Abstract
Exosomes as a unique subtype of small extracellular vesicles (sEVs) have attracted increasing interest in recent years in the fields of mesenchymal stromal cell (MSC) research. Studies have confirmed that exosomes derived from MSCs preserve immunosuppressive phenotype and can mimic therapeutic benefits of their parent cells. This review briefly summarizes most recent findings on the potential of exosomes as an alternative of therapeutic MSCs, focusing on the role of MSCs and their secreted exosomes in regulation of immune cells, preclinical and clinical evidence of therapeutic outcomes of MSC exosomes, and the biodistribution and pharmacokinetic profile of systemically administered exosomes. It is appreciated that exosomes from MSCs of different sources have variable contents including inflammatory mediators, tropic factors, signaling molecules, and nucleic acids (DNA, mRNA, microRNA and long non-coding RNA). Diverse functions of exosomes derived from different sources are expected. More importantly, exosomes isolated in vitro may not mirror that from in vivo where donor MSCs are exposed to specific disease or injury-related conditions. Simulating in vivo microenvironment by pretreatment of MSCs with relevant chemical mediators may lead to their secretion of therapeutically more efficient exosomes/sEVs. However, we know very little about the key molecules involved and the differences between exosomes released under different conditions. These issues would be tremendous interest to preclinical research that pursues exosome biology underlain therapeutic mechanisms of MSCs. Further studies are expected to demonstrate the superiority of MSC-derived exsomes/sEVs as a pharmaceutical entity with regard to efficacy, safety, and practicability.
Keywords: Bone marrow stromal cells, Mesenchymal stromal cells, extracellular vesicles, immune regulation, Tregs, pain
Introduction
Exosomes are nano-bioactive vesicles (≈40-150 nm) derived from fusion of multivesicular bodies/multivesicular endosomes (MVB/MVE) and the plasma membrane and release of intraluminal vesicles (ILVs) into the extracellular space. The extracellular release of these vesicles was originally observed as a way to remove obsolete transferrin receptors from reticulocytes during their maturation [1]. This phenomenon was further confirmed and the vesicles were named EXOSOME by Johnstone et al. [2, also see 3]. Initial work found that exosomes were not from other cellular elements in the blood such as matured red blood cells, platelets or white cells [2], but it is now appreciated that exosomes can also be secreted by almost all types of cells and they provide a vehicle to transfer chemical mediators as well as genetic materials between cells. Accordingly, the functional significance of exosomes is far beyond “externalization of ‘superfluous’ membrane proteins” [2], and their potential as biomarkers and therapeutic surrogate of stem cells are being extensively explored. It is now recognized that exosomes belong to a class of small extracellular membrane-bound vesicles (sEVs) released from multiple types of cells. Since the purity of exosomes is often uncertain in a particular study, the more generic term EVs are frequently used.
There has been an intense interest in mesenchymal stromal cells (MSCs) related to dental medicine as dental tissues are rich sources of MSCs [4-7]. Preclinical and clinical studies have shown therapeutic potential of MSCs in numerous diseases including those related to orofacial injury. For instance, application of bone marrow stromal cells (BMSCs), a major type of MSCs, produced long-lasting relief from orofacial pain of myogenic origin [8,9]. Gingiva-derived MSCs help to facilitate wound healing in the gingiva [10]. The beneficial effect of MSCs is underlain by interactions of transfused cells with the host immune system [11]. It is noted that the therapeutic outcome of MSCs could be duplicated by MSC-conditioned medium that contains secretome of MSCs [12, 13]. Lai et al. [14] initially identified that cardioprotective effect of MSCs was mediated by their secreted exosomes. Further studies demonstrate that immune suppression by MSC-derived exosome-like EVs is closely correlated with their internalization by immune effector cells [15] and the effect of exosomes is dose-dependent [16]. Ample recent studies support that the therapeutic efficacy of MSCs is related to exosomes derived from MSCs [10, 17-19]. Exosomes seem to be able to produce effects comparable to their parent cells via similar mechanisms [20]. This essay will briefly discuss recent literature on the therapeutic mechanisms of MSCs with a focus on related exosomes and the potential of these extracellular vesicles in MSC-based therapy.
Exosomes as extracellular vesicles [21-25]
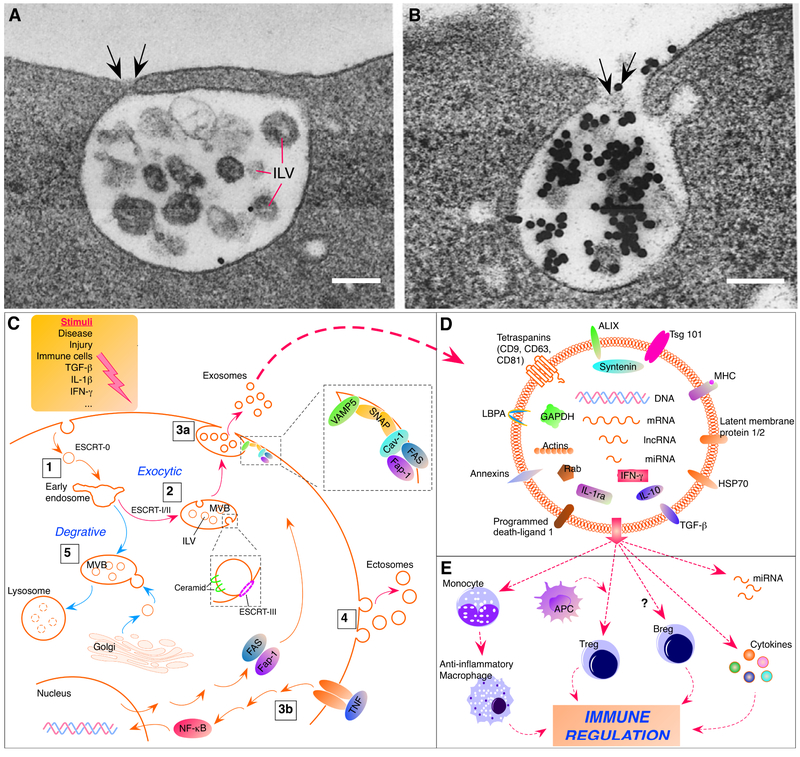
Cell-secreted membrane vesicles may be collectively called sEVs, including exosomes and ectosomes [24]. Although through different selection and retention mechanisms, both types of sEVs package content from the cytosol and may perform similar functions. Exosomes are considered distinct in the mechanisms of formation and possess specific marker proteins, or exosome-enriched proteins, such as tetraspanins CD63, CD9 and CD81 [10,26]. CD63 is enriched in MVB/MVE and considered hallmark of exosomes [26]. Although less specific, ALIX [apoptosis-linked gene 2 (ALG-2) interacting protein X] and TSG101 (tumor susceptibility gene 101 protein) are involved in sorting cargo into exosomes and also used as exosomal markers [27]. On the other hand, intracellular proteins associated with compartments other than endosomes are usually not found on exosomes [see 23]. Thus, exosomes are from the intracellular endosome compartment [1] (Fig. 1. A, B), which is different from ectosomes, the other major type of sEVs, that are shed directly from certain regions of the plasma membrane, or some microvesicles shed from apoptotic cells. The formation of exosomes involves enrichment of sorted molecules in small membrane domains of late endosomes, accumulation of cargoes, and inward membrane budding to form ILVs, which leads to maturation of MVB/MVE [see 24] (Fig. 1C).
