Abstract
Background and Aims
Altering the lipid component in diets may affect the incidence of metabolic bone disease in patients dependent on parenteral nutrition. Consumption of polyunsaturated fatty acids (PUFA) can impact bone health by modulating calcium metabolism, prostaglandin synthesis, lipid oxidation, osteoblast formation, and osteoclastogenesis. The aim of this study was to evaluate the dietary effects of PUFA on murine bone health.
Methods
Three-weeks-old male (n=30) and female (n=30) C57BL/6J mice were randomized into one of three dietary groups. The diets differed only in fat composition: soybean oil (SOY), rich in ω−6 PUFA; docosahexaenoic acid alone (DHA), an ω−3 PUFA; and DHA with arachidonic acid, an ω−6 PUFA, at a 20:1 ratio (DHA/ARA). After 9 weeks of dietary treatment, femurs were harvested for micro-computed tomographic analysis and mechanical testing via 3-point bending. Separate mice from each group were used solely for serial blood draws for measurement of biomarkers of bone formation and resorption.
Results
At the microstructural level, although some parameters in cortical bone reached differences that were statistically significant in female mice, these were too small to be considered biologically relevant. Similarly, trabecular bone parameters in male mice were statistically different in some dietary groups, although the biological interpretation of such subtle changes translate into a lack of effect in favor of any of the experimental diets. No differences were noted at the mechanical level and in blood-based biomarkers of bone metabolism across dietary groups within gender.
Conclusions
Subtle differences were noted at the bones’ microstructural level, however these are likely the result of random effects that do not translate into changes that are biologically relevant. Similarly, differences were not seen at the mechanical level, nor were they reflected in blood-based biomarkers of bone metabolism. Altogether, dietary consumption of PUFA do not seem to affect bone structure or metabolism in a healthy model of growing mice.
Keywords: bone, bone strength, bone micro-architecture, polyunsaturated fatty acids
INTRODUCTION
Infants and children who are unable to adequately absorb nutrients through their intestinal tract are dependent on parenteral nutrition (PN) to sustain life. However, PN is associated with significant complications such as intestinal failure-associated liver disease (IFALD), sepsis, and metabolic bone disease (MBD) [1–3]. Several factors have been implicated in the pathogenesis of MBD in the setting of long-term PN administration. These include the presence, or lack thereof, of components in PN formulations that are either toxic or essential to bone health, respectively [4]. The lack of enteral nutrition, co-morbidities, prolonged immobilization, and the use of certain medications can also put these particular patients at risk for development of MBD [4,5]. Additionally, the amount of calcium and phosphorus that can be added to PN formulations is limited given the risk of precipitation in the solution to be infused [6]. Abnormal bone mineral accrual so early in life may result in permanent bone damage and pose problems for those affected later in life, ranging from growth failure to increased risk of osteoporosis and fractures [7,8].
PN-associated MBD affects patients of all ages. In a single-institution study, Diamanti et al described a prevalence of 83% in PN-dependent children [3]. Seventeen percent of these cases were complicated by fractures, similar to the 4–24% reported in PN-dependent adults [9–11]. Currently, one of the most widely used lipid emulsions available in the United States is Intralipid® (Baxter/Fresenius Kabi, Deerfield, Illinois, USA), a soybean oil-based lipid emulsion (SOLE). We previously reported results from a retrospective review of PN-dependent premature neonates (gestational age ≤ 37 weeks receiving PN for at least 4 weeks) [12]. Findings showed a significant decrease in the incidence of bone fractures in children receiving a fish oil-based lipid emulsion (FOLE; Omegaven®, Fresenius Kabi, Bad Homburg, Germany) compared to those receiving Intralipid® (5.3 vs 12%, respectively). These findings occurred despite uniformity in all other aspects of nutrition, including calcium and vitamin D intake [12].
FOLE contains high concentrations of omega-3 polyunsaturated fatty acids (ω−3 PUFAs) such as eicosapentaenoic and docosahexaenoic acids (EPA and DHA, respectively). DHA has been shown to have beneficial effects in maintaining bone health in both animal and human studies [13–17]. Some ω−3 PUFAs have a bigger effect on bone mass, while others affect mostly the tissue’s architecture [18]. The aim of this study was to evaluate the effects of dietary ω−3 and ω−6 PUFAs on bone’s structural, mechanical, and metabolic properties in male and female healthy growing mice. We hypothesize that provision of a diet rich in ω−3 PUFAs will result in improved bone health as determined by results from microstructural imaging, mechanical testing, and measurement of blood-based biomarkers.
MATERIALS AND METHODS
Murine Model and Diets
The animal protocol was approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital. Male and female 3-week old mice of the C57BL/6 strain were obtained from Jackson Laboratories (Bar Harbor ME, USA). Animals were assigned to receive one of three diets that differed only in the source of fat used for its formulation (n=10 per group, 3 groups with female mice, and 3 groups with male mice, for a total of 6 groups and 60 animals). All experimental diets contained equal caloric and food weight components with 10% of calories provided by fat (Table 1). The fat content for the SOY diet (AIN-93M Purified Rodent Diet #110900, Dyets Inc., Bethlehem PA, USA) was derived purely from soybean oil. This diet is rich in ω−6 PUFAs and served as a control. The DHA/arachidonic acid (ARA) diet (AIN-93M Purified Rodent Diet #102723, Dyets Inc., Bethlehem PA, USA) contains both ω−3 and ω−6 PUFAs at a 20:1 ratio (DHA:ARA). This ratio is similar to that present in cold water fish and has been used by our group in the past to investigate the effects of ω−3 PUFAs in a model of murine hepatosteatosis [19]. The third group received a DHA diet (AIN-93M Purified Rodent Diet #102716, Dyets Inc., Bethlehem PA, USA) that contains only ω−3 PUFAs and no ω−6 PUFAs. The SOY and DHA/ARA diets were utilized, as their fatty acid content reflected those of lipid emulsions provided to PN-dependent children. The DHA diet aimed to mimic the effects of dietary ω−3 PUFA supplementation without addition of ω−6 PUFAs.
Table 1.
Composition of experimental diets.
| Ingredient | SOY | DHA/ARA | DHA |
|---|---|---|---|
| Casein (g/kg) | 140 | 140 | 140 |
| L-Cysteine (g/kg) | 1.8 | 1.8 | 1.8 |
| Sucrose (g/kg) | 100 | 100 | 100 |
| Cornstarch (g/kg) | 465.7 | 465.7 | 465.7 |
| Dyetrose (g/kg) | 155 | 155 | 155 |
| Soybean Oil (g/kg) | 40 | 0 | 0 |
| Docosahexaenoic Acid (DHA) (g/kg) | 0 | 8 | 8.4 |
| Arachidonic Acid (ARA) (g/kg) | 0 | 0.4 | 0 |
| Hydrogenated Coconut Oil (g/kg) | 0 | 31.6 | 31.6 |
| t-Butylhydroquinone (g/kg) | 0.008 | 0.008 | 0.008 |
| Cellulose (g/kg) | 50 | 50 | 50 |
| Calcium Pantothenate (g/kg) | 1.6 | 1.6 | 1.6 |
| Calcium Carbonate (g/kg) | 357 | 357 | 357 |
| Vitamin D3 (400000 IU/g) (g/kg) | 0.25 | 0.25 | 0.25 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 |
Given their high content of PUFAs, which are particularly prone to oxidation, all pelleted diets were subjected to oxidation tests (Covance Laboratories, Madison WI, USA). These aimed to ensure the integrity of the PUFAs provided in the experimental diets and included the peroxide and anisidine values (PV and AV, respectively). The former measures the primary and secondary products of oxidation, whereas the latter measures only secondary products of oxidation. The Totox value (AV+2PV) indicates an oil’s overall oxidation state (Table 2). According to the International Fish Oil Standard (IFOS), values below 19.5 are considered optimal. All Totox values obtained were deemed appropriate to proceed with our studies. Although that of the SOY diet was borderline, we decided to proceed with this formulation, as this is not especially formulated but rather used extensively as a commercially available control diet.
