Abstract
Mitochondria are attractive cellular organelles which are so interesting in both basic and clinical research, especially after it was found that they were arisen as a bacterial intruder in ancient cells. Interestingly, even now, they are the focus of many investigations and their function and relevance to health and disease have remained open questions. More recently, research on mitochondria have turned out their potential application in medicine as a novel therapeutic intervention. The importance of this issue is highlighted when we know that mitochondrial dysfunction can be observed in a variety of diseases such as cardiovascular diseases, neurodegenerative diseases, ischemia, diabetes, renal failure, skeletal muscles disorders, liver diseases, burns, aging, and cancer progression. In other words, transplantation of viable mitochondria into the injured tissues would replace or augment damaged mitochondria, allowing the rescue of cells and restoration of the normal function. Therefore, mitochondrial transplantation would be revolutionary for the treatment of a variety of diseases in which conventional therapies have proved unsuccessful. Here, we describe pieces of evidence of mitochondrial transplantation, discuss and highlight the current and future directions to show why mitochondrial transplantation could be a master key for treatment of a variety of diseases or injuries.
Keywords: Mitochondria dysfunction, Reactive oxygen species, Neurodegenerative diseases, Heart failure, Mitochondrial transplantation
Introduction
Mitochondria are found in all mammalian cells except for mature red blood cells (Hoppel et al. 2009; Huang et al. 2013; McCully et al. 2016). Mitochondria have a variety of functions in the cells (Rub et al. 2017). It was classically believed that mitochondria were involved only in producing energy and were famous as energy chambers. However, they are involved in other important multiple cell functions such as heat regulation, calcium homeostasis, biogenesis and assembly of iron-sulfur proteins, control of apoptosis, reactive oxygen species ROS production, cell survival and proliferation, production of metabolites and coordination of metabolic pathways (Loureiro et al. 2017; McCully et al. 2016; Salimi et al. 2015, 2017). Of note is that they are key organelles in signaling platform and regulating many cells activity (Kabekkodu et al. 2015). It has been believed that mitochondria have been evolved from aerobic bacteria and/or early eukaryotic cells over a billion years ago. The mitochondria have their own DNA, mtDNA, with 16,569 bp length and encode 13 proteins for electron transport chain, 2 mitochondrial rRNAs and 22 mitochondrial tRNAs (Kuznetsova et al. 2017; Siira et al. 2017). Table 1 illustrates the proteins encoded by mtDNA. Although the mitochondria have their own genome, their biosynthetic pathways depend completely on nuclear DNA-encoded proteins (n-mitoproteins) (Berridge et al. 2018). For example, cytochrome c oxidase (COX), a very important enzyme in the function of mitochondria, consists of 14 subunits in which three of them encoded by mitochondrial DND and the rest eleven subunits encoded by nuclear DNA (Sinkler et al. 2017). Glycyl-tRNA synthetase gene (GARS) is another nuclear-encoded protein which is essential for protein translation in both cytoplasm and mitochondria. Orchestration of mitochondria-encoded and nuclear-encoded subunits or their crosstalk is necessary for the normal functions of mitochondria such as electron transport, ATP production and mitochondrial membrane potential (Boczonadi et al. 2018).
Table 1.
Genes and proteins which are expressed by mitochondria DNA
| Gene | Protein |
|---|---|
| MT-ATP6 | ATP synthaseSubunit 6 (complex V) |
| MT-ATP8 | ATP synthaseSubunit 8 (complex V) |
| MT-CYB | Cytochrome b (Complex III) |
| MT-CO1 | Cytochrome c oxidaseSubunit 1 (complex IV) |
| MT-CO2 | Cytochrome c oxidaseSubunit 2 (complex IV) |
| MT-CO3 | Cytochrome c oxidaseSubunit 3 (complex IV) |
| MT-RNR2 | Humanin |
| MT-ND1 | NADH dehydrogenase Subunit 1 (complex I) |
| MT-ND2 | NADH dehydrogenase Subunit 2 (complex I) |
| MT-ND3 | NADH dehydrogenaseSubunit 3 (complex I) |
| MT-ND4 | NADH dehydrogenaseSubunit 4 (complex I) |
| MT-ND5 | NADH dehydrogenaseSubunit 5 (complex I) |
| MT-ND6 | NADH dehydrogenase Subunit 6 (complex I) |
| MT-ND4L | NADH-ubiquinone oxidoreductase chain 4L |
MT-ATP mitochondria adenosine triphosphate, MT-CYB cytochrome b, MT-CO cytochrome c oxidase, RNR2 mitochondrially encoded 16S RNA, NADH nicotinamide adenine dinucleotide
Some tissues such as cardiac muscles, skeletal muscles, neurons, and oocyte have plenty of mitochondria but mammalian hepatocytes have only 800 mitochondria. Mitochondria not only are different in terms of number but also their size, shape, distribution, and feature vary from cell to cell. Moreover, the characters are changed and adapted depending on the cell physiological or pathological conditions (Bragoszewski et al. 2017).
Mitochondria have a variety of shapes. For example, they represent long, elongated, and tubular mitochondrial network shapes and small grain-shaped morphology. Interestingly, they can alter under the patho-physiological condition of cells (Simula and Campello 2018). This process is referred to mitochondrial dynamic or biogenesis (Chen and Chan 2009; Liesa et al. 2009). Fission and fusion are two important processes that control shape, size, number, motility, and inheritance of mitochondria. Fission and fusion occur during cell division, cell migration, cell proliferation, apoptosis and the localization of mitochondria throughout the cells (Simula and Campello 2018).
In fusion, two mitochondria are fused together by two steps. Firstly, the outer mitochondrial membranes (OMM) are fused together. Then, the inner mitochondrial membranes (IMM) are fused as well. In liver tissues, the mitochondria are fused and make a mega mitochondrion while in other tissues they have an arrangement in a network system. Mitochondrial fission is a multi-step process in which one mitochondrion is divided in two daughter mitochondria. These two processes define the structural and functional status of mitochondria. Fusion maintains the functionality and genetic and biochemical homogeneity of mitochondria. It also facilitates the communication between them and involved in cell homeostasis. On the other side, the fission is responsible to control number and distribution of the mitochondria. Of note, fission has a critical role in the removal of damaged mitochondria through mitophagy. In highly polarized cells such as neurons, mitochondrial dynamic is very important in synaptic transmission and vesicle recycling. Mitochondrial dynamic also involves in neurogenesis during the development of the brain (Cantó 2018; Cid-Castro et al. 2018; Simula and Campello 2018; Tilokani et al. 2018).
The main proteins which are involved in mammalian mitochondria fusion are Mitofusion1 (Mfn1), Mitofusion2 (Mfn2), and optic atrophy type1 (Opa1); and the main ones for mitochondria fission are Dynamin-related protein 1 (Drp1), Fis1 (mitochondrial fission protein 1), and Mitochondrial Protein 18 (MTP18) (Arnoult et al. 2005; Bach et al. 2003; Ehses et al. 2009; Griparic et al. 2007; Liesa et al. 2009). It is noteworthy that the expression of these proteins and the balances between fusion and fission statuses of mitochondria alters in many mitochondrial dysfunctions and under pathological conditions (Chen et al. 2017; Mathis et al. 2017; Onyango et al. 2016; Schirone et al. 2017; Simula et al. 2017; You et al. 2017).
Mitochondrial dysfunction can be traced in a variety of diseases including cardiovascular diseases, diabetes, neurodegenerative diseases, ischemia, renal failure, skeletal muscles disorders, peripheral nerve injury, spinal cord injury, liver injury, aging, cancer, infertility, burn injury and even extensive exercise (Kuwahara et al. 2016; Ma et al. 2017; McCully et al. 2016; Wang et al. 1986; Zhang et al. 2008).
