Abstract
Background
Critically ill patients require regular body position changes to minimize the adverse effects of bed rest, inactivity and immobilization. However, uncertainty surrounds the effectiveness of lateral positioning for improving pulmonary gas exchange, aiding drainage of tracheobronchial secretions and preventing morbidity. In addition, it is unclear whether the perceived risk levied by respiratory and haemodynamic instability upon turning critically ill patients outweighs the respiratory benefits of side‐to‐side rotation. Thus, lack of certainty may contribute to variation in positioning practice and equivocal patient outcomes.
Objectives
To evaluate effects of the lateral position compared with other body positions on patient outcomes (mortality, morbidity and clinical adverse events) in critically ill adult patients. (Clinical adverse events include hypoxaemia, hypotension, low oxygen delivery and global indicators of impaired tissue oxygenation.) We examined single use of the lateral position (i.e. on the right or left side) and repeat use of the lateral position (i.e. lateral positioning) within a positioning schedule.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5), MEDLINE (1950 to 23 May 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1937 to 23 May 2015), the Allied and Complementary Medicine Database (AMED) (1984 to 23 May 2015), Latin American Caribbean Health Sciences Literature (LILACS) (1901 to 23 May 2015), Web of Science (1945 to 23 May 2015), Index to Theses in Great Britain and Ireland (1950 to 23 May 2015), Trove (2009 to 23 May 2015; previously Australasian Digital Theses Program (1997 to December 2008)) and Proquest Dissertations and Theses (2009 to 23 May 2015; previously Proquest Digital Dissertations (1980 to 23 May 2015)). We handsearched the reference lists of potentially relevant reports and two nursing journals.
Selection criteria
We included randomized and quasi‐randomized trials examining effects of lateral positioning in critically ill adults. We included manual or automated turns but limited eligibility to studies that included duration of body position of 10 minutes or longer. We examined each lateral position versus at least one comparator (opposite lateral position and/or another body position) for single therapy effects, and the lateral positioning schedule (repeated lateral turning) versus other positioning schedules for repetitive therapy effects.
Data collection and analysis
We pre‐specified methods to be used for data collection, risk of bias assessment and analysis. Two independent review authors carried out each stage of selection and data extraction and settled differences in opinion by consensus, or by third party adjudication when disagreements remained unresolved. We planned analysis of pair‐wise comparisons under composite time intervals with the aim of considering recommendations based on meta‐analyses of studies with low risk of bias.
Main results
We included 24 studies of critically ill adults. No study reported mortality as an outcome of interest. Two randomized controlled trials (RCTs) examined lateral positioning for pulmonary morbidity outcomes but provided insufficient information for meta‐analysis. A total of 22 randomized trials examined effects of lateral positioning (four parallel‐group and 18 cross‐over designs) by measuring various continuous data outcomes commonly used to detect adverse cardiopulmonary events within critical care areas. However, parallel‐group studies were not comparable, and cross‐over studies provided limited data as the result of unit of analysis errors. Eight studies provided some data; most of these were single studies with small effects that were imprecise. We pooled partial pressure of arterial oxygen (PaO2) as a measure to detect hypoxaemia from two small studies of participants with unilateral lung disease (n = 19). The mean difference (MD) between lateral positions (bad lung down versus good lung down) was approximately 50 mmHg (MD ‐49.26 mmHg, 95% confidence interval (CI) ‐67.33 to ‐31.18; P value < 0.00001). Despite a lower mean PaO2 for bad lung down, hypoxaemia (mean PaO2 < 60 mmHg) was not consistently reported. Furthermore, pooled data had methodological shortcomings with unclear risk of bias. We had similar doubts regarding internal validity for other studies included in the review.
Authors' conclusions
Review authors could provide no clinical practice recommendations based on the findings of included studies. Available research could not eliminate the uncertainty surrounding benefits and/or risks associated with lateral positioning of critically ill adult patients. Research gaps include the effectiveness of lateral positioning compared with semi recumbent positioning for mechanically ventilated patients, lateral positioning compared with prone positioning for acute respiratory distress syndrome (ARDS) and less frequent changes in body position. We recommend that future research be undertaken to address whether the routine practice of repositioning patients on their side benefits all, some or few critically ill patients.
Plain language summary
Lateral positioning for critically ill adult patients
We reviewed the evidence on the effects of turning critically ill adults from side to side while lying on a hospital bed. We found 24 studies.
Background
Nurses change the body position of critically ill patients as frequently as every two hours to prevent bed sores and other complications associated with immobility. Turning from side to side may also help loosen and drain secretions accumulated within the lungs. Routine lateral repositioning is a relatively safe standard practice. However, if a patient's blood pressure or oxygen level drops to a dangerously low reading during the position change, urgent medical attention is required. Most events resolve quickly, but for some patients these events may be slow to resolve and are potentially life‐threatening. We wanted to discover whether routine lateral repositioning is better than other positioning strategies including less frequent turns, and whether a lateral position may cause more adverse events.
Search date
The evidence is current to May 2015.
Study characteristics
We included randomized studies of critically ill adults receiving treatment in intensive care units and in other critical care areas. We selected studies that included lateral positioning after a single turn or following repetitive turns. The duration of each body position was 10 minutes or longer. Comparisons included the other lateral position (opposite side), as well as supine (lying on your back), semi recumbent (lying on your back with your upper body elevated to a 45‐degree angle) and prone (lying on your stomach) positions.
Results
We found 24 eligible studies. No studies reported on mortality. Two studies reported on pulmonary morbidity following cardiac surgery, but available data were insufficient for analysis. The other studies reported measures that we included to identify clinical adverse events. Most of these studies did not report results in a way that could be combined for review of evidence, and trial design was often dissimilar. We compared two studies of critically ill adults with unilateral lung disease (one 'bad lung' and one 'good lung'). Oxygen levels within the blood were lower for 'bad lung down' (side lying with the 'bad lung' lowermost). However, the sample was small, both studies were of poor quality and very low oxygen levels in the blood were not consistently found across studies. Therefore, results need to be viewed with caution.
Conclusion
We found no clear evidence on the effectiveness of routine lateral repositioning or the effects of a single turn for critically ill patients. Good quality studies are needed to find out whether routine lateral repositioning is still recommended for most critically ill patients, and whether one body position is best avoided for some.
Summary of findings
Summary of findings for the main comparison. Lateral positioning compared with supine immobilization.
| Lateral positioning compared with supine immobilization for critically ill adult patients | |||
|
Patient or population: critically ill adult patients Settings: critical care areas Intervention: 2‐hourly lateral positioning schedule for 24 hours Comparison: supine position for 24 hours | |||
| Outcomes | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Mortality ‐ not measured | ‐ | See comment | No studies within search strategy |
| Morbiditya ‐ not reported | 85 (2 studies) |
See comment | Acute lung pathology data not availablea |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||
aSummary statistics not available from 2 RCTs (n = 85) that examined the incidence of atelectasis (including lobar, segmental or platelet‐like atelectasis), pneumonia or parenchymal infiltrates, pleural effusion, pulmonary oedema or pneumothorax present on chest x‐ray 1 to 3 days after cardiac surgery
Summary of findings 2. Right lateral position compared with left lateral position.
| Right lateral position compared with left lateral position for critically ill adult patients | |||
|
Patient or population: critically ill adult patients Settings: critical care areas Intervention: right lateral position Comparison: left lateral position | |||
| Outcomes | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Hypoxaemiaa Partial pressure of arterial oxygen (PaO2) < 60 mmHg Follow‐up: 10 to 30 minutes after turning |
70 (2 studiesb) | ⊕⊕⊝⊝ Lowc |
Studies could not be pooled because of variability and incomplete data reporting for meta‐analysisd |
| Hypotensione ‐ not reported | ‐ | See comment | Studies had incomplete data reporting for meta‐analysisf |
| Profound hypertensiong ‐ not reported | ‐ | See comment | Studies had incomplete data reporting for meta‐analysisf |
| Low oxygen delivery (DO2)h ‐ not reported | ‐ | See comment | Studies had incomplete data reporting for meta‐analysisi |
|
Global indicators of tissue oxygenation impairmentj Mixed venous saturation (SvO2) < 60% Follow‐up: 1 to 10 minutes after turning |
103 (3 studiesk) | ⊕⊕⊝⊝ Lowl |
Studies could not be pooled because of variability and incomplete data reporting for meta‐analysism |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||
aOutcome measures include oxygen saturation (SaO2) less than 90% (critical threshold for detecting hypoxaemia) bCross‐over trials (participants as their own control) cGRADE downgraded 5 levels because of methodological variability, including risk of bias (allocation concealment not described, unclear risk of performance bias, washout inadequate to rule out carryover effects in cross‐over trials), inconsistency (samples had clinical variability, outcome data were not available from all studies), indirectness (no dichotomous data, cross‐over studies with continuous data had mean values extracted to detect critical thresholds for each outcome, most studies had single diagnostic group; postoperative cardiac surgery), imprecision (wide confidence interval within available data, most cross‐over studies did not report within‐subject variance and too few similar studies provided data for meta‐analysis) and insufficient number of studies to test for publication bias dAvailable PaO2 data at 10 and 15 minutes after turning were not pooled because of clinical variability (differences in the location of unilateral lung disease). PaO2 data were not available from an additional four cross‐over studies (n = 194) measuring PaO2 10 to 30 minutes after turning. SaO2 data were not available from five cross‐over studies (n = 256) measuring SaO2 1 to 30 minutes after turning. None of these studies reported within‐subject variance for meta‐analysis eOutcome measures include mean arterial blood pressure (MABP) less than 60 mmHg and systolic blood pressure (SBP) less than 90 mmHg (critical thresholds for detecting hypotension).
fOutcome measures include diastolic blood pressure (DBP) greater than 120 mmHg (critical threshold for detecting profound hypertension) gMABP data were not available from three cross‐over studies (n = 54). SBP and DBP data were not available from two cross‐over studies (n = 150) measuring SBP and DBP. All measures taken within the first 30 minutes after turning. None of these studies reported within‐subject variance for meta‐analysis hOutcome measures include cardiac output (CO) less than 4 L/min, cardiac index (CI) less than 2.2 L/min/m2 and low arterial oxygen content (CaO2) (critical thresholds for detecting low DO2) iCO data were not available from four cross‐over studies (n = 129), and CI data were not available from two cross‐over studies (n = 24). Whole sample CaO2 data were not available from one cross‐over study (n = 15). All measures were taken within the first 30 minutes after turning. None of these studies reported within‐subject variance for meta‐analysis jOutcome measures include lactate levels, oxygen consumption (VO2), arterial‐venous oxygen content difference (C(a‐v)O2) and SvO2 as global indicators of an alteration in tissue oxygenation kTwo parallel‐group trials (n = 60) and one cross‐over trial (n = 42) lGRADE downgraded by four levels because of methodological variability, including risk of bias (no description of allocation concealment, unclear risk of performance bias, washout inadequate to rule out carryover effects in cross‐over trials, completeness of outcome data and reporting unclear), inconsistency (samples had clinical variability, outcome data were not available from all studies), indirectness (no dichotomous data, cross‐over studies with continuous data had mean values extracted to detect critical thresholds for each outcome; most studies had single diagnostic group; postoperative cardiac surgery), imprecision (most cross‐over studies did not report within‐subject variance, and too few similar studies provided data for meta‐analysis) and insufficient number of studies to test for publication bias mAvailable SvO2 data from two parallel‐group trials (n = 60) and one cross‐over trial (n = 42) were not pooled because of trial dissimilarities (application of co‐intervention in a parallel‐group study) and the unit of analysis between parallel‐group and cross‐over trials. SvO2 data were not available from an additional four cross‐over trials (n = 182) measuring SvO2 up to 25 minutes after turning. None of these studies reported within‐subject variance for meta‐analysis. Other unavailable data for global indicators of tissue oxygenation included lactate levels (one cross‐over trial), VO2 (two cross‐over trials) and C(a‐v)O2 (two cross‐over trials)
Summary of findings 3. Bad lung down compared with good lung down for critically ill patients with unilateral lung disease.
| Bad lung down compared with good lung down for critically ill adult patients with unilateral lung disease | |||||
|
Patient or population: critically ill adult patients with unilateral lung disease Settings: critical care areas Intervention: bad lung down Comparison: good lung down | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Good lung down | Bad lung down | ||||
|
Hypoxaemia PaO2 < 60 mmHg Follow‐up: 10 to 15 minutes after turninga |
Mean PaO2 for good lung down was 122.185 mmHgb | Mean PaO2 for bad lung down was 49.26 lower (67.33 to 31.18 lower) | 19 (2 studiesc) | ⊕⊕⊝⊝ Lowd |
Hypoxaemia detected in 1 study |
|
Global indicators of tissue oxygenation impairment Arterial‐venous oxygen content difference (C(a‐v)O2) ‐ not reported |
See comment | See comment | 30 (1 study) |
See comment | Sample data not available from single cross‐over study |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio (other abbreviations, e.g..OR) | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
aComposite time interval includes early turning (10 minutes) and short‐term turning (15 minutes) responses
bAverage of study means (good lung down 122.185 mmHg; bad lung down 73.12 mmHg; rounding to two decimal places)
cCross‐over trials with participants as their own control
dGRADE downgraded four levels because of methodological variability, including risk of bias (unclear risk of selection, performance, selective reporting biases, and unclear risk of other bias related to cross‐over designs including washout inadequate to rule out carryover effects), inconsistency (inconsistent finding of hypoxaemia for bad lung down between studies, small samples not representative of critically ill adults with unilateral lung disease (some participants were breathing room air, and one study included a child)), indirectness (no dichotomous data, cross‐over studies with continuous data had mean values extracted to detect critical thresholds for each outcome) and insufficient number of studies to test for publication bias.
Background
Description of the condition
The primary goal of repositioning immobile and critically ill patients is to reduce preventable complications associated with bed rest and inactivity without compromising oxygen delivery (DO2) and tissue oxygenation (Hamlin 2008a). Patient positioning is a fundamental nursing activity (Evans 1994; Hawkins 1999). However, lateral positioning performed routinely may not be suitable for all intensive care unit (ICU) patients. Some authors have called for its cautious use in patients susceptible to cardiopulmonary and circulatory dysfunction (Bein 1996; Wilson 1994; Winslow 1990; Yeaw 1996). Patients may exhibit hypoxaemia, dyspnoea, arrhythmias or hypotension upon turning (Banasik 2001; Gawlinski 1998; Summer 1989; Winslow 1990). In the past, ICU participants have been withdrawn from lateral positioning trials as the result of intolerance to a position change (Gavigan 1990; Shively 1988; Tidwell 1990). Even though position intolerance has not been sufficiently defined, the presence of respiratory and haemodynamic instability is commonly cited. Previous research acknowledges that some critically ill patients may experience significant transient changes in oxygen transport variables during repositioning. However, it is argued that for the vast majority of critically ill patients, the reduction in oxygen transport variables such as mixed venous oxygen saturation (SvO2) returns to baseline within five minutes and is unlikely to lead to adverse outcomes (Gawlinski 1998; Tidwell 1990; Winslow 1990). Currently, no systematic review on lateral positioning has examined the incidence of clinical adverse events that may contribute to impairment in tissue oxygenation. Furthermore, no current evidence‐based clinical practice guidelines suggest the best ways to manage ICU patients who demonstrate changes in their monitored variables upon turning.
Description of the intervention
Routine patient positioning in the ICU prophylactically promotes comfort, prevents pressure ulcer formation and may reduce the incidence of deep vein thrombosis, pulmonary emboli, atelectasis and pneumonia (Banasik 2001; Keller 2002; Krishnagopalan 2002; Nielsen 2003; Schallom 2005). Routine positioning usually involves moving the patient between right and left lateral positions. However, this side‐to‐side rotation is often interrupted by another body position such as the supine or semi recumbent position (Kim 2002; Shively 1988). Two‐hourly turns are standard practice for prevention of complications associated with prolonged bed rest (Ahrens 2004; Doering 1993; Krishnagopalan 2002). Yet, empirical research has not established the optimal frequency of routine positioning (Ahrens 2004; Shively 1988).
During routine positioning, clinician discretion often determines the sequence of body positions, which may be based on convenience or custom (Doering 1993; Evans 1994). However, for some critically ill patients, body position may be selected to provide therapeutic benefit, that is, in some instances, goal‐directed therapeutic positioning may take precedence over routine positioning to improve physiological function while facilitating recovery (Evans 1994; Griffiths 2005). The duration of the chosen therapeutic position may extend beyond the standard two hours or may be shortened, according to the effectiveness of the chosen position in improving outcomes. The lateral position is recommended as a therapeutic body position for patients with unilateral lung disease (Thomas 1998; Wong 1999), and it is known that lying on the side of the healthier lung with the relatively healthy lung lowermost (synonyms include better lung dependent or inferior, and 'good lung down') may improve arterial oxygenation. This finding has been consistently reported across numerous studies, regardless of whether participants were spontaneously breathing (Remolina 1981; Seaton 1979; Sonnenblick 1983; Zack 1974) or were mechanically ventilated (Banasik 1987; Banasik 1996; Gillespie 1987; Ibanez 1981; Kim 2002; Rivara 1984). However, the optimal length of time that patients should remain on their side for therapeutic benefit is unknown, as is the impact of changes in arterial oxygenation on the incidence of morbidity or mortality.
How the intervention might work
Frequent lateral turning attenuates the deleterious compressive effects of immobility on the integumentary, musculoskeletal and neuromuscular systems (Jones 2004) and aids tracheobronchial mobilization and drainage (Bassi 2012). Pressure injury prevention is a significant focus of routine positioning (National Pressure Ulcer Advisory Panel 2009). In addition, critically ill patients may have improved respiratory outcomes with routine lateral positioning. Postural drainage in lateral positions may increase sputum volume among patients with excessive secretions (Davis 2001). The gravitational effects of repetitive lateral positioning mobilize pulmonary secretions towards the large bronchus, in turn stimulating a cough sufficient to expectorate accumulated bronchial secretions or to facilitate their removal by suction (Dean 1992; Fink 1990; Ibanez 1981). Regularly alternating the side‐lying position may prevent pooling of bronchial secretions (Jastremski 2002). Frequent lateral positioning in unilateral lung disease may help keep tracheobronchial secretions within the central airway, making the airway accessible for suctioning while minimizing the gravitational movement of secretions into healthier lung regions (Ibanez 1981). Frequent turning may assist with re‐expansion of collapsed dependent alveoli (Fink 2002). Gravitational forces within the non‐dependent lung region, which contains more negative intra‐pleural pressures compared with the dependent lung region, are applied to collapsed alveoli (Fink 2002). Observational studies suggest that the lateral‐horizontal position (side‐lying without head elevation) may potentially reduce the incidence of ventilator‐associated pneumonia (VAP) (Mauri 2010). The theoretical premise for repetitive lateral positioning has not been challenged over the years, as patients left immobile in the supine position or in any other body position for long periods are considered at significant risk of dependent airway closure, atelectasis, pneumonia and arterial deoxygenation, in part because of accumulation of bronchial secretions (Fink 2002; Goldhill 2007).
Why it is important to do this review
Although lateral positioning is provided as a simple non‐invasive respiratory therapy, uncertainty about its effects in critically ill adult patients is ongoing. A previous systematic review (Thomas 2007b) on the effects of lateral positioning reported that meta‐analysis of haemodynamic variables frequently monitored in the ICU was not possible because of weaknesses in trial design and lack of adequate reporting within the included trials. The same review conducted a meta‐analysis of three randomized trials for oxygenation variables and found evidence supporting patient positioning with the good lung down in mechanically ventilated patients with unilateral lung disease. Higher oxygen tensions were found in this lateral position compared with the supine or opposite lateral position (Thomas 2007b). However, sample size and publication bias may have influenced the magnitude and direction of treatment effects. Results of non‐randomized trials have suggested that some individuals may demonstrate a paradoxical effect with the good lung down. These individuals demonstrate better oxygenation with the diseased lung lowermost in the lateral position (Chang 1989; Choe 2000; Seaton 1979; Zack 1974). Furthermore, this meta‐analysis identified that the primary condition varied across trials and included postoperative coronary artery bypass graft (CABG) and bilateral and unilateral lung disease (Thomas 2007b). However, these review authors performed no subgroup analysis, heterogeneity testing nor sensitivity analysis of methodological quality. Therefore, the strength of the evidence remains unclear.
Other qualitative overviews (Nielsen 2003; Wong 1999) report conclusions similar to those of the previous systematic review (Thomas 2007b). However, these overviews did not use systematic and rigorous methods to minimize bias. Both reviews included non‐randomized studies and did not assess study quality (Nielsen 2003) or based quality assessment on a level of evidence hierarchy without appraising trial design (Wong 1999). Furthermore, systematic reviews and meta‐analyses examining the related area of continuous lateral positioning have not examined outcomes specifically attributed to the right or left lateral position (Choi 1992; Delaney 2006; Goldhill 2007). To this point, no systematic review has comprehensively and rigorously examined the effects of right and left lateral positions used as single or repeated therapy for critically ill adult patients. This review will investigate the incidence of mortality, morbidity and clinical adverse events during and after lateral positioning to provide the best available evidence on body positioning during critical illness. Results of the present review may inform the development of evidence‐based clinical practice guidelines and identify areas for future research.
Objectives
To evaluate effects of the lateral position compared with other body positions on patient outcomes (mortality, morbidity and clinical adverse events) in critically ill adult patients. (Clinical adverse events include hypoxaemia, hypotension, low oxygen delivery and global indicators of impaired tissue oxygenation.) We examined single use of the lateral position (i.e. on the right or left side) and repeat use of the lateral position (i.e. lateral positioning) within a positioning schedule.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized and quasi‐randomized clinical trials including those of cross‐over design conducted to evaluate the effects of the lateral position as a single or repetitive therapy for patients in a critical care area.
Types of participants
We included trials involving adult patients (aged 16 years and older) classified as critically ill.
We defined critically ill participants as:
patients diagnosed with acute impairment of one or more of the vital organ systems that may be life‐threatening (e.g. acute respiratory failure due to pneumonia, pulmonary oedema or acute respiratory distress syndrome, acute cardiac failure due to myocardial infarction, acute liver failure due to fulminant hepatitis); or
patients diagnosed with an acute disease, injury or condition requiring admission to a critical care area (ICU, coronary care unit (CCU) or cardiothoracic unit (CTU)) for advanced physiological monitoring, support or intervention (e.g. diabetic ketoacidosis, severe burns, blunt abdominopelvic trauma, postoperative cardiopulmonary bypass surgery).
In addition, we considered a trial eligible for inclusion if investigators provided their own definition of critical illness or described the eligible population as critically ill without providing a specific definition. In this case, we considered only trials located in a critical care area.
We excluded trials investigating children, pregnant women or patients with spinal cord injury exclusively, or inclusively with these subgroups exceeding 10%. We also excluded trials conducted within the operating theatre.
Types of interventions
Use of the lateral position as a single or repeated therapy for critically ill adult patients was the intervention of interest for this review.
We considered trials eligible for inclusion if they compared at least one lateral position (i.e. right lateral or left lateral position) or used other descriptors such as good lung down or bad lung down (i.e. relatively diseased lung lowermost) versus one of the following body positions (definitions are tabulated in Additional Table 4).
1. Pertinent definitions of the body positions of interest.
| Body position | Definition |
| Lateral position | The lateral position is described as side‐lying with pillows strategically placed along the patient's back, and possibly buttocks, and a pillow placed between the patient's flexed legs to prevent adduction and internal rotation of the hip. Patients are rolled to the right or left side, but the degree of rotation from the horizontal plane may vary in clinical practice. Rotation may be between 30 and 60 degrees, but up to 90 degrees. The head of the bed may also be elevated, while the patient is on his or her side. Synonyms include lateral dependent position, lateral decubitus position, lateral recumbent position, lateral tilt, lateral rotation and side‐lying. A lateral positioning schedule repeatedly utilizes the right and left lateral position. However, lateral rotation from side to side may be interrupted with another body position such as the supine position or semi recumbent position, and the order of sequence may vary. Furthermore, a specialized automated bed may perform continuous lateral positioning in the form of kinetic therapy (> 40 degrees rotation on each side) or continuous lateral rotational therapy (CLRT) (< 40 degrees rotation on each side). CLRT synonyms include continuous postural oscillation and continuous axial rotation |
| Supine position | The supine position is described as the patient lying flat on his or her back with the face looking upwards. Synonyms include flat backrest position and dorsal recumbent position |
| Semi Fowler's position or semi recumbent position | Semi Fowler's position is described as the supine position with 30 degrees head elevation, whereas the semi recumbent position may increase the degree of head elevation up to 45 degrees. Synonyms include 30 to 45 degrees head elevation, head of bed (HOB) elevation or backrest elevation |
| Fowler's position or high Fowler's position | Fowler's position is the supine position with 60 degrees head elevation; whereas high Fowler's position is sitting upright in bed at 90 degrees |
| Prone position | The prone position is described as front‐lying with the person lying on his or her abdomen with 1 or both arms at the sides and head turned towards 1 side. The Sims position is a modified prone position (semi prone). Synonyms for the prone position include ventral decubitus position |
| Trendelenburg position | The Trendelenburg position is described as the supine position with the head of the bed lower than the foot; the bed is inclined downwards, usually by 10 degrees. This position elevates the feet, legs and trunk above the person's head. A modified Trendelenburg position involves elevating the legs only, up to 30 degrees. Synonyms include head‐down tilt |
| Reverse Trendelenburg position | The reverse Trendelenburg position is described as elevating the head while lowering the legs without hip flexion (i.e. the bed is not jack‐knifed). The bed is inclined approximately 30 to 45 degrees in reverse to the Trendelenburg position. In this position, the head is elevated above the trunk, legs and feet, with the feet at the lowest point of the sloping bed. Synonyms include vertical positioning |
| Positioning schedule | For this review, a positioning schedule is defined as a sequence of pre‐determined body positions utilized in succession. The total duration of the positioning schedule and the time spent in each body position may vary between trials |
Opposite lateral.
Supine.
Semi Fowler's or semi recumbent.
Fowler's or high Fowler's (sitting).
Prone.
Reverse Trendelenburg.
Trendelenburg.
We had set a minimum duration for the intervention. Trials must have maintained the position of interest for 10 minutes or longer to be eligible for inclusion. We considered kinetic therapy and continuous lateral rotation therapy if separate data were provided for right and left lateral positions. The optimal degree of rotation from the horizontal plane and the degree of head of bed (HOB) elevation in the lateral position remain unknown; therefore, we included all descriptions of the lateral position and its synonyms. We included trials with co‐interventions applied equally across all groups.
We excluded trials with co‐interventions applied to only one randomized group.
Types of outcome measures
Primary outcomes
In‐hospital mortality (mortality within the critical care area and mortality before the time of discharge from the hospital).
Incidence of morbidity (with particular focus on pulmonary and cardiovascular morbidity).
-
Clinical adverse events during or after repositioning (with particular focus on cardiopulmonary events), for example:
hypoxaemia (including arterial oxygen saturation (SaO2) and/or partial pressure of arterial oxygen (PaO2) critical thresholds);
cardiac arrhythmias;
profound hypertension (including diastolic blood pressure (DBP) critical threshold);
hypotension (including mean arterial blood pressure (MABP) and/or systolic blood pressure (SBP) critical thresholds); and
other indicators of haemodynamic compromise such as alterations in oxygen delivery determinants (including cardiac output (CO) or cardiac index (CI), arterial oxygen content (CaO2) and/or oxygen delivery index (DO2I) critical thresholds) or global indicators of tissue oxygenation (including mixed venous oxygen concentration (SvO2) and oxygen consumption index (VO2I) critical thresholds).
Continuous and dichotomous outcome data were collected for clinical adverse events. In an effort to try to standardize interpretation of clinical adverse events from continuous variable(s), we set critical thresholds (see Additional Table 5 for critical threshold values for each type of clinical adverse event).
2. Critical threshold values for detecting clinical adverse events.
| Clinical adverse event | Critical threshold within continuous data |
| Hypoxaemia | Mean PaO2 < 60 mmHg, or Mean SaO2 < 90% |
| Hypotension | Mean SBP < 90 mmHg, or Mean MABP < 60 mmHg |
| Profound hypertension (severe, refractory or hypertensive crisis) | Mean DBP ≥ 120 mmHg, or any definition given by investigators |
| Low oxygen delivery | Mean DO2I < 500 mL O2/min or any definition given by investigators |
| Low oxygen delivery (single determinants) | Mean CO < 4 L/min or mean CI < 2.2 L/min/m2, or CaO2 reflective of low SaO2 (< 90%) with or without significant anaemia (Hb < 8 g/dL), or any definition given by investigators |
| Global indicator of tissue oxygenation impairment (imbalance between oxygen supply vs demand) | Mean SvO2 < 60 mmHg, or Mean VO2I < 100 mL O2/min or any definition given by investigators |
Secondary outcomes
Pulmonary physiology (oxygenation as measured by oxygenation index (OI) or hypoxia score (partial pressure of arterial oxygen to fraction of inspired oxygen ratio (P/F ratio)) and pulmonary artery pressures).
Vital signs (respiratory rate, heart rate, blood pressure, temperature).
Duration of assisted ventilation (all forms of positive‐pressure ventilation).
Length of stay in the critical care area.
Length of stay in hospital.
Differences in participant comfort or satisfaction (any measure reported by trial investigators).
We considered for inclusion trials that reported at least one primary or secondary outcome of interest; however, we focused on primary outcomes in this review.
We excluded trials that included pressure ulcer formation as the sole primary outcome.
Search methods for identification of studies
Electronic searches
We conducted a systematic search of the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 5), MEDLINE (the Institute for Scientific Information (ISI)) (1950 to 23 May 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) (1937 to 23 May 2015), the Allied and Complementary Medicine Database (AMED) (EBSCOhost) (1984 to 23 May 2015), Latin American Caribbean Health Sciences Literature (LILACS) (Virtual Health Library) (1901 to 23 May 2015) and the ISI Web of Science (1945 to 23 May 2015).
We searched the following electronic databases of higher‐degree theses for relevant unpublished trials: Index to Theses in Great Britain and Ireland (1950 to 2 January 2014), Trove (1 January 2009 to 23 May 2015; previously Australasian Digital Theses Program (1997 to 31 December 2008)) and Proquest Dissertations and Theses (1 January 2009 to 23 May 2015; previously Proquest Digital Dissertations (1980 to 31 December 2008)).
We used major subject headings and text words with truncation (*) for each database.
We entered the search terms 'lateral position*', 'lateral turn*', 'lateral rotation*', 'side lying', 'postur*', 'critical care', 'intensive care', 'critical* ill*' and 'ventilat*' as single terms or in combination to identify potentially relevant citations in databases with limited search functions.
We developed a comprehensive search strategy to locate participants, interventions and comparisons of interest through MEDLINE. We combined the search strategy with a randomized controlled trial (RCT) filter to identify relevant trials. We adapted this search to other databases with more advanced search functions (see Appendix 1 for database searches). We took the RCT filter from a previous version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006).
We imposed no language restrictions.
Searching other resources
We handsearched the reference lists of relevant articles for additional trials. The master handsearching database of The Cochrane Collaboration did not include two journals of interest ‐ American Journal of Critical Care (1992 to 2015, May Issue 3) and Australian Critical Care (1991 to 2015, May Issue 2). We handsearched both journals to identify potentially relevant reports, including studies reported in conference proceedings. Furthermore, we contacted experts in the field to help identify additional references or unpublished reports.
Data collection and analysis
Review authors (NH, NF) and review contributors (DG, RB) worked independently to search for relevant trials within the search strategy and to assess their eligibility for inclusion using specific inclusion and exclusion criteria (see Appendix 2). Review authors (NH, NF) and contributors (DG, RB, LG) independently performed data extraction and quality assessment of eligible trials using a Cochrane Anaesthesia Review Group standardized data extraction form adapted for this review (see Appendix 3). The primary review author (NH) and another person (review author or contributor) completed each stage independently. We piloted the standardized forms using a representative sample of trials to ensure consistency of reporting between trial authors. We revised these tools when we found inconsistencies or misinterpretations. We resolved disagreements by consensus, with adjudication by a third party (TB) if consensus was not reached. We extracted from the primary study reference additional information and data presented within duplicate reports. If information was insufficient for review authors to extract relevant data, we contacted trial authors, when possible, to request missing information.
Selection of studies
We screened titles and abstracts extracted through the search strategy for relevancy to the review. We excluded bibliographic citations that clearly did not meet the inclusion criteria. We retrieved full‐text versions of reports that we considered potentially eligible, to assess them for inclusion in the review against the eligibility criteria. We compared the results of independent screening and eligibility assessment and determined the final selection of trials for inclusion by consensus.
Data extraction and management
We summarized in tables trials that met the inclusion criteria to enable comparison of participant and trial characteristics and to facilitate assessment of each study’s risk of bias. We tabulated separately trials excluded from the review and documented the reasons for exclusion. Extracted data included types of participants, standard management applied, interventions provided, types of outcomes and results of comparisons of body positions. The duration of the intervention and data collection intervals varied between trials. Such differences in trial design may account for differences in outcomes; therefore, we chose to examine outcomes at different time points during and after the intervention.
We used the following composite time intervals (i.e. turning responses or positioning schedule responses) to group findings across a range of time points (minutes, hours or days) commonly reported within the literature for primary outcomes.
Immediately at 0 minutes (immediate turning response).
Between 1 and 10 minutes (early turning response).
Between 11 and 30 minutes (short‐term turning response).
Between 31 and 119 minutes (intermediate‐term turning response).
At two hours (benchmark turning response).
After longer than two hours but before the next position change (delayed turning response).
After cessation of positioning therapy (positioning schedule response).
If data were insufficient, we pooled relevant outcome data across composite time intervals.
The primary review author entered extracted data into the Review Manager computer programme (RevMan 5.3), and data were verified independently (CW, DG, JG, NF).
Assessment of risk of bias in included studies
We appraised the risk of bias for each study by using a standardized checklist adapted from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We judged the risk of bias as high, low or unclear for key domains (random sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting and other biases) and tabulated the rationale. We assessed inadequate random sequence generation and allocation sequence concealment (selection bias) at the study level, and inadequate blinding and incomplete outcome data (performance, detection and attrition bias) at the outcome‐specific level. Selective outcome reporting (reporting bias) and other biases (such as carryover effects in cross‐over studies) may affect study‐specific and outcome‐specific levels of assessment; therefore, we assessed these domains at both levels. In addition, we separated blinding of participants and caregivers from outcome assessor blinding because controlling for performance differences within positioning trials is inherently difficult. A participant’s spatial awareness of posture and a clinician’s participation in turning procedures and ongoing management limit concealment of allocated body positions during a trial. Overall, we made judgements for seven domains within the risk of bias assessment.
Unit of analysis issues
We extracted paired data for meta‐analysis if the unit of analysis was appropriate, or if individual participant data were available for calculation of relevant summary statistics. For cross‐over trials, this calculation included within‐subject variance to avoid a unit of analysis error. We collected data from cross‐over trials that provided three or more treatments (body positions); therefore, we extracted three or more pair‐wise comparisons. However, we paired for analysis only treatment data that ‘crossed over’ (i.e. allocated to each period with counterbalance).
Assessment of heterogeneity
We visually inspected summary tables of included trials to identify substantial clinical heterogeneity amongst trials. Clear evidence of poor homogeneity between studies resulted in a narrative summary of findings for extracted outcome data. If we identified two or more randomized trials with comparable populations undergoing similar interventions, we implemented a meta‐analysis of extracted data by using the DerSimonian and Laird random‐effects model within RevMan 5.3 software. We tested for homogeneity between trials for each outcome by using the Cochran's Q statistic with P value less than or equal to 0.10. We formally tested the impact of heterogeneity by using the I2 test (Higgins 2002). We set an I2 threshold greater than 50% to indicate that variation across trials due to heterogeneity was substantial.
Data synthesis
We quantitatively estimated each trial's treatment effect with 95% confidence intervals (95% CIs). We graphically represented point estimates within forest plots by using the inverse variance method. If combined data revealed minimal statistical heterogeneity, pooled outcome data provided a summary statistic of effect, with mean difference (MD) with 95% CI provided for continuous outcomes. Other pre‐planned summary statistics of effect included risk ratio (RR) for dichotomous outcomes and standardized mean difference (SMD) for different continuous outcome scales across trials. Forest plots of parallel‐group trials display the mean and standard deviation (SD) for continuous data, whereas mean values from cross‐over studies are not displayed within forest plots of continuous data. Therefore, to enable detection, narrative reporting and interpretation of clinical adverse events, we reported central tendency measures for each treatment for studies entered for meta‐analysis.
The quality of the evidence guided the inferences drawn. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) approach presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to rate the quality of the body of evidence. The GRADE system rates studies according to comparisons and outcomes and may downgrade evidence from high quality on the basis of study limitations (risk of bias), indirectness of evidence, inconsistency of results, imprecision of effect estimates and potential publication bias (Guyatt 2011). We planned to use GRADE for all reported primary outcomes, with main comparisons presented within 'Summary of findings' tables using GRADEprofiler software (GRADEPro). Discussion of review findings includes a critique of the strength of evidence and identification of possible limitations of individual studies. We discuss the clinical implications of the findings along with identified gaps within research, and we provide recommendations for future research.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity may be present because of the nature of the inclusion criteria. Body position effects may differ between disease states and severity of illness amongst participants. Positive‐pressure ventilation may alter the effects of turning compared with spontaneous unassisted breathing. In addition, differences in the angle of lateral rotation may contribute to variation. We planned to perform subgroup analyses for data pooled within a meta‐analysis, had we identified sufficient studies (refer to the Differences between protocol and review section for details). We also planned to examine possible sources of clinical variability when an I2 statistic was less than 50% but heterogeneity remained statistically significant.
Sensitivity analysis
We planned to examine methodological shortcomings of review findings by performing a sensitivity analysis of pooled data within a meta‐analysis. We planned to compare results with and without studies that adequately addressed randomization, allocation concealment, outcome assessor blinding, standard management and co‐interventions applied equally across groups, and to perform intention‐to‐treat analysis with loss to follow‐up of less than 20%. We also planned to perform sensitivity analysis on the basis of choice of summary statistic and presence of outlying trial results. It is not feasible to blind healthcare professionals providing the intervention, and it is impractical to blind participants in a procedural trial on positioning; therefore, participant and caregiver blinding was not subject to sensitivity analysis. In addition, requests for missing data from trial authors were not always successful. If study authors did not respond, or if it was not possible to find them, we included the study in question in the review but planned to analyse study inclusion and exclusion for overall effects on findings, as part of the sensitivity analysis.
For detection of publication bias, a large number of studies are required to provide moderate power (Sterne 2011). Therefore, we planned assessment of publication bias through inspection of funnel plots with a set threshold of 10 or more included studies for each outcome.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search strategy elicited 3917 database citations and 252 citations from other sources. We retrieved full‐text reports for 160 selected citations, including several that included insufficient information in the title and/or abstract to enable a decision about relevancy. We conducted further screening and assessment of relevancy within eligibility assessment. We found 91 reports to be irrelevant upon eligibility assessment, including five non‐English reports. A search update revealed two citations awaiting classification; we contacted study authors and received no response (see Characteristics of studies awaiting classification). We selected 34 reports for inclusion and 33 for exclusion. The adjudicator assessed four studies, resulting in inclusion of all four. We identified additional sources for three included studies following primary investigator contact (Chan 1991; Reed 2002) and retrieved a conference proceeding abstract not located during the search (Staudinger 2004). Once duplicate reports were taken into account, we determined that 24 randomized trials met the inclusion criteria (six parallel‐group studies and 18 cross‐over studies) (see Figure 1).
1.
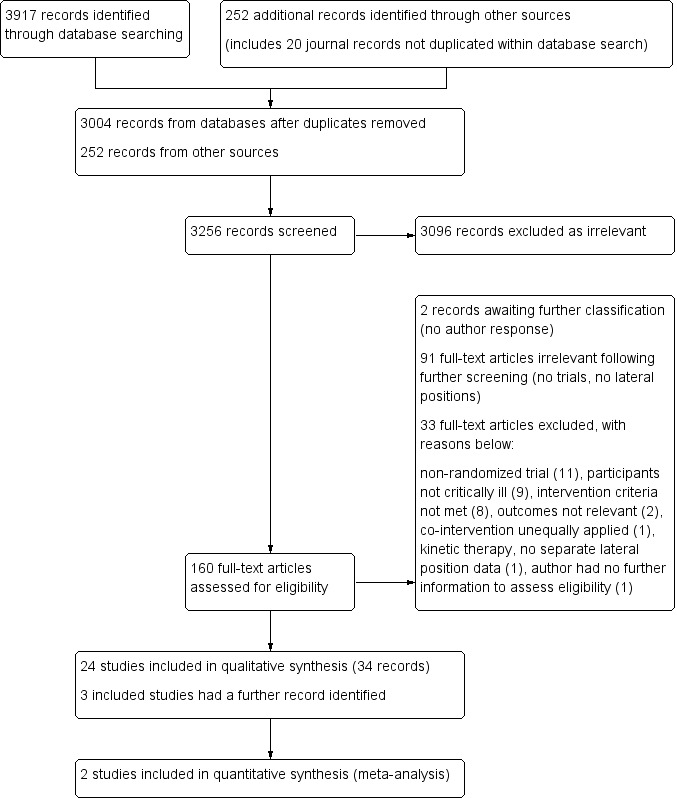
Study flow diagram of records searched, screened and selected.
We collected data from all study sources and nominated the first full‐text publication as the primary reference, with the exception of two studies: an unpublished thesis (Reed 2002) that conducted pre‐specified secondary analysis of phase 1 data (Jesurum‐Urbaitis 2002), and a citation (Banasik 1996) nominated as the primary reference by the principal investigator (Dr. Jacquelyn Banasik, personal communication, 27 March 2010). We contacted several primary investigators for additional information regarding unclear randomization methods and other elements of trial design that may influence results. We received additional information on random sequence generation from the primary investigator of one study (Dr. Jorge Ibañez, personal communication, 23 June 2010). Another primary investigator confirmed that intention‐to‐treat analysis was conducted but provided no other details and indicated that additional data for extraction were no longer available (Marianne Chulay, personal communication, 29 June 2010). We established contact but received no further information about trial design and no additional data from several investigators (Margaret Gavigan, personal communication, 26 May 2010; Gayle Whitman, personal communication, 29 March 2010; Patricia Lewis, personal communication; 20 July 2010; Sandy Tidwell, personal communication, 22 July 2010). We established contact with the author of an unpublished thesis (Carroll 1991) because the conference proceeding abstract of the same study (Carroll 1992) provided insufficient information, and we could not retrieve an American university thesis copy despite our efforts to do so. The study author provided additional information on the intervention and comparators and confirmed that no individual participant data are available to enable calculation of appropriate summary statistics for meta‐analysis (Karen Carroll, personal information, 5 May 2010). We learned that for three studies (Banasik 1987; Banasik 1996; Banasik 2001) conducted by the same investigator, additional information was not available for data extraction purposes (Dr. Jacquelyn Banasik, personal communication, 15 May 2010). We sought to contact the corresponding authors and/or primary investigators for five other studies (Bein 1996; Chan 1992; de Laat 2007; Kim 2002; Remolina 1981). We retrieved the last known contact details through the World Wide Web, and in some cases, we contacted an affiliated university to trace contact details. However, we received no response to our enquiries. All studies with missing information remained eligible for inclusion, and we identified relevant data within the results (see 'Notes' within the Characteristics of included studies table for specifics regarding unavailable data).
