Abstract
Cuminum cyminum L. (cumin) seed is used as a spice in various countries. Although several functions of the components in cumin seed have been reported, the anti-allergic effect of the water-soluble component in cumin seed has not been reported yet. In this study, we focused on the suppressive effect of cumin seed aqueous extract on degranulation in order to reveal the anti-allergic effect of cumin. Cumin seed aqueous extract significantly suppressed the antigen-induced degranulation of rat basophilic leukemia cell line RBL-2H3 cells in a dose-dependent manner without cytotoxicity. The extract also inhibited the elevation of the intracellular calcium ion concentration induced by antigen. Immunoblot analysis revealed that the extract suppresses phosphorylation of phosphatidylinositol 3-kinase, Bruton’s tyrosine kinase, phospholipase C-γ1/2, and Akt in the signaling pathways activated by antigen induction via FcεRI. Furthermore, the extract suppressed microtubule formation induced by antigen. In addition, oral administration of cumin seed aqueous extract significantly suppressed the passive cutaneous anaphylaxis reaction in BALB/c mice. Our findings suggest that cumin seed contains water-soluble components with the anti-allergic effect. Therefore, cumin seed has potential as anti-allergic functional food.
Electronic supplementary material
The online version of this article (10.1007/s10616-019-00309-2) contains supplementary material, which is available to authorized users.
Keywords: Anti-allergic effect, Cuminum cyminum L., Degranulation, β-Hexosaminidase, Passive cutaneous anaphylaxis, RBL-2H3 cells
Introduction
Allergies, also known as allergic diseases, are caused by hypersensitivity of the immune system to harmless substances in the environment. Allergic symptoms are mainly divided into four types based on the difference in their mechanisms to generate immune responses. Type I is a group of immediate allergy, in which immunoglobulin E (IgE) is involved. The typical diseases caused by type I allergic reaction are atopic bronchial asthma, allergic rhinitis, urticaria, allergic conjunctivitis, atopic dermatitis, anaphylactic shock, and others (Johansson et al. 2004). The reaction is induced by binding of allergens to IgE bound to the high-affinity IgE receptor FcεRI on the cell surface of mast cells and basophils (Stone et al. 2010). The binding causes aggregation of FcεRI and activates the Src family non-receptor tyrosine kinases such as Lyn and Fyn. Activated Lyn provokes Ca2+ mobilization through the activation of spleen tyrosine kinase (Syk) and LAT (linker for activation of T cells)-SLP76 (SH2 domain containing leukocyte protein of 76 kDa)-phospholipase C-γ (PLCγ) complex. In parallel, activated Fyn provokes microtubule formation through the activation of GRB2-associated-binding protein 2 (Gab2) that phosphorylates phosphatidylinositol 3-kinase (PI3K). These activations lead to granule translocation and granule-membrane fusion, resulting in the release of chemical mediators, such as histamine, leukotriene, and proteases, called degranulation (Nishida et al. 2005; Kraft and Kinet 2007; Gilfillan and Rivera 2009). The released chemical mediators induce smooth muscle contraction, facilitation of vascular permeability and of gland secretion, and others involved in allergic symptoms (White 1999). Thus, prevention of the activation of mast cells and basophils has therapeutic potential of allergic diseases.
Cuminum cyminum L. (cumin) is a plant of the family Apiaceae. Its seed has a unique scent and is used as a spice all over the world. It contains cuminaldehyde, a main component of the scent, γ-terpinene, β-pinene, p-coumaric acid, luteolin, and others (Iacobellis et al. 2005; Rebey et al. 2012; Pandey et al. 2015; Al-Snafi 2016). These components are fat-soluble and there are reports that cumin essential oil has many effects such as anti-inflammatory (Wei et al. 2015), anti-oxidative (Rebey et al. 2012; Pandey et al. 2015; Bag and Chattopadhyay 2015), and anti-bacterial (Iacobellis et al. 2005; Bag and Chattopadhyay 2015) effects. Although a large number of studies have been made on hydrophobic substances in cumin, little is known about hydrophilic substances. In addition, we found that 100% ethanol extract of cumin seed has no effect on degranulation. Therefore, we focused on the cumin seed aqueous extract and examined its anti-allergic effect.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), penicillin, streptomycin, fetal bovine serum (FBS), bovine serum albumin (BSA), mouse anti-dinitrophenyl (DNP) monoclonal IgE, DNP-human serum albumin (HSA) conjugate, Triton X-100, Evans blue, Pefabloc SC, Aprotinin, cOmplete EDTA-free, and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pluronic F-127 and Fluo 3-AM were purchased from Dojindo Laboratories (Mashiki, Japan). Goat anti-actin antibody and horseradish peroxidase (HRP)-labeled anti-goat IgG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Alexa Fluor 488-labeled anti-α-tubulin antibody, Alexa Fluor 488-labeled anti-mouse IgG antibody, HRP-labeled anti-rabbit IgG antibody and rabbit antibodies against anti-Lyn antibody, anti-phosphorylated Lyn antibody, anti-Syk antibody, anti-phosphorylated Syk antibody, anti-PI3K p55 antibody, anti-phosphorylated PI3K p85/p55 antibody, anti-Bruton’s tyrosine kinase (Btk) antibody, anti-phosphorylated Btk antibody, anti-PLCγ1 antibody, anti-phosphorylated PLCγ1 antibody, anti-PLCγ2 antibody, anti-phosphorylated PLCγ2 antibody, anti-Akt antibody and anti-phosphorylated Akt antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). All other chemicals were purchased from Fujifilm Wako Pure Chemical (Osaka, Japan) or Nacalai Tesque (Kyoto, Japan) unless otherwise noted.
