Abstract
The optimal management strategy for children with immune-tolerant chronic hepatitis B virus (HBV) infection remains unknown. The purpose of this clinical trial was to determine the safety and efficacy of therapy with entecavir and peginterferon in a group of children in the immune-tolerant phase of HBV infection. Children with immune-tolerant features of chronic hepatitis B received entecavir once daily in a dose of 0.015 mg/kg (0.5 mg maximum) for 48 weeks; peginterferon alfa-2a (180 μg/1.73m2 subcutaneously) once weekly was added at the end of week 8 and continued until week 48. The primary endpoint was lack of detectable HBeAg with HBV DNA levels ≤1,000 IU/mL 48 weeks after stopping therapy. Sixty children (75% female), median age 10.9 (range 3.4–17.9) years, were enrolled. All were positive for HBsAg and HBeAg and had high levels of HBV DNA with normal or minimally elevated levels of alanine aminotransferase (ALT). Fifty-five children completed the entire 48-week course of therapy. At 48 weeks after treatment ended (week 96), two children (3%) achieved the primary endpoint and were also HBsAg negative and anti-HBs positive. One child was HBeAg positive but HBsAg negative at week 60; another was HBeAg negative but HBsAg positive at week 72 which were their last clinic visits. In the remaining children, serum ALT and HBV DNA levels at week 96 were similar to baseline. Thirty-seven children experienced adverse events (AE) and one had a serious AE. Conclusion: The combination of entecavir and peginterferon for up to 48 weeks rarely led to loss of HBeAg with sustained suppression of HBV DNA levels in children in the immune-tolerant phase of HBV infection, and treatment was associated with frequent AEs.
Keywords: treatment, antiviral trial, adverse events, viral load, HBeAg seroconversion, pediatrics
Chronic hepatitis B virus (HBV) infection remains an important cause of chronic liver disease worldwide resulting in significant morbidity and mortality with estimates suggesting that 25% of infected individuals will ultimately develop cirrhosis or hepatocellular carcinoma. (1, 2) Universal HBV vaccination and prenatal screening in the United States and Canada have decreased the incidence of chronic HBV infection in children born in these countries. New cases of hepatitis B, however, are seen in North America among children of immigrants and children adopted internationally (3–5). On average, an estimated 53,800 people with chronic HBV immigrated to the United States annually during 2004–2008. (4)
The immune-tolerant phase of chronic HBV infection is defined by the presence of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) with high levels of HBV DNA but normal levels of alanine aminotransferase (ALT) in serum and no or only mild liver inflammation on liver biopsy. (6, 7) Many children with chronic HBV remain in the immune-tolerant phase during much of childhood. Treatment with interferon alone in such children is rarely effective as loss of HBV DNA or HBeAg occurs in less than 10% of those treated. (8, 9) Similarly, use of oral nucleoside analogs alone in these children is also ineffective. (9, 10) More encouraging results have been reported with a combination of interferon and nucleoside analogs, although from small single center studies only using lamivudine and standard interferon alfa. (11, 12) The aims of this open-label, single-arm multi-center study, were to evaluate the safety and efficacy of a lead-in phase of 8 weeks of entecavir monotherapy followed by 40 weeks of its combination with peginterferon in a large group of children in the immune-tolerant phase of chronic HBV infection.
The Hepatitis B Research Network (HBRN) is a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded multicenter, prospective study of chronic hepatitis B in both children and adults followed at 28 clinical sites in the United States and Canada. The goals of the HBRN are to better define the natural history, pathogenesis and therapy of chronic hepatitis B. The HBRN includes both a pediatric and an adult cohort study and has initiated 3 therapy trials: two trials of entecavir and peginterferon (one in children and one in adults) with immune-tolerant chronic hepatitis B and one trial of tenofovir and peginterferon in adults with immune-active chronic hepatitis B. This report is a description of the design and results of the open-label pediatric immune-tolerant study that was carried out at 7 pediatric clinical sites in the states of California, Maryland, Minnesota, Missouri, Texas, and Washington, and in Ontario, Canada. The Data Coordinating Center for the HBRN was located at the University of Pittsburgh, Pittsburgh, Pennsylvania.
Patients and Methods
Human subjects:
No study activities were conducted until the parent(s) or legal guardian(s) had signed the informed consent. Children at least 12 years old signed an assent form per the requirements of the individual Institutional Review Boards. Interpreters were provided for non-English speakers. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and approval was provided by the appropriate institutional review committee at each study site, the Data Coordinating Center and by a central Data Monitoring and Safety Board for the HBRN appointed by the NIDDK. The ClinicalTrials.gov Identifier was NCT01368497
Clinical Trial Design:
Children with immune-tolerant chronic hepatitis B infection who fulfilled the entry criteria were enrolled. Key inclusion criteria at screening were: age 3 to 17 years, presence of HBeAg and HBsAg in serum, HBV DNA >107 IU/mL, ALT ≤ 60 U/L in males, ≤40 U/L in females, and no evidence of hepatocellular carcinoma, liver decompensation or other serious co-morbidities. Detailed inclusion and exclusion criteria are listed in Supplemental Tables 1 and 2.
