Abstract
Background
In people who have had a stroke, upper limb paresis affects many activities of daily life. Reducing disability is therefore a major aim of rehabilitative interventions. Despite preserving or recovering movement ability after stroke, sometimes people do not fully realise this ability in their everyday activities. Constraint‐induced movement therapy (CIMT) is an approach to stroke rehabilitation that involves the forced use and massed practice of the affected arm by restraining the unaffected arm. This has been proposed as a useful tool for recovering abilities in everyday activities.
Objectives
To assess the efficacy of CIMT, modified CIMT (mCIMT), or forced use (FU) for arm management in people with hemiparesis after stroke.
Search methods
We searched the Cochrane Stroke Group trials register (last searched June 2015), the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library Issue 1, 2015), MEDLINE (1966 to January 2015), EMBASE (1980 to January 2015), CINAHL (1982 to January 2015), and the Physiotherapy Evidence Database (PEDro; January 2015).
Selection criteria
Randomised control trials (RCTs) and quasi‐RCTs comparing CIMT, mCIMT or FU with other rehabilitative techniques, or none.
Data collection and analysis
One author identified trials from the results of the electronic searches according to the inclusion and exclusion criteria, three review authors independently assessed methodological quality and risk of bias, and extracted data. The primary outcome was disability.
Main results
We included 42 studies involving 1453 participants. The trials included participants who had some residual motor power of the paretic arm, the potential for further motor recovery and with limited pain or spasticity, but tended to use the limb little, if at all. The majority of studies were underpowered (median number of included participants was 29) and we cannot rule out small‐trial bias. Eleven trials (344 participants) assessed disability immediately after the intervention, indicating a non‐significant standard mean difference (SMD) 0.24 (95% confidence interval (CI) ‐0.05 to 0.52) favouring CIMT compared with conventional treatment. For the most frequently reported outcome, arm motor function (28 studies involving 858 participants), the SMD was 0.34 (95% CI 0.12 to 0.55) showing a significant effect (P value 0.004) in favour of CIMT. Three studies involving 125 participants explored disability after a few months of follow‐up and found no significant difference, SMD ‐0.20 (95% CI ‐0.57 to 0.16) in favour of conventional treatment.
Authors' conclusions
CIMT is a multi‐faceted intervention where restriction of the less affected limb is accompanied by increased exercise tailored to the person’s capacity. We found that CIMT was associated with limited improvements in motor impairment and motor function, but that these benefits did not convincingly reduce disability. This differs from the result of our previous meta‐analysis where there was a suggestion that CIMT might be superior to traditional rehabilitation. Information about the long‐term effects of CIMT is scarce. Further trials studying the relationship between participant characteristics and improved outcomes are required.
Keywords: Humans, Stroke Rehabilitation, Upper Extremity, Exercise Movement Techniques, Exercise Movement Techniques/methods, Immobilization, Immobilization/methods, Paresis, Paresis/etiology, Paresis/rehabilitation, Randomized Controlled Trials as Topic, Stroke, Stroke/complications, Time Factors
Plain language summary
Constraint‐induced movement therapy for upper limb (arm) recovery after stroke
Review question
We wanted to assess the effects of constraint‐induced movement therapy (CIMT) on ability to manage daily activities and on the recovery of movement in paralysed arms after a stroke.
Background
After a stroke, people can suffer from paralysis of an arm, and, even if some movement control remains, use it less than the unaffected arm. The paralysis makes arm movements, such as reaching, grasping, and manipulating objects difficult. In turn, this causes many difficulties in activities of daily life, such as bathing, dressing, eating and using the toilet. During CIMT the unaffected arm is restrained so it cannot be used, which means the affected arm has to be used instead. The unaffected arm and hand are prevented from moving with a glove or a special arm rest. CIMT is supposed to be a useful tool for recovering the ability to perform everyday activities.
Study characteristics
We, a team of Cochrane researchers, searched widely through the medical literature and identified 42 relevant studies involving 1453 participants. The evidence is current to January 2015. The participants in these studies had some control of their affected arm and were generally able to open their affected hand by extending the wrist and fingers. CIMT treatments varied between studies in terms of the time for which the participants' unaffected arm was constrained each day, and the amount of active exercise that the affected arm was required to do. CIMT was compared mainly to active physiotherapy treatments, and sometimes to no treatment.
Key results
The 42 studies assessed different aspects of recovery from stroke, and not all measured the same things. Eleven studies (with 344 participants) assessed the effect of CIMT on disability (the effective use of the arm in daily living) and found that the use of CIMT did not lead to improvement in ability to manage everyday activities such as bathing, dressing, eating, and toileting. Twenty‐eight trials (with 858 participants) tested whether CIMT improved the ability to use the affected arm. CIMT appeared to be more effective at improving arm movement than active physiotherapy treatments or no treatment.
Quality of the evidence
The quality of the evidence for each outcome is limited due to small numbers of study participants and poor reporting of study details. We considered the quality of the evidence to be low for disability and very low for the ability to use the affected arm.
Summary of findings
for the main comparison.
| Constraint‐induced movement therapy (CIMT) or modified CIMT (mCIMT) or Forced Use (FU) compared with usual care or no treatment for the recovery of affected upper limb in people with stroke | |||||
| Patient or population: people with stroke receiving upper limb rehabilitation Settings: inpatient and outpatients Intervention: CIMT or mCIMT or FU Comparison: usual care or no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Usual care or no treatment | CIMT or mCIMT or FU | ||||
|
Disability
different scales assessing disability or dependence in activities of daily living Follow‐up: at the end of treatment |
The mean disability in the intervention groups was 0.24 standard deviations higher (‐0.05 lower to 0.52 higher) | 344 (11 studies) | A standard deviation of 0.24 represents a small difference between the groups The estimated effect is non significant because its 95% interval confidence includes the null effect |
||
|
Arm Motor Function
different scales assessing motor ability and functioning of upper extremity in functional tasks Follow‐up: at the end of treatment |
The mean arm motor function in the intervention groups was 0.34 standard deviations higher (0.12 to 0.55 higher) | 858 (28 studies) | A standard deviation of 0.34 represents a small difference between the groups | ||
|
Perceived Arm Motor Function (Quality of Use)
Motor Activity Log scale. Follow‐up: at the end of treatment |
The mean perceived arm motor function (quality of use) ranged across control groups from 0.14 to 1.4 points | The mean perceived arm motor function (quality of use) in the intervention groups was 0.68 higher (0.47 to 0.88 higher) | 891 (24 studies) | The minimal clinically important difference for this scale assessing the quality of use is 1 or 1.1 points depending on the dominance of the affected arm (Lang 2008). | |
| Perceived Arm Motor Function (Amount of Use) Motor Activity Log scale Follow‐up: at the end of treatment | The mean perceived arm motor function (amount of use) ranged across control groups from ‐0.07 to 1.6 points | The mean perceived arm motor function (amount of use) in the intervention groups was 0.79 higher (0.50 to 1.08 higher) | 851 (23 studies) | ||
|
Arm Motor Impairment
different scales assessing the impairment Follow‐up: at the end of treatment |
The mean arm motor impairment in the intervention groups was 0.82 standard deviations higher (0.31 to 1.34 higher) | 372 (16 studies) | A standard deviation of 0.82 represents a large difference between the groups | ||
|
Quality of life
Stroke Impact Scale Follow‐up: at the end of treatment |
The mean quality of life score ranged across control groups from ‐3.46 to 7.5 points | The mean quality of life in the intervention groups was 6.54 higher (‐1.2 lower to 14.28 higher) | 96 (3 studies) | ||
|
Dexterity
Different tests assessing dexterity Follow‐up: at the end of treatment |
The mean dexterity in the intervention groups was 0.42 standard deviations higher (0.04 lower to 0.79 higher) | 113 (4 studies) | A standard deviation of 0.42 represents a small difference between the groups. | ||
| *The assumed risk is based on the highest and the lowest estimate of the scores in the control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | |||||
Background
Description of the condition
Stroke is a health concern worldwide and one of the main causes of disability (Albert 2012; WHO 2011). In Europe, stroke costs around EUR 64.1 billion, and in the United Kingdom around GBP 8.9 billion per annum is spent on community care and rehabilitation of people after stroke (Gustavsson 2010; Saka 2009). In fact, only 12% of people that experience a stroke are independent in basic activities of daily living (ADL) one week after stroke onset (Wade 1987); in the long‐term, up to 74% of them have to rely on assistance for basic ADLs like feeding, self‐care, and mobility (Miller 2010).
Description of the intervention
To restore independence to stroke survivors and reduce the cost of therapy and care, a number of approaches are now being investigated in an attempt to increase the effectiveness of stroke rehabilitation techniques for the recovery of the upper extremity (Pollock 2014). The management of upper extremity in people with stroke can involve a number of different treatments, which include: bilateral arm training (McCombe Waller 2008), biofeedback (Crow 1989; Moreland 1994; Rathkolb 1990; Sathian 2000), brain stimulation (Dayan 2013; Kagan 2012), electrical stimulation/functional electrical stimulation (Pomeroy 2006), mental practice (Page 2005a; Page 2007a), mirror therapy (Michielsen 2010), robot assistance (Hesse 2003; Lum 2002; Masiero 2007; Mehrholz 2012), repetitive task training (French 2007), virtual reality (Laver 2011), and constraint‐induced movement therapy (CIMT; Miltner 1999; Page 2001; Page 2002a; Taub 1993; Taub 1994; Taub 1999).
CIMT, as described by the first authors (Miltner 1999; Taub 1994; Taub 1999), is based on two fundamental principles.
Forced use of the affected arm by restraining the unaffected arm, with a sling or a hand splint, during dedicated exercise sections or usual ADLs (90% of waking hours).
Massed practice (several hours of exercise) of the affected arm through a shaping method, where shaping involves a commonly operant conditioning method in which a behavioural objective (in this case 'movement') is approached in small steps of progressively increasing difficulty. The participant is rewarded with enthusiastic approval for improvement, but never blamed or punished for failure.
The initial report of the use of CIMT proposed extensive and intensive training (six to eight hours per day; Miltner 1999; Taub 1994; Taub 1999); over the years, though, others have developed different forms of constraint therapy, reducing the training during the period of restraint (Page 2001; Page 2002a; Page 2002b), or concentrating only on the use of restraint (forced use), with no additional treatment of the affected arm (Burns 2007; Ploughman 2004).
How the intervention might work
The rationale for CIMT is based on the theory of 'learning non‐use' from experiments on monkeys. Researchers observed that after upper limb de‐afferentation (interruption of nerves), monkeys did not use their affected limb even though their motor ability was nearly normal (Knapp 1963; Taub 1977; Taub 1980). This 'non‐use' was an acquired behaviour learned during the spinal shock period and, as a consequence of its origin, could be reversed by behavioural measures such as, for example, constraint of the sound limb. Thus the learned 'non‐use' theory predicts that people after stroke have, in fact, greater movement ability than they show in their everyday tasks. If this is correct, constraint of the unaffected arm would be a useful tool for realising this ability in everyday activities (Sterr 2006).
Why it is important to do this review
Over recent years, the neuroplasticity and cortical reorganisation of the central nervous system (CNS) has been observed and described in trials with people after stroke undergoing CIMT (Kim 2004; Levy 2001; Liepert 2000; Liepert 2001; Lin 2010; Ro 2006; Schaechter 2002; Szaflarski 2006). The preliminary findings suggest that the functional improvements produced by CIMT are accompanied by plastic brain reorganisation associating noticeable brain changes with functional improvements related to CIMT. Our initial review published in 2008 identified 19 studies, now several new studies have been published and an update of our review was necessary in order to define better the effect of constraining therapies on stroke recovery.
Objectives
To assess the efficacy of CIMT, modified CIMT (mCIMT), or forced use (FU) for arm management in people with hemiparesis after stroke.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs comparing CIMT or mCIMT or FU with other rehabilitative techniques (occupational therapy or physiotherapy), or none.
Types of participants
We examined trials of adults (aged over 18 years) with a clinical diagnosis of stroke, either ischaemic or haemorrhagic (World Health Organization (WHO) definition; Hatano 1976), with paresis of an arm.
Types of interventions
The studies included all used CIMT or mCIMT or FU for the treatment of the affected upper limb compared with other rehabilitative techniques (occupational therapy or physiotherapy) or none.
For the purpose of this review we used the following definitions (as described in Hoare 2007):
CIMT: restraint of the unaffected upper limb, with more than three hours per day of therapy;
mCIMT: restraint of the unaffected upper limb, with three hours or less per day of therapy;
FU: restraint of the unaffected upper limb but no specific treatment of the affected upper limb.
We considered all interventions, irrespective of:
number of hours of training per day;
number of hours of restraint per day;
duration of treatment;
type of exercise used in training sessions.
Types of outcome measures
If a study presented more than one measure for the same outcome category, we included the measure most frequently used across studies in the analysis.
Primary outcomes
Disability
Functional Independence Measure (FIM), Barthel Index (BI).
Secondary outcomes
Arm motor function
Wolf Motor Function Test (only score; WFMT), Arm Research Arm Test (ARAT), Arm Motor Ability Test (AMAT), Emory Function Test (EMF), Assessment of motor and process skills (AMPS).
Perceived arm motor function
Motor Activity Log (MAL): Amount of Use (AoU) and Quality of Use (QoU).
Arm motor impairment
Fugl Meyer Assessment (FMA), Chedoke McMaster Impairment Inventory (CMII), hand strength.
Quality of life
Stoke Impact Scale (SIS).
Dexterity*
Nine‐Hole Peg Test (9HPT), Sixteen‐Hole Peg Test (16HPT), Grooved Pegboard Test (GPT).
* a low score in scales assessing this item indicates a positive outcome and indicates a better performance.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched June 2015), the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2015, Issue 1; Appendix 1), MEDLINE Ovid (1966 to January 2015; Appendix 2), EMBASE Ovid (1980 to January 2015; Appendix 3), CINAHL Ebsco (1982 to January 2015; Appendix 4), AMED Ovid (1985 to January 2015; Appendix 5), and in January 2015 the Physiotherapy Evidence Database (PEDro; http://ptwww.cchs.usyd.edu.au/pedro/; Appendix 6).
In addition, we searched the following trials registries:
National Institute of Health Clinical Trials Database (http://www.clinicaltrials.gov; 1 June 2015);
Stroke Trials Registry (www.strokecenter.org/trials/; 1 June 2015).
Searching other resources
We also searched the reference lists of relevant papers.
Data collection and analysis
Selection of studies
One review author (DC) read the titles of identified references and eliminated obviously irrelevant studies. We obtained abstracts for the remaining studies and then, on the basis of the inclusion criteria, two review authors (DC and VS) independently ranked these as 'relevant', 'irrelevant' or 'unsure'. We retrieved and reviewed the full text articles for those ranked as relevant and those ranked as unsure. We resolved disagreements by consensus, and consulted a third review author (RG) if disagreements persisted.
We have documented the reasons for the exclusion of studies in Characteristics of excluded studies. When studies published in non‐English languages appeared relevant, we retrieved the full text and asked a native speaker to translate it in order to ascertain whether the study met the inclusion criteria.
Data extraction and management
Four review authors (DC, VS, GC and RG) independently extracted data. We recorded all data on a standardised checklist, incorporating: methods (e.g. randomisation, blinding, completeness of follow‐up, reliability and validity of scales), details of participants (e.g. age, sex, time since stroke, side affected), interventions, inclusion and exclusion criteria, and all assessed outcomes. We resolved disagreements by consensus. In some cases we contacted study authors by email for clarification. When not clearly reported or imputable, we extracted numeric data from graphs through the use of Engauge Software 5.1.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies using the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Methods of randomisation
We regarded a randomisation method as appropriate if it meant that each study participant had the same chance of receiving each intervention. We considered the following methods of allocation appropriate: using random number tables, a computer random number generator, coin tossing, or card shuffling.
Allocation concealment (when the investigators cannot predict which treatment comes next)
We scored this as:
low risk of bias — when the method of allocation was clearly described (e.g. central randomisation, serially numbered opaque, sealed envelopes);
unclear risk of bias — when the authors did not report any allocation concealment approach at all, or did not describe it clearly;
high risk of bias — when the method of allocation was not concealed.
Potential for selection bias after allocation
We scored this as:
low risk of bias — trials where an intention‐to‐treat analysis was possible and there were few losses to follow up;
unclear risk of bias — trials reporting exclusions (less than 10% exclusions);
high risk of bias — no reporting of exclusions, or more than 10% exclusions, or wide differences in exclusions between groups.
Blinding with reference only to the outcome assessor
We scored this as:
low risk of bias — blinded;
unclear risk of bias — information not reported;
high risk of bias — not blinded.
Follow‐up
We scored this as:
low risk of bias — if the numbers and reasons for dropouts and withdrawals in all intervention groups were described and if 90% or more of the randomised participants were included in the analysis, or if it was specified that there were no dropouts or withdrawals;
unclear risk of bias — if the report gave the impression there were no dropouts or withdrawals, but it was not specifically stated;
high risk of bias — if less than 90% of the randomised participants were included in the analysis or the number or reasons for dropouts and withdrawals were not described.
Scales to measure outcomes
Scales had to be supported by studies about their psychometric properties. We classified the scales as:
low risk of bias — if studies support the reliability and validity of the scale;
unclear risk of bias — if supporting data were not provided, or the scale has never been tested;
high risk of bias — if there was evidence of insufficient reliability or validity.
Measures of treatment effect
Two review authors (DC and VS) independently classified outcome measures in terms of the domain assessed (disability, arm motor function, perceived arm motor function, arm motor impairment, quality of life and dexterity). When a study presented more than one outcome measure for the same domain, we used the measure most frequently utilised across studies for the analysis. We converted continuous data to mean difference (MD) and, if different scales were used, we first computed a standardised mean difference (SMD), and second, an overall MD and overall SMD.
Dealing with missing data
When standard deviations of the changes were not reported, we estimated them in the treatment and control groups from the variances, or through the use of Engauge Software 5.1 as needed for data analysis.
If data for the estimation of standard deviation of changes were unreported, we contacted study authors by email to request the information. If we did not receive a reply, we contacted the study authors again.
Assessment of heterogeneity
We did a statistical summary of treatment effects only if there was no major clinical heterogeneity in terms of participants' characteristics. We assessed the degree of heterogeneity among the trials by the I2 statistic for each outcome. We judged an I2 value greater than 50% to be indicative of substantial heterogeneity (Higgins 2011). We calculated overall estimates using the fixed‐effect or random‐effects model, depending on the I2 heterogeneity test results and on clinical heterogeneity related to the implementation of interventions and to the characteristics of the participants.
Assessment of reporting biases
We addressed publication bias by means of visual inspection of funnel plots for signs of asymmetry, and generated the funnel plots using Review Manager 5 (RevMan 2014). We explored publication bias on arm motor function instead of disability, as arm motor function was the most frequent outcome assessed by the included studies.
Data synthesis
We pooled outcomes measured with different instruments using SMD. In all analyses with the exception of the subgroup analyses, we used the random‐effects model with 95% CI using Review Manager 5 in order to take into account the clinical heterogeneity among studies (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
There were four possible post‐hoc subgroup analyses (Table 2).
1. Criteria for subgroup analysis.
| Study ID | Dosage of practice | Anatomical restraint | Constraint effect | Time since stroke |
| 1 = 3 hour or less; 2 = more than 3 hours | 1 = only hand; 2 = both arm and hand | 1 = restraint; 2 = restraint plus exercise | 1 = 0 to 3 months; 2 = 3 to 9 months; 3 = more than 9 months; 4 = wide range (from 0.5 to 37 months) | |
| Alberts 2004 | 2 | 1 | 2 | 2 |
| Atteya 2004 | 1 | 2 | 2 | 2 |
| Azab 2009 | 1 | 1 | 2 | 1 |
| Bergheim 2010 | 1 | 1 | 2 | 1 |
| Boake 2007 | 2 | 1 | 2 | 1 |
| Brogårdh 2009 | 1 | 1 | 2 | 1 |
| Brunner 2012 | 1 | 1 | 2 | 1 |
| Dahl 2008 | 2 | 1 | 2 | 4 |
| Dromerick 2000 | 1 | 1 | 2 | 1 |
| Dromerick 2009 | 1 | 1 | 2 | 1 |
| Hammer 2009 | 1 | 2 | 1 | 1 |
| Hayner 2010 | 2 | 1 | 2 | 3 |
| Huseyinsinoglu 2012 | 1 | 1 | 2 | 2 |
| Khan 2011 | 2 | 1 | 2 | 4 |
| Kim 2008 | 1 | 1 | 1 | 3 |
| Krawczyk 2012 | 2 | 2 | 2 | 3 |
| Lin 2007 | 1 | 1 | 2 | 3 |
| Lin 2009a | 1 | 1 | 2 | 4 |
| Lin 2010 | 1 | 1 | 2 | 4 |
| Myint 2008 | 2 | 2 | 2 | 1 |
| Page 2001 | 1 | 2 | 2 | 2 |
| Page 2002b | 1 | 2 | 2 | 2 |
| Page 2004 | 1 | 2 | 2 | 3 |
| Page 2005b | 1 | 1 | 2 | 1 |
| Page 2008 | 1 | 2 | 2 | 3 |
| Ploughman 2004 | 1 | 1 | 2 | 1 |
| Singh 2013 | 1 | 1 | 2 | 1 |
| Smania 2012 | 1 | 1 | 2 | 2 |
| Tariah 2010 | 1 | 1 | 2 | 2 |
| Taub 1993 | 2 | 2 | 2 | 3 |
| Treger 2012 | 1 | 1 | 2 | 1 |
| Van Delden 2013 | 1 | 1 | 2 | 2 |
| Wang 2011 | 1 | 1 | 2 | 1 |
| Wittenberg 2003 | 2 | 2 | 2 | 3 |
| Wolf 2006 | 2 | 1 | 2 | 2 |
| Wu 2007a | 1 | 1 | 2 | 4 |
| Wu 2007b | 1 | 1 | 2 | 4 |
| Wu 2007c | 1 | 1 | 2 | 4 |
| Wu 2011 | 1 | 1 | 2 | 4 |
| Wu 2012a | 1 | 1 | 2 | 4 |
| Yoon 2014 | 2 | 2 | 2 | 1 |
'Dosage of task practice': on the basis of the cut‐off of three hours, which is the difference between CIMT and mCIMT (see 'Types of interventions'), we calculated the dosage of exercise by multiplying the number of weeks by the number of sessions per week by the session duration in hours. We divided trials into those providing more than 30 hours of training, and those providing 30 hours of training or less.
Anatomical region restraint: we divided studies in to those constraining the unaffected arm only at the hand by a mitt, and those constraining both hand and arm by a sling and mitt.
Restraint effect: we included only the studies where the only independent variable between groups was restraint (e.g. where constraint was not accompanied by additional exercise, or the number of hours and type of treatment in the control and constraint groups were the same).
Time since stroke: we used mean time since stroke at recruitment to classify trials into three categories: zero to three months, three months to nine months, and over nine months.
To investigate differences between subgroups, we used the approach for a significance test described by Deeks 2001. This method is implemented in the Review Manager software for fixed‐effect analyses based on the inverse‐variance method (RevMan 2014).
Sensitivity analysis
We conducted sensitivity analyses for the primary outcome to explore the effects of the methodological quality of the included studies on overall effect.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies.
Results of the search
The database searches identified 5863 records, while the searches of the trial registers identified nine records of ongoing, completed or terminated studies.
On the basis of information presented in titles and abstracts, we identified 33 studies as potentially relevant and we obtained the full text papers. Seven papers did not meet at least one of our inclusion criteria: firstly, most studies compared different forms of CIMT, and secondly, they reported data from trials already included in the review.
We included 24 papers that reported 23 trials, and added these to the 19 trials identified in the previous version of this review to give a total of 42 included trials (Figure 1).
1.
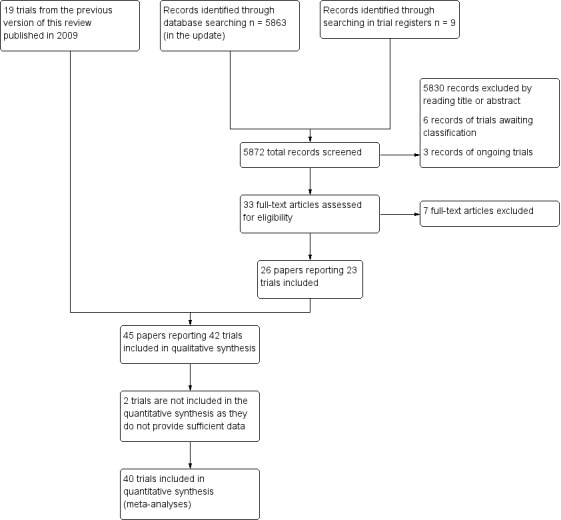
Study flow diagram.
Included studies
A total of 42 published RCTs met the inclusion criteria (Alberts 2004; Atteya 2004; Azab 2009; Boake 2007; Bergheim 2010; Brogårdh 2009; Brunner 2012; Dahl 2008; Dromerick 2000; Dromerick 2009; Hammer 2009; Hayner 2010; Huseyinsinoglu 2012; Khan 2011; Kim 2008; Krawczyk 2012; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Singh 2013; Smania 2012; Suputtitada 2004; Tariah 2010; Taub 1993; Treger 2012; Van Delden 2013; Wang 2011; Wittenberg 2003; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011, Wu 2012a; Yoon 2014).
In 13 studies, participants were randomised to three interventions:
mCIMT, traditional rehabilitation (training without restriction of the sound limb), and no treatment (Atteya 2004; Page 2001; Page 2002b ; Page 2004; Page 2008);
CIMT at low dose versus CIMT at high dose versus control (Dromerick 2009);
mCIMT versus conventional therapy versus therapeutic climbing (Khan 2011);
mCIMT versus bilateral arm training (BAT) versus control (Lin 2009a);
mCIMT versus modified bilateral arm training with rhythmic auditory cueing (BATRAC) versus dose‐matched conventional treatment Van Delden 2013);
CIMT plus mirror therapy versus CIMT versus control (Yoon 2014);
mCIMT versus conventional treatment versus intensive conventional treatment (Wang 2011);
mCIMT versus BAT versus control (Wu 2011);
mCIMT plus trunk restraint versus mCIMT versus control (Wu 2012a).
In order to reduce the heterogeneity among studies and to preserve the equipoise principle, we considered only the data from arms comparing CIMT or mCIMT of FU with traditional rehabilitation (Edwards 1998). For Dromerick 2009 we combined the two experimental groups working at two different regimens into a single group performing mCIMT; in Wang 2011 we considered the intensive conventional group to be the control group.
For more details, see the Characteristics of included studies table.
The studies were conducted in the USA (14 studies), Asia (14 studies) and Europe (14 studies).
Nine were identified as pilot RCTs (Alberts 2004; Brogårdh 2009; Dromerick 2000; Hammer 2009; Khan 2011; Myint 2008; Page 2002b; Page 2005b; Ploughman 2004), although it is not clear whether 'pilot' referred to examination of new CIMT characteristics, to the feasibility of the study, or to the small sample and lack of sample size calculation. Nineteen studies were multicentre.
