Abstract
Infant-mother behavioral synchrony is thought to scaffold the development of self-regulation in the first years of life. During this time, infants’ and mothers’ physiological regulation may contribute to dyadic synchrony and, in infants, dyadic synchrony may support infants’ physiological regulation. Because the sympathetic (SNS) and parasympathetic (PNS) nervous systems serve different regulatory functions, the current study aimed to elucidate relations between infants’ and mothers’ SNS and PNS functioning and dyadic behavioral synchrony. Skin conductance (SC; SNS index), respiratory sinus arrhythmia (RSA; PNS index), heart period (HP; index of joint SNS and PNS arousal), and behavioral synchrony were assessed in six-month-old infants (N = 140) and their mothers during a mild social stressor, the Face-to-Face Still-Face Paradigm (FFSF; Tronick, Als, Adamson, Wise & Brazelton, 1978). Synchrony was related to infants’ and mothers’ PNS and to mothers’ broad autonomic arousal but not to SNS-specific arousal. Higher levels of behavioral synchrony were associated with lower infant RSA but with higher mother HP and RSA at baseline and in each FFSF episode. Therefore, lower-RSA infants may have required more synchronous engagement with mothers to support regulation, while higher-RSA, less-aroused mothers may have been particularly well-attuned to infants’ emotions. Findings suggest that each individuals’ physiological state may contribute to the behavioral functioning of the dyad.
Keywords: behavioral synchrony, sympathetic nervous system, parasympathetic nervous system, infants, mothers
The sympathetic (SNS) and parasympathetic (PNS) nervous systems, which are thought to underlie self-regulation, both facilitate and develop in the context of interactions with caregivers (Armony & Vuilleumier, 2013; Porges & Furman, 2011). Specifically, infant-mother interactions during distress and recovery are thought to simultaneously shape and require infants’ SNS and PNS (Busuito & Moore, 2017; Enlow et al., 2014). Likewise, mothers to draw on their own physiological resources when interacting with their infants (Moore et al., 2009). One important quality of dyadic interactions is the degree of coordination of infants’ and mothers’ behaviors over time, or dyadic behavioral synchrony. Behavioral synchrony is related to infant and mother physiological functioning, and predicts self-regulation in early childhood (Feldman, Greenbaum, & Yirmiya, 1999; Moore & Calkins, 2004). Because the SNS and PNS play unique roles in the regulation of behavior (Porges, 2007), the current study seeks to elucidate each of these physiological systems’ unique roles during infant-mother interaction and associations with behavioral synchrony.
SNS & PNS Functioning
The SNS and PNS operate jointly but orthogonally (Berntson et al., 1991) and contribute distinct functions to self-regulation and behavior (Porges, 2007). SNS (“flight or fight”) activation reflects heightened arousal, exerting an excitatory effect on the sweat glands and heart while down-regulating metabolism to conserve energy. PNS (“rest and digest”) activation facilitates a calm state, slowing heart rate (Thayer & Brosschot, 2005). At rest, lower SNS and higher PNS tones indicate a high degree of PNS control, reflecting a calm, adaptive homeostatic state. While increases in SNS activation and decreases in PNS activation both mobilize the organism for action, the PNS is theorized to have evolved in mammals to allow for the dynamic modulation of physiological arousal required during social engagement and emotion regulation (Porges, 2007), making it particularly relevant for the study of infant-mother interaction. Broad measures of cardiac activity, including heart rate (HR) and heart period (HP), are jointly determined by the SNS and PNS and therefore reflect general autonomic arousal but do not allow for specific identification of SNS versus PNS contributions.
While a growing body of work has examined either a single system (or a single system together with HR or HP) in infants and mothers or both the SNS and PNS within an individual, to our knowledge, only two studies to date have examined concurrent SNS and PNS functioning in infants and mothers (Ham & Tronick, 2006; 2009). Because the SNS and PNS serve different regulatory functions, examining the concurrent operations of both systems in infants and in mothers may improve our understanding of how these systems function a) during infancy and b) in the dyadic context.
The Face-to-Face Still-Face Paradigm.
The FFSF has been used for 40 years to examine infants’ and mothers’ responses to social interaction and unexpected disengagement (Tronick, Als, Adamson, Wise & Brazelton, 1978). The FFSF is comprised of three episodes: the normal play, during which infants and mothers interact as they usually would; the still-face, during which mothers disengage from the interaction; and the reunion episode, during which infants and mothers resume interacting. Mothers’ still-face disengagement withdraws regulatory support and is considered a mild stressor for infants, and the reunion episode offers an opportunity to observe how dyads work together to regain a mutually positive interaction.
Infant behavioral responses to the FFSF have been well-documented: infants show decreases in positive and increases in negative affect from the normal play to still-face episodes and then increases in positive and decreases in negative affect from the still-face to reunion episodes (Mesman, van IJzendoorn, Bakermans-Kranenburg, 2009). More recently, infants’ broad autonomic and PNS responses have been documented. Infants show increases in HR (Conradt & Ablow, 2010; Gunning, Halligan & Murray, 2013; Mattson et al., 2013) and decreases in HP (Bazhenova, Plonskaia & Porges, 2001; Moore & Calkins, 2004; Stewart et al., 2013) from normal play to still-face episodes, suggesting increases in arousal. Few studies report changes in HR or HP from still-face to reunion, although the general trend is slight decreases in arousal (Moore & Calkins, 2004).
Neither HR nor HP can be used to distinguish between SNS and PNS contributions to arousal changes. However, largely due to methodological challenges, few studies have examined infant SNS functioning during the FFSF. While one study found increases in infant SNS tone across the FFSF, including from still-face to reunion episodes (Ham & Tronick, 2006), another found no changes (Enlow et al., 2014). A more recent study found that SNS arousal increased across the still-face only for infants in high-risk environments (i.e., low SES, maternal psychopathology; Suurland et al., 2017).
In contrast to this inconsistent and limited research on the SNS, a growing body of work has identified a typical PNS response in infants that coincides with changes in mothers’ support (Bazhenova et al., 2001; Conradt & Ablow, 2010; Moore & Calkins, 2004; Moore et al., 2009; Shahrestani, Stewart, Quintana, Hickie, Guastella, 2014). During the still-face episode, when mothers are unresponsive, infants show decreases in PNS tone from normal play, suggesting active physiological self-regulation. During the reunion, when interaction resumes, most infants show PNS increases, although not to pre-still-face levels.
Despite evidence that parenting is a self-regulatory task that requires autonomic regulation (Leerkes, Su, Calkins, Supple & O’Brien, 2016; Mills-Koonce et al., 2009), few studies have examined mothers’ autonomic functioning during the FFSF (c.f., Moore et al., 2009; Oppenheimer, Measelle, Laurent, & Ablow, 2013). On average, mothers’ PNS tone has been found to increase from baseline to still-face episodes and then to return to baseline levels (Moore et al., 2009; Oppenheimer et al., 2013). To date, although two studies have examined mother SC in the FFSF (Ham & Tronick, 2006; 2009), neither of these reported mean patterns of change in mothers’ SC across the procedure and both were based on small samples of infant-mother pairs (ns = 12 and 18).
Infant and Mother SNS & PNS Functioning within a Dyadic Context
The FFSF affords an opportunity to examine physiological functioning in the context of the dyadic behaviors that contribute to children’s self-regulatory development. Because their self-regulatory capacities are not fully developed, infants rely on caregivers’ support to regulate emotion: With caregivers’ attunement and responsiveness to their behavioral signals, infants navigate situations that are beyond their developmental capabilities. With supported practice, infants’ self-regulatory abilities are expanded over time and they learn to independently regulate distress (Kopp, 1982; Tronick, 1989).
Behavioral synchrony is thought to scaffold this repeated regulation of emotions and recovery from distress. Behavioral synchrony measures coordination between two individuals’ affective behaviors, without regard for the type of behavior that is expressed. For example, mothers are typically more positive and engaged than infants but may modulate their behaviors, becoming more or less positive in response to changes in infants’ behaviors (e.g., Busuito & Moore, 2017; Conradt & Ablow, 2010; Ham & Tronick, 2006). Therefore, synchrony reflects an attunement between infants and mothers that facilitates active regulation of distress; in this way, it may build a repertoire for regulating stress that facilitates the transition from mutual regulation to self-regulation (Cohn & Tronick, 1988; Feldman, Greenbaum & Yirmiya, 1999). Indeed, behavioral synchrony during infancy predicts competence across a number of domains later in childhood: Greater behavioral synchrony is related to secure attachment (Lindsay & Caldera, 2014; Lundy, 2002), greater empathy (Feldman, 2007), and better self-regulation (Davis et al., 2016; Lindsey et al., 2009) in early childhood.
