Abstract
Background
Reconstruction of the anterior cruciate ligament (ACL) commonly involves patellar tendon (PT) or hamstring tendon(s) (HT) autografts. There is no consensus with respect to the choice between these two grafts in ACL surgery.
Objectives
This review compared the outcomes of ACL reconstruction using PT versus HT autografts in ACL deficient patients.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (April 2008), the Cochrane Central Register of Controlled Trials (2008, Issue 2), MEDLINE (1966 to April 10 2008), EMBASE (1980 to April 10 2008), conference proceedings and reference lists. No language restrictions were applied.
Selection criteria
Randomized and quasi‐randomized controlled trials comparing outcomes (minimum two year follow‐up) following ACL reconstruction using either PT or HT autografts in skeletally mature adults, irrespective of the number of bundles, fixation method or incision technique.
Data collection and analysis
After independent study selection, the four authors independently assessed trial quality and risk of bias, and extracted data using pre‐developed forms. Trial authors were contacted for additional data and information. Risk ratios with 95% confidence intervals were calculated for dichotomous outcomes, and mean differences and 95% confidence intervals for continuous outcomes.
Main results
Nineteen trials providing outcome data for 1597 young to middle‐aged adults were included. Many trials were at high risk of bias reflecting inadequate methods of randomization, lack of blinding and incomplete assessment of outcome.
Pooled data for primary outcomes, reported in a minority of trials, showed no statistically significant differences between the two graft choices for functional assessment (single leg hop test), return to activity, Tegner and Lysholm scores, and subjective measures of outcome. There were also no differences found between the two interventions for re‐rupture or International Knee Documentation Committee scores. There were inadequate long‐term results, such as to assess the development of osteoarthritis.
All tests (instrumental, Lachman, pivot shift) for static stability consistently showed that PT reconstruction resulted in a more statically stable knee compared with HT reconstruction. Conversely, patients experienced more anterior knee problems, especially with kneeling, after PT reconstruction. PT reconstructions resulted in a statistically significant loss of extension range of motion and a trend towards loss of knee extension strength. HT reconstructions demonstrated a trend towards loss of flexion range of motion and a statistically significant loss of knee flexion strength. The clinical importance of the above range of motion losses is unclear.
Authors' conclusions
There is insufficient evidence to draw conclusions on differences between the two grafts for long‐term functional outcome. While PT reconstructions are more likely to result in statically stable knees, they are also associated with more anterior knee problems.
Keywords: Adult; Humans; Middle Aged; Young Adult; Anterior Cruciate Ligament Injuries; Anterior Cruciate Ligament; Anterior Cruciate Ligament/surgery; Patellar Ligament; Patellar Ligament/transplantation; Recovery of Function; Rupture; Rupture/surgery; Tendons; Tendons/transplantation; Thigh; Transplantation, Autologous; Treatment Outcome
Patellar or hamstring tendon grafts for ACL reconstruction in adults
The anterior cruciate ligament (ACL) is important for maintaining the stability in the knee, particularly in activities involving cutting, pivoting or kicking. People with ruptured ACLs have unstable knees that generally become more damaged over time. Reconstruction of ruptured ACLs commonly involves using autografts (grafts taken from the person undergoing surgery), obtained by removing part of the patellar tendon or the hamstring tendon. This review aimed to find out if one graft was better than the other.
This review included 19 studies reporting the outcomes of ACL reconstruction with patellar tendon versus hamstring tendon grafts in a total of 1597 young to middle‐aged adults. Many trials used flawed methods that might have affected their results.
The limited data available for functional outcomes including patient‐rated assessment did not show whether one graft was better than the other. Similarly, there were no differences found between the two types of graft for re‐rupture or in the results of an internationally used knee score. All tests for knee stability favoured patellar tendon grafts. Conversely, people had more anterior knee pain and discomfort with kneeling after patellar tendon reconstruction. After patellar tendon reconstruction, more people had some loss in their ability to straighten out their leg at the knee. In contrast, more people had some loss in their ability to bend their leg at the knee after hamstring tendon reconstruction. It is not clear how important these losses in range of motion of the knee were to the patients themselves.
The review concluded that the current evidence was insufficient to recommend which of the two types of graft was better for ACL reconstruction.
Background
The anterior cruciate ligament (ACL) of the knee acts to maintain joint stability by restraining anterior translation of the tibia relative to the femur (Seitz 1996). It also prevents abnormal rotational motion and varus/valgus angulation at full knee extension. Its role is particularly important in athletes, such as footballers, when performing activities involving cutting (i.e. sudden change in direction), pivoting, and kicking.
Description of the condition
ACL injury is a common orthopaedic problem with an annual incidence of approximately 200,000 cases per year in the United States (Miyasaka 1991). The classic mechanism of ACL injury involves non‐contact deceleration, such as sudden stopping or changes in direction. ACL tears are commonly associated with meniscal and articular cartilage injury (Shelbourne 1991; Smith 2001).
An ACL deficient knee is defined by the absence or loss of function of the ligament, resulting in biomechanical loss of stability. Though the natural history of ACL deficiency is poorly defined, studies have reported that a person with an ACL deficient knee presents with pain, recurrent symptoms of instability (Noyes 1983; Noyes 1989) and the discontinuation or limitation of pre‐injury sporting activities (Barrack 1990). ACL injury predisposes the knee to chronic instability, further meniscal and chondral damage and an impaired quality of life (Jackson 1993; Mohtadi 1998). It may also predispose to osteoarthritis (Daniel 1994; Sherman 1988).
Description of the intervention
Where available and clinically‐indicated, the most commonly recommended treatment for ACL deficiency is a surgical procedure called ACL reconstruction. This involves the use of a tendon graft to reconstruct the torn or deficient ligament. The primary goal of surgery is to achieve a functionally stable knee while minimizing morbidity and complications associated with the procedure. More than 70,000 ACL reconstructions are performed annually in the United States (Lyman 2009). While ACL reconstruction is a clinically accepted intervention, non‐operative management is often indicated for people who are less active, have minimal instability symptoms, and who are unable or unwilling to follow the demanding post‐surgical rehabilitation protocols. It is noteworthy that a Cochrane review comparing surgical versus non‐surgical intervention for ACL injuries found no evidence from randomized controlled trials to inform current practice (Linko 2005). Nevertheless, surgical ACL reconstruction remains the current standard of care.
An ACL reconstruction can use several options for the tendon graft. One option is to use allograft tissue from a cadaver donor (Frank 1997) or, less commonly, an artificial graft (Grontvedt 1996; Mirza 2000). More commonly, surgeons use the patient's own tendon tissue (autograft). The two most common autografts are the patellar tendon (PT) and hamstring tendon(s) (HT) (Johnson 1992). A PT graft involves surgically harvesting the central one third of the tendon with the attached bone from the patella and tibia. The HT graft involves harvesting the tendonous portion of the patient's semitendinosus and/or gracilis muscles.
Possible benefits and risks of patellar tendon and hamstring grafts
Proponents of the PT autograft cite superior graft strength, secure fixation and ease of harvest as advantages over the HT autograft, which is associated with increased graft incorporation time, possible hamstring weakness and inferior fixation. Those favouring the HT autograft cite smaller incisions, decreased donor site morbidity, multi‐bundled structure and larger surface area for incorporation. These proponents often state concern over patellar tendonitis and tendon rupture, patellar fracture and anterior knee pain with use of the PT graft. In the absence of specific contraindications to the use of one graft type over the other, the autograft offering the greatest likelihood of superior outcome and minimal morbidity and complication remains an unresolved issue (Biau 2006; Biau 2007; Grant 2003; Spindler 2004).
Why it is important to do this review
Thirteen recently published reviews (Biau 2006; Biau 2007; Dauty 2005; Forster 2005; Freedman 2003; Goldblatt 2005; Grant 2003; Herrington 2005; Prodromos 2005; Schultz 2002; Spindler 2004; Thompson 2005; Yunes 2001) compare PT and HT autografts in ACL reconstruction. These reviews vary in their methodology, resulting in potentially biased conclusions. For example, three reviews (Goldblatt 2005; Freedman 2003; Yunes 2001) used search strategies limited to MEDLINE and the English language, which may be evidence of a publication bias. In addition, these authors did not restrict their reviews to randomized clinical trials. The most recent publication addressing this topic analyzes the previously published systematic reviews (Poolman 2007). The purpose of this "review of systematic reviews" was to address the discrepancies and contradictory recommendations. Amongst their conclusions were that the existing reviews were of variable quality, sensitivity analyses were inconsistently applied, and that only two reviews (Biau 2006; Dauty 2005) were found to be methodologically sound. A key reason for performing this Cochrane review was to include more recent trials that utilize modern surgical techniques. In addition, the Cochrane Library facilitates a dynamic process for inclusion of future trials.
Our review compares the effectiveness of PT and HT autografts for the reconstruction of the ACL deficient knee using a comprehensive literature search to identify the evidence from recent and internationally published and unpublished randomized controlled trials. Given the controversy and uncertainty over which graft type should be used, our goal was to provide a definitive, unbiased and reproducible systematic review.
Objectives
The aim of this review was to compare the outcomes of patellar tendon versus hamstring tendon autografts for ACL reconstruction in people with ACL deficiency.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized controlled trials (for example, allocation by hospital record number or date of birth) that compared PT and HT grafts as stated in the 'Objectives'.
Types of participants
The population of interest was skeletally mature patients with documented ACL deficiency of the knee, requiring ACL reconstruction.
Types of interventions
Arthroscopically assisted ACL reconstruction using either a patellar tendon (PT) or a hamstring tendon (HT) autograft. HT autografts could be double or multiple stranded grafts. Single or double incision techniques were included as well as any method of fixation of the graft. Excluded interventions included allografts, synthetic materials and revision ACL reconstructions.
Types of outcome measures
Specific outcomes were not used to include or exclude trials. The outcomes were reported as follows:
Primary outcomes
1. Functional assessments 2. Return to activity / level of sport participation (including Tegner (Tegner 1985) and Lysholm (Lysholm 1982) scores) 3. Subjective knee scores (patient satisfaction, Cincinnati score (Noyes 1983; Noyes 1989), Anterior Cruciate Ligament Quality of Life (Mohtadi 1998))
Secondary outcomes
1. Complications (i.e. infection, arthrofibrosis), adverse outcomes, recurrent injury with and without reoperation 2. Static stability measures (KT arthrometer or other stability assessment devices) 3. Clinical composite scores; i.e. IKDC (International Knee Documentation Committee) (Irrgang 2001) 4. Range of motion 5. Strength testing (Cybex muscle testing or equivalent) 6. Pain / anterior knee symptomatology
Timing of outcome assessment
Outcome assessment was analyzed based on long term follow‐up, which was defined as greater than two years following ACL reconstruction. Subsequent to the protocol, we stipulated that a minimum two year follow‐up was necessary for trial inclusion. This was to ensure that there was consistency across the trials in the report of functional outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialized Register (April 2008), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2008 Issue 2), MEDLINE (1966 to April 10 2008), EMBASE (1980 to April 10 2008), and reference lists of articles. There were no constraints based on language or publication status.
In MEDLINE (OVID ONLINE), the first three levels of the optimal trial search strategy (Higgins 2005) were combined with the subject specific search. The complete search strategy is shown in Appendix 1. The search strategies used in the EMBASE (OVID ONLINE) database and The Cochrane Library (Wiley InterScience) are also shown in Appendix 1.
Searching other resources
The bibliographies of all papers identified by the search strategy were handsearched.
Specific proceedings of knee surgery, arthroscopic surgery and sport medicine meetings and conferences (1997 to 2006) were searched from the following organizations: European Society of Sports Traumatology Knee Surgery and Arthroscopy (ESSKA), American Orthopaedic Society for Sports Medicine (AOSSM), International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS), American Academy of Orthopaedic Surgeons (AAOS), World Congress on Orthopaedic Sports Trauma, and Arthroscopy Association of North America (AANA). To avoid publication bias, the investigators of the trials identified from the proceedings were contacted to obtain results and data of any unpublished studies.
Data collection and analysis
Selection of studies
All randomized or quasi‐randomized controlled trials directly comparing outcomes following ACL reconstruction using either PT or HT (gracilis and semitendinosus) autografts in adults were considered. Four review authors from two centres (NM and DC ‐ Calgary, DW and KD ‐ Toronto) independently applied the inclusion criteria to select citations in MEDLINE, other databases and reference lists for retrieval of full articles. Where there was disagreement or doubt, the full article was retrieved. Each centre independently assessed each full study report to see if it met the review inclusion criteria.
Data extraction and management
Four review authors (NM, DC, DW and KD) used pre‐developed data‐extraction forms to independently extract the data. The review authors compared the data extracted for each study to achieve consensus between the two centres. Where required, corresponding authors of individual trials were contacted for additional data or clarification of methodology.
Assessment of risk of bias in included studies
Risk of bias tables for the included studies were completed for the six domains of The Cochrane Collaboration's 'Risk of bias' tool: sequence generation, allocation concealment, blinding, completeness of outcome data, selective reporting and other bias. Many of the judgements drew on our ratings for methodological quality, which was assessed previously using the Cochrane Bone, Joint and Muscle Trauma Group's former quality assessment tool (Madhok 2006) (seeAppendix 2), and the Detsky scale (Detsky 1992) (seeAppendix 3), which has been used previously to grade orthopaedic RCTs (Bhandari 2002).
Four review authors (NM, DC, DW and KD) independently scored the methodological quality of included studies. The next level of review involved discussion within each centre and the third level of review was between centres. Consensus agreement was achieved between centres.
Measures of treatment effect
Risk ratios with accompanying 95% confidence intervals were calculated for dichotomous outcomes, and mean differences and 95% confidence intervals for continuous outcomes. In general, unfavourable outcome data are presented for dichotomous outcomes. However, for continuous outcomes such as strength and range of motion where higher values represent a better outcome, the descriptors 'Favours PT' or 'Favours HT' in the analyses were switched to reflect the correct direction of effect.
Dealing with missing data
Trial authors were contacted for missing data. Where standard deviations were not reported for a trial that otherwise could be included in a meta‐analysis, the mean standard deviations for the treatment groups from the other trials were used in an exploratory analysis.
Assessment of heterogeneity
Heterogeneity was evaluated by visual inspection and tested using a chi‐squared test (significance P < 0.10) and the I‐squared statistic (> 50%) (Higgins 2003).
Data synthesis
The results of comparable groups of trials were pooled using a fixed‐effect model. In the presence of heterogeneity, a random‐effects model was used.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed comparing number of strands for HT grafts (< 4 strands versus 4 strands), and the method of hamstring tendon femoral fixation (endobutton versus screws).
In the published protocol of this review, we specified the following subgroups:
1. Duration of ACL deficiency (acute: less than three months; or chronic: greater than three months) 2. Method used in graft fixation (e.g. screw, button, staple) 3. Type of hamstring graft preparation (double or quadruple stranded) 4. Surgical approach used in ACL reconstruction (single or two incisions)
Sensitivity analysis
Sensitivity analyses are a method of investigating the importance of some of the assumptions and decisions made during a systematic review. For this review, we planned sensitivity analyses to explore the effect of methodological quality on the aggregate estimate of treatment effect, assuming that studies of lower quality would produce an exaggerated estimate of treatment effect. We performed two types of sensitivity analyses, one comparing randomized to quasi‐randomized controlled trials and the other examining the effects of using imputed standard deviations.
Results
Description of studies
Results of the search
The electronic search of the databases resulted in a total of 326 references. An additional 39 references were identified by handsearching conference proceedings from the European Society of Sports Traumatology Knee Surgery and Arthroscopy (ESSKA), American Orthopaedic Society for Sports Medicine (AOSSM), International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS), American Academy of Orthopaedic Surgeons (AAOS), World Congress on Orthopaedic Sports Trauma, and Arthroscopy Association of North America (AANA). No new references were identified by handsearching the bibliographies of identified studies.
Seventy‐nine of the 365 identified references discussed patellar tendon (PT) and hamstring tendon (HT) autografts for ACL reconstruction in the form of reviews, meta‐analyses, prospective clinical trials, case series, or randomized and quasi‐randomized clinical trials. One Spanish study and three German studies were identified and required translation. After removing duplicate references and selecting only randomized and quasi‐randomized clinical trials, 26 studies were identified. Only 19 of these studies, which were reported in 22 publications, were included in the final analysis. Seven studies, four of which were insufficiently reported in conference abstracts, were excluded for reasons listed in the Characteristics of excluded studies. Of the three trials reported in full, Beard 2001 alone was excluded because of insufficient follow‐up, whereas Carter 1999 and Sato 2005 reported only on intermediate outcomes as well. One ongoing study (Taylor 2006) was not included in the review because minimum two year follow‐up data were not yet available at the cut‐off time for study selection.
Included studies
Nineteen studies were included. All 19 studies reported long‐term (24 months or more) results with an average follow‐up ranging from a minimum of 24 months to 102 months. Additionally, short‐term (< 6 months) follow‐up results were reported in three studies (Aglietti 2004; Feller 2003; Maletis 2007) and intermediate‐term (6 months to < 24 months) results in five studies (Aglietti 2004; Aune 2001; Beynnon 2002; Feller 2003; Maletis 2007). For three studies, the findings were reported in two publications. Aglietti (Aglietti 1994) reported two and five year follow‐up information on the same group of randomized patients (Aglietti 1994; Aglietti 1997). Jansson and Harilainen (Jansson 2003) reported results on the same population of patients at a minimum of 21 months and minimum of three years (median of five years) respectively (Jansson 2003; Harilainen 2006). O'Neill (O'Neill 1996) initially reported results on 125 patients at a mean follow‐up of 42 months in 1996, then on an additional 101 patients (one died) for a total of 225 patients (with minimum six year follow‐up) in 2001 (O'Neill 1996; O'Neill 2001). However, O'Neill's 2001 publication did not report on all of the original outcomes analyzed in the 1996 publication (O'Neill 1996; O'Neill 2001).
One relevant study was translated from the German language to provide information for use in the review (Ropke 2001). One trial, which was identified by handsearching conference proceedings, represented an unpublished manuscript received after contacting the author. This study was subsequently published in 2007 with the information being essentially identical (Maletis 2007). The remaining studies were published between 1994 and 2007.
Design
All included studies used some form of randomization. Eight studies used computer‐generated randomization, random numbers tables, or sealed envelopes for the randomization process (Anderson 2001; Aune 2001; Beynnon 2002; Ejerhed 2003; Eriksson 2001; Feller 2003; Laxdal 2005; Maletis 2007). The remaining 11 trials used a quasi‐randomization method for treatment allocation, including alternating sequences (Aglietti 1994; Aglietti 2004; Zaffagnini 2006), birth date (Ibrahim 2005; Jansson 2003; Marder 1991; Matsumoto 2006; O'Neill 1996; Shaieb 2002), surgery date or surgical register sequence (Ropke 2001; Sajovic 2006).
The majority of the studies compared two treatment groups. However, four studies compared three treatment groups with different variations on the reconstructive technique (Anderson 2001; Laxdal 2005; O'Neill 1996; Zaffagnini 2006). Anderson 2001 included a third comparison group that combined an intra‐articular double‐stranded semitendinosus/gracilis hamstring with an extra‐articular reconstruction using the ilio‐tibial band. This group of patients was not included in this review. Laxdal 2005 included two hamstring groups (3‐strand semitendinosus graft and a quadruple semitendinosus/gracilis graft), both of which were included in the review. O'Neill 1996 included two patellar tendon reconstruction groups, which differed only in the incision technique (single or two‐incision); both patellar tendon groups were included in the review. Zaffagnini 2006 also included a second hamstring treatment group with an extra‐articular component to the reconstructive technique; this group was not included in the review.
Sample sizes
The sample sizes for the 19 trials ranged from 40 participants (Ropke 2001) to 229 participants (O'Neill 1996; O'Neill 2001). Sample size was determined a priori in only six trials (Aglietti 2004; Anderson 2001; Aune 2001; Beynnon 2002; Jansson 2003; Maletis 2007). Five of these trials based sample size calculations on KT arthrometer measurements; however, there were significant discrepancies between the definitions for clinically important differences. Aglietti 2004 based their sample size calculations on the KT arthrometer measurement (134 N), with a side to side difference of 1.0 mm and a standard deviation of 1.5 mm. Their calculated sample size was 48 patients per group. They subsequently entered 60 patients per group to account for a 20% loss to follow‐up rate. Based on additional information provided by Anderson 2001, a calculated "sample size of 35 [patients per group] was necessary to determine a difference of 0.8 standard deviations for KT‐1000 values. For nominal data, such as the IKDC score, a power of 0.8 detected a 36% difference in the groups." Therefore, Anderson 2001 enrolled 105 patients into three separate groups; 102 were seen at an average of 35 months follow‐up. As described by Aune 2001, the calculated sample size of 22 patients per group was based on a 3 mm difference in joint laxity on the KT arthrometer (manual maximum) and standard deviation of 3 mm (alpha 0.05, beta 0.01). (However, the beta value claimed by Aune 2001 is unusually small and inconsistent with the small sample size.) Seventy‐two patients were enrolled in Aune 2001 to account for patients lost due to no shows or injuries to the uninvolved knee, with 61 patients analyzed at a minimum of 24 months follow‐up. The sample size estimate reported for Beynnon 2002 was based on the primary outcome of translation, as determined by KT arthrometer measurement at 133 N, with a side to side difference of 2.5 mm and a variation of 4.0 mm. This resulted in a calculated sample size of 24 patients per group. Beynnon 2002 entered 56 patients in total, but only evaluated 22 in each group at final follow‐up. Maletis 2007 also used the KT arthrometer measurement (manual maximum) as their primary outcome measure, but used a clinically important side to side difference of 1.5 mm with a standard deviation of 2.0 mm. They calculated a sample size of 39 patients per group, subsequently entered a total of 99 patients and evaluated 96 patients at a mean follow‐up of 26 months. Jansson 2003 used the Lysholm score as, "one of our main outcome measurements" to calculate sample size and defined a 10‐point difference as clinically significant. They did not report a standard deviation, but stated that a total sample size of 54 for both treatment groups was sufficient. They randomized 99 patients, but evaluated 89 patients at a minimum of 21 months follow‐up. We were unable to replicate all sample size calculations based on the available information. There was no consistency with respect to what was defined as the minimal clinically important difference, standard deviation of the KT arthrometer measurement, and the necessary power to show a difference between groups.
