Abstract
Objectives:
This study sought to determine whether salt-induced angiotensin II suppression contributes to impaired cerebral blood flow (CBF) autoregulation.
Methods:
Cerebral autoregulation was evaluated with laser-Doppler flowmetry during graded reductions of blood pressure. Autoregulatory responses in rats fed high salt (HS; 4% NaCl) diet vs low salt (LS; 0.4% NaCl) diet were analyzed using linear regression analysis, model-free analysis, and a mechanistic theoretical model of blood flow through cerebral arterioles.
Results:
Autoregulation was intact in LS-fed animals as mean arterial pressure (MAP) was reduced via graded hemorrhage to approximately 50 mmHg. Short term (3 days) and chronic (4 weeks) HS diet impaired CBF autoregulation, as evidenced by progressive reductions of laser-Doppler flux with arterial pressure reduction. Chronic low dose angiotensin II infusion (5 mg/kg/min, i.v.) restored CBF autoregulation between the pre-hemorrhage MAP and 50 mm Hg in rats fed short term HS diet. Mechanistic-based model analysis showed a reduced myogenic response and reduced baseline vascular smooth muscle tone with short-term HS diet, which was restored by angiotensin II infusion.
Conclusions:
Short term and chronic HS diet lead to impaired autoregulation in the cerebral circulation, with salt-induced ANG II suppression as a major factor in the initiation of impaired CBF regulation.
Keywords: Autoregulation, Salt, Angiotensin II, Cerebral Blood Flow, Hemorrhage
INTRODUCTION
Hypertension is a major health concern in the United States and throughout the world, and exposure to a high salt diet is a well-recognized risk factor for hypertension (34, 72). It is well-documented that high blood pressure impairs physiologic vascular reactivity in humans (5, 16, 66) and both impaired microvessel relaxation (17, 45, 53) and an elevated sensitivity to pressor stimuli (3, 26, 27, 41) contribute to the pathophysiology of hypertension in humans and in experimental animal models.
Exposure to a high salt diet is known to induce hypertension, especially in salt sensitive individuals and in a disproportionate number of individuals of African American descent (11). Humans who are salt sensitive exhibit a higher mortality rate than salt-insensitive counterparts, even if they do not develop hypertension (71, 73), and may experience other adverse effects on the cardiovascular system and other organs (44).
In addition to its role in the development of many forms of hypertension, it is now known that high salt (HS) diet can lead to impaired vascular relaxation in the absence of an increase in blood pressure. For example, impaired vascular relaxation in response to a variety of endothelium-dependent (and –independent) vasodilator agonists has been demonstrated in arteries and arterioles of normotensive animals fed a HS diet (19, 40, 42, 46, 54, 69, 70, 78). Human subjects that are salt-insensitive for blood pressure (23); and healthy young humans with a short term (5 days) exposure to moderate increases in dietary salt intake (8, 65) also exhibit impaired endothelial function with elevated dietary salt intake.
In HS fed animals, impaired relaxation of multiple arteries (and arterioles) is accompanied by reduced NO levels and increased vascular oxidative stress (37, 39, 54, 76–78). Paradoxically, this increase in vascular oxidative stress and endothelial dysfunction in HS-fed animals is due to salt-induced ANG II suppression, as chronic i.v. infusion of a low dose of ANG II (to restore normal plasma ANG II levels) (24, 76) restores endothelium dependent vasodilator responses, increases vascular NO levels, and reduces vascular oxidative stress in HS-fed animals (46, 47, 54, 69, 70, 76, 77). Even more surprising, the protective effect of low dose ANG II infusion in HS-fed animals is mediated via activation of the AT1 receptor as it is eliminated by the AT1 receptor blocker losartan and is unaffected by AT2 receptor blockade with PD-123319. This protective effect of low dose ANG II infusion in HS-fed animals appears to be due to activation of antioxidant defenses controlled by the master antioxidant and cell protective transcription factor nuclear factor (erythroid-derived 2)-like-2 (NRF2), as it is absent in NRF2 knockout rats fed HS diet (55). In contrast to the effect of low dose ANG II infusion, infusion of a higher dose of ANG II increases vascular oxidative stress in mesenteric resistance arteries of Sprague-Dawley (S-D) rats fed HS diet (77), as expected from the well-known ability of ANG II to increase superoxide production by NADPH oxidase via activation of the ANG II AT1 receptor. However, infusion of the same low dose ANG II that ameliorates endothelial dysfunction in HS fed rats eliminates acetylcholine (ACh)-induced dilation in middle cerebral arteries of S-D rats fed normal salt diet (K. Fink and J.H. Lombard, unpublished).
Under normal conditions, the cerebral vasculature can tolerate large changes in arterial blood pressure via local autoregulatory mechanisms that dilate arterioles and resistance arteries during periods of diminished blood pressure and constrict the arteries to maintain constant blood flow when arterial pressure is elevated (15, 51). However, in contrast to the extensive research investigating endothelial function and responses to vasoactive agonists with HS diet, much less is known regarding the impact of elevated dietary salt intake on autoregulatory mechanisms that maintain local blood flow constant during changes in arterial blood pressure. This is especially true in the cerebral circulation. The minimal blood pressure needed to preserve autoregulation of brain blood flow, frequently termed the “lower limit of autoregulation” (LLA), is the arterial blood pressure at which cerebral blood flow decreases significantly from resting values (2, 61) and then falls linearly with reduced pressure (49). In general, this is the blood pressure at which the cerebral circulation is incapable of increasing blood flow via relaxation of cerebral resistance vessels. Based on previous observations showing that HS diet impairs vascular relaxation mechanisms mediated via NO and ACh in the salt-insensitive Sprague-Dawley rat (40, 42), we hypothesized that HS diet would impair the ability of the cerebral circulation to maintain blood flow via autoregulatory vasodilation during hemorrhage-induced reductions in arterial blood pressure, and would shift the lower limit of autoregulation of cerebral blood flow to a higher blood pressure in these animals. Such a reduction in autoregulatory ability could have profoundly adverse effects, e.g. increasing the risk and severity of ischemic injury following myocardial infarction or ischemic stroke. (62, 63)
In the present study, we utilized the thinned-skull laser Doppler flowmetry (LDF) technique (22, 28) to determine the effect of chronic HS diet on cerebral autoregulation, as assessed by the ability of the cerebral circulation to maintain a constant tissue blood flow during graded reductions in perfusion pressure produced via successive systemic blood volume withdrawals. We also conducted studies to determine whether short term HS diet (3–5 days), which leads to vascular oxidant stress (54, 76, 78) and impaired vascular relaxation in response to multiple endothelium-dependent and-independent vasodilator agonists in several different animal models (19, 40, 42, 46, 54, 69, 70, 78), also impairs cerebral vascular autoregulation; and whether this may be related to salt-induced ANG II suppression. In the latter studies, we hypothesized that any impairment in the autoregulation of blood flow during exposure to HS diet could be prevented by chronic infusion of a low dose of ANG II to prevent salt-induced ANG II suppression (14, 69, 70, 76). Both a model-free and a mechanistic-based theoretical model were used to analyze LDF values in the low salt (LS) control, short-term HS (ST-HS), short-term HS with saline infusion (ST-HS + saline) and short-term HS with ANG II infusion (ST-HS + ANG II infusion), to understand the observed responses.