Included studies
Most studies were conducted in the USA (n = 16). Other study sites included Austria (Schellongowski 2007), Australia (Thomas 2007a), Canada (Chan 1992), Germany (Bein 1996), the Netherlands (de Laat 2007), Nepal (Tripathi 2009), Spain (Ibanez 1981) and South Korea (Kim 2002). Sample size ranged from nine to 120 participants (mean sample 35.04 ± 25.42 standard deviation (SD)). Most participants were male (76.13% across 20 studies, four studies unspecified). Mean age in parallel‐group trials ranged from 52 years (10 SD) (Chulay 1982) to 68.2 years (9.9 SD) (Reed 2002). Mean age within cross‐over studies spanned four decades; the lowest mean was 33.5 years (13.89 SD) and a child aged 10 years was included in the sample (Ibanez 1981), and the highest mean was 69.95 years (8.64 SD) (Banasik 1996). Three cross‐over studies did not report age (Carroll 1992; Pena 1989; Whitman 1982); another cross‐over study reported an age range (Remolina 1981).
Fifteen studies included mechanically ventilated participants exclusively. The other nine studies consisted of a mixture of mechanically ventilated and spontaneously breathing participants (Doering 1988; Lewis 1997; Remolina 1981), participants extubated during data collection (72 hours) (Chulay 1982; Gavigan 1990), spontaneously breathing participants (Gawlinski 1998; Shively 1988) and participants for whom the mode of breathing was not indicated (de Laat 2007; Whitman 1982). All parallel‐group trials included cardiac surgical patients within hours of surgery as an inclusion criterion, with the main diagnostic group undergoing coronary artery revascularization. One cross‐over study described its eligible population as 'critically ill' with low cardiac ejection fraction (< 30%) and admitted to a coronary care or cardiac observation unit (Gawlinski 1998). Another cross‐over study included critically ill participants with mixed causes of illness with low PaO2 (< 70 mmHg) and/or low cardiac index (CI) (< 2.2 L/min/m2) (Banasik 2001). The other cross‐over studies enrolled participants after they had undergone cardiac surgery (Banasik 1987; Banasik 1996; Carroll 1992; Chan 1992; Doering 1988; Pena 1989; Tidwell 1990; Whitman 1982) or single lung transplant surgery (George 2002), or had received a diagnosis of acute respiratory failure (ARF) (Bein 1996; Ibanez 1981; Kim 2002; Remolina 1981; Schellongowski 2007; Thomas 2007a; Tripathi 2009). Five studies with ARF participants pre‐specified acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS) criteria for inclusion (Bein 1996; Kim 2002; Schellongowski 2007; Thomas 2007a; Tripathi 2009). Four of these studies reported severity of illness at baseline by using one or more prognostic scoring systems (Bein 1996; Schellongowski 2007; Thomas 2007a; Tripathi 2009). Participants with ARF were diagnosed predominantly with bilateral lung disease (Bein 1996; Schellongowski 2007; Tripathi 2009) or were stratified into groups for analysis on the basis of the presence of bilateral lung disease or unilateral lung disease (Kim 2002; Thomas 2007a). One study (Tripathi 2009) classified lung infiltrate asymmetry within bilateral lung disease and regrouped data according to better lung down and better lung up (i.e. lower lung infiltration score (LIS) for regrouped data). Two studies exclusively examined participants with unilateral lung disease (Ibanez 1981; Remolina 1981). Other cross‐over studies reported subgroup analysis of participants with unilateral atelectasis (Banasik 1987; Chan 1992) or conducted post hoc analysis based on the presence of bilateral lung disease or unilateral lung disease (Banasik 1996; Banasik 2001). In these studies, most participants were without unilateral lung disease (Banasik 1987; Banasik 1996; Chan 1992), or half the sample had unilateral atelectasis or pleural effusion (Banasik 2001). Investigators in another study (Kim 2002) stratified results for all body positions into diagnostic groups and reported them separately without total sample analysis. Nonetheless, we considered only data from whole samples for meta‐analysis.
Angle of lateral rotation and degree of head of bed (HOB) elevation
Angle of lateral rotation from the horizontal plane and degree of head of bed (HOB) elevation varied across included studies. Eighteen studies reported an angle of lateral rotation of 20 degrees (Whitman 1982), 30 degrees (Chan 1992; de Laat 2007), 45 degrees (Banasik 1987; Banasik 1996; Banasik 2001; Carroll 1992; Chulay 1982; Doering 1988; Gavigan 1990; Gawlinski 1998; George 2002; Reed 2002; Tidwell 1990; Tripathi 2009), 62 degrees (Bein 1996; Schellongowski 2007) and 90 degrees from the horizontal plane (Thomas 2007a). Only three studies reported use of a protractor to verify the angle of lateral rotation (Banasik 1996; Banasik 2001; Doering 1988); two of these studies also used a commercial wedge (Banasik 1996; Banasik 2001). Another 11 studies set the degree of lateral rotation with a commercial and/or foam wedge (Banasik 1987; Carroll 1992; Chulay 1982; de Laat 2007; Gavigan 1990; Gawlinski 1998; George 2002; Reed 2002; Tidwell 1990) or Rotorest Kinetic Treatment table or bed (Bein 1996; Schellongowski 2007) without reporting whether the angle of rotation had been verified. Six studies did not report the degree of lateral rotation (Ibanez 1981; Kim 2002; Lewis 1997; Pena 1989; Remolina 1981; Shively 1988).
Six studies applied HOB elevation equally to all body positions, with elevation set at 20 degrees (de Laat 2007; Doering 1988; Gawlinski 1998; Reed 2002) or 30 degrees (Chan 1992; George 2002). Five studies reported that the HOB elevation was not undertaken, except for a single pillow placed under the head (Banasik 1987; Banasik 1996; Banasik 2001; Carroll 1992), or used the prefix ‘decubitus’ to describe body positions (Pena 1989). Seven studies provided no description of HOB elevation for any of the body positions of interest (Chulay 1982; Gavigan 1990; Ibanez 1981; Lewis 1997; Remolina 1981; Schellongowski 2007; Tripathi 2009). Another three studies did not report HOB elevation for lateral positions but reported a supine position angle of HOB elevation of zero (Bein 1996), 15 degrees (Kim 2002) or less than 20 degrees (Thomas 2007a). Other studies described varying angles of HOB elevation for lateral, supine and other backrest positions (Tidwell 1990; Whitman 1982; Shively 1988).
Trial characteristics
Parallel‐group trials
Two RCTs (Chulay 1982; Gavigan 1990) (n = 85) examined use of a repetitive lateral positioning schedule (two‐hourly turning between supine position and alternating lateral positions) versus supine position for 24 hours after cardiac surgery. Investigators did not clearly identify nor consistently report primary versus secondary outcomes (Chulay 1982; Gavigan 1990). However, the main outcomes of interest were incidence of acute lung pathology (particularly atelectasis) identified by daily chest radiograph at days one, two and three (consistent with 24, 48 and 72 hours in the other study). Other reported outcomes included number of hours with fever (temperature > 38.0ºC) within 72 hours after surgery and ICU length of stay (LOS) (Chulay 1982; Gavigan 1990), duration of intubation (Chulay 1982) and length of hospital stay (Gavigan 1990).
The other four parallel‐group studies examined effects of the lateral position on SvO2 (n = 118) (Lewis 1997; Reed 2002; Shively 1988) and CI (n = 69) (de Laat 2007) within hours after cardiac surgery. However, trial characteristics differed considerably. Shively 1988 (n = 30) compared one‐hourly versus two‐hourly turning frequencies, utilizing four body positions provided sequentially. Investigators performed comparative analysis of these four body positions within the first hour, but randomized groups received an identical sequence of treatments (body positions) without counterbalance. Therefore, within‐subject comparisons were not valid for inclusion. Other parallel‐group studies (n = 157) compared the right lateral position versus the left lateral position within the first 10 minutes after turning (Lewis 1997; Reed 2002), or up to two hours in each allocated lateral position, followed by two hours in the supine position (de Laat 2007). Two studies (de Laat 2007; Lewis 1997) used a split‐plot design with co‐interventions, which included timing of the first turn applied after surgery (de Laat 2007) and timing of a one‐minute backrub applied after the lateral turn (Lewis 1997). One study (de Laat 2007) introduced a non‐randomized group after study commencement, and this invalidated data extraction. Another study (Reed 2002) measured DO2I, oxygen consumption index (VO2I), oxygen extraction ratio (O2ER) and CI, but we did not extract these outcomes for this review, as analysis was based on stratification of baseline haemoglobin (Hb) levels without total sample analysis according to randomization.
Cross‐over trials
All 18 cross‐over studies examined the effects of lateral position as single therapy, with random assignment to the treatment sequence. All participants received all treatments (body positions) for comparison. Duration within each body position ranged from 10 minutes to two hours, and most studies reported body position duration less than 30 minutes. One cross‐over study (Pena 1989) included two comparators but did not identify the specific body position provided after the initial lateral turn, and we could obtain no additional information (Maria Peña, personal communication, 8 June 2010). The other 17 cross‐over studies provided a single application of the supine position and each lateral position (right lateral position and left lateral position; bad lung down and good lung down in unilateral lung disease; better lung down and worse lung down in bilateral lung disease; or native lung down and allograft lung down after single lung transplant). Two studies included other body positions as comparators: the prone position (Kim 2002) and an additional supine position with 30‐degree HOB elevation (Tidwell 1990). Review authors anticipated data extraction and comparative analysis of paired data from periods that were ‘crossed over’. None of the cross‐over studies used adequate methods to evaluate the change from baseline or initial body position within a randomized trial design to warrant data extraction based on this unit of measurement. All cross‐over studies except for one (Pena 1989) showed uniformity among lateral positions within each allocated period. Seven cross‐over studies used variations of the Latin squares design or the Williams design to examine cross‐over differences (period contrasts) between all body positions of interest (Banasik 1987; Banasik 1996; Banasik 2001; Chan 1992; George 2002; Kim 2002; Whitman 1982), but one study reported double entry of supine position results (Chan 1992). Risk of bias assessment reveals further detail of this anomaly within the design (see Chan 1992, Characteristics of included studies). Eleven cross‐over studies did not show period uniformity for supine position data. Investigators in these studies did not allocate the supine position to the same treatment period(s) as other comparators (lateral positions) (Bein 1996; Carroll 1992; Doering 1988; Gawlinski 1998; Ibanez 1981; Schellongowski 2007; Thomas 2007a; Tidwell 1990) or did not reveal the treatment sequence (Pena 1989; Remolina 1981; Tripathi 2009). Data extracted from these 11 cross‐over studies for paired comparisons involving the supine position or the 30‐degree HOB position were not valid.
Detection of hypoxaemia
Eleven cross‐over studies reported at least one continuous data measure (SaO2 and/or PaO2) for detection of hypoxaemia (see Characteristics of included studies). Investigators reported single measures taken at 10 minutes (Banasik 1987; Banasik 1996; Remolina 1981), 15 minutes (Banasik 2001; Ibanez 1981), 20 minutes (Tripathi 2009) and 30 minutes after turning (Chan 1992; Kim 2002) or repeated measures taken up to 25 minutes (Tidwell 1990) and 30 minutes after turning (George 2002; Schellongowski 2007). Five studies included both measures (PaO2 and SaO2) as study outcomes (Banasik 1987; Banasik 1996; Banasik 2001; Chan 1992; Tripathi 2009).
In terms of pair‐wise comparisons of data from these cross‐over studies, five studies (n = 256) compared SaO2 between right lateral and left lateral positions (Banasik 1987; Banasik 1996; Banasik 2001; Chan 1992; Tidwell 1990). Six studies (n = 264) compared PaO2 between right lateral and left lateral positions (Banasik 1987; Banasik 1996; Banasik 2001; Chan 1992; Ibanez 1981; Kim 2002). Researchers in another study collected but did not report data on SaO2 (n = 12) (Schellongowski 2007). Several studies measured PaO2 according to lung orientation for lateral positions, including bad lung down versus good lung down for unilateral lung disease participants (n = 19) (Ibanez 1981; Remolina 1981) and allograft lung down versus native lung down for 15 single lung transplant participants (George 2002). Similarly, Tripathi 2009 compared SaO2 and PaO2 levels between better lung down and better lung up for 16 participants with bilateral lung disease.
A single study performed pair‐wise comparisons of PaO2 between the prone position and each of the lateral positions (right and left sides)(Kim 2002) (n = 32). Five studies compared the supine position and each lateral position (right and left side); four studies (n = 222) measured SaO2 (Banasik 1987; Banasik 1996; Banasik 2001; Chan 1992) and all five studies (n = 254) PaO2 (Banasik 1987; Banasik 1996; Banasik 2001; Chan 1992; Kim 2002). Another study (George 2002) (n = 15) compared PaO2 between allograft lung down and supine positions, and between native lung down and supine positions, after single lung transplant. Other studies measuring PaO2 and/or SaO2 (Ibanez 1981; Remolina 1981; Schellongowski 2007; Tidwell 1990; Tripathi 2009) did not show period uniformity for all treatments (body positions).
Detection of hypotension or profound hypertension
Seven cross‐over studies reported at least one continuous data measure (SBP, MABP and/or DBP) for detection of hypotension or profound hypertension (see Characteristics of included studies). One study (n = 120) conducted non‐invasive blood pressure (NIBP) measurements in each arm 10 minutes after turning, with SBP and DBP reported for each arm as co‐primary outcomes (Banasik 1996). Six studies (n = 119) conducted invasive blood pressure measurements of MABP at 15 minutes (Bein 1996) or 20 minutes after turning (Tripathi 2009), or conducted repeated measures up to 30 minutes (George 2002; Schellongowski 2007; Chan 1992) and 120 minutes after turning (Thomas 2007a). One study (Chan 1992) conducted other blood pressure (BP) measures (SBP and DBP) up to 30 minutes after turning, possibly as secondary outcomes.
In terms of pair‐wise comparisons from these cross‐over studies, three studies (n = 54) compared right lateral and left lateral positions by measuring MABP (Bein 1996; Chan 1992; Schellongowski 2007), and two studies (n = 150) measured SBP and DBP (Banasik 1996; Chan 1992). In addition, researchers compared MABP between better lung down and better lung up for 16 participants with bilateral lung disease (Tripathi 2009), and between allograft lung down and native lung down for 15 single lung transplant participants (George 2002). Another study (Thomas 2007a) did not provide separate MABP data for each lateral position (see Thomas 2007a in Characteristics of included studies).
Pair‐wise comparisons involving other body positions included comparison between the supine position and each lateral position (right and left sides) for two studies (n = 150) measuring SBP and DBP (Banasik 1996; Chan 1992), and for one study (n = 30) measuring MABP (Chan 1992). However, the latter study performed dual entry of supine position data (see Chan 1992, Risk of bIas table in Characteristics of included studies). Another study (n = 15) measured MABP between supine position and allograft lung down, and between supine position and native lung down, after single lung transplant (George 2002). As previously described, four studies (Bein 1996; Schellongowski 2007; Thomas 2007a; Tripathi 2009) did not perform valid pair‐wise comparisons involving supine position data.
Detection of inadequate oxygen delivery
Eight cross‐over studies measured at least one oxygen delivery (DO2) determinant (i.e. CaO2 or CO) or determinant indexed to body mass (i.e. CI) (see Characteristics of included studies). Investigators measured outcomes to detect inadequate DO2 at 15 minutes (Banasik 2001; Bein 1996; Doering 1988; Whitman 1982) and 25 minutes after turning (George 2002), or repeated measures up to 15 minutes (Carroll 1992), 30 minutes (Schellongowski 2007) and 120 minutes after turning (Thomas 2007a). A single study measured all DO2 determinants (Banasik 2001).
In terms of pair‐wise comparisons from these cross‐over trials, one study (n = 12) measured CaO2 to compare right lateral and left lateral positions (Banasik 2001), and six studies (n = 153) measured CO or CI as a primary or co‐primary outcome (Banasik 2001; Bein 1996; Carroll 1992; Doering 1988; Schellongowski 2007; Whitman 1982). Another study (Thomas 2007a) (n = 34) measured CO and CI for a subgroup (ALI/ARDS group) without performing total sample analysis or comparative analysis between right and left lateral positions. A single study (George 2002) (n = 15) compared CO between allograft lung down and native lung down for single lung transplant participants. Two studies (n = 62) performed comparison of CO between supine position and each lateral position (right and left sides) (Banasik 2001; Whitman 1982), with one study (n = 12) also measuring CaO2 (Banasik 2001). George 2002 also measured CO between the supine position and each lateral position (allograft lung down and native lung down). Other studies did not provide valid supine position data for paired comparisons (Bein 1996; Carroll 1992; Doering 1988; Schellongowski 2007; Thomas 2007a).
Global indicators of the adequacy of tissue oxygenation
Eight cross‐over studies measured one or more global indicators of tissue oxygenation, including lactate levels (Banasik 2001), oxygen consumption (VO2) (Banasik 2001; Tidwell 1990), arterial‐venous oxygen content difference (C(a‐v)O2, also known as arteriovenous oxygen difference (a‐vDO2)) (Banasik 1996; Chan 1992) and SvO2 (Banasik 1996; Banasik 2001; Carroll 1992; Gawlinski 1998; George 2002; Pena 1989; Tidwell 1990). One study (n = 12) used an unknown comparator for repeated measures of SvO2 taken up to 120 minutes after turning (Pena 1989). Other studies measured global indicators of tissue oxygenation at 10 minutes (Banasik 1996) and 15 minutes after turning (Banasik 2001), and still other studies conducted repeated measures up to 15 minutes (Carroll 1992; George 2002), 25 minutes (Gawlinski 1998; Tidwell 1990) or 30 minutes after turning (Chan 1992).
In terms of pair‐wise comparisons from these cross‐over trials, comparison between right lateral and left lateral positions included measures of lactate (n = 12) (Banasik 2001), VO2 (n = 46) (Banasik 2001; Tidwell 1990), C(a‐v)O2 (n = 150) (Banasik 1996; Chan 1992) and SvO2 (n = 224) (Banasik 1996; Banasik 2001; Carroll 1992; Gawlinski 1998; Tidwell 1990) as primary or co‐primary outcomes. However, one study (Banasik 2001) removed VO2 data from outcome reporting because some data were missing. Neither study measuring C(a‐v)O2 measured or imputed VO2 (Chan 1992) or Hb levels (Banasik 1996) for the derived formula. Another study (Banasik 1996) reported a measurement error, with central venous oxygen saturation (ScvO2) sampled but labelled as SvO2. A single study (George 2002) compared SvO2 between allograft lung down and native lung down for 15 single lung transplant participants. This study also compared SvO2 between the supine position and each lateral position (allograft lung down and native lung down). Pair‐wise comparisons between the supine position and each lateral position (right and left sides) included studies measuring lactate (n = 12) (Banasik 2001), VO2 (n = 12) (Banasik 2001), C(a‐v)O2 (n = 150) (Chan 1992; Banasik 1996) and SvO2 (n = 132) (Banasik 1996; Banasik 2001). However, as has been mentioned, Banasik 2001 did not report VO2, and Chan 1992 performed dual entry of supine position data and used an incomplete C(a‐v)O2 formula (Banasik 1996; Chan 1992). The other studies (Carroll 1992; Gawlinski 1998; Tidwell 1990) did not conduct a valid paired comparison between the supine position and each lateral position.
Secondary outcomes in cross‐over studies
Secondary outcomes of interest included P/F ratio, vital signs other than blood pressure measures reported previously and pulmonary pressures. Four cross‐over studies (n = 72) reported P/F ratio along with variable body position duration and measurement intervals (Ibanez 1981; Schellongowski 2007; Thomas 2007a; Tripathi 2009). Two cross‐over studies provided sufficient data on the fraction of inspired oxygen (FiO2) for P/F ratio conversion of reported PaO2 at 30 minutes and 10 minutes, respectively (Kim 2002; Remolina 1981). Two of these studies did not conduct total sample analysis between body positions (Thomas 2007a; Kim 2002). No study performed valid comparisons involving supine position data. For comparison between lateral positions, one study (Ibanez 1981) reported P/F ratio results for right and left lateral positions, but these results were based on unequal proportions of participants with right and left dominant unilateral lung disease. The same cross‐over study conducted analysis between lateral positions according to the worst lateral position and the best lateral position (Ibanez 1981), which, in their data set, corresponded to bad lung down and good lung down ‐ the same comparison positions reported by another study (Remolina 1981).
For other secondary outcomes, five studies (n = 175) measured heart rate (HR) as a co‐primary outcome at 10 minutes (Banasik 1996), 15 minutes (Banasik 2001; Bein 1996) or 20 minutes after turning (Tripathi 2009), or conducted repeated measures within the first 30 minutes after turning (George 2002). Doering 1988 (n = 51) reported HR at 15 minutes after turning as a secondary outcome. Another study (Chan 1992) (n = 30) conducted repeated measures of HR, respiratory rate (resp. rate), pulmonary capillary wedge pressure (PCWP) and systolic, mean and diastolic (S, M, D) pulmonary artery pressures (PAPs) up to 30 minutes after turning, but these might have been secondary outcomes. Resp. rate was an outcome in two other studies (n = 132) (Banasik 1996; Banasik 2001). Three studies (Bein 1996; Doering 1988; Tripathi 2009) provided invalid supine position data for paired comparisons.
Risk of bias in included studies
Researchers stated that they performed random assignment to treatment groups (parallel‐group studies) or treatment sequences (cross‐over studies) (see Characteristics of included studies). However, only nine studies adequately described the method of randomization used (i.e. random sequence generation) (Banasik 1987; Banasik 1996; Banasik 2001; Chulay 1982; Doering 1988; Gawlinski 1998; Ibanez 1981; Reed 2002; Thomas 2007a). Fourteen studies did not describe the randomization method (Bein 1996; Carroll 1992; Chan 1992; Gavigan 1990; George 2002; Kim 2002; Lewis 1997; Pena 1989; Remolina 1981; Schellongowski 2007; Shively 1988; Tidwell 1990; Tripathi 2009; Whitman 1982). One study (de Laat 2007) had high risk of selection bias, attributed to inadequate random sequence generation. This study deviated from pre‐specified randomization by adding a non‐randomized reference (control) group to the study design after performing interim analysis of the first 15 participants. Overall, most included studies (except Reed 2002) had two or more domains with unclear risk of bias (see Figure 2; Figure 3). However, three studies (de Laat 2007; Gavigan 1990; Shively 1988) were seriously flawed. Two studies had high risk of attrition bias (Gavigan 1990; Shively 1988) and the third study had high risk of selection bias as mentioned above. The next section provides a summary of the risk of bias under each domain and highlights important flaws within single studies.
2.
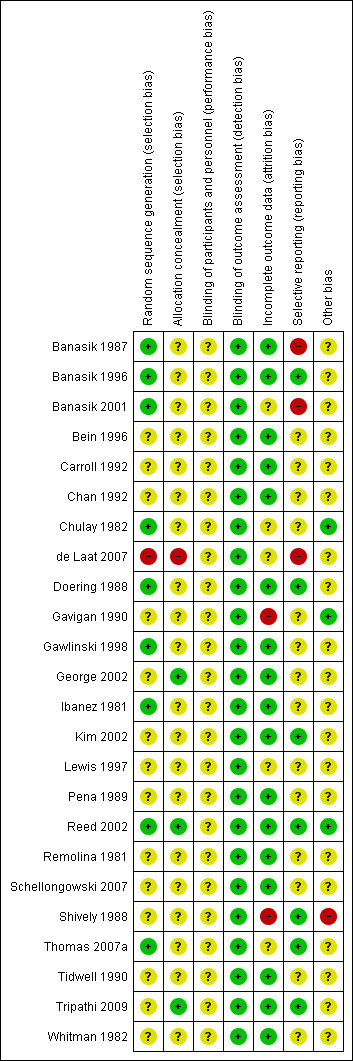
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
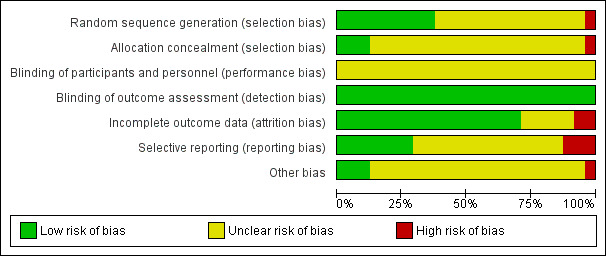
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All but two studies provided an inadequate description of concealment procedures (George 2002; Reed 2002). Only one of these studies was protected against selection bias (Reed 2002).
Blinding
We assessed blinding of participants and caregivers (performance bias) separately from blinding of outcome assessment (detection bias).
Blinding of participants and caregivers
All lateral positioning trials had unclear risk of performance bias, although most studies reported objective measures. Controlling for performance differences is inherently difficult within positioning trials. Caregivers and non‐sedated participants are aware of group assignment and/or treatments provided (body positions) throughout the course of a study. One RCT (Chulay 1982) attempted to minimize risks of performance and detection bias. Investigators did not inform nursing and medical staff of the dependent variables being studied, and investigators did not participate in management decisions nor in participant care (Chulay 1982). Nonetheless, without blinding of caregivers to treatment and comparators, parallel groups may have been managed differently unintentionally. Furthermore, unbalanced cross‐over designs with treatments not allocated to all periods may exaggerate performance bias. Conversely, uniform cross‐over trials of short duration and balanced period design are unlikely to have substantial bias that can be attributed to performance differences between periods. We describe performance issues further within the discussion on methodological limitations.
Blinding of outcome assessment
Two RCTs investigating lateral positioning as repetitive therapy conducted outcome assessor blinding to the main outcome (acute lung pathology identified on chest x‐ray daily for 72 hours) (Chulay 1982; Gavigan 1990). No other study described outcome assessor blinding procedures. However, all other outcomes were objective measures taken from blood gas analysers or real‐time physiological monitoring systems, or length of stay retrieved from medical records (Chulay 1982; Gavigan 1990). Therefore, knowledge of group assignment during the study was unlikely to lead to plausible outcome assessor bias. We judged all studies as having low risk of detection bias.
Incomplete outcome data
Incomplete outcome data within parallel‐group trials
Reed 2002 had low risk of attrition bias, with no missing data. Three other parallel‐group trials (Chulay 1982; de Laat 2007; Lewis 1997) had unclear risk of attrition bias, with data completeness not transparent. Researchers did not report group size for results and comparative analysis; therefore, it was unclear whether all repeated measures within each treatment group were accounted for. The other two parallel‐group trials (Gavigan 1990; Shively 1988) had high risk of attrition bias, with neither study conducting intention‐to‐treat analysis. Gavigan 1990 withdrew one‐third of the intervention group because of haemodynamic instability, leading to results based on uneven group size. Investigators defined haemodynamic instability but did not pre‐specify that it was a reason for study protocol termination (Gavigan 1990). Shively 1988 conducted ‘as treated’ analysis between two groups of similar size (i.e. one‐hourly versus two‐hourly turning). However, after the study commenced, the investigator withdrew 36% of the eligible sample on the basis of pre‐specified termination criteria (n = 11) or equipment failure (n = 6). Shively 1988 cited haemodynamic instability and inability to maintain a position as the main reasons for protocol termination. It appears that the investigator assessed both criteria subjectively, as they did not provide set parameters for termination. Nonetheless, whether withdrawals were evenly distributed across both groups, remains unknown (Shively 1988).
Incomplete outcome data within cross‐over trials
Fifteen cross‐over studies reported between‐subject central tendencies and measures of dispersion for each treatment (body position) (Banasik 1987; Banasik 1996; Banasik 2001; Bein 1996; Chan 1992; Doering 1988; Gawlinski 1998; George 2002; Ibanez 1981; Kim 2002; Remolina 1981; Schellongowski 2007; Thomas 2007a; Tidwell 1990; Tripathi 2009). The other cross‐over studies provided insufficient summary data (Carroll 1992; Pena 1989; Whitman 1982). Overall, investigators reported only one within‐subject comparison (right lateral versus left lateral position for PaO2) (Banasik 1987). Furthermore, nine cross‐over studies did not report the sample size of outcome data within results and analysis (Banasik 1996; Banasik 2001; Carroll 1992; Doering 1988; Pena 1989; Schellongowski 2007; Thomas 2007a; Tidwell 1990; Whitman 1982). In these cases, it remained unclear whether all data points and/or repeated measures were accounted for, within reported outcomes. Attrition and unit of analysis errors within cross‐over designs do not lead to systematic bias but may exaggerate imprecision (Higgins 2011). Attrition may influence the power to detect a difference, as incomplete individual data are withdrawn from cross‐over studies intending to conduct within‐subject analysis. Although studies reported between‐subject results and analysis of treatment effects, most cross‐over studies have low risk of attrition bias for within‐subject comparisons, except for two studies with unclear risk of attrition bias (Banasik 2001; Thomas 2007a). Banasik 2001 removed all VO2 data from results and analysis because some data were missing. Attrition contributed to the risk of reporting bias. Thomas 2007a rearranged position data across multiple periods into independent groups for analysis; one group (lateral positioning) reported missing data without the period specified. Although study authors stated that they conducted intention‐to‐treat analysis, they did not provide clear information on how they handled attrition. Therefore, the design features and method of analysis conducted led to unclear risk of attrition bias (see Thomas 2007a in Characteristics of included studies).
Selective reporting
Nine studies had low risk of reporting bias for primary outcomes. Four of these studies presented results consistent with the intended method of analysis, including all pre‐specified secondary outcomes and/or subgroup analyses (Kim 2002; Reed 2002; Shively 1988; Thomas 2007a). The other five studies with low risk of reporting bias for primary outcomes did not clearly pre‐plan secondary analysis (Chan 1992; Doering 1988; Gawlinski 1998; Tripathi 2009) or omitted some outcomes from pre‐specified subgroup analyses (Banasik 1996). Lewis 1997 had unclear risk of reporting bias associated with ambiguous results. This split‐plot RCT reported main effects testing for primary outcomes but provided unclear information on which split‐plot groups were involved in the analysis. Therefore, it was difficult for review authors to ascertain whether comparisons were reported accurately, or if selective reporting was involved (see Lewis 1997 in Characteristics of included studies). For diverse reasons, another 11 studies (parallel‐group trials and cross‐over studies) had unclear risk of reporting bias (Bein 1996; Carroll 1992; Chulay 1982; Gavigan 1990; George 2002; Ibanez 1981; Pena 1989; Remolina 1981; Schellongowski 2007; Tidwell 1990; Whitman 1982). We have provided specific details about the judgement for each study in the risk of bias tables (Characteristics of included studies). We included in the meta‐analyses two of these cross‐over studies with unclear risk of reporting bias (Ibanez 1981; Remolina 1981). One study did not explicitly state that the method and analysis were pre‐planned (Remolina 1981). The other study reported different gas exchange measures (i.e. P/F ratio) compared with those indicated within the method (Ibanez 1981). We found high risk of reporting bias in three studies (Banasik 1987; Banasik 2001; de Laat 2007). Banasik 2001 selectively reported tissue oxygenation measures and removed one co‐primary outcome measure (VO2) from the analysis as the result of missing data. Banasik 1987 did not report all pre‐specified comparisons. The other study did not indicate which method of analysis investigators planned to use before changing the research design during the trial (de Laat 2007).
Other potential sources of bias
Two parallel‐group studies (de Laat 2007; Lewis 1997) had unclear risk of bias because of possible baseline differences between groups and standard management practices during data collection. In addition, Lewis 1997 reported a statistically significant difference in the primary outcome (SvO2) at baseline for participants turned left versus those turned right (P value < 0.05). One study (Chan 1992) increased the risk of detection (measurement) bias by using different methods of detection between supine and lateral positions. Outcomes reported for the supine position included average score from two measurements taken at baseline and during the treatment sequence. Furthermore, Chan 1992 modified the treatment sequence for two groups (sequences). Modification after random assignment did not lead to group assignment violations but may pose other risks of bias related to the unbalanced design, omission of all pre‐specified sequences and unequal group size. Analysis may lead to erroneous conclusions about group (treatment sequence) differences. Another cross‐over study (Gawlinski 1998) presented ambiguous numerators for group size, standard medication management and a baseline parameter (PCWP) (see Gawlinski 1998 in Characteristics of included studies). Nonetheless, these discrepancies were not likely to influence within‐subject analysis of the primary outcome (SvO2). Other potential sources of bias included unknown carryover and period effects within cross‐over designs. All cross‐over studies had unclear risk of bias for this domain.
Carryover (residual) effects
Cross‐over designs require a sufficiently long washout between treatments to avoid the possibility that carryover effects may seriously influence the validity of effect estimates (Senn 2002). Only one study (Shively 1988) mentioned the threat of carryover effects but applied no cross‐over of treatments. Notably, three cross‐over studies included a passive washout period that was shorter than that afforded the treatment periods (Gawlinski 1998; Ibanez 1981; Schellongowski 2007). In addition, Gawlinski 1998 took measurements at the end of the passive washout period for inclusion in the comparative analysis ‐ similar to ‘baseline change’ analysis without an explicitly stated intention.
Six cross‐over studies reported a stabilization period (i.e. active washout) before a single measurement was taken during each treatment period. Amongst these studies, the duration of stabilization before data collection ranged from 10 minutes (Banasik 1996) to 15 minutes (Banasik 2001; Bein 1996; Doering 1988; Whitman 1982) to 30 minutes for primary outcomes (Chan 1992). None of these studies provided a rationale for the duration of stabilization or active washout. Another five cross‐over studies made no mention of washout before a single measurement at 10 minutes (Banasik 1987; Remolina 1981), 15 minutes (Ibanez 1981), 20 minutes (Tripathi 2009) and 30 minutes in each body position (Kim 2002). Most of these 11 cross‐over studies turned participants to the next body position in the treatment sequence immediately following data collection. Two studies extended the duration for each body position beyond data collection by 10 minutes and five minutes, respectively (Banasik 1996; Banasik 2001). Another two studies did not report treatment duration (Remolina 1981; Tripathi 2009). These studies may have had insufficient washout before the time of data collection. Therefore, cross‐over studies conducting a single measurement during each treatment period had unclear risk of bias due to carryover effects.
Seven cross‐over studies took repeated measurements within each treatment period (Carroll 1992; Gawlinski 1998; George 2002; Pena 1989; Schellongowski 2007; Thomas 2007a; Tidwell 1990). Data collection commenced immediately or within a few minutes after turning, with repeated measures conducted up to 25 or 30 minutes before turning to the next body position within the sequence (Gawlinski 1998; George 2002; Schellongowski 2007; Tidwell 1990). Another two studies (Carroll 1992; Thomas 2007a) had an unbalanced design and varied the duration of treatment (lateral versus supine position). In addition, one of these studies obtained fewer measurements in the supine position, which was the shorter treatment (Thomas 2007a). Pena 1989 measured outcome data continuously for two hours but reported no extractable results, and contact with study authors yielded no additional information. None of the cross‐over studies with repeated measures within each treatment period reported active washout after data collection. Therefore, all seven cross‐over studies had unclear risk of bias due to carryover effects.
Period effects or treatment by period interactions
Investigators in 12 cross‐over studies did not describe baseline body position before the time of data collection (Banasik 1987; Banasik 1996; Banasik 2001; Bein 1996; Doering 1988; Gawlinski 1998; George 2002; Ibanez 1981; Kim 2002; Remolina 1981; Tripathi 2009; Whitman 1982). Without knowledge of the previous body position at baseline, it is unclear whether few, many or all participants were manually turned to the allocated body position, or stayed in the same position to which they were randomly allocated during the first period. Five studies had a non‐uniform unbalanced design, with all treatment sequences commencing in the supine position (first period) (Bein 1996; Doering 1988; Gawlinski 1998; Ibanez 1981; Schellongowski 2007). Participants who stayed in the same body position as allocated may have had data collected without a turn, leading to high risk of intervention bias when treatments were of different duration. Furthermore, the clinical status of participants and/or their management may have intentionally or unintentionally differed in the first period compared with other treatment periods; therefore, the risk of bias due to a period effect remains unclear. Six cross‐over studies did not clearly minimize period effects or treatment‐by‐period interactions through their design; these studies showed lack of uniformity and balance (Carroll 1992; Thomas 2007a; Tidwell 1990) or provided no information on uniformity and balance (Pena 1989; Remolina 1981; Tripathi 2009).
Sequence effects
Four cross‐over studies (Banasik 1987; Banasik 1996; Banasik 2001; Kim 2002) were variance‐balanced for the effect of sequence, therefore within‐subject effects were unlikely to be substantially biased because of differences in treatment sequence. In contrast, eight cross‐over studies (Bein 1996; Carroll 1992; Doering 1988; Gawlinski 1998; Ibanez 1981; Schellongowski 2007; Thomas 2007a; Tidwell 1990) were not designed to minimize potential sources of bias. Four cross‐over studies (Pena 1989; Remolina 1981; Tripathi 2009; Whitman 1982) provided unclear information on balance. Another two studies with uniform cross‐over designs had incomplete balance (Chan 1992; George 2002). In addition, George 2002 reported a group (treatment sequence) difference for PaO2 as a co‐primary outcome of interest. One group had statistically significantly higher PaO2 levels compared with the other two groups (George 2002). Researchers offered no explanation about whether the phenomenon was due to chance alone, baseline differences or sequencing effects.
Effects of interventions
See: Table 1; Table 2; Table 3
Primary outcomes
Mortality
No studies reported mortality as an outcome.
Morbidity
Two RCTs (Chulay 1982; Gavigan 1990) were comparable, but neither study reported acute lung pathology frequencies within allocated groups. Meta‐analysis was not possible because data were unavailable. No other study reported pulmonary, cardiovascular or any other types of morbidity as outcomes of interest (see Table 1).
Clinical adverse events
No study reported dichotomous data (i.e. present or absent data) to reveal the frequency of hypoxaemia, hypotension, profound hypertension, arrhythmias, low oxygen delivery or alterations in global indicators of tissue oxygenation.
Continuous data extraction for detection of hypoxaemia, hypotension, profound hypertension, low oxygen delivery or alterations in tissue oxygenation was limited. Overall, only one cross‐over study (Banasik 1987) reported within‐subject variance, and one parallel‐group study (Lewis 1997) reported summary statistics for some but not all repeated measures. Six other studies revealed variance, calculated from individual participant data (Chan 1992; Gawlinski 1998; George 2002; Ibanez 1981; Reed 2002; Remolina 1981).
Hypoxaemia
Four cross‐over studies (Banasik 1987; George 2002; Ibanez 1981; Remolina 1981) (94 participants) provided whole sample PaO2 data. Another cross‐over study (Chan 1992) reported only subgroup PaO2 data (n = 9). Furthermore, review authors adjusted PaO2 data from one study (Remolina 1981) to avert a unit of analysis error within the effect estimate (see Remolina 1981 in Characteristics of included studies). We grouped extracted pair‐wise comparisons to detect hypoxaemia according to the relevant time period (see Tables 4.1 to 4.11, Appendix 4). We found no available SaO2 data for meta‐analysis. We report paired comparison results in the sections below.
Right lateral position versus left lateral position on PaO2 as a measure for detecting hypoxaemia
We identified no studies for meta‐analysis (see Table 2). Clinical heterogeneity was apparent in two cross‐over studies with extractable PaO2 data (Banasik 1987; Ibanez 1981). Each study had unequal numbers of participants with right and left lung disease. One study (Ibanez 1981) included participants with predominantly right unilateral lung disease (n = 7/10), and the other (Banasik 1987) included a subgroup with left lung atelectasis (n = 10/60). The location of unilateral lung disease was a confounding factor and may contribute to contradictory findings. In terms of single study results, one study (Banasik 1987) had a small precise MD in PaO2 at 10 minutes (an early turning response; MD 5.2 mmHg, 95% CI 0.89 to 9.51; P value = 0.02). However, removal of subgroup data (participants with left lung atelectasis) from the analysis led to a small imprecise MD for the remaining sample (participants with bilateral atelectasis or no atelectasis) (see 4.1.1. in Table 4.1, Appendix 4). The other study (Ibanez 1981) reported an imprecise MD in PaO2 at 15 minutes (short‐term turning response) (see 4.3.1. in Table 4.3, Appendix 4). Mean results did not reveal hypoxaemia (lowest mean PaO2 107 mmHg on right side (Ibanez 1981), 111.5 mmHg on left side (Banasik 1987)).
Bad lung down versus good lung down on PaO2 as a measure for detecting hypoxaemia in patients with unilateral lung disease
Two cross‐over studies (Ibanez 1981; Remolina 1981) were comparable for data pooling and estimation of a summary effect for bad lung down versus good lung down for critically ill patients with unilateral lung disease. We did not detect statistical heterogeneity across time points (at 10 minutes and 15 minutes after turning; P value = 0.38, I2= 0%). The summary effect suggests that mean PaO2 was significantly lower for bad lung down (MD ‐ 49.26 mmHg, 95% CI ‐67.33 to ‐31.18; P value < 0.00001; Analysis 1.1). The direction and magnitude of treatment effects were consistent between studies, but the meta‐analysis was based only on data from 19 participants (see Table 3). Average PaO2 level for bad lung down across both studies was approximately 73 mmHg. However, one study (Remolina 1981) detected hypoxaemia for bad lung down, with a mean PaO2 of 59.78 mmHg (range 49 to 77 mmHg for adjusted results). The same study found that mean PaO2 for good lung down was well above the hypoxaemia threshold of 60 mmHg (mean PaO2 100.67 mmHg, range 58 to 167 mmHg for adjusted results) (Remolina 1981). Conversely, Ibanez 1981 had a larger MD but reported central tendency measures above the critical threshold for hypoxaemia (mean PaO2 86.5 mmHg for bad lung down, range 37 to 192 mmHg; mean PaO2 143.7 mmHg for good lung down, range 92 to 305 mmHg).
