open circle) local sweat rate values are shown.
DISCUSSION
This investigation is the first to simultaneously assess thermoregulatory effector responses in donor and non-injured sites during exercise-heat stress among well-healed burn survivors. Our study revealed that the combination of prolonged moderate-intensity exercise in hot/dry ambient conditions evokes comparable elevations in skin blood flow (Fig. 1) and local sweat rates (Fig. 2) within a donor site and an adjacent non-injured control skin. Given the lack of any thermoregulatory impairment in donor sites, these findings suggest that donor sites should not be considered part of a burn injury when determining whether a U.S. Army soldier or recruit meets the Standards of Medical Fitness.
The present findings are in agreement with those of Davis et al. (9, 10), who demonstrated similar elevations in local skin blood flow and sweat rate within donor and non-injured control sites during whole-body heating. The principal distinctions between the current and prior investigations are the mode of heat stress and the environmental conditions to which the assessed sites were exposed. In the studies by Davis et al. (9, 10), heat loss responses from donor skin were assessed during passive whole-body heating using a water-perfused suit. Although this approach permits tight control of mean skin and core temperatures, the combination of externally-applied conductive heat gain and restricted evaporative heat dissipation by the garment leads to extreme elevations in mean skin temperature (~38°C) that are uncharacteristic of most exercise and/or environmental heat stress conditions. Additionally, encapsulation in a water-perfused suit permits the evaluation of only reflex heat loss responses from a skin area of lower surface temperature exposed to the thermoneutral laboratory environment. In the current study, heat stress was imposed through combined exercise (i.e., elevated metabolic heat production) and exposure to a high air temperature, the combination of which offers greater ecological validity in circumstances involving exercise-heat stress such as military training or operational settings (18). Core temperature increased by a magnitude similar to that reported by Davis et al. (+0.9°C from baseline) (9, 10); however, mean skin temperature was ~3°C lower in our study owing to greater evaporative heat dissipation and thus skin cooling afforded by the minimal clothing ensemble. Additionally, since thermoregulatory responses were measured from skin areas exposed to hot ambient conditions, observed skin blood flow and sweating responses represent both thermoregulatory reflex and direct local warming effects (19, 20). Notwithstanding these methodological differences between studies, both the current investigation and those of Davis et al. (9, 10) consistently observed elevations in skin blood flow and local sweat rates that were not different between donor and non-injured control sites.
The lack of a significant impairment in donor site thermoregulatory function may reflect an absence of any major post-surgical disruption to the anatomy and innervation of cutaneous arterioles and sweat glands within the donor site. Following a deep partial-thickness (2nd degree) or full-thickness (3rd degree) burn injury, devitalized skin is excised, and a skin graft is transplanted from a non-injured donor site onto the wound. Since first reported in 1942 (21), the vast majority of deep burn injuries have been treated with ‘split-thickness’ skin grafts (22, 23), consisting of only the epidermis and a partial thickness of the dermis (24–26). Although removal of these superficial skin layers disrupts the structure and innervation of remaining cutaneous vessels and sweat glands in the harvested split-thickness graft (24), skin remaining within the donor site retains most of its dermal layer, including deeper thermoregulatory end-organ structures such as the reticular plexus of cutaneous arterioles, the secretory coils and lower ducts of sweat glands, as well as the neural connections to those structures that are imperative for appropriate autonomic activation of heat loss responses (27, 28). As a result, normal elevations in skin blood flow and sweating would be expected in well-healed donor sites. The present findings are in line with this expectation (Fig. 1–2), and affirm that the structural integrity and innervation of thermoregulatory end-organs are largely retained in donor sites.