MATERIALS AND METHODS
Separate groups of 9–11 week-old Sprague-Dawley rats were placed on either a low salt diet (0.4% NaCl/Dyets) or a high salt diet (4% NaCl/Dyets) with water to drink ad libitum. Our initial studies sought to determine whether chronic high salt diet (30 days) would lead to impaired cerebral vascular autoregulation (see below). Because short-term exposure to high salt diet increases vascular oxidant stress (54, 77, 78) and leads to a surprisingly rapid impairment of vascular relaxation mechanisms in rats (40, 42, 46, 69, 70), hamsters (54), and healthy human volunteers (8, 65), we conducted a second series of studies to determine whether short-term (3 days) high salt diet would also impair cerebral autoregulation; and, if so, whether prevention of salt-induced angiotensin II suppression (76) would restore cerebrovascular autoregulation in these animals. All experiments were approved prior to the initiation of the study by the Institutional Animal Care and Use Committee and all surgeries were performed using either aseptic (survival surgery) or clean (non-survival surgery) surgical techniques in a designated surgical area.
Laser Doppler Flowmetry Measurements:
The cerebral blood flow experiments utilized a previously-described thinned-skull laser Doppler flowmetry (LDF) protocol (21, 22, 28, 75). After the animal was anesthetized (see below) and placed in the stereotaxic apparatus (Stoelting Co.), a dorsal midline incision was made longitudinally at the top of the skull. Connective tissue was removed with fine forceps and scissors, and the exposed parietal bone was thinned to translucency over the somatosensory cortex just caudal to the bregma using a dissecting microscope and a high-speed drill and dental burr attachment. Room temperature saline was applied liberally during the drilling procedure to prevent heat buildup from damaging the underlying microvessels. Residual bleeding was quickly resolved with pressure and, if necessary, Gelfoam gelatin absorbent material (Johnson & Johnson). Any animal that bled excessively or had the skull penetrated during drilling was immediately excluded from the experiment. A heating pad supplied by a circulating warm water pump (T-Pump, Gaymar Industries) was placed under the rat to maintain the animal’s body temperature at 37°C during all procedures performed in the stereotaxic apparatus.
After thinning the skull to translucency, a laser probe was positioned over the exposed cerebral microcirculation, using microscopic guidance to avoid placing the probe over any large vessels. Because LDF measurements reflect the number and velocity of moving particles (i.e., RBC) under a specific set of conditions (rather than absolute blood flow), a micromanipulator was used to fix the position of the LDF probe and insure that no movement of the probe occurred during the experiment.
To assure that microvessel flow was being adequately tracked, the animal was briefly ventilated (~ 3 minutes) with a hypercapnic gas mixture (5%CO2, 21%O2, balance N2), which raises the expired end tidal CO2 (ETCO2) to ~60 mmHg. During this preliminary evaluation, blood flow, as assessed by the laser Doppler probe, increased by ~50%−150% if the probe was placed over a suitable area of the cerebral microcirculation. A transient decrease in laser Doppler flow reading during this procedure indicated that the probe may have inadvertently been placed over a large blood vessel or vein. If this occurred, the probe was repositioned, the same steps were repeated, and blood flow was allowed to return to baseline values.
Hemorrhage Protocol—Long Term Studies:
For the long term studies, groups of male Sprague-Dawley rats were placed on diets consisting of either high salt (4.0% NaCl; Dyets, HS) or low salt (0.4% NaCl; Dyets, LS) for 30 days with water to drink ad libitum. All groups were age-matched at 12–13 weeks of age at the time of the experiment. In the long-term experiments, animals were anesthetized initially with 50 mg/kg i.p. sodium pentobarbital. A digital scale was used to assess the mass of each animal, and the recorded mass was used to calculate intravenous infusion rate of supplemental sodium pentobarbital anesthesia (approximately 10 mg·kg−1·hour−1) via a femoral vein throughout the experiment, after the initial letdown period. Accurate and stable intravenous (i.v.) infusion was achieved using a digitally-programmable syringe pump (Harvard Apparatus), and the anesthetic (Nembutal) was diluted to 50% strength with 0.9% sterile isotonic saline solution to maintain patency of the i.v. infusion line.
Polyethylene cannulas (PE 50) were inserted into both femoral arteries to permit recording of systemic arterial blood pressure from one cannula and blood volume withdrawal from the other. The trachea was exposed to insert and secure a PE 240 catheter to maintain a patent airway. After the rat had been placed in a stereotaxic apparatus, the airway tube was securely attached to a small animal ventilator and CO2 analyzer (Harvard Apparatus) that was used to ventilate the animal. A carbon dioxide monitor (CAPSTAR-100) was connected in conjunction with the inhaled gas mixture and mechanical ventilator, which allowed the respiratory rate and inspiratory volume to be monitored and continually adjusted so that the expired end tidal CO2 was maintained at 35 mmHg throughout the procedure.
After preparation for LDF measurements, the animals were allowed approximately 45 minutes to equilibrate after placement of the laser probe; and a 5-minute period was then recorded as a resting control for all values. After the control period, the animals were bled through one femoral artery catheter to decrease systemic blood pressure by 10 mmHg increments, as measured by a catheter in the other femoral artery. Blood was withdrawn every five minutes until a mean arterial pressure of approximately 20 mmHg was reached, at which time the animal was euthanized by an anesthetic overdose and a bilateral pneumothorax.
Because previous studies (35, 43) performed in other laboratories have demonstrated a significant decrease of cerebral blood flow autoregulation occurring at a systemic arterial blood pressure of approximately 50 mmHg under similar experimental conditions in normotensive animals, we added an additional five-minute recording period at a mean arterial pressure of 55 mmHg to define the lower limit of autoregulation (LLA) between our normotensive experimental groups on chronic LS and HS diet more precisely. This additional recording period was added to increase the resolution of our recordings at 55 mm Hg, where we hypothesized a significant difference may occur between animals fed low salt vs. high salt diet.
Hemorrhage Protocol-Short Term Studies:
In the short-term studies, rats were maintained on either a low salt (LS; 0.4% NaCl/Dyets) diet or switched to a high salt (HS; 4% NaCl/Dyets) diet for 3–5 days prior to the experiment. The short-term studies included two additional groups of age-matched Sprague-Dawley rats that were fitted with chronic catheters, housed individually in the chronic monitoring facility, and allowed a 2–3 day recovery period. Following the recovery period, the animals were placed on a high salt diet (4% NaCl/Dyets) and the saline infusion was continued for an additional three days. At this point, the animals were divided into 2 groups with one group continuing the saline infusion while the second group was infused with ANG II (5 ng/kg/min) at the same rate for three more days to prevent salt-induced ANG II suppression (14, 69, 70, 76), after which the animals were anesthetized for the acute studies of cerebral autoregulation.
For the short-term studies, the animals were weighed and then anesthetized with isoflurane by first placing them in an induction chamber through which 5% isoflurane was administered along with a 30% O2 (balance N2) gas mixture. Once the animal was in a deep plane of anesthesia, it was removed from the induction chamber, placed on a surgical table warmed to 37° C, and fitted with a nose cone that delivered a 3% isoflurane concentration with the same supplemental oxygen mixture used for the preparatory surgery (cannulation of a femoral artery and insertion of a tracheal cannula).
A precision-valve flowmeter (Aalborg Instruments & Controls, Inc.) connected in series with the inhalational gas mixture and mechanical ventilator attached to the tracheal cannula allowed precise control of minute ventilation. Inspiratory rate and minute ventilatory volume were monitored and carefully adjusted in real time to maintain the expired end tidal CO2 (ETCO2), an estimate of arterial pCO2, which is a crucial determinant of cerebral blood flow, at a constant value of approximately 35 mmHg. Supplemental oxygen (30% O2, balance N2) was provided in the inhalational gas, as described previously (40).
After the probe was correctly placed over the microvasculature, the animal was allowed to equilibrate for 45 minutes after exposure to hypercapnic air and prior to the hemorrhage experiments. Mean arterial pressure (MAP) (mmHg) was measured with a pressure transducer that was calibrated immediately prior to each use, and baseline laser Doppler readings (LDF) were recorded using a Windaq data acquisition system. Baseline readings were recorded for two minutes followed by successive 1.5 ml blood withdrawals to reduce arterial pressure via a femoral artery catheter that was also used to monitor blood pressure. Use of a single catheter in these experiments was necessary because scarring from placement of the chronic venous line prevented access to the femoral artery. Following a two-minute acclimation period, measurements were recorded every 30 seconds for two minutes. This process was repeated until the animal reached a MAP of 20 mmHg or less.