Fig. 1.
Exosome formation and role in immune regulation. A. View of a MVB or MVE sparsely labeled with colloidal gold-transferrin. Note ILV and the apparent fusion of the MVB/MVE (arrows) and the plasma membrane. This may represent incipient MVE exocytosis. Scale=100 nm, × 107,000. B. View of MVE exocytosis in an unfixed reticulocyte incubated with gold-transferrin. Note release of ILV with associated gold-labeled transferrin (arrows). Scale=200 nm, × 61,000. C. Biosynthesis pathway of exosomes. 1. Endocytotic vesicles become early endosomes after shedding clathrin coat. The ESCRT-0 helps to recruit ubiquitinated proteins (not drawn) that are important for cargo selection [29]. 2. Early endosomes develop into later endosomes (not drawn) and MVB. In the exocytic pathway of MVB, ILVs are formed by inward budding involving activities of ESCRT-I/II [24]. ESCRT-III plays a role in deubiquitination and final separation of budding membrane into ILVs (enlarged inset). In oligodendroglial cell line, ceramide, but not ESCRT complexes, is required for cargo sorting into MVB-ILVs. 3a. Shown in gingiva-derived MSCs, exocytosis of ILVs involves Fas/Fap-1/Cav-1 complex and SNAP25/VAMP5 (enlarged to the right) [10]. 3b. FAS/Fap-1 are upregulated by TNF involving activation of NF-κB pathway, associated with increased ILV release. 4. Ectosomes are the other type of sEVs formed by outward budding a piece of the plasma membrane. 5. MVBs in the degrative pathway receive specific enzyme-carrying vesicles from the Golgi apparatus and become lysosomes. Dashed circles in lysosome indicate degraded content. Also note that different stimuli (top right) can condition MSCs and impact on cargo content of secreted exosomes. D. Schematic representation of an exosome containing cargo molecules. E. The effects of exosomes on immune cells. Most exosomes are internalized by monocytes that may become anti-infllammatory macropohages. APC participate in exosome-induced Tregs. IL-10-secreting Bregs may be induced by exosomes. Exosomal miRNAs and cytokines are released after internalization into target cells. See text for abbreviations.
Panels A and B are adapted from Figures 12 and 13, Harding et al., 1983. Originally published in The Journal of Cell Biology by American Society for Cell Biology; Rockefeller Institute, Vol. 97, page 336, Reproduced with permission of Rockefeller University Press via Copyright Clearance Center (License ID: 4400840432590).
The four highly conserved protein assemblies of endosomal sorting complex required for transport (ESCRT) drive the key steps during biogenesis of ILVs. In general, ESCRT-0 (and ALIX) helps sorting ubiquitinated proteins, ESCRT-I/II are involved in luminal budding of ILVs, and ESCRT-III filaments take care of scission of ILVs [see 24, 28, 29]. Additionally, nonubiquitinated proteins can be sorted into MVBs through Cos (yeast MVB sorting factors) that creates distinct limiting membrane domains within the endosome [30]. Sorting of glycoproteins into exosomes requires N-linked glycosylation [31]. There may be cell type-dependent differences in exosome biogenesis. In a mouse oligodendroglial cell line, budding of exosome-ILVs is promoted by ceramide, a cone-shaped lipid generated from sphingomyelin by sphingomyelinases, and ESCRT-independent [32]. In MCF-7 (Michigan Cancer Foundation-7) cell line, the formation of exosomes involves syndecan-syntenin-ALIX interactions and ESCRT-dependent [33]. Unlike ILVs in the endolysosomal pathway, ILVs of precursor exosomes are not destined for degradation. Upon fusion of MVB/MVE and the plasma membrane, ILVs are exocytosed as exosomes. After traveling to the target site, exosomes are taken up by target cells and their cargo released to activate or suppress cellular activities.
In search for mechanisms underlying beneficial effects of MSCs, exosomes secreted from MSCs have gained much attention in recent years [14, 17, 34]. A recent report demonstrates the molecular machinery involved in exosome release from MSCs [10]. From gingiva-derived MSCs, exosomes are released via activation of a common soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE)-mediated membrane fusion mechanism. The signaling of this process requires Fas binding with Fas-associated phosphatase-1 (Fap-1) and caveolin-1 (Cav-1), followed by the formation of a complex with the synaptosome-associated protein of 25 kDa/vesicle-associated membrane protein 5 (SNAP25/VAMP5) (Fig. 1C). Tumor necrosis factor (TNF) selectively promotes this process by upregulating FAS/Fap-1 via the activation of the nuclear factor kappa B (NF-κB) pathway. The released vesicles contain interleukin-1 receptor antagonist (IL-1ra) that facilitates wound healing.
To achieve functional communication between cells, exosomes carry distinct cargoes including nucleic acids [DNA, mRNA, long non-coding RNA (lncRNA), microRNA (miRNA)], proteins [tetraspanins, ESCRT-associated proteins, heat shock proteins (HSP)] [35], anti-inflammatory cytokines [IL-10, transforming growth factor beta (TGF-β)], and lipids [sphingomyelin, lysobisphosphatidic acid (LBPA)] [36, 37] (Fig. 1D). Importantly, cytosol soluble proteins that are not processed through the endoplasmic reticulum-Golgi pathway can be incorporated into the exosome via inward budding of endosome membrane. Exosomal proteins involved in a spectrum of functions and pathologic activities have been identified through proteomic analyses [38]. Exosomes from a certain cell type may only carry a specific set of molecules. For example, LBPA is not found in exosomes from oligodendroglial cell line and B-cell [32, 39]. The content of exosomes including nucleic acids is affected by disease and may be developed into biomarkers [40-43]. The consequences of exosome-mediated intercellular communication can be physiological, pathological, or to the best of our interest, diagnostic and therapeutic.
MSCs, exosomes and immune regulation
MSCs have been known to be immune suppressive and shown promise in the treatment of immune-related diseases such as graft-versus-host disease (GvHD) [44-46]. The beneficial effects of MSCs are largely attributed to their ability to regulate immune responses (anti-inflammation) and secrete trophic factors (regeneration) [34, 47-49]. It is known that systemically infused MSCs are largely trapped in the lungs and do not appear to stay long in the host [11, 50-52]. However, infused MSCs would first interact with circulating immune cells and impact on subsequent responses, which is essential for their lasting beneficial effects. Likewise, MSC-relevant exosomes are capable of regulating immune responses that favor the resolution of disease conditions (Fig. 1E).
Monocytes/macrophages
MSCs can interact with cells of the innate immune system [53]. MSCs are able to attract macrophages [54], promote monocyte survival [55], and polarize macrophages toward an anti-inflammatory profile [11, 55-59]. In a rat model of persistent pain, BMSC-produced pain relief is attenuated after clodronate- induced depletion of monocytes/macrophages [11]. BMSCs improve survival and organ function in a mouse model of sepsis, but the improvement is lost after depletion of monocytes/macrophages [60]. These findings point to monocyte/macrophage population as an important relay of MSCs’ action.