The trial was originally designed as a prospective randomized controlled trial but due to low enrollment the study was revised to a single-arm treatment design. Some parents and guardians expressed their unwillingness to enroll their child knowing they might be randomized to observation and not treatment. Participants initially randomized to the control group were offered enrollment in the treatment arm providing they still met enrollment criteria. Subjects received entecavir as monotherapy orally once daily in a dose of 0.015 mg/kg (0.5 mg maximum) for 8 weeks at which point peginterferon alfa-2a (180 μg/1.73m2) subcutaneously once weekly was added and the combination continued through week 48. Children were followed for 48 weeks after discontinuation of therapy (96 weeks from initiation of treatment). Participants who exhibited adverse events (AEs) deemed to be related to therapy underwent dose adjustment or discontinuation of therapy as detailed in the protocol, and continued to complete the study follow-up protocol. Entecavir was provided by Bristol Myers Squibb and peginterferon alfa-2a by Genentech, Inc. under separate Clinical Trial Agreements with the NIDDK.
Assessments were undertaken at an initial screening visit and at baseline (week 0), weeks 4, 8, 10, 12, 14, 16, 20 and 24 and then every 4 weeks until week 48, and at 4, 8, 12, 24, 36, and 48 weeks after drug discontinuation corresponding to weeks 52, 56, 60, 72, 84 and 96 after treatment initiation for those who completed the course of treatment. A diagram of the study design is shown in Figure 1. Data collected include baseline demographics, symptoms of liver disease, intercurrent illnesses, and findings on physical examination. Adverse events were monitored throughout the study. Blood work was drawn to measure markers of viral and liver disease status, assessment of AEs, and for research biospecimen banking.
Figure 1.
Study design. Weeks 52, 56, 60, 72, 84, and 96 correspond to 4, 8, 12, 24, 36, and 48 weeks after end of treatment, respectively, for those who discontinued treatment early.
Laboratory tests for routine hematology, clinical chemistries, qualitative HBsAg, HBeAg, anti-HBs and anti-HBe were done locally at clinical sites. We did not use a central laboratory for routine hematology, clinical chemistries, and anti-HBe to save costs and to get faster turn-around of the results. Quantitative HBsAg and HBeAg were performed by automated immunoassays (Elecsys: Roche Molecular Systems, Branchburg, NJ) with a lower limit of quantitation 0.3 IU/mL for HBeAg and 0.05 IU/mL for HBsAg,- and HBV DNA by real-time polymerase chain reaction (lower limit of quantification (LLOQ) of 20 IU/mL and a lower limit of detection of 10 IU/mL) (COBAS Ampliprep/COBAS TaqMan HBV v2.0 test: Roche Molecular Systems) at the HBRN central virology laboratory at the University of Washington. These assays were provided by Roche Molecular Systems via a Cooperative Research and Development Agreement with NIDDK. When there was a discrepancy between measurements provided by the local and central laboratories, the results from the central virology laboratory were used. HBV genotyping was performed at the Molecular Epidemiology and Bioinformatics Laboratory in the Division of Viral Hepatitis at the Centers for Disease Control and Prevention using mass spectrometry (13)
Endpoints:
The primary efficacy endpoint was chosen a priori as lack of detectable HBeAg and HBV DNA levels ≤1,000 IU/mL 48 weeks after end-of-treatment. The primary safety endpoints were AEs and serious AEs (SAEs). Secondary endpoints at the end of treatment and 48 weeks post-treatment included HBsAg loss, HBeAg loss, HBeAg seroconversion, HBsAg seroconversion, normal ALT levels (≤40 U/L for males and ≤35 U/L for females), HBV DNA ≤1000 IU/mL and <20 IU/mL, growth parameters (weight, height, body mass index [BMI], and Tanner scores).
Statistics:
Descriptive statistics (median, range) were used to characterize distributions of continuous variables. Bootstrapped 95% confidence intervals about medians were constructed from 1000 bootstrap replications for continuous variables over time. Frequencies and percentages were used for categorical variables. The percentages of participants achieving the efficacy endpoints were summarized with the point estimate and the 95% exact binomial confidence interval. Participants who did not have primary endpoint data were considered as treatment failures. Safety endpoints are presented as rates of adverse and serious adverse events per 100 person-years with 95% confidence intervals. SAS 9.3 (SAS Institute, Cary NC, USA) and R 3.3.1 were used for statistical analysis and graphical display.
