Abstract
Background And Objectives:
Alloimmunization is common following transfusion with platelet rich plasma (PRP) and can cause complications such as platelet refractoriness or transplant rejection. It has previously been shown that pathogen reduction of PRP with riboflavin and UV light (UV+R) can protect against alloimmunization in mice and induce partial tolerance to subsequent transfusions.
Materials and Methods:
Using B6 H2d congenic mice, this study evaluated the relative contributions of major histocompatibility (MHC) antigens and minor antigens to both the alloresponse to PRP transfusion and the partial tolerance induced by UV+R treatment.
Results:
Both total and MHC specific alloantibody responses were highest when both MHC and minor antigens were mismatched, with lower alloantibody responses observed with MHC mismatch alone, demonstrating that allogeneic minor antigens can enhance the response to allogeneic MHC. There was a weak, but significant alloantibody response to minor antigens only. UV+R treatment protected against both major and minor antigen alloimmunization. Both allogeneic MHC and minor antigens primed an enhanced cytokine response ex vivo, though this was weaker with minor antigens, and both responses were blocked with UV+R treatment.
Conclusion:
Allogeneic MHC is both necessary and sufficient to induce the partial tolerance associated with UV+R treatment.
Keywords: Alloimmunization, MHC, minor antigens, pathogen reduction, tolerance
INTRODUCTION:
Alloimmunization is a common outcome of platelet transfusion and can result in negative outcomes for recipients such as refractoriness to subsequent transfusions or rejection of transplants.[1–3] Measured rates of alloantibody generation vary among study populations, but range from 7–55% for antibodies against major histocompatibility (MHC) antigens,[1, 4–11] and between 0–2% for antibodies against human platelet antigens (HPA).[12–15] While platelets express low levels of class I MHC antigens, high levels of MHC antigens can be found on white blood cells (WBCs), and leukoreduction of platelet products has been shown to significantly reduce, though not eliminate, the risk of alloimmunization to MHC antigens.[4, 6, 8–11, 16]
Previous work has demonstrated that pathogen reduction of platelet products with riboflavin and UVB light (UV+R), a treatment designed to reduce the risk of transfusion-transmission of infectious agents, can also efficiently block alloimmunization in animal models.[17–19] In vitro work with human peripheral blood mononuclear cells (PBMCs) demonstrated that the UVB treatment results in rapid cell death, slight reductions in surface HLA and costimulatory molecule expression, massive downregulation of surface adhesion molecules, and failure to form productive conjugates with allogeneic T cells, blocking mixed lymphocyte reactions.[20, 21]
In addition to protecting against alloimmunization to the UV+R treated products themselves, transfusion with UV+R treated platelet rich plasma (PRP) modulates responses to subsequent exposures of untreated PRP, inducing a partial tolerance that is both antigen specific and dependent on the presence of WBCs in the treated product.[17] This tolerance effect, while not reducing antibody levels, diminishes cytokine responses from alloantigen-stimulated splenocytes. Cells treated with various doses and wavelengths of UV light were shown to induce tolerance both clinically and in some animal models. Extracoporeal photochemotherapy, in which autologous blood is treated with psoralen (a photoactivator) and UVA light, has been used clinically to down-modulate damaging immune responses associated with various autoimmune diseases or graft-versus-host disease.[22–24] Repeated infusion of allogeneic PBMCs treated with low-dose UVB light has been shown to induce donor specific humoral tolerance that can be transferred to naïve mice via CD4+ CD25+ T cells.[25, 26]
The antigens driving the partial tolerance induction with UV+R PRP have not been defined. Herein, we utilized congenic B6.C-H2d/bByJ (B6 H2d) mice expressing BALB/c MHC on a C57BL/6 background as donors into both BALB/cJ (BALB/c) and C57BL/6J (B6) recipients to evaluate if allogeneic MHC antigens, minor antigens, or both are required for partial tolerance induction, as well as to evaluate the role of different antigens in the response to allogeneic platelet transfusion.
MATERIALS AND METHODS:
Mice
Female BALB/c and B6 mice ages 8–12 weeks were used as recipients, and male and female BALB/c, B6, or B6 H2d mice aged 2–6 months were used as donors. All mice were purchased from the Jackson Laboratory (Bar Harbor, Maine), and B6 H2d mice were bred on site at Vitalant Research Institute under barrier conditions in a specific-pathogen-free vivarium. All research was performed with approval and oversight of the Institutional Animal Care and Use Committee at Covance Laboratories, Inc. (San Carlos, CA) under Animal Welfare Assurance A3367–01.
Transfusions
PRP was prepared as previously described.[17] WBCs from buffy coats were added back to the PRP yielding a mean 5.7×106 WBCs/mL and 4.8×108 platelets/mL. Pathogen reduced PRP was treated with the Mirasol pathogen reduction system (Terumo BCT, Lakewood, CO).[17] Transfusions consisted of 100μL freshly prepared PRP administered via tail vein.
Antibody screening
Serum was collected 2 weeks after final transfusion and antibodies were screened by flow cytometry as previously described[17, 19] using BALB/c, B6, or B6 H2d splenocytes as target cells. Median fluorescence intensity (MFI) values were normalized by dividing all MFIs by the mean MFI of the non-transfused controls for that experiment.