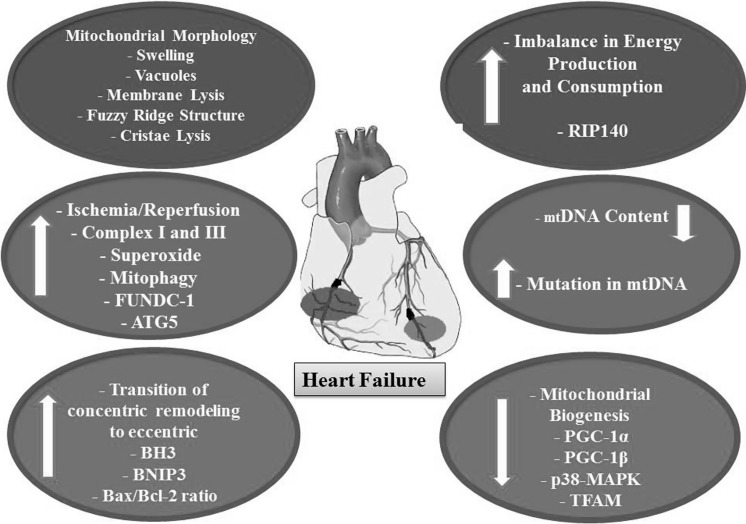
In tissues with high demand for energy such as cardiomyocytes, one-third of the cell’s volume is occupied with mitochondria and approximately 30 kg of ATP/day is produced (Moreno-Lastres et al. 2012; Sabbah 2016; Tymoczko et al. 2011; von Hardenberg and Maack 2017). With mitochondrial dysfunction, the heart is like an engine without fuel. The higher the number of mitochondria, the more the level of the reactive oxygen species ROS produced. Figure 1 illustrates the role of mitochondria dysfunction in heart diseases (Aimo et al. 2016; Kanaan and Harper 2017; Lopez-Crisosto et al. 2017).
Fig. 1.
Schematic representation of mitochondrial dysfunctions in heart failure. Mitochondria present abnormal configurations such as swelling and vacuoles, membrane lysis, fuzzy ridge structure (indistinct) and cristae lysis. Abbreviation: RIP140 receptor-interacting protein 140, PGC-1a Peroxisome proliferator-activated receptor gamma coactivator-1 a, PGC-1b Peroxisome proliferator-activated receptor gamma coactivator-1 b, p38-MAPK p38 mitogen-activated protein kinases, TFAM mitochondrial transcription factor A, FUNDC-1 Fun14 domain containing 1, ATG5 Autophagy related gene 5
Tissues of the central nervous system (CNS) have a plenty of mitochondria, therefore it would be expectable to produce higher levels of metabolites and ROS (Onyango et al. 2017). Supporting this notion, mitochondrial dysfunction has been reported in several neurodegenerative diseases such as Alzheimer, Multiple Sclerosis (MS), Huntington (HD) and Amyotrophic Lateral Sclerosis (ALS), and Parkinson disease (PD), or other nervous system diseases such as spinal cord injury, peripheral nerve injury, and ischemic brain injury traces of mitochondrial dysfunctions can be found as well (Bonafede and Mariotti 2017; Di Domenico et al. 2017; Faizi et al. 2016; Ganie et al. 2016; Giannoccaro et al. 2017; Gollihue et al. 2017; Grimm et al. 2016; Kim et al. 2017; Kuo et al. 2017; Picone et al. 2016; Shaki et al. 2017). It is noteworthy that neurons are very sensitive to ROS and they consume 20% of the body’s total basal oxygen (Grimm et al. 2016).
Another high prevalence and incurable disease, in which mitochondrial dysfunctions play an important role in the pathogenesis of the disease, is acute kidney injury (AKI) (Roushandeh et al. 2017). Kidneys are second to the heart in oxygen consumption and mitochondrial number. Therefore, it is obvious that mitochondria dysfunction is a key contributor to renal tubular cell death during acute kidney injury (Forbes 2016; Ralto and Parikh 2016). Therefore, restoring mitochondrial dysfunction in the aforementioned example diseases would be regarded as a new potential strategy for the treatment of a variety of diseases. The present review discusses highlights and describes pieces of evidence with regard to the restoration of mitochondrial dysfunction. We will try to introduce mitochondrial transplantation as a novel master key for treatment of various incurable diseases in the future.
Mitochondrial transplantation
Mitochondria are the organelles playing fundamental roles in the cellular function and metabolism and are also viewed as a key player in the cell pathology and death (McCully et al. 2016). Recently, the term “mitochondrial transplantation” opens a novel horizon for many diseases (Elliott et al. 2015; Emani et al. 2017; McCully et al. 2016). As described above, the mitochondrial dysfunction threatens cell homeostasis, disturbs energy production and finally leads to cell death and diseases (Lim et al. 2015; Morris and Berk 2015; Onyango et al. 2017; Ralto and Parikh 2016; Sasaki and Iwata 2007; Simula et al. 2017). “Mitochondrial transplantation” seeks to find the strategies to replace or restore damaged mitochondria.
The concept of mitochondrial transplantation originated from fact that the mitochondria can be transferred from one cell to another cell. This especially occurs when the mitochondria are damaged to support the injured cells (Hayakawa et al. 2016, 2018; McCully et al. 2016; Rocca et al. 2017).
Mitochondria are transferred between cells by various contact modes including; junction, cell fusion, and tunneling nanotube formation (Lu et al. 2017; Paliwal et al. 2018).
It has been shown that when the neurons encounter the ischemia, astrocytes deliver generously their own healthy mitochondria to the neurons in order to prevent them from death. More recently, it has been revealed that the extracellular mitochondrial particles which were released by astrocytes were mediated by a calcium-dependent mechanism involving CD38 and cyclic ADP ribose signaling. Moreover, microglia, other supporting cells of the nervous system, followed this pattern (Hayakawa et al. 2016; Rocca et al. 2017). It has also been shown that endothelial progenitor cells-derived conditioned medium containing functionally viable mitochondria was successfully incorporated into normal brain endothelial cells. It promoted angiogenesis and decreased the trans-cellular permeability of brain endothelial cells as well. Furthermore, it enhanced the copy number of mtDNA, mitochondrial proteins expression and, ATP production (Hayakawa et al. 2018).
Macropinocytosis is another mechanism underlying mitochondria internalize into the cells. Several studies have also been suggested that mitochondria enter into cells by an actin-dependent macropynocytosis; however, the precise mechanism and the endocytosis pathway are still unclear. Of note, it has been reported that < 10% of injected mitochondria was taken up by cardiomyocytes in an animal model of cardiac ischemia. Nonetheless, they exerted their therapeutic properties and improved ATP production (Kumar 2017; Pacak et al. 2015).
It is obscure whether the delivering of mitochondria into the cells is active or passive? Some studies have been indicated that mitochondrial internalization is passive because when the internalization of molecular pathways were inhibited with specific blockers, mitochondria delivered to the cells. However, the endocytosis was dependent on the cell types. It is noteworthy that when the mitochondria transplanted, they might be delivered into the different cells including macrophages, vessels endothelial cells, pericytes, neurons and neuroglia such as oligodendrocytes, microglia, and astrocytes (Paliwal et al. 2018). It is interesting to mention that mitochondria were detected in CSF of patients with subarachnoid hemorrhage or in experimental subarachnoid hemorrhage rat model. It has been revealed that mitochondria in the CSF were originated from astrocytes. This findings suggest that it would be feasible to transplant the isolated mitochondria through CSF when the brain or spinal cord are injured in order to restore the damaged tissues (Zussman et al. 2017).
It has been reported that some strategies such as peptide-mediated mitochondrial delivery (PMD) can be employed to enhance mitochondrial intake and increase the transplantation efficiency especially in the patients who suffer from a mitochondrial disease. The PMD strategy positively had impacts on the rescue of the myoclonic epilepsy and ragged-red fiber (MERRF) model cell line. It is expecting that transplantation of mitochondria by PMD would have a better outcome for clinical use in the future. Although this approach is promising, however, further studies are required (Liu et al. 2014).
Dextran, a biocompatible polymer, can increase the delivery rates of isolated mitochondria. However, this is also in the beginning steps and requires further investigations (Wu et al. 2018).
Sources and methods of mitochondrial isolation
Isolation of mitochondria from skeletal muscles has been suggested as an appropriate candidate for mitochondrial transplantation. Pectoralis major, rectus abdominis, gastrocnemius and even neck strap muscles such as sternohyoid or deltoid and lattissimus dorsi are the suitable sources to obtain mitochondria (Emani et al. 2017; Masuzawa et al. 2013; McCully et al. 2016, 2017). Due to cosmetic reason, pectoralis major muscles are not a suitable source to isolate mitochondria for women (Table 2).