Computational Methods:
A model free analysis was performed to see if an average fit to the normalized flow versus pressure data can show differences between the four groups from this study, LS, ST-HS, ST-HS + ANG II and ST-HS + saline. The model free expression used to fit the lower region of the autoregulation curve is a simple polynomial of the form:
The least squares error fit is performed in Matlab (MathWorks Inc, Natick, MA) using the polyfit function. The ST-HS and LS groups are compared first and then the ST-HS + ANG II and ST-HS + saline infusion groups are compared.
In addition to the model-free analysis, a second analysis was performed using a mechanistic computational model of the cerebral vasculature. This computational model of cerebral blood flow regulation was developed based on models of Spronck et al. (60) and Carlson and Secomb (7). As with the model free analysis, the short-term HS versus control experimental groups are analyzed separately from the short-term HS with ANG II infusion versus short-term HS with saline infusion groups. The mechanistic cerebral vasculature model is represented in the schematic shown in Figure 1 and the model equations are given in the Supplement in full detail along with definitions of all the variables and parameters used in this model.
Figure 1:
Schematic representation of the mechanistic model of cerebral blood flow used to analyze the control and short-term high salt groups and the short-term high salt with saline infusion and short-term high salt with ANG II infusion groups. PA is the input arterial pressure, RAB is the resistance to flow of the cerebral arteries, CAB is the capacitance of the cerebral arteries, RATLB is the varying resistance to flow in the cerebral arterioles, CVB is the capacitance of the cerebral veins, RVB is the resistance to flow in the cerebral veins, and PVC is the pressure in the vena cava.
In the short-term perturbations, we hypothesize that any network and vessel structural changes do not have sufficient time to occur so that the changes in autoregulatory function will be reflected solely by changes in active vasoregulation governed by endothelial and vascular smooth muscle function. Because of this, parameters in the model that relate to the vascular passive response and some elements of the active vascular smooth muscle response can be held constant at values optimized for all eleven animals in the LS and short term high salt (ST-HS) groups and then separately for the ten animals in the ST-HS + saline and ST-HS + ANG II groups. What is thought to change between groups in the two analyses is the sensitivity of the cerebral regulatory vessels to vessel wall stress and baseline vascular tone. These parameters are optimized to fit the autoregulatory data of flow versus pressure in a two-step process. First the LS and the ST-HS groups (or the ST-HS + saline and the ST-HS + ANG II groups) are fit together with one set of parameters defining the vascular smooth muscle (VSM) strength and length dependence (Cact0, Cact1 and Cact2) and either 11 (LS and ST-HS) or 10 (ST-HS + saline and ST-HS + ANG II) sets of parameters defining the baseline myogenic activation and sensitivity of the VSM to stress (Ctone0, Ctone1) for each animal. The optimization is then performed to fit each individual dataset again with Cact0, Cact1 and Cact2 fixed at their values from the first optimization and only Ctone0 and Ctone1 allowed to vary for each animal. This ensures the best possible fit to the data for each animal. All parameters for the passive response of the cerebral vessel compartment are fixed to fit control data from a previous set of S-D rats. The fixed parameters used in the analysis and the adjustable parameters along with their ranges are given in Table 1.
Table 1.
Fixed parameter values and adjustable parameters with their ranges used in the mechanistic model analysis of control, short-term HS, short-term HS with saline infusion and short-term HS with ANG II infusion.
| Fixed Parameters | |||
| Parameter Name | Description | Value | Units |
| RAB | Cerebral arterial resistance to flow | 500 | (mmHg*s)/mL |
| RVB | Cerebral venous resistance to flow | 1700 | (mmHg*s)/mL |
| RAtlB,0 | Cerebral arteriolar reference resistance to flow | 2500 | (mmHg*s)/mL |
| CAB | Cerebral arterial vasculature capacitance | 0.0105 | mL/mmHg |
| CVB | Cerebral venous vasculature capacitance | 0.0500 | mL/mmHg |
| AENcol | Arteriolar non-collagen content-stiffness product | 2.01e3 | mmHg |
| AECol | Arteriolar collagen content-stiffness product | 8.77e6 | mmHg |
| α | Arteriolar collagen distribution characteristic strain | 1.24 | unitless |
| γ | Arteriolar collagen distribution shape factor | 7.11 | unitless |
| DAtl,0 | Arteriolar reference diameter | 189.8 | μm |
| AWall | Arteriolar circumferential wall area | 8405 | μm2 |
| TDAtl | Arteriolar diameter time constant | 1 | s |
| TAAtl | Arteriolar activation time constant | 15 | s |
| Adjustable Parameters | |||
| Parameter Name | Description | Range | Units |
| CAct0 | Arteriolar strength of smooth muscle | 1–10 | mmHg*μm |
| CActl | Arteriolar position of peak tension generation | 0.5–1.3 | unitless |
| CAct2 | Arteriolar width of smooth muscle diameter-tension curve | 0.1–1.0 | unitless |
| CTone0 | Arteriolar smooth muscle activation sensitivity to stress | 0–25 | 1/(mmHg*μm) |
| CTonel | Arteriolar smooth muscle activation baseline tone factor | 0–25 | unitless |
Statistical Analysis:
Data was recorded for later analysis by automated data acquisition systems (WINDAQ & BIOPAC systems) connected to a personal computer. Recorded parameters were available for arterial blood pressure, expired pCO2, ventilation rate in breaths per minute, and laser Doppler blood flow signal in blood perfusion units (BPU). Baseline LDF readings obtained after the animal was euthanized were also evaluated to eliminate the influence of any non-specific LDF signal influencing measured flow rates. In our data analysis, average baseline LDF readings were determined to be <5% of recorded blood flow signals obtained at 100 mmHg of systemic arterial blood pressure.
Linear regression analysis was used to evaluate the correlation between all the LDF values within an experimental group and their corresponding arterial pressure. Slopes of the LDF vs. arterial pressure relationship were calculated for individual animals and summarized as mean ±SEM for the animals in that group. All other data were summarized mean ± SEM. Statistical analysis was performed using 1-way analysis of variance (ANOVA), with a Student Newman-Keuls (SNK) post-hoc analysis and an unpaired Student’s t-test, where appropriate. Differences were judged to be significant at P<0.05.
RESULTS
Effect of Long-Term HS Diet on Cerebral Blood Flow Autoregulation:
Calculation of the slope of cerebral autoregulation is a frequently-used measure of the ability of the microvasculature to maintain constant blood flow during variations in systemic arterial pressure (29, 56). In these studies, we initially determined the effect of stepwise reductions in arterial blood pressure on microvascular blood flow, as assessed by laser Doppler flowmetry (LDF) in the cerebral circulation of Sprague-Dawley (S-D) rats fed long term (4 weeks) HS diet. When linear regression analysis was performed on cerebral blood flow between arterial pressures of 40 and 100 mmHg, rats fed long term HS diet exhibited a significantly greater negative slope of the flow vs. pressure relationship (−0.61±0.1, n=7) compared to controls maintained on LS diet (0.31 ±0.09, n=7) (P<0.05). Cerebral blood flow in HS-fed rats was also significantly lower than the pre-hemorrhage value at an arterial pressure of 60 mmHg, compared to approximately 40 mmHg in low-salt fed rats—indicative of a shift in the lower limit of autoregulation to a higher arterial pressure. Taken together, these observations are consistent with an impaired ability of the cerebral circulation to regulate its blood flow following chronic HS exposure.