Despite statistically similar elevations in skin blood flow and local sweat rate between donor and non-injured control sites, smaller increases in skin blood flow and local sweat rate were observed in donor sites (Fig. 1–2). Sweat mapping studies with high spatial resolution of local sweat rates have observed median local sweat rate responses that differed by as much as 0.34 mg cm−2 min−1 between lateral and midline aspects of the torso in males and by as much as 0.58 mg cm−2 min−1 between immediately-adjacent lateral and midline aspects of the upper back in females exercising at 60% of VO2max under temperate environmental conditions (29–31). Additionally, high inter-site heterogeneity in local sweat rates was also observed in the lower limb, with differences in median local sweat rate values of up to 0.18 mg cm−2 min−1 between medial and mid-anterior segments of the thigh, and up to 0.26 mg cm−2 min−1 between medial and lateral aspects of the lower leg (30, 31). Although not as comprehensively characterized in the literature, intra-regional variation in skin blood flow has also been documented (32). In the present study, donor and non-injured control sites unavoidably spanned some of the aforementioned skin areas that exhibit high intra-segmental variability in local sweat rate, including the medial vs. lateral sides of the lower leg and thigh (Table 2). Therefore, the small differences in skin blood flow (14 AU) and local sweat rate (0.07 mg cm−2 min−1) observed between donor and non-injured site may simply reflect typical intra-segmental variation in thermoregulatory effector responses rather than permanent damage from the removal of skin grafts.
Perspectives
The present findings may contribute to an update of the U.S. Army’s Standards of Medical Fitness pertaining to burn survivors. Under the current Standard, recruits and soldiers with combined burned and donor skin areas ≥40% of TBSA, but having a burned area <40% of TBSA would be excluded from Army service. By omitting donor sites from the Standard, the Army may prevent the needless exclusion of recruits and personnel with burned and donor sites totalling ≥40% of TBSA, but burned areas of <40% of TBSA, without risking the physical performance and safety of these individuals during military activities. Given that burn injuries requiring medical intervention affect nearly 500,000 Americans annually (33), and have constituted up to 10% of casualties in military conflicts since World War II (34–37), such an update to the Standards of Medical Fitness may improve the recruitment and/or retention of otherwise qualified Army personnel with burn injuries.
Application of the present findings is not limited to military personnel with burn injuries. Numerous occupations with a high risk of burn injury also involve physical labor and/or exposure to hot conditions (e.g., firefighting, construction, electrical utilities). Additionally, exercise training constitutes an important part of long-term rehabilitation in burn survivors. For these individuals, clinicians should emphasize that the presence/absence of donor sites, and their size, are unlikely to contribute to the risk of heat-related exhaustion, illness, or injury during prolonged periods of physical activity and/or environmental heat stress.
Considerations
Our experimental conditions were limited to a single combination of moderate-intensity work, ambient temperature, and relative humidity, resulting in modest elevations in skin blood flow and local sweat rate. It is possible that greater exercise intensities and/or environmental temperatures yielding greater thermolytic requirements could unmask differences in the capacity to increase skin blood flow and sweating between donor and non-injured control sites. Although such an impairment would theoretically impact maximum whole-body heat dissipation and thereby exacerbate the increase in core temperature during prolonged exercise-heat stress, the effect would likely be marginal since donor sites typically constitute a small area of the body surface (38). Nevertheless, this is a possibility that deserves future examination.
For individuals with extensive burns, donor sites may be re-harvested for skin grafts following re-epithelialization of skin within the donor area (23, 39). It is possible that multiple harvesting procedures performed within the same donor site contribute to some of the variability in the observed sweating and skin blood flow responses. However, the burn survivors tested in the present study were unaware as to whether their donor sites had been harvested for skin grafts. Thus, a link between the number of harvesting procedures and the magnitude of the difference in heat loss responses between donor and non-injured control sites cannot be made based on the present data.
CONCLUSIONS
In burn survivors, well-healed donor sites retain the capability to increase skin blood flow and sweat rate during exercise-heat stress. Given the lack of any discernable thermoregulatory impairment within donor sites, these findings suggest that donor sites should not be included with the amount of burned skin when considering whether the burn injury of a recruit or active duty soldier meets the U.S. Army’s Standards of Medical Fitness.