Bypassing MSCs, exosomes can directly impact macrophages. In the spinal injury rat model, MSC- derived exosomes can reach selectively to the site of injury after systemic administration and target CD206-expressing macrophages [61]. Willis et al. [62] isolated exosomes from MSC-conditioned medium and showed that MSC-derived exosomes modulated macrophage phenotype toward anti-inflammation. MSC-derived EVs reduced macrophage activation, an effect associated with reduced inflammation in abdominal aortic aneurysm [63]. Monocytes/macrophages are key immune cells for BMSC-produced pain relief [11]. Interestingly, exosome-like and immunosuppressive EVs from MSCs are primarily internalized by monocytes [15]. This would be consistent with a hypothesis that monocytes are programed to relay beneficial effects of MSCs.
Regulatory T cells
The regulatory T cells (Tregs) of CD4 cell lineage are immune suppressor cells. In patients with systemic lupus erythematosus, BMSCs induced Treg cells and downregulated follicular T helper cells [64]. In mice with injury of the infraorbital nerve and increased pain sensitivity, the percentage of CD4+CD25+Foxp3+ Tregs among the CD4+ population was decreased compared to naive mice, but increased to above the naïve level after receiving BMSCs; and the restore of Tregs was associated with pain relief [65]. In vitro studies showed that BMSCs induced T regulatory 1 (Tr1), T helper 3 (Th3) [66] and CD4+CD25+Foxp3+ [55] Tregs. Exposing Tregs to MSCs increased their immunosuppression activity [67]. Interestingly, BMSCs induced CD4+CD25+Foxp3+ Tregs in human peripheral blood mononuclear cells (PBMC) in a contact-independent fashion [55], implying contribution of soluble factors and other medium such as EVs. Supporting this view, induction of CD4 T cells into functional Tregs by MSCs could be mediated by secreted TGF-β via paracrine signaling [68]. Immunoregulative EVs from BMSCs were internalized by PBMCs from patients with type 1 diabetes [69].
Mesenchymal stromal cell-derived exosomes/sEVs can induce Tregs (Fig. 1E). When treated PBMCs or THP-1 cells with MSC-derived exosomes/sEVs, CD4+CD25+Foxp3+ Tregs were induced [70-72]. In PBMCs from asthmatic patients, it is noted that MSC exosomes do not seem to directly contact CD4 cells. They rather are internalized by CD14+ monocytes and CD19+ B cells, followed by polarization of Tregs [72]. Zhang et al. [73] showed that induction of CD4+CD25+Foxp3+ Tregs from CD4+ T cells was dependent upon antigen presenting CD11C cells and MSC exosomes, while MSC exosomes alone cannot convert T cells to Tregs [71]. Thus, T cell activation appears to be necessary for the induction of Tregs by MSC exosomes. It is important to note that not only Tregs are induced, the immunosuppressive function of Tregs are also enhanced by MSC exosomes [72].
B lymphocytes
Human MSCs suppress antibody-producing effector B-cell proliferation in culture [74]. This effect can be fully reproduced by membrane vesicles from MSCs’ cell culture supernatant [75]. IL-10 secreting CD19+/CD38hi/CD24hi/IL 10+ B-regulatory cells (Bregs) can be induced by co-culturing with adipose tissue-derived MSCs [76]. Suppression of B-cell-mediated immune responses by transplantation of human palatine tonsil-derived MSCs was associated with induction of Bregs [77]. It is unclear whether induction of Bregs could also be achieved by MSC exosomes. Cosenza et al. [16] show that the anti-inflammatory effect of MSC exosomes is associated with an increase in CD19+IL-10+ Breg-like cells in lymph nodes.
Cytokines
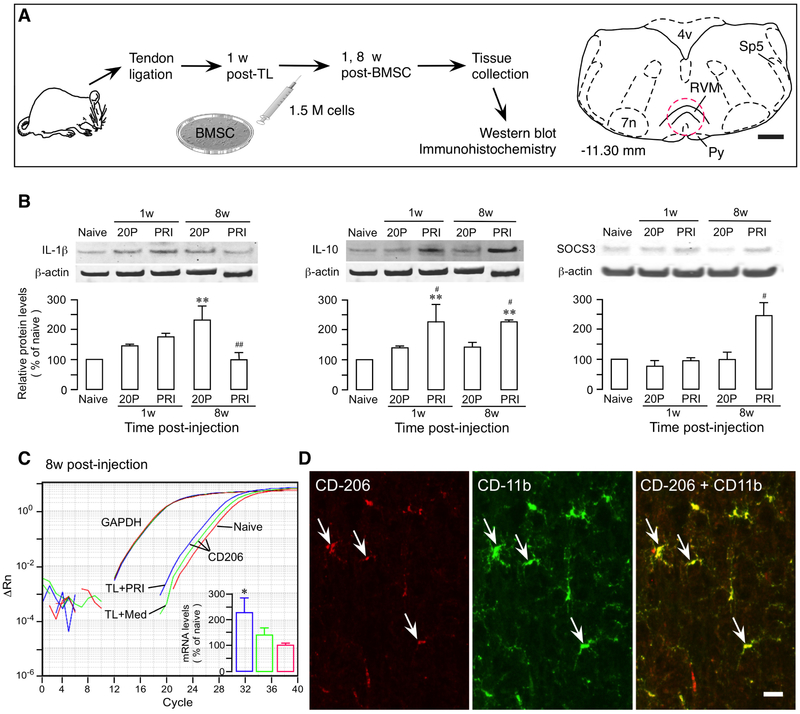
Inflammatory cytokines including chemokines are mediators of MSC-induced immune regulation. MSC- secreted anti-inflammatory TGF-β is critically important for pain-attenuating effect of BMSC [78]. With high-passage BMSCs that showed low levels of cytokine/chemokine expression including TGF-β, the pain-relieving efficacy of BMSCs was lost [11]. Numerous studies have demonstrated the role of MSCs in promoting an anti-inflammatory phenotype in MSC recipients. In a rat model of persistent pain induced by ligation injury of the masseter muscle tendon, transplantation of BMSCs downregulated pro-inflammatory IL-1β and upregulated anti-inflammatory IL-10 and CD206 in the rostral ventromedial medulla (RVM), a key site of brain pain modulatory circuitry (Fig. 2) [80]. It is also noted that a pro-inflammatory environment favors MSC-mediated immune regulation. MSC-induced inhibition of B cells requires T-cell-derived interferon gamma (IFN-γ) [81]. Priming MSCs with inflammatory cytokines enhances immunosuppression by MSC-derived EVs [15]. Pretreatment of BMSCs with IL-1β led to stronger pain inhibition in animal neuropathic pain model [82]. Tumor necrosis factor promotes IL-1ra release from MSCs [10].
Fig. 2.