Participants
A total of 1453 participants were enrolled in the 42 trials. There were more men (n = 934; 64%) than women. The mean age ranged from 37 years to 87 years (Page 2004; Wu 2007c, respectively), with the majority between 55 and 70 years. Time since stroke was zero to three months for 13 trials (Azab 2009; Bergheim 2010; Boake 2007; Brogårdh 2009; Brunner 2012; Dromerick 2000; Dromerick 2009; Myint 2008; Page 2005b; Ploughman 2004; Singh 2013; Treger 2012; Yoon 2014); three to nine months for six trials (Alberts 2004; Atteya 2004; Hammer 2009; Page 2001; Page 2002b; Wolf 2006), and more than nine months for five trials (Lin 2007; Page 2004; Page 2008; Taub 1993; Wittenberg 2003). Eight studies reported time since stroke onset vaguely: in the next days (Khan 2011), more than 1.5 months (Krawczyk 2012), more than two months (Tariah 2010), more than three months (Lin 2010), more than six months (Hayner 2010; Lin 2009a; Wu 2011), and more than one year (Kim 2008). One trial considered participants in which stroke onset varied between 0 to six months (Wang 2011), three trials between one to 37 months (Wu 2007a; Wu 2007b; Wu 2007c), one between one to six months (Van Delden 2013), two between three to 24 months (Huseyinsinoglu 2012; Smania 2012), one study between six to 59 months (Wu 2012a), one trial considered people in which the stroke onset varied between one to 92 months (Dahl 2008), and one between one and 10 years (Suputtitada 2004).
Thirty‐six studies with a total of 1298 participants described the type of stroke: 15 studies included only people with ischaemic stroke (Alberts 2004; Bergheim 2010; Dromerick 2000; Hammer 2009; Hayner 2010; Krawczyk 2012; Lin 2010; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Tariah 2010; Taub 1993; Treger 2012), while the remaining 21 trials enrolled people with haemorrhagic and ischaemic stroke (Boake 2007; Brunner 2012; Dahl 2008; Dromerick 2009; Huseyinsinoglu 2012; Kim 2008; Lin 2007; Lin 2009a; Myint 2008; Ploughman 2004; Singh 2013; Smania 2012; Suputtitada 2004; Van Delden 2013; Wang 2011; Wolf 2006; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a; Yoon 2014).
Fifty‐six per cent (n = 729) of the participants had an ischaemic stroke, the remaining 44% (n = 569) had a haemorrhagic stroke.
Thirty‐three studies, with a total of 1011 participants, reported the number of people with the right‐side affected (n = 627; 62%; Alberts 2004; Atteya 2004; Azab 2009; Boake 2007; Bergheim 2010; Brogårdh 2009; Brunner 2012; Dromerick 2000; Dromerick 2009; Hammer 2009; Khan 2011; Krawczyk 2012; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Smania 2012; Suputtitada 2004; Tariah 2010; Taub 1993; Van Delden 2013; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a; Yoon 2014).
Nine studies, with a total of 524 participants, reported the number of people presenting with paresis of pre‐stroke dominant side (n = 260; 50%; Alberts 2004; Huseyinsinoglu 2012; Myint 2008; Taub 1993; Van Delden 2013; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2007c).
The main inclusion criteria reported were as follows.
-
Movement capacity of the upper arm:
ability to extend actively the metacarpophalangeal and interphalangeal joints at least 10°, and the wrist 20° (Alberts 2004; Atteya 2004; Bergheim 2010; Boake 2007; Dahl 2008; Hammer 2009; Huseyinsinoglu 2012; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Taub 1993; Suputtitada 2004; Tariah 2010; Wang 2011; Wittenberg 2003; Wolf 2006; Wu 2007a);
ability to extend actively the metacarpophalangeal and interphalangeal joints and the wrist at least 10° (Singh 2013; Smania 2012);
ability to extend the metacarpophalangeal and interphalangeal joints of two digits and the wrist 10°, plus 10° of thumb abduction/extension (Alberts 2004; Brogårdh 2009; Brunner 2012; Smania 2012; Van Delden 2013; Wolf 2006; Yoon 2014);
trace of movements of the hand and some fingers dexterity preserved (Azab 2009; Hayner 2010; Kim 2008);
ability to lift a floppy disc off the table top and to release it afterwards (Krawczyk 2012);
score 1 to 3 on the motor arm items of the National Institute of Health Stroke Scale (NIHSS; Boake 2007; Dromerick 2000);
stage 3 or above in the reach Brunnstrom for the proximal part of the upper extremity (Lin 2007; Lin 2009a; Wu 2007b; Wu 2007c; Wu 2012a);
stage 2 to 6 on the Chedoke McMaster Impairment Inventory (CMII; Khan 2011; Ploughman 2004);
score 0 to 2 on Modified Rankin Scale before the stroke (Dahl 2008).
-
Absence of cognitive impairment:
Mini Mental State Examination (MMSE) or modified MMSE more than 24 or 70 respectively (Alberts 2004; Atteya 2004; Brogårdh 2009; Brunner 2012; Dahl 2008; Dromerick 2009; Hammer 2009; Hayner 2010; Huseyinsinoglu 2012; Krawczyk 2012; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Singh 2013; Smania 2012; Suputtitada 2004; Tariah 2010; Taub 1993; Van Delden 2013; Wang 2011; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a);
no neglect or speech comprehension difficulties (Boake 2007; Brogårdh 2009; Dahl 2008; Hammer 2009; Huseyinsinoglu 2012; Kim 2008; Krawczyk 2012; Singh 2013; Suputtitada 2004; Taub 1993; Treger 2012; Van Delden 2013; Wang 2011; Yoon 2014);
score ≤ 1 on the consciousness, communication and neglect item of the NIHSS (Dromerick 2000).
Non‐use of the affected arm in the real world: score < 2.5 on the MAL (Alberts 2004; Huseyinsinoglu 2012; Lin 2007; Lin 2009a; Page 2005b; Page 2008; Smania 2012; Tariah 2010; Wittenberg 2003; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2011; Wu 2012a).
No balance problems including walking (Alberts 2004; Brogårdh 2009; Hammer 2009; Hayner 2010; Huseyinsinoglu 2012; Kim 2008; Krawczyk 2012; Lin 2007; Lin 2009a; Myint 2008; Smania 2012; Suputtitada 2004; Tariah 2010; Taub 1993; Wang 2011; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2011; Wu 2012a).
No excessive pain in the affected arm: score < 4 on the visual analogue scale (Alberts 2004; Atteya 2004; Huseyinsinoglu 2012; Khan 2011; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Singh 2013; Tariah 2010; Wang 2011; Wolf 2006).
No excessive spasticity: score ≤ 2 (in any joint) respectively on the Ashworth Scale or on the modified Ashworth Scale (Atteya 2004; Hammer 2009; Huseyinsinoglu 2012; Lin 2007; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Singh 2013; Tariah 2010; Taub 1993; Wang 2011; Wu 2007a; Wu 2007c).
No joint limitation of the affected arm (Alberts 2004; Boake 2007; Wolf 2006).
Intervention
Nine studies, with a total of 416 participants, focused on the efficacy of CIMT (Alberts 2004; Dahl 2008; Hayner 2010; Krawczyk 2012; Myint 2008; Taub 1993; Wang 2011; Wittenberg 2003; Wolf 2006), while 29 studies, with a total of 943 participants, focused on the efficacy of mCIMT (Atteya 2004; Azab 2009; Bergheim 2010; Boake 2007; Brunner 2012; Dromerick 2000; Dromerick 2009; Huseyinsinoglu 2012; Khan 2011; Lin 2007; Lin 2009a; Lin 2010; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Singh 2013; Smania 2012; Suputtitada 2004; Tariah 2010; Treger 2012; Van Delden 2013; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a; Yoon 2014). Four studies, with 94 participants, investigated the efficacy of FU (Brogårdh 2009; Hammer 2009; Kim 2008; Ploughman 2004).
Time of restraint:
During waking hours for one study (Wittenberg 2003);
90% of waking hours for eleven studies (Alberts 2004; Boake 2007; Brogårdh 2009; Dahl 2008; Huseyinsinoglu 2012; Myint 2008; Singh 2013; Smania 2012; Taub 1993; Wang 2011; Wolf 2006);
from six hours per day to 90% of waking hours for one study (Dromerick 2009);
from six to seven hours per day for two studies (Azab 2009; Bergheim 2010);
six hours per day for 14 studies (Dromerick 2000; Hammer 2009; Hayner 2010; Lin 2007; Lin 2009a; Lin 2010; Suputtitada 2004; Van Delden 2013; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a; Yoon 2014);
five hours per day for eight studies (Atteya 2004; Kim 2008; Krawczyk 2012; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008);
four to five hours per day for one study (Khan 2011);
four hours per day for two studies ( Brunner 2012; Treger 2012);
two hours per day for one study (Tariah 2010);
a mean effective restraint time of 2.7 hours per day was reported by one study (Ploughman 2004).
Time of exercise with the affected arm:
between 30 and 45 hours/week in seven studies (Alberts 2004; Dahl 2008; Hayner 2010; Suputtitada 2004; Taub 1993; Wittenberg 2003; Wolf 2006);
between 10 and 25 hours/week in 20 studies (Boake 2007; Brunner 2012; Dromerick 2000; Dromerick 2009; Huseyinsinoglu 2012; Khan 2011; Krawczyk 2012; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Singh 2013; Smania 2012; Tariah 2010; Wang 2011; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Yoon 2014);
five hours/week or less in 11 studies (Atteya 2004; Azab 2009; Bergheim 2010; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Treger 2012; Van Delden 2013).
Treatment duration:
two weeks in 19 studies (Alberts 2004; Bergheim 2010; Boake 2007; Brogårdh 2009; Dahl 2008; Dromerick 2000; Dromerick 2009; Hammer 2009; Hayner 2010; Huseyinsinoglu 2012; Myint 2008; Singh 2013; Smania 2012; Suputtitada 2004; Taub 1993; Treger 2012; Wittenberg 2003; Wolf 2006; Yoon 2014);
three weeks for nine studies (Krawczyk 2012; Lin 2007; Lin 2009a; Lin 2010; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a);
four weeks for three studies (Azab 2009; Brunner 2012; Wang 2011);
six weeks for one study (Van Delden 2013);
eight weeks for two studies (Kim 2008; Tariah 2010);
10 weeks for six studies (Atteya 2004; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008).
One study did not report the treatment duration (Khan 2011).
Types of exercise:
all studies used functional or ADL tasks: in 19 studies this was done through shaping techniques (Alberts 2004; Bergheim 2010; Brunner 2012; Boake 2007; Dromerick 2009; Hayner 2010; Huseyinsinoglu 2012; Kim 2008; Lin 2010; Myint 2008; Page 2002b; Page 2004; Page 2005b; Page 2008; Smania 2012; Tariah 2010; Van Delden 2013; Wolf 2006; Wu 2007a);
two studies included proprioceptive neuromuscular facilitation (PNF; Atteya 2004; Page 2001);
one study used conventional treatment for upper extremity, which involved the facilitation of proximal motor control progressing to skilled‐task training, without shaping therapy (Ploughman 2004).
Anatomical region restraint:
both hand and arm in 12 studies (Atteya 2004; Hammer 2009; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2008; Ploughman 2004; Taub 1993; Wang 2011; Wittenberg 2003; Yoon 2014);
only the hand in the remaining 30 studies.
Intervention delivery
In all studies the interventions were delivered and supervised by trained physiotherapists or occupational therapists, and each participant assigned to an intervention group participated in individual therapy sessions, except in Dahl 2008 and Suputtitada 2004 where the participants exercised in groups of four. The wearing of the constraint was checked by questioning the participants every two weeks about satisfaction with the protocol (Atteya 2004), keeping a log of the hours of restraint per day (Azab 2009; Brogårdh 2009; Brunner 2012; Hammer 2009; Lin 2009a; Myint 2008; Page 2002a; Page 2004; Page 2005b; Ploughman 2004; Singh 2013; Smania 2012; Tariah 2010; Treger 2012; Wang 2011; Wu 2011; Wu 2012a), and through a physical sensor and timer placed in the mitt and by a home diary (Wolf 2006). Supervision of the constraint was not described in the other studies.
Twenty‐four studies included outpatients (Alberts 2004; Atteya 2004; Azab 2009; Hayner 2010; Huseyinsinoglu 2012; Kim 2008; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Smania 2012; Suputtitada 2004; Tariah 2010; Taub 1993; Wang 2011; Wolf 2006; Wu 2007b; Wu 2011; Wu 2012a), 11 studies included only inpatients (Bergheim 2010; Brogårdh 2009; Dahl 2008; Dromerick 2000; Dromerick 2009; Khan 2011; Krawczyk 2012; Singh 2013; Treger 2012; Wittenberg 2003; Yoon 2014), six studies included both inpatients and outpatients (Boake 2007; Brunner 2012; Hammer 2009; Ploughman 2004; Wu 2007a; Wu 2007c), and one study did not specify (Van Delden 2013).
Outcomes
All studies considered pre‐treatment and post‐treatment outcome measures. Seventeen studies had longer follow‐up:
one month (Van Delden 2013);
one and three months (Hammer 2009);
three months (Bergheim 2010; Boake 2007; Brogårdh 2009; Dromerick 2009; Smania 2012);
four months (Tariah 2010);
six months (Azab 2009; Dahl 2008; Hayner 2010; Khan 2011; Wittenberg 2003);
12 months (Krawczyk 2012; Myint 2008);
at four, eight and 12 months (Wolf 2006);
up to three years (Taub 1993).
The 42 included trials considered similar outcome categories. We attributed measures used in the studies to each outcome category as detailed below and in Table 3.
2. Outcome measures used in the included studies.
| Study ID | Arm motor function | Perceived motor function | Dexterity | Arm motor impairment | Activities of daily living measures | Quality of life | Kinematics | Neurophysiologics | Strength |
| Alberts 2004 | Wolf Motor Function Test | Fugl Meyer Assessment | Hand dynamometer | ||||||
| Atteya 2004 | Action Research Arm Test, Wolf Motor Function Test | Motor Activity Log | Fugl Meyer Assessment | ||||||
| Azab 2009 | Bartel Index | ||||||||
| Bergheim 2010 | Wolf Motor Function Test, Motor Assessment Scale |
||||||||
| Boake 2007 | Motor Activity Log | Grooved Pegboard Test | Fugl Meyer Assessment | Transcranial magnetic stimulation | |||||
| Brogårdh 2009 | Motor Assessment Scale, Sollerman Hand Function Scale |
Motor Activity Log | |||||||
| Brunner 2012 | Action Research Arm Test | Nine‐Hole Peg Test | |||||||
| Dahl 2008 | Wolf Motor Function Test | Motor Activity Log | Functional Independence Measure | Stroke Impact Scale | |||||
| Dromerick 2000 | Action Research Arm Test | ||||||||
| Dromerick 2009 | Action Research Arm Test | Functional Independence Measure | Stroke Impact Scale | ||||||
| Hammer 2009 | Action Research Arm Test, Motor Assessment Scale |
Motor Activity Log | Sixteen‐Hole Peg Test | Fugl Meyer Assessment | Grippit | ||||
| Hayner 2010 | Wolf Motor Function Test | ||||||||
| Huseyinsinoglu 2012 | Wolf Motor Function Test | Motor Activity Log | Functional Independence Measure | ||||||
| Khan 2011 | Wolf Motor Function Test | Motor Activity Log | |||||||
| Kim 2008 | Manual Function Test | Motor Activity Log | Perdue Pegboard Test | ||||||
| Krawczyk 2012 | Rivermead motor assessment arm scale | Motor Activity Log | |||||||
| Lin 2007 | Motor Activity Log | Functional Independence Measure | Yes | ||||||
| Lin 2009a | Motor Activity Log | Fugl Meyer Assessment | Functional Independence Measure | Stroke Impact Scale | |||||
| Lin 2010 | Motor Activity Log | Fugl Meyer Assessment | Functional magnetic resonance | ||||||
| Myint 2008 | Action Research Arm Test | Motor Activity Log | Nine‐Hole Peg Test | Bartel Index | |||||
| Page 2001 | Action Research Arm Test, Wolf Motor Function Test | Motor Activity Log | Fugl Meyer Assessment | ||||||
| Page 2002b | Action Research Arm Test | Motor Activity Log | Fugl Meyer Assessment | ||||||
| Page 2004 | Action Research Arm Test | Motor Activity Log | Fugl Meyer Assessment | ||||||
| Page 2005b | Action Research Arm Test | Motor Activity Log | Fugl Meyer Assessment | ||||||
| Page 2008 | Action Research Arm Test | Motor Activity Log | Fugl Meyer Assessment | ||||||
| Ploughman 2004 | Action Research Arm Test | Chedoke McMaster Impairment Inventory | Functional Independence Measure | Jamar | |||||
| Singh 2013 | Wolf Motor Function Test (only time) | Fugl Meyer Assessment | |||||||
| Smania 2012 | Wolf Motor Function Test | Motor Activity Log | |||||||
| Tariah 2010 | Wolf Motor Function Test | Motor Activity Log | Fugl Meyer Assessment | ||||||
| Taub 1993 | Emory Motor Function Test | Motor Activity Log | |||||||
| Treger 2012 | Manual Function Test | Functional Independence Measure | |||||||
| Van Delden 2013 | Action Research Arm Test | Motor Activity Log | Nine‐Hole Peg Test | Fugl Meyer Assessment, Motricity Index |
Stroke Impact Scale | ||||
| Wang 2011 | Wolf Motor Function Test | ||||||||
| Wittenberg 2003 | Wolf Motor Function Test | Motor Activity Log | Transcranial magnetic stimulation, positron emission tomography | ||||||
| Wolf 2006 | Wolf Motor Function Test | Motor Activity Log | Stroke Impact Scale | ||||||
| Wu 2007a | Motor Activity Log | Functional Independence Measure | Stroke Impact Scale | Yes | |||||
| Wu 2007b | Motor Activity Log | Fugl Meyer Assessment | Yes | ||||||
| Wu 2007c | Motor Activity Log | Fugl Meyer Assessment | Functional Independence Measure | Stroke Impact Scale | |||||
| Wu 2011 | Wolf Motor Function Test | Motor Activity Log | Yes | ||||||
| Wu 2012a | Action Research Arm Test | Motor Activity Log | Stroke Impact Scale | ||||||
| Yoon 2014 | Wolf Motor Function Test | Nine‐Hole Peg Test, Box and Block Test |
Fugl Meyer Assessment | Bartel Index | Hand Dynamometer |
Primary outcomes
-
Disability:
Functional Independence Measure (FIM): nine studies (Dahl 2008; Dromerick 2009; Huseyinsinoglu 2012; Lin 2007; Lin 2009a; Ploughman 2004; Treger 2012; Wu 2007a; Wu 2007c);
Barthel Index (BI): three studies (Azab 2009; Myint 2008; Yoon 2014).
Secondary outcomes
-
Arm motor function:
Action Research Arm Test (ARAT): 14 studies (Atteya 2004; Brunner 2012; Dromerick 2000; Dromerick 2009; Hammer 2009; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Van Delden 2013; Wu 2012a);
Wolf Motor Function Test (WMFT): 14 studies (Alberts 2004; Atteya 2004; Dahl 2008; Hayner 2010; Huseyinsinoglu 2012; Khan 2011; Singh 2013; Smania 2012; Tariah 2010; Wittenberg 2003; Wang 2011; Wolf 2006; Wu 2011; Yoon 2014);
Emory Motor Function test (EMF): one study (Taub 1993);
Manual Function Test (MFT): two studies (Kim 2008; Treger 2012);
The Rivermead Motor Assessment Arm scale: one study (Krawczyk 2012);
Motor Assessment Scale: one study (Brogårdh 2009).
-
Perceived motor function, amount of use and quality of use:
Motor Activity Log (MAL): 29 studies (Atteya 2004; Boake 2007; Brogårdh 2009; Dahl 2008; Hammer 2009; Huseyinsinoglu 2012; Khan 2011; Kim 2008; Krawczyk 2012; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Smania 2012; Tariah 2010; Taub 1993; Van Delden 2013; Wittenberg 2003; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a).
-
Arm motor impairment:
Fugl‐Meyer Assessment (FMA): 17 studies (Alberts 2004; Atteya 2004; Boake 2007; Hammer 2009; Lin 2009a; Lin 2010; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Singh 2013; Tariah 2010; Van Delden 2013; Wu 2007b; Wu 2007c; Yoon 2014);
Chedoke McMaster Impairment Inventory (CMII): three studies (Ploughman 2004; Tariah 2010; Van Delden 2013);
Birgitta Lind Marks Assessment Motor (BLMA): one study (Krawczyk 2012);
Jamar hand dynamometer: one study (Ploughman 2004);
maximal grip strength with a force transducer: three studies (Alberts 2004; Van Delden 2013; Yoon 2014);
shoulder and elbow isometric force: one study (Khan 2011).
-
Dexterity:
Grooved Pegboard Test (GPT): one study (Boake 2007);
Nine‐Hole Peg Test (NHPT): four studies (Brunner 2012; Myint 2008; Van Delden 2013; Yoon 2014);
Sixteen‐Hole Peg Test: one study (Hammer 2009);
Box and block test: one study (Yoon 2014);
Perdue Pegboard Test: one study (Kim 2008).
-
Quality of life:
Stroke Impact Scale (SIS): seven studies (Dahl 2008; Dromerick 2009; Lin 2009a; Van Delden 2013; Wolf 2006; Wu 2007c; Wu 2012a).
Excluded studies
We excluded 12 studies after reading the full text as they did not meet our inclusion criteria. We have provided all the reasons for these exclusions in Characteristics of excluded studies.
Risk of bias in included studies
Refer to Figure 2 or Figure 3 and Characteristics of included studies. If required, we contacted the corresponding author of the relevant studies for further information.
2.
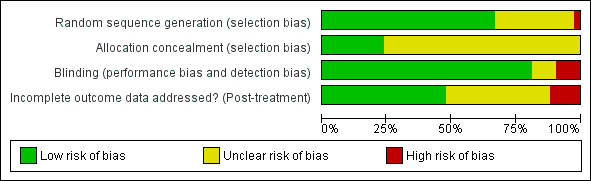
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
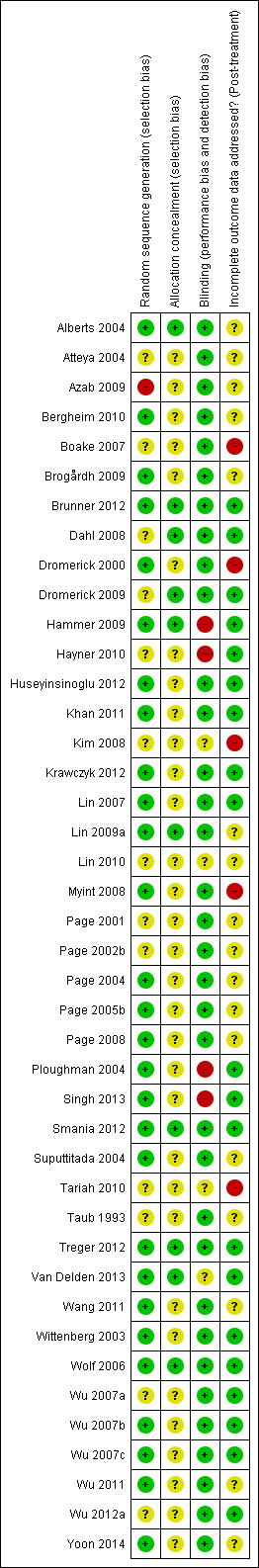
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation
The sequence of randomisation was described and appropriate in 27 studies (Alberts 2004; Bergheim 2010; Brogårdh 2009; Brunner 2012; Dromerick 2000; Hammer 2009; Huseyinsinoglu 2012; Khan 2011; Krawczyk 2012; Lin 2007; Lin 2009a; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Singh 2013; Smania 2012; Suputtitada 2004; Treger 2012; Van Delden 2013; Wang 2011; Wittenberg 2003; Wolf 2006; Wu 2007b; Wu 2007c; Wu 2011; Yoon 2014). Lin 2007 used a randomisation stratified by side of stroke; Alberts 2004 and Wolf 2006 balanced the randomisation with respect to gender, premorbid handedness, side of stroke and level of function; Boake 2007 stratified by age and NIHSS score, and Dromerick 2009 balanced for age, total NIHSS score, pretest ARAT and days from stroke onset. Prestratification was applied to the participants based on whether they had received botulinum A injection in Huseyinsinoglu 2012. Van Delden 2013 stratified the participants according to whether they had higher functional ability or lower functional ability of the arm. In Hayner 2010 study participants were stratified into more and less affected.
We considered one study at high risk of bias because only a keyword of the article referred to randomisation (Azab 2009). We considered other studies at unclear risk of bias mainly because they provided insufficient data.
Allocation concealment
Allocation concealment was described and appropriate in 10 studies (Alberts 2004; Brunner 2012; Dahl 2008; Hammer 2009; Khan 2011; Lin 2009a; Smania 2012; Treger 2012; Van Delden 2013; Wolf 2006); the remaining studies did not report sufficient information.
Blinding
Outcome assessors were blinded in 34 studies. In Hammer 2009, Hayner 2010, Ploughman 2004 and Singh 2013 the assessor was not blinded, and blinding was not described in the remaining four studies (Kim 2008; Lin 2010; Tariah 2010; Van Delden 2013).
Incomplete outcome data
Sixteen studies provided complete information about participants who withdrew and their reasons (Boake 2007; Brunner 2012; Hammer 2009; Hayner 2010; Huseyinsinoglu 2012; Khan 2011; Kim 2008; Krawczyk 2012; Lin 2007; Myint 2008; Ploughman 2004; Singh 2013; Smania 2012; Treger 2012; Van Delden 2013; Wolf 2006); four studies provided numbers of withdrawals but not reasons (Azab 2009; Dromerick 2000; Dromerick 2009; Tariah 2010); 16 studies presented unclear information about withdrawals: none of these clearly stated that there were no dropouts (Alberts 2004; Atteya 2004; Brogårdh 2009; Lin 2009a; Lin 2010; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Suputtitada 2004; Tariah 2010; Taub 1993; Wang 2011; Wu 2011; Yoon 2014 ). In one study one participant was excluded from the analyses post‐hoc because he had received botulinum toxin type A in the more affected limb less than three months before the study (Page 2004).
The remaining six studies had no drop‐outs.
By post‐treatment follow‐up nine studies had lost less than 10% of participants (Brogårdh 2009; Brunner 2012; Dromerick 2009; Hayner 2010; Huseyinsinoglu 2012; Khan 2011; Lin 2007; Van Delden 2013; Wolf 2006); six studies had lost between 10% and 20% of participants (Dromerick 2000; Hammer 2009; Krawczyk 2012; Myint 2008; Ploughman 2004; Smania 2012); and two studies had lost more than 20% of participants (Boake 2007; Kim 2008).
At long‐term follow‐up, Myint 2008 and Hammer 2009 had lost less than 10% of participants, while Azab 2009, Boake 2007, Brogårdh 2009, Krawczyk 2012, and Wolf 2006 had lost between 10% and 20% of participants.