Cytokine screening
Spleens were collected 2 weeks after final transfusion. WBCs were obtained by mincing, straining, light density separation, and washing. Cells were cultured for 48 hours in the presence of mitomycin C-treated donor type stimulator cells (either BALB/c, B6, or B6 H2d, as indicated) as previously described.[17] Culture supernatants were screened for cytokines using a custom Milliplex Map Mouse Cytokine/Chemokine 13-plex containing IFN-γ, GM-CSF, MCP-1, MIP-1β, TNF-α, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, and IL-13 as previously described.[17]
Statistical analysis
Experiments were repeated two times with data pooled between paired experiments, and 5 mice per group per experiment. Groups were compared by ANOVA with Tukey’s post-test for comparisons between groups accounting for multiple comparisons. Analysis was done in Prism Version 7.0c (GraphPad Software, Inc., La Jolla, CA).
RESULTS:
To determine if allogeneic MHC is sufficient to induce both transfusion alloimmunization and the partial tolerance induction observed with pathogen reduction in our mouse model, congenic B6 H2d mice were used as donors into B6 recipients. These mice have the H2d MHC loci of BALB/c mice on a B6 background, so when transfused into B6 mice, the only alloantigens in the blood products are derived from the MHC region (Figure 1A). B6 recipients were given no transfusion, an untreated B6 PRP transfusion (syngeneic negative control), an untreated BALB/c PRP transfusion (full allogeneic positive control), an untreated B6 H2d PRP transfusion, a pathogen reduced B6 H2d PRP transfusion, or a pathogen reduced B6 H2d PRP transfusion followed 2 weeks later by an untreated B6 H2d PRP transfusion (Figure 1B). Two weeks after final transfusion, blood and spleen were collected, with antibodies screened from the serum and cell cultures setup with splenocytes for cytokine production.
Figure 1. Allogeneic minor antigens enhance the antibody response to allogeneic MHC.
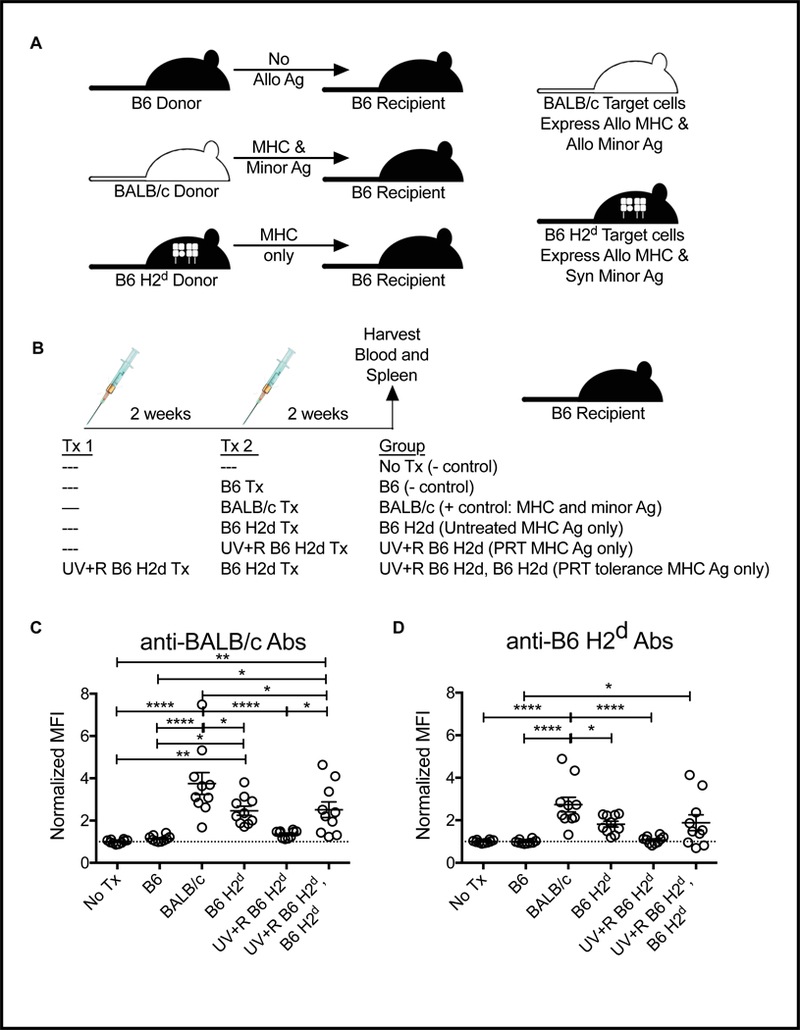
(A) Schematic of genetic differences between donor and recipient. (B) Schematic of experimental groups: B6 recipient mice received either no transfusion (No Tx) or were given a single transfusion with untreated PRP from syngeneic B6 donors, untreated PRP from fully allogeneic BALB/c donors (where both MHC and minor antigens are allogeneic), or PRP from congenic B6 H2d donors (BALB/c MHC on B6 background, only MHC alloantigens present) with or without pathogen reduction with UV+R. The final group was given a first transfusion of UV+R PRP from B6 H2d donors followed 2 weeks later by second transfusion with untreated B6 H2d PRP. Two weeks after last transfusion, serum from recipients was screened for antibodies against (C) BALB/c target cells (antibodies against both MHC and minor antigen) and (D) B6 H2d target cells (antibodies against MHC only) by flow cytometry. *p<0.05, **p<0.01, ****p<0.0001.