Table 2.
Summary of the mitochondrial dysfunction in nervous system diseases
| Nervous system diseases | Mitochondrial dysfunction |
|---|---|
| Alzheimer | Mitochondria fragmentation |
| Abnormal cristae | |
| Quantity of the mitochondria | |
| NDUFA2, NDUFB3, UQCR11, COX7C, ATPD, ATP5L, ATP50 | |
| NRF1, NRF2, TFAM and PGC-1α hippocampal tissue↓ | |
| Mutation of mtDNA (mitochondrial 5-methylcytosine in entorhinal cortex) | |
| Complex IV and Δψm index↓ | |
| Multiple Sclerosis | NAA↓ |
| Complexes I, III and IV↓ | |
| mtHSP70↑ | |
| Mitochondrial membrane potential↓ | |
| Cytochrome C expression↓ | |
| Parkinson | mtDNA mutations↑ |
| PINK-1↓ | |
| PARKIN-1↓ | |
| MDVs↓ | |
| PARK genes↓ | |
| Huntington | Abnormal Huntingtin gene↑ |
| Ca++ loading capacity↓ | |
| Activation of the excitotoxicity↑ | |
| PGC-1 a↓ | |
| Cytochrome b and c oxidase 1↑ | |
| 8-hydroxy-2-deoxyguanosine↑ | |
| Amyotrophic Lateral Sclerosis | Swelling, Fragmented and Vacuolated mitochondria |
| Intermembrane space dilation, disorganized crista↑ | |
| HK1↑ | |
| SOD1↓ | |
| Spinal Cord Injury | Ca++ loading capacity↓ |
| Activation of the excitotoxicity↑ | |
| CtBP gene↓ | |
| PDHC, complex I, complex IV disturbance↑ | |
| Permeability of mitochondria membrane | |
| Drp1, Fis1↑ | |
| Peripheral Nerve Injury | Cycling of futile proton↑ |
| ATP synthase↓ | |
| Electron transport chain | |
| Mutation in OPA1, MFN2 and mitofusin 2↑ | |
| Interaction of mitochondria, microtubules and endoplasmic reticulum↓ | |
| Swollen and vacuolated mitochondria | |
| Mutation of mtDNA↑ | |
| Ischemic Brain | Depolarization and swollen disturbance in calcium homeostasis↑ |
| ROS and MDA↑ | |
| Mfn2 gene expression↓ |
NDUFA2 NADH dehydrogenase (ubiquinone)1 alpha subcomplex subunit 2, UQCR11 ubiquinol-cytochrome c reductase complex, COX7C cytochrome c oxidase subunit 7c, ATP5L synthase subunit g, NRF1 nuclear respiratory factor1, NRF2 nuclear respiratory factor 2, TFAM transcription factor A, PGC-1α Peroxisome proliferator-activated receptor gamma coactivator-1 a, NAA N-acetyl aspartate, mtHSP70 mitochondrial heat shock protein 70, PINK1 PTEN-induced kinase 1, MDV mitochondria delivery vesicle, HK1 Hexokinase 1, SOD1 super oxide dismutase1, CtBP C-terminal-binding protein, PDHC pyruvate dehydrogenase complex, Drp1 dynamin-related protein1, Fis1 fusion protein 1, OPA1 optic atrophy type1, MFN2 Mitofusion 2, MDA malondialdehyde, ROS reactive oxygen species
Mesenchymal stem cells (MSCs) are multipotent and attractive cells in regenerative medicine that can be obtained from a variety of sources (Roudkenar et al. 2018). Recently it has been suggested that stem cells, especially MSCs, are suitable and substitute source to isolate mitochondria (Wang et al. 2018). Interestingly, it has been shown that the therapeutic effects of MSCs can be exerted by secretome which are containing various molecules including mitochondria (Abbasi-Malati et al. 2018). Moreover, MSCs donate their mitochondria to injured cells via tunneling nanotube (Wang et al. 2018). Stem cell-derived mitochondrial transplantation could be a novel strategy for tissue injury especially in the patients with mitochondrial diseases (Paliwal et al. 2018; Wang et al. 2018). Another, the source of mitochondria could be spermatozoa. They have a plenty of mitochondria. However, further studies are required to clarify whether it would be possible to isolate healthy mitochondria from these cells and utilize them in the clinic or not?
A number of studies have been demonstrated that the efficacy of mitochondrial transplantation highly depends on viable and functional mitochondria. Non-viable mitochondria, previously frozen mitochondria, mitochondrial fractions (proteins, complex I–V), mitochondrial DNA and RNA, and exogenous ATP or ADP do not provide any cytoprotection (Masuzawa et al. 2013; McCully et al. 2009; Preble et al. 2013).
Recently, Mc Cully and his team established a rapid isolation protocol using different filters that took less than half an hour. Therefore, this is valuable for clinical application. For clinical application of mitochondria transplantation, it is essential to meet the criteria of good manufacturing practice (GMP). Quality control of size, number, purity, shape, viability, and function of the organelles must be analyzed. Tissue dissociator and multisizer counter are essential and helpful devices for isolation, counting, control the shape, and size of the organelles. All parts of the isolation procedure must be performed on the ice. In some laboratories, the hemocytometer could be helpful but it is prone to personal mistakes. The viability of the organelles can be assayed by mitochondrial fluorescent probes such as MitoTracker Orange CMTMRos (Preble et al. 2014b).
The function of the mitochondria can be evaluated by polarographic or spectrophotometric techniques. Moreover, Clark electrode measurements, oxygen consumption rate (OCR), are the reliable and suitable method for the evaluation of the function of mitochondria. Finally, ATP measurement is the another and versatile method to evaluate the functional activity of isolated mitochondria (Preble et al. 2013).
Labeling of mitochondria
Fluorescent dyes are used to visualize intercellular transplanted mitochondrial. Rosamine-based dyes (MitoTracker Red CMXRos and MitoTracker Red CMH2XRos) and carbocyanine-based dyes (MitoTracker Green FM, MitoTracker Red FM, and MitoTracker Deep Red FM) are usually employed for mitochondrial labeling. Mitochondrial fluorescent proteins (MFP) are other options for mitochondrial labeling. MitoGFP, mitoRFP, mitoYFP, and mitoDsRed are examples of MFP. To visualize the stained mitochondria confocal microscope or time-lapse confocal microscope is necessary (Berridge et al. 2018).
Delivery methods of mitochondrial transplantation
Mitochondrial transplantation can be performed by several routes. In situ and systemic transplantations are two main routes could be employed in the clinic. By in situ approaches, it is assured that all the isolated mitochondria can be delivered to the tissues. However, over-accumulation of the organelle would be challenging. Several studies have been tried to inject the mitochondria directly into the cardiac tissue using a suitable syringe. Supporting this notion, mitochondria have been directly injected into the myocardium of ischemic heart patients by a 27–32 gauge syringe (Emani et al. 2017; McCully et al. 2017). Direct transplantation of mitochondrial usually requires surgery in order to access the injured tissues. However, transplantation of mitochondria during surgery, for example in the ischemic heart, may not be repeated for several times. In situ transplantation of mitochondria into CNS by syringe and steriotax are feasible in preclinical studies, but their application in the clinic would be difficult (Gollihue et al. 2017).
The sonography-guided catheter carrying mitochondria through a carotid system for brain and renal artery for kidney might be applicable. Systemic injection such as intravenous transplantation is another way for mitochondrial transplantation. By systemic injection, it would be possible to transplant mitochorndria several times. However, it not only needs a high number of mitochondria but also the transplanted mitochondria might distribute vastly throughout the body including in the injured tissue. As mentioned above, recently it has been found that after subarachnoid hemorrhage, the extracellular mitochondria were found in CSF that in turn, could highlight and recommend a new route for mitochondrial transplantation for brain deficits and spinal cord injuries i.e. through subarachnoid space or brain ventricles (Chou et al. 2017).
Mitochondria can be transferred in vitro by different protocols as well. Co-culture of healthy cells with cells containing damaged mitochondria resulted in delivering of the healthy mitochondria into injured cells (Ahmad et al. 2014; Jiang et al. 2016; Wang and Gerdes 2015). Microinjection is another method for delivering isolated mitochondria into the recipient cells (Oktay et al. 2015).