Table 2.
Summary of results from oxidation tests performed on experimental diets.
| Oxidation Test | SOY | DHA/ARA | DHA |
|---|---|---|---|
| Peroxide Value (PV) (mEq/kg of fat) | 8.25 | 6.33 | 6.45 |
| Anisidine Value (AV) | 4.28 | 4.08 | 2.5 |
| Totox Value (AV+2PV) | 20.78 | 16.74 | 15.4 |
Animals were earmarked for identification and housed in regular vented cages (5 mice per cage) within a barrier room with a 12-hour light and dark cycle. They were given full and uninhibited access to water and food. Animals were weighed every 3 days, and growth and appearance were closely monitored. Food consumption was also recorded as grams/cage/3 days. All animals were maintained on the experimental diets for 9 consecutive weeks during a period of rapid growth (from week 3 to week 12), after which they were euthanized. Left femurs were harvested, cleaned of all excess soft tissue, wrapped in normal saline (0.9% sodium chloride)-soaked gauze and stored at −20ºC until micro-computed tomographic (μCT) analysis and three-point bending mechanical testing. For determination of blood-based biomarkers of bone resorption and formation, blood samples were collected after a period of overnight fasting. As repeated blood draws may induce a bone marrow reaction that could ultimately alter the mechanical properties of rapidly growing bone [20], five additional animals from each group were used to obtain blood samples via retro-orbital puncture at baseline and one month into the study. A third set of blood samples were obtained immediately prior to euthanasia in five animals from each of the original groups of 10. Blood samples obtained at euthanasia were also used for serum fatty acid analysis.
μCT Imaging Analysis
Bone microarchitecture indices were measured using a high-resolution μCT imaging system (μCT, Scanco Medical, Brüttisellen, Switzerland) following the guidelines from the American Society for Bone and Mineral Research for the use of μCT in rodents [21]. For female diet groups, n=9 for SOY (one animal was excluded from analysis given poor oral intake from malocclusion), and n=10 for DHA/ARA and DHA groups each. For male diet groups, n=10 for SOY, DHA/ARA, and DHA. Femurs were placed in a scanning tube containing normal saline and stabilized proximally and distally with foam pieces. Sequential transaxial images were acquired using a 10 μm3 isotropic voxel size with an X-ray tube voltage and current of 70 kVp and 114 mA, respectively, integration time of 200 ms, and subjected to Gaussian filtration. Based on a previously described protocol [22], the distance from the femoral head to the distal end of the femoral condyles was used to define the femoral length on the μCT scans. The microarchitecture of cortical bone was determined using the images obtained from the mid-diaphysis of the specimen, starting at 55% of the length below the femoral head and extending distally for 50 slices. The following cortical bone indices were measured: total cross-sectional area (TA, mm2), bone area (BA, mm2), cortical bone area fraction (BA/TA), thickness (mm), and tissue mineral density (TMD, mgHA/mm3). For trabecular bone, the distal metaphyseal region was identified beginning 20 slices above the peak of the distal growth plate and extended proximally for 130 slices. The trabecular bone indices included bone volume fraction (BV/TV), trabecular thickness (mm), separation (mm), and number (1.mm−1), connectivity density (1.mm−3), Structure Model Index (SMI), and tissue mineral density (TMD, mgHA/mm3). The appropriate thresholds were used and calculated based on adaptive-iterative thresholding (AIT) performed on the SOY group (control). The intra-specimen variability of μCT assessment of three-dimensional microstructural and densitometric indices of excised bone samples is less than 0.5% in our laboratory. Following imaging, the specimens were wrapped once again in saline-soaked gauzed and stored at −20ºC until mechanical testing.
Mechanical Testing
Specimens were thawed to room temperature and subjected to three-point bending using a Bose Electroforce® 3200 (TA Instruments, Wintest v4.1, Wakefield MA, USA) load frame under displacement control conditions with an 8 mm support span. Femurs were placed with their posterior aspect facing up on the support span and secured to their position to avoid rolling prior to testing with a polydimethylsiloxane silicone polymer. Each femur was subjected to three-point mono-cyclic loading to failure at a displacement rate of 0.01 mm.s-1.
Extrinsic and intrinsic structural properties were assessed from the load-displacement curves. Yield and ultimate load, stiffness, and Young’s modulus were calculated using μCT-based area moment of inertia measures and an in-house developed MATLAB program (MathWorks, Natick MA, USA).
Blood-Based Biomarkers and Fatty Acid Analyses
Blood samples were collected after overnight fasting on days 0 (baseline), 32, and 63 (euthanasia) via retro-orbital puncture under inhaled isoflurane. For all experimental groups, n=5 in each time point. Following collection, whole blood was centrifuged at 4ºC for 15 minutes at 2255 g and stored at −80ºC for subsequent analysis. Based on recommendations from the International Osteoporosis Foundation, we elected to assess levels of procollagen type 1 propeptide (P1NP) as a surrogate marker of bone formation, and C-terminal telopeptide of type I collagen (CTX) as a marker of bone resorption [23]. Enzyme-linked immunosorbent assay (ELISA) kits were used on the obtained serum samples: P1NP (mouse P1NP ELISA kit #MP0585, NeoScientific, Woburn MA, USA) and CTX (mouse CTX-1 ELISA kit #MC0850, NeoScientific, Woburn MA, USA).
For analysis of fatty acid composition (n=3 per experimental group), total lipids were extracted from the different serum samples based on the methods of Folch [24]. Briefly, heptadecanoic acid (C17:0) was used as an internal standard (Nu-Chek Prep, Inc., Elysian MN, USA) and added to 30–50 μL of each serum sample. A mixture of chloroform and methanol at a 2:1 ratio was then added to allow for creation of a biphasic system that would allow for lipid separation and subsequent extraction. Once separated, the solvent was evaporated under nitrogen conditions to minimize oxidation, followed by saponification with 0.5 N methanolic sodium hydroxide (Ricca Chemical Company, Arlington, Texas USA), and methylation with 12% boron trifluoride (1.5M) in methanol (Acros Organics, Geel, Belgium). Fatty acid methyl ester profiles were acquired by gas liquid chromatography using an Agilent Technologies 6890N gas chromatograph (Agilent Technologies, Inc., Wilmington DE, USA) coupled to an Agilent-5975B mass spectrometer equipped with a Supelcowax SP-10 capillary column (Agilent Technologies, Inc.). The fatty acid profiles were expressed as percentage of the total fatty acids.