Setting
The included studies were truly international in scope. The 10 countries represented were Australia, Finland, Germany, Italy, Japan, Kuwait, Norway, Slovenia, Sweden and USA. All settings represented referral based practices.
Participants
A total of 1748 patients with a confirmed diagnosis of ACL deficiency were randomized between 1989 and 2003, where reported. Twenty‐five patients from one trial (Ibrahim 2005) were not accounted for and two patients died in O'Neill's trial (O'Neill 1996), but no group designation was provided for these. Data from these patients and a further 124 patients (lost to follow‐up = 89; re‐rupture = 24; contralateral rupture = 9; withdrawal = 1; and previous reconstruction = 1) were unavailable for analysis. This left a total of 1597 participants with data for analysis (seeTable 2). A comparison of the number of analyzed patients in each treatment group showed little difference in the proportions available in each group (seeAnalysis 1.25: risk ratio (RR) 0.99; 95% CI 0.96 to 1.03). Based on the definition of chronic ACL deficiency being greater than three months from time of injury, seven trials (Aglietti 1994; Beynnon 2002; Ibrahim 2005; Jansson 2003; Marder 1991; Matsumoto 2006; Ropke 2001) only included patients with chronic ACL deficiency. Seven studies provided baseline data for numbers of patients with acute ACL deficiency (seeAnalysis 1.26) but did not provide separate follow‐up data for these patients. Therefore, it was not possible to separate out acute reconstructions in the analysis.
Table 1.
Randomized versus analyzed
| Study | Randomized | Lost to follow‐up | Re‐ruptures | Contralateral ruptures | Withdrawal | Previous ACL Recons | Analyzed |
| Aglietti 1994 / Aglietti 1997 | 63 Total | 3 Total | NR | NR | NR | NR | 30 PT 30 HT |
| Aglietti 2004 | 60 PT 60 HT | 0 | NR | NR | NR | NR | 60 PT 60 HT |
| Anderson 2001 | 35 PT 35 HT | 0 PT 2 HT | NR | NR | NR | NR | 35 PT 33 HT |
| Aune 2001 | 35 PT 37 HT | 8 Total | 1 PT* 2 HT* | 3 Total | NR | NR | 29 PT 32 HT |
| Beynnon 2002 | 28 PT 28 HT | 6 PT 6 HT | NR | NR | NR | NR | 22 PT 22 HT |
| Ejerhed 2003 | 34 PT 37 HT | 1 PT 1 HT | 1 PT 2 HT | NR | NR | NR | 32 PT 34 HT |
| Eriksson 2001 | 84 PT 80 HT | 2 PT 2 HT | 2 PT 3 HT + 1 extra surgery | NR | NR | NR | 80 PT 74 HT |
| Feller 2003 | 31 PT 34 HT | 4 PT 2 HT | 1 PT 0 HT | NR | 0 PT 1 HT | NR | 26 PT 31 HT |
| Ibrahim 2005 | 110 total | 25 | NR | NR | NR | NR | 40 PT 45 HT |
| Jansson 2003 / Harilainen 2006 | 51 PT 48 HT | 13 PT 3 HT | 0 PT 4 HT | 1 PT 4 HT | NR | NR | 37 PT 37 HT |
| Laxdal 2005 | 134 Total | 9 Total | 1 PT 2 HT + 2 early septic failure | 1 PT 0 HT | NR | 0 PT 1 HT | 40 PT 78 HT |
| Maletis 2007 | 46 PT 53 HT | 0 PT 2 HT | 0 PT 1 HT | 2 PT* 1 HT* | NR | NR | 46 PT 50 HT |
| Marder 1991 | 40 PT 40 HT | 3 PT 5 HT | 1 PT* 1 HT* | NR | NR | 2 PT* 0 HT* | 37 PT 35 HT |
| Matsumoto 2006 | 40 PT 40 HT | 3 PT 5 HT | NR | NR | NR | NR | 37 PT 35 HT |
| O'Neill 1996 / O'Neill 2001 | 229 Total | 2 PT 0 HT 2 Deaths | 2 PT* 2 HT* | NR | NR | NR | 150 PT 75 HT |
| Ropke 2001 | 20 PT 20 HT | NR | NR | NR | NR | NR | 20 PT 20 HT |
| Sajovic 2006 | 32 PT 32 HT | 2 PT 1HT | 1 PT 1HT | 3 PT 2 HT | NR | NR | 26 PT 28 HT |
| Shaieb 2002 | 82 Total | 12 Total | 2 PT 2 HT | NR | NR | NR | 31 PT 35 HT |
| Zaffagnini 2006 | 25 PT 25 HT | 0 | 0 | NR | NR | NR | 25 PT 25 HT |
| TOTAL | 1748 |
0* 89 + 2 deaths (excluded) 25 unaccounted (excluded) |
9* 24 (excluded) | 3* 9 (excluded) | 0* 1 (excluded) | 2* 1 (excluded) | 1597 |
| NR = None reported * = included in analysis; all other patients for lost to follow‐up, re‐ruptures, contralateral ruptures and withdrawals were excluded from the analysis |
Analysis 1.25.
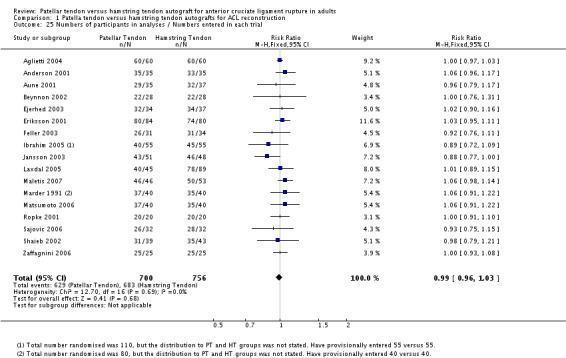
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 25 Numbers of participants in analyses / Numbers entered in each trial.
Analysis 1.26.
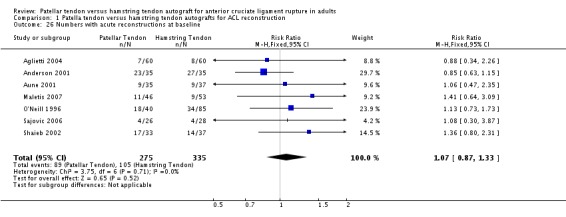
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 26 Numbers with acute reconstructions at baseline.
The studies typically included patients of similar age ranges (14 to 59 years). The mean age in individual trials ranged from 21.5 years to 32 years. None of the studies included skeletally immature patients. Based on data from 17 trials, there were nearly twice as many males as females (946 versus 477). The gender comparison between patellar tendon and hamstring tendon reconstructions was almost identical (seeAnalysis 1.27: RR 0.98; 95% CI 0.91 to 1.05).
Analysis 1.27.
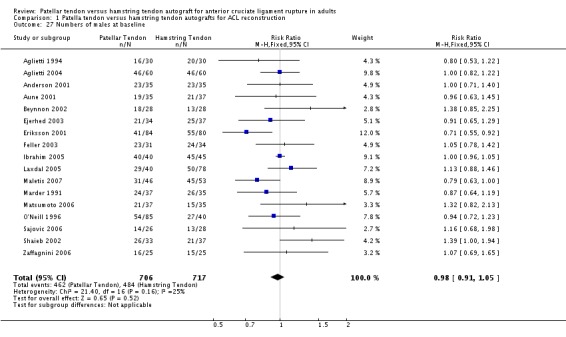
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 27 Numbers of males at baseline.
Interventions
All of the included studies compared a single‐stranded patellar tendon graft to a single‐bundle hamstring graft. Extra‐articular procedures were excluded from this review. Three studies (Anderson 2001; Beynnon 2002; O'Neill 1996) used a double hamstring construct combining the semitendinosus (ST) and gracilis (G) tendons. Ropke 2001 used a double‐looped ST tendon construct. A triple‐ or quadruple‐looped semitendinosus hamstring graft was used as a comparison in two trials (Ejerhed 2003; Laxdal 2005). The quadruple‐stranded hamstring graft (double‐loop ST and G) represented the comparison group in the majority of trials (Aglietti 1994; Aglietti 2004; Aune 2001; Feller 2003; Ibrahim 2005; Jansson 2003; Maletis 2007; Marder 1991; Sajovic 2006; Shaieb 2002; Zaffagnini 2006). Eriksson 2001 used a quadruple hamstring graft with the ST tendon alone. Matsumoto 2006 used a novel technique utilizing a five‐stranded hamstring construct with bone plugs on either end.
Outcome measures
In general, the outcomes were reported as proportions or means. In situations where the standard deviations were not reported, the mean of the standard deviations from the other trials that reported this statistic was imputed. Standard deviations were reported in only seven trials (Anderson 2001; Aune 2001; Feller 2003; Marder 1991 ; Matsumoto 2006; Sajovic 2006; Zaffagnini 2006) or were calculated from raw data in six studies (Ejerhed 2003; Eriksson 2001; Feller 2003; Laxdal 2005; Maletis 2007; Sajovic 2006).
Primary outcomes
Functional assessments
Various measures of objectively‐assessed knee function were reported by individual trials. Nine studies (Aune 2001; Beynnon 2002; Ejerhed 2003; Eriksson 2001; Laxdal 2005; Maletis 2007; O'Neill 1996; Sajovic 2006; Zaffagnini 2006) reported on the single hop test but in several different ways; five studies provided data for the proportion of patients achieving at least 90% of the results for the opposite leg.
Return to activity/level of sport participation
Return to activity was reported in various ways: as a proportion of patients based on the Cincinnati score (Feller 2003), a proportion of patients with light or sedentary activity according to the International Knee Documentation Committee (IKDC) score (Aglietti 2004; Anderson 2001; Beynnon 2002; Marder 1991), a study‐specific questionnaire (Ibrahim 2005; Maletis 2007) and as a proportion of patients returning to pre‐injury levels (Marder 1991; O'Neill 1996; Sajovic 2006). Only the four trials reporting the IKDC score (Aglietti 2004; Anderson 2001; Beynnon 2002; Marder 1991) provided consistent data for pooling. The Tegner activity level (Tegner 1985) reported in nine studies (Beynnon 2002; Ejerhed 2003; Eriksson 2001; Ibrahim 2005; Jansson 2003; Laxdal 2005; Maletis 2007; Ropke 2001; Zaffagnini 2006) and the Lysholm (Lysholm 1982) scores reported in nine studies (Ejerhed 2003; Eriksson 2001; Ibrahim 2005; Jansson 2003; Laxdal 2005; Maletis 2007; O'Neill 1996; Sajovic 2006; Shaieb 2002) were reported as means, medians or proportions of patients achieving a defined score or level. A comparison of the mean scores allowed for the greatest number of studies to be pooled for each outcome.
Subjective knee scores
Patient satisfaction
Patient satisfaction was assessed in only six studies, and reported in various ways. Aglietti 1994 used a subjective satisfaction rating on a 100‐point scale and Aglietti 2004 used a subjective scale for knee complaints on a 10‐point scale. Eriksson 2001 asked patients to rate how their knee affected function and activity level on a visual analogue scale and reported the results as a median score out of 100. Maletis 2007 and Shaieb 2002 both used four point Likert scales to assess patient satisfaction. However, patients in Maletis 2007 rated their knee from 0 to 100 and ranked their knee as much better, slightly better, not better or worse at the follow‐up periods, whereas Shaieb 2002 asked patients to rate their surgical results as excellent, good, fair or poor. Ibrahim 2005 reported that patients completed a detailed questionnaire that included questions about satisfaction with surgery, episodes of giving way, and episodes of pain, but only provided a summary statement of the overall results.
Cincinnati and Anterior Cruciate Ligament Quality of Life (ACL‐QOL)
Mean Cincinnati scores were reported by two studies (Aune 2001; Feller 2003). The ACL‐QOL was not reported in any study.
Secondary outcomes
Complications /adverse outcomes/recurrent injury with and without reoperation
Intra‐operative and peri‐operative complications were inconsistently reported across the studies. One trial (Aglietti 2004) reported that there were no intra‐operative or post‐operative complications and that none of the patients underwent additional surgery during the study period. Four studies (Beynnon 2002; Ibrahim 2005; Ropke 2001; Zaffagnini 2006) did not report intra‐operative or peri‐operative complications.
Several rare adverse outcomes were reported in single trials. Two patients in the HT group of Aune 2001 suffered complications relating to graft harvesting: a lesion of saphenous nerve with permanent sensory loss and a rupture of the sartorius tendon causing severe flexion‐strength deficit. Eriksson 2001 reported one intra‐operative case of a blow‐out fracture of the posterior femoral tunnel in the PT group. Although Marder 1991 reported no peri‐operative complications, one patient required closed manipulation under anaesthesia for limited flexion. Shaieb 2002 reported one case of reflex sympathetic dystrophy in the PT group.
Re‐ruptures were specifically reported in 13 trials (Aune 2001; Ejerhed 2003; Eriksson 2001; Feller 2003; Jansson 2003; Laxdal 2005; Maletis 2007; Matsumoto 2006; Marder 1991; O'Neill 1996; Sajovic 2006; Shaieb 2002; Zaffagnini 2006). However, the cause of re‐rupture was not clearly described in any of these studies. In addition, many trials did not specify whether the trial participants with re‐ruptures received revision ACL reconstruction surgery.
Static stability measures
In most studies, static instability outcomes were based on some measure of translation, including an instrumented arthrometer, the Lachman test, and related findings such as the pivot shift test. The KT arthrometer was used in 17 out of the 19 studies. The other two trials used a CA‐4000 arthrometer (Jansson 2003) and the Stryker laxity test (Eriksson 2001). However, there was no consistency in how these arthrometer outcome measurements were recorded or classified; therefore, no more than seven studies could be pooled for the analyses for the KT arthrometer outcome.
The Lachman test was reported as a proportion of patients with a defined translation grade or millimetre range; however, four different grading schemes were used: A / B / C (Sajovic 2006), 0 / +1 / +2 / +3 (Aglietti 2004; Ejerhed 2003; Zaffagnini 2006), 0 or > 1 (Eriksson 2001) and positive / negative test (Jansson 2003; Ibrahim 2005). Shaieb 2002 reported the Lachman test as a mean side to side difference.
The pivot shift test was also reported as the proportion of patients by grade; however the grades varied between studies: O / I / II / III (Aglietti 1994), 0 / +1 / +2 / +3 (Aglietti 2004; Anderson 2001; Beynnon 2002; Eriksson 2001; Laxdal 2005; Maletis 2007; Marder 1991; Shaieb 2002; Zaffagnini 2006), A / B / C (Sajovic 2006), ‐ / + / ++ (Jansson 2003) and as a positive / negative test (Feller 2003; Ibrahim 2005).
Clinical composite scores (IKDC ‐ International Knee Documentation Committee)
The International Knee Documentation Committee (IKDC) subjective knee form and group grading scheme from grade A (Normal) to grade D (Severely abnormal) were used in 15 studies (Aglietti 1994; Aglietti 2004; Anderson 2001; Ejerhed 2003; Eriksson 2001; Feller 2003; Ibrahim 2005; Jansson 2003; Laxdal 2005; Maletis 2007; Matsumoto 2006; O'Neill 1996; Ropke 2001; Sajovic 2006; Zaffagnini 2006). However, the results were reported as total overall median or average scores, or by the proportion of patients with grades A, B, C, D, or a combination of these grades. For example, the subjective knee form score was reported as a median by Feller 2003, and as a mean by Aglietti 2004 and Matsumoto 2006; thus making a meaningful outcome very difficult to compare with only two studies reporting the IKDC scores in the same way. It is important to note that the studies included in this review utilized two different versions of the IKDC knee form, which differ in their scoring systems. The scoring system used in the original (1995) version provides an overall grade (A, B, C or D) that incorporates the patient's subjective score (Hefti 1993; Irrgang 1998). The newer (2000) version provides an overall group grade (A, B, C or D) in addition to a patient‐based subjective score out of 100 (Irrgang 2001).
Range of motion
Range of motion assessment was reported in several ways, including different degree ranges for extension and flexion deficits, or as heel height differences. Therefore, in order to compare the various studies, commonly‐reported degree ranges were selected. The proportion of patients achieving extension deficits greater than three degrees was pooled in 14 trials (Aglietti 1994; Aglietti 2004; Anderson 2001; Ejerhed 2003; Eriksson 2001; Feller 2003; Ibrahim 2005; Laxdal 2005; Marder 1991; Matsumoto 2006; O'Neill 1996; Sajovic 2006; Shaieb 2002; Zaffagnini 2006) and flexion deficits greater than five degrees were reported in 12 trials (Aglietti 1997; Aglietti 2004; Anderson 2001; Ejerhed 2003; Eriksson 2001; Feller 2003; Laxdal 2005; Marder 1991; Matsumoto 2006; O'Neill 1996; Sajovic 2006; Zaffagnini 2006). Three studies reported heel height differences (Feller 2003; Maletis 2007; Matsumoto 2006).
Strength testing
Strength testing was a common outcome, but with inconsistently reported results between the studies. The speeds at which the strength was tested varied from 60, 120, 180, 240 and 300 degrees per second. The dynamometers used included the Cybex I and II for the majority of studies, the Lido Multijoint II for one study (Jansson 2003), as well as the Biodex for two trials (Maletis 2007; O'Neill 1996). Results were reported as average strength measurements (Aglietti 1994; Anderson 2001; Aune 2001; Beynnon 2002; Matsumoto 2006) normal strength deficits (Aglietti 2004), median measurements (Ejerhed 2003), percentage of the opposite side (Jansson 2003; Maletis 2007; Marder 1991), and proportion of patients within defined deficit ranges (O'Neill 1996).
Pain /Anterior knee symptomatology
Pain scores were also inconsistently reported between the studies as anterior knee pain, kneeling pain, and patellofemoral problems. Pain was assessed using the IKDC scale and a Likert scale for patellofemoral crepitation (Aglietti 1994), the Patellofemoral Pain Score (Eriksson 2001), the Kujala Patellofemoral score (Ibrahim 2005), a visual analogue scale for severity of anterior knee pain and pain on kneeling (Aglietti 2004; Ejerhed 2003; Feller 2003; Laxdal 2005), and by measuring the disturbance area of anterior knee sensitivity (Laxdal 2005). Anterior knee pain was reported based on visual analogue scales of severity (Feller 2003), incidence of knee pain (Aune 2001; Beynnon 2002; Ejerhed 2003; Feller 2003; Marder 1991; Ropke 2001; Sajovic 2006; Shaieb 2002), patellofemoral problems (Aglietti 1994) or measured area on the knee reported as a median (Laxdal 2005). Matsumoto 2006 reported complaints of anterior knee pain and Zaffagnini 2006 reported the presence of anterior knee and kneeling pain.
Risk of bias in included studies
The risk of bias of each study was assessed for allocation (sequence generation and allocation concealment), blinding (of outcome assessors), completeness of outcome data, selection of outcomes reported and other sources of bias. Figure 1 shows the judgements for these six items for individual trials. Many of the judgements drew on our ratings for methodological quality.
Figure 1.
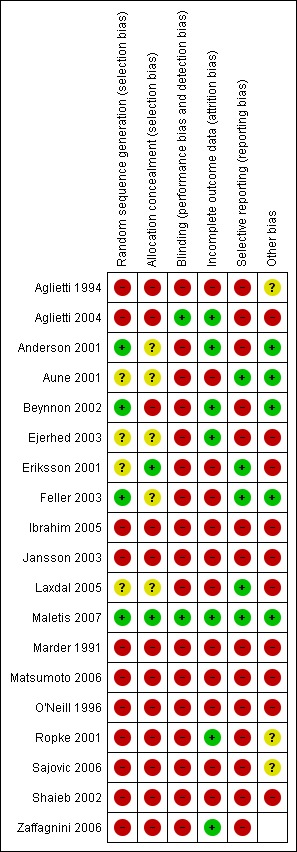
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The consensus scores for each item of the Cochrane Bone, Joint and Muscle Trauma Group's former quality assessment tool (seeAppendix 2) and the Detsky scale (Detsky 1992) (seeAppendix 3) are shown in Appendix 4 and Appendix 5 respectively.
Allocation
Only one trial (Maletis 2007) was rated at low risk of bias reflecting adequate sequence generation (computer‐generated) and allocation concealment (closed envelopes opened at time of surgery). Adequate methods of sequence generation were described in three other trials (computer generated: Anderson 2001; Feller 2003; random number table: Beynnon 2002). Four other trials reported using closed/sealed envelopes (Aune 2001; Ejerhed 2003; Eriksson 2001; Laxdal 2005), but concealment of allocation was judged adequate only in Eriksson 2001, where the envelopes were opened close to surgery. The remaining 11 trials used various forms of quasi‐randomization and were considered at high risk of bias from inadequate sequence generation and lack of allocation concealment. Notably, in Aglietti 2004, though the randomization was described as alternating, there appeared to be some other process involved given the identical distributions of gender, and left and right knees in the two groups. None of the included studies reported any type of stratification in the randomization process to account for differences in chronicity of the ACL deficiency, age, gender, or any other factors that may be associated with outcome.
Blinding
It is not possible to blind the surgeon in surgical trials comparing patellar tendon to hamstring tendon reconstructions and very difficult to guarantee blinding of the patients. However, it should be possible to have an independent assessor who is not involved with the randomization process or surgical procedure to measure the outcomes. Blinded, independent assessors were involved in only two trials (Aglietti 2004; Maletis 2007). In both studies, blinding was achieved using a stockinette sleeve to cover the surgical incisions at each follow‐up visit. No mention of how this may have affected the ability to assess the knees was reported. The following outcomes were blinded during assessment in each of these studies: KT arthrometer testing, tenderness, effusion, pivot shift, patellofemoral crepitus, sensory deficits, range of motion, single‐leg hop test, strength testing, and questionnaires (Aglietti 2004; Maletis 2007). In Aglietti 2004, radiographic outcomes were also assessed; however, it is unclear whether the measurements for tunnel positioning were performed independently. Independent, but non‐blinded assessments of the patients were performed in eight trials (Aune 2001; Ejerhed 2003; Eriksson 2001; Feller 2003; Laxdal 2005; O'Neill 1996; Ropke 2001; Shaieb 2002). The remaining studies did not have blinded or independent assessment.