MitoCeption is a new and efficient protocol for delivering of mitochondria in vitro. In this method, the isolated mitochondria enforced to deliver into cells by centrifuging of culture plates followed by 24 h incubation. For the first time, Caicedo et al. in 2015 successfully delivered the mitochondria which were isolated from mesenchymal stem/stromal cells to cancer cells by mitoCeption method (Caicedo et al. 2015; Nzigou et al. 2017).
More recently, a simple and quick method was introduced for delivering of exogenous mitochondria into culture cells. The new protocol only requires centrifugation of the cells and mitochondria at 1500×g for 5 min without additional incubation (Kim et al. 2018).
Doses of mitochondria for transplantation
It is noteworthy that the suitable dose of mitochondria for transplantation has not been optimized so far. Moreover, almost all of available information in this regard is based on preclinical studies. However, according to McCulley and et al. study, administration of approximately 2 × 105 mitochondria per gram of tissue wet weight is a suitable dose for treatment of ischemic heart. Of noted, they revealed that only 10% of injected mitochondria can be found in the cardiomyocytes however, they had therapeutic effects (Emani et al. 2017). Administration doses of mitochondria both in vitro and in vivo studies are summarized in Table 3. It is worth to mention that the healthy, function and viability of the isolated mitochondria are much more important than the dose of administration.
Table 3.
Summary of some preclinical studies dealing with mitochondrial transplantation
| Mitochondrial isolation from: | Mitochondria transplantation to: | Animal model Or Human disease | Route of transplantation | Dose of injected mitochondria | Mitochondrial transplantation outcomes | Reference |
|---|---|---|---|---|---|---|
| Rectus Abdominis | Heart | Human heart ischemic/reperfusion | In situ to ischemic area | 1 × 107 | Improved cardiac functions | Emani et al. (2017) |
| Independent to ECMO support | ||||||
| Pectoralis major | Heart | Ischemic/reperfusion of heart in Pig | Subendocardial injection | 9.9 × 107 | Decreased infarct size | Kaza (2017) |
| Improve the cardiac tissue histologically | ||||||
| Long survival of transplanted mitochondria | ||||||
| Non-ischemic skeletal muscle | Heart | Occlusion of left descending artery in rabbit | In situ injection into ischemic area | 9.7 × 106 ± 1.7 × 106/ml | Decrease Infarct size | Akihiro Masuzawa et al. (2013) |
| Improve cardiac function | ||||||
| Decrease cardiomyocyte apoptosis and necrosis | ||||||
| PC12 cell line | Spinal cord | Spinal cord injury rat | Intraspinal injection | 12.5 μg mitochondria | Presence of transplanted mitochondria in spinal cord | Gollihue et al. (2017) |
| BHK-21 cells | Sciatic nerve | Sciatic nerve crush model in rat | Injection into epineurium | 195 μg mitochondria | Improve the cytoskeleton of injured sciatic nerve | Kuo et al. (2017) |
| Decrease the ROS production | ||||||
| Improved the muscles function and nerve conduction | ||||||
| Develop neurobehavioral activity | ||||||
| Hamster cells | Brain | Ischemic/reperfusion of brain in rat | Intra cerebral and intra-arterial injection | 75 μg of mitochondria | Restore the motor function of the ischemic rat brain | Huang et al. (2016) |
| Decrease of apoptotic cells and infarct size | ||||||
| Allogenic peptid-labled mitochondria from PC12 cells and human osteosarcoma cybrids (xenogeneic source) | Brain | 6-OHDA induced Parkinson model | Local injection to MFB | 1.05 μg of Pep-1–conjugated mitochondria | Protects neurons in substantia nigra and nigristriate circuit | Chang et al. (2016) |
| Improve motor function | ||||||
| Decrease cytotoxic effects of 6-OHDA | ||||||
| Increase of mitochondrial function | ||||||
| Oogonial precursor cells | Human oocyte | Infertile woman | Intracytoplasmic injection | Not mentioned | Increase of IVF rate | Oktay et al. (2015) |
| Young donor oocyte cytoplasm | Human oocyte | Infertile woman DOR | Intracytoplasmic injection | Not mentioned | Increase pregnancy | Woods and Tilly (2015) |
| Increase oocyte quality | ||||||
| Decrease maternally inherited diseases | ||||||
| Granular cell | Human oocyte | Infertile woman | Intracytoplasmic injection | Not mentioned | Two normal babies were born | Kong et al. (2003a, b) |
| Granular cell | Human oocyte | Infertile and old woman | Intracytoplasmic injection | Not mentioned | Formation of embryo | Kong et al. (2003a, b) |
| Gastrocnemius and quadriceps femoris muscle | Syngeneic injection (isolated from BALB/cJ mice donors) or allogeneic injection mitochondria (isolated from C57BL/6J mice donors) | Skin graft | IP | 1 × 105, 1 × 106 or 1 × 107 mitochondria | No immuonological rsponese and DAMPs were seen | Ramirez-Barbieri et al. (2018) |
| Epstein–Barr virus transformed lymphocytes or rat brain | Co-culture in in vitro | Rodent model of SZ | In situ | In vitro: 10–50 µg protein/106 cells | Long-lasting improvement in mitochondrial functions | Robicsek et al. (2017) |
| Intracerebral in in vivo | In vivo: 100 µg/4.5 µl in in vivo | Attenuated SZ-related deficits |
ECMO extracorporeal membrane oxygenation, ROS reactive oxygen species, BHK-21 baby hamster kidney, 6-OHDA 6-Hydroxydopamine hydrobromide, IVF in vitro fertilization, DOR diminished ovary reservoir, IP intraperitoneal, SZ schizophrenia, DAMPs damage-associated molecular pattern molecules
Clinical trial of mitochondria transplantation
Clinical application of mitochondria transplantation is very limited and needs more preclinical studies to know about its optimization and develop its efficiency and safety with improvements in its quality control protocols. The first attempt in this regard was initiated in 2016 and conducted by Mc Cully research team in Boston children hospital at Harvard University in the United States. The primary results were released in 2017 and opened a new hopeful window for many patients. A novel strategy termed “mitochondrial auto transplantation” helps to treat the patients suffering from heart ischemia/reperfusion. Five children patients, age from 2 days to 2 years old, with cardiac ischemia participated in this study. Epicardial echocardiography was performed to detect akinesias or hypo kinesis. The mitochondria were extracted during 20–30 min from 2 pieces of samples obtained from rectus abdominis muscle. 1 × 107 ± 1 × 104 mitochondria were injected using a tuberculin syringe directly into 10 ischemic areas according to echocardiography findings. After the transplantation, none of the patients had bleeding or arrhythmia because of the injection. In four of the patients, cardiac functions improved according to echocardiographic findings and were separated from extracorporeal membrane oxygenation (ECMO) support. Two of them passed away for cardiac problems but also died for respiratory and other disorders. The dose applied in this study was based on previous animal studies and needs to be developed. The authors proposed more clinical trials in the future in other diseases to optimize the protocol and efficiency (Emani et al. 2017). It is worth mentioning, according to www.clinicaltrail.gov, only one clinical trial dealing with mitochondria transplantation can be found.
Preclinical trial of mitochondria transplantation
Our knowledge about preclinical studies of mitochondrial transplantation is also owing to Mc Cully research group. In 2016, they injected viable, functional autologous mitochondria to ischemic pig heart. They isolated the healthy mitochondria from the non-ischemic area, pectoralis major muscle. 9.9 3 × 107 ± 1.4 3 × 107/mL; 1.3 3 × 107 mitochondria per injection site was transplanted by sub-endocardial injection into eight areas at risk. The results showed that there was no significant difference in terms of heart and left ventricle weight. There was no immune response in animals receiving the mitochondria. Myocardial damage significantly was higher in vehicle animals according to the results of a creatine kinase-MB isoenzyme analysis. Infarct size in the group receiving mitochondria was significantly less that the vehicle. Histopathological and ultrastructural findings showed an increase in the longitudinal and transverse interfibrillar separation and mitochondrial damage in the vehicle heart compared to the mitochondria transplanted group. However, the transplanted mitochondria did not change the heart fibrosis (Kaza et al. 2017).
