Abstract
Recently, our group has contrasted an endothelial cell-smooth muscle cell (EC-SMC) co-culture model with 3D-cultured SMCs and found that SMCs could respond to high shear stress (SS), which has not been explored before. SMCs were not directly exposed to the flow but were under an EC monolayer; therefore, it is necessary to explore the influence of EC on SMC behaviors under high SS for understanding the mechanism of SMC response to various magnitudes of SS. In the present study, TGF-β1 expression in ECs in an EC-SMC co-culture model was suppressed by an siRNA transfection method. Next, phenotypic changes were observed and MMP-2 and -9 productions were measured in SMCs in the co-culture model after 72-h flow exposure to different SS levels. We confirmed that TGF-β1 expression in ECs could influence SMC phenotypic change under SS conditions and that TGF-β1 expression in ECs could also change MMP-2 production but not MMP-9 production in SMCs under SS conditions in the co-culture model. These results could be useful for understanding the mechanisms of SMC response to SS, particularly for understanding signal transduction emanating from ECs.
Keywords: Co-culture model, Smooth muscle cell, Endothelial cell, Shear stress, TGF-β1, siRNA transfection
Introduction
Vascular smooth muscle cells (SMCs) are important for blood vessel remodeling processes during cardiovascular disease (e.g., atherosclerosis and cerebral aneurysm) (Andres 1998; Frösen 2014; Penn et al. 2014; Starke et al. 2014; Tabas 2017); however, the factors influencing SMC behaviors remain unclear. Using endothelial cell (EC)-SMC co-culture models, previous studies have revealed that SMCs could change their phenotype and migration ability under a low fluid shear stress (SS) environment at less than 1 Pa (Chiu et al. 2004; Davies et al. 1985; Isakson and Duling 2005; Wang et al. 2006a, b). Recently, our group contrasted an EC-SMC co-culture model with 3D-cultured SMCs, wherein the SMCs were randomly cultured in a collagen gel (Han et al. 2017). Using this co-culture model, SMCs maintained in a contractile phenotype under a physiological level of SS (2 Pa), and SMCs could turn into a synthetic phenotype under a lower level of SS (0.2 Pa)—this finding is agreed with researches mentioned above. And for the first time, we found that a high level of SS (10 Pa) could change SMCs from a contractile phenotype to a synthetic phenotype in the co-culture model. Phenotype changes of SMC could be related to blood vessel dysfunction. Therefore, we furthermore investigated expressions of matrix metalloproteinase (MMP)-2 and -9, which have the capacity to degrade all components of the extracellular matrix. And the results showed that high SS at 10 Pa induced higher MMP-2 and -9 productions in SMCs in the co-culture model. These results showed that SMCs could respond to high SS in phenotypical change and MMPs productions in the co-culture model, which could be related to blood vessel dysfunctions under high SS and has not yet been explored.
However, SMCs were not directly exposed to the flow but were under an EC monolayer; therefore, it is possible that SMCs react to SS stimulations through EC-induced signal transduction complexes. To understand the mechanism of SMC response to various magnitudes of SS, it is necessary to explore the signal pathways from EC to SMC behaviors under high SS.
In the present study, we explored the influence of transforming growth factor beta 1 (TGF-β1) from ECs on phenotypic change and MMP production in SMCs in a co-culture model. It is reported that TGF-β1 signaling in ECs was influenced by SS (Cucina et al. 1998; Uchida and Haas 2013; Walshe et al. 2013). Further, TGF-β1 could promote SMC phenotypic change to a contractile state (Adam et al. 2000; Powell et al. 1998). Therefore, we hypothesized that SS influences TGF-β1 activation in ECs and that TGF-β1 expression in ECs modulates SMC phenotypes in the co-culture model.
To suppress gene expressions from cells in co-culture models, ECs are pretreated with siRNA transfection, specific inhibitors or conditioned media in the previous researches (Qi et al. 2011; Davenport et al. 2016; Wang et al. 2014; Xu et al. 2009). However, they focused on cells responses under statically culturing for no longer than 24 h, which are all quite different experimental conditions from the long time (72 h) flow-exposure experiment condition in the present study. Here, we tried to suppress TGF-β1 expression from ECs in the co-culture model by an siRNA transfection method; and phenotypic change of SMCs in the co-culture model was observed after flow-exposure experiments. To understand SMC functional changes, MMP-2 and -9 production in SMCs was also observed in the same conditions. As a result, we confirmed that TGF-β1 expression in ECs could influence SMC phenotypic change under SS conditions. Further, TGF-β1 expression in ECs could also change MMP-2 production but not MMP-9 production in SMCs under SS conditions in the co-culture model. These results could be helpful for understanding the mechanisms of SMC behaviors under different SS conditions, particularly under high SS.
