Abstract
Background
Hormone therapy (HT) is widely provided for control of menopausal symptoms and has been used for the management and prevention of cardiovascular disease, osteoporosis and dementia in older women. This is an updated version of a Cochrane review first published in 2005.
Objectives
To assess effects of long‐term HT (at least 1 year's duration) on mortality, cardiovascular outcomes, cancer, gallbladder disease, fracture and cognition in perimenopausal and postmenopausal women during and after cessation of treatment.
Search methods
We searched the following databases to September 2016: Cochrane Gynaecology and Fertility Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and PsycINFO. We searched the registers of ongoing trials and reference lists provided in previous studies and systematic reviews.
Selection criteria
We included randomised double‐blinded studies of HT versus placebo, taken for at least 1 year by perimenopausal or postmenopausal women. HT included oestrogens, with or without progestogens, via the oral, transdermal, subcutaneous or intranasal route.
Data collection and analysis
Two review authors independently selected studies, assessed risk of bias and extracted data. We calculated risk ratios (RRs) for dichotomous data and mean differences (MDs) for continuous data, along with 95% confidence intervals (CIs). We assessed the quality of the evidence by using GRADE methods.
Main results
We included 22 studies involving 43,637 women. We derived nearly 70% of the data from two well‐conducted studies (HERS 1998; WHI 1998). Most participants were postmenopausal American women with at least some degree of comorbidity, and mean participant age in most studies was over 60 years. None of the studies focused on perimenopausal women.
In relatively healthy postmenopausal women (i.e. generally fit, without overt disease), combined continuous HT increased the risk of a coronary event (after 1 year's use: from 2 per 1000 to between 3 and 7 per 1000), venous thromboembolism (after 1 year's use: from 2 per 1000 to between 4 and 11 per 1000), stroke (after 3 years' use: from 6 per 1000 to between 6 and 12 per 1000), breast cancer (after 5.6 years' use: from 19 per 1000 to between 20 and 30 per 1000), gallbladder disease (after 5.6 years' use: from 27 per 1000 to between 38 and 60 per 1000) and death from lung cancer (after 5.6 years' use plus 2.4 years' additional follow‐up: from 5 per 1000 to between 6 and 13 per 1000).
Oestrogen‐only HT increased the risk of venous thromboembolism (after 1 to 2 years' use: from 2 per 1000 to 2 to 10 per 1000; after 7 years' use: from 16 per 1000 to 16 to 28 per 1000), stroke (after 7 years' use: from 24 per 1000 to between 25 and 40 per 1000) and gallbladder disease (after 7 years' use: from 27 per 1000 to between 38 and 60 per 1000) but reduced the risk of breast cancer (after 7 years' use: from 25 per 1000 to between 15 and 25 per 1000) and clinical fracture (after 7 years' use: from 141 per 1000 to between 92 and 113 per 1000) and did not increase the risk of coronary events at any follow‐up time.
Women over 65 years of age who were relatively healthy and taking continuous combined HT showed an increase in the incidence of dementia (after 4 years' use: from 9 per 1000 to 11 to 30 per 1000). Among women with cardiovascular disease, use of combined continuous HT significantly increased the risk of venous thromboembolism (at 1 year's use: from 3 per 1000 to between 3 and 29 per 1000). Women taking HT had a significantly decreased incidence of fracture with long‐term use.
Risk of fracture was the only outcome for which strong evidence showed clinical benefit derived from HT (after 5.6 years' use of combined HT: from 111 per 1000 to between 79 and 96 per 1000; after 7.1 years' use of oestrogen‐only HT: from 141 per 1000 to between 92 and 113 per 1000). Researchers found no strong evidence that HT has a clinically meaningful impact on the incidence of colorectal cancer.
One trial analysed subgroups of 2839 relatively healthy women 50 to 59 years of age who were taking combined continuous HT and 1637 who were taking oestrogen‐only HT versus similar‐sized placebo groups. The only significantly increased risk reported was for venous thromboembolism in women taking combined continuous HT: Their absolute risk remained low, at less than 1/500. However, other differences in risk cannot be excluded, as this study was not designed to have the power to detect differences between groups of women within 10 years of menopause.
For most studies, risk of bias was low in most domains. The overall quality of evidence for the main comparisons was moderate. The main limitation in the quality of evidence was that only about 30% of women were 50 to 59 years old at baseline, which is the age at which women are most likely to consider HT for vasomotor symptoms.
Authors' conclusions
Women with intolerable menopausal symptoms may wish to weigh the benefits of symptom relief against the small absolute risk of harm arising from short‐term use of low‐dose HT, provided they do not have specific contraindications. HT may be unsuitable for some women, including those at increased risk of cardiovascular disease, increased risk of thromboembolic disease (such as those with obesity or a history of venous thrombosis) or increased risk of some types of cancer (such as breast cancer, in women with a uterus). The risk of endometrial cancer among women with a uterus taking oestrogen‐only HT is well documented.
HT is not indicated for primary or secondary prevention of cardiovascular disease or dementia, nor for prevention of deterioration of cognitive function in postmenopausal women. Although HT is considered effective for the prevention of postmenopausal osteoporosis, it is generally recommended as an option only for women at significant risk for whom non‐oestrogen therapies are unsuitable. Data are insufficient for assessment of the risk of long‐term HT use in perimenopausal women and in postmenopausal women younger than 50 years of age.
Plain language summary
Long‐term hormone therapy for perimenopausal and postmenopausal women
Review question
In perimenopausal and postmenopausal women, what are the clinical effects of using hormone therapy (HT) for a year or longer?
Background
HT is given for control of menopausal symptoms. It has also been used for the management and prevention of chronic diseases such as cardiovascular disease, osteoporosis and dementia.
Study characteristics
This review included 22 double‐blinded randomised controlled trials (RCTs) (43,637 women). The evidence is current to September 2016.
Key results
In relatively healthy postmenopausal women, using combined continuous HT for 1 year increased the risk of a heart attack from about 2 per 1000 to between 3 and 7 per 1000, and increased the risk of venous thrombosis (blood clot) from about 2 per 1000 to between 4 and 11 per 1000. With longer use, HT also increased the risk of stroke, breast cancer, gallbladder disease and death from lung cancer.
Oestrogen‐only HT increased the risk of venous thrombosis after 1 to 2 years' use: from 2 per 1000 to 2 to 10 per 1000. With longer use, it also increased the risk of stroke and gallbladder disease, but it reduced the risk of breast cancer (after 7 years' use) from 25 per 1000 to between 15 and 25 per 1000.
Among women over 65 years of age taking continuous combined HT, the incidence of dementia was increased.
Risk of fracture was the only outcome for which results showed strong evidence of clinical benefit from HT (both types).
Women with intolerable menopausal symptoms may wish to weigh the benefits of symptom relief against the small absolute risk of harm arising from short‐term use of low‐dose HT, provided they do not have specific contraindications. HT may be unsuitable for some women, including those at increased risk of cardiovascular disease, increased risk of thromboembolic disease (such as those with obesity or a history of venous thrombosis) or increased risk of some types of cancer (such as breast cancer, in women with a uterus). The risk of endometrial cancer for women with a uterus who take oestrogen‐only HT is well documented.
HT is not indicated for primary or secondary prevention of cardiovascular disease or dementia, nor for preventing deterioration of cognitive function in postmenopausal women. Although HT is considered effective for prevention of postmenopausal osteoporosis, it is generally recommended as an option only for women at significant risk, for whom non‐oestrogen therapies are unsuitable. Data are insufficient for assessment of the risk of long‐term HT use in perimenopausal women or postmenopausal women younger than 50 years of age.
Quality of the evidence
For most studies, risk of bias was low in most domains and the overall quality of the evidence was moderate. The main limitation was that only about 30% of women were 50 to 59 years old at baseline ‐ the age at which women are likely to consider HT for vasomotor symptoms.
Summary of findings
Background
Description of the condition
The median age at onset of menopause varies across geographical regions. In Europe, it ranges from about 50 to 53 years, in North America from 50 to 51 years, in Latin America from 44 to 53 years and in Asia from 42 to 49 years (Palacios 2010). Most women experience menopause (the last menstrual period) after a phase of changing ovarian function (the perimenopause) that may last several years and is characterised by irregular menstrual cycles (Greendale 1999). Women are said to be postmenopausal when menstruation has ceased for 12 months. Many (although not all) perimenopausal and postmenopausal women report a variety of symptoms, including hot flushes and vaginal dryness, which probably relate to the natural decline in oestrogen levels. Symptoms tend to fluctuate and their severity varies greatly between individuals, with some reporting intense discomfort and a substantial reduction in quality of life. Most research has focused on white women, but the experience of menopause differs between women of different races and ethnicities, as well as by menopausal stage (Avis 2001; Palacios 2010). The duration of regular hot flushes is highly variable. Most women report that hot flushes last from 6 months to 2 years (Kronenberg 1994), but longitudinal research suggests that the time from onset to resolution of symptoms is often considerably longer (Guthrie 2005).
Description of the intervention
Hormone therapy (HT) consists of oestrogen alone (oestrogen‐only HT) or oestrogen combined with a progestogen (combined HT). It is available in a variety of formulations and doses that can be taken orally, vaginally or intranasally, or as an implant, skin patch, cream or gel. Clinical effects vary according to the type of HT and its duration of use.
The addition of a progestogen reduces the risk of endometrial hyperplasia associated with the use of oestrogen alone in women with a uterus (Furness 2012), but the issue is problematic because progestogens have adverse effects on blood lipids and may have the potential to cause symptoms such as headache, bloating and breast tenderness (McKinney 1998). Progestogens used for HT include synthetic derivatives of progesterone, synthetic derivatives of testosterone and natural progesterones derived from plants. These differ in their metabolic action and potential for adverse effects, and it is currently unclear which type of progestogen has the best risk‐benefit profile for use in HT. In combined HT, progestogen can be taken continuously (every day), sequentially (for part of each month) or less frequently.
Hormone therapy (HT) has been utilised for over 50 years for the treatment of women with hot flushes and other menopausal symptoms, and its efficacy is well established, as evidenced by a Cochrane systematic review of 24 randomised controlled studies of HT for hot flushes that was published between 1971 and 2000 (MacLennan 2004).
During the past 25 years, HT has also been used for the management or prevention of chronic disease. Oestrogens and progestogens affect most body systems and have been proposed as causing or preventing a wide range of conditions. Recommendations for use have varied over time, but through the 1990s, commonly held expert opinion was that most postmenopausal women could benefit from HT (Hemminki 2000a). This view was based on strong and consistent observational evidence that HT reduced the risk of coronary heart disease (CHD) by at least 30%. A meta‐analysis of 25 cohort, case‐control and angiographic studies published up to 1997 revealed a risk ratio of 0.70 (95% confidence interval (CI) 0.65 to 0.75) for CHD among oestrogen users compared with never‐users.
Other benefits reported in observational studies of HT include strong evidence of a reduction in osteoporotic fractures, a possible preventive or delaying effect on cognitive decline or dementia and even a reduction in overall mortality for current users (Barrett‐Connor 1998).
How the intervention might work
Oestrogen has a favourable effect on some biomarkers, including indicators of cardiovascular disease and disorders of bone metabolism. It has been shown to improve endothelial vasodilator function, promote angiogenesis and modulate autonomic function. Thus cardioprotective benefits of oestrogen have some biological plausibility (Miller 2008). However, biomarkers interact via multiple complex pathways, and the overall effect of oestrogen on clinical outcomes cannot be predicted with any certainty. Therefore, trials with clinical endpoints such as myocardial infarction (MI) are necessary (Banks 2009a).
Why it is important to do this review
Observational studies have revealed a range of adverse effects of HT, including doubling or tripling of the risk of thromboembolic events, a large increase in endometrial cancer risk among women taking oestrogen without progestogen, an increased incidence of gallbladder disease and a possible link between HT and breast cancer. The suggestion that HT might increase the risk of breast cancer was supported by evidence of an increase in breast density in a high proportion of women taking oestrogen, but findings have been inconsistent and controversial (Barrett‐Connor 1998). The results of a very large observational study conducted in the UK (Beral 2003) raised concerns that current users of both combined and oestrogen‐only HT were at increased risk of both incident and fatal breast cancer after relatively short periods of use. The increase in risk was greatest among users of combined HT, with no large variations reported between the effects of specific oestrogens or specific progestogens. Risks were greater if HT use started at around the time of menopause than if it started later. Breast cancer rates were highest among current users of combined HT who began use within 5 years of menopause (Beral 2011).
Coronary heart disease (CHD) is the most common cause of death and morbidity in older women, and it was held that a significant reduction in CHD risk from HT would outweigh any potential adverse effects. However, these uncontrolled studies showed strong potential for selection or compliance bias, or both, with oestrogen‐takers more likely to be healthy, well‐educated, compliant women with a lower baseline risk of cardiovascular disease. The need for randomised controlled trials has been recognised (Barrett‐Connor 2001; Hemminki 2000a). It has been suggested that wide prescribing of HT in the 1990s, despite the lack of randomised evidence of its efficacy and safety, might reflect a conflict between commercial and professional interest groups and good public policy (Hemminki 2000). Randomised controlled trials (RCTs) have failed to demonstrate the marked CHD benefits of HT seen in observational studies and have raised questions about its overall risk‐benefit profile.
Other Cochrane reviews have found strong evidence that HT is effective in treating women with menopausal symptoms. One review reported a 75% reduction in the frequency of hot flushes among perimenopausal and postmenopausal women taking HT, relative to placebo, and a statistically significant reduction in symptom severity for the HT group (odds ratio (OR) 0.13, 95% CI 0.07 to 0.23) (MacLennan 2004). Another review found that local oestrogens were more effective in relieving the symptoms of vaginal atrophy among postmenopausal women when compared with placebo or non‐hormonal gel (Suckling 2006). However, women contemplating the use of HT for menopausal symptoms must be aware of negative findings in other areas, as discussed below.
Previous and forthcoming Cochrane systematic reviews of HT in perimenopausal and postmenopausal women will explore the following topics.
Cardiovascular disease (Boardman 2015).
Dementia and cognitive function (Hogervorst 2009; Lethaby 2008).
Endometrial hyperplasia (Furness 2012).
Hot flushes (MacLennan 2004).
Pelvic organ prolapse (Ismail 2010).
Sexual function (Nastri 2012).
Urinary incontinence (Cody 2009).
Vaginal atrophy (Suckling 2006).
Weight and body fat distribution (Kongnyuy 1999).
In view of the large number of reviews on individual aspects of HT, review authors recognised the need for a systematic review that would provide an overview of all relevant long‐term clinical outcomes, thereby providing assistance to women and their clinicians who must make informed judgements about the use of HT. An a priori decision was made to exclude studies of duration shorter than 1 year and not to include as outcomes menopausal symptom control, early‐onset side effects of HT and surrogate measures such as endometrial hyperplasia and bone mineral density. This review is not intended to replace other Cochrane reviews on HT, including those listed above. These reviews remain an important source of evidence on individual aspects of HT and will continue to be updated regularly.
This is an updated version of the original Cochrane review first published in 2005.
Objectives
To assess effects of long‐term HT (at least 1 year's duration) on mortality, cardiovascular outcomes, cancer, gallbladder disease, fracture and cognition in perimenopausal and postmenopausal women during and after cessation of treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blinded studies, which we defined as provided blinding of participants and all researchers and outcome assessors.
For cross‐over studies, we intended to use only results from the end of the first phase (before the treatment cross‐over) because of the potential carry‐over effect of HT therapy from the first treatment phase. However, we identified no cross‐over studies for inclusion.
Types of participants
Eligible participants were perimenopausal or postmenopausal women recruited from any healthcare setting or a population‐based sample.
Perimenopausal women were defined as women who had not yet had their final menstrual period but were in the transitional period between more‐or‐less regular cycles of ovulation and menstruation and complete cessation of these cycles.
Postmenopausal women were defined as women with surgical menopause (removal of both ovaries) and women with spontaneous menopause and amenorrhoea for longer than 12 months.
Studies included women both with and without a prior history of disease (e.g. cardiovascular disease, fracture, osteoporosis).
Types of interventions
All oestrogens, with and without progestogens, administered by oral, transdermal, subcutaneous or intranasal routes, and given as perimenopausal or postmenopausal therapy for any reason for 12 months or longer, compared with placebo.
Exclusion criteria
We excluded studies with co‐interventions that might potentially affect the outcomes being measured and studies of topical vaginal HT creams, topical tablets and rings. These interventions are covered in another Cochrane review (Suckling 2006).
Our rationale for excluding trials of less than 1 year's duration is that we considered such trials unlikely to be long enough for investigators to report intervention‐related clinical events.
Types of outcome measures
We considered only studies reporting at least one of the following outcomes for inclusion in this review.
Death from any cause (total mortality).
Cause‐specific mortality.
Coronary events (myocardial infarction or coronary death).
Stroke (ischaemic or haemorrhagic) or transient ischaemic attack (TIA).
Venous thromboembolism (pulmonary embolism or deep vein thrombosis).
Breast cancer.
Colorectal cancer.
Lung cancer.
Endometrial cancer.
Ovarian cancer.
Gallbladder disease.
Fractures (hip fracture, clinically diagnosed vertebral fracture, total clinically diagnosed fracture).
Cognitive function (using global measures) or dementia (including Alzheimer's disease) as measured in the included studies.
We planned to restrict our focus to long‐term clinical outcomes and to not include menopausal symptom control and early‐onset side effects of HT as outcomes. HT for control of hot flushes is the topic of another systematic review (MacLennan 2004).
We restricted inclusion to studies reporting one of our outcomes of interest because HT may be studied in the same population for different purposes, and we wished to ensure that we included only relevant studies.
Search methods for identification of studies
We searched for all published and unpublished double‐blinded RCTs of HT versus placebo, without language restriction and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We performed electronic searches of the Cochrane Gynaecology and Fertility Group (CGF) Trials Register (5 September 2016; Procite platform; Appendix 1), the Cochrane Central Register of Controlled Trials (CENTRAL) Online (5 September 2016; CRSO platform; Appendix 2), MEDLINE (1966 to 5 September 2016; Ovid platform; Appendix 3), Embase (1980 to 5 September 2016; Ovid platform; Appendix 4), PsycINFO (2010 to 5 September 2016; Ovid platform; Appendix 5) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to September 2016; EBSCO platform; Appendix 6). We did not restrict the search by language. The GGF Information Specialist designed the search strategy.
We also searched the following trial registers for ongoing and registered trials (Appendix 7).
http://www.clinicaltrials.gov (a service of the US National Institutes of Health).
http://www.who.int/trialsearch/Default.aspx (search portal of the World Health Organization International Trials Registry Platform).
Searching other resources
We checked the reference lists of relevant publications returned by the above searches.
We contacted the following pharmaceutical companies in December 2003, via their websites or by letter, to request data from any published or unpublished randomised controlled trials of HT included in their files: Schering AG, Novartis, NovoNordisk, Paines and Byrnes/NZMS, 3M Pharmaceuticals, Organon, Wyeth. We received reprints of published studies from one company (NovoNordisk), another company reported that it had no unpublished studies with completed study reports available (Wyeth), and two companies (3M Pharmaceuticals, Organon) acknowledged our request.
We documented the search flow in a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) chart (Moher 2009).
Data collection and analysis
Selection of studies
One review author screened the titles or abstracts, or both, of all publications obtained by the search strategy to identify potentially eligible studies. If the abstract suggested that the study might be eligible for inclusion, we obtained the full article. Two review authors checked potentially eligible studies against the inclusion criteria of the review. They performed this assessment while unblinded and resolved any uncertainty by discussion. If necessary, we sought additional information from the corresponding author of the study.
Data extraction and management
When studies had multiple publications, the review authors collated multiple reports on the same study, so that each study rather than each report is the unit of interest in the review, and assigned such studies a single study ID with multiple references. Owing to the very large number of publications for some studies (e.g. WHI 1998), we have on occasion referred in the text to specific papers that are listed under additional references or as sub‐publications under the single study ID, to make clear where specific outcomes and time frames are reported.
When no events occurred in either comparison group for a particular outcome, we did not enter data in the Data and analyses tables for that outcome, in keeping with Cochrane recommendations (Higgins 2011).
Extracted data included study characteristics and outcome data (see data extraction table for details; Appendix 8). We corresponded with study investigators to request additional data on methods and/or results, as required.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias using the Cochrane risk of bias assessment tool (Higgins 2011) to examine selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. We resolved disagreements by discussion, or we sought assistance from a third review author. We described all judgements fully and presented our conclusions in the 'Risk of bias table' (Figure 1), which we incorporated into our interpretation of review findings by performing sensitivity analyses (see below).
1.
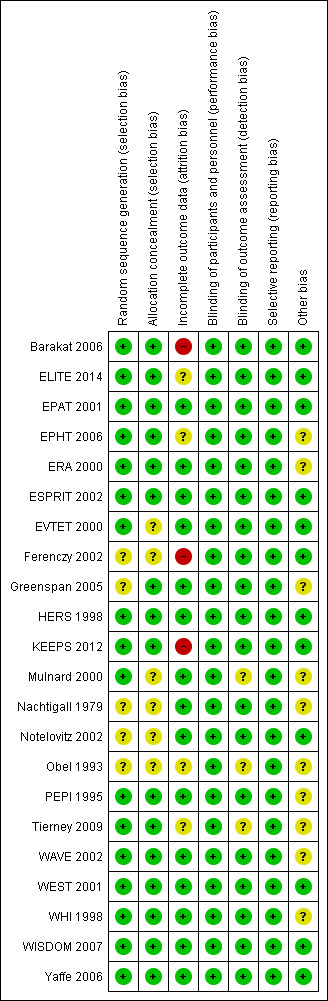
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We performed statistical analysis according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We analysed treatment effects by comparing outcomes for each group measured at the end of therapy, during ongoing follow‐up or at both time points.
For dichotomous data, we generated 2 × 2 tables for each study and expressed data as risk ratios (RRs) with 95% confidence intervals (CIs). If studies reported the number of events occurring in each comparison group at a mean follow‐up time (i.e. not all women had been followed up for that duration of time, and others had been followed up longer), we made the simplifying assumption that risk was constant across the follow‐up period and reported data as dichotomous data at a fixed time point. If risk varied significantly across the follow‐up period, we noted this variation in the Results section.
We expressed continuous data as mean differences (MDs) with 95% CIs.
For outcomes for which studies reported no events in the HT nor the placebo group, we did not enter results into data tables.
Unit of analysis issues
The unit of analysis was per woman.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the original trialists. Where these could not be obtained, we analysed only available data.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary, and if this was not the case, we planned to refrain from pooling the data. We assessed statistical heterogeneity by measuring the I2statistic. We regarded I2 greater than 50% as indicating substantial heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If we included 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We planned to pool the results of individual studies (meta‐analyse) only when they were clinically similar with respect to study population, intervention and outcome of interest. If an individual study pooled the results of study arms that used different types of HT, we did not include the pooled results in this review.
We combined data for meta‐analysis by using RevMan software and the Peto‐modified Mantel‐Haenszel method. We could reach no consensus about whether a fixed‐effect or a random‐effects model should be used for meta‐analysis, so we performed both types of analysis. This could be viewed as a sensitivity analysis performed to assess the impact of the choice of model on results of the analysis; unless results proved robust to both models, we would have to treat them with caution. Published graphs display results obtained with the fixed‐effect model.
We planned to combine continuous data for meta‐analysis, had any such data been available for pooling. Meta‐analytical methods for continuous data assume that the underlying distribution of measurements is normal. The ratio of the mean to its standard deviation serves as a crude method of assessing skew if this ratio was less than 1.65 for any study group; unless original data were available for log transformation, we did not include the results in analysis tables but reported them in 'Other data' tables. We reported data in the 'Other data' section if they were clearly skewed and if investigators reported results in the publication as median values and ranges with non‐parametric tests of significance.
We conducted separate analyses according to the type of HT used (oestrogen only or combined HT). For some outcomes (death, cardiovascular disease, cognition scores), we anticipated that the effect of the intervention might differ according to the clinical status of the participant, and so we conducted separate analyses for studies of women without major health problems and for studies of women with specific health conditions. For other outcomes (cancer, cholecystic disease, fractures), we combined all available study results.
We calculated pooled RRs for all outcomes and translated our main findings into rates per thousand on the basis of rates and confidence intervals per thousand as reported in the 'Summary of findings' tables.
Subgroup analysis and investigation of heterogeneity
If we detected substantial heterogeneity (> 50%), we planned to explore possible explanations by performing subgroup analyses (e.g. different populations) and/or sensitivity analyses (e.g. by risk of bias). We planned to take any statistical heterogeneity into account when interpreting the results, especially if we noted variation in the direction of effect.
Sensitivity analysis
As noted above, we checked whether use of a random‐effects model for each analysis materially influenced our findings.
We planned to conduct sensitivity analyses to examine effects of methodological differences between studies provided we identified sufficient studies (> 5). These analyses might help to explain any substantial statistical heterogeneity that may be detected. We planned to explore the following specific differences.
Restricting analysis to studies with adequate methods: defined for this purpose as adequate allocation concealment, analysis by intention to treat and losses to follow‐up < 10%.
Differences among studies with respect to participants, interventions or clinical criteria for defining outcomes, although as noted above, we planned to refrain from combining studies that were obviously dissimilar in these respects.
We planned to conduct additional subgroup or sensitivity analyses if other possible sources of heterogeneity became evident during preparation of the review; however, we would have to interpret the results of any such post hoc analyses with great caution.
Overall quality of the body of evidence: 'Summary of findings' tables
We prepared 'Summary of findings' tables using GRADEpro (GRADEpro GDT 2014) and Cochrane methods (Higgins 2011). This table shows the overall quality of the body of evidence for the main comparisons (combined HT and oestrogen‐only HT vs placebo) and for the most clinically relevant outcomes (coronary events, stroke, venous thromboembolism, breast cancer, lung cancer, gallbladder disease, clinical fractures) in accordance with GRADE (Atkins 2004) criteria (study risk of bias, consistency of effect, imprecision, indirectness and publication bias). We justified judgements about evidence quality (high, moderate, low or very low) and documented and incorporated these judgments into reporting of results for each outcome.
Results
Description of studies
Results of the search
Results of search to 2012
We retrieved 57 studies through searches conducted up to 2012 and considered them for inclusion. We included 23 studies and excluded 34 studies.
Search update 2017
We screened 3046 records, discarded 3041 as clearly irrelevant and retained 45 articles, which we checked in full text. From these 44 articles, we identified two new studies: KEEPS 2012 (15 articles) and ELITE 2014 (5 articles). We also identified 20 articles that were additional publications related to studies already included (19 articles for WHI 1998 and one article for EPHT 2006) and five studies that we excluded (AHT 2015; Paoletti 2015; Rasgon 2014; Schierbeck 2012; SMART 2016).
For this update of the review, we also excluded three studies that were included in previous versions of the review but that no longer meet our eligibility criteria because we have decided to report fewer outcomes (Haines 2003; Nielsen 2006; Pefanco 2007). See Differences between protocol and review.
Thus we have included 22 studies and have excluded 42 studies from this review (see Figure 2 for study flow).
2.
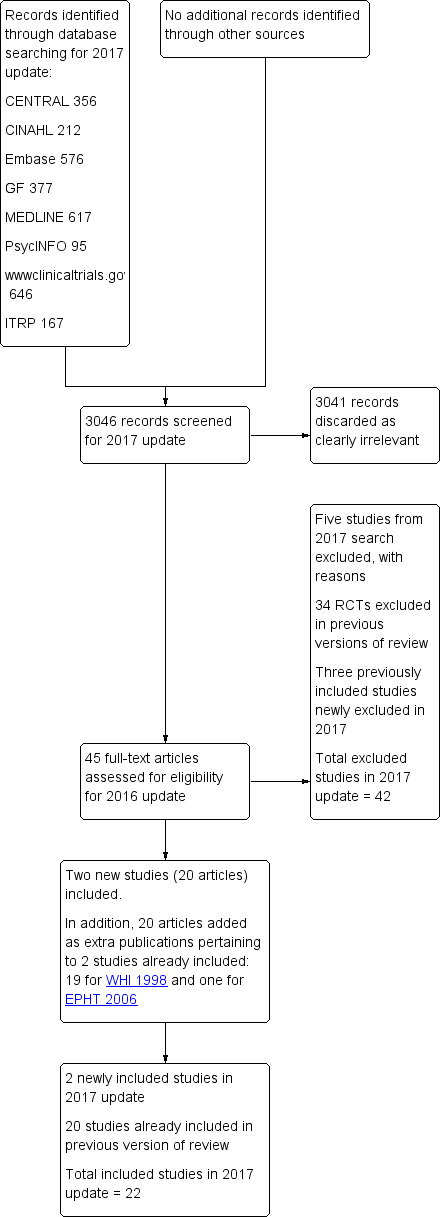
Study flow diagram.
Included studies
The 22 eligible studies are based on one very large study (WHI 1998). WHI 1998 incorporated randomised comparisons of two different HT regimens versus placebo and published these results separately. One study (WHI 2002) compared combined oestrogen and progesterone versus placebo and is referred to in this review as WHI 1998 (combined HT arm); the other compared oestrogen‐only HT versus placebo and is referred to in this review as WHI 1998 (oestrogen‐only HT arm). WHI 1998 also included a subgroup study known as the Women's Health Initiative Memory Study (WHIMS), which measured cognitive outcomes in older women (aged 65 to 79 years at study entry) from both arms of WHI 1998 and is referred to in this review as WHI 1998 (WHIMS) (Shumaker 1998). An additional ancillary study ‐ WHI 1998 (WHISCA) ‐ enrolled women from WHI 1998 (WHIMS) who were free of dementia to investigate the effects of HT on domain‐specific cognitive function in older women (Resnick 2004).
The 22 identified studies included 43,637 randomised women: 22,693 randomised to some form of HT and 20,928 to placebo (treatment allocation was unclear for 16 women in one study (Ferenczy 2002)). WISDOM 2007 included 1307 additional women who were randomised to a comparison of two active hormone therapies but are not included in this review. Investigators analysed results for more than 99% of these women by intention to treat. Although some studies used biological measures as their primary outcome (e.g. lumen of carotid artery), we included them because they also reported clinical endpoints relevant to this review as prespecified secondary outcomes.
The studies varied dramatically in size. The largest was WHI 1998, which randomised 27,347 participants, and the other studies varied in sample size from 40 (Tierney 2009) to 5692 (WISDOM 2007) participants. Investigators included 8000 women in each group in WHI 1998 (combined HT arm) and more than 5000 in each group in WHI 1998 (oestrogen‐only HT arm), along with more than 1400 in each group on the oestrogen‐only HT arm of WHI 1998 (WHIMS) and more than 2200 in each group on the combined arm of WHI 1998 (WHIMS). HERS 1998 included about 1380 women in each comparison group, ESPRIT 2002 included more than 500 in each group, EPHT 2006 included around 400 women in each group and KEEPS 2012 included 220 to 275 per group. Otherwise, none of the studies included more than 210 women in each group. Five of the smaller studies were single‐centred (ELITE 2014; EPAT 2001; Nachtigall 1979; Obel 1993; Tierney 2009), and it is unclear whether one study (EVTET 2000) enlisted more than one trial centre. The other 10 studies involved between 7 and 40 trial centres.
Fourteen studies were conducted in the USA, and one in each of the following countries: UK, Estonia, Norway, Canada and Denmark; three studies were international in scope (one in the USA and Canada, one in Canada and the Netherlands and one in the UK, Australia and New Zealand). Two studies (EPHT 2006; WISDOM 2007) were originally planned as part of a larger international project, but planning was beset with delays, and in the meantime, WHI 1998 began in the USA when other countries were no longer prepared to commit funds to a second study with similar objectives. Both of these studies were prematurely closed as a result of publication of early WHI 1998 findings.
We attempted to contact investigators for the following studies to request more information about their methods or outcomes: Barakat 2006; ELITE 2014; EPAT 2001; EVTET 2000; Ferenczy 2002; HERS 1998; KEEPS 2012, Mulnard 2000; Notelovitz 2002; Obel 1993; PEPI 1995; WAVE 2002; WEST 2001; WHI 1998, WISDOM 2007. Investigators from the following studies kindly supplied clarification or additional unpublished data, or both: Barakat 2006; ELITE 2014; ERA 2000; EPHT 2006; HERS 1998; Obel 1993; PEPI 1995; WAVE 2002; WISDOM 2007.
Participants
The women included in these studies were predominantly postmenopausal, spontaneously or surgically. The age of participants ranged from 26 to 91 years, with mean or median age of each study ranging from 48 to 76 years (no age was stated in Obel 1993). In more than half of the studies, mean participant age was over 60 years. Inclusion criteria varied according to the primary objectives of individual studies. Some were designed to investigate the use of HT for treatment of women with menopausal symptoms or for disease prevention and thus enrolled women in reasonably good health. Others were designed to assess whether HT was beneficial for women with a history of cancer or established disease, including heart disease, thromboembolic disease, stroke, Alzheimer's disease or long‐term medical conditions requiring hospitalisation; these studies restricted entry to women who had received a diagnosis of the condition of interest.
Studies of women without established medical conditions
Thirteen studies enrolled relatively healthy women (ELITE 2014; EPAT 2001; EPHT 2006; Ferenczy 2002; Greenspan 2005; KEEPS 2012, Notelovitz 2002; Obel 1993; PEPI 1995; Tierney 2009; WHI 1998; WISDOM 2007; Yaffe 2006). Women in some of these studies had risk factors (such as raised cholesterol), and a small minority within individual studies had a history of cardiovascular disease, but most participants were fit women without overt disease. Most of these studies were interested in the use of HT for disease prevention.
Three studies were large and investigated the use of HT to prevent cardiovascular disease while also reporting a wide range of other endpoints; researchers provided highly detailed lists of inclusion and exclusion criteria (PEPI 1995; WHI 1998, WISDOM 2007). In WHI 1998, enrolment was targeted to establish set fractions for baseline age categories and to achieve representation of racial and ethnic groups in the proportions recorded by the US census for individuals 50 to 79 years of age.
The WHI 1998 (combined HT arm) investigators noted that prevalence of prior cardiovascular disease in participants was low: 4.4% had a history of myocardial infarction, coronary revascularisation, stroke or transient ischaemic attack. They also commented that levels of cardiovascular risk factors were consistent with a generally healthy population of postmenopausal women: 2.9% reported a history of angina, 36% were hypertensive (or were being treated for hypertension), 13% were being treated for high cholesterol, 4.4% were being treated for diabetes and 10.5% were current smokers (Manson 2003). Similarly, in WHI 1998 (oestrogen‐only HT arm), participants in general were considered healthy, although 4.1% had a history of myocardial infarction or coronary revascularisation, 5.8% had a history of angina, 1.4% had a history of stroke,1.6% had a history of venous thrombosis, 48% were hypertensive (or were being treated for hypertension), 15% were receiving treatment for high cholesterol, 7.7% were being treated for diabetes and 10.5% were current smokers (Stefanick 2003).
PEPI 1995 compared the characteristics of their cohort with values returned in large US surveys and concluded that although the PEPI 1995 cohort was generally in better health than the wider US population, these individuals were not so markedly different as to limit the generalisability of study results. Both KEEPS 2012 and ELITE 2014 were designed to test whether menopausal HT initiated soon after menopause could delay progression of atherosclerosis. Two other 'prevention' studies aimed to test the possible beneficial effects of HT on arterial wall density (EPAT 2001) and bone density (Notelovitz 2002). Four much smaller studies also enrolled women without stated health problems who were in early menopause (Obel 1993) or were postmenopausal and aimed to assess the effects of HT on endometrial safety (Ferenczy 2002; Obel 1993) and other clinical outcomes (Greenspan 2005; Tierney 2009).
WISDOM 2007 recruited women with no known major health problems from general practice registers in countries with free or low fee healthcare systems. Investigators designed recruitment to target older women first; as a result, median participant age was 63 years and few women in the younger age group were included when the study closed prematurely.
Studies of women with established medical conditions or a history of cancer
Six studies included women with established cardiovascular disease (ERA 2000; ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002; WEST 2001). ERA 2000 and WAVE 2002 included women who had coronary artery stenosis evident on angiogram. HERS 1998 and ESPRIT 2002 randomised women who had had a myocardial infarction or (in the case of HERS 1998) coronary artery surgery. EVTET 2000 and WEST 2001 included women who had had a thromboembolic (pulmonary embolism (PE) or deep vein thrombosis (DVT)) or cerebrovascular event (stroke or TIA). The largest of these six studies (HERS 1998) compared its cohort of women with a similar group of women presumed to have coronary heart disease, who were participants in a survey designed to produce nationally representative data: The HERS 1998 cohort included significantly fewer smokers, women with hypertension and women with diabetes than the comparison group, but individuals were comparable with respect to blood pressure, body mass index, physical activity and cholesterol levels.
One study (Mulnard 2000) included women with Alzheimer's disease, and an older study (Nachtigall 1979) included women with a range of medical conditions such as diabetes, need for custodial care, arteriosclerosis and chronic neurological disorders: All participants in this study were hospitalised for the duration of the 10‐year study.
One study enrolled women after surgery (including bilateral salpingo‐oophorectomy) for early‐stage endometrial cancer (Barakat 2006).
Interventions
The included studies used a wide variety of oestrogen‐alone or oestrogen and progestogen combinations as interventions; some included more than one intervention arm, each with a different dose, formulation or route of HT. Most comparisons used a moderate dose of oestrogen (e.g. oestradiol 1 mg, conjugated equine oestrogen (CEE) 0.625 mg daily, transdermal oestradiol 0.05 mg twice weekly). Nachtigall 1979 used a much higher dose than the other included studies, reflecting the fact that it was conducted many years earlier than the others.
The range of interventions used follows here.
Oestrogen‐only HTs
These included the following.
Oestradiol (17‐B oestradiol), an oestrogen derived from Mexican wild yam, 1 mg orally (ELITE 2014; EPAT 2001; WEST 2001).
Oestradiol valerate, which is a pro‐drug for oestradiol (meaning that it is converted in the body into the active form); the dose used was 2 mg (ESPRIT 2002).
Transdermal oestradiol skin patches; doses used were 0.014 mg (Yaffe 2006) and 0.025 mg, 0.05 mg or 0.075 mg daily (Notelovitz 2002).
Intranasal 17‐B oestradiol, delivered by a puff via each nostril once a day, at a dose of 0.15 mg or 0.3 mg daily (Nielsen 2006).
Conjugated equine oestrogen (CEE), a blend of equine oestrogens; 0.625 mg (Barakat 2006; ERA 2000; Greenspan 2005; Mulnard 2000; PEPI 1995; WAVE 2002; WHI 1998 (oestrogen‐only HT arm)) and 1.25 mg daily (Mulnard 2000). One study (Barakat 2006) allowed doubling of the dose for women who were symptomatic. WISDOM 2007 also included an oestrogen‐only arm, but the comparison group was taking combined therapy, and this comparison is not relevant to this review.
Most studies using oestrogen‐only HT did not randomise women to this comparison unless they had had a hysterectomy (Greenspan 2005; Mulnard 2000; Nachtigall 1979; Notelovitz 2002; WAVE 2002; WEST 2001; WHI 1998 (oestrogen‐only HT arm)).
Combined HT regimens
Combined regimens included one of the above types of oestrogen in combination with one of the following progestogens.
Medroxyprogesterone acetate (MPA), a synthetic progestogen structurally related to progesterone.
Dydrogesterone, a synthetic progestogen structurally related to progesterone.
Norethisterone (norethindrone), a synthetic progestogen structurally related to testosterone.
Micronised progesterone, a natural progestogen synthesised from plant sources and finely ground to improve its absorption.
Drosperinone, a synthetic progestogen structurally related to spironolactone.
Continuous combined regimens
These included the following.
CEE 0.625 mg with MPA 2.5 mg daily (EPHT 2006; ERA 2000; Greenspan 2005; HERS 1998; PEPI 1995; WAVE 2002; WHI 1998 (combined arm); WISDOM 2007).
CEE 2.5 mg with MPA 10 mg daily (Nachtigall 1979).
Oestradiol 2 mg with 1 mg norethisterone daily (EVTET 2000).
Combined sequential regimens
These included the following.
Oestradiol 1 mg daily with MPA 5 mg for 12 days once a year (WEST 2001).
Oestradiol 1 mg daily for 4 days, oestradiol 1 mg plus 0.35 mg norethindrone daily for 3 days each week (Tierney 2009).
Oestradiol 2 mg days 1 to 22, 1 mg days 22 to 28, with norethisterone 1 mg days 13 to 22 (Obel 1993).
Oestradiol 1 mg daily with dydrogesterone 5 mg or 10 mg days 14 to 28 (Ferenczy 2002).
Oestradiol 2 mg daily with 10 to 20 mg dydrogesterone days 14 to 28 (Ferenczy 2002).
Oestradiol 0.05 mg patch with cyclic micronised progesterone 200 mg daily for 12 days a month (KEEPS 2012).
CEE 0.425 mg daily with cyclic micronised progesterone 200 mg daily for 12 days a month (KEEPS 2012).
CEE 0.625 with MPA 10 mg days 1 to 12 (PEPI 1995).
CEE 0.625 mg with micronised progesterone 200 mg days 1 to 12 (PEPI 1995).
Oral oestradiol 1 mg daily, plus 40 mg cyclic micronised progesterone as 4% vaginal gel for 10 days per 30‐day cycle for women with an intact uterus only (ELITE 2014).
The control arm of each study received placebo tablets, patches or nasal spray, as appropriate.
The duration of HT use varied, with the longest study lasting 10 years (Nachtigall 1979). Three studies reported outcomes after HT use for around 1 year (EVTET 2000; Mulnard 2000; WISDOM 2007); seven measured outcomes after 2 years (EPAT 2001; ESPRIT 2002; Ferenczy 2002; Notelovitz 2002; Obel 1993; Tierney 2009; Yaffe 2006), eight at around 3 years (Barakat 2006; EPHT 2006; ERA 2000; Greenspan 2005; PEPI 1995; WAVE 2002; WEST 2001) and 1 at 4 years (KEEPS 2012). HERS 1998 measured outcomes after 4.1 years and continued the study unblinded for 2.7 additional years. ELITE 2014 measured outcomes after 2.5 years and subsequently at 5 years of HT use.
Investigators planned that interventions in the WHI study would continue for 8.5 years, but both arms of the study were terminated early. WHI 1998 (combined HT arm) was stopped early owing to net harm. Researchers reported outcomes at 5.6 years and over 4 subsequent months of follow‐up for primary and selected outcomes, incorporating events up to the date that participants were instructed to stop their study pills. WHI 1998 (oestrogen‐only HT arm) was also stopped early when it was decided that the prospect of obtaining more precise evidence about effects of the intervention was unlikely to outweigh potential harms, although no predefined safety boundaries had been crossed. Investigators reported results in the oestrogen‐only arm for a mean follow‐up of 7.1 years for primary outcomes: Median time receiving treatment was 5.9 years in the intervention group and 5.8 years in the placebo group. Additional poststudy follow‐up occurred in WHI 1998, as noted below.
Two other studies also closed prematurely in response to WHI 1998 findings (EPHT 2006; WISDOM 2007).
Outcomes
The outcomes measured by individual studies varied according to study objectives. Major clinical events were not primary outcomes for several of these studies but were measured as adverse effects, for example, cardiovascular events or the incidence of cancer and fracture in the study population, or both. Eight studies used biological measures as their primary outcome (ELITE 2014; EPAT 2001; ERA 2000; KEEPS 2012; Notelovitz 2002; PEPI 1995; WAVE 2002; Yaffe 2006).
The largest study in the review (WHI 1998) was concerned mainly with the cardioprotective role of HT in relatively healthy women, and study authors reported cardiovascular clinical endpoints as the primary outcome. They designated invasive breast cancer as a primary adverse outcome and included the incidence of other cancers, fractures, gallbladder disease and death as secondary outcomes. Two other studies (EPHT 2006; WISDOM 2007) measured similar outcomes.
WHI 1998 also conducted a number of analyses not specified in the study protocol. Lung cancer was not a prespecified outcome but was investigated in both arms of the study in post hoc analyses, which included additional follow‐up periods after the planned completion date of the study.
