Abstract
Lysozyme is an anti-bacterial protein that is widely distributed in nature. Our previous studies revealed that lysozyme shows anti-inflammatory effect on hyperinflammatory macrophages in vitro. The effect of lysozyme on lipopolysaccharide-induced inflammation model mice was examined in this study. Oral administration of lysozyme at 2250 mg/kg body weight/day (high-dose group) significantly suppressed interleukin (IL)-6 and tumor necrosis factor-α levels in the serum. IL-6 level in the spleen was significantly suppressed by lysozyme at 450 mg/kg body weight/day (middle-dose group) and high-dose group due to the suppression of gene expression level. The gene expression levels of IL-1β and IL-12 were also decreased by lysozyme in the high-dose group. In addition, lysozyme significantly suppressed IL-6 level in the liver in the high-dose group. Our findings suggest that lysozyme mitigates inflammatory condition in vivo by suppressing inflammatory cytokine levels in serum and organs from LPS-induced inflammation model mice.
Keywords: Anti-inflammation, Lysozyme, Lipopolysaccharide-induced systemic inflammation, Inflammatory cytokine
Introduction
Inflammation is an important biological defense system, which occurs when individuals are infected with pathogens such as viruses and bacteria. Leukocytes, such as macrophages, neutrophils, mast cells, and T lymphocytes, are related to inflammation. Lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria, is recognized by macrophages and promotes the release of various mediators such as cytokines, chemokines, and prostaglandins. These pro- and anti-inflammatory mediators are subtly balanced. The balance between these mediators controls inflammatory response (Cavaillon and Annane 2006). Excessive inflammatory reactions by losing the balance between these mediators induce many diseases, such as rheumatoid arthritis, inflammatory bowel disease, and arteriosclerosis promoted by insulin resistance (Keffer et al. 1991; Wellen and Hotamisligil 2003; Xu et al. 2003; Xavier and Podolsky 2007; Nishimoto et al. 2016).
Systemic inflammatory response syndrome (SIRS) is an acute inflammatory reaction induced by bacterial invasion, injury, and hemorrhagic shock. Symptoms of SIRS include the change in body temperature and leukocyte count and the increase in heart rate, respiratory rate, and serum inflammatory cytokine levels (Bone et al. 1992; Cavaillon and Annane 2006). Sepsis is an infectious disease accompanied by SIRS, and approximately 60% of sepsis is caused by Gram-negative bacteria. Since LPS plays an important part to initiate the inflammatory reaction, intraperitoneal administration of LPS is commonly used to induce SIRS in animal models (Copeland et al. 2005; Rampanelli et al. 2013).
Lysozyme is an antibacterial protein that breaks down bacterial cell walls and widely distributed in various biological fluids and tissues, such as tears, saliva, and respiratory secretions, as well as in polymorphonuclear leukocytes (Jollès and Jollès 1984). Hen egg white lysozyme is a basic protein (pI = 11) composed of a single polypeptide chain with 129 amino acid residues. Lysozyme has been reported to promote antibody production by lymphocytes and to enhance the anti-bacterial and immunostimulatory activities by heat treatment (Murakami et al. 1997; Sugahara et al. 2000, 2002; Carrillo et al. 2016). Lysozyme also has an anti-inflammatory effect by binding LPS to form a complex and inhibits inflammatory reactions (Takada et al. 1994). Moreover, our previous study also revealed that lysozyme inhibits phosphorylation of c-Jun N-terminal kinase (JNK) to suppress the expression of pro-inflammatory cytokine genes in hyperinflammatory macrophages (Tagashira et al. 2018). These facts suggest that lysozyme has immune-regulatory effects through various mechanisms in vitro. Because lysozyme has been reported to be one of the molecules absorbed as an intact form from the proximal intestinal tract (Yokooji et al. 2013), it is surmised that lysozyme, which is not degraded in the digestive tract, may be absorbed as an intact form and show the anti-inflammatory effect in vivo. Based on the above, we investigated the effect of lysozyme from hen egg white on LPS-induced systemic inflammation model mice to clarify the anti-inflammatory effect of oral administration of lysozyme in vivo.
Materials and methods
Reagents
Lysozyme from chicken egg white (purity ≥ 96%) and LPS from Escherichia coli 026/B6 were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were purchased from Fujifilm Wako Pure Chemical (Osaka, Japan) or Nacalai Tesque (Kyoto, Japan) unless otherwise noted.