Methods
Construction of the co-culture model
Human carotid artery ECs and SMCs from passages 4–9 (Cell Applications, Inc., San Diego, CA, USA) were used in all the experiments.
The co-culture model was constructed in the same way as in a previous study (Fig. 1a, Han et al. 2017). Briefly, the co-culture model comprised SMCs (three-dimensionally cultured in a collagen layer), a membrane filter, a spacer ring, and ECs. The ECs were cultured on the membrane filter with a proliferation medium (PM), and treated with siRNA as described later. SMCs were seeded into a type I collagen solution, and then incubated at 37 °C for 60 min to induce polymerization. SMC differentiation was controlled with a Quiescent Medium (QM) to induce the contractile phenotype.
Fig. 1.
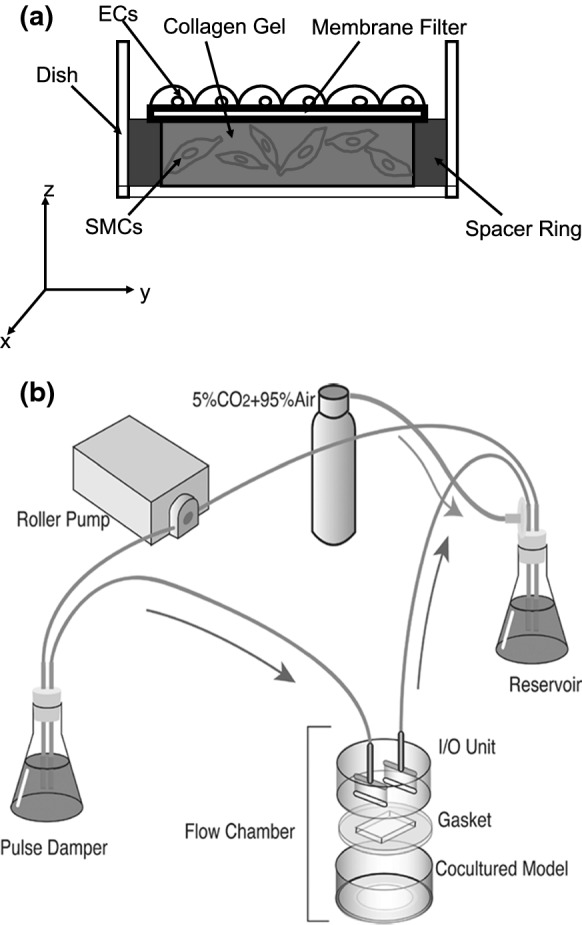
A diagram of a the EC-SMC co-culture model. The x–y–z coordinate represents the planes of observation by the confocal laser scanning microscope. b The flow system. [modified from Han et al. (2017)]
siRNA transfection to ECs
ON-TARGETplus Human TGF-β1 siRNA-SMART pool was purchased from Thermo Scientific Dharmacon (Lafayette, CO, USA). It contains four types of siRNA with the following target sequences: GGACUAUCCACCUGCAGA, GCAGAGUACACACAGCAUA, CCGAGAAGCGGUACCUGAA and AUUGAGGGCUUUCGCCUUA. SiRNA was resuspended and transfected into ECs strictly following the protocol provided by the manufacturer. In Fig. 2, TGF-β1 expression in ECs at 72 h after siRNA transfection is shown, as a quality control measure. Compared to normal ECs (wild type, WT), the expression level of TGF-β1 is suppressed to approximately 50%.
Fig. 2.

PCR results of the changes in TGF-β1 expression in WT ECs, represent by (−), or siRNA-transfected ECs, represent by (+). a A typical agarose gel result. b Expression levels of TGF-β1 (normalized to expression levels of GAPDH)
After transfection and just before flow-exposure experiments, ECs on the membrane filter were layered over the SMC layer to complete the co-culture model.
