Abstract
The Hedgehog (Hh) family of morphogens direct cell fate decisions during embryogenesis and signal to maintain tissue homeostasis after birth. Hh ligands harbor dual lipid modifications that anchor the proteins into producing cell membranes, effectively preventing ligand release. The transporter-like protein Dispatched (Disp) functions to release these membrane tethers and mobilize Hh ligands to travel toward distant cellular targets. The molecular mechanisms by which Disp achieves Hh deployment are not yet fully understood, but a number of recent publications provide insight into the complex process of Hh release. Herein we review this literature, integrate key discoveries, and discuss some of the open questions that will drive future studies aimed at understanding Disp-mediated Hh ligand deployment.
Keywords: Dispatched, Hedgehog, Sonic Hedgehog, Cytonemes, Morphogen, Membrane trafficking
Hedgehog proteins at a glance
Hedgehog (Hh) family proteins are evolutionarily conserved morphogens that provide cells with positional information and fate instruction during early embryonic development. After development, Hh ligands contribute to tissue homeostasis and wound healing [1,2]. Consistent with these roles, developmental pathologies such as holoprosencephaly (HPE) (see Glossary) (Box 1) and Pallister Hall Syndrome, and cancers including medulloblastoma, basal cell carcinoma and rhabdomyosarcoma, can result from Hh pathway dysregulation [3–5]. Thus, a clear understanding of the molecular mechanisms driving release, delivery and interpretation of the Hh signal in both physiological and pathophysiological contexts is essential.
Box 1: Disp and Holoprosencephaly.
Holoprosencephaly (HPE) is a developmental syndrome in which the forebrain fails to separate into left and right cerebral hemispheres. It is a spectral disorder categorized into four classes of increasing severity: microform, lobar, semi-lobar, and alobar, which typically results in near complete midline failure (reviewed in [57]). Although the majority of HPE mutations are sporadic, some are familial, and are inherited in an autosomal-dominant manner. Pathology of affected individuals in a family harboring the same HPE mutation can range from asymptomatic to alobar. This variable etiology likely results from complex genetic interactions that are not yet well understood, as many genetic loci and developmental signaling pathways including Nodal, BMP, SHH and FGF are linked to HPE. SHH pathway genes contributing to HPE include SHH, PTCH1, GLI2, GAS1 and DISP1 [4].
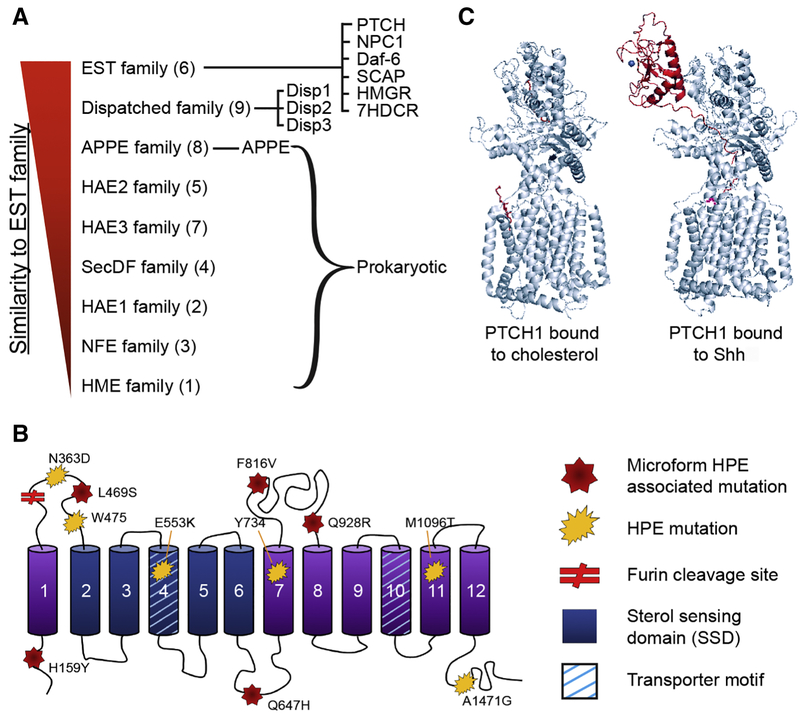
Consistent with its crucial role during forebrain development, SHH gene mutations account for approximately 17% of familial and 4% of sporadic HPE cases [57]. Although not a ‘major’ HPE gene like SHH, several DISP1 variants have been identified in patients with confirmed HPE diagnosis (Figure 1B). Although the majority of these alterations are thought to be benign, two identified truncation mutations, W475X and Y734X, which terminate in EC1 and carboxyl-terminal to TM6, do ablate Disp activity. These variants were identified in patients presenting with a milder microform HPE, manifesting as craniofacial malformation without forebrain alteration [58]. Compound heterozygous mutation of DISP1 (N363D and E553K), or DISP1 M1096T mutation in combination with a P347Q mutation in SHH were more recently identified in microform HPE [59]. How these specific mutations compromise Disp function is not clear, but effects may result from their proximity to established Disp regulatory domains. N363D localizes to the first EC loop carboxyl to the Furin cleavage site, and E553K localizes to TM4 of the SSD. M1906T falls within TM11, so could potentially affect activity by shifting TM topology. Threonine typically localizes to β-sheets, rather than α-helical domains, due to it having two non-hydrogen attachments at its C-beta carbon position. This additional volume at the Disp protein backbone could potentially alter a TM11 α-helix, thereby shifting TM positioning. Although this alteration may not be severe enough to impact Disp function in isolation, its deleterious effects when combined with SHH mutation reveal that minor perturbations to DISP physiology can have context-specific functional consequences.
