Abstract
Areca nut chewing habits are associated with several oral manifestations like leukoplakia, submucous fibrosis and oral squamous cell carcinoma. Although numerous evidence on areca toxicity is known but the mechanistic pathway of disease causation is to be studied. Aqueous areca nut extract treated A549 cells showed reduced cell viability by 48 h with IC50 value of 0.50%. The toxic nature of areca nut induced the production of reactive oxygen species with decreased anti-oxidant glutathione S transferase levels lead to altered redox homeostasis. PCR studies showed decreased mRNA levels of Jun and Fos AP-1 subunits on extract treatment by 48 h. The protein levels of PCNA, CDK4, RB, p53, c-Jun and c-Fos were found to be downregulated with upregulated CDK inhibitor p21 on extract treatment as compared to control. Results of FACS analysis further confirm G1/S phase cell cycle arrest on areca nut extract exposure. The regulation of downstream AP-1 subunits by MAPKs was studied by using specific inhibitors of ERK, JNK and p38 along with areca nut extract. Results showed the redox activation of MAP kinases down regulated the mRNA levels of AP-1 subunits in aqueous areca nut extract treated cells. Hence the present study aids in elucidating the role of MAP kinases in regulating the AP-1 subunits and their implications on target genes that are involved regulation of various cellular processes. Further, it would help in understanding the mechanistic aspects of the diseased state which may facilitate in designing of new therapeutic modalities that could help in cancer management.
Keywords: AP-1 factors, Areca nut extract, A549 cells, Cell cycle regulators, MAPK
Introduction
The International Agency for Research on Cancer (IARC) has classified betel quid with or without tobacco as group I human carcinogens that are closely associated with an elevated risk of orally potential malignant disorders (OPMDs) and cancers of the oral cavity. Worldwide there are around 600 million betel quid chewers (Chen et al. 2018). The concurrent use of areca nut has increased the occurrence of oral cancer in some Western Pacific island countries and is associated with significant morbidity and mortality rates. Globally, mortality rate from oral cancer, is less than 50% based on a 5 yr. mortality rate, however, mortality rates was as high as 67 to 80% have been reported from some countries in the Western Pacific Region (World Health Organization 2012). Studies have shown the cytotoxic and genotoxic effect of areca nut extract on cultured human oral epithelial cells (Sundqvist et al. 1989). Increased production of reactive oxygen species (ROS) in several types of cells were observed on areca nut exposure (Chang et al. 2001; Liu et al. 1996). Excessively produced free radical further depletes anti-oxidants and thereby generates oxidative stress and leads to alteration in bio-chemical processes like cell growth, gene expression, and apoptosis. Further increased ROS mediates various signaling cascade relating to survival, proliferation, resistance to apoptosis, angiogenesis and metastases in pre-cancerous cells as well as cancer cells, which may promote cancer initiation and progression (Halliwell 2007).
Among which redox activation of certain transcription factors like Activator Protein-1(AP-1), Nuclear factor Kappa B (NF-κB) through mitogen activated protein kinases (MAPKs) and its components like extracellular regulated kinase (ERK), Jun N terminal kinase (JNK) and p38 which play important role in maintaining various biological processes. AP-1 is the first dimeric mammalian transcription factor to be identified (Angel and Karin 1991) which includes a menagerie of basic leucine zipper (bZIP) proteins that belong to the Jun, Fos, Maf and ATF sub-families (Chinenov and Kerppola 2001). These factors are activated in response to an incredible array of stimuli, including mitogenic growth factors, inflammatory cytokines, UV and ionizing irradiation, cellular stress, and neoplastic transformation (Angel and Karin 1991). The diverse combinational dimers of AP-1 subunits are known to regulate several biological or pathological status of an organism, which participates in various signaling pathways stimulated by great variety of extracellular signals (Wisdom 1999). Although there exists numerous evidence on the biological significance and physiological functions of AP-1 and its components but it’s still being explicated. Previous studies states that AP-1 activity with that of growth factors and tumor promoters prompts the relatedness of regulatory act in cellular growth control and neoplastic transformation (Hsu et al. 1993). Despite of increasing awareness about the physiological functions of AP-1 but their function of target genes are not always obvious (Shaulian and Karin 2002). There are evidences that AP-1 proteins, mostly those that belong to the Jun group, control cell survival and death through their ability to regulate the expression and function of cell cycle regulators such as Cyclin D1, p53, p21cip1/waf1, p19ARF and p16 (Shaulian and Karin 2001, 2002). Amongst the Jun proteins, c-Jun is unique in its ability to positively regulate cell proliferation through the repression of tumor suppressor gene expression and induction of cyclin D1. c-Jun actions are antagonized by Jun B, which upregulates tumor suppressor genes and represses cyclin D1. The tumor suppressor p53, an important target of AP-1 are known to regulate their effects on cell survival and death, whose expression as well as transcriptional activity is modulated by AP-1 proteins (Shaulian et al. 2000).
Analysis of in vitro cell culture models that exhibit altered expression of individual AP-1 members revealed unique and crucial roles for each Jun proteins, but some functional redundancy appears among the Fos proteins. Inhibition of Fos and Jun expression in mouse fibroblasts and erythroleukaemia cells using antisense RNA demonstrated their requirement in proliferation and cell cycle progression (Shaulian and Karin 2001). Similarly, microinjection of antibodies against Fos and Jun prevents serum stimulated quiescent mouse fibroblasts from re-entering the cell cycle (Kovary and Bravo 1991). Interestingly, non-cross reactive antibodies against any Jun protein, including Jun B, inhibit cell cycle entry, whereas simultaneous inactivation of several Fos proteins is required to achieve a similar effect (Kovary and Bravo 1991), suggesting a unique and critical role for each AP-1 protein. Hence in the present study human lung A549 cells were used has an in vitro model system to study the effect of aqueous areca nut extract on viability, on redox homeostasis and their influence on activation of mitogen activated protein kinases in regulation of downstream AP-1 transcription factors and their influence on target genes which further decides the cellular fate.
Materials and Methods
A549 cells (human lung adenocarcinoma cancer cells) were purchased from NCCS (Pune, India); TRIzol, and Oligo’s forward and reverse primers for AP-1 factors (Table 1) were purchased from Sigma-Aldrich (St. Louis, USA). Fetal bovine serum (FBS), penicillin, streptomycin, glutamine, RPMI 1640 medium, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), dichlorofluorescein diacetate (DCFDA), 1-Chloro-2,4-dinitrobenzene (CDNB),Tris base, Sodium Chloride, Glycine, Ammonium per sulphate, tween 20, sodium dodecyl sulphate, acrylamide, bis acrylamide, sodium acetate were purchased from Himedia (Mumbai, India). Oligo dTs and superscript reverse transcriptase were obtained from Invitrogen Bio Services India Pvt. Ltd. (Bangalore, India). Taq DNA polymerase, BrdU ELISA Kit and anti-rabbit/mouse secondary antibody HRP conjugate was purchased from Merck-Millipore (Mumbai, India). Antibodies to PCNA, RB, CDK4, p21, p53, c-Jun, and c-Fos were purchased from Neo Biolabs (MA, USA).