Sample preparation
Cumin seed powder was provided by S&B Foods Inc. (Tokyo, Japan). The powder was suspended in 10 mM sodium phosphate buffer (NaPB; pH 7.4) at 0.05 g/mL at 12 °C for 24 h. After centrifugation at 15,000×g at 4 °C for 20 min, the supernatant was collected and ultracentrifuged at 200,000×g at 4 °C for 30 min. After ultracentrifugation, the supernatant was collected and ultrafiltrated with a membrane of molecular weight cut off (MWCO) 3000 (Merck Millipore, Darmstadt, Germany). The filtrate was then collected and the pH was adjusted to 7.4. Finally, the extract was sterilized through a 0.45 μm membrane and used as ultrafiltrated cumin seed aqueous extract (UCAE). To determine the UCAE concentration, a portion of UCAE was freeze-dried and the solute was weighed. In order to inactive endogenous enzymes that affect the β-hexosaminidase release assay described below, UCAE was heated at 100 °C for 5 min before use. In addition, to evaluate the heat stability of the bioactive components, UCAE was heated at 100 °C for 30 min. The heated-UCAE was used for the β-hexosaminidase release assay. Since a large amount of the cumin seed aqueous extract was necessary for the in vivo experiment described below, a cumin seed extract was prepared without ultrafiltration that causes loss of the sample. Cumin seed powder was suspended in distilled water at 0.05 g/mL at 12 °C for 24 h. After centrifugation at 15,000×g at 4 °C for 20 min, the supernatant was collected and ultracentrifuged at 200,000×g at 4 °C for 30 min. After ultracentrifugation, the supernatant was collected and freeze-dried to make a concentrated sample. The freeze-dried powder was dissolved in 10 mM NaPB and used as cumin seed aqueous extract (CAE) for the in vivo experiment.
Cells and cell culture
RBL-2H3 cells were obtained from American Type Culture Collection (Rockville, MD, USA) and cultured in DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 5% FBS at 37 °C in a humidified incubator with a 5% CO2-95% air atmosphere.
Mice
Six-week-old female BALB/c mice were obtained from Japan SLC (Hamamatsu, Japan) and kept in an animal room under a 12 h light/dark cycle at 24 ± 1 °C. They received standard food and water ad libitum. Animal experiments were approved by the Animal Experiment Committee of Ehime University and were performed in accordance with the Guidelines of Animal Experiments of Ehime University.
β-Hexosaminidase release assay
Histamine in granules is released from basophils and mast cells by antigen-stimulation via IgE on cell surface FcεRI. Since β-hexosaminidase is present in the granules as well as histamine, degranulation of RBL-2H3 cells was evaluated by measuring the activity of the granule-stored β-hexosaminidase secreted in the extracellular medium. RBL-2H3 cells suspended in DMEM containing 5% FBS were seeded into a 96-well culture plate (Corning, Corning, NY, USA) at 4 × 104 cells/well and cultured at 37 °C for 18 h. After incubation, the cells were sensitized with 50 ng/mL anti-DNP IgE at 37 °C for 2 h. After washing with the modified Tyrode’s (MT) buffer (20 mM HEPES, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.05% BSA, pH 7.4) twice, anti-DNP IgE-sensitized cells were treated with 120 μL of MT buffer containing various concentrations of UCAE or 5 mM NaPB as a control at 37 °C for 10 min. Then, sample solutions were removed and 120 μL of MT buffer containing 5 mM NaPB and 10 μL of 0.625 μg/mL DNP-HSA solution diluted in MT buffer were added to each well and incubated at 37 °C for 30 min. After incubation, the plate was placed on ice for 10 min to stop the reaction. The supernatant was then collected from each well, and the cells were sonicated in 130 μL of MT buffer containing 0.1% Triton X-100 for 5 s on ice. Both the supernatant and the cell lysate were transferred into a new 96-well microplate at 50 μL/well and preincubated at 37 °C for 5 min. Then, 100 μL of 3.3 mM p-nitrophenyl-2-acetamido-2-deoxy-β-d-glucopyranoside dissolved in 0.1 M citrate buffer (pH 4.5) was added to each well and incubated at 37 °C for 25 min. The enzyme reaction was terminated by the addition of 100 μL of 2 M glycine buffer (pH 10.4), and the absorbance was measured at 405 nm using a microplate reader (SH-8000Lab; Corona Electric, Hitachinaka, Japan). For the blank, 100 μL of 2 M glycine buffer was added before the addition of 100 μL of the substrate solution. The β-hexosaminidase release rate was calculated as follows: [(Asupernatant − Ablank of supernatant)/{(Asupernatant − Ablank of supernatant) + (Acell lysate − Ablank of cell lysate)}] × 100, in which “A” is the absorbance of each well.