Animals
Seven-week-old female BALB/c mice were obtained from Japan SLC (Hamamatsu, Japan) and housed in an animal room under a 12 h light/dark cycle at a temperature of 24 ± 2 °C. Animals received a pelleted basal diet and water ad libitum. Animal experiments were approved by the Animal Experiment Committee of Ehime University and were performed in accordance with the Guidelines of Animal Experiments of Ehime University.
LPS-induced systemic inflammation model mice
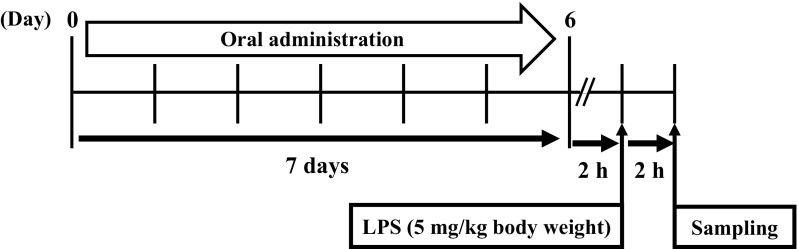
The assay was performed by the method of Clark et al. (2007) with some modifications. Treatment of mice was conducted along the schedule shown in Fig. 1. After acclimatization to their housing environment for 1 week, 8-week-old female BALB/c mice were placed into 5 groups (7 mice per group). On day 0, mice were orally administered 200 µL of 10 mM sodium phosphate buffer (NaPB) containing lysozyme at 4.5 mg/kg body weight/day for the low-dose group, at 450 mg/kg body weight/day for the middle-dose group, or at 2250 mg/kg body weight/day for the high-dose group using a feeding needle (Natsume Seisakusho, Tokyo, Japan) for seven consecutive days from day 0 to day 6. Mice in the intact and control groups were administered 200 µL of 10 mM NaPB alone. Two hours after oral administration on day 6, all mice except for the intact group were injected with 20 µL of phosphate-buffered saline (PBS) containing LPS (5 mg/kg body weight) into the peritoneum to induce systemic inflammation. The intact group was injected with 20 µL of PBS alone. Two hours after intraperitoneal administration, all mice were euthanized to collect whole blood, spleen, and liver.
Fig. 1.
Experiment schemes generating LPS-induced systemic inflammation model mice. Seven-week-old female BALB/c mice were orally administrated with lysozyme of various concentration and NaPB (intact and control group). On 6 days, all mice except intact group were injected with LPS (5 mg/kg body weight). In contrast, intact group was injected with PBS alone. After that, all mice were collected serum, spleen, and liver
Leukocytes counting
An equal volume of PBS containing 4.5% dextran 200,000 (Fujifilm Wako Pure Chemical) was added to an aliquot of the collected blood. After mixing by inversion, the mixture was allowed to stand for 30 min to precipitate erythrocytes. The supernatant was then collected in new tubes and centrifuged at 200×g for 5 min at 4 °C. After discarding the supernatant, sterilized distilled water was added to the cell pellet to hemolyze erythrocytes. After 5–10 s, 10 × PBS was added at 10% to restore osmotic pressure and mixed by inversion. After centrifugation at 200×g for 5 min at 4 °C, the supernatant was discarded and the cells were suspended in PBS for cell counting using a hemocytometer.
Cytokine measurements
The blood samples stored at 4 °C overnight were centrifuged at 2000×g for 20 min at 4 °C, and the serum samples were used to measure the concentration of cytokines. Collected spleen and liver were flash frozen in liquid nitrogen. A piece of each organ sample was homogenized and sonicated in RIPA buffer at 20 mg/mL. After centrifugation at 12,000×g for 15 min at 4 °C, the supernatant was collected and stored at − 35 °C. The concentrations of interleukin (IL)-6 and tumor necrosis factor (TNF)-α in samples were measured by enzyme-linked immunosorbent assay (ELISA) using mouse IL-6 ELISA kit (BioLegend, San Diego, CA, USA) and mouse TNF-α ELISA kit (eBioscience, San Diego, CA, USA), respectively, according to the manufacturer’s instructions.