Flow-exposure experiments
A flow loop was constructed by connecting a pulse damper, flow chambers, a reservoir and a roller pump with silicone tubes (Fig. 1b, Han et al. 2017). The co-culture model was exposed to various magnitudes of SS (0.2, 2, 6 and 10 Pa) in parallel plate flow chambers for 72 h using a mixture of 25% PM and 75% QM (Co-culture Medium: CM) at 37 °C in a 95% air and 5% CO2 atmosphere.
Western blotting
After flow exposure, the membrane filter was carefully detached to leave SMCs undisturbed. Type I collagenase (Wako Pure Chemical Industries, Ltd., Japan) was added to melt the collagen gel; subsequently, SMCs were extracted and added to a lysis buffer [10 mM MgCl2, 5 mM EGTA, 50 mM b-glycerophosphate, 0.1% sodium deoxycholate, 1% NP-40, 1% sodium dodecyl sulfate, 1 mM sodium orthovanadate and 1% protease inhibitor cocktail (P2714, Sigma-Aldrich)] to prepare cell lysates for western blot analysis. After electrophoresis and transfer to PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA), two typical contractile proteins (α-SMA and calponin) and a reference protein (β-actin) were detected with their primary antibodies (Sigma-Aldrich) and an AP-conjugated secondary antibody substrate kit (Bio-Rad Laboratories), which are same as the previous study (Han et al. 2017). The band intensities of α-SMA or calponin were quantified with ImageJ (https://imagej.nih.gov/ij/, version: 2.0.0) and normalized to β-actin. To quantify the band intensities, we used “Gels” function in “Analyze” tab, and measured areas using the Wand (tracing) tool in the software.
Reverse transcription-polymerase chain reaction (RT-PCR)
Following the previous study (Han et al. 2017), MMP-2 and -9 RNA expression rates were measured after flow-exposure experiments by an RT-PCR method. Total RNAs were directly isolated from gel embedded SMCs using NucleoSpin RNA II (Takara Bio, Inc., Shiga, Japan). RNA concentrations and purity were determined by absorbance at 260 and 280 nm with a NanoVue spectrophotometer (GE Healthcare Life Sciences, Chicago, IL, USA). The ratios of A260 to A280 were between 1.75 and 1.80. RNA samples were treated by a QuantiTect Reverse Transcription kit followed by two-step RT-PCR. MMP-2 and -9 RT-PCR primers were purchased from Origene (Rockville, MD, USA), and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) RT-PCR primers were used as the internal control. The GenBank nucleotide sequence accession numbers are as follows: MMP-2, NM_004530; MMP-9, NM_004530 and GAPDH, M33197. According to the user manual of the Rotor-Gene Q (Qiagen, Hilden, Germany), the RT-PCR thermal cycling conditions were 95 °C for 5 min, followed by 40 cycles at 95 °C for 5 s and 60 °C for 10 s. Approximately 10 µl of the PCR product was separated by electrophoresis. The band density was analyzed with ImageJ. The RT-PCR results are presented as the ratio of the target band density to the corresponding GAPDH band density. QM15 sample is set as fold 1, and samples under different SS are presented as fold to QM15 in the figures.
Statistics
Results are expressed as mean ± SD determined from at least three independent experiments. The Student’s t test was used for comparison between pairs of groups. A value of p < 0.05 was considered to be statistically significant. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were used to compare MMP results and study the influence of SS. A value of p < 0.05 was considered to be statistically significant.
Results
Phenotypic change of SMCs after flow-exposure
After siRNA transfection into ECs, the co-culture model was exposed to SS of 0.2, 2, 6 and 10 Pa as described above. Alpha-SMA is one of the earliest markers of differentiation of SMC lineages and the most abundant contractile protein present in SMCs, and calponin is another early marker protein to regulate actin/myosin interaction during SMC differentiation; therefore, the expressions of these two marker protein are considered as SMC phenotype in the present study. Figure 3 shows the relative expression levels of α-SMA and calponin in the SMCs after 72-h flow exposure. The expression of α-SMA and calponin showed no obvious difference under each SS condition.
Fig. 3.