Three studies performed intention‐to‐treat analyses (Alberts 2004; Smania 2012; Wolf 2006). Twenty‐five studies that did not have apparent withdrawals performed analyses on all included participants (Atteya 2004; Azab 2009; Bergheim 2010; Brogårdh 2009; Brunner 2012; Dromerick 2009; Dahl 2008; Krawczyk 2012; Lin 2009a; Lin 2010; Page 2001; Page 2002b; Page 2005b; Page 2008; Singh 2013; Suputtitada 2004; Treger 2012; Wang 2011; Wittenberg 2003; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a; Yoon 2014). One study mixed intention‐to‐treat and per‐protocol analyses (Boake 2007). The others performed per‐protocol analyses (Dromerick 2000; Hammer 2009; Hayner 2010; Huseyinsinoglu 2012; Khan 2011; Kim 2008; Lin 2007; Myint 2008; Page 2004; Ploughman 2004;Taub 1993; Tariah 2010; Van Delden 2013).
Validity of scales
All scales used in the studies for primary and secondary outcomes were supported by references to their psychometric properties, and were considered able to quantify performance in individuals after stroke with motor characteristics similar to the people enrolled in the included studies. The study on clinimetric properties of the MAL scale reports relatively stable internal consistency in a population of chronic stroke patients, a correlation with ARAT score at baseline (Spearman's rho was 0.63 for AoU and QoU), but considerable doubts remain about the longitudinal construct validity of the instrument, and the study does not recommend its use as a primary outcome measure in trials (Van der Lee 2004).
Other potential sources of bias
Six trials based their sample size on prior statistical power calculations (Brogårdh 2009; Brunner 2012; Smania 2012; Treger 2012; Van Delden 2013; Wolf 2006). Most studies were very small; the median sample size was 29 randomised participants (interquartile range 16 to 44). Small sample sizes are related to type 2 errors (Altman 1990; Hotopf 1997; Hotopf 1999), so if the median number of participants randomised is 29, then the complete analysis will only include around 15 participants per group.
Publication bias and small study effects
Visual inspection of the funnel plot indicated that pooled data might have been influenced by publication bias (Figure 4). Slight asymmetry of the plot is possible, with few studies characterised by extreme statistically significant results, largely favouring CIMT. It is also possible that others studies are 'missing' from the opposite area, which is in favour of the control. Another possible reason for slight asymmetry could be related to the large number of small trials we identified. Their methodological components for random sequence generation, allocation concealment and double blinding might have been inadequate. The reporting of most studies was largely unsatisfactory, preventing us from making full judgements of methods. These potential methodological shortcomings can be associated with exaggerated estimates of benefits of treatment.
4.
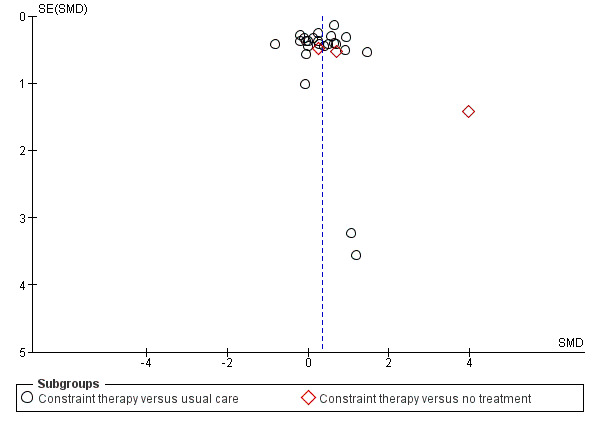
Funnel plot of comparison: 3 Constraint versus control: secondary outcomes, outcome: 3.1 Arm Motor Function.
Studies awaiting assessment
Six studies are awaiting assessment because information that is currently available about them is insufficient to determine whether they would be eligible for inclusion in this review. Five studies are labelled as 'completed' or 'terminated' on ClinicalTrials.gov (Barzel 2015; Boe 2014; Dos Santos 2012; Olivier 2012; Uswatte 2014), and one has been published as a poster (Jansa 2007).
Three studies are ongoing and recruiting (Gautier 2015; Padovani Do Santos 2015; Pereira 2015).
Effects of interventions
See: Table 1
We conducted meta‐analyses when at least two studies provided sufficient data. We included trials that compared the intervention versus no treatment, or no active treatment, in a specific subgroup to show how the estimated overall effect was based on information provided by these studies (Alberts 2004; Kim 2008; Taub 1993; Wittenberg 2003). In consideration of the clinical heterogeneity among studies, which related to variability in the interventions included and in the patient case‐mix, we considered it appropriate to perform random‐effects meta‐analyses to incorporate heterogeneity, except within subgroup analyses.
Fourteen trials monitored the presence of adverse events or medical complications leading to dropouts (Boake 2007; Brunner 2012; Dahl 2008; Dromerick 2000; Dromerick 2009; Hammer 2009; Khan 2011; Kim 2008; Krawczyk 2012; Myint 2008; Page 2008; Ploughman 2004; Smania 2012; Wolf 2006). Six of these studies monitored and reported on adverse events (Boake 2007; Dahl 2008; Dromerick 2000; Page 2008; Ploughman 2004; Wolf 2006), and four stated that none occurred (Boake 2007; Dahl 2008; Page 2008; Ploughman 2004). Rates of adverse events among these studies appeared not to differ between CIMT and the comparison groups, and CIMT appeared to have no adverse effects.
Primary outcomes
Comparison 1.1: Disability post‐intervention
Twelve studies with 411 participants measured disability immediately after the experimental and control interventions (Azab 2009; Dahl 2008; Dromerick 2009; Huseyinsinoglu 2012; Lin 2007; Lin 2009a; Myint 2008; Ploughman 2004; Treger 2012; Wu 2007a; Wu 2007c; Yoon 2014). Data were available for 344 participants (84%) from 11 studies. The impact of CIMT on disability indicated a non‐significant effect (SMD 0.24, 95% CI ‐0.05 to 0.52; Analysis 1.1).
1.1. Analysis.
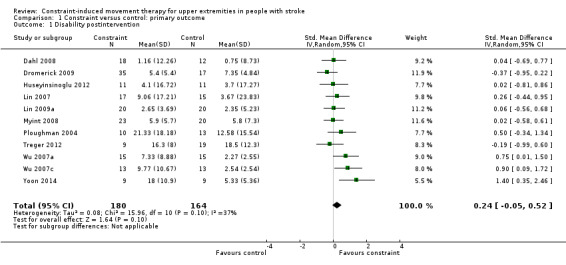
Comparison 1 Constraint versus control: primary outcome, Outcome 1 Disability postintervention.
Sixty‐nine participants contributing to this meta‐analysis were recruited from studies with more than a 10% loss to follow‐up.
Comparison 1.2: Disability at three‐ and six‐month follow‐up
Three studies recruiting 125 participants measured disability at three months (Dromerick 2009; Myint 2008), or at six months after treatment (Dahl 2008). The impact of CIMT on disability indicated a non‐significant effect (SMD ‐0.21, 95% CI ‐0.57 to 0.16, Analysis 1.2).
1.2. Analysis.
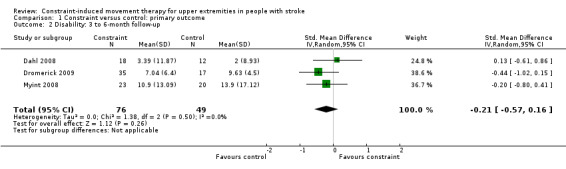
Comparison 1 Constraint versus control: primary outcome, Outcome 2 Disability: 3 to 6‐month follow‐up.
Subgroup analysis: Disability
We carried out analyses for the following three subgroups considering data availability.
'Dosage of task practice': we grouped trials according to whether they provided 30 or more hours of exercise, or up to 30 hours of exercise.
Anatomical region restraint: we grouped trials according to whether both arm and hand were restrained, or only the hand.
Time since stroke: we grouped trials according to whether they recruited within three months, three to nine months, or more than nine months post stroke.
Comparison 2.1: Amount of task practice
Three studies with 91 participants reported over 30 hours of exercise (Dahl 2008; Myint 2008; Yoon 2014); eight studies with 253 participants reported 30 hours or less of exercise (Dromerick 2009; Huseyinsinoglu 2012; Lin 2007; Lin 2009a; Ploughman 2004; Treger 2012; Wu 2007a; Wu 2007c). Longer exercise for upper limb function showed no statistically significant effect size (SMD 0.25, 95% CI ‐0.18 to 0.67); shorter exercise had a non‐significant effect size (SMD 0.18, 95% CI ‐0.07 to 0.44; Analysis 2.1). The difference between the two groups of trials was not significant (P value 0.8).
2.1. Analysis.
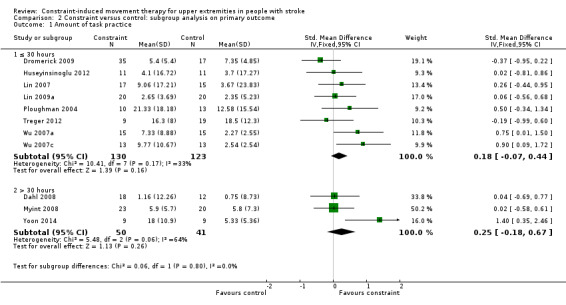
Comparison 2 Constraint versus control: subgroup analysis on primary outcome, Outcome 1 Amount of task practice.
Comparison 2.2: Anatomical region restraint
Two studies with 61 participants reported both arm and hand restriction (Myint 2008; Yoon 2014); nine studies including 283 participants reported only hand restriction (Dahl 2008; Dromerick 2009; Huseyinsinoglu 2012; Lin 2007; Lin 2009a; Ploughman 2004; Treger 2012; Wu 2007a; Wu 2007c). The restriction of both arm and hand for upper limb function showed a non‐statistically significant effect size (SMD 0.35, 95% CI ‐0.17 to 0.87); restriction of the hand only was non‐statistically significant (SMD 0.17, 95% CI ‐0.08 to 0.41; Analysis 2.2). The difference between the effect estimates for the two groups of trials was not significant (P value 0.53).
2.2. Analysis.
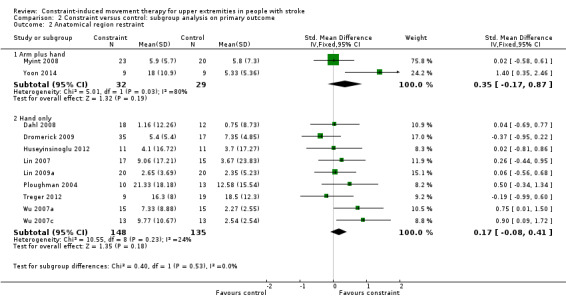
Comparison 2 Constraint versus control: subgroup analysis on primary outcome, Outcome 2 Anatomical region restraint.
Comparison 2.3: Time since stroke
Five studies with 164 participants measured disability on people with stroke at zero to three months (Myint 2008; Ploughman 2004; Treger 2012; Yoon 2014); five studies with 176 participants measured it at more than nine months (Dahl 2008; Huseyinsinoglu 2012; Lin 2007; Lin 2009a; Wu 2007a).
No studies measured disability on people with subacute stroke at three to nine months. We did not include four studies in this subgroup analysis because of the wide range of chronicity of participants (Dahl 2008; Huseyinsinoglu 2012; Lin 2009a; Wu 2007c). People with acute and chronic stroke showed no statistically significant effect size: for zero to three months (SMD 0.07, 95% CI ‐0.26 to 0.39) or more than nine months (SMD 0.49 CI ‐0.02 to 1.00; Analysis 2.3). The difference between the effect estimates for the two groups of trials was not significant (P value 0.17). We did not find heterogeneity among studies (I2 = 47.2%).
2.3. Analysis.
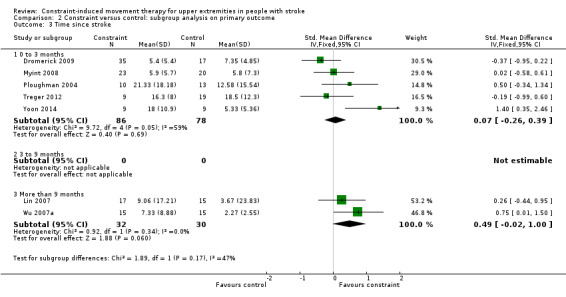
Comparison 2 Constraint versus control: subgroup analysis on primary outcome, Outcome 3 Time since stroke.
The comparison for the restraint effect could not be performed because of insufficient data.
Secondary outcomes
Comparison 3.1: Arm motor function
Thirty‐four studies with 988 participants measured arm motor function (Alberts 2004; Atteya 2004; Bergheim 2010; Brogårdh 2009; Brunner 2012; Dahl 2008; Dromerick 2000; Dromerick 2009; Hammer 2009; Hayner 2010; Huseyinsinoglu 2012; Khan 2011; Kim 2008; Krawczyk 2012; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Singh 2013; Smania 2012; Tariah 2010; Taub 1993; Treger 2012; Van Delden 2013; Suputtitada 2004; Wang 2011; Wittenberg 2003; Wolf 2006; Wu 2011; Wu 2012a; Yoon 2014). Data were available for 858 participants (87%). The impact of CIMT on upper limb function indicated a significant effect size (SMD 0.34, 95% CI 0.12 to 0.55; Analysis 3.1). We found moderate heterogeneity among studies (I2 = 47%).
3.1. Analysis.
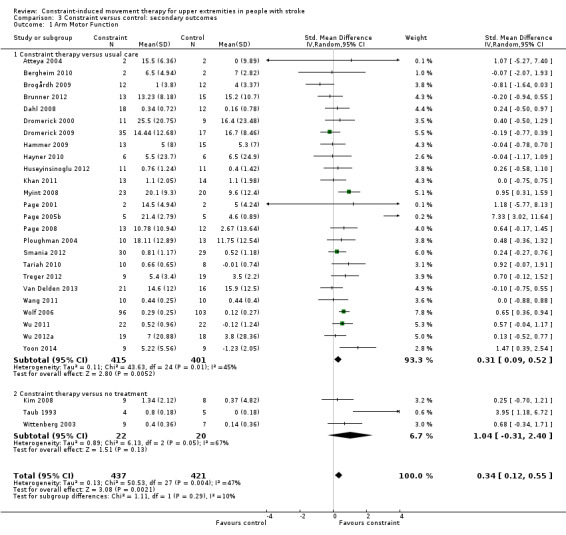
Comparison 3 Constraint versus control: secondary outcomes, Outcome 1 Arm Motor Function.
Comparison 3.2: Perceived arm motor function (quality of use (QoU))
Twenty‐nine studies with 1086 participants measured perceived arm motor function QoU (Atteya 2004; Boake 2007; Brogårdh 2009; Brunner 2012; Dahl 2008; Hammer 2009; Huseyinsinoglu 2012; Khan 2011; Kim 2008; Krawczyk 2012; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Smania 2012; Tariah 2010; Taub 1993; Van Delden 2013; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a); data were available for 891 participants (82%). The impact of CIMT on perceived upper limb function QoU indicated a large and significant effect (MD 0.68, 95% CI 0.47 to 0.88; Analysis 3.2). We found considerable heterogeneity among studies (I2 = 74%).
3.2. Analysis.
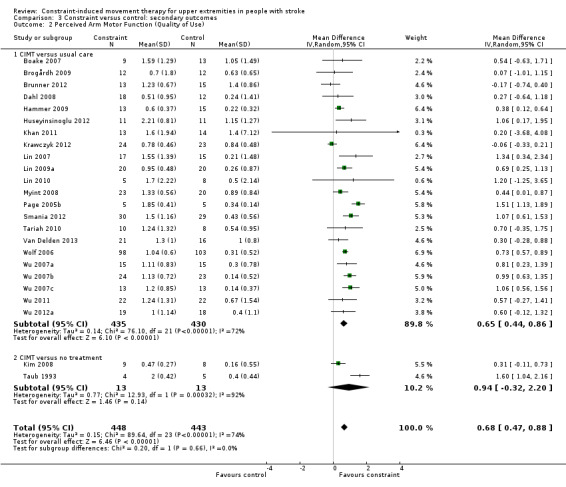
Comparison 3 Constraint versus control: secondary outcomes, Outcome 2 Perceived Arm Motor Function (Quality of Use).
Comparison 3.3: Perceived arm motor function (amount of use (AoU))
Twenty‐eight studies with 1046 participants measured perceived arm motor function (AoU; Atteya 2004; Boake 2007; Brogårdh 2009; Brunner 2012; Dahl 2008; Hammer 2009; Huseyinsinoglu 2012; Khan 2011; Kim 2008; Lin 2007; Lin 2009a; Lin 2010; Myint 2008; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Smania 2012; Tariah 2010; Van Delden 2013; Wittenberg 2003; Wolf 2006; Wu 2007a; Wu 2007b; Wu 2007c; Wu 2011; Wu 2012a); data were available for 851 participants (81%). The impact of CIMT on perceived upper limb function AoU indicated a large and significant effect (MD 0.79, 95% CI 0.50 to 1.08; Analysis 3.3). We found considerable heterogeneity among studies (I2 = 87%).
3.3. Analysis.
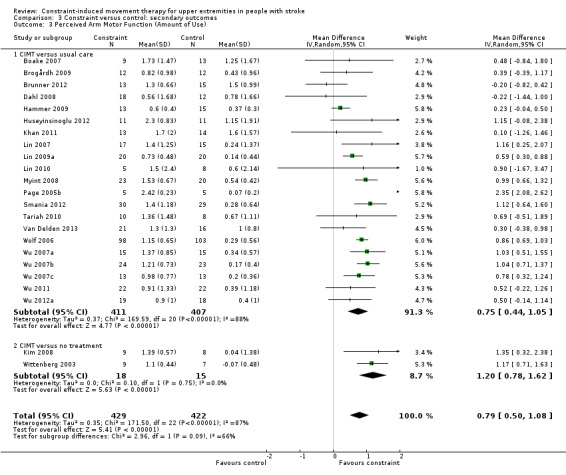
Comparison 3 Constraint versus control: secondary outcomes, Outcome 3 Perceived Arm Motor Function (Amount of Use).
Comparison 3.4: Arm motor impairment
Eighteen studies with 451 participants measured arm motor impairment (Alberts 2004; Atteya 2004; Boake 2007; Hammer 2009; Lin 2009a; Lin 2010; Page 2001; Page 2002b; Page 2004; Page 2005b; Page 2008; Ploughman 2004; Singh 2013; Tariah 2010; Van Delden 2013; Wu 2007b; Wu 2007c; Yoon 2014); data were available for 372 participants (82%). The impact of CIMT on upper limb impairment indicated a significant effect (SMD 0.82, 95% CI 0.31 to 1.34; Analysis 3.4). We found considerable heterogeneity among studies (I2 = 77%).
3.4. Analysis.
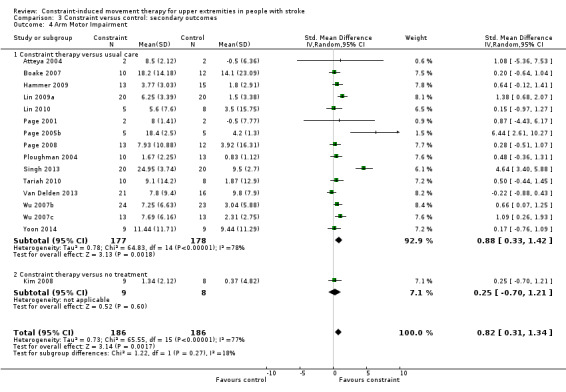
Comparison 3 Constraint versus control: secondary outcomes, Outcome 4 Arm Motor Impairment.
Comparison 3.5: Quality of life
Eight studies with 537 participants measured quality of life (Dahl 2008; Dromerick 2009; Lin 2009a; Van Delden 2013; Wolf 2006; Wu 2007b; Wu 2007c; Wu 2012a); data were available for 96 participants (18%). The impact of CIMT on quality of life indicated a non‐significant effect (MD 6.54, 95% CI ‐1.2 to 14.28; Analysis 3.5). We found no statistical heterogeneity (I2 = 0%).
3.5. Analysis.
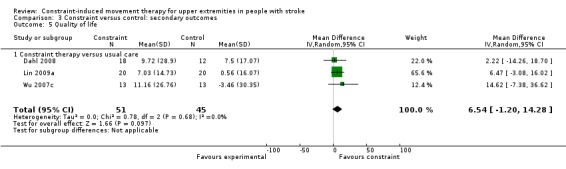
Comparison 3 Constraint versus control: secondary outcomes, Outcome 5 Quality of life.
Comparison 3.6: Dexterity
Seven studies with 229 participants included a measure of dexterity (Boake 2007; Brunner 2012; Hammer 2009; Kim 2008; Myint 2008; Van Delden 2013; Yoon 2014); data were available for 113 participants (49%). The impact of CIMT on upper limb dexterity indicated a significant effect (SMD 0.42, 95% CI 0.04 to 0.79; Analysis 3.6). We found no statistical heterogeneity (I2 = 0%).
3.6. Analysis.
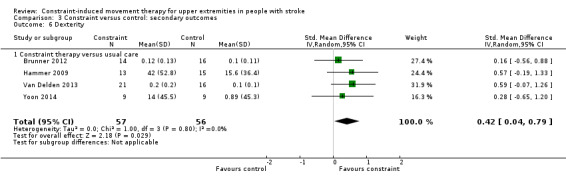
Comparison 3 Constraint versus control: secondary outcomes, Outcome 6 Dexterity.
Discussion
Summary of main results
This work updates the previous Cochrane review published in 2008 on the efficacy of CIMT, mCIMT and FU. The review now includes 42 trials with 1453 participants. All studies enrolled people who had compromised, but residual, ability of upper arm and hand, participants were able to extend the wrist and the metacarpophalangeal joints at least 10° and 20° respectively, or presented a Brunnstrom stage > 3 and with limited pain or spasticity. Moreover, people with cognitive impairment were excluded.
Results of this review show a superiority of CIMT in comparison with other rehabilitation approaches on the recovery from motor impairment and motor function (secondary outcomes) but not in disability (primary outcome).
Effect of CIMT on disability
Eleven trials with 344 participants measured disability and we included their results in the analysis.
The impact of CIMT on disability indicates a non‐significant effect if compared with active rehabilitation approaches (SMD 0.24, 95% CI ‐0.05 to 0.52). Also, at the longest follow‐up, no superiority of CIMT is documented and subgroup analyses do not show interactions between disability and amount of task practice, anatomical region restraint or time since stroke. The main active rehabilitation approaches used by the control groups consisted of occupational therapy and techniques of adaptation to motor impairment (Dahl 2008; Dromerick 2009; Lin 2009a; Myint 2008), functional task practice (Lin 2007; Ploughman 2004; Treger 2012; Wu 2007a; Wu 2007c), Bobath principles (Huseyinsinoglu 2012), and unspecified conventional rehabilitation (Yoon 2014). The treatment duration was well balanced among studies except in that of Huseyinsinoglu 2012, in which CIMT treatment lasted three times as long as the treatment performed by the control group, and in the Yoon 2014 study, in which there was a similar four‐fold imbalance between the groups.
In summary, these studies showed that the use of constraining approaches (CIMT, mCIMT and FU) compared with a similar dose of rehabilitation targeting the practice of functional tasks did not result in a demonstrable improvement in disability.
Secondary outcomes
Twenty‐eight studies with a total of 848 participants measured arm motor function and we included them in the analysis. CIMT was always compared with active rehabilitation approaches, and showed a limited effect in improving arm motor function.
The majority of trials used a mCIMT, eight studies used CIMT (Dahl 2008; Hayner 2010; Khan 2011; Myint 2008; Taub 1993; Wittenberg 2003; Wolf 2006; Yoon 2014), and only three studies used FU (Hammer 2009; Kim 2008; Ploughman 2004). Comparison groups performed the same dose of treatment with the exception of five studies in which the control groups' dose was lower (Dromerick 2009; Taub 1993; Wittenberg 2003; Wolf 2006; Yoon 2014), one study in which the dose was smaller in the treatment group (Huseyinsinoglu 2012), and two studies in which it was not clearly specified (Dahl 2008; Kim 2008).
Twenty‐three and 24 of the included studies with a total of 851 and 891 participants, respectively, measured the perceived arm motor function (AoU and QoU, respectively) and we included them in the analysis. In three studies the control groups did not perform treatments (Kim 2008, Taub 1993, Wittenberg 2003). The estimated effect of CIMT led to a significant and clinically relevant improvement in the perceived arm motor function of the paretic arm (Lang 2008).
Sixteen of the included studies with a total of 372 participants measured arm motor impairment and we included them in the analysis. In one study the control group did not perform treatments (Kim 2008). The estimated effect of CIMT was considered to be large in modifying the arm motor impairment of the affected arm.
Four of the included studies with a total of 113 participants measured dexterity and we included them in the analysis. The estimated effect of CIMT led to a significant small effect in improving upper limb dexterity.
CIMT does not appear to have a better effect than other rehabilitation approaches in improving quality of life; this was measured in three studies.
It is worth noting the considerable heterogeneity of the studies included in the review, regarding the way in which CIMT was applied and the characteristics of the control treatments. Considering this heterogeneity, and some differences among the outcome measures used by the authors, the results of these analyses should be interpreted with caution.
When reported, rates of adverse events among included studies do not appear to differ between CIMT and the comparison groups, and CIMT appears to have no adverse effects.
Sixteen studies declared dropout levels of 4% to 23%, including losses for non‐medical reasons, with the exception of one study in which four of the 13 participants in the experimental group did not complete the programme due to difficulties in performing the ADLs (Kim 2008).
Overall completeness and applicability of evidence
In 2009 this review concluded that "the impact of CIMT on disability indicated a modest significant benefit". With the increase in the number of included studies, the effect of CIMT on disability decreased and became non‐significant (SMD 0.24, 95% CI ‐0.05 to 0.52; Analysis 1.1). We classified the magnitude of effect sizes as proposed by Juni 2006. The effect size of 0.24 standard deviation units obtained for disability is considered small. It corresponds to an overlap in the distribution of participants allocated in the experimental or control interventions of about 85% of cases, indicating that only 15% of people would benefit from CIMT treatment after stroke. Also, the sample sizes of the 42 included studies were generally small.
It has been argued that only individuals presenting with mild to moderate paresis of the upper limb (Nijland 2010; Smania 2007), as well as those who are more motivated, would be eligible for CIMT treatment (Wissink 2014). Actually, from reports of included trials there is a clear difficulty in finding eligible participants. Sixty‐one per cent for Lin 2010, and 93% for Smania 2012, of people assessed for eligibility were excluded because they did not fit the inclusion criteria. Moreover, about 20% of eligible people refused to participate in the study. This means that only a small number of the people who were screened were included in these eligible trials. Moreover, the presence of movement requested as part of the inclusion criteria could have allowed for selection of those people with less severe stroke. Transcranial magnetic stimulation and diffusion tensor imaging studies show that voluntary wrist and finger extension are associated with the integrity of the corticospinal tract system (Butler 2007; Stinear 2007; Stinear 2010). Consequently, the characteristics of people to include in these trials raise questions about the application of this intervention in a wide range of stroke survivors.