Behavioral synchrony may shape infants’ physiological functioning and, simultaneously, infants’ physiological functioning may contribute to behavioral synchrony via infants’ individual behavior (Busuito & Moore, 2017; Enlow et al., 2014; Feldman, Magori-Cohen, Galili, Singer, & Louzoun, 2011; Moore & Calkins, 2004; Provenzi et al., 2015). Compared with infants of sensitive mothers, infants of insensitive mothers showed greater increases in SNS arousal across the FFSF (Enlow et al., 2014), suggesting that the quality of caregiving supports the regulation of SNS arousal.
With respect to PNS functioning, infants in dyads that showed higher levels of behavioral synchrony in the normal play exhibited decreases in PNS tone during the still-face episode, suggesting that infants who had more dyadic support prior to the still-face were later able to rely on physiological resources to self-regulate (Moore & Calkins, 2004). In another study, infants of more sensitive mothers showed PNS decreases, relative to baseline, in the reunion episode, perhaps reflecting active attempts to reengage with their mothers (Moore et al., 2009). However, infants in more affectively flexible dyads and infants whose mothers showed greater concurrent sensitivity showed greater increases in PNS tone in the reunion episode than other infants (Busuito & Moore, 2017; Conradt & Ablow, 2010). Likewise, also in the reunion episode, behavioral synchrony and infant PNS tone have been positively related (Ham & Tronick, 2009).
These apparently contradictory findings may be due to the different measures of dyadic behavior examined (i.e., maternal sensitivity, behavioral synchrony, flexibility), which capture different qualities of the interaction. It may also be due to the timing of the behavioral measurement, with studies using earlier measures of interaction quality capturing different relations with infant physiology (e.g., preparing infants to call on physiological self-regulation) than concurrent dyadic behavior (e.g., obviating the need for physiological self-regulation or reflecting contributions from physiological regulation).
The one study that has examined relations between behavioral synchrony and mothers’ physiology found no relations with PNS functioning and did not report findings for mothers’ individual SNS functioning (Ham & Tronick, 2009). However, mothers’ PNS functioning has been associated with parenting behavior, with more-sensitive mothers showing greater withdrawal in the reunion, relative to baseline, than less-sensitive mothers (Moore et al., 2009). Additionally, mothers’ physiology may be contingent upon dyadic context: Infant distress has been found to predict greater maternal SNS arousal (Ham & Tronick, 2006) and PNS withdrawal (Oppenheimer et al., 2013). PNS withdrawal predicted heightened sensitivity to infant distress only for mothers of infants who developed avoidant attachments (Mills-Koonce et al., 2007).
The Current Study
Although SNS and PNS functioning is theorized to contribute to and be shaped by dyadic exchanges (Armony & Vuilleumier, 2013; Porges, 2007), particularly during distress and recovery, little work has examined relations between behavioral synchrony and infants’ and mothers’ physiological functioning. The current study sought to described normative patterns of infants’ and mothers’ SNS and PNS functioning and clarify relations between behavioral synchrony and individual’s patterns of physiological responding.
Aim 1.
Describe patterns of infants’ and of mothers’ broad autonomic functioning (HP), SNS functioning (SC), and PNS functioning (RSA) across baseline and FFSF episodes, replicating and extending existing pilot work (Ham & Tronick, 2006). Because intrapersonal and socio-cultural factors have been associated autonomic functioning, including race and socioeconomic status (Alkon et al., 2014; DiPietro et al., 2004; Hill et al., 2015), infant age and sex (Bar-Haim, Marshall, & Fox, 2000; Korkushko, Shatilo, Plachinda, & Shatilo, 1991), and infant feeding method (Quigley, Moore, Propper, Goldman, & Cox, 2017), effects of factors that could potentially obscure or contribute to patterns of infants’ and of mothers’ autonomic responses were examined. Based on prior work (Ham & Tronick, 2006) and because the FFSF is a mild social stressor, we expected infants to show increasing arousal (i.e., SC increases and HP decreases) between the normal play and still-face, and decreases in arousal (i.e., SC decreases and HP increases) from still-face to reunion episodes (Ham & Tronick, 2006; Moore & Calkins, 2004). Also consistent with prior work (e.g., Moore & Calkins, 2004; Shahrestani et al., 2014), we expected infants to show RSA decreases from normal play to still-face and RSA increases from still-face to reunion episodes. In accordance with limited prior work, we expected mothers to show increases in arousal (i.e., SC increases and HP decreases) across the FFSF (Ham & Tronick, 2006), and RSA decreases from still-face to reunion episodes, reflecting their active attempts to soothe and reengage infants (Moore et al., 2009).
Aim 2.
Examine whether dyadic behavioral synchrony was related to patterns of infants’ or mothers’ broad autonomic functioning (HP) or to SNS (SC) or PNS (RSA) functioning across baseline and FFSF episodes. Because prior research has not examined relations between infants’ and mothers’ SNS functioning and dyadic behavior, these analyses were exploratory. However, given associations between maternal insensitivity and SNS arousal in both infants and mothers (Enlow et al., 2014; Leerkes et al., 2015), we expected behavioral synchrony to be inversely related to SC levels in both infants and mothers. Consistent with prior research, we expected infants in more-synchronous dyads to show greater PNS withdrawal during the still-face and reunion episodes, which would indicate active attempts to self-regulate (Moore & Calkins, 2004) and then to reengage with their mothers (Moore et al., 2009), relative to other infants. Relative to mothers in less-synchronous dyads, we expected mothers in more-synchronous dyads to show greater PNS withdrawal, which theoretically supports behavioral responsiveness, during normal play and reunion.
Method
Participants
Mothers and infants in the current study were drawn from postnatal follow-up of a larger, prospective prenatal cohort examining ontogeny of the mother-child relationship. The study (Fetal Neurobehavioral Development and Postnatal Continuity; Protocol # 00000856) was approved by the Institutional Review board at Johns Hopkins Bloomberg School of Public Health. Recruitment was restricted to low-risk, non-smoking women carrying singleton pregnancies. Of the 158 participants eligible for the 6 month follow-up (exclusive of preterm delivery, n = 8, and other undetected conditions of prenatal origin, n = 3), 7 had moved out of the area and 11 either declined or had scheduling conflicts within the target postnatal window, resulting in a sample of 140 infants and mothers.
Women were relatively mature, M (SD) age = 32.0 (1.7), well educated, M (SD) years of completed education = 17.1 (4.4), and married (95%). Most (n = 105, 75%) identified as non-Hispanic White, 11 (8%) identified as African-American, and 24 (17%) identified as Asian, Hispanic or more than one race. Over half (57.6%) were primiparous. Forty-three percent (n = 60) of infants were girls. Feeding method was categorized as: exclusively breastfed (44%), exclusively formula fed (30%), or a combination of breast milk and formula (26%). During the visit, mothers reported on the time that their infants were last fed, and a variable was computed to measure the amount of time that had elapsed between the last feeding and the visit. This was included in the current study because hydration may influence SC levels (Fowles, 2007).
Procedures
Mothers and their infants participated in a laboratory visit when infants were 6 months of age, M (SD) days postpartum = 191.8 (9.1). Almost all (96%) visits occurred in the morning commencing near 1000 h. Following consent procedures and collection of demographic information, women were asked to change infants’ diapers during which time a 3-lead electrocardiogram was applied to infants’ chests to capture cardiac measures and two gelled silver-silver chloride electrodes were applied to the sole of infants’ left feet to index electrodermal activity. Non-latex wrap was applied over the electrodes and a snap front sleeper and pair of socks were provided to dress the infant. Following confirmation of acquisition of infant physiological signals, mothers were similarly fitted with a 3-lead electrocardiogram and two gelled electrodes to the base of their non-dominant hand. A non-latex wrap was applied to women’s hands to ensure continued adherence of electrodes during interaction with their infants.
A 4-minute baseline interaction followed, in which infants were in a seated position in their mother’s lap. Picture board books were provided and mothers were asked to show or read a book(s) to their infants. Physiological data from infants and mothers were recorded continuously across the baseline period utilizing Biolab Acquisition software 3.0 (Mindware Technologies Ltd., Gahanna OH) and behavior was video-recorded using a split-screen procedure, with one camera recording the mother and one recording the infant. Infant and mother behavior was coded offline (see Measures). Women were asked to place their infants in an infant seat and sit in a chair directly in front of their infants for face-to-face interaction.