Measurement of cell viability
RBL-2H3 cells suspended in DMEM containing 5% FBS were seeded into a 96-well culture plate at 5 × 103 cells/well and cultured at 37 °C for 24 h. After removal of the medium, the cells were treated with 120 μL of DMEM containing 5% FBS and various concentrations of UCAE or 5 mM NaPB as a control at 37 °C for 24 h. After washing with phosphate-buffered saline (PBS; pH 7.4), 110 μL of DMEM and 10 μL of the Cell Count Reagent SF (Nacalai Tesque), which contains WST-8, were added to each well of the plate and incubated at 37 °C for 90 min. After incubation, the absorbance was measured at 450 nm using a microplate reader (Model 680; Bio-Rad Laboratories, Hercules, CA, USA).
Measurement of intracellular calcium ion concentration ([Ca2+]i)
RBL-2H3 cells were seeded into a white 96-well culture plate (Nunc, Roskilde, Denmark) and sensitized with anti-DNP IgE as described in the β-hexosaminidase release assay. The anti-DNP IgE-sensitized cells were then washed with PBS twice and incubated with 100 μL of the recording buffer (20 mM HEPES, 115 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 13.8 mM glucose, pH 7.4) containing 5 μg/mL Fluo 3-AM, 1.25 mM probenecid, and 0.04% Pluronic F-127 at 37 °C for 1 h. After washing with PBS, the cells were treated with 100 μL of the recording bufer containing 1.25 mM probenecid and 3.2 mg/mL UCAE or 5 mM NaPB as a control and incubated at 37 °C for 10 min. After removal of the solution, 120 μL of the recording buffer containing 1.25 mM probenecid and 5 mM NaPB was added, and the cells were stimulated with 10 μL of 0.625 μg/mL DNP-HSA diluted in MT buffer. The fluorescent intensity was immediately monitored with an excitation wavelength of 480 nm and an emission wavelength of 530 nm using a microplate reader (SH-8000Lab).
Immunoblot analysis
RBL-2H3 cells were seeded into a 35 mm tissue culture dish (Corning) at 5 × 105 cells/dish and cultured at 37 °C for 18 h. The cells were sensitized with 50 ng/mL anti-DNP IgE at 37 °C for 2 h. After washing with MT buffer twice, anti-DNP IgE-sensitized cells were treated with 980 μL of MT buffer containing 3.2 mg/mL UCAE or 5 mM NaPB as a control at 37 °C for 10 min. Then, 20 μL of 2.5 μg/mL DNP-HSA solution diluted in MT buffer was added to each dish and incubated at 37 °C for 5 min. After washing with PBS, the cells were lysed with a cell lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 30 mM Na4P2O7, and 1% Triton X-100) containing Pefabloc SC, Aprotinin, cOmplete EDTA-free, and a phosphatase inhibitor cocktail. The cell lysates were collected with a cell scraper (Corning) and placed on ice for 30 min. The cell lysates were then centrifuged at 12,000×g at 4 °C for 15 min, and the supernatants were equalized to the same protein concentration. Proteins in a total cell lysate were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Buckinghamshire, UK). The membranes were blocked with 5% skim milk dissolved in Tris-buffered saline (pH 7.4) containing 0.1% Tween 20 (TBS-T) at room temperature for 1 h. After washing with TBS-T three times, the membranes were incubated with each primary antibody in TBS-T containing 5% BSA or in TBS-T containing 5% skim milk for anti-actin antibody at 4 °C overnight. After washing with TBS-T three times, the membranes were incubated with HRP-labeled anti-rabbit IgG antibody or HRP-labeled anti-goat IgG antibody for anti-actin antibody in TBS-T containing 5% skim milk at room temperature for 1 h. After washing with TBS-T three times, the blots were developed by ImmunoStar LD (Fujifilm Wako Pure Chemical). Bands were visualized using a ChemiDoc XRS Plus apparatus (Bio-Rad Laboratories), and the chemiluminescent intensity was quantified using Image Lab software (Bio-Rad Laboratories).
Immunofluorescent staining
RBL-2H3 cells were seeded into a 35 mm tissue culture dish at 4 × 105 cells/dish with 50 ng/mL anti-DNP IgE and cultured at 37 °C for 18 h. After washing with MT buffer twice, anti-DNP IgE-sensitized cells were treated with 980 μL of MT buffer containing 3.2 mg/mL UCAE or 5 mM NaPB as a control at 37 °C for 10 min. Then, the cells were stimulated with 20 μL of 2.5 μg/mL DNP-HSA solution diluted in MT buffer and incubated at 37 °C for 5 min. After washing with PBS, the cells were fixed with 4% paraformaldehyde at room temperature for 15 min. After washing with PBS for 5 min three times, the cells were blocked with PBS containing 0.3% Triton X-100 and 5% BSA for 1 h. To visualize tubulin, the cells were stained with Alexa Fluor 488-labeled anti-α-tubulin antibody diluted in PBS containing 0.3% Triton X-100 and 1% BSA at 4 °C overnight. After washing with PBS for 5 min three times, the cells were stained with Alexa Fluor 488-labeled anti-mouse IgG antibody diluted in PBS containing 0.3% Triton X-100 and 1% BSA at room temperature for 1 h. After washing with PBS for 5 min three times, the cells were treated with DAPI solution diluted in PBS at room temperature for 15 min. After washing with PBS for 5 min three times, the cells were then treated with an anti-fading agent SlowFade (Molecular Probes, Eugene, OR, USA) to prevent fading and observed with a confocal microscope (Fluoview FV10i, Olympus, Tokyo, Japan). The images were analyzed with FV10-ASW software (Olympus).