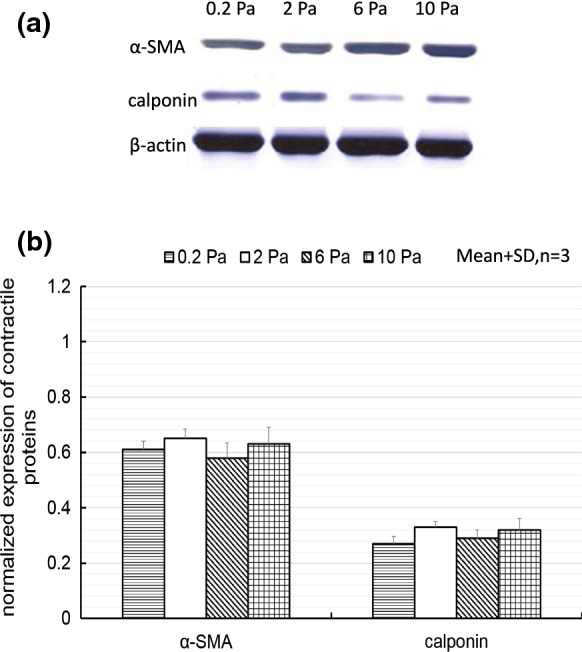
Western blotting results of changes in the expression of α-SMA and calponin in SMCs in the co-culture model with siRNA-transfected ECs under different SS conditions. a A typical western blot result. b Expression levels of contractile proteins (normalized to expression levels of β-actin)
In the present study, we focused on SMC phenotypic change under various magnitude of SS. We would like to compare these data to a co-culture model under statically condition at future, for better understanding of SMC response to SS conditions.
MMP production of SMCs after flow exposure
Figure 4 shows MMP-2 and -9 production in SMCs in the co-culture model as determined at RNA level by RT-PCR after flow exposure to SS of 0.2, 2, 6 or 10 Pa for 72 h, when TGF-β1 expression in ECs was suppressed by siRNA transfection. MMP-2 production in SMCs showed no obvious difference under each SS condition. After exposure to SS of 10 Pa, MMP-9 production in SMCs increased in comparison with cells exposed to other SS conditions (0.2, 2 or 6 Pa).
Fig. 4.

RT-PCR results of the changes in MMP-2 and -9 production in SMCs in the co-culture model with siRNA-transfected ECs under SS conditions. a A typical agarose gel result. b Fold changes of MMP-2 and -9. Data from QM15 was set as onefold. *p < 0.05 vs. 2 Pa in one-way ANOVA with Tukey’s post hoc test
Discussion
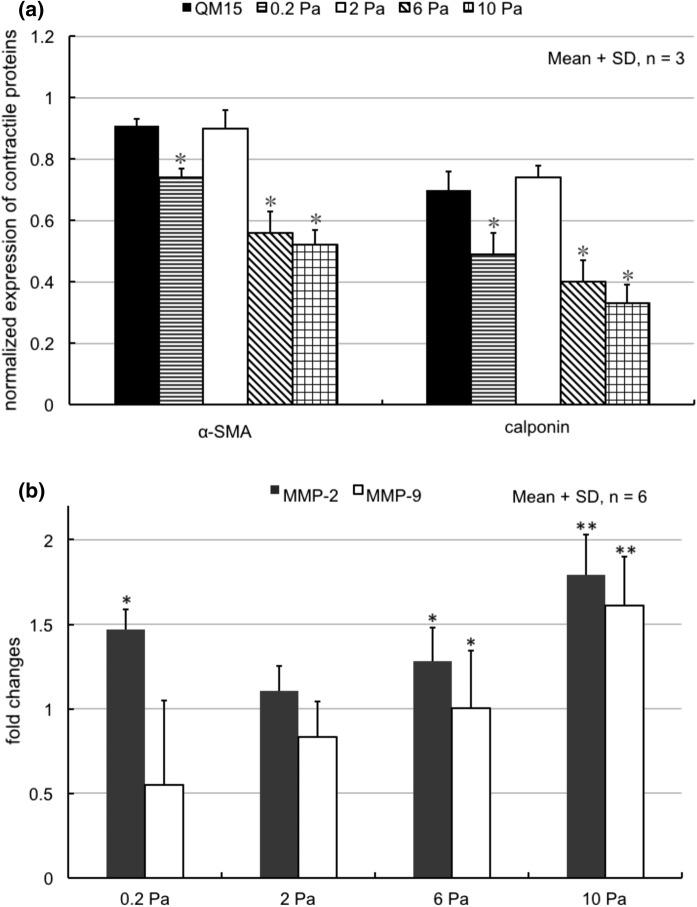
As introduced above, our group previously constructed an EC-SMC co-culture model with SMCs having a contractile phenotype when cultured in collagen gel. Using this co-culture model, SMCs maintained in a contractile phenotype under a physiological level of SS (2 Pa), and SMCs could turn into a synthetic phenotype under a lower level of SS (0.2 Pa). And we found that a high level of SS (10 Pa) could change SMCs from a contractile phenotype to a synthetic phenotype in the co-culture model. Furthermore, we found that high SS at 10 Pa induced higher MMP-2 and -9 productions in SMCs in the co-culture model, as shown in Fig. 5a, b (Han et al. 2017). These results showed that SMCs could respond to high SS in phenotypical change and MMPs productions in the co-culture model, which could be related to blood vessel dysfunction. In this study, TGF-β1 expression in ECs was suppressed by siRNA transfection. We then observed the phenotypic change and MMP production in SMCs in the co-culture model.
