Abstract
Nanosized extracellular vesicles (EVs) possess the natural machinery needed to enter selectively, and transmit complex molecular messages efficiently into targeted cells. The intracellular fate of the vesicular cargos depends on the route of internalization. Therefore, understanding the mechanism of attachment and subsequent intake of these vesicles (before and after exerting any modification) is imperative. Here the extent of communication, the uptake kinetics and the pathways of endothelial EVs into endothelial cells in the presence of specific pharmacological inhibitors were assessed by imaging flow cytometry. The results showed that the uptake of endothelial EVs into endothelial cells was largely an energy dependent process using predominantly a receptor-mediated, clathrin-dependent pathway.
Keywords: Exosome, extracellular vesicles, endothelial cells, endocytosis, nanomedicine
Graphical Abstract

TOC: Effect of temperature (37°C and 4°C) (top panel, imaging flow cytometry) and pharmacologic pathway specific inhibitors (Chlorpromazine, Bafilomycin A1 and Nystatin; bottom panel, fluorescence microscopy) on the internalization of DiO-labeled endothelial EVs into endothelial cells.
Introduction
Extracellular vesicles (EVs) are naturally occurring membrane bound particles carrying proteins and various forms of nucleic acids (1). A growing body of evidence indicates that EVs play an important role as messengers in cell to cell communication and participate in regulatory processes (1–3). EVs have the capability to selectively enter cells and efficiently deliver a molecular cargo. The tissue specific binding properties, efficient intracellular delivery of content and favorable size of EVs make them attractive tools as novel carriers for drug and exogenic nucleic acid delivery (4–6) in cancer therapy as well as in regenerative medicine (7–8). The intracellular fate of EVs designed for therapeutic purposes depends on the pathway used to internalize the vesicles. At least five uptake mechanisms are involved in the cellular internalization of EVs, including clathrin-dependent, micropinocytosis, lipid-raft, membrane fusion and caveolin-dependent endocytosis as summarized in Figure 1 (9). The contribution of an individual pathway to the internalization of EVs varies among the cell type, cell cycle, culture medium and the origin of the EVs (9). As EVs are a mixture of particles with various intracellular origins, it is not surprising that multiple pathways participate in their uptake into the cells. Therefore, it is critical to investigate and understand the various mechanisms of internalization (9–10).
Figure 1.

Potential interactions and uptake mechanisms of extracellular vesicles (EVs) into cells. At least five communication pathways are distinguished between EVs and cells: direct fusion of the vesicles with plasma membrane, micropinocytosis, receptor-mediated clathrin-dependent internalization, caveolin-dependent pathway and lipid raft endocytosis.
Endothelial cells have been the target of various therapeutic strategies to destroy tumor vasculature or inhibit angiogenesis in neoplasms and restore endothelial lining in vascular diseases (11–13). EVs derived from a variety of cells, including those of endothelial origin, have been shown to communicate with the endothelium, but the current knowledge concerning the EV-endothelial cell interaction and ensuing intracellular events is limited (12). We previously showed that endothelial EVs interact with parental cells and are promising delivery vectors (14). Here we investigated potential internalization pathways of endothelial EVs into endothelial cells using multiple imaging modalities including the state-of-the-art imaging flow cytometry.
Materials and Methods
Reagents
Fast DiO, 3,3′-Dilinoleyloxacarbocyanine Perchlorate, fluorescent membrane dye and Slow Fade Mounting media were obtained from Life Technologies (Carlsbad, CA). Cell culture media and supplements, Fetal Bovine Serum (FBS), Phosphate Saline Buffer (PBS), Trypsin, and antibiotics were from Fisher Scientific International, Inc. (Hampton, NH). MicroBCA Protein Assay kits were from Thermo Fisher Scientific Inc. (Waltham, MA). All blocking agents including Bafilomycin A1, Nystatin and Chlorpromazine were purchased from Sigma-Aldrich (St. Louis, MO).
