Abstract
Background
Anophthalmia is the absence of one or both eyes, and it can be congenital (i.e. a birth defect) or acquired later in life. There are two main types of orbital implant: integrated, whereby the implant receives a blood supply from the body that allows for the integration of the prosthesis within the tissue; and non‐integrated, where the implant remains separate. Despite the remarkable progress in anophthalmic socket reconstruction and in the development of various types of implants, there are still uncertainties about the real roles of integrated (hydroxyapatite (HA), porous polyethylene (PP), composites) and non‐integrated (polymethylmethacrylate (PMMA)/acrylic and silicone) orbital implants in anophthalmic socket treatment.
Objectives
To assess the effects of integrated versus non‐integrated orbital implants for treating anophthalmic sockets.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 7), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2016), Embase (January 1980 to August 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to August 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 8 August 2016.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of integrated and non‐integrated orbital implants for treating anophthalmic sockets.
Data collection and analysis
Two authors independently selected relevant trials, assessed methodological quality and extracted data.
Main results
We included three studies with a total of 284 participants (250 included in analysis). The studies were conducted in India, Iran and the Netherlands. The three studies were clinically heterogenous, comparing different materials and using different surgical techniques. None of the included studies used a peg (i.e. a fixing pin used to connect the implant to the prosthesis). In general the trials were poorly reported, and we judged them to be at unclear risk of bias.
One trial compared HA using traditional enucleation versus alloplastic implantation using evisceration (N = 100). This trial was probably not masked. The second trial compared PP with scleral cap enucleation versus PMMA with either myoconjunctival or traditional enucleation (N = 150). Although participants were not masked, outcome assessors were. The last trial compared HA and acrylic using the enucleation technique (N = 34) but did not report comparative effectiveness data.
In the trial comparing HA versus alloplastic implantation, there was no evidence of any difference between the two groups with respect to the proportion of successful procedures at one year (risk ratio (RR) 1.02, 95% confidence interval (CI) 0.95 to 1.09, N = 100, low‐certainty evidence). People receiving HA had slightly worse horizontal implant mobility compared to the alloplastic group (mean difference (MD) −3.35 mm, 95% CI −4.08 to −2.62, very low‐certainty evidence) and slightly worse vertical implant motility (MD −2.76 mm, 95% CI −3.45 to −2.07, very low‐certainty evidence). As different techniques were used – enucleation versus evisceration – it is not clear whether these differences in implant motility can be attributed solely to the type of material. Investigators did not report adverse events.
In the trial comparing PP versus PMMA, there was no evidence of any difference between the two groups with respect to the proportion of successful procedures at one year (RR 0.92, 95% CI 0.84 to 1.01, N = 150, low‐certainty evidence). There was very low‐certainty evidence of a difference in horizontal implant motility depending on whether PP was compared to PMMA with traditional enucleation (MD 1.96 mm, 95% CI 1.01 to 2.91) or PMMA with myoconjunctival enucleation (−0.57 mm, 95% CI −1.63 to 0.49). Similarly, for vertical implant motility, there was very low‐certainty evidence of a difference in the comparison of PP to PMMA traditional (MD 3.12 mm 95% CI 2.36 to 3.88) but no evidence of a difference when comparing PP to PMMA myoconjunctival (MD −0.20 mm 95% CI −1.28 to 0.88). Four people in the PP group (total N = 50) experienced adverse events (i.e. exposures) compared to 6/100 in the PMMA groups (RR 17.82, 95% CI 0.98 to 324.67, N = 150, very low‐certainty evidence).
None of the studies reported socket sphere size, cosmetic effect or quality of life measures.
Authors' conclusions
Current very low‐certainty evidence from three small published randomised controlled trials did not provide sufficient evidence to assess the effect of integrated and non‐integrated material orbital implants for treating anophthalmic sockets. This review underlines the need to conduct further well‐designed trials in this field.
Plain language summary
Integrated compared with non‐integrated orbital implants for treating anophthalmic sockets
What is the aim of this review? The aim of this Cochrane Review was to find out if integrated orbital implants are better than non‐integrated orbital implants for treating anophthalmic sockets. Cochrane researchers collected and analysed all relevant studies to answer this question and found three studies.
Key messages There is uncertainty as to the benefits and harms of integrated compared with non‐integrated orbital implants. What was studied in the review? 'Anophthalmia' is the absence of the eye in the orbit. This can occur in childhood (because of problems with development) or it can happen during the course of life (due to an accident or other eye disease).
Doctors can put an implant in the orbit to fill the void left by the removal of the eye and this together with an external prosthesis can improve the patient's appearance. This orbital implant can be made of two types of materials – integrated or non‐integrated material. If the material is integrated, then new blood vessels can grow into the implant material. If the material is non‐integrated, then the orbital implant remains separate from the rest of the orbit's tissue.
The review authors looked to see if the type of implant material affected the success of the surgery or, in other words, if integrated implants can provide better results than non‐integrated implants. They were also interested in how much the external prosthesis could move better after surgery – using integrated or non‐integrated orbital implants. Also, the authors wanted to know if the type of orbital implant material can affect people's quality of life. Were there any adverse (harmful) effects of using integrated or non‐integrated orbital implants?
What are the main results of the review? The type of material used for the orbital implant may not affect the success of the surgery (low‐certainty evidence). The review authors judged the evidence on prosthesis movement and adverse effects as providing very little certainty about the true effects. There was no information on quality of life.
How up‐to‐date is this review? The Cochrane researchers searched for studies that had been published up to 8 August 2016.
Summary of findings
Summary of findings 1. Summary of findings.
| Integrated versus non‐integrated orbital implants for treating anophthalmic sockets | |||||||
|
Patient or population: people with anophthalmic sockets Intervention: integrated implants Comparison: non‐integrated implants | |||||||
| Outcomes | Comparison | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty (quality) of the evidence (GRADE) | Comment | |
| Risk with non‐integrated material | Risk with integrated material | ||||||
| Proportion of successful procedures at 1 year and up to 5 years after surgery (no exposure/extrusion) | PP (scleral cap enucleation) versus PMMA (traditional and myoconjunctival enucleation) | 960 per 1000 | 883 per 1000 (806 to 1000) | RR 0.92 (0.84 to 1.01) | 150 (1) | ⊕⊕⊝⊝ Lowa | — |
| HA (traditional enucleation) versus alloplastic (evisceration) | 960 per 1000 | 980 per 1000 (912 to 1000) | RR 1.02 (0.95 to 1.09) | 100 (1) | ⊕⊕⊝⊝ Lowb |
— | |
| Socket and prosthesis motility (horizontal) | PP (scleral cap enucleation) versus PMMA (traditional enucleation) | The mean socket and prosthesis motility (horizontal) score was 5.14 mm | The mean socket and prosthesis motility (horizontal) score in the intervention group was on average 1.96 mm more (1.01 mm more to 2.91 mm more) | — | 100 (1) | ⊕⊝⊝⊝ Very lowa,c | — |
| PP (scleral cap enucleation) versus PMMA (myoconjunctival enucleation) | The mean socket and prosthesis motility (horizontal) score was 7.67 mm | The mean socket and prosthesis motility (horizontal) score in the intervention group was on average 0.57 mm less (1.63 mm less to 0.49 mm more) |
— | 100 (1) | ⊕⊝⊝⊝ Very lowa,c | — | |
| HA (traditional enucleation) versus alloplastic (evisceration) | The mean socket and prosthesis motility (horizontal) score was 10.25 mm | The mean socket and prosthesis motility (horizontal) score in the intervention group was on average 3.35 mm less (4.08 mm less to 2.62 mm less) | — | 100 (1) | ⊕⊝⊝⊝ Very lowb,c | — | |
| Socket and prosthesis motility (vertical) | PP (scleral cap enucleation) versus PMMA (traditional enucleation) | The mean socket and prosthesis motility (vertical) score was 2.68 mm | The mean socket and prosthesis motility (vertical) score in the intervention group was on average 3.12 mm more (2.36 mm more to 3.88 mm more) | — | 100 (1) | ⊕⊝⊝⊝ Very lowa,c | — |
| PP (scleral cap enucleation) versus PMMA (myoconjunctival enucleation) | The mean socket and prosthesis motility (vertical) score was 6 mm | The mean socket and prosthesis motility (vertical) score in the intervention group was on average 0.20 mm less (1.28 mm less to 0.88 mm more) | — | 100 (1) | ⊕⊝⊝⊝ Very lowa,c | — | |
| HA (traditional enucleation) versus alloplastic (evisceration) | The mean socket and prosthesis motility (vertical) score was 8.45 mm | The mean socket and prosthesis motility (vertical) score in the intervention group was on average 2.76 mm less (3.45 mm less to 2.07 mm less) | — | 100 (1) | ⊕⊝⊝⊝ Very lowb,c | — | |
| Any adverse outcomes (e.g. extrusions or migration, infections, ectropion) | PP (scleral cap enucleation) versus PMMA (traditional or myoconjunctival enucleation) | 1 per 1000 | 18 per 1000 (1 to 325 per 1000) | RR 17.82 (0.98 to 324.67) | 150 (1) | ⊕⊝⊝⊝ Very lowa,c | Adverse outcomes not reported for HA versus alloplastic comparison |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HA: hydroxyapatite; PMMA: polymethylmethacrylate; PP: porous polyethylene; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
aDowngraded for risk of bias (−1) and indirectness (−1). The trial was poorly reported, and it was difficult to judge risk of bias for many domains. It was conducted in one specific setting, and it is unclear if the findings are generalisable to other settings. bDowngraded for risk of bias (−1) and indirectness (−1). The trial was poorly reported, and it was difficult to judge risk of bias for most domains. It was a quasi‐randomised study and probably not masked. It was conducted in one specific setting, and it is unclear if the findings are generalisable to other settings. cDowngraded for imprecision (−1): confidence intervals include clinically unimportant effect.
Background
Description of the condition
Anophthalmia is the absence of one or both eyes, and it can be congenital (i.e. a birth defect) or acquired later in life (such as by trauma, tumour, glaucoma, etc.).
Description of the intervention
History
The development of techniques for enucleation (the surgical removal of the eyeball leaving the eye muscles and orbital contents intact) and evisceration (the removal of the eye's contents) occurred almost concurrently with the development of the materials used in the manufacture of prostheses and implants for aesthetic and functional repair of the anophthalmic socket. The first implants were very light and made of a type of very thin glass (Tonkelaar 1991). Since the destruction of glass implant manufacturing plants during World War II, other types of non‐integrated (non‐porous) materials, such as silicone and polymethylmethacrylate (PMMA), have emerged and gained popularity. These types of implants still dominate the world market in anophthalmic socket repair.
Since 1987, the landscape of eye socket reconstruction has changed substantially, with integrated (porous) implants made of natural hydroxyapatite becoming widely available (Perry 1990). The BioEye (Hydroxyapatite Orbital Implant; Integrated Orbital Implants Inc., San Diego, CA, USA) is an implant built using natural hydroxyapatite and composed of calcium carbonate, the same mineral that forms the hard parts of bones. A hydrothermal reaction converts calcium carbonate into calcium phosphate during BioEye manufacture (Massry 1995). This material is extremely porous, allowing for vascular and fibrovascular ingrowth; thus, it can become "part of the patient's body" as an integrated implant (Flanagan 1990). Synthetic hydroxyapatites were introduced soon after natural hydroxyapatite in several countries (Jordan 1998), including Brazil (Jordan 2000; Schellini 2000).