Incomplete outcome data
Bias can result from the loss of data from the final analysis of patients who were lost to follow‐up or otherwise excluded. Overall, 91.4% of the total participants recruited into the included studies were included in the follow‐up analyses. No studies included an intention‐to‐treat analysis, and cross‐overs to the opposite treatment arm did not occur. There were a variety of reasons for the loss of patients, including two deaths (seeTable 2). In all studies, the largest category of patients excluded from the analysis were reported as lost to follow‐up. Feller 2003 reported one withdrawal, who was excluded from the analysis. Only three (Aune 2001; Marder 1991; O'Neill 1996) out of 12 studies reporting re‐ruptures included the data of these patients in the analysis. Maletis 2007 was the only study out of the three studies reporting contralateral ruptures to include data from these patients in the analysis. Laxdal 2005 excluded one patient from the analysis who had undergone previous ACL reconstruction on the study knee.
In several studies, fewer patients contributed data for some outcomes than the overall number of patients available at final follow‐up. An extreme example is that of O'Neill 1996, where data were available for IKDC, KT arthrometer measurements, strength and radiographic outcomes for only 125 participants, despite being reported as recorded in the later report of this trial (O'Neill 2001) that included 225 participants in the follow‐up analyses.
Shaieb 2002 randomized 82 patients, of whom 12 were lost to follow‐up and 13 were unavailable for clinical examinations. Therefore, while subjective questionnaire information (Lysholm) was available for 70 patients (85%), clinical information was only available for a maximum of 57 patients (69.5%). Furthermore, the published results for each outcome were based on varying numbers of patients in this trial.
Laxdal 2005 randomized 134 patients, but nine participants were lost to follow‐up and seven more participants were excluded leaving 118 participants in the follow‐up analysis. However, data were missing for a further six participants for Tegner and Lysholm scores, and flexion and extension deficit outcomes.
Selective reporting
One potential source of bias in the trials included in this review may be due to selective reporting of some outcomes and not others. This selective reporting may be dependent on the nature and direction of the results, or due to a high loss‐to‐follow‐up rate. For example, in order to include comparable results for the Tegner Activity outcome score, data from Jansson 2003 were taken from the original two‐year publication and follow‐up period because data for these for the later follow‐up period were not reported (Harilainen 2006). A selective reporting bias may also be evident in studies publishing results where no patients were lost to follow‐up. The population of ACL deficient patients is typically very mobile and therefore one would expect at least a small percentage of patients to be lost in follow‐up, particularly beyond two years. In this review, two studies (Aglietti 2004; Zaffagnini 2006) reported on patients randomized and followed to the end of the trial, and state they had no lost to follow‐ups. Aglietti 1994 excluded the three patients who were lost to follow‐up from their analysis. Ropke 2001 also only reported data on all 40 patients they randomized and did not report any lost to follow‐ups, re‐ruptures, contralateral ruptures or withdrawals (Ropke 2001).
Other potential sources of bias
The bases for judgement on the 'other source of bias' were varied, but often involved an assessment of the potential for serious imbalance in baseline characteristics.
Many of the trials did not adequately describe the population in which they conducted the study, including the number of patients that declined participation, or met clinical and intra‐operative exclusion criteria.
All authors of the included studies were contacted for additional information regarding study methodology and unanalyzed data. Ten authors responded, seven of whom provided their original raw data (Ejerhed 2003; Eriksson 2001; Feller 2003; Laxdal 2005; Maletis 2007; Sajovic 2006; Zaffagnini 2006); however, raw data were unavailable for three studies (Anderson 2001; Aune 2001; Shaieb 2002). Anderson 2001 provided additional information on study methodology. The lack of additional data for the remaining studies included in this review is one potential source of bias because the data included in the analyses can only be based on published and reported results.
Effects of interventions
The primary outcomes that were originally defined in the protocol, including functional assessment, return to activity and sport, and patient satisfaction, were not well reported or were inconsistently measured. No trials utilized patient‐based validated outcome measures. There was no universally reported outcome in the literature. The results were not analyzed according to shorter follow‐up intervals for outcome assessment because insufficient data were available. Only four studies (Aglietti 2004; Aune 2001; Feller 2003; Maletis 2007) reported selected outcomes at less than six months follow‐up, and five studies (Aglietti 2004; Aune 2001; Beynnon 2002; Feller 2003; Maletis 2007) reported 12 month follow‐up data. All studies included in this meta‐analysis report outcomes at a minimum of 24 months of follow‐up.
Sensitivity analyses were performed to investigate the differences in the results for randomized and quasi‐randomized trials; and where standard deviations have been imputed. Where sufficient data were available, subgroup analyses were presented for studies using < 4‐strand versus 4‐strand grafts; and hamstring femoral fixation with endobutton versus interference screw. Other planned subgroup analyses (seeSubgroup analysis and investigation of heterogeneity) were not performed.
Primary outcomes
Functional assessments
The hop test was reported in nine studies. Pooled data from five trials (Eriksson 2001; Laxdal 2005; Maletis 2007; O'Neill 1996; Sajovic 2006) for the numbers of trial participants who were able to hop less than 90% of the non‐operative side showed no significant difference between the PT and HT groups at two years or more follow‐up (seeAnalysis 1.1: RR 1.17, 95% confidence interval (CI) 0.84 to 1.63). None of the remaining four trials (Aune 2001; Beynnon 2002; Ejerhed 2003; Zaffagnini 2006) measuring this outcome reported statistically significant differences between the two groups at two year or more follow‐up.
Analysis 1.1.
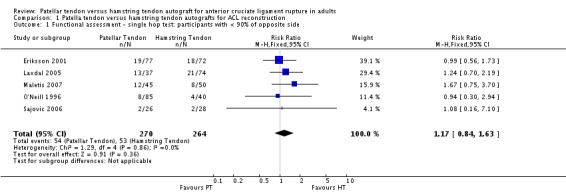
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 1 Functional assessment ‐ single hop test: participants with < 90% of opposite side.
Return to activity/level of sport participation
Pooled data from four trials (Aglietti 2004; Anderson 2001; Beynnon 2002; Marder 1991) showed no statistically significant differences between the patellar tendon and hamstring groups with respect to the proportion of trial participants who returned to light or sedentary activity only (seeAnalysis 1.2: RR 1.23, 95% CI 0.81 to 1.85). One trial that could not be pooled in this analysis (O'Neill 1996) found that patients with a two‐incision patellar tendon reconstruction (95%) returned to a statistically significant greater level of activity, compared to the patients with hamstrings (88%) or one‐incision patellar tendon (89%) reconstruction groups.
Analysis 1.2.
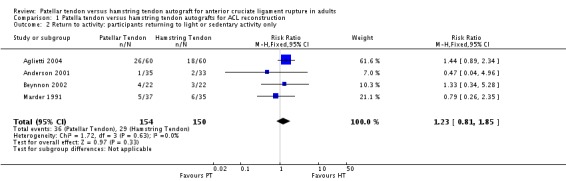
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 2 Return to activity: participants returning to light or sedentary activity only.
Only four trials reporting the Tegner activity score provided full data for pooling (Ejerhed 2003; Laxdal 2005; Maletis 2007; Zaffagnini 2006). This analysis showed no statistically significant difference between the two graft types (mean difference (MD) 0.23, 95% CI ‐0.12 to 0.59; seeAnalysis 1.3). An exploratory analysis where the data for three trials (Ibrahim 2005; Jansson 2003; Ropke 2001) with imputed standard deviations are included also showed no difference; as did a sensitivity analysis showing the results for the three randomized trials only. Two trials not included in the above analysis (Beynnon 2002; Eriksson 2001) reported no statistically significant differences between groups for the Tegner activity score.
Analysis 1.3.
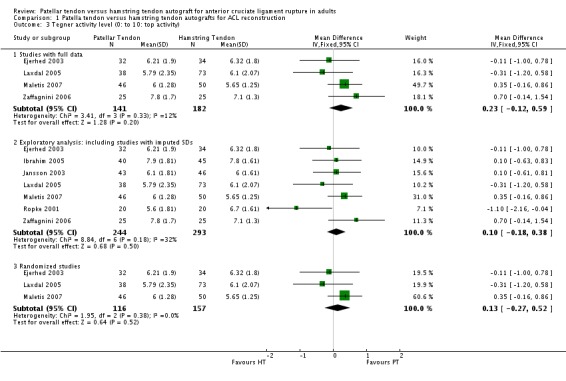
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 3 Tegner activity level (0: to 10: top activity).
The Lysholm score, pooled in five trials with full data, showed no difference between groups (seeAnalysis 1.4). An exploratory analysis where the data for two quasi‐randomized trials (Ibrahim 2005; Shaieb 2002) with imputed standard deviations are included also showed no difference; as did a sensitivity analysis showing the results for the four randomized trials only. While Jansson 2003 reported a significantly greater (P = 0.022) increase in scores from pre‐operative to two year follow‐up period for the HT group (23 points) compared with the PT group (15 points), they also reported no difference between groups in the percentages of patients in the four categories (excellent, good, fair, poor). O'Neill 1996 found no difference between groups in the proportion of patients with greater than 90% Lysholm scores.
Analysis 1.4.
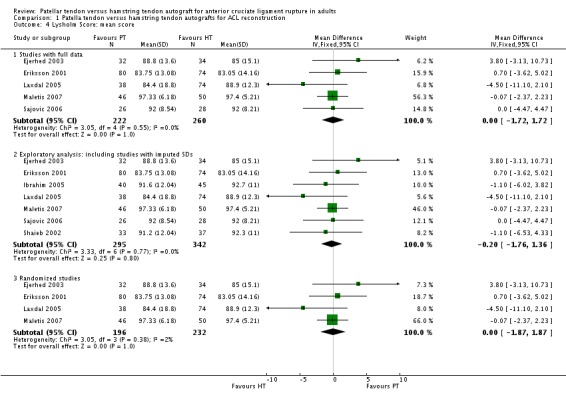
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 4 Lysholm Score: mean score.
Subjective knee scores
A pooled analysis of patient satisfaction was not possible because of the lack of a standard patient satisfaction outcome across the studies.
Based on a 100‐point scale measuring patient satisfaction compared with the opposite knee (i.e rated as 100), the PT group scored 85 and the HT group scored 79 in Aglietti 1994, who reported there was no statistically significant difference. Aglietti 2004 reported no difference between the two groups in subjective outcome based on a 10‐point visual analogue scale.
Aglietti 2004 also reported no difference between groups in the Knee Injury and Osteoarthritis Outcome Score (KOOS) (Roos 1998).
Pooled data from two studies showed no statistical significant difference between the two groups in the Cincinnati knee scores (seeAnalysis 1.5).
Analysis 1.5.
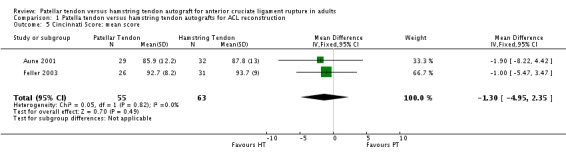
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 5 Cincinnati Score: mean score.
Secondary outcomes
Complications/adverse outcomes/recurrent injury with and without reoperation
As well as the inconsistent reporting of complications/adverse outcomes among the included studies, none of these occurred at a sufficient frequency to make a meaningful comparison between PT and HT reconstructions.
Re‐ruptures were reported in 13 trials. The event rates were small, with 2.6% (15) of the reported 575 PT reconstructions suffering a re‐rupture compared with 3.3% (19) of the 581 reported HT reconstructions. There was no statistically significant difference between the two groups (RR 0.78, 95% CI 0.41 to 1.50; seeAnalysis 1.6). Note, due to missing data, that the denominators for O'Neill 1996 and Shaieb 2002 were the numbers of participants included in the analyses rather than the numbers randomized in each trial.
Analysis 1.6.
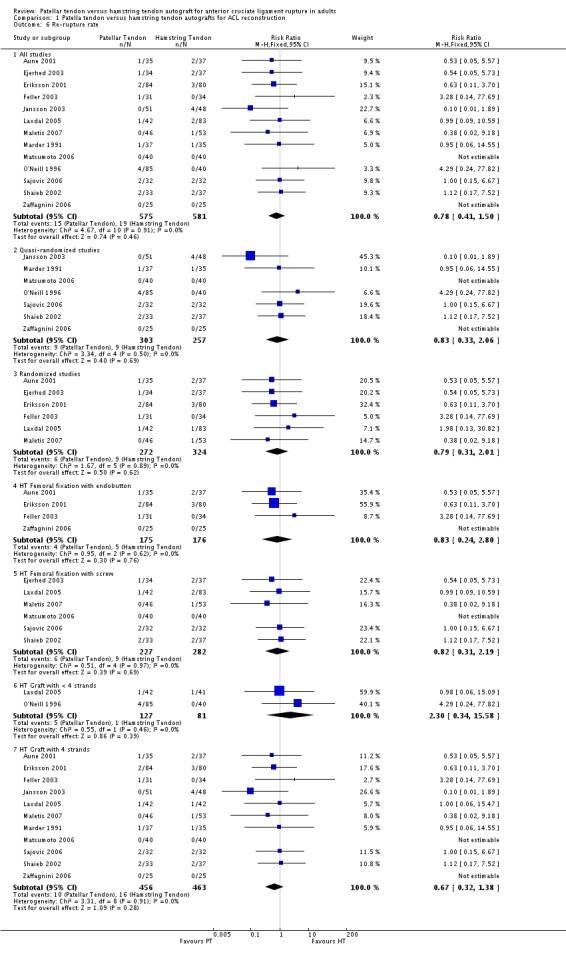
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 6 Re‐rupture rate.
Static stability measures
Although favouring the PT group, the differences between the two groups was not statistically significant for the proportions of patients with translation measurements greater than 5 mm at 134 N (4 trials; RR 0.40; 95% CI 0.07 to 2.33; seeAnalysis 1.7), or at maximum force (6 trials; RR 0.53; 95% CI 0.25 to 1.09; seeAnalysis 1.8). For continuous outcome data, the PT group was favoured for all KT arthrometer measurement forces (seeAnalysis 1.9 and Analysis 1.10). It was not possible to pool the data from Aglietti 1994 because the KT arthrometer categories are not inclusive of all possible measurements. Despite this discrepancy, there were no reported differences. Jansson 2003, which measured static stability using the CA‐4000 arthrometer at an unspecified force, reported no statistically significant difference between groups. There was no difference between groups based on the Stryker side‐to‐side laxity test at 20 lb and 30 lb in Eriksson 2001.
Analysis 1.7.
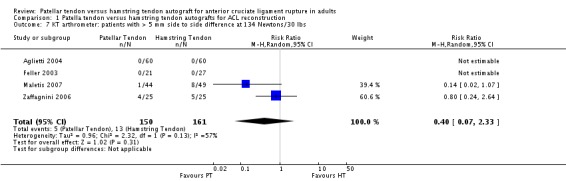
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 7 KT arthrometer: patients with > 5 mm side to side difference at 134 Newtons/30 lbs.
Analysis 1.8.
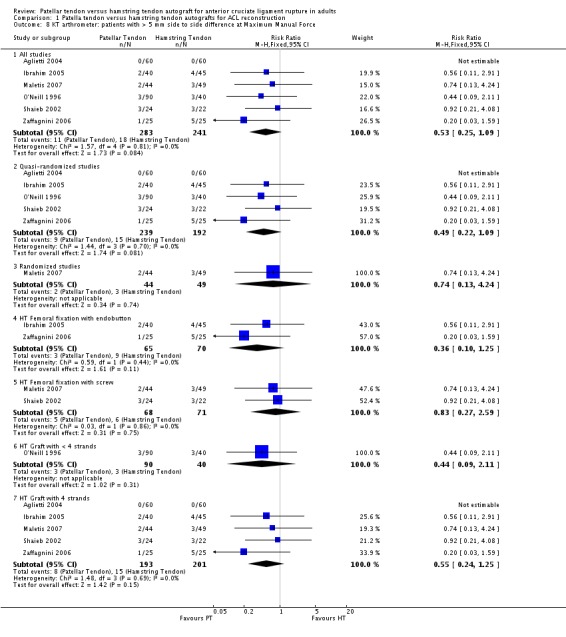
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 8 KT arthrometer: patients with > 5 mm side to side difference at Maximum Manual Force.
Analysis 1.9.
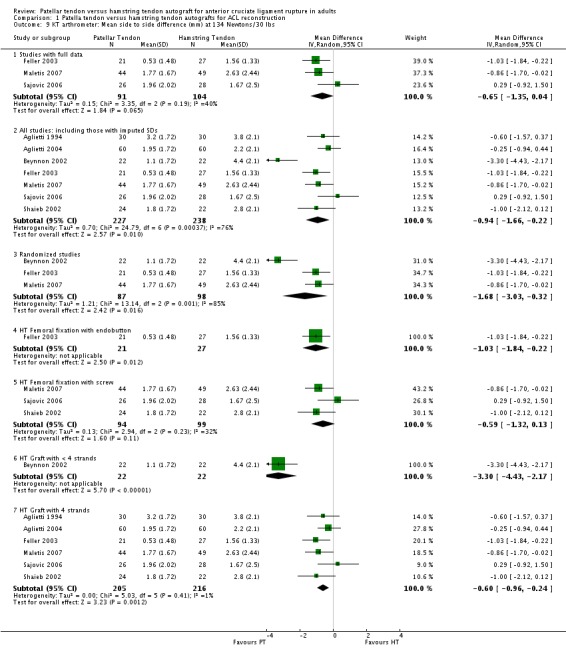
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 9 KT arthrometer: Mean side to side difference (mm) at 134 Newtons/30 lbs.
Analysis 1.10.
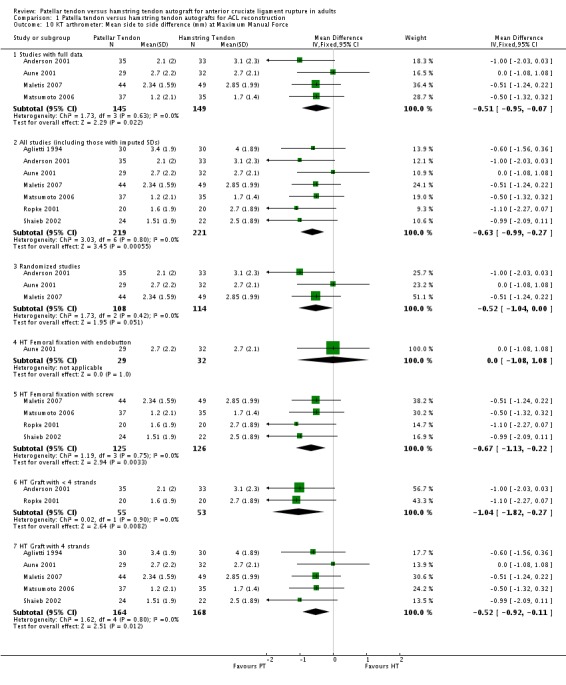
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 10 KT arthrometer: Mean side to side difference (mm) at Maximum Manual Force.
The Lachman test was reported in 13 trials, but only 10 studies defined the Lachman test in a consistent way to allow pooling of the results (i.e. the proportion of patients with greater than 2 mm or a positive (+) translation). These showed a statistically significant effect in favour of patellar tendon reconstruction (RR 0.83; 95% CI 0.71 to 0.99; seeAnalysis 1.11). In the remaining three studies, Aglietti 2004 reported that all patients in both groups were restored to within 5 mm (1+) with a firm endpoint, as measured by the Lachman test; Jansson 2003 reported no difference between groups, based on a positive or negative Lachman test; and Shaieb 2002 reported the Lachman test as a mean side to side difference in each group, but did not define the grading scale or provide statistical interpretation of the results.
Analysis 1.11.
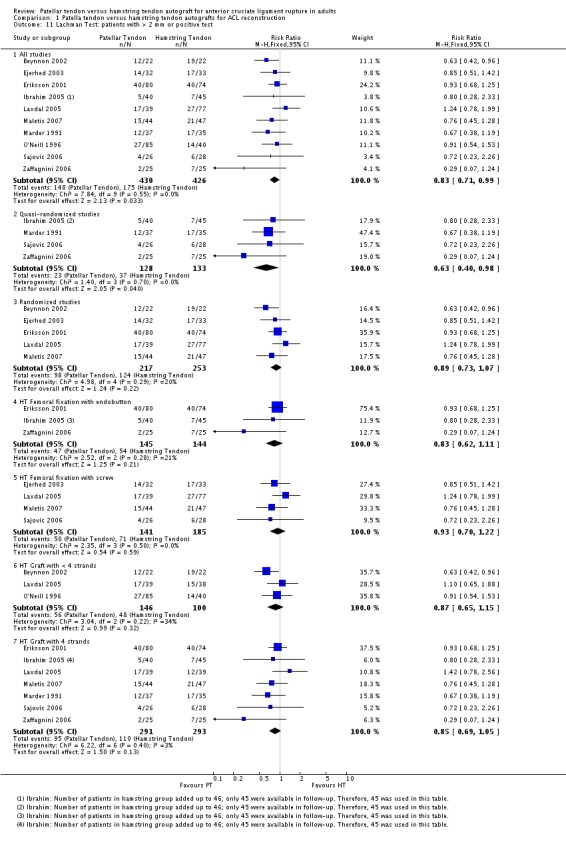
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 11 Lachman Test: patients with > 2 mm or positive test.
Pooled data from 14 studies of patients with a positive pivot shift test at follow‐up significantly favoured patellar tendon reconstruction (RR 0.70; 95% CI 0.54 to 0.89; seeAnalysis 1.12).
Analysis 1.12.
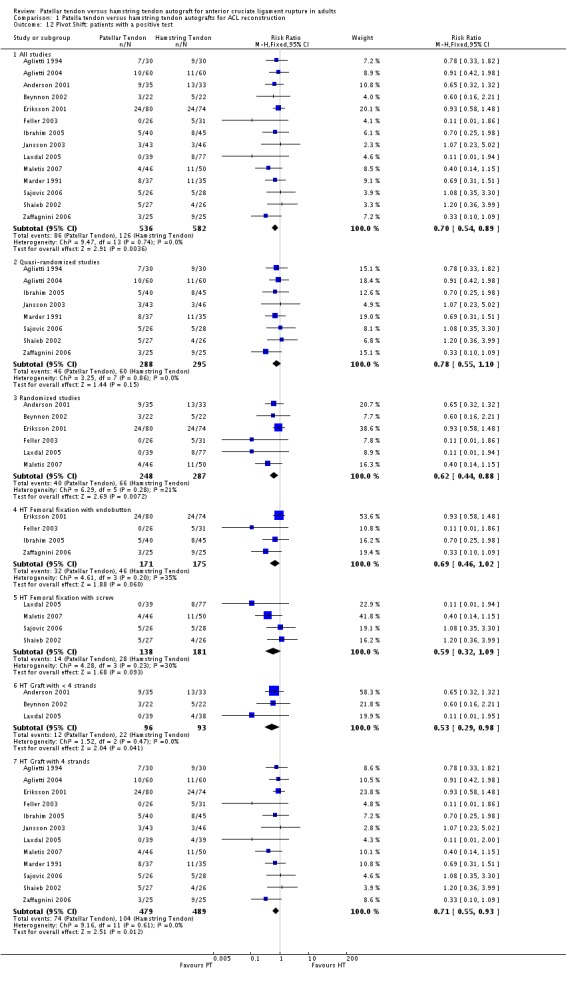
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 12 Pivot Shift: patients with a positive test.