Real-time reverse transcription-polymerase chain reaction (real-time RT-PCR)
A piece of frozen spleen and liver samples was used for RNA extraction. Total RNA was isolated using Sepasol-RNA I Super G (Nacalai Tesque) according to the manufacturer’s instructions and used as a template for cDNA synthesis with MMLV-reverse transcriptase (Promega, Madison, WI, USA) and an oligo-(dT)20 primer (Toyobo, Osaka, Japan). Real-time PCR was performed using Thunderbird SYBR qPCR Mix (Toyobo), 10 pmol of a forward primer, 10 pmol of a reverse primer, and 0.1 µg of a cDNA sample as previously described (Nishi et al. 2011) with some modifications. Thermal cycling conditions were 20 s at 95 °C, and 40 cycles of 3 s at 95 °C and 30 s at 60 °C. PCR products were measured on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), and relative gene expression was calculated based on the comparative CT method using StepOne Software v2.1 (Applied Biosystems). Expression of the glyceraldehyde-3-phosphate (GAPDH) gene was used as an endogenous control. Specific oligonucleotide sequences for each gene are as follows. Mouse GAPDH: sense, 5′-AAAGTGGAGATTGTTGCCAT-3′ and antisense, 5′-TTGACTGTGCCGTTGAATT-3′; mouse IL-6: sense, 5′-AAGCCAGAGTCCTTCAGAGAGAT-3′ and antisense, 5′-TTGGATGGTCTTGGTCCTTAGC-3′; mouse TNF-α: sense, 5′-CTACTCCCAGGTTCTCTTCAA-3′ and antisense, 5′-GCAGAGAGGAGGTTFACTTTC-3′; mouse IL-1β: sense, 5′-GAAGTCAAGAGCAAAGTGG-3′ and antisense, 5′-ACAGTCCAGCCCATACTTT-3′; mouse IL-12: sense, 5′-GGAAGCACGGCAGAATA-3′ and antisense, 5′-AACTTGAGGGAGAAGTAGGAATGG-3′.
Statistical analysis
Data obtained were expressed as mean ± standard deviation. One-way ANOVA followed by the Tukey–Kramer test was used to assess the statistical significance of the difference. Values with *p < 0.05 or **p < 0.01 were considered statistically significant.
Results
Effect of lysozyme on body weight and leukocyte numbers in the blood
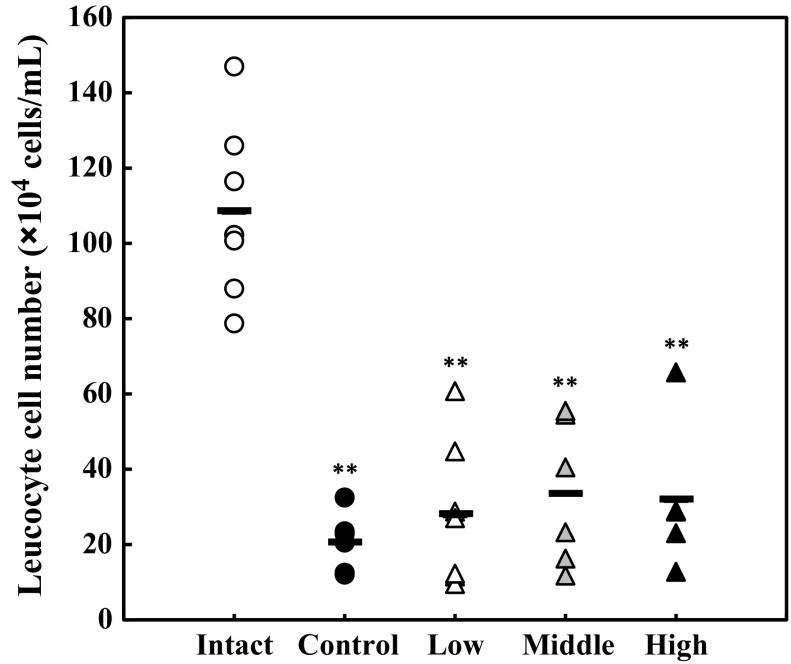
Firstly, we investigated the effect of lysozyme on the body weight of mice. Mice were orally administered various doses of lysozyme or 10 mM NaPB alone (vehicle) for seven consecutive days. As the result, oral administration of lysozyme did not affect the body weight of mice in all lysozyme-administered groups compared with the control group (data not shown). Systemic inflammation was induced by intraperitoneal injection of LPS at 5 mg/kg body weight. Two hours after LPS injection, symptoms of the LPS-induced systemic inflammatory response were observed: decreased mobility, piloerection, and weeping eyes (data not shown). Moreover, the number of leukocytes in the blood was measured. As shown in Fig. 2, the number of leukocytes in the blood from the control group and LPS-administered groups significantly declined compared with the intact group, suggesting that systemic inflammation was successfully induced by intraperitoneal administration of LPS. On the other hand, there was no statistical significance in the leukocyte number in the blood between the control group and any of lysozyme-administered groups, suggesting that lysozyme does not affect the decrease in the plasma leukocyte number induced by intraperitoneal injection of LPS.