Other biomaterials, such as porous polyethylene (Medpor; Porex Technologies Corporation, Fairburn, GA, USA), became available for the repair of anophthalmic sockets in mid‐1991 (Karesh 1994). Experimental research in Brazil also made use of similar materials, showing good results (Schellini 2000).
Different types of implants are used to reconstruct the anophthalmic cavity. These implants can be classified according to their shape (spherical, oval or conical), the material used (integrated or non‐integrated) and the surface type (smooth/non‐porous or porous). Non‐porous (non‐integrated) implants are most often composed of PMMA or silicone, while porous (integrated) implants are made of hydroxyapatite, porous polyethylene and composites (including bioceramics).
In 1995, a study from the USA reported that most American surgeons were using spheres made of natural hydroxyapatite (Hornblass 1995). By 2004, this preference had changed, with American surgeons preferring to use porous polyethylene implants without coupling pegs between the implant and the external prosthesis (Su 2004). A Canadian study in 2006 revealed that porous polyethylene implants were the most widely used prostheses in Canada (Jordan 2006). In Brazil, despite a lack of studies on anophthalmic socket implants, integrated implants are rarely used, most likely due to their higher cost; however, actually PMMA is still the preferred choice among 62.75% of Brazilian ophthalmologists (Schellini 2015; Sousa 2012).
Physicians originally considered that the use of pegs conferred advantages for integrated implants, enabling the anchoring of implants to the external prothesis. Nowadays, pegs have fallen out of use, as there have been observational reports of complications, although the evidence for this is scarce.
With the increased use of integrated implants, studies have observed complications in 10% to 22% of patients, including conjunctival dehiscence, implant exposure or extrusion, and the necessity to remove the implant (Buettner 1992; Goldberg 1994; Nunery 1993; Rubin 1994; Shields 1992; Shields 1994). These complication rates are based on the longest periods of observation in the individuals who received the implants. Several reports have been published on the lack of success of integrated implants, and these failures have been related to the failure of the surgical technique, the use of external prostheses that create too much pressure against the surface of the implant (Shields 1994), and the use of uncoated spheres (Rubin 1994). This research led to the conclusion that hydroxyapatite spheres had a higher risk of failure than spheres made of silicone (Nunery 1993).
Thus, despite the remarkable progress in anophthalmic socket reconstruction and the development of various types of implants, there are still uncertainties about the real roles of integrated and non‐integrated orbital implants in anophthalmic socket treatment.
Enucleation surgery
Enucleation surgery refers to removing the entire eyeball, that is, without cutting into or dissecting the globe. It consists of the following steps.
Determination of general or local anaesthesia.
A 360° conjunctival peritomy (the conjunctiva is separated from the sclera with scissors close to the limbus).
Identification and sectioning of the extrinsic ocular rectus muscle and sectioning of the optic nerve.
Removal of the entire eye and the inspection of Tenon's capsule.
Determination of the implant size.
Insertion of the orbital implant.
If necessary, wrapping of the implant to be attached to the extrinsic ocular rectus muscles.
Suturing of Tenon's fascia.
Suturing of the conjunctiva (attaching the upper conjunctiva to the lower conjunctiva).
Evisceration surgery
Evisceration surgery removes the eye's contents but leaves the scleral shell, Tenon's capsule, the orbital fat and the extraocular muscles intact. The operation consists of the following steps.
Determination of general or local anaesthesia.
A 360° peritomy (the conjunctiva is separated from the sclera using scissors).
Penetration into the anterior chamber using a no. 11 scalpel blade and enlargement of the corneo‐scleral opening with scissors.
Removal of the eye contents using an ocular evisceration spoon.
Evaluating the size of the implant to be placed into the scleral shell.
Suturing of the sclera and Tenon's fascia.
Suturing of the conjunctiva (attaching the upper conjunctiva to the lower conjunctiva).
How the intervention might work
The main factors that favour the use of integrated implants are the mobility of the external prosthesis and the presence of immune cells within implants that receive a blood supply from the host, allowing for the integration of the prosthesis with the host's tissue, which in turn results in a lower risk of migration and implant extrusion (Flanagan 1990; Rubin 1994). In contrast, the non‐integrated implants contain no unique apparatus for attachments to the extraocular muscles and do not allow in‐growth of organic tissue into their inorganic substance.
Enucleation surgery is used in cases of intraocular tumours, in patients at risk of sympathetic ophthalmia and in cases of severe atrophy of the scleral layer (phthisis bulbi). Evisceration surgery is used in cases of virulent endophthalmitis. However, both enucleation and evisceration surgeries are used for painful and blind eyes, and there are different arguments as to whether these techniques should be the procedure of choice. Both procedures should include implant placement to replace the lost volume and to restore the facial aesthetics. The implants can be non‐integrated (PMMA, silicone) or integrated (i.e. hydroxyapatite, polyethylene or composites).
Why it is important to do this review
Various autologous tissues, such as bone, fat and dermis, have been used to restore anophthalmic sockets, as have organic and alloplastic materials, including acrylics, silicone, hydroxyapatite and porous polyethylene. The high incidence of complications has prompted new research aiming to identify the gold standard material. However, the best technique in the management of anophthalmic sockets is currently unclear, and there is variation in trends around the world. A systematic review of integrated and non‐integrated orbital implants would be useful to patients, ophthalmologists and other professionals involved in providing eye care to evaluate the efficacy of procedures and to reduce complications.
Objectives
To assess the effects of integrated versus non‐integrated orbital implants for treating anophthalmic sockets.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which investigators determined allocation to treatment by alternation, use of alternate medical records, date of birth, or other predictable methods). This decision was due to our anticipation of not finding many trials in this area.
Types of participants
Participants were people affected by anophthalmia, regardless of age or sex. We also considered patients who had an implant rejection.
Types of interventions
We included the following interventions in this review.
Intervention group: porous/integrated materials (hydroxyapatite, porous polyethylene, composites).
Comparator: non‐porous/non‐integrated materials (polymethylmethacrylate (PMMA)/acrylic and silicone).
We also planned to investigate composite integrated/non‐integrated implants such as the Gutthoff implant as per our published protocol (Schellini 2013).
We considered both the enucleation and evisceration reconstruction of the anophthalmic socket.
Types of outcome measures
Primary outcomes
The primary outcomes for this review were:
the proportion of successful procedures at one year and up to five years after surgery; and
the proportion of successful procedures after five years.
We considered a successful procedure as involving no need for secondary reconstruction of the anophthalmic socket or implant extrusion. We also considered implant removal and implant exposure needing implant removal as an implant extrusion.
Secondary outcomes
Secondary outcomes for this review were:
Horizontal and vertical socket and prosthesis motility measured by evaluating the prosthesis excursion in different eye gaze positions or any validated measurement aiming to measure the impact of motility function loss;
degree of vascularisation measured by magnetic resonance imaging (MRI);
sphere size before and after surgery;
cosmetic effect (self‐reported);
quality of life measures: any validated measurement scale aiming to measure the impact of visual function loss on quality of life of participants;
economic data: we planned to perform comparative cost analysis if data were available; and
adverse events: any adverse outcomes as reported in trials, particularly extrusions or migration, infections, ectropion, ptosis, significant inflammatory responses or exposures.
We wanted to measure the secondary outcome measures at one year or more after surgery.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 7), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2016), Embase (January 1980 to August 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to August 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 8 August 2016.
See appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We handsearched the reference lists of the identified relevant studies for additional citations. We also contacted specialists in the area and the main authors of included trials about unpublished data as well as pharmaceutical companies for further details of published and unpublished trials.
Data collection and analysis
Selection of studies
Two authors (RED and EJC) independently screened the trials identified by the literature search. We obtained full copies of all potentially or definitely relevant articles. We resolved disagreements by discussion and consulted each other for quality assurance of the processes. We documented reasons for exclusion for any study we rejected after viewing full copies in the 'Characteristics of excluded studies' table.
Data extraction and management
Two authors (RED and EJC) independently extracted data. We resolved any discrepancies by discussion. We used a standard data extraction form to record the following information: characteristics of the study (design, methods of randomisation), participants, interventions and outcomes (types of outcome measures, adverse events). One author (ECJ) entered all data into RevMan 5 (RevMan 2014). The second author (RED) independently checked the data entered.
Assessment of risk of bias in included studies
We assessed risk of bias using the methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the following six separate criteria: random sequence generation, allocation concealment, masking (blinding), incomplete outcome data, selective reporting and other types of bias. See Table 2 for further information on each parameter.
1. Risk of bias criteria.
| Random sequence generation | Was the allocation sequence adequately generated, for example with random number tables or computer‐generated random numbers? We assessed trials as being at low risk of bias (the method used is either adequate or unlikely to introduce confounding), uncertain risk of bias (there is insufficient information to assess whether the method used is likely to introduce confounding) or high risk of bias (the method used (e.g. quasi‐randomised trials) is improper and likely to introduce confounding). |
| Allocation concealment | Was allocation adequately concealed in a way that would not allow either the investigators or the participants to know or influence allocation to an intervention group before an eligible participant was entered into the study (for example using central randomisation or sequentially numbered, opaque, sealed envelopes held by a third party)? We judged trials to be at low risk of bias (the method used (e.g. central allocation) is unlikely to induce bias in the final observed effect), uncertain risk of bias (there is insufficient information to assess whether the method used is likely to induce bias in the estimate of effect) or high risk of bias (the method used (e.g. open random allocation schedule) is likely to induce bias in the final observed effect). |
| Masking (blinding) | We judged the possibility of masking being done on both participants and outcome assessors. We did not consider masking of personnel as this is not feasible in these trials. Were the participants and study outcome assessors masked from knowledge of which intervention a participant received? We judged trials to be at low risk of bias (the outcome measurement is not likely to be influenced by lack of masking), uncertain risk of bias (there is insufficient information to assess whether the type of masking used is likely to induce bias in the estimate of effect) or high risk of bias (the outcome or the outcome measurement is likely to be influenced by lack of masking). |
| Incomplete outcome data | Were incomplete outcome data adequately addressed? Incomplete outcome data essentially include: attrition, exclusions and missing data. If any withdrawals occurred, were they described and reported by treatment group with reasons given? We recorded whether or not there were clear explanations for withdrawals and dropouts in the treatment groups. An example of an adequate method to address incomplete outcome data is the use of an intention to‐treat analysis (ITT). We judged trials to be at low risk of bias (the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data), uncertain risk of bias (there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data is likely to induce bias in the estimate of effect), or high risk of bias (the crude estimate of effects (e.g. complete case estimate) clearly was biased due to the underlying reasons for missing data, and the methods used to handle missing data are unsatisfactory). |
| Selective reporting | Are reports of the study free from any suggestion of selective outcome reporting? This was interpreted as no evidence that statistically non‐significant results might have been selectively withheld from publication, for example selective under‐reporting of data or selective reporting of a subset of data. We judged trials to be at low risk of bias (the trial protocol is available and all of the trials prespecified outcomes that are of interest in the review have been reported or similar), uncertain risk of bias (there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting), or high risk of bias (not all of the trials prespecified primary outcomes have been reported or similar). |
| Other sources of bias | Was trial funded from parties that might not have conflicting interests (e.g. an antibacterial agent manufacturer), or any academic, professional, financial or other benefits to the person responsible for the trial were independent of the direction or statistical significance of trial results? We assessed trials as being at low risk of bias (if trial funding did not come), unclear risk of bias (if the source of funding was not clear, or if it was unclear whether the person responsible for the trial stands to benefit according to the direction or statistical significance of trial results) or high risk of bias (if the trial’s source of funding had a conflict of interest, or if any academic, professional, financial or other benefits to the person responsible for the trial are dependent on the direction or statistical significance of trial results). |
Firstly, we copied information that was relevant for making a judgment on a criterion from the original publication into an assessment table. If study authors provided additional information, we entered it in the table along with an indication that this was unpublished information. Two review authors (RED and ECJ) independently made a judgment as to whether the risk of bias for each criterion was low, uncertain, or high. We resolved disagreements by discussion.