Clinical composite scores ‐ IKDC (International Knee Documentation Committee)
Fifteen out of the 19 trials reported the IKDC rating as a proportion of patients who were Normal or Nearly Normal. Studies using the 1995 version and the 2000 version showed results that were similar between the PT and HT reconstructions, with no statistically significant differences between the two groups (seeAnalysis 1.13).
Analysis 1.13.
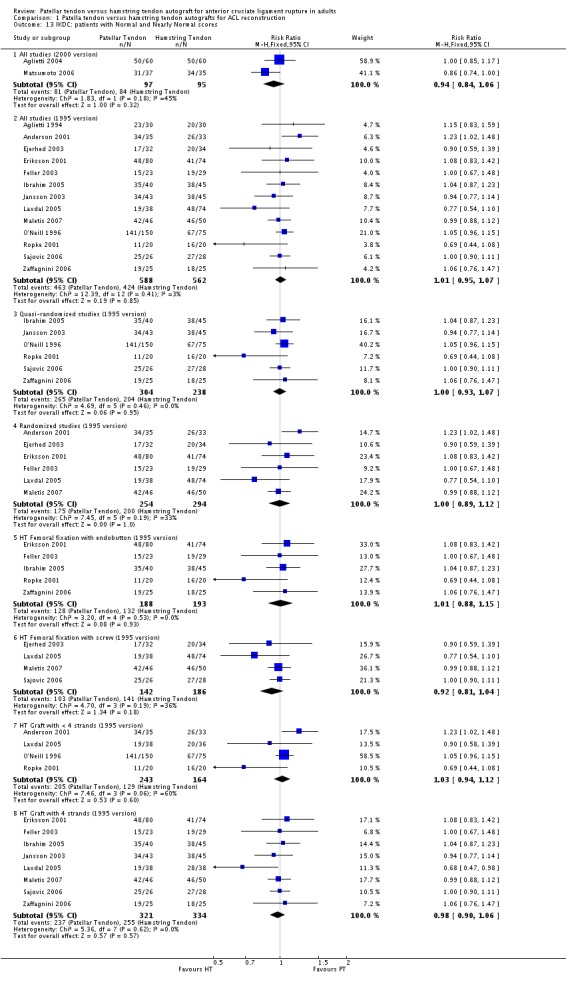
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 13 IKDC: patients with Normal and Nearly Normal scores.
Because the items determining the overall grades in each version of the IKDC knee forms are different, the versions used in each study must be accounted for in the analysis of overall grades and subjective scores. Subjective IKDC scores showed no difference between the two groups when presented in terms of patients with Normal (A) and Nearly Normal (B) grades in the six studies using the 1995 IKDC version (seeAnalysis 1.14). The two trials using the subjective score from the 2000 version favoured the HT grafts; however, the difference did not reach statistical significance (seeAnalysis 1.15).
Analysis 1.14.
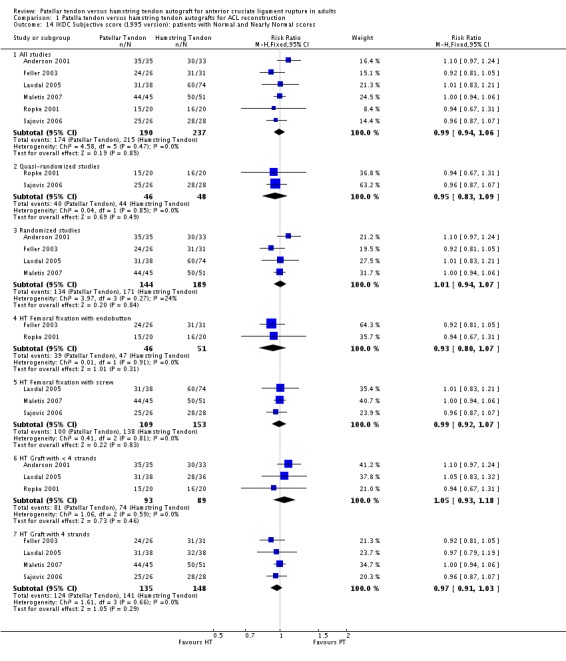
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 14 IKDC Subjective score (1995 version): patients with Normal and Nearly Normal scores.
Analysis 1.15.
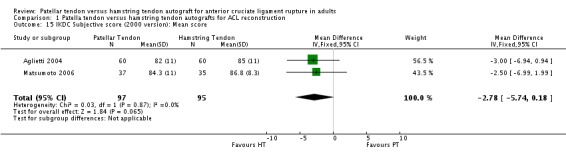
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 15 IKDC Subjective score (2000 version): Mean score.
Range of motion
Range of motion was reported in various ways, with heel height difference and extension deficit of more that three degrees representing extension loss, and flexion deficit of more than five degrees representing flexion loss.
Pooled data from three studies (Feller 2003; Maletis 2007; Matsumoto 2006) reporting heel height differences favoured the hamstring tendon group, However, but the result was not statistically significant when the random‐effects model was used (MD 3.72 mm; 95% CI ‐1.38 mm to 8.82 mm, seeAnalysis 1.16).
Analysis 1.16.
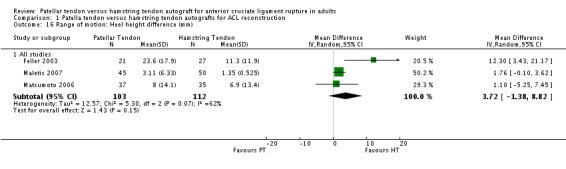
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 16 Range of motion: Heel height difference (mm).
Pooled data from 14 studies reporting the proportion of patients with extension deficits greater than three degrees showed a statistically significant difference favouring the hamstring tendon group (RR 1.71; 95% CI 1.25 to 2.33; seeAnalysis 1.17). Jansson 2003 reported a statistically significant, but clinically insignificant, mean extension deficit in the patellar tendon group. Pooled data from 12 studies for flexion deficits greater than five degrees tended to favour the patellar tendon group (RR 0.88; 95% CI 0.70 to 1.10; seeAnalysis 1.18). Three other studies reported flexion deficits in different ways. Maletis 2007 and Shaieb 2002 reported mean flexion deficits, which were reported not to be statistically significant between the treatment groups. Ibrahim 2005 reported that five patients in the PT group and one patient in the hamstring tendon group had less than 15 degrees flexion deficit.
Analysis 1.17.
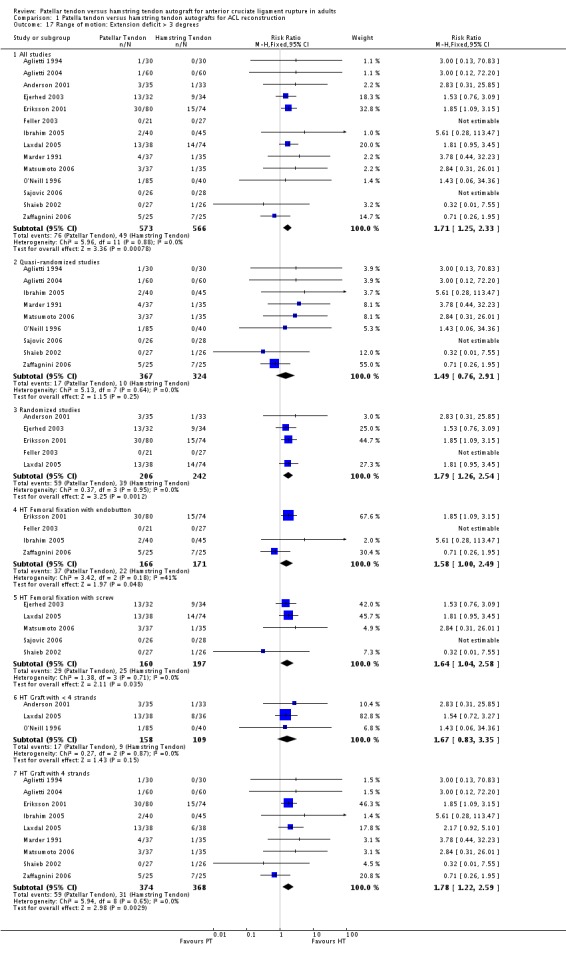
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 17 Range of motion: Extension deficit > 3 degrees.
Analysis 1.18.
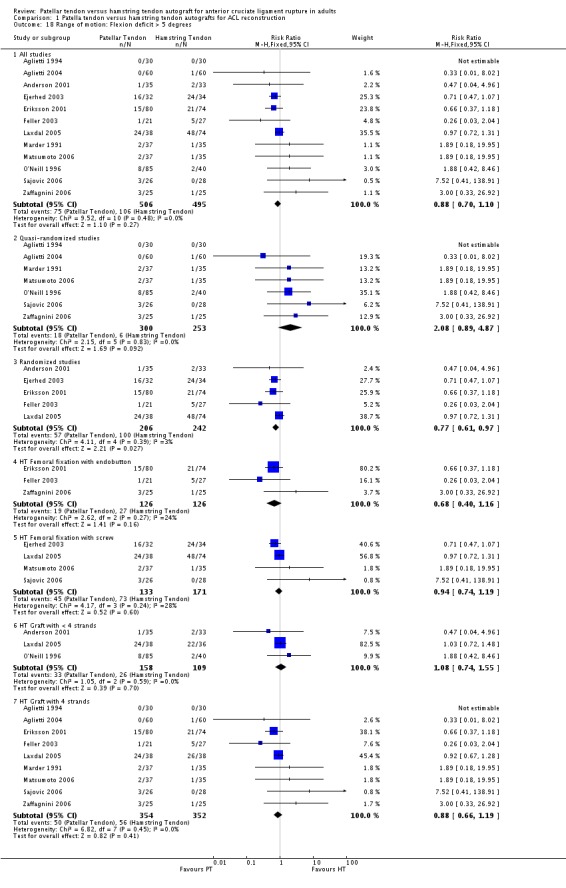
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 18 Range of motion: Flexion deficit > 5 degrees.
Strength testing
Only the studies reporting strength using common speeds and measurement units were compared. Strength was analyzed as an average flexion and extension torque at speeds of 60 and 180 degrees per second using a Cybex Dynanometer (Anderson 2001; Aune 2001; Beynnon 2002; Marder 1991; Matsumoto 2006). PT reconstructions appear to result in a loss of extension and preservation of flexion strength. It is the opposite for HT reconstructions. The flexion strength deficits were greater and represented statistically significant differences. Based on data from five trials, of which three had imputed SDs, the mean difference was 6.63% (95% CI 3.12% to 10.13%) at 60 degrees per second (seeAnalysis 1.19). Based on data from three trials, the mean difference was 5.58% (95% CI 1.52% to 9.65%) at 180 degrees per second (see Analysis 1.20). Note that the designations, "Favours HT" and "Favours PT", are switched in these forest plots to reflect that a higher number is favourable. Including trials with imputed SDs, the extension strength deficits at 60 and 180 degrees per second were not statistically significant: 5 trials; MD ‐2.97%, 95% CI ‐8.10% to 2.16% (seeAnalysis 1.21); 3 trials, MD ‐1.48%, 95% CI ‐6.45% to 3.48% (seeAnalysis 1.22). Aglietti 1994 reported flexion and extension muscle strength deficits based on the Cybex II dynamometer, with no significant difference between groups. Aglietti 2004 reported no strength deficits on the Cybex at 60, 120 and 180 degrees per second. Ejerhed 2003 reported median peak torque values at 60 degrees per second, with no significant differences in strength between groups. Jansson 2003 reported no significant differences between groups using the Lido Multijoint II dynamometer; however, the data to support this conclusion were not published. Maletis 2007 measured flexion and extension strength using the Biodex and concluded that the patellar tendon group had extension strength deficits compared with the hamstring tendon group at all measured speeds; conversely, the hamstring tendon group had flexion strength deficits compared with the patellar tendon group at all measured speeds. Significant differences were present for extension at 60 degrees per second and for flexion at 180 degrees per second (Maletis 2007). O'Neill 1996 reported the proportion of patients with more than 10% strength deficits. The authors concluded that 34% of patients in the patellar tendon group had at least a 10% quadriceps muscle strength deficit, compared with 13% patients in the hamstring tendon group. Nineteen percent of patients in the hamstring tendon group had a hamstring muscle strength deficit greater than 10%, compared with 10% of patients in the patellar tendon group. Both differences were statistically significant (O'Neill 1996).
Analysis 1.19.
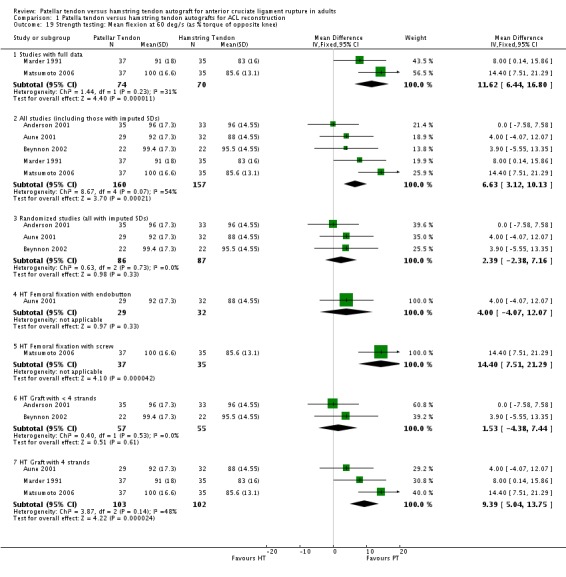
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 19 Strength testing: Mean flexion at 60 deg/s (as % torque of opposite knee).
Analysis 1.20.
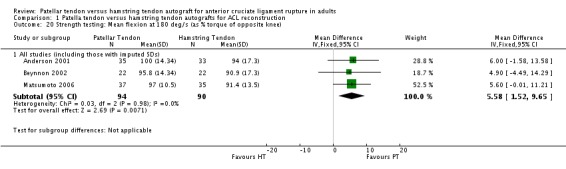
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 20 Strength testing: Mean flexion at 180 deg/s (as % torque of opposite knee).
Analysis 1.21.
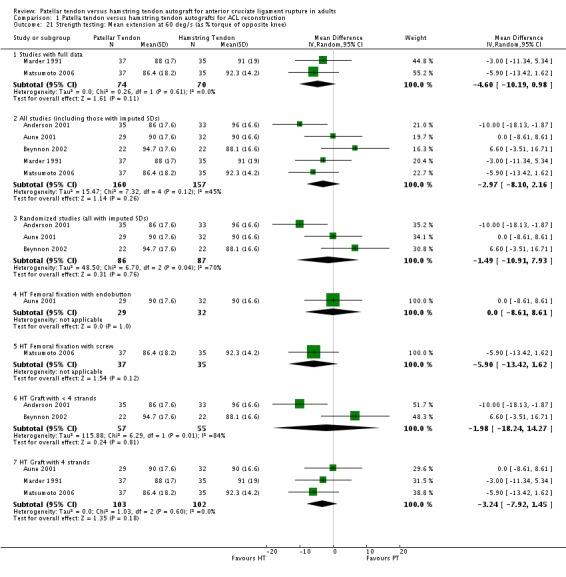
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 21 Strength testing: Mean extension at 60 deg/s (as % torque of opposite knee).
Analysis 1.22.
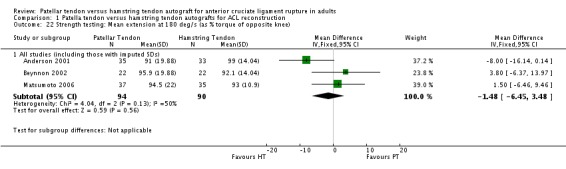
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 22 Strength testing: Mean extension at 180 deg/s (as % torque of opposite knee).
Pain/Anterior knee symptomatology
Because of the variation in how pain outcomes were reported, data for two outcomes were pooled: proportions of patients with general anterior knee symptoms (Aune 2001; Beynnon 2002, Ejerhed 2003; Feller 2003; Marder 1991; Ropke 2001; Sajovic 2006; Shaieb 2002) and kneeling discomfort (Aglietti 2004; Ejerhed 2003; Feller 2003; Laxdal 2005). The general incidence of anterior knee symptomatology was statistically significantly higher in PT patients (RR 1.45, 95% CI 1.05 to 2.01; seeAnalysis 1.23). Kneeling discomfort was also statistically significantly higher in the PT group (RR 4.46, 95% CI 2.97 to 6.69; seeAnalysis 1.24).
Analysis 1.23.
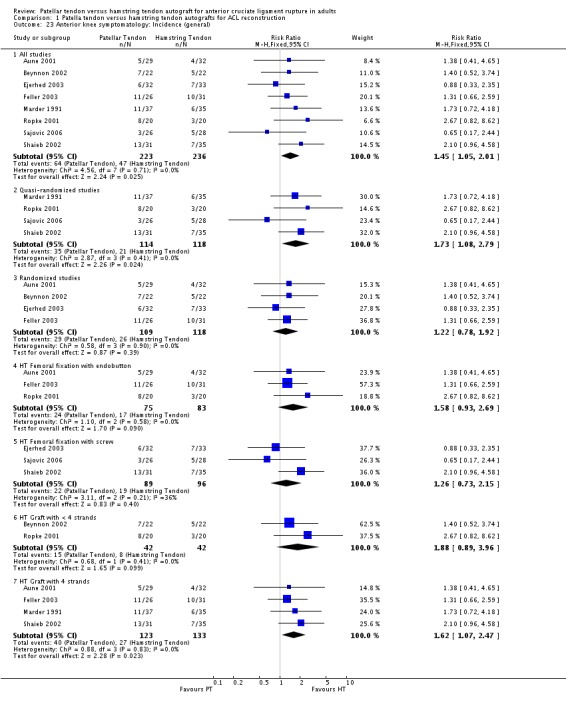
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 23 Anterior knee symptomatology: Incidence (general).
Analysis 1.24.
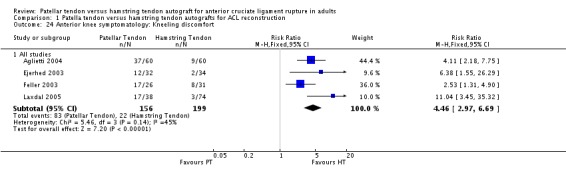
Comparison 1 Patella tendon versus hamstring tendon autografts for ACL reconstruction, Outcome 24 Anterior knee symptomatology: Kneeling discomfort.
Six other studies measured some form of anterior knee symptomatology, but their data could not be presented in the analyses. Aglietti 1994 reported on anterior knee symptomatology as patellofemoral problems and concluded that there was no statistical significance between groups. Eriksson 2001 reported anterior knee symptomatology using a modified patellofemoral score found no difference between the median scores for the two groups. Ibrahim 2005 measured anterior knee symptomatology using the Kujala Patellofemoral score and found no significant difference between the groups. Laxdal 2005 reported the disturbance of anterior knee sensitivity as a median area, with no statistically significant difference between groups. Knee walking ability was reported as being statistically significantly better in the HT group (Laxdal 2005). Matsumoto 2006 reported that complaints of anterior kneeling pain were less common in the HT group, which approached statistical significance. Zaffagnini 2006 reported anterior knee pain was present in 36% of the PT group and 12% of the HT group; and kneeling pain was present in 72% of PT group compared to 44% in the HT group. Both differences were reported to be statistically significant (Zaffagnini 2006).
General comparisons
There were no differences with respect to the number of patients, gender distribution or chronicity of ACL deficiency at the minimum two year follow‐up period.
Sensitivity analyses
Sensitivity analyses were performed to check the robustness of the results when randomized clinical trials only were included. These analyses were performed to avoid making erroneous conclusions because of the inclusion of methodologically poor trials, here represented by quasi‐randomized trials.
Compared with the overall main analysis, pooled data from randomized clinical trials gave visually consistent findings for most outcomes, and supported statistically significant differences favouring PT reconstructions for the following outcomes: KT arthrometer (mean side to side differences at 134 N) (Analysis 1.9), KT arthrometer (mean side to side differences at maximum manual force) (Analysis 1.10) and pivot shift (Analysis 1.12). Similarly, pooled data from randomized clinical trials supported statistically significant differences favouring HT reconstructions for extension range of motion deficit (> 3 degrees) (Analysis 1.17). It is noteworthy that whereas the effect in favour of PT reconstruction for flexion range of motion deficit of > 5 degrees was not statistically significant in the overall main analysis, it was when the results were pooled from randomized trials only (seeAnalysis 1.18). For this outcome, the results from quasi‐randomized trials are consistently and significantly different from those of the randomized trials and tend to favour HT reconstruction.
Subgroup analyses
Subgroup analyses were performed where comparisons could be made between < 4‐strands and 4‐strands hamstring tendon grafts, and HT femoral fixation using an endobutton versus screw fixation. Visual inspection shows no indication of statistically significant differences in effects between the subgroups of either comparison for the various outcomes tested (seeAnalysis 1.6,Analysis 1.8,Analysis 1.10,Analysis 1.11,Analysis 1.2,Analysis 1.13,Analysis 1.14,Analysis 1.17,Analysis 1.18,Analysis 1.19,Analysis 1.21 and Analysis 1.23). An exception could be drawn for KT Arthrometer results (seeAnalysis 1.9) for the < 4 versus 4‐strands comparison but data, including imputed SDs, from only one small trial (Beynnon 2002) were available for the < 4 strands subgroup.
Discussion
Summary of main results
This review includes seven randomized and 12 quasi‐randomized clinical trials that compared patellar tendon (PT) and hamstring tendon (HT) reconstructions of ACL deficient knees. Outcome data at two year follow‐up were available for 1597 out of the 1748 patients randomized into these 19 trials.