Cell lines and isolation of EVs
Endothelial cells isolated from the aorta of C57BL/6 ApoE−/− mice were cultured as described previously (14–15). EVs were isolated from supernatant of endothelial cells as described (14). Briefly, serial centrifugations of the supernatant from cultured endothelial cells were applied, followed by filtration through 0.45μm and then 0.2 μm PVDF membranes to remove large cellular components. EVs were obtained by ultracentrifugation of the filtered supernatant at 120,000 × g. The pellet was washed in buffered PBS, sedimented at 120,000 × g and resuspended in PBS.
DiO labeling of endothelial cell EVs:
Labeling EVs with DiO was performed as previously described (14). Briefly, purified endothelial exosomes (109 CD63 positive particles) were incubated with Fast DiO green fluorescent membrane dye at a final concentration of 2 μg/ml for 1 hour at room temperature. Labeled exosomes were diluted with PBS and spun at 120,000 × g for 90 min to sediment labeled exosomes and remove unbound dye. The washing step was repeated, and the resultant exosome pellet was suspended in PBS.
Size, protein content and particle concentration of Endothelial EVs
Total protein content of the purified original and DiO-labeled EVs was determined by using the MicroBCA Protein Assay Kit according to manufacturer’s instructions (14). The concentration, size, and size distribution of vesicles before and after DiO labeling were analyzed using the NanoSight NS-300 particle analyzer by averaging five readings for each sample.
Transmission Electron Microscopy of Endothelial EVs
Purified original and DiO-labeled EVs were fixed in 2% paraformaldehyde, mounted onto formvar-coated copper grids (200) and incubated for 5 minutes at room temperature. Following removal of the excess suspension of EVs, grids were stained with 2% uranyl-acetate for 1 minute and imaged by a Tecnai F20 Twin transmission electron microscope in our Core Facility. Images were collected at a magnification of 29,000X and recorded by a Gatan US4000 CCD camera.
Fluorescence microscopy imaging
Endothelial cells were grown on glass slides in 10 cm dishes at a density of × 105 cells/ml for 24 hours. Cells were rinsed twice with PBS then treated with 1 μM Bafilomycin A1, 25 μM Nystatin and 25 μM Chlorpromazine, respectively, for 30 minutes. Cells were then incubated with DiO-labeled EVs in Opti-MEM at 37°C for an additional hour. Unbound particles were removed with two washes of PBS. Cells were fixed in 4 % paraformaldehyde for 5 minutes and washed twice with 2 % BSA-PBS for 2 minutes, followed by DAPI staining for 5 minutes and then one rinse of PBS. Slides were sealed with a cover glass and Slow Fade mounting media. Cells were subsequently imaged with a Zeiss Axio Imager Z1 microscope equipped with an Axio Cam HRm digital monochromatic camera.
Study of uptake kinetics of extracellular vesicles by imaging flow cytometry
Endothelial cells were seeded in 6-well plates at 50,000 cells/well for 24 hours in DMEM supplemented with 5 % FBS. Cells were washed twice with PBS followed by treatment with purified DiO-labeled EVs at concentrations ranging from 75 × 108 to 75 × 103 particles/well in Opti-MEM. Cells were incubated at either 37°C or 4°C for one hour. After two washes with PBS, cells were trypsinized, and detached cells were then centrifuged at 300 × g for 7 minutes at 4°C followed by one extra wash cycle with PBS. Cell pellet was re-suspended in 50 μl PBS supplemented with 10 % FBS and kept on ice until image acquisition. 10,000 cells from each group were analyzed by the Amnis ImageStreamX platform and InspireTM software in our Flow Cytometry Core Facility (16).
Pathway specific blocking of endocytosis of extracellular vesicles
Endothelial cells were grown in 6-well plates at a density of 1.6 × 105 cells/well for 24 hours. After rinsed twice with PBS, cells were treated with Opti-MEM containing 0.025, 0.1, and 1 μM Bafilomycin A1; 1, 10 and 25 μM Nystatin or 1, 10 and 25 μM Chlorpromazine for 30 min. Then, cells were incubated with 1.6 × 109 DiO-labeled EVs in Opti-MEM at 37°C for an additional hour in the presence of the above blocking agents. Unbound particles were removed by wash with PBS. Cells were detached with trypsin spun down and subject to imaging flow cytometry analysis as above.