Table 1.
Sequence of primers (F: forward and R: reverse) used for the amplification of different AP-1 factors
| Gene | Primer sequence | Annealing temp. (°C) | Product size. (bp) | PCR cycles |
|---|---|---|---|---|
| AP-1 factors | ||||
| c-Jun | F: GCCTACAGATGAACTCTTTCTGGC R: CCTGAAACATCGCACTATCCTTTG |
64 | 525 | 30 |
| Jun-D | F: CGCAGCCTCAAACCCTGCCTTTCC R: AAACAGGAATGTGGACTCGTAG |
64 | 500 | 30 |
| Jun-B | F: CCAGTCCTTCCACCTCGACGTTTACAAG R: GACTAAGTGCGTGTTTCTTTTCCACAGTAC |
58 | 257 | 30 |
| c-Fos | F: TCTTCCTTCGTCTTCACC R: AATCAGAACACACTATTGCC |
58 | 577 | 30 |
| Fra-1 | F: AGGAAGGAACTGACCGAC R: GAAGGGGAGGAGACATTG |
60 | 497 | 30 |
| β-actin | F: TACCACTGGCATCGTGATGGACT R: TCCTTCTGCATCCTGTCGGCAAT |
62 | 516 | 30 |
F forward, R reverse
Culturing of A549 cells
A549 cells were grown in 25 cm2 culture flask using RPMI-1640 media with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin and 2 mM l-glutamine. Cells were cultured in a humidified atmosphere at 37 °C by supplying 5% CO2 in an incubator. The 80–90% confluent flask containing cells were trypsinised and sub cultured to 96 or 6 well plates for further treatments (Kiran Kumar et al. 2016).
Preparation of aqueous extract of areca nut
Areca nut was collected from Uttara Kannada district, Karnataka, India. Areca nut was finely powered using pestle and mortar, 1 g of powdered areca nut was suspended in 10 ml of sterile water to prepare 10% stock (w/v) and placed in orbitary shaker at room temperature for 24 h and the extract was filtered using Whatmann no. 1 filter paper. The main stock (10%) was further diluted with media (e.g. 0.1 ml of areca nut main stock in 9.90 ml media gives 0.1% (v/v) concentration) to treat cells with different concentrations or stored at − 20 °C until further use (Nagesh et al. 2016).
Cell viability assay (MTT)
The viable cell percentage was measured by MTT assay as described earlier (Mosmann 1983). A549 cells were seeded at density of 3 × 103 cells/well in a 96-well plate and incubated overnight in a CO2 incubator. Cells were then exposed to fresh media containing different concentration of aqueous areca nut extract (0.1, 0.25, 0.5, 0.75 and 1.0%) for 48 h. After the incubation period, 50 µl (2 mg/ml) of MTT was added into each well and incubated for 4 h, insoluble formazan formed by viable cells were finally dissolved in DMSO (100 µl) and read against blank using a microplate reader (Perkin Elmer) at 540 nm.
Reactive oxygen species (ROS) assay
ROS generated in cells by the action of toxic substances were measured as per the protocol described earlier (Periyakaruppan et al. 2009). A549 cells (3 × 103 cells/well) were cultured overnight in a black colored 96-well plate, washed with phosphate buffered saline (PBS) and treated with 10 µM DCFDA in 1 N NaOH for 3 h. Further, the cells were washed with PBS and incubated with different concentration of aqueous extract of areca nut in media for 15 min. The fluorescent intensity was recorded using a multimode plate reader (Perkin Elmer) at excitation wavelength of 485 nm and emission of 527 nm.
Glutathione S-transferase (GST) assay
GST assay was carried out as per the protocol described earlier (Mannervik 1985). A549 cells (5 × 105 cells/well) were cultured in a 6-well plate and incubated overnight. Cells were treated with or without different concentration of aqueous extract of areca nut and further incubated for 24 h. Cell lysate was prepared using 200 µl lysis buffer/well. 100 µl cell lysate was added to 900 µl enzyme cocktail containing PBS of pH 6.5, 100 mM CDNB in ethanol and 100 mM reduced glutathione in water. Reaction mixture was incubated at room temperature for 5 min and absorbance was measured spectrophotometrically at 340 nm.
Cell cycle analysis by flow cytometry using propidium iodide staining
A549 cells were cultured in a 6 well plate and treated with or without different concentration of areca nut extract (0.25 and 0.5%) for 24 h. The cells residing at different phases of cell cycle in treated samples were measured by flow cytometric analysis as per the manufacturers protocol. After incubation the cells were harvested, fixed in ethanol and stained with propidium iodide (50 µg/ml) and the cells residing in different phases were analyzed using a FACS Scan laser flow cytometric analysis system (Scientific Instruments Centre, Indian Institute of Science, Bengaluru). The percentage of cells residing in different phases of cell cycle was compared with control.
RNA isolation and semi-quantitative RT-PCR analysis
A549 cells (5 × 105 cells/well) were seeded into 6-well plate and incubated with or without aqueous areca nut extract (0.25 and 0.5%) for 48 h. Total RNA was isolated from control and treated cell samples using TRIzol reagent as per the manufacturer instructions. Reverse transcription of RNA and PCR analysis were carried out as per the protocol described earlier (Sharma et al. 1999). Total RNA (2 μg) from different samples were reverse-transcribed using Oligo (dT) primers and superscript reverse transcriptase. The cDNA was subjected to 30 cycles of PCR in a gradient Eppendorf thermo cycler using different forward and reverse primers of AP-1 factors (Table 1). The β-actin was used as a positive control and for normalization. Amplified PCR products were analyzed by electrophoresis using 1% agarose gels and 1X TAE buffer.