Pooled data for primary outcomes, reported in a minority of trials, showed no statistically significant differences between the two graft choices for the single leg hop test (the only commonly reported functional assessment measure), return to activity, Tegner and Lysholm scores, and subjective measures of outcome. There were also no differences found between the two interventions for re‐rupture (other complications and adverse events were inconsistently reported across the studies) or the IKDC composite score (knee laxity, swelling and loss of motion).
The main findings of this review, based on secondary outcome measures, are that patellar tendon reconstruction for anterior cruciate ligament deficiency demonstrates better stability as determined by instrumented measures (KT arthrometer at 134 N and manual maximum forces), Lachman and the pivot shift tests, compared with hamstring tendon reconstructions. Conversely, PT reconstructions result in a higher incidence of kneeling problems and trends towards other measures of pain, discomfort, tenderness and problems in the anterior aspect of the operated knee. Similarly, by harvesting through the extensor mechanism, PT reconstructions consequently have a significant loss of extension motion and a trend to extensor weakness. The HT reconstructions demonstrate the opposite effects, with flexion motion loss and flexion weakness.
Sensitivity analyses showed that pooled data from randomized clinical trials only produced results that were consistent with the above findings. It was noted that for the outcome of flexion deficit, randomized trials favoured PT reconstruction whereas quasi‐randomized trials favoured HT reconstruction. The reason for this disparity is not clear.
Only two of the prespecified subgroup analyses were attempted for outcomes where there were sufficient data. Neither subgroup analysis (< 4‐strands versus 4‐strands hamstring tendon grafts; HT femoral fixation using an endobutton versus screw fixation) revealed significant differences resulting from these differences in methods of HT reconstruction. There is a complete lack of data to allow for subgroup analyses investigating differences in the outcomes between acute (i.e. < 3 months from original injury) ACL reconstruction surgery compared with chronic reconstructions.
Overall completeness and applicability of evidence
The original objective of this systematic review was to compare outcomes between patellar tendon and hamstring tendon ACL reconstructions. The patient populations were similar across the studies in terms of age. More males were included overall, but there was no bias to one procedure over the other. We purposely excluded those patients who had a concomitant extra‐articular procedure, since these additional procedures are not commonly used anymore. Most of the trials had clearly defined inclusion and exclusion criteria at the time of patient recruitment.
The outcomes measured and reported are relevant to some aspects of the evaluation of ACL reconstructions. However, there is an emphasis on static laxity measurements, a lack of consistency with how the outcomes are measured and not enough long‐term follow‐up data. Subjective knee scores were rarely reported and, in particular, the ACL‐QOL (Mohtadi 1998) was not used as an outcome in any of the included studies, despite being the only validated disease‐specific measure of health‐related quality of life for ACL deficiency.
While conducted in several countries, all trial settings represented referral based practices. None of the studies were population based; therefore, specific selection biases for surgical indications could not be determined and may be a potential source of clinical heterogeneity between the studies. Furthermore, regional geographical practice patterns in terms of operative technique, access to care and rehabilitation may also contribute to variability between trials. In each centre, it is possible that eligible patients were not recruited into their trial. In some trials, the authors reported the numbers of these patients and those who refused to participate; however, the reasons for refusal or lack of participation were not consistently identified. Therefore, the current standard of reporting clinical trials (CONSORT) (Moher 2001) was not followed in the majority of studies. This has important implications on the generalizability of the results. However, overall the existing literature reflects current practice and the cross‐section of studies from various countries supports the external validity of the results and conclusions.
Quality of the evidence
With the exception of Maletis 2007, all trials were judged at high risk of bias in at least one of the seven listed domains. The 12 quasi‐randomized trials, some conducted relatively recently, were particularly at high risk of bias. While blinding of the surgeons is impossible for these trials and it will be obvious to the patients which tendons are harvested and used, blinding of outcome assessors is possible but was performed in only two of the 19 trials (Aglietti 2004; Maletis 2007).
Another concern relates to incomplete outcome reporting. While the majority of trials accounted for all of the randomized patients throughout the follow‐up period, there are some puzzling discrepancies in terms of exclusions and reporting of patients lost to follow‐up. For example, Aglietti conducted two separate studies using "a strictly alternating manner" of quasi‐randomization. Aglietti 1994 had three participants (5%) lost to follow‐up out of the 63 randomized. In Aglietti 2004, the sample size was based upon a 20% lost to follow‐up rate. This a priori estimate of 20% is inconsistent with their reported experience and, moreover, in Aglietti 2004 a 100% follow‐up was reported in the final analysis at a minimum of two years. Two other studies (Ropke 2001; Zaffagnini 2006) also reported 100% follow‐up at a minimum of two years. While it is possible that perfect follow‐up can be achieved, perhaps reflecting cultural differences, this does not match typical experience with conducting randomized clinical trials in similar populations (Grant 2005).
Potential biases in the review process
All attempts were made to identify studies, both published and unpublished, which compared PT and HT grafts in ACL reconstruction. However, publication bias cannot be totally avoided as one can never know whether unpublished studies exist beyond the search strategies employed in this review.
Various delays, of which the rigour of the review process involving collaboration from two centres with an imperative on achieving consensus played a large role, have resulted in the date of last search being three years before publication of this review. A search of CENTRAL and the Cochrane Bone, Joint and Muscle Trauma Group Specialized Register carried out in November 2010 was done to gauge whether these delays could have a potentially substantial effect on the review findings. From this, admittedly incomplete, search we identified five articles that would potentially contribute to review findings. One report (Holm 2010) presents 10 year follow‐up results for Aune 2001; and two reports (Alden 2009; Liden 2007) present seven year follow‐up results for Ejerhed 2003; both trials are included already in this review. The response by Liden et al to a letter by Taylor (Taylor 2008) identified that 14 patients of Ejerhed 2003, a single‐centre trial, were also recruited into Laxdal 2005, a multicentre trial. While a disconcerting find, which we will document appropriately in a future update, sensitivity analyses involving the removal of the results for these two trials in turn for primary outcomes do not reveal this to be serious. The fifth article (Taylor 2009) is the published report of Taylor 2006, listed as ongoing and unpublished in this review. Without wanting to pre‐empt the findings of a future update, based on this albeit rapid and non‐rigorous appraisal we consider that it is unlikely that the inclusion of the more recently available evidence would dramatically affect our review findings.
As described above, a key strength of this review was the rigour with which the studies were selected. This process was performed independently at two centres and consensus was reached on inclusions. Similarly, quality assessments and data extraction were performed independently, then agreed upon by consensus.
Although all authors of the included studies were contacted for additional information on study methodology and data, these attempts were only partially successful. In those situations where the authors were contacted, the ability to receive data was not consistent and therefore further attempts were not made. Although the lack of raw data presents a potential bias with respect to this review, it more accurately reflects the information available in the public domain. This also applies to the lack of clarification on methods of randomization, which is of particular concern given that inappropriate studies could have been inadvertently included.
The criteria applied for study selection can dramatically affect the quality and quantity of data available for the review. An important change in the inclusion criteria from that in the protocol was the exclusion of trials with less than two year follow‐up. We consider this is justified on clinical terms. While most patients will have returned to their activities and sports within the first year following ACL reconstruction, our clinical experience is that many patients will not stress their knees or achieve full sport participation until two years. We considered that studies with only six and 12 month follow‐up data give an inadequate picture of outcome and one that would not be consistent with trials with a minimum of two years follow‐up. In the event, this resulted in the exclusion of five trials. However, three of these were reported in abstract only and no further information was forthcoming. The fourth trial (Sato 2005) reported only on anterior tibial translation during isokinetic motion. Thus, the only potential loss to the review was Beard 2001, which reported 12 month follow‐up. As reported above the data available from the included trials for 12 month follow‐up were insufficient for presenting in this review. This again supports our changed inclusion criteria.
One major issue in this review is the decision to pool of results where there is clinical and methodological heterogeneity. Although the patients were of a similar demographic, there were differences in the study populations such as in the inclusion of acute ACL reconstructions, differences in surgical technique, and rehabilitation protocols. In addition to the differences in study methods and associated risk of bias, there was variation in outcomes used, and how measurements were made and reported, and the lack of a universal method of assessment. However, the results were consistent (I‐squared values were zero or low) for most of the outcomes presented in the forest plots. There was also reassurance from the results of sensitivity and subgroup analyses.
Agreements and disagreements with other studies or reviews
The current review can be directly compared with a similar meta‐analysis published in 2006 and 2007 (Biau 2006; Biau 2007). The overall results of this review were similar also to other reviews on this topic (Spindler 2004; Yunes 2001). A more detailed approach to assessing study quality was used in the present study. The review by Biau included 18 trials, compared with 19 in this Cochrane review. Three of the trials included in Biau's review were excluded from our review because only one‐year data were available (Beard 2001; Hantes 2004) or there were insufficient data available (Callaway 1994). Four more recently published trials appear only in our review (Maletis 2007; Matsumoto 2006; Sajovic 2006; Zaffagnini 2006).
Authors' conclusions
The currently available evidence from randomized and quasi‐randomized trials comparing patellar tendon (PT) and hamstring tendon (HT) reconstructions of ACL deficient knees provides an insufficient basis for drawing strong clinical recommendations with respect to the choice between these two grafts for ACL reconstruction surgery.
Evidence from a minority of trials indicated that neither graft was superior in terms of functional assessment (single leg hop test), return to activity, Tegner and Lysholm scores, subjective measures of outcome, re‐rupture or International Knee Documentation Committee scores at a minimum of two years follow‐up. Irrespective of how stability was measured, ACL reconstructions performed with a patellar tendon are more likely to result in a statically stable knee compared with a hamstring tendon reconstruction. Conversely, the evidence supports previous findings that patients having a PT reconstruction are more likely to experience problems in the anterior aspect of their knees, particularly problems with kneeling.
The clinical importance of the findings of loss in extension range of motion and strength after PT reconstruction and loss in flexion range of motion and knee flexion strength after HT reconstruction is uncertain.
This review has highlighted the ongoing controversy regarding the issue of choice of graft type for surgical reconstruction of the ACL deficient knee. Unfortunately, this meta‐analysis has failed to resolve this controversy, partly because of several methodological concerns. Therefore, there remains a need for future methodologically sound research on the question of type of graft choice, including use of newer techniques such as double‐bundle ACL reconstruction.
Future randomized controlled trials should adhere to established standards for methodology, conduct and reporting. These include using allocation concealment, assessor blinding and consistency with respect to choosing and performing outcome assessment. The standardization and use of validated patient‐reported primary outcomes and longer term follow‐up is absolutely necessary for this topic area. Also, the definitions of graft failure and re‐ruptures must be agreed upon to allow consistent reporting. Patients should be stratified into their respective groups based on gender, acuity of the ACL deficiency, patient activity level and associated knee pathologies. The long‐term follow‐up of patients to a minimum of five years is critical to determine if there is any chance of affecting the "natural history" of ACL deficiency.
Acknowledgements
The authors greatly appreciate the considerable editorial contribution provided by Helen Handoll, as well as editorial feedback from Paul Jenkins, Vicki Livingstone and Janet Wale.
We thank the following authors who provided additional unpublished information for their studies: AK Aune, AF Anderson, L Ejerhed, K Eriksson, JA Feller, G Laxdal, GB Maletis, M Sajovic, and S Zaffagnini.
The authors would like to thank the editorial team of the Cochrane Bone, Joint and Muscle Trauma Group for their valuable comments on earlier versions, including the protocol. In particular, we acknowledge Lesley Gillespie for her guidance and contribution to the development of the search strategies and Lindsey Elstub for her assistance throughout the editorial review process.
Appendices
Appendix 1. Search strategies
MEDLINE (OVID ONLINE)
1. Anterior Cruciate Ligament/ 2. (anterior adj2 cruciate$ adj2 ligament$).tw. 3. or/1‐2 4. Reconstructive Surgical Procedures/ 5. Transplants/ 6. Transplantation, Autologous/ 7. Tendons/tr or Tendons, Para‐Articular/tr 8. (graft$ or reconstruct$ or autograft$).tw. 9. or/4‐8 10. patella$.tw. 11. (hamstring$ or gracilis or semi tend?nos$ or semitend?nos$).tw. 12. and/10‐11 13. and/3,9,12 14. randomized controlled trial.pt. 15. controlled clinical trial.pt. 16. Randomized Controlled Trials/ 17. Random Allocation/ 18. Double Blind Method/ 19. Single Blind Method/ 20. or/14‐19 21. Animals/ not Human/ 22. 20 not 21 23. clinical trial.pt. 24. exp Clinical Trials as topic/ 25. (clinic$ adj25 trial$).tw. 26. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 27. Placebos/ 28. placebo$.tw. 29. random$.tw. 30. Research Design/ 31. or/23‐30 32. 31 not 21 33. 32 not 22 34. exp Clinical Trials as topic/ 35. Evaluation Studies.pt. 36. Follow Up Studies/ 37. Prospective Studies/ 38. (control$ or prospectiv$ or volunteer$).tw. 39. or/34‐38 40. 39 not 21 41. 40 not (22 or 33) 42. 22 or 33 or 41 43. and/13,42
EMBASE (OVID ONLINE)
1. Anterior Cruciate Ligament Rupture/ 2. Anterior Cruciate Ligament/ 3. (anterior adj2 cruciate$ adj2 ligament$).tw. 4. or/1‐3 5. Tendon Graft/ or Tissue Graft/ or Muscle Graft/ 6. Autograft/ 7. exp Transplantation/ 8. (graft$ or reconstruct$ or autograft$).tw. 9. or/5‐8 10. Hamstring/ 11. Gracilis Tendon Autograft/ or Gracilis Muscle/ 12. Semitendinosus Tendon Autograft/ or Semitendinosus Muscle/ 13. (hamstring$ or gracilis or semi tend?nos$ or semitend?nos$).tw. 14. or/10‐13 15. Patella Tendon/ 16. Patella Tendon Autograft/ 17. patella$.tw. 18. or/15‐17 19. and/14,18 20. and/4,9,19 21. exp Randomized Controlled trial/ 22. exp Double Blind Procedure/ 23. exp Single Blind Procedure/ 24. exp Crossover Procedure/ 25. Controlled Study/ 26. or/21‐25 27. ((clinical or controlled or comparative or placebo or prospective$ or randomi#ed) adj3 (trial or study)).tw. 28. (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$ or comparis$)).tw. 29. ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw. 30. (cross?over$ or (cross adj1 over$)).tw. 31. ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. 32. (alternat$ adj15 group$1).tw. 33. or/27‐32 34. or/26,33 35. limit 34 to human 36. and/20,35
The Cochrane Library (Wiley InterScience)
#1 MeSH descriptor Anterior Cruciate Ligament explode all trees #2 (anterior in Record Title near/6 cruciate* in Record Title near/6 ligament* in Record Title) #3 (anterior in Abstract near/6 cruciate* in Abstract near/6 ligament* in Abstract) #4 (#1 or #2 or #3) #5 MeSH descriptor Reconstructive Surgical Procedures this term only #6 MeSH descriptor Transplants explode all trees #7 MeSH descriptor Transplantation, Autologous explode all trees #8 ( (graft* in Record Title near/6 reconstruct* in Record Title near/6 autograft* in Record Title) or (graft* in Abstract near/6 reconstruct* in Abstract near/6 autograft* in Abstract) ) #9 (#5 or #6 or #7 or #8) #10 (patella* in Record Title or patalla* in Abstract) #11 ( (hamstring* in Record Title or gracilis in Record Title or (semi in Record Title and tendinos* in Record Title) or (semi in Record Title and tendonos* in Record Title) or semitendinos* in Record Title or semitendonos* in Record Title) or (hamstring* in Abstract or gracilis in Abstract or (semi in Abstract and tendinos* in Abstract) or (semi in Abstract and tendonos* in Abstract) or semitendinos* in Abstract or semitendonos* in Abstract) ) #12 (#10 and #11) #13 (#4 and #9 and #12)
Appendix 2. Cochrane Bone, Joint and Muscle Trauma Group's quality assessment tool
| Items and scores |
| A. Was the assigned treatment adequately concealed prior to allocation? 2 = method did not allow disclosure of assignment. 1 = small but possible chance of disclosure of assignment or unclear. 0 = quasi‐randomized or open list/tables. |
| B. Were the outcomes of patients/participants who withdrew described and included in the analysis (intention to treat)? 2 = withdrawals well described and accounted for in analysis. 1 = withdrawals described and analysis not possible. 0 = no mention, inadequate mention, or obvious differences and no adjustment. |
| C. Were the outcome assessors blinded to treatment status? 2 = effective action taken to blind assessors. 1 = small or moderate chance of unblinding of assessors. 0 = not mentioned or not possible. |
| D. Were the treatment and control groups comparable at entry? 2 = good comparability of groups, or confounding adjusted for in analysis. 1 = confounding small; mentioned but not adjusted for. 0 = large potential for confounding, or not discussed. |
| E. Were the participants blind to assignment status after allocation? 2 = effective action taken to blind participants. 1 = small or moderate chance of unblinding of participants. 0 = not possible, or not mentioned (unless double‐blind), or possible but not done. |
| F. Were the treatment providers blind to assignment status? 2 = effective action taken to blind treatment providers. 1 = small or moderate chance of unblinding of treatment providers. 0 = not possible, or not mentioned (unless double‐blind), or possible but not done. |
| G. Were care programmes, other than the trial options, identical? 2 = care programmes clearly identical. 1 = clear but trivial differences. 0 = not mentioned or clear and important differences in care programmes. |
| H. Were the inclusion and exclusion criteria clearly defined? 2 = clearly defined. 1 = inadequately defined. 0 = not defined. |
| I. Were the interventions clearly defined? 2 = clearly defined interventions are applied with a standardized protocol. 1 = clearly defined interventions are applied but the application protocol is not standardized. 0 = intervention and/or application protocol are poorly or not defined. |
| J. Were the outcome measures used clearly defined? (by outcome) 2 = clearly defined. 1 = inadequately defined. 0 = not defined. |
| K. Were diagnostic tests used in outcome assessment clinically useful? (by outcome) 2 = optimal. 1 = adequate. 0 = not defined, not adequate. |
| L. Was the surveillance active, and of clinically appropriate duration? (by outcome) 2 = active surveillance and appropriate duration. 1 = active surveillance, but inadequate duration. 0 = surveillance not active or not defined. |
Appendix 3. Detsky Quality Scale for randomized trials
| Assessment |
| 1. a) Were the patients assigned randomly? 1 = Yes 0 = No b) Randomization adequately described? 2 = Yes 1 = Partly 0 = No c) Was treatment group concealed to investigator? 1 = Yes 0 = No |
| 2. a) Description of outcome measurement adequate? 1 = Yes 0 = No b) Outcome measurements objective? 2 = Yes 1 = Partly 0 = No c) Were the assessors blind to treatment? 1 = Yes 0 = No |
| 3. a) Were inclusion /exclusion criteria well defined? 2 = Yes 1 = Partly 0 = No b) Number of patients excluded and reason? 2 = Yes 1 = Partly 0 = No |
| 4. a) Was the therapy fully described for the treatment group? 2 = Yes 1 = Partly 0 = No b) Was the therapy fully described for the controls? 2 = Yes 1 = Partly 0 = No |
| 5. a) Was the test stated and was there a P value? 1 = Yes 0 = No b) Was the statistical analysis appropriate? 2 = Yes 1 = Partly 0 = No c) If the trial was negative, were confidence intervals or post hoc power calculations performed? 1 = Yes 0 = No d) Sample size calculation before the study? 1 = Yes 0 = No |
Appendix 4. Methodological quality assessment using the Cochrane BJMT tool: Results for individual trials
| Items (defined in Table 2) | ||||||||||||
| Study ID | A | B | C | D | E | F | G | H | I | J | K | L |
| Aglietti 1994 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| Aglietti 2004 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| Anderson 2001 | 1 | 1 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| Aune 2001 | 2 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 2 |
| Beynnon 2002 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| Ejerhed 2003 | 2 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 1 |
| Eriksson 2001 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 1 | 1 |
| Feller 2003 | 1 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 2 |
| Ibrahim 2005 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 1 | 1 | 1 |
| Jansson 2003 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 1 | 1 |
| Laxdal 2005 | 2 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 2 |
| Maletis 2007 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 1 | 2 |
| Marder 1991 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 2 | 1 | 1 |
| Matsumoto 2006 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 2 |
| O'Neill 1996 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 1 | 2 |
| Ropke 2001 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 1 |
| Sajovic 2006 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| Shaieb 2002 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 1 | 2 |
| Zaffagnini 2006 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 1 |
Appendix 5. Methodological quality assessment using the Detsky Quality Scale: Results of individual trials
| Study ID | Item | |||||||||||||
| 1 | 2 | 3 | 4 | 5 | ||||||||||
| a) | b) | c) | a) | b) | c) | a) | b) | a) | b) | a) | b) | c) | d) | |
| Aglietti 1994 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 2 | 0 | 0 |
| Aglietti 2004 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 2 | 2 | 1 | 2 | 0 | 1 |
| Anderson 2001 | 1 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 0 |
| Aune 2001 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 1 | 0 | 0 |
| Beynnon 2002 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 2 | 0 | 1 |
| Ejerhed 2003 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 2 | 0 | 0 |
| Eriksson 2001 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 0 |
| Feller 2003 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 2 | 0 | 0 |
| Ibrahim 2005 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| Jansson 2003 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 2 | 0 | 1 |
| Laxdal 2005 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 2 | 0 | 0 |
| Maletis 2007 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 1 |
| Marder 1991 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 0 |
| Matsumoto 2006 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 2 | 0 | 0 |
| O'Neill 1996 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 1 | 0 | 0 |
| Ropke 2001 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 2 | 0 | 0 |
| Sajovic 2006 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 2 | 0 | 0 |
| Shaieb 2002 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 0 |
| Zaffagnini 2006 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 1 | 0 |
Data and analyses
Comparison 1.