Fig. 2.
Change in the cell numbers of leukocytes in the blood by intraperitoneal administration of LPS. The cell numbers of leukocytes in the blood of mice were measured using a hemocytometer. Opened circle, intact group; closed circle, control group; opened triangle, low-dose group; shaded triangle, middle-dose group; closed triangle, high-dose group. Data are represented as mean ± standard deviation (n = 7). **p < 0.01 against intact by Tukey–Kramer test
Effect of lysozyme on inflammatory cytokine levels in serum
The effect of lysozyme on inflammatory cytokine levels in serum was examined. The serum concentrations of IL-6 and TNF-α were measured by ELISA. As the results, serum IL-6 and TNF-α levels of the high-dose group decreased by 40% compared with those of the control group with a statistically significant difference (Fig. 3). Further, the amounts of IL-6 and TNF-α tended to be suppressed in the middle-dose group. These results suggested that oral administration of lysozyme alleviates the systemic inflammation in vivo.
Fig. 3.
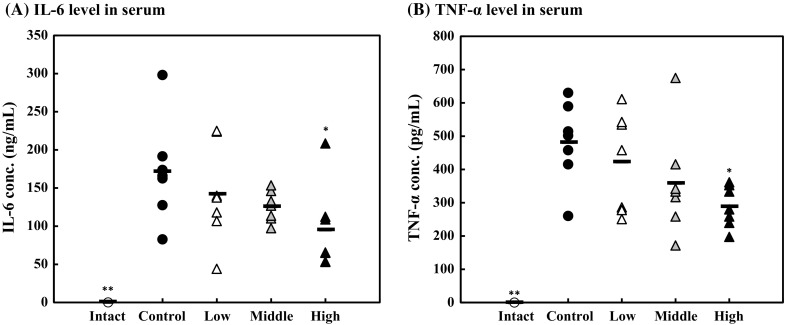
Effect of lysozyme on the amounts of inflammatory cytokines in serum. IL-6 (a) and TNF-α (b) levels in the serum analyzed using ELISA kits. Opened circle, intact group; closed circle, control group; opened triangle, low-dose group; shaded triangle, middle-dose group; closed triangle, high-dose group. Data are represented as mean ± standard deviation (n = 7). *p < 0.05 or **p < 0.01 against control by Tukey–Kramer test
Effect of lysozyme on the production and gene expression of inflammatory cytokines in the spleen
The effect of lysozyme on inflammatory cytokine production in the organs from LPS-induced systemic inflammation model mice was examined. At first, inflammatory cytokine levels in the spleen were measured by ELISA. As shown in Fig. 4, the IL-6 and TNF-α levels in the spleen were significantly suppressed in the high-dose and middle-dose groups. IL-6 and TNF-α levels in the spleen were suppressed by 40% in the high-dose group compared with the control group.
Fig. 4.