Electrocardiography (EKG), recorded 2 h and 4 weeks after injection, did not show fibrillation, bradycardia or conducting system defects in the ventricle of two groups. Interestingly, transplanted mitochondria were detected 4 weeks after injection in the pig’s heart (Kaza et al. 2017).
Masuzawa et al. (2013) extracted the functional mitochondria from the non-ischemic skeletal muscle and injected then into regional ischemic area induced with occlusion of rabbit left anterior descending artery. They injected 9.7 ± 1.7 × 106/mL mitochondria immediately before reperfusion in eight ischemic regions and recovered the animals for 4 weeks. The mitochondria resided in interstitial spaces surrounding cardiomyocytes at 0, 2, 4 and 24 h after the injection. The results revealed that no arrhythmogenic was detected after mitochondrial transplantation. Infarct size and cardiomyocytes necrosis decreased in the experimental group and the cardiac function developed. Infarct size did not increase after 28 days indicating the effectiveness of mitochondrial transplantation. Dimensional echocardiography demonstrated that after mitochondrial transplantation the myocardial function increased significantly and diastolic and systolic blood pressure did not change. Of note is that cardiomyocyte apoptosis decreased in the group that received mitochondria after 28 days. Proteomic analysis showed that after mitochondrial transplantation 26 proteins were upregulated and 23 were down-regulated. These proteins were precursors for energy production and cell respiration. Overall, their results revealed that administration of mitochondria enhanced cardiac function and decreased necrosis and apoptosis in cardiomyocytes (Masuzawa et al. 2013).
More recently, Gollihue et al. designed a strategy in which the isolated mitochondria were labeled with turbo-green fluorescent protein (tGFP) in vivo. The labeled mitochondria were injected into the spinal cord injury model of a rat and were detected in the rat tissue. The transplanted mitochondria were found within microglia and neurons of the spinal cord 24 h and 48 h after the injection (Gollihue et al. 2017, 2018).
Kuo et al. (2017) tried to know mitochondrial transplantation potential in neuroprotection and regeneration after sciatic nerve crush in a rat model. They also co-cultured the sciatic nerve explants with mitochondria in in vitro where the results were exciting. Mitochondria improved the cytoskeleton of injured sciatic nerve explants and decreased the ROS production. The electrophysiology findings showed that the ttransplanted mitochondria into the perineurium improved the muscles function and nerve conduction. The ROS production decreased significantly and animal neurobehavioral activity developed. This will be good news for those suffering from peripheral neuropathies (Kuo et al. 2017).
Some studies have demonstrated that the exogenous mitochondria transplantation has protective effects on brain and liver after ischemia. In a study, the mitochondria isolated from hamster cells were injected into the brain ischemic model in a rat (Huang et al. 2016). The findings revealed that transplanted mitochondria restored the motor function of the ischemic rat brain. Furthermore, apoptotic cells and infarct size significantly decreased in the brain tissue of rat which received exogenous mitochondria. Based on these observations, it was strongly suggested that mitochondria transplantation has protective effects on neurons of CNS after ischemia (Huang et al. 2016).
In another study conducted by Chang et al. (2016), the exogenous and allogenic peptide-labeled mitochondria that were transplanted to an animal model of Parkinson’s disease induced with 6-hydroxydopamine (6-OHDA) showed protective effects on neurons in the substantia nigra and nigristriate circuit. The isolated mitochondria were transplanted into medial forebrain bundle of PD model. After a three-month follow up, the motor function of PD model improved, the mitochondrial function increased and the cytotoxic effects of 6-OHDA decreased (Chang et al. 2016).
Immune cell therapy is one of the new and well-known approaches to cancer therapy. However, the metabolic functions of adult stem cells, immune cells, and somatic cells are attenuated with aging. More recently, Kim et al. demonstrated that the combination of immunotherapy and mitochondrial transplantation increased two folds cytotoxicity effects of immunotherapy against cancer cells (Kim et al. 2018).
More recently mitochondria transplantation were performed in the experimental rat model of schizophrenia (SZ). Mitochondria were isolated from lymphocytes (lymphoblasts) of three healthy persons or from whole brains (except the cerebellum) of 10 rats using a percoll gradient method. The isolated mitochondria were transferred to the lymphoblasts of schizophrenia patients and also were transplanted to the experimental rat model. Moreover, the isolated mitochondria were transferred to differentiated dopaminergic neurons of iPCs of healthy and SZ patients. The results revealed that approximately 50% of the lymphoblasts up took the isolated active normal mitochondria and recovered the impaired respiration of lymphoblasts of SZ. This study demonstrated that heterologous isolated active normal mitochondria entered into different cell types and induced long-lasting improvement in mitochondrial functions and differentiation of SZ-iPSCs into neurons. Furthermore, in the animal model of SZ, Intra-prefrontal cortex transplantation of isolated active normal mitochondria in young rats exposed prenatally to a viral mimic prevented mitochondrial Δψ m and attenuated SZ-related deficits (Robicsek et al. 2017).
More recently, Moskowitzova et al. reported that mitochondrial transplantation prolonged cold preservation time and increased the heart transplantation outcomes. In their study, donor’s hearts received 1 × 108 mitochondria or respiration buffer, as a vehicle group, before excision and at the beginning of reperfusion during graft to the recipient mice. The donor’s hearts maintained for 27–30 h in saline before graft. The results were promising. Ejection fraction and beating scores improved in the hearts that received mitochondria transplantation compared to the vehicle group. In addition, apoptosis and histopathological changes were decreased in the heart of the mitochondrial transplanted group. Overall, these findings strongly suggested that mitochondria transplantation might be employed to improve organ transplantation outcomes (Moskowitzova et al. 2018).
The oocyte is one of the richest cells in terms of mitochondria in women (Van Blerkom 2011). It has been believed that mitochondria have a crucial role in the oogenesis and in the early stages of development as well (Oktay et al. 2015). The mitochondria can be considered as a landmark to estimate the quality of the oocyte (May-Panloup et al. 2016). Aging affects the mitochondria quality and increases the mtDNA mutations and results in defects in the granulosa and oocyte development (May-Panloup et al. 2016).
Based on a study on 72 infertile men, ten types of nucleotide variants were found in their mitochondrial DNA. These nucleotide variants lead to disturbance in mitochondria energy production and in turn interfere with sperm motility and function (Heidari et al. 2016).
In another study, it was found that mitochondrial maternal mito-nuclear incompatibility leads to severe effects on oogenesis and embryo survival (Zhang et al. 2017). Overall, mitochondria replacement might be considered as a new strategy for infertility treatment and ovary juvenilization (May-Panloup et al. 2016).
It has been proved that aging decreases the oocyte quality and decreases the rate of pregnancy. In a clinical trial that was conducted by Oktay K in 2015, the healthy mitochondria isolated from oogonial precursor cells were injected with a needle during intracytoplasmic sperm injection. Again, the result was amazing. In autologous mitochondrial injection group, the rate of the in vitro fertilization (IVF) outcome was higher than the group received no mitochondrial injection (Oktay et al. 2015).
Diminished ovarian reserve (DOR) leads to a low response to stimulation in IVF cycle (Alborzi et al. 2015). It has been suggested that mitochondrial transplantation could be a new hope for the treatment of infertility in assisted reproductive technology (ART) outcome (Alborzi et al. 2015). Transplantation of young donor oocyte cytoplasm including healthy mitochondria into aged fertilized eggs is a promising and interesting strategy in ART field and would increase the rate of pregnancy improve the quality of the oocyte and decrease maternally inherited diseases especially mitochondrial disorders (Woods and Tilly 2015).
Kong et al. (2003a, b) had two successful experiences for autologous granular cell mitochondria transfer. In the first study, a 37 years old woman with several times failure in ART, received autologous granular cell mitochondria transfer in five oocytes in which four of them fertilized but three of the embryos implanted. One of the embryos was aborted at 4th week of pregnancy and finally, two normal babies were born for the first time by this method in China (Kong et al. 2003a). In the other case, a 46-year-old woman underwent an autologous granular cell mitochondria transfer for her MII oocytes. The oocytes fertilized and one of them was transferred to the uterus. The fetus was formed but spontaneous abortion occurred at 9th week of pregnancy. This was the first pregnancy with this method in an aged woman (Kong et al. 2003b).