Fig. 5.
a SMC phenotypic change and b MMP-2 and -9 production in SMCs in the co-culture model, with WT ECs under different SS conditions. a *p < 0.05 versus QM15 in the Student’s t test. b *p < 0.05 versus 2 Pa, **p < 0.01 versus 2 Pa. in one-way ANOVA with Tukey’s post hoc tests. [modified from Han et al. (2017)]
After exposure to various magnitudes of SS (0.2, 2, 6 or 10 Pa) for 72 h, the expression of α-SMA and calponin in SMCs showed no obvious difference. Compared to the contractile phenotype of SMCs (QM15) in Fig. 5a, the expression of α-SMA and calponin was at a lower level, shown in Fig. 3, indicating SMCs could change to a synthetic state after flow exposure. In the co-culture model with WT ECs, SS of 2 Pa could maintain SMCs in the contractile phenotype as shown in Fig. 5a; while in the present study, SMCs changed to a synthetic state under 2 Pa in the co-culture model with siRNA transfected ECs as shown in Fig. 3. This result suggested that TGF-β1 expression in ECs could influence SMC phenotypic change in response to SS in the co-culture model. Previous studies have reported that TGF-β1 expression in ECs could promote SMC contractile phenotype differentiation in the co-culture model under static culture conditions (Adam et al. 2000; Powell et al. 1998). Therefore, it is possible that SS of 2 Pa could induce higher expression of TGF-β1 in WT ECs to maintain the contractile phenotype in SMCs; while SS of 0.2, 6 or 10 Pa could suppress TGF-β1 expression in WT ECs, resulting in SMCs changing their phenotype to a synthetic state, considering that TGF-β1 expression suppression by siRNA did not change α-SMA and calponin expression in SMCs under these SS conditions (0.2, 6 or 10 Pa). However, further experiments are needed to confirm TGF-β1 expression in ECs under different SS conditions.
When TGF-β1 expression in ECs was suppressed by siRNA, MMP-2 production in SMCs showed no obvious difference after flow exposure to SS amounts of 0.2, 2, 6 or 10 Pa for 72 h, as shown in Fig. 4. In the co-culture model with WT ECs, MMP-2 production in SMCs decreased under 2 Pa SS, compared to the other SS conditions (0.2, 6 and 10 Pa), as shown in Fig. 5b. Therefore, TGF-β1 expression in ECs could influence MMP-2 production in SMCs under SS in the co-culture model. It is reported that TGF-β1 activation was partly related to the increase of MMP-2 activity in an animal study (Wang et al. 2006a, b), which agrees with the present study.
Compared to Fig. 5b, it seems that MMP-9 production in SMCs showed no obvious difference, whether TGF-β1 expression in ECs was suppressed or not. A higher SS of 10 Pa could induce higher MMP-9 production in SMCs in both conditions. Therefore, TGF-β1 expression from ECs did not influence MMP-9 production in SMCs under SS in the co-culture model.