Statistical Analyses
Growth and food intake curves were generated for each of the experimental groups. A broken-stick mixed model was used to analyze the data to accommodate a change in slope at day 12. The optimal covariance structure to model the repeated measures within each animal was determined to be autoregressive with lag 1, based on Akaike’s information criterion [25]. Modeling was performed with SAS version 9.4 (Cary NC, USA). Microstructural and mechanical indices, and fatty acid profiles were compared within genders across the three dietary groups with two-way analysis of variance (ANOVA) with post-hoc Tukey correction for multiple comparisons. Levels of blood based-biomarkers were compared within genders across the three dietary groups at the three different time points (days 0, 32, and 63) with two-way ANOVA with post-hoc Tukey correction for multiple comparisons. Statistical analyses were conducted with Prism 7 (GraphPad Software, Inc., La Jolla CA, USA). P-values below 0.05 were considered statistically significant for all analyses.
RESULTS
On average, there was no difference in weight by diet (P=0.63 after adjusting for sex). As expected, there was a difference in weight by sex (P<0.0001 after adjusting for diet), although the effect on sex did not depend on the type of diet (P=0.92) (Figure 1). Of note, one animal from the female group assigned to the DHA/ARA diet, which had stunted growth, was excluded given the fact that it was later found to be from dental malocclusion that led to suboptimal food intake. Samples from this mouse were similarly excluded from all tests and analyses.
Figure 1.
Weight (grams) for n=89 animals. Shown are males (filled circles) and females (empty circles), with means from a broken-stick mixed model shown for soy, DHA/ARA, and DHA. Data are jittered right/left to prevent male/female overlap.
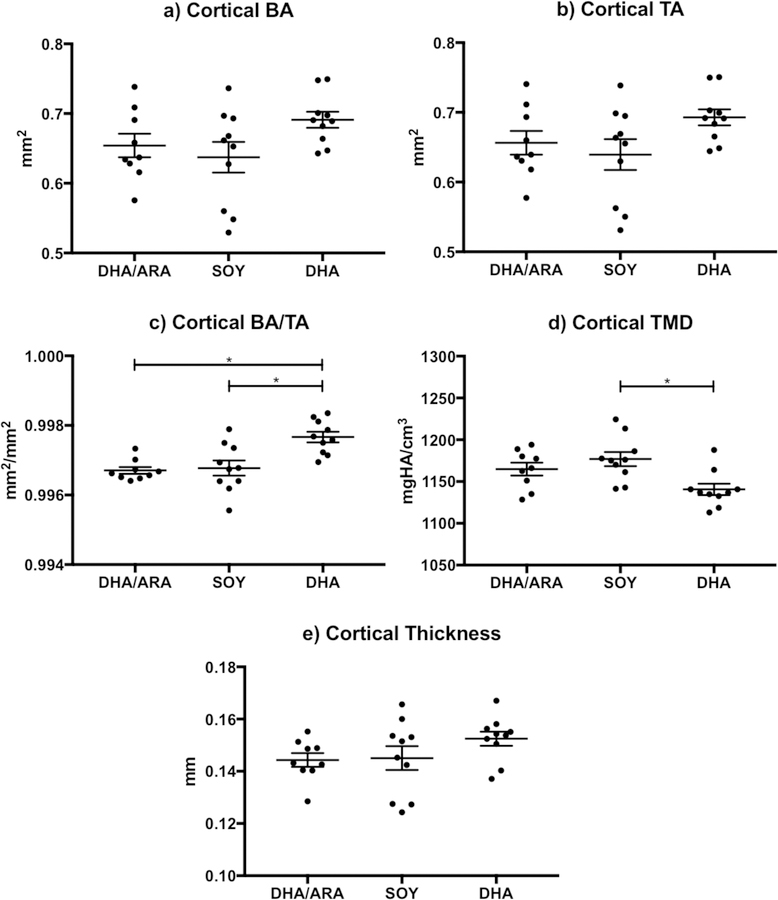
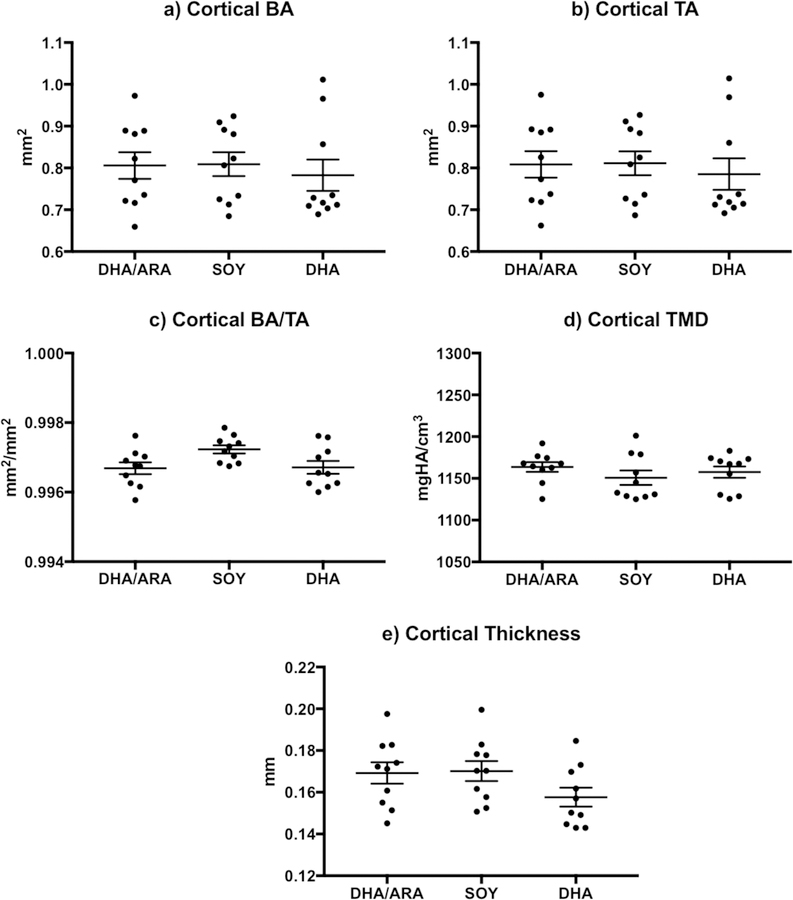
Mid-diaphyseal cortical bone morphometric analysis of female animals (Figure 2) showed trends of significantly higher TA and BA in animals on the DHA group, compared to those in the SOY and DHA/ARA groups. However, the BA/TA reached a P-value below 0.05 and was statistically significant. Conversely, animals in the DHA group had lower cortical TMD that was statistically significant only when compared to the SOY group. There were no differences in cortical thickness measurements across the groups (Table 3). In male specimens, none of the cortical bone indices evaluated showed significant differences across the experimental groups (Figure 3 and Table 3).
Figure 2.