1.1. Analysis.
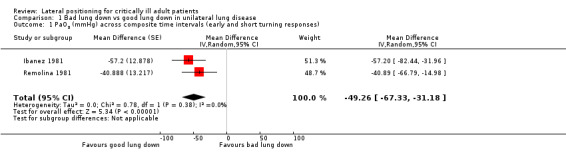
Comparison 1 Bad lung down vs good lung down in unilateral lung disease, Outcome 1 PaO2 (mmHg) across composite time intervals (early and short turning responses).
Native lung down versus allograft lung down on PaO2 as a measure for detecting hypoxaemia after single lung transplant
The only study of single lung transplant participants (George 2002) reported that MDs in PaO2 between allograft lung down and native lung down at five minutes (an early turning response) and at 15 minutes (short‐term turning response) were small, imprecise and in the opposite direction at each time point (see Tables 4.2 and 4.4, Appendix 4). Investigators found no hypoxaemia within mean results (lowest mean PaO2 116.93 mmHg for allograft lung down, five minutes after turning) for the 15 participants.
Effect of each lateral position versus a comparison body position other than lateral on PaO2 as a measure for detecting hypoxaemia
Only one study with extractable PaO2 data compared lateral and supine positions (George 2002). Single study results revealed a small imprecise MD in PaO2 between lateral positions (allograft lung down, native lung down) and supine position at 5 minutes and 15 minutes (early turning and short‐term turning responses) for 15 single lung transplant participants (George 2002) (see Tables 4.6 and 4.7, Appendix 4). Researchers found no hypoxaemia within mean results (lowest mean PaO2 114.93 mmHg for supine position, five minutes after turning). Furthermore, one must exercise caution when interpreting paired comparisons between supine position and native lung down because of the unbalanced design used for this comparison (see George 2002 in Characteristics of included studies).
Hypotension or profound hypertension
Only one study provided extractable blood pressure (BP) data (George 2002) (see Appendix 5). Single study results favoured no body position, and the direction of treatment effect for MABP varied across time points for each pair‐wise comparison during early turning and short‐term turning responses (at five, 15 and 30 minutes) (George 2002). Furthermore, the small to negligible MD in MABP was imprecise (see Appendix 5). Hypotension was not found within mean results for 14 single lung transplant participants (lowest mean MABP 75.21 mmHg for the native lung down, 15 minutes after turning). A 3 mmHg difference separated the lowest and highest mean values (highest mean MABP was recorded for the allograft lung down position, 30 minutes after turning).
Low oxygen delivery
Only one study (George 2002) provided extractable CO and/or CI data (see Appendix 6). In terms of single study results, the MD in CO was negligible for pair‐wise comparisons between allograft lung down, native lung down and supine positions 25 minutes after turning (George 2002). Low CO was not found within mean results (lowest mean CO 4.91 L/min in the supine position) for 15 single lung transplant participants. However, one must view the results with caution, as investigators presented no rationale for differences in analytical methods between other blood flow measures (HR and MABP) and CO. The unpublished thesis of the same study reported CO range for each body position without a central tendency measure or inferential statistics, representing a possible source of reporting bias.
Global indicators of the adequacy of tissue oxygenation
We derived data for global indicators of the adequacy of tissue oxygenation from a cross‐over study (Chan 1992) with extracted subgroup C(a‐v)O2 data (n = 9) tabulated separately (see Appendix 7), and we obtained whole sample SvO2 data from two cross‐over studies (Gawlinski 1998; George 2002) and two parallel‐group studies (Lewis 1997; Reed 2002) (see Table 2). We analysed SvO2 data according to each method reported for consistency with the unit of analysis (see Tables 8.1 to 8.6, Appendix 8; see additional 'Summary of findings' tables for single studies in Appendix 9). We have provided paired comparison results below.
Right lateral position versus left lateral position on SvO2 as a global indicator of tissue oxygenation
Meta‐analyses of SvO2 data were not possible, as we identified insufficient studies with available data and/or similarities in trial design and participant characteristics. One cross‐over study (Gawlinski 1998) reported SvO2 data for the comparison between right lateral versus left lateral positions during early turning responses (i.e. one minute intervals for five minutes) and short‐term turning responses (i.e. 15th and 25th minutes after turning). In terms of single study results, MDs in SvO2 for all turning responses were small (Gawlinski 1998) (see Tables 8.1 to 8.2, Appendix 8). Confidence intervals were narrow without a precise treatment effect, with the exception of the paired comparison at four minutes. At this time point, data favoured the right lateral position with a mean SvO2 level 1.786% higher than that of the left lateral position (MD 1.79, 95% CI 0.18 to 3.39; P value = 0.03). However, the difference of less than 2% is unlikely to signify clinical importance.
In terms of parallel‐group trials, the split‐plot RCT (Lewis 1997) reported summary statistics for three time points (5th, 6th and 10th minute) during the 10 minutes of repeated measures. Extracted data at minute 6 and minute 10 after turning reflected an applied co‐intervention (backrub) (Lewis 1997). In contrast, Reed 2002 applied no co‐intervention to each lateral position during the 10 minutes of data collection after turning. We did not pool SvO2 data at 5 minutes because we could not clearly identify the result with and without the co‐intervention (backrub). Therefore, we reported single study results. Each study reported a small MD between lateral positions during early turning responses (up to 10 minutes), which lacked precision (Lewis 1997; Reed 2002) (see Table 8.6, Appendix 8).
Native lung down versus allograft lung down on SvO2 as a global indicator of tissue oxygenation following single lung transplant
In the single study of single lung transplant participants (George 2002), the MD in SvO2 was negligible to small and was imprecise for paired comparisons between native lung down and allograft lung down at five and 15 minutes after turning (see Table 8.3, Appendix 8).
Effect of each lateral position versus a comparison body position other than lateral on SvO2 as a global indicator of tissue oxygenation
Only one study provided SvO2 data for a comparison body position other than the opposite lateral position (George 2002). In this study, MDs in SvO2 were negligible to small and results imprecise at five and 15 minutes after turning for paired comparisons between each lateral position (native lung down and allograft lung down) and the supine position (see Tables 8.4 and 8.5 in Appendix 8).
Other adverse events
Two studies (Gavigan 1990; Thomas 2007a) pre‐specified adverse events as outcomes of interest but did not report the numbers and types of events for each group or treatment. One study (Gavigan 1990) reported no differences between groups. The other study (Thomas 2007a) reported five adverse events of moderate severity (requiring medical intervention with changes in ventilation or inotropic therapy) and nine minor (transient) events over the study duration. Investigators noted haemodynamic events on return to the supine position and minor events associated with agitation. However, this cross‐over study did not report differences between treatments or periods. Furthermore, it was unclear whether adverse events occurred more often than once for each participant. No other study reported adverse events during data collection as outcome measures.
Secondary outcomes
Pulmonary physiology
No included studies measured mean airway pressure or oxygenation index (OI). Pulmonary artery pressures including PCWP were not available.
Hypoxia score
For two cross‐over studies (Ibanez 1981; Remolina 1981), we calculated P/F ratio data from individual participant data (see Table 10.2, Appendix 10). No other study provided P/F ratio data. We pooled P/F ratio data for the comparison between bad lung down and good lung down in participants with unilateral lung disease, as we detected no statistically significant heterogeneity across composite time intervals (early turning response and short‐term turning response) (P value = 0.71, I2 = 0%) (Analysis 1.2). The meta‐analysis found that results favoured good lung down in participants with unilateral lung disease (MD ‐85.33 points, 95% CI ‐107.14 to ‐63.53; P value < 0.00001). The finding was precise, but the meta‐analysis was based on only 19 participants. The two studies within the meta‐analysis reported mean P/F ratio below 200 for bad lung down; 121.82 points (range 46.25 to 192 points) and 191.56 points (range 54 to 309.52 points), respectively (Ibanez 1981; Remolina 1981). Neither study reported mean P/F ratio above 300 for good lung down, with mean P/F ratio of 203.75 points (range 109 to 283 points) 15 minutes after turning (Ibanez 1981) and 281.83 points (range 167 to 390.48 points) 10 minutes after turning (Remolina 1981).
1.2. Analysis.
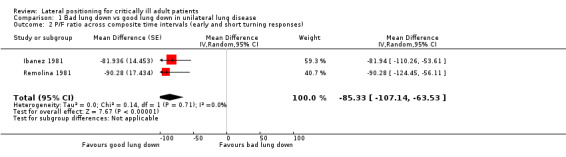
Comparison 1 Bad lung down vs good lung down in unilateral lung disease, Outcome 2 P/F ratio across composite time intervals (early and short turning responses).
Vital signs
Only two studies presented summary data suitable for extraction of vital signs data other than BP (Chulay 1982; George 2002). Six cross‐over studies measuring HR (Banasik 1996; Banasik 2001; Bein 1996; Chan 1992; Doering 1988; Tripathi 2009), three cross‐over studies measuring resp. rate (Banasik 1996; Banasik 2001; Chan 1992) and an RCT (Gavigan 1990) measuring temperature for the outcome of number of hours with fever did not provide data.
Blood pressure
Researchers examined BP measures under clinical adverse events.
Heart rate
In terms of single study results for HR, one study (George 2002) reported two time points that reached statistical significance: the comparison at 30 minutes after turning for supine position versus allograft lung down (MD ‐7.64, 95% CI ‐13.00 to ‐2.29; P value = 0.005) and the comparison at five minutes after turning for supine position versus native lung down (MD 3.36, 95% CI 0.29 to 6.42; P value = 0.03). However, the direction and magnitude of changes were not consistent across comparisons. The probability of a type 1 error cannot be ruled out. Data favoured no other time points for the other pair‐wise comparisons between allograft lung down, native lung down and supine position, and effect estimates lacked precision in this underpowered study (see Appendix 11).
Respiratory rate
No study provided respiratory rate data sufficient for analysis.
Temperature
In terms of single study results for temperature, one RCT (Chulay 1982) reported 17.60 fewer hours with fever for repetitive lateral positioning versus supine positioning at 72 hours (MD ‐17.60, 95% CI ‐26.12 to ‐9.08; P value < 0.00001). Data also revealed fewer hours with fever for lateral positioning on day two (48 hours) (see Appendix 12). However, investigators provided no summary statistics for day one (24 hours) and selectively reported only statistically significant results.
Duration of mechanical ventilation
One RCT (Chulay 1982) reported the duration of endotracheal intubation. This single study favoured repetitive lateral positioning over supine immobilization, with approximately five fewer hours of intubation (MD‐4.80 , 95% CI ‐9.57 to ‐0.03; P value = 0.05) (see Appendix 13).
Length of stay
One RCT (Chulay 1982) reported summary data suitable for extraction on length of ICU stay. This single study favoured repetitive lateral positioning over supine immobilization for length of ICU stay, with eighteen and a half fewer hours in the ICU (MD ‐18.60, 95% CI ‐33.07 to ‐4.13; P value = 0.01) (see Appendix 14).
Pain score or participant satisfaction
No eligible studies examined pain scores and participant satisfaction as outcomes of interest.
Subgroup analysis
Due to limitations with available data, we could not conduct subgroup analysis of primary or secondary outcomes to test for differences in effect size due to primary disease and condition, severity of illness, presence of positive‐pressure ventilation or differences in angle of lateral rotation (≤ 45 degrees versus > 45 degrees). Most studies provided unsuitable outcome reporting for meta‐analysis, including unit of analysis errors for cross‐over studies and/or insufficient studies of similar trial design and outcomes.
Sensitivity analysis
We did not assess publication bias, as too few studies provided extractable and comparable data on the same outcomes of interest for a funnel plot analysis to be valid.
The strength of evidence for the effect of lateral positioning as single and repetitive therapy is inadequate to permit conclusions. For the two meta‐analyses conducted, we extracted pooled data for each comparison from the same two studies with a small sample size (Ibanez 1981; Remolina 1981). However, neither study had low risk of bias. In both studies, risks of selection bias, reporting bias and other biases were unclear. Notably, each cross‐over trial did not sufficiently control for confounding of treatment effects by carryover effects or period‐by‐treatment interactions. Removal of these two studies from meta‐analyses resulted in no available evidence from comparable studies with low risk of bias for any outcome of interest.
Discussion
Summary of main results
Review authors identified three main types of study design amongst 24 studies. Trials included randomized controlled trials (RCTs) comparing a lateral positioning schedule versus supine positioning; parallel‐group trials comparing right lateral versus left lateral positions; and cross‐over trials comparing both lateral positions with the supine position. Two cross‐over trials included more than three body positions (Kim 2002; Tidwell 1990). Body position duration for lateral positions and comparators ranged from 10 minutes to two hours. Two RCTs (Chulay 1982; Gavigan 1990) included lateral positions (right and left side) more than once within a sequence. Postoperative cardiac surgery was the most common inclusion criterion, and the location of lung disease (none, right unilateral lung disease, left unilateral disease or bilateral lung disease) varied greatly within and between samples. Whether cross‐over studies examined within‐subject variability remains largely unknown. Many studies did not provide sample size calculations. Therefore, many studies may have lacked the power to detect differences between body positions.
Common methodological issues and differences in trial characteristics limited the ability of review authors to summarize studies. Cross‐over studies reported data with unit of analysis errors for meta‐analysis, and parallel‐group studies were largely non‐comparable. Inadequate reporting of summary statistics was a major limitation in reports of overall findings. Primary investigator contact was often unsuccessful and older study data no longer available. Study differences including whether cross‐over designs were variance‐balanced; types of co‐interventions provided in parallel‐group studies; differences in measurement intervals reported; and outcomes of interest and planned analysis that may limit comparability regardless of the data available for paired comparisons. Supine position data from unbalanced non‐uniform cross‐over studies were invalid for analysis. Other variabilities in design included angle of lateral rotation; degree of head of bed (HOB) elevation and, for haemodynamic outcomes influenced by hydrostatic pressure (systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MABP), pulmonary artery pressure (PAP) and pulmonary capillary wedge pressure (PCWP)) ‐ a valid reference point for lateral positions. We considered variations in trial characteristics when assessing heterogeneity.
Lateral positioning as repetitive therapy
Overall, evidence was insufficient to support or refute the two‐hour standard of turning critically ill patients for prevention or treatment of pulmonary morbidity or any other outcome. Two RCTs (Chulay 1982; Gavigan 1990) comparing lateral positioning versus supine immobilization in postoperative cardiac surgical patients reported no differences in the incidence of atelectasis and other acute lung pathologies. However, data were unsuitable for meta‐analysis, and power analysis was not reported. Other reported outcomes (fewer hours with fever, reduced hours intubated/mechanically ventilated and reduced length of intensive care unit (ICU) stay) favoured the lateral positioning schedule, but we extracted data from only one RCT (Chulay 1982). Both studies had unclear risk of selection, performance and reporting biases (Chulay 1982; Gavigan 1990), along with high risk of attrition bias for all outcomes (Gavigan 1990).
Lateral position as single therapy
We conducted very limited quantitative analysis and found low‐quality evidence of the effects of lateral positions on partial pressure of arterial oxygen (PaO2) as a measure to detect hypoxaemia in two small studies (Ibanez 1981; Remolina 1981). The summary effect for critically ill participants with unilateral lung disease showed an approximately 50 mmHg difference in PaO2 between lateral positions (bad lung down versus good lung down), with lower mean PaO2 for bad lung down (Ibanez 1981; Remolina 1981). However, both studies had unclear risk of bias and included only 19 participants for meta‐analysis; only one study (Remolina 1981) had mean PaO2 for bad lung down less than 60 mmHg (hypoxaemia). Moreover, external validity of the meta‐analyses may not extend beyond the two study samples. In particular, sample heterogeneity in the Remolina 1981 study may have contributed to attainment of the threshold for hypoxaemia. This study included only one mechanically ventilated participant and one‐third of participants (n = 3) on room air, with significantly lower mean PaO2 and lower mean fraction of inspired oxygen (FiO2) (approximately 30% difference) compared with the Ibanez 1981 sample. Room air participants are not representative of critically ill patients with unilateral lung disease. The other 20 studies reported insufficient extractable and/or comparable data. We extracted single study results for continuous data measures to detect clinical adverse events and obtained insufficient evidence to determine lateral position effects (Banasik 1987; Gawlinski 1998; George 2002; Lewis 1997; Reed 2002). We could draw no conclusions on the effect of each lateral position (right side versus left side, better lung down versus worse lung down in bilateral lung disease, and native lung down versus allograft lung down for single lung transplant patients) over a relatively short period (i.e. 10 minutes to two hours). Only one study sample provided extractable supine position data for comparison with each lateral position, but results were small and imprecise (George 2002). We could draw no conclusions on the effect of each lateral position versus the supine position.
Overall completeness and applicability of evidence
Causal inferences from lateral positioning trials are largely in doubt. It is unclear whether the meta‐analysis for bad lung down versus good lung down in unilateral lung disease was subject to publication bias. None of the other eligible studies limited inclusion to unilateral lung disease. Insufficient studies were available for preparation of a funnel plot. However, a search of other non‐English databases may yield additional studies. Nonetheless, both studies entered for meta‐analysis had methodological shortcomings that diminished the robustness of findings with small study effects. In addition, FiO2 variation is likely a confounding factor for detection of hypoxaemia. Future studies of critically ill patients with unilateral lung disease may yield different results.
Quality of the evidence
International peer‐reviewed journals have adopted Consolidated Standards of Reporting Trials (CONSORT) or similar standards to improve the quality of RCT reporting (Bennett 2005; Smith 2008). However, most studies in this review were published before the CONSORT guidelines were revised (Moher 2001), and two‐thirds of studies were conducted more than 15 years ago. Therefore, selective reporting of primary outcomes and inadequate reporting of procedures that minimize selection, performance, detection and attrition bias may not have been intentional. Only one study (Reed 2002), a secondary analysis between lateral positions, had low risk of bias across domains, with the exception of blinding of participants and caregivers. Most included studies (n = 18) used a cross‐over design, but none sufficiently acknowledged methodological limitations associated with this design. Cross‐over trials often under‐report important methodological domains for assessment of risk of bias (Elbourne 2002; Mills 2009). Mills 2009 selected cross‐over studies from the PubMed database during a single month in 2000. These investigators found that reports of two‐treatment, two‐period cross‐over studies frequently omitted allocation concealment, issues around carryover effects and within‐subject effects. Similar issues of inadequate outcome reporting of within‐subject effects as well as inconsistencies in reporting study procedures for minimizing bias were prominent in this systematic review of cross‐over studies providing more than two treatments.
A meta‐epidemiological study that investigated evidence of bias associated with inadequate allocation concealment and lack of blinding in parallel‐group studies reported that the effect of inadequate or unclear allocation concealment on treatment estimates may vary, depending on whether the trial reported subjectively or objectively assessed outcomes, with the latter less likely to lead to substantial bias (Wood 2008). In this review, many studies with objective measures inadequately reported random sequence generation to minimize risk of selection bias. Furthermore, investigators did not usually describe concealment of random allocation. Uniform and balanced cross‐over studies that inadequately concealed allocation to groups (sequences) may introduce negligible bias for within‐subject differences in objectively assessed outcomes. However, most trials with uniform and balanced designs did not report within‐subject differences, and sources of bias associated with group allocation were difficult to unravel. If period effects are associated with possible group‐by‐period interactions, inadequate concealment may increase the risk of bias, even for cross‐over studies with objectively assessed measures. Furthermore, it may be difficult to distinguish group differences due to a genuine sequencing effect or due to inadequate concealment of allocation for non‐uniform unbalanced cross‐over studies.
The meta‐epidemiological study (Wood 2008) also found no evidence of bias attributed to lack of blinding of objective outcome data in parallel‐group trials. Review authors did not judge inadequate descriptions of outcome assessor blinding as introducing major risk for objectively assessed physiological variables. Overall, risk of detection bias was low. However, the type of intervention under investigation (body positioning) makes it difficult to control for performance bias. Performance differences during data collection may contribute to group (sequence) and period effects, but substantial performance bias may be unlikely for uniform and balanced cross‐over studies of relatively short duration.
Unclear risk of carryover bias in cross‐over designs
Carryover effects (positive, negative or indifferent residual effects) due to inadequate washout may seriously distort treatment effects (Elbourne 2002; Senn 2002). Cross‐over trials did not acknowledge carryover effects as a source of bias. Although some investigators may have performed tests for carryover, these tests cannot be used to distinguish simple carryover from more complex interactions (Jones 2003). Linear model analysis may express carryover effects in alternative ways, including carryover (residual) effects, group (sequence) effects or treatment‐by‐period interactions, depending on the type of design (Jones 2003). Interpreting such tests is problematic, as carryover effects and treatment‐by‐period interactions are confounded within designs that include three or more treatments and periods (a term known as 'aliasing', as each effect cannot be separately analysed) (Jones 2003).
Residual effects, period effects and sequence effects
Senn 2002 recommends that investigators should not test for carryover because this provides poor power to detect residual effects, but emphasizes that designs need to include sufficient washout between periods in the first place if investigators are to be satisfied that carryover effects are unlikely. Most cross‐over studies collected data just before turning to the next position within the sequence. The first measurement in each body position was taken within the first 30 minutes after turning, and most studies collected data 10 to 15 minutes after turning. Data‐free intervals (active or passive washout) before or after data collection according to the design did not match recommendations for washout four times as long as the measurable duration of effect (Senn 2002). In principle, return to baseline values may indicate the end of a measurable effect. However, uncertainty remains about the duration of a measurable effect for cardiopulmonary and haemodynamic variables upon turning. Turning in quick succession may confer carryover effects.
In the absence of sufficient washout between treatments, an optimal (uniformity and balanced) cross‐over design may account for the presence of carryover effects if period effects and treatment‐by‐period interactions are ruled out (Jones 2003; Senn 2002). In this case, residual effects are equally applied to all treatments for within‐subject analysis and are not a major contributor to biased effect estimates. However, plausible examples of secular changes that may contribute to a period effect during data collection of one or more oxygen transport variables include titration of fluid replacement, inotropic or vasoactive therapy, ventilatory care changes and decreased haemoglobin levels after cardiac surgery. Any one of these changes may not have transcended all periods. Only seven cross‐over studies (Banasik 1987; Banasik 1996; Banasik 2001; Chan 1992; George 2002; Kim 2002; Whitman 1982) reported a uniform design and a relative short study duration and thus were balanced for the effects of periods. Furthermore, period effect testing with multi‐variate and/or univariate analysis of variance (ANOVA) was not reported, and studies conducting tests other than ANOVA did not consider period effects or treatment‐by‐period interactions as possible sources of bias (see other biases section in the Characteristics of included studies). Lack of information on period differences within trials makes identification of possible sources of bias difficult when allocation concealment and performance bias are unknown, clinical management is not standardized and washout periods are inadequate to prevent carryover effects.
Sequence effects (i.e. simple group effects and more complex treatment‐by‐period interactions) are sources of bias in studies of cross‐over design with three or more treatments, but whether they contribute to substantial bias (large distortion in magnitude and/or direction of the effect estimate) depends on the design (Jones 2003; Senn 2002). Most cross‐over studies did not test for sequencing effects. Notably, unbalanced designs do not include all possible combinations of sequences involving the same treatments. Therefore, unexamined sequences of treatments may yield different results.
Potential biases in the review process
Assessment of trial quality as pre‐specified in the protocol was superseded by the risk of bias tools developed by The Cochrane Collaboration (Higgins 2011). However, the change in methodological assessment of individual studies is unlikely to lead to significant changes in findings, as the risk of bias assessment was implemented before data were collected, and sensitivity analysis criteria remained unchanged.
The published protocol did not pre‐specify thresholds for detecting a clinical adverse event. As such, review authors may have levied risk of detection bias if conclusions were based on meta‐analyses. However, review authors made every effort to minimize detection bias, including pre‐specifying clinical adverse events of interest and reporting only threshold results of studies that met conditions for meta‐analysis. We did not plan inferences unless studies demonstrated precision and low risk of bias and met threshold values for clinical adverse events of interest. Physiological variables such as blood pressure (BP) and arterial oxygen saturation (SaO2) are often referred to as surrogate outcomes or secondary clinical endpoints (Higgins 2011). However, critical thresholds for continuously monitored variables in the ICU are widely reported within the critical care literature, as they are clinical triggers for instigating therapy or changes in clinical management to avoid tissue hypoperfusion and organ dysfunction.
The Cochrane Handbook for Systematic Reviews of Interventios recommends separate analysis of parallel‐group studies and cross‐over trials because of differences in the unit of analysis (Higgins 2011). Furthermore, meta‐analyses may exclude cross‐over trials because of inherent difficulties with data extraction and statistical pooling when available data includes parallel‐group studies (Elbourne 2002). However, this systematic review did not avoid cross‐over trials, as the intention was to summarize all randomized studies that met our inclusion criteria to provide clarity about the strength of evidence. Furthermore, we did not conduct multiple meta‐analyses in this review; therefore we did not make statistical adjustments to account for multiplicity. However, multiplicity within analysis may pose a risk within future systematic reviews.
Agreements and disagreements with other studies or reviews
The most recent literature review (Johnson 2009) and integrative review (Winklelman 2010) on the effects of body positioning for critically ill patients failed to give weight to the numerous methodological issues that may limit the validity and applicability of reported studies to real‐world situations. Furthermore, findings from this systematic review were incongruent with findings from a previous systematic review on lateral positioning (Thomas 2007b). Thomas 2007b acknowledged the paucity of research but concluded that individual variation in lateral positions was large, greatest improvement in oxygenation was derived from the good lung down and haemodynamic compromise may be associated with lateral positioning in patients treated with coronary artery bypass grafting (CABG) during the 24‐hour postoperative period or in the extreme right lateral position. Authors from this systematic review could draw no such conclusions. However, differences between the two systematic reviews extended to differences in methodological approach. Thomas 2007b reported standardized mean differences (SMDs) with Hedge’s g statistic for pooled data from cross‐over designs. However, this summary statistic requires a pooled standard deviation (SD) for meta‐analyses. In addition, Thomas 2007b did not acknowledge carryover effects as a potential source of bias. In contrast, this systematic review has considered the appropriate unit of analysis for extracting data from cross‐over studies (i.e. within‐subject differences for paired comparisons). In addition, we considered risk of bias assessment, but statistical and clinical heterogeneity limited causal inferences. Nonetheless, this systematic review concurs with the previous systematic review in suggesting that long‐term outcomes should be investigated in future studies examining lateral positioning to aid clinical decision making.
Authors' conclusions
Implications for practice.
No longitudinal randomized studies that examine the benefits of regular lateral positioning for the duration of mechanical ventilation and beyond were found for this review. The effectiveness of lateral positioning compared with other positioning practices (i.e. supine positioning, semi recumbent positioning or prone positioning schedules) for pulmonary morbidity, other types of morbidity, clinical adverse events (potential threats to survival) and mortality remains unclear. Researchers have not been able to answer questions about lateral positioning (repetitive therapy) for critically ill adults.
Respiratory and/or haemodynamic instability in the ICU may lead to different positioning decisions by clinicians. Variations in turning response between lateral positions for patients with unilateral lung disease have been reported, including a paradoxical effect of better oxygenation with the bad lung down. However, it is not known whether this paradoxical effect is rare, frequent or widespread within certain subgroups of patients with unilateral lung disease. Futhermore, it is unclear whether labile cardiopulmonary or haemodynamic variables upon turning pose a perceived or direct threat (i.e. harm). Research has not provided strong evidence that critically ill patients have a higher incidence of haemodynamic and/or respiratory instability upon turning to lateral positions compared with other body positions.
Whether some critically ill patients may exhibit clinical adverse events of greater frequency or severity in a lateral position compared with other body positions, or in the first five minutes after turning compared with other time points throughout the duration of therapy, remains uncertain. Therefore, clinical decisions about whether to return the patient immediately to a more horizontal position or to semi recumbency when physiological variables become labile during lateral positioning must be considered on a case‐by‐case basis with reference to local guidelines for acceptable parameters. The effect of lateral positioning on morbidity, consequences of delays in turning patients and recognition of clinically important signs of haemodynamic and/or respiratory instability upon turning are important clinical considerations that remain to be resolved by researchers.
Implications for research.
Clinical practice utilizes various positioning schedules, but the optimal combination of the most effective body positions for treating morbidity, while preventing complications and minimizing clinical adverse events, remains unknown. Trials on prone positioning and semi recumbent positioning have not reported comparisons with routine lateral positioning as the clinical standard. It is unknown whether repositioning delays may increase the incidence of pulmonary and other morbidities. Further research is required to assess the effects of lateral positioning as a simple, inexpensive and easy to execute positioning practice compared with other positioning schedules (semi recumbent positioning and prone positioning) on respiratory outcomes such as acute lung injury (ALI), ventilator‐associated pneumonia (VAP) and severe atelectasis (involving several segments of a lung lobe or the whole lobe with associated opacification and loss of lung volume on chest radiograph) and mortality. Other outcomes such as resolution of dyspnoea, pain and discomfort and the incidence of adverse events or complications including pressure injury are of interest and help investigators weigh benefit versus harm in comparisons of critically ill patients or specific subgroups within this population.
The fact that some individuals may experience clinical adverse events in one or more body positions requires identification of the nature of those events and the participant characteristics most likely to be associated with benefit from regular turning versus those not associated with benefit, as risks (severe clinical adverse events, morbidity and mortality) may outweigh perceived benefits. Findings from this systematic review support the need for additional studies of routine patient positioning of critically ill adults with high acuity or severity of illness, to identify the nature and incidence of clinical adverse events during and after turning or repositioning. In addition, future studies (parallel‐group or cross‐over studies) may need to consider whether the chosen study design is best suited for examining specific postural effects (differences between body positions) or turning effects (differences in baseline changes), with investigators of future cross‐over studies cognizant of design features that minimize carryover bias.
All future researchers may need to consider consistent reporting of trial methods, participant characteristics and outcomes appropriate for the design to improve data collection and extraction for meta‐analyses of the quantitative evidence of effectiveness of lateral positioning as single or repetitive therapy. Such steps may lead to development of evidence‐based clinical guidelines for this fundamental nursing therapy as the result of unbiased quality research.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Nicola Petrucci (Content Editor), Nathan Pace (Statistical Editor), Dolores Matthews (Copy Editor), Tom J. Overend and Suzanne Robertson‐Malt (Peer Reviewers), Suzanne Cunliffe (Consumer Referee), and Jane Cracknell (Managing Editor) for help and editorial advice provided during preparation of this systematic review. Thank you to David Glanville (DG), Rebecca Brady (RB) and Laura Gittings (LG) for their contributions as independent reviewers (screening, selection, data abstraction and quality appraisal of papers), Margaret Staples for biostatistician advice, Kat Pawley for verification of handsearched journal entries and Claire Weeden (CW) and Jessica Guinane (JG) for their contributions in verifying extracted data.
Appendices
Appendix 1. Search strategies for electronic databases
1 MEDLINE (ISI) search strategy
| # | Search history |
| #1 | (MH:exp=Critical Care) or (MH:exp=Life Support Care) or (MH:exp=Critical Illness) or (TS="critical care") or (TS="intensive care") or (TS="coronary care") or (TS="cardiothoracic unit") or (TS=( ICU or ITU or CCU or CTU )) or (TS="critical* ill*") |
| #2 | (MH:exp=Respiration, Artificial) or (MH:exp=Ventilators, Mechanical) or (TS="artificial* respirat*") or (TS="mechanical* ventilat*") or (TS="positive pressure ventilat*") or (TS="non invasive ventilat*") |
| #3 | (#1 or #2) |
| #4 | (TS="lateral position*") or (TS= "lateral rotat*") or (TS= "lateral recumben*") or (TS= "lateral turn*") or (TS= "lateral decubit*") or (TS= "lateral tilt*") or (TS="side lying") or (TS= "side position*") or ((TI=lateral or AB=lateral) and MH:exp=Posture) or (TI="dependent position*" or AB="dependent position*") |
| #5 | (MH=Prone Position) or (MH=Supine Position) or (MH=Head‐down Tilt) or (TS="supine position*") or (TS="dorsal position*") or (TS=recumben*) or (TS="horizontal position*") or (TS="prone position*") or (TS="ventral decubit*") or (TS="head down*") or (TS="head tilt*") or (TS=Trendelenburg) or (TS="vertical position*") or (TS="degree* position*") or (TS="backrest elevat*") or (TS= "head elevat*") or (TS=(semi‐Fowler* or Fowler*)) or (TS=(semi‐recumben* or semirecumben*)) or (TS=sitting) or (TS="upright position*") or ((TI= position* or AB= position*) and MH:exp=Posture) |
| #6 | (#4 or #5) |
| #7 | (#3 and #6) |
| #8 | (#7) AND Document Types=(Randomized Controlled Trial) |
| #9 | (MH=(randomized controlled trials) OR MH=(Random Allocation) OR MH=(Double Blind Method) OR MH=(Single Blind Method)) |
| #10 | #7 and #9 |
| #11 | #8 or #10 |
| #12 | MH=animals not (MH=human and MH=animals) |
| #13 | #11 not #12 |
| #14 | (#7) AND Document Types=(Clinical Trial) |
| #15 | ((MH:exp= Clinical Trials) or (TI= (clin* SAME trial*)) or (AB=(clin* SAME trial*)) or (TI=((singl* or doubl* or trebl* or tripl*) SAME (blind* or mask*))) or (AB=((singl* or doubl* or trebl* or tripl*) SAME (blind* or mask*))) or (MH=Placebos) or (TI=placebo* or AB=placebo*) or (TI=random* or AB=random*) or (MH=Research Design)) |
| #16 | #7 and #15 |
| #17 | #14 or #16 |
| #18 | #17 not #12 |
| #19 | (MH=Comparative Study) or (MH:exp=Evaluation studies) or (MH=Follow Up Studies) or (MH=Prospective Studies) or (TI=(control* or prospectiv* or volunteer*) or AB=(control* or prospectiv* or volunteer*)) |
| #20 | #7 and #19 |
| #21 | #20 not #12 |
| #22 | #21 OR #18 OR #13 |
2 Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
| # | Search history |
| #1 | MeSH descriptor: [Critical Care] explode all trees |
| #2 | MeSH descriptor: [Life Support Care] this term only |
| #3 | MeSH descriptor: [Critical Illness] this term only |
| #4 | (critical care) or (intensive care) or (coronary care) or (cardiothoracic unit) or (ICU or ITU or CCU or CTU) or (critical* ill*) |
| #5 | MeSH descriptor: [Respiration, Artificial] this term only |
| #6 | MeSH descriptor: [Ventilators, Mechanical] explode all trees |
| #7 | (artificial* respirat*) or (mechanical* ventilat*) or (positive pressure ventilat*) or (non invasive ventilat*) |
| #8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 in Trials |
| #9 | (lateral position*) or (lateral rotat*) or (lateral recumben*) or (lateral turn*) or (lateral decubit*) or (lateral tilt) or (side lying) or (side position*) or (dependent position*) |
| #10 | MeSH descriptor: [Posture] explode all trees |
| #11 | lateral and #10 |
| #12 | #9 or #11 in Trials |
| #13 | MeSH descriptor: [Prone Position] this term only |
| #14 | MeSH descriptor: [Supine Position] this term only |
| #15 | MeSH descriptor: [Head‐Down Tilt] this term only |
| #16 | (supine position) or (dorsal position) or (recumbent*) or (horizontal position*) or (prone position) or (ventral decubuit*) or (head down*) or (head tilt*) or (Trendelenburg) or (vertical position*) or (degree* position*) or (backrest elevat*) or (head elevat*) or ((semi‐Fowler*) or (Fowler*)) or ((semi‐recumben*) or (semirecumben*)) or (sitting) or (upright position*) |
| #17 | position* and #10 |
| #18 | #13 or #14 or #15 or #16 or #17 in Trials |
| #19 | #12 or #18 |
| #20 | #8 and #19 |
3 CINAHL (EBSCOhost) search strategy
| S | Search history |
| S1 | (MH "Critical Care+") or (MH "Critical Care Nursing+") or (MH "Life Support Care") or (MH "Critical Illness") or (MH "Critically Ill Patients") or (MH "Intensive Care Units+") or "critical care" or "intensive care" or "coronary care" or "cardiothoracic unit" or (ICU or ITU or CCU or CTU) or "critical* ill*" |
| S2 | (MH "Ventilation, Mechanical+") or "mechanical ventilat*" or "artificial* respirat*" or "positive pressure ventilat*" or "noninvasive ventilat*" or "non invasive ventilat*" |
| S3 | S1 or S2 |
| S4 | "lateral position*" or "lateral rotat*" or "lateral recumben*" or "lateral turn*" or "lateral decubit*" or "lateral tilt*" or "side lying" or "side position*" or ((TI lateral or AB lateral) and MH "POSTURE+") or ((TI lateral or AB lateral ) and MH "Patient Positioning") or ((TI dependent position* or AB dependent position*)) |
| S5 | (MH "Patient Positioning+") or "supine position*" or "dorsal position*" or recumben* or "horizontal position*" or "prone position*" or "ventral decubit*" or "head down*" or "head tilt*" or Trendelenburg or "vertical position*" or degree* position* or "backrest elevat*" or "head elevat*" or semi‐Fowler* or Fowler* or semi recumben* or semirecumben* or sitting or "upright position*" or ((TI position* or AB position*) and MH "POSTURE+") |
| S6 | S4 or S5 |
| S7 | S3 and S6 |
| S8 | (PT Clinical Trial) or "random* control* trial*" or (MH "Random Sample+") or (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Clinical Trials+") or (TI clinical trial* or AB clinical trial*) or (TI (singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or (AB (singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or (MH Placebos) or (TI placebo* or AB placebo*) or (TI random* or AB random*) or (MH "Quantitative Studies+") or (MH "Crossover Design") or (MH "Quasi‐Experimental Studies+") or (MH "Comparative Studies") or (MH "Evaluation Research") or (MH "Prospective Studies") or (TI (control* or prospectiv* or volunteer*) or AB (control* or prospectiv* or volunteer*)) |
| S9 | S7 and S8 |
4 AMED (EBSCOhost) search strategy
| S | Search history |
| S1 | (SU "Critical Care") or (SU "Intensive Care") or (SU "Intensive Care Units") or (SU "Coronary Care") or (SU "Life Support Care") or (SU "Critical Illness") or (TX "critical care") or (TX "intensive care") or (TX "coronary care") or (TX "cardiothoracic") or (TX (ICU or ITU or CCU or CTU)) or (TX "critical* ill*") |
| S2 | (SU "Ventilators Mechanical") or (SU "Respiration Artificial") or (TX "mechanical* ventilat*") or (TX "artificial* respirat*") or (TX "positive pressure ventilat*") or (TX "non‐invasive ventilat*" or "non invasive ventilat*") |
| S3 | S1 or S2 |
| S4 | (TX "lateral position*") or (TX "lateral rotat*") or (TX "lateral recumben*") or (TX "lateral turn*") or (TX "lateral decubit*") or (TX "lateral tilt*") or (TX "side lying") or (TX "side position*") or (( TI "lateral" or AB "lateral" ) and SU "Posture") or (TI "dependent position*" or AB "dependent position*") |
| S5 | (SU "Prone Position") or (SU "Supine Position") or (SU "Head‐down Tilt") or (TX "supine position*") or (TX "dorsal position*") or (TX "recumben*") or (TX "horizontal position*") or (TX "prone position*") or (TX "ventral decubit*") or (TX "head down*") or (TX "head tilt*") or (TX "Trendelenburg*") or (TX "vertical position*") or (TX "degree* position*") or (TX "backrest elevat*") or (TX "head elevat*") or (TX "Fowler*") or (TX "semi recumben*") or (TX "sitting") or (TX "upright position*") or (( TI position* or AB position* ) and SU "Posture") |
| S6 | S4 or S5 |
| S7 | S3 and S6 |
5 LILACS (Virtual Health Library) search strategy
| # | Search history |
| 1 | critical$ AND lateral AND position$ |
| 2 | intensive AND lateral AND position$ |
| 3 | ventilat$ AND lateral AND position$ |
| 4 | critical$ AND lateral AND turn$ |
| 5 | intensive AND lateral AND turn$ |
| 6 | ventilat$ AND lateral AND turn$ |
| 7 | critical$ AND lateral AND rotation$ |
| 8 | intensive AND lateral AND rotation$ |
| 9 | ventilat$ AND lateral AND rotation$ |
| 10 | critical$ AND side AND lying |
| 11 | intensive AND side AND lying |
| 12 | ventilat$ AND side AND lying |
| 13 | critical$ AND postur$ |
| 14 | intensive AND postur$ |
| 15 | ventilat$ AND postur$ |
| 16 | Print out of searches 1 to 15 |
6 Web of Science (ISI) search strategy
| # | Search history |
| #1 | (TS="critical care") or (TS="life support") or (TS="intensive care") or (TS="coronary care") or (TS="cardiothoracic unit") or (TS=(ICU or ITU or CCU or CTU)) or (TS="critical* ill*") |
| #2 | (TS="Respiration, Artificial") or (TS="Ventilators, Mechanical") or (TS="artificial* respirat*") or (TS="mechanical* ventilat*") or (TS="positive pressure ventilat*") or (TS="non invasive ventilat*") |
| #3 | #1 or #2 |
| #4 | (TS="lateral position*") or (TS="lateral rotat*") or (TS="lateral recumben*") or (TS="lateral turn*" ) or (TS="lateral decubit*") or (TS="lateral tilt*") or (TS="side lying") or (TS="side position*") or (TS="lateral" and TS=Posture) or (TS="dependent position*") |
| #5 | (TS="prone position*") or (TS="supine position*") or (TS="head down tilt") or (TS="dorsal position*") or (TS= recumben*) or (TS="horizontal position*") or (TS="ventral decubit*" ) or (TS="head tilt") or (TS=Trendelenburg) or (TS="vertical position*") or (TS="degree* position*") or (TS="backrest elevat*") or (TS="head elevat*") or (TS=Fowler*) or (TS="semi recumben*" or TS="semirecumben*") or (TS=sitting) or (TS="upright position*") or (TS=position* and TS=Posture) |
| #6 | #4 or #5 |
| #7 | #3 and #6 |
| #8 | (TS="random* control* trial*") or (TS="clinical trial*") or (TS=((singl* or doubl* or trebl* or tripl*) SAME (blind* or mask*))) or (TS=placebo*) or (TS=random*) or (TS=(control* or prospectiv* or volunteer*)) |
| #9 | #7 and #8 |
7 Index to Theses search strategy
| S | Search history |
| 1 | ((ti contains critical care) OR (ti contains intensive care) OR (ti contains critical* ill*) OR (ti contains ventilat*)) AND (ti contains lateral*) |
| 2 | ((ti contains critical care) OR (ti contains intensive care) OR (ti contains critical* ill*) OR (ti contains ventilat*)) AND (ti contains position*) |
| 3 | ((ti contains critical care) OR (ti contains intensive care) OR (ti contains critical* ill*) OR (ti contains ventilat*)) AND (ti contains turn*) |
| 4 | ((ti contains critical care) OR (ti contains intensive care) OR (ti contains critical* ill*) OR (ti contains ventilat*)) AND (ti contains rotation*) |
| 5 | ((ti contains critical care) OR (ti contains intensive care) OR (ti contains critical* ill*) OR (ti contains ventilat*)) AND (ti contains side lying) |
| 6 | ((ti contains critical care) OR (ti contains intensive care) OR (ti contains critical* ill*) OR (ti contains ventilat*)) AND (ti contains postur*) |
| 7 | Printout 1 to 6 |
8 Trove search strategy (limited to theses and Australian content), previously Australasian Digital Theses Program
| # | Search history |
| #1 | "critical care" and lateral |
| #2 | "intensive care" and lateral |
| #3 | "critically ill" and lateral |
| #4 | "critical illness" and lateral |
| #5 | ventilat* and lateral |
| #6 | "critical care" and position |
| #7 | "intensive care" and position |
| #8 | "critically ill" and position |
| #9 | "critical illness" and position |
| #10 | ventilat* and position |
| #11 | "critical care" and turn |
| #12 | "intensive care" and turn |
| #13 | "critically ill" and turn |
| #14 | "critical illness" and turn |
| #15 | ventilat* and turn |
| #16 | "critical care" and rotation |
| #17 | "intensive care" and rotation |
| #18 | "critically ill" and rotation |
| #19 | "critical illness" and rotation |
| #20 | ventilat* and rotation |
| #21 | "critical care" and "side lying" |
| #22 | "intensive care" and "side lying" |
| #23 | "critically ill" and "side lying" |
| #24 | "critical illness" and "side lying" |
| #25 | ventilat* and "side lying" |
| #26 | "critical care" and postur* |
| #27 | "intensive care" and postur* |
| #28 | "critically ill" and postur* |
| #29 | "critical illness" and postur* |
| #30 | ventilat* and postur* |
| #31 | List of #1 to #30 |
9 ProQuest Dissertations and Theses search strategy, previously ProQuest Digital Dissertations
| # | Search history |
| #1 | ("critical care" and "lateral position*") or ("intensive care" and "lateral position*") or ("critical* ill*" and "lateral position*") or ("ventilat*" and "lateral position*") |
| #2 | ("critical care" and "lateral turn*") or ("intensive care" and "lateral turn*") or ("critical* ill*" and "lateral turn*") or ("ventilat*" and "lateral turn*") |
| #3 | ("critical care" and "lateral rotation*") or ("intensive care" and "lateral rotation*") or ("critical* ill*" and "lateral rotation*") or ("ventilat*" and "lateral rotation*") |
| #4 | ("critical care" and "side lying") or ("intensive care" and "side lying") or ("critical* ill*" and "side lying") or ("ventilat*" and "side lying") |
| #5 | ("critical care" and "body postur*") or ("intensive care" and "body postur*") or ("critical* ill*" and "body postur*") or ("ventilat*" and " body postur*") |
| #6 | Marked records (#1‐ #5) |
Appendix 2. Assessment of eligibility tool
Study Eligibility Form
(screening and selection of trials for inclusion in the review)
| Review number | Date completed | |||||||
| Name of reviewer | Abstract | |||||||
| Full text | ||||||||
| Notes and changes after consensus agreement (recorded in red ink on primary reviewer’s form only) | ||||||||
| Decision: Return to this section upon completion of eligibility screening and indicate the following status | ||||||||
| Awaiting further assessment | To be included in review | To be excluded from review | ||||||
| Reason for exclusion | ||||||||
| Summary of trial eligibility | ||||||||
| RCT/Quasi‐randomized trial | Critically ill adult participants | Right and/or left lateral position compared with other body positions (each body position maintained for ≥ 10 minutes) | Trial includes mortality, morbidity or clinical adverse events as primary outcomes, or trial includes ≥ 1 secondary outcome | |||||
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | |||||
| First study author | Journal article/Thesis/Conference proceedings (include source, volume (issue), page numbers) | Year | ||||||
Specific Inclusion Criteria
| A. The research design is a randomized controlled trial or a quasi‐randomized trial? | ||
| Yes | No | Unclear |
| B. The study population is human? | ||
| Yes | No | Unclear |
| C. The study population is adult (> 16 years of age)? A mixture of adult and children participants will be considered if individual data are provided for each participant and the proportion of children enrolled in the trial does not exceed 10% | ||
| Yes | No | Unclear |
| D. Trial is located in a critical care area (e.g. intensive care unit (ICU), coronary care unit (CCU), cardiothoracic unit (CTU))? | ||
| Yes | No | Unclear |
E. The trial population is critically ill? At least 1 of the following statements must be met for the trial to be eligible for inclusion
| ||
| Yes 1 2 3 4 (circle number) |
No | Unclear 1 2 3 4 (circle number) |
F. The trial’s intervention of interest (right and/or left lateral position) involves comparison with ≥ 1 of the following body positions
With regards to the intervention of interest (the lateral position) and comparisons 2. Each body position should be maintained for ≥ 10 minutes before the next position change to be eligible for inclusion. Kinetic therapy and continuous lateral rotation therapy (CLRT) may be eligible, if separate data are provided for right and left lateral position with duration ≥ 10 minutes in each position | ||
| Yes 1 2 (circle number) |
No 1 2 (circle number) |
Unclear 1 2 (circle number) |
G. The trial reports ≥ 1 of the following outcome measures
| ||
| Yes 1 2 3 4 5 6 7 8 9 (circle number) |
No | Unclear 1 2 3 4 5 6 7 8 9 (circle number) |
Specific Exclusion Criteria – excluded trials are marked NO
H. With regards to the following subgroup of participants
| ||
| Yes | No 1 2 3 (circle number) |
Unclear 1 2 3 (circle number) |
| I. With regards to outcome measures The trial measured more than pressure ulcer formation as a primary outcome of interest (i.e. tick No to exclude trials investigating pressure ulcer formation as the sole primary outcome) | ||
| Yes | No | Unclear |
A trial will be eligible for inclusion if all inclusion criteria questions (A to G) and all exclusion criteria questions (H to I) are marked yes.