Effect of Short-Term HS Diet (± ANG II Infusion) on Cerebral Blood Flow Autoregulation:
In the short-term experiments, animals fed LS diet (representative animal shown in Figure 2A) maintained cerebral blood flow from the pre-hemorrhage value of MAP down to 50 mmHg MAP. In those experiments, LDF values were not significantly correlated with MAP between the pre-hemorrhage arterial pressure and a MAP of 50 mmHg (r=0.0283, n=26), indicating that autoregulation of cerebral blood flow is intact over this pressure range. However, at pressures ≤50 mmHg, LDF fell progressively with each drop in arterial pressure and was significantly correlated with MAP (r=0.6079, n=25; P<0.01), as expected for blood flow responses below the lower limit of autoregulation. By contrast, LDF in the HS group (representative animal shown in Figure 2B) was significantly correlated with arterial pressure in the pressure ranges both above (r=0.6776, n=28, P<0.01) and below (r=0.8231, n=37; P<0.01) 50 mmHg, indicating that short term exposure to HS diet leads to a rapid impairment of cerebral blood flow autoregulation. Mean slopes of the pressure/flow relationship from individual rats between the pre-hemorrhage value and 50 mmHg MAP were significantly greater in the HS-fed rats (−0.8±0.1, n=6) than in the LS-fed rats (0.33±0.39, n=5). Slopes of the pressure/flow relationship below 50 mm Hg in HS-fed rats (−1.79±0.13, n=5) and LS-fed rats (−1.82±0.38, n=5) were significantly greater than those in the range from pre-hemorrhage MAP to 50 mmHg but were not significantly different from each other.
Figure 2:
Representative blood pressure and cerebral blood flow response to serial blood withdrawals for control animals on a low salt diet (A) and a short-term (3 day) high salt diet (B). Each measurement is averaged using a moving window to see the trends in response of pressure and flow. In addition, during each of the blood withdrawals the pressure catheter was disconnected to allow blood to be withdrawn, so the periods during the actual blood withdrawal have been removed from the time course. Note that flow and pressure for the control animal (LS diet) does not change much over the first three blood withdrawals compared with changes observed in the short-term high salt animal. In addition, the control animal showed only ~ 25% drop in flow compared to ~ 75% drop in the short-term high salt animal over the course of the experiment. The shorter duration of the experimental period in the HS-fed rat vs. the LS-fed control reflects the reduced ability of the HS animal to compensate for the reduction in blood pressure following blood volume withdrawal.
Computational Analysis of Cerebral Autoregulatory Responses in Rats Fed LS Diet vs. Short Term High Salt (ST-HS) Diet:
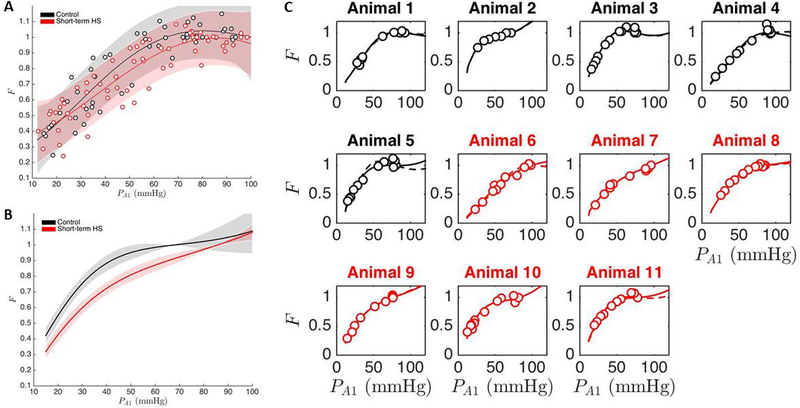
Figure 3 summarizes the computational analysis of cerebral vascular autoregulation in rats fed low salt diet vs. short term HS diet. The model free polynomial fit to each group is shown in Figure 3A while Figure 3B shows the predicted average response of each group along with the predicted confidence intervals. The predicted average response and confidence intervals are calculated using the Matlab nonlinear fit function, nlinfit, and the associated predicted confidence interval function, nlpredci. These two functions consider all of the optimized simulations in each group to generate an average response and the predicted confidence interval for each group. The optimized simulations of individual animal experiments are presented in Figure 3C and show that simulations of individual LS control rats predict a more distinct autoregulatory plateau than the simulations of the individual ST-HS rats, again consistent with a rapid onset of impaired cerebral blood flow regulation following short term exposure to high salt diet.
Figure 3:
Computational analysis of cerebral vascular autoregulation in rats fed low salt diet vs. short term HS diet. Panel A: Model free polynomial fit to each group. Panel B: Predicted average response of each group along with the predicted confidence intervals. Panel C: Optimized simulations of individual animal experiments showing that simulations of individual LS control rats predict a more distinct autoregulatory plateau than the simulations of the individual ST-HS rats, consistent with a rapid onset of impaired cerebral blood flow regulation following short term exposure to high salt diet (See text for details).
Earlier studies by Frisbee et al. (18) showed that HS diet does not change the structural properties or passive mechanical properties of skeletal muscle arterioles of Sprague-Dawley rats. Based on those observations, we used the modified Spronck et al. model (60) to fit the data using the argument that the short-term high salt treatment did not change the structural properties of the vessel so parameters associated with structural features (Cact0 - number and strength of SMCs; Cact1 - relationship between passive and active vessel wall components in terms of stress generation; and Cact2 - range of VSM force generation as a function of strain) were optimized across all groups. Parameters associated with sensitivity to stress and baseline VSM tone (Ctone0 and Ctone1, respectively) were allowed to vary from individual to individual. The results of those calculations, summarized in Table 2, showed that Ctone0 is just below the 95% confidence level on the p-value where Ctone1 was significantly different between the two groups at 95% confidence.
Table 2.
Model parameters for LS and STHS. Ctone0 and Ctonel indicate the sensitivity of the vessels to vessel wall strain as induced by pressure. Large values of Ctone0 indicate a higher sensitivity of the vessel to stress and larger values of Ctone1 indicate a higher basal tone in the vessel.
| LS | ST HIGH SALT | P VALUE | |
|---|---|---|---|
| Ctone0 | 6.56 ± 1.23 | 4.68 ± 1.61 | 0.0616 |
| Ctone1 | 5.37 ± 1.13 | 3.56 ± 0.65 | 0.0087 |
ANG II Infusion Experiments:
Similar to noninfused rats fed HS diet, cerebral autoregulation was impaired in short-term HS rats infused with the isotonic saline vehicle for ANG II (representative animal shown in Figure 4A). LDF in the HS+saline group was significantly correlated with arterial pressure between the pre-hemorrhage MAP and 50 mm Hg (r=0.8335, n=24; p<0.01) but LDF in the HS+ANG II group (representative animal shown in Figure 4B) was not (r=0.2304, n=24; ns). For pressures ≤ 50 mm Hg, LDF and arterial pressure values were significantly correlated in both the HS+ saline group (r=0.877, n=23; p<0.01) and in the HS + ANG II infused group (r=0.4995, n=23; p<0.05). Similar to non-infused animals fed LS or HS diet, the slopes of the pressure/flow relationship below 50 mm Hg were not significantly different in the HS + saline group (−1.98±0.1, n=5) and the HS + ANG II infused group (−1.56±0.74, n=5).
