Abstract
Bone mesenchymal stem cells (BMSCs) have the capacity to differentiate into germ cells (GCs). This study was conducted to develop a non-integrated method of using RNA transfection to derive putative male GCs from goat BMSCs (gBMSCs) in vitro by overexpressing STRA8, BOULE and DAZL. The gBMSCs were induced by co-transfection these three mRNAs together (mi-SBD group) or sequential transfection according to their expression time order in vivo (mi-S + BD group). After transfection, a small population of gBMSCs transdifferentiated into early germ cell-like cells and had the potential to enter meiosis. These cells expressed primordial germ cell specific genes STELLA, C-KIT and MVH, as well as premeiotic genes DAZL, BOULE, STRA8, PIWIL2 and RNF17. Importantly, the expression level of meiotic marker synaptonemal complex protein 3 significantly increased in these transfected two groups compared with control cells by qRT-PCR, immunofluorescence and western blot analysis (P < 0.05). Moreover, the protein expression of MVH was significantly higher in mi-S + BD group than that in mi-SBD group (P < 0.05). In addition, compared with control group, the methylation rate of imprinted gene H19 decreased in these two transfected group (P < 0.05), and the rate was significantly lower in mi-S + BD group compared with mi-SBD group (P < 0.05). This study helps to understand the mechanisms of action of key genes in GCs differentiation and also provides a novel system for in vitro induction of male GCs from stem cells.
Keywords: BMSCs, Germ cells, STRA8, DAZL, BOULE, Differentiation
Introduction
Stem cells provide a powerful tool for studying the mechanisms of germ cell development in vitro. Several studies have shown that embryonic stem cells (ESCs) have the ability to differentiate into haploid male germ cells in vitro (Geijsen et al. 2004; Kee et al. 2009b; Kerkis et al. 2007). However, due to ethical constraints for human ESC and difficult establishment of other species ES cell lines, it limits the application of ESCs. Meanwhile, induced pluripotent stem cells (iPSCs) are another potential cells to induce differentiation of germ cells in vitro (Li et al. 2014; Easley et al. 2012), while the efficiency of iPSC induction still remains low. The BMSCs are pluripotent adult stem cells that exhibit differentiation capacity into different cell types of both mesenchymal and non-mesenchymal lineages, including germ cells (Nayernia et al. 2006;Drusenheimer et al. 2007; Hua et al. 2009; Ghasemzadeh-Hasankolaei et al.2016). More importantly, BMSCs is relatively easier to isolate and expand extensively without losing their multipotency, which make them ideal model cells for in vitro differentiation and study gametogenesis. To date, induction methods by treatment with exogenous factors like retinoic acid (RA), transforming growth factor-beta superfamily growth factors and bone morphogenetic protein (BMP4) are reported to induce BMSCs differentiation into GC-like lineages (Shirazi et al. 2012; Ghasemzadeh-Hasankolaei et al. 2014; Yan et al. 2015). However, in both the mouse and human in vitro systems, progression of meiosis has limited the success of production of mature gametes.
Germ cell development is controlled by unique gene expression programs. Some genes have been shown to be critical for germ cell development, with several demonstrating conservation across species. STAR8 (stimulated by retinoic acid gene 8) is a specific expression gene in mammalian germ cells transition from mitosis to meiosis, which is indispensable for premeiotic DNA replication and subsequent entry into the prophase of meiosis I (Mark et al. 2008). STRA8 was also usually used to construct the germ cell specific reporting system (Anderson et al. 2008). The RNA-binding protein DAZL and BOULE, highly conserved between species, play important roles in animal germ cell development. DAZL is a master gene controlling germ cell differentiation and ectopic expression of DAZL promotes the dynamic differentiation of mouse ES cells into gametes in vitro (Yu et al. 2009). Forced expression of DAZ gene family (DAZL, DAZ and BOULE) induces human ESCs to differentiate to PGCs and haploid gametes in vitro (Kee et al. 2009a). Also, co-overexpression of DAZL and VASA could promote meiotic progression of human-derived germ cells from human ESCs and iPSCs in vitro (Medrano et al. 2012). Taken together, co-overexpression of multiple germ cell special genes might be able to improve in vitro germ cell differentiation. However, most of the research about in vitro derivation germ cells form BMSCs focused on mice and human, other species are still limited.