Patella tendon versus hamstring tendon autografts for ACL reconstruction
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional assessment ‐ single hop test: participants with < 90% of opposite side | 5 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.84, 1.63] |
| 2 Return to activity: participants returning to light or sedentary activity only | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.85] |
| 3 Tegner activity level (0: to 10: top activity) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Studies with full data | 4 | 323 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.12, 0.59] |
| 3.2 Exploratory analysis: including studies with imputed SDs | 7 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.38] |
| 3.3 Randomized studies | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.27, 0.52] |
| 4 Lysholm Score: mean score | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Studies with full data | 5 | 482 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.72, 1.72] |
| 4.2 Exploratory analysis: including studies with imputed SDs | 7 | 637 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.76, 1.36] |
| 4.3 Randomized studies | 4 | 428 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.87, 1.87] |
| 5 Cincinnati Score: mean score | 2 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.95, 2.35] |
| 6 Re‐rupture rate | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 All studies | 13 | 1156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.41, 1.50] |
| 6.2 Quasi‐randomized studies | 7 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.33, 2.06] |
| 6.3 Randomized studies | 6 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.31, 2.01] |
| 6.4 HT Femoral fixation with endobutton | 4 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.80] |
| 6.5 HT Femoral fixation with screw | 6 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.31, 2.19] |
| 6.6 HT Graft with < 4 strands | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.34, 15.58] |
| 6.7 HT Graft with 4 strands | 11 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.38] |
| 7 KT arthrometer: patients with > 5 mm side to side difference at 134 Newtons/30 lbs | 4 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.07, 2.33] |
| 8 KT arthrometer: patients with > 5 mm side to side difference at Maximum Manual Force | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 All studies | 6 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.09] |
| 8.2 Quasi‐randomized studies | 5 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.22, 1.09] |
| 8.3 Randomized studies | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.13, 4.24] |
| 8.4 HT Femoral fixation with endobutton | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.25] |
| 8.5 HT Femoral fixation with screw | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.27, 2.59] |
| 8.6 HT Graft with < 4 strands | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.09, 2.11] |
| 8.7 HT Graft with 4 strands | 5 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.25] |
| 9 KT arthrometer: Mean side to side difference (mm) at 134 Newtons/30 lbs | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Studies with full data | 3 | 195 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.35, 0.04] |
| 9.2 All studies: including those with imputed SDs | 7 | 465 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.66, ‐0.22] |
| 9.3 Randomized studies | 3 | 185 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐3.03, ‐0.32] |
| 9.4 HT Femoral fixation with endobutton | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.84, ‐0.22] |
| 9.5 HT Femoral fixation with screw | 3 | 193 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.32, 0.13] |
| 9.6 HT Graft with < 4 strands | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐4.43, ‐2.17] |
| 9.7 HT Graft with 4 strands | 6 | 421 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.96, ‐0.24] |
| 10 KT arthrometer: Mean side to side difference (mm) at Maximum Manual Force | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Studies with full data | 4 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.95, ‐0.07] |
| 10.2 All studies (including those with imputed SDs) | 7 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.99, ‐0.27] |
| 10.3 Randomized studies | 3 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.04, 0.00] |
| 10.4 HT Femoral fixation with endobutton | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.08, 1.08] |
| 10.5 HT Femoral fixation with screw | 4 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.13, ‐0.22] |
| 10.6 HT Graft with < 4 strands | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.82, ‐0.27] |
| 10.7 HT Graft with 4 strands | 5 | 332 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.92, ‐0.11] |
| 11 Lachman Test: patients with > 2 mm or positive test | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 All studies | 10 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.99] |
| 11.2 Quasi‐randomized studies | 4 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 11.3 Randomized studies | 5 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.07] |
| 11.4 HT Femoral fixation with endobutton | 3 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.11] |
| 11.5 HT Femoral fixation with screw | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.22] |
| 11.6 HT Graft with < 4 strands | 3 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.15] |
| 11.7 HT Graft with 4 strands | 7 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.05] |
| 12 Pivot Shift: patients with a positive test | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 All studies | 14 | 1118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.89] |
| 12.2 Quasi‐randomized studies | 8 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.10] |
| 12.3 Randomized studies | 6 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.88] |
| 12.4 HT Femoral fixation with endobutton | 4 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.02] |
| 12.5 HT Femoral fixation with screw | 4 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.09] |
| 12.6 HT Graft with < 4 strands | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.29, 0.98] |
| 12.7 HT Graft with 4 strands | 12 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.93] |
| 13 IKDC: patients with Normal and Nearly Normal scores | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 All studies (2000 version) | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.06] |
| 13.2 All studies (1995 version) | 13 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 13.3 Quasi‐randomized studies (1995 version) | 6 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 13.4 Randomized studies (1995 version) | 6 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 13.5 HT Femoral fixation with endobutton (1995 version) | 5 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.15] |
| 13.6 HT Femoral fixation with screw (1995 version) | 4 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 13.7 HT Graft with < 4 strands (1995 version) | 4 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.12] |
| 13.8 HT Graft with 4 strands (1995 version) | 8 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| 14 IKDC Subjective score (1995 version): patients with Normal and Nearly Normal scores | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 All studies | 6 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.06] |
| 14.2 Quasi‐randomized studies | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.09] |
| 14.3 Randomized studies | 4 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.07] |
| 14.4 HT Femoral fixation with endobutton | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.07] |
| 14.5 HT Femoral fixation with screw | 3 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.07] |
| 14.6 HT Graft with < 4 strands | 3 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 14.7 HT Graft with 4 strands | 4 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |
| 15 IKDC Subjective score (2000 version): Mean score | 2 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐2.78 [‐5.74, 0.18] |
| 16 Range of motion: Heel height difference (mm) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 All studies | 3 | 215 | Mean Difference (IV, Random, 95% CI) | 3.72 [‐1.38, 8.82] |
| 17 Range of motion: Extension deficit > 3 degrees | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 All studies | 14 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.25, 2.33] |
| 17.2 Quasi‐randomized studies | 9 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.76, 2.91] |
| 17.3 Randomized studies | 5 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.26, 2.54] |
| 17.4 HT Femoral fixation with endobutton | 4 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.00, 2.49] |
| 17.5 HT Femoral fixation with screw | 5 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.04, 2.58] |
| 17.6 HT Graft with < 4 strands | 3 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.83, 3.35] |
| 17.7 HT Graft with 4 strands | 9 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.22, 2.59] |
| 18 Range of motion: Flexion deficit > 5 degrees | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 All studies | 12 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.10] |
| 18.2 Quasi‐randomized studies | 7 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.89, 4.87] |
| 18.3 Randomized studies | 5 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.97] |
| 18.4 HT Femoral fixation with endobutton | 3 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.16] |
| 18.5 HT Femoral fixation with screw | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.19] |
| 18.6 HT Graft with < 4 strands | 3 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.55] |
| 18.7 HT Graft with 4 strands | 9 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.19] |
| 19 Strength testing: Mean flexion at 60 deg/s (as % torque of opposite knee) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 Studies with full data | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 11.62 [6.44, 16.80] |
| 19.2 All studies (including those with imputed SDs) | 5 | 317 | Mean Difference (IV, Fixed, 95% CI) | 6.63 [3.12, 10.13] |
| 19.3 Randomized studies (all with imputed SDs) | 3 | 173 | Mean Difference (IV, Fixed, 95% CI) | 2.39 [‐2.38, 7.16] |
| 19.4 HT Femoral fixation with endobutton | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐4.07, 12.07] |
| 19.5 HT Femoral fixation with screw | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 14.40 [7.51, 21.29] |
| 19.6 HT Graft with < 4 strands | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 1.53 [‐4.38, 7.44] |
| 19.7 HT Graft with 4 strands | 3 | 205 | Mean Difference (IV, Fixed, 95% CI) | 9.39 [5.04, 13.75] |
| 20 Strength testing: Mean flexion at 180 deg/s (as % torque of opposite knee) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 All studies (including those with imputed SDs) | 3 | 184 | Mean Difference (IV, Fixed, 95% CI) | 5.58 [1.52, 9.65] |
| 21 Strength testing: Mean extension at 60 deg/s (as % torque of opposite knee) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Studies with full data | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐4.60 [‐10.19, 0.98] |
| 21.2 All studies (including those with imputed SDs) | 5 | 317 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐8.10, 2.16] |
| 21.3 Randomized studies (all with imputed SDs) | 3 | 173 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐10.91, 7.93] |
| 21.4 HT Femoral fixation with endobutton | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐8.61, 8.61] |
| 21.5 HT Femoral fixation with screw | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐5.90 [‐13.42, 1.62] |
| 21.6 HT Graft with < 4 strands | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐18.24, 14.27] |
| 21.7 HT Graft with 4 strands | 3 | 205 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐7.92, 1.45] |
| 22 Strength testing: Mean extension at 180 deg/s (as % torque of opposite knee) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 All studies (including those with imputed SDs) | 3 | 184 | Mean Difference (IV, Fixed, 95% CI) | ‐1.48 [‐6.45, 3.48] |
| 23 Anterior knee symptomatology: Incidence (general) | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 All studies | 8 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.05, 2.01] |
| 23.2 Quasi‐randomized studies | 4 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.08, 2.79] |
| 23.3 Randomized studies | 4 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.78, 1.92] |
| 23.4 HT Femoral fixation with endobutton | 3 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.93, 2.69] |
| 23.5 HT Femoral fixation with screw | 3 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.73, 2.15] |
| 23.6 HT Graft with < 4 strands | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.89, 3.96] |
| 23.7 HT Graft with 4 strands | 4 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.07, 2.47] |
| 24 Anterior knee symptomatology: Kneeling discomfort | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 All studies | 4 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [2.97, 6.69] |
| 25 Numbers of participants in analyses / Numbers entered in each trial | 17 | 1456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| 26 Numbers with acute reconstructions at baseline | 7 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.87, 1.33] |
| 27 Numbers of males at baseline | 17 | 1423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.05] |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 9, 2011
| Date | Event | Description |
|---|---|---|
| 4 May 2008 | Amended | Converted to new review format. |
Differences between protocol and review
Subsequent to the protocol, we stipulated that a minimum two year follow‐up was necessary for trial inclusion. This was to ensure that there was consistency across the trials in the report of functional outcomes.
We restructured the list of outcomes measures so that activity and subjective knee scores that had been listed separately were listed under primary outcomes, and merged complications, adverse effects and recurrent injury into one category.
Only scores for individual items rather than total scores for methodological quality are presented. Risk of bias tables are completed for all trials.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aglietti 1994
| Methods | Treatment allocation by alternating Non blinded assessment Mean follow‐up: 28 months (1994 publication); 5.7 years (1997 publication) Loss to follow‐up: 3 (excluded from analysis) Graft re‐ruptures: not reported | |
| Participants | Italy 63 participants with chronic (> 6 months), isolated unilateral ACL tears Group allocation data for 60 participants. PT: n = 30, mean 21.5 years (14 to 33 years), 16 males / 14 females HT: n = 30, mean 23.7 years (14 to 36 years), 20 males / 10 females | |
| Interventions | Arthroscopic ACL reconstruction with: 1. Patellar tendon (number of incisions not specified; 20 mm bone blocks, proximal fixation ‐ sutures, cortical bone screw, washer; tibial fixation ‐ interference screw, sutures and cortical bone screw) versus 2. Hamstring tendon: doubled semitendinosus/gracilis tendon (4 strands; number of incisions not specified; proximal fixation ‐ cortical bone screw, washer; tibial fixation ‐ staple, sutures and cortical bone screw (in 24 cases), or sutures, screw and washer (in 6 cases)) | |
| Outcomes | Mean 28 months follow‐up for: Subjective evaluation, symptoms, range of motion, joint stability; extension loss; flexion loss; static stability with KT‐2000 (15 lb, 20 lb, 30 lb); muscle strength with Cybex II (60, 120, 180 deg/sec); level of activity (based on function, intensity, and return to sport); objective stability; patellofemoral symptoms; pivot shift (absent/glide/jerk/subluxation) | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating patients. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three patients lost to follow‐up not accounted for in analysis. |
| Selective reporting (reporting bias) | High risk | Did not report re‐ruptures, contralateral ruptures or withdrawals. |
| Other bias | Unclear risk | Unclear |
Aglietti 2004
| Methods | Treatment allocation by alternating Independent & blinded assessment Minimum follow‐up: 24 months Loss to follow‐up: None Graft re‐ruptures: not reported | |
| Participants | Italy 120 patients with chronic (> 30 days), isolated unilateral ACL tears PT: n = 60, mean 25 years (16 to 39 years), 46 males / 14 females HT: n = 60, mean 25 years (15 to 39 yrs), 46 males / 14 females | |
| Interventions | Arthroscopically assisted ACL reconstruction with: 1. Patellar tendon (single incision; proximal fixation ‐ Tunneloc screw; tibial fixation ‐ Soft threaded interference screw) versus 2. Hamstring tendon: Semitendinosus/gracilis tendons (4 strands; single incision; proximal fixation ‐ Bone Mulch screw; tibial fixation ‐ WasherLoc device) | |
| Outcomes | Minimum 4, 12 and 24 months follow‐up for: Static stability with KT‐1000 (134N & MM); Lachman (defined by the IKDC 2000); pivot shift (defined by IKDC 2000); IKDC (2000); return to sports/activity; strength with Cybex (60, 120, 180 deg/sec); KOOS; range of motion; anterior knee pain | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Independent blinded assessor for follow‐up outcome assessment. Patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | Did not report re‐ruptures, contralateral ruptures or withdrawals. |
| Other bias | High risk | It is difficult to understand how 60 patients randomly assigned to each group resulted in identical numbers of left and right sided injured knees, the same sex distribution and mean age. There were no patients lost to follow‐up despite an a priori sample size estimate, which allowed for a 20% lost to follow‐up. In their previous published trial, only 3 of 60 patients were lost to follow‐up, which suggests that accounting for a 20% lost to follow‐up rate in the sample size estimate was excessive. |
Anderson 2001
| Methods | Computer‐generated random assignment Non blinded assessment Mean follow‐up: 35.4 months Loss to follow‐up: 2 HT (these were excluded from the analysis) Graft re‐ruptures: not reported | |
| Participants | USA Study recruitment period: 1991 to 1993 70 participants with acute (< 12 weeks) or chronic (> 12 weeks) ACL deficiency PT: n = 35, mean 23.6 years (14 to 44 years), 23 males / 12 females HT: n = 35, mean 20.1 years (14 to 38 years), 23 males / 12 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision; proximal fixation ‐ interference screw (7 x 25 mm); tibial fixation ‐ barbed staples) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (2 strands; single incision; proximal fixation ‐ barbed staples; tibial fixation ‐ non‐absorbable sutures) | |
| Outcomes | Mean 35 months follow‐up for: Static stability with KT‐1000; range of motion; pivot shift; IKDC; return to sport/activity; strength with Cybex II (60 & 180 deg/s); patellofemoral crepitus | |
| Notes | A third intervention group involving HT with extra‐articular procedure (semitendinosus/gracilis tendons with a Losee extra‐articular iliotibial band tenodesis) in 35 patients is not included in this review. Additional methodological information received from Anderson comprised a description of the sample size calculation, patient recruitment / inclusion / exclusion numbers, and the number or re‐ruptures and revisions. Individual patient data were unavailable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | Did not report re‐ruptures, contralateral ruptures or withdrawals. |
| Other bias | Low risk | |
Aune 2001
| Methods | Randomization method: sealed envelopes Independent, non blinded assessment Minimum follow‐up: 24 months Loss to follow‐up: 8 patients in total (excluded from the analysis) Exclusions: 3 patients in total with ACL injury of the uninvolved knee (excluded from the analysis) Graft re‐ruptures: 1 PT and 2 HT had traumatic failures requiring revision sx (included in analysis) | |
| Participants | Norway 72 participants with subacute (as soon as inflammation was over and full range of motion was achieved) & chronic (failed non‐operative treatment) ACL deficiency PT: n = 35, mean 25 years, 19 males / 16 females HT: n = 37, mean 27 years, 21 males / 16 females | |
| Interventions | ACL reconstruction with: 1. Patellar tendon (single incision; proximal fixation ‐ Titanium interference screw (7 x 25 mm); tibial fixation ‐ interference screw (7 x 25 mm)) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (4 strands; single incision; proximal fixation ‐ EndoButton and interference screw; tibial fixation ‐ barbed staples) | |
| Outcomes | Minimum 24 months (also 6 and 12 months) follow‐up for: Cincinnati score; static stability with KT‐1000 (mm); stairs hopple test; single leg hop; patient satisfaction; kneeling test; strength with Cybex 6000 (60 & 240 degrees/sec) | |
| Notes | Additional methodological information received from Aune comprised a description of allocation concealment and the sample size calculation. Individual patient data were unavailable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used for randomization, but allocation process to preserve concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Independent assessor. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eight patients were lost to follow‐up and three patients had contra‐lateral ruptures. The authors failed to report to which groups these patients belonged at baseline. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Beynnon 2002
| Methods | Treatment allocation using a random numbers table Non blinded assessment Mean follow‐up: 39 months Loss to follow‐up: 6 PT and 6 HT (excluded from analyses) Graft re‐ruptures: not reported | |
| Participants | USA 56 participants with chronic (> 6 months), isolated unilateral ACL tears PT: n = 28, mean 28.5 years (18 to 46 years), 18 males / 10 females HT: n = 28, mean 29.9 years (19 to 42 yrs), 13 males / 15 females | |
| Interventions | Arthroscopic ACL reconstruction with: 1. Patellar tendon (2 incisions; proximal and tibial fixation ‐ interference screw) versus 2. Hamstring tendon: semitendinosus/gracilis graft (2 strands; 2 incisions; proximal fixation ‐ Belt‐buckle type double‐staple technique; tibial fixation ‐ staple) | |
| Outcomes | Mean 12 and 39 months follow‐up for: Static stability with KT‐1000 (90 N & 133 N); Lachman (0/1+2+3+); pivot shift; knee function (single‐leg hop, weight bearing, squats, stairs, run in place, duck walk); anterior knee pain; Tegner; return to sport/activity; strength with Cybex (60, 180, 240 deg/s); stiffness | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Both groups had 6 patients lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Did not report re‐ruptures, contralateral ruptures or withdrawals. |
| Other bias | Low risk | |
Ejerhed 2003
| Methods | Sealed envelopes, but randomization method not described Independent, but non blinded assessment Minimum follow‐up: 24 months Loss to follow‐up: 1 PT and 1 HT (excluded from analysis) Graft re‐ruptures: 1 PT and 2 HT (excluded from analysis) | |
| Participants | Sweden 71 participants with chronic (> 2 months), unilateral ACL tears PT: n = 34, mean 26 years (14 to 49 years), 21 males / 11 females HT: n = 37, mean 29 years (15 to 59 years), 25 males / 9 females | |
| Interventions | Arthroscopic ACL reconstruction with: 1. Patellar tendon (2 incisions; proximal fixation ‐ "Silk" interference screw (7 mm); tibial fixation ‐ "Silk" interference screw (9 mm)) versus 2. Hamstring tendon: semitendinosus tendon (3 or 4 strands; single incision; proximal and tibial fixation ‐ soft‐threaded RCI interference screw (7 mm)) | |
| Outcomes | Minimum 24 months follow‐up for: Lachman (0/1+2+3+); Lysholm; Tegner; range of motion; static stability with KT‐1000 (89N); single leg hop test; IKDC; anterior knee pain; knee walking test; strength with Cybex (60 deg/s) | |
| Notes | Additional information received from Ejerhed comprised individual pre‐ and post‐operative patient data for the following outcomes: anterior knee pain, knee walking, kneeling, Lysholm, Cybex extension and flexion of injured and non‐injured sides, KT‐1000, range of motion (extension & flexion), IKDC, Tegner activity level. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but allocation process to preserve concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Independent assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | Excluded re‐ruptures in final analysis. Did not report contra‐lateral ruptures or withdrawals. |
| Other bias | High risk | Lack of explicit inclusion and exclusion criteria, patient sampling strategy and 14 patient overlap with Laxdal's trial. |
Eriksson 2001
| Methods | Closed envelopes, but randomization method not described Independent, but non blinded assessment Mean follow‐up: 33 months (median 31 months) Loss to follow‐up: 4 patients in total (excluded from analysis) Graft re‐ruptures (all traumatic): 2 PT (no revision surgery), 3 HT (no revision surgery), 1 HT requiring revision surgery (total of 10 patients excluded from the analysis) | |
| Participants | Sweden 164 participants with chronic (> 2 months), isolated unilateral ACL tears and an overall mean age of 25.7 years (+/‐6.9 years), PT: n = 84, mean 25 years (15 to 40 years), 41 males / 43 females HT: n = 80, mean 27 years (16 to 45 years), 55 males / 25 females | |
| Interventions | Arthroscopic ACL reconstruction with: 1. Patellar tendon (single incision; proximal fixation ‐ non‐absorbable interference screw (7 x 20 mm); tibial fixation ‐ non‐absorbable interference screw (9 x 20 mm)) versus 2. Hamstring tendon: semitendinosus tendon (4 strands; single incision; proximal fixation ‐ Endobutton; tibial fixation ‐ screws) | |
| Outcomes | Mean 33 months follow‐up for: Stryker laxity test (9.08 kg, 18.16 kg); range of motion; pivot shift; Lachman (as defined by the IKDC 1995); single‐leg hop; Tegner activity level; Lysholm; patellofemoral pain; IKDC; patient satisfaction; knee function (VAS) | |
| Notes | Additional information received from Eriksson comprised a description of the allocation concealment methodology, lack of a sample size calculation, and the mean age for each group. Eriksson also provided individual patient data for the following outcomes: Tegner activity level, Lysholm, single leg hop, IKDC, ROM of index and contralateral knees, flexion and extension deficits, Stryker strength (20lbs) for index and contralateral knees; Lachman; anterior and posterior drawer tests; pivot shift test; MCL and LCL stability; patellofemoral, medial and lateral crepitus; harvest site pathology; thigh circumference of the index and contralateral legs; pain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Closed envelopes opened within one hour of surgery. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Independent assessor. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors excluded patients who were lost to follow‐up and those who suffered a re‐rupture, of the ACL graft. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | Gender distribution was different between the two groups at baseline. |