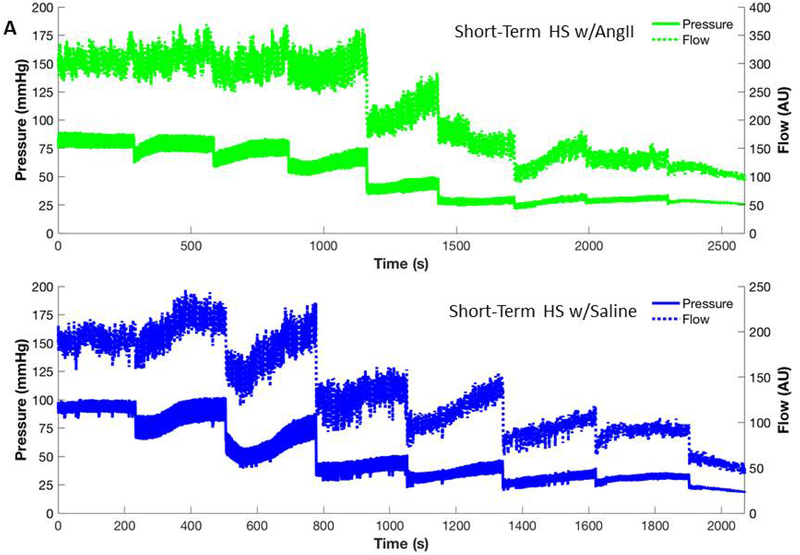
Figure 4:
Representative blood pressure and cerebral blood flow response to serial blood withdrawals for animals on a short-term high salt diet with low dose ANG II infusion (A) and animals on a short-term high salt diet with saline infusion (B). Each measurement is averaged using a moving window to see the trends in response of pressure and flow. In addition, during each of the blood withdrawals the pressure catheter was disconnected to allow blood to be withdrawn so the periods during the actual blood withdrawal have been removed from the time course. Note that infusion of ANG II yields a response similar to the low salt control in Figure 2A, seemingly reversing the effects of a short-term high salt diet exhibited in saline-infused rats. The shorter duration of the experimental period in the HS animal receiving saline infusion vs. the HS-fed rat receiving low dose ANG II infusion reflects the improved ability of the ANG II-infused HS group to compensate for the reduction in blood pressure following blood volume withdrawal.
Computational Analysis of Cerebral Autoregulatory Responses in Rats Fed ST-HS Diet with Saline Infusion or ANG II infusion:
The same analysis that was performed on the LS and ST-HS groups was also performed on the ST-HS + saline and ST-HS + ANG II infusion groups. Model free analysis is shown in Figure 5A, and the mechanistic model results of autoregulation in the two groups and in each individual animal are shown in Figure 5B and 5C, respectively. As in the previous analysis, the two groups are hard to distinguish with the model free analysis but are distinctly different when represented with a mechanistic model. Optimized parameter values of Ctone0 and Ctone1 are shown in Table 3. The average response of the ST-HS + ANG II infusion group shows a distinct leftward shift in autoregulatory function when compared to the ST-HS + saline infusion group when analyzed with the mechanistic cerebral vasculature model (Figure 5B), while this distinction cannot be clearly seen in the model free analysis (Figure 5A).
Figure 5:
Computational analysis of cerebral vascular autoregulation in HS-fed rats receiving continuous i.v. infusion of a low dose of ANG II or continuous i.v. infusion of saline vehicle. Panel A: Model free polynomial fit to each group. Panel B: Predicted average response of each group along with the predicted confidence intervals. Panel C: Optimized simulations of individual animal experiments. The average response of the ST-HS + ANG II infusion group shows a distinct leftward shift in autoregulatory function when compared to the ST-HS + saline infusion group when analyzed with the mechanistic cerebral vasculature model (Figure 5B), while this distinction cannot be clearly seen in the model free analysis (Figure 5A) (See text for details).
Table 3.
Model parameters for STHS w/saline infusion and STHS w/ANG II infusion. Ctone0 and Ctone1 indicate the sensitivity of the vessels to vessel wall strain as induced by pressure. Large values of Ctone0 indicate a higher sensitivity of the vessel to stress and larger values of Ctone1 indicate a higher basal tone in the vessel.
| STHS w/saline | STHS w/ANG II | P VALUE | |
|---|---|---|---|
| Ctone0 | 6.07 ± 1.95 | 18.42 ± 5.67 | 0.0017 |
| Ctone1 | 6.05 ± 2.04 | 18.14 ± 7.48 | 0.0082 |
DISCUSSION
As noted above, both short term and chronic exposure to high salt diet lead to impaired vascular relaxation, increased oxidant stress, and reduced nitric oxide availability in arterioles, resistance arteries, and conduit vessels from multiple vascular beds (8, 14, 19, 37–40, 42, 46, 54, 65, 69, 70, 76–78), including the cerebral circulation. Because of the similarities between functional data in individual arteries of HS-fed animals (14, 40, 46, 69, 70) and existing evidence of an elevated risk of ischemic stroke due to impairment of NO-mediated vascular relaxation, we decided to examine the effect of HS diet on autoregulation of blood flow using laser-Doppler flowmetry (LDF) in the in vivo cerebral circulation. This is important because, compared to the responses of the vasculature to different vasoactive agonists, much less is known regarding the effect of HS diet and salt-induced ANG II suppression on the ability of local autoregulatory mechanisms to maintain cerebral blood flow during reductions in perfusion pressure. Based on the extensive findings demonstrating that high salt diet leads to impaired vascular relaxation in cerebral arteries (and other vascular beds), we hypothesized that cerebral blood flow autoregulation in response to controlled stepwise reductions of arterial pressure would be impaired in rats maintained on either a long term or a short-term high salt diet.
Consistent with that hypothesis, our initial experiments demonstrated that chronic exposure to HS diet significantly impaired the ability of the rats to maintain cerebral blood flow in response to reductions in arterial pressure. These findings are consistent with previous observations in our laboratory indicating that cerebral resistance arteries (40, 42) and pial arterioles (40) exhibit impaired relaxation to a variety of vasodilator stimuli in Sprague-Dawley rats following HS diet; and that blood flow assessed by LDF in the pial microcirculation of HS-fed S-D rats fails to increase in response to a bolus infusion of Ach (47). However, the increase in cerebral blood flow in response to a brief test exposure to the non-endothelium dependent stimulus of hypercapnia (36, 59) was intact in the HS fed rats (47), demonstrating that the loss of cerebral blood flow autoregulation in HS-fed rats does not result from a general and nonspecific inability of the vessels to dilate.
Although a strict definition of the lower limit of autoregulation (LLA) is still elusive, the LLA is generally recognized as the blood pressure at which blood flow falls progressively below resting values as arterial pressure is reduced due to an inability of the arterioles to dilate to maintain blood flow despite the reduction in perfusion pressure. (32) In the present study, cerebral blood flow was maintained relatively constant in LS-fed rats during sustained reductions in arterial pressure to values as low as 40–50 mmHg, a finding consistent with previous estimations of the lower limit of autoregulation in healthy rats (35, 43). However, cerebral blood flow in rats fed the long-term HS diet decreased progressively with successive reductions in arterial pressure showing that HS diet eliminates the plateau phase of blood flow regulation that is normally present in healthy rats. In addition, cerebral blood flow relative to pre-hemorrhage control in rats fed long term HS diet was significantly lower than that in LS-fed animals at pressures as high as 60 mmHg, indicative of an upward shift in the lower limit of autoregulation.
In addition to identifying the lower limit of autoregulation, the slope of the relationship between blood pressure and cerebral blood flow is a valuable indicator of autoregulatory capacity and cerebral vascular health (56). In the present study, there was a significant increase in the slope of the LDF flow vs. arterial pressure relationship during hemorrhagic hypotension in rats fed long term HS diet compared to rats maintained on LS diet. In this regard, our observations in rats fed long-term HS diet are strikingly similar to those obtained in several models of hypertension. For example, autoregulation of cerebral blood flow during hypotension is impaired in spontaneously hypertensive rats (SHR) (4, 52) and in stroke-prone SHR rats (SP-SHR) (57). HS-induced hypertension in Dahl salt-sensitive (SS) rats also shifts the upper limit of autoregulation toward higher arterial blood pressures (58). Taken together, these observations strongly indicate that dysfunction of the cerebral vasculature is present in normotensive animals consuming a high salt diet for 4 weeks, and that this vascular dysfunction adversely affects the ability of the cerebral circulation to maintain tissue perfusion in the face of reductions in blood pressure (20).