Graphs representing cortical bone indices (mean ± standard error of the mean) measured in female mice (DHA/ARA, n=9; SOY, n=10; DHA, n=10): a) cortical BA (bone area); b) cortical TA (total cross-sectional area); c) cortical BA/TA (bone area fraction); d) cortical TMD (tissue mineral density); and e) cortical thickness. Where indicated, mean value differences between treatment groups are considered statistically significant (* P < 0.05).
Table 3.
Results of cortical bone parameters obtained on from micro-computed tomography with comparisons across experimental groups within sex (shown are mean ± standard error of the mean). Only P-values below 0.05 are given in detail. Abbreviations: ARA, arachidonic acid; BA/TA, bone area fraction; DHA, docosahexaenoic acid; NS, not statistically significant (P < 0.05); TMD, tissue mineral density.
| Parameter | DHA/ARA | SOY | DHA | DHA vs SOY | DHA vs DHA/ARA | DHA/ARA vs SOY | Sex |
|---|---|---|---|---|---|---|---|
| Bone area (mm2) | 0.65±0.02 | 0.64±0.02 | 0.69±0.01 | NS | NS | NS | Female |
| Total area (mm2) | 0.66±0.02 | 0.64±0.02 | 0.69±0.01 | NS | NS | NS | |
| BA/TA | 0.997±0.00 | 0.997±0.00 | 0.998±0.00 | NS | 0.0012 | 0.002 | |
| Cortical TMD (mgHA/cm3) | 1165±7.7 | 1177±8.4 | 1141±6.8 | 0.0059 | NS | NS | |
| Cortical Thickness (mm) | 0.14±0.003 | 0.15±0.005 | 0.15±0.003 | NS | NS | NS | |
| Bone area (mm2) | 0.81±0.03 | 0.81±0.03 | 0.78±0.04 | NS | NS | NS | Male |
| Total area (mm2) | 0.81±0.03 | 0.81±0.03 | 0.79±0.04 | NS | NS | NS | |
| BA/TA | 0.997±0.00 | 0.997±0.00 | 0.997±0.00 | NS | NS | NS | |
| Cortical TMD (mgHA/cm3) | 1164±5.8 | 1151±8.6 | 1158±6.8 | NS | NS | NS | |
| Cortical Thickness (mm) | 0.17±0.005 | 0.17±0.005 | 0.16±0.005 | NS | NS | NS | |
Figure 3.
Graphs representing cortical bone indices (mean ± standard error of the mean) measured in male mice (DHA/ARA, n=10; SOY, n=10; DHA, n=10): a) cortical BA (bone area); b) cortical TA (total cross-sectional area); c) cortical BA/TA (bone area fraction); d) cortical TMD (tissue mineral density); and e) cortical thickness. Where indicated, mean value differences between treatment groups are considered statistically significant (* P < 0.05).
None of the indices evaluating trabecular bone microstructure in female mice were significantly different (Figure 4 and Table 4). Distal metaphyseal trabecular bone morphometric analysis of male animals showed SOY mice to have significantly lower trabecular number and higher trabecular separation compared to the other two experimental groups (Figure 5). Only when compared to the DHA group, the SOY mice had significantly higher trabecular thickness values. The other indices, including BV/TV, connectivity density, SMI, and trabecular TMD were not different across the different experimental groups (Table 4).
Figure 4.
Graphs representing trabecular bone indices (mean ± standard error of the mean) measured in female mice (DHA/ARA, n=9; SOY, n=10; DHA, n=10): a) BV/TV (bone volume fraction); b) connectivity density; c) SMI (structure model index); d) trabecular number; e) trabecular thickness; f) trabecular separation; g) trabecular TMD (tissue mineral density). Where indicated, mean value differences between treatment groups are considered statistically significant (* P < 0.05).
Table 4.
Results of trabecular bone parameters obtained on from micro-computed tomography with comparisons across experimental groups within sex (shown are mean ± standard error of the mean). Only P-values below 0.05 are given in detail. Abbreviations: ARA, arachidonic acid; BA, bone area; DHA, docosahexaenoic acid; NS, not statistically significant (P < 0.05); TA, total area; TMD, tissue mineral density.
| Parameter | DHA/ARA | SOY | DHA | DHA vs SOY | DHA vs DHA/ARA | DHA/ARA vs SOY | Sex |
|---|---|---|---|---|---|---|---|
| BV/TV | 0.04±0.004 | 0.05±0.004 | 0.05±0.004 | NS | NS | NS | Female |
| Connectivity Density (1/mm3) | 29.5±6.7 | 45.4±8.8 | 36.4±6.2 | NS | NS | NS | |
| SMI | 3.28±0.06 | 3.06±0.09 | 3.16±0.06 | NS | NS | NS | |
| Trabecular Number (1/mm) | 3.61±0.11 | 3.83±0.12 | 3.72±0.1 | NS | NS | NS | |
| Trabecular Thickness (mm) | 0.038±0.00 | 0.037±0.00 | 0.037±0.00 | NS | NS | NS | |
| Trabecular Separation (mm) | 0.28±0.01 | 0.26±0.01 | 0.27±0.01 | NS | NS | NS | |
| Trabecular TMD (mgHA/cm3) | 977.3±5.8 | 980.5±3.8 | 970±3.7 | NS | NS | NS | |
| BV/TV | 0.12±0.01 | 0.12±0.01 | 0.1±0.01 | NS | NS | NS | Male |
| Connectivity Density (1/mm3) | 182.8±17.8 | 156.4±8.8 | 168.2±15.3 | NS | NS | NS | |
| SMI | 2.47±0.11 | 2.43±0.07 | 2.57±0.09 | NS | NS | NS | |
| Trabecular Number (1/mm) | 5.63±0.07 | 5.14±0.1 | 5.52±0.11 | 0.0173 | NS | 0.0022 | |
| Trabecular Thickness (mm) | 0.039±0.00 | 0.043±0.00 | 0.036±0.00 | 0.0313 | NS | NS | |
| Trabecular Separation (mm) | 0.18±0.00 | 0.19±0.00 | 0.18±0.00 | 0.0295 | NS | 0.0038 | |
| Trabecular TMD (mgHA/cm3) | 971.1±4.2 | 976.2±4.3 | 968.8±3.2 | NS | NS | NS | |
Figure 5.
Graphs representing trabecular bone indices (mean ± standard error of the mean) measured in male mice (DHA/ARA, n=10; SOY, n=10; DHA, n=10): a) BV/TV (bone volume fraction); b) connectivity density; c) SMI (structure model index); d) trabecular number; e) trabecular thickness; f) trabecular separation; g) trabecular TMD (tissue mineral density). Where indicated, mean value differences between treatment groups are considered statistically significant (* P < 0.05).
No statistically significant differences were seen between experimental groups in any of the indices evaluated by mechanical testing (Table 5). No differences were seen in any of the time points where blood-based biomarkers of bone formation (P1NP) and resorption (CTX) were measured (Figure 6). Results from fatty acid analysis are summarized in Table 6. Differences across groups reflected changes that are typically seen according to their dietary group. None of the animals tested showed biochemical essential fatty acid deficiency, defined by a triene to tetraene ratio (Mead acid/ARA), equal or greater than 0.2.
Table 5.
