Abstract
Purpose
To model prediction of undetected glaucoma in a predominantly white population, based on intraocular pressure (IOP) and subject age.
Methods
In 1992–1997, a population screening for glaucoma was performed at Malmö University Hospital where individuals between 55–79 years of age (n = 46 614) living in Malmö, were invited to a free eye health examination. Recently examined patients were not invited (n = 4117). IOP and age were recorded for all screened subjects. Subjects who screened positive were further examined to establish or reject a glaucoma diagnosis. We performed multiple regression analysis of the combined effect of age and IOP on the likelihood of undetected glaucoma.
Results
In all, 32 918 subjects attended the screening (77.5% of invited), 22 218 women and 11 700 men, while 9579 refrained from participation. Glaucoma was diagnosed in 406 subjects. The proportion of subjects with glaucoma increased exponentially with increasing IOP and older age. Still, the majority of subjects with glaucoma (57%) had ≤IOP 21 mmHg. The predicted rate of undetected glaucoma was low, <5%, for subjects with IOP <25 mmHg, but rose rapidly with higher IOP, reaching 81% in the group with IOP >35 mmHg and age 75–79 years. The model fit well to the data (R 2 = 0.97).
Conclusion
We created a model estimating the combined effect of IOP and age on the likelihood of undetected glaucoma. The model may facilitate case‐finding in European‐derived populations. Despite the important impact of IOP on the risk of glaucoma, a large proportion of subjects with undetected glaucoma had IOP ≤ 21 mmHg.
Keywords: age, glaucoma, IOP, prediction, screening, undetected
Introduction
Several risk factors for glaucoma have been identified (Leske 2007), and higher intraocular pressure (IOP) and older age are frequently reported in this context (Hollows & Graham 1966; Bankes et al. 1968; Kahn et al. 1977; Bengtsson 1981; Sommer et al. 1991; Klein et al. 1992; Dielemans et al. 1994; Mitchell et al. 1996; Wensor et al. 1998; Gordon et al. 2002; Iwase et al. 2004; Quigley & Broman 2006; Nemesure et al. 2007; Heijl et al. 2013). Half of all glaucoma cases in developed countries are undetected (Rudnicka et al. 2006), and population screening would seem to be ideal for detecting glaucoma, due to its relatively high prevalence, severity of disease and asymptomatic initial stage. However, population screening for glaucoma is not generally recommended, because available methods are time‐consuming and expensive, and not sufficiently specific. Nevertheless, it may be cost effective to screen high‐risk groups (Mowatt et al. 2008), and there is a need for improved case detection (Quigley 2011).
Community optometrists/opticians represent a valuable resource for the detection of glaucoma. Indeed, conducting opportunistic glaucoma case‐finding during regular optician visits can help reach a large proportion of the population at risk of developing glaucoma (Stoutenbeek & Jansonius 2006).
Many opticians already screen their customers for elevated IOP, as recommended by, for example, AAOs Preferred Practice Pattern for POAG Suspects (Prum et al. 2016). Individuals with elevated intraocular pressure (IOP) are recommended to have a comprehensive medical eye evaluation.
A very sizeable data set is needed to calculate the combined effect of more than one factor on the likelihood of glaucoma. We have access to data from a large population screening of 32 918 subjects that can be used to estimate the combined effect of age and IOP on the likelihood of undetected glaucoma and to develop a diagnostic prediction model for risk assessment that may improve case detection and provide a valuable tool for recommendations of referrals to ophthalmologists. Accordingly, we conducted the present study to develop such a model.
Materials and Methods
Source of data
A population screening was performed at Malmö University Hospital in Sweden between October 1992 and January 1997 to identify individuals with undiagnosed manifest glaucoma for possible inclusion in a randomized controlled treatment study, the Early Manifest Glaucoma Trial (EMGT) (Leske et al. 1999). The screening was approved by the Ethics Committee of the University of Lund.