Statistical analysis
Comparisons between two groups were performed using Student T test. P values of < 0.05 were considered statistically significant. Error bars represent standard deviations.
Results
In vitro characterization of endothelial EVs
Exosomal membranes are rich in tetraspanins (CD9, CD63, and CD81) and are widely considered as specific exosomal markers. In this study, ELISA confirmed the abundancy of CD63 and CD9 (particle concentrations positive for CD63 and CD9 were 1.56 × 1010 and 2.27 × 109 particles/ml, respectively) in the purified vesicles. The correlation between total protein and particle concentrations was established, resulting in 4 × 1011 endothelial EVs per mg of protein. DiO-labeled particles did not show morphological differences from unstained vesicles. NanoSight analysis of the particles did not reveal significant changes in the size distribution of the vesicles before and after DiO labeling (Figure 2.). The mean particle diameter was 125 +/− 48 nm before labeling and 125 +/− 63nm following labeling.
Figure 2.

Characterization of EVs. Transmission electron micrographs show endothelial EVs before (A) and after (B) labeling with DiO membrane dye. Average of five recorded curves represents the size distribution of endothelial EVs before (C) and after (D) labeling with DiO membrane dye determined by NanoSight platform. Scale bar represents 100 nanometers.
Kinetics of concentration- and temperature-dependent uptake of endothelial EVs by endothelial cells
Imaging flow cytometry was employed to assess the uptake kinetics of endothelial EVs by endothelial cells at various particle concentrations at 37°C or 4°C, respectively. Numerous individual events recorded by ImageStreamX were directly linked to high resolution microscopic images. Cells were gated to focused, live single cells (shown in Supplementary Figure 1). Scatter plots in Figure 3A, lower panel, compare cell-associated fluorescence intensities from cells treated with the highest concentration of labeled EVs at 37°C and 4°C. Cells incubated with the highest concentration of labeled particles resulted in a 90.5 % fluorescence positivity at 37°C but only 3.04 % at 4°C. The upper panel of Figure 3A shows representative cells with average fluorescence intensity at 37°C and 4°C, respectively. The fluorescence intensities corresponding to labeled particles were observed throughout the cells at 37°C, while at 4°C cells demonstrated only a few fluorescence dots localized to the rim rather than the deep intracellular space. The graph in Figure 3B depicts the cell-associated fluorescence intensities at 37°C and 4°C as a function of concentration of labeled particles. As seen on the semi-log graph, the curve with intensity values showed a saturable pattern at 37°C, while intensity values from cells incubated with EVs at 4°C remained constantly low at all EV concentrations. These results implied that low temperature restricted the uptake of labeled vesicles; furthermore, they suggest that the uptake of EVs into endothelial cells is an active, likely receptor mediated process.
Figure 3.

Internalization kinetics of DiO-labeled endothelial EVs analyzed by imaging flow cytometry. Representative images (upper panel, A) and scatter plots (lower panel, A) demonstrate endothelial cells treated with DiO-labeled EVs at 37°C and 4°C temperatures recorded by Amnis ImageStreamX Flow Cytometer. Graph depicts uptake kinetics of DiO-labeled EVs in the function of concentration of EVs at 37°C and 4°C temperatures (B).
Effect of pharmacological inhibitors on the uptake of endothelial EVs to endothelial cells
Three specific blocking agents were used to evaluate possible endocytotic pathways involved in the internalization of endothelial EVs by endothelial cells. Chlorpromazine inhibits clathrin-dependent endocytosis, nystatin is known to interfere with caveolin-1 related uptake, and Bafilomycin A1 causes decreased acidification of the endocytotic vesicles and consequently inhibits macropinocytosis (17–18). Conventionally, fluorescence microscopy imaging is used to investigate intracellular localization of labeled particles in the absence or presence of the highest non-cytotoxic concentration of inhibitory agents. Figure 4 shows high power images of representative endothelial cells treated with Chlorpromazine, Bafilomycin A1 and Nystatin respectively in the presence of DiO-labeled endothelial EVs. Green dot fluorescence corresponds to DiO-labeled EVs that interact with the cells, either attached to the membrane surface or internalized into the cells. As shown in Figure 4, none of the applied inhibitors was able to completely block the uptake of labeled vesicles. To quantify the extent of internalized endothelial vesicles into endothelial cells, the ImageStreamx platform was applied.
