Abstract
Purpose:
To provide long-term, natural history data of a case of a subclinical choroidal neovascular membrane (CNVM) in the setting of age-related macular degeneration.
Methods:
Retrospective review of the 10-year clinical course of a patient including multimodal imaging.
Patient:
A 75-year-old White female with macular degeneration referred for an exudative CNVM who was also found to have a subclinical CNVM in the contralateral eye.
Results:
On presentation, visual acuity was 20/25 in the right eye and 20/40 in the left eye. In the left eye, a retinal pigment epithelial detachment with associated subretinal and intraretinal fluid was found on spectral-domain optical coherence tomography (OCT). Fluorescein angiography (FA) was consistent with a predominately classic CNVM, which was well-visualized on indocyanine green angiography (ICG-A). Treatment was initiated with bevacizumab for 10 months which reduced the amount of subretinal and intraretinal fluid, but progressive geographic atrophy developed over the subsequent 9 years reducing vision to 20/100. Interestingly, at initial presentation a non-exudative fibrovascular pigment epithelial detachment was detected in the right (contralateral) eye. This was monitored with multimodal imaging twice yearly for 10 years without any signs of exudation, and vision remained 20/25. OCT angiography revealed a remarkably similar appearance of the subclinical CNVM compared to ICG-A 10-years prior, suggesting anatomic stability.
Discussion and Conclusion:
The advent of OCT angiography has increased detection of subclinical CNVMs. Recent evidence suggests that subclinical CNVMs have a high rate of progression to exudation over 1 year, which raises the question of whether early treatment is beneficial. This case provides 10-year follow-up with multimodal imaging (FA, ICG-A, OCT, OCT-A) of a subclinical CNVM which remained stable and without exudation, suggesting that they may be closely observed.
Keywords: Age-related macular degeneration, Choroidal neovascular membrane, Fibrovascular retinal pigment epithelial detachment, Optical coherence topography angiography
Summary
A 75-year-old white female with age-related macular degeneration presented with an exudative choroidal neovascular membrane (CNVM) which was treated with bevacizumab. Indocyanine green angiography revealed a subclinical CNVM in the contralateral eye. After 10 years follow-up, the subclinical CNVM remained non-exudative and the patient retained excellent vision in that eye.
BACKGROUND
Age-related macular degeneration (AMD) is one of the leading causes of blindness in the developed world. It is projected to affect as many as 196 million people by 2020 and 288 million people by 2040.1 Most vision loss occurs in the late stages of AMD, defined as the presence of neovascular disease or central geographic atrophy. Neovascular AMD is characterized by the presence of a choroidal neovascular membrane (CNVM) which can exudate resulting in hemorrhage and fluid leakage which damages the retina.2 Fluorescein angiography (FA) is the gold standard for diagnosing CNVMs, as it may allow visualization of the membrane in the early phase of the angiogram, and can assess leakage in the late phase.3 Classic, minimally classic and occult leakage patterns have been described.3 Indocyanine green angiography (ICG-A) is also helpful for visualizing occult CNVMs as it may better assess deeper structures, especially in the presence of hemorrhage.3,4 Optical coherence tomography (OCT) is an imaging modality which is often used to quantify the degree of exudation and is helpful in monitoring response to anti-vascular endothelial growth factor (anti-VEGF) treatment.3 More recently, however, OCT angiography was developed as a rapid, non-invasive technique to image the vasculature using laser light reflectance to detect blood flow through tissues. This has resulted in the detection of subclinical CNVMs, or fibrovascular pigment epithelial detachments (PED),5 which appear quiescent but may carry risk of future exudation.4,6 Here we present a case of a patient with an incidentally discovered subclinical CNVM which remains stable and without exudation over 10-years of follow-up.
CASE REPORT
A 75-year-old white woman with a history of age-related macular degeneration was referred for evaluation of neovascular disease in her left eye after receiving 3 monthly bevacizumab injections. Her ocular history included one episode of optic neuritis in her right eye, as well as a retinal tear in her right eye which was treated with laser retinopexy. Her medical history was unremarkable except for hypothyroidism for which she was taking levothyroxine. She did not have a family history of eye disease and was a non-smoker.