Participants
A free eye health examination was offered to all female residents in Malmö aged 55–79 years and all male residents aged 60–79 years. Those who had recently been examined at the department of Ophthalmology in Malmö, and individuals already having a glaucoma diagnosis, were not invited to the screening (n = 4117). Individuals with a glaucoma diagnosis who had received the diagnosis elsewhere and came to the screening were excluded from the analysis. Subjects with IOP missing in both eyes, for example, if they declined tonometry, were excluded.
Outcome
Subjects who fulfilled any of the following criteria were invited to one or two post‐screening visits: intraocular pressure >25 mmHg with Goldmann applanation tonometry and/or suspected glaucomatous optic disc changes (vertically elongated cupping of the disc, localized narrowing of the optic disc rim, nerve fibre layer defect, optic disc haemorrhages) in the photographs, and/or those who had a self‐reported family history of glaucoma in siblings. The post‐screening examinations were intended to establish or reject the diagnosis of glaucoma and eligibility for the EMGT. At these visits, a full eye examination was performed including standard automated perimetry (SAP) with the 24‐2 Full Threshold program of the Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA, USA).
The glaucoma diagnosis was based on the presence of repeatable visual field defects compatible with glaucoma and not explained by other causes. In subjects with only one visual field test, corresponding defects in the optic nerve head (as evaluated by at least one glaucoma specialist) and/or in the retinal nerve fibre layer (RNFL) were required. If no visual field was available (e.g., due to physical disability or blindness), or if the visual field was erratic (e.g., a clover‐leaf field), obvious glaucomatous damage in the optic nerve head and/or RNFL was required.
Predictors
At the screening examination, IOP was measured and fundus colour photographs were obtained. In the present study, the predictors used were as follows: Screening IOP divided into 5‐mmHg intervals ranging from 10 to 34 mmHg. We used the IOP of the eye with the higher IOP of the two for analysis, and subjects were categorized into two groups, glaucoma in at least one eye versus no glaucoma. Eyes with IOP values <10 and ≥35 mmHg were assigned to two separate groups. The other predictor was subject age categorized in age groups at 5‐year intervals, from 55 to 79 years. The proportions of newly detected subjects with glaucoma were calculated for each combination of the seven IOP groups and the five age groups.
Populations size and missing data
A total of 42 497 individuals within the target age intervals, with the exception of individuals who had visited the department within one year prior to the screening, were invited to the screening. Those not attending the screening, 9579 individuals or 22.5% of all invited, were not considered in the current analysis, no imputation method was applied. The mean age of those not attending was 66.7 years (SD 5.6 years) and for those attending 67.1 years (SD 5.6 years).
Statistical analysis
A multivariate regression analysis was performed in order to model the association between age and IOP and the interaction between age and IOP on one hand, and the proportion of previously undetected glaucoma on the other. Both age and IOP showed exponential relationships to the proportion of newly detected glaucomatous subjects. To obtain a linear relationship, a logarithmic (ln) transformation was performed on the dependent variable: proportion of glaucomatous subjects at different levels of IOP and age. To facilitate conversion of data back to the original scale, cells with no glaucoma were regarded as missing values rather than adding an arbitrary constant to the data. A multiple linear regression including residual analysis was performed:
The coefficients from the regression analysis were used to model the combined effect of age and IOP level on the predicted proportion of glaucomatous subjects for each combination of age and IOP groups.
Results
Participants
The flow of participants is shown in Fig. 1. In all, 77.5% of the 42 497 individuals who were invited attended the screening, resulting in 32 918 screened subjects. Recently examined individuals, a total of 4117 individuals, were not invited. Eighty‐three subjects with a prior diagnosis of glaucoma unknown to us were screened, but excluded from the current analysis. We identified 545 undiagnosed glaucomatous eyes in 406 subjects, and 231 (57%) had an IOP value ≤21 mmHg in the eye with the higher IOP. In 86% of the newly detected glaucomatous eyes, the diagnosis was based on repeatable visual field defects compatible with glaucoma and not explained by other causes; considering the remaining 14%, the diagnosis was based on a single field with corresponding optic disc changes in 9% and on optic disc appearance alone in 5%.