Passive cutaneous anaphylaxis (PCA) reaction in mice
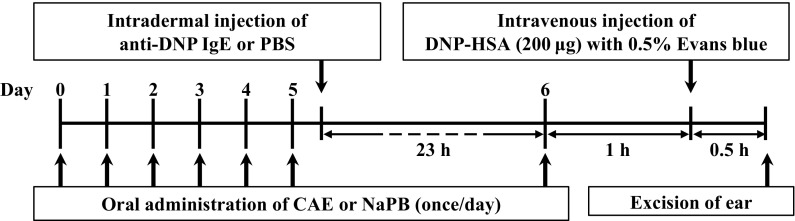
The experiment was conducted as scheduled in Fig. 1. Six-week-old female BALB/c mice were orally administrated 20 μL of 10 mM NaPB as a control or CAE (25 mg/kg body weight/day or 125 mg/kg body weight/day) for seven consecutive days. Twenty-three hours before the final oral administration, the left and right ears were intradermally injected with anti-DNP IgE and PBS, respectively. One hour after the final oral administration, mice were intravenously injected with 200 μg of DNP-HSA diluted in PBS containing 0.5% Evans blue. After 30 min, the ears of mice were evaluated. Evans blue dye was extracted from each ear with 700 μL of formamide at 63 °C overnight. After the extraction, the absorbance of the dye was measured at 620 nm using a spectrophotometer (Ultrospec 3000; Amersham Pharmacia Biotech, Uppsala, Sweden). The absorbance of the dye extracted from the left ear was subtracted with that from the right ear.
Fig. 1.
The experimental design used to examine the effect of CAE on passive cutaneous anaphylaxis (PCA) model mice
Statistical analysis
Data obtained were expressed as the mean ± standard deviation (SD). Statistical analysis were performed using GraphPad Prism version 7.02 (GraphPad Software, La Jolla, CA, USA). One-way analysis of variance followed by Dunnett’s multiple comparison tests was used to assess the statistical significance. Values of *P < 0.05 and **P < 0.01 were considered statistically significant.
Results
Effect of UCAE on degranulation of RBL-2H3 cells
Firstly, we conducted the β-hexosaminidase release assay to examine the effect of UCAE on degranulation of RBL-2H3 cells. Anti-DNP IgE-sensitized RBL-2H3 cells were treated with various concentrations of UCAE, and then degranulation was induced by antigen stimulation. As shown in Fig. 2a, degranulation was suppressed by treating the cells with UCAE in a dose-dependent manner. Statistically significant differences against control in the β-hexosaminidase release rate were observed at equal to or higher than 1.6 mg/mL UCAE, suggesting that UCAE has a degranulation-suppressive activity on RBL-2H3 cells. In addition, the effect of UCAE on the viability of RBL-2H3 cells was examined. RBL-2H3 cells were treated with various concentrations of UCAE for 24 h. After the addition of WST-8 solution, the absorbance was measured at 450 nm. As shown in Fig. 2b, UCAE was found to exhibit no cytotoxicity at any concentrations tested. From these results, 3.2 mg/mL UCAE showed a strong degranulation-suppressive activity without cytotoxicity and thus used for further experiments.
Fig. 2.
Effect of UCAE on degranulation and cell viability of RBL-2H3 cells. a Anti-DNP IgE-sensitized cells were treated with various concentrations of UCAE or with 5 mM NaPB, and then degranulation was induced by antigen stimulation. Released β-hexosaminidase was used as a marker of degranulation. b The cells were treated with various concentrations of UCAE or with 5 mM NaPB for 24 h, and then the cell viability was measured using WST-8 solution. Open circle indicates NaPB-treated cells as a control; closed circles indicate UCAE-treated cells. Data are represented as the mean ± SD of three independent experiments (a) or quadruplicate of wells (b). Dunnett’s test was used to assess the statistical significance of the deference (**P < 0.01) against control
Properties of bioactive component in UCAE
To examine the properties of the bioactive component in UCAE involved in degranulation, UCAE was heated at 100 °C for 5 min or 30 min and used for the β-hexosaminidase release assay. As shown in Fig. 3, there was little difference in the degranulation-suppressive activity between UCAE heated for 5 min and 30 min. The result suggested that the bioactive component in UCAE is stable to heat.
Fig. 3.