After the intervention phase of WHI 1998 had been completed, investigators followed up major clinical outcomes in surviving participants (i.e. those who consented), comprising 78% of participants in the oestrogen‐only arm and 83% in the combined HT arm. Median cumulative follow‐up (intervention phase plus extended follow‐up) was 13.2 years in the oestrogen‐only arm (including median postintervention follow‐up of 6.6 years) and 13 years in the combined HT group (including median postintervention follow‐up of 6.6 years) (Manson 2013).
WHI 1998 (WHIMS) comprised a large subset of older women from WHI 1998 who were evaluated for probable dementia (the planned primary outcome) and for mild cognitive impairment (as a planned secondary outcome). Researchers also reported global cognitive function, although this was not a formally preplanned endpoint. WHI 1998 (WHIMS) reported separate results for the two study arms and also pooled study results, but we did not include the pooled results in this review (see Methods).
Two smaller studies reported endometrial cancer as a primary outcome (Barakat 2006; Ferenczy 2002), and two (Obel 1993; Tierney 2009) reported as primary outcomes clinical events that were not of interest for this review, but researchers measured outcomes of interest as adverse events.
Five other studies were concerned with the effect of HT on established clinical disease. Four reported cardiovascular outcomes: Primary outcomes were myocardial infarction or death (ESPRIT 2002; HERS 1998), thromboembolism (EVTET 2000) and stroke (WEST 2001). The larger studies also measured a range of other major clinical events such as the incidence of cancer, fracture and gallbladder disease (ESPRIT 2002; HERS 1998). One study reported the effect of HT on global cognitive function (Greenspan 2005) and one on progression of symptoms in women with Alzheimer's disease (Mulnard 2000); another study measured a wide range of clinical outcomes over a treatment period of 10 years with HT in women who were receiving long‐term hospital care for a range of medical conditions (Nachtigall 1979).
Excluded studies
We excluded 42 studies from this review for the following reasons.
29 reported no outcomes of interest for this review.
5 were not double‐blinded.
4 used an intervention of less than 1 year's duration or reported only short‐term (3‐month) outcomes.
3 did not include a placebo group.
1 used a co‐intervention in the HT group.
See Excluded studies.
Risk of bias in included studies
3.
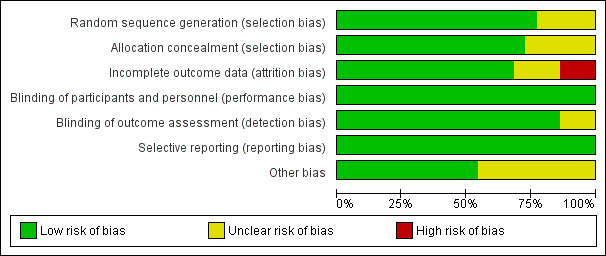
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Seventeen of the 22 studies described a satisfactory method of randomisation, which in all cases was computer generated. Sixteen described a satisfactory method of allocation concealment: In these studies, researchers entered information about an eligible participant, or they accomplished this via remote contact between the recruiting centre and the study coordinating centre or pharmacy. One of these studies (EPHT 2006) randomised women who expressed an interest in participating but did not open the randomisation envelope until their eligibility had been checked and they had consented. Two studies described using computer‐generated randomisation but did not provide details of the procedure for allocation to treatment (EVTET 2000; Mulnard 2000). Three studies supplied no detailed information about randomisation nor allocation concealment (Ferenczy 2002; Nachtigall 1979; Notelovitz 2002).
We rated 17 studies as having low risk of bias related to sequence generation and 16 as having low risk of bias related to allocation concealment. We rated remaining studies as having unclear risk of bias in these domains.
Blinding
All studies described themselves as (at least) double‐blinded. Eighteen studies explicitly stated that all participants, clinical staff and outcome assessors or research staff were blinded to treatment allocation, or they reported 'hard' outcomes unlikely to be influenced by blinding. In the WHI study, 331 women randomised to receive active treatment were unblinded and changed arms from WHI 1998 (oestrogen‐only HT arm) to WHI 1998 (combined HT arm) according to a change in protocol. Three studies apparently blinded participants and clinical staff but did not explicitly state whether outcomes assessors were also blinded (Mulnard 2000; Obel 1993; Tierney 2009)
The larger studies described an unblinded mechanism to be used when required for management of adverse effects. PEPI 1995 unblinded 39 women (4%) during the course of the study, 32 of whom were taking oestrogen‐only HT. WHI 1998 (combined HT arm) reported that during 5.6 years of follow‐up, 3444 women in the combined HT group (40%) and 548 women in the placebo group (6%) were unblinded; whereas in WHI 1998 (oestrogen‐only HT arm), only 100 women in the active group (< 2%) and 83 in the placebo group (< 2%) were unblinded. Nachtigall 1979 reported that 13 women in the HT group and 17 in the control group were unblinded. Two women were unblinded in WISDOM 2007. The other studies did not report such information.
One randomised blinded study (HERS 1998) completed 4.1 years of follow‐up and was then extended for a further duration 2.7 years unblinded.
We rated all studies as having low risk of performance bias and 19 as having low risk of detection bias. We rated three studies as having unclear risk of detection bias.
Incomplete outcome data
For the purposes of this review, we defined losses to follow‐up as participants for whom outcomes of interest were unknown (and who may or may not have had outcomes imputed in statistical analysis). We defined drop‐outs as participants who stopped their allocated treatment (and in some cases changed to a different off‐trial treatment) but had known clinical outcomes and were included in the analysis. Adherence to treatment refers to the number of tablets actually taken, which we often assessed by pill counts (Table 3). We defined intention to treat as analysis of all randomised participants in the groups to which they were randomised.
1. Adherence to treatment.
| Study | How defined | Assessment | HT group | Placebo group | Note |
| Barakat 2006 | Discontinuation of therapy for longer than a month (or use of HT in placebo group) | Not stated | 41.1% compliant for whole follow‐up period (median 3 years) | 50.1% compliant for whole follow‐up period (median 3 years) | |
| ELITE 2014 | > 80% of prescribed treatment taken | Pill counts | Median > 98% over median of 5 years | Median > 98% over median of 5 years | |
| EPAT 2001 | Percentage of study medication consumed | Pill counts | Level of adherence 95% in the 87% of participants evaluated | Level of adherence 92% in the 92% of participants evaluated | |
| EPHT 2006 | > 80% of prescribed treatment taken | Number of collected and returned drugs and clinic reports | < 40% compliant at 3 years (estimated from graph) | < 30% compliant at 3 years (estimated from graph) | |
| ERA 2000 | Percentage of study medication taken | Pill counts | Level of adherence at 3.2 years:
Women on unopposed oestrogen, measured in 79% of participants only: 74% Women on combined HRT, measured in 82% of participants only: 84% |
Level of adherence at 3.2 years: Measured in 80% of participants only: 86% 5 women initiated treatment outside study | |
| ESPRIT 2002 | "Regular tablet use" | Self‐report to family doctor. Self‐report to study nurse at 6 weeks and whenever in contact with trial staff | Number non‐adherent: 51% at 12 months 57% at 24 months | Number non‐adherent: 31% at 12 months 337% at 24 months | Triallists attribute higher non‐compliance in HRT group to prevalence of vaginal bleeding (reported by 56% in HRT group, 7% in controls) |
| EVTET 2000 | Adherence not described | ||||
| Ferenczy 2002 | Adherence not described | ||||
| Greenspan 2005 | "Taking at least 80% of medication for at least 80% of entire study period" | Pill counts 6‐monthly | 90% adherent at 3 years | 94% adherent at 3 years | |
| HERS 1998 | Taking at least 80% of study medication | Pill counts | 79% adherent at 1 year 70% adherent at 3 years 3% initiated treatment outside study About 50% continued to use open‐label HRT during unblinded follow up (4.2‐6.8 years) | 91% adherent at 1 year 81% non‐adherent at 3 years Less than 10% used HRT during unblinded follow‐up (4.2‐6.8 years) | Proportion of women who reported taking study medication at 1 year: HRT group: 82% Placebo group: 91% |
| KEEPS 2012 | Pill or patch counts, percentage used | Pill counts or weights | 94%‐95% in all groups, among women who completed trial at 4 years | ||
| Mulnard 2000 | Taking at least 80% of study medication | Plasma oestradiol level evaluation at each visit Pill counts at each visit | No information given in publication | ||
| Nachtigall 1979 | Adherence not described | ||||
| Notelovitz 2002 | Adherence not described | ||||
| Obel 1993 | Adherence not described | ||||
| PEPI 1995 | Taking at least 80% of study medication | Study diary reviewed at clinic visits Pill counts | Number adherent at 36 months:
Women without uterus: 80%‐89% at 36 months Women with uterus: 1. On unopposed CEE: 44% 2. On combined therapy: 80% |
Number adherent at 36 months:
Women without uterus: 67% Women with uterus: 76% |
|
| Tierney 2009 | Taking at least 80% of study medication | Pill counts weekly | No information given in publication | ||
| WAVE 2002 | Percentage of study medication taken | Pill counts | At 2.8 years: Adherence 67% in the 78% of women analysed | At 2.8 years: Adherence 70% in the 81% of women analysed | |
| WEST 2001 | Percentage of study medication taken | Self‐report to study nurse 3‐monthly Computer chip in medication bottle records opening date and time Pill counts | At 2.8 years:
Mean adherence including drop‐outs: 70% Mean adherence excluding dropouts: 90% 35% discontinued medication by 2.8 years, of whom 1% initiated treatment outside study |
At 2.8 years:
Mean adherence including dropouts: 74% over 2.8 years Mean adherence excluding dropouts: 90% 24% discontinued medication 2% initiated treatment outside study |
|
| WHI 1998 (unopposed oestrogen arm) | Taking at least 80% of study medication. Temporary discontinuation (e.g. during surgery) permitted | Weighing of returned medication bottles | At 6.8 years, about 53.8% of women were non‐adherent In addition, 5.7% of women had initiated hormone use through their own physician | At 6.8 years, about 53.8% of women were non‐adherent In addition 9.1% of women had initiated hormone use through their own physician | |
| WHI 1998 (combined arm) | Taking at least 80% of study medication. Temporary discontinuation (e.g. during surgery) permitted | Weighing of returned medication bottles | 42% non‐adherent by 5.2 years Of these, 6.2% initiated HRT outside study | 10.7% crossed to active treatment by 5.2 years | Analyses censoring events 6 months after non‐adherence increased effect sizes |
| WISDOM 2007 | Supply of study medication | Time at risk minus temporary interruptions and time after withdrawal from treatment | 73% of time | 86% of time | Women had a 3 month run‐in period on placebo. Only women who took 80% of tablets were randomised |
| Yaffe 2006 | Supply of study medication | Patch counts: 75% use over 2 years counted as compliance | 84% | 84% of time | Women had a 1 week run‐in period. Only compliant women were randomised. |
Drop‐out rates were generally high, particularly in the active treatment groups, and they increased over time. In WHI 1998 (combined HT arm), 42% of the active treatment group and 38% of the placebo group were no longer taking their allocated treatment at 5 years, and a further 10.7% of the placebo group had crossed to active therapy. In WHI 1998 (oestrogen‐only HT arm), 53% of participants overall were no longer taking their allocated treatment at 6.8 years, and a further 5.7% had initiated hormone use outside the study. See the Characteristics of included studies table and Table 3 for details on drop‐outs and non‐adherence in other studies.
Losses to follow‐up were low in most studies, with no women lost to follow‐up in seven studies (EPAT 2001; ERA 2000; ESPRIT 2002; EVTET 2000; Mulnard 2000; Nachtigall 1979; WEST 2001), and 1% to 5.2% lost in five other studies, all of which were large and of long duration (3 to 6.8 years) (Greenspan 2005; HERS 1998; PEPI 1995; WAVE 2002; WHI 1998). Only five women (0.01%) were lost to follow‐up in WISDOM 2007. The Estonian study monitored outcomes by means of linkages to a national health insurance database and national cancer registry, and study authors stated that the probability of missing data in these databases was small (EPHT 2006). However, different publications for this study (EPHT 2006) reported slightly different numbers of randomised participants. In six smaller studies of 1 to 5 years' duration, a higher proportion of women (8.5% to 21%) were lost to follow‐up (ELITE 2014; KEEPS 2012; Notelovitz 2002; Obel 1993; Tierney 2009; Yaffe 2006), and in Ferenczy 2002, results were unavailable for 34% of participants for the outcome of interest for this review. It was unclear whether any women were lost to follow‐up in Barakat 2006 (see Description of studies).
Fourteen of the included studies supplied sufficient data to permit an intention‐to‐treat (ITT) analysis, at least for all reported outcomes of interest for this review (EPAT 2001; ERA 2000; ESPRIT 2002; EVTET 2000; Greenspan 2005; HERS 1998; KEEPS 2012; Mulnard 2000; Nachtigall 1979; Notelovitz 2002; WEST 2001; WHI 1998; WISDOM 2007; Yaffe 2006), or such data were extractable, and a further two studies analysed more than 97% of participants by intention to treat (PEPI 1995; WAVE 2002). Five studies did not include all participants in an ITT analysis for outcomes of interest (ELITE 2014; EVTET 2000; Ferenczy 2002; Obel 1993; Tierney 2009). It was unclear whether one study used ITT analysis because investigators provided no description of participants other than those that were "eligible and assessable" (Barakat 2006), and one study had slightly differing participation rates across trial publications (EPHT 2006).
WHI 1998 (combined HT arm) and WHI 1998 (WHISCA) continued follow‐up beyond the planned study completion date (March 2005) for women who consented to continue follow‐up. All women had already been instructed to stop taking their assigned study medication in July 2002. Seventeen per cent of surviving women in WHI 1998 (combined HT arm) declined to provide re‐consent, and their data were censored for the additional follow‐up period. Baseline characteristics were similar in the two groups, and imputation analyses suggested that this loss to follow‐up did not significantly influence study findings. Fifteen per cent of women in WHI 1998 (WHISCA) declined to continue follow‐up. The study extension phase ran from April 2005 to September 2010. WHI 1998 (oestrogen‐only HT arm) also conducted extended follow‐up (in 78% of surviving participants) from April 2005 to September 2010; among women who provided additional consent, baseline characteristics were similar to those of the original randomised group.
We rated 16 studies as having low risk of attrition bias, four as having unclear risk and three as having high risk.
Selective reporting
All studies reported all expected outcomes, and we rated them as having low risk of selective reporting.
Other potential sources of bias
Eleven of the included studies had other potential sources of bias (ELITE 2014; EPHT 2006; ERA 2000; Greenspan 2005; Mulnard 2000; Nachtigall 1979; Obel 1993; PEPI 1995; Tierney 2009; WAVE 2002; WHI 1998); we rated them as havng unclear risk of this bias. In most cases, potential bias was related to baseline imbalance between participants in individual prognostic characteristics and did not appear likely to have a marked effect on outcomes. We rated the other studies as having low risk of bias in this domain.
Effects of interventions
Summary of findings for the main comparison. Combined continuous hormone therapy (HT) compared with placebo for postmenopausal women.
| Combined continuous hormone therapy (HT) compared with placebo for perimenopausal and postmenopausal women | ||||||
|
Population: relatively healthy postmenopausal women Setting: community Intervention: combined continuous HT (moderate‐dose oestrogen) ‐ CEE 0.625 mg + MPA 2.5 mg Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk* | Corresponding risk | |||||
| Placebo | Combined continuous hormone therapy (HT) | |||||
|
Coronary events (MI or cardiac death) Follow‐up: mean/median 1 year |
2 per 1000 | 4 per 1000 (3 to 7) | RR 1.89 (1.15 to 3.10) | 20,993 (2 studies) | ⊕⊕⊕⊝ Moderatea | |
| Stroke Follow‐up: mean 3 years | 6 per 1000 | 8 per 1000 (6 to 12) | RR 146 (1.02 to 2.09) | 17,585 (2 studies) | ⊕⊕⊕⊝ Moderatea | |
|
Venous thromboembolism (DVT or PE) Follow‐up: mean/median 1 year |
2 per 1000 | 7 per 1000 (4 to 11) | RR 4.28 (2.49 to 7.34) | 20,993 (2 studies) | ⊕⊕⊕⊝ Moderatea | |
| Breast cancer Follow‐up: median 5.6 years | 19 per 1000 | 24 per 1000 (20 to 30) | RR 1.27 (1.03 to 1.56) | 16,608 (1 study) | ⊕⊕⊕⊝ Moderatea | |
|
Death from lung cancer Follow‐up: median 8 yearsb |
5 per 1,000 |
9 per 1000 (6 to 13) |
RR 1.74 (1.18 to 2.55) |
16,608 (1 study) | ⊕⊕⊕⊝ Moderatea | |
|
Gallbladder disease Follow‐up: mean 5.6 years |
16 per 1000 | 27 per 1000 (21 to 34) |
RR 1.64 (1.30 to 2.06) |
14,203 (1 study) |
⊕⊕⊕⊝ Moderatea | |
| All clinical fractures Follow‐up: mean 5.6 years | 111 per 1000 | 87 per 1000 (79 to 96) | RR 0.78 (0.71 to 0.86) | 16,608 (1 study) | ⊕⊕⊕⊝ Moderatea | |
| *The basis for the assumed risk is the mean risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded one level for questionable applicability: Only about 33% of the study sample was 50‐59 years of age at baseline (i.e. the age women are most likely to consider HT for vasomotor symptoms); mean participant age was 63 years.
b5.6 years' intervention plus postintervention follow‐up: post hoc analysis.
Summary of findings 2. Oestrogen‐only hormone therapy (HT) compared with placebo for postmenopausal women.
| Oestrogen‐only hormone therapy (HT) compared with placebo for perimenopausal and postmenopausal women | ||||||
|
Population: relatively healthy postmenopausal women Setting: community Intervention: oestrogen‐only HT (moderate dose) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oestrogen‐only hormone therapy (HT) | |||||
| Coronary events (MI or cardiac death) Follow‐up: mean 7.1 yearsa | 41 per 1000 | 38 per 1000 (32 to 46) | RR 0.94 (0.78 to 1.13) | 10,739 (1 study) | ⊕⊕⊕⊝ Moderateb | |
| Stroke Follow‐up: mean 7.1 yearsa | 24 per 1000 | 32 per 1000 (25 to 40) | RR 1.33 (1.06 to 1.67) | 10,739 (1 study) | ⊕⊕⊕⊝ Moderateb | |
|
Venous thromboembolism (DVT or PE) Follow up 1‐2 years |
2 per 1000 |
5 per 1000 (2 to 10) |
RR 2.22 (1.12 to 4.39) |
10,739 (1 study) | ⊕⊕⊕⊝ Moderateb | |
|
Venous thromboembolism (DVT or PE): CEE 0.625 mg (moderate dose) Follow‐up: mean 7.1 yearsa |
16 per 1000 | 21 per 1000 (16 to 28) | RR 1.32 (1.00 to 1.74) | 10,739 (1 study) | ⊕⊕⊕⊝ Moderateb | |
| Breast cancer Follow‐up: mean 7.1 yearsa | 25 per 1000 | 20 per 1000 (15 to 25) | RR 0.79 (0.61 to 1.01) | 10,739 (1 study) | ⊕⊕⊕⊝ Moderateb | |
|
Gallbladder disease Follow‐up: mean 7.1 yearsa |
27 per 1000 |
47 per 1000 (38 to 60) |
RR 1.78 (1.42 to 2.24) |
8376 (1 study) | ⊕⊕⊕⊝ Moderateb | |
| All clinical fractures Follow‐up: mean 7.1 yearsa | 141 per 1000 | 103 per 1000 (92 to 113) | RR 0.73 (0.65 to 0.80) | 10,739 (1 study) | ⊕⊕⊕⊝ Moderateb | |
| *The basis for the assumed risk is the mean risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aMedian use of CEE 5.9 years (LaCroix 2011). bDowngraded one level for questionable applicability: Only 31% of study sample was 50‐59 years of age at baseline (i.e. the age women are most likely to consider HT for vasomotor symptoms); mean participant age was 63 years.
We present the results below. In most cases, details of effect measures are reported in the text only when results were statistically significant. For full results of all comparisons, see Data and analyses. See also Table 1 and Table 2.
We grouped results as follows.
-
By outcome.
We grouped outcomes such as death, cardiovascular events, cognitive measures and quality of life according to the clinical status of participant groups, in the following order: relatively healthy women, women with a history of cardiovascular disease, women hospitalised with chronic illness and women with dementia.
For outcomes such as cancer, fracture and gallbladder disease, we grouped all participants together as 'all women'.
-
By intervention.
Oestrogen‐only HT.
Combined continuous HT regimens.
Combined sequential regimens.
Within these categories, we have grouped interventions according to the oestrogen dose used, with equivalence between doses based on the Australian Menopause Society guide to equivalent HT doses (AMS 2016), which classifies HT as low dose (e.g. oral oestradiol 1 mg), medium dose (e.g. oral oestradiol 2 mg, transdermal oestradiol 50 µg, conjugated equine oestrogen 0.065 mg) or higher dose (e.g. transdermal oestradiol 75 µg).
Meta‐analysis
Although comparisons with similar oestrogen doses are grouped together, we pooled comparisons (meta‐analysed) only if they used the same combination of oestrogen and progestogen for the same (or a similar) length of time. WHI 1998 and PEPI 1995 used the same HT regimen and reported several of the same clinical outcomes at 3 years, but in most cases, PEPI 1995 reported no events in either arm. We combined three studies (ERA 2000; HERS 1998; WAVE 2002) for some 3‐year (2.8 to 3.2) outcomes, but otherwise meta‐analysis was inappropriate for most outcomes because the studies used different types or doses of oestrogen or progestogen, or both, and these do not necessarily have the same metabolic effects; or they used different durations of HT, which might have led to different effects as the result of trends over time.
Very few results were suitable for pooling; therefore, statistical heterogeneity was not a major issue in this review. One meta‐analysis displayed statistically significant heterogeneity (I2 = 66.2%), but it involved only two small studies with few events, and we attributed the heterogeneity to chance (Analysis 2.21).
2.21. Analysis.
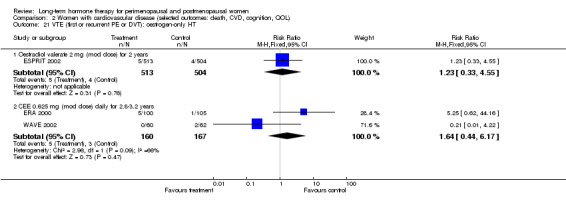
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 21 VTE (first or recurrent PE or DVT): oestrogen‐only HT.
Time points for reporting results
In some cases, we rounded up or down time points for reporting results, as follows.
WHI 1998 (oestrogen‐only HT arm) reported results after a mean follow‐up of 7.1 or 7.9 years. Among women who consented (78% of those surviving), follow‐up was extended (for a median of 6.6 years) after the predefined study termination date to achieve a cumulative median follow‐up of 13.2 years. The median duration of active treatment in this arm of the study was 5.8 to 5.9 years (LaCroix 2011). We have reported results at mean or median follow‐up points as reported by the study publications.
WHI 1998 (combined HT arm) reported results after a mean of 5.6 years of active treatment (intervention phase) or at a mean of 7.9 years. The 7.9‐year follow‐up included 2.4 years of postintervention follow‐up and continued up to the predefined study termination date (31 March 2005). Among women who consented (83% of those surviving), follow‐up was extended after the predefined study termination date for a median of 6.6 years to achieve median cumulative follow‐up of 13 years. This arm of the study also reported selected clinical outcomes for each year of follow‐up: All women had been enrolled for at least 3.5 years at the time of the study publication, so we used these data to calculate outcomes on an ITT basis after 1, 2 and 3 years of use of HT, with all randomised participants inserted as the denominator (Chlebowski 2009). We have reported results at mean or median follow‐up points as reported by the study publications.
EPHT 2006 reported results for most outcomes at a mean follow‐up of 3.43 years, with a range of 2 to 5 years. Results for quality of life were reported at a mean of 3.6 years. We have reported results in our tables as if all women underwent 3 years of follow‐up.
WISDOM 2007 reported results after a median follow‐up of 11.9 months (range 7.1 to 19.6). We have reported results in our tables as if all women had undergone 1 year's follow‐up.
Barakat 2006 reported results after a median follow‐up of 35.7 months. We have reported results in our tables as if all women had undergone 3 years of follow‐up.
HERS 1998 reported results from the blinded portion of the study after a mean follow‐up of 4.1 years, which we mentioned above (see Methods). These results were presented as dichotomous data, and investigators reported selected clinical outcomes for each year of follow‐up. All women had been enrolled for at least 3 years at the time of the report, so for this review, we have used these data to calculate outcomes on an ITT basis after 1, 2 and 3 years of HT use, with all randomised participants inserted as the denominator.
Results for outcomes of interest
We derived all of the statistically significant findings of this review from the two biggest studies ‐ HERS 1998 and WHI 1998 ‐ both of which reported adequate methods of allocation concealment, analysed all participants by intention to treat and reported small losses to follow‐up (1% to 5.2%).
1. Death from any cause (total mortality)
Relevant comparisons
Seven studies (ELITE 2014; EPHT 2006; EPAT 2001; KEEPS 2012; PEPI 1995; WHI 1998; WISDOM 2007) with a total of eight different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome in healthy women.
Five studies of women with cardiovascular disease (ERA 2000; ESPRIT 2002; HERS 1998; WAVE 2002; WEST 2001) with a total of four different interventions, comprising comparisons of oestrogen‐only HT and combined continuous HT versus placebo for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured death from any cause.
Two other studies measured this outcome: one comparing oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer (Barakat 2006), and one (Nachtigall 1979) comparing combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
Results of analysis show no statistically significant difference between HT and placebo for this outcome in any population group (Analysis 1.1; Analysis 1.2; Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 4.1; Analysis 5.1).
1.1. Analysis.
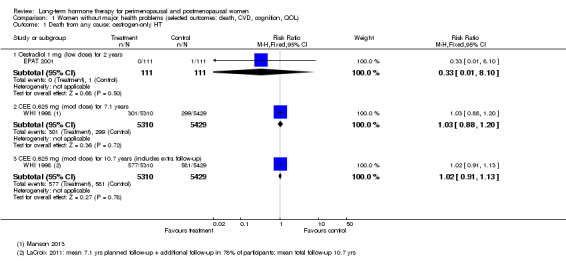
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 1 Death from any cause: oestrogen‐only HT.
1.2. Analysis.
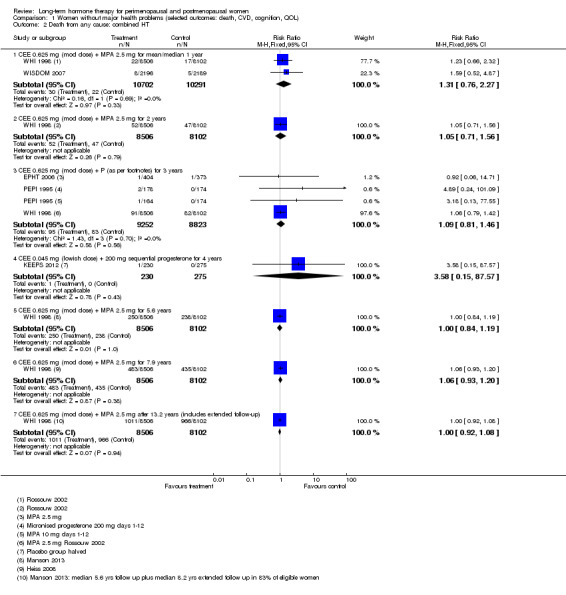
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 2 Death from any cause: combined HT.
2.1. Analysis.
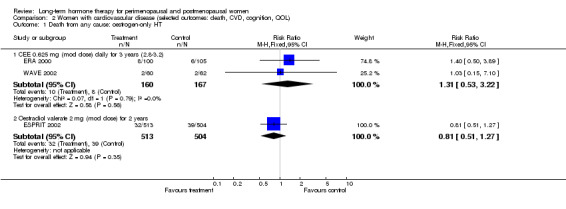
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 1 Death from any cause: oestrogen‐only HT.
2.2. Analysis.
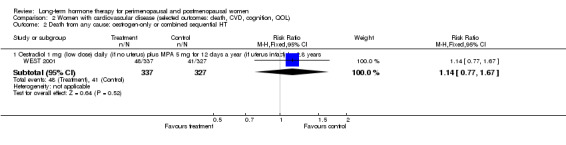
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 2 Death from any cause: oestrogen‐only or combined sequential HT.
2.3. Analysis.
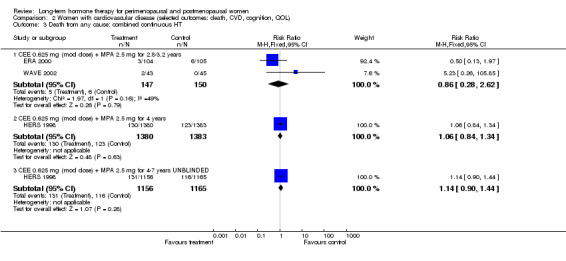
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 3 Death from any cause: combined continuous HT.
4.1. Analysis.
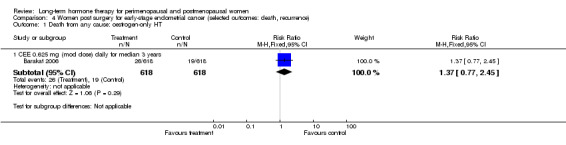
Comparison 4 Women post surgery for early‐stage endometrial cancer (selected outcomes: death, recurrence), Outcome 1 Death from any cause: oestrogen‐only HT.
5.1. Analysis.
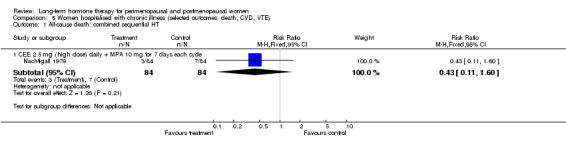
Comparison 5 Women hospitalised with chronic illness (selected outcomes: death, CVD, VTE), Outcome 1 All‐cause death: combined sequential HT.
2. Cause‐specific mortality
2.1 Death from coronary heart disease
Relevant comparisons
Four studies (EPAT 2001; Tierney 2009; WHI 1998; WISDOM 2007) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome in relatively healthy women.
Five studies of women with cardiovascular disease (ERA 2000; ESPRIT 2002; HERS 1998; WAVE 2002; WEST 2001) with a total of four different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured death from coronary heart disease.
In addition, the study comparing oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer measured this outcome (Barakat 2006).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome in any population group (Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 4.3).
1.4. Analysis.
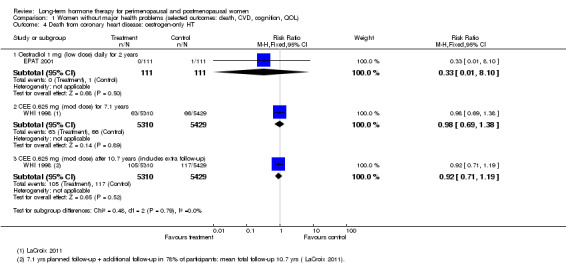
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 4 Death from coronary heart disease: oestrogen‐only HT.
1.5. Analysis.
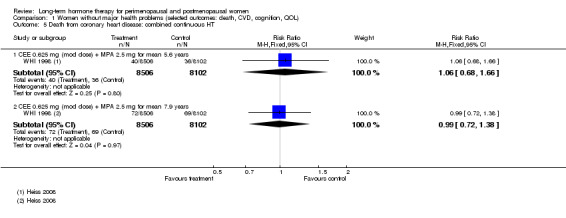
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 5 Death from coronary heart disease: combined continuous HT.
1.6. Analysis.
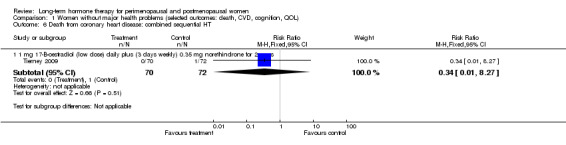
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 6 Death from coronary heart disease: combined sequential HT.
2.4. Analysis.
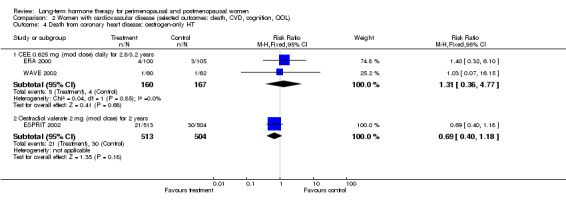
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 4 Death from coronary heart disease: oestrogen‐only HT.
2.5. Analysis.
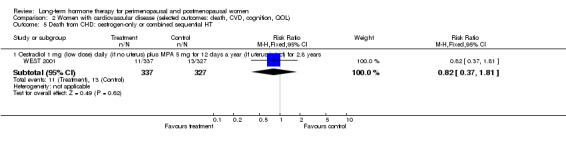
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 5 Death from CHD: oestrogen‐only or combined sequential HT.
2.6. Analysis.
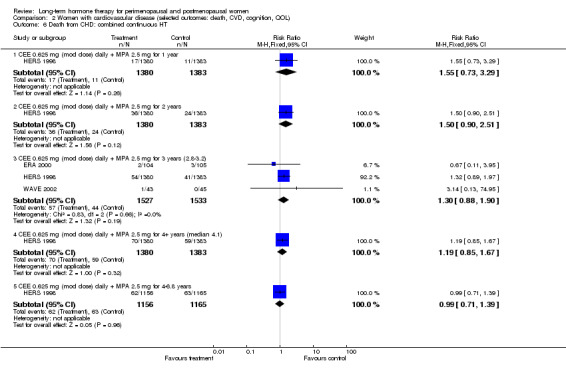
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 6 Death from CHD: combined continuous HT.
4.3. Analysis.
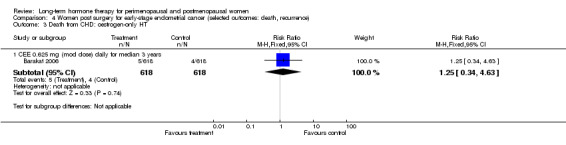
Comparison 4 Women post surgery for early‐stage endometrial cancer (selected outcomes: death, recurrence), Outcome 3 Death from CHD: oestrogen‐only HT.
2.2 Death from stroke
Relevant comparisons
Four comparisons of relatively healthy women taking combined continuous HT for 1 year (WISDOM 2007) and for 5.6 years (WHI 1998 (combined HT arm)), or taking oestrogen‐only HT for 7.1 years (WHI 1998 (oestrogen‐only HT arm)) or taking combined sequential HT for 2 years (Tierney 2009), reported this outcome. One study of women with a history of stroke who were taking oestrogen‐only HT (with annual progesterone for women who had a uterus) for 2.8 years (WEST 2001) also reported this outcome.
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 1.7; Analysis 1.9; Analysis 1.8; Analysis 2.8).
1.7. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 7 Death from stroke: oestrogen‐only HT.
1.9. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 9 Death from stroke: combined continuous HT.
1.8. Analysis.
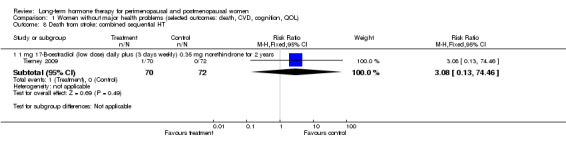
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 8 Death from stroke: combined sequential HT.
2.8. Analysis.
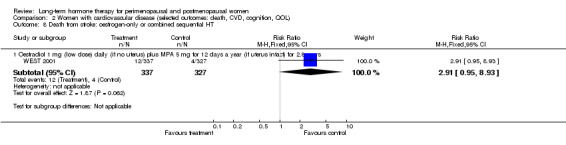
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 8 Death from stroke: oestrogen‐only or combined sequential HT.
2.3 Death from breast cancer
Relevant comparisons
One study of comparatively healthy women taking oestrogen‐only HT for a median of 7.2 years (WHI 1998 (oestrogen‐only HT arm)) with postintervention follow‐up for a median of 4.7 years reported this outcome, as did two studies of relatively healthy women taking combined continuous HT for 1 year (WISDOM 2007) and for 5.6 years (WHI 1998). Follow‐up for breast cancer outcomes was continued for a mean total of 11 years among women in WHI 1998 (combined HT arm) who agreed to continue follow‐up after the planned study completion date (Chlebowski 2010).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome at 1 or 5.6 years.
Among women taking oestrogen‐only HT, after a median of 11.8 years (7.2 years' intervention plus postintervention follow‐up), the death rate from breast cancer was lower in the HT arm (risk ratio (RR) 0.38, 95% confidence interval (CI) 0.15 to 0.98) (WHI 1998 (oestrogen‐only HT; Analysis 1.12).
1.12. Analysis.
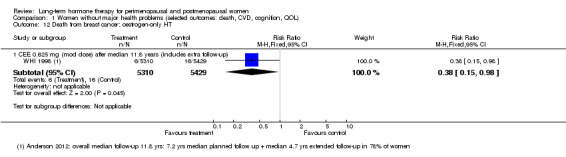
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 12 Death from breast cancer: oestrogen‐only HT.
At 11 years' follow‐up, WHI 1998 (combined HT arm) reported more deaths from breast cancer in the HT group than in the placebo group; this finding was of borderline statistical significance (RR 1.98, 95% CI 1.00 to 3.95; Analysis 1.11). Absolute risk of breast cancer increased from 1 per 1000 in the control group to 3 per 1000 (95% CI 1 to 6) in the HT group.
1.11. Analysis.
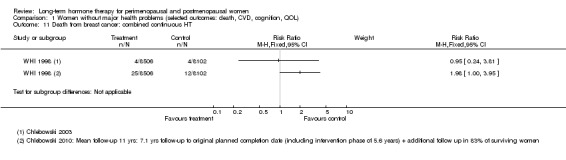
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 11 Death from breast cancer: combined continuous HT.
At 11 years' follow‐up, researchers also found that significantly more deaths resulted from all causes after a breast cancer diagnosis in the combined HT group than in the placebo group (published hazard ratio (HR) 1.57, 95% CI 1.01 to 2.48; P = 0.045) (Chlebowski 2010).
2.4 Death from colorectal cancer
Relevant comparisons
Investigators reported this outcome in relatively healthy women in the oestrogen‐alone group of WHI 1998 after mean follow‐up of 7.1 years, as well as in the WHI 1998 (combined HT arm) at mean follow‐up of 5.6 and 7.1 years. Researchers also reported on this after 11.6 years' follow‐up, including a mean of 5.6 years' intervention plus postintervention follow‐up after the study ended, in 83% of participants (Simon 2012).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 1.10; Analysis 1.13).
1.10. Analysis.
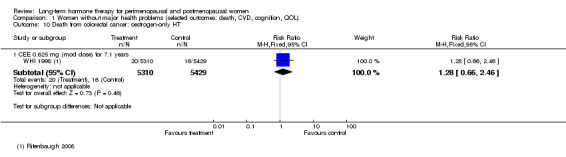
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 10 Death from colorectal cancer: oestrogen‐only HT.
1.13. Analysis.
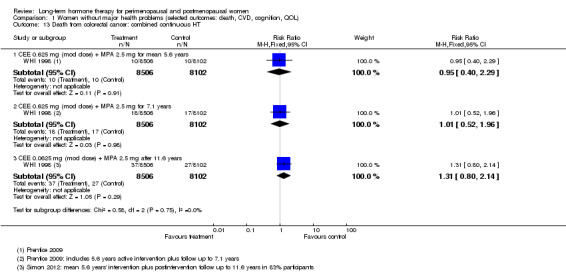
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 13 Death from colorectal cancer: combined continuous HT.
2.5 Death from endometrial cancer
Relevant comparisons
The study comparing oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer reported this outcome (Barakat 2006).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 4.2).
4.2. Analysis.
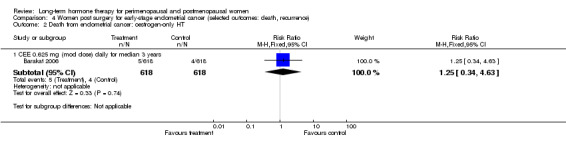
Comparison 4 Women post surgery for early‐stage endometrial cancer (selected outcomes: death, recurrence), Outcome 2 Death from endometrial cancer: oestrogen‐only HT.
2.6 Death from lung cancer
Relevant comparisons
WHI 1998 reported this outcome in relatively healthy women in the oestrogen‐only HT group in a post hoc analysis after mean follow‐up of 7.9 years (including 8 months' follow‐up post intervention) (Chlebowski 2010b), and in the combined HT arm of WHI 1998 in a post hoc analysis after mean follow‐up of 8 years (including 2.4 years' follow‐up post intervention) (Chlebowski 2009). Study authors reported lung cancer overall, non‐small cell lung cancer and small cell lung cancer separately. One much smaller study (Tierney 2009) reported this outcome in women taking combined sequential HT or placebo.
Results
Results of analysis show no statistically significant differences between HT and placebo for any of these outcomes among women in the oestrogen‐only HT arm of WHI 1998 (Analysis 1.14). However, in the combined HT arm of WHI 1998, women in the intervention group were significantly more likely to die of lung cancer overall (RR 1.74, 95% CI 1.18 to 2.55), or of non‐small cell lung cancer (RR 1.91, 95% CI 1.24 to 2.93), than women in the placebo arm (Analysis 1.15). Absolute risk of lung cancer increased from 5 per 1000 in the control group to 9 per 1000 (95% CI 6 to 13) in the HT group. This finding was independent of smoking status. The mortality rate for small cell lung cancer did not differ significantly between groups. Review authors noted no statistically significant findings in the combined sequential HT study (Analysis 1.16).
1.14. Analysis.
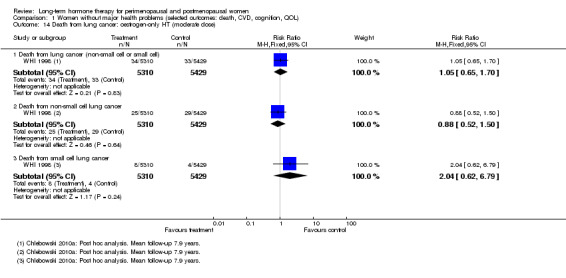
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 14 Death from lung cancer: oestrogen‐only HT (moderate dose).
1.15. Analysis.
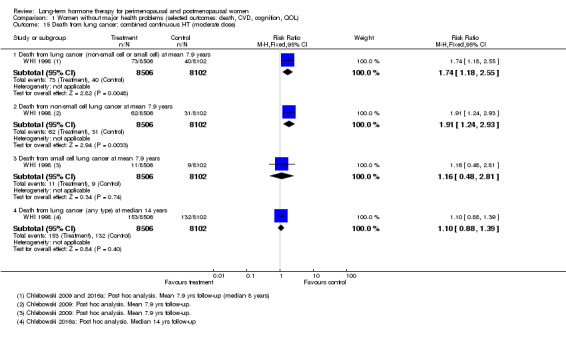
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 15 Death from lung cancer: combined continuous HT (moderate dose).
1.16. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 16 Death from lung cancer: combined sequential HT (low dose oestrogen).
2.7 Death from any cancer
Relevant comparisons
Two studies of relatively healthy women taking continuous HT for 1 year (WISDOM 2007) and for 5.6 years (WHI 1998 (combined HT arm)) and one study of women with cardiovascular disease taking combined continuous HT for 4.1 years, with unblinded follow‐up to 6.8 years (HERS 1998) reported this outcome.
Results
Results of analysis showed no statistically significant differences between HT and placebo for this outcome (Analysis 1.17; Analysis 2.9).
1.17. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 17 Death from any cancer: combined continuous HT.
2.9. Analysis.
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 9 Death from cancer: combined continuous HT.
3. Coronary events (myocardial infarction or cardiac death)
Relevant comparisons
Eight studies (ELITE 2014; EPAT 2001; EPHT 2006; KEEPS 2012; PEPI 1995; Tierney 2009; WHI 1998, WISDOM 2007) with a total of nine different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for varying durations from 1 year to over 7 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm) (LaCroix 2011) and to 13.2 years in the combined HT arm (Manson 2013), reported this outcome in relatively healthy women.
Six studies (ERA 2000; ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002; WEST 2001) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured coronary events as an outcome in women with cardiovascular disease.