Results
Participant Characteristics:
Between September 20, 2012 and December 30, 2014, 77 HBeAg-positive children were screened for eligibility of whom 17 were excluded and 60 enrolled. Reasons for exclusion included refusal of consent (n=12) and HBV DNA or ALT levels (n=5) (Supplemental Figure 1). Sixteen children had originally enrolled in the control arm and all chose to enroll in the open-label treatment arm when the study design was revised. Participants included 45 girls (75%) and 15 boys (25%) ranging in age from 3.4 to 17.9 years (median = 10.9 years) (Table 1). All except six children were Asian (90%) and 83% were born outside of the US or Canada. HBV genotypes varied by race, the Asian children being largely genotypes B (52%, n=31) and C (37%, n=22); while the 3 black children were infected with HBV genotypes A (n=1) or E (n=2), the single white child with genotype D and the 2 mixed race children with genotypes D and B. In keeping with the immune-tolerant phenotype and the eligibility criteria, at screening all children were HBsAg and HBeAg positive, had high levels of HBV DNA (median = 8.3 log10 IU/mL), and had normal or minimally elevated ALT levels (median 37 U/L in boys and 24 U/L in girls) by the local laboratory. We also applied the “more stringent” ALT values recommended as a consequence of the obesity epidemic.(14–16) As indicated in Table 1, there were 24 (40%) participants whose screening ALT values were below the “more stringent” upper limit of normal (25 U/L for males and females 1-<13 years old, 25 U/L for males 13-<18 years old, 22 U/L for females 13-<18 years old) and 23 (38%) whose baseline ALT values were below those limits. Baseline virology and serology results are shown in Table 1.
Table 1.
Baseline Characteristics
| Characteristics | Total |
|---|---|
| n=60 n (%) or median (range) | |
| Age (years) | 10.9 (3.4, 17.9) |
| Sex | |
| Girls | 45 (75%) |
| Boys | 15 (25%) |
| BMI (kg/m2) | 16.7 (13.8–30.6) |
| Race | |
| White | 1 (2%) |
| Black/African-American | 3 (5%) |
| Asian | 54 (90%) |
| Mixed | 2 (3%) |
| Born in the US/Canada | |
| Yes | 10 (17%) |
| No | 50 (83%) |
| HBV genotype | |
| A | 1 (2%) |
| B | 32 (53%) |
| C | 22 (37%) |
| D | 3 (5%) |
| E | 2 (3%) |
| HBV DNA (log10 IU/mL) - Screening | 8.3 (7.3–9.1) |
| HBV DNA (log10 IU/mL) - Baseline | 8.2 (7.5–9.1) |
| Quantitative HBeAg (log10 IU/mL) | 3.2 (2.1–3.8) |
| Quantitative HBsAg (log10 IU/mL) | 4.7 (3.2–5.4) |
| ALT (U/L) - Screening | 28 (10–57) |
| Boys | 37 (23–57) |
| Girls | 24 (10–40) |
| ALT ≤ new ULN* - Screening | 24 (40%) |
| ALT (U/L) - Baseline | 29.0 (12–112) |
| Boys | 39.0 (21–112) |
| Girls | 26.0 (12–71) |
| ALT ≤ new ULN* - Baseline | 23 (38%) |
| AST (U/L) | 30.0 (14–92) |
| Total bilirubin (mg/dL) | 0.4 (0.1–1.0) |
| Albumin (g/dL) | 4.4 (3.5–5.1) |
| Platelets (x103/mm3) | 269.0 (134–492) |
| White Blood Cells (x103/mm3) | 5.8 (3.4–13.8) |
Therapy:
All 60 children initiated treatment with entecavir and 45 (75 %) completed the full 48 weeks of entecavir and 40 weeks of peginterferon treatment without dose reduction or early discontinuation. Five of the other 15 discontinued treatment early: 1 participant who started entecavir was withdrawn at week 4 before starting peginterferon because of seizures. Four other children discontinued both therapies early; in one because of virological breakthrough (week 33), in two for thyroid conditions (weeks 36 and 44), and in one because of investigator discretion (week 44). There were also 10 children who had at least one peginterferon dose reduction without discontinuation because of mild to moderate interferon side effects.
In the 55 children who started and did not discontinue peginterferon early, the total peginterferon dose over the 40 weeks of treatment ranged from 90 to 180 μg per 1.73m2 body surface area (BSA). The average weekly dose was 175 μg per 1.73m2 BSA for the 55 children, representing 97% of the total expected dose. Entecavir was stopped after 48 weeks of therapy because entecavir monotherapy had been shown in other studies in adults to have very low rates of HBeAg loss and to avoid selection of resistance variants in these children who may, someday, need therapy.
Laboratory test results:
As shown graphically in Figures 2a and 2b, ALT and AST levels were stable during the initial 8 weeks of entecavir monotherapy, but rose within 4 weeks of starting peginterferon therapy. Median ALT and AST values were nearly twice the baseline values throughout the period of peginterferon treatment and promptly decreased to the pre-treatment values once peginterferon was stopped. ALT values at least doubled from baseline and to above the upper limit of normal (ULN) in 41 children (68%) and rose to above 5 times the ULN in 7 (12%). During these ALT elevations no bilirubin value increased more than 0.5 mg/dL from baseline, and no alkaline phosphatase increased by more than 50 U/L from baseline.