The Hh gene was discovered in Drosophila melanogaster through a genetic screen aimed at identifying drivers of embryonic patterning [6]. Subsequent studies revealed vertebrates to have three Hh protein orthologs, referred to as Sonic Hh (Shh), Desert Hh and Indian Hh [7]. For the purposes of this review, we will refer to the Drosophila ligand as Hh, use Shh when discussing studies involving vertebrate Hh proteins, and Hedgehogs (Hhs) when referring to ligand release or transport processes shared between all Hh family members.
A unique aspect of Hhs that influences their cellular release and activity is that they are dually lipid modified by a long chain fatty acid on the amino-terminus, and by a covalently-linked cholesterol moiety at the carboxyl-terminus [8,9]. These hydrophobic lipid modifications behave as lipid anchors that tether Hhs to producing cell membranes. Because Hhs must signal to target cells that can be situated up to ~300 μm from their site of production in vertebrates, and ~50 μm in flies, cellular machinery capable of alleviating the lipid anchors is necessary [10,11]. A crucial component of this machinery is the transporter-like protein Dispatched (Disp), which is an evolutionarily-conserved pathway regulator that was first identified in Drosophila nearly 20 years ago [12]. The importance of Disp for Hhs deployment and morphogen gradient formation is supported by multiple genetic studies in both flies and vertebrates [12–16]. However, until recently, molecular mechanisms controlling Disp activity, and facilitating release of Hhs from producing cell membranes have remained unclear. A number of recent biochemical analyses of Disp, combined with cryo-EM studies of the structurally homologous Shh receptor Patched (Ptch), have begun to shed light on cellular processes that can impact Disp regulation and function. Herein we review these studies to summarize current understanding of how Disp facilitates release and distribution of Hh family members.
Dispatched Shapes the Hhs Morphogen Gradient
Cholesterol modification of Hh ligands is essential for establishment of their morphogen gradients, which provide both short and long-range signals across developing tissues [17–19]. Hhs lacking cholesterol are secreted from producing cells in an unregulated manner, leading to inappropriate distribution across target fields of cells [17]. The direct mechanism by which cholesterol contributes to Hhs morphogen gradients is unclear, but it is well established that cholesterol-modified Hhs require Disp to deploy for long-range signaling. In the absence of Disp function, Hhs fail to release from signal producing cells, and the morphogen gradients collapse (Box 2) [12,13]. Juxtacrine signaling to cells directly adjacent to an Hhs source can be maintained without Disp, but long-range targets do not receive ligand. Thus, cells situated far from the morphogen source fail to adopt appropriate fates, which corrupts tissue patterning and leads to early embryonic lethality. Disp knockout mice show overt left/right asymmetry defects and die around ~E9.5 [12–15]. These mice phenocopy animals lacking the Hh pathway signal transducing protein Smoothened (Smo), underscoring the importance of Disp for Shh pathway activity during early development.
Box 2: The Hedgehog Morphogen Gradient.
During embryogenesis, the organization of cells into tissues is controlled by signaling proteins called morphogens. Morphogen gradients form as developmental signaling proteins spread across fields of target cells. These gradients allow for cells within the signal-receiving field to receive distinct thresholds of ligand exposure relative to their distances from the morphogen source. Shh gradients form during early development and contribute to patterning of numerous tissues and organs. Cellular responses to Shh gradients have been studied extensively in the developing nervous system and limb bud, allowing for these tissues to serve as paradigms for how morphogen gradients are established and interpreted during organogenesis (reviewed in [60,61]).
In the developing nervous system, formation of the Shh gradient in the neural tube is initiated by ligand release from the notochord, which signals to the adjacent floor plate to express and secrete Shh throughout the ventral neural tube. Shh signals in a ventral to dorsal trajectory to regulate expression of distinct cross-repressive transcription factors in cells located at stereotypical distances from the floor plate. Each transcription factor specifies a distinct progenitor domain, which eventually gives rise to multiple classes of post-mitotic neurons [60]. Formation of this gradient is dependent upon Disp, as evidenced by a complete failure of the neural tube to organize in Disp knockout mice [13].
The long range Shh signal is crucial during limb bud development, and acts across a broad gradient to pattern the anterior-posterior axis of the tissue. Shh originates from the zone of polarizing activity (ZPA) in the posterior region of the limb bud, and signals toward the anterior to control digit identity [61]. Perturbation of the gradient in this developmental context can lead to duplication or loss of specific digits. Expression of a Shh protein lacking its cholesterol modification, which renders its release to be Disp-independent, results in pronounced expansion of the gradient and development of multiple ectopic digits [62]. Conversely, abbreviation of the Shh gradient due to Disp loss leads to loss of intermediate digit identities [63], underscoring the importance of Shh morphogen gradient control by Disp in this system.
Structural Homology and Functional Implications
Amino acid sequence analyses of Disp and the Shh receptor Ptch indicate that both proteins share structural homology with a family of bacterial efflux pumps that function in resistance, nodulation and division (RND) transporter complexes (Figure 1A) [12,13]. In bacteria, these antiporters work as trimers, and use the proton motive force to move a range of small molecule substrates across membranes [20]. A characteristic of such proteins is the topology of their twelve transmembrane (TM) domains, which are arranged into two pseudo-symmetrical halves, each containing six TM segments and one large extracellular globular domain (Figure 1B) [20,21]. Although the Disp structure has not yet been solved, recent cryo-electron microscopy (cryoEM) studies revealed that Ptch possesses stereotypical RND membrane topology, and closely resembles structures of the bacterial Aerobic Respiration Control Sensor Protein (ArcB) transporter and the vertebrate cholesterol transporter Niemann-Pick type C1 (NPC1) (Figure 1C) [22–25].
Figure 1: Disp homology and structural analysis.