In fact, we previously demonstrated that ectopic expression of DAZL gene enhanced goat BMSCs trans-differentiation into male germ cells compared to the RA treated cells (Yan et al. 2015). Our recent study also has revealed the co-transfection with plasmids of STRA8, BOULE, and DAZL was able to promote gBMSCs trans-differentiate into to early goat germ cell–like cells in vitro (Li et al. 2017). However, compared with plasmid DNA based method, RNA does not require cell proliferation for expression of the encoded protein, on the other hand, mRNAs are translated within minutes following entry into the cytoplasm, whereas plasmids require time-consuming nuclear import and transcription, which indicate more efficient transduction of primary cells than DNA (Mclenachan et al. 2013). Therefore, here we sought to determine whether co-overexpression of STRA8, BOULE and DAZL using RNA synthesized in vitro would efficiently induce gBMSCs to trans-differentiate into germ cells and also compare the induction efficiency of co-transfection these three mRNAs together or sequential transfection according to their expression time order in vivo. To our knowledge, this is the first study using a non-integrated method of RNA transfection to derive putative male GCs from BMSCs.
Methods and materials
Ethics statement
This study was approved by the ethical committee of the College of Animal Science and Technology, Nanjing Agricultural University, China. All protocols were conducted in strict accordance with the Animal Research Committee guidelines of the College of Animal Science and Technology, Nanjing Agricultural University, China.
STRA8, BOULE and DAZL mRNA preparation by in vitro transcription
The plasmids pEX-4-STRA8, pEX-4-DAZL and pEGFP-DAZL were constructed in our previous study (Li et al. 2017) (Fig. 1a). There primers including the T7 promoter sequence were designed and used amplified STRA8, BOULE and DAZL, the PCR product were purified and followed by in vitro RNA transcription (IVT) using the mMESSAGE mMACHINE T7 kit (Applied Biosystems/Ambion, TX, USA) that allows 5′cap formation. The transcribed RNA was recovered on MEGAclear™ Transcription Clean-Up Kit according to the RNA Cleanup protocol of the manufacturer. RNA integrity and quantity was monitored by agarose gel electrophoresis and Nanodrop, respectively. PCR primers were as follows:
T7-STRA8-F: TAATACGACTCACTATAGGGATGGAGACTGCAGGAGAAGGTG
T7-STRA8-R: TCACAGATCTTCATCGTCAAAT
T7-BOULE-F: TAATACGACTCACTATAGGGATGGAGACCGAGTCCGGGGCGC
T7-BOULE-R: TTAATAATGAATGCTCCACACT
T7-DAZL-F: TAATACGACTCACTATAGGGATGGCGCCCTCCTCTCTGAACC
T7-DAZL-R: TCAAACAGACTTAAGCACTGCC
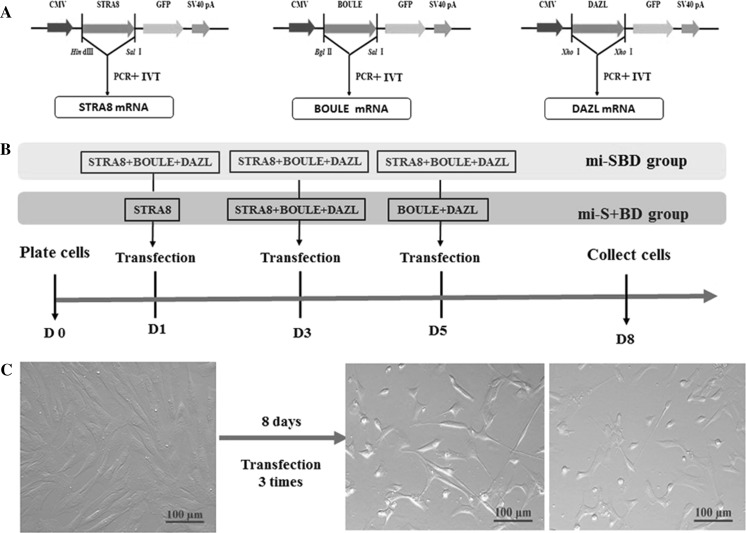
Fig. 1.