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
WHI 1998 (oestrogen‐only HT arm) reported no statistically significant difference between the two groups for this outcome (Analysis 1.18). However, WHI 1998 (combined HT arm) reported that relatively healthy women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) were at significantly higher risk of a coronary event after taking HT for 1, 2 and 3 years (at 1 year: RR 1.74 (95% CI 1.05 to 2.89); at 2 years: RR 1.49 (95% CI 1.05 to 2.12); at 3 years: RR 1.43 (95% CI 1.05 to 1.95)). At mean follow‐up of 5.6 years, researchers noted no statistically significant differences between groups (RR 1.17, 95% CI 0.95 to 1.44), and they observed no differences between groups after extended follow‐up to 13.2 years. WISDOM 2007 and EPHT 2006 reported data for this outcome at 1 year and at 3 years, respectively. Pooling these data with data from WHI 1998 (combined HT arm) resulted in a risk ratio at 1 year of 1.89 (95% CI 1.15 to 3.10) and at 3 years of 1.45 (95% CI 1.07 to 1.98; Analysis 1.19). Absolute risk of a coronary event increased after 1 year from 2 per 1000 in the control group to 4 per 1000 (95% CI 3 to 7) in the HT group; after 2 years from 6 per 1000 in the control group to 9 per 1000 (95% CI 7 to 13) in the HT group; and after 4 years from 8 per 1000 in the control group to 11 per 1000 (95% CI 8 to 13) in the HT group.
1.18. Analysis.
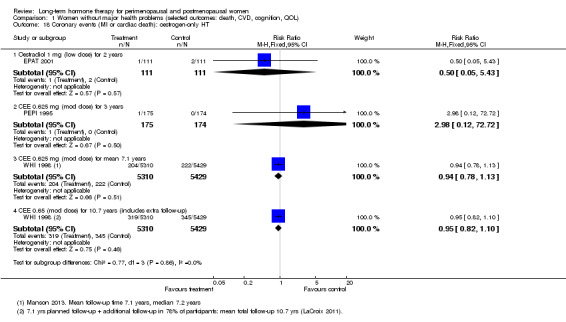
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 18 Coronary events (MI or cardiac death): oestrogen‐only HT.
1.19. Analysis.
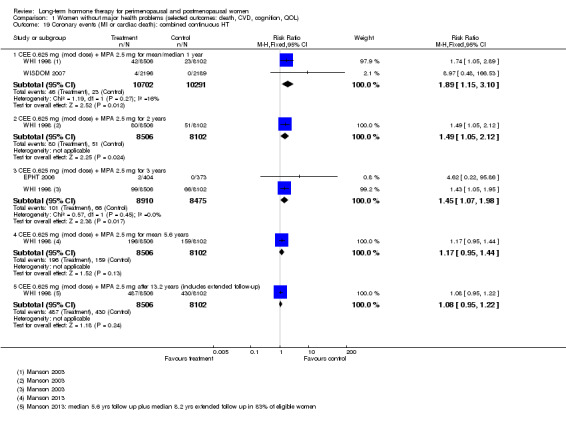
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 19 Coronary events (MI or cardiac death): combined continuous HT.
No other studies found statistically significant differences between HT and placebo for this outcome (Analysis 1.20; Analysis 2.10; Analysis 2.7; Analysis 2.11; Analysis 2.12; Analysis 5.2). HERS 1998 reported results of borderline statistical significance at 1 year, suggesting increased risk for women with cardiovascular disease taking combined continuous therapy (RR 1.5, 95% CI 1.00 to 2.25; Analysis 2.12), and initial analysis of time trends in HERS 1998 suggested a trend towards increased risk in the HT group that diminished over time. However, subsequent analysis based on the entire 6.8 years of follow‐up (blinded and unblinded) showed no statistically significant variation in risk over time.
1.20. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 20 Coronary events (MI or cardiac death): combined sequential HT.
2.10. Analysis.
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 10 Coronary event (MI or cardiac death): oestrogen‐only HT.
2.7. Analysis.
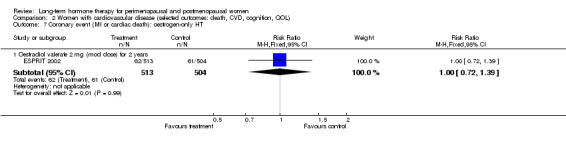
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 7 Coronary event (MI or cardiac death): oestrogen‐only HT.
2.11. Analysis.
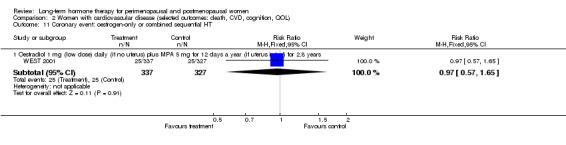
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 11 Coronary event: oestrogen‐only or combined sequential HT.
2.12. Analysis.
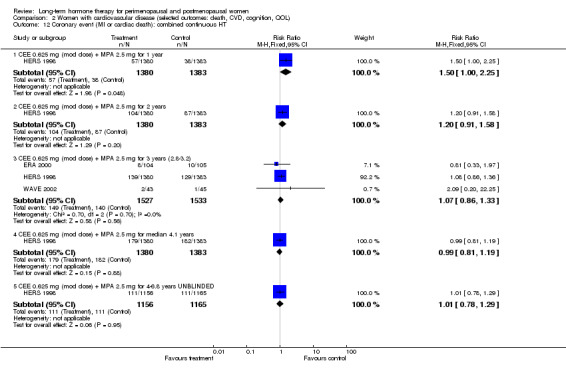
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 12 Coronary event (MI or cardiac death): combined continuous HT.
5.2. Analysis.
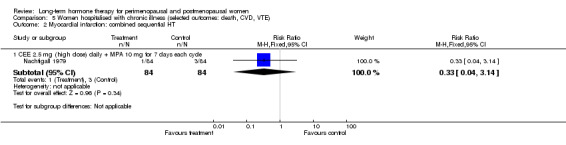
Comparison 5 Women hospitalised with chronic illness (selected outcomes: death, CVD, VTE), Outcome 2 Myocardial infarction: combined sequential HT.
4. Stroke and transient ischaemic attack
4.1 Stroke
Relevant comparisons
Six studies (EPAT 2001; EPHT 2006; KEEPS 2012; PEPI 1995; Tierney 2009; WHI 1998) with a total of seven different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, reported this outcome in relatively healthy women; WHI 1998 (oestrogen‐only arm) extended follow‐up to 10.7 years, and Manson 2013 extended follow‐up to 13.2 years in the combined HT arm.
Five studies (ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002; WEST 2001) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured this outcome in women with cardiovascular disease.
Results
WHI 1998 (oestrogen‐only HT arm) reported a statistically significant increase in the incidence of stroke at 7.1 years' follow‐up (RR 1.33, 95% CI 1.06 to 1.67; Analysis 1.22). Absolute risk of a stroke increased from 23 per 1000 in the control group to 32 per 1000 (95% CI 25 to 40) in the HT group. Study authors noted that the excess in the intervention arm was due to increased risk of ischaemic rather than haemorrhagic stroke, and that the excess risk became apparent after 4 years' follow‐up (Hendrix 2006). However, increased risk was not maintained during extended follow‐up (overall 10.7 years; RR 1.17, 95% CI 0.97 to 1.40) (LaCroix 2011). Although WHI 1998 (combined HT arm) reported no statistically significant differences between groups in the incidence of stroke during the first 2 years of the study, women taking combined continuous HT were at significantly higher risk of stroke after taking HT for 3 or more years (at 3 years: RR 1.47, 95% CI 1.02 to 2.11; at a mean of 5.6 years: RR 1.39, 95% CI 1.09 to 1.77; at a mean of 7.9 years: RR 1.29, 95% CI 1.06 to 1.56). A statistically significant difference between groups was no longer evident at 13.2 years (RR 1.15, 99% CI 0.99 to 1.33). EPHT 2006 also reported data for this outcome at 3 years; pooling these data with data from WHI 1998 (combined HT arm) resulted in a risk ratio at 3 years of 1.46 (95% CI 1.02 to 2.09; Analysis 1.23). Absolute risk of a stroke increased at 3 years from 6 per 1000 in the control group to 8 per 1000 (95% CI 6 to 12) in the HT group; at 5.6 years from 14 per 1000 in the control group to 19 per 1000 (95% CI 15 to 24) in the HT group; and at 7.9 years from 21 per 1000 in the control group to 28 per 1000 (95% CI 23 to 34) in the HT group.
1.22. Analysis.
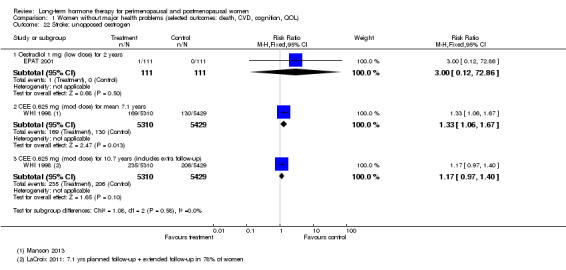
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 22 Stroke: unopposed oestrogen.
1.23. Analysis.
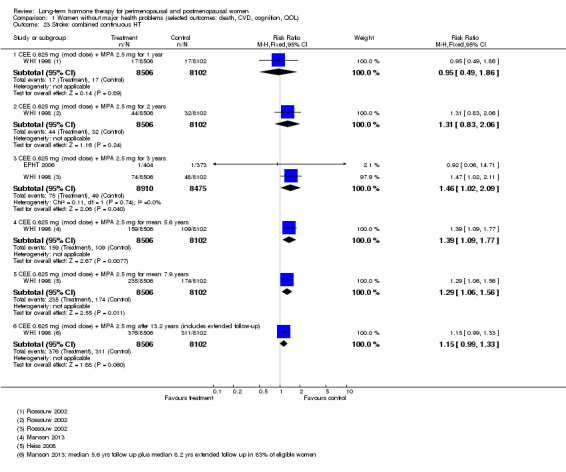
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 23 Stroke: combined continuous HT.
None of the other studies found any statistically significant differences between HT and placebo for this outcome (Analysis 1.22; Analysis 1.24; Analysis 1.25; Analysis 2.14; Analysis 2.13; Analysis 2.15). As noted above, most of the relevant studies were small.
1.24. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 24 Stroke: combined sequential HT.
1.25. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 25 Stroke: combined sequential HT.
2.14. Analysis.
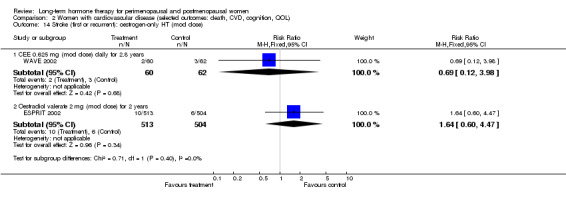
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 14 Stroke (first or recurrent): oestrogen‐only HT (mod dose).
2.13. Analysis.
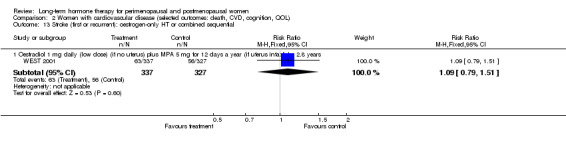
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 13 Stroke (first or recurrent): oestrogen‐only HT or combined sequential.
2.15. Analysis.
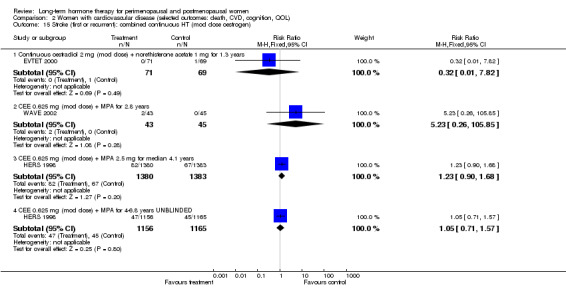
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 15 Stroke (first or recurrent): combined continuous HT (mod dose oestrogen).
4.2 Transient ischaemic attack (TIA)
Relevant comparisons
Four studies (ELITE 2014; EPAT 2001; PEPI 1995; Tierney 2009) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for 2 or 3 years, reported this outcome in relatively healthy women.
Three studies (ESPRIT 2002; HERS 1998; WEST 2001) of women with cardiovascular disease with a total of three different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), also measured this outcome.
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 1.26; Analysis 1.27; Analysis 2.16; Analysis 2.17; Analysis 2.18).
1.26. Analysis.
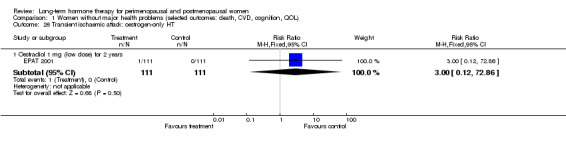
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 26 Transient ischaemic attack: oestrogen‐only HT.
1.27. Analysis.
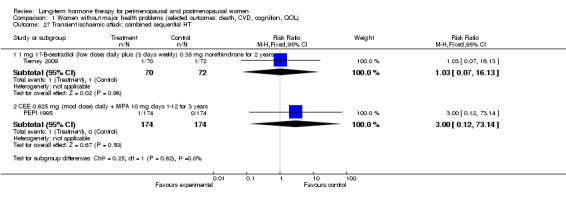
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 27 Transient ischaemic attack: combined sequential HT.
2.16. Analysis.
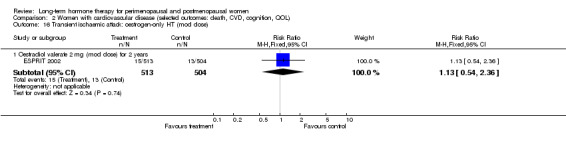
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 16 Transient ischaemic attack: oestrogen‐only HT (mod dose).
2.17. Analysis.
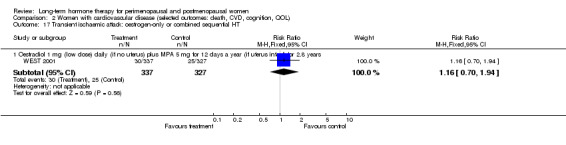
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 17 Transient ischaemic attack: oestrogen‐only or combined sequential HT.
2.18. Analysis.
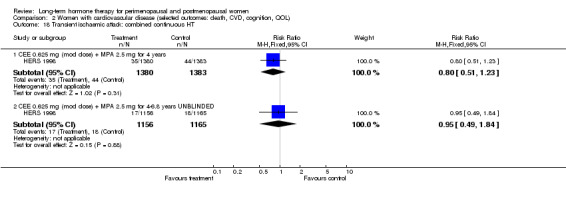
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 18 Transient ischaemic attack: combined continuous HT.
4.3 Stroke or transient ischaemic attack
Relevant comparisons
One study of relatively healthy women (WISDOM 2007) taking combined continuous HT or placebo for a median of 1 year reported stroke or TIA as a combined outcome. Another study (ERA 2000) of women with known coronary disease taking oestrogen‐only HT, combined continuous therapy or placebo also reported this combined outcome at 3.2 years' mean follow‐up.
Results
Neither study found a statistically significant difference for this outcome between women taking HT and women taking placebo (Analysis 1.29; Analysis 2.20; Analysis 2.19).
1.29. Analysis.
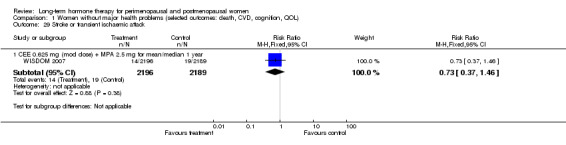
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 29 Stroke or transient ischaemic attack.
2.20. Analysis.
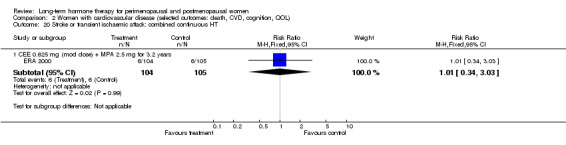
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 20 Stroke or transient ischaemic attack: combined continuous HT.
2.19. Analysis.
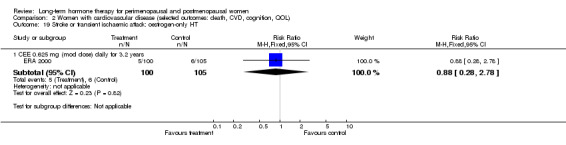
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 19 Stroke or transient ischaemic attack: oestrogen‐only HT.
5. Venous thromboembolism (pulmonary embolus or deep vein thrombosis)
Relevant comparisons
Six studies (ELITE 2014; EPAT 2001; PEPI 1995; Tierney 2009; WHI 1998; WISDOM 2007) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome in relatively healthy women.
Five studies of women with cardiovascular disease (ERA 2000; ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), also measured venous thromboembolism.
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they needed custodial care.
Results
WHI 1998 (oestrogen‐only HT arm) reported that relatively healthy women taking oestrogen‐only HT (CEE 0.625 mg) were at higher risk of a thromboembolic event than women taking placebo. Risk was highest within the first 2 years and was statistically significant during this time period (RR 2.22, 95% CI 1.12 to 4.39). Absolute risk of an event increased from 2 per 1000 in the control group to 5 per 1000 (95% CI 2 to 10) in the HT group. At a mean follow‐up of 7 years, risk was lower, but the intervention group was still at higher risk bordering on statistical significance (RR 1.32, 95% CI 1.00 to 1.74). The increased risk disappeared during extended follow‐up (overall 10.7 years' follow‐up: RR 1.05, 95% CI 0.84 to 1.31). When deep vein thrombosis was considered as a single outcome (without pulmonary embolism), the rate was significantly lower in the HT group during extended follow‐up (RR 0.63, 95% CI 0.41 to 0.98), although the rate over the entire 10.7 years' intervention and extended follow‐up did not differ significantly between the two groups (RR 1.04, 95% CI 0.84 to 1.29; Analysis 1.30).
1.30. Analysis.
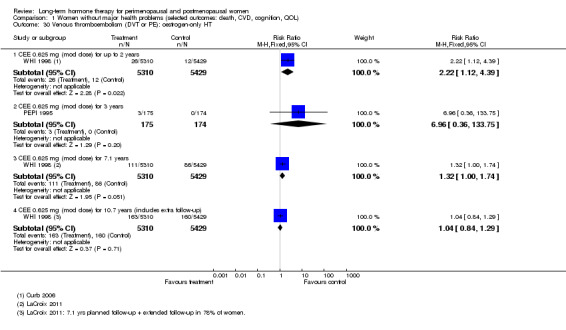
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 30 Venous thromboembolism (DVT or PE): oestrogen‐only HT.
WHI 1998 (combined HT arm) reported that relatively healthy women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) were at significantly higher risk of a thromboembolic event than women taking placebo; this applied at 1 to nearly 8 years' follow up (at 1 year: RR 3.59, 95% CI 1.95 to 6.61; at 2 years: RR 2.98, 95% CI 1.88 to 4.71; at 3 years: RR 2.54, 95% CI 1.73 to 3.72; at a mean of 5.6 years: RR 2.03, 95% CI 1.55 to 2.64; at a mean of 7.9 years: RR 1.65, 95% CI 1.32 to 2.05). Analysis of this comparison revealed a statistically significant time trend for diminishing risk of venous thromboembolism over time. WISDOM 2007 also reported data for this outcome at 1 year; pooling these data with data from WHI 1998 (oestrogen‐only HT arm) resulted in a risk ratio at 1 year of 4.28 (95% CI 2.49 to 7.34) (Analysis 1.32). Absolute risk of an event increased at 1 year from 2 per 1000 in the control group to 7 per 1000 (95% CI 4 to 11) in the HT group; at 2 years from 3 per 1000 in the control group to 9 per 1000 (95% CI 6 to 14) in the HT group; and at 5.6 years from 10 per 1000 in the control group to 20 per 1000 (95% CI 15 to 26) in the HT group.
1.32. Analysis.
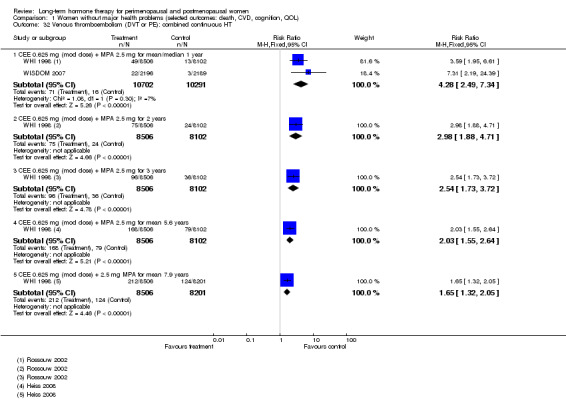
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 32 Venous thromboembolism (DVT or PE): combined continuous HT.
Similarly, in HERS 1998, women with cardiovascular disease who were taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) for 1 to 4 years were significantly more likely to experience a venous thromboembolism than women on placebo (at 1 year: RR 3.26, 95% CI 1.06 to 9.96; at 2 years: RR 3.51, 95% CI 1.42 to 8.66; at 3 years: RR 3.01, 95% CI 1.50 to 6.04; at a mean of 4.1 years: RR 2.62, 95% CI 1.39 to 4.94; Analysis 2.22). Absolute risk of an event increased at 1 year from 3 per 1000 in the control group to 9 per 1000 (95% CI 3 to 29) in the HT group; at 2 years from 4 per 1000 in the control group to 15 per 1000 (95% CI 6 to 38) in the HT group; and at 4.1 years from 9 per 1000 in the control group to 13 per 1000 (95% CI 6 to 28) in the HT group.
2.22. Analysis.
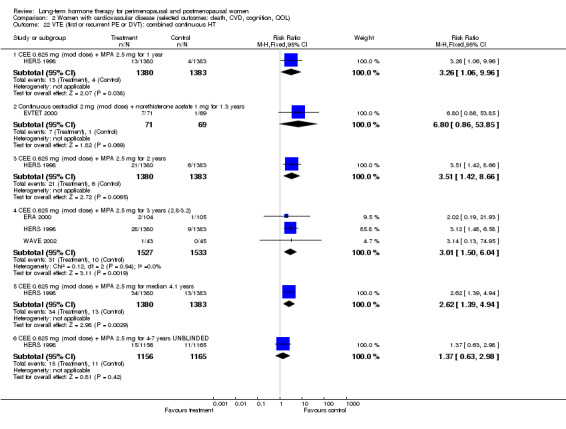
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 22 VTE (first or recurrent PE or DVT): combined continuous HT.
None of the other studies found any statistically significant differences between HT and placebo for this outcome (Analysis 1.31; Analysis 2.21; Analysis 5.3).
1.31. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 31 Venous thromboembolism (DVT or PE): combined sequential HT.
5.3. Analysis.
Comparison 5 Women hospitalised with chronic illness (selected outcomes: death, CVD, VTE), Outcome 3 Venous thromboembolism (DVT or PE): combined sequential HT.
6. Breast cancer
Relevant comparisons
Nine studies (ELITE 2014; EPAT 2001; EPHT 2006; Greenspan 2005; KEEPS 2012; Notelovitz 2002; PEPI 1995; WHI 1998; WISDOM 2007) with a total of 11 different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, reported this outcome in relatively healthy women. WHI 1998 (combined HT arm) extended follow‐up beyond the planned completion date to achieve a mean 11‐year follow‐up for this outcome in the 85% of women who consented to stay in the study; WHI 1998 (oestrogen‐only arm) extended follow‐up to a total of 10.7 years in the 78% of women who agreed to continue.
Four studies (ERA 2000; ESPRIT 2002; HERS 1998; WAVE 2002) with a total of four different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 2 to 4 years, with unblinded follow‐up to 7.1 years (HERS 1998), measured this outcome in women with cardiovascular disease .
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
WHI 1998 (oestrogen‐only HT arm) reported a non‐statistically significant decrease in risk of breast cancer at 7.1 years' follow‐up among relatively healthy women taking oestrogen‐only HT (CEE 0.625 mg) compared with women taking placebo (RR 0.79, 95% CI 0.61 to 1.01). Follow‐up continued for a median of 5.8 years after the intervention phase. The overall cumulative breast cancer incidence over the 10.7 years' mean follow‐up (median 11.8 years) showed a significantly lower rate in the HT group (RR 0.78, 95% CI 0.63 to 0.96). Absolute risk of breast cancer decreased over 10.7 years' follow‐up from 37 per 1000 in the control group to 29 per 1000 (95% CI 23 to 35) in the HT group. The overall cumulative rate remained lower after a median of 13 years' follow‐up (RR 0.80, 95% CI 0.65 to 0.97). Study authors noted that when event rates in the early and late postintervention periods were compared, hazard ratios (HRs) for breast cancer differed significantly (P = 0.04). The significant difference between groups in HR for breast cancer diminished over time and disappeared at approximately 4.5 years post intervention (Chlebowski 2015a)
WHI 1998 (combined HT arm) reported this outcome at yearly intervals. Results showed no statistically significant differences between groups in the incidence of breast cancer during the first 4 years of follow‐up, but the HT group was at significantly higher risk of breast cancer after taking HT for 5 or more years (at a mean of 5.6 years' follow‐up: RR 1.27, 95% CI 1.03 to 1.56; at a mean of 7.9 years' follow‐up: RR 1.27, 95% CI 1.07 to 1.52). Absolute risk of breast cancer increased at 5.6 years' follow‐up from 19 per 1000 in the control group to 23 per 1000 (95% CI 19 to 29) in the HT group; and at 7.9 years' follow‐up from 26 per 1000 in the control group to 33 per 1000 (95% CI 28 to 40) in the HT group. Analysis in this arm of WHI 1998 revealed a statistically significant trend for increasing breast cancer risk over time in the group taking HT. WISDOM 2007 also reported data for this outcome at a median follow‐up of 1 year. Pooling these data with data from WHI 1998 (combined HT arm) resulted in significantly reduced risk of breast cancer at 1 year in the HT arm (RR 0.53, 95% CI 0.28 to 0.96). However, at a mean of 11 years' follow‐up in WHI 1998 (combined HT arm), the rate of invasive breast cancer was significantly higher in the HT arm (RR 1.25, 95% CI 1.08 to 1.45). Rates remained higher in the intervention arm at a median of 13.2 years' follow‐up (RR 1.28, 95% CI 1.11 to 1.47) (Analysis 6.3). Breast cancers diagnosed in the HT group were of similar histology and stage to those diagnosed among controls but were more likely to be node positive (P = 0.03).
6.3. Analysis.
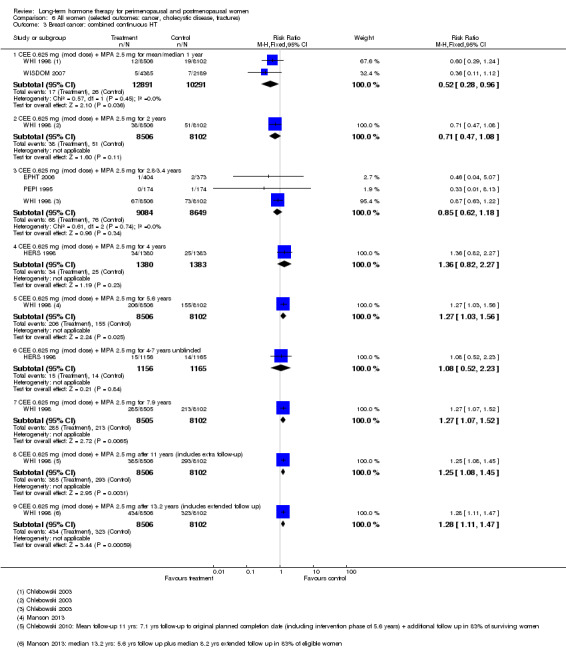
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 3 Breast cancer: combined continuous HT.
Results of analysis show no statistically significant differences between any other type of HT and placebo for this outcome, although (as noted above) relevant studies were small (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4).
6.1. Analysis.
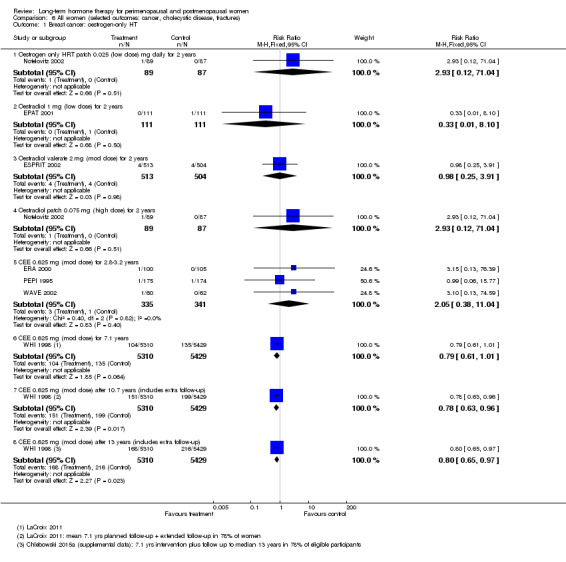
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 1 Breast cancer: oestrogen‐only HT.
6.2. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 2 Breast cancer: oestrogen‐only or combined HT.
6.4. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 4 Breast cancer: combined sequential HT.
7. Colorectal cancer
Relevant comparisons
Seven studies (ELITE 2014; EPAT 2001; HERS 1998; Greenspan 2005; PEPI 1995; Tierney 2009; WHI 1998, WISDOM 2007) with a total of seven different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome. Investigators also reported this outcome after extended follow‐up in WHI 1998 (combined HT arm) at 11.6 years (Simon 2012) and at 13.2 years (Manson 2013).
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
WHI 1998 (combined HT arm) reported no statistically significant differences in the incidence of colorectal cancer among relatively healthy women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) compared with women taking placebo, at 1 to 4 years' follow‐up. However, women taking combined continuous HT had a significantly lower incidence of colon cancer at a mean follow up of 5.6 years (RR 0.64, 95% CI 0.44 to 0.91). Absolute risk of colorectal cancer decreased from 9 per 1000 in the control group to 6 per 1000 (95% CI 4 to 8) in the HT group. Rates tended to favour the HT group over extended follow‐up: The difference was not statistically significant at 7.9 years (RR 0.76, 95% CI 0.57 to 1.01) nor at 13.2 years (RR 0.80, 95% CI 0.63 to 1.01) but did reach statistical significance at 11.6 years (RR 0.78, 95% CI 0.61 to 0.99).
Results of analysis show no statistically significant differences between any other type of HT and placebo for this outcome (Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.9).
6.6. Analysis.
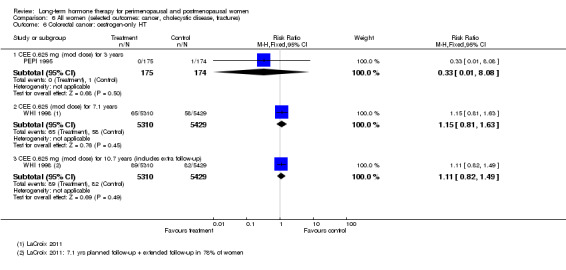
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 6 Colorectal cancer: oestrogen‐only HT.
6.7. Analysis.
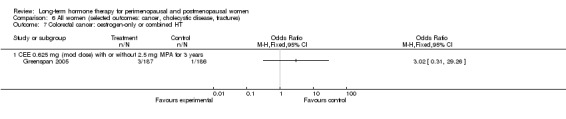
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 7 Colorectal cancer: oestrogen‐only or combined HT.
6.8. Analysis.
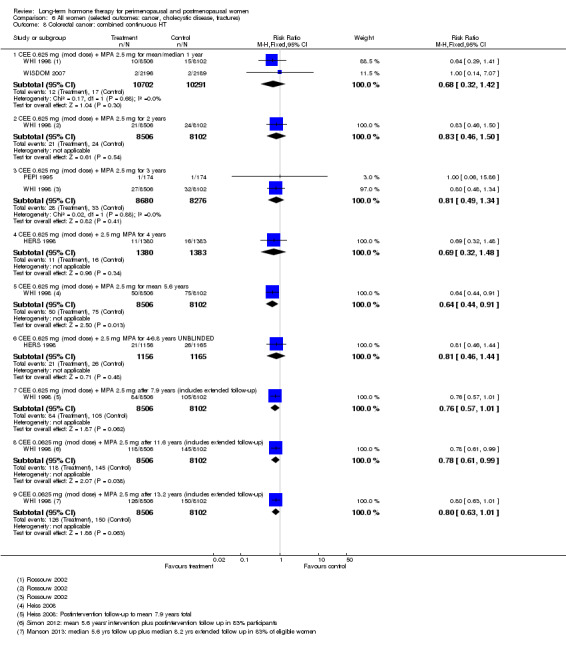
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 8 Colorectal cancer: combined continuous HT.
6.9. Analysis.
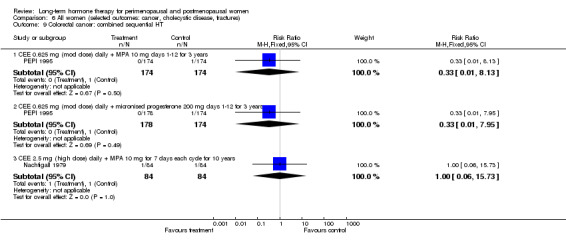
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 9 Colorectal cancer: combined sequential HT.
8. Lung cancer
Relevant comparisons
WHI 1998 (oestrogen‐only arm) reported this outcome in relatively healthy women after a mean follow‐up of 7.1 years; WHI 1998 (combined HT arm) reported this outcome at 5.6 years and after extended follow‐up at 7.9 years and 14 years.
Results
Results of analysis show no statistically significant differences between HT and placebo groups for this outcome (Analysis 6.12; Analysis 6.11; Analysis 6.13).
6.12. Analysis.
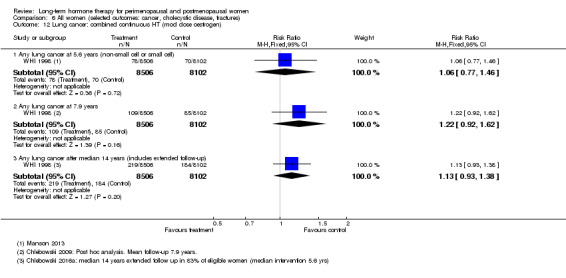
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 12 Lung cancer: combined continuous HT (mod dose oestrogen).
6.11. Analysis.
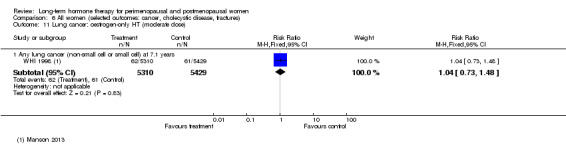
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 11 Lung cancer: oestrogen‐only HT (moderate dose).
6.13. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 13 Lung cancer: combined sequential HT.
9. Endometrial cancer
Relevant comparisons
Nine studies (EPAT 2001; ESPRIT 2002; Ferenczy 2002; HERS 1998; KEEPS 2012; Nachtigall 1979; Obel 1993; PEPI 1995; WHI 1998 (combined HT arm)) with a total of 13 different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 10 years, reported this outcome. WHI 1998 (combined HT arm) also reported this outcome after extended follow‐up, at 13.2 years. The study of oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer (Barakat 2006) measured recurrent endometrial cancer. In comparisons of oestrogen‐only HT versus placebo (EPAT 2001; ESPRIT 2002; PEPI 1995), all women with a uterus were monitored closely for endometrial hyperplasia, and two studies specified that study medications were withdrawn if atypical hyperplasia was detected (ESPRIT 2002; PEPI 1995).
Results
At 13 years' median follow‐up, rates of endometrial cancer were lower in the combined HT group (RR 0.66, 95% CI 0.48 to 0.90) in WHI 1998.
Results of analysis showed no other statistically significant differences between HT and placebo for this outcome (Analysis 6.14; Analysis 6.15; Analysis 6.14; Analysis 6.16), and no statistically significant differences between groups in rates of recurrent endometrial cancer (Analysis 6.17). One study (Obel 1993) reported no events.
6.14. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 14 Endometrial cancer: oestrogen‐only HT.
6.15. Analysis.
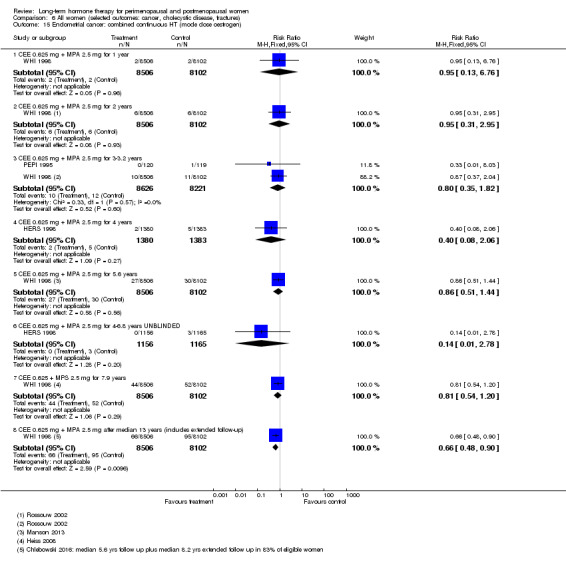
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 15 Endometrial cancer: combined continuous HT (mode dose oestrogen).
6.16. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 16 Endometrial cancer: combined sequential HT.
6.17. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 17 Recurrent endometrial cancer: oestrogen‐only HT.
10. Ovarian cancer
Relevant comparisons
WHI 1998 (combined HT arm), which used combined continuous CEE 0.625 mg + MPA 2.5 mg at 5.6 years' mean follow‐up and again after extended follow‐up, at 13.2 years (Manson 2013), reported ovarian cancer incidence, and ELITE 2014, which utilised oestrogen with or without sequential progesterone vaginal gel, reported a single event.
Results
Results of analysis showed no statistically significant differences between groups for this outcome (Analysis 6.18).
6.18. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 18 Ovarian cancer: combined continuous HT.
11. Gallbladder disease
Relevant comparisons
Four studies (ERA 2000; HERS 1998; PEPI 1995; WHI 1998), which compared oestrogen‐only HT, combined continuous HT and sequential combined HT versus placebo for 3 to over 7 years, reported gallbladder disease requiring surgery. For this outcome, the two largest studies stated that they excluded from analysis women who had had their gallbladder removed (HERS 1998), who reported a history of gallbladder disease, or both (WHI 1998).
Results
Meta‐analysis of the three studies comparing oestrogen‐only HT versus placebo for the outcome of gallbladder disease requiring surgery (ERA 2000; PEPI 1995; WHI 1998) showed a statistically significant increase in risk in the HT group (RR 1.75, 95% CI 1.40 to 2.19); these studies had a mean follow‐up ranging from 3 to 7.1 years. Absolute risk of an event increased from 26 per 1000 in the control group to 45 per 1000 (95% CI 36 to 57) in the HT group. Meta‐analysis of the four studies comparing combined continuous HT versus placebo (ERA 2000; HERS 1998; PEPI 1995; WHI 1998) showed significantly increased risk in the HT group (RR 1.55, 95% CI 1.29 to 1.86); these studies had a mean follow‐up ranging from 3 to 5.6 years. Absolute risk of an event increased from 27 per 1000 in the control group to 47 per 1000 (95% CI 38 to 60) in the HT group. Although these studies had differing lengths of follow‐up, review authors noted no statistical heterogeneity in either meta‐analysis. Similarly, during unblinded follow‐up, HERS 1998 reported an increase in events in the HT group that reached borderline statistical significance (RR 1.63, 95% CI 1.00 to 2.70; Analysis 6.20; Analysis 6.21; Analysis 6.22). WHI 1998 investigators reported that hazard estimates for risk in active and placebo groups started to diverge during the first year of follow‐up, with the oestrogen group separating earlier than the combined continuous HT group.
6.20. Analysis.
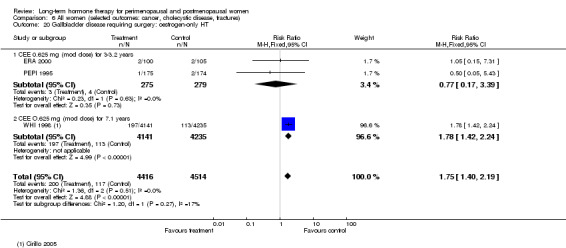
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 20 Gallbladder disease requiring surgery: oestrogen‐only HT.
6.21. Analysis.
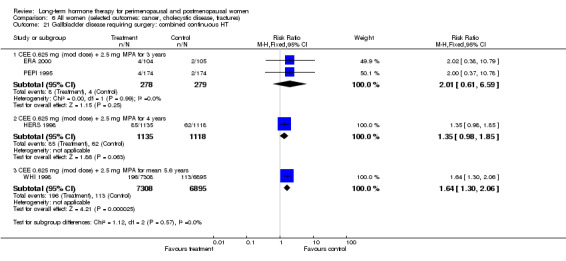
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 21 Gallbladder disease requiring surgery: combined continuous HT.
6.22. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 22 Gallbladder disease requiring surgery: combined sequential HT.
12. Fractures
12.1 Hip fracture
Relevant comparisons
Five studies, which compared combined continuous HT (HERS 1998; WHI 1998; WISDOM 2007), combined sequential HT (Tierney 2009; WEST 2001) and oestrogen‐only HT (WEST 2001; WHI 1998) versus placebo for between 1 and 7.9 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), and with extended follow‐up to 13.2 years in both arms of WHI 1998, reported the incidence of hip fracture.
Results
Both arms of WHI 1998 found a statistically significant reduction in the risk of hip fracture for women taking HT. WHI 1998 (oestrogen‐only HT arm) reported a statistically significant reduction in the risk of hip fracture for women taking HT (CEE 0.625 mg) at 7.1 years' mean follow‐up (RR 0.64, 95% CI 0.45 to 0.93; Analysis 6.23). Absolute risk of a hip fracture decreased from 14 per 1000 in the control group to 9 per 1000 (95% CI 6 to 13) in the HT group. Benefit derived from HT was not maintained during extended follow‐up (to 13.2 years). WHI 1998 (combined HT arm) reported this outcome at yearly intervals and found no statistically significant differences in the incidence of hip fracture during the first 4 years' follow‐up, but at 5.6 years' mean follow‐up, reduction in the risk of hip fracture among women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) was statistically significant (RR 0.68, 95% CI 0.48 to 0.97; Analysis 6.25). Absolute risk of hip fracture decreased from 9 per 1000 in the control group to 6 per 1000 (95% CI 4 to 9) in the HT group. This risk remained significantly lower in the HT group at mean follow‐up of 7.9 years (RR 0.77, 95% CI 0.60 to 0.99) and 13.2 years (RR 0.82, 95% CI 0.69 to 0.97).
6.23. Analysis.
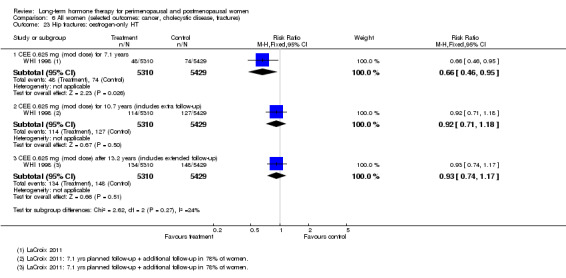
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 23 Hip fractures: oestrogen‐only HT.
6.25. Analysis.
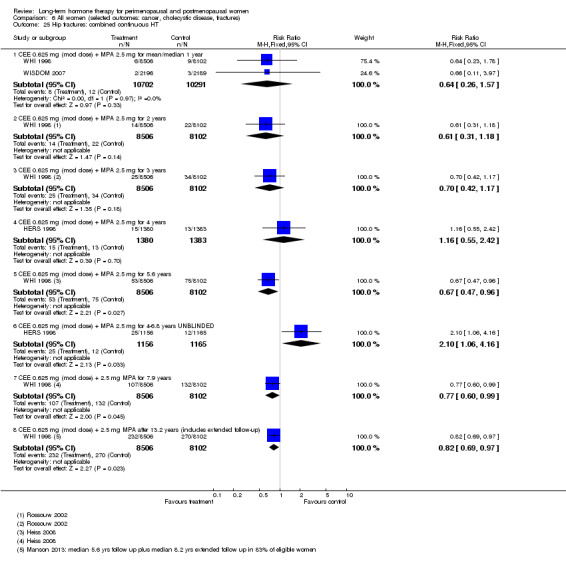
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 25 Hip fractures: combined continuous HT.
However, HERS 1998 found no statistically significant differences between combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) and placebo for this outcome, and the unblinded extension of this study reported a statistically significantly increased risk in the group taking HT from years 4.1 to 6.8 (post randomisation) (RR 2.10, 95% CI 1.06 to 4.16; Analysis 6.25).
Other studies found no statistically significant differences between groups (Analysis 6.24; Analysis 6.26).