A) A homology diagram showing the degree of similarity between various RND transporter families and the EST transporter family, which includes PTCH1 and NPC1. Most similar is at the top and least similar at the bottom. The RND transporter subfamily number is indicated in parenthesis to the right of the family name. All RND transporters except for the EST and Disp families are found in prokaryotic organisms. B) Structural diagram of Disp. Transmembrane domains are shown as cylinders. HPE-associated mutations of Disp are indicated with a red star if documented in microform HPE, or a yellow asterisk if in symptomatic HPE. Amino acid changes are indicated [59,64,65]. C) The structure of PTCH1 with cholesterol (red molecule), or in the presence of palmitoylated Shh (red protein). Structures were obtained from the protein structure data bank using structures provided from [22,24] and rendered in The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC..
Disp, Ptch and NPC1 all contain sterol sensing domains (SSD), which are unique to proteins that bind, transport or respond to cellular sterols [26]. In both Ptch and Disp the SSD spans TM2-TM6 and contains a conserved GxxDD motif in TM4 (Figure 1B). In bacterial RND efflux pumps, similar motifs work with a second motif in TM10 to coordinate protons and move substrate [20,27]. A second conserved GxxxD motif is present in Disp TM10, and when disrupted along with the TM4 motif, prevented release of Hh from ligand-producing cells. Thus, it was initially hypothesized that Disp would use a TM4/TM10 proton-binding mechanism similar to RND transporters to release Hh ligands [13]. However, more recent work demonstrated that while mutation of the TM4 motif was sufficient to impair Hh release, mutation of the TM10 GxxxD on its own was not [28]. As such, compromised function of the TM4/TM10 compound mutant may be the result of disrupted SSD function, rather than by blocking a TM4/TM10-mediated proton-driven transport activity.
Despite their predicted structural homology, sequence homology between Disp, Ptch and NPC1 is limited predominantly to their SSDs [26]. The recent Ptch structures support that, similar to NPC1, its SSD binds and potentially transports a sterol molecule (Figure 1C) [22–25]. Structural examination of Shh in complex with Ptch revealed the primary interface between Shh and Ptch to occur by the amino-terminal fatty acid modification of the ligand inserting into a channel formed between the two large extra-cellular domains (ECDs) of Ptch [22]. SSD mutations that block sterol binding to Ptch shifted conformation of the ECDs to compromise Shh association, potentially indicating that sterol loading into the SSD may impact fatty acid access to the ECD channel [22,24].
The role of the Disp SSD in Shh release is not yet clear. However, a recent biochemical interrogation suggested that the SSD may facilitate Disp-Shh binding by directly associating with the carboxyl-terminal cholesterol modification. To release ligand, Disp was hypothesized to transfer the sterol tag to the secreted glycoprotein Scube2 (Signal Peptide, CUB Domain, Epidermal Growth Factor-like protein 2), a vertebrate-specific Disp co-factor that significantly enhances Shh membrane extraction [29]. This model is supported by the ability of both Disp and Scube2 to individually co-immunoprecipitate with cholesterol-modified Shh, but not with a truncated ShhN molecule that lacks the carboxyl-terminal sterol. Further, Shh modified with photoactivatable cholesterol co-immunoprecipitated with Disp and Scube2 under denaturing conditions post-crosslinking, consistent with Shh being attached to each of the deployment proteins by sterol [29]. As such, Disp was proposed to transfer cholesterol-modified Shh to Scube2 in a manner analogous to how NPC1 transfers cholesterol to NPC2 [29,30]. Although this is a logical hypothesis, results from a second study of Disp and Scube2 indicated that the amino terminal fatty acid modification on Shh may be the Scube2-associated lipid [31]. Shh binding to Scube2 was not strictly dependent upon fatty acylation, but biochemical analysis of their association revealed that palmitoylated Shh bound Scube2 more tightly than a mutant lacking the palmitate moiety. Additionally, unmodified Shh was released from membranes by Disp and Scube2 more slowly than fatty acylated ligand, supporting a direct contribution of palmitate to Scube2-assisted membrane extraction [31]. These results suggest an alternative model in which the Disp SSD could bind the cholesterol modification, allowing Scube2 to collect Shh from Disp by binding the amino-terminal fatty acid. Given the recent discovery that free cholesterol binding to the Ptch SSD promotes Shh binding [22,24], it should also be considered that free cholesterol could bind the Disp SSD to similarly influence its ability to bind Hhs or transfer them to Scube2.
Dispatched Cleavage and Membrane Trafficking
How Disp is regulated in Shh-producing cells to control ligand deployment has, until recently, remained limited. Early studies examining Disp function in MDCK cells and Drosophila tissue suggested that it likely assembles into trimers, and localizes to the basolateral membrane of polarized cells to release ligand [32,33]. Deletion mutagenesis of Disp revealed that both localization and trimerization were dependent upon the intracellular carboxyl-terminal tail [32]. This suggests the carboxyl-terminal domain could function as a control point for Disp activity. However, post-translational modifications occurring within the tail have yet to be reported, making potential regulatory behaviors of the domain difficult to predict.
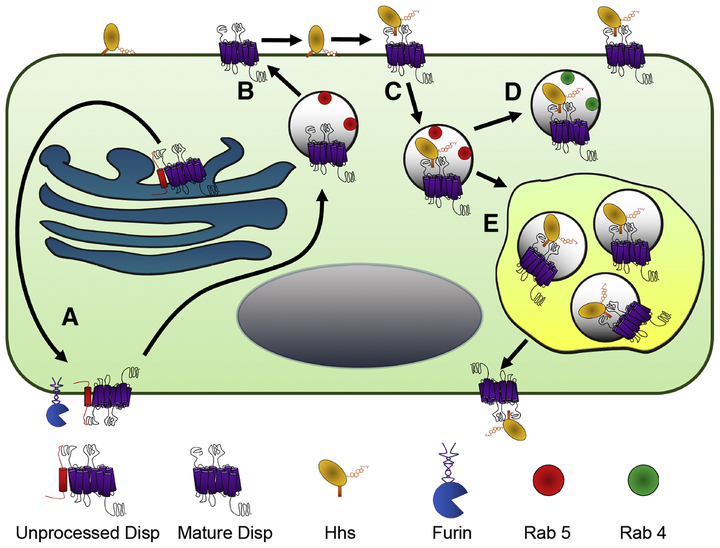
Based upon its predicted structural homology with Ptch, which binds Shh through its large EC loops, it is possible that Disp may also engage ligand through its EC domains [22,24,25,29]. The EC loops of Disp could therefore provide another potential control point for regulation of Hhs release. Consistent with this hypothesis, a conserved Furin cleavage site was recently identified in the predicted first EC loop of Disp (Figure 1B). Cleavage by Furin occurs on the cell surface to generate the ~150 kDa carboxyl-terminal functional domain, and to release a ~35 kDa fragment containing the first TM domain and a portion of EC1 (Figure 2A). Mutation of the cleavage site, or genetic loss of Furin, disrupted Disp cleavage and compromised Hhs release, confirming a functional link between Disp processing and functionality [34].