Antibodies against both BALB/c target cells (MHC and minor alloantigens) and B6 H2d target cells (MHC alloantigens only) were evaluated. Antibodies against BALB/c were highest in mice receiving BALB/c transfusions, but those receiving B6 H2d PRP also had a significant, though slightly reduced, antibody response against BALB/c antigens, demonstrating that MHC alone is sufficient to drive an alloantibody response, but that minor antigens contribute to the alloantibody response (Figure 1C). Pathogen reduction of the PRP with riboflavin and UV light blocked the alloantibody response to B6 H2d PRP, but did not block alloantibody responses to subsequent exposure (Figure 1C). Surprisingly, the same pattern was observed using B6 H2d target cells to measure the anti-MHC antibody response directly, with mice transfused with BALB/c PRP making stronger anti-MHC antibody responses than mice receiving B6 H2d PRP (Figure 1D). Anti-MHC antibodies were blocked with pathogen reduction, and exposure to pathogen reduced PRP did not block anti-MHC antibody responses to subsequent untreated PRP transfusion (Figure 1D).
To evaluate priming of cells in vivo to allogeneic MHC and minor antigens or allogeneic MHC antigens alone, splenocytes from the recipient mice described above (Figure 1B) were cultured with either BALB/c or B6 H2d stimulator cells, and cytokine production was measured. Production of IFN-γ, GM-CSF, and IL-10 are shown in Figure 2A, and IL-4, IL-5, and IL-13 in Figure 2B. Unlike what was seen with antibody production, similar responses were observed with the BALB/c and B6 H2d stimulations, and no significant differences were detected between the mice transfused with BALB/c and B6 H2d PRP, suggesting that MHC, not minor antigens, are the dominant contributors to the T cell alloresponse. Consistent with our earlier work with a complete donor mismatch, transfusion of UV+R treated B6 H2d PRP blocked cytokine priming following transfusion, and also blocked responses to subsequent untreated PRP transfusion, demonstrating that allogeneic MHC alone is sufficient to induce the partial tolerance associated with pathogen reduction.
Figure 2. Allogeneic MHC is sufficient for PRT induced tolerance.
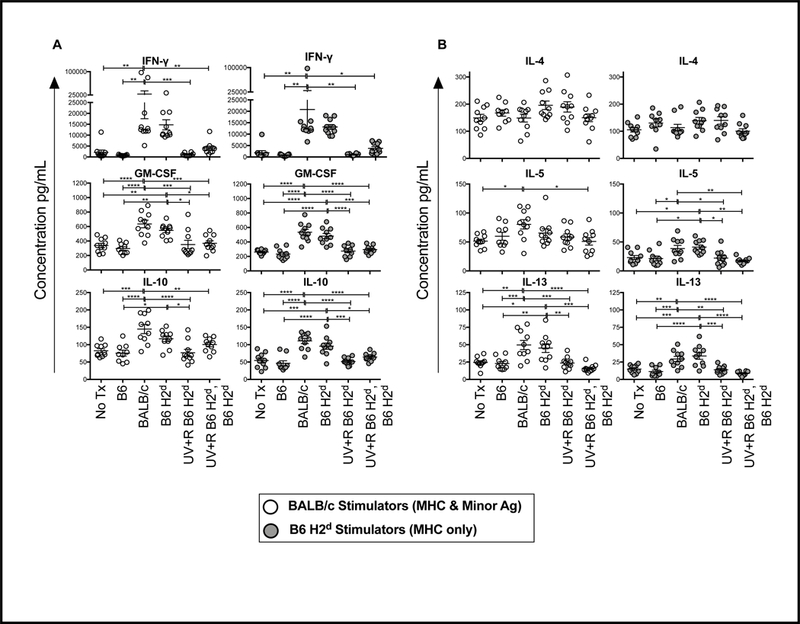
Splenocytes from mice described in Figure 1 were collected 2 weeks after final transfusion and cultured with mitomycin C treated splenocytes from BALB/c (open circle) or B6 H2d (grey filled circle) mice for 48 hours. Culture supernatants were collected and screened for cytokines. (A) IFN-γ, GM-CSF, IL-10, (B) IL-4, IL-5, and IL-13 are plotted as examples. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
To determine if allogeneic MHC is necessary to induce both transfusion alloimmunization and the partial tolerance induction observed with pathogen reduction in our mouse model, congenic B6 H2d mice were used as donors into BALB/c recipients. As these 2 strains both carry the H2d MHC loci, but on 2 different backgrounds, the only alloantigens in the blood products are non-MHC minor antigens (Figure 3A). BALB/c recipients were given no transfusion, an untreated B6 PRP transfusion (full allogeneic positive control), an untreated B6 H2d PRP transfusion, a pathogen reduced B6 H2d PRP transfusion, or a pathogen reduced B6 H2d PRP transfusion followed 2 weeks later by an untreated B6 H2d PRP transfusion (Figure 3B). Blood and spleen were collected 2 weeks after final transfusion to screen for antibodies and cell priming.
Figure 3. Transfusion of allogeneic minor antigens induces a small alloantibody response that is blocked with PRT.