Western blotting
The total cell protein extracts of A549 cells from control and aqueous areca nut extract treated samples were prepared and specific protein levels were analyzed by Western blotting as per the protocol described earlier (Sharma et al. 1999). The control and treated cells present in 6-well plate were washed thrice with PBS and 0.2 ml of ice cold cell lysis buffer was added. Cells were scraped and suspension was gently transferred to a pre-cooled Eppendorf tube, mixed gently by swirling on ice and centrifuged at 15,000×g for 20 min at 4 °C. Supernatant was used to determine protein concentration by Bradford’s method (Bradford 1976). The cell lysates containing equal amount of protein (60 µg) of different samples were separated on SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred on to PVDF membrane. The membrane was blocked with 5% Carnation fat-free milk at room temperature for 1 h. Primary Antibody (PCNA or CDK4 or RB or p53 or p21 or c-Jun or c-Fos) in blocking solution (1:1000) was added and incubated for overnight, washed and incubated with anti-rabbit/mouse secondary antibody-HRP (1:500), and further incubated for 1 h at room temperature. Immunoreactive proteins were visualized using Luminata Forte Western HRP substrate as per the specifications provided by the supplier in Syngene Gel Documentation system (MD, USA). Immunoreactive bands were quantified using image analysis software (Image J). GAPDH was used as a positive control and for normalization.
Statistical analysis
Experimental data shown as mean ± standard deviation from three independent experiments. Statistical analysis for MTT, ROS and GST assays was carried out by Student’s t test, mRNA and protein analysis was done by one-way ANOVA followed by post hoc tukey test. Difference between control and aqueous areca nut treated cell samples were considered significant if the level was *P < 0.05, **P < 0.01 and ***P < 0.001 and #P < 0.05 when compared to aqueous areca nut extract treated samples.
Results
Aqueous areca nut extract reduces the viability of A549 cells
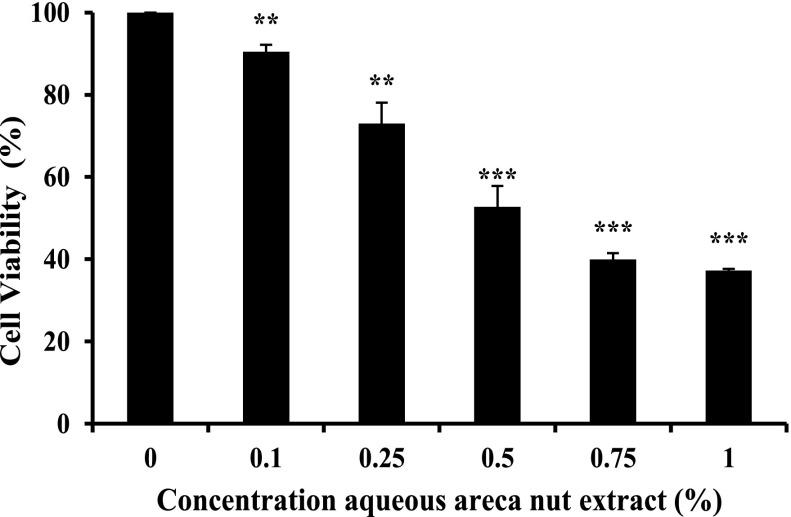
A549 cells were treated with or without different concentration of aqueous areca nut extract for 48 h and the viability were determined by MTT assay. Results show that the aqueous areca nut extract decreased cell viability in a dose dependent manner by 48 h. More than 50% decrease in viability was found at 0.5% aqueous areca nut extract in A549 cells as compared to control (Fig. 1).
Fig. 1.
Effect of aqueous extract of areca nut on the viability of A549 cells. Values were expressed as mean ± SD (n = 4). Results were significantly different from control if *P < 0.05; **P < 0.01 and ***P < 0.001 by using student’s t-test
Aqueous extract of areca nut induces reactive oxygen species (ROS) in A549 cells
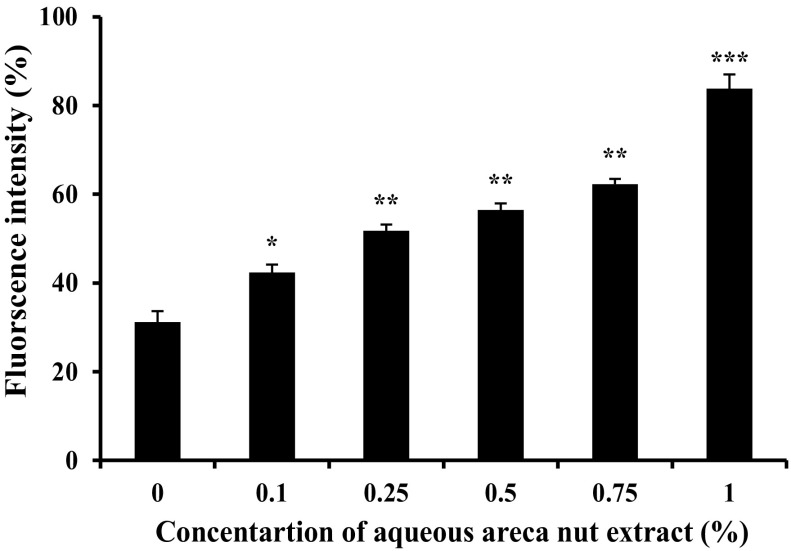
A549 cells were treated with or without different concentration of aqueous areca nut extract and total ROS produced in cells were measured. Results show that aqueous areca nut extract induced ROS in a dose dependent manner. More than 60% increase in production of ROS at 1% aqueous areca nut extract treated cells was observed as compared to control (Fig. 2).
Fig. 2.
Effect of aqueous extract of areca nut on ROS production in A549 cells. Values were expressed as mean ± SD (n = 4). Results are significantly different from control if *P < 0.05; **P < 0.01 and ***P < 0.001 by using student’s t-test
Areca nut extract decreases glutathione S-transferase activity in A549 cells
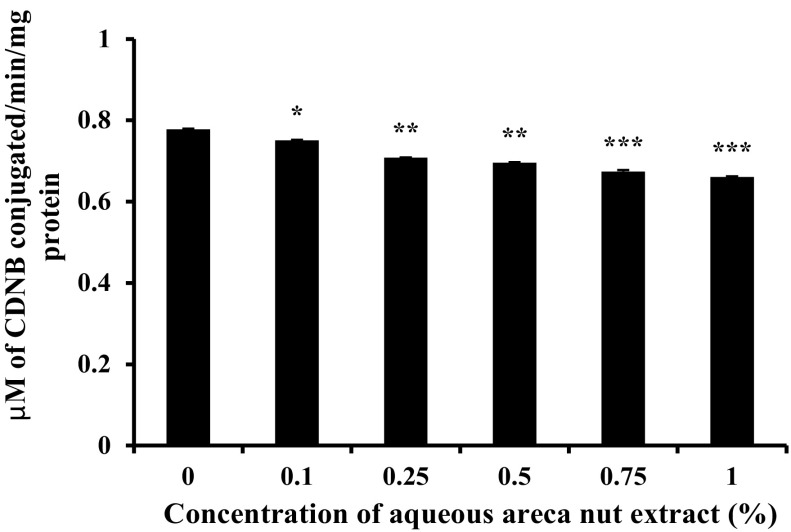
A549 cells were treated with or without different concentration of aqueous areca nut extract for 24 h and GST enzyme activity were assayed. Results show that the cells treated with aqueous areca nut extract decreased the GST enzyme activity in a dose dependent manner. Cells treated with 1% aqueous areca nut extract showed 15% decrease in GST enzyme activity as compared to control (Fig. 3).