ACKNOWLEDGEMENTS
This work was supported by the Department of Defense (W81XWH-15–1-0647 to CGC), a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship (to MNC), and the National Institutes of Health (R01GM068865–11S1 to CGC and GM). We are sincerely grateful to the volunteers who participated in this study, and to Amy Adams, Sarah Bailey, Manall Jaffery, Naomi Kennedy, and Kelly Lenz for their technical assistance with the study.
MNC and CGC were involved in conception and design of the experimental protocol. MNC, GM, and MH were responsible for data collection. Data analysis and interpretation were performed by MNC and CGC. MNC drafted the manuscript. All authors critically revised the manuscript and approved its final version.
Footnotes
Publisher's Disclaimer: Medicine & Science in Sports & Exercise® Published ahead of Print contains articles in unedited manuscript form that have been peer reviewed and accepted for publication. This manuscript will undergo copyediting, page composition, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered that could affect the content.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Shapiro Y, Epstein Y, Ben-Simchon C, Tsur H. Thermoregulatory responses of patients with extensive healed burns. J Appl Physiol. 1982;53:1019–22. [DOI] [PubMed] [Google Scholar]
- 2.Ganio MS, Schlader ZJ, Pearson J, et al. Nongrafted Skin Area Best Predicts Exercise Core Temperature Responses in Burned Humans. Med Sci Sports Exerc. 2015;47(10):2224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of the Army. Army Regulation 40–501. Standards of Medical Fitness. 2007.
- 4.Conway H Sweating Function in Transplanted Skin. Surg Gynec Obstet. 1939;69:756–61. [Google Scholar]
- 5.Ferguson JC, Martin CJ. A study of skin temperatures, sweat rate and heat loss for burned patients. Clin Phys Physiol Meas. 1991;12(4):367–75. [DOI] [PubMed] [Google Scholar]
- 6.Freund PR, Brengelmann GL, Rowell LB, Engrav L, Heimbach DM. Vasomotor control in healed grafted skin in humans. J Appl Physiol. 1981;51(1):168–71. [DOI] [PubMed] [Google Scholar]
- 7.McGibbon B, Beaumont WV, Strand J, Paletta FX. Thermal regulation in patients after the healing of large deep burns. Plast Reconstr Surg. 1973;52(2):164–70. [DOI] [PubMed] [Google Scholar]
- 8.Ponten B Grafted skin. Acta Chir Scand Suppl. 1960;257:1–78. [PubMed] [Google Scholar]
- 9.Davis SL, Shibasaki M, Low DA, et al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res. 2007;28(3):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis SL, Shibasaki M, Low DA, et al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res. 2009;30(4):675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuBois D, DuBois EF. A formula to estimate surface area if height and weight are known. Arch Intern Med. 1916;17:863–71. [Google Scholar]
- 12.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66(4):1586–92. [DOI] [PubMed] [Google Scholar]
- 13.Cramer MN, Jay O. Biophysical aspects of human thermoregulation during heat stress. Auton Neurosci Basic Clin. 2016;196:3–13. [DOI] [PubMed] [Google Scholar]
- 14.Morris NB, Cramer MN, Hodder SG, Havenith G, Jay O. A comparison between the technical absorbent and ventilated capsule methods for measuring local sweat rate. J Appl Physiol. 2013;114:816–23. [DOI] [PubMed] [Google Scholar]
- 15.Cramer MN, Jay O. Selecting the correct exercise intensity for unbiased comparisons of thermoregulatory responses between groups of different mass and surface area. J Appl Physiol. 2014;116(9):1123–32. [DOI] [PubMed] [Google Scholar]
- 16.Kenefick RW, Cheuvront SN. Hydration for recreational sport and physical activity. Nutr Rev. 2012;70 Suppl 2:S137–42. [DOI] [PubMed] [Google Scholar]