Figure 1.
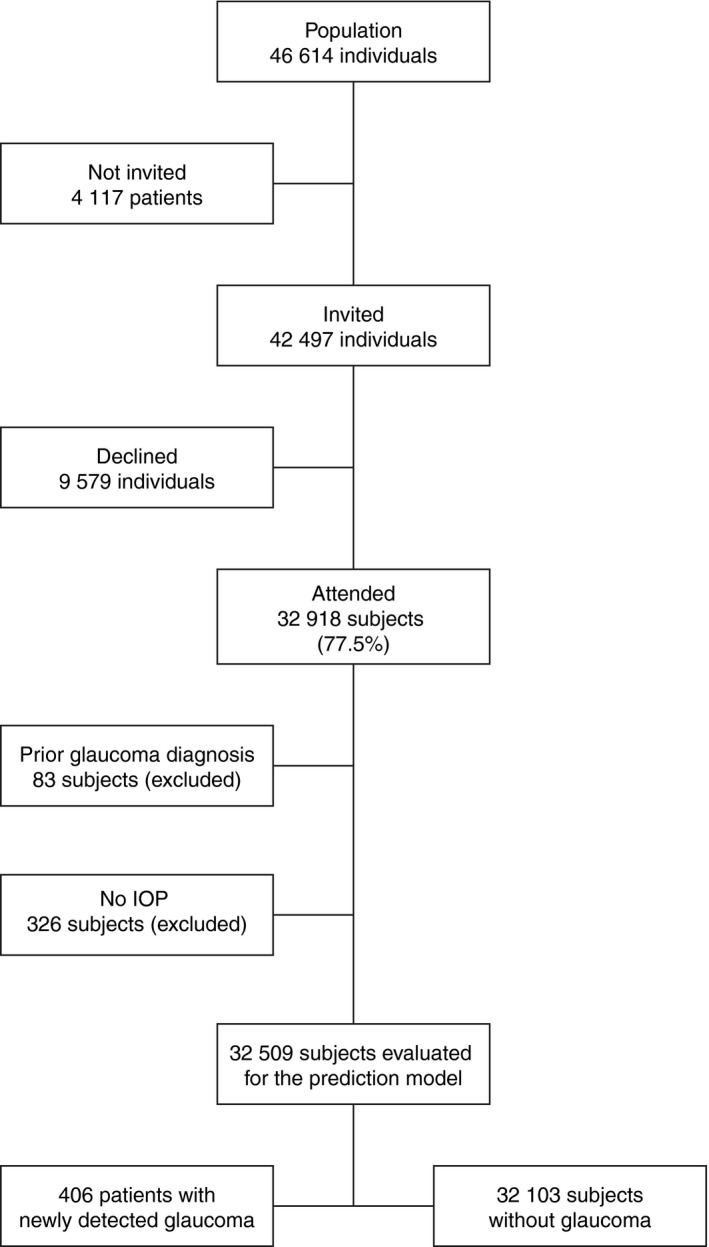
Flow chart of screened subjects.
A total of 32 509 subjects were evaluated. Intraocular pressure (IOP) measurements were missing for 726 eyes (1.1% of all eyes) in 400 subjects, but none of those eyes were glaucomatous. In 326 subjects, the IOP for both eyes was missing and thus not used in the model development. In 74 subjects, the IOP for one eye was missing. The most common explanation for a missing IOP measurement was that the subject declined tonometry. The largest group of screened subjects, 34%, were between 65 to 69 years of age followed by the group between 60 to 64 years of age. The distribution of age of screened subjects can be seen in Table 1. A large majority (30 261 subjects) of the screened subjects had IOP within statistically normal limits in the eye with higher IOP.