Promotion of anti-inflammatory phenotype by BMSCs. A. Flowchart of the experiment. Tendon ligation of the rat induces pain hypersensitivity that can be attenuated by systemic BMSCs. A drawing of brainstem transverse section to the right at −11.30 caudal to bregma illustrates rostral ventromedial medulla (RVM) [79]. Dashed circle indicates the area punched for analysis. 4v, fourth ventricle; 7n, facial nucleus; Py, pyramidal tract; Sp5, spinal trigeminal tract; TL, tendon ligation. Scale = 1 mm. B. BMSCs facilitate anti-inflammatory immune reactions. The 20-Passage (20P) BMSCs were used as a control, since primary BMSCs (PRI) produced pain relief and 20P BMSCs were ineffective. IL-1β (Left) showed a trend of upregulation at 1w post-BMSC. At 8w, the level of IL-1β was at the naïve level in PRI BMSC-treated while IL-1β in 20P BMSC-treated rats remained at a higher level. IL-10 (Middle) was upregulated at 1w and 8w after PRI BMSC treatment. SOCS3 (anti-suppressor of cytokine signaling 3) proteins (Right), a feedback inhibitor of signaling pathways related to cytokine transcription, was significantly upregulated at 8w, consistent with suppression of IL-1β. β-actin was a loading control. C. qPCR plot illustrating levels of CD206 genes associated with microglia of anti-inflammatory phenotype. Note leftward shift of the amplification curves for TL+PRI BMSC (blue) samples, compared to TL+Med (diluted culture medium) (green) and Naïve (red), indicating increased expression. CD206 mRNA levels were quantified (inset). GAPDH was an endogenous control. D. Double immunofluorescence staining illustrates co-localization of CD206 with CD11b (arrows), a microglia marker. Scale=0.04 mm. *, p<0.05, **, p<0.01, vs. Naïve; #, p<0.05, ##, p<0.01, vs. 20P, n=3-4. See [11] for details of methods.
The cytokine profile has similar significance in MSC exosomes. Exosome preparations from human BMSC donors express both anti- and pro-inflammatory cytokines including IL-10, TGF-β, and IFN-γ [83]. MSC exosomes also are capable of inducing IL-10 and TGF-β and suppressing IL-1β, IL-6 and TNF in the THP cell line [71]. MSC sEVs increased IL-10 in PBMC culture medium [84]. MSC exosomes improve Treg polarization via upregulation of IL-10 and TGF-β1 in PBMCs [72]. Small extracellular vehicles (likely exosomes) from IL-1β-activated MSCs induce higher expression of anti-inflammatory cytokines from splenic mononuclear cells derived from experimental autoimmune encephalomyelitis-affected mice when compared to that of sEVs from non-treated MSCs [70]. Exosomes from MSCs primed with IFN-γ and TGF-β are more effective in converting mononuclear cells to Tregs [85]. Thus, exosomes derived from MSCs are positioned to have MSC-like anti-inflammatory performance.
Cargo RNAs
Abundant RNA species are found in exosomes, including miRNA, mRNA and lncRNA. It is interesting to note that exosomes can carry RNAs that are not present in parent cells [86, 87]. Exosomal RNAs are secured in the lumen of vesicles as they are trypsin-treatment resistant [86]. RNAs transported by exosomes are functional after reaching target cells, allowing regulation of gene expression and synthesis of novel proteins in recipient cells.
MicroRNAs
Selected miRNAs have been shown to be necessary for MSCs’ beneficial effects. MSCs secrete miRNA- containing exosomes [88]. Accordingly, exosomes’ cargo includes the same miRNAs from donor cells, even with higher amount [15]. In the cecal ligation and puncture sepsis model, knockout of miR-223 from MSCs eliminated their protection against sepsis-induced injury; and exosomes isolated from miR-223 knockout MScs also lost their protection against sepsis [89]. Both MSCs and their derived exosomes are cardioprotective against myocardial infarction in animal models. However, anti-miR-125b treatment of exosomes significantly attenuated their protective effect [90]. MiR-181c in human umbilical cord MSC-derived exosomes is key to anti-inflammatory effects in burned rat inflammation model [91]. Exosomal miR-29c plays a role in therapeutic effects of placenta-derived MSCs in Duchenne muscular dystrophy mice [92]. Exogenous miRNAs can be incorporated into exosomes. Lee et al. [93] showed that miR-124 and miR-145 mimics regulated target gene expression in neural progenitor cells-astrocyte culture via delivery in MSC-derived exosomes. Exosomes from lipopolysaccharide (LPS)-treated MSCs specifically carry let-7b [94]. Exosomal shuttling of let-7b plays an important role in wound healing in diabetic animals.
On the other hand, exosomal miRNAs have been associated with disease conditions, such as allergic rhinitis [95], chronic myeloid leukemia patients with musculoskeletal pain [96], and lumbar radicular pain [97]. Disease-specific exosomal-associated miRNAs have potential to be developed into diagnostic markers as well as therapeutic targets. MiR-663 associated with systemic lupus erythematosus suppresses beneficial effects of BMSCs in this condition and accordingly, inhibition of miR-663 improves BMSCs’ therapeutic effect [64]. MSC-exosomes can also be used to deliver miRNA treatment. EVs derived from MSCs transfected with miR-147 mimic reduced aortic inflammation in elastase-treated mice model of abdominal aortic aneurysm [63].
mRNAs
One interesting phenomenon in exosome biology is that mRNAs can be shuttled to cells where new proteins of therapeutic interest can be made. For example, BMSC-derived sEVs containing a specific set of mRNAs are similarly effective as BMSCs in improving acute kidney injury in mice [98]. Human BMSC-derived exosomes contain insulin-like growth factor-1 receptor (IGF-1R) mRNA. Exosomal transfer of IGF-1R mRNA to damaged renal tubular cells promoted their proliferation and repair and this effect was significantly reduced when IGF-1R transcription in donor cells was silenced [99]. MSC-derived exosomes are able to shuttle mRNAs to different cell types, unlike somatic cells such as mast cells whose exosomes selectively transfer mRNAs to mast cells but not CD4 cells [86].
IncRNAs
Long non-coding RNAs are transcripts longer than 200 nucleotides that are generally not translated into proteins, but involved in epigenetic regulation of gene expression through diverse mechanisms. Although there has been no direct evidence linking lncRNAs with MSC-derived exosomes, cancer-derived exosomal lncRNAs are considered promising to be cancer biomarkers [40, review].
Therapeutic exosomes
Practically, there has been no definitive way to distinguish exosomes from other subsets of sEVs isolated from different sources with different isolation protocols. It is appreciated that unfractionated microvesicles are used in some studies where contribution of exosomes was not separated from other sEVs [100]. Even with characterization by exosome-specific markers, the used vesicles are still cautiously termed sEVs when contribution of other types of EVs cannot be excluded with confidence; or we may call them exosome-like vesicles since they do express some common exosome markers. Direct comparison showed that both exosomes and larger EVs (microparticles) derived from MSCs are immunosuppressive, but exosomes show greater anti-inflammatory effects in the in vivo models [16]. This is understandable as each EV subtype undergoes different genesis process and carry distinct cargo, and should be entitled to different functions. Thus, in understanding the literature of EV-related therapy and comparing findings, the nature of sEVs and variables related to specific study settings need to be carefully considered.
There has been numerous preclinical evidence that indicates efficacy of MSC-derived exosomes or EVs in treating modeled disease conditions [100-102, reviews]. Human embryonic stem cell-derived MSCs reduced infarct size in a mouse model of myocardial ischemia/reperfusion injury. The cardioprotective effect of these MSCs is attributable to their secreted exosomes [14]. Mice with carbon tetrachloride- induced liver failure are rescued by human umbilical cord MSC-derived exosomes [103]. The effect of MSCs on gingiva wound healing is mimicked by exosome-like sEVs secreted by gingiva-derived MSCs [10]. The anti-inflammatory effect of MSC-derived exosomes is recently observed in the collagen-induced arthritis mouse model [16] and antigen-induced synovitis pig model [17]. In the endotoxin-induced acute lung injury mouse model, intratracheal injection of human BMSC-derived microvesicles (200 nm) reduced inflammation and injury [104]. Rat MSC-derived exosomes improved functional recovery after middle cerebral artery occlusion [105]. There seems donor-specificity of exosome’s effect. MSC-derived exosomes improved retinal injury and inflammation, but fibroblasts-derived exosomes did not have this effect [106].