6.24. Analysis.
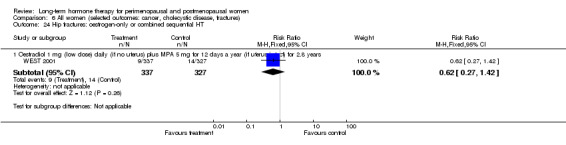
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 24 Hip fractures: oestrogen‐only or combined sequential HT.
6.26. Analysis.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 26 Hip fractures: combined sequential HT.
12.2 Clinical vertebral fractures
Relevant comparisons
WHI 1998 (oestrogen‐only HT arm) reported the incidence of vertebral fracture at follow‐up of 7.1 years. Two studies of combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) versus placebo (HERS 1998; WHI 1998 (combined HT arm)) also reported the incidence of vertebral fracture at follow‐up from 4 to nearly 8 years.
Results
At a mean of 7.1 years' follow‐up, WHI 1998 (oestrogen‐only HT arm) reported significantly fewer fractures in the oestrogen‐only HT group (CEE 0.625 mg) than in the placebo group (RR 0.64, 95% CI 0.44 to 0.94; Analysis 6.27). Absolute risk of a clinical vertebral fracture decreased from 13 per 1000 in the control group to 8 per 1000 (95% CI 6 to 12) in the HT group. Similarly, at a mean of 5.6 years' follow‐up, WHI 1998 (combined HT arm) reported significantly fewer fractures in the HT group than in the placebo group (RR 0.68, 95% CI 0.48 to 0.97; Analysis 6.28). Absolute risk of a clinical vertebral fracture decreased from 10 per 1000 in the control group to 7 per 1000 (95% CI 5 to 10) in the HT group. At a mean of 7.9 years' follow‐up, WHI 1998 (combined HT arm) no longer observed significant differences between groups. HERS 1998 found no significant differences between groups during follow‐up.
6.27. Analysis.
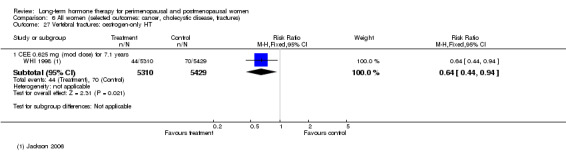
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 27 Vertebral fractures: oestrogen‐only HT.
6.28. Analysis.
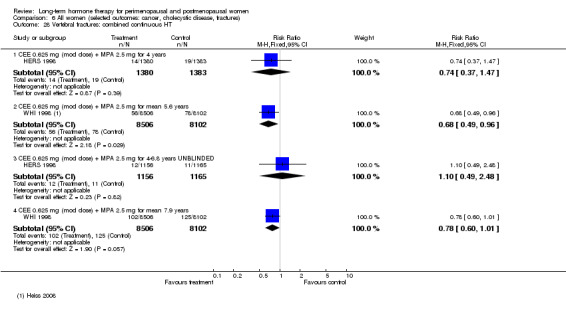
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 28 Vertebral fractures: combined continuous HT.
12.3 Any fractures
Relevant comparisons
Nine studies (EPHT 2006; ERA 2000; ESPRIT 2002; Greenspan 2005; HERS 1998; Tierney 2009; WEST 2001; WHI 1998; WISDOM 2007) comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for 1 to nearly 8 years reported the incidence of any fracture.
Results
Both arms of WHI 1998 showed a statistically significant reduction in the risk of any fracture for women taking HT. Investigators reported this at 5.6 and 7.9 years' mean follow‐up in women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) (at 5.6 years: RR 0.78, 95% CI 0.71 to 0.86; at 7.9 years: RR 0.82, 95% CI 0.76 to 0.89) and at 7.1 years' mean follow‐up in women taking oestrogen‐only HT (CEE 0.625 mg) (RR 0.73, 95% CI 0.65 to 0.80) (Analysis 6.30; Analysis 6.32). At 5.6 years, in WHI 1998 (combined HT arm), absolute risk of any fracture decreased from 111 per 1000 in the control group to 86 per 1000 (95% CI 79 to 94) in the HT group, and in WHI 1998 (oestrogen‐only HT arm) at 7.1 years, absolute risk of any fracture decreased from 140 per 1000 in the control group to 102 per 1000 (95% CI 91 to 112) in the HT group. None of the other studies found any statistically significant differences between HT and placebo for this outcome (Analysis 6.29; Analysis 6.31; Analysis 6.33).
6.30. Analysis.
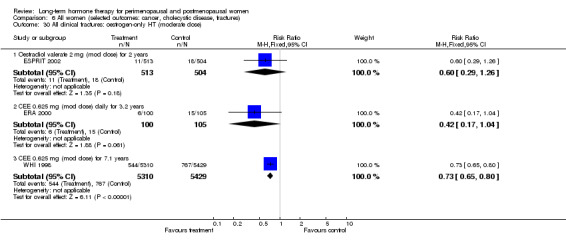
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 30 All clinical fractures: oestrogen‐only HT (moderate dose).
6.32. Analysis.
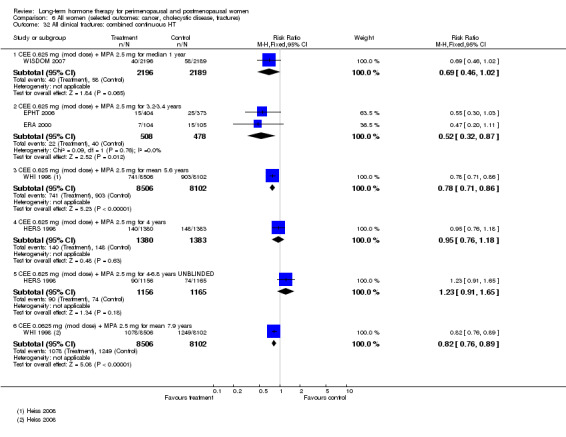
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 32 All clinical fractures: combined continuous HT.
6.29. Analysis.
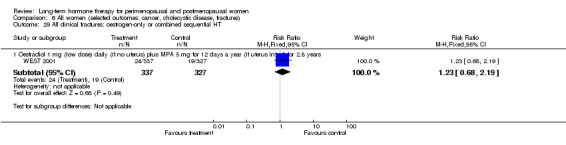
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 29 All clinical fractures: oestrogen‐only or combined sequential HT.
6.31. Analysis.
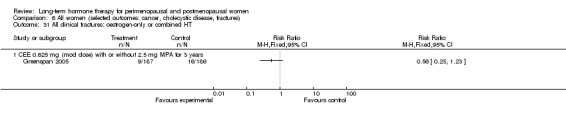
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 31 All clinical fractures: oestrogen‐only or combined HT.
6.33. Analysis.
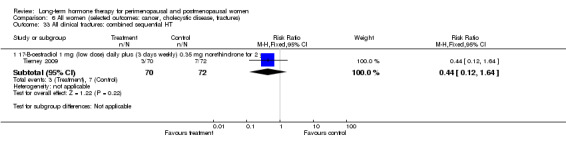
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 33 All clinical fractures: combined sequential HT.
13. Cognitive function
13.1 Global cognitive function
Relevant comparisons
Five studies, which compared low‐dose oestrogen patches versus placebo for 2 years (Yaffe 2006) and combined continuous CEE 0.625 mg with or without MPA 2.5 mg versus placebo for 3 years (Greenspan 2005), oestradiol 1 mg daily with or without intermittent vaginal progesterone gel with follow‐up at 2.5 and 5 years (ELITE 2014), 0.45 mg oral or 0.05 mg transdermal oestrogen with intermittent progesterone 200 mg versus placebo for 4 years (KEEPS 2012), oestrogen‐only HT versus placebo for a mean of 5.6 years (WHI 1998 (WHIMS)) and continuous CEE 0.625 mg + MPA 2.5 mg versus placebo for a mean of 4.2 years (WHI 1998 (WHIMS)), reported this outcome. Researchers measured global cognitive function using a cognitive screening test known as the Modified Mini‐Mental State Examination (3MSE), on which a higher score reflects better cognitive functioning. KEEPS 2012 included women 42 to 58 years of age at randomisation, WHI 1998 (WHIMS) included only women over 65 years of age and Yaffe 2006 included only women over 60 years of age.
Results
Over 2 years' follow‐up in Yaffe 2006, 3 years' in Greenspan 2005, 4 years' in and 2.5 years' or 5 years' in ELITE 2014, investigators noted no significant difference in cognitive function between intervention and placebo groups. Nor did they observe any difference in the effect of treatment when women in Yaffe 2006 were stratified according to cognitive status at baseline (3MSE ≤ 90 or > 90) (Analysis 1.34; Table 4). Inbestigators in ELITE 2014 subgrouped comparisons according to when oestradiol was initiated (within 6 years of menopause vs 10 or more years after menopause). Study results showed no evidence of differences between the two subgroups in the effect of HT on cognition at 2.5 years.
1.34. Analysis.
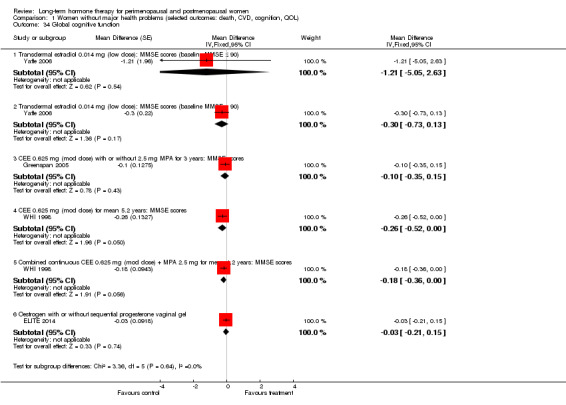
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 34 Global cognitive function.
2. Other data.
| Study | Comparison | Instrument | Measure | Outcome | Intervention | Effect |
| KEEPS 2012 | Oestrogen (CEE or oestradiol) + cyclic oral micronised progesterone 200 mg/d × 12 days per month vs placebo (n = 275) for 48 months |
Modified Mini Mental State Examination (MMSE) | Differences between intervention and placebo groups in mean rate of change over time | Global cognition | 0.45 mg/d oral CEE (n = 230) | P = 0.178 |
| 0.05 mg/d transdermal oestradiol (n = 222) | P = 0.840 |
In both treatment groups and in both placebo groups of WHI 1998 (WHIMS), mean 3MSE scores increased from baseline and continued to increase for 3 to 5 years before they started to decline. Results showed a pattern of higher increases from baseline in 3MSE scores in the placebo groups, which emerged after 1 to 2 years and were maintained throughout the study. The mean difference between groups in 3MSE score changes was of borderline statistical significance in both arms of the study, with results favouring the placebo group; however, in both cases, the lower boundary of the confidence interval was zero (oestrogen‐only HT arm: weighted mean difference (WMD) ‐0.25, 95% CI ‐0.52 to 0.00; combined HT arm: WMD ‐0.18, 95% CI ‐0.35 to 0.00) (Analysis 1.34).
In the WHI 1998 (WHIMS) combined HT arm, a decline of 10 points or more in 3MSE scores (which represents > 2 standard deviations from baseline mean scores) was significantly more likely to occur among women in the active treatment group (RR 1.57, 95% CI 1.10 to 2.24; Analysis 1.31). Study results showed the same trend in the oestrogen‐only HT group, but this finding was not of statistical significance.
1.3. Analysis.
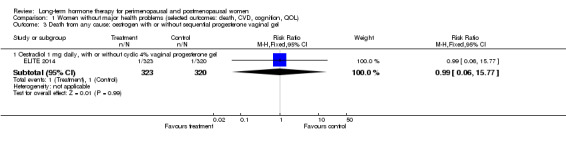
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 3 Death from any cause: oestrogen with or without sequential progesterone vaginal gel.
13.2 Probable dementia
Relevant comparisons
WHI 1998 (WHIMS), which included only women over 65 years of age and compared oestrogen‐only HT (CEE 0.625 mg) versus placebo for a mean of 5.6 years, and combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) versus placebo for a mean of 4.2 years, reported this outcome.
Results
In the oestrogen‐only HT arm, researchers noted no statistically significant differences between groups. In the combined HT arm, the incidence of probable dementia was significantly higher in the group taking combined continuous HT than in the placebo group (RR 1.97, 95% CI 1.16 to 3.33). At 4.2 years, absolute risk of probable dementia increased from 9 per 1000 in the control group to 18 per 1000 (95% CI 11 to 30) in the HT group (Analysis 1.35).
1.35. Analysis.
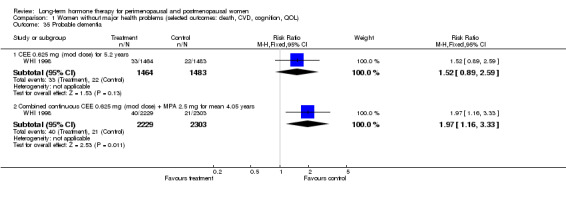
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 35 Probable dementia.
13.3 Change in dementia status
Relevant comparisons
One small study (Mulnard 2000) included women with mild to moderate Alzheimer's disease. Researchers compared unopposed oestrogen for 1 year versus placebo and examined the primary outcome of change in overall status with relation to Alzheimer's disease, as measured by the Clinical Global Impression of Change Scale.
Results
Results of analysis show no statistically significant differences between groups (Analysis 3.1).
3.1. Analysis.

Comparison 3 Women with dementia, Outcome 1 Worsening of dementia on treatment (by ADCS‐CGIC score): oestrogen‐only HT.
Other analyses
Studies included in any one analysis were insufficient to allow construction of a funnel plot.
Use of a random‐effects model had no material effect on any analyses. Studies in any one analysis were insufficient to permit sensitivity analysis by study risk of bias. Nor did any analyses include studies with marked clinical differences.
Discussion
Summary of main results
Cardiovascular disease
No evidence indicates that hormone therapy (HT) has a role in the treatment or prevention of cardiovascular disease. On the contrary, HT significantly increases the incidence of stroke and venous thromboembolism. and combined continuous HT also significantly increases the risk of coronary events (myocardial infarction or cardiac death). Oestrogen‐only HT does not appear to have any statistically significant effect (positive or negative) on coronary disease.
An increase in the risk of coronary events and in venous thromboembolism was evident during the first year of treatment among women taking combined continuous HT in both HERS 1998 and WHI 1998. Although a significant trend in both arms of WHI 1998 and in the blinded phase of HERS 1998 showed diminution of cardiovascular risk in the HT group over time, subsequent analysis of HERS 1998 data, which included both blinded and unblinded follow‐up, revealed no statistically significant variation in risk over time. WHI 1998 investigators suggest that the apparent decline in cardiovascular risk in later years may be due to an acceleration of events during earlier years among susceptible women in the HT group, and they point out that with longer duration of treatment, the risk of breast cancer is increased.
WHI 1998 (combined HT arm) conducted prespecified subgroup analyses to evaluate whether any clinical characteristics of the study population might plausibly modulate the coronary effects of HT: Variables included age, time since menopause, presence or absence of vasomotor symptoms, prior hormone use, coronary heart disease (CHD) risk factor status and presence or absence of preexisting cardiovascular disease. However, none of these variables significantly affected results.
Among women taking combined HT in WHI 1998, those who had factor V Leiden mutation (a blood coagulation disorder) were at higher risk of venous thromboembolism (Cushman 2004). Statistical power was insufficient to allow investigators to determine whether significant excess risk was associated with a history of venous thromboembolism (among women taking combined HT). The incidence of thromboembolism was higher among older and obese women, although this was related to their higher baseline risk of an event, and their risk ratio did not differ from that of other women taking combined HT.
It has been suggested that vascular effects of HT may differ according to a woman's age or time since onset of menopause. Thus oestrogen may counteract the early stages of atherosclerosis in recently menopausal women by inhibiting lipid deposits within the endothelium. However, HT may have adverse effects on more advanced disease, by facilitating an increase in enzymes that tend to disrupt atherosclerotic lesions, and by encouraging clot formation (Manson 2013;Reslan 2012). Research findings on the effect of HT in early menopause on intermediate outcomes of CVD are variable (ELITE 2014; KEEPS 2012), and research findings on this topic are continuing (Manson 2015).
Breast cancer
In WHI 1998 (combined HT arm), breast cancer rates in the HT group were initially lower than in the placebo group, and when WHI 1998 and WISDOM 2007 data were combined, at 1 year's follow‐up, the difference reached statistical significance, favouring the intervention group. However, by the fourth year of use, more events occurred in the HT group, and a statistically significant trend showed increasing risk over time. At a mean of 11 years' follow‐up in WHI 1998, women in the combined HT group had a significantly higher rate of invasive breast cancer than controls, and longer follow‐up (to a median of 13.2 years) showed no evidence of attenuation of risk (Chlebowski 2015a). At 11 years, the trend toward a higher rate of death from breast cancer approached statistical significance (Chlebowski 2010). This long‐term increase in risk was apparent despite evidence that the risk of breast cancer associated with combined HT declined markedly over the first 2 years after discontinuation of hormones (Chlebowski 2009a).
WHI 1998 investigators commented that breast cancers in the combined HT group were diagnosed at a similar grade but at a more advanced stage and suggested that combined HT may stimulate breast cancer growth while delaying diagnosis. Evidence shows that combined HT increases the frequency of abnormal mammograms and indications for breast biopsy but compromises the diagnostic performance of both of these tests (Chlebowski 2008). These factors would account for the lower incidence of breast cancer among women taking combined therapy during the first 2 years in WHI 1998. Subgroup analyses of prior hormone use in WHI 1998 revealed that the cumulative incidence of breast cancer over time in women taking combined HT increased at a greater rate than in women taking placebo after about 3 years for prior hormone users and after about 5 years for women with no prior use. Interference with mammography precluded the possibility of defining with any reliability a time frame for the safe use of combined HT (Anderson 2006).
WHI 1998 reported a decrease in the risk of breast cancer in the unopposed oestrogen arm of the trial, which reached statistical significance when investigators took into account the entire 10.7 years of intervention and extended follow‐up. Cumulative event rates still differed significantly between groups over 13 years' follow‐up, and risk of death from breast cancer was lower in the HT group at nearly 12 years. Comparison of hazard ratios during early and late postintervention periods showed that lower risk of breast cancer in the oestrogen arm persisted for about 4.5 years after the intervention was provided, at which point a significant difference between the interventions was no longer evident (Chlebowski 2015a;Chlebowski 2015b). Subgroup analyses showed significantly fewer early cancers and significantly fewer ductal carcinomas in the intervention group, although the incidence of lobular tumours did not differ significantly. Results showed that the reduction in breast cancer risk was concentrated in women without benign breast disease (P = 0.01) or a first‐degree family history of breast cancer (P = 0.02) (Anderson 2012). Oestrogen‐only HT appears to increase the number of women needing repeat mammography or breast biopsy but (in contrast to combined HT) does not appear to substantially compromise breast cancer detection (Chlebowski 2010a).
WHI 1998 researchers stated that differences between participants across the two arms of the study did not explain differences in breast cancer incidence and suggested that increased risk in the combined group might be due to progestogen. Similar trends in other studies (Beral 2003; HERS 1998) support this theory. A nested case‐control study comparing pair‐matched controls of women who developed breast cancer in either arm of WHI 1998 (Zhao 2014) suggested that post‐treatment changes in serum oestrogens and concentrations of sex hormone‐binding globulin, or changes in the association of such concentrations with disease risk, might explain both the increased breast cancer risk noted with combined HT and the reduction in risk seen with unopposed oestrogen. It has been observed that exposure to oestrogen after a sustained period of oestrogen deprivation reduces the risk of breast cancer (Jordan 2015; Obiorah 2013).
Colorectal cancer
The significantly reduced incidence of colorectal cancer in women taking combined continuous HT in WHI 1998 was offset by the finding that colorectal cancers diagnosed in such women tended to be more advanced, with greater likelihood of lymphatic or metastatic involvement. Moreover, the reduced incidence in the HT group did not lead to a reduced death rate from colorectal cancer over extended follow‐up (7.1 years), although investigators noted that an even longer period of follow‐up might be required to observe a mortality benefit from a reduction in the incidence of small, localised cancers. Women taking oestrogen‐only HT in WHI 1998 did not have a reduced incidence of or death rate from colorectal cancer over 7 years' follow‐up nor during extended follow‐up to 10.7 years. Overall, no strong evidence suggests a clinically meaningful reduction in colorectal cancer rates with oestrogen‐alone or oestrogen plus progestin. Findings in the WHI observational study supported this conclusion (Prentice 2009).
Lung cancer
Post hoc analysis of WHI 1998 data revealed that combined HT did not significantly increase the incidence of lung cancer over 8 years' follow‐up but did increase mortality from lung cancer, independent of smoking status. Study authors (Chlebowski 2009) suggested that this might be so because combined HT stimulates the growth of preexisting small cell lung cancers.
Gynaecological cancers
None of the included studies showed an increase in the incidence of endometrial cancer in the group taking HT. Three studies randomised women with a uterus to oestrogen‐only HT (EPAT 2001; ESPRIT 2002; PEPI 1995). As endometrial cancer is well documented as an adverse effect of unopposed oestrogen (Kurman 1985), these women were closely monitored for atypical endometrial hyperplasia and received treatment (and discontinuation of study medications) if it was detected. PEPI 1995 reported that women in the oestrogen‐only HT group were significantly more likely to develop atypical endometrial hyperplasia than women in the placebo group, whereas women in the combined HT groups in the same study showed no increased risk of hyperplasia. After more than 13 years' extended follow‐up, rates of endometrial cancer were lower in the combined HT group in WHI 1998.
The study of oestrogen‐only therapy in women who had undergone surgery for stage I or II endometrial cancer was underpowered owing to early discontinuation and could not conclusively refute or support the safety of this therapy with regard to risk of recurrence. Study authors note that recurrence rates were low, at 1.9% in the placebo group and 2.3% in the intervention group (Barakat 2006).
Results showed a trend towards increased risk of ovarian cancer in WHI 1998 (combined HT arm), which did not reach statistical significance (Anderson 2003). As noted above, a systematic review of (mainly) observational studies (Greiser 2006) suggests that both oestrogen‐only and combined therapy may be associated with increased risk of ovarian cancer.
A randomised study with 4‐year follow‐up of 130 women with a history of ovarian cancer (Guidozzi 1999) found that oestrogen‐only hormone therapy did not negatively affect disease‐free or overall survival time compared with no hormone therapy. A similar randomised study (AHT 2015) showed that women with severe menopausal symptoms after ovarian cancer who took HT (of varying types, according to consultant preference) had improved overall and relapse‐free survival compared with controls not taking HT. The present systematic review did not include these studies because they lacked a placebo control group.
The apparent reduction in risk of endometrial cancer associated with combined HT is offset by the suggestion of increased risk of ovarian cancer (Manson 2013).
Fractures
Evidence on HT and fractures is not consistent. WHI 1998 found a significantly reduced risk of fractures in women taking combined continuous HT or oestrogen‐only HT over nearly 8 years' follow‐up, but HERS 1998 reported no benefit for women on continuous combined HT. Moreover, unblinded continuation of HERS 1998 revealed a significantly increased risk of hip fracture among such women. Study authors attributed this finding to chance, noting that the effect was considerably smaller in the as‐treated analysis, and that such a finding lacks biological plausibility (Hulley 2004). WHI 1998 (combined HT arm) investigators tested the hypothesis that the beneficial effect of HT on fracture incidence differed according to fracture risk factors. They found that the reduction in risk provided by HT was no greater in women at high risk of fracture (Cauley 2003). However, WHI 1998 excluded women with severe osteoporosis and did not routinely collect bone mineral density; thus the benefits of HT may outweigh the risks for some women with severe osteoporosis. Reduced risk of hip fracture associated with HT did not persist in the extended follow‐up phase of WHI 1998 (oestrogen‐only HT).
Our analyses of hip fracture may have had insufficient power to reach conclusive findings. The risk of hip fracture rises steeply from the age of about 60 years but is still under 0.5% among women in the UK 65 to 69 years of age (Banks 2009).
Most women who need treatment for low bone mineral density require lifelong therapy, but the highest risk of cardiovascular events with combined HT occurs during the first year of use. Overall, although HT is considered effective for prevention of postmenopausal osteoporosis, it is generally recommended as an option only for women at significant risk, for whom non‐oestrogen therapies are unsuitable (Cranney 2002; NIH 2004).
Cognitive outcomes
WHI 1998 (WHIMS) found that neither combined HT nor oestrogen‐only HT improved global cognitive function in women over 65 years of age. Improvement in global cognitive function (Modified Mini‐Mental State Examination (3MSE) scores) that occurred in all participant groups over the first few years of WHIMS was attributed to a learning effect resulting from repeated administration of cognitive tests (Espeland 2004). The difference in mean scores between active therapy and placebo groups was of borderline statistical significance and consistently favoured placebo groups, although the difference was too small to be clinically meaningful. However, a marked decrease in 3MSE scores (defined as > 2 standard deviations from the baseline mean) was more frequent in the active treatment groups, and this trend reached statistical significance in the combined HT group. Moreover, in both arms, HT had greater detrimental effects in women whose baseline 3MSE scores were lowest (Espeland 2004).
Similarly, for the outcome of probable dementia, a negative trend in both active treatment groups reached statistical significance in the combined HT group. Evidence of increased risk in this group began to appear as early as 1 year after randomisation and persisted over 5 years' follow‐up. The overall risk of dementia in women taking combined HT was twice that in women in the corresponding placebo group. Investigators noted that the absolute risk of dementia remained relatively small, at 45 per 10,000 postmenopausal women over 65 years of age who took combined HT for 1 year (Shumaker 2004).
These findings were unexpected and contrast sharply with findings reported in earlier research. WHI 1998 (WHIMS) investigators suggested that this might be due to the healthy user bias seen in observational studies (whereby HT users had a better prognosis at baseline than the control groups), to the differential effects of HT on specific domains of cognition not measured individually by 3MSE, or both. Alternatively, they suggested that HT might need to be initiated during a critical period, such as menopause, to protect cognitive function at a later age. The mean age of the WHIMS population was 71 years, and the study could not address this theory, although previous users of HT in WHIMS did not have higher scores (Espeland 2004). Moreover, results of extended follow‐up in WHI 1998 (WHISCA) show no evidence of any benefit in domain‐specific cognitive function from oestrogen‐alone or combined HT.
A post hoc comparison of global cognitive function among younger women in WHI 1998 (Espeland 2013;Vaughan 2013) included women randomised to either active arm of WHI 1998 (CEE or combined HT) versus women in the placebo arm. Scores were similar in the two groups (P = 0.66). The study included 1376 women 50 to 55 years of age when randomised for WHI 1998. Cognitive testing was conducted an average of 7.2 years following trial completion, when women had a mean age of 67.2 years, and was repeated 1 year later. Study investigators concluded that "CEE‐based therapies produced no overall sustained benefit or risk to cognitive function when administered to postmenopausal women aged 50–55 years". However, among 2880 women who had enrolled for WHI 1998 at the age of 65 to 79 years, long‐term decrements in global cognitive function were noted in the HT groups (CEE or combined HT) relative to the placebo group, which consisted of older women (P < 0.05). Effects were small, and decrements were fairly stable. Findings did not vary according to type of HT, prior use or time since last menstrual period (Espeland 2016).
Gallbladder disease
Researchers have noted a statistically significant association between HT and gallbladder disease, with excess risk related to both oestrogen‐only and combined continuous HT. Although most of the statistical power for this outcome was derived from WHI 1998, findings with respect to combined continuous HT were strongly supported by data from both blinded and unblinded follow‐up in HERS 1998. WHI 1998 investigators noted that the risk started to increase in the active group during the first year and appeared to increase over time. They calculated that for one excess case of gallbladder disease, 323 women would need to take oestrogen‐only HT, or 500 women would need to take combined continuous HT for a year.
Overall completeness and applicability of evidence
Type of HT
Nearly all statistically significant findings described in this review derived from the two biggest studies ‐ HERS 1998 and WHI 1998. Both studies evaluated oral conjugated equine oestrogen (CEE) 0.625 mg, with or without continuous methoxyprogesterone (MPA 2.5 mg). Smaller studies using other types of HT reported very few or no major clinical events. We were generally unable to combine results from individual studies because they used different types of HT, which may not be equivalent in effect, or they differed with respect to the study population, or both. Controversy surrounds the degree to which the findings of WHI 1998 apply to any type of HT other than continuous combined oral CEE 0.625 mg with or without MPA 2.5 mg. Effects may vary with different oestrogens and progestogens, different time frames for the use of HRT and different doses and routes of administration (e.g. unopposed oestrogen and intrauterine progestogen). Observational evidence shows that transdermal oestrogen differs from oral oestrogen in that it is not associated with increased risk of venous thromboembolism and suggests that some types of progestogen are thrombogenic but others are safe in this respect (Canonico 2007).
Population characteristics
It is important to consider any increased risk to health in absolute rather than relative terms. This review is inevitably dominated by the findings of WHI 1998, which was designed to evaluate the efficacy of HT in preventing major causes of morbidity and mortality among older women (Matthews 1997). It was not designed to evaluate the risks and benefits of hormone therapy for treatment of menopausal symptoms, and it specifically excluded women who reported menopausal symptoms severe enough to preclude assignment to placebo treatment (Anderson 2003). Moreover, WHI 1998 did not include women younger than 50 years of age, and study findings may not apply to young surgically menopausal women, for example, a woman who has had both ovaries removed while in her forties (Kaunitz 2002).
Evidence is lacking on the long‐term effects of HT on healthy younger women, who are most likely to use it for menopausal symptoms. Such women are likely to be in their early fifties, when the absolute risk of a life‐threatening event is low; it has been estimated that absolute risk for many diseases approximately doubles with each decade of age (Hulley 2004). Subgroup analyses of women 50 to 59 years of age in WHI 1998 (combined HT arm)revealed that for relatively healthy women taking combined continuous HT, the only increase in risk that reached statistical significance was risk of venous thrombosis. Risk in the HT group increased from eight venous thromboses per 10,000 women per year to 19 per 10,000 women per year. This increase in risk was highest during the first year of therapy but continued over 5 years of treatment, and it was particularly high in obese women (i.e. women with a body mass index greater than 30), who had a 5‐year risk of 1.4% compared with 0.5% among women of normal weight. In the oestrogen‐only arm of WHI 1998, over the full 10.7 years of intervention and extended follow‐up, younger women (aged 50 to 59 years) randomised to HT had significantly more favourable outcomes than those randomised to placebo. The HT group had significantly lower hazard ratios for coronary heart disease, myocardial infarction and death when compared with the placebo group. Findings were similar for both coronary outcomes when data were stratified by time since menopause rather than by age. This contrasted with findings in older women, among whom those in the HT group showed a trend for higher rates of coronary heart disease, myocardial infarction and death, and significantly higher rates of colorectal cancer and chronic disease. WHI 1998 authors noted that study participants took unopposed oestrogen for a median duration of less than 6 years, and that study results cannot be extrapolated to longer or shorter treatment durations. Moreover, it is important to note that oestrogen‐only HT is contraindicated for women with an intact uterus, as use from 1 to 5 years has been estimated to increase the risk of endometrial cancer threefold (from a baseline lifetime risk of about 3% for a woman of 50), with effects persisting for several years after oestrogen is stopped (Grady 1995).
It has been suggested that effects of HT may differ according to whether it is initiated soon after menopause or after a lengthy gap (Barret‐Connor 2007). Analysis of randomised (Prentice 2009a) and observational (WHI 1998) data revealed that for most clinical outcomes, effects of HT did not vary by HT timing; this applied to both oestrogen‐only and combined HT. One exception was breast cancer, for which risk was higher among women who initiated HT soon after menopause than in those who had a longer time gap. Their overall risk of cancer was also higher.
HT appears to carry increased risk of recurrence for women with a history of breast cancer. Two unblinded studies conducted in Sweden randomised breast cancer survivors with menopausal symptoms to HT or non‐hormonal treatment. Both studies were terminated early owing to a statistically significant increase in the incidence of recurrent breast cancer in the hormonal group in one of the studies (risk ratio (RR) 3.5, 95% confidence interval (CI) 1.5 to 8.1) (Chlebowski 2004; Holmberg 2004). After a median of 4 years' follow‐up in this study, a clinically and statistically significantly increased risk of a new breast cancer event continued in the HT arm (RR 2.4, 95% CI 1.3 to 4.2) (Holmberg 2008). A similar study initiated in the UK terminated recruitment prematurely in January 2004 (ICR 2001).
For cardiovascular outcomes, results of HERS 1998 largely support the results of WHI 1998 (combined HT arm), suggesting that these findings can be generalised to older women taking combined continuous HT, whether or not they have known cardiovascular risk factors (although their findings differ with respect to fracture risk).
Health benefits and risks after stopping HT
WHI 1998 (combined HT arm) reported health outcomes at a mean of 2.4 years’ extended follow‐up after the planned intervention period (Heiss 2008). Follow‐up data for this period were available for 95% of women, few of whom were using HT during extended follow‐up (4.3% in the intervention arm and 1.2% in the placebo arm at 1 year after the study was stopped). Over the course of follow‐up, risk of coronary events, stroke and venous thromboembolism decreased in the group that had been randomised to combined HT, and reached a level comparable with that of the placebo group. Similarly, results showed no significant differences between groups in risk of fractures or of colorectal cancer by the end of postintervention follow‐up. However, in the group that had been randomised to combined HT, the hazard ratio (HR) for the outcome “all cancer” increased from 1.03 (95% CI 0.92 to 1.15) during the intervention phase to 1.24 (95% CI 1.04 to 1.48) in the postintervention period. This increase in risk was attributable in part to the disappearance of previous apparent protection from colorectal cancer, with some continued excess risk of breast cancer, along with added risk of lung cancer in the HT group. Study authors noted that clinical vigilance appears to be warranted with regard to sustained higher risk of malignancy following termination of combined HT therapy.
WHI 1998 (oestrogen‐only arm) reported health outcomes at a mean of 3.9 years' extended follow‐up (LaCroix 2011). Follow‐up data for this period were available for 78% of women, of whom only a small minority were using hormone therapy (up to 4.7% in the intervention arm and 3% in the placebo arm). Over the course of extended follow‐up, results continued to show no significant difference between groups in rates of coronary events. Increases in risk of stroke and venous thromboembolism in the HT arm rapidly disappeared, as did reduced risk of hip fracture in this group. As noted above, the lower incidence of breast cancer persisted and became statistically significant with extended follow‐up to 10.7 years (i.e. including both planned intervention and extended follow‐up periods).
Quality of the evidence
Most of the included studies were at low risk of bias in most domains (Figure 1). We rated the overall quality of evidence as moderate, and the main limitation involved questions about applicability of the evidence because most of the data were provided by WHI 1998, in which only about 33% of the study sample was 50 to 59 years of age at baseline (i.e. the age at which women are most likely to consider HT for vasomotor symptoms); mean participant age was 63 years.
A high proportion of women in these studies did not receive the treatment to which they were randomised. In general, the number of women who discontinued their medication or who took less than 80% of their medication was disproportionately high in the HT groups, presumably because of a higher incidence of adverse effects such as vaginal bleeding. WHI 1998 noted that if discontinuation of treatment and initiation of non‐study treatment occurred independently of risk factors for clinical outcomes, their intention‐to‐treat analysis underestimates both harms and benefits of HT among women who adhere to treatment (WHI 2002). This study included a disproportionate number of women who were unblinded in the HT group compared with the placebo group (40% vs 6%), primarily to manage persistent vaginal bleeding, and it has been suggested that this differential unblinding may have resulted in higher detection rates of otherwise undetectable myocardial infarction in the HT group (Shapiro 2003). However, it has been suggested that detection bias on a scale that would explain the differences between groups for coronary heart disease could not have occurred, and that any bias was more likely to have occurred in the opposite direction, mitigating against detection of effects (Tucker 2003).
Potential biases in the review process
This review is subject to patient selection bias. Most of the included studies had a mean participant age over 60 years, and none focused on perimenopausal women. In all but one of the 20 studies that reported mean participant age, mean age at enrolment was over 50 years. This does not reflect usual clinical practice with respect to prescribing of HT, which is most likely to occur for treatment of vasomotor symptoms at the time women reach menopause (Pedersen 2003). Moreover, participants described as 'relatively healthy' in this review were derived largely from WHI 1998. Investigators reported a high frequency of obesity and hypertensive disorders among WHI 1998 participants; only 30% were of normal weight, and 30% were morbidly obese (body mass index (BMI) > 30 kg/m2); 36% were receiving treatment for hypertension or had blood pressure exceeding 140/90 mmHg at enrolment.
Despite extensive searching, we may have failed to identify all relevant studies. However, it is unlikely that we missed any study large enough to substantially influence our overall findings, given dominance of the review by WHI 1998. Similarly, although our data could conceivably have been organised in a different way (e.g. for categorising of study populations and HT doses), again the dominance of WHI 1998 makes it extremely unlikely that this would have influenced our findings. We chose not to pool studies that used different types of HT, and this approach is supported by contrasting findings in the two arms (combined vs oestrogen‐only HT) of WHI 1998 for some outcomes.
The choice of 1 year's duration of HT as a cut‐off point for inclusion of studies was arbitrary but is unlikely to have introduced bias, as it was a prespecified criterion.
Agreements and disagreements with other studies or reviews
Our findings are consistent with those of a Cochrane review of HT for preventing cardiovascular disease in postmenopausal women (Boardman 2015), which concluded that use of HT in postmenopausal women has little if any benefit for primary or secondary prevention of cardiovascular disease and causes an increase in the risk of stroke and venous thromboembolic events. Boardman 2015 differed from the current review in that review authors pooled data related to unopposed oestrogen with data for combined HT.
However, our findings differ from those of some older systematic reviews.
A systematic review of available randomised evidence was conducted in 1997 and was updated with unpublished evidence in 2000 (Hemminki 1997; Hemminki 2000). Review authors reported a conservatively estimated odds ratio for cardiovascular events of 1.34 (95% CI 0.55 to 3.30) among those taking HT. However, this result was based on only 15 secondary events in those allocated to HT and seven among the control groups and provided insufficient evidence to exclude potential benefit from HT.
Beral 2002 pooled the results of four randomised controlled trials of HT published between 1998 and 2002 (EVTET 2000; HERS 1998; WEST 2001; WHI 1998). They reported no significant excess or reduction in the risk ratio of CHD, and their findings negated the large beneficial effect of HT reported for cardiovascular outcomes in earlier observational studies. Moreover, they described excess risk of stroke, pulmonary embolus and breast cancer. Review authors found that risk of colorectal cancer or fractured neck of femur was significantly reduced for the HT group, and the findings for endometrial cancer risk were inconclusive. The authors of this review pooled the results of studies that used different types of HT over variable time frames. The most notable difference between the current review and Beral 2002 is that the current review found a statistically significant increase in risk of coronary heart disease among women taking combined continuous HT, particularly in the first year. Unlike the current review, Beral 2002 pooled results from studies of differing participant groups and types of HT; this appears to explain why overall findings differ.
Salpeter 2004 meta‐analysed 17 RCTs of HT that reported at least 1 death and concluded that risk of death was significantly reduced in women with a mean age under 60 years who were taking HT compared with those taking a placebo, although results showed no difference when older women were compared. This meta‐analysis pooled studies that differed widely with respect to the type of HT used and the clinical status of participants; in several studies, death was not a prespecified outcome. Moreover, women with poor prognosis for ovarian cancer accounted for 60% of events in the meta‐analysis of studies of younger women. In the current review, evidence shows no survival advantage for women taking HT, although only one of the included studies (WHI 1998 (oestrogen‐only HT)) analysed younger women as a subgroup for this outcome. Of the 17 studies that Salpeter et al included in their meta‐analysis of younger women, only two met the inclusion criteria for the present review; the other 15 studies included in the earlier review were not blinded, did not report mortality as a primary or secondary outcome or were of less than 1 year's duration.
A systematic review of studies of hormone therapy and ovarian cancer (Greiser 2006) included 30 case‐control studies, seven cohort studies, one randomised controlled study and four cancer registry studies. This review found increased risk of ovarian cancer associated with the use of oestrogen‐only HT (RR 1.28, 95% CI 1.18 to 1.40) or combined hormone therapy (RR 1.11, 95% CI 1.02 to 1.21). This risk applied to a range of common histological subtypes of ovarian cancer. Review authors noted that this review was limited by reliance on observational data; however, heterogeneity was low or moderate in most analyses.
Another systematic review (Bath 2005) meta‐analysed 28 RCTs of HT that reported stroke events. HT was associated with a statistically significant excess risk of stroke, particularly ischaemic stroke. Moreover, participants in the HT group who had a stroke seemed to have a worse outcome. This review had very broad inclusion criteria and pooled a wide range of studies, which used different types of HT for a range of indications, some with male participants and some without placebo control. It is unclear to what extent these findings apply to perimenopausal and postmenopausal women.
A Danish study (Schierbeck 2012) of 1006 recently postmenopausal or perimenopausal women (aged 45 to 58 years) was not eligible for this review owing to lack of blinding. Investigators reported that after 10 years of treatment, women taking triphasic 17‐B oestradiol with norethisterone acetate or (in those who had had a hysterectomy) oestradiol alone were at lower risk of experiencing their primary outcome ‐ a composite of death and admission to hospital for heart failure or myocardial infarction (hazard ratio 0.48, 95% CI 0.26 to 0.87; P = 0.015). No increase in risk of cancer, venous thromboembolism or stroke was apparent. Study authors attributed the difference between their findings and those of WHI 1998 to the younger age and proximity to menopause of their participants. This study was limited by lack of blinding and by the use of a composite primary outcome that was not prespecified in the study protocol (Marjoribanks 2012; Schroll 2012; Shapiro 2003). It has been noted that the cardiovascular outcomes were not ascertained by researchers but were based on data from individual clinicians entered into a national database (Abelin 2012).
We found no randomised studies or systematic reviews that provided evidence about the risks of long‐term HT in perimenopausal women or those younger than 50 years of age.
Current recommendations favour the use of low‐dose HT for relief of vasomotor symptoms among women within 10 years of their last period, taken for the shortest possible time required to achieve treatment goals, with doses individually tailored and reviewed regularly (NAMS 2012; NICE 2015; RANZCOG 2012).
Authors' conclusions
Implications for practice.
HT for women with menopausal symptoms
Women with intolerable menopausal symptoms may wish to weigh the benefits of symptom relief against the small absolute risk of harm arising from short‐term use of low‐dose HT, provided they do not have specific contraindications. HT may be unsuitable for some women, including those at increased risk of cardiovascular disease, increased risk of thromboembolic disease (e.g. obesity, history of venous thrombosis) or increased risk of some types of cancer (e.g. breast cancer in women with a uterus). The risk of endometrial cancer among women with a uterus taking oestrogen‐only HT is well documented.
Although none of the studies included in this review focused specifically on women in the age group most likely to require menopausal symptom relief, subgroup analyses in WHI 1998 suggested that among relatively healthy women in their 50s taking oestrogen‐only or combined HT, the only significant risk was increased incidence of venous thromboembolism in those taking combined HT. Absolute risk of venous thromboembolism was low, at 0.5% overall for a woman taking HT for 5 years. For women in their 50s without a uterus, taking oestrogen‐only HT for 5 to 6 years appears relatively safe and may even confer some health benefits. However, safety over longer‐term use is unknown.
HT for other indications
HT is not indicated for primary or secondary prevention of cardiovascular disease or dementia, nor for preventing deterioration of cognitive function in postmenopausal women.
Although HT is considered effective for prevention of postmenopausal osteoporosis, it is generally recommended as an option only for women at significant risk, for whom non‐oestrogen therapies are unsuitable. Strong evidence suggests that both oestrogen‐only HT and combined therapy significantly increase the risk of stroke and gallbladder disease, and that long‐term use of combined continuous therapy also increases the risk of coronary events, breast cancer, death from lung cancer and (in women over 65 years of age) dementia.
HT for women with previous disease or smoking history
HT is not recommended for use in women with cardiovascular disease or with a history of venous thrombosis or breast cancer. Randomised evidence provides no specific contraindications for its use in women with a history of endometrial cancer or ovarian cancer, although data are scanty. Women at high risk of lung cancer (current smokers or long‐term past smokers) should be aware that combined HT increases the risk of death from lung cancer.
Implications for research.