Feller 2003
| Methods | Computer‐generated random assignment Independent, but unblinded assessment Mean follow‐up: 36 months Loss to follow‐up: 4 PT and 2 HT (excluded from analysis) Withdrawals: 1 HT (excluded from analysis) Graft re‐ruptures (traumatic): 1 PT (excluded from analysis) | |
| Participants | Australia 65 participants with acute (3 weeks to 12 months), isolated unilateral ACL tears PT: n = 31, mean 25.8 years (SD = 6 years), 23 males / 8 females HT: n=34, mean 26.3 years (SD = 6 years), 24 males / 10 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision; proximal fixation ‐ EndoButton; tibial fixation ‐ Cannulated metallic interference silk screw) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (4 strands; single incision; proximal fixation ‐ EndoButton; tibial fixation ‐ whip stitched suture and fixation post) | |
| Outcomes | Minimum 36 months (also of 4, 8, 12 and 24 months) follow‐up for: Static stability with KT‐1000 (67N & 134N); range of motion; pivot shift; IKDC, Cincinnati; anterior knee pain (VAS); strength with Cybex II (60 & 240 deg/s); return to sport/activity; kneeling; radiographic assessment | |
| Notes | Additional information received from Feller comprised individual pre‐ and post‐operative patient data for the following outcomes: IKDC, pain, effusion, pain with kneeling, flexion and extension deficits, heel height difference, KT‐1000 (67N, 134N, max manual), Cincinnati | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients who were lost to follow‐up, withdrew or suffered a re‐rupture were excluded from the final analysis. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Ibrahim 2005
| Methods | Treatment allocation by birth date Non blinded assessment Mean follow‐up: 81 months (60 to 96 months) Loss to follow‐up: 25 patients in total (information on group allocation unavailable) Graft re‐ruptures: not reported | |
| Participants | Kuwait 110 participants Group allocation data for 85 male participants (mean age 22.3 years (17 to 34 years)) with chronic ACL deficiency (mean time from injury to surgery was 9.7 months (4 to 13 months)) PT: n = 40 (mean age unavailable) HT: n = 45 (mean age unavailable) |
|
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision; proximal fixation ‐ EndoButton; tibial fixation ‐ interference screw) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (4 strands; single incision; proximal fixation ‐ EndoButton; tibial fixation ‐ screw and spiked washer or small plate 2 screws and staple) | |
| Outcomes | Mean 81 months follow‐up for: Subjective questionnaire (return to work, satisfaction, giving way episodes, anterior knee pain, return to activity); loss of extension and flexion, Lachman (reported as the proportion of patients with a Grade 1 or negative test); pivot shift (reported as the proportion of patients a pivot glide or negative shift); anterior drawer sign (proportion of patients with grade one or negative test) ; KT‐1000 arthrometer (maximum manual test reported as a proportion of patients); Lysholm (proportion of patients with excellent, good, fair; and as a mean score); Tegner (reported as an average activity level); IKDC (1995); radiographic examination | |
| Notes | No information on loss to follow‐up. Unable to get additional information or data from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Birthdate. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not account for lost to follow‐up patients or describe the group allocation for these lost to follow‐up patients. |
| Selective reporting (reporting bias) | High risk | Did not report standard deviations for mean values (e.g. Lysholm and Tegner scores). No detail with respect to how radiographic assessment was determined. |
| Other bias | High risk | No information presented to determine any selection bias since inclusion and exclusion criteria were not described. All patients with reported data and included in follow‐up were males. The description of the baseline characteristics (e.g. no average age for each group) between the two groups was insufficient to determine if the groups were comparable. |
Jansson 2003
| Methods | Treatment allocation by birth year Non blinded assessment Minimum follow‐up: 21 months (21 to 38 months, 2003 publication); 47 months (median 5 years, 2006 publication) Loss to follow‐up (excluded from analysis): 2003 ‐ 8 PT; 2006 ‐ 13 PT and 3 HT Graft re‐ruptures (high energy trauma): 2 HT (excluded from analysis) Failures: 2 HT (excluded from analysis) |
|
| Participants | Finland 99 participants with acute or chronic (1 week to 20 years), isolated unilateral ACL tears PT: n = 51 (no age or gender information) HT: n = 48 (no age or gender information) | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (2 incisions; proximal and tibial fixation ‐ interference metal screws (8 or 9 mm)) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (one incision; 4 strands; proximal fixation ‐ titanium plate attached with a Dacron loop; tibial fixation ‐ cortical screw (4.5 mm) and spiked washer) | |
| Outcomes | Minimum 21 months and median 60 months follow‐up for: Lachman (positive or negative test); pivot shift (positive or negative); stability with CA‐4000 arthrometer; strength with Lido Multijoint II dynamometer (60 & 180 degrees/second); IKDC; Lysholm; Tegner; Kujala patellofemoral score form; range of motion (mean extension deficit); radiographic assessment | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Treatment allocation by birth year |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients who were lost to follow‐up and who suffered graft re‐ruptures and failures were excluded. |
| Selective reporting (reporting bias) | High risk | For the Tegner Activity outcome score, data from Jansson 2003 were taken from the original two‐year publication and follow‐up period because this data for the later follow‐up period was not reported (Harilainen 2006). |
| Other bias | High risk | No baseline information on age and gender distribution was provided |
Laxdal 2005
| Methods | Closed envelopes, but randomization method not described Independent, but non blinded assessment Mean follow‐up: 26 months (24 to 43 months) Loss to follow‐up: 9 patients in total (excluded from analysis) Exclusions from analysis (2 patients in total): 1 PT (contralateral rupture) + 1 HT(ST/G) (previous ACL reconstruction) Graft re‐ruptures after return to sport (excluded from analysis): 1 HT(ST/G); 1 HT(ST) and 1 PT. Failures due to sepsis (excluded from analysis): 1 HT(ST/G) and 1 HT(ST) | |
| Participants | Sweden 134 participants with chronic (> 2 months), isolated unilateral ACL tears Group allocation data for 118 participants. PT: n = 40, mean 28 years (16 to 52 years), 29 males / 11 females HT(ST): n = 39, mean 24 years (12 to 41 years), 22 males / 17 females HT(ST/G): n = 39, mean 26 years (15 to 35 years), 28 males / 11 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision, transtibial technique, proximal and tibial fixation ‐ Acufex interference screw) versus 2. Hamstring tendon: 3‐strand semitendinosus graft (ST) (single incision, soft‐threaded, round‐headed RCI interference screw (7 mm) ‐ tibial and femoral); or 4‐strand semitendinosus/gracilis graft (ST/G) (single incision, soft‐threaded, round‐headed RCI interference screw ‐ tibial and femoral) | |
| Outcomes | Mean 26 months follow‐up for: Tegner, Lysholm, KT‐1000 (89N), range of motion, single‐leg hop, knee walking test, anterior knee pain, IKDC, Cybex | |
| Notes | The original sample size was 50 per group, but recruitment ended early. Additional information received for trial comprised individual pre‐ and post‐operative patient data for the following outcomes: KT‐1000, Lysholm, Tegner, single‐leg hop, IKDC, knee walk test, re‐rupture, patellofemoral pain, Lachman (0/1+/2+), pivot shift (1/2/3), range of motion (flexion and extension), Cybex flexion and extension strength |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Closed envelopes, but allocation process to preserve concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients excluded from analysis due to lost to follow‐up, re‐ruptures and sepsis. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | Recruitment was curtailed at 134 patients due to "time factor". Original sample size was determined a priori to be 150 patients. There is an overlap of 14 patients that were also included in Ejerhed's trial. |
Maletis 2007
| Methods | Closed envelopes, computer‐generated randomization at time of surgery. Trained and experienced blinded assessment Mean follow‐up: 26.2 months Loss to follow‐up: 2 HT (no data for 24‐month analysis only) Exclusions: 2 PT + 1 HT (3 patients in total ruptured their contralateral ACL; therefore were excluded from the KT‐1000 and 1‐leg hop analyses only) Graft re‐ruptures: 1 HT with no follow‐up (excluded from analyses) | |
| Participants | USA 99 patients with acute or chronic (0 to 3 months, 3.1 to 6 months, > 6 months), isolated unilateral ACL tears PT: n = 46, mean 27.2 years (15 to 42 years), 31 males / 15 females HT: n = 53, mean 27.7 years (14 to 48 years), 45 males / 8 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision, proximal fixation ‐ bioabsorbable interference screw (7 x 23 mm); tibial fixation ‐ bioabsorbable interference screw (9 x 23 mm)) versus 2. Hamstring tendon: semitendinosus/gracilis graft (4 strands; single incision, proximal fixation ‐ 1 screw; tibial fixation ‐ 2 bioabsorbable screws) | |
| Outcomes | Minimum 24 months (also 12 months) follow‐up for: Tegner; IKDC; Lysholm: ROM; single leg hop; KT‐1000 (manual maximum) (3 and 6 months reported; SF‐36; patient knee rating; pivot shift (0/1/2/3); kneeling; return to activity; Biodex strength testing (60, 180, 300, degrees/second) (6 months reported) | |
| Notes | Additional information received from Maletis comprised individual pre‐ and post‐operative patient data for the following outcomes: IKDC, Lysholm, Tegner activity level, range of motion (flexion and extension deficits), heel height difference, single‐leg hop, KT‐1000 (maximum manual force and 134N), SF‐36 score, pivot shift test, Lachman; patellofemoral crepitus, strength testing (60 and 180 degrees/second). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization. |
| Allocation concealment (selection bias) | Low risk | Closed envelopes opened at time of surgery. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Independent and blinded assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Marder 1991
| Methods | Treatment allocation by one‐to‐one alternating sequence Unblinded assessment Mean follow‐up: 29 months (24 to 42 months) Loss to follow‐up: 3 PT and 5 HT (excluded from analysis) Graft re‐ruptures: 1 PT and 1 HT (included in analysis) | |
| Participants | USA 80 participants with chronic ACL deficiency, including 2 patients with previous ACL repair in the PT group PT: n = 37, mean 21.6 years (16 to 35 years), 24 males / 13 females HT: n = 35, mean 23.8 years (17 to 41 years), 26 males / 9 females | |
| Interventions | Arthrocopic ACL reconstruction with: 1. Patellar tendon (2 incisions, proximal and tibial fixation ‐ posts and washers) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (4 strands; 2 incisions; proximal and tibial fixation ‐ posts and washers) | |
| Outcomes | Mean 29 months (24 to 42 months) follow‐up for: Zarins‐Rowe subjective rating score, average out of 50 points; proportion of patients with giving way or apprehension and knee pain; proportion of patients who returned to their pre‐injury activity level, had 2+ or greater patellofemoral crepitus or presence of an effusion; range of motion reported as a proportion of patients with a loss of extension or flexion; Lachman test (reported as a proportion of patients by grade (0/1+/2+/3+/4+) and as a mean value; pivot shift test (reported as a proportion of patients by grade (0/1/2/3/3+) and as a mean value; static stability with KT‐1000 at 90 N reported as a proportion of patients by grade and as a mean value; isokinetic testing using Cybex dynamometer at 60 degrees/sec. | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | One‐to‐one alternating sequence. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attempt to blind assessors or patients. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on lost to follow‐up patients. |
| Selective reporting (reporting bias) | High risk | Static stability testing using KT arthrometer only at 90 Newtons. Ordinal data were analysed as continuous and parametric statistics utilized (e.g. Lachman, pivot shift and KT arthrometer testing). No baseline information on patients lost to follow‐up. |
| Other bias | High risk | No inclusion or exclusion criteria were specified. Two patients in the patellar tendon group had a previous ACL repair prior to the reconstruction. |
Matsumoto 2006
| Methods | Treatment allocation by birth date Non blinded assessment Mean follow‐up by group: PT 87 months (+/‐ 12.2 months), HT 80.7 months (+/‐ 13.2 months) Loss to follow‐up: 3 PT and 5 HT (excluded from analysis) Graft re‐ruptures: none reported | |
| Participants | Japan 80 participants with chronic ( > 60 months), isolated unilateral ACL tears PT: n = 40, mean 23.7 years (+/‐ 7.7 years), 21 males / 16 females HT: n = 40, mean 24.4 years (+/‐9.7 years), 15 males / 20 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision, proximal fixation ‐ interference screws (7 x 20 mm); tibial fixation ‐ interference screws (9 x 20 mm)) 2. Hamstring tendon: semitendinosus/gracilis tendons (5 strands; single incision, technique used bone graft; proximal fixation ‐ interference screws (7 x 20 mm); tibial fixation ‐ interference screws (9 x 20 mm)) | |
| Outcomes | Mean 80 months follow‐up for: KT‐1000 (Manual Maximum), range of motion, heel height difference, kneeling, anterior knee pain, IKDC; return to activity; Cybex II (60, 180 degrees/second) | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Treatment allocation by birth date |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients lost to follow‐up were excluded. |
| Selective reporting (reporting bias) | High risk | Did not report graft re‐ruptures. |
| Other bias | High risk | There was an imbalance in the gender distribution with more males in the PT group and more females in the HT group. |
O'Neill 1996
| Methods | Treatment allocation by birth month Independent, non blinded assessment Follow‐up: mean 42 months (24 to 60 months) [1996 publication]; mean 102 months (72 to 121 months) [2001 publication] Loss to follow‐up: 1 PT (2 incision), 1 HT (2 patients in total; excluded from analysis) Graft re‐ruptures (traumatic): 2 PT (1 incision), 2 HT (4 patients in total; included in analysis) | |
| Participants | USA 127 participants (1996 publication) / 229 participants (2001 publication) with acute (< 3 weeks) or chronic (> 3 weeks), isolated unilateral ACL tears Group allocation data for 125 participants (1996 publication) / 225 participants (2001 publication) PT (1 incision): n = 45, mean 28 years (14 to 56 years), 28 males / 17 females (1996 publication); n = 75 (2001 publication) PT (2 incision): n = 40, mean 26 years (15 to 49 years), 26 males / 14 females (1996 publication); n = 75 (2001 publication) HT: n = 40, mean 27 years (14 to 56 years), 27 males / 13 females (1996 publication); n = 75 (2001 publication) Loss to follow‐up: 1 PT (1 incision) and 1 PT (2 incision) and 2 deaths (group assignment not reported). Failures (traumatic, included in the analysis) : 2 PT (2 incision) and 2 HT (1996 publication) / 5 PT (1 incision), 4 PT (2 incision) and 1 HT(2001 publication). |
|
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon: 1 or 2 incisions, (proximal fixation ‐ interference screw (9 x 25 mm); tibial fixation ‐ interference screw, or barbed staples for long grafts (17 cases)) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (2 strands; 2 incisions; proximal and tibial fixation ‐ barbed staples) | |
| Outcomes | Mean 42 months follow‐up for: Lysholm, KT‐2000 (Maximum Manual), range of motion, Lachman (0/1/2/3); single‐leg hop, return to activity; failures; strength with Biodex, patellofemoral crepitus; IKDC; radiographs. Mean 102 months follow‐up for: KT‐2000 (Maximum Manual), return to activity; failures; strength with Biodex, patellofemoral crepitus; IKDC; radiographs. |
|
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by birth month. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The two patients lost to follow‐up and two deaths were excluded from the analysis. |
| Selective reporting (reporting bias) | High risk | The authors failed to report the following outcomes in their 2001 publication on the larger groups of patients: Return to activity; Lachman; range of motion; patellofemoral crepitus; Lysholm score and single leg hop. |
| Other bias | High risk | Recruitment in this trial continued beyond the point where the results of the original publication (1996) were likely analysed. This may have resulted in a selection bias in the 2001 publication. |
Ropke 2001
| Methods | Treatment allocation by surgery date Independent, non blinded assessment Minimum follow‐up: 2 years Loss to follow‐up: not reported Graft re‐ruptures: not reported | |
| Participants | Germany 40 participants (total 32 males / 8 females) with chronic (> 3 months), isolated unilateral ACL tears PT: n = 20, mean 27.4 years (17 to 37 years) HT: n = 20, mean 27.7 years (15 to 43 years) | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision; proximal and tibial fixation ‐ titanium interference screws (7 x 20 mm)) versus 2. Hamstring tendon: semitendinosus tendon (2 strands; single incision; proximal fixation ‐ Endobutton and MerseleneBand; tibial fixation ‐ ligament staples (8 mm)) | |
| Outcomes | Minimum 24 months follow‐up for: IKDC (subjective evaluation, symptoms, range of motion, joint stability); extension loss; flexion loss; static stability with KT‐2000 (15 lb, 20 lb, 30 lb); muscle strength with Cybex II (60, 120, 180 degrees/second); return to sports/activity; objective stability; patellofemoral symptoms; pivot shift | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Treatment allocation by surgery date |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Independent assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | The authors did not provide any information on graft re‐ruptures, contra‐lateral ruptures and patients lost to follow‐up. |
| Other bias | Unclear risk | There was no information to determine gender distribution for each group. |
Sajovic 2006
| Methods | Treatment allocation by operative registration position Non blinded, not independent assessment Minimum follow‐up: 60 months Loss to follow‐up: 2 PT and 1 HT (excluded from analysis) Exclusions from analysis (5 patients in total): 3 PT and 2 HT (due to contralateral ruptures) Graft re‐ruptures: 1 PT and 1 HT (revision surgery) | |
| Participants | Slovenia 64 participants with acute or chronic isolated unilateral ACL tears PT: n = 32 randomized / n = 26 analyzed (4 acute, 22 chronic), mean 27 years (16 to 46 years), 14 males / 12 females HT: n = 32 randomized / n = 28 analyzed (4 acute, 24 chronic), mean 24 years (14 to 42 years), 13 males / 15 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision; proximal fixation ‐ metal round‐head cannulated interference (RCI) screw; tibial fixation ‐ bioabsorbable interference screws) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (4 strands; single incision; proximal fixation ‐ custon left‐threaded or right‐thread round‐head cannulated interference (RCI) screw; tibial fixation ‐ bioabsorbable interference screw) | |
| Outcomes | Minimum 60 months follow‐up for: Lysholm, KT‐2000 (89 N and 134 N), range of motion, Lachman (A/B/C); pivot shift; single‐leg hop, anterior knee pain, IKDC, return to activity, radiographs | |
| Notes | Additional information received from Sajovic comprised a description of the lack of a sample size calculation, and individual pre‐ and post‐operative patient data for the following outcomes: IKDC, Lysholm, anterior knee pain, patellofemoral crepitation, flexion and extension deficits, Lachman, pivot shift, KT‐2000, donor side morbidity, single‐leg hop, x‐ray arthrosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Treatment allocation by operative registration position. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients were excluded due to graft re‐ruptures, contra‐lateral ruptures and lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Baseline information was not provided for the patients excluded from the final analysis. |
| Other bias | Unclear risk | Insufficient information was available to understand the eligible population and how patients were included and excluded from the trial. |
Shaieb 2002
| Methods | Treatment allocation by birth date Independent, non blinded assessment Mean follow‐up: 33 months Loss to follow‐up: 12 patients in total (excluded from analysis) Graft re‐ruptures (4 patients in total, excluded from analysis): 1 PT (traumatic), 1 PT (atraumatic) and 2 HT (traumatic) | |
| Participants | USA 82 participants with acute or chronic isolated unilateral ACL tears Group allocation data for 66 participants (after excluding lost to follow‐ups and graft re‐ruptures) PT: n = 31 (17 acute, 14 chronic), mean 32 years (14 to 48 years), 26 males / 7 females HT: n = 35 (14 acute, 21 chronic), mean 30 years (14 to 53 years), 21 males / 16 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision, proximal fixation ‐ round‐head cannulated, noncutting, metal interference screw; tibial fixation ‐ interference screw) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (4 strands; single incision, proximal fixation ‐ round‐head cannulated, noncutting, metal interference screw; tibial fixation ‐ interference screw) | |
| Outcomes | Mean 33 months follow‐up for: Lysholm, KT‐1000 (89N,134N, manual maximum), range of motion, pivot shift; return to activity, patient satisfaction, Cincinnati, Lachman (mean side to side difference), return to sport, thigh circumference, patellofemoral pain | |
| Notes | No additional methodological information or individual patient data were received from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Treatment allocation by birth date. |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete clinical data was not available for 13 of the patients available for follow‐up. These patients completed questionnaires by mail only. |
| Selective reporting (reporting bias) | High risk | |
| Other bias | High risk | Baseline characteristics for all patients entered into the trial were not reported. |
Zaffagnini 2006
| Methods | Treatment allocation by alternate systematic sampling Not independent, non blinded assessment Minimum follow‐up: 60 months Loss to follow‐up: none Exclusions from analysis: none Graft re‐ruptures: none | |
| Participants | Italy 50 participants with acute or chronic (1 to 13 months) isolated unilateral ACL tears PT: n = 25, mean 30.5 years (22 to 47 years), 16 males / 9 females HT: n = 25, mean 31.3 years (26 to 49 years), 15 males / 10 females HT and extra‐articular plasty: n = 25, mean 26.7 years (15 to 44 years), 18 males / 7 females | |
| Interventions | Arthroscopically‐assisted ACL reconstruction with: 1. Patellar tendon (single incision, proximal and tibial fixation ‐ interference screws) versus 2. Hamstring tendon: semitendinosus/gracilis tendons (4 strands; single incision; proximal fixation ‐ EndoButton; tibial fixation ‐ interference screw) | |
| Outcomes | Minimum 60 months follow‐up for: Tegner, KT‐2000 (maximum manual force and 134 N); range of motion, Lachman (0/1+/2+/3+); pivot shift; single‐leg hop; time required to return to sport and activity, complications, anterior knee pain, IKDC, radiographic, thigh circumference | |
| Notes | A third intervention group involving HT with extra‐articular plasty procedure in 25 patients is not included in this systematic review and meta‐analysis. Additional methodological information received comprised a confirmation of the number of re‐ruptures, graft failures and contralateral ruptures, and individual patient data for the following outcomes: Lachman, pivot shift test, re‐rupture/revisions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Treatment allocation by alternate systematic sampling. The authors did not define what is meant by "alternate systematic sampling." |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | Did not report contralateral ruptures or withdrawals. |
IKDC: International Knee Documentation Committee
Methods: if study does not specify assessment, assumed unblinded and not independent assessors
Lost to follow‐up: patients have unknown outcome; unclear on re‐ruptures in this group
Participants: n sizes listed are original randomized, not patients at follow‐up (unless indicated)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bach 2000 | Abstract only that reported interim results: two year follow‐up data for only 41% of the trial participants. No response from trial author. |
| Beard 2001 | Twelve month follow‐up only. |
| Callaway 1994 | Abstract only with insufficient data for analysis. No response from trial author. |
| Carter 1999 | Only six month follow‐up and only reported on isokinetic testing. |
| Hantes 2004 | Twelve month follow‐up only. Insufficient information available in abstract. |
| Mologne 1996 | Abstract only reporting preliminary results at six months. No response from trial author. |
| Sato 2005 | Less than two year follow‐up; only measured anterior tibial translation during isokinetic concentric contraction exercises. |
Characteristics of ongoing studies [ordered by study ID]
Taylor 2006
| Trial name or title | A comparison of patellar tendon and hamstring tendons for ACL reconstruction using similar femoral and tibial fixation methods: A randomized clinical trial |
| Methods | Randomized controlled trial |
| Participants | 64 Patients: 32 PT 32 HT Military patients 11 females, 53 males |
| Interventions | PT 10 mm endobutton and bioscrew femoral fixation; Tibial fixation bioscrew and sutures over screw and washer HT quadruple ST and Grac with same fixation as PT |
| Outcomes | Lysholm; SANE; IKDC subjective and objective; KOOS; recurrence of instability; re‐injury; return to activity; heel height diff; ROM; thigh girth; single leg hop distance and time; Biodex at 60 and 300 deg/sec; KT 2000; X‐rays at 2 years; |
| Starting date | August 2000 to May 2003 |
| Contact information | dean.taylor@duke.edu; Phone (919) 684‐6603 |
| Notes | Still getting follow‐up information; not yet published (in 2008). |
Contributions of authors
DC, KD, NM and DW were equally involved in the conception and design of study, the analysis and interpretation of data, drafting and revising the review, and providing final approval of the document to be published. DC is the guarantor of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Calgary Orthopaedic Research and Education Fund, Canada.