Figure 4.

Fluorescence microscopy images showing interaction of DiO-labeled EVs with endothelial cells in the absence or presence of Chlorpromazine, Bafilomycin A1 and Nystatin, respectively. Green fluorescence represents DiO-labeled endothelial EVs, blue is DAPI nuclear staining. Images were recorded with 100 × oil objective.
Figure 5 depicts the dose-dependent inhibitory effect of Chlorpromazine, Bafilomycin A1 and Nystatin on the uptake of EVs by endothelial cells determined by imaging flow cytometry. 25 μM and 10 μM Chlorpromazine reduced the uptake of EVs by 89% and 62%respectively (p<0.05). Bafilomycin A1 resulted in significant decreases at all applied concentrations, 40.42 to 61.68% in the internalization of endothelial vesicles (p<0.05). When various doses of Nystatin were applied, only the highest, 25 μM reduced EV-uptake significantly, by 41.62% (p<0.05). None of the inhibitors was able to achieve complete blockage of the uptake of labeled vesicles. Under the above conditions, Chlorpromazine exerted the highest inhibitory effect, suggesting that clathrin-dependent pathway may be the main intracellular route for EV intake.
Figure 5.
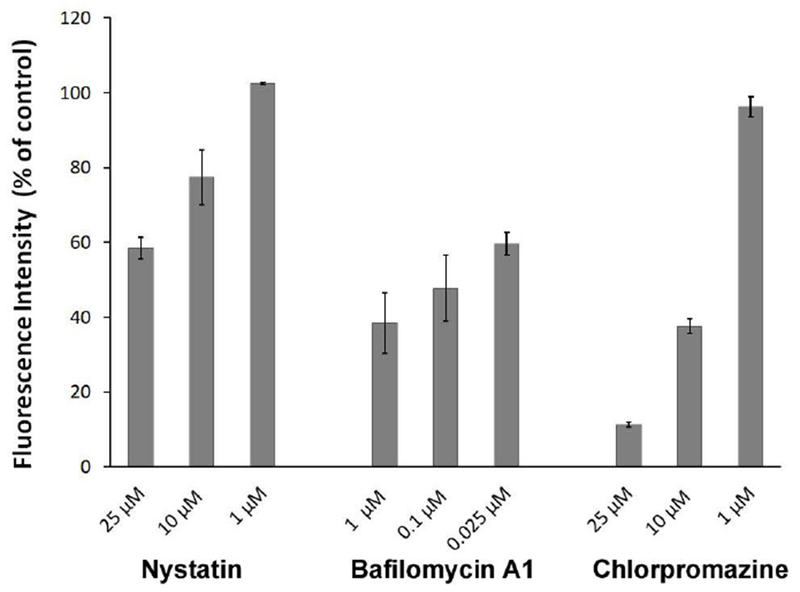
Effect of pharmacologic pathway specific inhibitors on the internalization of DiO-labeled endothelial EVs into endothelial cells. Graph depicts dose-dependent inhibitory effect of the Chlorpromazine, Bafilomycin A1 and Nystatin, respectively on the relative uptake of EVs obtained from imaging flow cytometry.
Discussion
Potential uptake mechanisms underlying endothelial extracellular vesicles by endothelial cells were explored by employing an in vitro cultured endothelial cell model. A variety of EVs communicate with endothelial cells but little is known about the mode of uptake mechanism and effect of these vesicles including EV entry into endothelial cells. Understanding the mechanism leading to the uptake into endothelial cells is crucial to determine the fate of EVs and the cargos they carry.
In this study the pathways of endothelial EVs entering into endothelial cells were investigated by multiple modalities, including imaging flow cytometry. ImageStreamX combines high resolution image analysis with flow cytometry. As seen in other studies with exosomes and viruses, we found that the internalization of endothelial EVs into parent cells was abolished at a low temperate (4°C), however surface binding to the cells still occurred. In contrast, both surface binding and extensive internalization were observed in cells incubated at 37°C. The kinetics of uptake exhibited a saturable pattern, suggesting involvement of receptor(s) in the process similar to other reports on the uptake of EVs by ovarian cancer cells and macrophages (19–20).