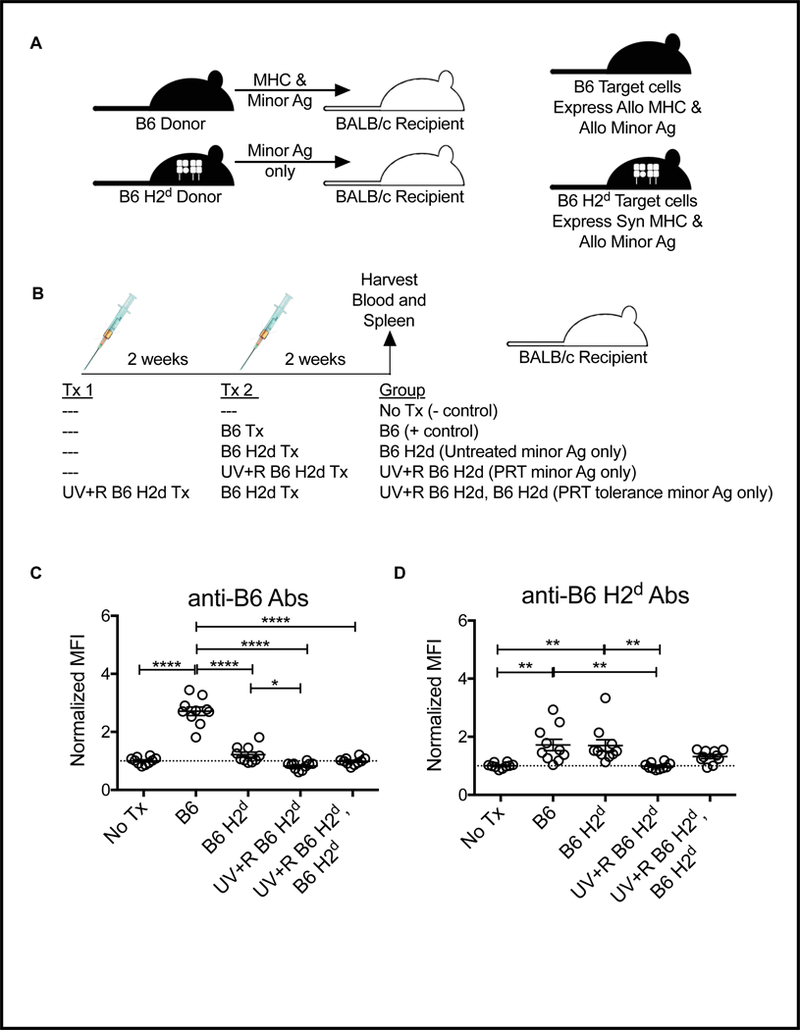
(A) Schematic of genetic differences between donor and recipient with different donor types. (B) Schematic of experimental groups: BALB/c recipient mice received either no transfusion (No Tx) or were given a single transfusion with untreated PRP from fully allogeneic B6 donors (where both MHC and minor antigens are allogeneic), or PRP from congenic B6 H2d donors (BALB/c MHC on B6 background, only minor alloantigens present) with or without pathogen reduction with UV+R. The final group was given a first transfusion of UV+R PRP from B6 H2d donors followed 2 weeks later by second transfusion with untreated B6 H2d PRP. Two weeks after last transfusion, serum from recipients was screened for antibodies against (B) B6 target cells (antibodies against both MHC and minor antigen) and (C) B6 H2d target cells (antibodies against allogeneic minor antigens only) by flow cytometry. *p<0.05, **p<0.01, ****p<0.0001.
Antibodies against both B6 target cells (MHC and minor alloantigens) and B6 H2d target cells (minor alloantigens only) were evaluated. As expected, BALB/c mice receiving B6 PRP transfusions mounted a robust anti-B6 antibody response, but those receiving an untreated B6 H2d PRP transfusion did not have significantly increased antibodies against B6 target cells compared with non-transfused controls (Figure 3C). There was, however, a weak, but significant increase observed in the recipients of untreated B6 H2d PRP compared with recipients of UV+R treated B6 H2d PRP, and no detectable antibodies in the group receiving UV+R treated B6 H2d PRP followed by untreated B6 H2d PRP (Figure 3C). In contrast, when B6 H2d target cells were used, a weak, but significant antibody response was detected against B6 minor antigens in recipients of either the B6 or B6 H2d PRP transfusions when compared to non-transfused controls, and there was no significant difference between these 2 groups (Figure 3D). Treatment of the PRP with UV+R blocked this antibody response, but recipients of UV+R treated B6 H2d PRP followed by untreated B6 H2d PRP did not differ significantly from any of the other groups (Figure 3D).
To evaluate priming of cells in vivo to allogeneic MHC and minor antigens or allogeneic minor antigens alone, splenocytes from the recipient mice described above (Figure 3A) were cultured ex vivo for 48 hours with either B6 or B6 H2d stimulator cells, and cytokines were measured in culture supernatants. As with experiments described above, IFN-γ, GM-CSF, and IL-10 (Figure 4A), and IL-4, IL-5, and IL-13 (Figure 4B) responses are plotted. When B6 stimulator cells were used, primed responses were only detected in the group transfused with untreated B6 PRP (full mismatch). In contrast, stimulation with B6 H2d cells induced production of IFN-γ, GM-CSF, and IL-10 by the groups transfused with either untreated B6 or B6 H2d PRP (Figure 4A). These secondary responses to allogeneic minor antigens were roughly equivalent to the primary responses against allogeneic MHC (see non transfused group stimulated with B6 cells), but were significantly higher than the non-transfused control group (Figure 4A). For IFN-γ and IL-10 there was no difference between the groups receiving B6 versus B6 H2d PRP, though levels of GM-CSF were slightly higher in B6 recipients (Figure 4A). While UV+R treatment blocked cell priming to allogeneic minor antigens in the primary response, the group receiving UV+R treated B6 H2d PRP followed by untreated B6 H2d PRP did not exhibit tolerance to exposed allogeneic minor antigens (Figure 4A). Interestingly, when stimulated with B6 minor antigens only, elevated levels of the TH2 cytokines IL-4, IL-5, and IL-13 were seen in the group transfused with untreated B6 PRP, but not the group receiving untreated B6 H2d PRP (Figure 4B).