Face-to-Face Still-Face.
The FFSF (Tronick et al., 1978) was used to elicit infants’ and mothers’ physiological and behavioral responses to mild social stress. Mothers were told that the task would be terminated if their infants cried for 20 seconds or if mothers indicated they would like to stop at any time. In the normal play episode of the FFSF, mothers were instructed to play with their babies as they normally would (without toys) for 2 minutes. After the normal play episode, a research assistant instructed mothers to face away from their infants for 15 seconds and then to turn back and maintain a neutral expression for the 2-minute still-face episode. Mothers were asked to refrain from responding to their infants in any way during this episode, including vocalization, facial expression, and touch. Mothers were then instructed to turn away from their infants again for 15 seconds and then resume interacting with them in whatever manner they chose (again without toys) during the 2-minute reunion episode without taking infants out of the infant seat.
Measures
Physiological measures.
Physiological data were continuously collected during the 4-minute baseline episode and during each episode of the FFSF; the 4 channels were amplified using MindWare’s Bionex system and transmitted to a computer in an adjacent room and captured using BioLab Acquistion Software 3.013 (Mindware Technologies Ltd, Gahanna, OH).
Skin Conductance.
SC data were edited using MindWare’s Electrodermal Activity Analysis software version 3.1.0 (MindWare Technologies, Ltd.). The software editing algorithm identifies peaks and troughs of the SC waveform (peaks and troughs indicate SC responses), detects artifacts, and provides graphical display for visual inspection and manual editing of any remaining outlier points. The mean SC values (expressed in microSiemens (μS)), within each episode (baseline, normal play, still-face, reunion) were calculated and used for analyses in the current study. Larger values of SC indicated greater sympathetic arousal.
Respiratory Sinus Arrhythmia and Heart Period.
Heart rate data files were processed and edited using MindWare Heart Rate Variability Analysis software version 3.0 (MindWare Technologies Ltd.). The software collects heart interbeat intervals (IBI) by identifying R-wave peaks. It detects and corrects outlier points due to movement artifacts and provides graphical display for visual inspection and manual editing of any remaining outlier points. Measures of HP and RSA are then derived from edited IBI files. The software quantifies RSA using an algorithm to extract heart rate variability within the frequency band of spontaneous respiration in young children (.24 Hz – 1.04 Hz) and reports RSA in units of msec2. Data were edited using the R-peak Editor system, which uses an algorithm to systematically insert missed or correct outlier R-peaks. RSA was calculated in 30-second epochs for the 4-minute baseline episode and for each 2-minute episode of the FFSF. These epoch lengths are typical and valid for parent-infant paradigms of this duration (Bar-Haim et al., 2000). The means of RSA epochs within each episode (baseline, normal play, still-face, reunion) were calculated and used for analyses in the current study. Larger values of RSA indicated higher parasympathetic tone.
Infant and mother affective behaviors.
Infants’ and mothers’ behaviors during the FFSF were coded by trained research assistants who were naïve to the hypotheses of the study. Facial affect and gaze were coded in 1-second intervals using a modified Monadic phase coding system (Tronick et al., 1980). Facial affect was coded as either positive, negative, or neutral. If a face could not be seen (e.g., infant turned away from the camera, mother’s face turned towards the ground), affect was coded as missing. Gaze was coded as away or toward partner in 1-second intervals. Gaze was coded as missing only if the face was obscured to the extent that it could not be determined if the head was turned away or towards the partner (e.g., infant’s head was obscured by mother). The validity of this system has been demonstrated in relation to other discrete-emotion coding systems (Matias, Cohn & Ross, 1990; Weinberg & Tronick, 1994). Coders were trained to reliability using a large pool of videotaped FFSF interactions from a separate study and a subset of interactions from the current study (15% of full sample) was randomly selected for double coding. Inter-coder reliability between coders was defined as coders observing the same behavior within one second of each other and was quantified using kappa to correct for chance agreement (κ = .89 for infants’ and mothers’ affect, κ = .80 for infants’ gaze, κ = .73 for mothers’ gaze). To compare infants’ and mothers’ affective responses to those reported in prior studies, we computed positive and negative affect scores for infants and mothers separately, as the percentage of valid time (i.e., not missing) that the affect was displayed during each FFSF episode.
The goal of infant-mother interactions is to maximize time spent in a state of positive engagement (i.e., smiling and looking at the other), although they typically move among a variety of states during the interaction. Therefore, following prior research (Cohn & Tronick, 1988; Moore & Calkins, 2004) the second-by-second affect and gaze codes were combined to calculate a 6-point social engagement score for infants and mothers separately at each second of the interaction. A value of 1 was assigned if the individual displayed negative affect and gaze away from partner, 2 if negative affect and gaze towards, 3 if neutral affect and gaze away, 4 if neutral affect and gaze toward, 5 if positive affect and gaze away, and 6 if positive affect and gaze toward. As expected, mothers’ scores were moderately skewed (normal play M = 5.63, skewness = −2.23, kurtosis = 4.23; reunion M = 5.52; skewness = −2.15, kurtosis = 3.65), whereas infants’ scores were normally distributed (normal play M = 3.76, skewness = 0.03, kurtosis = −0.76; reunion M = 3.52, skewness = 1.87, kurtosis = −0.87). Values reflect normative responses to the FFSF, in which mothers are largely positive and looking at infants and infants exhibit a wider range of affective behaviors.
Dyadic Behavioral Synchrony.
The Monadic phase coding system has been widely used to conceptualize synchrony as coordination of change along this social engagement continuum, without necessarily matching in affective states (Cohn & Tronick, 1987; Feldman et al., 1999; Moore & Calkins, 2004). Synchrony was computed as the Pearson correlation coefficient between infants’ and mothers’ social engagement scores, reflecting the degree to which mothers and infants moved synchronously among the 6 different social engagement states. Variables were computed separately for the normal play and the reunion episodes of the FFSF. Fisher’s r to z transformation was used to transform the synchrony scores for use in preliminary correlations and main analyses.
Missing data.
Eleven dyads were missing behavioral and physiological data due to infants becoming too distressed to complete the FFSF. Other physiological data were missing primarily due poor electrode connectivity and movement artifact. As a result, HP, SC, and RSA data are missing at different rates for infants and mothers and in the various episodes (i.e., baseline, normal play, still-face, and reunion (see below)). Therefore, analytic sample size differed across main analyses—reflecting cases with complete physiological data across the FFSF—due to listwise deletion employed in general linear models.
Across measures, data were missing at the lowest rates for baseline and the highest rates for reunion (with the exception of mothers’ SC, which had the lowest missing rate for normal play). Missing HP ranged from 4% to 27% for infants and from 1% to 16% for mothers; missing SC ranged from 40% to 58% for infants and from 44% to 49% for mothers; missing RSA ranged from 4% to 32% for infants and from 3% to 17% for mothers. There were no differences in infant sex, infant or mother age, infant or mother baseline HR or HP, behavioral synchrony, or observed infant fussiness between infants or mothers with complete FFSF physiological data and those with missing data.
Data Analysis Plan
Aim 1.
Repeated measures general linear models (GLMs) were conducted to examine patterns of infants’ and of mothers’ HP, SC, and RSA across baseline and FFSF episodes. Separate models were conducted for infants’ HP, SC, and RSA and for mothers’ HP, SC, and RSA. In the event of a significant episode effect, indicating changes in physiological responses as the social demands of the FFSF changed, repeated within-subjects contrast tests were used to examine episode-to-episode change. Based on preliminary analyses and prior research, demographic and infant state (i.e., time since last feeding) variables were then added to the GLMs as covariates (continuous variables) or between-subjects factors (categorical variables) to test for effects on infants’ and mothers’ patterns of physiological responses.
Aim 2.
To test whether behavioral synchrony was related to patterns of infants’ and mothers’ HP, SC, and RSA responses, behavioral synchrony in the reunion episode was added as a covariate to each of the GLMs specified above. Only synchrony in the reunion episode was used in main analyses, because, consistent with prior work (Conradt & Ablow, 2010; Leerkes et al., 2015; Leerkes et al., 2016), synchrony in the normal play episode was not correlated with infants’ or mothers’ HP, SC, and RSA (see Preliminary Analyses).
Results
Preliminary Analyses
Preliminary analyses were conducted to examine associations among study variables using IBM SPSS for Windows, Version 24.0 (SPSS Inc., 2016).
Affective behaviors during the FFSF.