The included studies were heterogeneous in participant and intervention characteristics for both CIMT and the control group. However, none of the subgroup analyses performed in this review (dose of treatment, time since stroke, anatomical region restraint) revealed a group of better responders. Although no evidence exists that the dose of CIMT influences the results, it does not imply that it is not important. Consequently, it is not possible to exclude the possibility that the high dose of CIMT reported in the Yoon 2014 study introduced heterogeneity in the analysis, thus providing overestimation of the effect of CIMT on disability. Finally, the results of this meta‐analysis do not show that the first weeks after stroke onset are the most important for the application of CIMT, as studies on neuroplasticity might suggest (Sunderland 2005).
Improvements introduced by CIMT are mainly based on learning to optimise the use of end‐effectors through compensatory strategies. The effects documented in this meta‐analysis involve motor impairment and motor function without a translation in disability. This could be considered as surprising, as the rationale for CIMT is based on decreasing the learned non‐use phenomenon, however, it could be due to the characteristics of the measures of disability.
The number of RCTs and the data that inform this review have increased over the past few years. However, the included studies were generally poor in terms of relevance of findings and quality of reporting. Only 11 out of 42 studies (with 344 participants) reported data on the most relevant clinical outcome – disability – comparing CIMT with an active control intervention. Reporting was often incomplete, which made some studies uninformative.
The applicability of cumulative evidence characterised by a large number of small trials of uncertain quality challenges definitive conclusions about the role of CIMT; however, the findings of this review suggest that CIMT does not show relevant benefits for the outcomes that may matter most to people after their stroke.
Quality of the evidence
Three‐quarters of the included trials can be considered to be at unclear risk of bias (see Risk of bias in included studies) for at least one key bias area. In fact, key methodological information was often not reported for sequence generation, allocation concealment, blinding, and missing data. Blinding of study personnel, particularly outcome assessors, was reported in the majority of studies.
Many trials were likely to be underpowered, likely to approach analyses on a per protocol basis, and had a strong inclination to perform multiple testing on function scales.
Recent meta‐reporting studies showed promising improvements in the reporting of rehabilitation trials (Abdul Latif 2011; Villamar 2013), and reviews (DiSilvestro 2015; Gianola 2013). In the cohort of trials that have evaluated the effectiveness of CIMT, there were a few recent trials that adopted robust methods and accurate reporting of clinical and methodological aspects (Brunner 2012; Smania 2012; Treger 2012; Wolf 2006). These trials represent the next generation in terms of methodological issues, and a major step forward in research to understand fully the benefit and safety of rehabilitation techniques in comparative studies.
Potential biases in the review process
We chose disability as the primary outcome, although it was considered by a minority of eligible studies (11 studies with a total of 344 participants), whereas arm motor function was used as the primary outcome in the majority of included studies (28 studies with 858 participants).
Although the analysis showed the largest effect of CIMT on perceived arm motor function, caution is needed in interpreting this result, because of the lack of consistency in the MAL scale, as described in the Risk of bias in included studies section. Its clinimetric properties need further investigation in order to define its use in longitudinal studies.
Most trials were small, with some trials enrolling only six or 10 participants. This is unacceptable, given the high incidence of stroke and the opportunity to recruit a large sample. Our sample of trials may therefore have been influenced by publication bias, which tends to exaggerate the effect of treatment. The randomisation methods were described only in about half of the included trials. It is not possible to determine if some studies excluded participants after randomisation, or whether blinding was not adequately maintained. These weaknesses could be expected to lead to bias in favour of a treatment effect. The reporting of the data was poor; for example, many trials only reported that there were no significant differences between the intervention and the control groups. This lack of proper reporting could also be expected to lead to bias in favour of a treatment effect. It should also be noted that many authors of trials have a cultural and professional interest in disseminating positive results about the rehabilitative techniques they propose.
Finally, only one author of the review scanned the titles obtained from electronic databases searching in order to exclude irrelevant studies and this could have introduced bias.
Authors' conclusions
Implications for practice.
Compared with traditional rehabilitation, constraint‐induced movement therapy (CIMT) is associated with limited improvements in motor impairment and motor function, but these benefits do not convincingly reduce disability. These results differ from our previous meta‐analysis, which suggested a possible improvement in disability with CIMT. The recent studies included in this review did not confirm these findings, and data about the long‐term effects of CIMT are limited.
CIMT can be considered a multifaceted intervention where the restriction of the less affected limb is accompanied by an increase in the amount and quality of exercise for the affected limb. The impact on arm impairment and motor function may not be due solely to the constraint, but also to the type and amount of exercise. However, this review could not identify which of these factors is more important.
The selection of participants for the included studies focused on people with stroke who had at least some active extension of the wrist and fingers, with limited pain or spasticity, plus good compliance with rehabilitation treatment. It appears that the review results apply most appropriately to this patient group. Many studies were underpowered with a high risk of small trial bias and publication bias. It is not clear if the apparent benefits on motor impairment and motor function can be translated into improvements in activities of daily living. Moreover, it is not possible to comment on the long‐term effects of CIMT.
Implications for research.
It is likely that additional randomised controlled trials (RCTs) investigating CIMT as a rehabilitation technique would be worthwhile if they:
involve a control group under active treatment, since CIMT involves a certain amount of exercise;
consider disability or arm motor function as the primary outcomes;
include a validated quality of life measure as one of the outcomes; and
determine and report the sample size and power analysis transparently.
CIMT trials do not make it clear which people might most benefit from this treatment. Participants in the RCTs were those with at least some active extension of wrist and fingers and with limited pain or spasticity. Researchers involved in future studies should analyse the correlation between participant characteristics and outcome improvements in order to identify responders to CIMT. Clinicians who aspire to offer their patients a tailored programme of CIMT need to examine individual characteristics carefully to identify potential factors that are likely to increase the limited chance of success of CIMT.
Feedback
Risk of bias, 25 May 2017
Summary
Date of Submission: 25‐May‐2017 Name: Martin Vuillème Email Address: martin.vuilleme@gmail.com Role: Volunteer translator Comment: (Singh 2013) is assessed by the authors as being at low risk of bias in the "performance bias and detection bias" domain. The support for this judgement is [Quote: "the rater ... was not blinded to the study"]. This is not coherent with the assessment of the authors, as their methods explicitly say that studies with no blinding will be scored as high risk. The full quote from Singh is : "There are few limitations of our study like: Small sample size due to limited stroke subjects, the rater who was not blinded to the study.". Singh P, Pradhan B (2013). Study to assess the effectiveness of modified constraint‐induced movement therapy in stroke subjects: a randomized controlled trial. Annals of Indian Academy of Neurology, 16(2),180. doi:10.4103/0972‐2327.112461
Reply
Dear Martin Vuillème
Thank you for reporting back to us the incoherent evaluation of the risk of bias of the study by Singh et al (1) in the text of our review. The judgment of the "Performance bias and detection bias" domain in the Risk of bias table for this study has been corrected to "high risk". The text of the review on risk of bias has also been corrected.
The overall quality of evidence or the conclusions of the review have not changed (2).
Best regards,
Valeria Sirtori, Davide Corbetta, Greta Castellini, Lorenzo Moja, Roberto Gatti
References:
1. Singh P, Pradhan B. Study to assess the effectiveness of modified constraint‐induced movement therapy in stroke subjects: A randomized controlled trial. Annals of Indian Academy of Neurology. 2013; 16(2): 180.
2. Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint‐induced movement therapy for upper extremities in people with stroke. Cochrane Database of Systematic Reviews 2015, Issue 10. Art.No.:CD004433. DOI: 10.1002/14651858.CD004433.pub3.”
Contributors
Martin Vuillème: commentator
Valeria Sirtori, Davide Corbetta, Greta Castellini, Lorenzo Moja, Roberto Gatti: review authors
What's new
| Date | Event | Description |
|---|---|---|
| 1 September 2017 | Feedback has been incorporated | A correction has been made to the 'Risk of bias' table for Singh 2013 and the text of the review amended accordingly. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 31 May 2015 | New search has been performed | We updated the searches to January 2015 and have included several new trials in the review; the previous review included 19 trials while the current version includes 42 trials involving 1453 participants |
| 31 May 2015 | New citation required and conclusions have changed | New trials included in the review led to changes of the estimated effects of treatment. Statistical significance and meaningful differences were lost for clinically relevant outcomes, changing our interpretation of results. Our conclusions are now more conservative |
| 24 April 2008 | Amended | Converted to new review format. |
Acknowledgements
We renew our acknowledgements for all those people who helped in developing the protocol and the first version of this systematic review: we would like to thank Hazel Fraser for constant help and assistance, Brenda Thomas for help developing the search strategy, and the Cochrane Stroke Group Editorial Team.
Thanks to all the editors and reviewers that worked to improve the quality of this review: Peter Langhorne, Alex Pollock, Valentina Assi, Ruth Barclay, Brenda Thomas, and Kedar K Mate.
Thanks to Elizabeth Royle for her valuable copy‐edit comments, to Francesca Nicastro for English language support, to Prof Winstein for her availability and support.
Thanks also to Profs Alberts, Atteya, Azab, Bergheim, Boake, Brogardh, Brunner, Dahl, Dromerick, Hammer, Hayner, Huseyinsinoglu, Khan, Kim, Krawczyk, Lin, Myint, Page, Ploughman, Singh, Smania, Suputtittada, Tariah, Taub, Treger, Van Delden, Wang, Wittenberg, Wolf, Wu, and Yoon for their availability and collaboration, and especially for their work and efforts to provide better care for people who have experienced a stroke.
Appendices
Appendix 1. CENTRAL search strategy
The Cochrane Central Register of Controlled Trials (CENTRAL) (onlinelibrary.wiley.com)
#1 MeSH descriptor: [Cerebrovascular Disorders] explode all trees
#2 MeSH descriptor: [Brain Injuries] this term only
#3 MeSH descriptor: [Brain Injury, Chronic] this term only
#4 #1 or #2 or #3
#5 stroke* or cva or poststroke or post‐stroke (Word variations have been searched)
#6 cerebrovasc* or "cerebral vascular" (Word variations have been searched)
#7 cerebral or cerebellar or brain* or vertebrobasilar (Word variations have been searched)
#8 infarct* or isch?emi* or thrombo* or emboli* or apoplexy (Word variations have been searched)
#9 #7 and #8
#10 cerebral or brain or subarachnoid (Word variations have been searched)
#11 hamorrhage or hemorrhage or haematoma or hematoma or bleed* (Word variations have been searched)
#12 #10 and #11
#13 MeSH descriptor: [Hemiplegia] this term only
#14 MeSH descriptor: [Paresis] explode all trees
#15 #13 or #14
#16 hempar* or hemipleg* or paresis or paretic or "brain injur*" (Word variations have been searched)
#17 #4 or #5 or #6 or #9 or #12 or #15 or #16
#18 MeSH descriptor: [Upper Extremity] explode all trees
#19 "upper limb*" or "upper extremit*" or "arm" or "shoulder" or "hand" or "axilla" or "elbow*" or "forearm*" or "finger*" or "wrist*" (Word variations have been searched)
#20 #18 or 19
#21 MeSH descriptor: [Restraint, Physical] this term only
#22 MeSH descriptor: [Exercise Movement Techniques] this term only
#23 MeSH descriptor: [Exercise] this term only
#24 MeSH descriptor: [Exercise Therapy] this term only
#25 MeSH descriptor: [Immobilization] this term only
#26 MeSH descriptor: [Physical Therapy Modalities] this term only
#27 "constrain*" or "restrain*" or "immobili*" (Word variations have been searched)
#28 "mCIMT" or "CIT" or "CI therapy" or "forced use" (Word variations have been searched)
#29 MeSH descriptor: [Recovery of Function] this term only
#30 MeSH descriptor: [Splints] this term only
#31 MeSH descriptor: [Casts, Surgical] this term only
#32 #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31
#33 #17 and #20 and #32 in Trials
Appendix 2. MEDLINE (Ovid) search strategy
The following search strategy, which was developed by the Cochrane Stroke Group Trials Search Coordinator, was used for MEDLINE (Ovid) and was adapted for the Cochrane Central Register of Controlled Trials (CENTRAL).
1. exp cerebrovascular disorders/ or brain injuries/ or brain injury, chronic/ 2. (stroke$ or cva or poststroke or post‐stroke).tw. 3. (cerebrovasc$ or cerebral vascular).tw. 4. (cerebral or cerebellar or brain$ or vertebrobasilar).tw. 5. (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy).tw. 6. 4 and 5 7. (cerebral or brain or subarachnoid).tw. 8. (haemorrhage or hemorrhage or haematoma or hematoma or bleed$).tw. 9. 7 and 8 10. hemiplegia/ or exp paresis/ 11. (hempar$ or hemipleg$ or paresis or paretic or brain injur$).tw. 12. 1 or 2 or 3 or 6 or 9 or 10 or 11 13. exp upper extremity/ 14. (upper limb$ or upper extremit$ or arm or shoulder or hand or axilla or elbow$ or forearm$ or finger$ or wrist$).tw. 15. 13 or 14 16. restraint, physical/ 17. exercise movement techniques/ or exercise/ or exercise therapy/ 18. immobilization/ 19. physical therapy techniques/ 20. (constrain$ or restrain$ or immobili$).tw. 21. (mCIMT or CIT or "CI therapy" or "forced use").tw. 22. recovery of function/ 23. splints/ or casts, surgical/ 24. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 12 and 15 and 24
Appendix 3. EMBASE search strategy
EMBASE (Ovid)
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or cerebral artery disease/ or exp cerebrovascular accident/ or stroke/ or exp carotid artery disease/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp brain injury/ or stroke patient/ or stroke unit/ 2. (stroke$ or cva or poststroke or post‐stroke).tw. 3. (cerebrovasc$ or cerebral vasc$).tw. 4. (cerebral or cerebellar or brain$ or vertebrobasilar).tw. 5. (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy).tw. 6. 4 and 5 7. (cerebral or brain or subarachnoid).tw. 8. (haemorrhage or hemorrhage or haematoma or hematoma or bleed$).tw. 9. 7 and 8 10. hemiplegia/ or hemiparesis/ or paresis/ 11. (hemipleg$ or hemipar$ or paresis or paretic or brain injur$).tw. 12. 1 or 2 or 3 or 6 or 9 or 10 or 11 13. exp arm/ 14. (upper limb$ or upper extremit$ or arm or shoulder or hand or axilla or elbow$ or forearm$ or finger$ or wrist$).tw. 15. 13 or 14 16. constraint induced therapy/ or exp exercise/ or exp kinesiotherapy/ or physiotherapy/ or immobilization/ 17. (restrain$ or constrain$ or immobili$).tw. 18. (mCIMT or CIT or CI therapy or "forced use").tw. 19. dynamic splint/ or plaster cast/ or splint/ 20. (splint$ or cast or casts).tw. 21. or/16‐20 22. 12 and 15 and 21
Appendix 4. CINAHL search strategy
CINAHL (Ebsco)
S1 .(MH "Cerebrovascular Disorders+") or (MH "stroke patients") or (MH "stroke units") S2 .TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) S3 .TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) S4 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) S5 .S3 and S4 S6 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) S7 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S8 .S6 and S7 S9 .(MH "Hemiplegia") S10 .TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S11 .(MH "Left Hemisphere Injuries") OR (MH "Right Hemisphere Injuries") OR (MH "Brain Injuries") S12 .(MH "Upper Extremity+") S13 .TI ( upper limb* or upper extremit* or arm or shoulder or hand or axilla or elbow* or forearm* or finger* or wrist* ) or AB ( upper limb* or upper extremit* or arm or shoulder or hand or axilla or elbow* or forearm* or finger* or wrist* ) S14 .(MH "Constraint‐Induced Therapy") S15 .(MH "Restraint, Physical") S16 .(MH "Immobilization") S17 .(MH "Taping and Strapping") S18 .(MH "Exercise+") S19 .(MH "Therapeutic Exercise+") S20 .(MH "Physical Therapy/MT") S21 .(MH "Slings") OR (MH "Splints") S22 .(MH "Casts") S23 .(MH "Task Performance and Analysis") S24 .TI ( constrain* or restrain* or immobil* ) or AB ( constrain* or restrain* or immobil* ) S25 .TI ( mCIT or CIT or "CI therapy" or "forced use" or splint* or cast or casts ) or AB ( mCIT or CIT or "CI therapy" or "forced use" or splint* or cast or casts ) S26 .S1 or S2 or S5 or S8 or S9 or S10 or S11 S27 .S12 or S13 S28 .S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S29 .S26 and S27 and S28
Appendix 5. AMED (Ovid) search strategy
AMED (Ovid)
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. brain injuries/ or hemiplegia/
3. (stroke$ or cva or poststroke or post‐stroke).tw.
4. (cerebrovasc$ or cerebral vascular).tw.
5. (cerebral or cerebellar or brain$ or vertebrobasilar).tw.
6. (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy).tw.
7. 5 and 6
8. (cerebral or brain or subarachnoid).tw.
9. (haemorrhage or hemorrhage or haematoma or hematoma or bleed$).tw.
10. 8 and 9
11. (hempar$ or hemipleg$ or brain injur$).tw.
12. 1 or 2 or 3 or 4 or 7 or 10 or 11
13. exp arm/
14. (upper limb$ or upper extremit$ or arm or shoulder or hand or axilla or elbow$ or forearm$ or finger$ or wrist$).tw.
15. 13 or 14
16. restraint physical/
17. exercise/ or exercise movement techniques/ or exercise therapy/
18. immobilization/ or casting/ or splinting/
19. physical therapy modalities/
20. splints/
21. "recovery of function"/
22. (constrain$ or restrain$ or immobili$).tw.
23. (mCIT or CIT or "CI therapy" or "forced use" or splint$ or cast or casts).tw.
24. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25. 12 and 15 and 24
Appendix 6. PEDro search strategy
PEDro is a web‐based database of randomised controlled trials and systematic reviews relevant to physiotherapy. The following search strategy was used.
Abstract and Title: constraint, stroke, cva, poststroke, hemi, brain injur, *matoma, bleed, cerebrovasc, cerebral, brain, infarct, thrombo. Body part: upper arm, shoulder or shoulder girdle / forearm or elbow / hand or wrist. All search terms in the title or abstract were combined with body part descriptors using the AND operator.
Data and analyses
Comparison 1. Constraint versus control: primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disability postintervention | 11 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.05, 0.52] |
| 2 Disability: 3 to 6‐month follow‐up | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.57, 0.16] |
Comparison 2. Constraint versus control: subgroup analysis on primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amount of task practice | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 ≤ 30 hours | 8 | 253 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.07, 0.44] |
| 1.2 > 30 hours | 3 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.18, 0.67] |
| 2 Anatomical region restraint | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Arm plus hand | 2 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.17, 0.87] |
| 2.2 Hand only | 9 | 283 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.08, 0.41] |
| 3 Time since stroke | 7 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 0 to 3 months | 5 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.26, 0.39] |
| 3.2 3 to 9 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 More than 9 months | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.02, 1.00] |
Comparison 3. Constraint versus control: secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Arm Motor Function | 28 | 858 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.12, 0.55] |
| 1.1 Constraint therapy versus usual care | 25 | 816 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.09, 0.52] |
| 1.2 Constraint therapy versus no treatment | 3 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 1.04 [‐0.31, 2.40] |
| 2 Perceived Arm Motor Function (Quality of Use) | 24 | 891 | Mean Difference (IV, Random, 95% CI) | 0.68 [0.47, 0.88] |
| 2.1 CIMT versus usual care | 22 | 865 | Mean Difference (IV, Random, 95% CI) | 0.65 [0.44, 0.86] |
| 2.2 CIMT versus no treatment | 2 | 26 | Mean Difference (IV, Random, 95% CI) | 0.94 [‐0.32, 2.20] |
| 3 Perceived Arm Motor Function (Amount of Use) | 23 | 851 | Mean Difference (IV, Random, 95% CI) | 0.79 [0.50, 1.08] |
| 3.1 CIMT versus usual care | 21 | 818 | Mean Difference (IV, Random, 95% CI) | 0.75 [0.44, 1.05] |
| 3.2 CIMT versus no treatment | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 1.20 [0.78, 1.62] |
| 4 Arm Motor Impairment | 16 | 372 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.31, 1.34] |
| 4.1 Constraint therapy versus usual care | 15 | 355 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.33, 1.42] |
| 4.2 Constraint therapy versus no treatment | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.70, 1.21] |
| 5 Quality of life | 3 | 96 | Mean Difference (IV, Random, 95% CI) | 6.54 [‐1.20, 14.28] |
| 5.1 Constraint therapy versus usual care | 3 | 96 | Mean Difference (IV, Random, 95% CI) | 6.54 [‐1.20, 14.28] |
| 6 Dexterity | 4 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.04, 0.79] |
| 6.1 Constraint therapy versus usual care | 4 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.04, 0.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alberts 2004.
| Methods | Randomisation automated and balanced with respect to sex, premorbid handedness, side of stroke and level of function Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | USA Recruited from 247 facilities spanning the 7 participating sites participating in a multi‐site trial 10 participants: 5 intervention, 5 control Inclusion criteria: cerebrovascular accident between 3 and 9 months, 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist, minimum passive range of motion of 90° for shoulder flexion and abduction Exclusion criteria: score of < 24 on the MMSE, physician‐determined major medical problems that would interfere with participation Mean age (SD): intervention group: 65 (8.2) years, control group: 63.4 (15.5) years % women: intervention group 60%, control group: 40% Stroke details: only ischaemic, 20% with right hemiparesis in each group Time since stroke, mean (SD): intervention group 6.4 (1.1) months, control group 5.6 (1.5) months |
|
| Interventions | CIMT versus no treatment
CIMT: shaping or adaptive task practice and repetitive task practice techniques
Amount of restraint: 90% of waking hours per day
Anatomical region restraint: hand Session duration: 6 hours per day, 5 days per week for 2 weeks |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation by random automated generator Quote: "Ten patients were randomly assigned to 1 of 2 groups" |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "An evaluator blinded to group assignment performed pre‐ and post‐ WMFT and FMA assessments" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Atteya 2004.
| Methods | Randomisation details were not reported Blinded outcome assessor No information about withdrawals Single centre, outpatients | |
| Participants | Saudi Arabia Recruited via the King Saud Univerity 6 participants: 2 intervention, 2 control, 2 no treatment Inclusion criteria: cerebrovascular accident between 1 and 6 months; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: significant cognitive impairment, haemorraghic lesion, significant spasticity, significant pain of the upper limb Mean age (SD): intervention group: 55 (2.8) years, control group: 52 (4.2) years, no treatment group: 56 (15.5) years % women: intervention group 50%, control group: 50%, no treatment group: 50% Stroke details: only ischaemic, 50% with right hemiparesis in each group Time since stroke, mean (SD): intervention group 5.6 (0.3) months, control group 3.95 (2.3) months, no intervention group 4.65 (1.2) months |
|
| Interventions | mCIMT versus control versus no treatment CIMT: physical and occupational therapy focused on PNF with emphasis on ADL tasks, compensatory techniques with the unaffected side, 2 functional tasks of the WMFT with shaping techniques Amount of restraint: 5 waking hours per day Anatomical region restraint: arm and hand Control: physical and occupational therapy focused on PNF with emphasis on ADL tasks, compensatory techniques with the unaffected side Session duration: 1 hour per day, 3 days per week, 10 weeks for each treatment group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all subjects were randomly assigned ... with an equal probability" Comment: insufficient information to make a judgment |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of outcome assessor |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Azab 2009.
| Methods | Randomisation details were not reported Blinded outcome assessor No withdrawals Single centre, outpatients |
|
| Participants | Jordan Recruited from King Abudallah University Hospital 37 participants: 20 intervention, 17 control Inclusion criteria: ability to voluntarily extend fingers and wrist slightly Exclusion criteria: severe cognitive disabilities Mean age (SD): 56 (9.9) years for all participants % women: 24% of all participants Stroke details: only ischaemic, 57% with right hemiparesis Time since stroke, mean (SD): 2.75 (0.7) months for all participants |
|
| Interventions | mCIMT versus control mCIMT: active range of motion of bilateral upper extremities, stretching exercises, hand‐eye co‐ordination activities, ambulation, and strengthening exercises for bilateral upper extremities Amount of restraint: 6 to 7 hours per day Anatomical region restraint: hand Control: active range of motion of bilateral upper extremities, stretching exercises, hand‐eye co‐ordination activities, ambulation, and strengthening exercises for bilateral upper extremities Session duration: 4 hours per week (in 3 day/week) for 4 weeks for both groups |
|
| Outcomes | Measures pre/post treatment and follow‐up at 6 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Information provided only in the abstract Quote: "Key words: Barthel Index, CIMT, stroke randomized control study" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The occupational therapist and the two physical therapists were double‐blinded to the therapy and group assignment of the patients" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | Quote: "The BI was measured at the beginning of the rehabilitation program and at the discharge from rehabilitation. The BI was also re‐evaluated at 6 months post discharge in 18 patients (64% of the initial experimental group)" |
Bergheim 2010.
| Methods | Randomisation by computer Blinded outcome assessor No withdrawals Single centre, inpatients |
|
| Participants | Norway Recruited from stroke unit and the neurological department of geriatric medicine of Ullevaal University Hospital 4 participants: 2 intervention, 2 control Inclusion criteria: cerebrovascular accident between 14 and 21 days; 10° of active extension in the finger and 20° in the wrist; ability to walk indoors without the use of walking aids; sufficient cognitive function Exclusion criteria: cerebral haemorrhage, prior stroke, unstable medical status, second cerebral diseases that were difficult to differentiate from a stroke, and previous illness/injury that significantly impaired function in arms Mean age (SD): intervention group: 70.5 (13.4) years, control group: 76.5 (4.9) years % women: intervention group 50%, control group: 50% Stroke details: only ischaemic, 0% with right hemiparesis with 0% paresis of the dominant side in treatment group, 50% with right hemiparesis with 50% paresis of the dominant side in control group Time since stroke: 14‐21 days after stroke onset |
|
| Interventions | mCIMT versus control
mCIMT: functional activities through shaping approach Amount of restraint: 6‐7 hours per day Anatomical region restraint: hand Control: mono and bilateral activities Session duration: 1 hour per day, 5 days/week, 2 weeks for both groups |
|
| Outcomes | Measures pre/post treatment and follow‐up at 3 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation was performed from a computer generated list" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Enrolled patients consented in writing and orally and were randomized by closed numbered envelopes participation respectively group mCIMT or TF [traditional physiotherapy]" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The outcome was examined by a physiotherapist blinded to therapy patients received" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | No missing outcome data |
Boake 2007.