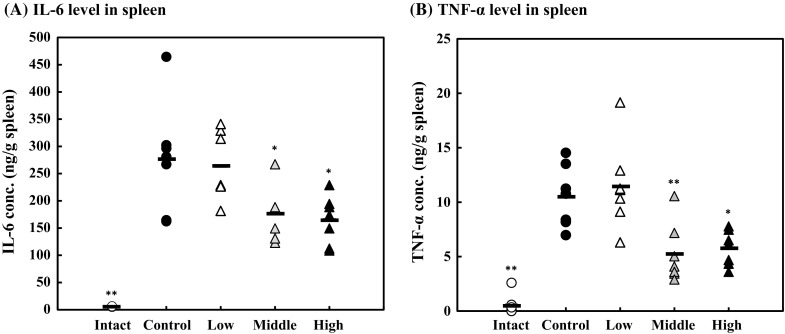
Effect of lysozyme on inflammatory cytokine levels in the spleen. IL-6 (a) and TNF-α (b) levels in the spleen analyzed using ELISA kits. Opened circle, intact group; closed circle, control group; opened triangle, low-dose group; shaded triangle, middle-dose group; closed triangle, high-dose group. Data are represented as mean ± standard deviation (n = 7). *p < 0.05 or **p < 0.01 against control by Tukey–Kramer test
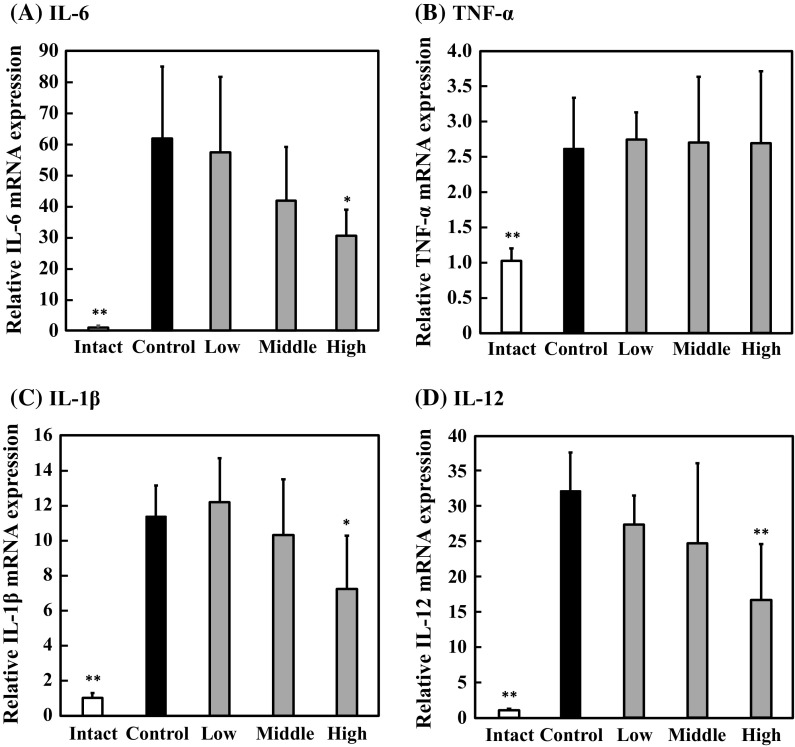
Next, the effect of lysozyme on the expression of IL-6 and TNF-α genes in the spleen was examined by real-time RT-PCR. As the results, the IL-6 gene expression was significantly suppressed in the high-dose group compared with that in the control group (Fig. 5a). Moreover, the tendency to suppress IL-6 gene expression was observed in the middle-dose group. These results indicated that lysozyme downregulates the IL-6 gene expression in the spleen, leading to the decreased IL-6 concentration. However, lysozyme did not affect TNF-α gene expression level in the spleen (Fig. 5b). These results suggested that lysozyme suppresses the TNF-α production in the spleen in a different way from IL-6 production. In addition, we measured the expression levels of IL-1β and IL-12 genes. As indicated in Fig. 5c, d, the expression levels of IL-1β and IL-12 genes in the spleen from the high-dose group were suppressed by 40–50% compared with those from the control group with a statistically significant difference. These results suggested that oral administration of lysozyme inhibits the expression of inflammatory cytokine genes in the spleen.
Fig. 5.
Effect of lysozyme on inflammatory cytokine gene expressions in the spleen. Expressions of IL-6 (a), TNF-α (b), IL-1β (c) and IL-12 (d) genes in the spleen was analyzed using real-time RT-PCR. Data are represented as mean ± standard deviation (n = 7). *p < 0.05 or **p < 0.01 against control by Tukey–Kramer test
Effect of lysozyme on inflammatory cytokines levels in the liver
Furthermore, the effect of lysozyme on inflammatory cytokine levels in the liver was examined. As shown in Fig. 6, the IL-6 level in the liver was suppressed by 40% in the high-dose group compared with that in the control group with a statistically significant difference. On the other hand, the TNF-α level in the liver was not affected by lysozyme. These results suggest that oral administration of lysozyme is particularly effective in suppressing IL-6.
Fig. 6.