Results from mechanical testing indices and comparisons across experimental groups within animal sex (shown are mean ± standard error of the mean). Abbreviations: ARA, arachidonic acid; DHA, docosahexaenoic acid; NS, not statistically significant (P < 0.05).
| Index | DHA/ARA | SOY | DHA | DHA vs SOY | DHA vs DHA/ARA | DHA/ARA vs SOY | Sex |
|---|---|---|---|---|---|---|---|
| Yield load (N) | 7.3±0.5 | 8.0±0.6 | 7.4±0.3 | NS | NS | NS | Female |
| Ultimate load (N) | 14.1±0.5 | 14.6±0.3 | 14.0±0.5 | NS | NS | NS | |
| Young’s modulus (Mpa) | 86.0±5.3 | 93.3±3.9 | 79.7±5.5 | NS | NS | NS | |
| Stiffness (N/mm) | 74.7±1.9 | 78.6±3.1 | 75.3±2.3 | NS | NS | NS | |
| Yield load (N) | 8.7±0.4 | 7.6±0.6 | 9.1±0.4 | NS | NS | NS | Male |
| Ultimate load (N) | 16.9±0.8 | 17.2±1.1 | 17.6±0.7 | NS | NS | NS | |
| Young’s modulus (Mpa) | 143.7±17.8 | 148.2±28.4 | 142.3±12.5 | NS | NS | NS | |
| Stiffness (N/mm) | 90.1±4.6 | 88.4±6.6 | 95.0±3.7 | NS | NS | NS | |
Figure 6.
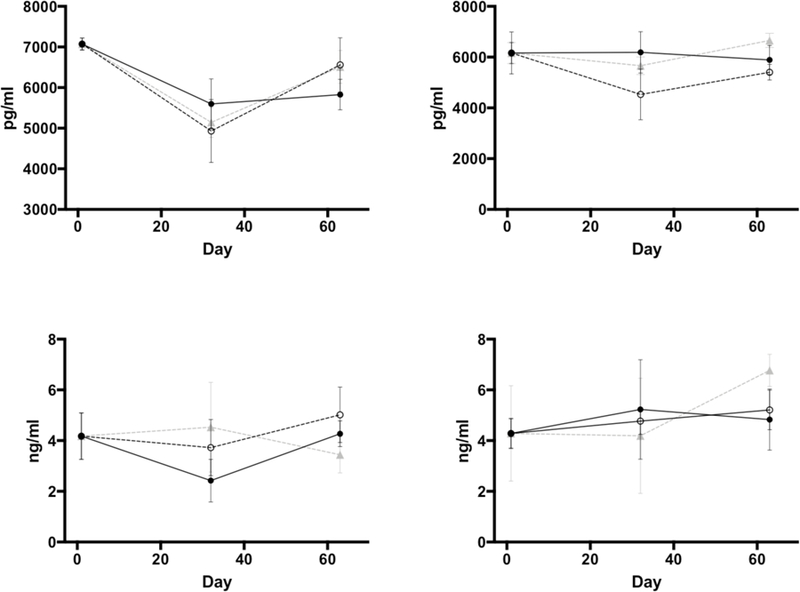
Changes (mean ± standard error of the mean) in levels of P1NP (procollagen type 1 propeptide, top panels) and CTX (C-terminal telopeptide of type I collagen, bottom panels) in female (left-sided panels) and male (right-sided panels) animals. Blood samples collected at baseline (day 0), day 32, and prior to euthanasia (day 63). Filled circles and full lines represent animals in the DHA/ARA group; empty circles and interrupted line belong to animals in the SOY group; and gray triangles and gray interrupted lines belong to the DHA group.
Table 6.
Fatty acid analyses expressed in percentage from n=3 per experimental group at the time of euthanasia (shown are mean ± standard deviation). Where indicated, mean value differences are considered statistically significant (P < 0.05) across all dietary groups (*) or between DHA/AA and DHA compared to SOY (#).
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| DHA/ARA | SOY | DHA | Comparison | DHA/ARA | SOY | DHA | Comparison | |
| 14:0 | 0.76±0.05 | 0.29±0.03 | 0.56±0.04 | 0.83±0.05 | 0.27±0.02 | 0.73±0.09 | ||
| 15:0 | 0.14±0.02 | 0.09±0.01 | 0.1±0.01 | 0.14±0.02 | 0.1±0.01 | 0.1±0.01 | ||
| 16:0 | 26.74±0.66 | 22.14±0.28 | 26.76±0.3 | # | 28.3±0.34 | 22.2±0.16 | 28.59±0.17 | # |
| 16:1 | 5.66±0.32 | 3.4±0.55 | 6.19±0.49 | # | 7.56±0.54 | 4.28±0.21 | 7.56±0.35 | # |
| 18:0 | 15.3±1.04 | 14.61±0.9 | 13.32±0.82 | 13.84±0.89 | 12.52±0.63 | 13.11±0.14 | ||
| 18:1ω9 | 19.83±1.18 | 14.85±1.67 | 19.75±0.27 | # | 16.5±0.9 | 14.08±0.3 | 18.78±1.51 | * |
| 18:1ω7 | 1.5±0.09 | 1.75±0.09 | 1.53±0.13 | 1.15±0.06 | 2.11±0.05 | 1.31±0.12 | ||
| 18:2ω6 | 5.17±0.19 | 20.64±0.62 | 7.99±0.23 | * | 5.93±0.27 | 21.43±0.56 | 8.71±0.29 | * |
| 19:0 | 0.1±0.01 | 0.14±0.02 | 0.11±0.01 | 0.19±0.01 | 0.19±0.03 | 0.19±0.01 | ||
| 18:3ω6 | 0.02±0.01 | 6.31±5.85 | 0.02±0.02 | 0 | 0.56±0.01 | 0 | ||
| 18:3ω3 | 0.08±0.01 | 0.46±0.05 | 0.09±0.02 | 0.08±0.02 | 0.51±0.02 | 0.07±0.03 | ||
| 20:0 | 0.1±0.02 | 0.06±0.01 | 0.11±0.03 | 0.18±0.04 | 0.12±0.03 | 0.17±0.02 | ||
| 20:1ω9 | 0.34±0.11 | 0.18±0.01 | 0.45±0.16 | 0.56±0.14 | 0.39±0.18 | 0.51±0.1 | ||
| 20:1ω7 | 0.03±0.01 | 0.02±0.01 | 0.05±0.02 | 0.1±0.02 | 0.06±0.02 | 0.09±0.03 | ||
| 20:2ω6 | 0.02±0.02 | 0.03±0.02 | 0.1±0.06 | 0.03±0.03 | 0.13±0.05 | 0.04±0.04 | ||
| 20:3ω9 | 0.13±0.04 | 0.14±0.01 | 0.06±0.01 | 0.07±0.05 | 0.18±0.03 | 0.04±0.02 | ||
| 20:3ω6 | 0.22±0.03 | 1.11±0.15 | 0.23±0.11 | 0.16±0.07 | 1.42±0.19 | 0.25±0.09 | ||
| 20:4ω6 | 6.14±0.55 | 13.84±0.59 | 1.45±0.15 | * | 5.35±0.1 | 13.4±0.47 | 1.17±0.14 | * |
| 20:5ω3 | 6.76±0.81 | 0.41±0.05 | 7.94±0.16 | # | 4.21±0.16 | 0.48±0.04 | 5.85±0.37 | * |
| 22:0 | 0.05±0.01 | 0.03±0.01 | 0.05±0.01 | 0.06±0.02 | 0.04±0.02 | 0.04±0.01 | ||
| 22:1ω9 | 0.2±0.02 | 0.13±0.02 | 0.26±0.02 | 0.22±0.01 | 0.12±0.04 | 0.23±0.01 | ||
| 22:5ω6 | 0 | 0.07±0.04 | 0 | 0 | 0.17±0.01 | 0 | ||
| 22:5ω3 | 0.45±0.06 | 0.21±0.03 | 0.54±0.01 | 0.46±0.08 | 0.33±0.02 | 0.5±0.06 | ||
| 22:6ω3 | 10.27±1.43 | 4.93±0.21 | 12.35±0.5 | * | 14.07±1.21 | 4.86±0.27 | 11.99±2.17 | * |
DISCUSSION
The finding that FOLE, rich in DHA, is associated with a reduced incidence of bone fracture rates in infants was first suggested in a retrospective clinical study by our group [12]. Given these findings, coupled with those available in the literature suggesting the beneficial effects of ω−3 PUFAs in bone, we sought to determine how dietary DHA influences bone structural integrity and strength in healthy growing mice. Results from this study suggest that dietary consumption of omega-3 ω−3 PUFAs does not have a particular effect on bone health and metabolism in healthy mice.