As seen in Table 2, consistent with a large body of research (see Mesman et al., 2009 for review), infants showed increased negative and decreased positive affect in the still-face episode compared with normal play, and subsequently increased in positive and decreased in negative affect in the reunion. Mothers showed high levels of positive and low levels of negative affect in both interactive episodes. Consistent with prior work on behavioral synchrony (Moore & Calkins, 2004; Feldman et al., 2011), dyads showed low levels of synchrony on average (Table 2), and levels were not significantly different between the normal play and reunion episodes.
Table 2.
Descriptive statistics for infant and mother affect and dyadic behavioral synchrony in each episode of the FFSF
| Normal Play | Still-Face | Reunion | ||||
|---|---|---|---|---|---|---|
| N | M (SD) | N | M (SD) | N | M (SD) | |
| Infant % Positive | 126 | .27 (.23) | 123 | .06 (.13) | 116 | .23 (.24) |
| Infant % Negative | 126 | .16 (.24) | 123 | .36 (.36) | 116 | .25 (.32) |
| Mother % Positive | 125 | .84 (.16) | 115 | .78 (.21) | ||
| Mother % Negative | 125 | .01 (.05) | 115 | .03 (.10) | ||
| Behavioral Synchrony | 122 | .20 (.19) | 107 | .20 (.16) | ||
Note. % Positive and % Negative refer to the percentage of valid time infants or mothers spent displaying positive or negative affect. Mother behavior was not coded during the still-face episode, when mothers are instructed to pose a still-face.
Table 3 presents correlations among behavioral variables. Positive and negative affect showed moderate to high stability across episodes of the FFSF for infants and for mothers. With the exception of infant and mother negative affect in the reunion, infant and mother affect were moderately to highly correlated in the normal play and reunion episodes. Infant positive affect showed only a small positive correlation with behavioral synchrony in the normal play episode, whereas mothers’ positive affect was inversely correlated with behavioral synchrony in both the normal play and reunion and mothers’ negative affect in the reunion was moderately and positively correlated with behavioral synchrony in the reunion. This suggests that mothers were the main contributors to dyadic synchrony and that lower levels of positive affect may have reflected empathic responses to infant neutral and negative infant affect.
Table 3.
Correlations among infant and mother behavioral variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. % Infant Positive NP | - | ||||||||||
| 2. % Infant Positive SF | .27** | - | |||||||||
| 3. % Infant Positive RN | .50** | .38** | - | ||||||||
| 4. % Infant Negative NP | −.39** | .00 | −.19* | - | |||||||
| 5. % Infant Negative SF | −.15 | −.29** | −.18* | .43** | - | ||||||
| 6. % Infant Negative RN | −.06 | −.05 | −.35** | .31** | .49** | - | |||||
| 7. % Mother Positive NP | .25** | .05 | .17 | −.29** | −.09 | −.18 | - | ||||
| 8. % Mother Positive RN | .30** | .05 | .34** | −.25** | −.20* | −.34** | .61** | - | |||
| 9. % Mother Negative NP | −.23* | −.06 | .04 | .48** | .17 | .00 | −.42** | −.20* | - | ||
| 10. % Mother Negative RN | −.20* | −.05 | −.08 | .36** | .23* | −.15 | −.07 | −.39** | .58** | - | |
| 11. NP Behavioral Synchrony | .20* | −.22* | .01 | −.05 | .04 | .05 | −.33** | −.19* | .15 | .02 | - |
| 12. RN Behavioral Synchrony | −.16 | .04 | .09 | .10 | −.03 | −.06 | −.25* | −.41** | .05 | .29** | .18 |
Note.
p < .05;
p < .01
Relations between individuals’ physiology, individuals’ behavior, and dyadic behavioral synchrony. With the exception of HP, there were few associations between physiological and behavioral variables. In normal play, infant HP was related to infant positive, r(110) = .31, p < .01, and infant negative, r(110) = −.43, p < .001, affect. In the still-face, infant HP was related to infant negative affect, r(106) = −.54, p < .001. In the reunion, infant HP was related to infant positive, r(94) = .28, p < .01, and infant negative, r(94) = −.57, p < .001, affect. With the exception of infant RSA and infant positive affect in the normal play, r(109) = .23, p < .05, infant SC and RSA were uncorrelated with infant behavior within episodes. Mothers’ physiology and behavior were uncorrelated within each FFSF episode.
Infants’ HP, SC, or RSA and behavioral synchrony were uncorrelated in the normal play, as were mothers’ HP, SC, or RSA responses and behavioral synchrony in the normal play. Behavioral synchrony in the reunion was correlated with mothers’ reunion RSA, r(102) = .22, p < .05 and with mothers’ reunion HP, r(103) = .24, p < .05.
Relations between infants’ and mothers’ physiology.
Infant-mother SC was correlated within all episodes (r’s from .40 – .45, p’s < .10). Infant-mother HP and RSA were uncorrelated.
Relations between physiology and demographic and infant state variables.
Infant age was unrelated to infant SC or RSA but was positively correlated with infant HP in the normal play, r(120) = .23, p < .05, still-face, r(116) = .25, p < .01, and reunion, r(102) = .29, p < .01. The amount of time that elapsed between infants’ last feeding and the lab visit was related to infant SC, such that more recent feeding was associated with lower SC in the still-face, r(68) = .30, p < .05, and reunion, r(59) = .29, p < .05, episodes. Because time since last feeding was related only to infant SC and may theoretically influence SC via hydration level (Fowles, 2007), it was included as a covariate in analyses of infants’ SC only. Based on preliminary analyses and on prior work finding differences in RSA based on age (Bornstein & Suess, 2000), infant age was included as a covariate in all analyses of infant physiology during the FFSF.
Male infants had higher RSA than female infants at baseline, F(1, 133) = 6.17, p < .05, and in the reunion episode, F(1, 93) = 4.52, p < .05. Differences as a function of marital status were not examined given the very small number of unmarried mothers in the sample. Parity, race, feeding method, and whether or not the infant was fed during the visit were unrelated to infants’ HP, SC, and RSA at baseline or in any episode. Therefore, because infant sex was related only to RSA, and because differences in child RSA as a function of sex have been found in prior research (Bar-Haim et al., 2000; Korkushko et al., 1991), infant sex was included as a potential moderator of response patterns across the FFSF in analyses of infant RSA only.
Maternal age was inversely related to mothers’ RSA in the still-face, r(127) = −.24, p < .01, and maternal education was positively correlated with mothers’ baseline RSA, r(136) = .21, p < .05, and with mothers’ HP at baseline, r(138) = .28, p < .01, and in the normal play, r(129) = .30, p < .001, still-face, r(128)= .21, p < .05, and reunion, r(117) = .34, p < .001. Therefore, maternal age and education were added as covariates to all analyses of mothers’ physiology.
Although mothers’ reunion HP differed as a function of feeding method, F(2, 114) = 3.39, p < .05, Tukey’s HSD post hoc tests showed only trend-level differences between groups, with breastfeeding mothers (695.27 ± 79.63) and combination-feeding mothers (702.19 ± 108.36) showing marginally greater HP than non-breastfeeding mothers (649.52 ± 75.55). Because there were no relations between feeding method and either mother SC or RSA, and because post hoc tests were not significant, feeding method was not included as a covariate in main analyses. Race, infant sex, and whether or not the infant was fed during the visit were unrelated to mothers’ HP, SC, and RSA at baseline or in any episode of the FFSF.
Based on these preliminary analyses and on prior research, infant age was included in all models of infant physiology (HP, SC, RSA), time since last feeding was included in models predicting infant SC, and infant sex was included in models predicting infant RSA. Mother age and education were included as covariates in all models predicting mother physiology.
Main Analyses
Aim 1.
Examine patterns of infants’ and of mothers’ autonomic responses across baseline and FFSF episodes, replicating and extending existing work. Descriptive statistics for infant and mother physiology at baseline and in each FFSF episode are presented in Table 1.
Table 1.