When we classified trials as being at low risk of bias in sequence generation, allocation concealment, masking, incomplete data and selective outcome reporting, we considered them to be trials at low risk of bias. If the protocol was available, we compared the pre‐specified outcomes with the outcomes reported in the Results to assess selective reporting bias. Otherwise, we used the primary trial report to compare outcomes listed in the Methods section with those in the Results.
We recorded this information for each included trial in 'Risk of bias' tables in RevMan 5 (RevMan 2014) and summarised the risk of bias for each study in a summary 'Risk of bias' figure and graph.
Measures of treatment effect
Binary outcomes
For dichotomous data (proportion of successful procedures and adverse outcomes), we used the risk ratio (RR) as the effect measure with 95% confidence intervals (CIs).
Continuous outcomes
For continuous data (socket motility and prosthesis motility, degree of vascularisation, sphere size, cosmetic effect, and quality of life), we presented the results as mean differences (MDs) with 95% CIs. When pooling data across studies we would have estimated the MD if different trials measured the outcomes in the same way. We would have used the standardised mean difference (SMD) to combine trials that measure the same outcome but used different methods.
Unit of analysis issues
The unit of analysis was each participant, as only a minority would be anophthalmic in both eyes.
Dealing with missing data
An intention‐to‐treat analysis (ITT) considers data from all trial participants allocated to an intervention whether they received the intervention or not. We planned to impute an outcome for a dropout rate of 5%. For each trial we planned to report whether or not the investigators stated if the analysis was performed according to the ITT principle. If participants were excluded after allocation, we planned to report any details provided in full.
Furthermore, we planned to perform the analysis on an ITT basis whenever possible (Newell 1992). Otherwise, we adopted the available case analysis.
We attempted to contact study authors for missing data if necessary.
Assessment of heterogeneity
We quantified inconsistency among the pooled estimates using the Chi2 test for heterogeneity. This illustrates the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling error (Higgins 2003). I2 = [(Q ‐ df )/Q] x 100% test, where Q is the Chi2 statistic and df its degrees of freedom. We assessed heterogeneity between the trials by visual examination of the forest plot to check for overlapping CIs, using the Chi2 test for heterogeneity with a 10% level of significance, and the I2 statistic. We classified heterogeneity using the following I2 values.
0 to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
Apart from assessing the risk of selective outcome reporting (see Assessment of risk of bias in included studies), we would have assessed the likelihood of potential publication bias using funnel plots had there been at least 10 trials. If small studies in a meta‐analysis showed larger treatment effects, we would have considered other causes including selection biases, poor methodological quality, heterogeneity, artefactual causes and chance.
Data synthesis
We ana lysed data as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If three or more studies had been included in a meta‐analysis, we would have used the random‐effects model, and if there were substantial heterogeneity, we would have investigated the source of heterogeneity by conducting a subgroup analysis. As there were fewer than three studies, we described the clinical characteristics of the included studies in tables.
Subgroup analysis and investigation of heterogeneity
In the case of excessive clinical heterogeneity (I2 > 50%), we would have used subgroup analyses to pool the results. Subgroup analyses are secondary analyses in which the participants are divided into groups according to shared characteristics, and outcome analyses are conducted to determine if any significant treatment effect occurs according to that characteristic. If data had permitted, we would have carried out the following subgroup analyses, as we hypothesised that the effect of the interventions might be different due to variations in the surgical techniques, type or size of material used, and age.
Types of anophthalmic reconstructions (enucleation and evisceration).
Different implant sizes (small, medium, and large, approximating the volume of a 16 mm, 18 mm and 20 mm sphere).
Different types of non‐integrated and integrated materials.
Different ages: adults (aged 18 to 65 years) versus older adults (66 to 70 years) versus children and adolescents (17 years old or younger).
Sensitivity analysis
Had there been an adequate number of studies, we would have performed a sensitivity analysis to assess methodological decisions and the robustness of the results. We would have included the following factors in the sensitivity analysis, separating studies according to:
trials with low risk of bias versus those with high risk of bias; and
rates of withdrawal for each outcome (greater than 5%).
Summary of findings and assessment of the certainty of the evidence
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (the proportion of successful procedures at one year or more after surgery; the proportion of successful procedures after five years and; socket and prosthesis motility; cosmetic effect; any adverse outcomes and quality of life) in our review and construct a 'Summary of findings' table using the GRADE software (GRADEpro 2015). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The assessment of the quality of a body of evidence considers within‐study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 407 references (Figure 1). The Cochrane Information Specialist removed 69 duplicate records, and we screened the remaining 338 reports. We rejected 259 records after reading the abstracts and obtained the full‐text reports of 79 references for further assessment. We identified three studies that met the inclusion criteria (Colen 2000; Shome 2010; Tari 2009). We assessed and excluded a further 76 references; see Characteristics of excluded studies for details.
1.
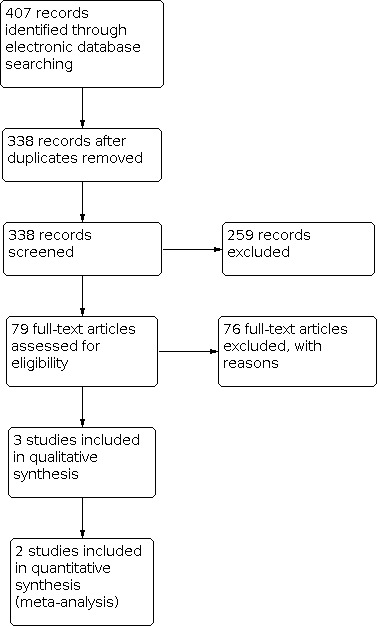
Study flow diagram.
Included studies
See Characteristics of included studies and Table 3.
2. Clinical characteristics of the included studies.
| Study ID | No of participants randomised | Material | Technique used | Mean follow‐up period (months) | |
| Colen 2000 | 34 | Integrated | Hydroxyapatite | Enucleation | 3a |
| Non‐integrated | Acrylic | Enucleation | |||
| Shome 2010 | 150 | Integrated | Porous polyethylene | Enucleation | PP: 15.6 |
| Non‐integrated | Polymethylmethacrylate | Enucleation (myoconjunctival or traditional) | Traditional: 16.4 Myoconjuntival: 17.3 |
||
| Tari 2009 | 100 | Integrated | Hydroxyapatite | Enucleation | 13.16 |
| Non‐integrated | Alloplastic | Evisceration | 11.56 | ||
aAbsolute number.
We included three trials with a total of 284 participants (Colen 2000; Shome 2010; Tari 2009).
Design
Colen 2000 was a multicentre randomised clinical trial, while Tari 2009 was a single‐centre randomised clinical trial, and Shome 2010, a randomised trial did not report whether it took place in one or more centres.
Participants
Tari 2009 took place in Tehran University Eye Research Center, Tehran, Iran. The quadrisection group consisted of 50 participants: 35 men and 15 women with a mean age of 42.32 years. The HA group consisted of 50 participants: 41 men and 9 women with a mean age of 39.56 years. The reason(s) for operation in the quadrisection group were: a painful blind eye (n = 36), cosmetic unacceptability (n = 9) and endophthalmitis (n = 8). In the other group, there were 36 participants who had a painful blind eye; 6 cited cosmetic unacceptability; 5, acute trauma; and three were attributed to the other group.
Shome 2010 took place in Hyderabad, India; however, authors did not specify the institution, nor did they report the age, sex or health status details of the 150 participants.
Colen 2000 was a multicentre trial in the Rotterdam Eye Hospital, Rotterdam, and in the University Medical Center, Utrecht, Netherlands. The hydroxyapatite group consisted of 14 participants: seven men and seven women with a mean age of 64.0 years. In this group, six participants had the implant on the right side and eight on the left side. The acrylic group consisted of 16 participants: nine men and seven women with a mean age of 57.8 years. In this group, nine participants had the implant on the right side and seven on the left side. Thirty‐four participants were eligible for the study, and 30 were ana lysed. All participants had intraocular melanoma.
Interventions
Colen 2000 studied hydroxyapatite (integrated group) (n = 14) and acrylic implants (non‐integrated group) (n = 16). All participants underwent the enucleation technique. The follow‐up was three months.
Shome 2010 compared PMMA (non‐integrated group) (n = 50) versus PMMA myoconjunctival (non‐integrated group) (n = 50) versus integrated porous polyethylene (integrated group) (n = 50). The mean follow‐up of the participants was 16.4 months in the traditional PMMA group, 17.3 in the myoconjunctival PMMA group, and 15.6 in the porous polyethylene group.
Tari 2009 compared evisceration plus quadrisection of sclera with alloplastic implantation (non‐integrated group) (n = 50) versus enucleation with HA (integrated group) (n = 50). The mean follow‐up was 11.6 months for quadrisection and 13.2 months for HA.
Outcomes
Colen 2000 evaluated motility, saccadic gain and saccadic symmetry.
Shome 2010 measured implant and prosthesis movement as well as implant displacement and exposure.
Tari 2009 evaluated the presence or absence of exposure or extrusion and deep superior sulcus deformity along with implant motility.
Excluded studies
We excluded 76 studies after obtaining full‐text reports, as they did not meet the inclusion criteria. See the Characteristics of excluded studies for study names and the reasons for exclusion.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We classified Colen 2000 and Shome 2010 as being at unclear risk of bias for random sequence allocation due to the lack of information reported in the papers. However, Tari 2009 used an alternative method to randomise participants, and we judged the risk of bias to be high. With regard to allocation concealment, only Colen 2000 reported the use of an envelope to mask the randomisation (low risk of bias) while Shome 2010 and Tari 2009 did not report this domain (unclear risk of bias).
Blinding
The masking of personnel does not apply for the clinical question under study, so we did not evaluate it in this review.
Only Colen 2000 reported that participants were unaware of the type of implant (low risk of bias), while Shome 2010 and Tari 2009 did not report information related to this domain (unclear risk of bias).
In both Colen 2000 and Shome 2010, the investigators who recorded the outcomes were unaware of the type of implant, so we assessed these studies as being at low risk of detection bias.
Incomplete outcome data
With regard to incomplete outcome data, in Colen 2000 there was only 11.77% of dropouts and in Tari 2009 all participants completed the study (low risk of bias). Shome 2010 did not report on the number of participants completing the study (unclear risk of bias).