List below any potentially relevant reports found within the reference list.
| First author | Journal article (include source, volume (issue), page numbers) | Year of publication |
Appendix 3. Data collection and extraction tool
Data Collection Form
(Data extraction and risk of bias assessment of included trials)
|
STUDY ID (first author of primary reference and year of publication) |
Reviewer’s name | ||
| Date completed | |||
| Notes: Freehand space for writing actions such as contact with trial authors, translation required and changes made after consensus agreement (changes recorded in red ink on primary reviewer’s form only) | |||
| Re‐verification of eligibility |
| Has an eligibility form been completed for this trial? Yes/No If no, complete an eligibility form before proceeding |
Check other references identified in searches. If further references to this trial are known, link the papers now and list the source below, starting with the paper listed as the primary reference.
| Review number (as stated on eligibility form) | Study author(s) | Journal/Thesis/Conference proceedings, etc (include the language of non‐English papers in brackets) | Year |
Data Extraction
| Participant characteristics | |
| Characteristic | Description/Detail from report |
| 1. Age (range, mean/SD, median, etc., within each group, if provided. Report on the number of children younger than age 16) | |
| 2. Sex of participants (numbers, %, etc., within each group, if provided) | |
| 3. Primary diagnosis(disease, condition or injury) and co‐morbidities reported at baseline (description, numbers, %, etc., within each group, if provided) | |
| 4. Severity of illness score/scale at baseline (type of measurement tool, description of scale/score including lower and upper limits, numbers, %, mean/SD, median, range, etc., within each group, if provided) | |
| 5. Physiological, immunological, biochemicalmeasurements or diagnostic findings reported at baseline as indicators of participant’s diagnosis and/or severity of illness (description of measurements and findings, number, %, mean/SD, median, range, etc., within each group, if provided) | |
| Participant characteristics | Description/Detail from report |
| 6. Ventilation status (report description, numbers, %, mean/SD, median, range, etc., within each group, if provided). Include data on the following a. type of ventilation mode, e.g. · spontaneous unassisted breathing (SB) · non‐invasive CPAP (CPAP) · non‐invasive ventilation (NIV) · mechanical ventilation (MV), state specific mode of MV reported b. ventilation settings, if provided · mean airway pressure · tidal volume (Vt) · pressure support or IPAP · PEEP or EPAP · FiO2 c. If ventilatory changes were made during the trial, such as changes in ventilatory settings, cessation of mechanical ventilation or extubation |
|
| 7. Standard management and care (report type of standard management and care applied across groups, e.g. sedation, muscle relaxants, inotropes or vasoactive drugs used, including dose rate and titration protocol, as well as suctioning procedures during the trial, etc.) | |
| 8. Other (additional information that maybe relevant e.g. report numbers, % etc and provide details on any deviation from the protocol, co‐intervention other than standard management and care not applied equally across all groups of the trial, adverse events other than the outcomes of interest reported after group allocation). |
| Trial Characteristics | Description/Detail from Report |
| 9. What was the trial’s design (parallel, cross‐over, other etc). (Parallel trial – participants assigned to two or more treatment groups via randomization and remain in allocated group for remainder of trial. Crossover trial – participants are randomized to a particular sequence of treatments and serve as their own control). |
|
| 10. What was the setting and country of the trial? (Specify specialty (ICU, CCU or CTU etc) and type of hospital or level of trauma centre (tertiary or level I, II or III hospital etc in the country of origin). | |
| 11. How was the reference population defined? (i.e. What were the inclusion criteria prior to group allocation?) | |
| 12. What were the exclusion criteria before group allocation? | |
| 13. Was the sample size justified by a priori calculation of effect size/power? If not, state how study author justified sample size | |
| 14. How many people were eligible to participate from the reference population (i.e. those who met inclusion criteria)? |
| Trial characteristics | Description/Detail from report |
| 15. How many participants were randomized in the trial? | |
| 16. State the title/name given to each randomly allocated treatment group by investigators/authors of the trial (use direct quotes) and the number of participants in each group | |
| 17. Number of participants in each group who received the intended treatment | |
| 18. Number of participants reported in the analysis for each outcome (Provide details of any differences in numbers of participants between intended and actual analysis of outcomes) | |
| 19. Number of excluded participants (i.e. investigators/trialists withdrew participants from trial after randomization and group allocation. Report numbers, %, etc., and study author’s rationale for exclusion) | |
| 20. Number of drop‐outs or loss to follow‐up (participants who intentionally or unintentionally withdrew from the trial after treatment allocation. Report numbers, %, etc., and rationale if provided) | |
| 21. Length of follow‐up reported in the trial | |
| 22. Duration of the trial (i.e. from first to last participant) | |
| 23. Describe the positioning schedule and/or sequence for each allocated treatment group (e.g. group 1 ‐ 2‐hourly lateral positioning vs group 2 ‐ supine positioning (parallel‐group design) or randomized sequence of SLR for all participants serving as own controls with 6 possible combinations of SLR (cross‐over design). Report on the following · duration in each body position, including baseline position · total number of turns undertaken by participants in each group during the trial · total duration of the positioning schedule (from baseline/first position to last measurement in the last position). If cross‐over design, give duration in each arm and total duration |
| Trial characteristics | Description/Detail from report |
| 24.Description of the positioning technique for the intervention (i.e. lateral position(s)), including a. degree of rotation b. degree of head or feet elevation c. presence of hip flexion d. method of verification |
|
| 25.Description of the positioning technique for the comparison body position(s), including if applicable e. degree of rotation f. degree of head or feet elevation g. presence of hip flexion h. method of verification |
|
| 26.Was a stabilization or washout period reported before, during or after a position change? Describe the study author’s rationale for stabilization or washout, length of time (in minutes), whether it was used for all groups before or after a particular position or at any stage during the trial, etc. |
|
| 27.Were outcomes measures validated? List instruments or measurements that were validated and the method of validation used |
|
| Trial characteristics | Description/Detail from report |
| 28.Were specific definitions given for each outcome measure (including values, formulas, thresholds, events or complications)? | |
| 29.Is there any other relevant information? (e.g. industry or other sources of funding, description of outliers, any other relevant information such as unclear aims or objectives, outcome measures taken but not discussed in the results section) |
| Outcomes of interest | |
|
Outcomes of interest for this review are as follows 30. in‐hospital mortality 31. morbidity (pulmonary, cardiovascular or other) 32. clinical adverse events (see instructions) 33. oxygenation index ((mean airway pressure × FiO2 × 100/PaO2 or able to be calculated from individual data) or hypoxia score (PaO2/FiO2 ratio stated or able to be calculated from individual data) 34. pulmonary artery pressures 35. vital signs 36. duration of mechanical ventilation 37. length of stay in the critical care area 38. length of stay in hospital 39. patient comfort or satisfaction measures | |
| State the outcome of interest reported in the paper (usually reported in abstract/introduction and/or methods section). Any event or outcome described in the analysis section only, which is not an intended outcome under investigation, describe under trial characteristics |
Time points of measurement or time to follow‐up (see instructions) |
| Number _____ Provide description of outcome _____________________________ |
|
| Number _____ Provide description of outcome _____________________________ |
|
| Number _____ Provide description of outcome _____________________________ |
|
| Number _____ Provide description of outcome _____________________________ |
|
| Number _____ Provide description of outcome _____________________________ |
|
Risk of Bias Assessment
| Domain | Judgement (circle) | Description (for quotation, use exclamation marks) |
|
Was the allocation sequence adequately generated? Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. For cross‐over trials, was the order of treatment randomized? |
Yes/No/Unclear | |
|
Was allocation adequately concealed? Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment |
Yes/No/Unclear | |
|
Was knowledge of the allocated intervention adequately prevented during the study? For participants, caregivers and outcome assessors: Describe the methods used to blind awareness of group assignment for each main outcome Provide any information related to whether the intended blinding was effective |
Yes/No/Unclear | |
|
Were incomplete outcome data adequately addressed? Describe the completeness of data for each main outcome including attrition and exclusions from the analysis. Are any data points missing? Were withdrawals and exclusions detailed separately and included in the intention‐to‐treat analysis? |
Yes/No/Unclear |
| Domain | Judgement (circle) | Description (for quotation, use exclamation marks) |
|
Are reports of the study free of suggestion of selective outcome reporting? Have the outcome data been analysed according to the method/protocol stated? If not, please specify the differences, including outcome measures not reported in the analysis |
Yes/No/Unclear | |
|
Was the study apparently free of other problems that could put it at high risk of bias? Were groups comparable on trial entry? If not, specify the differences. Is there likelihood of serious carryover effects between treatments in cross‐over trials, i.e. was the washout period between treatments adequate? Did all participants in all groups have outcomes measured in the same way? If not, please specify the differences. Were measurement tools validated appropriately? If not, specify why the outcome measure may be prone to bias. Did intervention and comparison groups receive identical management and standard care during the trial, except for the intervention of interest? If not, specify differences in management or standard care |
Yes/No/Unclear |
| For continuous data | ||||||||
| State outcome of interest | Unit of measurement # State whether outcome is final value (fv) or change from baseline value (cbv) |
Time point (refer to guide) |
State subgroup for pair‐wise comparison. e.g. R) lung disease | Results | Notes (include related statistics and descriptive results if no statistics provided) | |||
| State body position or intervention for comparison ____________________ |
State body position or intervention for comparison ___________________ |
|||||||
| n | Mean (SD)a | n | Mean (SD)a | |||||
| State body position or intervention for comparison ____________________ |
State body position or intervention for comparison ___________________ |
|||||||
| n | Mean (SD)a | n | Mean (SD)a | |||||
| State body position or intervention for comparison ____________________ |
State body position or intervention for comparison ___________________ |
|||||||
| n | Mean (SD)a | n | Mean (SD)a | |||||
aFor cross‐over trials, report standard error (SE) or standard deviation of the mean difference (SDdiff ) for pair‐wise comparisons. Alternatively, standard error (SE) of the mean difference (MD) or confidence intervals for the MD or paired t‐statistic or P value from paired t test (if any of these statistics are reported). # Convert kPa to mmHg, divide kPa by 0.133 as the conversion factor. State any other conversion factors used
| For dichotomous data | |||||
| State group or subgroup e.g. L) lung disease. |
State outcome of interest | Time point (refer to guide) |
Results | ||
| State intervention/group 1 ____________________ |
State intervention/control/ group 2 or comparison ___________________ |
||||
| n/N n = number of participants with outcome, not number of events N = total number of participants in group Write both n and N for each outcome |
n/N n = number of participants with outcome, not number of events N = total number of participants in group Write both n and N for each outcome |
||||
|
Other information relevant to the results Indicate whether any data were obtained from the primary study author or if results were estimated from graphs, etc., or were calculated by you using a formula (this should be stated and the formula given). In general, if results are not reported in paper(s) obtained, this should be made clear here to be cited in the review |
|||||
Source acknowledgment
Cochrane Anaesthesia Review Group (CARG) Data Extraction Form, version 3, January 2007
The Cochrane Collaboration tool for assessing risk of bias
Appendix 4. Lateral position versus a comparison body position: estimate of treatment effect from single cross‐over studies with extractable partial pressure of arterial oxygen (PaO2) data
4.1 MD in PaO2 (mmHg) with 95% CI (generic inverse variance (GIV), random‐effects model (REM)) between right lateral (R) and left lateral (L) positions during an early turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (R–L) | SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in PaO2 at 10 minutes after turn | 1 | 60 | 5.2 | 2.2 | 5.20 (0.89 to 9.51) | 2.36 (P value = 0.02) |
| 4.1.1. Subgroup without left lung atelectasis (i.e. bilateral or no atelectasis) |
Banasik 1987 | 50/60 | 3.9 | 2.4 | 3.90 (‐0.80 to 8.60) | 1.63 (P value = 0.10) |
| 4.1.2. Subgroup with left lung atelectasis | Banasik 1987 | 10/60 | 11.8 | 4.6 | 11.80 (2.78 to 20.82) | 2.57 (P value = 0.01) |
Footnote a Reported MD and SE (MD). Subgroup data entered intoRevMan 5.3generated a weighted mean difference (WMD) of 6.88 mmHg (CI 95% ‐0.63 to 14.38, P value = 0.07), with 62.3% weighting for the subgroup without left lung atelectasis. Weighting explains the difference between reported MD and calculated MD using subgroup data
4.2 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between native lung down (N) and allograft lung down (A) for postoperative single lung transplant participants during an early turning response
| Outcome/Subgroup | Studies | Number | MD (N–A) | SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in PaO2 at 5 minutes after turn | George 2002 | 15 | 2.6 | 7.183 | 2.60 (‐11.48 to 16.68) | 0.36 (P value = 0.72) |
Footnote: aTreatment effect estimate calculated from individual participant data
4.3 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between right lateral (R) and left lateral (L) positions during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (R–L) | SE (MD) | Effect estimate (95% CI) | Z test |
|
MD in PaO2 during a short‐term turning response |
2 | 40 | No pooled data because of participant dissimilarities and insufficient data |
|||
| 4.3.1. aMD in PaO2 at 15 minutes after turn | Ibanez 1981 | 10 | ‐16.2 | 22.366 | ‐16.20 (‐60.04 to 27.64) | 0.72 (P value = 0.47) |
| 4.3.2. aMD in PaO2 at 30 minutes after turn | Chan 1992 | 30 | Insufficient data | |||
| 4.3.2.1. Subgroup with unilateral atelectasis | Chan 1992 | 6/30 | 0.2 | 5.544 | 0.20 (‐10.67 to 11.07) | 0.15 (P value = 0.88) |
| 4.3.2.2. Subgroup with no atelectasis | Chan 1992 | 3/30 | 6.333 | 7.881 | 6.33 (‐9.11 to 21.78) | 0.8 (P value = 0.42) |
| 4.3.2.3. Subgroup with bilateral atelectasis | Chan 1992 | 21/30 | Effect estimate unable to be calculated |
Footnote: aTreatment effect estimates calculated from individual participant data
4.4 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between native lung down (N) and allograft lung down (A) for postoperative single lung transplant participants during a short‐term turning response
| Outcome/Subgroup | Studies | Number | MD (N–A) | SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in PaO2 at 15 minutes after turn | George 2002 | 15 | ‐4.533 | 9.284 | ‐4.53 (‐22.73 to 13.66) | 0.49 (P value = 0.63) |
Footnote: aTreatment effect estimate calculated from individual participant data
4.5 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between bad lung down (BLD) and good lung down (GLD) for unilateral lung disease participants across composite time intervals
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (BLD–GLD) |
SE (MD) | Effect estimate (95% CI) | Z test |
| MD in PaO2 after turning | 4 | 35 | See meta‐analysis (subgroup data not included). |
|||
| 4.5.1. subgroup with left lung atelectasis at 10 minutes after turn |
Banasik 1987 | 10/60 | ‐11.8 | 4.6 | ‐11.80 (‐20.82 to ‐2.78) | 2.57 (P value = 0.01) |
| 4.5.2. a,bMD in PaO2 at 10 minutes after turn | Remolina 1981 | 9 | ‐40.888 | 13.217 | ‐40.89 (‐66.79 to ‐14.98) | 3.09 (P value = 0.002) |
| 4.5.3. aMD in PaO2 at 15 minutes after turn | Ibanez 1981 | 10 | ‐57.2 | 12.878 | ‐57.20 (‐82.44 to ‐31.96) | 4.44 (P value < 0.00001) |
| 4.5.4. aSubgroup with unilateral atelectasis at 30 minutes after turn |
Chan 1992 | 6/30 | 0.333 | 4.558 | 0.33 (‐8.60 to 9.27) | 0.07 (P value = 0.94) |
Footnotes: aTreatment effect estimates calculated from individual participant data. bSample had an adjusted calculation with second data set removed to avoid a unit of analysis error
4.6 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between supine position (S) and allograft lung down (A) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number | MD (S–A) | SE (MD) | Effect estimate (95% CI) | Z test |
| 4.6.1. aMD in PaO2 at 5 minutes after turn | George 2002 | 15 | ‐2.0 | 5.126 | ‐ 2.00 (‐12.05 to 8.05) | 0.39 (P value = 0.7) |
| 4.6.2.aMD in PaO2 at 15 minutes after turn | George 2002 | 15 | ‐5.67 | 4.552 | ‐5.67 (‐14.59 to 3.25) | 1.24 (P value = 0.21) |
Footnote: aTreatment effect estimates calculated from individual participant data
4.7 MD in PaO2 (mm Hg) with 95% CI (GIV, REM) between supine position (S) and native lung down (N) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number | MD (S–N) | SE (MD) | Effect estimate (95% CI) | Z test |
| 4.7.1. aMD in PaO2 at 5 minutes after turn | George 2002 | 15 | ‐4.60 | 5.459 | ‐4.60 (‐15.30 to 6.10) | 0.84 (P value = 0.4) |
| 4.7.2. aMD in PaO2 at 15 minutes after turn | George 2002 | 15 | ‐1.13 | 6.453 | ‐1.13 (‐13.78 to 11.51) | 0.18 (P value = 0.86) |
Footnote: aTreatment effect estimates calculated from individual participant data
4.8 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between supine position (S) and left lateral (L) position during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–L) |
SE (MD) |
Effect estimate (95% CI) | Z test |
| a,bMD in PaO2 at 30 minutes after turn | 1 | 30 | Insufficient data | |||
| 4.8.1. Subgroup with left lower lobe atelectasis | Chan 1992 | 5/30 | ‐4.8 | 2.691 | ‐4.80 (‐10.07 to 0.47) | 1.78 (P value = 0.07) |
| 4.8.2. Subgroup with no atelectasis | Chan 1992 | 3/30 | 2.667 | 9.333 | 2.67 (‐15.63 to 20.96) | 0.29 (P value = 0.078) |
| 4.8.3. Subgroup with right lower lobe atelectasis | Chan 1992 | 1/30 | Effect estimate unable to be calculated. |
|||
| 4.8.4. Subgroup with bilateral atelectasis | Chan 1992 | 21/30 | Effect estimate unable to be calculated. |
Footnotes: aTreatment effect estimates calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
4.9 MD in PaO2(mmHg) with 95% CI (GIV, REM) between supine position (S) and right lateral (R) position during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–R) | SE (MD) | Effect estimate (95% CI) | Z test |
| a,bMD in PaO2 at 30 minutes after turn | 1 | 30 | Insufficient data | |||
| 4.9.1. Subgroup with left lower lobe atelectasis | Chan 1992 | 5/30 | ‐5.00 | 5.683 | ‐5.00 (‐16.14 to 6.14) | 0.88 (P value = 0.38) |
| 4.9.2. Subgroup with no atelectasis | Chan 1992 | 3/30 | ‐3.67 | 2.728 | ‐3.67 (‐9.01 to 1.68) | 1.34 (P value = 0.18) |
| 4.9.3. Subgroup with right lower lobe atelectasis | Chan 1992 | 1/30 | Effect estimate unable to be calculated. |
|||
| 4.9.4. Subgroup with bilateral atelectasis | Chan 1992 | 21/30 | Effect estimate unable to be calculated. |
Footnotes: aTreatment effect estimates calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
4.10 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between supine position (S) and bad lung down (BLD) for unilateral lung disease participants during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–BLD) |
SE (MD) |
Effect estimate (95% CI) | Z test |
|
a,bMD in PaO2 at 30 minutes after turn (subgroup with unilateral atelectasis) |
Chan 1992 | 6/30 | ‐4.0 | 2.338 | ‐4.00 (‐8.58 to 0.58) | 1.71 (P value = 0.09) |
Footnotes: aTreatment effect estimate calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
4.11 MD in PaO2 (mmHg) with 95% CI (GIV, REM) between supine position (S) and good lung down (GLD) for unilateral lung disease participants during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–GLD) |
SE (MD) |
Effect estimate (95% CI) | Z test |
|
a,bMD in PaO2 at 30 minutes after turn (subgroup with unilateral atelectasis) |
Chan 1992 | 6/30 | ‐3.666 | 4.828 | ‐3.67 (‐13.13 to 5.80) | 0.76 (P value = 0.45) |
Footnotes: aTreatment effect estimate calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
Appendix 5. Lateral position versus a comparison body position: estimate of treatment effect from single cross‐over studies with extractable mean arterial blood pressure (MABP) data
5.1 MD in MABP (mmHg) with 95% CI (GIV, REM) between native lung down (N) and allograft lung down (A) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number | MD (N–A) |
SE (MD) |
Effect estimate (95% CI) | Z test |
| 5.1.1. aMD in MABP at 5 minutes after turn | George 2002 | 14 | 1.929 | 2.893 | 1.93 (‐3.74 to 7.60) | 0.67 (P value = 0.50) |
| 5.1.2. aMD in MABP at 15 minutes after turn | George 2002 | 14 | ‐1.286 | 2.121 | ‐1.29 (‐5.44 to 2.87) | 0.61 (P value = 0.54) |
| 5.1.3. aMD in MABP at 30 minutes after turn | George 2002 | 14 | ‐2.429 | 2.053 | ‐2.43 (‐6.45 to 1.59) | 1.18 (P value = 0.24) |
Footnote: aTreatment effect estimates calculated from individual participant data
5.2 MD in MABP (mmHg) with 95% CI (GIV, REM) between supine position (S) and allograft lung down (A) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (S–A) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 5.2.1. aMD in MABP at 5 minutes after turn | George 2002 | 14 | 0.071 | 3.498 | 0.07 (‐6.78 to 6.93) | 0.02 (P value = 0.98) |
| 5.2.2. aMD in MABP at 15 minutes after turn | George 2002 | 14 | ‐0.357 | 3.081 | ‐0.36 (‐6.40 to 5.68) | 0.21 (P value = 0.91) |
| 5.2.3. aMD in MABP at 30 minutes after turn | George 2002 | 14 | ‐1.643 | 2.301 | ‐1.64 (‐6.15 to 2.87) | 0.71 (P value = 0.48) |
Footnote: aTreatment effect estimates calculated from individual participant data
5.3 MD in MABP (mmHg) with 95% CI (GIV, REM) between supine position (S) and native lung down (N) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (S–N) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 5.3.1. aMD in MABP at 5 minutes after turn | George 2002 | 14 | ‐1.857 | 2.849 | ‐1.86 (‐7.44 to 3.73) | 0.65 (P value = 0.51) |
| 5.3.2. aMD in MABP at 15 minutes after turn | George 2002 | 14 | 0.929 | 2.760523 | 0.93 (‐4.48 to 6.34) | 0.34 (P value = 0.74) |
| 5.3.3. aMD in MABP at 30 minutes after turn | George 2002 | 14 | 0.786 | 2.4 | 0.79 (‐3.92 to 5.49) | 0.33 (P value = 0.74) |
Footnote: aTreatment effect estimates calculated from individual participant data
Appendix 6. Lateral position versus a comparison body position: estimate of treatment effect from single cross‐over studies with extractable cardiac output (CO) data
6.1 MD in CO (L/min) with 95% CI (GIV, REM) between native lung down (N) and allograft lung down (A) for postoperative single lung transplant participants during a short‐term turning response
| Outcome/Subgroup | Studies | Number | MD (N–A) | SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in CO at 25 minutes after turn | George 2002 | 14 | ‐0.0070 | 0.15 | ‐0.01 (‐0.30 to 0.29) | 0.05 (P value = 0.96) |
Footnote: aTreatment effect estimates calculated from individual participant data
6.2 MD in CO (L/min) with 95% CI (GIV, REM) between supine position (S) and allograft lung down (A) for postoperative single lung transplant participants during a short‐term turning response
| Outcome/Subgroup | Studies | Number | MD (S–A) | SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in CO at 25 minutes after turn | George 2002 | 14 | ‐0.021 | 0.202 | ‐0.02 (‐0.42 to 0.37) | 0.10 (P value = 0.92) |
Footnote: aTreatment effect estimate calculated from individual participant data
6.3 MD in CO (L/min) with 95% CI (GIV, REM) between supine position (S) and native lung down (N) for postoperative single lung transplant participants during a short‐term turning response
| Outcome/Subgroup | Studies | Number | MD (S–N) | SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in CO at 25 minutes after turn | George 2002 | 15 | ‐0.013 | 0.207 | ‐0.01 (‐0.42 to 0.39) | 0.06 (P value = 0.95) |
Footnote: aTreatment effect estimate calculated from individual participant data
Appendix 7. Lateral position versus a comparison body position: estimate of treatment effect from single cross‐over studies with extractable arterial‐venous oxygen content difference (C(a‐v)O2) data
7.1 MD in C(a‐v)O2 (mL O2/100 mL) with 95% CI (GIV, REM) between right lateral (R) and left lateral (L) positions during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (R–L) |
SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in C(a‐v)O2 as inverse indicator of CO at 30 minutes after turn | 1 | 30 | Insufficient data | |||
| 7.1.1. Subgroup with left lower lobe atelectasis | Chan 1992 | 5/30 | 0.15 | 0.23 | 0.15 (‐0.30 to 0.60) | 0.65 (P value = 0.51) |
| 7.1.2. Subgroup with no atelectasis | Chan 1992 | 3/30 | ‐0.06 | 0.278 | ‐0.06 (‐0.60 to 0.48) | 0.22 (P value = 0.83) |
| 7.1.3. Subgroup with right lower lobe atelectasis | Chan 1992 | 1/30 | Effect estimate unable to be calculated | |||
| 7.1.4. Subgroup with bilateral atelectasis | Chan 1992 | 21/30 | Effect estimate unable to be calculated |
Footnote: aTreatment effect estimates calculated from individual participant data
7.2 MD in C(a‐v)O2 (mL O2/100 mL) with 95% CI (GIV, REM) between supine position (S) and left lateral (L) position during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–L) |
SE (MD) |
Effect estimate (95% CI) | Z test |
| a,bMD in C(a‐v)O2 as inverse indicator of CO at 30 minutes after turn | 1 | 30 | Insufficient data | |||
| 7.2.1. Subgroup with left lower lobe atelectasis | Chan 1992 | 5/30 | 0.528 | 0.666 | 0.53 (‐0.78 to 1.83) | 0.79 (P value = 0.43) |
| 7.2.2. Subgroup with no atelectasis | Chan 1992 | 3/30 | 0.0 | 0.222 | 0.00 (‐0.44 to 0.44) | 0 (P value = 1.0) |
| 7.2.3. Subgroup with right lower lobe atelectasis | Chan 1992 | 1/30 | Effect estimate unable to be calculated | |||
| 7.2.4. Subgroup with bilateral atelectasis | Chan 1992 | 21/30 | Effect estimate unable to be calculated |
Footnotes: aTreatment effect estimates calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
7.3 MD in C(a‐v)O2 (mL O2/100 mL) with 95% CI (GIV, REM) between supine position (S) and right lateral (R) position during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–R) |
SE (MD) |
Effect estimate (95% CI) | Z test |
| a,bMD in C(a‐v)O2 as inverse indicator of CO at 30 minutes after turn | 1 | 30 | Insufficient data | |||
| 7.3.1. Subgroup with left lower lobe atelectasis | Chan 1992 | 5/30 | 0.378 | 0.577 | 0.38 (‐0.75 to 1.51) | 0.66 (P value = 0.51) |
| 7.3.2. Subgroup with no atelectasis | Chan 1992 | 3/30 | 0.06 | 0.093 | 0.06 (‐0.12 to 0.24) | 0.65 (P value = 0.52) |
| 7.3.3. Subgroup with right lower lobe atelectasis | Chan 1992 | 1/30 | Effect estimate unable to be calculated | |||
| 7.3.4. Subgroup with bilateral atelectasis | Chan 1992 | 21/30 | Effect estimate unable to be calculated |
Footnotes: aTreatment effect estimates calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
7.4 MD in C(a‐v)O2 (mL O2/100 mL) with 95% CI (GIV, REM) between bad lung down (BLD) and good lung down (GLD) for unilateral lung disease participants during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (BLD–GLD) |
SE (MD) |
Effect estimate (95% CI) | Z test |
|
aMD in C(a‐v)O2 as inverse indicator of CO at 30 minutes after turn (subgroup with unilateral atelectasis) |
Chan 1992 | 6/30 | ‐0.15 | 0.188 | ‐0.15 (‐0.52 to 0.22) | 0.8 (P value = 0.42) |
Footnote: aTreatment effect estimate calculated from individual participant data
7.5 MD in C(a‐v)O2 (mL O2/100 mL) with 95% CI (GIV, REM) between supine position (S) and good lung down (GLD) for unilateral lung disease participants during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–GLD) |
SE (MD) |
Effect estimate (95% CI) | Z test |
|
a,bMD in C(a‐v)O2 as inverse indicator of CO at 30 minutes after turn (subgroup with unilateral atelectasis) |
Chan 1992 | 6/30 | 0.258 | 0.471 | 0.26 (‐0.67 to 1.18) | 0.55 (P value = 0.58) |
Footnotes: aTreatment effect estimate calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
7.6 MD in C(a‐v)O2 (mL O2/100 mL) with 95% CI (GIV, REM) between supine position (S) and bad lung down (BLD) during a short‐term turning response
| Outcome/Subgroup | Studies |
Number (n/N) |
MD (S–BLD) |
SE (MD) |
Effect estimate (95% CI) | Z test |
|
a,bMD in C(a‐v)O2 as inverse indicator of CO at 30 minutes after turn (subgroup with unilateral atelectasis) |
Chan 1992 | 6/30 | 0.408 | 0.558 | 0.41 (‐0.69 to 1.50) | 0.73 (P value = 0.46) |
Footnotes: aTreatment effect estimate calculated from individual participant data. bSupine position data were not combined with baseline supine data as conducted in the original study
Appendix 8. Lateral position versus a comparison body position: estimate of treatment effect from single studies with extractable mixed venous oxygen saturation (SvO2) data
8.1 Cross‐over trials: MD in SvO2 (%) with 95% CI (GIV, REM) between right lateral position (R) and left lateral position (L) during an early turning response
| Outcome/Subgroup | Studies | Number |
MD (R – L) |
SE (MD) |
Effect estimate (95% CI) | Z test |
| 8.1.1. aMD in SvO2 at 1 minute after turn | Gawlinski 1998 | 42 | 0.786 | 0.993 | 0.79 (‐1.16 to 2.73) | 0.79 (P value = 0.43) |
| 8.1.2. aMD in SvO2 at 2 minutes after turn | Gawlinski 1998 | 42 | 1.809 | 1.082 | 1.81 (‐0.31 to 3.93) | 1.67 (P value = 0.09) |
| 8.1.3. aMD in SvO2 at 3 minutes after turn | Gawlinski 1998 | 42 | 1.738 | 0.933 | 1.74 (‐0.09 to 3.57) | 1.86 (P value = 0.06) |
| 8.1.4. aMD in SvO2 at 4 minutes after turn | Gawlinski 1998 | 42 | 1.786 | 0.819 | 1.79 (0.18 to 3.39) | 2.18 (P value = 0.03) |
| 8.1.5. aMD in SvO2 at 5 minutes after turn | Gawlinski 1998 | 42 | 1.0 | 0.814 | 1.00 (‐0.60 to 2.60) | 1.23 (P value = 0.22) |
Footnote: aTreatment effect estimates calculated from individual participant data
8.2 Cross‐over trials: MD in SvO2 (%) with 95% CI (GIV, REM) between right lateral position (R) and left lateral position (L) during a short‐term turning response
| Outcome/Subgroup | Studies | Number |
MD (R–L) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 8.2.1.aMD in SvO2 at 15 minutes after turn | Gawlinski 1998 | 42 | 1.381 | 0.906 | 1.38 (‐0.39 to 3.16) | 1.52 (P value = 0.13) |
| 8.2.2. aMD in SvO2 at 25 minutes after turn | Gawlinski 1998 | 38 | 1.0 | 0.905 | 1.00 (‐0.77 to 2.77) | 1.10 (P value = 0.27) |
Footnote: aTreatment effect estimates calculated from individual participant data
8.3 Cross‐over trials: MD in SvO2 (%) with 95% CI (GIV, REM) between native lung down (N) and allograft lung down (A) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (N–A) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 8.3.1. aMD in SvO2 at 5 minutes after turn | George 2002 | 15 | 1.0 | 1.467 | 1.00 (‐1.88 to 3.88) | 0.68 (P value = 0.5) |
| 8.3.2. aMD in SvO2 at 15 minutes after turn | George 2002 | 15 | ‐1.8 | 1.184 | ‐1.80 (‐4.12 to 0.52) | 1.52 (P value = 0.13) |
Footnote: aTreatment effect estimates calculated from individual participant data
8.4 Cross‐over trials: MD in SvO2 (%) with 95% CI (GIV, REM) between supine position (S) and allograft lung down (A) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (S–A) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 8.4.1. aMD in SvO2 at 5 minutes after turn | George 2002 | 15 | 2.4 | 1.444 | 2.40 (‐0.43 to 5.23) | 1.66 (P value = 0.10) |
| 8.4.2. aMD in SvO2 at 15 minutes after turn | George 2002) | 15 | 0.2 | 1.5 | 0.20 (‐2.74 to 3.14) | 0.13 (P value = 0.89) |
Footnote: aTreatment effect estimates calculated from individual participant data
8.5 Cross‐over trials: MD in SvO2 (%) with 95% CI (GIV, REM) between supine position (S) and native lung down (N) for postoperative single lung transplant participants across composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (S–N) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 8.5.1. aMD in SvO2 at 5 minutes (early turning response) | George 2002 | 15 | 1.4 | 1.473 | 1.40 (‐1.49 to 4.29) | 0.95 (P value = 0.34) |
| 8.5.2. aMD in SvO2 at 15 minutes (short‐term turning response) | George 2002 | 15 | 2.0 | 1.384 | 2.00 (‐0.71 to 4.71) | 1.45 (P value = 0.15) |
Footnote: aTreatment effect estimates calculated from individual participant data
8.6 Parallel‐group trials: MD in SvO2 (%) with 95% CI (IV, REM) between right lateral position (R) and left lateral position (L) during an early turning response
| Outcome/Subgroup | Studies | Right lateral position | Left lateral position | Effect estimate (95% CI) | Z test | ||||
| Mean (%) | SD (%) | Total | Mean (%) | SD (%) | Total | ||||
| 8.6.1. aMD in SvO2 at 1 minute after turn | Reed 2002 | 60.2 | 6.06 | 15 | 57.81 | 9.57 | 16 | 2.39 (‐3.21 to 7.99) | 0.84 (P value = 0.40) |
| 8.6.2. aMD in SvO2 at 2 minutes after turn | Reed 2002 | 60.6 | 6.74 | 15 | 58.0 | 9.29 | 16 | 2.60 (‐3.09 to 8.29) | 0.9 (P value = 0.37) |
| 8.6.3. aMD in SvO2 at 3 minutes after turn | Reed 2002 | 61.2 | 6.19 | 15 | 59.0 | 8.53 | 16 | 2.20 (‐3.02 to 7.42) | 0.83 (P value = 0.41) |
| 8.6.4. aMD in SvO2 at 4 minutes after turn | Reed 2002 | 61.73 | 6.4 | 15 | 58.94 | 8.15 | 16 | 2.79 (‐2.35 to 7.93) | 1.06 (P value = 0.26) |
| 8.6.5. MD in SvO2 at 5 minutes after turn | 2 | 29 | 31 | No pooled data because of trial dissimilarities | |||||
| 8.6.5.1. bPossibly delayed backrub subgroup with co‐intervention (backrub) not applied | Lewis 1997 | 64.6 | 8.9 | 14 | 64.5 | 6 | 15 | ‐0.10 (‐5.86 to 5.66) | 0.03 (P value = 0.97) |
| 8.6.5.2. aMD in SvO2 at 5 minutes after turn | Reed 2002 | 63.0 | 6.48 | 15 | 59.31 | 8.95 | 16 | 3.69 (‐1.79 to 9.17) | 1.32 (P value = 0.19) |
| 8.6.6. MD in SvO2 at 6 minutes after turn | 2 | 29 | 31 | No pooled data because of trial dissimilarities | |||||
| 8.6.6.1. bPossibly delayed backrub subgroup with co‐intervention (backrub) applied | Lewis 1997 | 60.5 | 11.2 | 14 | 56.3 | 7.4 | 15 | ‐4.20 (‐11.16 to 2.76) | 1.18 (P value = 0.24) |
| 8.6.6.2. aMD in SvO2 at 6 minutes after turn | Reed 2002 | 63.53 | 6.7 | 15 | 59.31 | 9.5 | 16 | 4.22 (‐1.54 to 9.98) | 1.44 (P value = 0.15) |
| 8.6.7.aMD in SvO2 at 7 minutes after turn | Reed 2002 | 63.87 | 7.08 | 15 | 61.0 | 8.73 | 16 | 2.87 (‐2.71 to 8.45) | 1.01 (P value = 0.31) |
| 8.6.8. aMD in SvO2 at 8 minutes after turn | Reed 2002 | 63.8 | 7.51 | 15 | 60.81 | 8.98 | 16 | 2.99 (‐2.82 to 8.80) | 1.01 (P value = 0.31) |
| 8.6.9. aMD in SvO2 at 9 minutes after turn | Reed 2002 | 64.13 | 6.93 | 15 | 61.56 | 9.13 | 16 | 2.57 (‐3.11 to 8.25) | 0.89 (P value = 0.38) |
| 8.6.10. MD in SvO2 at 10 minutes after turn | 2 | 29 | 31 | No pooled data because of trial dissimilarities | |||||
| 8.6.10.1. bPossibly delayed backrub subgroup with co‐intervention (backrub) applied | Lewis 1997 | 65.0 | 9.1 | 14 | 63.4 | 7.2 | 15 | ‐1.60 (‐7.60 to 4.40) | 0.52 (P value = 0.60) |
| 8.6.10.2. aMD in SvO2 at 10 minutes after turn | Reed 2002 | 63.87 | 7.39 | 15 | 62.13 | 8.97 | 16 | 1.74 (‐4.03 to 7.51) | 0.59 (P value = 0.55) |
Footnotes: aTreatment effect estimates calculated from individual participant data. bAmbiguity noted between sample results and subgroup results within the split‐plot design
Appendix 9. Additional 'Summary of findings' tables for single studies
| Supine position compared with right lateral position for critically ill adult patients | ||||||
| Patient or population: critically ill adult patients Settings: critical care areas Intervention: supine position Comparison: right lateral position | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Right lateral position | Supine position | |||||
| Hypoxaemia Partial pressure of arterial oxygen (PaO2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available |
| Global indicator of alteration in tissue oxygenation Arterial‐venous oxygen content difference (C(a‐v)O2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
| Footnote: aCross‐over trial with participants as their own controls | ||||||
| Supine position compared with left lateral position for critically ill adult patients | ||||||
| Patient or population: critically ill adult patients Settings: critical care areas Intervention: supine position Comparison: left lateral position | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Left lateral position | Supine position | |||||
| Hypoxaemia Partial pressure of arterial oxygen (PaO2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available |
| Global indicator of alteration in tissue oxygenation Arterial‐venous oxygen content difference (C(a‐v)O2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
| Footnote: aCross‐over trial with participants as their own controls | ||||||
| Supine position compared with bad lung down position for critically ill adult patients with unilateral lung disease | ||||||
| Patient or population: critically ill adult patients with unilateral lung disease Settings: critical care areas Intervention: supine position Comparison: bad lung down position | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bad lung down position | Supine position | |||||
| Hypoxaemia Partial pressure of arterial oxygen (PaO2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available |
| Global indicator of alteration in tissue oxygenation Arterial‐venous oxygen content difference (C(a‐v)O2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
| Footnote: aCross‐over trial with participants as their own controls | ||||||
| Supine position compared with good lung down for critically ill adult patients with unilateral lung disease | ||||||
| Patient or population: critically ill adult patients with unilateral lung disease Settings: critical care areas Intervention: supine position Comparison: good lung down | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Good lung down | Supine position | |||||
| Hypoxaemia Partial pressure of arterial oxygen (PaO2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available. |
| Global indicator of alteration in tissue oxygenation Arterial‐venous oxygen content difference (C(a‐v)O2) ‐ not reported | See comment | See comment | ‐ | 30 (1 studya) | See comment | Single cross‐over study, whole sample data not available. |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
| Footnote: aCross‐over trial with participants as their own controls | ||||||
| Native lung down compared with allograft lung down for critically ill adult patients following single lung transplant | ||||||
| Patient or population: critically ill adult patients following single lung transplant Settings: critical care areas Intervention: native lung down Comparison: allograft lung down | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Allograft lung down | Native lung down | |||||
| Hypoxaemia PaO2 < 60 mmHg Follow‐up: 5 minutes after turninga | Mean PaO2 for allograft lung down was 116.93 mmHg | Mean PaO2 for native lung down was 2.60 higher (11.48 lower to 16.68 higher). | ‐ | 15 (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresc |
| Hypotension MABP < 60 mmHg Follow‐up: 5 minutes after turningd | Mean MABP for allograft lung down was 76.07 mmHg | Mean MABP for native lung down was 1.93 higher (3.74 lower to 7.60 higher) | ‐ | 14e (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresf |
| Cardiac output (CO) as a measure of low oxygen delivery (DO2) CO < 4 L/min Follow‐up: 25 minutes after turning | Mean CO for allograft lung down was 5.01 L/min | Mean CO for native lung down was 0.01 higher (0.3 lower to 0.29 higher) | ‐ | 14e (1 study) | ⊕⊕⊝⊝ Lowb | |
| Mixed venous oxygen saturation as a global indicator of tissue oxygenation SvO2 < 60% Follow‐up: 5 minutes after turninga | Mean SvO2 for allograft lung down was 68.26% | Mean SvO2 for native lung down was 1.00 higher (1.88 lower to 3.88 higher) | ‐ | 15 (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresg |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
| Footnotes: aSingle cross‐over study had two time points (5 and 15 minutes after turning). First result is provided within the table bOnly study with single lung transplant participants. GRADE downgraded four levels because of methodological variability, including risk of bias (method of randomization not described, unclear risk of performance bias, reported difference due to sequence (group) effect, inadequate washout to rule out carryover effects), indirectness (no dichotomous data, cross‐over study with continuous data had mean values extracted to detect critical thresholds for each outcome), imprecision (imprecise single study results across time points) and insufficient number of studies to test for publication bias cOther time point: at 15 minutes after turning, mean PaO2 for allograft lung down was 122 mmHg; native lung down was 4.53 lower (95% CI 22.73 lower to 13.66 higher) dSingle cross‐over study had three time points (5, 15 and 30 minutes after turning). First result is provided in the table eMissing data from 1 participant fOther time points: At 15 minutes after turning, mean MABP for allograft lung down was 76.5 mmHg; native lung down was 1.29 lower (95% CI 5.44 lower to 2.87 higher). At 30 minutes after turning, mean MABP for allograft lung down was 78.21 mmHg; native lung down was 2.43 lower (95% CI 6.45 lower to 1.59 higher) gOther time point: At 15 minutes after turning, mean SvO2 for allograft lung down was 69.2%, native lung down was 1.80 lower (95% CI 4.12 lower to 0.52 higher) | ||||||
| Supine position compared with allograft lung down for critically ill adult patients following single lung transplant | ||||||