Figure 4. Allogeneic MHC is necessary for PRT induced tolerance.
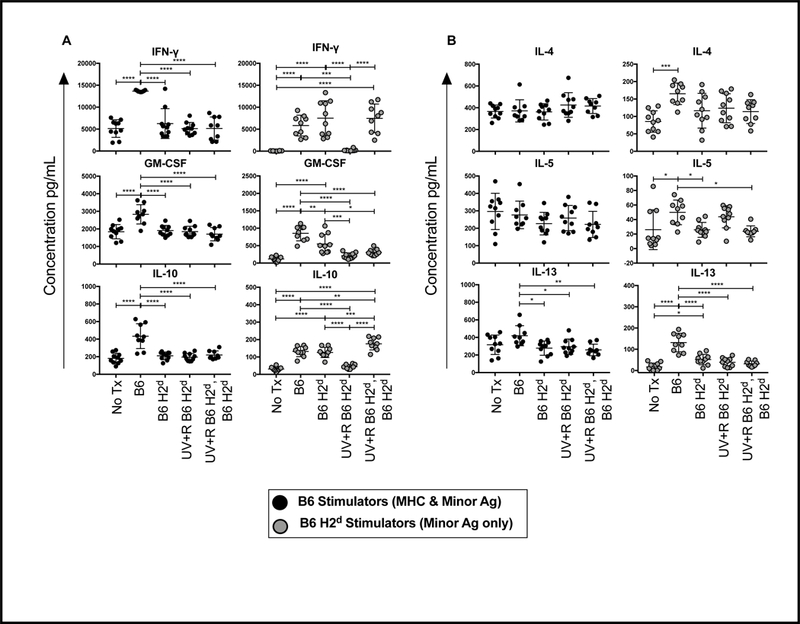
Splenocytes from mice described in Figure 3 were collected 2 weeks after final transfusion and cultured with mitomycin C treated splenocytes from B6 (black filled circle) or B6 H2d (grey filled circle) mice for 48 hours. Culture supernatants were collected and screened for cytokines. (A) IFN-γ, GM-CSF, IL-10, (B) IL-4, IL-5, and IL-13 are plotted as examples. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
DISCUSSION:
We demonstrate that while transfusion of allogeneic minor antigens can induce both antibody and cellular immune responses, allogeneic MHC is both necessary and sufficient for induction of partial tolerance with transfusion of UV+R treated PRP. Pathogen reduction with UV+R was able to block alloimmunization directed at both MHC and minor alloantigens. Furthermore, we have shown that while the response to allogeneic minor antigens following transfusion is relatively small compared with the response to allogeneic MHC, the presence of allogeneic minor antigens boosts the antibody response targeting allogeneic MHC. Results are summarized in Figure 5, which plots all antibodies and cytokines screened as fold changes over non-transfused controls for each experimental setup.
Figure 5. Global changes by group and stimulation type.
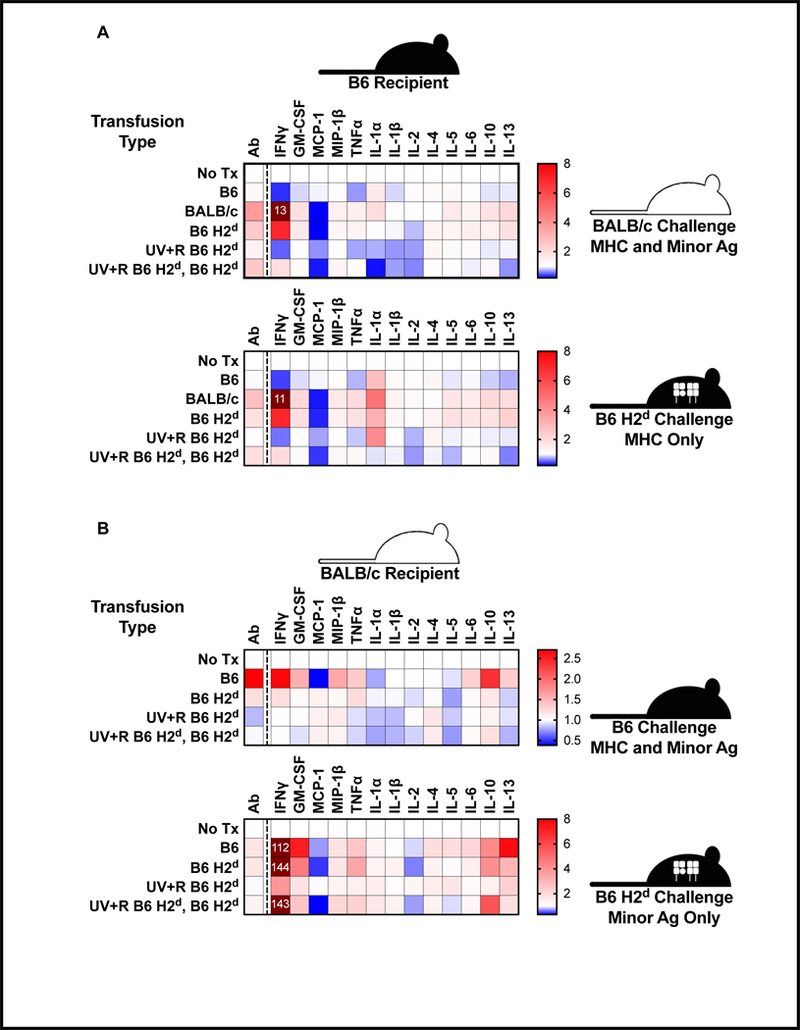
For each antibody or cytokine measurement, values for each animal were divided by the mean value for non-transfused controls, and the means of these fold changes values are plotted as heat maps. Values exceeding the upper limit of the map range labeled with fold-change values. (A) The experiments using B6 recipients are plotted for BALB/c target/stimulatory cells (allogeneic MHC and minor antigens) in the upper panel and B6 H2d target/stimulatory cells (allogeneic MHC only) in the lower panel. (B) The experiments using BALB/c recipients are plotted for B6 target/stimulatory cells (allogeneic MHC and minor antigens) in the upper panel and B6 H2d target/stimulatory cells (allogeneic minor antigens only) in the lower panel.