Figure 2: Models of Disp intracellular trafficking.
A) Newly synthesized Disp is trafficked to the basolateral surface of cells to be cleaved at the cell surface by Furin [32–34,66]. B) Cleavage is thought to allow for internalization of mature Disp with the early endosomal marker Rab5 [34], for eventual apical trafficking. C) Apical Disp may then associate with membrane tethered Hhs for reinternalization in Rab5-positive endosomes [33,38]. Disp/Hhs containing vesicles may then be, D) recycled back to the apical surface in Rab4 positive vesicles [38] for Hh release, or E) sent to the MVB for basolateral surface presentation [33,39].
The mechanism by which cleavage promotes Hhs release is not yet clear. One possibility is that cleavage governs Disp-Shh binding. However, wild type and cleavage-deficient Disp proteins bound Shh with similar efficiencies, indicating that cleavage is not a prerequisite for ligand association [34]. The ability of Disp to form large molecular weight complexes was also unaffected by cleavage site mutation, making Furin-mediated Disp processing unlikely to control self-association and/or potential binding partner engagement.
A behavior of Disp that was observed to be altered by cleavage disruption was its membrane trafficking. Examination of subcellular localization of wild type and cleavage-compromised Disp proteins in vivo in Drosophila revealed a change from a predominantly basal and basolateral membrane enrichment of the wild type protein to a uniform distribution of the cleavage site mutant throughout all cellular membrane domains [34]. Cleavage-compromised Disp showed reduced colocalization with the early endosomal marker Rab5, suggesting that Furin-mediated cleavage may guide membrane trafficking by controlling Disp endocytosis and/or membrane retargeting [34]. This possibility is supported by in vivo studies in Drosophila salivary glands. Whereas co-expression of Hh-GFP with wild type Disp in salivary gland cells depleted GFP signal from the tissue, cleavage deficient Disp co-expression led to ligand retention on salivary gland cell membranes [34]. Moreover, overexpression of wild type Disp in Drosophila wing imaginal discs triggered anterior wing over-growth, suggesting potentiation of the long-range signal. Over-expression of non-cleavable Disp did not induce wing over-growth, suggestive of attenuated Hh release. As such, Furin processing is crucial for Disp to effectively deploy Hh to elicit long-range effects in vivo [34].
Whether Disp is cleaved by additional proteases has not yet been reported. Biochemical and genetic studies suggest a role for protein sheddases in Hhs release, raising the possibility that Disp could undergo further processing by this class of proteases. However, in these studies, Hh ligands were found to be the substrates for the sheddases. The sheddases clipped the ligands’ membrane-embedded cholesterol and palmitate modifications to free Hhs from their lipid membrane tethers [35–37]. The role of Disp in this model is still unclear. It could function by situating Hhs in the membrane such that sheddases can access the ligands’ lipid modifications. It is also possible that Disp may assist by recruiting Scube2, which is proposed to aid in ligand release by influencing Hhs interaction with sheddase proteins [35,36]. Future investigations are needed to determine whether Disp is also clipped by sheddases, and to reconcile the proposed release of unlipidated Hhs with the role of the amino-terminal palmitate during Ptch binding [22–25].
Disp-Mediated Hh Membrane Recycling
Due to its cholesterol modification, Drosophila Hh enriches on sphingolipid-rich apical membranes of polarized epithelial cells [33,38,39]. Genetic studies examining Hh release from wing imaginal disc epithelia suggest that Hh must be endocytosed from the apical cell surface in a Disp-dependent manner prior to its eventual release for long range signaling (Figure 2B–C) [33,38]. Based upon an observed sub-apical colocalization with the early endosomal marker Rab5, Disp was proposed to collect Hh from the cell surface and into recycling endosomes (Figure 2C). Expression of a dominant negative Rab5 mutant blocked Hh endocytosis, and decreased long-range target gene expression, consistent with Disp-mediated endosomal recycling being required for long-range signaling activity [33]. The effects of Disp were specific for endocytosis of Hh because internalization of the endocytic reporters FM-64 and dextran were unaffected by Disp loss. Hh was also found to localize to Rab4-positive recycling endosomes, further supporting that Hh undergoes membrane recycling as part of its release mechanism (Figure 2D) [38]. How Disp selectively promotes Hh membrane recycling is not currently understood, but one logical hypothesis is that Disp might couple Hh to endocytic machinery. Unfortunately, due to a lack of information regarding cellular Disp binding partners, prediction of candidates for Disp-mediated endocytic coupling is not currently feasible.