- 17.Sawka MN, Pandolf KB. Physical Exercise in Hot Climates: Physiology, Performance, and Biomedical Issues Medical Aspects of Harsh Environments. Falls Church, VA: Office of the Surgeon General, U.S. Army; 2002. p. 87–133. [Google Scholar]
- 18.Welles AP, Buller MJ, Richter MW, McCarthy S, Hoyt RW. Thermal-Work Strain and Energy Expenditure during Marine Rifle Squad Operations in Afghanistan (August 2013). Natick, MA: U.S. Army Research Institute of Environmental Medicine; 2015. [Google Scholar]
- 19.Nadel ER, Cafarelli E, Roberts MF, Wenger CB. Circulatory regulation during exercise in different ambient temperatures. J Appl Physiol. 1979;46:430–7. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol. 2014;4(1):33–89. [DOI] [PubMed] [Google Scholar]
- 21.Brown JB, McDowell F. Massive repairs of burns with thick split-skin grafts: Emergency “dressings” with homografts. Ann Surg. 1942;115(4):658–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crandall CG, Davis SL. Cutaneous vascular and sudomotor responses in human skin grafts. J Appl Physiol. 2010;109(5):1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wooldridge M, Surveyer JA. Skin Grafting for Full-Thickness Burn Injury. Am J Nurs. 1980;80(11):2000–4. [PubMed] [Google Scholar]
- 24.Ratner D Skin grafting. From here to there. Dermatol Clin. 1998;16(1):75–90. [DOI] [PubMed] [Google Scholar]
- 25.Skouge JW. Techniques for Split-Thickness Skin Grafting. J Dermatol Surg Oncol. 1987;13(8):841–50. [DOI] [PubMed] [Google Scholar]
- 26.Thornton JF. Skin Grafts and Skin Substitutes. Sel Reasings Plast Surg. 2004;10(1):1–23. [Google Scholar]
- 27.Shibasaki M, Wilson TE, Crandall CG. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol. 2006;100:1692–701. [DOI] [PubMed] [Google Scholar]
- 28.Smith CJ, Johnson JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton Neurosci Basic Clin. 2016;196:25–36. [DOI] [PubMed] [Google Scholar]
- 29.Havenith G, Fogarty A, Bartlett R, Smith CJ, Ventenat V. Male and female upper body sweat distribution during running measured with technical absorbents. Eur J Appl Physiol. 2008;104:245–55. [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, Havenith G. Body mapping of sweating patterns in athletes: a sex comparison. Med Sci Sports Exerc. 2012;44:2350–61. [DOI] [PubMed] [Google Scholar]
- 31.Smith CJ, Havenith G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur J Appl Physiol. 2011;111:1391–404. [DOI] [PubMed] [Google Scholar]
- 32.Tenland T, Salerud EG, Nilsson GE, Oberg PA. Spatial and temporal variations in human skin blood flow. Int J Microcirc Clin Exp. 1983;2(2):81–90. [PubMed] [Google Scholar]
- 33.American Burn Association. 2016 National Burn Repository: Report of Data from 2006–2015. Chicago, IL: 2016. [Google Scholar]
- 34.Cancio LC, Horvath EE, Barillo DJ, et al. Burn support for Operation Iraqi Freedom and related operations, 2003 to 2004. J Burn Care Rehabil. 2005;26(2):151–61. [DOI] [PubMed] [Google Scholar]
- 35.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma. 2003;54(5 Suppl):S13–19. [DOI] [PubMed] [Google Scholar]
- 36.Shafir R, Nili E, Kedem R. Burn injury and prevention in the Lebanon War, 1982. Isr J Med Sci. 1984;20(4):311–3. [PubMed] [Google Scholar]
- 37.Shirani K, Becker W, Rue L, Mason A Jr, Pruitt B Jr. Burn care during Operation Desert Storm. J US Army Med Dep. 1992;PB 8–92-1/2:37–9. [Google Scholar]
- 38.Pripotnev S, Papp A. Split thickness skin graft meshing ratio indications and common practices. Burns. 2017;43(8):1775–81. [DOI] [PubMed] [Google Scholar]
- 39.MacFarlane DF. Current techniques in skin grafting. Adv Dermatol. 2006;22:125–38. [DOI] [PubMed] [Google Scholar]