Selective reporting
There was no evidence of selective reporting in any of the included studies (low risk of bias) (Colen 2000; Shome 2010; Tari 2009).
Other potential sources of bias
There was no evidence of any conflict of interest in Shome 2010 and Tari 2009 (low risk of bias), and Colen 2000 did not report on it (unclear risk of bias).
Effects of interventions
See: Table 1
Please see Table 4 and Table 1.
3. Clinical outcome.
| Study ID | Type of material (surgical technique) | Proportion of successful procedures at 1‐5 years after surgery (no exposure/extrusion) | Adverse outcomes (i.e.infection or migration) | Horizontal implant motility (mm) | Vertical implant motility (mm) | |
| Integrated | Non‐integrated | RR (95% CI) | RR (95% CI) | MD (95% CI) | MD (95% CI) | |
| Colen 2000 | HA (enucleation) | Acrylic (enucleation) | — | — | — | — |
| Shome 2010 | PP (enucleation) | PMMA traditional (enucleation) | 0.92 ( 0.84 to 1.01) N = 150 |
17.82 (0.98 to 324.67) N = 150 |
1.96 (1.01 to 2.91) N = 100 |
(3.12 (2.36 to 3.88) N = 100 |
| PP (enucleation) |
PMMA myoconjunctival (enucleation) |
−0.57 ( −1.63 to 0.49) N = 100 |
(−0.20 (−1.28 to 0.88) N = 100 |
|||
| Tari 2009 | HA (enucleation) |
Alloplastic (evisceration) |
1.02 (0.95 to 1.09) N = 100 |
— | −3.35 ( −4.08 to −2.62) N = 100 | (−2.76 (−3.45 to −2.07) N = 100 |
HA: hydroxyapatite; MD: mean difference; PMMA: polymethylmethacrylate; PP: porous polyethylene; RR: risk ratio.
We were unable to pool data from Shome 2010 and Tari 2009 in a meta‐analysis because of the different surgical techniques used (evisceration versus enucleation) and due to the heterogeneity among the clinical outcomes ana lysed.
We did not include Colen 2000 in the meta‐analysis as it did not report comparative effectiveness data. The authors compared vertical versus horizontal saccades gain, symmetry and curvilinearity between the operated and fellow eye instead of comparing it between both the intervention and comparator groups.
Proportion of successful procedures at one year and up to five years after surgery (no exposure/extrusion)
We found no statistically significant difference in the proportion of participants needing secondary reconstruction of the anophthalmic socket, implant extrusion or implant removal in the groups receiving integrated versus non‐integrated implants at one year or more after surgery. We compared HA versus alloplastic evisceration in one trial (RR 1.02, 95% CI 0.95 to 1.09, P = 0.56, Tari 2009; N = 100), and in another single trial, we compared both PMMA traditional and myoconjunctival versus porous polyethylene (RR 0.92, 95% CI 0.84 to 1.01, P = 0.07, Shome 2010; N = 150).
Proportion of successful procedures after five years
None of the included studies reported on this outcome.
Socket and prosthesis motility
Two studies reported on socket and prosthesis motility (Shome 2010; Tari 2009). Follow‐up for Shome 2010 was 16.4 months in the PMMA traditional group, 17.3 months in the PMMA myoconjunctival group, and 15.6 months in the porous polyethylene group; for Tari 2009, follow‐up was 11.6 months for non‐integrated and 13.2 months for integrated implants.
There was a statistically significant difference favouring the non‐integrated (alloplastic) implant compared to the integrated (HA) with regard to horizontal implant motility in one trial (MD −3.35 mm, 95% CI −4.08 to −2.62, P < 0.001; Tari 2009; N = 100). In contrast, there was a statistically significant difference favouring the integrated (PP) implant compared to the non‐integrated (PMMA traditional) implant in another (MD 1.96 mm, 95% CI 1.01 to 2.91, P < 0.001; Shome 2010; N = 100). There was no statistically significant difference with regard to horizontal implant motility between the integrated (PP) and non‐integrated implants (PMMA myoconjunctival) in a single trial (MD −0.57 mm, 95% CI −1.63 to 0.49, P = 0.29; Shome 2010; N = 100).
With regard to vertical implant motility, there was a statistically significant difference favouring the non‐integrated (alloplastic) implant compared to the integrated (HA) implant in one trial (MD −2.76 mm, 95% CI −3.45 to −2.07, P < 0.001, Tari 2009; N = 100). However, there was a statistically significant difference favouring the integrated (PP) implant compared to the non‐integrated (PMMA traditional) implant in another (MD 3.12 mm, 95% CI 2.36 to 3.88, P < 0.001, Shome 2010; N = 100). The same single trial showed no statistically significant difference for vertical implant motility between the integrated (PP) and non‐integrated (PMMA myoconjunctival) implants (MD −0.20 mm, 95% CI −1.28 to 0.88, P = 0.72, Shome 2010; N = 100).
Degree of vascularisation measured by magnetic resonance imaging
None of the included studies reported on this outcome.
Sphere size before and after surgery
None of the included studies reported on this outcome.
Cosmetic effect (self‐reported)
None of the included studies reported on this outcome.
Quality of life measures
None of the included studies reported on this outcome.
Economic data
Two studies reported on economic data (Shome 2010; Tari 2009).
Shome 2010 reported that the costs of the porous polyethylene implant (integrated) is approximately USD 300.00 compared to the PMMA non‐integrated implant that costs around USD 2.00.
Tari 2009 also quoted a lesser cost in the alloplastic compared to porous implant (integrated).
Adverse events
Shome 2010 was the only trial to report on this outcome.
There was no statistically significant difference between the non‐integrated implant (PMMA traditional and myoconjunctival) versus the integrated (PP) implant (RR 17.82, 95% CI 0.98 to 324.67, P = 0.05, Shome 2010; N = 150).
Furthermore, Shome 2010 reported that 6/50 participants from the traditional PMMA group presented significant superior sulcus deformity, and Tari 2009 reported that 10/50 and 7/50 participants in the alloplastic and HA groups, respectively, presented the same deformity.
Discussion
Summary of main results
This review examined the effect of integrated versus non‐integrated implants for treating anophthalmic sockets. We included three studies with a total of 280 participants. Overall, the quality of the included studies was low. Given the limited number of included studies, it was not possible to assess reporting bias, perform sensitivity analyses or explore all the planned subgroup analyses according to type of reconstruction and material, implant size and age. Because of our comprehensive search strategy and contact with experts in the field, we are confident that we have mapped all clinical trials of integrated versus non‐integrated implants for treating anophthalmic sockets.
We saw very different results and effect estimates in these two trials; in the individual trials, a pooled average effect is not informative. There are several characteristics of these trials that could perhaps explain the differences: Shome 2010 used two different enucleation techniques (i.e. traditional versus myoconjunctival) while Tari 2009 used enucleation and scleral quadrisection evisceration. In addition, Tari 2009 presented a high risk of bias related to the random sequence generation, while Shome 2010 did not report the methods of the randomisation process.
Overall completeness and applicability of evidence
We think that there is not enough evidence to draw a robust conclusion, and this is not useful to guide clinical practice.
Quality of the evidence
The methodological quality of the included studies was generally low. Methodological aspects of all studies had an unclear risk of bias for masking of participants and personnel (performance bias) (Shome 2010; Tari 2009); only Colen 2000 masked personnel and participants. Only Colen 2000 and Shome 2010 masked outcome assessors to protect the reliability of results. For sequence generation, we assessed Tari 2009 as being at high risk of bias. Only Colen 2000 reported adequate methods of allocation concealment. Shome 2010 did not report whether the participants completed the study (unclear risk of bias); however, the remaining trials were at low risk of bias for this domain (Colen 2000; Tari 2009). We judged all the studies to be at low risk of bias for selective reporting and two studies to be at low risk of other bias (Shome 2010; Tari 2009).
Overall, this review contains very low‐certainty evidence for the following reasons: the risk of bias was generally unclear for two of the three included trials; one trial was at high risk of bias for random sequence generation, as it used an alternative method (Tari 2009); the directness of the evidence was limited by the fact that there were only two studies that dealt with a population from low‐ and middle‐income countries, there were different effects results between the included studies in the analysis due to the different surgical techniques used (enucleation and evisceration); we could not assess risk of publication bias due to the small number of studies; and the precision of effect estimates was very large.
Potential biases in the review process
We made every attempt to reduce the risk of bias in the review process, using broad inclusion criteria and a comprehensive search strategy to identify eligible trials. There were no language restrictions, and we obtained translations of non‐English trials wherever possible. However, unclear reporting of trial methods and data limited the extent to which we could meaningfully compare all relevant data from the identified trials.
Agreements and disagreements with other studies or reviews
One published review aimed to evaluate biomaterials for orbital implants and ocular prostheses (Baino 2014). The authors described in a qualitative way the different types of implants and confirmed the need for well‐designed, long‐term clinical trials on orbital implants.
Although the first descriptions of integrated implants appeared in the 1940s, the introduction of natural hydroxyapatite in the 1980s marked a rise in their popularity. The main advantage seen in this material was the increased mobility it conferred due to the placement of a peg that fixed the prothesis to the eye socket (Perry 1990). However, complications with the coupled implants began to emerge almost immediately, and pegs have since fallen out of use. Thus, the main motivation for integrated implants has ceased to exist.
Since then, various new implants have become available, making it very important to determine if integrated implants are superior to and safer than non‐integrated ones. Available literature supports the superiority of integrated implants, but most of these studies are from the 1990s and usually used retrospective, non‐randomised or case study methodology (Ashworth 1996; Blázquez 1998). Many of them do not compare implants of different categories. However, a recent case series proportional meta‐analysis showed lower chance of exposure with the use of PP implants compared to bioceramic implant for anophthalmic socket reconstruction (Schellini 2016).
Although there were few randomised studies in general, the greatest difficulty was in finding studies that dealt with non‐integrated implants, especially those involving PMMA or silicone implants.
None of the three studies included in this review is categorical about the superiority of the tested implants. Thus, doubt about the superiority of integrated implants persists. And a tangible point that favours non‐integrated implants is the dramatic price difference, as Shome 2010 highlighted that the porous polyethylene (integrated) implant costs approximately USD 300.00 compared USD 2.00 for the PMMA (non‐integrated) implant.
The most important contribution of our review is our finding that non‐integrated implants may be superior with regard to exposure or extrusion implants. Another study (not included in this review) also comments on higher exposures rates in the porous implants (Custer 2003).
However, given the quality of the included studies, we believe it is very important to have more studies that employ appropriate randomisation and masking in order to definitively determine which kind(s) of implants are better.
Authors' conclusions
Implications for practice.
Current very low‐certainty evidence from three small published randomised controlled trials does not provide sufficient evidence to assess the effect of integrated and non‐integrated material orbital implants for treating anophthalmic sockets. This review underlines the need to conduct further well‐designed trials in this field.
Implications for research.