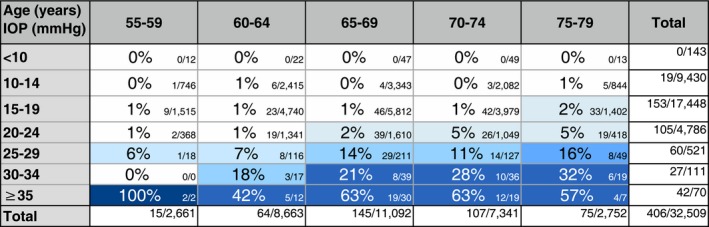
Table 1.
Observed proportions (%) of subjects with glaucoma detected at population screening in each age and IOP group. The number of subjects with glaucoma/number of screened subjects is shown to the right. Relatively few subjects were screened in the youngest and the oldest group compared to the other three groups
Model development
The observed proportions of subjects with newly detected glaucoma in at least one eye and the number of glaucomatous subjects versus screened subjects in each age and IOP group are shown in Table 1. No subjects with glaucoma were detected at IOP levels <10 mmHg in the eye with the higher pressure and only 19 subjects with glaucoma had IOP in the interval between 10 to 14 mmHg. Although the majority of subjects with glaucoma detected by the screening had pressures of 21 mmHg or less (231 subjects, 57%), this resulted in a proportion of glaucoma of 0.8% of all screened subjects at ages 55–79 years with IOP 21 mmHg or less. Forty‐six subjects with glaucoma had IOP 22–24 mmHg, resulting in a proportion of glaucoma of 2.4% of all screened subjects with IOP 22–24 mmHg, considering all age groups. Proportions of glaucoma were thus small up to the 25 mmHg level. The proportion increased slightly with age up to the 25 mmHg level. Higher proportion (18.3%) was seen if IOP was ≥25 mmHg. The proportion increased with age and was high (i.e., ≥20%) at IOP levels ≥30 mmHg in subjects aged ≥65 years.
Model specifications
The likelihood of undiagnosed glaucoma increased exponentially with both age and IOP level, and the combined effect of age and IOP was highly significant (p = 0.000). The effect of IOP alone was greater than that of age alone, although the effects of both factors were highly significant (p = 0.000). Table 2 presents the proportions predicted when using the following formula:
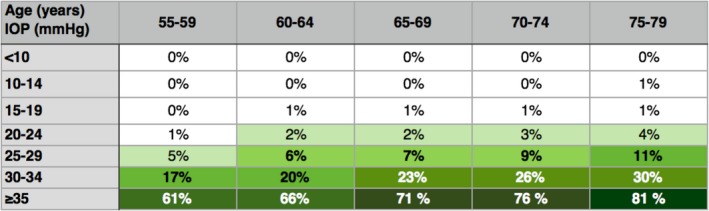
Table 2.
Predicted proportions of undetected glaucoma at different levels of age and IOP in the population
Model performance
The regression model fit well to the data, with a coefficient of determination (R2) of 0.97. The residuals were normally distributed and were randomly dispersed around the horizontal zero line. The 95% confidence interval was ± 0.30 for the age coefficient, ±0.21 for the IOP coefficient, and ±0.06 for the interaction between the two.
Discussion
We were able to study the combined effect of IOP and age on the likelihood of undetected glaucoma, because we had access to data from a large‐scale population screening of almost 33 000 subjects. Although 57% of all subjects with previously undetected glaucoma had an IOP of ≤21 mmHg in the eye with the higher IOP, the influence of IOP was considerably greater than that of age. Relatively few screened subjects had pressures above 24 mmHg (2.2%), but the proportion of glaucomatous eyes with pressure above 24 mmHg was markedly higher than in those with lower IOP values.