Owing to the immunosuppressive property of MSCs, MSCs have shown impressive effect in GvHD cases. Le Blanc et al. [44] were the first to show that haploidentical MSCs improved severe acute GvHD of the gut and liver in a treatment-resistant patient. Inspired by preclinical work that suggests a role of exosomes in mediating therapeutic effect of MSCs [14], MSC-derived exosomes were used in a therapy-esistant GvHD patient [83]. Exosome preparations from MSC donors were carefully characterized. They are sized at ≈100-140 nm, express exosomal markers CD81 and Tsg101, and contain high amount of IL-10 and TGF-β. Except IFN-γ, they do not contain pro-inflammatory cytokines such as IL-1β, IL-17a and TNF. These exosomes produced immunosuppressive effects on patient immune cells, as seen by a decreased number of PBMCs and natural killer cells that release proinflammatory cytokines upon stimulation. Escalated in a two-week period, the patient received a total exosome dosage of 8 units (exosomes from the supernatant of 4 × 107 MSCs were defined as 1 unit) without side effects. The MSC-exosomes clearly improved GvHD symptoms and the patient was stable for several months. This is the first clear clinical case supporting that MSC-derived exosomes are promising surrogate of MSC-based therapy. A phase I clinical study comparing the effects of umbilical cord blood MSC-derived exosomes (40-180 nm) and microvesicles (180-1000 nm) on type I diabetes has been registered (NCT02138331).
Most studies agree that MSC-derived exosomes produce comparable, but not necessarily greater, therapeutic benefit as that of parent MSCs [106]. For instance, MSC-derived EVs produced neuroprotection similar to MSCs in a mouse stroke model [107]. Del Fattore et al. [84] report that MSC-derived sEVs may be more immunoregulative than the parent cells. BMSC-EVs, but not MSCs, increased the CD4+CD25+CD127low Tregs/CD4+CD25-CD127high Teffector cells ratio in PBMCs and the immunosuppressive cytokine IL-10 concentration in culture medium [84]. Two recent studies report different effect of exosomes on Treg induction. Cosenza et al. [16] showed that MSC-derived exosomes tended to increase the CD4+CD25+Foxp3+ Treg population in splenocytes while parent MSCs did not have this effect. In contrast, MSC-exosomes were significantly less efficient in inducing CD4+CD25+Foxp3+ Tregs in PBMC culture from both asthma patients and healthy controls, compared to MSCs [72]. These results suggest differential immunoregulatory properties of MSCs and their exosomes under different experimental settings or disease conditions [Also see 108].
One advantage of using exosomes is to get around MSCs’ side effects. For example, exosomes are nanoparticles that can penetrate blood brain barrier. The use of MSC exosomes can avoid potential pulmonary embolism related to transplantation of MSCs [109]. Systemic DiR-labeled MSC exosomes are found in the injured rat spinal cord at 3 and 24 h after injection, while MSCs are trapped in the lungs and cannot reach the injured site [61]. Additionally, exosomes and other sEVs are potentially capable of targeted drug delivery and function as diagnostic biomarkers [93, 110-112].
Fate of injected exosomes
It has long been recognized that MSCs are trapped in the lungs immediately following systemic infusion and mostly removed from the body within days or a few weeks [11, 50, 51, 113]. However, do MSC- derived exosomes or sEVs have a favorable biodistribution and pharmacokinetic profile over their parent cells? In an intracerebral hemorrhage rat model, Dil-labeled MSC-derived exosomes reached brain, liver, lung and spleen after intravenous injection, although the distribution was not quantified [114]. Studies with different labeling and tracking strategies consistently show that intravenous exosomes/sEVs dominantly appear in the liver within minutes, with significant but lesser amount in the spleen and lungs [115-121]. DiR-labeled unmodified tumor-derived exosomes were almost completely removed from the circulation at 24 h after i.v. injection [118]. Significant tissue presence of DiR-labeled sEVs from HEK cells was observed at 48 h after injection [119]. Other study showed that luciferase-biotin-labeled EVs from HEK cells were eliminated via liver and kidney within six hours [121]. Apparently, the pharmacokinetics of systemically applied exosomes is much similar to their parent cells, i.e., they are removed from the circulation and body in a short period of time. Accumulation of exosomes/EVs in the liver and spleen is consistent with removal of exogenously applied organells by the immune system involving complement opsonization and monocytes/macrophages. Exosomes appear to be able to home to the injury site [114, 116]. Similar to MSCs [122], intranasal administration led to better brain accumulation of exosomes at the injured brain site, compared to i.v. injection [123].
Priming effect and “Environment” factors
One important difference between employing MSC exosomes and MSCs is that MSCs are put into an environment unique to a disease state and have opportunity to interact with host immune cells and release trophic factors/immune mediators in the light of circumstances. The state of the host appears important for MSC’s action. BMSCs produce pain relief in injured animals but have no effect on pain sensitivity in non-injured [8], likely due to the fact that injury and pain hypersensitivity are associated with activated immune system [124] that is targeted by transplanted MSCs. Interestingly, inflammatory priming of MSCs leads to stronger therapeutic effect. IL-1β pretreated MSCs are more efficacious in attenuating neuropathic pain and suppressing glial hyperactivity [82]. In fact, human MSCs are subject to differential TLR priming that leads to different phenotypes. Waterman et al. [125]. show that TLR4-primed MSCs are pro-inflammatory and TLR3-primed MSCs are immunosuppressive. Apparently, MSC exosomes released in vivo under disease conditions may possess properties different from those derived from the cultures. Although exosomes/sEVs obtained in vitro tend to diffuse to the injured site [e.g. 116] and are subject to inflammatory environment, their impact on the host may be limited. There is an impression that culture MSC-derived sEVs are not as efficient as their parent cells [see 108 for a review], as in vivo conditions may not be reliably duplicated in vitro and culture-derived exosomes do not have benefits of host immune stimulation before secretion.
Exosomes from primed MSCs appear more effective. Pretreatment of MSCs with inflammatory cytokines enhanced their sEVs’ immunosuppression [15]. In splenic mononuclear cells co-culture, exosome-like EVs from IL-1β-primed MSCs induced significantly higher amount of IL-10 and TGF-β than EVs from untreated MSCs [70]. Exosomes from MSCs pretreated with TGF-β/IFN-γ were more efficient in transforming mononuclear cells to Tregs [85]. Exosomes from LPS-pretreated MSCs were superior to those from untreated MSCs in immune regulation by polarizing macrophages to anti-inflammatory phenotype in a rat diabetic model [94]. The priming effect is also observed in exosomes from macrophages. LPS stimulation of macrophages culture (RAW 264.7 cells) led to secretion of exosomes containing an increased amount of miRNA involved in resolution of inflammation [126].