This review highlights the need for continued research into the selection of the appropriate implants to treat anophthalmic sockets. The low statistical power of available data provides strong justification for designing new randomised trials that can fill the gap in existing knowledge. These trials in particular should compare integrated versus non‐integrated orbital implants with the same technique (i.e. enucleation or evisceration) for the treatment of anophthalmic socket with a minimum follow‐up of one year and larger sample size to evaluate proportion of successful procedures and adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 16 June 2021 | Feedback has been incorporated | The Plain language summary has been amended in response to feedback about the use of the wording 'artificial eyes'. |
History
Protocol first published: Issue 1, 2013 Review first published: Issue 11, 2016
Acknowledgements
We acknowledge the support of Anupa Shah, Jennifer Evans and Iris Gordon from Cochrane Eyes and Vision (CEV) in the preparation of the review. We thank Daniel Ezra, Catey Bunce, Sajid Ataullah and Alicia Galindo Ferreiro for their comments on the protocol, review or both. Regina El Dib received a Brazilian Research Council (CNPq) scholarship (CNPq 310953/2015‐4).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Anophthalmos #2 anophthalm* #3 MeSH descriptor Eye Enucleation #4 MeSH descriptor Eye Evisceration #5 MeSH descriptor Orbit Evisceration #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Orbital Implants #8 MeSH descriptor Eye, Artificial #9 MeSH descriptor Prostheses and Implants #10 ((eye* or ocular or socket or orbit* or integrated or nonintegrated or non‐integrated or non integrated) near/4 implant*) #11 ((eye* or ocular or socket or orbit* or integrated or nonintegrated or non‐integrated or non integrated) near/4 reconstruct*) #12 MeSH descriptor Hydroxyapatites #13 MeSH descriptor Durapatite #14 durapatite* or hydroxylapatite* #15 BioEye or Bio‐Eye #16 ((integrated or nonintegrated or non‐integrated or non integrated) near/2 sphere*) #17 Interpore‐500 or Interpore 500 or Interpore500 #18 Interpore‐200 or Interpore 200 or Interpore200 #19 Alveograf or Calcitite or Ossopan or Osteogen or Periograf or Osprovit #20 MeSH descriptor Polymethyl Methacrylate #21 MeSH descriptor Methacrylates #22 Polyethylene or Polythene or Medpor or LDPE or HDPE or Polymethyl Methacrylate or Polymethylmetacrylate or PMMA or Acron or Implast #23 Methyl Acrylic Plastic or Kallocryl or Lucite or Palacos or Plexiglas or Plexiglass or Superacryl or Palavit or Perspex or Silicone or silicones #24 MeSH descriptor Bone Cements #25 bone cement near/3 (CMW or Surgical Simplex or Acrylic Bone) #26 material* near/3 (integrated or nonintegrated or non‐integrated or non integrated or solid or porous or wrapped) #27 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 #6 AND #27
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. Anophthalmos/ 14. anophthalm$.tw. 15. Eye Enucleation/ 16. Eye Evisceration/ 17. Orbit Evisceration/ 18. or/13‐17 19. Orbital Implants/ 20. Eye, Artificial/ 21. "Prostheses and Implants"/ 22. ((eye$ or ocular or socket or orbit$ or integrated or nonintegrated or non‐integrated or non integrated) adj4 implant$).tw. 23. ((eye$ or ocular or socket or orbit$ or integrated or nonintegrated or non‐integrated or non integrated) adj4 reconstruct$).tw. 24. Hydroxyapatites/ 25. Durapatite/ 26. (durapatite$ or hydroxylapatite$).tw. 27. (BioEye or Bio‐Eye).tw. 28. ((integrated or nonintegrated or non‐integrated or non integrated) adj2 sphere$).tw. 29. (Interpore‐500 or Interpore 500 or Interpore500).tw. 30. (Interpore‐200 or Interpore 200 or Interpore200).tw. 31. (Alveograf or Calcitite or Ossopan or Osteogen or Periograf or Osprovit).tw. 32. Polymethyl Methacrylate/ 33. Methacrylates/ 34. (Polyethylene or Polythene or Medpor or LDPE or HDPE or Polymethyl Methacrylate or Polymethylmetacrylate or PMMA or Acron or Implast).tw. 35. (Methyl Acrylic Plastic or Kallocryl or Lucite or Palacos or Plexiglas or Plexiglass or Superacryl or Palavit or Perspex or Silicone or silicones).tw. 36. Bone Cements/ 37. (bone cement adj3 (CMW or Surgical Simplex or Acrylic Bone)).tw. 38. (material$ adj3 (integrated or nonintegrated or non‐integrated or non integrated or solid or porous or wrapped)).tw. 39. or/19‐38 40. 18 and 39 41. 12 and 40
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. anophthalmia/ 34. anophthalm$.tw. 35. enucleation/ 36. or/33‐35 37. orbit implant/ 38. visual prosthesis/ 39. ((eye$ or ocular or socket or orbit$ or integrated or nonintegrated or non‐integrated or non integrated) adj4 implant$).tw. 40. ((eye$ or ocular or socket or orbit$ or integrated or nonintegrated or non‐integrated or non integrated) adj4 reconstruct$).tw. 41. hydroxyapatite/ 42. (durapatite$ or hydroxylapatite$).tw. 43. (BioEye or Bio‐Eye).tw. 44. ((integrated or nonintegrated or non‐integrated or non integrated) adj2 sphere$).tw. 45. (Interpore‐500 or Interpore 500 or Interpore500).tw. 46. (Interpore‐200 or Interpore 200 or Interpore200).tw. 47. (Alveograf or Calcitite or Ossopan or Osteogen or Periograf or Osprovit).tw. 48. "poly(methyl methacrylate)"/ 49. (Polyethylene or Polythene or Medpor or LDPE or HDPE or Polymethyl Methacrylate or Polymethylmetacrylate or PMMA or Acron or Implast).tw. 50. (Methyl Acrylic Plastic or Kallocryl or Lucite or Palacos or Plexiglas or Plexiglass or Superacryl or Palavit or Perspex or Silicone or silicones).tw. 51. bone cement/ 52.(bone cement adj3 (CMW or Surgical Simplex or Acrylic Bone)).tw. 53. (material$ adj3 (integrated or nonintegrated or non‐integrated or non integrated or solid or porous or wrapped)).tw. 54. or/37‐53 55. 36 and 54 56. 32 and 55
Appendix 4. LILACS search strategy
anophthalmi$ or enucleation or evisceration and eye$ or ocular or socket or orbit$ or integrated or nonintegrated or non‐integrated or non integrated and implant$ or reconstruct$
Appendix 5. ISRCTN search strategy
Anophthalmia or Anophthalmic
Appendix 6. ClinicalTrials.gov search strategy
Anophthalmia or Anophthalmic
Appendix 7. ICTRP search strategy
Anophthalmia or Anophthalmic
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Colen 2000.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Multicentre or single‐centre: multicentre (two centres) Setting: the Rotterdam Eye Hospital, Rotterdam, and in the University Medical Center, Utrecht, Netherlands Period: February 1997 to May 1999 Sample size: a difference of 0.10 or more between the two types of implants would be clinically relevant Follow up: 3 months |
|
| Participants | 34 patients eligible for the study; 30 ana lysed Mean age: 64.0 years for hydroxyapatite group and 57.8 years for acrylic group Sex: 7 men and 7 women for hydroxyapatite group; 9 men and 7 women in acrylic group Inclusion criteria: patients with intraocular melanoma, without extrascleral extension on ultrasound examination Exclusion criteria: patients with a visual acuity of less than 6/12 (20/40) in the remaining eye and patients with a history of abnormal eye motility, strabismus, any eye surgery, and chronic inflammatory ocular or orbital disorders |
|
| Interventions | Hydroxiapatite (integrated group) (n = 14) versus acrylic (non‐integrated group) (n = 16) There was no peg device. All participants underwent the enucleation technique (scleral‐covered spherical orbital implant). |
|
| Outcomes | Motility; saccadic gain (i.e. dividing the saccadic amplitude by the target amplitude) and saccadic symmetry (i.e. artificial eye amplitude divided by health eye amplitude) | |
| Notes | 21 healthy volunteers served as control participants. The reason for surgery was intraocular melanoma. This study was supported by Rotterdam Eye Hospital Research Fund, Rotterdam, Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Selection by opening an envelope with a previously randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were unaware of the type of enucleation implant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators who recorded the eye movements were unaware of the type of enucleation implant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11.77% dropouts |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | No evidence; no report of conflict of interest. |
Shome 2010.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Multicentre or single‐centre: not reported Setting: Hyderabad, India Period: July 2004 to June 2007 Sample size: to ascertain a 30% difference with 80% power and 5% error in between the 3 groups, inclusion of a minimum of 39 patients in each group was necessary. Follow up: mean follow‐up in months per group: PMMA traditional, 16.4; PMMA myoconjunctival, 17.3 and; porous polyethylene, 15.6. |
|
| Participants | 150 participants Mean age: not reported Sex: not reported Inclusion criteria: not reported Exclusion criteria: participants who had undergone prior radiotherapy and periocular chemotherapy |
|
| Interventions | Integrated porous polyethylene (PP) (integrated group) (n = 50) versus PMMA traditional (non‐integrated group) (n = 50) versus PMMA myoconjunctival (non‐integrated group) (n = 50) There was no peg device. All patients underwent enucleation technique. In the PP group, the enucleation was performed using the scleral cap technique. In the PMMA traditional group, the enucleation was done with muscle imbrication. In the PMMA myoconjunctival group, the enucleation was done with myoconjunctival technique, which is an alternative to muscle imbrication. |
|
| Outcomes | The primary outcome measured was to compare and evaluate implant and prosthesis movement among these 3 groups of patients. The secondary outcomes were implant displacement and exposure. | |
| Notes | There was no report of the reasons for surgery. This study was supported by Hyderabad Eye Institute, Hyderabad, India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked observer |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence. There was no proprietary or commercial interest in any materials discussed in this article. |
Tari 2009.