The ability of Disp to prompt Hh internalization was lost upon mutation of the conserved transporter motifs in TM4 and TM10 [33]. This result could suggest that Disp either 1) uses the proton motive force to promote Hh recycling, or 2) the TM4 SSD is required for recycling to occur. The second hypothesis may be suggested by function of another SSD family member, the endoplasmic reticulum (ER) cholesterol monitor SCAP. Cholesterol membrane depletion prompts SCAP to transport SREBP transcription factors from the ER to Golgi [40]. The SCAP response to changing sterol levels is dependent upon its SSD, which releases SCAP protein from Insig ER tethers upon sensing sterol reduction [26,40]. Insig release allows SCAP to associate with COPII proteins to load SREBP into budding, Golgi-bound vesicles [41]. It is tempting to speculate that the Disp SSD might respond to a specific sterol environment surrounding apically-localized Hh to load ligand into recycling endosomes for membrane cycling and subsequent release.
Although genetic support for Disp-mediated Hh membrane recycling in Drosophila is relatively clear, why Hh re-internalization is required for Disp to release the long-range signal is not. Over-expression studies in cultured cells suggest that Hhs are deployed on lipoproteins, so it is possible that endosomal recycling could allow for Disp-mediated loading of ligand onto these complexes [42]. More recent experimental evidence indicates that Hhs are released from producing cells in exovesicles in both flies and vertebrates, raising the possibility that Disp-controlled recycling facilitates Hhs exovesicle packaging (Figure 2E) [39,43–45]. Immuno-electron microscopy revealed Disp and Hh-positive punctae in multivesicular bodies (MVB) and exovesicles along basolateral membranes in Drosophila epithelia [39]. Disp was therefore hypothesized to initiate internalization of Hh from apical membranes to redirect it basolaterally for exovesicle-mediated release.
An alternative model posits that rather than directing Hh basally, Disp recycles Hh back to the apical membrane to be released for long range signaling. In a study supporting this model, expression of dominant negative Rab4 drove accumulation of Hh basolaterally and reduced long-range target gene induction, suggesting that Hh cannot deploy from basolateral membranes for long-range transport [38]. Nevertheless, both studies agree that Hh must package into exovesicles and undergo Disp-dependent membrane recycling for long-range signaling to occur [33,38,39,43]. Further investigation will be needed to clarify whether Disp preferentially directs Hh for apical or basolateral membrane release through the recycling process.
Biochemical and genetic studies indicate that the endosomal sorting complex required for transport (ESCRT) machinery is crucial for Hh exovesicle packaging and morphogen gradient function [39,43,44]. In vertebrates, ESCRT proteins promote release of Shh exovesicles to maintain progenitor cell pools during brain development [44]. In flies, knockdown of ESCRT or exovesicle proteins reduced long-range Hh signaling activity, consistent with an evolutionarily conserved role for ESCRT in Hhs release [39,43]. Imaging of Drosophila wing imaginal discs revealed punctae containing both Disp and Hh on the surface of extracellular exovesicles (Figure 3A) [39]. These exovesicles purified from cultured Drosophila cells were competent to activate Hh reporter gene expression when provided exogenously, indicating that vesicular Hh is operative for signaling [39]. Although effects of Disp loss on Hh exovesicle localization were not tested, its presence with ligand inside the structures argues for its involvement in this release mechanism.
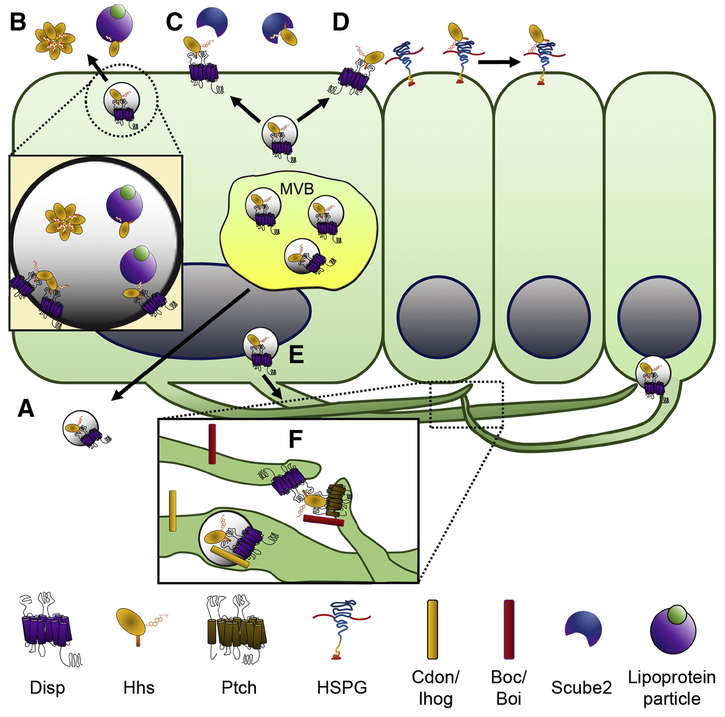
Figure 3, Key Figure: Models of Disp mediated Hhs release.
A) Disp/Hh positive exovesicles originating from MVBs are secreted from the basolateral surface of cells and spread across tissue [33,39]. B) Disp/Hhs positive exovesicles targeted to the apical surface of cells may provide a conducive environment for oligomerization of Hhs to form multimers, or allow for the hand off to lipoprotein particles for effective secretion into the extracellular space [38,42,46,47]. C) Disp transfers Shh to Scube2 for release from the producing cell [29,31,67]. D) Disp distributes Hhs by interactions with HSPGs allowing for the passage of Hhs across neighboring cell surfaces [48–50]. E) Disp/Hh exovesicles, which may also contain the Hh co-receptor Ihog, are loaded into cytonemes and transported to cytoneme tips for release to target cells [39]. F) Cytonemes containing Disp, Hhs, and co-receptors may pass Hhs to Ptch at receiving cell cytoneme tips [49,51,54].