The general experimental procedure. a The scheme of the in vitro synthesis of STRA8, BOULE and DAZL mRNA. b The transfection method scheme. c The morphology of non-transfected and transfected gBMSCs
In vitro induction of gBMSCs to putative germ cells by transfection
One day prior to transfection, the in vitro isolated and cultured goat BMSCs at passage 4 were seeded in a 12-well plate at 70% confluency. The detailed culture information on gBMSCs was described in our previous study (Li et al. 2017; Yan et al. 2015). The transfection method was followed the protocol of Lipofectamine® MessengerMAX (Thermo Fisher Scientific, USA). To optimize the induction efficiency of STRA8, BOULE and DAZL mRNA, gBMSCs were transfected with these three mixture together (mi-SBD group, the ratio was 1:1:1), or with the mRNA according their chronological expression order in vivo (named mi-S + BD group, the ratio was 1:1:1). For mi-SBD group, the mRNA mixtures of STRA8, BOULE and DAZL were transfected three times on Day 1, Day 3 and Day 5, respectively. For mi-SBD group, only STRA8 mRNA were first transfected on Day 1, followed by STRA8, BOULE and DAZL mRNA on Day 3, and finally BOULE and DAZL mRNA were transfected on Day 5. The transfected BMSCs on Day 8 were collected for further analysis. The experiment design was shown as Fig. 1b.
Quantitative RT-PCR analysis
Total RNA was isolated from the cells and the goat testis using PureLink RNA Mini Kit and TRIzol reagent (Invitrogen Life Technologies), respectively. The cDNA was then synthesized using a reverse transcription reagent kit with gDNA Eraser (Takara, Dalian, China). qRT-PCR was performed to detect the expression of genes on a StepOnePlus Real-Time PCR System (Life Technologies, USA) using SYBR Green Master mix (Roche Applied Science, Mannheim, Germany). Comparative quantification of the expression levels of targeted genes was done by the 2-ΔΔCt method with the expression of the target genes normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The gene primers were designed as our previous paper (Li et al. 2017).
Immunofluorescence analysis
Cells were seeded in glass-bottom dishes and fixed with ice-cold methanol for 10 min at room temperature (RT) and permeabilized (0.25% Triton X-100) (Sigma) for 10 min. Then they were blocked with Immunol Staining Blocking Buffer (Beyotime) for 60 min at RT on a rocking platform. The primary rabbit anti-DAZL antibody (1:100 dilution, ab34139; Abcam) and rabbit anti-SCP3 antibody (1:100 dilution; ab150292, Abcam) were added to cells and incubated for overnight at 4◦C. Afterward, the cells were washed with PBS for 3 times, then the secondary antibody, 594-conjugated donkey anti-rabbit antibody (1:200 dilution, ab96921; Abcam) was added to cells and incubated in dark for 2 h at RT. Finally, the nuclei of samples were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime) for 10 min and samples were examined with a confocal laser-scanning microscope (Zeiss LSM 710 META, Germany).
Western blotting
Total protein was extracted from the cells with RIPA lysis buffer (Beyotime Biotechnology, China) and quantified by BCA method. The Western blot standard protocol were used as our previously described (Li et al. 2017). The primary rabbit anti-MVH antibody (1:300 dilution, ab13840; Abcam) and rabbit anti-SCP3 antibody (1:300 dilution, ab150292; Abcam) were used. Horseradish peroxidase-labeled goat antirabbit (1:1000 dilution, A0208; Beyotime) was used as a secondary antibody. Samples were also incubated for ACTIN (β-actin, internal protein) (1:200 dilution, AA128; Beyotime) with secondary antibody (1:1000 dilution, A0216;Beyotime). Band intensities were estimated by densitometry and normalized to ACTIN. Bands were quantified using Image J software (Wayne Rasband, Maryland, USA).
Methylation analyses of H19 imprinting gene
After 8 days transfection, total genomic DNA was extracted from the gBMSCs using TIANamp Genomic DNA kit (Tiangen, Beijing, China). First, the putative differentially methylated regions (DMRs) of H19 were identified using the online software MethyPrimer with the following restrictive conditions: GC percentage ≥ 50.0% and CpG observe/expect ≥ 0.6. Bisulfite PCR specific primers were M-H19-F: 5′-GTATTAGGTTTTTGGTGGTATAGAG-3′; M-H19-R: 5′-CTCTCCTCTCCCAACTTCAAC-3′. Second, bisulfite treatment and recovery of DNA samples were performed using the EZ DNA Methylation-Direct kit (Zymo Research, Irvine, CA, USA) following the instructions. Finally, bisulfite PCR and sequencing was performed. The Methylation patterns were analyzed using the BIQ Analyzer software and sequences derived from greater than 10 clones with 99% cytosine conversions only. The methylation percentage of every sample was obtained using BIQ Analyzer software.
Statistical analyses
Statistical analyses were done using SPSS software (version 19.0). Values were expressed as Mean ± standard error of mean (SEM). Data were analyzed with analysis of variance and Fisher protected least significant difference tests. The difference of methylation rate was analyzed by Chi square test. For all analysis, P < 0.05 was considered statistically significant. All experiments were repeated at least 3 times.