| Methods | Randomisation: stratified by age and NIHSS, other details were not reported Blinded outcome assessor Post‐treatment withdrawals 22%, follow‐up withdrawals: 11% Single centre, inpatients and outpatients | |
| Participants | USA Recruited from admissions to the University Hospital of Memorial Hermann 23 participants: 10 intervention, 13 control Inclusion criteria: cerebrovascular accident within 14 days; score 1 to 3 on the motor arm item of the NIHSS; 10° of active movement in the thumb and 2 or more fingers of the affected hand. Exclusion criteria: not reported Mean age (SD): intervention group: 63.1 (14.3)years, control group: 58.9 (14) years % women: intervention group 30%, control group: 38% Stroke details: ischaemic or haemorrhagic, 40% with right hemiparesis in treatment group, 54% with right hemiparesis in control group Time since stroke, mean (range): intervention group 3.3 (3 to 4.1) months, control group 3.3 (3 to 4.3) months |
|
| Interventions | mCIMT versus control mCIMT: functional tasks with shaping techniques Amount of restraint: 90% of waking hours per day Anatomical region restraint: hand Control: ADL with either hand, improvement of strength, muscle tone and range of motion of the affected arm Session duration: 3 hours per day, 6 days per week, 2 weeks for each group |
|
| Outcomes | Measures pre/post treatment, follow up at 3 to 4 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients underwent baseline testing and were randomly allocated to either CIMT or traditional therapy" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Outcome evaluations were performed by personnel from outside ... who were blind to treatment assignment" |
| Incomplete outcome data addressed? (Post‐treatment) | High risk | 1/10 missing from intervention group (due to incomplete data), 4/13 missing from control group (due incomplete data and injuries). Reasons for missing data outcomes possibly related to the true effect, with imbalance across intervention and control groups |
Brogårdh 2009.
| Methods | Randomisation by computer Blinded outcome assessor Follow‐up withdrawals: < 5% Single centre, inpatients |
|
| Participants | Sweden Recruited from the Department of Rehabilitation at Lund University Hospital 24 participants: 12 intervention, 12 control Inclusion criteria: stroke onset between 1 and 3 months; 10° of active extension at wrist at least 10° of active extension of 2 fingers and 10° of active movement in the thumb Exclusion criteria: deformity of the more affected arm due to previous injury, drug abuse, epilepsy, mental disorder and botulinum toxin injections for spasticity treatment Mean age (SD): intervention group: 58.5 (6.3) years, control group: 56.7 (10.5) years % women: intervention group 17%, control group: 33% Stroke details: 58% with right hemiparesis with 75% paresis of the dominant side in treatment group, 75% with right hemiparesis with 83% paresis of the dominant side in control group Time since stroke, mean (SD): intervention group 1.56 (0.53) months, control group 1.7 (0.7) months |
|
| Interventions | mCIMT versus control mCIMT: task practise, fine motor practise, muscle strength training, muscle stretching, swimming pool training, general activity training. Activity of upper arm was delivered through shaping approach Amount of restraint: 90% of waking hours per day Anatomical region restraint: hand Control: task practise, fine motor practise, muscle strength training, muscle stretching, swimming pool training, general activity training. Activity of upper arm was delivered through shaping approach Session duration: 3 hours per day for 2 weeks for both groups |
|
| Outcomes | Measures pre/post treatment, follow‐up at 3 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed from a computer‐generated list of consecutive random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All patients were assessed by independent and blinded assessors" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | 1 participant missed the three months follow‐up |
Brunner 2012.
| Methods | Randomisation by computer Blinded outcome assessor Post‐treatment withdrawals 6% Multicentre, inpatients and outpatients |
|
| Participants | Norway Recruited from 2 hospitals in the City of Bergen 30 participants: 14 intervention, 16 control Inclusion criteria: cerebrovascular accident between 2 and 16 weeks; ability to extend the affected wrist and fingers at least 10° Exclusion criteria: additional neurological diseases, unstable medical conditions, musculoskeletal disorders affecting arm mobility and severe cognitive impairment Mean age (SD): intervention group: 61 (10) years, control group: 64.8 (12.8) years % women: intervention group 21%, control group: 50% Stroke details: ischaemic or haemorrhagic; 43% with right hemiparesis in treatment group, 37% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 1.6 (1.3) months, control group 1.23 (0.8) months |
|
| Interventions | mCIMT versus control mCIMT: task‐related arm training, strength training, mobility training with shaping approach and self training focusing on unilateral activities Amount of restraint: 4 hours per day Anatomical region restraint: hand Control: task‐related arm training, strength training, mobility training with shaping approach and self training focusing on bilateral activities Session duration: 4 hours a week with physiotherapist plus 2‐3 hours everyday of self‐training for 4 weeks for both groups |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomized controlled trial was applied. A computerized random numbers generator was used for randomising the patients in blocks of four patients into a modified constraint‐induced movement therapy or a bimanual training group" |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque, sealed envelopes were prepared by a person not involved in the study, classifying the patients into one of the two groups" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The randomizations led to a balanced allocation, and blinded raters secured unbiased assessments" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | Quote: "There was two drop‐outs, one in each group, due to other medical problems" |
Dahl 2008.
| Methods | Block randomisation, other details were not reported Blinded outcome assessor No withdrawals Single centre, inpatients | |
| Participants | Norway Recruited from the Stroke Unit at Trondheim University Hospital and by announcement at hospitals and rehabilitation institutions in the neighbouring countries 30 participants: 18 intervention, 12 control Inclusion criteria: time from onset of stroke > two weeks; score 0 to 2 points before the stroke on the modified Ranking Scale; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: presence of other neurological diseases, unstable cardiovascular disease, severe depression (> 12 points on Montgomery and Aasberg Depression Rating Scale), marked neglect (line bisection more than 2 cm over the midline), life expectancy < 6 months, sequel from a previous stroke and clinically evaluated insufficient endurance to participate Mean age (SD): intervention group: 62 (8) years, control group: 60 (12) years % women: intervention group 11%, control group: 42% Stroke details: ischaemic or haemorrhagic; 78% paresis of dominant side in treatment group, 58% paresis of the dominant side in control group Time since stroke, mean (SD): intervention group 21 (18) months, control group 26 (27) months |
|
| Interventions | CIMT versus control CIMT: personalised ADL task training of the paretic limb, training difficulty was updated with daily progress Amount of restraint: 90% of waking hours per day Anatomical region restraint: hand Control: treatment given according to each patient's need, involving both upper and lower extremity with various occupational and physical therapy approaches Session duration: 6 hours per day in the CIMT group, unspecified duration for control group for 10 consecutive weekdays |
|
| Outcomes | Measures pre/post treatment and follow‐up at 6 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were block‐randomised into a CIMT group or a control group" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes were used for randomisation and the procedure was carried out by an external office" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Two independent and blinded assessors performed the assessments" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | No missing data |
Dromerick 2000.
| Methods | Randomisation by random number table, other details were not reported Blinded outcome assessor Post‐treatment withdrawals: 13% Single centre, inpatients | |
| Participants | USA Recruited from the acute stroke and brain injury rehabilitation service 20 participants: 11 intervention, 9 control Inclusion criteria: admission to inpatient rehabilitation within 14 days of ischemics stroke; score 1 or 2 on the motor arm item of the NIHSS; preserved cognitive function Exclusion criteria: no upper extremity injury or conditions that limited use before the stroke Mean age (SD): intervention group: 61.5 (13.7) years, control group: 71.4 (5.3) years % women: intervention group 25%, control group: 63% Stroke details: only ischaemic; 75% with right hemiparesis in treatment group, 63% with right hemiparesis in control group Time since stroke, mean (SD): 6 (2.6) days for both groups (range 4 to 14 days) |
|
| Interventions | CIMT versus control CIMT: ADL and functional tasks with the affected limb Amount of restraint: at least 6 hours per day Anatomical region restraint: hand Control: compensatory techniques for ADL, upper extremity strength, range of motion and traditional positioning Session duration: 2 hours per day, 5 days per week, 2 weeks for both groups |
|
| Outcomes | Measures pre/post treatment
Measures post‐treatment only
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were individually randomized into experimental or control groups by using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All posttreatment assessments were performed by blinded testers" |
| Incomplete outcome data addressed? (Post‐treatment) | High risk | 3/23 dropouts, reasons not reported |
Dromerick 2009.
| Methods | Randomisation balanced for age, total NIHSS score, ARAT score and days from stroke onset, other details were not reported
Blinded outcome assessor Follow‐up withdrawals: < 4% Single centre, inpatients |
|
| Participants | USA Recruitment from acute stroke admissions at Barnes‐Jewish Hospital in St Louis 52 participants: 35 intervention, 17 control Inclusion criteria: cerebrovascular accident within 28 days; score ≥ 3 on the upper arm item of the MAS, but no necessary movements in the hand Exclusion criteria: inability to give informed consent; clinically significant fluctuations in mental status within 3 days of enrolment; not independent prior to stroke; hemispatial neglect; sensory loss; not expected to survive 1 year due to other illnesses Mean age (SD): intervention group: 63.6 (14.38) years, control group: 64.7 (14.6) years % women: intervention group 57%, control group: 63% Stroke details: ischaemic or haemorrhagic; 51% with right hemiparesis with 45% paresis of the dominant side in treatment group, 52.9% with right hemiparesis with 44.2% paresis of the dominant side in control group Time since stroke, mean (SD): intervention group 9.3 (4.6) days, control group 10.4 (5.7) days |
|
| Interventions | This trial had 3 arms: 2 of the intervention groups performed mCIMT; 1 of the mCIMT groups performed a low intensity treatment (Low mCIMT) and the other group performed a high intensity treatment (High mCIMT) mCIMT (Low mCIMT versus High mCIMT) versus control mCIMT: functional activities of basic ADL with shaping approach for both groups Amount of restraint: Low mCIMT 6 hours per day, High mCIMT 90% of waking hours Anatomical region restraint: hand Control: traditional occupational therapy, involving compensatory techniques for ADL range of motion, and strengthening and upper extremity bilateral training activities Session duration: 2 hours per day for Low mCIMT, 3 hours per day for High mCIMT and 2 hours per day for control group for 5 days a week for 2 weeks |
|
| Outcomes | Measures pre/post treatment, follow‐up at 3 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... we adaptively randomized the group balancing for age, total NIHSS score, pretest ARAT and days from stroke onset" |
| Allocation concealment (selection bias) | Low risk | Quote: "The study clinical team met weekly to assure adherence to protocols" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Trained raters performed all blinded evaluations" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | Quote: "All but two participants were available for assessment at the 90‐day primary endpoint" |
Hammer 2009.
| Methods | Randomisation through marked ballots of paper
Unblinded outcome assessor
Post‐treatment withdrawals 13% Single centre, inpatients and outpatients |
|
| Participants | Sweden Recruited from the departments of rehabilitation medicine, geriatrics, and neurology at a university hospital in central Sweden 30 participants: 15 intervention, 15 control Inclusion criteria:cerebrovascular accident between 1 and 6 months; 10° of active extension in the finger and 20° in the wrist Exclusion criteria: no severe cognitive impairment (score of 20 points in the MMSE); ability to understand and follow instructions Mean age (SD): intervention group: 66.3 (10.3) years, control group: 60.4 (11.1) years % women: intervention group 7%, control group: 40% Stroke details: 73% with right hemiparesis with 80% paresis of the dominant side in treatment group, 53% with right hemiparesis with 46% paresis of the dominant side in control group Time since stroke, mean (SD): intervention group 2.6 (1.5) months, control group 2.3 (1.2) months |
|
| Interventions | mCIMT versus control mCIMT: conventional rehabilitation consisting of task‐oriented activities, facilitation of proximal and distal motor control and improvement of strength and endurance, skilled task training (moving objects, writing or typing) and daily tasks Amount of restraint: 6 hours per day, 5 days a week Anatomical region restraint: arm and hand Control: conventional rehabilitation consisting of task‐oriented activities, facilitation of proximal and distal motor control and improvement of strength and endurance, skilled task training (moving objects, writing or typing) and daily tasks Session duration: 3 hours per day, 5 days per week, 2 weeks for both groups |
|
| Outcomes | Measures pre/post treatment and follow‐up at 1 and 3 months:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a restricted block randomisation was used. Thirty pieces of paper had been prepared with the letter E (experimental group ...) on 15 of them and the letter K (conventional group ...) on the other 15. A block size of 10 was used (5 "E" plus 5 "K")" |
| Allocation concealment (selection bias) | Low risk | Quote: "The pieces of paper were folded twice, and the first block of 10 was placed in a metal box, while the rest were stored in 2 sealed envelopes with 1 block in each. For each participant in the study, the metal box was shaken, and an arbitrarily chosen staff member drew a piece of paper to determine the group allocation" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: " ... the present study had ... lack of blinding" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | Quote: "There were a total of 4 dropouts during the study. Two participants in the FU group discontinued the 2‐week intervention period; one dropped out on the first day of intervention because of refusal to continue, and the other was discharged on day 5 of the intervention. The other 2 participants dropped out before follow‐up because of illness (forced‐use group) and because of refusal to continue (standard training group)" |
Hayner 2010.
| Methods | Randomisation balanced for WMFT score, other details were not reported
Unblinded outcome assessor
Post‐treatment withdrawals 8% Single centre, outpatients |
|
| Participants | Canada Recruited through information disseminated to participants in a free clinic at Samuel Merritt University, clinics in the vicinity, and a local CVA support group 12 participants: 6 intervention, 6 control Inclusion criteria: cerebrovascular accident least 6 months; ability to place the affected hand on a table surface, trace movements in the hand and had sufficient endurance to participate in therapy 6 hours per day for 10 consecutive weekdays Exclusion criteria: inability to refrain from smoking (because a smoking area was unavailable), inability to tolerate a regular diet (because making lunch was a part of the therapeutic design) Mean age (SD): intervention group: 54 (11.62) years, control group: 559.5 (11.77) years % women: intervention group 67%, control group: 50% Stroke details: ischaemic Time since stroke, mean (SD): intervention group 21.1 (13.8) months, control group 67 (30.4) months |
|
| Interventions | CIMT versus control
CIMT: functional activities with 1 hand Amount of restraint: at least 6 hours per day Anatomical region restraint: hand Control: functional activities with 2 hands Session duration: 6 hours per day for 10 consecutive weekdays for both groups |
|
| Outcomes | Measures pre/post treatment and follow‐up at 6 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were stratified into more and less affected UE [upper extremity] groups as determined by the WMFT total score and then blindly randomized into the CIMT or bilateral group" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were ... blindly randomized into the CIMT or bilateral group" Quote: "To ensure that intervention was truly of the same intensity and to avoid organizational confounds, all participants were treated simultaneously, in the same location, and by the same therapists" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Raters were not blinded" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | Quote: "One participant, randomized to the CIMT group, injured his affected UE at home before posttesting during a non–study‐related activity and was dropped from the study" |
Huseyinsinoglu 2012.
| Methods | Randomisation by computer, stratified by people who received injections of botulinum toxin‐A Blinded outcome assessor Post‐treatment withdrawals: 8% Single centre, outpatients | |
| Participants | Turkey Recruited from the outpatient clinic of the Stroke Unit of the Florence Nightingale Hospital 24 participants: 13 intervention, 11 control Inclusion criteria: cerebrovascular accident between 3 and 24 months; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: no serious cognitive disorders; no excessive pain that would interfere with the ability to participate in the treatment; no excessive spasticity in any joint of the affected arm Mean age (SD): intervention group: 49.1 (13.7) years, control group: 48.2 (15.4) years % women: intervention group 36%, control group: 54% Stroke details: ischaemic or haemorrhagic; 64% paresis of dominant side in treatment group, 27% paresis of the dominant side in control group Time since stroke, mean (SD): intervention group 10.6 (6.1) months, control group 13.1 (6.3) months |
|
| Interventions | mCIMT versus control mCIMT: behavioural techniques, shaping and task activities Amount of restraint: 90% of waking hours for 12 consecutive days Anatomical region restraint: hand Control: control muscle, tone quality of movements, weight bearing and stability of trunk arm activity in functional situation following Bobath principles Session duration: mCIMT: 3 hours per day for 10 consecutive days; control group: 1 hour per day for 10 consecutive days |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects ... were randomly assigned to either ... group by using a randomisation function of Microsoft Office Excel software. Blocked randomisation was used. Treatment and random number columns were created and each part of the treatment column was (pre‐assigned as B and C subjects, respectively) given a random number between 0 and 1 by the Microsoft Excel software random number generator. The sort and filter menu was used to sort the random number row from smallest to largest so that treatment groups were randomly ordered. Pre‐stratification was applied to the subjects based on whether they had received injections of botulinum toxin‐A within past three months" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Before and after the interventions, measurements were obtained by a rater blinded to the group assignment. The blinded rater was trained to administer these tests before the beginning of the study" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | Quote: "Two dropped out of the constraint‐induced movement therapy group during the intervention period; both were personal choice. 22 participants completed the two‐week treatment" |
Khan 2011.
| Methods | Randomisation with stratification by age, time since stroke, arm/hand function Blinded outcome assessor Post‐treatment withdrawals 4%, follow‐up withdrawals: 7% Single centre, inpatients | |
| Participants | Switzerland Recruitment from stroke patients referred for inpatient rehabilitation in the Neurorehabilitation Center Valens 42 participants: 13 intervention, 14 control, 15 therapeutic climbing Inclusion criteria: people with acute, subacute and chronic stroke; minimal to moderate arm and hand function stage 2‐6 on the Chedoke McMaster Impairment Inventory sub scale and hand control Exclusion criteria: shoulder pain, other neurological disorders or other serious co‐morbidities Mean age (SD): intervention group: 60.4 (16.1) years, control group: 60.4 (14.8) years, therapeutic climbing 62.2 (13.5) years % women: intervention group 23%, control group: 50%, therapeutic climbing 33% Stroke details: 61% with right hemiparesis with 61% paresis of the dominant side in treatment group, 43% with right hemiparesis with 50% paresis of the dominant side in control group, and 67% with right hemiparesis with 73% paresis of the dominant side in therapeutic climbing group Time since stroke, mean (SD): intervention group 5.2 (10.9) months, control group 15.7 (40.4) days, therapeutic climbing 11 (21.3) months |
|
| Interventions | This trial had 3 arms: the intervention group performed CIMT; a comparison group performed therapeutic climbing (TC) and the control group CIMT versus control versus TC CIMT: task‐oriented training Amount of restraint: during the exercises Anatomical region restraint: hand Control: postural control, inhibition of synergistic movements, facilitation of economic movements, conventional therapy TC: climbing‐specific exercises performed at the climbing wall inside the clinic Session duration: CIMT: 5 hours of individual physiotherapy and occupational therapy per week plus 5 hours of group exercises and 5 hours of self training per week; Control group: 7.5 hours of individual physiotherapy and occupational therapy plus 5 hours of group exercises per week; TC: 3,5 hours of individual physiotherapy and occupational therapy plus 4 hours of TC per week plus 5 hours of group exercises per week. Total duration of treatment was not reported |
|
| Outcomes | Measures pre/post treatment and follow‐up at 6 months:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent and blinded research assistant performed concealed randomization using a randomization schedule with blocks of three generated by the primary researcher" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "An independent and blinded research assistant performed concealed randomization using a randomization schedule with blocks of three generated by the primary researcher" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The same independent and blinded assessor performed all outcome measurements" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | 1/15 missing participant from conventional neurological therapy (thrombosis) 1/14 missing participant from CIMT (home sickness) 3/15 missing participants from climbing at 6 months follow‐up (1 participant died, 1 suffered another stroke, 1 refused to turn up) |
Kim 2008.
| Methods | Randomisation details were not reported Blinding of outcome assessor not reported Post‐treatment withdrawals: 23% Single centre, outpatients |
|
| Participants | Republic of Korea Participant recruitment information not provided 17 participants: 9 intervention, 8 control Inclusion criteria: cerebrovascular accident > 12 months; mild weakness of the affected upper limb (key muscle can move against some resistance) some fine motor ability of the affected hand Exclusion criteria: balance problems, severe visual impairments, cognitive deficits and aphagia Mean age (SD): intervention group: 51.7 (9.5) years, control group: 59.6 (10.3) years % women: intervention group 44%, control group: 50% Stroke details: ischaemic or haemorrhagic Time since stroke, mean (SD): intervention group 23.8 (7) months, control group 33.3 (18.5) months |
|
| Interventions | Forced use versus control Forced use: no exercises Amount of restraint: 5 hours day, 7 days per week Anatomical region restraint: hand Control: no exercises Total duration of treatment: 8 weeks |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to either the control group or the CIMT group" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not reported |
| Incomplete outcome data addressed? (Post‐treatment) | High risk | Quote: "Four of the 13 patients in the CIMT group did not complete [the] program. It seems that all 4 patients discontinued participation due to difficulties in performing some ADLs such as eating, dressing, dialling the phone, opening a door or operating a remote control" |
Krawczyk 2012.
| Methods | Randomisation by computer, stratified by age, gender, affected side of the body, time between the onset of stroke and the beginning of the study and severity of the arm motor deficit Blinded outcome assessor Follow‐up withdrawals: 19% Single centre, inpatients | |
| Participants | Poland Recruited stroke patients consecutively admitted to the inpatient neurorehabilitation unit in the Institute of Psychiatry and Neurology Hospital 47 participants: 24 intervention, 23 control Inclusion criteria: cerebrovascular accident more than 6 weeks before starting the study, presence of a motor deficit in the arm as assessed with the RMAAS Exclusion criteria: permanent use of the involved arm in life situations and coexisting lack of well‐defined treatment goals by the patient; excessive pain, spasticity or ataxia; presence of a severe or uncontrolled medical condition; orthopaedic or neurological limitations prior to the stroke that could affect outcome; bilateral or brainstem stroke Mean age (SD): intervention group: 48 (14) years, control group: 46 (13) years % women: intervention group 21%, control group: 25% Stroke details: ischaemic or haemorrhagic; 46% with right hemiparesis in treatment group, 43% with right hemiparesis in control group Time since stroke: 53% of participants were within 6 months post stroke |
|
| Interventions | mCIMT versus control mCIMT: task‐oriented training MAL activities applied with shaping Amount of restraint: 5 hours per day Anatomical region restraint: arm plus hand Control: task‐oriented training MAL activities applied with shaping Session duration: 6 hours per day, 5 days a week for 3 weeks each group |
|
| Outcomes | Measures pre/post treatment and follow‐up at 1 year
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated by a computer program" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "A trained investigator who was blinded to the study group … carried out all three clinical assessments" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | At 1 year follow‐up 3/24 in CIMT group (1 died, 1 changed address, 1 refused to participate) and 6/23 participants in voluntary‐constraint group (1 died, 3 changed address, 2 refused to participate) did not participate |
Lin 2007.
| Methods | Randomisation by random number table stratified by side of stroke, allocation by sealed envelopes Blinded outcome assessor Post‐treatment withdrawals: 6% Multicentre, outpatients | |
| Participants | Taiwan Recruited from rehabilitation departments of 3 medical centres 32 participants: 17 intervention, 15 control Inclusion criteria: cerebrovascular accident > 12 months; Brunnstrom Stage > 3 on arm section; amount of use < 2.5 on the MAL, no serious cognitive deficits, no excessive spasticity in any joints of the affected upper limb Exclusion criteria: history of stroke or other neurological, neuromuscular or orthopaedic disease Mean age (SD): intervention group: 57.11 (18.3) years, control group: 58.77 (15.5) years % women: intervention group 35%, control group: 33% Stroke details: ischaemic or haemorrhagic; 53% with right hemiparesis in treatment group, 60% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 15.97 (3.46) months, control group 16.61 (2.89) months |
|
| Interventions | mCIMT versus control mCIMT: ADL activity with the affected arm Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: strength, balance, fine motor dexterity training, functional task practice, stretching/weight‐bearing by the affected arm Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a table of random numbers, 10 randomly selected numbers in the range from 1 to 20 were assigned to [the] modified constraint‐induced movement therapy group and the remaining 10 numbers to [the] traditional rehabilitation group" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients with left stroke were randomized using two sets of sealed envelopes and those with right stroke using another two sets of sealed envelopes. For each two sets of envelopes, one unmarked set of 20 envelopes were presented to a patient to choose one. The unmarked envelopes contained a single sheet of paper with a number ranging from 1 to 20. In the second set of envelopes, which were marked with numbers from 1 to 20, modified constraint‐induced movement therapy or traditional rehabilitation sheets were sealed" Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Two occupational therapists blind to group allocation provided the evaluations" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | 2/17 missing participants from the control group (due to unstable medical condition) |
Lin 2009a.
| Methods | Randomisation by computer stratified according to participating hospital Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | Taiwan Recruited on the basis of brain imaging identifying unilateral stroke in 3 medical centres 60 participants: 20 intervention, 20 control, 20 bilateral arm training group Inclusion criteria:cerebrovascular accident > 12 months; Brunnstrom Stage > 3 on arm section; amount of use < 2.5 on the MAL, no serious cognitive deficits, no excessive spasticity in any joints of the affected upper limb Exclusion criteria: not reported Mean age (SD): intervention group: 55.28 (9.34) years, control group: 58.77 (15.5) years, bilateral arm training group 51.58 (8.67) years % women: intervention group 45%, control group: 45%, bilateral arm training group 40% Stroke details: ischaemic or haemorrhagic; 40% with right hemiparesis in treatment group, 60% with right hemiparesis in control group; 55% with right hemiparesis in bilateral arm training group Time since stroke, mean (SD): intervention group 21.25 (21.59) months, control group 21.9 (20.51) months, bilateral arm training group 18.5 (17.4) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a comparison group that performed bilateral arm training; and the control group mCIMT versus bilateral arm training versus control mCIMT: functional tasks by shaping techniques with the affected arm Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: training for hand function, co‐ordination, balance and compensatory practice on functional tasks Bilateral arm training: simultaneous movements of both upper extremities in functional tasks Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... participants were individually randomized into the distributed CIT, BAT, or control intervention groups, with the computerized (block) randomizations scheme, including pre stratification according to participating hospital" |
| Allocation concealment (selection bias) | Low risk | Quote: "One set of opaque, numbered envelopes was prepared for each site containing cards indicating the allocated group. When a new patient was registered, a card was extracted and the relevant occupational therapist informed of the group allocation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "... raters were blinded to the participant group and trained to properly administer the outcome measures" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Lin 2010.
| Methods | Randomisation details were not reported Blinding of outcome assessor not reported No information about withdrawals Multicentre, outpatients | |
| Participants | Taiwan Recruited from 2 medical centres 13 participants: 5 intervention, 8 control Inclusion criteria: cerebrovascular accident > 3 months; ability to extend actively at least 20° at the wrist and 10° at the metacarpophalangeal and interphalangeal joints on the last 4 fingers of the affected hand; sufficient cognitive ability Exclusion criteria: not reported Mean age (SD): intervention group: 46.04 (26) years, control group: 51.06 (12.4) years % women: intervention group 40%, control group: 0% Stroke details: ischaemic or haemorrhagic; 20% with right hemiparesis in treatment group, 62% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 21.5 (12.3) months, control group 16.3 (18.3) months |
|
| Interventions | mCIMT versus control mCIMT: functional tasks delivered through shaping approach Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: neurodevelopmental treatments focusing on balance training, stretching and weight‐bearing with the affected limb, fine‐motor tasks and practice of compensatory activities of daily living Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomized to the dCIT [distributed Constraint‐induced therapy] or the CI [control intervention] group" |
| Allocation concealment (selection bias) | Unclear risk | Information not provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not provided |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | Information not provided |
Myint 2008.