Thus, a selectively activated immune environment benefits MSC/exosome-involved immune regulation. MSC-derived exosomes induced Tregs in mouse spleen CD4 cells that were activated by allogeneic APC- enriched CD11C+ cells, but not by stimulation with anti-CD3/CD28 antibodies [73]. EVs from TLR4- primed pro-inflammatory MSCs were ineffective in improving indices of nerve regeneration after sciatic axotomy, while EVs from rest or TLR3-primed anti-inflammatory MSCs significantly improved regeneration [127]. Exosomes isolated from serum of injured animals treated with BMSCs can produce relief of persistent pain in tendon-ligated animals when focally injected into the brain pain modulatory site RVM [Guo unpublished observations]. Compared to EVs from healthy MSCs, EVs separated from MSCs of leukemic patients exhibited greater effects on migration of leukemic cells and gene modification [128]. Hypoxia inducible factor-1α (HIF-1α) mediates adaptive responses to hypoxia and facilitates angiogenesis. Overexpression of HIF-1α in dental pulp MSCs led to enhanced exosome secretion and angiogenesis in vivo, compared to untreated MSCs [129].
However, IFN-γ priming of MSCs did not affect their exosomes in decreasing the proliferation of ConA-activated splenocytes [16]. Kilpinen et al. [130] showed that IFN-γ stimulation of human umbilical cord blood-derived MSCs led to changes in cargo proteins in their secreted exosome-like EVs including loss of complements (C3, C4A, C5) and some lipid binding proteins; and loss of protective effect against kidney reperfusion injury in rats. Kou et al. [10] showed that TNF, but not IFN-γ increased secretion of IL-1ra containing vesicles. These results suggest that the content of secreted exosomes is variable, depending on the microenvironment that their parent MSCs were facing. Inflammatory priming of MSCs by a single IFN-γ treatment may not be effective or even counterintuitive to the purpose of enhancing immunoregulation.
Concluding remarks
Exosomes as a unique subtype of sEVs have attracted increasing interest in the recent eight years in the fields of MSC research. Most studies have confirmed that exosomes derived from MSCs preserve immunosuppressive phenotype and can mimic therapeutic benefits of their parent cells. Their potential as a surrogate of therapeutic MSCs has been explored rigorously. Their role as a medicinal modality is systematically considered [20, 110, 120]. It is appreciated that exosomes from MSCs of different sources have variable contents including inflammatory mediators, tropic factors, signaling molecules, mRNAs, miRNAs and lncRNAs. Diverse functions of exosomes derived from different sources are expected. More importantly, exosomes isolated in vitro may not mirror that from in vivo where parent MSCs are exposed to specific disease or injury-related conditions. Simulating in vivo microenvironment by pretreatment of MSCs with relevant chemical mediators may lead to their secretion of therapeutically more efficient exosomes/sEVs. However, we know very little about the key molecules involved and the differences between exosomes released under different conditions. These issues would be tremendous interest to preclinical research that pursues exosome biology underlain therapeutic mechanisms of MSCs. There have also been concerns on the certainty of exosomes being used in different studies, as there are no definitive ways to separate them from other subtypes of sEVs that are also membrane bound [23]. These concerns are being addressed by developing improved isolation and labeling strategies [120, 131, 132]. Further studies are expected to demonstrate the superiority of MSC-derived exosomes/sEVs as a pharmaceutical entity with regard to efficacy, safety, and practicability.
Acknowledgement
The author’s work was supported by the Maryland Stem Cell Foundation grant 2014-MSCRFI-0584 and National Institutes of Health grant DE025137.
Footnotes
Conflict of interest statement
The author declares no conflicts of interest.
References
- 1.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983; 97:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987; 262:9412–20. [PubMed] [Google Scholar]
- 3.Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981; 645:63–70. [DOI] [PubMed] [Google Scholar]
- 4.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009; 88:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yildirim S, Zibandeh N, Genc D, Ozcan EM, Goker K, Akkoc T. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016; 2016:4682875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima K, Kunimatsu R, Ando K, Ando T, Hayashi Y, Kihara T, Hiraki T, Tsuka Y, Abe T, Kaku M, Nikawa H, Takata T, Tanne K, Tanimoto K. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2018; 497(3):876–882. doi: 10.1016/j.bbrc.2018.02.156. [DOI] [PubMed] [Google Scholar]
- 7.Stanko P, Altanerova U, Jakubechova J, Repiska V, Altaner C. Dental Mesenchymal Stem/Stromal Cells and Their Exosomes. Stem Cells Int. 2018; 2018:8973613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Wang H, Zou S, Gu M, Watanabe M, Wei F, Dubner R, Huang GT, Ren K. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells. 2011; 29(8):1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W, Chu YX, Imai S, Yang JL, Zou S, Mohammad Z, Wei F, Dubner R, Ren K. Further observations on the behavioral and neural effects of bone marrow stromal cells in rodent pain models. Mol Pain. 2016. June 21;12 pii: 1744806916658043. doi: 10.1177/1744806916658043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y, Giraudo C, Le A, Shi S. The Fas/Fap-1/Cav- 1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018; 10(432). pii: eaai8524. doi: 10.1126/scitranslmed.aai8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Imai S, Yang JL, Zou S, Watanabe M, Chu YX, Mohammad Z, Xu H, Moudgil KD, Wei F, Dubner R, Ren K. In vivo immune interactions of multipotent stromal cells underlie their long- lasting pain-relieving effect. Sci Rep. 2017; 7:10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007; 1(2):129–37. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Gama KB, Santos DS, Evangelista AF, Silva DN, de Alcântara AC, Dos Santos RR, Soares MBP, Villarreal CF. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells Int. 2018; 2018:8179013. doi: 10.1155/2018/8179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010; 4(3):214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016; 6:24120. doi: 10.1038/srep24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C1, Noël D Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8:1399–1410. doi: 10.7150/thno.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casado JG, Blázquez R, Vela FJ, Álvarez V, Tarazona R, Sánchez-Margallo FM. Mesenchymal Stem Cell-Derived Exosomes: Immunomodulatory Evaluation in an Antigen-Induced Synovitis Porcine Model. Front Vet Sci. 2017; 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells. 2017; 35:1747–1759. [DOI] [PubMed] [Google Scholar]
- 19.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC Exosome- Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol Ther. 2017; 25(2):465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017; 35:851–858. [DOI] [PubMed] [Google Scholar]
- 21.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009; 9:581–93. [DOI] [PubMed] [Google Scholar]
- 22.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014; 3:26913. doi: 10.3402/jev.v3.26913. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015; 25:364–72. [DOI] [PubMed] [Google Scholar]
- 25.Meldolesi J Exosomes and Ectosomes in Intercellular Communication. Curr Biol. 2018; 28:R435–R444. [DOI] [PubMed] [Google Scholar]
- 26.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016; 113(8):E968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtiö J, El Andaloussi S, Wood MJ, Vader P. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016; 6:22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissig C, Gruenberg J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014; 24:19–25. [DOI] [PubMed] [Google Scholar]
- 29.Frankel EB, Audhya A. ESCRT-dependent cargo sorting at multivesicular endosomes. Semin Cell Dev Biol. 2018; 74:4–10. doi: 10.1016/j.semcdb.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald C, Payne JA, Aboian M, Smith W, Katzmann DJ, Piper RC. A family of tetraspans organizes cargo for sorting into multivesicular bodies. Dev Cell. 2015; 33(3):328–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Y, Eng WS, Colquhoun DR, Dinglasan RR, Graham DR, Mahal LK. Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J Biol Chem. 2014; 289(47):32526–37. doi: 10.1074/jbc.M114.606269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319:1244–1247. [DOI] [PubMed] [Google Scholar]