Figure 2.
Median and 95% confidence intervals by timepoints for ALT, AST, white blood cell count, and platelets. Weeks 52, 60, 72, 84, and 96 correspond to 4, 12, 24, 36, and 48 weeks after end of treatment, respectively, for those who discontinued treatment early.
Addition of peginterferon therapy was also accompanied by a decrease in white blood cell and platelet counts (Figure 2c and 2d). For the children with white blood cell counts available at Week 0 and Week 48, the median decline was 30%. The lowest value was 1,800 cells/mm3. No child developed a serious bacterial infection and blood counts returned to baseline promptly once peginterferon was stopped. Platelet counts also declined between week 8 (the start of peginterferon therapy) to the end of peginterferon therapy (week 48) with median platelet decline of 36% (and lowest value of 93,000/ mm3 at week 48). No child had abnormal bleeding or bruising episodes during therapy and platelet counts returned to baseline within 4 weeks of stopping peginterferon.
HBV DNA and hepatitis B antigen levels:
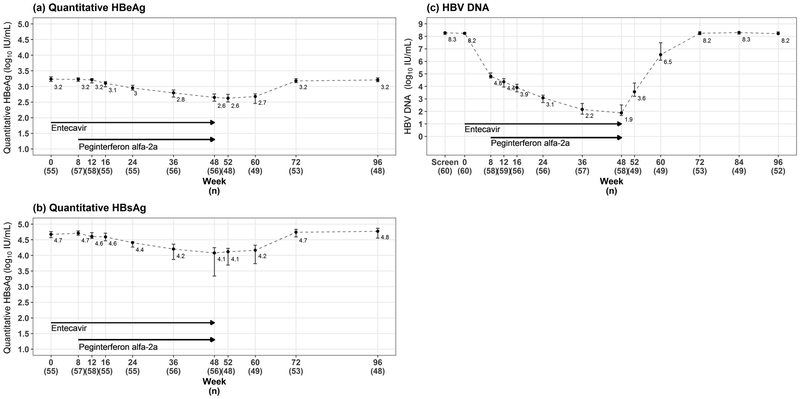
Quantitative levels of HBsAg and HBeAg decreased in all children generally beginning by week 24 and continuing to week 48. The decreases were mild to moderate in most children (Figure 3a and 3b). Median HBeAg fell by 0.6 logs and HBsAg by 0.6 logs during therapy, and in all except 4 participants (see below) essentially returned to their baseline by week 96, values being within 0.1 log10 of baseline. Median HBV DNA levels demonstrated a similar but more dramatic pattern during therapy (Figure 3c). HBV DNA levels decreased in all subjects during treatment, the median HBV DNA levels decreasing from 8.2 to 4.8 log10 IU/mL at 8 weeks during entecavir monotherapy and then to 3.1 log10 IU/mL at 24 weeks and 1.9 log10 IU/mL at 48 weeks during combination therapy. Forty-five children (75%, 95%CI: 62.1 to 85.3%) had HBV DNA ≤1,000 IU/mL at the end of treatment and levels were <20 IU/mL in 14 (23%, 95%CI: 13.4 to 36.0%) (Table 2). During the post-treatment follow-up, HBV DNA levels rose to near baseline values in most participants, but remained undetectable or below the lower level of quantitation in 3 children and below 1000 IU/mL in a fourth child. Details regarding these four children are as follows:
Figure 3.
Median and 95% confidence intervals by timepoints for quantitative HBeAg, HBsAg, and HBV DNA. Weeks 52, 60, 72, 84, and 96 correspond to 4, 12, 24, 36, and 48 weeks after end of treatment, respectively, for those who discontinued treatment early.
Table 2.
Efficacy endpoints*
| Endpoints | End of treatment | End of follow-up | ||
|---|---|---|---|---|
| n=60 (%) | 95% Confidence Intervals (%) | n=60 (%) | 95% Confidence Intervals (%) | |
| HBeAg Loss** | 2 (3) | (0.4–11.5) | 2 (3) | (0.4–11.5) |
| HBeAg seroconversion | 2 (3) | (0.4–11.5) | 2 (3) | (0.4–11.5) |
| HBsAg Loss** | 3 (5) | (1.0–13.9) | 2 (3) | (0.4–11.5) |
| HBsAg seroconversion*** | 2 (3) | (0.4–11.5) | 2 (3) | (0.4–11.5) |
| ALT ≤40 U/L for males, ≤35 U/L for females | 21 (35) | (23.1–48.4) | 39 (65) | (51.6–76.9) |
| HBV DNA (IU/mL) ≤ 1000 IU/mL | 45 (75) | (62.1–85.3) | 2 (3) | (0.4–11.5) |
| HBV DNA (IU/mL) < 20 IU/mL | 14 (23) | (13.4–36.0) | 2 (3) | (0.4–11.5) |
|
Primary Endpoint:
HBeAg loss AND HBV DNA ≤ 1000 IU/mL |
2 (3) | (0.4–11.5) | 2 (3) | (0.4–11.5) |
Analyses were performed using all 60 enrolled participants.