| Study characteristics | ||
| Methods | Study design: quasi‐randomised controlled trial Multicentre or single‐centre: single‐centre Setting: Tehran University Eye Research Center, Tehran, Iran Period: January 2006 to June 2007 Sample size: not reported Follow up: 11.56 months for non‐integrated group and 13.16 months for integrated group |
|
| Participants | 100 patients. Mean age: 42.32 years for evisceration group and 39.56 years for enucleation group Sex: 15 women and 35 men for evisceration group; and 9 women and 41 men for evaluation group Inclusion criteria: not reported Exclusion criteria: significant preoperative motility abnormalities, cases of severe phthisis, and cases that were not followed up for at least 4 months |
|
| Interventions | Enucleation with HA (integrated group) (n = 50) versus evisceration plus quadrisection of sclera with alloplastic implantation (non‐integrated group) (n = 50) There was no peg device. Enucleation via the traditional technique and evisceration plus scleral quadrisection |
|
| Outcomes | The primary outcome was the presence or absence of exposure or extrusion and deep superior sulcus deformity, and the secondary outcome was implant motility measured by detecting the amount of overlying conjunctival movement. | |
| Notes | Reasons for surgery were: painful blinded eye, cosmetic unacceptability, acute trauma, and endophthalmitis. The reason for the surgery was the same for both groups. However, all the trauma patients had enucleation and all the endophthalmitis patients had evisceration. There was no financial support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly divided in 2 groups for alternate surgical plans including enucleation with HA implantation or evisceration plus quadrisection of sclera with alloplastic implantation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence. The authors declare no financial support or relationships that may pose a conflict of interest. |
HA: hydroxyapatite; PMMA: polymethylmethacrylate; PP: porous polyethylene.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agahan 2004 | RCT, but evaluated two types of PMMA (non‐integrated) implants |
| Alwitry 2007 | Case series |
| Arat 2003 | Retrospective study |
| Ashworth 1996 | Case series |
| Ashworth 1998 | Case series |
| Blázquez 1998 | Non‐randomised trial |
| Chen 1996 | Case series |
| Chen 2000 | Case series |
| Chen 2006 | Retrospective study |
| Choi 2005 | Retrospective study |
| Chuah 2004 | Retrospective study |
| Clauser 2004 | Case series |
| Custer 1999 | Non‐randomised clinical trial |
| Custer 2006 | Case series |
| De Potter 1994 | Case series |
| Downes 1992 | Case series |
| Dutton 1991 | Case series |
| Filatova 2008 | Case series. |
| Genevois 2004 | Retrospective study |
| Georgiadis 1998a | Case series |
| Georgiadis 1998b | Case series |
| Georgiadis 1999 | Case series |
| González‐Candial 2007 | Retrospective study |
| Guillinta 2003 | Case series |
| Gupta 2007 | Another clinical question (wrapping material for hydroxyapatite implants) |
| Hashimoto 1994 | Cross‐sectional study |
| Hoyama 2000 | Retrospective study |
| Iordanidou 2004 | Case series |
| Jordan 2004 | Retrospective study |
| Jordan 2010 | Retrospective study |
| Kamal 2012 | Retrospective study |
| Karesh 1994 | Case series |
| Kassaee 2006 | Case series |
| Kim 2004 | Case series |
| Klapper 2003 | Retrospective study |
| Krastinova 2001 | Retrospective study |
| Lee 2002 | Retrospective study |
| Li 2001 | Retrospective study |
| Li 2010 | RCT, but evaluated only integrated orbital implants |
| Liang 2006 | Retrospective study |
| Liu 2005 | Non‐randomised clinical trial |
| Liu 2012 | Retrospective study |
| Long 2003 | Another clinical question (wrapped and unwrapped hydroxyapatite orbital) |
| Lopes 2011 | Retrospective study |
| Lucci 2007 | Another clinical question (porous polyethylene either spherical or quad‐motility orbital implant) |
| Lukáts 2002 | Case series |
| Lyle 2007 | Retrospective study |
| Manteiga 2006 | Case series |
| Massry 1995 | Retrospective study |
| Massry 2001 | Retrospective study |
| Moura 2007 | Case series. |
| Naik 2007 | Another clinical question (Medpor and Medpor‐Plus orbital implants) |
| Narikawa 2011 | Retrospective study |
| Nikolaenko 2006a | Case series |
| Nikolaenko 2006b | Case series |
| Ortiz 2012 | Cross‐sectional |
| Pan 2003 | Retrospective study |
| Perry 2002 | Case series |
| Perry 2004 | Retrospective study |
| Saeed 2000 | Case series |
| Schellini 2000 | Retrospective study |
| Schellini 2007 | Retrospective study |
| Sebastiá 2000 | Non‐randomised clinical trial |
| Shields 19993 | Case series |
| Shkromida 1989 | Case series |
| Sires 1998 | Case series |
| Song 2002 | Case series |
| Soto 2003 | Cross‐sectional study |
| Stephen 1999 | Case series |
| Tabatabaee 2011 | Retrospective study |
| Van Acker 2001 | Case series |
| Villarroel 2001 | Case series |
| Vittorino 2007 | Retrospective study |
| Wang 2009 | Retrospective study |
| Woog 2004 | Case series |
| Yoon 2008 | Retrospective study |
PMMA: polymethylmethacrylate; RCT: randomised controlled trial.
Differences between protocol and review
None.
Contributions of authors
Protocol Conceiving the protocol: Silvana Schellini (SS) and Regina El Dib (RED) Co‐ordinating the protocol: RED Writing drafts of the protocol: SS and RED Responding to peer review comments: SS and RED Responding to comments from the editorial base: SS and RED Person responsible for reading and checking the protocol before submission: SS and RED
Review Conceiving the review: Silvana Schellini (SS), Regina El Dib (RED) and Eliane C Jorge (ECJ) Co‐ordinating the review: RED Undertaking manual searches: ECJ and RED Screening search results: Leandro Ramos e Silva (LRS), RED and ECJ Organizing retrieval of papers: LRS and Joyce Farah (JF) Screening retrieved papers against inclusion criteria: SS, RED and ECJ Appraising quality of papers: SS, RED and ECJ Abstracting data from papers: Yuqing Zhang (YZ) and JF Writing to authors of papers for additional information: YZ and LRS Obtaining additional data about papers: ECJ and LRS Obtaining and screening data on unpublished studies: LRS and JF Data management for the review: RED and ECJ Data entry: RED and ECJ Statistical data analysis: RED Interpretation of data: SS, RED and ECJ Statistical inferences: SS, RED and ECJ Writing the review: SS, ECJ, LRS and RED Guarantor for the review (one author): RED and ECJ Person responsible for reading and checking review before submission: SS, RED and ECJ
Sources of support
Internal sources
No source, Brazil
External sources
No source, Brazil
Declarations of interest
Silvana Schellini: none known. Regina El Dib: none known. Leandro Ramos e Silva: none known. Joyce Farah: none known. Yuqing Zhang: none known. Eliane C Jorge: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Colen 2000 {published data only}
- Colen TP, Paridaens DA, Lemij HG, Mourits MP, Van Den Bosch WA. Comparison of artificial eye amplitudes with acrylic and hydroxyapatite spherical enucleation implants. Ophthalmology 2000;107(10):1889-94. [DOI] [PubMed] [Google Scholar]
Shome 2010 {published data only}
- Shome D, Honavar SG, Raizada K, Raizada D. Implant and prosthesis movement after enucleation: a randomized controlled trial. Ophthalmology 2010;117(8):1638-44. [DOI] [PubMed] [Google Scholar]
Tari 2009 {published data only}
- Tari AS, Malihi M, Kasaee A, Tabatabaie SZ, Hamzedust K, Musavi MF, et al. Enucleation with hydroxyapatite implantation versus evisceration plus scleral quadrisection and alloplastic implantation. Ophthalmic Plastic and Reconstructive Surgery 2009;25(2):130-3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agahan 2004 {published data only}
- Agahan AL, Tan AD. Use of hollow polymethylmethacrylate as an orbital implant. Philippine Journal of Ophthalmology 2004;29(1):21-5. [Google Scholar]
Alwitry 2007 {published data only}
- Alwitry A, West S, King J, Foss AJ, Abercrombie LC. Long-term follow-up of porous polyethylene spherical implants after enucleation and evisceration. Ophthalmic Plastic and Reconstructive Surgery 2007;23(1):11-5. [DOI] [PubMed] [Google Scholar]
Arat 2003 {published data only}
- Arat YO, Shetlar DJ, Boniuk M. Bovine pericardium versus homologous sclera as a wrapping for hydroxyapatite orbital implants. Ophthalmic Plastic and Reconstructive Surgery 2003;19(3):189-93. [DOI] [PubMed] [Google Scholar]
Ashworth 1996 {published data only}
- Ashworth JL, Rhatigan M, Sampath R, Brammar R, Sunderland S, Leatherbarrow B. The hydroxyapatite orbital implant: a prospective study. Eye 1996;10(Pt 1):29-37. [DOI] [PubMed] [Google Scholar]
Ashworth 1998 {published data only}
- Ashworth J, Brammar R, Inkster C, Leatherbarrow B. A study of the hydroxyapatite orbital implant drilling procedure. Eye 1998;12(1):37-42. [DOI] [PubMed] [Google Scholar]
Blázquez 1998 {published data only}
- Blázquez GP, Santos RG, Diaz LA, Bravo MS, Acosta, JLO, Pérez JR. HAP-200 porous hydroxyapatite used as a biocompatible spherical implant in surgical anophthalmos [Hidroxiapatita Porosa HAP-200 como bioimplante esférico integrado en el anoftalmos quirúrgico]. Revista Cubana de Oftalmología 1998;11(1):5-13. [Google Scholar]
Chen 1996 {published data only}
- Chen Q, Yi J, Liao H. Clinical application of a new mobile integrated orbital implant. Zhonghua Yanke Zazhi [Chinese Journal of Ophthalmology] 1996;32(3):182-4. [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen X, Gao D, Yin S. Application of polyester fiber heart patches to secondary intraorbital implantation of hydroxyapatite spheres. Zhonggua Zhengxing Waike Zazhi [Chinese Journal of Plastic Surgery] 2000;16(5):270-2. [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen YH, Cui HG. High density porous polyethylene material (Medpor) as an unwrapped orbital implant. Journal of Zhejiang University Science B 2006;7(8):679-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2005 {published data only}
- Choi BH, Lee SH, Chung WS. Correction of superior sulcus deformity and enophthalmos with porous high-density polyethylene sheet in anophthalmic patients. Korean Journal of Ophthalmology 2005;19(3):168-73. [DOI] [PubMed] [Google Scholar]
Chuah 2004 {published data only}
- Chuah CT, Chee SP, Fong KS, Por YM, Choo CT, Luu C, et al. Integrated hydroxyapatite implant and non-integrated implants in enucleated Asian patients. Annals, Academy of Medicine, Singapore 2004;33(4):477-83. [PubMed] [Google Scholar]
Clauser 2004 {published data only}
- Clauser L, Sarti E, Dallera V, Galiè M. Integrated reconstructive strategies for treating the anophthalmic orbit. Journal of Cranio-Maxillofacial Surgery 2004;32(5):279-90. [DOI] [PubMed] [Google Scholar]