In addition to the well-known deleterious effects of long term exposure to HS diet on the vasculature (47), there is increasing evidence that HS diet leads to a surprisingly rapid loss of endothelium-dependent vasodilation in cerebral arteries and other vascular beds in both animals (14, 40, 54, 69, 70) and humans (8, 65); and that salt-induced ANG II suppression is a major contributor to the oxidative stress and impaired vascular relaxation that occurs in response to elevated dietary salt intake (14, 54, 69, 70, 76, 77). For example, isolated arteries of Sprague-Dawley rats fed short term HS diet exhibit an impaired vascular relaxation in response to reduced PO2 and receptor-mediated vasodilator stimuli (42, 69, 70, 76–78) that is reversed by preventing the reduction in plasma ANG II levels via chronic intravenous infusion of low (sub-pressor) dose of ANG II (5 ng·kg−1·min−1) to prevent salt-induced ANG II suppression (14, 46, 69, 70, 76). Similar observations have been reported in cheek pouch arterioles of golden hamsters fed HS diet for 3 days (54). In HS-fed rats and hamsters, the protective effect of ANG II infusion to restore vascular relaxation is eliminated by simultaneous administration of the AT1 receptor antagonist losartan (14, 46), indicating that physiological levels of ANG II play an important role in maintaining normal vascular relaxation mechanisms via tonic interaction of ANG II with AT1 receptors, and that these mechanisms are compromised in the presence of salt-induced ANG II suppression. Importantly, AT1 receptor blockade with losartan not only eliminates the protective effect of low dose ANG II infusion to restore normal vasodilator responses in arterioles [54] and resistance arteries [69], but also eliminates the ability of ANG II infusion to reduce oxidative stress in the aortas of HS-fed hamsters [54].
Consistent with earlier findings showing impaired responses to acute exposure to vasodilator stimuli in normotensive rats fed short-term HS diet, we found that autoregulatory responses to arterial pressure reduction were impaired in the pial microcirculation of HS-fed rats and that chronic i.v. infusion of a sub-pressor dose of ANG II restored the autoregulation of cerebral blood flow in rats fed short-term HS diet. The latter finding is consistent with earlier studies showing that prevention of salt-induced ANG II suppression ameliorates endothelial dysfunction (42, 54, 69, 70, 76–78) and vascular oxidative stress (12, 54, 76, 77) in HS-fed rats and hamsters. Taken together, these observations support the hypothesis that physiological levels of ANG II in the plasma play an important role in maintaining the ability of the cerebral circulation to autoregulate its blood flow, and that salt-induced suppression of plasma ANG II levels results in an impaired ability of local autoregulatory mechanisms to maintain blood flow to the brain when arterial pressure is reduced in rats fed HS diet. While the mechanisms by which HS-induced ANG II suppression may contribute to increased oxidative stress and an impaired ability to autoregulate cerebral blood flow during reductions in arterial blood pressure remain to be determined, a likely candidate is a downregulation of antioxidant defense mechanisms mediated by the master antioxidant and cell-protective transcription factor NRF2, as the protective effect of low dose ANG II infusion to restore ACh-induced dilation is lost in HS fed Nrf2(−/−) knockout rats [55].
One potential complicating factor in the present experimental design is related to the expected increase in plasma angiotensin II levels with blood volume withdrawal, even in the presence of the initial salt-induced ANG II suppression in normotensive rats. Any hemorrhage-induced increase in plasma ANG II levels could either contribute to a compensatory vasoconstrictor response or actively dilate cerebral blood vessels. Because blood loss is a potent stimulus for angiotensin II release (30, 50), we expect plasma ANG II levels to increase in response to blood volume withdrawal, although the relative magnitude of this increase (compared to that animals fed low salt diet) is uncertain. In this regard, an important area for future study will be to measure plasma ANG II levels in LS- and HS-fed animals subjected to hemorrhagic stress.
Any potential effect of a hemorrhage-induced increase in plasma angiotensin II levels is further complicated by the actual effect of elevated ANG II levels on active tone in cerebral arterioles and resistance arteries. For example, some studies have reported that angiotensin II causes dilation of cerebral arterioles (48) mediated by prostaglandins (25) or endothelium-derived hyperpolarizing factor (68). Other authors have reported that angiotensin II causes constriction in the cerebral circulation via AT1 receptor activation that overrides the vasodilator action of AT2 receptors (67). Still others have reported vasodilation mediated via AT1 receptor activation in the cerebral circulation (64). If the vasodilator effect of any elevation in plasma angiotensin II levels predominates, any suppression of plasma ANG II levels in HS-fed rats (compared to LS-fed animals) could theoretically contribute to a reduced dilation of the arterioles and impair autoregulation in the HS-fed animals. However, in light of our previous observation that direct infusion of an ACh bolus fails to dilate the pial microcirculation in normotensive rats fed high salt diet [47], we believe that the effect of chronic salt-induced ANG II suppression on vascular relaxation mechanisms (rather than any acute effects of elevated plasma ANG II levels) may be the primary driving force leading to impaired autoregulatory responses in the HS-fed animals. The latter conclusion is consistent with the impaired relaxation to multiple vasodilator stimuli (12, 14, 40, 54, 69, 70, 76, 77), including reduced PO2 (19, 42, 46), in isolated cerebral arteries of normotensive animals fed HS diet.
An interesting area for future investigation would be the effect of high salt diet on tissue levels of angiotensin II in the brain, in light of an earlier report (6) that an increase in dietary salt content identical to the one used in the present study increased components of the renin-angiotensin system (RAS) in the brain of Sprague-Dawley rats with chronic kidney disease produced by 5/6 nephrectomy. However, expression of the brain RAS was unaffected by HS diet in sham-operated controls in that study, arguing against a potential effect of the local brain RAS on cerebral autoregulation in the present study.
While all these possibilities are clearly worthy of investigation, we believe the most likely explanation for the impaired ability of cerebral arteries and pial microvessels to dilate in response to the autoregulatory stimuli of reduced blood pressure and reduced tissue perfusion in the present study is the deleterious effect of chronic suppression of plasma angiotensin II levels that occurs with high salt diet in normotensive animals. For example, high salt diet impairs cerebral vascular responses to multiple vasodilators (nitric oxide, prostacyclin, etc.), including the ability of the pial microcirculation to dilate in response to intra-arterial infusion of a bolus of acetylcholine, (although the pial microcirculation from high salt fed rats still exhibits a large increase in blood flow in response to the powerful vasodilator response of hypercapnia) (47).
The results of the present study evaluating the effect of HS diet on the autoregulation of cerebral blood flow raise some interesting questions regarding the role of HS diet and ANG II suppression in affecting vascular regulation in humans, where endothelial dysfunction develops very rapidly and without hypertension in young normotensive human males (65) and females (8) following a moderate increase in dietary salt intake. Endothelial dysfunction is an important prognostic indicator of multiple adverse cardiovascular events, including death (74); and long-term follow-up studies have shown that salt-sensitive (for blood pressure) individuals who remained normotensive have a significantly higher mortality rate than salt-resistant normotensive counterparts.(73) In this regard, the impaired ability of the cerebral microcirculation to autoregulate its blood flow in HS-fed animals during reductions in arterial perfusion pressure (regardless of the underlying mechanisms) may be indicative of an additional danger of high salt diets in humans.