Results
Morphological changes of goat BMSCs after induction with STRA8, BOULE and DAZL mRNA
The STRA8, BOULE and DAZL gene CDS fragments were amplified from the plasmids pEX-4-STRA8, pEX-4-BOULE and pEGFP-DAZL by primers containing the T7 promoter sequence, which was 1104 bp, 840 bp and 966 bp, respectively. After in vitro transcription, gBMSCs were induced for 3 times every 3 days by transfection of STRA8, BOULE and DAZL mRNA. After 8 days of induction, some of the gBMSCs in mi-SBD and mi-S + BD groups manifested as long spindle and irregular scale shaped, and the nucleoplasm ratio increased, but no obvious morphological change differences were seen the mi-SBD and mi-S + BD groups (Fig. 1c).
Expression of germ cell-specific genes in goat BMSCs after induction with STRA8, BOULE and DAZL mRNA
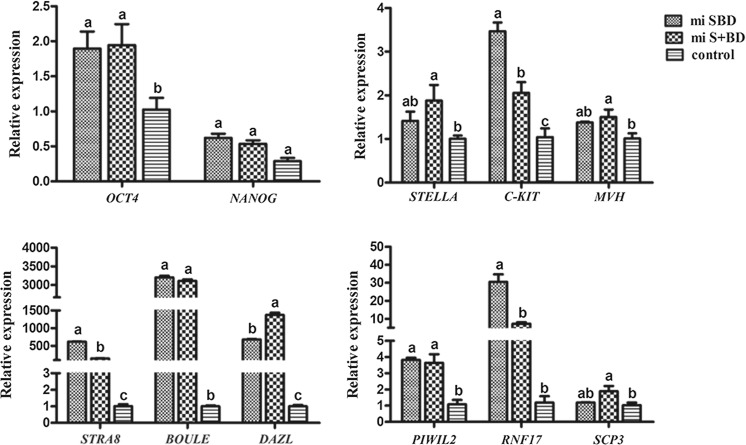
After induction gBMSCs with STRA8, BOULE and DAZL mRNA, we used qRT-PCR to quantitate the expression of early and late germ cell-specific genes (Fig. 2). First, we observed that mi-SBD and mi-S + BD groups showed upregulation in two pluripotency genes OCT4 and NANOG compared with non-transfected gBMSCs, and the OCT4 expression levels significantly increased (P < 0.05). Next, to test whether the transfected cells undergo the progress of PGCs stage. we analyzed three PGCs markers, STELLA, C-KIT and MVH. As expected, the expressions of these three genes were notably increased in both mi-SBD and mi-S + BD groups. STELLA and MVH increased significantly in the miS + BD group compared with the control group (P < 0.05). To further investigate whether meiosis happens in the case of our differentiation process, we then focused on the gene expression levels for several genes STRA8, DAZL, BOULE, PIWIL2, RNF17 and SCP3, all these 6 genes showed significant higher levels in the mi-SBD and mi-S + BD group than that in control group (P < 0.05). SCP3 is required for synapsis phase of meiosis. Importantly, we also observed the expression level of the meiotic gene SCP3 was elevated in mi-S + BD group compared with mi-SBD and control group (P < 0.05).
Fig. 2.
The expression level of germ cell markers in transfected gBMSCs by qRT-PCR
The germ cell-specific proteins exhibited expression in transdifferentiated gBMSCs
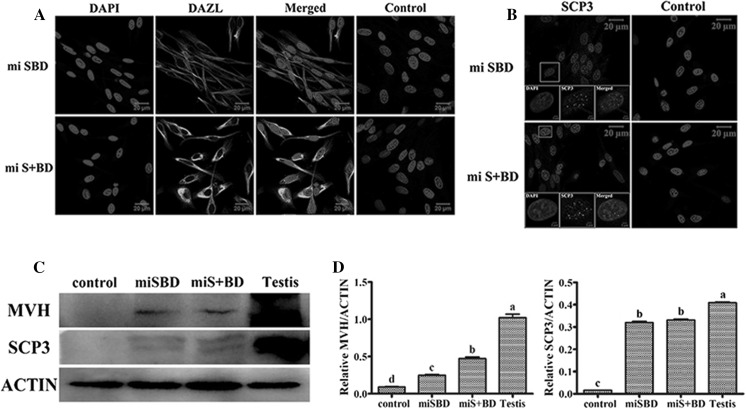
Although the gBMSCs in mi-SBD and mi-S + BD groups expressed some germ cell markers in mRNA levels, whether the translation level was concomitantly regulated remained to be studied. Therefore, we investigated the differentiation by immunofluorescence and western blot to detect the protein expression of early germ cell and meiotic germ cell marker DAZL, MVH, and SCP3. Immunofluorescence results showed the mi-SBD and mi-S + BD groups revealed a strong positive fluorescence signal for the DAZL protein, while negative signal staining was detected in the control gBMSCs (Fig. 3a). On the other hand, signaling for anti-SCP3 antibodies was also positive in the mi-SBD and mi-S + BD groups compared with the control (Fig. 3b). Furthermore, western blot analysis showed that MVH and SCP3 protein levels were significantly higher in mi-SBD and mi-S + BD groups (P < 0.05, Fig. 3c, d). As shown, the mi-S + BD group had the highest expression level of MVH, compared with control gBMSCs (P < 0.05).