Prevention of alloimmunization with UV+R pathogen reduction has yet to be observed in the clinical setting, and it remains to be seen if the protective effects observed in our mouse model will apply to human transfusion. A recent randomized clinical trial did evaluate alloimmunization risk with and without UV+R treatment, but was underpowered, and was unable to detect a difference between groups.[27] There are major challenges in evaluating this question in clinical trials, including the already reduced risks associated with leukoreduction, the variability in the immunogenicity of particular donor recipient MHC combinations, and perhaps most importantly, the administration of other untreated allogeneic blood products (such as red cells) along with treated platelets. It is reassuring, however, that in vitro, treated human PBMCs show an almost complete loss of immunogenicity.[20, 21]
MHC antigens are named for their potent allogeneic stimulatory capacity, and are critically important alloantigens in transfusion and transplantation. Much of their potency as alloantigens stems from their unique ability to directly stimulate high frequencies of T cells via T cell receptor binding. This direct presentation may play a role in the tolerance induction observed in our model. Earlier work in vitro demonstrated that pathogen reduction with UV+R led to impaired conjugate formation with allogeneic responder cells due to down-regulation of adhesion molecules on the cell surface.[20] While we never detected tolerance induction in vitro, it is possible that weak or more transient T cell interactions with treated cells could result in tolerance induction in vivo. Class I MHC is expressed on both WBCs and platelets, and while expression on platelets is much lower than on WBCs, platelet numbers are approximately 2 logs higher than WBC numbers in our transfused PRP.[28] Both are sources of class I MHC alloantigen, though not necessarily equivalent. An earlier UV tolerance model found that humoral tolerance to MHC could be induced in mice by transfusion of UVB treated WBCs, but that the presence of platelets or plasma interfered with this tolerance induction.[25] In our model, transfusion with untreated leukoreduced platelets was insufficient to induce ex vivo cytokine priming even with multiple transfusions, suggesting that alloantigens on WBCs may be more important for inducing T cell responses.[17, 19]
The enhanced anti-MHC response observed when allogeneic minor antigens are present was interesting as the inverse was not observed, that is, the antibody response directed towards minor antigens was equivalent in the presence and absence of allogeneic MHC. One explanation is that peptides derived from the allogeneic minor antigens may be presented by the allogeneic MHC antigens, leading to conformational changes that increase the immunogenicity of these MHC antigens. Allorecognition by the innate immune system may also help to explain the enhanced antibody response. SIRP1α polymorphisms between donor and recipient that impact binding to CD47, for example, have been shown to impact DC infiltration into the graft in a different transplant model, driving innate allorecognition, with the polymorphism associated with BALB/c leading to innate activation in recipients with a B6 background.[29] In addition, a recent study using mouse models of RBC alloimmunization has demonstrated that antibody responses to one RBC antigen (HOD) can be enhanced if transfused RBCs coexpress a second alloantigen (KEL) to which the recipient has been primed by prior transfusion.[30] In this RBC transfusion model, however, the boosting effect required priming through an earlier transfusion event, as the anti-HOD response was not significantly different between mice receiving a single exposure to RBCs expressing HOD only versus HOD and KEL. The frequency of alloantibodies targeting non-MHC platelet antigens has also been shown to be significantly higher among anti-HLA antibody positive platelet recipients with rates ranging from 9–25% (compared with overall rates of 0–2%).[12, 31, 32] This may, however, have more to do with other confounding variables, such as number of transfusions (or other alloexposures), patient characteristics, etc.