Similar to Sprague-Dawley rats fed high salt diet, Dahl salt-sensitive (SS) rats maintained on a low salt diet are normotensive but are exposed to chronically low plasma ANG II levels in the blood due to impaired regulation of their plasma renin activity (1, 9, 31). Like Sprague-Dawley rats, MCA of LS-fed SS rats exhibit endothelial dysfunction and impaired vascular relaxation responses that can be ameliorated by chronic i.v. infusion of a sub-pressor dose of ANG II (10, 13). The association of endothelial dysfunction with adverse cardiovascular events in humans (74) raises the question of whether chronic exposure to low levels of ANG II in the plasma (due to elevated dietary salt intake or in low-renin forms of salt-sensitive hypertension) play a role in the higher long-term mortality rate in salt-sensitive individuals who remain normotensive (73) or in the rapid onset of endothelial dysfunction in healthy young human subjects subjected to short term elevations in dietary salt intake (8) (65).
While controlled blood volume withdrawal was used to reduce arterial pressure to evaluate cerebral autoregulation in the present study, this raises intriguing questions regarding the potential impact of high salt diet and low angiotensin levels on clinical conditions characterized by low blood pressure, notably circulatory shock. A recent study (33) evaluated the use of angiotensin II infusion (versus placebo) as an adjunct to infusion of conventional vasopressor agents (norepinephrine, AVP) in patients with distributive shock characterized by widespread vasodilation and low blood pressure. That study suggested that infusion of angiotensin II increases blood pressure and allows a reduction in the doses of conventional vasoconstrictors used to raise blood pressure in that patient population. In that study, ANG II infusion also lowered cardiovascular sequential organ failure assessment (SOFA) scores, indicating a reduction in organ dysfunction. The latter observation suggests that ANG II infusion could have some beneficial actions under the conditions of widespread vasodilation in distributive shock. The results of that study raise the possibility that angiotensin II infusion exerts these beneficial effects by increasing the perfusion of vulnerable organs during distributive shock. However, the relative contribution of elevated perfusion pressure due to additional vasoconstriction in angiotensin II-infused patients vs. the maintenance of local autoregulatory mechanisms in vulnerable organs in that study remains to be determined.
Supplementary Material
Acknowledgements:
The authors thank Lynn Dondlinger for her outstanding technical assistance, Kaleigh Kozak for her assistance in preparing the manuscript, and Dr. David Harder for his advice and assistance in LDF protocols.
Grant Support: NIH #R01-HL128242 and NIH #1 P50 GM094503.
List of Abbreviations:
- Acetylcholine
ACh
- ANG II
angiotensin II
- CBF
cerebral blood flow
- ETCO2
expiratory end tidal CO2
- HS
high salt
- i.v.
intravenous
- LDF
laser-Doppler flowmetry/laser Doppler flow
- LLA
lower limit of autoregulation
- LS
low salt
- MAP
mean arterial pressure
- NRF2
Nuclear factor (erythroid-derived 2)-like-2
- S-D
Sprague-Dawley
- SHR
spontaneously hypertensive rat
- SMCs
smooth muscle cells
- SP-SHR
stroke-prone spontaneously hypertensive rat
- ST-HS
short term high salt
- VSM
vascular smooth muscle
REFERENCES
- 1.Amaral SL, Roman RJ, and Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension 37: 386–390, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Barry DI, Strandgaard S, Graham DI, Braendstrup O, Svendsen UG, Vorstrup S, Hemmingsen R, and Bolwig TG. Cerebral blood flow in rats with renal and spontaneous hypertension: resetting of the lower limit of autoregulation. J Cereb Blood Flow Metab 2: 347–353, 1982. [DOI] [PubMed] [Google Scholar]
- 3.Bohlen HG. Arteriolar closure mediated by hyperresponsiveness to norepinephrine in hypertensive rats. J Appl Physiol 236: H157–H164, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Cai H, Yao H, Ibayashi S, Zhao G, Kitazono T, Nagao T, and Fujishima M. Effects of long-acting angiotensin-converting enzyme inhibitor, imidapril, on the lower limit of cerebral blood flow autoregulation in hypertensive rats. Eur J Pharmacol 341: 73–77, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, and Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 40: 754–760, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, Wilcox CS, and Hou FF. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol: 26: 1619–1633, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson BE, and Secomb TW. A theoretical model for the myogenic response based on the length-tension characteristics of vascular smooth muscle. Microcirculation 12: 327–338, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cavka A, Cosic A, Jukic I, Jelakovic B, Lombard JH, Phillips SA, Seric V, Mihaljevic I, and Drenjancevic I. The role of cyclo-oxygenase-1 in high-salt diet-induced microvascular dysfunction in humans. J Physiol 593: 5313–5324, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley AW, Jr., Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, and Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Drenjancevic-Peric I, and Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension 45: 687–691, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Dries DL, Strong MH, Cooper RS, and Drazner MH. Efficacy of angiotensin-converting enzyme inhibition in reducing progression from asymptomatic left ventricular dysfunction to symptomatic heart failure in black and white patients. J Am Coll Cardiol 40: 311–317, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Durand MJ, and Lombard JH. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dimutase expression. Am J Hypertens 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand MJ, Moreno C, Greene AS, and Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. Am J Physiol 299: H1865–1874, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durand MJ, Raffai G, Weinberg BD, and Lombard JH. Angiotensin-(1–7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol 299: H1024–1033, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faraci FM, Baumbach GL, and Heistad DD. Cerebral circulation: humoral regulation and effects of chronic hypertension. J Am Soc Nephrol 1: 53–57, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Forstermann U, and Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Frisbee JC, and Lombard JH. Development and reversibility of altered skeletal muscle arteriolar structure and reactivity with high salt diet and reduced renal mass hypertension. Microcirculation 6: 215–225, 1999. [PubMed] [Google Scholar]
- 18.Frisbee JC, and Lombard JH. Reduced renal mass hypertension, but not high salt diet, alters skeletal muscle arteriolar distensibility and myogenic responses. Microvascular Research 59: 255–264, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Frisbee JC, Sylvester FA, and Lombard JH. High-salt diet impairs hypoxia-induced cAMP production and hyperpolarization in rat skeletal muscle arteries. Am J Physiol 281: H1808–H1815, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Gao E, Young WL, Pile-Spellman J, Ornstein E, and Ma Q. Mathematical considerations for modeling cerebral blood flow autoregulation to systemic arterial pressure. Am J Physiol 274: H1023–1031, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, and Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Gerrits RJ, Stein EA, and Greene AS. Laser-Doppler flowmetry utilizing a thinned skull cranial window preparation and automated stimulation. Brain Res Protocols 3: 14–21, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, and Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590: 5519–5528, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross V, Kurth TM, Skelton MM, Mattson DL, and Cowley AW, Jr. Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol 274: R1317–R1323, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Haberl RL, Anneser F, Villringer A, and Einhaupl KM. Angiotensin II induces endothelium-dependent vasodilation of rat cerebral arterioles. Am J Physiol 258: H1840–H1846, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Hermsmeyer K Electrogenesis of increased norepinephrine sensitivity of arterial vascular muscle in hypertension. Circ Res 38: 362–367, 1976. [DOI] [PubMed] [Google Scholar]
- 27.Holloway ET, and Bohr DF. Reactivity of vascular smooth muscle in hypertensive rats. Circ Res 33: 678–685, 1973. [DOI] [PubMed] [Google Scholar]