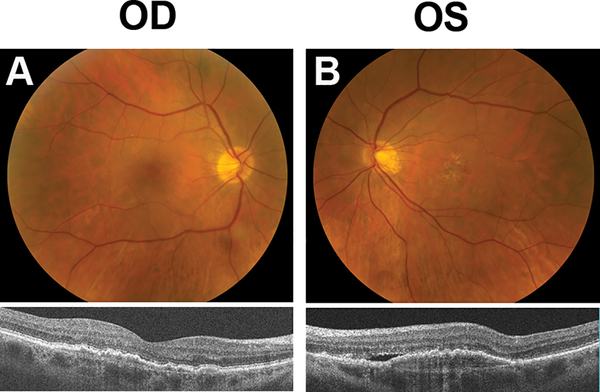
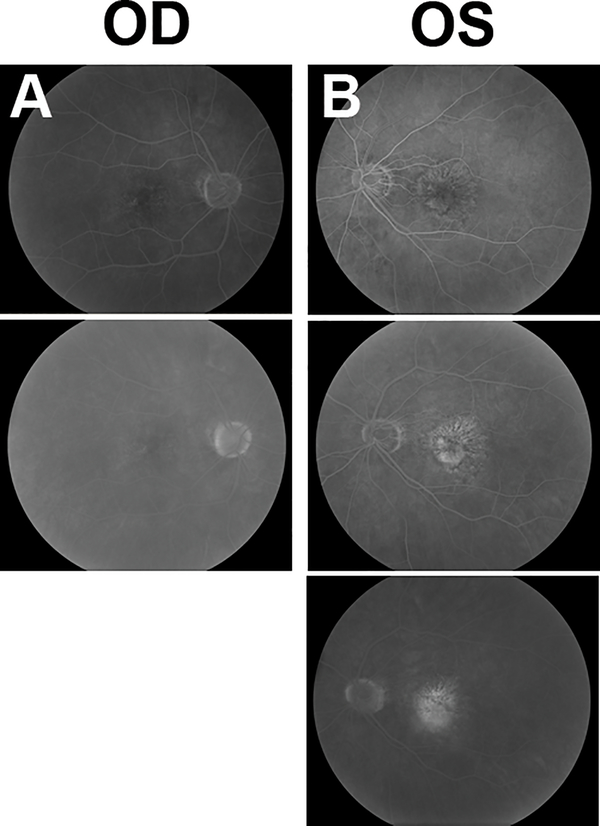
On examination, visual acuity was 20/25 in the right eye and 20/40 in the left eye. Intraocular pressure was 16 mmHg in both eyes. Slit lamp biomicroscopy was notable for 1+ nuclear sclerotic cataracts in both eyes. Fundoscopy showed a few intermediate drusen in the right eye, and a low-lying irregular elevation of the retinal pigment epithelial (RPE) was seen on OCT (Model 5000, Carl Zeiss Meditec, Inc, Dublin CA) (Figure 1a, Supplemental Digital Content 1, which demonstrates drusen and fundus changes in each eye with color fundus photograph and SD-OCT at baseline examination). The left eye had a moderate number of intermediate drusen with extensive RPE changes centrally, with associated yellow lipid exudate and early fibrotic changes. OCT revealed an irregular RPE detachment with associated subretinal and intraretinal fluid (Figure 1b, Supplemental Digital Content 1, which demonstrates drusen and fundus changes in each eye with color fundus photograph and SD-OCT at baseline examination). Fluorescein angiography (FA) revealed a predominately classic CNVM in the left eye with extensive late leakage (Figure 2b, Supplemental Digital Content 2, which demonstrates fluorescein angiography of both eyes at baseline), which was well-visualized on video indocyanine green angiography (ICG-A) (Video 1, Supplemental Digital Content 3, which demonstrates leakage of the choroidal neovascular membrane in the left eye on indocyanine green angiography). Video ICG-A of the contralateral (right) eye revealed a large subfoveal choroidal neovascular complex with late leakage of ICG dye, but a normal appearance on FA. (Figure 2a, Supplemental Digital Content 2, which demonstrates fluorescein angiography of both eyes at baseline, Video 2, Supplemental Digital Content 4, which demonstrates leakage of the choroidal neovascular membrane in the right eye on indocyanine green angiography). The neovascular complex seen on ICG in the right eye corresponds to the area of irregular elevation of the RPE on OCT suggesting a fibrovascular RPE detachment without exudation.