| Patient or population: critically ill adult patients following single lung transplant Settings: critical care areas Intervention: supine position Comparison: allograft lung down | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Allograft lung down | Supine position | |||||
| Hypoxaemia PaO2 < 60 mmHg Follow‐up: 5 minutes after turninga | Mean PaO2 for allograft lung down was 116.93 mmHg | Mean PaO2 for supine position was 2.0 lower (12.05 lower to 8.05 higher) | ‐ | 15 (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresc |
| Hypotension MABP < 60 mmHg Follow‐up: 5 minutes after turningd | Mean MABP for allograft lung down was 76.07 mmHg | Mean MABP for supine position was 0.07 higher (6.78 lower to 6.93 higher) | ‐ | 14e (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresf |
| Cardiac output (CO) as a measure of low oxygen delivery (DO2) CO < 4 L/min Follow‐up: 25 minutes after turning | Mean CO for allograft lung down was 5.01 L/min | Mean CO for supine position was 0.02 lower (0.42 lower to 0.37 higher) | ‐ | 14e (1 study) | ⊕⊕⊝⊝ Lowb | |
| Mixed venous oxygen saturation as a global indicator of tissue oxygenation SvO2 < 60% Follow‐up: 5 minutes after turning1 | Mean SvO2 for allograft lung down was 68.26% | Mean SvO2 for supine position was 2.40 higher (0.43 lower to 5.23 higher) | ‐ | 15 (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresg |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
| Footnotes: aSingle cross‐over study had two time points (5 and 15 minutes after turning). First result is provided within the table bOnly study with single lung transplant participants. GRADE downgraded four levels because of methodological variability, including risk of bias (method of randomization not described, unclear risk of performance bias, reported difference due to sequence (group) effect, inadequate washout to rule out carryover effects), indirectness (no dichotomous data, cross‐over study with continuous data had mean values extracted to detect critical thresholds for each outcome), imprecision (imprecise single study results across time points) and insufficient number of studies to test for publication bias cOther time point: At 15 minutes after turning, mean PaO2 for allograft lung down was 122 mmHg; supine position was 5.67 lower (95% CI 14.59 lower to 3.25 higher) dSingle cross‐over study had three time points (5, 15 and 30 minutes after turning). First result is provided in the table eMissing data from one participant fOther time points: At 15 minutes after turning, mean MABP for allograft lung down was 76.5 mmHg; supine position was 0.36 lower (95% CI 6.40 lower to 5.68 higher). At 30 minutes, mean MABP for allograft lung down was 78.21 mmHg; supine position was 1.64 lower (95% CI 6.15 lower to 2.87 higher) gOther time points: At 15 minutes after turning, mean SvO2 for allograft lung down was 69.2%, supine position 0. 20 higher (95% CI 2.74 lower to 3.14 higher) | ||||||
| Supine position compared with native lung down for critically ill adult patients following single lung transplant | ||||||
| Patient or population: critically ill adult patients following single lung transplant Settings: critical care areas Intervention: supine position Comparison: allograft lung down | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Native lung down | Supine position | |||||
| Hypoxaemia PaO2 < 60 mmHg Follow‐up: 5 minutes after turninga | Mean PaO2 for native lung down was 119.53 mmHg | Mean PaO2 for supine position was 4.60 lower (15.30 lower to 6.10 higher). | ‐ | 15 (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresc |
| Hypotension MABP < 60 mmHg Follow‐up: 5 minutes after turningd | Mean MABP for native lung down was 78 mmHg | Mean MABP for supine position was 1.86 lower (7.44 lower to 3.73 higher) | ‐ | 14e (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresf |
| Cardiac output (CO) as a measure of low oxygen delivery (DO2) CO < 4 L/min Follow‐up: 25 minutes after turning | Mean CO as a measure of low oxygen delivery for native lung down was 4.92 L/min | Mean CO for supine position was 0.01 lower (0.42 lower to 0.39 higher) | ‐ | 14e (1 study) | ⊕⊕⊝⊝ Lowb | |
| Mixed venous oxygen saturation as a global indicator of tissue oxygenation SvO2 < 60% Follow‐up: 5 minutes after turning1 | Mean SvO2 for native lung down was 69.26% | Mean SvO2 for supine position was 1.40 higher (1.49 lower to 4.29 higher) | ‐ | 15 (1 study) | ⊕⊕⊝⊝ Lowb | Single study with repeated measuresg |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
| Footnotes: aSingle cross‐over study had two time points (5 and 15 minutes after turning). First result is provided within the table bOnly study with single lung transplant participants. GRADE downgraded four levels because of methodological variability, including risk of bias (method of randomization not described, unclear risk of performance bias, reported difference due to sequence (group) effect, inadequate washout to rule out carryover effects), indirectness (no dichotomous data, cross‐over study with continuous data had mean values extracted to detect critical thresholds for each outcome), imprecision (imprecise single study results across time points) and insufficient number of studies to test for publication bias cOther time point: At 15 minutes after turning, mean PaO2 for native lung down was 117.46 mmHg; for supine position was 1.13 lower (95% CI 13.78 lower to 11.51 higher) dSingle cross‐over study had three time points (5, 15 and 30 minutes after turning). First result is provided in the table eMissing data from one participant fOther time points: At 15 minutes after turning, mean MABP for native lung down was 75.21 mmHg; for supine position was 0.93 higher (95% CI 4.48 lower to 6.34 higher). At 30 minutes after turning, mean MABP for native lung down was 75.78 mmHg; for supine position was 0.79 higher (95% CI 3.92 lower to 5.49 higher) gOther time points: At 15 minutes after turning, mean SvO2 for native lung down was 67.4%, for supine position was 2.0 higher (95% CI 0.71 lower to 4.71 higher) | ||||||
Appendix 10. Lateral position versus a comparison body position: estimate of treatment effect from single cross‐over studies with extractable hypoxia score (P/F ratio) data
10.1 MD in hypoxia score (P/F ratio) with 95% CI (GIV, REM) between right lateral (R) and left lateral (L) positions for unilateral lung disease participants during a short‐term turning response
| Outcome/Subgroup | Studies | Number | MD (R–L) | SE (MD) | Effect estimate (95% CI) | Z test |
| aMD in P/F ratio at 15 minutes after turn | Ibanez 1981 | 10 | ‐24.036 | 29.843 | ‐‐24.04 (‐82.53 to 34.46) | 0.81 (P value = 0.42) |
Footnote: aTreatment effect estimate calculated from individual participant data
10.2 MD in hypoxia score (P/F ratio) with 95% CI (GIV, REM) between bad lung down (BLD) and good lung down (GLD) for unilateral lung disease participants across composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (BLD–GLD) |
SE (MD) | Effect estimate (95% CI) | Z test |
| MD in P/F ratio after turning | 2 | 19 | See meta‐analysis | |||
| 10.2.1. a,bMD in P/F ratio at 10 minutes after turn | Remolina 1981 | 9 | ‐90.28 | 17.434 | ‐90.28 (‐124.45 to ‐56.11) |
5.18 (P value < 0.00001) |
| 10.2.2. aMD in P/F ratio at 15 minutes after turn | Ibanez 1981 | 10 | ‐81.936 | 14.453 | ‐81.94 (‐110.26 to ‐53.61) | 5.67 (P value < 0.00001) |
Footnotes: aTreatment effect estimates calculated from individual participant data. bSample had an adjusted calculation with second data set removed to avoid a unit of analysis error
Appendix 11. Lateral position versus a comparison body position: estimate of treatment effect from single cross‐over studies with extractable heart rate (HR) data
10.1 MD in HR (beats/min) with 95% CI (GIV, REM) between native lung down (N) and allograft lung down (A) for postoperative single lung transplant participants across all composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (N–A) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 11.1.1. aMD in HR at 5 minutes after turn | George 2002 | 14 | ‐3.0 | 5.18 | ‐3.00 (‐13.15 to 7.15) | 0.56 (P value = 0.58) |
| 11.1.2. aMD in HR at 15 minutes after turn | George 2002 | 14 | 1.357 | 1.485 | 1.36 (‐1.55 to 4.27) | 0.91 (P value = 0.36) |
| 11.1.3. aMD in HR at 30 minutes after turn | George 2002 | 14 | ‐5.071 | 3.068 | ‐5.07 (‐11.08 to 0.94) | 1.65 (P value = 0.10) |
Footnote: aTreatment effect estimates calculated from individual participant data
11.2 MD in HR (beats/min) with 95% CI (GIV, REM) between supine position (S) and allograft lung down (A) for postoperative single lung transplant participants across all composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (S–A) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 11.2.1. aMD in HR at 5 minutes after turn | George 2002 | 14 | 0.357 | 5.486 | 0.36 (‐10.40 to 11.11) | 0.07 (P value = 0.95) |
| 11.2.2. aMD in HR at 15 minutes after turn | George 2002 | 14 | 1.286 | 1.982 | 1.29 (‐2.60 to 5.17) | 0.65 (P value = 0.52) |
| 11.2.3. aMD in HR at 30 minutes after turn | George 2002 | 14 | ‐7.643 | 2.733 | ‐7.64 (‐13.00 to ‐2.29) | 2.80 (P value = 0.005) |
Footnote: aTreatment effect estimates calculated from individual participant data
11.3 MD in HR (beats/min) with 95% CI (GIV, REM) between supine position (S) and native lung down (N) in postoperative single lung transplant participants across all composite time intervals
| Outcome/Subgroup | Studies | Number |
MD (S–N) |
SE (MD) | Effect estimate (95% CI) | Z test |
| 11.3.1. aMD in HR at 5 minutes after turn | George 2002 | 14 | 3.357 | 1.564 | 3.36 (0.29 to 6.42) | 2.13 (P value = 0.03) |
| 11.3.2. aMD in HR at 15 minutes after turn | George 2002 | 14 | ‐0.071 | 2.562 | ‐0.07 (‐5.09 to 4.95) | 0.03 (P value = 0.98) |
| 11.3.3. aMD in HR at 30 minutes after turn | George 2002 | 14 | ‐2.571 | 2.531 | ‐2.57 (‐7.53 to 2.39) | 1.02 (P value = 0.31) |
Footnote: aTreatment effect estimates calculated from individual participant data
Appendix 12. Lateral positioning versus supine positioning: estimate of treatment effect from single studies with extractable temperature data
12.1 MD in number of hours with fever (temperature >38°C) with 95% CI (inverse variance (IV), REM) between supine positioning and repetitive lateral positioning (24‐hour therapy)
| Outcome/Subgroup | Studies |
2‐hour turns, alternating lateral positions |
Supine immobilization | Effect estimate (95% CI) | Z test | ||||
| Mean | SD | Total | Mean | SD | Total | ||||
| 12.1.1. MD in number of hours with fever during first 72 hours postop | Chulay 1982 | 26.4 | 14.1 | 17 | 44.0 | 11.4 | 18 | ‐17.60 (‐26.12 to ‐9.08) |
4.05 (P value < 0.0001) |
| 12.1.1.1. MD in number of hours with fever during day 1 | Chulay 1982 | No data | 17 | No data | 18 | No data reported | |||
| 12.1.1.2. MD in number of hours with fever during day 2 | Chulay 1982 | 10.4 | 7.0 | 17 | 14.8 | 6.4 | 18 | ‐4.4 (‐8.85 to 0.05) |
1.94 (P value = 0.05) |
| 12.1.1.3. MD in number of hours with fever during day 3 | Chulay 1982 | 3.1 | 4.7 | 17 | 13.9 | 7.3 | 18 | ‐10.8 (‐14.85 to ‐6.75) |
5.23 (P value < 0.00001) |
Appendix 13. Lateral positioning versus supine positioning: estimate of treatment effect from single studies with extractable data on the duration of mechanical ventilation
13.1 MD in duration of intubation/mechanical ventilation (hours) with 95% CI (IV, REM) between supine positioning and repetitive lateral positioning (24‐hour therapy)
| Outcome/Subgroup | Studies |
2‐hour turns, alternating lateral positions |
Supine immobilization | Effect estimate (95% CI) | Z test | ||||
| Mean (%) | SD (%) | Total | Mean (%) | SD (%) | Total | ||||
| MD in intubation/mechanical ventilation duration (hours) | Chulay 1982 | 14.4 | 5.4 | 17 | 19.2 | 8.7 | 18 | ‐4.80 (‐9.57 to ‐0.03) | 1.97 (P value = 0.05) |
Appendix 14. Lateral positioning versus supine positioning: estimate of treatment effect from single studies with extractable data on length of stay (LOS) in the intensive care unit (ICU)
14.1 MD in LOS in ICU (hours) with 95% CI (IV, REM) between supine positioning and repetitive lateral positioning (24‐hour therapy)
| Outcome/Subgroup | Studies |
2‐hour turns, alternating lateral positions |
Supine immobilization | Effect estimate (95% CI) | Z test | ||||
| Mean (%) | SD (%) | Total | Mean (%) | SD (%) | Total | ||||
| MD in LOS in ICU (hours) | Chulay 1982 | 39.7 | 14.2 | 17 | 58.3 | 27.7 | 18 | ‐18.60 (‐33.07 to ‐4.13) | 2.52 (P value = 0.01) |
Data and analyses
Comparison 1. Bad lung down vs good lung down in unilateral lung disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PaO2 (mmHg) across composite time intervals (early and short turning responses) | 2 | Mean Difference (Random, 95% CI) | ‐49.26 [‐67.33, ‐31.18] | |
| 2 P/F ratio across composite time intervals (early and short turning responses) | 2 | Mean Difference (Random, 95% CI) | ‐85.33 [‐107.14, ‐63.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Banasik 1987.
| Methods |
Blocked randomized cross‐over trial (3‐treatment, 6‐sequence, 3‐period design) Pre‐specified analysis: 1‐way ANOVA of position, treatment order (sequence/group) and repeated turning, with paired t tests for position |
|
| Participants | 60 mechanically ventilated adults within 3 hours of elective coronary revascularization surgery Sex (M/F) 47/13, mean age 62.4 years ± 7.7 Mean FiO2 0.5 ± 0.1, no PEEP (n = 12), mean PEEP 4.3 cmH2O ± 0.9 (n = 48), mean Vt 839 mL ±108 Subgroups (location of atelectasis on CXR): bilateral (n = 14), left lung (n = 10) and no right lung atelectasis Exclusion criteria: emergency CABG, concomitant pulmonary disease or prior lung surgery, simultaneous valvular surgery Setting: 325‐bed medical centre, Spokane, Washington, USA |
|
| Interventions | Left lateral, right lateral and supine positions for 10 minutes Sequences/groups: SLR, SRL, RLS, RSL, LRS, LSR |
|
| Outcomes | Arterial oxygenation measures (PaO2 and SaO2) at 10 minutes | |
| Standard management | Ventilator settings unchanged during trial, no tracheal suctioning | |
| Position description | 45 degrees lateral rotation, commercially available foam wedge, angle verification method not described No HOB elevation (horizontal for all positions) |
|
| Washout period | Not described | |
| Notes | Comments: no sample size calculation described. Except for 1 paired analysis, no extractable summary statistics for meta‐analysis due to unit of analysis error (reported mean and SDa for each body position). Additional data were no longer available (personal communication from contact investigator Associate Professor Jacquelyn Banasik; primary investigator identified within the correspondence as Dr. Margaret Bruya) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "pre‐established, computer generated randomized turning schedule", 6 participants per block, 10 blocks in total. Participants assigned study number and turning schedule when eligible |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described (primary outcomes) Subgroup classification: CXR assessed independently without radiologist awareness of study purpose Comment: objective outcome measures taken from calibrated blood gas analyser; therefore lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data points. Numerator for each outcome identified in table of results, consistent with sample size |
| Selective reporting (reporting bias) | High risk | One paired analysis reported for PaO2 (within‐subject difference between lateral positions) Comment: selective reporting of paired analysis likely, as no report of SaO2 data as co‐primary outcome and no report of other pair‐wise comparisons (within‐subject differences between each lateral position and supine position) for PaO2 Other reporting issues: PaCO2 reported (ANOVA tables) although this outcome was not pre‐specified within aims or methods. However, PaCO2 was not relevant for this review Unclear whether subgroup analysis "with and without unilateral atelectasis" was pre‐planned, but this analysis was unlikely to influence primary reporting |
| Other bias | Unclear risk | No baseline position described. Uniform cross‐over design with balance (each body position preceded each other body position twice, 10 participants allocated to each sequence (group) because of block design). No statistical differences reported between 6 sequences Quotation: Participants "did not consistently demonstrate an increase or decrease in PaO2 or SaO2 from the first to second to the last position". Unclear whether treatment‐by‐period interactions were investigated Comment: Tests for carryover and sequence effects have low power to detect differences, carryover effects may be due to short active and possibly insufficient washout. Unclear risk of carryover bias due to inadequate washout |
Banasik 1996.
| Methods |
Blocked randomized cross‐over trial (3‐treatment, 6‐sequence, 3‐period design) Pre‐specified analysis: multi‐variate ANOVA for arterial and venous blood gases measures, univariate ANOVA with repeated measures and independent t tests for haemodynamic variables, subgroup analyses with and without valvular surgery and preoperatively diagnosed lung disease |
|
| Participants | 120 mechanically ventilated haemodynamically stable adults within 3 hours of non‐emergent CABG or valvular surgery Sex (M/F) 83/37, mean age 69.95 years ± 8.64 Mean FiO2 0.54 ± 0.1, mean PEEP 4.2 cmH2O ± 2, mean Vt 810 mL ± 123 with A/C mode Subgroups: valvular surgery (n = 25, concomitant CABG for 14/25), preoperative lung disease (n = 38) Post hoc subgroups (atelectasis location, diagnosed within 24 hours of surgery): bilateral (n = 20), left lung (n = 27) and right lung (n = 5) Exclusion criteria: emergency cases Setting: critical care unit (ICU and coronary care), 300‐bed hospital in urban area of northwestern USA |
|
| Interventions | Left lateral, right lateral and supine positions for 20 minutes Sequences/groups: SLR, SRL, RLS, RSL, LRS, LSR |
|
| Outcomes | Arterial and venous blood gases measures (PaO2, SaO2, SvO2, a‐vDO2) and resp. rate Haemodynamic measures (HR and systolic and diastolic NIBP recorded on right and left arms) All measures taken after 10 minutes Other co‐primary outcomes reported but not relevant for this review were PaCO2, pH, HCO3‐, CVP and PvO2 |
|
| Standard management | No changes in ventilation settings, no tracheal suctioning, no change in dose rates of IV vasoactive medication | |
| Position description | 45 degrees lateral rotation, passively turned, foam wedge pillow to maintain angle Angle verification method: protractor No HOB elevation with single pillow under the head for all positions |
|
| Washout period | 10 minute stabilization period reported, with approximately 20‐minute duration for each body position | |
| Notes | A priori sample size calculation for subgroups (large effect size (0.85); 1‐β and α not stated) 1996 publication (primary reference) reported arterial and venous blood gas measures 1994 publication reported haemodynamic measures Both published reports described the same study (personal communication with principal investigator) Venous gas sampling (a‐vDO2 and SvO2) collected from a subset because of cost constraints (subset: last 40 CABG participants without preoperative lung disease) Measurement unit/errors: SvO2 reported in mmHg (error), not %. Furthermore, mixed venous sample taken from CVC line without a PA catheter (i.e central venous oxygen saturation (ScvO2)). No unit given for a‐vDO2, no Hb level collected for a‐vDO2 Comment: no extractable summary statistics for meta‐analysis because of unit of analysis error (reported mean and SD for each body position). Additional data no longer available (personal communication from principal investigator) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized turning sequence, 6 participants per block, 20 blocks in total. Participants assigned sequential study numbers and positioning sequence in order of admission |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from digital display of continuous and intermittent physiological monitoring systems or calibrated blood gas analyser; therefore, lack of outcome assessor blinding was unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numerator for each outcome listed: PaO2 (n = 118), SaO2 (n = 118), HR (n = 119), BP (n = 113) and resp. rate (n = 120) Subset for venous gas measures (a‐vDO2 and SvO2) (n = 40) Comment: subset data likely to be equally distributed across groups (sequences) as the result of blocked randomization. Therefore, missing whole sample data unlikely to bias within‐subject comparisons |
| Selective reporting (reporting bias) | Low risk | Comment: Analysis methods differed between publications, with paired comparison of all outcomes not pre‐specified nor conducted. On the basis of method of analysis reported for each outcome, selective reporting of primary outcomes not evident within each report Other reporting issues: Only 1994 publication reported all pre‐specified subgroup analyses. Unclear selective reporting of post hoc subgroup analysis based on presence of atelectasis. Although selective reporting was unclear for subgroups, such analyses were unlikely to influence primary reporting |
| Other bias | Unclear risk | Baseline characteristics inconsistently reported across publications. Rationale for lack of baseline outcome data stated: "the aim was to study the effect of particular positions, not the effect of turning", unclear whether the description refers to the cross‐over design and unit of analysis. Statistical difference in Vt (i.e. baseline demographics) was reported between CABG subgroup and valvular subgroup, associated with weight differences between subgroups, as Vt set at mL/kg Comment: Allocation concealment is unclear. Individual baseline differences unlikely to bias within‐subject analysis of outcome data. However, unclear whether any baseline differences between groups (sequences) occurred by chance No baseline position described. Uniform cross‐over design with balance (each body position preceding each other body position twice, 20 participants allocated to each sequence (group) as the result of block design) Comment: Carryover effects may be due to short active and possibly insufficient washout; therefore, unclear risk of carryover bias Unclear whether all treatments (body positions) were measured in the same way (i.e. time elapsed before measurement) after 10 minutes but before the next turn at 20 minutes Comment: small differences in measurement intervals between participants (as possible source of bias) unlikely to yield clinically important differences within individuals (within‐subject variability) |
Banasik 2001.
| Methods |
Randomized cross‐over trial (3‐treatment, 6‐sequence, 3‐period design) Pre‐specified analysis: univariate ANOVA with repeated measures for all dependent variables |
|
| Participants | 12 mechanically ventilated adults with PA catheter who met critical illness criteria (PaO2 ≤ 70 mmHg on current ventilator settings with supplemental oxygen or cardiac index ≤ 2.0 L/min/m2) Sex (M/F) 7/5, mean age 65 years ± 15.5 Mean FiO2 0.75 ± 0.18, mean PEEP 8.0 cmH2O ± 3.5 (n = 8), mean Vt 775 mL ± 89 with A/C mode Baseline: mean PaO2 60.5 mmHg ± 8.7 (n = 8), mean CI 1.5 L/min/m2 ± 0.3 (n = 5), 1 participant met both inclusion criteria Diagnosis: ischaemic heart disease (n = 3), valvular heart disease (n = 2), pericarditis (n = 1), respiratory failure (n = 3), hyponatraemia (n = 1), liver failure (n = 1), pancreatitis (n = 1) Pulmonary co‐morbidity/possible subgroup analysis (based on pre‐study CXR): bilateral infiltrates or atelectasis (n = 5), right‐sided atelectasis or effusion (n = 4), left‐sided atelectasis or effusion (n = 2) and normal CXR (n = 1) Setting: ICU and cardiac ICU in urban 450‐bed hospital in northwestern USA |
|
| Interventions | Left lateral, right lateral and supine positions for 20 minutes Sequences/groups: SLR, SRL, RLS, RSL, LRS, LSR |
|
| Outcomes | Tissue oxygen delivery measures (HR, CO, arterial and mixed venous blood gases (PaO2, SaO2, SvO2), CaO2 and VO2), serum lactate as a measure of the adequacy of tissue oxygenation, resp. rate (possibly secondary outcome) All measures taken between 15th and 20th minutes Other co‐primary outcomes reported but not relevant for this review were pH, PaCO2 and HCO3 |
|
| Standard management | Ventilator settings unchanged during trial and no tracheal suctioning. All participants received sedative and pain medication, but not during data collection. No muscle relaxants given and no change in vasoactive medication dose rates | |
| Position description | 45 degrees lateral rotation, passively turned with participants instructed not to assist, commercial foam wedge to maintain angle Angle verification method: protractor No HOB elevation with single pillow under the head for all positions |
|
| Washout period | 15‐minute stabilization period (data collected over 5 minutes before next position change) | |
| Notes | Post hoc power analysis performed (effect size 0.558 to detect mean difference in CaO2 of 2.3%, CO of 6.7% and lactate of 7.5%, 1‐β = 0.8, α = 0.05) CaO2 calculations included Hb level taken from first arterial line sample only, no active bleeding reported Comments: no extractable summary statistics for meta‐analysis because of unit of analysis error (reported mean and SD for each body position). Additional data no longer available (personal communication from principal investigator) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized positioning sequence. Participants assigned study number and associated position sequence based on order of entry into the study |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from digital display of continuous physiological monitoring system or calibrated blood gas analyser; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants completed study. Numerator for each outcome stated within ANOVA tables, consistent with sample size except for mixed venous data Quotation: "because of some missing data there was insufficient power to evaluate the effect of position on venous blood gases and VO2" Comment: impact of missing VO2 and other venous gas measures (SvO2) unclear, as measures were part of the evaluation of tissue oxygen delivery |
| Selective reporting (reporting bias) | High risk | Mixed venous sampling (i.e. VO2 and SvO2) not included within results or analysis because of missing data Comment: high risk of selective reporting bias for outcomes measuring tissue oxygen delivery Other reporting issue: unclear whether subgroup analysis based on location of lung pathology (unilateral vs bilateral) was pre‐planned, but such analysis was unlikely to influence primary reporting |
| Other bias | Unclear risk | No baseline position described. Uniform cross‐over design with unclear balance (no sequence group numbers, small sample size (n = 12) for 6 sequences). No baseline characteristics presented except for inclusion criteria. Unclear whether any baseline differences between groups (sequences) occurred by chance, as allocation concealment is unclear Comment: unclear whether sequence effects or treatment‐by‐period interactions may be sources of bias. If carryover present, unclear whether equally applied across treatments because of unclear balance. Carryover effects may be due to short active and possibly insufficient washout. Unclear risk of carryover bias Data collection between 15th and 20th minutes following each turn; therefore, unclear whether all treatments (body positions) were measured in the same way (i.e. time elapsed before measurement) Comment: small differences in measurement intervals between participants (as possible source of bias) unlikely to yield clinically important differences within individuals (within‐subject variability) |
Bein 1996.
| Methods |
Cross‐over trial (3‐treatment, 2‐sequence, 3‐period design) Method of analysis: non‐parametric tests (Wilcoxon test) due to non‐normality of continuous data |
|
| Participants | 12 mechanically ventilated adults with ARF requiring positive inotropic support who were eligible for kinetic treatment on the basis of clinical findings (P/F ratio ≤ 225) and CXR (Murray lung injury score ≥ 9) All participants had pneumonia based on definition of Centers for Disease Control and Prevention (USA) and SIRS criteria of hyperdynamic circulation (CI > 3.5 L/min/m2 after fluid resuscitation (PCWP ≥ 14 mmHg) and vasopressor requirements (i.e. dopamine > 6 μg/kg/min) to maintain mean arterial pressure > 70 mmHg), hyperthermia > 38.5°C and abnormal white blood cell count < 3 or > 12 cu/mm Sex (M/F) 11/1, mean age 48.7 years ± 18.5 Mean baseline P/F ratio 178.8 ± 49.2 I:E ratio 1:1 with inspiratory peak pressure (Pmax) ≤ 30 mbar and PEEP ≤ 10 mbar with pressure control mode Diagnosis: multiple trauma (n = 3), carcinoma resection (n = 3), intracerebral haemorrhage (n = 2), cardiopulmonary resuscitation (n = 1), spinal cord injury (SCI) (n = 1), Chlamydia infection (n = 1), abdominal aorta aneurysm (n = 1) Severity of illness: mean APACHE II score 19.6 ± 5.1, mean EVLW 15.4 mL/kg ± 6.2 Setting: 8‐bed ICU, University Hospital (study authors from Germany) |
|
| Interventions | Extreme left lateral, extreme right lateral and supine positions for 15 minutes Sequences/groups: SLR, SRL |
|
| Outcomes | Cardiovascular measures (CI, MABP, HR, MPAP, PCWP) at 15 minutes Other co‐primary outcomes reported but not relevant for this review were CVP, RV ejection fraction (REF), RV end‐diastolic volume (RVEDV), intrathoracic blood volume (ITBV), RV stroke work index (RVSWI), systemic vascular resistance index (SVRI), pulmonary vascular resistance index (PVRI) and concentration of atrial natriuretic peptide (ANP) |
|
| Standard management | All participants sedated continuously | |
| Position description | 62 degrees lateral rotation, angle verification method not described HOB elevation: supine position 0 degrees, no further descriptions Comment: all participants on Rotorest Kinetic Treatment Table (Kinetic Concepts, San Antonio, Texas, USA), angle likely to be automatically set |
|
| Washout period | 15‐minute stabilization period (static body position) before data collection. No description of rotation between static body positions | |
| Notes | No sample size calculation described Double indicator (thermal and dye) dilution system (Pulsion) used to measure haemodynamic data with fibreoptic and thermistor capability of femoral arterial catheter. Transducers maintained at left atrium level throughout study Comments: SCI diagnosis < 10% of sample, lateral turning not contraindicated; therefore, study met inclusion criteria. No extractable lateral position data for meta‐analysis because of unit of analysis error (reported median and range for each body position). Supine position order not randomized; therefore, within‐subject differences between each lateral position and supine position not valid for extraction Email request for further information regarding study design and results to enable data transformation, no response from principal investigator |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "randomized order" of left dependent position or right dependent position. Participants randomized to sequences. No further description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from digital display of continuous and intermittent physiological monitoring systems and blood sampling for radioimmunoassay; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. Median reported for 12 participants, consistent with sample size |
| Selective reporting (reporting bias) | Unclear risk | Study aim and hypothesis focused on lateral position effects. Research objective not clear about intended comparisons of interest, but method of analysis indicated that data from left lateral and right lateral positions were compared with data recorded in supine position Comment: Inclusion of supine position as a comparator was not explicitly clear. Unclear whether omissions in reporting the study design occurred, or if comparisons between lateral and supine positions were conducted posteriori. Furthermore, no explanation given for skewed continuous data within the sample |
| Other bias | Unclear risk | No baseline position described. Non‐uniform unbalanced cross‐over design. Number of participants allocated to each sequence unknown Comments: unknown whether participants were in the same body position, before commencing the study, as their first treatment period (If no turning was required for the first period, possible bias due to differences in treatment duration and data collection methods). No information provided about whether vasoactive medication and other standard management practices were consistently applied across groups (sequence) and periods Comment: unclear whether sequence effects, period effects or treatment‐by‐period interactions may have been sources of bias. If carryover present, unlikely to be equally applied to treatments because of lack of balance and uniformity. Carryover effects may be due to short active and possibly insufficient washout. Unclear risk of carryover bias |
Carroll 1992.
| Methods |
Cross‐over trial (3‐treatment, 2‐sequence, 4‐period design) Pre‐specified analysis: repeated measures 2‐way ANOVA |
|
| Participants | 16 patients within 2 to 4 hours after CABG surgery Setting: not described (study author from USA) |
|
| Interventions | Left lateral and right lateral positions for 20 minutes and supine position (varied, see sequence schema) Sequences/groups: S(baseline), LT20, ST60, RT20, ST15 S(baseline), RT20, ST60, LT20, ST15 (T in minutes) (personal communication from principal investigator) |
|
| Outcomes | CO and SvO2 measured at baseline (for lateral positions, before turning), at 0 minutes (immediately after turning) and at 15 minutes after each turn (personal communication with principal investigator) | |
| Standard management | Not described | |
| Position description | 45 degrees lateral rotation, foam wedge, angle verification method not described No HOB elevation (bed remained flat in all positions) |
|
| Washout period | Not described Comment: active washout in supine position after data collection, before the second set of baseline data (immediately before contralateral position) |
|
| Notes | Conference proceeding abstract (primary reference). Unpublished thesis not accessible via inter‐library loan system Unknown duration of baseline (supine) position. In abstract, reported comparisons (between supine and left lateral position, supine and right lateral position, and lateral positions and supine position) were without summary data or findings from statistical tests. Unclear whether paired comparison between lateral positions was conducted Comments: supine position order not randomized; therefore within‐subject differences between each lateral position and supine position not valid for extraction. No extractable lateral position data, and individual participant data were not available for calculation of SE (MD) for paired comparisons (personal communication from principal investigator) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Insufficient information Quotation from communication: "Randomly assigned to turn initially to the left or the right" (personal communication from principal investigator). Participants randomized to sequences |
| Allocation concealment (selection bias) | Unclear risk | Not described. Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not described. Insufficient information Comment: missing data points, if present, unlikely to bias within‐subject comparisons |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Non‐uniform unbalanced cross‐over design. Insufficient information Comment: unclear whether sequence effects, period effects or treatment‐by‐period interactions may have been sources of bias. Carry effects may be due to short active and possibly insufficient washout in some periods. Unclear risk of carryover bias |
Chan 1992.
| Methods |
Randomized cross‐over trial (3‐treatment, 6‐sequence, 3‐period design) Pre‐specified analysis: 1‐way ANOVA, subgroup analysis identified |
|
| Participants | 30 mechanically ventilated adults between 6 and 10 hours after elective CABG surgery via sternotomy Other inclusion criteria: English speaking, presence of arterial line and PA catheter, postop SBP > 90 mmHg and temperature > 36.5°C Sex (M/F) 29/1, mean age 61.70 years ± 9.46 Mean FiO2 0.44 ± 0.13, mean PEEP 6.50 cmH2O ± 1.81, mean Vt 1242.13 mL [sic] ± 144.22 (15 mL/kg of preop weight) with SIMV mode Subgroups: fluid balance in excess of 25% (from surgery to study) (n = 21), left lung atelectasis (n = 5) and no atelectasis (n = 3) on immediate postop CXR (other CXR groups: right lung (n = 1) and bilateral (n = 21) atelectasis) Exclusion: preop pulmonary disease, MI in past 6 weeks, valvular disease or previous valvular replacement, external cardiac assist device Setting: 10‐bed adult cardiovascular ICU of major tertiary referral hospital in Edmonton, Western Canada |
|
| Interventions | Left lateral, right lateral and supine positions for 30 minutes Sequences/groups: 1 = RLS (n = 6), 2 = RSL (n = 4), 3 = SRL (n = 1), 4 = SLR (n = 1), 5 = LSR (n = 10), 6 = LRS (n = 8) Note: 30 minutes of baseline data collection for initial body position in which patients were found before the study (all participants found in the supine position for baseline data) |
|
| Outcomes | PaO2 and C(a‐v)DO2 (as inverse indicator of CO) at 30 minutes SaO2 at 30 minutes and cardiorespiratory outcomes (BP (SMD), HR, resp. rate, PAP (SMD), PCWP) taken at 0, 5, 15 and 30 minutes (possibly secondary outcomes for post hoc analysis) Relative pulmonary shunt (co‐primary outcome) reported but not relevant for this review |
|
| Standard management | No ventilator changes. Suctioning during trial (30%), hourly suction (n = 3), with 30 minutes elapsed after suctioning before data collection resumed. Pariticpants received IV analgesia before study procedures and titration of inotropic and vasoactive medications (i.e. dopamine (n = 28), NTG (n = 25) and SNP) | |
| Position description | 30 degrees lateral rotation, 30 degrees hard foam, single pillow between legs, angle verification method not described 30 degrees HOB elevation for all positions (20 degrees head rest and 20 degrees thorax elevation set with single pillow under head to achieve angle of 30 degrees); verification method: protractor (30‐degree angular ruler). Investigators acknowledged participants slid down (position difficult to maintain) |
|
| Washout period | 30 minutes chosen as conservative rest period to allow for equalization of gases | |
| Notes | Post hoc power calculation (effect size 0.2, 1‐β 0.12, α 0.05), power of 0.51 required a sample of 200 (stated to be beyond study scope) Comment: study underpowered to detect clinically important differences in primary outcomes. Post hoc analysis of BP and PAPs in lateral positions may be subject to measurement error because transducers were levelled to phelebostatic axis for all body positions Mean duration from surgery to study 8 hours 50 minutes ± 5 hours 22 minutes Correlational analysis (influence of preop and postop variables on dependent variables) and FiO2 co‐variance analysis reported, but not relevant for this review Mean Vt (demographics) showed typographic discrepancy between text and table (postop ventilator settings). Sequence modified for groups 3 and 4 (i.e. 2 participants; 1 allocated to each group) Comment: Modification occurred as participant's "initial position" was the same as the first period position (supine position). Study commenced with second period position for both groups, with sequence ending with allocated first period position Comments: no extractable summary statistics for meta‐analysis because of unit of analysis error (sample and subgroup means reported for each body position, no variance provided) PaO2 and C(a‐v)DO2 data from subgroups with unilateral atelectasis; no atelectasis calculated and extracted from individual participant data. No raw data for bilateral atelectasis group (n = 21); therefore no extractable sample data Email request for contact details of principal investigator; no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Participants randomly assigned to 1 of 6 sequences |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described. Subgroup classification: CXRs assessed independently without radiologist awareness of study purpose Comment: objective outcome measures taken from real‐time physiological monitoring systems and blood gas analyser; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No numerator for each outcome Comment: degree of freedom (df 5, 24) in ANOVA tables consistent with sample size (2 of the 6 groups included only 1 participant in each group). No missing data apparent |
| Selective reporting (reporting bias) | Unclear risk | Study conducted different data collection practices between treatments. Average score for supine position data (baseline data taken after extended period in initial supine position combined with supine position data during treatment sequence) compared with each lateral position. Rationale for combining supine data provided ‘to differentiate changes due to pathology or due to treatment effect’. Reported that no measured pathology occurred during the time of the study, as indicated by a small clinically non‐significant difference between baseline and supine position data. However, no statistics provided Comment: unclear risk of selective reporting bias, as unclear whether duplicate supine data entry and analysis within ANOVA were intended Cardiopulmonary variables appears to be part of post hoc analysis, as hypothesis testing focused on PaO2, relative shunt and C(a‐v) DO2 as outcomes of interest. Furthermore, unclear whether results (mean value for each cardiopulmonary variable) were averaged from all repeated measures (i.e. 0, 5, 15 and 30 minutes) or were taken from single time point at the end of the period (i.e. 30 minutes), similar to primary outcomes. Unclear selective reporting of secondary outcomes, but such analysis unlikely to influence primary reporting |
| Other bias | Unclear risk | Cross‐over design with non‐uniformity of periods after sequence modification and lack of balance (unequal sequence size, not all body positions preceded each other the same number of times after modification). No statistical difference based on treatment sequence (between‐group analysis conducted according to the group to which each participant was initially allocated, despite treatment order modification for 2 sequences). Repeated turning did not demonstrate consistent increase or decrease in primary outcomes. Unknown whether treatment‐by‐period interactions were investigated Comment: Tests for carryover and sequence effects have low power to detect differences. If carryover present, unlikely to be equally applied across treatments because of lack of balance and non‐uniformity with lack of standard management controls. Carryover effects may be due to short active and possibly insufficient washout. Unclear risk of carryover bias |
Chulay 1982.