Although there were parameters assessing cortical bone structure that reached statistical significance in favor of one experimental group or the other, these were so small that ultimately do not translate into an effect that is biologically relevant. Furthermore, all values obtained seem to fall within a range that is considered normal in healthy mice. This is further proven by the lack of differences in any of the indices assessed via mechanical testing in female mice. The same conclusion can be drawn from the differences seen in trabecular bone in male mice. Although some of our findings showed lower trabecular number and higher trabecular separation in animals allocated to the SOY group, a finding that is seen in animal models that simulate bone loss patterns [26], the lack of differences across groups based on mechanical testing further suggest that these changes may have been observed at random.
Our findings are similar to those reported by Sirois et al, who studied the effects of administering fish oil or a control soybean oil-based diet in 3-week old male and female rats for 5 weeks [27]. Results from this study showed no differences in lumbar bone mineral content (BMC) or density (BMD), or in femoral BMC, BMD, yield load, resilience, peak load, or toughness between the diet groups within each sex. Of note, the fish oil-based diet contained 10 g/kg of soybean oil in addition to the 60 g/kg of menhaden oil (versus 70 g/kg of soybean oil in the control group), which may have confounded the effects of DHA. Results from our study further confirm the fact that dietary supplementation of DHA does not affect the bone at the microstructural level and, in consequence, does not improve its intrinsic stiffness as determined by mechanical testing.
Recently, a study by Kohut et al found that small for gestational age piglets fed formula supplemented with ARA and DHA had higher lumbar spine BMC, with lower weight animals requiring even larger amounts of ARA and DHA [28]. In this study, however, piglets were still given a standard formula and the DHA:ARA ratio was 6:1 for both groups, thus not allowing for an accurate assessment of which fatty acid may have had the positive influence on bone. In contrast, our present study strictly controlled for the amount of fatty acids provided in the experimental diets yet still failed to show a beneficial effect of DHA on bone in healthy animals.
It is still unclear how FOLE may prevent and/or decrease the risk of bone fractures in PN-dependent infants. The beneficial effects of DHA at the microstructural level in disease states may be due to its effect on bone structure and composition either via inflammatory mediators and/or through changes in balance between osteoblast and osteoclast activity. Both ω−3 PUFAs and ω−6 PUFAs serve as lipid precursors of inflammatory mediators; however, the type of response is dependent on the type of mediator that is predominantly produced. The metabolism of ω−6 PUFAs lead to the production of pro-inflammatory mediators, whereas ω−3 PUFAs lead to the production of molecules that are less potent in their pro-inflammatory effects and also have anti-inflammatory activity [29]. Watkins et al demonstrated how the dietary ratio of ω−6:ω−3 PUFAs alters the fatty acid composition of bone compartments in rats by altering the local concentrations of ARA, and modulating the production of prostaglandin E2 and other factors that affect bone modeling [30]. Furthermore, this study showed how the activity of serum alkaline phosphatase isoenzymes were greater in animals fed a diet high in ω−3 PUFAs or with a low ratio of ω−6:ω−3 PUFAs.
It has been shown that the derivatives of ω−3 PUFAs, including DHA, actively resolve inflammation through other mediators called resolvins, protectins, and maresins [31–33]. Herrera et al studied osteoclast cultures derived from murine bone marrow cells and found that cells treated with the inflammation-resolving lipid mediator resolvin E1 had inhibition of osteoclast growth and bone resorption [34]. Similarly, Bhattacharya et al found that the higher BMD levels of 18-month old mice fed a fish oil-based diet versus an ω−6 PUFA-rich corn oil-based diet, were associated with the presence of decreased inflammatory cytokines [35]. Although this does not demonstrate causation, it suggests that inflammation plays a key role in bone metabolism. More recently, Williams-Bey et al studied the effects of DHA, and how it can limit the activation of inflammasomes, molecules that serve as central regulators of innate immunity and inflammation [36]. Therefore, by reducing the production of interleukin 1β through its interaction with G-protein coupled receptor 120 (GPR120), DHA suppressed inflammasome activation by two distinct mechanisms: suppression of nuclear translocation of nuclear factor -κB, and enhancing autophagy in cells.
Oh el al demonstrated that GPR120 functions as an ω−3 PUFA receptor in pro-inflammatory macrophages and mature adipocytes [37]. Pre-treatment with DHA of macrophages from wild-type mice inhibited chemotaxis by 80%, whereas the same treatment had no effect in macrophages from GPR120 knockouts. Being osteoclasts a type of specialized macrophage, these findings explain the significant effect of ω−3 PUFAs on bone resorption. Other studies have linked the inhibition of osteoclasts as a potential mechanism for the beneficial effects of ω−3 PUFAs in bone [38,39].
Similar to GPR120, GPR40 is activated by medium-chain fatty acids as well as long-chain fatty acids such as DHA [40]. GPR120 is expressed in osteoblastic cells and GPR120/40 are expressed in osteoclasts [41]. Cornish et al found that a GPR120/40 agonist mimicked the anti-osteoclastogenic actions of fatty acids, identifying a link between lipid and bone metabolism [41]. More recently, Kasonga et al studied the effects of DHA and ARA on osteoclast formation and activity by isolating CD14+ monocytes [42]. Results from this study revealed that both types of long-chain PUFAs decrease the formation potential of osteoclasts in a dose-dependent manner at early stages of differentiation. Also, these fatty acids seemed to affect the potential for bone resorption in mature osteoclasts without affecting its numbers. Although these results can prove the beneficial effects of anti-inflammatory ω−3 PUFAs in bone health, they seem to contradict those that suggest that ω−6 PUFAs increase bone loss through its pro-inflammatory mediators [17,30]. The presence of other studies suggesting an opposite beneficial effect of ω−6 PUFAs in bone is proof that much of the action of PUFAs on bone metabolism remain to be elucidated [43]. The latter statement is supported by findings in our study, in which all diets exerted similar effects at the bone’s microstructural level. This was regardless of the presence (DHA/ARA) or not (DHA) of ω−6 PUFAs. We did not observe any differences in blood-based biomarkers of bone turnover during our study period. These findings are in conflict with the mentioned studies that argue that the effects of ω−3 PUFAs may be in part related to their role in osteoclasts and bone resorption.