Fig. 3.
Effect of aqueous extract of areca nut on GST activity in A549 cells. Values were expressed as mean ± SD (n = 3). Results are significantly different from control if *P < 0.05; **P < 0.01 and ***P < 0.001 by using student’s t-test. Units: µM of CDNB conjugated/min/mg protein
Areca nut extract induces G1/S phase cell cycle arrest
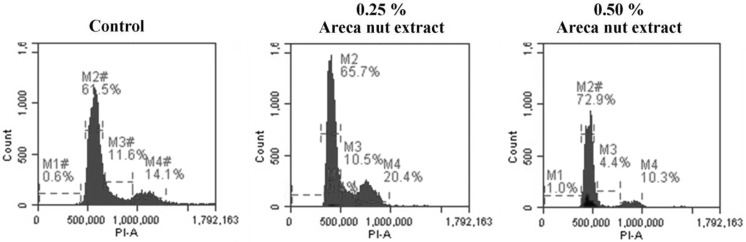
Cells treated with different concentration of aqueous extract of areca nut (0.25 and 0.5%) for 24 h and the cells were harvested and fixed in chilled ethanol. Further fixed cells were subjected to propidium iodide stain for 15–20 min and were analyzed for the cells residing in different phase of the cell cycle. Results of flow cytometric analysis showed cells on treatment with 0.25% extract showed 65.7% cells residing at G1 phase further with increased areca nut concentration i.e., 0.5% showed 72.9% cells residing at G1 phase of cell cycle. Further, the treated cells showed decrease in the cell number that entered S phase by 10.5% with 0.25 and 4.4% with 0.5% concentrations of aqueous areca nut extract as compared to control cells (Fig. 4). Flow cytometric analysis suggest induction of G1/S phase cell cycle arrest in aqueous areca nut extract treated A549 cells.
Fig. 4.
Effect of areca nut extract on induction of cell cycle arrest in A549 cells. Results were expressed as % of cells residing in different phase of cell cycle such as Sub G0, G1/S and G2/M as compared to control
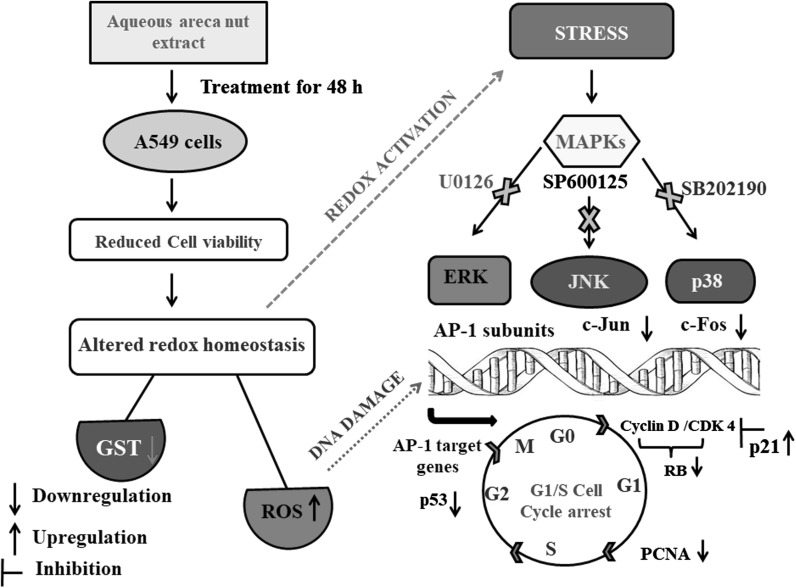
Areca nut extract induces p21 and inhibits p53, CDK4, RB and PCNA protein levels in A549 cells
Cells treated with or without different concentration of aqueous areca nut extract showed dose dependent decrease in protein levels of major cell cycle regulators. Areca nut extract treated A549 cells showed decreased protein levels of PCNA by 36 and 68%, CDK4 by 39 and 61%, RB by 59 and 75% and p53 by 51 and 61% with 0.25 and 0.50% areca nut extract respectively as compared to control. Whereas CDK inhibitor p21 showed increased protein level by twofold with 0.50% areca nut extract (Fig. 5).
Fig. 5.
Effect of areca nut extract on protein levels of cell cycle regulators in A549 cells. Data shown are mean ± SD (n = 3) and differences in protein levels of cell cycle regulators are statistically significant: if *P < 0.05 compared with control using one-way ANOVA followed by post hoc Tukey test. The bar graphs represent the respective densitometric analysis
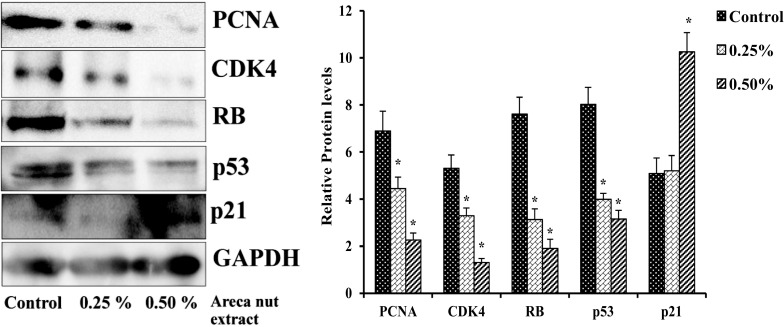
Down regulation of AP-1 (Jun and Fos) transcripts in areca nut extract treated A549 cells
A549 cells showed the mRNAs expression of Jun (c-Jun, Jun B, and Jun D) and Fos (Fra-1, Fra-2, and c-Fos) family members at different levels. A549 cells treated with aqueous areca nut extract decreases the expression of Jun and Fos family members of AP-1 factors in dose dependent manner when compared to control. There was a significant decrease in mRNA expression of c-Jun by 18 and 20%, Jun B by 31 and 59% and Jun D by 10 and 14%, similarly Fos family member also show significant decrease in expression of c-Fos by 65 and 83%, Fra-1 by 34 and 62% and Fra-2 by 9 and 71% with 0.25 and 0.5% areca nut extract respectively when compared to control (Fig. 6). The results suggested that the Jun and Fos family members are probably involved in down regulation of major cell cycle regulator in areca nut extract treated cells leads to decrease in the cell viability.
Fig. 6.