Disp Transports Hhs on Cytonemes
How Disp directs Hh family ligands to establish their morphogen gradients and initiate long range responses remains a topic of significant debate. Early models proposed that Hh family morphogen gradients formed by free diffusion, and that Disp might function to package Hh molecules into multimers that could shield its lipid modifications from the aqueous extracellular environment (Figure 3B) [19,46]. Chaperone assisted diffusion and cell-to-cell movement, in which Hh sequentially shuttles along neighboring cell membranes via Heparan Sulfate Proteoglycans (HSPGs), have also been proposed as possible distribution mechanisms (Figure 3C–D) [42,47–50]. In such cases, the contribution of Disp would likely be limited to transferring Hhs from producing cell membranes to extracellular chaperones or adjacent cell HSPGs (Figure 3C–D).
More recently, evidence has accumulated in support of a model in which Hhs-producing cells actively distribute morphogen along specialized filopodia called cytonemes (Figure 3E–F). These actin-based cellular extensions contain Disp and the Hh co-receptors Cdon/Ihog and Boc/Boi, and provide a conduit upon which Hhs can be transported far from their site of synthesis [28,33,39,51–53]. Disp and Hh containing exovesicles are found along cytonemes that reach across basal sections of Drosophila wing imaginal discs (Figure 3E–F) [39]. In addition, cytonemes containing the Hh receptor Ptch have been documented to extend from signal receiving cells in both fly and vertebrate systems. These Ptch-containing extensions connect with signal-producing cell cytonemes to receive Hhs across cytoneme tips through what might function as a ‘morphogenetic synapse’ (Figure 3F) [49,53–55].
Recent studies performed using both cultured cells and Drosophila imaginal discs supports an active role for Disp in controlling cytoneme dynamics. Disp over-expression increased cytoneme occurrence rates, and its knockdown reduced occurrence [28,33]. How Disp promotes cytoneme occurrence is not yet clear. However, Disp-expressing cells showed slower cytoneme retraction rates than non-expressing cells, raising the possibility that Disp might influence actin dynamics or filopodial tip adhesion molecules to increase cytoneme durations [28]. Disruption of actin nucleation in Drosophila by knockdown of the formin protein Diaphanous shortened cytonemes and disrupted long-range signaling, indicating that targeting the actin cytoskeleton could be a feasible mechanism to control cytoneme activity [52,54,55]. As such, determination of the intermediaries facilitating communication between Disp and actin regulators will likely be an important step toward understanding cytoneme contribution to Hh morphogen gradient formation.
Concluding Remarks
One of the most perplexing questions in Hh biology is how a dually lipid-modified protein can exit the membrane of a ligand-producing cell and navigate the extracellular milieu to establish a morphogen gradient. Although the discovery of Disp was a significant advance toward answering this question, the precise molecular mechanism(s) by which it facilitates ligand deployment have remained unclear. Because of this, a number of seemingly incompatible models for Hhs release have emerged. However, with insights gained through continued biochemical, cell biological and genetic interrogations of Disp, we are poised to adjust the model and reconcile discordant observations regarding Hhs mobilization (see Outstanding Questions).
It is clear that Hhs release does not occur through a classical transport mechanism, but is instead a complex process that is influenced by Disp protein cleavage, membrane trafficking, cofactor collaboration and cytoneme mobilization. As such, it is unlikely that a single molecular mechanism is operative during Hhs release and morphogen gradient establishment in all cell types and tissue contexts. Whereas tissues patterned through broad Hhs gradients might require multiple deployment systems to drive morphogenesis, tissues patterned predominantly by juxtacrine signaling or shorter gradients might require only one. In some contexts, apically localized Hhs might engage in Disp-independent juxtacrine signaling through sheddase-mediated release or HSPG association. In other contexts, long-range signaling might be governed by Disp cleavage promoting Hhs localization to basolateral membranes for exosome loading and cytoneme-based transport. Future studies are needed to clarify whether differing routes of Hhs deployment can occur through distinct molecular mechanisms functioning in coordination, and to determine whether different Disp post-translational modifications or binding partners contribute to route selection.
Detailed examination of Disp function in cytonemes will be crucial for understanding how it actively contributes to and reinforces Hhs morphogen gradients. Determination of Disp structure will be necessary for understanding its molecular mechanism(s) of action during gradient establishment. Structural studies will likely provide insights into function of the Disp SSD, reveal how Furin cleavage impacts Disp activity, and may also define the basis of Disp collaboration with Scube2. Structural studies could also provide information about how deleterious HPE disease mutations compromise Disp activity (Box 1).
In addition to narrowing a wide knowledge gap in Hh developmental biology research, improved understanding of Disp structure and function will likely also be relevant to cancer. A growing body of evidence supports that Shh facilitates tumor-stroma communication in a range of cancers to influence tumor growth (reviewed in [56]). The ability of Disp to modulate cytoneme dynamics or deployment route selection might therefore be exploited by tumors to enhance such communication. Thus, delineation of the molecular mechanisms by which Disp facilitates Hh family ligand transport during tissue morphogenesis may reveal novel opportunities for therapeutic intervention against Shh-secreting tumors.
Highlights.
The recently solved structure of the Shh receptor Ptch may provide insight into how Disp binds and releases Hh family ligands.
Disp is activated by Furin-mediated processing.
The secreted glycoprotein Scube2 collaborates with Disp during Shh deployment.
Disp directs endosomal recycling of Hh to facilitate release of the long-range signal.
Disp localizes to, and can modulate activity of cytonemes to affect Hh delivery to target cells.
Outstanding Questions.
Will Disp show structural homology to Ptch and NPC1? Will structural analysis reveal the role of the Disp SSD?
How does Disp cleavage promote Hhs release?
How does Disp control Hh membrane trafficking?
What is the molecular mechanism by which Disp and Scube2 extract lipid modified Shh from producing cell membranes?
Are there additional Disp functional partners promoting Shh deployment?
How does Disp influence cytoneme-mediated Hhs distribution across developing tissues?
Does Disp modulate cytoneme-based Shh delivery during tumor-stroma communication?
Does Disp have therapeutic potential for targeting tumor-stroma communication?