A weakness of the current investigation is that some of the proportions presented here may be lower than the true numbers. All subjects with IOP >25 mmHg underwent visual field testing, but eyes with IOP ≤25 mmHg screened negative unless disc or RNFL findings were suspicious or if subjects had a positive family history of glaucoma. The reason for using 25 mmHg was that the original purpose of the screening was to identify previously undetected subjects with glaucoma to be included in EMGT. Thus, glaucomatous eyes with small optic discs may have been missed, since glaucoma eyes with small discs often appear healthy (Heijl & Molder 1993). Another reason that suggests that the number of subjects with glaucoma and IOP ≤ 21 mmHg is underestimated in the current study due to the screening criteria is that Springelkamp et al. (2017) showed that one out of four newly detected glaucoma cases had discs within normal limits according to the (strict) ISGEO criteria and the mean IOP of those cases was 16.3 mmHg. Another study showed higher proportions of normal‐tension glaucoma detected when screened with visual fields and optic disc evaluation for all subjects (Stoutenbeek et al. 2008).
Interpretation
Predicted proportions of undetected glaucoma were relatively small in subjects with IOP levels up to 25 mmHg but increased slightly with age. Markedly higher proportions of ≥17% were noted at IOP levels ≥30 mmHg. At IOP levels of ≥35 mmHg, the predicted proportions of glaucoma ranged from 61% in the youngest age group to 81% in the oldest age group, although the number of subjects with those IOPs was small, representing only 0.2% of all screened subjects.
The strengths of this study are the large size of the screened population and the fact that the diagnosis was confirmed with visual field tests in most eyes (95%). Due to the considerable size of the material, the denominator was relatively large in most cells: 51% of the cells (18/35) included ≥100 subjects, and 31% (11/35) comprised ≥1000 screened subjects, Table 1.
Many studies have shown increasing rates of glaucoma at higher IOP values (Sommer et al. 1991; Mitchell et al. 1996; Iwase et al. 2004) or older age (Bengtsson 1981; Dielemans et al. 1994; Leske et al. 1994; Wensor et al. 1998; Quigley & Broman 2006). We have presented a model that shows the combined effect of age and IOP and the interaction of age and IOP on the presence of undetected glaucoma in the community.
Implications
Considering possible general applicability of our results, it can be noted that the proportion of undetected glaucoma in the Malmö screening was very similar to rates previously reported in other developed countries (Kahn et al. 1977; Sommer et al. 1991; Coffey et al. 1993; Dielemans et al. 1994; Mitchell et al. 1996; Wensor et al. 1998).
Ocular hypertension is a well‐known risk factor for glaucoma (Gordon et al. 2002) and as recommended by the Preferred Practice Pattern for POAG Suspects of the AAO, eye care providers should measure IOP in all individuals over 40 years of age (Prum et al. 2016). False‐positive test results lead to a reduction in the predictive power of positive testing and increase the number of patients that require hospital care and thus add to both the workload at outpatient departments and the costs of health care. Since the prevalence of glaucoma increases with age and people are living longer, the burden of disease to society are increasing. The model could reduce the number of individuals referred to an ophthalmologist when referral is based solely on age and IOP, although there is still a considerable rate of false positives using the model, even at higher IOP values. Table 3 shows the number needed to screen to detect one new case of glaucoma in each age‐IOP group. We can see an inverted exponential relationship, with more subjects needed to be screened to detect one subject with glaucoma with lower IOP and younger age.
Table 3.