Effect of areca nut extract on the mRNAs expression of AP-1 factors in A549 cells. Data shown are mean ± SD (n = 3) and differences in mRNA levels of AP-1 factors are statistically significant, if *P < 0.05 compared with control, as analyzed using one-way ANOVA followed by post hoc Tukey test. The bar graph represents their respective densitometric analysis
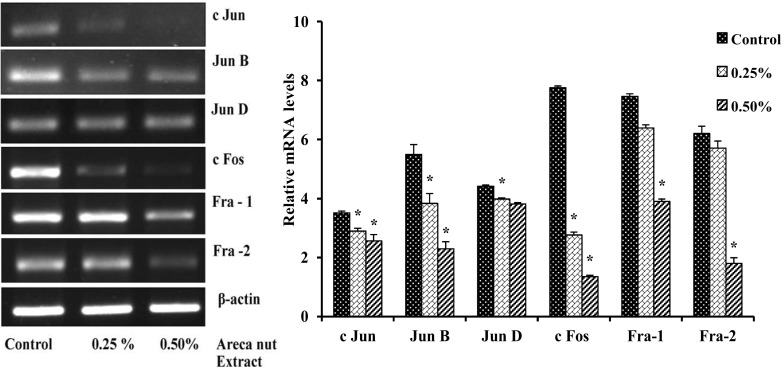
Aqueous areca nut extract down regulates the protein levels of AP-1 factors (c-Jun and c-Fos)
A549 cells treated with or without different concentration (0.25 and 0.5%) of areca nut extract showed decrease in protein levels of c-Jun and c-Fos in a dose dependent manner. Results shows decrease in c-Jun protein levels by 59 and 61% and c-Fos by 37 and 53% in cells treated with 0.25 and 0.5% areca nut extract respectively when compared to control (Fig. 7).
Fig. 7.
Effect of areca nut extract on protein levels of AP-1 factors in A549 cells. Data shown are mean ± SD (n = 3) and differences in protein levels of AP-1 factors are statistically significant: if *P < 0.05 compared with control using one-way ANOVA followed by post hoc Tukey test. The bar graph represents their respective densitometric analysis
ERK inhibitor U0126 decreased the mRNA levels of AP-1 factors in areca nut extract treated cells
To study the role of ERK MAPK pathway on downstream expression pattern of AP-1 subunits in areca nut extract treated cells. A549 cells were pretreated with or without U0126 for 1 h before treating it with or without areca nut extract. Cells treated with areca nut extract showed decreased expression of c Jun by 52%, Jun B by 13%, Jun D was non-significant, c Fos by 14%, Fra 1 by 37% and Fra 2 by 37% with 0.5% areca nut extract treated A549 cells respectively when compared to control. Whereas cells treated in combination with U0126 and areca nut extract showed decrease in mRNA levels of c Jun by 18%, Jun B by 25%, Jun D by non-significant, c Fos by 52%, Fra 1 by 09% and Fra 2 by 48% with 10 μM U0126 along with 0.5% areca nut extract treated A549 cells respectively when compared to areca nut extract treated cells (Fig. 8).
Fig. 8.
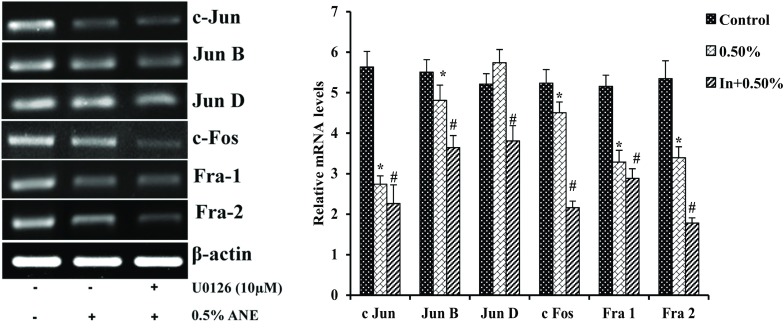
Effect of U0126 on the mRNA transcripts of AP-1 factors in areca nut extract treated cells. Data shown are mean ± SD (n = 3) and differences in mRNA levels of AP-1 factors are statistically significant: if *P < 0.05 compared with control and #P < 0.05 as compared to areca nut extract values using one-way ANOVA followed by post hoc Tukey test. The bar graph represents their respective densitometric analysis
JNK inhibitor SP600125 downregulated the mRNA levels of Jun AP-1 factors in areca nut extract treated cells
To study the role of JNK MAPK pathway on downstream expression pattern of AP-1 subunits in areca nut extract treated cells. A549 cells were pretreated with or without SP600125 for 1 h before treating it with or without areca nut extract. Cells treated with arecoline showed decreased expression of c Jun by 38%, Jun B 33%, Jun D 38%, c Fos by 54%, Fra 1 by 18% and Fra 2 by non-significant with 0.5% areca nut extract treated A549 cells respectively when compared to control. Whereas cells treated in combination with SP600125 and areca nut extract showed decrease in mRNA levels of c Jun by 17%, Jun B by 37%, Jun D by non-significant, c Fos by 13%, Fra 1 by 09% and Fra 2 by non-significant with 10 μM SP600125 along with 0.5% areca nut extract treated A549 cells respectively when compared to alone areca nut extract treated cells (Fig. 9).
Fig. 9.
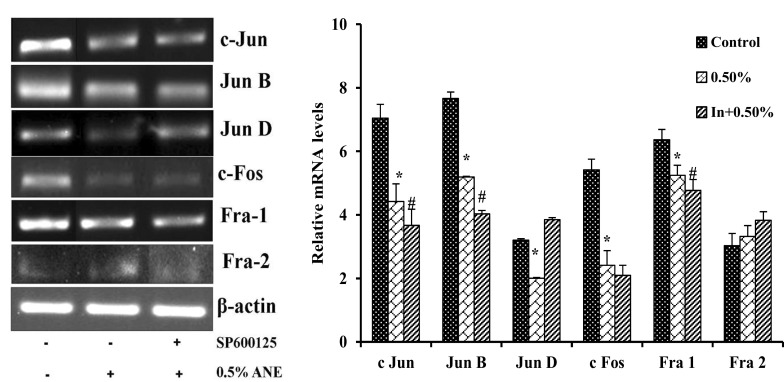
Effect of SP600125 on mRNA transcripts of AP-1 factors in arecanut extract treated cells. Data shown are mean ± SD (n = 3) and differences in mRNA levels of AP-1 factors are statistically significant: if *P < 0.05 compared with control and #P < 0.05 as compared to areca nut extract values using one-way ANOVA followed by post hoc Tukey test. The bar graph represents their respective densitometric analysis
p38 inhibitor SB202190 decreased mRNA levels of AP-1 subunits in areca nut extract treated cells
To study the role of p38 MAPK pathway on downstream expression pattern of AP-1 subunits in areca nut extract treated cells with. A549 cells were pretreated with or without SB202190 for 1 h before treating it with or without areca nut extract. Cells treated with areca nut extract showed decreased expression of c Jun by 54%, Jun B by 15%, Jun D 18%, c Fos by 74%, Fra 1 by 61% and Fra 2 by non-significant with 0.5% areca nut extract treated A549 cells respectively when compared to control. Whereas cells treated in combination with SB202190 and areca nut extract showed decrease in mRNA levels of c Jun by 17%, Jun B by non-significant, Jun D by 21%, c Fos by 17%, Fra 1 by 20% and Fra 2 by 29% with 10 μM SB202190 along with 0.5% areca nut extract treated A549 cells respectively when compared to alone areca nut extract treated cells (Fig. 10).