No studies have adequately assessed the safety of HT used for symptom relief by perimenopausal women, women younger than 50 years or women with temporary or permanent iatrogenic ovarian failure. Not enough is known about factors that may modulate the risks involved, such as clinical characteristics or biomarkers affecting individual women, different oestrogens and progestogens, different time frames for the use of HT and different doses and routes of administration (e.g. unopposed oestrogen and intrauterine progestogen, whether the risk of thromboembolism is diminished by the use of patches). Reliable evidence is needed to show the efficacy and safety of alternatives to HT for control of menopausal symptoms among women who may wish to avoid using HT, or for whom its use is unsuitable.
What's new
| Date | Event | Description |
|---|---|---|
| 6 January 2017 | New citation required and conclusions have changed | The addition of 2 new studies and changes in review outcomes have led to changes in the conclusions of this review. |
| 6 January 2017 | New search has been performed |
New included studies: New excluded studies:
WHI changes: 1. Updated WHI 1998 to include data from follow‐up publications 2. Mean intervention period of combined WHI 1998 corrected from 5.2 years to 5.6 years (corresponding with the date when women were instructed to stop taking the medication) (see Chlebowski 2003) |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 31 October 2012 | Amended | Correction to 'Characteristics of included studies' table and description of studies, related to sample numbers for ESPRIT 2002 and HERS 1998 No change to data or analyses |
| 3 February 2012 | New citation required and conclusions have changed | Search updated to February 2012 New studies: Greenspan 2005;Nielsen 2006;Pefanco 2007;Tierney 2009 Updated WHI 1998 for the following outcomes: lung cancer (Chlebowski 2009, Chlebowski 2010a), breast cancer (Chlebowski 2010), cognition in older women (Espeland 2010; Resnick 2009), colorectal cancer (Prentice 2009a; Ritenbaugh 2008), clinical outcomes at 7.1 and 10.7 yrs in oestrogen‐only HT arm (LaCroix 2011) and at 7.9 years in combined arm (Heiss 2008) Updated EPHT for quality of life (Veerus 2008) Updated WISDOM for quality of life (Welton 2008) Reclassified equivalent HT doses in line with Australian Menopause Society recommendations: amended table of comparisons for studies using oral oestradiol (n = 10) Added 'Summary of findings' table and absolute risks, updated Discussion and Conclusions sections |
| 11 February 2009 | New citation required but conclusions have not changed | Review updated June 2008 |
| 31 May 2008 | New search has been performed | Added the following new studies: Barakat 2006, EPHT 2006, WISDOM 2007, Yaffe 2006. WHI 1998oestrogen‐only arm, data added: Venous thromboembolism at 2 & 7.1 years' follow‐up Coronary heart disease, venous thromboembolism, stroke, breast cancer, fracture and quality of life at 7.1 years' follow‐up WHI 1998combined arm, data added: Subgroup analysis of breast cancer risk by prior hormone summarised in the text (Discussion section) Data on main outcomes after 3 years post intervention (Discussion section) Results from WHI 1998 (WHISCA) on specific cognitive functions in older women added to the text No substantial changes to overall findings of this review. Statistically significant risk of venous thromboembolism for WHI oestrogen‐only arm now evident at follow‐up "up to 2 years" |
| 18 May 2008 | Amended | Converted to new review format |
| 20 November 2003 | New citation required and conclusions have changed | Substantive amendments made |
Acknowledgements
Thanks to Marian Showell (CFG Information Specialist) who designed and ran the search strategy for the 2012 and 2017 updates of this review, and Josie Rishworth, who helped format the labelling of the analysis tables for the 2012 update. Thanks also to Quirine Lamberts, Jane Suckling and the following Cochrane Review Groups, all of whom helped with the first version of this review: Breast Group, Colorectal Cancer Group, Dementia and Cognitive Improvement Group, Gynaecological Cancer Group, Heart Group, Gynaecology and Fertility Group, Musculoskeletal Group, Peripheral Vascular Diseases Group and Stroke Group. See Contributions of authors for details. Martha Hickey kindly provided comments and suggestions on drafts of the 2008 update.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
From inception to 5th September 2016
PROCITE platform
Keywords CONTAINS "menopausal" or "Menopause" or "menopause‐surgical" or "perimenopausal" or "perimenopause" or "postmenopausal" or "postmenopause" or "climacteric" or Title CONTAINS "menopausal" or "menopause‐surgical" or "perimenopausal" or "perimenopause" or "postmenopausal" or "postmenopause" or "climacteric" or "Menopause"
AND
Keywords CONTAINS "HRT" or "HT "or "hormone replacement therapy" or "Hormone Substitution" or "hormone therapy" or "progestagen" or "Progesterone" or "progestin" or "progestins" or "progestogen" or "progestogens" or "medroxyprogesterone" or "MPA" or "dydrogesterone" or "dydrogestrone" or"*Estradiol" or "Estriol‐"or "estrogen" or "*Estrogens" or "oestrodiol" or "oestrogen" or "CEE" or "CEE + MPA" or "conjugated equine "or "premarin" or "17‐beta estradiol" or "17beta‐estradiol + norethisterone acetate" or Title CONTAINS "HRT" or "HT "or "hormone replacement therapy" or "Hormone Substitution" or "hormone therapy" or "progestagen" or "Progesterone" or "progestin" or "progestins" or "progestogen" or "progestogens" or "medroxyprogesterone" or "MPA" or "dydrogesterone" or "dydrogestrone" or"*Estradiol" or "Estriol‐"or "estrogen" or "*Estrogens" or "oestrodiol" or "oestrogen" or "CEE" or "CEE + MPA" or "conjugated equine "or "premarin" or "17‐beta estradiol" or "17beta‐estradiol + norethisterone acetate"
(3443 hits)
Appendix 2. CENTRAL search strategy
From inception to 5th September 2016
CRSO web platform
#1 MESH DESCRIPTOR Climacteric EXPLODE ALL TREES (6208) #2 MESH DESCRIPTOR Menopause EXPLODE ALL TREES (5986) #3 MESH DESCRIPTOR perimenopause EXPLODE ALL TREES (92) #4 MESH DESCRIPTOR Postmenopause EXPLODE ALL TREES (4016) #5 MESH DESCRIPTOR Hot Flashes EXPLODE ALL TREES (571) #6 (postmenopaus* or post‐menopaus* or post menopaus*):TI,AB,KY (12065) #7 (perimenopaus* or peri‐menopaus* or peri menopaus*):TI,AB,KY (512) #8 (climacter* or menopaus*):TI,AB,KY (6621) #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 (15564) #10 MESH DESCRIPTOR Hormone Replacement Therapy EXPLODE ALL TREES (2716) #11 (HT or HRT):TI,AB,KY (3774) #12 MESH DESCRIPTOR Progestins EXPLODE ALL TREES (1959) #13 progestogen*:TI,AB,KY (675) #14 progesterone:TI,AB,KY (4345) #15 (medroxyprogesterone acetate or MPA):TI,AB,KY (2402) #16 dydrogesterone:TI,AB,KY (221) #17 (norethisterone or norethindrone):TI,AB,KY (1053) #18 (hormone adj1 therap*):TI,AB,KY (4934) #19 (estrogen* or oestrogen*):TI,AB,KY (9441) #20 estradiol:TI,AB,KY (7032) #21 CEE:TI,AB,KY (332) #22 (conjugated equine estrogen*):TI,AB,KY (585) #23 premarin:TI,AB,KY (165) #24 estriol:TI,AB,KY (329) #25 oestradiol:TI,AB,KY (1022) #26 (mestranol or estrone):TI,AB,KY (761) #27 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 (21577) #28 #9 AND #2777 (23)
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) Epub Ahead of Print, In Process & Other Non‐Indexed Citations, Ovid MEDLINE (R) Daily, and Ovid MEDLINE (R)
1946 to 5th September 2016
Ovid platform 1 exp climacteric/ or exp menopause/ or exp perimenopause/ or exp postmenopause/ (54052) 2 (postmenopaus$ or post‐menopaus$ or post menopaus$).tw. (53161) 3 (perimenopaus$ or peri‐menopaus$ or peri menopaus$).tw. (3986) 4 (climacter$ or menopaus$).tw. (44202) 5 or/1‐4 (94884) 6 exp hormone replacement therapy/ or exp estrogen replacement therapy/ (22896) 7 HT.tw. (56334) 8 exp Progestins/ (64617) 9 progestogen.tw. (3549) 10 progesterone$.tw. (74873) 11 (medroxyprogesterone acetate or MPA).tw. (25898) 12 dydrogesterone.tw. (413) 13 (norethisterone or norethindrone).tw. (3167) 14 HRT.tw. (9055) 15 (hormone adj1 therap$).tw. (11962) 16 (estrogen$ or oestrogen$).tw. (142386) 17 estradiol$.tw. (73298) 18 CEE.tw. (1055) 19 conjugated equine estrogen$.tw. (1157) 20 premarin.tw. (506) 21 estriol.tw. (4005) 22 oestradiol.tw. (12633) 23 (mestranol or estrone).tw. (9155) 24 or/6‐23 (346991) 25 5 and 24 (38329) 26 randomized controlled trial.pt. (429552) 27 controlled clinical trial.pt. (91634) 28 randomized.ab. (368786) 29 placebo.tw. (183239) 30 clinical trials as topic.sh. (179204) 31 randomly.ab. (262645) 32 trial.ti. (161272)
33 (crossover or cross‐over or cross over).tw. (71021) 34 or/26‐33 (1089025) 35 exp animals/ not humans.sh. (4306043) 36 34 not 35 (1002924) 37 25 and 36 (8009)
Appendix 4. Embase search strategy
Ovid platform
From inception to 5th September 2016
1 exp "menopause and climacterium"/ or exp menopause/ or exp postmenopause/ or exp premenopause/ (100290)
2 (postmenopaus$ or post‐menopaus$ or post menopaus$).tw. (70911)
3 (perimenopaus$ or peri‐menopaus$ or peri menopaus$).tw. (5362)
4 (climacter$ or menopaus$).tw. (60743)
5 or/1‐4 (135983)
6 exp hormone substitution/ (45945)
7 exp estrogen therapy/ (15437)
8 HT.tw. (68139)
9 exp gestagen/ (147348)
10 Progestin$.tw. (11594)
11 progestogen$.tw. (5353)
12 progesterone$.tw. (80542)
13 (medroxyprogesterone acetate or MPA).tw. (28766)
14 dydrogesterone.tw. (517)
15 (norethisterone or norethindrone).tw. (2885)
16 HRT.tw. (12399)
17 (hormone adj1 therap$).tw. (15869)
18 (estrogen$ or oestrogen$).tw. (162017)
19 estradiol$.tw. (79497)
20 CEE.tw. (1377)
21 conjugated equine estrogen$.tw. (1319)
22 premarin.tw. (2850)
23 estriol.tw. (3812)
24 oestradiol.tw. (12169)
25 or/6‐24 (452498)
26 5 and 25 (55119)
27 Clinical Trial/ (866278)
28 Randomized Controlled Trial/ (416151)
29 exp randomization/ (71920)
30 Single Blind Procedure/ (22979)
31 Double Blind Procedure/ (131292)
32 Crossover Procedure/ (48506)
33 Placebo/ (280258)
34 Randomi?ed controlled trial$.tw. (142786)
35 Rct.tw. (21407)
36 random allocation.tw. (1556)
37 randomly allocated.tw. (25534)
38 allocated randomly.tw. (2152)
39 (allocated adj2 random).tw. (762)
40 Single blind$.tw. (17901)
41 Double blind$.tw. (165293)
42 ((treble or triple) adj blind$).tw. (588)
43 placebo$.tw. (238306)
44 prospective study/ (349547)
45 or/27‐44 (1614380)
46 case study/ (39807)
47 case report.tw. (313200)
48 abstract report/ or letter/ (971946)
49 or/46‐48 (1317703)
50 45 not 49 (1572737)
51 26 and 50 (14243)
Appendix 5. PsycINFO search strategy
From 1806 to 5th September 2016
Ovid platfrom
1 exp menopause/ (3311) 2 (postmenopaus$ or post‐menopaus$ or post menopaus$).tw. (2599) 3 (perimenopaus$ or peri‐menopaus$ or peri menopaus$).tw. (635) 4 (climacter$ or menopaus$).tw. (4484) 5 or/1‐4 (6036) 6 exp hormone therapy/ (1853) 7 HT.tw. (11590) 8 exp progestational hormones/ (2155) 9 Progestin$.tw. (576) 10 progestogen.tw. (107) 11 progesterone$.tw. (3764) 12 (medroxyprogesterone acetate or MPA).tw. (572) 13 dydrogesterone.tw. (9) 14 (norethisterone or norethindrone).tw. (41) 15 HRT.tw. (549) 16 (hormone adj1 therap$).tw. (922) 17 (estrogen$ or oestrogen$).tw. (7529) 18 estradiol$.tw. (5322) 19 CEE.tw. (147) 20 conjugated equine estrogen$.tw. (72) 21 premarin.tw. (38) 22 estriol.tw. (52) 23 oestradiol.tw. (440) 24 or/6‐23 (25236) 25 5 and 24 (2144) 26 random*.ti,ab,hw,id. (156444) 27 trial*.ti,ab,hw,id. (145619) 28 controlled stud*.ti,ab,hw,id. (10362) 29 placebo*.ti,ab,hw,id. (34986) 30 ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id. (24854) 31 (cross over or crossover or factorial* or latin square).ti,ab,hw,id. (24751) 32 (assign* or allocat* or volunteer*).ti,ab,hw,id. (135461) 33 treatment effectiveness evaluation/ (20144) 34 mental health program evaluation/ (1962) 35 exp experimental design/ (51656) 36 "2000".md. (33542) 37 or/26‐36 (435984) 38 25 and 37 (615)
Appendix 6. CINAHL search strategy
From 1982 to 5th September 2016
Ebsco platform
| # | Query | Results |
| S38 | S25 AND S37 | 3,344 |
| S37 | S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 | 1,071,060 |
| S36 | TX allocat* random* | 5,230 |
| S35 | (MH "Quantitative Studies") | 14,815 |
| S34 | (MH "Placebos") | 9,793 |
| S33 | TX placebo* | 39,352 |
| S32 | TX random* allocat* | 5,230 |
| S31 | (MH "Random Assignment") | 41,527 |
| S30 | TX randomi* control* trial* | 109,343 |
| S29 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 848,664 |
| S28 | TX clinic* n1 trial* | 189,022 |
| S27 | PT Clinical trial | 79,712 |
| S26 | (MH "Clinical Trials+") | 202,243 |
| S25 | S6 AND S24 | 9,967 |
| S24 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 | 58,243 |
| S23 | TX mestranol or estrone | 242 |
| S22 | TX oestriol | 15 |
| S21 | TX estriol | 326 |
| S20 | TX premarin | 53 |
| S19 | TX conjugated equine estrogen* | 246 |
| S18 | TX CEE | 812 |
| S17 | TX estradiol | 4,345 |
| S16 | TX estrogen* or TX oestrogen* | 14,167 |
| S15 | TX hormone N1 therap* | 19,141 |
| S14 | TX norethisterone or TX norethindrone | 182 |
| S13 | TX dydrogesterone | 39 |
| S12 | TX medroxyprogesterone acetate or TX MPA | 16,317 |
| S11 | TX progesterone | 4,342 |
| S10 | TX progestogen | 459 |
| S9 | (MM "Progestational Hormones+") OR (MM "Medroxyprogesterone Acetate") OR (MM "Estrogens+") | 6,252 |
| S8 | TX HT or TX HRT | 10,069 |
| S7 | (MM "Hormone Replacement Therapy+") OR (MM "Hormone Therapy+") | 5,753 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 25,078 |
| S5 | TX hot flush* or TX hot flash* | 2,572 |
| S4 | TX climacter* or TX menopaus* | 14,243 |
| S3 | TX perimenopaus* or TX peri‐menopaus* or TX peri menopaus* | 3,419 |
| S2 | TX postmenopaus* or TX post‐menopaus* or TX post menopaus* | 13,673 |
| S1 | (MM "Climacteric+") OR (MM "Perimenopause") OR (MM "Perimenopausal Symptoms+") OR (MM "Menopause+") OR (MM "Hot Flashes") | 10,339 |
Appendix 7. Trials registers search strategy
www.clinicaltrials.gov:
hormone and menopausal and random
The World Health Organisation International Trials Registry Platform search portal:
hormone + menopausal
Appendix 8. Data extraction domains
Data were extracted from the primary studies on the following study characteristics:
Trial characteristics
1. Method of randomisation 2. Method of allocation concealment 3. Use of stratification 4. Adequacy of double‐blinding (i.e. an explicit statement that therapies could not be distinguished by appearance or administration route, or both) 5. Number of participants screened for eligibility, randomised, analysed, excluded, lost to follow‐up or dropped out (i.e. withdrew from the study but were followed up) 6. Level of adherence to therapy 7. Whether an intention‐to‐treat analysis was done 8.The use of a power calculation to estimate sample size 9. Duration, timing and location of the study 10. Study design (e.g. parallel or cross‐over, single‐centre or multi‐centre) 11. Source of funding
Characteristics of study participants
1. Inclusion and exclusion criteria 2. Age and any other recorded characteristics of women in the study 3. Menopausal status (i.e. peri‐ or postmenopausal and how status was defined, surgical or natural menopause) of the women in the study 4. Baseline equality of treatment groups 5. Means of recruitment
Data and analyses
Comparison 1. Women without major health problems (selected outcomes: death, CVD, cognition, QOL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause: oestrogen‐only HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 1.2 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 1.3 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.13] |
| 2 Death from any cause: combined HT | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.76, 2.27] |
| 2.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.71, 1.56] |
| 2.3 CEE 0.625 mg (mod dose) + P (as per footnotes) for 3 years | 3 | 18075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.46] |
| 2.4 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [0.15, 87.57] |
| 2.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 2.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.20] |
| 2.7 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 3 Death from any cause: oestrogen with or without sequential progesterone vaginal gel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.77] |
| 4 Death from coronary heart disease: oestrogen‐only HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestradiol 1 mg (low dose) daily for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 4.2 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.38] |
| 4.3 CEE 0.625 mg (mod dose) after 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.19] |
| 5 Death from coronary heart disease: combined continuous HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
| 5.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.38] |
| 6 Death from coronary heart disease: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.27] |
| 7 Death from stroke: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 CEE 0.625 mg (low dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.58, 2.32] |
| 8 Death from stroke: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 9 Death from stroke: combined continuous HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 1 year | 1 | 4385 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.12, 73.37] |
| 9.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.46, 2.35] |
| 10 Death from colorectal cancer: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.66, 2.46] |
| 11 Death from breast cancer: combined continuous HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Death from breast cancer: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 CEE 0.625 mg (mod dose) after median 11.8 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.98] |
| 13 Death from colorectal cancer: combined continuous HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.40, 2.29] |
| 13.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 7.1 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.52, 1.96] |
| 13.3 CEE 0.0625 mg (mod dose) + MPA 2.5 mg after 11.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.80, 2.14] |
| 14 Death from lung cancer: oestrogen‐only HT (moderate dose) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Death from lung cancer (non‐small cell or small cell) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.70] |
| 14.2 Death from non‐small cell lung cancer | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.52, 1.50] |
| 14.3 Death from small cell lung cancer | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.62, 6.79] |
| 15 Death from lung cancer: combined continuous HT (moderate dose) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Death from lung cancer (non‐small cell or small cell) at mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.18, 2.55] |
| 15.2 Death from non‐small cell lung cancer at mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.24, 2.93] |
| 15.3 Death from small cell lung cancer at mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.48, 2.81] |
| 15.4 Death from lung cancer (any type) at median 14 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.39] |
| 16 Death from lung cancer: combined sequential HT (low dose oestrogen) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 17 Death from any cancer: combined continuous HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 CEE O.625 mg daily (mod dose) + MPA 2.5 mg for 3 years | 1 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.11, 67.80] |
| 17.2 CEE 0.625 mg daily (mod dose) + MPA 2.5 mg for mean 5.2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.53] |
| 18 Coronary events (MI or cardiac death): oestrogen‐only HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.43] |
| 18.2 CEE 0.625 mg (mod dose) for 3 years | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.12, 72.72] |
| 18.3 CEE 0.625 mg (mod dose) for mean 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.13] |
| 18.4 CEE 0.65 (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 19 Coronary events (MI or cardiac death): combined continuous HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.15, 3.10] |
| 19.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.05, 2.12] |
| 19.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 2 | 17385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.07, 1.98] |
| 19.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.95, 1.44] |
| 19.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| 20 Coronary events (MI or cardiac death): combined sequential HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.09] |
| 20.2 1 mg (low dose) 17‐B‐oestradiol daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.27] |
| 20.3 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.71 [0.15, 90.70] |
| 21 Coronary events (MI or cardiac death): oestrogen with or without sequential progesterone vaginal gel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.16] |
| 22 Stroke: unopposed oestrogen | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.86] |
| 22.2 CEE 0.625 mg (mod dose) for mean 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.67] |
| 22.3 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.97, 1.40] |
| 23 Stroke: combined continuous HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 1 year | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.86] |
| 23.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.83, 2.06] |
| 23.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 2 | 17385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.02, 2.09] |
| 23.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.09, 1.77] |
| 23.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.06, 1.56] |
| 23.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.99, 1.33] |
| 24 Stroke: combined sequential HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 1 mg (low dose) 17‐B‐oestradiol daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 24.2 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.14] |
| 25 Stroke: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.51] |
| 26 Transient ischaemic attack: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.86] |
| 27 Transient ischaemic attack: combined sequential HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.13] |
| 27.2 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.14] |
| 28 Transient ischaemic attack: oestrogen with or without sequential progesterone vaginal gel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Oestradiol 1 mg daily,with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.44] |
| 29 Stroke or transient ischaemic attack | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 1 | 4385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.46] |
| 30 Venous thromboembolism (DVT or PE): oestrogen‐only HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 CEE 0.625 mg (mod dose) for up to 2 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.12, 4.39] |
| 30.2 CEE 0.625 mg (mod dose) for 3 years | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.96 [0.36, 133.75] |
| 30.3 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.00, 1.74] |
| 30.4 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 31 Venous thromboembolism (DVT or PE): combined sequential HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 31.2 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.14] |
| 31.3 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.73] |
| 31.4 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.08, 19.69] |
| 32 Venous thromboembolism (DVT or PE): combined continuous HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.28 [2.49, 7.34] |
| 32.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.88, 4.71] |
| 32.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.73, 3.72] |
| 32.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.55, 2.64] |
| 32.5 CEE 0.625 mg (mod dose) + 2.5 mg MPA for mean 7.9 years | 1 | 16707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.32, 2.05] |
| 33 Venous thromboembolism (DVT or PE): oestrogen with or without sequential progesterone vaginal gel | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 33.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.25, 8.83] |
| 34 Global cognitive function | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 34.1 Transdermal estradiol 0.014 mg (low dose): MMSE scores (baseline MMSE ≤ 90) | 1 | Mean Difference (Fixed, 95% CI) | ‐1.21 [‐5.05, 2.63] | |
| 34.2 Transdermal estradiol 0.014 mg (low dose): MMSE scores (baseline MMSE > 90) | 1 | Mean Difference (Fixed, 95% CI) | ‐0.3 [‐0.73, 0.13] | |
| 34.3 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years: MMSE scores | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.35, 0.15] | |
| 34.4 CEE 0.625 mg (mod dose) for mean 5.2 years: MMSE scores | 1 | Mean Difference (Fixed, 95% CI) | ‐0.26 [‐0.52, 0.00] | |
| 34.5 Combined continuous CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 4.2 years: MMSE scores | 1 | Mean Difference (Fixed, 95% CI) | ‐0.18 [‐0.36, 0.00] | |
| 34.6 Oestrogen with or without sequential progesterone vaginal gel | 1 | Mean Difference (Fixed, 95% CI) | ‐0.03 [‐0.21, 0.15] | |
| 35 Probable dementia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 CEE 0.625 mg (mod dose) for 5.2 years | 1 | 2947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.89, 2.59] |
| 35.2 Combined continuous CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 4.05 years | 1 | 4532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.16, 3.33] |
1.21. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 21 Coronary events (MI or cardiac death): oestrogen with or without sequential progesterone vaginal gel.
1.28. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 28 Transient ischaemic attack: oestrogen with or without sequential progesterone vaginal gel.
1.33. Analysis.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 33 Venous thromboembolism (DVT or PE): oestrogen with or without sequential progesterone vaginal gel.
Comparison 2. Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause: oestrogen‐only HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 CEE 0.625 mg (mod dose) daily for 3 years (2.8‐3.2) | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.53, 3.22] |
| 1.2 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.27] |
| 2 Death from any cause: oestrogen‐only or combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.67] |
| 3 Death from any cause: combined continuous HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2.8‐3.2 years | 2 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.28, 2.62] |
| 3.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 3.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐7 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
| 4 Death from coronary heart disease: oestrogen‐only HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 CEE 0.625 mg (mod dose) daily for 2.8‐3.2 years | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.36, 4.77] |
| 4.2 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.18] |
| 5 Death from CHD: oestrogen‐only or combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.81] |
| 6 Death from CHD: combined continuous HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 1 year | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.73, 3.29] |
| 6.2 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 2 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.90, 2.51] |
| 6.3 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 3 years (2.8‐3.2) | 3 | 3060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.88, 1.90] |
| 6.4 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 4+ years (median 4.1) | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.85, 1.67] |
| 6.5 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 4‐6.8 years | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.39] |
| 7 Coronary event (MI or cardiac death): oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 8 Death from stroke: oestrogen‐only or combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.95, 8.93] |
| 9 Death from cancer: combined continuous HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 CEE 0.625 mg daily (mod dose) + MPA 2.5 mg for 4+ years (median 4.1) | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.49, 1.57] |
| 9.2 CEE 0.625 mg daily (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.86, 2.65] |
| 10 Coronary event (MI or cardiac death): oestrogen‐only HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 CEE 0.625 (mod dose) daily for 2.8‐3.2 years | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.54, 2.40] |
| 11 Coronary event: oestrogen‐only or combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.57, 1.65] |
| 12 Coronary event (MI or cardiac death): combined continuous HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 1 year | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.00, 2.25] |
| 12.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.91, 1.58] |
| 12.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years (2.8‐3.2) | 3 | 3060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 12.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 4.1 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.19] |
| 12.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.29] |
| 13 Stroke (first or recurrent): oestrogen‐only HT or combined sequential | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Oestradiol 1 mg daily (low dose) (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 14 Stroke (first or recurrent): oestrogen‐only HT (mod dose) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 CEE 0.625 mg (mod dose) daily for 2.8 years | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 14.2 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.60, 4.47] |
| 15 Stroke (first or recurrent): combined continuous HT (mod dose oestrogen) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Continuous oestradiol 2 mg (mod dose) + norethisterone acetate 1 mg for 1.3 years | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.82] |
| 15.2 CEE 0.625 mg (mod dose) + MPA for 2.8 years | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.23 [0.26, 105.85] |
| 15.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 4.1 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.90, 1.68] |
| 15.4 CEE 0.625 mg (mod dose) + MPA for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.71, 1.57] |
| 16 Transient ischaemic attack: oestrogen‐only HT (mod dose) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.54, 2.36] |
| 17 Transient ischaemic attack: oestrogen‐only or combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.70, 1.94] |
| 18 Transient ischaemic attack: combined continuous HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.23] |
| 18.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.84] |
| 19 Stroke or transient ischaemic attack: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 CEE 0.625 mg (mod dose) daily for 3.2 years | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.28, 2.78] |
| 20 Stroke or transient ischaemic attack: combined continuous HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3.2 years | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.34, 3.03] |
| 21 VTE (first or recurrent PE or DVT): oestrogen‐only HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.33, 4.55] |
| 21.2 CEE 0.625 mg (mod dose) daily for 2.8‐3.2 years | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.44, 6.17] |
| 22 VTE (first or recurrent PE or DVT): combined continuous HT | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 1 year | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [1.06, 9.96] |
| 22.2 Continuous oestradiol 2 mg (mod dose) + norethisterone acetate 1 mg for 1.3 years | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.80 [0.86, 53.85] |
| 22.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.51 [1.42, 8.66] |
| 22.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years (2.8‐3.2) | 3 | 3060 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [1.50, 6.04] |
| 22.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 4.1 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.39, 4.94] |
| 22.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐7 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.63, 2.98] |
Comparison 3. Women with dementia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Worsening of dementia on treatment (by ADCS‐CGIC score): oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Unopposed CEE 0.625 mg (mod dose) or 1.25 mg (high dose) daily for 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Women post surgery for early‐stage endometrial cancer (selected outcomes: death, recurrence).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death from any cause: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 CEE 0.625 mg (mod dose) daily for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.77, 2.45] |
| 2 Death from endometrial cancer: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 CEE 0.625 mg (mod dose) daily for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.63] |
| 3 Death from CHD: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 0.625 mg (mod dose) daily for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.63] |
Comparison 5. Women hospitalised with chronic illness (selected outcomes: death, CVD, VTE).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause death: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.60] |
| 2 Myocardial infarction: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.14] |
| 3 Venous thromboembolism (DVT or PE): combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.07] |
Comparison 6. All women (selected outcomes: cancer, cholecystic disease, fractures).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breast cancer: oestrogen‐only HT | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen only HRT patch 0.025 (low dose) mg daily for 2 years | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.04] |
| 1.2 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 1.3 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.91] |
| 1.4 Oestradiol patch 0.075 mg (high dose) for 2 years | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.04] |
| 1.5 CEE 0.625 mg (mod dose) for 2.8‐3.2 years | 3 | 676 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.38, 11.04] |
| 1.6 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.01] |
| 1.7 CEE 0.625 mg (mod dose) after 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.63, 0.96] |
| 1.8 CEE 0.625 mg (mod dose) after 13 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.97] |
| 2 Breast cancer: oestrogen‐only or combined HT | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Breast cancer: combined continuous HT | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 23182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.96] |
| 3.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.47, 1.08] |
| 3.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2.8‐3.4 years | 3 | 17733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.18] |
| 3.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.82, 2.27] |
| 3.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.03, 1.56] |
| 3.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐7 years unblinded | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.52, 2.23] |
| 3.7 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 7.9 years | 1 | 16607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.07, 1.52] |
| 3.8 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 11 years (includes extra follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.08, 1.45] |
| 3.9 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.11, 1.47] |
| 4 Breast cancer: combined sequential HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.85] |
| 4.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [0.44, 34.64] |
| 4.3 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.30, 10.64] |
| 4.4 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.31, 11.02] |
| 4.5 CEE 2.5 mg daily (high dose) + MPA 10 mg for 7 days each cycle for 10 years | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.03] |
| 5 Breast cancer: oestrogen with or without sequential progesterone vaginal gel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.10] |
| 6 Colorectal cancer: oestrogen‐only HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 CEE 0.625 mg (mod dose) for 3 years | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.08] |
| 6.2 CEE 0.625 (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.81, 1.63] |
| 6.3 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.82, 1.49] |
| 7 Colorectal cancer: oestrogen‐only or combined HT | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Colorectal cancer: combined continuous HT | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.32, 1.42] |
| 8.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.50] |
| 8.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 2 | 16956 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.49, 1.34] |
| 8.4 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.32, 1.48] |
| 8.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.91] |
| 8.6 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.46, 1.44] |
| 8.7 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 7.9 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.57, 1.01] |
| 8.8 CEE 0.0625 mg (mod dose) + MPA 2.5 mg after 11.6 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.99] |
| 8.9 CEE 0.0625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 9 Colorectal cancer: combined sequential HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.13] |
| 9.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 9.3 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle for 10 years | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.73] |
| 10 Colorectal cancer: oestrogen with or without sequential progesterone vaginal gel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.83] |
| 11 Lung cancer: oestrogen‐only HT (moderate dose) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Any lung cancer (non‐small cell or small cell) at 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.48] |
| 12 Lung cancer: combined continuous HT (mod dose oestrogen) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Any lung cancer at 5.6 years (non‐small cell or small cell) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 12.2 Any lung cancer at 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.92, 1.62] |
| 12.3 Any lung cancer after median 14 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.93, 1.38] |
| 13 Lung cancer: combined sequential HT | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 17‐B‐oestradiol 1 mg (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 78.13] |
| 14 Endometrial cancer: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 CEE 0.625 mg (mod dose) for 3‐3.2 years | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 15 Endometrial cancer: combined continuous HT (mode dose oestrogen) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 CEE 0.625 mg + MPA 2.5 mg for 1 year | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.13, 6.76] |
| 15.2 CEE 0.625 mg + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.31, 2.95] |
| 15.3 CEE 0.625 mg + MPA 2.5 mg for 3‐3.2 years | 2 | 16847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.35, 1.82] |
| 15.4 CEE 0.625 mg + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 15.5 CEE 0.625 mg + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.51, 1.44] |
| 15.6 CEE 0.625 mg + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.78] |
| 15.7 CEE 0.625 + MPS 2.5 mg for 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.20] |
| 15.8 CEE 0.625 mg + MPA 2.5 mg after median 13 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.90] |
| 16 Endometrial cancer: combined sequential HT | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 17‐B‐oestradiol 1 mg (low dose) + dydrogesterone 5 mg days 14‐28 for 2 years | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.08, 45.95] |
| 16.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 16.3 Oestradiol 2 mg (mod dose) + dihydrogesterone 20 mg for 2 years | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [0.16, 67.59] |
| 16.4 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.97 [0.29, 123.81] |
| 16.5 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.71 [0.15, 90.70] |
| 16.6 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle for 10 years | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.07] |
| 17 Recurrent endometrial cancer: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Oestrogen (type and dose not stated) for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.54, 2.50] |
| 18 Ovarian cancer: combined continuous HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.76, 2.69] |
| 18.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 19 Ovarian cancer: oestrogen with or without sequential progesterone vaginal gel | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.08] |
| 20 Gallbladder disease requiring surgery: oestrogen‐only HT | 3 | 8930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.40, 2.19] |
| 20.1 CEE 0.625 mg (mod dose) for 3‐3.2 years | 2 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.17, 3.39] |
| 20.2 CEE O.625 mg (mod dose) for 7.1 years | 1 | 8376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.42, 2.24] |
| 21 Gallbladder disease requiring surgery: combined continuous HT | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 3 years | 2 | 557 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.61, 6.59] |
| 21.2 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 4 years | 1 | 2253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.98, 1.85] |
| 21.3 CEE 0.625 mg (mod dose) + 2.5 mg MPA for mean 5.6 years | 1 | 14203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.30, 2.06] |
| 22 Gallbladder disease requiring surgery: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.37, 10.78] |
| 22.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.67] |
| 23 Hip fractures: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.95] |
| 23.2 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 23.3 CEE 0.625 mg (mod dose) after 13.2 years (includes extended follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.74, 1.17] |
| 24 Hip fractures: oestrogen‐only or combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.42] |
| 25 Hip fractures: combined continuous HT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.26, 1.57] |
| 25.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.18] |
| 25.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.17] |
| 25.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.55, 2.42] |
| 25.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.47, 0.96] |
| 25.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.06, 4.16] |
| 25.7 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 25.8 CEE 0.625 mg (mod dose) + 2.5 mg MPA after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 26 Hip fractures: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 17‐B‐oestradiol 1 mg (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.27] |
| 27 Vertebral fractures: oestrogen‐only HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.94] |
| 28 Vertebral fractures: combined continuous HT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.37, 1.47] |
| 28.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.96] |
| 28.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.49, 2.48] |
| 28.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.01] |
| 29 All clinical fractures: oestrogen‐only or combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.68, 2.19] |
| 30 All clinical fractures: oestrogen‐only HT (moderate dose) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| 30.2 CEE 0.625 mg (mod dose) daily for 3.2 years | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 30.3 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.80] |
| 31 All clinical fractures: oestrogen‐only or combined HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 31.1 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 32 All clinical fractures: combined continuous HT | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 1 year | 1 | 4385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.02] |
| 32.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3.2‐3.4 years | 2 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.32, 0.87] |
| 32.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.71, 0.86] |
| 32.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 32.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.91, 1.65] |
| 32.6 CEE 0.0625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.76, 0.89] |
| 33 All clinical fractures: combined sequential HT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 17‐B‐oestradiol 1 mg (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.64] |
6.5. Analysis.
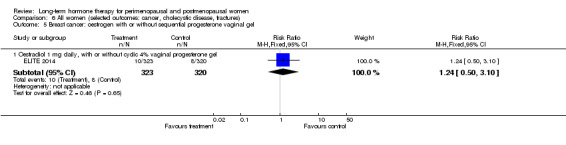
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 5 Breast cancer: oestrogen with or without sequential progesterone vaginal gel.
6.10. Analysis.
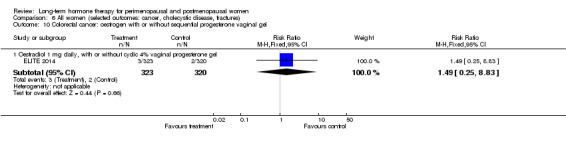
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 10 Colorectal cancer: oestrogen with or without sequential progesterone vaginal gel.
6.19. Analysis.
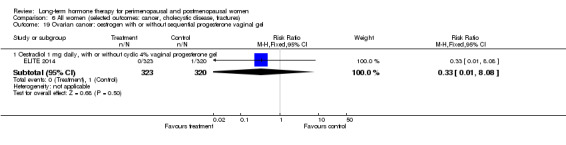
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 19 Ovarian cancer: oestrogen with or without sequential progesterone vaginal gel.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barakat 2006.
| Methods | Stated purpose: to determine the effect of oestrogen therapy on recurrence rate and survival in women who have undergone surgery for stage I or II endometrial cancer Stratification: stratified by stage No, of women screened for eligibility: unclear No. randomised: 1236 (see Notes) No. analysed: 1236 Losses to follow‐up: none stated Adherence to treatment: 41% in HT group, 50% in placebo group at trial end Analysis by intention to treat: yes No. of centres: not stated Years of recruitment: June 1997 to January 2003 Design: parallel Funding: National Cancer Institute grant | |
| Participants | Included Women post total hysterectomy and bilateral salpingo‐oophorectomy (at least) for surgically staged stage I or II endometrial cancer within 20 weeks of study entry, with indication for use of oestrogen therapy including hot flushes, vaginal atrophy, increased risk of CHD or increased risk of osteoporosis. Had to have undergone clinical exam with history, pelvic exam and chest X‐ray before study entry. Normal hepatic function and normal mammogram or negative breast biopsy within previous year Excluded Women with history or suspicion of breast cancer or other malignancy with exception of non‐melanoma skin cancer within past 5 years or with history of acute liver disease or thromboembolic disease Median age: 57 Age range: 26‐91 Means of recruitment: not stated Baseline equality of treatment groups: well balanced Country: USA | |
| Interventions | HT arm: 0.625 mg CEE (unopposed oestrogen) Control arm: placebo Duration: planned for 3 years with 2 years' additional follow‐up. Closed early with median follow‐up 35.7 months | |
| Outcomes | Total deaths CHD deaths Coronary event deaths Endometrial cancer deaths Endometrial cancer (recurrence) | |
| Notes | Enrolment decreased after WHI was published in July 2002. Study closed prematurely owing to poor accrual. In addition, preponderance of participants had low risk profile, so low event rate meant power unlikely to be reached with original power calculation. This study planned to enrol 2108 women. Numbers randomised not entirely clear: Study refers to 1236 "eligible and assessable women". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remotely generated |
| Allocation concealment (selection bias) | Low risk | Remotely dispensed drugs |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No losses to follow‐up reported, but numbers randomised not entirely clear: Study refers to 1236 "eligible and assessable women". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and physicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Review authors believe risk of bias low owing to 'hard' nature of outcomes |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
ELITE 2014.
| Methods | 2 × 2 double‐blinded placebo‐controlled parallel‐group RCT Stated purpose: to assess the effect of HT on progression of subclinical atherosclerosis and cognitive effects initiated between early and late postmenopause Stratification: not stated No. of women screened for eligibility: 3061 (n = 2166 via telephone, n = 895 in person) No. of women randomised: 643 (323 to HT, 320 to placebo; subgrouped by time since menopause with respect to initiation of HT) No. of women analysed: 567 underwent cognitive baseline assessment, total of 567 women provided cognitive outcomes at 2.5 years and 455 women provided outcomes at 5 years. Losses to f/u: 2.2% of women were lost to follow‐up, and another 10.0% discontinued participation before cognitive outcomes were assessed (14 lost to follow‐up, 22 dropouts due to adverse events, 40 discontinued for other reasons before contributing to cognitive outcomes at 2.5 years). Adherence to treatment: Mean adherence for oestradiol or placebo was ≥ 98% for early and late group women. Analysis by intention to treat: yes No. of centres: 1 Years of recruitment: July 2005 and September 2008 Design: parallel Funding: supported by National Institutes of Health grant for initial and supplemental funding of ELITE and ELITE‐Cog. Study drugs and placebo were supplied without charge or restriction by Teva Pharmaceuticals, Watson Pharmaceuticals and Abbott Laboratories. |
|
| Participants | 643 healthy postmenopausal women with clinical evidence of CVD or diabetes, subgrouped by time since menopause (< 6 years since menopause (n = 271) or > 10 years since menopause (n = 372)) Included Women with a serum oestradiol level < 25 picogram/mL and cessation of regular menses > 6 months who are < 6 years and > 10 years postmenopausal Excluded Clinical signs, symptoms or personal history of cardiovascular disease, indeterminate time since menopause, DM or fasting serum glucose ≥ 140 mg/dL, uncontrolled hypertension (diastolic blood pressure ≥ 110 mmHg), untreated thyroid disease, serum creatinine > 2.0 mg/dL, plasma triglyceride levels > 500 mg/dL, life‐threatening disease with prognosis < 5 years, cirrhosis or liver disease, hx of deep vein thrombosis or pulmonary embolism, history of breast cancer, current HT Median age: 53.4 years for early, 63.6 years for late Age range: 41‐84 Means of recruitment: telephone and in person Baseline equality of treatment group: no statistically significant difference in baseline characteristics. Women not contributing to analysis were similar to other women in most but not all comparisons. Country: USA |
|
| Interventions | 1. Oral 17β‐oestradiol 1 mg daily with (uterine intact) or without (hysterectomy) vaginal micronised progesterone gel 4% (45 mg) 10 days per month: Study publication does not state how many women were in each group. 2. Placebo Originally planned for 5 years, extended to 7.5 years |
|
| Outcomes |
Primary study outcome Progression of subclinical atherosclerosis ‐ not relevant for current review Secondary outcomes Cognitive function at 2.5 and 5 years, cardiovascular events (fatal or nonfatal MI, silent MI, sudden death), stroke, venous thromboembolism (DVT or PE), cancer (breast, uterine, ovarian, gastrointestinal, lung), bone fracture, all‐cause mortality, non‐coronary mortality |
|
| Notes | Power calculation: 506 sample size provides power of 80% needed to detect difference in rate of change in carotid artery intima media thickness and effect size of 0.22 in early and late groups combined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generated by computer by study statistician |
| Allocation concealment (selection bias) | Low risk | "Stratified randomization list [was] used to prepare the study products. After determining a participant’s eligibility, clinic staff pulled the next study product in sequence from the appropriate stratum, recorded the product identification number, and dispensed the product". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 567/643 women (88%) analysed for cognitive outcomes at 2.5 years, 455/643 (71%) at 5 years |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, participants, clinic staff and data monitors were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated whether outcome assessor blinded, but most probably, as study author mentioned trial was extended before blinding was unmasked after receiving supplementary funding |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential source of bias identified |
EPAT 2001.
| Methods | Stated purpose: to determine the effect of oestrogen‐alone HT on progression of subclinical atherosclerosis in healthy postmenopausal women without preexisting cardiovascular disease, as measured by changes in thickness of carotid artery wall
Stratification: by low‐density lipoprotein cholesterol level (threshold < 4.15 mmol/L), previous duration of HT (threshold < 5 years) and diabetes mellitus status
Blinding: participants, gynaecologists, clinical staff and image analysts. The data monitor and the data analyst were blinded to treatment assignment until analyses were completed.