Acknowledgements:
This work was supported by National Institutes of Health Grant R35GM122546 (SKO) and by ALSAC of St. Jude Children’s Research Hospital. We thank members of the Ogden lab for comments on the manuscript.
Glossary:
- Aerobic respiration control sensor protein (ArcB)
prokaryotic efflux pump and a member of the resistance nodulation cell division superfamily.
- Coatomer II (COPII)
coats membrane-bound transport vesicles from the endoplasmic reticulum to the Golgi apparatus.
- Cryogenic electron microscopy (Cryo-EM)
technique used for protein structure determination.
- Dispatched (Disp)
Discovered in 1999, a 12-transmembrane protein responsible for hedgehog release from producing cells.
- Extracellular domain (ECD)
portion of membrane-bound proteins external to the cell interior.
- Endosomal sorting complexes required for transport (ESCRT)
cellular machinery that remodels membranes, and are essential for membrane bending, budding, and vesicle transport.
- Holoprosencephaly (HPE)
Developmental disorder in which separation of the forebrain into left and right hemispheres is affected.
- Insulin induced gene 1 (INSIG)
binds to SCAP/SREBP complex to retain SCAP in the ER.
- Madin-Darby Canine Kidney cells (MDCK)
isolated from epithelial cells of the kidney tubule of an adult Cocker Spaniel, used in molecular biology studies for cell polarity and cell adhesion.
- Multivesicular bodies (MVB)
specialized endosomes that contain membrane-bound vesicles in their lumen, and can function as a vesicle trafficking hub in cells.
- Niemann-Pick disease, type C1 (NPC1)
membrane-bound protein that is responsible for intracellular cholesterol trafficking.
- Patched (Ptch)
functions as the receptor for Hhs and is predicted to share structural homology with Disp.
- Ras-related protein 4 or 5 (Rab4 and Rab5)
small GTPase proteins that localize to endosomes and regulate endosomal trafficking.
- Resistance nodulation cell division (RND)
A superfamily of transporters that transports substrates across membranes.
- Sterol regulatory element-binding protein cleavage-activating protein (SCAP)
an escort protein which is required for cholesterol homeostasis; shuttles SCAP/SREBF complex from the ER in response to decreased membrane cholesterol.
- Signal peptide, CUB and EGF-like domain-containing protein 2 (SCUBE2)
aides in Shh release from the membrane and trafficking to receiving cells.
- Smoothened (Smo)
class F G protein-coupled receptor that functions as the signal transducer of the Hh pathway.
- Sterol regulatory element-binding proteins (SREBP)
transcription factors that bind sterol regulatory elements.
- Sterol-sensing domain (SSD)
transmembrane component of some membrane proteins capable of binding or responding to sterols.
- Transmembrane (TM)
a domain within a membrane-bound protein that spans the lipid bilayer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ingham PW and McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 [DOI] [PubMed] [Google Scholar]
- 2.Jiang J and Hui C-C (2008) Hedgehog signaling in development and cancer. Dev. Cell 15, 801–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barakat MT et al. (2010) Learning from Jekyll to control Hyde: Hedgehog signaling in development and cancer. Trends Mol. Med 16, 337–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MM Jr. (2010) Hedgehog signaling update. Am. J. Med. Genet. Part A 152A, 1875–1914 [DOI] [PubMed] [Google Scholar]
- 5.Hill P et al. (2007) The molecular basis of Pallister Hall associated polydactyly. Hum. Mol. Genet 16, 2089–2096 [DOI] [PubMed] [Google Scholar]
- 6.Nüsslein-Volhard C and Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801 [DOI] [PubMed] [Google Scholar]
- 7.Echelard Y et al. (1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–30 [DOI] [PubMed] [Google Scholar]
- 8.Porter JA et al. (1996) Cholesterol modification of hedgehog signaling proteins in animal development. Science 274, 255–9 [DOI] [PubMed] [Google Scholar]
- 9.Pepinsky RB et al. (1998) Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem 273, 14037–45 [DOI] [PubMed] [Google Scholar]
- 10.Strigini M and Cohen SM (1997) A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124, 4697–705 [DOI] [PubMed] [Google Scholar]
- 11.Wang B et al. (2000) Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–34 [DOI] [PubMed] [Google Scholar]
- 12.Burke R et al. (1999) Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99, 803–15 [DOI] [PubMed] [Google Scholar]
- 13.Ma Y et al. (2002) Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell 111, 63–75 [DOI] [PubMed] [Google Scholar]
- 14.Kawakami T et al. (2002) Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development 129, 5753–65 [DOI] [PubMed] [Google Scholar]
- 15.Caspary T et al. (2002) Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr. Biol 12, 1628–32 [DOI] [PubMed] [Google Scholar]
- 16.Tian H et al. (2004) Dose dependency of Disp1 and genetic interaction between Disp1 and other hedgehog signaling components in the mouse. Development 131, 4021–4033 [DOI] [PubMed] [Google Scholar]
- 17.Li Y et al. (2006) Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc. Natl. Acad. Sci. U. S. A 103, 6548–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrero I and Chiang C (2007) A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trends Cell Biol. 17, 1–5 [DOI] [PubMed] [Google Scholar]
- 19.Gallet A et al. (2003) Cholesterol modification of hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to hedgehog. Dev. Cell 4, 191–204 [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi A et al. (2015) Structural basis of RND-type multidrug exporters. Front. Microbiol 6, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viljoen A et al. (2017) The diverse family of MmpL transporters in mycobacteria: from regulation to antimicrobial developments. Mol. Microbiol 104, 889–904 [DOI] [PubMed] [Google Scholar]
- 22.Qi X et al. (2018) Structures of human Patched and its complex with native palmitoylated sonic hedgehog. Nature 560, 128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi X et al. (2018) Two Patched molecules engage distinct sites on Hedgehog yielding a signaling-competent complex. Science (80-.). 362, eaas8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong X et al. (2018) Structural basis for the recognition of Sonic Hedgehog by human Patched1. Science (80-.). 361, eaas8935. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y et al. (2018) Structural Basis for Cholesterol Transport-like Activity of the Hedgehog Receptor Patched. Cell 175, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuwabara PE and Labouesse M (2002) The sterol-sensing domain: multiple families, a unique role? Trends Genet. 18, 193–201 [DOI] [PubMed] [Google Scholar]