Fig. 10.
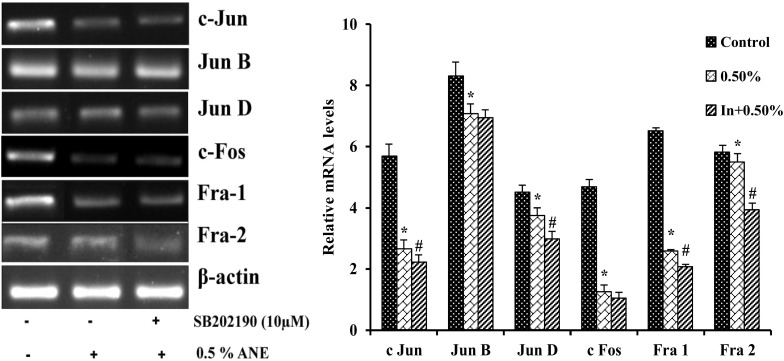
Effect of SB202190 on the mRNA transcripts of AP-1 factors in extract treated cells. Data shown are mean ± SD (n = 3) and differences in mRNA levels of AP-1 factors are statistically significant: if *P < 0.05 compared with control and #P < 0.05 as compared to areca nut extract values using one-way ANOVA followed by post hoc Tukey test. The bar graph represents the densitometric analysis
Discussion
Although there exist abundant evidence that show strong correlation between betel quid chewing and the incidence of oral cancer, leukoplakia and oral submucous fibrosis (OSF) (Canniff et al. 1986; IARC 1985). Areca nut and its component arecoline have long been reflected to be the key etiologic factors in the pathogenesis of oral cancer and OSF (Canniff et al. 1986; Jeng et al. 2001). Arecoline, an alkaloid of areca nut also demonstrates genotoxic effects in both bacterial test systems as well as cultured mammalian cells (IARC 1985; Jeng et al. 2001). However, the effects of areca nut extract on cellular toxicity, oxidative stress, cell cycle regulation and the downstream activation and regulation of AP-1 transcription factors through MAP kinases and their controlling mechanism in deciding the cellular fate will be discussed below. MTT assay showed decreased viability of A549 cells by 48 h on areca nut extract treatment these are in agreement with previous reports, where areca nut extract and arecoline were found to be cytotoxic and genotoxic to various kinds of cells thereby inhibited the growth of oral mucosal fibroblasts and gingival fibroblasts (Jeng et al. 1999, 2001). Further extract treated cells showed increasing DCF fluorescence as an indication of induced ROS in a dose dependent manner followed by decreased enzyme activity of glutathione S transferase. Previous studies (Chang et al. 2001; Lu et al. 2010) have shown excessively produced ROS brings about morphological changes on areca nut extract treatment. Recently, production of ROS (Clutton 1997; Shackelford et al. 1999) and depletion of cellular glutathione (Schnelldorfer et al. 2000; Vahrmeijer et al. 1999) have been shown to influence on cell cycle progression, apoptosis and chemical toxicity.
Deregulation of cell cycle control is one of the major causes of cancer induction (Laird and Shalloway 1997). Impairment of cell cycle by toxic chemicals usually leads to growth retardation, cytotoxicity and apoptosis (Li and Brooks 1999; Smith and Fornace 1996). Therefore, it is of interest to know whether areca nut extract affects cell cycle progression. Cyclins dependent kinases and cyclins are the regulators players which controls the progression on cell cycle. Cyclin D1 along with CDK4/6 kinases initiate the phosphorylation of the RB (retinoblastoma) and this complex is inhibited by p16 as well as p21 inhibitor which plays a key role in cell cycle arrest at the G1 checkpoint in response to DNA damage. Areca nut extract treated A549 cells showed decreased protein levels of PCNA (proliferative cell nuclear antigen), CDK4, RB and p53 with increased p21 levels. Similar results were observed in normal human oral keratinocytes with increased p16 and p21 levels (Lu et al. 2006) along with decreased mRNA levels of Cyclin E1, Cyclin D1, CDK4, RB, p53 and upregulated p16 and p21 levels in human A549 cells on areca nut extract exposure (Nagesh et al. 2016). Correspondingly, Lee et al. (2005) showed increased levels of PCNA in lesions of oral lichen planus in areca quid chewing patients. The upregulated p21 can be a major element underlying G1 phase cell cycle arrest which is further confirmed by FACS analysis. Similar results were observed in oral carcinoma 3 cells on areca nut extract exposure for 24 h showed G1/S phase cell cycle arrest (Lu et al. 2010). Active tumor suppressor p53 are known to protect cells from DNA damages by inducing its target genes. The molecules activated by p53 induce apoptosis, cell cycle arrest and DNA repair to conserve the genome (Blagosklonny and Pardee 2002). However, the activation of p53 will be reflected by the amount of protein or the phosphorylation level of Ser-15, but it was not in the case of areca nut extract treatment. Thus it would state that the damage caused by areca extract may bypass the surveillances of p53 and undergo genetic pathogenesis prompting tumorigenesis and suggested that the activation of p21 by areca nut is p53-independent (Lu et al. 2006). Results of the study put forward that CDK inhibitor (p21/p16) act as negative regulators in modulating cell arrest on areca-mediated cellular toxicity. The regular impairments of such regulatory genes in areca nut associated oral squamous cell carcinoma might substantiate the fact that keratinocyte lacking such genomic protection may confer tumorigenic propensity (Chang et al. 2000; Lin et al. 2000). Further Chang et al. (2001) also observed deregulated cell cycle arrest with areca nut and arecoline exposure which would lead to numerous oral carcinogenesis. The mechanism behind the up-regulation of p21 is not well understood. It is hypothesized that ROS elicited by areca nut extract could be an important trigger (Liu et al. 1996). Induced production of ROS facilitates error prone DNA replication with increased expression of CDK inhibitor p21 and further leads to G1/S phase cell cycle arrest in arecoline treated which may contribute to OSCC (Ji et al. 2012). The modification of gene expression by ROS has direct effects on cell proliferation and apoptosis through the activation of MAPKs and its regulation of transcription factors like AP-1 and NF-κB (Waris and Ahsan 2006). Studies on genetically modified mice and cells have highlighted a crucial role for AP-1 in a variety of cellular events involved in normal development or neoplastic transformation causing cancer. However, emerging evidence indicates that the contribution of AP-1 for determination of cellular fates critically depends on the relative abundance of AP-1 subunits, the composition of AP-1 dimers, the quality of stimulus, the cell type and the cellular environment. Therefore, AP-1-mediated regulation of various cellular processes should be considered within the context of a complex dynamic network of signaling pathways and other nuclear factors that respond simultaneously.