Limitations of this study include the lack of use of single-housing and paired-feeding techniques to control for food intake in every mouse. However, the growth and intake curves obtained throughout our study period were equivalent across the experimental groups within males and females. Also, the activity level of each mouse was not controlled for, a factor that can significantly impact bone health. More importantly, the model used in this study is that of healthy mice, different to what is seen in patients afflicted with MBD.
Results from this study reject our hypothesis that dietary provision of ω−3 PUFAs can have a beneficial effect on bone in healthy growing mice. Perhaps the beneficial effects of ω−3 PUFAs on bone become evident in the setting of disease states. For this reason, our group aims to study the effects of dietary ω−3 PUFAs in diseased animal models to further test this hypothesis in future studies.
ACKNOWLEDGEMENTS
This work was funded by the Children’s Hospital Surgical Foundation within Boston Children’s Hospital (L.A-B., E.C., D.T.D., G.LF., M.A.B., A.P., P.N., and M.P.), the Beth Israel Deaconess Medical Center Department of Orthopedic Surgery (J.C.V-C., M.B.C., A.M., and A.N.), the National Institutes of Health Institutional Training Grants 5T32HD007466–16/17 (E.C.), 5T32HL007734–23 (D.T.D), 5T32HL007734–22 (M.A.B.), and 1F32DK104525–01 (G.L.F.). Special thanks to Daniel Brooks, MSc from the Center for Advanced Orthopedic Studies for his support and help in setting up the protocols for micro-CT scanning, and to Dr. Bruce Bistrian, MD PhD from Beth Israel Deaconess Medical Center, for his thoughtful input in fatty acid metabolism required to design the study.
ABBREVIATIONS (in alphabetical order)
- AIT
adaptive iterative thresholding
- AV
anisidine value
- ARA
arachidonic acid
- BA
bone area
- BA/TA
cortical bone area fraction
- BMC
bone mineral content
- BMD
bone mineral density
- BV/TV
trabecular bone volume fraction
- CTX
C-terminal telopeptide of type I collagen
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FOLE
fish oil-based lipid emulsion
- GPR
G-protein coupled receptor
- IFALD
intestinal failure-associated liver disease
- MBD
metabolic bone disease
- μCT
micro-computed tomography
- P1NP
procollagen type 1 propeptide
- PN
parenteral nutrition
- PUFA
polyunsaturated fatty acid
- PV
peroxide value
- SOLE
soybean oil-based lipid emulsion
- TA
total cross-sectional area
- TMD
tissue mineral density
Footnotes
DISCLOSURE
A license agreement for the use of Omegaven® has been signed by Boston Children’s Hospital and Fresenius Kabi. Mark Puder holds an issued patent on the treatment of parenteral nutrition-associated liver disease. He serves on the Scientific Advisory Boards for Pronova-BASF and Sancilio and Company Inc.
REFERENCES
- [1].Jeejeebhoy KN, Parenteral nutrition in the intensive care unit, Nutr. Rev 70 (2012) 623–630. doi: 10.1111/j.1753-4887.2012.00538.x. [DOI] [PubMed] [Google Scholar]
- [2].Rangel SJ, Calkins CM, Cowles RA, Barnhart DC, Huang EY, Abdullah F, Arca MJ, Teitelbaum DH, Parenteral nutrition–associated cholestasis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review, J. Pediatr. Surg 47 (2012) 225–240. doi: 10.1016/j.jpedsurg.2011.10.007. [DOI] [PubMed] [Google Scholar]
- [3].Diamanti A, Bizzarri C, Bizzarri C, Basso MS, Gambarara M, Cappa M, Daniele A, Noto C, Castro M, How does long-term parenteral nutrition impact the bone mineral status of children with intestinal failure?, J. Bone Miner. Metab 28 (2010) 351–8. doi: 10.1007/s00774-009-0140-0. [DOI] [PubMed] [Google Scholar]
- [4].Buchman AL, Moukarzel A, Metabolic bone disease associated with total parenteral nutrition, Clin. Nutr 19 (2000) 217–231. doi: 10.1054/clnu.1999.0083. [DOI] [PubMed] [Google Scholar]
- [5].Klein GL, Metabolic bone disease of total parenteral nutrition., Nutrition 14 (1998) 149–52. [DOI] [PubMed] [Google Scholar]
- [6].MacKay M, Jackson D, Eggert L, Fitzgerald K, Cash J, Practice-based validation of calcium and phosphorus solubility limits for pediatric parenteral nutrition solutions., Nutr. Clin. Pract 26 (2011) 708–13. doi: 10.1177/0884533611426435. [DOI] [PubMed] [Google Scholar]
- [7].Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA, A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study., J. Bone Miner. Res 14 (1999) 1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- [8].Pichler J, Chomtho S, Fewtrell M, Macdonald S, Hill SM, Growth and bone health in pediatric intestinal failure patients receiving long-term parenteral nutrition., Am. J. Clin. Nutr 97 (2013) 1260–9. doi: 10.3945/ajcn.112.057935. [DOI] [PubMed] [Google Scholar]
- [9].Pironi L, Tjellesen L, De Francesco A, Pertkiewicz M, Morselli Labate AM, Staun M, Przedlacki J, Lezo A, Orlandoni P, Pasanisi F, ESPEN-home artificial nutrition working group, Bone mineral density in patients on home parenteral nutrition: a follow-up study, Clin. Nutr 23 (2004) 1288–1302. doi: 10.1016/j.clnu.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [10].Haderslev KV, Tjellesen L, Haderslev PH, Staun M, Assessment of the longitudinal changes in bone mineral density in patients receiving home parenteral nutrition., JPEN. J. Parenter. Enteral Nutr 28 (2004) 289–94. doi: 10.1177/0148607104028005289. [DOI] [PubMed] [Google Scholar]
- [11].Raman M, Aghdassi E, Baun M, Yeung M, Fairholm L, Saqui O, Allard JP, Metabolic bone disease in patients receiving home parenteral nutrition: a Canadian study and review., JPEN. J. Parenter. Enteral Nutr 30 (2006) 492–6. doi: 10.1177/0148607106030006492. [DOI] [PubMed] [Google Scholar]
- [12].Fallon EM, Nazarian A, Nehra D, Pan AH, O’Loughlin AA, Nose V, Puder M, The effect of docosahexaenoic acid on bone microstructure in young mice and bone fracture in neonates., J. Surg. Res 191 (2014) 148–55. doi: 10.1016/j.jss.2014.04.005. [DOI] [PubMed] [Google Scholar]
- [13].Kruger MC, Coetzee M, Haag M, Weiler H, Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone., Prog. Lipid Res 49 (2010) 438–49. doi: 10.1016/j.plipres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- [14].Li Y, Seifert MF, Lim S-Y, Salem N, Watkins BA, Bone mineral content is positively correlated to n-3 fatty acids in the femur of growing rats., Br. J. Nutr 104 (2010) 674–85. doi: 10.1017/S0007114510001133. [DOI] [PubMed] [Google Scholar]