Multiple technical methods exist to investigate the types of endocytotic pathways involved in carrying EVs into the cells (21–23). Chemical inhibitions are preferred and widely applied due to simplicity and effectiveness. In this study we investigated three major pathways of endocytosis using chemical inhibitors. Chlorpromazine is known to block specifically receptor mediated clathrin-dependent internalization (9, 24). Chlorpromazine exerted ~90 % inhibition of the uptake of EVs into parent cells when the highest non-cytotoxic concentration was applied. Bafilomycin A1, which blocks micropinocytosis, and Nystatin, which inhibits caveolin-dependent internalization, was able to reduce intracellular uptake of EVs by ~40 and ~60 %, respectively. These results suggest that clathrin-dependent uptake is the main route for EVs to enter into the cells. However, the receptors participating in the process remain to be identified. Noticeably, clathrin-dependent endocytosis is not the only pathway by which endothelial EVs enter into cells. Micropinocytosis, and to a lesser extent, caveolin-dependent uptake contributed to the internalization of EVs to parent cells.
It is noteworthy that the small molecule inhibitors used to study the pathway of EV-uptake by endothelial cells may have side effects or toxicity to EVs or cells. In this study we used three inhibitors at various concentrations: Bafilomycin A1 0.025, 0.1, 1, 2 μM; Nystatin 1, 10, 25, 100 μM; or Chlorpromazine 1, 10, 25, 100 μM. The cytotoxicity effect of the above inhibitors was evaluated by imaging flow cytometry to detect relative permeability of DAPI or To-Pro3 in the imaging flow cytometry studies which are non-permeable for live cells.
In terms of cell viability, the three inhibitors at the above concentrations did not result in significant toxic effect except for Bafilomycin A1 which showed a minimal decrease in viability (less than 2% change compared to the control) at the highest dose applied (Supplement Figure 2). As DMSO was used as solvent for both Nystatin and Bafilomycin A1, toxic effect of DMSO on cells was also evaluated. DMSO was not toxic at the concentrations used with measurement of cell viability (data not shown). Lipophilic membrane dyes are widely used to label EVs for both in vitro and in vivo tracking of the particles. Lipophilic dyes are incorporated into the membrane of the vesicles and they are thought not to interfere with the structure or function of the labeled particles (9).
At least five cellular mechanisms exist in the uptake of nanosize particles including EVs into cells. Internalization of nanoparticles and vesicles is a highly dynamic process and depends on the size, physical and chemical characteristics of the particles; type and life cycle of the cell and possibly cellular environment i.e. in vitro or in vivo. Therefore, it is critical to study further of the pathways or internalization routs for each EVs system for particular applications.
Conclusions:
These results indicate that receptor mediated clathrin-dependent pathway is the major route for endothelial cell derived EVs to enter into endothelial cells while all the pathways tested play roles in the uptake of EVs. These results also demonstrated that imaging flow cytometry is a powerful tool to elucidate the internalization kinetics and pathways involved and to facilitate the development of efficient EVs based drug delivery.
Supplementary Material
Acknowledgements
Research reported in this publication was partially supported by the National Cancer Institute (CCSG P30 CA044579) and the National Heart, Lung, and Blood Institute (R21HL120003) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supporting Information: Gating strategy for imaging flow cytometry and cell toxicity of inhibitors at varied concentrations.