| Methods | RCT with 2‐group design, stratified randomization by sex and number of coronary artery bypass grafts (< 3 or ≥ 3) | |
| Participants | 35 postoperative mechanically ventilated adults within 2 hours of elective CABG surgery with systolic BP > 90 mmHg Sex (M/F) 30/5, mean age 52 years ± 10 for both groups Mean number of grafts 3.0 ± 0.98 (experimental group), 3.3 ± 0.97 (control group) PEEP set at 5 cmH2O, Vt range 12 to 15 mL/kg with SIMV mode via volume‐cycled ventilators Exclusion: aneurysmectomy, valve replacement, vasopressor therapy, intra‐aortic balloon assistance Setting: surgical ICU (study authors from USA) |
|
| Interventions |
Experimental group (n = 17): lateral positioning schedule for first 24 postoperative hours (turned systematically every 2 hours between supine and alternating left lateral and right lateral positions) Control group (n = 18): supine immobilization for first 24 postoperative hours (maintained in supine position without turning) |
|
| Outcomes | Data collected on (1) presence of CXR abnormalities (evidence of lobar, segmental or platelet‐like atelectasis, pulmonary oedema, pleural effusion, parenchymal infiltrates or pneumothorax) on admission, then daily for 72 hours; (2) temperature recorded 1‐ to 2‐hourly for 72 hours (with number of hours > 38°C reported within results); (3) PaO2 recorded after 4 hours for first 24 hours; (4) other vital signs (resp. rate, HR, BP) and PAPs recorded at 15 and 60 minutes each hour for first 24 hours; and (5) duration of intubation and LOS in SICU Quotation from communication: "Primary end point was atelectasis. Presence of fever and chest x‐ray evidence of atelectatic areas were the measures for the primary outcome measure." It was not intended to measure PaO2 over the 24‐hour period. Vital signs except temperature were obtained for the purpose of safety monitoring. PAPs also were not primary endpoints (personal communication from principal investigator) Other outcomes reported but not relevant for this review were P(A‐a)O2 on 100% on admission to SICU and 24 hours postop |
|
| Standard management | Reported identical medical and nursing care, including respiratory management. Similar amount of analgesic medication given in both groups. However, control group received more antipyretic medication within first 72 hours postop compared with experimental group (not statistically significant) FiO2 adjusted to maintain PaO2 at 85 mmHg or above. Weaning from ventilator accomplished over 10 to 18 hour period on the basis of ventilatory mechanics and ABGs. No routine chest physiotherapy or intermittent positive‐pressure breathing (IPPB) after extubation |
|
| Position description | 45 degrees lateral rotation, foam wedge during 2‐hour periods, angle verification method not described No other descriptions |
|
| Washout period | Not applicable for design | |
| Notes | No sample size calculation described Quotation: "no complications could be directly attributed to changing position" Instrument validation and reliability not reported Retrospective analysis of medical notes and charts conducted to identify rationale for difference in LOS between groups Left lung atelectasis in experimental group (72%) and control group (68%) with follow‐up periods and baseline not specified Comments: no extractable dichotomous data on CXR abnormalities for meta‐analysis. Number of participants in each group ‘with and without’ atelectasis or any other chest abnormalities not reported for any follow‐up period. Additional data no longer available (personal communication from principal investigator) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by sex and number of bypass grafts and randomly assigned to 1 of 2 groups by drawing from a hat |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | For all outcomes, nurses and house staff physicians responsible for care were aware of the study but were not informed of dependent variables being studied, and investigators did not participate in management decisions or care. Personnel unaware of outcomes, but unclear whether groups may have been managed differently unintentionally |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CXRs assessed independently without observer awareness of group assignment Comment: objective outcome measures taken from real‐time physiological monitoring system and ABG analyser. Duration of intubation and length of stay recorded, but personnel not informed of dependent variables being studied. Therefore, lack of outcome assessor blinding unlikely to bias these results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described Quotation from personal communication: "Intent to treat analysis was performed" Comment: unclear whether data were complete, or if data points were missing. No further description of study methods and results was available |
| Selective reporting (reporting bias) | Unclear risk | Quotation: Aim was "to determine whether immobility or systematic turning is of clinical, physiological, or economic value after CAB surgery" The aim and methods included insufficient information to clarify primary outcomes versus secondary outcomes, as well as length of intended follow‐up for each outcome. Summary statistics and statistical testing for 1 vital sign (temperature), LOS and length of intubation time reported. No results presented on the incidence of CXR abnormalities other than atelectasis. Atelectasis reported as a percentage for each group, without indicating follow‐up period (discussion implied that the incidence of atelectasis was unchanged from baseline). Other outcomes (PaO2 and other vital signs (resp. rate, HR, BP)) briefly reported without summary statistics or statistical analysis. Investigators reported that ABGs were not uniformly available after 24 hours. No outcome reporting for PAPs. Economic costing stated as an outcome without mention within methods or results section. However, this outcome was not relevant for this review. Overall, risk of selective reporting unclear as the result of ambiguity between study purpose and intended primary outcomes |
| Other bias | Low risk | Minimal data presented on participant characteristics; both groups stated to be similar |
de Laat 2007.
| Methods |
Initially an RCT with split‐plot design, randomized to time after surgery (first tier), then body position (second tier). However, non‐randomized reference group included after interim analysis Pre‐specified method of analysis before interim analysis not described. Study reported model of bioequivalence for comparison between randomized groups and non‐randomized reference group |
|
| Participants | 69 postoperative myocardial revascularization adults with PA catheter and arterial line in situ and haemodynamic values within safe ranges (safe range defined as CI > 1.5 L/min/m2 or MAP, PCWP and RAP not exceeding baseline > 15% or MABP not < 15% below baseline) Sex (M/F) 52/17, mean age from 63.4 to 68 years between groups Exclusion: no ventricular assist device Termination criteria: values that fall outside the ‘safe range’ (described above) in the lateral position. In addition, protocol indicated that participants in pain during lateral position would be turned back Setting: 14‐bed ICU, Radboud University, Nijmegen Medical Centre, The Netherlands |
|
| Interventions |
Group A (n = 27) commenced lateral position 2 hours after surgery, turned to right lateral (n = 13) versus left lateral (n = 14) positions Group B (n = 28) commenced lateral position 4 hours after surgery, turned to right lateral (n = 14) versus left lateral (n = 14) positions Group C (n = 14) maintained in supine position for 6 hours commencing 2 hours after surgery (non‐randomized reference group) Group A and B period schema: baseline positionT15, lateral positionT120, supine positionT120 (T in minutes) |
|
| Outcomes | Groups A and B: CI at 30, 120, 150 and 240 minutes (equivalent to 30 and 120 minutes in lateral position, then 30 and 120 minutes in supine position) Group C: CI at 30 minutes, 2 hours, 2 hours 30 minutes, 4 hours, 4 hours 30 minutes and 6 hours |
|
| Standard management | Mechanical ventilation not described but implied within text Quotation "The 30° lateral position ... was adapted to the specific situation of sedated and ventilated patients in cooperation with a physiotherapist" IABP (n = 6), antihypertensives (n = 28), inotropes or vasopressors (n = 21), with minimal high‐dose inotropes (n = 1). Analgesics given according to prescription, with no additional analgesia required. Changes in medication or dose rate to be recorded for interpretation of changes in CI on a participant level Comment: Statement implies that medication may have been titrated during the study |
|
| Position description | 30 degrees lateral rotation, 30 degrees wedge cushion, angle verification method not described 20 degrees HOB elevation for lateral position, no description for supine position. Positioning instructions given to nurses |
|
| Washout period | Not applicable for design | |
| Notes | No sample size calculation described Length of duration in each body position for Groups A and B was mean 117 ± 8 minutes (range 104 to 158 minutes), with some participants turned later than protocol because of other ICU priorities Supine position data for Groups A and B analysed according to group allocation Comment: termination criteria possibly subjected to measurement error, as transducers measuring MABP, PCWP and RAP were levelled to phlebostatic axis for all body positions Unclear whether any percentage change was associated with difference in hydrostatic pressure Quotation: "no signs of discomfort in lateral position in both groups were observed" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random assignment by drawing sealed envelopes for 4 positioning conditions (Groups A and B). Unclear random sequence generation method for participants randomized to groups. Nonetheless, high risk of selection bias, as not all eligible participants were randomized to groups. Non‐randomized reference group (Group C) selected from eligible population following interim analysis of data (first 15 participants). Investigators acknowledged selection bias, but reported groups were comparable, as baseline CI data and starting values for initiating the study were not significantly different between groups Comment: Group differences other than baseline CI may confound results. Unclear how eligible participants were selected for randomization procedures vs non‐randomization following interim analysis. Investigator knowledge of haemodynamic status of participants as a pre‐requisite for eligibility may have influenced unintentional or intentional group selection |
| Allocation concealment (selection bias) | High risk | Group C allocation not concealed. No description for other groups (except use of sealed envelopes) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from real‐time physiological monitoring system; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 75 participants eligible (3 refused consent, 3 excluded because of postop complications before group allocation). Trial profile figure indicates that 69 participants (A, B, C groups) completed trial. Missing data point for lateral position (n = 1) because participant turned back early at 31 minutes as the result of an adverse event (pneumothorax later identified), but not explicitly clear whether all data points for supine position were available for analysis. No group/subgroup numbers provided for results or analysis |
| Selective reporting (reporting bias) | High risk | High risk of selective reporting after interim analysis, with primary analysis performed according to post hoc design change and findings from subgroup analysis forming major conclusions of the study. Post hoc subgroup analysis conducted on number of participants receiving vasoactive medication at the start of the lateral position who demonstrated a decrease in CI > 15% for the lateral position (all groups, including reference group). Analysis ambiguous, as supine reference group (C) was not measured in the lateral position, and participant measurement intervals were split into 2 groups (CA and CB) to correspond with Group A and Group B time points, with frequencies analysed separately (i.e. CA vs group A, CB vs group B). Data from reference group likely to be reported more than once within the analysis (unit of analysis error) |
| Other bias | Unclear risk | No withdrawals based on termination criteria. Primary outcome reporting (CI) unlikely to be biased by potential measurement error of termination criteria variables (i.e. MABP, PCWP and RAP) Descriptive statistics tabulated for each group (A, B, CA, CB) for baseline haemodynamics and inotropic and vasoactive medication, with dose rate for each drug 5 minutes before turning to the lateral position. Group participants appear to be using more than 1 drug Comment: Unclear whether all participants received the same management for ventilation, titration of medication and fluid replacement. Unclear if group differences in baseline variables and standard management |
Doering 1988.
| Methods |
Cross‐over trial (3‐treatment, 2‐sequence, 3‐period design) Pre‐specified analysis: 1‐way repeated measures ANOVA analysis and post hoc paired t testing of significant differences (P < 0.05) within ANOVA |
|
| Participants | 51 postoperative cardiac surgery adults following use of pump‐oxygenator (CABG, aortic or mitral valve replacement or combination, with 4 participants undergoing additional aneurysmectomy, epicardial mapping and implantation of automatic defibrillator leads) Other inclusion criteria: CVP ≥ 5 cmH2O ≥ 1 hour after fluid expansion and ≥ 2 hours after administration of diuretics Sex (M/F) 37/14, mean 63.1 years Mean PEEP 5.29 cm [sic] (range 0 to 20 cm [sic]) in mechanically ventilated participants (n = 42), otherwise extubated (n = 9) Exclusion: congestive heart failure (New York Heart Association Class III or IV), postural hypotension, presence of valvular disorders not repaired during surgical procedure, presence of atrial fibrillation Setting: large Western medical center (study authors from USA) |
|
| Interventions | Left lateral, right lateral and supine positions for approximately 15 minutes Sequences/groups: 1 (n = 26)= SRL, 2 (n = 25) = SLR |
|
| Outcomes | CO after 15 minutes (mean 16.3 minutes in each position before data collection) HR (secondary outcome) after 15 minutes Stroke volume (secondary outcome) reported but not relevant for this review |
|
| Standard management | Vasodilators, vasopressors, inotropes and antihypertensives administered (n = 22), with no changes made during data collection | |
| Position description | 45 degrees lateral rotation, 20 degrees HOB elevation for all body positions, verification method (rotation angle and HOB elevation): standard protractor with lateral angle standardized from shoulder level, and HOB standardized from bed frame level | |
| Washout period | Quotation: "Waiting period" of at least 15 minutes before data collection "to allow for the re‐establishment of any hemodynamic parameters that might have been altered by the effect of repositioning" | |
| Notes | No sample size calculation described Study commenced between 4 and 24 hours (mean 10.6 hours) after surgery, with positioning schedule completed within mean 55 minutes (range 51 to 70 minutes) CO determined by pulmonary artery thermodilution method, with 2 injectates averaged for each result Frequency of CO variation > 10% (subgroup analysis) with typographic discrepancy between text and Table 2 (relationship between CO variation and baseline variables and characteristics) Comments: no extractable lateral position data for meta‐analysis because of unit of analysis error (reported mean and SD for each body position). Additional data no longer available (personal communication with principal investigator). Supine position order not randomized; therefore, within‐subject difference between each lateral position and supine position not valid for extraction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned by coin toss to 1 of 2 positioning sequences |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from real‐time physiological monitoring system; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eligible participants (n = 59), exclusions 8.5% (calculated) for clinical instability (n = 3) and unavailability of the researcher (n = 2), withdrawals 5.1% (calculated) because of inability to complete study protocol (n = 3), with rationale provided (unco‐operative, required IV sedation, equipment failure in 1 body position). No primary CO data missing for those who completed the protocol (n = 51). HR data missing for 3 participants based on df (2, 47) in ANOVA Comment: withdrawals and missing data unlikely to bias within‐subject comparisons |
| Selective reporting (reporting bias) | Low risk | Primary outcome reported as intended, but unclear whether secondary analyses was pre‐planned. Secondary analyses (1‐way ANOVA and post hoc paired t testing) conducted between subgroups according to degree of CO variation (< 10% vs ≥ 10%) to position change, and presence of 7 demographic and clinical variables at baseline Comment: unclear selective reporting of secondary/subgroup analyses; however, analyses not relevant for the review and unlikely to influence primary outcome reporting |
| Other bias | Unclear risk | No baseline position described. Non‐uniform unbalanced cross‐over design Comments: unknown whether participants were in the same body position before commencing the study and during the first treatment period (If no turning was required for the first period, possible bias due to differences in treatment duration and data collection methods). Unclear whether sequence effects, period effects or treatment‐by‐period interactions may have been sources of bias. If carryover present, unlikely to be equally applied to treatments because of lack of balance and uniformity. Carryover effects may be due to short active and possibly insufficient washout. Unclear risk of carryover bias Study purpose was to compare effects of supine position with those of right lateral and left lateral positions, but results may be subject to interpretation bias, as frequencies and summary data reported were based on a directional change in body position, with no data collected following a turn from lateral position to supine position |
Gavigan 1990.
| Methods |
RCT with 2‐group design Pre‐specified analysis: ANOVA, quotation: “for any differences between and within groups” |
|
| Participants | 50 mechanically ventilated elective CABG surgery adults within 2 hours of surgery (each group included 1 participant with both CABG and aortic valve replacement); other inclusion criteria: SBP 90 to180 mmHg, DBP < 100 mmHg, HR < 120 beats/min, PEEP ≤ 5cmH2O (range 0 to 5) Sex (M/F) 42/8, mean 62.12 years ± 10.33 FiO2 (0.4 or 0.5) adjusted for optimal PO2, Vt 10 to 15 mL/kg with A/C mode (volume‐cycled MA1 device) Exclusion: asthma, COPD, tuberculosis, lung cancer, pneumothorax Termination criteria: PEEP > 5 cmH2O Setting: surgical CCU, Thomas Jefferson Unversity Hospital, Philadephia, Pennsylvania, USA, for 5‐month period |
|
| Interventions |
Experimental group (n = 18): lateral positioning schedule for first 24 postoperative hours (turned every 2 hours between supine and alternating left lateral and right lateral positions) Control group (n = 32): supine immobilization for first 24 postoperative hours (maintained in supine position without turning) |
|
| Outcomes |
Other data collected and reported included presence of temperature > 38.2°C measured rectally (collected 1‐ to 4‐hourly over first 3 postop days) and length of ICU stay Other data collected and not reported included other vital signs, haemodynamic monitoring recorded hourly, time of extubation and post‐extubation ABG (unclear whether secondary outcomes or taken for monitoring purposes) |
|
| Standard management | Quotation: "Patients in both groups were treated the same in all aspects of care with the exception of turning". Post extubation, all participants began incentive spirometry once an hour for first postop day and chest physiotherapy immediately and every 4 hours, with morphine sulphate offered to all every 3 hours and up to a half‐hour before chest physiotherapy | |
| Position description | 45 degrees lateral rotation, commercial foam wedge, angle verification method not described. No other descriptions | |
| Washout period | Not applicable for design | |
| Notes | No sample size calculation described No description about reliability or validity of atelectasis scoring system Study tabulated frequencies according to postop day (days 1 to 3), type of atelectasis (lobar, segmental, discoid) and percentage with right‐ and left‐sided atelectasis of each type Comments: unclear whether participants were represented more than once within the results table. Atelectasis incidence reported (74% of sample, but unclear if at baseline or during any of the follow‐up periods) with no numerator with and without atelectasis for each allocated group at follow‐up periods. No extractable dichotomous data on CXR abnormalities for meta‐analysis. No summary statistics for any other outcome. Study information no longer accessible; attempt made to find study file (personal communication with principal investigator). No further information received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to experimental or control group, with no further description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described Comment: Caregiver awareness of group allocation may influence length of stay; unclear if lack of blinding may bias this result |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Radiologist documented evidence of lung pathology for CXR without awareness of group assignment. Single assessor of atelectasis scoring system unaware of group allocation. Blinding of other outcomes not described Comment: temperature and other objective outcome measures taken from real‐time physiological monitoring system; therefore, lack of outcome assessor blinding unlikely to bias these results |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis conducted. Uneven group size (18 vs 32) due to withdrawal of one‐third of original experimental group (actual numerator not described) Withdrawals due to haemodynamic compromise following a turn (described as a transient drop in SBP < 100 mmHg that returned to baseline when immediately returned to the supine position) Comment: PEEP limit was sole termination criterion |
| Selective reporting (reporting bias) | Unclear risk | Aims, methods and results provided insufficient information for identification of all primary outcomes from secondary outcomes, including temperature and other vital signs Comment: inadequate reporting of dichotomous group data for postop atelectasis. No other data on CXR abnormalities. No summary data reported for length of stay in ICU or hospital. Frequency of temperature > 38.2°C analysed (P value and df reported), with no other vital signs analysed. Overall, unclear risk of selective reporting bias for primary vs secondary outcomes, as ambiguity between study purpose, outcomes collected and outcomes reported |
| Other bias | Low risk | Method of randomization considered adequate by investigators, as no difference found in baseline demographics and characteristics |
Gawlinski 1998.
| Methods |
Cross‐over trial (3‐treatment, 2‐sequence, 3‐period design) with single passive washout period between lateral positions Pre‐specified analysis: repeated measures MANOVA of “position order” (group 1 vs group 2), position (treatment) and timing of measurement. Pre‐specified post hoc analysis |
|
| Participants | 42 critically ill adults with < 30% ejection fraction (documented by 2‐dimensional echocardiography or radionuclide ventriculography) who had an existing fibreoptic PA catheter in situ Sex (M/F) 32/10, mean age 53.93 years ± 11.75 Diagnosis: dilated cardiomyopathy (n = 21), ischaemic cardiomyopathy (n = 19), coronary artery disease (n = 1), MI (n = 1) Exclusion: documented septic shock Setting: cardiac care unit (CCU) or coronary observation unit (COU), UCLA Medical Center, Los Angeles, California, USA |
|
| Interventions | Left lateral, right lateral and supine positions for 25 minutes Sequences/groups Group 1 (n = 17)= S, R, S(washout T15), L Group 2 (n = 25)= S, L, S(washout T15), R (T in minutes) Comment: group size discrepancy within text (numerator stated above) and tables (group 1 = 23, group 2 = 19) |
|
| Outcomes | SvO2 at 0 minutes (baseline), 1 to 5 minutes (each minute), 15 and 25 minutes Note: 0‐minute data for each lateral position collected in supine position (i.e. end of first period and passive washout period) Secondary outcomes for secondary analysis: CO, DO2 (CO, Hb and SaO2), VO2 and other variables (RAP, PAP, PCWP, HR) at 0 and 3 minutes, with the exception of SaO2 measured at all time points) |
|
| Standard management | Dose rate of medication (laxis, inotropes, vasodilators or combination) during the study (n = 14) was constant, without titration 30 minutes before the study (no medications up to 6 hours before commencement of study (n = 28)) Comment: Group numbers for vasoactive medication showed typographic discrepancies between text and tables |
|
| Position description | 45 degrees lateral rotation, wedge, single pillow between flexed legs, angle verification method not described 20 degrees HOB elevation for all body positions, with single pillow under head |
|
| Washout period | Quotation: "Stablization period of 15 minutes in the supine position...occurred before and after each position change" Comment: 15‐minute period in supine position between lateral positions, without inclusion as a treatment, equivalent to passive washout |
|
| Notes | Post hoc power analysis of treatment sequence (moderate to large effect 0.63, 1‐β = 0.8, α = 0.05) and position (moderate effect 0.44, 1‐β = 0.8, α = 0.05). 44 participants enrolled, 2 excluded, as did not meet inclusion criteria. Funding support identified and no apparent conflict of interest Pre‐specified post hoc/secondary analysis included stepwise regression of SvO2 in left lateral position at 3 minutes with DO2 determinants and VO2 as independent variables; and correlation coefficient analysis between SvO2 and CO at baseline (0 minutes) and at 3 minutes. Other analyses not pre‐specified but reported were "the effect of medication on the response of SvO2 to positioning" (subgroups with or without vasoactive medication) and SaO2, CO, HR and VO2 response to positioning Comment: all post hoc analyses not relevant for this review Other comments: Lateral position data included unit of analysis error (line graph showed mean values for each time point in each body position without SEM presented as reported variance). However, within‐subject SvO2 difference between lateral positions was calculated and was extracted from individual participant data (without group allocation) Supine position order not randomized; therefore, within‐subject difference between each lateral position and supine position not valid for extraction. Data not extracted at 0 minutes (data collected in supine position) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to 1 of 2 groups (sequences) via coin toss |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from real‐time physiological bedside monitoring; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotation: "missing data points were coded as missing rather than substituting mean values". Raw data table for SvO2 indicate that 2 participants had missing data at 25 minutes in all body positions, and 2 other participants had blanks at 25 minutes in left lateral position, but according to ANOVA tables, df was given as 38. Unclear whether 2 blanks in left lateral position indicated a typographical or printing error Comment: missing data unlikely to bias within‐subject comparisons |
| Selective reporting (reporting bias) | Unclear risk | Paired analysis of SvO2 between supine position and each lateral position at each time point not pre‐specified but reported within results Comment: unclear whether paired analysis was selectively reported, or if an omission occurred Published report limited reporting to the first 2 hypotheses, whereas unpublished thesis reported results of all 3 hypotheses (third hypothesis investigated SvO2 and CO relationship) Comment: Analysis conducted was not relevant for this review Secondary post hoc analysis was not pre‐specified but was unlikely to influence primary outcome reporting |
| Other bias | Unclear risk | No baseline position described. Non‐uniform unbalanced cross‐over design Comments: unknown whether participants were in the same body position before commencing the study for their first treatment period (if no turning was required for the first period, possible bias due to differences in treatment duration and data collection methods). Groups reported as comparable at baseline except for PCWP (statistical difference, mean PCWP > 18 mmHg for both groups, highest baseline PCWP between groups indicated a typographical discrepancy between text and tables). Investigators reported group difference in single parameter unlikely to influence SvO2 when no difference found for baseline CO or other baseline parameters that may alter SvO2 Comments: individual PCWP variation unlikely to bias within‐subject SvO2 difference. No statistical difference found for main effect testing of group (sequence of supine, right lateral vs supine, left lateral), interaction effects of group and position or group and time (measurement intervals) or interaction effects of group, position and time. However, group effect tests did not include sequences with lateral positions preceding supine position, and the test has low power to detect differences according to treatment order Quotation: "medication therapy affecting cardiopulmonary status was not controlled"; investigators acknowledge limitation Comments: Uncontrolled titration of medication may lead to unknown period or higher‐order sequence effects. Unclear whether period effects or treatment‐by‐period interactions may have been sources of bias. If carryover present, unclear if equally applied across periods and sequences because of lack of balance and non‐uniformity within periods. Carryover effects may be due to short active and passive washout that were possibly insufficient. Unclear risk of carryover bias |
George 2002.
| Methods |
Randomized cross‐over trial (3‐treatment, 3‐sequence, 3‐period design), with stratification by diagnosis 3:2 (emphysema: fibrosis) Pre‐specified analysis: MANOVA of group, time (measurement intervals) and diagnosis (hypothesis testing); univariate ANOVA of group and position (with no time point specified) |
|
| Participants | 15 mechanically ventilated adults within 24 hours of single lung transplant (SLT) surgery, with functional arterial catheter and oximeter catheter (no cardiopulmonary bypass), who were haemodynamically stable (no unstable arrhythmias or hypotension, not on ECMO) Sex (M/F) 8/7, mean age 54 years ± 8 Mean FiO2 0.43 ± 0.06, mean PEEP 8.5 cmH2O ± 3.1, mean Vt 600 mL ± 190 with SIMV mode (n = 11) or double‐lumen endobronchial tube with differential lung ventilation (n = 4) Diagnosis: emphysema (n = 9), lung fibrosis (n = 6); right lung allograft (n = 5), left lung allograft (n = 10) Termination criteria: ectopy, desaturation (SpO2 < 85%), hypotension (MABP < 90 mmHg) Setting: Cardiothoracic ICU, University Medical Center, Southwestern Pennsylvania, USA, September 1997 to December 1998 Comment: discrepancy in number of females. Possible typographical error for MABP < 90 mmHg as exclusion/termination criterion |
|
| Interventions | Left lateral, right lateral and supine positions for 30 minutes Lateral positions described according to locations of transplanted (allograft) lung and non‐transplanted (native) lung lowermost in lateral position (i.e. allograft lung down (A) and native lung down (N)) Sequences/groups: 1 (n = 5)= NAS, 2 (n = 5)= SAN, 3 (n = 5)= NSA |
|
| Outcomes | Oxygenation effects (PaO2 and SvO2) at 5 and 15 minutes; blood flow effects (MABP and HR) at 5, 15 and 30 minutes; blood flow effects (CO) at 25 minutes. All outcome measures recorded at baseline Other co‐primary outcomes reported but not relevant for this review were PaCO2 and minute ventilation (Vmin) (ventilation variables) |
|
| Standard management | Pre‐planned ventilation changes based on measured PaCO2 and Vmin (0.1 FiO2 increase before second turn (n = 1)). Vasoactive medication not controlled for (commenced NTG at turn 3 for non‐specific ST changes on ECG (n = 1)). Likert pain score with threshold set for standardization of pain medication during data collection and rationale provided. Pain relief delivered (n = 3) on the basis of pain score, otherwise at the discretion of bedside nurse (11 participants received analgesia (IV or epidural) before or during study) No further description provided |
|
| Position description | 45 degrees lateral rotation, 45 degrees wedge, angle verification method not described 30 degrees HOB elevation for all body positions, HOB verification method: angle indicator on bed frame |
|
| Washout period | Not described | |
| Notes | Study commenced mean 5 hours 37 minutes ± 4 hours 58 minutes after surgery (calculated from minutes, 1 outlier at 1289 minutes) A priori sample size calculation (effect size 0.185 for position, 0.551 for time and 1.16 for interaction with α = 0.05 and 1‐β = 0.8). Post hoc power analysis: 60 to 80 participants required to achieve statistical significance Comment: study underpowered to detect differences in primary outcomes CXR obtained closest to data collection (2 hours to 24 hours postop) for ischaemic‐reperfusion injury identification. Scoring system for degree of infiltration developed by one of the investigators. Pre‐specified effects of ischaemic‐reperfusion injury score on dependent variables, but not relevant for this review Other descriptions for analysis: immediate response (1 to 5 minutes), short‐term response (15 minutes), long‐term response (25 to 30 minutes) to turning. Discrepancy in baseline measurement (unclear whether taken for each body position or once before the first turn) Comments: Summary statistics (mean and SE for each body position) included unit of analysis errors for meta‐analysis. Within‐subject differences for all pair‐wise comparisons were calculated and extracted from individual patient data (group allocation identified) Discrepancy between mean values for PaO2 and SvO2 was reported within the tables and raw data calculations for each body position (differences included single time point and average score for all time points) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described Quotation: "stratified randomized design", participants "randomly assigned to three different sequencing patterns of turning" with 3:2 stratification for each sequence (group), "randomized to group I, II, or III using prepared assignment cards". Unclear random sequence generation for sequentially numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Individual, not associated with data collection; placed cards in sealed envelopes. Envelopes coded by diagnosis (stratified randomization) and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described for primary outcomes. CXR assessor for ischaemic‐reperfusion injury score blinded to group allocation Comment: objective outcome measures taken from real‐time physiological monitoring systems and calibrated blood gas and oximeter analysers; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 consecutive SLT patients recruited; 4 did not meet inclusion criterion of haemodynamic stability. Missing data acknowledged and left blank (2 dependent variables for 1 participant, and single data entry point for another participant). Unclear whether investigator conducted a review of potential bias' from missing data, as stated within the methods Comment: missing data unlikely to bias within‐subject difference for primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Most participants met termination criterion (MABP) and were not excluded or withdrawn Comment: possible typographical error, but not explicitly clear whether SBP < 90 mmHg was intended parameter to indicate hypotension Analysis does not appear to be conducted in accordance with protocol for blood flow effects. Results and analyses of HR and MABP were conducted separately from CO for hypothesis testing of blood flow effects. CO range for each group was reported only, without analysis. CO as an outcome measure was not reported in the publication Comment: unclear selective reporting of blood flow effects due to ambiguity between hypotheses, methods of data collection and analysis for all measures |
| Other bias | Unclear risk | No baseline position described. Baseline ventilator settings presented separately for each group (mean and SD) with comparable group settings; other dependent variables taken at baseline but not reported Uniform cross‐over design without balance (not all body positions preceded each other the same number of times, and the supine position did not precede native lung down). Sequence (group) effect reported for PaO2 (univariate analysis), with higher mean PaO2 for group 3 for all body positions compared with other groups. However, not all possible sequence combinations examined in the design. No explanation for group effect provided. Unknown whether period effects or treatment‐by‐period interactions were investigated Comment: unclear whether group (sequence) differences due to baseline differences (random error in small sample and/or possible selection bias as unclear randomization procedures) or differences in standard care (unclear performance bias) or a genuine sequencing effect. Overall, unclear whether groups were comparable on trial entry. Carryover effects may be due to short active and possibly insufficient washout, with unclear carryover bias |
Ibanez 1981.
| Methods |
Cross‐over trial (3‐treatment, 2‐sequence, 3‐period design), with passive washout period between lateral positions Pre‐specified analysis: paired t‐student tests |
|
| Participants | 10 continuous mechanical ventilation (CMV) patients with ARF secondary to unilateral lung disease Sex (M/F) 7/3, mean age 33.5 years ± 13.898 including 1 child (10‐year‐old) Mean FiO2 0.72 ± 0.239 (calculated), mean PEEP 12 ± 4.853 cmH2O (calculated), mean Vt 750 mL (650 to 1000 mL) with MA‐1 ventilator Diagnosis: pneumonia (n = 9), lung contusion/haemorrhage (n = 1) Other diagnostic characteristics at baseline: right unilateral lung disease (n = 7) and left unilateral lung disease (n = 3) Setting: ICU (study authors from Spain) |
|
| Interventions | Left lateral, right lateral and supine positions for 15 minutes. Lateral positions also reported as 'worse' and 'best' lateral positions Sequences/groups S, L, S(washout T5), R S, R, S( washout T5), L (T in minutes) (personal communication from principal investigator) |
|
| Outcomes | Gas exchange measures (PaO2 and P/F ratio) at 15 minutes Other co‐primary outcomes reported but not relevant for this review were PaCO2 and D(A‐a)O2 |
|
| Standard management | Ventilator settings unchanged during trial, no tracheal suctioning performed during study, all participants sedated (diazepam) and paralysed (pancuronium) | |
| Position description | Lateral 'decubitus' position, otherwise no further descriptions | |
| Washout period | Not described Quotation from communication: "...After each lateral position, the patient was returned to supine position for a period of 5 minutes and after that, the patient was moved to the other lateral decubitus for 15 minutes" Comment: 5‐minute passive washout (personal communication from principal investigator) |
|
| Notes | No sample size calculation described ABG analyser reliability not described Four participants died (3 sepsis, 1 pulmonary haemorrhage) Child participant (worse overall values, lowest PaO2 37 mmHg for right lateral position) Comment: Possible physiological differences between adults and children may distort variance within this small study Individual FiO2 levels reported. Worse lateral position was 'bad lung down' in all cases. Pair‐wise comparisons of right lateral vs left lateral, and bad lung down vs good lung down, calculated and extracted from individual participant data (with no group/sequence allocation) Comments: Summary statistics (mean and SE for each body position) included a unit of analysis error for meta‐analysis. Supine position order not randomized; therefore, within‐subject difference between each lateral position and supine position not valid for extraction Discrepancy between mean P/F ratio reported within text (from worse to best lateral position, 112 and 189, respectively) and extracted data (from worse (bad lung down) to best (good lung down) lateral position, 121.8 and 203.75, respectively) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "the order in which body positions was assumed was randomly determined" Quotation from communication: "We used a random sampling number tables...first body position of each patient was assigned according to random numbers (RLD odd, LLD, even)", "supine position was not included in the random assignment and it was the first position allocated for a period of 15 minutes" (personal communication from principal investigator) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. Participants sedated and paralysed, therefore unlikely to be aware of interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described. Radiologist unaware of results when interpreting CXRs to determine diagnostic group (right or left unilateral lung disease) at baseline Comment: objective outcome measures taken from ABG analyser; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data points, outcome data for each individual presented |
| Selective reporting (reporting bias) | Unclear risk | Only ‘ABG sampling’ described in the methods section for gas exchange measures, with PaO2, D(AaO2), PaCO2 and P/F ratio reported within the results. No description within the methods of intention to undertake specific analysis of 'worse' vs 'best' lateral position, or left lung disease vs right lung disease. Unclear whether selective reporting or an omission occurred |
| Other bias | Unclear risk | No baseline position described. Non‐uniform unbalanced cross‐over design. Number of participants allocated to each sequence unknown Comments: unknown whether participants were in the same body position before commencing the study with their first treatment period (If no turning was required for the first period, possible bias due to differences in treatment duration and data collection methods). Unclear whether sequence effects, period effects or treatment‐by‐period interactions were investigated or may have been sources of bias. If carryover was present, unlikely to be equally applied across treatments because of lack of balance and non‐uniformity. Carryover effects may be due to short, possibly insufficient, active and passive washout Unclear risk of carryover bias |
Kim 2002.
| Methods |
Randomized cross‐over study with 4 ×4 Latin square design (4‐treatment, 24‐sequence, 4‐period design), with participants stratified by disease/diagnostic group for Latin square experimental set, determined before participation Pre‐specified analysis: ANOVA with Scheffe test of statistically different means (P value < 0.05, 2‐sided) |
|
| Participants | 32 mechanically ventilated adults with ALI and/or ARDS who met ABG inclusion criteria before intubation (PaO2 < 60 mmHg, PaCO2 > 50 mmHg and pH < 7.3) Sex (M/F) 23/9, mean age 65 years ± 11 FiO2 0.5 for unilateral lung disease and 0.8 for bilateral lung disease, PEEP range 4 to 12 cmH2O, mean Vt 7.7 mL/kg ± 0.9 with A/C or SIMV modes Disease/diagnosis stratification: dominant right lung disease (pneumonia n = 11, pulmonary oedema n = 1), dominant left lung disease (pneumonia n = 8), bilateral lung disease (pneumonia n = 4, pulmonary oedema n = 3, ARDS n = 5) Setting: Medical ICU, Kangnam St Mary's Hospital of Catholic University, Seoul, South Korea |
|
| Interventions | Left lateral, right lateral, supine and prone positions for 30 minutes Sequences: see Notes below |
|
| Outcomes | PaO2 at 30 minutes (P/F ratio calculated as the result of standardization of FiO2 for unilateral and bilateral lung disease groups) Other co‐primary outcomes reported but not relevant for this review were PaCO2, respiratory static compliance and resistance |
|
| Standard management | Ventilation settings unchanged during trial. Tracheal suctioning performed during study, duration < 15 seconds, suction power < 20 kPa (150 mmHg) and suction catheter inner/outer diameter ratio < 2 | |
| Position description | Lateral recumbent position, no further description For prone position, quotation: "abdomen, thorax and pelvis supported to allow rib cage to move freely during respiration and face supported with special pad". No further description 15 degrees HOB elevation for supine position, verification method not described |
|
| Washout period | Not described Quotation: Participants were "placed in the supine position between lateral and prone positions" for a "few minutes" for minor therapeutic manoeuvres before turning to next position in sequence (no outcome measures taken) |
|
| Notes | No sample size calculation described No description of ABG analyser reliability Notes from investigators on Latin square design: up to 24 possible sequences in 4 × 4 Latin square design. Four participants in same disease group who underwent different random position changes form an experimental set. "Four body positions and order of position changes balanced in such a way that each patient of an experimental set experiences each body position once and each body position occurs once in each order". Sequence for each individual was tabulated Comment: example of an experimental set; SLRP, LSPR, RPSL, PRLS, i.e. 4 sequences out of the possible 24 sequences. Comments: measurement conversion from kPa to mmHg (formula = kPa/0.1333) for extracted data. However, no extractable summary statistics for meta‐analysis because of unit of analysis error (reported mean and SD for each body position within each stratified diagnostic group, with no results or analyses provided for the total sample) Email requests for further information regarding study design and results, with no response from contacting author or affiliated university of the principal investigator |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described Quotation: "A randomized clinical study was performed using a 4 × 4 Latin square design. A complete cycle of four positions ...according to a preplanned random order ..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described. Radiologist unaware of results when interpreting CXRs to determine diagnostic group at baseline Comment: objective outcome measures taken from ABG analyser; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data points. Numerator stated for results of each disease/diagnostic group, consistent with sample size |
| Selective reporting (reporting bias) | Low risk | Outcome reporting consistent with intended method of analysis |
| Other bias | Unclear risk | No baseline position described. Uniform and balanced cross‐over design. Unclear whether extra turn for suctioning and other manoeuvres occurred before turning prone or before turning to a lateral position Comments: Carryover effects may be due to short active and possibly insufficient washout. Additional turn and manoeuvres may lead to period effects (performance bias may give rise to period‐by‐treatment interactions) and carryover effects (inadequate passive washout). Unclear whether results were biased by unequal standard care and/or carryover bias |
Lewis 1997.