Effect of lysozyme on inflammatory cytokine levels in the liver. IL-6 (a) and TNF-α (b) levels in the liver analyzed using ELISA kits. Opened circle, intact group; closed circle, control group; opened triangle, low-dose group; shaded triangle, middle-dose group; closed triangle, high-dose group. Data are represented as mean ± standard deviation (n = 7). **p < 0.01 against control by Tukey–Kramer test
Discussion
In previous study, we revealed that lysozyme from hen egg white suppresses production of inflammatory cytokines through inhibiting phosphorylation of JNK, one of MAP kinase family, in LPS-induced hyperinflammatory macrophages in vitro (Tagashira et al. 2018). Therefore, we investigated the anti-inflammatory effect of lysozyme in vivo. Previous studies have reported that intravenous administration of lysozyme inhibited polyphosphate-mediated vascular inflammatory responses in mice (Chung et al. 2016). Moreover, lysozyme attenuated vascular inflammation through inhibiting of high mobility group box 1 signaling pathways in human endothelial cells and in mice (Lee et al. 2015). It has been reported that intravenous administration of lysozyme alleviates vascular inflammation in vivo. On the other hand, this research aims at the application of lysozyme from hen egg white as a functional food. Since it has been reported that lysozyme is absorbed from the intestinal tract without being decomposed (Yokooji et al. 2013), we investigated the effect of oral administration of lysozyme in mice. Figure 1 showed that lysozyme did not affect body weight of all mice after oral administration for 7 consecutive days, suggesting that the lysozyme doses used in this experiment shows no toxicity. We induced the systemic inflammatory response by intraperitoneal administration of LPS to the control group and lysozyme administration groups on day 6. Inducing inflammation by intraperitoneal administration of LPS has been used in many papers (Turrin et al. 2001; Kabir et al. 2002; Gao et al. 2013). However, there are few reports on the effect of lysozyme on LPS-induced systemic inflammatory model mice. Therefore, we examined the anti-inflammatory effect of oral administration of lysozyme from hen egg white on LPS-induced systemic inflammation model mice. Because leukocytes migrate to sites of inflammation such as organs, and the number of leukocytes in the blood changes when systemic inflammation is induced (Cavaillon and Annane 2006; Nierthaus et al. 2013), we measured leukocyte counts in serum. We found that the number of leukocytes in the blood of the control group and LPS-administered groups significantly declined compared with that of the intact group and there was no alleviation of leukocyte count fluctuation due to the administration of lysozyme, suggesting that lysozyme did not affect the fluctuation of leukocyte by LPS-induced systemic inflammation. Since it has been reported that cytokines are produced in excess in systemic inflammatory response (Cavaillon and Annane 2006), we analyzed serum inflammatory cytokines levels. As shown in Fig. 3, lysozyme significantly inhibited amounts of IL-6 and TNF-α levels in serum. It has been revealed that lysozyme suppresses production of IL-6 and TNF-α in hyperinflammatory macrophages (Tagashira et al. 2018). These outcomes suggest that lysozyme could act on macrophages and suppress them in vivo.
Systemic inflammation acts on various organs such as lung, heart, liver, spleen, and kidney and contributes to the release of inflammatory cytokines (Saito et al. 2000; Cavaillon and Annane 2006; Qin et al. 2007). For example, acute kidney injury is induced by intraperitoneally injection of LPS (Chen et al. 2015). In this study, we examined the effect of lysozyme on spleen and liver from LPS-induced systemic inflammation model mice. The spleen traps and removes blood-borne antigens, and is involved in innate and adaptive immunity against pathogens. Leukocytes in the spleen include T cells, B cells, dendritic cells, monocytes, and macrophages, and the spleen regulates immune responses harmful to the host. We found that IL-6 and TNF-α levels in the spleen were significantly suppressed by lysozyme. In previous studies, it has been reported that IL-6 and TNF-α gene expression levels increase in the spleen by LPS-induced inflammation (Saito et al. 2000; Qin et al. 2007). We also found that IL-6 gene expression is inhibited by lysozyme in the spleen, suggesting that lysozyme suppresses IL-6 production in the spleen through inhibiting IL-6 gene expression. In contrast, lysozyme did not affect TNF-α gene expression, suggesting that TNF-α production in the spleen suppressed by the different way from IL-6 production. Furthermore, lysozyme inhibited the expression of IL-1β and IL-12 genes. IL-1β is an inflammatory cytokine produced by various cells such as monocytes, tissue macrophages, and keratinocytes and is known to play central roles in the inflammatory response and to activate various kinds of cells (Dinarello 1996). For example, IL-1β activates neutrophils and enhances superoxide release, chemotaxis, and degranulation (Brandolini et al. 1996; Suzuki et al. 2001). On the other hand, IL-12 is mainly produced monocytes/macrophages, B cells, and connective tissue mast cells (Vignali and Kuchroo 2012). IL-12 also induces production of interferon γ (IFN-γ) by NK cells and T cells, which promotes Th1 differentiation. Although IL-12 plays an important role in biological defense, it is also involved in the organ-specific autoimmune diseases in humans such as diabetes, arthritis, and inflammatory bowel disease (Oppmann et al. 2000). In addition, it is suggested that the production of these inflammatory cytokines suppressed by lysozyme may inhibit to activate other cells. Moreover, it is expected that lysozyme ameliorates not only systemic inflammation but also organ-specific autoimmune diseases because IL-12 is involved in organ-specific autoimmune diseases. In fact, it has been reported that lysozyme modulates the expression of IL-8, IL-12, IL-17, and IFN-γ genes in colonic tissues in a porcine model of dextran sodium sulfate-induced colitis (Lee et al. 2009). In this study, lysozyme suppressed the expression of inflammatory cytokine genes in the spleen from LPS-induced systemic inflammation model mice, suggesting that oral administration of lysozyme could attenuate organ-specific inflammation.