Fig. 3.
Immunofluorescence analysis for cellular localization of DAZL and SCP3 in transfected gBMSCs. a Some of the gBMSCs in mi-SBD and mi-S + BD groups showed DAZL-positive clusters (green) in the cytoplasm. b Some of the gBMSCs in mi-SBD and mi-S + BD groups showed SCP3-positive clusters (green) in the nuclear. Normal rabbit serum was used in place of primary antibody in control groups, bar = 20 μm. c, d Western blot analysis for MVH and SCP3 proteins in different transfected gBMSCs. (Color figure online)
The methylation status of H19 imprinted gene in transdifferentiated gBMSCs
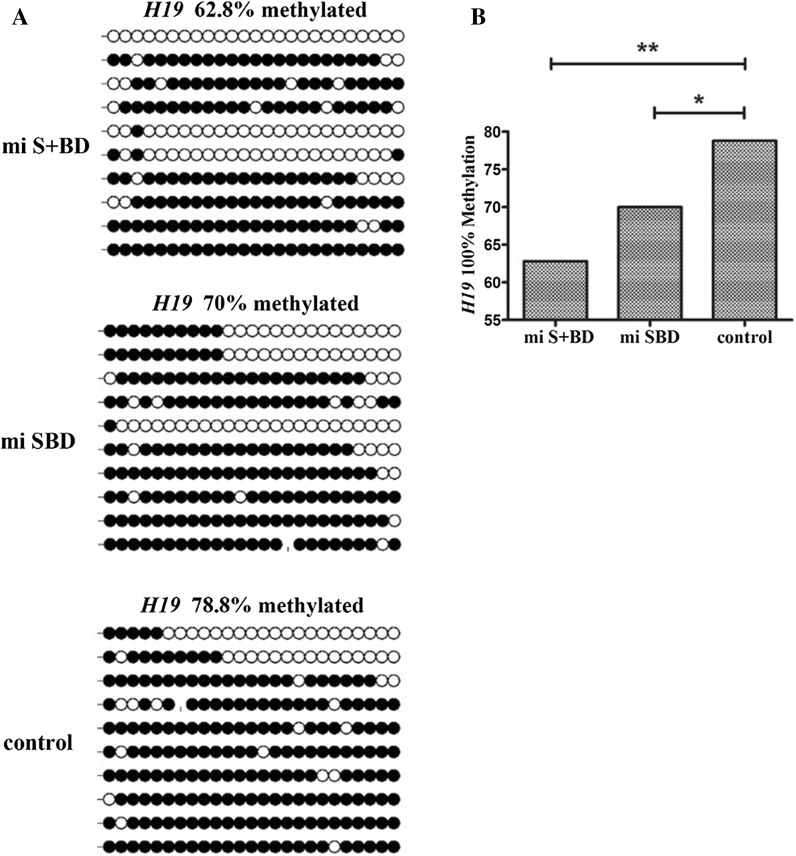
Erasure of epigenetic imprints and their subsequent sex-specific re-establishment is a critical and diagnostic process in germ cell development that is necessary for embryo development. To determine if ectopic expression of STRA8, BOULE and DAZL mRNA alters epigenetic programs, we analyzed methylation status at the CpG islands of the imprinted, maternally expressed gene H19 by BIQ software. We observed that the H19 locus was 78.8% methylated in undifferentiated control cells. Overexpression of STRA8, BOULE and DAZL mRNA led to a significant decrease in the methylation ratio of H19 in mi-SBD and mi-S + BD groups compared with the control, which was 62.8% and 70%, respectively (P < 0.05, Fig. 4).