No. of women screened for eligibility: 1161 prescreened by phone, 422 screened on site, of whom 52% randomised
No. randomised: 222
No. analysed: 222 for clinical outcomes Losses to follow‐up: 33 women were not evaluable for primary study endpoints, but clinical endpoints were reported for all. Adherence to treatment in evaluable women: During the trial, mean pill adherence was 95% in the oestradiol group and 92% in the placebo group (P = 0.08). Analysis by intention to treat: yes No. of centres: 1 Years of recruitment: 1994‐1998 Design: parallel Funding: National Institute on Aging |
|
| Participants |
Included
Postmenopausal women aged > 45 years, no preexisting cardiovascular disease, low‐density lipoprotein levels > 3.37 mmol/L
Excluded Women with previous breast or gynaecological cancer, frequent hot flushes, diastolic blood pressure > 110, uncontrolled diabetes or thyroid disease, abnormal bloods, smokers Mean age: 61.15 Age range: 51.4‐69.2 Means of recruitment: not stated Baseline equality of treatment groups: no significant differences in demographics or clinical variables Country: USA |
|
| Interventions | HT arm: unopposed micronised 17B‐oestradiol 1 mg daily Control arm: placebo Duration: 2 years | |
| Outcomes |
Primary outcomes Carotid artery wall thickness on ultrasound Myocardial infarction Cerebrovascular accident Transient ischaemic attack Deep vein thrombosis Pulmonary embolism |
|
| Notes | Power calculation: sample size of 200 required to detect treatment effect size (difference in carotid artery wall thickness) of 0.40 or greater with 80% power | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Blinded medication packets assigned sequentially and remotely after eligibility confirmed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33 women were not able to be evaluated for primary (physiological) study endpoints, but clinical endpoints were reported for all by ITT analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, gynaecologists, clinical staff and image analysts. The data monitor and the data analyst were blinded to treatment assignment until analyses were completed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Adverse clinical symptoms and bleeding were assessed by the study gynaecologist, who was blinded to treatment assignment". |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
EPHT 2006.
| Methods | Stated purpose: to ascertain harms and benefits of combined HT among healthy postmenopausal Estonian women
Stratification: by centre
No. of women screened for eligibility: 39,713 (whole female population aged 50‐64 from 2 areas of Estonia)
No. randomised and consented: 777 for clinical outcomes (Veerus 2006 publication), 796 for quality of life (Veerus 2008 publication) (see Notes)
No. analysed: 777 for clinical outcomes (HT 404, placebo 373), 796 for quality of life (HT 415, placebo 381)
Losses to follow‐up: none stated
Adherence to treatment: < 40% in HT group and < 30% in placebo group by 3 years (estimated from graph)
Analysis by intention to treat: yes
No. of centres: 3
Years of recruitment: 1999‐2001
Design: parallel
Funding: academic and government grants www.controlled‐trials.com/ ISRCTN35338757/35338757 |
|
| Participants | Included Postmenopausal women > 12 months since last period Excluded Women who had used hormone therapy during the past 6 months; with untreated endometrial adenomatosis or atypical hyperplasia of the endometrium; history of breast cancer, endometrial cancer or ovarian cancer; any other cancer treated less than 5 years ago; history of meningioma; myocardial infarction within the past 6 months; history of hepatitis or functional liver disorders in the past 3 months; history of deep vein thrombosis, pulmonary embolism or cerebral infarction; porphyria; hypertension greater than 170/110 mmHg despite medication; laparoscopically or histologically confirmed endometriosis Mean age: 59 Age range: 50‐70 Means of recruitment: invitation sent to whole female population aged 50‐64 from 2 areas of Estonia Baseline equality of treatment groups: more prior use of oral contraceptive in HT group ‐ 9.2% vs 6.4%; HT group older (59 vs 58.5) Country: Estonia | |
| Interventions | HT arm: combined oestrogen and progesterone as 1 daily tablet containing CEE 0.625 mg and medroxyprogesterone acetate 2.5 mg Control arm: matching placebo Duration: mean follow up 3.43 years (range 2‐5). Planned for 10‐year follow‐up but closed early | |
| Outcomes | Death
CHD
Cancers
Fractures
CVD Quality of life measured with EuroQol‐5D questionnaire at 3 years (also measured with Women's Health Questionnaire at 1 year), but no baseline measure, and results pooled for blinded and unblinded HT arms (data not reported in this review) |
|
| Notes | Women randomised before eligibility and consent checked ‐ envelopes opened only once these processes were completed. Additional 1001 women in unblinded trial arms Designed as part of international WISDOM trial Mean follow‐up only 3.43 years (range 2‐5) for clinical outcomes, 3.6 years for quality of life. Planned for 10‐year follow‐up but closed early |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remotely randomised in permuted blocks |
| Allocation concealment (selection bias) | Low risk | Non‐transparent sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No stated losses to follow‐up or drop‐outs, analysed by intention to treat. However, stated participation rates differ slightly across trial publications (796 vs 777). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed by entries in cancer registry ‐ review authors believe low risk of bias |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Quality of life not measured (with EuroQol‐5D) at baseline ‐ possible baseline differences |
ERA 2000.
| Methods | Stated purpose: to evaluate effects of HT on progression of coronary atherosclerosis
Stratification: according to lipid lowering therapy at baseline and hospital where angiogram was performed
Blinding: participants, clinic staff and all outcome assessors blinded
Unblinding: treatment assignment available to designated member of data management staff. Questions related to adverse effects directed to gynaecology physician and nurse not connected with study
No. of women screened for eligibility: not stated
No. randomised: 309
No. analysed: 309 (for clinical events)
Losses to follow‐up: none (for clinical events)
Adherence to treatment in 248 participants evaluated was as follows: Unopposed oestrogen group took 74.5% of their prescribed medication, and combined HT group took 84%; placebo group took 85.8%. 5 women in the placebo group started to take HT. Analysis by intention to treat: Although only 248 participants were available for the primary trial endpoint (which was biological), clinical adverse events, including outcomes of interest to this review, were reported for all participants at 3.2 years by intention to treat. No. of centres: 6 Years of recruitment: January 1996‐December 1997 Design: parallel Funding: grants from National Heart, Lung and Blood Institute and National Center for Research Resources General Clinical Research Center, study medications from Wyeth‐Ayerst Research |
|
| Participants |
Included Postmenopausal women aged 55‐80 years (non‐natural menses for at least 5 years, or for 1 year and FSH > 40 mu/mL or oophorectomy) with at least 1 stenosis > 30% in any single coronary artery confirmed by coronary angiography within 4 months of randomisation, baseline gynaecological examination normal Excluded Failure to achieve > 80% compliance during 4‐week placebo run‐in phase, breast or endometrial cancer, history of DVT or PE, symptomatic gallstones or elevated liver enzymes, fasting plasma triglycerides > 400 mg/dL, MI within 4 weeks, renal insufficiency, dye allergy, > 70% stenosis of coronary artery, uncontrolled hypertension, uncontrolled diabetes, planned or prior coronary artery bypass graft, revascularisation of only qualifying lesion (for study), inadequate baseline angiogram for study, other non‐CHD disease likely to be fatal or to prevent adequate follow‐up, participation in other intervention studies, plans to leave area within 3 years Mean age: 66 Age range: 41.8‐79.9 Means of recruitment: media announcements, contact through hospital records and admissions, screening logs from other studies Baseline equality of treatment groups Country: USA Follow‐up: 3 months, 6 months, then 6‐monthly clinic visits; annual smear and mammography, annual endometrial aspiration |
|
| Interventions | HT arm: 1 of the following 1. 0.625 mg CEE (unopposed oestrogen) 2. 0.625 mg CEE plus 2.5 mg MPA (combined continuous therapy) Control arm: placebo Duration: 3.2 years mean | |
| Outcomes | Primary outcome angiographic MI Stroke Death DVT PE | |
| Notes | Power calculation: 80% power for primary angiographic outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised in random blocks |
| Allocation concealment (selection bias) | Low risk | Computer‐displayed treatment assignment after eligible participant details entered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up for clinical adverse events. Analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | More in unopposed oestrogen group using nitrates at baseline; otherwise prognostic balance between groups |
ESPRIT 2002.
| Methods | Stated purpose: to ascertain whether unopposed oestrogen reduces the risk of further cardiac events in postmenopausal women who survive a first myocardial infarction Stratification: by clinical centre Blinding: participants, clinicians, outcome assessors. Pharmaceutical company dispensed medication/placebo in identical numbered packages. Unblinding: on request of family doctor or if participant withdrew from treatment (in later stages of study, only if withdrawing participant had not had a hysterectomy). Outcome assessors remained blinded throughout. No. of women screened for eligibility: 3121 met inclusion criteria for MI (reasons for non‐participation listed in study). No. randomised: 1017 No. analysed: 1017 Drop‐outs: Drop‐outs included 43 women in the HT group (8%) and 57 in the placebo group (11%) who did not take any of the trial medication. Losses to follow‐up: none Known non‐adherence with allocated treatment was as follows: At 1 year, 51% of participants on the HT arm and 31% on the placebo arm were not taking their allocated tablets regularly. At 2 years, 57% of participants on the HT arm and 37% on the placebo arm were not taking their allocated tablets regularly. Analysis by intention to treat: yes No. of centres: 35 Years of recruitment: July 1996‐Feb 2000 Design: parallel Funding: Schering AG provided medication. | |
| Participants |
Included Postmenopausal women admitted to coronary care units or general medical wards at participating centres, who met diagnostic criteria for myocardial infarction, were discharged alive within 31 days of admission Excluded Women with previously documented MI who had used HT or had vaginal bleeding in the 12 months before admission, history of breast, ovarian or endometrial cancer, active thrombophlebitis, history of DVT or PE, liver disease, Rotor syndrome, Dubin‐Johnson syndrome or severe renal disease Mean age: 62 years (SD 5) Means of recruitment: Research nurses checked hospital case notes and approached potentially eligible women if their family doctor agreed to collaborate. Baseline equality of treatment groups: yes Countries: England and Wales |
|
| Interventions | HT arm: unopposed oestradiol valerate 2 mg daily Control arm: placebo Duration: 2 years | |
| Outcomes | Recurrent MI Cardiac death All‐cause death Endometrial cancer Breast cancer Stroke Thromboembolism | |
| Notes | Power calculation: needed 1700 participants to give 80% power to detect 33.3% decrease in incidence of non‐fatal reinfarction or cardiac death (2‐sided P = 0.05) Accrual lower than anticipated: Study closed with only 1017 participants, giving 56% power to detect above‐mentioned outcomes, assuming full compliance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers generated by trial statistician in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Women assigned consecutively to numbers kept on list accessible to statistician only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up; analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
EVTET 2000.
| Methods | Stated purpose: to determine if HT alters risk of venous thromboembolism in high‐risk women Randomisation method: computer‐generated 1:1 block randomisation with fixed block sizes of 10 Allocation method: not described Stratification: by age < 60 years or ≥ 60 years; 37 (23 HT and 14 placebo) women did not attend all visits owing to premature termination of the study Blinding: double‐blind No. of women screened for eligibility: 328 No. randomised: 140 (71 HT, 69 placebo) No. analysed: 140 Losses to follow‐up: nil, although 37 (23 HT and 14 placebo) women did not attend all visits owing to premature termination of the study Adherence to treatment: 33 dropouts: 10 in HT group (2 wanted to be sure of being treated with oestrogen for postmenopausal symptoms, 8 had adverse effects), 23 in the placebo group (11 wanted to be sure of being treated with oestrogen for postmenopausal symptoms, 10 had adverse effects, 2 no reason stated) Analysis by intention to treat: Main findings were not reported by intention to treat because dropouts from the placebo group were not included in the denominator for the rate of recurrent thromboembolism. We included all randomised participants in analysis for this review. No. of centres: not stated Years of recruitment: February 1996‐March 1999 Design: stratified double triangular sequential design Funding: Novo‐Nordisk Pharmaceutical and research forum Ulleval University Hospital | |
| Participants |
Included Postmenopausal women with history of VTE, aged < 70 years, previous VTE verified by objective means (i.e. venography or ultrasonography in cases of DVT; lung scan, helical computed tomography or angiography in cases of PE) Excluded Current use or use of anticoagulants within past 3 months, familial antithrombin deficiency, any type of malignant disease including known, suspected or past history of carcinoma of the breast; acute or chronic liver disease or history of liver disease in which liver function tests had failed to return to normal; porphyria; known drug abuse or alcoholism; life expectancy less than 2 years; women who had taken part in other clinical trials within 12 weeks before study entry Mean age: 55.8 years Age range: 42‐69 years Means of recruitment: letters to family doctors, gynaecologists and hospitals, health bulletins and media Baseline equality of treatment groups: Baseline characteristics were similar for HT group and placebo group with regard to previous disease (coronary heart disease, hypertension, stroke, diabetes), smoking habits and serum lipids. All women had previously suffered at least 1 VTE, and the total number of previous VTEs was 75 in the placebo group and 77 in the HT group. Country: Norway |
|
| Interventions | HT arm: 2 mg oestradiol plus 1 mg norethisterone acetate 1 mg Control arm: placebo Duration: planned 2 years, stopped prematurely at median 1.3 years' follow‐up | |
| Outcomes | Venous thrombosis
Myocardial infarction Transient ischaemic attacks Stroke |
|
| Notes | Study was terminated early; only 140 women enrolled of 240 planned Power calculation: At a significance level of 5% and a power of 90%, sample size was estimated to a maximum of 240 women . After publication of results of the HERS study, which showed as a secondary endpoint increased risk of VTE, recruitment of women was discontinued in September 1998, until reviewed by the safety monitoring committee. The committee was also concerned about a non‐significant clustering of endpoints in 1 study group, without knowing treatment allocation. The committee advised on premature termination of the study, even though formal boundaries showing excess risk of VTE were not reached. The final decision on termination of the study was made in February 1999, and by the end of March 1999, all participants had completed a final follow‐up visit. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 1:1 block randomisation with fixed block sizes of 10 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Main findings were not reported by intention to treat because drop‐outs from the placebo group were not included in the denominator for the rate of recurrent thromboembolism. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded participants and personnel ‐ "equal‐looking" placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Selective reporting (reporting bias) | Low risk | Retrospectively registered protocol on trials register. Reports all expected outcomes |
| Other bias | Low risk | No apparent source of other bias |
Ferenczy 2002.
| Methods | Stated purpose: to assess endometrial safety and bleeding patterns of 17B‐oestradiol sequentially combined with dydrogesterone Stratification: not mentioned Blinding: double‐blinded No. of women screened for eligibility: 844 No. randomised: 595 (HT group 1: 117, HT group 2: 114, HT group 3: 117, HT group 4: 118, placebo group: 113 (see Interventions)) No. analysed: 442 (for endometrial cancer, which is the only outcome of interest for this review) Losses to follow‐up: Endometrial status was evaluated by a biopsy, which was available only for women who remained on active treatment for over a year, or who received placebo and completed the 2‐year study. This resulted in 153 losses to follow‐up for this outcome (87 from active treatment groups (24%) and 50 from the placebo group (44%), plus another 16 who received no study medication). Adherence to treatment: not reported Analysis by intention to treat: no No. of centres: multi‐centre (number not stated) Years of recruitment: not stated Design: parallel Funding: Solvay Pharmaceutical | |
| Participants |
Included Postmenopausal women with a uterus with amenorrhoea of at least 6 months or surgically postmenopausal (following bilateral oophorectomy without hysterectomy, more than 3 months before enrolment), FSH within normal postmenopausal range Excluded Abnormal (uninvestigated bleeding) vaginal bleeding, use of oestrogens and/or progestogens and/or androgens in the preceding 6 months or more, and any previous use of oestradiol pellet/implant therapy Age range: 45‐65 Baseline equality of treatment groups: yes Countries: Canada and Netherlands |
|
| Interventions |
HT arm 1 mg/d 17B‐oestradiol/ 5 mg dydrogesterone for the last 14 days of each 28‐day cycle 1 mg/d 17B‐oestradiol/10 mg dydrogesterone for the last 14 days of each 28‐day cycle 2 mg/d 17B‐oestradiol/10 mg dydrogesterone for the last 14 days of each 28‐day cycle 2 mg/d 17B‐oestradiol/20 mg dydrogesterone for the last 14 days of each 28‐day cycle Control arm: placebo Duration: 26 cycles (104 weeks) |
|
| Outcomes | Endometrial cancer | |
| Notes | Power calculation: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Endometrial status was evaluated by a biopsy, which was available only for women who remained on active treatment for over a year, or who received placebo and completed the 2‐year study. This resulted in 153 losses to follow‐up (26%) for this outcome. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blinded ‐ review authors believe risk of bias low owing to 'hard' nature of outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Review authors believe risk of bias low owing to 'hard' nature of outcomes |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
Greenspan 2005.
| Methods | Stated purpose: to determine effects of HT on clinical outcomes, including cognitive function, in elderly women Stratification: no Blinding: double‐blinded Unblinding: not described No. of women screened for eligibility: 573 No. randomised: 373 (187 to HT, 186 to placebo) No. analysed: 373 Losses to follow‐up: 8 (6 in HT group, 2 in placebo group) Adherence to treatment: 61% on HT, 67% on placebo Analysis by intention to treat: yes No of centres: 1 Years of recruitment: study conducted January 1996‐May 2001 Design: parallel Funding: academic research funding. Pharmaceutical companies provided the drugs. | |
| Participants |
Included Community‐dwelling women aged 65 years or older with (n = 243) or without (n = 130) a uterus, with complete medical history, physical examination and lab evaluation; tolerated HT in run‐in phase Excluded Women with any illnesses or taking medications that could affect bone mineral metabolism within past year, or with known contraindication to HT Mean age: 71 years Age range: 65‐90 Means of recruitment: advertisements, presentations, physical referrals Baseline equality of treatment groups: yes Country: USA; n = 373 healthy women, mean age 71.3 years, 243 with a uterus and 130 without a uterus |
|
| Interventions | Three‐month open run‐in phase on HT 1. CEE oral (0.625 mg/d) or CEE oral (0.625 mg/d) + medroxyprogesterone acetate (2.5 mg/d) in women with a uterus 2. Placebo |
|
| Outcomes | MMSE, breast cancer, DVT, clinical fractures, colon cancer (and other outcomes not relevant to this review) | |
| Notes | Half of participants also took alendronate; all took calcium and vitamin D supplement and a multi‐vitamin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised lists "prepared by study statistician" |
| Allocation concealment (selection bias) | Low risk | Research pharmacist assigned treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis; low losses to follow‐up (6/373) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Those who assessed the outcomes were blinded to treatment assignment... block sizes randomly determined to enhance blinding of study staff" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Possibly some potential for confounding from 3‐month run‐in phase on HT and concurrent use of alendronate |
HERS 1998.
| Methods | Stated purpose: to determine if combined HT alters risk for CHD events in postmenopausal women with established coronary disease
Stratification: by clinical centre
Blinding: participants, clinical centre staff, outcome assessors, data analysts, funders blinded
Unblinding: When required for safety or symptom control, participants reported directly to gynaecology staff, who were located separately from clinical staff, did not communicate with them about breast or gynaecological problems and were not involved in outcome ascertainment.
No. of women screened for eligibility: 3463, of whom 43% were excluded (ineligible, declined to participate, did not return for appointment or did not comply with placebo run‐in therapy)
No. randomised: 2763
No. analysed: 2763
Losses to follow‐up: vital status known for all women at end of trial. 59 women did not complete follow‐up (32 in experimental arm, 27 in placebo arm).
Adherence to treatment by women evaluated: by self‐report: at 1 year: 82% HT arm, 91% control arm; at 3 years: 75% HT arm, 81% control arm; by pill count in HT arm: at 1 year: 79%; at 3 years: 70% HT arm
Analysis by intention to treat: yes (also analysed by treatment received, with inclusion limited to women with > 80% compliance)
No. of centres: 20
Years of recruitment: February 1993‐September 1994
Design: parallel
Funding: pharmaceutical (Wyeth‐Ayerst) UNBLINDED CONTINUATION OF HERS 1998: N.B. Follow‐up continued unblinded, as an open‐label observational study 2321 women (93% of 2510 surviving HERS participants) followed up for a further 2.7 years ‐ originally planned for additional 4 years, but executive committee decided no further useful information likely to emerge No. analysed: 2311 for vital status Losses to follow‐up: 10 women (1%) not contacted at final follow‐up (2 in HT arm, 8 in control arm); of these, vital status known for 5 Adherence to treatment: Among women originally assigned to the HT group, 45% reported at least 80% compliance during the sixth year of follow‐up. Among women originally assigned to placebo, 8% reported taking HT at 6 years. |
|
| Participants |
Included Postmenopausal women younger than 80 years, with a uterus, with coronary disease (myocardial infarction, coronary artery bypass surgery, percutaneous coronary revascularisation or angiographic evidence of at least 50% narrowing of 1 or more major arteries, as documented by baseline ECG or hospital discharge summary), likely to be available for follow‐up for at least 4 years Excluded Women whose coronary event occurred within 6 months of randomisation, use of hormone therapy within 3 months of randomisation, serum triglycerides ≥ 300 mg/dL, history or baseline findings suggestive of venous thromboembolism, breast cancer, endometrial cancer, cervical cancer, uncontrolled hypertension, uncontrolled diabetes, severe congestive heart failure, other life‐threatening disease, alcoholism, drug abuse, history of intolerance of HT, any preexisting condition indicating unsuitability for long‐term HT or placebo therapy, > 80% compliance with placebo medication during run‐in phase Mean age: 67 years (SD 7) Age range: 44‐79 Means of recruitment: lists of cardiac patients, mass mailing, direct advertising Baseline equality of treatment groups: more women in control arm on statins at randomisation (67% vs 54%). When adjusted in analyses ‐ made no statistically significant difference Country: USA |
|
| Interventions | HT arm: conjugated equine oestrogen 0.625 mg with medroxyprogesterone acetate 2.5 mg
Control arm: placebo identical in appearance
Continuous oral regimen
Adherence to treatment defined as > 80% compliance with medication or placebo
Duration: 4.2 years, mean FOR UNBLINDED CONTINUATION OF HERS 1998: Continuation planned for an additional 4 years but stopped after mean additional 2.7 years, as no additional useful data anticipated |
|
| Outcomes | Coronary events (MI or coronary death) Venous thromboembolism Fracture Gallbladder disease Endometrial, breast or ovarian cancer Death | |
| Notes | Power calculation: 90% power to observe 24% reduction in coronary events at an average of 4.2 years' (P = 0.05) follow‐up Further unblinded follow‐up 2.7 years (HERS II) ‐ see below | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Computer displayed after participant details entered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status known for all women at end of trial. 59 women did not complete follow‐up (32 in experimental arm, 27 in placebo arm). Analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, clinical centre staff, data analysts and funders blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | More women in control arm on statins at randomisation (67% vs 54%). When adjusted in analyses ‐ made no statistically significant difference |
KEEPS 2012.
| Methods | Stated purpose: to test whether menopausal HT initiated within 3 years of menopause can delay progression of atherosclerosis
Stratification: by study centre
No. of women screened for eligibility: 4532
No. randomised: 727 (oral HT: 230, transdermal HT: 222, placebo: 275)
No. analysed: 580
Losses to follow‐up: in oral HT, transdermal HT and placebo groups, respectively, for the following reasons: withdrawals for AEs = 16/230, 9/222, 12/275; personal reasons 11/230, 8/222, 24/275; non‐adherence 1/230, 4/222, 3/275; unknown reasons 15/230, 22/222, 18/275
Drop‐outs/adherence to treatment: 11% of women non‐adherent with treatment but included in analysis (116/580) For KEEPS‐COG study, sample sizes were oral HT: 220, transdermal HT: 211, placebo: 262. Analysis by intention to treat: no ‐ data not imputed for women lost to follow‐up No. of centres: 9 Years of recruitment: 2005‐2008 Design: double‐blinded parallel‐group RCT Funding: Aurora Foundation (not‐for‐profit) and other academic grants. Study medications provided in part by pharmaceutical companies |
|
| Participants |
Included Women aged 42‐58 within 6‐36 months of final menses Excluded Women post hysterectomy, BMI > 35 kg/m2, low‐density lipoprotein cholesterol > 160 mg/dL, coronary artery calcium over 50 Agaston units at baseline, smoking over 10 cigarettes per day, history of diabetes, myocardial infarction, stroke, thromboembolic disease or cancer Mean age: 52.7 years, mean 1.8 years since menopause Age range: 42‐58 Means of recruitment: mass mailings, posters, print and online advertising, Internet web page Baseline equality of treatment groups: high‐density lipoprotein cholesterol lower in placebo group, otherwise no statistically significant difference in baseline characteristics Country: USA |
|
| Interventions |
HT arm 0.45 mg/d oral CEE + cyclic oral micronised progesterone 200 mg/d × 12 days per month 0.05 mg/d transdermal oestradiol + cyclic oral micronised progesterone 200 mg/d × 12 days per month Control: placebo Duration: 4 years (original protocol was for 5 years, shortened during first year after reconsideration of study design) |
|
| Outcomes | Primary outcome: carotid artery intima media thickness Outcomes relevant to this review: quality of life, clinical CVD events (including MI, stroke) reported as adverse events Cognition: KEEPS‐COG ancillary study enrolled 93% of women in KEEPS 2012 (participation by invitation). Measured with MMSE Global cognitive function also reported in a subset of participants (CEE 29, combined HT 59, placebo 36) in conjunction with magnetic resonance imaging monitoring of brain structure: data not included in this review (Kantarci 2015) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomly sequenced blocks of 13 in a ratio 4:4:5 (oral CEE/transdermal CEE/placebo) |
| Allocation concealment (selection bias) | Low risk | Remotely generated sequence; database key not accessible to study personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 80% of women included in analysis for primary clinical endpoint at 4 years (580/727): 43 withdrew in each HT group, 57 withdrew in placebo group 619/693 (89%) women in KEEPS‐COG were included in analysis (85% of total KEEPS sample) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study pharmacist provided blinded packets of study drugs for each participant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to study allocation |
| Selective reporting (reporting bias) | Low risk | Reports all expected outcomes and all outcomes planned in protocol. Reporting of AEs actively solicited |
| Other bias | Low risk | No other source of potential bias identified |
Mulnard 2000.
| Methods | Stated purpose: to determine whether oestrogen‐only HT affects global, cognitive or functional decline in women with mild to moderate Alzheimer's disease Stratification: not mentioned No. of women screened for eligibility: 153 No. randomised: 120 (CEE 0.625 mg: 42, CEE 1.25 mg: 39, placebo: 39) No. analysed: 120 Losses to follow‐up: nil Adherence to treatment: 23 drop‐outs (7 in placebo group, 7 in CEE 0.625 mg group, 9 in CEE 1.25 mg/d). Adherence to treatment was measured and was defined as the proportion of individuals who ingested at least 80% of the study medication but was not reported in the trial publication. Analysis by intention to treat: yes No. of centres: 32 Years of recruitment: not stated Design: parallel placebo‐controlled Funding: National Institute on Aging, Wyeth Ayerst | |
| Participants |
Included Women with a diagnosis of probable Alzheimer's disease according to National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer Disease and Related Disorders Association Criteria in mild or moderate stage (study protocol specified MMSE score of 14‐28; several exceptions were made by the project director to allow for participants with MMSE scores as low as 12); female sex; previous hysterectomy (oophorectomy not required); older than 60 years; absence of major clinical depressive disorder (as measured by score < 17 on the Hamilton Depression Rating Scale (Ham D); normal gynaecological, breast and mammography results Excluded Myocardial infarction within 1 year, history of thromboembolic disease or hypercoagulable state, hyperlipidaemia, use of excluded medications (i.e. oestrogens within 3 months; current use of antipsychotics, anticonvulsants, anticoagulants, beta‐blockers, narcotics, methyldopa, clonidine or prescription cognitive‐enhancing or antiparkinson medications, including experimental medications within 60 days before baseline. Stable doses of neuroleptics, antidepressants, anxiolytics, sedatives and hypnotics were allowed). At initiation of the protocol, individuals treated with donepezil or tacrine were excluded, but a protocol amendment after 20 months of enrolment allowed stable use (minimum of 4 weeks) of these medications before screening for the study Mean age: 75 Age range: 56‐91 Means of recruitment: not stated Baseline equality of treatment groups: no significant differences between the 3 groups in terms of baseline and demographic characteristics Country: USA |
|
| Interventions | HT arm CEE oral 0.625 mg/d CEE 1.25 mg/d Control: placebo Duration: 1 year | |
| Outcomes |
Primary outcome Progression of Alzheimer's disease (Alzheimer's Disease Co‐operative Study version of the Clinical Global Impression of Change Scale) |
|
| Notes | Power calculation: 81% to detect a 29% difference in the proportion of participants who worsened in the 2 groups (60% worse in the placebo group vs 31% worse in the oestrogen group) using a 2‐tailed (alpha) =.05 (based on data from a similar trial, with 40 participants receiving placebo and 80 receiving oestrogen) * Inclusion criteria state > 60 years, but age range at baseline was 56‐91 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated in blocks of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up stated. Analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blinded; used "identically appearing tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States double‐blinded; no further details |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Inclusion criteria state > 60 years, but age range at baseline 56‐91 |
Nachtigall 1979.
| Methods | Stated purpose: to evaluate effects of HT Stratification: not mentioned Unblinding: code broken if a major medical complication or death occurred (13 times in HT group, 17 times in control group) No. of women screened for eligibility: 403 (235 excluded: 74 ineligible, 31 refused, 130 no match for pair found) No. randomised: 168 No. analysed: 168 Losses to follow‐up: none Adherence to treatment: not mentioned Analysis by intention to treat: yes, although any events occurring after unblinding were not recorded No. of centres: 1 Years of recruitment: unclear ‐ study lasted 10 years and was complete by 1976 Design: parallel Funding: not stated | |
| Participants |
Included Postmenopausal inpatients with chronic disease (last menstrual period > 2 years previously, FSH > 105.5 mU, total urinary oestrogen < 10 micrograms/dl), never taken HT. All hospitalised for entire study period; screened with history, physical examination, medical record review; matched on the basis of chronic disease diagnosis, as follows: diabetes mellitus (14 pairs), custodial care (20 pairs), arteriosclerosis (9 pairs). Other pairs matched on the basis of chronic neurological disorders Excluded Acute heart disease, hypertension (blood pressure > 160/94), apparent malignancy, hysterectomy Mean age: 55 Baseline equality of treatment groups: Correlation for diagnosis was identical. Correlation for some other risk factors was low between individual pairs, but group means were similar. Country: New York Hospital for Chronic Diseases |
|
| Interventions | HT arm: CEE 2.5 mg daily, plus MPA 10 mg for 7 days each month Control arm: placebo Duration: 10 years | |
| Outcomes | Death, myocardial infarction, "serious embolism" (pulmonary embolus), breast cancer, colon cancer, endometrial cancer, gallstones | |
| Notes | Power calculation: not mentioned Re generalisability: Study authors point out that almost all women had long‐term chronic disease, were hospitalised for the entire study period, had much lower than normal overall parity and had more prolonged bed rest than the average woman. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women matched for diagnosis of chronic disease. From matched pairs, research nurse randomly selected which member would be assigned to which group. Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up described. Analysed by intention to treat, but any events occurring after unblinding not recorded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States participants and research physicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States participants and research physicians blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Correlation for some baseline prognostic factors was low between individual pairs, but group means were similar. |
Notelovitz 2002.
| Methods | Stated purpose: to determine the lowest effective dose of an oestradiol transdermal delivery system for preventing bone loss in postmenopausal women Stratification: not described Unblinding: not described No. of women screened for eligibility: not stated No. randomised: 355 (0.025 mg dose: 89; 0.05 mg dose: 90; 0.075 mg dose: 89; placebo: 87) No. analysed: 355 (data imputed for losses to follow‐up) Losses to follow‐up: 34 (9.6%) Adherence to treatment: 125 drop‐outs: 125 (35%) did not complete 2 years' treatment (88 in active treatment arms, 37 in placebo arm). One participant was withdrawn for failure to adhere to the treatment schedule. Overall level of adherence to treatment in women who continued with their allocated treatment is not described. Analysis by intention to treat: yes No. of centres: 22 Years of recruitment: not stated Design: parallel Funding: Proctor and Gamble Pharmaceuticals | |
| Participants |
Included Postmenopausal, non‐osteoporotic, ambulatory women younger than 70 years of age who had had a hysterectomy, with or without bilateral oophorectomy, at least 12 months earlier. Postmenopausal status documented by serum oestrogen < 23 picograms/mL and FSH serum levels > 40 mlU/mL. Non‐osteoporotic status defined by dual energy x‐ray absorptiometry (DXA) minimum T‐score of ‐2.5 Excluded Participants who had received oral oestrogens within 2 months of enrolment, or who had contraindications to oestrogen therapy or history of oestrogen intolerance, women with clinically significant systemic or psychiatric disorders; history of cancer (other than basal cell carcinoma in remission or uterine cancer treated by hysterectomy); history of osteomalacia, hyperparathyroidism or untreated hyperthyroidism, abnormal serum lipids, creatinine or liver enzymes; use of medications within 3 months of enrolment that could modify BMD, radiographic abnormalities of the lumbar spine on anterior/posterior or lateral view, which would preclude precise DXA measurements Mean age: not stated Age range: not stated Means of recruitment: not stated Baseline equality of treatment groups: yes Country: USA |
|
| Interventions | HT arm: 2 patches, delivering daily dose of oestradiol: 0.025 mg, 0.05 mg or 0.075 mg Control arm: 2 placebo patches Duration: 2 years (26 cycles) | |
| Outcomes | Breast cancer (regular mammograms) Fractures | |
| Notes | Power calculation: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34 (9.6%) losses to follow‐up. Analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blinded, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States double‐blinded, double‐dummy; "hard" outcomes |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
Obel 1993.
| Methods | Stated purpose: to compare combined and sequential therapy with respect to relief of climacteric symptoms, effects on the endometrium and on vaginal cellular maturation, steroid metabolism and side effects Stratification: not mentioned Unblinding: not described No. of women screened for eligibility: 176, of whom 21 unwilling to take placebo, 2 found not postmenopausal, 2 excluded for private reasons No. randomised: 151 (combined HT: 50, sequential HT: 50, placebo: 51) No. analysed: 129 (in the groups to which they were allocated) Losses to follow‐up: 22 (11 from combined group, 5 from sequential group, 6 from placebo group) Adherence to treatment: not described Analysis by intention to treat: no No. of centres: 1 Years of recruitment: not stated Design: parallel Funding: Pharmaceutical Division, Novo Nordisk | |
| Participants |
Included Women in early menopause (last spontaneous vaginal bleeding > 6 and < 24 months earlier), no HT within preceding 24 months Excluded Women with previous or current oestrogen‐dependant neoplasia, thromboembolic disease, liver or pancreatic disease, diabetes mellitus, severe obesity, disease with high or low bone turnover and medication known to influence bone metabolism or provoke induction of liver enzymes Mean age: not stated Age range: not stated Means of recruitment: All 5800 women born between 1930 and 1933 in Frederiksborg County, Denmark, were invited to participate. Baseline equality of treatment groups: yes Country: Denmark |
|
| Interventions | HT arm Oral oestradiol 2 mg + norethisterone 1 mg Oral oestradiol 2 mg days 1‐22 + norethisterone acetate days 13‐22, then oestradiol 1 mg days 22‐28 Control arm: placebo Duration: 2 years | |
| Outcomes | Only outcomes of interest to this review: endometrial cancer, quality of life | |
| Notes | Power calculation: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22 (15%) losses to follow‐up for endometrial cancer (11 from combined group, 5 from sequential group, 6 from placebo group), analysed by ITT. However, only 70% of women included for quality of life outcomes (loss to follow‐up rates similar across groups) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded ‐ identical placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States double‐blinded ‐ no further details |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Baseline quality of life scores on several measures appear substantially lower for placebo group. |
PEPI 1995.
| Methods | Stated purpose: to investigate effects of oestrogen‐only and combined therapies on cardiovascular disease risk factors, as well as on endometrial status, breast changes, bone density, menopausal symptoms and quality of life factors Stratification: by clinical centre and hysterectomy status Blinding: participants, clinical and laboratory personnel blinded; medication packages visually indistinguishable Unblinding: unblinding officer at each trial centre or by phone call to co‐ordinating centre; referral gynaecologist at each centre not directly involved with data collection or patient care; able to access treatment assignment for management of safety issues No. of women screened for eligibility: approximately 1460 (states that 60% of women screened were randomised) No. randomised: 875 No. analysed: 847 (97%) Losses to follow‐up: 28 (CEE‐only group: 5/170, CEE + MPA sequential group: 5/174, CEE + MPA continuous group: 4/174, CEE + MPA sequential group: 5/178, placebo group: 9/174) Adherence to treatment: drop‐out rate disproportionately high in women with a uterus assigned unopposed oestrogen: 55% had to discontinue assigned therapy, largely owing to endometrial hyperplasia. Of 847 women who attended 3‐year follow‐up, 75% with a uterus and 80% without a uterus had at least 80% adherence to treatment. (Note: 55% of women with a uterus assigned unopposed oestrogen were required to discontinue assigned therapy owing to endometrial hyperplasia.) Analysis by intention to treat: no ‐ but 97% of women analysed by ITT No. of centres: 7 Years of recruitment: December 1989‐February 1990 Design: parallel Funding: research grants from National Heart, Lung and Blood Institute, National Institute of Child Health and Human Development, National Institute of Health and Human Development, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Aging, USA | |
| Participants |
Included Healthy postmenopausal women 45‐65 years of age, with or without a uterus; ceased menstruation 12 months before entry or had hysterectomy at least 2 months before entry and FSH levels < 40 mU/mL Excluded Women who had used hormones within past 3 months, women treated with thyroid hormone unless stabilised on treatment, serious illness including heart or thromboembolic disease, previous endometrial or breast cancer, contraindications to oestrogen Mean age: 56 years (SD 4) Age range: 45‐64 Means of recruitment: through mass media and community efforts Baseline equality of treatment groups: Women assigned to placebo had higher mean levels of fibrinogen and low density lipoprotein‐C at baseline. Country: USA |
|
| Interventions | HT arm: 1 of the following regimens CEE 0.625 mg daily (unopposed oestrogen) CEE 0.625 mg daily plus MPA 10 mg daily for first 10 days (combined sequential treatment) CEE 0.625 mg plus MPA 2.5 mg daily (combined continuous treatment) CEE 0.625 mg plus MP 200 mg daily for first 12 days (combined sequential treatment) Control arm: placebo Duration: 3 years | |
| Outcomes | Primary endpoints: biological markers, not relevant to this review; however, the following prespecified outcomes were also measured.
Breast cancer
Endometrial cancer
Cardiovascular disease Thromboembolism Gallbladder disease |
|
| Notes | Power calculation: based on primary (biological) outcome: A sample of 840 women was projected to provide minimum power of 0.92 to detect differences of 5 mg/dL in HDL cholesterol for any pair‐wise comparison of treatment arms at 3 years. 55% of women with a uterus assigned unopposed oestrogen were required to discontinue assigned therapy owing to endometrial hyperplasia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocks of variable length |
| Allocation concealment (selection bias) | Low risk | Allocation assignments on encrypted file loaded on computer at clinical centre and issued once eligibility confirmed (or by phone to co‐ordinating centre in case of computer failure) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28 (3%) lost to follow‐up; 97% of women analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, clinical and laboratory personnel blinded; medication packages visually indistinguishable |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical and laboratory personnel blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Placebo group had higher levels of fibrinogen and low‐density lipoprotein C at baseline; otherwise, groups prognostically balanced |
Tierney 2009.
| Methods | Stated purpose: to determine whether oestradiol and norethindrone HT prevents decline in delayed verbal recall in older women with normal to mildly impaired memory functioning Stratification: none reported Unblinding: adverse effects managed by data safety monitoring board, which did not have access to study participants No. of women screened for eligibility: 987 No. randomised: 142 No. analysed: 128 at 2 years Losses to follow‐up: 14 (8 in HT group, 6 in placebo group) Adherence to treatment: 26 discontinued intervention in HT group, 16 discontinued in placebo group Analysis by intention to treat: no, but 128/142 analysed by ITT (90%) No. of centres: 1 Years of recruitment: 2000‐2004 Design: parallel Funding: Canadian Institutes of Health Research, Insitute of Neurosciences Mental Health and Addiction, Shire Biochemistry. Pharmaceutical companies provided tablets. | |
| Participants |
Included Women at least 60 years of age, at least 12 months since last menstrual cycle, normal to below‐normal scores on screening for short‐delay verbal recall, fluent in English and with normal reading and hearing abilities Excluded Women with dementia or history of a condition that would affect cognition; women with conditions considered to be exacerbated by oestrogen, or taking specific medications (listed in the publication) including HT within past 2 years; women received neuropsychological testing to rule out dementia Mean age: not stated Age range: 61‐87 Means of recruitment: advertisements, display booths (e.g. in hospitals, seniors' clubs), family physician referrals Baseline equality of treatment groups: similar baseline scores Country: Canada |
|
| Interventions | HT group: 1 mg 17‐B oestradiol daily for 4 days a week followed by 1 combined oestrogen/progestin ampoule (1 mg 17‐B oestradiol and 0.35 mg norethindrone) per day for 3 days a week Control: placebo Duration: 2 years All women were given the same intervention, whether or not they had a uterus. |
|
| Outcomes | Short‐delay verbal recall of the California Verbal Learning Test Adverse events, including cardiovascular events, cancer, fractures |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Pharmacy allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% losses to follow‐up by 2 years; those lost to follow‐up did not differ on baseline short‐delay recall scores from those who stayed in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study personnel and participants were blinded to treatment assignment for the duration of the study; placebo capsules were identical in appearance to the active capsule. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States that study personnel and participants were blinded ‐ no specific statement about outcome assessment |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | More women in HT group than in placebo group reported breast tenderness, vaginal bleeding and discharge, suggesting that they may have been aware that they were receiving HT. |
WAVE 2002.
| Methods | Stated purpose: to determine whether HT or antioxidant vitamin supplements, alone or in combination, influence the progress of coronary artery disease in postmenopausal women, as measured by angiography Stratification: clinical centre, hysterectomy status Unblinding: adverse effects managed by gynaecologist not involved in outcome assessment who had access to treatment assignment if necessary, with permission of co‐ordinating centre No. randomised: 211 No. analysed: 206 for clinical status at end of study Losses to follow‐up: 5 (3 in HT group, 2 in placebo group) Adherence to treatment: evaluated for 159/211 who had angiographic follow‐up: HT group took 67% of medication, placebo group took 70%; 9/108 women in placebo group crossed to open‐label oestrogen Analysis by intention to treat: no ‐ but 98% of women analysed by ITT No. of centres: 7 Years of recruitment: July 1997‐August 1999 Design: parallel Funding: National Heart, Lung and Blood Institute contract, General Clinical Research Center grant, USA | |
| Participants |
Included Postmenopausal women with 1 or more 15% to 75% coronary stenoses in an artery not subjected to intervention, seen on angiogram within 4 months of study entry. Postmenopausal defined as post bilateral oophorectomy, younger than 55 years of age with an FSH of 40 Mu/mL or higher or older than 55 years Excluded HT use within 3 months, concurrent use of more than 60 mg/d of vitamin C or 30 IU daily of vitamin E and unwilling to stop taking them; suspected breast, uterine or cervical cancer; uncontrolled diabetes or hypertension, MI within 4 weeks, elevated triglycerides or creatinine levels, symptomatic gallstones, heart failure, history of haemorrhagic stroke, bleeding diathesis, PE, DVT or untreated osteoporosis Mean age: 65 Age range: 56‐74 Means of recruitment: recruited at clinical sites in USA and Canada Baseline equality of treatment groups: higher prevalence of diabetes and higher fasting blood glucose levels in the HT group Countries: USA and Canada |
|
| Interventions |
HT arm: 1 of the following regimens
CEE 0.625 (oestrogen‐only therapy) ‐ for women who had had a hysterectomy
CEE 0.625 and MPA 2.5 mg daily (continuous combined therapy) ‐ for women who had not had a hysterectomy
Control arm: placebo Duration: 3 years In addition, this study included women who were prescribed a regimen of vitamins E and C or placebo vitamins. The only comparison considered in this review was HT/placebo vitamins vs placebo HT/placebo vitamins. |
|
| Outcomes | Primary outcome biological: change in minimum lumen diameter of qualifying coronary lesions Outcomes of interest to review All‐cause death Total mortality Cardiovascular events Venous thromboembolism Stroke Breast cancer Quality of life | |
| Notes | Study publication pools results for women receiving unopposed and combined therapies. Power calculation: based on primary (biological) outcome: 423 women provide 90% power to detect an effect size of at least 0.33 (corresponding to a change in minimum lumen diameter of 0.1 mm and assuming 20% of women would not undergo a follow‐up angiogram) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐randomised, permuted block design with random blocks of 2 and 4 |
| Allocation concealment (selection bias) | Low risk | Remotely by phone call to study co‐ordinating centre |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 5 (3 in HT group, 2 in placebo group); 98% of women analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators and staff at clinical centres blinded, except (when necessary) the study gynaecologist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators and staff at clinical centres blinded ‐ main outcomes "hard" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Active group had higher prevalence of diabetes and higher fasting blood glucose levels; otherwise, groups were prognostically balanced. |
WEST 2001.
| Methods | Stated purpose: to determine whether 17B‐oestradiol reduces risk of recurrent stroke or death among postmenopausal women who have experienced a transient ischaemic attack or a non‐disabling ischaemic stroke Stratification: by trial centre and risk level (3 levels) Unblinding: study internist unblinded in the case of overriding concern about a woman's clinical care No. of women screened for eligibility: 5296 (2772 ineligible, 1843 declined to participate, 17 unable to be randomised within protocol time frame) No. randomised: 664 (HT: 337, placebo: 327) No. analysed: 664 Losses to follow‐up: nil Adherence to treatment: 34% of the oestradiol group and 24% of the placebo group dropped out. Non‐adherence to allocated treatment: overall mean: HT group: 44%; placebo: 36%. Among women who continued with treatment, adherence to treatment was 90% in both groups. Analysis by intention to treat: yes No. of centres: 21 (single recruitment hub) Years of recruitment: December 1993‐May 1998 Design: parallel Funding: National Institute of Neurological Disorders and Stroke grant, Medical Research Council of Canada grant. Mead Johnson Laboratories provided support and study drug. | |
| Participants |
Included Postmenopausal women (i.e. amenorrhoea for at least 12 months, or having undergone hysterectomy and > 55 years of age) over 44 years of age within 90 days of a qualifying ischaemic stroke or transient ischaemic attack Excluded Women whose index event was disabling or occurred while taking oestrogen; women with history of breast or endometrial cancer, who had had a venous thromboembolic event while receiving oestrogen replacement therapy, had had a neurological or psychiatric disease that could complicate evaluation of endpoints or had a coexisting condition that limited life expectancy Mean age: 71 Age range: 46‐91 Means of recruitment: admissions to 20 largest regional hospitals in Connecticut and Massachusetts; also via contact with selected neurology groups and direct referral from physicians Baseline equality of treatment groups: yes Country: USA |
|
| Interventions | HT arm: 17‐beta oestradiol 1 mg daily plus, for women with a uterus, a course of medroxyprogesterone acetate once a year, 5 mg daily for 12 days Control arm: placebo Duration: 2.8 years | |
| Outcomes | Death or recurrent stroke Myocardial infarction Cognitive function | |
| Notes | Study publication pools results for women receiving unopposed and combined therapies. Power calculation: 652 women required to give 80% power to detect a reduction in the rate of death or non‐fatal stroke from 25% in the placebo group to 15% in the HT group (2‐tailed P = 0.05) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated at pharmacy, in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | By remote contact with trial pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up; analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Endpoint assessors blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
WHI 1998.
| Methods | Stated purpose: to test the hypothesis that women taking HT will have lower rates of coronary heart disease and osteoporosis‐related fractures
COMBINED HT ARM:
Stratification: by clinical centre site and age group
Re blinding: all participants, clinic staff and outcome assessors blinded, with the exception of 331 participants who were unblinded from the unopposed oestrogen arm and were reassigned to the combined HT arm owing to change in the protocol (see Notes). A further 432 women (248 in the experimental arm and 183 in the placebo arm) had a hysterectomy after randomisation (for reasons other than cancer) and were switched to unopposed oestrogen or corresponding placebo in the unopposed oestrogen study arm.