- [15].Lau BYY, Ward WE, Kang JX, Ma DWL, Femur EPA and DHA are correlated with femur biomechanical strength in young fat-1 mice., J. Nutr. Biochem 20 (2009) 453–61. doi: 10.1016/j.jnutbio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [16].Paunescu A-C, Ayotte P, Dewailly E, Dodin S, Pedersen HS, Mulvad G, Côté S, Polyunsaturated fatty acids and calcaneal ultrasound parameters among Inuit women from Nuuk (Greenland): a longitudinal study., Int. J. Circumpolar Health 72 (2013) 20988. doi: 10.3402/ijch.v72i0.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Högström M, Nordström P, Nordström A, n-3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: the NO2 Study., Am. J. Clin. Nutr 85 (2007) 803–7. [DOI] [PubMed] [Google Scholar]
- [18].Lukas R, Gigliotti JC, Smith BJ, Altman S, Tou JC, Consumption of different sources of omega-3 polyunsaturated fatty acids by growing female rats affects long bone mass and microarchitecture., Bone 49 (2011) 455–62. doi: 10.1016/j.bone.2011.05.029. [DOI] [PubMed] [Google Scholar]
- [19].Le HD, Fallon EM, Kalish BT, de Meijer VE, Meisel JA, Gura KM, Nose V, Pan AH, Bistrian BR, Puder M, The effect of varying ratios of docosahexaenoic acid and arachidonic acid in the prevention and reversal of biochemical essential fatty acid deficiency in a murine model, Metabolism 62 (2013) 499–508. doi: 10.1016/j.metabol.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moseley JE, Skeletal changes in the anemias., Semin. Roentgenol 9 (1974) 169–84. [DOI] [PubMed] [Google Scholar]
- [21].Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R, Guidelines for assessment of bone microstructure in rodents using micro-computed tomography, J. Bone Miner. Res 25 (2010) 1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- [22].Ko FC, Karim L, Brooks DJ, Bouxsein ML, Demay MB, Bisphosphonate Withdrawal: Effects on Bone Formation and Bone Resorption in Maturing Male Mice, J. Bone Miner. Res 32 (2017) 814–820. doi: 10.1002/jbmr.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA, IOF-IFCC Bone Marker Standards Working Group, Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards, Osteoporos. Int 22 (2011) 391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- [24].Folch J, Lees M, Stanley GHS, A simple method for the isolation and purification of total lipides from animal tissues, J. Biol. Chem 226 (1957) 497–509. doi: 10.1371/journal.pone.0020510. [DOI] [PubMed] [Google Scholar]
- [25].Bozdogan H, Model selection and Akaike’s Information Criterion (AIC): The general theory and its analytical extensions, Psychometrika 52 (1987) 345–370. doi: 10.1007/BF02294361. [DOI] [Google Scholar]
- [26].Yu EW, Carmody JS, Brooks DJ, LaJoie S, Kaplan LM, Bouxsein ML, Cortical and trabecular deterioration in mouse models of Roux-en-Y gastric bypass., Bone 85 (2016) 23–8. doi: 10.1016/j.bone.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sirois I, Cheung AM, Ward WE, Biomechanical bone strength and bone mass in young male and female rats fed a fish oil diet., Prostaglandins. Leukot. Essent. Fatty Acids 68 (2003) 415–21. [DOI] [PubMed] [Google Scholar]
- [28].Kohut J, Watkins B, Weiler H, Enhanced lumbar spine bone mineral content in piglets fed arachidonic acid and docosahexaenoic acid is modulated by severity of growth restriction., Br. J. Nutr 102 (2009) 1117–20. doi: 10.1017/S0007114509371780. [DOI] [PubMed] [Google Scholar]
- [29].Wall R, Ross RP, Fitzgerald GF, Stanton C, Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids, Nutr. Rev 68 (2010) 280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- [30].Watkins BA, Li Y, Allen KG, Hoffmann WE, Seifert MF, Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats., J. Nutr 130 (2000) 2274–84. [DOI] [PubMed] [Google Scholar]
- [31].Serhan CN, Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolving mediators, J. Thromb. Haemost 7 (2009) 44–48. doi: 10.1111/j.1538-7836.2009.03396.x. [DOI] [PubMed] [Google Scholar]
- [32].Serhan CN, Petasis NA, Resolvins and protectins in inflammation resolution., Chem. Rev 111 (2011) 5922–43. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kalish BT, Le HD, Fitzgerald JM, Wang S, Seamon K, Gura KM, Gronert K, Puder M, Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators, AJP Gastrointest. Liver Physiol 305 (2013) G818–G828. doi: 10.1152/ajpgi.00106.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara MN, Serhan CN, Van Dyke TE, Gyurko R, An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption., Br. J. Pharmacol 155 (2008) 1214–23. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bhattacharya A, Rahman M, Sun D, Fernandes G, Effect of fish oil on bone mineral density in aging C57BL/6 female mice, J. Nutr. Biochem 18 (2007) 372–379. doi: 10.1016/j.jnutbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [36].Williams-Bey Y, Boularan C, Vural A, Huang N-N, Hwang I-Y, Shan-Shi C, Kehrl JH, Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy., PLoS One 9 (2014) e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM, GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects, Cell 142 (2010) 687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rahman MM, Bhattacharya A, Fernandes G, Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid., J. Cell. Physiol 214 (2008) 201–9. doi: 10.1002/jcp.21188. [DOI] [PubMed] [Google Scholar]
- [39].Shen C-L, Yeh JK, Rasty J, Chyu M-C, Dunn DM, Li Y, Watkins BA, Improvement of bone quality in gonad-intact middle-aged male rats by long-chain n-3 polyunsaturated fatty acid., Calcif. Tissue Int 80 (2007) 286–93. doi: 10.1007/s00223-007-9010-8. [DOI] [PubMed] [Google Scholar]
- [40].Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI, The Orphan G Protein-coupled Receptor GPR40 Is Activated by Medium and Long Chain Fatty Acids, J. Biol. Chem 278 (2003) 11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- [41].Cornish J, MacGibbon A, Lin J-M, Watson M, Callon KE, Tong PC, Dunford JE, van der Does Y, Williams GA, Grey AB, Naot D, Reid IR, Modulation of osteoclastogenesis by fatty acids., Endocrinology 149 (2008) 5688–95. doi: 10.1210/en.2008-0111. [DOI] [PubMed] [Google Scholar]
- [42].Kasonga AE, Deepak V, Kruger MC, Coetzee M, Arachidonic acid and docosahexaenoic acid suppress osteoclast formation and activity in human CD14+ monocytes, in vitro., PLoS One 10 (2015) e0125145. doi: 10.1371/journal.pone.0125145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL, Dietary intakes of arachidonic acid and alpha-linolenic acid are associated with reduced risk of hip fracture in older adults., J. Nutr 141 (2011) 1146–53. doi: 10.3945/jn.110.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]