One other participant had HBsAg loss and another HBeAg loss but their last visits were at 60 and 72 weeks and there were no results at week 96 for assessment of end-of-follow up responses.
One participant with HBsAg seroconversion at the end of treatment did not have results for assessment of end-of-follow up responses.
Responder:
At 48 weeks after discontinuation of treatment, two participants (3%, 95%CI: 0.4 to 11.5%) (Cases #2 and 23) had achieved the primary endpoint of lack of detectable HBeAg and HBV DNA levels ≤1000 IU/mL and had normal ALT levels (11 and 14 U/L). Strikingly, these two participants also lost HBsAg by the end of treatment and developed both anti-HBs and anti-HBe and remained so at the end of post-treatment follow-up. Both responders were Asian females, ages 10.1 and 5.1 years, with suspected mother to child transmission; one with genotype B and one genotype C. HBV DNA was undetectable (<10 IU/mL) in one and present but unquantifiable (<20 IU/mL) in the other at week 96. The pattern and timing of decrease and loss of HBV markers were remarkably similar in the two children (Figure 4) with loss of HBeAg by week 36, loss of HBsAg at week 48 and accompanying mild to moderate rises in ALT levels (peak values 80 and 129 U/L) during treatment that resolved rapidly with stopping. Neither child had symptoms or bilirubin elevation during the ALT elevations.
>Figure 4.
Pattern of response in the two patients who achieved the primary endpoint
Two other children had a virologic response but did not meet the primary endpoint criteria (Figure 5). A 5.2-year-old black male with genotype E (Case #19) became HBeAg negative and anti-HBe positive by week 52 week and remained so until his last visit at week 72 at which point HBV DNA was 94 IU/mL, HBsAg positive, and ALT 13 U/L. An 11-year-old Asian female with genotype B (Case #34) became HBsAg negative by week 48 with HBV DNA 23 IU/mL. At the time, she was weakly positive for HBeAg by the quantitative test (0.4 IU/mL), but negative for HBeAg by the local qualitative assay. She was last seen as a part of the trial at week 60, at which time she was still HBsAg negative with HBV DNA below the limit of quantification, but had low levels of HBeAg (0.8 IU/mL). When seen in routine clinical follow up 4 months after completion of the trial, she was negative for HBeAg but weakly positive for quantitative HBsAg (0.5 IU/mL) while HBV DNA was undetectable. Neither child had a week 96 visit, so they did not meet the criteria for a primary outcome.
>Figure 5.
Pattern of response in two atypical patients with a virologic response who did not reach the primary endpoint.
The remaining 56 participants had decreases in HBV DNA levels during therapy, but levels returned to near baseline within 12 to 36 weeks of treatment discontinuation and none became HBeAg or HBsAg negative at any time during or after treatment. At week 96, HBV DNA, HBeAg and HBsAg levels were similar to those at baseline. Despite the marked rise in HBV DNA levels following withdrawal of therapy, there were no accompanying flares in ALT levels, indeed, values decreased to baseline while HBV DNA levels rose.
Safety:
Seventy-six AEs were reported by 37 children (Table 3 and Supplemental Table 3). Most were mild-to-moderate in severity and considered to be related to peginterferon (Supplemental Table 3c). The most common AEs were hematologic (n=16, 21%), infectious (n=12, 16%), and hepatic (elevated bilirubin or ALT) (n=8, 11%). The treatment emergent AE rate was 70 per 100 person-years (95% CI: 56 to 87 per 100 person-years). As previously noted, two children discontinued therapy early because of thyroid abnormalities, one because of ALT elevation, and one because of presumed virologic breakthrough. The treatment emergent SAE rate was 1 per 100 person-years (95% CI: 0 to 7 per 100 person-years). Only one child had an SAE that was considered possibly related to therapy (peginterferon), consisting of new onset ptosis thought by his pediatric neurologist to be myasthenia gravis despite negative test results. After discontinuation of therapy the ptosis remained but he showed no other signs of progression of myasthenia gravis. Z-scores for weight, height, and body mass index tended to be lower at the end of treatment than at baseline, as did height 48 weeks after treatment (Supplemental Figure 2a, 2b and 2c). Tanner staging was recorded based upon patient self-report. During the study period, most patients progressed through puberty as expected.
Table 3.