Fig. 4.
DNA methylation status of H19 DMR in gBMSCs. a Diagrams represent methylation status of each CpG dinucleotides in individual DNA clones of mi-S + BD group, mi-SBD group and control (un-treated gBMSCs). Unfilled (white) and filled (black) circles represent unmethylated and methylated CpGs, respectively. Horizontal line of circles represent one single clone that was sequenced. b Percentage of methylated CpGs found in each condition at the DMR of H19 (*P < 0.05, **P < 0.01)
Discussion
Previous studies demonstrated that BMSCs can form germ cells in vitro, albeit with different efficiencies observed between different induction methods (Shirazi et al. 2012; Yan et al. 2015; Ghasemzadeh-Hasankolaei et al. 2016; Li et al. 2017). Here we examined the effect of ectopic expression of STRA8, BOULE and DAZL on goat germ cell formation and progression through meiosis. The administration of synthetic mRNA has proven more efficient to reprogram cell fate (Rosa and Brivanlou 2010). Although mRNA transfection of fibroblasts (Yakubov et al. 2010) and iPS cells (Preskey et al. 2016) have been described, mRNA delivery to bone marrow stem cells to make them trans-differentiation has not been previously reported. We speculated that prolonged transfection of the multiple genes mRNA probably enhanced the trans-differentiation to a greater extent. Therefore, this study was conducted to develop a non-integrated method of using RNA transfection to derive putative male GCs from gBMSCs by overexpressing STRA8, BOULE and DAZL. Meanwhile, we also optimized the transfection system in case of degradation of mRNA. In this study, we observed that gBMSCs with ectopic expression of STRA8, BOULE and DAZL mRNA expressed most of the germ cell markers that we analyzed, including marker characteristic of germ cells in both early and later stages of maturation, similar to our previous results reported by DNA-based methods (Li et al. 2017) and other reports in different model system.
OCT4 is primarily expressed in pluripotent lineages and regarded to have a role in germ cell development. OCT4 expression is increased in PGCs and gradually decreased during development towards SSCs and A1–A4 spermatogonia (Kehler et al. 2004). qRT-PCR showed an enhanced expression of the OCT4 in gBMSCs derived GC cells. A low level of OCT4 expression was observed in untreated gBMSCs, which was consistent with previous study on mouse BMSCs (Nayernia et al. 2006). Furthermore, we analyzed three PGCs markers, STELLA, C-KIT and MVH. STELLA is involved in initiating germ cell competence and specification, and in the demarcation of PGCs from their somatic neighbors (Saitou et al. 2002). C-KIT has a similar effect, which is also essential to the process of germ cell migration, proliferation and survival in the gonadal anlage, and it is also involved in the reprogramming of PGCs (Høyer et al. 2005). Our findings indicated that STELLA and C-KIT expression levels increased in mi-SBD and mi-S + BD groups compared with non-transfected group. MVH is an important germ cell marker that expresses from migratory PGCs in the genital ridge to post meiotic germ cells. Loss of MVH function causes a deficiency in the proliferation and differentiation of male germ cells (Tanaka et al. 2000). In our study, MVH showed upregulated in mRNA and protein expression levels compared with the control group, which showed that cells with the highest expression in mi-S + BD group.
The data above suggested that overexpression of STRA8, BOULE and DAZL mRNA promotes PGC formation. We next examined if overexpression of these genes promotes germ-cell differentiation beyond the PGC stage. Furthermore, we observed PIWIL2 and RNF17 expression greatly upregulated in the mi-S + BD and mi-SBD groups compared with the control group. The Piwi-like 2 (PIWIL2) gene, a member of the AGO/PIWI gene family, which function in development and maintenance of germline stem cells. PIWIL2 has a low expression in pre-migratory and migratory stages and an almost increased expression during the development of GCs from post-migratory PGCs to spermatogonia stages (Deng and Lin 2002). RNF17, a component of the mammalian germ cell nuage, is essential for spermatogenesis. SCP3 is a specific meiosis marker that indicates synaptonemal complex formation in meiotic prophase (Fraune et al. 2016). Immunohistochemistry showed that SCP3 is highly expressed in in goat BMSC-GC cells, but not in untreated BMSCs. Furthermore, both SCP3 mRNA and protein expression levels significantly increased in the mi-SBD and mi-S + BD groups, and especially in the mi-S + BD group, which had the highest expression level. More importantly, we observed punctate and elongated SCP3 staining foci using mRNA transfection methods in this present study, indicating the initiation of meiosis, which were not observed when using plasmids transfection in our previous pulished study (Li et al. 2017). Taken together, these results suggested that maturation and progression through meiosis of a small subpopulation of transfected gBMSCs were enhanced, but it has not yet been determined whether these cells can complete meiosis and form functional spermatozoa.