The signaling cascades elicited by ROS culminates the activation of AP-1 factors, c-Jun and c-Fos subunits involved in regulation of cellular proliferation. Results of the study showed decreased mRNA expression of both Jun and Fos family members of AP-1 on areca nut extract exposure. Similarly, protein levels of c-Jun and c-Fos were found to be down regulated followed by areca nut extract treatment. Studies have shown that ROS triggered transcription factor showed inhibition of thioredoxins which in turn inhibits several pro-survival transcription factors such as Egr-1, AP-1 and NF-κB resulting in a G1 phase arrest (Cook et al. 2004). AP-1 proteins control cell cycle progression by regulating the expression of key components of the cell cycle machinery. Normally c-Jun stimulates G1 to S phase transition by repressing p53, which in turn reduces p21 levels which later induces cyclin D1and CDK4 complex. c-Fos and Fos B have redundant functions in the stimulation of S phase entry and the induction of cyclin D1 expression. Jun B inhibits G1 to S phase transition by inducing p16 and repressing cyclin D1. Jun D inhibits S phase entry and increases the numbers of resting cells by modulating the Ras/p53 pathway (Jochum et al. 2001; Mechta et al. 1997; Shaulian et al. 2000). Further, reduced expression of c-Jun on areca nut treatment may be one such factor responsible for decreased Cyclin D1 levels. Due to the toxic effect of areca nut, the levels of Jun subunit have come down along with down regulated cyclins and CDKs which would aid in transition of cells from G1 to S phase of cell cycle with induction of p21 an inhibitor of CDKs. (Passegue and Wagner 2000). Further, studies were carried to confirm the involvement of MAPKs in regulation of downstream AP-1 transcription factors by using specific inhibitors of ERK (U0126), JNK (SP600125) and p38 (SB202190) along with areca nut extract. Results showed decreased mRNA expression on both Jun and Fos family members on pretreatment of ERK inhibitor with areca extract, while JNK inhibitor with extract showed significant decrease in mRNA levels of Jun family members of AP-1 whereas p38 inhibitor with areca extract showed decrease mRNA levels of most of the AP-1 players. These results are in agreement with studies carried by (Tseng et al. 2007), on normal oral keratinocytes have shown the activation of p38 MAP kinases and a low or transient activation of JNK by areca nut was observed in SAS cells (Lu et al. 2010). Similarly areca ingredients activated MEK pathway which in turn regulate the downstream transcription factors that are involved in modulating various pathophysiological processes (Chang et al. 2004). Thus, results of the study show the active involvement of stress active JNK and p38 MAPKs regulate the expression of downstream AP-1 transcription factors. Therefore, the present study aids in better understanding the role MAPKs in regulation of AP-1 transcription factors and their influence on major cell cycle regulators which in turn helps in deciding the cellular fate in areca nut extract treated human lung cancer A549 cells. This would further help in designing new therapeutic approach to suppress the oncogenic act of specific AP-1 subunits, which would facilitate to provide a better treatment strategy for cancer management.
Acknowledgements
The authors wish to express their gratitude to the University Grant Commission-Centre with Potential for Excellence Area (UGC-CPEPA) [8-2/2008(NS/PE)] and Department of Science and Technology-Promotion of University Research and Scientific Excellence (DST-PURSE) [SR/59/Z-23/2010/38(c)], New Delhi for providing financial support. Author RN is grateful to UGC-CPEPA for providing fellowships. Authors also wish to express their gratitude to the Department of Microbiology and Biotechnology, Bangalore University, Bengaluru, for providing the DST-FIST, UGC-SAP and department instrumentation facility.
Abbreviations
- ANE
Areca nut extract
- AP-1
Activator protein-1
- CDK
Cyclin dependent kinase
- PCNA
Proliferative cell nuclear antigen
- RB
Retinoblastoma
- RT-PCR
Semi quantitative reverse transcriptase-Polymerase Chain Reaction
Compliance with ethical standards
Conflict of interest
Authors declare that there are no conflicts of interest.