Unblinding: when required for safety or symptom management, unblinding officer, unblinded clinical gynaecologist, who was not involved with outcomes assessment. At average 5.2‐year follow‐up, 3444 women in experimental group and 548 women in placebo group had been unblinded, mainly to manage persistent vaginal bleeding.
No. randomised: 16,608 (8506 to experimental group, 8102 to placebo group)
No. analysed: 16,608
Losses to follow‐up: 583 participants (3.5%) ‐ i.e. no outcome data for > 18 months: 307 in HT arm (3%), 276 in control group (3.5%). Vital status known for 96.5%
Drop‐outs/Non‐adherence to allocated treatment: Women with adherence to treatment less than 80% (by pill count) were counted as drop‐outs. Drop‐out rates at 5.6 years were 42% in the experimental arm and 38% in the placebo group. In addition, 10.7% of women in the placebo group crossed to receive active treatment.
Analysis by intention to treat: yes (analysed with and without unblinded group in experimental arm)
No. of centres: 40
Power calculation: Sample gives 80%‐95% power for primary endpoint comparisons at 5% significance, assuming an intervention effect of 20% for CHD and 21% for combined fractures at 6‐9 year follow‐up, and an intervention effect of 22% for breast cancer at 14‐year follow up (risk ratio of 1.3 assumed for increased risk of breast cancer in intervention group).
Years of recruitment: 1993‐1998
Note: planned 8.5 years' follow‐up. Trial was stopped after mean of 5.6 years, as test statistic for breast cancer exceeded predetermined stopping boundary, and global risk index indicated risks exceeding benefits. This study continued follow‐up for breast cancer outcomes beyond the planned trial completion date for women who consented to continue follow‐up (n = 12,788: 83% of those eligible, of whom 2.7% dropped out (Manson 2013)). Seventeen percent of surviving women declined to be re‐consented, and their data were censored for the additional follow‐up period. Baseline characteristics were evenly distributed between the 2 groups. WHI 1998 UNOPPOSED OESTROGEN ARM: Stratification: as above Blinding: as above Unblinding: as above No. randomised: 10,739 (including 248 in experimental arm, 183 in placebo arm) joined this study after randomisation to corresponding arms in WHI 2002 and having subsequently had hysterectomy (for reasons other than cancer). No. analysed: 10,739 Losses to follow‐up: 563 Drop‐outs/Non‐adherence to allocated treatment: Women with adherence to treatment of under 80% by pill count were counted as dropouts. The drop‐out rate was 53.8% by the end of the study (6.8 years) and did not vary significantly between study arms. In addition, 9.1% of women in the placebo arm and 5.7% in the active treatment group initiated hormone use outside of the study through their own physician. Analysis by intention to treat: yes No. of centres: 40 Power calculation: 12,375 participants needed to detect a 21% reduction in CHD rates over projected 9‐year average follow‐up Years of recruitment: 1993‐1998 N.B. This arm of WHI 1998 was stopped early after a mean follow‐up of 7.1 years (planned for 9), when it was determined that the prospect of obtaining more precise evidence about effects of the intervention was unlikely to outweigh potential harms, although no predefined safety boundaries had been crossed. This study continued follow‐up for all outcomes beyond the planned trial completion date for women who consented to continue follow‐up (n = 7645; 78% of those eligible, of whom 3% dropped out). Twenty‐two percent of surviving women declined to be re‐consented, and their data were censored for the additional follow‐up period. Baseline characteristics were similar in the 2 groups, and imputation analyses suggested that this loss to follow‐up did not significantly influence study findings. WHIMS ANCILLARY STUDY: WHI 1998 enrolled 7479 participants who were free of probable dementia and were 56‐79 years of age. Of these, 4532 were from the combined HT arm of WHI 1998 [WHI 1998 (WHIMS:combined arm) and 2947 were from the unopposed oestrogen arm [WHI 1998 (WHIMS:unopposed oestrogen arm)]. Overall, 92.4% of eligible women participated. Years of recruitment: May 1996‐December 1999 Analysis by intention to treat: all analysed by ITT for planned primary and secondary outcomes. For a third outcome (global cognitive function), which was not formally preplanned, 178 participants (3.9%) were excluded from the combined arm (151 because relevant follow‐up data were missing, and 27 because they consented to join WHIMS more than 6 months after WHI treatment assignment, by which time treatment effects may already have been under way), and 139 (4.7%) were excluded from the unopposed oestrogen arm (109 owing to missing follow‐up data and 30 as the result of enrolment 6 months or more after randomisation). Adherence to allocated treatment (i.e. proportion taking > 80% of study medication): unopposed oestrogen arm: year 1: 77.2% in HT group vs 84.1% in placebo group; year 6: 42%% in HT group vs 47.8% in placebo group; combined HT arm: year 1: 71% in HT group vs 83% in the placebo group; year 4: 49% in HT group vs 61% in placebo group. Power: designed to provide > 80% power to detect an observed 40% relative reduction in the incidence rate of clinically diagnosed all‐cause dementia Duration: Mean time from randomisation to WHI 1998 to the last WHIMS cognitive screening examination was 4.05 years for women in the combined HT arm and 5.21 years for women in the unopposed oestrogen arm. WHISCA ANCILLARY STUDY Randomised in 1999 Enrolled 2304 women who had been enrolled in WHIMS for a mean of 3 years (1106 women from WHIMS: combined arm; 886 from WHIMS: unopposed oestrogen arm) Re‐randomised in 2004‐2005 for further follow‐up to September 2007; 84% of the original cohort agreed to continue (n = 1933). Those who participated were more likely than those who did not to be younger, non‐smokers, free of diabetes and cardiovascular disease and prior users of oral contraceptives, and to have higher MMSE scores. Among ongoing participants, active and placebo groups had similar characteristics. |
|
| Participants | COMBINED HT ARM
Included Postmenopausal women (no vaginal bleeding for 6 months, or for 12 months for 50‐54 year olds; any use of postmenopausal hormones), with a uterus, aged 50‐79 at initial screening, likely to reside in area for 3 years, provision of written informed consent Excluded Medical condition predictive of survival time < 3 years, invasive cancer in past 10 years (except non‐melanoma skin cancer), breast cancer at any time or suspicion of breast cancer at baseline screening, acute myocardial infarction, stroke, transient ischaemic attack in previous 6 months, known chronic active hepatitis or severe cirrhosis, blood counts indicative of disease, severe hypertension or current use of oral corticosteroids, femoral neck bone mineral density more than 3 standard deviations below the corresponding age‐specific mean, endometrial cancer or endometrial hyperplasia at baseline, malignant melanoma, pulmonary embolism or deep vein thrombosis that was non‐traumatic or had occurred in the previous 6 months, bleeding disorder, lipaemic serum and hypertriglyceridaemia diagnosis, current use of anticoagulants or tamoxifen, PAP smear or pelvic abnormalities, unwillingness or inability to complete baseline study requirements, alcoholism, drug dependency, mental illness, dementia, severe menopausal symptoms inconsistent with assignment to placebo, inability or unwillingness to discontinue current HT use or oral testosterone use, inadequate adherence with placebo run‐in, unwillingness to have baseline or follow‐up endometrial aspirations, active participant in another randomised clinical trial Mean age: 63 years (SD 7) Age range: 50‐79. Age ratio of 33%:45%:21% for baseline age categories of 50‐59, 60‐69 and 70‐79, respectively (enrolment targeted to achieve ratio of 30:45:25) Recruitment: letter of invitation in conjunction with media awareness programme. Sampling method gave women from minority groups 6‐fold higher odds of selection than Caucasian women and resulted in a sample with 84% racially/ethnically designated "white", and 16% non‐"white" Screening: Interested women were screened by phone or mail for eligibility, then attended 3 screening visits for history, clinical exam and tests. Three‐month washout period before baseline evaluation of women using postmenopausal hormones at baseline screening. Lead‐in placebo pills given for at least 4 weeks during screening process to establish compliance with pill taking Baseline equality of treatment groups: no substantive differences between study groups at baseline Country: USA UNOPPOSED OESTROGEN ARM Included Postmenopausal women who had undergone hysterectomy (therefore considered postmenopausal for enrolment purposes), aged 50‐79 at initial screening, likely to reside in area for 3 years, provision of written informed consent Excluded As above Mean age: 64 Age range: 50‐79. Age ratio of 33%:45%:21% for baseline age categories of 50‐59, 60‐69 and 70‐79, respectively (enrolment targeted to achieve ratio of 30:45:25) Recruitment: as above Screening: as above Baseline equality of treatment groups: no substantive differences between study groups at baseline Country: USA WHIMS ancillary study Included Participants in either arm of WHI 1998, at least 65 years of age and free of probable dementia WHISCA ANCILLARY STUDY Women from 14 of the WHIMS clinical sites |
|
| Interventions | COMBINED HT ARM
Experimental group: combined oestrogen and progesterone as 1 daily tablet containing conjugated equine oestrogen 0.625 mg and medroxyprogesterone acetate 2.5 mg
Control group: matching placebo
Duration: 5.6 years (mean duration of treatment) Permanent discontinuation of medication: women who developed breast cancer, endometrial hyperplasia not responsive to treatment, endometrial atypia, endometrial cancer, deep vein thrombosis, pulmonary embolus, malignant melanoma, meningioma, triglyceride level over 1000 mg/dL, prescription of oestrogen, testosterone or selective oestrogen‐receptor modulators by their personal physician Temporary discontinuation of medication: women who had acute MI, stroke, fracture, major injury involving hospitalisation, surgery involving anaesthesia, illness resulting in immobilisation for longer than 1 week, other severe illness for which hormone use is temporarily inappropriate N.B. WHI 1998 (WHIMS) investigators reported outcomes according to study arm (unopposed oestrogen or combined HT therapy) and also (as per protocol) reported results pooled across the 2 arms. However, results showed significant baseline prognostic differences between the 2 arms (see Quality Table). We have not pooled the results in this review. UNOPPOSED OESTROGEN ARM Experimental group: 0.635 mg CEE daily Control arm: placebo Permanent discontinuation of medication: as above WHIMS ANCILLARY STUDY As for either arm of WHI 1998 above |
|
| Outcomes | COMBINED HT ARM
Cardiovascular disease: acute MI, silent MI, coronary death, stroke, pulmonary embolus
Cancer: breast, colorectal, endometrial, other cancers
Fractures: hip, vertebral, osteoporotic UNOPPOSED OESTROGEN ARM As above (with the exception of endometrial cancer) WHIMS ANCILLARY STUDY Cognitive function Mild cognitive impairment Dementia For assessment of outcomes, women in WHIMS underwent up to 4 phases of testing as follows. 1. Participants underwent cognitive screening with the Modified MMSE at baseline and annually. 2. Women who scored below an education‐adjusted cut‐off point proceeded to a battery of psychoneurological tests and standardised interviews, plus interviews with a designated informant (friend or relative). 3. Clinical assessments by local physicians. 4. CT and blood tests to rule out reversible pathology. All cases judged locally as probable dementia were independently evaluated by 2 adjudicators blinded to the diagnosis, as were 50% of cases of mild cognitive impairment and 10% of all cases without dementia. Mild cognitive impairment defined as per current DSM IV criteria ‐ operationally defined as follows: poor performance (< 10th percentile) on a battery of neuropsychological tests, report of mild functional impairment from designated informant, no evidence of a psychiatric or medical explanation for the cognitive decline, absence of dementia Dementia defined as per DSM‐IV criteria |
|
| Notes | N.B. The original WHI protocol allowed women with a uterus to be randomised to receive unopposed oestrogen. As evidence emerged (from the PEPI trial) that this could be unsafe, 331 participants with a uterus in the intervention group in the unopposed oestrogen arm were reassigned to the intervention group in the combined HT arm. Both arms closed early: Combined arm at 5.6 years (8.5 planned); oestrogen‐only arm at 6.8 years (9 planned) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised by permuted block algorithm |
| Allocation concealment (selection bias) | Low risk | By local access to remote study database |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Combined HT arm: 583 participants (3.5%) lost to follow‐up. Vital status known for 96.5% For 11‐year breast cancer outcomes in the combined HT arm, 17% of women had withdrawn. Imputation analyses and comparison of baseline characteristics suggested that this did not significantly influence effect estimates. Oestrogen‐only arm: 563 (5%) ‐ analysed by intention to treat WHISCA: for ongoing follow‐up (2004‐2007), 16% of women withdrew. Clinical and demographic characteristics of those continuing differed from those discontinuing, but active and placebo groups did not differ significantly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and clinic staff were blinded, with the exception of 331 participants who were unblinded from the unopposed oestrogen arm and reassigned to the combined HT arm owing to a change in protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | In the unopposed oestrogen arm, greater use of aspirin at bedtime in the placebo group at baseline In the combined HT arm, lower prevalence of stroke and higher percentage of participants using statins in the active treatment group at baseline |
WISDOM 2007.
| Methods | Stated purpose: to assess long‐term benefits and risks of HT Stratification: by hysterectomy status and intended use of HT: women with no uterus and unwilling to take placebo randomised to CEE or combined HT. Equal probability of any treatment within each stratum No. of women screened for eligibility: 14,203 No. randomised: 4385; 2196 on combined therapy, 2189 on placebo (see Notes) No. analysed: 4385 Losses to follow‐up: 5 Adherence to treatment: 615 (14%) had dropped out from randomised treatment by trial closure. Trial treatment delivered 73% of time to women in combined HT arm and 86% of time to women on placebo Analysis by intention to treat: yes No. of centres: 384 UK, 91 Australian and 24 New Zealand general practitioner practices Years of recruitment: 1999‐2002 Design: parallel Funding: non‐commercial medical research funding | |
| Participants |
Included Postmenopausal women 50‐69 years of age Excluded History of breast cancer, any other cancer in past 10 years except basal or squamous cell skin cancer, endometriosis or endometrial hyperplasia, venous thromboembolism, gallbladder disease, MI, unstable angina, cardiovascular accident, subarachnoid haemorrhage, transient ischaemic attack, use of HT within past 6 months, unlikely to be able to give informed consent Mean age: 63 Age range: 50‐69 Means of recruitment: general practitioner practice registers Countries: UK, Australia, New Zealand |
|
| Interventions | HT arm: daily CEE 0.625 mg plus medroxyprogesterone acetate 2.5 mg (for women with or without a uterus), or daily CEE 0.625 (for women without a uterus)
Control arm: placebo
Duration: planned for median 10 years, but prematurely closed after median 11.9 months (range 7.1‐19.6) Women with a uterus within 3 years of last period, those aged 50‐53 and older women with unacceptable breakthrough bleeding took medroxyprogesterone acetate 5.0 mg. Women with a uterus who experienced unacceptable spotting or bleeding on the above therapy were offered open‐label CEE 0.625 mg plus medroxyprogesterone acetate 10.0 mg daily for the last 14 days of a 28‐day cycle. All women took placebo medication during run‐in: Those who achieved 80% compliance were randomised. A further 1307 women were randomised to a comparison of oestrogen‐only vs combined HT: These results are not reported here. |
|
| Outcomes | Major cardiovascular disease (primary)
Osteoporotic fractures (primary)
Breast cancer
Mortality
VTE
CVD
Dementia (no follow‐up data collected)
Adverse events Quality of life (reported among 3721 women with an intact uterus or subtotal hysterectomy, among whom 1862 were randomised to combined HT and 1859 to placebo). A variety of overall and symptom‐specific measures were used, including EuroQoL‐5D (which measures health‐related quality of life) and a generic VAS scale (which measures quality of all aspects of life) ‐ only these 2 measures are included in this review |
|
| Notes | Powered in protocol to detect 25% reduction in CHD over 10 years ‐ this assumed an 18,000 sample size, but trial stopped early with 26% of target A further 1307 women were included in comparison of combined therapy vs oestrogen only and were not included in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remote computer‐generated |
| Allocation concealment (selection bias) | Low risk | Remote computer‐generated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 615 (14%) had withdrawn from randomised treatment by trial closure; analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and clinic staff blinded except when vaginal bleeding triggered a code break |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessors blinded except when vaginal bleeding triggered a code break |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
Yaffe 2006.
| Methods | Stated purpose: to investigate effects of unopposed ultra‐low‐dose transdermal oestradiol on cognition and health‐related quality of life in postmenopausal women Stratification: by clinical centre No. of women screened for eligibility: 1509 (of whom 605 had 1‐week run in phase. 10/605 were non‐compliant and 1678 were found ineligible or refused to continue screening) No. randomised: 417 (treatment group: 208; placebo group: 209) No. analysed: using a time × treatment interaction. 388 at year 1, 376 at year 2 Losses to follow‐up: 40 Adherence to treatment: drop‐outs: 41. Among those who completed treatment, 84% used at least 75% of study drug during the entire 2 years. No. of centres: 9 Years of recruitment: 1999‐2000 Design: parallel Funding: industry funded | |
| Participants |
Included Women 60‐80 years of age with intact uterus, at least 5 years post menopause, normal bone density Excluded Women with unexplained uterine bleeding, endometrial hyperplasia, endometrium >mm double thickness on ultrasonography, abnormal mammogram suggestive of breast cancer, history of metabolic bone disease, cancer, coronary disease, stroke, transient ischaemic attack, VTE, uncontrolled hypertension, uncontrolled thyroid disease, liver disease, abnormal fasting triglyceride or fasting glucose, ever taken fluoride, calcitonin or bisphosphates, oestrogen or progestin within past 3 months Median age: 67 Means of recruitment: not stated Baseline equality of treatment groups: mean MMSE scores slightly higher in intervention group (P = 0.04) Country: USA |
|
| Interventions | HT arm: oestradiol patch delivering approx 0.014 mg oestradiol daily, applied to abdomen weekly Control arm: identical placebo patch Duration: 2 years All participants also received 400 mg calcium twice daily and 400 IU vitamin D daily. | |
| Outcomes | Preplanned secondary outcomes
Changes in global cognition (MMSE)
Short‐Form Health Survey (SF‐36): Physical Component Scale and Mental Component Scale Bone mineral density was primary outcome (not reported here). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remotely by computer |
| Allocation concealment (selection bias) | Low risk | By pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40/417 (9.5%) women lost to follow‐up; analysed by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
Abbreviations
BMD: bone mineral density.
BMI: body mass index.
CEE: conjugated equine oestrogen.
CHD: coronary heart disease.
CVD: cardiovascular disease.
DM: diabetes mellitus.
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
DVT: deep vein thrombosis.
DXA: dual‐energy X‐ray absorptiometry.
EuroQol‐5D: quality of life questionnaire.
ET: oestrogen therapy.
FSH: follicle‐stimulating hormone.
Ham D: Hamilton Depression Rating Scale.
HDL: high‐density lipoprotein.
HT: hormone therapy.
ITT: intention to treat: analysis of all randomised participants in the groups to which they were randomised.
IU: International Units.
LDL: low‐density lipoprotein.
mg: milligram.
mL: millilitre.
MI: myocardial infarction.
MMSE: Mini Mental State Examination.
MP: micronised progesterone.
MPA: medroxyprogesterone acetate.
mu: milliunits.
PE: pulmonary embolism.
RCT: randomised controlled trial.
SD: standard deviation.
VAS: visual analogue scale.
VTE: venous thromboembolism.
WHI: Women's Health Initiative.
WHIMS: Women's Health Initiative Memory Study.
Definitions
Adherence to treatment refers to the number of tablets actually taken, which is often assessed by pill counts (see Additional Table 4). Drop‐outs: Participants who stopped their allocated treatment (and in some cases changed to a different off‐trial treatment) but have known clinical outcomes and were included in the analysis. Intention to treat: Analysis of all randomised participants in the groups to which they were randomised. Losses to follow‐up: Participants for whom outcomes of interest were unknown (and who may or may not have had outcomes imputed by statistical analysis).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AHT 2015 | Not blinded |
| Aitken 1971 | No outcomes of interest measured |
| Aitken 1973 | No outcomes of interest measured |
| Angerer 2000 | Duration less than 1 year |
| Bloch Thomsen 2002 | No outcomes of interest measured |
| Chen 2001 | No placebo, no outcomes of interest measured |
| Christiansen 1981 | No outcomes of interest measured |
| Corrado 2002 | No placebo, no outcomes of interest measured |
| Corson 1999 | No placebo, no outcomes of interest measured |
| de Roo 1999 | No outcomes of interest measured |
| Eiken 1996 | No outcomes of interest measured |
| Estratab 1977 | No outcomes of interest measured |
| EWA 2000 | No placebo, no outcomes of interest measured |
| Genant 1990 | No outcomes of interest measured |
| Graser 2001 | Duration less than 1 year, no outcomes of interest measured |
| HABITS 2004 | Not double‐blinded |
| Haines 2003 | No outcomes of interest measured |
| Hall 1998 | Not double‐blinded |
| Jensen 1985 | No outcomes of interest measured |
| Kuopio 1998 | Not blinded |
| Lufkin 1992 | No outcomes of interest measured |
| Maki 2004 | No outcomes of interest measured |
| Mizunuma 2010 | No comparisons of interest comparing HT vs placebo only |
| Newhouse 2000 | Duration less than 1 year |
| Ng 1992 | No outcomes of interest measured |
| Nielsen 2006 | No outcomes of interest measured |
| Ory 1998 | No outcomes of interest measured |
| Os 2002 | No placebo, no outcomes of interest measured |
| Paoletti 2015 | No outcomes of interest measured |
| Papworth 2002 | No placebo |
| Pefanco 2007 | No outcomes of interest measured |
| Post 2001 | No outcomes of interest measured |
| Rasgon 2014 | No placebo group, interim outcomes measured |
| Saitta A 2001 | Duration less than 1 year |
| Sanchez‐Guerrero 2007 | No outcomes of interest measured |
| Schierbeck 2012 | Not blinded |
| SMART 2016 | Co‐intervention (bazedoxifene) in the HT group |
| Steiner 2007 | Combines EPAT and WELL‐HART data. No outcomes of interest measured |
| Teede 2002 | No outcomes of interest measured |
| ULTRA 2005 | No outcomes of interest measured |
| Virtanen 1999 | Duration less than 1 year, no outcomes of interest measured |
| Wharton 2011 | Planned duration of 1 year. Owing to high drop‐out rate, results were reported only at 3 months. |
Differences between protocol and review
For the 2017 update, we decided to omit quality of life as an outcome and to focus on adverse events only, to make the review more concise. This meant that we excluded two previously included studies (Haines 2003; Nielsen 2006).
We also decided for the 2017 update to limit the outcome "Cognitive function" to studies of global measures of cognition, also to keep the review as concise as possible. This did not change any of our data.
For the 2017 update, we did not include studies that did not report any events in either group for a particular outcome in the meta‐analysis for that outcome because they did not add useful data (Higgins 2011).
Contributions of authors
For the 2017 update of the review, Jane Marjoribanks (JM) extracted and entered data and drafted the text, and Jasmine Lee (JL) checked study selection and data extraction. Helen Roberts (HR), Cindy Farquhar (CF) and Anne Lethaby (AL) commented on and contributed to the drafts.
For the 2008 and 2012 updates of the review, JM extracted and entered data and drafted the text, and CF checked study selection and data extraction. HR, CF and AL commented on and contributed to the drafts.
CF and AL developed the original protocol and circulated it to members of the Cochrane HT Study Group for comment. The following people contributed specifically to the protocol: Professor Shah Ebrahim, Dr Peter Tugwell, Teresa Moore and Maria Judd. For the original version of the review, JM and Jane Suckling searched for relevant studies and selected studies for inclusion, and JM extracted and entered data that were checked by Quirine Lamberts. JM drafted the review, circulated it to other members of the Cochrane HRT Study Group for comment and edited the draft.
The following individuals commented on the draft of the original review: Breast Cancer Group: Sue Carrick, Sue Lockwood (Editor); Dementia and Cognitive Improvement Group: Professor Leon Flicker (Editor), Professor Lon Schneider (Editor); Heart Group: Lee Hooper (Editor), Theresa Moore (Review Group Coordinator); Gynaecology and Fertility Group: Cindy Farquhar (Co‐ordinating Editor), Anne Lethaby (Editor); Stroke Group: Professor Ale Agra (Editor), Steff Lewis (Statistical Editor).
Sources of support
Internal sources
University of Auckland, New Zealand.
External sources
None, Other.
Declarations of interest
Cindy Farquhar is a director/shareholder of a gynaecology clinic and undertakes private practice within those premises. She has received travel/accommodation/meeting expenses from ESHRE or ASRM for attendance at scientific meetings.
JL, AL, JM and HR have no interests to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Barakat 2006 {published data only}
- Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS. Randomized double‐blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2006;24:587‐92. [DOI] [PubMed] [Google Scholar]
ELITE 2014 {published and unpublished data}
- Henderson VW, John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive effects of estradiol after menopause ‐ a randomized trial of the timing hypothesis. Neurology 2016;87(7):699‐708. [DOI: 10.1212/WNL.0000000000002980] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis HN, Mack MJ, Shoupe D, Azen SP, Stanczyk FZ, Hwang‐Levine J, et al. Methods and baseline cardiovascular data from the early versus late intervention trial with estradiol testing the menopausal timing hypothesis. Menopause 2015;22(4):391‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis HN, Mack MJ, Shoupe D, Azen SP, Stanczyk FZ, et al. Testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol. Circulation 2014;130:no pagination. [Google Scholar]
- Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang‐leving J, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. New England Journal of Medicine 2016;374(13):1221‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Hodis HN, John JA, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive outcomes from the early versus late intervention trial with estradiol: a test of the critical window hypothesis. Menopause 2014;21(12):1330. [Google Scholar]
EPAT 2001 {published data only}
- Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis: a randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 2001;135:939‐53. [DOI] [PubMed] [Google Scholar]
- Karim R, Mack WJ, Lobo RA, Hwang J, Liu C, Liu C. Determinants of the effect of estrogen on the progression of subclinical atherosclerosis: Estrogen in the Prevention of Atheroscerosis Trial. Menopause 2005;12(4):366‐73. [DOI] [PubMed] [Google Scholar]
EPHT 2006 {published data only}
- Veerus P, Fischer K, Hovi SL, Karro H, Rahu M, Hemminki E. Symptom reporting and quality of life in the Estonian Postmenopausal Hormone Therapy Trial. BMC Women's Health 2010;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerus P, Hovi S, Fischer K, Rahu M, Hakama M, Hemminki E. Results from the Estonian postmenopausal hormone therapy trial (IRSRCTN35338757). Maturitas 2006;55:162‐73. [DOI] [PubMed] [Google Scholar]
- Vorobjov S, Hovi S, Veerus P, Pisarev H, Rahu M, Hemminki E. Treatment adherence in the Estonian postmenopausal hormone therapy (EPHT) trial (ISRCTN35339757). Maturitas 2005;52:286‐95. [DOI] [PubMed] [Google Scholar]
ERA 2000 {published data only}
- Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, et al. Effects of oestrogen replacement on the progression of coronary artery atherosclerosis. New England Journal of Medicine 2000;343:522‐9. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Reboussin DM, Potvin Klein K, Sharp PC, Shumaker SA, Snyder TE, et al. The estrogen replacement and atherosclerosis (ERA) study: study design and baseline characteristics of the cohort. Controlled Clinical Trials 2000;21:257‐85. [DOI] [PubMed] [Google Scholar]
- Nair GV, Herrington DM. The ERA trial: findings and implications for the future. Climacteric 2000;3:227‐32. [DOI] [PubMed] [Google Scholar]
ESPRIT 2002 {published data only}
- The Esprit Team. Oestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trial. The Lancet 2002;360:2001‐8. [DOI] [PubMed] [Google Scholar]
EVTET 2000 {published data only}
- Hoibraaten E, Mowinckel MC, Ronde H, Bertina RG, Sandset PM. Hormone replacement therapy and acquired resistance to activated protein C: results of a randomized, double‐blind, placebo‐controlled trial. British Journal of Haematology 2001;115:415‐20. [DOI] [PubMed] [Google Scholar]
- Hoibraaten E, Qvigstad E, Andersen IO, Mowinckel MC, Sandset PM. The effects of hormone replacement therapy (HRT) on hemostatic variables in women with previous venous thromboembolism ‐ Results from a randomized, double‐blind, clinical trial. Thrombosis and Haemostasis 2001;85:775‐81. [PubMed] [Google Scholar]
- Hoibraaten E, Qvigstad E, Andersen TO, Mowinckel MC, Sandset PM. Increased risk of recurrent venous thromboembolism during hormone replacement therapy. Thrombosis and Haemostasis 2000;84:961‐7. [PubMed] [Google Scholar]
Ferenczy 2002 {published data only}
- Ferenczy A, Gelfand MM, Weijer PHM, Rioux JE. Endometrial safety and bleeding patterns during a 2‐year study of 1 or 2 mg 17 Beta‐estradiol combined with sequential 5‐20 mg dydrogesterone. Climacteric 2002;5:26‐35. [PubMed] [Google Scholar]
Greenspan 2005 {published data only}
- Greenspan SL, Resnick NM, Parker RA. The effect of hormone replacement on physical performance in community‐dwelling elderly women. American Journal of Medicine 2005;118:1232‐9. [DOI] [PubMed] [Google Scholar]
HERS 1998 {published data only}
- Bibbins‐Domingo K, Lin F, Vittinghoff E, Barrett‐Connor E, Hulley SB, Grady D. Effect of hormone therapy among women with heart failure and coronary artery disease. American Journal of Cardiology 2005;95(2):289‐91. [DOI] [PubMed] [Google Scholar]
- Byington RP, Furberg CD, Herrington DM, Herd JA, Hunninghake D, Lowery M, et al. Effect of oestrogen plus progestin on progression of carotid atherosclerosis in postmenopausal women with heart disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2002;22:1692‐7. [DOI] [PubMed] [Google Scholar]
- Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley SB. Heart and Estrogen/progestin Replacement Study (HERS): design, methods and baseline characteristics. Controlled Clinical Trials 1998;19:314‐35. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy. Journal of the American Medical Association 2002;288(1):49‐57. [DOI] [PubMed] [Google Scholar]
- Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. Annals of Internal Medicine 2000;132(9):689‐96. [DOI] [PubMed] [Google Scholar]
- Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett‐Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Americal Journal of Medicine 2002;113(7):543‐8. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Fong J, Sempos CT, Black DM, Schrott HG, Rautaharju P, et al. Comparison of the Heart and Oestrogen Replacement Study (HERS) cohort with women with coronary disease from the National Health and Nutrition Examination Survey III (NHANES III). American Heart Journal 1998;136(1):115‐24. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Vittinghoff E, Lin F, Fong J, Harris F, Hunninghake D, et al. Statin therapy, cardiovascular events and total mortality in the Heart and Estrogen/progestin Replacement Study (HERS). Circulation 2002;105(25):2962‐7. [DOI] [PubMed] [Google Scholar]
- Hulley S, Furberg C, Barrett‐Connor E, Cauley J, Grady D, Haskell, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy. Journal of the American Medical Association 2002;288(1):58‐66. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280(7):605‐13. [DOI] [PubMed] [Google Scholar]
- Khan MA, Hlatky MA, Liu MW, Lin F, Rogers WJ, Shlipak MG. Effect of postmenopausal hormone therapy on coronary heart disease events after percutaneous transluminal coronary angioplasty. American Journal of Cardiology 2003;91:989‐91. [DOI] [PubMed] [Google Scholar]
- Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen‐progestin Replacement Study (HERS). Circulation 2001;103(5):638‐42. [DOI] [PubMed] [Google Scholar]
- Simon JA, Hunninghake DB, Agarwal SK, Lin F, Cauley JA, Ireland CC, et al. Effect of estrogen plus progestin on risk for biliary tract surgery in postmenopausal women with coronary artery disease. Annals of Internal Medicine 2001;135:493‐501. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, Shlipak MG, Varosy PD, Furberg CD, Ireland CC, Khan SS, et al. Risk factors and secondary prevention in women with heart disease: the Heart and Estrogen/progestin Replacement Study. Annals of Internal Medicine 2003;138:81‐9. [DOI] [PubMed] [Google Scholar]
KEEPS 2012 {published and unpublished data}
- Asthana S, Gleason CE, Wharton W, Dowling MN, Carlsson CM, et al. The Kronos Early Estrogen Prevention Study: results of the cognitive and affective substudy (KEEPS‐Cog). Menopause: 23rd Annual meeting of North American Menopause Society, October 3‐October 6 2012, Orlando 2012;19(12):1365‐404. [Google Scholar]
- FIles JA, Miller VM, Cha S, Pruthi S. Effects of different hormone therapies on breast pain in recently postmenopausal women: findings from the Mayo Clinic KEEPS Breast Pain Ancillary Study. Journal of Womens Health 2014;23(10):801‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason C, Wharton W, Dowling M, Brinton E, Santoro N, et al. The Kronos Early Estrogen Prevention Study ‐ Cognitive and Affective Sub‐study (KEEPS‐CA): menopausal hormone therapy effects on mood, quality of life and memory complaints. Menopause: 23rd Annual meeting of North American Menopause Society, October 3‐October 6 2012, Orlando 2012;19(12):1402 (P‐89). [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized controlled KEEPS‐Cognitive and Affective Study. PLoS Medicine 2015;12(6):e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM. Effects of oral conjugated estrogen or transdermal estradiol plus oral progesterone treatment on common carotid artery intima media thickness (CIMT) & coronary artery calcium (CAC) in menopausal women: initial results from the Kronos Early Estrogen Prevention Study (KEEPS). Menopause: 23rd Annual meeting of North American Menopause Society, October 3‐October 6 2012, Orlando 19;12:1365‐1404. 2012;19(12):1365‐404. [Google Scholar]
- Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women. Annals of Internal Medicine 2014;161:249‐60. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Gunter JL, Gleason CE, et al. Effects of hormone therapy on brain structure in recently postmenopausal women: a randomized controlled trial. Neurology 2016;87(9):887‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling JM, Lahr BA, Bailey KR, Harman SM, Miller VM. Endothelial function in women of the Kronos Early Estrogen Prevention Study. Climacteric 2015;18(2):187‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE 2012. The KRONOS early prevention study (KEEPS): rationale, design and baseline characteristics of the study population. Menopause: 23rd Annual meeting of North American Menopause Society, October 3‐October 6 2012, Orlando 2012;19(12):1365‐1404. [Google Scholar]
- NCT00154180. Kronos early estrogen prevention study (KEEPS). https://clinicaltrials.gov/ct2/show/NCT00154180.
- Naftolin F, Harman SM, Black D, Brinton E, Budoff M, et al. Adverse events during four years of daily oral conjugated estrogen or transdermal estradiol plus cyclic oral progesterone treatment in recently menopausal women: the KRONOS early estrogen prevention study (KEEPS). Menopause: 23rd Annual meeting of North American Menopause Society, October 3‐October 6 2012, Orlando 2012;19(12):1402‐3 (P‐90). [Google Scholar]
- Raz L, Dowling NM, Gleason C, Jayachandran M, Asthana S, et al. Effects of menopausal hormone therapy on serum serotonin and self‐report depression in healthy, recently menopausal women. Journal of Womens Health 2013;22(3):18. [Google Scholar]
- Rexrode KM, Manson JE, Kalan K, Lobo R, Freeman R, et al. Effects of oral conjugated estrogen or transdermal estradiol plus oral progesterone treatment on breast outcomes among menopausal women in the Kronos Early Estrogen Prevention Study (KEEPS). Menopause: 23rd Annual meeting of North American Menopause Society, October 3‐October 6 2012, Orlando 2012;19(12):1403 (P‐92). [Google Scholar]
- Wharton W, Gleason CE, Dowling NM, Carlsson CM, Brinton EA, Santoro MN, et al. The KEEPS‐Cognitive and Affective Study: baseline associations between vascular risk factors and cognition. Journal of Alzheimer's Disease 2014;2:331‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulnard 2000 {published data only}
- Mulnard R, Cotman C, Kawas C, Dyck C, Sano M, Doody R, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. JAMA 2000;283(8):1007‐15. [DOI] [PubMed] [Google Scholar]
Nachtigall 1979 {published data only}
- Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstetrics and Gynecology 1979;54(1):74‐9. [DOI] [PubMed] [Google Scholar]
Notelovitz 2002 {published data only}
- Notelovitz M, John VA, Good WR. Effectiveness of Alora estradiol matrix transdermal delivery system in improving lumbar bone mineral density in health, postmenopausal women. Menopause 2002;9(5):343‐53. [DOI] [PubMed] [Google Scholar]
Obel 1993 {published and unpublished data}
- Bech P. Personal communication with J. Marjoribanks. December 2003.
- Bech P, Munk‐Jensen N, Obel EB, Ulrich LG, Eiken P, Pors Nielsen SP. Combined versus sequential hormonal replacement therapy: a double blind placebo‐controlled study on quality of life‐related measures. Psychotherapy and Psychosomatics 1998;67:259‐65. [DOI] [PubMed] [Google Scholar]
- Obel EB, Munk‐Jensen N, Svenstrup B, Bennett P, Micic S, Henrik‐Nielsen R, et al. A two‐year double‐blind controlled study of the clinical effect of combined and sequential postmenopausal replacement therapy and steroid metabolism during treatment. Maturitas 1993;16:13‐21. [DOI] [PubMed] [Google Scholar]
PEPI 1995 {published data only}
- Barrett‐Connor E, Slone S, Greendale G, Kritz‐Silverstein D, Espeland M, Johnson SR, et al. The postmenopausal estrogen/progestin interventions study: primary outcomes in adherent women. Maturitas 1997;27:261‐74. [DOI] [PubMed] [Google Scholar]
- Cushman M, Legault C, Barrett‐Connor E, Stefanick ML, Kessler C, Judd HL, et al. Effect of postmenopausal hormones on inflammation‐sensitive proteins. Circulation 1999;100:717‐22. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Bush TL, Mebane‐Sims I, Stefanick ML, Johnson S, Sherwin R, et al. Rationale, design and conduct of the PEPI trial. Controlled Clinical Trials 1995;16:3S‐19S. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. Journal of the National Cancer Institute 2003;951(1):30‐7. [DOI] [PubMed] [Google Scholar]
- Miller VT, Byington RL, Espeland MA, Langer R, Marcus R, Shumaker S, et al. Baseline characteristics of the PEPI particpants. Controlled Clinical Trials 1995;16:54S‐65S. [DOI] [PubMed] [Google Scholar]
- Reboussin BA, Greendale GA, Espeland MA. Effect of hormone replacement therapy on self‐reported cognitive symptoms: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. Climacteric 1998;13(3):172‐9. [DOI] [PubMed] [Google Scholar]
- Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA 1995;273(3):199‐208. [PubMed] [Google Scholar]
- Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. JAMA 1996;275(5):370‐5. [DOI] [PubMed] [Google Scholar]
Tierney 2009 {published data only}
- Tierney MC, Oh P, Moineddin R, Greenblatt EM, Snow WG, Fisher RH, et al. A randomized double‐blind trial of the effects of hormone therapy on delayed verbal recall in older women. Psychoneuroendocrinology 2009;34(7):1065‐74. [DOI] [PubMed] [Google Scholar]
WAVE 2002 {published data only}
- Bittner V, Tripputi M, Hsia J, Gupta H, Steffes M. Remnant‐like lipoproteins, hormone therapy and angiographic and clinical outcomes: the Women's Angiographic Vitamin and Estrogen trial. American Heart Journal 2004;147:293‐9. [DOI] [PubMed] [Google Scholar]
- Hsia J, Alderman EL, Verter JI, Rogers WJ, Thompson P, Howard BV, et al. Women's angiographic vitamin and estrogen trial: design and methods. Controlled Clinical Trials 2002;23:708‐27. [DOI] [PubMed] [Google Scholar]
- Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women. JAMA 2002;288(19):2432‐40. [DOI] [PubMed] [Google Scholar]
WEST 2001 {published data only}
- Kernan WN, Brass LM, Viscoli CM, Sarrel PM, Makuch R, Horowitz RI. Estrogen after ischemic stroke: dlinical basis and design of the Women's Estrogen for Stroke trial. Journal of Stroke and Cardiovascular Diseases 1998;7(1):85‐95. [DOI] [PubMed] [Google Scholar]
- Stefanick ML, Anderson GL, Margolis KL, Hendix SL, Rodabough RJ, Paskett ED, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA 2006;295:1647‐57. [DOI] [PubMed] [Google Scholar]
- Viscoli C, M, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horowitz RI. Estrogen therapy and risk of cognitive decline: results from the Women's Estrogen for Stroke Trial (WEST). American Journal of Obstetrics and Gynecology 2004;192:387‐93. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horowitz RI. A clinical trial of estrogen‐replacement therapy after ischemic stroke. New England Journal of Medicine 2001;345(17):1243‐9. [DOI] [PubMed] [Google Scholar]
WHI 1998 {published data only}
- Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow‐up of the Women's Health Initiative randomised placebo‐controlled trial. The Lancet 2012;13:476‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GL, Chlebowski RT, Rossouw JE, Rodabough RJ, McTiernan A, Margolis KL, et al. Prior hormone therapy and breast cancer risk in the Women's Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006;55:103‐15. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SAA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures. Journal of the American Medical Association 2003;290(13):1739‐48. [DOI] [PubMed] [Google Scholar]
- Barnabei VM, Cochrane BB, Aragaki AK, Nygaard I, Willliams RS, McGovernn PG, et al. Menopausal symptoms and treatment‐related effects of estrogen and progestin in the Women's Health Initiative. Obsterics and Gynecology 2005;105(5 Part 1):1063‐73. [DOI] [PubMed] [Google Scholar]
- Brunner RL, Gass M, Aragaki A, Hays J, Granek I, Woods N, et al. Effects of conjugated equine estrogen on health‐related quality of life in postmenopausal women with hysterectomy. Archives of Internal Medicine 2005;165:1976‐86. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density. Journal of the American Medical Association 2003;290(13):1729‐38. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Anderon GL, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010;304(15):1684‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL. Menopausal hormone therapy and breast cancer mortality: clinical implications. Therapeutic Advances in Drug Safety 2015;6(2):45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL, Manson JE, Schwartz AG, Wakelee H, Gass M, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. Journal of the National Cancer Institute 2010;102(18):1413‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL, Sarto GE, Haque R, Runowicz CD, Aragaki AK, et al. Continuous combined estrogen plus progestin and endometrial cancer. Journal of the National Cancer Institute 2016;108(3):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Aragaki AK, Anderson GL. Menopausal hormone therapy influence on breast cancer outcomes in the Women's Health Initiative. Journal of the National Comprehensive Cancer Network 2015;13(7):917‐24. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Barrington W, Aragaki AK, Manson JE, Sarto G, O'Sullivan MJ, et al. Estrogen alone and health outcomes in black women by African ancestry. Journal of Clinical Oncology. Conference: 2015 Annual Meeting of the American Society of Clinical Oncology, ASCO Chicago. 2015; Vol. 33 (15 Supplement 1).