It is obvious that autologous transplantation of mitochondria could have more efficient outcomes. However, in some cases including mitochondrial related diseases or in some sickest patients, isolation of their own mitochondria is not feasible. On the other hand, some patients need several series of injections. Therefore, in this regard heterologous mitochondria transplantation is inevitable. The main problems of heterogenous or allergenic mitochondria transplantation are immune system responses and damage-associated molecular pattern (DAMPs). Of note, in all previous studies, only a single injection of mitochondria has been reported. What would happen following serial injections of mitochondria into the damaged tissues? Mc Cully in 2018 conducted a study to know the immune system behavior after direct or indirect autogenetic and allogeneic injections, single and serial injections and also a different number of isolated mitochondria (1 × 105, 1 × 106 or 1 × 107 mitochondria). The findings have been demonstrated that the level of immune system profiles including IL-1, IL-4, IL-6, IL-12, IL-18, IP-10, macrophage inflammatory protein MIP-1 α, and MIP-1 β did not change. Single or serial injections of mitochondria did not show any DAMP in recipient tissues. Based on previous studies, circulating free mtDNA activated DAMPs and induced an immune response and cell injury which resulted in remarkable damages to lung or heart tissues (Kuck et al. 2015; Simmons and Gillespie 2015; Cloonan and Choi 2016). However, more recently it has been demonstrated that there was no increase in circulating free mtDNA following 10 days of recovery from serial i.p. injections of either syngeneic or allogeneic mitochondria compared with the control mice, received only respiration buffer. In addition, as histopathological analysis revealed, no evidence of inflammation, cellular damages such as necrosis and fibrosis in heart and lung tissues were observed (Ramirez-Barbieri et al. Ramirez-Barbieri 2018).
Challenges of mitochondrial transplantation
It is exciting for a scientific committee to use mitochondrial transplantation in the clinic for the treatment of several diseases. However, there are many unsolved problems including safety and efficiency. The isolation of healthy and functional mitochondria is very important and crucial. It is noteworthy that only functional mitochondria can exert their therapeutic efficiency. Therefore, the quality control of healthy mitochondria is crucial (McCully et al. 2016; Preble et al. 2014a). We also do not exactly know for how long mitochondria can be functional after transplantation. The transplanted mitochondria can exert their therapeutic effects both extracellular and intracellular. The transplanted mitochondria in the ischemic heart immediately increased myocardial ATP levels, rapidly altered the myocardial proteome to upregulate pathways for energy generation, cellular respiration and upregulated cardioprotective cytokines (Masuzawa et al. 2013; Pacak et al. 2015). These cytokines are associated with reduced cardiomyocyte apoptosis and enhanced functional cardiac recovery and cardiac remodeling independent of cardiac myocyte regeneration (Masuzawa et al. 2013). The mitochondria were rapidly internalized (2.5–60 min) into myocardial cells by actin-dependent endocytosis where they improved cellular function (Cowan et al. 2017). The transplanted, mitochondria maintained viable and had function for at least 28 days and had no pro-arrhythmic, inflammatory, immune or auto-immune response (Emani and McCully 2018).
It has been recommended to obtain tissue biopsy from non-ischemic skeletal muscle mainly from pectoralis major or rectus abdominis. Usually, 6 mm tissue biopsy is suitable to take enough mitochondria for injection. Approximately, the number of mitochondria which can be obtained from a 6 mm biopsy is 2.4 × 1010 ± 0.1 × 1010 (Emani et al. 2017; McCully et al. 2016; Preble et al. 2014a). The availability of other tissues to replace skeletal muscle for the sake of obtaining functional and higher number of mitochondria requires further studies. Some considerations in terms of quality control must be applied before clinical application of mitochondria including; the number of mitochondria, mitochondrial viability, mitochondrial purity and mitochondrial function (Preble et al. 2014a, c). Although mitochondrial transplantation has been optimized for cardiac transplantation, the method should get optimized and revised for other tissues as well.
The route of injection could be another challenge. The clinical trial and preclinical studies used the in situ injection. In 2017, Gollihue optimized intraspinal delivery of mitochondria in SCI model of rat (Gollihue et al. 2017). However, clinical application of in situ injection into the spinal cord or other parts of CNS such as hippocampus or substantia nigra seems not feasible. Therefore, it might be feasible to inject the mitochondria through other routes such as intravenous or intramuscular or even orally.
Currently, autologous transplantation is recommended for the clinical trial (Emani et al. 2017). However, we do not know what would happen if heterogonous mitochondrial transplantation is employed. Isolation of the healthy mitochondria from patients suffering from ischemic diseases is limited. Therefore, finding another source more healthy organs and easier isolation process would be helpful. Currently, the storage of mitochondria is impossible and it must be injected immediately after isolation. Therefore, addressing this challenge would be revolutionary in mitochondrial medicine.
Conclusion
All body cells except red blood cells contain mitochondria which are involved in a large number of important cellular and metabolic processes. The importance of mitochondrion in the maintenance and regulation of cellular homeostasis and function is well-established and there are sufficient supporting studies showing that mitochondrial injury or loss of function is harmful. In other words, mitochondrial dysfunction plays a crucial role in the pathogenesis of a variety of diseases. Therefore, transplantation of viable mitochondria isolated from a healthy tissue and then its delivery into the injured organ/tissue/cells would replace or augment the damaged mitochondria, allowing the rescue of cells and restoration of normal function. Supporting our notion, more recently, the efficacy of mitochondrial transplantation for cardio-protection has been shown in a clinical trial which strongly suggests that mitochondria might be considered beyond their initial role to supply energy i.e. as a novel therapeutic intervention. Overall, mitochondrial transplantation might not only act as a novel unique therapeutic strategy but also could be applicable for the treatment of many diseases/injuries in which conventional therapies have proved unsuccessful. However, mitochondrial transplantation is in the beginning steps of development and further and comprehensive studies including the precise mechanisms of protection are required.