Treatment emergent adverse events by system (n=76) reported by 37 participants.
| System | # of AEs (%) |
|---|---|
| Hematological | 16 (21%) |
| Infection | 12 (16%) |
| Hepato-biliary | 8 (11%) |
| Dermatologic | 7 (9%) |
| Ear/nose/throat | 6 (8%) |
| Endocrine | 4 (5%) |
| Ophthalmic | 4 (5%) |
| Allergy/Immunology | 3 (4%) |
| Neurological | 2 (3%) |
| Other neuropsychological | 2 (3%) |
| Cardiac | 1 (1%) |
| Severe respiratory | 1 (1%) |
| Gastrointestinal | 1 (1%) |
| Urological | 1 (1%) |
| Gynecological | 1 (1%) |
| Seizure* | 1 (1%) |
| Abscess | 1 (1%) |
| Oral | 1 (1%) |
| Musculoskeletal | 1 (1%) |
| Constitutional symptoms | 1 (1%) |
| Metabolic | 1 (1%) |
| Lymphatic | 1 (1%) |
Febrile seizure during entecavir therapy thought related to viral illness.
Discussion
Experience in treating chronic hepatitis B in adults has suggested that response rates to antiviral therapy are highest in patients with high serum aminotransferase and low HBV DNA levels (17), features uncharacteristic of HBV infection in children with the immune tolerant phenotype (18–20). The scientific rationale of this trial was to evaluate the use of a highly potent nucleoside analogue in combination with peginterferon as a means of inducing a permanent remission in immune tolerant chronic HBV Infection in children. The protocol design was based upon two single center studies done with a first-generation oral nucleoside and standard interferon alfa. Therapy was initiated with an 8-week course of oral entecavir monotherapy to lower HBV DNA levels, followed by the addition of weekly injections of peginterferon alfa-2a for 40 weeks to provide further antiviral activity as well as immune stimulation in an attempt to induce sustained clearance of HBeAg. This approach, however, yielded a minimal response rate with only 2 of 60 children (3%) achieving the pre-established primary efficacy endpoint. Two other children had virologic and biochemical evidence of response as well, but were not seen at the critical post-treatment time point to qualify as reaching the efficacy endpoint. Yet even if one were to count these 2 participants as responders, the overall response rate was still low (7%) and more than three-fold less than response rates reported in the previous smaller studies from Europe: 22% (11) and 39% (12). A more recent trial from Asia utilizing several regimens of interferon with or without lamivudine for 72 weeks, also reported high response rates, with loss of HBeAg in 33% and loss of HBsAg in 22% (21).
There were three striking findings in our study: (1) a rise in serum aminotransferase (aspartate aminotransferase [AST] and ALT) levels during peginterferon therapy; (2) a lack of flares or rises in ALT and AST levels upon withdrawal of antiviral therapy; and (3) the similar clinical pattern and completeness of the few responses that were observed.
Serum ALT and AST levels, which were normal or near normal in all children before initiation of treatment, were stable during the initial 8 weeks of entecavir monotherapy, but promptly rose to a median value of twice baseline values once peginterferon was started (Figure 2). The ALT and AST increases were mild to moderate in degree and were not progressive, the average levels remaining stable for the entire 40 weeks of peginterferon therapy and promptly returning to baseline once it was stopped. The elevations in ALT and AST were not accompanied by increases in alkaline phosphatase or bilirubin levels nor in clinical symptoms. While the increases accompanied declines in HBV DNA, HBeAg and HBsAg, they also accompanied declines in white blood cell and platelet counts and were seen in patients with and without HBeAg loss. Such mild-to-moderate ALT elevations during interferon therapy have been reported in previous studies of children with immune tolerant HBV infection (11) as well as in adults without liver disease receiving interferon therapy for cancer (20, 22).
A second striking finding was the lack of aminotransferase elevations or flare of disease when therapy was stopped. In patients with immune active chronic hepatitis B (both HBeAg positive and negative), flares in disease activity occur in almost half of patients when antiviral therapy is stopped. These flares can be severe and symptomatic and instances of acute liver failure and death or need for emergency transplantation have been reported (17). In contrast, in the children who did not clear HBeAg or HBsAg with combination therapy, serum ALT and AST levels did not rise, but instead fell promptly into the normal or near normal range after discontinuation of therapy despite rapid increase in serum HBV DNA levels. This lack of withdrawal flares is perhaps the most distinctive and telling feature of the so-called “immune tolerant” phenotype of chronic HBV infection and suggest the lack of host-specific immune factors controlling cell injury in the face of high viral replication. The children who did not have a beneficial response to treatment did not have a worsening of disease, such as transition to immune active disease (HBeAg-positive or -negative) (23).