Effect of heat-treated UCAE on degranulation of RBL-2H3 cells. Anti-DNP IgE-sensitized cells were treated with various concentrations of UCAE heated for 5 min or 30 min or with 5 mM NaPB, and then degranulation was induced by antigen stimulation for 30 min. Released β-hexosaminidase was used as a marker of degranulation. Open circle indicates NaPB-treated cells as a control; closed circles indicate 5 min-heated UCAE-treated cells; open triangles indicate 30 min-heated UCAE-treated cells. Data are represented as the mean ± SD of three independent experiments
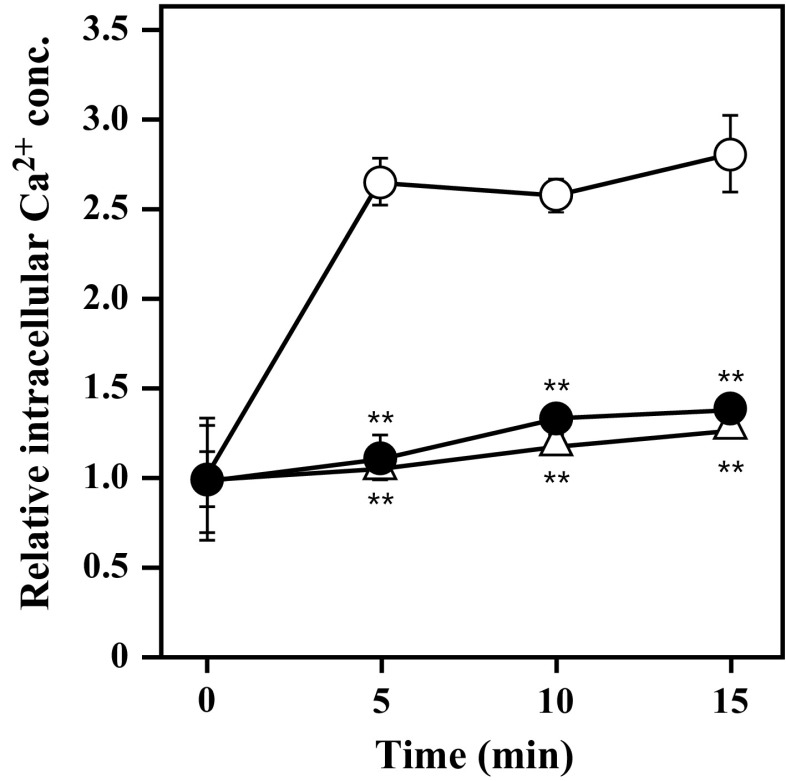
Effect of UCAE on the [Ca2+]i in RBL-2H3 cells
Because the increase in [Ca2+]i is well known to be required for mast cell degranulation, we evaluated the effect of UCAE on the elevation of [Ca2+]i in antigen-stimulated RBL-2H3 cells. As shown in Fig. 4, [Ca2+]i in the RBL-2H3 cells increased rapidly by antigen stimulation, whereas the elevation was significantly suppressed by UCAE. The result indicated that UCAE would suppress degranulation by modulating the elevation of [Ca2+]i caused by antigen stimulation.
Fig. 4.
Effect of UCAE on [Ca2+]i in RBL-2H3 cells. Anti-DNP IgE-sensitized cells were incubated with Fluo-3 AM for 1 h and then treated with UCAE (3.2 mg/mL) or with 5 mM NaPB. The cells were then stimulated with antigen and the fluorescence intensity was measured. Open triangles indicate NaPB-treated cells not stimulated with antigen; open circles indicate NaPB-treated cells stimulated with antigen as a control; closed circles indicate UCAE-treated cells stimulated with antigen. Data are represented as the mean ± SD of triplicate of wells. Dunnett’s test was used to assess the statistical significance of the deference (**P < 0.01) against control
Effect of UCAE on the intracellular signaling pathways involved in degranulation
Immunoblot analysis was conducted to evaluate the effect of UCAE on signaling pathways involved in the antigen-induced degranulation. As shown in Fig. 5a, b, phosphorylation of PI3K, Btk, PLCγ1/2, and Akt was suppressed by treating RBL-2H3 cells with UCAE, although that of Lyn and Syk was not affected. Especially, the phosphorylation level of PI3K was significantly decreased. Because PI3K is located on the signaling pathways upstream of Btk, PLCγ1/2, and Akt, these results implied that the suppressive effect of UCAE on degranulation of RBL-2H3 cells seems to be caused by inhibited phosphorylation of PI3K.
Fig. 5.