| Methods | Randomisation by drawing sealed envelopes, other details were not provided Blinded of outcome assessor Post‐treatment withdrawals: 10%; follow‐up withdrawals: 7.5% Single centre, outpatients | |
| Participants | China Recruited from 3 hospitals with rehabilitation facilities 43 participants: 23 intervention, 20 control Inclusion criteria: cerebrovascular accident between 2 to 16 weeks; 10° of active extension to the metacarpophalangeal and interphalangeal joints 20° at wrist Exclusion criteria: severe aphasia, high risk of fall, cerebellar stroke and severe shoulder pain affecting therapy Mean age (SD): intervention group: 63.4 (13.6) years, control group: 63.9 (12.2) years % women: intervention group 56%, control group: 60% Stroke details: ischaemic or haemorrhagic; 48% with right hemiparesis in treatment group, 70% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 1.27 (0.7) months, control group 1.5 (0.95) months |
|
| Interventions | CIMT versus control CIMT: adaptive task practice (shaping) Amount of restraint: 90% of waking hours Anatomical region restraint: arm and hand Control: bimanual task, compensatory techniques for ADL strength, range of motion, positioning and mobility training Session duration: 4 hours per day, 5 days per week, 2 weeks for each group |
|
| Outcomes | Measures pre/post treatment and follow‐up at 12 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized by drawing sealed envelopes which were filled at random with indication of which intervention group the patient was allocated to" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The observer was blinded" |
| Incomplete outcome data addressed? (Post‐treatment) | High risk | 5/28 missing from intervention group (due to transport problem, inadequate home support; others changed their mind about trial participation); 0/20 missing participants in the control group. Reasons for missing data outcomes possibly related to the true effect, with imbalance across intervention and control groups |
Page 2001.
| Methods | Randomisation details were not provided Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | USA Recruited through letters sent to people who experienced a cerebrovascular accident and were discharged from outpatients therapy provided at 4 rehabilitation hospitals 6 participants: 2 intervention, 2 control, 2 no treatment Inclusion criteria: stroke between 4 weeks and 6 months, 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: severe cognitive impairment, excessive spasticity and pain Mean age (SD): intervention group: 55 (4.24) years, control group: 52 (5.65) years, no intervention group 60.5 (23.33) years % women: intervention group 50%, control group 50%, no treatment group 50% Stroke details: ischaemic or haemorrhagic; 50% with right hemiparesis in treatment group, 50% with right hemiparesis in control group, 100% with right hemiparesis in no treatment group Time since stroke, mean (SD): intervention group 5.65 (0.21) months, control group 3.75 (2.47) months, no treatment group 4.5 (0.7) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a control group performed usual care; and the third group performed no treatment mCIMT versus control versus no treatment mCIMT: physical and occupational therapy focused on PNF with emphasis on ADL tasks, compensatory techniques with the unaffected side, two functional task of the WMFT with shaping techniques Amount of restraint: 5 waking hours per day Anatomical region restraint: arm and hand Control: physical and occupational therapy focused on PNF with emphasis on ADL tasks, compensatory techniques with the unaffected side Session duration: 1 hour per day, 3 days per week, 10 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all subjects were randomly assigned ... with an equal probability" Comment: insufficient information to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "A blinded examiner administered all instruments" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Page 2002b.
| Methods | Randomisation details were not provided Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | USA Recruited through letters sent to people who experienced a cerebrovascular accident and were discharged from outpatients therapy provided at 4 rehabilitation hospitals 14 participants: 4 intervention, 5 control, 5 no treatment Inclusion criteria: stroke between 4 weeks and 6 months; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: severe cognitive impairment, excessive spasticity and pain Mean age (SD): intervention group: 73.5 (6.35) years, control group: 67.4 (13.8) years, no intervention group 68.2 (14.13) years % women: intervention group 0%, control group 20%, no treatment group 80% Stroke details: only Ischaemic; 50% with right hemiparesis in treatment group, 20% with right hemiparesis in control group, 60% with right hemiparesis in no treatment group Time since stroke, mean (SD): intervention group 5 (0.8) months, control group 4.9 (0.9) months, no treatment group 4.3 (0.67) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a control group performed usual care; and the third group performed no treatment mCIMT versus control versus no treatment mCIMT: physical therapy and occupational therapy focused on functional tasks by the more affected limb, stretching, stand/balance, gait training, shaping techniques on 2 or 3 functional tasks Amount of restraint: 5 waking hours per day Anatomical region restraint: arm and hand Control: physical and occupational therapy focused on functional tasks by the more affected limb and PNF Session duration: 1 hour per day, 3 days per week, 10 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... all subjects randomly assigned ... with equal probability" Comment: insufficient information to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...a blinded rater again administered the instruments to all subjects" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Page 2004.
| Methods | Randomisation by computer random number table, other details were not provided Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | USA Recruited thorough advertisements placed in therapy clinics and given to therapists in hospitals 17 participants: 7 intervention, 4 control, 6 no treatment Inclusion criteria: stroke > 1 year; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: severe cognitive impairment, excessive spasticity and pain Mean age (SD): intervention group: 54.6 (12.77) years, control group: 60.75 (13.6) years, no intervention group 63.6 (9.81) years % women: intervention group 29%, control group 0%, no treatment group 17% Stroke details: only ischaemic; 71% with right hemiparesis in treatment group, 50% with right hemiparesis in control group, 50% with right hemiparesis in no treatment group Time since stroke, mean (SD): intervention group 25.42 (6.53) months, control group 38 (23.9) months, no treatment group 36.5 (26) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a control group performed usual care; and the third group performed no treatment mCIMT versus control versus no treatment mCIMT: functional task with the affected arm, strengthening, stretching, compensatory techniques, shaping techniques on 2 or 3 functional tasks Amount of restraint: 5 waking hours per day Anatomical region restraint: arm and hand Control: physical and occupational therapy focused on PNF, stretching and compensatory techniques Session duration: 1 hour per day, 3 days per week, 10 weeks for both treatment groups |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomly assigned to 1 of 3 condition groups with equal probability by using a computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "examiner was blinded in that he was unaware of the patients' randomized grouping" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | One participant had received botulinum toxin type A in the more affected limb < 3 months before the study and was excluded from post hoc analysis |
Page 2005b.
| Methods | Randomisation by random number table, other details were not provided Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | USA Recruited volunteers; other details not provided 10 participants: 5 intervention, 5 control Inclusion criteria: stroke < 14 days; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist, more affected limb non use, defined as an amount of use score of < 2.5 on the MAL Exclusion criteria: severe cognitive impairment, excessive spasticity and pain Mean age (SD): intervention group: 58.6 (6.35) years, control group: 62.2 (10.3) years % women: intervention group 20%, control group 20% Stroke details: only Ischaemic; 80% with right hemiparesis in each group Time since stroke, mean (SD): intervention group 4 (1.6) days, control group 4.8 (3.03) days |
|
| Interventions | mCIMT versus control mCIMT: shaping techniques on 3 functional tasks, range of motion Amount of restraint: 5 waking hours per day Anatomical region restraint: hand Control: stretching, weight bearing, manual dexterity exercise with the affected arm, compensatory techniques Session duration: 1 hour per day, 3 days per week, 10 weeks for each treatment group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random numbers table, patients were then randomly assigned to either 1) mCIT (n = 5) or 2) traditional rehabilitation (TR) (n = 5)" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The Fugl‐Meyer, ARA, and MAL were administered by the same examiner who performed pretests, blinded to group assignment" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Page 2008.
| Methods | Randomisation by computer‐generated random numbers table, other details were not provided Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | USA Recruited thorough advertisements placed in neurology and physical therapy clinics 35 participants: 13 intervention, 12 control, 10 no treatment Inclusion criteria: stroke > 12 months; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist; more affected limb non‐use, defined as an amount of use score of < 2.5 on the MAL Exclusion criteria: severe cognitive impairment, excessive spasticity and pain Mean age (SD): 57.9 (8.4) years for all groups % women: 37% for all groups Stroke details: only ischaemic; 66% with right hemiparesis Time since stroke, mean: 39.8 months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a control group performed usual care; and the third group performed no treatment mCIMT versus control versus no treatment mCIMT: functional task by shaping techniques Amount of restraint: 5 waking hours per day Anatomical region restraint: arm and hand Control: PNF, stretching Session duration: 30 minutes per day, 3 days per week, 10 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned to 1 of 3 groups with equal probability of assignment to any of the groups using a computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of outcome assessor |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Ploughman 2004.
| Methods | Randomisation by random number generation, other details were not provided Blinded outcome assessor only on admission to treatment Post‐treatment withdrawals: 11% Single centre, inpatients and outpatients | |
| Participants | Canada Recruited from people admitted to multidisciplinary rehabilitation services from June 2001 to February 2003 23 participants: 10 intervention, 13 control Inclusion criteria: no more than 16 weeks post‐stroke at inclusion; > stage 2 but ≤ stage 6 on the CMII for the arm and hand Exclusion criteria: evidence of cognitive impairment Mean age (SD): intervention group: 57.8 (10.65) years, control group: 61.62 (5.68) years % women: intervention group 30%, control group 38% Stroke details: ischaemic or haemorrhagic; 60% with right hemiparesis in treatment group, 31% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 1.2 (0.75) months, control group 1.3 (0.78) months |
|
| Interventions | FU therapy ('FUT' in trial report) plus usual care versus usual care Usual care: facilitation of the proximal motor control progressing to skilled‐task training, strength and endurance training, functional electric stimulation, gait training Amount of restraint: average 2.7 hours per day Anatomical region restraint: hand (only thumb) Session duration: mean therapy 58.9 ± 41.45 minutes per day control group, and 61.74 ± 23.68 minutes per day intervention group, duration of study not specified |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned, by using random number generation, to either conventional rehabilitation or conventional rehabilitation plus FUT" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The ARAT admission and discharge assessments were performed by the principal investigator who was blinded to the treatment condition only on admission assessment" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | 3/13 missing from intervention group due to assessment being too stressful; 1/14 missing from control group for same reason |
Singh 2013.
| Methods | Randomisation through lottery method Unblinded outcome assessor No withdrawals Single centre, inpatients | |
| Participants | India Recruited via Central Referral Hospital and STNM Hospital in Sikkim 40 participants: 20 intervention, 20 control Inclusion criteria: cerebrovascular accident between 2 and 4 weeks; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 10° at wrist Exclusion criteria: severe aphasia, severe shoulder pain affecting therapy or any comorbid condition that could limit upper extremity function Mean age (SD): intervention group: 55.2 (9.27) years, control group: 21.9 (20.51) years % women: intervention group 30%, control group: 45% Stroke details: ischaemic or haemorrhagic Time since stroke, mean (SD): intervention group 0.6 (0.11) months, control group 0.65 (0.13) months |
|
| Interventions | mCIMT versus control mCIMT: shaping Amount of restraint: 10 hours per day Anatomical region restraint: hand Control: standard physical therapy, compensatory technique for daily activities, strengthening, and range of motion exercises for the affected arm Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were individually randomized into intervention and control groups by using lottery method" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "There are few limitations of our study like: Small sample size due to limited stroke subjects, the rater who was not blinded to the study." |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | Quote: "Since no follow‐up and less time was kept for restraint of the unaffected upper extremity so no drop out during the study" |
Smania 2012.
| Methods | Randomisation automated Blinded outcome assessor Post‐treatment withdrawals 10%, follow‐up withdrawals: 35% Multicentre, outpatients |
|
| Participants | Italy Recruited from 9 clinical sites. 66 participants: 34 intervention, 32 control Inclusion criteria: cerebrovascular accident occurred between 3 to24 months earlier; 10° of active wrist extension, at least 10° of thumb abduction/extension, and at least 10° of extension at the level of the metacarpophalangeal and interphalangeal joints in at least 2 digits Exclusion criteria: severe cognitive impairment, amount of use ≥ 2.5 on the MAL Mean age (SD): intervention group: 63.93 (9.56) years, control group: 68.25 (12.68) years % women: intervention group 13%, control group: 21% Stroke details: ischaemic or haemorrhagic; 47% with right hemiparesis with 53% paresis of the dominant side in treatment group, 45% with right hemiparesis with 48% paresis of the dominant side in control group Time since stroke, mean (SD): intervention group 11.1 (8.91) months, control group 9.38 (7.78) months |
|
| Interventions | mCIMT versus control mCIMT: passive mobilisation, task practise, ADL activities through shaping approach and household activities consisting in functional activities Amount of restraint: 12 hours per day Anatomical region restraint: hand Control: passive mobilisation and stretching, active motility tasks, ADL activities and household activities consisting in functional activities Session duration: 2 hours per day, 5 days per week, 2 weeks for each group |
|
| Outcomes | Measures pre/post treatment and follow‐up at 3 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "If eligible, patients were allocated to the experimental group (EG) or the control group (CG) by means of an automated randomizations system" |
| Allocation concealment (selection bias) | Low risk | Quote: "The group allocation was concealed using sealed numbered envelopes that were sent to the clinical hospital where the treatment was delivered. The randomizations list was locked in a desk drawer accessible only to the main investigator" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "At each research centre the same examiner, who was blinded with regard to treatment allocation, evaluated patients enrolled in the study" Quote: " Examiners were requested to inform their research coordinator if they discovered to which group a patient belonged, and they were periodically questioned by the coordinator about this" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | 4/30 missing participants from mCIMT (1 for unco‐operativeness, 3 for medical complications) 3/32 missing participants from control group (1 for unco‐operativeness, 2 for medical complications) Quote: "An intention‐to‐treat analysis was used" |
Suputtitada 2004.
| Methods | Randomisation by random‐number table, other details were not provided Blinded outcome assessor No information about withdrawals Single centre, outpatients | |
| Participants | Thailand Recruited from the Department of Rehabilitation Medicine of King Chulalongkorn Memorial Hospital 69 participants: 36 intervention, 33 control Inclusion criteria: 20° of active extension at wrist, 10° at metacarpophalangeal and interphalangeal joints Exclusion criteria: balance problems; severe aphasia; sensory disorder; severe cognitive impairments Mean age (SD): intervention group: 60.1 (4.8) years, control group: 58.7 (4.2) years % women: intervention group 33.3%, control group: 30.6% Stroke details: ischaemic or haemorrhagic; 91% with right hemiparesis in treatment group, 94% with right hemiparesis in control group Time since stroke: 1‐3 years in both groups |
|
| Interventions | CIMT versus control mCIMT: not described Amount of the restraint: 6 hours per day plus time not structured at home Anatomical region restraint: hand Control: neurodevelopmental treatment Session duration: 2 hours per day, 5 days per week, 2 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " ... patients were randomized individually into 2 groups by using the table of randomizations" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "This was a[n] ... observer‐blinded clinical trial" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | No information provided |
Tariah 2010.
| Methods | Randomisation details were not reported Blinded outcome assessor No information about withdrawals Single centre, home‐based treatment for experimental group and outpatients for control group | |
| Participants | Jordan Participant recruitment information not provided 18 participants: 10 intervention, 8 control Inclusion criteria: > 2 months post‐stroke at inclusion, 20° of active extension at wrist, 10° at metacarpophalangeal and interphalangeal joints Exclusion criteria: cognitive impairment; amount of use ≥ 2.5 on the MAL; excessive spasticity and pain Mean age (SD): intervention group: 54.8 (10.9) years, control group: 60.6 (4.9) years % women: intervention group 20%, control group: 50% Stroke details: only ischaemic; 70% with right hemiparesis in treatment group, 50% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 9.2 (5.79) months, control group 9.4 (4) months |
|
| Interventions | mCIMT versus control mCIMT: training activities focused on patients’ ADLs, instrumental activities of daily living, and leisure activities (e.g. playing cards, chess, crafts, gardening) Amount of restraint: 4 hours per day Anatomical region restraint: hand Control: weight‐bearing and facilitation of arm movement based on conventional neurodevelopmental procedures Session duration: 2 hours per day, 7 days per week, 2 months for both groups |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants ... were randomly numbered from one to twenty. Participants with odd numbers were allocated to CIMT group and those with even numbers were allocated to Neurodevelopmental Treatment NDT [control] group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The investigators, who were blind to the allocation of the groups, provided the evaluation tests" |
| Incomplete outcome data addressed? (Post‐treatment) | High risk | 2/10 in the NDT group dropped out after randomisation. Reasons not provided. |
Taub 1993.
| Methods | Randomisation details were not reported Blinded outcome assessor No information about withdrawals Multicentre, outpatients | |
| Participants | USA Recruited from the Spain Rehabilitation Center and the Departement of Neurology of the University of Alabama 9 participants: intervention 4, control 5 Inclusion criteria: stroke > 1 year; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: balance problems, extensive use of the affected arm, cognitive deficits, medical problems, > 75 years of age, left dominance or left hemiplegia Median age: intervention group: 65 years, control group: 63 years % women: intervention group 75%, control group: 80% Stroke details: only right side affected and right arm dominance for each group Median time since stroke: intervention 4.1 years, control: 4.5 years |
|
| Interventions | CIMT versus usual care CIMT: functional activity with the affected arm Amount of restraint: 90% of waking hours per day Anatomical region restraint: arm and hand Usual care: exhorted to focus attention on using the affected arm; range of self‐movement with the aid of the unaffected arm Session duration: Intervention: 6 hours per day, 5 days per week, 2 weeks Control: 15 minutes per day, 5 days per week, 2 weeks |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of outcome assessor |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Treger 2012.
| Methods | Randomisation by computer random numbers table, other details not provided
Blinded outcome assessor No withdrawals Single centre, inpatients |
|
| Participants | Israel Recruited from people admitted to the Department of Neurological Rehabilitation, the Loewenstein Hospital Rehabilitation Center 28 participants: 9 intervention, 19 control Inclusion criterion: active movement in most joints of the affected upper limb (grade ≥ 16 of the manual function test) Exclusion criteria: neurological or orthopedic disorders prohibiting the use of the paretic arm, neglect, apraxia, and cognitive disorders impeding collaboration Mean age (SD): intervention group: 62 (28.4) years, control group: 61.5 (8.4) years % women: intervention group 55%, control group: 16% Stroke details: only ischaemic Time since stroke, mean (SD): intervention group 1.32 (0.94) months, control group 0.77 (0.8) months |
|
| Interventions | mCIMT versus control mCIMT: training of the affected upper limb based on a task‐oriented approach, emphasising repetitive practice of functional activities and behavioural shaping Amount of restraint: 4 hours per day Anatomical region restraint: hand Control: training of the affected upper limb based on a task‐oriented approach, emphasizing repetitive practice of functional activities and behavioural shaping Session duration: 1 hour and 45 minutes per day, 5 days per week, 2 weeks for both groups |
|
| Outcomes | Measures pre/post treatment:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed allocation was performed by a computer‐generated randomized table of numbers created prior to the study" |
| Allocation concealment (selection bias) | Low risk | Quotes: "Concealed allocation was performed by a computer‐generated randomized table of numbers created prior to the study" "Individual, sequentially numbered index cards with the random assignment were prepared, folded, and placed in sealed opaque envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The assessor of the upper limb function tests was blinded to the type of intervention. The same assessor performed baseline and follow‐up tests" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | No participants lost to follow‐up |
Van Delden 2013.
| Methods | Randomisation by using the minimisation method Blinding of outcome assessor not reported Follow‐up withdrawals: 8% Single centre |
|
| Participants | The Netherlands Recruited from the Reade rehabilitation centre in Amsterdam 60 participants: 22 intervention, 19 control, 19 bilateral arm training with rhythmic auditory cueing Inclusion criteria: cerebrovascular accident between 1 and 6 months; 10° of active wrist extension, 10° af active thumb abduction/extension and 10° active extension in at least 2 additional digits; motivated to participate Exclusion criteria: upper‐limb orthopaedic limitations; cognitive impairment. Mean age (SD): intervention group: 59.8 (13.8) years, control group: 56.9 (12.7) years, bilateral arm training with rhythmic auditory cueing 62.6 (9.8) years % women: intervention group 36%, control group: 58%, bilateral arm training with rhythmic auditory cueing 42% Stroke details: ischaemic or haemorrhagic; 45% with right hemiparesis with 50% paresis of the dominant side in treatment group, 58% with right hemiparesis with 37% paresis of the dominant side in control group, 58% with right hemiparesis with 47% paresis of the dominant side in bilateral arm training with rhythmic auditory cueing (BATRAC) group Time since stroke, mean (SD): intervention group 2.14 (1.6) months, control group 2.6 (1.6) months, BATRAC group 1.8 (1.14) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a comparison group performed bilateral arm training with rhythmic auditory cueing (BATRAC); and the control group mCIMT versus BATRAC versus control mCIMT: functionally oriented task practice; Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: exercise therapy based on existing guidelines for upper extremity treatment after stroke as presented by the Dutch Society of Occupational Therapy BATRAC: bilateral movements that targeted rhythmic flexion and extension movements about the wrist rather than movements of proximal parts of the upper limb Session duration: 1 hour per day, 3 days per week, 6 weeks for each group |
|
| Outcomes | Measures pre/post treatment and follow up at 1 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted from supplementary materials: "After stratification, participants were randomized in permuted blocks and allocated to 1 of the 3 intervention groups" "Concealed allocation was effectuated online using the minimization method" |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed allocation was effectuated online using the minimization method" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not provided |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | 1/22 participants lost from mCIMT (moved to another place) 1/19 participants lost from BATRAC (intervention refused after allocation) 3/19 participants lost from control (moved to another place) |
Wang 2011.
| Methods | Randomisation by computer random‐numbers table, other details were not provided
Blinded outcome assessor No information about withdrawals Single centre, outpatients |
|
| Participants | China Recruited from people admitted to the Affiliated Hospital of Medical School Qingdao University 30 participants: 10 intervention, 10 control high‐intensity, 10 control low‐intensity Inclusion criteria: 20° of active extension at wrist, 10° at metacarpophalangeal and interphalangeals joints Exclusion criteria: excessive pain in the affected limb; aphasia; cognitive impairment Mean age (SD): intervention group: 59.4 (10.89) years, control group high‐intensity: 63.5 (9.63) years, control group control low‐intensity: 67 (7.45) years % women: intervention group 50%, control group high‐intensity 60%, control group control low‐intensity 30% Stroke details: ischaemic or haemorrhagic; 53% with right hemiparesis in treatment group, 60% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 2.7 (2.2) months, control group high‐intensity: 2.9 (2.2), control group control low‐intensity: 2.2 (1.2) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a control group performed high‐intensity training; and the another control group performed low‐intensity training mCIMT versus high‐intensity training group versus low‐intensity training group mCIMT: functional activities through shaping approach Amount of restraint: 90% of waking hours Anatomical region restraint: hand Low‐intensity and high‐intensity groups: strength, balance, manual dexterity exercises, functional task practice, stretching and weight‐bearing exercises Session duration: mCIMT and high‐intensity group: 3 hours per day; low‐intensity group: 45 minutes per day, all groups: 5 days a week for 4 weeks |
|
| Outcomes | Measures pre/post treatment:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants ... were subsequently assessed at random (using a random numbers table) into 3 groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The Wolf Motor Function Test (WMFT) was administered before therapy, and 2 and 4 weeks after the intervention period by the same rater, who was blinded to the group assignment" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
Wittenberg 2003.
| Methods | Randomisation by random‐numbers table, other details were not provided Blinded outcome assessor No withdrawals Single centre, inpatients | |
| Participants | USA Recruited mainly from referral by community physicians and therapists 16 participants: 9 intervention, 7 control Inclusion criteria: cerebrovascular accident > 12 months, 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist Exclusion criteria: not reported Mean age (range): intervention group: 65 (41‐81) years, control group: 63 (50‐75) years % women: intervention group 11%, control group: 28% Stroke details: only ischaemic Time since stroke, mean (SD): intervention group 34 (16‐86) months, control group 28 (12‐48) months |
|
| Interventions | CIMT versus control CIMT: task‐oriented exercise of the affected arm Amount of restraint: waking hours Anatomical region restraint: arm and hand Control: task performance with the unaffected side, passive therapy for the affected arm Session duration: Intervention: 6 hours per day, 4 days per week, 4 hours on weekend days, 2 weeks Control: 3 hours per day, 5 days per week, for 2 weeks |
|
| Outcomes | Measures pre/post treatment and follow up at 6 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random number table, patients were randomized into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "... thoroughly blinded raters" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | No missing data |
Wolf 2006.
| Methods | Randomisation automated, balanced with respect to sex, premorbid handedness, side of stroke and level of function Blinded outcome assessor Post‐treatment withdrawals: 8%; follow‐up withdrawals: 17% Multicentre, outpatients | |
| Participants | USA Recruited from 247 facilities spanning the 7 participating sites 222 participants: 106 intervention, 116 control Inclusion criteria: cerebrovascular accident between 3 and 9 months; 10° of active extension to the metacarpophalangeal and interphalangeal joints and 20° at wrist or 10° of active extension to the metacarpophalangeal and interphalangeal joints of two digits, and at wrist, 10° of thumb abduction/extension Exclusion criteria: scored less than 24 on the MMSE; physician‐determined medical problems could interfere with participation; excessive pain of the paretic extremity; substantial use of the paretic arm in daily life as determined by a score ≥ 2.5 on the Motor Activity Log Mean (SD) age: intervention group: 61 (13.5), control group: 63.43 (12.6) years % women: intervention group 34.9, control group: 37.1 Stroke details: ischaemic or haemorrhagic; 47.2% with hemiparesis of the dominant side in treatment group, 51.75% with hemiparesis of the dominant side in control group Time since stroke, mean (SD): intervention group 5.9 (2.1), control group 6.2 (2.3) months |
|
| Interventions | CIMT versus control CIMT: adaptive task practice (shaping) and standard task training of the paretic limb Amount of restraint: 90% of waking hours Anatomical region restraint: hand Control: usual and customary care ranged from no treatment to the application of mechanical interventions or various occupational and physical therapy approaches in the home Session duration: CIMT: 6 hours per day, 7 days per week, 2 weeks; control: not provided. |
|
| Outcomes | Measures pre/post treatment and follow up at 4, 8, and 12 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to the experimental (CIMT) or control condition using an automated, centralized system administered by the data management centre" |
| Allocation concealment (selection bias) | Low risk | Centralised |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of outcome assessor |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | 8/106 missing from intervention group (5 withdrew, 1 moved, 1 stroke, 1 poor health), 15/116 missing from control group (7 withdrew, 2 moved, 2 died) |
Wu 2007a.
| Methods | Randomisation details were not reported Blinded outcome assessor No withdrawals Multicentre, inpatients/outpatients | |
| Participants | Taiwan Recruited from the rehabilitation departments of 2 medical centres (Chang Gung Memorial Hospital and National Taiwan University Hospital) 30 participants: 15 intervention, 15 control Inclusion criteria: cerebrovascular accident between 12 and 36 months; 10° of active extension to the finger and 20° at wrist; non‐use of the more affected upper extremity (AoU score < 2.5 on the MAL); no serious cognitive deficits Exclusion criteria: balance problems sufficient to compromise safety when wearing the study’s constraint device; excessive spasticity in any joint of the affected upper extremity Mean age (SD): intervention group: 54.66 (8.63) years, control group: 53.31 (6.29) years % women: intervention group 47%, control group: 40% Stroke details: 40% with right hemiparesis in treatment group, 33% with right hemiparesis in control group Time since stroke, mean (SD): intervention group 18.53 (6.92) months, control group 17.61 (7.55) months |
|
| Interventions | mCIMT versus control mCIMT: functional tasks by shaping techniques with the affected arm, normalisation of muscle tone Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: neurodevelopmental therapy emphasising balance training, stretching/weight bearing of the affected arm, fine‐motor dexterity training in addition to practice on ADL with the less affected side Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... subjects were randomized with equal probability" Comment: insufficient information to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "A certified occupational therapist blind to study hypothesis and subject allocation was trained to administer the assessments" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | No missing data |
Wu 2007b.