- Chlebowski RT, Cirillo DJ, Eaton CB, Stefanick ML, Pettinger M, Carbone KD, et al. Estrogen alone and joint symptoms in the Women's Health Initiative randomized trial. Menopause 2013;20(6):600‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. JAMA 2003;289(24):3243‐53. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, et al. for the WHI Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. New England Journal of Medicine 2009;6(360):573‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Rohan TE, Manson JE, Aragaki AK, Kaunitz A, Stephanick ML, et al. Breast cancer after use of estrogen plus progestin and estrogen alone. JAMA 2015;1(3):296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Schwartz AG, Wakelee H, Anderson GKL, Stefanick ML, Manson JE, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post‐hoc analysis of a randomised controlled trial. The Lancet 2009;374(9697):1243‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Wactawski‐Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough R, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. New England Journal of Medicine 2004;350(10):991‐1004. [DOI] [PubMed] [Google Scholar]
- Cirillo DJ, Wallace RB, Rodabough RJ, Greenland P, Lacroix AZ, Limacher MC, et al. Effect of estrogen therapy on gallbladder disease. JAMA 2005;293(3):330‐9. [DOI] [PubMed] [Google Scholar]
- Curb JD, Prentice RL, Bray PF, Langer RD, Horn L, Barnabei VN, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Archives of Internal Medicine 2006;166:772‐80. [DOI] [PubMed] [Google Scholar]
- Cushman M, Kuller LH, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292(13):1573‐80. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Brinton RD, Hugenschimidt C, Manson JE, Craft S, Yaffe K, et al. Impact of type 2 diabetes and postmenopausal hormone therapy on incidence of cognitive impairment in older women. Diabetes Care 2015;38:2316‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Brinton RD, Manson JE, Yaffe K, Hugenschmidt C. Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology 2015;85:1131‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Brunner RL, Hogan PE, Rapp SR, Coker LH, Legault C, et al. Long‐term effects of conjugated equine estrogen therapies on domain‐specific cognitive function: results from the Women's Health Initiative study of cognitive aging extension. Journal of the American Geriatrics Society 2010;58(7):1263‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Manson JE, Goveas JS, Shumaker SA, Hayden KM, et al. Long‐term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. Journals of Gerontology Series A‐Biological Sciences and Medical Sciences 2016 Aug 9 [Epub ahead of print]. [DOI: 10.1093/gerona/glw156] [DOI] [PMC free article] [PubMed]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA 2004;291(24):2959‐3007. [DOI] [PubMed] [Google Scholar]
- Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Annals of Epidemiology 2003;13:S18‐77. [DOI] [PubMed] [Google Scholar]
- Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, et al. Effects of estrogen plus progestin on health‐related quality of life. New England Journal of Medicine 2003;348(19):1‐16. [DOI] [PubMed] [Google Scholar]
- Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SAA, Brzyski R, et al. Health risks and benefit 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008;299(9):1036‐45. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil‐Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation 2006;113:2425‐34. [DOI] [PubMed] [Google Scholar]
- Hsia J, Langer RD, Manson JE, Keller L, Johnson KC, Hendrix SL, et al. Conjugated equine estrogens and coronary heart disease. Archives of Internal Medicine 2006;166:357‐65. [DOI] [PubMed] [Google Scholar]
- Jackson RD, Wactawski‐Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women's Health Initiative randomized trial. Journal of Bone and Mineral Research 2006;21(6):817‐28. [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. for the WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA 2011;305(13):1305‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani S, Chlebowski RT, Prentice RL, Kato I, Wactawski‐Wende J, Johnson KC, et al. Estrogen and colorectal cancer incidence and mortality. Cancer 2015;121:3261‐71. [DOI] [PubMed] [Google Scholar]
- Maalouf NM, Sato AH, Welch BJ, Howard BV, Cochrane BB, Sakhaee K, et al. Postmenopausal hormone use and the risk of nephrolithiasis. Archives of Internal Medicine 2010;170(18):1678‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post‐stopping phases of the Women's Health Intiative randomized trials. JAMA 2013;310(13):1353‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. New England Medical Journal 2003;349:523‐34. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Shumaker SA, Bowen DJ, Langer RD, Hunt JR, Kaplan RM, et al. Women's Health Initiative: Why now? What is it? What's new?. American Psychologist 1997;52(2):101‐16. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. NIH News: NIH asks participants in Women's Health Initiative estrogen‐alone study to stop study pills, begin follow‐up phase. National Institutes of Health website: www.nih.gov/news (accessed 26 March 2004). [DOI] [PubMed]
- Prentice RL, Pettinger M, Beresford SAA, Wactawski‐Wende J, Hubbell FA, Stefanick ML, et al. Colorectal cancer in relation to postmenopausal estrogen and estrogen plus progestin in the Women's Health Initiative clinical trial and observational study. Cancer Epidemiology, Biomarkers & Prevention 2009;18(5):1531‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. Journal of the American Medical Association 2003;289(20):2663‐72. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age‐associated cognitive decline. Clinical Trials 2004;1:440‐50. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, An Y, Maki PM, Coker Laura H, Jackson R, et al. for the Women's Health Initiative Study of Cognitive Aging Investigators. Effects of conjugated equine oestrogens on cognition and affect in postmenopausal women with prior hysterectomy. Journal of Clinical Endocrinology and Metabolism 2009;94(11):4152‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. Journal of Clinical Endocrinology and Metabolism 2006;91:1802‐10. [DOI] [PubMed] [Google Scholar]
- Ritenbaugh C, Stanford JL, Wu LL, Shikany JM, Schoen RE, Stefanick ML, et al. Women's Health Initiative Investigators. Conjugated equine estrogens and colorectal cancer incidence and survival: the Women's Health Initiative randomized clinical trial. Cancer Epidemiology, Biomarkers & Prevention 2008;17(10):2609‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297(13):1465‐77. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Fillit H, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291(24):2947‐58. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. JAMA 2003;289(20):2651‐62. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of esyrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials 1998;19:604‐21. [DOI] [PubMed] [Google Scholar]
- Simon MS, Chlebowski RT, Wactawski‐Wende J, Johnson KV, Muskovitz A, Kato I, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. Journal of Clinical Oncology 2012;30(32):3983‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, Rodabough RJ, Paskett ED, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA 2006;295(14):1647‐57. [DOI] [PubMed] [Google Scholar]
- Stefanick ML, Cochrane BB, Hsia J, Barad D, Liu J, Johnson S. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13:S78‐86. [DOI] [PubMed] [Google Scholar]
- Tang J, Spaunhurst KM, Chlebowski RT, Wactawski‐Wende J, Keiser E, Thomas F, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: Women’s Health Initiative randomized trials. Journal of the National Cancer Institute 2011;103:1469‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004;291(14):1701‐12. [DOI] [PubMed] [Google Scholar]
- The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19:61‐109. [DOI] [PubMed] [Google Scholar]
- Toh S, Hernandez‐Diaz S, Logan R, Rossouw JE, Hernan MA. Coronary heart disease in postmenopausal recipients of estrogen plus progestin therapy: does the increased risk ever disappear? A randomized trial. Annals of Internal Medicine 2010;152(4):211‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L, Espeland MA, Snively B, Shumaker SA, Rapp SR, et al. The rationale, design, and baseline characteristics of the Women's Health Initiative Memory Study of Younger Women (WHIMS‐Y). Brain Research 2013;1514:3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitt B, Pettinger M, Weinstein A, Katz J, Torner J, Wasko MC, et al. Women's Health Initiative Investigators. Effects of postmenopausal hormone therapy on rheumatoid arthritis: the women's health initiative randomized controlled trials. Arthritis & Rheumatism 2008;59(3):302‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassertheil‐Smoller AM, Kaplan RC, Salazar CR. Stroke findings in the Women's Health Initiative. Seminars in Reproductive Medicine 2014;32:438‐46. [DOI] [PubMed] [Google Scholar]
- Wassertheil‐Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. JAMA 2003;289(20):2673‐84. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women's Health Initiative investigators. Oestrogen plus progestogen increased coronary heart disease and breast cancer events in postmenopausal women. Therapeutics 2002;288:321‐33. [Google Scholar]
- Writing group for the Women's Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA 2002;288(3):321‐33. [DOI] [PubMed] [Google Scholar]
- Zhao A, Chlebowski RT, Anderson GL, Kuller LH, Manson JE, Gass M, et al. Sex hormone associations with breast cancer risk and the mediation of randomized trial postmenopausal hormone therapy effects. Breast Cancer Research 2014;16:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
WISDOM 2007 {published data only}
- Vickers MR, MacLennan AH, Lawton B, Ford D, Martin J, Meredith SK, et al. Main morbidities recorded in the women's international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ Online First 2007;335:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MR, Martin J, Meade TW, the WISDOM Study Team. The women's international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial. BMC Women's Health 2007;7(2):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton AJ, Vickers MR, Kim J, Ford D, Lawton BA, MacLennan AH, et al. the Wisdom Team. Health related quality of life after combined hormone replacement therapy: randomised controlled trial. BMJ 2008;337(212):a1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaffe 2006 {published data only}
- Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, et al. Effects of ultra‐low‐dose transdermal estradiol on cognition and health‐related quality of life. Archives of Neurology 2006;63:945‐50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AHT 2015 {published and unpublished data}
- Eeles RA, Morden JP, Gore M, Mansi J, Glees J, Wenzel M, et al. Adjuvant hormone therapy may improve survival in epithelial ovarian cancer: results of the AHT randomized trial. Journal of Clinical Oncology 2015;33(35):4138‐44. [DOI] [PubMed] [Google Scholar]
Aitken 1971 {published data only}
- Aitken JM, Lorimer AR, McKay Hart D, Lawrie TDV, Smith DA. The effects of oophorectomy and long‐term mestranol therapy therapy on the serum lipids of middle‐aged women. Clinical Science 1971;41:597‐603. [DOI] [PubMed] [Google Scholar]
- Aitken JM, McKay Hart D. Oestrogen in oophorectomized women. British Medical Journal 1971;2(5758):400‐1. [PMC free article] [PubMed] [Google Scholar]
Aitken 1973 {published data only}
- Aitken JM, Hart DM, Lindsay R, Anderson JB, Smith DA, Wilson GM. Prevention of bone loss following oophorectomy in premenopausal women: a retrospective assessment of the effects of oophorectomy and a prospective controlled trial of the effects of mestranol therapy. Israel Journal of Medical Sciences 1976;12(7):607‐14. [PubMed] [Google Scholar]
- Aitken JM, Lindsay R, Hart DM. Long‐term oestrogens for the prevention of post‐menopausal osteoporosis. Postgraduate Medical Journal 1976;52 Suppl 6:18‐25. [PubMed] [Google Scholar]
- Aitken M, Hart DM, Lindsay R. Oestrogen replacement therapy for prevention of osteoporosis after oophorectomy. British Medical Journal 1973;3:515‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay R, Aitken JM, Anderson JB, Hart DM, MacDonald EB, Clarke AC. Long‐term prevention of postmenopausal osteoporosis by oestrogen. The Lancet 1976;1(7968):1038‐40. [DOI] [PubMed] [Google Scholar]
Angerer 2000 {published data only}
- Angerer P, Stork S, Kothny W, Schmitt P, Schacky C. Effect of oral postmenopausal hormone replacement on progression of atherosclerosis: a randomised controlled trial. Arteriosclerosis, Thrombosis and Vascular Biology 2001;21(2):262‐8. [DOI] [PubMed] [Google Scholar]
Bloch Thomsen 2002 {published data only}
- Bloch Thomsen A, Silvestri S, Haarbo J, Christiansen C, Bjarnason N. Association between target organ responses during hormone replacement therapy. Abstracts of posters:10th World Congresss on Menopause, Climacteric 2002;5 Suppl 1:57. [Google Scholar]
Chen 2001 {published data only}
- Chen FP, Lee N, Soong YK, Huang KE. Comparison of transdermal and oral estrogen‐progestin replacement therapy: effects on cardiovascular risk factors. Menopause: The Journal of the North American Menopause Society 2001;8(5):347‐52. [DOI] [PubMed] [Google Scholar]
Christiansen 1981 {published data only}
- Christiansen C, Christensen MS, Jensen J, Hagen C, Stocklund K, Transbol I. Effects of natural oestogen/gestagen and thiazide on coronary risk factors in normal postmenopausal women: a 2 year double‐blind placebo study [Naturlig ostrogen/gestagen og tiazids virkning pa kardiovaskulaere risikofaktorer hos postmenopausale kvinder]. Ugeskrift for Laeger 1981;143:2230‐4. [PubMed] [Google Scholar]
Corrado 2002 {published data only}
- Corrado F, Altavilla D, D'Anna R, Cancellieri F, Cannata ML. Effects of the phytoestrogen genistein and hormone replacement therapy on bone mineral density and metabolism in early post‐menopausal women: a randomised double blind placebo‐controlled study. Abstracts of Posters: 10th World Congress on Menopause: Climacteric 2002;5 (Supplement 1):173. [Google Scholar]
Corson 1999 {published data only}
- Corson SL, Richart RM, Caubel P, Lim P. Effect of a unique constant‐estrogen, pulsed‐progestin hormone replacement therapy containing 17‐Beta estradiol and norgestimate on endometrial histology. International Journal of Fertility 1999;44(6):279‐85. [PubMed] [Google Scholar]
de Roo 1999 {published data only}
- Vogelvang TE, Mijatovic V, Kamp O, Netelenbos JC, Neele SJM, Pines A, et al. Neither long‐term treatment with raloxifene nor hormone replacement therapy modulate cardiac function in health postmenopausal women: two randomized, placebo‐controlled, 2‐year studies. American Journal of Obstetrics and Gynaecology 2002;186:729‐36. [DOI] [PubMed] [Google Scholar]
- Valk‐de Roo GW, Stehouwer CDA, Meijer P, Mijatovic V, Kluft C, Kenemans P, et al. Both raloxifene and estrogen reduce major cardiovascular risk factors in healthy postmenopausal women: a 2‐year placebo‐controlled study. Arteriosclerosis, Thrombosis and Vascular Biology 1999;19:2993‐3000. [DOI] [PubMed] [Google Scholar]
Eiken 1996 {published data only}
- Eiken P, Kolthoff N, Pors Nielsen SP. Effect of 10 years' hormone replacement therapy on bone mineral content in postmenopausal women. Bone 1996;5 Suppl:191S‐193S. [DOI] [PubMed] [Google Scholar]
Estratab 1977 {published data only}
- Genant HK, Lucas J, Weiss S, Akin M, Emkey R, McNaney‐Flint H, et al. Low‐dose esterified estrogen therapy. Archives of Internal Medicine 1997;157:2609‐15. [DOI] [PubMed] [Google Scholar]
- Trabal JF, Lenihan JP, Melchione TE, Stoltz RR, Khairi S, Yang HNM, et al. Low‐dose unopposed estrogens: preliminary findings on the frequency and duration of vaginal bleeding in postmenopausal women receiving esterified estrogens over a two‐year period. Menopause: The Journal of the North Amercian Menopause Society 4;3:130‐8. [Google Scholar]
- Watts NB, Nolan JC, Brennan JJ, Yang HM, ESTRATAB/Osteoporosis Study Group. Esterified estrogen therapy in postmenopausal women. Relationships of bone marker changes and plasma estradiol to BMD changes: a two year study. Menopause 2000;7(6):375‐82. [DOI] [PubMed] [Google Scholar]
EWA 2000 {published data only}
- Os I, Hofstad AE, Brekke M, Abdelnoor M, Nesheim BI, Jacobsen AF, et al. The EWA (Estrogen in Women with Atheroscelerosis) Study: a randomized study of the use of hormone replacement therapy in women with angiographically verified coronary artery disease. Characteristics of the study population. Effects on lipids and lipoproteins. Journal of Internal Medicine 2000;247:433‐41. [DOI] [PubMed] [Google Scholar]
Genant 1990 {published data only}
- Genant HK, Baylink DJ, Gallagher JC, Harris ST, Steiger P, Herber M. Effect of estrone sulfate on postmenopausal bone loss. Obstetrics and Gynaecology 76;4:579‐84. [PubMed] [Google Scholar]
Graser 2001 {published data only}
- Graser T, Muller A, Druckman R, Oettel M. Effects of a combination of 2 mg estradiol valerate and 3 mg dienogest on coagulation, lipid profile and glucose metabolism in postmenopausal women. Drugs of Today 2001;37 Suppl:87‐99. [Google Scholar]
HABITS 2004 {published data only}
- Holmberg L, Anderson H, for the HABITS Steering and Data Monitoring Committees. HABITS (hormonal replacement therapy after breast cancer ‐ is it safe?), a randomised comparison: trial stopped. The Lancet 2004;363:453‐5. [DOI] [PubMed] [Google Scholar]
Haines 2003 {published data only}
- Haines CJ, Fan Yim S, Chung TKH, Lan CWK, Lau EWC, Ng MHL, et al. A prospective, randomized, placebo‐controlled study of the dose effect of oral oestradiol on menopausal symptoms, psychological well being and quality of life in postmenopausal Chinsese women. Maturitas 2003;44:207‐14. [DOI] [PubMed] [Google Scholar]
Hall 1998 {published data only}
- Hall G, Pripp U, Schenck‐Gustafsson K, Landgren B‐M. Longterm effects of hormone replacement therapy on symptoms of angina pectoris, quality of life and compliance in women with coronary artery disease. Maturitas 1998;28:235‐42. [DOI] [PubMed] [Google Scholar]
Jensen 1985 {published data only}
- Jensen J, Christiansen C, Rodbro P. Cigarette smoking, serum estrogens and bone loss during hormone‐replacement therapy early after menopause. New England Journal of Medicine 1985;313:973‐5. [DOI] [PubMed] [Google Scholar]
Kuopio 1998 {published data only}
- Komulainen MH, Kroger H, Tuppurainen MY, Heikkinen A‐M, Alhava E, Honkanen R, et al. HRT and vit D in prevention of non‐vertebral fractures in postmenopausal women: a 5 year randomized trial. Maturitas 1998;31:45‐54. [DOI] [PubMed] [Google Scholar]
Lufkin 1992 {published data only}
- Lufkin EG, Wahner HW, O'Fallon WM, Hodgson SF, Kotowicz MA, Lane AW, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Annals of Internal Medicine 1992;117(1):1‐9. [DOI] [PubMed] [Google Scholar]
Maki 2004 {published data only}
- Maki PM, Resnick SM, Brandt J, Dobs AR, Durso SC, McCrae RR. Changes in self‐reported personality coincident with declines in cognition: results from parallel hormone therapy trials in elderly men and women. Neurobiology of Aging 2004;25 Suppl 2:106. [Google Scholar]
Mizunuma 2010 {published data only}
- Mizunuma H, Taketani Y, Ohta H, Honjo H, Gorai I, Itabashi A, et al. Dose effects of oral estradiol on bone mineral density in Japanese women with osteoporosis. Climacteric 2010;13:72‐83. [DOI] [PubMed] [Google Scholar]
Newhouse 2000 {unpublished data only}
- Newhouse 2000. Effects of Estrogen on Memory in Post‐Menopausal Women and Patients With Alzheimer Disease. Clinical trials.gov.2003.
Ng 1992 {published data only}
- Ng HT, Chang SP, Yanfg TZ, Cho MP, Wei TC. Estradiol administered in a percutaneous gel for the prevention of postmenopausal bone loss. Asia‐Oceania Journal of Obstetrics and Gynaecology 1993;19(2):115‐9. [DOI] [PubMed] [Google Scholar]
Nielsen 2006 {published data only}
- Nielsen T, Ravn P, Pitkin J, Christiansen C. Pulsed estrogen therapy improves postmenopausal quality of life: a 2‐year placebo‐controlled study. Maturitas 2006;53(2):184‐90. [DOI] [PubMed] [Google Scholar]
- Nielsen TF, Ravn P, Bagger YZ, Warming L, Christiansen C. Pulsed estrogen therapy in prevention of postmenopausal osteoporosis. A 2‐year randomized, double blind, placebo‐controlled study. Osteoporosis International 2004;15(2):168‐74. [DOI] [PubMed] [Google Scholar]
Ory 1998 {published data only}
- Ory SJ, Field CS, Herrmann RR, Zinsmeister AR, Riggs BL. Effects of long‐term transdermal administration of estradiol on serum lipids. Mayo Clinic Proceedings 1998;73:735‐8. [DOI] [PubMed] [Google Scholar]
Os 2002 {published data only}
- Os I, Os A, Sandset PM, Bolling S, Seljeflot I, Djurovic S, et al. Hormone replacement therapy does not affect plasma homocysteine in postmenopausal women with coronary artery disease. Cardiology 2002;98:6‐12. [DOI] [PubMed] [Google Scholar]
Paoletti 2015 {published data only}
- Paoletti AM, Cagnacci A, Carlo C, Orru MM, Neri M, D'Alterio MN, et al. Clinical effect of hormonal replacement therapy with estradiol associated with noretisterone or drosperinone. A prospective randomized placebo controlled study. Gynecological Endocrinology 2015;31(5):384‐9. [DOI] [PubMed] [Google Scholar]
Papworth 2002 {published data only}
- Clarke SC, Kelleher J, Lloyd‐Jones H, Slack M, Schofield PM. A study of hormone‐replacement therapy in postmenopausal women with ischaemic heart disease: the Papworth HRT atherosclerosis study. British Journal of Obstetrics and Gynaecology 2002;109:1056‐62. [DOI] [PubMed] [Google Scholar]
Pefanco 2007 {published data only}
- Pefanco MA, Kenny AM, Kaplan RF, Kuchel G, Walsh S, Kleppinger A, et al. The effect of 3‐year treatment with 0.25 mg/day of micronized 17beta‐estradiol on cognitive function in older postmenopausal women. Journal of the American Geriatrics Society 2007;55(3):426‐31. [DOI] [PubMed] [Google Scholar]
Post 2001 {published data only}
- Post MS, Mooren MJ, Baal WM, Neel SJM, Netelenbos JC, Kenemans P. Raloxifene reduces impedance to flow within the uterine artery in early postmenopausal women: a 2‐year randomized placebo‐controlled comparative study. American Journal of Obstetrics and Gynaecology 2001;185:557‐62. [DOI] [PubMed] [Google Scholar]
- Vogelvang TE, Mijatovic V, Kamp O, Netelenbos JC, Neele SJM, Pines A, et al. Neither long‐term treatment with raloxifene nor hormone replacement therapy modulate cardiac function in health postmenopausal women: two randomized, placebo‐controlled, 2‐year studies. American Journal of Obstetrics and Gynaecology 2002;186:729‐36. [DOI] [PubMed] [Google Scholar]
Rasgon 2014 {published data only}
- Rasgon NL, Geist CL, Kenna HA, Wroolie TE, Williams KE, Silverman DH. Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One 2014;9(3):e89095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saitta A 2001 {published data only}
- Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, et al. Randomized, double‐blind, placebo‐controlled study on effects of raloxifene and hormone replacement therapy on plasma NO concentrations, endothelin‐1 levels, and endothelium‐dependent vaso‐dilation in postmenopausal women. Arteriosclerosis, Thrombosis and Vascular Biology 2001;21(9):1512‐9. [DOI] [PubMed] [Google Scholar]
Sanchez‐Guerrero 2007 {published data only}
- Sanchez‐Guerrero J, Gonzalez‐Perez M, Durand‐Carbajal M, Lara‐Reyes P, Jimenez‐Santana L, Romero‐Diaz J, et al. Menopause hormonal therapy in women with systemic lupus erythematosus. Arthritis and Rheumatism 2007;56(9):3070‐9. [DOI] [PubMed] [Google Scholar]
Schierbeck 2012 {published data only}
- Scheirbeck LL, Rejnmakr L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012;345(e6409):1‐11. [DOI] [PubMed] [Google Scholar]
SMART 2016 {published and unpublished data}
- Mirkin S, Pinkerton JV, Kagan R, Thompson JR, Pan K, Pickar JH, et al. Gynaecologic safety of conjugated estrogens plus bazedoxifene: pooled analysis of five phase 3 trials. Journal of Womens Health 2016;25(5):431‐9. [DOI] [PubMed] [Google Scholar]
Steiner 2007 {published data only}
- Steiner AZ, Xiang M, Mack WJ, Shoupe D, Felix JC, Lobo RA, et al. Unopposed estradiol therapy in postmenopausal women. Obstetrics and Gynecology 2007;109:581‐7. [DOI] [PubMed] [Google Scholar]
Teede 2002 {published data only}
- Teede HJ, Liang YL, Kotsopoulos D, Zoungas S, Craven R, McGrath BP. Placebo‐controlled trial of transdermal estrogen therapy alone in postmenopausal women: effects on arterial compliance and endothelial function. Climacteric 2002;5:160‐69. [PubMed] [Google Scholar]
ULTRA 2005 {published data only}
- Diem S, Grady D, Quan J, Vittinghoff E, Wallace R, Hanes V, et al. Effects of ultralow‐dose transdermal estradiol on postmenopausal symptoms in women aged 60 to 80 years. Menopause 2006;1:130‐8. [DOI] [PubMed] [Google Scholar]
- Waetjen LE, Brown JS, Vittinghoff E, Ensrud KE, Pinkerton J, Wallace R, et al. The effect of ultralow‐dose transdermal estradiol on urinary incontinence in postmenopausal women. Obstetrics and Gynecology 2005;1:946‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virtanen 1999 {published data only}
- Virtanen I, Polo‐Kantola P, Erkkola R, Polo O, Ekholm E. Climacteric vasomotor symptoms do not imply autonomic dysfunction. British Journal of Obstetrics and Gynaecology 1999;106:155‐64. [DOI] [PubMed] [Google Scholar]
Wharton 2011 {published data only}
- Wharton W, Baker LD, Gleason CE, Dowling M, Barnet JH, Johnson S, et al. Short‐term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer's disease: results of a randomized controlled trial. Journal of Alzheimer's Disease 2011;26:495‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abelin 2012
- Abelin AP, Nunes CM, Camazzola FE, Santos RP. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
AMS 2016
- Australian Menopause Society. AMS guide to equivalent HRT/MHT doses. https://www.menopause.org.au/for‐women/information‐sheets/426‐ams‐guide‐to‐equivalent‐hrt‐mht‐doses, 2016; Vol. Accessed 6 January 2017.
Anderson 2003
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SAA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures. JAMA 2003;290(13):1739‐48. [DOI] [PubMed] [Google Scholar]
Anderson 2006
- Anderson GL, Chlebowski RT, Rossouw JE, Rodabough RJ, McTiernan A, Margolis KL, et al. Prior hormone therapy and breast cancer risk in the Women's Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006;55:103‐15. [DOI] [PubMed] [Google Scholar]
Anderson 2012
- Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow‐up of the Women's Health Initiative randomised placebo‐controlled trial. The Lancet 2012;13:476‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avis 2001
- Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Social Science and Medicine 2001;52:345‐6. [DOI] [PubMed] [Google Scholar]
Banks 2009
- Banks E, Reeves GK, Beral V, Balkwill A, Liu B, Roddam A, for the Million Women Study collaborators. Hip fracture incidence in relation to age, menopausal status, and age at menopause: prospective analysis. PLoS Medicine 2009;6(11):e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banks 2009a
- Banks E, Canfell K. Invited Commentary: Hormone therapy risks and benefits ‐ The Women's Health Initiative findings and the postmenopausal estrogen timing hypothesis. American Journal of Epidemiology 2009;1:24‐8. [DOI] [PubMed] [Google Scholar]
Barret‐Connor 2007
- Barrett‐Connor E. Hormones and heart disease: the timing hypothesis. American Journal of Epidemiology 2007;166(5):506‐10. [DOI] [PubMed] [Google Scholar]
Barrett‐Connor 1998
- Barrett‐Connor E, Grady D. Hormone replacement therapy, heart disease and other considerations. Annual Review of Public Health 1998;19:55‐72. [DOI] [PubMed] [Google Scholar]
Barrett‐Connor 2001
- Barrett‐Connor E, Stuenkel CA. Hormone replacement therapy (HRT) ‐ risks and benefits. International Journal of Epidemiology 2001;30:423‐6. [DOI] [PubMed] [Google Scholar]
Bath 2005
- Bath PMW, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta‐analysis. BMJ 2005;330:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beral 2002
- Beral V, Banks E, Reeves G. Evidence from randomised trials on the long‐term effects of hormone replacement therapy. The Lancet 2002;360:942‐4. [DOI] [PubMed] [Google Scholar]
Beral 2003
- Beral V, Million Women Study Collaborators. Breast cancer and hormone‐replacement therapy in the Million Women Study. The Lancet 2003;362:419‐27. [DOI] [PubMed] [Google Scholar]
Beral 2011
- Beral V, Reeves G, Bull D, Green J, for the Million Women Study Collaborators. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. Journal of the National Cancer Institute 2011;103:296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boardman 2015
- Boardman HMP, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post‐menopausal women. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD002229.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Canonico 2007
- Canonico M, Oger E, Plu‐Bureau G, Conard J, Meyer G, Levesque H, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007;115(7):840‐5. [DOI] [PubMed] [Google Scholar]
Cauley 2003
- Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density. JAMA 2003;290(13):1729‐38. [DOI] [PubMed] [Google Scholar]
Chlebowski 2003
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. JAMA 2003;289(24):3243‐53. [DOI] [PubMed] [Google Scholar]
Chlebowski 2004
- Chlebowski RT, Col N. Menopausal hormone therapy after breast cancer. The Lancet 2004;363:410‐1. [DOI] [PubMed] [Google Scholar]
Chlebowski 2008
- Chlebowski RT, Anderson G, Pettinger M, Lane D, Langer RD, Gillian MA, et al. for the Women’s Health Initiative Investigators. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Archives of Internal Medicine 2008;168(4):370‐77. [DOI] [PubMed] [Google Scholar]
Chlebowski 2009
- Chlebowski RT, Schwartz AG, Wakelee H, Anderson GKL, Stefanick, ML, Manson JE, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post‐hoc analysis of a randomised controlled trial. Lancet 2009;374(9697):1243‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2009a
- Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, for the WHI Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. New England Journal of Medicine 2009;57:3‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2010
- Chlebowski RT, Anderson G, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Women's Health Initiative Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010;304(15):1719‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2010a
- Chlebowski, Anderson G, Manson JE, Pettinger M, Yasmeen S, Lane D, et al. Estrogen alone in postmenopausal women and breast cancer detection by means of mammography and breast biopsy. Journal of Clinical Oncology 2010;28:2690‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2010b
- Chlebowski RT, Anderson GL, Manson JE, Schwartz AG, Wakelee H, Gass M, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. Journal of the National Cancer Institute 2010;102(18):1413‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2015a
- Chlebowski RT, Rohan TE, Manson JE, Aragaki AK, Kaunitz A, Stefanick ML, et al. Breast cancer after use of estrogen plus progestin and estrogen alone. JAMA 2015;1(3):296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2015b
- Chlebowski RT, Aragaki AK, Anderson GL. Menopausal hormone therapy influence on breast cancer outcomes in the Women's Health Initiative. Journal of the National Comprehensive Cancer Network 2015;13(7):917‐24. [DOI] [PubMed] [Google Scholar]
Cody 2009
- Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in post‐menopausal women. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001405.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cranney 2002
- Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C, et al. Summary of meta‐analyses of therapies for postmenopausal osteoporosis. Endocrine Reviews 2002;23(4):570‐8. [DOI] [PubMed] [Google Scholar]
Cushman 2004
- Cushman M, Kuller LH, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292(13):1573‐80. [DOI] [PubMed] [Google Scholar]
Espeland 2004
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA 2004;291(24):2959‐3007. [DOI] [PubMed] [Google Scholar]
Espeland 2013
- Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM. Long term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50‐55 years. JAMA Internal Medicine 2013;173(15):1429‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Espeland 2016
- Espeland MA, Rapp SR, Manson JE, Goveas JS, Shumaker SA, Hayden KM, et al. Long‐term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. Journals of Gerontology Series A‐Biological Sciences and Medical Sciences 2016;00(00):11‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furness 2012
- Furness S, Roberts H, Marjoribanks J, Lethaby A. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000402.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version 1 September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grady 1995
- Grady G, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta‐analysis. Obstetrics and Gynaecology 1995;85(2):304‐13. [DOI] [PubMed] [Google Scholar]
Greendale 1999
- Greendale GA, Lee NP, Arriola ER. The menopause. The Lancet 1999;353:571‐80. [DOI] [PubMed] [Google Scholar]
Greiser 2006
- Greiser CM, Greiser EM, Dören M. Menopausal hormone therapy and risk of ovarian cancer: systematic review and meta‐analysis. Human Reproduction Update 2007;13(5):453‐63. [DOI] [PubMed] [Google Scholar]
Guidozzi 1999
- Guidozzi F, Daponte A. Estrogen replacement for ovarian cancer survivors: a randomized controlled trial. Cancer 1999;86:1013‐8. [DOI] [PubMed] [Google Scholar]
Guthrie 2005
- Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. Hot flushes during the menopause transition: a longitudinal study in Australian‐born women. Menopause 2005;12(4):460‐7. [DOI] [PubMed] [Google Scholar]
Heiss 2008
- Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SAA, Brzyski R, et al. Health risks and benefit 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008;299(9):1036‐45. [DOI] [PubMed] [Google Scholar]
Hemminki 1997
- Hemminki E, McPherson K. Impact of post menopausal hormone therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 1997;315:149‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemminki 2000
- Hemminki E, McPherson K. Value of drug‐licensing documents in studying the effect of postmenopausal hormone therapy on cardiovascular disease. Lancet 2000;355:566‐9. [DOI] [PubMed] [Google Scholar]
Hemminki 2000a
- Hemminki E. Hormone replacement therapy: discrepancies between evidence and recommendations. Scandinavian Journal of Public Health 2000;28:81‐3. [DOI] [PubMed] [Google Scholar]
Hendrix 2006
- Hendrix SL, Wassertheil‐Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation 2006;113:2425‐34. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011 ]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hogervorst 2009
- Hogervorst E, Yaffe K, Richards M, Huppert FAH. Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003799.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holmberg 2004
- Holmberg L, Anderson H, for the HABITS Steering and Data Monitoring Committees. HABITS (hormonal replacement therapy after breast cancer ‐ is it safe?), a randomised comparison: trial stopped. The Lancet 2004;363:453‐5. [DOI] [PubMed] [Google Scholar]
Holmberg 2008
- Holmberg L, Iversen OE, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J, et al. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. Journal of the National Cancer Institute 2008;100(7):475‐82. [DOI] [PubMed] [Google Scholar]
Hulley 2004
- Hulley SB, Grady D. The WHI estrogen‐alone trial ‐ do things look any better?. JAMA 2004;291:1769‐71. [DOI] [PubMed] [Google Scholar]
ICR 2001
- Institute of Cancer Research. UK Randomised Trial of Hormone Replacement Therapy (HRT) in Women with a History of Early Breast Cancer UK Randomised Trial of Hormone Replacement Therapy (HRT) in Women with a History of Early Breast Cancer. Online at: http://www.icr.ac.uk/ctsu/hrt.htm (accessed 8 March 2005).
Ismail 2010
- Ismail SI, Bain C, Hagen S. Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007063.pub2] [DOI] [PubMed] [Google Scholar]
Jordan 2015
- Jordan VC. The new biology of estrogen induced apoptosis applied to treat and prevent breast cancer. Endocrine‐Related Cancer 2015;22(1):R1‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaunitz 2002
- Kaunitz AM. Use of combination hormone replacement therapy in light of recent data from the Women's Health Initiative. Medscape Women's Health e‐journal at: http://medscape.com 2002; Vol. 7, issue 4. [PubMed]
Kongnyuy 1999
- Kongnyuy EJ, Norman RJ, Flight IHK, Rees MCP. Oestrogen and progestogen hormone replacement therapy for peri‐menopausal and post‐menopausal women: weight and body fat distribution. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001018] [DOI] [PubMed] [Google Scholar]
Kronenberg 1994
- Kronenberg F. Hot flashes:phenomenology, quality of life and search for treatment options. Experimental Gerontology 1994;29(3/4):319‐36. [DOI] [PubMed] [Google Scholar]
Kurman 1985
- Kurman RJ, Norris HJ. Evaluation of criteria for distinguishing atypical endometrial hyperplasia from well‐differentiated carcinoma. Cancer 1985;56(2):403‐12. [DOI] [PubMed] [Google Scholar]
LaCroix 2011
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. for the WHI Investigators. Health outcomes after stopping conjugated equine esotrogens among postmenopausal women with prior hysterectomy. JAMA 2011;305(13):1305‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lethaby 2008
- Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003122.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacLennan 2004
- MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD002978.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manson 2003
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. New England Medical Journal 2003;349:523‐34. [Google Scholar]
Manson 2013
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post‐stopping phases of the Women's Health Intiative randomized trials. JAMA 2013;310(13):1353‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manson 2015
- Manson JE. The ‘timing hypothesis’ for estrogen therapy in menopausal symptom management. Womens Health 2015;11(4):437‐40. [DOI] [PubMed] [Google Scholar]
Marjoribanks 2012
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Trial does not change the conclusions of Cochrane review of long term hormone therapy for perimenopausal and postmenopausal women. BMJ 2012;345:e8141. [DOI] [PubMed] [Google Scholar]
Matthews 1997
- Matthews KA, Shumaker SA, Bowen DJ, Langer RD, Hunt JR, Kaplan RM, et al. Women's Health Initiative: Why now? What is it? What's new?. American Psychologist 1997;52(2):101‐16. [DOI] [PubMed] [Google Scholar]
McKinney 1998
- McKinney K A, Thompson W. A practical guide to prescribing hormone replacement therapy. Drugs 1998;56(1):49‐57. [DOI] [PubMed] [Google Scholar]
Miller 2008
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacological Reviews 2008;60:210‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
NAMS 2012
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 2012;19(3):257‐71. [DOI] [PubMed] [Google Scholar]
Nastri 2012
- Nastri CO, Lara LA, Ferriani RA, Rosa‐e‐Silva ACJS, Figueiredo JBP, Martins WP. Hormone therapy for sexual function in perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009672.pub2] [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. Menopause. https://www.nice.org.uk/guidance/ng23/evidence/full‐guideline‐559549261, 2015 (accessed September 2016).
NIH 2004
Obiorah 2013
- Obiorah I, Jordan VC. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause 2013;20(4):372‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palacios 2010
- Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 2010;13:419‐28. [DOI] [PubMed] [Google Scholar]
Pedersen 2003
- Pedersen AT. Issues to debate on the Women's Health Initiative (WHI) study. Epidemiology or randomized clinical trials ‐ time out for hormone replacement therapy studies?. Human Reproduction 2003;18(11):2241‐4. [DOI] [PubMed] [Google Scholar]
Prentice 2009
- Prentice RL, Pettinger M, Beresford SAA, Wactawski‐Wende J, Hubbell FA, Stefanick ML, et al. Colorectal cancer in relation to postmenopausal estrogen and estrogen plus progestin in the Women's Health Initiative clinical trial and observational study. Cancer Epidemiology, Biomarkers and Prevention 2009;18(5):1531‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prentice 2009a
- Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. American Journal of Epidemiology 2009;170:12‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
RANZCOG 2012
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Menopausal hormone therapy advice (C‐Gyn 16). https://www.ranzcog.edu.au/document.../hormone‐replacement‐therapy‐advice.html, 2012 (accessed September 2016).
Reslan 2012
- Reslan OM, Khalil RA. Vascular effects of estrogenic menopausal hormone therapy. Review of Recent Clinical Trials 2012;7(1):47‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Resnick 2004
- Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age‐associated cognitive decline. Clinical Trials 2004;1:440‐50. [DOI] [PubMed] [Google Scholar]
Salpeter 2004
- Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women. Journal of General Internal Medicine 2004;19:701‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroll 2012
- Schroll J. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
Shapiro 2003
- Shapiro S. Risks of estrogen plus progestin therapy: a sensitivity analysis of findings in the Women's Health Initiative randomized controlled trial. Climacteric 2003;6:302‐10. [PubMed] [Google Scholar]
Shumaker 1998
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials 1998;19:604‐21. [DOI] [PubMed] [Google Scholar]
Shumaker 2004
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Fillit H, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291(24):2947‐58. [DOI] [PubMed] [Google Scholar]
Simon 2012
- Simon MS, Chlebowski RT, Wactawski‐Wende J, Johnson KV, Muskovitz A, Kato I, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. Journal of Clinical Oncology 2012;30(32):3983‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stefanick 2003
- Stefanick ML, Cochrane BB, Hsia J, Barad D, Liu J, Johnson S. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13:S78‐86. [DOI] [PubMed] [Google Scholar]
Suckling 2006
- Suckling J, Kennedy R, Lethaby A, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001500.pub2] [DOI] [PubMed] [Google Scholar]
Tucker 2003
- Tucker G. Risks of estrogen plus progestin therapy: a sensitivity analysis of findings in the Women's Health Initiative randomized trial: comments from reviewer. Climacteric 2003;6:302‐10. [PubMed] [Google Scholar]
Vaughan 2013
- Vaughan L, Espeland MA, Snively B, Shumaker SA, Rapp SR, Shupe R, et al. The rationale, design, and baseline characteristics of the Women's Health Initiative Memory Study of Younger Women (WHIMS‐Y). Brain Research 2013;1514:3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHI 2002
- Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomised controlled trial. JAMA 2002;288:321‐33. [DOI] [PubMed] [Google Scholar]
Zhao 2014
- Zhao A, Chlebowski RT, Anderson GL, Kuller LH, Manson JE, Gass M, et al. Sex hormone associations with breast cancer risk and the mediation of randomized trial postmenopausal hormone therapy effects. Breast Cancer Research 2014;16:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cochrane HRT Study Group 2003
- Cochrane HRT Study Group. Hormone replacement therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004143] [DOI] [Google Scholar]
Farquhar 2005
- Farquhar C, Marjoribanks J, Lethaby A, Suckling JA, Lamberts Q. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2005, Issue 7. [DOI: 10.1002/14651858.CD004143.pub2] [DOI] [PubMed] [Google Scholar]
Farquhar 2009
- Farquhar C, Marjoribanks J, Lethaby A, Suckling JA, Lamberts Q. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004143.pub3] [DOI] [PubMed] [Google Scholar]
Marjoribanks 2012a
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004143.pub4] [DOI] [PubMed] [Google Scholar]