Number needed to screen to detect one new case of glaucoma in each age/IOP group using the prediction model
| Age (years) IOP (mmHg) | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 |
|---|---|---|---|---|---|
| <10 | 3334 | 2000 | 1429 | 834 | 527 |
| 10–14 | 1000 | 625 | 435 | 286 | 189 |
| 15–19 | 271 | 193 | 137 | 98 | 70 |
| 20–24 | 76 | 58 | 44 | 34 | 26 |
| 25–29 | 21 | 18 | 14 | 12 | 10 |
| 30–34 | 6 | 6 | 5 | 4 | 4 |
| 35 or more | 2 | 2 | 2 | 2 | 2 |
As previously mentioned, although very small proportions of subjects with IOP ≤21 mmHg had glaucoma, they represented 57% of all subjects found to have glaucoma in the screening. The explanation could be, or at least in part be, that individuals with undetected glaucoma and high IOP are more likely to be discovered in routine clinical practice or being referred to an ophthalmologist from an optician than individuals with IOP within the statistically normal limits (Grødum et al. 2002). It has been shown that individuals with undetected glaucoma and ≤IOP 21 mmHg are often overlooked in routine clinical practice (Grødum et al. 2002). Another reason could be that the natural course of the disease is slower on a group level when the IOP is ≤21 mmHg, than with high untreated IOP (Heijl et al. 2009), and more time could pass before symptoms develop that make the patient seek ophthalmologic care.
The specificity of the model would be 99% when using a cutoff at 25 mmHg for referral. However, the sensitivity would be low, only 32%.
Here, we have presented a model for the prediction of undiagnosed glaucoma based on the combined effect of IOP and age and the interaction between the two factors. By knowing the subjects age and measuring the IOP the model can be used to calculate the probability of the subject to have undetected glaucoma. Our results may prove useful when updating guidelines for referrals to ophthalmologists from primary eye care professionals i.e., opticians, optometrists or general practitioners.
Contributorship: All authors contributed according to the ICMJE Recommendations. Sigridur Oskarsdottir: Analysis of the data, manuscript writing. Anders Heijl: Data gathering and design of the screening, assisted in manuscript writing. Boel Bengtsson: Statistical analysis, assisted in manuscript writing.
Anders Heijl serves as a consultant for Zeiss and Allergan and has received speaker honoraria from Allergan and Santen. Boel Bengtsson is a consultant to Zeiss. No conflicting relationships exist for Sigridur Oskarsdottir.
This study was supported by grants from the following: the Swedish Research Council (grant K2011‐63X‐10426‐19‐3), the National Eye Institute, Bethesda, Maryland (grant no U10EY10260), the Herman Järnhardt Foundation, the Foundation for Visually Impaired in Former Malmöhus County, and Crown Princess Margareta's Foundation. The National Eye Institute participated in the design of the study. Other sponsors or funding organizations had no role in the design or performance of this research.
References
- Bankes JL, Perkins ES, Tsolakis S & Wright JE (1968): Bedford glaucoma survey. BMJ 1: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson B (1981): The prevalence of glaucoma. British J Ophthalmol 65: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey M, Reidy A, Wormald R, Xian WX, Wright L & Courtney P (1993): Prevalence of glaucoma in the west of Ireland. British J Ophthalmol 77: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE & de Jong PT (1994): The prevalence of primary open‐angle glaucoma in a population‐based study in The Netherlands. The Rotterdam Study. Ophthalmology 101: 1851–1855. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD et al. (2002): The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open‐angle glaucoma. Arch Ophthalmol (Chicago, IL: 1960) 120: 714–720; discussion 829‐730. [DOI] [PubMed] [Google Scholar]
- Grødum K, Heijl A & Bengtsson B (2002): A comparison of glaucoma patients identified through mass screening and in routine clinical practice. Acta Ophthalmol Scand 80: 627–631. [DOI] [PubMed] [Google Scholar]
- Heijl A & Molder H (1993): Optic disc diameter influences the ability to detect glaucomatous disc damage. Acta Ophthalmol 71: 122–129. [DOI] [PubMed] [Google Scholar]