Descriptive statistics for infant and mother mean heart period, skin conductance levels, and respiratory sinus arrhythmia at baseline and in each episode of the FFSF
| Baseline | Normal Play | Still-Face | Reunion | |||||
|---|---|---|---|---|---|---|---|---|
| N | M (SD) | N | M (SD) | N | M (SD) | N | M (SD) | |
| Infant HP (msec) | 135 | 433.69 (33.46) | 120 | 436.52 (34.16) | 116 | 414.43 (35.71) | 102 | 415.90 (37.95) |
| Mother HP (msec) | 138 | 728.35 (103.19) | 129 | 683.05 (97.42) | 128 | 763.93 (111.50) | 117 | 685.77 (89.00) |
| Infant SC (uS) | 84 | 9.27 (7.04) | 71 | 13.13 (8.91) | 68 | 14.31 (9.05) | 59 | 16.52 (10.04) |
| Mother SC (uS) | 73 | 7.22 (5.05) | 79 | 11.49 (7.21) | 75 | 11.20 (7.23) | 72 | 12.59 (8.08) |
| Infant RSA (msec2) | 135 | 3.18 (.85) | 118 | 3.87 (1.15) | 109 | 3.85 (1.13) | 95 | 3.79 (1.19) |
| Mother RSA (msec2) | 136 | 5.86 (1.04) | 124 | 5.75 (1.27) | 127 | 5.63 (1.08) | 116 | 5.47 (.97) |
Note. SC = mean skin conductance level; RSA = respiratory sinus arrhythmia; HP = heart period.
Infants’ HP responses.
Multivariate tests indicated a significant episode effect on infants’ HP, F(3, 99) = 20.88, p < .001, η2 = .39. Infants’ HP did not change significantly from baseline to normal play or from still-face to reunion but decreased from normal play to still-face, F(1, 101) = 47.16, p < .001, η2 = .32, suggesting an increase in arousal. Next, infant age was added to the model predicting infants’ HP. There was a significant main effect of infant age, F(1, 100) = 10.62, p < .01, η2 = .10, such that older infants had higher HP at baseline and during each episode of the FFSF compared with younger infants, suggesting lower arousal. With age included in the model, episode effects became non-significant, perhaps due to decreased observed power to detect an effect (from 1.00 to .26).
Mothers’ HP responses.
Because the assumption of sphericity was not met, the Greenhouse-Geisser test of within-subjects effects was interpreted and showed a significant episode effect on mothers’ HP, F(2.49, 288.96) = 132.49, p < .001, η2 = .53. Within-subjects repeated contrast tests revealed that mean levels of mothers’ HP decreased from baseline to normal play, F(1, 116) = 119.94, p < .001, η2 = .51, increased from normal play to the still-face, F(1, 116) = 242.07, p < .001, η2 = .68, and decreased from the still-face to the reunion, F(1, 116) = 231.03, p < .001, η2 = .67, indicating that mothers showed heightened arousal during both interactive episodes. Next, maternal age and education were added to the model as covariates. A main effect of maternal education on mother HP, F(1, 114) = 15.03, p < .001, η2 = .12, indicated that, on average, more highly-educated mothers had greater HP at baseline and in each FFSF episode relative to less-educated mothers, suggesting lower arousal. Addition of these covariates changed the following findings: the previously statistically significant decreases in HP between baseline and normal-play, F(1, 114) = 3.53, p < .10, η2 = .03, and between still-face and reunion, F(1, 114) = 2.86, p < .10, η2 = .02, became marginally significant.
Infants’ SC responses.
Because the assumption of sphericity was not met, the Greenhouse-Geisser test of within-subjects effects was interpreted and showed a significant episode effect on infants’ SC, F(1.60, 81.39) = 52.55, p < .001, η2 = .51. Within-subjects contrast tests found that mean levels of infants’ SC increased from baseline to the normal play, F(1, 51) = 46.41, p < .001, η2 = .48, from normal play to still-face, F(1, 51) = 10.72, p < .01, η2 = .17, and from still-face to reunion, F(1, 51) = 19.07, p < .001, η2 = .27, episodes, indicated increasing SNS arousal across the task. There were no main or interaction effects involving age or time since last feeding on infant SC; therefore, results reported were for the most parsimonious model.
Mothers’ SC responses.
Because the assumption of sphericity was not met, the Greenhouse-Geisser test of within-subjects effects was interpreted and showed a significant episode effect on mothers’ SC, F(2.09, 125.56) = 131.18, p < .001, η2 = .69. Within-subjects contrast tests found that mean levels of mothers’ SC increased from baseline to normal play, F(1, 60) = 202.50, p < .001, η2 = .77, decreased from normal play to the still-face, F(1, 60) = 5.05, p < .05, η2 = .08, and increased from the still-face to the reunion, F(1, 60) = 30.69, p < .001, η2 = .34, suggesting increases in SNS arousal during the interactive episodes. Mothers’ level of SC during the reunion was higher than during baseline, F(1, 60) = 202.90, p < .001, η2 = .77, and normal play, F(1, 60) = 11.44, p < .01, η2 = .16. Next, maternal age and education were added as covariates. There were no significant main or interaction effects involving maternal age or education, therefore, results reported were for the most parsimonious model without covariates.
Infants’ RSA responses.
Multivariate tests indicated a significant episode effect on infants’ RSA, F(3, 84) = 19.83, p < .001, η2 = .42. Within-subjects contrasts indicated that mean levels of infants’ RSA increased from baseline to normal play, F(1, 86) = 51.28, p < .001, η2 = .37. Infants showed the expected RSA decreases from normal play to still-face and increases from still-face to reunion, but these changes were not statistically significant. Next, infant sex was added to the GLM as a between-subjects factor and infant age was added as a covariate to test possible moderation of patterns of infants’ RSA. There were no significant effects of covariates or moderator, therefore, results reported are for the most parsimonious model.
Mothers’ RSA responses.
Because the assumption of sphericity was not met, the Greenhouse-Geisser test of within-subjects effects was interpreted and showed a significant episode effect on mothers’ RSA, F(2.61, 284.54) = 6.60, p < .01, η2 = .06. Within-subjects contrasts revealed that mothers’ mean levels of RSA showed no significant changes from baseline to normal play or from normal play to still-face, but decreased significantly from the still-face to reunion, F(1, 109) = 4.61, p < .05, η2 = .04, suggesting a need for increased regulation, perhaps to support active attempts to reengage and soothe their infants following the still-face. Consistent with this, mothers’ mean level of RSA was significantly greater in the normal play than reunion, F(1, 109) = 5.67, p < .05, η2 = .05. Next, maternal age and education were added to the model as covariates. There was a main effect of maternal age, F(1, 107) = 6.44, p < .05, η2 = .06, indicating older mothers had lower RSA than younger mothers at baseline and in each FFSF episode. With addition of these covariates, there was no longer a significant episode effect; however, observed power to detect an episode effect (.31) was now attenuated.
Aim 2.
Examine whether dyadic behavioral synchrony is related to patterns of infants’ or mothers’ autonomic responses across baseline and FFSF episodes. Behavioral synchrony in the reunion was then added as a covariate to each of the most parsimonious GLMs reported above. Covariates and moderators from the previous aim were retained only if they were found to be significant. Figure 1 illustrates infants’ and mothers’ autonomic responses across baseline and FFSF episodes as a function of behavioral synchrony.
Figure 1.
Infant and mother heart period (HP), skin conductance (SC), and respiratory sinus arrhythmia (RSA) as a function of dyadic behavioral synchrony.