Footnotes
S. Chidananda Sharma—Deceased.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochem Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell Cycle. 2002;1:103–110. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canniff JP, Harvey W, Harris M. Oral submucous fibrosis: its pathogenesis and management. Br Dent J. 1986;160:429–434. doi: 10.1038/sj.bdj.4805876. [DOI] [PubMed] [Google Scholar]
- Chang KW, Lin SC, Kwan PC, Wong YK. Association of aberrant p53 and p21(WAF1) immunoreactivity with the outcome of oral verrucous leukoplakia in Taiwan. J Oral Pathol Med. 2000;29:56–62. doi: 10.1034/j.1600-0714.2000.290202.x. [DOI] [PubMed] [Google Scholar]
- Chang MC, et al. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis. 2001;22:1527–1535. doi: 10.1093/carcin/22.9.1527. [DOI] [PubMed] [Google Scholar]
- Chang MC, et al. The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J Biol Chem. 2004;279:50676–50683. doi: 10.1074/jbc.M404465200. [DOI] [PubMed] [Google Scholar]
- Chen G, Hsieh M-Y, Chen AW-G, Kao NH-L, Chen M-K. The effectiveness of school educating program for betel quid chewing: a pilot study in Papua New Guinea. J Chin Med Assoc. 2018;81:352–357. doi: 10.1016/j.jcma.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 2001;20:2438. doi: 10.1038/sj.onc.1204385. [DOI] [PubMed] [Google Scholar]
- Clutton S. The importance of oxidative stress in apoptosis. Br Med Bull. 1997;53:662–668. doi: 10.1093/oxfordjournals.bmb.a011637. [DOI] [PubMed] [Google Scholar]
- Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14:259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Hsu JC, Cressman DE, Taub R. Promoter-specific trans-activation and inhibition mediated by JunB. Cancer Res. 1993;53:3789–3794. [PubMed] [Google Scholar]
- IARC Betel-quid and areca-nut chewing. IARC Monogr Eval Carcinog Risk Chem Hum. 1985;37:137–202. [PubMed] [Google Scholar]
- Jeng JH, Hahn LJ, Lin BR, Hsieh CC, Chan CP, Chang MC. Effects of areca nut, inflorescence piper betle extracts and arecoline on cytotoxicity, total and unscheduled DNA synthesis in cultured gingival keratinocytes. J Oral Pathol Med. 1999;28:64–71. doi: 10.1111/j.1600-0714.1999.tb01998.x. [DOI] [PubMed] [Google Scholar]
- Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001;37:477–492. doi: 10.1016/S1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- Ji WT, Yang SR, Chen JY, Cheng YP, Lee YR, Chiang MK, Chen HR. Arecoline downregulates levels of p21 and p27 through the reactive oxygen species/mTOR complex 1 pathway and may contribute to oral squamous cell carcinoma. Cancer Sci. 2012;103:1221–1229. doi: 10.1111/j.1349-7006.2012.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum W, Passegue E, Wagner EF. AP-1 in mouse development and tumorigenesis. Oncogene. 2001;20:2401–2412. doi: 10.1038/sj.onc.1204389. [DOI] [PubMed] [Google Scholar]
- Kiran Kumar KM, et al. Cadmium induces oxidative stress and apoptosis in lung epithelial cells. Toxicol Mech Methods. 2016;26:658–666. doi: 10.1080/15376516.2016.1223240. [DOI] [PubMed] [Google Scholar]
- Kovary K, Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991;11:4466–4472. doi: 10.1128/MCB.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AD, Shalloway D. Oncoprotein signalling and mitosis. Cell Signal. 1997;9:249–255. doi: 10.1016/S0898-6568(96)00176-3. [DOI] [PubMed] [Google Scholar]
- Lee JJ, et al. Higher expressions of p53 and proliferating cell nuclear antigen (PCNA) in atrophic oral lichen planus and patients with areca quid chewing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:471–478. doi: 10.1016/j.tripleo.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Li JM, Brooks G. Cell cycle regulatory molecules (cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors) and the cardiovascular system; potential targets for therapy? Eur Heart J. 1999;20:406–420. doi: 10.1053/euhj.1998.1308. [DOI] [PubMed] [Google Scholar]
- Lin SC, Chang KW, Chang CS, Liu TY, Tzeng YS, Yang FS, Wong YK. Alterations of p16/MTS1 gene in oral squamous cell carcinomas from Taiwanese. J Oral Pathol Med. 2000;29:159–166. doi: 10.1034/j.1600-0714.2000.290403.x. [DOI] [PubMed] [Google Scholar]
- Liu TY, Chen CL, Chi CW. Oxidative damage to DNA induced by areca nut extract. Mutat Res. 1996;367:25–31. doi: 10.1016/S0165-1218(96)90018-X. [DOI] [PubMed] [Google Scholar]
- Lu SY, Chang KW, Liu CJ, Tseng YH, Lu HH, Lee SY, Lin SC. Ripe areca nut extract induces G1 phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis. 2006;27:1273–1284. doi: 10.1093/carcin/bgi357. [DOI] [PubMed] [Google Scholar]
- Lu HH, Kao SY, Liu TY, Liu ST, Huang WP, Chang KW, Lin SC. Areca nut extract induced oxidative stress and upregulated hypoxia inducing factor leading to autophagy in oral cancer cells. Autophagy. 2010;6:725–737. doi: 10.4161/auto.6.6.12423. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Mechta F, Lallemand D, Pfarr CM, Yaniv M. Transformation by ras modifies AP1 composition and activity. Oncogene. 1997;14:837–847. doi: 10.1038/sj.onc.1200900. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nagesh R, et al. Aqueous areca nut extract induces oxidative stress in human lung epithelial A549 cells: probable role of p21 in inducing cell death. Gene Rep. 2016;6:103–111. doi: 10.1016/j.genrep.2016.12.008. [DOI] [Google Scholar]
- Passegue E, Wagner EF. JunB suppresses cell proliferation by transcriptional activation of p16(INK4a) expression. EMBO J. 2000;19:2969–2979. doi: 10.1093/emboj/19.12.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyakaruppan A, et al. Uranium induces apoptosis in lung epithelial cells. Arch Toxicol. 2009;83:595–600. doi: 10.1007/s00204-008-0396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer. 2000;89:1440–1447. doi: 10.1002/1097-0142(20001001)89:7<1440::AID-CNCR5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Shackelford RE, Kaufmann WK, Paules RS. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ Health Perspect. 1999;107:5–24. doi: 10.1289/ehp.99107s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SC, Clemens JW, Pisarska MD, Richards JS. Expression and function of estrogen receptor subtypes in granulosa cells: regulation by estradiol and forskolin. Endocrinology. 1999;140:4320–4334. doi: 10.1210/endo.140.9.6965. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Schreiber M, Piu F, Beeche M, Wagner EF, Karin M. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell. 2000;103:897–907. doi: 10.1016/S0092-8674(00)00193-8. [DOI] [PubMed] [Google Scholar]
- Smith ML, Fornace AJ., Jr Mammalian DNA damage-inducible genes associated with growth arrest and apoptosis. Mutat Res. 1996;340:109–124. doi: 10.1016/S0165-1110(96)90043-3. [DOI] [PubMed] [Google Scholar]
- Sundqvist K, Liu Y, Nair J, Bartsch H, Arvidson K, Grafstrom RC. Cytotoxic and genotoxic effects of areca nut-related compounds in cultured human buccal epithelial cells. Cancer Res. 1989;49:5294–5298. [PubMed] [Google Scholar]
- Tseng YH, Chang CS, Liu TY, Kao SY, Chang KW, Lin SC. Areca nut extract treatment down-regulates involucrin in normal human oral keratinocyte through P13K/AKT activation. Oral Oncol. 2007;43:670–679. doi: 10.1016/j.oraloncology.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vahrmeijer AL, van Dierendonck JH, Schutrups J, van de Velde CJ, Mulder GJ. Effect of glutathione depletion on inhibition of cell cycle progression and induction of apoptosis by melphalan (l-phenylalanine mustard) in human colorectal cancer cells. Biochem Pharmacol. 1999;58:655–664. doi: 10.1016/S0006-2952(99)00130-6. [DOI] [PubMed] [Google Scholar]
- Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom R. AP-1: one switch for many signals. Exp Cell Res. 1999;253:180–185. doi: 10.1006/excr.1999.4685. [DOI] [PubMed] [Google Scholar]
- World Health Organization ROftWP . Review of areca (betel) nut and tobacco use in the Pacific: a technical report WHO Regional Office for the Western Pacific. Manila: Philippine; 2012. [Google Scholar]