Effect of UCAE on the signaling pathways involved in degranulation of RBL-2H3 cells. Anti-DNP IgE-sensitized cells were treated with UCAE (3.2 mg/mL) or with 5 mM NaPB and stimulated with antigen for 5 min. Each cell lysate was then used for immunoblot analysis. a p-Lyn, p-Syk, p-PI3K, p-Btk, p-PLCγ1, p-PLCγ2, and p-Akt indicate phosphorylated Lyn, phosphorylated Syk, phosphorylated PI3K, phosphorylated Btk, phosphorylated PLCγ1, phosphorylated PLCγ2, and phosphorylated Akt, respectively. A representative blot from three independent experiments is shown. b The ratio of phosphorylation in each cell lysate relative to that in the control cells. Gray bars indicate NaPB-treated cells not stimulated with antigen; open bars indicate NaPB-treated cells stimulated with antigen as a control; closed bars indicate UCAE-treated cells stimulated with antigen. Data are represented as the mean ± SD of three independent experiments. Dunnett’s test was used to assess the statistical significance of the deference (*P < 0.05; **P < 0.01) against control
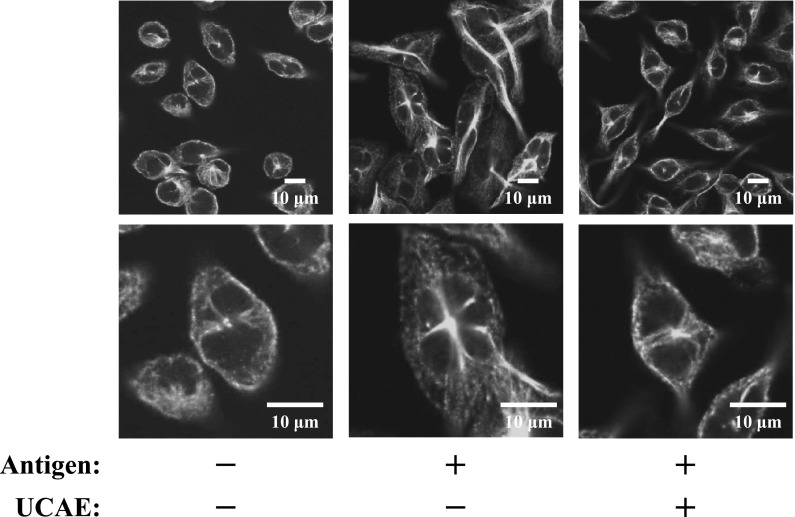
Effect of UCAE on microtubule formation
Microtubule formation is a crucial process for degranulation. Thus, we evaluated the effect of UCAE on microtubule formation by immunofluorescent staining of α-tubulin. As shown in Fig. 6, in the cells stimulated by the antigen, elongation of a thick and long fibrous structure was observed. On the other hand, the cells treated with UCAE had few fibrous structures as same as non-stimulated cells and tubulin length was shorter than NaPB-treated cells. Therefore, it is suggested that UCAE would suppress the degranulation by the inhibition of microtubule formation.
Fig. 6.
The effect of UCAE on microtubule formation of RBL-2H3 cells. Anti-DNP IgE-sensitized cells were treated with 3.2 mg/mL of UCAE or with 5 mM NaPB, and then degranulation was induced by stimulation with antigen for 5 min. The cells were fixed with 4% paraformaldehyde, and then the cells were blocked with PBS containing 5% BSA. After staining with Alexa Fluor 488-labeled anti-α-tubulin antibody, the cells were observed with a confocal microscope
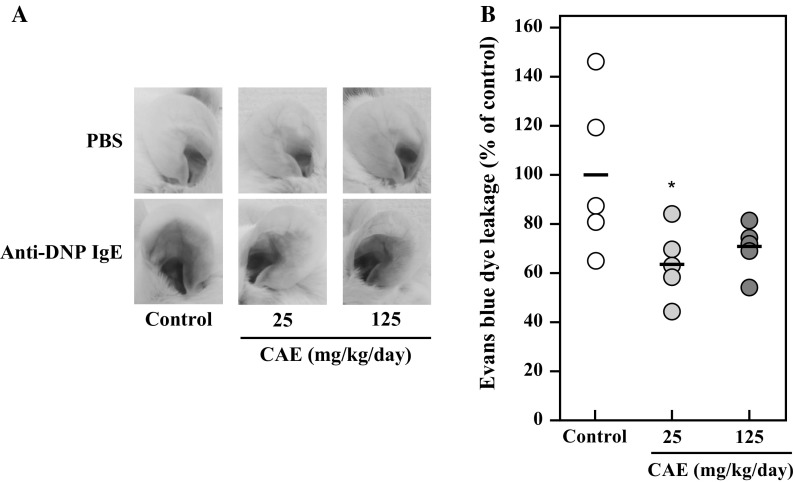
Effect of CAE on PCA reaction in mice
As described above, UCAE showed the anti-allergic effect in vitro. Therefore, the effect of cumin seed aqueous extract was evaluated in vivo. Before the in vivo experiment, the difference of the inhibitory effect of cumin seed aqueous extract with/without an ultrafiltration step on degranulation of RBL-2H3 cells was examined. The result showed that the non-ultrafiltrated extract (i.e. CAE) suppressed the degranulation as well as the ultrafiltrated extract (i.e. UCAE) (Supplementary data). Hence, CAE was used for the in vivo experiment. PCA is an in vivo model most widely used to study the anaphylactic response. Therefore, the effect of orally administered CAE on the PCA reaction in BALB/c mice was investigated. According to the schedule shown in Fig. 1, two different doses of CAE were orally administered for seven consecutive days, and the PCA reaction was induced. Figure 7a shows that Evans blue dye leakage at ears is suppressed by oral administration of CAE. Figure 7b represents the percentage of dye leakage against control, and the significant difference was observed at 25 mg/kg body weight/day of CAE. This result suggested that the cumin seed aqueous extract exhibits the anti-allergic effect in vivo as well as in vitro.
Fig. 7.