Declarations of interest
None known.
New
References
References to studies included in this review
- Aglietti P, Buzzi R, Zaccherotti G, Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. American Journal of Sports Medicine 1994;22(2):211‐7; discussion 217‐8. [DOI] [PubMed] [Google Scholar]; Aglietti P, Zaccherotti G, Buzzi R, Biase P. A comparison between patellar tendon and double semitendinosus/gracilis tendon for anterior cruciate ligament reconstruction. A minimum five‐year followup. Journal of Sports Traumatology & Related Research 1997;19(2):58‐68. [Google Scholar]
- Aglietti P, Giron F, Buzzi R, Biddau F, Sasso F. Anterior cruciate ligament reconstruction: bone‐patellar tendon‐bone compared with double semitendinosus and gracilis tendon grafts. A prospective, randomized clinical trial. Journal of Bone and Joint Surgery ‐ American Volume 2004;86(10):2143‐55. [PubMed] [Google Scholar]
- Anderson A. Personal communication (email) August 10 2007. ; Anderson AF, Snyder RB, Lipscomb AB, Jr. Anterior cruciate ligament reconstruction. A prospective randomized study of three surgical methods. American Journal of Sports Medicine 2001;29(3):272‐9. [DOI] [PubMed] [Google Scholar]
- Aune AK. Personal communication (email) April 3 2008. ; Aune AK, Holm I, Risberg MA, Jensen HK, Steen H. Four‐strand hamstring tendon autograft compared with patellar tendon‐bone autograft for anterior cruciate ligament reconstruction. A randomized study with two‐year follow‐up. American Journal of Sports Medicine 2001;29(6):722‐8. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, et al. Anterior cruciate ligament replacement: comparison of bone‐patellar tendon‐bone grafts with two‐strand hamstring grafts. A prospective, randomized study. Journal of Bone and Joint Surgery ‐ American Volume 2002;84(9):1503‐13. [DOI] [PubMed] [Google Scholar]
- Ejerhed L. Personal communication (email) March 26 2004. ; Ejerhed L, Kartus J, Sernert N, Kohler K, Karlsson J. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? A prospective randomized study with a two‐year follow‐up. American Journal of Sports Medicine 2003;31(1):19‐25. [DOI] [PubMed] [Google Scholar]
- Erickson K. Personal communication (email) November 14 2007. ; Eriksson K, Anderberg P, Hamberg P, Lofgren AC, Bredenberg M, Westman I, et al. A comparison of quadruple semitendinosus and patellar tendon grafts in reconstruction of the anterior cruciate ligament. Journal of Bone and Joint Surgery ‐ British Volume 2001;83(3):348‐54. [DOI] [PubMed] [Google Scholar]
- Feller J. Personal communication (email) March 1 2004. ; Feller JA, Webster KE. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. American Journal of Sports Medicine 2003;31(4):564‐73. [DOI] [PubMed] [Google Scholar]
- Ibrahim SA, Al‐Kussary IM, Al‐Misfer AR, Al‐Mutairi HQ, Ghafar SA, Noor TA. Clinical evaluation of arthroscopically assisted anterior cruciate ligament reconstruction: patellar tendon versus gracilis and semitendinosus autograft. Arthroscopy 2005;21(4):412‐7. [DOI] [PubMed] [Google Scholar]
- Harilainen A, Linko E, Sandelin J. Randomized prospective study of ACL reconstruction with interference screw fixation in patellar tendon autografts versus femoral metal plate suspension and tibial post fixation in hamstring tendon autografts: 5‐year clinical and radiological follow‐up results. Knee Surgery, Sports Traumatology, Arthroscopy 2006;14:517‐28. [DOI] [PubMed] [Google Scholar]; Jansson KA, Linko E, Sandelin J, Harilainen A. A prospective randomized study of patellar versus hamstring tendon autografts for anterior cruciate ligament reconstruction. American Journal of Sports Medicine 2003;31(1):12‐8. [DOI] [PubMed] [Google Scholar]
- Kartus J. Personal communication (email) July 29 2007. ; Laxdal G, Kartus J, Hansson L, Heidvall M, Ejerhed L, Karlsson J. A prospective randomized comparison of bone‐patellar tendon‐bone and hamstring grafts for anterior cruciate ligament reconstruction. Arthroscopy 2005;21(1):34‐42. [DOI] [PubMed] [Google Scholar]
- Maletis GB. Personal communication (email) August 20 2007. ; Maletis GB, Cameron SL, Tengan JJ, Burchette RJ. A prospective randomized study of ACL reconstruction: A comparison of patellar tendon and quadruple strand semitendinosis/gracilis tendons fixed with bioabsorbable interference screws. American Orthopaedic Society for Sports Medicine Specialty Day Conference Proceedings; 2006 Mar 25; Chicago (IL). 2006. [DOI] [PubMed] [Google Scholar]; Maletis GB, Cameron SL, Tengan JJ, Burchette RJ. A prospective randomized study of anterior cruciate ligament reconstruction: a comparison of patellar tendon and quadruple‐strand semitendinosus/gracilis tendons fixed with bioabsorbable interference screws. American Journal of Sports Medicine 2007;35(3):384‐94. [DOI] [PubMed] [Google Scholar]
- Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. American Journal of Sports Medicine 1991;19(5):478‐84. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Yoshiya S, Muratsu H, Yagi M, Iwasaki Y, Kurosaka M, et al. A comparison of bone‐patellar tendon‐bone and bone‐hamstring tendon‐bone autografts for anterior cruciate ligament reconstruction. American Journal of Sports Medicine 2006;34(2):213‐9. [DOI] [PubMed] [Google Scholar]
- O'Neill DB. Arthroscopically assisted reconstruction of the anterior cruciate ligament. A prospective randomized analysis of three techniques. Journal of Bone and Joint Surgery ‐ American Volume 1996;78(6):803‐13. [PubMed] [Google Scholar]; O'Neill DB. Arthroscopically assisted reconstruction of the anterior cruciate ligament: A follow‐up report. Journal of Bone and Joint Surgery ‐ American Volume 2001;83(9):1329‐32. [DOI] [PubMed] [Google Scholar]
- Ropke M, Becker R, Urbach D, Nebelung W. Semitendinosus tendon vs. patellar ligament. Results of a prospective randomized study after anterior cruciate ligament reconstruction [Semitendinosussehne vs. ligamentum patellar. Klinische ergebniusse einer prospektiven randomisierten studie nach vorderer kreuzbansplastik]. Der Unfallchirurg 2001;104(4):312‐6. [DOI] [PubMed] [Google Scholar]
- Sajovic M. Personal communication (email) July 29 2007. ; Sajovic M, Vengust V, Komadina R, Tavcar R, Skaza K. A prospective, randomized comparison of semitendinosus and gracilis tendon versus patellar tendon autografts for anterior cruciate ligament reconstruction: five‐year follow‐up. American Journal of Sports Medicine 2006;34(12):1933‐40. [DOI] [PubMed] [Google Scholar]
- Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. American Journal of Sports Medicine 2002;30(2):214‐20. [DOI] [PubMed] [Google Scholar]
- Zaffagnini S, Bruni D. Personal communication (email) August 2 2007. ; Zaffagnini S, Marcacci M, Lo Presti M, Giordano G, Iacono F, Neri MP. Prospective and randomized evaluation of ACL reconstruction with three techniques: a clinical and radiographic evaluation at 5 years follow‐up. Knee Surgery, Sports Traumatology, Arthroscopy 2006;14(11):1060‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bach T, Hoher J, Schutz C, Tiling T. Frontal cruciate ligament replacement with semitendinosus 4‐ply transplant versus patellar transplant [Vordere Kreuzbandersatzplastik mit Semitendinosus 4‐fach Transplantat versus Patellarsehnentransplantat]. Hefte zur Zeitschrift "Der Unfallchirurg". 2000; Vol. 275:245. [Google Scholar]
- Beard DJ, Anderson JL, Davies S, Price AJ, Dodd CA. Hamstrings vs. patella tendon for anterior cruciate ligament reconstruction: a randomised controlled trial. Knee 2001;8(1):45‐50. [DOI] [PubMed] [Google Scholar]
- Callaway GH, Nicholas SJ, Cavanaugh JT, Cavo C, Pavlov H, Wickiewicz TL, et al. Hamstring augmentation versus patella tendon reconstruction of acute anterior cruciate ligament disruption: A randomized, prospective study (Abstract). Orthopaedic Transactions 1994;18:1017. [Google Scholar]
- Carter TR, Edinger S. Isokinetic evaluation of anterior cruciate ligament reconstruction: hamstring versus patellar tendon. Arthroscopy 1999;15(2):169‐72. [DOI] [PubMed] [Google Scholar]
- Hantes, M Zibis A, Zachos B, Basdekis G, Malikos K. Donor site morbidity in the first year after ACL reconstruction: A comparative study between patellar tendon and hamstrings (Abstract 241). 11th ESSKA 2000 Congress. 2004. [Google Scholar]
- Mologne TS, Crabb I, Grover I, Watson BT. Hamstring versus patellar tendon ACL reconstruction in the active duty patient: preliminary results of a prospective randomized study (Abstract). Orthopaedic Transactions 1996;20(4):912. [Google Scholar]
- Sato N, Higuchi H, Terauchi M, Kimura M, Takagishi K. Quantitative evaluation of anterior tibial translation during isokinetic motion in knees with anterior cruciate ligament reconstruction using either patellar or hamstring tendon grafts. International Orthopaedics 2005;29(6):385‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Taylor D, DeBerardino T, Nelson B, Tenuta J, Mountcastle S. A comparison of patellar tendon and hamstring tendons for ACL reconstruction using similar femoral and tibial fixation methods: A randomized study. 12th ESSKA 2000 Congress Conference Proceedings. May 24‐27, 2006. [Google Scholar]
Additional references
- Aglietti P, Zaccherotti G, Buzzi R, Biase P. A comparison between patellar tendon and double semitendinosus/gracilis tendon for anterior cruciate ligament reconstruction. A minimum five‐year followup. Journal of Sports Traumatology & Related Research 1997;19(2):58‐68. [Google Scholar]
- Ahlden M, Kartus J, Ejerhed L, Karlsson J, Sernert N. Knee laxity measurements after anterior cruciate ligament reconstruction, using either bone‐patellar‐tendon‐bone or hamstring tendon autografts, with special emphasis on comparison over time. Knee Surgery, Sports Traumatology, Arthroscopy 2009;17(9):1117‐24. [DOI] [PubMed] [Google Scholar]
- Barrack RL, Bruckner JD, Kneisl J, Inman WS, Alexander AH. The outcome of nonoperatively treated tears of the anterior cruciate ligament in active young adults. Clinical Orthopaedics and Related Research 1990;(259):192‐9. [PubMed] [Google Scholar]
- Bhandari M, Richards RR, Sprague S, Schemitsch EH. The quality of reporting of randomized trials in the Journal of Bone and Joint Surgery from 1988 through 2000. Journal of Bone and Joint Surgery ‐ American Volume 2002;84:388‐96. [DOI] [PubMed] [Google Scholar]
- Biau DJ, Tournoux C, Katsahian S, Shranz PJ, Nizard RS. Bone‐patellar tendon‐bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta‐analysis. BMJ 2006;332(7548):995‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biau DJ, Tournoux C, Katsahian S, Schranz P, Nizard R. ACL reconstruction: A meta‐analysis of functional scores. Clinical Orthopaedics and Related Research 2007;(458):180‐7. [DOI] [PubMed] [Google Scholar]
- Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL‐injured patient. A prospective outcome study. American Journal of Sports Medicine 1994;22:632‐44. [DOI] [PubMed] [Google Scholar]
- Dauty M, Tortellier L, Rochcongar P. Isokinetic and anterior cruciate ligament reconstruction with hamstrings or patella tendon graft: analysis of literature. International Journal of Sports Medicine 2005;26(7):599‐606. [DOI] [PubMed] [Google Scholar]
- Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbe KA. Incorporating variations in the quality of individual randomized trials into meta‐analysis. Journal of Clinical Epidemiology 1992;45:225‐65. [DOI] [PubMed] [Google Scholar]
- Forster MC, Forster IW. Patellar tendon or four‐strand hamstring? A systematic review of autografts for anterior cruciate ligament reconstruction. Knee 2005;12(3):225‐30. [DOI] [PubMed] [Google Scholar]
- Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. Journal of Bone and Joint Surgery ‐ American Volume 1997;79:1556‐76. [DOI] [PubMed] [Google Scholar]
- Freedman KB, D'Amato MJ, Nedeff DD, Kaz A, Bach BR. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. American Journal of Sports Medicine 2003;31:2‐11. [DOI] [PubMed] [Google Scholar]
- Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruciton of the anterior cruciate ligament: meta‐analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy 2005;21:791‐803. [DOI] [PubMed] [Google Scholar]
- Grant JA, Mohtadi NG. ACL reconstruction with autografts. Weighing performance considerations and postoperative care. Physician and Sportsmedicine 2003;31(4):www.physsportsmed.com/issues/2003/2004/grant.htm. [DOI] [PubMed] [Google Scholar]
- Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy‐supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. American Journal of Sports Medicine 2005;33(9):1288‐97. [DOI] [PubMed] [Google Scholar]
- Grontvedt T, Engebretsen L, Benum P, Fasting O, Molster A, Strand T. A prospective, randomized study of three operations for acute rupture of the anterior cruciate ligament. Five‐year follow‐up of one hundred and thirty‐one patients. Journal of Bone and Joint Surgery ‐ American Volume 1996;78(2):159‐68. [DOI] [PubMed] [Google Scholar]
- Harilainen A, Linko E, Sandelin J. Randomized prospective study of ACL reconstruction with interference screw fixation in patellar tendon autografts versus femoral metal plate suspension and tibial post fixation in hamstring tendon autografts: 5‐year clinical and radiological follow‐up results. Knee Surgery, Sports Traumatology, Arthroscopy 2006;14:517‐28. [DOI] [PubMed] [Google Scholar]
- Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surgery, Sports Traumatology, Arthroscopy 1993;1:226‐34. [DOI] [PubMed] [Google Scholar]
- Herrington L, Wrapson C, Matthews M, Matthews H. Anterior cruciate ligament reconstruction, hamstring versus bone‐patella tendon‐bone grafts: a systematic literature review of outcome from surgery. Knee 2005;12(1):41‐50. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]; Appendix 5b. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
- Holm I, Oiestad BE, Risberg MA, Aune AK. No difference in knee function or prevalence of osteoarthritis after reconstruction of the anterior cruciate ligament with 4‐strand hamstring autograft versus patellar tendon‐bone autograft: a randomized study with 10‐year follow‐up. American Journal of Sports Medicine 2010;38(3):448‐54. [DOI] [PubMed] [Google Scholar]
- Irrgang JJ, Ho H, Harner CD, Fu FF. Use of the International Knee Documentation Committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy 1998;6:107‐14. [DOI] [PubMed] [Google Scholar]
- Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD. Development and validation of the international knee documentation committee subjective knee form. American Journal of Sports Medicine 2001;29(5):600‐13. [DOI] [PubMed] [Google Scholar]
- Mohtadi NG. Quality of life assessment as an outcome in anterior cruciate ligament reconstructive surgery. In: Jackson DW, Arnoczky SP editor(s). The anterior cruciate ligament: current and future concepts. New York: Raven Press, 1993. [Google Scholar]
- Johnson RJ, Beynnon BD, Nichols CE, Renstrom PA. The treatment of injuries of the anterior cruciate ligament. Journal of Bone and Joint Surgery ‐ American Volume 1992;74(1):140‐51. [PubMed] [Google Scholar]
- Liden M, Ejerhed L, Sernert N, Laxdal G, Kartus J. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction: a prospective, randomized study with a 7‐year follow‐up. American Journal of Sports Medicine 2007;35(5):740‐8. [DOI] [PubMed] [Google Scholar]
- Linko E, Harilainen A, Malmivaara A, Seitsalo S. Surgical versus conservative interventions for anterior cruciate ligament ruptures in adults. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001356.pub3] [DOI] [PubMed] [Google Scholar]
- Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. Journal of Bone and Joint Surgery ‐ American Volume 2009;91(10):2321‐8. [DOI] [PubMed] [Google Scholar]
- Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. American Journal of Sports Medicine 1982;10(3):150‐4. [DOI] [PubMed] [Google Scholar]
- Madhok R, Shaw LJ, Gillespie LD. Bone, Joint and Muscle Trauma Group. About The Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2006, Issue 1. [DOI] [PubMed]
- Mirza F, Mai DD, Kirkley A, Fowler PJ, Amendola A. Management of injuries to the anterior cruciate ligament: results of a survey of orthopaedic surgeons in Canada. Clinical Journal of Sport Medicine 2000;10(2):85‐8. [DOI] [PubMed] [Google Scholar]
- Miyasaka KC, Daniel DM, Stone ML. The incidence of knee ligament injuries in the general population. American Journal of Knee Surgery 1991;4:43. [Google Scholar]
- Moher, D Schulz, KF Altman, G CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357:1191‐4. [PubMed] [Google Scholar]
- Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. American Journal of Sports Medicine 1998;26:350‐9. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic Anterior cruciate‐deficient knee. Part 1: The long‐term functional disability in athletically active individuals. Journal of Bone and Joint Surgery ‐ American Volume 1983;65:154‐62. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Barber SD, Mooar LA. A rationale for assessing sports activity levels and limitations in knee disorders. Clinical Orthopaedics and Related Research 1989;(246):238‐49. [PubMed] [Google Scholar]
- O'Neill DB. Arthroscopically assisted reconstruction of the anterior cruciate ligament: A follow‐up report. Journal of Bone and Joint Surgery ‐ American Volume 2001;83(9):1329‐32. [DOI] [PubMed] [Google Scholar]
- Poolman RW, Abouali JAK, Conter HJ, Bhandari M. Overlapping systematic reviews of anterior cruciate ligament reconstruction comparing hamstring autograft with bone‐patellar tendon‐bone autograft: Why are they different?. Journal of Bone and Joint Surgery ‐ American Volume 2007;89:1542‐52. [DOI] [PubMed] [Google Scholar]
- Prodromos CC, Joyce BT, Shi K, Keller BL. A meta‐analysis of stability after anterior cruciate ligament reconstruction as a function of hamstring versus patellar tendon graft and fixation type. Arthroscopy 2005;21(10):1202. [DOI] [PubMed] [Google Scholar]
- Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS) ‐ development of a self‐administered outcome measure. Journal of Orthopaedic & Sports Physical Therapy 1998;28(2):88‐96. [DOI] [PubMed] [Google Scholar]
- Schultz WR, Carr CF. Comparison of clinical outcomes of reconstruction of the anterior cruciate ligament: autogenous patellar tendon and hamstring grafts. American Journal of Orthopedics 2002;31(11):613‐20. [PubMed] [Google Scholar]
- Seitz H, Schlenz I, Muller E, Vecsei V. Anterior instability of the knee despite an intensive rehabilitation program. Clinical Orthopaedics and Related Research 1996;(328):159‐64. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Nitz PA. The O'Donoghue triad revisited. Combined knee injuries involving anterior cruciate and medial collateral ligament tears. American Journal of Sports Medicine 1991;19:474‐7. [DOI] [PubMed] [Google Scholar]
- Sherman MF, Warren RF, Marshall JL, Savatsky GJ. A clinical and radiographical analysis of 127 anterior cruciate insufficient knees. Clinical Orthopaedics and Related Research 1988;(227):229‐37. [PubMed] [Google Scholar]
- Smith JP, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament‐deficient knees. A prospective analysis of 575 tears. American Journal of Sports Medicine 2001;29:415‐9. [DOI] [PubMed] [Google Scholar]
- Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE. Anterior cruciate ligament reconstruction autograft choice: bone‐tendon‐bone versus hamstring: does it really matter? A systematic review. American Journal of Sports Medicine 2004;32:1986‐95. [DOI] [PubMed] [Google Scholar]
- Taylor DC. Re: patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction: a prospective, randomized study with a 7‐Year follow‐up [comment]. American Journal of Sports Medicine 2008;36(10):e1. [DOI] [PubMed] [Google Scholar]
- Taylor DC, DeBerardino TM, Nelson BJ, Duffey M, Tenuta J, Stoneman PD, et al. Patellar tendon versus hamstring tendon autografts for anterior cruciate ligament reconstruction: a randomized controlled trial using similar femoral and tibial fixation methods. American Journal of Sports Medicine 2009;37(10):1946‐57. [DOI] [PubMed] [Google Scholar]
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clinical Orthopaedics and Related Research 1985;(198):43‐9. [PubMed] [Google Scholar]
- Thompson J, Harris M, Grana WA. Patellofemoral pain and functional outcome after anterior cruciate ligament reconstruction: an analysis of the literature. American Journal of Orthopedics 2005;34(8):396‐9. [PubMed] [Google Scholar]
- Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: A meta‐analysis. Arthroscopy 2001;17:248‐57. [DOI] [PubMed] [Google Scholar]