- 28.Hudetz AG, Lee JG, Smith JJ, Bosnjak ZJ, and Kampine JP. Effects of volatile anesthetics on cerebrocortical laser Doppler flow: hyperemia, autoregulation, carbon dioxide response, flow oscillations, and role of nitric oxide. Adv Pharmacol 31: 577–593, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Immink RV, van den Born BJ, van Montfrans GA, Koopmans RP, Karemaker JM, and van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation 110: 2241–2245, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Jakschik BA, Marshall GR, Kourik JL, and Needleman P. Profile of circulating vasoactive substances in hemorrhagic shock and their pharmacologic manipulation. J Clin Invest 54: 842–852, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang J, Stec DE, Drummond H, Simon JS, Koike G, Jacob HJ, and Roman RJ. Transfer of a salt-resistant renin allele raises blood pressure in Dahl salt-sensitive rats. Hypertension 29: 619–627, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Jones SC, Radinsky CR, Furlan AJ, Chyatte D, Qu Y, Easley KA, and Perez-Trepichio AD. Variability in the magnitude of the cerebral blood flow response and the shape of the cerebral blood flow-pressure autoregulation curve during hypotension in normal rats [corrected]. Anesthesiology 97: 488–496, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Khanna A, Ostermann M, and Bellomo R. Angiotensin II for the treatment of vasodilatory shock. New Eng J Med 377: 2604, 2017. [DOI] [PubMed] [Google Scholar]
- 34.Kotchen TA, and McCarron DA. Dietary electrolytes and blood pressure: A statement for healthcare professionals from the American Heart Association Nutrition Committee. Circulation 98: 613–617, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Lee JG, Hudetz AG, Smith JJ, Hillard CJ, Bosnjak ZJ, and Kampine JP. The effects of halothane and isoflurane on cerebrocortical microcirculation and autoregulation as assessed by laser-Doppler flowmetry. Anesth Analg 79: 58–65, 1994. [PubMed] [Google Scholar]
- 36.Lee JG, Smith JJ, Hudetz AG, Hillard CJ, Bosnjak ZJ, and Kampine JP. Laser-Doppler measurement of the effects of halothane and isoflurane on the cerebrovascular CO2 response in the rat. Anesth Analg 80: 696–702, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Lenda DM, and Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol 282: H395–H402, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Lenda DM, and Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res 39: 41–50, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Lenda DM, Sauls BA, and Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol 279: H7–H14, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Rusch NJ, and Lombard JH. Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension 33: 686–688, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Lombard JH, Hess ME, and Stekiel WJ. Enhanced response of arterioles to oxygen during development of hypertension in SHR. Am J Physiol 250: H761–H764, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Lombard JH, Sylvester FA, Phillips SA, and Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol 284: H1124–H1133, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Lu H, Werner C, Engelhard K, Scholz M, and Kochs E. The effects of sevoflurane on cerebral blood flow autoregulation in rats. Anesth Analg 87: 854–858, 1998. [DOI] [PubMed] [Google Scholar]
- 44.MacGregor GA. Salt--more adverse effects. Am J Hypertens 10: 37S–41S, 1997. [PubMed] [Google Scholar]
- 45.Mayhan WG, Faraci FM, and Heistad DD. Impairment of endothelium-dependent responses of cerebral arterioles in chronic hypertension. Am J Physiol 253: H1435–H1440, 1987. [DOI] [PubMed] [Google Scholar]
- 46.McEwen ST, Balus SF, Durand MJ, and Lombard JH. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. Am J Physiol 297: H1296–H1303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEwen ST, Schmidt JR, Somberg L, de la Cruz L, and Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation 16: 220–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng W, and Busija DW. Comparative effects of angiotensin-(1–7) and angiotensin II on piglet pial arterioles. Stroke 24: 2041–2045, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Merzeau S, Preckel MP, Fromy B, Lefthériotis G, and Saumet JL. Differences between cerebral and cerebellar autoregulation during progressive hypotension in rats. Neurosci Lett 280: 103–106, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Michailov ML, Schad H, Dahlheim H, Jacob IC, and Brechtelsbauer H. Renin-angiotensin system responses of acute graded hemorrhage in dogs. Circ Shock 21: 217–224, 1987. [PubMed] [Google Scholar]
- 51.Naveri L The role of angiotensin receptor subtypes in cerebrovascular regulation in the rat. Acta Physiol Scand Suppl 630: 1–48, 1995. [PubMed] [Google Scholar]
- 52.Nishimura Y, Ito T, and Saavedra JM. Angiotensin II AT1 blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke 31: 2478–2486, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Panza JA, Casino PR, Kilcoyne CM, and Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation 87: 1468–1474, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Priestley JR, Buelow MW, McEwen ST, Weinberg BD, Delaney M, Balus SF, Hoeppner C, Dondlinger L, and Lombard JH. Reduced angiotensin II levels cause generalized vascular dysfunction via oxidant stress in hamster cheek pouch arterioles. Microvasc Res 89: 134–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Priestley JR, Kautenburg KE, Casati MC, Endres BT, Geurts AM, and Lombard JH. The NRF2 knockout rat: a new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am J Physiol 310: H478–487, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozet I, Vavilala MS, Lindley AM, Visco E, Treggiari M, and Lam AM. Cerebral autoregulation and CO2 reactivity in anterior and posterior cerebral circulation during sevoflurane anesthesia. Anesth Analg 102: 560–564, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Smeda JS, and McGuire JJ. Effects of poststroke losartan versus captopril treatment on myogenic and endothelial function in the cerebrovasculature of SHRsp. Stroke 38: 1590–1596, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Smeda JS, and Payne GW. Alterations in autoregulatory and myogenic function in the cerebrovasculature of Dahl salt-sensitive rats. Stroke 34: 1484–1490, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Smith JJ, Lee JG, Hudetz AG, Hillard CJ, Bosnjak ZJ, and Kampine JP. The role of nitric oxide in the cerebrovascular response to hypercapnia. Anesth Analg 84: 363–369, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Spronck B, Martens EG, Gommer ED, and van de Vosse FN. A lumped parameter model of cerebral blood flow control combining cerebral autoregulation and neurovascular coupling. Am J Physiol 303: H1143–1153, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Strandgaard S Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation 53: 720–727, 1976. [DOI] [PubMed] [Google Scholar]
- 62.Strandgaard S Autoregulation of cerebral circulation in hypertension. Acta Neurol Scand Suppl 66: 1–82, 1978. [PubMed] [Google Scholar]
- 63.Strandgaard S, and Tominaga S. Abnormal cerebrovascular regulation in hypertensive patients. Br Med J 2: 1230–1231, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takao M, Kobari M, Tanahashi N, Tomita M, Yokoyama M, Tomita Y, Otomo M, Inoue K, and Fukuuchi Y. Dilatation of cerebral parenchymal vessels mediated by angiotensin type 1 receptor in cats. Neuroscience Letters 318: 108–112, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Tzemos N, Lim PO, Wong S, Struthers AD, and MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Vanhoutte PM, Feletou M, and Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol 144: 449–458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vincent JM, Kwan YW, Chan SL, Perrin-Sarrado C, Atkinson J, and Chillon JM. Constrictor and dilator effects of angiotensin II on cerebral arterioles. Stroke 36: 2691–2695, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Wackenfors A, Vikman P, Nilsson E, Edvinsson L, and Malmsjo M. Angiotensin II-induced vasodilatation in cerebral arteries is mediated by endothelium-derived hyperpolarising factor. Eur J Pharmacol 531: 259–263, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Weber DS, and Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol 280: H2196–H2202, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Weber DS, and Lombard JH. Elevated salt intake impairs dilation of rat skeletal muscle resistance arteries via ANG II suppression. Am J Physiol 278: H500–506, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clinical Hyperten (Greenwich, Conn) 4: 274–276, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weinberger MH. Sodium, potassium, and blood pressure. Am J Hyperten 10: 46S–48S, 1997. [PubMed] [Google Scholar]
- 73.Weinberger MH, Fineberg NS, Fineberg SE, and Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. [DOI] [PubMed] [Google Scholar]
- 74.Widlansky ME, Gokce N, Keaney JF, Jr., and Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Zagorac D, Yamaura K, Zhang C, Roman RJ, and Harder DR. The effect of superoxide anion on autoregulation of cerebral blood flow. Stroke 36: 2589–2594, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, and Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol 291: H929–H938, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Zhu J, Huang T, and Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries J Vasc Res 44: 382–390, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Zhu J, Mori T, Huang T, and Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol 286: H575–H583, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.