A final striking feature in this trial was that the clinical responses, while uncommon, were complete in that both of the responders not only became HBeAg negative, but also cleared HBsAg and developed both anti-HBe and anti-HBs. During the 48 weeks of follow up, both children also had normal serum aminotransferase levels and had undetectable HBV DNA or detected but unquantifiable levels. These features suggest that they were cured of their chronic viral infection despite having had extremely high levels of HBV DNA, HBeAg and HBsAg before treatment. A third child also had a complete response with loss of HBsAg and HBeAg during long-term follow up. Three times higher rates of loss of HBsAg were reported in previous studies of combination antiviral therapy of children with immune tolerant phenotype, rates of HBsAg loss being 4 of 23 (17%) (11) and 6 of 28 (21%) (12) during or within 24 weeks of stopping therapy. In contrast, in a parallel study conducted by the HBRN in adults, none of 27 patients treated with a similar regime of entecavir and peginterferon met primary endpoint. These results suggest the possibility of cure of chronic HBV infection at least in children and call for increased efforts to find antiviral agents for hepatitis B that might be combined with nucleoside analogs with or without peginterferon. Clearly, identifying other viral and host targets for antiviral therapy of hepatitis B is an important challenge and should be a priority in the search for a “cure” of this important viral infection for both children and adults (24).
The shortcomings of the current study need to be mentioned. The study was initially planned as a randomized controlled trial but was converted to a single arm study due to the low recruitment. Sixteen of the children in the control group were re-enrolled into the treatment arm. As a result, this study was an open-label trial and lacked controls. Because there is a spontaneous rate of loss of HBeAg and even HBsAg in chronic hepatitis B, the outcomes observed in this study might have occurred by chance. Arguing against this conclusion was the similarity in sequence and timing of the virologic responses in the responders, all of them occurring during the last few months of therapy or immediately thereafter. Another shortcoming was that the majority of participants were Asian and had genotypes B or C. Differences in response rates may be seen in children of European or African descent though worldwide majority of children with immune tolerant chronic HBV infection are Asians. Interestingly, in previous studies that included few non-Asian children, the majority of complete responses (loss of HBsAg) were reported in Asian children. In our trial, both responders were Asians while the one patient who became HBeAg-negative without loss of HBsAg was black and had genotype E.
The strengths of the current study were that it was a multicenter study, using modern potent therapies for hepatitis B and had rigorous design of evaluation, monitoring and follow up. Furthermore, highly sensitive serologic and virologic assays were used and frequent testing performed demonstrating the timing and potency of antiviral activity and patterns of response. The difficulties in interpretation of results at several time points were due to slightly more sensitive quantitative HBsAg and HBeAg assays used in the central laboratory compared to the qualitative assays used locally.
In summary, in this open label study of the combination of entecavir and peginterferon in 60 children with immune tolerant chronic HBV infection, the overall response rate was low (3%) while adverse side effects were common, indicating that this approach is of limited and unsatisfactory efficacy. Nevertheless, the few responses that occurred were complete and convincing which argues for increased efforts to identify better treatments for hepatitis B, that might result in cure of this infection in a higher proportion of patients including children with immune tolerant phenotype of disease.
Supplementary Material
Acknowledgements:
We would like to thank Jay Hoofnagle, M.D. for critical review of the manuscript, and research coordinators, patients and families for participation in the trial.
Financial support: The Hepatitis B Research Network (HBRN) is funded by a U01 grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to the following investigators Kathleen B. Schwarz (U01 DK082916), Steven H. Belle PhD (U01 DK082864), Harry L. A. Janssen, MD, PhD (U01 DK082874), Norah A. Terrault, MD, MPH (U01 DK082944), Lewis R. Roberts, MB, ChB, PhD (U01 DK 082843), Adrian M. Di Bisceglie, MD (U01 DK082871), and an interagency agreement between NIDDK and the Center for Disease Control and Prevention: Lilia Milkova Ganova-Raeva, PhD (A-DK-3002–001). Entecavir was provided by Bristol Myers Squibb, and peginterferon alfa-2a was provided by Genentech, Inc. through respective Clinical Trial Agreements with NIDDK. Additional support was provided by Roche Molecular Systems, Inc. through a Cooperative Research and Development Agreement with NIDDK.
Duality of Interest: K.F.M. receives funding from Gilead and Shire for studies related to hepatitis; N.R.-B. receives funding from Gilead for studies related to hepatitis; P.R. receives funding from Gilead, Vertex, BMS, Abbvie, and Roche/Genentech for research studies related to hepatitis and Gilead, Abbvie and Roche/Genentech for consulting related to hepatitis; J.T. receives funding from Gilead, Alnylam, Arrowhead Research, GLG Pharma, Genkyotex, Dicerna, and RxCelerate; K.B.S. also receives funding from Gilead, Bristol Myers Squibb, and Roche Therapeutics for clinical trials in hepatitis and consulting from Gilead and Roche Therapeutics relating to hepatitis.