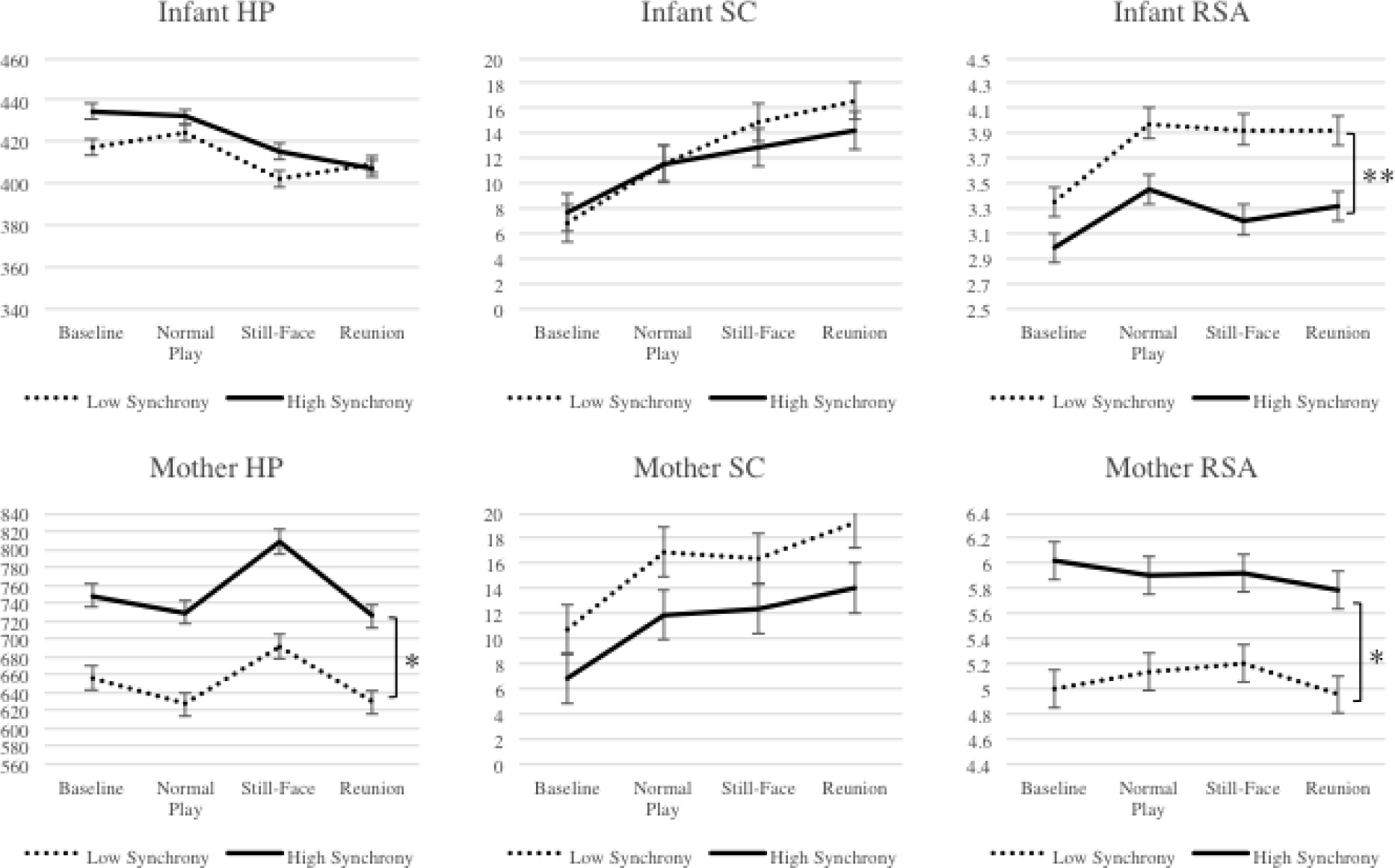
Note. Asterisks denote significant differences in physiology at each time point. * p <.05; ** p < .01.
Infants’ HP responses.
There were no main or interactive effects of behavioral synchrony in the reunion on infants’ HP. The main effect of infant age remained, F(1, 85) = 6.06, p < .05, η2 = .07, indicating that older infants had higher HP at baseline and in FFSF episode.
Mothers’ HP responses.
A main effect of behavioral synchrony in the reunion, F(1, 100) = 6.14, p < .05, η2 = .06, indicated that mothers in dyads with lesser behavioral synchrony had lower HP at baseline and in each FFSF episode (indicating higher arousal) than mothers in dyads with greater behavioral synchrony. The main effect of maternal education on mother HP remained, F(1, 100) = 11.07, p < .01, η2 = .10, indicating that more highly-educated mothers, on average, had greater HP at baseline and in each FFSF episode relative to less-educated mothers, suggesting lower arousal.
Infants’ SC responses.
There were no main or interaction effects involving behavioral synchrony in the reunion on infants’ SC.
Mothers’ SC responses.
There were no main or interaction effects involving behavioral synchrony in the reunion on mothers’ SC.
Infants’ RSA responses.
Because the assumption of sphericity was not met, the Greenhouse-Geisser test of within-subjects effects was interpreted and showed a significant main effect of behavioral synchrony on infants’ RSA, F(2.72, 204.04) = 6.34, p < .01, η2 = .08, indicating that infants in dyads with lesser behavioral synchrony had higher RSA at baseline and in each FFSF episode relative to infants in dyads with greater behavioral synchrony.
Mothers’ RSA responses.
A main effect of behavioral synchrony in the reunion, F(1, 94) = 3.98, p < .05, η2 = .04, indicated that mothers in dyads with lesser behavioral synchrony had lower RSA at baseline and in each FFSF episode relative to mothers in dyads with greater behavioral synchrony. There was a significant main effect of maternal age, F(1, 94) = 4.55, p < .05, η2 = .05, indicating that older mothers had lower RSA at baseline and in each FFSF episode.
Discussion
The current study examined the operation of multiple physiological regulatory systems in the context of a stressful infant-mother interaction. We investigated associations between infants’ and mothers’ physiology and behavioral synchrony, a measure of dyadic regulation thought to scaffold the development of infants’ self-regulation, to elucidate possible functional relations between individuals’ internal regulatory resources and dyadic behavior.
Patterns of Individuals’ Physiological Responses
Infants showed a robust pattern of SNS increases across the FFSF, supporting the proposition that the task is a mild stressor (Tronick, 1980; Tronick, 1989). Infants’ simultaneous increases in SNS and PNS tones from baseline to normal play indicated heightening arousal coupled with a decreased need for physiological regulation, which may have been obviated by regulatory support provided by mothers during the normal play. From normal play to still-face, infants showed increases in SNS arousal and, consistent with prior work, increases in broad autonomic arousal (i.e., HP; Bazhenova, Plonskaia & Porges, 2001; Moore & Calkins, 2004; Stewart et al., 2013) but no significant changes in PNS tone. From still-face to reunion, infants showed increases in SNS arousal but no changes in broad autonomic arousal or PNS tone. Although this was unexpected, as it did not replicate the expected PNS still-face response (e.g., Bazhenova, Plonkaia & Porges, 2001; Moore & Calkins, 2004), infants’ mean RSA in each episode (Table 1) suggests expected PNS withdrawal from normal play to still-face and PNS increases from still-face to reunion, although these did not achieve statistical significance. These slight PNS changes may explain why broad autonomic arousal increased from normal play to still-face but not from still-face to reunion, despite increases in SNS arousal during the latter period.
Like infants, mothers showed increases in SNS tone from baseline to normal play, suggesting that arousal accompanies initiation of dyadic interaction. Unlike infants, however, mothers showed a decrease in arousal from normal play to still-face that was apparent in decreases in SC and increases in HP. This decrease in physiological arousal was likely due to the lessor demands for mothers in the still-face episode, both socially and physically, relative to interactive episodes. Still, it is notable that mothers did not exhibit an arousal response to witnessing their children’s distress during this episode, as prior work has speculated about the degree of emotional challenge posed by the still-face to mothers (Mesman et al., 2009).
Consistent with the theory that simultaneous SNS increases and PNS withdrawal facilitates mobilization to meet environmental demands (Beauchaine, 2001; Berntson et al., 1991), mothers exhibited heightened arousal (i.e., SC increases and marginal HP decreases) accompanied by PNS withdrawal when tasked with reengaging their infants in the reunion. Prior work has suggested that PNS withdrawal supports mothers’ efforts to provide regulatory support to their infants (Moore et al., 2009). These findings suggest either or both of the following: First, SNS activation may also facilitate behavioral responsiveness (i.e., this may not be a unique function of the PNS, as theorized by Porges, 2007). And second, because the reunion episode is theoretically the most challenging for mothers, PNS withdrawal may not be sufficient, and simultaneous SNS activation may facilitate mothers’ capacities to soothe their infants.
Infants and mothers exhibited similar pattern of SNS increases but distinct PNS patterns across the task. Within-episode correlations between infant and mother SNS tones suggest a sort of physiological concordance within dyads that may be due to genetic influences or attunement to the arousal level of the other. However, the same kind of physiological concordance was not observed in PNS tone. This may reflect the different demands of the FFSF for infants versus mothers (Moore et al., 2009), particularly during the still-face episode, when infants are tasked with self-regulating while mothers are required to disengage and sit still.
Dyadic Behavioral Synchrony
Behavioral synchrony was associated exclusively with PNS functioning in infants and with both PNS functioning and broad autonomic arousal—but not SNS-specific arousal—in mothers. Higher levels of behavioral synchrony in the reunion were associated with lower levels of infant PNS tone but with higher mother PNS tone and HP at baseline and in each FFSF episode. Although direction of causality cannot be inferred, several factors may have contributed to these results, which may speak to distinct physiological conditions in infants and mothers that support mutual regulation following a challenge.