- 33.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012; 14:677–85. [DOI] [PubMed] [Google Scholar]
- 34.Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016; 7:131. doi: 10.1186/s13287-016-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005; 280(24):23349–55. [DOI] [PubMed] [Google Scholar]
- 36.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014; 1841:108–20. [DOI] [PubMed] [Google Scholar]
- 37.Lai RC, Tan SS, Yeo RW, Choo AB, Reiner AT, Su Y, Shen Y, Fu Z, Alexander L, Sze SK, Lim SK. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016; 5:29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010; 73:1907–20. [DOI] [PubMed] [Google Scholar]
- 39.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Möbius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003; 278(13):10963–72. [DOI] [PubMed] [Google Scholar]
- 40.Dragomir M, Chen B, Calin GA. Exosomal lncRNAs as new players in cell-to-cell communication. Transl Cancer Res. 2018; 7(Suppl 2):S243–S252. doi: 10.21037/tcr.2017.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Momen-Heravi F, Getting SJ, Moschos SA. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol Ther. 2018. August 3 pii: S0163–7258(18)30134–7. doi: 10.1016/j.pharmthera.2018.08.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Tsilioni I, Theoharides TC. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J Neuroinflammation. 2018;15:239. doi: 10.1186/s12974-018-1275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Li Y, Guan X, Zhao J, Shen L, Liu J. Exosomal double-stranded DNA as a biomarker for the diagnosis and preoperative assessment of pheochromocytoma and paraganglioma. Mol Cancer. 2018; 17(1):128. doi: 10.1186/s12943-018-0876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363(9419):1439–41. [DOI] [PubMed] [Google Scholar]
- 45.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008; 371(9624):1579–86. [DOI] [PubMed] [Google Scholar]
- 46.Trento C, Bernardo ME, Nagler A, Kuçi S, Bornhäuser M, Köhl U, Strunk D, Galleu A, Sanchez-Guijo F, Gaipa G, Introna M, Bukauskas A, Le Blanc K, Apperley J, Roelofs H, Van Campenhout A, Beguin Y, Kuball J, Lazzari L, Avanzini MA, Fibbe W, Chabannon C, Bonini C, Dazzi F Manufacturing mesenchymal stromal cells for the treatment of graft-versus-host disease: a survey amongst centers affiliated to the European Group of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018. July 19 pii: S1083–8791(18)30402–6. doi: 10.1016/j.bbmt.2018.07.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pleumeekers MM, Nimeskern L, Koevoet JLM, Karperien M, Stok KS, van Osch GJVM. Trophic effects of adipose-tissue-derived and bone-marrow-derived mesenchymal stem cells enhance cartilage generation by chondrocytes in co-culture. PLoS One. 2018; 13:e0190744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017; 35:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teixeira GQ, Pereira CL, Ferreira JR, Maia AF, Gomez-Lazaro M, Barbosa MA, Neidlinger-Wilke C, Goncalves RM. Immunomodulation of human mesenchymal stem/stromal cells in intervertebral disc degeneration: insights from a proinflammatory/degenerative ex vivo model. Spine; (Phila Pa: 1976). 2018. June 15;43(12): E673–E682. doi: 10.1097/BRS.0000000000002494. [DOI] [PubMed] [Google Scholar]
- 50.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009; 5:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, Cox CS. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009; 110:1189–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011; 106:1299–310. [DOI] [PubMed] [Google Scholar]
- 53.Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol Lett. 2015; 168:140–6. [DOI] [PubMed] [Google Scholar]
- 54.Neirinckx V, Agirman G, Coste C, Marquet A, Dion V, Rogister B, Franzen R, Wislet S. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther. 2015; 6:211. doi: 10.1186/s13287-015-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013; 31(9):1980–91. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 56.Blázquez R, Sánchez-Margallo FM, Álvarez V, Usón A, Casado JG. Surgical meshes coated with mesenchymal stem cells provide an anti-inflammatory environment by a M2 macrophage polarization. Acta Biomater. 2016; 31:221–230. [DOI] [PubMed] [Google Scholar]
- 57.Blázquez R, Sánchez-Margallo FM, Álvarez V, Usón A, Marinaro F, Casado JG. Fibrin glue mesh fixation combined with mesenchymal stem cells or exosomes modulates the inflammatory reaction in a murine model of incisional hernia. Acta Biomater. 2018; 71:318–329. doi: 10.1016/j.actbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Park HJ, Kim J, Saima FT, Rhee KJ, Hwang S, Kim MY, Baik SK, Eom YW, Kim HS. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem Biophys Res Commun. 2018; 498(4):988–995. [DOI] [PubMed] [Google Scholar]
- 59.Siniscalco D, Giordano C, Galderisi U, Luongo L, de Novellis V, Rossi F, Maione S Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front. Integr. Neurosci. 2011, 5:79 DOI: 10.3389/fnint.2011.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009; 15:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018; 13(1):e0190358. doi: 10.1371/journal.pone.0190358. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA, Kourembanas S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med. 2018; 197:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spinosa M, Lu G, Su G, Bontha SV, Gehrau R, Salmon MD, Smith JR, Weiss ML, Mas VR, Upchurch GR Jr, Sharma AK. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J. 2018. May 29:fj201701138RR. doi: 10.1096/fj.201701138RR. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geng L, Tang X, Zhou K, Wang D, Wang S, Yao G, Chen W, Gao X, Chen W, Shi S, Shen N, Feng X, Sun L. MicroRNA-663 induces immune dysregulation by inhibiting TGF-β1 production in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Cell Mol Immunol. 2018. March 26. doi: 10.1038/cmi.2018.1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo W, Imai S, Zou S-P, Wei F, Dubner R, Ren K Immune regulation and mesenchymal stromal cell-produced pain relief: 2 Role of NFκB signaling and regulatory T cells. 2016. Program No. 445.11, San Diego: Society for Neuroscience. [Google Scholar]
- 66.Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011; 117(18):4826–35. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 67.Yan Z, Zhuansun Y, Chen R, Li J, Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res. 2014; 324(1):65–74. doi: 10.1016/j.yexcr.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 68.Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012; 53(2):786–93. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Favaro E, Carpanetto A, Lamorte S, Fusco A, Caorsi C, Deregibus MC, Bruno S, Amoroso A, Giovarelli M, Porta M, Perin PC, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 2014; 57:1664–73. [DOI] [PubMed] [Google Scholar]
- 70.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012. September;147(1–2):47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014; 23:1233–44. [DOI] [PubMed] [Google Scholar]
- 72.Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018; 363(1):114–120. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B, Yeo RWY, Lai RC, Sim EWK, Chin KC, Lim SK. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy. 2018; 20(5):687–696. [DOI] [PubMed] [Google Scholar]
- 74.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107(1):367–72. [DOI] [PubMed] [Google Scholar]
- 75.Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22(2):369–79. doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- 76.Gupte KS, Vanikar AV, Trivedi HL, Patel CN, Patel JV. In-vitro generation of interleukin-10 secreting B-regulatory cells from donor adipose tissue derived mesenchymal stem cells and recipient peripheral blood mononuclear cells for potential cell therapy. Biomed J. 2017; 40(1):49–54. doi: 10.1016/j.bj.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho KA, Lee JK, Kim YH, Park M, Woo SY, Ryu KH. Mesenchymal stem cells ameliorate B-cell- mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell Mol Immunol. 2017. doi: 10.1038/cmi.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest. 2015; 125:3226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7th ed. Amsterdam: Elsevier; 2014. [Google Scholar]
- 80.Imai S, Guo W, Zou S-P, Wei F, Dubner R, Ren K Immune regulation and mesenchymal stromal cell-produced pain relief: 1 promotion of anti-inflammatory phenotype. 2016. Program No. 445.16, San Diego: Society for Neuroscience. [Google Scholar]