Custer 1999 {published data only}
- Custer PL, Trinkaus KM, Fornoff J. Comparative motility of hydroxyapatite and alloplastic enucleation implants. Ophthalmology 1999;106(3):513-6. [DOI] [PubMed] [Google Scholar]
Custer 2006 {published data only}
- Custer PL, McCaffery S. Complications of sclera-covered enucleation implants. Ophthalmic Plastic and Reconstructive Surgery 2006;22(4):269-73. [DOI] [PubMed] [Google Scholar]
De Potter 1994 {published data only}
- De Potter P, Shields CL, Shields JA, Singh AD. Use of the hydroxyapatite ocular implant in the pediatric population. Archives of Ophthalmology 1994;112(2):208-12. [DOI] [PubMed] [Google Scholar]
Downes 1992 {published data only}
- Downes R, Lavin M, Collin R. Hydrophilic expanders for the congenital anophthalmic socket. Advances in Ophthalmic Plastic and Reconstructive Surgery 1992;9:57-61. [PubMed] [Google Scholar]
Dutton 1991 {published data only}
- Dutton JJ. Coralline hydroxyapatite as an ocular implant. Ophthalmology 1991;98(3):370-7. [DOI] [PubMed] [Google Scholar]
Filatova 2008 {published data only}
- Filatova IA, Kataev MG, Harb Ali H. Exposure of orbital implants: causes and treatment. Vestnik Oftalmologii 2008;124(3):36-41. [PubMed] [Google Scholar]
Genevois 2004 {published data only}
- Genevois O, Millet P, Retout A, Quintyn JC. Comparison after 10 years of two 100-patient cohorts operated on for eviscerations or enucleations. European Journal of Ophthalmology 2004;14(5):363-8. [DOI] [PubMed] [Google Scholar]
Georgiadis 1998a {published data only}
- Georgiadis NS, Terzidou CD, Dimitriadis AS. Restoration of the anophthalmic socket with secondary implantation of a coralline hydroxyapatite sphere. Ophthalmic Surgery and Lasers 1998;29(10):808-14. [PubMed] [Google Scholar]
Georgiadis 1998b {published data only}
- Georgiadis NS, Terzidou CD, Dimitriadis AS. Restoration of the anophthalmic socket with secondary implantation of a coralline hydroxyapatite sphere. Ophthalmic Surgery and Lasers 1998;29(10):808-14. [PubMed] [Google Scholar]
Georgiadis 1999 {published data only}
- Jordan DR, Brownstein S, Faraji H. Clinicopathologic analysis of 15 explanted hydroxyapatite implants. Ophthalmic Plastic and Reconstructive Surgery 2004;20(4):285-90. [DOI] [PubMed] [Google Scholar]
González‐Candial 2007 {published data only}
- González-Candial M, Umaña MA, Galvez C, Medel R, Ayala E. Comparison between motility of biointegratable and silicone orbital implants. American Journal of Ophthalmology 2007;143(4):711-2. [DOI] [PubMed] [Google Scholar]
Guillinta 2003 {published data only}
- Guillinta P, Vasani SN, Granet DB, Kikkawa DO. Prosthetic motility in pegged versus unpegged integrated porous orbital implants. Ophthalmic Plastic and Reconstructive Surgery 2003;19(2):119-22. [DOI] [PubMed] [Google Scholar]
Gupta 2007 {published data only}
- Gupta M, Lyon F, Singh AD, Rundle PA, Rennie IG. Bovine pericardium (Tutopatch) wrap for hydroxyapatite implants. Eye 2007;21(4):476-9. [DOI] [PubMed] [Google Scholar]
Hashimoto 1994 {published data only}
- Hashimoto M, Rodrigues AC, Silva MRBM, Schellini SA. Anophtalmic socket; cases, surgery and complications [Cavidade anoftálmica; causas, reconstruçäo e complicações]. Revista Brasileira de Oftalmologia 1994;56(6):73-8. [Google Scholar]
Hoyama 2000 {published data only}
- Hoyama E, Schellini SA, Ferreira VL, Rossa R, Padovani CR. Porous polyethylene sphere used in anophthalmic cavity [Uso de esferas de polietileno poroso em cavidade anoftálmica]. Revista Brasileira de Oftalmologia 2000;59(1):40-4. [Google Scholar]
Iordanidou 2004 {published data only}
- Iordanidou V, De Potter P. Porous polyethylene orbital implant in the pediatric population. American Journal of Ophthalmology 2004;138(3):425-9. [DOI] [PubMed] [Google Scholar]
Jordan 2004 {published data only}
- Jordan DR, Brownstein S, Faraji H. Clinicopathologic analysis of 15 explanted hydroxyapatite implants. Ophthalmic Plastic and Reconstructive Surgery 2004;20(4):285-90. [DOI] [PubMed] [Google Scholar]
Jordan 2010 {published data only}
- Jordan DR, Klapper SR, Gilberg SM, Dutton JJ, Wong A, Mawn L. The bioceramic implant: evaluation of implant exposures in 419 implants. Ophthalmic Plastic and Reconstructive Surgery 2010;26(2):80-2. [DOI] [PubMed] [Google Scholar]
Kamal 2012 {published data only}
- Kamal S, Bodh SA, Goel R, Kumar S. Long-term surgical outcomes of porous polyethylene orbital implants: a review of 314 cases. British Journal of Ophthalmology 2012;96(8):1153. [DOI] [PubMed] [Google Scholar]
Karesh 1994 {published data only}
- Karesh JW, Dresner SC. High-density porous polyethylene (Medpor) as a successful anophthalmic socket implant. Ophthalmology 1994;101(10):1688-95. [PubMed] [Google Scholar]
Kassaee 2006 {published data only}
- Kassaee A, Kashkouli MB, Panjtanpanah M, Sadeghi A, Tabatabaee Z. Mersilene mesh versus sclera in wrapping hydroxyapatite orbital implants. Ophthalmic Plastic and Reconstructive Surgery 2006;22(1):41-4. [DOI] [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim JH, Khwarg SI, Choung HK, Yu YS. Management of porous polyethylene implant exposure in patients with retinoblastoma following enucleation. Ophthalmic Surgery, Lasers and Imaging 2004;35(6):446-52. [PubMed] [Google Scholar]
Klapper 2003 {published data only}
- Klapper SR, Jordan DR, Ells A, Grahovac S. Hydroxyapatite orbital implant vascularization assessed by magnetic resonance imaging. Ophthalmic Plastic and Reconstructive Surgery 2003 ;19(1):46-52. [DOI] [PubMed] [Google Scholar]
Krastinova 2001 {published data only}
- Krastinova D, Kelly MB, Mihaylova M. Surgical management of the anophthalmic orbit, part 1: congenital. Plastic and Reconstructive Surgery 2001;108(4):817-26. [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee SY, Jang JW, Lew H, Kim SJ, Kim HY. Complications in motility peg placement for hydroxyapatite orbital implant in anophthalmic socket. Japanese Journal of Ophthalmology 2002;46(1):103-7. [DOI] [PubMed] [Google Scholar]
Li 2001 {published data only}
- Li T, Shen J, Duffy MT. Exposure rates of wrapped and unwrapped orbital implants following enucleation. Ophthalmic Plastic and Reconstructive Surgery 2001;17(6):431-5. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li HY, Yang QC, Yan QC, Qiu H, Zhao Y, Zhang JS. Clinical comparison of two kinds of orbital implants and discussion of surgical technique. International Journal of Ophthalmology 2010;10(3):558-60. [Google Scholar]
Liang 2006 {published data only}
- Liang T, Zhao GQ, Meng XX, Zhang LY. Clinical analysis of hydroxyapatite orbital implantation after ocular trauma in 211 cases. Zhonghua Chuangshang Zazhi [Chinese Journal of Traumatology] 2006;9(5):282-7. [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu D. A comparison of implant extrusion rates and postoperative pain after evisceration with immediate or delayed implants and after enucleation with implants. Transactions of the American Ophthalmological Society 2005;103:568-91. [PMC free article] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu XF, Ding YN, Huo J. Clinical observation of two different kinds of hydroxyapatite orbital implantation surgeries. International Journal of Ophthalmology 2012;12(10):2014-5. [Google Scholar]
Long 2003 {published data only}
- Long JA, Tann TM, Bearden WH, Callahan MA. Enucleation: is wrapping the implant necessary for optimal motility? CN-00488763. Ophthalmic Plastic and Reconstructive Surgery 2003;19(3):194-7. [DOI] [PubMed] [Google Scholar]
Lopes 2011 {published data only}
- Lopes N, Castela G, Andres R, Lisboa M, Castela R, Loureiro R. Reconstruction of anophthalmic socket. Journal Francais d'Ophtalmologie 2011;34(9):608-14. [DOI] [PubMed] [Google Scholar]
Lucci 2007 {published data only}
- Lucci LM, Höfling-Lima AL, Erwenne CM, Toledo Cassano EM. Artificial eye amplitudes and characteristics in enucleated socket with porous polyethylene spherical and quad-motility implant [Amplitude de movimento e características das próteses oculares em cavidadesenucleadas com implante de polietileno poroso esférico e “quad-motility”]. Arquivos Brasileiros de Oftalmologia 2007;70(5):831-8. [DOI] [PubMed] [Google Scholar]
Lukáts 2002 {published data only}
- Lukáts O. Contracted anophthalmic socket repair. Orbit 2002;21(2):125-30. [PubMed] [Google Scholar]
Lyle 2007 {published data only}
- Lyle CE, Wilson MW, Li CS, Kaste SC. Comparison of orbital volumes in enucleated patients with unilateral retinoblastoma: hydroxyapatite implants versus silicone implants. Ophthalmic Plastic and Reconstructive Surgery 2007;23(5):393-6. [DOI] [PubMed] [Google Scholar]
Manteiga 2006 {published data only}
- Manteiga MB, Caballero FJ, Montesino-lvarez, Isis, Soto RG. Evisceration with double scleral coverage [Evisceración con doble cobertura escleral]. Revista Cubana de Investigaciones Biomédicas 2006;25(2). [ISSN 1561-3011] [Google Scholar]
Massry 1995 {published data only}
- Massry GG, Holds JB. Coralline hydroxyapatite spheres as secondary orbital implants in anophthalmos. Ophthalmology 1995;102(1):161-6. [DOI] [PubMed] [Google Scholar]
Massry 2001 {published data only}
- Massry GG, Holds JB. Evisceration with scleral modification. Ophthalmic Plastic and Reconstructive Surgery 2001;17(1):42-7. [DOI] [PubMed] [Google Scholar]
Moura 2007 {published data only}
- Moura Eda M, Vieira GS. Use of Medpor spherical implant: analysis of 61 orbital surgeries [Uso do implante esférico Medpor™: análise de 61 cirurgias orbitárias]. Arquivos Brasileiros de Oftalmologia 2007;70(1):7-12. [DOI] [PubMed] [Google Scholar]
Naik 2007 {published data only}
- Naik MN, Murthy RK, Honavar SG. Comparison of vascularization of Medpor and Medpor-Plus orbital implants: a prospective, randomized study. Ophthalmic Plastic and Reconstructive Surgery 2007;23(6):463-7. [DOI] [PubMed] [Google Scholar]
Narikawa 2011 {published data only}
- Narikawa S, Schellini SA, Padovani CR. Dermofat graft in secondary anophthalmic socket: a retrospective study and literature review [Enxerto dermoadiposo em cavidades anoftálmicas secundárias: estudo retrospectivo e revisão da literatura]. Revista Brasileira de Oftalmologia 2011;70(6):411-5. [Google Scholar]
Nikolaenko 2006a {published data only}
- Nikolaenko VP, Astakhov IuS. Implantation of porous polytetrafluoroethylene orbital inserts: complications. Communication 2. Vestnik Oftalmologii 2006;122(2):21-4. [PubMed] [Google Scholar]
Nikolaenko 2006b {published data only}
- Nikolaenko VP, Astakhov IuS. Implantation of porous polytetrafluoroethylene orbital inserts: techniques and outcomes. Communication 1. Vestnik Oftalmologii 2006;122(2):18-21. [PubMed] [Google Scholar]
Ortiz 2012 {published data only}