The higher PNS tone observed in higher-synchrony mothers is thought to indicate a greater physiological capacity, and lessor need, to regulate (Berntson et al., 1991), which makes sense in light of these mothers’ lower physiological arousal (i.e., higher HP) relative to lower-synchrony mothers. Higher behavioral synchrony was also associated with lower levels of concurrent positive and higher levels of concurrent negative affect in mothers. Therefore, it may be that higher-synchrony mothers’ higher PNS tones and lower arousal allowed them to reflect infants’ emotions rather than relying more heavily on positive affect to help infants recover from distress. This is consistent with prior work on this sample finding that high maternal verbal positivity and greater anxiety during pregnancy independently predicted lower behavioral synchrony at six months postpartum (blinded for review). The authors speculated that high levels of expressed positive affect may reflect a tendency to avoid negative emotion—a characteristic that might impede synchronous engagement during infant distress. Therefore, in the current study, mothers with higher PNS tone may have been better equipped to tolerate actual or anticipated infant distress without attempting to distract or uplift infants with their own positive affect. Consistent with this interpretation, higher PNS tone has been shown to prospectively predict less frequent use of avoidant regulatory strategies in young adults (Geisler, Kubiak, Siewert, & Weber, 2013). Conversely, mothers who exhibited a lessor capacity for PNS regulation (i.e., lower RSA) and heightened broad autonomic arousal (i.e., lower HP) at baseline and across the task may not have had the physiological space to mobilize further to meet the new challenge of reengaging their infants—and potentially providing support for the regulation of distress—in the reunion episode (Berntson et al., 1991; Moore et al., 2009). Together, these findings suggest that maternal proneness to arousal at multiple levels of functioning (i.e., physiological, emotional, or cognitive) may constrain mothers’ ability to interact synchronously with infants, particularly in the face of distress.
Of note, results of the current study did not bear out hypotheses with respect to mothers’ physiology-dyadic behavior relations, which were based on limited prior work finding that PNS withdrawal—not tonic levels—were related to more sensitive behavior in mothers (Moore et al., 2009). However, they are in keeping with the broader literature on adult autonomic functioning, in which high PNS tone is consistently associated with social engagement and emotional health (Geisler et al., 2013; see Thayer & Brosschot, 2005 for a review). According to this work, high PNS tone reflects space for PNS withdrawal when such regulation is needed (Berntson et al., 1991). Higher-synchrony mothers in the current study exhibited higher PNS tone concurrent with lower arousal, suggesting that, although they had physiological space to regulate, they had little need to. Therefore, higher PNS tone when arousal is low—as associated with higher levels of behavioral synchrony in the current study—and PNS withdrawal when regulation is needed, as found in prior work (Moore et al., 2009; Oppenheimer et al., 2013), may both support sensitive or synchronous behavior in mothers.
Inverse relations between infant PNS tone and behavioral synchrony, which were contrary to hypotheses based on existing research (e.g., Fox, 1989; Moore & Calkins, 2004; see Beauchaine, 2001 for a review), may speak to infants’ reliance on caregivers for external regulation of internal states. Because lower PNS tone is thought to indicate lesser capacity to regulate (Berntson et al., 1991), infants with lower PNS tone may have had a greater need for external support (i.e., behavioral synchrony) than infants with higher PNS tone. This proposal is supported by findings suggesting that mothers were the drivers of behavioral synchrony, as indicated by correlations between behavioral synchrony and mother, but not infant, affect. As discussed above, mothers who were less aroused and had greater capacity to regulate may have been particularly well equipped to engage in synchronous interactions with their infants: Higher levels of behavioral synchrony may have reflected mothers’ attempts to provide scaffolding for infants with limited capacities for PNS regulation by modulating their affect in attunement with infants’ cues.
It is also possible that lower PNS tone in higher-synchrony infants reflected earlier withdrawal—perhaps in response to the diaper change or to being in an unfamiliar setting—that was then maintained across the task, such that infants presenting with lower PNS tone may actually have been exhibiting the PNS withdrawal associated with higher synchrony and prosocial behavior in other studies (Moore & Calkins, 2004; see Beauchaine, 2001 for a review). According to this explanation, and consistent with Porges’ (2007) theory that PNS withdrawal evolved to support social engagement, higher-synchrony infants’ lower PNS tone in each episode may indicate use of physiological resources to contribute to behavioral synchrony in the interactive episodes and to regulate during the still-face episode.
In sum, higher levels of behavioral synchrony may reflect mothers’ attunement and responsivity to infants who need greater external support to regulate given their physiological resources. Mothers with relatively greater capacity for PNS regulation who are experiencing low levels of arousal may be particularly well-equipped to reflect infants’ emotions. In this way, the physiological resources of each individual may support the regulation of the dyadic system.
Limitations and Future Directions
Several limitations of the current study should be noted: First, research that has identified links between autonomic functioning and later mental and physical health (see Beauchaine, 2001; Thayer & Brosschot, 2005 for reviews) has found that measurement of SNS and PNS functioning in different organs may point to distinct clinical implications. Because the current study measured SNS functioning via the skin (SC) and PNS functioning via the heart (RSA), future work examining concurrent cardiac SNS and PNS functioning will be better equipped to investigate the orthogonal functioning of these two systems and its prediction of mental and physical health outcomes. Second, our aggregate measure of synchrony may have lacked the sensitivity to capture some nuances of dyadic behavior (e.g., lead-lag structure, time-lag to synchrony). Future research could use dynamic measures of synchrony to elucidate specific characteristics of dyadic behavior that are related to physiological functioning and the development of self-regulation. Third, synchrony in the normal play was not correlated with infants’ or mothers’ physiology, consistent with prior work suggesting that links between individual physiology and dyadic behavior are most apparent in the context of infant distress when dyads are working towards the joint goal of regaining mutually positive interactions (Conradt & Ablow, 2010; Leerkes et al., 2016). Because of this, we only examined behavioral synchrony in the reunion. Future work should examine dyadic regulation during a wider range of contexts and using a higher-risk samples, both of which may increase variability in dyadic behavior. Finally, future studies will need to employ longitudinal designs to investigate when and how individual physiology supports dyadic behavior versus when dyadic behavior shapes individuals’ physiological functioning.
Conclusions
SNS and PNS functioning are thought at once to facilitate and be shaped by dyadic behavior, particularly between infants and their caregivers (Armony & Vuilleumier, 2013; Porges, 2007). However, the focus in existing research on behavioral and PNS responses during infant-mother social interactions has limited understanding of the unique functioning of and relations between the regulatory systems involved (i.e., SNS, PNS, and dyadic behavior). Therefore, in the current study, we examined infants’ and mothers’ SNS and PNS functioning and behavioral synchrony during an infant-mother interaction. Infants exhibiting lower PNS tone may have required greater behavioral synchrony from their mothers to support their regulation. In mothers, relatively high PNS tone (indicating capacity for PNS regulation) concurrent with low autonomic arousal may facilitate maintenance of a synchronous interaction in the face of actual or anticipated infant distress. In this way, behavioral synchrony may reflect a call and response between infants and mothers composed of each individual’s physiological needs and capacities.
Acknowledgements
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant (R01 HD 27592) awarded to J.A. DiPietro and National Science Foundation Graduate Research Fellowships (No. DGE1255832) to A. Busuito and to K.M. Quigley. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors’ and do not necessarily reflect the views of the National Science Foundation. We thank the dedication and generosity of our study families without whom this work would not be possible.
Contributor Information
Alex Busuito, The Pennsylvania State University.