In the present study, we used siRNA transfection to suppress gene expression in ECs, which has been rarely used in co-culture model related flow experiments before. As mentioned in the introduction, cells responses under statically culturing for 8 h or 24 h were observed in co-culture models, while ECs are pretreated with siRNA transfection, specific inhibitors or conditioned media to suppress gene expression in the previous researches (Qi et al. 2011; Davenport et al. 2016; Wang et al. 2014; Xu et al. 2009). However, SMC in the co-culture model could response to SS stimulation in a different way from the static condition, as reported in our former study (Han et al. 2017). The present study provided a more direct view of the influence of gene expression in ECs on SMC function in the co-culture model under long time SS exposure conditions than the previous studies. Our group was the first to apply high SS to a co-culture model with 3D-cultured SMCs, as previously introduced (Han et al. 2017). In addition, SMCs were cultured at the bottom of a dish as a two-dimensional monolayer in most of previous studies (Sakamoto et al. 2011), whereas in vivo SMCs were mixed with extracellular matrix in the tunica media of blood vessels. Considering of such structure, we constructed the present co-culture model with SMC 3-D cultured in collagen gel, as introduced in our former study (Han et al. 2017). Therefore, the author suggested a method to investigate signal transduction between ECs and SMCs under high SS in the present study. The present result revealed the role of TGF-β1 in ECs on the mechanisms of SMC response to high SS. In the future, we would like to test the expression of other gene (e.g., PDGF and VEGF) in ECs, which might be related to SMC functional changes (Nakazawa et al. 2000; Qi et al. 2011; Wu et al. 2010), using the same method used in the present study. We also would like to test if it is possible to perform the flow-exposure experiment with both the siRNA transfected co-culture model and the untreated model in once time in the future, for better understanding of the current method.
Conclusions
In the present study, TGF-β1 expression in ECs in an EC-SMC co-culture model was suppressed by siRNA transfection. Then, phenotypic changes and MMP-2 and -9 production in SMCs in the co-culture model was measured after 72-h flow exposure to various magnitudes of SS (0.2, 2, 6 or 10 Pa).
There is no obvious difference in the expression of α-SMA and calponin in SMCs under each SS condition when TGF-β1 expression in ECs was suppressed. In addition, MMP-2 production in SMCs showed no obvious difference, while a higher SS of 10 Pa induced high MMP-9 production in SMCs. Compared to the co-culture model with WT ECs, these results confirmed that TGF-β1 expression in ECs could influence SMC phenotypic change under SS conditions. Under a SS of 2 Pa, TGF-β1 suppression could induce SMC phenotypical change to a synthetic state, which is different from non-transfected co-culture models. In addition, suppressing TGF-β1 expression in ECs could also change MMP-2 productions under different SS conditions, compared to co-culture model with WT ECs. However, MMP-9 production in SMCs under SS conditions in the co-culture model showed same tendency in co-culture models with siRNA transfected ECs or WT ECs.
We believed that these results could be useful for understanding the mechanisms of SMC response to SS, particularly for understanding signal transduction emanating from ECs.
Acknowledgements
The authors would like to thank Dr. Jennifer M. Dolan for technical advices on cell culturing and flow-exposure experiment. We also thank Prof. Takehiko Sato, Prof. Makoto Kanzaki and Dr. Daisuke Yoshino for support during the experiments. This work was partly supported by grants from the JSPS Core-to-Core Program, A. Advanced Research Networks, ‘International research core on smart layered materials and structures for energy saving’ and the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (B), 2013-2015 (Grant No. 25282140 awarded to Makoto Ohta).