| Methods | Randomisation by random‐numbers table, other details were not provided Blinded outcome assessor No withdrawals Multicentre, outpatients | |
| Participants | Taiwan Recruited from 2 stroke rehabilitation units 47 participants: 24 intervention, 23 control Inclusion criteria: cerebrovascular accident between 3 weeks and 37 months; Brunnstrom stage > 3 on arm section; non‐use of the more affected upper extremity (amount‐of‐use score < 2.5 on the MAL); no serious cognitive deficits Exclusion criteria: balance problems sufficient to compromise safety when wearing the study's constraint device Mean age (SD): intervention group: 53.93 (11.2) years, control group: 56.77 (12.9) years % women: intervention group 33%, control group: 30% Stroke details: ischaemic or haemorrhagic; 46% with right hemiparesis in treatment group, 48% with right hemiparesis in control group, all participants had right‐hand dominance Time since stroke, mean (SD): intervention group 12.51 (9.64) months, control group 11.98 (11.72) months |
|
| Interventions | mCIMT versus control mCIMT: ADL training with the affected arm Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: neurodevelopmental therapy emphasising functional task practice, stretching/weight‐bearing, fine‐motor dexterity training Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned to the CIMT or traditional intervention group by using a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Clinical evaluation were administered in random order by a blinded rater" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | No missing data |
Wu 2007c.
| Methods | Randomisation by random‐numbers table, other details were not provided Blinded outcome assessor No withdrawals Multicentre, inpatients/outpatients | |
| Participants | Taiwan Recruited from the rehabilitation departments of 3 medical centres 26 participants: 13 intervention, 13 control Inclusion criteria: cerebrovascular accident between 0.5 and 31 months; Brunnstrom stage > 3 on arm section; non use of the more affected upper extremity (amount‐of‐use score < 2.5 on the MAL); no serious cognitive deficits Exclusion criteria: balance problems sufficient to compromise safety when wearing the study's constraint device Mean age (SD): intervention group: 71.44 (6.42) years, control group: 71.94 (16.79) years % women: intervention group 38%, control group: 46% Stroke details: ischaemic or haemorrhagic; 46% with right hemiparesis in treatment group, 54% with right hemiparesis in control group, all participants had right hand dominance Time since stroke, mean (SD): intervention group 6.70 (8.99) months, control group 8.32 (7.97) months |
|
| Interventions | mCIMT versus usual care
mCIMT: functional tasks by shaping techniques with the affected arm, normalisation of muscle tone
Amount of restraint: 6 hours per day Anatomical region restraint: hand Usual care: neurodevelopmental therapy emphasising functional task practice, stretching/weight‐bearing, fine‐motor dexterity training Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were individually randomized into the mCIMT or the traditional rehabilitation group by using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Before and after the 3‐week intervention period, the tests were administered in random order by a blinded rater" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | No missing data |
Wu 2011.
| Methods | Randomisation by computer Blinded outcome assessor No information about withdrawals Multicentre, outpatients |
|
| Participants | Taiwan Recruited from 4 stroke rehabilitation units 66 participants: 22 intervention, 22 control, 22 BAT group Inclusion criteria: cerebrovascular accident > 12 months; Brunnstrom Stage > 3 on arm section; amount of use < 2.5 on the MAL, no serious cognitive deficits, no excessive spasticity in any joints of the affected upper limb Exclusion criteria: not reported Mean age (SD): intervention group: 51.91 (11.93) years, control group: 55.19 (2.5) years, bilateral arm training group 52.22 (10.72) years % women: intervention group 32%, control group: 72%, BAT group 18% Stroke details: ischaemic or haemorrhagic; 64% with right hemiparesis in treatment group, 54% with right hemiparesis in control group; 45% with right hemiparesis in BAT group Time since stroke, mean (SD): intervention group 14.91 (12.04) months, control group 17.77 (12.45) months, BAT group 15.92 (13.74) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a comparison group performed bilateral arm training; and the control group mCIMT versus bilateral arm training versus control mCIMT: functional tasks by shaping techniques with the affected arm Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: neurodevelopmental therapy emphasising functional task practice, stretching/weight bearing, fine‐motor dexterity training BAT: simultaneous movements of both upper extremities in functional tasks Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote. "Eligible participants were randomized to ... treatment groups using the computerized (block) randomizations scheme" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Before and after the 3‐week intervention period, ... outcome measures were administered by 2 certified, trained occupational therapists blinded to the participant group" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals Analyses were performed on 21/22 participants for kinematics in BAT group, 21/22 participants for WMFT in mCIMT and control groups |
Wu 2012a.
| Methods | Randomisation prestratified on the basis of participating hospital Blinded outcome assessor No withdrawals Multicentre, outpatients |
|
| Participants | Taiwan Recruited from the rehabilitation departments of 4 hospitals 57 participants: 19 intervention, 18 control, 20 arm plus trunk restraint Inclusion criteria: cerebrovascular accident > 12 months; residual motor ability of the affected upper extremity (score on the arm motor subscale of the FMA of ≥15); amount of use < 2.5 on the MAL, no serious cognitive deficits, no excessive spasticity in any joints of the affected upper limb Exclusion criteria: not reported Mean age (SD): intervention group: 56.3 (12.2) years, control group: 58.6 (11.6) years, arm plus trunk restraint group 54 (9.7) years % women: intervention group 26%, control group: 22%, arm plus trunk restraint group 20% Stroke details: ischaemic or haemorrhagic; 37% with right hemiparesis in treatment group, 28% with right hemiparesis in control group; 60% with right hemiparesis in arm plus trunk restraint group Time since stroke, mean (SD): intervention group 13.7 (7.3) months, control group 17.7 (13.4) months, arm plus trunk restraint 15.7 (13.5) months |
|
| Interventions | This trial had 3 arms: the intervention group performed mCIMT; a comparison group performed arm plus trunk restraint; and the control group mCIMT versus arm plus trunk restraint versus control mCIMT: functional tasks by shaping techniques with the affected arm Amount of restraint: 6 hours per day Anatomical region restraint: hand Control: neurodevelopmental therapy emphasising functional task practice, stretching/weight bearing, fine‐motor dexterity training, arm plus trunk restraint: functional tasks by shaping techniques with the affected arm with restraining of trunk anterior and rotation movements Session duration: 2 hours per day, 5 days per week, 3 weeks for each group |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All participants were unaware of the study hypotheses and were randomized to the dCIT‐TR [distribuited constraint‐induced therapy combined with trunk restraint], dCIT [distribuited constraint‐induced therapy], or control group by a pre stratification strategy based on the participating site" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The outcome measures were administered before and after a 3‐week intervention by 3 certified occupational therapists who were unaware of group allocation" |
| Incomplete outcome data addressed? (Post‐treatment) | Low risk | No missing data |
Yoon 2014.
| Methods | Randomisation by random card Blinded outcome assessor No information about withdrawals Single centre, inpatients |
|
| Participants | Korea Recruited from the Department of Rehabilitation Medicine at Pusan National University Yangsan Hospital 26 participants: 9 intervention, 9 control, 8 arm restraint plus mirror therapy Inclusion criteria: 10° of active wrist extension, 10° of active thumb abduction/extension and 10° active extension in at least 2 additional digits; possibility of simple communication; patients who could maintain a sitting position for more than 30 minutes Exclusion criteria: depression; inability to co‐operate in the treatment; inability to perform the active task training for musculoskeletal problems; spasticity; complex regional pain syndrome or secondary adhesive capsulitis Mean age (SD): intervention group: 64.33 (8.54) years, control group: 60.56 (16.94) years, arm restraint plus mirror therapy group 47.36 (14.4) years % women: intervention group 33%, control group: 55%, arm restraint plus mirror therapy group 25% Stroke details: ischaemic or haemorrhagic; 67% with right hemiparesis in treatment group, 44% with right hemiparesis in control group; 62% with right hemiparesis in arm restraint plus mirror therapy group Time since stroke, mean (SD): intervention group 0.6 (0.3) months, control group 0.8 (0.4) months, arm restraint plus mirror therapy group 0.8 (0.38) months |
|
| Interventions | This trial had 3 arms: the intervention group performed CIMT; a comparison group performed CIMT plus mirror therapy; and the control group CIMT versus CIMT plus mirror therapy versus control CIMT: fine motor exercise under the supervision of occupational therapist plus conventional physiotherapy plus self exercise Amount of restraint: 6 hours per day Anatomical region restraint: arm plus hand CIMT plus mirror therapy: fine motor exercise under the supervision of occupational therapist plus conventional physiotherapy plus mirror therapy with flexion/extension of the shoulder, elbow, wrist, finger, and pronation/supination of the forearm Control: self‐exercise program Session duration: CIMT: 6 hours of exercise, plus 40 minutes of conventional physiotherapy plus 30 minutes of self exercise daily; CIMT plus mirror therapy: 6 hours of exercise, plus 40 minutes of conventional physiotherapy, plus 30 minutes of mirror therapy daily; Control group: 60 minutes of self exercise, plus 40 minutes of conventional physiotherapy All for 5 days a week for 2 weeks |
|
| Outcomes | Measures pre/post treatment
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "they were assigned into three groups by picking a random card with numbers on them" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "... the results were compared between the three groups by the blinded observers" |
| Incomplete outcome data addressed? (Post‐treatment) | Unclear risk | The study provided no information about withdrawals |
9HPT: Nine‐Hole Peg Test, a test measuring finger‐hand co‐ordination in terms of the time it takes a patient to place nine pegs in a 5‐in by 5‐in board then remove them
16HPT: Sixteen‐Hole Peg Test: the time needed to place 16 pegs (2.15.9 cm) in a pegboard with 16 holes determined with a stopwatch
ADL: activities of daily living
AMAT: Arm Motor Activity Test, 16 timed items
AMPS: Assessment of Motor and Process Skills, a real‐time test in which patients do prescribed functional tasks that are videotaped and scored by a viewer
AoU: amount of use
ARAT: Action Research Arm Test, 19 items, 57‐point test divided into four categories (grasp, grip, pinch and gross movement), each item graded on a 4‐point ordinal scale (anchored 0 = can perform no part of the test, 3 = performs the test normally)
BAT: Bilateral arm training
BATRAC: Bilateral arm training with rhythmic auditory cueing
BI: Barthel Index
BLMA: Birgitta Lindmarks Motor Assessment
Box and Block Test: assesses unilateral gross manual dexterity
CI: control intervention
CIMT: constraint‐induced movement therapy
CIT: constraint‐induced therapy
CMII: Chedoke‐McMaster Impairment Inventory, a 7‐point scale ranging from 1 to 7 that presents 7 stages of motor recovery for arm, hand, postural control and shoulder pain, assessed with a severity scale
COPM: Canadian Occupational Performance Measure, a structured clinical assessment that allows participants to self‐rate goals of therapy in the categories of self‐care, productivity, and leisure
CVA: Cerebrovascular accident
dCIT: distributed constraint‐induced therapy
EMF: Emory Motor Function test, 16 timed items (2 strength items and 1 quality of movement item)
EmNSA: Erasmus modification of the Nottingham Sensory Assessment to measure the sense of touch, pressure, proprioception, and sharp‐blunt discrimination in the upper limb
FAI: Frenchay Activities Index, a self‐report scale, measures a person's perception of instrumental ADL participation at 3 or 6 months. It contains 15 items that can be separated into 3 factors: domestic chores, leisure/work, and outdoor activities. Each item is scored on a 0 to 3 point scale. Higher scores indicate better performance.
FIM: Functional Independence Measure, 5 items that specifically assess upper extremity function. Each item is scored on a 7‐point ordinal scale
FIM2: Functional Independence Measure, 18 items grouped into six sub scales. Each item is scored on a 7‐point ordinal scale
FIM3: Functional Independence Measure, 6 items that specifically assess upper extremity function. Each item is scored on a 7‐point ordinal scale
FMA: Fugl‐Meyer Assessment, a 66‐point upper extremity section of the Fugl‐Meyer Assessment of Motor Recovery After Stroke which assesses impairment using a 3‐point ordinal scale ( 0 = cannot perform to 2 = can perform fully)
FMA2: Fugl‐Meyer Assessment, a 33‐point upper extremity section of the Fugl‐Meyer Assessment of Motor Recovery After Stroke Assessment, which assesses impairment using a 3‐point ordinal scale (0 = cannot perform to 2 = can perform fully)
FU: Forced use
GPT: Grooved Pegboard Test, a test of dexterity that evaluates the speed with which the patient grasps and inserts 25 pegs (3 cm long, 5 mm diameter) into a grid of vertical holes in a horizontal 10 cm2 surface. It indicates the number of pegs placed per second for each hand
MAL: Motor Activity Log, a semi‐structured interview comprising 30 ADL tasks graded on a 6‐point AoU scale and a 6‐point Quality of Movement (QoM) scale
MAL2: Motor Activity Log, a semi‐structured interview comprising 14 ADL tasks graded on a 6‐point AoU scale and a 6‐point Quality of Movement (QoM) scale
MAL3: Motor Activity Log, a semi‐structured interview comprising 28 ADL tasks graded on a 6‐point AoU scale and a 6‐point Quality of Movement (QoM) scale
MAS: Motor Assessment Scale, a performance‐based scale developed for assessing everyday motor function in patients with stroke. Eight areas of motor function are assessed using a 7‐point scale (0 to 6)
MAS2: Modified Motor Assessment Scale, items used for upper extremity only; both arms were tested, consisting of 15 tasks from gross arm to fine finger movements in a 0–5 point scale
MASh: Modified Ashworth Scale, grades spasticity on the International Classification of Functioning level of body functions (muscle tone functions)
mCIMT: modified constraint‐induced movement therapy
MESUPES: Motor Evaluation Scale for Arm in Stroke Patients, a scale that takes the quality of upper limb movement into account during the evaluation of arm performance after stroke
MFT: Manual Function Test, assess various functions of the paralysed upper limb in hemiplegic patients post stroke in performing simple tasks
MI: Motricity Index, to measure strength in the upper limbs
MMSE: Mini Mental State Examination
NIHSS: The National Institute of Health Stroke Scale. assesses cognitive, sensory, and motor impairments as an indicator of overall stroke severity
PET: positron emission tomography
PNF: proprioceptive neuromuscular facilitation
RMAAS: Rivermead Motor Assessment Arm Scale: motor performance test
SD: standard deviation
SHFT: Sollerman Hand Function Test, consisting of 20 sub‐tests reflecting daily hand activities (type of grasp, quality of movement and speed of performance assessed in a 0–4 point scale)
SIS: Stroke Impact Scale
TC: Therapeutic climbing
TF: Traditional physiotherapy
TMS: transcranical magnetic stimulation
UE: upper extremity
WMFT: Wolf Motor Function Test, 17 simple limb movements and tasks with the affected arm. 15 items are timed and two assess strength
WMFT2: Wolf Motor Function Test, 19 simple limb movements and tasks with the affected arm. 17 items are timed and two assess strength
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brogårdh 2006 | RCT; the study authors explored the extended mitt used alone after CIMT and 4‐years follow‐up |
| Fuzaro 2012 | RCT; the study authors compare mCIMT and a modified FU |
| Gautier 2008 | RCT; the study authors compare 2 different forms of CIMT |
| Lin 2008 | RCT; the study authors compare mCIMT and a modified FU |
| Lin 2009b | RCT; the study authors compare mCIMT and a modified FU |
| Sawaki 2014 | RCT; some participants were also included in another study (Wolf 2006) |
| Tan 2012 | Not an RCT, controls were matched to subject receiving CIMT |
| Van der Lee 1999 | Not an RCT; computer‐generated randomisation, but with 21 aberrations (11 participants who should have received the experimental treatment were allocated to the reference group and 10 vice versa) |
| Wu 2012b | RCT; it included participants from the included study of Wu 2012a |
CIMT: constraint‐induced movement therapy FU: forced use mCIMT: modified constraint‐induced movement therapy RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Barzel 2015.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | mCIMT |
| Outcomes | MAL‐QOM and WMFT |
| Notes | Protocol for a completed study (clinicaltrials.gov) |
Boe 2014.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | mCIMT |
| Outcomes | ARAT, MAL, Satisfaction with Stroke Care Questionnaire, Re‐integration to Normal Living Index |
| Notes |
Dos Santos 2012.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | Restraint of the less affected upper limb |
| Outcomes | Fugl‐Meyer Scale, FIM |
| Notes |
Jansa 2007.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | CIMT |
| Outcomes | Assesment of motor and process skills |
| Notes | Presented as a poster at 11th Congress of the EFNS, Brussels, Belgium, 2007 |
Olivier 2012.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | Light constraint‐induced therapy |
| Outcomes | MAL‐QOM and WMFT |
| Notes | This study has been terminated (departure of the investigator co‐ordinator to another country) |
Uswatte 2014.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | Expanded Constraint Induced therapy |
| Outcomes | MAL |
| Notes |
ARAT: Action Research Arm Test CIMT: constraint‐induced movement therapy FIM: Functional Independence Measure MAL: Motor Activity Log MAL‐QOM: Motor Activity Log ‐ Quality Of Movement mCIMT: modified constraint‐induced movement therapy RCT: randomised controlled trial WMFT: Wolf Motor Function Test
Characteristics of ongoing studies [ordered by study ID]
Gautier 2015.
| Trial name or title | Examining mechanisms of neuroplasticity following motor rehabilitation in stroke ‐ examining how motor rehabilitation promotes brain reorganization following stroke, an MRI Study |
| Methods | RCT |
| Participants | People with stroke |
| Interventions | Constraint‐induced therapy |
| Outcomes | Brain structure, WMFT, ARAT, MAL |
| Starting date | July 2012 |
| Contact information | Gauthier.33@osu.edu |
| Notes |
Padovani Do Santos 2015.
| Trial name or title | Checking a security protocol of modified forced use therapy and efficacy reducing the constriction of the movement time in 12 hours |
| Methods | RCT |
| Participants | People with stroke |
| Interventions | FU therapy |
| Outcomes | Root mean square activity through surface electromyography and strength handgrip |
| Starting date | May 2015 |
| Contact information | Tamyris Padovani dos Santos, University of Sao Paulo |
| Notes |
Pereira 2015.
| Trial name or title | Effects of constraint‐induced therapy for the scapular kinematics and related to the quality of movement in patients with severe chronic hemiparesis |
| Methods | RCT |
| Participants | People with stroke |
| Interventions | Constraint‐induced therapy |
| Outcomes | Movement of the scapula and trunk through kinematic, WMFT, MAL |
| Starting date | January 2015 |
| Contact information | nat_duarte@yahoo.com.br |
| Notes |
ARAT: Action Research Arm Test FU: forced use MAL: Motor Activity Log RCT: randomised controlled trial WMFT: Wolf Motor Function Test
Differences between protocol and review
In 2003, based on the‐then existing evidence about CIMT, the protocol for this review was published in The Cochrane Library (Sirtori 2003); subsequently, the same authors found that the protocol did not reflect the increasing variability among potentially relevant primary studies and the review was out of date in terms of systematic review methodology. The main shortcomings in the protocol related to:
the inclusion criteria in terms of participants, interventions and outcome measures, as they were too restrictive and narrowly focused, being de facto a subgroup analysis (Higgins 2011). Outcome measures added during the systematic review process were not present in the original protocol of this review. These items were perceived as being of importance for physiotherapists and people with stroke, and offer a more complete picture about the efficacy of this technique;
the Methods section, which did not provide enough detail to ensure replicability.
We have now revised these sections extensively, with the following main amendments.
Background: to reflect what is known in 2015.
Objectives: to include studies investigating not only CIMT but also modified CIMT (mCIMT) and Forced Use (FU) therapy, which are closely related and belong to one specific class of intervention.
Types of interventions: to include interventions that differ widely in duration and intensity.
Types of outcome measures: new secondary outcomes were added in order to offer physiotherapists and people with stroke a more complete picture of the efficacy of this technique.
Methods of the review: to provide enough detail to allow repetition of the review by other researchers.
We considered these legitimate reasons to modify the original protocol.