Abbreviations:
- AE
Adverse event
- ALT
alanine aminotransferase
- BMI
body mass index
- CI
confidence interval
- DNA
deoxyribonucleic acid
- HBRN
Hepatitis B Research Network
- HBV
hepatitis B virus
- LLOQ
lower limit of quantification
- SAE
serious adverse event
- ULN
upper limit of normal
References
- 1.MacLachlan JH, Locarnini S, Cowie BC. Estimating the global prevalence of hepatitis B. Lancet 2015; 386: 1515–7. [DOI] [PubMed] [Google Scholar]
- 2.Bortolotti F, Calzia R, Cadrobbi P, Giacchini R, Ciravegna B, Armigliato M, et al. Liver cirrhosis associated with chronic hepatitis B virus infection in childhood. J Pediatr 1986; 108: 224–7. [DOI] [PubMed] [Google Scholar]
- 3.Stadler LP, Mezoff AG, Staat MA. Hepatitis B virus screening for internationally adopted children. Pediatrics 2008; 122: 1223–8. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B–United States, 1974–2008. PLoS One 2011; 6: e27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz KB, Cloonan YK, Ling SC, Murray KF, Rodriguez-Baez N, Schwarzenberg S, et al. Children with chronic hepatitis B in the United States and Canada. J Pediatr 2015; 167:1287–94. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerkar N Hepatitis B in children: complexities in management. Pediatr Transplant 2005; 9: 685–91. [DOI] [PubMed] [Google Scholar]
- 7.Tran TT. Immune tolerant hepatitis B: a clinical dilemma. Gastroenterol Hepatol (NY) 2011; 7: 511–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Jonas MM, Block JM, Haber B, Karpen SJ, London WT, Murray KF, et al. Treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology 2010; 52:2192–2205. [DOI] [PubMed] [Google Scholar]
- 9.Clemente MG, Vajro P. An update on the strategies used for the treatment of chronic hepatitis B in children. Expert Rev Gastroenterol Hepatol 2016; 10: 649–58. [DOI] [PubMed] [Google Scholar]
- 10.Artan R Lamivudine monotherapy in children with immune-tolerant chronic hepatitis B virus. J Chemother 2005; 17: 198–202. [DOI] [PubMed] [Google Scholar]
- 11.D’Antiga L, Aw M, Atkins M, Moorat A, Vergani D, Mieli-Vergani G. Combined lamivudine/ interferon-α treatment in ‘immunotolerant’ children perinatally infected with hepatitis B: A pilot study. J Pediatr 2006; 148: 228–33. [DOI] [PubMed] [Google Scholar]
- 12.Poddar U, Yachha SK, Agarwal J, Krishnani N. Cure for immune-tolerant hepatitis B in children: is it an achievable target with sequential combo therapy with lamivudine and interferon? J Viral Hepat 2013; 20: 311–6. [DOI] [PubMed] [Google Scholar]
- 13.Ganova-Raeva L, Ramachandran S, Honisch C, Forbi JC, Zhai X, Khudyakov Y. Robust hepatitis B virus genotyping by mass spectrometry. Journal of clinical microbiology. 2010;48(11):4161–4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012; 58:854–868. [DOI] [PubMed] [Google Scholar]
- 15.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010; 138:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bussler S, Vogel M, Pietzner D, Harms K, Buzek T, Penke M et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase): effects of age, sex, body mass index, and pubertal stage Hepatology In Press. [DOI] [PubMed] [Google Scholar]
- 17.Lok AS, McMahon BJ. Chronic Hepatitis B: Update 2009. AASLD Practice Guideline. Hepatology 2009; 50:661–662. [DOI] [PubMed] [Google Scholar]
- 18.Shah U, Kelly D, Chang MH, Fujisawa T, Heller S, González-Peralta RP, et al. Management of chronic hepatitis B in children. J Pediatr Gastroenterol Nutr 2009; 48: 399–404. [DOI] [PubMed] [Google Scholar]
- 19.Kobak GE, MacKenzie T, Sokol RJ, Narkewicz MR. Interferon treatment for chronic hepatitis B: enhanced response in children 5 years old or younger. J Pediatr 2004; 145: 340–5. [DOI] [PubMed] [Google Scholar]
- 20.Jones GJ, Itri LM. Safety and tolerance of recombinant interferon alfa-2a(Roferon-A) in cancer patients. Cancer 1986; 57(8 Suppl): 1709–15. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Zhang H, Dong Y, Wang L, Xu Z, Liu W, et al. Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: A pilot open-label randomized study. Journal of Hepatology 2018:68(6):1123–1128. [DOI] [PubMed] [Google Scholar]
- 22.Quesada JR, Talpaz M, Rios A, Kurzrock R, Gutterman JU. Clinical toxicity of interferons in cancer patients: a review. J Clin Oncol 1986; 4: 234–43. [DOI] [PubMed] [Google Scholar]
- 23.Di Bisceglie AM, Lombardero M, Teckman J, Roberts L, Janssen HLA, Belle SH, et al. Determination of hepatitis B phenotype using biochemical and serological markers. J Viral Hepat 2017; 24: 320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block TM, Alter H, Brown N, Brownstein A, Brosgart C, Chang KM, et al. Research priorities for the discovery of a cure for hepatitis B: report of a workshop. Antiviral Res 2018; 150: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.