Figure 1. Baseline Photographs.
(A) Color fundus photograph of the right eye showing few intermediate drusen (top) and SD-OCT demonstrating low-lying irregular elevation of the retinal pigment epithelium (bottom).
(B) Color fundus photograph of left eye showing large druse, retinal pigment epithelial changes and yellow exudate consistent with neovascular AMD (top). SD-OCT shows a retinal pigment epithelial detachment with associated subretinal fluid (bottom).
Figure 2. Fluorescein angiography (FA) of both eyes at baseline.
(A) FA images taken at 1 minute 2 seconds (top) and 7 minutes 46 seconds (bottom). Right eye shows a branching pattern of hyperfluorescence on FA without any leakage.
(B) FA images taken at 19 seconds (top), 45 seconds (middle), and 6 minutes 22 seconds (bottom). Left eye shows a branching hyperfluorescence on FA with late leakage consistent with a predominantly classic CNVM.
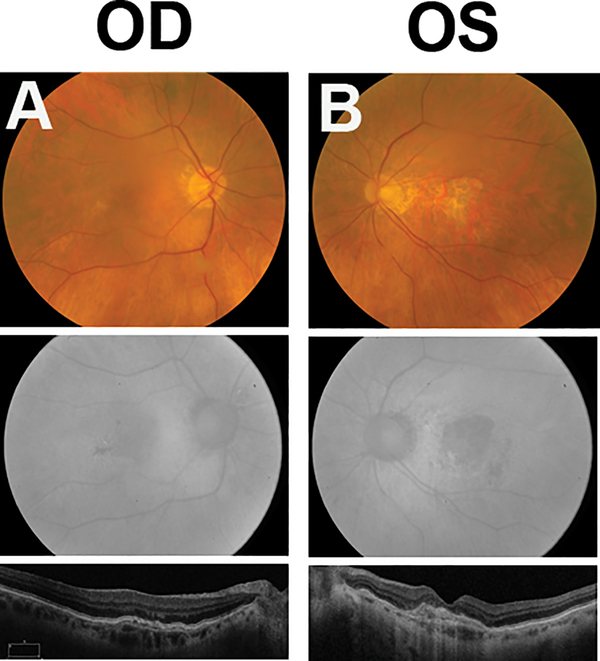
The patient received 5 additional intravitreal bevacizumab injections in the left (exudative) eye over the next 10 months, with the last 2 injections using a double dose of 2.5 mg to treat residual intraretinal fluid. Vision improved to 20/25 and the patient was observed over the following 9 years during which there was no recurrence of exudation. Unfortunately, geographic atrophy slowly developed during follow-up and vision decreased to 20/100 at year 8 when the fovea became involved (Figure 3b, Supplemental Digital Content 5, which demonstrates fundus appearance 10 years after presentation in both eyes on color fundus photograph, fundus autofluorescence, and OCT images).
Figure 3. Fundus photograph, fundus autofluorescence, and OCT taken 10 years after presentation.
(A) The right eye remains stable with no change in the fundus appearance, autofluorescence, or OCT.
(B) The left eye shows signs of central scarring and geographic atrophy on fundus photography, fundus autofluorescence, and OCT.