Contributions of authors
This systematic review has been written on the basis of the review authors' clinical experience (VS, DC and RG). All review authors contributed to all stages of the review. Three review authors (VS, DC and GC) independently assessed study selection, data extraction and methodological quality. We resolved disagreements by consensus, and consulted a fourth review author (RG) if disagreement persisted. LM provided insight into epidemiological and statistical methods. VS, DC, RG and LM drafted different parts of the manuscript.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Incentive Award funding to the Cochrane Stroke Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Davide Corbetta: none known. Valeria Sirtori: none known. Greta Castellini: none known. Lorenzo Moja: none known. Roberto Gatti: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Alberts 2004 {published data only}
- Alberts JL, Butler AJ, Wolf SL. The effects of constraint‐induced therapy on precision grip: a preliminary study. Neurorehabilitation Neural Repair 2004;18(4):250‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atteya 2004 {published data only}
- Atteya AAA. Effects of modified constraint induced movement therapy on upper limb function in subacute stroke patients. Neurosciences 2004;9(1):24‐9. [PubMed] [Google Scholar]
Azab 2009 {published data only}
- Azab M, Al‐Jarrah M, Nazzal M, Maayah M, Abu Sammour M, Jamous M. Effectiveness of constraint‐induced movement therapy (CIMT) as home‐based therapy on Barthel Index in patients with chronic stroke. Topics in Stroke Rehabilitation 2009;16(3):207‐11. [DOI] [PubMed] [Google Scholar]
Bergheim 2010 {published data only}
- Bergheim A. Modified constraint induced movement therapy versus traditional physiotherapy for cerebral infarction: a pilot study [Modifisert constraint‐induced movement therapy versus tradisjonell fysioterapietter hjerneinfarkt: en pilot studie]. Fysioterapeuten 2010;2(10):16‐22. [Google Scholar]
Boake 2007 {published data only}
- Boake C, Noser EA, Ro T, Baraniuk S, Gaber M, Johnson R, et al. Constraint‐induced movement therapy during early stroke rehabilitation. Neurorehabilitation and Neural Repair 2007;21(1):14‐24. [DOI] [PubMed] [Google Scholar]
Brogårdh 2009 {published data only}
- Brogårdh C, Vestling M, Sjölund BH. Shortened constraint‐induced movement therapy in subacute stroke ‐ no effect of using a restraint: a randomized controlled study with independent observers. Journal of Rehabilitation Medicine 2009;41:231‐6. [DOI] [PubMed] [Google Scholar]
Brunner 2012 {published data only}
- Brunner IC, Skouen JS, Strand LI. Is modified constraint‐induced movement therapy more effective than bimanual training in improving arm motor function in the subacute phase post stroke? A randomized controlled trial. Clinical Rehabilitation 2012;26(12):1078‐86. [DOI] [PubMed] [Google Scholar]
Dahl 2008 {published data only}
- Dahl AE, Askim T, Stock R, Langørgen E, Lydersen S, Indredavik B. Short‐ and long‐term outcome of constraint‐induced movement therapy after stroke: a randomized controlled feasibility trial. Clinical Rehabilitation 2008;22(5):436‐47. [PUBMED: 18441040 ] [DOI] [PubMed] [Google Scholar]
Dromerick 2000 {published data only}
- Dromerick AW, Edwards DF, Hahn M, Dromerick AW, Edwards DF, Hahn M. Does the application of constraint‐induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke?. Stroke 2000;31(12):2984‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dromerick 2009 {published data only}
- Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, et al. Very early constraint‐induced movement during stroke rehabilitation (VECTORS): a single‐center RCT. Neurology 2009;73:195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammer 2009 {published and unpublished data}
- Hammer AM, Lindmark B. Effects of forced use on arm function in the subacute phase after stroke: a randomized, clinical pilot study. Physical Therapy 2009;89(6):526‐39. [DOI] [PubMed] [Google Scholar]
- Hammer AM, Lindmark B. Is forced use of the paretic upper limb beneficial? A randomized pilot study during subacute post‐stroke recovery. Clinical Rehabilitation 2009;23(5):424‐33. [DOI] [PubMed] [Google Scholar]
Hayner 2010 {published data only}
- Hayner K, Gibson G, Giles GM. Comparison of constraint‐induced movement therapy and bilateral treatment of equal intensity in people with chronic upper‐extremity dysfunction after cerebrovascular accident. American Journal of Occupational Therapy 2010;64:528‐39. [DOI] [PubMed] [Google Scholar]
Huseyinsinoglu 2012 {published data only}
- Huseyinsinoglu BE, Ozdincler AR, Krespi Y. Bobath Concept versus constraint‐induced movement therapy to improve arm functional recovery in stroke patients: a randomized controlled trial. Clinical Rehabilitation 2012;26(8):705‐15. [DOI] [PubMed] [Google Scholar]
Khan 2011 {published data only}
- Khan CM, Oesch PR, Gamper UN, Kool JP, Beer S. Potential effectiveness of three different treatment approaches to improve minimal to moderate arm and hand function after stroke – a pilot randomized clinical trial. Clinical Rehabilitation 2011;25(11):1032‐41. [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim DG, Cho YW, Hong JH, Song JC, Chung HA, Bai D, et al. Effect of constraint‐induced movement therapy with modified opposition restriction orthosis in chronic hemiparetic patients with stroke. NeuroRehabilitation 2008;23:239‐44. [PubMed] [Google Scholar]
Krawczyk 2012 {published data only}
- Krawczyk M, Sidaway M, Radwan´ska A, Zaborska J, Ujma R, Członkowska A. Effects of sling and voluntary constraint during constraint‐induced movement therapy for the arm after stroke: a randomized, prospective, single‐centre, blinded observer rated study. Clinical Rehabilitation 2012;26(11):990‐8. [DOI] [PubMed] [Google Scholar]
Lin 2007 {published and unpublished data}
- Lin KC, Wu CY, Wei TH, Lee CY, Liu JS. Effects of modified constraint‐induced movement therapy on reach‐to‐grasp movements and functional performance after chronic stroke: a randomized controlled study. Clinical Rehabilitation 2007;21:1075‐86. [DOI] [PubMed] [Google Scholar]
Lin 2009a {published data only}
- Lin K, Chang Y, Wu C, Chen Y. Effects of constraint‐induced therapy versus bilateral arm training on motor performance, daily functions, and quality of life in stroke survivors. Neurorehabilitation and Neural Repair 2009;23(5):441‐8. [DOI] [PubMed] [Google Scholar]
- Wu C, Hsieh Y, Lin K, Chuang L, Chang Y, Liu H, et al. Brain reorganization after bilateral arm training and distributed constraint‐induced therapy in stroke patients: a preliminary functional magnetic resonance imaging study. Chang Gung Medical Journal 2010;33(6):628‐38. [PubMed] [Google Scholar]
Lin 2010 {published data only}
- Lin K, Chung H, Wu C, Liu H, Hsieh Y, Chen I, et al. Constraint‐induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. American Journal of Physical Medicine and Rehabilitation 2010;89(3):177‐85. [DOI] [PubMed] [Google Scholar]
Myint 2008 {published data only}
- Myint JMWW, Yuen GFC, Yu TKK, Kng CPL, Wong AMY, Chow KKC, et al. A study of constraint‐induced movement therapy in subacute stroke patients in Hong Kong. Clinical Rehabilitation 2008;22:112‐24. [DOI] [PubMed] [Google Scholar]
Page 2001 {published data only}
- Page SJ, Sisto SA, Levine P, Johnston MV, Hughes M, Ge SJ, et al. Modified constraint induced therapy: a randomized feasibility and efficacy study. Journal of Rehabilitation Research and Development 2001;38(5):583‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Page 2002b {published data only}
- Page SJ, Sisto S, Johnston MV, Levine P. Modified constraint‐induced therapy after subacute stroke: a preliminary study. Neurorehabilitation and Neural Repair 2002;16(3):290‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Page 2004 {published data only}
- Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint‐induced movement therapy in chronic stroke: a single‐blinded randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2004;85(1):14‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Page 2005b {published data only}
- Page SJ, Levine P, Leonard AC. Modified constraint‐induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabilitation and Neural Repair 2005;19(1):27‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Page 2008 {published data only}
- Page SJ, Levine P, Leonard A, Szaflarski JP, Kissela BM. Modified constraint‐induced therapy in chronic stroke: results of a single‐blinded randomized controlled trial. Physical Therapy 2008;88(3):333‐40. [DOI] [PubMed] [Google Scholar]
Ploughman 2004 {published and unpublished data}
- Ploughman M, Corbett D. Can forced‐use therapy be clinically applied after stroke? An exploratory randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2004;85(9):1417‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2013 {published data only}
- Singh P, Pradhan B. Study to assess the effectiveness of modified constraint‐induced movement therapy in stroke subjects: a randomized controlled trial. Annals of Indian Academy of Neurology 2013;16(2):180‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smania 2012 {published data only}
- Smania N, Gandolfi M, Paolucci S, Iosa M, Ianes P, Recchia S, et al. Reduced‐intensity modified constraint‐induced movement therapy versus conventional therapy for upper extremity rehabilitation after stroke: a multicenter trial. Neurorehabilitation and Neural Repair 2012;26(9):1035‐45. [DOI] [PubMed] [Google Scholar]
Suputtitada 2004 {published data only}
- Suputtitada A, Suwanwela NC, Tumvitee S. Effectiveness of constraint‐induced movement therapy in chronic stroke patients. Journal of the Medical Association of Thailand 2004;87(12):1482‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Tariah 2010 {published data only}
- Tariah HA, Almalty A, Sbeih Z, Al‐Oraibi S. Constraint induced movement therapy for stroke survivors in Jordon: a home‐based model. International Journal of Therapy and Rehabilitation 2010;17(12):638‐46. [Google Scholar]
Taub 1993 {published and unpublished data}
- Taub E, Miller NE, Novack TA, Cook EW 3rd, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Archive of Physical Medicine and Rehabilitation 1993;74:347‐54. [MEDLINE: ] [PubMed] [Google Scholar]
Treger 2012 {published data only}
- Treger I, Aidinof L, Lehrer H, Kalichman L. Modified constraint‐induced movement therapy improved upper limb function in subacute poststroke patients: a small‐scale clinical trial. Topics in Stroke Rehabilitation 2012;19(4):287‐93. [DOI] [PubMed] [Google Scholar]
Van Delden 2013 {published data only}
- Delden AEQ, Peper CE, Nienhuys KN, Zijp NI, Beek PJ, Kwakkel G. Unilateral versus bilateral upper limb training after stroke. the Upper Limb Training After Stroke clinical trial. Stroke 2013;44:2613‐6. [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang Q, Zhao J, Zhu Q, Li J, Meng P. Comparison of conventional therapy, intensive therapy and modified constraint‐induced movement therapy to improve upper extremity function after stroke. Journal of Rehabilitation Medicine 2011;43:619‐25. [DOI] [PubMed] [Google Scholar]
Wittenberg 2003 {published and unpublished data}
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, et al. Constraint‐induced therapy in stroke: magnetic‐stimulation motor maps and cerebral activation. Neurorehabilitation and Neural Repair 2003;17(1):48‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolf 2006 {unpublished data only}
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint‐induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006;296(17):2095‐104. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, et al. Retention of upper limb function in stroke survivors who have received constraint‐induced movement therapy: the EXCITE randomised trial. Lancet Neurology 2008;7:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2007a {published and unpublished data}
- Wu CY, Lin KC, Chen HC, Chen IH, Hong WH. Effects of modified constraint‐induced movement therapy on movement kinematics and daily function in patients with stroke: a kinematic study of motor control mechanisms. Neurorehabilitation and Neural Repair 2007;21(5):460‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2007b {published and unpublished data}
- Wu CY, Chen CL, Tang SF, Lin KC, Huang YY. Kinematic and clinical analyses of upper‐extremity movements after constraint‐induced movement therapy in patients with stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88:964‐70. [DOI] [PubMed] [Google Scholar]
Wu 2007c {published and unpublished data}
- Wu CY, Chen CL, Tsai WC, Lin KC, Chou SH. A randomized controlled trial of modified constraint‐induced movement therapy for elderly stroke survivors: changes in motor impairment, daily functioning and quality of life. Archives of Physical Medicine and Rehabilitation 2007;88:273‐8. [DOI] [PubMed] [Google Scholar]
Wu 2011 {published data only}
- Wu C, Chuang L, Lin K, Chen H, Tsay P. Randomized trial of distributed constraint‐induced therapy versus bilateral arm training for the rehabilitation of upper‐limb motor control and function after stroke. Neurorehabilitation and Neural Repair 2011;25(2):130‐9. [DOI] [PubMed] [Google Scholar]
Wu 2012a {published data only}
- Wu C, Chen Y, Lin K, Chao C, Chen Y. Constraint‐induced therapy with trunk restraint for improving functional outcomes and trunk‐arm control after stroke: a randomized controlled trial. Physical Therapy 2012;92(4):483‐92. [DOI] [PubMed] [Google Scholar]
Yoon 2014 {published data only}
- Yoon JA, Koo BI, Shin MJ, Shin YB, Ko H, Shin Y. Effect of constraint‐induced movement therapy and mirror therapy for patients with subacute stroke. Annals of Rehabilitation Medicine 2014;38(4):458‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brogårdh 2006 {published data only}
- Brogardh C, Flansbjer U. What is the long‐term benefit of constraint‐induced movement therapy? A four‐year follow‐up. Clinical Rehabilitation 2009;23:418‐23. [DOI] [PubMed] [Google Scholar]
- Brogårdh C, Sjölund BH. Constraint‐induced movement therapy in patients with stroke: a pilot study on effects of small group training and of extended mitt use. Clinical Rehabilitation 2006;20(3):218‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fuzaro 2012 {published data only}
- Fuzaro AC, Guerreiro CT, Galetti FC, Jucá RBVM, Araujo JE. Modified constraint‐induced movement therapy and modified forced‐use therapy for stroke patients are both effective to promote balance and gait improvements. Revista Brasileira de Fisioterapia 2012;16(2):157‐65. [DOI] [PubMed] [Google Scholar]
Gautier 2008 {published data only}
- Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke 2008;39(5):1520‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin KC, Wu CY, Liu JS. A randomized controlled trial of constraint‐induced movement therapy after stroke. Acta Neurochirurgica. Supplement 2008;101:61‐4. [DOI] [PubMed] [Google Scholar]
Lin 2009b {published data only}
- Lin K, Wu C, Liu J, Chen Y, Hsu C. Constraint‐induced therapy versus dose‐matched control intervention to improve motor ability, basic/extended daily functions, and quality of life in stroke. Neurorehabilitation and Neural Repair 2009;23(2):160‐5. [DOI] [PubMed] [Google Scholar]
Sawaki 2014 {published data only}
- Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols‐Larsen DS, Wolf SL, Good DC, Wittenberg GF. Differential patterns of cortical reorganization following constraint‐induced movement therapy during early and late period after stroke: A preliminary study. NeuroRehabilitation 2014;35(3):415‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2012 {published data only}
- Tan C, Tretriluxana J, Pitsch E, Runnarong N, Winstein CJ. Anticipatory planning of functional reach‐to‐grasp: a pilot study. Neurorehabilitation and Neural Repair 2012;26(8):957‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Lee 1999 {published data only}
- Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single‐blind randomized clinical trial. Stroke 1999;30(11):2369‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2012b {published data only}
- Wu C, Chen Y, Chen H, Lin K, Yeh I. Pilot trial of distributed constraint‐induced therapy with trunk restraint to improve poststroke reach to grasp and trunk kinematics. Neurorehabilitation and Neural Repair 2012;26(3):247‐55. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Barzel 2015 {published data only}
- Barzel A, Ketels G, Tetzlaff B, Krüger H, Haevernick K, Daubmann A, et al. Enhancing activities of daily living of chronic stroke patients in primary health care by modified constraint‐induced movement therapy (HOMECIMT): study protocol for a cluster randomized controlled trial. Trials 2013;14:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boe 2014 {unpublished data only}
- Boe S. Atlantic Canada Modified Constraint Induced Movement Therapy Trial. ClinicalTrials.gov (accessed 1 June 2015). [NCT01283620]
Dos Santos 2012 {unpublished data only}
- dos Santos LB. Impact of the restriction of the non paretic upper limb in rehabilitation of patients post‐stroke: randomized clinical trial. ClinicalTrials.gov (accessed 1 June 2015). [NCT01623973]
Jansa 2007 {unpublished data only}
- Jansa J, Puh U, Angleitner K, Sicherl Z, Mesec A. Functional evaluation of constraint‐induced movement therapy (CIMT) in acute stroke. European Journal of Neurology 2007; Vol. 14, issue 1 Suppl:152‐3.
Olivier 2012 {unpublished data only}
- Olivier S. Effects of a modified constraint induced therapy intervention in stroke patients: a multicenter, randomized controlled trial. ClinicalTrials.gov (accessed 1 June 2015). [NCT00839670]
Uswatte 2014 {unpublished data only}
- Uswatte G. Constraint‐induced therapy modified for rehabilitating arm function in stroke survivors w/plegic hands. ClinicalTrials.gov (accessed 1 June 2015). [NCT00366210]
References to ongoing studies
Gautier 2015 {unpublished data only}
- Gautier L. Examining how motor rehabilitation promotes brain reorganization following stroke, an MRI study. ClinicalTrials.gov (accessed 1 June 2015). [NCT01725919]
Padovani Do Santos 2015 {published data only}
- Padovani dos Santos T. Checking a security protocol of modified forced use therapy and efficacy reducing the constriction of the movement time in 12 hours. ClinicalTrials.gov (accessed 1 June 2015).
Pereira 2015 {unpublished data only}
- Pereira ND, Camargo PR, Furtado CH. Effects of constraint‐induced therapy for the scapular kinematics and related to the quality of movement in patients with severe chronic hemiparesis. ClinicalTrials.gov (accessed 1 June 2015). [NCT02373436]
Additional references
Abdul Latif 2011
- Abdul Latif L, Daud Amadera JE, Pimentel D, Pimentel T, Fregni F. Sample size calculation in physical medicine and rehabilitation: a systematic review of reporting, characteristics, and results in randomized controlled trials. Archives of Physical Medicine and Rehabilitation 2011;92(2):306‐15. [DOI] [PubMed] [Google Scholar]
Albert 2012
- Albert SJ, Kesselring J. Neurorehabilitation of stroke. Journal of Neurology 2012;259(1):817‐32. [DOI] [PubMed] [Google Scholar]
Altman 1990
- Altman DG, Dore CJ. Randomisation and baseline comparisons in clinical trials. Lancet 1990;335:149‐53. [DOI] [PubMed] [Google Scholar]
Burns 2007
- Burns A, Burridge J, Pickering R. Does the use of a constraint mitten to encourage use of the hemiplegic upper limb improve arm function in adults with subacute stroke?. Clinical Rehabilitation 2007;21(10):895‐904. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Butler 2007
- Butler A, Wolf S. Putting the brain on the map: use of transcranial magnetic stimulation to assess and induce cortical plasticity of upper‐extremity movement. Physical Therapy 2007;87:719–36. [DOI] [PubMed] [Google Scholar]
Crow 1989
- Crow JL, Lincoln NB, Nouri FM, Weerdt W. The effectiveness of EMG biofeedback in the treatment of arm function after stroke. International Disability Studies 1989;11(4):155‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dayan 2013
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nature Neuroscience 2013;16(7):838‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DiSilvestro 2015
- DiSilvestro KJ, Tjoumakaris FP, Maltenfort MG, Spindler KP, Freedman KB. Systematic reviews in sports medicine. American Journal of Sports Medicine 2015 Apr 21 [Epub ahead of print]. [PUBMED: 25899433] [DOI] [PubMed] [Google Scholar]
Edwards 1998
- Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J. Ethical issues in the design and conduct of randomized controlled trials. Health Technology Assessments 1998;2:1‐130. [PubMed] [Google Scholar]
Engauge Software 5.1 [Computer program]
- http://digitizer.sourceforge.net/. Engauge Digitizer. http://digitizer.sourceforge.net/, 2011.
French 2007
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006073.pub2] [DOI] [PubMed] [Google Scholar]
Gianola 2013
- Gianola S, Gasparini M, Agostini M, Castellini G, Corbetta D, Gozzer P, et al. Survey of the reporting characteristics of systematic reviews in rehabilitation. Physical Therapy 2013;93(11):1456‐66. [DOI] [PubMed] [Google Scholar]
Gustavsson 2010
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E. Cost of disorders of the brain in Europe 2010. European Neuropsychopharmacology 2011;21:718‐79. [DOI] [PubMed] [Google Scholar]
Hatano 1976
- Hatano S. Experience from a multicenter stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54:541‐53. [PMC free article] [PubMed] [Google Scholar]
Hesse 2003
- Hesse S, Schulte‐Tigges G, Konrad M, Bardeleben A, Werner C. Robot‐assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Archives of Physical Medicine and Rehabilitation 2003;84(6):915‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoare 2007
- Hoare BJ, Wasiak J, Imms C, Carey L. Constraint ‐induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004149.pub2] [DOI] [PubMed] [Google Scholar]
Hotopf 1997
- Hotopf M, Lewis G, Normand C. Putting trials on trial ‐ the costs and consequences of small trials in depression: a systematic review of methodology. Journal of Epidemiology and Community Health 1997;51(4):354‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotopf 1999
- Hotopf M, Churchill R, Lewis G. Pragmatic randomised controlled trials in psychiatry. British Journal of Psychiatry 1999;175:217‐23. [DOI] [PubMed] [Google Scholar]
Juni 2006
- Juni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Practice and Research. Clinical Rheumatology 2006;20(4):721‐40. [DOI] [PubMed] [Google Scholar]
Kagan 2012
- Kagan A, Korner‐Bitensky N. Virtual reality ‐ upper extremity. Stroke Engine Intervention. Montreal: McGill University. Available at: http://www.strokengine.ca/intervention/virtual‐reality‐upper‐extremity/ 2009 (accessed 30 May 2015).
Kim 2004
- Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Plastic changes of motor network after constraint‐induced movement therapy. Yonsei Medical Journal 2004;45(2):241‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knapp 1963
- Knapp HD, Taub E, Berman AJ. Movements in monkeys with deafferented forelimbs. Experimental Neurology 1963;7:305‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lang 2008
- Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper‐extremity measures early after stroke. Archives of Physical Medicine and Rehabilitation 2008;89(9):1693‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laver 2011
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008349.pub2] [DOI] [PubMed] [Google Scholar]
Levy 2001
- Levy CE, Nichols DS, Schmalbrock PM, Keller P, Chakeres DW. Functional MRI evidence of cortical reorganization in upper‐limb stroke hemiplegia treated with constraint‐induced movement therapy. American Journal of Physical Medicine and Rehabilitation 2001;80(1):4‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liepert 2000
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment‐induced cortical reorganization after stroke in humans. Stroke 2000;31(6):1210‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liepert 2001
- Liepert J, Uhnde I, Graf S, Leidner O, Weiller C. Motor cortex plasticity during forced‐use therapy in stroke patients: a preliminary study. Journal of Neurology 2001;248(4):315‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lum 2002
- Lum PS, Burgar CG, Shor PC, Majmundar M, Loos M. Robot‐assisted movement training compared with conventional therapy techniques for the rehabilitation of upper‐limb motor function after stroke. Archives of Physical Medicine and Rehabilitation 2002;83(7):952‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Masiero 2007
- Masiero S, Celia A, Rosati G, Armani M. Robotic‐assisted rehabilitation of the upper limb after acute stroke. Archives of Physical Medicine and Rehabilitation 2007;88(2):142‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCombe Waller 2008
- McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits?. NeuroRehabiltiation 2008;23:29‐41. [PMC free article] [PubMed] [Google Scholar]
Mehrholz 2012
- Mehrholz J, Platz T, Kugler J, Pohl M. Electromechanical and robot‐assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD006876.pub3] [DOI] [PubMed] [Google Scholar]
Michielsen 2010
- Michielsen ME, Selles RW, Geest JN, EckhardtM, Yavuzer G, Stam HJ, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients. Neurorehabilitation and Neural Repair 2011;25(3):223‐33. [DOI] [PubMed] [Google Scholar]
Miller 2010
- Miller E, Murray L, Richards L, Zorowitz R, Bakas T. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke 2010;41:2402–48. [DOI] [PubMed] [Google Scholar]
Miltner 1999
- Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E. Effects of constraint‐induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke 1999;30(3):586‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moreland 1994
- Moreland J, Thomson MA. Efficacy of electromyographic biofeedback compared with conventional physical therapy for upper‐extremity function in patients following stroke: a research overview and meta‐analysis. Physical Therapy 1994;74(6):534‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nijland 2010
- Nijland RH, Wegen EE, Harmeling‐van der Wel BC, Kwakkel G, EPOS Investigators. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke 2010;41(4):745‐50. [DOI] [PubMed] [Google Scholar]
Page 2002a
- Page SJ, Sisto S, Johnston MV, Levine P, Hughes M. Modified constraint‐induced therapy in subacute stroke: a case report. Archive of Physical Medicine and Rehabilitation 2002;82(3):286‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Page 2005a
- Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Archives of Physical Medicine and Rehabilitation 2005;86(3):399‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Page 2007a
- Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo‐controlled trial. Stroke 2007;38(4):1293‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pollock 2014
- Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010820.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pomeroy 2006
- Pomeroy VM, King L, Pollock A, Baily‐Hallam A, Langhorne P. Electrostimulation for promoting recovery of movement of functional ability after stroke. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003241.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathkolb 1990
- Rathkolb O, Baykoushev S, Baykousheva V. Myobiofeedback in motor reeducation of wrist and fingers after hemispherical stroke. Electromyography and Clinical Neurophysiology 1990;30(2):89‐92. [MEDLINE: ] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ro 2006
- Ro T, Noser E, Boake C, Johnson R, Gaber M, Speroni A, et al. Functional reorganization and recovery after constraint‐induced movement therapy in subacute stroke: case reports. Neurocase 2006;12(1):50‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saka 2009
- Saka Ö, Mcguire A, Wolfe C. Cost of stroke in the United Kingdom. Age and Ageing 2009;38:27‐32. [DOI] [PubMed] [Google Scholar]
Sathian 2000
- Sathian K, Greenspan AI, Wolf SL. Doing it with mirrors: a case study of a novel approach to neurorehabilitation. Neurorehabilitation and Neural Repair 2000;14(1):73‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schaechter 2002
- Schaechter JD, Kraft E, Hilliard TS, Dijkhuizen RM, Benner T, Finklestein SP, et al. Motor recovery and cortical reorganization after constraint‐induced movement therapy in stroke patients: a preliminary study. Neurorehabilitation and Neural Repair 2002;16(4):326‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sirtori 2003
- Sirtori V, Gatti R, Corbetta D. Constraint induced movement therapy for upper extremities in stroke patients. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004433] [DOI] [PubMed] [Google Scholar]
Smania 2007
- Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, et al. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke 2007;38(3):1088‐90. [DOI] [PubMed] [Google Scholar]
Sterr 2006
- Sterr A, Szameitat A, Shen S, Freivogel S. Application of the CIT concept in the clinical environment: hurdles, practicalities, and clinical benefits. Cognitive and Behavioral Neurology 2006;19(1):48‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stinear 2007
- Stinear C, Barber P, Smale P, Coxon J, Fleming M, Byblow W. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170–80. [DOI] [PubMed] [Google Scholar]
Stinear 2010
- Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurology 2010;9:1228–32. [DOI] [PubMed] [Google Scholar]
Sunderland 2005
- Sunderland A, Tuke A. Neuroplasticity, learning and recovery after stroke: a critical evaluation of constraint‐induced therapy. Neuropsychological Rehabilitation 2005;15(2):81‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szaflarski 2006
- Szaflarski JP, Page SJ, Kissela BM, Lee JH, Levine P, Strakowski SM. Cortical reorganization following modified constraint‐induced movement therapy: a study of 4 patients with chronic stroke. Archives of Physical Medicine and Rehabilitation 2006;87(8):1052‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taub 1977
- Taub E, Heitmann RD, Barro G. Alertness, level of activity, and purposive movement following somatosensory deafferentation in monkeys. Annals of the New York Academy of Sciences 1977;290:348‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taub 1980
- Taub E, Harger M, Grier HC, Hodos W. Some anatomical observations following chronic dorsal rhizotomy in monkeys. Neuroscience 1980;5(2):389‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taub 1994
- Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW, DeLuca SC, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. Journal of the Experimental Analysis of Behavior 1994;61(2):281‐93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taub 1999
- Taub E, Uswatte G, Pidikiti R. Constraint‐induced movement therapy: a new family of techniques with broad application to physical rehabilitation ‐ a clinical review. Journal of Rehabilitation Research and Development 1999;36(3):237‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Van der Lee 2004
- Lee JH, Beckerman H, Knol DL, Vet HCW, Bouter LM. Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke 2004;35:1410‐4. [DOI] [PubMed] [Google Scholar]
Villamar 2013
- Villamar MF, Contreras VS, Kuntz RE, Fregni F. The reporting of blinding in physical medicine and rehabilitation randomized controlled trials: a systematic review. Journal of Rehabilitation Medicine 2013;45(1):6‐13. [DOI] [PubMed] [Google Scholar]
Wade 1987
- Wade D, Hewer R. Functional abilities after stroke: measurement, natural history and prognosis. Journal of Neurology, Neurosurgery, and Psychiatry 1987;50:177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. The atlas of heart disease and stroke. http://www.who.int/cardiovascular_diseases/en/cvd_atlas_15_burden_stroke.pdf?ua=1 (accessed on 1 June 2015).
Wissink 2014
- Wissink KS, Spruit‐van Eijk M, Buijck BI, Koopmans RT, Zuidema SU. Stroke rehabilitation in nursing homes: intensity of and motivation for physiotherapy. Tijdschrift voor Gerontologie en Geriatrie 2014;45(3):144‐53. [PUBMED: 24801121] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bjorklund 2006
- Bjorklund A, Fecht A. The effectiveness of constraint‐induced therapy as a stroke intervention: a meta‐analysis. Occupational Therapy in Health Care 2006;20(2):31‐49. [DOI] [PubMed] [Google Scholar]
Bonaiuti 2007
- Bonaiuti D, Rebasti L, Sioli P. The constraint induced movement therapy: a systematic review of randomised controlled trials on the adult stroke patients. Europa Medicophysica 2007;43(2):139‐46. [MEDLINE: ] [PubMed] [Google Scholar]
Corbetta 2010
- Corbetta D, Sirtori V, Moja L, Gatti R. Constraint‐induced movement therapy in stroke patients: systematic review and meta‐analysis. European Journal of Physical and Rehabilitation Medicine 2010;46:537‐44. [PubMed] [Google Scholar]
French 2008
- French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al. A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness. Health Technology Assessment 2008;12(30):1‐4. [DOI] [PubMed] [Google Scholar]
Hakkennes 2005
- Hakkennes S, Keating JL. Constraint‐induced movement therapy following stroke: a systematic review of randomised controlled trials. Australian Journal of Physiotherapy 2005;51(4):221‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kwakkel 2015
- Kwakkel G, Veerbeek JM, Wegen EE, Wolf SL. Constraint‐induced movement therapy after stroke. Lancet Neurology 2015;14(2):224‐34. [PUBMED: 25772900] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mark 2006
- Mark VW, Taub E, Morris DM. Neuroplasticity and constraint‐induced movement therapy. Europa Medicophysica 2006;42:269‐84. [PubMed] [Google Scholar]
McIntyre 2012
- McIntyre A, Viana R, Janzen S, Mehta S, Pereira S, Teasell R. Systematic review and meta‐analysis of constraint‐induced movement therapy in the hemiparetic upper extremity more than six months post stroke. Topics in Stroke Rehabilitation 2012;19(6):499‐513. [DOI] [PubMed] [Google Scholar]
Nijland 2011
- Nijland R, Kwakkel G, Bakers J, Wegen E. Constraint‐induced movement therapy for the upper paretic limb in acute or sub‐acute stroke: a systematic review. International Journal of Stroke 2011;6:425‐33. [DOI] [PubMed] [Google Scholar]
Pelton 2012
- Pelton T, Vliet P, Hollands K. Interventions for improving coordination of reach to grasp following stroke: a systematic review. International Journal of Evidence‐based Healthcare 2012;10:89‐102. [DOI] [PubMed] [Google Scholar]
Peurala 2011
- Peurala Sh, Kantanen MP, Sjögren T, Paltamaa J, Karhula M, Heinonen A. Effectiveness of constraint‐induced movement therapy on activity and participation after stroke: a systematic review and meta‐analysis of randomized controlled trials. 2011 26;3:209‐23. [DOI] [PubMed] [Google Scholar]
Shi 2011
- Shi YX, Tian JH, Yang KH, Zhao Y. Modified constraint‐induced movement therapy versus traditional rehabilitation in patients with upper‐extremity dysfunction after stroke: a systematic review and meta‐analysis. Archives of Physical Medicine and Rehabilitation 2011;92:972‐82. [DOI] [PubMed] [Google Scholar]
Sirtori 2009
- Sirtori V, Corbetta D, Moja L, Gatti R. Constraint‐induced movement therapy for upper extremities in stroke patients. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004433] [DOI] [PubMed] [Google Scholar]
Stevenson 2012
- Stevenson T, Thalman L, Christie H, Poluha W. Constraint‐induced movement therapy compared to dose‐matched interventions for upper‐limb dysfunction in adult survivors of stroke: a systematic review with meta‐analysis. Physiotherapy Canada 2012;64(4):397‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thrane 2014
- Thrane G, Friborg O, Anke A, Indredavik B. A meta‐analysis of constraint‐induced movement therapy after stroke. Journal of Rehabilitation Medicine 2014;46(9):833‐42. [DOI] [PubMed] [Google Scholar]