Acknowledgements
Part of this study was supported by National Institute for Medical Research Development (Grant No. 962134).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi-Malati Z, Roushandeh AM, Kuwahara Y, Roudkenar MH. Mesenchymal stem cells on horizon: a new arsenal of therapeutic agents. Stem Cell Rev Rep. 2018;14:484–499. doi: 10.1007/s12015-018-9817-x. [DOI] [PubMed] [Google Scholar]
- Ahmad T, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimo A, et al. Targeting mitochondrial dysfunction in chronic heart failure: current evidence and potential approaches. Curr Pharm Des. 2016;22:4807–4822. doi: 10.2174/1381612822666160701075027. [DOI] [PubMed] [Google Scholar]
- Alborzi S, Madadi G, Samsami A, Soheil P, Azizi M, Alborzi M, Bakhshaie P. Decreased ovarian reserve: any new hope? Minerva Ginecol. 2015;67:149–167. [PubMed] [Google Scholar]
- Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005;280:35742–35750. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- Bach D, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- Berridge M, Herst P, Rowe M, Schneider R, McConnell M. Mitochondrial transfer between cells: methodological constraints in cell culture and animal models. Anal Biochem. 2018;552:75–80. doi: 10.1016/j.ab.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Boczonadi V, et al. Mutations in glycyl-tRNA synthetase impair mitochondrial metabolism in neurons. Hum Mol Genet. 2018;27:2187–2204. doi: 10.1093/hmg/ddy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafede R, Mariotti R. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front Cell Neurosci. 2017;11:80. doi: 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragoszewski P, Turek M, Chacinska A. Control of mitochondrial biogenesis and function by the ubiquitin–proteasome system. Open Biol. 2017;7:170007. doi: 10.1098/rsob.170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073. doi: 10.1038/srep09073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C. Mitochondrial dynamics: shaping metabolic adaptation. Int Rev Cell Mol Biol. 2018;340:129–167. doi: 10.1016/bs.ircmb.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Chang J-C, et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl Res. 2016;170:40–56. doi: 10.1016/j.trsl.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Receptor-interacting protein 140 overexpression impairs cardiac mitochondrial function and accelerates the transition to heart failure in chronically infarcted rats. Transl Res. 2017;180:91–102. doi: 10.1016/j.trsl.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Chou SH, et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke. 2017;48:2231–2237. doi: 10.1161/STROKEAHA.117.017758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid-Castro C, Hernández-Espinosa DR, Morán J. ROS as regulators of mitochondrial dynamics in neurons. Cell Mol Neurobiol. 2018;38:995–1007. doi: 10.1007/s10571-018-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloonan SM, Choi AM. Mitochondria in lung disease. J Clin Invest. 2016;126:809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan DB, Yao R, Thedsanamoorthy JK, Zurakowski D, Pedro J, McCully JD. Transit and integration of extracellular mitochondria in human heart cells. Sci Rep. 2017;7:17450. doi: 10.1038/s41598-017-17813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Barone E, Perluigi M, Butterfield DA. The triangle of death in Alzheimer’s disease brain: the aberrant cross-talk among energy metabolism, mammalian target of rapamycin signaling, and protein homeostasis revealed by redox proteomics. Antioxid Redox Signal. 2017;26:364–387. doi: 10.1089/ars.2016.6759. [DOI] [PubMed] [Google Scholar]
- Ehses S, et al. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Jiang X, Head J. Mitochondria organelle transplantation: a potential cellular biotherapy for cancer. J Surg. 2015;S(2):9. [Google Scholar]
- Emani SM, McCully JD. Mitochondrial transplantation: applications for pediatric patients with congenital heart disease. Transl Pediatr. 2018;7:169. doi: 10.21037/tp.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2017;154:286–289. doi: 10.1016/j.jtcvs.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Faizi M, Seydi E, Abarghuyi S, Salimi A, Nasoohi S, Pourahmad J. A search for mitochondrial damage in Alzheimer’s disease using isolated rat brain mitochondria. Iran J Pharm Res. 2016;15:185. [PMC free article] [PubMed] [Google Scholar]
- Forbes JM. Mitochondria-power players in kidney function? Trends Endocrinol Metab. 2016;27:441–442. doi: 10.1016/j.tem.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Ganie SA, Dar TA, Bhat AH, Dar KB, Anees S, Zargar MA, Masood A. Melatonin: a potential anti-oxidant therapeutic agent for mitochondrial dysfunctions and related disorders. Rejuvenation Res. 2016;19:21–40. doi: 10.1089/rej.2015.1704. [DOI] [PubMed] [Google Scholar]
- Giannoccaro MP, La Morgia C, Rizzo G, Carelli V. Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson’s disease. Mov Disord. 2017;32:346–363. doi: 10.1002/mds.26966. [DOI] [PubMed] [Google Scholar]
- Gollihue JL, et al. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J Neurotrauma. 2018;35:1800–1818. doi: 10.1089/neu.2017.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue JL, Patel SP, Mashburn C, Eldahan KC, Sullivan PG, Rabchevsky AG. Optimization of mitochondrial isolation techniques for intraspinal transplantation procedures. J Neurosci Methods. 2017;287:1–12. doi: 10.1016/j.jneumeth.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A, Mensah-Nyagan AG, Eckert A. Alzheimer, mitochondria and gender. Neurosci Biobehav Rev. 2016;67:89–101. doi: 10.1016/j.neubiorev.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, et al. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells. 2018;36:1404–1410. doi: 10.1002/stem.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari MM, Khatami M, Danafar A, Dianat T, Farahmand G, Talebi AR. Mitochondrial genetic variation in iranian infertile men with varicocele. Int J Fertil Steril. 2016;10:303–309. doi: 10.22074/ijfs.2016.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppel CL, Tandler B, Fujioka H, Riva A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol. 2009;41:1949–1956. doi: 10.1016/j.biocel.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, et al. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc Natl Acad Sci. 2013;110:2846–2851. doi: 10.1073/pnas.1300741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PJ, et al. Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transpl. 2016;25:913–927. doi: 10.3727/096368915X689785. [DOI] [PubMed] [Google Scholar]
- Jiang D, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016;7:e2467. doi: 10.1038/cddis.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabekkodu SP, Chakrabarty S, Shukla V, Varghese VK, Singh KK, Thangaraj K, Satyamoorthy K. Mitochondrial biology: from molecules to diseases. Mitochondrion. 2015;24:93–98. doi: 10.1016/j.mito.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Kanaan GN, Harper ME. Cellular redox dysfunction in the development of cardiovascular diseases. Biochim Biophys Acta. 2017;2:30241–30244. doi: 10.1016/j.bbagen.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Kaza AK, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153:934–943. doi: 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]
- Kim YJ, et al. Association between Mitofusin 2 gene polymorphisms and late-onset Alzheimer’s disease in the Korean population. Psychiatry Investig. 2017;14:81–85. doi: 10.4306/pi.2017.14.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Hwang JW, Yun C-K, Lee Y, Choi Y-S. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep. 2018;8:3330. doi: 10.1038/s41598-018-21539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LH, Liu Z, Li H, Zhu L, Chen SL, Xing FQ. First twins born in Mainland China by autologous granular cell mitochondria transfer. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:990–991. [PubMed] [Google Scholar]
- Kong LH, Liu Z, Li H, Zhu L, Xing FQ. Pregnancy in a 46-year-old woman after autologous granular cell mitochondria transfer. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:743–747. [PubMed] [Google Scholar]
- Kuck JL, Obiako BO, Gorodnya OM, Pastukh VM, Kua J, Simmons JD, Gillespie MN. Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am J Phys Lung Cell Mol Phys. 2015;308:1078–1085. doi: 10.1152/ajplung.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SR. Mitochondrial transplantation: another miracle of molecular medicine? J Thorac Cardiovasc Surg. 2017;154:284–285. doi: 10.1016/j.jtcvs.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Kuo C-C, et al. Prevention of axonal degeneration by perineurium injection of mitochondria in a sciatic nerve crush injury model. Neurosurgery. 2017;80:475–488. doi: 10.1093/neuros/nyw090. [DOI] [PubMed] [Google Scholar]
- Kuwahara Y, Roudkenar MH, Suzuki M, Urushihara Y, Fukumoto M, Saito Y, Fukumoto M. The involvement of mitochondrial membrane potential in cross-resistance between radiation and docetaxel. Int J Radiat Oncol Biol Phys. 2016;96:556–565. doi: 10.1016/j.ijrobp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Kuznetsova I, Siira SJ, Shearwood AJ, Ermer JA, Filipovska A, Rackham O. Simultaneous processing and degradation of mitochondrial RNAs revealed by circularized RNA sequencing. Nucl Acids Res. 2017;45:5487–5500. doi: 10.1093/nar/gkx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- Lim TK, Rone MB, Lee S, Antel JP, Zhang J. Mitochondrial and bioenergetic dysfunction in trauma-induced painful peripheral neuropathy. Mol Pain. 2015;11:58. doi: 10.1186/s12990-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-S, Chang J-C, Kuo S-J, Liu K-H, Lin T-T, Cheng W-L, Chuang S-F. Delivering healthy mitochondria for the therapy of mitochondrial diseases and beyond. Int J Biochem Cell Biol. 2014;53:141–146. doi: 10.1016/j.biocel.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Lopez-Crisosto C, et al. Sarcoplasmic reticulum–mitochondria communication in cardiovascular pathophysiology. Nat Rev Cardiol. 2017;14:342–360. doi: 10.1038/nrcardio.2017.23. [DOI] [PubMed] [Google Scholar]