Kelsey M. Quigley, The Pennsylvania State University
Ginger A. Moore, The Pennsylvania State University
Kristin M. Voegtline, Johns Hopkins School of Medicine
Janet A. DiPietro, Johns Hopkins Bloomberg School of Public Health
References
- Armony J, & Vuilleumier P (Eds.). (2013). The Cambridge handbook of human affective neuroscience Cambridge university press. [Google Scholar]
- Alkon A, Boyce WT, Tran L, Harley KG, Neuhaus J, & Eskenazi B (2014). Prenatal adversities and Latino children’s autonomic nervous system reactivity trajectories from 6 months to 5 years of age. PloS one, 9(1), e86283 10.1371/journal.pone.0086283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, & Fox NA (2000). Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental psychobiology, 37(1), 44–56. [DOI] [PubMed] [Google Scholar]
- Bazhenova OV, Plonskaia O, & Porges SW (2001). Vagal reactivity and affective adjustment in infants during interaction challenges. Child Development, 72(5), 1314–1326. [DOI] [PubMed] [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and psychopathology, 13(2), 183–214. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1991). Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological review, 98(4), 459. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, & Suess PE (2000). Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology, 36(1), 54. [PubMed] [Google Scholar]
- Busuito A, & Moore GA (2017). Dyadic flexibility mediates the relation between parent conflict and infants’ vagal reactivity during the Face-to-Face Still-Face. Developmental psychobiology, 59(4), 449–459. 10.1002/dev.21508 [DOI] [PubMed] [Google Scholar]
- Cohn JF, & Tronick EZ (1988). Mother-infant face-to-face interaction: Influence is bidirectional and unrelated to periodic cycles in either partner’s behavior. Developmental psychology, 24(3), 386. [Google Scholar]
- Conradt E, & Ablow J (2010). Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development, 33(3), 251–265. 10.1016/j.infbeh.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Davis M, Bilms J, & Suveg C (2017). In sync and in control: A meta-analysis of parent–child positive behavioral synchrony and youth self-regulation. Family process, 56(4), 962–980. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RH, Zavaleta N, & Gurewitsch ED (2004). Fetal neurobehavioral development: a tale of two cities. Developmental psychology, 40(3), 445. [DOI] [PubMed] [Google Scholar]
- Enlow MB, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T, & Wright RJ (2014). Maternal sensitivity and infant autonomic and endocrine stress responses. Early human development, 90(7), 377–385. 10.1016/j.earlhumdev.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2007). On the origins of background emotions: From affect synchrony to symbolic expression. Emotion, 7(3), 601. [DOI] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, & Yirmiya N (1999). Mother–infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology, 35(1), 223–231. 10.1037/0012-1649.35.1.223 [DOI] [PubMed] [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, & Louzoun Y (2011). Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development, 34(4), 569–577. 10.1016/j.infbeh.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Fowles DC (2007). 10 The Measurement of Electrodermal Activity in Children. Developmental psychophysiology: Theory, systems, and methods, 286. [Google Scholar]
- Geisler FC, Kubiak T, Siewert K, & Weber H (2013). Cardiac vagal tone is associated with social engagement and self-regulation. Biological psychology, 93(2), 279–286. 10.1016/j.biopsycho.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Gunning M, Halligan SL, & Murray L (2013). Contributions of maternal and infant factors to infant responding to the Still Face paradigm: A longitudinal study. Infant Behavior and Development, 36(3), 319–328. 10.1016/j.infbeh.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Ham J, & Tronick ED (2006). Infant Resilience to the Stress of the Still-Face. Annals of the New York Academy of Sciences, 1094(1), 297–302. [DOI] [PubMed] [Google Scholar]
- Ham J, & Tronick E (2009). Relational psychophysiology: Lessons from mother–infant physiology research on dyadically expanded states of consciousness. Psychotherapy Research, 19(6), 619–632. 10.1080/10503300802609672 [DOI] [PubMed] [Google Scholar]
- Hill LK, Hu DD, Koenig J, Sollers JJ III, Kapuku G, Wang X, … & Thayer JF (2015). Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosomatic medicine, 77(1), 16 10.1097/PSY.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB (1982). Antecedents of self-regulation: a developmental perspective. Developmental psychology, 18(2), 199. [Google Scholar]
- Korkushko OV, Shatilo VB, Plachinda YI, & Shatilo TV (1991). Autonomic control of cardiac chronotropic function in man as a function of age: assessment by power spectral analysis of heart rate variability. Journal of the autonomic nervous system, 32(3), 191–198. [DOI] [PubMed] [Google Scholar]
- Leerkes EM, Su J, Calkins SD, Supple AJ, & O’brien M (2016). Pathways by which mothers’ physiological arousal and regulation while caregiving predict sensitivity to infant distress. Journal of Family Psychology, 30(7), 769 10.1037/fam0000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Supple AJ, O’Brien M, Calkins SD, Haltigan JD, Wong MS, & Fortuna K (2015). Antecedents of maternal sensitivity during distressing tasks: Integrating attachment, social information processing, and psychobiological perspectives. Child development, 86(1), 94–111. 10.1111/cdev.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey EW, & Caldera YM (2015). Shared affect and dyadic synchrony among secure and insecure parent–toddler dyads. Infant and Child Development, 24(4), 394–413. [Google Scholar]
- Lindsey EW, Cremeens PR, Colwell MJ, & Caldera YM (2009). The structure of parent–child dyadic synchrony in toddlerhood and children’s communication competence and Self-control. Social Development, 18(2), 375–396. [Google Scholar]
- Lundy BL (2002). Paternal socio-psychological factors and infant attachment: The mediating role of synchrony in father–infant interactions. Infant Behavior and Development, 25(2), 221–236. [Google Scholar]
- Matias R, Cohn JF, & Ross S (1989). A comparison of two systems that code infant affective expression. Developmental Psychology, 25(4), 483. [Google Scholar]
- Mattson WI, Ekas NV, Lambert B, Tronick E, Lester BM, & Messinger DS (2013). Emotional expression and heart rate in high-risk infants during the face-to-face/still-face. Infant Behavior and Development, 36(4), 776–785. 10.1016/j.infbeh.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesman J, van IJzendoorn MH, & Bakermans-Kranenburg MJ (2009). The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review, 29(2), 120–162. 10.1016/j.dr.2009.02.001 [DOI] [Google Scholar]
- Mills-Koonce WR, Gariépy JL, Propper C, Sutton K, Calkins S, Moore G, & Cox M (2007). Infant and parent factors associated with early maternal sensitivity: A caregiver-attachment systems approach. Infant Behavior and Development, 30(1), 114–126. 10.1016/j.infbeh.2006.11.010 [DOI] [PubMed] [Google Scholar]
- Moore GA, & Calkins SD (2004). Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology, 40(6), 1068. [DOI] [PubMed] [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, & Cox MJ (2009). Mother–infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development, 80(1), 209–223. [DOI] [PubMed] [Google Scholar]
- Moore GA, Quigley KM, Voegtline KM, & DiPietro JA (2016). Don’t worry, be (moderately) happy: Mothers’ anxiety and positivity during pregnancy independently predict lower mother–infant synchrony. Infant Behavior and Development, 42, 60–68. 10.1111/j.1467-8624.2008.01255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer JE, Measelle JR, Laurent HK, & Ablow JC (2013). Mothers’ vagal regulation during the Still-Face Paradigm: Normative reactivity and impact of depression symptoms. Infant Behavior and Development, 36(2), 255–267. 10.1016/j.infbeh.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological psychology, 74(2), 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, & Furman SA (2011). The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant and child development, 20(1), 106–118. 10.1002/icd.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzi L, Casini E, De Simone P, Reni G, Borgatti R, & Montirosso R (2015). Mother–infant dyadic reparation and individual differences in vagal tone affect 4-month-old infants’ social stress regulation. Journal of experimental child psychology, 140, 158–170. 10.1016/j.jecp.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Quigley KM, Moore GA, Propper CB, Goldman BD, & Cox MJ (2017). Vagal regulation in breastfeeding infants and their mothers. Child development, 88(3), 919–933. 10.1111/cdev.12641 [DOI] [PubMed] [Google Scholar]
- Shahrestani S, Stewart EM, Quintana DS, Hickie IB, & Guastella AJ (2014). Heart rate variability during social interactions in children with and without psychopathology: A meta-analysis. Journal of Child Psychology and Psychiatry, 55(9), 981–989. 10.1111/jcpp.12226 [DOI] [PubMed] [Google Scholar]
- Stewart AM, Lewis GF, Heilman KJ, Davila MI, Coleman DD, Aylward SA, & Porges SW (2013). The covariation of acoustic features of infant cries and autonomic state. Physiology & behavior, 120, 203–210. 10.1016/j.physbeh.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurland J, van der Heijden KB, Smaling HJ, Huijbregts SC, van Goozen SH, & Swaab H (2017). Infant autonomic nervous system response and recovery: Associations with maternal risk status and infant emotion regulation. Development and psychopathology, 29(3), 759–773. 10.1017/S0954579416000456 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Brosschot JF (2005). Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology, 30(10), 1050–1058. [DOI] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, & Brazelton TB (1978). The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child psychiatry, 17(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Tronick EZ (1980). On the primacy of social skills. The exceptional infant, 4, 144–158. [Google Scholar]
- Tronick EZ (1989). Emotions and emotional communication in infants. American psychologist, 44(2), 112. [DOI] [PubMed] [Google Scholar]
- Weinberg MK, & Tronick EZ (1994). Beyond the face: An empirical study of infant affective configurations of facial, vocal, gestural, and regulatory behaviors. Child development, 65(5), 1503–1515. [DOI] [PubMed] [Google Scholar]