Importantly, erasure of the epigenetic marks at imprinted loci is a process that occurs specifically during mammalian germ cell development just prior to, or shortly after, germ cells arrive to the gonadal ridges. Erasure and re-establishment of epigenetic signatures of imprinted loci is a germline-specific process (Clark 2015). Medrano et al. (2012)found that overexpression of VASA in human ESCs and iPSCs led to a significant decrease in the methylation ratio of H19 (Medrano et al. 2012). Nolte et al. (2010)showed the mouse spermatogenic cells induced by RA induction differentiate into haploid male germ cells, whose H19 methylation level also appeared to decline (Nolte et al. 2010). Similar to those previous research, in this study, we also observed that imprinted H19 locus methylation level showed a significant decrease when cells were subjected to ectopic expression of STRA8, BOULE and DAZL mRNA. The gBMSCs in mi-SBD group showed lower H19 methylation level than that of gBMCs in mi-S + BD group. These results suggested that erasure of imprints may be a specialization of VASA in direct or indirect regulation of RNAs implicated in DNA methylation/demethylation. Further studies should examine the role of possible targets of VASA that may be implicated in germline epigenetic reprogramming.
To summarize, after co-transfected or sequential transfected mRNA of STRA8, BOULE, and DAZL into gBMSCs, these cells showed increased expression levels of early- and late-stage germ cell markers and lower H19 methylation level, which indicated that gBMSCs had the ability of differentiation into putative germ cells in vitro. Furthermore, these cells had the potential to enter meiosis. Moreover, the mi-S + BD method of sequential transfection was more efficient than the miSBD co-transfection, indicating that the gene regulation in time order is essential for gametogenesis. These results suggested that maturation and progression through meiosis of a small subpopulation of transfected gBMSCs were enhanced, but it has not yet been determined whether these cells can complete meiosis and form functional spermatozoa. This study provides a novel system for in vitro induction of male GCs from stem cells, and also contributes to understanding the study of molecular mechanisms for spermatogenesis.
Acknowledgements
We thank all the participants of stem cell group to conduct the experiment and revise the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (No. 31201802) and Natural Science Foundation of Jiangsu Province (No. BK20161444).
Author’s contribution
YLZ and PZL conceived and conducted the study and wrote the manuscript, JP, WYJ, WZY, FYX and ZGM helped to performe the experiments, NHT revised the manuscript, FW supervised the project. All authors contributed to discuss the results.
Abbreviations
- gBMCs
Goat bone mesenchymal stem cells
- ESCs
Embryonic stem cells
- GCs
Germ cells
- iPSCs
Induced pluripotent stem cells
- PGCs
Primordial germ cells
- qRT-PCR
Quantitative real-time PCR
- RA
Retinoic acid
- BMP4
Bone morphogenetic protein 4
- DMRs
Differentially methylated regions
Compliance with ethical standards
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anderson EL, Baltus AE, Roepersgajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AT. DNA methylation remodeling in vitro and in vivo. Curr Opin Genet Dev. 2015;34:82–87. doi: 10.1016/j.gde.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Lin H. Miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819. doi: 10.1016/S1534-5807(02)00165-X. [DOI] [PubMed] [Google Scholar]
- Drusenheimer N, Wulf G, Nolte J, Lee JH, Dev A, Dressel R, Gromoll J, Schmidtke J, Engel W, Nayernia K. Putative human male germ cells from bone marrow stem cells. Soc Reprod Fertil Suppl. 2007;63:69–76. [PubMed] [Google Scholar]
- Easley CA, 4th, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, Schatten GP. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2:440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraune J, Brochierarmanet C, Alsheimer M, Volff JN, Schücker K, Benavente R. Evolutionary history of the mammalian synaptonemal complex. Chromosoma. 2016;125:355–360. doi: 10.1007/s00412-016-0583-8. [DOI] [PubMed] [Google Scholar]
- Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh-Hasankolaei M, Sedighi-Gilani MA, Eslaminejad MB. Induction of ram bone marrow mesenchymal stem cells into germ cell lineage using transforming growth factor-beta superfamily growth factors. Reprod Domest Anim. 2014;49:588–598. doi: 10.1111/rda.12327. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh-Hasankolaei M, Eslaminejad MB, Sedighi-Gilani M. Derivation of male germ cells from ram bone marrow mesenchymal stem cells by three different methods and evaluation of their fate after transplantation into the testis. Vitro Cell Dev Biol Anim. 2016;52:49–61. doi: 10.1007/s11626-015-9945-4. [DOI] [PubMed] [Google Scholar]
- Høyer PE, Byskov AG, Møllgård K. Stem cell factor and c-Kit in human primordial germ cells and fetal ovaries. Mol Cell Endocrinol. 2005;234:1. doi: 10.1016/j.mce.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Hua J, Pan S, Yang C, Dong W, Dou Z, Sidhu KS. Derivation of male germ cell-like lineage from human fetal bone marrow stem cells. Reprod Biomed Online. 2009;19:99–105. doi: 10.1016/S1472-6483(10)60052-1. [DOI] [PubMed] [Google Scholar]
- Kee K, Angeles VT, Flores M, Nguyen HN, Pera RAR. Human DAZL, DAZ and BOULE genes modulate primordial germ cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomelí H, Nagy A, McLaughlin KJ, Schöler HR, Tomilin A. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkis A, Fonseca SA, Serafim RC, Lavagnolli TM, Abdelmassih S, Abdelmassih R, Kerkis I. In vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytes. Cloning Stem Cells. 2007;9:535. doi: 10.1089/clo.2007.0031. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Feng X, Liao S, Zhang D, Cui X, Gao F, Han C. Generation of male germ cells from mouse induced pluripotent stem cells in vitro. Stem Cell Res. 2014;12:517. doi: 10.1016/j.scr.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Li PZ, Yan GY, Han L, Pang J, Zhong BS, Zhang GM, Wang F, Zhang YL. Overexpression of STRA8, BOULE, and DAZL genes promotes goat bone marrow-derived mesenchymal stem cells in vitro transdifferentiation toward putative male germ cells. Reprod Sci. 2017;24:300–312. doi: 10.1177/1933719116654990. [DOI] [PubMed] [Google Scholar]
- Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Féret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121:3233–3242. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- Mclenachan S, Zhang D, Palomo AB, Edel MJ, Chen FK. mRNA transfection of mouse and human neural stem cell cultures. PLoS ONE. 2013;8:e83596. doi: 10.1371/journal.pone.0083596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano JV, Ramathal C, Nguyen HN, Simon C, Reijo Pera RA. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells. 2012;30:441–451. doi: 10.1002/stem.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J, Engel W. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86:654–663. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- Nolte J, Michelmann HW, Wolf M, Wulf G, Nayernia K, Meinhardt A, Zechner U, Engel W. PSCDGs of mouse multipotent adult germline stem cells can enter and progress through meiosis to form haploid male germ cells in vitro. Differentiation. 2010;80:184–194. doi: 10.1016/j.diff.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Preskey D, Allison T, Jones M, Mamchaoui K, Unger C. Synthetically modified mRNA for efficient and fast human iPS cell generation and direct transdifferentiation to myoblasts. Biochem Biophys Res Commun. 2016;473:743–751. doi: 10.1016/j.bbrc.2015.09.102. [DOI] [PubMed] [Google Scholar]
- Rosa A, Brivanlou AH. Synthetic mRNAs: powerful tools for reprogramming and differentiation of human cells. Cell Stem Cell. 2010;7:549. doi: 10.1016/j.stem.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Kashani IR. BMP4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biol Int. 2012;36:1185. doi: 10.1042/CBI20110651. [DOI] [PubMed] [Google Scholar]
- Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, Noce T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000;14:841. [PMC free article] [PubMed] [Google Scholar]
- Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394:189–193. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- Yan G, Fan Y, Li P, Zhang Y, Wang F. Ectopic expression of DAZL gene in goat bone marrow-derived mesenchymal stem cells enhances the trans-differentiation to putative germ cells compared to the exogenous treatment of retinoic acid or bone morphogenetic protein 4 signalling molecules. Cell Biol Int. 2015;39:74–83. doi: 10.1002/cbin.10348. [DOI] [PubMed] [Google Scholar]
- Yu Z, Ji P, Cao J, Zhu S, Li Y, Zheng L, Chen X, Feng L. Dazl promotes germ cell differentiation from embryonic stem cells. J Mol Cell Biol. 2009;1:93. doi: 10.1093/jmcb/mjp026. [DOI] [PubMed] [Google Scholar]