Antibodies against allogeneic minor antigens were difficult to detect above background when target cells contained allogeneic MHC as well, only becoming apparent when target cells expressing the allogeneic minor antigens only were used. Similarly, unlike MHC antigens, allogeneic minor antigens alone did not induce much of a cytokine response in naïve cells in mixed lymphocyte cultures. When cells were primed with minor alloantigens in vivo, however, ex vivo cytokine responses were significantly increased, and for many cytokines were roughly equivalent to primary responses to allogeneic MHC. This is consistent with what is known about allogeneic minor antigens in transplant, that they are sufficient to induce rejection even when MHC is matched, but that the rejection is slower than with allogeneic MHC.[33] As most minor antigens are not nearly as diverse as MHC antigens, the likelihood of repeated exposure to some of these minor antigens with subsequent transfusions is likely higher than that of MHC.[34] Some of these minor antigens are expressed on platelets and can result in platelet refractoriness,[32, 35, 36] and many of these minor antigens could increase the risk of rejection of other transplanted cells or tissues for transfusion recipients who go on to receive transplants. Indeed, highly transfused bone marrow transplant recipients have been shown to be at increased risk of transplant rejection, in spite of MHC matching.[37–39] While WBCs are thought to be the predominant source of these minor antigens, a study using minor antigen mismatched strains in a murine model of bone marrow transplant found that transfusion of stringently leukoreduced platelets was sufficient to induce bone marrow transplant rejection, suggesting that platelets are also an important source of allogeneic minor antigens.[40]
Finding ways to prevent alloimmunization and induce tolerance to alloantigens would be of great benefit to transfusion and transplant recipients. Here, we have shown that pathogen reduction with UV+R is able to block alloresponses targeting both MHC and minor antigens. Furthermore, we have demonstrated that allogeneic MHC is both necessary and sufficient for induction of partial tolerance with transfusion of UV+R pathogen reduced PRP, providing insight into the mechanisms regulating this response. While tolerance is not induced towards allogeneic minor antigens using UV+R pathogen reduction, the immediate prevention of an alloresponse against these antigens still protects from priming responses to these antigens, and may help to eliminate the increased risk of transplant rejection associated with pre-transplant transfusion, as well as platelet refractoriness.
ACKNOWLEDGMENTS:
The authors thank NHLBI for support via NIH R01 HL133024 and Terumo BCT for providing pathogen reduction supplies. R.P.J. designed research, collected, analyzed and interpreted data, performed statistical analysis, and wrote manuscript. J.Q.T. collected and interpreted data and reviewed manuscript. M.O.M. designed research, collected and interpreted data, and reviewed manuscript. J.W.H. collected data.
Sources of support: This work was supported by NIHR01HL133024, and pathogen reduction materials were provided by Terumo BCT.
REFERENCES
- 1.Howard JE, Perkins HA: The natural history of alloimmunization to platelets. Transfusion 1978; 18: 496–503. [DOI] [PubMed] [Google Scholar]
- 2.Slichter SJ, Davis K, Enright H, et al. : Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005; 105: 4106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itescu S, Tung TC, Burke EM, et al. : Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation 1998; 98: 786–93. [DOI] [PubMed] [Google Scholar]
- 4.Andreu G, Dewailly J, Leberre C, et al. : Prevention of HLA immunization with leukocyte-poor packed red cells and platelet concentrates obtained by filtration. Blood 1988; 72: 964–9. [PubMed] [Google Scholar]
- 5.Dutcher JP, Schiffer CA, Aisner J, et al. : Alloimmunization following platelet transfusion: the absence of a dose-response relationship. Blood 1981; 57: 395–8. [PubMed] [Google Scholar]
- 6.Fisher M, Chapman JR, Ting A, et al. : Alloimmunisation to HLA antigens following transfusion with leucocyte-poor and purified platelet suspensions. Vox sanguinis 1985; 49: 331–5. [DOI] [PubMed] [Google Scholar]
- 7.Karpinski M, Pochinco D, Dembinski I, et al. : Leukocyte reduction of red blood cell transfusions does not decrease allosensitization rates in potential kidney transplant candidates. J Am Soc Nephrol 2004; 15: 818–24. [DOI] [PubMed] [Google Scholar]
- 8.Murphy MF, Metcalfe P, Thomas H, et al. : Use of leucocyte-poor blood components and HLA-matched-platelet donors to prevent HLA alloimmunization. British journal of haematology 1986; 62: 529–34. [DOI] [PubMed] [Google Scholar]
- 9.Schiffer CA, Dutcher JP, Aisner J, et al. : A randomized trial of leukocyte-depleted platelet transfusion to modify alloimmunization in patients with leukemia. Blood 1983; 62: 815–20. [PubMed] [Google Scholar]
- 10.van Marwijk Kooy M, van Prooijen HC, Moes M, et al. : Use of leukocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective, randomized trial. Blood 1991; 77: 201–5. [PubMed] [Google Scholar]
- 11.Jackman RP, Deng X, Bolgiano D, et al. : Leukoreduction and ultraviolet treatment reduce both the magnitude and the duration of the HLA antibody response. Transfusion 2014; 54: 672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kickler T, Kennedy SD, Braine HG: Alloimmunization to platelet-specific antigens on glycoproteins IIb-IIIa and Ib/IX in multiply transfused thrombocytopenic patients. Transfusion 1990; 30: 622–5. [DOI] [PubMed] [Google Scholar]
- 13.Kiefel V, Konig C, Kroll H, et al. : Platelet alloantibodies in transfused patients. Transfusion 2001; 41: 766–70. [DOI] [PubMed] [Google Scholar]