- Silveira MO, García MJG, Arias JCS, Díaz MG, Torres HO. Surgical-prosthetic rehabilitation of patients with atypical anophthalmic cavities [Rehabilitación quirúrgico-protésica de pacientes con cavidades anoftálmicas atípicas]. Medisan 2012;16(1):75-80. [Google Scholar]
Pan 2003 {published data only}
- Pan MH, Wu YW, Yen RF, Tzen KY, Liao SL, Kao CH. Different fibrovascularization rate between coralline hydroxyapatite and high density porous polyethylene (Medpore) measured by 99mTc-MDP bone scintigraphy 6 months after intraorbital implantation. Nuclear Medicine Communications 2003;24(12):1237-41. [DOI] [PubMed] [Google Scholar]
Perry 2002 {published data only}
- Perry JD, Goldberg RA, McCann JD, Shorr N, Engstrom R, Tong J. Bovine hydroxyapatite orbital implant: a preliminary report. Ophthalmic Plastic and Reconstructive Surgery 2002;18(4):268-74. [DOI] [PubMed] [Google Scholar]
Perry 2004 {published data only}
- Perry JD, Tam RC. Safety of unwrapped spherical orbital implants. Ophthalmic Plastic and Reconstructive Surgery 2004;20(4):281-4. [DOI] [PubMed] [Google Scholar]
Saeed 2000 {published data only}
- Saeed M, Monis M, Cheema AM, Mughal MA. Surgical treatment of anophthalmic socket - an experience with 42 intra orbital implants. Journal of the College of Physicians and Surgeons Pakistan 2000;10(5):175-8. [Google Scholar]
Schellini 2000 {published data only}
- Schellini SA, Hoyama E, Padovani CR, Ferreira VR, Roça R. Complications with nonintegrated and integrated spheres in anophthalmic socket reconstruction [Complicações com uso de esferas não integráveis e integráveis na reconstrução da cavidade anoftálmica]. Arquivos Brasileiros de Oftalmologia 2011;63(3):175-8. [Google Scholar]
Schellini 2007 {published data only}
- SchelliniI SA, IchidaII FK, PadovaniII CR. Anophthalmic cavity and implant extrusion [Extrusão dos implantes em portadores de cavidade anoftálmica]. Arquivos Brasileiros de Oftalmologia 2007;70(5):752-5. [DOI] [PubMed] [Google Scholar]
Sebastiá 2000 {published data only}
- Sebastiá R, Lessa S, Flores E. Reconstruction of the unophthalmic socket with spherical implant covered by fascia lata autograft [Reconstrução da cavidade anoftálmica com implante esférico revestido de enxerto autólogo de fascia lata]. Revista Brasileira de Oftalmologia 2000;59(2):132-4. [Google Scholar]
Shields 19993 {published data only}
- Shields CL, Shields JA, De Potter P, Singh AD. Lack of complications of the hydroxyapatite orbital implant in 250 consecutive cases. Transactions of the American Ophthalmological Society 1993;901:177-89. [PMC free article] [PubMed] [Google Scholar]
Shkromida 1989 {published data only}
- Shkromida MI. The results of cosmetic prosthesis in patients in relation to the method of formation of a moveable base for the prosthesis following removal of the eyeball. Oftalmologicheskii Zhurnal 1989;6:357-9. [PubMed] [Google Scholar]
Sires 1998 {published data only}
- Sires BS, Holds JB, Archer CR. Postimplantation density changes in coralline hydroxyapatite orbital implants. Ophthalmic Plastic and Reconstructive Surgery 1998;14(5):318-22. [DOI] [PubMed] [Google Scholar]
Song 2002 {published data only}
- Song D, Gao F, Su S, Sun GZ. Effect research of the hydroxyapatite orbital implant on orbital development in children. Chinese Journal of Clinical Rehabilitation 2002;6(6):918-9. [Google Scholar]
Soto 2003 {published data only}
- Soto MH, Márquez IF, Centelles IA, Cabrera CG. Use of dermis fat graft in patients with retraction of the anophthalmic cavity [Utilización de injerto dermo-graso en pacientes con retracción de la cavidad anoftálmica]. Revista Cubana de Oftalmología 2003;16(2):1. [Google Scholar]
Stephen 1999 {published data only}
- Stephen BE. The glass spherical hollow orbital implant: a prospective study. Ceylon Medical Journal 1999;44(2):74-80. [PubMed] [Google Scholar]
Tabatabaee 2011 {published data only}
- Tabatabaee Z, Mazloumi M, Rajabi MT, Khalilzadeh O, Kassaee A, Moghimi S, et al. Comparison of the exposure rate of wrapped hydroxyapatite (Bio-Eye) versus unwrapped porous polyethylene (Medpor) orbital implants in enucleated patients. Ophthalmic Plastic and Reconstructive Surgery 2011;27(2):114-8. [DOI] [PubMed] [Google Scholar]
Van Acker 2001 {published data only}
- Van Acker E, De Potter P. Porous polyethylene (Medpor) orbital implant. Prospective study of 75 primary implantations. Journal Francais d'Ophtalmologie 2001;24(10):1067-73. [PubMed] [Google Scholar]
Villarroel 2001 {published data only}
- Villarroel F, Valenzuela A, Valdivia H. Porous orbital Implant: clinical experience [Implantes orbitarios porosos: experiencia clínica]. Archivos Chilenos de Oftalmología 2001;58(1/2):137-43. [Google Scholar]
Vittorino 2007 {published data only}
- Vittorino M, Serrano F, Suárez F. Enucleation and evisceration: 370 cases review. Results and complications [Enucleación y evisceración: estudio de 370 casos. Resultados y complicaciones]. Archivos de la Sociedad Española de Oftalmología 2007;82(8):495-9. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang JK, Lai PC, Liao SL. Late exposure of the bioceramic orbital implant. American Journal of Ophthalmology 2009;147(1):162-70. [DOI] [PubMed] [Google Scholar]
Woog 2004 {published data only}
- Woog JJ, Dresner SC, Lee TS, Kim YD, Hartstein ME, Shore JW, et al. The smooth surface tunnel porous polyethylene enucleation implant. Ophthalmic Surgery, Lasers and Imaging 2004;35(5):358-62. [PubMed] [Google Scholar]
Yoon 2008 {published data only}
- Yoon JS, Lew H, Kim SJ, Lee SY. Exposure rate of hydroxyapatite orbital implants a 15-year experience of 802 cases. Ophthalmology 2008;115(3):566-72. [DOI] [PubMed] [Google Scholar]
Additional references
Baino 2014
- Baino F, Perero S, Ferraris S, Miola M, Balagna C, Verné E, et al. Biomaterials for orbital implants and ocular prostheses: Overview and future prospects. Acta Biomater 2014;10(3):1064-87. [DOI] [PubMed] [Google Scholar]
Buettner 1992
- Buettner H, Bartley GB. Tissue breakdown and exposure associated with orbital hydroxyapatite implants. American Journal of Ophthalmology 1992;113(6):669-73. [DOI] [PubMed] [Google Scholar]
Custer 2003
- Custer PL, Kennedy RH, Woog JJ, Kaltreider SA, Meyer DR. Orbital implants in enucleation surgery: a report by the American Academy of Ophthalmology. Ophthalmology 2003;110(10):2054-61. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Flanagan 1990
- Flanagan JC. A new orbital implant to increase prosthetic motility following enucleation. Transactions - Pennsylvania Academy of Ophthalmology and Otolaryngology 1990;42:974-6. [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Goldberg 1994
- Goldberg RA, Dresner SC, Braslow RA, Kossovsky N, Legmann A. Animal model of porous polyethylene orbital implants. Ophthalmic Plastic and Reconstructive Surgery 1994;10(2):104-9. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADEpro GDT. Version accessed prior to 12 September 2016. Hamilton, ON: GRADE Working Group, McMaster University, 2015.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hornblass 1995
- Hornblass A, Biesman BS, Eviatar JA. Current techniques of enucleation: a survey of 5,439 intraorbital implants and a review of the literature. Ophthalmic Plastic and Reconstructive Surgery 1995;11(2):77-86. [PubMed] [Google Scholar]
Jordan 1998
- Jordan DR, Munro SM, Brownstein S, Gilberg SM, Grahovac SZ. A synthetic hydroxyapatite implant: the so-called counterfeit implant. Ophthalmic Plastic and Reconstructive Surgery 1998;14(4):244-9. [DOI] [PubMed] [Google Scholar]
Jordan 2000
- Jordan DR, Mawn LA, Brownstein S, McEachren TM, Gilberg SM, Hill V, et al. The bioceramic orbital implant: a new generation of porous implants. Ophthalmic Plastic and Reconstructive Surgery 2000;16(5):347-55. [DOI] [PubMed] [Google Scholar]
Jordan 2006
- Jordan DR, Klapper SR. Surgical techniques in enucleation: the role of various types of implants and the efficacy of pegged and nonpegged approaches. International Ophthalmology Clinics 2006;46(1):109-32. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837-41. [DOI] [PubMed] [Google Scholar]
Nunery 1993
- Nunery WR, Heinz GW, Bonnin JM, Martin RT, Cepela MA. Exposure rate of hydroxyapatite spheres in the anophthalmic socket: histopathologic correlation and comparison with silicone sphere implants. Ophthalmic Plastic and Reconstructive Surgery 1993;9(2):96–104. [DOI] [PubMed] [Google Scholar]
Perry 1990
- Perry AC. Integrated orbital implants. Advances in Ophthalmic Plastic and Reconstructive Surgery 1990;8:75-81. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubin 1994
- Rubin PA, Popham JK, Bilyk JR, Shore JW. Comparison of fibrovascular ingrowth into hydroxyapatite and porous polyethylene orbital implants. Ophthalmic Plastic and Reconstructive Surgery 1994;10(2):96-103. [DOI] [PubMed] [Google Scholar]
Schellini 2015
- Schellini SA, El Dib R, Limongi RM, Mörschbächer R. Anophthalmic socket: choice of orbital implants for reconstruction. Arq Bras Oftalmol 2015;78(4):260-3. [DOI] [PubMed] [Google Scholar]
Schellini 2016
- Schellini S, Jorge E, Sousa R, Burroughs J, El-Dib R. Porous and nonporous orbital implants for treating the anophthalmic socket: A meta-analysis of case series studies. Orbit 2016;35(2):78-86. [DOI] [PubMed] [Google Scholar]
Shields 1992
- Shields CL, Shields JA, De Potter P. Hydroxyapatite orbital implant after enucleation. Experience with initial 100 consecutive cases. Archives of Ophthalmology 1992;110(3):333-8. [DOI] [PubMed] [Google Scholar]
Shields 1994
- Shields CL, Shields JA, De Potter P, Singh AD. Problems with the hydroxyapatite orbital implant: experience with 250 consecutive cases. British Journal of Ophthalmology 1994;78(9):702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sousa 2012
- Sousa RL, Schellini SA, Zornoff DC, Padovani CR. Pipelines repair anophthalmic cavity in Brazil [Condutas para reparação da cavidade anoftálmica no Brasil]. Arq Bras Oftalmol 2012;75(6):394-7. [DOI] [PubMed] [Google Scholar]
Su 2004
- Su GW, Yen MT. Current trends in managing the anophthalmic socket after primary enucleation and evisceration. Ophthalmic Plastic and Reconstructive Surgery 2004;20(4):274–80. [DOI] [PubMed] [Google Scholar]
Tonkelaar 1991
- Den Tonkelaar I, Henkes HE, Van Leersum GK. Herman Snellen (1834-1908) and Müller's 'reform-auge'. A short history of the artificial eye. Documenta Ophthalmologica 1991;77(4):349-54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schellini 2013
- Schellini S, El Dib R. Integrated and non-integrated orbital implants for treating anophthalmic sockets. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD010293. [DOI: 10.1002/14651858.CD010293] [DOI] [PMC free article] [PubMed] [Google Scholar]