Effect of oral administration of CAE on IgE-mediated passive cutaneous anaphylaxis model mice. Mice were orally administered 10 mM NaPB as a control or CAE (25 mg/kg body weight/day or 125 mg/kg body weight/day) for seven consecutive days. Twenty-three hours before the final oral administration, the left and right ears were intradermally injected with anti-DNP IgE and PBS, respectively. Twenty-four hours after the intradermal injection, mice were intravenously injected with 200 μg of DNP-HSA diluted in PBS containing 0.5% Evans blue. After 30 min, the ears of mice were evaluated. a A representative ear picture from each group is shown. b Evans blue dye was extracted from each ear, and the absorbance of the dye was measured at 620 nm. The absorbance of the dye extracted from the left ear was subtracted with that from the right ear. Lines indicate the average of the dye leakage in each group (n = 5). Dunnett’s test was used to assess the statistical significance of the deference (*P < 0.05) against control
Discussion
In this study, we found that the cumin seed aqueous extract has an anti-allergic effect. UCAE downregulated phosphorylation of PI3K and Btk, resulting in inhibition of elevation in [Ca2+]i and of microtubule formation; however, there are several points that need to be clarified. The first question is that what component in the cumin seed aqueous extract is involved in the degranulation-suppressive effect. UCAE used in this study was extracted with NaPB and the substances larger than 3000 Da were removed by ultrafiltration. In addition, heat treatment did not affect the inhibitory effect of UCAE on degranulation (Fig. 3). From these facts, it is suggested that the bioactive component in the cumin seed aqueous extract is a heat-stable and water-soluble substance with the molecular weight lower than 3000 such as a phenolic glycoside, peptide, or low-molecular-weight polysaccharide. Actually, there are some papers reporting that the substances shown above have the anti-allergic effect (Kim et al. 2008a; Shimoda and Hamada 2010; Tomochika et al. 2011).
The second question is how the cumin seed aqueous extract inhibits degranulation. As mentioned in the introduction, there are two degranulation-signaling pathways: calcium-dependent Lyn-Syk-LAT pathway and calcium-independent Fyn-Gab2-PI3K pathway (Nishida et al. 2005; Kraft and Kinet 2007; Gilfillan and Rivera 2009). Aggregation of FcεRI by binding antigen through IgE activates the Src family non-receptor tyrosine kinases such as Lyn and Fyn. The activated Lyn provokes phosphorylation of Syk, which induces phosphorylation of LAT. The activated LAT recruits several proteins containing PLCγ and forms a complex (Turner and Kinet 1999; Silverman et al. 2006; Tan et al. 2017). In addition, Btk activated by Lyn phosphorylates PLCγ. Phosphorylated PLCγ catalyzes the hydrolysis of phosphatidylinositol bisphosphate (PIP2) to yield inositol trisphosphate, which liberates intracellular Ca2+, and diacylglycerol, which activates protein kinase C (Cho et al. 2004; Kraft and Kinet 2007). The activation causes granule-membrane fusion (Woska and Gillespie 2012). In parallel, the activated Fyn phosphorylates the adaptor protein Gab2 that activates PI3K (Gu et al. 2001; Parravicini et al. 2002). PI3K catalyzes the phosphorylation of PIP2 to phosphatidylinositol trisphosphate (PIP3) (Koyasu 2003; Kim et al. 2008b). The activation via PI3K causes the microtubule formation and induces granule translocation (Nishida et al. 2005; Oka et al. 2005; Miyata et al. 2008). Moreover, PIP3 activates Akt that is involved in degranulation (Takayama et al. 2013). Activation of these two pathways results in degranulation. As shown in Fig. 5, UCAE suppressed the phosphorylation of PI3K, Btk, PLCγ1/2, and Akt although not affecting the phosphorylation of Lyn or Syk. In addition, UCAE inhibited the increase in [Ca2+]i (Fig. 4) and microtubule formation induced by antigen stimulation (Fig. 6). These results suggested that UCAE has the suppressive effect on the activation of both pathways by downregulated phosphorylation of PI3K and Btk involved in degranulation (Fig. 8). Because the bioactive component in UCAE is water-soluble, it would not be easily penetrated into the cell. Thus, it is assumed that UCAE is involved in the upstream events before PI3K activation, such as Fyn activation, through immunoreceptor tyrosine-based inhibition motif (ITAM) on FcεRI. Further investigations are required to clarify the mechanism underlying the inhibitory activity of UCAE on degranulation.
Fig. 8.

The scheme of inhibitory effect of the cumin seed aqueous extract on degranulation of RBL-2H3 cells
Consecutive oral administration of CAE for 7 days inhibited the PCA reaction in mice as shown in Fig. 7; however, a single oral administration of CAE did not (data not shown). The results suggested that continuous ingestion of CAE is necessary to obtain the suppressive effect on PCA reaction. In addition, the higher dose of CAE (125 mg/kg body weight/day) strongly tended to suppress the PCA reaction (P = 0.0855). There might be substances that impede the inhibition of allergic responses in the high-dose condition or some CAE metabolites may impede the inhibition of allergic responses.
Because cumin is known to contain some ingredients with an adverse effect such as allergen, it seems necessary to devise a method such as extraction of bioactive components or removal of substances with an adverse effect when cumin seeds are used as functional foods.
Conclusion
Although a large number of studies have been made on hydrophobic substances in cumin seeds, no anti-allergic effect of hydrophilic substances in cumin seeds has been reported so far. Therefore, this is the first report describing the anti-allergic effect of cumin seed aqueous extract. UCAE significantly suppressed the degranulation of RBL-2H3 cells through the inhibition of signaling cascade via FcεRI. In addition, consecutive oral administration of CAE inhibited PCA reaction in mice. From these results, we conclude that the cumin seed has potential as an anti-allergic functional food.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by S&B Foods Inc. Animal experiments and fluorescence microscopic observation were accomplished at the Division of Genetic Research of the Advanced Research Support Center (ADRES), Ehime University.