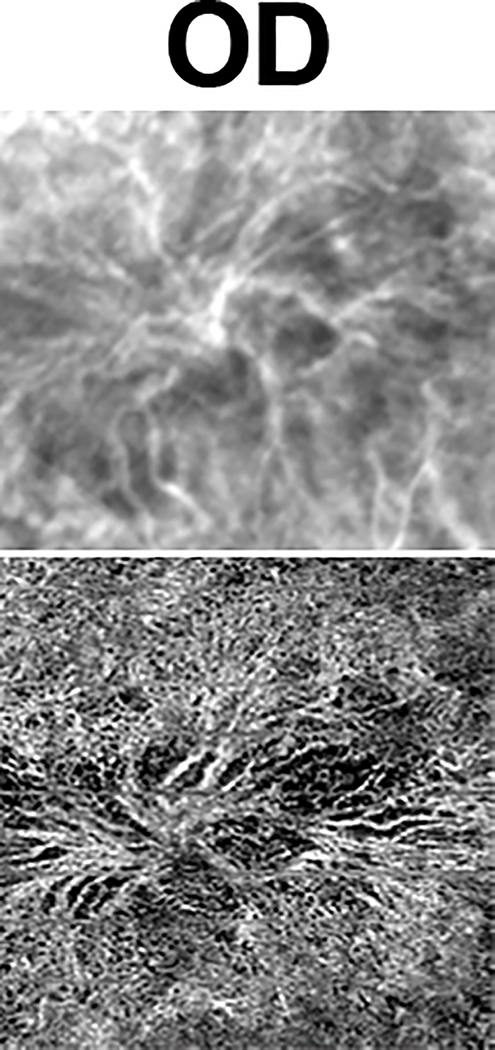
Her right eye, which had a subclinical CNVM on initial presentation, remained stable over the subsequent 10 years both clinically (20/25 vision) and on multimodal imaging (OCT, FA, ICG-A) (Figure 3a, Supplemental Digital Content 5, which demonstrates fundus appearance 10 years after presentation in both eyes on color fundus photograph, fundus autofluorescence, and OCT images). When OCT angiography became available, the right eye was imaged which showed a choroidal neovascular complex which was remarkably similar in size and branching pattern as the initial ICG-A (Figure 4, Supplemental Digital Content 6, which demonstrates similar anatomic appearance of the subclinical choroidal neovascular complex in the right eye on indocyanine green angiography at baseline and SD-OCT 10 years after presentation).
Figure 4.
ICG-A at baseline (top) and an OCT-A (bottom) 10 years later shows a remarkable similarity in the anatomic appearance of the subclinical choroidal neovascular complex.
DISCUSSION
The advent of OCT angiography (OCT-A) has revealed the presence of subclinical CNVMs in asymptomatic macular degeneration patients. There is a paucity of evidence as to the natural history of these CNVMs given the inability to detect them without advanced imaging, which is usually reserved for patients with exudative disease.
Recently, De Oliveira Dias et al used swept-source OCT-A to follow a cohort of 160 sequential patients with intermediate AMD or geographic atrophy in the study eye and exudative AMD in the fellow eye.6 Subclinical CNVM was detected using OCT-A in 23 eyes, corresponding to a prevalence of 14%. The cumulative incidence of developing exudation at 1-year of these eyes was 21%, corresponding to a 15 times greater risk of exudation in these eyes compared to those without evidence of CNVM on OCT-A at baseline.6 Subclinical CNVMs may be a precursor to exudative disease, and clinicians may question whether prophylactic treatment is warranted to prevent exudation. To date, there are insufficient data to answer this question. The Prophylactic Ranibizumab for Exudative Age-Related Macular Degeneration (PREVENT Study NCT02140151) is a multicenter, randomized trial which is underway and provides quarterly ranibizumab to patients with intermediate AMD to prevent exudation, given a history of exudative disease in the fellow eye. However, in this study, eyes being treated with intermediate AMD may or may not have a subclinical CNVM.
Our case illustrates that a subclinical CNVM confirmed with multimodal imaging may remain stable over a 10-year interval with remarkable anatomical stability on ICG-A and OCT-A, and excellent visual acuity. The appearance of CNVM complexes on ICG-A and OCT-A have recently been correlated,4 and OCT-A may be an effective surrogate for monitoring subclinical CNVMs. More research is required to understand the pathophysiology of subclinical CNVMs, their biological role, and their relationship to exudative disease. It is interesting that our patient had an unchanged fundus appearance (few intermediate drusen) in the eye with a subclinical CNVM over 10-years, which does not keep with the natural history of AMD. This contrasts with the contralateral eye which developed progressive geographic atrophy after receiving treatment for an exudative CNVM. It has been hypothesized that CNVMs may have an adaptive role to provide nourishment to the overlying retinal pigment epithelial cells and photoreceptors, and that treatment with anti-VEGF may precipitate geographic atrophy.6,7 Given this, a reasonable option includes close observation of such subclinical CNVMs and treatment may be offered when exudation is present.