- 27.Tseng TT et al. (1999) The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol 1, 107–25 [PubMed] [Google Scholar]
- 28.Bodeen WJ et al. (2017) A fixation method to preserve cultured cell cytonemes facilitates mechanistic interrogation of morphogen transport. Development 144, 3612–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tukachinsky H et al. (2012) Dispatched and Scube Mediate the Efficient Secretion of the Cholesterol-Modified Hedgehog Ligand. Cell Rep. 2, 308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon HJ et al. (2009) Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137, 1213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creanga A et al. (2012) Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes Dev. 26, 1312–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etheridge LA et al. (2010) Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development 137, 133–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callejo A et al. (2011) Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl. Acad. Sci. U. S. A 108, 12591–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart DP et al. (2018) Cleavage activates dispatched for Sonic Hedgehog ligand release. Elife 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobs P et al. (2016) Bridging the gap: heparan sulfate and Scube2 assemble Sonic hedgehog release complexes at the surface of producing cells. Sci. Rep 6, 26435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakobs P et al. (2017) Ca 2+ coordination controls sonic hedgehog structure and its Scube2-regulated release. J. Cell Sci 130, 3261–3271 [DOI] [PubMed] [Google Scholar]
- 37.Ohlig S et al. (2011) Sonic Hedgehog Shedding Results in Functional Activation of the Solubilized Protein. Dev. Cell 20, 764–774 [DOI] [PubMed] [Google Scholar]
- 38.D’Angelo G et al. (2015) Endocytosis of Hedgehog through Dispatched Regulates Long-Range Signaling. Dev. Cell 32, 290–303 [DOI] [PubMed] [Google Scholar]
- 39.Gradilla A-C et al. (2014) Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat. Commun 5, 5649. [DOI] [PubMed] [Google Scholar]
- 40.Yang T et al. (2002) Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 110, 489–500 [DOI] [PubMed] [Google Scholar]
- 41.Sun L-P et al. (2005) Insig Required for Sterol-mediated Inhibition of Scap/SREBP Binding to COPII Proteins in Vitro. J. Biol. Chem 280, 26483–26490 [DOI] [PubMed] [Google Scholar]
- 42.Panáková D et al. (2005) Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435, 58–65 [DOI] [PubMed] [Google Scholar]
- 43.Matusek T et al. (2014) The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature 516, 99–103 [DOI] [PubMed] [Google Scholar]
- 44.Coulter ME et al. (2018) The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep. 24, 973–986.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vyas N et al. (2015) Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci. Rep 4, 7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng X et al. (2001) A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411, 716–720 [DOI] [PubMed] [Google Scholar]
- 47.Vyas N et al. (2008) Nanoscale Organization of Hedgehog Is Essential for Long-Range Signaling. Cell 133, 1214–1227 [DOI] [PubMed] [Google Scholar]
- 48.Eugster C et al. (2007) Lipoprotein-Heparan Sulfate Interactions in the Hh Pathway. Dev. Cell 13, 57–71 [DOI] [PubMed] [Google Scholar]
- 49.González-Méndez L et al. (2017) Cytoneme-mediated cell-cell contacts for Hedgehog reception. Elife 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsbottom S and Pownall M (2016) Regulation of Hedgehog Signalling Inside and Outside the Cell. J. Dev. Biol 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders TA et al. (2013) Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 497, 628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischoff M et al. (2013) Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat. Cell Biol 15, 1269–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kornberg TB (2014) Cytonemes and the dispersion of morphogens. Wiley Interdiscip. Rev. Dev. Biol 3, 445–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen W et al. (2017) Essential basal cytonemes take up Hedgehog in the Drosophila wing imaginal disc. Development 144, 3134–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roy S et al. (2014) Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein. Science (80-.). 343, 1244624–1244624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skoda AM et al. (2018) The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. basic Med. Sci 18, 8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng X and Oliver G (2009) Pathogenesis of holoprosencephaly. J. Clin. Invest 119, 1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kauvar EF and Muenke M (2010) Holoprosencephaly: recommendations for diagnosis and management. Curr. Opin. Pediatr 22, 687–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mouden C et al. (2016) Complex mode of inheritance in holoprosencephaly revealed by whole exome sequencing. Clin. Genet 89, 659–668 [DOI] [PubMed] [Google Scholar]
- 60.Dessaud E et al. (2008) Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489–503 [DOI] [PubMed] [Google Scholar]
- 61.Tickle C and Towers M (2017) Sonic Hedgehog Signaling in Limb Development. Front. Cell Dev. Biol 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y et al. (2006) Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc. Natl. Acad. Sci 103, 6548–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harfe BD et al. (2004) Evidence for an Expansion-Based Temporal Shh Gradient in Specifying Vertebrate Digit Identities. Cell 118, 517–528 [DOI] [PubMed] [Google Scholar]
- 64.Roessler E et al. (2009) The mutational spectrum of holoprosencephaly-associated changes within the SHH gene in humans predicts loss-of-function through either key structural alterations of the ligand or its altered synthesis. Hum. Mutat 30, E921–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kantarci S et al. (2010) Characterization of the chromosome 1q41q42.12 region, and the candidate gene DISP1, in patients with CDH. Am. J. Med. Genet. A 152A, 2493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simmen T et al. (1999) Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol. Cell. Biol 19, 3136–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson J-LFA et al. (2012) Scube activity is necessary for Hedgehog signal transduction in vivo. Dev. Biol 368, 193–202 [DOI] [PubMed] [Google Scholar]