- 14.Kurz M, Knobl P, Kalhs P, et al. : Platelet-reactive HLA antibodies associated with low posttransfusion platelet increments:a comparison between the monoclonal antibody-specific immobilization of platelet antigens assay and the lymphocytotoxicity test. Transfusion 2001; 41: 771–4. [DOI] [PubMed] [Google Scholar]
- 15.Sanz C, Freire C, Alcorta I, et al. : Platelet-specific antibodies in HLA-immunized patients receiving chronic platelet support. Transfusion 2001; 41: 762–5. [DOI] [PubMed] [Google Scholar]
- 16.Sintnicolaas K, van Marwijk Kooij M, van Prooijen HC, et al. : Leukocyte depletion of random single-donor platelet transfusions does not prevent secondary human leukocyte antigen-alloimmunization and refractoriness: a randomized prospective study. Blood 1995; 85: 824–8. [PubMed] [Google Scholar]
- 17.Jackman RP, Muench MO, Heitman JW, et al. : Immune modulation and lack of alloimmunization following transfusion with pathogen-reduced platelets in mice. Transfusion 2013; 53: 2697–709. [DOI] [PubMed] [Google Scholar]
- 18.Slichter SJ, Pellham E, Bailey SL, et al. : Leukofiltration plus pathogen reduction prevents alloimmune platelet refractoriness in a dog transfusion model. Blood 2017; 130: 1052–61. [DOI] [PubMed] [Google Scholar]
- 19.Muench MO, Heitman JW, Inglis H, et al. : Reduced alloimmunization in mice following repeated transfusion with pathogen-reduced platelets. Transfusion 2016. [DOI] [PubMed] [Google Scholar]
- 20.Jackman RP, Heitman JW, Marschner S, et al. : Understanding loss of donor white blood cell immunogenicity after pathogen reduction: mechanisms of action in ultraviolet illumination and riboflavin treatment. Transfusion 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fast LD, Dileone G, Li J, et al. : Functional inactivation of white blood cells by Mirasol treatment. Transfusion 2006; 46: 642–8. [DOI] [PubMed] [Google Scholar]
- 22.Babic AM: Extracorporeal photopheresis: Lighting the way to immunomodulation. American journal of hematology 2008; 83: 589–91. [DOI] [PubMed] [Google Scholar]
- 23.Knobler R: Extracorporeal photochemotherapy--present and future. Vox sanguinis 2000; 78 Suppl 2: 197–201. [PubMed] [Google Scholar]
- 24.Oliven A, Shechter Y: Extracorporeal photopheresis: a review. Blood reviews 2001; 15: 103–8. [DOI] [PubMed] [Google Scholar]
- 25.Kao KJ: Induction of humoral immune tolerance to major histocompatibility complex antigens by transfusions of UVB-irradiated leukocytes. Blood 1996; 88: 4375–82. [PubMed] [Google Scholar]
- 26.Kao KJ, Huang ES, Donahue S: Characterization of immunologic tolerance induced by transfusion of UV-B--irradiated allogeneic mononuclear leukocytes. Blood 2001; 98: 1239–45. [DOI] [PubMed] [Google Scholar]
- 27.Norris PJ, Kaidarova Z, Maiorana E, et al. : Ultraviolet light-based pathogen inactivation and alloimmunization after platelet transfusion: results from a randomized trial. Transfusion 2018. [DOI] [PubMed] [Google Scholar]
- 28.Jackman RP, Muench MO, Inglis H, et al. : Reduced MHC alloimmunization and partial tolerance protection with pathogen reduction of whole blood. Transfusion 2017; 57: 337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai H, Friday AJ, Abou-Daya KI, et al. : Donor SIRPalpha polymorphism modulates the innate immune response to allogeneic grafts. Sci Immunol 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SR, Bennett A, Girard-Pierce K, et al. : Recipient priming to one RBC alloantigen directly enhances subsequent alloimmunization in mice. Blood Adv 2018; 2: 105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kekomaki S, Volin L, Koistinen P, et al. : Successful treatment of platelet transfusion refractoriness: the use of platelet transfusions matched for both human leucocyte antigens (HLA) and human platelet alloantigens (HPA) in alloimmunized patients with leukaemia. Eur J Haematol 1998; 60: 112–8. [DOI] [PubMed] [Google Scholar]
- 32.Novotny VM, van Doorn R, Witvliet MD, et al. : Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: a prospective study. Blood 1995; 85: 1736–41. [PubMed] [Google Scholar]
- 33.Goulmy E: Human minor histocompatibility antigens. Current opinion in immunology 1996; 8: 75–81. [DOI] [PubMed] [Google Scholar]
- 34.Sommer S: The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool 2005; 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langenscheidt F, Kiefel V, Santoso S, et al. : Platelet transfusion refractoriness associated with two rare platelet-specific alloantibodies (anti-Baka and anti-PlA2) and multiple HLA antibodies. Transfusion 1988; 28: 597–600. [DOI] [PubMed] [Google Scholar]
- 36.Pappalardo PA, Secord AR, Quitevis P, et al. : Platelet transfusion refractoriness associated with HPA-1a (Pl(A1)) alloantibody without coexistent HLA antibodies successfully treated with antigen-negative platelet transfusions. Transfusion 2001; 41: 984–7. [DOI] [PubMed] [Google Scholar]
- 37.Champlin RE, Horowitz MM, van Bekkum DW, et al. : Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood 1989; 73: 606–13. [PubMed] [Google Scholar]
- 38.Deeg HJ, Self S, Storb R, et al. : Decreased incidence of marrow graft rejection in patients with severe aplastic anemia: changing impact of risk factors. Blood 1986; 68: 1363–8. [PubMed] [Google Scholar]
- 39.Gluckman E, Horowitz MM, Champlin RE, et al. : Bone marrow transplantation for severe aplastic anemia: influence of conditioning and graft-versus-host disease prophylaxis regimens on outcome. Blood 1992; 79: 269–75. [PubMed] [Google Scholar]
- 40.Patel SR, Cadwell CM, Medford A, et al. : Transfusion of minor histocompatibility antigen-mismatched platelets induces rejection of bone marrow transplants in mice. The Journal of clinical investigation 2009; 119: 2787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