Compliance with ethical standards
Conflict of interest
Hiroyuki Onda and Takuya Sugahara are inventors in a patent application filed by Ehime University, which disclose bioactive agents targeting mast cell degranulation described in the present article. The remaining authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Snafi AE. The pharmacological activities of Cuminum cyminum—a review. IOSR J Pharm. 2016;6:46–65. [Google Scholar]
- Bag A, Chattopadhyay RR. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE. 2015;10:e0131321. doi: 10.1371/journal.pone.0131321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Woo CH, Yoon SB, Kim JH. Protein kinase Cδ functions downstream of Ca2+ mobilization in FcεRI signaling to degranulation in mast cells. J Allergy Clin Immunol. 2004;114:1085–1092. doi: 10.1016/j.jaci.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Saito K, Klaman LD, Shen J, Fleming T, Wang Y, Pratt JC, Lin G, Lim B, Kinet JP, Neel BG. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- Iacobellis NS, Lo Cantore P, Capasso F, Senatore F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J Agric Food Chem. 2005;53:57–61. doi: 10.1021/jf0487351. [DOI] [PubMed] [Google Scholar]
- Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TAE, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim Y, Kim HY, Ro JY, Jeoung D. Inhibitory mechanism of anti-allergic peptides in RBL2H3 cells. Eur J Pharmacol. 2008;581:191–203. doi: 10.1016/j.ejphar.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Kim MS, Rådinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- Miyata N, Gon Y, Nunomura S, Endo D, Yamashita K, Matsumoto K, Hashimoto S, Ra C. Inhibitory effects of parthenolide on antigen-induced microtubule formation and degranulation in mast cells. Int Immunopharmacol. 2008;8:874–880. doi: 10.1016/j.intimp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, Yamamoto T, Yagi T, Hirano T. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Hori M, Ozaki H. Microtubule disruption suppresses allergic response through the inhibition of calcium influx in the mast cell degranulation pathway. J Immunol. 2005;174:4584–4589. doi: 10.4049/jimmunol.174.8.4584. [DOI] [PubMed] [Google Scholar]
- Pandey S, Patel MK, Mishra A, Jha B. Physio-biochemical composition and untargeted metabolomics of cumin (Cuminum cyminum L.) make it promising functional food and help in mitigating salinity stress. PLoS One. 2015;10:e0144469. doi: 10.1371/journal.pone.0144469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- Rebey IB, Zakhama N, Karoui IJ, Marzouk B. Polyphenol composition and antioxidant activity of cumin (Cuminum cyminum L.) seed extract under drought. J Food Sci. 2012;77:C734–C739. doi: 10.1111/j.1750-3841.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Hamada H. Enzymatic synthesis and anti-allergic activities of curcumin oligosaccharides. Biochem Insights. 2010;3:1–5. doi: 10.4137/BCI.S2768. [DOI] [Google Scholar]
- Silverman MA, Shoag J, Wu J, Koretzky GA. Disruption of SLP-76 interaction with Gads inhibits dynamic clustering of SLP-76 and FcεRI signaling in mast cells. Mol Cell Biol. 2006;26:1826–1838. doi: 10.1128/MCB.26.5.1826-1838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama G, Ohtani M, Minowa A, Matsuda S, Koyasu S. Class I PI3K-mediated Akt and ERK signals play a critical role in FcεRI-induced degranulation in mast cells. Int Immunol. 2013;25:215–220. doi: 10.1093/intimm/dxs105. [DOI] [PubMed] [Google Scholar]
- Tan JW, Israf DA, Md Hashim NF, Cheah YK, Harith HH, Shaari K, Tham CL. LAT is essential for the mast cell stabilising effect of tHGA in IgE-mediated mast cell activation. Biochem Pharmacol. 2017;144:132–148. doi: 10.1016/j.bcp.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Tomochika K, Shimizu-Ibuka A, Tamura T, Mura K, Abe N, Onose J, Arai S. Effects of peanut-skin procyanidin A1 on degranulation of RBL-2H3 cells. Biosci Biotechnol Biochem. 2011;75:1644–1648. doi: 10.1271/bbb.110085. [DOI] [PubMed] [Google Scholar]
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor FcεRI. Nature. 1999;402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhang X, Bi Y, Miao R, Zhang Z, Su H (2015) Anti-inflammatory effects of cumin essential oil by blocking JNK, ERK, and NF-κB signaling pathways in LPS-stimulated RAW 264.7 cells. Evid Based Complement Alternat Med Vol. 2015, Article ID 474509, 8 pages 10.1155/2015/474509 [DOI] [PMC free article] [PubMed]
- White M. Mediators of inflammation and the inflammatory process. J Allergy Clin Immunol. 1999;103:S378–S381. doi: 10.1016/S0091-6749(99)70215-0. [DOI] [PubMed] [Google Scholar]
- Woska JR, Jr, Gillespie ME. SNARE complex-mediated degranulation in mast cells. J Cell Mol Med. 2012;16:649–656. doi: 10.1111/j.1582-4934.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.