References
- Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J Biol Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- Andres V. Control of vascular smooth muscle cell growth and its implication in atherosclerosis and restenosis (review) Int J Mol Med. 1998;2:81–89. doi: 10.3892/ijmm.2.1.81. [DOI] [PubMed] [Google Scholar]
- Chiu JJ, Chen LJ, Chen CN, Lee PL, Lee CI. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J Biomech. 2004;37:531–539. doi: 10.1016/j.jbiomech.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Cucina A, Sterpetti AV, Borrelli V, Pagliei S, Cavallaro A, D’Angelo LS. Shear stress induces transforming growth factor-beta 1 release by arterial endothelial cells. Surgery. 1998;123:212–217. doi: 10.1016/S0039-6060(98)70260-0. [DOI] [PubMed] [Google Scholar]
- Davenport C, Harper E, Forde H, Rochfort KD, Murphy RP, Smith D, Cummins PM. RANKL promotes osteoblastic activity in vascular smooth muscle cells by upregulating endothelial BMP-2 release. Int J Biochem Cell Biol. 2016;77:171–180. doi: 10.1016/j.biocel.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Davies PF, Truskey GA, Warren HB, O’Connor SE, Eisenhaure BH. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985;101:871–879. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frösen J. Smooth muscle cells and the formation, degeneration, and rupture of saccular intracranial aneurysm wall–a review of current pathophysiological knowledge. Transl Stroke Res. 2014;5:347–356. doi: 10.1007/s12975-014-0340-3. [DOI] [PubMed] [Google Scholar]
- Han X, Sakamoto N, Tomita N, Meng H, Sato M, Ohta M (2017). Influence of shear stress on phenotype and MMP production of smooth muscle cells in a co-culture model. J Biorheol (accepted) [DOI] [PMC free article] [PubMed]
- Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Yasuhara H, Shigematsu K, Shigematsu H. Smooth muscle cell migration induced by shear-loaded platelets and endothelial cells. Enhanced platelet-derived growth factor production by shear-loaded platelets. Int Angiol. 2000;19:142–146. [PubMed] [Google Scholar]
- Penn DL, Witte SR, Komotar RJ, Sander Connolly E., Jr The role of vascular remodeling and inflammation in the pathogenesis of intracranial aneurysms. J Clin Neurosci. 2014;21:28–32. doi: 10.1016/j.jocn.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Powell RJ, Bhargava J, Basson MD, Sumpio BE. Coculture conditions alter endothelial modulation of TGF-beta 1 activation and smooth muscle growth morphology. Am J Physiol. 1998;274:H642–H649. doi: 10.1152/ajpheart.1998.274.2.H642. [DOI] [PubMed] [Google Scholar]
- Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, Jiang ZL. PDGF-BB and TGF-{beta}1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci USA. 2011;108:1908–1913. doi: 10.1073/pnas.1019219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N, Kiuchi T, Sato M. Development of an endothelial-smooth muscle cell coculture model using phenotype-controlled smooth muscle cells. Ann Biomed Eng. 2011;39:2750–2758. doi: 10.1007/s10439-011-0372-8. [DOI] [PubMed] [Google Scholar]
- Starke RM, Chalouhi N, Ding D, Raper DM, Mckisic MS, Owens GK, Hasan DM, Medel R, Dumont AS. Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res. 2014;5:338–346. doi: 10.1007/s12975-013-0290-1. [DOI] [PubMed] [Google Scholar]
- Tabas I. 2016 Russell ross memorial lecture in vascular biology: molecular-cellular mechanisms in the progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:183–189. doi: 10.1161/ATVBAHA.116.308036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida C, Haas TL. Endothelial cell TIMP-1 is upregulated by shear stress via Sp-1 and the TGFβ1 signaling pathways. Biochem Cell Biol. 2013;92:77–83. doi: 10.1139/bcb-2013-0086. [DOI] [PubMed] [Google Scholar]
- Walshe TE, dela Paz NG, D’Amore PA. The role of shear-induced transforming growth factor-β signaling in the endothelium. Arterioscler Thromb Vasc Biol. 2013;33:2608–2617. doi: 10.1161/ATVBAHA.113.302161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HQ, Huang LX, Qu MJ, Yan ZQ, Liu B, Shen BR, Jiang ZL. Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium. 2006;13:171–180. doi: 10.1080/10623320600760282. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- Wang L, Han Y, Shen Y, Yan ZQ, Zhang P, Yao QP, Shen BR, Gao LZ, Qi YX, Jiang ZL. Endothelial insulin-like growth factor-1 modulates proliferation and phenotype of smooth muscle cells induced by low shear stress. Ann Biomed Eng. 2014;42:776–786. doi: 10.1007/s10439-013-0957-5. [DOI] [PubMed] [Google Scholar]
- Wu S, Wu X, Zhu W, Cai WJ, Schaper J, Schaper W. Immunohistochemical study of the growth factors, aFGF, bFGF, PDGF-AB, VEGF-A and its receptor (Flk-1) during arteriogenesis. Mol Cell Biochem. 2010;343:223–229. doi: 10.1007/s11010-010-0517-3. [DOI] [PubMed] [Google Scholar]
- Xu S, He Y, Vokurkova M, Touyz RM. Endothelial cells negatively modulate reactive oxygen species generation in vascular smooth muscle cells: role of thioredoxin. Hypertension. 2009;54:427–433. doi: 10.1161/HYPERTENSIONAHA.109.133983. [DOI] [PubMed] [Google Scholar]