References
- 1.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J and Amigorena S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol 2001, 166(12), 7309–7318. [DOI] [PubMed] [Google Scholar]
- 2.Barros FM, Carneiro F, Machado JC, Melo SA. Exosomes and Immune Response in Cancer: Friends or Foes? Front. Immunol 2018, 9, 730. doi: 10.3389/fimmu.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karasu E, Eisenhardt SU, Harant J, Huber-Lang M. Extracellular Vesicles: Packages Sent With Complement. Front. Immunol 2018, 9, 721. doi: 10.3389/fimmu.2018.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Erviti L, Seow YQ, Yin HF, Betts C, Lakhai S, Wood MJA Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes, Nature Biotechnol. 2011, 29, 341. [DOI] [PubMed] [Google Scholar]
- 5.Marcus ME, Leonard JN, FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals. 2013, 6, 659–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vader P, Mol EA, Pasterkamp G, Schiffelers RM, Extrcellular vesicles for drug delivery. Adv. Drug Deliv. Rev 2016, 106, 148–156. [DOI] [PubMed] [Google Scholar]
- 7.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, Oakley R.M.El, Pasterkamp G, De Kleijn DP, Lim SK, Exosomes secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010, 4, 214–222. [DOI] [PubMed] [Google Scholar]
- 8.DeJong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC, Extracellular vesicles: potential roles in regenerative medicine. Front. Immunol 2014, 5, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulcahy LA, Pink RC, and Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 10.3402/jev.v3.24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdera HC, Gitz-Francois JJ, Schiffelers RM, Vader P, Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and micropinocytosis. J. Controlled Release 2017, 266, 100–108. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer E, Dunmore BJ, Hassan D, Ormiston ML, Moore S, Deighton J, Long L, Yang XD, Stewart DJ, Morrell NW. A Potential Role for Exosomal TCTP Export in Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol 2018. doi: 10.1165/rcmb.2017-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol 2018, 6, 18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bern MM. Extracellular vesicles: how they interact with endothelium, potentially contributing to metastatic cancer cell implants. Clin. Transl. Med 2017, 6(1), 33. doi: 10.1186/s40169-017-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, Shi W, He J. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int. J. Nanomedicine 2014, 9, 4223–30. doi: 10.2147/IJN.S64267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manichaikul A, Wang Q, Shi YL, Zhang Z, Leitinger N, Shi W. Characterization of Ath29, a major mouse atherosclerosis susceptibility locus, and identification of Rcn2 as a novel regulator of cytokine expression. Am. J. Physiol. Heart Circ. Physiol 2011, 301(3), H1056–61. doi: 10.1152/ajpheart.00366.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lannigan J, Erdbruegger U. Imaging flow cytometry for the characterization of extracellular vesicles. Methods. 2017, 112, 55–67. [DOI] [PubMed] [Google Scholar]
- 17.Bannunah AM, Vllasaliu D, Lord J, Stolnik S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: effect of size and surface charge. Mol. Pharm 2014, 11(12), 4363–73. [DOI] [PubMed] [Google Scholar]
- 18.Foster H, Reynolds A, Stenbeck G, Dong J, Thomas P, Kartells E. Internalisation of membrane progesterone receptor-α after treatment with progesterone: Potential involvement of a clathrin-dependent pathway. Mol. Med. Rep 2010, 3(1), 27–35. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Bucan V, Baehre H, von der Ohe J, Otte A, Hass R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int. J. Oncol 2015, 47(1), 244–52. [DOI] [PubMed] [Google Scholar]
- 20.Lee HD, Kim YH, Kim DS. Exosomes derived from human macrophages suppress endothelial cell migration by controlling integrin trafficking. Enr. J. Immunol 2014, 44(4), 1156–69. [DOI] [PubMed] [Google Scholar]
- 21.Schneider DJ, Speth JM, Penke LR, Wettlaufer SH, Swanson JA, Peters-Golden M. Mechanisms and modulation of microvesicle uptake in a model of alveolar cell communication. J. Biol. Chem 2017, 292(51), 20897–20910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delenclos M, Trendafilova T, Mahesh D, Baine AM, Moussaud S, Yan IK, Patel T, McLean PJ. Investigation of Endocytic Pathways for the Internalization of Exosome-Associated Oligomeric Alpha-Synuclein. Front. Nenrosci 2017, 11, 172. doi: 10.3389/fnins.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazan-Halevy I, Rosenblum D, Weinstein S, Bairey O, Raanani P, Peer D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett 2015, 364(1), 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linares J, Matesanz MC, Vila M, Feito MJ, Gonqalves G, Vallet-Regí M, Marques PA, Portolés MT. Endocytic mechanisms of graphene oxide nanosheets in osteoblasts, hepatocytes and macrophages. ACS Appl. Mater. Interfaces 2014, 6(16), 13697–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