| Methods |
RCT with split‐plot design, randomized to body position (first tier), then timing of a 1‐minute backrub (second tier) Pre‐specified analysis: repeated measures 2 × 2 × 3 × 5 ANOVA (timing of backrub, positions, repeated measures (baseline, backrub and rest) and time intervals (1 to 5 minutes)), Scheffe tests (P value < 0.05) for significant differences in time |
|
| Participants | 57 critically ill men who had fibreoptic PA catheter, indwelling arterial line and baseline SvO2 ≥ 50% (baseline SvO2 < 60% (n = 8)) Mean age 60.9 years ± 8.6 Mechanical ventilation (n = 4), PEEP 5 cm H2O (n = 3), otherwise extubated at time of data collection (n = 53), with oxygen therapy applied via facemask (n = 22) or nasal cannula (n = 27), and no supplemental oxygen (n = 4) Diagnosis: aortocoronary bypass (n = 49), aortic aneurysm resection (n = 6), atrial septal repair (n = 1) and oesophagogastrectomy (n = 1) Exclusion criteria: younger than 18 years old, sepsis, pneumonectomy or lobectomy, mechanical assist device, organ transplantation, use of neuromuscular blocking agents Setting: Surgical ICU, Veterans Affairs Medical Center, Houston, Texas, USA |
|
| Interventions | Left lateral position vs right lateral position for 10 minutes Co‐intervention (1‐minute backrub) timed immediately (applied from 0 to 1 minute after turn) or delayed (applied from 5th to 6th minute after turn) Groups: left lateral with immediate backrub (n = 15), left lateral with delayed backrub (n = 15), right lateral with immediate backrub (n = 13), right lateral with delayed backrub (n = 14) All groups had supine position with 20 to 40 degrees HOB elevation for 5 minutes as baseline |
|
| Outcomes | SvO2 every minute for 10 minutes (analysed at 5‐minute periods labelled as rest or backrub according to allocated group). Baseline SvO2 data reported every minute for 5 minutes | |
| Standard management | Dopamine (n = 39) and NTG (n = 33) equally distributed across groups; dopamine (renal perfusion dose) not titrated | |
| Position description | Angle of lateral rotation, HOB elevation or verification methods not described Single data collector turned participants, placed 2 folded standard pillows (1 behind back, 1 between knees) for lateral position |
|
| Washout period | Not applicable for design | |
| Notes | Power analysis conducted (α = 0.05, β = 0.8, medium effect, indicated sample size 23), recruited 23 in each group for groupings of position and immediacy of backrub SvO2 via fibreoptic PA catheter First research question not relevant for the review (SvO2 change after 1‐minute backrub given immediately vs delayed) Second research question relevant for the review, "What is the effect of right and left lateral position on SvO2 in critically ill patients" Extracted SvO2 data from 3 time points without total sample data or all time points reported. Group size ambiguity for 1 extracted time point (5 minutes) (see risk of bias assessment) Contact established with primary investigator to request additional information regarding study design and clarification of results. No further correspondence received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described Quotation: "randomly assigned to right and left lateral position and then to..." timing of backrub |
| Allocation concealment (selection bias) | Unclear risk | “Sealed envelope technique” used to assign position, then timing of backrub. No further description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measure taken from continuous fibreoptic venous oximeter recordings; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotation: " no participants asked to be withdrawn". All participants appear to have completed the study. However, numerator for each time point under applied conditions (position, timing of backrub) not stated within results or analysis; therefore, unclear whether data points were missing |
| Selective reporting (reporting bias) | Unclear risk | Analysis was consistent with the trial protocol. No numerator for comparisons between lateral positions within text. Summary statistics incomplete, with only statistically significant results reported within text. However, results presented graphically. Figure 2 graph labelled sequential mean scores for each time point from baseline (5 time points) followed by the turn (5 time points) followed by a backrub (5 time points). Caption for Figure 2 graph listed 57 participants for the title 'effect of position' on SvO2 at baseline, after a turn and after the backrub Comment: Figure 2 graph and results are ambiguous. Sequential mean SvO2 scores at each time point for left lateral group vs right lateral group may represent data from delayed backrub groups (n = 29), despite the graph caption including the total sample. No results reported between lateral positions for participants with immediate backrub and lateral turn (i.e. dual intervention). Given that study had a split‐plot design, numerator omissions for specific results make interpretation of the line graph difficult. Information was insufficient for review authors to judge low risk of bias due to ambiguity within the report |
| Other bias | Unclear risk | DO2 determinants at baseline were similar between groups, but a statistical difference in SvO2 at baseline between position groups was reported (SvO2 slightly higher in participants turned left than those turned right) (P value < 0.05) Inotropes and vasoactive medication reported to be equally distributed, but titration of medication not controlled during data collection. Unclear whether a small number of mechanically ventilated participants (n = 4) were distributed equally across groups, and whether suctioning was performed. Overall, unclear risk of bias due to baseline SvO2 differences and unclear standard management practices |
Pena 1989.
| Methods | Cross‐over trial (2‐treatment, 2‐sequence, 2‐period design) | |
| Participants | 12 mechanically ventilated postoperative participants within first 6 hours after open heart surgery Setting: not described (study author from USA) |
|
| Interventions | First turn (lateral position), second turn (unclear whether lateral or supine position) Period schema: baseline (unknown duration), lateral positionT120, comparator body positionT120 (T in minutes) |
|
| Outcomes | SvO2 measured continuously at baseline, immediately after turning until next turn (continuous recording up to 2 hours) | |
| Standard management | Insufficient information | |
| Position description | Turned 2‐hourly for a total of 2 turns. First turn was lateral decubitus position Quotation from communication: "both lateral positions were used", but investigator cannot confirm whether the sequence was lateral then contralateral position, as the original data were no longer available (personal communication from principal investigator). Wording in the abstract introduction suggests that turning may refer to 'turning patients side to side after surgery' |
|
| Washout period | Not described | |
| Notes | Primary reference was an abstract from conference proceedings SvO2 system not described, except continuous real‐time measurements (possibly fibreoptic PA catheter with oximetry capability) No data (personal communication from principal investigator) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described Quotation from communication: "study participants were allocated to each body position according to a random list of numbers ranging from 1 to total number of positions in the study" Comment: insufficient information. Sequence generation and other trial features could not be recalled. Data no longer available |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measure taken from continuous SvO2 monitoring system; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not described Comment: attrition or missing data points unlikely to bias within‐subject comparisons |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information. Unclear risk of bias due to carryover, period or sequencing effects |
Reed 2002.
| Methods |
Two‐group RCT Pre‐specified secondary analysis of phase 1 data: repeated measure ANOVA, independent sample t tests, stratified analysis of subgroups |
|
| Participants | 31 mechanically ventilated anaemic postoperative cardiovascular surgery adults (≥ 18 years) Other inclusion criteria: ≥ 6 hours after surgery with extracorporeal circulation, stable haemodynamics (HR 60 to 125, SBP ≥ 90 mmHg), sustained low oxygen delivery (DO2I < 500 mL/min/m2), PEEP < 10 cm and FiO2 < 0.8, functional arterial catheter and continuous SvO2/CO PA catheter, no morphine sulphate allergy, English speaking Sex (M/F) 16/15, mean age 68.2 years ± 9.9 Mean FiO2 0.4839 ± 0.0073, mean PEEP 5.65 cmH2O ± 1.29, mean Vt 779.35 mL ± 156.89 with SIMV (n = 27) or A/C mode (n = 4) Diagnosis: CABG (n = 14), heart valve replacement (n = 10), CABG and valve replacement (n = 6), aneurysm repair (n = 1) Stratified subgroups according to Hb levels: < 9.99 g/dL (n = 14), > 10.00 g/dL (n = 17) Termination criteria: (1) cardiopulmonary instability (defined as SBP < 90 mmHg for > 1 minute or lethal dysrhythmia), (2) dosage change in vasoactive or inotropic infusion, (3) blood transfusion administration, (4) required IV resuscitation defined as > 200 mL delivered in < 30 minutes, (5) diuretic or cardiac drug administration, (6) change in ventilator setting (i.e. FIO2, rate, volume, PEEP), (7) procedure or treatment that required participant movement or acknowledgement other than study procedures (e.g. endotracheal suctioning), (8) disruption in study protocol by participant care activities or procedures, (9) analgesic, anxiolytic, anaesthetic or paralytic agent administration or dosage change of continuous infusion of an anaesthetic agent (e.g. propofol) and (10) participant requests withdrawal Setting: 16‐bed adult cardiovascular surgery ICU at a not‐for‐profit private teaching hospital, Seattle, Washington, USA |
|
| Interventions |
Left lateral position (n = 16) vs right lateral position (n = 15) for 10 minutes All participants were in the baseline supine position for 30 minutes before turning |
|
| Outcomes | SvO2 (primary outcome), all other outcomes (second study aim) for stratified subgroup analysis SvO2 and VO2I measured minutely from 1 to 10 minutes; DO2I, CI and O2ER measured at 3, 5 and 10 minutes. All outcome data collected at baseline |
|
| Standard management | No participants withdrawn on the basis of termination criteria (i.e. no deviation from standard management during data collection) Comment: Standard management appears to be equally applied to groups |
|
| Position description | 45 degrees lateral rotation, commercial foam wedge, participants instructed not to assist with turning, angle verification method not described 20 degrees HOB elevation for all body positions, with single pillow under head; HOB verification method: 20 degrees protractor placed at the bed frame with bed adjusted until angle between horizontal stationary frame and mobile vertical frame was 20 degrees |
|
| Washout period | Not applicable for design | |
| Notes | No sample size calculation described Primary investigation (Jesurum‐Urbaitis 2002) using a pre‐test/post‐test design compared lateral positioning effects without morphine (phase 1) and with morphine (phase 2) (no comparison between lateral positions reported). Secondary analysis of phase 1 data (Reed 2002) included 3 study aims conducted prospectively with the primary investigation Stop clock commenced immediately after foam wedge positioned correctly for all measures SvO2 and CI measured by flow‐directed thermodilution fibreoptic continuous cardiac output PA catheter. Measurement reliability and validity adequately described Reed 2002 reported mean SvO2 ± SD at each time point, with individual participant data presented within Appendices (line graphs of SvO2 with group allocations). No meaningful data extracted for CI, DO2I, VO2I and O2ER (data regrouped according to Hb level for stratified analysis (second aim) without summary statistics for randomized groups) 16.1% (n = 5) died of postoperative complications Comments: comparison between supine baseline position and lateral positions (first aim), and observation of SvO2 recovery time to baseline (third aim), not relevant for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lateral position determined by lottery, drawing from opaque envelopes with slips of paper titled "left lateral" and "right lateral". Participants had equal opportunity to be rotated to left or right lateral positions. Random sequence generation was probably done |
| Allocation concealment (selection bias) | Low risk | Quotation: "blindly selected a paper from the envelope marked "lateral position random assignment" ‐ located in the study cart. Allocation was concealed up until the time of baseline data collection Comment: allocation concealment probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from real‐time physiological monitoring system and fibreoptic pulmonary artery catheter; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. Numerator stated for all results. Raw SvO2 data for each individual presented graphically with all raw scores accounted for |
| Selective reporting (reporting bias) | Low risk | Outcome reporting consistent with intended method of analysis. However, for stratified subgroup analysis based on Hb level, no point estimates for CI and other derived calculations. However, these omissions were unlikely to influence primary outcome reporting |
| Other bias | Low risk | No apparent risks identified. Study identified similar characteristics and baseline variables between groups |
Remolina 1981.
| Methods |
Cross‐over trial (3‐treatment, 3‐period design), unclear sequence order Method of analysis: 1‐way AVOVA Quotation: "Difference[s] between groups were tested with the Newman‐Keuls tests" |
|
| Participants | 9 consecutive hospitalized patients with indwelling arterial catheter in situ who had unilateral or predominantly unilateral lung disease (atelectasis, consolidation, infiltrates, pleural effusion) on CXR Sex not described, age range 36 to 72 years Mean FiO2 (calculated) 0.407 ± 0.231, Puritan Bennett MA‐1 volume‐limited ventilator (n = 1), otherwise spontaneously breathing (n = 8) Diagnosis: pneumonia ± aspiration (n = 6), bronchogenic or metastatic cancer (n = 2), bronchogenic cancer with pneumonia (n = 1) Subgroup classification: right lung pathology (n = 2), left lung pathology (n = 7) Setting: not described (study authors from USA) |
|
| Interventions | Right lateral, left lateral and supine positions (duration and sequence unknown) Lateral positions analysed according to bad lung down and good lung down only |
|
| Outcomes | Arterial blood gas pressures (PaO2) at 10 minutes (calculated P/F ratio for this review, as raw data for FiO2 and PaO2 were tabulated) Other co‐primary outcomes reported but not relevant for this review were PaCO2 and pH |
|
| Standard management | FiO2 unchanged from level set as part of management of the disease (FiO2 monitored with fuel‐cell oxygen analyser) | |
| Position description | Not described | |
| Washout period | Not described | |
| Notes | No sample size calculation described For each body position, 11 results from 9 participants (data collected on 2 consecutive days for 2 participants; all other participants (n = 7) had data collected once for each body position) Summary statistics (mean and SE for each body position) included unit of analysis error for meta‐analysis. Comparison between lateral positions (within‐subject difference) was calculated from individual participant data Comments: extracted data adjusted with second set of data from 2 participants with repeated measures removed from analysis to avoid a unit of analysis error (i.e. effect estimate from first day of data collection). Unclear supine position order; therefore, comparison between each lateral position and supine position not valid for extraction No description of informed consent or review by ethics committee No reply to fax correspondence sent to primary investigator requesting further information about study design and results |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random order of positions Quotation: "...supine, right lateral or left lateral position; which were assumed in random order". No further description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from ABG analyser; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data, as all raw data presented in a table |
| Selective reporting (reporting bias) | Unclear risk | Difference in data collection methods between participants, with no explanation given. All pre‐specified outcomes reported. However, test statistic provided for each body position did not identify paired comparison Comments: unclear whether planned paired analysis of ‘groups’ referred to sequence or specific body positions. Unclear whether analysis of data from consecutive days was planned for all participants. Unclear if selective outcome reporting occurred, as method of analysis provided insufficient information |
| Other bias | Unclear risk | No baseline position described. Insufficient detail on sequence. Sequence (group) size unknown. Body position duration not stated; therefore, unknown whether washout was applied after data collection Comment: unknown whether participants were in the same body position before commencing the study and during the first treatment period (If no turning was required for the first period, possible bias due to differences in treatment duration and data collection methods). Comments: Unclear whether cross‐over design was uniform and balanced. Highly probably that the study did not control for carryover nor sequence effects because details are lacking. Therefore, unclear if carryover, sequence or period effects or treatment‐by‐period interactions were sources of bias |
Schellongowski 2007.
| Methods |
Phase 2 study: cross‐over trial (3‐treatment, 2‐sequence, 5‐period design), with continuous rotation (passive washout) between all treatments Method of analysis: Friedman test for differences between body positions and Dunn's multiple comparison post‐test for pairs of time points |
|
| Participants | 12 mechanically ventilated adults with ARF due to ALI or ARDS diagnosed within 96 hours of inclusion (ARDS defined, based on guidelines from the American European Consensus Conference) Other inclusion criteria: decision to treat participant with CLRT within 48 hours of inclusion, haemodynamic stability during rotation over maximal angle at least 12 hours before inclusion Sex (M/F) 10/2, median age 54 years (range 22 to 81 years) Median PEEP 10 mbar (range 6 to 15 mbar), median Vt 556 mL (range 326 to 756 mL) with time‐cycled pressure‐controlled mode Diagnosis: pneumonia (n = 11), near drowning causing ARDS (n = 1), 7 of 12 had sepsis Severity of illness: median APACHE II 17 (range 7 to 37), median SAPS II 46 (range 31 to 85), median Murray lung injury score 2.63 (range 2 to 3.5) Termination criteria: haemodynamic or respiratory instability (defined as sustained decline in BP necessitating vasopressor therapy or dose rate increase of vasopressor and/or decline in SaO2 measured by pulse oximetry < 88%) Setting: ICU of University Hospital, Vienna, Austria (location of study authors) |
|
| Interventions | Steep left lateral and steep right lateral positions for 30 minutes, supine position (S1, S2, S3) for 10 minutes. Note: full cycle of continuous rotation for 8 minutes after all treatments, except S3 Sequences/groups S1(baseline),L, S2, R, S3 S1(baseline),R, S2, L, S3 |
|
| Outcomes | Pulmonary gas exchange (SaO2, P/F ratio, SvO2) and haemodynamics (MABP, CI) measures at 10, 20 and 30 minutes for steep lateral positions, and at 10 minutes for all supine positions Other co‐primary outcomes reported but not relevant for this review were pulmonary shunt fraction, PaCO2 and respiratory mechanics measures (Vt, PIP, PEEP, static compliance) |
|
| Standard management | All ventilation settings kept unchanged during data collection. FiO2 set to achieve SaO2 of 92% to 96%, PEEP (increments of 2 mbar between 5 and 20 mbar) set to maintain FiO2 ≤ 0.6 and SaO2 > 91%, frequency set to keep PaCO2 < 60 torr and to avoid dynamic hyperinflation, PIP kept to lowest level to apply Vt of approx 8 mL/kg body weight, haemodynamics stabilized by adequate volume substitution to keep PCWP of 12 to 15 torr and vasopressors if necessary. All participants received continuous infusion of analgo‐sedation (midazolam and sufentanil and/or ketamine) and sedation titrated to achieve Ramsay sedation score of 5 and to suppress spontaneous breathing. No muscle relaxants given | |
| Position description | 62 degrees static lateral rotation, Rotorest KCl Medisus bed paused in supine position for 10 minutes (baseline), then paused in each body position of interest during data collection. No further descriptions except continuous rotation for 1 hour before phase 2 study protocol | |
| Washout period | Not described Comment: continuous lateral rotation of the whole body along its longitudinal axis from 1 lateral position to the other, with maximum angle of 124 degrees (full cycle for 8 minutes) between body positions (i.e. passive washout period without data collection) |
|
| Notes | No sample size calculation described CI determination by intermittent thermodilution technique Pressure transducers fixed to moveable portion of kinetic system close to the participant to guarantee that the position of the tip was always at the level of the left atrium, with pressure transducers zeroed to mid‐axillary level Results table (Table 3) stated 'measured values' with ± symbol only Comments: Summary statistics ambiguous (unclear whether mean and SD were intended, all baseline measures were reported in median and non‐parametric statistical tests were conducted). Data transformation possibly required for meta‐analysis. However, no extractable lateral position data because of unit of analysis error. Supine position order not randomized; therefore, comparison between each lateral position and supine position not valid for extraction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described Quotation: "patients were randomized to stop either in left or right steep position first" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from real‐time physiological monitoring systems, ABG and mixed venous gas analyser; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 participants completed the study, with 2 withdrawals during phase 2 study as termination criteria met (SaO2 < 80% in left lateral position, concomitant decrease in BP, Vt and compliance). Numerator for each reported outcome was not stated; unclear whether data points were missing and how missing data were handled within the analysis (Friedman test) Comment: missing data points, if present, unlikely to bias within‐subject differences |
| Selective reporting (reporting bias) | Unclear risk | No explanation was given as to why non‐parametric statistical analysis (Friedman test) was conducted for continuous data. Primary endpoints for pulmonary gas exchange were unclear; P/F ratio was reported within results and analysis but was not mentioned within the methods section, whereas, SaO2 and mixed venous gases were indicated as measures at each time point but were not reported within the results or analysis Comment: unclear whether pulmonary gas exchange measures were selectively reported Furthermore, P/F ratio results within a line graph appear to be misleading. P/F ratios were presented sequentially from S1 to right lateral to S2 to left lateral to S3 (at each time point for each period (treatment) for each individual). However, half the group was rotated to the left lateral position (L) in the second period (first lateral position); therefore, sequence and changes between body positions were not accurately represented graphically |
| Other bias | Unclear risk | Non‐uniform unbalanced cross‐over design. Sequence effects and treatment‐by‐period interactions not reported Comments: unclear whether sequence effects, period effects or treatment‐by‐period interactions were a source of bias. If carryover effects were present, they were unlikely to be equally applied across treatments because of lack of balance and uniformity. Carryover effects may be due to short active washout and continuous rotation during passive washout period. Possibly insufficient washout before and after data collection. Overall, unclear risk of carryover bias Possible intervention bias for comparisons between supine and lateral positions due to unequal period duration and numbers of measures taken within each period (comparisons not equivalent) |
Shively 1988.
| Methods |
RCT with 2‐group design (frequency of turning). In addition, comparative analysis of body positions without counterbalance/cross‐over Pre‐specified analysis: 3‐factor mixed‐model ANOVA for group (turning frequency), position and time of measurement (main effect and interactions), univariate ANOVA for position and time of measurement. Contrast comparisons of all significant ANOVA results. Pre‐specified within‐subject analysis of good lung down vs bad lung down for unilateral lung disease subgroup (paired t test) |
|
| Participants | 30 coronary artery bypass surgery adults within 24 hours of surgery (mean 9 hours ± 2.34) who were haemodynamically stable, had oximeter system in situ for continuous monitoring of SvO2 and were extubated (mean 3 hours ± 2.18 before the study) Sex (M/F) 23/7 (all females in group 2), mean age 59 years ± 9.7 Mean FiO2 (calculated) 0.426 ± 0.069 Exclusion criteria: chronic or terminal pulmonary disease such as COPD, tuberculosis or lung cancer or any portion of the lung removed, or cardiac arrest in postoperative period Subgroup classification (CXR within 24 hours of surgery): left unilateral lung pathology (group 1 = 6, group 2 = 6), right unilateral lung pathology (group 1 = 0, group 2 = 1), bilateral lung pathology (group 1 = 4, group 2 = 5), normal CXR (group 1 = 5, group 2 = 3) Termination criteria: (1) at participant's request, (2) unable to maintain a position for the specified time or (3) haemodynamically unstable during study (arterial pressures, venous pressures and CO could not be maintained within the participant's normal range) Setting: 3 critical care units in 3 major hospitals in Austin, Texas, USA, from June to December 1985 |
|
| Interventions | Group 1 (n = 15): 1‐hourly turns (lateral positioning schedule) for 4 hours Group 2 (n = 15): 2‐hourly turns (lateral positioning schedule) for 8 hours Periods: supine position (baseline) followed by lateral positioning schedule of right lateral, 45 degrees sitting, left lateral, and supine positions. Note: lateral positioning schedule sequential and identical for both groups |
|
| Outcomes | SvO2 at 0 minutes, 15 minutes and 1 hour (both groups), with SvO2 at 2 hours for group 2 | |
| Standard management | Same oxygen level throughout study (n = 29). All medications listed with numerator for each medication. Nipride infusion (n = 18), ≥ 1 dose of morphine given during study (n = 18), decreasing use of intravenous nitrates throughout study Comment: vasoactive medication not controlled during study |
|
| Position description | Degree of lateral rotation and angle verification method not described 20 degrees HOB elevation for all body positions, except sitting position had 45 degrees HOB elevation. HOB elevation verification method: checked with goniometer |
|
| Washout period | Not described | |
| Notes | No sample size calculation described. Individual participant data tabulated. Unclear whether 2‐hour data were entered in real time or were extrapolated for 4 participants (group 2) turned 10 to 20 minutes earlier than 2‐hour limit (turned because of discomfort) For subgroup analysis (n = 13), unclear whether repeated measures (3 or 4 depending on allocated group) were averaged Comment: Sequence was not randomized; therefore comparison between body positions (within‐subject difference) was not valid for extraction. No data were extracted for meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "randomly assigned the patients to one of two groups". No further description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comments: unclear whether subgroup classification (CXR assessment of lung pathology) was blinded, but omission unlikely to bias primary outcome reporting. Furthermore, objective outcome measure taken from digital display of continuous physiological monitoring system; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 68 consented, 26 excluded (could not start protocol) and 12 withdrawn (could not finish protocol); unclear whether exclusion before randomization. Even group numbers, no missing data points for 30 “as treated” participants. However, withdrawals > 20% (group allocation not identified). Rationale for exclusion/withdrawal provided (extended intubation or haemodynamic instability did not meet inclusion criteria (n = 21), inability to maintain position or haemodynamic instability met termination criteria (n = 9), restlessness or nausea (n = 2), technical problems with SvO2 monitor (n = 6)) Comment: no intention‐to‐treat analysis; therefore, high risk of attrition bias for parallel‐group (turning frequency) analysis |
| Selective reporting (reporting bias) | Low risk | Method of analysis conducted as pre‐specified and consistent with stated aims and hypotheses |
| Other bias | High risk | Non‐uniform unbalanced study design for analysis of 'body position' effects. Standard management may not have been equally applied across groups and periods, as titration of vasoactive medications/fluids, use of PRBC and volume expanders were not controlled for Sequence and carryover effects acknowledged as major threats to internal validity. Vital signs and additional haemodynamic data gathered before and after each position change to assess for threats Comments: 'Sequence effect' testing (in context of body position effects) was meaningless (identical sequence order). Methods to minimize sequence and carryover effects bias were inadequate, with contradictory statements reported. Reported carryover effects were not apparent, but investigators also reported that group 2 ‘possibly experienced carryover effects’ (interaction found in univariate but not multi‐variate analyses). Group 2 had lower mean SvO2 values for sitting 45‐degree and supine 20‐degree positions compared with group 1, with a rationale for the effect accredited to extent and occurrence of pulmonary pathology (group 2 had fewer normal CXRs, 3 vs 5). Furthermore, risk of intervention bias for examining body position effects was high, as groups 1 and 2 had different duration of treatment and measurement intervals, with no control for group or period effects. Unclear whether group effects were confounded by selection and/or performance bias. Unclear if period effects or treatment‐by‐period interactions were sources of bias. If carryover was present, unlikely to be equally applied across treatments because of lack of balance and uniformity |
Thomas 2007a.
| Methods |
Cross‐over trial (3‐treatment, 2‐sequence, 4‐period design) Pre‐specified analysis: linear mixed‐model analysis of group, side, time (measurement intervals) and their interactions, a priori paired contrasts of time (data combined from right lateral and left lateral positions, i.e. grouped as 'lateral positioning' for comparison with data combined from supine position periods). Pre‐specified subgroup analyses included position (within‐subject factor) for unilateral lung pathology (UniLP) (comparison between bad lung down and good lung down) |
|
| Participants | 34 mechanically ventilated adults who were haemodynamically stable (baseline HR 60 to 130 beats/min, MABP 70 to 120 mmHg, no compromising arrhythmias, ICP < 20 mmHg, mean PAP < 30 mmHg, PCWP 8 to 17 mmHg) and met subgroup classification Sex (M/F) 22/12, mean age 46.1 years ± 17.3 Subgroup classification (lung pathology locality on CXR): no lung pathology (NoLP) (n = 4), UniLP (n = 13), bilateral lung pathology (BilatLP) consistent with ALI/ARDS criteria based on American‐European consensus conference on ARDS (n = 17) Mean PEEP (NoLP = 5.3 ± 0.5 cmH2O, UniLat = 7.5 ± 3.0 cmH2O, BilatLP = 8.8 ± 2.8 cmH2O), mean Vt (NoLP = 10.2 ± 1.6 mL/kg, UniLat = 9.1 ± 1.9 mL/kg, BilatLP = 7.4 ± 1.5 mL/kg) with Bilevel mode (n = 8), SIMV mode (n = 25) and CPAP mode (n = 1) Diagnosis: respiratory sepsis (n = 15), neurological (n = 9), postop neurosurgical (n = 5), postop abdominal (n = 2), abdominal sepsis (n = 1), non‐operative cardiogenic (n = 1), bacteraemia (n = 1) Severity of illness at baseline: mean APACHE II score (NoLP 16 ± 4.2, UniLP 18.2 ± 5.7, BilatLP 26.5 ± 9.7), mean SOFA score and MFI scores for each subgroup also given Exclusion criteria: < 18 years, pre‐existing severe chronic respiratory disease (FEV < 40%), burn injuries, chest wall abnormalities, pulmonary barotrauma (e.g. pneumothorax), paralysis medications, nitric oxide, contraindications to lateral positioning (e.g. unstable spinal fractures) and unilateral changes on CXR due to effusions or pulmonary masses Setting: ICU, Royal Brisbane and Women's Hospital (tertiary referral university affiliated metropolitan centre) Brisbane, Australia |
|
| Interventions | Left lateral and right lateral positions for 120 minutes, first supine position for 60 minutes, second supine position for ≥ 30 minutes Sequences/groups S(baseline T 30),R, S, L, S S(baseline T 30),L, S, R, S (T in minutes) |
|
| Outcomes | (1) P/F ratio, (2) MABP and HR, (3) CO and CI (BilatLP subgroup only), (4) adverse events Measurement intervals for each sequence reported as T0, T30, T120, T150, T0, T30, T120, T150 (T in minutes). Lateral positions measured at T30 and T120. Supine position data measured at T0 (before each lateral turn) and T150 (equivalent to 30 minutes in the supine position) |
|
| Standard management | Ventilator settings constant (pre‐planned adjustment of FiO2 ≤ 0.1 to alleviate severe hypoxaemia (SpO2 < 90% or PaO2 < 60 mmHg), change not required). Physiotherapy (manual hyperinflation) withheld. Pre‐oxygenation (hyperoxygenation) with closed circuit suctioning, 20 minutes allowed for equilibrium (minimum) before ABG sampling. Vasopressor medication and sedation altered as deemed clinically necessary by medical staff; changes documented (vasopressor commenced before protocol (n = 4) and during study (n = 1) for hypotension, vasopressor commenced before protocol (n = 1) and during study (n = 1) with 500 mL of fluid bolus for CPP control) | |
| Position description | 90 degrees lateral rotation, HOB elevation not described for lateral positions and < 20 degrees for supine position, angle verification method not described for rotation or HOB elevation Other descriptions: pillow placed in front of participant's thorax for cuddling, with care taken to ensure that pelvis and shoulder girdles were at 90 degrees to the support surface; investigator monitored position during study to ensure position maintained and minor adjustment made as necessary |
|
| Washout period | Not described | |
| Notes | Sample size calculation for P/F ratio (20 in each group, moderate effect with magnitude of P/F change 20, σ = 35, α = 0.05, 1‐β = 0.8). Recalculation during interim analysis, with a requirement for 128 participants stated. Recruitment ceased with reason provided (beyond the capacity of single‐centre study) Comment: Study was underpowered to detect differences among primary outcomes CO and CI determined by oesophageal Doppler calculations (single outcome testing (CO/CI) in ALI/ARDS subgroup only) Schema for analysis included baseline, lateral positioning, recovery. Data from the 2 groups (sequences) were combined at each time point (T0, T30, T120, T150) (i.e. lateral position data (right and left side) combined, and supine position periods combined before (i.e. 0 minutes) and after (i.e. 150 minutes) lateral positioning). Paired contrast analysis conducted from supine to lateral or from lateral to supine positions. Comparisons between lateral positioning, baseline and recovery (supine position periods) were not equivalent in terms of duration in each body position (120 minutes vs 30 minutes) and number of measurements taken within each body position (2 repeated measures vs 1 measure for each period) Comments: no extractable lateral position data for meta‐analysis because no period data were provided for each lateral position (right vs left; total sample) and for a unit of analysis error (mean and SD for time points). Supine position order was not randomized within the design; therefore, within‐subject comparisons involving the supine position were not valid for meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotations: "prospective, within subject randomized cross‐over study" .."order of right or left 90° lateral turn was randomized"..."originally generated from a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Quotation: "concealed allocation" stated. No further description, except principal investigator was unaware of participant's previous responses to position change to reduce selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from digital display of continuous and intermittent physiological monitoring systems and calibrated blood gas analyser; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No numerator reported for outcome results. Intention‐to‐treat analysis stated, with data from participants who did not complete protocol included in the analysis. Lateral position data had 11 turns missing, with rationale provided (9 participants did not complete entire protocol) Comments: missing data points unlikely to bias within‐subject differences in primary outcomes. However, given the specific design features and methods of analysis of this study (within‐participant lateral position data collated and analysed as an independent group for comparison with supine position data), it remains unclear how missing data were handled. It was not explicitly clear that supine position data were complete. Unclear whether each group (sequence) and/or each period (body position) had similar rates of attrition |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes reported; analysis consistent with pre‐specified method stated |
| Other bias | Unclear risk | Non‐uniform unbalanced cross‐over design with unit of analysis errors and design ambiguity. Methods section did not make it explicitly clear that participants returned to supine position for data collection at 150 minutes after the second lateral position, but results were presented Unclear whether subgroup analysis (comparison between bad lung down vs good lung down in UnilatLP group) was based on average of repeated measures or a single measurement at 30 minutes or 120 minutes after turning. However, discrepancy unlikely to influence main effect testing of group, side and time Comments: Unclear whether carryover, sequence or period effects or other treatment‐by‐period interactions may have been sources of bias. Matched pairs and linear mixed‐model analyses did not account for possible period or carryover effects within the design or analysis. Carryover effects may be due to short active and possibly insufficient washout within some periods. Unclear risk of carryover bias |
Tidwell 1990.
| Methods |
Phase 2 of study: cross‐over trial (4‐treatment, 2‐sequence, 6‐period design) Method of analysis: repeated measure ANOVA between T0 and TI (T0 = immediately before turning to next position within the sequence, T1 = 1 minute after body position change) |
|
| Participants | 34 mechanically ventilated postoperative CABG surgery adults with FiO2 ≤ 0.6 Sex (M/F) 30/6 (phase 1), mean age 64 years (range 49 to 77 years) Exclusion: concomitant cardiac valvular disease, PEEP required post surgery Setting: ICU, major Pacific Northwest medical centre, December 1988 to September 1989 (study authors from USA) |
|
| Interventions | Six body position changes: S (baseline) to 30° HOB, 30° HOB to S, S to L, S to R, L to S, R to S Each body position maintained for 30 minutes, except baseline (unknown duration) Sequences/groups S(baseline),30° HOB, S, R, S, L, S S(baseline),30° HOB, S, L, S, R, S |
|
| Outcomes | SvO2, VO2 and SaO2 at 0 minutes (T0, recorded before body position of interest), then minutely from 1 to 5 minutes (T1‐5), at 15 minutes (T15) and at 25 minutes (T25) in each body position | |
| Standard management | Ventilator settings not described. Unclear whether standard management (reported in phase 1 study) applied within phase 2. Phase 1 study standards included participants sedated with morphine and diazepam, rewarmed to 37 to 38°C within first 4 hours postop (phase 2 study conducted 4 to 8 hours after surgery) | |
| Position description | 45 degrees lateral rotation, commercial rigid foam wedge, passive position changes, HOB elevation: recumbent (flat) for lateral positions, HOB position set at 30 degrees elevation, angle verification methods not described for rotation or HOB elevation | |
| Washout period | Not described | |
| Notes | Phase 2 study method reported in Osguthorpe 1990 No sample size calculation described SvO2 via fibreoptic oximetry thermodilution catheter VO2 calculation: participants connected to an in‐line computerized metabolic cart and blender system with Boehringer 1‐way valve connected to T‐piece of the ETT to isolate inspired and expired gas Pre‐specified Pearson's correlation between dependent variables not relevant for the review Pre‐specified ANOVA analysis between 2 body positions (treatment effect), but non‐equivalent time points (T0 vs T1) Summary statistics (mean and SD) for each time point (i.e. 7 data points) for each body position, including time point for prior body position (i.e. T0) and other time points (T1 to T25) presented within results table Comments: no extractable lateral position data for meta‐analysis because of unit of analysis error. Paired comparisons involving supine or HOB positions were not valid for extraction, as position order was not randomized Contact established with primary investigator to request additional information regarding study design and clarification of results. However, no further correspondence received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described Quotation: "The sequence of position changes was randomized to eliminate ordering effect as source of bias" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from real‐time physiological monitoring systems; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Phase 1 study (n = 36), 1 withdrawal before data collection (due to inability to haemodynamically tolerate position change), minor discrepancy (n = 1) for phase 2 sample size (n = 34). No numerator stated for outcome reporting. Unclear completeness of outcome data Comment: missing data points, if present, unlikely to bias within‐subject differences for primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified paired data analysis between T0 and T1 reported. Mean scores for repeated measures tabulated for all body positions and mean scores presented within a line graph of each time point (T0 to T25), changing from supine to each lateral position. No statistical tests or analyses reported for time points other than T0 and T1 Comment: Discussion and conclusion sections reported other results (table and line graph) and a descriptive inference of a correlation between dependent variables based on 4 individuals with greatest variation in SvO2, when no statistically significant correlation was found within the sample. Insufficient information within the report to rule out selective reporting of outcomes and possible risk of interpretation bias |
| Other bias | Unclear risk | Non‐uniform unbalanced cross‐over design Quotation: "The sequence of position changes was randomized to eliminate ordering effect as source of bias." No testing of sequence or period effects reported. Unclear whether participants within each group were comparable on trial entry or had similar management and care (ventilation settings, fluid management, vasoactive medication administration and titration), as group differences were not examined and minimal sample demographics and participant characteristics were described Comment: Bias associated with selection and/or performance may confound sequencing effects within an unbalanced non‐uniform trial. Unclear whether sequence effects, period effects or treatment‐by‐period interactions may have been sources of bias. Carryover effects may be due to short active and possibly insufficient washout. Carryover, if present, was unlikely to be equally applied across all treatments because of lack of balance and uniformity. Overall, unclear risk of carryover bias |
Tripathi 2009.
| Methods |
Cross‐over trial (3‐treatment, 3‐period design), unknown sequence Method of analysis: 2‐tailed Pearson's correlation co‐efficient for regrouped data based on lung infiltration score (LIS) Quotation: “We regrouped patients on the basis of LIS differences between the two lungs, i.e., LIS ≤ 2 (n = 6); for 3 or 4 (n = 7); and ≥ 5 (n = 3) and compared oxygenation parameters to find out the significant lung infiltrates asymmetry related with the postural hypoxemia.” Secondary analysis (post hoc ANOVA and Student t test) for "regrouped data" (better lung down vs supine vs better lung up) |
|
| Participants | 16 mechanically ventilated adults with ARDS (ARDS criteria: resp. rate ≥ 30/min, chest x‐ray with bilateral infiltrates, PaO2/FiO2 < 150, PaO2/FiO2 < 200 with PEEP and hypocarbia (PaCO2 < 32 mm Hg)) Exclusion: haemodynamic instability and/or on inotropes Sex (M/F) 8/8, mean age 41.188 years ± 15.048 (calculated) (range 18 to 68 years) Mean PEEP 11.188 cmH2O ± 2.903 (calculated), mean Vmin 7.244 L/min ± 1.834 (calculated) Diagnosis: abdominal sepsis (n = 8), aspiration infiltrates (n = 2), pneumonitis (n = 3), scorpion sting (n = 3) Severity of illness: mean APACHE II score 21.625 ± 5.353 (calculated) Subgroup classification (lung infiltration score (LIS)): left LIS ≥ right LIS (n = 9), right LIS ≥ left LIS (n = 6), equal bilateral LIS (n = 1) Setting: ICU for 2‐month period (study authors from Nepal) |
|
| Interventions | Right lateral, left lateral and supine positions Lateral positions described according to lower LIS, data regrouped as better lung down position (group 1), supine position (group 2), better lung up position (group 3) Sequence: unknown |
|
| Outcomes | ABG (PaO2, SaO2), P/F ratio, haemodynamic parameters (MABP and HR) after 20 minutes in each body position Other co‐primary outcomes reported but not relevant for the review were alveolar‐arterial O2 pressure difference (AaDpO2), CVP, PaCO2, pH, bicarbonate |
|
| Standard management | All participants sedated, given muscle relaxant to maintain identical ventilation settings, FiO2 increased for SpO2 < 90% as a rescue measure only | |
| Position description | 45 degrees lateral tilt, otherwise no further description | |
| Washout period | Not described | |
| Notes | A priori sample size calculation (50% change in P/F ratio taken as clinically significant difference, assuming SD of 50, α = 0.05, 1‐β = 0.95, 15 participants desired on the basis of a priori t test for 2 independent means). However, a reporting anomaly for a priori sample size calculation was noted, with no statement in the original pdf (downloaded 2 August 2013). Statement was present in electronic Web printout (13 October 2013), with publication date stated as 9 September 2009 Reported additional observational study: quotation: "cyclic change (4 hourly) of patient position from supine to right or left lateral...in the subsequent days", with grading of pressure sores over the week following the initial study Comments: Observational study of pressure sores was not relevant for the review No extractable summary statistics for meta‐analysis because of unit of analysis error (reported mean and SD for each body position). Unclear supine position order; therefore, comparison between supine position and each lateral position not valid for extraction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: sequence of 3 body positions "in random fashion" using the "sealed‐envelope technique" Comment: unknown method of allocation sequence generation |
| Allocation concealment (selection bias) | Low risk | Sealed envelope technique with no further description Comment: 3 sequences unknown at time of enrolment, allocation concealment probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. Participants sedated and given muscle relaxant |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding procedures unclear Quotations: "The observer was kept blinded for the LIS of the chest x‐rays”...“The LIS grading was done by the nursing staff (6 nurses scored 2 chest x‐rays and 1 nurse scored 4 x‐rays) attending to the patients.” LIS used for classification of better lung down and better lung up for comparisons Comment: unclear whether nurses were aware of LIS and outcomes. However, objective outcome measures taken from ABG analysers and physiological monitoring systems in the ICU; therefore, lack of outcome assessor blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numerator stated for each body position, consistent with sample size |
| Selective reporting (reporting bias) | Low risk | All outcomes were listed, without differentiation between primary and secondary outcomes. Title, statistical methods described and conclusion suggest that primary outcomes were oxygenation parameters for correlation analysis. Method of analysis also reports pressure sores, but evaluation of pressure scores from additional observational study was not relevant for this review. Post hoc secondary analysis reported for all listed outcomes except pressure sores. Unclear whether haemodynamic outcomes were secondary outcomes |
| Other bias | Unclear risk | No baseline position described. Unclear whether cross‐over design was uniform and balanced as unknown sequence (group) size and sequence order. Unclear body position duration; therefore, unclear if washout may have been applied after data collection Comments: unknown whether participants were in the same body position before commencing the study and during the first treatment period (If no turning was required for the first period, possible bias due to differences in treatment duration and data collection methods). Comments: highly probably that the study did not control for carryover nor sequence effects based on lack of detail. Therefore, unclear whether carryover, sequence or period effects or treatment‐by‐period interactions were sources of bias Two participants desaturated (SaO2 < 90%) with FiO2 increased as per protocol. Unclear if all participants received the same management during the study Quotation: “All patients were sedated and muscle relaxant was given to maintain identical ventilation settings during the study period.” However, unclear whether sedation was titrated during the study. Unclear risk of performance bias for all outcomes related to unclear uniformity and balance in the design |
Whitman 1982.
| Methods |
Randomized cross‐over design (3‐treatment, 6‐sequence, 3‐period design) Method of analysis: 2‐way ANOVA, Newman‐Keuls multiple range test of paired differences between body positions (labelled as position groups) |
|
| Participants | 50 postoperative adult cardiac surgery patients Setting: not described (study author from USA) |
|
| Interventions | Left lateral, right lateral and supine positions for 15 to 20 minutes Sequences/groups: SLR, SRL, RLS, RSL, LRS, LSR |
|
| Outcomes | CO at 15 to 20 minutes | |
| Standard management | Quotation: "no alteration in therapeutic management were undertaken during data collection period" | |
| Position description | 20 degrees lateral rotation, HOB elevation: recumbent (flat) for lateral positions and 20 degrees for supine position, angle verification methods not described for rotation or HOB elevation | |
| Washout period | Quotation: "15 minutes were allowed to elapse for restabilization of any haemodynamic parameters that might have altered owing to the movement of repositioning" | |
| Notes | Primary reference was a conference proceeding abstract Data collection did not exceed 75 minutes PA catheter thermodilution technique was used for CO studies No extractable summary statistics for meta‐analysis because of unit of analysis error (reported mean without variance) and insufficient information on the mean difference. Additional data were no longer accessible; principal investigator attempted to locate study file (personal communication). No further information was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Randomly assigned to position sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described. Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described Comment: objective outcome measures taken from real‐time physiological monitoring systems; therefore, lack of outcome assessor blinding was unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not described. Insufficient information Comment: missing data, if present, unlikely to influence within‐subject differences for the primary outcome |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
aStandard deviation (SD) is the measure of dispersion following each stated mean value unless otherwise stated.