- Heijl A, Bengtsson B, Hyman L, Leske MC & EMGT Group (2009): Natural history of open‐angle glaucoma. Ophthalmology 116: 2271–2276. [DOI] [PubMed] [Google Scholar]
- Heijl A, Bengtsson B & Oskarsdottir SE (2013): Prevalence and severity of undetected manifest glaucoma: results from the early manifest glaucoma trial screening. Ophthalmology 120: 1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollows FC & Graham PA (1966): Intra‐ocular pressure, glaucoma, and glaucoma suspects in a defined population. British J Ophthalmol 50: 570–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Suzuki Y, Araie M et al. (2004): The prevalence of primary open‐angle glaucoma in Japanese: the Tajimi Study. Ophthalmology 111: 1641–1648. [DOI] [PubMed] [Google Scholar]
- Kahn HA, Leibowitz HM, Ganley JP, Kini MM, Colton T, Nickerson RS & Dawber TR (1977): The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol 106: 17–32. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Sponsel WE, Franke T, Cantor LB, Martone J & Menage MJ (1992): Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 99: 1499–1504. [DOI] [PubMed] [Google Scholar]
- Leske MC (2007): Open‐angle glaucoma – an epidemiologic overview. Ophthalmic Epidemiol 14: 166–172. [DOI] [PubMed] [Google Scholar]
- Leske MC, Connell AM, Schachat AP & Hyman L (1994): The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol (Chicago, IL: 1960) 112: 821–829. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L & Bengtsson B (1999): Early manifest glaucoma trial: design and baseline data. Ophthalmology 106: 2144–2153. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Smith W, Attebo K & Healey PR (1996): Prevalence of open‐angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 103: 1661–1669. [DOI] [PubMed] [Google Scholar]
- Mowatt G, Burr JM, Cook JA, Siddiqui MA, Ramsay C, Fraser C, Azuara‐Blanco A & Deeks JJ (2008): Screening tests for detecting open‐angle glaucoma: systematic review and meta‐analysis. Invest Ophthalmol Vis Sci 49: 5373–5385. [DOI] [PubMed] [Google Scholar]
- Nemesure B, Honkanen R, Hennis A, Wu SY & Leske MC (2007): Incident open‐angle glaucoma and intraocular pressure. Ophthalmology 114: 1810–1815. [DOI] [PubMed] [Google Scholar]
- Prum BE Jr, Lim MC, Mansberger SL et al. (2016): Primary open‐angle glaucoma suspect preferred practice Pattern® Guidelines. Ophthalmology 123: 112–151. [DOI] [PubMed] [Google Scholar]
- Quigley HA (2011): Glaucoma. Lancet (London, England) 377: 1367–1377. [DOI] [PubMed] [Google Scholar]
- Quigley HA & Broman AT (2006): The number of people with glaucoma worldwide in 2010 and 2020. British J Ophthalmol 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicka AR, Mt‐Isa S, Owen CG, Cook DG & Ashby D (2006): Variations in primary open‐angle glaucoma prevalence by age, gender, and race: a Bayesian meta‐analysis. Invest Ophthalmol Vis Sci 47: 4254–4261. [DOI] [PubMed] [Google Scholar]
- Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J & Singh K (1991): Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol (Chicago, IL: 1960) 109: 1090–1095. [DOI] [PubMed] [Google Scholar]
- Springelkamp H, Wolfs RC, Ramdas WD, Hofman A, Vingerling JR, Klaver CC & Jansonius NM (2017): Incidence of glaucomatous visual field loss after two decades of follow‐up: the Rotterdam Study. Eur J Epidemiol 32: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoutenbeek R & Jansonius NM (2006): Glaucoma screening during regular optician visits: can the population at risk of developing glaucoma be reached? Br J Ophthalmol 90: 1242–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoutenbeek R, de Voogd S, Wolfs RC, Hofman A, de Jong PT & Jansonius NM (2008): The additional yield of a periodic screening programme for open‐angle glaucoma: a population‐based comparison of incident glaucoma cases detected in regular ophthalmic care with cases detected during screening. Br J Ophthalmol 92: 1222–1226. [DOI] [PubMed] [Google Scholar]
- Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM & Taylor HR (1998): The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology 105: 733–739. [DOI] [PubMed] [Google Scholar]