Finally, as a result of examining inflammatory cytokine levels in the liver, lysozyme suppressed IL-6 levels, whereas TNF-α did not affect by lysozyme. IL-6 is produced by various cells including T cells, B cells, macrophages, adipose cells, endothelial cells, and fibroblasts, and acts on various cells such as T cells and B cells (Kang et al. 2015). IL-6 is not expressed in healthy individuals but expressed by infection and tissue damage. On the other hand, chronic expression of IL-6 gene is known to be involved in the pathogenesis of many autoimmune and inflammatory diseases (Tanaka and Kishimoto 2014). Therefore, inhibition of IL-6 production is expected to be a therapeutic strategy for various diseases characterized by overexpression of IL-6 gene. In this study, lysozyme significantly suppressed IL-6 level in serum, spleen, and liver from LPS-induced systemic inflammation model mice, expecting that lysozyme could be applied as an effective functional material.
Conclusion
Lysozyme suppressed IL-6 and TNF-α levels in serum from LPS-induced systemic inflammation model mice. In addition, lysozyme inhibited the expression of IL-6 gene in the spleen, resulting in the suppressed IL-6 levels in the spleen and serum. Moreover, the expression of IL-1β and IL-12 genes were also inhibited by oral administration of lysozyme. On the other hand, lysozyme suppressed TNF-α levels in the spleen, whereas the expression of TNF-α gene was not affected by lysozyme. These results suggest that lysozyme inhibited TNF-α levels in the spleen by a different way from the IL-6 pathway. Furthermore, lysozyme decreased IL-6 levels in the liver, suggesting that lysozyme inhibits the expression and production of IL-6 in LPS-induced systemic inflammation model mice. Taken together, our data indicated that oral administration of lysozyme from hen egg white ameliorates LPS-induced systemic inflammation, could be effective in organ-specific inflammation.
Acknowledgements
This work was supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research C (15K07432). Animal experiments were accomplished at the Division of Genetic Research of the Advanced Research Support Center (ADRES), Ehime University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RMH, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Brandolini L, Bertini R, Bizzarri C, Sergi R, Caselli G, Zhou D, Locati M, Sozzani S. IL-1β primes IL-8-activated human neutrophils for elastase release, phospholipase D activity, and calcium flux. J Leukoc Biol. 1996;59:427–434. doi: 10.1002/jlb.59.3.427. [DOI] [PubMed] [Google Scholar]
- Carrillo W, Spindola H, Ramos M, Recio I, Carvalho JE. Anti-inflammatory and anti-nociceptive activities of native and modified hen egg white lysozyme. J Med Food. 2016;19:978–982. doi: 10.1089/jmf.2015.0141. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12:151–170. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- Chen Y, Du Y, Li Y, Wang X, Gao P, Yang G, Fang Y, Meng Y, Zhao X. Panaxadiol saponin and dexamethasone improve renal function in lipopolysaccharide-induced mouse model of acute kidney injury. PLoS ONE. 2015;10:e0134653. doi: 10.1371/journal.pone.0134653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Ku SK, Lee S, Bae JS. Suppressive effects of lysozyme on polyphosphate-mediated vascular inflammatory responses. Biochem Biophys Res Commun. 2016;474:715–721. doi: 10.1016/j.bbrc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 2007;21:3747–3755. doi: 10.1096/fj.06-7460com. [DOI] [PubMed] [Google Scholar]
- Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D, Inflammation and the Host Response to Injury Investigators Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Gao LN, Cui YL, Wang QS, Wang SX. Amelioration of Danhong injection on the lipopolysaccharide-stimulated systemic acute inflammatory reaction via multi-target strategy. J Ethnopharmacol. 2013;149:772–782. doi: 10.1016/j.jep.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Jollès P, Jollès J. What’s new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984;63:165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Kabir K, Gelinas JP, Chen M, Chen D, Zhang D, Luo X, Yang JH, Carter D, Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002;17:300–303. doi: 10.1097/00024382-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Kang S, Tanaka T, Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27:21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]
- Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Agric Food Chem. 2009;57:2233–2240. doi: 10.1021/jf803133b. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Na DH, Bae JS. Anti-inflammatory effects of lysozyme against HMGB1 in human endothelial cells and in mice. Inflammation. 2015;38:1911–1924. doi: 10.1007/s10753-015-0171-8. [DOI] [PubMed] [Google Scholar]
- Murakami F, Sasaki T, Sugahara T. Lysozyme stimulates immunoglobulin production by human–human hybridoma and human peripheral blood lymphocytes. Cytotechnology. 1997;24:177–182. doi: 10.1023/A:1007936629501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierthaus A, Klatte S, Linssen J, Eismann NM, Wichmann D, Hedke J, Braune SA, Kluge S. Revisiting the white blood cell count: immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis—a prospective, observational study. BMC Immunol. 2013;14:1–8. doi: 10.1186/1471-2172-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K, Kondo A, Okamoto T, Nakano H, Daifuku M, Nishimoto S, Ochi K, Takaoka T, Sugahara T. Immunostimulatory in vitro and in vivo effects of a water-soluble extract from kale. Biosci Biotechnol Biochem. 2011;75:40–46. doi: 10.1271/bbb.100490. [DOI] [PubMed] [Google Scholar]
- Nishimoto S, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Murata C, Kim-Kaneyama JR, Sato F, Bando M, Yagi S, Soeki T, Hayashi T, Imoto I, Sakaue H, Shimabukuro M, Sata M. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv. 2016;25:e1501332. doi: 10.1126/sciadv.1501332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu MR, Gorman D, Wagner J, Zurawski S, Liu YJ, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampanelli E, Dessing MC, Claessen N, Teske GJD, Joosten SPJ, Pals ST, Leemans JC, Florquin S. CD44-deficiency attenuates the immunologic responses to LPS and delays the onset of endotoxic shock-induced renal inflammation and dysfunction. PLoS ONE. 2013;8:e84479. doi: 10.1371/journal.pone.0084479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Patterson C, Hu Z, Runge MS, Tipnis U, Sinha M, Papaconstantinou J. Expression and self-regulatory function of cardiac interleukin-6 during endotoxemia. Am J Physiol Heart Circ Physiol. 2000;279:H2241–H2248. doi: 10.1152/ajpheart.2000.279.5.H2241. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Murakami F, Yamada Y, Sasaki T. The mode of actions of lysozyme as an immunoglobulin production stimulating factor. Biochim Biophys Acta. 2000;1475:27–34. doi: 10.1016/S0304-4165(00)00041-6. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Yamada Y, Yano S, Sasaki T. Heat denaturation enhanced immunoglobulin production stimulating activity of lysozyme from hen egg white. Biochim Biophys Acta. 2002;1572:19–24. doi: 10.1016/S0304-4165(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hino M, Kutsuna H, Hato F, Sakamoto C, Takahashi T, Tatsumi N, Kitagawa S. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1β. J Immunol. 2001;167:5940–5947. doi: 10.4049/jimmunol.167.10.5940. [DOI] [PubMed] [Google Scholar]
- Tagashira A, Nishi K, Matsumoto S, Sugahara T. Anti-inflammatory effect of lysozyme from hen egg white on mouse peritoneal macrophages. Cytotechnology. 2018;70:929–938. doi: 10.1007/s10616-017-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Ohno N, Yadomae T. Detoxification of lipopolysaccharide (LPS) by egg white lysozyme. FEMS Immunol Med Microbiol. 1994;9:255–263. doi: 10.1111/j.1574-695X.1994.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2:288–294. doi: 10.1158/2326-6066.CIR-14-0022. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salamán CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull. 2001;54:443–453. doi: 10.1016/S0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- Vignali DAA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokooji T, Hamura K, Matsuo H. Intestinal absorption of lysozyme, an egg-white allergen, in rats: kinetics and effect of NSAIDs. Biochem Biophys Res Commun. 2013;438:61–65. doi: 10.1016/j.bbrc.2013.07.024. [DOI] [PubMed] [Google Scholar]