- 81.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006; 24(2):386–98. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Deng G, Wang H, Yang M, Yang R, Li X, Zhang X, Yuan H. Interleukin-1β pre-treated bone marrow stromal cells alleviate neuropathic pain through CCL7-mediated inhibition of microglial activation in the spinal cord. Sci Rep. 2017; 7:42260. doi: 10.1038/srep42260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014; 28:970–3. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 84.Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, Fierabracci A, Muraca M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015; 24(12):2615–27. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, Fu L, Liang Y, Guo Z, Wang L, Ma C, Wang H. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J Cell Physiol. 2018; 233(9):6832–6840. [DOI] [PubMed] [Google Scholar]
- 86.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9(6):654–9. [DOI] [PubMed] [Google Scholar]
- 87.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Peréz Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015; 6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015. October 7;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B, Fan GC. Exosomal miR-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci Rep. 2015. September 8;5:13721. doi: 10.1038/srep13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y, Zhong Z, Zhao J, Li Q, Zhu D, Ke C, Zhong S, Wu X, Yu H, Zhu W, Chen J, Zhang J, Wang J, Hu X. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of mir-125b. Circ Res. 2018. pii: CIRCRESAHA.118.312758. doi: 10.1161/CIRCRESAHA.118.312758. [DOI] [PubMed] [Google Scholar]
- 91.Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016; 8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bier A, Berenstein P, Kronfeld N, Morgoulis D, Ziv-Av A, Goldstein H, Kazimirsky G, Cazacu S, Meir R, Popovtzer R, Dori A, Brodie C Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials. 2018; 174:67–78. doi: 10.1016/j.biomaterials.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 93.Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev. 2014; 23(23):2851–61. doi: 10.1089/scd.2014.0146. [DOI] [PubMed] [Google Scholar]
- 94.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015; 13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu G, Yang G, Zhang R, Xu G, Zhang L, Wen W, Lu J, Liu J, Yu Y. Altered microRNA Expression Profiles of Extracellular Vesicles in Nasal Mucus From Patients With Allergic Rhinitis. Allergy Asthma Immunol Res. 2015; 7(5):449–57. doi: 10.4168/aair.2015.7.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asano M, Umezu T, Katagiri S, Kobayashi C, Tauchi T, Gotoh M, Ando K, Okabe S, Ohyashiki JH, Ohyashiki K. Up-regulated exosomal miRNA-140–3p in CML patients with musculoskeletal pain associated with discontinuation of tyrosine kinase inhibitors. Int J Hematol. 2017; 105(4):419–422. doi: 10.1007/s12185-017-2199-z. [DOI] [PubMed] [Google Scholar]
- 97.Moen A, Jacobsen D, Phuyal S, Legfeldt A, Haugen F, Røe C, Gjerstad J. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J Transl Med. 2017; 15(1):89. doi: 10.1186/s12967-017-1194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009; 20(5):1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013; 22(5):772–80. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2015; 11(1):150–60. doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 101.Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant. 2018; 27:349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng G, Huang R, Qiu G, Ge M, Wang J, Shu Q, Xu J. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018. June 28. doi: 10.1007/s00441-018-2871-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC Exosome- Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol Ther. 2017; 25:465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014; 32:116–25. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013; 33(11):1711–5. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N, Volarevic V. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. Adv Exp Med Biol. 2018. May 18. doi: 10.1007/5584_2018_219. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 107.Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015; 4(10):1131–43. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front Cell Dev Biol. 2016; 4:83. doi: 10.3389/fcell.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jung JW, Kwon M, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013; 54: 1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O'Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Krämer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Théry C, Rohde E, Giebel B. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015; 4:30087. doi: 10.3402/jev.v4.30087. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D, Chen X, Chen Y, Yang Z, Jin L, Jiang W, Tian C, Zhou G, Zen K, Zhang J, Zhang Y, Li J, Zhang CY. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015; 5:17543. doi: 10.1038/srep17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shao H, Chung J, Lee K, Balaj L, Min C, Carter BS, Hochberg FH, Breakefield XO, Lee H, Weissleder R. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun. 2015; 6: 6999. doi: 10.1038/ncomms7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guo W, Imai S, Dubner R, Ren K. Multipotent stromal cells for arthritic joint pain therapy and beyond. Pain Manag. 2014; 4:153–162. [DOI] [PubMed] [Google Scholar]
- 114.Otero-Ortega L, Gómez de Frutos MC, Laso-García F, Rodríguez-Frutos B, Medina-Gutiérrez E, López JA, Vázquez J, Díez-Tejedor E, Gutiérrez-Fernández M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2018; 38(5):767–779. doi: 10.1177/0271678X17708917. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013; 165(2):77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014; 33(5):1055–63. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morishita M, Takahashi Y, Nishikawa M, Sano K, Kato K, Yamashita T, Imai T, Saji H, Takakura Y. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J Pharm Sci. 2015; 104(2):705–13. doi: 10.1002/jps.24251. [DOI] [PubMed] [Google Scholar]
- 118.Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015; 199:145–55. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015; 4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di Rocco G, Baldari S, Toietta G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int. 2016; 2016:5029619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014; 8(1):483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Danielyan L, Beer-Hammer S, Stolzing A, Schäfer R, Siegel G, Fabian C, Kahle P, Biedermann T, Lourhmati A, Buadze M, Novakovic A, Proksch B, Gleiter CH, Frey WH, Schwab M. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer's and Parkinson's disease. Cell Transplant. 2014; 23 Suppl 1:S123–39. doi: 10.3727/096368914X684970. [DOI] [PubMed] [Google Scholar]
- 123.Betzer O, Perets N, Angel A, Motiei M, Sadan T, Yadid G, Offen D1, Popovtzer R In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano. 2017; 11(11):10883–10893. doi: 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- 124.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010; 16:1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010. April 26;5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R, Aradillas Lopez E, Alexander GM, Sacan A, Fortina P, Ajit SK. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain. 2014; 155(8):1527–39. doi: 10.1016/j.pain.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raisi A, Azizi S, Delirezh N, Heshmatian B, Farshid AA, Amini K. The mesenchymal stem cell-derived microvesicles enhance sciatic nerve regeneration in rat: a novel approach in peripheral nerve cell therapy. J Trauma Acute Care Surg. 2014; 76(4):991–7. doi: 10.1097/TA.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 128.Crompot E, Van Damme M, Pieters K, Vermeersch M, Perez-Morga D, Mineur P, Maerevoet M, Meuleman N, Bron D, Lagneaux L, Stamatopoulos B. Extracellular vesicles of bone marrow stromal cells rescue chronic lymphocytic leukemia B cells from apoptosis, enhance their migration and induce gene expression modifications. Haematologica. 2017; 102(9):1594–1604. doi: 10.3324/haematol.2016.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells. 2017; 35:1747–1759. [DOI] [PubMed] [Google Scholar]
- 130.Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J, Finckenberg P, Siljander P, Kankuri E, Mervaala E, Laitinen S. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013; 2. doi: 10.3402/jev.v2i0.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One. 2017; 12(1):e0170628. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017; 7(3):789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]