Abbreviations: acute lung injury (ALI), Acute Physiology And Chronic Health Evaluation II (APACHE II), acute respiratory distress syndrome (ARDS), acute respiratory failure (ARF), alveolar arterial oxygen difference (D(AaO2)), analysis of variance (ANOVA), arterial blood gas (ABG), arterial oxygen content (CaO2), arterial‐venous oxygen difference (a‐vDO2), assist‐control ventilation (A/C), bicarbonate (HCO3), bilateral lung disease (BLD), blood pressure (BP), cardiac index (CI), cardiac output (CO), central venous catheter (CVC), central venous pressure (CVP), chest x‐ray (CXR), chronic obstructive pulmonary disease (COPD), coronary artery bypass graft (CABG), degrees of freedom (df), diastolic blood pressure (DBP), endotracheal tube (ETT), extracorporeal membrane oxygenation (ECMO), extravascular lung water (EVLW), fraction of inspired oxygen (FiO2), haemoglobin (Hb), heart rate (HR), head of bed (HOB), inspiratory‐to‐expiratory ratio (I:E ratio), intensive care unit (ICU), intra‐aortic balloon pump (IABP), intravenous (IV), left internal mammary artery (LIMA), left lateral position (L), length of stay (LOS), nitroglycerin (NTG), male/female (M/F), mean difference (MD), mean pulmonary artery pressure (MPAP), minute (min), minute ventilation (Vmin), mixed venous oxygen saturation, (SvO2), multi‐variate analysis of variance (MANOVA), myocardial infarction (MI), non‐invasive blood pressure (NIBP), number (no), oxygen delivery (DO2), oxygen consumption (VO2), oxygen saturation by pulse oximetry (SpO2), packed red blood cell (PRBC), partial pressure of arterial carbon dioxide (PaCO2), partial pressure of arterial oxygen (PaO2), partial pressure of arterial oxygen to fraction of inspired oxygen ratio (P/F ratio), partial pressure of venous oxygen (PvO2), positive end‐expiratory pressure (PEEP), postoperative (postop), pulmonary artery (PA), pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP), respiratory rate (resp. rate), right lateral position (R), saturation of arterial oxygen (SaO2), sequential organ failure assessment (SOFA), simplified acute physiology score (SAPS), sodium nitroprusside (SNP), standard error (SE) or standard error of the mean (SEM), standard error of the mean difference (SE (MD)), supine position (S), synchronized intermittent mandatory ventilation (SIMV), systemic inflammatory response syndrome (SIRS), systolic blood pressure (SBP), systolic, mean and diastolic (S,M,D), temperature (temp), tidal volume (Vt), time (T), unilateral lung disease (ULD)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahrens 2004 | Intervention does not meet criteria (duration < 10 minutes in each body position). Experimental group had a 10‐minute pause in each lateral position and a 5‐minute pause in the supine position during kinetic therapy No separate outcome data reported between body positions during kinetic therapy |
| Aitken 1995 | Non‐randomized study |
| Aitken 2000 | Non‐randomized study |
| Bridges 2000 | Intervention does not meet criteria (duration < 10 minutes in each body position) for phase 2 study |
| Briones 1991 | Insufficient information about study design within conference proceeding abstract for assessment of eligibility for inclusion. Study author contacted and responded (original report may be difficult to track, university was to be contacted). No further response received (personal communication, Dr. Tess Briones, 9 October 2009) |
| Chang 1989 | Participants not critically ill |
| Chang 1993 | Participants not critically ill |
| Dhainaut 1980 | Non‐randomized study |
| Enright 1997 | Non‐randomized study (Participants were examined in 4 body positions including right and left lateral positions, with the sequence of treatment randomized. However, the study conducted separate descriptive and inferential analyses of each body position, with no comparative analysis between body positions) |
| Gillespie 1987 | Non‐randomized study |
| Groom 1990 | Non‐randomized study |
| Hamlin 2008b | Intervention does not meet criteria (duration < 10 minutes in each body position) Two‐hourly turning with the sequence 'supine‐left lateral‐supine‐right lateral' compared with automated continuous lateral turning. Automated turning did not meet inclusion criteria Information provided by principal investigator (personal communication, Prof. Sandra Hanneman, 6 March 2010), who clarified the design of the primary (parent) study on preventable pulmonary complications and the substudy on haemodynamic outcomes (as a report was not available at the time of personal communication) |
| Lange 1988 | Participants not critically ill (elective cardiac catheterization for evaluation of chest pain) Randomized design violations (last 7 participants had fewer comparisons between body positions and fewer outcome measures taken than the first 17 participants, despite randomization of position order) |
| Ledwith 2010 | Outcomes not relevant (brain tissue oxygen, intracranial pressure, cerebral perfusion pressure) |
| Mauri 2010 | Non‐randomized study |
| McLean 2001 | No separate outcome data reported between positions during kinetic therapy (Experimental group had 10‐minute pause in each body position, with control group turned 2‐hourly accordingly to unit protocol, minimal description) |
| Murphy 1977 | Intervention does not meet criteria (duration < 10 minutes in each body position) 8 adult males allocated to 3 groups according to position of the PA catheter insertion site. Thereafter, participants within each group randomized to a subgroup with a pre‐specified sequence (supine‐lateral‐supine vs lateral‐supine‐lateral). Positioning sequence was repeated for each participant 4 times during the study |
| Neagley 1985 | Participants not critically ill. Non‐randomized study (not all body positions for comparison were randomized) |
| Nelson 1989 | Intervention does not meet criteria (duration < 10 minutes in each body position/positioning schedule for comparison) Data collected during "continuous full side‐to‐side rotation" and "compared to three static positions after 30 minutes in the static position", including right lateral, left lateral and supine positions (personal communication, Prof. Loren Nelson, 5 March 2010) |
| Porto 2008 | Outcomes not relevant (respiratory system compliance) |
| Rivara 1984 | Non‐randomized study |
| Romero 1995 | Participants not critically ill |
| Ross 1995 | Intervention does not meet criteria (duration < 10 minutes in each body position) Outcome data collected after 5 minutes, but further correspondence indicated that body position changes may have occurred within a minimum of 10 minutes as the result of transducer adjustment, instrument checks and other protocol procedures performed before data collection at the 3 different transducer positions; "exact time spent in each position was not recorded" (personal communication, Associate Prof. Carol Ross, 4 June 2010) |
| Seaton 1979 | Participants not critically ill (preop and after 24 hours postop for unilateral lobectomy/thoracotomy for resection of lung tumour) |
| Shinners 1993 | Non‐randomized study (Group allocation according to timing of enrolment i.e. first half allocated to one sequence (supine‐side lying‐side lying), and second half allocated to other sequence (side lying‐supine‐supine). Thereafter, participants randomly assigned to right or left lateral position for the side‐lying period) |
| Simonis 2012 | Intervention does not meet criteria (duration < 10 minutes in each body position), no separate outcome data reported between body positions during kinetic therapy |
| Sonnenblick 1983 | Participants not critically ill |
| Staudinger 2010 | Intervention does not meet criteria (duration < 10 minutes in each body position), no separate outcome data were reported between body positions during CLRT |
| Williams 1997 | Co‐intervention (mattress type) not equally applied to groups (Conference proceeding abstract of randomized study of 50 critically ill participants, comparing 2‐hourly turns on standard hospital mattress vs 8‐hourly turns on air‐suspension mattress for P/F ratio and numerical chest x‐ray score from day 0 to day 3) |
| Wilson 1994 | Non‐randomized study |
| Winslow 1990 | Non‐randomized study |
| Yeaw 1996 | Participants not critically ill |
| Zack 1974 | Not all participants were critically ill (most were ambulatory) |
Characteristics of studies awaiting assessment [ordered by study ID]
Daihua 2012.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Email sent to contact author on 23 December 2013 to request information for assessment of eligibility for inclusion. No response |
Samir 2010.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Inter‐library loans and Infortrieve unable to supply full‐text journal article in Australia. No journal response to journal registration to access email details of contact author. Sent email to potential trial author to confirm authorship on 23 December 2013. No response |
Differences between protocol and review
We made the following changes to the published protocol (Hewitt 2008).
The Australasian Digital Theses Program database ceased operation in 2011. We accessed content from this database and other Australian theses through the Trove service of the National Library of Australia. We adapted the search methods accordingly, using 'theses' and 'Australian content' as limiters within this database. ProQuest Digital Dissertations was changed to the Proquest Dissertations and Theses database. We adapted the keyword 'postur*' to 'body postur*' to improve specificity in this database from 2009.
We indicated in the protocol that we planned to conduct subgroup analysis to examine differences in populations based on:
primary disease, injury or condition;
severity of illness (only trials with validated definitions, scales or scoring systems will be analysed for differences in findings due to differences in severity of illness);
presence of assisted ventilation (for this review, assisted ventilation is considered to be any form of positive pressure, including non‐invasive ventilation and continuous positive airway pressure (CPAP)); and
variations in positioning techniques (trials that rotate participants 45 degrees or more from the horizontal plane will be compared with trials that rotate patients less than 45 degrees).
Furthermore, we intended to analyse outcome data from study populations rather than individuals to explain possible sources of variability. However, meta‐analyses was limited to two studies, with no further analysis of variation possible.
We considered a fixed‐effect model of analysis in the protocol. However, studies included in the review were without clear homogeneity. We made the assumption that differences in effect estimates may not be due to chance alone, and that heterogeneity across studies examining critically ill patients may also contribute. In the two studies combined for meta‐analysis (Ibanez 1981; Remolina 1981), information about angle of rotation was insufficient, as were details of treatment sequence and possible differences between spontaneous breathing and mechanical ventilation, for review authors to be certain about homogeneity for a fixed‐effect model of analysis. Reporting both random‐effects and fixed‐effect models would not have been sufficiently meaningful within this review.
We had planned to examine possible sources of substantial statistical heterogeneity through a narrative summary of trial characteristics and risk of bias. In addition, if we found clear evidence of poor homogeneity between trials, we planned to undertake a narrative summary of the findings rather than a meta‐analysis. However, we found that most data were not available in a form suitable for data extraction for meta‐analysis. The only meta‐analysis conducted for a primary outcome (partial pressure of arterial oxygen (PaO2) for detection of hypoxaemia) included two studies without statistical heterogeneity (Ibanez 1981; Remolina 1981).
Our intention was to consider grouping and analysing relevant morbidity data within categories (compound pulmonary morbidity, cardiovascular morbidity and any other system morbidity) when studies provided insufficient outcome data for each measure. However, morbidity data were not available for this review. In addition, clinical adverse events reported as dichotomous or continuous data within the meta‐analysis were intended to be grouped for narrative analysis according to the following classification of severity adapted from Vohra 2007: severe (unintentional event or complication that is associated with morbidity and mortality), moderate (unintentional event or complication requiring immediate medical intervention but not directly associated with morbidity and mortality) and mild (self limiting event or complication that is transient in nature, requiring no medical intervention). After sensitivity analysis was performed, we identified no studies that could be included to evaluate the severity of adverse events.
At the time of protocol publication, The Cochrane Collaboration did not use the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) approach. However, this review incorporates the recommendations for utilizing GRADE to evaluate quality of evidence and presents findings within 'Summary of findings' tables.
Contributions of authors
Conceiving of and designing the review: Nicky Hewitt (NH), Tracey Bucknall (TB). Co‐ordinating the review: NH. Undertaking manual searches: NH. Screening search results: NH, Nardene Faraone (NF). Organizing retrieval of papers: NH. Screening retrieved papers against inclusion criteria: NH, NF, TB. Appraising quality of papers: NH, NF. Abstracting data from papers: NH, NF. Writing to authors of papers for additional information: NH. Providing additional data about papers: NH. Obtaining and screening data on unpublished studies: NH, NF. Managing data for the review: NH. Entering data into Review Manager (RevMan): NH. Analysing RevMan statistical data: NH. Performing other statistical analysis not using RevMan: NH Performing double entry of data: data entered by person one: NH; data verified by person two: NF. Interpreting data: NH, TB. Making statistical inferences: NH. Writing the review: NH, TB. Securing funding for the review: NH, TB. Serving as guarantor for the review (one review author): NH. Taking responsibility for reading and checking the review before submission: NH, TB.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Joanna Briggs Institute Collaborating Centre Grant, Australia.
AUS $5,000 received for biostatistician advice
Declarations of interest
Nicky Hewitt: none known.
Tracey Bucknall: none known.
Nardene Faraone: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Banasik 1987 {published data only}
- Banasik JL, Bruya MA, Steadman RE, Demand JK. Effect of position on arterial oxygenation in postoperative coronary revascularization patients. Heart & Lung 1987;16(6 Pt 1):652‐7. [PUBMED: 3500152] [PubMed] [Google Scholar]
Banasik 1996 {published data only}
- Banasik J. The effect of position on peripheral oxygenation in postoperative CABG patients. Heart & Lung 1990;19(3):302. [Google Scholar]
- Banasik JL, Emerson RJ. Effect of lateral position on arterial and venous blood gases in postoperative cardiac surgery patients. American Journal of Critical Care 1996;5(2):121‐6. [PUBMED: 8653163] [PubMed] [Google Scholar]
- Emerson RJ, Banasik JL. Effect of position on selected hemodynamic parameters in postoperative cardiac surgery patients. American Journal of Critical Care 1994;3(4):289‐99. [PUBMED: 7920958] [PubMed] [Google Scholar]
Banasik 2001 {published data only}
- Banasik JL, Emerson RJ. Effect of lateral positions on tissue oxygenation in the critically ill. Heart & Lung 2001;30(4):269‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Emerson R, Banasik J. Effect of position on oxygen delivery in the critically ill. American Journal of Critical Care 1999;8(4):273. [Google Scholar]
Bein 1996 {published data only}
- Bein T, Metz C, Keyl C, Pfeifer M, Taeger K. Effects of extreme lateral posture on hemodynamics and plasma atrial natriuretic peptide levels in critically ill patients. Intensive Care Medicine 1996;22(7):651‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carroll 1992 {published data only (unpublished sought but not used)}
- Carroll K. The effects of early position changes on cardiac output and SvO2 in the coronary artery bypass graft surgery patient. Heart & Lung 1992;21(3):286. [ ISSN 0147‐9563] [Google Scholar]
- Carroll, KC. The effects of early position changes on cardiac output and venous oxygen saturation in the coronary artery bypass graft surgery patient. Unpublished M.S. thesis, San Diego State University, San Diego, United States of America, 1991.
Chan 1992 {published and unpublished data}
- Chan M. The effects of three different positions on arterial oxygen and relative pulmonary shunt in post‐operative coronary arterial bypass graft patients. Unpublished master's thesis, University of Alberta, Alberta, Canada,. Canada: University of Alberta, 1991.
- Chan M, Jensen L. Positioning effects on arterial oxygen and relative pulmonary shunt in patients receiving mechanical ventilation after CABG. Heart & Lung 1992;21(5):448‐56. [PUBMED: 1399664] [PubMed] [Google Scholar]
Chulay 1982 {published data only}
- Chulay M, Brown J, Summer W. Effect of postoperative immobilization after coronary artery bypass surgery. Critical Care Medicine 1982;10(3):176‐9. [PUBMED: 7037302] [DOI] [PubMed] [Google Scholar]
de Laat 2007 {published data only}
- Laat E, Schoonhoven L, Grypdonck M, Verbeek A, Graaf R, Pickkers P, Achterberg T. Early postoperative 30° lateral positioning after coronary artery surgery: influence on cardiac output. Journal of Clinical Nursing 2007;16(4):654‐61. [PUBMED: 17402946] [DOI] [PubMed] [Google Scholar]
Doering 1988 {published data only}
- Doering L, Dracup K. Comparisons of cardiac output in supine and lateral positions. Nursing Research 1988;37(2):114‐8. [PUBMED: 3347519] [PubMed] [Google Scholar]
Gavigan 1990 {published data only}
- Gavigan M, Kline‐O'Sullivan C, Klumpp‐Lybrand B. The effect of regular turning on CABG patients. Critical Care Nursing Quarterly 1990;12(4):69‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gawlinski 1998 {published and unpublished data}
- Gawlinski A, Dracup K. Effect of positioning on SvO2 in the critically ill patient with a low ejection fraction. Nursing Research 1998;47(5):293‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gawlinski AF. Effect of positioning on venous oxygen saturation in the critically ill patient with a low ejection fraction. Unpublished doctoral dissertation, University of California, Los Angeles, United States,. California, United States: University of California, Los Angeles, 1993.
George 2002 {published and unpublished data}
- George E, Hoffman L, Boujoukos A, Zullo T. The effect of position on oxygenation in single lung transplants. American Journal of Critical Care 2000;9(3):211. [ISSN 1062‐3264] [PubMed] [Google Scholar]
- George EL. The effect of positioning on oxygenation in single lung transplant recipients. Unpublished PhD thesis, University of Pittsburgh, Pennsylvania, United States,. Pennsylvania, United States: University of Pittsburgh, 1999. [ISBN 978‐0‐599‐61071‐2]
- George EL, Hoffman LA, Boujoukos A, Zullo TG. Effect of positioning on oxygenation in single‐lung transplant recipients. American Journal of Critical Care 2002;11(1):66‐75. [PUBMED: 11785558] [PubMed] [Google Scholar]
Ibanez 1981 {published data only}
- Ibañez J, Raurich JM, Abizanda R, Claramonte R, Ibañez P, Bergada J. The effect of lateral positions on gas exchange in patients with unilateral lung disease during mechanical ventilation. Intensive Care Medicine 1981;7(5):231‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2002 {published data only}
- Kim MJ, Hwang HJ, Song HH. A randomized trial on the effects of body positions on lung function with acute respiratory failure patients. International Journal of Nursing Studies 2002;39(5):549‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lewis 1997 {published data only}
- Lewis P, Nicols E, Mackey G, Fadol A, Sloane L, Villagomez E, et al. The effect of turning and backrub on mixed venous oxygen saturation in critically ill patients. American Journal of Critical Care 1997;6(2):132‐40. [PUBMED: 9172850] [PubMed] [Google Scholar]
Pena 1989 {published data only (unpublished sought but not used)}
- Pena M. The effect of position change on mixed venous oxygen saturation measurements in open heart surgery patients during the immediate postoperative period. Heart & Lung 1989;18(3):305. [Google Scholar]
Reed 2002 {published data only}
- Jesurum‐Urbaitis JT. Oxygen consumption modulation: effects of an opioid agonist on tissue oxygenation following lateral positioning in cardiovascular surgical patients with low oxygen delivery. Unpublished PhD thesis, Texas Woman's University, Texas, United States of America,. Texas, United States: Texas Woman's University, 2002. [ISBN 978‐0‐493‐93047‐3]
- Reed S, Jesurum‐Urbaitis J, Kumpula J, Motzer S, Simpson T, Burr R, et al. The effect of lateral positioning on tissue oxygenation in cardiovascular surgical patients with anemia. American Journal of Critical Care 2003;12(3):279‐80. [Google Scholar]
- Reed SL. The effect of lateral positioning on tissue oxygenation in cardiovascular surgical patients with anemia. Unpublished MN thesis, University of Washington, Seattle, Washington, United States of America, 2002.
Remolina 1981 {published data only}
- Remolina C, Khan AU, Santiago TV, Edelman NH. Positional hypoxemia in unilateral lung disease. New England Journal of Medicine 1981;304(9):523‐5. [PUBMED: 6779161] [DOI] [PubMed] [Google Scholar]
Schellongowski 2007 {published data only}
- Schellongowski P, Losert H, Locker GJ, Laczika K, Frass M, Holzinger U, et al. Prolonged lateral steep position impairs respiratory mechanics during continuous lateral rotation therapy in respiratory failure. Intensive Care Medicine 2007;33(4):625‐31. [DOI: 10.1007/s00134-006-0513-y] [DOI] [PubMed] [Google Scholar]
- Staudinger T, Losert H, Schellongowski P, Locker GJ, Laczika K. Physiological effects of lateral steep positioning in critically ill patients suffering from acute respiratory distress syndrome (ARDS). Critical Care 2004;8(Suppl 1):P35. [DOI: 10.1186/cc2502] [DOI] [Google Scholar]
Shively 1988 {published data only}
- Shively M. Effect of position change on mixed venous oxygen saturation in coronary artery bypass surgery patients. Heart & Lung 1988;17(1):51‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Shively MJ. The effect of position change on oxygenation in critically ill adults. Unpublished PhD thesis, University of Texas, Austin, United States of America, 1986. [UMI Order PUZ8910655]
Thomas 2007a {published and unpublished data}
- Thomas PJ. Examination of the role of postural changes in ventilated intensive care patients. Current practice, investigation and guidelines. Unpublished PhD thesis, University of Queensland, Queensland, Australia,. Queensland, Australia: School of Health and Rehabilitation Sciences, University of Queensland, 2006:103‐129.
- Thomas PJ, Paratz JD, Lipman J, Stanton WR. Lateral positioning of ventilated intensive care patients: a study of oxygenation, respiratory mechanics, hemodynamics, and adverse events. Heart & Lung 2007;36(4):277‐86. [PUBMED: 17628197] [DOI] [PubMed] [Google Scholar]
Tidwell 1990 {published data only}
- Osguthorpe SG, Tidwell SL, Ryan WJ, Paull DL, Smith TL. Evaluation of the patient having cardiac surgery in the postoperative rewarming period. Heart & Lung 1990;19(5 Pt 2):570‐4. [PUBMED: 2211170] [PubMed] [Google Scholar]
- Tidwell SL, Williams JR, Osguthrope SG, Paull DL, Smith TL. Effects of position changes on mixed venous oxygen saturation in patients after coronary revascularization. Heart & Lung 1990;19(5 Pt 2):574‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Tripathi 2009 {published data only}
- Tripathi M, Pandey M, Nepal B, Rai H, Bhattarai B. Evaluation of lung infiltration score to predict hypoxemia in ventilated acute respiratory distress syndrome patients and the lateralization of skin pressure sore. Indian Journal of Medical Sciences 2009;63(9):392‐401. [PUBMED: 19805918] [PubMed] [Google Scholar]
Whitman 1982 {published data only (unpublished sought but not used)}
- Whitman GR, Howaniak DL, Verga TS. Comparison of cardiac output measurements in 20‐degree supine and 20‐degree right and left lateral recumbent positions. Heart & Lung 1982;11(3):256‐7. [Google Scholar]
References to studies excluded from this review
Ahrens 2004 {published data only}
- Ahrens T, Kollef M, Stewart J, Shannon W. Effect of kinetic therapy on pulmonary complications. American Journal of Critical Care 2004;13(5):376‐83. [MEDLINE: ] [PubMed] [Google Scholar]
- Ahrens T, Sherman G, Stewart J, Kollef MH. Kinetic therapy is associated with reductions in pulmonary complications amongst patients with acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 2001;163:A754. [Google Scholar]
Aitken 1995 {published data only}
- Aitken L. Comparison of pulmonary artery pressure measurements in the supine and 60° lateral positions. Australian Critical Care 1995;8(4):21‐9. [PUBMED: 8704390] [DOI] [PubMed] [Google Scholar]
Aitken 2000 {published data only}
- Aitken LM. Reliability of measurements of pulmonary artery pressures obtained with patients in the 60° lateral position. American Journal of Critical Care 2000;9(1):43‐51. [PUBMED: 10631390] [PubMed] [Google Scholar]
Bridges 2000 {published and unpublished data}
- Bridges E, Woods SL, Brengelmann GL, Mitchell P, Laurent‐Bopp D. Effect of the 30° lateral recumbent position on pulmonary artery and pulmonary artery wedge pressures in critically ill adult cardiac surgery patients. American Journal of Critical Care 2000;9(4):262‐75. [PUBMED: 10888149] [PubMed] [Google Scholar]
- Bridges EJ. Effect of the 30‐degree lateral recumbent position on pulmonary artery and pulmonary artery wedge pressures in critically ill adults. Unpublished PhD dissertation, University of Washington, United States of America, 1998. [UMI Order AAI9907881] [PubMed]
Briones 1991 {published data only (unpublished sought but not used)}
- Briones T, Dickenson S, Bieberitz R. Effects of positioning on SvO2 and hemodynamic measurements. Heart & Lung 1991;20(3):297. [Google Scholar]
Chang 1989 {published data only}
- Chang S, Shiao G, Perng R. Postural effect on gas exchange in patients with unilateral pleural effusions. Chest 1989;96(1):60‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chang 1993 {published data only}
- Chang SC, Chang HI, Shiao GM, Perng RP. Effect of body position on gas exchange in patients with unilateral central airways lesions. Down with good lung. Chest 1993;103(3):787‐91. [DOI] [PubMed] [Google Scholar]
Dhainaut 1980 {published data only}
- Dhainaut, JF, Bons J, Bricard C, Monsallier JF. Improved oxygenation in patients with extensive unilateral pneumonia using the lateral decubitus position. Thorax 1980;35(10):792‐3. [PUBMED: 7466727] [DOI] [PMC free article] [PubMed] [Google Scholar]
Enright 1997 {unpublished data only}
- Enright S. Cardiorespiratory alterations following positional adjustment in critically ill mechanically ventilated patients with acute respiratory failure. Unpublished M.Phil. thesis, University of Manchester, Manchester, United Kingdom, 1997.
Gillespie 1987 {published data only}
- Gillespie DJ, Rehder K. Body position and ventilation‐perfusion relationships in unilateral pulmonary disease. Chest 1987;91(1):75‐9. [PUBMED: 3792089] [DOI] [PubMed] [Google Scholar]
Groom 1990 {published data only}
- Groom L, Frisch SR, Elliott M. Reproducibility and accuracy of pulmonary artery pressure measurements in supine and lateral positions. Heart & Lung 1990;19(2):147‐51. [PUBMED: 2318657] [PubMed] [Google Scholar]
Hamlin 2008b {published data only (unpublished sought but not used)}
- Hamlin S. Multi‐site randomized clinical trial of horizontal positioning to prevent and treat pulmonary complications in mechanically ventilated critically ill patients: hemodynamic substudy. Southern Online Journal of Nursing Research 2008;8(2):2. [ISSN 1538‐0696] [Google Scholar]
Lange 1988 {published data only}
- Lange RA, Katz J, McBride W, Moore DM, Hillis LD. Effects of supine and lateral positions on cardiac output and intracardiac pressures. The American Journal of Cardiology 1988;62(4):330‐3. [PUBMED: 3400615] [DOI] [PubMed] [Google Scholar]
Ledwith 2010 {published data only}
- Ledwith M, Bloom S, Maloney‐Wilensky E, Coyle B, Polomano RC, Roux PD. Effect of body position on cerebral oxygenation and physiologic parameters in patients with acute neurological conditions. Journal of Neuroscience Nursing 2010;42(5):280‐7. [PUBMED: 20968224] [DOI] [PubMed] [Google Scholar]
Mauri 2010 {published data only}
- Mauri T, Berra L, Kumwilaisak K, Pivi S, Ufberg JW, Kueppers F, et al. Lateral‐horizontal patient position and horizontal orientation of the endotracheal tube to prevent aspiration in adult surgical intensive care unit patients: a feasibility study. Respiratory Care 2010;55(3):294‐302. [PUBMED: 20196878] [PubMed] [Google Scholar]
McLean 2001 {published data only}
- McLean B. Rotational kinetic therapy for ventilation/perfusion mismatch. Connect:Critical Care Nursing in Europe 2001;1(4):113‐8. [ISSN 1748‐6254] [Google Scholar]
Murphy 1977 {unpublished data only}
- Murphy CE. The effect of lateral positioning on pulmonary artery and pulmonary capillary wedge pressures in critically ill patients. Unpublished MN thesis, The University of Oregon, Oregon, United States of America, 1977.
Neagley 1985 {published data only}
- Neagley SR, Zwillich CW. The effect of positional changes on oxygenation in patients with pleural effusions. Chest 1985;88(5):714‐7. [PUBMED: 3902387] [DOI] [PubMed] [Google Scholar]
Nelson 1989 {published data only}
- Nelson LD, Anderson RRT. Physiologic effects of steep positioning in the surgical intensive care unit. Archives of Surgery 1989;124(3):352‐5. [PUBMED: 2919968] [DOI] [PubMed] [Google Scholar]
Porto 2008 {published data only}
- Porto EF, Castro AAM, Leite JRO, Miranda SV, Lancauth A, Kumpel C. Comparative analysis of respiratory system compliance in three different positions (lateral, supine and sitting) of patients on long‐term invasive mechanical ventilation [Análise comparativa da complacência do sistema respiratório em três diferentes posições no leito (lateral, sentada e dorsal) em pacientes submetidos à ventilação mecânica invasiva prolongada]. Revista Brasileira de Terapia Intensiva 2008;20(3):213‐9. [PubMed] [Google Scholar]
Rivara 1984 {published data only}
- Rivara D, Artucio H, Arcos J, Hiriart C. Positional hypoxemia during artificial ventilation. Critical Care Medicine 1984;12(5):436‐8. [PUBMED: 6370598] [DOI] [PubMed] [Google Scholar]
Romero 1995 {published data only}
- Romero S, Martin C, Hernandez L, Arriero JM, Benito N, Gil J. Effect of body position on gas exchange in patients with unilateral pleural effusion: influence of effusion volume. Respiratory Medicine 1995;89(4):297‐301. [PUBMED: 7597270] [DOI] [PubMed] [Google Scholar]
Ross 1995 {published data only (unpublished sought but not used)}
- Ross CJM. A comparison of pulmonary artery pressures in supine and lateral positions using three adjustments of the pressure transducer. Unpublished MN thesis, University of Alberta, Alberta, Canada, 1988.
- Ross CJM, Jones R. Comparisons of pulmonary artery pressure measurements in supine and 30 degree lateral positions. Canadian Journal of Cardiovascular Nursing 1995;6(3‐4):4‐8. [ISSN 0843‐6096 ] [PubMed] [Google Scholar]
Seaton 1979 {published data only}
- Seaton D, Lapp NL, Morgan WK. Effect of body position on gas exchange after thoracotomy. Thorax 1979;34(4):518‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinners 1993 {published data only}
- Shinners PA, Pease MO. A stabilization period of 5 minutes is adequate when measuring pulmonary artery pressures after turning. American Journal of Critical Care 1993;2(6):474‐7. [PUBMED: 8275153] [PubMed] [Google Scholar]
Simonis 2012 {published data only}
- Simonis G, Steiding K, Schaefer K, Rauwolf T, Strasser RH. A prospective, randomized trial of continuous lateral rotation ("kinetic therapy") in patients with cardiogenic shock. Clinical Research in Cardiology 2012;101(12):955‐62. [PUBMED: 22729756] [DOI] [PubMed] [Google Scholar]
Sonnenblick 1983 {published data only}
- Sonnenblick M, Melzer E, Rosin AJ. Body positional effect on gas exchange in unilateral pleural effusion. Chest 1983;83(5):784‐6. [PUBMED: 6839822] [DOI] [PubMed] [Google Scholar]
Staudinger 2010 {published data only}
- Staudinger T, Bojic A, Holzinger U, Meyer B, Rohwer M, Mallner F, et al. Continuous lateral rotation therapy to prevent ventilator‐associated pneumonia. Critical Care Medicine 2010;38(2):486‐90. [PUBMED: 19789440] [DOI] [PubMed] [Google Scholar]
Williams 1997 {published data only}
- Williams S, Williams K, Breadmore R, Vincent J. Effects of infrequent turning on chest complications in critically ill patients. Australian Critical Care 1997;10(1):29. [Google Scholar]
Wilson 1994 {published data only}
- Wilson J, Woods I. Changes in oxygen delivery during lateral rotational therapy. Care of the Critically Ill 1994;10(6):262‐5. [ISSN 0266‐0970] [Google Scholar]
Winslow 1990 {published data only}
- Winslow EH, Clark AP, White KM, Tyler DO. Effects of a lateral turn on mixed venous oxygen saturation and heart rate in critically ill adults. Heart & Lung 1990;19(5 Pt 2):557‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Yeaw 1996 {published data only}
- Yeaw EMJ. The effect of body positioning upon maximal oxygenation of patients with unilateral lung pathology. Journal of Advanced Nursing 1996;23(1):55‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zack 1974 {published data only}
- Zack MB, Pontoppidan H, Kazemi H. The effect of lateral positions on gas exchange in pulmonary disease: a prospective evaluation. American Review of Respiratory Disease 1974;110(1):49‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Daihua 2012 {published data only}
- Daihua Y, Wei C, Xude S, Linong Y, Changjun G, Hui Z. The effect of body position changes on stroke volume variation in 66 mechanically ventilated patients with sepsis. Journal of Critical Care 2012;27(4):416.e7‐12. [PUBMED: 22459159] [DOI] [PubMed] [Google Scholar]
Samir 2010 {published data only}
- Samir EM, Hamed HMF, Elgohary MM. The effect of different positioning after lung recruitment with CPAP hold maneuver on oxygenation and lung mechanics in acute respiratory distress syndrome. Egyptian Journal of Anaesthesia 2010;26(3):249‐59. [Google Scholar]
Additional references
Bassi 2012
- Bassi GL, Bertral R, Martí JD, Rodriguez‐Romero D, Torres A. New insights in positioning tracheally intubated and mechanically ventilated patients. Clinical Pulmonary Medicine 2012;19(4):174‐82. [Google Scholar]
Bennett 2005
- Bennett JA. The consolidated standards of reporting trials (CONSORT): guidelines for reporting randomized trials. Nursing Research 2005;54(2):128‐32. [DOI] [PubMed] [Google Scholar]
Carroll 1991
- Carroll, KC. The effects of early position changes on cardiac output and venous oxygen saturation in the coronary artery bypass graft surgery patient. Unpublished M.S. thesis, San Diego State University, San Diego, United States of America, 1991.
Chan 1991
- Chan M. The effects of three different positions on arterial oxygen and relative pulmonary shunt in post‐operative coronary arterial bypass graft patients. Unpublished master's thesis, University of Alberta, Alberta, Canada,. Canada: University of Alberta, 1991.
Choe 2000
- Choe K, Kim Y, Shim T, Lim C, Lee S, Koh Y, et al. Closing volume influences the postural effect on oxygenation in unilateral lung disease. American Journal of Respiratory and Critical Care Medicine 2000;161(6):1957‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choi 1992
- Choi S, Nelson L. Kinetic therapy in critically ill patients: combined results based on meta‐analysis. Journal of Critical Care 1992;7(1):57‐62. [ISSN: 0883‐9441] [Google Scholar]
Davis 2001
- Davis KJ, Johannigman JA, Campbell RS, Marraccini A, Luchette FA, Frame SB, et al. The acute effects of body position strategies and respiratory therapy in paralysed patients with acute lung injury. Critical Care 2001;5(2):81‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dean 1992
- Dean E, Ross J. Oxygen transport: the basis for contemporary cardiopulmonary physical therapy and its optimization with body positioning and mobilization. Physical Therapy Practice 1992;1(4):34‐44. [Google Scholar]
Delaney 2006
- Delaney A, Gray H, Laupland KB, Zuege D. Kinetic bed therapy to prevent nosocomial pneumonia in mechanically ventilated patients: a systematic review and meta‐analysis. Critical Care 2006;10(3):R70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doering 1993
- Doering LV. The effects of positioning on hemodynamics and gas exchange in the critically ill: a review. American Journal of Critical Care 1993;2(3):208‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Evans 1994
- Evans D. The use of position during critical illness: current practice and review of the literature. Australian Critical Care 1994;7(3):16‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fink 1990
- Fink MP, Helsmoortel CM, Stein KL, Lee PC, Cohn SM. The efficacy of an oscillating bed in the prevention of lower respiratory tract infection in critically ill victims of blunt trauma. A prospective study. Chest 1990;97(1):132‐37. [DOI] [PubMed] [Google Scholar]
Fink 2002
- Fink JB. Positioning versus postural drainage. Respiratory Care 2002;47(7):769‐77. [PubMed] [Google Scholar]
Goldhill 2007
- Goldhill DR, Imhoff M, McLean B, Waldmann C. Rotational bed therapy to prevent and treat respiratory complications: a review and meta‐analysis. American Journal of Critical Care 2007;16(1):50‐61. [MEDLINE: ] [PubMed] [Google Scholar]
GRADEPro [Computer program]
- McMaster University. GRADEPro. McMaster University, Version [30 March 2014].
Griffiths 2005
- Griffiths H, Gallimore D. Positioning critically ill patients in hospital. Nursing Standard 2005;19(42):56‐64. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Hamlin 2008a
- Hamlin SK, Hanneman SK, Wachtel S, Gusick G. Adverse hemodynamic effects of lateral rotation during mechanical ventilation. Dimensions of Critical Care Nursing 2008;27(2):54‐61. [DOI] [PubMed] [Google Scholar]
Hawkins 1999
- Hawkins S, Stone K, Plummer L. An holistic approach to turning patients. Nursing Standard 1999;14(3):51‐2, 54‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 15 April 2007).
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org, 2011. [Google Scholar]
Jastremski 2002
- Jastremski CA. Back to basics: can body positioning really make a difference in the intensive care unit?. Critical Care Medicine 2002;30(11):2607‐8. [DOI] [PubMed] [Google Scholar]
Jesurum‐Urbaitis 2002
- Jesurum‐Urbaitis JT. Oxygen consumption modulation: effects of an opioid agonist on tissue oxygenation following lateral positioning in cardiovascular surgical patients with low oxygen delivery. Unpublished PhD thesis, Texas Woman's University, Denton, Texas, United States of America, 2002.
Johnson 2009
- Johnson KL, Meyenburg T. Physiological rationale and current evidence for therapeutic positioning in critically ill patients. AACN Advanced Critical Care 2009;20(3):228‐40. [DOI] [PubMed] [Google Scholar]
Jones 2003
- Jones B, Kenward MG. Design and Analysis of Cross‐over Trials. 2nd Edition. Boca Raton: Chapman & Hall/CRC, 2003. [Google Scholar]
Jones 2004
- Jones A, Dean E. Body position change and its effect on hemodynamic and metabolic status. Heart & Lung 2004;33(5):281‐90. [DOI] [PubMed] [Google Scholar]
Keller 2002
- Keller BPJA, Wille J, Ramshorst B, Werken C. Pressure ulcers in intensive care patients: a review of risks and prevention. Intensive Care Medicine 2002;28(10):1379‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krishnagopalan 2002
- Krishnagopalan S, Johnson EW, Low LL, Kaufman L. Body positioning of intensive care patients: clinical practice versus standards. Critical Care Medicine 2002;30(11):2588‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mills 2009
- Mills EJ, Chan A, Wu P, Vail A, Guyatt G. Design, analysis and presentation of crossover trials. Trials 2009;10(4):27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Annals of Internal Medicine 2001;134(8):657‐62. [DOI] [PubMed] [Google Scholar]
National Pressure Ulcer Advisory Panel 2009
- National Pressure Ulcer Advisory Panel & European Pressure Ulcer Advisory Panel. Pressure ulcer prevention recommendations. Prevention and treatment of pressure ulcers: clinical practice guideline 2009. http://www.guideline.gov (accessed 27 September 2011).
Nielsen 2003
- Nielsen KG, Holte K, Kehlet H. Effects of posture on postoperative pulmonary function. Acta Anaesthesiologica Scandinavica 2003;47(10):1270‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Osguthorpe 1990
- Osguthorpe SG, Tidwell SL, Ryan WJ, Paull DL, Smith TL. Evaluation of the patient having cardiac surgery in the postoperative rewarming period. Heart & Lung 1990;19(5 Pt 2):570‐4. [PUBMED: 2211170] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schallom 2005
- Schallom L, Metheny NA, Stewart J, Schnelker R, Ludwig J, Sherman G, et al. Effect of frequency of manual turning on pneumonia. American Journal of Critical Care 2005;14(6):478‐9. [PubMed] [Google Scholar]
Senn 2002
- Senn, SJ. Cross‐Over Trials in Clinical Research. Chichester: Wiley, 2002. [Google Scholar]
Smith 2008
- Smith BA, Lee H, Lee JH, Choi, M, Jones DE, Bausell RB, et al. Quality of reporting randomized controlled trials (RCTs) in the nursing literature: application of the consolidated standards of reporting trials (CONSORT). Nursing Outlook 2008;56(1):31‐7. [DOI] [PubMed] [Google Scholar]
Staudinger 2004
- Staudinger T, Losert H, Schellongowski P, Locker GJ, Laczika K. Physiological effects of lateral steep positioning in critically ill patients suffering from acute respiratory distress syndrome (ARDS). Critical Care 2004;8(Suppl 1):P35. [DOI: 10.1186/cc2502] [DOI] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011}. www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Summer 1989
- Summer WR, Curry P, Haponik EF, Nelson S, Elston R. Continuous mechanical turning of intensive care unit patients shortens length of stay in some diagnostic‐related groups. Journal of Critical Care 1989;4(1):45‐53. [ISSN: 0883‐9441] [Google Scholar]
Thomas 1998
- Thomas AR, Bryce TL. Ventilation in the patient with unilateral lung disease. Critical Care Clinics 1998;14(4):743‐73. [DOI] [PubMed] [Google Scholar]
Thomas 2007b
- Thomas PJ, Paratz JD. Is there evidence to support the use of lateral positioning in intensive care? A systematic review. Anaesthesia and Intensive Care 2007;35(2):239‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vohra 2007
- Vohra S, Johnston BC, Cramer K, Humphreys K. Adverse events associated with pediatric spinal manipulation: a systematic review. Pediatrics 2007;119(1):e275‐83. [DOI] [PubMed] [Google Scholar]
Winklelman 2010
- Winkelman C, Chiang LC. Manual turns in patients receiving mechanical ventilation. Critical Care Nurse 2010;30(4):36‐44. [DOI] [PubMed] [Google Scholar]
Wong 1999
- Wong WP. Use of body positioning in the mechanically ventilated patient with acute respiratory failure: application of Sackett's rules of evidence. Physiotherapy Theory and Practice 1999;15(1):25‐41. [AMED AN: 9174060] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hewitt 2008
- Hewitt N, Bucknall T, Glanville D. Lateral positioning for critically ill adult patients. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007205] [DOI] [PMC free article] [PubMed] [Google Scholar]