Supplementary Material
Video 2. Indocyanine green angiography (ICG-A) of the right eye at baseline showing leakage of the choroidal neovascular membrane.
Video 1. Indocyanine green angiography (ICG-A) of the left eye at baseline showing leakage of the choroidal neovascular membrane.
Supplemental Digital Content 1. Figure that demonstrates drusen and fundus changes in each eye with color fundus photograph and SD-OCT at baseline examination. tif
Supplemental Digital Content 2. Figure that demonstrates fluorescein angiography of both eyes at baseline. tif
Supplemental Digital Content 3. Video that demonstrates leakage of the choroidal neovascular membrane in the left eye on indocyanine green angiography. m4v
Content Supplemental Digital Content 4. Video that demonstrates leakage of the choroidal neovascular membrane in the right eye on indocyanine green angiography. m4v
Supplemental Digital Content 5. Figure that demonstrates the fundus appearance of both eyes10 years after presentation on color fundus photograph, fundus autofluorescence, and OCT images. tif
Supplemental Digital Content 6. Figure that demonstrates the similar anatomic appearance of the subclinical choroidal neovascular complex in the right eye on indocyanine green angiography at baseline and SD-OCT 10 years after presentation. tif
Acknowledgments
This work was completed at the National Eye Institute, National Institutes of Health, Bethesda, MD.
This work has been supported by the National Eye Institute Intramural Research Program, National Institutes of Health (NIH), Bethesda, Maryland; and the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, e Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
Footnotes
None of the authors have proprietary or financial interests related to the material in this manuscript.
REFERENCES
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–116. [DOI] [PubMed] [Google Scholar]
- 2.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. [DOI] [PubMed] [Google Scholar]
- 3.Gess AJ, Fung AE, Rodriguez JG. Imaging in neovascular age-related macular degeneration. Semin Ophthalmol. 2011;26(3):225–233. [DOI] [PubMed] [Google Scholar]
- 4.Roisman L, Zhang Q, Wang RK, et al. Optical Coherence Tomography Angiography of Asymptomatic Neovascularization in Intermediate Age-Related Macular Degeneration. Ophthalmology. 2016;123(6):1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariri A, Heussen FM, Nittala MG, Sadda SR. Optical coherence tomographic correlates of angiographic subtypes of occult choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54(13):8020–8026. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural History of Subclinical Neovascularization in Nonexudative Age-Related Macular Degeneration Using Swept-Source OCT Angiography. Ophthalmology. 2018;125(2):255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunwald JE, Pistilli M, Daniel E, et al. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2017;124(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 2. Indocyanine green angiography (ICG-A) of the right eye at baseline showing leakage of the choroidal neovascular membrane.
Video 1. Indocyanine green angiography (ICG-A) of the left eye at baseline showing leakage of the choroidal neovascular membrane.
Supplemental Digital Content 1. Figure that demonstrates drusen and fundus changes in each eye with color fundus photograph and SD-OCT at baseline examination. tif
Supplemental Digital Content 2. Figure that demonstrates fluorescein angiography of both eyes at baseline. tif
Supplemental Digital Content 3. Video that demonstrates leakage of the choroidal neovascular membrane in the left eye on indocyanine green angiography. m4v
Content Supplemental Digital Content 4. Video that demonstrates leakage of the choroidal neovascular membrane in the right eye on indocyanine green angiography. m4v
Supplemental Digital Content 5. Figure that demonstrates the fundus appearance of both eyes10 years after presentation on color fundus photograph, fundus autofluorescence, and OCT images. tif
Supplemental Digital Content 6. Figure that demonstrates the similar anatomic appearance of the subclinical choroidal neovascular complex in the right eye on indocyanine green angiography at baseline and SD-OCT 10 years after presentation. tif