- Loureiro R, Mesquita KA, Magalhaes-Novais S, Oliveira PJ, Vega-Naredo I. Mitochondrial biology in cancer stem cells. Semin Cancer Biol. 2017 doi: 10.1016/j.semcancer.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget. 2017;8:15539. doi: 10.18632/oncotarget.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, et al. SIRT1 Activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev. 2017;4602715:13. doi: 10.1155/2017/4602715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa A, et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304:H966–H982. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis S, Couratier P, Julian A, Corcia P, Le Masson G. Current view and perspectives in amyotrophic lateral sclerosis. Neural Regen Res. 2017;12:181. doi: 10.4103/1673-5374.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Panloup P, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22:725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296:H94–H105. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully JD, Levitsky S, Pedro J, Cowan DB. Mitochondrial transplantation for therapeutic use. Clin Transl Med. 2016;5:16. doi: 10.1186/s40169-016-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully JD, Cowan DB, Emani SM, Del Nido PJ. Mitochondrial transplantation: from animal models to clinical use in humans. Mitochondrion. 2017;34:127–134. doi: 10.1016/j.mito.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Moreno-Lastres D, Fontanesi F, García-Consuegra I, Martín MA, Arenas J, Barrientos A, Ugalde C. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012;15:324–335. doi: 10.1016/j.cmet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13:68. doi: 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitzova K, et al. Mitochondrial transplantation prolongs cold preservation time in murine cardiac transplantation. J Heart Lung Transpl. 2018;37:S22–S23. doi: 10.1016/j.healun.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzigou BM, Gerbal-Chaloin S, Bokus A, Daujat-Chavanieu M, Jorgensen C, Hugnot J-P, Vignais M-L. MitoCeption: transferring isolated human MSC mitochondria to glioblastoma stem cells. J Vis Exp. 2017 doi: 10.3791/55245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, et al. Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22:1612–1617. doi: 10.1177/1933719115612137. [DOI] [PubMed] [Google Scholar]
- Onyango IG, Dennis J, Khan SM. Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis. 2016;7:201. doi: 10.14336/AD.2015.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango IG, Khan SM, Bennett JP., Jr Mitochondria in the pathophysiology of Alzheimer’s and Parkinson’s diseases. Front Biosci. 2017;22:854–872. doi: 10.2741/4521. [DOI] [PubMed] [Google Scholar]
- Pacak CA et al (2015) Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biology open:BIO201511478 [DOI] [PMC free article] [PubMed]
- Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25:31. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone P, et al. Biological and biophysics aspects of metformin-induced effects: cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging. 2016;8:1718. doi: 10.18632/aging.101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble JM, Kondo H, Levitsky S, McCully JD. Quality control parameters for mitochondria transplant in cardiac tissue. Mol Biol. 2013;2:1008. [Google Scholar]
- Preble J, Kondo H, Levitsky S, James D, McCully J. Quality control parameters for mitochondria transplant in cardiac tissue JSM Biochem. Mol Biol. 2014;2:1008. [Google Scholar]
- Preble JM, Pacak CA, Kondo H, MacKay AA, Cowan DB, McCully JD. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp. 2014 doi: 10.3791/51682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble JM, Pacak CA, Kondo H, MacKay AA, Cowan DB, McCully JD. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp. 2014;5:51682. doi: 10.3791/51682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralto KM, Parikh SM. Mitochondria in acute kidney injury. Semin Nephrol. 2016;36:8–16. doi: 10.1016/j.semnephrol.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Barbieri G, et al. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion. 2018 doi: 10.1016/j.mito.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Robicsek O, et al. Isolated mitochondria transfer improves neuronal differentiation of schizophrenia-derived induced pluripotent stem cells and rescues deficits in a rat model of the disorder. Schizophr Bull. 2017;44:432–442. doi: 10.1093/schbul/sbx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca CJ, et al. Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich’s ataxia. Sci Transl Med. 2017;9:eaaj2347. doi: 10.1126/scitranslmed.aaj2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudkenar MH, Halabian R, Tehrani HA, Amiri F, Jahanian-Najafabadi A, Roushandeh AM, Abbasi-Malati Z. Lipocalin 2 enhances mesenchymal stem cell-based cell therapy in acute kidney injury rat model. Cytotechnology. 2018;70:103–117. doi: 10.1007/s10616-017-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roushandeh AM, Bahadori M, Roudkenar MH. Mesenchymal stem cell-based therapy as a new horizon for kidney injuries. Arch Med Res. 2017;48:133–146. doi: 10.1016/j.arcmed.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Rub C, Wilkening A, Voos W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017;367:111–123. doi: 10.1007/s00441-016-2485-8. [DOI] [PubMed] [Google Scholar]
- Sabbah HN. Targeting mitochondrial dysfunction in the treatment of heart failure. Expert Rev Cardiovasc Ther. 2016;14:1305–1313. doi: 10.1080/14779072.2016.1249466. [DOI] [PubMed] [Google Scholar]
- Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, Pourahmad J. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox Biol. 2015;6:461–471. doi: 10.1016/j.redox.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi A, Roudkenar MH, Seydi E, Sadeghi L, Mohseni A, Pirahmadi N, Pourahmad J. Chrysin as an anti-cancer agent exerts selective toxicity by directly inhibiting mitochondrial complex II and V in CLL B-lymphocytes. Cancer Invest. 2017;35:174–186. doi: 10.1080/07357907.2016.1276187. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2007;66:10–16. doi: 10.1097/nen.0b013e31802c396b. [DOI] [PubMed] [Google Scholar]
- Schirone L, et al. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid Med Cell Longev. 2017;3920195:2. doi: 10.1155/2017/3920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaki F, Shayeste Y, Karami M, Akbari E, Rezaei M, Ataee R. The effect of epicatechin on oxidative stress and mitochondrial damage induced by homocycteine using isolated rat hippocampus mitochondria. Res Pharm Sci. 2017;12:119. doi: 10.4103/1735-5362.202450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siira SJ, Shearwood AJ, Bracken CP, Rackham O, Filipovska A. Defects in RNA metabolism in mitochondrial disease. Int J Biochem Cell Biol. 2017;85:106–113. doi: 10.1016/j.biocel.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Simmons JD, Gillespie MN. Plasma nuclear and mitochondrial DNA levels in acute myocardialinfarction patients. Coron Artery Dis. 2015;26:286–288. doi: 10.1097/MCA.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simula L, Campello S. Monitoring the mitochondrial dynamics in mammalian cells. Methods Mol Biol. 2018;1782:267–285. doi: 10.1007/978-1-4939-7831-1_15. [DOI] [PubMed] [Google Scholar]
- Simula L, Nazio F, Campello S. The mitochondrial dynamics in cancer and immune-surveillance. Semin Cancer Biol. 2017;47:29–42. doi: 10.1016/j.semcancer.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Sinkler CA, Kalpage H, Shay J, Lee I, Malek MH, Grossman LI, Hüttemann M. Tissue-and condition-specific isoforms of mammalian cytochrome c oxidase subunits: from function to human disease. Oxid Med Cell Longev. 2017 doi: 10.1155/2017/1534056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymoczko JL, Berg JM, Stryer L. Biochemistry: a short course. New York: Macmillan; 2011. [Google Scholar]
- Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- von Hardenberg A, Maack C. Mitochondrial therapies in heart failure. Handb Exp Pharmacol. 2017;243:491–514. doi: 10.1007/164_2016_123. [DOI] [PubMed] [Google Scholar]
- Wang X, Gerdes H-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22:1181. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Chen KM, Wang Y, Shi SP. Functional changes in rat-liver mitochondria during the early phase of burn injury. Burns Incl Therm Inj. 1986;12:461–464. doi: 10.1016/0305-4179(86)90069-0. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Stem cell-derived mitochondria transplantation: a novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res Therapy. 2018;9:106. doi: 10.1186/s13287-018-0832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DC, Tilly JL. Autologous Germline Mitochondrial Energy Transfer (AUGMENT) in human assisted reproduction. Semin Reprod Med. 2015;33:410–421. doi: 10.1055/s-0035-1567826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, et al. Polymer functionalization of isolated mitochondria for cellular transplantation and metabolic phenotype alteration. Adv Sci. 2018;5:1700530. doi: 10.1002/advs.201700530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, et al. Receptor-interacting Protein 140 represses Sirtuin 3 to facilitate hypertrophy, mitochondrial dysfunction and energy metabolic dysfunction in cardiomyocytes. Acta Physiol. 2017;220:58–71. doi: 10.1111/apha.12800. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Lu NH, Xie Y, Guo GH, Zhan JH, Chen J. Influence of heat shock preconditioning on structure and function of mitochondria in gastric mucosa of severely burned animals: experiment with rats. Zhonghua Yi Xue Za Zhi. 2008;88:564–567. [PubMed] [Google Scholar]
- Zhang C, Montooth KL, Calvi BR. Incompatibility between mitochondrial and nuclear genomes during oogenesis results in ovarian failure and embryonic lethality. Development. 2017;144:2490–2503. doi: 10.1242/dev.151951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zussman B, Weiner G, Ducruet A. Mitochondrial transfer into the cerebrospinal fluid in the setting of subarachnoid hemorrhage. Neurosurgery. 2017;82:N11–N13. doi: 10.1093/neuros/nyx528. [DOI] [PubMed] [Google Scholar]